- Vocational School of Technical Sciences, Recep Tayyip Erdogan University, Rize, Türkiye
This study evaluated the concentrations, sources, and health risks of trace metals and metalloids in the effluents of 15 wastewater treatment plants (WWTPs) located along the Black Sea and the Sea of Marmara, ecologically and economically vital regions of Türkiye. Effluent samples were collected in winter and autumn, and metal concentrations were analyzed using ICP-MS to assess seasonal variations and potential risks. Results showed notable seasonal and regional differences, with aluminium (Al) and nickel (Ni) as the most abundant metals. The highest total metal concentration was recorded in autumn at station S2 (326.09 mg/L). Non-carcinogenic risks were negligible (HI< 1) across all stations, but low carcinogenic risks (10-6< CRi ≤ 10-4) for chromium (Cr) and nickel (Ni) were detected at some locations. Source apportionment using Principal Component Analysis revealed mixed geogenic and anthropogenic origins, primarily from industrial activities and urban runoff. While effluents generally complied with national standards, several metals exceeded international limits, highlighting risks to ecosystems and human health. These findings underscore the urgent need for stricter discharge regulations, improved treatment technologies, and continuous monitoring to mitigate the environmental and health impacts of WWTP discharges.
1 Introduction
Urban wastewater includes rainwater and a mix of household, agricultural, and industrial wastes that are released into the ocean, especially in coastal communities. When this discharge is either left untreated or undergoes standard treatment, significant amounts of contaminants, including metals, are released, posing serious threats to human health and ecosystems (Akdemir and Dalgıc, 2020; Manasa and Mehta, 2020; Ahmed et al., 2021; Singh et al., 2024). Metals are non-biodegradable substances that are toxic to living organisms and have the potential to accumulate in tissues and reach higher levels of the food chain (Nnaji et al., 2023; Kumar et al., 2023). The monitoring of these substances in municipal wastewater is of great utility in identifying common sources of emissions. The corrosion roofs results in the release of copper (Cu), zinc (Zn) and aluminium (Al) while in addition runoff from streets introduces cadmium (Cd), lead (Pb) from asphalt, tires, and brake pads. Mercury (Hg) from amalgam dental fillings and other metal pollutants in household and industrial waste can also result in wastewater contamination (De Buyck et al., 2021; Fiala and Hwang, 2021; Fairbanks et al., 2021; Shrestha et al., 2021; Singh and Devi, 2023). The elements chromium (Cr) and nickel (Ni), which are widely used in various industrial processes, are significant contributors to wastewater contamination. Cr is used in metallurgy, electroplating, and the production of paints, pigments, preservatives, and paper. Ni is used in stainless steel, electronics, and coins (Jaishankar et al., 2014; Kinuthia et al., 2020). The metalloid arsenic (As) is released through natural processes, volcanic emissions, and human activities such as mining, fossil fuel combustion, and the use of As based pesticides, herbicides, and feed additives (Satyapal and Kumar, 2021). As a versatile metal manganese (Mn) is an integral part of various industrial and metallurgical applications (Nkele et al., 2022). In the present era, a variety of techniques are employed in wastewater treatment plants to remove these metals from urban wastewater, with some plants even being adapted for urban metal mining. Nevertheless, it is not possible to assert that these techniques are 100% effective. It is estimated that only 24% of the world’s domestic and industrial wastewater undergoes a treatment process prior to disposal and subsequent reuse (Ida and Eva, 2021; Varennes et al., 2023; Lakshmi and Reddy, 2017). Furthermore, research on wastewater treatment plants (WWTPs) in Turkiye has largely focused on operational issues, metal removal technics and local environmental impacts, with a lack of studies systematically addressing both environmental and human health risks on a broader scale (Maryam and Büyükgüngör, 2019; Yesil and Tugtas, 2019; Arslan et al., 2023; Abu Amr et al., 2023; Molaey et al., 2024).
The Black Sea and the Sea of Marmara, the focus of this study, are of vital importance to Turkey’s fisheries sector (TSI, 2024). With a rich biodiversity and a significant economic impact even on neighboring countries, the protection of this important ecosystem is of utmost importance and requires sustainable policies (FAO, 2023). The coastal cities of these seas at the center of this study exhibit significant tourism potential, offering a variety of marine tourism and water sports opportunities such as swimming, sailing, yachting and sport fishing (MCT, 2023).
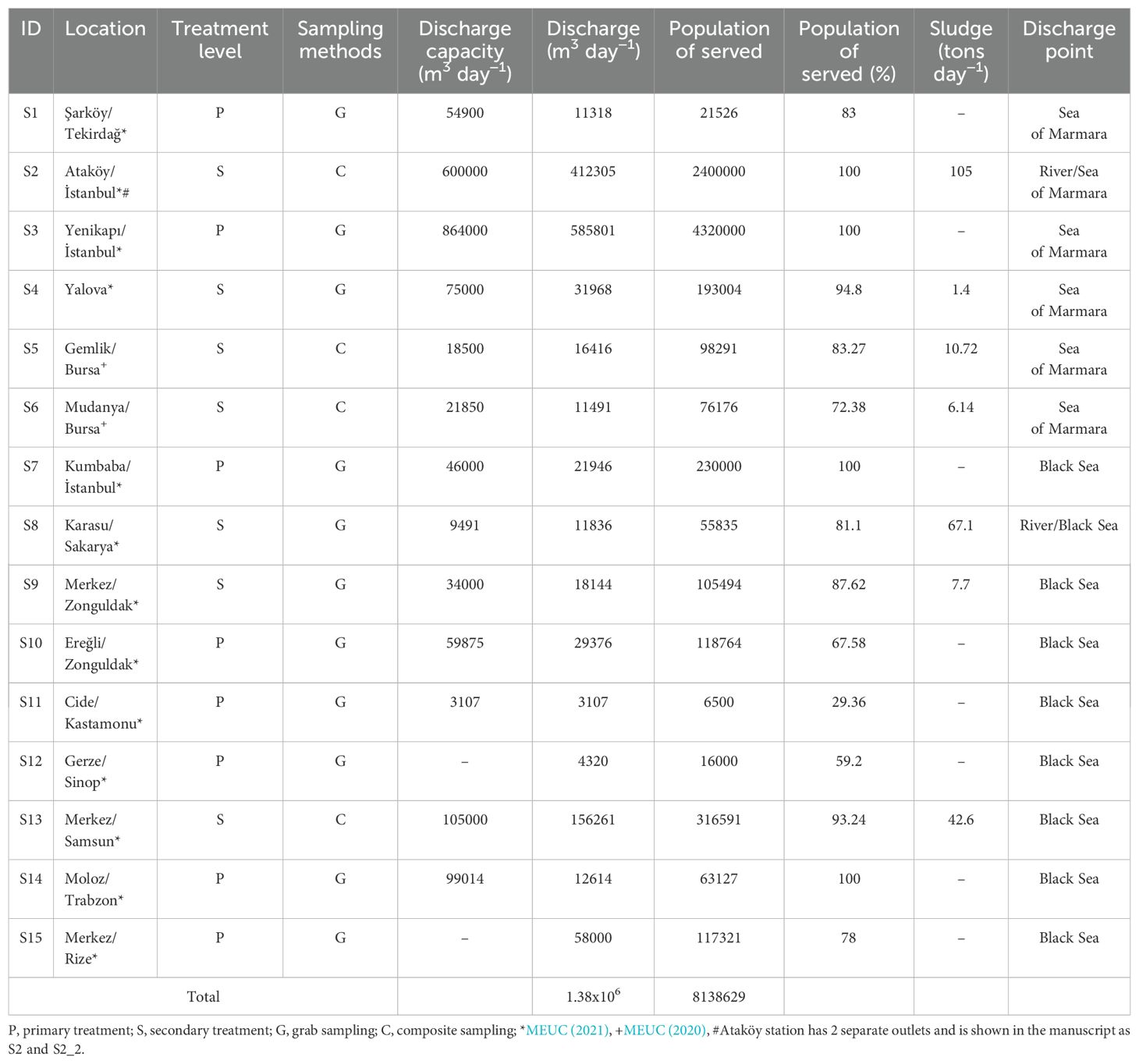
This study primarily aims to: (i) determine the concentrations of metal(loid)s in WWTPs across cities that collectively represent a significant portion of the country’s population through instantaneous measurements taken at random intervals, and (ii) evaluate the cumulative impacts and potential risks to human health. Metal(loid) levels were analyzed in effluents from 15 WWTPs, with daily flow rates ranging from 3,107 to 585,801 m³ and serving populations between 6,500 and 4,320,000 (MEUC, 2021, 2020), with 9 of these WWTPs discharging into the Black Sea and 6 into the Sea of Marmara, representing regions with varying population densities and diverse treatment methods.
2 Materials and methods
2.1 Study area
To monitor metal(loid) concentrations in wastewater discharged from wastewater treatment plants in Türkiye into the Black Sea and the Sea of Marmara, 15 different treatment plants were selected with a total treatment capacity of 1.38 × 106 m3 per day (Figure 1). The total volume of wastewater discharged from these facilities constitutes 12% of the water discharged from wastewater treatment facilities in Türkiye. While 2 out of 6 WWTPs discharged into Marmara have primary treatment features, 4 out of 6 have secondary treatment features. 7 of the WWTPs that discharge wastewater into the Black Sea have primary treatment capacity and 2 of them have secondary treatment capacity. Thirteen plants discharge wastewater directly into the Sea of Marmara and the Black Sea, while two plants discharge wastewater into the sea through rivers Ayamama and Sakarya, less than 1 km away from sea (Table 1).
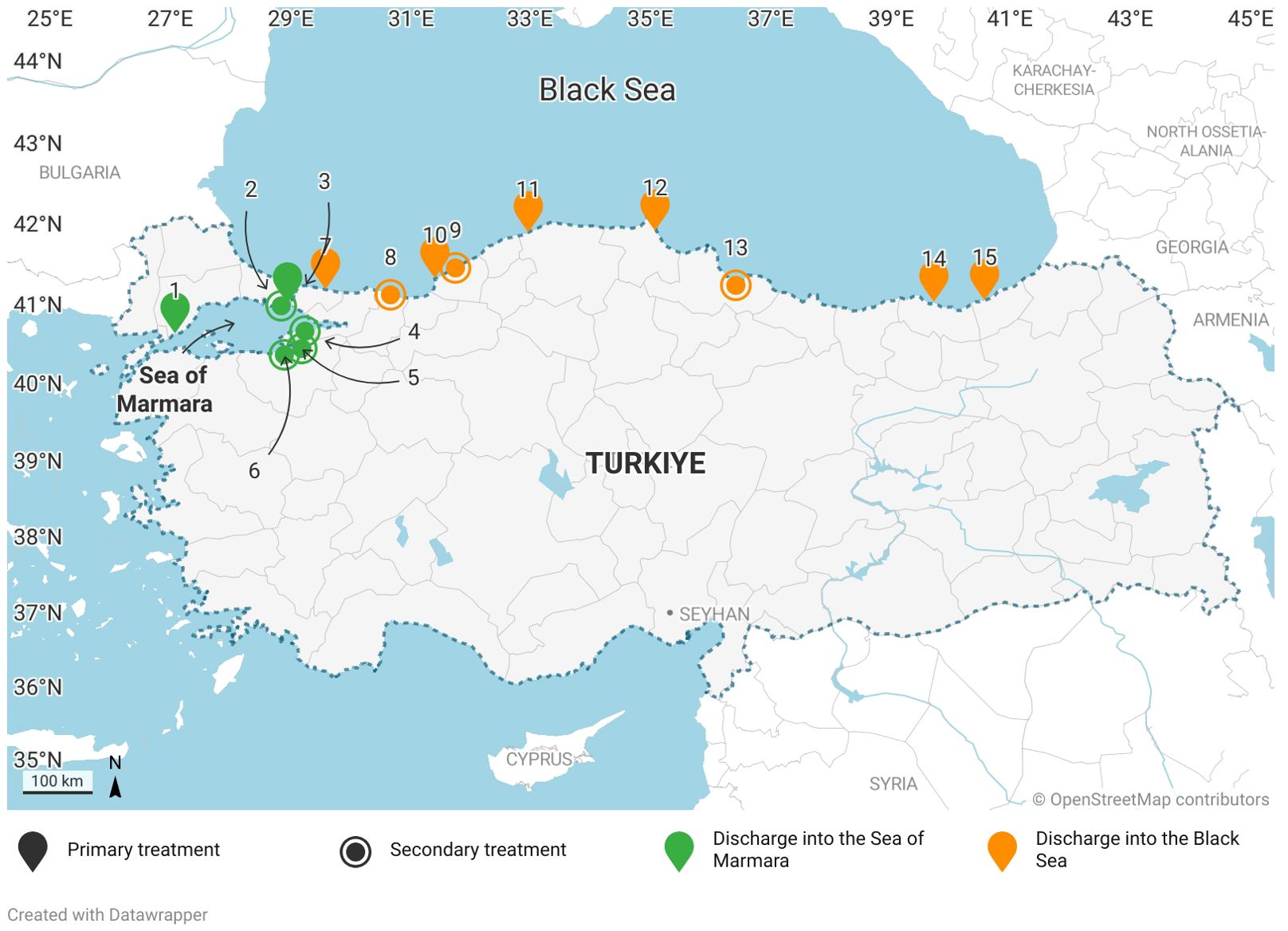
Figure 1. Sampling locations map. 9 of the wastewater samples collected from sewage treatment plants are discharged into the Black Sea and 6 into the Sea of Marmara.
2.2 Sample collection and determination of metal(loid)s
Sampling was carried out twice, depending on the capacity of WWTP facilities, between, February 12–20, 2023 (winter) using scoop or composite sampling methods, and September 24–30, 2023 (autumn) using only scoop sampling method. In the summer and spring months, tourism activities are the most intense period, so the increasing population in the coastal areas causes the treatment plants to operate over capacity. Since the intensity during the tourism season does not fully reflect the dynamics of the region and the calculations cannot be found accurately, the sampling was carried out in winter and autumn months when mostly local residents are present, and the facilities operate at their normal capacity. The samples were collected in 1 liter glass bottles that were previously cleaned with ultrapure water in the laboratory. The samples taken were brought to Recep Tayyip Erdoğan University Vocational School of Technical Sciences Research Laboratory to be studied in a portable cooler. Total Suspended Solids (TSS) were filtered (Millipore HA filters, 0.45 μm) from 1 L of wastewater samples. Samples were prepared with a preliminary digesting process via a Berghof Speedwave XPERT model microwave instrument according to US EPA (2007). Metal determinations of all the samples were carried out using an Inductively Coupled Plasma Mass Spectrometer ICP-MS (Agilent 7800 ICP-MS) at BUMER- Bayburt University. Labmix24 LM24-CUS-70050 and LM24-CP-M424.5N were used as ICP-MS Calibration Standard.
2.3 Quality assurance and quality control
Quality assurance and control was ensured by blank runs, triplicate analysis of each sample and comparing measurements of the reference material prepared with the calibration standard. To ensure the accuracy of this method, a recovery experiment in which samples were spiked with mixed standards of trace elements has been conducted. The recoveries were from 95% to 99%, indicating that this method is accurate. Metal contents were expressed in terms of µg/L. The detection limit (LOD) and quantitation limit (LOQ), defined as three and ten times the signal to noise ratio respectively. LOD values were found as Al: 3,32; Cr: 0,02; Mn: 0,05; Ni: 0,07; Cu: 0,09; Zn: 1,00; As: 0,01; Cd: 0,004; Hg: 0,09; Pb: 0,01 µg/L for metals and LOQ values were found as 11,06; 0,06; 0,15; 0,25; 0,29; 3,32; 0,32; 0,01; 0,31; 0,03 µg/L, respectively.
2.4 Health risk assessment (non-carcinogenic and carcinogenic)
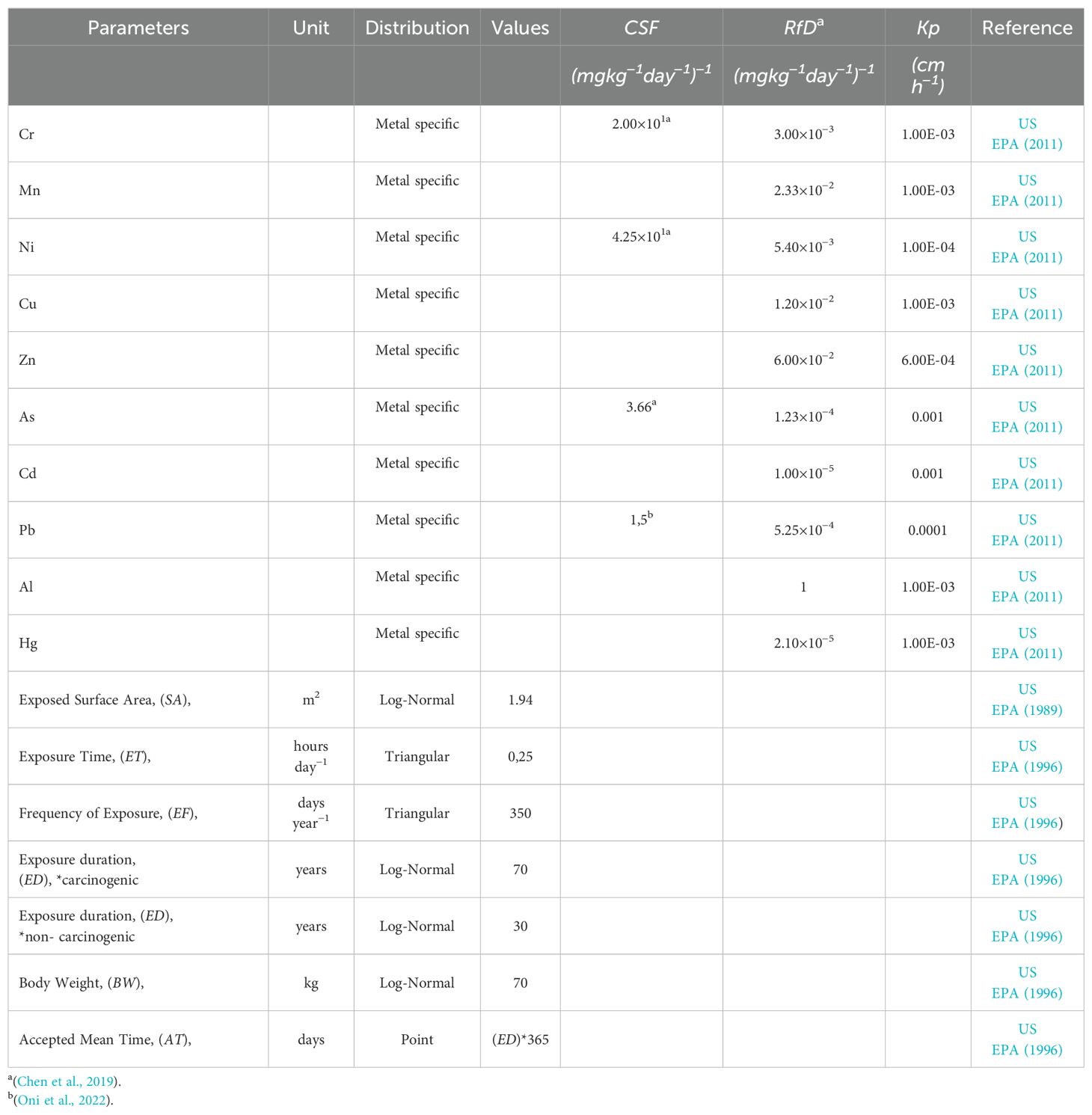
The location where the discharge occurs, whether a sea or river, is a space that is heavily utilized by the general public for recreational activities such as swimming, scuba diving, spearfishing, etc., thereby increasing the likelihood of dermal exposure. In order to make a valid risk assessment for human health, it is important to have a good definition of the problem and to know the dose and duration of exposure for a stepwise risk assessment. The current study used the US Environmental Protection Agency’s (US EPA) human health risk assessment potential method to quantitatively characterize both non-carcinogenic and carcinogenic risks in adults (US EPA, 2001). The aforementioned elements (Cr, Ni, Pb and As) have been classified as possible carcinogens by the International Agency for Research on Cancer (IARC) whereas the others (Al, Cr, Mn, Ni, Cu, Zn, As, Cd, Hg, Pb) are classified as non-carcinogens (Oni et al., 2022; IARC, 2024). The calculations were conducted exclusively on adult individuals, with a focus on dermal exposure. Hazard Index (HI) and Carcinogenic risk (CRi) are computed respectively using Equations 1, 2;
The non- carcinogenic risk was determined using the following formula;
The carcinogenic risk was determined using the following formula;
For both equations , is the concentration of the chemical (mg L−1), , is exposed surface area (m2), , is coefficient of permeability for skin (cm h−1), , is exposure time (h day−1), , is frequency of exposure (days year−1), , is exposure duration (years), , is body weight (kg), , is the accepted mean time for carcinogens is 70 years, and 30 years for non-carcinogens and , represents reference dose (mg kg−1day−1)−1. The constant values and the slope factor () values derived from the existing literature and employed in the risk formula presented in this study are provided in Table 2 (Chen et al., 2019; Oni et al., 2022; Özkaynak et al., 2022; Saha et al., 2017; US EPA, 2011, 1996, 1989). In accordance with the US EPA (1989) report, a Hazard Index (HI) value of less than 1 (HI< 1) signifies that non-carcinogenic health effects are either minimal or absent. Conversely, an HI value greater than 1 indicates the presence of non-carcinogenic health risks. When assessed in terms of carcinogenic risk, CRi is categorized as follows: very low cancer risk (CRi ≤ 10−6), low cancer risk (10−6< CRi ≤ 10−4), moderate cancer risk (10−4< CRi ≤ 10−3), higher cancer risk (10−3< CRi ≤ 0.1), very high cancer risk (CRi ≥ 0.1) (ATSDR, (Agency for Toxic Substances and Disease Registry), 1995; Ge et al., 2013).
2.5 Statistical analyses
Statistical analysis of the metal concentrations data set was performed using the JMP® 17 Statistical Software (SAS Institute Inc., Cary, USA) package. One-way analysis of variance (ANOVA) followed by Tukey Test was used to compare sampling stations among each other. Student’s T-test was used to reveal the difference between seasons. The significance level was determined as p<0.05. Metal levels and risk assessment graphics were created using Origin(Pro), 2024b. OriginLab Corporation, Northampton, MA, USA.
Multivariate statistical tool; Principal Component Analysis (PCA) were used to evaluate the relationship between different pollutants. Principal Component Analyzes (PCA) were fulfilled using SPSS 29.0 (SPSS Inc., USA) software. The Kaiser–Meyer–Olkin (KMO) measurement value of the data used for PCA analysis was 0.68, and the significance level was p<0.05. The principal component analysis (PCA) was used in the data set to determine the relationships and common origins between metals (Lu et al., 2012; Yang et al., 2011). PCA was performed with Varimax rotation, which facilitated the interpretation of output results by minimizing the number of variables that loaded high loads on each component. According to the results obtained from PCA, possible sources of chemical elements were interpreted (Kelepertzis, 2014; Peris et al., 2008; Yuan et al., 2013).
3 Results
3.1 Metal(loid) levels
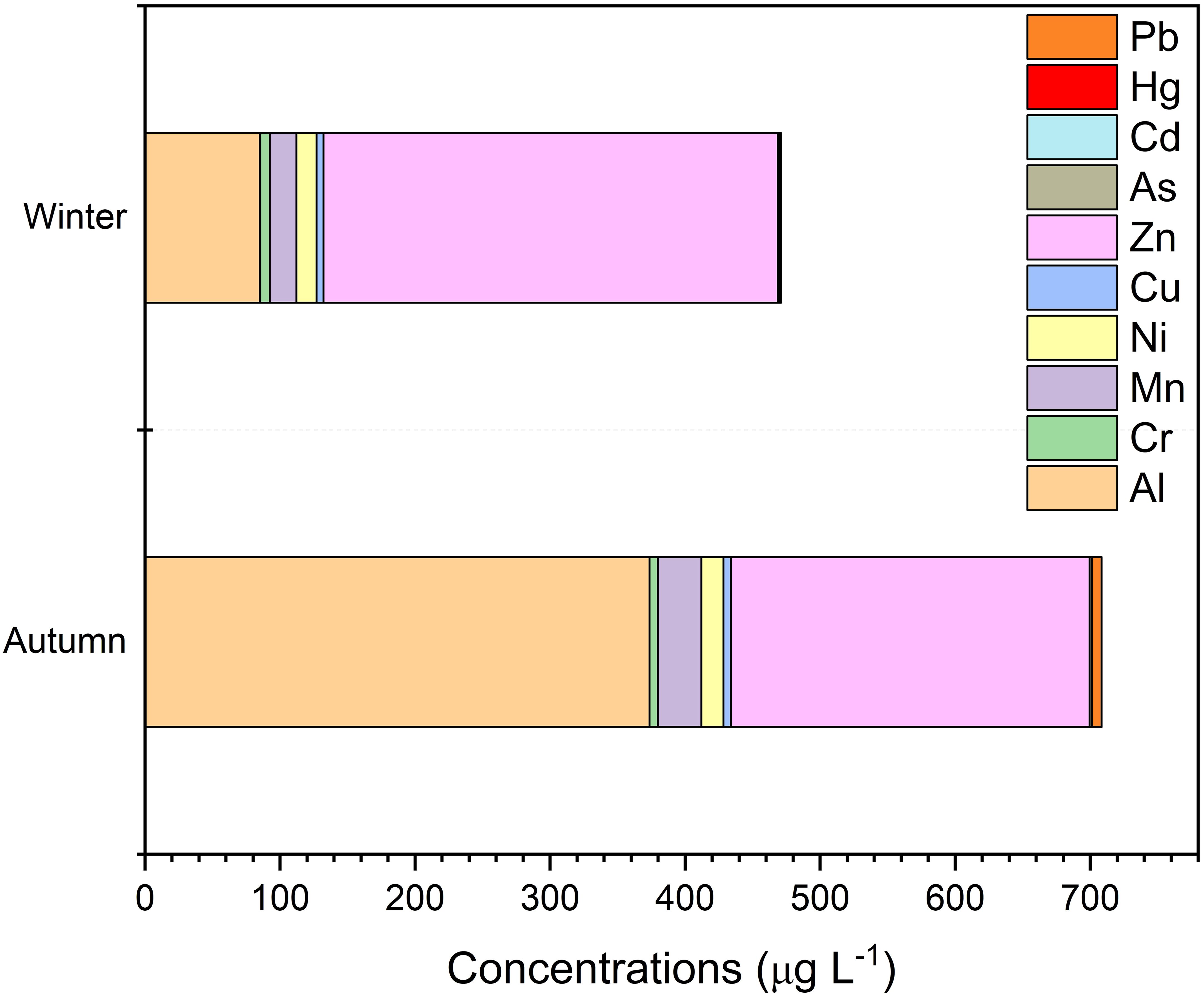
The concentration of Hg varied from below detection levels (ND) to 0,42 µg/L, with an average concentration of 0,12 µg/L. Pb concentrations ranged from a minimum of 0.02 µg/L at station S4 to a maximum of 28,93 µg/L at station S2_2. Ni concentrations spanned from 0,07 µg/L at S10 to 123,41 µg/L at S2, with an average concentration of 15,63 ± 0,22 µg/L. Aluminium (Al) levels were recorded between 11,51 µg/L at S12 and 1804,33 µg/L at S2, with an average concentration of 229,49 ± 3,48 µg/L. Lithium (Li) concentrations ranged from a minimum of 1,27 µg/L at S15 to a peak of 52,22 µg/L at S1. Cr levels varied from ND to 44,02 µg/L at station S3. Seasonal total metal concentrations also varied significantly across stations. During the autumn season, the highest total metal concentration was recorded at station S2 (326,09 mg/L), while the lowest was observed at station S7 (19,19 mg/L). In the winter season, the highest total metal concentration was measured at station S3 (131,24 mg/L), and the lowest at station S13 (2,90 mg/L). Detailed metalloid concentrations and the results of statistical analyses of wastewater samples from WWTPs are provided in Supplementary Material (SM1) and illustrated in Figure 2. Aggregated seasonal metal concentrations are presented in Figure 3.
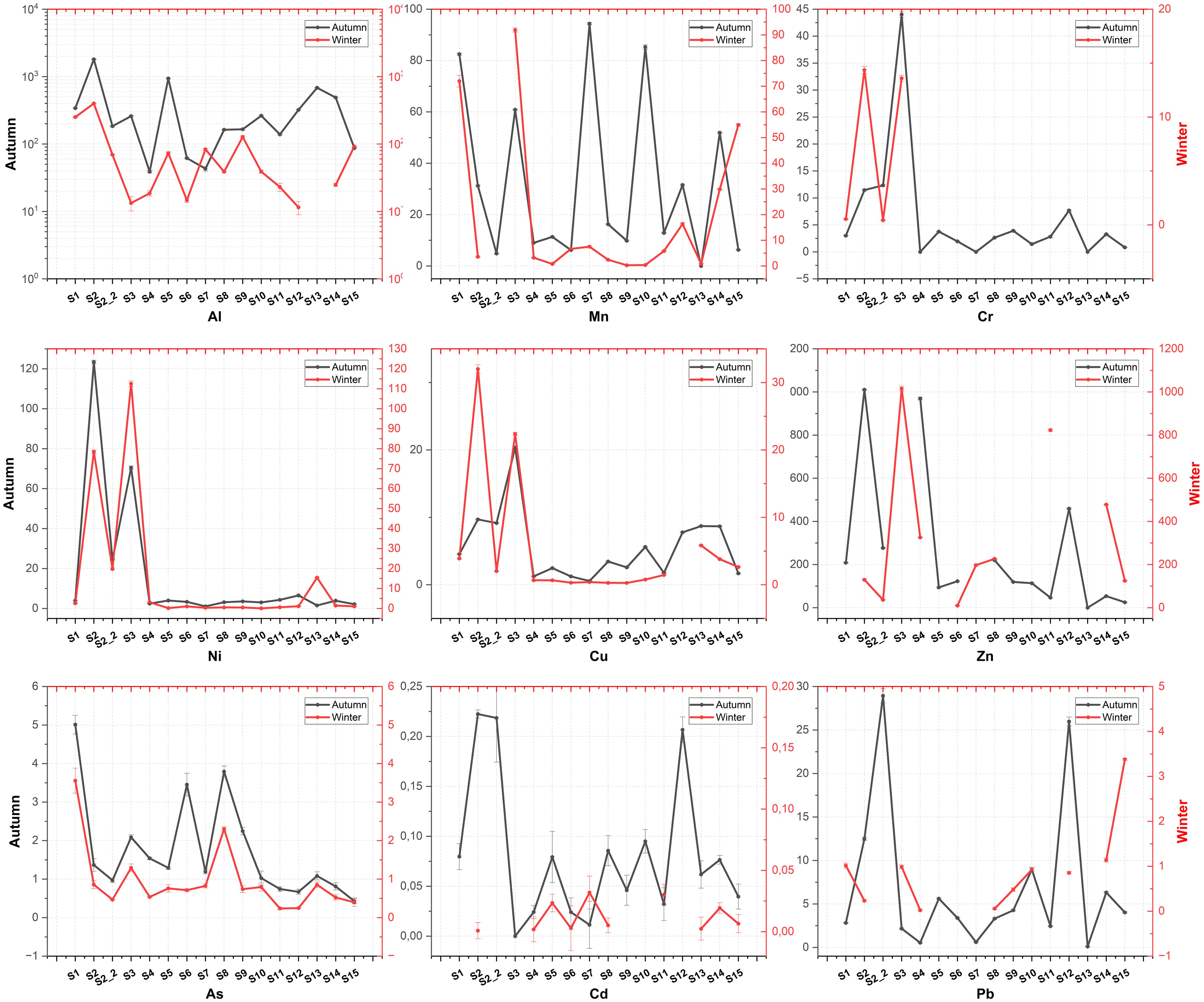
Figure 2. Trace elements concentrations (average ± standard error) in WWTP effluent along the coastline of the Black Sea and Sea of Marmara both for the autumn and winter seasons.
The discharge of wastewater into the environment is subject to a number of restrictions, which are designed to ensure that the quality of the receiving environment is not compromised. In Türkiye, the wastewater standards foreseen for the discharge of wastewater from wastewater infrastructure facilities are determined by the Ministry of Environment, Urbanization and Climate Change ‘Water Pollution Control Regulation- (WPCR) (WPCR, 2022). Therewithal WHO-World Health Organization (Aneyo et al., 2016), CMOH-China Ministry of Health (CMOH, 2019), WB-World Bank (WB and IFC, 2008), and US EPA-United States, Environmental Protection Agency (Babel and Kurniawan, 2003) such standards set by various organizations are summarized in Table 3. for comparing international standards (Kinuthia et al., 2020).
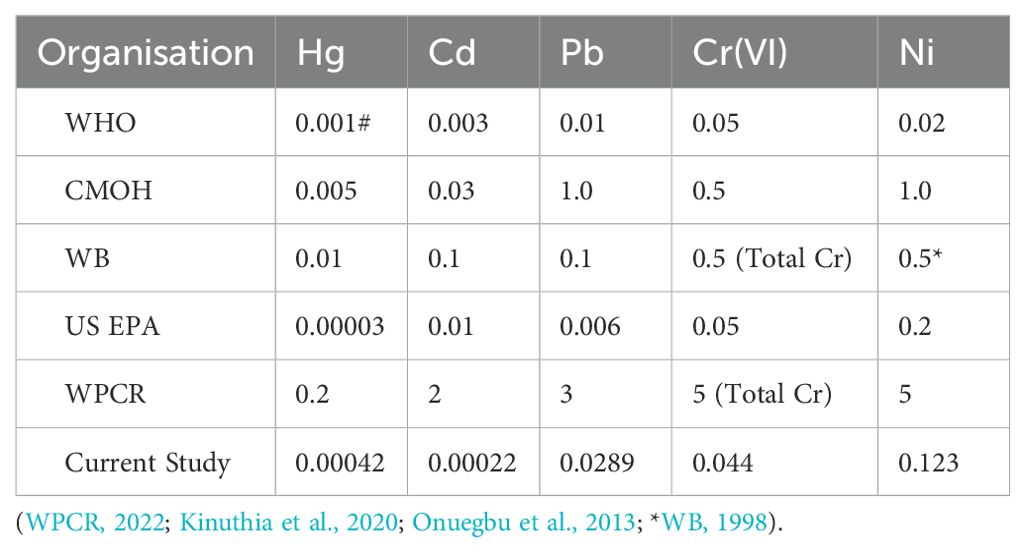
Table 3. Standards set by various organizations regarding wastewater discharge and maximum values of current study (mg L-1).
3.2 Source apportionment
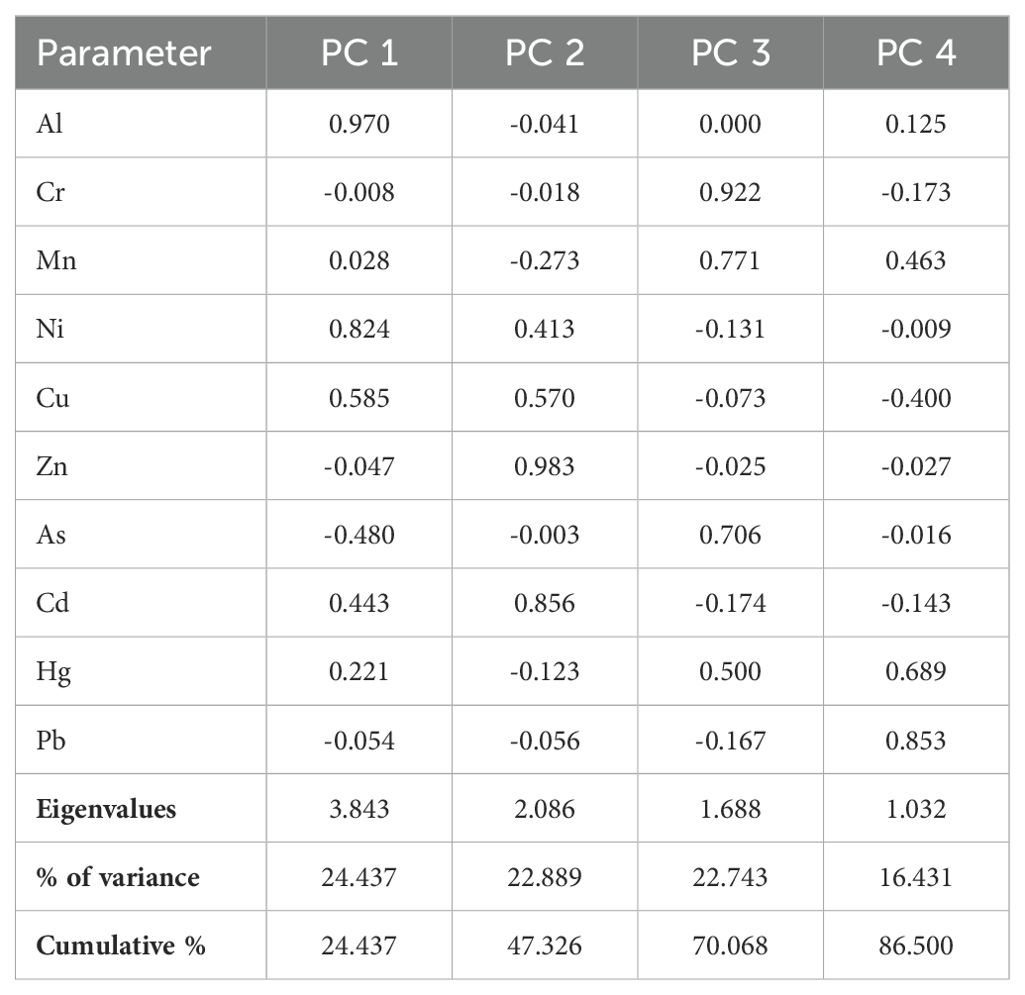
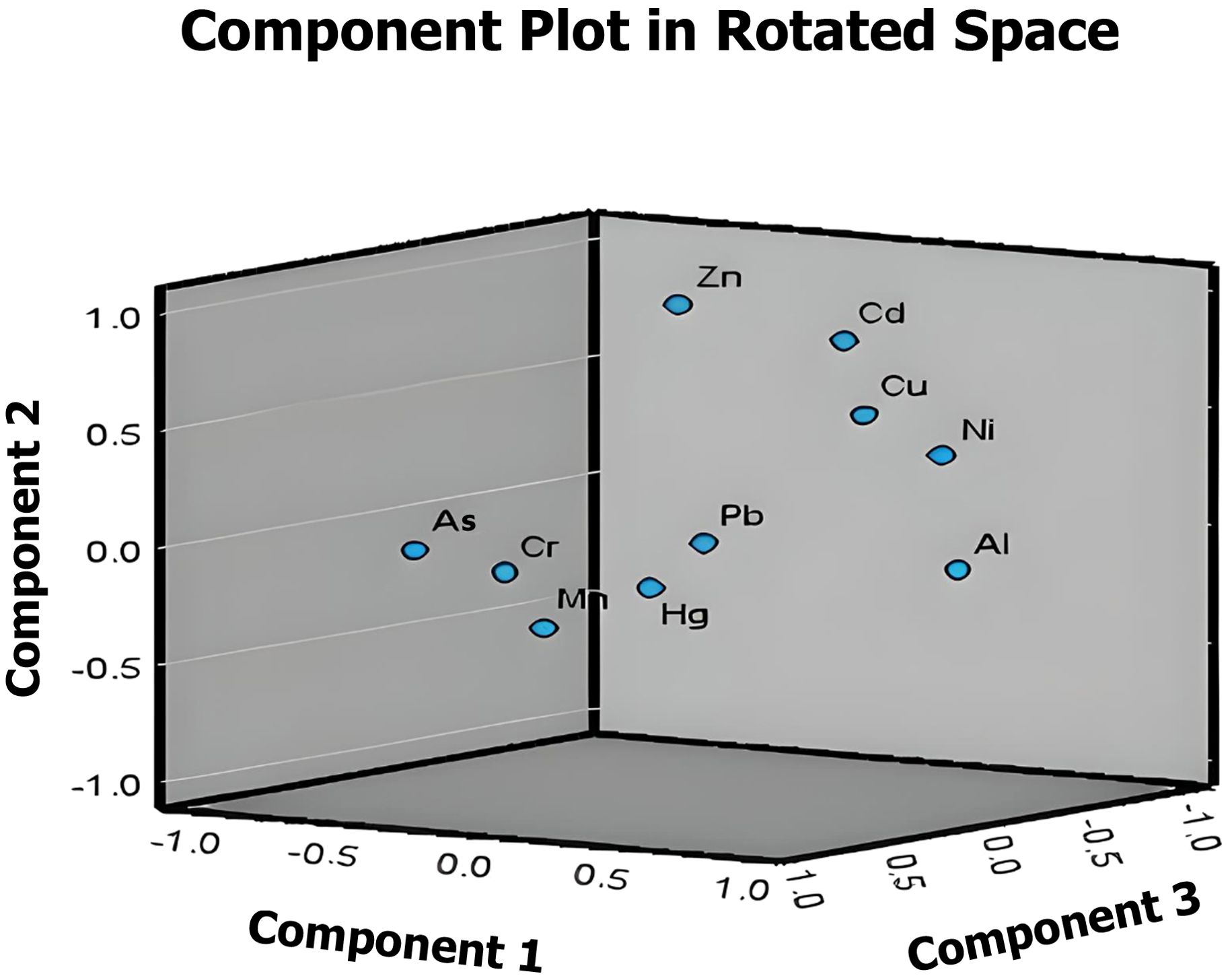
PCA analysis, a multivariate statistical method, was utilized to unravel the relationships between metals in wastewater and their sources. The Kaiser–Meyer–Olkin (KMO) measurement value of the data used for PCA analysis was 0.68, and the significance level was p<0.05. Therefore, PCA can be used as a reliable tool to analyze the source of metals in wastewater (Ji et al., 2019; Liu et al., 2023). Four principal components with eigenvalues exceeding 1 were identified, collectively explaining 86.500% of the system variance (Table 4). The graphical representation of these components depicting the interrelations among metals is illustrated in Figure 4.
3.3 Health risk assessment (non-carcinogenic and carcinogenic)
In consideration of the typical data set with mean values, Figure 5 presents data regarding both the non-carcinogenic risks associated with metals (Al, Cr, Mn, Ni, Cu, Zn, Cd, Hg, Pb) and the metalloid As, as well as the carcinogenic risks associated with metals (Cr, Ni, Pb) and the metalloid As. The HI for adults ranged from 1.72E−07 to 2.71E−03 for basis on each station, with metal(loid)s HI values ranked in the following order: Al > As > Cd > Zn > Hg > Cr > Mn > Pb > Ni > Cu. The CRi values for adults ranged from 1.99E−09 to 6.97E−05 basis on each station, with Cr and Ni having a higher calculated CRi value than Pb and As.
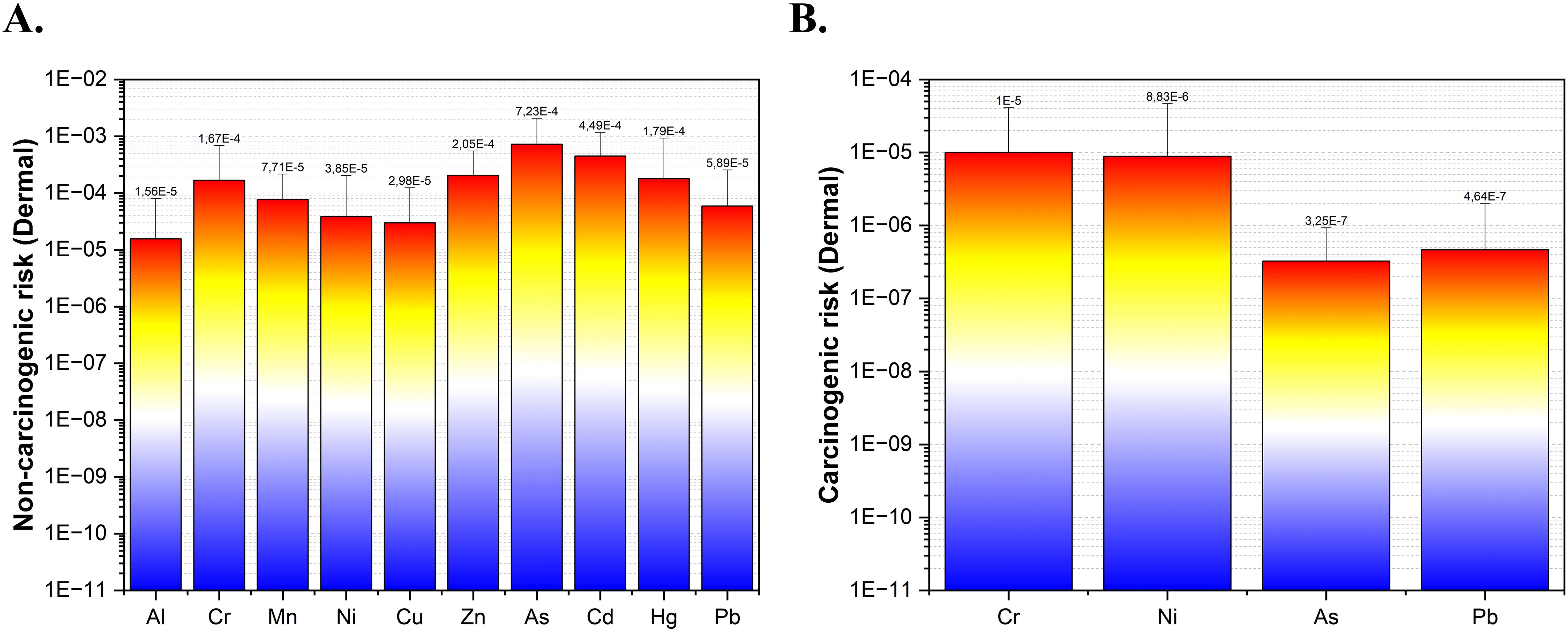
Figure 5. (A) Evaluation of non-carcinogenic risks associated with dermal exposure to various metal(loid)s. (B) Assessment of carcinogenic risks from dermal exposure to specific metal(loid)s.
4 Discussion
In this study, the wastewater discharged to the Sea of Marmara and the Black Sea from 15 WWTPs in 11 different provinces in Türkiye was examined. Sampling was done on random days that could not be manipulated by any inference.
4.1 Metal(loid) levels
According to WPCR, when the standards regarding metals in wastewater infrastructure facilities that result in deep sea discharge are accepted as criteria, the values measured from all stations are within the discharge limits. According to Figures 2, 3, the metal concentrations increase the most in the Autumn season, while the largest contribution comes from the element Al. In a study conducted by Edokpayi et al. (2017), it was stated that metal enrichment in the water environment would decrease with the increasing water volume in the winter season, and metal enrichment in the water environment would increase with the effect of evaporation in the summer season (Edokpayi et al., 2017). Another study conducted by (Islam et al., 2015) reported that metal concentration was expected to be low during the rainy season due to the dilution effect on metals, but some site–specific activities and sources of metal pollution may cause an exception to this general trend (Islam et al., 2015). In this study, similar to other studies in the literature, more enrichment was detected in the Autumn season compared to the winter season (Rajeshkumar et al., 2018; Zhao et al., 2014). Except for S3 for the autumn season, this result coincides proportionally with the population density that the facilities serve, but when the results are considered for the winter season, S13 station has lower metal levels in both instant and composite samples, although it serves a relatively more crowded population.
Exposure to Hg and Hg compounds in humans causes various health problems; cancer, damage to the brain, lungs and kidneys, damage to developing fetuses, high blood pressure or abnormal heart rate, vomiting and diarrhea, skin rashes and eye irritation are among the important health problems associated with Hg (Kinuthia et al., 2020; Martin and Griswold, 2009). The highest value was determined in S1 station. Similar results were revealed in a study conducted in Brazil (Sao Paulo) and the Hg value was found to be 0.13 µg/L (Oliveira et al., 2007). Hg values measured at the S1 station in winter and respectively S11, S1, S8, S2, and S9 stations in autumn were measured above the limit values set by the United States Environmental Protection Agency (US EPA) (Babel and Kurniawan, 2003). In addition, the value measured at S3 station is equal to the limit value. Sorme and Lagerkvist, 2002, mentioned in their study that 70% of the sources of Ni and Hg metals have been identified, and that the Hg contribution to wastewater comes mainly from pipe sediments and amalgam used in dental fillings, which is less common today in Türkiye than in the past. The contamination of waterways and other aquatic environments can be attributed to a number of factors, including the improper disposal of lighting systems such as fluorocarbons, mercury-operated thermometers, and similar devices. Additionally, the release of mercury-containing medical waste, cleaners, or other chemicals into the environment can also contribute to this phenomenon.
According to Kinuthia et al., 2020, Pb poisoning in humans damages the kidneys, liver, heart, brain, skeleton and nervous system (Flora et al., 2006). In case of Pb exposure, the first symptoms of poisoning include headache, dullness, memory loss and irritability (CDC, 2002). Pb poisoning can cause impaired hemoglobin synthesis and anemia. It has been claimed that chronic exposure to low levels of Pb in children can reduce their mental capacity. According to the International Agency for Research on Cancer (IARC), Pb is a possible human carcinogen (IARC, 2024; Järup, 2003; Kinuthia et al., 2020; Oni et al., 2022). Pb values measured from S2 and S12 stations in the autumn season were measured above the limit values determined by both the World Health Organization (WHO) and the United States Environmental Protection Agency (US EPA). In addition, the Pb values measured at S10 and S14 stations are above the limit values determined by the United States Environmental Protection Agency (US EPA). In light of the fact that the primary sources of Pb contamination are industrial activities, the findings of the study are deemed to be valid for all stations with the exception of S12. It is acknowledged that another significant source is from urban vehicle traffic and car washes. However, given the relatively low population density at S12 station, it is challenging to ascertain the precise origin of this elevated value. On the contrary, mean concentrations of Pb were lower than other studies (Chanpiwat et al., 2010; Karvelas et al., 2003; Oliveira et al., 2007).
The negative effects of Ni on human health include dermatitis, allergy, organ diseases and respiratory system cancer (Kinuthia et al., 2020; Seilkop and Oller, 2003). Ni observations of this study was comparatively lower than the content recorded in the Bangkok (Chanpiwat et al., 2010) and Thessaloniki (Karvelas et al., 2003). Ni values measured in both autumn and winter samples from S2 and S3 stations are above the limit values determined by the World Health Organization (WHO). A variety of natural processes, including rock and soil erosion, contribute to the presence of Ni in natural water bodies (Pratap et al., 2023). The results of the study indicate that Ni and Al are of natural (geogenic) origin, as determined by PCA analysis. Nevertheless, as both stations where the highest values were recorded (S2 and S3) are situated in regions where industrial and production activities are particularly prevalent, it is evident that industrial waste is a significant contributor to the pollution in these stations.
Aluminium (Al) coagulants can be used to reduce phosphate loads discharged into surface waters during wastewater treatment. Al is considered potentially toxic as it causes gill damage in fish under some pH conditions (Comber et al., 2005). According to Environmental Quality Standards (EQS), the limiting concentration is 10 µg/L for waters with pH values below 6.5 and 25 µg/L for waters with pH values above 6.5 (Comber et al., 2005). The autumn sampling results indicate that the Al value measured for all stations is significantly higher than the determined threshold values, even for those undergoing primary treatment. For winter sampling, only the values measured at stations S14, S13, S12, S11, S6 and S4 are below the limit values.
When examined from a human health perspective, exposure to Li ions can cause damage to the nervous system, kidneys, liver, brain, cardiovascular and endocrine systems (Ivkovic and Stern, 2014; Luo et al., 2015; McKnight et al., 2012; Yamaguchi et al., 2013). Both stations where the lowest and highest lithium values were measured, discharge is carried out with mechanical treatment. When examining why this value is highest in S1. Although there is currently no Li resource of economic value in Türkiye, it has been mentioned in various studies that boron deposits contain Li in certain amounts (Sensöz et al., 2021). Whether there is Li contamination in urban wastewater due to geological processes is a different research topic. There are studies on obtaining Li from wastewater (Luo et al., 2015). Today, classical purification systems do not have any effect on the removal of Li pollution (Choi et al., 2019).
Prior research has indicated a correlation between Cr released from chromium production and tanning activities and the development of respiratory tract cancer (ATSDR, (Agency for Toxic Substances and Disease Registry), 2012; Rai et al., 2019). It can be ascertained that there are leather manufacturing facilities in the vicinity of the relevant station (S3). In alignment with the available literature, it is hypothesized that there is a potential contribution to the Cr concentration. As, which has its origins in the Earth’s crust, mining, metal smelting and fossil fuel combustion, has been identified as a carcinogenic substance to the skin (skin cancer) and internal organs. It has also been found to affect the central nervous system, cause pigmentation, hard spots (hyperkeratosis) and skin lesions on the palms and feet. However, the concentrations present are below the WHO threshold of 10 µg/L for all stations in both seasons (Pratap et al., 2023).
4.2 Source apportionment
Factor loadings were classified by Liu et al. (2003) as strong (< 0.75) and moderate (0.75–0.50). PC1, PC2, PC3 and PC2 explained 24.437%, 22.889%, 22.743% and 16.431% of the total variance, respectively. PC1 is strongly loaded on Al and Ni and this component can be explained as natural (geogenic) sources. On the other hand, PC2 is loaded on Cu, Zn and Cd and PC3 is loaded on Cr, Mn, As and Mn and therefore the sources of these components can be explained as multiple sources, natural (geogenic) and anthropogenic. PC4 is strongly and moderately predominantly loaded on Hg and Cd and the source of this component can be explained as anthropogenic such as industrial activities (Moloi et al., 2020; Saha et al., 2017).
4.3 Health risk assessment (non-carcinogenic and carcinogenic)
In light of findings of this study, the calculated HI values were found to be less than one (HI< 1), which suggests that the non-cancer risks associated with metal(loid)s would not be taken into account for adults in the study area. In consideration of the mean values within the scope of this study, the CRi values calculated for Ni and Cr were found to be within the range of low cancer risk (10−6< CRi ≤ 10−4) while for As and Pb emerged very low cancer risk (CRi ≤ 10−6) as described in (ATSDR, (Agency for Toxic Substances and Disease Registry), 1995; Ge et al., 2013). A risk assessment based on the analysis of individual stations (SM 2) revealed that only S2 and S3 stations exhibited a low cancer risk associated with Cr and Ni, both in the autumn and winter seasons. Furthermore all stations except S1 in winter showed a very low cancer risk (CRi ≤ 10-6) for Cr both in the autumn and winter seasons. Upon evaluation of the other stations in terms of Ni, it was observed that the majority exhibited a very low cancer risk (CRi ≤ 10-6), with the exception of S7 and S13 in the autumn season. During the winter season, S1, S4, and S13 also demonstrated a very low cancer risk (CRi ≤ 10-6). In the newsworthy existing literature, probabilistic health risk studies have predominantly focused on the potential contamination of fish (Ramish et al., 2024), municipal sewage sludge from a wastewater treatment plants (Nyashanu et al., 2023), drinking water derived from wastewater (Manyepa et al., 2024), and agricultural crops irrigated with wastewater (Abou Fayssal et al., 2024; Mohammadi et al., 2024). A paucity of studies has been conducted on the wastewater discharged directly from wastewater treatment plants in Türkiye (Arslan-Alaton et al., 2007; Birtek et al., 2022; Salihoglu, 2013). In their study on the wastewater of ships, Özkaynak et al. (2022) ranked the dermal intake of metals in the carcinogenic risk calculation from highest to lowest as follows: Ni > As > Cr (Özkaynak et al., 2022). This ranking is not the same as the ranking that was obtained in the current study.
From another point of view it is anticipated that wastewater discharged to the marine environment via outfalls will be retained on the seabed. Although wastewater is effectively trapped in the Sea of Marmara except for some specific conditions due to the presence of a stable pycnocline layer (Özturk, 1996), when considering temperature, salinity and Sigma-theta (σT) profile (Agirbas et al., 2014) it is probable that in the Black Sea, wastewater will mix with surface waters throughout the year, with the exception of the summer months, given the current discharge depths (Akdemir, 2021). It can be reasonably deduced that planktonic organisms and other living organisms, which play a significant role in the Black Sea ecosystem and are known to be highly susceptible to pollutants, will be directly affected. Furthermore, it can be reasonably inferred that this is closely associated with the health of those who engage in recreational activities in the Black Sea. Upon examination of the characteristics of the treatment plants, it becomes evident that the majority of WWTPs in the Black Sea have only undergone primary treatment.
5 Conclusion
Based on the findings of this research and measured values, although the wastewater discharged from the facilities seems to meet the national quality criteria, there are differences with some international criteria. In this form, 1.38 × 106 m3 (per day) of wastewater is discharged from the facilities to the receiving environment. However, this does not mean that the wastewater discharged from the stations does not have negative effects on the health of the ecosystem or that it will not harm the receiving body when evaluated cumulatively. Both the Black Sea and the Sea of Marmara are the two important seas from which we benefit most from fishing resources (TSI, 2024). The metal(loid)s subject to this study pose serious life risks for the top step of the food pyramid as a result of their bioaccumulation and biomagnification effects. Furthermore, the receiving body also presents significant risks for recreational users who engage in intense activities, prompting concerns about potential dangers. The results of this study suggest that it is necessary to determine permissible discharge limits at least according to international standards, establish control mechanisms to reduce pollutants at their sources, and enhance the effectiveness of treatment systems. The reduction of pollutants at their sources is a complex undertaking that requires a multifaceted approach. It is only through social cooperation, the implementation of incentives, technological advancement and innovation, coupled with an emphasis on education and awareness, that the aforementioned benefits, including cost-effectiveness, environmental protection, compliance with environmental legislation and sustainability, can be achieved.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
TA: Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research funded by FBA-2021-1269 project supported by Research Funds of the Recep Tayyip Erdogan University.
Acknowledgments
I would like to extend my gratitude to the Water and Sewerage Administrations of Rize, Trabzon, Samsun, Gerze, Cide, Zonguldak, Ereğli, Sakarya, Yalova, Bursa, Tekirdağ and Istanbul Municipalities for their invaluable support. I would like to express my gratitude to Dr. Tanju Mutlu, Dr. Kenan Gedik, Dr. Murat Şirin and Dr. Mert Minaz for providing me with invaluable motivation and support. This study has been supported by the Recep Tayyip Erdoğan University Development Foundation (Grant number: 02024010031140).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1521449/full#supplementary-material
References
Abou Fayssal S., Kumar P., Popescu S. M., Khanday M.ud d., Sardar H., Ahmad R., et al. (2024). Health risk assessment of heavy metals in saffron (Crocus sativus L.) cultivated in domestic wastewater and lake water irrigated soils. Heliyon 10. doi: 10.1016/j.heliyon.2024.e27138
Abu Amr S. S., Abujazar M. S. S., Karaağaç S. U., Mahfud R., Alazaiza M. Y. D., Hamad R. J. A. (2023). Application of plant-based natural coagulant for sustainable treatment of steel and iron industrial wastewater, Karabuk, Turkey. Desalination Water Treat 287, 39–45. doi: 10.5004/dwt.2023.29393
Agirbas E., Feyzioglu A. M., Kopuz U. (2014). Seasonal Changes of Phytoplankton Chlorophyll a, Primary Production and their Relation in the Continental Shelf Area of the South Eastern Black Sea. Turk J. Fish Aquat Sci. 14, 713–726. doi: 10.4194/1303-2712-v14_3_14
Ahmed J., Thakur A., Goyal A. (2021). “Biological treatment of industrial wastewater,” in Biological Treatment of Industrial Wastewater. Ed. Shah M. P. (The Royal Society of Chemistry, Cambridge), 1–14.
Akdemir T. (2021). Derin Deniz Deşarjı Yapılarının Faunası ve Toksikolojik Olarak Değerlendirilmesi (Rize, Türkiye: Recep Tayyip Erdogan University Institute of Graduate Studies, Rize).
Akdemir T., Dalgic G. (2020). The impact of the marine sewage outfalls on the sediment quality: The Black Sea and the Marmara case. Saudi J. Biol. Sci. 28 (1), 238–246. doi: 10.1016/j.sjbs.2020.09.055
Aneyo I. A., Doherty F. V., Adebesin O. A., Hammed M. O. (2016). Biodegradation of pollutants in waste water from pharmaceutical, textile and local dye effluent in Lagos, Nigeria. J. Health pollut. 6, 34–42. doi: 10.5696/2156-9614-6.12.34
Arslan E., Demir T., Üstünyıldız B., Baldan Pakdil N. (2023). Operational issues and proposed solutions in wastewater treatment plants in the Western Black Sea basin of Turkey. Materials Today: Proc. 81, 59–67. doi: 10.1016/j.matpr.2022.12.173
Arslan-Alaton I., Tanik A., Ovez S., Iskender G., Gurel M., Orhon D. (2007). Reuse potential of urban wastewater treatment plant effluents in Turkey: a case study on selected plants. Desalination 215, 159–165. doi: 10.1016/j.desal.2006.11.019
ATSDR, (Agency for Toxic Substances and Disease Registry) (1995). Public health assessment (New York, USA: Johnstown City Landfill, Johnstown, Fulton Country).
ATSDR, (Agency for Toxic Substances and Disease Registry) (2012). Toxicological Profile for Chromium (Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service).
Babel S., Kurniawan T. A. (2003). Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J. Hazard Mater 97, 219–243. doi: 10.1016/S0304-3894(02)00263-7
Birtek R. I., Karpuzcu M. E., Ozturk I. (2022). Occurrence of priority substances in urban wastewaters of Istanbul and the estimation of the associated risks in the effluents. Environ. Monit Assess. 194, 426. doi: 10.1007/s10661-022-09840-w
CDC (2002). Cover illustration courtesy of the Los Angeles County Childhood Lead Poisoning Prevention Program. Los Angeles Public Health, LA, USA.
Chanpiwat P., Sthiannopkao S., Kim K.-W. (2010). Metal content variation in wastewater and biosludge from Bangkok’s central wastewater treatment plants. Microchemical J. 95, 326–332. doi: 10.1016/j.microc.2010.01.013
Chen G., Wang X., Wang R., Liu G. (2019). Health risk assessment of potentially harmful elements in subsidence water bodies using a Monte Carlo approach: An example from the Huainan coal mining area, China. Ecotoxicol Environ. Saf. 171, 737–745. doi: 10.1016/j.ecoenv.2018.12.101
Choi H., Ryu J. S., Shin W. J., Vigier N. (2019). The impact of anthropogenic inputs on lithium content in river and tap water. Nat. Commun. 10. doi: 10.1038/s41467-019-13376-y
CMOH (2019). Effluent Standards (China Ministry of Environment, Laws & Regulations Database of The Republic of China: Taiwan).
Comber S. D. W., Gardner M. J., Churchley J. (2005). Aluminium speciation: implications of wastewater effluent dosing on river water quality. Chem. Speciation Bioavailability 17, 117–128. doi: 10.3184/095422905782774874
De Buyck P. J., Van Hulle S. W. H., Dumoulin A., Rousseau D. P. L. (2021). Roof runoff contamination: A review on pollutant nature, material leaching, and deposition. Rev. Environ. Sci. Biotechnol. 20 (2), 549–606. doi: 10.1007/s11157-021-09567-z
Edokpayi J. N., Odiyo J. O., Popoola E. O., Msagati T. A. M. (2017). Evaluation of temporary seasonal variation of heavy metals and their potential ecological risk in Nzhelele River, South Africa. Open Chem. 15, 272–282. doi: 10.1515/chem-2017-0033
Fairbanks S. D., Pamanik S. K., Thomas J. A., Das A., Martin N. (2021). The management of mercury from dental amalgam in wastewater effluent. Environ. Technol. Rev. 10 (1), 213–223. doi: 10.1080/21622515.2021.1957884
FAO (2023). The State of Mediterranean and Black Sea Fisheries 2023 (Rome, Italy: FAO). doi: 10.4060/cc8888en
Fiala M., Hwang H. M. (2021). Influence of highway pavement on metals in road dust: A case study in Houston, Texas. Water Air Soil pollut. 232, 185. doi: 10.1007/s11270-021-05139-7
Flora S. J. S., Flora G., Saxena G. (2006). Environmental occurrence, health effects and management of lead poisoning. In: Cascas SB, Sordo J editors. Lead: Chemistry, Analytical Aspects, Environmental Impacts and Health Effects. Netherlands: Elsevier Publication), 158–228. doi: 10.1016/B978-044452945-9/50004-X
Ge J., Woodward L. A., Li Q. X., Wang J. (2013). Composition, distribution and risk assessment of organochlorine pesticides in soils from the Midway Atoll, North Pacific Ocean. Sci. Total Environ. 452–453, 421–426. doi: 10.1016/j.scitotenv.2013.03.015
IARC (2024). IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online at: https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (Accessed 8.28.24).
Islam Md., Uddin M., Tareq S., Shammi M., Kamal A., Sugano T., et al. (2015). Alteration of water pollution level with the seasonal changes in mean daily discharge in three main rivers around Dhaka City, Bangladesh. Environments 2, 280–294. doi: 10.3390/environments2030280
Ivkovic A., Stern T. A. (2014). Lithium-induced neurotoxicity: clinical presentations, pathophysiology, and treatment. Psychosomatics 55, 296–302. doi: 10.1016/j.psym.2013.11.007
Jaishankar M., Tseten T., Anbalagan N., Mathew B. B., Beeregowda K. N. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 7, 60–72. doi: 10.2478/intox-2014-0009
Järup L. (2003). Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182. doi: 10.1093/bmb/ldg032
Ji Z., Zhang H., Zhang Y., Chen T., Long Z., Li M., et al. (2019). Distribution, ecological risk and source identification of heavy metals in sediments from the Baiyangdian Lake, Northern China. Chemosphere 237, 124425. doi: 10.1016/j.chemosphere.2019.124425
Karvelas M., Katsoyiannis A., Samara C. (2003). Occurrence and fate of heavy metals in the wastewater treatment process. Chemosphere 53, 1201–1210. doi: 10.1016/S0045-6535(03)00591-5
Kelepertzis E. (2014). Accumulation of heavy metals in agricultural soils of Mediterranean: Insights from Argolida basin, Peloponnese, Greece. Geoderma 221–222, 82–90. doi: 10.1016/j.geoderma.2014.01.007
Kinuthia G. K., Ngure V., Beti D., Lugalia R., Wangila A., Kamau L. (2020). Levels of heavy metals in wastewater and soil samples from open drainage channels in Nairobi, Kenya: community health implication. Sci. Rep. 10. doi: 10.1038/s41598-020-65359-5
Kumar D., Malik S., Rani R., Kumar R., Duhan J. S. (2023). Behavior, risk, and bioremediation potential of heavy metals/metalloids in the soil system. Rendiconti Lincei. Sci. Fisiche e Naturali 34 (3), 809–831. doi: 10.1007/s12210-023-01166-0
Lakshmi K. S., Reddy M. A. (2017). Wastewater - an overview. Int. J. Contemp. Res. Rev. 08 (08), 20254–20262. doi: 10.15520/ijcrr/2017/8/08/287
Liu C.-W., Lin K.-H., Kuo Y.-M. (2003). Application of factor analysis in the assessment of groundwater quality in a blackfoot disease area in Taiwan. Sci. Total Environ. 313, 77–89. doi: 10.1016/S0048-9697(02)00683-6
Liu X., Sheng Y., Liu Q., Li Z. (2023). Ecological and environmental risks of heavy metals in sediments in Dingzi Bay, South Yellow Sea. Mar. pollut. Bull. 188, 114683. doi: 10.1016/j.marpolbul.2023.114683
Lu A., Wang J., Qin X., Wang K., Han P., Zhang S. (2012). Multivariate and geostatistical analyses of the spatial distribution and origin of heavy metals in the agricultural soils in Shunyi, Beijing, China. Sci. Total Environ. 425, 66–74. doi: 10.1016/j.scitotenv.2012.03.003
Luo X., Guo B., Luo J., Deng F., Zhang S., Luo S., et al. (2015). Recovery of lithium from wastewater using development of li ion-imprinted polymers. ACS Sustain Chem. Eng. 3, 460–467. doi: 10.1021/sc500659h
Manasa R. L., Mehta A. (2020). “Wastewater: Sources of Pollutants and Its Remediation,” in Environmental Biotechnology Vol. 2. Environmental Chemistry for a Sustainable World, vol 45. Eds. Gothandam K., Ranjan S., Dasgupta N., Lichtfouse E. (Springer, Cham). doi: 10.1007/978-3-030-38196-7_9
Manyepa P., Gani K. M., Seyam M., Banoo I., Genthe B., Kumari S., et al. (2024). Removal and risk assessment of emerging contaminants and heavy metals in a wastewater reuse process producing drinkable water for human consumption. Chemosphere 361. doi: 10.1016/j.chemosphere.2024.142396
Martin S., Griswold W. (2009). Environmental science and technology briefs for citizens human health effects of heavy metals. Environmental Science and Technology Briefs for Citizens. Issue 15, Kansas State University, Center for Hazardous Substance Research.
Maryam B., Büyükgüngör H. (2019). Wastewater reclamation and reuse trends in Turkey: Opportunities and challenges. J. Water Process Eng. 30, 100501. doi: 10.1016/j.jwpe.2017.10.001
McKnight R. F., Adida M., Budge K., Stockton S., Goodwin G. M., Geddes J. R. (2012). Lithium toxicity profile: a systematic review and meta-analysis. Lancet 379, 721–728. doi: 10.1016/S0140-6736(11)61516-X
MCT (2023). Border Statistics 2023 (Ankara, Türkiye: Ministry of Culture and Tourism of the Republic of Türkiye, Tourism Statistics Yearly Bulletins).
MEUC (2020). Republic of Türkiye, Ministry of Environment, Urbanisation and Climate Change 2020 Provincial Environmental Status Reports. Ankara, Türkiye.
MEUC (2021). Republic of Türkiye, Ministry of Environment, Urbanisation and Climate Change 2021 Provincial Environmental Status Reports. Ankara, Türkiye.
Mohammadi M. J., Kiani F., Farhadi M., Ghanbari S., Jalili D., Mirzaei L. (2024). Evaluation of carcinogenic risk of heavy metals due to consumption of rice in Southwestern Iran. Toxicol. Rep. 12, 578–583. doi: 10.1016/j.toxrep.2024.05.005
Molaey R., Appels L., Yesil H., Tugtas A. E., Çalli B. (2024). Sustainable heavy metal removal from sewage sludge: A review of bioleaching and other emerging technologies. Sci. Total Environ. 955, 177020. doi: 10.1016/j.scitotenv.2024.177020
Moloi M., Ogbeide O., Voua Otomo P. (2020). Probabilistic health risk assessment of heavy metals at wastewater discharge points within the Vaal River Basin, South Africa. Int. J. Hyg Environ. Health 224, 113421. doi: 10.1016/j.ijheh.2019.113421
Nkele K., Mpenyana-Monyatsi L., Masindi V. (2022). Challenges, advances and sustainabilities on the removal and recovery of manganese from wastewater: A review. J. Cleaner Production 377, 134152. doi: 10.1016/j.jclepro.2022.134152
Nnaji N. D., Onyeaka H., Miri T., Ugwa C. (2023). Bioaccumulation for heavy metal removal: a review. SN Appl. Sci. 5, 125. doi: 10.1007/s42452-023-05351-6
Nyashanu P. N., Shafodino F. S., Mwapagha L. M. (2023). Determining the potential human health risks posed by heavy metals present in municipal sewage sludge from a wastewater treatment plant. Sci. Afr 20. doi: 10.1016/j.sciaf.2023.e01735
Oliveira A. D. S., Bodo A., Beltramini Trevilato T. M., Magosso Takayanagui A. M., Domingo J. L., Segura-Muñoz S. I. (2007). Heavy metals in untreated/treated urban effluent and sludge from a biological wastewater treatment plant. Environ. Sci. pollut. Res. 14, 483–489. doi: 10.1065/espr2006.10.355
Oni A. A., Babalola S. O., Adeleye A. D., Olagunju T. E., Amama I. A., Omole E. O., et al. (2022). Non-carcinogenic and carcinogenic health risks associated with heavy metals and polycyclic aromatic hydrocarbons in well-water samples from an automobile junk market in Ibadan, SW-Nigeria. Heliyon 8, e10688. doi: 10.1016/j.heliyon.2022.e10688
Onuegbu T. U., Umoh E. T., Onwuekwe I. T. (2013). Physico–chemical analysis of effluents from Jacbon chemical industries limited, makers of bonalux emulsion and gloss paints. Int J. Sci. Tech. 2 (2), 169–173.
Özkaynak Ö.H., İçemer G. T., Merdun H. (2022). Determination of the risk on human health of heavy metals contained by ship source bilge and wastewater discharged to the sea on the mediterranean by monte carlo simulation. Sustainability (Switzerland) 14. doi: 10.3390/su14148408
Özturk İ. (1996). Atıksu Ön Arıtma ve Deniz Deşarjı Sistemleri. 1st ed (İstanbul: İstanbul Technical University).
Peris M., Recatalá L., Micó C., Sánchez R., Sánchez J. (2008). Increasing the knowledge of heavy metal contents and sources in agricultural soils of the european mediterranean region. Water Air Soil pollut. 192, 25–37. doi: 10.1007/s11270-008-9631-1
Pratap B., Kumar S., Nand S., Azad I., Bharagava R. N., Romanholo Ferreira L. F., et al. (2023). Wastewater generation and treatment by various eco-friendly technologies: Possible health hazards and further reuse for environmental safety. Chemosphere 313, 137547. doi: 10.1016/j.chemosphere.2022.137547
Rai P. K., Lee S. S., Zhang M., Tsang Y. F., Kim K.-H. (2019). Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int. 125, 365–385. doi: 10.1016/j.envint.2019.01.067
Rajeshkumar S., Liu Y., Zhang X., Ravikumar B., Bai G., Li X. (2018). Studies on seasonal pollution of heavy metals in water, sediment, fish and oyster from the Meiliang Bay of Taihu Lake in China. Chemosphere 191, 626–638. doi: 10.1016/j.chemosphere.2017.10.078
Ramish, Atif Irshad M., Nawaz R., Nasim I., Irfan A., Hussain A., et al. (2024). Bioaccumulation of carcinogenic metals in river fish: A quantitative investigation of public health risk. Ecol. Indic 162. doi: 10.1016/j.ecolind.2024.112057
Saha N., Rahman M. S., Ahmed M. B., Zhou J. L., Ngo H. H., Guo W. (2017). Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manage 185, 70–78. doi: 10.1016/j.jenvman.2016.10.023
Salihoglu N. K. (2013). Assessment of urban source metal levels in influent, effluent, and sludge of two municipal biological nutrient removal wastewater treatment plants of bursa, an industrial city in Turkey. Clean (Weinh) 41, 153–165. doi: 10.1002/clen.201100518
Satyapal G. K., Kumar N. (2021). “Arsenic: Source, distribution, toxicity, and bioremediation,” in Arsenic Toxicity: Challenges and Solutions. Ed. Kumar N. (Springer, Singapore), 153–163. doi: 10.1007/978-981-33-6068-6_6
Seilkop S. K., Oller A. R. (2003). Respiratory cancer risks associated with low-level nickel exposure: an integrated assessment based on animal, epidemiological, and mechanistic data. Regul. Toxicol. Pharmacol. 37, 173–190. doi: 10.1016/S0273-2300(02)00029-6
Sensöz H., Sayın Z. E., Savaş M., Erdoğan Y. (2021). Emet bor üretim tesisleri atıklarının lityum içeriğinin incelenmesi. Afyon Kocatepe Univ. J. Sci. Eng. 21, 1460–1469. doi: 10.35414/akufemubid.933125
Shrestha R., Ban S., Devkota S., Sharma S., Joshi R., Tiwari A. P., et al. (2021). Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 9, 105688. doi: 10.1016/j.jece.2021.105688
Singh S., Devi N. L. (2023). “Heavy metal pollution in atmosphere from vehicular emission,” in Heavy Metal Toxicity: Environmental Concerns, Remediation and Opportunities. Eds. Singh R. P., Singh P., Srivastava A. (Springer, Singapore). doi: 10.1007/978-981-99-0397-9_9
Singh P. K., Kumar U., Kumar I., Dwivedi A., Singh P., Mishra S., et al. (2024). Critical review on toxic contaminants in surface water ecosystem: Sources, monitoring, and its impact on human health. Environ. Sci. pollut. Res. 31 (45), 56428–56462. doi: 10.1007/s11356-024-34932-0
Sorme L., Lagerkvist R. (2002). Sources of heavy metals in urban wastewater in Stockholm. Sci. Total Environ. 298 (1-3), 131–145. doi: 10.1016/S0048-9697(02)00197-3
Sylwan I., Thorin E. (2021). Removal of heavy metals during primary treatment of municipal wastewater and possibilities of enhanced removal: A review. Water 138 (8), 1121. doi: 10.3390/w13081121
TSI (2024). Fishery Statistics 2023 (Ankara, Türkiye: Turkish Statistical Institute Fishery Statistics), 2023.
US EPA (1989). Risk Assessment: Guidance for Superfund Volume 1 Human Health Evaluation Manual (Part A).
US EPA (1996). Quantitative Uncertainty Analysis of Superfund Residential Risk Pathway Models for Soil and Groundwater: White Paper. U.S. Environmental Protection Agency, Washington, D.C.
US EPA (2001). Risk Assessment Guidance for Superfund: Volume III-Part A, Process for Conducting Probabilistic Risk Assessment. U.S. Environmental Protection Agency, USA.
US EPA (2007). United States Environmental Protection Agency, Method 3015A (SW-846): Microwave Assisted Acid Digestion of Aqueous Samples and Extracts,” Revision 1 (Washington DC).
US EPA (2011). “Risk assessment guidance for superfund,” in Part A: Human Health Evaluation Manual; Part E, Supplemental Guidance for Dermal Risk Assessment; Part F, Supplemental Guidance for Inhalation Risk Assessment, vol. 1. U.S. Environmental Protection Agency, Office of emergency and remedial response, Washington, DC.
Varennes E., Blanc D., Azaïs A., Choubert J. M. (2023). Upgrading wastewater treatment plants to urban mines: Are metals worth it? Resour Conserv. Recycl 189. doi: 10.1016/j.resconrec.2022.106738
WB (1998). Project guidelines: Industry sector guidelines. Pollution Prevention and abatement Handbook (WorldBank). The World Bank Group, Washington, D.C.
WB, IFC (2008). Environmental, Health, and Safety Guidelines for Thermal Power Plants. International Finance Corporation, World Bank Group, Washington, D.C.
WPCR (2022). Ministry of Environment, Urbanization and Climate Change ’Water Pollution Control Regulation- (WPCR). Available online at: https://www.resmigazete.gov.tr/eskiler/2022/12/20221217-8.htm (accessed November 1, 2024).
Yamaguchi H., Inoshita M., Shirakami A., Hashimoto S., Ichimiya C., Shigekiyo T. (2013). A case of severe hypothyroidism causing cardiac tamponade associated with lithium intoxication. J. Cardiol. cases 8, e42–e45. doi: 10.1016/j.jccase.2013.03.011
Yang Z., Lu W., Long Y., Bao X., Yang Q. (2011). Assessment of heavy metals contamination in urban topsoil from Changchun City, China. J. Geochem Explor. 108, 27–38. doi: 10.1016/j.gexplo.2010.09.006
Yesil H., Tugtas A. E. (2019). Removal of heavy metals from leaching effluents of sewage sludge via supported liquid membranes. Sci. Total Environ. 693, 133608. doi: 10.1016/j.scitotenv.2019.133608
Yuan G.-L., Sun T.-H., Han P., Li J. (2013). Environmental geochemical mapping and multivariate geostatistical analysis of heavy metals in topsoils of a closed steel smelter: Capital Iron & Steel Factory, Beijing, China. J. Geochem Explor. 130, 15–21. doi: 10.1016/j.gexplo.2013.02.010
Keywords: deep sea discharge, coastal pollution, anthropogenic input, urban runoff, metal
Citation: Akdemir T (2024) Trace element concentrations in effluent of municipal wastewater treatment plants along the Turkish coasts and assessment of human health risk. Front. Mar. Sci. 11:1521449. doi: 10.3389/fmars.2024.1521449
Received: 01 November 2024; Accepted: 02 December 2024;
Published: 19 December 2024.
Edited by:
Jia Li, Yibin University, ChinaReviewed by:
Levent Bat, Sinop University, TürkiyeAbhay B. Fulke, Council of Scientific and Industrial Research (CSIR), India
Tamer Akkan, Giresun University, Türkiye
Copyright © 2024 Akdemir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tolga Akdemir, dG9sZ2EuYWtkZW1pckBlcmRvZ2FuLmVkdS50cg==
 Tolga Akdemir
Tolga Akdemir