- Marine Science Program, Biological and Environmental Science and Engineering Division (BESE), King Abdullah University of Science and Technology (KAUST), Thuwal, Saudi Arabia
Coloniality and clonality in marine sessile organisms offer several advantages, such as better space occupation and directional growth. In coral colonies, species-specific functional connections are maintained among polyps, allowing for resource translocation and colony architecture coordination. A potential whole-colony integration mechanism is apical dominance, a phenomenon controlling branching patterns through hormonal signaling in plants and seagrass, yet unconfirmed in scleractinian corals. This study aims at investigating the occurrence of apical dominance in corals, hypothesizing that highly integrated species exhibit this mechanism. We experimentally tested this hypothesis in situ by removing the apical tip in three different species (Stylophora sp., Acropora hemprichii, A. pharaonis), presenting two contrasting levels of integration and monitoring their branching morphogenesis over time. After 74 days, the null hypothesis that apical dominance does not occur could not be rejected for A. hemprichii and Stylophora sp., likely due to experimental limitations. However, A. pharaonis exhibited accelerated apical regrowth and increased lateral branching after tip removal, suggesting that apical dominance-like mechanisms may operate in this species. These findings highlight the importance of addressing potential Type 1 and Type 2 errors in experimental design to improve reliability while addressing the emergence of apical dominance in highly integrated coral colonies. Further long-term experiments are needed to capture morphometric changes in slow-growing species, such as A. hemprichii. These findings suggest novel endogenous mechanisms coordinating complex three-dimensional morphogenesis in clonal organisms and offer valuable application in the growing field of coral farming and restoration.
1 Introduction
Colonial clonal invertebrates developed from solitary ancestors, when the budding rate exceeded the rate of bud separation (Mackie, 1986). Clonality and coloniality have been gained and lost several times across metazoan lineages (Hiebert et al., 2021). Such convergent events support the view of their adaptive value (Hiebert et al., 2021), such as improved space competition (Jackson, 1979), directional growth to escape predators and physical disturbances (Buss, 1979), and regeneration after predation or partial damage (Berning, 2008; Luz et al., 2021). The extent of clonal integration depends on the existence of chemical connectivity that allows organisms to respond to changes in environment and impacts experienced by a member or a section of the colony (Song et al., 2013). Clonal integration ultimately guides colony growth and shape in an adaptive way. Whereas clonal integration is prevalent in clonal plants (Song et al., 2013), it has been demonstrated in some clonal invertebrates (Berking et al., 2002; Berking, 2006), but not in scleractinian corals.
In corals, colonies are built by the iteration of a single unit, the polyp. During colony growth, functional connections among polyps and intra-colonial integration are maintained through an intricate gastrovascular system (e.g., Acropora cervicornis, Gladfelter, 1983; Bouderlique et al., 2022) or are limited to the coenenchyma tissue among neighboring polyps and a simpler gastrovascular system (e.g., Pocillopora damicornis, Li et al., 2020). Colony integration is species-specific and can be inferred from skeletal features (Table 1, from Swain et al., 2018). Roles of colony integration include resource translocation among polyps, especially towards injured areas (Oren et al., 1997, 2001; Rinkevich and Loya, 1983a, b), bleaching resistance (Swain et al., 2018), and colony morphogenesis (Loya, 1976).
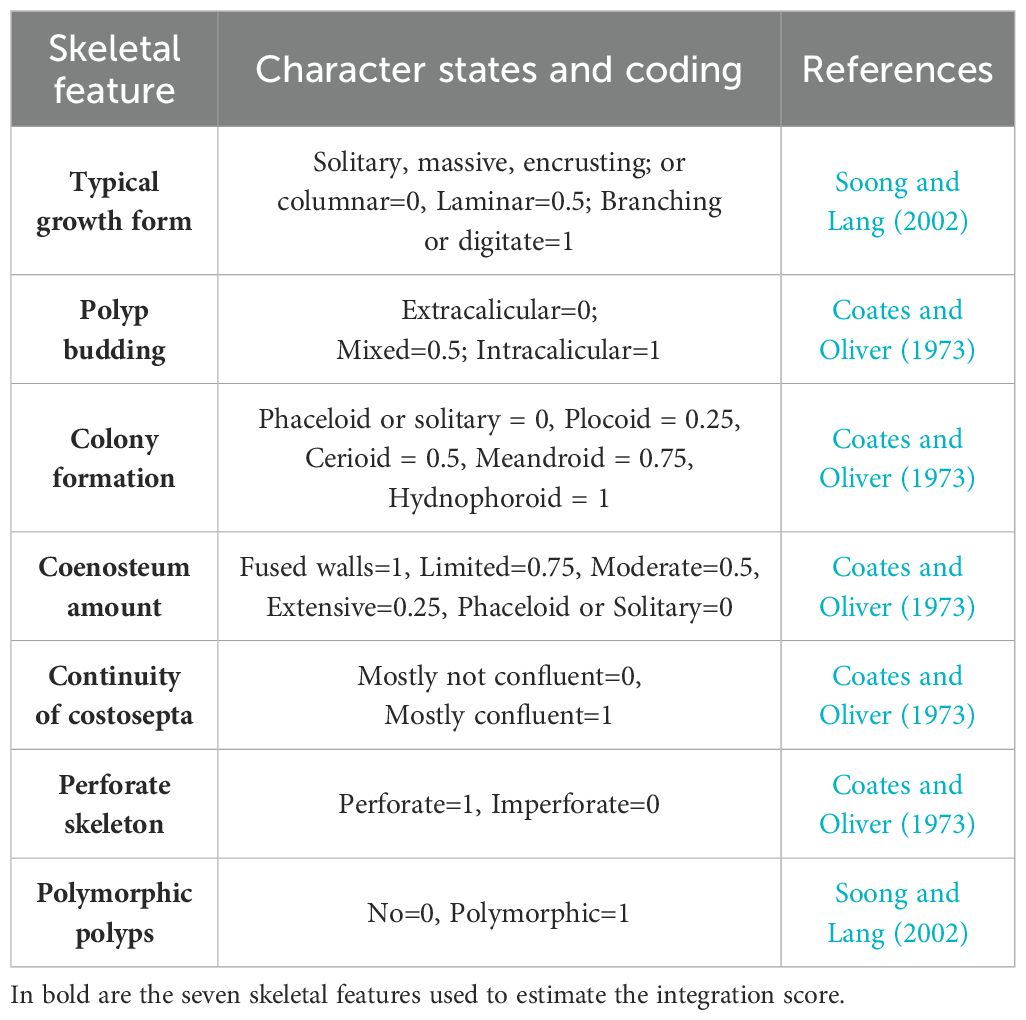
Table 1. Description of the seven skeletal features employed in Swain et al. (2018), to estimate coral colonies integration level.
Complex branching coral species present a unique case. Previous research has suggested a close association between coral morphogenesis, branch development, and colony integration, enabling colonies to maintain buffer distances among branch modules and prevent tip fusion (Oliver, 1984; Rinkevich and Loya, 1985). A relationship between integration and the development of complex three-dimensional structures has been hypothesized (Jackson, 1979; Silén, 1981). In scleractinian corals, the emergence of mesh-like complex canals, connecting polyps within the same colony, promoted the development of more complex coral geometrical structures, such as branching growth forms (Li et al., 2023). This suggests the occurrence of whole-colony integrative mechanisms, such as apical dominance, a phenomenon well studied in marine and terrestrial plants (Schwarzschild and Zieman, 2008) but still largely ignored in research on coral biology and ecology (i.e., Sánchez and Lasker, 2004; Shaish and Rinkevich, 2009). In plants, apical dominance refers to the control exerted by the apical shoot through the production of hormones, primarily auxins, which suppress the growth of lateral buds below it (Cline, 1997). Apical dominance is tightly linked to the regeneration capacity and overcompensation mechanisms in plants, which can exceed normal growth performances after partial mortality (Ramula et al., 2019), and in controlling the branching process, it ultimately controls the shape of the tree or clone, as well as the resource allocation to support growth of the apical meristem (Beveridge et al., 2023).
Thus far, apical dominance has never been demonstrated in scleractinian corals (i.e., Stylophora pistillata, Shaish and Rinkevich, 2009). However, it has been hypothesized as a mechanism modulating branching in the Octocorallia Pseudopterogorgia bipinnata (Sánchez and Lasker, 2004), characterized by equally diametric-sized hollow stem canals all along the branches (solenia, Bayer, 1973) connecting the whole colony. As demonstrated in plants, apical growth is dependent on clonal integration (i.e., Syringodium filiforme, Schwarzschild and Zieman, 2008), to allow for resources and hormone translocation. Furthermore, Berking (2002, 2006) proposed a branching mechanism in other cnidarian organisms. Hydrozoan branching is organized by a pattern-forming system, including a set of morphogens, with self-organizing properties. The system determines the positional value of the tissue, creating a gradient of values, which favor or inhibit the onset of new buds (Berking, 2006).
In corals, the presence of a hypothetical morphogenetic “isomone” released in the water to prevent branch fusion has been postulated (Rinkevich and Loya, 1985), but the chemical class of molecules involved is yet to be identified. This hypothesis has been recently questioned due to the energetic requirements for releasing a pheromone that is to maintain critical concentration levels in a highly hydrodynamic environment (Re et al., 2024). In contrast, the possible presence of an isomone circulating through the gastrovascular system, and exerting morphogenetic controlling roles remains surprisingly underexplored in corals. Such an internal control mechanism would avoid the dilution of an external delivery mechanism and would, therefore, be energetically possible. Indeed, hormonal apical control in plants is exerted through chemical messengers that are transported through the internal transport systems of plants (Tamas, 1995; Aloni et al., 2006), where apical dominance has been studied for over a century (Beveridge et al., 2023). Existing evidence also indicates that the required connectivity is possible in corals, such as in species with a complex gastrovascular system (Gladfelter, 1983). Hence, we propose that such processes are likely to occur in certain coral species presenting high three-dimensional complexity and connectivity among single polyps (Sánchez and Lasker, 2004).
Here, we test experimentally in situ the occurrence of apical dominance across three species of scleractinian branching corals, two presenting high apparent level of colony integration (Acropora) and one with a low level (Stylophora). We first evaluated the average level of colony integration across different coral growth forms to test our hypothesis that more intricate shapes exhibit higher levels of integration. Subsequently, we experimentally tested apical dominance in branching corals with different levels of integration, drawing on established experimental approaches testing for apical dominance in plants, which involve the apical bud removal and observation of the subsequent effects on the dormant buds lower down the branch (Schwarzschild and Zieman, 2008; Terrados et al., 1997). Here, coral nubbins underwent a tip removal treatment, and their branch growth patterns were monitored over time in a reef setting to determine any potential impact on lower branches and colony architecture against control colonies with intact tips.
2 Materials and methods
2.1 Linking integration score to colony growth form
Following Swain et al. (2018) (Table 1), we related the integration index score (I-score ranging from 0 to 7, with 0 being the lowest integration and 7 the highest) with different growth forms of coral colonies to assess if branching and more complex 3D forms present higher integration values. The integration index score of 88 species was estimated in Swain et al. (2018). For each species, the typical growth form (i.e., Submassive, Massive, Laminar, Hispidose, Encrusting, Columnar, Digitate, Corymbose, Branching_Open, Branching_Closed, Tables_Or_Plates) was reported from the Coral Trait Database (downloaded on March 9, 2022; CTD, Madin et al., 2016). Growth forms were grouped in “branching” (Digitate, Corymbose, Branching_Open, Branching_Closed, Tables_Or_Plates, Hispidose) versus “non-branching” (i.e., Submassive, Massive, Laminar, Encrusting, Columnar) categories, following the textual descriptions from Veron (2000) (Supplementary Table 1). For each group, the weighted average integration score was calculated. To test for significant differences between the groups, the non-parametric test Mann–Whitney U was performed.
2.2 In situ experiment
We chose the Red Sea branching coral species Acropora hemprichii, Acropora pharaonis, and Stylophora sp. to represent two levels of integration index (Table 2). The integration indices used are those reported in the original work of Swain et al. (2018), where available. For those not reported, estimates were made in the current study, yielding results similar to those for congeneric species (Table 2, Supplementary Table 1). Acropora hemprichii presents a branching open morphotype with dome-shaped apical corallites and large conical radial polyps (Wallace et al., 2012). Acropora pharaonis grows in large horizontal tables with abundant axial corallites (Wallace et al., 2012). The emergence of lateral branches presents a regularity in the internode length (Figure 1). Stylophora sp. grows in a spherical shape (Loya, 1976) with thick rounded branches and small polyps (Arrigoni et al., 2016) Up-growing bifurcating branches originate through dichotomous splitting at the branch tip (Shaish et al., 2007).

Table 2. Table showing the total integration score (I-score) for the three species analyzed and the score assigned to each skeletal feature.

Figure 1. Acropora pharaonis colony in Al Fahal reef (Saudi Arabia). This species exhibits a regular spacing between consecutive branches, contributing to its distinctive growth pattern.
On 2 June 2024, nine coral colonies were collected between 2 m and 10 m in Al Fahal reef, offshore Thuwal (22° 18′ 20.4474″N, 38° 57′ 54.5904″E; Saudi Arabia). Three colonies per species were collected at a minimum distance of 5 m to provide better genetic diversity. From each colony, 10 nubbins of 3 cm–8 cm were fragmented with a Gryphon C-40 Diamond saw for high precision. Five nubbins per colony were used as controls, leaving the apical tip intact, whereas five nubbins underwent apical tip removal (~1 cm from the top) (Figure 2). This treatment aimed at eliminating any potential biological input form the apical portion. A total of 90 coral nubbins (45 for control and 45 for treatment) were prepared and glued with BSI IC-GEL™ (Cyanoacrylate coral safe glue) to ceramic plugs (2.48 cm in diameter). Each sample was identified by an alphanumerical code (A. pharaonis = T; A. hemprichii = B; Stylophora sp. = S; the first number identify the colony and the second number the fragment; tr. and ctr. codes were used for identifying the treatment and control, respectively). For each sample, images were acquired with an Olympus TG-6 camera to reconstruct the three-dimensional model in Agisoft Metashape Professional (version 1.8.4., PhotoScan, Agisoft).

Figure 2. Comparison of control (left) and treatment “Tip Removal” (right) samples in the three species selected at the beginning of the experiment.
The colonies were then fixed on a PVC tree structure deployed in Al Fahal reef at 9-m depth. The 90 fragments were randomly assigned to a specific position on the tree at a minimum distance of 4 cm to avoid interference, ensuring similar microenvironmental conditions. A miniDOT® logger (Fondriest Environmental, Inc.) was secured to the structure, monitoring temperature (°C), dissolved oxygen (mg/L), and dissolved oxygen saturation (%) (results are displayed in Supplementary Figure 1).
After 74 days from the beginning of the experiment, the tree structure was recovered due to increasing temperature and signs of bleaching, which recommended terminating the experiment. The samples were photographed to reconstruct the 3D mesh with the software Agisoft Metashape Professional version 1.8.4. (complete workflow can be found in Supplementary Table 2). Before uploading the picture in the software, image quality was checked and brightness adjusted.
2.3 Statistical analysis
Seven different morphometric parameters were measured on the 3D models obtained (Table 3) at the beginning of the experiment (T0) and the end (Tf) using the software MeshLab version 2023.12 (Million and Kenkel, 2020; complete workflow Supplementary Table 3).
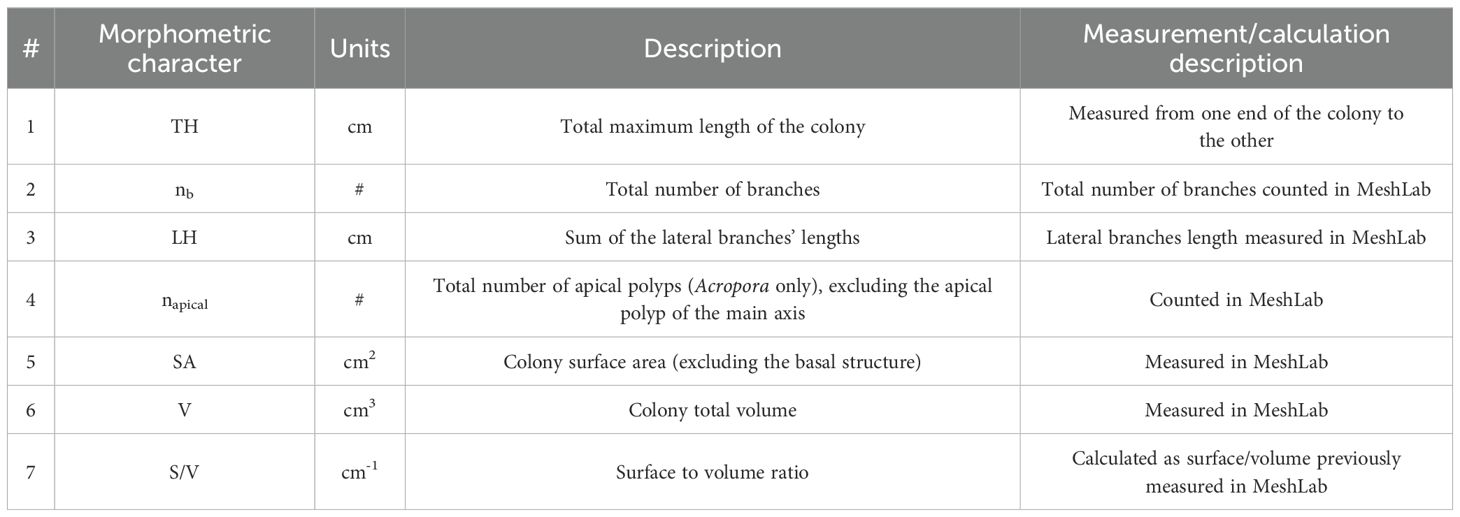
Table 3. List of the seven morphometric parameters measured in the object mesh of the samples obtained at T0 and Tf.
For the analysis of morphometric changes, each coral species was treated independently to account for species-specific responses. The difference between the initial and final measurements for each morphometric characteristic was calculated. Before conducting statistical tests, we first assessed the assumptions of homogeneity of variance and normality using the Levene’s test. We performed a “Pearson Correlation” to remove parameters that are related (p>0.9). Afterward, a one-way ANOVA was carried out on the selected parameters to test for significant differences between control and treatment. The level of significance was calculated using Bonferroni correction to avoid type I error. A post-hoc test allowed to determine which parameter contributed to the observed differences. The null hypotheses tested that, if rejected, would provide support for the hypothesis of apical dominance, are the following: the number of lateral branches, lateral branch length, and count of apical polyps should not increase in the “removed-tip” group, suggesting that the release from the apical control is not accelerating the lateral extension and generation of new branches and apical polyps in Acropora. Moreover, the apical growth should show no differences between the “Tip Removal” group compared with the “Control”, as the resources should be redistributed uniformly. No significant changes are expected in surface-to-volume ratio between the “Tip Removal” and “Control” groups.
3 Results
3.1 Linking integration score to colony growth form
Integration level cannot be directly measured but it is rather inferred based on several skeletal features, which are postulated to be related to colony integration, resulting into a continuum of integration score values (I-score). Results from relating the I-score with a complex three-dimensional structure (branching versus non-branching) resulted in an average I-score in branching species of 3.11 (spanning between 1.75 and 4.25) and 2.32 (spanning between 0 and 4.25) in non-branching colonies. The non-parametric test Mann–Whitney U resulted in a significant difference between the groups (p-value 0.00045), with the branching species scoring higher values (Figure 3).
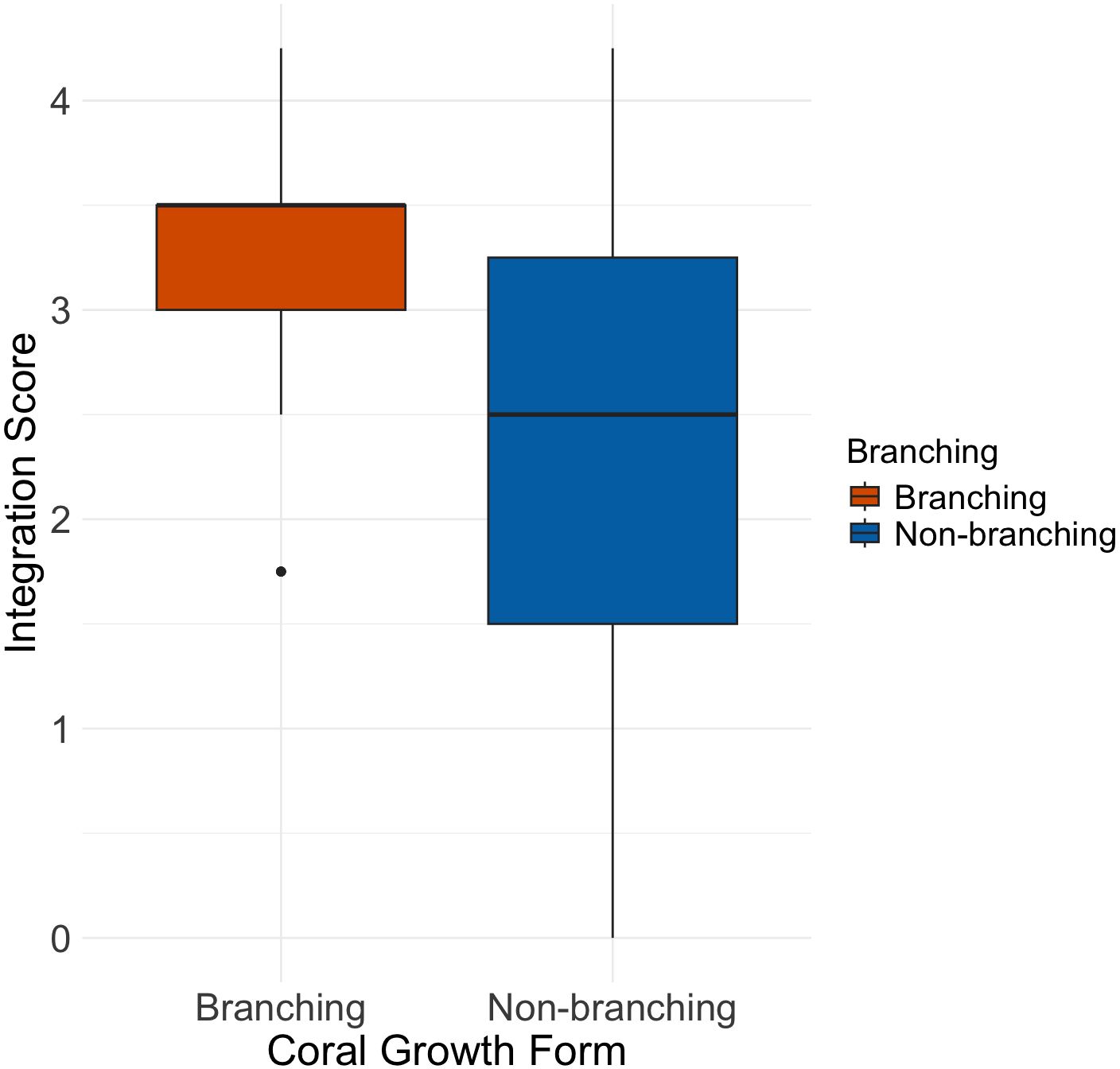
Figure 3. Boxplot showing the average integration score in Non-branching (n =55) versus Branching (n =33) coral species.
These findings are in line with the hypothesis that high colony integration is related to complex 3D shapes (Jackson, 1979; Silén, 1981). However, within the branching group, species of the Pocilloporidae family (Pocillopora, Seriatopora, and Stylophora, Supplementary Table 1) exhibited lower integration levels, suggesting that not all branching patterns necessarily reflect a highly integrated colony-wide mechanism. Conversely, some species without a typical branching form showed high integration scores, indicating that the inferred colony integration level may not solely dependent on morphological complexity.
3.2 Experimental results
At the end of the experiment, a total of 66 colonies out of 90 were retrieved, where the remaining 24 colonies were lost due to predation or detachment from the structure. Unfortunately, due to increase in seawater temperature over the last week of the experiment (Supplementary Figure 1), samples were showing signs of bleaching and tissue loss, mostly in the two Acropora species. However, no difference in survival rate within the same species has been detected between control and treatment groups, and this event at the end of the experiment would not have possibly affected prior growth. Indeed, all the samples showed tissue and calcification growth, both at the tip and on the basal plate. For the analysis, the material growing on the tile was excluded. Moreover, the “number of branches” parameter was removed due to the limited duration of the experiment compared with the typical growth rate of the species selected (median growth rate value from coraltraits.org downloaded on the 16 January 2025, Madin et al., 2016: Acropora hemprichii = 6.9 mm year−1, Acropora pharaonis = 13.4 mm year−1, Stylophora sp. = 19.3 mm year−1), which did not allow sufficient time for lateral branch generation, thereby reducing a Type II error in the statistical test. However, the differentiation of a radial polyp into an apical corallite leads to the initiation of a new branch and therefore a new growth direction (Oliver, 1984). Given the limited time of the experiment, we investigated the emergence of apical polyps as a proxy for the formation of lateral branching. All the assumptions of the ANOVA were met, and no data transformation was needed for the three species. The full dataset of the result is shown in Supplementary Table 4.
Acropora pharaonis showed significant differences in the total colony length extension (TH), with treated colonies presenting increased growth compared with control samples (Figure 4A, Supplementary Table 5). No significant differences were found in the other five parameters investigated (Figures 4B–F). However, heterogeneity in the lateral branch growth response was observed. Although the lateral branch length extension (LH) showed insignificant results (Figure 4C), we observed that preexisting lateral formed branches near the tip exhibited accelerated growth compared with the rest of the colony in some “Tip removed” samples (Figure 5A). The same phenomenon did not appear in treated samples without already formed lateral branches (Figure 5B) and in the control group (Figure 5C). The high variability in the number of lateral branches at the beginning of the experiment in the coral nubbins might have led to limited power to detect responses, and, hence, a likelihood of Type 2 error. Similarly, in one control sample, we noticed elevated LH growth in one lateral branch. The branch was cut during the fragging operation, potentially triggering the accelerated growth (Figure 6A). Another relevant observation was the development of apical polyps in a control sample of A. pharaonis that was accidentally trimmed during the fragmentation process (Figure 6B). During the experiment, the skeleton was covered by tissue and started producing apical polyps.
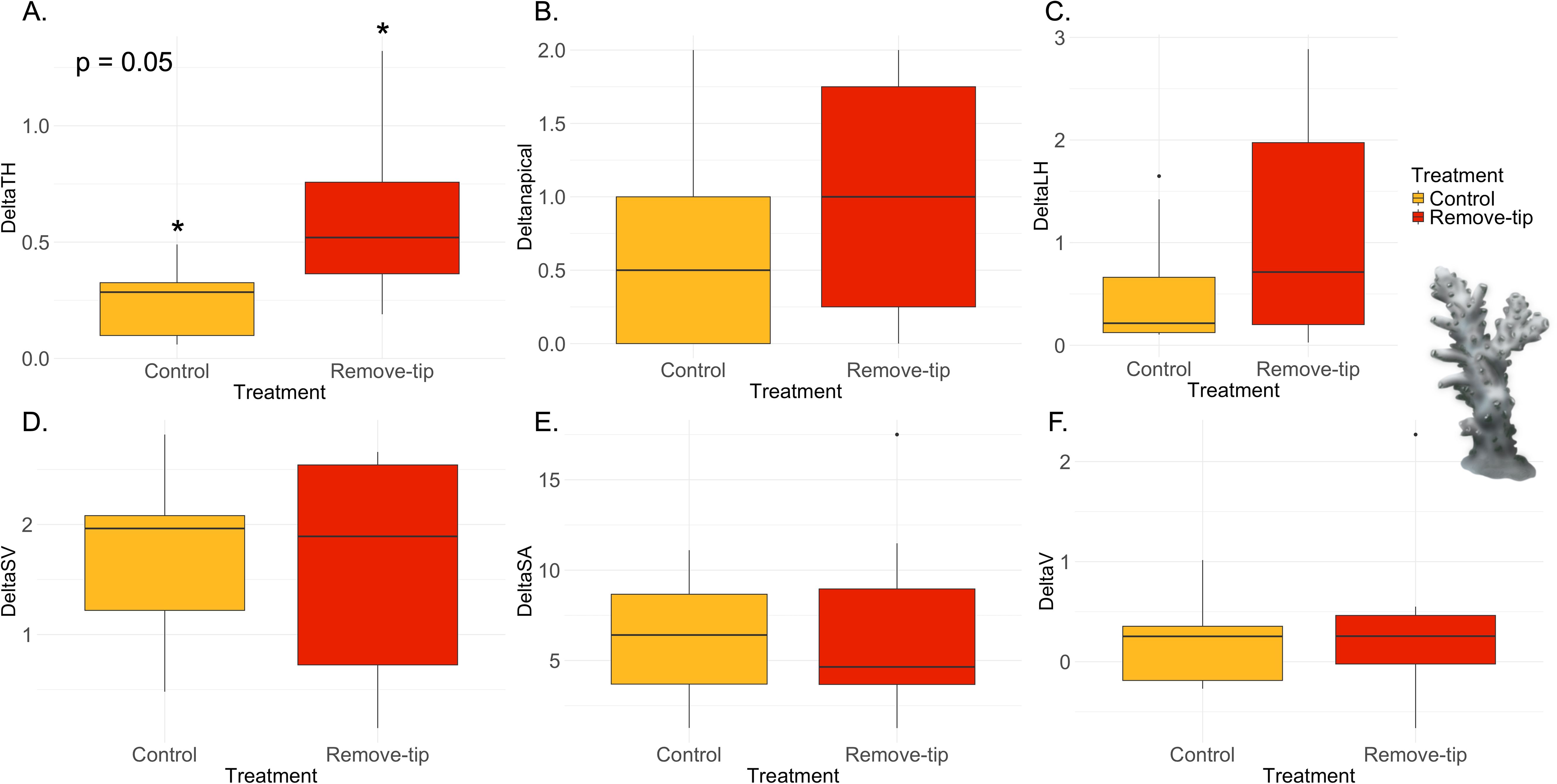
Figure 4. Results of the ANOVA with Bonferroni correction in Acropora pharaonis. Control versus remove-tip comparison for each parameter calculated as Tf − T0 are shown in the panels. (A) Total maximum length of the colony (ΔTH) in cm, which resulted significant (p-value 0.05); (B) total number of apical polyps (Δnapical); (C) total sum of the later branches (ΔLH) in cm; (D) surface-to-volume-ratio (ΔSV) in cm−1; (E) total surface area (ΔSA) in cm2; and (F) total volume (ΔV) in cm3.

Figure 5. CloudComapare images of (A) Acropora pharaonis sample (T1.1_tr) showing the accelerated growth rate of lateral branches already in the upper part of the colony. (B) A. pharaonis colony (T1.4_tr) without lateral branches at the beginning of the experiment did not show any significant lateral growth. (C) A. pharaonis (T.5_ctr) colony control with expanded lateral branches does not show any increase in growth in the branches. Color bar identifies the point-to-point distances between the clouds at the beginning and end of the experiment. Blue indicates low growth; Red is the maximum growth (Red = 1.25 cm in A; Red= 0.2 cm in B).
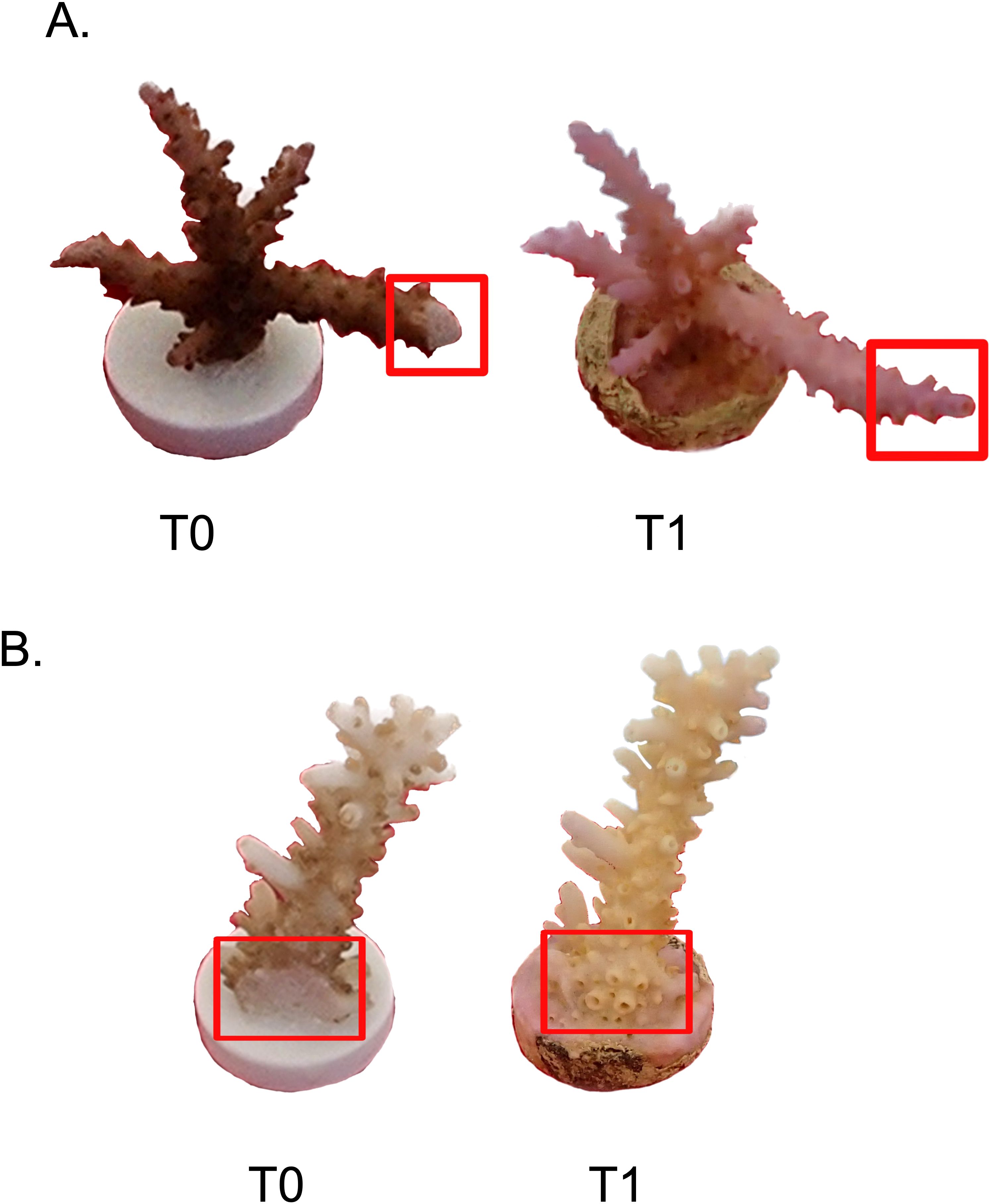
Figure 6. (A) Acropora pharaonis control (T3.3_ctr) at the beginning (left) and end of the experiment (right). This sample presented a considerable lateral growth. Note that one of the side branches was accidentally trimmed during the fragmenting operations. (B) Acropora pharaonis control (T2.5_ctr) at the beginning (left) and end of the experiment (right). In the red square is visible the cut portion of the colony that developed apical polyps.
Stylophora sp. does not present apical polyps; hence, the term apical dominance is applied more broadly to indicate the top side of coral nubbins. Hence, the variable “apical polyps” was not measured, resulting in only five morphometric parameters studied. This species showed significant increase in the surface-to-volume ratio (S/V) in the control group compared with treatment (Figure 7C, Supplementary, Table 5), although no differences were found in surface area (SA) and volume (V) (Figures 7D, E). The other variable measured did not show any statistical difference between groups (Figures 7A, B; Supplementary Table 5). Finally, Acropora hemprichii did not show any significant differences in the variable measured between treatment and control group (Figure 8, Supplementary Table 5).
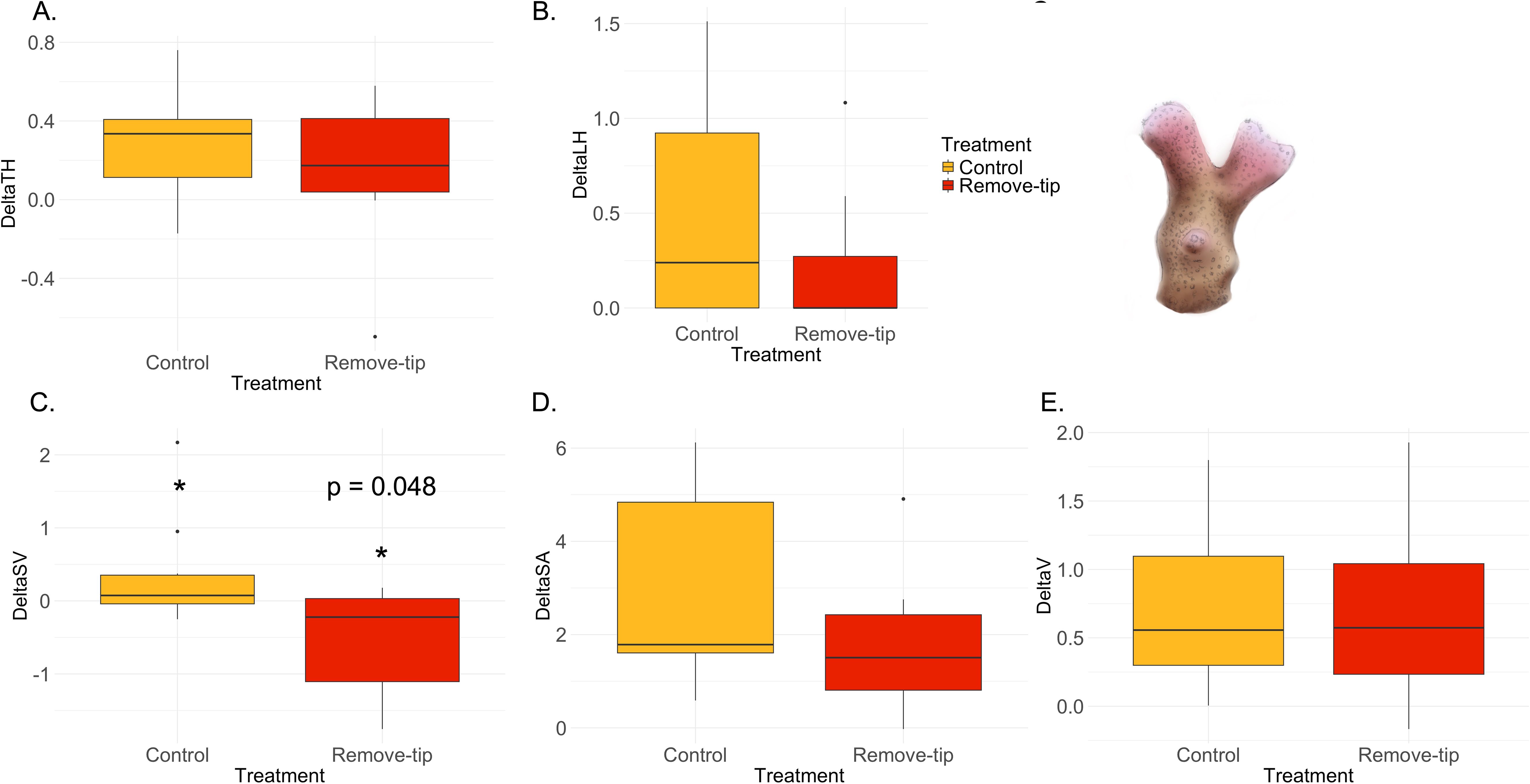
Figure 7. Results of the ANOVA with Bonferroni correction in Stylophora sp. Control versus remove-tip comparison for each parameter calculated as Tf − T0 are shown in the panels. (A) Total maximum length of the colony (ΔTH) in cm; (B) total sum of the later branches (ΔLH) in cm; C) surface-to-volume-ratio (ΔSV) in cm−1, which resulted significant (p-value 0.048); (D) total surface area (ΔSA) in cm2; and E) total volume (ΔV) in cm3.
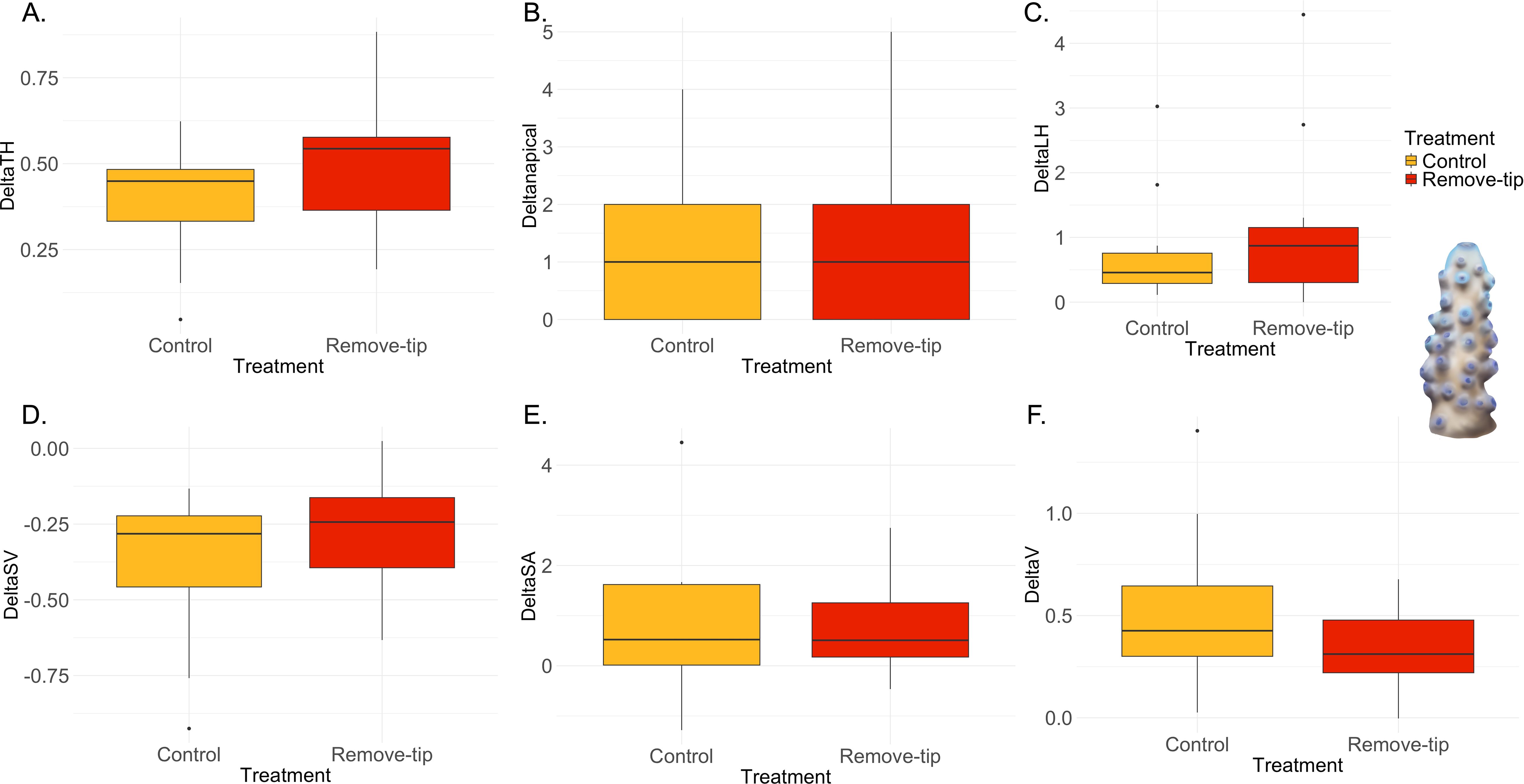
Figure 8. Results of the ANOVA with Bonferroni correction in Acropora hemprichii. Control versus remove-tip comparison for each parameter calculated as Tf − T0 are shown in the panels. (A) Total maximum length of the colony (ΔTH) in cm; (B) total number of apical polyps (Δnapical); (C) total sum of the later branches (ΔLH) in cm; (D) surface-to-volume-ratio (ΔSV) in cm−1; (E) total surface area (ΔSA) in cm2; and (F) total volume (ΔV) in cm3.
4 Discussion
Our results aligned with the prediction that more integrated colonies, as the coral of the genus Acropora with an intricate gastrovascular system (Gladfelter, 1983), may display apical dominance-like mechanisms compared with species presenting limited internal connections (i.e., Stylophora sp.).
Colony growth form is one of the seven skeletal features considered in calculating the integration score (Table 1), where branching and digitate colonies account for the highest value. Similarly, the presence of polymorphic polyps (i.e., Acropora) contributes to a highest I-score. Although the formation of complex colonies seems to be related to the overall colony integration level (Figure 3), branching species belonging to the family Pocilloporidae showed a lower I-score. In young colonies of Stylophora pistillata, the development of vertical growth and branches formation seems to emerge as an allometric response (Guerrini et al., 2021). Specifically, branching occurs when segments between polyps are shorter than the average inter-polyp distance, suggesting that spatial constraints drive this architectural adaptation as a solution for colony expansion (Guerrini et al., 2021).
Upon the removal of the apical portion of the colony, the three species exhibited distinct morphometric responses. Unfortunately, the abrupt interruption of the study due to a strong marine heatwave, limited the sampling to only two time points. In Stylophora sp., colonies subjected to tip removal displayed a lower surface-to volume ratio increment compared with the control group (Figure 7C). In the treated group, resources appeared to be primarily redirected towards reskinning the exposed skeleton, whereas the control group showed signs of calcification, lateral branch extension, and surface area growth (Figure 7D). However, differences between the two groups in surface area and lateral branch extension were not statistically significant, likely due to the limited duration of the experiment. These observations might indicate an oriented translocation of resources towards the injured part of the colony (Oren et al., 2001), which could be otherwise equally distributed for colony expansion. Furthermore, a recent study tracking particle trajectory within the gastrovascular system of Stylophora pistillata revealed a “selfish sharing” model among the polyps of this species. Signaling molecules or food particles tend to remain longer in the vicinity of an individual polyp, which initially obtained or produced them (Bouderlique et al., 2022). These findings seem to point out the absence of apical dominance mechanisms in this species, possibly related to poorly interconnected polyps, aligning with previous results from a 1-year experiment by Shaish and Rinkevich (2009).
Acropora pharaonis exhibited an accelerated regrowth of the trimmed apical polyp compared with control group (Figure 4A, Supplementary Table 5). Similar to Stylophora sp., this result could be attributed to a reallocation of resources towards the injured area (Oren et al., 2001). However, the complex gastrovascular system of Acropora allows for an enhanced transport of photosynthates from distant polyps within the colony, as demonstrated in A. cervicornis (Pearse and Muscatine, 1971; Taylor, 1977; Gladfelter, 1983). Indications of apical dominance mechanisms in A. pharaonis arise from the observation of increased lateral branching growth in some treated colonies presenting formed branching (Figure 5A). Released from the control exerted by the apical polyp, lateral branches incremented their extension, redirecting resource allocation driving the colony growth. In a longer-term experiment, we would expect to observe similar branching patterns in smaller nubbins where lateral branches had not yet formed. In contrast, Acropora hemprichii did not show any statistical differences between the treatment and the control group in any of the variables measured. Almost certainly, the time was not sufficient for the colony to express any morphometric changes as A. hemprichii presents a lower growth rate compared with the congeneric A. pharaonis. Hence, we consider the results obtained for Acropora hemprichii to be inconclusive, insufficient to reject or support the null hypothesis tested here.
Similarly, in Octocorallia, increased lateral branches growth was observed near the injured apical portion of the colonies (Sánchez and Lasker, 2004). Indeed, the presence of a complex structure of canals and vessels in this coral group allows for resource translocation towards injured areas (Pearse and Muscatine, 1971). Nevertheless, corals do not present storage organs similar to dormant buds and meristems found in plants and seagrasses. Dormant branch structures in corals, receiving a surplus of resources, might exceed normal growth (Sánchez and Lasker, 2004), allowing the colony to survive and redirect its growth in case of physical damage (Liddle and Kay, 1987). The close relationship between apical dominance and clonal integration has been largely observed in plants. For example, in the seagrass Syringodium filiforme, Schwarzschild and Zieman (2008) demonstrated that removing rhizome apical meristems (RAMs) led to increased branching. Their study also highlighted the importance of clonal integration, as the growth of RAMs depended on resource translocation from older ramets. In our experiment, Acropora pharaonis showed hints of apical dominance mechanisms, exhibiting enhanced growth of the apical portion in treated colonies, redirecting the development towards lateral formed branches (Figure 5A). Indications of apical dominance-like mechanisms in this species were also apparent from the internode regularity, resembling the pattern commonly observed in plants (Figure 1) and the intricate gastrovascular system (Gladfelter, 1983).
The null hypothesis that apical dominance does not occur could not be rejected by the experimental results obtained for Acropora hemprichii and Stylophora sp. However, it was indeed rejected for Acropora pharaonis, suggesting that the occurrence of apical dominance for this species cannot be discarded. Indeed, all experiments have limitations, including a risk of Type I and Type II error, which holds in the case of our experiments, which should be considered a first effort to test this important question in coral clonal biology. Type I error (i.e., the null hypothesis not having rejected due to lack of power) is indeed possible, as the duration of the experiment was impacted by the massive bleaching event that decimated corals at the reef where the experiment was being conducted. As a result, as discussed, some of the species, particularly the slow-growing A. hemprichii, may not have expressed the branching pattern leading, therefore, to inconclusive results. On the contrary, Type II, rejecting the null hypothesis when it should have been upheld, may derive from the experimental results obtained for Acropora pharaonis, possibly derived from mechanisms other than apical dominance, but yielding apparent outcomes consistent with those expected under the presence of apical dominance.
The overall colony architecture in branching species has been found to be dictated by light (Kaniewska et al., 2009) and water flow (Graus et al., 1977; Bottjer, 1980). Particularly, in Acropora species, the direction and quality of the light spectrum (Kaniewska et al., 2009) and the polyp position within the colony (Oliver, 1984) govern the production of apical polyps. The presence of a morphogen could be considered as an endogenous mechanism to dictate branching patterns (Oliver, 1984; Guerrini et al., 2021) and maintain a buffer exclusion zone around branches (Oliver, 1984; Rinkevich and Loya, 1985), although the molecule has not been identified yet. The recent single-polyp metabolomics study by Roach et al. (2023) revealed significant biochemical variability at multiple spatial and organizational levels, from individual polyps to whole colonies. This work highlighted the spatial structuring of metabolite profiles in Montipora capitata and may reflect the differentiation of growing apical polyps. Such findings provide an important molecular perspective on the spatial heterogeneity within coral colonies, offering a powerful tool to investigate processes like colony integration and branching regulation at unprecedented resolution. Applying these approaches to branching species such as Acropora could uncover the biochemical underpinnings of morphogenetic patterns and apical dominance-like mechanisms.
Alternative, but not mutually exclusive, hypotheses to explain the highly coordinated growth in corals have been formulated. Polyps’ tentacles might be able to sense the surrounding environment and recognize clonal individuals, regulating branching frequency and growth direction (i.e., Dendrophyllia cribrosa, Sentoku et al., 2015). However, this hypothesis is yet to be tested. In fact, microscale investigations at the polyp level in corals remain relatively underexplored, despite significant advancements in recent years. These studies began with the description of vertical ciliary flow by Shapiro et al. (2014) and the subsequent development of coral-on-a-chip technology, which enabled unprecedented spatiotemporal resolution (Shapiro et al., 2016). Such advancements have highlighted the importance of active boundary layer control above coral tissues in maintaining the delicate balance of oxygen concentrations around polyps (Pacherres et al., 2022). More recently, Bouderlique et al. (2022) detailed particle flow across the coral surface and within the gastrovascular systems of various scleractinian species. This research uncovered a novel connecting system among polyps, suggesting the formation of integrated polyp groups that may facilitate intra-colonial communication. These findings underscore the need for further studies on colony integration and branching patterns at finer spatial scales. Future research should incorporate tools like single-polyp metabolomics (Roach et al., 2023) to provide deeper insights into these mechanisms.
Isolated branches and coral nubbins seem to be able to sense their spatial position (Shaish and Rinkevich, 2009), similar to the mechanism governing branching in hydrozoans (Berking et al., 2002; Berking, 2006). Kücken et al. (2011) proposed a model for coral morphogenesis based on dissolved nutrients and photosynthetic products serving as cues for positional information. This mechanism drives cellular differentiation during the embryogenesis (Wolpert, 1996) and plays a role in the origin of the dorso-ventral axis of cnidarian (Holstein, 2022). Understanding the morphogenetic cue could reveal its potential role in dictating the branching pattern in corals.
The evidence presented here cannot be considered conclusive. Progressing from this initial step toward a more robust assessment of apical dominance in corals requires a strong inference approach (Platt, 1964) to advancing this research further. This requires improvements to the experimental design conducted here, to constrain the likelihood of Type I and II errors, as well as discovering the mechanistic basis for apical dominance. On the first effort, we recommend future experiments be extended to other species and also be extended in duration. In addition, we recommend the experimental design used here be expanded to include further treatment levels, such as varying degrees of apical trimming and radial polyp removal at different scales (from small nubbins to whole colonies). On the second effort, hormones, particularly auxin and cytokinins, have been identified to be responsible for apical dominance in plants (Beveridge et al., 2023). Similarly, peptides have been postulated to act as neurohormonal signals for corals (Tarrant, 2005, 2007), but never demonstrated. Experimental metabolomics, coupled with the improved experimental design recommended, may be able to put forward a number of candidate signaling molecules, which role can then be tested in subsequent experiments.
The investigation of apical dominance in scleractinian corals remains largely unexplored, with this study representing one of the few morphometry-based approach to examine endogenous mechanisms governing colony branching (Sánchez and Lasker, 2004; Shaish and Rinkevich, 2009; Guerrini et al., 2021), complementing more recent molecule-based efforts (Roach et al., 2023). Understanding these processes in colonial organisms like corals is essential to addressing fundamental questions about the emergence of complex biological hierarchies and how higher-level individuals arise from the integration of lower-level units (Hiebert et al., 2021). Moreover, the understanding of apical dominance and the potential trade-offs may open a new possibility to accelerate coral growth and find applicable solutions in coral farming and restoration (Page, 2013). This study provides insights into the role of colony integration and the potential relation to apical dominance in scleractinian corals. Nonetheless, further long-term studies and complementary molecular approaches are required to fully understand this phenomenon and the biological mechanism underpinning it.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
ER: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. CD: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing, Project administration.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. ER acknowledges the funding from King Abdullah University of Science and Technology (KAUST) baseline funding BAS/1/1071-01-01.
Acknowledgments
ER thanks Nayra Pluma, Sofia Carlotto, David Atienza, Sebastian Schmidt-Roach, and CMOR boat crew for fieldwork support. AI tool ChatGPT 4o was employed for improving the manuscript flow.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. AI tool ChatGPT 4o was employed for improving the manuscript flow.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1531462/full#supplementary-material
References
Aloni R., Aloni E., Langhans M., Ullrich C. I. (2006). Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 97, 883–893. doi: 10.1093/aob/mcl027
Arrigoni R., Benzoni F., Terraneo T. I., Caragnano A., Berumen M. L. (2016). Recent origin and semi-permeable species boundaries in the scleractinian coral genus Stylophora from the Red Sea. Sci.Rep 6, 34612. doi: 10.1038/srep34612
Berking S. (2006). Principles of branch formation and branch patterning in Hydrozoa. J. Dev. Biol. 50, 123–134. doi: 10.1387/ijdb.052043sb
Berking S., Hesse M., Herrmann K. (2002). A shoot meristem-like organ in animals; monopodial and sympodial growth in Hydrozoa. Int. J. Dev. Biol. 46 (3), 301–308.
Berning B. (2008). Evidence for sublethal predation and regeneration among living and fossil ascophoran bryozoans. Bryozoan Studies 15, 1–7.
Beveridge C. A., Rameau C., Wijerathna-Yapa A. (2023). Lessons from a century of apical dominance research. J. Exp. Bot. 74, 3903–3922. doi: 10.1093/jxb/erad137
Bottjer D. J. (1980). Branching morphology of the reef coral Acropora cervicornis in different hydraulic regimes. J. Paleontol. 1102–1107.
Bouderlique T., Petersen J., Faure L., Abed-Navandi D., Bouchnita A., Mueller B., et al. (2022). Surface flow for colonial integration in reef-building corals. Curr. Biol. 32, 2596–2609.e7. doi: 10.1016/J.CUB.2022.04.054
Budd A. F., Johnson K. G., Klaus J. S. (2006). Neogene Marine Biota of Tropical America. Available online at: http://nmita.iowa.uiowa.edu/database/corals/coralmnu.htm (Accessed January 03, 2025).
Buss L. W. (1979). “Habitat selection, directional growth and spatial refuges: Why colonial animals have more hiding places,” in Biology and systematics of colonial organisms. Eds. Larwood G., Rosen B. R. (Academic Press, London, UK), 459–497.
Cline M. G. (1997). Concepts and terminology of apical dominance. Am. J. Bot. 84, 1064–1069. doi: 10.2307/2446149
Coates A. G., Oliver W. A. J. (1973). “Coloniality in zoantharian corals,” in Animal colonies: development and function through time., 3–27.
Gladfelter E. H. (1983). Circulation of fluids in the gastrovascular system of the reef coral Acropora cervicornis. Biol. Bull. 165, 619–636. doi: 10.2307/1541469
Graus R. R., Chamberlain J. A. Jr., Boker A. M. (1977). Structural modification of corals in relation to waves and currents: reef biota. AAPG Special Volumes 45, 135–153. doi: 10.1306/St4393C11
Guerrini G., Shefy D., Shashar N., Shafir S., Rinkevich B. (2021). Morphometric and allometric rules of polyp’s landscape in regular and chimeric coral colonies of the branching species Stylophora pistillata. Dev. Dynam. 250 (5), 652–668. doi: 10.1002/dvdy.290
Hiebert L. S., Simpson C., Tiozzo S. (2021). Coloniality, clonality, and modularity in animals: The elephant in the room. JEZ-B 336, 198–211. doi: 10.1002/jez.b.v336.3
Holstein T. W. (2022). The role of cnidarian developmental biology in unraveling axis formation and Wnt signaling. Dev. Biol. 487, 74–98. doi: 10.1016/j.ydbio.2022.04.005
Jackson J. B. C. (1979). Morphological strategies of sessile animals. Biology Systematic Colonial Organisms. 499–555.
Kaniewska P., Campbell P. R., Fine M., Hoegh-Guldberg O. (2009). Phototropic growth in a reef flat acroporid branching coral species. J. Exp. Biol. 212, 662–667. doi: 10.1242/jeb.022624
Kücken M., Rinkevich B., Shaish L., Deutsch A. (2011). Nutritional resources as positional information for morphogenesis in the stony coral Stylophora pistillata. J. Theor. Biol. 275, 70–77. doi: 10.1016/j.jtbi.2011.01.018
Liddle M. J., Kay A. M. (1987). Resistance, survival and recovery of trampled corals on the Great Barrier Reef. Biol. Conserv. 42, 1–18. doi: 10.1016/0006-3207(87)90049-8
Li Y., Han T., Bi K., Liang K., Chen J., Lu J., et al. (2020). The 3D reconstruction of Pocillopora colony sheds light on the growth pattern of this reef-building coral. Iscience. 23 (6).
Li Y., Liao X., Wang X., Li Y., Zhao H., Zhao Y., et al. (2023). Polyp-canal reconstruction reveals evolution 430 towards complexity in corals. Research 6, 0166.
Loya Y. (1976). Skeletal regeneration in a Red Sea scleractinian coral population. Nature 261 (5560), 490–491. doi: 10.1038/261490a0
Luz B. L. P., Miller D. J., Kitahara M. V. (2021). High regenerative capacity is a general feature within colonial dendrophylliid corals (Anthozoa, Scleractinia). JEZ-B 336, 281–292. doi: 10.1002/jez.b.v336.3
Mackie G. O. (1986). From aggregates to integrates: physiological aspects of modularity in colonial animals. Philos. Trans. R. Soc. London. B. Biol. Sci. 313 (1159), 175–196. doi: 10.1098/rstb.1986.0032
Madin J. S., Anderson K. D., Andreasen M. H., Bridge T. C., Cairns S. D., Connolly S. R., et al. (2016). The coral trait database, a curated database of trait information for coral species from the global oceans. Scientific Data. 3 (1), 1–22.
Million W. C., Kenkel C. (2020). Phenotyping 3D coral models in MeshLab. protocols.io. doi: 10.17504/protocols.io.bgbpjsmn
Oliver J. K. (1984). Intra-colony variation in the growth of Acropora formosa: extension rates and skeletal structure of white (zooxanthellae-free) and brown-tipped branches. Coral. Reefs. 3, 139–147. doi: 10.1007/BF00301958
Oren U., Benayahu Y., Lubinevsky H., Loya Y. (2001). Colony integration during regeneration in the stony coral Favia favus. Ecology 82, 802–813. doi: 10.1890/0012-9658(2001)082[0802:CIDRIT]2.0.CO;2
Oren U., Rinkevich B., Loya Y. (1997). Oriented intra-colonial transport of 14C labeled materials during coral regeneration. Mar. Ecol. Prog. Ser. 161, 117, 122. doi: 10.3354/meps161117
Pacherres C. O., Ahmerkamp S., Koren K., Richter C., Holtappels M. (2022). Ciliary flows in corals ventilate target areas of high photosynthetic oxygen production. Curr. Biol. 32 (19), 4150–4158. doi: 10.1201/9781003477518-6
Page C. (2013). Reskinning a reef: Mote Marine Lab scientists explore a new approach to reef restoration. Coral. Magazine. 10, 72–80.
Pearse V. B., Muscatine L. (1971). Role of symbiotic algae (zooxanthellae) in coral calcification. Biol. Bull. 141, 350–363. doi: 10.2307/1540123
Platt J. R. (1964). Strong Inference: Certain systematic methods of scientific thinking may produce much more rapid progress than others. Science 146, 347–353. doi: 10.1126/science.146.3642.347
Ramula S., Paige K. N., Lennartsson T., Tuomi J. (2019). Overcompensation: a 30-year perspective. Ecology 100, e02667. doi: 10.1002/ecy.2019.100.issue-5
Re E., Schmidt-Roach S., Llabres E., Sintes T., Duarte C. M. (2024). Clonal growth patterns in colonial anthozoan corals oceanography and marine biology: an annual review (62), 248–266. doi: 10.1201/9781003477518-6
Rinkevich B., Loya Y. (1983a). “Oriented translocation of energy in grafted reef corals,” in Coral Reefs, 1, 243–247.
Rinkevich B., Loya Y. (1983b). Short-term fate of photosynthetic products in a hermatypic coral. J. Exp. Mar. Biol. Ecol. 73, 175–184. doi: 10.1016/0022-0981(83)90082-5
Rinkevich B., Loya Y. (1985). Coral isomone: a proposed chemical signal controlling intraclonal growth patterns in a branching coral. Bull. Mar. Sci. 36, 319–324.
Roach T. N. F., Matsuda S. B., Martin C., Huckeba G., Kahkejian V., et al. (2023). Single-polyp metabolomics reveals biochemical structuring of the coral holobiont at multiple scales. Commun. Biol. 6, 984. doi: 10.1038/s42003-023-05342-8
Sánchez J. A., Lasker H. R. (2004). Do multi-branched colonial organisms exceed normal growth after partial mortality? Proc. R. Soc. B.: Biol. Sci. 271 (SUPPL. 3), S117–S120. doi: 10.1098/rsbl.2003.0108
Schwarzschild A. C., Zieman J. C. (2008). Apical dominance and the importance of clonal integration to apical growth in the seagrass Syringodium filiforme. Mar. Ecol. Prog. Ser. 360, 37–46. doi: 10.3354/meps07408
Sentoku A., Morisaki H., Masumoto S., Ohno R., Tomiyama T., Ezaki Y. (2015). Internal skeletal analysis of the colonial azooxanthellate scleractinian Dendrophyllia cribrosa using microfocus X-ray CT images: Underlying basis for its rigid and highly adaptive colony structure. J. Struct. Biol. 189, 37–43. doi: 10.1016/j.jsb.2014.11.002
Shaish L., Abelson A., Rinkevich B. (2007). How plastic can phenotypic plasticity be? The branching coral Stylophora pistillata as a model system. PloS One 2, e644. doi: 10.1371/journal.pone.0000644
Shaish L., Rinkevich B. (2009). Critical evaluation of branch polarity and apical dominance as dictators of colony astogeny in a branching coral. PloS One 4 (1), e4095. doi: 10.1371/journal.pone.0004095
Shapiro O. H., Fernandez V. I., Garren M., Guasto J. S., Debaillon-Vesque F. P., Kramarsky-Winter E., et al. (2014). Vortical ciliary flows actively enhance mass transport in reef corals. Proc. Natl. Acad. Sci. 111, 13391–13396. doi: 10.1073/pnas.1323094111
Shapiro O. H., Kramarsky-Winter E., Gavish A. R., Stocker R., Vardi A. (2016). A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat. Commun. 7, 10860. doi: 10.1038/ncomms10860
Silén L. (1981). Colony structure in Flustra foliacea (Linnaeus) Bryozoa, Cheilostomata). Acta Zool. 62 (4), 219–232. doi: 10.1111/j.1463-6395.1981.tb00631.x
Song Y. B., Yu F. H., Keser L. H., Dawson W., Fischer M., Dong M., et al. (2013). United we stand, divided we fall: a meta-analysis of experiments on clonal integration and its relationship to invasiveness. Oecologia 171, 317–327. doi: 10.1007/s00442-012-2430-9
Soong K., Lang J. C. (1992). Reproductive integration in reef corals. Biol. Bull. 183, 418–431. doi: 10.2307/1542018
Swain T. D., Bold E. C., Osborn P. C., Baird A. H., Westneat M. W., Backman V., et al. (2018). Physiological integration of coral colonies is correlated with bleaching resistance. Mar. Ecol. Prog. Ser. 586, 1–10. doi: 10.3354/meps12445
Tamas I. A. (1995). “Hormonal regulation of apical dominance,” in Plant hormones: physiology, biochemistry and molecular biology (Springer Netherlands, Dordrecht), 572–597.
Tarrant A. M. (2005). Endocrine-like signaling in cnidarians: current understanding and implications for ecophysiology. Integr. Comp. Biol. 45, 201–214. doi: 10.1093/icb/45.1.201
Tarrant A. M. (2007). Hormonal signaling in cnidarians: do we understand the pathways well enough to know whether they are being disrupted? Ecotoxicology 16, 5–13. doi: 10.1007/s10646-006-0121-1
Taylor D. L. (1977). Intra-colonial transport of organic compounds and calcium in some Atlantic reef corals. In International Coral Reef Symposium 3. Proc.; Miami, Fla.; 1977; Miami; Rosentiel Sch. Mar. Atmos. Sci.; Da. 1977; 1, 431–436; Bibl. 10 Ref.
Terrados J., Duarte C. M., Kenworthy W. J. (1997). Is the apical growth of Cymodocea nodosa dependent on clonal integration? Mar. Ecol. Prog. Ser. 158, 103–110. doi: 10.3354/meps158103
van Woesik R., Van Woesik K., Van Woesik L., Van Woesik S. (2013). Effects of ocean acidification on the dissolution rates of reef-coral skeletons. PeerJ 1, e208. doi: 10.7717/peerj.208
Wallace C. C., Done B. J., Muir P. R. (2012). Revision and catalogue of worldwide staghorn corals Acropora and Isopora (Scleractinia: Acroporidae) in the Museum of Tropical Queensland. Memoirs. Queensland. Museum. - Nat. 57, 1–255. doi: 10.17082/j:2204-1478-56-2.2013-42
Keywords: apical dominance, clonal integration, overcompensation, branching pattern, coordinated growth, scleractinian coral
Citation: Re E and Duarte CM (2025) Emerging evidence for apical dominance in colonial branching Acropora corals. Front. Mar. Sci. 12:1531462. doi: 10.3389/fmars.2025.1531462
Received: 20 November 2024; Accepted: 06 February 2025;
Published: 05 March 2025.
Edited by:
Baruch Rinkevich, Israel Oceanographic and Limnological Research, IsraelReviewed by:
Isabelle Domart-Coulon, Muséum National d’Histoire Naturelle, FranceNadav Shashar, Ben-Gurion University of the Negev, Israel
Andrew Heyward, Australian Institute of Marine Science (AIMS), Australia
Copyright © 2025 Re and Duarte. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleonora Re, ZWxlb25vcmEucmVAa2F1c3QuZWR1LnNh
 Eleonora Re
Eleonora Re Carlos M. Duarte
Carlos M. Duarte