
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 17 February 2025
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1542173
This article is part of the Research Topic Development of Sustainable Aquafeed: Novel Ingredients and Formulations View all 3 articles
To investigate the ameliorative effects of Lycium barbarum polysaccharide (LBP) on growth performance, oxidative stress, and glucolipid metabolism in largemouth bass (Micropterus salmoides) fed with high-carbohydrate diets. Fish (41.81 ± 0.36) g were fed on high-carbohydrate diets (15% α-starch) supplemented with 0% (15% α-Starch and no LBP, HLBP), 0.2g/kg LBP (LBP0.2), 0.4g/kg LBP (LBP0.4), 0.6g/kg LBP (LBP0.6) and 0.8g/kg LBP (LBP0.8) for 56 days. An additional low-carbohydrate (10% α-starch) dietary group (10% α-Starch and no LBP, NLBP) was set up and fed for the same 56 days. The final body weight (FBW), weight gain ratio (WGR), and specific growth rate (SGR) of fish in the LBP0.6 group were the highest, and the treatment groups were all significantly higher than in the HLBP group (P < 0.05). The activities of AMS, LPS, and TPS in the intestine and liver were all highest in the LBP0.6 group and significantly higher than in the HLBP group (P < 0.05). In the intestine and liver, the activities of T-AOC, CAT, GSH-PX, and SOD were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of CAT, SOD, and GPX genes were highest in the LBP0.8 group and significantly higher than in the HLBP group (P < 0.05). In the intestine and liver, the expression of IL-1β, IL-8, and TNF-α genes were significantly lower in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05); the expression of IL-10 and TGF-β1 genes were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of GK, PFK, and G6P genes were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of ACC, CPT-1, and FAS genes were significantly higher in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05). In summary, the addition of 0.6 g/kg LBP was effective in ameliorating the negative effects of a high-carbohydrate diet on largemouth bass.
Fish require protein as a necessary macronutrient to sustain their regular lifestyle. In aquatic feed, protein is the most expensive macronutrient (Wang et al., 2023). In actual production, the cheapest carbohydrate is frequently employed to promote protein-sparing action and improve feed physical qualities in order to lower feed costs (Li et al., 2020a). However, fish’s capacity to digest and metabolize carbohydrates is restricted, and the utilization efficiency of dietary carbohydrates varied among different fish species (Kamalam et al., 2012). Therefore, a diet high in carbohydrates may be harmful to fish health (Wang et al., 2022a). It has been reported that supplementing diets with 20% dietary starch can negatively affect largemouth bass (Lin et al., 2018; Zhang Y. et al., 2020; Romano et al., 2022). It has also been reported that the addition of 15% dietary carbohydrates resulted in the accumulation of glycogen and liver lipids in largemouth bass (Ma et al., 2019). As a result, there is increasing interest in ways to mitigate the negative effects of high-carbohydrate diets on carnivorous fish.
Studies have reported that adding plant extracts to high-carbohydrate diets can improve growth, immunity, and hepatic glucolipid metabolism in fish (Wang et al., 2024). Additionally, numerous plant extracts are known to promote growth, enhance immunity, and combat pathogens in aquaculture (Liu et al., 2023). Lycium barbarum is a plant in the Solanaceae family that is widely distributed in northwestern China. It has also been imported to North and South America as well as Western Europe (Liu H. et al., 2022). Because Lycium barbarum is rich in nutrients and active components such as amino acids, polysaccharides, polyphenols, vitamins, and trace elements, it is used as a frequent tonic and in traditional Chinese medicine in China (Amagase and Farnsworth, 2011; Liu et al., 2024). More than 200 different components have been identified, characterized, and analyzed to date. Polysaccharides, vitamins, betaine, and mixed extracts from goji berries are considered beneficial to health, offering anti-aging properties, improved vision, and fatigue resistance (Yao et al., 2018). Lycium barbarum polysaccharide (LBP), a water-soluble polysaccharide extracted from Lycium barbarum, is primarily composed of various monosaccharides, including arabinose, galactose, glucose, mannose, rhamnose, and xylose (Wu et al., 2010), and has been identified as one of the active ingredients responsible for biological activities (Shan et al., 2011). Previous studies have shown that LBP has antioxidant (Jin et al., 2013), immunomodulatory (Zhang et al., 2011), hypoglycemic (Tang et al., 2015), and anti-inflammatory (Tian et al., 2019). Lycium barbarum polysaccharides, as immunomodulatory agents, can enhance the immune defense capabilities of fish, thereby increasing their resistance to pathogens and reducing the incidence of diseases (Tan et al., 2019a). The regulation of carbohydrate and lipid metabolism is crucial for maintaining energy balance and preventing metabolic-related diseases. LBP can modulate these processes by influencing key enzymes involved in carbohydrate and lipid metabolism, thereby helping to maintain normal physiological functions and enhance growth performance (Jia et al., 2024). Furthermore, studies have shown that LBP may reduce liver injury and oxidative stress in the organism caused by lipid deposition (Li et al., 2007; Wu et al., 2024). The antioxidative capacity is particularly important for neutralizing oxidative stress induced by high-carbohydrate diets. Oxidative stress is a key factor leading to cellular damage and aging. The antioxidative components in LBP can effectively scavenge free radicals and mitigate inflammatory responses, thereby protecting cells from damage (Tian et al., 2019). Therefore, we speculated that LBP could mitigate the damage of high carbohydrate diets on fish organisms.
The largemouth bass (Micropterus salmoides), originally from the United States, was introduced into China in the 20th century (Yang et al., 2023). Its production has reached 0.7 million tons due to its rapid growth, high quality meat, high yields and strong adaptability in China (Li et al., 2020b; Zhou et al., 2022). The consumer market for largemouth bass has great potential in China (Lin et al., 2020). However, under the current intensive farming conditions, high-carbohydrate diets burden the growth performance and organismal health of largemouth bass. Therefore, we chose LBP as a functional additive to explore its ability to alleviate the negative effects of high-carbohydrate diets on largemouth bass.
Healthy fish and base feed were supplied by Zhejiang Huzhou Hai huang Biotechnology Co. (Huzhou, China). The fish were given a 7-day acclimatization period before the experiment to become used to the environment and amenities. The initial weight of the fish was (41.81 ± 0.36) g. The feed composition of each experimental group is shown in Table 1. The experimental groupings are as follows: 10% α-Starch and no LBP(NLBP); 15% α-Starch and no LBP(HLBP); 15% α-Starch and 0.2% LBP(LBP0.2); 15% α-Starch and 0.4% LBP(LBP0.4); 15% α-Starch and 0.6% LBP(LBP0.6); 15% α-Starch and 0.8% LBP(LBP0.8).
The trial was carried out in an indoor system. After acclimatization and being fasted for 24 h, fish were randomly distributed into 18 cement tanks (1.50 m × 1.50 m × 1.0 m, the height of water 0.8 m). These tanks were randomly divided into six treatments with three replicates each, with 30 fish for each tank. During the eight weeks of feeding trial, fish were fed two times per day at 7:00 and 16:00, the feeding rate was 5% of body weight. Two-third of the water in each tank was renewed twice a week. During the feeding trial, the temperature in the water was (28 ± 0.5) °C; dissolved oxygen was at least 6.0 mg/L; pH was 7.5–8.3, and nitrate level was under 0.03 mg/L.
At the end of the feeding trial, the fish were fasted for 24 h. Fish in each tank were weighted and counted individually. Blood was collected from the tail vein (1 mL), and centrifuged (4°C, 1600×g for 10 min) after preserved in 24°C for 4 hours. The serum was collected and stored at −20°C. The intestine and liver of fifteen fish per treatment (five fish per tank) were dissected using sterilized surgical tweezers and scissors, then immediately frozen in liquid nitrogen and stored at refrigerated (−80°C) until further analysis was conducted. All experimental fish were anaesthetized with tricaine methanesulfonate (MS-222) before collecting the samples. The general culturing and sampling of experimental fish were in accordance with Shanghai Ocean University’s ethical guidelines for the care and use of laboratory animals.
Serum biochemical indices was determined as follows: triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL), alanine (ALT) and aspartate transaminase (AST), alkaline phosphatase (AKP) activities were quantified in the serum. The test kits are provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Digestive enzyme activity was determined as follows: Amylase (AMS), Lipase (LPS), and Trypsin (TPS) activities were quantified in the intestinal and liver of fish by the commercial detection kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer.
Antioxidant capacity was determinate as follows: total antioxidant capacity (T-AOC), catalase (CAT), glutathione peroxidase (GSH-PX), superoxide dismutase (SOD) activities, and malondialdehyde (MDA) content were quantified in the intestinal and liver of fish by the commercial detection kits of Nanjing Jiancheng Bioengineering Institute (Nanjing, China) according to the manufacturer.
According to the manufacturer’s instructions (Takara, China), total RNA was isolated from intestinal and liver by using Trizol reagent. (Takara, China). Nanodrop 2000 (Thermo Scientific, USA) was used to quantitate the concentration of RNA. The obtained RNA samples were reversely transcribed to cDNA by NovoScript® Plus All-in-one 1st Strand cDNA Synthesis SuperMix. (Novoprotein, China). Real-time quantitative RT-qPCR was performed on LightCycler (Roche, China). All RT-qPCR primers were designed by the Primer 5 software. The primers for these genes are listed in Table 2. The 2−ΔΔCt comparative CT method was employed to quantitate expression levels for CAT, SOD, GPX, IL-1β, IL-8, TNF-α, IL-10, TGF-β1, GK, PFK, G6P, ACC, CPT-1, and FAS genes relative to the β-actin gene.
• Calculation formula of growth indexes:
● Weight gain rate (WGR, %) = 100 × (final weight - initial weight)/initial weight
● Specific growth rate (SGR, %/day) = 100 × [ln (final weight (g)) - ln (initial weight (g))]/days
● Hepatosomatic index (HSI, %) = 100 × [liver weight (g)/body weight (g)]
● Survival rate (SR, %) = 100 × (number of final fish/numbers of initial fish)
● Feed coefficient ratio (FCR) = feed intake (g)/wet weight gain (g)
Results were presented as mean ± standard error (SE). SPSS 25.0 software (IBM, Chicago, IL, USA) was used for all statistical analyses. All the data were tested for normality, homogeneity and independence of variance prior to ANOVA tests. Student’s t-test was used to compare the data between the NLBP and HLBP groups. One-way analysis of variance (ANOVA) followed by Tukey’s multiple-range tests were used for analyzing the data among different LBP levels in the 15% α-Starch diet. In all statistical tests used, p < 0.05 was considered significantly different.
The effects of adding LBP to the diet on the growth performance of largemouth bass are shown in Table 3. FBW, WGR, and SGR were significantly higher in the NLBP group than in the HLBP group (P < 0.05). HSI was significantly lower in the NLBP group than in the HLBP group (P < 0.05). The FBW, WGR, and SGR of fish in the LBP0.6 group were the highest, and the treatment groups were all significantly higher than in the HLBP group (P < 0.05). The quadratic polynomial regression results of Lycium barbarum polysaccharide levels with WGR and SGR are shown in Figures 1, 2. HSI was highest in the HLBP group and was significantly higher than in the LBP0.4, LBP0.6, and LBP0.8 groups (P < 0.05). FCR was highest in the HLBP group.
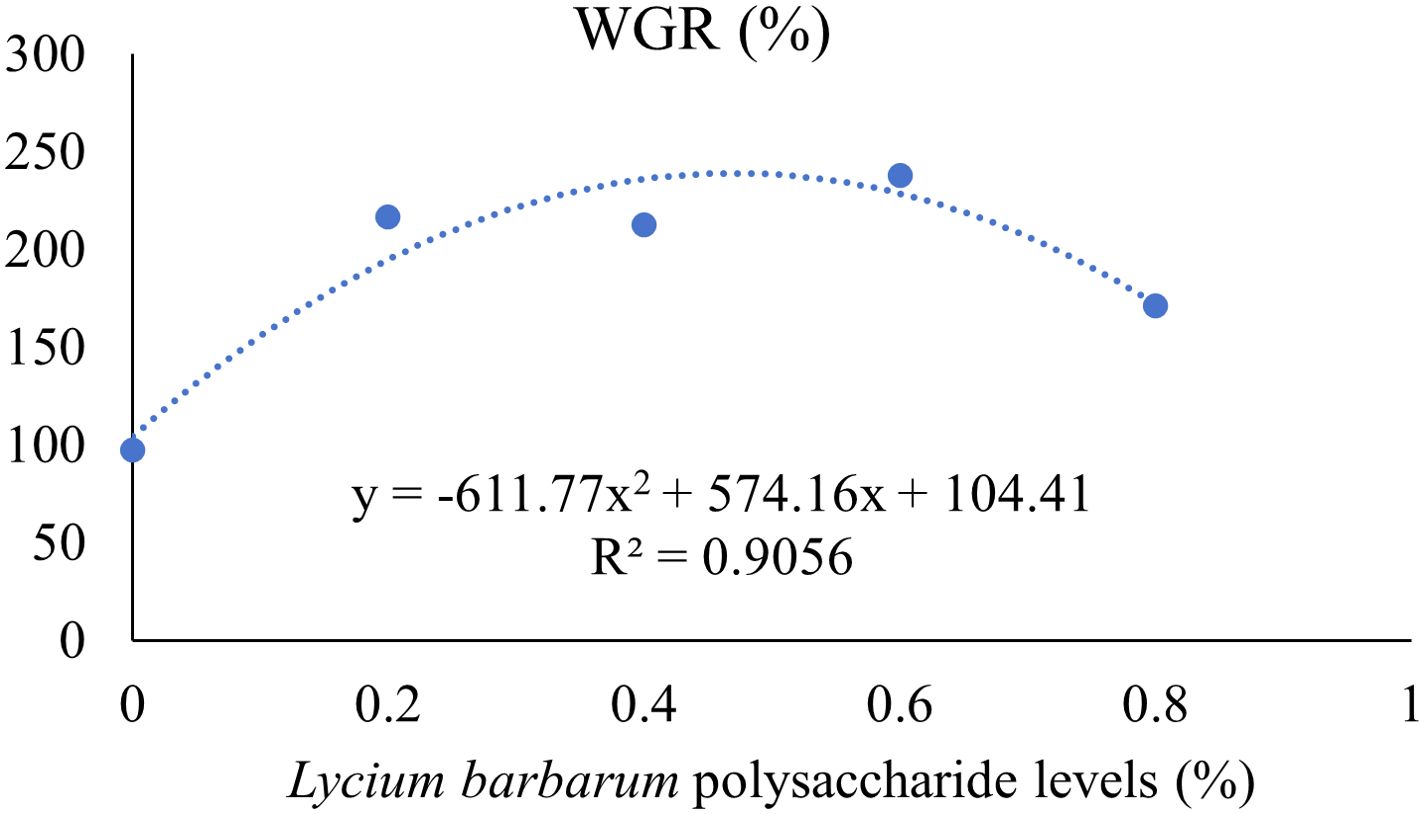
Figure 1. Quadratic polynomial regression of LBP levels in feed and weight gain rate of largemouth bass. Different groups are as follows: the control (0); 0.2% Lycium barbarum polysaccharides (0.2); 0.4% Lycium barbarum polysaccharides (0.4); 0.6% Lycium barbarum polysaccharides (0.6); 0.8% Lycium barbarum polysaccharides (0.8).
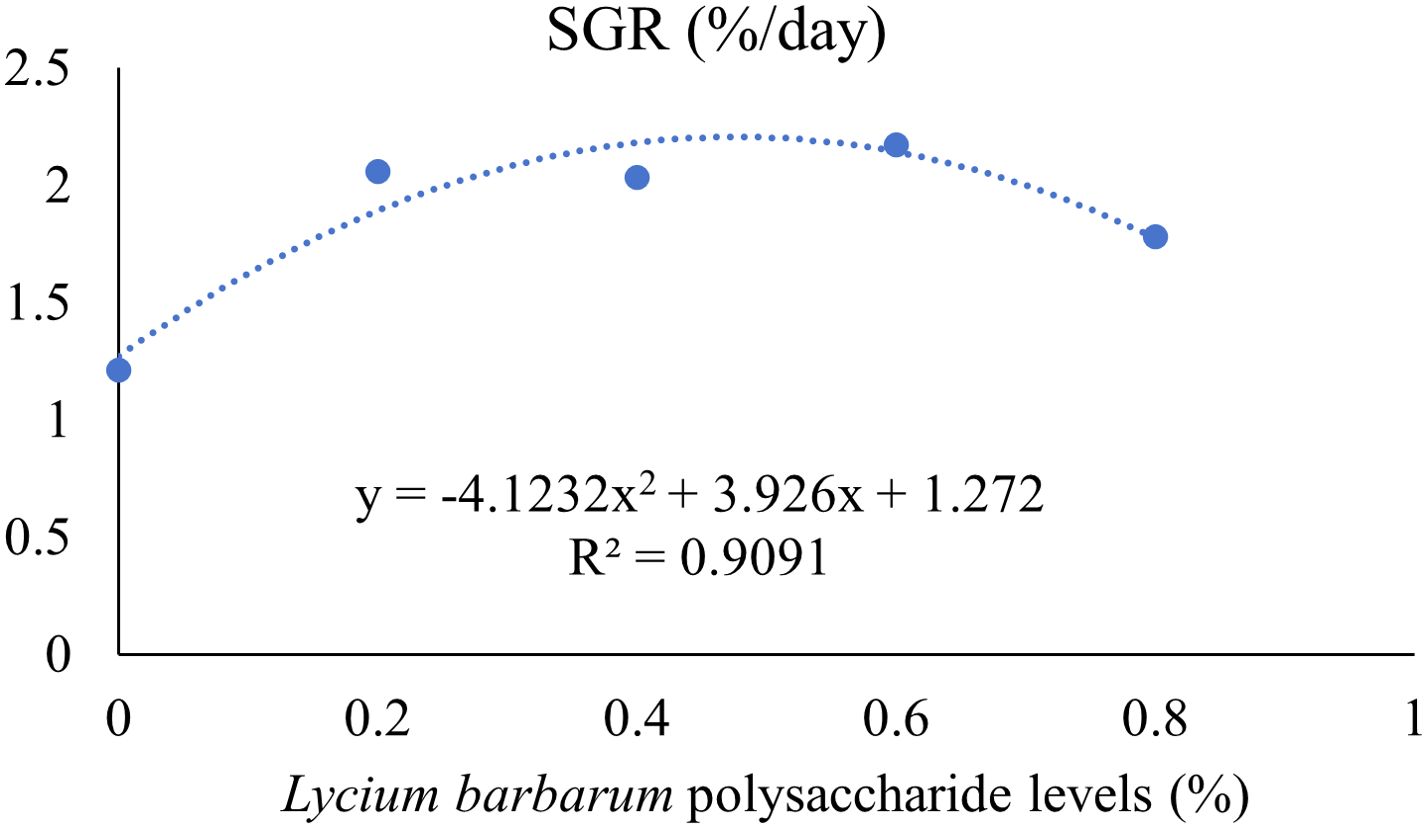
Figure 2. Quadratic polynomial regression of LBP levels in feed and specific growth rate of largemouth bass. Different groups are as follows: the control (0); 0.2% Lycium barbarum polysaccharides (0.2); 0.4% Lycium barbarum polysaccharides (0.4); 0.6% Lycium barbarum polysaccharides (0.6); 0.8% Lycium barbarum polysaccharides (0.8).
Effects of dietary LBP on digestive enzymes activities of the intestinal and liver of fish are shown in Figure 3. The activities of AMS, LPS, and TPS in the intestine were all highest in the LBP0.6 group and significantly higher than in the HLBP group (P < 0.05). In the liver, the activities of AMS, LPS, and TPS were all highest in the LBP0.6 group and significantly higher than in the HLBP group (P < 0.05).
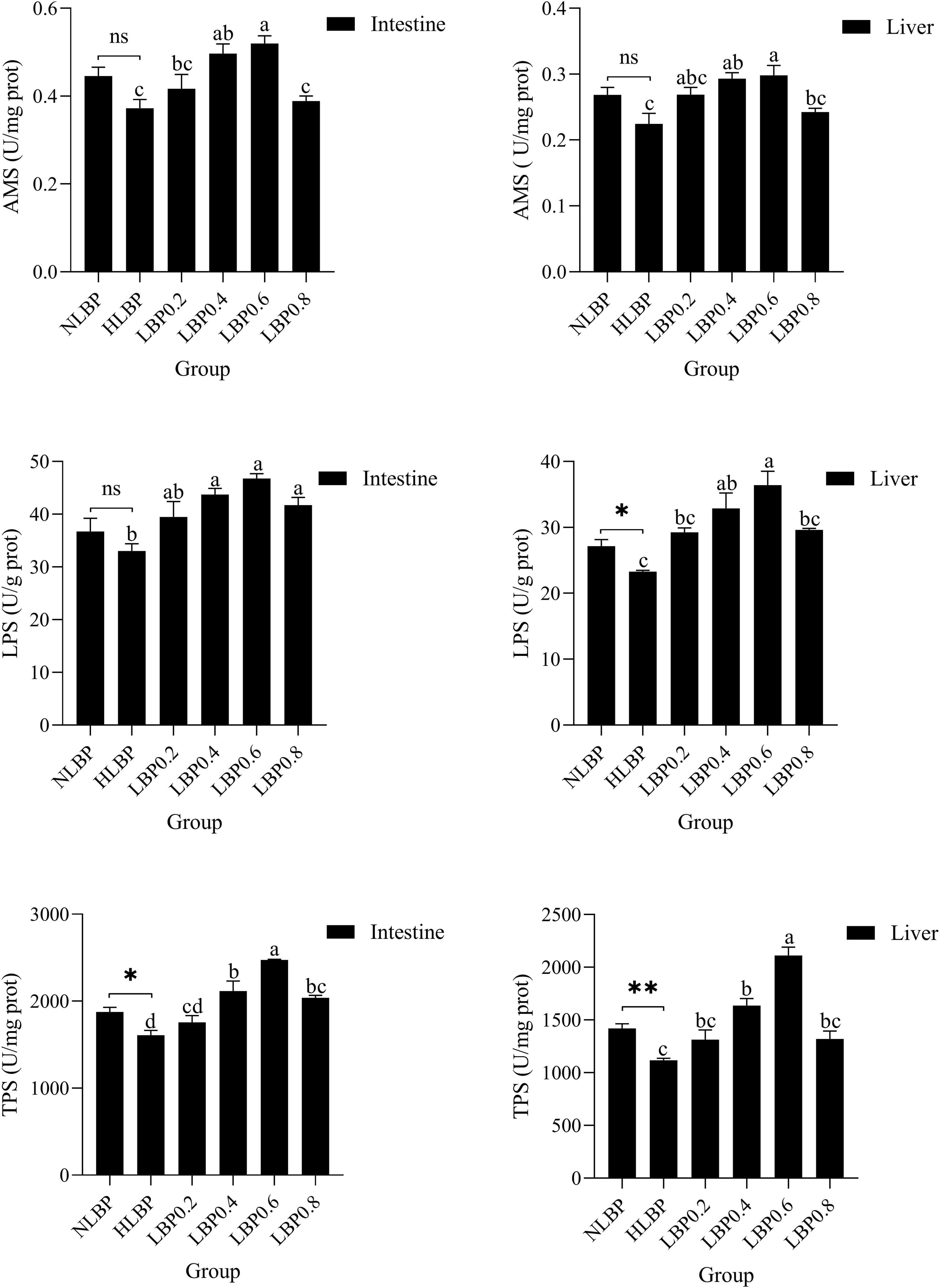
Figure 3. Effects of dietary Lycium barbarum polysaccharides on AMS, LPS, and TPS activities in the intestinal and liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
As shown in Table 4, serum levels of TG content and ALT activity were significantly higher in the HLBP group than in the NLBP group (P < 0.05). TC and LDL contents were extremely significantly higher in the HLBP group than in the NLBP group (P < 0.01). HDL content and AKP activity in the HLBP group were significantly lower than in the NLBP group (P < 0.05). In the experimental group, TC and LDL contents were significantly lower than in the HLBP group, and ALT and AST activities were significantly lower than in the HLBP group (P < 0.05). The content of HDL and AKP activity were significantly higher in the LBP0.8 group than in the HLBP group (P < 0.05).
The antioxidant capacity of the intestine and liver is shown in Figure 4. In the intestine and liver, the activities of T-AOC and GSH-PX were significantly higher in the NLBP group than in the HLBP group (P < 0.05); the activity of CAT was extremely significantly higher in the NLBP group than in the HLBP group (P < 0.01); the content of MDA was significantly lower in the NLBP group than in the HLBP group (P < 0.05). In the intestine and liver, the activities of T-AOC, CAT, GSH-PX, and SOD were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). In the intestines of the experimental groups, except for the LBP0.2 group, the MDA content was significantly lower than that of the HLBP group (P < 0.05). In the liver, MDA content was significantly lower in the experimental groups than in the HLBP group (P < 0.05).

Figure 4. Effects of dietary Lycium barbarum polysaccharides on T-AOC, CAT, GSH-PX, SOD activities and MDA content in the intestinal and liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
As shown in Figure 5, the relative expression levels of the CAT, SOD, and GPX genes in the intestines. There was a significant difference in CAT gene expression between the NLBP and HLBP groups (P < 0.05). The expression of SOD gene in the NLBP group was extremely significantly higher than that in the HLBP group (P < 0.01). The expression of CAT, SOD, and GPX genes were significantly higher in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05). As shown in Figure 6, the relative expression levels of the CAT, SOD, and GPX genes in the liver. The expression of CAT and SOD genes were significantly higher in the NLBP group than in the HLBP group (P < 0.05). The expression of CAT, SOD, and GPX genes were highest in the LBP0.8 group and significantly higher than in the HLBP group (P < 0.05).
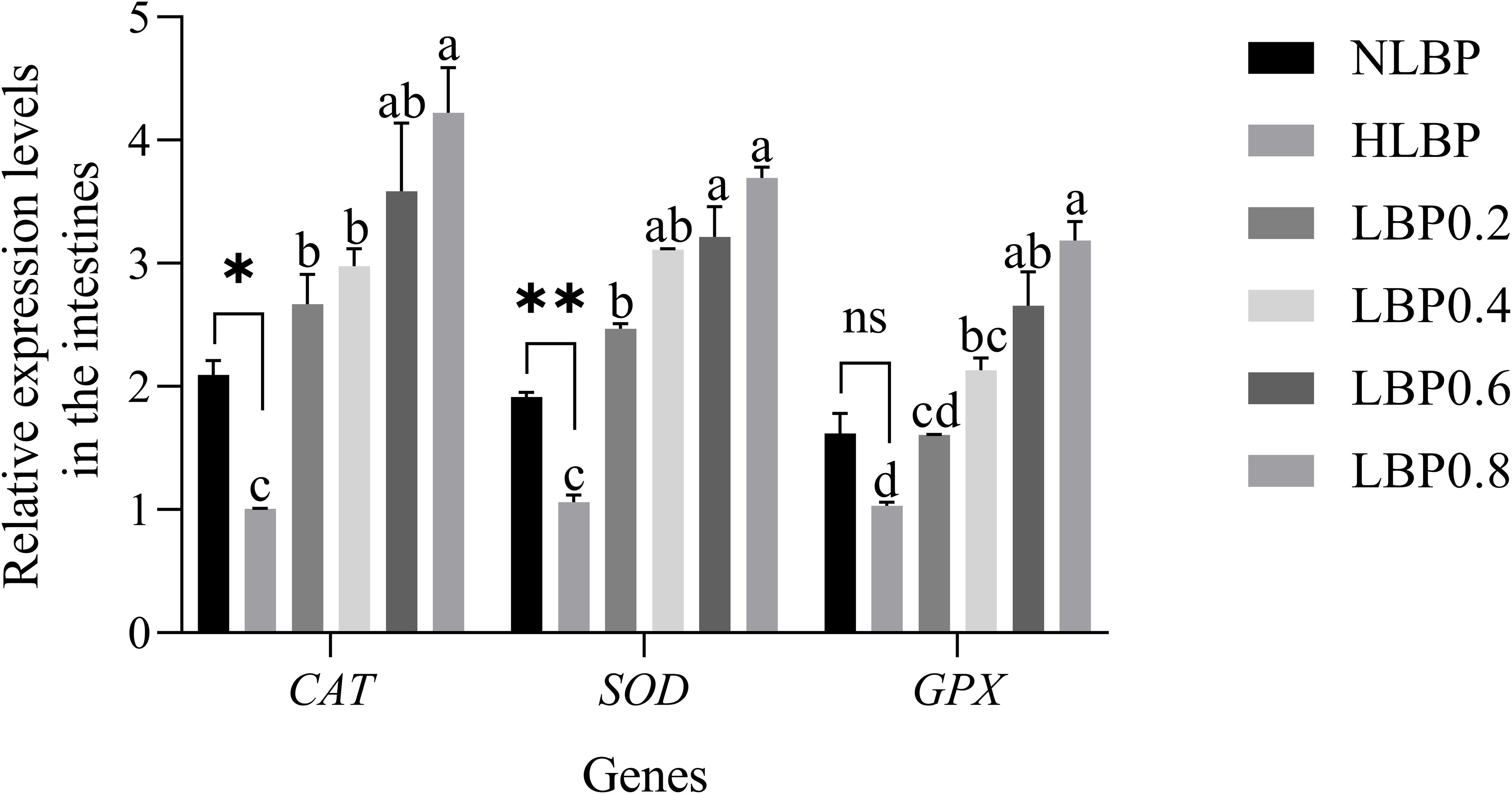
Figure 5. The relative expression levels of CAT, SOD, and GPX genes in the intestines of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
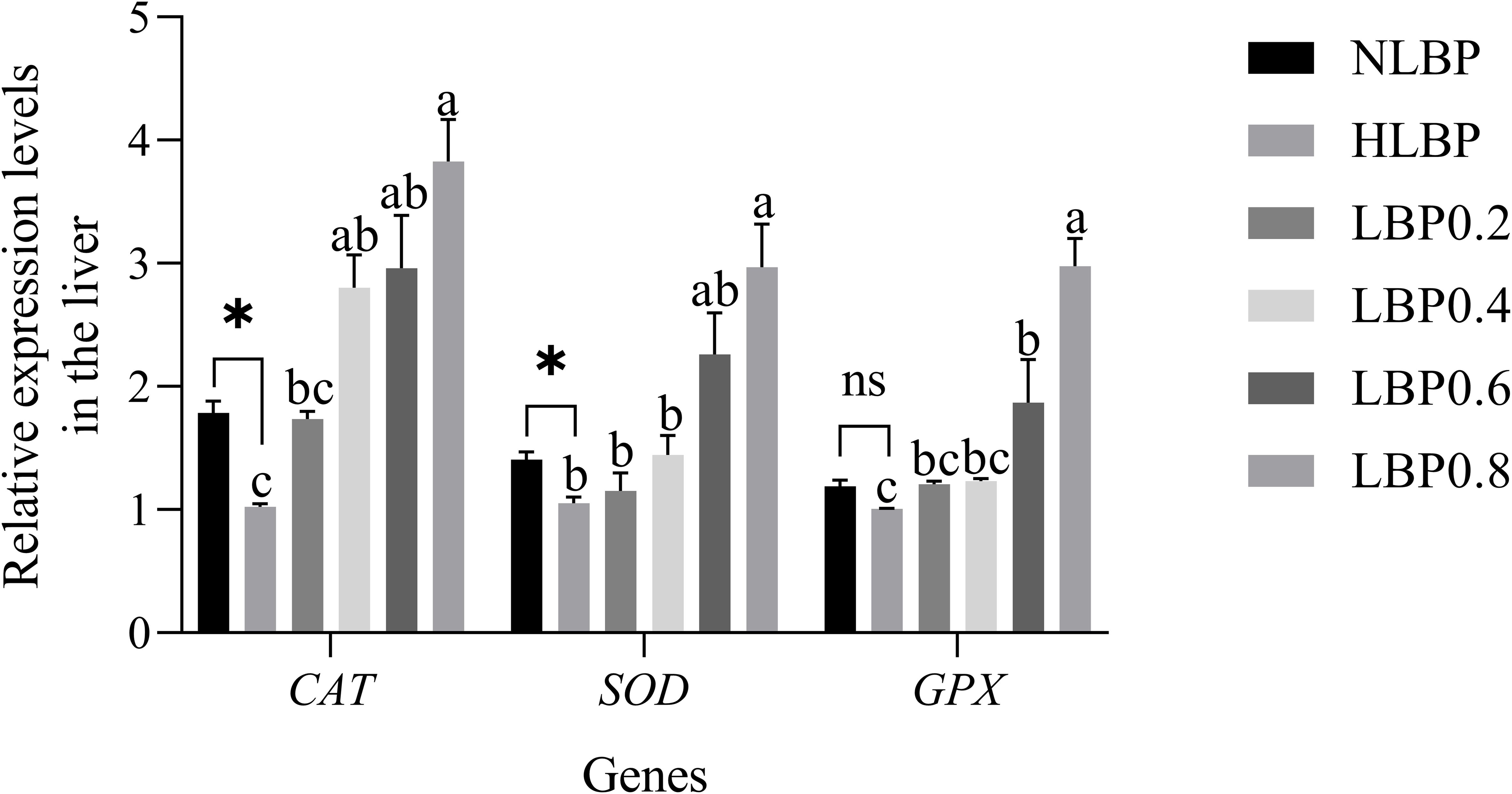
Figure 6. The relative expression levels of CAT, SOD, and GPX genes in the liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
The expression of inflammatory factors in the intestine is shown in Figure 7. IL-1β and TNF-α genes expression were significantly lower in the NLBP group than in the HLBP group (P < 0.05). IL-10 and TGF-β1 genes expression were significantly higher in the NLBP group than in the HLBP group (P < 0.05). The expression of IL-1β, IL-8, and TNF-α genes were significantly lower in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of IL-10 and TGF-β1 genes were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of inflammatory factors in the liver is shown in Figure 8. IL-8 and TNF-α genes expression were significantly lower in the NLBP group than in the HLBP group (P < 0.05). TGF-β1 gene expression was significantly higher in the NLBP group than in the HLBP group (P < 0.05). The expression of IL-1β, IL-8, and TNF-α genes were significantly lower in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of IL-10 and TGF-β1 genes were significantly higher in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05).
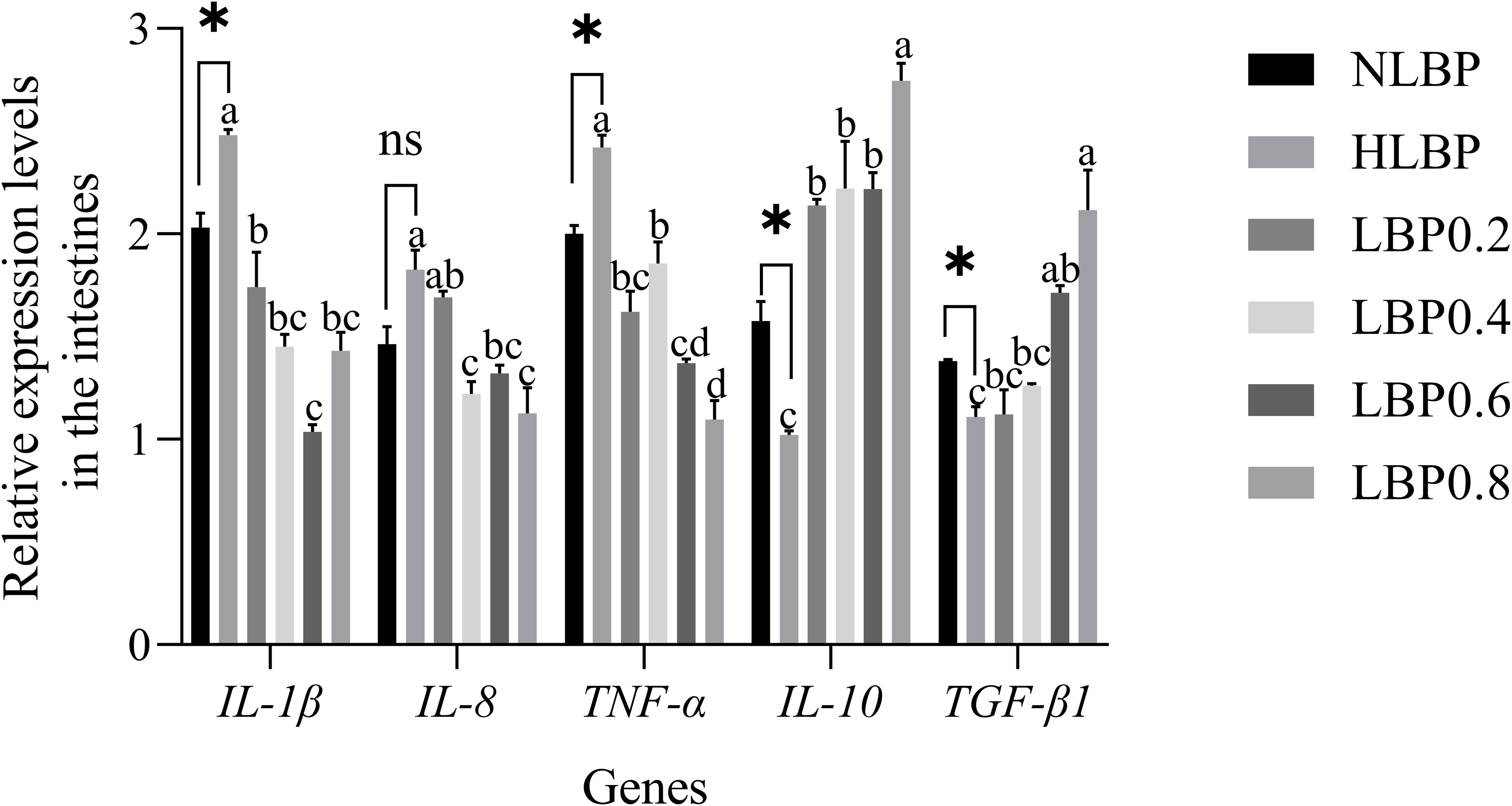
Figure 7. The relative expression levels of IL-1β, IL-8, TNF-α, IL-10, and TGF-β1 genes in the intestines of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
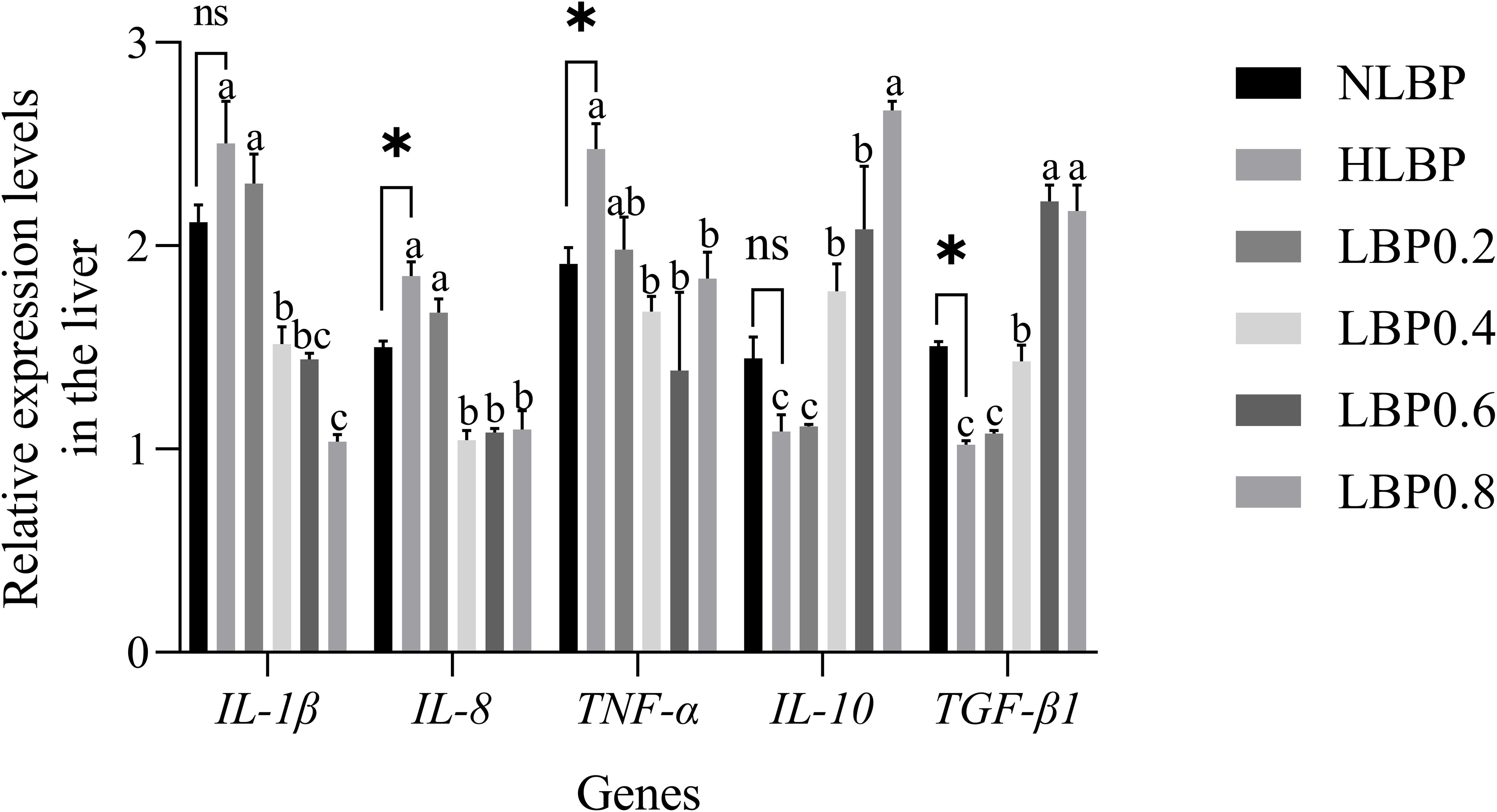
Figure 8. The relative expression levels of IL-1β, IL-8, TNF-α, IL-10, and TGF-β1 genes in the liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
The expression of genes related to glucose metabolism in the liver is shown in Figure 9. GK gene expression was significantly lower in the NLBP group than in the HLBP group (P < 0.05). PFK gene expression in the NLBP group was extremely significantly lower than that in the HLBP group (P < 0.01). The expression of GK, PFK, and G6P genes were significantly higher in the LBP0.6 and LBP0.8 groups than in the HLBP group (P < 0.05). The expression of genes related to lipid metabolism in the liver is shown in Figure 10. ACC and FAS genes expression were significantly lower in the NLBP group than in the HLBP group (P < 0.05). The expression of ACC, CPT-1, and FAS genes were significantly higher in the LBP0.4, LBP0.6, and LBP0.8 groups than in the HLBP group (P < 0.05). There was no significant difference in G6P and CPT-1 genes expression between the NLBP and HLBP groups (P > 0.05).
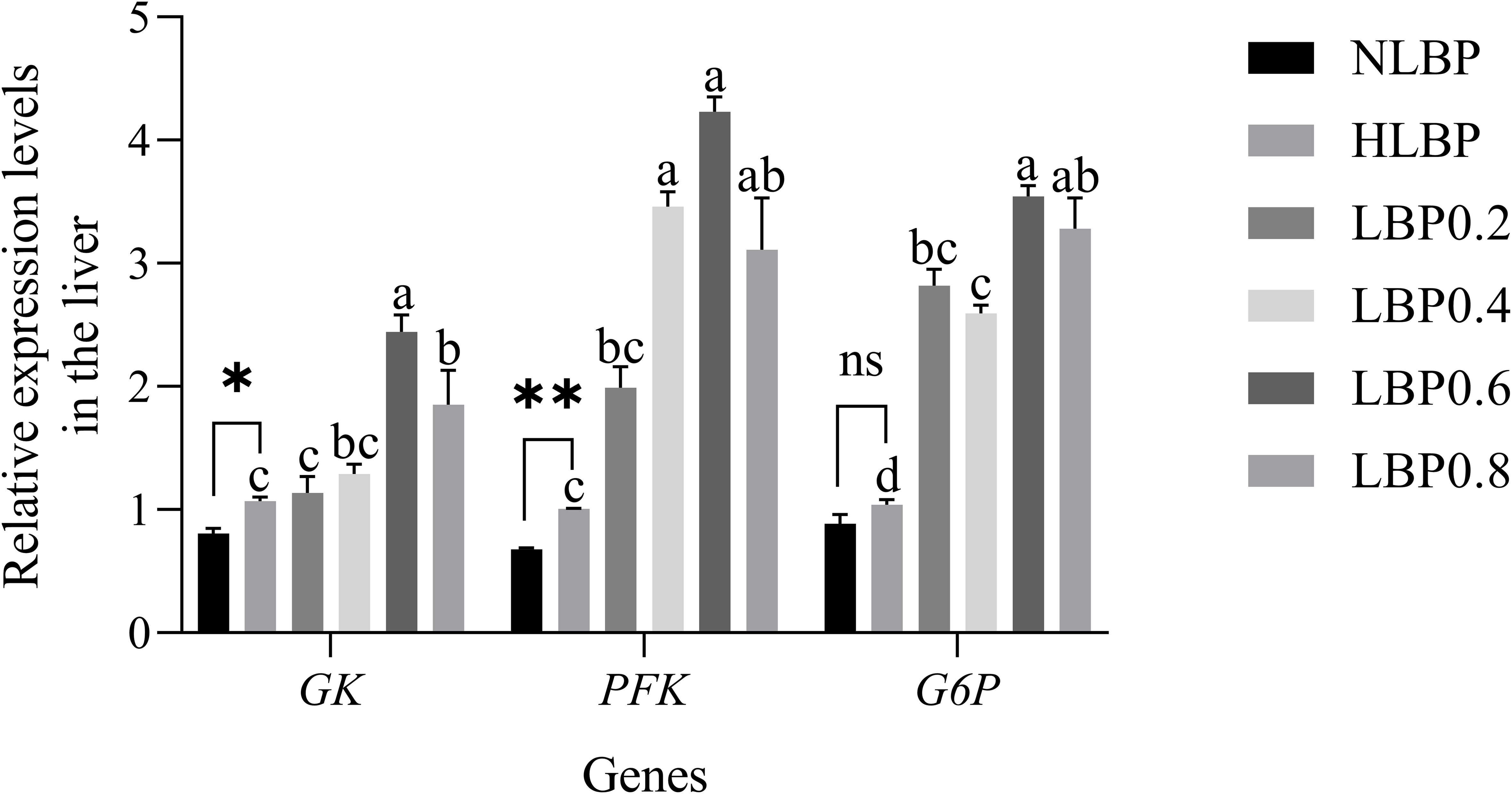
Figure 9. The relative expression levels of GK, PFK, and G6P genes in the liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).

Figure 10. The relative expression levels of ACC, CPT-1, and FAS genes in the liver of largemouth bass. “*” represents statistical difference between the NLBP group and the HLBP group, using Student’s t-test (* p < 0.05, ** p < 0.01 or ns p > 0.05). “abcd” represents statistical difference among different LBP levels in the high glucose feed diet. Data expressed as a mean ± standard error. Different groups are as follows: 10% glucose feed without LBP (NLBP); 15% glucose feed without LBP (HLBP); 0.2% Lycium barbarum polysaccharides (LBP0.2); 0.4% Lycium barbarum polysaccharides (LBP0.4); 0.6% Lycium barbarum polysaccharides (LBP0.6); 0.8% Lycium barbarum polysaccharides (LBP0.8).
Research has demonstrated that high-carbohydrate diets can have adverse effects on carnivorous fish (Liu Y. et al., 2022; Zheng et al., 2023), and largemouth bass have a low utilization of carbohydrates (Li S. et al., 2020). In this study, a high-carbohydrate diet was found to significantly impair the growth performance of largemouth bass. FBW, WGR, and SGR were significantly lower in the high-carbohydrate diet group than in the low-carbohydrate diet group. This finding suggests that the adaptability of largemouth bass to high-carbohydrate diets may be limited, potentially due to the specificity of its intestinal metabolism and enzymatic activity. Known for their excellent safety and low toxicity, polysaccharides are a type of natural extract from plants made up of many monosaccharides with the same or distinct structures joined together by glycosidic linkages (Tian et al., 2019). It is now widely used in the aquaculture industry. Numerous studies have shown that dietary supplementation with 0.5-2.0 g/kg LBP significantly improves the growth performance of aquatic animals such as Turkestan barbel (Sun et al., 2023), spotted sea bass (Huang Z. et al., 2023), Nile tilapia (Zhang X. et al., 2020) and hybrid grouper (Tan et al., 2019b). In this study, the dietary inclusion of LBP was found to be effective in mitigating the adverse effects of a high-carbohydrate diet on the growth performance of largemouth bass. Dietary addition of 0.6 g/kg LBP showed the best growth performance, even better than the low-carbohydrate dietary group. This is an interesting phenomenon that may be related to the ability of LBP to increase carbohydrate utilization by largemouth bass (Ooi et al., 2004). LBP may have the potential to enhance carbohydrate metabolism and improve energy utilization, a function which could be achieved by modulating the intestinal microenvironment and directly influencing metabolic pathways. Furthermore, it has been demonstrated that plant extracts can enhance lipid metabolism in fish (Xiao et al., 2017). Another significant role of LBP may be related to their impact on lipid metabolism. In this study, LBP addition above 0.4 g/kg was effective in reducing the hepatosomatic index of largemouth bass. This suggests that LBP may reduce hepatic fat accumulation by regulating lipid metabolism. These data suggest that LBP positively regulates the growth performance of largemouth bass. Most studies show that improvements in growth performance and feed consumption efficiency are correlated with increased digestive enzyme activity (Najdegerami et al., 2017; Zheng et al., 2018; Huang et al., 2020). In this experiment, the addition of LBP significantly increased feed utilization by largemouth bass, and the addition of 0.4–0.6 g/kg of LBP significantly increased the digestive enzyme activity of largemouth bass. Polysaccharides can enhance the digestive enzyme activity of aquatic animals (Wu et al., 2020; Yu et al., 2022); this may be due to their ability to influence the structure of the intestinal flora of aquatic organisms (Liu et al., 2020). Firstly, several species in the gut have the ability to produce exogenous digestive enzymes directly, which aids in the host’s improvement of digestion (Latorre et al., 2016). Furthermore, polysaccharides may be broken down by intestinal bacteria and transformed into healthy compounds like short-chain fatty acids. In addition to enhancing the action of digestive enzymes and controlling the pH of the animal intestines, these chemicals also benefit intestinal epithelial cells, which in turn promotes intestinal function (Huang Z. et al., 2023). In this study, the addition of 0.6 g/kg LBP significantly ameliorated the negative effects of a high-carbohydrate diet on largemouth bass and significantly improved growth performance and digestibility.
The blood absorbs and digests the nutrients in the diet. As a result, serum biochemical parameters can reveal an aquatic animal’s health in addition to its clinical and physiological conditions (Mu et al., 2018; Abdel-Latif et al., 2021). Variations in those factors have shown how aquatic animals may adapt to different food formulations and surroundings (Li M. et al., 2020), as well as how such changes might influence their immune systems (Chekani et al., 2021; Zhong et al., 2024). In the present study, a high-carbohydrate diet significantly increased serum TG, TC, and LDL levels in largemouth bass. This is consistent with metabolic disorders observed in other aquatic animals under similar dietary conditions (Jia et al., 2024). Such alterations in metabolism may lead to decreased energy utilization efficiency and fat accumulation, thereby impacting the health and growth of fish. This suggests that a high carbohydrate diet appears to negatively affect glycolipid metabolism in largemouth bass. In general, HDL facilitates the transport of cholesterol from peripheral tissues to hepatic cells for further metabolism, whereas LDL mediates the transfer of cholesterol from the liver to various tissues. But elevated LDL levels are not a good sign (Jafari et al., 2018). Addition of LBP to a high-carbohydrate diet effectively reduced serum TG, TC, and LDL levels, suggesting that LBP can help largemouth bass utilize carbohydrates efficiently and regulate glycolipid metabolism. Serum ALT and AST activities are widely recognized as important indicators of liver damage, reflecting the extent of hepatocellular injury (Liu et al., 2012). In addition, serum AKP activity also reflects the health status of the liver in largemouth bass (Huang H. et al., 2023). In this study, the activities of serum ALT, AST, and AKP demonstrate that a high-carbohydrate diet can adversely impact the liver health of largemouth bass. However, the supplementation with LBP effectively mitigates these negative effects. This may be associated with the hepatoprotective and anti-inflammatory effects of LBP, suggesting their potential application in the prevention of liver diseases. High-carbohydrate diets have the potential to negatively impact the antioxidant capacity of largemouth bass, potentially leading to increased oxidative stress and associated health issues (Lin et al., 2018; Guo et al., 2020). SOD, CAT, T-AOC, GSH-PX, and MDA were often used to assess antioxidant capacity in fish (Tan et al., 2019a). SOD, CAT, and GSH-PX were important antioxidant enzymes and T-AOC directly reflected the ability of fish to scavenge oxygen free radicals, while MDA content showed the severity of oxidative stress (Tan et al., 2016). In the present study, a high-carbohydrate diet significantly decreased the activity of antioxidant enzymes and the expression levels of antioxidant-related genes in both the intestine and liver. This indicates that the high carbohydrate diet significantly impacted the antioxidant capacity of largemouth bass. However, the addition of LBP significantly improved the situation. According to some research, the LBP exhibited hepatoprotective and antioxidant abilities, improving the liver damage caused by carbon tetrachloride by raising the activity of antioxidant enzymes in common carp (Liu et al., 2015). This has similar conclusions to the present study. In the present study, the supplementation of LBP was found to significantly enhance the activities of antioxidant enzymes and upregulate the expression of antioxidant genes in both the intestinal and hepatic tissues of largemouth bass. These findings suggest that LBP has the potential to markedly improve the antioxidant capacity of largemouth bass when they are fed a high-carbohydrate diet.
In aquaculture species, eels, Nile tilapia, and largemouth bass experienced intestinal and hepatic inflammation when fed a diet high in carbohydrates (Lin et al., 2018; Shi et al., 2022; Wang et al., 2022b). In summary, inflammation is a healthy physiological process that restores tissue vitality and removes foreign particles interfering with tissue homeostasis (Kiecolt-Glaser et al., 2010). However, when proinflammatory chemicals have the upper hand, persistent inflammation can lead to tissue damage (Shimoda et al., 2016). Pro-inflammatory cytokines, including, IL-1β, IL-8, and TNF-α are useful indicators for assessing inflammation and have significant functions in fish immunological response (Zhang et al., 2018). Anti-inflammatory factors such as IL-10 and TGF-β1 can likewise be used as indicators of the inflammatory response in fish (Tan et al., 2019a). In the present study, chronic high-carbohydrate diets significantly increased the expression of pro-inflammatory factors in the intestine and liver, in contrast to significantly decreasing the expression of anti-inflammatory factors. This suggests that a high-carbohydrate diet may exacerbate tissue inflammation by promoting the expression of inflammatory factors, thereby posing a threat to the intestinal and hepatic health of fish. Likewise, some studies show that a diet containing 15% starch induced intestinal inflammation in largemouth bass (Zhou et al., 2021). A high-carbohydrate diet may trigger inflammatory responses by affecting the balance of gut microbiota, increasing intestinal permeability, or directly stimulating immune cells. In addition, it has been shown that oxidative stress leads to an inflammatory response in the body (Zhang et al., 2022), which is similar to the conclusions obtained in this study. The antioxidant properties of LBP may contribute to their anti-inflammatory effects. Oxidative stress has been widely recognized as associated with inflammatory states, and antioxidants can alleviate inflammation induced by oxidative stress. Therefore, LBP may indirectly suppress inflammatory responses by enhancing the activity of antioxidant enzymes and reducing oxidative stress. Interestingly, in this experiment, the expression of pro-inflammatory factors in the intestine and liver of largemouth bass decreased significantly and the expression of anti-inflammatory factors increased significantly with increasing LBP addition. This shows the same trend as antioxidant-related genes and there seems to be a dose effect. This also seems to validate oxidative stress as one of the factors contributing to the body’s inflammatory response. In conclusion, LBP was effective in ameliorating the largemouth bass intestinal and liver inflammatory responses induced by a high-carbohydrate diet.
Fish that consume excessive starch may develop hyperglycemia and glycogen accumulation (Zhang et al., 2019). In this study, a high-carbohydrate diet significantly increased the expression of GK and PFK genes, which are key regulators of glucose metabolism. The upregulation of these genes suggests that consumption of a high-carbohydrate diet enhances glucose metabolic activity. But in comparison to growth performance, largemouth bass did not effectively utilize carbohydrates. This may indicate issues with metabolic efficiency or potential inhibition of related glucose metabolic pathways. Dietary addition of LBP can effectively regulate the abundance of intestinal flora, thereby affecting the ability of organisms to metabolize glycolipids (Cui et al., 2020; Zhu et al., 2020). In this study, the addition of LBP to a high-carbohydrate diet significantly increased the expression of glucose metabolism genes and was highest in the LBP0.6 group. This suggests that the addition of appropriate dose of LBP can help largemouth bass improve the utilization of carbohydrates. ACC and FAS are the key enzymes involved in fatty acid synthesis. Acetyl-CoA is carbonylated to malonyl-CoA by ACC, and malonyl-CoA is converted to palmitic acid by FAS, which is then esterified to triglycerides (Wu and Huang, 2020). One of the important enzymes in lipolytic metabolism is CPT-1. PPAR-α mediates CPT1’s catalytic conversion of lipoyl CoA to lipoyl carnitine, which starts the mitochondrial β-oxidation of long-chain fatty acids (Song et al., 2015). In the present study, the high-carbohydrate diet significantly increased the expression of ACC and FAS genes, but there was no significant difference in the expression of CPT-1 gene. This suggests that a high-carbohydrate diet increased the risk of lipid deposition in the liver of largemouth bass. This is not good information for the state of health of largemouth bass. However, the addition of LBP to a high-carbohydrate diet can effectively alleviate this condition. In this study, the addition of LBP significantly increased the expression of lipid synthesis genes and lipolysis genes, which appeared to reach a state of equilibrium, which appeared to be more favorable to largemouth bass when comparing growth performance. This suggests that LBP may reduce adverse lipid accumulation by modulating the balance between lipid synthesis and breakdown, thereby maintaining liver health. This balance may be due to the role of Lycium barbarum polysaccharides in regulating lipid metabolism, particularly through their potential involvement in PPAR-α mediated metabolic pathways. In summary, LBP can help largemouth bass utilize high carbohydrates efficiently.
In summary, high-carbohydrate diets can negatively affect the growth performance, digestibility, immune status, and metabolism of largemouth bass. However, the addition of LBP to the high-carbohydrate diet can effectively improve this situation, and LBP can effectively improve the growth performance, digestive ability, immune status, and glycolipid metabolism of largemouth bass. Under the conditions of this experiment, the most suitable additive amount of LBP was 0.6 g/kg.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The animal study was approved by Shanghai Ocean University’s ethical guidelines. The study was conducted in accordance with the local legislation and institutional requirements.
LQ: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YH: Data curation, Formal analysis, Investigation, Software, Writing – review & editing. GZ: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Experimental funding was provided by the “Research on the application of functional additives in aquatic healthy aquaculture (D-8006-25)”.
Research on the application of functional additives in aquatic healthy aquaculture (D-8006-25).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Latif H. M. R., Hendam B. M., Shukry M., El-Shafai N. M., El-Mehasseb L. M., Dawood M. A. O., et al. (2021). Effects of sodium butyrate nanoparticles on the hemato-immunological indices, hepatic antioxidant capacity, and gene expression responses in Oreochromis niloticus. Fish Shellfish Immunol. 119, 516–523. doi: 10.1016/j.fsi.2021.10.039
Amagase H., Farnsworth N. R. (2011). A review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Res. Int. 44, 1702–1717. doi: 10.1016/j.foodres.2011.03.027
Chekani R., Akrami R., Ghiasvand Z., Chitsaz H., Jorjani S. (2021). Effect of dietary dehydrated lemon peel (Citrus limon) supplementation on growth, hemato-immunolological and antioxidant status of rainbow trout (Oncorhynchus mykiss) under exposure to crowding stress. Aquaculture 539. doi: 10.1016/j.aquaculture.2021.736597
Cui F., Shi C.-L., Zhou X.-J., Wen W., Gao X.-P., Wang L.-Y., et al. (2020). Lycium barbarum polysaccharide extracted from Lycium barbarum leaves ameliorates asthma in mice by reducing inflammation and modulating gut microbiota. J. Medicinal Food 23, 699–710. doi: 10.1089/jmf.2019.4544
Guo J.-L., Kuang M.-W., Zhong Y.-F., Zhou Y.-F., Chen Y.-J., Lin S.-M. (2020). Effects of supplemental dietary bile acids on growth, liver function and immunity of juvenile largemouth bass (Micropterus salmoides) fed high-starch diet. Fish Shellfish Immunol. 97, 602–607. doi: 10.1016/j.fsi.2019.12.087
Huang Z.-F., Li Z.-B., Xu A.-L., Zheng D.-J., Ye Y.-L., Wang Z. (2020). Effects of exogenous multienzyme complex supplementation in diets on growth performance, digestive enzyme activity and non-specific immunity of the Japanese seabass, Lateolabrax japonicus. Aquac. Nutr. 26, 306–315. doi: 10.1111/anu.12991
Huang Z.-F., Ye Y.-L., Xu A.-L., Li Z.-B. (2023). Effects of dietary crude polysaccharides from Lycium barbarum on growth performance, digestion, and serum physiology and biochemistry of spotted sea bass Lateolabrax maculatus. Aquac. Rep. 32. doi: 10.1016/j.aqrep.2023.101710
Huang H., Zhuang Y., Tang X.-S., Wang N.-A., Yuan K., Zhong G.-F. (2023). Dietary acidic calcium sulfate enhances growth, digestive enzyme activities, intestinal histology and resistance against Aeromonas hydrophila in juvenile largemouth bass, Micropterus salmoides. Aquac. Rep. 29. doi: 10.1016/j.aqrep.2023.101467
Jafari F., Agh N., Noori F., Tokmachi A., Gisbert E. (2018). Effects of dietary soybean lecithin on growth performance, blood chemistry and immunity in juvenile stellate sturgeon (Acipenser stellatus). Fish Shellfish Immunol. 80, 487–496. doi: 10.1016/j.fsi.2018.06.023
Jia X.-W., Yu H.-Y., Du B., Shen Y.-B., Gui L., Xu X.-Y., et al. (2024). Incorporating Lycium barbarum residue in diet boosts survival, growth, and liver health in juvenile grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol. 149. doi: 10.1016/j.fsi.2024.109573
Jin M.-L., Huang Q.-S., Zhao K., Shang P. (2013). Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int. J. Biol. Macromol. 54, 16–23. doi: 10.1016/j.ijbiomac.2012.11.023
Kamalam B. S., Medale F., Kaushik S., Polakof S., Skiba-Cassy S., Panserat S. (2012). Regulation of metabolism by dietary carbohydrates in two lines of rainbow trout divergently selected for muscle fat content. J. Exp. Biol. 215, 2567–2578. doi: 10.1242/jeb.070581
Kiecolt-Glaser J. K., Gouin J.-P., Hantsoo L. (2010). Close relationships, inflammation, and health. Neurosci. Biobehav. Rev. 35, 33–38. doi: 10.1016/j.neubiorev.2009.09.003
Latorre J. D., Hernandez-Velasco X., Wolfenden R. E., Vicente J. L., Wolfenden A. D., Bielke L. R., et al. (2016). valuation and selection of bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Veterinary Sci. 3. doi: 10.3389/fvets.2016.00095
Li X.-Y., Zheng S.-X., Ma X.-K., Cheng K.-M., Wu G.-Y. (2020a). Effects of dietary starch and lipid levels on the protein retention and growth of largemouth bass (Micropterus salmoides). Amino Acids 52, 999–1016. doi: 10.1007/s00726-020-02869-6
Li X., Wei X., Guo X., Mi S., Hua X., Li N., et al. (2020b). Enhanced growth performance, muscle quality and liver health of largemouth bass (Micropterus salmoides) were related to dietary small peptides supplementation. Aquac. Nutr. 26, 2169–2177. doi: 10.1111/anu.13155
Li S., Wang A., Li Z., Zhang J., Sang C., Chen N. (2020). Antioxidant defenses and non-specific immunity at enzymatic and transcriptional levels in response to dietary carbohydrate in a typical carnivorous fish, hybrid grouper (Epinephelus fuscoguttatus ♀ x. lanceolatus ♂). Fish Shellfish Immunol. 100, 109–116. doi: 10.1016/j.fsi.2020.03.015
Li M.-M., Xu C., Ma Y.-C., Ye R.-K., Chen H.-Y., Xie D.-Z., et al. (2020). Effects of dietary n-3 highly unsaturated fatty acids levels on growth, lipid metabolism and innate immunity in juvenile golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 105, 177–185. doi: 10.1016/j.fsi.2020.06.060
Li X. M., Li X. L., Zhou A. G. (2007). Evaluation of antioxidant activity of the polysaccharides extracted from Lycium barbarum fruits in vitro. Eur. Polymer J. 43, 488–497. doi: 10.1016/j.eurpolymj.2006.10.025
Lin S.-M., Shi C.-M., Mu M.-M., Chen Y.-J., Luo L. (2018). Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 78, 121–126. doi: 10.1016/j.fsi.2018.04.046
Lin S.-M., Zhou X.-M., Zhou Y.-L., Kuang W.-M., Chen Y.-J., Luo L., et al. (2020). Intestinal morphology, immunity and microbiota response to dietary fibers in largemouth bass, Micropterus salmoide. Fish Shellfish Immunol. 103, 135–142. doi: 10.1016/j.fsi.2020.04.070
Liu B., Xie J., Ge X., Miao L., Wang G. (2012). Effect of high dietary carbohydrate on growth, serum physiological response, and hepatic heat shock cognate protein 70 expression of the top-mouth culter Erythroculter ilishaeformis Bleeker. Fish. Sci. 78, 613–623. doi: 10.1007/s12562-012-0486-4
Liu H., Cui B., Zhang Z. (2022). Mechanism of glycometabolism regulation by bioactive compounds from the fruits of Lycium barbarum: A review. Food Res. Int. 159. doi: 10.1016/j.foodres.2022.111408
Liu H.-L., Huang Y., Huang X.-L., Li M.-H., Chen D.-F., Geng Y., et al. (2023). Eucommia ulmoides Oliver enhances the antioxidant capacity and protects Micropterus salmoides from liver damage and immune function impairment caused by a high starch diet. J. Funct. Foods 101. doi: 10.1016/j.jff.2023.105424
Liu Y., Cao L., Du J., Jia R., Wang J., Xu P., et al. (2015). Protective effects of Lycium barbarum polysaccharides against carbon tetrachloride-induced hepatotoxicity in precision-cut liver slices in vitro and in vivo in common carp (Cyprinus carpio L.). Comp. Biochem. Physiol. C-Toxicol, Pharmacol. 169, 65–72. doi: 10.1016/j.cbpc.2014.12.005
Liu W.-C., Zhou S.-H., Balasubramanian B., Zeng F.-Y., Sun C.-B., Pang H.-Y., et al. (2020). Dietary seaweed (Enteromorpha) polysaccharides improves growth performance involved in regulation of immune responses, intestinal morphology and microbial community in banana shrimp Fenneropenaeus merguiensis. Fish Shellfish Immunol. 104, 202–212. doi: 10.1016/j.fsi.2020.05.079
Liu Y., Liu N., Wang A., Chen N., Li S. (2022). Resveratrol inclusion alleviated high-dietary-carbohydrate-induced glycogen deposition and immune response of largemouth bass, Micropterus salmoides. Br. J. Nutr. 127, 165–176. doi: 10.1017/S0007114521000544
Liu L., Zhao Y., Huang Z., Long Z., Qin H., Lin H., et al. (2024). Effects of Lycium barbarum polysaccharides supplemented to high soybean meal diet on immunity and hepatic health of spotted sea bass Lateolabrax maculatus. Front. Immunol. 15. doi: 10.3389/fimmu.2024.1333469
Ma H.-J., Mou M.-M., Pu D. -C., Lin S.-M., Chen Y.-J., Luo L. (2019). Effect of dietary starch level on growth, metabolism enzyme and oxidative status of juvenile largemouth bass, Micropterus salmoides. Aquaculture 498, 482–487. doi: 10.1016/j.aquaculture.2018.07.039
Mu H., Shen H., Liu J., Xie F., Zhang W., Mai K. (2018). High level of dietary soybean oil depresses the growth and anti-oxidative capacity and induces inflammatory response in large yellow croaker Larimichthys crocea. Fish Shellfish Immunol. 77, 465–473. doi: 10.1016/j.fsi.2018.04.017
Najdegerami E. H., Bakhshi F., Tokmechi A., Harzevili A.-S., Sorgeloos P., Bossier P. (2017). Dietary effects of poly–hydroxybutyrate on the growth performance, digestive enzyme activity, body composition, mineral uptake and bacterial challenge of rainbow trout fry (Oncorhynchus mykiss). Aquac. Nutr. 23, 246–254. doi: 10.1111/anu.12386
Ooi G. T., Tawadros N., Escalona R. M. (2004). Pituitary cell lines and their endocrine applications. Mol. Cell. Endocrinol. 228, 1–21. doi: 10.1016/j.mce.2004.07.018
Romano N., Fischer H., Rubio-Benito M.-M., Overtuf K., Sinha A.-K., Kumar V. (2022). Different dietary combinations of high/low starch and fat with or without bile acid supplementation on growth, liver histopathology, gene expression and fatty acid composition of largemouth bass, Micropterus salmoides. Comp. Biochem. Physiol. a-Molecular Integr. Physiol. 266. doi: 10.1016/j.cbpa.2022.111157
Shan X., Zhou J., Ma T., Chai Q. (2011). Lycium barbarum polysaccharides reduce exercise-induced oxidative stress. Int. J. Mol. Sci. 12, 1081–1088. doi: 10.3390/ijms12021081
Shi Y., Zhong L., Zhong H., Zhang J., Liu X., Peng M., et al. (2022). Taurine supplements in high-carbohydrate diets increase growth performance of Monopterus albus by improving carbohydrate and lipid metabolism, reducing liver damage, and regulating intestinal microbiota. Aquaculture 554. doi: 10.1016/j.aquaculture.2022.738150
Shimoda M., Horiuchi K., Sasaki A., Tsukamoto T., Okabayashi K., Hasegawa H., et al. (2016). Epithelial cell-derived a disintegrin and metalloproteinase-17 confers resistance to colonic inflammation through EGFR activation. Ebiomedicine 5, 114–124. doi: 10.1016/j.ebiom.2016.02.007
Song Y.-F., Wu K., Tan X.-Y., Zhang L.-H., Zhuo M.-Q., Pan Y.-X., et al. (2015). Effects of recombinant human leptin administration on hepatic lipid metabolism in yellow catfish Pelteobagrus fulvidraco: in vivo and in vitro studies. Gen. Comp. Endocrinol. 212, 92–99. doi: 10.1016/j.ygcen.2015.01.022
Sun Y., Meng X., Hu X., Liu R., Zhao Z., Wang S., et al. (2023). Dietary supplementation with Lycium barbarum polysaccharides conducive to maintaining the health of Luciobarbus capito via the enhancement of enzyme activities and the modulation of gut microbiota. Int. J. Biol. Macromol. 232. doi: 10.1016/j.ijbiomac.2023.123500
Tan X., Lin H., Huang Z., Zhou C., Wang A., Qi C., et al. (2016). Effects of dietary leucine on growth performance, feed utilization, non-specific immune responses and gut morphology of juvenile golden pompano Trachinotus ovatus. Aquaculture 465, 100–107. doi: 10.1016/j.aquaculture.2016.08.034
Tan X., Sun Z., Ye C., Lin H. (2019a). The effects of dietary Lycium barbarum extract on growth performance, liver health and immune related genes expression in hybrid grouper (Epinephelus lanceolatus♂x. fuscoguttatus♀) fed high lipid diets. Fish Shellfish Immunol. 87, 847–852. doi: 10.1016/j.fsi.2019.02.016
Tan X., Sun Z., Ye C. (2019b). Dietary Lycium barbarum extract administration improved growth, meat quality and lipid metabolism in hybrid grouper (Epinephelus lanceolatus ♂ x. fuscoguttatus ♀) fed high lipid diets. Aquaculture 504, 190–198. doi: 10.1016/j.aquaculture.2019.01.044
Tang H.-L., Chen C., Wang S.-K., Sun G.-J. (2015). Biochemical analysis and hypoglycemic activity of a polysaccharide isolated from the fruit of Lycium barbarum L. Int. J. Biol. Macromol. 77, 235–242. doi: 10.1016/j.ijbiomac.2015.03.026
Mohan K., Ravichandran S., Muralisankar T., Uthayakumar V., Chandirasekar R., Seedevi P., et al. (2019). Potential uses of fungal polysaccharides as immunostimulants in fish and shrimp aquaculture: a review. Aquaculture 500, 250–263. doi: 10.1016/j.aquaculture.2018.10.023
Wang T., Xu R., Qiao F., Du Z.-Y., Zhang M.-L. (2022a). Effects of mannan oligosaccharides (MOS) on glucose and lipid metabolism of largemouth bass (Micropterus salmoides) fed with high carbohydrate diet. Anim. Feed Sci. Technol. 292. doi: 10.1016/j.anifeedsci.2022.115449
Wang T., Wu H.-X., Li W., Xu R., Qiao F., Du Z.-Y., et al. (2022b). Effects of dietary mannan oligosaccharides (MOS) supplementation on metabolism, inflammatory response and gut microbiota of juvenile Nile tilapia (Oreochromis niloticus) fed with high carbohydrate diet. Fish Shellfish Immunol. 130, 550–559. doi: 10.1016/j.fsi.2022.09.052
Wang K.-W., Liu Q.-Q., Zhu J., Deng X., Luo L., Lin S.-M., et al. (2023). Transcriptome analysis provides insights into the molecular mechanism of liver inflammation and apoptosis in juvenile largemouth bass Micropterus salmoides fed low protein high starch diets. Comp. Biochem. Physiol. D-Genomics Proteomics 45. doi: 10.1016/j.cbd.2022.101047
Wang Y., Wu J., Li L., Yao Y., Chen C., Hong Y., et al. (2024). Effects of tannic acid supplementation of a high-carbohydrate diet on the growth, serum biochemical parameters, antioxidant capacity, digestive enzyme activity, and liver and intestinal health of largemouth bass, Micropterus salmoides. Aquac. Nutr. 668. doi: 10.1155/2024/6682798
Wu H.-T., He X. J., Hong Y.-K., Ma T., Xu Y.-P., Li H.-H. (2010). Chemical characterization of Lycium barbarum polysaccharides and its inhibition against liver oxidative injury of high-fat mice. Int. J. Biol. Macromol. 46, 540–543. doi: 10.1016/j.ijbiomac.2010.02.010
Wu C., Shan J., Feng J., Wang J., Qin C., Nie G., et al. (2020). Effects of dietary Radix Rehmanniae Preparata polysaccharides on the digestive enzymes, morphology, microbial communities and mucosal barrier function of the intestine of Luciobarbus capito. Aquac. Res. 51, 1026–1037. doi: 10.1111/are.14448
Wu D., Li J.-N., Fan Z., Sun Z.-P., Zheng X.-H., Zhang H.-T., et al. (2024). Dietary Lycium barbarum Polysaccharide Modulates Growth Performance, Antioxidant Capacity, and Lipid Metabolism in Common Carp (Cyprinus carpio) Fed with High-Fat Diet. Antioxidants 13. doi: 10.3390/antiox13050540
Wu X., Huang T. (2020). Recent development in acetyl-CoA carboxylase inhibitors and their potential as novel drugs. Future Medicinal Chem. 12, 533–561. doi: 10.4155/fmc-2019-0312
Xiao P., Ji H., Ye Y., Zhang B., Chen Y., Tian J., et al. (2017). Dietary silymarin supplementation promotes growth performance and improves lipid metabolism and health status in grass carp (Ctenopharyngodon idellus) fed diets with elevated lipid levels. Fish Physiol. Biochem. 43, 245–263. doi: 10.1007/s10695-016-0283-6
Yang W., Wu J.-J., Song R., Li Z., Jia X.-W., Qian P.-C., et al. (2023). Effects of dietary soybean lecithin on growth performances, body composition, serum biochemical parameters, digestive and metabolic abilities in largemouth bass Micropterus salmoides. Aquac. Rep. 29. doi: 10.1016/j.aqrep.2023.101528
Yao R., Heinrich M., Weckerle C.-S. (2018). The genus Lycium as food and medicine: A botanical, ethnobotanical and historical revie. J. Ethnopharmacol. 212, 50–66. doi: 10.1016/j.jep.2017.10.010
Yu W., Yang Y., Zhou Q., Huang X., Huang Z., Li T., et al. (2022). Effects of dietary Astragalus polysaccharides on growth, health and resistance to Vibrio harveyi of Lates calcarifer. Int. J. Biol. Macromol. 207, 850–858. doi: 10.1016/j.ijbiomac.2022.03.176
Zhang X.-R., Zhou W.-X., Zhang Y.-X., Qi C.-H., Yan H.-G., Wang Z.-F., et al. (2011). Macrophages, rather than T and B cells are principal immunostimulatory target cells of Lycium barbarum L. polysaccharide LBPF4-OL. J. Ethnopharmacol. 136, 465–472. doi: 10.1016/j.jep.2011.04.054
Zhang C., Rahimnejad S., Wang Y., Lu K., Song K., Wang L., et al. (2018). Substituting fish meal with soybean meal in diets for Japanese seabass (Lateolabrax japonicus): Effects on growth, digestive enzymes activity, gut histology, and expression of gut inflammatory and transporter genes. Aquaculture 483, 173–182. doi: 10.1016/j.aquaculture.2017.10.029
Zhang W., Liu K., Tan B., Liu H., Dong X., Yang Q., et al. (2019). Transcriptome, enzyme activity and histopathology analysis reveal the effects of dietary carbohydrate on glycometabolism in juvenile largemouth bass, Micropterus salmoides. Aquaculture 504, 39–51. doi: 10.1016/j.aquaculture.2019.01.030
Zhang Y., Xie S., Wei H., Zheng L., Liu Z., Fang H., et al. (2020). High dietary starch impaired growth performance, liver histology and hepatic glucose metabolism of juvenile largemouth bass, Micropterus salmoides. Aquac. Nutr. 26, 1083–1095. doi: 10.1111/anu.13066
Zhang X., Huang K., Zhong H., Ma Y., Guo Z., Tang Z., et al. (2020). Effects of Lycium barbarum polysaccharides on immunological parameters, apoptosis, and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 97, 509–514. doi: 10.1016/j.fsi.2019.12.068
Zhang Q., Xue Y., Fu Y., Bao B., Guo M. (2022). Zinc deficiency aggravates oxidative stress leading to inflammation and fibrosis in lung of mice. Biol. Trace Element Res. 200, 4045–4057. doi: 10.1007/s12011-021-03011-7
Zheng X., Duan Y., Dong H., Zhang J. (2018). Effects of dietary lactobacillus plantarum on growth performance, digestive enzymes and gut morphology of Litopenaeus vannamei. Probiotics Antimicrobial Proteins 10, 504–510. doi: 10.1007/s12602-017-9300-z
Zheng L., Wang Z., Zhang B., Yan L., Wang P., Zhao C., et al. (2023). Effects of high dietary carbohydrate levels on growth performance, enzyme activities, expression of genes related to liver glucose metabolism, and the intestinal microbiota of lateolabrax maculatus juveniles. Fishes 8. doi: 10.3390/fishes8090431
Zhong G.-F., Zhang L.-F., Zhuang Y., Li Q., Huang H., Cao C., et al. (2024). Effects of brown fishmeal on growth performance, digestibility, and lipid metabolism of the Chinese soft-shelled turtle (Pelodiscus sinensis). Mar. Biotechnol. 26, 92–102. doi: 10.1007/s10126-023-10274-9
Zhou Y.-L., He G.-L., Jin T., Chen Y.-J., Dai F.-Y., Luo L., et al. (2021). High dietary starch impairs intestinal health and microbiota of largemouth bass, Micropterus salmoides. Aquaculture 534. doi: 10.1016/j.aquaculture.2020.736261
Zhou X., Wang Y., Yu J., Li J., Wu Q.-H., Bao S.-S., et al. (2022). Effects of dietary fermented Chinese herbal medicines on growth performance, digestive enzyme activity, liver antioxidant capacity, and intestinal inflammatory gene expression of juvenile largemouth bass (Micropterus salmoides). Aquac. Rep. 25. doi: 10.1016/j.aqrep.2022.101269
Keywords: Lycium barbarum polysaccharide, largemouth bass (Micropterus salmoides), growth performance, antioxidant capacity, inflammation response, glucolipid metabolism
Citation: Qi L, He Y and Zhong G (2025) Dietary Lycium barbarum polysaccharide modulates growth performance, antioxidant capacity, and glucolipid metabolism in largemouth bass (Micropterus salmoides) fed with high- carbohydrate diet. Front. Mar. Sci. 12:1542173. doi: 10.3389/fmars.2025.1542173
Received: 09 December 2024; Accepted: 27 January 2025;
Published: 17 February 2025.
Edited by:
Christian Larbi Ayisi, University of Environment and Sustainable Development, GhanaReviewed by:
Yun Wang, Chinese Academy of Fishery Sciences (CAFS), ChinaCopyright © 2025 Qi, He and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guofang Zhong, Z2Z6aG9uZ0BzaG91LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.