- 1Institute for the Study of Nanostructured Materials, National Research Council, Rome, Italy
- 2Departamento Académico de Física del Estado Sólido, Universidad Nacional Mayor de San Marcos, Lima, Peru
- 3Museo Señora de Cao and Fundacion Wiese, Trujillo, Peru
- 4Surface Analysis Laboratory, Istituto Nazionale di Fisica Nucleare (INFN) & Department of Mathematics and Physics, Roma Tre University, Rome, Italy
A large number of metal artifacts with exceptional artistic value of the Moche culture have been found in the tombs of the Lords of Sipán (Lambayeque, Peru) and of the Lady of Cao (El Brujo, Peru) characterized by different burial conditions. Some of the objects, dated around 300–400 AD, are constituted by substrates of Cu- or Ag-based alloys coated by uniformly distributed thin films of precious metal (1–4 microns) that create also polymetallic bicolored surfaces with “gold” and “silver” areas. In order to investigate the corrosion product structure and composition as well as to identify the techniques used to give the gold or silver appearance, an integrated analytical approach has been adopted. The selected complementary methodologies were scanning electron microscopy (SEM) coupled with energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and optical microscopy (OM). The findings reveal that the substrates are mainly composed of Cu-Ag-Au alloys that at the site of Sipán have been almost completely corroded during the burial. Furthermore, the results show that the main aggressive agent is Cl– coming from the soil and that the degradation phenomena were likely enhanced by the galvanic coupling between the precious metal layer and the less noble substrate. The degradation products have formed mainly layered structures containing chloroargyrite (AgCl), cuprite (Cu2O), nantokite (CuCl), and atacamite [CuCl2.3Cu(OH)2] polymorphs. These latter species warn that dangerous copper cyclic corrosion is occurring, a harmful phenomenon, commonly defined as “bronze disease,” which must be firmly mitigated. Finally, the findings reveal that the Moche metal workers used the depletion gilding to selectively modify the surface chemical composition of the artifacts to produce the Ag or Au thin films. According to this subtractive method, the surface of the Cu-Au-Ag alloys was enriched with a layer of precious metal by means of cycles of thermal treatments and removal of Cu or both Cu and Ag from the outermost region by using pickling solutions.
Introduction
In 1987, great treasures composed of spectacular metal artifacts were discovered in the unlooted tombs of the Lords of Sipán (Huaca Rajada, Lambayeque, Peru), and in 2006, in the tomb of the Lady of Cao (El Brujo, Trujillo, Peru). These sites are characterized by different burial conditions that have influenced the long-term corrosion behavior. Indeed, the artifacts found at Sipán are extensively corroded, while the objects found at El Brujo are generally in a good state of preservation. The extraordinary artifacts found there, mainly jewels and ceremonial ornaments, were produced by the metal smiths of the Moche culture that flourished on the northern coast of present-day Peru between about 0 and 600 AD. The discovery of these amazing artifacts confirms that the Moche metal workers were sophisticated pre-Columbian producers of unequaled metal objects. Some of them show a bicolored aspect since characterized by a polymetallic surface with thin “gold” and “silver” areas likely achieved by using manufacturing methods able to precisely manipulate the surface chemical composition at a nanoscale dimension.
In order to investigate the microchemical surface and bulk structure and the degradation products formed during the long-term burial, we have adopted an integrated analytical approach by using complementary methodologies such as scanning electron microscopy (SEM) combined with energy-dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), and optical microscopy (OM).
It is worth noting that the interaction between these polymetallic (Ag-Cu-Au) artifacts and the soil constituents has lasted many hundred years, and the information on the resulting products could be of great interest in the study of long-term environmentally driven degradation phenomena of polymetallic materials, phenomena that until now have been investigated by few authors (Scott, 1986, 1998; Hörz and Kallfass, 2000; Ingo et al., 2013a, b).
Our effort was also aimed to gain further insights into methods used by the Moche metallurgists to modify the surface chemical composition (Lechtman, 1971, 1979; Lechtman et al., 1982; Lechtman, 1984a, b; Scott, 1986, 1998, 2000; Hörz and Kallfass, 1998, 2000). We point out that the selected artifacts were not objects of common use but ornaments for an elite exhibiting power and rank. They include high-status ceremonial objects and spectacular nose ornaments, i.e., the narigueras, large jewels to be hooked to the nostrils. These artifacts clearly demonstrate the outstanding virtuosity of the Moche artisans and the impressive quality of their metal production as well as reflect the most sophisticated metal production techniques of that period in the New World.
Experimental Details
Archeological Artifacts
The archeological artifacts to be analyzed have been selected by the archeologists. The samples are mainly fragments detached from the artifacts likely being crushed by pressure from the soil sediments during the long-term burial or small parts accidentally broken during the cleaning and conservation procedures. For XRD measurements, low quantities of patina have been also carefully removed from the objects and properly ground. The samples were subjected to metallographic, microchemical, and structural investigations in as-received conditions by means of the abovementioned analytical techniques. Furthermore, some fragments also have been cross-sectioned to study the structure of the corrosion products and of the thin Ag or Au surface layers. In order to prepare the cross sections, representative shards have been embedded in a suitable two-component resin (curing time: 1 day) and sectioned by using a diamond saw able to retain the outermost layer’s features and the bulk microchemical structure (Ingo et al., 2013a). The polishing procedure was performed by using silicon carbide papers until 1,200 grit, while the final treatment was carried out with diamond pastes up to 0.1 μm.
Scanning Electron Microscopy – Energy-Dispersive Spectroscopy Analysis
The surface morphology and microchemical structure have been investigated by means of a thermionic scanning electron microscope (SEM) Stereoscan 360 (Cambridge, United Kingdom) and a high-spatial resolution LEO 1530 field emission scanning electron microscope (FE-SEM). SEM and FESEM instruments are equipped with an INCA 250 and INCA 450 energy-dispersive spectrometer (EDS), respectively, and back-scattered electron (BSE) detectors. SEM investigations were performed by using both secondary (SE) and BSE electrons and selecting an acceleration voltage of 20 kV, while the FE-SEM characterizations were carried out by varying the acceleration voltages from 1 to 20 kV in order to better disclose the micromorphological features.
In order to investigate the powered materials, a suitable double-faced adhesive carbon tape was used to collect and fix the matter. These carbon tapes are commonly and conveniently used for SEM-EDS microchemical investigations (Ingo et al., 2001). In order to avoid charging effects induced on the sample by the electron beam, the surfaces of the samples were coated with a thin layer of Cr or C, deposited by using a Bal-Tech SCD 500 equipment at a pressure of about 5 × 10–3 mbar to achieve a conductive and uniform film, a few nanometers thick.
X-Ray Diffraction Investigation
XRD investigations were carried out by analyzing directly the artifact surfaces or the small amount of powdered patina by using a Siemens 5000 X-ray powder diffractometer with Ni-filtered Cu Kα radiation (λ = 0.154056 nm). The following experimental parameters were selected: angular values between 10 and 90° in additive mode, step size 0.05°, and sampling time of 20 s. XRD patterns were analyzed by using specific electronic databases and compared with the literature information.
Optical Microscopy Study
OM morphological studies were performed by using a Leica MZFLIII and a Leica Application Suite (LAS) multifocus stereo microscope. Furthermore, at the Museum of the Lady of Cao, a Zeiss optical microscope equipped with a digital camera was used for in situ morphological investigation. The metallurgical features of the cross-sectioned artifacts have been studied by means of a Leica MEF IV optical microscope.
X-Ray Photoelectron Spectroscopy
The XPS technique has been used to ascertain the surface possible presence of alloying elements such as As or Sn (Lechtman and Klein, 1999). Measurements were carried out by using an ESCALAB Mk II spectrometer equipped with a hemispherical electron energy analyzer and a twin anode X-ray source emitting not monochromatized Al Kα and Mg Kα radiations, 1,486.6 and 1,253.6 eV, respectively. The binding energy (BE) accuracy has been measured to be ± 0.1 eV. The values of the BEs of the Au 4f7/2 signal from Ar+-cleaned Au 99.99%, of the Zr 3d5/2 peak from zirconia, and of Sn 3d5/2 peak from cassiterite (SnO2) were 84.0, 182.3, and 487.0 eV, respectively (Paparazzo et al., 1988; Ingo et al., 1992; Ingo and Padeletti, 1994; Curulli et al., 2005). In order to avoid, or reduce, any eventual sample damage potentially provoked by the x-ray irradiation during the XPS measurements, short acquiring times were imposed and a copper sample holder cooled by liquid N2 was used (Schlesinger et al., 2000). Other experimental details are reported elsewhere (Ingo et al., 2000, 2002).
Results and Discussion
The paper presents some highlighting cases of study describing the microchemical features of long-term buried Cu-Ag-Au alloy artifacts whose outermost chemical composition was intentionally modified to achieve a local accentuated Ag or Au surface amount. This latter in some cases formed polymetallic bicolored surfaces with “gold” and “silver” areas likely for symbolic, shamanic, or religious reasons.
The aim of the ancient goldsmiths was to give to the objects the appearance of solid precious metal or to create an elaborate and fascinating specific visual effect combining the color of different metals (Oddy, 1981, 1990; Raub, 1986a, b; Schorsch, 1988; Bray, 1993; Merkel et al., 1995; Hörz and Kallfass, 1998, 2000; Grimwade, 1999; Centeno and Schorsch, 2000; Ingo et al., 2013a).
The first case describes the surface and bulk microchemical and morphological features of a gilded semicircular diadem, an owl crown, almost completely mineralized) found in the tomb T15 at Sipán (Peru) where a young warrior was interred.
The OM images and the XRD pattern reported in Figure 1 show areas of the bare original gilded surface embedded in light green-blue corrosion products, i.e., the patina. This structure suggests to the conservators to pay a great deal of attention during the cleaning procedure to avoid the loss of the thin gold layer that could be peeled away (Scott, 1990, 1991, 2002; Ingo et al., 2013b).
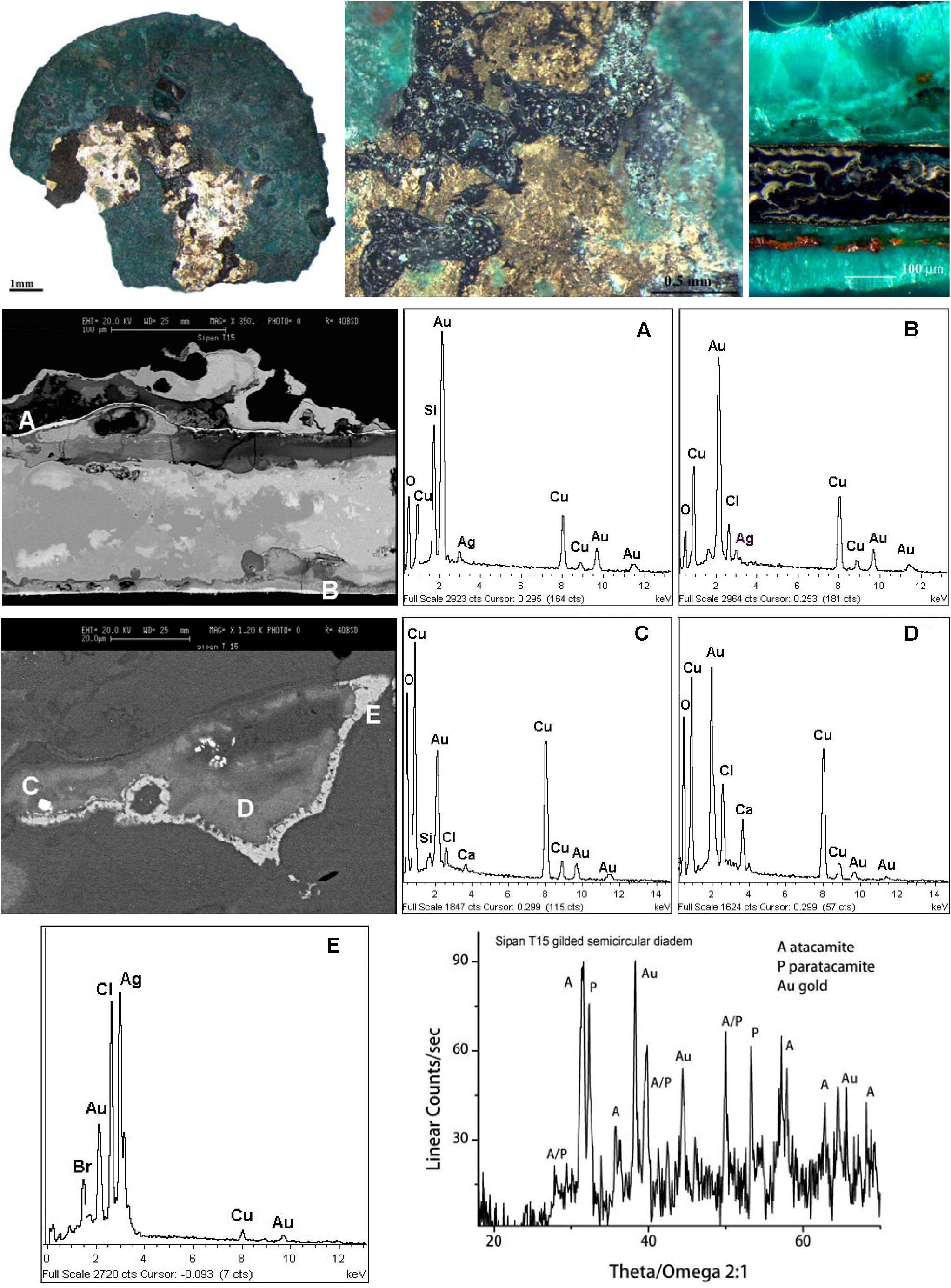
Figure 1. SEM, EDS (spectrum A–E), OM and XRD results describing the micro-chemical of a fragment of a gilded semicircular diadem (tomb T15 of a young warrior, Sipán). OM images and XRD pattern show the green patina composed of acicular atacamite [Cu2Cl(OH)3] with areas of the bare original gilded surface. The OM image discloses also layered particles of reddish cuprite (Cu2O) and yellow-orange nantokite (CuCl).
The results reveal that the corrosion products are mainly constituted by atacamite and its polymorph paratacamite [basic copper oxy-chloride, Cu2Cl(OH)3], thus demonstrating that the main aggressive agent coming from the burial environment is chlorine. Furthermore, the findings show the presence of a layer or randomly scattered particles of reddish cuprite (Cu2O) and yellow-orange nantokite (CuCl) inside the patina.
Since the presence of atacamite corrosion products in bronze archeological objects is a symptom of the detrimental “bronze disease” degradation phenomenon, great attention must be paid to avoid its insurgence and development. This relentless and cyclic corrosion process is caused by the presence of chlorine in the burial environment that corrodes copper forming nantokite (Scott, 1990, 1991, 2002; Bastidas et al., 2010). When this latter specie is exposed to moisture and oxygen, it gives rise to the formation of atacamite or its polymorphs [2Cu2(OH)3Cl] and hydrochloric acid which in turn reacts with copper to form new nantokite in a cyclic and self-sustaining process that converts copper in atacamite.
As a consequence of this phenomenon, the surface of the object is transformed into a light greenish powder, thus inducing the progressive loss of its original form and integrity (Ingo et al., 2013b). At present, different conservation materials and methods are used to hinder the degradation process. Unfortunately, some of these procedures are based on the use of toxic inhibitors (Stupnišek-Lisac et al., 1998; Antonijevic and Petrovic, 2008) but tailored innovative solutions based on nanostructured materials are emerging for bronze conservation and cleaning similar to those used to stop degradation of other classes of ancient works of art (Chelazzi et al., 2014; Baglioni et al., 2015; Poggi et al., 2016).
In order to monitor the presence of Pb, Sn, or As also as surface enrichment (Lechtman and Klein, 1999) from the Cu-Ag-Au substrate caused by corrosion phenomena, we have used the XPS technique (Ingo et al., 2002, 2013b; Hayez et al., 2004). The results (not shown) reveal that neither Pb nor As is present in the metallic state or as oxides and further exclude the presence in the corrosion products of cassiterite (SnO2) or romarkite (SnO) (Paparazzo et al., 1988; Ingo et al., 1992), thus suggesting the absence of As, Pb, and Sn in the Cu-Ag-Au alloy.
Other information is given by the SEM images and EDS spectra of the cross-sectioned fragment shown in Figure 1 that describe the microchemical structure of the artifact (~0.2 mm thick). The object is almost completely mineralized, and the only survived pristine metal is the thin gold layer originally on the surface and now almost entirely embedded in the Cu corrosion products.
The gold layer (2–3 μm thick; Figure 1 and Supplementary Figure S1) contains a low amount of silver and copper and is uniformly distributed over the entire surface on both sides of the hammered sheet substrate marking the original surface of the object.
The presence of Au and Ag is well documented also in the inner part of the object as shown by the BSE SEM images and the EDS spectra of the light gray areas scattered everywhere inside the mineralized substrate. These local enrichments of precious metals indicate the use of a “tumbaga” Cu-based ternary alloy containing an appreciable content of gold and a small amount of silver.
As a consequence of the long-term interaction with the surrounding environment, silver has reacted with chlorine from the soil and it has been transformed in chlorargyrite (AgCl), while gold has formed small metallic nanoparticles distributed in the mineralized substrate. Furthermore, EDS spectra confirm the absence of Pb, As, and Sn in the substrate as already documented by XPS for the external surface (Lechtman and Klein, 1999).
The severe corrosion process occurring during the long-term burial does not allow determining precisely the chemical composition of the tumbaga Ag-Cu-Au ternary alloy (Twilley and Boyles, 1981). Therefore, it cannot be possible to determine the relative proportions of metals which constitute the alloy substrate. However, the gold content may be estimated to be around 10–15 weight percent (hereafter wt%).
It is worth noting that the corrosion of copper is likely enhanced by the presence of the precious metal layer since a relevant degradation driving force could be the difference between the electrochemical potentials of Cu and Au or Ag along with the soil characteristics (pH, humidity, presence of aggressive ions) that could increase the transformation of Cu and Ag into minerals (Ingo et al., 2013a, b).
The severe alteration phenomena could have been probably exacerbated by the galvanic corrosion, also known as dissimilar metal corrosion or bimetallic corrosion. This latter destructive reaction is an electrochemical process occurring when two or more metals are in contact in the presence of an electrolyte and a corrosive environment.
Under these conditions, the less noble metal acts as an anode and is preferentially corroded at an accelerated rate if compared with the uncoupled condition (Ingo et al., 2013a, b). On the contrary, the other more noble metal acts as a cathode and remains protected until the less noble metal is completely transformed in corrosion products. As mentioned above, the extent of corrosion depends on the difference between the electrochemical potentials of the involved metals and makes gilded-metal artworks particularly unstable from a chemico-physical and structural point of view.
Our findings suggest that the electrochemical oxidation–reduction process could have enhanced the degradation of these polymetallic objects having created a galvanic cell formed by the precious metal layer connected to the substrate with water and dissolved ions that provide a means for ion and electron migration (Ingo et al., 2013a, b).
Concerning the manufacturing techniques used by the Moche metallurgists, the above shown results exclude the application of a thin gold film via mechanical or metallurgical methods and suggest the use of the gilding process to remove copper from a tumbaga substrate to create an Au-looking surface. According to this bottom-up subtractive method, an artifact appearance could be substantially modified, rendering the objects golden or silvery even if the amount of Au or Ag in the alloy was relatively low.
As described by other scholars, the depletion gilding is based first on the surface oxidation of Cu from the near-surface region that is favored as compared to Au or Ag (Lechtman, 1971, 1979; Forty, 1979; Forty and Durkin, 1980; Lechtman et al., 1982; Lechtman, 1984a, b; Scott, 1986, 1998, 2000; Hörz and Kallfass, 1998, 2000; Sáenz-Samper and Martinón-Torres, 2017).
The removal of Cu oxides was carried out by immersing the oxidized artifact in a poultice of an acid plant juice containing likely other appropriate corrosive chemical compounds that progressively dissolved copper oxides from the surface and created a fine outermost layer of precious metal. In this way, an Ag or Au spongy crust on the surface was formed and then burnished, leaving a brilliant finish.
The success of the process depends mainly on the skill of the artisans that empirically selected not only the alloy chemical composition but also the pickling solutions (Forty, 1979; Forty and Durkin, 1980; Lechtman et al., 1982; Hultquist, 1985; Guisbiers et al., 2014).
The artifacts found at Sipán and Trujillo demonstrate that the Moche artisans were able to effectively control the selective removal of copper oxides (and sometimes silver) from the surface, creating a homogeneous distribution of Au or Ag along the surface (Ingo et al., 2013a, b). This ability was based only on the long-term cognitive skill of the Moche metal workers who realized that an alloy surface could be modified by empirically optimized treatments and that unique symbolic and esthetic features could be achieved.
It is worth pointing out that the aim of the Moche metallurgists was not fraudulent as frequently observed in the Old World (Lins and Oddy, 1975; Lechtman, 1979, 1984a, b; Scott, 1986, 1998, 2000; Schorsch, 1988; Oddy, 1991; La Niece, 1993; Zwicker et al., 1993; Cooke et al., 2008; Ingo et al., 2013b). Their aim was only finalized to modify color and esthetic appearance of an object combining different metals as Cu, Au, and Ag on the artifact surface, thus stimulating the development of sophisticated methods acting on a nanoscale as the electrochemical replacement plating discovered by Lechtman (1971, 1979, 1984a, 1984b) and Lechtman et al. (1982). Other techniques were also developed to alter the surface chemical composition such as the foil gilding or the fusion gilding the use of which can be determined only by examining the ancient gilt surfaces via microchemical investigations (Scott, 1986, 1998, 2000; Cooke et al., 2008).
The second case study is a gilded plaque, completely mineralized, found in the tomb T16 of a warrior found with a “Pututo,” i.e., a ceremonial trumpet produced by using a great seashell.
The high-spatial resolution SEM images shown in Figure 2 (first row) describe the morphology of the surface gold-enriched layer present on both sides, while the other SEM and EDS results allow to evaluate the internal corroded structure of the artifact.
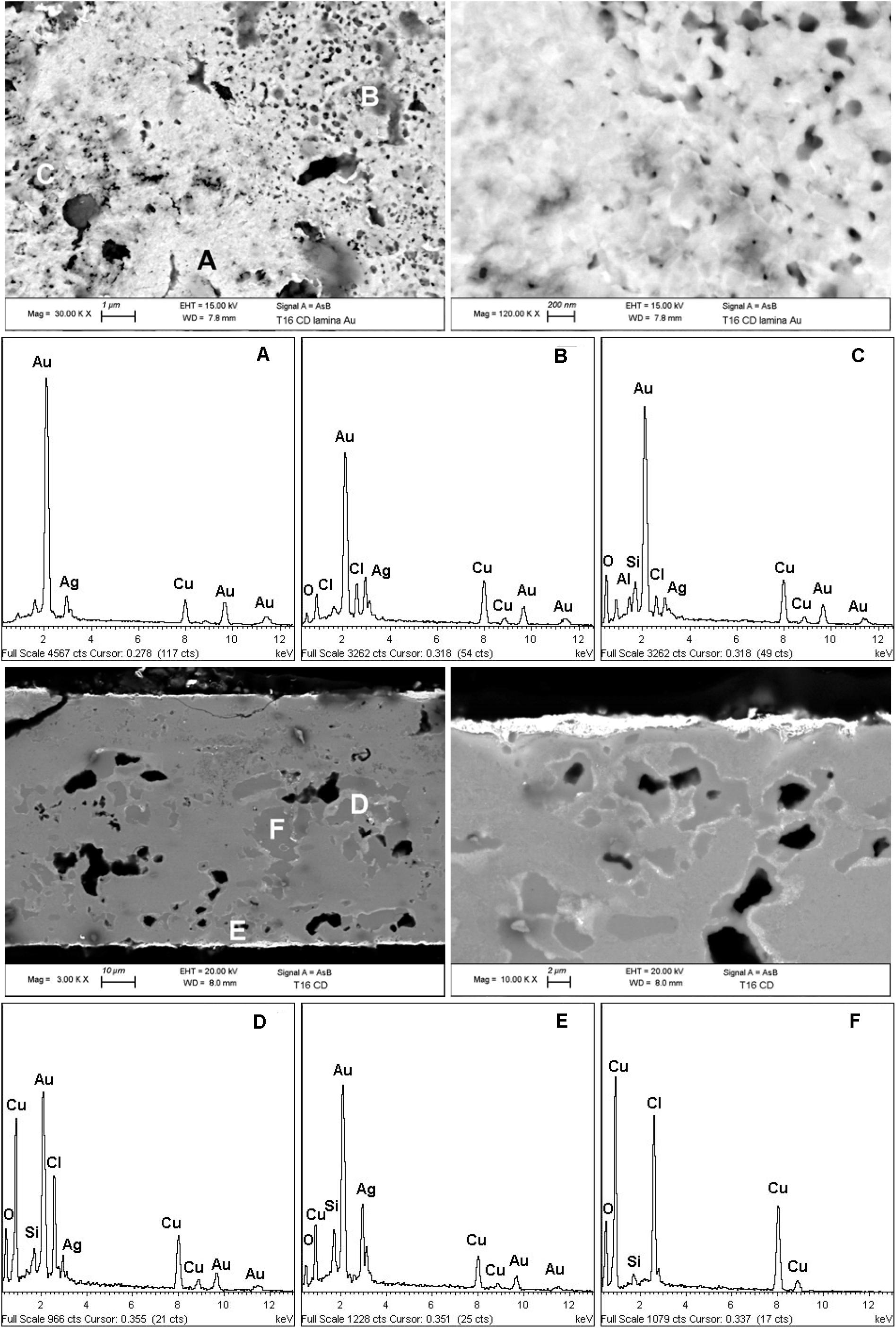
Figure 2. SEM images and the EDS spectra (A–F) of a fragment of a gilded plaque completely mineralized found in the tomb T16 of the warrior with a ceremonial trumpet called Pututo. The results describe the microchemistry and the morphology of the surface gold-enriched layer present on both sides as well as show the internal corroded structure of the artifact.
The high-spatial resolution SEM images (first row) reveal the presence of not uniformly distributed micro and nano pitlike pores on the surface of the gilding layer. The development of this morphological feature could be related to the procedures adopted for the formation and removal of oxides such as temperature and duration of thermal treatments as well as to the pickling conditions.
The thickness of the gilding layer is not uniform and ranges from about 2–4 μm and is mainly composed of Au with a consistent presence of Ag and Cu. Also for this object the Moche metalworkers used a tumbaga Cu-based alloy with gold and some silver as documented by the BSE SEM images and the EDS spectra of the cross-sectioned fragment. These results also reveal the presence of consistent amounts of Ag and Au in the light gray areas of the completely mineralized substrate and of chlorine as main degrading agent.
Another interesting object found at Sipán is a silver-plated nariguera found in the tomb T16 of the warrior with a Pututo. The SEM-EDS results shown in Figure 3 reveal that the artifact is constituted by an Ag-based alloy whose surface was deliberately enriched in silver. The SEM and OM images and EDS spectra show that it is almost completely mineralized and describe the microchemical structure of the surface silver-enriched layer as well as show the internal corroded structure of the artifact. The XRD pattern of the green copper corrosion product (third row, Figure 3) also in this artifact reveals the presence of harmful atacamite that is present also inside the artifact (see the OM image shown in the third row of Figure 3), thus confirming the role of chlorine in the corrosion process.
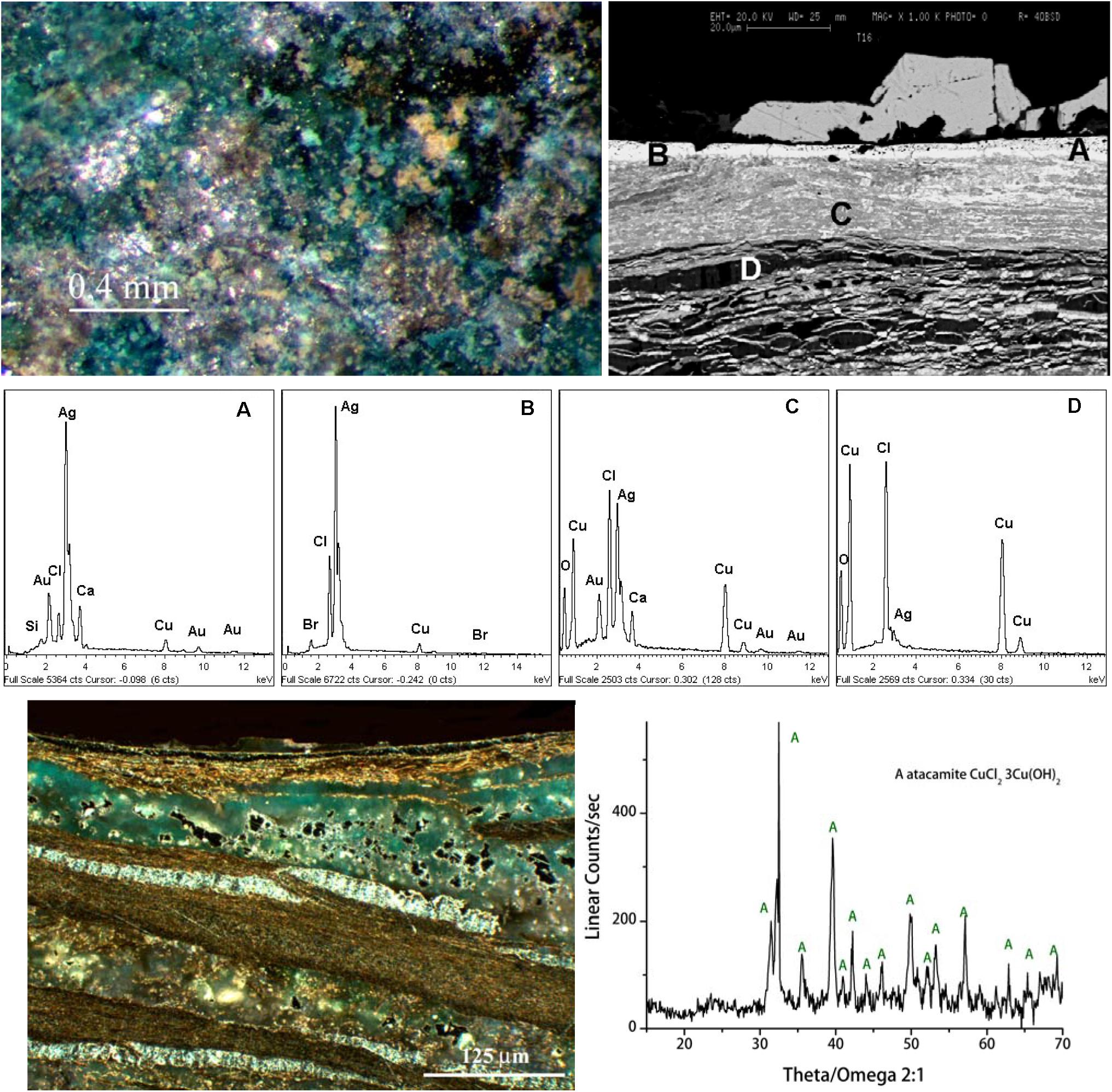
Figure 3. SEM and OM images and EDS spectra (A–D) of a fragment of a silver plated nariguera almost completely mineralized found in the tomb T16 of the warrior with a ceremonial trumpet called Pututo. The results describe the micro-chemical structure of the surface silver-enriched layer as well as show the internal corroded structure of the artifact. The XRD pattern of the green copper corrosion product (third row) reveals the presence of harmful atacamite that is present also inside the artefact (see the OM image shown in the third row).
Unfortunately, the conditions of the investigated artifacts found at Sipán do not allow the determination of detailed information on the chemical composition and metallurgical features of the Ag-Cu-Au alloys used by the Moche metallurgists; therefore, our investigations have been focused also on the well-preserved artifacts found in the tomb of the lady of Cao whose environment was different with respect to that of Sipán being desert-arid.
In particular, we have investigated some narigueras, i.e., large jewels to be hooked to the nostrils, that have been found with the mummy of the Lady of Cao; she was also well-preserved by the arid burial condition.
The narigueras are generally in a very good state of conservation and are generally characterized by well-evident “silver” and “gold” surfaces as depicted in Figures 4, 5 where the front and back of the nariguera F4-22, F4-24, and F4-03 are shown.
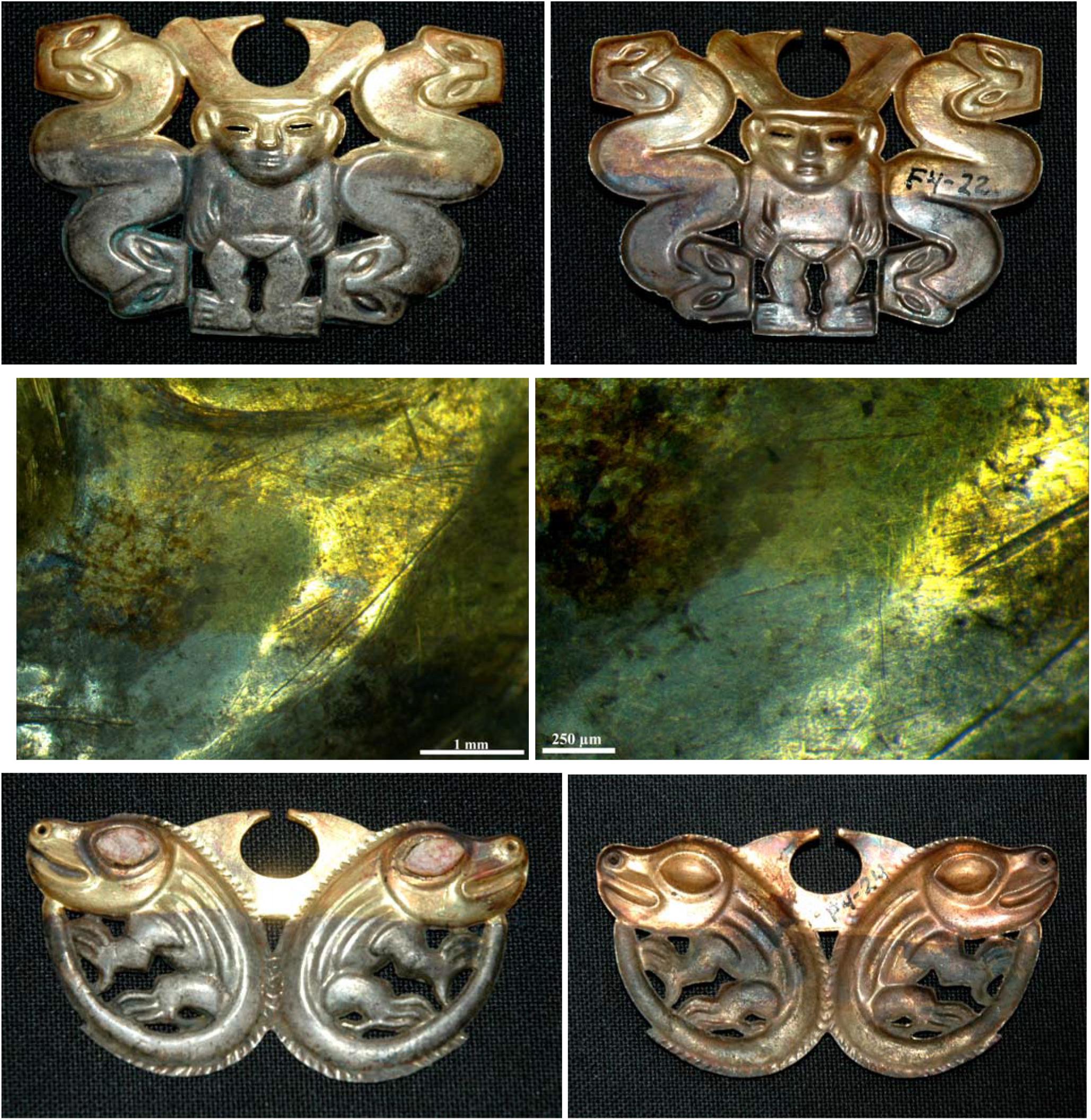
Figure 4. The front and back of the nariguera F4-22 from the tomb of the Lady of Cao and optical images of the interface between the gold- and the silver-enriched areas, on the first and second rows, respectively. In the third row is shown the front and back of the nariguera F4-24. The images of the narigueras clearly reveal the color change from the “silver” and “gold” surface.
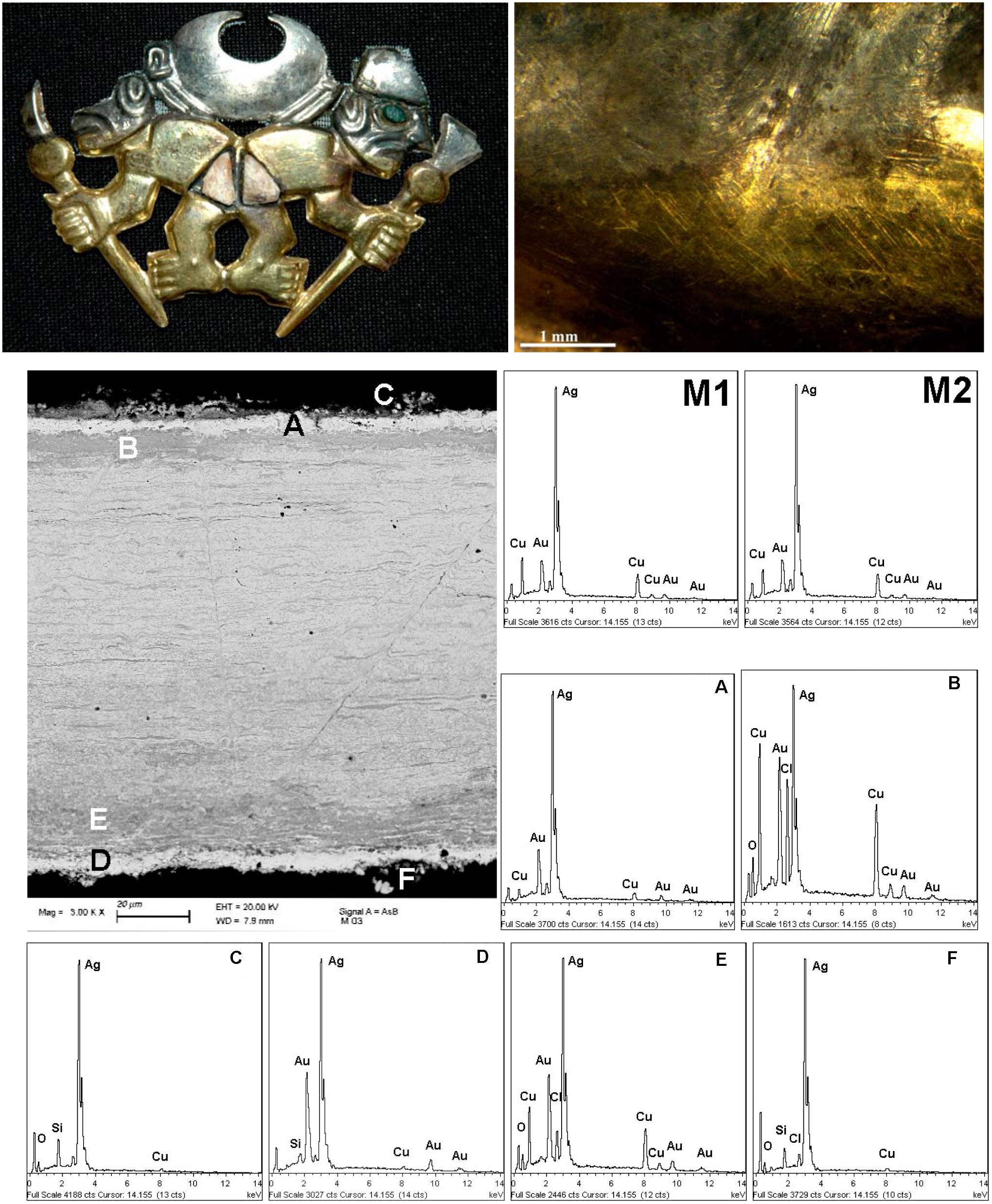
Figure 5. The nariguera F4-03 from the tomb of the Lady of Cao and an optical image the interface between the “gold” and the “silver” areas, on the left and right side of the first raw, respectively. SEM images and EDS spectra (M1, M2, A–F) of the different areas of a cross-sectioned fragment of the nose ornament taken in the silvered area of the head of the animal on the left, second and third raw, respectively. The EDS elemental semi-quantitative composition (expressed as wt%) are reported in Table 1.
Some morphological details are shown by the optical images reported in Figures 4, 5 that disclose the smooth interface between the “gold” and the “silver” areas characterized by a color change variation quite sharp without a morphological variation.
We have had the possibility to investigate in detail a small fragment detached from the “silver” area of the head of the animal on the left of the narigeura F4-03 (Figure 5). The FE-SEM images and the EDS spectra of the cross-sectioned fragment shown in Figures 5, 6 and Supplementary Figure S2 reveal the metallurgical features of the single sheet constituting the nariguera while in Table 1 are reported the EDS elemental semiquantitative compositions expressed as weight percent (wt%). These latter data reveal the use of a deliberate ternary alloy of Ag, Cu, and Au to produce the narigeura F4-003.
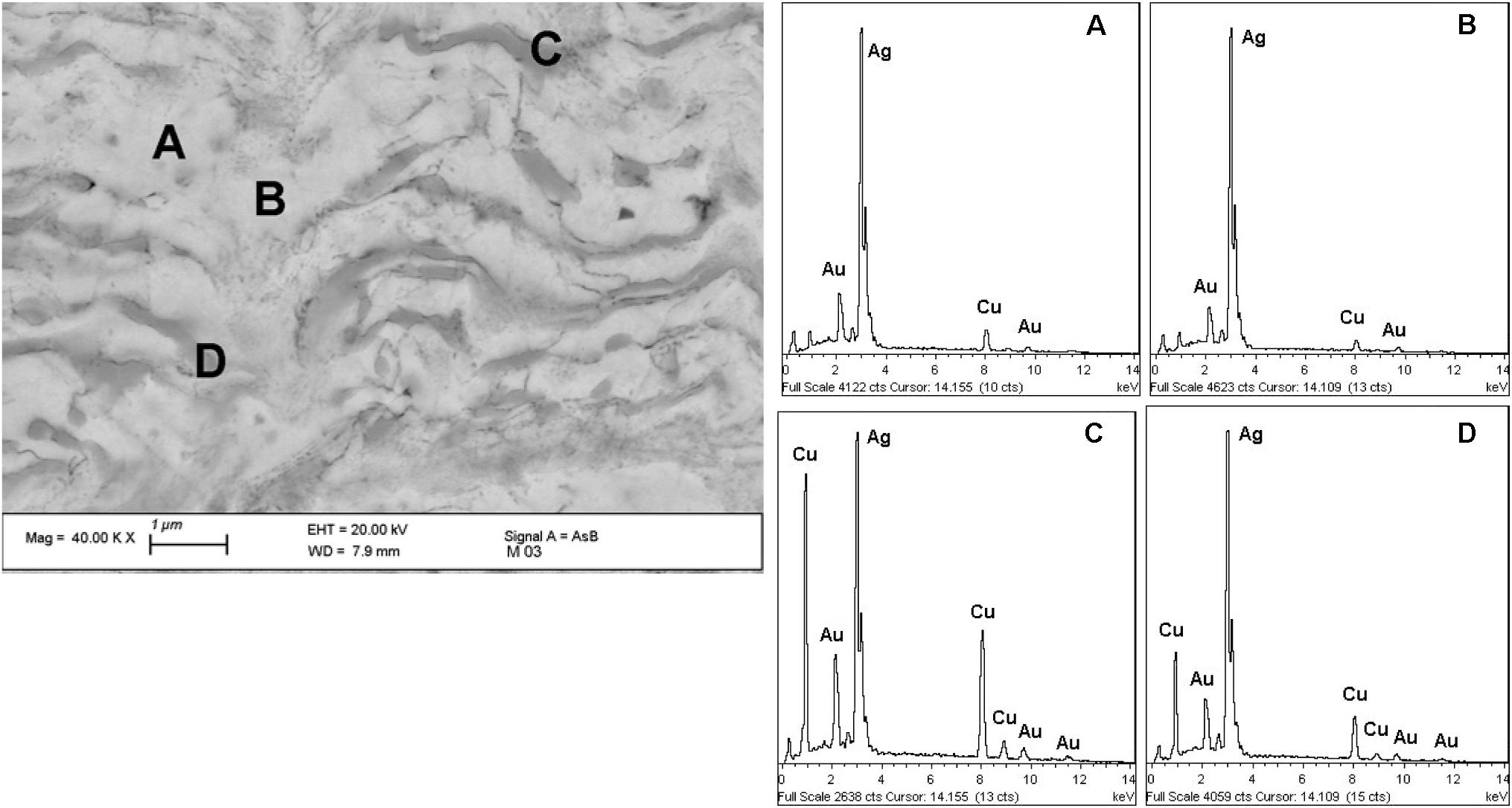
Figure 6. SEM image and EDS spectra (A–D) of the different areas of the alloy matrix of the nose ornament (nariguera) F4-03 from the tomb of the Lady of Cao. The EDS elemental semi-quantitative chemical composition (expressed as wt%) are reported in Table 2.
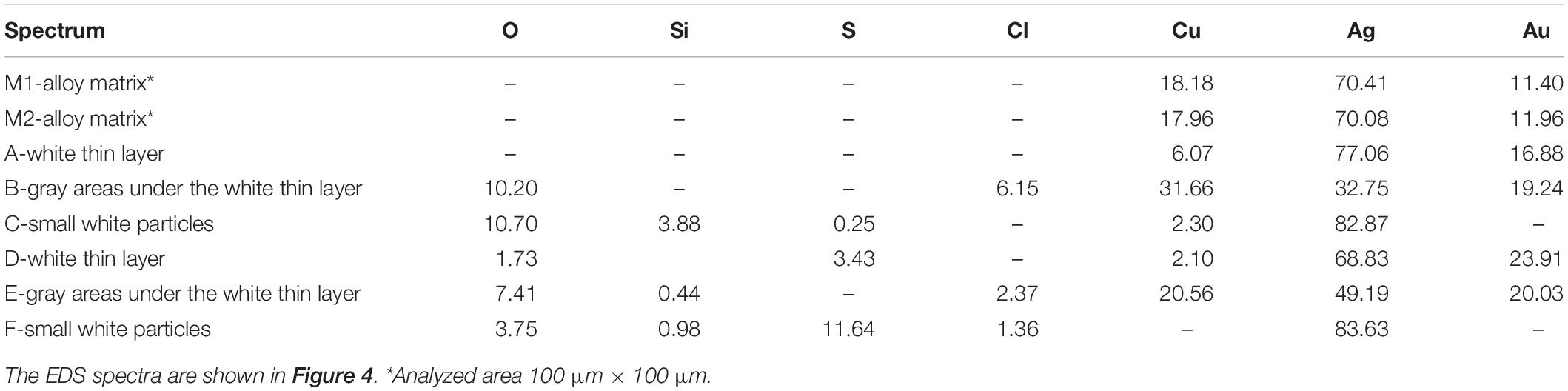
Table 1. Energy-dispersive spectroscopy (EDS) elemental composition [expressed as weight percent (wt%)] of the different areas of the cross-sectioned fragment of the nariguera F4-03 from the tomb of the Lady of Cao.
The BSE SEM and the EDS results of the cross-sectional view through the nariguera show a lamellar-fibrous structure with an array of periodical alternating light and gray semi-aligned layers quite parallel to the surface.
This structure is similar to that observed in the nariguera found at Sipán in the tomb 16 and shown by the SEM and OM images reported in Figure 3; therefore, from the comparisons between these structures, we can evaluate the effect of the long-term corrosion phenomena that occurred in the soil to the buried Ag-Cu-Au artifacts.
As evidenced by the EDS results reported in Figure 6 and Tables 1, 2, the alternated layers of the bulk structure are characterized by different amounts of Au, Cu, and Ag being the bright areas Ag-enriched, while the gray ones are Cu-enriched.
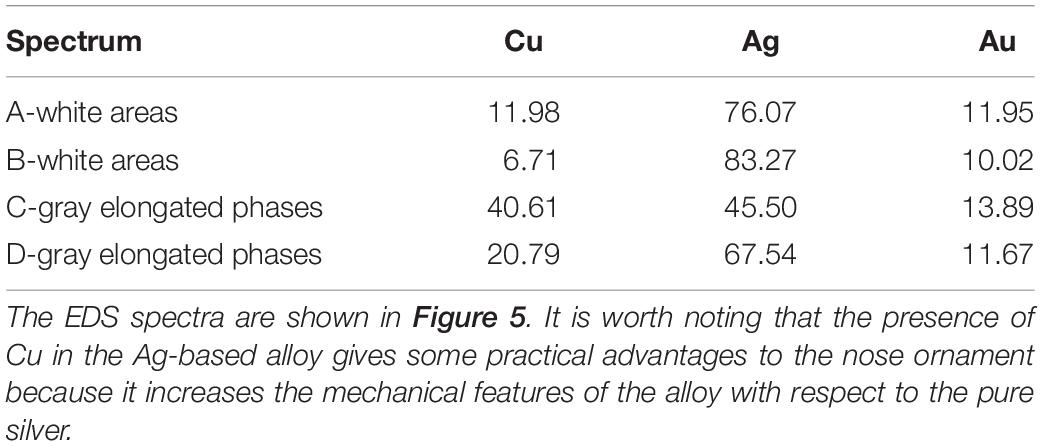
Table 2. Energy-dispersive spectroscopy (EDS) elemental composition of the different areas present in the alloy matrix of the nariguera F4-03 from the tomb of the Lady of Cao.
The data reported in Tables 1, 2 are in agreement with the information achieved by Cesareo et al. (2016, 2017) and Cesareo (2019), private communication during an in situ investigation carried out at the Museum of the Lady of Cao by using a portable X-ray fluorescence (XRF) apparatus. The reliability and utility of this analytical approach have been already largely demonstrated in the study of cultural heritage artifacts being a rapid and useful method to evaluate the elemental surface content of metals and degraded surfaces (Angelini et al., 2006; Figueiredo et al., 2010; Galli et al., 2011; Trojek and Hlozek, 2012).
SEM images shown in Figures 5, 6 also reveal that the metallurgical structure reasonably has been originated by the drastic deformation of a heterogeneous material where segregation areas have been formed. This phenomenon presumably occurred during the solidification of the cast Ag-based alloy (Hörz and Kallfass, 1998, 2000) and has given rise to the formation of Cu-enriched areas in the melt containing more precious metals and less Cu.
SEM images clearly show that the material was subjected to heavy deformation by mechanical working carried out to reduce the thickness of the sheet and by the embossing and punching during the manufacture of the nariguera. The intense hammering process have elongated the Cu-enriched areas to form thin parallel layers whose shape was slightly modified by the thermal treatment of annealing carried out to restore ductility before the final depletion gilding process.
After repeated cycles of hammering and annealing treatments of a single Ag-based alloy sheet, the depletion gilding finally removed Cu or Cu and Ag from the near-surface region forming an outermost silver- or gold-enriched layer, respectively. The formation of a “gold” or a “silver” surface was achieved likely by tuning the pickling procedures and agents.
The presence of these inhomogeneous structures has clearly influenced the environmentally driven alteration phenomena, thus giving rise to the formation of peculiar corrosion products and structures.
Our results provide some other interesting information on the pickling procedures. The images shown in Figure 4 reveal that the narigueras have been dipped up to half its height in a solution or wrapped in a poultice that removed, in a targeted manner, copper and silver from different areas of the object to achieve a seemingly bicolored metallic surface characterized by a spectacular dual esthetic effect.
As shown by the OM images of the front and the back of the nariguera F4-24 (Figure 4 and Supplementary Figure S3), the color change from the “silver” and “gold” surface is quite sharp and the interface smooth without any morphological variation.
The reasons of combining gold and silver surfaces in these nose ornaments, sometimes rarely also with copper, with a typical duality, could be presumably ascribed to a symbolic and spiritual meaning, perhaps to be related to an astral or religious rationale. Some scholars believe that Au was considered to be the rain or the sweat of the sun and Ag the rain or the tears of the moon (Lechtman et al., 1982; Jones and Heidi, 2002; Cooke et al., 2008) or that gold could be related to the masculinity and the right side of the humans while silver could be associated to the femininity and the left side (Hörz and Kallfass, 1998, 2000). However, it seems that gold and silver were associated in some way to the sun and the moon, respectively, with a religious symbolism likely related to the nature duality.
However, the production of bicolored polymetallic surfaces was made possible by the local availability of metal resources and mainly by the sophisticated ability of the Moche metallurgists in the alloying and surface chemical manipulation processes. By means of empirically optimized methods, they modified at a nanoscale dimension the surface chemical composition of Cu-Au-Ag ternary alloys often containing only small percentages of precious metals to give them locally an accentuated appearance up to change their aspect where this desired specific effect was required.
This advanced skill was employed to produce elaborate artifacts with polymetallic surfaces and complex shapes of spectacular beauty with symbolic, shamanic, or religious values to be worn by an elite of the highest status, exhibiting power and rank.
Conclusion
This study highlights the analytical ability of micro-destructive surface and bulk techniques in providing a detailed information on the long-term interaction between polymetallic artifacts and surrounding environment that has caused at the Sipán site the almost complete mineralization of the objects.
The integrated analytical approach based on the use of complementary techniques such as SEM-EDS, XPS, OM, and XRD allows to describe the naturally grown degradation products resulting from interactions between soil species and Ag-Cu-Au ternary alloys and disclose that the main aggressive agent is Cl– from the burial soil.
The microchemical information suggests also that the degradation phenomena were probably enhanced by the galvanic coupling between the precious metal layer and the less noble substrate.
The corrosion process formed mainly layered structures containing chloroargyrite (AgCl), cuprite (Cu2O), nantokite (CuCl), and atacamite [CuCl2⋅3Cu(OH)2] polymorphs. This information is useful to select tailored conservation materials and procedures for a long-term reliable conservation since the presence of atacamite warns that the dangerous copper cyclic corrosion is occurring and must be firmly mitigated to transmit these fascinating artifacts to future generations.
Finally, the combined use of different investigation methodologies has given information useful to identify the manufacturing methods used by the Moche goldsmiths to chemically modify the surface of Cu-Au-Ag alloys in some case achieving the contemporaneous presence of “gold” and “silver” areas. The depletion gilding was the method used by the Moche metallurgists to create these amazing esthetic effects by manipulating the surface of Cu-Au-Ag ternary alloys at a nanoscale dimension for creating adherent precious metal layers with a thickness ranging from one to a few micrometers.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
GI, MA, AB, SZ, AF, CG, EM, MP, CR, PS, GD, and LT equally contributed to preparing the samples, performing the research, analyzing the artifacts, discussing the data, and critically giving a substantial intellectual contribution.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The activities have been carried out in the framework of the Bilateral Italian–Peruvian project between CNR and CONCYTECH (2009–2011 and 2012–2014). Dr. Erica Isabella Parisi is gratefully acknowledged for her technical contribution.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmats.2020.00230/full#supplementary-material
References
Angelini, E., Ingo, G. M., Grassini, S., Corbellini, S., De Caro, T., Riccucci, C., et al. (2006). Potentialities of XRF and ELS portable instruments for the characterisation of ancient artefacts. Appl. Phys. A Mat. Sci. Process. 83, 643–649. doi: 10.1007/s00339-006-3546-8
Antonijevic, M. M., and Petrovic, M. B. (2008). Copper corrosion inhibitors. a review. Int. J. Electrochem. Sci. 3, 1–28.
Baglioni, P., Bonelli, N., Chelazzi, D., Chevalier, A., Dei, L. G., Domingues, J., et al. (2015). Organogel formulations for the cleaning of easel paintings. Appl. Phys. A-Mat. Sci. & Process. 121, 857–868. doi: 10.1007/s00339-015-9364-0
Bastidas, D. M., Criado, M., Fajardo, S., La Iglesia, V. M., Cano, E., and Bastidas, J. M. (2010). Copper deterioration: causes, diagnosis and risk minimisation. Int. Mat. Rev. 55, 99–127. doi: 10.1179/095066009X12506721665257
Bray, W. (1993). “Techniques of gilding and surface-enrichment in pre-Hispanic American metallurgy,” in Metal Plating and Patination, eds P. T. Craddock, and S. La Niece, (Butterworth-Heinemann), 174–192.
Centeno, S. A., and Schorsch, D. (2000). “The characterisation of gold layers on copper artifacts from the Piura Valley (Peru) in the early intermediate period,” in Gilded Metals: History, Technology and Conservation, ed. T. Drayman-Weisser (London: Archetype Publications Ltd).
Cesareo, R. (2019). Gold, gildings and tumbaga from the Moche tomb of the Lady of Cao: an EDXRF test for the internal ratio method. X Ray Spectrom. 48, 202–207. doi: 10.1002/xrs.3021
Cesareo, R., Bustamante, A. D., Azeredo, S., Lopes, R. T., Jordan, R. F., Fernandez, A., et al. (2017). Radiography and transmission measurements on gold and silver from the Moche tomb “Señora de Cao”. Radiogr. Diagn. Imaging 2017, 1–6. doi: 10.15761/RDI.1000106
Cesareo, R., Jordan, R. F., Fernandez, A., Bustamante, A. D., Fabian, J., Zambrano, S. D., et al. (2016). Analysis of the spectacular gold and silver from the Moche tomb “Señora de Cao”. X Ray Spectrom. 45, 138–154. doi: 10.1002/xrs.2680
Chelazzi, D., Chevalier, A., Pizzorusso, G., Giorgi, R., Menu, M., and Baglioni, P. (2014). Characterization and degradation of poly(vinyl acetate)-based adhesives for canvas paintings. Polym. Degradation Stabil. 107, 314–320. doi: 10.1016/j.polymdegradstab.2013.12.028
Cooke, C. A., Abbott, M. B., and Wolfe, A. P. (2008). “Metallurgy in Southern South America,” in Encyclopaedia of the History of Science, Technology, and Medicine in Non-Western Cultures, ed. H. Selin (Cham: Springer), 1658–1662. doi: 10.1007/978-1-4020-4425-0_9628
Curulli, A., Valentini, F., Padeletti, G., Cusmà, A., Ingo, G. M., Kaciulis, S., et al. (2005). Gold nanotubules arrays as new materials for sensing and biosensing: synthesis and characterisation. Sens. Actuators B 111, 526–531. doi: 10.1016/j.snb.2005.03.084
Figueiredo, E., Silva, R. J. C., Araújo, M. F., and Senna-Martinez, J. C. (2010). Identification of ancient gilding technology and late bronze age metallurgy by EDXRF, Micro-EDXRF, SEM-EDS and metallographic techniques. Microchim. Acta 168, 283–291. doi: 10.1007/s00604-009-0284-6
Forty, A. J. (1979). Corrosion micromorphology of noble metal alloys and depletion gilding. Nature 282, 597–598. doi: 10.1038/282597a0
Forty, A. J., and Durkin, P. (1980). A micromorphological study of the dissolution of silver-gold alloys in nitric acid. Philos. Mag. A 42, 295–318. doi: 10.1080/01418618008239360
Galli, A., Bonizzoni, L., Sibilia, E., and Martini, M. (2011). EDXRF analysis of metal artefacts from the grave goods of the Royal Tomb 14 of Sipan, Peru. X Ray Spectrom. 40, 74–78. doi: 10.1002/xrs.1298
Grimwade, M. (1999). “The surface enrichment of carat gold alloys- depletion gilding,” in Proceedings of the Thirteenth Santa Fe Symposium 16-19 May, 1999, Albuquerque, NM, 293–318.
Guisbiers, G., Mejia-Rosales, S., Khanal, S., Ruiz-Zepeda, F., Whetten, R. L., and José-Yacaman, M. (2014). Gold–copper nano-alloy, “Tumbaga”, in the era of nano: phase diagram and segregation. Nano Lett. 14, 6718–6726. doi: 10.1021/nl503584q
Hayez, V., Franquet, A., Hubin, A., and Terryn, H. (2004). XPS study of the atmospheric corrosion of copper alloys of archaeological interest. Surf. Interf. Anal. 36, 876–879. doi: 10.1002/sia.1790
Hörz, G., and Kallfass, M. (1998). Pre-columbian metalworking in peru - ornamental and ceremonial objects from the royal tombs of Sipan. JOM J. Min. Met. Mat. Soc. 50, 8–16. doi: 10.1007/s11837-998-0298-2
Hörz, G., and Kallfass, M. (2000). The treasure of gold and silver artifacts from the Royal Tombs of Sipán, Peru - a study on the Moche metalworking techniques. Mat. Character. 45, 391–420. doi: 10.1016/S1044-5803(00)00093-0
Hultquist, G. (1985). Surface enrichment of low gold alloys. Gold Bull. 18, 53–57. doi: 10.1007/BF03214686
Ingo, G. M., Angelini, E., Bultrini, G., De Caro, T., Pandolfi, L., and Mezzi, A. (2002). Contribution of surface analytical techniques for the microchemical study of archaeological artefacts. Surf. Interf. Analysis 34, 328–336. doi: 10.1002/sia.1311
Ingo, G. M., Bultrini, G., De Caro, T., and Del Vais, C. (2000). Microchemical study of the black gloss on red and black-figured Attic vases. Surf. Interf. Anal. 30, 101–105. doi: 10.1016/0169-4332(92)90326-S
Ingo, G. M., Bustamante, A. D., Alva, W., Angelini, E., Cesareo, R., Gigante, G. E., et al. (2013a). Gold coated copper artifacts from the Royal Tombs of Sipán (Huaca Rajada, Perù): manufacturing techniques and corrosion phenomena. Appl. Phys. A Mat. Sci. Process. 113, 877–887. doi: 10.1007/s00339-013-7711-6
Ingo, G. M., Giorgi, L., Zacchetti, N., and Azzerri, N. (1992). Electrochemical and XPS studies on lacquer-low tinplated steel adhesion. Corr. Sci. 33, 361–377. doi: 10.1016/0010-938X(92)90066-C
Ingo, G. M., Guida, G., Angelini, E., Di Carlo, G., Mezzi, A., and Padeletti, G. (2013b). Ancient mercury-based plating methods: combined use of surface analytical techniques for the study of manufacturing process and degradation phenomena. Acc. Chem. Res. 46, 2365–2375. doi: 10.1021/ar300232e
Ingo, G. M., and Padeletti, G. (1994). Segregation aspects at the fracture surfaces of 8 wt% yttria-zirconia thermal barrier coatings. Surf. Interf. Anal. 21, 450–454. doi: 10.1002/sia.740210623
Ingo, G. M., Riccucci, C., Bultrini, G., Dirè, S., and Chiozzini, G. (2001). Thermal and microchemical characterization of sol-gel SiO2, TiO2 and x SiO2-(1-x) TiO2 powders. J. Therm. Anal. Calorim. 66, 37–46. doi: 10.1023/A:1012471112566
La Niece, S. (1993). “Silvering,” in Metal Plating and Patination, eds P. T. Craddock and S. La Niece (Oxford: Butterworth-Heinemann), 201–210.
Lechtman, H. (1971). “Ancient methods of gilding silver: examples from the old and the. new worlds,” in Science and Archaeology, ed. R. H. Brill (Cambridge, MA: MIT press), 2–30.
Lechtman, H. (1979). Pre-Columbian technique for electrochemical replacement plating of gold and silver on copper objects. J. Metals 31, 154–160. doi: 10.1007/BF03354479
Lechtman, H. (1984a). Andean value systems and the development of prehistoric metallurgy. Technol. Cult. 25, 1–36. doi: 10.2307/3104667
Lechtman, H. (1984b). Pre-Columbian surface metallurgy. Sci. Am. 250, 56–63. doi: 10.1038/scientificamerican0684-56
Lechtman, H., Erlij, A., and Barry, E. J. (1982). New perspective on Moche metallurgy - techniques of gilding copper at Loma-Negra, Northern Peru. Amer. Antiquity 47, 3–30. doi: 10.2307/280051
Lechtman, H., and Klein, S. (1999). The production of copper-arsenic alloys (arsenic bronze) by cosmelting: modern experiment, ancient practice. J. Archaeol. Sci. 26, 497–526. doi: 10.1006/jasc.1998.0324
Lins, P. A., and Oddy, W. A. (1975). The origins of mercury gilding. J. Archaeol. Sci. 2, 365–373. doi: 10.1016/0305-4403(75)90007-2
Merkel, J. F., Seruya, A. I., Griffiths, D., and Shimada, I. (1995). Metallography and microanalysis of precious metalobjectsfrom the middle Sican elite tombs at batan Grande, Peru. Mat. Res. Soc. Symp. Proc. 352, 105–126.
Oddy, W. A. (1990). Gilding: an outline of the technological history of the plating of gold on to silver or copper in the Old World. Endeavour 15, 29–33. doi: 10.1016/0160-9327(91)90085-P
Oddy, W. A. (1991). Gilding: an outline of the technological history of the plating of gold on to silver or copper in the Old World. Endeavour 5, 29–33. doi: 10.1016/0160-9327(91)90085-P
Paparazzo, E., Fierro, G., Ingo, G. M., and Zacchetti, N. (1988). XPS studies on the surface thermal modifications of tin oxides Surf. Interf. Anal. 12, 438–439. doi: 10.1002/sia.740120720
Poggi, G., Toccafondi, N., Chelazzi, D., Canton, P., Giorgi, R., and Baglioni, P. (2016). Calcium hydroxide nanoparticles from solvothermal reaction for the deacidification of degraded waterlogged wood. J. Coll. Interf. Sci. 473, 1–8. doi: 10.1016/j.jcis.2016.03.038
Raub, C. J. (1986b). The development of gilding from antiquity to the middle ages, Materials Australasia, 18 (9), November/December. 7–11.
Sáenz-Samper, J., and Martinón-Torres, M. (2017). Depletion gilding, innovation and life-histories: the changing colours of Nahuange metalwork. Antiquity 91, 1253–1267. doi: 10.15184/aqy.2017.97
Schlesinger, R., Klewe-Nebenius, H., and Bruns, M. (2000). Characterization of artificially produced copper and bronze patina by XPS. Surf. Interf. Anal. 30, 135–139. doi: 10.1002/1096-9918(200008)30:1<135::aid-sia720>3.0.co;2-v
Schorsch, D. (1988). Silver- and gold- Moche artifacts from Loma Negra, Peru. Metropol. Museum J. 33, 109–136. doi: 10.2307/1513009
Scott, D. A. (1986). Gold and silver alloy coatings over copper: an examination of some artefacts from Ecuador and Colombia. Archaeometry 28, 33–50. doi: 10.1111/j.1475-4754.1986.tb00372.x
Scott, D. A. (1990). Bronze disease: a review of some chemical problems and the role of relative humidity. J. Am. Instit. Conserv. 29, 193–206. doi: 10.2307/3179583
Scott, D. A. (1991). Metallography and Microstructure of Ancient and Historic Metals. Malibou: The J. Paul Getty Conservation Institute.
Scott, D. A. (1998). Technical examination of ancient South American metals: some examples from Colombia, Peru and Argentina. Boletín Museo del Oro N 44, 79–105.
Scott, D. A. (2000). “A review of gilding techniques in South America,” in Gilded Metals: History, Technology and Conservation, ed. T. Drayman-Weisser (London: Archetype Publications Ltd), 203–222.
Scott, D. A. (2002). “Corrosion and environment,” in Copper and Bronze in Art, ed. T. Paul (Malibou, CA: Getty Conservation Institute), 10–80.
Stupnišek-Lisac, E., Lonèariæ Božiæ, A., and Cafuk, I. (1998). Low-toxic copper corrosion inhibitors. Corrosion 54, 713–720. doi: 10.5006/1.3284890
Trojek, T., and Hlozek, M. (2012). X-ray fluorescence analysis of archaeological finds and art objects: recognizing gold and gilding. Appl. Radiat. Isot. 70, 1420–1423. doi: 10.1016/j.apradiso.2012.03.033
Twilley, J. W., and Boyles, J. (1981). Compositional analysis of columbian Tumbaga by X-ray fluorescence spectroscopy. Revue d’Archéométrie 1, 303–312. doi: 10.3406/arsci.1981.1160
Keywords: Cu-Ag-Au ternary alloys, long-term burial soil corrosion, surface analytical techniques, metal surface chemical modification, galvanic coupling, tumbaga
Citation: Ingo GM, Albini M, Bustamante AD, Zambrano Alva SdP, Fernandez A, Giuliani C, Messina E, Pascucci M, Riccucci C, Staccioli P, Di Carlo G and Tortora L (2020) Microchemical Investigation of Long-Term Buried Gilded and Silvered Artifacts From Ancient Peru. Front. Mater. 7:230. doi: 10.3389/fmats.2020.00230
Received: 29 November 2019; Accepted: 23 June 2020;
Published: 29 July 2020.
Edited by:
Tadeusz Hryniewicz, Koszalin University of Technology, PolandReviewed by:
Jude Mary Runge, CompCote International, Inc., United StatesEmma Paola Angelini, Politecnico di Torino, Italy
Copyright © 2020 Ingo, Albini, Bustamante, Zambrano Alva, Fernandez, Giuliani, Messina, Pascucci, Riccucci, Staccioli, Di Carlo and Tortora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel M. Ingo, Z2FicmllbG1hcmlhLmluZ29AY25yLml0
 Gabriel M. Ingo
Gabriel M. Ingo Monica Albini
Monica Albini Angel D. Bustamante2
Angel D. Bustamante2 Elena Messina
Elena Messina Cristina Riccucci
Cristina Riccucci Gabriella Di Carlo
Gabriella Di Carlo Luca Tortora
Luca Tortora