- 1State Key Laboratory of Biocontrol, Guangdong Provincial Key Laboratory for Aquatic Economic Animals and Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), School of Life Sciences, Sun Yat-sen University, Guangzhou, China
- 2Hunan Micrograss Biotechnology Co., Ltd., Xiangtan, China
- 3Laboratory of Aquatic Animal Nutrition and Feed, Fisheries College, Guangdong Ocean University, Zhanjiang, China
The research investigated the nutritional physiology effect of ginseng saponins on Litopenaeus vannamei (L. vannamei) under low-fishmeal diets. In total, five experimental groups were arranged, with 21% fishmeal (high-fishmeal) serving as the positive control (PC), 11% fishmeal (low-fishmeal) serving as the negative control (NC), and 11% fishmeal serving as the addition in all three other groups. Similarly, ginseng saponins (GSP, purity of 2%) were added in the order of 0.1%, 0.3%, and 0.5% (GSP0.1, GSP0.3, and GSP0.5), with an 8-week growth cycle. Both GSP0.1 and GSP0.3 showed significantly higher growth performance (final body weight, FBW; weight gain rate, WGR; specific growth rate, SGR) than the NC group, but significantly lower growth performance than the PC group (P<0.05). However, it was found that there was no significant difference in the body composition of the whole shrimp between the experimental groups. Compared to the PC group, the GSP0.3 group exhibited significantly elevated levels of antioxidant enzymes, total antioxidant capacities (T-AOC), superoxide dismutase (SOD), and glutathione peroxidase (GSH-Px) (P<0.05). Additionally, significant differences were observed between the PC and GSP0.3 groups regarding the expression levels of sod, cat, and gsh-px (P<0.05). And there was a better morphological organization of shrimp hepatopancreas in the GSP0.3 group than in all other groups. In comparison with the PC group, there was no significant difference in shrimp survival rates after ammonia nitrogen stress with ginseng saponins added (P>0.05). Whereas, in terms of the relative expression levels of the corresponding genes, in shrimp of the GSP0.3 group, the relative expression of antioxidant-related genes sod, cat, and gsh-px were significantly higher than that of the PC group (P<0.05). Caspase3 and p53, along with bcl-2 and bax, were found to be significantly more expressed in shrimp of the GSP0.3 group than in all other groups (P<0.05). These findings imply that in addition to improving growth performance, adding ginseng saponins at a concentration of 11% fishmeal could improve the antioxidant capacity of L. vannamei as well as its resistance to stress. Therefore, ginseng saponins can be utilized as a functional additive to increase L. vannamei growth performance, enhance antioxidant capacity, and reduce stress in low-fishmeal diets, 0.3% of ginseng saponins is optimal.
1 Introduction
There are many reasons why fishmeal is recognized as the best source of protein in aquafeeds. These include its high palatability, balanced nutrient profile, easy digestibility, and absorption properties. The current change in global climate, however, that has resulted in a decrease in the catch of capture fisheries, contributing to fishmeal shortages and increased prices (Hodar et al., 2020; Jannathulla et al., 2019). Several studies have been conducted in search of suitable protein sources to replace fishmeal, but a high percentage of fishmeal substitution may negatively affect aquatic animal growth and immunocompetence. As a result of 45% replacement of fishmeal with defatted yellowtail worm, Larimichthys crocea exhibits decreased growth performance and immunocompetence (Zhang et al., 2022). It is reported that Oreochromis niloticus and Sarotherodon galilaeus suffer from poorer growth performance and liver health after 20% and 30% replacements with vegetable meal (Sallam et al., 2021). Enhancing the antioxidant and immune capacities of aquatic animals under low-fishmeal diets is considered as one of the ways to reduce the negative impacts of low-fishmeal diets. Therefore, the aquaculture industry needs feed additives that are cost-effective, environmentally safe, and capable of improving shrimp immune capacity.
An herbaceous perennial plant in the Nansingidae family, Ginseng has a long history of medicinal use and is believed to treat a number of ailments, it regulates the immune system particularly well, and is therefore considered an immunomodulator (Kang and Min, 2012). Among its benefits are the enhancement of immunity, improves blood circulation, antioxidant properties, and anticancer properties (Kim, 2018, 2012; Ratan et al., 2021). A wide range of diseases have been treated with ginseng extracts in aquatic animals. It is found that dietary supplementation of ginseng extract significantly improved the survival rate of juvenile Oncorhynchus mykiss (Bulfon et al., 2017). Supplementing Nile tilapia diets with ginseng extract improves growth performance, feed utilization, and hematological indices (Goda, 2008). The survival of fish attacked by Aeromonas hydrophila increases with increasing levels of ginseng in their diets (Abdel-Tawwab, 2015). The addition of ginseng extract to Nile tilapia diets can be used as a natural alternative to hygromycin as a growth promoter and also as an immunoregulator (Elsayed et al., 2014). The main reason for the medicinal value of ginseng is believed to be its main active ingredient, ginsenoside (Guo et al., 2021; Murthy et al., 2014). A number of studies have demonstrated its immunomodulatory and antioxidant properties, along with its anti-inflammatory and anti-stress effects (Elekofehinti, 2015; Güçlü-Ustündağ and Mazza, 2007). Inflammatory responses may be modulated by ginseng saponins, their metabolites or derivatives, including Rb1, Re, Rg1, Rg3, Rg5, Rh2, and Rp1, which modulates inflammatory signaling pathways (Kim et al., 2017; Riaz et al., 2019).
As one of the most common hazardous substances in the aquaculture environment, ammonia nitrogen (NH3-N) is derived from feed residues, animal excreta, and microbial metabolism (Abdelfatah, 2022; Edwards et al., 2024; Zhang et al., 2023). Ammonia nitrogen is present mostly as free ammonia (NH3) and as ammonium ions (NH4+) in water bodies. Free ammonia is less toxic than ammonium ions. Ammonia nitrogen concentrations in the environment cause a wide range of negative effects on aquatic animals, including shrimps (Duan et al., 2024; Lu et al., 2016; Páez-Osuna, 2001). Shrimps will experience physiological stress due to ammonia nitrogen, resulting in an increase in their respiratory rate and blood concentration of ammonia nitrogen, consequently, oxygenation is affected, the acid-base balance and osmotic pressure regulation of the body are affected, and normal metabolism is affected (Valencia-Castañeda et al., 2018). When shrimp are exposed to high concentrations of nitrogen, their growth will be inhibited, resulting in impaired feeding and digestion, slow growth, weight loss, and shortening of their bodies, among other problems (Frías-Espericueta et al., 2000; Lin et al., 1993). Additionally, ammonia nitrogen stress weakens the shrimp’s immune system, making it less resistant to pathogenic microorganisms and susceptible to infection with virus-causing diseases such as white spot syndrome virus (WSSV) (Kathyayani et al., 2019; Ma et al., 2023). Ammonia stress poses a serious threat to shrimp survival and health. To reduce the harm caused by ammonia-nitrogen stress, aquaculture managers need to implement various strategies (Emerenciano et al., 2022). To optimize feed management and minimize the production of feed residues, it is essential to avoid overfeeding. Strengthening water quality management through regular water changes is necessary to maintain adequate dissolved oxygen levels. Additionally, it is critical to reasonably control stocking density to prevent water quality deterioration caused by excessive density. Regular monitoring of key parameters such as ammonia nitrogen concentration, pH, and dissolved oxygen is also imperative, with timely control measures implemented as needed. To protect the health and sustainable development of aquaculture, these methods of scientific management and rational water quality control can effectively reduce ammonia nitrogen stress (Zhang Y. et al., 2020). Current studies indicate that incorporating functional additives into the diet can enhance the stress resistance of aquatic animals under ammonia nitrogen conditions (Jin et al., 2018; Kaleo et al., 2019; Sallam et al., 2020; Yilmaz, 2019).
Ginseng extract has been found to enhance growth and immunity in Nile Tilapia (Oreochromis niloticus) and hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) (Ahmed et al., 2022; Riaz et al., 2019; Sun et al., 2018). Due to its fast growth rate, long breeding season, and tasty meat, L. vannamei has been introduced and cultivated in many countries (Zhang et al., 2019). However, there is still a lack of information on the application of ginseng saponins in the diet of L. vannamei. In this study, we explored the application of ginseng saponins in the diet of L. vannamei and investigated the effects of ginseng saponins on growth performance, antioxidant capacity, and ammonia nitrogen stress resistance of L. vannamei fed with low-fishmeal diet, which provided a theoretical basis for the development of functional feed additives.
2 Materials and methods
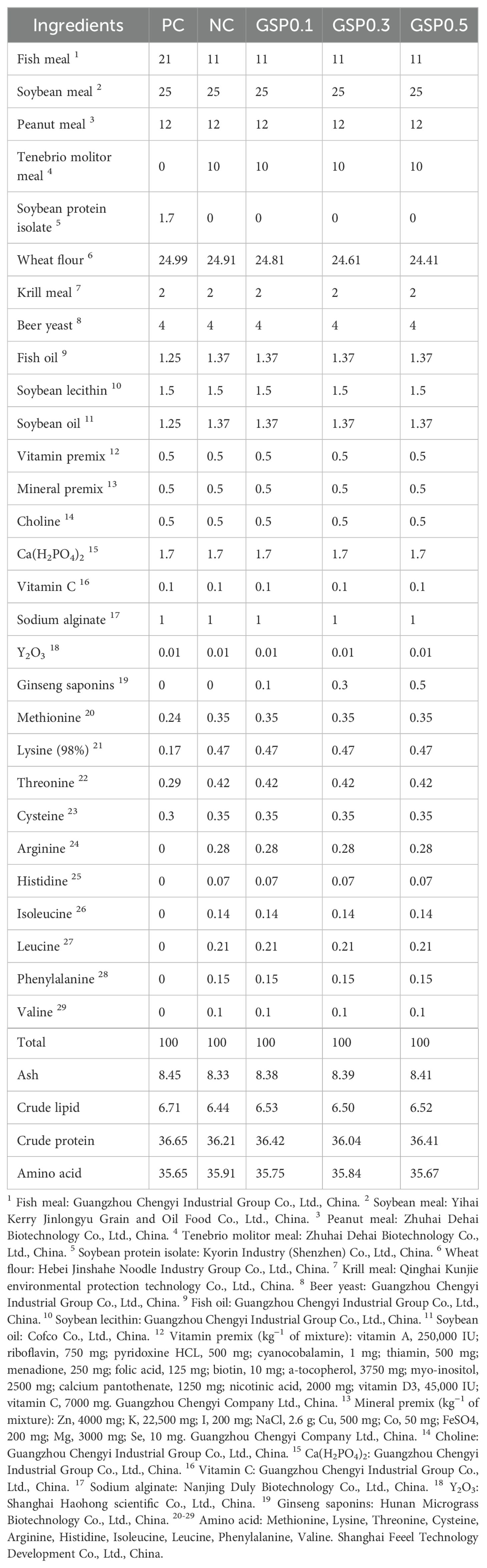
2.1 Diet preparation
According to the diet formula in Table 1, all ingredients were crushed and sieved, weighed and mixed completely, fish oil and soybean oil were added in turn, then water was added and stirred well. An extruder made long strips of the mixed ingredients and a pelletizer made them into feed pellets. Afterwards, the feed was placed in the oven to mature, removed and air-dried, and then packed in sealed plastic bags and stored in the refrigerator at -20°C.
2.2 Culture experiment
For the experiment, shrimp larvae were staged for six weeks prior to being selected and assigned to culture tanks randomly. At the commencement of the study, shrimp of uniform size (approximately 0.45 g in weight) were chosen and randomly assigned to experimental groups. Prior to commencing the formal experiment, the initial sample of 200 shrimp were obtained. Four replicates were established for each treatment, with thirty shrimp allocated to each aquarium (1 m3). Throughout the experimental duration, the water quality within the culture system was meticulously regulated to ensure optimal conditions for shrimp survival, with a consistent temperature maintained at 28°C. The shrimp were fed three times a day and initially 5% of their body weight was fed to them during the experiment. Following feeding, the feeding rate was increased by 20% if there were no surpluses, while decreased by 10% if there were surpluses.
2.3 Sampling
The feeding was stopped for 24 h at the end of the 8-week culture experiment. Survival rates and the other growth performance were calculated using live shrimp. To analyze the whole shrimp composition, five shrimp were randomly selected from each bucket, stored at -20°C in the refrigerator. In addition, four shrimp were randomly selected for dissection to examine their H&E sections, gene expression, and enzyme activity.
2.4 Ammonia nitrogen stress tests
After routine sampling, ammonia nitrogen stress tests were performed according to the experimental method (Liu and Chen, 2004), To determine the amount of ammonium chloride to add to the formal experiments, one culture tank per treatment group was selected for the ammonia nitrogen stress pre-experiment. The concentration of NH3 in the water was determined to be 4.5 mg/L, at 4-hour intervals, the ammonia concentration in the water was measured to maintain the NH4Cl concentration, the stress tests kept 12 h. After the ammonia nitrogen stress tests, the survival rate was recorded, four shrimp from each group were randomly selected and sampled for dissection to examine their gene expression, and enzyme activity.
2.5 Proximate composition determination
Moisture, crude protein, crude lipid, and crude ash of whole shrimp were determined by using standard methods (Association of Official Analytical Chemists, AOAC. In order to calculate the moisture content of whole shrimp, the shrimp were dried at 105°C, and their dry weight were measured at constant weight. The dried whole shrimp were ground, and the crude protein and crude lipid contents were determined by using a fully automated Dumas nitrogen tester (N pro (DT Ar/He Basic), Gerhardt GMBH & CO.KG, Germany) and an automated lipid analyzer (Soxtec System HT6, Tecator, Sweden).
2.6 Total RNA extraction and cDNA synthesis
Animal RNA Extraction Kit (Beyotime Biotech Inc, Shanghai, China) was used to extract total RNA from hepatopancreas, and a microspectrophotometer was used to determine its concentration and quality (Thero Scientific, USA). Reverse transcription of cDNA was carried out using the Evo MMLV Reverse Transcription Kit II (Accurate Biotechnology, Hunan, China), which was stored in the refrigerator at -20°C for storage.
2.7 Real-time quantitative PCR (qRT-PCR)
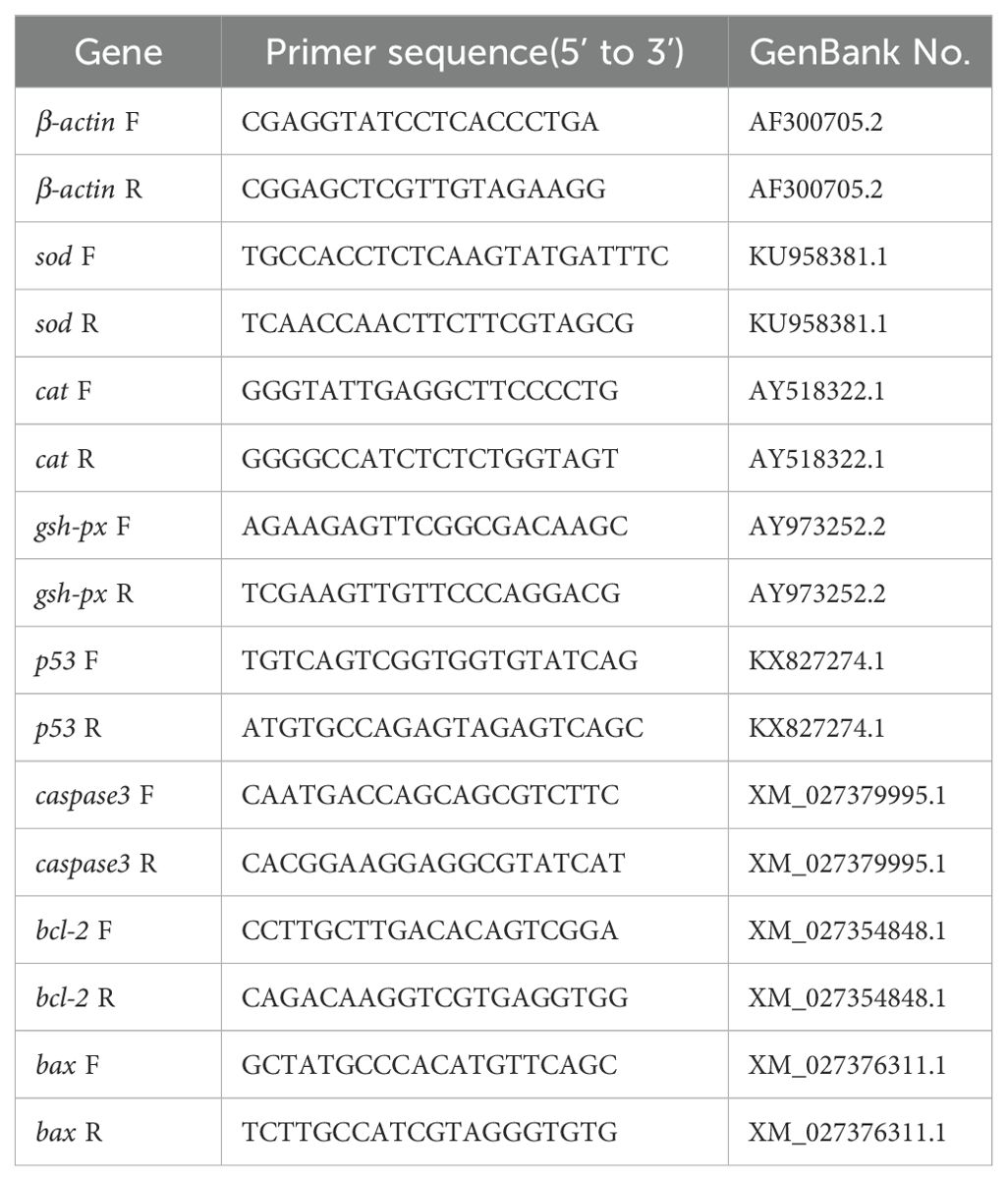
All genes were amplified by qRT-PCR using a Roche real-time fluorescence quantitative PCR system (LightCycler 480 II, Roche Diagnostics, Basel, Switzerland). The reagents used were SYBR Green Pro Taq HS premixed qPCR kit (Accurate Biotechnology, Hunan, China), with a reaction system of 10 μL. The PCR reaction conditions consisted of an initial denaturation step at 95°C for 30 s, followed by 40 cycles of denaturation at 95°C for 5 s, annealing at 60°C for 34 s, and extension at 95°C for 15 s. A final extension step was performed at 60°C for 60 s, followed by a melt curve analysis. Three biological replicates were performed using the β-actin gene as the internal reference gene, and primer sequences are shown in Table 2. Using the 2-ΔΔct method (Livak and Schmittgen, 2001), three replica wells were set up for each reaction, and the relative expression of genes was calculated.
2.8 Enzyme activity assay
A volume of PBS solution was added to approximately 0.5 g of hepatopancreatic sample, and the sample was ground. After centrifugation at 4000 rpm for 15 min at 4°C, the supernatant was extracted. Afterwards, the supernatant was used to detect hepatopancreatic antioxidant enzymes and digestive enzymes. Superoxide dismutase (SOD), total antioxidant capacity (T-AOC), lipid oxidation (MDA), glutathione peroxidase (GSH-Px) were detected using the kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.9 Morphological microscopy of hepatopancreatic tissues
The samples were initially fixed in a 4% paraformaldehyde solution, followed by fixation in a 70% ethanol solution after 24 h. Subsequently, the samples underwent dehydration by using ethanol solutions of varying concentrations, were embedded in paraffin. This experiment used embedded tissue samples cut into 3 μm slices, observing and photographing H&E sections by using a Nikon orthomicroscope (Eclipse Ni-E, Nikon, Japan). The NIS-Elements viewer software (National Institutes of Health, Bethesda, USA) was used to measure and analyze the photographs.
2.10 Statistical analysis
The formula for calculating the parameters of this test includes the following equations, Initial body weight (IBW, g)=initiaotal wet weight/initial number of tails; Final body weight (FBW, g)=final total wet weight/final number of tails; Weight gain (WG, %)=100×(final body weight-initial body weight)/initial body weight; Specific growth rate (SGR, %/day)=100×(Ln final mean weight-Ln initial mean weight)/number of days; Feed conversion rate (FCR)=dry diet fed/wet weight gain; Survival rate (SR, %)=100×number of terminal surviving tails/number of initial tails.
The results of the experiment were expressed as “mean ± standard error”. Data were analyzed using SPSS 26.0, and differences between groups were compared by one-way analysis of variance (ANOVA) and Tukey’s honestly significant difference (Tukey HSD) test (P<0.05), before running ANOVA, normality tests had been performed.
3 Results
3.1 Growth performance and feed conversion rate
Table 3 showed the growth performance of L. vannamei after supplementation with ginseng saponins under low-fishmeal diets. There were significant differences, between the positive control group and the other groups in terms of final body weight, weight gain, specific growth rate and feed conversion rate (P<0.05). It was found that the GSP0.1 and GSP0.3 groups had significant improvements in final body weight, weight gain, and specific growth rate when compared with NC group (P<0.05). And Shrimp FCR were significantly lower in the GSP0.1, GSP0.3, and GSP0.5 groups compared with the NC group (P<0.05).
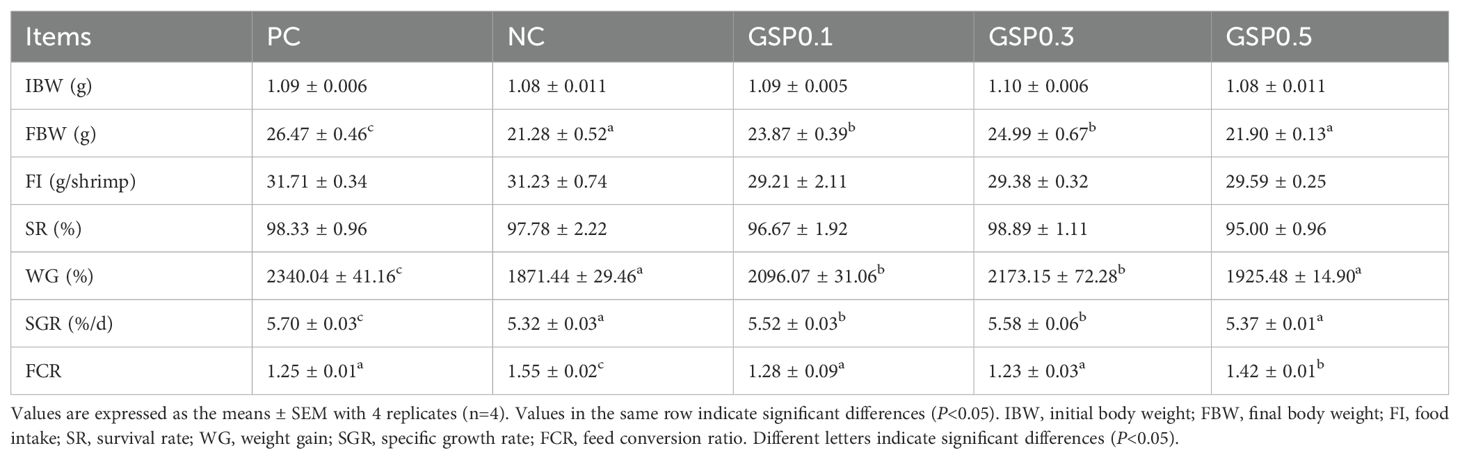
Table 3. Effect of the addition of ginseng saponins at low-fishmeal level on growth performance and feed utilization of L. vannamei.
3.2 Whole shrimp proximate composition
Table 4 showed how ginseng saponins affect the body composition of L. vannamei after addition of ginseng saponins to diets in low-fishmeal. The crude lipid of shrimp in the GSP0.3 group increased after ginseng saponins were added, and there was no significant difference between the GSP0.3 group and the PC group. And moisture and crude protein contents were not significantly different between groups.
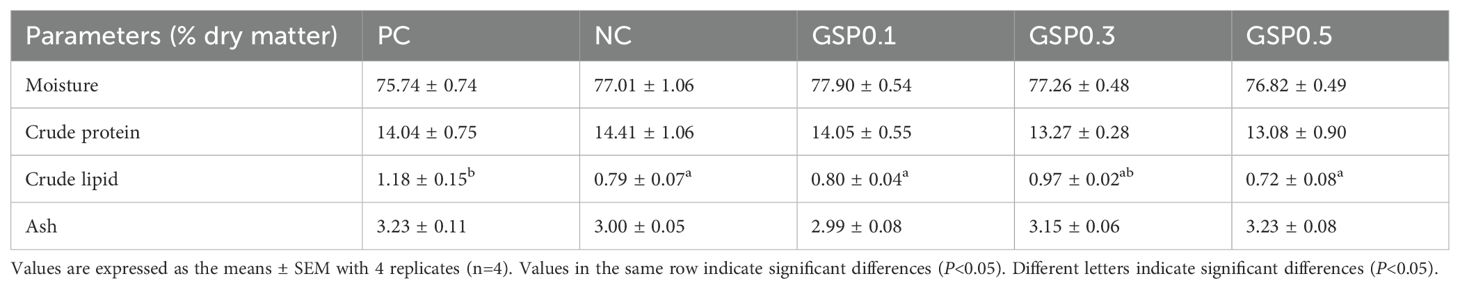
Table 4. Effect of the addition of ginseng saponins at low-fishmeal level on the whole body composition of L. vannamei (% dry weight).
3.3 Hepatopancreas morphology
Figure 1 showed the hepatopancreas tissue sections of L. vannamei. Shrimp in the GSP0.3 group had better hepatopancreatic health than shrimp in any other groups based on the hepatopancreas microsomal compactness, basement membrane integrity, and regularity of stellate duct lume. Hepatopancreatic histomorphology was improved by the addition of ginseng saponins compared to NC group.
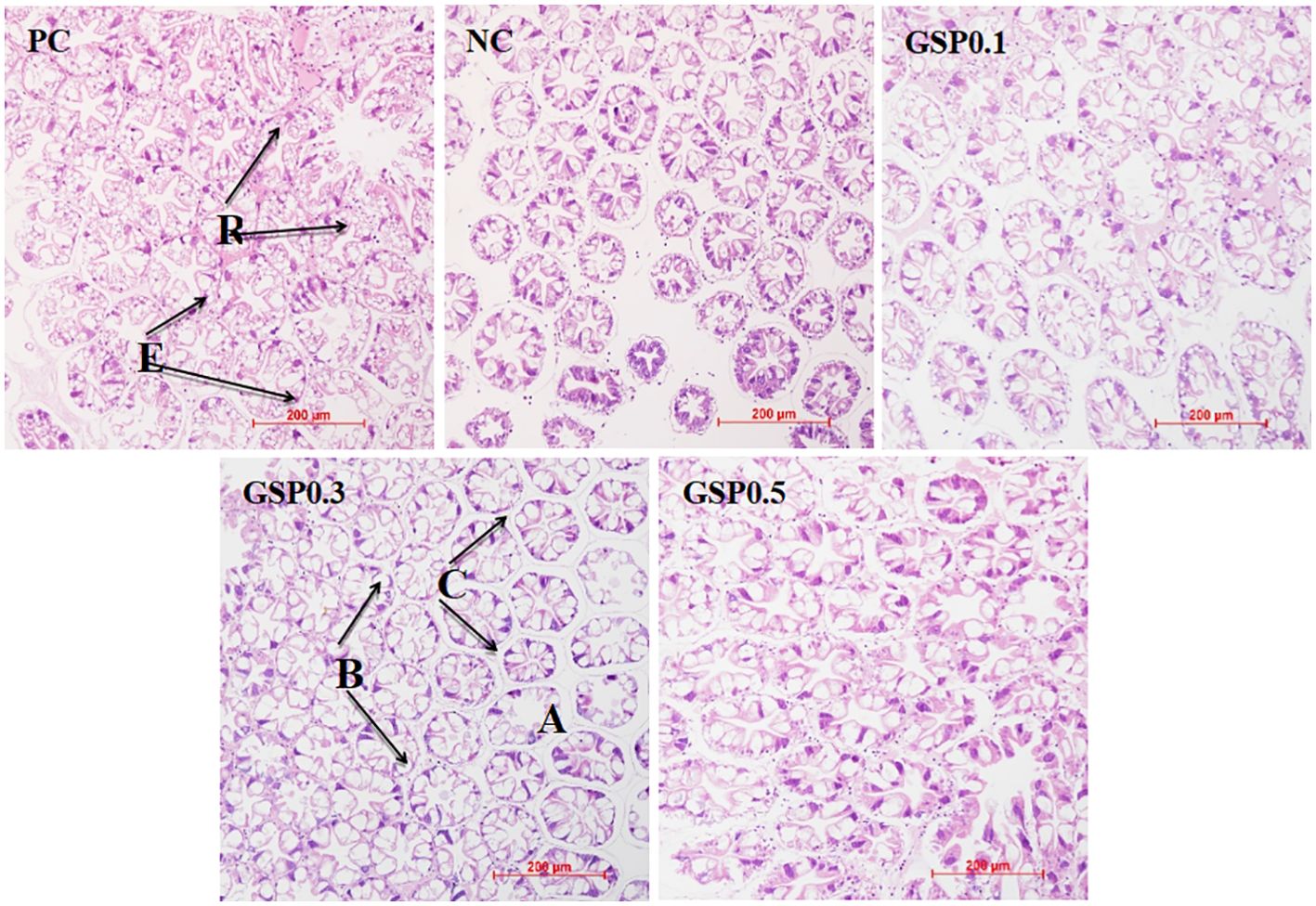
Figure 1. Effect of the addition of ginseng saponins at low-fishmeal level on the hepatopancreas morphology of L. vannamei. Scale bar: 200 μm. Magnification: 20×.
3.4 Antioxidant enzyme activities
According to Table 5, ginseng saponins added to low-fishmeal diets increased antioxidant capacity of L. vannamei’s hepatopancreas. Compared to NC, GSP0.1, GSP0.3, and GSP0.5 groups had significantly lower MDA levels in the hepatopancreas (P<0.05). There was a significant difference between the GSP0.3, GSP0.1, and GSP0.5 groups in terms of hepatopancreatic MDA levels (P<0.05). Compared with the other groups, the GSP0.3 group shown significantly higher activities of T-AOC, SOD, and GSH-Px activities (P<0.05). With SOD and T-AOC activities in hepatopancreas were significantly higher in GSP0.1 and GSP0.5 than in NC (P<0.05).
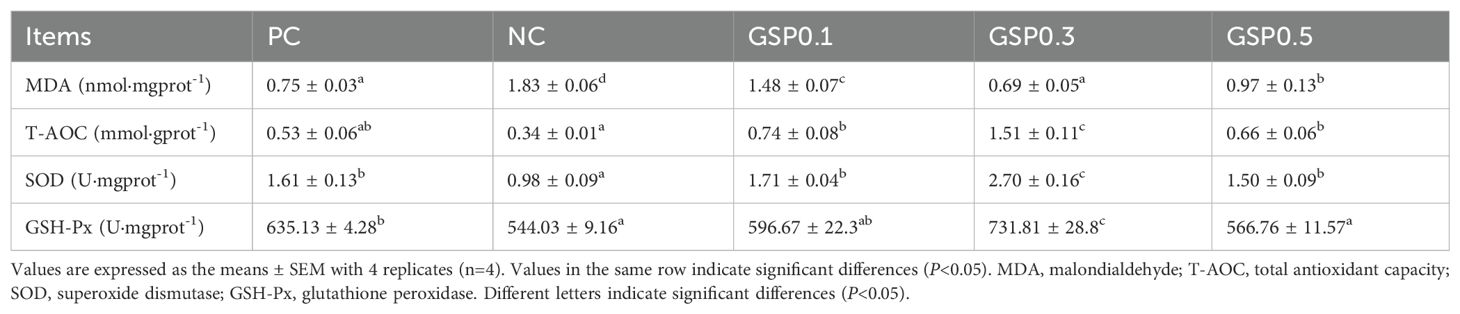
Table 5. Effect of the addition of ginseng saponins at low-fishmeal level on the hepatopancreatic antioxidant enzyme activities of L. vannamei.
3.5 Antioxidant gene expression
Figure 2 showed the expression of the hepatopancreatic antioxidant genes in L. vannamei, after the addition of ginseng saponins. A significant difference was found between the PC and NC groups in terms of the relative expression level of sod gene in the GSP0.3 group (P<0.05). Compared to the NC group, the cat gene expression level in GSP0.3 was significantly higher (P<0.05). It was significant that the relative expression of gsh-px gene was higher than that of PC and NC groups (P<0.05).
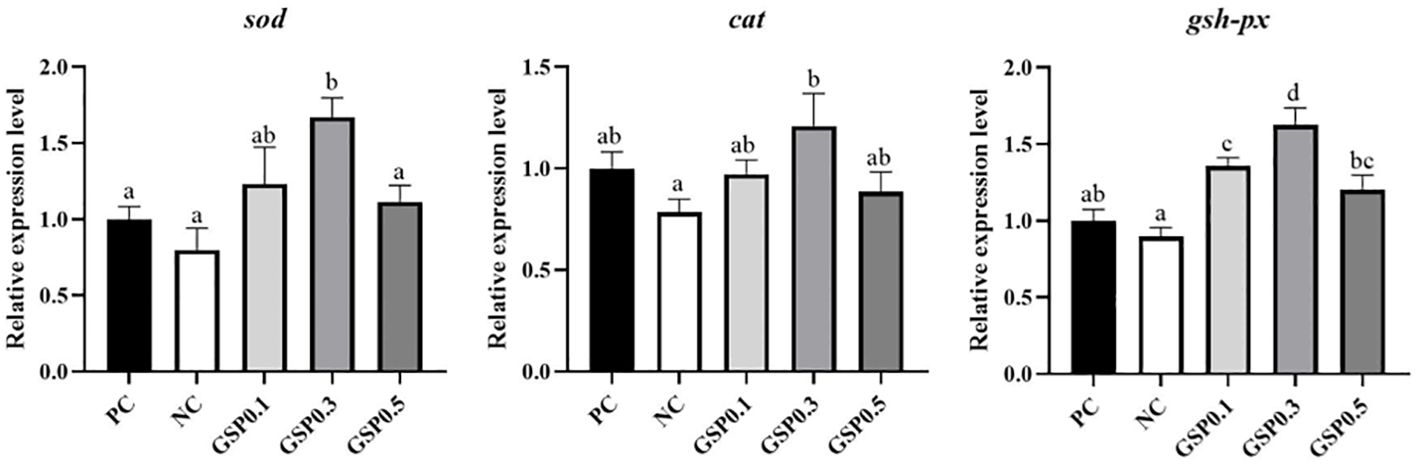
Figure 2. Effect of the addition of ginseng saponins at low-fishmeal level on the expression of antioxidant genes in the hepatopancreas of L. vannamei. Data represent the means ± SEM (n = 4). Different letters indicate significant differences (P<0.05).
3.6 Survival rate after ammonia nitrogen stress
In Figure 3, compared to the low-fishmeal control group NC, the survival rate of L. vannamei after ammonia nitrogen stress, which was shown to have been improved significantly with 0.3% of ginseng saponins added (P<0.05). In addition, there were no significant differences between the GSP0.1, GSP0.3, and GSP0.5 groups and the PC group in terms of L. vannamei survival rate.
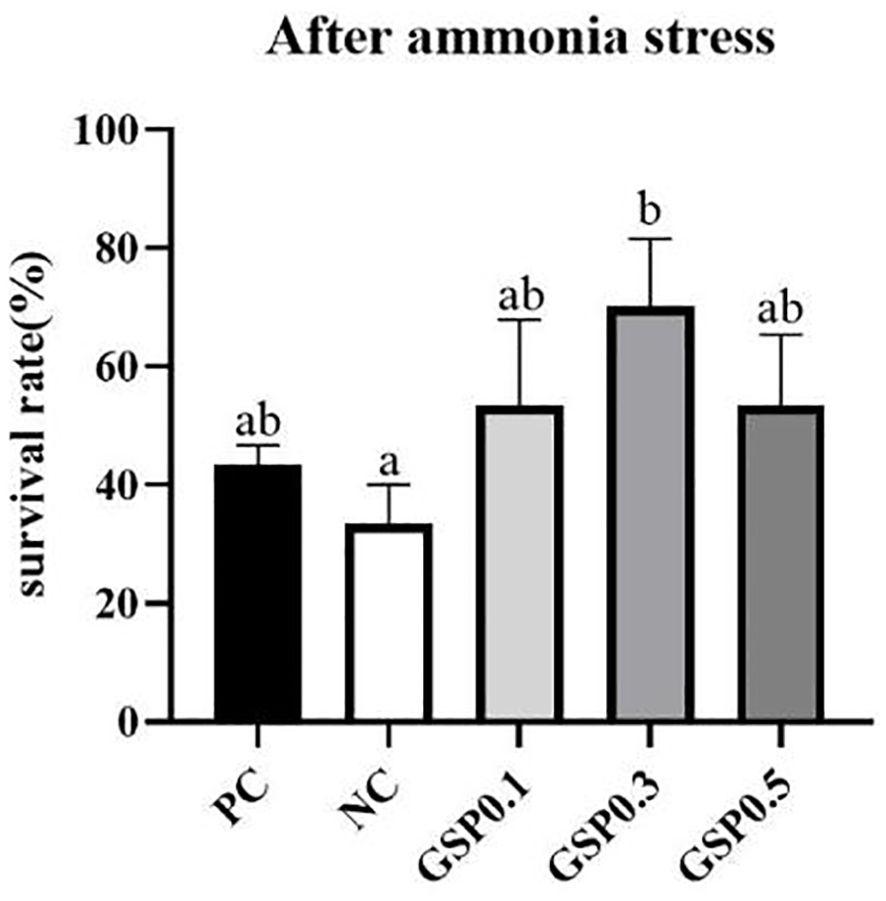
Figure 3. Effect of the addition of ginseng saponins at low-fishmeal level on the survival rate of L. vannamei under ammonia nitrogen stress. Data represent the means ± SEM (n = 4). Different letters indicate significant differences (P<0.05).
3.7 Gene expression after ammonia nitrogen stress
According to Figures 4, 5, the relative expression of antioxidant, and apoptosis genes were altered in L. vannamei under ammonia nitrogen stress. The relative expression levels of sod, cat, and gsh-px genes were significantly higher in the GSP0. 3 group than in the other groups (P<0.05). After the addition of ginseng saponins, there was a significant increase in p53 gene relative expression in the GSP0.3 group compared to other groups (P<0.05), and in the GSP0.3 group, caspase3, bcl-2, and bax genes shown significantly higher expression levels than those in other groups (P<0.05).
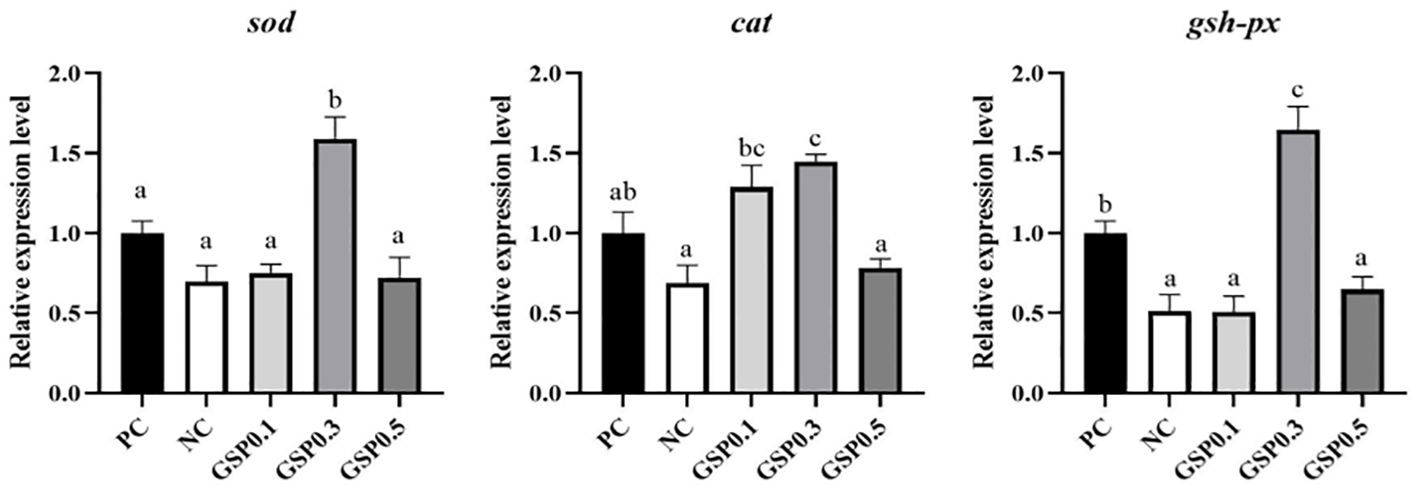
Figure 4. Effect of the addition of ginseng saponins at low-fishmeal level on the expression of antioxidant genes in the hepatopancreas of L. vannamei under ammonia nitrogen stress. Data represent the means ± SEM (n = 4). Different letters indicate significant differences (P<0.05).
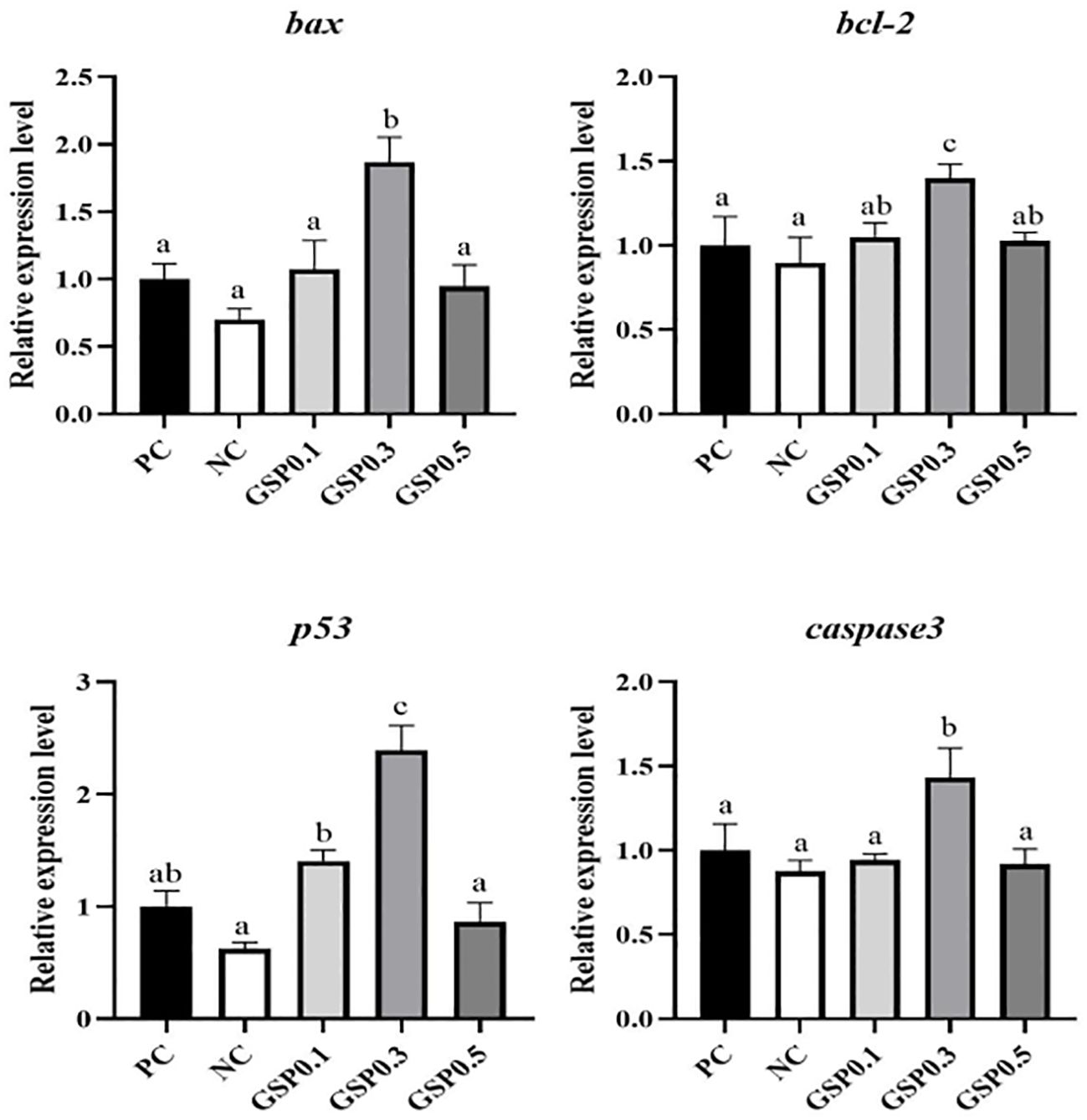
Figure 5. Effect of the addition of ginseng saponins at low-fishmeal level on the expression of apoptotic genes in the hepatopancreas of L. vannamei under ammonia nitrogen stress. Data represent the means ± SEM (n = 4). Different letters indicate significant differences (P<0.05).
4 Discussion
As far as growth performance is concerned, L. vannamei in the 0.1% and 0.3% ginseng saponins-supplemented groups outperformed the low-fishmeal negative control. Additionally, the growth performance of L. vannamei in the 0.3% ginseng saponins-supplemented groups was highly similar to that of the high-fishmeal positive control. L. vannamei increased growth performance by adding 0.1% to 0.3% ginseng saponins to low-fishmeal diets, which was close to the PC group. Ginseng saponins may be one of the most important ingredients in shrimp growth. In the similar studies, that triterpenoid, Quillaja saponins has a favorable effect on the growth performance of Cyprinus carpio and Oreochromis niloticus (Francis et al., 2002, 2001). Furthermore, no significant differences in whole shrimp proximate composition were found between the PC group and the low-fishmeal diets supplemented with ginseng saponins.
There are many different kinds of organisms that produce SOD, an enzyme that scavenges superoxide radicals in their cytosol (Chen et al., 2013; Wang et al., 2015). In shrimp hepatopancreas, the MDA content was not significantly different between the 0.3% ginseng saponins-added diet and the 20% fishmeal-added diet, and was significantly lower than the other three treatment groups, and a significantly higher level of enzyme activity was observed in the SOD, T-AOC, and GSH-Px than in the PC group. These results showed that addition of the 0.3% ginseng saponins to a diet low in fishmeal improved the antioxidant capacity of L. vanname. In related studies, ginseng polysaccharide complex has been shown to improve SOD activity in the hepatopancreas of L. vannamei, and to enhance antioxidant activity (Liu et al., 2011). Using the polysaccharide extract from S. fusiforme, Vibrio harveyi was inhibited in shrimp, Fenneropenaeus chinensis, and SOD activity in the shrimp’s muscle increased (Huang et al., 2006). A significant increase in SOD activity and cyt-SOD mRNA expression has been observed in tiger shrimp, Penaeus monodon, after the addition of sodium alginate (Liu et al., 2006). On the other hand, the relative expression of sod, gsh-px, and cat in shrimp of the 0.3% ginseng saponins group was significantly higher than that of shrimp from the PC group in terms of relative expression, that indicated that adding ginseng saponins to low-fishmeal diets could enhance the antioxidant capacity of shrimp from L. vannamei. The study has found that the hepatopancreas mRNA level of sod, cat, and gsh-px can be improved by addition of 0.04% ginseng polysaccharide in diet of L. vannamei (Liu et al., 2011). Furthermore, ginseng saponins added to low fish meal diets improved the morphology of hepatopancreatic tissue of L. vannamei, with 0.3% ginseng saponins being more effective. It was found that under immune stimulation, ginseng saponins increase antioxidant genes expression in the liver of broilers, alleviate liver histology changes, inhibit inflammatory responses, and promote antioxidant activity (Hu et al., 2023). And the protodiol saponin ginsenoside Rk1, isolated from ginseng, has been suggested to have significant antitumor properties (Wu et al., 2024). These studies were consistent with the results of the present experiment. In shrimp, ginseng saponins were found to be beneficial in alleviating the effects of lipid peroxidation damage and improving their antioxidant capacity, especially when 0.3% ginseng saponins were added.
Shrimp is highly susceptible to ammonia stress. Not only does it damage their health, but it may also cause economic losses for aquaculture as well (Chen et al., 2019; Li et al., 2023). Aquatic animals’ survival rate decreased as ammonia concentration and exposure time increased, but different species of aquatic animals tolerated ammonia differently (Frances et al., 2000; Kır et al., 2004; Wang and Walsh, 2000). An increase in the resistance of shrimp to ammonia stress was observed in those treated with immune potentiating agents, such as gracilaria tenuistipitata extract, astaxanthin and mannan oligosaccharide (Fu et al., 2007; Pan et al., 2003; Zhang et al., 2012). In terms of stress resistance, following ammonia-nitrogen stress, shrimp supplemented with 0.3% ginseng saponins survived just as well as shrimp in the high-fishmeal control group, as well as significantly more than shrimp in the low-fishmeal control group. A variety of environmental factors, including pH, nitrites, stress, and temperature, can increase oxidative stress and activate antioxidant genes expression (Guo et al., 2013; Wang et al., 2009; Zhou et al., 2008). In the present study, as with survival rate, the relative expression levels of antioxidant genes sod, cat, and gsh-px were significantly higher in shrimp from the low-fishmeal diet supplemented with 0.3% ginseng saponins than in shrimp from the high-fishmeal diet. A key component of the apoptosis process, caspase3 cleaves a wide range of structural and regulatory proteins through its proteolytic activity (Chang et al., 2009). It is known that oxidative stress activates downstream target genes and induces apoptosis using p53 as a tumor suppressor (Sun et al., 2016). Nitrite exposure has been shown to increase Scylla paramamosain and Pelteobagrus fulvidraco caspase3 and p53 expression in previous studies (Cheng et al., 2020; Zhang M. et al., 2020). Previous study had shown that sensitive to apoptosis genes loss increased pro-apoptotic bax and bcl-2 imbalances in mitochondria. Caspase9 and caspase3 were activated, thereby promoting cell death (Chang and Ding, 2014). As for apoptosis-related genes, shrimp from the 0.3% ginseng saponins supplemented group showed significantly higher expression levels of p53, caspase3, bcl-2, and bax than shrimp from the PC group. As a result of these findings, shrimp stress resistance may be improved by adding 0.3% ginseng saponins to low-fishmeal diets. Ginsenoside Rk1 was found to promote autophagy-dependent apoptosis (Wu et al., 2024). The process of apoptosis is a kind of programmed cell death that is managed by genes. Upregulation of apoptosis may signal that the cells in an organism are still within control. This suggested that shrimp were becoming more stress resistant due to the upregulation of apoptosis genes.
5 Conclusion
In this experiment, different amounts of ginseng saponins were added to high percentage fishmeal replacement diets of L. vannamei to investigate the effects of ginseng saponins on the nutritional physiology of L. vannamei fed with low fish meal diets. Overall, ginseng saponins, as a natural additive, has a number of positive effects on aquatic animal nutrition, including promoting growth performance, improving antioxidant capacity, and improving anti-stress ability. Ginseng saponins has an extensive application potential in the aquatic feed industry because of its unique characteristics.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the Care and Use of Laboratory Animals of the National Institutes of Health (NIH) and approved by Experimental Animal Ethics Committee of Sun Yat-sen University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SL: Writing – original draft, Visualization, Formal analysis, Conceptualization. RY: Writing – review & editing, Investigation, Formal analysis. XC: Writing – review & editing, Investigation. YG: Writing – review & editing, Investigation. DH: Writing – review & editing, Resources, Conceptualization. BZ: Writing – review & editing, Resources, Conceptualization. ZZ: Writing – review & editing, Resources, Conceptualization. XH: Writing – review & editing, Resources, Conceptualization. ZL: Writing – review & editing, Resources, Conceptualization. BT: Writing – review & editing, Supervision, Project administration. JN: Writing – review & editing, Supervision, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research received funding from multiple sources, including the National Key Research and Development Program of China (2023YFD2402000), Project of Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai) (SML2023SP236), Project of Hunan Micrograss Biotechnology Co., Ltd. (HT99982022-0099), Project of Science and Technology of Guangxi Province (AA23062047), and Project of China Agriculture Research System of MOF and MARA 48 (CARS 48).
Conflict of interest
Authors DH, BZ, and ZZ were employed by Hunan Micrograss Biotechnology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdelfatah A. (2022). Recent used techniques and promised solutions for biofiltration treatment of fish wastewater. Egypt. J. Chem. 65, 181–197. doi: 10.21608/ejchem.2022.116302.5268
Abdel-Tawwab M. (2015). The use of American Ginseng (Panax quinquefolium) in practical diets for Nile tilapia (Oreochromis niloticus): resistance to waterborne copper toxicity. Aquacult. Res. 46, 1001–1006. doi: 10.1111/are.12237
Ahmed M. M., Mohammed A. T., Farag M. R., Hassan M. A., Mawed S. A., Alagawany M., et al. (2022). Dietary Supplementation of Nile Tilapia (Oreochromis niloticus) With Panax ginseng Essential Oil: Positive Impact on Animal Health and Productive Performance, and Mitigating Effects on Atrazine- Induced Toxicity. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.920057
Bulfon C., Bongiorno T., Messina M., Volpatti D., Tibaldi E., Tulli F. (2017). Effects of Panax ginseng extract in practical diets for rainbow trout (Oncorhynchus mykiss) on growth performance, immune response and resistance to Yersinia ruckeri. Aquacult. Res. 48, 2369–2379. doi: 10.1111/are.13072
Chang C.-C., Yeh M.-S., Cheng W. (2009). Cold shock-induced norepinephrine triggers apoptosis of haemocytes via caspase-3 in the white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immunol. 27, 695–700. doi: 10.1016/j.fsi.2009.08.010
Chang S. C., Ding J. L. (2014). Ubiquitination by SAG regulates macrophage survival/death and immune response during infection. Cell Death Differ. 21, 1388–1398. doi: 10.1038/cdd.2014.54
Chen S., Yu Y., Gao Y., Yin P., Tian L., Niu J., et al. (2019). Exposure to acute ammonia stress influences survival, immune response and antioxidant status of pacific white shrimp (Litopenaeus vannamei) pretreated with diverse levels of inositol. Fish. Shellfish. Immunol. 89, 248–256. doi: 10.1016/j.fsi.2019.03.072
Chen X., Lin H.-Z., Jiang S.-G., Wu K.-C., Liu Y.-J., Tian L.-X., et al. (2013). Dietary supplementation of honeysuckle improves the growth, survival and immunity of Penaeus monodon. Fish. Shellfish. Immunol. 35, 161–169. doi: 10.1016/j.fsi.2013.04.020
Cheng C.-H., Su Y.-L., Ma H.-L., Deng Y.-Q., Feng J., Chen X.-L., et al. (2020). Effect of nitrite exposure on oxidative stress, DNA damage and apoptosis in mud crab (Scylla paramamosain). Chemosphere 239, 124668. doi: 10.1016/j.chemosphere.2019.124668
Duan Y., Nan Y., Zhu X., Yang Y., Xing Y. (2024). The adverse impacts of ammonia stress on the homeostasis of intestinal health in Pacific white shrimp (Litopenaeus vannamei). Environ. pollut. 340, 122762. doi: 10.1016/j.envpol.2023.122762
Edwards T. M., Puglis H. J., Kent D. B., Durán J. L., Bradshaw L. M., Farag A. M. (2024). Ammonia and aquatic ecosystems – A review of global sources, biogeochemical cycling, and effects on fish. Sci. Total. Environ. 907, 167911. doi: 10.1016/j.scitotenv.2023.167911
Elekofehinti O. O. (2015). Saponins: Anti-diabetic principles from medicinal plants - A review. Pathophysiology 22, 95–103. doi: 10.1016/j.pathophys.2015.02.001
Elsayed S., abd elgalil S., rashed N. (2014). Immunomodulatory and growth performance effects of ginseng extracts as a natural growth promoter in comparison with oxytetracycline in the diets of nile tilapia (Oreochromis niloticus). Int. J. Livestock. Res. 4, 130. doi: 10.5455/ijlr.20140109084346
Emerenciano M. G. C., Rombenso A. N., Vieira F. D. N., Martins M. A., Coman G. J., Truong H. H., et al. (2022). Intensification of penaeid shrimp culture: an applied review of advances in production systems, nutrition and breeding. Anim. (Basel). 12, 236. doi: 10.3390/ani12030236
Frances J., Nowak B. F., Allan G. L. (2000). Effects of ammonia on juvenile silver perch. Aquaculture 183, 95–103. doi: 10.1016/S0044-8486(99)00286-0
Francis G., Makkar H. P., Becker K. (2001). Effects of Quillaja saponins on growth, metabolism, egg production and muscle cholesterol in individually reared Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 129, 105–114. doi: 10.1016/s1532-0456(01)00189-2
Francis G., Makkar H. P. S., Becker K. (2002). Dietary supplementation with a Quillaja saponin mixture improves growth performance and metabolic efficiency in common carp (Cyprinus carpio L.). Aquaculture. 203, 311–320. doi: 10.1016/S0044-8486(01)00628-7
Frías-Espericueta M. G., Harfush-Melendez M., Páez-Osuna F. (2000). Effects of ammonia on mortality and feeding of postlarvae shrimp Litopenaeus vannamei. Bull. Environ. Contam. Toxicol. 65, 98–103. doi: 10.1007/s0012800100
Fu Y.-W., Hou W.-Y., Yeh S.-T., Li C.-H., Chen J.-C. (2007). The immunostimulatory effects of hot-water extract of Gelidium amansii via immersion, injection and dietary administrations on white shrimp Litopenaeus vannamei and its resistance against Vibrio alginolyticus. Fish. Shellfish. Immunol. 22, 673–685. doi: 10.1016/j.fsi.2006.08.014
Goda A.M.A.-S. (2008). Effect of dietary ginseng herb (Ginsana® G115) supplementation on growth, feed utilization, and hematological indices of nile tilapia, oreochromis niloticus (L.), fingerlings. J. World Aquacult. Soc. 39, 205–214. doi: 10.1111/j.1749-7345.2008.00153.x
Güçlü-Ustündağ O., Mazza G. (2007). Saponins: properties, applications and processing. Crit. Rev. Food Sci. Nutr. 47, 231–258. doi: 10.1080/10408390600698197
Guo H., Xian J.-A., Li B., Ye C.-X., Wang A.-L., Miao Y.-T., et al. (2013). Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol. 157, 366–371. doi: 10.1016/j.cbpc.2013.03.001
Guo M., Shao S., Wang D., Zhao D., Wang M. (2021). Recent progress in polysaccharides from Panax ginseng C. A. Meyer. Food Funct. 12, 494–518. doi: 10.1039/d0fo01896a
Hodar A., Vasava R., Mahavadiya D., Joshi N. (2020). Fish meal and fish oil replacement for aqua feed formulation by using alternative sources: a review. J. Exp. Zool. India 23, 13–21.
Hu W., Bi S., Shao J., Qu Y., Zhang L., Li J., et al. (2023). Ginsenoside Rg1 and Re alleviates inflammatory responses and oxidative stress of broiler chicks challenged by lipopolysaccharide. Poult. Sci. 102, 102536. doi: 10.1016/j.psj.2023.102536
Huang X., Zhou H., Zhang H. (2006). The effect of Sargassum fusiforme polysaccharide extracts on vibriosis resistance and immune activity of the shrimp, Fenneropenaeus chinensis. Fish. Shellfish. Immunol. 20, 750–757. doi: 10.1016/j.fsi.2005.09.008
Jannathulla R., Rajaram V., Kalanjiam R., Ambasankar K., Muralidhar M., Dayal J. S. (2019). Fishmeal availability in the scenarios of climate change: Inevitability of fishmeal replacement in aquafeeds and approaches for the utilization of plant protein sources. Aquacult. Res. 50, 3493–3506. doi: 10.1111/are.14324
Jin M., Xiong J., Zhou Q.-C., Yuan Y., Wang X.-X., Sun P. (2018). Dietary yeast hydrolysate and brewer’s yeast supplementation could enhance growth performance, innate immunity capacity and ammonia nitrogen stress resistance ability of Pacific white shrimp (Litopenaeus vannamei). Fish. Shellfish. Immunol. 82, 121–129. doi: 10.1016/j.fsi.2018.08.020
Kaleo I. V., Gao Q., Liu B., Sun C., Zhou Q., Zhang H., et al. (2019). Effects of Moringa oleifera leaf extract on growth performance, physiological and immune response, and related immune gene expression of Macrobrachium rosenbergii with Vibrio Anguillarum and ammonia stress. Fish. Shellfish. Immunol. 89, 603–613. doi: 10.1016/j.fsi.2019.03.039
Kang S., Min H. (2012). Ginseng, the “Immunity boost”: the effects of panax ginseng on immune system. J. Ginseng. Res. 36, 354–368. doi: 10.5142/jgr.2012.36.4.354
Kathyayani S. A., Poornima M., Sukumaran S., Nagavel A., Muralidhar M. (2019). Effect of ammonia stress on immune variables of Pacific white shrimp Penaeus vannamei under varying levels of pH and susceptibility to white spot syndrome virus. Ecotoxicol. Environ. Saf. 184, 109626. doi: 10.1016/j.ecoenv.2019.109626
Kim J.-H. (2012). Cardiovascular diseases and panax ginseng: A review on molecular mechanisms and medical applications. J. Ginseng. Res. 36, 16–26. doi: 10.5142/jgr.2012.36.1.16
Kim J.-H. (2018). Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J. Ginseng. Res. 42, 264–269. doi: 10.1016/j.jgr.2017.10.004
Kim J. H., Yi Y.-S., Kim M.-Y., Cho J. Y. (2017). Role of ginsenosides, the main active components of Panax ginseng, in inflammatory responses and diseases. J. Ginseng. Res. 41, 435–443. doi: 10.1016/j.jgr.2016.08.004
Kır M., Kumlu M., Eroldoğan O. T. (2004). Effects of temperature on acute toxicity of ammonia to Penaeus semisulcatus juveniles. Aquaculture 241, 479–489. doi: 10.1016/j.aquaculture.2004.05.003
Li H., Li Q., Wang S., He J., Li C. (2023). Ammonia nitrogen stress increases susceptibility to bacterial infection via blocking IL-1R–Relish axis mediated antimicrobial peptides expression in shrimp. Aquaculture 563, 738934. doi: 10.1016/j.aquaculture.2022.738934
Lin H.-P., Thuet P., Trilles J.-P., Mounet-Guillaume R., Charmantier G. (1993). Effects of ammonia on survival and osmoregulation of various development stages of the shrimp Penaeus japonicus. Mar. Biol. 117, 591–598. doi: 10.1007/BF00349770
Liu C.-H., Chen J.-C. (2004). Effect of ammonia on the immune response of white shrimpLitopenaeus vannamei and its susceptibility to Vibrio alginolyticus. Fish. Shellfish. Immunol. 16, 321–334. doi: 10.1016/S1050-4648(03)00113-X
Liu C.-H., Yeh S.-P., Kuo C.-M., Cheng W., Chou C.-H. (2006). The effect of sodium alginate on the immune response of tiger shrimp via dietary administration: Activity and gene transcription. Fish. Shellfish. Immunol. 21, 442–452. doi: 10.1016/j.fsi.2006.02.003
Liu X.-L., Xi Q.-Y., Yang L., Li H.-Y., Jiang Q.-Y., Shu G., et al. (2011). The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immunol. 30, 495–500. doi: 10.1016/j.fsi.2010.11.018
Livak K. J., Schmittgen T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408. doi: 10.1006/meth.2001.1262
Lu X., Kong J., Luan S., Dai P., Meng X., Cao B., et al. (2016). Transcriptome Analysis of the Hepatopancreas in the Pacific White Shrimp (Litopenaeus vannamei) under Acute Ammonia Stress. PloS One 11, e0164396. doi: 10.1371/journal.pone.0164396
Ma R., Yang Y., Cao H., Li P. (2023). Editorial: Aquaculture animal diseases: pathogens and control. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1223046
Murthy H. N., Georgiev M. I., Kim Y.-S., Jeong C.-S., Kim S.-J., Park S.-Y., et al. (2014). Ginsenosides: prospective for sustainable biotechnological production. Appl. Microbiol. Biotechnol. 98, 6243–6254. doi: 10.1007/s00253-014-5801-9
Páez-Osuna F. (2001). The environmental impact of shrimp aquaculture: causes, effects, and mitigating alternatives. Environ. Manage. 28, 131–140. doi: 10.1007/s002670010212
Pan C.-H., Chien Y.-H., Hunter B. (2003). The resistance to ammonia stress of Penaeus monodon Fabricius juvenile fed diets supplemented with astaxanthin. J. Exp. Mar. Biol. Ecol. 297, 107–118. doi: 10.1016/j.jembe.2003.07.002
Ratan Z. A., Haidere M. F., Hong Y. H., Park S. H., Lee J.-O., Lee J., et al. (2021). Pharmacological potential of ginseng and its major component ginsenosides. J. Ginseng. Res. 45, 199–210. doi: 10.1016/j.jgr.2020.02.004
Riaz M., Rahman N. U., Zia-Ul-Haq M., Jaffar H. Z. E., Manea R. (2019). Ginseng: A dietary supplement as immune-modulator in various diseases. Trends Food Sci. Technol. 83, 12–30. doi: 10.1016/j.tifs.2018.11.008
Sallam A. E., Mansour A. T., Alsaqufi A. S., El-Sayed Salem M., El-Feky M. M. M. (2020). Growth performance, anti-oxidative status, innate immunity, and ammonia stress resistance of Siganus rivulatus fed diet supplemented with zinc and zinc nanoparticles. Aquacult. Rep. 18, 100410. doi: 10.1016/j.aqrep.2020.100410
Sallam E. A., Matter A. F., Mohammed L. S., Azam A. E., Shehab A., Mohamed Soliman M. (2021). Replacing fish meal with rapeseed meal: potential impact on the growth performance, profitability measures, serum biomarkers, antioxidant status, intestinal morphometric analysis, and water quality of Oreochromis niloticus and Sarotherodon galilaeus fingerlings. Vet. Res. Commun. 45, 223–241. doi: 10.1007/s11259-021-09803-5
Sun S., Gu Z., Fu H., Zhu J., Ge X., Xuan F. (2016). Molecular cloning, characterization, and expression analysis of p53 from the oriental river prawn, Macrobrachium nipponense, in response to hypoxia. Fish. Shellfish. Immunol. 54, 68–76. doi: 10.1016/j.fsi.2016.03.167
Sun Z., Tan X., Ye H., Zou C., Ye C., Wang A. (2018). Effects of dietary Panax notoginseng extract on growth performance, fish composition, immune responses, intestinal histology and immune related genes expression of hybrid grouper (Epinephelus lanceolatus ♂ × Epinephelus fuscoguttatus ♀) fed high lipid diets. Fish. Shellfish. Immunol. 73, 234–244. doi: 10.1016/j.fsi.2017.11.007
Valencia-Castañeda G., Frías-Espericueta M. G., Vanegas-Pérez R. C., Pérez-Ramírez J. A., Chávez-Sánchez M. C., Páez-Osuna F. (2018). Acute toxicity of ammonia, nitrite and nitrate to shrimp litopenaeus vannamei postlarvae in low-salinity water. Bull. Environ. Contam. Toxicol. 101, 229–234. doi: 10.1007/s00128-018-2355-z
Wang Y., Li Z., Li J., Duan Y.-F., Niu J., Wang J., et al. (2015). Effects of dietary chlorogenic acid on growth performance, antioxidant capacity of white shrimp Litopenaeus vannamei under normal condition and combined stress of low-salinity and nitrite. Fish. Shellfish. Immunol. 43, 337–345. doi: 10.1016/j.fsi.2015.01.008
Wang Y., Walsh P. J. (2000). High ammonia tolerance in fishes of the family Batrachoididae (Toadfish and Midshipmen). Aquat. Toxicol. 50, 205–219. doi: 10.1016/S0166-445X(99)00101-0
Wang W.-N., Zhou J., Wang P., Tian T.-T., Zheng Y., Liu Y., et al. (2009). Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. Part C.: Toxicol. Pharmacol. 150, 428–435. doi: 10.1016/j.cbpc.2009.06.010
Wu H., Qu L., Bai X., Zhu C., Liu Y., Duan Z., et al. (2024). Ginsenoside Rk1 induces autophagy-dependent apoptosis in hepatocellular carcinoma by AMPK/mTOR signaling pathway. Food Chem. Toxicol. 186, 114587. doi: 10.1016/j.fct.2024.114587
Yilmaz E. (2019). Effects of dietary anthocyanin on innate immune parameters, gene expression responses, and ammonia resistance of Nile tilapia (Oreochromis niloticus). Fish. Shellfish. Immunol. 93, 694–701. doi: 10.1016/j.fsi.2019.08.033
Zhang J., Dong Y., Song K., Wang L., Li X., Tan B., et al. (2022). Effects of the Replacement of Dietary Fish Meal with Defatted Yellow Mealworm (Tenebrio molitor) on Juvenile Large Yellow Croakers (Larimichthys crocea) Growth and Gut Health. Anim. (Basel). 12, 2659. doi: 10.3390/ani12192659
Zhang J., Liu Y., Tian L., Yang H., Liang G., Xu D. (2012). Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish. Shellfish. Immunol. 33, 1027–1032. doi: 10.1016/j.fsi.2012.05.001
Zhang M., Yin X., Li M., Wang R., Qian Y., Hong M. (2020). Effect of nitrite exposure on haematological status, oxidative stress, immune response and apoptosis in yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 238, 108867. doi: 10.1016/j.cbpc.2020.108867
Zhang Y., Cao J., Meng X., Wang W., Wang J., Wang F., et al. (2023). Chronic ammonia toxicity disturbed energy homeostasis and damaged the hepatopancreas of swimming crab Portunus trituberculatus. Aquacult. Rep. 32, 101680. doi: 10.1016/j.aqrep.2023.101680
Zhang Y., Lu R., Qin C., Nie G. (2020). Precision nutritional regulation and aquaculture. Aquacult. Rep. 18, 100496. doi: 10.1016/j.aqrep.2020.100496
Zhang Y., Yu J., Su Y., Du Y., Liu Z. (2019). Long-term changes of water quality in aquaculture-dominated lakes as revealed by sediment geochemical records in Lake Taibai (Eastern China). Chemosphere 235, 297–307. doi: 10.1016/j.chemosphere.2019.06.179
Keywords: Litopenaeus vannamei, ginseng saponins, growth performance, antioxidant capacity, ammonia nitrogen stress
Citation: Lin S, Yao R, Cui X, Guo Y, Hu D, Zhou B, Zhou Z, He X, Liao Z, Tan B and Niu J (2024) Optimizing shrimp nutrition and health: ginseng saponins as functional additives in low-fishmeal diets on Litopenaeus vannamei. Front. Mar. Sci. 11:1479921. doi: 10.3389/fmars.2024.1479921
Received: 13 August 2024; Accepted: 07 October 2024;
Published: 28 October 2024.
Edited by:
Keshuai Li, BioMar, NorwayCopyright © 2024 Lin, Yao, Cui, Guo, Hu, Zhou, Zhou, He, Liao, Tan and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beiping Tan, YnB0YW5AMTI2LmNvbQ==; Jin Niu, bml1ajNAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Sihan Lin
Sihan Lin Rong Yao1†
Rong Yao1† Beiping Tan
Beiping Tan