Introduction
Cone snails are a diverse and fascinating group of marine animals with unique characteristics and a long evolutionary history. The shell is typically cone-shaped, toxoglossan radula is their feeding apparatus, which is unique to cone snails. The radula contains modified teeth that function like harpoons, used to inject venom into their prey (Díaz et al., 2005). Over 700 species of cone snails exist in the world. These species have evolved over a significant period, approximately 50 million years, indicating their long-standing presence and adaptation within marine ecosystems (Zhao and Antunes, 2022; Duda et al., 2001). Conus snails have evolved highly targeted hunting strategies compared to other molluscs. They use a diverse arsenal of venoms called conotoxins to paralyze and capture prey.
Cone snails primarily inhabit warm, tropical and subtropical waters. Despite their regional preference, cone snails are still widely distributed throughout tropical oceans, covering around 25% of the Earth’s ocean area. The Indo-West Pacific region reigns supreme in cone snail diversity, hosting a staggering 60% of all known species (Filmer, 2001; Kumar et al., 2015). Around 93 species of cone snails have been recorded in India and, the studies on taxonomy and ecology of Conidae is well documented (Stoliczka, 1868; Winckworth, 1943; Tryon, 1883; Cernohorsky, 1964; Kohn, 1978; Coomans et al., 1979; Franklin et al., 2013; Kumar et al., 2015; Ravinesh et al., 2018; Venkatesan et al., 2019; Rout et al., 2022).
In 2011, an IUCN workshop evaluated threats to over 630 cone snail species, raising awareness about their potential vulnerability (Peters et al., 2013; Tenorio Jimenez et al., 2020). Some of the threats to Conus species are most diverse (High number of species increases chances of overexploitation), wide distribution (makes them susceptible to diverse threats across vast areas), endemism (many species only exist in specific locations, increasing their risk of extinction), depth distribution (some species inhabit deeper waters, challenging conservation efforts), trade (unregulated collection for shells by amateurs and traders poses a threat). Despite these threats, cone snails used as occasional food in some Pacific communities; their venoms hold potential pharmacological use (Peters et al., 2013). The beauty and diversity of their shells fuel commercial and amateur collecting markets (Ravinesh et al., 2018). Precise identification of species is crucial due to their venoms are used in neurobiology and drug discovery (Terlau and Olivera, 2004) and for conservation needs. Unsustainable harvesting practices by shell collectors and commercial traders pose a significant threat to these unique creatures. Comprehensive study focusing abundance and vertical distribution of Conidae in the intertidal areas of these Islands is limited. The objectives of study are to elucidate the spatial and vertical distribution of cone snails in the intertidal zone along the South Andaman Islands, and to estimate the taxonomic diversity and their habitat preference.
Materials and methods
Andaman and Nicobar Islands can be considered as biological laboratories, housing a diversified fauna, especially in the marine environment (Subba Rao, 1980). These islands (6°45’ N to 13°45’ N and 92°12’ E to 93°57’ E) are located in the South-eastern Bay of Bengal (Figure 1A), with a coastline of about 1962 km. The Andaman Archipelago is broadly divided into three regions viz. North Andaman, Middle Andaman and South Andaman. The climate of these islands is typically tropical, with hot and humid conditions (Pandey et al., 2018). Conus species were collected from the selected 9 locations viz., Kodiyaghat (KG), Burmanallah (BN), Beodnabad (BD), Brookshabad Beach (BB), Corbyn’s Cove (CC), Horn Bill (HB), Science Centre (SC), Phoenix Bay (PB), Chatham (CH) (Figure 1A). Sampling was carried out bi-monthly (for two years) during low tides from August 2019 to November 2022. To understand the vertical distribution of Conids, the intertidal area was divided into three zones, high-, mid- and low- waterline zones. Triplicate samples were collected using 1m2 quadrate from each waterline. Specimens which imposed difficulties in identification were brought to the laboratory washed and preserved in 10% buffered formalin and were identified based on taxonomic identification keys up to species level (Subba Rao, 2003; Abbott et al., 1983; Franklin and Apte, 2021). Only photographs were taken for the scheduled species and rare species without disturbing their natural habitat. Univariate and multivariate measures were performed using ecology software PRIMER v.7 (Clarke et al., 2014).
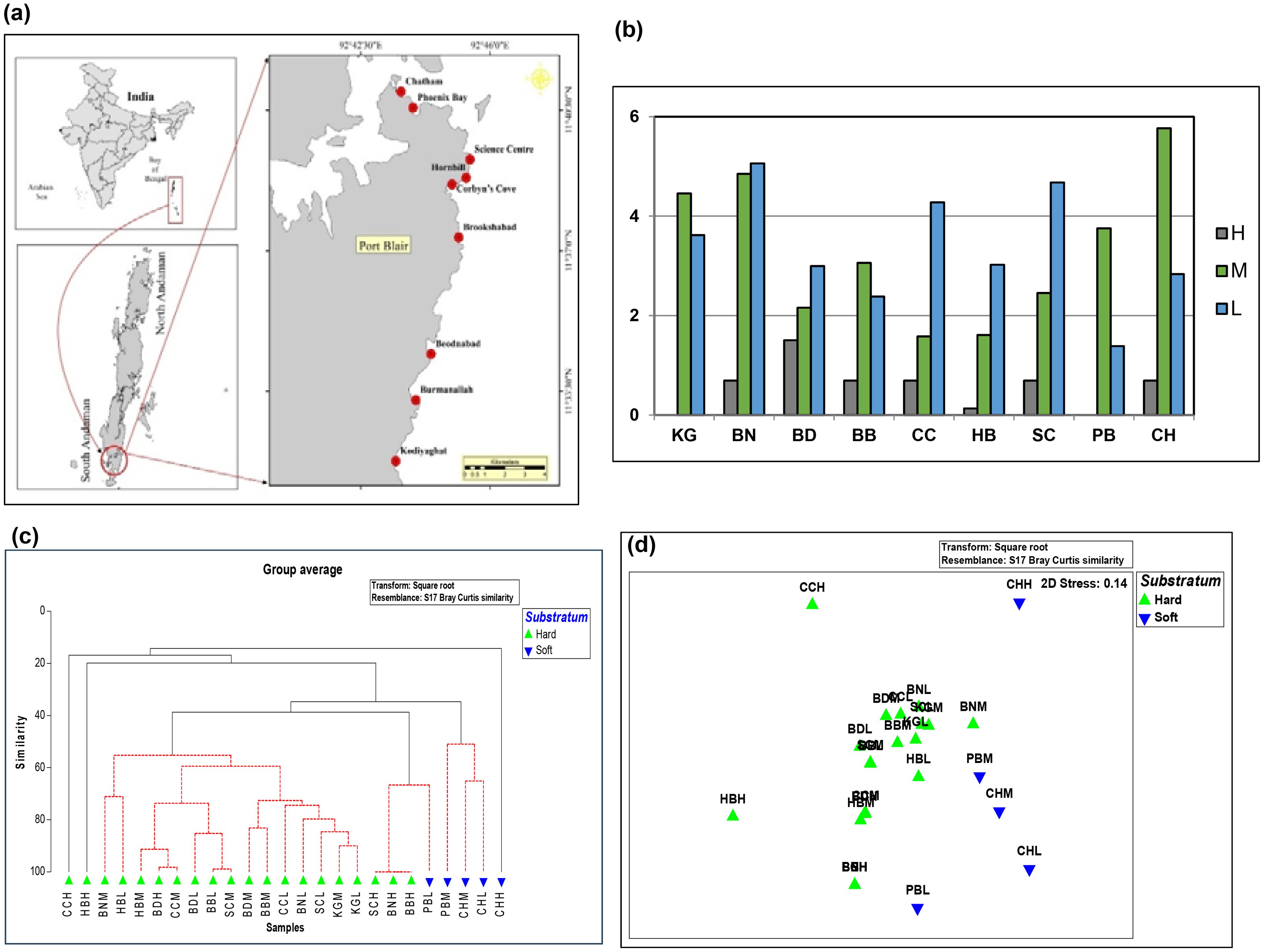
Figure 1. (A) Study locations of the present study. (B) Abundance of Conus species at selected locations along the Port Blair Coast (log transformed data) depicting the vertical distribution pattern (h-high, m-mid. l- low water lines). (C) Bray-Curtis similarity-based dendrogram showing the Conus species assemblage pattern in the study area. (D) nMDS plots of Conus species assemblage in the study area.
Results
This research explores the dynamics of the cone snail (Conidae) community in the South Andaman Islands. To achieve this, samples were collected across nine locations, KG, BN, BD, BB, CC, HB, SC, PB and CH from August 2019 to November 2022, encompassing varying seasons (southwest monsoon, northeast monsoon and non-monsoon). This extensive data collection addresses a significant knowledge gap in our understanding of these region’s cone snail populations. A summarized geographical, geomorphological and ecological information is provided in the Table 1.
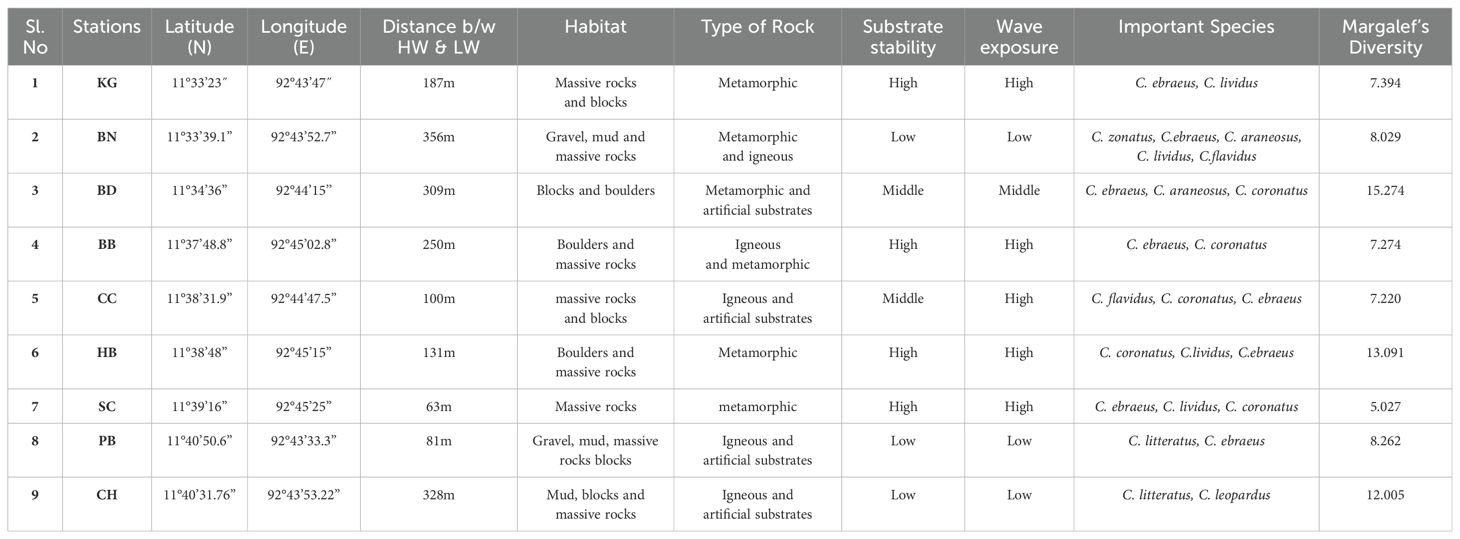
Table 1. Geographical information, geomorphology and ecological characteristics of sampling sites during the study period (August, 2019 to November, 2022). HW-high water, LW-low waterline.
Species composition
The study identified a diverse cone snail community of the South Andaman Islands, encompassing 348 individuals represented by 15 species. Among these, Conus ebraeus exhibited the highest incidence across all sampling stations, indicating its ubiquity within the study area. C. flavidus demonstrated a similar widespread presence, occurring at all stations. Conversely, C. parvatus and C. virgo manifested as rare taxa, restricted to stations HB and CH, respectively. C. litteratus displayed a limited distribution, observed solely at stations PB and CH. Temporal variability appeared minimal during the study. C. ebraeus maintained its presence across all three seasons. C. flavidus flourished during the North-East monsoon and non-monsoon periods, while displaying reduced abundance during the South-west monsoon. C. parvatus and C. nussatella were encountered exclusively during the South-west monsoon and non-monsoon seasons, respectively. Vertical Distribution: C. ebraeus and C. coronatus demonstrated a wider vertical distribution, present across all waterline zones (high, mid, and low). C. lividus exhibited a preference for mid and low waterline zones, with a single observation in the high waterline. C. flavidus, C. araneosus, C. zonatus, C. sponsalis, C. chaldaeus, C. litteratus, C. leopardus, and C. eburneus were restricted to the mid and low waterlines. C. parvatus was encountered only once in the low tidal zone, absent from the high and mid tidal zones. C. distans and C. virgo were exclusively observed within the mid waterlines.
Abundance
Conus ebraeus reigns as the most abundant species across all stations, with high abundance observed at SC and BB. C. parvatus, often misidentified as C. coronatus, was exclusively found at HB (Supplementary Table S1), showing habitat specificity. C. zonatus exhibited localized distribution, occurring only in stations BN, BD, and SC. Whereas, C. litteratus was found primarily at CH and PB, displayed a preference for specific habitats (sea grass and mud cover). Among these, C. ebraeus emerged as the pre-dominant species, constituting 56% of the total observed individuals. Based on the abundance, the ascending order of species includes, C. coronatus (15%), C. flavidus (9%), and C. lividus (8%), together contributing 32% of the total density. Several other species were present in low abundances, viz., C. litteratus (4%), C. chaldaeus (2%), and C. araneosus (1%). Significantly, C. virgo, C. parvatus, and C. nussatella were rarely encountered, with numerical contributions falling below 1%.
The northeast monsoon witnessed the highest overall abundance (49%), followed by the non-monsoon season (26%) and South-west monsoon season (25%). C. ebraeus maintained its dominance throughout all seasons. C. ebraeus predominant at Stations BB and SC, followed by C. coronatus at stations SC and HB. The least abundant species were C. nussatella, C. parvatus, and C. virgo. Mid waterline and low waterline zones harboured the highest overall abundance, with C. ebraeus and C. leopardus thriving in both areas. High waterline zone of all locations harboured relatively low numbers.
Species richness
The highest species richness, encompassing 5.33 was observed at Station BN. Station CH followed closely with 4.67 species, highlighting its diverse cone snail community. Stations BD and PB exhibited the lowest species richness suggesting specific environmental factors influencing the species composition. Seasonal Fluctuations: Northeast monsoon season was witnessed the peak species richness with 7 species. The mid waterline zone of station CH boasted the highest species richness (9 species), indicating favourable habitat conditions for a variety of cone snail species. Mid waterline zone of BN (7 species) displayed notable richness as well, highlighting the importance of mid waterline zones for cone snail communities. Interestingly, high waterline zones at Stations PB and KG were completely devoid of cone snails, suggesting ecological constraints for their presence in these areas (Figure 1B).
Species diversity
Station CH harboured the most diverse species based on Margalef’s diversity (d). Notably, it’s peaked occurred during the non-monsoon season (10.43). This suggests CH provides unique environmental conditions like soft and muddy substratum, lush sea grass patch (protecting from high temperatures), rain fall, temperature, dissolve oxygen fostering high species richness and evenness within the community, influenced by seasonal variations. KG and PB showed the lowest Margalef’s d values, indicating low species richness in these periods. These findings highlight potential environmental factors in these locations or seasons limiting species diversity and community structure. Mid-waterline zone of BD displayed the highest d (9.49). This reinforces the trend of mid waterline zones harbouring greater species richness and evenness compared to high-waterline zones. Interestingly, all high waterline zones across different stations exhibited the lowest d values, suggesting unfavourable conditions for cone snail diversity at these elevations.
Community assemblages
Cluster analysis delineated two primary groups among the study sites. Majority of the samples from the study sites namely BN, HB, BD, CC, BB, SC, and KG constituted one group, characterized by predominantly hard, rocky substratum. This assemblage is characterized by Conus ebraeus, C. lividus, C. coronatus, C. flavidus, C. araneosus, and C. zonatus. Conversely, the second group, encompassing samples of PB and CH, exhibited a soft, muddy substrate with the station CH featuring a luxuriant seagrass meadow. Notably, Conus litteratus and C. leopardus were the common occurrences of PB and CH. However, the dendrogram showed that the highwater line samples (e.g. CH, CC & HB) were mostly outliers (Figure 1C), suggesting the low abundance and diversity of conids in the highwater intertidal areas. In general, the conids were exhibiting the high diverse and abundance in mid- and low-waterline sites relative to high-waterline sites (particularly CH, CC & HB). Multi-dimensional scaling (nMDS) pattern confirms the similar observation (Figure 1D).
Discussion
Although ecologically significant, quantitative data on gastropods in the Andaman and Nicobar Islands remains scarce (Pandey et al., 2018), The present study addresses this knowledge gap by analysing cone snail diversity, distribution, and dynamics across nine locations along the eastern coastline of South Andaman Islands. The fringing coral reef ecosystems that encircle the Andaman and Nicobar Islands provide a haven for a diverse assemblage of molluscs, encompassing a spectrum of microclimatic and macroclimatic niches (Subba Rao, 1980). These reefs function as microcosms, harbouring a multitude of distinct environmental zones within a relatively confined area. The intricate topography of the coral structures creates a mosaic of light availability, water flow patterns, and nutrient gradients, fostering a remarkable heterogeneity at the microclimatic scale. The interplay between these micro and macroclimatic factors creates a unique selective pressure, favouring the establishment of a rich and prominent molluscan fauna that has adapted to exploit the diverse ecological niches within the reef complex (Kohn, 1983; Kohn and Leviten, 1976; Leviten, 1978; Leviten and Kohn, 1980).
Rocky coastlines exhibit a significant degree of micro environmental heterogeneity, making them some of the most ecologically diverse habitats within the marine realm (Tait and Dipper, 1998; Díaz et al., 2005). This intricate tapestry of microenvironments arises from the interplay of various physical factors, including wave action, tidal exposure, and the topography of the rocky substrate itself. The resulting variability in factors like light penetration, water movement, and nutrient availability creates a mosaic of ecological niches, each fostering a unique assemblage of marine life (Sommer et al., 2002). The inherent stability and firmness of the rocky substrate provide a crucial advantage for a multitude of organisms, including molluscs. Unlike the ever-shifting sands of soft-bottom habitats, rocky coastlines offer a secure foundation for sessile organisms to attach themselves and establish their habitat. This stability also allows for the development of complex crevices, tide pools, and overhangs, further enriching the available microhabitats and providing additional refuges for a wider variety of marine life (Araujo et al., 2005). The predominance of rocky coastlines along the Andaman Islands likely contributes to the remarkable abundance and diversification in this region.
The observed discrepancies in the abundance and diversity of cone snail species across different regions likely stem from their specific habitat preferences. Coral reefs, for example, demonstrably foster an exceptional richness of cone snail life, as evidenced by the comprehensive research of (Kohn and Leviten, 1976; Leviten, 1978; Leviten and Kohn, 1980). Locations with high levels of habitat heterogeneity, characterized by a complex interplay of microenvironments, are particularly conducive to supporting a thriving cone snail population. This is likely due to the confluence of two key factors, a readily available food source and a multitude of microhabitats fulfilling various ecological needs (Franklin et al., 2009). The study sites, BN and CH coincide their observation. The physical characteristics and microhabitat diversity within these locations encompassing elements like sea grass meadows, rocky outcrops, and soft-bottom substrates evidently contribute to the flourishing of a wider variety of Conus species. This can be attributed to the presence of a mosaic of ecological niches, each providing unique resources and fostering the establishment of distinct cone snail populations. The increased complexity of the substrate at CH, with its diverse habitat assemblage, further amplifies this effect, leading to a demonstrably greater species richness (2.16 H’) compared to areas with less varied environments. In essence, the intricate interplay between habitat complexity and resource availability plays a pivotal role in dictating the distribution and diversity of cone snail populations across various regions. Localities with a rich tapestry of microhabitats and readily accessible food sources create an ideal environment for a plethora of cone snail species to thrive. Despite comparable environmental factors, such as the existence of mangrove patches, pebbles, and muddy substrates in the mangrove area, and the profusion of seaweed, sea grass, and mixed substrates in BN, KG exhibits a significantly lower diversity. Notably, mangroves are entirely absent in KG, and the majority of its substrate comprises boulder rocks. Moreover, the intertidal zone in KG is less extensive than in BN. These disparities likely account for the observed difference in biodiversity between the two locations.
An analysis of substrate composition revealed a fascinating correlation between habitat complexity and cone snail diversity. Station BN, boasting a heterogeneous mixture of rocks, pebbles, coral, and sea grass, exhibited the greatest species richness which coincided with previous studies (Pandey et al., 2018). Cone snails being particularly prominent around coral reefs and shallow-water tropical marine habitats (Kohn and Nybakken, 1975; Muttenthaler et al., 2012).This suggests that a mosaic of microhabitats plays a critical role in supporting a wider variety of Conus species.
Conus litteratus on the other hand, exhibits a restricted distribution, demonstrating a particular preference for sandy substrates devoid of coral reefs and areas with minimal water movement (Muttenthaler et al., 2012). Interestingly, Conus litteratus and C. leopardus were found exclusively at Stations CH and PB, which were characterized by soft or muddy substrates. However, it is important to note that both these stations are heavily influenced by anthropogenic activities viz., oil slicks, domestic waste, and sewage discharges which provides a resilient behaviour from these two species to thrive in polluted conditions. Apart from living in polluted waters, they also managed to grow in areas of high anthropogenic activity, with frequent disturbances in water, quite unlike the results of Muttenthaler et al. (2012) claiming the need for minimal water disturbances. This distribution pattern warrants further investigation to understand the potential tolerance or specific adaptations of these species to survive in such environments.
The most abundant species, Conus ebraeus, demonstrated a clear preference for hard-bottom substrates with crevices, particularly in shallow tropical waters. This species exclusively feed on errant polychaetes of the families, Nereididae and Euncidae (Duda et al., 2009) that are abundant in intertidal areas of Andamans (Nosad et al., 2021; Sahu et al., 2022). This might be one of the reasons for this species dominance. This habitat selection aligns perfectly with its ecological niche. The presence of Conus coronatus alongside Conus ebraeus is likely due to their shared feeding habits. Both species appear to favour stations with rocky or mixed substrates, which offer a dual benefit: providing shelter from predators and sunlight while also supporting populations of prey within crevices and coral structures. As mentioned in the earlier studies by Díaz et al., 2005, female cone snails lay their eggs arranged in rows usually on the undersurface of rocks, empty shells or other hard substrate (Rolan and Massilia, 1995). This might be one of the reasons for Conidae snails preferring rocky or hard substratum. The exclusive presence of Conus litteratus at CH and PB, where it exhibited a good size and growth, presents an intriguing ecological puzzle. Potential explanations for this localized distribution include reduced predation pressure due to the soft substrate and the presence of sea grass bed offering protection from sunlight and temperature extremes (South-west monsoon: 27.75±, North-east monsoon: 27.5± and Non-monsoon: 31.5±), coupled with an abundance of food sources within this specific habitat type (Nosad et al., 2021).
The peak numerical abundance of cone snails coincided with the northeast monsoon season, suggesting a potential causal relationship with the associated increase in rainfall and decrease in temperature. These environmental fluctuations may influence factors such as prey availability, primary productivity, and water currents, ultimately leading to a high population. Cone snail distribution patterns exhibited a distinct preference for mid-waterline and low-waterline zones. This targeted selection can likely be attributed to a confluence of favourable ecological conditions: partial submergence, reduced exposure to harsh sunlight, and an abundance of food sources. Furthermore, the presence of crevices within these intertidal zones provides vital shelter for cone snails, enabling them to evade predators or seek refuge during unfavourable conditions. Conversely, high-waterline areas, characterized by extended exposure to direct sunlight, elevated temperatures, and potentially limited food resources, were observed to have a sparser Conus population. This suggests that these environmental factors may act as stressors, driving cone snails to seek refuge in the more hospitable conditions offered by the mid-waterline and low-waterline zones. However, the scenario seemed reversed in stations HB and SC where high waterline regions boasted higher evenness. This could indicate specific environmental factors at these locations promoting more equitable abundance distribution among the present species.
C. sponsalis resembles C. parvatus (Walls, 1979) which might lead to misidentifications that are extremely common in malacology. Hence utilization of novel technologies like molecular taxonomy using DNA barcoding may help to eliminate such misidentifications, which is crucial to develop conservation strategies, as carried out by Sarhan et al. (2021) who confirmed the efficacy of using DNA barcoding for specimen identification of different cone snails, which provided better opportunities of exploring Conus species and enlighten their status better. Apart from their ecology and distribution, an in-depth study of their venoms and a comparative analysis of the chemical composition of the venom in different species may also provide insights into their adaptability, resilience, and feeding strategies. Such species-wise analysis may provide a plethora of information that has overlooked so far, especially in the remote islands of Andaman and Nicobar.
Conclusion
During this study, Conus ebraeus emerged as the predominant species among the nine study sites. Conus litteratus and Conus leopardus were exclusively confined to stations PB and CH, characterized by soft, muddy substrates. The overwhelming majority of cone snail species exhibited a preference for mid and low waterline zones, in contrast to the high waterline intertidal areas. These findings underscore the pivotal roles of substrate type, waterline or tidal amplitude, and habitat characteristics in shaping the distribution patterns of cone snails. The Conus is a highly complex genus that still has various in-depth studies to be undertaken regarding their adaptations, individual species-wise preferences of habitat, feedings, etc. This study establishes a foundation for understanding how habitat complexity, substrate type affect the diversity and abundance of cone snails The findings contribute to a better understanding of these ecologically important gastropods in the Andaman and Nicobar Islands and could be used for comparing similar studies in other tropical coastal environments in addition to conservation aspects. It also calls for responsible management and research practices to ensure their conservation and potential benefits for coastal communities.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
PG: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft. AB: Data curation, Investigation, Visualization, Writing – review & editing. KM: Data curation, Resources, Visualization, Writing – review & editing. GT: Conceptualization, Investigation, Methodology, Software, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude to the authorities of Pondicherry University for laboratory facilities and the conferral of the University fellowship to PG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1477472/full#supplementary-material
References
Abbott R. T., Dance S. P., Abbott T. (1983). Compendium of seashells () (New York: EP Dutton), 248pp.
Araujo R., Barbara I., Sousa-Pinto I., Quintino V. (2005). Spatial variability of intertidal rocky shore assemblages in the northwest coast of Portugal. Estuar. Coast. Shelf Sci. 64, 658–670. doi: 10.1016/j.ecss.2005.03.020
Clarke K. R., Gorley R. N., Somerfield P. J., Warwick R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation. 3rd edition (Plymouth, UK: PRIMER-E Ltd), 256pp.
Coomans H. E., Moolenbeek R. G., Wils E. (1979). Alphabetical revision of the (sub) species in recent Conidae 2 abbas to adansonii, Zoological Museum, Amsterdam. Basteria 43, 9–23.
Díaz J. M., Gracia A. M., Cantera J. R. (2005). Checklist of the cone shells (Mollusca: gastropoda: neogastropoda: conidae) of Colombia. Biota Colombiana 6, 73–85.
Duda T., Kohn A. J., Matheny A. M. (2009). Cryptic species differentiated in Conus ebraeus, a widespread tropical marine gastropod. Biol. Bull. 217, 292–305. doi: 10.1086/bblv217n3p292
Duda T., Kohn A. J., Palumbi S. R. (2001). Origins of diverse feeding ecologies within Conus, a genus of venomous marine gastropods. Biol. J. Linn. Soc. 73, 391–409. doi: 10.1006/bijl.2001.0544
Filmer R. M. (2001). A catalogue of nomenclature and taxonomy in the living Conidae 1758-1998 (Leiden: Backhuys Publishers), 388p.
Franklin J. B., Apte D. A. (2021). Three new distribution records of Conidae (Gastropoda: Neogastropoda: Conoidea) from the Andaman Islands, India. J. Threatened Taxa 13, 18378–18384. doi: 10.11609/jott.6891.13.5.18378-18384
Franklin J. B., Subramanian K. A., Fernando S. A., Krishnan K. S. (2009). Diversity and distribution of conidae from the tamil nadu coast of India (Mollusca: caenogastropoda: conidae). Zootaxa 2250, 1–63. doi: 10.11646/zootaxa.2250.1.1
Franklin J. B., Venkateshwaran P., Vinithkumar N. V., Kirubagaran R. (2013). Four new records of family Conidae (Caenogastropoda: Mollusca) from Andaman Islands. Zootaxa. 3635, 81–86. doi: 10.11646/zootaxa.3635.1.8
Kohn A. J. (1978). The conidae (Mollusca: gastropoda) of India. J. Natural History 12, 295–335. doi: 10.1080/00222937800770171
Kohn A. J. (1983). Microhabitat factors affecting abundance and diversity of Conus on coral reefs. Oecologia 60, 293–301. doi: 10.1007/bf00376841
Kohn A. J., Leviten P. J. (1976). Effect of habitat complexity on population density and species richness in tropical intertidal predatory gastropod assemblages. Oecologia 25, 199–210. doi: 10.1007/bf00345098
Kohn A. J., Nybakken J. W. (1975). Ecology of Conus on eastern Indian Ocean fringing reefs: Diversity of species and resource utilization. Mar. Biol. 29, 211–234. doi: 10.1007/bf00391848
Kumar P. S., Kumar D. S., Umamaheswari S. (2015). A perspective on toxicology of Conus venom peptides. Asian Pacific J. Trop. Med. 8, 337–351. doi: 10.1016/s1995-7645(14)60342-4
Leviten P. J. (1978). Resource partitioning by predatory gastropods of the genus Conus on subtidal Indo-Pacific coral reefs: the significance of prey size. Ecology 59, 614–631. doi: 10.2307/1936589
Leviten P. J., Kohn A. J. (1980). Microhabitat resource use, activity patterns, and episodic catastrophe: Conus on tropical intertidal reef rock benches. Ecol. Monograph 50, 55–75. doi: 10.2307/2937246
Muttenthaler M., Dutertre S., Wingerd J. S., Aini J. W., Walton H., Alewood P. F., et al. (2012). Abundance and diversity of Conus species (Gastropoda: Conidae) at the northern tip of New Ireland province of Papua New Guinea. Nautilus-Sanibel 126, p.47. doi: 10.1179/nau.2012.126.2.47
Nosad S., Ganesh T., Lakra R. K. (2021). Efficiency of sampling gears (quadrate and core) and taxonomic resolution on the soft bottom intertidal macrobenthic community of Port Blair coast. Res. J. Chem. Environ. 25, 93–103. doi: 10.25303/2511rjce93103
Pandey V., Thiruchitrambalam G., Satyam K. (2018). Habitat heterogeneity determines structural properties of intertidal gastropod assemblages in a pristine tropical island ecosystem. Indian J. Geo Mar. Sci. 04, 846–853 pp.
Peters H., O’Leary B. C., Hawkins J. P., Carpenter K. E., Roberts C. M. (2013). Conus: first comprehensive conservation Red List assessment of a marine gastropod mollusc genus. PloS One 8, e83353. doi: 10.1371/journal.pone.0083353
Ravinesh R., Bijukumar A., Kohn A. J. (2018). Conidae (Mollusca, gastropoda) of lakshadweep, India. Zootaxa 4441, 467–494. doi: 10.11646/zootaxa.4441.3.3
Rolan E., Massilia G. R. (1995). Spawning and development of Mediterranean Conus: aquarium observations (Prosobranchia: Conidae). Argonauta 9, 9–22.
Rout S. S., Dash B., Subba Rao N. V., Surya Rao K. V., Raman A. V., Raut D. (2022). New records of Conidae (Mollusca, Gastropoda) from Andhra Pradesh, east coast of India. Indian J. Geo-Marine Sci. 50, 641–647. doi: 10.56042/ijms.v50i08.36599
Sahu N., Lakra R. K., Thiruchitrambalam G. (2022). Effect of cyclonic storm Vardah on the community structure and ecosystem functioning of macrobenthic fauna in the intertidal region of South Andaman Islands. Frontiers in Marine Science. 9, 953–985. doi: 10.3389/fmars.2022.953985
Sarhan M., Abdel-Wahab M., Aly H., Fouda M. (2021). DNA barcoding of seven cone snail species from Red Sea coast of Egypt. Egyptian J. Aquat. Res. 47, 93–99. doi: 10.1016/j.ejar.2020.10.012
Sommer U., Worm B., Worm B., Karez R. (2002). Competition, coexistence and diversity on rocky shores. Competition coexistence 161, 133–163.
Subba Rao N. V. (1980). On the conidae of andaman and nicobar islands. Records Zoological Survey India. 77, 39–50. doi: 10.26515/rzsi/v77/i1-4/1979/161839
Subba Rao N. V. (2003). Indian seashells (Part-I): polyplacopora and gastropoda (Kolkata: Records of the Zoological Survey of India, Occasional Publications, Hoogly printing Co. Ltd. (A Government of India Enterprises), 416.
Tait R. V., Dipper F. (1998). Elements of marine ecology. Butterworth-Heinemann. 4th Edition (A division of Reed Educational and Professional Publishing Ltd. Elements of Marine Ecology).
Tenorio Jimenez M., Abalde S., Pardos-Blas J. R., Zardoya R. (2020). Taxonomic revision of West African cone snails (Gastropoda: Conidae) based upon mitogenomic studies: implications for conservation. Eur. J. Taxonomy 663, 1–89. doi: 10.5852/ejt.2020.663
Terlau H., Olivera B. M. (2004). Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 84, 41–68.
Tryon G. W. Jr. (1883). Family Conidae. Manual of conchology, structural and systematic, with illustrations of the species Vol. 1 (Academy of Natural Sciences of Philadelphia), 3–150.
Venkatesan R., Barua S., Hafiz M. (2019). Contribution to the knowledge on Indian marine molluscs: Family Conidae. Records Zoological Survey India 119 (2), 165–184. doi: 10.26515/rzsi/v119/i2/2019/144125
Walls J. G. (1979). Cone shells: A synopsis of the living Conidae (Hong Kong: T.F.H. Publications), 972–973.
Winckworth R. (1943). Holten’s systematic list of the shells of Chemnitz. Proc. Malacological Soc. London 25, 146–150.
Keywords: conidae, spatial distribution, Port Blair, intertidal organisms, gastropods, multivariate analysis
Citation: Gutthavilli PR, Bharne AM, Marimuthu K and Thiruchitrambalam G (2024) Unveiling the enigmatic cone snails along the coastal environments of the South Andaman Islands: diversity, distribution and their habitat preference. Front. Mar. Sci. 11:1477472. doi: 10.3389/fmars.2024.1477472
Received: 09 August 2024; Accepted: 16 September 2024;
Published: 03 October 2024.
Edited by:
Bragadeeswaran Sunramanian, Annamalai University, IndiaReviewed by:
Padmakumar Kb, Cochin University of Science and Technology, IndiaP.K Karuppasamy, Presidency College, India
Muthusamy Anand, Madurai Kamaraj University, India
Copyright © 2024 Gutthavilli, Bharne, Marimuthu and Thiruchitrambalam. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ganesh Thiruchitrambalam, Z2FuZXNodC5vbWJAcG9uZGl1bmkuZWR1Lmlu
 Pridvi Raj Gutthavilli
Pridvi Raj Gutthavilli Ayushi Mahendra Bharne
Ayushi Mahendra Bharne Kumaresh Marimuthu
Kumaresh Marimuthu Ganesh Thiruchitrambalam
Ganesh Thiruchitrambalam