- 1School of Life Science, East China Normal University, Shanghai, China
- 2East China Sea Fisheries Research Institute, Chinese Academy of Fishery Sciences, Shanghai, China
In recent years, as anthropogenic activities the alkalinity of water bodies has intensified, which has seriously affected the development of aquaculture. Cross breeding can inherit the good traits of parents and develop stronger resistance to stress. Therefore, we investigated the advantages of the hybrid population (TH) of Litopenaeus vannamei over the normal variety (TC) in terms of survival rate, morphological changes of gill tissue, ion transport, and energy metabolism. After culture in the same environment, two species of shrimp were subjected to acute exposure to alkalinity levels of 50 mg/L, 200 mg/L, and 350 mg/L for 24 hours, and samples were taken at 0, 4, 8, 12, and 24 h, respectively. The study showed that under alkalinity stress, the TH group had a higher survival rate, greater hemolymph urea nitrogen content, and better gill tissue integrity compared to the TC group. The TH group also exhibited increased activities of key enzymes such as Na+/K+ ATPase and Ca2+/Mg2+ ATPase, along with elevated levels of urea nitrogen, arginase. Additionally, the expression of key genes, including NKA (Na+/K+-ATPase), CA (carbonic anhydrase), and HSPs (heat shock proteins) was upregulated in the TH group, that suggests that these genes may play a crucial role in improving tolerance to high-alkalinity environments. Our results demonstrated that under the same concentration of alkaline stress, the TH population had stronger nitrogen metabolism ability and stronger stress resistance than the TC population. This study can provide a theoretical reference for breeding high-alkalinity tolerance varieties of L. vannamei.
1 Introduction
The global area of saline-alkali land, which is approximately 950 million hectares, includes 99.13 million hectares in China, of which about 55% is saline-alkali water (surface water), covering 45.87 million hectares, and this represents a significant underutilized resource (Wang et al., 2023). Since saline-alkali water typically has a high pH and is primarily composed of high concentrations of carbonate (including bicarbonate and carbonate) and complex ions such as sodium, potassium, calcium, and chloride ions, these conditions can have adverse effects on the survival, growth, development, and reproduction of organisms (Cheng et al., 2022). In the natural environment, saline water also affects the organisms living in it in various ways. Increased salinity can directly affect the pH of water bodies, and eventually extreme alkalinity will lead to the mass death of aquatic organisms (Wilkie and Wood, 1996). In addition, excess carbonate in saline water exhibits acute toxicity and is negatively correlated with the DNA methylation level of Scylla paramamosain (Wang et al., 2023).The high-alkaline environment will affect the osmoregulatory ability of L. vannamei by altering the activity of Na+/K+-ATPase and heat shock proteins (altered gene expression) (Giffard-Mena et al., 2024). Besides, it was found that carbonate alkalinity stress interferes with ion transport, antioxidant defense and energy metabolism by affecting proline metabolism (especially pycr gene expression), leading to liver injury and decreased survival of Oreochromis niloticus (Wang et al., 2024). Current studies showed that the China rate of salinization will still expanding at an average annual rate of 10 % (Huang et al., 2025). Therefore, studying the ecological and physiological changes of aquatic organisms in saline-alkali water environment has important reference value for the development of saline-alkali water aquaculture, the protection of the ecological environment, and the improvement of water resource utilization.
As an important respiratory organ, gills have functions such as ion balance, osmoregulation, gas exchange, and filtration in aquatic animals (McNamara, 2012). Therefore, gill tissue is commonly analyzed to assess the extent of environmental stress-induced damage in shrimp (da Mota Araujo et al., 2019). It was found that high alkalinity stress may lead to inhibition of anti-inflammatory and excretory regulation function in the gills of Eriocheir sinensis (Zhang et al., 2023b). Additionally, study by Zhao et al. (2020) found that Oreochromis niloticus, under carbonate alkalinity stress, activated pathways related to ammonia metabolism, regulating ammonia levels through ion transport. Among them, ion transport enzymes including Na+/K+-ATPase (NKA) and carbonic anhydrase (CA) play an important role (Yao et al., 2015). NKA is one of the main driving enzymes involved in active transepithelial ion exchange processes on the cell membrane (Charmantier et al., 2008). CA primarily facilitates the conversion of carbon dioxide to bicarbonate and carbonate, and at the tissue and cellular levels, it facilitates gas exchange, ion transport, and acid-base balance (Deng et al., 2023). Although there are different subtypes of CA in crustaceans, consistent results appear in response to changes in salinity (Pan et al., 2016). Furthermore, studies have shown that important ion transporters such as V(H)-atpase, Na+K+-atpase, and Na+/H+ and Cl−/HCO3− exchangers play a role in maintaining ion regulation (Gocha et al., 1987). In addition, gills also have a certain detoxifying effect. Aquatic organisms produce glutamate and glutamine through glutamate dehydrogenase (GDH) and glutamine synthetase (GS), which were crucial for the regulation of nitrogen assimilation (Anderson et al., 2002; Sinha et al., 2013). By producing glutathione to improve the body’s antioxidant and detoxification ability (Kim and Kwak, 2022). The ability of organisms to resist adverse environments is regulated by various pathways, including a variety of important genes, such as nitric oxide synthase (NOS), thioredoxin (Trx), heat shock protein, etc. NOS is a key enzyme that catalyzes the synthesis of nitric oxide (NO), which is known as an antimicrobial molecule that fights pathogen infection (Jeong et al., 2016). Thioredoxin (Trx) is a ubiquitous small redoreductase involved in disulfide bond reduction of a large number of target proteins (Traverso et al., 2007). Intracellular-resident heat shock proteins (HSPs) with molecular weights of approximately 70 and 90 kDa function as chaperones, helping proteins to fold/unfold and transport across membranes, and to prevent protein aggregation after environmental stresses (Shevtsov and Multhoff, 2016). Therefore, it is necessary to study the effects of high alkalinity on ion transport and stress resistance in aquatic organisms.
Hybridization, which includes interspecies, intraspecies, and subspecies crosses, is a commonly used method for producing superior offspring (Chan et al., 2019). For intraspecific genetic improvement, aquaculture typically uses two main traditional breeding methods: cross breeding and selective breeding (Hulata, 2004). Hybrids usually have higher hybrid viability, such as survival rate, food conversion rate, heat tolerance, disease resistance and other traits, such as hybrid abalone have a higher response to the immune response and have higher heat tolerance (Liang et al., 2014), oysters obtained by hybridization have a higher survival rate (Ma et al., 2022), and hybridization significantly increased the survival rate of L. vannamei (Ye et al., 2023). In addition, these methods used in the cultivation of oysters, scallops, carp, catfish, salmon, carp, sunfish, etc (Bartley et al., 2000; Guo, 2009). Overall, hybridization is associated with improved stress resistance. Therefore, it is the most effective and practical way to obtain high-alkalinity resistant L. vannamei varieties by hybridization.
L. vannamei is an important species in the world’s aquaculture industry, with the advantages of delicious meat, high nutritional value and fast growth rate (Sirirustananun et al., 2011). In addition, it can tolerate a wide range of temperature and salinity conditions, allowing it to be used in aquaculture in many parts of the world (Roy et al., 2010). However, its alkalinity resistance is unstable, and its survival rate significantly decreases at pH levels above 8.3 (Fu-yi, 2004). Current research on alkaline stress focuses on growth, metabolism and immunity. In this study, the physiological mechanism of the normal vareity of L. vannamei and the hybrid population under high alkalinity stress was compared and analyzed, in order to confirm that the hybrid population has certain advantages in alkalinity stress resistance. This study provides a theoretical basis for breeding alkalinity-resistant aquatic species in saline-alkali environments.
2 Materials and methods
2.1 Source of experimental organisms and management
The L. vannamei hybrid group (TH) used in this experiment were provided by Shanghai Ocean University (Shanghai, China), hybridization strategy used was described previously (Ye et al., 2024b, hybridization was performed between EB♂ × CE♀. Shrimp of the normal variety from the Lutai Company (Wenchang, China) were selected as the control group (TC). Both groups of shrimps were previously raised in exactly the same conditions (salinity of 1 ppt, pH of 8.2 ± 0.1, temperature of 25 ± 0.5°C, and dissolved oxygen >6.5 mg/L). Then, healthy shrimp of uniform size (body weight 4.89 ± 0.31g) were randomly selected from the two groups (180 shrimp in each group, a total of 360 shrimp) for follow-up experiment.
2.2 Acute carbonate exposure experiment in shrimps
According to the research conducted by (Zongli et al., 2012), they explored the effects of varying levels of carbonate alkalinity—termed simply as “alkalinity” throughout their work—on the L. vannamei when subjected to toxicity-induced stress. Consequently, our experimental design encompasses three distinct alkalinity levels: a control group maintained at 50 mg/L, an intermediate alkalinity group adjusted to 200 mg/L, and a high alkalinity stress group calibrated to 350 mg/L. For the control scenario, the baseline water with its inherent alkalinity of 50 mg/L was utilized without modification. In contrast, the intermediate (200 mg/L) and high (350 mg/L) alkalinity groups required Na2CO3 to be dissolved in deionized water and then added to the base water under stirring. The pH was monitored with a calibrated pH meter throughout the process to ensure stability. This was followed by a 24-hour period to ensure complete stabilization of the solutions. The specific carbonate alkalinity was quantified employing an acid-base titration method (Wang et al., 1997), specifically by using 0.02 N hydrochloric acid (HCl) as the titrant and a pH meter to detect the endpoint at pH 4.5.
All 360 shrimps were divided into the following six groups: TC-50, TC-200, TC-350, TH-50, TH-200 and TH-350. Each group was randomly assigned to three replicate tanks with 20 shrimps and 60 L water each. Except for the alkalinity settings, the water quality parameters were the same as those in the previous culture. Then a 24h acute alkalinity stress experiment was carried out. Shrimp were fasted throughout the experimental period. Sampling times were set at 0, 4, 8, 12, and 24 h for detailed physiological measurements. In the TC-350 and TH-350 mg/L stress group, three shrimp from each group were randomly selected at 0, 4, 8, 12 and 24 h for measurement of ammonia excretion and oxygen consumption, and hemolymph was sampled for subsequent analysis. After 24 h, the death rate of shrimp was recorded and the survival rate of each group was calculated. Then, ten individuals from each treatment group were anesthetized on ice, and intact gills were collected and stored in 1.5 mL centrifuge tubes frozen in liquid nitrogen at -80°C for subsequent biochemical and gene expression analyses. Three individuals from the TC-50, TC-350, TH-50 and TH-350 treatments were fixed in Bouin’s solution and stored at 4°C for histological analysis.
2.3 Determination of physiological parameters
Hemolymph samples were centrifuged at 4°C and 7100g for 10 minutes, and 20 μL of each supernatant was analyzed using a 110 Osmometer (Fiske, Norwood, MA, USA). Besides, for the rest of the supernatant, the commercial kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China) were used to determine hemolymph ammonia (A086-1-1) and hemolymph urea nitrogen (C013-2-1). In the above determination of physiological parameters, the measurement procedures followed the manufacturer’s instructions provided in the kit.
2.4 Determination of histopathology observation
Gill tissues were harvested from both the TH and TC groups, which correspond to the control (50 mg/L) and the alkalinity stress (350 mg/L) groups, respectively, 24 hours subsequent to the onset of alkalinity stress. These tissue samples were meticulously cleansed using normal saline. Following this, they were immersed in Bouin’s solution to undergo fixation for 48 hours. Subsequent processing included dehydrated with a gradient ethanol series, embedded in paraffin, and sectioned. Histological sections were then stained utilizing the hematoxylin and eosin (H&E). Finally, prepared sections were observed and photographed under a Nikon ECLIPSE light microscope (Tokyo, Japan).
2.5 Determination of the biochemical parameters
Gill tissues from each group were placed in sterile tubes with 0.86% saline. After homogenization, samples were centrifuged at 2500 rpm for 20 min at 4°C, and the supernatant was collected for biochemical assays. Kits for ion concentration [Na+ (C002-1-1), K+ (C001-2-1), Cl- (C003-2-1), Ca2+ (C004-2-1)], ATPase activity [Na+/K+ ATPase (A070-2-2), Ca2+/Mg2+ ATPase (A016-2-2), total ATPase (A070-1-1)], indicators related to ammonia metabolism [arginase (H321-1), urea nitrogen (C013-2-1), glutamic acid (A074-1-1), glutamate dehydrogenase (A125-1-1), glutaminase (A124-1-1), glutamine synthetase (A047-1-1)] were purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All procedures were conducted according to the manufacturer’s instructions.
2.6 Quantitative real-time PCR analysis
Gene expression levels in gill tissues were quantified using quantitative real-time PCR (qRT-PCR) with commercial kits. The process, following our previously outlined protocol (Ye et al., 2024a), comprised RNA extraction (Aidlab, Beijing, China), cDNA synthesis (TaKaRa, Shiga, Japan), construction of fluorescence quantitative system (Vazyme Biotechnology, Nanjing, China), and detection using a CFX96 qRT-PCR instrument (Bio-Rad, USA). Specific primers for the target genes were designed based on their coding sequences (Table 1) and synthesized by Shanghai Sangon Biotechnology Co., Ltd. (Shanghai, China), primers were validated by melting curve analysis to confirm specificity. β-actin served as the internal reference gene, and relative gene expression was calculated using the 2−ΔΔCt method (Livak and Schmittgen, 2001).
2.7 Statistical analysis
All data are presented as means ± SD. Data were analyzed using SPSS 22.0 software (IBM, USA). One-way ANOVA followed by Duncan’s post hoc test for each variety in different environmental treatments, with significance set at P < 0.05. An independent samples t-test was used to detect differences between TC and TH under the same environmental treatments, with significance set at *P < 0.05, **P < 0.01 ***P < 0.001. After correlation analysis and Mantel tests between the biochemical parameter matrix and the shrimp species factor matrix, the R package linkET was used to display associations between shrimp species and biochemical parameters, as well as correlations among the biochemical parameters, through network plots and correlation heatmaps.
3 Results
3.1 Survival rate and physiological parameters analysis under acute alkalinity stress
In this study, the survival rate of shrimp in the alkalinity stress groups (200 mg/L and 350 mg/L) significantly decreased by 8 h and declined further over time (Figure 1A). Notably, at 12 h and 24 h, the survival rate in the TH group was significantly higher than that in the TC group under 350 mg/L alkalinity stress (P < 0.05). Additionally, the results for hemolymph osmolarity under high alkalinity stress indicated an initial increase followed by a decrease over time (Figure 1B). At 8 h and 12 h, the osmolarity in the TC group was significantly higher than that in the TH group (P < 0.05).
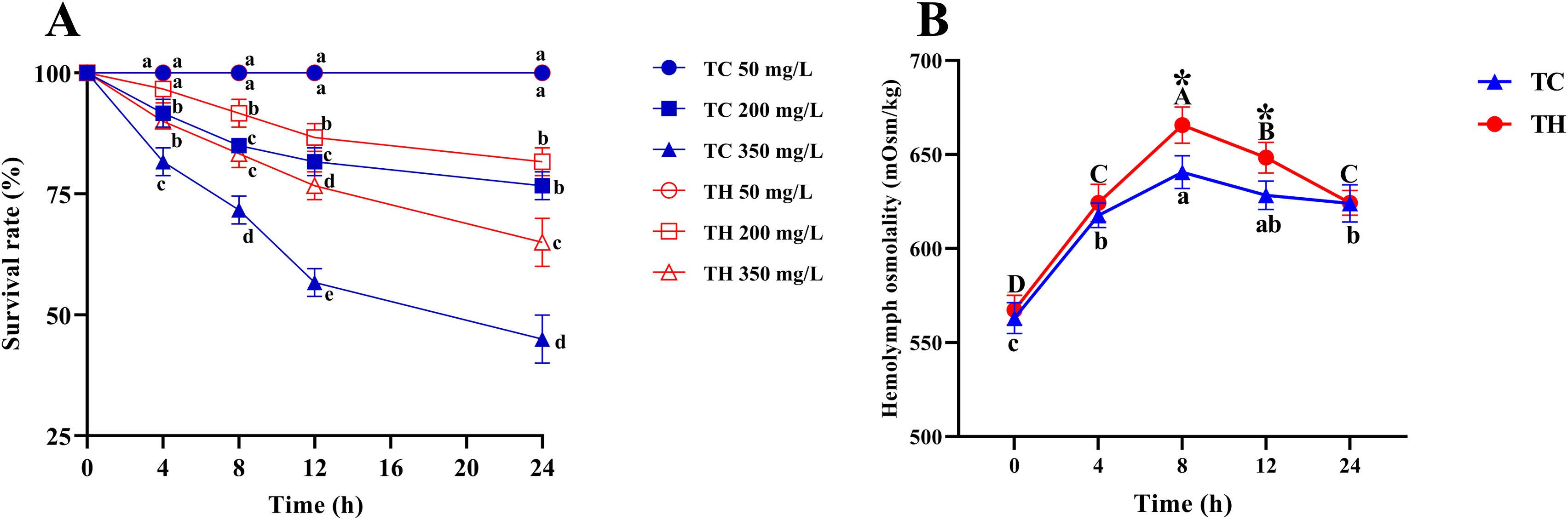
Figure 1. (A) Survival rate of each group in different alkalinity at 4, 8, 12, 16, 20 and 24 h, different letters above the bars of the same series indicate significant differences (P < 0.05) among the different populations (mean ± SD, n = 3); (B) Changes in hemolymph osmolality of TH and TC under 350 mg/L alkalinity treatment for 24 hours, uppercase letters indicate significant differences within TH, and lowercase letters indicate significant differences within TC (P < 0.05). The asterisk (*) denotes a significant difference between TH and TC (mean ± SD, n = 3). TH, the hybridization group; TC, the control group.
Compared to control alkalinity levels, high alkalinity stress significantly decreased the nitrogen excretion rate, but increased oxygen consumption rate, hemolymph ammonia content, and hemolymph urea nitrogen content in shrimp (Figure 2). No significant differences in these physiological parameters were observed between the two groups during the early stages of stress (P > 0.05). As stress duration increased, the nitrogen excretion rate and oxygen consumption rate were significantly higher in the TH group compared to the TC group at 8, 12, and 24 h (P < 0.05). Moreover, the hemolymph urea nitrogen content was significantly higher in the TH group than in the TC group at 12 and 24 h (P < 0.05). After 24 hours of high alkalinity treatment, the hemolymph ammonia content in the TC group was significantly higher than in the TH group (P < 0.05).
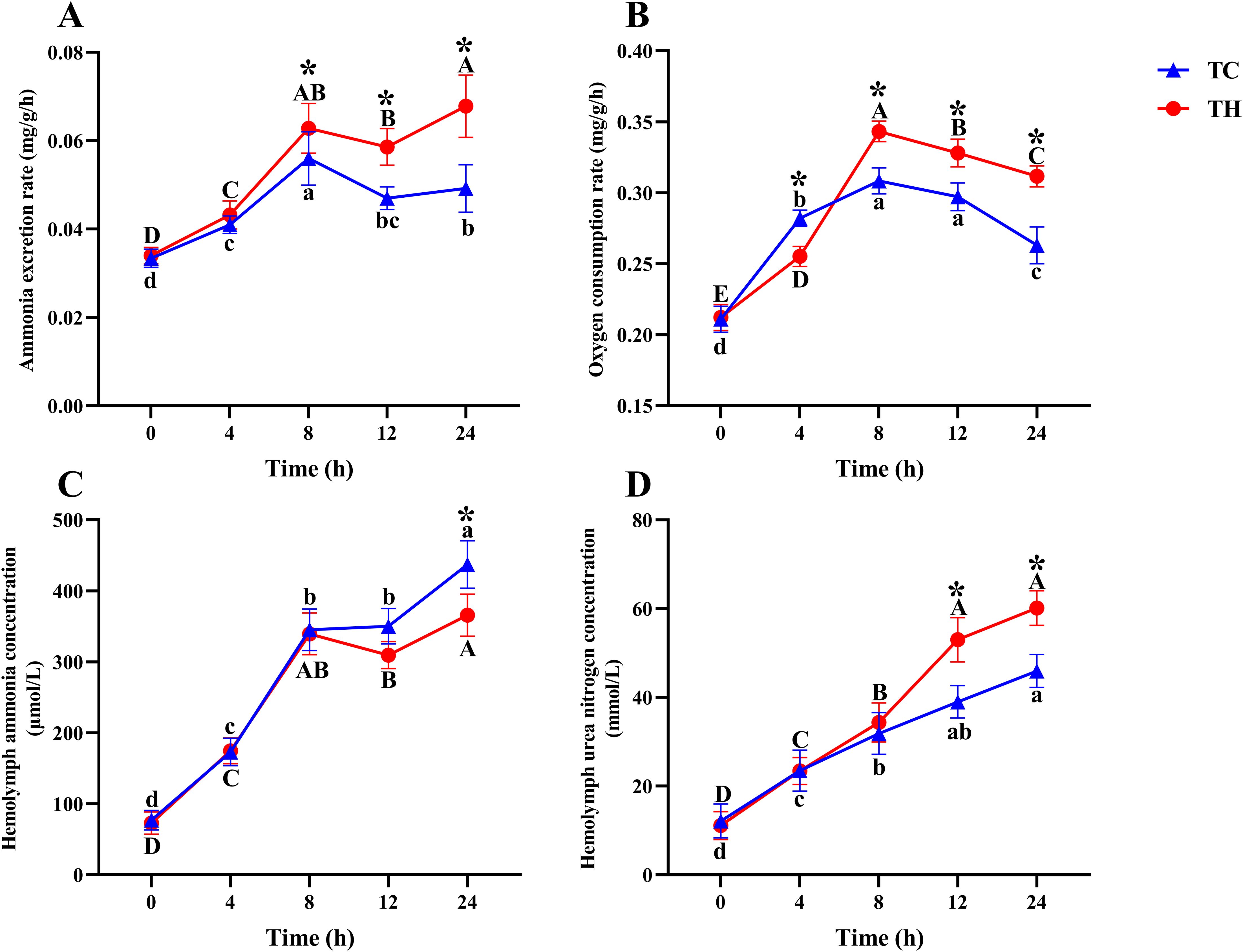
Figure 2. Changes in (A) ammonia excretion rate, (B) oxygen consumption rate, (C) hemolymph ammonia nitrogen, (D) hemolymph urea nitrogen of TH and TC under 350 mg/L alkalinity treatment for 24 hours. Data are presented as the mean ± SD (n =3). Uppercase letters indicate significant differences within TH, and lowercase letters indicate significant differences within TC (P < 0.05). The asterisk (*) denotes a significant difference between TH and TC. TH, the hybridization group; TC, the control group.
3.2 Histological analysis of gill
At 50 mg/L alkalinity, the gill filament structures of both the TC and TH groups were complete and clear, with uniformly distributed blood cells and intact marginal channel structures (Figures 3A, C). Under 350 mg/L alkalinity stress, the TC group gill filaments exhibited epithelial cell damage, including narrowing, crumpling, rupture of the stratum corneum, and reduced blood cell presence, with notable structural damage (Figure 3B). Similarly, under 350 mg/L alkalinity stress, the gill filaments in the TH group exhibited wrinkling of epithelial cells and partial rupture of the stratum corneum, but blood cells remained abundant and some areas retained their structural integrity (Figure 3D).
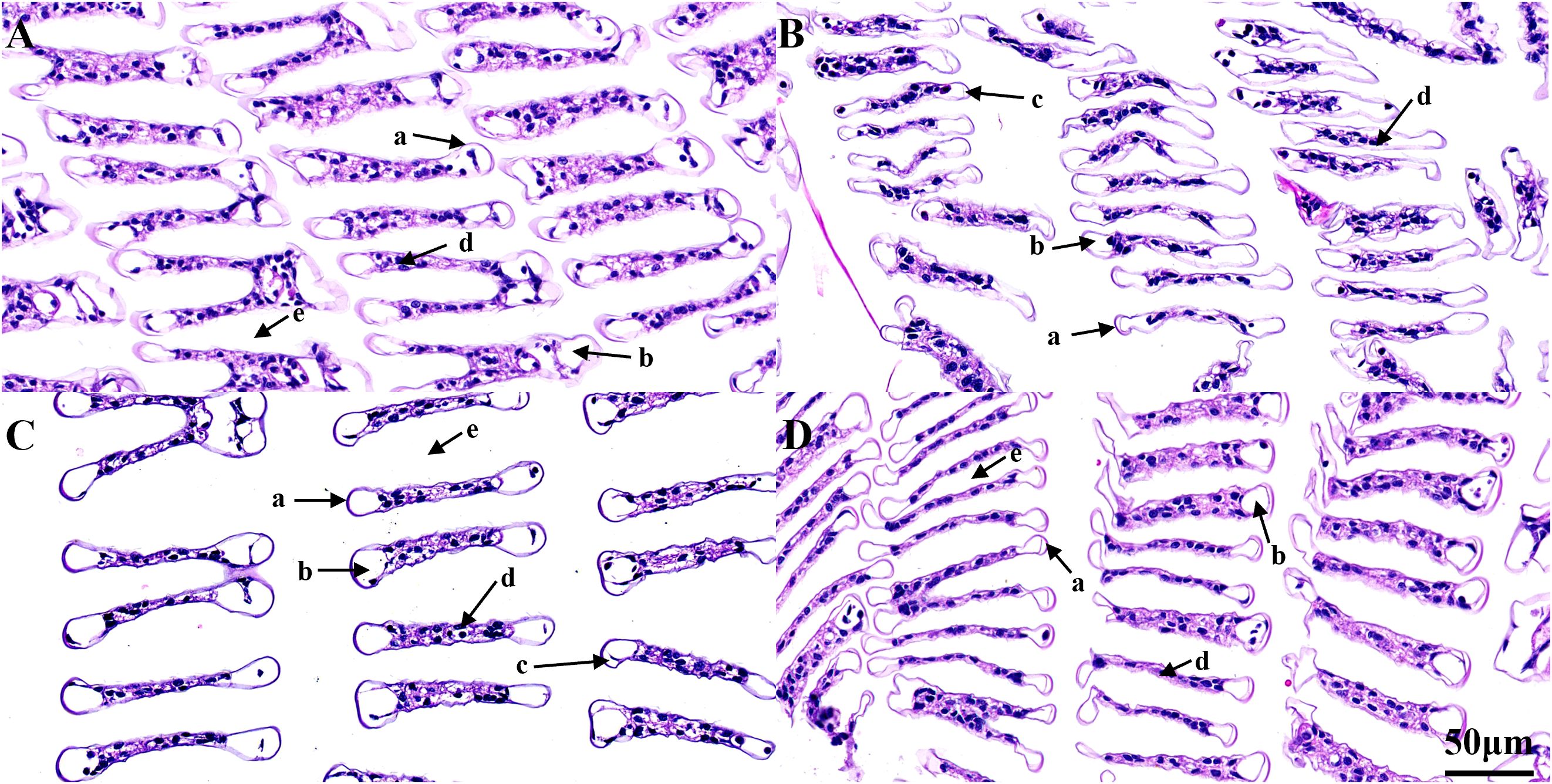
Figure 3. Histological structure of gill in TC group and TH group. (A) TC under 50 mg/L alkalinity treatment; (B) TC under 350 mg/L alkalinity treatment; (C) TH under 50 mg/L alkalinity treatment; (D) TH under 350 mg/L alkalinity treatment. The key structural included: a = angular cortex; b = epithelial cells; c = subcutaneous space, d = blood cells; e= diaphragm. All scale bars are 50 μm. TH, the hybridization group; TC, the control group.
3.3 Ion concentration and ATPase activity analysis of gill under acute alkalinity stress
Compared to the control alkalinity, high alkalinity stress significantly increased the Cl-, K+ and Na+ contents while decreased calcium ion contents in shrimp (P < 0.05, Figure 4). In the 50 mg/L alkalinity environment, no significant differences in ion contents were observed between the TH and TC groups (P > 0.05). Under high alkalinity stress, the Cl- (P < 0.01), K+ (P < 0.05) and Na+ contents (P < 0.05) in the gill tissues of the TH group were significantly higher, while the calcium (P > 0.05) were no significant, compared to the TC group.
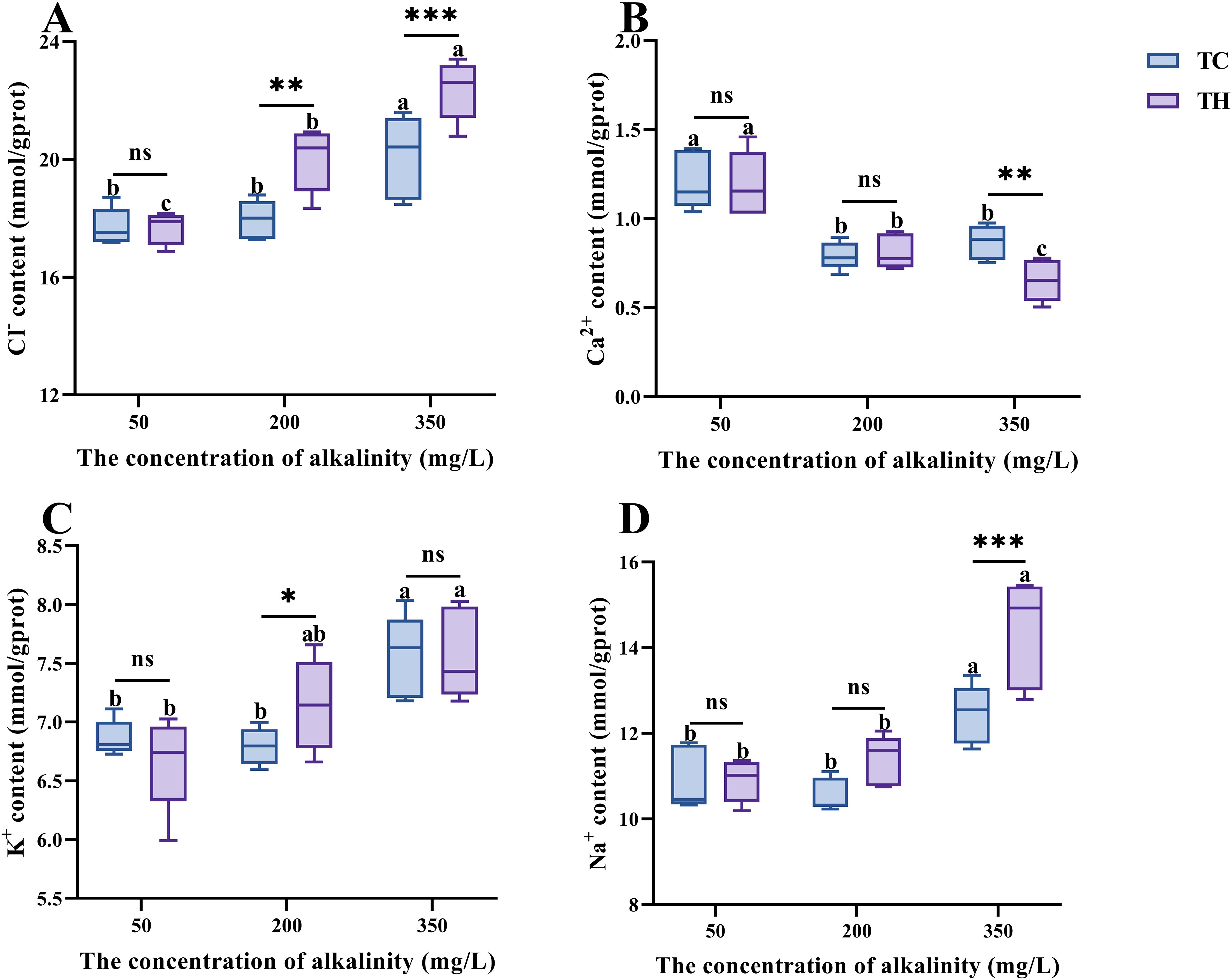
Figure 4. (A-D) Effects of different concentrations of alkalinity on ions content in the gills of L. vannamei. Data are presented as the mean ± SD (n =5). Different letters denote statistical differences between alkalinity treatments (P < 0.05). nsP>0.05, *P < 0.05, **P < 0.01 ***P < 0.001. TH, the hybridization group; TC, the control group.
For ATPase activities, Na+/K+ ATPase, Ca2+/Mg2+ ATPase, and total ATPase activities significantly increased with rising alkalinity in TH group (P < 0.05, Figure 5). The activity of these three enzymes in TC group increased first and then decreased with the increase of alkalinity. Under high alkalinity stress, the TH group exhibited higher Na+/K+ ATPase (P < 0.01), Ca2+/Mg2+ ATPase (P < 0.05), and total ATPase activities (P < 0.05) in gill tissues compared to the TC group, with Na+/K+ ATPase significant differences already observed under intermediate alkalinity (P < 0.05). This is closely related to the activation of sensitive molecular biomarkers (NKA, etc.) in aquatic organisms under osmotic stress (Su et al., 2022).
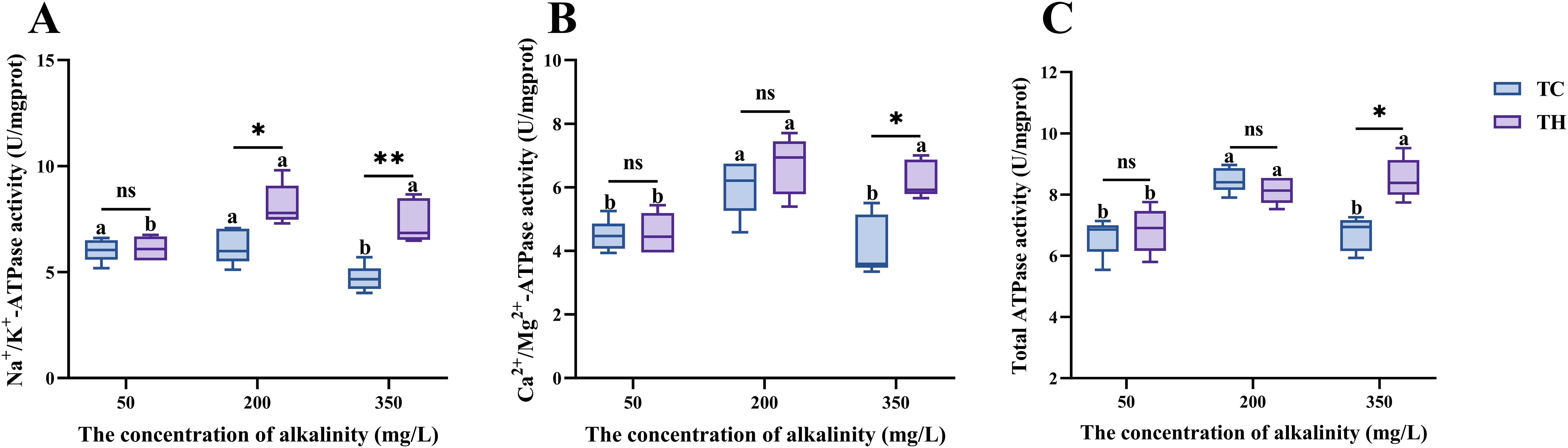
Figure 5. (A-C) Effects of different concentrations of alkalinity on ions transport ATPase activity in the gills of L. vannamei. Data are presented as the mean ± SD (n =5). Different letters denote statistical differences between alkalinity treatments (P < 0.05). nsP>0.05, *P < 0.05, **P < 0.01. TH, the hybridization group; TC, the control group.
3.4 Analysis of parameters related to ammonia metabolism
To investigate the effects of alkalinity stress on ammonia metabolism in shrimp, relevant indicators were measured. Results showed that, compared to the control alkalinity, high alkalinity stress significantly increased the BUN, Glu content, and GDH, GLS, arginase activity in gill tissues (P < 0.05, Figure 6). However, at 50 mg/L alkalinity, no significant differences in these indicators were observed between the TH and TC groups (P > 0.05). Under high alkalinity stress, compared to the TC group, the TH group showed significantly higher urea nitrogen content (P < 0.05), arginase activity (P < 0.01), while showed significantly lower in Glu content (P < 0.05), GDH (P < 0.01), GS (P < 0.01) and GLS (P < 0.01) activity in gill tissues.
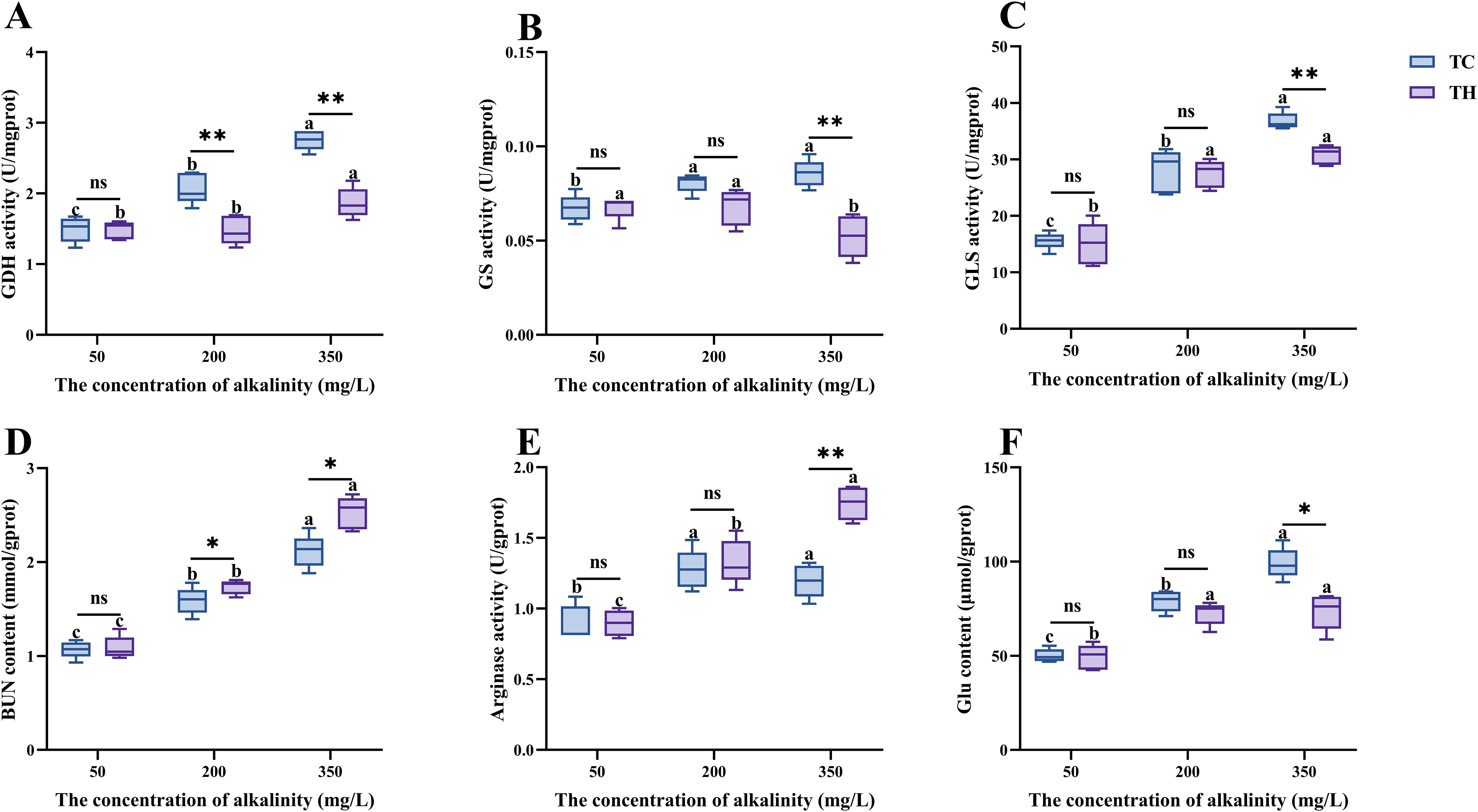
Figure 6. (A-F) Effects of different concentrations of alkalinity on nitrogen metabolism index in the gills of L. vannamei. Data are presented as the mean ± SD (n =5). Different letters denote statistical differences between alkalinity treatments (P < 0.05). nsP>0.05, *P < 0.05, **P < 0.01. TH, the hybridization group; TC, the control group.
3.5 Gene expressions
Ion transport-related genes are shown in Figures 7A–D. Compared to the 50 mg/L alkalinity treatment, high alkalinity stress significantly upregulated the gene expression levels of NKA, CA, AMPK, and ABCT3 (P < 0.05). At 200 mg/L alkalinity, there were no significant differences in CA, AMPK and ABCT3 expression levels between the TC and TH groups (P > 0.05), but NKA expression levels showed significant differences (P < 0.05). Additionally, under high alkalinity, the TH group had significantly higher expression levels of NKA (P < 0.01), CA (P < 0.01), AMPK (P < 0.05), and ABCT3 (P < 0.01) compared to the TC group. Antioxidant-related genes are shown in Figures 7E–H. Compared to the 50 mg/L alkalinity treatment, high alkalinity stress significantly upregulated the gene expression levels of NOS, Trx, HSP60, and HSP70 (P < 0.05). The NOS expression level in the TH group was significantly lower than in the TC group at 200 mg/L (P < 0.05) and 350 mg/L alkalinity (P < 0.01). The Trx expression level in the TH group was significantly lower than in the TC group only at 350 mg/L (P < 0.05). Furthermore, the HSP 70 expression levels in the TH group were significantly higher than in the TC group across all three alkalinity concentrations (P < 0.05). For the HSP60, the TH group showed significantly higher expression levels only at 350 mg/L alkalinity compared to the TC group (P < 0.05).
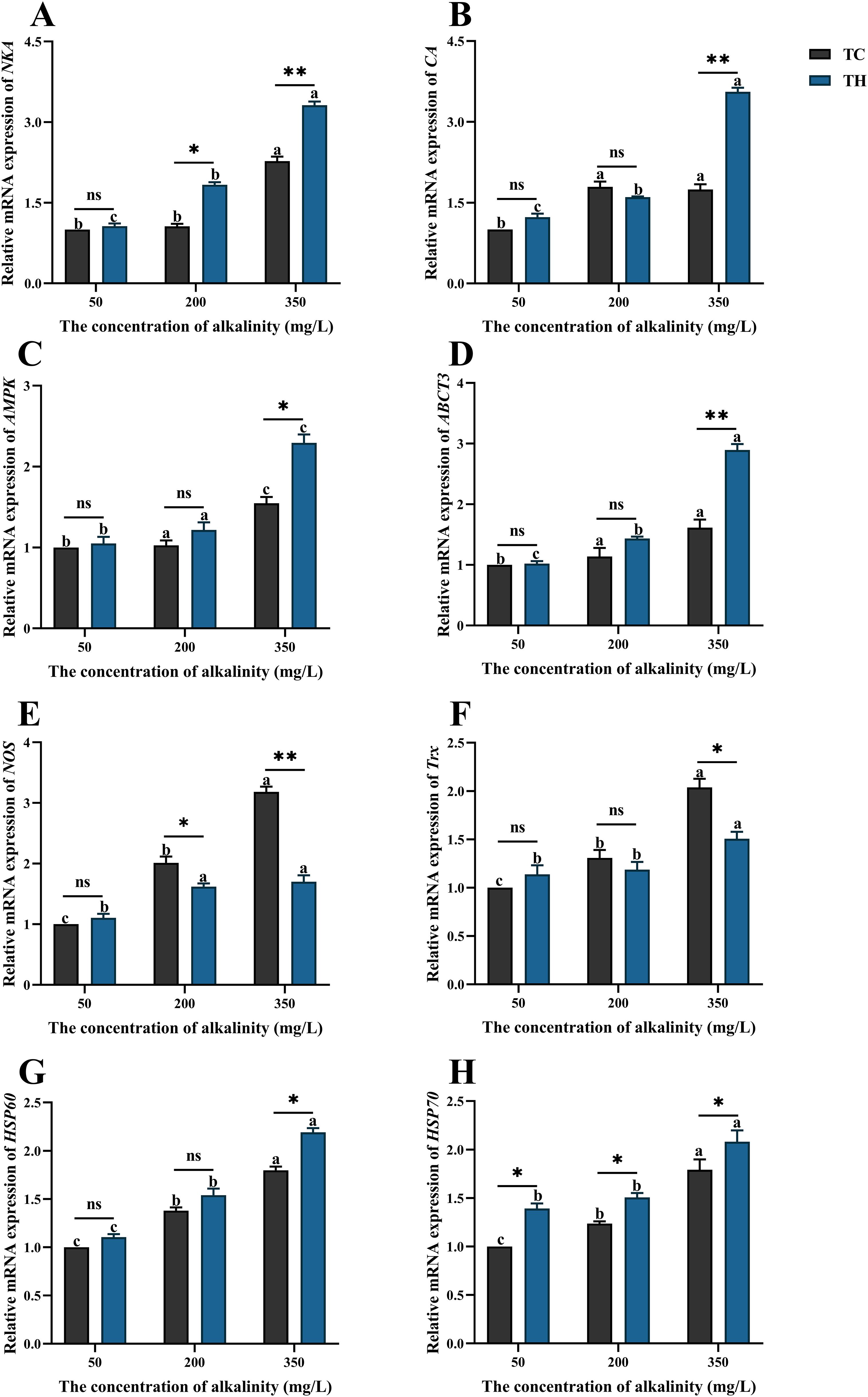
Figure 7. Effects of different concentrations of alkalinity on the gene expression of ion transport-related genes and antioxidant-related genes in the gills of L. vannamei. (A) NKA (Na+/K+-ATPase); (B) CA (carbonic anhydrase); (C) AMPK (adenosine 5 ‘-monophosphate (AMP) activates protein kinase); (D) ABCT3 (ATP-binding cassette transporter 3); (E) NOS (nitric oxide synthetase); (F) Trx (thioredoxin); (G) HSP 60 (Heat shock protein 60); (H) HSP 70 (Heat shock protein 70). Data are presented as the mean ± SD (n =3). Different letters denote statistical differences between alkalinity treatments (P < 0.05). nsP>0.05, *P < 0.05, **P < 0.01. TH, the hybridization group; TC, the control group.
3.6 Correlation analysis
To elucidate potential regulatory mechanisms, a ggcor correlation plotwas used (Figure 8). Analysis revealed notable differences in the correlations between alkalinity and the measured parameters (ion transport, ammonia metabolism, and key genes) in the TC group versus the TH group. Alkalinity influenced almost all the parameters in the TH group (P < 0.05), indicating a comprehensive activation of ion transport and ammonia metabolism under alkalinity stress. In contrast, the TC group exhibited no significant correlation between alkalinity and ion transport enzyme activities (P > 0.05), while the TH group showed comprehensive activation of ion transport and ammonia metabolism (P < 0.05). The correlation analysis among the measured parameters revealed that the osmoregulatory key gene NKA in both the TH and TC groups was significantly positively correlated with other genes (P < 0.05). Specifically, in the TH group, Na+/K+-ATPase activity showed significant correlations with chloride ions, calcium ions, Glu, GLS, and NOS, but not with other parameters (P > 0.05). Conversely, in the TC group, Na+/K+-ATPase activity displayed significant negative correlations with the majority of the parameters (P < 0.05).
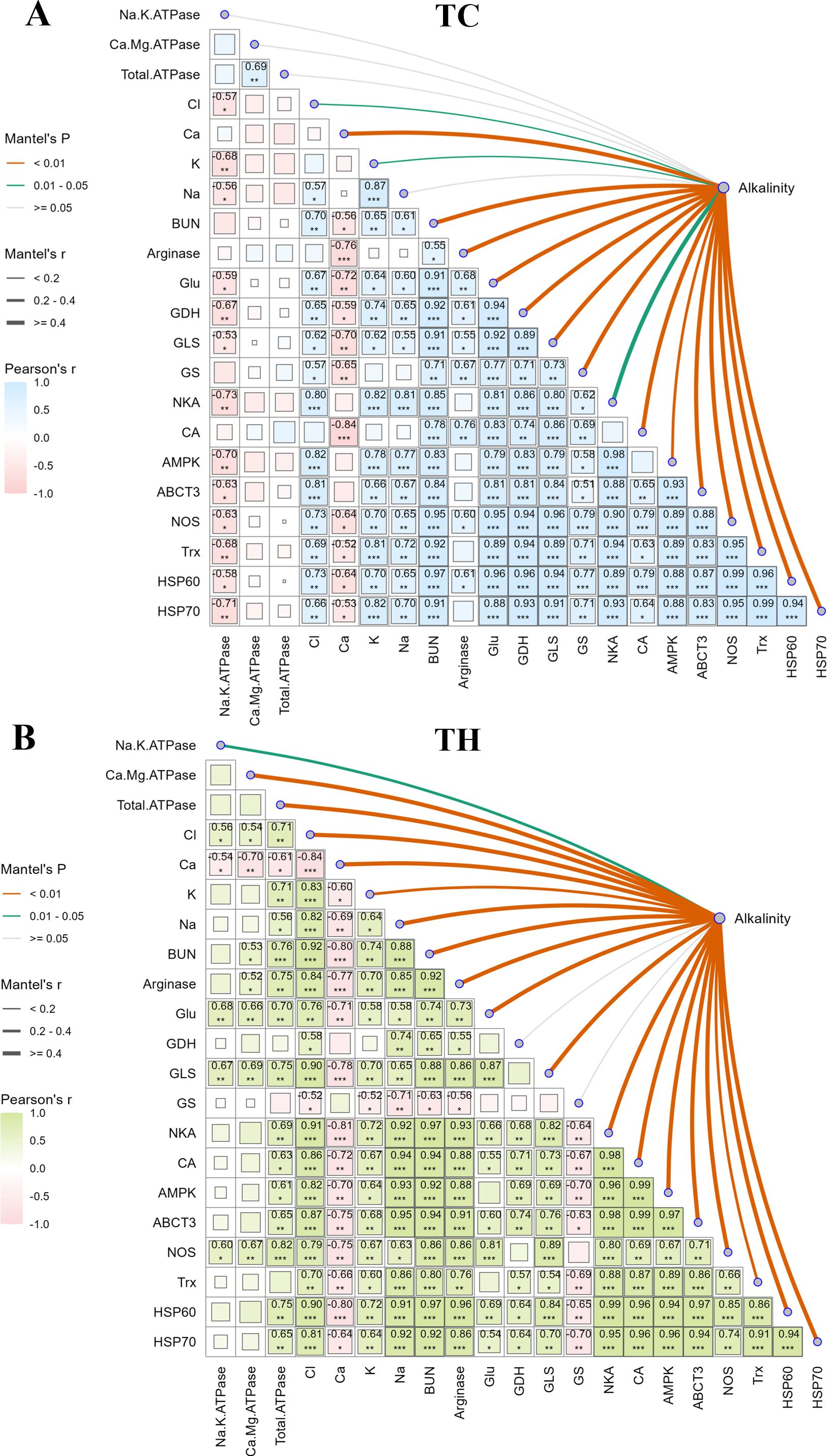
Figure 8. Ggcor correlation combination plots and regulatory network in L. vannamei at 24 h post alkalinity exposure. (A) The indicators correlation in the TC; (B): The indicators correlation in the TH. Rows and columns correspond to the genes, and each cell contains the corresponding correlation and P-value (*P < 0.05, **P < 0.01 ***P < 0.001). Pearson’s R-values are color-coded according to the color legend. The curve width corresponds to the mantel’s r statistic for the correlations between the alkalinity exposure and indicators. The curve color corresponds to the mantel’s P statistic for the correlations between the alkalinity exposure and indicators. TH, the hybridization group; TC, the control group.
4 Discussion
With the development and utilization of resources, the use of saline-alkali water has become increasingly important for the sustainability of fisheries (Geng et al., 2016). Alkalinity is a critical ecological factor in aquatic environments, capable of causing physiological and biochemical functions in aquatic organisms and, in severe cases, can result in death (Zhang et al., 2024). Our previous studies (Ye et al., 2024b; Ye et al., 2023) have shown that the TH group exhibits excellent growth performance and antioxidant immunity under low salinity, showing good tolerance to low salt environment, but its related mechanisms of alkali resistance has not been studied. Therefore, we set up experiments under alkalinity stress to evaluate the dominant traits and regulatory mechanisms of hybrid populations. In this study, the survival rate of shrimp under acute 24-hour alkalinity stress showed that the TH group showed a considerable benefit whereas the survival rate of high alkalinity group declined significantly over time. Li et al. (2022b) reported that hybrid Macrobrachium nipponense had higher survival under ammonia-nitrogen stress, indicating enhanced resistance through hybridization. Therefore, we speculated that the survival results of the TH group in this experiment also had heterosis—the enhanced function or growth of hybrid organisms.
4.1 Ion transport
The extent of osmoregulation in aquatic animals can be quantified by measuring the osmotic pressure and ion levels in the hemolymph/plasma, as well as the responses of related enzymatic systems (Romano et al., 2014). Under conditions of high alkalinity stress, the plasma concentration and osmotic pressure increase due to passive water loss (Geng et al., 2016). Na+/K+-ATPase contains binding sites for Na+, K+, and ATP, playing a crucial role in osmoregulation and ion balance in crustaceans, with typical ATPase catalytic and ion transport functions (Huang et al., 2019). Thus, the activity of Na+/K+-ATPase in gill tissue rises, activating the ion discharge mechanism, which leads to a gradual reduction in osmotic pressure until it stabilizes (He et al., 2009; LeBreton and Beamish, 1998). Based on this, we speculated that the TH group demonstrated a stronger ability to adapt to alkaline conditions due to display higher levels of mRNA expression of NKA gene and Na+/K+-ATPase activity during the regulatory process, as evidenced by a 46% upregulation in NKA mRNA expression and a 57% increase in Na+/K+-ATPase activity in TH group under high alkalinity conditions (350 mg/L) compared to the TC group. This allowed for a faster rate of osmotic pressure regulation than the TC group. The results show that hybrid tilapia (Oreochromis mossambicus female × O. urolepis hornorum male) has a mechanism of osmotic regulation adapted to salt-alkali stress (Su et al., 2020), which is similar to our results. Therefore, we speculated that hybridization makes TH have more excellent osmoregulation ability. Additionally, under high alkalinity stress, we observed that the concentration of K+ in the TH gill tissue was still higher than that in the control alkalinity group, which may also be related to the activity of Na+/K+-ATPase. K+ concentration had significant effects on shrimp growth, molting, feeding and nutrient retention (Zhu et al., 2006). Therefore, higher K+ concentration is conducive to good stress resistance of L. vannamei. Carbonic anhydrase (CA), which functions in ion transport and acid-base balance, is highly sensitive to changes in environmental salinity, facilitates gill ion uptake by providing H+ and HCO3− for the Na+/H+ and Cl−/HCO3− exchangers (Ge et al., 2023; Roy et al., 2007). In this experiment, the hybrid population showed higher expression of carbonic anhydrase (CA), suggesting a more robust exchange function of Na+ and Cl- to maintain osmotic pressure balance. This finding confirms that the levels of Na+ and Cl- in the gills of the TH group were significantly higher compared to those in the TC group. Ca2+-Mg2+-ATPase is considered an appropriate indicator for measuring the balance of Ca2+ and Mg2+ in aquatic organisms (Liao et al., 2021). Studies have shown that Ca2+ are not involved in regulating the osmotic concentration in prawns but are consumed from Ca2+ stores during the molting process (Huong et al., 2010). Therefore, we concluded that the decrease in calcium ions of L. vannamei observed in the high alkalinity conditions is due to a strategic response where the shrimp reduce their molting activity to avoid external stimuli. And it leads to the accumulation of calcium.
4.2 Histological analysis
The primary function of gill tissue is gas exchange and the maintenance of ion balance, and it is particularly susceptible to attacks by toxic substances (Shi et al., 2022). Under environmental stress, the gill tissues of crustaceans often undergo structural abnormalities accompanied by inflammatory responses (Zhang et al., 2023a). In this study, it was found that under the stress of high alkalinity, the gill tissue structure of L. vannamei was damaged, and the epithelial cells shrank and hemocytopenia appeared. However, the gill morphology of the TH group was significantly better than that of the TC group under high alkalinity conditions. This suggests that while excessive alkalinity can damage gill tissue structure, the TH group evidently possesses a more effective stress-resistance system compared to the TC group. However, how high alkalinity damages gill tissue and how the TH group avoids excessive tissue damage has not been understood. We hypothesize that this phenomenon is related to ammonia toxicity. Therefore, we measured the indicators related to nitrogen metabolism.
4.3 Ammonia metabolism
Oxygen consumption rate and ammonia nitrogen excretion are considered to be important indicators to evaluate energy metabolism and anti-stress in aquatic animals under stress environment (Chen and Chia, 1995). Ammonia, a highly toxic molecule to organisms produced through the catabolism of proteins and amino acids, is primarily excreted through the gills or converted into urea and glutamine to diminish its toxicity to the body (Liu et al., 2022; Ren et al., 2016). Our study found that in the early stage of alkalinity stress, the ammonia excretion rate in prawn, resulting in ammonia accumulation. It may be due to the high pH value of the alkaline water environment, NH3 was prevented from binding to H+, thus preventing NH3 conversion to NH4+ excretion and promoting the accumulation of ammonia in hemolymph (Tao et al., 2024). Therefore, we speculated that the excessive accumulation of ammonia led to the damage of gill tissue structure of shrimp.
At the same time, we also observed an increase in the oxygen consumption rate of the shrimp. ATP-binding cassette transporters (ABCT), as one of the largest and most ubiquitous families of membrane proteins, are involved in the transport of a wide variety of endogenous substrates and exogenous compounds across lipid membranes, playing a role in cellular detoxification, lipid homeostasis, and the regulation of ion channels (Dean et al., 2001; Higgins, 1992). AMP-activated protein kinase (AMPK) is a key regulator of cellular energy and metabolism, primarily by activating ATP-producing pathways (Xu et al., 2016). Moreover, studies have shown that AMPK signaling pathway is significantly activated in osmoregulation of Scylla paramamosain (Jin et al., 2024). Our results indicated that total ATPase activity and the gene expression levels of ABCT3 and AMPK significantly increased under high alkalinity conditions. Therefore, we speculated that when shrimp experience stress, AMPK signaling pathway is activated and enhance their internal energy supply systems and increase oxygen consumption, thereby generating more energy to facilitate the transport of metabolic waste. Among the groups, compared to the TC group, the TH group exhibits a stronger energy supply, which contributes to its superior performance in the process of ammonia expulsion and helps to mitigate ammonia toxicity. The same results were found in the reciprocal hybrids of Suminoe oysters (Crassostrea ariakensis), which significantly improved aerobic metabolism and provided more sufficient energy, showing heterosis (Zhang et al., 2023).
Studies have shown that when ammonia levels rise in aquatic organisms, ammonia is converted to urea, a less toxic form of nitrogen waste, to reduce ammonia toxicity (Zhao et al., 2024). Consequently, we detected and observed a continuous increase in urea nitrogen in shrimp hemolymph and gill under high alkaline stress conditions. In addition, studies have shown that in an alkaline water environment, urea, when supplemented with other osmotic pressure regulating ions, can enhance the osmotic regulation ability of Luciobarbus capito in saline water, thereby improving its tolerance to such environments (Geng et al., 2016). Compared with the TC group, the BUN content in the TH group is higher, which also indicated that hybridization brought stronger osmoregulation ability to TH group. Urea is produced through the ornithine urea cycle (OUC) via the degradation and hydrolysis of arginine, while arginase, acting as a key enzyme in this cycle, plays a critical role in crustaceans (Geng et al., 2020). Thus, reducing ammonia toxicity by enhancing urea synthesis with OUC may be a way to improve shrimp tolerance. In our research findings, in contrast to the TC group, the TH group significantly increased the activity of arginase under high alkalinity stress, thus strengthened the urea cycle. In the study conducted by Li et al. (2022a) on Macrobrachium nipponense, it was demonstrated that hybridization improved the nitrogen metabolism capacity. Hence, we speculated that the nitrogen metabolism capacity of low-salt shrimp cultured in this experiment was improved by crossbreeding.
GDH, GS, and GLS are key enzymes involved in the metabolism of glutamine within organisms. These enzymes help reduce the ammonia concentration in vivo through regulation, thereby decreasing ammonia toxicity within the organism (Weis, 2014). Studies have shown that GDH works by converting alpha-ketoglutaric acid and ammonia to glutamate, which is then catalyzed by GS to glutamine (Duan et al., 2018). Then glutamine is transported to the ammonia excretion site, where it is broken down by GLS to produce ammonia, which is then excreted outside the body by transporters (Wei et al., 2023). Our results showed that, compared with control alkalinity, high alkalinity stress led to the up-regulation of glutamine-metabolizing enzyme activity and an increase in the content of the intermediate product (glutamate) in the L. vannamei. This result suggests that when ammonia concentration increases, the glutamine metabolic pathway tends to move ammonia in the direction of the reaction between glutamate and glutamine to slow down ammonia toxicity (Qiu et al., 2018). However, not all hybridizations produced better phenotypic character, at the same high alkalinity, glutamine metabolizing enzyme activity in TH group was significantly lower than that in TC group, which could depend on environmental conditions, variety difference, and evolutionary divergence (Qin et al., 2022; Ren et al., 2019; Yao et al., 2015). Therefore, we speculated that, compared with the TH group, the TC group alleviated ammonia stress primarily through the activation of the glutamine pathway, a key mechanism in this context.
NOS produces NO by catalyzing the conversion of l-arginine to l-citrulline, with concomitant oxidation of NADPH (Bogdan, 2001). NO has signal transduction function, can affect transcription factor activity, regulate upstream signal cascade and processing of major gene products (Bogdan, 2001). Therefore, with the increase of alkaline concentration, the expression of NOS genes increases, thus stabilizing the organism’s transcription and translation of stress. Ammonia can induce an excess of free radicals, particularly reactive oxygen species (ROS), leading to oxidative stress in aquatic species (Hegazi et al., 2010; Liang et al., 2016). Trx, which stands for thioredoxin, serves as a substrate for peroxide-reducing proteins and antioxidant proteins and is involved in maintaining cellular redox homeostasis (Ren et al., 2010), and its expression increased with the increase of alkaline stress concentrations. Heat shock proteins (HSPs) can maintain the normal function of cells, decompose damaged cells, improve the body’s ability to resist the external environment, and maintain its own health (Giffard-Mena et al., 2024). We speculated that NOS and Trx could reduce damage at the gene level and increase the expression of related stress resistance genes in L. vannamei in response to high acute alkalinity stress. While high HSPs expression levels could better help L. vannamei protein to maintain a stable structure, support and repair damaged cytoskeletal elements, assist in the production and folding of intracellular proteins, enzymes and hormone receptors, and improve the stress resistance at the protein level (Roberts et al., 2010). In this study, we observed that the expression levels of HSP60 and HSP70 in the TH group were significantly higher than those in the TC group under high alkalinity stress. The expression of NOS and Trx is completely opposite, reflect the diversity of varieties. In our previous study, we found that hybrid populations cultured in low-salt environments had stronger expression of HSPs gene to TC groups (Ye et al., 2024). These findings suggest that the enhanced antioxidant immunity observed in hybrids under low salinity conditions also applies to alkalinity stress.
Based on these findings, we recommend focusing future breeding programs on enhancing alkali tolerance through selective breeding of strains that exhibit higher Na+/K+-ATPase activity and better osmoregulation capabilities. Specifically, breeding programs should prioritize individuals with elevated Na+/K+-ATPase activity as this trait appears critical for maintaining ion balance under high alkalinity conditions.
5 Conclusion
This study revealed the impacts of acute alkalinity exposure on gill histology, osmoregulatory capacity, and nitrogen metabolic function in both hybrid and control populations. The results indicated that under high alkalinity stress, the TH group exhibited superior osmotic regulation ability and enhanced energy metabolism functions compared to the TC group. Overall, the hybrid population demonstrated hybridization advantages by exhibiting enhanced tolerance to alkalinity stress. Our research provides a robust theoretical basis for the cultivation and breeding of L. vannamei in saline-alkaline conditions. However, the specific regulatory mechanisms underlying the stress resistance advantages require further investigation. Future studies should focus on elucidating these pathways and assessing long-term sustainability in such environments. Additionally, exploring genetic stability over multiple generations is crucial. By addressing these aspects, future research can enhance our understanding and application of hybrid advantages.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Academic Ethics and Law Committee, East China Normal University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
YY: Conceptualization, Formal analysis, Investigation, Resources, Visualization, Writing – original draft, Writing – review & editing. HL: Conceptualization, Formal analysis, Investigation, Visualization, Writing – review & editing. HY: Conceptualization, Formal analysis, Writing – review & editing. XD: Conceptualization, Writing – review & editing. JH: Visualization, Writing – review & editing. YJZ: Visualization, Writing – review & editing. YL: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing. YLZ: Conceptualization, Formal analysis, Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the Agriculture Research System of Shanghai, China (Grant No. 202205) and the Young Elite Scientists Sponsorship Program by CAST (2022QNRC001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Anderson P. M., Broderius M. A., Fong K. C., Tsui K. N. T., Chew S. F., Ip Y. K. (2002). Glutamine synthetase expression in liver, muscle, stomach and intestine of Bostrichthys sinensis in response to exposure to a high exogenous ammonia concentration. J. Exp. Biol. 205, 2053–2065. doi: 10.1242/jeb.205.14.2053
Bartley D. M., Rana K., Immink A. J. (2000). The use of inter-specific hybrids in aquaculture and fisheries. Rev. Fish Biol. Fisheries 10, 325–337. doi: 10.1023/A:1016691725361
Bogdan C. (2001). Nitric oxide and the regulation of gene expression. Trends Cell Biol. 11, 66–75. doi: 10.1016/S0962-8924(00)01900-0
Chan W. Y., Hoffmann A. A., van Oppen M. J. H. (2019). Hybridization as a conservation management tool. Conserv. Lett. 12, e12652. doi: 10.1111/conl.12652
Charmantier G., Charmantier-Daures M., Towle D. W. (2008). Osmotic and Ionic Regulation in Aquatic Arthropods. (Boca Raton, Frorida, USA: University of California Press).
Chen J.-C., Chia P.-G. (1995). Effects of unilateral eyestalk ablation on oxygen consumption and ammonia excretion of juvenile Penaeus japonicus bate at different salinity levels. J. Crustacean Biol. 15, 434–443. doi: 10.2307/1548765
Cheng Y., Zhao J., Ayisi C. L., Cao X. (2022). Effects of salinity and alkalinity on fatty acids, free amino acids and related substance anabolic metabolism of Nile tilapia. Aquaculture Fisheries 7, 389–395. doi: 10.1016/j.aaf.2020.06.005
da Mota Araujo H. R., Fernandes M. N., da Cruz A. L. (2019). Gill morphology and Na+/K+-ATPase activity of Gobionellus oceanicus (Teleostei: Gobiidae) in an estuarine system. Biol. Trace Element Res. 187, 526–535. doi: 10.1007/s12011-018-1393-z
Dean M., Hamon Y., Chimini G. (2001). The human ATP-binding cassette (ABC) transporter superfamily. J. Lipid Res. 42, 1007–1017. doi: 10.1016/S0022-2275(20)31588-1
Deng Z., Zhang Z., Zhao R., Sun J., Hao P., Zhang L., et al. (2023). Effects of high-salinity on the expression of aquaporins and ion transport-related genes in Chinese shrimp (Fenneropenaeus chinensis). Aquaculture Rep. 30, 101577. doi: 10.1016/j.aqrep.2023.101577
Duan Y., Wang Y., Zhang J., Sun Y., Wang J. (2018). Dietary effects of succinic acid on the growth, digestive enzymes, immune response and resistance to ammonia stress of Litopenaeus vannamei. Fish Shellfish Immunol. 78, 10–17. doi: 10.1016/j.fsi.2018.04.008
Fu-yi Y. (2004). Adaptability of whiteleg shrimp Penaeus vannamei to carbonate saline-alkali inland water environment I. Adaptive capacity of desalinated juvenile shrimp to alkalinity. Fisheries Sci. Technol. Inf.
Ge Q., Wang J., Li J., Li J. (2023). Effect of high alkalinity on shrimp gills: Histopathological alternations and cell specific responses. Ecotoxicology Environ. Saf. 256, 114902. doi: 10.1016/j.ecoenv.2023.114902
Geng Z., Liu Q., Wang T., Ma S., Shan H. (2020). Changes in physiological parameters involved in glutamine and urea synthesis in Pacific white shrimp, Litopenaeus vannamei, fed Ampithoe sp. meal and exposed to ammonia stress. Aquaculture Res. 51, 2725–2734. doi: 10.1111/are.14611
Geng L., Tong G., Jiang H., Xu W. (2016). Effect of salinity and alkalinity on luciobarbus capito gill Na+/K+-ATPase enzyme activity, plasma ion concentration, and osmotic pressure. BioMed. Res. Int. 2016, 4605839. doi: 10.1155/2016/4605839
Giffard-Mena I., Ponce-Rivas E., Sigala-Andrade H. M., Uranga-Solís C., Re A. D., Díaz F., et al. (2024). Evaluation of the osmoregulatory capacity and three stress biomarkers in white shrimp Penaeus vannamei exposed to different temperature and salinity conditions: Na+/K+ ATPase, Heat Shock Proteins (HSP), and Crustacean Hyperglycemic Hormones (CHHs). Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 271, 110942. doi: 10.1016/j.cbpb.2024.110942
Gocha N., Pequeux A., Wanson S., Gilles R. (1987). Cl– fluxes across isolated, perfused gills of the chinese crab Eriocheir sinensis (M.Edw.) acclimated to fresh water. Comp. Biochem. Physiol. Part A: Physiol. 88, 581–584. doi: 10.1016/0300-9629(87)90085-5
Guo X. (2009). Use and exchange of genetic resources in molluscan aquaculture. Rev. Aquaculture 1, 251–259. doi: 10.1111/j.1753-5131.2009.01014.x
He X., Zhuang P., Zhang L., Xie C. (2009). Osmoregulation in juvenile Chinese sturgeon (Acipenser sinensis Gray) during brackish water adaptation. Fish Physiol. Biochem. 35, 223–230. doi: 10.1007/s10695-008-9230-5
Hegazi M. M., Attia Z. I., Ashour O. A. (2010). Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 99, 118–125. doi: 10.1016/j.aquatox.2010.04.007
Higgins C. F. (1992). ABC transporters: from microorganisms to man. Annu. Rev. Cell Dev. Biol. 8, 67–113. doi: 10.1146/annurev.cb.08.110192.000435
Huang G., Liu B., Jiang X., Liang Y., Cai J., Huang L. (2025). The application of amendments improves properties of salt-affected soils across China. Soil Tillage Res. 248, 106431. doi: 10.1016/j.still.2024.106431
Huang Y., Liu Z., Li Y., Wu D., Zhang M., Zhao Y. (2019). Cloning and characterisation of Na+/K+-ATPase and carbonic anhydrase from oriental river prawn Macrobrachium nipponense. Int. J. Biol. Macromolecules 129, 809–817. doi: 10.1016/j.ijbiomac.2019.02.098
Hulata G. (2004). Genetic manipulations in aquaculture: a review of stock improvement by classical and modern technologies. Genetica 111, 155–173. doi: 10.1023/a:1013776931796
Huong D. T. T., Jasmani S., Jayasankar V., Wilder M. (2010). Na/K-ATPase activity and osmo-ionic regulation in adult whiteleg shrimp Litopenaeus vannamei exposed to low salinities. Aquaculture 304, 88–94. doi: 10.1016/j.aquaculture.2010.03.025
Jeong C. B., Kang H. M., Seo J. S., Park H. G., Rhee J. S., Lee J. S. (2016). Identification and molecular characterization of nitric oxide synthase (NOS) gene in the intertidal copepod Tigriopus japonicus. Gene 577, 47–54. doi: 10.1016/j.gene.2015.11.019
Jin M., Luo J., Zhu T., Fang F., Xie S., Lu J., et al. (2024). Examination of role of the AMP-activated protein kinase (Ampk) signaling pathway during low salinity adaptation in the mud crab, Scylla paramamosain, with reference to glucolipid metabolism. Aquaculture 593, 741329. doi: 10.1016/j.aquaculture.2024.741329
Kim W.-S., Kwak I.-S. (2022). EDCs trigger immune-neurotransmitter related gene expression, and cause histological damage in sensitive mud crab Macrophthalmus japonicus gills and hepatopancreas. Fish Shellfish Immunol. 122, 484–494. doi: 10.1016/j.fsi.2022.02.014
LeBreton G. T. O., Beamish F. W. H. (1998). The influence of salinity on ionic concentrations and osmolarity of blood serum in lake sturgeon, Acipenser fulvescens. Environ. Biol. Fishes 52, 477–482. doi: 10.1023/A:1007421410090
Li Y., Xu W., Zhu B., Jiang Q., Ye Y., Zhao Y. (2022a). Comparison of detoxification capacity between broodstock and hybrid offspring in the gills of juvenile shrimp (Macrobrachium nipponense): Response to chronic ammonia stress. Aquaculture Res. 53, 4487–4496. doi: 10.1111/are.v53.12
Li Y., Ye Y., Jiang Q., Che X., Zhao Y. (2022b). Comparative proteome analysis reveals possible heterosis for growth, immunity and antioxidation mechanisms in Macrobrachium nipponense hybrid offspring and parent populations. Aquaculture Res. 53, 6878–6889. doi: 10.1111/are.v53.18
Liang Z., Liu R., Zhao D., Wang L., Sun M., Wang M., et al. (2016). Ammonia exposure induces oxidative stress, endoplasmic reticulum stress and apoptosis in hepatopancreas of pacific white shrimp (Litopenaeus vannamei). Fish Shellfish Immunol. 54, 523–528. doi: 10.1016/j.fsi.2016.05.009
Liang S., Luo X., You W., Luo L., Ke C. (2014). The role of hybridization in improving the immune response and thermal tolerance of abalone. Fish Shellfish Immunol. 39, 69–77. doi: 10.1016/j.fsi.2014.04.014
Liao B., Wang J., Xiao B., Yang X., Xie Z., Li D., et al. (2021). Effects of acute microplastic exposure on physiological parameters in Tubastrea aurea corals. Mar. pollut. Bull. 165, 112173. doi: 10.1016/j.marpolbul.2021.112173
Liu Y., Yao M., Li S., Wei X., Ding L., Han S., et al. (2022). Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 822, 153622. doi: 10.1016/j.scitotenv.2022.153622
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Ma H., Lv W., Qin Y., Li J., Li X., Liao Q., et al. (2022). Aquaculture potential of two Kumamoto oyster (Crassostrea sikamea) populations and their reciprocal hybrids in southern China. Aquaculture 546, 737301. doi: 10.1016/j.aquaculture.2021.737301
McNamara J. C. (2012). Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: a review. J. Comp. Physiol. B 182, 997–1014. doi: 10.1007/s00360-012-0665-8
Pan L., Hu D., Liu M., Hu Y., Liu S. (2016). Molecular cloning and sequence analysis of two carbonic anhydrase in the swimming crab Portunus trituberculatus and its expression in response to salinity and pH stress. Gene 576, 347–357. doi: 10.1016/j.gene.2015.10.049
Qin Y., Liao Q., Shi G., Yang Y., Zhou Y., Li J., et al. (2022). Comparison of growth, survival and fertility of the southern and northern populations of Crassostrea ariakensis and their hybrids in southern China. Aquaculture 549, 737744. doi: 10.1016/j.aquaculture.2021.737744
Qiu L., Shi X., Yu S., Han Q., Diao X., Zhou H. (2018). Changes of ammonia-metabolizing enzyme activity and gene expression of two strains in shrimp Litopenaeus vannamei under ammonia stress. Front. Physiol. 9. doi: 10.3389/fphys.2018.00211
Ren J. S., Fox S. P., Howard-Williams C., Zhang J., Schiel D. R. (2019). Effects of stock origin and environment on growth and reproduction of the green-lipped mussel Perna canaliculus. Aquaculture 505, 502–509. doi: 10.1016/j.aquaculture.2019.03.011
Ren Q., Li M., Yuan L., Song M., Xing X., Shi G., et al. (2016). Acute ammonia toxicity in crucian carp Carassius auratus and effects of taurine on hyperammonemia. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 190, 9–14. doi: 10.1016/j.aquaculture.2019.03.011
Ren Q., Zhang R.-R., Zhao X.-F., Wang J.-X. (2010). A thioredoxin response to the WSSV challenge on the Chinese white shrimp, Fenneropenaeus chinensis. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 151, 92–98. doi: 10.1016/j.cbpc.2009.08.012
Roberts R., Agius C., Saliba C., Bossier P., Yeong ys. (2010). Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. fish Dis. 33, 789–801. doi: 10.1111/j.1365-2761.2010.01183.x
Romano N., Wu X., Zeng C., Genodepa J., Elliman J. (2014). Growth, osmoregulatory responses and changes to the lipid and fatty acid composition of organs from the mud crab, Scylla serrata, over a broad salinity range. Mar. Biol. Res. 10, 460–471. doi: 10.1080/17451000.2013.819981
Roy L. A., Davis D. A., Saoud I. P., Boyd C. A., Pine H. J., Boyd C. E. (2010). Shrimp culture in inland low salinity waters. Rev. Aquaculture 2, 191–208. doi: 10.1111/j.1753-5131.2010.01036.x
Roy L. A., Davis D. A., Saoud I. P., Henry R. P. (2007). Branchial carbonic anhydrase activity and ninhydrin positive substances in the Pacific white shrimp, Litopenaeus vannamei, acclimated to low and high salinities. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 147, 404–411. doi: 10.1016/j.cbpa.2007.01.003
Shevtsov M. A., Multhoff G. (2016). Heat shock protein–peptide and HSP-based immunotherapies for the treatment of cancer. Front. Immunol. 7. doi: 10.3389/fimmu.2016.00171
Shi K., Li M., Qin Z., Wang J., Liu P., Li J., et al. (2022). The mechanism of carbonate alkalinity exposure on juvenile Exopalaemon carinicauda with the transcriptome and microRNA analysis. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.816932
Sinha A. K., Giblen T., AbdElgawad H., De Rop M., Asard H., Blust R., et al. (2013). Regulation of amino acid metabolism as a defensive strategy in the brain of three freshwater teleosts in response to high environmental ammonia exposure. Aquat. Toxicol. 130-131, 86–96. doi: 10.1016/j.aquatox.2013.01.003
Sirirustananun N., Chen J. C., Lin Y. C., Yeh S. T., Liou C. H., Chen L. L., et al. (2011). Dietary administration of a Gracilaria tenuistipitata extract enhances the immune response and resistance against Vibrio alginolyticus and white spot syndrome virus in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 31, 848–855. doi: 10.1016/j.fsi.2011.07.025
Su M., Liu N., Zhang Z., Zhang J. (2022). Osmoregulatory strategies of estuarine fish Scatophagus argus in response to environmental salinity changes. BMC Genomics 23, 545. doi: 10.1186/s12864-022-08784-2
Su H., Ma D., Zhu H., Liu Z., Gao F. (2020). Transcriptomic response to three osmotic stresses in gills of hybrid tilapia (Oreochromis mossambicus female × O. urolepis hornorum male). BMC Genomics 21, 110. doi: 10.1186/s12864-020-6512-5
Tao S., Li X., Wang J., Bai Y., Wang J., Yang Y., et al. (2024). Examination of the relationship of carbonate alkalinity stress and ammonia metabolism disorder-mediated apoptosis in the Chinese mitten crab, Eriocheir sinensis: Potential involvement of the ROS/MAPK signaling pathway. Aquaculture 579, 740179. doi: 10.1016/j.aquaculture.2023.740179
Traverso J., Vignols F., Cazalis R., Pulido A., Sahrawy M., Cejudo F., et al. (2007). PsTRXh1 and PsTRXh2 are both pea h-type thioredoxins with antagonistic behavior in redox imbalances. Plant Physiol. 143, 300–311. doi: 10.1104/pp.106.089524
Wang X., Fang W., Liu L., Fu Y., Zhou Y., Zhou D., et al. (2023). Molecular characterization and DNA methylation analysis of carbonic anhydrase (Sp-CA) in the mud crab Scylla paramamosain: Its potential osmoregulation role under carbonate alkalinity stress. Aquaculture Rep. 30, 101591. doi: 10.1016/j.aqrep.2023.101591
Wang X., Ma Y., Su Y. (1997). Determining surface areas of marine alga cells by acid-base titration method. Chemosphere 35, 1131–1141. doi: 10.1016/S0045-6535(97)00177-X
Wang M., Yan Y., Liu W., Fan J., Li E., Chen L., et al. (2024). Proline metabolism is essential for alkaline adaptation of Nile tilapia (Oreochromis niloticus). J. Anim. Sci. Biotechnol. 15, 142. doi: 10.1186/s40104-024-01100-w
Wei S., Liu T., Zhao Y., Xiao Y., Zhou D., Zheng J., et al. (2023). Combined effects of dietary carbohydrate levels and ammonia stress on growth, antioxidant capacity and glucose metabolism in juvenile oriental river prawn (Macrobrachium nipponense). J. Exp. Zoology Part A: Ecol. Integr. Physiol. 339, 978–993. doi: 10.1002/jez.v339.10
Weis J. S. (2014). “Bioaccumulation/storage/detoxification,” in Physiological, Developmental and Behavioral Effects of Marine Pollution (Springer Netherlands, Dordrecht), 355–392.
Wilkie M. P., Wood C. M. (1996). The adaptations of fish to extremely alkaline environments. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 113, 665–673. doi: 10.1016/0305-0491(95)02092-6
Xu C., Li E., Xu Z., Wang S., Chen K., Wang X., et al. (2016). Molecular characterization and expression of AMP-activated protein kinase in response to low-salinity stress in the Pacific white shrimp Litopenaeus vannamei. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 198, 79–90. doi: 10.1016/j.cbpb.2016.04.005
Yao Z., Lai Q., Hao Z., Chen L., Lin T., Zhou K., et al. (2015). Carbonic anhydrase 2-like and Na+-K+-ATPase α gene expression in medaka (Oryzias latipes) under carbonate alkalinity stress. Fish Physiol. Biochem. 41, 1491–1500. doi: 10.1007/s10695-015-0101-6
Yao T., Zhang Y., Yan X., Wang Z., Li D., Su J., et al. (2015). Interspecific hybridization between Crassostrea angulata and C. ariakensis. J. Ocean Univ. China 14, 710–716. doi: 10.1007/s11802-015-2546-8
Ye Y., Li S., Zhu B., Yang Y., Du X., Li Y., et al. (2024a). Effects of dietary melatonin on growth performance, nutrient composition, and lipid metabolism of Pacific white shrimp (Penaeus vannamei). Aquaculture 578, 740095. doi: 10.1016/j.aquaculture.2023.740095
Ye Y., Zhu B., Yun J., Yang Y., Tian J., Xu W., et al. (2024b). Comparison of antioxidant capacity and immune response between low salinity tolerant hybrid and normal variety of Pacific white shrimp (Litopenaeus vannamei). Aquaculture Int. 32, 1879–1894. doi: 10.1007/s10499-023-01248-8
Ye Y., Zhu B., Zhang J., Yang Y., Tian J., Xu W., et al. (2023). Comparison of growth performance and biochemical components between low-salinity-tolerant hybrid and normal variety of pacific white shrimp (Penaeus vannamei). Animals 13, 2837. doi: 10.21203/rs.3.rs-2735736/v1
Zhang Z., Li A., Zhang K., Wang C., Wang W., Zhang G., et al. (2023). Accelerated energy metabolism plays an important role in Heterosis and maternal effect of hybrids bred from southern and northern Suminoe oysters (Crassostrea ariakensis). Aquaculture 566, 739214. doi: 10.1016/j.aquaculture.2022.739214
Zhang R., Shi X., Guo J., Mao X., Fan B. (2024). Acute stress response in gill of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquaculture 586, 740766. doi: 10.1016/j.aquaculture.2024.740766
Zhang R., Shi X., Liu Z., Sun J., Sun T., Lei M. (2023a). Histological, physiological and transcriptomic analysis reveal the acute alkalinity stress of the gill and hepatopancreas of Litopenaeus vannamei. Mar. Biotechnol. 25, 588–602. doi: 10.1007/s10126-023-10228-1
Zhang R., Zhao Z., Li M., Luo L., Wang S., Guo K., et al. (2023b). Metabolomics analysis reveals the response mechanism to carbonate alkalinity toxicity in the gills of Eriocheir sinensis. Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 263, 109487. doi: 10.1016/j.cbpc.2022.109487
Zhao X. F., Huang J., Li W., Wang S. Y., Liang L. Q., Zhang L. M., et al. (2024). Rh proteins and H+ transporters involved in ammonia excretion in Amur Ide (Leuciscus waleckii) under high alkali exposure. Ecotoxicology Environ. Saf. 273, 116160. doi: 10.1016/j.ecoenv.2024.116160
Zhao Y., Zhang C., Zhou H., Song L., Wang J., Zhao J. (2020). Transcriptome changes for Nile tilapia (Oreochromis niloticus) in response to alkalinity stress. Comp. Biochem. Physiol. Part D: Genomics Proteomics 33, 100651. doi: 10.1016/j.cbd.2019.100651
Zhu C.-B., Dong S.-L., Wang F., Zhang H.-H. (2006). Effects of seawater potassium concentration on the dietary potassium requirement of Litopenaeus vannamei. Aquaculture 258, 543–550. doi: 10.1016/j.aquaculture.2006.03.038
Keywords: alkaline stress, gene expression, ion transport, Litopenaeus vannamei, nitrogen metabolism
Citation: Ye Y, Liu H, Yuan H, Du X, Huang J, Zhou Y, Li Y and Zhao Y (2025) Comparative mechanisms of acute high-alkalinity stress on the normal and hybrid populations of pacific white shrimp (Litopenaeus vannamei). Front. Mar. Sci. 12:1559292. doi: 10.3389/fmars.2025.1559292
Received: 12 January 2025; Accepted: 18 February 2025;
Published: 07 March 2025.
Edited by:
Vikash Kumar, Central Inland Fisheries Research Institute (ICAR), IndiaReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanSoumya Prasad Panda, Central Inland Fisheries Research Institute (ICAR), India
Copyright © 2025 Ye, Liu, Yuan, Du, Huang, Zhou, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yunlong Zhao, eWx6aGFvNDI2QDE2My5jb20=; Yiming Li, bGl5bUBlY3NmLmFjLmNu
 Yucong Ye1
Yucong Ye1 Xinglin Du
Xinglin Du Yiming Li
Yiming Li Yunlong Zhao
Yunlong Zhao