
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 25 February 2025
Sec. Discoveries
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1514216
Information on coral reproductive biology and coral spawning times is crucial to advance basic and applied research and inform strategies for coral reef conservation and restoration. Important efforts have been made to collate coral spawning times and reproductive patterns in global and regional datasets. Here, we report and document the first in situ spawning of Pavona maldivensis Gardiner, 1905, observed in the Red Sea. A medium size colony was observed releasing sperm on the full moon night on 23 May 2024, at sunset time. Our observations suggest that the widespread Indo-Pacific P. maldivensis is likely gonochoric. This first report on the in situ spawning timing for P. maldivensis contributes to expanding coral spawning databases and provides valuable data on its reproductive biology, which is relevant for coral restoration and conservation efforts.
The current global coral crisis has pushed forward the development of a wide range of strategies to improve coral resilience to withstand the pressing anthropogenic impacts (Voolstra et al., 2021). Some of these interventions rely on successfully collecting gametes to sexually propagate corals. Therefore, detailed information on coral spawning is crucial to advance basic and applied research and inform strategies for coral reef conservation and restoration. Currently, reproduction data is available for over 300 scleractinian species in 61 genera in the Indo-Pacific (Harrison, 2011; Baird et al., 2021), where over 600 hard coral species are reported (Veron et al., 2015). In the Red Sea, where research on coral reproduction historically started in the Gulf of Aqaba (Rinkevich and Loya, 1979; Shlesinger and Loya, 1985; Shlesinger et al., 1998; Rapuano et al., 2017), synchronous spawning of scleractinian corals at central latitudes has been observed around full moon nights in spring (Bouwmeester et al., 2015; Osman et al., 2024). In the Indo-Pacific region, most spawning observations are on species of the genus Acropora, with 38% of the total spawning records, while information on other coral genera is relatively scarce (Baird et al., 2021).
Pavona Lamarck, 1801, is commonly observed in shallow Indo-Pacific coral reefs, including the Red Sea (Terraneo et al., 2017), but few studies have described its reproductive biology. Species of Pavona are almost exclusively gonochoric and broadcast spawners (Shlesinger et al., 1998; Harrison, 2011). In situ spawning has been reported two and three days after the full moon of November in Pavona cactus Forskål, 1775, in the Great Barrier Reef (Marshall and Stephenson, 1933), of March in Pavona gigantea Verrill, 1869, in the Galapagos Islands, Ecuador (Glynn et al., 1996), from January to April for Pavona varians Verrill, 1864, and Pavona sp. (later formally described as Pavona chiriquiensis Glynn, Maté & Stemann, 2001) in the Gulf of Chiriquí, Panama (Glynn et al., 2000), and in October for Pavona clavus Dana, 1846, in Contadora Island, Panama (Glynn et al., 2011). In situ spawning was observed six days after full moon of August in Pavona decussata Dana, 1846, in Kochi Prefecture, Japan (Mezaki et al., 2014). In the Red Sea, no in situ spawning observations have been reported so far for Pavona; however, April has been inferred as the spawning month in P. varians, based on the oocyte maturity (Bouwmeester et al., 2015). Here, we report the first in situ spawning observation of Pavona maldivensis Gardiner, 1905.
In April and May 2024, in situ surveys to document coral spawning were conducted in Al Fahal Reef (22°18’21” N; 38°57’44” E), a midshore reef located in the central Red Sea (Garcias-Bonet et al., 2024). Underwater surveys took place by SCUBA divers during two consecutive months on 21–25 April 2024 and 20–24 May 2024, covering the full moon nights on 24 April and 23 May 2024. Surveys were conducted from 17:30 to 19:00 and from 21:30 to 23:00 at depths from 8–10 meters using red light torches to minimize the negative impact of light on coral spawning. Spawning observations and timing were noted and pictures and videos were taken with an Olympus TG-6 camera equipped with an Olympus PT-059 underwater housing (OM System). In situ seawater temperature was monitored using a Multiparameter CTD probe (Ocean Seven 310, Idronaut) as part of a sustained environmental data observation platform (Garcias-Bonet et al., 2024).
We report and document the first in situ spawning observation of Pavona maldivensis Gardiner, 1905 (Figure 1) in the central Red Sea (Al Fahal Reef) on the full moon night, on 23 May 2024 at 18:49h, ten minutes before sunset time (18:59h), when in situ daily mean seawater temperature was 29.68°C. A medium size P. maldivensis colony (80 cm in diameter) at a depth of 9 m was observed releasing sperm in a dense cloud surrounding the entire colony for seven minutes (Figure 1, Supplementary Movie 1). No eggs seemed to be released from the colony, suggesting that P. maldivensis is likely gonochoric, in agreement with reproductive data available for other Pavona species. For instance, Pavona varians has been reported as gonochoric in the Red Sea (Bouwmeester et al., 2015; Shlesinger et al., 1998) and mostly gonochoric with some hermaphrodite colonies in Eastern Pacific (Glynn et al., 2000).
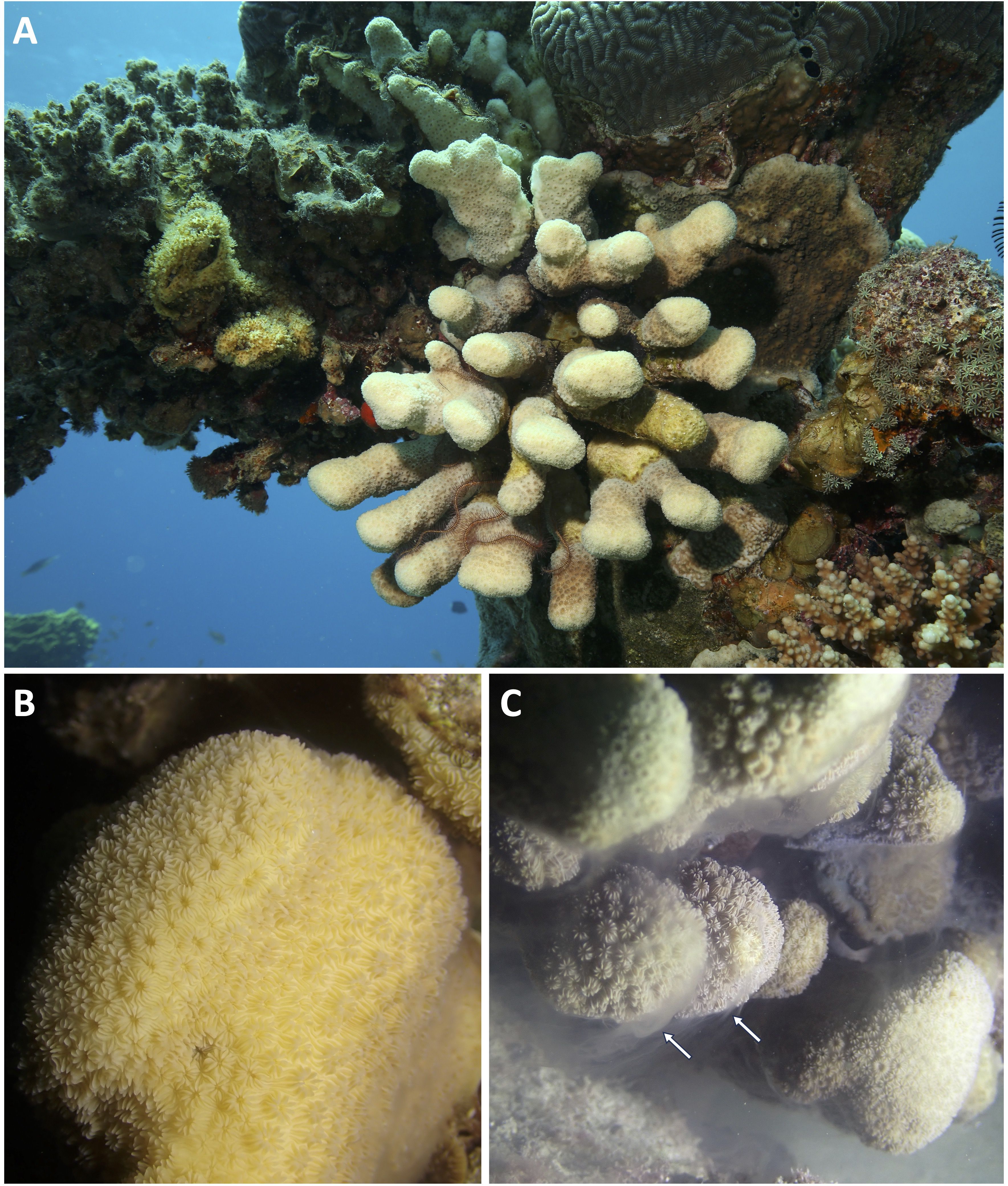
Figure 1. (A) Picture of a Pavona maldivensis colony in the Red Sea. Image by Francesca Benzoni. (B) Detail of a P. maldivensis colony in vivo. The colony exhibits the characteristic knob-shaped structure, with corallites defined by the species’ distinct raised, rounded, and well-defined wall. The corallite septa are alternating in height and thickness. Image by Marco Casartelli. (C) In situ spawning observation, with a dense cloud of sperm being released (arrows), from P. maldivensis in Al Fahal Reef in central Red Sea. Image by Neus Garcias-Bonet.
Contrary to the spawning synchrony observed in many scleractinian corals (Baird et al., 2009, 2021), spawning times in Pavona genus doesn’t seem to be synchronized, with in situ observations ranging from midday to few hours before sunrise around full moon nights across different months. Similarly to our spawning observation at sunset time in P. maldivensis, Pavona sp. spawning was reported shortly after sunset in Panamá (Glynn et al., 2000), P. gigantea spawning was reported in the late afternoon (two hours before sunset) in Galapagos Islands, Ecuador (Glynn et al., 1996), and the release of eggs in Pavona explanulata was observed three hours after sunset in Taiwan (Lin and Nozawa, 2017). Contrary, Pavona sp. spawning was reported at midday in Thailand (Plathong et al., 2006), male and female colonies of P. varians were observed spawning two hours before sunrise in Panamá (Glynn et al., 2000) and P. decussata was observed spawning about one hour before sunrise in Japan (Mezaki et al., 2014).
The spawning observation of P. maldivensis reported here is based on a single male colony; therefore, further research, increasing the observation time window and number of monitored colonies, is needed to fully describe the spawning patterns, characterize the reproductive biology and confirm the gonochorism of P. maldivensis in the Red Sea. P. maldivensis is known to be widespread from the Red Sea to the East Pacific Region (Sheppard, 1991; Veron, 2000; Salvat et al., 2016). In the Red Sea, it can be commonly observed from very shallow to upper mesophotic low-light environments such as under reef crest ledges and underhangs (Al Tawaha et al., 2019), with Pavona genus accounting on average for less than 1% of the benthic cover in the central Red Sea (Monroe et al., 2018). This first report on the in situ spawning timing for P. maldivensis in the Red Sea contributes to expanding global coral spawning databases and provides valuable data on its reproductive biology, which is relevant for coral restoration and conservation efforts.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
The manuscript presents research on animals that do not require ethical approval for their study.
NG-B: Conceptualization, Methodology, Writing – original draft. MC: Conceptualization, Methodology, Writing – review & editing. SV: Methodology, Writing – review & editing. FB: Conceptualization, Writing – review & editing. RP: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. NG-B and RP acknowledge funding from KAUST (grants FCC/1/1973-51- 01, REI/1/4984-01-01 and BAS/1/1095-01-01).
We would like to thank the Coastal and Marine Resources Core Lab team at KAUST.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1514216/full#supplementary-material
Supplementary Movie 1 | In situ spawning observation from a Pavona maldivensis colony in Al Fahal Reef in central Red Sea on the full moon night in May, on the 23rd of May 2024, after sunset time (18:50h). File format: Apple QuickTime Movie (MOV).
Al Tawaha M., Benzoni F., Eid E., Abu Awali A. (2019). The Hard Corals of JORDAN: A Field Guide. Available online at: https://www.boa.unimib.it/handle/10281/232270 (Accessed September 9, 2024).
Baird A. H., Guest J. R., Edwards A. J., Bauman A. G., Bouwmeester J., Mera H., et al. (2021). An indo-pacific coral spawning database. Sci. Data 8, 35. doi: 10.1038/s41597-020-00793-8
Baird A. H., Guest J. R., Willis B. L. (2009). Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. System. 40, 551–715. doi: 10.1146/annurev.ecolsys.110308.120220
Bouwmeester J., Baird A. H., Chen C. J., Guest J. R., Vicentuan K. C., Berumen M. L. (2015). Multi-species spawning synchrony within scleractinian coral assemblages in the red sea. Coral Reefs 34, 65–77. doi: 10.1007/s00338-014-1214-6
Garcias-Bonet N., Villela H., García F., Duarte G., Delgadillo-Ordoñez N., Raimundo I., et al. (2024). The coral probiotics village: an underwater laboratory to tackle the coral reefs crisis. Authorea. doi: 10.22541/au.172511515.58013062/v1
Glynn P. W., Colley S. B., Gassman N. J., Black K., Cortés J., Maté J. L. (1996). Reef coral reproduction in the eastern pacific: Costa Rica, Panamá, and Galápagos islands (Ecuador). III. Agariciidae (Pavona gigantea and gardineroseris planulata). Mar. Biol. 125, 579–601. doi: 10.1007/BF00353270
Glynn P. W., Colley S. B., Guzman H. M., Enochs I. C., Cortés J., Maté J. L., et al. (2011). Reef coral reproduction in the eastern pacific: Costa Rica, Panamá, and the Galápagos islands (Ecuador). VI. Agariciidae, pavona clavus. Mar. Biol. 158, 1601–1617. doi: 10.1007/s00227-011-1673-z
Glynn P. W., Colley S. B., Ting J. H., Maté J. L., Guzmán H. M. (2000). Reef coral reproduction in the eastern pacific: Costa Rica, Panamá and Galápagos islands (Ecuador). IV. Agariciidae, recruitment and recovery of pavona varians and pavona sp.a. Mar. Biol. 136, 785–805. doi: 10.1007/s002270000286
Harrison P. L. (2011). “Sexual Reproduction of Scleractinian Corals,” in Coral Reefs: An Ecosystem in Transition. Eds. Dubinsky Z., Stambler N. (Springer Netherlands, Dordrecht), 59–85. doi: 10.1007/978-94-007-0114-4_6
Lin C.-H., Nozawa Y. (2017). Variability of spawning time (Lunar day) in acropora versus merulinid corals: A 7-yr record of in situ coral spawning in Taiwan. Coral Reefs 36, 1269–1785. doi: 10.1007/s00338-017-1622-5
Marshall S. M., Stephenson T. A. (1933). The breeding of reef animals. PART I. The corals. Sci. Reports/Great Barrier Reef Exped. 1928-29 3, 219–455.
Mezaki T., Keshavmurthy S., Chen C. A. (2014). An old and massive colony of pavona decussata is sexually active at high latitude (32°N) in Japan. Coral Reefs 33, 97–975. doi: 10.1007/s00338-013-1080-7
Monroe A. A., Ziegler M., Roik A., Röthig T., Hardenstine R. S., Emms M. A., et al. (2018). In situ observations of coral bleaching in the central Saudi Arabian red sea during the 2015/2016 global coral bleaching event. PloS One 13, e01958145. doi: 10.1371/journal.pone.0195814
Osman E. O., Suggett D. J., Attalla T. M., Casartelli M., Cook N., El-Sadek I., et al. (2024). Spatial variation in spawning timing for multi-species acropora assemblages in the red sea. Front. Mar. Sci. 11, 1333621. doi: 10.3389/fmars.2024.1333621
Plathong S., Baird A. H., Chen C. A., Chanmethakul T., Suwonno V., Buaphet P., et al. (2006). Daytime gamete release from the reef-building coral, pavona sp., in the gulf of Thailand. Coral Reefs 25, 72–72. doi: 10.1007/s00338-005-0065-6
Rapuano H., Brickner I., Shlesinger T., Meroz-Fine E., Tamir R., Loya Y. (2017). Reproductive strategies of the coral turbinaria reniformis in the northern gulf of aqaba (Red sea). Sci. Rep. 7, 426705. doi: 10.1038/srep42670
Rinkevich B., Loya Y. (1979). The reproduction of the red sea coral stylophora pistillata. I. Gonads and planulae. Mar. Ecol. Prog. Ser. 1, 133–144. doi: 10.3354/meps001133
Salvat B., Petek S., Folcher E., Debitus C., Benzoni F., Pichon M., et al. (2016). “Invertébrés Benthiques Des Marquises,” in Biodiversité Terrestre et Marine Des Îles Marquises, Polynésie Française (Société Française d’Ichtyologie), 221–258. Available at: https://boa.unimib.it/bitstream/10281/152989/2/2016%20Salvat%20et%20al%20Marquises.pdf (Accessed September 16, 2024).
Sheppard C. R. C., Sheppard A. L. S. (1991). Corals and Coral Communities of Arabia. Buttiker W., Krupp F.. Eds., Fauna of Saudi Arabia Vol. 12 (Marine Management (Holdings) Limited). Basle: Natural History Museum. p. 3–170
Shlesinger Y., Goulet T. L., Loya Y. (1998). Reproductive patterns of scleractinian corals in the northern red sea. Mar. Biol. 132, 691–701. doi: 10.1007/s002270050433
Shlesinger Y., Loya Y. (1985). Coral community reproductive patterns: red sea versus the great barrier reef. Science 228, 1333–1335. doi: 10.1126/science.228.4705.1333
Terraneo T. I., Arrigoni R., Benzoni F., Tietbohl M. D., Berumen M. L. (2017). Exploring the genetic diversity of shallow-water agariciidae (Cnidaria: anthozoa) from the Saudi Arabian red sea. Mar. Biodivers. 47, 1065–1785. doi: 10.1007/s12526-017-0722-3
Veron J. E. N. (2000). Corals of the World. Available online at: https://cir.nii.ac.jp/crid/1370848662412845447 (Accessed September 16, 2024).
Veron J., Stafford-Smith M., DeVantier L., Turak E. (2015). Overview of distribution patterns of zooxanthellate scleractinia. Front. Mar. Sci. 1. doi: 10.3389/fmars.2014.00081
Keywords: coral reproductive biology, coral gametes, broadcast spawners, coral sperm release, Red Sea
Citation: Garcias-Bonet N, Casartelli M, Vimercati S, Benzoni F and Peixoto RS (2025) First spawning record of the widespread Indo-Pacific Pavona maldivensis observed in the Red Sea. Front. Mar. Sci. 12:1514216. doi: 10.3389/fmars.2025.1514216
Received: 20 October 2024; Accepted: 06 February 2025;
Published: 25 February 2025.
Edited by:
Masaya Morita, University of the Ryukyus, JapanReviewed by:
Saki Harii, University of the Ryukyus, JapanCopyright © 2025 Garcias-Bonet, Casartelli, Vimercati, Benzoni and Peixoto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neus Garcias-Bonet, bmV1cy5nYXJjaWFzYm9uZXRAa2F1c3QuZWR1LnNh; Raquel S. Peixoto, cmFxdWVsLnBlaXhvdG9Aa2F1c3QuZWR1LnNh
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.