- 1Particle Accelerator for Medical Application Laboratory, Italian National Agency for New Technologies, Energy and Sustainable Economic Development (ENEA), Rome, Italy
- 2Department of Astronautical, Electrical and Energy Engineering (DIAEE), Sapienza University of Rome, Rome, Italy
- 3Research Center for Nanotechnology Applied to Engineering (CNIS), Rome, Italy
- 4Department of Chemical Sciences and Technologies, Tor Vergata University of Rome, Rome, Italy
- 5Department of Industrial Engineering, Tor Vergata University of Rome, Rome, Italy
- 6Department of Biology and Biotechnology “C. Darwin,” Sapienza University of Rome, Rome, Italy
Microorganisms often cause significant damage on historical objects. The archive or library materials as well as textile or leather artifacts suffer serious attacks that need appropriate care treatments. Several biocide processes have been implemented but often their application does not preserve the material of the good. The objective of this work is the disinfection through ionizing radiation of leather wallpaper from the museum building Palazzo Chigi in Ariccia (Rome, Italy). The controlled sterilization treatments were carried out using X-ray beams to eliminate the microorganisms present on the leather and maintaining unchanged the properties of the constituent material. Some fragments of decorated leather wallpaper, dating back to the 1700s, were irradiated with X-rays up to 5,000 Gy. The amount of microorganisms was evaluated by microbiological analysis before and after X-ray irradiation treatments to identify the dose that inhibits the bacterial load. It will be shown how the results obtained by the application of different chemical-physical techniques (Scanning Electron Microscopy, Fourier Transform Infrared spectroscopy and Light Transmission Analysis) have helped in the evaluation of the impact of the X-rays on leather chemical and physical integrity.
Introduction
The Palazzo Chigi of Ariccia (Rome) is an ancient residence of princes having the rooms enriched and decorated with precious seventeenth-century leather products: worked and printed leather with decorative motifs used in the form of panels for furnishing. The wallpapers in impressed leather are commonly called “cordovani,” from the manufacturing arts imported to Cordoba, Spain, from the east. These wallpapers still cover the walls of many rooms of the building and are a unique case in this genre (Petrucci, 2014). The whole covered rooms of the Palazzo Chigi and the numerous fragmented and incomplete wallpapers preserved in their archives could constitute a real “leather museum” (with pieces that can also be traced back to different types of use: table covers, for “dresser beds,” chair covers, valances, hat covers, etc.).
The realization of leather wallpapers dates back to ancient times and had a slow decline during the eighteenth century (replaced by fabrics and then printed papers): at the end of the century it seems that laboratories were active only in Barcelona, Mechelen, Venice, and Amsterdam (Contadini, 1989).
As most of the collagen-based artifacts, leather is frequently involved in bio-deterioration caused by microorganisms (Strzelczyk et al., 1989). The non-adequate environmental conditions of artifacts conservation constitute a crucial factor in the promotion and development of the bio-deterioration favored, in particular, by altered values of temperature and/or relative humidity (Strzelczyk et al., 1987, 1997; Montanari et al., 2012). The bio-deteriorating agents can cause structural and chromatic alterations inducing hydrolysis of the collagen fibers, modifying the leather's inorganic components, producing pigments, and organic acids causing chromatic alteration or discolouration of the support (Pinzari et al., 2012; Piñar et al., 2015a,b; Cicero et al., 2018; Mercuri et al., 2018). In some cases, they induce the crack and the partial detachments of the surface layers (Migliore et al., 2017, 2019).
High energy irradiation is a powerful tool for disinfection: archived and retained artifacts can be attacked and destroyed by microorganisms but they can be successfully treated in irradiation facilities, conventionally made with Co-60 gamma-ray sources (Adamo et al., 1998, 2001; Magaudda, 2004; da Silva et al., 2006; D'Almeida et al., 2009; Nunes et al., 2012; Sendrea et al., 2015, 2017).
Recently, the possibility to employ the X-rays to inhibit the microbial growth on bio-deteriorated parchment artifacts has been presented (Vadrucci et al., 2019a). The encouraging results obtained on this kind of collagenous material are due to the possibility to operate with a minimal intervention, the non-contact method and to reach the inside of 3-dimension items, by the high penetration power of the beams. All that suggests the possibility that X-ray irradiation treatment could be employed as a sterilization method more efficacious in terms of germicidal power, lowering of exposition time, better preservation of the substrate and less interference of the environment (powder, humiditiy) also in the case of the historical leather coming from the Palazzo Chigi of Ariccia.
Within the ADAMO project (developed thanks to the Excellence's Center of the Technological District for Culture (DTC) of Regione Lazio), it has been possible to perform biodeteriogens removal campaigns by the use of X-ray irradiation as efficacious, non-toxic and non-invasive system to inhibit bio-degradation and, at the same time, to make the characterization of the chromatic richness (Fantoni et al., 2019; Iorio et al., 2019; Vadrucci et al., 2019b).
The developed analytical methodology will be presented and discussed. The use of the nuclear technique for the bacterial growth inhibition on historical artcraft employing low dose levels, the use of microbiology procedures to control biodeteriogens and the use of surface and bulk physical analysis for material monitoring and characterization will be described in order to test the applicability of the irradiation treatment on these historical leathers.
Fragments of leather wall coverings were subjected to X-ray irradiation that inhibits bacterial growth at doses lower than 1 kGy which did not affect the characteristics of the substrate, as shown by the physical analysis carried out for the material monitoring.
Materials and Methods
Artifacts Description
The leather wallpapers in the Palazzo Chigi have been designed to furnish the home. They have been made with “green and gold,” “red and gold,” “red, green,” and “gold and silver” decorations, showing patterns from the Renaissance tradition in the Venetian manner, or embossed in the Dutch manner.
Many fragments of the leather panels are widespread on the antiquarian market and others are preserved in the warehouse halls where restoration laboratories were set up. Some of the pieces not worked by the restorers were made available for this work. They were all placed together in shelves not exposed to the sun but certainly not in optimal storage conditions.
The dimensions of the fragments that were the object of our research were a few tens of cm2.
Isolation and Characterization of Bacterial Strains
Bacteria from the leather samples were collected using sterile cotton swabs and then placed in sterile tube containing 2 mL of Nutrient Broth (NB) and finally incubated in laboratory at 30°C for 24 h. The collected samples were then plated on NB agar plates and, after further incubation at 30°C for 24 h, morphologically different colonies were purified.
DNA was extracted and amplified according to Bergkessel and Guthrie (2013). A region of about 1,400 bp from the 16S rRNA gene was amplified using the primers F8 (5′-AGAGTTTGATCCTGGCTCAG-3′) and R1492 (5′-GGTTACCTTGTTACGACTT-3′). The PCR reaction was performed utilizing the Taq DNA polymerase from Accuzyme DNA Polymerase (Bioline). BMR Genomics (Padova, Italy) sequenced the amplified region and the obtained sequences were analyzed with BLAST database. Bacterial strains isolated from the leather wallpapers and used in this study were the Gram-positive Bacillus cereus (Accession number: MN173590) and the Gram-negative Massilia timonae (Accession number: MN173589). They were grown in 2 mL of NB at 30°C overnight.
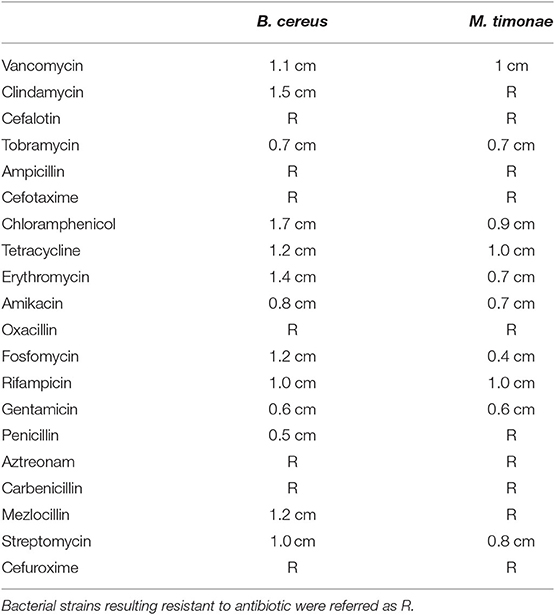
Antibiotic susceptibility tests were performed according to Schifano et al. (2019). One hundred microliters of overnight cultures of B. cereus or M. timonae were spread onto NB agar plates, in which the antibiotic discs were placed. The plates were then incubated for 24 h at 30°C. The zones of inhibition were measured from the center of the disc and recorded.
The biofilm formation was evaluated according to Zanni et al. (2017) with some differences: each well was filled with 200 μL of NB broth and B. cereus or M. timonae (1 × 107 cells/mL) in triplicate. Wells without bacteria were utilized as controls. Next, plates were incubated at 30°C for 24 h and Crystal Violet assay was performed. Absorbance at 600 nm was then measured by using a multiplate reader (Promega, GloMax multi+detection system). The experiment was repeated three times in triplicates.
Preparation of bio-deteriorated leather samples for irradiation treatments is performed cutting 1 × 1 cm2 pieces from the original leather fragment, than UV sterilized in both sides and stored in 3.5 cm Petri dishes at room-temperature until the load of bacteria for the irradiation tests (Figure 1A).
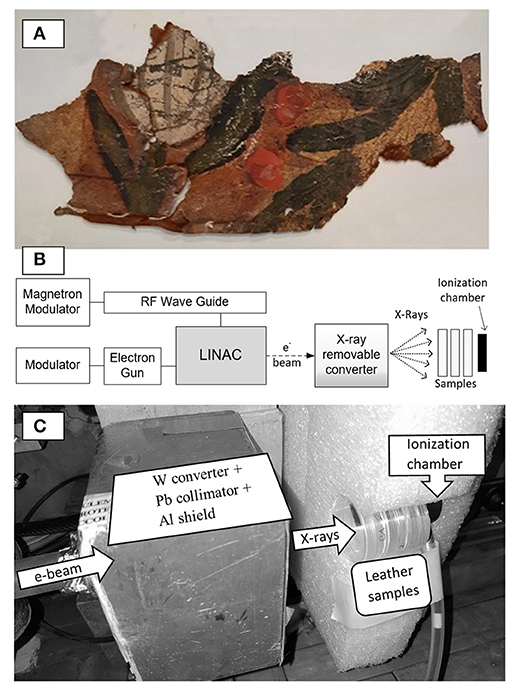
Figure 1. Leather specimens coming from Chigi Palace, Ariccia (Italy) and REX Irradiation System. (A) Fragment of ancient Spanish leather; (B) scheme of the REX facility and the irradiation geometrical layout set for experimental campaigns of leather treatment; (C) View of the irradiation layout inside the treatment chamber of the REX Source.
Irradiation Treatment
The irradiation care treatments were performed using the new REX source capable of emitting MeV energy X-ray beams (Vadrucci et al., 2019c). The REX setup consists of a linear electron accelerator, an irradiation chamber and a removable X-rays/electrons conversion system (Figures 1B,C).
The accelerator conditions used for the irradiation treatment were: 3 μs pulse length, 20 Hz repetition rate, and 120 mA output peak current. The X-rays dose and the dose rate measurements were performed by a parallel plate ionization chamber (mod. PPC05 from IBA). The samples were irradiated with different doses as indicated.
Antimicrobial Tests
Microbiological analysis were used to compare the total microbial count of bacteria cells before (untreated control samples, Ctr) and after (treated samples, Tr) the irradiation treatment; each dose was tested on three leathers fragment replicates. Liquid cultures of bacterial cells were spotted on specimens at the concentration of 7 × 103 cells/mL. After the irradiation, the microbes were collected by dipping the leather samples in 2 mL of PBS 1X, shaked for 2 min and spread an aliquot on the NB solid media. Plating was performed in triplicate and the microbial colonies on the plates were counted after 24 h at 25 ± 2°C. The differences in the colonization rate (in percentage) between untreated control samples and treated samples were statistically investigated by one-way ANOVA analysis with the Bonferroni post-test (GraphPad Prism 4.0 software, *p < 0.05, **p < 0.01, and ***p < 0.001).
Physical Analysis for Material Monitoring
Scanning Electron Microscopy Analysis
Scanning Electron Microscopy (SEM) was employed in order to evaluate morphological changes in the collagen fibers networking.
The microscopic analysis has been performed using a Zeiss Auriga Field Emission SEM (FE-SEM). In order to prevent charging, before each imaging session samples were sputter coated with a 30 nm Cr layer. The images were acquired at an accelerating voltage of 3 keV, using 1 kX and 5 kX magnifications. In order to evaluate the effects of the irradiation treatment on leather, SEM investigations have been performed on both pristine and irradiated specimens (untreated, 750, 1,000, and 5,000 Gy).
Fourier Transform Infrared Spectroscopy Attenuated Total Reflectance
The Fourier Transformed Infrared spectroscopy in the Attenuated Total Reflectance mode (FTIR-ATR) was used as surface technique to monitor the variations in secondary structures of the collagen protein in order to determine the variations induced by the deterioration processes eventually occurring in the sample after being irradiated (Badea et al., 2012).
FTIR absorption spectra were acquired on a Thermo-Scientific (mod. Is50) instrument (Thermo Scientific Inc., Madison WI) in Attenuated Total Reflectance (ATR) mode using a one reflection diamond cell, on which the sample was located during the measurement. Spectra were recorded from 4,000 to 750 cm−1, mediating over 32 scans with a resolution of 2 cm−1.
Light Transmission Analysis
The irradiated samples have been investigated also by the Light Transmission Analysis (LTA) (Cicero et al., 2019), as additional bulk analysis of the damage induced by the irradiations on the leather substrate, in order to determine the denaturation temperature, a parameter related to the deterioration degree of the collagenous material (Vadrucci et al., 2019a).
LTA was employed to analyze the hydrothermal denaturation process of the collagen fibers before and after irradiation procedure. The employed experimental configuration allows to characterize the phenomenon both in a qualitative and in a quantitative way enabling the imaging evaluation of the variations induced in the sample during the hydrothermal denaturation and, simultaneously, the recording of the variations of the optical properties of the investigated sample (Mercuri et al., 2016).
Results
In the following, the results obtained on the evaluation of the sterilization technique by X-ray irradiation as an alternative to the use of chemical biocides on historical leather artifacts are presented. The identification and characterization of the bacterial species together with their ability to form biofilms are reported. The data collected on the leather substrates subjected to increasing X-ray doses of the sterilization treatment are shown through the analysis carried out with the techniques presented in the previous paragraph.
Identification and Characterization of Bacterial Strains From Leather Samples
Different bacterial colonies were isolated from leather wallpaper of Palazzo Chigi in Ariccia and identified at the molecular level by the amplification of 16S rDNA. Among them, the comparison of the obtained sequences with those held in BLAST database allowed to identify the Gram-positive Bacillus cereus, and the Gram-negative Massilia timonae, which are involved in bio-deterioration, as reported in many works (Herrera and Videla, 2004; Jroundi et al., 2017). Antibiotic susceptibility test was performed using a panel of 20 antibiotics, including inhibitors of cell wall synthesis, protein synthesis, nucleic acid synthesis, and the results are reported in Table 1. The bacterial strains resulted resistant to different types of molecules, with M. timonae more resistant than B. cereus.
Evaluation of Biofilm Formation
The biodegradation of cultural heritage is mainly caused by the ability of microorganisms to form biofilms (Rivera et al., 2018). Indeed, bacteria can aggregate and adhere to a surface, forming a group of cells producing an extracellular matrix composed of DNA, proteins and polysaccharides. In these biofilms, microorganisms resist to adverse abiotic conditions (Dakal and Cameotra, 2012) and their metabolic activity, such as production of organic and inorganic acids, contributes to decay of different substrates (Scheerer et al., 2009; Sterflinger and Piñar, 2013). The biofilm on plastic surface was quantified after 24 h by Crystal Violet method. The biofilm formation capacity of B. cereus is about 80% lower than M. timonae (Figure 2A).
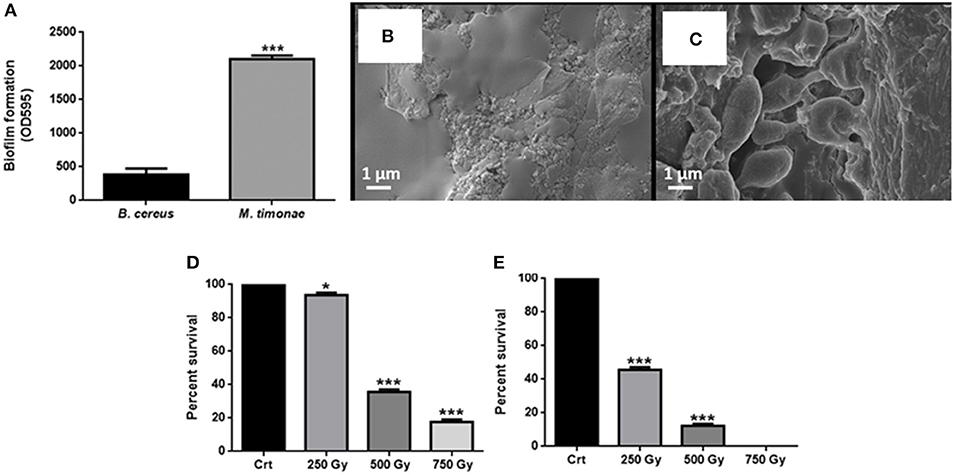
Figure 2. Evaluation of biofilm formation and cell viability after irradiation. (A) Biofilm formation capacity of B. cereus and M. timonae after 24 h of incubation at 30°C. For statistical analysis one-way ANOVA method coupled with the Bonferroni post-test was used (***p < 0.001). (B) FE-SEM micrograph of M. timonae biofilm after 24 h at 30°C, biofilm matrix, (C) particular of M. timonae cells. (D,E) Indicate respectively B. cereus and M. timonae cells recovered after irradiation. Crt represent the recovery from unexposed samples, set to 100%. To evaluate statistical significance a one-way ANOVA analysis with the Bonferroni post-test was used (*p < 0.5. and ***p < 0.001 with respect to Crt).
Since M. timonae resulted to be a higher biofilm producer, its capacity of forming biofilm was also evaluated on Spanish leather samples, through SEM analysis. In Figures 2B,C, it is possible to observe, as an example, the leather specimen covered by M. timonae biofilm. In particular, FE-SEM analysis allowed the observation of M. timonae biofilm matrix and, at higher magnification, single cells adhered on the substrate (Figures 2B,C, respectively).
Microbiological Tests After Irradiation
After irradiation of leather samples, the percentage of survival microorganisms collected from treated specimens were compared to untreated control sample. When leathers spread with B. cereus were irradiated, a bacterial growth reduction of 8, 66, and 84% was observed with 250, 500, and 750 Gy, respectively, as compared to control. The antibacterial activity was highlighted when samples were covered with the Gram-negative M. timonae culture and then treated. As the irradiation dose increased, a reduction of the number of cell recovery was obtained: the bacterial growth decreased of 56 and 89% when 250 and 500 Gy treatments were performed. Moreover, the total absence of growth was observed with 750 Gy treatment (Figures 2D,E).
SEM Characterization of the Morphological Changes in the Fibers Networking
SEM was employed in order to evaluate morphological changes in the fibers networking. The investigation was performed on all the irradiated samples with the aim of correlating the preservation conditions of the hierarchical structure to the irradiation dose employed in the treatment.
SEM micrographs of the non-irradiated sample (Figure 3A and magnification Figure 3B) show an intact fibers networking of the collagen, regardless of the long history of the specimen. Long and ordered bundles of fibers can be recognized within the pristine sample revealing, after all, a good preservation condition of the artifact. Comparing the obtained images of the untreated sample with the ones recorded on the leather sample irradiated at 750 Gy (Figures 3C,D) and 1,000 Gy (Figures 3E,F) dose levels, it is possible to notice that the ordered surface morphology is maintained also in the irradiated samples. Only slight differences can be observed in the sample treated with a dose of 1,000 Gy where some fibers start to shows a disordered structure due to the effects of a partial unwinding, revealing the onset of a gelatinization process.

Figure 3. Surface evaluation of the leather damage. SEM micrographs of the non-irradiated sample [(A) and its magnification (B)], of the leather irradiated with a dose of 750 Gy (C,D), with a dose of 1,000 Gy (E,F) and with the maximum dose of 5,000 Gy (G,H). Non-significant differences can be observed in the surface morphology of the sample irradiated with doses up to 1,000 Gy with respect to the non-irradiated one. A huge variation is instead visible in the fibers preservation condition of the sample irradiated with 5,000 Gy, where the surface shows wide areas of gelatinized materials [particularly visible in (G)] and the fibers bundles appear reduced in their dimension, frayed and fragmented (H).
Differently, the sample irradiated at the maximum dose of 5,000 Gy shows a completely different surface morphology with respect to the non-irradiated one. The image recorded at low magnification (Figure 3G) shows the total lack of the bundled organization of the fibers networking and the presence of areas of gelatinization with the typical melt-like appearance (Badea et al., 2008). At higher magnification (Figure 3H), the effect of the irradiation dose is clearly visible in the aspect of the remaining bundles where the ordered fibrillary structure is dramatically lost, the bundle dimension is heavily reduced and the remaining fibers appear frayed and fragmented.
FTIR-ATR Characterization of the Protein Induced Deterioration
FTIR-ATR is one of the most powerful, non-destructive techniques to monitor the changes in secondary structure (due to for example, denaturation) of proteins. The peptide groups are described in IR spectroscopy with nine characteristic bands named amide A, B, and I–VII in order of decreasing frequency (Haris and Chapman, 1995). Among them Amide I and amide II are the two major bands of the protein infrared spectrum; the amide I band falling in 1,680–1,610 cm−1 region, is mainly associated with the C=O stretching vibration related to the protein backbone and thus is sensitive to protein conformations and in turn, to water bound to the macromolecule. The amide II results essentially from the N–H bending vibration and from the C–N stretching vibration, and is in the 1,540–1,500 cm−1 region, depending on the conformation adopted by the peptide (Jackson and Mantsch, 1995; Tatulian, 2019). Due to the high sensitivity to conformation, an analysis on the relative intensities and shifts of these two bands furnishes information on the protein denaturation.
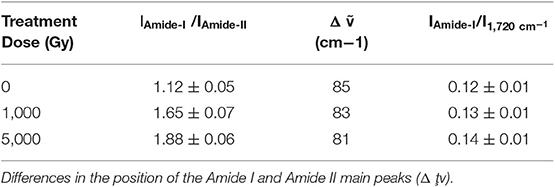
Data reported in the Table 2, indicates that the intensity of the Amide I band (IAmide I) decreases significantly after the irradiation treatment with respect to amide II band. This is ascribable to hydrolysis of the collagen present in the leather (Plavan et al., 2010; Vyskočilová et al., 2019). The slight difference between the position of the Amide I and Amide II main peaks, indicates that gelatinization does not occur even after high level treatment. Moreover, a slight increase of the band at about 1,720 cm−1, related to side chain oxided groups, is observed on increasing dose treatment (Figure 4A).
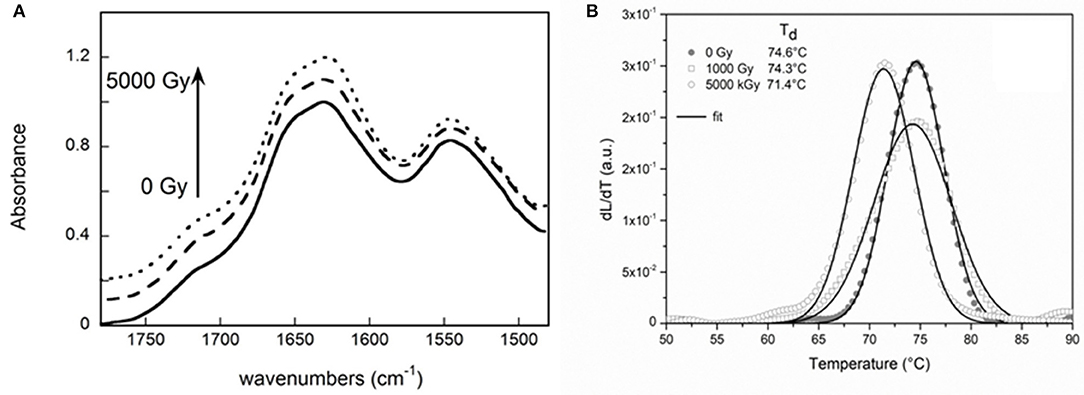
Figure 4. Structural evaluation of the leather damage and bulk evaluation of the leather damage. (A) FTIR spectra in the Amide I and Amide II region of not irradiated sample (black curve), of the leather irradiated with a dose of 1,000 Gy (dashed curve) and with a dose of 5,000 Gy (dotted curve); (B) LTA curves of the not irradiated sample (black dots curve), of the leather irradiated with a dose of 1,000 Gy (white squares curve) and with a dose of 5,000 Gy (white dots curve). A slight difference can be observed in the Td of the sample irradiated with 1,000 Gy with respect to the non-irradiated one while a large decrease of the denaturation temperature characterizes the sample irradiated with 5,000 Gy, thus revealing a significant deterioration induced by such irradiation dose.
LTA Characterization of the Deterioration Degree of Irradiated Leather
As mentioned in the previous paragraphs, the LTA allows to characterize the artificially induced hydrothermal denaturation of collagen-based materials and to determine the so-called denaturation temperature, the temperature at which, heating a fully hydrated specimen, the collagen fibers accomplish their thermal unfolding. When heated under condition of full hydration the collagen fibers lose their hierarchical well-structured arrangement turning into an amorphous one with a gel-like appearance. In a partially deteriorated collagen based material the maximum rate of the denaturation activity is reached at lower temperatures with respect to non-deteriorated and well-structured samples because these partially degraded collagen molecules require lower energy with respect to the intact collagen, while undergoing the gelatinization process. With respect to other collagen based materials such as parchment, leather accomplishes its hydrothermal denaturation at higher temperature values. This capability is mainly due to its peculiar manufacturing process: the tanning step gives the collagen fibers a higher and more uniform stabilization with respect to those obtained by the parchment manufacturing process, making them more resistant to the effect of deterioration agents (Cucos et al., 2014).
The analysis has been performed maintaining the samples immersed in water while heating them in the temperature range 25–100°C. The denaturation temperature recorded for the non-irradiated sample can be considered as the reference for the original preservation state of the leather samples while the variation from this starting value in the recorded Td for the irradiated ones can be considered as indication of a possible effects of the irradiation dose.
In Figure 4B the curves of the variation rate of the LTA signal as a function of the sample temperature for all the analyzed leather samples are reported. The curves describe the rate at which the hydrothermal denaturation takes place. Unlike curves describing the denaturation process of parchment, in the case of leather only one well-defined symmetric peak can be recognized. This is mainly due to the presence, in this kind of collagen based artifact, of a unique stabilized collagen population with a general more uniform and higher hydrothermal stability (Cucos et al., 2014) recognizable by higher values of Td with respect to the ones recorded for parchment (Vadrucci et al., 2019a). As it is shown in the graph, only a slight difference can be observed between the temperature of the sample irradiated with a dose of 1,000 Gy (ΔTd = 0.3°C) and the non-irradiated one. Typical intrinsic inhomogeneity of the sample microstructure has to be considered and such a small variation cannot be ascribed to the effect of the irradiation dose with absolute confidence. Differently, the large Td variation observed between the sample irradiated with a dose of 5,000 Gy and the reference one (ΔTd = 2.9°C) reveals a substantial deteriorating effect of the irradiation dose on the collagen structural stability.
Therefore, these preliminary results show that, with respect to parchment the leather manifests to better tolerate irradiation doses till 1,000 Gy that do not seem to induce significant variations of the thermal stability.
Discussion and Conclusions
Bio-deterioration is one of the main problems encountered in the conservation of cultural heritage, in all types of historic and modern relics (Sterflinger et al., 2018). There are several methods to control this phenomenon such as physical, mechanical and biochemical.
However, commercial biocides show often their effect only for a limited time, since they can be utilized as a nutrient source by autochthonous microorganisms that develop resistance (Kakakhel et al., 2019).
In this work, an alternative method to chemical biocides has been evaluated in the case of ancient leathers. In order to get insight on the efficacy of the treatment, two different bacterial isolated from the leather samples were used to contaminate the surface. B. cereus and M. timonae were isolated for this analysis because of their ability to form biofilm on the surface, a bacterial aggregation resistant to many biocides and able to deteriorate manufactures (Stewart, 2002; Rivera et al., 2018). This was confirmed also in this case by the antibiotic susceptibility test, revealing several resistances for both strains. The bacterial cells were spotted on the specimens and irradiated by X-ray. A linear accelerator for the production of X-rays allows numerous advantages compared to the traditional gamma-ray radioactive sources traditionally employed in the treatment of collagen based artifacts (Nunes et al., 2012; Sendrea et al., 2015, 2017). In fact, radioactive sources such as the Co-60 require massive shielding, compliance with stringent regulations for use and management, obtaining authorization for use, costly procedures for controlled disposal of the source at end of life. As mentioned in the previous paragraphs, this facility was already employed on the COBRA project (COBRA, 2017) for the preservation treatment of cultural assets, with the case studies of processes biodegradation removal from fabric, wood and parchment writing supports (Borgognoni et al., 2017).
Concerning the evaluation of the effects of the irradiation dose on the structural stability of the collagen molecule it is possible to say that, although its locally-destructive nature, SEM analysis remains a reference technique in order to collect images of the surface morphology and preservation condition of collagenous materials. It allows the evaluation of the eventual disruption of the surface fibers networking and the appearance of areas of gelatinization of the collagen fibers (Della Gatta et al., 2005; Badea et al., 2008, 2012). This preliminary surface analysis puts in evidence that the variation in the fibers morphology and dimension can be slightly appreciated starting from the dose of 1,000 Gy highlighting this dose as the threshold one to be not exceeded in order to not induce further deterioration factors in the treated substrate.
The preliminary test of the potential damage induced by the irradiations on differently irradiated leather substrates performed by the LTA gives us the possibility to obtain an indication of the average damage induced over the entire leather sample by different irradiation doses. The slight variation in the denaturation temperature obtained for the sample treated with the dose of 1,000 Gy confirms the surface information obtained by the SEM investigation and still indicates this dose as the threshold one.
Further and decisive information has been obtained by the employment of the FTIR-ATR analysis on the deterioration processes induced by the different irradiation doses with respect to the ones obtained by the previous analysis. This technique provides further and specific information on the characteristic deterioration processes induced in the treated substrate by the different irradiation doses. Specifically, it confirms the results obtained by the previous presented techniques regarding the gelatinization processes occurring noticeably only at high doses of irradiation. Furthermore, it gives important information also on the hydrolysis of the collagen molecule already occurring at the lowest investigated dose of irradiation (1,000 Gy).
In conclusion our data point out the irradiation method to be exploited as alternative to biocides for historical leathers since it doesn't lead to unacceptable changes in their functional or decorative properties.
Further researches are still in progress in order to discriminate damages eventually induced in collagen within ancient and modern leather manufactured with different tanning processes.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
MV: designed the study and organized the database. MV and DU: contributed to the conception of experimental campaigns. MV and FB: performed the irradiation tests. DU and ES: the microbiological studies. GD and CC: the SEM measurements. CM: the FTIR spectroscopy. FM and CC: the LTA analysis. MV: wrote the first draft of the manuscript. GD, CM, FB, ES, DU, and CC: wrote sections of the manuscript. All authors: contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was partially supported by the project ADAMO B86C18001220002 of the Excellence Center at the Lazio Technological District for Cultural Heritage (DTC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Francesco Petrucci, conservator of Palazzo Chigi in Ariccia (Rome), for the possibility to perform the analysis.
References
Adamo, M., Brizzi, M., Magaudda, G., Martinelli, G., Plossi-Zappalà, M., Rocchetti, F., et al. (2001). Gamma radiation treatment of paper in different environmental conditions: chemical, physical and microbiological analysis. Restaurator 22:107. doi: 10.1515/REST.2001.107
Adamo, M., Giovannotti, M., Magaudda, G., Plossi Zappalà, M., Rocchetti, F., and Rossi, G. (1998). Effect of gamma rays on pure cellulose paper as a model for the study of a treatment of “biological recovery” of biodeteriorated books. Restaurator 19, 41–59. doi: 10.1515/rest.1998.19.1.41
Badea, E., Della Gatta, G., and Usacheva, T. (2012). Effects of temperature and relative humidity on fibrillar collagen within parchment: a micro Differential Scanning Calorimetry (micro DSC) study. Polym. Degrad. Stabil. 97, 346–353. doi: 10.1016/j.polymdegradstab.2011.12.013
Badea, E., Miu, L., Budrugeac, P., Giurginca, M., Mašić, A., Badea, N., et al. (2008). Study of deterioration of historical parchments by various thermal analysis techniques complemented by SEM, FTIR, UV-Vis-NIR and unilateral NMR investigations. J. Thermal Anal. Calor. 91, 17–27. doi: 10.1007/s10973-007-8513-x
Bergkessel, M., and Guthrie, C. (2013). Colony PCR. Methods Enzymol. 529, 299–309. doi: 10.1016/b978-0-12-418687-3.00025-2
Borgognoni, F., Vadrucci, M., Bazzano, G., Ferrari, P., Massa, S., Moretti, R., et al. (2017). X-ray sterilization of insects and microorganisms for cultural heritage applications. Nuclear Instr. Methods Phys. Res. Sect. B 406, 309–313. doi: 10.1016/j.nimb.2017.03.033
Cicero, C., Mercuri, F., Paoloni, S., Orazi, N., Zammit, U., Glorieux, C., et al. (2019). Integrated adiabatic scanning calorimetry, light transmission and imaging analysis of collagen deterioration in parchment. Thermochim. Acta 676, 263–270. doi: 10.1016/j.tca.2019.05.007
Cicero, C., Pinzari, F., and Mercuri, F. (2018). 18th century knowledge on microbial attacks on parchment: analytical and historical evidence. Int. Biodeter. Biodegrad. 134, 76–82. doi: 10.1016/j.ibiod.2018.08.007
COBRA (2017). Project (lr13/2008 project n. 1031), 2015-2018 Methods, Technologies and Advanced Tools for the Conservation of Cultural Heritage, Based on the Application of Radiation and Enabling Technologies. Available online at: http://cobra.enea.it/english (accessed July 21, 2015).
Contadini, A. (1989). ““Cuoridoro”: tecnica e decorazione di cuoi dorati veneziani e italiani con influssi islamici,” In: Arte veneziana e arte islamica: atti del Primo simposio internazionale sull'arte veneziana e l'arte islamica, ed E. J. Grube (Venice: Edizioni l'Altra Riva, 231–251.
Cucos, A., Budrugeac, P., and Miu, L. (2014). DMA and DSC studies of accelerated aged parchment and vegetable-tanned leather samples. Thermochim. Acta 583, 86–93. doi: 10.1016/j.tca.2014.03.022
da Silva, M., Moraes, A. M. L., Nishikawa, M. M., Gatti, M. J. A., Vallim de Alencar, M. A., Brandão, L. E., et al. (2006). Inactivation of fungi from deteriorated paper materials by radiation. Int. Biodet. Biodegrad. 57, 163–167. doi: 10.1016/j.ibiod.2006.02.003
Dakal, T. C., and Cameotra, S. S. (2012). Microbially induced deterioration of architectural heritages: routes and mechanisms involved. Environ. Sci. Europe 24:36. doi: 10.1186/2190-4715-24-36
D'Almeida, M. L. O., Barbosa, P., d. S. M., Boaratti, M. F. G., and Borrely, S.I. (2009). Radiation effects on the integrity of paper. Radiat. Phys. Chem. 78, 489–492. doi: 10.1016/j.radphyschem.2009.03.032
Della Gatta, G., Badea, E., Ceccarelli, R., Usacheva, T. Maši, A., and Coluccia, S. (2005). Assessment of damage in old parchments by DSC and SEM. J. Therm. Anal. Calorim. 82, 637–649. doi: 10.1007/s10973-005-0944-7
Fantoni, R., Lazic, V., Vadrucci, M., Sorrentino, B., Chiari, M., Mazzinghi, A., et al. (2019). “Complementary characterization of ancient Roman frescoes by PIXE and LIBS techniques,” in EMSLIBS-2019 (Brno).
Haris, P. I., and Chapman, D. (1995). The conformational analysis of peptides using fourier transform IR spectroscopy. Biopolymers 37, 251–263. doi: 10.1002/bip.360370404
Herrera, L., and Videla, H. (2004). The importance of atmospheric effects on biodeterioration of cultural heritage constructional materials. Int. Biodet. Biodegrad. 54, 125–134. doi: 10.1016/j.ibiod.2004.06.002
Iorio, M., Graziani, V., Lins, S., Ridolfi, S., Branchini, P., Fabbri, A., et al. (2019). Exploring manufacturing process and degradation products of gilt and painted leather. J. Appl. Sci. 9:3016. doi: 10.3390/app9153016
Jackson, M., and Mantsch, H. H. (1995). The use and misuse of FTIR spectroscopy in the determination of protein structure. Crit. Rev. Biochem. Mol. Biol. 30, 95–120. doi: 10.3109/10409239509085140
Jroundi, F., Schiro, M., Ruiz-Agudo, E., Elert, K., Martín-Sánchez, I., González-Muñoz, M. T., et al. (2017). Protection and consolidation of stone heritage by self-inoculation with indigenous carbonatogenic bacterial communities. Nat. Commun. 8:279. doi: 10.1038/s41467-017-00372-3
Kakakhel, M. A., Wu, F., Gu, J.-D., Feng, H., Shah, K., and Wang, W. (2019). Controlling biodeterioration of cultural heritage objects with biocides: a review. Int. Biodet. Biodegrad. 143:104721. doi: 10.1016/j.ibiod.2019.104721
Magaudda, G. (2004). The recovery of biodeteriorated books and archive documents through gamma radiation: some considerations on the results achieved. J. Cult. Heritage 5, 113–118. doi: 10.1016/j.culher.2003.07.003
Mercuri, F., Buonora, P., Cicero, C., Helas, P., Manzari, F., Marinelli, M., et al. (2018). Metastructure of illuminations by infrared thermography. J. Cult. Heritage 31, 53–62. doi: 10.1016/j.culher.2017.10.008
Mercuri, F., Zammit, U., Paoloni, S., Cicero, C., and Orazi, N. (2016). Apparato e Metodo per l'analisi della Denaturazione di Collagene Strutturato in Materiali Membranacei. Patent pending - 102016000079558.
Migliore, L., Perini, N., Mercuri, F., Orlanducci, S., Rubechini, A., and Thaller, M. C. (2019). Three ancient documents solve the jigsaw of the parchment purple spot deterioration and validate the microbial succession model. Sci. Rep. 9, 1623–1623. doi: 10.1038/s41598-018-37651-y.
Migliore, L., Thaller, M. C., Vendittozzi, G., Mejia, A. Y., Mercuri, F., Orlanducci, S., et al. (2017). Purple spot damage dynamics investigated by an integrated approach on a 1244 A.D. parchment roll from the Secret Vatican Archive. Sci. Rep. 7:9521. doi: 10.1038/s41598-017-05398-7
Montanari, M., Melloni, V., Pinzari, F., and Innocenti, G. (2012). Fungal biodeterioration of historical library materials stored in Compactus movable shelves. Int. Biodet. Biodegrad. 75, 83–88. doi: 10.1016/j.ibiod.2012.03.011.
Nunes, I., Mesquita, N., Cabo Verde, S., João Trigo, M., Ferreira, A., Manuela Carolino, M., et al. (2012). Gamma radiation effects on physical properties of parchment documents: assessment of Dmax. Rad. Phys. Chem. 81, 1943–1946. doi: 10.1016/j.radphyschem.2012.07.016
Petrucci, F. (2014). “Vita e arte negli arredi dei palazzi barocchi tra tradizione e modernità,” in Edizioni Musei Vaticani: Città del Vaticano, eds P. Dentro Il, R. A. Volpi, and C. Vatican, 249–282.
Piñar, G., Sterflinger, K., Ettenauer, J., Quandt, A., and Pinzari, F. (2015b). A combined approach to assess the microbial contamination of the archimedes palimpsest. Microb. Ecol. 69, 118–134. doi: 10.1007/s00248-014-0481-7
Piñar, G., Sterflinger, K., and Pinzari, F. (2015a). Unmasking the measles-like parchment discoloration: molecular and microanalytical approach. Environ. Microbiol. 17, 427–443. doi: 10.1111/1462-2920.12471
Pinzari, F., Cialei, V., and Piñar, G. (2012). “A case study of ancient parchment bio-deterioration using variable pressure and high vacuum scanning electron microscopy,” in Historical Technology, Materials and Conservation: SEM and Microanalysis, eds N. Meeks, C. Cartwright, A. Meek, A. Mongiatti (London: Archetype Publications, 93–99.
Plavan, V., Giurginca, M., Budrugeac, P., Vilsan, M., and Miu, L. (2010). Evaluation of the physico-chemical characteristics of leather samples of some historical objects from kiev. Rev. Chim. 61, 627–631.
Rivera, L. E. C., Ramos, A. P., Sánchez, J. I. C., and Serrano, M. E. D. (2018). “Origin and Control Strategies of Biofilms in the Cultural Heritage,” in Antimicrobials, Antibiotic Resistance, Antibiofilm Strategies and Activity Methods, ed S. Kirmusaoğlu (IntechOpen). doi: 10.5772/intechopen.79617
Scheerer, S., Ortega-Morales, O., and Gaylarde, C. (2009). Microbial deterioration of stone monuments–an updated overview. Adv. Appl. Microbiol. 66, 97–139. doi: 10.1016/s0065-2164(08)00805-8.
Schifano, E., Zinno, P., Guantario, B., Roselli, M., Marcoccia, S., Devirgiliis, C., et al. (2019). The foodborne strain Lactobacillus fermentum MBC2 triggers pept-1-dependent pro-longevity effects in Caenorhabditis elegans. Microorganisms 7:45. doi: 10.3390/microorganisms7020045
Sendrea, C., Badea, E., Stănculescu, I., Lucreţia, M., and Horia, I. (2015). Dose-dependent effects of gamma irradiation on collagen in vegetable tanned leather by mobile NMR spectroscopy. Leather Footw. J. 15:139. doi: 10.24264/lfj.15.3.1
Sendrea, C., Carsote, C., Radu, M., Badea, E., and Miu, L. (2017). The effect of gamma irradiation on shrinkage activity of collagen in vegetable tanned leather. Revista Chim. 68, 1535–1538.
Sterflinger, K., Little, B., Pinar, G., Pinzari, F., de los Rios, A., and Gu, J.-D. (2018). Future directions and challenges in biodeterioration research on historic materials and cultural properties. Int. Biodet. Biodegrad. 129, 10–12. doi: 10.1016/j.ibiod.2017.12.007
Sterflinger, K., and Piñar, G. (2013). Microbial deterioration of cultural heritage and works of art — tilting at windmills? Appl. Microbiol. Biotechnol. 97, 9637–9646. doi: 10.1007/s00253-013-5283-1
Stewart, P. S. (2002). Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. 292, 107–113. doi: 10.1078/1438-4221-00196
Strzelczyk, A. B., Bannach, L., and Kurowska, A. (1997). Biodeterioration of archeological leather. Int. Biodet. Biodegrad. 39, 301–309. doi: 10.1016/S0964-8305(97)00026-7
Strzelczyk, A. B., Kuroczkin, J., and Krumbein, W. E. (1987). Studies on the microbial degradation of ancient leather bookbindings: part I. Int. Biodet. 23, 3–27. doi: 10.1016/0265-3036(87)90039-X
Strzelczyk, A. B., Kuroczkin, J., and Krumbein, W. E. (1989). Studies on the microbial degradation of ancient leather bookbindings. part 2. Int. Biodet. 25, 39–47. doi: 10.1016/0265-3036(89)90027-4
Tatulian, S. A. (2019). “FTIR analysis of proteins and protein–membrane interactions,” in Lipid-Protein Interactions: Methods and Protocols, ed J.H. Kleinschmidt (New York, NY: Springer New York), 281–325. doi: 10.1007/978-1-4939-9512-7_13
Vadrucci, M., Borgognoni, F., Cicero, C., Perini, N., Migliore, L., Mercuri, F., et al. (2019a). Parchment processing and analysis: ionizing radiation treatment by the REX source and multidisciplinary approach characterization. Appl. Rad. Isotopes 149, 159–164. doi: 10.1016/j.apradiso.2019.04.021
Vadrucci, M., Chiari, M., Giuntini, L., Picardi, L., Ronsivalle, C., and Sorrentino, B. (2019b). “PIXE spectroscopy for the ADAMO project,” in 3th European Conference on Accelerators in Applied Research and Technology, ECAART13 (Split, CR: Ruder Boškovic Institute).
Vadrucci, M., Ferrari, P., Borgognoni, F., and Campani, L. (2019c). The REX irradiation facility and its applications. Nuclear Inst. Methods Phys. Res. Sect. A 930, 126–131. doi: 10.1016/j.nima.2019.02.066
Vyskočilová, G., Ebersbach, M., Kopecká, R., Prokeš, L., and Príhoda, J. (2019). Model study of the leather degradation by oxidation and hydrolysis. Heritage Sci. 7:26. doi: 10.1186/s40494-019-0269-7
Zanni, E., Bruni, E., Chandraiahgari, C. R., De Bellis, G., Santangelo, M. G., Leone, M., et al. (2017). Evaluation of the antibacterial power and biocompatibility of zinc oxide nanorods decorated graphene nanoplatelets: new perspectives for antibiodeteriorative approaches. J. Nanobiotechnol. 15:57. doi: 10.1186/s12951-017-0291-4
Keywords: leather, bio-deterioration, antimicrobial, X-ray, SEM, FTIR-ATR, LTA, cultural heritage
Citation: Vadrucci M, De Bellis G, Mazzuca C, Mercuri F, Borgognoni F, Schifano E, Uccelletti D and Cicero C (2020) Effects of the Ionizing Radiation Disinfection Treatment on Historical Leather. Front. Mater. 7:21. doi: 10.3389/fmats.2020.00021
Received: 09 October 2019; Accepted: 20 January 2020;
Published: 11 February 2020.
Edited by:
Luca Tortora, Department of Science, Roma Tre University, ItalyReviewed by:
Vincenzo Palleschi, Istituto di Chimica dei Composti OrganoMetallici (ICCOM), ItalyGiuseppe Ciccarella, University of Salento, Italy
Copyright © 2020 Vadrucci, De Bellis, Mazzuca, Mercuri, Borgognoni, Schifano, Uccelletti and Cicero. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Monia Vadrucci, bW9uaWEudmFkcnVjY2lAZW5lYS5pdA==
 Monia Vadrucci
Monia Vadrucci Giovanni De Bellis
Giovanni De Bellis Claudia Mazzuca4
Claudia Mazzuca4 Fulvio Mercuri
Fulvio Mercuri Emily Schifano
Emily Schifano Daniela Uccelletti
Daniela Uccelletti Cristina Cicero
Cristina Cicero