- 1Department of Engineering and Architecture, University of Trieste, Trieste, Italy
- 2Fratelli d’Amico Armatori Spa, Rome, Italy
Climate change poses a global challenge related to the reduction of pollutant atmospheric emissions and the maritime transportation sector is directly involved, due to its significant impact on the production of Greenhouse Gases and other substances. While established technologies have effectively targeted emissions like Nitrogen Oxides (NOX) and Sulfur Oxides (SOX), the persistence of Carbon dioxide (CO2) emissions represents an ongoing and significant concern. Novel technologies targeting CO2 reduction have been lately studied and proposed for inland applications, and are now being developed for maritime applications. With this regard, the present study explores the potential of Carbon Capture Systems (CCS) to mitigate CO2 emissions produced by cargo ships. While the implementation of CCS faces challenges, including space limitations and logistical complexities, its possible integration onboard marks a significant step in the fight against climate change. The authors propose an innovative approach using a Calcium Hydroxide Ca(OH)2 based CCS, offering the dual benefit of CO2 reduction and the potential resolution of ocean acidification through Calcium carbonate (CaCO3), the final product resulting from the CO2 capture process. Additionally, the study examines the feasibility of the generated product for reuse in industry, promoting a circular economy and addressing environmental issues. This innovative solution underscores the urgent need for transformative measures to reduce maritime emissions, in line with efforts to safeguarding the marine environment and combat climate change.
1 Introduction
The effects of climate change emerge as a significant global challenge to be addressed. According to research (United Nations, 2023), over 80% of international cargo is transported by ships, which are responsible for about 3% of all global Greenhouse Gas (GHG) emissions. This is set to grow if no action is taken (IMO, 2020).
Specifically, ships are a substantial source of emissions released into the atmosphere, including Carbon Dioxide (CO2), Nitrogen Oxides (NOX), Carbon Monoxide (CO), Non-Methane Volatile Organic Compounds (NMVOC), Particulate Matter (PM), Sulphur Dioxide (SO2), and methane (CH4) (Shu et al., 2023; Aakko-Saksa et al., 2023).
While technologies such as Selective Catalytic Reduction (SCR), Scrubbers and Exhaust Gas Recirculation (EGR), are already established and proven to reduce NOX and SOX emissions, as reported by (Lehtoranta et al., 2015; Karatuğ et al., 2022; Andriiovych Kuropyatnyk and Victorovych Sagin, 2019), the situation differs concerning CO2 emissions.
Moreover, among these emissions, it is noteworthy that CO2 is the primary contributor to global warming (Yoro and Daramola, 2020). The escalating levels of CO2 have particularly led to a rise in global average temperatures, resulting in significant climate changes, including extreme weather events, alterations in precipitation patterns, and shifts in ecosystems (Nunes, 2023).
The consequences of CO2 emissions extend to the melting of polar ice caps and icebergs, contributing to rising sea levels that pose a direct threat to coastal communities and marine habitats (Durand et al., 2022).
Furthermore, the absorption of a substantial portion of emitted CO2 by the oceans leads to ocean acidification resulting in a decline of calcium carbonate (CaCO3) saturation state, causing a reduction in oceanic pH levels and severe repercussions for marine organisms (Kurihara, 2008; Jafari et al., 2023).
In order to address these pressing issues, regulations have been introduced to limit pollution emissions from ships. The International Maritime Organization (IMO) has implemented regulatory measures with the overarching goal of attaining net-zero GHG emissions from the shipping sector by or around 2050. Figure 1 illustrates essential regulatory measures and implementation support initiatives crucial for achieving the IMO’s emission reduction objectives (IMO, 2023).
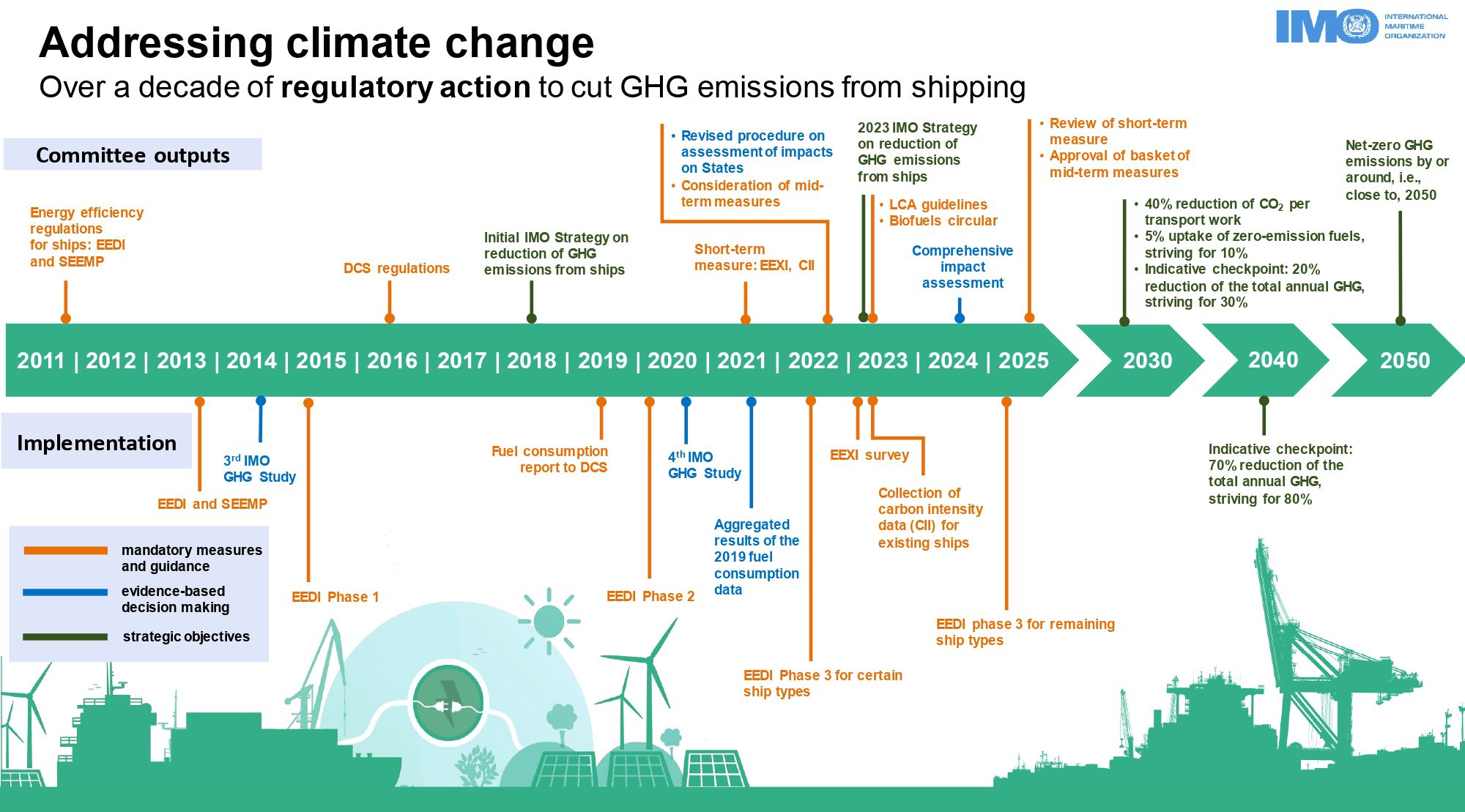
Figure 1. Timeline of IMO regulatory action to cut GHG emissions from shipping (IMO, 2023).
At the European level, the European Union (EU) has integrated shipping into the EU Emissions Trading System (ETS). Within this regulatory framework, shipping companies have to make financial contributions based on the emissions of CO2 they reported in the preceding year, for voyages and port stays within the EU/European Economic Area (EEA). In 2025, shipowners’ companies will be accountable for 40% of the emissions reported in 2024; this obligation will increase to 70% of their 2025 emissions in 2026. Starting from 2027, shipping companies will bear the entire cost, covering 100% of their reported emissions (DNV, 2023; European Commission, 2023).
Compliance with regulatory measures is imperative for the shipping industry, necessitating significant investments in technologies and alternative fuels (Balcombe et al., 2019). However, shipowners show less inclination to order new ships especially due to the uncertainties related to fuel technology and bunker costs. These factors significantly impact the decision-making process regarding solutions to reduce emissions for new ships (Zhang et al., 2021) and despite an ageing fleet, shipowners have opted to sell and purchase second-hand vessels. Because of this, while the transition to alternative fuels in the shipping industry is underway, the number of vessels operating on traditional fuels remains and is projected to be significantly high in the coming years, as evidenced by an analysis of the orderbook (Figure 2) (United Nations, 2023).
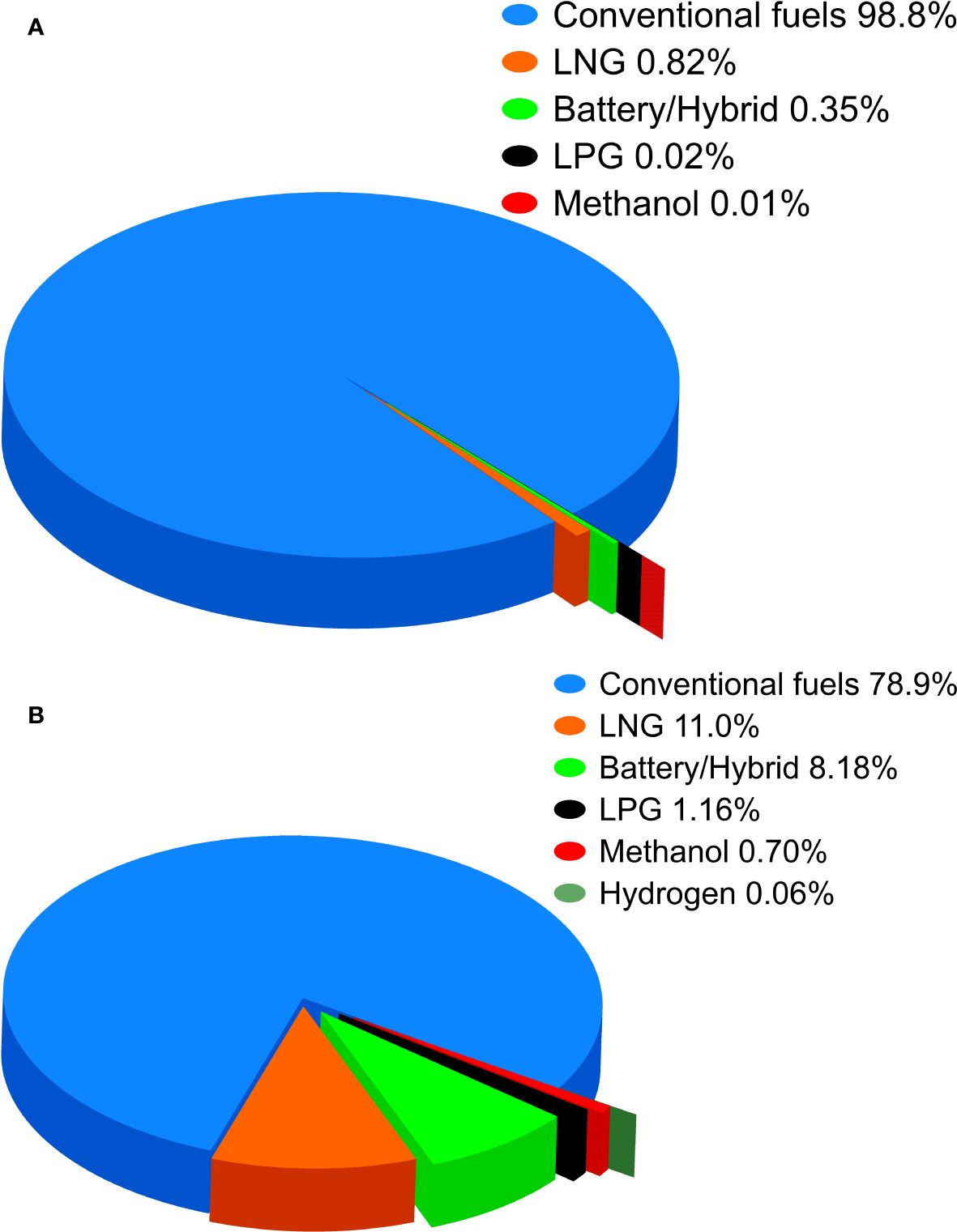
Figure 2. Percentage of vessels, in 2022 (A) and in orderbook (B), running with conventional and alternative fuels.
It emerges that the current fleet is predominantly composed of ships running on traditional fuels, and the prevailing order book continues to prioritize traditional fuel options. Therefore, the transition to low- and zero-impact alternative fuels is not imminent, and the maritime sector needs a technological shift to mitigate emissions. Postponing the adoption of technologies and fuels leading to climate neutrality to be fully ready is not feasible, as it would result in addressing a larger volume of emissions within a shorter timeframe (Galán-Martín et al., 2021).
Shipowners are compelled to promptly invest in onboard green technologies, given their significant influence on the emission profile of the global shipping fleet and its capacity to adhere to the GHG targets set by the IMO (Piccolo, 2023). Within this framework, it is imperative to discern the optimal solution for regulatory compliance that contributes to the immediate reduction of environmental emissions.
From the analysis of current regulations and future compliance measures in the shipping sector, aimed at addressing the challenges of climate change, CCSs emerge as a promising solution to immediately mitigate a significant amount of CO2 emissions, while facilitating a gradual transition to more sustainable energy sources like renewables (Hua et al., 2023; Tavakoli et al., 2024).
This technology employs different types of chemical and physical processes for capturing and storing CO2 emissions produced by the combustion of fossil fuel sources before they are released into the atmosphere and has proven effective for various inland industries (Leeson et al., 2017; Witte, 2021).
Nevertheless, implementing CCS onboard ships presents challenges due to limited space and stringent operational requirements. Additionally, the logistics of transporting captured CO2 from ships to permanent storage sites can be complex. This process requires specialized equipment, infrastructure, and adherence to strict safety and environmental regulations to ensure the secure and effective storage of CO2 (Al Baroudi et al., 2021).
Despite these challenges, the introduction of CCS technology in the maritime sector represents a significant innovation in combating climate change, aligning with international efforts towards decarbonization.
In this framework, although there are various studies on the implementation of this technology on land (Abanades et al., 2023; Han et al., 2011), the authors aim at addressing the challenges associated with its potential implementation onboard as well as the issues of CO2 storage and transportation. To this purpose, they propose a methodology to evaluate the main features of such innovative solution never tested onboard nor yet investigated in literature. This methodology enables a preliminary sizing of the CCS, which lacks established naval applications, through stoichiometric considerations. By employing relatively quick calculations, the method facilitates a preliminary assessment of the technology’s impact on the reference vessel, providing initial dimensional estimates that allow for an understanding of whether the system can be installed onboard and, subsequently, its broader impact on the vessel’s operations. Building on this methodology, the feasibility assessment for retrofitting an existing vessel design with a CCS with Calcium Hydroxide is conducted, with particular attention given to system-level integration and operational challenges rather than the detailed design of the CO2 scrubber itself. This system, as previously examined by the authors (Bortuzzo et al., 2023), provides distinct advantages over other types of CCSs. Notably, it simplifies onboard logistics by eliminating the need for CO2 liquefaction unit and the additional energy required for its operation (Risso et al., 2023).
The objective of the system implementation is to directly reduce CO2 emissions from ships while concurrently generating a product, CaCO3. This approach aligns with ocean alkalinity enhancement strategies, such as adding lime directly to seawater, which aim at mitigating atmospheric CO2 by enhancing the ocean’s natural carbon absorption capacity (Butenschön et al., 2021; Caserini et al., 2021a, 2022; Comes et al., 2024). The proposed CCS not only reduces CO2 emissions but also transforms the captured CO2 into calcium carbonate, which can be released into seawater to increase alkalinity and counteract ocean acidification. Unlike direct lime addition, which faces logistical challenges such as transportation and distribution, this system could offer a more sustainable and practical alternative.
The most relevant outcomes obtained from the study and their direct correlation with CO2 emission reduction within the maritime transportation sector and benefits for the marine environment are properly discussed.
2 Regulatory framework
To achieve zero emissions from ships promptly and protect the environment, IMO has adopted a strategy based on setting milestone GHG emissions reduction targets for the industry and aiming for a 20% - 30% reduction by 2030 to reach a remarkable reduction of 70% - 80% by 2040.
In this framework, ensuring the swift adoption of new technologies and alternative zero and near-zero GHG fuels to mitigate vessel emissions relies on two main strategies (IMO, 2023):
1. Developing standards for phased GHG emissions reduction: this involves establishing regulations to systematically decrease shipping GHG emissions over time:
The introduction of the Carbon Intensity Indicator (CII) by the IMO is part of these new regulations. The CII is an indicator that measures the carbon intensity of a ship’s operations, offering valuable insights into its environmental performance. It calculates CO2 emissions per unit of transport work, facilitating the comparison of emission efficiency between different routes, ship types and operating methods. By implementing the CII, regulators, shipping companies and stakeholders can effectively monitor and regulate carbon emissions in the maritime industry. However, this index would require some modifications, as highlighted by the study (Braidotti et al., 2023), due to inconsistencies between emissions and CII values, leading vessels to take routes with a higher environmental footprint. Furthermore, the gradual reduction of greenhouse gas emissions from shipping involves setting carbon intensity reduction targets over time. These targets aim to progressively decrease emission intensity by encouraging the adoption of cleaner fuels, energy efficient technologies and operational practices in the maritime sector.
2. Implementing maritime GHG emissions pricing mechanisms: introducing emissions taxes can incentivize immediate adoption of emission-reducing solutions for vessels.
A similar pricing mechanism has already been introduced by the European Union, incorporating shipping companies into the EU ETS. Shipping companies are required to by allowances on ETS for the 100% of emissions generated on voyages and port stays within the EU/European Economic Area (EEA), and 50% of emissions from voyages into or out of the EU/EEA. To ensure a gradual approach in 2025, they will pay 40% of reported emissions in 2024; in 2026, they will pay 70% of 2025 emissions; and from 2027, they will pay 100% of reported emissions. Such a pricing mechanism compels shipowners to minimize this additional cost while simultaneously fostering sustainable development to maintain their competitive edge in the market.
The challenge within this context lies in finding the best solution to meet current regulations while also complying with stricter limits in the future.
Therefore, it is important to gain a deep understanding of the technological roadmap. Currently, several initiatives are aligned with the International Maritime Organization’s objectives, such as the Norway-IMO Green Voyage 2050 Project, the EU-IMO GMN Project, the Republic of Korea-IMO GHG SMART Project, and the Singapore-IMO NEXT GEN Project (IMO, 2022), which aim to transform the shipping industry towards a low GHG emission future.
Shipping will undoubtedly need new technologies, new fuels, and innovation to meet the GHG targets (Bertagna et al., 2023). A promising technology under research involves the adoption of CCS onboard ships. These innovative systems have the potential to achieve substantial reductions in CO2 emissions produced by vessels. Key advantages include the possibility of reusing captured CO2, addressing compliance standards effectively, and achieving significant emissions reductions that may not be feasible with other existing technologies or fuels across the entire global fleet. This technology could be applied to both new and existing vessels and in particular the retrofitting of older units could improve their long-term economic viability by substantially reducing emissions.
So, as detailed above, IMO’s regulatory framework aims to reduce shipping GHG emissions by setting targets for 2030 and 2040, through strategies like phased emission reductions and emissions pricing mechanisms. These efforts are supported by initiatives and technologies such as CCS and the CII to promote cleaner fuels, energy efficiency, and sustainable shipping practices.
3 Impact of carbon capture systems on the marine environment
3.1 Technology description
Currently, existing technologies are not yet able to fully address CO2 emissions, despite significant progress in assessing the feasibility of implementing CCS onboard ships. These systems are designed to capture a significant percentage of CO2 emitted from fuel combustion thereby mitigating emissions into the atmosphere.
However, current research literature highlights technologies that require the CO2 liquefaction, onboard storage, and its disposal in ports, posing various challenges as outlined below (Visonà et al., 2024; Sridhar et al., 2024; Hua et al., 2023). In this context, the authors explore an alternative CCS tailored for onboard application, distinct from commonly encountered systems. This system, which eliminates the need for liquefaction and onboard storage, not only overcomes existing challenges but also offers additional advantages.
In fact, this approach signifies a concrete commitment to reducing emissions but also underscores the increasing awareness and urgency surrounding the imperative challenge of achieving decarbonization by 2050.
The amount of reduction achievable with CCS depends on their efficiency, expressed in %, as outlined in Equation 1 (Taipabu et al., 2023).
where CO2, in represents the total quantity of CO2 entering the system in a gaseous state, and CO2, out denotes the amount exiting the system after treatment, also in a gaseous state.
Based on the preliminary studies conducted by the authors (Bortuzzo et al., 2023), achieving a CCS efficiency of 25% appears feasible through retrofitting without compromising onboard space. While higher percentage of CO2 reductions could be achievable, especially through retrofit approaches involving reallocating space for larger CCS at the expense of other functions like cargo storage, opting for a 25% reduction aims to sidestep the necessity of compromising onboard space and to streamline the retrofit procedure.
Various CCS proposals are currently under research, including those examined by the authors (Bortuzzo et al., 2023), defined as post-combustion systems. These systems intervene after the engine combustion process, focusing on exhaust gas treatment to reduce CO2 emissions. The technologies explored include:
a. Carbon capture systems with molten carbonate fuel cells (MCFC);
b. Carbon capture systems with amines;
c. Carbon capture systems with calcium hydroxide.
Following the investigation conducted on the operation of the aforementioned CCS, it has been observed that the output post-CO2 treatment in the MCFC and Amine systems poses challenges concerning onboard liquefaction of CO2 and subsequent discharge in ports. These challenges give rise to concerns regarding environmental impact and the development of suitable disposal methods.
The imperative need for an adequate storage facility in ports, capable of maintaining CO2 in liquid form, necessitates pressurization and freezing systems. Additionally, a robust infrastructure for the secure and reliable transportation of CO2 is indispensable. Therefore, a well-organized network of pipelines should be instituted to facilitate the conveyance of stored CO2 from ports to industries or for confinement within geological sites. For shorter distances and smaller quantities, alternative means such as truck or train transport can also be considered (Chen and Morosuk, 2021). It is noteworthy that the transportation of CO2 requires special attention due to the high pressures and extremely low temperatures involved (International Energy Agency, 2020). The selection of transportation mode is influenced by economic considerations and regulatory frameworks, significantly affecting the decision-making process.
CO2 could find different applications across various products and services, including direct utilization without chemical alteration and its conversion into valuable products. Presently, approximately 230 million tons of CO2 are utilized globally each year (International Energy Agency, 2020), predominantly in the fertilizer and oil and gas industries. Emerging technologies present opportunities to exploit CO2 in sectors such as fuels, chemicals, and construction materials. However, the full realization of these possibilities depends on political support and the reduction of energy costs.
An additional alternative involves the storage of CO2 by injecting it into a deep underground geological reservoir comprising porous rocks covered by an impermeable layer, sealing the reservoir to prevent CO2 migration upward and dispersion into the atmosphere. Various types of reservoirs are suitable, including deep saline formations and depleted oil and gas reservoirs with greater capacity. When CO2 is injected into a reservoir, it spreads through it, occupying porous spaces. Typically, the gas is pre-compressed to increase density and convert it into a liquid state. The reservoir must be at a depth exceeding 800 meters to maintain CO2 in a dense liquid state (Chen and Morosuk, 2021).
The overall technical capacity for underground CO2 storage worldwide is uncertain, especially for saline formations where further site characterization and exploration are needed but potentially vast. The total global storage capacity has been estimated between 8,000 and 55,000 gigatons (Gt) (International Energy Agency, 2020). Moreover, the existing offshore potential is substantial, particularly within 300 km from the coast and depths less than 300 meters, with an estimated capacity spanning from 2,000 to 13,000 Gt.
However, it becomes evident that transferring CO2 to the storage site requires additional energy, leading to increased pollutant emissions associated with this operation, alongside the environmental concerns already highlighted. Moreover, the investments required to develop CO2 confinement infrastructures demand significant resources, including those allocated for economic studies conducted by governments and stakeholders in the maritime and other relevant sectors.
Due to the problems highlighted above, the CCSs based on molten carbonate fuel cells and amine present a difficult implementation on-board ship and may not give origin to effective carbon capture in terms of reduced pollutant emissions.
On the other hand, the third CCS under consideration, involving Calcium Hydroxide, has the advantage of not encountering the challenge of CO2 liquefaction and thus avoids the aforementioned consequences. This system presents potential environmental benefits, which will be examined by the authors in the subsequent section.
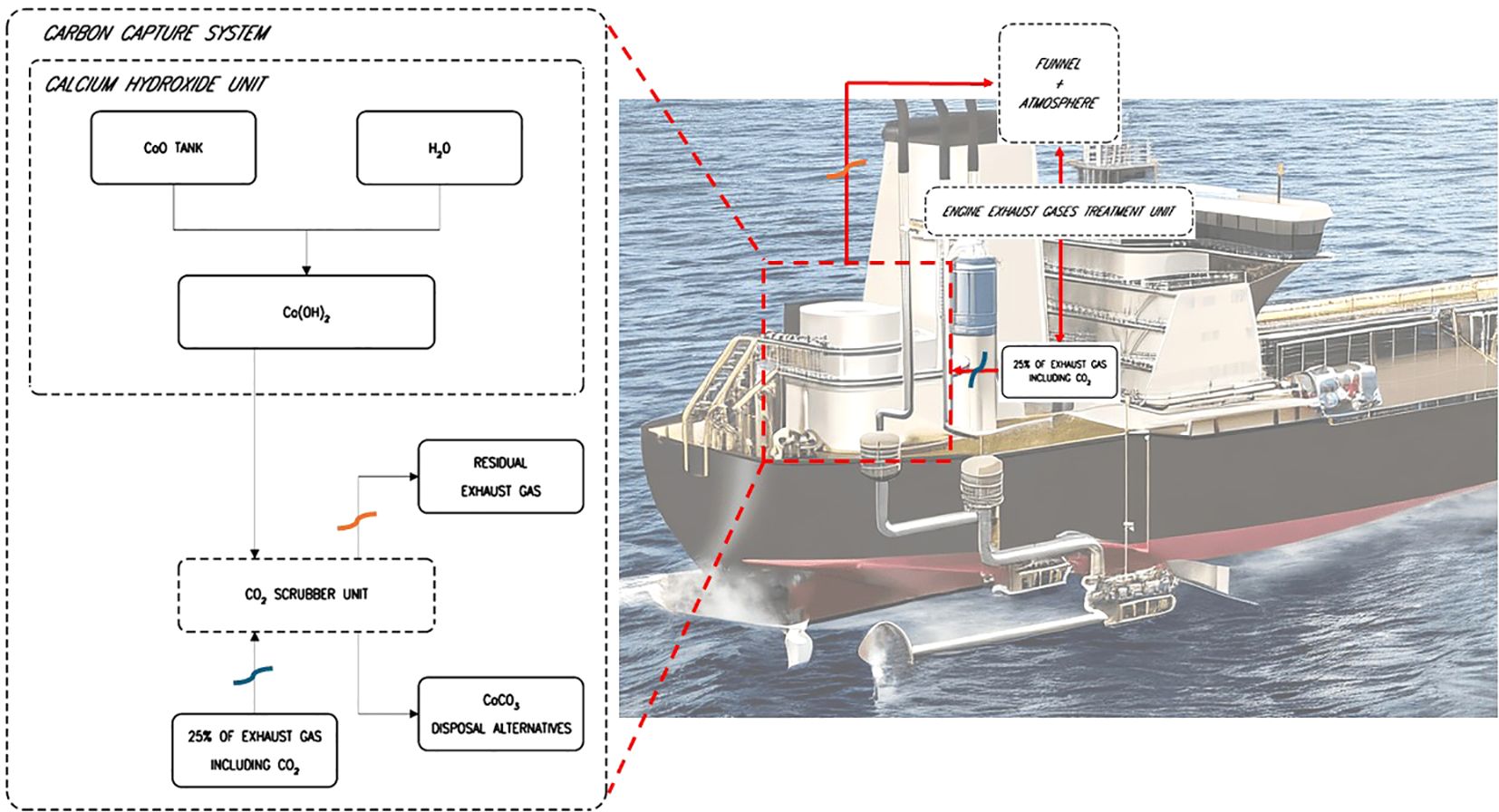
The operational principle of a CCS utilizing the Calcium Hydroxide is represented in Figure 3.
Specifically, the engine exhaust gas treatment unit is where exhaust gases are directed into an SOX scrubber. These gases then undergo a process where 25% are diverted to the CCS unit while the remainder is released into the funnel and into the atmosphere. Once the gases reach the CCS unit, they undergo a process outlined in the following steps (Bortuzzo et al., 2023):
1. initially, a quantity of calcium oxide (CaO), also known as quicklime, which is found in a solid state as a very fine powder, is extracted from a dedicated tank and reacted with H2O to form calcium hydroxide (Ca(OH)2):
This reaction occurs at specific temperatures that determine the purity of the produced Ca(OH)2. Studies indicate that the optimal temperature for the carbonation of Ca(OH)2 is approximately 200°C (Costagliola et al., 2022), ensuring better performance in absorbing CO2 in the subsequent reaction. The H2O used in this process is the same as that utilized in the SOX scrubber system.
2. Secondly, Ca(OH)2 is transferred to the designated scrubber for carbonation with CO2. Exhaust gases, including CO2, from the onboard engines are directed into this scrubber, and together with Ca(OH)2, they enable the capture of CO2 present in the gas stream. The product of carbonation is an inert inorganic compound, a suspension of CaCO3, which is a primary constituent of mollusk shells, along with water as a byproduct.
The choice to use slaking with Ca(OH)2 in onboard carbon capture systems, rather than direct injection of stack emissions into a CaO fluidized bed, involves several technical considerations. Slaking CaO to form Ca(OH)2 increases the surface area and reactivity of the sorbent, enhancing the capture efficiency of CO2 compared to using CaO directly (Ge et al., 2022). Additionally, the slaking process occurs at lower temperatures, which can be easier to manage onboard ships. Direct injection into a CaO fluidized bed requires maintaining high temperatures (around 650°C for carbonation and 950°C for calcination), which can be challenging in the variable conditions at sea. Using Ca(OH)2 also allows for more flexible operation, as the slaked lime can be stored and used as needed, whereas maintaining a fluidized bed requires continuous operation and precise control of gas flow and temperature (Valverde, 2018). However, the effectiveness of capturing CO2 can be influenced by temperature fluctuations, kinetics of the reactions, and impurities (Morse and Mackenzie, 1990; Zeebe and Wolf-Gladrow, 2001). Impurities like SOX and NOX can form stable compounds with calcium-based sorbents, decreasing their availability for CO2 capture and creating unwanted by-products (Lerman and Mackenzie, 2018). To mitigate the impact of impurities, the engine’s exhaust gas treatment unit includes an SOx scrubber capable of achieving a 98% reduction in sulfur oxides, placed upstream of the CO2 scrubber (Bortuzzo et al., 2024). As for NOx emissions, the lower combustion temperatures of a 2-stroke engine typically used onboard cargo ships minimize the risk of NOx interference with the CO2 scrubber, which is why the supplier does not include a Selective Catalytic Reduction (SCR) unit in the design. However, for other engine types where NOx emissions could pose a challenge, incorporating an SCR unit could be considered a viable solution to reduce NOx levels. These practices ensure that the CaCO3 formed is not impure and can potentially be discharged into the sea without causing pollution. In addition, reaction kinetics are affected by factors such as particle size, gas flow rates, and the partial pressure of CO2. Smaller particle sizes increase the surface area for reactions, enhancing capture efficiency, but maintaining optimal conditions for these variables can be challenging onboard ships. Stable operation of fluidized bed reactors is essential because variations in gas flow rates and temperature can lead to channeling or particle agglomeration, which reduces process efficiency (Zeebe and Wolf-Gladrow, 2001).
The CaCO3 obtained can be managed in two ways:
1. Storage in dedicated tanks onboard and subsequent disposal at ports;
2. Release overboard.
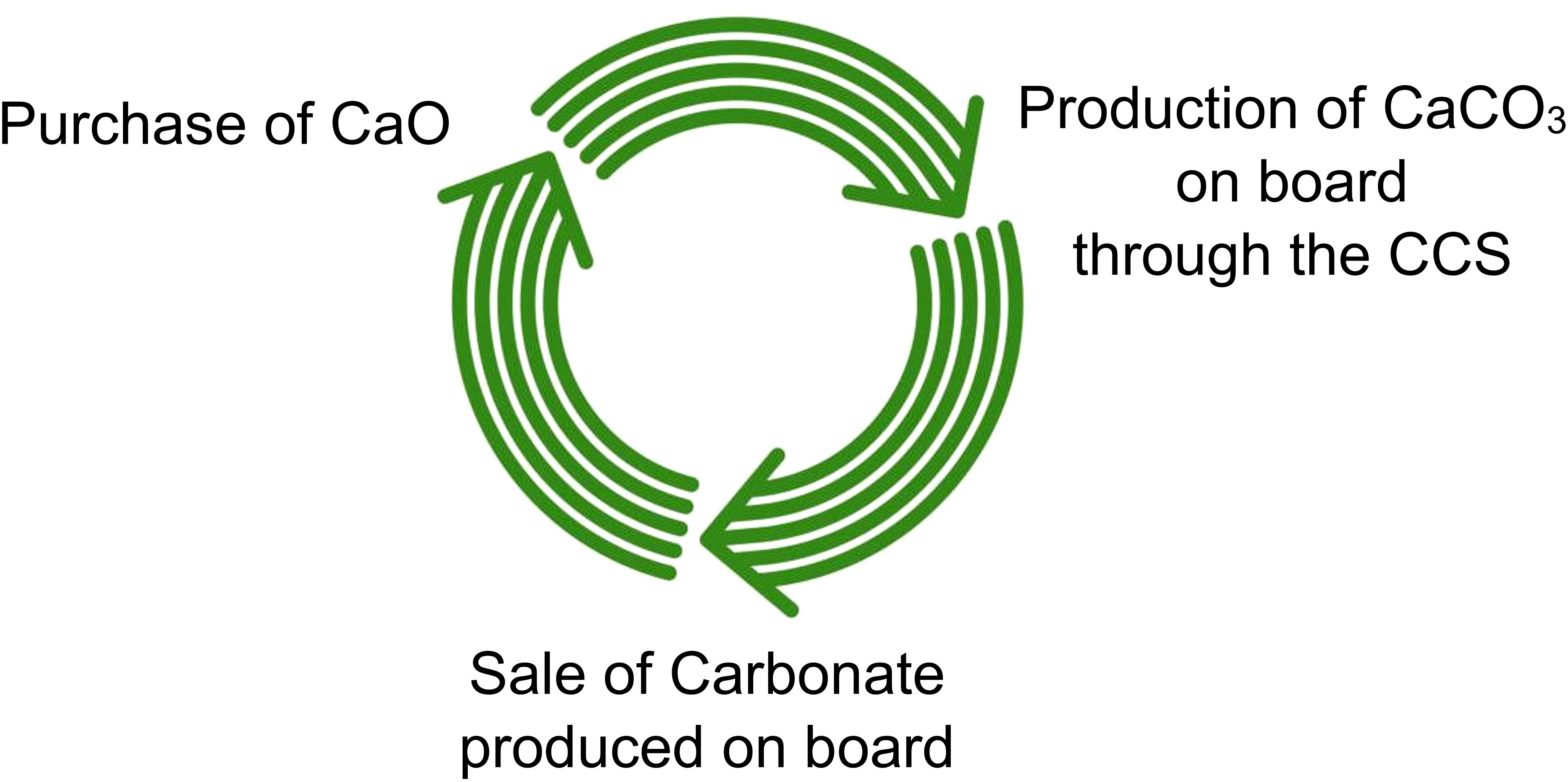
In the first scenario, a dedicated tank could be installed onboard for collecting CaCO3, which could then be disposed at ports for potential resale, thus creating a circular economy.
Indeed, the system described relies on CaO, which is stored on board in a dedicated tank. The production of CaO is attained through a process known as calcination. This essential thermochemical process consists in:
a. Raw material procurement: CaCO3 is extracted from quarries or limestone mines and ground into small particles;
b. Heating: subjecting CaCO3 to high temperatures, typically exceeding 900°C. CaCO3 thermally decomposes into CaO and CO2, according to the following chemical reaction:
Several studies are currently addressing how to avoid CO2 emissions from these land-based facilities and exploring potential mitigation strategies, which could serve as a pivotal benchmark for advancing carbon neutrality within the industry (Greco-Coppi et al., 2023; Wu et al., 2023). This is essential prerequisite to assure an effective application of CSS onboard, avoiding just relocation of shipborne emissions on land.
c. Cooling and Collection: Once the decomposition is complete, the material is cooled, and the final product, CaO, is collected for use in various applications.
Therefore, in this context, the initial step regarding raw material procurement could potentially change, considering the possibility of establishing circularity of the CaCO3 produced onboard the vessel through CCS with the producers of CaO, rather than sourcing it from quarries or limestone mines.
In the second scenario, releasing CaCO3 directly overboard, into the sea water, could potentially mitigate the increase in water acidity. In recent years, studies conducted on marine ecosystems have highlighted the repercussions of increased CO2 on the marine environment, leading to a phenomenon known as acidification (Hönisch et al., 2012). This process is caused by the absorption of atmospheric CO2 into seawater, where it reacts to form carbonic acid, thereby reducing the water’s pH. Marine acidification poses a serious threat to marine ecosystems, jeopardizing the survival of organisms such as corals and numerous fish species, as it can impede the formation of CaCO3 shells and other skeletal structures.
In this framework, the direct discharge of CaCO3 suspension, into water not only addresses pollution emission problems but also offers potential benefits, as it could help counteract the effects of ocean acidification, thus supporting marine ecosystems (Zhang et al., 2023). Some marine organisms can benefit from this process, as CaCO3 is utilized by many species to form and strengthen their shells or skeletons. This shell-building process involves the conversion of calcium carbonate into a solid structure through biological reactions (Rau, 2011). According to (Dupont and Pörtner, 2013), ocean acidification has already started and has the potential to significantly impact marine ecosystems. However, it is also essential to investigate how entire ecosystems will respond to acidification over long timescales.
(Rau and Caldeira, 1999) suggested a comparable approach where dissolving CaCO3 in seawater mixed with CO2 from industrial flue gases could increase ocean alkalinity. However, a significant drawback of this approach is the large volume of seawater needed, with thousands of tons required for each ton of CO2 captured (Renforth and Henderson, 2017). Additionally, there is a need for careful management of ocean alkalinity enhancement processes to maximize CO2 removal while minimizing unintended environmental impacts. For instance, as highlighted by (Moras et al., 2024), smaller grain sizes of Mg(OH)2 dissolve more quickly but also promote faster CaCO3 precipitation, which can reduce CO2 capture efficiency by causing CO2 outgassing.
Overall, implementing a CCS onboard a ship using calcium hydroxide involves the release of several tons of calcium carbonate in the form of suspension into the ocean. CaCO3 as a suspension form, poses no identified hazards. According to the IBC Code for bulk transport, it is classified as OS, and as Group C under the IMSBC Code for solid bulk transport. Since it is not listed among the prohibited wastes in Annex I and II of the London Convention, the disposal of a suspension of CaCO3 in the sea could be considered analogous to geological CO2 storage, resulting in an inert, non-toxic inorganic substance either suspended or deposited on the seabed. This could potentially be allowed based on the assessment outlined in Annex III of the same convention (RINA, 2023).
Once dispersed in the sea, CaCO3 suspension dissolves, helping to reduce water acidity (Harvey, 2008). The dissolution reaction is:
Currently, there are already practices of releasing CaCO3, for example, in various locations in the deep ocean, like in the northern Atlantic and near the Southern Ocean, where deep waters are rich in anthropogenic CO2 (Sulpis et al., 2021). Furthermore, CaCO3 dissolves more efficiently in water under higher pressure and lower temperature conditions and these factors vary considerably with the season and location in the surface layers (Caserini et al., 2021b).
Therefore, excluding some special restrictions that might be enforced in port or coastal areas, it can be reasonably expected that releasing CaCO3 into sea during navigation will be permitted or even incentivized by international bodies.
In summary, while innovative CCS solutions for onboard vessel applications are still under research, the use of calcium hydroxide for CO2 capture shows promise. This system avoids challenges like CO2 liquefaction and offers environmental benefits, including potential contributions to mitigating ocean acidification, while also addressing logistical and operational concerns for maritime emissions reduction.
3.2 Methodology for technology application onboard vessels
Given the absolute novelty of CCS with Calcium Hydroxide to be installed onboard a ship, its effective implementation is jeopardized by the lack of technical data coming from an actual case study. For such reason, the authors aim to provide a sound methodology that can allow sizing the system based on the chemical reactions, in order also to provide some reference data potentially useful for further technology developments.
The analyzed CCS is governed by the reaction Equations 2 and 3. To determine the quantities of CaO required for onboard storage on the vessel and thereby reduce CO2 emissions, a stoichiometric approach is utilized.
Equation 3 presents the stoichiometric ratio of the reactants as 1:1, indicating that the reactants act in equivalent molar quantities. By determining the amount of CO2 to be captured by the CCS, the number of moles of CO2 () can be calculated using the following equation:
where is the amount of CO2 (g/s), and is the molecular weight of CO2 (g/mol).
Subsequently, the number of moles of calcium carbonate () and calcium hydroxide () are calculated based on 1:1 stoichiometric ratio. The amount of calcium hydroxide () and calcium carbonate () are calculated:
Following the same procedure, the amount of CaO () and H2O () is determined starting from Equation 2. Since the reaction between CaO and H2O has a 1:1 stoichiometric ratio, the same number of moles calculated for CaO can be used to determine the amount of H2O used.
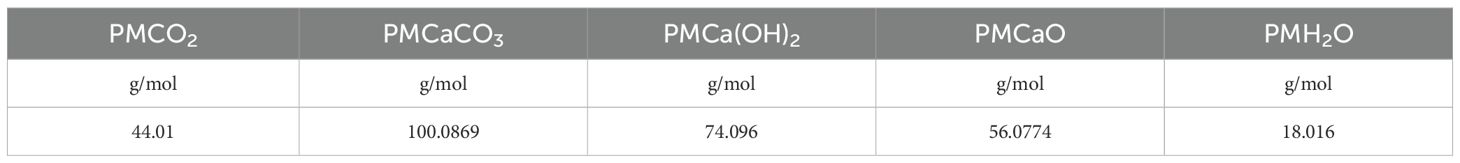
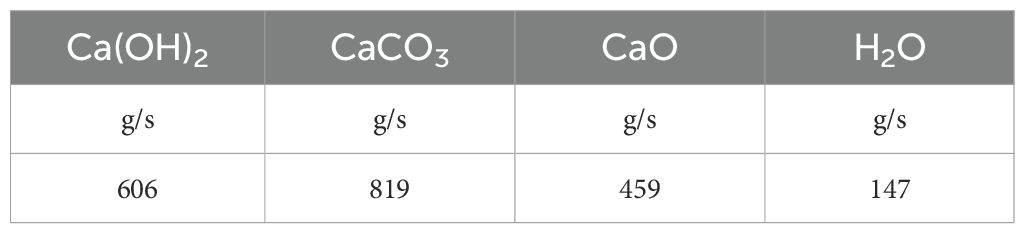
The values of the molecular weights (PM) for each reagent type are reported in Table 1.
3.3 Procedure for the feasibility assessment for onboard installation
Based on previous considerations, the process developed by the authors to perform the sizing of a Calcium Hydroxide based CCS for an existing vessel is outlined in the following.
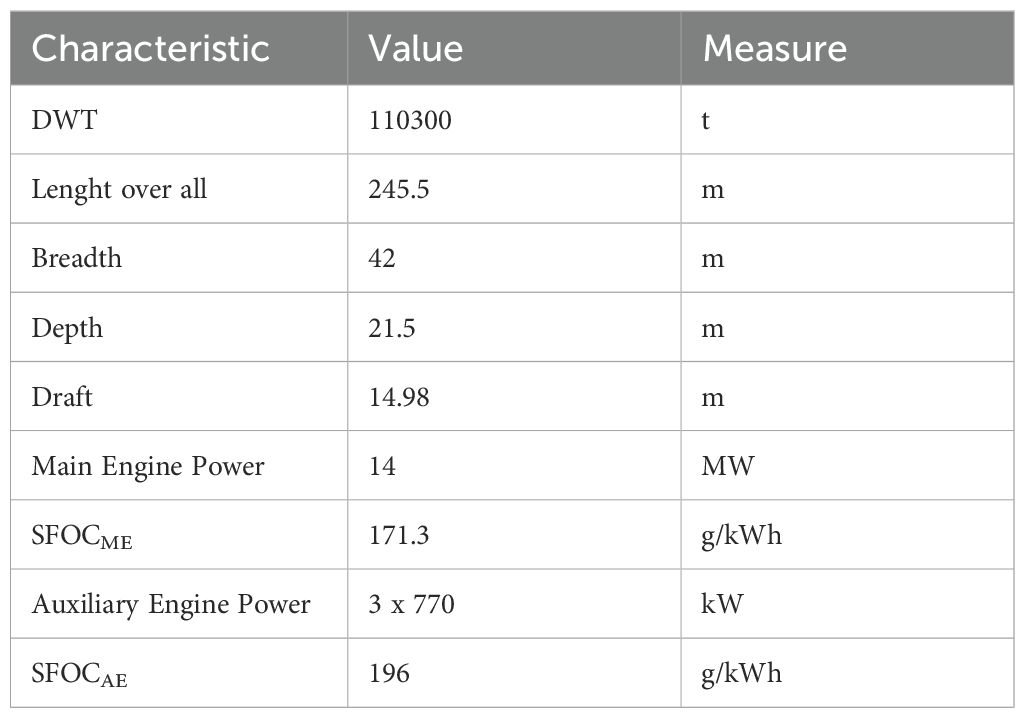
Firstly, it is imperative to consider the main characteristics of the vessel upon which the system is to be sized in order to identify available space and physical constraints for the installation of CCS. These characteristics include Deadweight tonnage (DWT), Length overall, Breadth, Depth, Draft, Main Engine and Auxiliary Engine specifications.
Secondly, the routes undertaken by the vessel have to be examined and the one necessitating the longest duration of navigation under loaded conditions should be selected. This ensures that the CCS is appropriately sized to effectively manage the most demanding voyage in terms of operation time of the CCS, thus assuring continuous capture of CO2 emitted by the vessels during its operations.
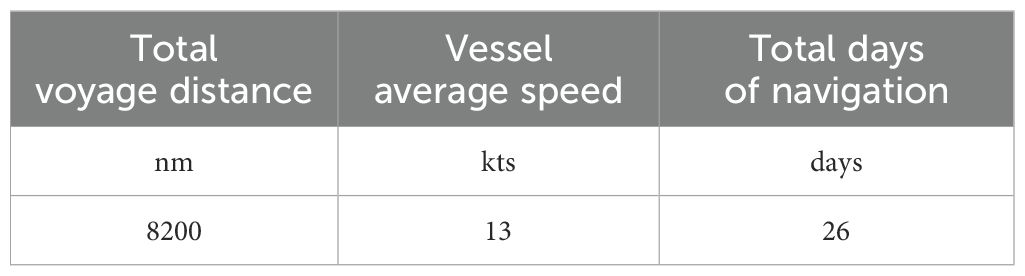
Based on the chosen route and real data extrapolated from the vessel under consideration, the total voyage distance in nm, the vessel average speed in kts and the total days of navigation (days), must be collected. following parameters must be collected.
With the aim of estimating the quantities of the elements required for the CCS with Calcium Hydroxide to react with CO2 and prevent its release into the atmosphere, the starting point is to understand the amount of CO2 that needs to be treated.
To accurately determine the system’s size, it’s crucial to calculate the CO2 emissions for the chosen voyage.
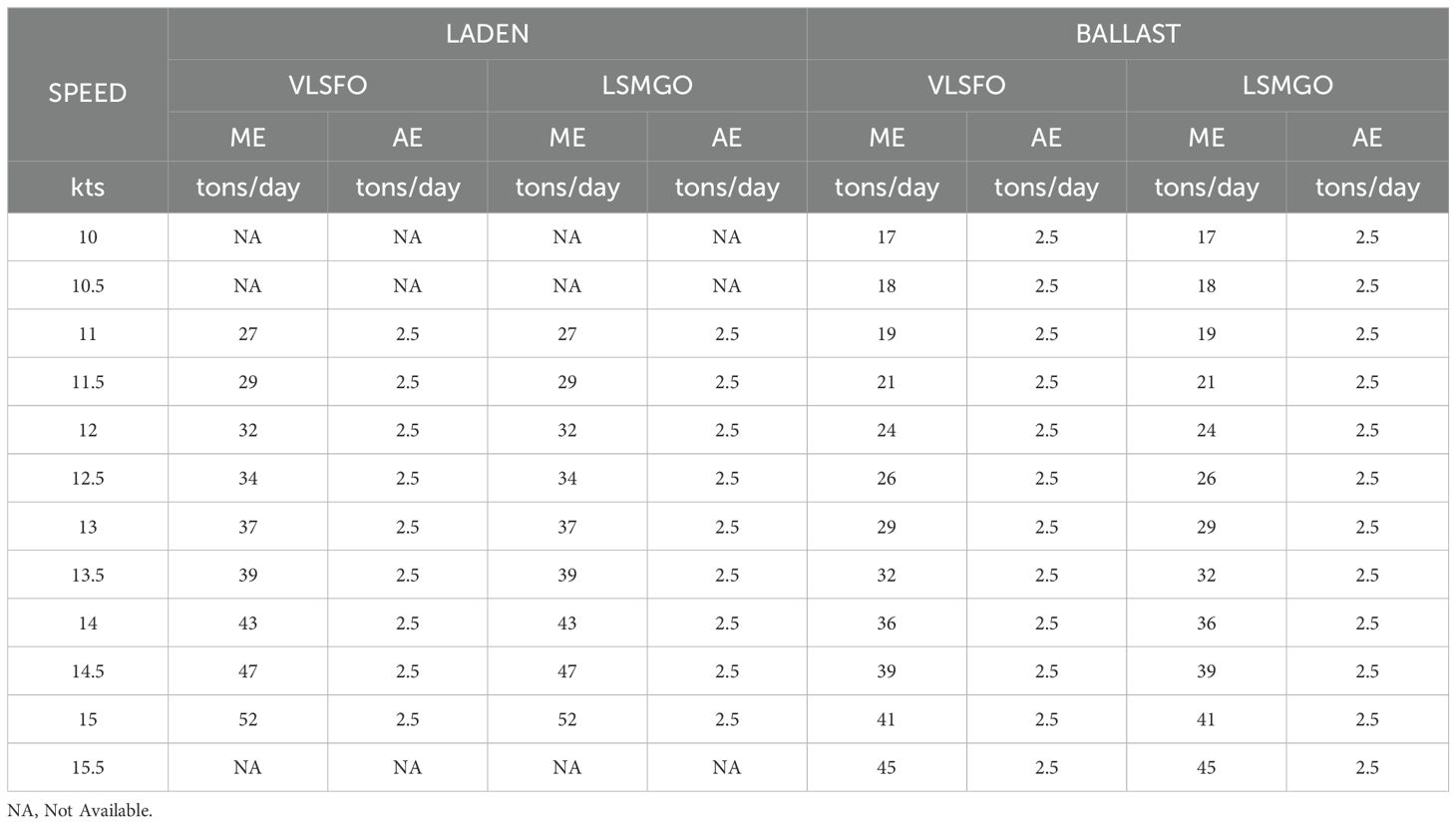
Based on total days of navigation and the vessel engine specifications at the selected average speed the daily fuel consumption (Cg), in tons/day, must be calculated as the sum of main engine fuel consumption (CgME) and auxiliary engines fuel consumption (CgAE).
where SFOC is the Specific Fuel Oil Consumption in g/kWh and PD is the delivered power in kW, for both Main Engine (ME) and Auxiliary Engines (AE), respectively.
From Cg, the Total Fuel Consumption for the selected voyage (CgTOT) can be derived and the total CO2 produced during the selected voyage (CO2Voyage) is calculated:
where CF is the Carbon Factor of the fuel in tonsCO2/tonsFuel.
From the CO2Voyage the CO2 flow rate production (), in g/s, can be calculated, along with the CO2 flow rate captured by CCS system, in g/s ().
The methodology described in Section 3.2 is applied to calculate, as a flow rate (g/s), the quantities of products necessary to achieve the desired % reduction in CO2 emissions compared to the capture plant’s efficiency assessing the information as , , , .
These results can be correlated with the number of navigation days of the ship along the selected route to obtain the quantities of products, in tons, required for the selected voyage.
Given the quantities of CaO essential for CO2 capture during navigation, the volumes of reactants required on board the ship can be calculated. The estimation of the volume containing the necessary amounts of reactants can be accomplished using the Equation 22:
Where represents the density for each reagent, defined as:
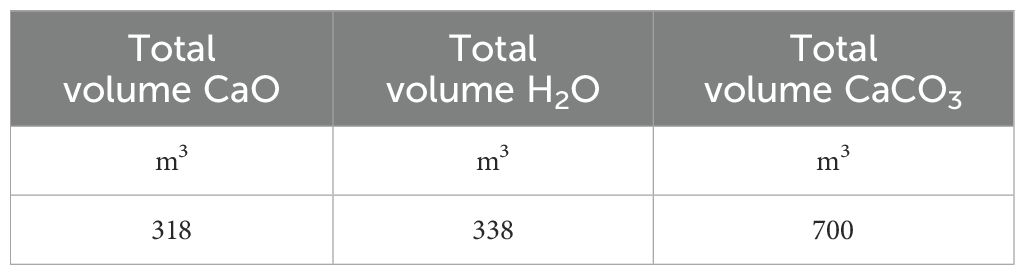
Total volume of CaO, H2O and CaCO3, that need to be stored onboard can be calculated.
Having established all the requisite quantities and volumes of substances essential for the operation of the CCS on board, the assessment of system installation can be conducted.
To underline the major advantages of the developed methodology, it outlines a stoichiometric approach for sizing a Calcium Hydroxide-based CCS for ships, determining the necessary quantities of reactants and assessing the feasibility of onboard installation based on vessel characteristics, voyage data, and emission calculations.
4 Test case
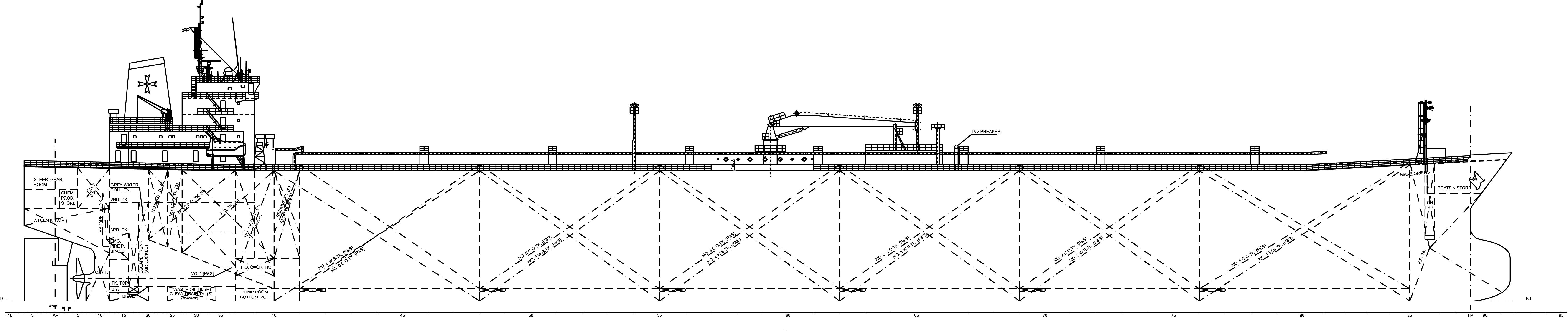
To thoroughly validate the presented methodology, the feasibility assessment of implementing a CCS utilizing Calcium Hydroxide on board a case study ship, consisting of an Oil Tanker Vessel depicted in Figure 4, has been conducted.
The primary characteristics of the vessel are outlined in Table 2.
The data of vessels’ voyages have been meticulously recording since 2020. This comprehensive dataset includes detailed information on performance and consumption. As a result, the compiled fuel consumption data correlated with vessel speed is available as reported and Table 3.
5 Results and discussion
5.1 Results
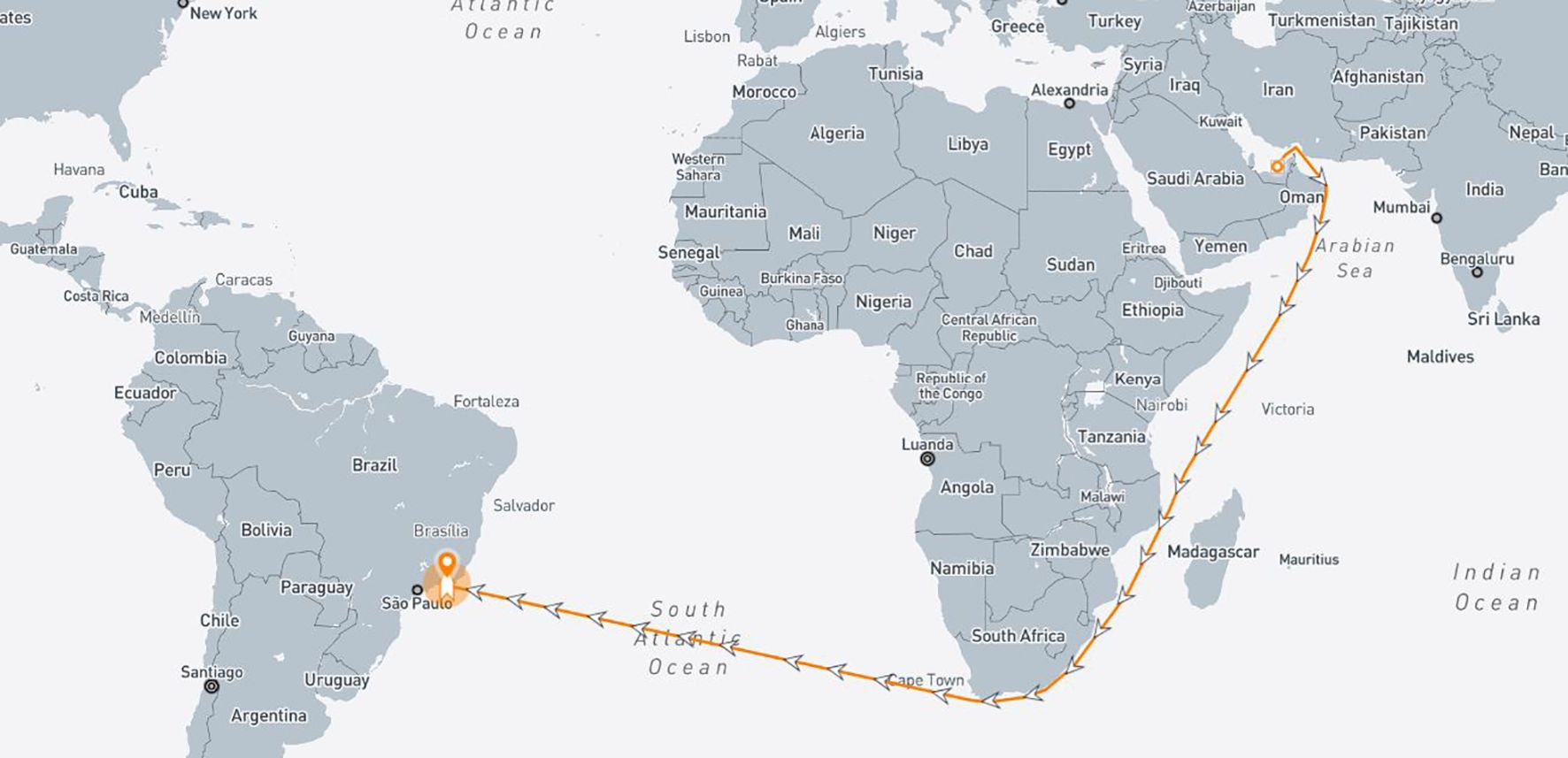
Analyzing the available records, the most demanding voyage is the route from Ruwais (Abu Dhabi, UAE) and reaching the terminal in Rio de Janeiro (Brazil) (Figure 5). Hence, this route has been selected to size the CSS.
Based on real data extrapolated from the ship’s case study, Table 4 presents the voyage characteristics.
A mentioned, initial research suggests that CCS could achieve a carbon capture efficiency (%) leading to a 25% reduction in CO2 emissions (Bortuzzo et al., 2023). This value has been assumed to further proceed.
The calculation of the CO2 produced in the selected voyage is conducted as reported in Table 5.
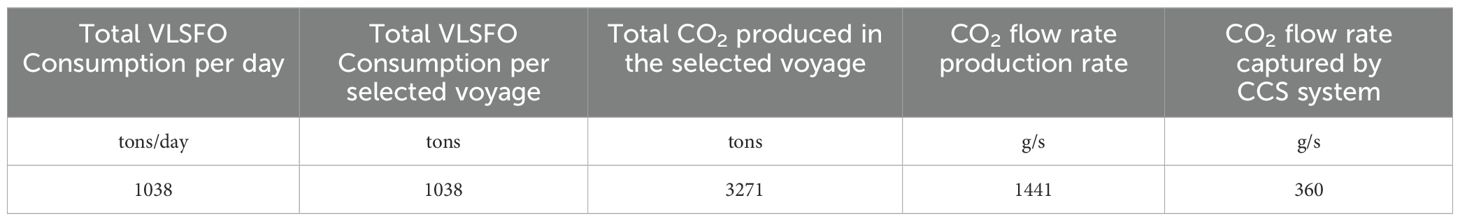
Table 5. Calculation to determine the 25% reduction of CO2 emissions produced during the selected voyage.
The quantities of reagents, necessary to achieve a 25% reduction in carbon dioxide emissions compared to the capture plant’s efficiency are calculated and reported in Table 6.
These results are correlated with the number of navigation days of the vessel along the selected route as reported in Table 7.
The volume containing the necessary amounts of reactants are calculated considering an allowance of approximately 2% and reported in Table 8.
Having all the requisite quantities and volumes of substances to operate the CCS on board, the authors proceeded to conduct an assessment of its installation.
Two lime (CaO) tanks were positioned on the main deck in a location where they do not potentially interfere with loading and unloading operations. However, the presence of a centrally located crane restricts the placement of the lime tanks further forward due to the maneuvering space required by the crane arm. The decision to install only two tanks onboard was made to expedite lime loading operations that could occur in port. Additionally, the choice was made not to incorporate tanks inside the ship from the early design stages, as this would have necessitated numerous modifications to the ship’s compartments, making the refitting complex and economically unsustainable.
Each tank’s characteristics are detailed as follows and depicted in Figure 6A: length equals to 15.9 m, width equals to 6 m, height equals to 3.5 and total capacity of 520 tons.
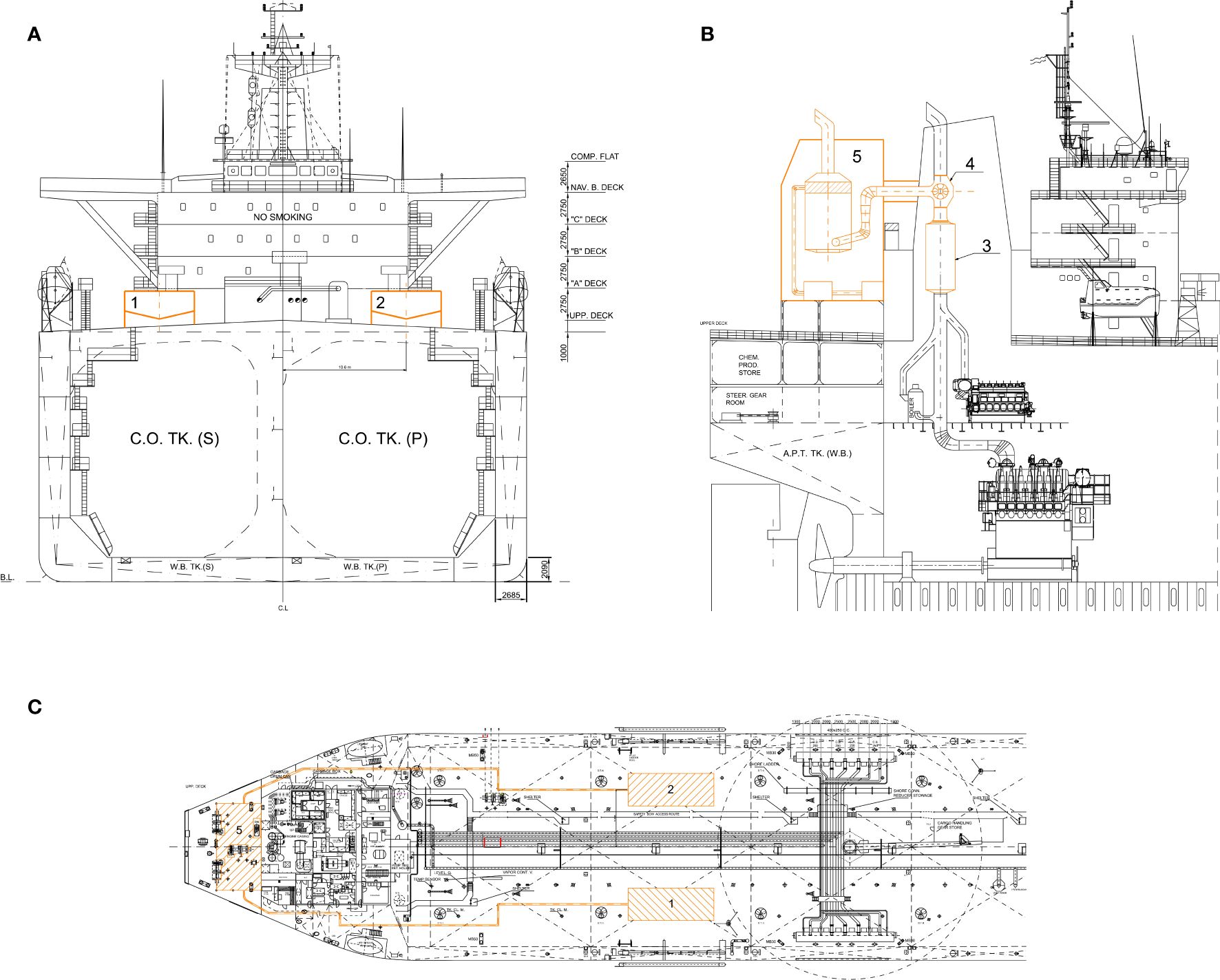
Figure 6. Implementation of CCS onboard case-study vessel (A–C), where 1 & 2 = CaO tanks; 3 = SOx scrubber; 4 = exhaust gas vent; 5 = CCS casing.
The total volume of CaO required to operate the system in order to capture 25% of CO2 produced during the selected voyage, which is the most demanding undertaken by the vessel, is quantified at 318 m³. This indicates that with this quantity, the ship will be able to undertake almost all other voyages, whose voyage length is less than one considered.
The height of 3.5 meters includes the support structure of the tanks and allows for sufficient space to enable a sloped configuration at the bottom of the tank, optimizing lime flow downwards through gravitational effects. Given the considerable length of the tanks, it is advisable to install a longitudinal bulkhead to limit load oscillations during rolling. Since CaO is a hygroscopic substance, the tanks housing must be equipped with insulation and a ventilation system to ensure a dry and well-ventilated internal environment.
The transfer of lime from the tanks to the CCS is managed through a system of volumetric pumps, with lime flow pipelines that can be positioned along the main deck.
The connection between the exhaust gas collector and the CCS is made through a duct originating from the ship’s funnel. To facilitate the transfer of exhaust gases from the duct inside the funnel to the one of CCS, an exhaust gas fan is installed, positioned downstream of the SOX scrubber. This arrangement ensures that the exhaust gas that flows into the carbon capture unit is free of sulfur and nitrogen oxides.
Within the necessary refitting operation to install the CCS, it enables the accommodation of all required machinery without substantial alteration to existing spaces on board the ship. The structure of the system extends approximately three superstructure decks in height and is supported by beams positioned on the main deck (Figure 6).
Additionally, the installation and operation of the CCS must consider the harsh marine environment, where factors such as humidity and salt exposure can accelerate corrosion and material degradation, affecting the system’s durability. To ensure long-term performance and reliable operation, insights from marine scrubber systems emphasize the importance of using durable, corrosion-resistant materials that can withstand high operational temperatures and energy demands. Regular maintenance is essential to maintain efficiency and address impurity buildup over time.
The main results are summed up as a 25% reduction in CO2 emissions achievable for the selected demanding voyage between Ruwais (UAE) and Rio de Janeiro (Brazil). The necessary reagents, system components, and tank design have been optimized for this reduction, ensuring system feasibility without substantial modifications to the ship. The installation plan takes into account operational constraints, including space limitations and environmental conditions, while maintaining durability and efficiency in a marine setting.
5.2 Discussion
The feasibility study on the implementation of a CCS using Calcium Hydroxide onboard the real case-ship has demonstrated that its components and the subsequent impact of their installation do not require complex procedures. Therefore, it is feasible to implement such systems even on older vessels, which may emit more pollutants compared to new buildings, without necessitating extensive modifications. Particularly noteworthy is the fact that there is no need to sacrifice space intended for cargo or other purposes, making retrofitting achievable within a short timeframe without significant structural alterations.
Regarding the stability of the vessel with the implementation of CCS onboard, future assessments will be essential, especially if the realistic implementation of this system on ships is considered. However, preliminary stability studies on vessels with different CCS implementations indicate that stability remains within acceptable limits with the original design (MMMCZCS, 2022). For the specific CCS under consideration, the additional installation of CaO tanks must be evaluated not only for its impact on overall stability but also from a structural perspective. It is important to assess the integration of the hull’s longitudinal strength and verify the support elements for the CaO tanks to prevent adverse effects on the vessel’s stability and safety. The CaO tanks should also undergo certification to ensure compliance with safety and security guidelines, given the material’s potential to cause hazardous reactions.
As evidenced by the case study, the system would be capable of reducing CO2 emissions from a ship’s voyage by approximately 25%. Given that the system has been sized for the most demanding voyage, it highlights the potential for emissions reduction across all voyages undertaken by the vessel. This undoubtedly offers significant and immediate benefits in addressing the global emissions challenge faced by the maritime sector.
In this study, no storage tank for the product discharged from the CCS, namely CaCO3, was designed onboard. This decision not only saved additional space onboard but also adopted a more environmentally sustainable approach by directly discharging the CaCO3 product overboard, mitigating ocean acidification.
Taking into account the potential adoption of this system throughout the global merchant fleet, which, as per the study conducted at the close of March 2022 (https://www.atlas-mag.net/en/category/tags/focus/the-world-merchant-fleet), encompasses approximately 57,000 merchant ships navigating voyages worldwide, the profound implications of this innovation cannot be overlooked. Even if only a portion of these vessels were to integrate this technology, the resultant production of CaCO3 could yield a dual benefit: mitigating CO2 emissions and promoting the health of our oceans and marine life.
However, this does not preclude the possibility of considering future onboard CaCO3 storage tanks, whether it involves all the produced CaCO3 or just a portion that is not discharged overboard. This substance could be disposed of in ports, to initiate a circular economy. This approach would necessitate the development of a green transport network for product distribution from the port to the utilization industry. This consideration applies even if other CCS, which require the discharge of liquefied CO2 into ports, were to be implemented.
Being the CaCO3 the substance from which CaO is produced, and the CaCO3 discharged by ships could potentially be utilized instead of sourcing it from quarries or limestone mines. Moreover, since the process of producing CaO generates CO2 emissions itself, this might be performed in an industry where CCS is already implemented. Given the widespread availability of CCS in land industries, which are already operational, highly efficient, and industrially established, this approach is promising.
Such integration could pave the way for a circular economy, as illustrated in Figure 7.
Another issue that could be addressed by implementing CCS onboard ships is certainly emissions of SOX and CO2 in ports. While the broader issue in ports includes more than just CO2 emissions, reducing these emissions could effectively mitigate the problem of large quantities of pollutants released especially in urban areas. Vessels that cannot use cold ironing could rely on CCS to achieve this goal.
Once further developments of the system are made, it will also be necessary to assess the additional amount of fuel required to operate the system as well as the associated operational expenses, including maintenance and materials such as CaO. However, in the absence of a CO2 liquefaction unit and the additional energy required for its operation, the extra energy demand typically needed for this unit in other types of CCSs is avoided (Bortuzzo et al., 2023; Hua et al., 2023).
However, it is worth noting that even if these costs are significant, these will be offset by savings from EU ETS or future pricing mechanisms, as well as tangible benefits for compliance with CII, EEXI, EEDI and GHG intensity regulations. In this framework, although various technologies can increase a ship’s energy efficiency and reduce CO2 emissions, such as wind-assist systems, air lubrication technology, and energy-saving devices like waste heat recovery systems, these are unlikely to achieve the same level of reduction as CCS. Consequently, CCS is expected to result in greater savings within carbon pricing mechanisms like the EU ETS.
5.3 Road map for future works
This study introduces a new framework to tackle challenges in marine environments and climate change mitigation. Future research could focus on detailed sensitivity analyses to validate assumptions about CCS efficiency and energy demands. These analyses would clarify the most influential variables and ensure the approach’s robustness under different conditions.
Building upon the insights gained from sensitivity analyses, further technical investigations could focus on enhancing the operational efficiency of the calcium looping process. This would involve addressing challenges such as optimizing CO2 capture under diverse operating conditions and minimizing associated risks. Pilot studies in real-world marine environments would also be invaluable for assessing the feasibility of the technology at scale and identifying practical barriers to implementation. Moreover, the implications related to the need to introduce new training activities for on-board personnel to operate with the novel technology must be foreseen.
Economic considerations represent another critical aspect for future exploration. By leveraging findings from sensitivity and technical analyses, a high-level economic evaluation could be conducted by analyzing costs related to equipment, maintenance, and energy use that are still not available due to the lack of full-scale systems installed onboard ships. This evaluation should aim to identify cost-reduction strategies and the potential financial benefits of the proposed approach. This evaluation would include projected savings from emissions credits and could inform decision-making for both policymakers and industry stakeholders.
Another essential area for future research lies in the ecological assessment of CaCO3 discharge and its potential impacts on marine ecosystems. Detailed studies should examine changes to biodiversity and water chemistry across various environments, complemented by predictive ecological modelling to forecast long-term consequences. These efforts should be coupled with the development of robust regulatory and monitoring frameworks capable of tracking direct and indirect impacts over time. Incorporating feedback mechanisms into such frameworks would help mitigate any unintended adverse effects, ensuring the sustainability of the intervention. Compared to other CCSs, this system eliminates the need for CO2 liquefaction, simplifying onboard operations and enhancing its life cycle assessment. By avoiding the complexities of discharging liquefied CO2 offshore, developing specialized port infrastructure, and transporting CO2 to underground storage sites, it offers a more streamlined and sustainable solution. However, for the system under study future research could explore innovative ship designs that integrate onboard CaCO3 storage tanks, enabling closed-loop solutions for future deployments. This approach would allow for the partial or complete containment of generated CaCO3 onboard, with subsequent disposal at ports to support a circular economy. Evaluating the feasibility of repurposing collected CaCO3 for industrial applications could further promote a sustainable green transport network by facilitating its distribution from ports to industries. These initiatives would address challenges related to onboard space constraints and land-based infrastructure, creating a comprehensive and sustainable strategy for CaCO3 management.
Additionally, CaCO3 is not listed among the prohibited wastes in Annex I and II of the London Convention, as outlined in this study. However, future research should consider new ecological insights and assess whether this approach aligns with existing regulatory frameworks or necessitates updates to international guidelines to accommodate novel strategies for climate mitigation.
Together, these avenues for further research provide a comprehensive roadmap for advancing the current study’s findings. They highlight the need for an interdisciplinary approach that integrates technical, economic, ecological and legal perspectives to maximize the potential of the proposed solution while addressing its limitations.
6 Conclusions
Drawing from prior research, it has been noted that among the investigated CCS methods, the calcium hydroxide-based approach stands out as the most effective in delivering environmental benefits. In particular, this system shows potential not only to reduce CO2 emissions, but also to help marine ecosystems and mitigate ocean acidification. Although applications on ships have yet to be implemented, the absence of a standardized method to assess dimensional requirements highlights the significance of the methodology developed by the authors. The stoichiometric approach, which involves relatively quick calculations, facilitates a preliminary assessment of the technology’s impact on the reference vessel. This methodology helps address system-level integration and operational challenges, while leaving the detailed design of the CO2 scrubber functionality to technology suppliers.
The stoichiometric approach employed in this study has enabled the determination of the required amounts of CaO for on-board storage, allowing the estimation of potential CO2 emission reductions. Equally significant is the calculation of the quantities of CaCO3 that could be produced through this system. The authors assessed that this product is suitable for addressing a critical environmental issue related to climate change—ocean acidification—or, alternatively, for reuse in industry, thereby promoting a circular economy.
The use of such a system could also be extended to ships’ port stays, addressing the port emissions issue. Currently, these emissions are managed through the cold ironing approach, but its implementation remains limited. Many ports and ships do not yet support it due to the necessary infrastructure and investments required from both ports and shipping companies. However, reducing port emissions would mark a significant stride forward for the ecosystem and human health.
Future research should focus on a more comprehensive examination of system feasibility for implementation and integration on board. This involves performing sensitivity analysis, advanced technical investigations, and economic assessments. Additionally, assessing the environmental impact of CaCO3 through a life cycle assessment is necessary to provide an understanding of the system’s overall benefits by considering everything from raw material extraction to final disposal, ensuring that the process meets sustainability goals. Furthermore, considering the absence of international regulations governing the discharge of CaCO3 into the marine ecosystem and the need for comprehensive research and statutory approval regarding the potential efficacy of releasing varying amounts of calcium carbonate into the sea, this issue undoubtedly deserves the attention of relevant maritime regulatory bodies in the near future.
These activities can give augmented scientific soundness in the transitional period necessary to have experimental data from established applications.
The research has the potential to highlight the proposed solution and evaluate its pre-feasibility on a widely used ship type, aiming at raising attention towards CCS based on Ca(OH)2 and encouraging further research in the field that could ultimately support the development of more sustainable practices within the maritime sector. In fact, despite its potential, this promising technology still faces challenges that must be addressed and it remains underexplored in terms of applications, with limited real-world implementations. By presenting these initial technical findings, the paper seeks to bridge a critical gap in the literature, stimulate broader discussion, and provide a foundation for advancing this field.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
VBo: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. LB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. VBu: Conceptualization, Investigation, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Research funded by the European Union through the NextGenerationEU program, National Recovery and Resilience Plan (NRRP), Missione 4, "ISTRUZIONE E RICERCA" - Componente 2, "DALLA RICERCA ALL'IMPRESA" - Linea di Investimento 1.4 - “CENTRI NAZIONALI”, Sustainable Mobility Center - Centro Nazionale per la Modalità Sostenibile – MOST (CNMS PROJECT - ID Code CN_00000023 - SPOKE 3 - WATERWAYS - CUP B43C22000440001, Open Call). This manuscript reflects only the Authors’ views and opinions and does not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them.
Conflict of interest
VBo was employed by Fratelli d’Amico Armatori Spa.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aakko-Saksa P. T., Lehto K., Kuittinen N., Järvinen A., Jalkanen J.-P., Johnson K., et al. (2023). Reduction in greenhouse gas and other emissions from ship engines: Current trends and future options. Prog. Energy Combust. Sci. 94, 101055. doi: 10.1016/j.pecs.2022.101055
Abanades J. C., Criado Y. A., White H. I. (2023). Direct capture of carbon dioxide from the atmosphere using bricks of calcium hydroxide. Cell Rep. Phys. Sci. 4, 101339. doi: 10.1016/j.xcrp.2023.101339
Al Baroudi H., Awoyomi A., Patchigolla K., Jonnalagadda K., Anthony E. J. (2021). A review of large-scale CO2 shipping and marine emissions management for carbon capture, utilization and storage. Appl. Energy. 287, 116510. doi: 10.1016/j.apenergy.2021.116510
Andriiovych Kuropyatnyk O., Victorovych Sagin S. (2019). Exhaust gas recirculation as a major technique designed to reduce NOх Emissions from marine diesel engines. NAŠE MORE: znanstveni časopis za more i pomorstvo 66, 1–9. doi: 10.17818/NM/2019/1.1
Balcombe P., Brierley J., Lewis C., Skatvedt L., Speirs J., Hawkes A., et al. (2019). How to decarbonize international shipping: Options for fuels, technologies and policies. Energy Convers. Manage. 182, 72–88. doi: 10.1016/j.enconman.2018.12.080
Bertagna S., Kouznetsov I., Braidotti L., Marinò A., Bucci V. (2023). A rational approach to the ecological transition in the cruise market: technologies and design compromises for the fuel switch. J. Mar. Sci. Eng. 11, 67. doi: 10.3390/jmse11010067
Bortuzzo V., Bertagna S., Bucci V. (2023). Mitigation of CO2 emissions from commercial ships: evaluation of the technology readiness level of carbon capture systems. Energies 16, 3646–3646. doi: 10.3390/en16093646
Bortuzzo V., Bertagna S., Marino A., Bucci V. (2024). Feasibility assessment for the application of carbon capture systems onboard cargo ships in response to the EU ETS regulation. Prog. Mar. Sci. Technol. 8, 82–93. doi: 10.3233/pmst240009
Braidotti L., Bertagna S., Rappoccio R., Utzeri S., Bucci V., Marinò A. (2023). On the inconsistency and revision of Carbon Intensity Indicator for cruise ships. Transp. Res. Part D Transp. Environ. 118, 103662–103662. doi: 10.1016/j.trd.2023.103662
Butenschön M., Lovato T., Masina S., Caserini S., Grosso M. (2021). Alkalinization scenarios in the mediterranean sea for efficient removal of atmospheric CO2 and the mitigation of ocean acidification. Front. Clim. 3. doi: 10.3389/fclim.2021.614537
Caserini S., Cappello G., Righi D., Raos G., Campo F., De Marco S., et al. (2021b). Buffered accelerated weathering of limestone for storing CO2: Chemical background. Int. J. Greenh. Gas Control. 112, 103517. doi: 10.1016/j.ijggc.2021.103517
Caserini S., Pagano D., Campo F., Abbà A., De Marco S., Righi D., et al. (2021a). Potential of maritime transport for ocean liming and atmospheric CO2 removal. Front. Clim. 3. doi: 10.3389/fclim.2021.575900
Caserini S., Storni N., Grosso M. (2022). The availability of limestone and other raw materials for ocean alkalinity enhancement. Glob. Biogeochem. Cycles. 36 (5). doi: 10.1029/2021gb007246
Chen F., Morosuk T. (2021). Exergetic and economic evaluation of CO2 liquefaction processes. Energies 14, 7174. doi: 10.3390/en14217174
Comes J., Islamovic E., Lizandara-Pueyo C., Seto J. (2024). Improvements in the utilization of calcium carbonate in promoting sustainability and environmental health. Front. Chem. 12. doi: 10.3389/fchem.2024.1472284
Costagliola M. A., Prati M. V., Perretta G. (2022). Post combustion CO2 capture with calcium and lithium hydroxide. Sci. Rep. 12, 10518. doi: 10.1038/s41598-022-14235-5
DNV. (2023). EU ETS: Preliminary Agreement to Include Shipping In The EU’s Emission Trading System from 2024. Technical and Regulatory News No 02/2023. Available online at: https://www.dnv.com/news/eu-ets-preliminary-agreement-to-include-shipping-in-theeu-s-emission-trading-system-from-2024-238068 (Accessed January 12, 2024).
Dupont S., Pörtner H.-O. (2013). A snapshot of ocean acidification research. Mar. Biol. 160, 1765–1771. doi: 10.1007/s00227-013-2282-9
Durand G., van den Broeke M. R., Le Cozannet G., Edwards T. L., Holland P. R., Jourdain N. C., et al. (2022). Sea-level rise: from global perspectives to local services. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.709595
European Commission. (2023). FAQ – Maritime transport in EU Emissions Trading System (ETS). Available online at: Climate.ec.europa.eu.https://climate.ec.europa.eu/euaction/transport/reducing-emissions-shipping-sector/faq-maritime-transport-euemissions-trading-system-ets_en (Accessed January 12, 2024).
Galán-Martín Á., Vázquez D., Cobo S., Mac Dowell N., Caballero J. A., Guillén-Gosálbez G. (2021). Delaying carbon dioxide removal in the European Union puts climate targets at risk. Nat. Commun. 12, 6490. doi: 10.1038/s41467-021-26680-3
Ge Z., Dou B., Wang L., Ding Y., Chen H., Xuan Y. (2022). Calcium-looping based energy conversion and storage for carbon neutrality –the way forward. Carbon Neutral. 1, 35. doi: 10.1007/s43979-022-00034-4
Greco-Coppi M., Hofmann C., Walter D., Strohle J., Epple B. (2023). Negative CO2 emissions in the lime production using an indirectly heated carbonate looping process. Mitig. Adapt. Strateg. Glob. Change 28, 30. doi: 10.1007/s11027-023-10064-7
Han S.-J., Yoo M., Kim D.-W., Wee J.-H. (2011). Carbon dioxide capture using calcium hydroxide aqueous solution as the absorbent. Energy Fuels 25, 3825–3834. doi: 10.1021/ef200415p
Harvey L. D. D. (2008). Mitigating the atmospheric CO2 increase and ocean acidification by adding limestone powder to upwelling regions. J. Geophys. Res. 113 (C4). doi: 10.1029/2007jc004373
Hönisch B., Ridgwell A., Schmidt D. N., Thomas E., Gibbs S. J., Slujis A., et al. (2012). The geological record of ocean acidification. Science 335, 1058–1063. doi: 10.1126/science.1208277
Hua W., Sha Y., Zhang X., Cao H. (2023). Research progress of carbon capture and storage (CCS) technology based on the shipping industry. Ocean Eng. 281, 114929. doi: 10.1016/j.oceaneng.2023.114929
IMO (2020). Forth IMO GHG study 2020. Available online at: https://wwwcdn.imo.org/localresources/en/OurWork/Environment/Documents/Fourth%20IMO%20GHG%20Study%202020%20-%20Full%20report%20and%20annexes.pdf (Accessed January 22, 2024).
IMO (2022). Future fuels and technology. Available online at: https://www.imo.org/en/OurWork/Environment/Pages/Future-Fuels-And-Technology.aspx (Accessed February 14, 2024).
IMO (2023). IMO’s work to cut GHG emissions from ships. Available online at: https://www.imo.org/en/MediaCentre/HotTopics/Pages/Cutting-GHG-emissions.aspx (Accessed March 13, 2024).
International Energy Agency. (2020). Special Report on Carbon Capture Utilisation and Storage CCUS in clean energy transitions. Energy Technology Perspectives. Available online at: https://iea.blob.core.windows.net/assets/181b48b4-323f-454d-96fb-0bb1889d96a9/CCUS_in_clean_energy_transitions.pdf.
Jafari F., Naeemi A. S., Sohani M. M., Noorinezhad M. (2023). Effect of elevated temperature, sea water acidification, and phenanthrene on the expression of genes involved in the shell and pearl formation of economic pearl oyster (Pinctada radiata). Mar. pollut. Bull. 196, 115603–115603. doi: 10.1016/j.marpolbul.2023.115603
Karatuğ Ç., Arslanoğlu Y., Guedes Soares C. (2022). Feasibility analysis of the effects of scrubber installation on ships. J. Mar. Sci. Eng. 10, 1838. doi: 10.3390/jmse10121838
Kurihara H. (2008). Effects of CO2-driven ocean acidification on the early developmental stages of invertebrates. Mar. Ecol. Prog. Ser. 373, 275–284. doi: 10.3354/meps07802
Leeson D., Mac Dowell N., Shah N., Petit C., Fennell P. S. (2017). A Techno-economic analysis and systematic review of carbon capture and storage (CCS) applied to the iron and steel, cement, oil refining and pulp and paper industries, as well as other high purity sources. Int. J. Greenh. Gas Control. 61, 71–84. doi: 10.1016/j.ijggc.2017.03.020
Lehtoranta K., Vesala H., Koponen P., Korhonen S. (2015). Selective catalytic reduction operation with heavy fuel oil: NOx, NH3, and particle emissions. Environ. Sci. Technol. 49, 4735–4741. doi: 10.1021/es506185x
Lerman A., Mackenzie F. T. (2018). Carbonate minerals and the CO2-carbonic acid system. Encycl. Earth Sci., 206–226. doi: 10.1007/978-3-319-39312-4_84
Mærsk Mc-Kinney Møller Center for Zero Carbon Shipping (MMMCZCS) (2022). The role of onboard carbon capture in maritime decarbonization. Available online at: https://www.zerocarbonshipping.com/publications/the-role-of-onboard-carbon-capture-in-maritime-decarbonization/ (Accessed February 22, 2024).
Moras C. A., Joannes-Boyau R., Bach L. T., Cyronak T., Schulz K. G. (2024). Carbon dioxide removal efficiency of iron and steel slag in seawater via ocean alkalinity enhancement. Front. Clim. 6. doi: 10.3389/fclim.2024.1396487
Nunes L. J. R. (2023). The rising threat of atmospheric CO2: A review on the causes, impacts, and mitigation Strategies. Environments 10, 66. doi: 10.3390/environments10040066
Piccolo V. (2023). GHG Emissions From Shipping: How to Overcome Persistent Challenges (Social Science Research Network). doi: 10.2139/ssrn.4506467
Rau G. H. (2011). CO2 mitigation via capture and chemical conversion in seawater. Environ. Sci. Technol. 45, 1088–1092. doi: 10.1021/es102671x
Rau G., Caldeira K. (1999). Enhanced carbonate dissolution: a means of sequestering waste CO2 as ocean bicarbonate. Energy Convers. Manage 40 (17), 1803–1813. doi: 10.1016/S0196-8904(99)00071-0
Renforth P., Henderson G. (2017). Assessing ocean alkalinity for carbon sequestration. Rev. Geophys. 55 (3), 636–674. doi: 10.1002/2016RG000533
RINA (2023). From today to 2050 challenges and opportunities for the maritime industry. Available online at: https://scresources.rina.org/media/From-Today-to-2050-Challenges-and-Opportunities-for-the-Maritime-Industry.pdf (Accessed January 29, 2024).
Risso R., Cardona L., Archetti M., Lossani F., Bosio B., Bove D. (2023). A review of on-board carbon capture and storage techniques: solutions to the 2030 IMO regulations. Energies 16, 6748. doi: 10.3390/en16186748
Shu Y., Hu A., Zheng Y., Gan L., Xiao G., Zhou C., et al. (2023). Evaluation of ship emission intensity and the inaccuracy of exhaust emission estimation model. Ocean Eng. 287, 115723–115723. doi: 10.1016/j.oceaneng.2023.115723
Sridhar P., Kumar A., Manivannan S., Farooq S., Karimi I. A. (2024). Technoeconomic evaluation of post-combustion carbon capture technologies on-board a medium range tanker. Comput. Chem. Eng. 181, 108545–108545. doi: 10.1016/j.compchemeng.2023.108545
Sulpis O., Jeansson E., Dinauer A., Lauvset S. K., Middelburg J. J. (2021). Calcium carbonate dissolution patterns in the ocean. Nat. Geosci. 14, 423–428. doi: 10.1038/s41561-021-00743-y
Taipabu M. I., Viswanathan K., Wu W., Handogo R., Mualim A., Huda H. (2023). New improvement of amine-based CO2 capture processes using heat integration and optimization. Chem. Eng. Process. 193, 109532–109532. doi: 10.1016/j.cep.2023.109532
Tavakoli S., Gamlem G. M., Kim D., Roussanaly S., Anantharaman R., Yum K. K., et al. (2024). Exploring the technical feasibility of carbon capture onboard ships. J. Clean. Prod. 452, 142032–142032. doi: 10.1016/j.jclepro.2024.142032
United Nations (2023). Review of Maritime Transport 2023 Vol. 2023 (Geneva: United Nations Conference on Trad and Development).
Valverde J. M. (2018). The Ca-looping process for CO2 capture and energy storage: role of nanoparticle technology. J. Nanopart. Res. 20, 39. doi: 10.1007/s11051-017-4092-3
Visonà M., Bezzo F., d’Amore F. (2024). Techno-economic analysis of onboard CO2 capture for ultra-large container ships. Chem. Eng. J. 485, 149982. doi: 10.1016/j.cej.2024.149982
Witte K. (2021). Social acceptance of carbon capture and storage (CCS) from industrial applications. Sustainability 13, 12278. doi: 10.3390/su132112278
Wu E., Wang Q., Ke L., Zhang G. (2023). Study on carbon emission characteristics and emission reduction measures of lime production—A case of enterprise in the Yangtze River Basin. Sustainability 15, 10185. doi: 10.3390/su151310185
Yoro K. O., Daramola M. O. (2020). Chpater 1 - CO2 emission sources, greenhouse gases, and the global warming effect. Adv. Carbon Capture, 3–28. doi: 10.1016/b978-0-12-819657-1.00001-3
Zeebe R. E., Wolf-Gladrow D. (2001). CO2 in seawater: equilibrium, kinetics, isotopes (Vol. 65). (Gulf Professional Publishing).
Zhang X., Bao Z., Ge Y.-E. (2021). Investigating the determinants of shipowners’ emission abatement solutions for newbuilding vessels. Transp. Res. Part D Transp. Environ. 99, 102989. doi: 10.1016/j.trd.2021.102989
Keywords: marine environment protection, atmospheric emissions reduction, ocean acidification mitigation, cargo ships, carbon capture system, calcium carbonate
Citation: Bortuzzo V, Bertagna S, Braidotti L and Bucci V (2025) Towards CO2 emissions reduction of shipping: Ca(OH)2 based carbon capture system for safeguarding the marine environment. Front. Mar. Sci. 12:1434342. doi: 10.3389/fmars.2025.1434342
Received: 17 May 2024; Accepted: 17 January 2025;
Published: 07 February 2025.
Edited by:
Yen-Chiang Chang, Dalian Maritime University, ChinaReviewed by:
Emilio Notti, National Research Council (CNR), ItalyAndrew Lockley, University College London, United Kingdom
Copyright © 2025 Bortuzzo, Bertagna, Braidotti and Bucci. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valentina Bortuzzo, dmFsZW50aW5hLmJvcnR1enpvMkBwaGQudW5pdHMuaXQ=
†These authors have contributed equally to this work and share first authorship
 Valentina Bortuzzo
Valentina Bortuzzo Serena Bertagna
Serena Bertagna Luca Braidotti
Luca Braidotti Vittorio Bucci
Vittorio Bucci