
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Mater., 22 July 2020
Sec. Ceramics and Glass
Volume 7 - 2020 | https://doi.org/10.3389/fmats.2020.00210
This article is part of the Research Topic2021 Retrospective: Ceramics and GlassView all 9 articles
In this article we present a short review of the main wet chemical methods developed for the preparation of Ce3+-doped Y3Al5O12 (YAG:Ce) nanocrystals for their use as nanophosphors in LED lighting technology: combustion, co-precipitation, sol-gel, modified-Péchini, and solvothermal routes. We highlight the key synthesis steps and discuss them in the view of the size, crystal quality, and agglomeration state of the obtained nanocrystals. The photoluminescence internal quantum yield of these nanocrystals is also discussed in light of their morphology. In addition, we report on other garnet-type nanophosphors [Gd3Sc2Al3O12, (Gd,Y)3Al5O12, etc.] doped with lanthanide ions (Ce3+, but also Eu3+ or Dy3+) developed with the goal of obtaining a warmer white light. The spectroscopic properties of these nanophosphors, in particular their emission range, is discussed in relation with the doping nature, doping concentration, and crystal field of the host matrices.
The advent of semiconductor technology has led to significant advances in lighting devices with the commercialization of white Light Emitting Diodes (wLEDs). Previously relegated to colored light applications, LEDs now successfully compete with conventional technologies in various general lighting applications and are leading products for the automotive market, for example. They are generally based on a GaN/InGaN chip, emitting in the blue, around 450 nm, coupled with a micron-sized luminescent garnet phosphor (Y3Al5O12 doped with Ce3+, YAG:Ce) (Nakamura and Fasol, 1997). This phosphor powder, encapsulated in an epoxy or silicone resin, is placed above the chip (remote phosphor) and partially absorbs the blue light to down-convert in the yellow range thus leading to white light emissions. However, the micron-size of YAG:Ce phosphors induces several drawbacks: (1) backscattering toward the blue chip, both reducing the external efficiency of the wLEDs at around 60% and damaging gradually the chip, thus reducing the device lifetime (Narendran et al., 2005); (2) need to encapsulate the phosphors in a resin to obtain a mechanically stable layer, the binder leading to accelerated aging of the wLEDs (Yang et al., 2012; Hang et al., 2013); (3) poor coupling with blue nanostructured chips that are now emerging, such as InGaN nanowire arrays for micro-displays (Ley et al., 2019). Moreover, with regard to the latter point in particular, the use of micron-sized phosphors is unsuitable to implement additive manufacturing techniques, which are quickly rising as a potential benefit to wLED developments. Indeed, the performance of modern LED devices can indeed be significantly improved by 2D and 3D printing, particularly by specially designed nano/micro-structures within LEDs requiring, which needs nanophosphors. Thus, to overcome these drawbacks, the use of nanophosphors with particle sizes smaller than 100 nm is required: (1) to control light scattering and enhance the light extraction that is directly associated with the emission efficiency of wLEDs devices; (2) to perform the phosphor shaping without any binder, as also targeted by researchers working on glass-ceramic phosphors (Chen et al., 2015), and to obtain a better coupling with nanostructured semi-conducting heterostructures (Schimpke et al., 2016); (3) to deposit phosphor layers by 2D and 3D printing on nanostructured diodes by ink-jet techniques, involving phosphor nanocrystals (NCs with diameter <100 nm) dispersed in solution with very good colloidal stability (Zhan et al., 2017). Another problem with the YAG:Ce phosphors, that is unrelated to particle size, is their yellow emission, leading to wLEDs exhibiting poor color rendering indexes (CRI), characterized by a cold white emission, uncomfortable for human eyes.
In this article, we review different chemical methods for the preparation of YAG:Ce NCs, pointing out their size and crystal quality, their aggregation or agglomeration state (Nichols et al., 2002) and finally their internal luminescence quantum yield (iQY). We then focus on other garnet-type nanophosphors whose spectroscopic properties are more appropriate to elaborate warm white LED lighting.
Solid-state reaction, used to synthesize commercial YAG:Ce powder, requires high temperature treatment (>1,500°C), which is energy-consuming and leads to micron-sized phosphors with an iQY of 87% (Xu et al., 2009; George et al., 2013b; Song et al., 2013). To synthesize YAG:Ce NCs, several approaches can be envisioned: (1) a top-down strategy, consisting in intensive grinding of micron-sized powder, but it induces a large number of surface defects acting as luminescence quencher; (2) bottom-up approaches, including Chemical Vapor Deposition (Murai et al., 2016), Pulsed Laser Deposition (Dejene, 2016), or wet chemical routes, such as combustion (Hess et al., 1994; Shi and Wang, 2001; Upasani et al., 2019), co-precipitation (Chen et al., 1999; Chiang et al., 2006; Kumar and Senthilselvan, 2017), sol-gel (Kareiva, 2011; Boukerika et al., 2014), modified Péchini (Belyakov and Kulikov, 2011), and solvothermal (Inoue et al., 1991; Nyman et al., 2009; Dantelle et al., 2018a) routes. In the following, we will detail the main wet chemical routes, which present the advantage of being cheaper than the others. Moreover, these wet chemical processes allow to achieve balanced mixture of precursors at the molecular level, resulting in the lowering of the nanoYAG crystallization temperature.
This YAG:Ce NC synthesis route consists in an exothermic reaction between a fuel, typically urea or glycine as reducing agent, and yttrium, aluminum, and cerium nitrates as oxidizers (Fu et al., 2008; Gupta et al., 2011; Wu et al., 2016). The combustion is initiated by an external heating (~500°C) followed by a high temperature treatment around 1,000°C to yield pure YAG and improve the Ce3+ incorporation into the matrix (Shi and Wang, 2001; Yang et al., 2006; Sawala et al., 2016). The combustion step leads to large and irregular particles containing lots of pores because of the gases liberated during the fuel combustion, while the high-temperature treatment required to crystallize the YAG phase results in strong particle sintering, which leads to highly agglomerated NCs (Shi and Wang, 2001; Abd et al., 2018). An alternative consists in mixing yttrium nitrate with glycine and aluminum nitrate with urea to avoid a subsequent heat treatment and reduce the NC agglomeration (Gupta et al., 2011; Dhadade et al., 2016; Sawala et al., 2016). It led to 40 nm particles agglomerated in 100–200 nm clusters, presenting an iQY of 54% (Haranath et al., 2006). The main issue of the combustion technique is the temperature inhomogeneity through the reacting mixture with the occurrence of the so-called “hot spots.” This leads to inhomogeneities in chemical composition with the formation of spurious phases such as YAlO3 and Y4Al2O9 (Hess et al., 1994; Yang et al., 2006), but also inhomogeneities in particle morphology with a broad size distribution. This is due to heterogeneous nucleation, growth and coalescence mechanisms, which are enhanced by the further high temperature treatment. In 2017, Abd et al. managed to synthesize YAG:Ce NCs through a shortened combustion synthesis, thanks to laser-assisted or microwave-assisted reaction (Abd et al., 2017, 2018). This latter yielded well-crystallized YAG:Ce NCs of about 60 nm in one-step with reduced, but still present, NC agglomeration (Figure 1A; Abd et al., 2019). To conclude, the combustion synthesis is a rapid and simple process requiring high temperature treatment to obtain crystalline NCs with high luminescence intensity, but with a severe agglomeration.
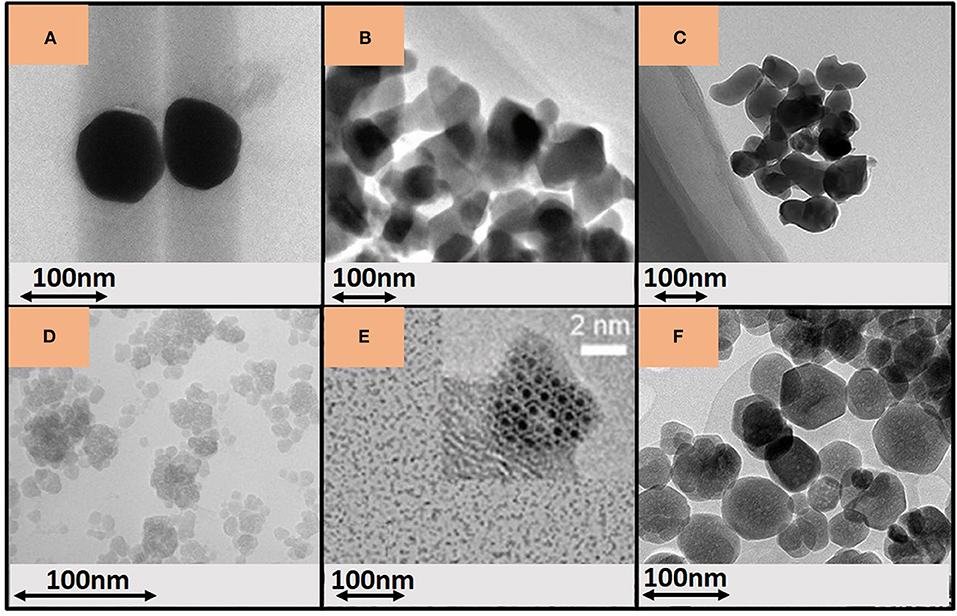
Figure 1. Microscopy images of YAG:Ce NCs prepared (A) by combustion, reproduced from Abd et al. (2019), with permission from the authors; (B) by co-precipitation, reproduced from Si et al. (2014), under the Creative Commons CC BY license; (C) by modified Péchini method, reproduced from He (2016), under the Creative Commons CC BY license; (D) by conventional solvothermal synthesis. Image reproduced from Kasuya et al. (2005), with permission from American Chemical Society, Copyright (2020); (E) by solvothermal synthesis after precursor dehydration and adding diethylene glycol. Image reproduced from Odziomek et al. (2017), with permission from The Royal Society of Chemistry; (F) by modified solvothermal synthesis, high pressure, and steady high temperature. Image reproduced from Dantelle et al. (2018a), under the Creative Commons CC BY license.
Chemical precipitation syntheses of YAG:Ce NCs have been commonly used by the normal strike technique or by the reverse strike route through the pH control of aqueous solutions. For normal strike, a basic solution (ammonium bicarbonate, ammonium hydroxide, etc., as precipitants) is added into an acidic one (nitrate precursor solution) or the other way around (reverse strike) (Yuan and Ryu, 2004; Caponetti et al., 2005; Zhang et al., 2008). Other routes used alcohol-water as precipitant solvent, the alcohol acting also as surfactant reducing initial NC aggregation (Tong et al., 2007). The obtained precipitate (hydroxides, carbonates, hydroxycarbonates…) requires a subsequent calcination at a temperature superior to 1,000°C to obtain pure nanoYAG with moderate to severe agglomeration and wide size dispersion: from a few hundreds of nanometers to micrometer NC agglomerates (Wang et al., 2016; Qiu et al., 2017; Tian et al., 2020). To tackle these issues, Qiu et al. showed that acidic conditions led to smaller YAG:Ce crystal size since it fosters the precipitation of Al3+ (aggregation of 65 nm crystals) and narrow size dispersion, alongside with higher emission intensity (Qiu et al., 2017). To reduce even more the agglomeration of YAG:Ce NCs, surfactants, such as steric stabilizers (polyethylene glycol, PEG10000), electric stabilizers [(NH4)2SO4, C12H25SO4Na], or other dispersing agents (e.g., graphene oxide nanosheets) (Li and Wang, 2009; Wang et al., 2009; Zhang and Yu, 2009; Que et al., 2017; Ji et al., 2018) were involved in the initial solutions, resulting after ~1,000°C calcination in nanoYAG (about 70 nm) with narrow particle size distribution, spherical shapes, moderate NC agglomeration, and enhanced PL emission intensity. With no surfactant but by combining ultrasonication during the addition of the initial solution to the precipitant and microwave heating, Si et al. managed to produce uniformly dispersed 18 nm (900°C) to 43 nm (1,100°C, Figure 1B) YAG particles depending on the annealing temperature, with reduced agglomeration (Si et al., 2014). Very recently, Gaiser et al. published an original two-step approach, including first the formation of particles in ionic liquid solution, followed by a heat-treatment at 600°C to crystallize YAG:Ce NCs embedded in a LiCl matrix, preventing their agglomeration. This process results in individual NCs with an average size below 100 nm, presenting a iQY of 51% (Gaiser et al., 2019).
Hence, the co-precipitation method allows the production of YAG:Ce NCs with relatively narrow size distribution. However, a high temperature calcination step is still required to either yield pure YAG phase and to improve emission properties, favoring agglomerated NCs if no care is taken.
The sol-gel synthesis of YAG:Ce NCs involves metal alkoxides as Y, Al precursors (which can be partly replaced by metal salts, generally Y or Ce nitrates) in alcoholic or aqueous solutions, that are converted into a gel through hydrolysis and condensation reactions (~60°C) (Veith et al., 1999; Devi et al., 2008; Boukerika et al., 2014; Zhang et al., 2017). The resulting gels are then dried into powders and further annealed at around 1,000°C, leading to YAG NCs with different agglomeration levels depending on temperature, duration and atmosphere used during heating treatments (Kareiva, 2011; Boukerika et al., 2016; Sundarakannan and Kottaisamy, 2016). Several conditions have been implemented to have a better control over the YAG:Ce particle size. In 2018, Singlard et al. managed to control the YAG:Ce NC size from 55 to 123 nm with metal concentration variation (Singlard et al., 2018). There are only few papers reporting iQY values for the sol-gel prepared YAG:Ce NCs. Murai et al. reported a thin YAG:Ce film formed of coalesced particles with a iQY of 65% via an epoxide-catalyzed sol-gel method with a 1,600°C heating treatment (Murai et al., 2012).
An important drawback of the sol-gel route is due to the high reactivity of the alkoxides precursors, with the risk of significant metal inhomogeneity in the gel. Thus, the modified Péchini method was used as an alternative to overcome the sol-gel route limitations, particularly to improve the control of YAG:Ce NC size. In the modified Péchini method, the metal ions are first chelated in aqueous solutions, usually by citric acid, which reacts then with a polyalcohol (typically ethylene glycol) giving viscous resins, through poly-esterification reactions, where metal ions are highly dispersed. After an annealing step, it leads to a lowered size of 50–70 nm NCs with agglomerates up to a few hundreds of nanometers (Figure 1C for a sample annealed at 1,030°C) (Zych et al., 2008; Zhang et al., 2009; Hassanzadeh-Tabrizi, 2012; He et al., 2016). Mamonova et al. synthesized freely dispersed individuals NCs of about 100 nm with increased emission intensity through an additional annealing in molten salts (Mamonova et al., 2017).
Although the sol-gel route involves low cost products and is broadly developed to synthesize rare-earth doped metal oxides (Danks et al., 2016), it is a complex method requiring control over a large number of parameters: pH, temperature, annealing parameters, reactant concentration, additives, complexing agent. It leads to poor control on the YAG:Ce NC size and do not allow to get YAG NC dispersion in a solvent, an essential step for further nanophosphor shaping. The modified Péchini method, a simpler alternative to sol-gel route allowing a deeper control over the NCs size, nevertheless requires high temperature treatments, favoring NC coalescence, and agglomeration, which are not usually sufficient to remove all the organic residues (Belyakov and Kulikov, 2011).
The conventional solvothermal route consists in heating in an autoclave (up to 300°C) a precursor solution composed of yttrium and cerium acetates, aluminum isopropoxide, and 1.4-butanediol used as solvent. An autogenous pressure builds inside the autoclave up to about 60 bars in a few hours (Kasuya et al., 2006; Kamiyama et al., 2010; Ramanujam et al., 2016). The combination of pressure and temperature leads to non-equilibrium conditions, allowing the nanocrystallization of YAG:Ce NCs, without any subsequent thermal treatment of nanopowders. This represents an important advantage to avoid NC coalescence and agglomeration that inevitably occur during the final high temperature treatments required for the previous synthesis routes. Depending on the reaction time and temperature, the conventional solvothermal method commonly yields YAG:Ce particles from 20 to 100 nm (Kasuya et al., 2005; Isobe, 2006; Nyman et al., 2009; Vorsthove and Kynast, 2011). These particles are usually composed of spherical NCs of a few nanometers with the formation of large cauliflower-shape agglomerates (Figure 1D; Kasuya et al., 2005) exhibiting PL iQY between 20 and 30% (Kasuya et al., 2005; Asakura et al., 2006; Nyman et al., 2009).
To improve the crystal quality and, subsequently the iQY of YAG:Ce NCs, Revaux et al. proposed a protected annealing method consisting in annealing at 1,000°C YAG:Ce NCs previously dispersed in a porous silica, which preserves their nanometer size of YAG:Ce (Revaux et al., 2011). This method led to 60 nm highly crystalline and well-dispersed NCs with an iQY of 60%. However, to retrieve the NCs, silica should be dissolved by hydrofluoric acid, highly toxic, which is not appropriate for any industrial development. In 2017, Odziomek et al. managed to synthesize ultra-small individual and monodispersed YAG:Ce NCs (4–5 nm) (Figure 1E) by dehydrating the starting precursors and adding diethylene glycol (Odziomek et al., 2017). The addition of a co-solvent contributes to size reduction and minimized NC aggregation. Recently, Dantelle et al. introduced a modified solvothermal process by combining high temperature (400°C) and steady high pressure (200 bar) to produce well-crystallized individuals YAG:Ce NCs with iQY of 40% (Figure 1F; Dantelle et al., 2018a).
The solvothermal method, scalable, and relatively cheap, appears as a highly promising route to synthesize highly crystalline YAG:Ce NCs that can be dispersed in ethanol without aggregation, opening the route to NC deposition through ink-jet printing and to the formation of 2D and 3D phosphor layers for white LED nanostructured devices.
Although YAG:Ce nanophosphors have been at the center of attention for the development of nanostructured LED lighting, other garnet-type nanophosphors are developed, using the synthesis methods presented above. Indeed, the combination of YAG:Ce and InGaN blue diode leads to a so-called “cold white” LED lighting, which can be responsible for eye discomfort and even, at high power, for retina damage (Behar-Cohen et al., 2011). A great amount of work has been made to propose phosphors, at the micron scale, that would present a red-shifted emission, leading to a warmer white light (George et al., 2013a; Li et al., 2015). Due to its stable chemical and optical properties, garnet-type structure provides excellent hosts, able to incorporate various lanthanide and transition metal ions (Setlur et al., 2008; Xia and Meijerink, 2017; Wang et al., 2019). Two strategies have been followed: (1) The first one consists in synthesizing new Ce3+-doped garnet compounds whose emission is shifted due to the crystal field effect of the host matrix. Indeed, Ce3+ emission is based on the radiative de-excitation from 5d levels, sensitive to the local environment, to 4f levels, and thus varies with the host nature; (2) The second strategy consists in modifying the nature of the doping ions and incorporating other lanthanide or transition metal ions whose electronic transitions fall in the red range.
With the need for miniaturization and better coupling with nanostructured diodes, the search for new nanophosphors with a redder emission has been launched (Cesaria and Di Bartolo, 2019), with the challenge to control the phase purity of these new compounds, as well as their NC size and crystal quality to obtain high PL efficiency.
According to Wu et al. (2007), and more recently to Ueda and Tanabe (Ueda and Tanabe, 2019), the emission wavelength of Ce3+ is directly related to the distortion of the dodecahedral site occupied by Ce3+, in the different garnet structures. Through thorough studies at the micron scale, it was shown that the bigger the site distortion, the more red-shifted the emission. Based on the numerous studies at the micron-sized level, several garnet-type nanophosphors have been developed. As shown on Figure 2, for nanophosphors, the substitution of Y3+ by Gd3+ ( = 1.019 Å vs. = 1.053 Å, coordination number, CN = 8) induces a red-shift of the emission of about 10 nm (Wako et al., 2016; Dantelle et al., 2017), whereas the Y3+ substitution by Lu3+ ( = 0.977 Å) shifts the emission toward the blue region (Praveena et al., 2011; Xu et al., 2014). Meanwhile, the substitution of octahedral aluminum ( = 0.535 Å, CN = 6) by Ga3+ ( = 0.66 Å, CN = 6) induces a 10 nm blue-shift of the emission (Dantelle et al., 2017) and, in Gd3Sc2Al3O12:Ce3+ NCs doped with 10 mol.% Ce3+, a significant red-shift of about 50 nm is observed (Dantelle et al., 2018b). These nanoscale evolutions follow the trends obtained at the micron scale and can be explained by the influence of the host crystal field, leading to different splittings of the 5d levels of Ce3+ responsible for the emission. Other garnets, such as Ce3+-doped silicate garnet Ca3Sc2Si3O12 (Levchuk et al., 2017), can be good alternatives to provide red emission. Note that, at present time, most of the garnet-type nanophosphor work reported in the literature deal with strongly agglomerate particles synthesized mainly by the sol-gel method, which is not entirely satisfactory for the use of NCs in nanostructured white LED lighting.
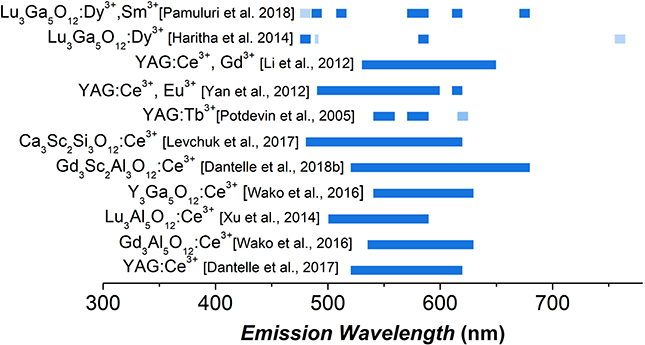
Figure 2. Emission range of different garnet-type nanophosphors reported in the literature. The emission range has been defined by considering all emission whose intensity is larger than Imax/2, with Imax the maximal PL emission intensity. The dark blue indicates a strong emission intensity, in comparison to the light blue. No emission intensity comparison can be done among the different nanophosphors.
Interestingly, as the synthesis methods employed to elaborate NCs involve non-equilibrium conditions, YAG:Ce NCs can be doped with higher concentration than their bulk equivalent. Thus, up to 13 mol.% Ce was introduced in YAG NCs produced by low-energy cluster beam deposition (Masenelli et al., 2013), and up to 30 mol.% using auto-combustion method (Haranath et al., 2006). Although the maximal quantum efficiency is usually obtained for a low doping concentration, high doping could allow to redshift the emission. Indeed, in Ce3+-doped YAG and Gd3Sc2Al3O12, emission is redshifted by increasing the cerium concentration from 1 mol. to 10 mol.% (Masenelli et al., 2013; Devys et al., 2017). This phenomenon can be an advantage for tuning the emission wavelength while using garnet-type nanophosphors and is explained by the fact that Ce3+ ions (rCe3+ = 1.143 Å) substitutes Y3+, inducing more distortion within the crystal lattice.
The second strategy to obtain phosphors with a redshifted emission consists in introducing other doping ions than Ce3+: other trivalent lanthanide ions, divalent lanthanide ions or transition metal ions. A large number of papers, dealing with garnets at the micron scale, report such doping modifications (Shang et al., 2014; Xia and Meijerink, 2017; Wang et al., 2019). At the nanoscale, the literature is scarcer and mainly reports nanophosphors doped with trivalent lanthanide ions (Gd3+, Eu3+, Sm3+, Dy3+, Tb3+), exhibiting electronic intra-configurational transitions in the red range. Such ions are incorporated as dopant or co-dopant in the garnet-type nanophosphors and provide red emission components (Figure 2; Potdevin et al., 2005; Li and Shen, 2012; Yan et al., 2012; Haritha et al., 2014; Pamuluri et al., 2018; Ali and Khedr, 2019).
The utility of these nanophosphors for wLED lighting can be evaluated by the calculation of their color coordinates (x,y), the correlated color temperature (CCT) and the color rendering index (CRI). The standard illuminant, corresponding to the average daylight, presents color coordinates of (0.31,0.33) and a CCT around 6,500 K (Noboru and Robertson, 2005). YAG:Ce-based wLEDs, give CCT values higher than 7,000 K (“cold white”) and a maximal CRI of ~80, according to Ce3+ concentration and phosphor thickness (Hua et al., 2016). Thanks to their red emission component, the above-mentioned compounds can be combined to semi-conducting diodes and provide better CCT and CRI. For instance, an optimized wLED combining YAG:Ce and YAG:Ce,Gd nanophosphors with a InGaN chip exhibits color coordinates of (0.31,0.32), a CCT of 6,564 K and a CRI of 86 (Li and Shen, 2012). Another example is Dy3+-doped Lu3Al5O12 nanophosphors, showing interesting color coordinates (0.317,0.321) and a CCT of 6,322 K, close to the ideal white light (Pamuluri et al., 2018).
In comparison to micron-sized phosphors, additional challenges appear when working with nanophosphors due to the crystal size and the synthesis methods involved. Indeed, if the Ln3+ emission wavelength is not affected by the size reduction, the PL efficiency and energy transfer between doping ions can be different due to surface traps and volume defects (Dantelle et al., 2018a; Liu et al., 2018). Moreover, the control of the oxidation state at the nanoscale is problematic. Indeed, when working with micron-sized powder, typical annealing under controlled atmosphere can be performed to stabilize the oxidation state of the doping ion, as for Ce3+-doped YAG (George et al., 2013b) and also in Eu2+-doped YAG (Havlák et al., 2016). To obtain NCs, post-annealing should be avoided, making the control of the oxidation state more complex. For instance, when using solvothermal method, Dantelle et al. showed by X-ray absorption spectroscopy that a non-negligible fraction of cerium ions (nearly 50%, depending on the synthesis temperature) were oxidized (Dantelle et al., 2018a). To preserve the right oxidation state while elaborating NCs, more complex strategies should be proposed. A recent study proposes the bubbling of a reducing gas in a solution prior to solvothermal treatment, preventing partially the oxidation of Ce3+ during the heat treatment (Cantarano et al., 2020).
Several solution-based methods (co-precipitation, sol-gel, solvothermal, etc.) have been developed for the synthesis of YAG:Ce NCs. However, most of them involve an annealing step at high temperature (typically above 800°C), which induces NC agglomeration and thus prevents their dispersion in solution and their further deposition onto blue LEDs. The solvothermal method, occurring completely in solution and requiring no further annealing, appears as an efficient way to produce non-agglomerated NCs. However, many parameters (pressure, temperature, solvent, etc.) should be controlled to control YAG:Ce nanocrystallization and to produce highly luminescent NCs. Aside the YAG:Ce nanophosphor, other garnet-type nanophosphors are developed with the goal of obtaining a red-shift emission. This emission shift can be obtained by considering either another garnet-type matrix or other doping ions, on the model of works performing at the micron scale. However, each new compound requires thorough nanocrystallization study to control the size, morphology, and PL properties. Hence, the synthesis of nanophosphors for white LED lighting still remains a real challenge. Perspectives also include nanophosphor shaping, using low cost techniques based on solution such as inkjet printing onto semi-conducting chips. The development of such wLED devices should allow better control of light scattering, minimization of optical losses, better coupling with nanostructured blue diodes for enhanced efficiency and miniaturization of the devices.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank the French Research Agency (ANR, grant: ANR-17-CE09-0035-01) for funding their research on nanophosphors.
Abd, H. R., Hassan, Z., Ahmed, N. M., Abdullah Almessiere, M., Omar, A. F., Alsultany, F. H., et al. (2018). Effect of annealing time of YAG:Ce3+ phosphor on white light chromaticity values. J. Electron. Mater. 47, 1638–1646. doi: 10.1007/s11664-017-5968-9
Abd, H. R., Hassan, Z., Ahmed, N. M., Alsultany, F. H., and Omar, A. F. (2019). Ce-doped YAG phosphor powder synthesized via microwave combustion and its application for white LED. Opt. Eng. 58:027110. doi: 10.1117/1.OE.58.2.027110
Abd, H. R., Hassan, Z., Ahmed, N. M., Omar, A. F., Alsultany, F. H., and Yusof, Y. (2017). Laser-induced solution combustion of nano-Y2.96Al5O12:0.04Ce phosphors and their fluorescent properties for white light conversion. J. Alloys Cmpd. 711, 42–50. doi: 10.1016/j.jallcom.2017.03.359
Ali, H., and Khedr, M. A. (2019). Energy transfer between Ce and Sm co-doped YAG nanocrystals for white light emitting devices. Results Phys. 12, 1777–1782. doi: 10.1016/j.rinp.2019.01.093
Asakura, R., Isobe, T., Kurokawa, K., Aizawa, H., and Ohkubo, M. (2006). Tagging of avidin immobilized beads with biotinylated YAG:Ce3+ nanocrystal phosphor. Anal. Bioanal. Chem. 386, 1641–1647. doi: 10.1007/s00216-006-0814-6
Behar-Cohen, F., Martinsons, C., Viénot, F., Zissis, G., Barlier-Salsi, A., Cesarini, J. P., et al. (2011). Light-emitting diodes (LED) for domestic lighting: any risks for the eye? Progr. Retinal Eye Res. 30, 239–257. doi: 10.1016/j.preteyeres.2011.04.002
Belyakov, A. V., and Kulikov, N. A. (2011). Production of nanopowders of yttrium-aluminum garnet by the Pechini method. Refract. Ind. Ceram. 52, 61–62. doi: 10.1007/s11148-011-9366-1
Boukerika, A., Guerbous, L., and Belamri, M. (2016). Effect of different annealing atmospheres on the structural and luminescence properties of Ce3+-doped YAG phosphors synthesized by sol-gel method. Optik 127, 5235–5239. doi: 10.1016/j.ijleo.2016.03.037
Boukerika, A., Guerbous, L., and Brihi, N. (2014). Ce-doped YAG phosphors prepared via sol-gel method: effect of some modular parameters. J. Alloys Compd. 61, 383–388. doi: 10.1016/j.jallcom.2014.06.133
Cantarano, A., Testemale, D., Sousa Nobre, S., Potdevin, A., Bruyère, R., Barbara, A., et al. (2020). Twofold advantage of gas bubbling for the advanced solvothermal preparation of efficient YAG:Ce nanophosphors. J. Mater. Chem. C. doi: 10.1039/D0TC02347G
Caponetti, E., Saladino, M. L., Chillura Martino, D., Pedone, L., Enzo, S., Russu, S., et al. (2005). Luminescence properties of neodymium-doped yttrium aluminium garnet obtained by the Co-precipitation method combined with the mechanical process. Solid State Phenom. 106, 7–16. doi: 10.4028/www.scientific.net/SSP.106.7
Cesaria, M., and Di Bartolo, B. (2019). Nanophosphors-based white light sources. Nanomaterials 9:1048. doi: 10.3390/nano9071048
Chen, D., Xiang, W., Liang, X., Zhong, J., Yu, H., Ding, M., et al. (2015). Advances in transparent glass–ceramic phosphors for white light-emitting diodes—a review. J. Euro. Ceram. Soc. 35, 859–869. doi: 10.1016/j.jeurceramsoc.2014.10.002
Chen, T. M., Chen, S. C., and Yu, C. J. (1999). Preparation and characterization of garnet phosphor nanoparticles derived from oxalate coprecipitation. J. Solid State Chem. 144, 437–441. doi: 10.1006/jssc.1999.8202
Chiang, C. C., Tsai, M. S., Hsiao, C. S., and Hon, M. H. (2006). Synthesis of YAG:Ce phosphor via different aluminum sources and precipitation processes. J. Alloys Compd. 416, 265–269. doi: 10.1016/j.jallcom.2005.08.041
Danks, A. E., Hall, S. R., and Schnepp, Z. (2016). The evolution of ‘sol-gel' chemistry as a technique for materials synthesis. Mater. Horizons. 3, 91–112. doi: 10.1039/C5MH00260E
Dantelle, G., Salaün, M., Bruyère, R., Kodjikian, S., and Ibanez, A. (2017). Luminescent coatings prepared from optimized YAG:Ce nanoparticles. Thin Solid Films 643, 36–42. doi: 10.1016/j.tsf.2017.05.001
Dantelle, G., Testemale, D., Homeyer, E., Cantarano, A., Kodjikian, S., Dujardin, C., et al. (2018a). A new solvothermal method for the synthesis of size-controlled YAG:Ce single-nanocrystals. RSC Adv. 8, 26857–26870. doi: 10.1039/C8RA05914D
Dantelle, G., Testemale, D., Kodjikian, S., and Ibanez, A. (2018b). Synthesis of high-quality garnet-type Gd3Sc2Al3O12:Ce3+ nanocrystals. SPIE Proc. 10533:153322. doi: 10.1117/12.2300166
Dejene, F. B. (2016). Structural and luminescence properties of yellow Y3Al5O12:Ce3+ thin-film phosphors prepared by pulsed laser deposition. Appl. Phys. A Mater. Sci. Process. 122:388. doi: 10.1007/s00339-016-9938-5
Devi, K., Choudhary, R., Satsangi, A. K., and Gupta, R. K. (2008). Sol-gel synthesis and characterisation of nanocrystalline yttrium aluminum garnet nanopowder. Def. Sci. J. 58, 545–549. doi: 10.14429/dsj.58.1675
Devys, L., Dantelle, G., Laurita, G., Homeyer, E., Gautier-Luneau, I., Dujardin, C., et al. (2017). A strategy to increase phosphor brightness: application with Ce3+-doped Gd3Sc2Al3O12. J. Lumin. 190, 62–68. doi: 10.1016/j.jlumin.2017.05.035
Dhadade, I. H., Moharil, S. V., Dhoble, S. J., and Rahangdale, S. R. (2016). One step combustion synthesis and thermoluminescence in Y3Al5O12:Ce3+. AIP Conf. Proc. 1728, 3–7. doi: 10.1063/1.4946588
Fu, Y. P., Wen, S. B., and Hsu, C. S. (2008). Preparation and characterization of Y3Al5O12:Ce and Y2O3:Eu phosphors powders by combustion process. J. Alloys Compd. 458, 318–322. doi: 10.1016/j.jallcom.2007.03.147
Gaiser, H. F., Kuzmanoski, A., and Feldmann, C. (2019). Y3Al5O12:Ce nanoparticles made by ionic-liquid assisted particle formation and LiCl-matrix-treated crystallization. RSC Adv. 9:10195. doi: 10.1039/C9RA01537J
George, N. C., Denault, K. A., and Seshadri, R. (2013a). Phosphors for solid-state white lighting. Ann. Rev. Mater. Res. 43, 481–501. doi: 10.1146/annurev-matsci-073012-125702
George, N. C., Pell, A. J., Dantelle, G., Page, K., Llobet, A., Balasubramanian, M., et al. (2013b). Local environments of dilute activator ions in the solid-state lighting phosphor Y3−xCexAl5O12. Chem. Mater. 25, 3979–3995. doi: 10.1021/cm401598n
Gupta, K. V. K., Muley, A., Yadav, P., Joshi, C. P., and Moharil, S. V. (2011). Combustion synthesis of YAG:Ce and related phosphors. Appl. Phys. B Lasers Opt. 105, 479–484. doi: 10.1007/s00340-011-4685-y
Hang, C., Fei, J., Tian, Y., Zhang, W., Wang, C., Zhao, S., et al. (2013). “The effects of humidity and temperature aging test on flexible packaging LED module,” in 14th International Conference on Electronic Packaging Technology (Dalian), 1126–1129. doi: 10.1109/ICEPT.2013.6756656
Haranath, D., Chander, H., Sharma, P., and Singh, S. (2006). Enhanced luminescence of Y3Al5O12:Ce3+ nanophosphor for white light-emitting diodes. Appl. Phys. Lett. 89:173118. doi: 10.1063/1.2367657
Haritha, P., Martin, I. R., Linganna, K., Monteseguro, V., Babu, P., Leon-Luis, S. F., et al. (2014). Optimizing white light luminescence in Dy3+-doped Lu3Ga5O12 nano-garnets. J. Appl. Phys. 116:174308. doi: 10.1063/1.4900989
Hassanzadeh-Tabrizi, S. A. (2012). Synthesis and luminescence properties of YAG:Ce nanopowder prepared by the Pechini method. Adv. Powder Technol. 23, 324–327. doi: 10.1016/j.apt.2011.04.006
Havlák, L., Bárta, J., Buryi, M., Jar,ý, V., Mihókov,á, E., Laguta, V., et al. (2016). Eu2+ stabilization in YAG structure: optical and electron paramagnetic resonance study. J. Phys. Chem. C 120, 21751–21761. doi: 10.1021/acs.jpcc.6b06397
He, X., Liu, X., Li, R., Yang, B., Yu, K., Zeng, M., et al. (2016). Effects of local structure of Ce3+ ions on luminescent properties of Y3Al5O12:Ce nanoparticles. Sci. Rep. 6:22238. doi: 10.1038/srep22238
Hess, N. J., Maupin, G. D., Chick, L. A., Sunberg, D. S., McCreedy, D. E., and Armstrong, T. R. (1994). Synthesis and crystallization of yttrium-aluminium garnet and related compounds. J. Mater. Sci. 29, 1873–1878. doi: 10.1007/BF00351307
Hua, S., Lua, C., Zhou, G., Liu, X., Qin, X., liu, G., et al. (2016). Transparent YAG:Ce ceramics for WLEDs with high CRI: Ce3+ concentration and sample thickness effects. Ceram. Int. 42, 6935–6941. doi: 10.1016/j.ceramint.2016.01.079
Inoue, M., Otsu, H., Kominami, H., and Inui, T. (1991). Synthesis of yttrium aluminum garnet by the glycothermal method. J. Am. Ceram. Soc. 74, 1452–1454. doi: 10.1111/j.1151-2916.1991.tb04129.x
Isobe, T. (2006). Low-temperature wet chemical syntheses of nanocrystal phosphors with surface modification and their characterization. Phys. Stat. Sol. 203, 2686–2693. doi: 10.1002/pssa.200669630
Ji, C., Gao, Q., Dai, P., Shen, L., Zhang, X., and Bao, N. (2018). Synthesis of high quality Ce:YAG nanopowders by graphene oxide nanosheet-assisted co-precipitation method. J. Rare Earths 36, 130–134. doi: 10.1016/j.jre.2017.08.003
Kamiyama, Y., Hiroshima, T., Isobe, T., Koizuka, T., and Takashima, S. (2010). Photostability of YAG:Ce3+ nanophosphors synthesized by glycothermal method. J. Electrochem. Soc. 157:J149. doi: 10.1149/1.3327907
Kareiva, A. (2011). Aqueous sol-gel synthesis methods for the preparation of garnet crystal structure compounds. Medziagotyra 17, 428–437. doi: 10.5755/j01.ms.17.4.782
Kasuya, R., Isobe, T., and Kuma, H. (2006). Glycothermal synthesis and photoluminescence of YAG:Ce3+ nanophosphors. J. Alloys Compd. 408–412, 820–823. doi: 10.1016/j.jallcom.2005.01.066
Kasuya, R., Isobe, T., Kuma, H., and Katano, J. (2005). Photoluminescence enhancement of PEG-modified YAG:Ce3+ nanocrystal phosphor prepared by glycothermal method. J. Phys. Chem. B 109, 22126–22130. doi: 10.1021/jp052753j
Kumar, S. A., and Senthilselvan, J. (2017). Effect of calcination temperatures on Green luminescence of Ce:YAG nanophosphor prepared by modified co-precipitation method. AIP Conf. Proc. 1832:050112. doi: 10.1063/1.4980345
Levchuk, I., Schroppel, F., Romling, L., Chepyga, L., Osvet, A., Khaidukov, N., et al. (2017). Highly luminescent Ca3Sc2Si3O12:Ce3+ silicate garnet nano- and microparticles with 50-70% photoluminescence quantum yields as efficient phosphor converters for white LEDs. TechConnect Brief 4, 194–197.
Ley, R., Chan, L., Shapturenka, P., Wong, M., Denbaars, S., and Gordon, M. (2019). Strain relaxation of InGaN/GaN multi-quantum well light emitters via nanopatterning. Opt. Express 27:30081. doi: 10.1364/OE.27.030081
Li, G., Tian, Y., Zhao, Y., and Lin, J. (2015). Recent progress in luminescence tuning of Ce3+ and Eu2+-activated phosphors for pc-WLEDs. Chem. Soc. Rev. 44, 8688–8713. doi: 10.1039/C4CS00446A
Li, K., and Shen, C. (2012). White LED based on nano-YAG:Ce3+/YAG:Ce3+,Gd3+ hybrid phosphors. Optik 123, 621–623. doi: 10.1016/j.ijleo.2011.06.005
Li, X., and Wang, W. (2009). Preparation of uniformly dispersed YAG ultrafine powders by co-precipitation method with SDS treatment. Powder Technol. 196, 26–29. doi: 10.1016/j.powtec.2009.06.013
Liu, S., Serrano, D., Fossati, A., Tallaire, A., Ferrier, A., and Goldner, P. (2018). Controlled size reduction of rare earth doped nanoparticles for optical quantum technologies. RSC Adv. 8, 37098–37104. doi: 10.1039/C8RA07246A
Mamonova, D. V., Kolesnikov, I. E., Manshina, A. A., Mikhailov, M. D., and Smirnov, V. M. (2017). Modified Pechini method for the synthesis of weakly-agglomerated nanocrystalline yttrium aluminum garnet (YAG) powders. Mater. Chem. Phys. 189, 245–251. doi: 10.1016/j.matchemphys.2016.12.025
Masenelli, B., Mollet, O., Boisron, O., Canut, B., Ledoux, G., Bluet, J. M., et al. (2013). YAG:Ce nanoparticle lightsources. Nanotechnology 24:165703. doi: 10.1088/0957-4484/24/16/165703
Murai, S., Sato, T., Yao, S., Kamakura, R., Fujita, K., and Tanaka, K. (2016). Fabrication of cerium-doped yttrium aluminum garnet thin films by a mist CVD method. J. Lumin. 170, 808–811. doi: 10.1016/j.jlumin.2015.10.048
Murai, S., Verschuuren, M. A., Lozano, G., Pirruccio, G., Koenderink, A. F., and Rivas, J. G. (2012). Enhanced absorption and emission of Y3Al5O12:Ce3+ thin layers prepared by epoxide-catalyzed sol-gel method. Opt. Mater. Express. 2:1111. doi: 10.1364/OME.2.001111
Nakamura, S., and Fasol, G. (1997). The Blue Laser Diode: GaN Based Light Emitters and Lasers. Heidelberg: Springer-Verlag, 317–320. doi: 10.1007/978-3-662-03462-0_16
Narendran, N., Gu, Y., Freyssinier-Nova, J. P., and Zhu, Y. (2005). Extracting phosphor-scattered photons to improve white LED efficiency. Phys. Status Solidi Appl. Mater. Sci. 202, 60–62. doi: 10.1002/pssa.200510015
Nichols, G., Byard, S., Bloxham, M. J., Botterill, J., Dawson, N. J., Dennis, A., et al. (2002). A review of the terms agglomerate and aggregate with a recommendation for nomenclature used in powder and particle characterization. J. Pharm. Sci. 91, 2103–2109. doi: 10.1002/jps.10191
Noboru, O., and Robertson, A. R. (2005). Standard and Supplementary Illuminants. Colorimetry. Chichester: Wiley, 92–96.
Nyman, M., Shea-Rohwer, L. E., Martin, J. E., and Provencio, P. (2009). Nano-YAG:Ce mechanisms of growth and epoxy-encapsulation. Chem. Mater. 21, 1536–1542. doi: 10.1021/cm803137h
Odziomek, M., Chaput, F., Lerouge, F., Sitarz, M., and Parola, S. (2017). Highly luminescent YAG:Ce ultra-small nanocrystals, from stable dispersions to thin films. J. Mater. Chem. C 5, 12561–12570. doi: 10.1039/C7TC03504G
Pamuluri, H., Rathaiah, M., Linganna, K., Jayasankar, C. K., Lavin, V., and Venkatramu, V. (2018). Role of Dy3+ - Sm3+ energy transfer in the tuning of warm to cold white light emission in Dy3+/Sm3+ co-doped Lu3Ga5O12 nano-garnets. New. J. Chem. 42, 1260–1270. doi: 10.1039/C7NJ04034B
Potdevin, A., Chadeyron, G., Boyer, D., Caillier, B., and Mahiou, R. (2005). Sol–gel based YAG:Tb3+ or Eu3+ phosphors for application in lighting sources. J. Phys. D38, 3251–3260. doi: 10.1088/0022-3727/38/17/S29
Praveena, R., Shi, L., Jang, K. H., Venkatramu, V., Jayasankar, C. K., and Seo, H. J. (2011). Sol–gel synthesis and thermal stability of luminescence of Lu3Al5O12:Ce3+ nano-garnet. J. Alloys Compd. 509, 859–886. doi: 10.1016/j.jallcom.2010.09.113
Qiu, Q., Huang, M., Zheng, W., Xuan, C., Wan, Y., Zhang, B., et al. (2017). Impact of molar ratio of total metal ions to precipitant on YAG:Ce nanophosphors synthesized by reverse titration coprecipitation. Ceram. Int. 43, 8730–8734. doi: 10.1016/j.ceramint.2017.04.004
Que, M., Que, W., Zhou, T., Shao, J., and Kong, L. (2017). Enhanced photoluminescence property of sulfate ions modified YAG:Ce3+ phosphor by co-precipitation method. J. Rare Earths 35, 217–222. doi: 10.1016/S1002-0721(17)60902-5
Ramanujam, P., Vaidhyanathan, B., Binner, J. G. P., Ghanizadeh, S., and Spacie, C. (2016). Solvothermal nanoYAG synthesis: mechanism and particle growth kinetics. J. Supercrit. Fluids 107, 433–440. doi: 10.1016/j.supflu.2015.09.031
Revaux, A., Dantelle, G., George, N., Seshadri, R., Gacoin, T., and Boilot, J. P. (2011). A protected annealing strategy to enhanced light emission and photostability of YAG:Ce nanoparticle-based films. Nanoscale 3, 2015–2022. doi: 10.1039/c0nr01000f
Sawala, N. S., Palan, C. B., Chauhan, A. O., and Omanwar, S. K. (2016). Photoluminescence properties of mixed fuel combustion synthesized Ce3+ ions doped Y3Al5O12 phosphor. Optik 127, 5120–5123. doi: 10.1016/j.ijleo.2016.02.064
Schimpke, T., Mandl, M., Stoll, I., Pohl-Klein, B., Bichler, D., Zwaschka, F., et al. (2016). Phys. Status Solidi A 213, 1577–1584. doi: 10.1002/pssa.201532904
Setlur, A. A., Heward, W. J., Hannah, M. E., and Happek, U. (2008). Incorporation of Si4+-N3− into Ce3+ -doped garnets for warm white LED phosphors. Chem. Mater. 20, 6277–6283. doi: 10.1021/cm801732d
Shang, M., Li, C., and Lin, J. (2014). How to produce white light in a single-phase host? Chem. Soc. Rev. 43, 1372–1386. doi: 10.1039/C3CS60314H
Shi, S., and Wang, J. (2001). Combustion synthesis of Eu3+ activated Y3Al5O12 phosphor nanoparticles. J. Alloys Compd. 327, 82–86. doi: 10.1016/S0925-8388(01)01399-8
Si, W., Ding, C., and Ding, S. (2014). Synthesis and characterization of YAG nanoparticles by ultrasound-assisted and ultrasound-microwave-assisted alkoxide hydrolysis precipitation methods. J. Nanomater. 408910. doi: 10.1155/2014/408910
Singlard, M., Rémondière, F., Oriol, S., Fiore, G., Vieille, B., Vardelle, M., et al. (2018). Sol-gel synthesis of yttrium aluminum garnet (YAG): effects of the precursor nature and concentration on the crystallization. J. Sol Gel Sci. Technol. 87, 496–503. doi: 10.1007/s10971-018-4722-y
Song, Z., Liao, J., Ding, X., Liu, X., and Liu, Q. (2013). Synthesis of YAG phosphor particles with excellent morphology by solid state reaction. J. Cryst. Growth. 365, 24–28. doi: 10.1016/j.jcrysgro.2012.12.022
Sundarakannan, B., and Kottaisamy, M. (2016). Sol-gel derived flux assisted synthesis of fine particles YAG:Ce3+ phosphor for remote phosphor converted white light emitting diodes. Mater. Res. Bull. 74, 485–490. doi: 10.1016/j.materresbull.2015.10.057
Tian, F., Chen, C., Liu, Y., Liu, Q., Ivanov, M., Wang, Q., et al. (2020). Fabrication of Nd:YAG transparent ceramics from co-precipitated powders by vacuum pre-sintering and HIP post-treatment. Opt. Mater. 101:109728. doi: 10.1016/j.optmat.2020.109728
Tong, S., Lu, T., and Guo, W. (2007). Synthesis of YAG powder by alcohol-water co-precipitation method. Matter. Lett. 61, 4287–4289. doi: 10.1016/j.matlet.2007.01.087
Ueda, J., and Tanabe, S. (2019). Review of luminescent properties of Ce3+-doped garnet phosphors: new insight into the effect of crystal and electronic structure. Opt. Mater. 1:100018. doi: 10.1016/j.omx.2019.100018
Upasani, M., Butey, B., and Moharil, S. V. (2019). Intensity improvement of YAG:Ce,Gd phosphor. AIP Conf. Proc. 2104:020010. doi: 10.1063/1.5100378
Veith, M., Mathur, S., Kareiva, A., Jilavi, M., Zimmer, M., and Huch, V. (1999). Low temperature synthesis of nanocrystalline Y3Al5O12 (YAG) and Ce-doped Y3Al5O12 via different sol-gel methods. J. Mater. Chem. 9, 3069–3079. doi: 10.1039/a903664d
Vorsthove, M., and Kynast, U. (2011). Efficiency issues in Ce3+ doped YAG nanocrystals. Mater. Res. Bull. 46, 1761–1765. doi: 10.1016/j.materresbull.2011.08.007
Wako, A. H., Dejene, F. B., and Swart, H. C. (2016). Effect of Ga3+ and Gd3+ ions substitution on the structural and optical properties of Ce3+-doped yttrium aluminium garnet phosphor nanopowders. Luminescence 31, 1313–1320. doi: 10.1002/bio.3108
Wang, J., Zheng, S., Zeng, R., Dou, S., and Sun, X. (2009). Microwave synthesis of homogeneous YAG nanopowder leading to a transparent ceramic. J. Amer. Ceram. Soc. 92, 1217–1223. doi: 10.1111/j.1551-2916.2009.03086.x
Wang, L., Zhao, F., Zhang, M., Hou, T., Li, Z., Pan, C., and Huang, H. (2016). Preparation and photoluminescence properties of YAG:Ce3+ phosphors by a series of amines assisted co-precipitation method. J. Alloys Cmpd. 661, 148–154. doi: 10.1016/j.jallcom.2015.11.106
Wang, X., Cao, Y., Wei, Q., Liu, X., Liao, X., Zhao, Z., et al. (2019). Insights into a novel garnet-based yellowish-green phosphor: structure, luminescence properties and application for warm white light-emitting diodes CrystEngComm 21, 6100–6108. doi: 10.1039/C9CE01163C
Wu, H., Yang, C., Zhang, Z., and Tang, Y. (2016). Photoluminescence and thermoluminescence of Ce3+ incorporated Y3Al5O12 synthesized by rapid combustion. Optik 127, 1368–1371. doi: 10.1016/j.ijleo.2015.10.228
Wu, J. L., Gundiah, G., and Cheetham, A. K. (2007). Structure–property correlations in Ce-doped garnet phosphors for use in solid state lighting. Chem. Phys. Lett. 441, 250–254. doi: 10.1016/j.cplett.2007.05.023
Xia, Z., and Meijerink, A. (2017). Ce3+-Doped garnet phosphors: composition modification, luminescence properties and applications. Chem. Soc. Rev. 46, 275–299. doi: 10.1039/C6CS00551A
Xu, J., Chen, W., Zeng, R., and Peng, D. (2014). A carbon-free sol–gel method for preparation of Lu3Al5O12:Ce3+ phosphors for potential applications in laser scintillators and LEDs. Mater. Lett. 133, 1–4. doi: 10.1016/j.matlet.2014.06.030
Xu, S., Sun, L., Zhang, Y., Ju, H., Zhao, S., Deng, D., et al. (2009). Effect of fluxes on structure and luminescence properties of Y3Al5O12:Ce3+ phosphors. J. Rare Earths 27, 327–329. doi: 10.1016/S1002-0721(08)60244-6
Yan, X., Li, W., Wang, X., and Sunz, K. (2012). Facile synthesis of Ce3+, Eu3+ co-doped YAG nanophosphor for white light-emitting diodes. J. Electrochem. Soc. 159, H195–H200. doi: 10.1149/2.101202jes
Yang, S. C., Kwak, S. Y., Jin, J. H., Kim, J. S., Choi, Y., Paik, K. W., et al. (2012). Thermally resistant UV-curable epoxy-siloxane hybrid materials for light emitting diode (LED) encapsulation. J. Mater. Chem. 22, 8874–8880. doi: 10.1039/c2jm16355a
Yang, Z., Li, X., Yang, Y., and Li, X. (2006). “The influence of Ce3+ doping concentration on luminescence of Y3Al5O12 :Ce3+ phosphors synthesized by combustion method,” in ICO20: Illumination, Radiation, and Color Technologies, 60330N. doi: 10.1117/12.668079
Yuan, F., and Ryu, H. (2004). Ce-doped YAG phosphor powders prepared by co-precipitation and heterogeneous precipitation. Mater. Sci. Eng. B 107, 14–18. doi: 10.1016/j.mseb.2003.10.002
Zhan, Z., An, J., Wei, Y., Tran, V. T., and Du, H. (2017). Inkjet-printed optoelectronics. Nanoscale 9, 965–993. doi: 10.1039/C6NR08220C
Zhang, K., Hu, W., Li, J., Tang, Y., and Liu, H. (2009). Matrix induced synthesis of Y3Al5O12:Ce phosphor through the Pechini method. Int. J. Mater. Res. 100, 238–242. doi: 10.3139/146.110004
Zhang, K., Liu, H. Z., Wu, Y. T., and Bin Hu, W. (2008). Co-precipitation synthesis and luminescence behavior of Ce-doped yttrium aluminum garnet (YAG:Ce) phosphor: the effect of precipitant. J. Alloys Compd. 453, 265–270. doi: 10.1016/j.jallcom.2006.11.101
Zhang, Y., Qiao, X., Wan, J., Wu, L. A., Chen, B., and Fan, X. (2017). Facile synthesis of monodisperse YAG:Ce3+ microspheres with high quantum yield via an epoxide-driven sol-gel route. J. Mater. Chem. C 5, 8952–8957. doi: 10.1039/C7TC02909H
Zhang, Y., and Yu, H. (2009). Synthesis of YAG powders by the co-precipitation method. Ceram. Int. 35, 2077–2081. doi: 10.1016/j.ceramint.2008.10.002
Keywords: nanocrystals, YAG:Ce, garnet, photoluminescence, LED lighting
Citation: Cantarano A, Ibanez A and Dantelle G (2020) Garnet-Type Nanophosphors for White LED Lighting. Front. Mater. 7:210. doi: 10.3389/fmats.2020.00210
Received: 23 April 2020; Accepted: 09 June 2020;
Published: 22 July 2020.
Edited by:
Stephane Parola, Université de Lyon, FranceReviewed by:
John Ballato, Clemson University, United StatesCopyright © 2020 Cantarano, Ibanez and Dantelle. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Géraldine Dantelle, Z2VyYWxkaW5lLmRhbnRlbGxlQG5lZWwuY25ycy5mcg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.