
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 27 February 2025
Sec. Marine Biogeochemistry
Volume 12 - 2025 | https://doi.org/10.3389/fmars.2025.1485853
Biological dinitrogen (N2) fixation is a major source of new N to surface seawater, sustaining ocean productivity. However, the fate of diazotroph-derived nitrogen (DDN), specifically its release and transfer, and the factors controlling these processes, remain poorly understood. Here, we established stable co-cultures of the major diazotrophs, filamentous Trichodesmium erythraeum IMS101 and unicellular Crocosphaera watsonii WH8501, with the pico-cyanobacterium Synechococcus sp. WH8102, to explore the intrinsic differences in DDN release and transfer between diazotroph strains. We found that T. erythraeum released similar amounts of DDN as C. watsonii, but had a significantly higher DDN transfer efficiency for supporting Synechococcus cell growth. These results implied a higher bioavailability of fixed N released by T. erythraeum than by C. watsonii. Additionally, we showed that elevated light levels significantly enhanced T. erythraeum DDN release and transfer. Our results provide new insights into the fate of N fixed by different diazotrophs and the environmental factors that control the process.
The availability of fixed nitrogen (N) is a key factor controlling phytoplankton growth throughout most of the oligotrophic oceans (Moore et al., 2013; Browning and Moore, 2023). In N-limited regions, diazotrophs convert the abundant N2 gas into ammonia, providing significant bioavailable N that fuels primary production (Gruber and Galloway, 2008). Several studies have been conducted in recent decades to determine the biogeographical distribution and controlling factors of diazotrophs in the global oceans (Mills et al., 2004; Sohm et al., 2011; Zehr, 2011; Wen et al., 2022). However, less attention has been given to the fate of the diazotroph-derived nitrogen (DDN) in marine ecosystems, particularly its release into the dissolved pool and its potential transfer to the other pelagic plankton. Understanding these processes and their controlling factors are essential for explaining the full impact of diazotrophs on marine N cycles, primary production, and carbon (C) export (Mulholland, 2007).
The filamentous cyanobacteria Trichodesmium and the free-living unicellular cyanobacterium (Crocosphaera) are the two main N2-fixers throughout the (sub)tropical oligotrophic ocean (Capone et al., 1997; Masuda et al., 2024). However, studies of the release and transfer of diazotroph fixed N have predominantly focused on Trichodesmium, which has been reported to release 6−90% of its newly fixed N into the dissolved pool (Konno et al., 2010; Benavides et al., 2013b; Berthelot et al., 2016; Bonnet et al., 2016a; Berthelot et al., 2017; Caffin et al., 2018; Lu et al., 2018). Previous studies showed that only 15−20% of cells within a Trichodesmium trichome are capable of fixing N (diazocytes), while the remaining cells (vegetative cells) in a filament rely heavily on the bioavailable N supplied by the diazocytes (Berman-Frank et al., 2003; Mulholland et al., 2004). This indicates that the newly fixed N by diazocytes could be actively released into the surrounding environment and subsequently taken up by the vegetative cells and potentially other co-existing plankton (Mulholland et al., 2004). In addition, the release of DDN in natural water is not solely tied to active physiological processes but also results from dying diazotrophic cells through viral lysis, sloppy feeding, programmed cell death, etc (ONeil et al., 1996; Hewson et al., 2004; Berman-Frank et al., 2004). The released DDN can be subsequently used by other plankton in the surrounding water. For example, several studies performed in the western tropical South Pacific Ocean (WTSP) showed that 6−12% of Trichodesmium fixed N was transferred to non-diazotrophic plankton using nanometer scale secondary ion mass spectrometry (nanoSIMS) coupled with 15N isotopic labelling and flow cytometry cell sorting (Bonnet et al., 2016c; Berthelot et al., 2016; Caffin et al., 2018).
Compared to Trichodesmium, less is known about the DDN release and transfer by Crocosphaera, which exhibits a distinctly different cell size, morphology and N2 fixation pattern (Berman-Frank et al., 2004; Masuda et al., 2024). For example, Crocosphaera fixes N at night while Trichodesmium fixes N during the day. Moreover, Crocosphaera has a higher competitive capability for combining N (Masuda et al., 2022), potentially affect the utilization of its released DDN by other non-diazotrophs. Consequently, DDN fixed by Crocosphaera may have a distinctly different fate in the marine ecosystem to that released from Trichodesmium.
There have been few direct comparative studies of DDN release and transfer between Trichodesmium and Crocosphaera. A field study conducted in the WTSP found that ∼20–40% of the fixed N was released to the dissolved pool when Trichodesmium dominated, while the DDN release was not quantifiable when Crocosphaera dominated (Caffin et al., 2018). In other studies, no significant difference was found in the DDN release (<10.3%) between Trichodesmium and Crocosphaera (Berthelot et al., 2015, 2016). For DDN transfer in artificially induced blooms, the efficiency of DDN transfer by Crocosphaera (4−5%) was only half that of Trichodesmium (~12%) (Berthelot et al., 2016). In contrast, field studies in similar regions reported higher transfer efficiencies for a Crocosphaera-dominated diazotroph community (15 ± 3%) than for a Trichodesmium-dominated diazotroph community (9 ± 3%) (Caffin et al., 2018). These contradictory results suggest that further comparative studies are needed to understand the intrinsic differences between the DDN release and transfer of the two types of diazotrophs.
Additionally, environmental factors such as temperature, nutrients, and light intensity regulate the N2 fixation in Trichodesmium (Bell and Fu, 2005; Breitbarth et al., 2008), and thus may also impact DDN release and transfer. For example, a Trichodesmium culture study showed that exposure to high-light levels significantly enhanced the release of fixed N in the form of ammonium (NH4+) and dissolved organic N (DON) (Wannicke et al., 2009). However, field incubation experiments have shown that the percentage of DDN released into the dissolved phase increases with a decline in light intensity (Lu et al., 2018). These results indicate that changes in light intensity modulate the release of fixed N from diazotrophs. It is unclear why the results vary between culture and field studies, and further studies are therefore needed to confirm how changes in light intensity impact diazotrophic DDN release and transfer.
Here, we established a co-culture of diazotrophs (Trichodesmium erythraeum IMS101 and Crocosphaera watsonii WH8501) with a non-diazotrophic pico-cyanobacteria Synechococcus sp. WH8102 under various light intensities. The ecological niches of the two diazotrophs and Synechococcus partially overlap (Campbell et al., 2005; Flombaum et al., 2013; Shao et al., 2023). The aim was to determine the differences in DDN release and transfer between T. erythraeum and C. watsonii, and then investigate the effect of light intensity on these processes using simple co-culture systems in a laboratory setting. We found that T. erythraeum was more efficient in transferring DDN to Synechococcus than C. watsonii, although the overall release and transfer of DDN were not significantly different between the two stains. These results imply a higher bioavailability of released fixed N by T. erythraeum than C. watsonii. Additionally, we found that an increase in light intensity significantly enhanced DDN release and transfer by T. erythraeum.
Two N2-fixing cyanobacteria, T. erythraeum IMS101 and C. watsonii WH8501, along with one non- N2-fixing pico-cyanobacterium, Synechococcus sp. WH8102, were cultured. T. erythraeum and C. watsonii were grown in Aquil-tricho medium (Hong et al., 2017) prepared with 0.22 µm-filtered and microwave-sterilized oligotrophic western North Pacific surface water. The medium was enriched with chelexed and filter-sterilized NaH2PO4, and supplied with filter-sterilized vitamins and trace metals, buffered with 5 µM EDTA. Synechococcus was also grown in Aquil-tricho medium but was enriched with 100 µM NaNO3.
All algae were pre-adapted to a light intensity of 200 µE m-2 s-1 by semi-continuous culturing for more than six months. The light level was monitored using a spherical light meter (QSL-2100, Biospherical Instruments Inc., San Diego CA, USA). The light intensity measured using the spherical light meter was approximately 2.5 times greater than that measured using a flat light meter (~80 µE m-2 s-1). Cultures were maintained in the exponential growth stage at 27°C with a 14:10 h light−dark cycle in an algal growth chamber (AGC-850, Firstek Corp, China) before starting the co-culture. Strict sterile techniques were applied for culturing and experimental manipulations.
Exponentially growing Synechococcus was transferred to an inorganic-N-free Aquil-tricho medium for a 2-day N-starvation acclimation, during which cell growth ceased (Supplementary Figure S1). The N-starved Synechococcus was then transferred separately into exponentially growing T. erythraeum and C. watsonii cultures, and co-cultured under the same conditions as the monoculture of T. erythraeum and C. watsonii. To achieve the exponential growth of diazotrophs and Synechococcus in the co-culture systems, inoculation ratios were optimized in the preliminary experiments. The optimized inoculation ratios were Synechococcus: T. erythraeum = ~5−8:1 (cell number: cell number), and Synechococcus: C. watsonii = 0.003:1 (cell number: cell number), respectively, which ensured a similar growth rate of Synechococcus in both co-cultures (Table 1). To minimize the impact of the inoculation process, the volume of the Synechococcus culture transferred was kept to less than 7% of the T. erythraeum and C. watsonii culture volumes. In the co-culture system, N fixed by diazotrophs was the sole N source supporting Synechococcus growth. Both the diazotrophs and Synechococcus in the co-cultures continued exponential growth for at least 3 days (as shown in Figure 1), after which an aliquots of the co-cultures were spiked with 15N2 gas (98.9 atom%, Cambridge Isotope Laboratories, Lot #: I-21065/AR0664758) and incubated for another 24 h to determine the total N2 fixation rate of diazotrophs, as well as the DDN release to the dissolved phase and DDN transfer to Synechococcus.
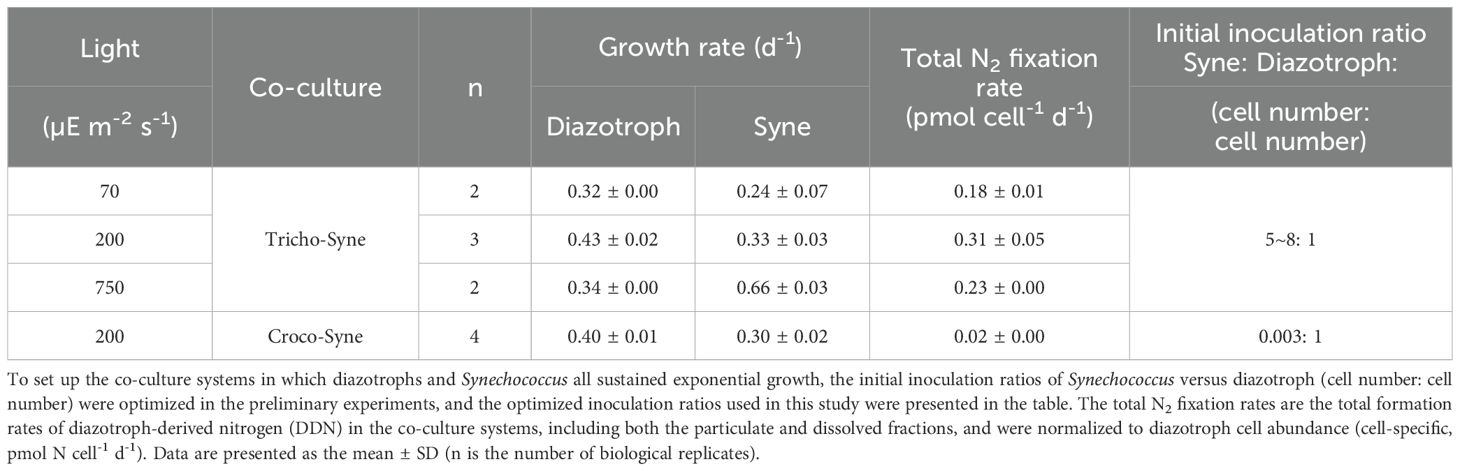
Table 1. Growth rates (d-1) of Synechococcus and two diazotrophs, Trichodesmium erythraeum and Crocosphaera watsonii, and the total N2 fixation rates (pmol N cell-1 d-1) of the two diazotrophs in the T. erythraeum−Synechococcus and C. watsonii−Synechococcus co-culture systems under different light intensities (70, 200, and 750 µE m-2 s-1).
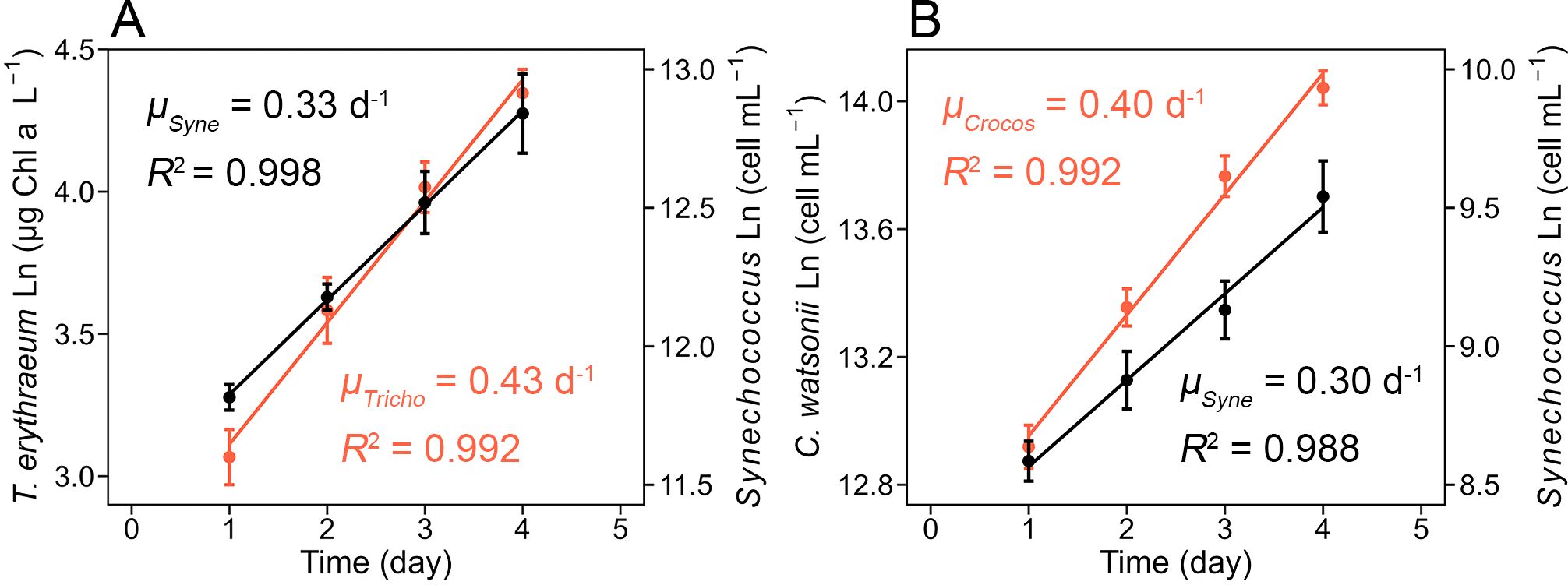
Figure 1. Exponential growth of Trichodesmium erythraeum IMS101, Crocosphaera watsonii WH8501, and Synechococcus sp. WH8102 in the T. erythraeum−Synechococcus and C. watsonii−Synechococcus co-culture systems under a light intensity of 200 μE m-2 s-1. (A) Growth rates of T. erythraeum (μTricho, red) and Synechococcus (μSyne, black) in their co-culture systems. (B) Growth rates of C. watsonii (μCrocos, red) and Synechococcus (μSyne, black) in their co-culture systems. The T. erythraeum−Synechococcus co-culture systems were set up at the optimized initial inoculation ratio of ~ 6:1 (Synechococcus cell number: T. erythraeum cell number), and C. watsonii−Synechococcus co-culture systems were 0.003:1 (Synechococcus cell number: C. watsonii cell number), to achieve the exponential growth of both strains. The growth of T. erythraeum was monitored daily by measuring the Chl a concentration, while the growth of C. watsonii and Synechococcus was monitored daily through the measurement of cell abundance. Error bars represent the standard deviation of biological replicates (n = 3 in the T. erythraeum−Synechococcus co-culture experiment, n = 4 in the C. watsonii−Synechococcus co-culture experiment).
To explore the impact of light intensity on DDN release and transfer, T. erythraeum and Synechococcus were adapted to a lower light intensity of 70 µE m-2 s-1, and a higher light intensity of 750 µE m-2 s-1 for at least 3 months. Subsequently co-cultures of T. erythraeum and Synechococcus were established as described above. Additionally, to examine DDN release and transfer at an even higher light intensity, e.g., 1100 and 1800 µE m-2 s-1 (approximately equal to full noon sunlight on a cloudless day) (Lu et al., 2018), at which the stable exponential growth of T. erythraeum and Synechococcus could not be achieved due to a strong light inhibition effect, we conducted short-term (24-h) mixed incubations that were enriched with 15N.
In the monoculture and co-cultures, T. erythraeum growth was monitored by daily measurements of the Chl a concentration. Briefly, T. erythraeum cells were collected by filtration onto 3-μm pore size polycarbonate membrane filters (PC, Millipore, Burlington, MA, USA). In the co-culture systems, because the cell size of Synechococcus was typically less than 1.5 μm, Synechococcus could not be trapped on the 3-μm PC filters. The filters were heated at 65°C for 6 min in 90% (vol/vol) methanol. After extraction, the filters were removed, and cell debris was pelleted by centrifugation. The Chl a concentration was subsequently determined by a spectrophotometric analysis following the method described by Demarsac and Houmard (1988). To count T. erythraeum cell numbers, photographs of T. erythraeum were taken using a camera (DS126281, Canon, Tokyo, Japan) connected to an inverted microscope (CKX41, Olympus, Tokyo, Japan). The total length of filaments in 1 mL culture were measured, and the cell number of ~20 filaments was counted. The average length of cells was obtained by dividing the total length of the measured filaments by their total cell number. The cell density of the culture was then calculated by dividing the total length of filaments in 1 mL culture by the average cell length. The Chl a per cell was calculated by dividing the Chl a concentration by the cell density.
The growth of C. watsonii and Synechococcus was monitored daily by measuring cell abundance using flow cytometry (Accuri™ C6, BD Biosciences, Franklin Lakes, NJ, USA). Briefly, samples were collected in 2 mL centrifuge tubes and preserved in freshly prepared 0.2-μm-filtered glutaraldehyde (0.5% vol/vol final concentration). After fixation in the dark for 15 mins, samples were frozen in liquid nitrogen and stored at -80°C until analysis. Synechococcus and C. watsonii cells were distinguished and quantified based on forward scatter and red fluorescence (670 nm).
Specific growth rates were calculated from the linear regressions of the natural logarithm of Chl a concentrations or cell densities versus time during the exponential growth phase. Each growth curve included four data points to ensure accuracy and reliability in the linear regressions.
Particulate N2 fixation was measured using the 15N gas dissolution method (Mohr et al., 2010), while concurrently, a C fixation assay was conducted utilizing NaH13CO3 (99 atom% 13C, Cambridge Isotope Laboratories, Cambridge, UK). The 15N2 gas (98.9 atom%, Cambridge Isotope Laboratories, Lot #: I-21065/AR0664758) was tested and confirmed to be non-contaminated following the method of Yu et al. (2024). To prepare the 15N-enriched water, 5 mL of 15N2 gas was dissolved into 500 mL of degassed seawater. Incubations were conducted in duplicate in acid-cleaned 1-L Nalgene polycarbonate bottles. Each bottle was spiked with 30 mL of 15N2-enriched water and NaH13CO3 solution to a final concentration of 200 μM, followed by incubation in an algae chamber for 24 h. The final 15N enrichment [100×15N/(15N+14N), atom%] of the N2 pool in the incubation bottles was measured using a Membrane Inlet Mass Spectrometer (MIMS), yielding a value of 1.29 ± 0.11 atom% (n = 11). Then, cells were filtered onto 25 mm pre-combusted (450°C for 4 h) GF/75 filters (Advantec, Eden Prairie, MN, USA). Cells not enriched with 15N and 13C were also collected to establish the baseline enrichments for biomass 15N and 13C. All filters were acid fumed, dried, and then analyzed using an EA IsoLink™ IRMS system (Flash IRMS elemental analyzer coupled to a Delta V isotope ratio mass spectrometer, Thermo Fisher Scientific, Waltham, MA, USA).
The N and C contents of the GF/75 filter blanks were 0.06 ± 0.00 μmol and 1.16 ± 0.06 μmol, respectively, consistently lower than the N (>1 µmol) and C (>10 µmol) contents of the measured samples. The natural 15N and 13C enrichments of co-culture samples were ~0.366 atom% and ~1.081 atom%, respectively, which are significantly lower than the values in the samples spiked with 15N2 and NaH13CO3 (15N>0.654 atom%, 13C>2.724 atom%). The average reproducibility of the 15N and 13C measurement of the USGS-40 standard was ± 0.0002 atom% and ± 0.0005 atom% (n = 18), respectively. The 15N and 13C fixation rates were calculated based on methods described by Montoya et al. (1996) and Hama et al. (1983), respectively.
The total N2 fixation rates were the total formation rates of DDN, including both the particulate and dissolved fractions.
To determine the DDN released to the dissolved fractions, 50 ml of the incubation waters, both with and without 15N enrichment, were filtered through 0.22 µm pore size Millex-GP syringe filters (Millipore Express PLUS membrane, Millipore). The filtrates were preserved at -20°C for subsequent measurement of the concentration and 15N enrichment of total dissolved nitrogen (TDN).
For measurement of TDN concentration, TDN was oxidized to NO3- using a purified persulfate oxidizing reagent (POR, ACS-grade, Merck, Rathway, NJ, USA) in a 12 mL 450°C pre-combusted borosilicate glass tube (Knapp et al., 2005). The POR was recrystallized four times and prepared as alkaline POR by dissolving 6 g K2S2O8 and 6 g NaOH (ACS-grade, Merck) in deionized water to a final volume of 100 mL. The residual NO3- concentration in the POR (POR blank) was determined to be less than 2 μmol L-1. Following oxidation, the sample pH was adjusted to 7–8 using 6 N HCl. The concentrations of the resulting NO3- were measured by a chemiluminescent analysis, with a detection limit of 0.5 μmol L-1 (Braman and Hendrix, 1989).
Isotopic analyses of 15N enrichment of TDN were conducted using the denitrifier method, which involves an isotopic analysis of the nitrous oxide produced by denitrifying Pseudomonas aureofaciens (Sigman et al., 2001). These analyses were performed on a Gasbench-Isotopic Ratio Mass Spectrometer (Delta V, Thermo Fisher Scientific). The rate of DDN released into the dissolved pool was calculated following Bonnet et al. (2016c):
where 15Nex is the 15N enrichment of the TDN fraction after 24 h of incubation relative to the time zero value, TDNcon is the measured TDN concentration, and 15Nsr is the 15N enrichment of the source N2 pool in the incubation bottles (as mentioned above).
For flow cytometry sorting (to separate the Synechococcus from the co-cultured diazotrophs), 0.3 to 0.7 L of co-culture algae were concentrated onto 0.22 µm pore size polycarbonate filters (47 mm) using a Nalgene polysulfone filtration unit (Item#: 300−4050, Thermo Fisher Scientific). Both the filtration unit and membrane were acid-cleaned prior to use. The cells on the filter were then resuspended into 4.5 mL of filtered seawater in a cryovial, where they were then fixed and preserved using glutaraldehyde (final concentration of 0.5% vol/vol) that had been filtered through a 0.2 μm filter. After fixation in the dark for 15 min, samples were frozen in liquid nitrogen and stored at -80°C until analysis.
Cell sorting was conducted following the method described by Baer et al. (2017), using a BD FACSAria™ III flow cytometer equipped with 488 and 561 nm lasers and detectors for forward and side scatter at 692 and 530 nm, respectively. Synechococcus populations were determined based on forward scatter and orange fluorescence (530 nm). Pre-filtered 30‰ NaCl (CAS: 7647−14−5, pure-grade, Sigma-Aldrich, St. Louis, MO, USA) solution was used as sheath fluid. The sorted populations were collected in 15 mL high-clarity polypropylene conical tubes (FALCON, Corning, NY, USA) and subsequently filtered onto pre-combusted GF/75 filters. Samples were then frozen in liquid nitrogen and stored at -80°C for further analysis.
The PON and 15N of the sorted cells were analyzed using the persulfate oxidation method coupled with the denitrifier method, respectively, as described above. The rate of DDN transferred to Synechococcus was calculated as follows:
where 15Nsyn is the 15N enrichment of Synechococcus after 24 h of incubation relative to the time zero value, PONcon is the particulate organic nitrogen content of the sorted Synechococcus (fmol cell-1), A is the abundance of Synechococcus, and 15Nsr is the 15N enrichment of the source N2 pool in the incubation bottles.
Filter and sheath blanks were measured (Supplementary Table S2) and subtracted from each analysis of mass. Based on the filter and sheath blank results, as well as the Synechococcus cellular N quota, a minimum of 2.5 × 107 Synechococcus cells were sorted to meet the quantifiable requirement.
The R software (version 4.3.0) was used to analyze data and establish the significance of differences based on a Welch two sample t-test or one-way ANOVA in combination with a Tukey post hoc test. A significance level of p < 0.05 was applied.
The T. erythraeum and C. watsonii monocultures were grown with similar growth rates of ~0.5 d⁻¹ under a light intensity of 200 μE m⁻² s⁻¹ (Table 2). The total N2 fixation rate (including fixed N in the particulate and dissolved phases) of T. erythraeum was either normalized to cell number (cell specific N2 fixation rate) or C biomass (C-specific N2-fixation rate), and was significantly higher than that of C. watsonii (t-test, p < 0.05, Table 2). Additionally, T. erythraeum had a significantly lower C:N and C-fix: N2-fix ratios compared to C. watsonii (t-test, p < 0.01, Table 2), suggesting that T. erythraeum was less efficient than C. watsonii in using the fixed N to support its own C fixation.
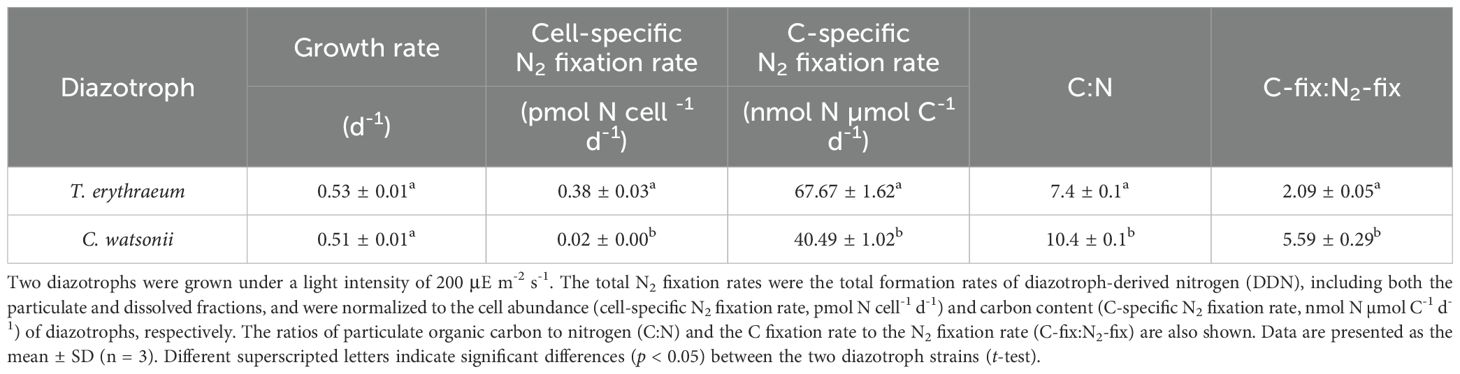
Table 2. Comparison of growth rates and total N2 fixation rates between Trichodesmium erythraeum IMS101 and Crocosphaera watsonii WH8501 in monocultures.
The monoculture of Synechococcus sp. grew exponentially with a rate of 0.91 d⁻¹ in the medium fortified with sufficient nitrate (100 μM) and under a light intensity of 200 μE m⁻² s⁻¹ (Supplementary Figure S1). Prior to inoculation into the exponentially growing T. erythraeum or C. watsonii cultures, Synechococcus was acclimated to an inorganic N-free medium for 2 days until cell growth ceased (Supplementary Figure S1).
To establish co-culture systems in which diazotrophs and Synechococcus all sustained exponential growth, the inoculation ratios were optimized in the preliminary experiments. It was found that at inoculation ratios of Synechococcus: T. erythraeum = ~6:1 (cell number: cell number), Synechococcus and T. erythraeum grew exponentially at growth rates of 0.33 ± 0.03 and 0.43 ± 0.02 d-1, respectively, under a light intensity of 200 μE m⁻² s⁻¹ (Figure 1). Therefore, the T. erythraeum fixed N supported the growth of Synechococcus and the growth of Synechococcus slightly inhibited the growth of T. erythraeum by about 20% (0.43 d-1 in co-culture vs 0.53 d-1 in monoculture). Additionally, the growth rate of Synechococcus in this co-culture system was one third of the maximum growth rate under N-replete conditions and the same light intensity (Supplementary Figure S1), suggesting a N-limitation of Synechococcus in the co-culture system. For the C. watsonii−Synechococcus co-culture system, the inoculation ratio of Synechococcus: C. watsonii was 0.003:1 (cell number: cell number), for both strains to sustain exponential growth at growth rates similar to those in the T. erythraeum-Synechococcus co-culture system (Figure 1). The huge difference in the inoculation ratios in the two co-culture systems was mainly due to the differences in the cell specific N2 fixation rates of the two diazotrophs, i.e., 0.31 pmol N cell-1 d-1 for T. erythraeum and 0.02 pmol N cell-1 d-1 for C. watsonii, respectively (Table 1). Additionally, it is likely that the two diazotrophs released different amounts and speciation of DDN to support the growth of non-diazotrophic phytoplankton.
On the 3rd day after initiating the T. erythraeum−Synechococcus and C. watsonii−Synechococcus co-culture systems (3rd day in Figure 1), 15N enriched seawater was added and the culture bottles were continually incubated under the same growing conditions for 24 h. The DDN released to the dissolved phase and DDN transferred to Synechococcus were measured. In the two co-culture systems, the diazotrophs reached a similar biomass on the 3rd day, i.e., 516 ± 12 and 619 ± 27 μmol C L-1 for T. erythraeum and C. watsonii, respectively. The 15N in diazotrophic (0.902 atom% and 0.738 atom% for T. erythraeum and C. watsonii respectively) and Synechococcus biomass (> 0.472 atom%), as well as in the dissolved pool (> 0.386 atom%) after 24 h of incubation were significantly enriched compared with the abundance of the natural isotope (~0.366 atom%, t-test, p < 0.05, Supplementary Table S1). The total volumetric N₂ fixation rate of T. erythraeum (34.9 ± 2.7 µmol N L-1 d-1), including the particulate fraction collected on the filter and the fraction released into the dissolved pool, was nearly double that of C. watsonii (18.9 ± 3.2 µmol N L-1 d-1). The majority of the total DDN (>95%) was contained within the diazotrophs themselves.
The rate of DD15N release into the dissolved pool (calculated by Equation 1) in the T. erythraeum−Synechococcus co-culture system was 507 ± 101 nmol N L-1 d-1, with no significant difference compared to that of the C. watsonii−Synechococcus co-culture (489 ± 49 nmol N L-1 d-1, t-test, Figure 2A). However, the percentage of the released DD15N relative to the total fixed N was slightly higher in C. watsonii (2.61%) compared to T. erythraeum (1.45%) (t-test, p < 0.01, Figure 2B). The volumetric rates of T. erythraeum DD15N transferred to Synechococcus (calculated by Equation 2) were significantly higher than those of the C. watsonii DD15N (t-test, p < 0.01, Figure 2A). Additionally, T. erythraeum had a significantly higher DD15N transfer efficiency (i.e., the proportion of the total fixed N, 0.36%) than C. watsonii (< 0.01%, t-test, p < 0.01, Figure 2B) in the co-cultures. Whereas, the total DDN release fractions (DDN released to the dissolved pool plus the DDN transferred to Synechococcus) did not differ significantly between the two co-culture systems.
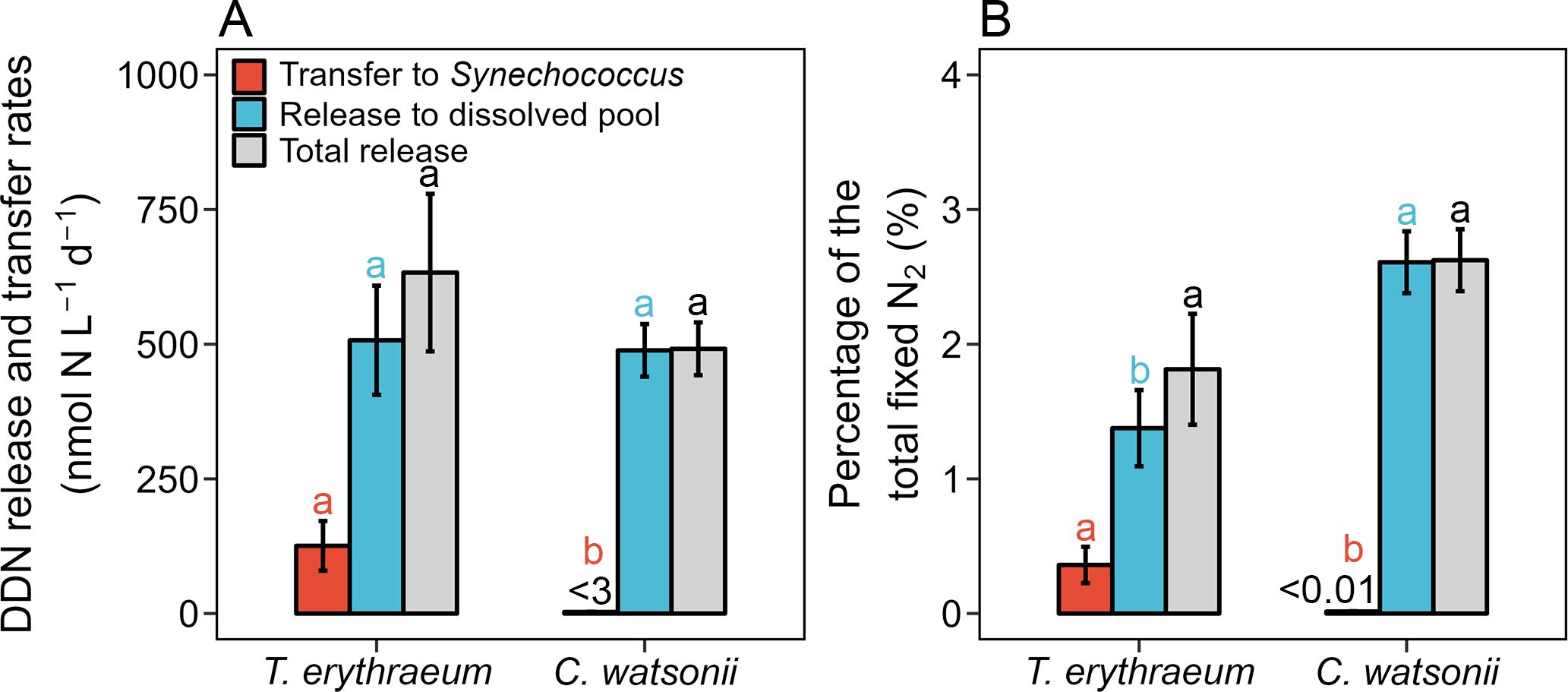
Figure 2. Comparison of diazotroph-derived nitrogen (DDN) release and transfer between Trichodesmium erythraeum IMS101 and Crocosphaera watsonii WH8501 in their co-culture systems under a light intensity of 200 μE m-2 s-1. (A) Rates of fixed N2 transferred to Synechococcus (red bars), released to the dissolved pool (blue bars), and total release (gray bars). (B) Percentage released and transferred DDN within the total fixed N2. The measurement was conducted on the 3rd day after initiating the co-culture systems (3rd day in Figure 1), when the biomass of diazotrophs T. erythraeum and C. watsonii reached 515 ± 12 and 619 ± 21 μmol C L-1, and the volumetric total N2 fixation rates were 34.9 ± 2.7 and 18.9 ± 3.2 μmol N L-1 d-1, respectively. The DDN transfer rate from C. watsonii to Synechococcus was < 3 nmol N L-1 d-1 and the percentage of total fixed N2 was < 0.01%. The error bars represent the standard deviation of biological replicates (n = 3 in the T. erythraeum−Synechococcus co-culture system, n = 4 in the C. watsonii−Synechococcus co-culture system). Different superscripted letters indicate significant differences (p < 0.05) between T. erythraeum and C. watsonii (t-test).
To explore the impact of light intensity on DDN release and transfer, we conducted additional co-cultures of T. erythraeum and Synechococcus under light intensities of 70 and 750 μE m-2 s-1, and compared the results to the treatment under the 200 μE m-2 s-1 condition (Table 1). The initial inoculation ratios of T. erythraeum and Synechococcus were kept similar under the different light treatments (5−8:1, Synechococcus cell number: T. erythraeum cell number, Table 1). T. erythraeum and Synechococcus grew exponentially under the three light intensities. The growth rates and total N2 fixation rates of T. erythraeum were significantly lower under 70 and 750 μE m-2 s-1 than under 200 μE m-2 s-1, suggesting that 70 and 750 μE m-2 s-1 were light-limited and light-inhibited conditions, respectively, for the growth and N2 fixation of T. erythraeum (Table 1). The growth rates of Synechococcus were lower under the low light conditions of 70 μE m-2 s-1 than 200 μE m-2 s-1, with consistently lower total N2 fixation rates of T. erythraeum under low light conditions. In contrast, the growth rates of Synechococcus increased when light intensity increased to 750 μE m-2 s-1, under which N2 fixation of T. erythraeum was inhibited by the high light conditions (Table 1). In line with these results, T. erythraeum exhibited a higher DD15N transfer efficiency under 750 μE m-2 s-1 than under 70 and 200 μE m-2 s-1 (Figure 3).
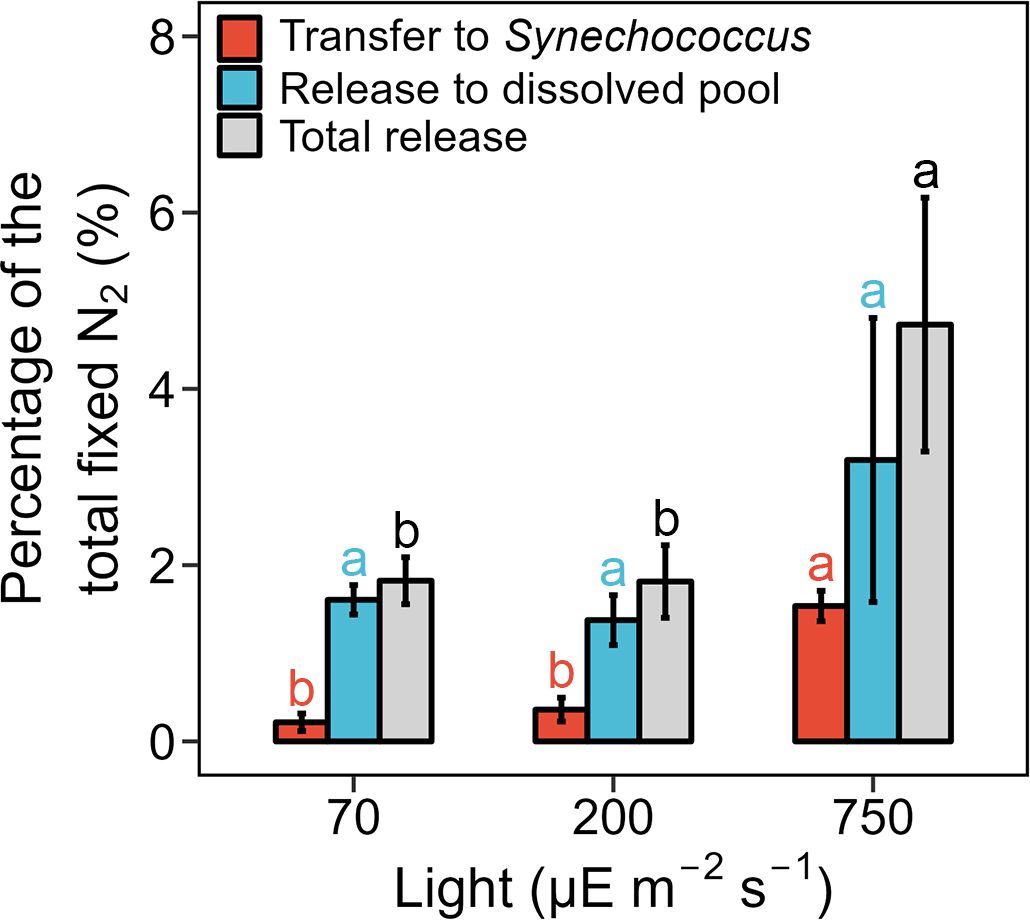
Figure 3. Percentage of diazotroph derived nitrogen (DDN) release and transfer in the Trichodesmium erythraeum IMS101 and Synechococcus sp. WH8102 co-culture system under different light intensities (70, 200, and 750 µE m-2 s-1). The measurement was conducted on the 3rd day after initiating the co-culture systems, when the biomass of T. erythraeum reached 212 ± 10, 516 ± 12, and 149 ± 7 μmol C L-1, and the volumetric total N2 fixation rates were 9.3 ± 1.0, 34.9 ± 2.7, and 9.8 ± 0.5 μmol N L-1 d-1, under the light intensities of 70, 200, and 750 µE m-2 s-1, respectively. Error bars represent the standard deviation of biological replicates (n = 2 in the 70 and 750 µE m-2 s-1 treatment groups, n = 3 in the 200 µE m-2 s-1 treatment group). Different superscripted letters indicate significant differences (p < 0.05) among the different light intensities (one-way ANOVA followed by a Tukey post hoc test).
Consistently, the highest percentage of DDN released into the dissolved phase was found under a light intensity of 750 μE m-2 s-1 (3.19%), although no statistically significant difference was found among the three light intensities (one-way ANOVA, p > 0.05, Figure 3). As a result, the total released fraction (release to the dissolved phase plus transfer to Synechococcus) was highest under 750 μE m-2 s-1 (Figure 3). To confirm that a high light intensity increased the DDN release, we conducted short-term (24-h) co-culture incubations under light intensities of 1100 and 1800 μE m-2 s-1, that were approximately equivalent to full noon sunlight on a cloudless day (Lu et al., 2018). Under these two high light conditions, T. erythraeum and Synechococcus could not grow exponentially due to strong light inhibition effects; therefore, only short-term incubations were conducted. The results showed that although the total N2 fixation rates were very low under the high light conditions of 1100 and 1800 μE m-2 s-1, the portion of fixed N released to the dissolved pool increased with increasing light intensity and reached ~25% under 1800 μE m-2 s-1 (Figure 4).
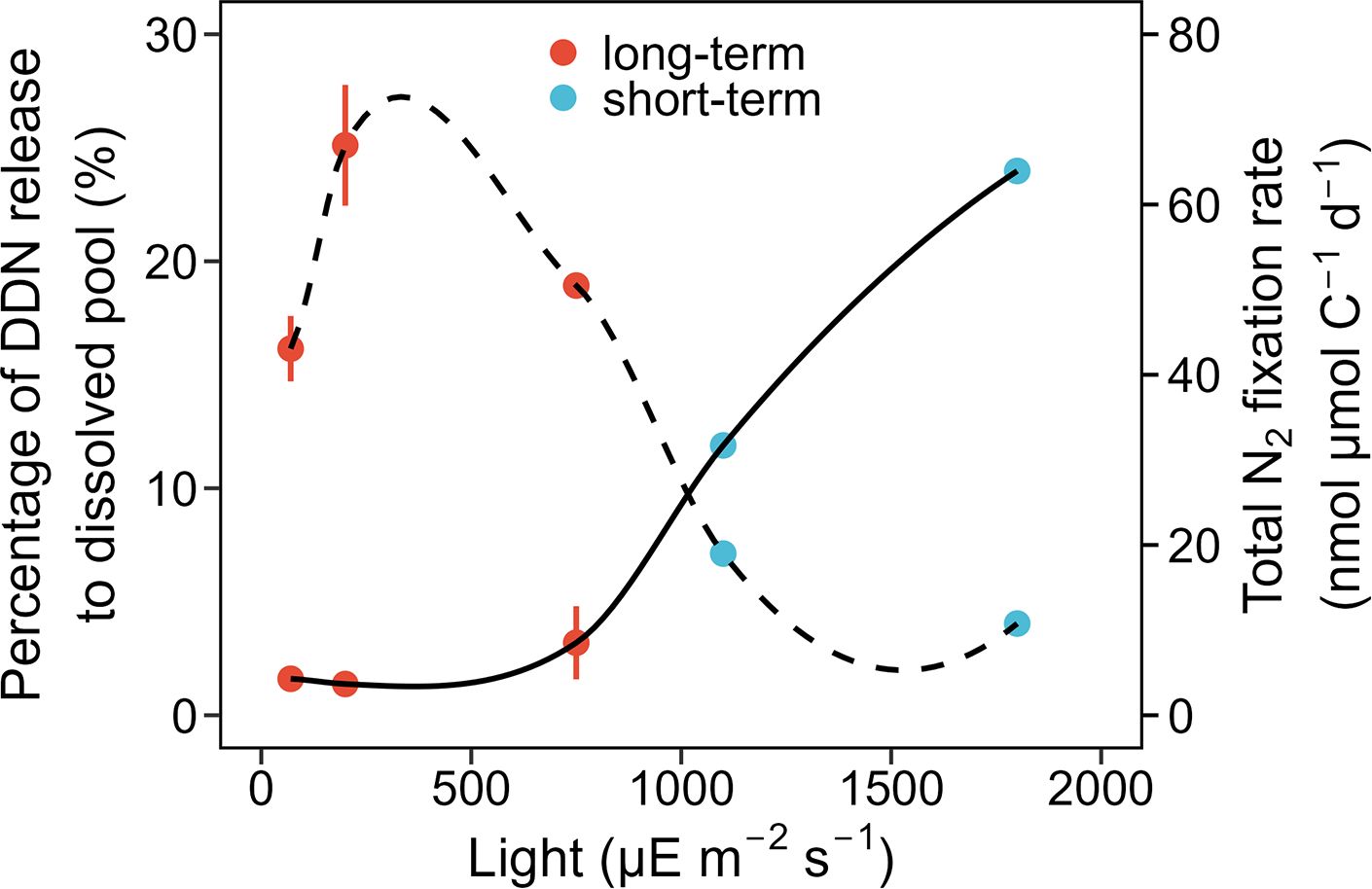
Figure 4. Percentage of total diazotroph-derived nitrogen (DDN) released to the dissolved pool (%) and total N2 fixation rate (nmol µmol C-1 d-1) of Trichodesmium erythraeum IMS101 in the co-cultures under different light intensities (70, 200, 750, 1100, and 1800 µE m-2 s-1). The solid line denotes the percentage of DDN released to the dissolved pool (%), while the dashed line indicates the N2 fixation rate. The total N2 fixation rates are the total formation rates of DDN in the co-culture systems, including both the particulate and dissolved fractions. The red dots represent the results from the long-term (4 days) co-culture experiments presented in Figure 3 (n = 2 in the 70 and 750 µE m-2 s-1 treatment group, 3 in the 200 µE m-2 s-1 treatment group). The blue dots represent the results from short-term (24-h) co-culture experiments (n = 1 in the 1100 and 1800 µE m-2 s-1 treatment groups).
Due to the importance of DDN in supporting phytoplankton productivity and C export in oligotrophic oceans, various studies have quantified the rate or fractions of DDN release and transfer by diazotrophic cyanobacteria (Benavides et al., 2013a; Bonnet et al., 2016b, c; Berthelot et al., 2017; Caffin et al., 2018). However, these studies produced substantial variations in their results, with the reasons behind these variations rarely reported. By establishing well-controlled co-cultures of representative marine diazotrophs with the pico-cyanobacterium Synechococcus, we compared the intrinsic differences in DDN release and transfer between the filamentous cyanobacterial diazotroph T. erythraeum and the unicellular C. watsonii. Additionally, we discussed the importance of light intensity in modulating DDN release and transfer.
In both the monoculture and co-cultures, T. erythraeum exhibited higher cell-specific and C-specific N2 fixation rates than C. watsonii under the same culture conditions (Tables 1; 2). These findings were aligned with previous reports in which N2 fixation rates were measured using the conventional acetylene reduction method (Knapp et al., 2012) and the novel nanoSIMS analysis (Berthelot et al., 2016). However, T. erythraeum was less efficient at using the fixed N to support its own C fixation than C. watsonii, as indicated by the lower C-fix:N2-fix ratios compared to C. watsonii (Table 2). As filamentous cyanobacterial diazotrophs, only 15−20% of its cells within a Trichodesmium trichome are diazocytes that are capable of N2 fixing. It was proposed that the new N fixed by diazocytes would be actively released into the surrounding environment and subsequently taken up by the vegetative cells in the rest of the filaments or by other phytoplankton (Berman-Frank et al., 2003; Mulholland et al., 2004). However, C. watsonii are unicellular N2 fixing cyanobacteria. Therefore, it is likely that T. erythraeum may release more of its fixed DDN to the surrounding environment and be less efficient in using its own fixed DDN.
After measuring DDN release, we did not find more DDN present in the dissolved phase in the T. erythraeum−Synechococcus co-culture compared to the C. watsonii−Synechococcus co-culture. The rate of DDN release was similar between the two species (Figure 2), even though the volumetric N2 fixation rate of T. erythraeum was twice as high as that of C. watsonii. Even when accounting for the DDN transferred to Synechococcus, the total amount of DDN excreted by T. erythraeum was similar to that of C. watsonii (Figure 2). However, the DDN excreted by T. erythraeum was more efficient in supporting Synechococcus cell growth in the co-cultures (Figure 2; Table 1). We propose that fixed N derived from T. erythraeum was likely more bioavailable for Synechococcus than that from C. watsonii. This was supported by the significantly higher DDN transfer efficiency of T. erythraeum (0.36%) compared to C. watsonii (< 0.01%) (Figure 2). It was also reported that in artificially induced blooms in the western South Pacific, a T. erythraeum bloom resulted in a significantly higher 15NH4+ enrichment in the dissolved pool than a C. watsonii bloom (Berthelot et al., 2016), suggesting a higher bioavailability of the fixed N released from T. erythraeum. Therefore, DDN released by T. erythraeum can contribute significantly to primary production by supporting the growth of non-diazotrophic phytoplankton (Campbell et al., 2005).
For the smaller unicellular C. watsonii, although the active release of its DDN played a minor role in supporting the growth of non-diazotrophic phytoplankton, it is more easily grazed by zooplankton than T. erythraeum and resulted in a more passive release of DDN (Caffin et al., 2018). Nevertheless, our co-culture systems provided a useful approach to compare the active DDN release and transfer by the two different diazotrophs. It should be noted that our observations of DDN release and transfer were the results obtained for 24-h of incubation after 15N enrichment. The turnover time of DON could be longer than 24-h (Bronk et al., 2007), therefore, the efficiency of DDN transfer may have been underestimated in our study systems.
Our study demonstrated the significant role of light intensity in controlling the release and transfer of DDN fixed by T. erythraeum. It was shown that T. erythraeum supported higher Synechococcus growth under a high light intensity of 750 μE m⁻² s⁻¹ (Table 1), which aligned well with the increased percentage of total DDN released to the dissolved pool and transferred to Synechococcus (Figure 3). Additionally, although even higher light intensities, e.g., 1100 and 1800 μE m⁻² s⁻¹, inhibited the growth and N2 fixation of T. erythraeum, they further increased the portion of fixed N released to the dissolved pool (Figure 4). It has been proposed that that the exudation of DDN in the form of NH4+ and DON could serve as a potential electron sink to protect cells from photo-damage (Wannicke et al., 2009).
However, previous field incubation experiments have found that the fraction of DDN in the dissolved phase increased when the light intensity decreased to a very low level, e.g., 15 μE m⁻² s⁻¹ (Lu et al., 2018). We found no significant difference in the fraction of DDN in the dissolved phase among the three light treatments of 70, 200, and 750 μE m⁻² s⁻¹. The DDN in the dissolved phase was the net result of diazotroph DDN excretion and uptake by phytoplankton. Light intensity not only affects the DDN excretion rate, but also the phytoplankton N uptake rate (Wannicke et al., 2009; Han et al., 2023). Under a very low light intensity, such as 15 μE m⁻² s⁻¹, although diazotrophs could decrease DDN excretion, phytoplankton may be short of energy for N uptake, leading to an increase in percentage of DDN in the dissolved fractions (Lu et al., 2018; Han et al., 2023). These studies highlight the important role of light intensity in controlling the diazotrophic N2 fixation rate and DDN release rate, as well as phytoplankton N uptake rates. Our results using the simple and well-controlled co-culture system clearly showed how light intensity affects T. erythraeum N2 fixation, DDN release, and transfer.
Factors other than light can also affect diazotrophic DDN release. For example, iron and phosphorus limitation can affect the DDN release of Azotobacter vinelandii, T. erythraeum, or Nodularia (McRose et al., 2019; Wannicke et al., 2009; Schoffelen et al., 2019). Moreover, top-down controls such as viral lysis and grazing could stimulate passive DDN release and promote C export (Bonnet et al., 2016a; Kuznecova et al., 2020). Future studies should investigate the effects of these potential factors to obtain a comprehensive understanding of the fate and role of DDN in supporting marine primary production.
By establishing co-cultures of diazotrophs with non-diazotrophic pico-cyanobacteria Synechococcus sp., this study provided new insights into the release and transfer of fixed N by two different strains of diazotrophs, and then investigated the impact of light intensity on these processes. First, we demonstrated that under identical culture conditions, T. erythraeum had a significantly higher efficiency in supporting Synechococcus growth than C. watsonii. This was evidenced by the notably higher DDN transfer efficiency of T. erythraeum than C. watsonii, although the overall exudation efficiency of fixed N (DDN release plus DDN transfer) revealed no significant differences between these two species. We therefore proposed that the higher bioavailability of the released N from T. erythraeum contributed to the more efficient DDN transfer of Synechococcus. Second, we showed that the elevated light intensity significantly increased DDN release and transfer. With the future warming of oceans, intensified seawater stratification may increase phytoplankton light exposure and could therefore alter the fate of DDN in the marine ecosystem.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
XH: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. ZW: Conceptualization, Formal Analysis, Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Investigation. TL: Methodology, Writing – original draft. HH: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The work was supported by the National Natural Science Foundation of China (42421004), the National Key Research and Development Program of China (2023YFF0805004, 2022YFE0136600), the National Natural Science Foundation of China (41925026, 42106041) and the PhD Fellowship of the State Key Laboratory of Marine Environmental Science at Xiamen University. Oligotrophic western North Pacific surface seawater for culturing was collected onboard of R/V Tan Kah Kee implementing the open research cruise NORC2022-306 supported by NSFC Shiptime Sharing Project (project number: 42149303).
We thank W. Lin and W. Zou for technical assistance with the analysis of PON and its isotopic composition.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1485853/full#supplementary-material
Baer S. E., Lomas M. W., Terpis K. X., Mouginot C., Martiny A. C. (2017). Stoichiometry of Prochlorococcus, Synechococcus, and small eukaryotic populations in the western North Atlantic Ocean. Environ. Microbiol. 19, 1568–1583. doi: 10.1111/1462-2920.13672
Bell P. R. F., Fu F. X. (2005). Effect of light on growth, pigmentation and N2 fixation of cultured Trichodesmium sp. from the Great Barrier Reef lagoon. Hydrobiologia 543, 25–35. doi: 10.1007/s10750-004-5713-2
Benavides M., Agawin N. S. R., Arístegui J., Peene J., Stal L. J. (2013a). Dissolved organic nitrogen and carbon release by a marine unicellular diazotrophic cyanobacterium. Aquat Microb. Ecol. 69, 69–80. doi: 10.3354/ame01621
Benavides M., Bronk D. A., Agawin N. S. R., Pérez-Hernández M. D., Hernández-Guerra A., Arístegui J. (2013b). Longitudinal variability of size-fractionated N2 fixation and DON release rates along 24.5°N in the subtropical North Atlantic. J. Geophy Res: Oceans 118, 3406–3415. doi: 10.1002/jgrc.20253
Berman-Frank I., Bidle K. D., Haramaty L., Falkowski P. (2004). The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway. Limnol Oceanogr 49, 997–1005. doi: 10.4319/lo.2004.49.4.0997
Berman-Frank I., Lundgren P., Falkowski P. (2003). Nitrogen fixation and photosynthetic oxygen evolution in cyanobacteria. Res. Microbiol. 154, 157–164. doi: 10.1016/s0923-2508(03)00029-9
Berthelot H., Benavides M., Moisander P. H., Grosso O., Bonnet S. (2017). High-nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas). Geophys Res. Lett. 44, 8414–8423. doi: 10.1002/2017gl073856
Berthelot H., Bonnet S., Camps M., Grosso O., Moutin T. (2015). Assessment of the dinitrogen released as ammonium and dissolved organic nitrogen by unicellular and filamentous marine diazotrophic cyanobacteria grown in culture. Front. Mar. Sci. 2. doi: 10.3389/fmars.2015.00080
B]erthelot H., Bonnet S., Grosso O., Cornet V., Barani A. (2016). Transfer of diazotroph-derived nitrogen towards non-diazotrophic planktonic communities: a comparative study between Trichodesmium erythraeum, Crocosphaera watsonii and Cyanothece sp. Biogeosci 13, 4005–4021. doi: 10.5194/bg-13-4005-2016
Bonnet S., Baklouti M., Gimenez A., Berthelot H., Berman-Frank I. (2016a). Biogeochemical and biological impacts of diazotroph blooms in a low-nutrient, low-chlorophyll ecosystem: Synthesis from the VAHINE mesocosm experiment (New Caledonia). Biogeosci 13, 4461–4479. doi: 10.5194/bg-13-4461-2016
Bonnet S., Berthelot H., Turk-Kubo K., Fawcett S., Rahav E., Stéphane L’Helguen, et al. (2016b). Dynamics of N2 fixation and fate of diazotroph-derived nitrogen in a low-nutrient, low-chlorophyll ecosystem: Results from the VAHINE mesocosm experiment (New Caledonia). Biogeosci 13, 2653–2673. doi: 10.5194/bg-13-2653-2016
Bonnet S., Berthelot H., Turk-Kubo K. A., Cornet-Barthaux V., Fawcett S., Berman-Frank I., et al. (2016c). Diazotroph derived nitrogen supports diatom growth in the South west Pacific: A quantitative study using NanoSIMS. Limnol Oceanogr 61, 1549–1562. doi: 10.1002/lno.10300
Braman R. S., Hendrix S. A. (1989). Nanogram nitrite and nitrate determination in environmental and biological materials by vanadium (III) reduction with chemiluminescence detection. Analytic Chem. 61, 2715–2718. doi: 10.1021/ac00199a007
Breitbarth E., Wohlers J., Klas J., LaRoche J., Peeken I. (2008). Nitrogen fixation and growth rates of Trichodesmium IMS101 as a function of light intensity. Mar. Ecol. Prog. Ser. 359, 25–36. doi: 10.3354/meps07241
Bronk D. A., See J. H., Bradley P., Killberg L. (2007). DON as a source of bioavailable nitrogen for phytoplankton. Biogeosci 4, 283–296. doi: 10.5194/bg-4-283-2007
Browning T. J., Moore C. M. (2023). Global analysis of ocean phytoplankton nutrient limitation reveals high prevalence of co-limitation. Nat. Commun. 14, 5014. doi: 10.1038/s41467-023-40774-0
Caffin M., Berthelot H., Cornet-Barthaux V., Bonnet S. (2018). Transfer of diazotroph-derived nitrogen to the planktonic food web across gradients of N2 fixation activity and diversity in the Western Tropical South Pacific. Biogeosci 15, 1–32. doi: 10.5194/bg-2017-572
Campbell L., Carpenter E. J., Montoya J. P., Kustka A. B., Capone D. G. (2005). Picoplankton community structure within and outside a Trichodesmium bloom in the southwestern Pacific Ocean. Vie Milieu/Life Environ. 55, 185–195.
Capone D. G., Zehr J. P., Paerl H. W., Badger M. R., Carpenter E. J. (1997). Trichodesmium, a globally significant marine cyanobacterium. Science 276, 1221–1229. doi: 10.1126/science.276.5316.1221
Demarsac N. T., Houmard J. (1988). Complementary chromatic adaptation – Physiological conditions and action spectra. Method Enzymol. 167, 318–328. doi: 10.1016/0076-6879(88)67037-6
Flombaum P., Gallegos J. L., Gordillo R. A., Rincon J., Zabala L. L., Jiao N., et al. (2013). Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proc. Natl. Acad. Sci. United States America 110, 9824–9829. doi: 10.1073/pnas.1307701110
Gruber N., Galloway J. N. (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296. doi: 10.1038/nature06592
Hama T., Miyazaki T., Ogawa Y., Iwakuma T., Takahashi M., Otsuki A., et al. (1983). Measurement of photosynthetic production of a marine phytoplankton population using a stable 13C isotope. Mar. Biol. 73, 31–36. doi: 10.1007/BF00396282
Han Q. Y., Qiu C. Y., Zeng W. X., Chen Y., Zhao M. Q., Shi Y. F., et al. (2023). Effect of DIN and DON sources on the nitrogen uptake of the seagrass and the macroalgae previously grown in different light levels. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1015323
Hewson I., Govil S. R., Capone D. G., Carpenter E. J., Fuhrman J. A. (2004). Evidence of Trichodesmium viral lysis and potential significance for biogeochemical cycling in the oligotrophic ocean. Aquat Microb. Ecol. 36, 1–8. doi: 10.3354/ame036001
Hong H., Shen R., Zhang F., Wen Z., Chang S., Lin W., et al. (2017). The complex effects of ocean acidification on the prominent N2-fixing cyanobacterium Trichodesmium. Science 356, 527–531. doi: 10.1126/science.aal2981
Knapp A. N., Dekaezemacker J., Bonnet S., Sohm J. A., Capone D. G. (2012). Sensitivity of Trichodesmium erythraeum and Crocosphaera watsonii abundance and N2 fixation rates to varying NO3– and PO43– concentrations in batch cultures. Aquat Microb. Ecol. 66, 223–236. doi: 10.3354/ame01577
Knapp A. N., Sigman D. M., Lipschultz F. (2005). N isotopic composition of dissolved organic nitrogen and nitrate at the Bermuda Atlantic time-series Study site. Global Biogeochem Cycle 19, GB1018. doi: 10.1029/2004gb002320
Konno U., Tsunogai U., Komatsu D. D., Daita S., Nakagawa F., Tsuda A., et al. (2010). Determination of total N2 fixation rates in the ocean taking into account both the particulate and filtrate fractions. Biogeosci 7, 2369–2377. doi: 10.5194/bg-7-2369-2010
Kuznecova J., Šulčius S., Vogts A., Voss M., Jürgens K., Šimoliūnas E. (2020). Nitrogen flow in diazotrophic cyanobacterium Aphanizomenon flos-aquae is altered by cyanophage infection. Front. Microbiol. 11. doi: 10.3389/fmicb.2020.02010
Lu Y., Wen Z., Shi D., Chen M., Zhang Y., Bonnet S., et al. (2018). Effect of light on N2 fixation and net nitrogen release of Trichodesmium in a field study. Biogeosc 15, 1–12. doi: 10.5194/bg-15-1-2018
Masuda T., Inomura K., Kodama T., Shiozaki T., Kitajima S., Armin G., et al. (2022). Crocosphaera as a major consumer of fixed nitrogen. Microbiol. Spectr. 10, e0217721. doi: 10.1128/spectrum.02177-21
Masuda T., Mareš J., Shiozaki T., Inomura K., Fujiwara A., Prášil O. (2024). Crocosphaera watsonii – A widespread nitrogen-fixing unicellular marine cyanobacterium. J. Phycol 60, 604–620. doi: 10.1111/jpy.13450
McRose D. L., Lee A., Kopf S. H., Baars O., Kraepiel A. M. L., Sigman D. M., et al. (2019). Effect of iron limitation on the isotopic composition of cellular and released fixed nitrogen in Azotobacter vinelandii. Geochimica Et Cosmochimica Acta 244, 12–23. doi: 10.1016/j.gca.2018.09.023
Mills M. M., Ridame C., Davey M., La Roche J., Geider R. J. (2004). Iron and phosphorus co-limit nitrogen fixation in the eastern tropical North Atlantic. Nature 429, 292–294. doi: 10.1038/nature02550
Mohr W., Großkopf T., Wallace D. W., LaRoche J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PloS One 5, e12583. doi: 10.1371/journal.pone.0012583.g001
Montoya J. P., Voss M., Kähler P., Capone D. G. (1996). A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl. Environ. Microbiol. 62, 986–993. doi: 10.1128/aem.62.3.986-993.1996
Moore C. M., Mills M. M., Arrigo K. R., Berman-Frank I., Bopp L., Boyd P. W., et al. (2013). Processes and patterns of oceanic nutrient limitation. Nat. Geosci 6, 701–710. doi: 10.1038/Ngeo1765
Mulholland M. R. (2007). The fate of nitrogen fixed by diazotrophs in the ocean. Biogeosci 4, 37–51. doi: 10.5194/bg-4-37-2007
Mulholland M. R., Bronk D. A., Capone D. G. (2004). Dinitrogen fixation and release of ammonium and dissolved organic nitrogen by Trichodesmium IMS101. Aquat Microb. Ecol. 37, 85–94. doi: 10.3354/ame037085
ONeil J. M., Metzler P. M., Glibert P. M. (1996). Ingestion of 15N2-labelled Trichodesmium spp and ammonium regeneration by the harpacticoid copepod Macrosetella gracilis. Mar. Biol. 125, 89–96. doi: 10.1007/Bf00350763
Schoffelen N. J., Mohr W., Ferdelman T. G., Duerschlag J., Littmann S., Ploug H., et al. (2019). Phosphate availability affects fixed nitrogen transfer from diazotrophs to their epibionts. Isme J. 13, 2701–2713. doi: 10.1038/s41396-019-0453-5
Shao Z., Xu Y., Wang H., Luo W., Wang L., Huang Y., et al. (2023). Global oceanic diazotroph database version 2 and elevated estimate of global oceanic N2 fixation. Earth Syst. Sci. Data 15, 3673–3709. doi: 10.5194/essd-15-3673-2023
Sigman D. M., Casciotti K. L., Andrieu M., Barford C., Galanter M., Böhlke J. K. (2001). A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Analyt Chem. 73, 4145–4153. doi: 10.1021/ac010088e
Sohm J. A., Webb E. A., Capone D. G. (2011). Emerging patterns of marine nitrogen fixation. Nat. Rev. Microbiol. 9, 499–508. doi: 10.1038/nrmicro2594
Wannicke N., Koch B. P., Voss M. (2009). Release of fixed N2 and C as dissolved compounds by Trichodesmium erythraeum and Nodularia spumigena under the influence of high light and high nutrient (P). Aquat Microb. Ecol. 57, 175–189. doi: 10.3354/ame01343
Wen Z., Browning T. J., Cai Y., Dai R., Zhang R., Du C., et al. (2022). Nutrient regulation of biological nitrogen fixation across the tropical western North Pacific. Sci. Adv. 8, eabl7564. doi: 10.1126/sciadv.abl7564
Yu X., Wen Z., Jiang R., Yang J.-Y. T., Cao Z., Hong H., et al. (2024). Assessing N2 fixation flux and its controlling factors in the (sub)tropical western North Pacific through high-resolution observations. Limnol Oceanogr Lett 9, 716–724. doi: 10.1002/lol2.10404
Keywords: Trichodesmium, Crocosphaera, DDN release, DDN transfer, light
Citation: Hu X, Wen Z, Luo T and Hong H (2025) Diazotroph-derived nitrogen release and transfer under varying light intensity: insights from co-culture studies. Front. Mar. Sci. 12:1485853. doi: 10.3389/fmars.2025.1485853
Received: 25 August 2024; Accepted: 10 February 2025;
Published: 27 February 2025.
Edited by:
Eric A. Webb, University of Southern California, United StatesCopyright © 2025 Hu, Wen, Luo and Hong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haizheng Hong, aG9uZ2h6QHhtdS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.