- 1Third Institute of Oceanography, Ministry of Natural Resources, Xiamen, China
- 2Observation and Research Station of Coastal Wetland Ecosystem in Beibu Gulf, Ministry of Natural Resources, Beihai, China
- 3Fujian Institute of Oceanography, Xiamen, China
- 4Key Laboratory of Underwater Acoustic Communication and Marine Information Technology of the Ministry of Education, College of Ocean and Earth Sciences, Xiamen University, Xiamen, China
The live sharksucker (Echeneis naucrates) is a bony fish that uses a specialized suction disk on its head to hitchhike on marine animals. Although live sharksuckers have been recorded attaching to various cetaceans worldwide, such associations between live sharksuckers and cetaceans have rarely been reported in the East Asia region. From 2011 to 2024, we conducted a long-term survey of Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, one of the key habitats for this cetacean in the seas around China. From 2022 to 2024, we recorded live sharksucker attachments on four Indo-Pacific humpback dolphin individuals, with a total of 11 events in Xiamen Bay. Specifically, we observed a live sharksucker temporarily detaching from the body of an Indo-Pacific humpback dolphin when the dolphin cleared its body out of the water and landed on one side multiple times. This reaction of the live sharksucker could be an adaptive characteristic to cope with the side breach behavior of their cetacean hosts. Considering the declining population of Indo-Pacific humpback dolphins, further investigation into the potential impacts of live sharksuckers on these dolphins is required.
1 Introduction
The live sharksucker (Echeneis naucrates) is one of eight species in the family Echeneidae, and these species are also known as remoras (Fertl and Landry, 1999; Brunnschweiler and Sazima, 2008). Echeneidae species use a specialized suction disk on their head to hitchhike on marine animals (Fertl and Landry, 1999; Souto et al., 2022). The live sharksucker is considered a host generalist (Flammang et al., 2020), with numerous documented hosts, including loggerhead turtles (Caretta caretta) (Sazima and Grossman, 2006), blacktip sharks (Carcharhinus limbatus) (Ritter, 2002), giant trevallies (Caranx ignobilis) (Brunnschweiler and Sazima, 2010), Guiana dolphins (Sotalia guianensis) (Souto et al., 2022), bottlenose dolphins (Tursiops truncatus) (Fertl and Landry, 1999), and other marine vertebrates.
The live sharksucker inhabits tropical and subtropical waters (Fertl and Landry, 1999) and ranges near and far from the coast (Akyol, 2013). The attachment of Echeneidae species to dolphins has been recorded in the Gulf of Mexico (Fertl and Landry, 1999), the Equatorial Atlantic Ocean (Wingert et al., 2021), the Gulf of California (Becerril-García et al., 2019), Northeastern Brazil (Souto et al., 2022), and elsewhere. Despite its global distribution (Collette et al., 2015), associations between Echeneidae species and cetaceans have rarely been reported in the East Asia region.
The Indo-Pacific humpback dolphin (Sousa chinensis) is a resident cetacean that lives along the coasts of southern China, Southeast Asia, the Bay of Bengal, and eastern India (Guo et al., 2022). This species of dolphin is typically found in coastal and estuarine habitats (Jefferson and Smith, 2016; Wu et al., 2014). The species is listed as vulnerable on the International Union for Conservation of Nature (IUCN) Red List (Jefferson et al., 2017) and has been classified as a first-class protected animal in China since 1988 (Wu et al., 2014). The population of Indo-Pacific humpback dolphins in Chinese seas is estimated to account for 83% of the species’ world population (Yong et al., 2023). However, its populations in this region have declined rapidly due to habitat decline (Huang et al., 2012; Wu et al., 2017; Guo et al., 2022), prey decline (Lin et al., 2021), and anthropic activities (Sun et al., 2022).
Ho et al. (2022) reported the first definite record of live sharksucker attachment to an Indo-Pacific humpback dolphin near Tai O, Lantau Island, Hong Kong, documenting that the attachment of a live sharksucker caused contusion-like lesions on the skin of the Indo-Pacific humpback dolphin host. Since associations between live sharksuckers and Indo-Pacific humpback dolphins have rarely been reported, current knowledge of the relationship between these two species is extremely limited.
Xiamen Bay is a key habitat for Indo-Pacific humpback dolphins in China (Wu et al., 2014), with an estimated population of 86 individuals in 2004 (Chen et al., 2008), 72 in 2007–2010 (Chen et al., 2018), and 54 in 2018–2019 (Zhong, 2021). In a previous study (Wang et al., 2015), two discrete Xiamen Bay communities of Indo-Pacific humpback dolphins (eastern and western) were recognized (Figure 1). The two communities showed strong geographic adherence and low-level intercommunity associations, which may be accelerating their path to local extinction (Wang et al., 2015). Chen et al. (2018) subsequently reported that the small population in Xiamen Bay met the critically endangered criterion of the IUCN Red List.
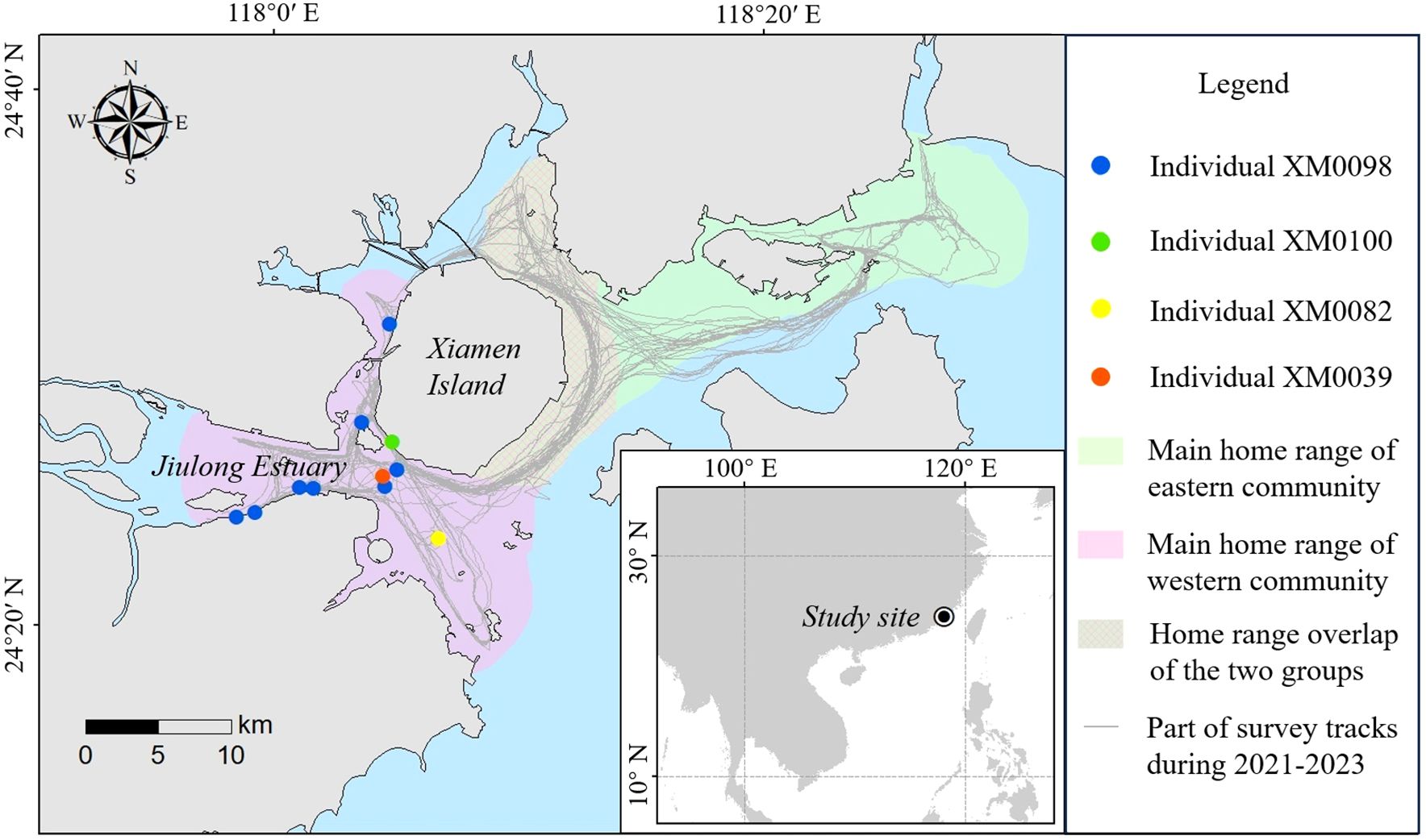
Figure 1. Locations of the live sharksucker attachments on Indo-Pacific humpback dolphins recorded in Xiamen Bay.
After 2 years since Ho et al. (2022) recorded the first case of live sharksucker attachment on an Indo-Pacific humpback dolphin in Hong Kong, we recorded a similar case in Xiamen Bay, located approximately 500 km from Hong Kong. Understanding the relationship between Indo-Pacific humpback dolphins and live sharksuckers is important for biological and ecological research on both species. Herein we report on our observations of live sharksuckers attached to Indo-Pacific humpback dolphins in Xiamen Bay and the interactions observed between the two species.
2 Materials and methods
2.1 Study area
Surveys of Indo-Pacific humpback dolphins were conducted in Xiamen Bay (24.45° N, 118.05° E), Fujian Province, southeastern China (Figure 1). Xiamen Bay covers an area of 750 km2 and has an average water depth of approximately 8 m (Kan et al., 2023; Zeng et al., 2020). Jiulong River is located west of Xiamen and is the most important local freshwater input source to Xiamen Bay.
2.2 Data collection
We conducted monthly surveys on Indo-Pacific humpback dolphins in Xiamen Bay from 2011 to 2024 using the systematic line-transect method. The survey covered most of the water areas of Xiamen Bay (Figure 1). We normally spent 3–6 days each month on conducting this survey and repeated the survey every month. Surveys were conducted only when permitted by the sea state and weather conditions. The researchers used small boats to search for Indo-Pacific humpback dolphins by traveling along previously designated lines at a speed of approximately 8–12 km/h. Indo-Pacific humpback dolphins were searched for with the naked eye or with the aid of binoculars from both sides of the boat. When the dolphins were sighted during the survey, the boat approached the dolphins from the side at a low speed, allowing the researchers to take photographs of the dolphins at appropriate distances. The photographers aimed to capture the dolphins’ dorsal fins and bodies.
Dolphin individuals were identified primarily based on the pattern of spots, nicks, notches, and pigmentation on and/or around the dorsal fins (Wang et al., 2016). Age classes were classified as neonates, calves, juveniles, and adults based on each individual’s skin color, body size, and behavior (Guo et al., 2020; Wu et al., 2014). The colors of the calves, juveniles, and adults were dark gray, light gray, and pinkish white, respectively.
During dolphin encounters, surface behavioral events, such as breaches, side breaches, spy hops, and tail slaps, were recorded (Lusseau, 2006; Serres et al., 2023). Other behavioral events are rare and could be easily missed by observers; therefore, they were not included (Serres et al., 2023). Tail slap behavior refers to a dolphin forcefully slapping the water surface with its tail flukes (Figure 2). Side breach behavior refers to a dolphin clearing its body out of the water and striking the water surface on one side (Figure 3). In addition, the monthly sea surface temperatures of Xiamen waters (24° N, 118° E) were downloaded from NOAA’s National Environmental Satellite, Data, and Information Service website (www.nnvl.noaa.gov).
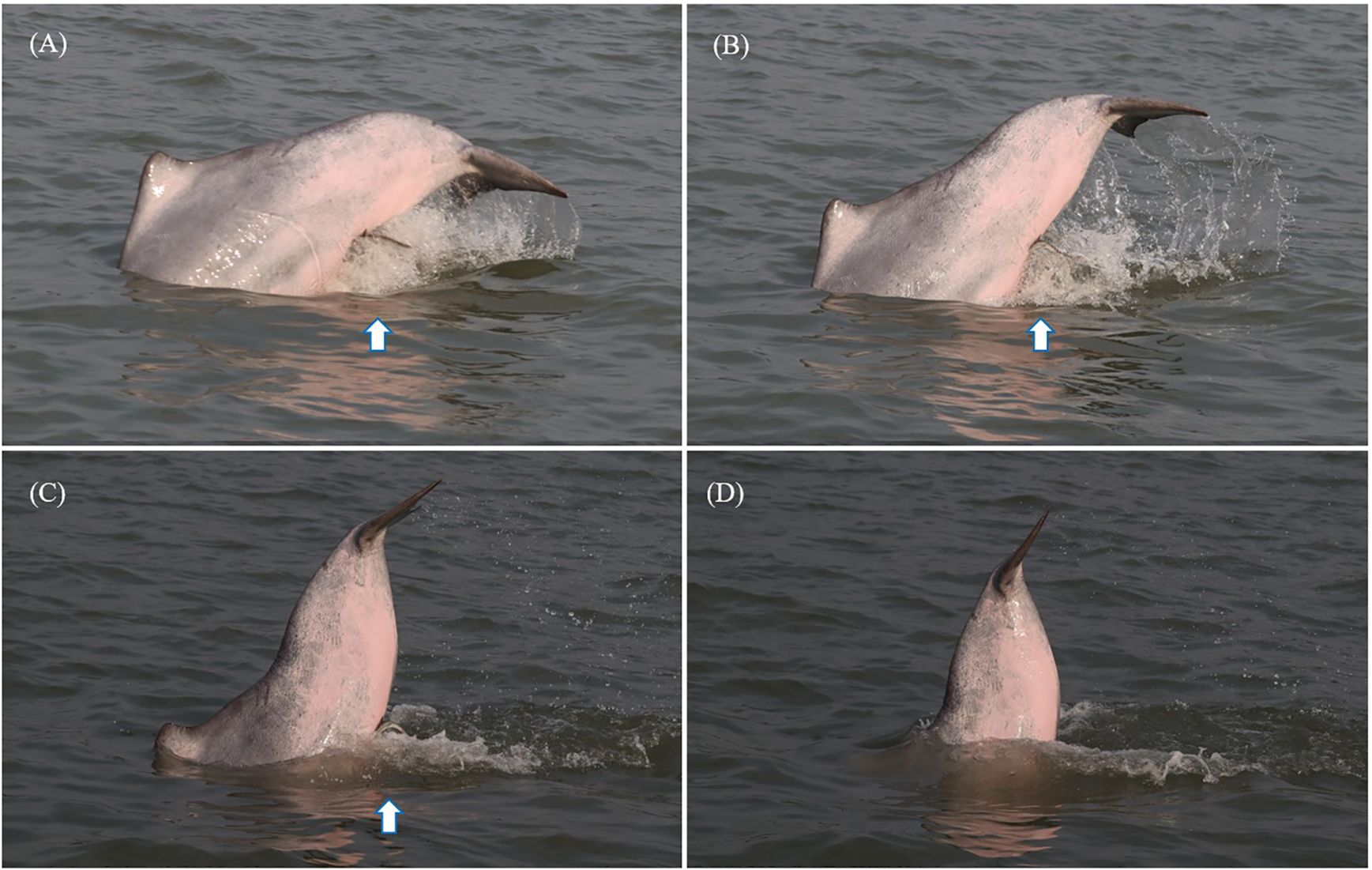
Figure 2. The Indo-Pacific humpback dolphin XM0100 slapping the water surface with its tail flukes. (A–D) Motions of the tail slap behavior. The arrows in this figure indicate the live sharksucker.
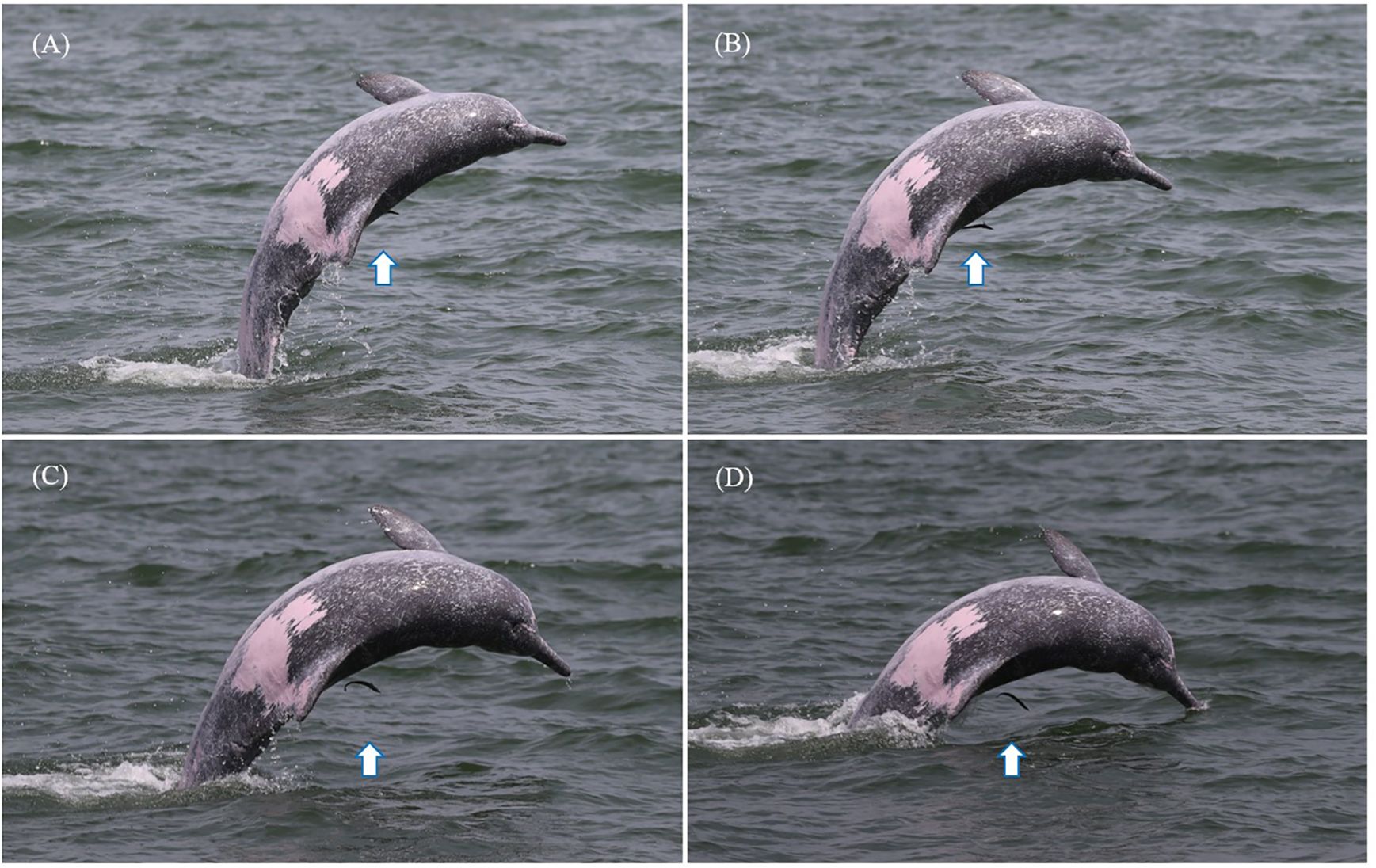
Figure 3. The adult Indo-Pacific humpback dolphin XM0082 jumping out of the water and striking the water surface on the body side. (A–D) Motions of the side breach behavior. The arrows in this figure indicate the live sharksucker.
3 Results
A total of 226 survey days were conducted in Xiamen Bay from January 2021 to August 2024. The monthly sea surface temperatures ranged from 14.6℃ to 28.7℃ during this period. We recorded 11 events of live sharksucker attachments on Indo-Pacific humpback dolphins in total. Among the 11 events, 10 were recorded with photos, and one was recorded by sight. The first record was on September 14, 2022, and the most recent record was on August 2, 2024. Live sharksucker attachments were recorded in February, March, August, September, October, November, and December.
A total of four identified Indo-Pacific humpback dolphin individuals were involved in these attachments, including two juveniles and two adults. These individuals were designated as XM098, XM0100, XM082, and XM0039, respectively. All four identified dolphins belonged to the western community in Xiamen Bay. The live sharksucker was identified at the species level based on its slender body and dark gray stripe, with white edges on each side of its body (Souto et al., 2022) (Figure 4). The attachment sites on the dolphin hosts included the front of the dorsal fin, the two flanks of the body, the underside of the body, and the tail stock (Figure 4). We did not notice any mark or contusion-like lesion on the skin where the live sharksucker had previously been attached.
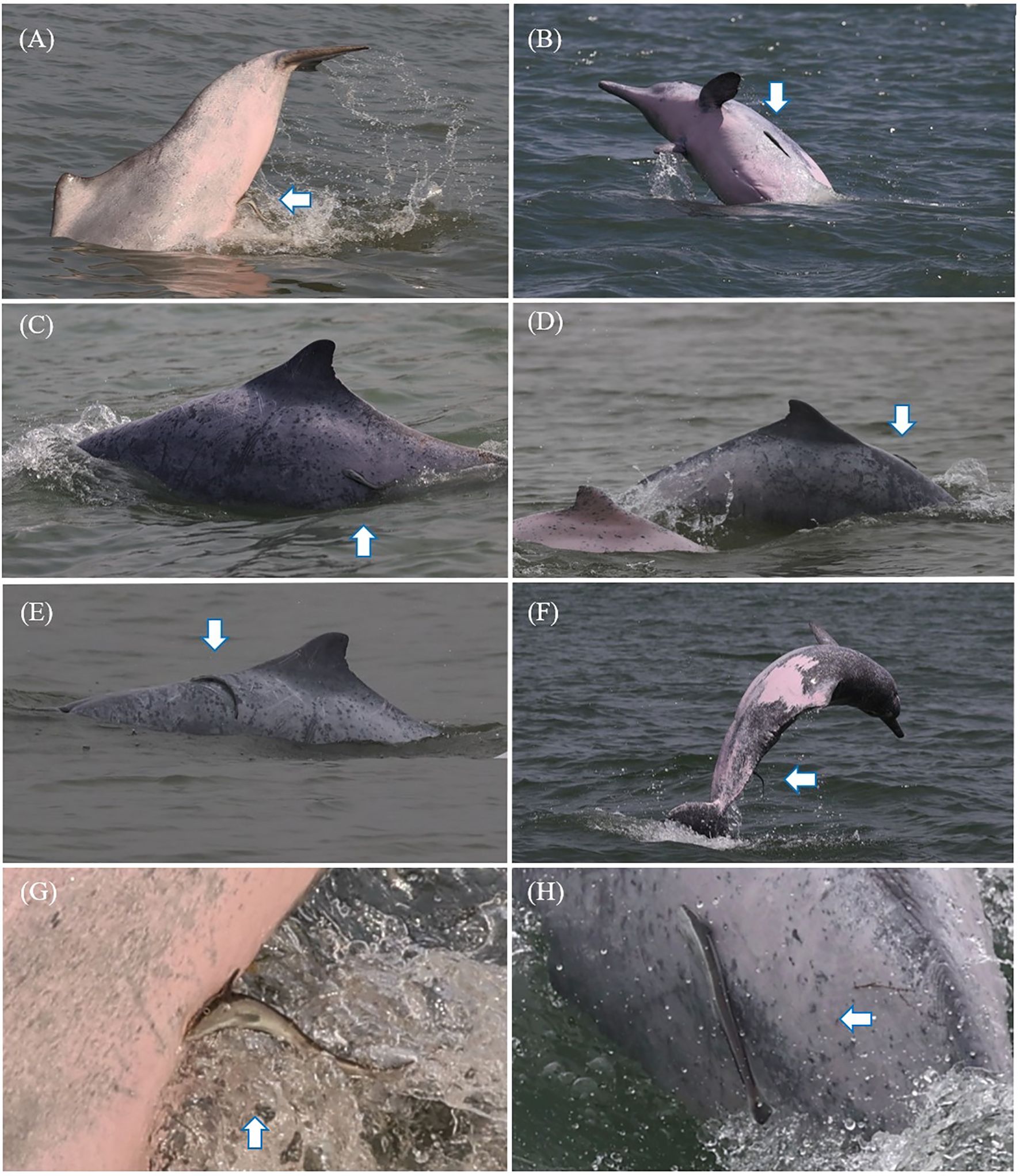
Figure 4. Attachment sites of live sharksuckers on Indo-Pacific humpback dolphins. (A) A live sharksucker attached to the underside of the body. (B, C) Live sharksuckers attached to the flank of the body. (D, E) Live sharksuckers attached to the front of the dorsal fin. (F) A live sharksucker attached to the tail stock. (G, H) Enlarged photographs of live sharksuckers. The arrows in this figure indicate the live sharksuckers.
We noticed that individual XM0100 displayed tail slap behavior when it was attached by a live sharksucker (Figure 2), including six tail slaps over the 18 min of observation. Whether XM0100 dislodged the live sharksucker by tail slaps on that day was unclear. At that time, the other dolphin individuals near XM0100 did not display a similar behavior.
XM0082 displayed side breach behavior when it was attached by a live sharksucker (Figure 3). During the 29 min of observation, XM0082 displayed side breach behavior 19 times. The other two dolphin individuals near XM0082 did not exhibit a similar behavior. We recorded that the live sharksucker left the body of its host when XM0082 displayed this side breaching behavior. Among the 19 side breaches, we recorded at least five events in which the live sharksucker detached from its host before striking the water surface (Figure 3, Supplementary Data Sheet 1). However, the next time XM0082 jumped out of the water, the live sharksucker was sighted on its body again.
Eight of the 11 sightings of live sharksucker attachments were on the same host, XM0098, a juvenile. XM0098 was still in the maternal care period and was following its mother at each sighting from September 14, 2022 to March 8, 2023. XM0098 was then no longer sighted, although its mother was seen several times thereafter. Consequently, XM0098 was assumed dead as it was not old enough to disperse. The other individuals were observed as having live sharksuckers attached for only one sighting event each (Supplementary Data Sheet 1).
During the 11 encounters, we took photos of 256 bouts of the focal Indo-Pacific humpback dolphin emerging from the water, which occurs relatively frequently as cetaceans need to breathe above the water surface. Live sharksuckers were documented by camera in only 21 of those 256 bouts (8.2% incidence rate). In all of these photos, an Indo-Pacific humpback dolphin individual was attached by only one live sharksucker each time.
4 Discussion
Herein we report the first event of live sharksuckers associated with the Indo-Pacific humpback dolphins in Xiamen Bay, and our work provides strong evidence that a live sharksucker temporarily detached from the body of its host when the dolphin exhibited aerial maneuvers. The association between live sharksuckers and Indo-Pacific humpback dolphins has been rarely reported. The specific relationship between these two species remains unclear due to the lack of relevant quantitative data.
The impacts of Echeneidae species attachment on their hosts are controversial. A number of studies have shown that remora attachments can negatively impact their hosts—for example, remora attachments could irritate the skin of their hosts (Weihs et al., 2007; Brunnschweiler, 2006). Ho et al. (2022) observed that live sharksucker attachments caused contusion-like lesions on the skin of an Indo-Pacific humpback dolphin. The attachment of remoras is considered to be a hydrodynamic burden to their hosts (Weihs et al., 2007; Norman et al., 2022). In addition, the attachment of sharksuckers could irritate their hosts and induce non-swimming-related behaviors (Ritter and Brunnschweiler, 2003; Brunnschweiler, 2006). However, other studies suggest that remoras may benefit their hosts by feeding on parasites on the hosts’ body surfaces (Flammang et al., 2020). Furthermore, Gayford (2024) suggested that the eco-evolutionary nature of the shark–remora relationship is poorly understood, and researchers should avoid categorizing this relationship as parasitism, mutualism, or commensalism without further robust evidence.
There are a few studies reporting that hosts attached by Echeneidae species attempted to dislodge these hitchhikers. Weihs et al. (2007) suggested that the aerial maneuvers displayed by spinner dolphins (Stenella longirostris) were associated with the removal of remoras. These aerial maneuvers during re-entry into the water could produce enough force to dislodge remoras (Fish et al., 2006). In addition, blacktip sharks occasionally attempted to dislodge sharksuckers (Ritter, 2002; Ritter and Brunnschweiler, 2003), and a blacktip shark was recorded to remove a remora from its body by displaying breaching behavior (Ritter and Brunnschweiler, 2003; Klimley et al., 2024).
It has previously been hypothesized that attempting to dislodge epibionts from their body was one of the reasons why dolphins evolved the behavior of jumping out of the water (Weihs et al., 2007). Herein we documented that a live sharksucker separated from its host (XM0082) when the host displayed side breach behavior. When XM0082 jumped out of the water, the live sharksucker stopped attaching and left its host (Figure 3, Supplementary Data Sheet 1). However, the next time its host jumped out of the water, the live sharksucker was again noted to be attached to the body, indicating that it attached to XM0082 again quickly after they returned to the water. The live sharksucker may have intentionally left its host before striking the water surface to avoid the powerful impact. The reaction could be an adaptive characteristic to cope with the side breach behavior of their cetacean hosts. It is also possible that the live sharksucker was not able to continue its attachment when the Indo-Pacific humpback dolphin jumped out of the water powerfully. Remoras are believed to have evolved abilities to adjust to their hitchhiking lifestyle. A study on blue whales (Balaenoptera musculus) indicated that remoras preferentially select regions offering lower drag forces (Flammang et al., 2020).
Herein we propose that cetacean researchers pay more attention to determining whether there are any epibionts on the bodies of cetaceans as such relationships can be easily ignored. Live sharksuckers can attach to the flanks and underside of the body (Figure 4). If Indo-Pacific humpback dolphins do not display a tail slap or side breach behavior, these attachments are difficult to observe. Consequently, the prevalence of Echeneidae species attachments among cetaceans could be underestimated.
This study provides valuable information on the poorly documented association between Indo-Pacific humpback dolphins and live sharksuckers. Our results indicate that the dolphins’ side breach behavior may serve to dislodge live sharksucker attachments, though more quantitative data are needed to support this opinion. Our study contributes to a better understanding of the newly discovered relationship between these two species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because This is a non-invasive observational study. No animals were contacted or harmed in this study.
Author contributions
FW: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft. BZ: Writing – original draft, Visualization. HW: Investigation, Writing – review & editing. YD: Investigation, Writing – review & editing. YZ: Writing – review & editing. ZS: Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the Natural Science Foundation of Fujian Province (no. 2022J05082), the Scientific Research Foundation of Third Institute of Oceanography, Ministry of Natural Resources (no. 2020017), the marine ecological early warning and monitoring Foundation of Ministry of Natural Resources (no. S-HR04-230701-24), the Natural Science Foundation of China (no. 42076159), and the Natural Science Foundation of Fujian Province (2021J01510).
Acknowledgments
We thank Yupeng Li, Nan Jin, and Xiangyu Zhao for their work in the field.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1516386/full#supplementary-material
References
Akyol O. (2013). Record of the live sharksucker, Echeneis naucrates Linnaeus 1758 (Osteichthyes: Echeneidae), from the northern Aegean Sea (Izmir Bay, Turkey). J. Appl. Ichthyol. 29, 263–264. doi: 10.1111/jai.12016
Becerril-García E. E., Rosales-Nanduca H., Paniagua-Mendoza A., Robles-Hernández R., Elorriaga-Verplancken F. R. (2019). Records of Whalesuckers (Remora australis) on Short-Beaked Common Dolphins (Delphinus delphis) in the Gulf of California, Mexico. Aquat. Mamm. 45, 299–302. doi: 10.1578/AM.45.3.2019.299
Brunnschweiler J. (2006). Sharksucker–shark interaction in two carcharhinid species. Mar. Ecol. 27, 89–94. doi: 10.1111/j.1439-0485.2005.00052.x
Brunnschweiler J., Sazima I. (2008). A new and unexpected host for the sharksucker (Echeneis naucrates) with a brief review of the echeneid—host interaction. Mar. Biodivers. Rec. 1, e41. doi: 10.1017/S1755267206004349
Brunnschweiler J., Sazima I. (2010). Swift swimming reef fish as hosts of small juvenile sharksuckers. Coral Reefs 29, 843–843. doi: 10.1007/s00338-010-0655-9
Chen B. Y., Gao H. L., Jefferson T., Lu Y., Wang L., Li S. S., et al. (2018). Survival rate and population size of Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, China. Mar. Mammal Sci. 34, 1018–1033. doi: 10.1111/mms.12510
Chen B. Y., Zheng D. M., Zhai F. F., Xu X., Sun P., Wang Q., et al. (2008). Abundance, distribution and conservation of Chinese White Dolphins (Sousa chinensis) in Xiamen, China. Mamm. Biol. 73, 156–164. doi: 10.1016/j.mambio.2006.12.002
Collette B., Curtis M., Williams J. T., Smith-Vaniz W. F., Pina Amargos F. (2015). Echeneis naucrates (errata version published in 2017) (The IUCN Red List of Threatened Species) (Accessed November 05, 2024).
Fertl D., Landry A. M. (1999). Sharksucker (Echeneis naucrates) on a bottlenose dolphin (Tursiops truncatus) and a review of other Cetacean-Remora associations. Mar. Mammal Sci. 15, 859–863. doi: 10.1111/j.1748-7692.1999.tb00849.x
Fish F. E., Nicastro A. J., Weihs D. (2006). Dynamics of the aerial maneuvers of spinner dolphins. J. Exp. Biol. 209, 590–598. doi: 10.1242/jeb.02034
Flammang B. E., Marras S., Lehmkuhl O., Anderson E. J., Mukherjee A., Cade D. E., et al. (2020). Remoras pick where they stick on blue whales. J. Exp. Biol. 223, jeb226654. doi: 10.1242/jeb.226654
Gayford J. H. (2024). The multidimensional spectrum of eco-evolutionary relationships between sharks and remoras. J. Fish Biol. 105, 4–9. doi: 10.1111/jfb.15759
Guo L., Lin W. Z., Zeng C., Luo D. Y., Wu Y. P. (2020). Investigating the age composition of Indo-Pacific humpback dolphins in the Pearl River Estuary based on their pigmentation pattern. Mar. Biol. 167, 50. doi: 10.1007/s00227-020-3650-x
Guo L., Luo D. Y., Yu R., Chen Z., Huang N. Y., Wang H. R., et al. (2022). Habitat decline of the largest known Indo-Pacific humpback dolphin (Sousa chinensis) population in poorly protected areas associated with the hypoxic zone. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1048959
Ho H. H. N., Kot B. C. W., Tsui H., Chung T. (2022). Visual Assessment of Contusion-Like Lesions Caused by Live Sharksucker (Echeneis naucrates) Attachment in an Indo-Pacific Humpback Dolphin (Sousa chinensis). J. Wildlife Dis. 58, 445–449. doi: 10.7589/JWD-D-21-00108
Huang S.-L., Karczmarski L., Chen J. L., Zhou R. L., Lin W. Z., Zhang H. F., et al. (2012). Demography and population trends of the largest population of Indo-Pacific humpback dolphins. Biol. Conserv. 147, 234–242. doi: 10.1016/j.biocon.2012.01.004
Jefferson T. A., Smith B. D. (2016). Re-assessment of the conservation status of the indo-pacific humpback dolphin (Sousa chinensis) using the IUCN red list criteria. Adv. Mar. Biol. 73, 1–26. doi: 10.1016/bs.amb.2015.04.002
Jefferson T. A., Smith B. D., Braulik G. T., Perrin W. (2017). Sousa chinensis (errata version published in 2018) (The IUCN Red List of Threatened Species) (Accessed December 13, 2024).
Kan Z. Y., Chen B., Yu W. W., Chen G. C., Ma Z. Y., Hu W. J., et al. (2023). Forecasting land-cover change effects on waterbirds in Xiamen Bay, China: Determining prospective species winners and losers. Mar. Environ. Res. 188, 106003. doi: 10.1016/j.marenvres.2023.106003
Klimley A. P., Curtis T. H., Johnston E. M., Kock A., Stevens G. M. W. (2024). A review of elasmobranch breaching behavior: why do sharks and rays propel themselves out of the water into the air? Environ. Biol. Fishes. doi: 10.1007/s10641-024-01584-5
Lin W. Z., Karczmarski L., Zhou R. L., Mo Y. Q., Guo L., Yiu S. K. F., et al. (2021). Prey decline leads to diet shift in the largest population of Indo-Pacific humpback dolphins? Integr. Zool. 16, 548–574. doi: 10.1111/1749-4877.12548
Lusseau D. (2006). The short-term behavioral reactions of bottlenose dolphins to interactions with boats in doubtful sound, New Zealand. Mar. Mammal Sci. 22, 802–818. doi: 10.1111/j.1748-7692.2006.00052.x
Norman B. M., Reynolds S. D., Morgan D. L. (2022). Three-way symbiotic relationships in whale sharks. Pac. Conserv. Biol. 28, 80–83. doi: 10.1071/PC20043
Ritter E. (2002). Analysis of Sharksucker, Echeneis naucrates, Induced Behavior Patterns in the Blacktip Shark, Carcharhinus limbatus. Environ. Biol. Fishes. 65, 111–115. doi: 10.1023/A:1019642221755
Ritter E., Brunnschweiler J. (2003). Do sharksuckers, echeneis naucrates, induce jump behaviour in blacktip sharks, carcharhinus limbatus? Mar. Freshw. Behav. Physiol. 36, 111–113. doi: 10.1080/1023624031000119584
Sazima I., Grossman A. (2006). Turtle riders: remoras on marine turtles in Southwest Atlantic. Neotrop. Ichthyol. 4, 123–126. doi: 10.1590/S1679-62252006000100014
Serres A., Lin W. Z., Liu B. S., Chen S. L., Li S. H. (2023). Context of breaching and tail slapping in Indo-Pacific humpback dolphins in the northern South China Sea. Behav. Ecol. Sociobiol. 77, 64. doi: 10.1007/s00265-023-03337-3
Souto L., Ross T., Sampaio C., Reis M. D. S., Bortolotto G. (2022). First record of sharksucker Echeneis naucrates (Perciformes, Echeneidae) associated with a young Guiana dolphin Sotalia guianensis (Cetartiodactyla, Delphinidae) in north-eastern Brazil. J. Mar. Biol. Assoc. U. K. 102, 386–389. doi: 10.1017/S0025315422000637
Sun X., Luo D. Y., Yu R. Q., Yu X. J., Liang Y. Q., Liu Z. W., et al. (2022). Long-term increase in mortality of Indo-Pacific humpback dolphins (Sousa chinensis) in the Pearl River Estuary following anthropic activities: Evidence from the stranded dolphin mortality analysis from 2003 to 2017. Environ. pollut. 307, 119526. doi: 10.1016/j.envpol.2022.119526
Wang X. Y., Wu F. X., Chang W.-L., Hou W., Chou L.-S., Zhu Q. (2016). Two separated populations of the Indo-Pacific humpback dolphin (Sousa chinensis) on opposite sides of the Taiwan Strait: Evidence from a larger-scale photo-identification comparison. Mar. Mammal Sci. 32, 390–399. doi: 10.1111/mms.12257
Wang X. Y., Wu F. X., Turvey S. T., Rosso M., Tao C. H., Ding X. H., et al. (2015). Social organization and distribution patterns inform conservation management of a threatened Indo-Pacific humpback dolphin population. J. Mammal. 96, 964–971. doi: 10.1093/jmammal/gyv097
Weihs D., Fish F. E., Nicastro A. J. (2007). Mechanics of remora removal by dolphin spinning. Mar. Mammal. Sci. 23, 707–714. doi: 10.1111/j.1748-7692.2007.00131.x
Wingert N., Milmann L., Baumgarten M., Danilewicz D., Sazima I., Ott P. (2021). Relationships between common bottlenose dolphins (Tursiops truncatus) and whalesuckers (Remora australis) at a remote archipelago in the Equatorial Atlantic Ocean. Aquat. Mamm. 47, 585–598. doi: 10.1578/AM.47.6.2021.585
Wu F. X., Wang X. Y., Ding X. H., Miao X., Zhu Q. (2014). Distribution Pattern of Indo-Pacific Humpback Dolphins (Sousa chinensis) along Coastal Waters of Fujian Province, China. Aquat. Mamm. 40, 341–349. doi: 10.1578/AM.40.4.2014.341
Wu H. P., Xu Y. H., Peng C. W., Liao Y. Y., Wang X. Y., Jefferson T. A., et al. (2017). Long-term habitat loss in a lightly-disturbed population of the Indo-Pacific humpback dolphin, Sousa chinensis. Aquat. Conserv. Mar. Freshw. Ecosyst. 27, 1198–1208. doi: 10.1002/aqc.2778
Yong L. M., Zhang Y. K., Zhao L. Y., Zeng Q. H., Lin L. S., Gao M. H., et al. (2023). Research advances on the ecology of Sousa chinensis. Biodivers. Sci. 31, 145–160. doi: 10.17520/biods.2022670
Zeng Q. H., Lin W. Z., Dai Y. F., Zhong M. D., Wang X. Y., Zhu Q. (2020). Modeling demographic parameters of an edge-of-range population of Indo-Pacific humpback dolphin in Xiamen Bay, China. Reg. Stud. Mar. Sci. 40, 101462. doi: 10.1016/j.rsma.2020.101462
Keywords: behavior, estuary, Indo-Pacific humpback dolphin, live sharksucker, interaction
Citation: Wu F, Zhou B, Wu H, Dai Y, Zhang Y and Song Z (2025) Attachment of live sharksuckers (Echeneis naucrates) to Indo-Pacific humpback dolphins (Sousa chinensis) in Xiamen Bay, China. Front. Mar. Sci. 12:1516386. doi: 10.3389/fmars.2025.1516386
Received: 24 October 2024; Accepted: 20 January 2025;
Published: 12 February 2025.
Edited by:
Menghong Hu, Shanghai Ocean University, ChinaReviewed by:
Natascha Wosnick, Federal University of Paraná, BrazilXian Sun, Sun Yat-sen University, China
Copyright © 2025 Wu, Zhou, Wu, Dai, Zhang and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuxing Wu, d3VmdXhpbmdAdGlvLm9yZy5jbg==; Zhongchang Song, c29uZ3pjQHhtdS5lZHUuY24=
 Fuxing Wu
Fuxing Wu Bing Zhou1,2
Bing Zhou1,2 Yu Zhang
Yu Zhang