
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 20 November 2024
Sec. Marine Ecosystem Ecology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1476223
This article is part of the Research Topic Understanding the Response of Ecosystems to Increasing Human Pressures and Climate Change – Management Options View all 24 articles
 Jimmy de Fouw1*
Jimmy de Fouw1* Peter W. van Horssen2
Peter W. van Horssen2 Johan Craeymeersch3
Johan Craeymeersch3 Mardik F. Leopold4
Mardik F. Leopold4 Jack Perdon3
Jack Perdon3 Karin Troost3
Karin Troost3 Ingrid Tulp5
Ingrid Tulp5 Jetze van Zwol3
Jetze van Zwol3 Catharina J. M. Philippart1
Catharina J. M. Philippart1Bivalves play a key role in coastal ecosystems by supporting food web, modifying habitats, and their economic value for fisheries. Many bivalve species are under pressure, showing large variations in population sizes and distributions, with climate change and human activities considered as important drivers. The Dutch North Sea hosts high densities of bivalve species, dominated by the cut trough shell Spisula subtruncata, with strong interannual variations and a patchy distribution. To explore the causes of this variation, data of an extensive long-term spatial benthic monitoring program (1995-2021) was analysed using a Bayesian spatio-temporal hurdle model. We considered indicators related to human activities, biological processes, climate change and habitat preference as explanatory variables for the observed long-term temporal and spatial variations. Results revealed that medium sediment grain size was key determinant of S. subtruncata occurrence and density. Increasing sea water temperatures during winter and the post-settlement phase positively affected annual population densities, while strong north-westerly winds led to lower densities. These climate change related factors had an overall positive effect on this species in the region. Human activities like shellfish dredging and sand nourishment had no measurable impact. However, shrimp and flatfish beam trawling overlapped with S. subtruncata occurrence and were negatively related to densities, suggesting higher beam trawling intensity in these areas may negatively impacts densities. Overall, the effects were stronger at medium to finer sediments where the highest densities occurred, indicating a strong habitat-dependent effect. Despite identifying multiple drivers, unexplained annual variation suggests other not included factors like predation pressure, also play a role. More detailed studies on the combined effects of climate change-driven environmental stressors and human activities are needed to fully understand the population dynamics. This knowledge is essential for developing more adequate fisheries and coastal management strategies to sustain biodiversity.
Many marine species exhibit large spatiotemporal variations in population sizes, distributions or other traits, driven by biological processes (e.g., predation, competition), local human activities (e.g., fisheries and coastal developments) and global climate change (oceanic warming and acidification, sea level rise and intensification of extreme weather events). Human-induced changes, including the global process of climate change and local activities, increasingly negatively affect marine ecosystems (Halpern et al., 2008; Waycott et al., 2009; Trégarot et al., 2024). Climate change effects often interact with local human related stressors that can be managed locally (Scheffer et al., 2015; Gissi et al., 2021). For example, reducing local fishing pressure can ensure that populations remain resilient to critical levels of climate change (e.g. Carpenter et al., 2017; Ramírez et al., 2021). The determination of boundaries, at which populations can withstand pressures due to climate change and ongoing intensification of local human activities, requires insights in which main factors are driving populations.
Bivalves, key benthic species in coastal ecosystems, are susceptible to effects of climate change and local human activities (Kroeker et al., 2010; Philippart et al., 2011; Gobler et al., 2014). They play important roles in ecosystems as food source and altering their physical environment (Norkko and Shumway, 2011; Sospedra et al., 2017; Ysebaert et al., 2019), facilitating themselves and many associated species (i.e. ecosystem engineers) (Bruno and Bertness, 2001; Rullens et al., 2019). Many bivalves are economically valuable for fisheries and often harvested in large amounts (Smaal et al., 2019). Without adequate local management measures, stocks are often at risk of overexploitation (Smaal et al., 2019; Huang et al., 2023). In addition, coastal development and pollution have resulted in decline of bivalve populations in the past (Lotze et al., 2006; Mackenzie, 2007). Besides local human activities, rising seawater temperature, linked to climate change, negatively affect recruitment and survival in many bivalve species (Sampaio et al., 2021; Kruft Welton et al., 2024). Despite considerable research, drivers of population dynamics particularly for sublittoral bivalve, remain unclear (but see: Weinberg, 2005; Narváez et al., 2015; Hofmann et al., 2018).
The cut trough shell Spisula subtruncata is a common bivalve in European sublittoral waters, from Norway, British Isles to northern Mediterranean (Hayward and Ryland, 2017). The species has an important role in the food web and is of economic value for fisheries, especially along the Dutch coast (Smaal and Lucas, 2000; Baptist and Leopold, 2009). Once dominant in Dutch coastal waters, its population collapsed after 2000, with a strong recovery in 2017 (Troost et al., 2023). Similar strong interannual variation has been observed for Spisula spp. in other regions (Fraschetti et al., 1997; Weissberger and Grassle, 2003; Degraer et al., 2007), likely driven by varying recruitment and mortality rates. The highest mortality occurs shortly after settlement, as in many bivalves (Gosselin and Qian, 1997), which is also the case for S. subtruncata (Ambrogi and Occhipinti Ambrogi, 1987; Degraer et al., 2007; Deval and Gokturk, 2008). In the Dutch North Sea, intensified human activities (such as coastal protection, harbour development, and shrimp fisheries) and climate change (Weijerman et al., 2005; Capuzzo et al., 2018; Herman et al., 2021; Wijsman et al., 2023) may drive the species’ dynamics, but their precise role remains uncertain.
Seawater temperature increases in the North Sea, a well-documented effect of climate change (Dulvy et al., 2008; Høyer and Karagali, 2016), plays a key role in bivalve recruitment success in temperate waters (e.g. Dekker and Beukema, 1993; Philippart et al., 2003; Beukema et al., 2009). Spawning is triggered by a water temperature threshold in spring (Cardoso et al., 2007; Nicolle et al., 2013). Variations in seawater temperature may then cause temporal shifts with regard to optimal conditions for the larvae and juvenile bivalves, such as food availability and/or predation pressure (Beukema et al., 2002; Philippart et al., 2010; Poloczanska et al., 2016). While wind conditions can affect the dispersal off pelagic eggs and larvae (Thiébaut, 1996; Ellien et al., 2004), increase mortality due to displacement during storms and reduce food uptake and subsequently growth (Witbaard et al., 2005; Raven, 2022).
With respect to biotic factors, juvenile and adult S. subtruncata are food sources for shrimp, starfish, demersal fish and diving sea ducks, potentially affecting the local population size (Braber and de Groot, 1973; Pihl and Rosenberg, 1984; Camphuysen et al., 2002; Fox, 2003). Interspecific competition for space and food with other bivalves may also influence the population dynamics of S. subtruncata. In the late 1970s, for example, the invasive razor clam Ensis leei established itself to become dominant across the northwest European coastline, after being presumably introduced as larvae from ballast water (Tulp et al., 2010). S. subtruncata may be affected locally by interspecific competition with E. leei and other co-occurring burrowing bivalve species with similar filter-feeding behaviour. Currently it is unclear whether these species interact by competing for space and food.
Shellfish dredge fisheries on S. subtruncata began in 1985 but were halted in 1999 after a strong decline in the local stocks (Craeymeersch et al., 2001). While shellfish dredging has been speculated to locally deplete S. subtruncata banks (Leopold, 1999; Camphuysen et al., 2002), its influence on the total Dutch population remains unclear. Beam trawl fisheries for shrimp and flatfish also physically impact the seabed (e.g. Eigaard et al., 2016; Hiddink et al., 2017; Tulp et al., 2020), affecting S. subtruncata densities (Bergman and van Santbrink, 2000).
Sand extraction and coastal nourishment, which increased in the past decade (Baptist and Leopold, 2009; van der Veer et al., 2015), can negatively affect the benthic biodiversity (Herman et al., 2021; Dauvin et al., 2022; Saengsupavanich et al., 2023). Some studies suggest benthic communities may take years to recover after nourishment (Wijsman et al., 2023), though others indicate a faster recovery (van Dalfsen and Essink, 1999). A large spatial link between sand nourishments and S. subtruncata could not be demonstrated (Baptist and Leopold, 2009).
To gain insight into the factors driving the dynamics of the S. subtruncata population in Dutch coastal waters we investigated i) the spatial patterns and temporal trends of S. subtruncata densities, and ii) the potential contributions of biotic-, anthropogenic- and climate-related factors. Using data from an annual benthic survey along the entire Dutch North Sea coast, we present two analyses covering different periods: one excluding fisheries effort (1995-2021) and one including fisheries effort (2009-2020). We gathered all relevant available data on a high spatiotemporal resolution and a statistical model was used to assess the effects of climate conditions (temperature, wind), habitat (sediment and tidal current), and human disturbance (fishing intensities and sand nourishments). Additionally, we explored the relationship between predation (e.g., shrimp, fish and birds) and S. subtruncata densities in a descriptive manner, as information on these parameters was not available at the same spatial resolution. We discuss how our findings offer insights for improving adequate fisheries and coastal management strategies to sustain biodiversity.
Since 1995, an annual survey of shellfish stocks is carried out by Wageningen Marine Research, covering all Dutch North Sea coastal waters. The main aim of the survey is to monitor densities and biomass of exploited shellfish species for stock assessments. The number of benthic sampling stations differs between years due to logistic circumstances (756-1405 stations; min - max), spread out along transect perpendicular to the coast (Figure 1). Samples were taken either with a towed bottom cutting dredge or with a modified hydraulic dredge (see for details: Troost et al., 2023). Mesh size was 5 millimetres and sampling depth 10 centimetres. Sampling distance was at most locations about 150 meters, width either 10 cm (towed dredge) or 20 cm (hydraulic dredge), covering 15 to 30m2 respectively. Surveys were carried out from April to June except in 2020 when the sampling was delayed due to the COVID restrictions and was conducted between 25 May and 9 June (Troost et al., 2023). For our analyses, sampling stations were linked to a 5km hexagonal grid resolution (Supplementary Figure A1) which enables us to spatially link all explanatory variables, available on different scales, to the survey data.
Figure 1. Study area with sampling grid with total sample stations (1995-2021) and coastal areas: Voordelta, West coast and Wadden coast.
Living bivalves were identified to species and representative subsamples were taken when the sample size was too large to process as a whole (see Troost et al., 2019). Species were grouped into length classes and weighted (fresh shell and flesh wet-weight to the nearest 0.1 gram). Broken shells were included when flesh and both sides of the hinge ligament was present, enabling species and length class determination. To test the potential effect of interspecific competition between S. subtruncata and other filter-feeding bivalve species, we included densities of Ensis spp., Lutraria lutraria, Donax vitattus and Chamelea striatula into the statistical analyses. Numbers of Ensis spp. and L. lutraria are underestimated in the survey as these species often burrow deeper than the sampling depth of 10 centimetres but relative comparison between years is possible as same methodology was used during the entire survey period (Troost et al., 2023).
Tidal current velocity data were obtained from a hydrodynamical model with a resolution of 0.02° longitude and 0.05° latitude (~2.3×3.5 km) (van der Molen et al., 2016) (Supplementary Figure A2). For our analyses we used the annual total mean current velocity m s-1 for each sampling point. Sediment median grain size (D50) was obtained from TNO Geological Survey of the Netherlands from Deltares OPeNDAP (https://opendap.deltares.nl/thredds/catalog/opendap/tno/ncp/catalog.html) (Supplementary Figure A2).
Sea surface temperature (SST) was used in our analyses, assuming that the coastal waters in our study area are permanently mixed and so that there are no differences in temperatures throughout the water column (van Leeuwen et al., 2015). Data on sea surface temperature (°C) was derived from daily values from 1995-2021 on a 0.05°× 0.05° spatial resolution (~5.5×3.5 km) from European Space Agency (ESA) SST Climate Change Initiative (CCI) project downloaded from the Copernicus Climate Change Service (Merchant et al., 2019) and linked to the hexagon grid. Following Cardoso (2007), we assume that S. subtruncata started to spawn at the day of the year (DY) when water temperature reached 16°C. At this temperature, egg development time takes about 3 days from fertilization until hatching, and the subsequent pelagic larval stage takes approximately 24 days, until settlement (Cardoso et al., 2007). Thus, we assume that the pelagic phase (from spawning to settlement) lasts 27 days. At ad libitum food availability, larval growth and survival correlates positively with seawater temperature (Verween et al., 2007; Enricuso et al., 2018). Therefore, we used the mean water temperature (daily values) between spawning and settlement as a proxy for the growth conditions during the pelagic phase (SSTp, subscript refers to larval pelagic stage).
Brown shrimp Crangon crangon is a dominant benthic predator on bivalve recruits in marine systems (Hamerlynck et al., 1993; van der Veer et al., 1998) affecting post-settlement survival rates (Hiddink et al., 2002; Andresen and van der Meer, 2010). For S. subtruncata, it has been estimated that once settled, it takes 77 days to reach a length of 4 mm, the size at which S. subtruncata has outgrown shrimp predation (Cardoso et al., 2007; Campos and van der Veer, 2008) (see Supplementary Figure A3). Therefore, we used the mean water temperature of 77 days after the settlement as a proxy for post-settlement growth conditions (SSTS, subscript refers to post-settlement).
We used mean sea surface temperature from November to February (SSTw) as a proxy for winter temperatures as a factor potentially impacting adult mortality by low temperatures or starvation at high temperatures when metabolic rates increase but phytoplankton densities are low (Compton et al., 2007).
The index for local wind-driven wave stress was calculated as the total number of days with strong winds (> 10 m s-1) per year from the northwest (direction between 280° and 10°). In our study area, north-western winds have the longest fetch for waves to build up, creating the most turbulence in coastal waters (Witbaard et al., 2005). Wind data was derived from The Copernicus European Regional ReAnalysis (CERRA) datasets (Schimanke et al., 2021).
Bathymetry was downloaded from EMODnet bathymetry portal at a resolution of 0.05°× 0.05° (https://emodnet.ec.europa.eu/en/bathymetry) and linked to the hexagon grid.
Fishing intensity was based on Vessel Monitoring system (VMS) and fishery logbook data to produce spatial data layers on fishing intensity of different categories expressed in Swept Area Ratio (SAR) (see for methods: Eigaard et al., 2016; ICES, 2021). Data was available from 2009 until 2020 at a spatial resolution of 0.05°×0.05° and converted to hexagon resolution. For the intensity of fisheries on molluscs, fisheries data for Ensis spp. and S. subtruncata are combined, as there is no species-specific fishing effort data available. Beam trawling on shrimp and flatfish (mainly sole and plaice) and demersal otter trawl differ in sea bottom surface impact, varying from sliding over the bottom surface (shrimp trawl) to completely overturning the upper layer of the sediment (flatfish beam trawl) (Eigaard et al., 2016). Because we assume that different fishing gears have different impacts on the benthos including S. subtruncata, we differentiate between four gear groups, being mollusc dredge (SARdredge), shrimp beam trawl (SARshrimp), flatfish beam trawl (SARdmf) and otter trawl (SARotter).
Data on coastal sand nourishments was supplied by ministerial institute Rijkswaterstaat (Supplementary Figure A4). Sand nourishment activities were conducted at the coastline and did not overlap with sampling points, however, sand from coastal nourishment is known to be redistributed along the coast and to deeper waters due to tidal currents and waves. Therefore, we calculated the distance from each benthos sampling point to the nearest active nourishment site (spatial polygon) on a yearly basis, and used this minimal distance as a covariate in the analyses to detect a possible effect. Sand extraction may also affect the benthos community, however, available data were insufficient to include in our statistical analyses.
Common scoter Melanitta nigra numbers were estimated from aerial midwinter surveys (since 1993) combined with ship based surveys and counts from the coast (Sluijter et al., 2022). Densities could not be converted to the hexagon grid spatial resolution due to the lack of resolution of the data at that scale. As there are large differences on larger spatial scale, we aggregated the data per coastal area (Figure 1).
Indices for predation pressure by shrimp and flatfish was derived from the demersal fish survey (DFS). The DFS has been conducted annually since 1970 in September-October and covers the entire Dutch coastal waters, down to 25 meters depth (Beek, 1997). A 6m-wide beam bottom trawl was used at low speed (2-3 knots) with a fine mesh of 20 mm which selects brown shrimp > 20 mm. Species densities were calculated from total shrimp C. crangon and flatfish (Pleuronectes platessa, Limanda limanda, Platichtys flesus and Solea solea) per haul (see for details: Tulp et al., 2012; Aarts et al., 2019). No correction for net selectivity was applied. Data on shrimp and flatfish densities were aggregated per coastal area and could not be converted to the hexagon grid spatial resolution due to the lack of small-scale resolution of the data.
Data exploration showed that the abundance data of S. subtruncata was spatially clustered and contained a high proportion of zero observations, on average per year 35.4% (sd=10.7%). We assume all zeros are from structural source (true zeros). To analyse these zeros and accommodate for zero inflation within the data, we used a two-step modelling approach (hurdle model) (Feng, 2021). The hurdle model fits two distributions; a probability of occurrence assuming a binomial distribution or Bernouilli (presence and absence) and a Gamma distribution (because the values were on a continuous scale in numbers m-2) at locations where S. subtruncata was present. This approach allows to separate effects on the occurrence (presence/absence, binomial part) and on the actual densities once S. subtruncata is present (gamma part). The combined distribution model is called a zero-altered Gamma (ZAG):
where Spisulais (Yis) is the density of S. subtruncata (m-2) at location i in year s. Covariates described in the previous section are used in the model are either used in the year i or the year before i-1 as some covariates have an effect on S. subtruncata during pre- and post-settlement phase the year before (Table 1). The ZAG distribution has three parameters: π is the Bernouilli part which denotes the probability of presence, µ and r are parameters of the Gamma part resembling the mean and the shape of the distribution respectively. The estimated value is linked with a logit link for the binomial part and a log-link for the Gamma part. Both sides of the hurdle model use the same fixed and random effects. For the analysis prior information is incorporated in the model. For the intercept and fixed effect these are the default priors (N(0,1000)). All covariates are modelled linearly, but year trend with non-linear smoother to describe the temporal dependence. To account for temporal correlation between samples, the non-linear year smoother is modelled with an autoregressive process AR1 and incorporated a Penalized Complexity Prior scaled on the mean and SD×3 of the S. subtruncata density (Wang et al., 2018). To account for residual spatial correlation a first order intrinsic conditional autoregressive random effect (iCAR) is used to model the data (Blangiardo and Cameletti, 2015; Zuur et al., 2017). On the spatial resolution of the hexagonal grid a spatial structured term ui is added where (first order) spatial adjacency defines the variance-covariance structure. In this hierarchical structure, information from neighbouring areas is utilized, leading to a smoothing effect that reduces extreme values and produces more balanced predictions.
Models were compared according to the Watanabe-Akaike’s Information Criterion (Watanabe, 2010), where the model with the lowest WAIC was considered as having the best support. Models of which WAIC differed less than 2 ΔWAIC from the best model are considered to be equally good (Zuur et al., 2017). Confidence intervals are reported as Bayesian Credibility Intervals (BCI) between 2.5–97.5%. When the BCI of an estimated coefficient does not contain zero we consider that an covariate has an effect on density or occurrence of S. subtruncata, and to be relevant of ‘significance’ in a Bayesian context (McElreath, 2020). Statistical modelling was performed in a Bayesian framework with INLA (Rue et al., 2009; Lindgren and Rue, 2015) in R (R Core Team version 4.1.1, 2021).
Our sea surface temperature covariates exhibited interdependence and showed correlation. Therefore, to investigate the potential effect of sea surface temperature related to timing of spawning (DY), the pelagic phase (SSTp) and the post-settlement phase (SSTs) in relation to all other covariates, three models with other independent covariates were considered. VMS fishery intensity was only available for the period 2009-2020. To make optimal use of the available data, we analysed two different sets of the temperature-related models: one covering the entire data period excluding fisheries effort (1995-2021) and one shorter series including fisheries effort (2009-2020).
Tidal current velocity and median grain size showed high correlation, as they are both related to hydrodynamic processes (Supplementary Figure A5). Due to the collinearity and interdependence, it was not possible to include both covariates in the analyses. Median grain size is known to be an important variable for S. subtruncata occurrence and was therefore included in the analyses (Table 1). To show predicted relative effects of a covariate, we used the lower and the upper values for median grain size (150-250 µm) where the highest densities of S. subtruncata occur and all other covariates were standardized with a mean of zero in each covariate plot.
Temporal trends of all covariates were analysed with a linear regression in R (R Core Team version 4.1.1, 2021).
The S. subtruncata population in the Dutch North Sea coastal zone showed strong interannual fluctuations, with different average densities but similar trends in the three coastal areas (Figure 2). High densities were observed from 1995 to 2000 in the West and Wadden coast, and between 1995 to 1997 in the Voordelta, with a strong decrease to lower densities thereafter. In all coastal areas, densities remained low until 2015 and increased again from 2016. Densities stayed rather high in subsequent years, but showed a general decrease in the Wadden coast (Figure 2) after 2018. During the entire period, persistently high-density clusters of S. subtruncata occurred in the northern part of the Voordelta, the northern part of the West coast area and north of the Wadden islands Terschelling and Ameland (Figure 3).
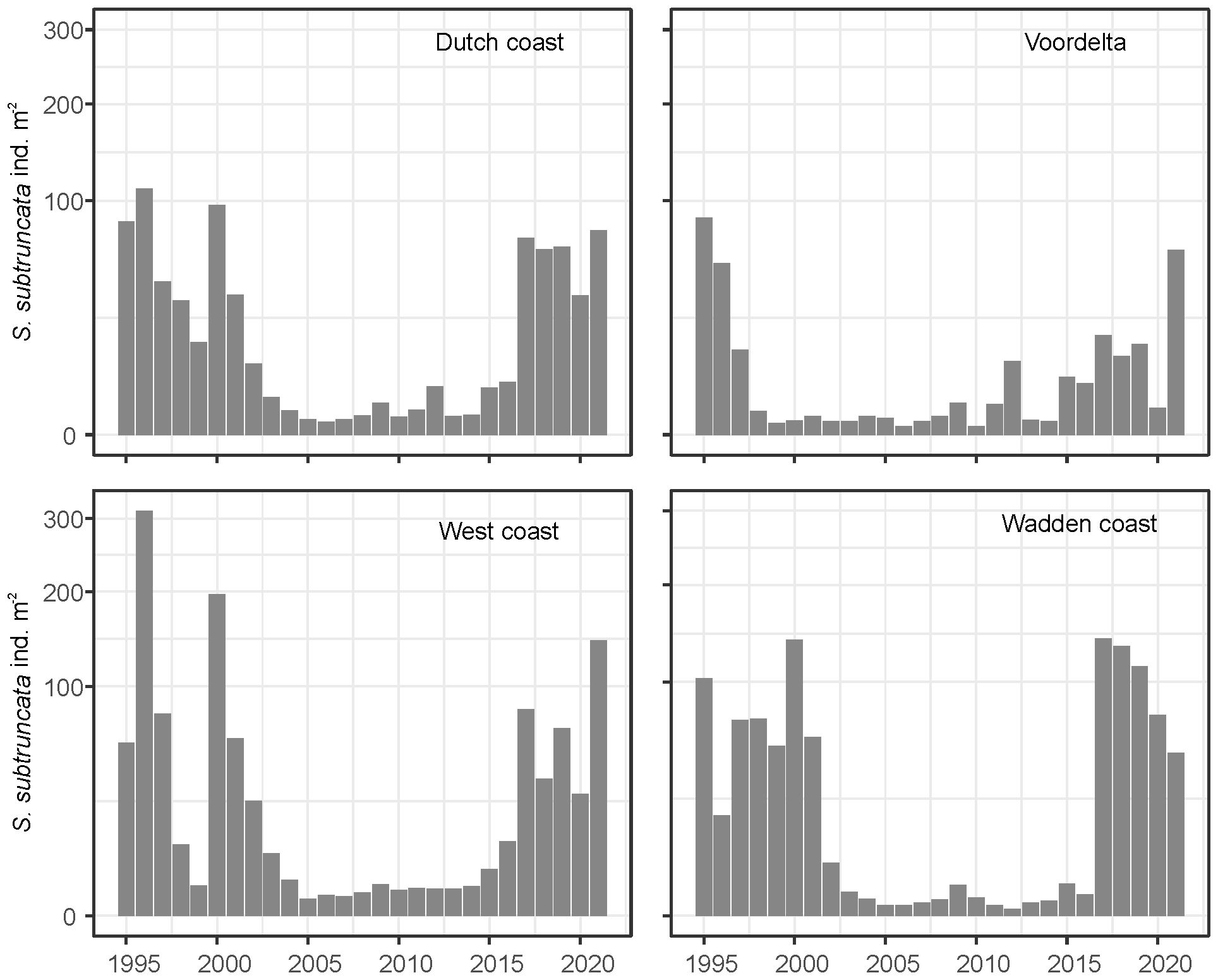
Figure 2. Total annual average S. subtruncata densities for the entire shallow Dutch North Sea coast (above left) and sub areas: Wadden coast, West coast and Voordelta.
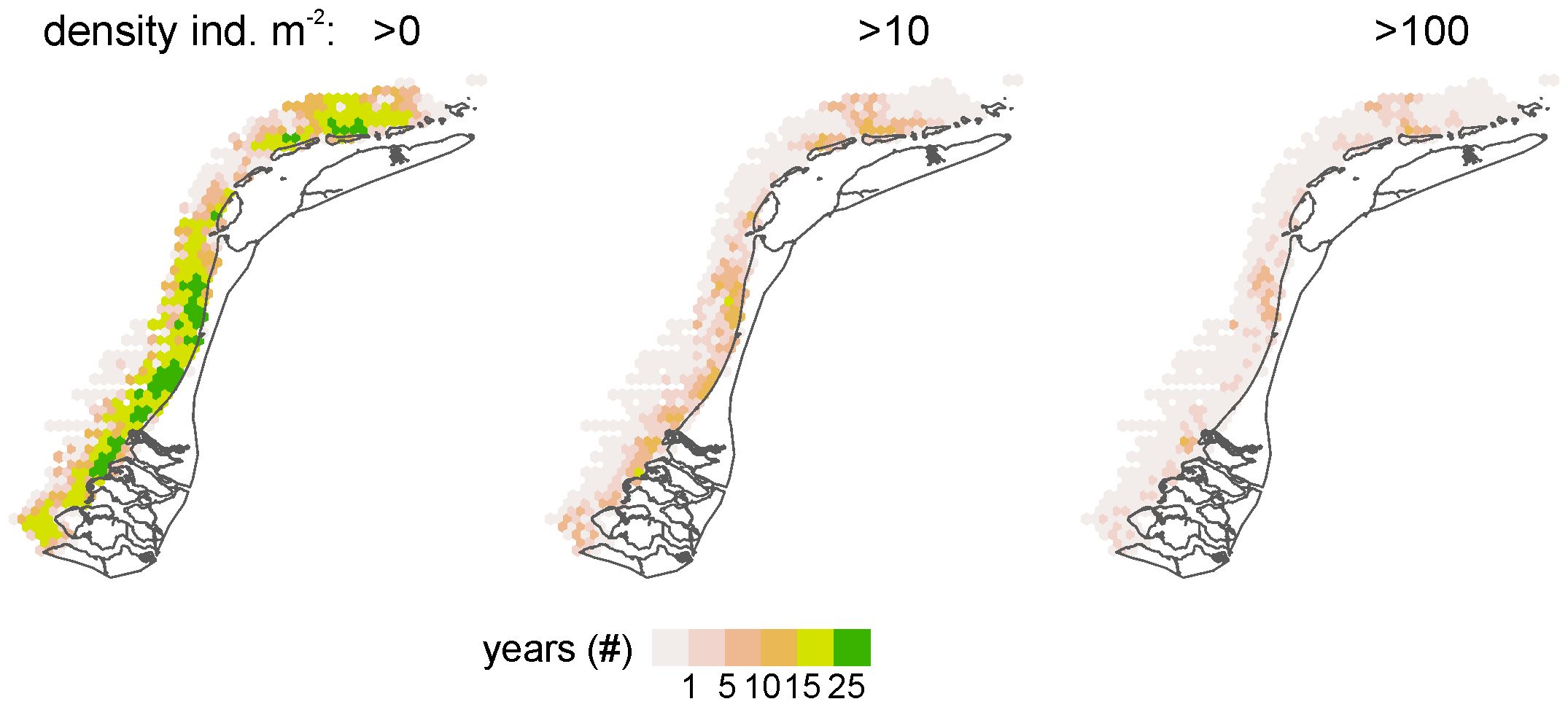
Figure 3. Spatial plots of the number of years when S. subtruncata densities reached >0, >10 or >100 individuals m2 per hexagon. Zero observations in grey.
The spawning threshold temperature of 16°C showed temporal and spatial variation, and was reached on average between 10 June (in 2017) and 22 July (in 1996) during the study period. The day at which the threshold was reached declined between 1995 and 2020 with 0.57-day year-1 (F1,25 = 8.97, P<0.01; Figure 4A) and tended to be later in deeper- and more northern waters (Supplementary Figure A6). For the pelagic larvae phase (defined as 27 days after the spawning date) no clear significant trend in average sea surface temperature was detected (F1,25 = 0.38, P=0.54; Figure 4B), but sea surface temperature during post-settlement phase (defined as 77 days after settlement, that starts 27 days after spawning) increased significantly, with 0.05°C year-1 (F1,25 = 6.21, P<0.05; Figure 4C). Winter sea surface temperature also showed an increasing trend, with 0.04°C year-1 (F1,25 = 5.68, P<0.05; Figure 4D). The annual number of days with strong (> 10 m s-1) north-western winds increased over time, but this increase was just not significant (F1,25 = 3.42, P=0.08; Figure 4E).
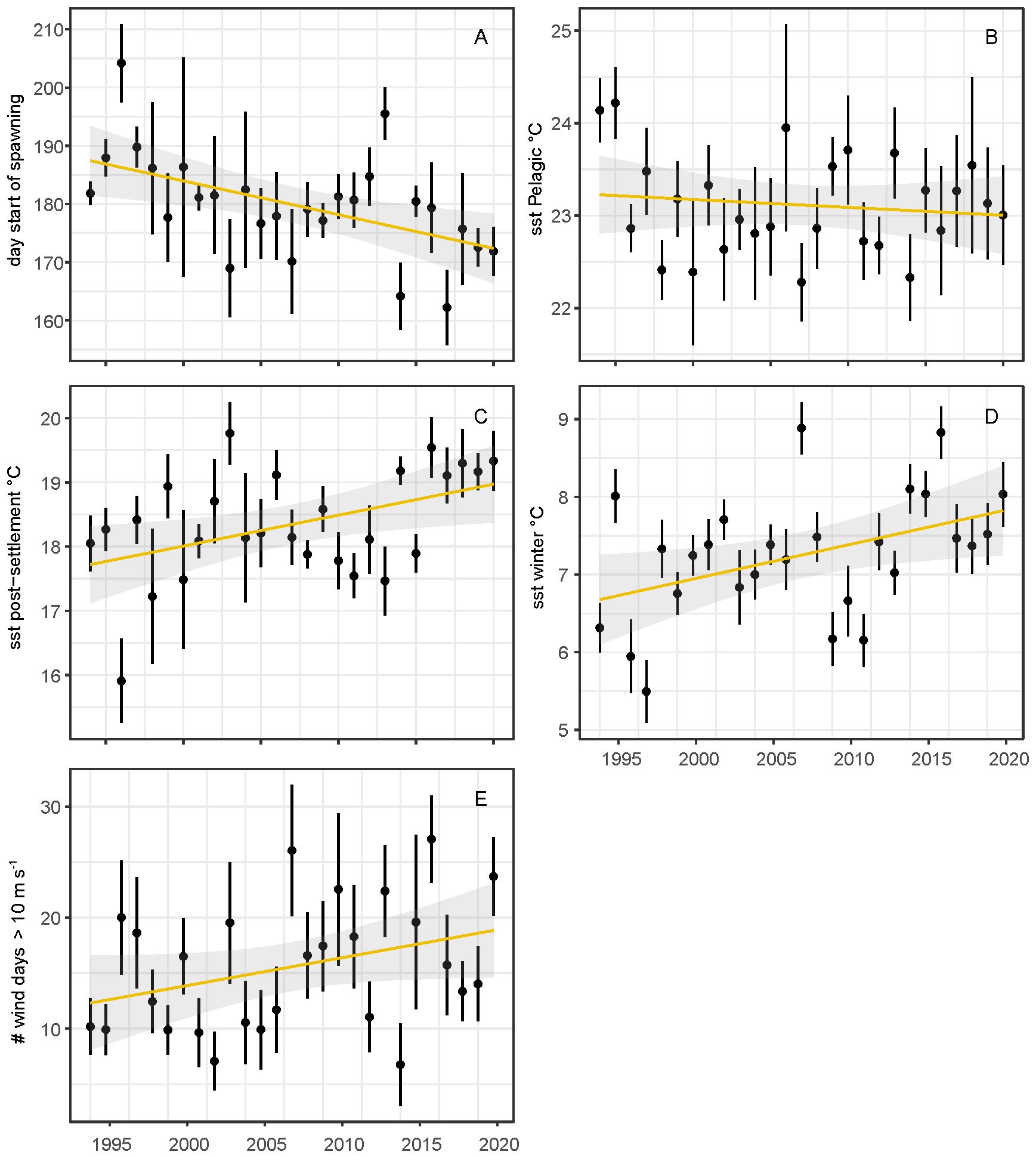
Figure 4. Average day of the year when the threshold for spawning was reached (16°C) (A) and average sea surface water temperature during pelagic phase (day: 0-27) (B), post settlement phase (day: 28-77) (C), sea surface water temperature in winter (D) and wind days with strong North-West winds > 10 m s-1 (E). Error bars represent standard errors.
The total density of suspension-feeding bivalve species increased during the study period with a yearly increase of 0.85 ind. m-2 to on average of 22.2 ind. m-2 (F1,25 = 21.22, P<0.001, Figure 5A). This trend was mainly due to the exponential increase of the invasive E. leei since 2000, with average densities reaching up to 100 m-2 in the Wadden coast area. Densities of E. leei fluctuated strongly between years for all coastal areas, but in general showed a decreasing trend after 2016 (Supplementary Figure A7).
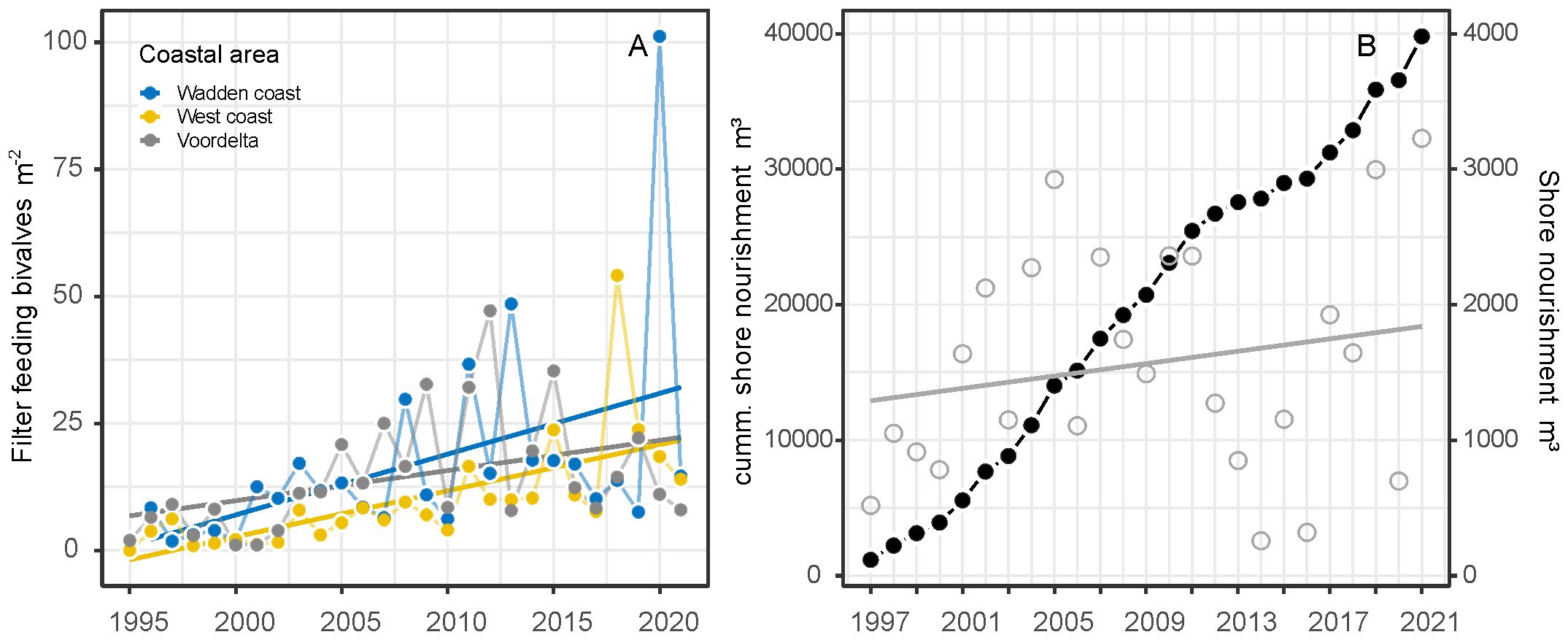
Figure 5. Trends of average densities of the sum of filter feeding bivalves (Ensis spp., Lutraria lutraria, Donax vitattus and Chamelea striatula) (A) and cumulative sand nourishment over the years (black dots) and yearly total shore nourishment volumes (grey circles) (B).
Fishing intensities (SAR) differed over time and between coastal areas between 2009 and 2020. Mollusc dredge fishery intensity differs between years (F1,27 = 19.66, P<0.001) and coastal areas (F2,27 = 34.28, P<0.001). Trends were different between coastal areas (F2,27 = 18.74, P<0.001), increased in the Voordelta and were stable in the West and Wadden coast (Figure 6A). Sole and place beam trawl fishing intensity declined over the years (F1,30 = 30.18, P<0.001) between areas (F2,30 = 74.74, P<0.001), were highest in the West coast, low and stable in the Voordelta over the years and very low in the Wadden coast (F2,30 = 17.04, P<0.001) (Figure 6B). Shrimp beam trawl intensity had the highest intensity of all fisheries, however no showed significant trend over the years (F1,32 = 0.32, P<0.57) but differed between coastal areas (F2,32 = 43.16, P<0.001). The fishing intensity was generally highest in the Wadden coast and was quite low in the West coast (Figure 6C). Total otter trawl fishing intensity declined over the years (F2,30 = 23.78, P<0.001) and differed between coastal areas (F2,30 = 8.45, P<0.01). Otter trawl fishing intensity was relatively high in the Wadden coast but strongly decreased after 2010 and fishing intensity was relatively low in the West coast and Voordelta (Figure 6D). Shore nourishment increased over the years along the entire Dutch coastline (F1,26 = 7.76, P<0.01), especially since 2000 (Figure 5B).
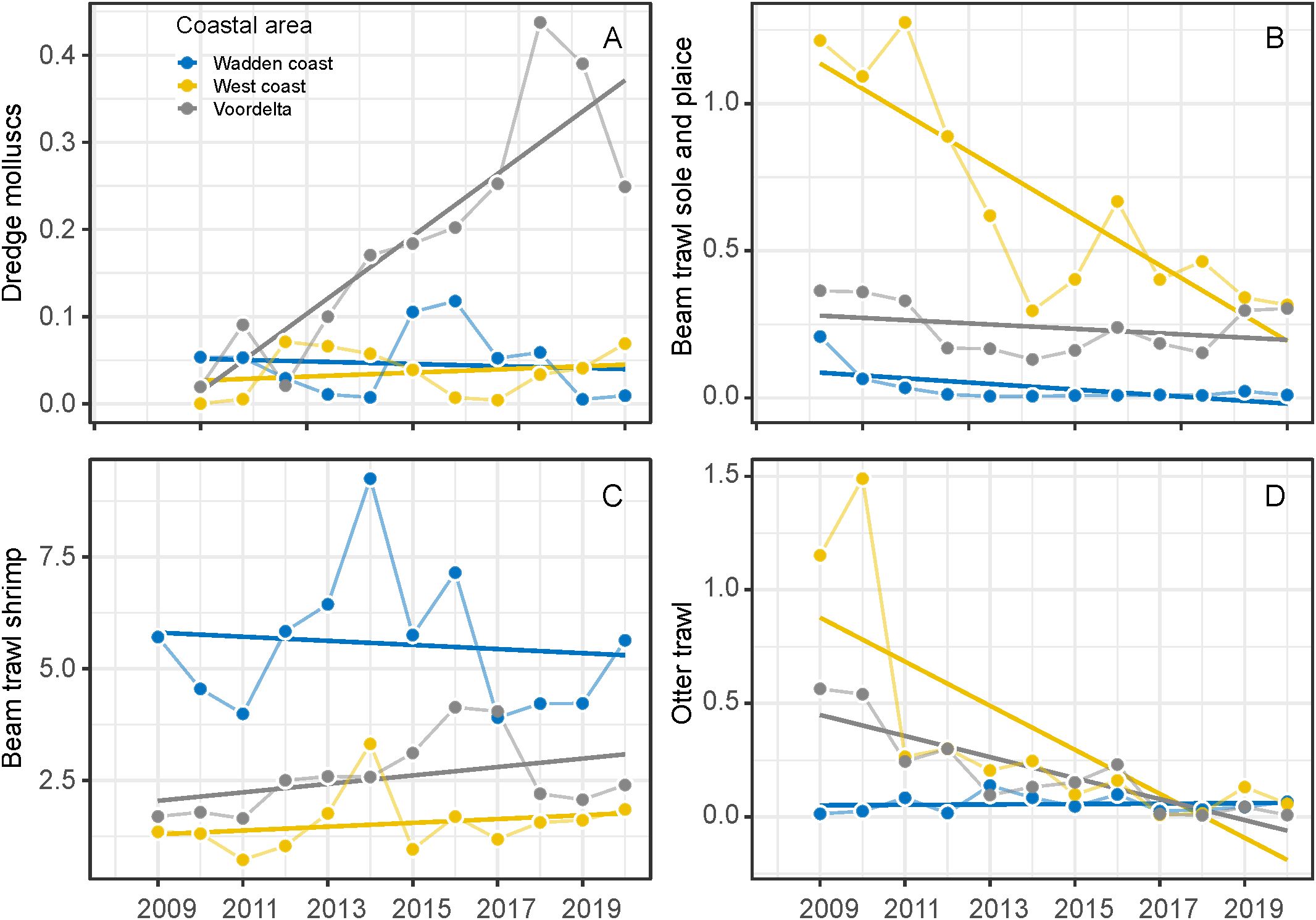
Figure 6. Fisheries intensity in the coastal areas expressed in Swept Area Ratio (SAR) in the coastal areas from 2009-2020. Dredge mollusc (S. subtruncata and E. leei) (A), beam shrimp trawl (B), beam sole and plaice trawl (C) and otter trawl fisheries (D).
Annual average shrimp densities and trends differed between coastal areas. In the Voordelta, shrimp densities increased over the years, while in the West coast and Wadden coast area they decreased (Figure 7A). Similar to shrimp, total flatfish numbers slightly increased in the Voordelta and decreased in the West and the Wadden coast (Figure 7B). The flatfish species plaice and dab were the numerically dominant species in the survey (Supplementary Figure A8). Common scoters are commonly found in all coastal areas but highest numbers were found in the eastern Wadden coast (Figure 7C). Incidentally, high numbers were also found in the other coastal areas.
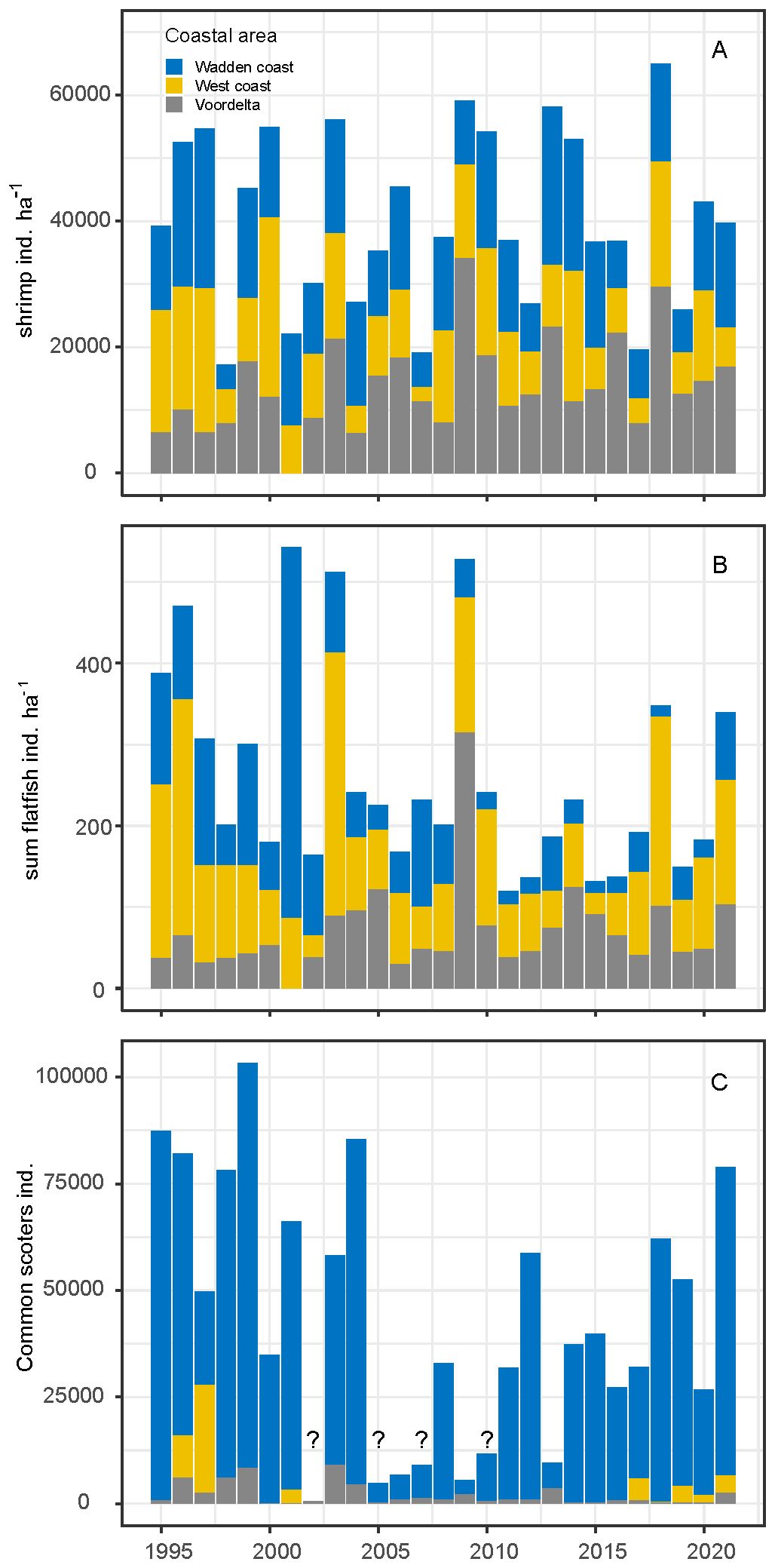
Figure 7. Trends of average densities of shrimp (A) and total number of flatfish species (B) and common scoter, uncomplete surveys are indicated with question mark (source: Sluijter et al., 2022) (C).
To investigate the effect of sea surface temperature from the moment of spawning three models (with respective temperature-effects at the moment of spawning, the pelagic phase and the post-settlement phase) were considered for the analyses for both sets of time series (Table 2). Based on the WAIC values model 1 and 2 were assumed similarly supporting the data for both time series, as they did not differ more than 2 ΔWAIC (Table 2). Based on model performance we conclude that model 2 had a satisfactory fit and correctly estimates occurrence and densities of S. subtruncata (Supplementary Material B). In addition, we considered this model biologically the most relevant and interpretable, because it includes average sea surface temperature in the post settlement phase (SSTs) instead the start of spawning date (DY). The post settlement phase is a critical period during which biological processes likely influence mortality and survival of S. subtruncata recruits, providing interpretability and stronger basis for causal understanding.
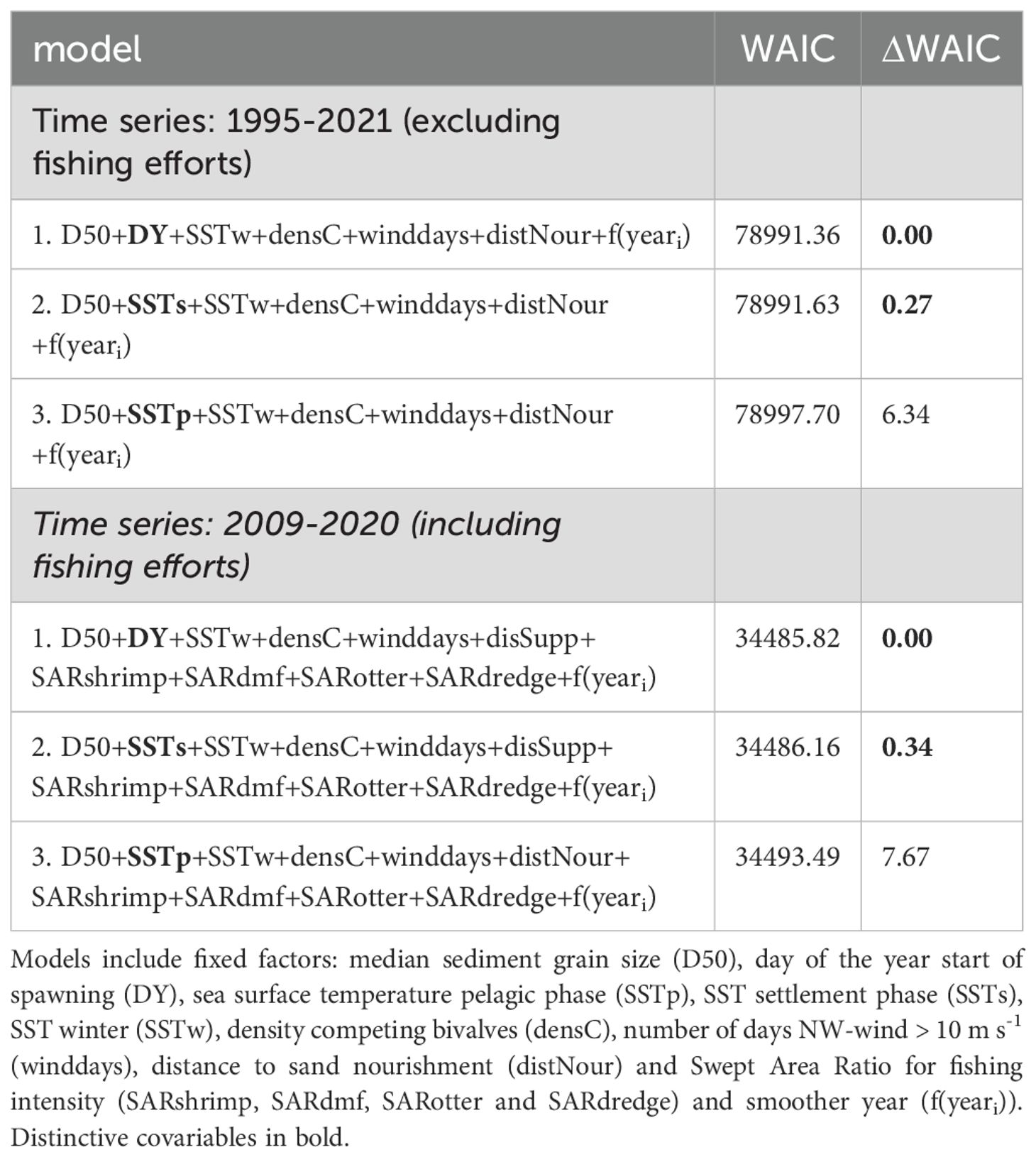
Table 2. Models ranked according Watanabe-Akaike information criterion (WAIC), with the ΔWAIC of the best (equally good) models printed in bold.
Model 2 shows that median grain size (D50) had the largest effect on densities and occurrence (Figure 8). The highest densities of S. subtruncata were at median grain sizes of 160-280 µm (Figure 9A). We used this range to standardise the effects of all other contributing covariates, as it indicates the preferred habitat for S. subtruncata based on median grain size (Figure 9A, between the blue and yellow lines). Median grain size showed a negative association (when all other variables were standardised) with occurrence and density, indicating higher abundances of S. subtruncata at intermediate fine compared to coarse sediments (Figure 9B). Sea surface temperature during the post settlement phase (SSTs) showed a positive association with densities (Figures 8, 9C). This effect became stronger with decreasing median grain size (Figure 9C). Likewise, increasing water temperature during winter had a positive effect on S. subtruncata densities (Figure 8) which also became stronger with decreasing median grain size (Figure 9D). The number of NW-wind days above 10 m s-1 (winddays) showed a negative effect on S. subtruncata densities and no effect on occurrence as the 95% confidence intervals overlap with zero (Figure 8). A negative association was found between densities and NW-wind days at high grain size, but a positive association at low grain size (Figure 9E). The cumulative density of filtering bivalves (densC) showed a positive effect on S. subtruncata densities but no effect on occurrence, as the 95% confidence intervals overlapped with zero (Figure 8). This positive effect with other filter-feeding bivalve species and S. subtruncata densities became stronger with increasing median grain size (Figure 9F). Shoreface sand nourishment (DistNour) did not contributed importantly to the models, as the 95% confidence intervals overlapped with zero (Figure 8).
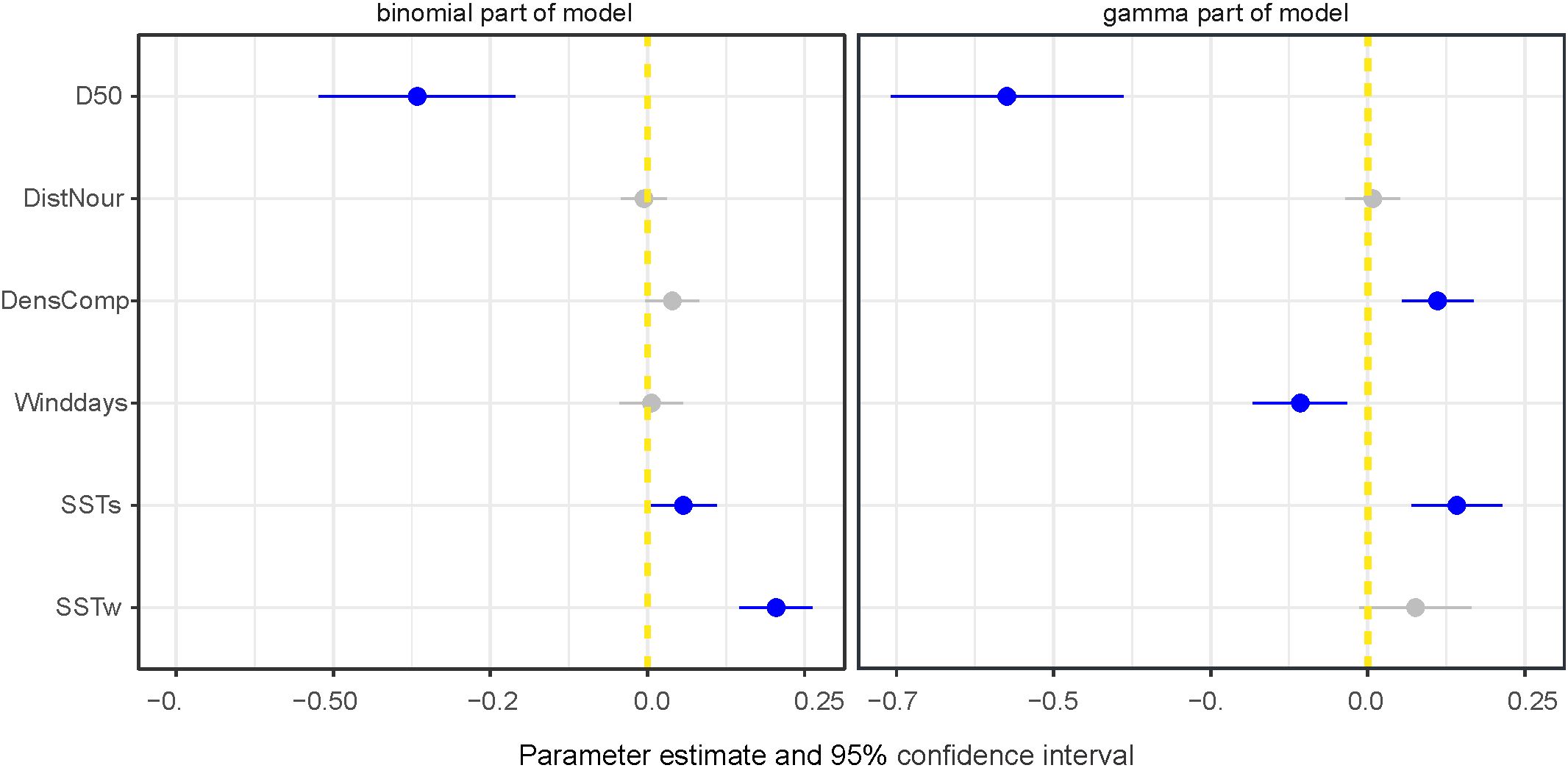
Figure 8. Estimated weights of the covariate effects indicated with posterior means with 95% confidence interval (CI) plotted for most parsimonious model excluding fisheries (for the dataset 1995-2021) for binomial and gamma part (best model in Table 2), blue points and lines show effects differing from zero with 95% CI. Median sediment grain size (D50), SST settlement phase (SSTs), SST winter (SSTw), density competing bivalves (DensCom), number of days NW wind > 10 m s-1 (winddays), distance to sand nourishment (DistNour).
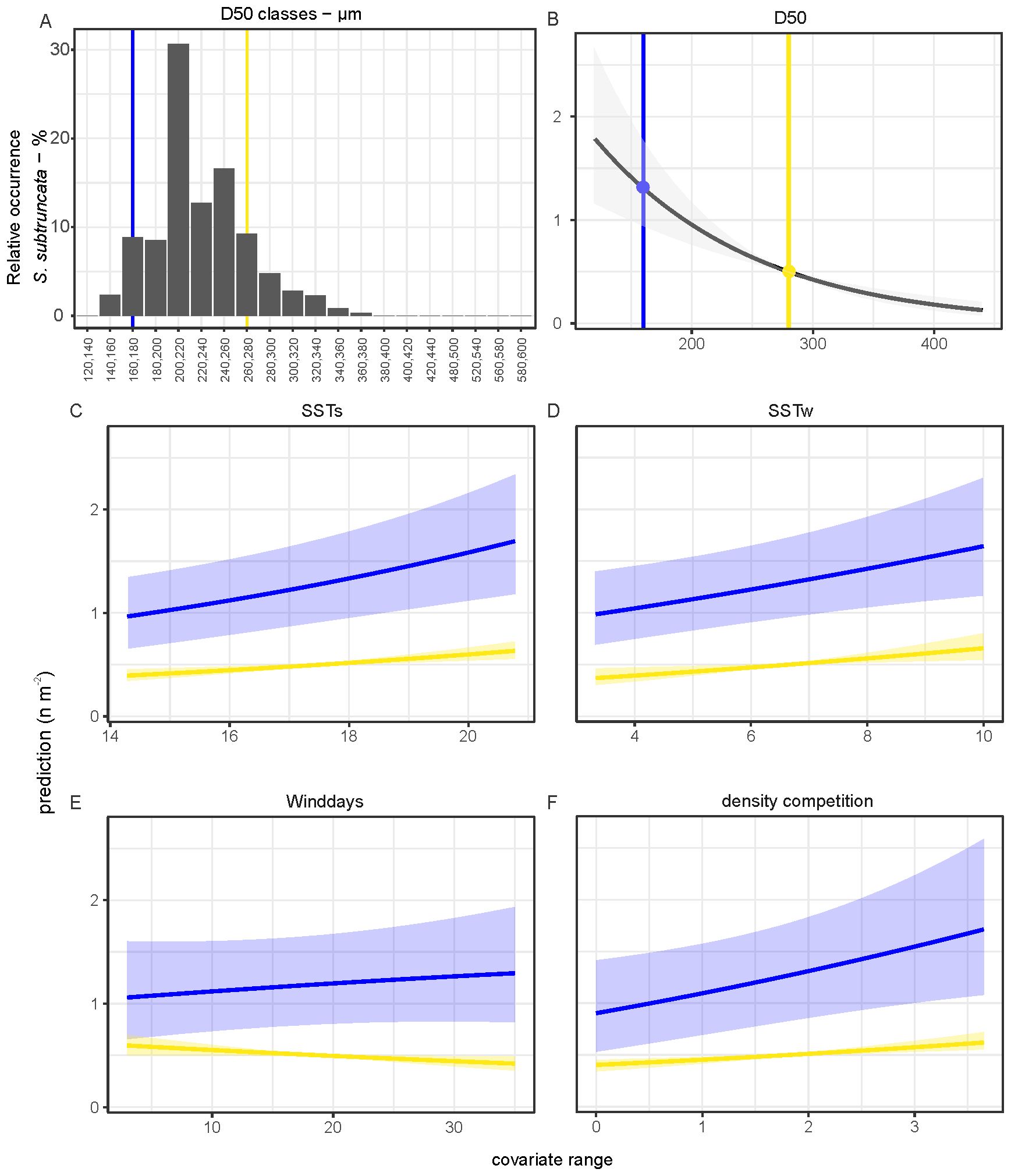
Figure 9. Frequency plot of sediment grain size where S. subtruncata occurred (A). Predicted contribution of covariates from the hurdle model. (B–F) Prediction per measured range of covariates with minimum (blue) and the maximum (yellow, for values see B) of the optimal range of occurrence of S. subtruncata for median grain size while all other covariates standardized with a mean of zero. Shaded areas represent 95% confidence interval around the prediction.
To investigate the effect of fishery intensity in combination with other covariates we used model 2, which was the most parsimonious model based on WAIC and included average sea surface temperature during the post settlement phase (SSTs) (Table 2). The fisheries covariates showed different effects in both parts of the model (Figure 10). Beam shrimp fishing intensity showed a positive association with occurrence of S. subtruncata but a negative with densities (Figure 10). This indicates that shrimp fisheries are associated with the species’ occurrence, but at locations where S. subtruncata is present, there is a negative association that became stronger with lower median grain size (Figure 11A). The effect of beam trawl fishing intensity on sole and plaice showed a similar association on occurrence and densities (Figure 10), with a negative association on S. subtruncata densities that was stronger at lower median grain size (Figure 11B). Otter trawling did not show a relevant association on occurrence as the 95% confidence interval overlapped with zero, implying no strong overlap between otter trawl fishing intensity and the presence of S. subtruncata (Figure 10). However, a weak positive effect of otter trawl fishing intensity on S. subtruncata densities was found (Figures 10, 11C). Mollusc dredge fisheries showed a positive association with the occurrence of S. subtruncata but no effect on densities (Figures 10, 11D). Most other (non-fishery) covariates within this model (DistNour, DensComp, SSTs and SSTw) showed similar results to the first model analyses on the longer time series (Figure 10). Only the number of NW-wind days above 10 m s-1 showed a positive association with S. subtruncata occurrence and no association with densities (Figure 10).
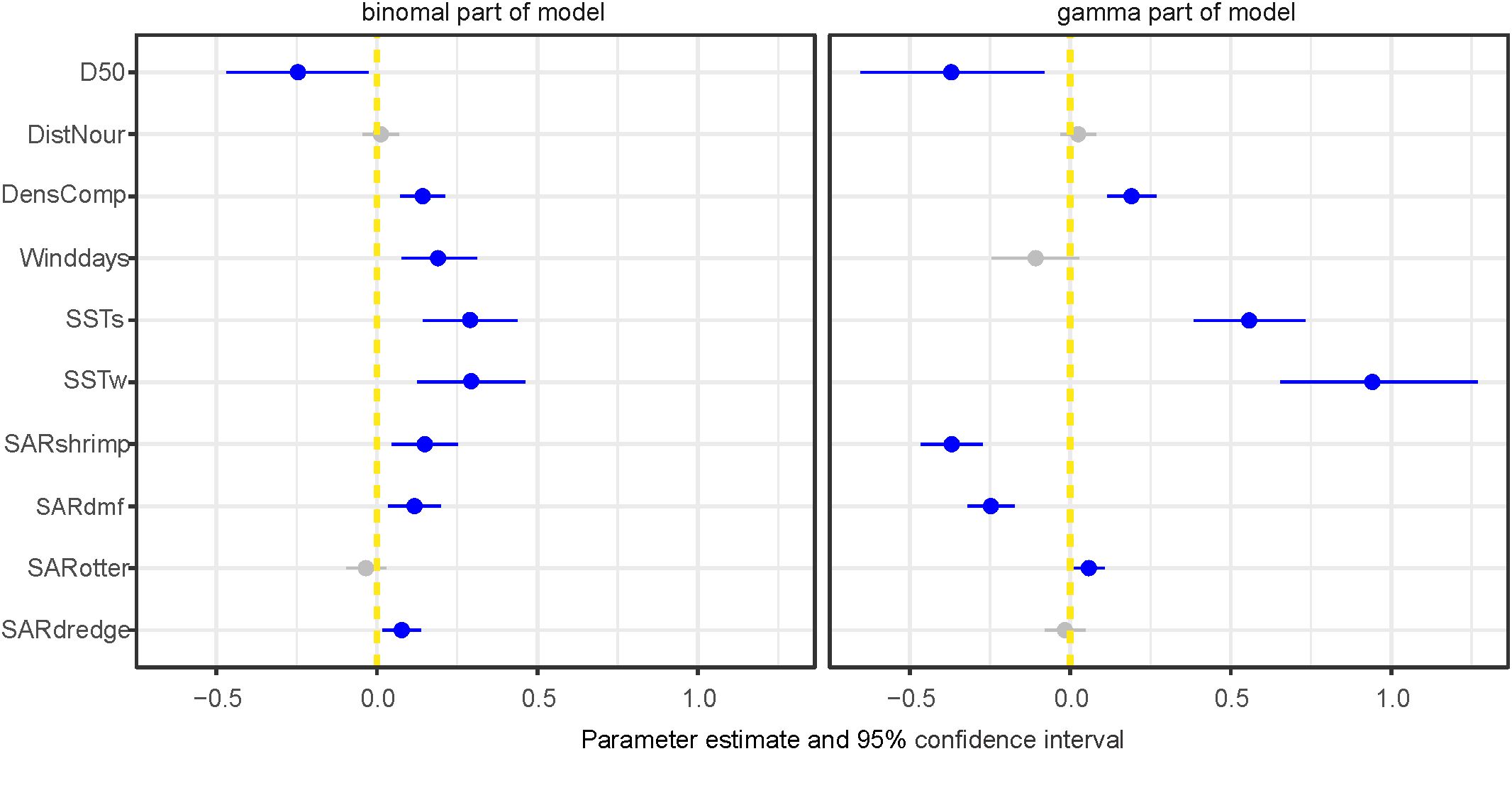
Figure 10. Estimated weights of the covariate effects indicated with posterior means with 95% confidence interval (CI) plotted for most parsimonious model including fisheries (for the dataset 2009-2020) for binomial and gamma part (best model in Table 2), blue points and lines show effects differing from zero with 95% CI. Median sediment grain size (D50), SST settlement phase (SSTs), SST winter (SSTw), density competing bivalves (DensComp), number of days wind > 10 m-s (Winddays), distance to sand nourishment (DistNour) and Swept area ratio for fishing intensity (SARshrimp, SARdmf, SARotter and SARdredge).
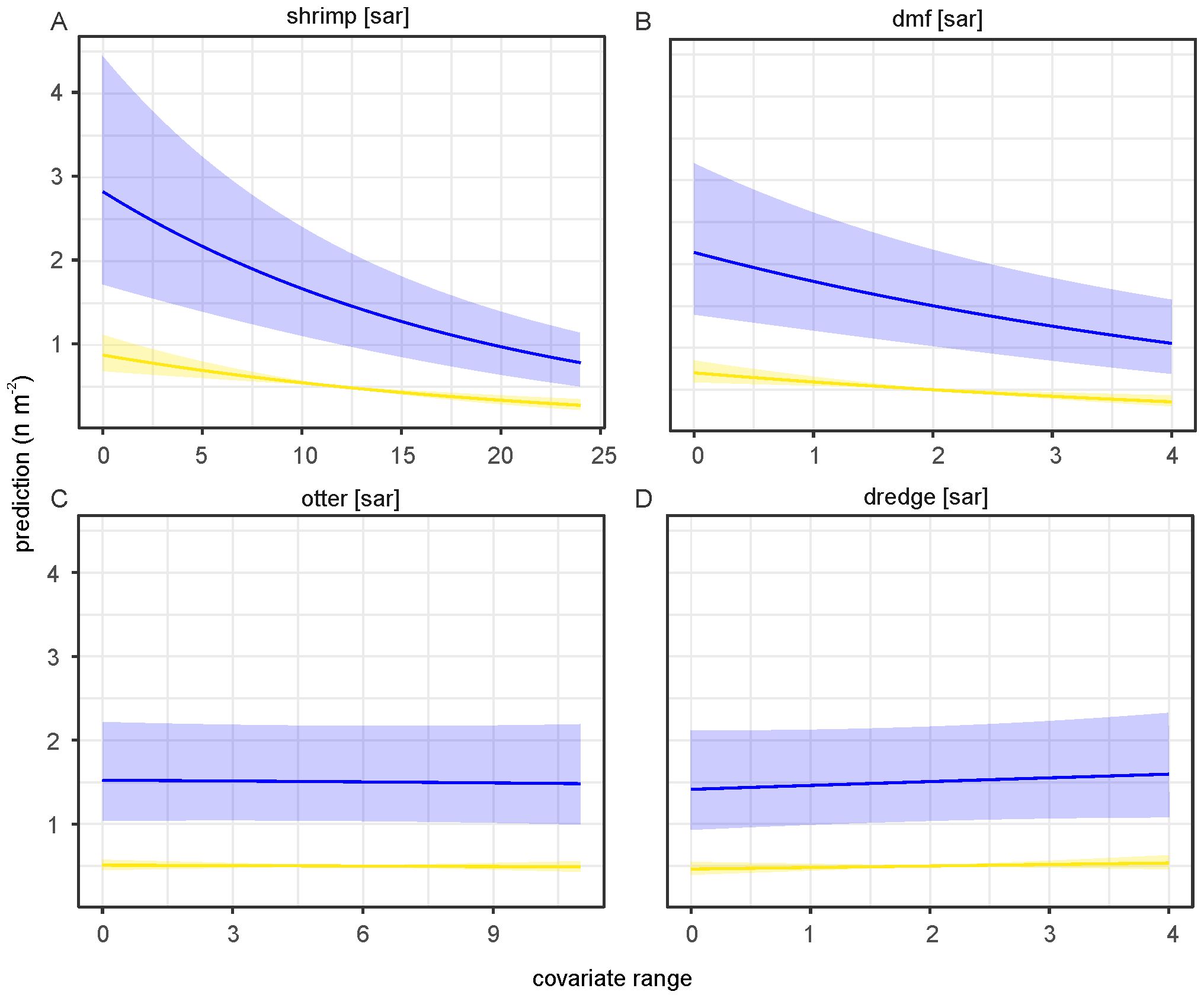
Figure 11. Predicted contribution of covariates the hurdle model. (A–D) Prediction per measured range of covariates with minimum (blue) and the maximum (yellow, for values see Figure 9B) of the optimal range of occurrence of S. subtruncata for median grain size while all other covariates standardized with a mean of zero. Shaded areas represent 95% confidence interval around the prediction.
Although several covariates were considered important in our models, the relative effect of the smoother year was high and captured the temporal trend of S. subtruncata densities to a large extent (Supplementary Material B). This was less apparent for the binomial part of the models. Year has no direct biological meaning as such. Therefore, there is some unexplained annual variation in densities and other factors which could not be captured by our model covariates should be considered.
Our study suggests that both local human activities and climate conditions are important factors influencing the S. subtruncata population dynamics in the Dutch North Sea. Water temperature in winter and after settlement, were positively linked to S. subtruncata occurrence and densities, while strong north-westerly winds (>10 m s-1) had a negative effect. Overall, recent climate conditions seem to have benefited the population. Shellfish dredging and sand nourishment showed no apparent effect in recent years. However, our analyses shows that shrimp and flatfish beam trawling were positively associated with S. subtruncata occurrence but negatively with densities, suggesting higher trawling intensity where the species is found, which then negatively affects densities. An unexplained year effect, indicates additional unknown factors may also affect recruitment and survival. While our observational, correlative analysis is no proof of causality, it sheds light on factors potentially driving the population. Further discussion of our model results, the year effect and, possible mechanisms is provided below.
Our analyses shows that medium sediment grain size (D50) is an important determinant explaining occurrence and the density of Spisula subtruncata, with highest densities found at medium fine sediments (160-280 μm), aligning with earlier studies (Degraer et al., 2006, 2007). Sediment grain size, shaped by tidal current velocities and wave action, is commonly linked to the distribution of macro zoobenthos (Ellis et al., 2017; Bosco Gusmao et al., 2022). Our study indicates that habitat, as indicted by grain size, is a good proxy for habitat preference for S. subtruncata, with stronger effect of stressors at lower sediment grain size. Similar sediment-related effects on benthic communities have been observed in the North Sea (van Denderen et al., 2014; Rijnsdorp et al., 2018; van der Reijden et al., 2018).
Changes in sediment texture can affect S. subtruncata densities, with beach and foreshore sand nourishment altering sediment texture and impacting benthic communities (Herman et al., 2021; Dauvin et al., 2022; Saengsupavanich et al., 2023; Wijsman et al., 2023). While our analysis treated median grain size as an annual static variable, potential changes from nourishment were not fully accounted for. Although no direct effect of sand nourishment was detected (cf., Baptist and Leopold, 2009), we may were unable to assess its potential impact as it is currently unknown how to examine nourishment effects on the spatiotemporal scale of our study. In addition, the lack of detailed sand extraction data hindered a comprehensive assessment of its impact on S. subtruncata population.
We found a positive association between winter temperatures and the occurrence and densities of S. subtruncata. Warmer winters may reduce mortality by increasing mobility, allowing S. subtruncata to bury themselves faster in the sediment to escape from storms and predation. Moreover, the absence of prolonged sub-zero water temperatures may avert direct mass mortality of shellfish as has been observed during severe winters (Armonies et al., 2001; Baptist and Leopold, 2009). Thus, increasing winter temperatures may led to higher adult survival of S. subtruncata.
The positive association between water temperature during the post-settlement phase (77 days after settlement) is likely related to increased survival of recruits. Warmer seawater increases growth rates of S. subtruncata, helping juveniles to outgrow predators like brown shrimp (van der Veer et al., 1998; Hiddink et al., 2002; Andresen and van der Meer, 2010), which feed on recruits up to 4 mm in size (Cardoso et al., 2007; Campos and van der Veer, 2008). Faster growth shortens the duration of the shrimp predation window, enhancing survival (Hiddink et al., 2002; Andresen and van der Meer, 2010). While shrimp reproduction and shoreward migration are also temperature-dependent (Boddeke, 1976; Beukema, 1992; Penning et al., 2021), rising North Sea temperatures could create a mismatch between shrimp and prey, potentially benefiting S. subtruncata. However, warming may also shift predator communities, with increasing cold water species in the coastal zone, negatively effecting bivalves. For example, flatfish such as sole and plaice, appear to be move from the Wadden Sea to the cooler coastal zone in the North Sea (van der Veer et al., 2022).
The positive association between post-settlement water temperature and S. subtruncata densities may also be related to food availability. Phytoplankton blooms play a crucial role for benthic fauna, driving rapid growth and changes in benthic communities (Zhang et al., 2015). There are indications that changes in North Sea phytoplankton have occurred in the recent and more distant past. Over the last century, for example, it is estimated that a reduced water transparency resulted in a delay of the spring bloom by 3 weeks (Opdal et al., 2019). Between 1988 and 2016, however, phytoplankton biomass declined and the timing of the spring bloom advanced (Desmit et al., 2020). While, shifts in phytoplankton species composition that have occurred (Baretta-Bekker et al., 2009), may have affected growth and survival of juvenile bivalves in Dutch North Sea coastal waters. Currently, the net consequences of altering phytoplankton bloom as a food source for benthic animals in the North Sea are difficult to evaluate due to the multifaceted changes in phytoplankton and unknown food preferences of the bivalves.
Strong north-westerly winds affected S. subtruncata densities negatively. Strong winds cause resuspension of fine sediments which might reduce filtering efficiency of feeding bivalves, hampering their growth (Witbaard et al., 2005). Additionally, the disturbance of waves can disturb the buried S. subtruncata and release them from the seafloor. As S. subtruncata live at the sediment surface, they are also vulnerable to strong under water currents which can transport them to the beach or other unfavourable habitat. Mass mortality events due to strandings are known to happen after strong north-westerly winds followed by wind from the land creating a reversed current at the bottom (Raven, 2022).
Our analyses showed a positive association between beam trawling for shrimp and flatfish and the presence of S. subtruncata, indicating that areas where the species is abundantly present, were more frequently fished. This aligns with observations that areas rich in bivalves attract shrimp and flatfish, and thus fisheries (van der Reijden et al., 2018; Hintzen et al., 2020). We also found a negative association between intensity of beam trawling for shrimp and flatfish and S. subtruncata density. While flatfish trawling, which penetrates the seabed, is known to increase bivalve mortality and affecting benthic communities (Tillin et al., 2006; Eigaard et al., 2016; Hiddink et al., 2017; Rijnsdorp et al., 2018), shrimp trawling, which lightly disturbs the seabed, is more frequent in the study area (Figure 6). However, studies on the impact of shrimp trawling on the benthic community in coastal waters are very limited (Tulp et al., 2020; Sala et al., 2023; van der Meer et al., 2024). The limited studies on the effects of shrimp beam trawling suggest it may shift benthic communities in the Wadden Sea (Tulp et al., 2020). This shift as observed in dredge sampling was mainly due to an increase in E. leei densities, likely able to relocate and quickly colonise disturbed shrimp trawl areas (Tulp et al., 2020), though a follow-up study based on box-core sampling within the same study did not find support for this hypothesis (van der Meer et al., 2024). A similar observation was found in an experimental study in the Wadden Sea, where E. leei preferred the manipulated unstable sediments (van der Heide et al., 2014).
The impact of bottom trawling on the benthic community varies between habitats. In highly dynamic habitats, with larger median grain size, benthic communities are less affected by trawling (van Denderen et al., 2014; Rijnsdorp et al., 2018; van der Reijden et al., 2018). Our study also found a stronger negative association between S. subtruncata and flatfish and shrimp trawl fisheries in areas with lower median grain size (Figures 11A, B), suggesting potential trawling effects are habitat dependent. In fine-medium sediment grain size, the bottom trawl gear may impact the seabed more strongly as it penetrates deeper into the seafloor, potentially increasing shellfish mortality and disrupting larval settlement. To disentangle the causal relationships and potential mechanisms, empirical research on the effects of beam trawl, especially shrimp fisheries, is needed, considering sediment characteristics.
Intensive fisheries on S. subtruncata can have strong effects on local abundances (Leopold, 1999; Smaal et al., 2001; Camphuysen et al., 2002), however, within our study, we did not find a relation between bivalve dredging and S. subtruncata densities. The absence of an effect may be because our analyses, covering fisheries data from 2009-2020, only includes two years of bivalve fisheries (2019-2020). In addition, the harvested quantities of adult S. subtruncata in these years were low, at 5.0 ×106 kg in 2019 and 6.5×106 kg in 2020, representing 0.3% and 0.6% of the estimated standing adult stocks respectively (Perdon et al., 2019; Troost et al., 2023). In earlier years (1995-1999), fishing pressure was much higher (1% to 22% of the total estimated standing adult stock) which might have contributed to the population decline at the end of the 1990s (estimated harvest data supplied by A. Seinen, Meromar; Troost et al., 2023).
The relative effect of the smoother year in our models was high and captured the temporal trend to a large extent. This means that the unexplained annual variation in densities is likely related to yearly recruitment fluctuations (Fogarty et al., 1991) which were not captured by our model covariates. Therefore, additional factors during pre- and post-settlement are likely responsible for the observed variation. Recruitment success of shellfish is highly dependent on factors that must be optimal during a critical ‘window of opportunity’ (Balke et al., 2011; Capelle et al., 2019). Here, we discuss factors not included in our model analyses that are potentially related to recruitment success.
Larval settlement largely depends on the wind conditions for survival, dispersal and local settlement conditions. Wind-induced mixing of the water column can affect the vertical distribution of phytoplankton, influencing food availability for fish and invertebrate larvae and in turn their survival (Corten and van de Kamp, 1996; Turley and Rykaczewski, 2019). Wind direction and force during the pelagic larval phase determine the dispersal and location of shellfish larvae settlement (Belgrano et al., 1995; Armonies et al., 2001). The optimum conditions for S. subtruncata during the pelagic and settlement phases are unknown, however the pelagic phase for S. subtruncata larvae is relatively long, making them vulnerable to predation and environmental fluctuations, leading to high recruitment variability (Fogarty et al., 1991; Cardoso et al., 2007).
We used shrimp densities in the North Sea (Tulp et al., 2012) (Figure 7A) and energy requirements of shrimp (Jung et al., 2017) to estimate shrimp predation pressure (Supplementary Figure A3). Based on average estimated shrimp densities of 1.35 m-2 (13.500 shrimp ha-1), with a maximum observed of 22 m-2 (220.000 ha-1) (Tulp et al., 2012) (Supplementary Figure A3), we estimate that brown shrimps reduce S. subtruncata densities by 1.800 to 30.000 recruits m-2 over a 77 day predation window respectively. The net effect depends on the starting settlement density of S. subtruncata recruits. For example, a recruit density of 15.000 m-2 may lead to a local reduction ranging from 10% to a potential complete depletion of recruit density. However, based on the maximum observed S. subtruncata recruit densities of 150.000 m-2 (Degraer et al., 2007), we estimate a local potential predation mortality by brown shrimp ranging from 1% to 20%. The trends in our descriptive data on shrimp predation did not provide a clear indication that they can explain the unexplained year effect. However, there is a large uncertainty on shrimp densities and are likely underestimated (Aarts et al., 2019; Supplementary Material A3). For other bivalve species it was shown that a predation pressure of this order of magnitude can drive population dynamics (Hiddink et al., 2002; Andresen and van der Meer, 2010; Weerman et al., 2014).
Flatfish also prey on bivalves, especially for dab and plaice it can be an important prey (Braber and de Groot, 1973; Rijnsdorp and Vingerhoed, 2001; Tulp et al., 2010). Stomach content analyses showed that S. subtruncata can be the dominant prey for plaice (Rijnsdorp and Vingerhoed, 2001). The same uncertainties as for brown shrimp apply for the estimation for flatfish densities. Moreover, larger flatfish (> 150 mm) are underrepresented in the DFS survey (Aarts et al., 2019) because the gear is not suited for larger, faster swimming fish, and diets are poorly known. Therefore, reliable estimates of flatfish predation pressure cannot be made.
Diving ducks, particularly common scoters, feed on adult S. subtruncata during winter in Dutch coastal waters (Camphuysen et al., 2002; Fox, 2003). Common scoters can potentially consume 4% to 20% of the local adult S. subtruncata (van de Wolfshaar et al., 2023). Since the ducks prefer larger specimens and are mainly present in winter, we can assume that scoter predation pressure on recruits during autumn is relatively low.
Our results showed that S. subtruncata was spatially aggregated on a large scale, occurring in high densities in distinct areas year after year. This spatial aggregation offers opportunities for conservation management. While European Union directives (e.g. Species and Habitats Directive 92/43/EEC and Birds Directive 79/409/EEC) permit anthropogenic activities within protected areas in the Dutch North Sea, provided that they do not significantly impact designated species and habitats. However, currently shrimp trawl fisheries, mollusc dredge fisheries and sand nourishment are occurring within protected areas (Natura 2000) and in areas with high densities of S. subtruncata (Hintzen, 2021; van de Wolfshaar et al., 2023), raising concerns about their potential impacts. Apart from the uncertainty about the impacts of shellfish dredging, shrimp trawl fisheries, there are uncertainties about the effects of sand extraction and nourishments. Due to the limitations of available data and methodological challenges for sand extraction and nourishments, we were unable to investigate potential impact over large spatiotemporal scales. The potential for climate effects to interact with local anthropogenic activities may complicate management, as they may amplify, neutralize or counteract each other’s impact on the benthic community (Wakelin et al., 2015). The net outcome of such interactions is currently unknown and may contribute to the strong temporal variations of the unexplained year effect.
The effects of potential drivers in our study appear to be context-dependent, with sediment grain size serving as a proxy for habitat. This implies that a nuanced understanding of habitat dependency at finer spatial scales could be critical. Additionally, further insights into what determines the critical window of opportunity driving S. subtruncata population dynamics are needed. Questions remain about how climate change and intensifying local human activities may significantly affect the S. subtruncata population. Addressing these uncertainties would benefit from focused empirical research, particularly observational and experimental studies during the early recruitment phases. This more in-depth empirical research on (a)biotic and anthropogenic effects, and their cumulative impacts is essential to develop adequate fisheries and coastal management strategies, to sustain biodiversity.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
JdF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft. PvH: Conceptualization, Data curation, Formal analysis, Methodology, Software, Visualization, Writing – review & editing. JC: Data curation, Investigation, Methodology, Writing – review & editing. ML: Data curation, Investigation, Methodology, Writing – review & editing. JP: Data curation, Investigation, Writing – review & editing. KT: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. IT: Data curation, Investigation, Methodology, Writing – review & editing. JvZ: Data curation, Investigation, Writing – review & editing. CP: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by Rijksdienst voor Ondernemend Nederland (RVO), project number: RVO 17684000005.
This work is based upon research and discussions within a consortium consisting (in addition to the authors) of Roef Mulder & Jonna van Ulzen (Vogelbescherming Nederland), DerkJan Berends, Johan Nooitgedacht, Egbert van der Tuin & Amerik Schuitemaker (Nederlandse Vissersbond), André Seinen (Meromar Seafoods BV), Afra Asjes (Wageningen Marine Research), and Loran Kleine Schaars, Marcel van der Linden and Tjitske Visscher (Royal Netherlands Institute of Sea Research). We are most grateful for their inputs. We acknowledge Rijkswaterstaat for providing data on nourishment and extraction of sand, Johan van der Molen (NIOZ) for providing data on tidal velocities, and Niels Hintzen (Wageningen University & Research) for updating of the VMS fishery data and his advice how to use them.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1476223/full#supplementary-material
Aarts G., Brasseur S., Poos J., Schop J., Kirkwood R., van Kooten T., et al. (2019). Top-down pressure on a coastal ecosystem by harbor seals. Ecosphere 10, 1–20. doi: 10.1002/ecs2.2538
Ambrogi R., Occhipinti Ambrogi A. (1987). Temporal variations of secondary production in the marine bivalve Spisula subtruncata off the Po River delta (Italy). Estuarine Coast. Shelf Sci. 25, 369–379. doi: 10.1016/0272-7714(87)90079-5
Andresen H., van der Meer J. (2010). Brown shrimp (Crangon crangon, L.) functional response to density of different sized juvenile bivalves Macoma balthica (L.). J. Exp. Mar. Biol. Ecol. 390, 31–38. doi: 10.1016/j.jembe.2010.04.027
Armonies W., Herre E., Sturm M. (2001). Effects of the severe winter 1995/96 on the benthic macrofauna of the Wadden Sea and the coastal North Sea near the island of Sylt. Helgoland Mar. Res. 55, 170–175. doi: 10.1007/s101520100077
Balke T., Bouma T. J., Horstman E. M., Webb E. L., Erftemeijer P. L. A., Herman P. M. J. (2011). Windows of opportunity: thresholds to mangrove seedling establishment on tidal flats. Mar. Ecol. Prog. Ser. 440, 1–9. doi: 10.3354/meps09364
Baptist M. J., Leopold M. F. (2009). The effects of shoreface nourishments on Spisula and scoters in The Netherlands. Mar. Environ. Res. 68, 1–11. doi: 10.1016/j.marenvres.2009.03.003
Baretta-Bekker J. G., Baretta J. W., Latuhihin M. J., Desmit X., Prins T. C. (2009). Description of the long-term, (1991–2005) temporal and spatial distribution of phytoplankton carbon biomass in the Dutch North Sea. J. Sea Res. 61, 50–59. doi: 10.1016/j.seares.2008.10.007
Beek C. L. (1997). Recruitment surveys on juvenile plaice and sole in continental nurseries in the North Sea by the Netherlands. ICES C.M 30.
Belgrano A., Legendre P., Dewarumez J. M., Frontier S. (1995). Spatial structure and ecological variation of meroplankton on the Belgian-Dutch coast of the North Sea. Mar. Ecol. Prog. Ser. 128, 51–59. doi: 10.3354/meps128051
Bergman M. J. N., van Santbrink J. W. (2000). Mortality in megafaunal benthic populations caused by trawl fisheries on the Dutch continental shelf in the North Sea in 1994. ICES J. Mar. Sci. 57, 1321–1331. doi: 10.1006/jmsc.2000.0917
Beukema J. (1992). Dynamics of juvenile shrimp Crangon crangon in a tidal-flat nursery of the Wadden Sea after mild and cold winters. Mar. Ecology-progress Ser. - Mar. ECOL-PROGR Ser. 83, 157–165. doi: 10.3354/meps083157
Beukema J. J., Cadée G. C., Dekker R. (2002). Zoobenthic biomass limited by phytoplankton abundance: evidence from parallel changes in two long-term data series in the Wadden Sea. J. Sea Res. 48, 111–125. doi: 10.1016/S1385-1101(02)00162-4
Beukema J. J., Dekker R., Jansen J. M. (2009). Some like it cold: populations of the tellinid bivalve Macoma balthica (L.) suffer in various ways from a warming climate. Mar. Ecol. Prog. Ser. 384, 135–145. doi: 10.3354/meps07952
Blangiardo M., Cameletti M. (2015). Spatial and spatio-temporal Bayesian models with R-INLA (Chichester, West Sussex, UK: John Wiley & Sons).
Boddeke R. (1976). The seasonal migration of the brown shrimp crangon crangon. Netherlands J. Sea Res. 10, 103–130. doi: 10.1016/0077-7579(76)90006-5
Bosco Gusmao J., Thieltges D. W., Dekker R., Govers L. L., Meijer K. J., Klemens Eriksson B. (2022). Comparing taxonomic and functional trait diversity in marine macrozoobenthos along sediment texture gradients. Ecol. Indic. 145, 109718. doi: 10.1016/j.ecolind.2022.109718
Braber L., de Groot S. J. (1973). The food of five flatfish species (Pleuronectiformes) in the southern north sea. Netherlands J. Sea Res. 6, 163–172. doi: 10.1016/0077-7579(73)90011-2
Bruno J. F., Bertness M. D. (2001). Habitat modification and facilitation in benthic marine communities. Mar. Community Ecol. 201–218.
Camphuysen C. J., Berrevoets C. M., Cremers H. J. W. M., Dekinga A., Dekker R., Ens B. J., et al. (2002). Mass mortality of common eiders (Somateria mollissima) in the Dutch Wadden Sea, winter 1999/2000: starvation in a commercially exploited wetland of international importance. Biol. Conserv. 106, 303–317. doi: 10.1016/S0006-3207(01)00256-7
Campos J., van der Veer H. (2008). Autecology of crangon crangon (L.) with an emphasis on latitudinal trends. Oceanogr. Mar. Biol. 46, 65–104. doi: 10.1201/9781420065756.ch3
Capelle J. J., Leuchter L., de Wit M., Hartog E., Bouma T. J. (2019). Creating a window of opportunity for establishing ecosystem engineers by adding substratum: a case study on mussels. Ecosphere 10, e02688. doi: 10.1002/ecs2.2688
Capuzzo E., Lynam C. P., Barry J., Stephens D., Forster R. M., Greenwood N., et al. (2018). A decline in primary production in the North Sea over 25 years, associated with reductions in zooplankton abundance and fish stock recruitment. Global Change Biol. 24, e352–e364. doi: 10.1111/gcb.13916
Cardoso J. (2007). Growth and Reproduction in Bivalves: an energy budget approach. PhD thesis. University of Groningen, Groningen, The Netherlands, 1–207.
Cardoso J., Witte J. I. J., van der Veer H. W. (2007). Growth and reproduction of the bivalve Spisula subtruncata (da Costa) in Dutch coastal waters. J. Sea Res. 57, 316–324. doi: 10.1016/j.seares.2006.12.002
Carpenter S. R., Brock W. A., Hansen G. J. A., Hansen J. F., Hennessy J. M., Isermann D. A., et al. (2017). Defining a Safe Operating Space for inland recreational fisheries. Fish Fish. 18, 1150–1160. doi: 10.1111/faf.12230
Compton T. J., Rijkenberg M. J. A., Drent J., Piersma T. (2007). Thermal tolerance ranges and climate variability: A comparison between bivalves from differing climates. J. Experiment. Marine Biol. Ecol. 352 (1), 200–211. doi: 10.1016/j.jembe.2007.07.010
Corten A., van de Kamp G. (1996). Variation in the abundance of southern fish species in the southern North Sea in relation to hydrography and wind. ICES J. Mar. Sci. 53, 1113–1119. doi: 10.1006/jmsc.1996.0137
Craeymeersch J., Leopold M. F., Wijk V. M. (2001). Halfgeknotte strandschelp en Amerikaanse zwaardschede: een overzicht van bestaande kennis over visserij, economische betekenis, regelgeving, ecologie van de beviste soorten en effecten op het ecosysteem (IJmuiden, The Netherlands: RIVO, Nederlands Instituut voor Visserijonderzoek).
Dauvin J.-C., Baux N., Lesourd S. (2022). Benthic impact assessment of a dredge sediment disposal in a dynamic resilient environment. Mar. pollut. Bull. 179, 113668. doi: 10.1016/j.marpolbul.2022.113668
Degraer S., Meire P., Vincx M. (2007). Spatial distribution, population dynamics and productivity of Spisula subtruncata: implications for Spisula fisheries in seaduck wintering areas. Mar. Biol. 152, 863–875. doi: 10.1007/s00227-007-0740-y
Degraer S., Wittoeck J., Appeltans W., Cooreman K., Deprez T., Hillewaert H., et al. (2006). The Macrobenthos Atlas of the Belgian Part of the North Sea. (Brussels, Belgium: Belgian Science Policy Office (BELSPO)).
Dekker R., Beukema J. J. (1993). Dynamics and growth of a bivalve, Abra tenuis, at the northern edge of its distribution. J. Mar. Biol. Assoc. United Kingdom 73, 497–511. doi: 10.1017/S0025315400033063
Desmit X., Nohe A., Borges A., Prins T., Cauwer K., Lagring R., et al. (2020). Changes in chlorophyll concentration and phenology in the North Sea in relation to de-eutrophication and sea surface warming. Limnol. Oceanogr. 65, 828–847. doi: 10.1002/lno.11351
Deval M. C., Gokturk D. (2008). Population structure and dynamics of the cut trough shell Spisula subtruncata (da Costa) in the Sea of Marmara, Turkey. Fish. Res. 89, 241–247. doi: 10.1016/j.fishres.2007.09.019
Dulvy N. K., Rogers S. I., Jennings S., Stelzenmüller V., Dye S. R., Skjoldal H. R. (2008). Climate change and deepening of the North Sea fish assemblage: a biotic indicator of warming seas. J. Appl. Ecol. 45, 1029–1039. doi: 10.1111/j.1365-2664.2008.01488.x
Eigaard O. R., Bastardie F., Breen M., Dinesen G. E., Hintzen N. T., Laffargue P., et al. (2016). Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES J. Mar. Sci. 73, i27–i43. doi: 10.1093/icesjms/fsv099
Ellien C., Thiébaut E., Dumas F., Salomon J.-C., Nival P. (2004). A modelling study of the respective role of hydrodynamic processes and larval mortality on larval dispersal and recruitment of benthic invertebrates: example of Pectinaria koreni (Annelida: Polychaeta) in the Bay of Seine (English Channel). J. Plankton Res. 26, 117–132. doi: 10.1093/plankt/fbh018
Ellis J. I., Clark D., Atalah J., Jiang W., Taiapa C., Patterson M., et al. (2017). Multiple stressor effects on marine infauna: responses of estuarine taxa and functional traits to sedimentation, nutrient and metal loading. Sci. Rep. 7, 12013. doi: 10.1038/s41598-017-12323-5
Enricuso O. B., Conaco C., Sayco S. L. G., Neo M. L., Cabaitan P. C. (2018). Elevated seawater temperatures affect embryonic and larval development in the giant clam Tridacna gigas (Cardiidae: Tridacninae). J. Molluscan Stud. 85, 66–72. doi: 10.1093/mollus/eyy051
Feng C. X. (2021). A comparison of zero-inflated and hurdle models for modeling zero-inflated count data. J. Stat. Distrib. Appl. 8, 8. doi: 10.1186/s40488-021-00121-4
Fogarty M. J., Sissenwine M. P., Cohen E. B. (1991). Recruitment variability and the dynamics of exploited marine populations. Trends Ecol. Evol. 6, 241–246. doi: 10.1016/0169-5347(91)90069-A
Fox A. (2003). Diet and habitat use of scoters Melanitta in the Western Palearctic - A brief overview. Wildfowl 54, 163–182.
Fraschetti S., Covazzi A., Chiantora M., Albertelli G. (1997). Life-history of the bivalve Spisula subtruncata (da Costa) in the Ligurian Sea (North-Western Mediterranean): the contribution of newly settled juveniles. J. Mar. Biol. Assoc. United Kingdom 80, 1152–1152.
Gissi E., Manea E., Mazaris A. D., Fraschetti S., Almpanidou V., Bevilacqua S., et al. (2021). A review of the combined effects of climate change and other local human stressors on the marine environment. Sci. Total Environ. 755, 142564. doi: 10.1016/j.scitotenv.2020.142564
Gobler C. J., DePasquale E. L., Griffith A. W., Baumann H. (2014). Hypoxia and acidification have additive and synergistic negative effects on the growth, survival, and metamorphosis of early life stage bivalves. PloS One 9, e83648. doi: 10.1371/journal.pone.0083648
Gosselin L., Qian P.-Y. (1997). Juvenile mortality in benthic marine invertebrates. Mar. Ecol. Prog. Ser. 146, 265–282. doi: 10.3354/meps146265
Halpern B. S., Walbridge S., Selkoe K. A., Kappel C. V., Micheli F., D’Agrosa C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Hamerlynck O., Hostens K., Arellano R. V., Mees J., Van Damme P. A. (1993). The mobile epibenthic fauna of soft bottoms in the Dutch Delta (south-west Netherlands): Spatial structure. Netherland J. Aquat. Ecol. 27, 343–358. doi: 10.1007/BF02334797
Hayward P. J., Ryland J. S. (2017). Handbook of the marine fauna of North-West Europe (Oxford, United Kingdom: Oxford university press).
Herman P. M. J., Moons J. J. S., Wijsman J. W. M., Luijendijk A. P., Ysebaert T. (2021). A mega-nourishment (sand motor) affects landscape diversity of subtidal benthic fauna. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.643674
Hiddink J. G., Jennings S., Sciberras M., Szostek C. L., Hughes K. M., Ellis N., et al. (2017). Global analysis of depletion and recovery of seabed biota after bottom trawling disturbance. Proc. Natl. Acad. Sci. 114, 8301–8306. doi: 10.1073/pnas.1618858114
Hiddink J. G., Marijnissen S. A. E., Troost K., Wolff W. J. (2002). Predation on 0-group and older year classes of the bivalve Macoma balthica: interaction of size selection and intertidal distribution of epibenthic predators. J. Exp. Mar. Biol. Ecol. 269, 223–248. doi: 10.1016/S0022-0981(02)00002-3
Hintzen N. (2021). Garnalenvisserij in Natura 2000 gebieden (IJmuiden, Netherlands: Wageningen Marine Research). doi: 10.18174/574233
Hintzen N. T., Aarts G., Poos J. J., van der Reijden K. J., Rijnsdorp A. D. (2020). Quantifying habitat preference of bottom trawling gear. ICES J. Mar. Sci. 78, 172–184. doi: 10.1093/icesjms/fsaa207
Hofmann E., Powell E., Klinck J., Munroe D., Mann R., Haidvogel D., et al. (2018). An overview of factors affecting distribution of the Atlantic surfclam (Spisula solidissima), a continental shelf biomass dominant, during a period of climate change. J. Shellfish Res. 37, 821–831. doi: 10.2983/035.037.0412
Høyer J. L., Karagali I. (2016). Sea surface temperature climate data record for the North sea and Baltic sea. J. Climate 29, 2529–2541. doi: 10.1175/JCLI-D-15-0663.1
Huang S., Edie S. M., Collins K. S., Crouch N. M. A., Roy K., Jablonski D. (2023). Diversity, distribution and intrinsic extinction vulnerability of exploited marine bivalves. Nat. Commun. 14, 4639. doi: 10.1038/s41467-023-40053-y
ICES (2021). OSPAR request on the production of spatial data layers of fishing intensity/pressure (ICES Advice: Technical Services).
Jung A. S., Dekker R., Germain M., Philippart C. J. M., Witte J. I. J., van der Veer H. W. (2017). Long-term shifts in intertidal predator and prey communities in the Wadden Sea and consequences for food requirements and supply. Mar. Ecol. Prog. Ser. 579, 37–53. doi: 10.3354/meps12263
Kroeker K. J., Kordas R. L., Crim R. N., Singh G. G. (2010). Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. doi: 10.1111/j.1461-0248.2010.01518.x
Kruft Welton R. A., Hoppit G., Schmidt D. N., Witts J. D., Moon B. C. (2024). Reviews and syntheses: The clam before the storm – a meta-analysis showing the effect of combined climate change stressors on bivalves. Biogeosciences 21, 223–239. doi: 10.5194/bg-21-223-2024
Leopold M. (1999). Spisula-vissers vissen wat ze vissen kunnen in mei 1999: nog steeds geen overheidsbeleid. Nieuwsbrief NZG 1, 5–6.
Lindgren F., Rue H. (2015). Bayesian spatial modelling with R-INLA. J. Stat. Softw. 63, 1–25. doi: 10.18637/jss.v063.i19
Lotze H. K., Lenihan H. S., Bourque B. J., Bradbury R. H., Cooke R. G., Kay M. C., et al. (2006). Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312, 1806–1809. doi: 10.1126/science.1128035
Mackenzie C. L. (2007). Causes underlying the historical decline in Eastern oyster (Crassostrea virginica Gmelin 1791) landings. J. Shellfish Res. 26, 927–938, 912. doi: 10.2983/0730-8000(2007)26[927:CUTHDI]2.0.CO;2
McElreath R. (2020). Statistical rethinking: A Bayesian course with examples in R and Stan (Boca Raton FL. USA: CRC press).
Merchant C. J., Embury O., Bulgin C. E., Block T., Corlett G. K., Fiedler E., et al. (2019). Satellite-based time-series of sea-surface temperature since 1981 for climate applications. Sci. Data 6, 223. doi: 10.1038/s41597-019-0236-x
Narváez D. A., Munroe D. M., Hofmann E. E., Klinck J. M., Powell E. N., Mann R., et al. (2015). Long-term dynamics in Atlantic surfclam (Spisula solidissima) populations: The role of bottom water temperature. J. Mar. Syst. 141, 136–148. doi: 10.1016/j.jmarsys.2014.08.007
Nicolle A., Dumas F., Foveau A., Foucher E., Thiebaut E. (2013). Modelling larval dispersal of the king scallop (Pecten maximus) in the English Channel: examples from the bay of Saint-Brieuc and the bay of Seine. Ocean Dynam. 63, 661–678. doi: 10.1007/s10236-013-0617-1
Norkko J., Shumway S. E. (2011). “Bivalves as Bioturbators and Bioirrigators,” in Shellfish Aquaculture and the Environment, vol. 297-317. (Hoboken, New Jersey, USA: Wiley-Blackwell).
Opdal A. F., Lindemann C., Aksnes D. L. (2019). Centennial decline in North Sea water clarity causes strong delay in phytoplankton bloom timing. Global Change Biol. 25, 3946–3953. doi: 10.1111/gcb.14810
Penning E., Govers L., Dekker R., Piersma T. (2021). Advancing presence and changes in body size of brown shrimp Crangon crangon on intertidal flats in the western Dutch Wadden Sea 1984-2018. Mar. Biol. 168, 1–12. doi: 10.1007/s00227-021-03967-z
Perdon K., Troost K., van Zwol J., Van Asch M., van der Pool J. (2019). “Schelpdierbestanden in de Nederlandse kustzone in 2019,” in Stichting Wageningen Research, Centrum voor Visserijonderzoek. doi: 1018174/497850
Philippart C. J. M., Anadón R., Danovaro R., Dippner J. W., Drinkwater K. F., Hawkins S. J., et al. (2011). Impacts of climate change on European marine ecosystems: Observations, expectations and indicators. J. Exp. Mar. Biol. Ecol. 400, 52–69. doi: 10.1016/j.jembe.2011.02.023
Philippart C. J., van Aken H. M., Beukema J. J., Bos O. G., Cadée G. C., Dekker R. (2003). Climate-related changes in recruitment of the bivalve Macoma balthica. Limnol. Oceanogr. 48, 2171–2185. doi: 10.4319/lo.2003.48.6.2171
Philippart C. J. M., van Iperen J. M., Cadee G. C., Zuur A. F. (2010). Long-term field observations on seasonality in chlorophyll-a concentrations in a shallow coastal marine ecosystem, the Wadden sea. Estuaries Coasts 33, 286–294. doi: 10.1007/s12237-009-9236-y
Pihl L., Rosenberg R. (1984). Food selection and consumption of the shrimp Crangon crangon in some shallow marine areas in western Sweden. Mar. Ecology-progress Ser. - Mar. ECOL-PROGR Ser. 15, 159–168. doi: 10.3354/meps015159
Poloczanska E. S., Burrows M. T., Brown C. J., García Molinos J., Halpern B. S., Hoegh-Guldberg O., et al. (2016). Responses of marine organisms to climate change across oceans. Front. Mar. Sci. 3. doi: 10.3389/fmars.2016.00062
Ramírez F., Pennino M. G., Albo-Puigserver M., Steenbeek J., Bellido J. M., Coll M. (2021). SOS small pelagics: A safe operating space for small pelagic fish in the western Mediterranean Sea. Sci. Total Environ. 756, 144002. doi: 10.1016/j.scitotenv.2020.144002
Raven H. (2022). Expansion of Crepidula fornicata drives the species into new niches. Nautilus -Greenville then Sanibel- 136, 56–62.
R Core Team version 4.1.1 (2021). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Rijnsdorp A. D., Bolam S. G., Garcia C., Hiddink J. G., Hintzen N. T., van Denderen P. D., et al. (2018). Estimating sensitivity of seabed habitats to disturbance by bottom trawling based on the longevity of benthic fauna. Ecol. Appl. 28, 1302–1312. doi: 10.1002/eap.1731
Rijnsdorp A., Vingerhoed B. (2001). Feeding of plaice Pleuronectes platessa L. and Sole Solea solea (L.) in relation to the effects of bottom trawling. J. Sea Res. 45, 219–229. doi: 10.1016/S1385-1101(01)00047-8
Rue H., Martino S., Chopin N. (2009). Approximate Bayesian inference for latent Gaussian models by using integrated nested Laplace approximations. J. R. Stat. Society: Ser. B (Statistical Methodology) 71, 319–392. doi: 10.1111/j.1467-9868.2008.00700.x
Rullens V., Lohrer A. M., Townsend M., Pilditch C. A. (2019). Ecological mechanisms underpinning ecosystem service bundles in marine environments – A case study for shellfish. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00409
Saengsupavanich C., Pranzini E., Ariffin E. H., Yun L. S. (2023). Jeopardizing the environment with beach nourishment. Sci. Total Environ. 868, 161485. doi: 10.1016/j.scitotenv.2023.161485
Sala A., Depestele J., Gümüş A., Laffargue P., Nielsen J. R., Polet H., et al. (2023). Technological innovations to reduce the impact of bottom gears on the seabed. Mar. Policy 157, 105861. doi: 10.1016/j.marpol.2023.105861
Sampaio E., Santos C., Rosa I. C., Ferreira V., Pörtner H.-O., Duarte C. M., et al. (2021). Impacts of hypoxic events surpass those of future ocean warming and acidification. Nat. Ecol. Evol. 5, 311–321. doi: 10.1038/s41559-020-01370-3
Scheffer M., Barrett S., Carpenter S. R., Folke C., Green A. J., Holmgren M., et al. (2015). Creating a safe operating space for iconic ecosystems. Science 347, 1317–1319. doi: 10.1126/science.aaa3769
Schimanke S., Ridal M., Le Moigne P., Berggren L., Undén P., Randriamampianina R., et al. (2021). “CERRA sub-daily regional reanalysis data for Europe on height levels from 1984 to present,” in Copernicus Climate Change Service (C3S) Climate Data Store (CDS).
Sluijter M., Arts F. A., Lilipaly S. J., Wolf P. A. (2022). Midwintertelling van zee-eenden in de Waddenzee en Nederlandse kustwateren in December 2021, Januari en Maart 2022 (Utrecht, Netherlands: Rijkswaterstaat, Rapport RWS-Centrale Informatievoorziening Rapport BM 22.21).
Smaal A. C., Craeymeersch J., Kamermans P., Stralen M. (2001). Is food shortage the cause of eider duck mortality? Shellfish and crab abundance in the Dutch Wadden Sea 1994 - 1999. Wadden Sea Newslett. 2001.
Smaal A. C., Ferreira J. G., Grant J., Petersen J. K., Strand Ø. (2019). Goods and services of marine bivalves (Cham, Switzerland: Springer Nature).
Smaal A. C., Lucas L. (2000). Regulation and monitoring of marine aquaculture in The Netherlands. J. Appl. Ichthyol. 16, 187–191. doi: 10.1046/j.1439-0426.2000.00266.x
Sospedra J., Falco S., Morata T., Rodilla M. (2017). Influence of organic enrichment and spisula subtruncata (da costa 1778) on oxygen and nutrient fluxes in fine sand sediments. Estuaries Coasts 40, 726–740. doi: 10.1007/s12237-016-0174-1
Thiébaut E. (1996). Distribution of Pectinaria koreniLarvae (Annelida: Polychaeta) in relation to the Seine river plume front (Eastern English Channel). Estuarine Coast. Shelf Sci. 43, 383–397. doi: 10.1006/ecss.1996.0077
Tillin H., Hiddink J., Jennings S., Kaiser M. (2006). Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea basin scale. Mar. Ecol. Prog. Ser. 318, 31–45. doi: 10.3354/meps318031
Trégarot E., D’Olivo J. P., Botelho A. Z., Cabrito A., Cardoso G. O., Casal G., et al. (2024). Effects of climate change on marine coastal ecosystems – A review to guide research and management. Biol. Conserv. 289, 110394. doi: 10.1016/j.biocon.2023.110394
Troost K., van Asch M., Brummelhuis E., van den Ende D., Perdon J., van Zweeden C., et al. (2019). Handboek bestandsopnames schelpdieren WOT Versie 3, december 2019. CVO rapport 18.
Troost K., Van Asch M., Cornelisse S., Glorius S., Van den Ende D., Van Es Y., et al. (2023). Schelpdierbestanden in de Nederlandse kustzone, Waddenzee en zoute deltawateren in 2022 (IJmuiden, Netherlands: Stichting Wageningen Research, Centrum voor Visserijonderzoek (CVO).
Tulp I., Bolle L. J., Meesters E., de Vries P. (2012). Brown shrimp abundance in northwest European coastal waters from 1970 to 2010 and potential causes for contrasting trends. Mar. Ecol. Prog. Ser. 458, 141–154. doi: 10.3354/meps09743
Tulp I., Craeymeersch J., Leopold M., van Damme C., Fey F., Verdaat H. (2010). The role of the invasive bivalve Ensis directus as food source for fish and birds in the Dutch coastal zone. Estuarine Coast. Shelf Sci. 90, 116–128. doi: 10.1016/j.ecss.2010.07.008
Tulp I., Glorius S., Rippen A., Looije D., Craeymeersch J. (2020). Dose-response relationship between shrimp trawl fishery and the macrobenthic fauna community in the coastal zone and Wadden Sea. J. Sea Res. 156, 101829. doi: 10.1016/j.seares.2019.101829
Turley B., Rykaczewski R. (2019). Influence of wind events on larval fish mortality rates in the Southern California Current Ecosystem. Can. J. Fish. Aquat. Sci. 76, 2418–2432. doi: 10.1139/cjfas-2018-0458
van Dalfsen J., Essink K. (1999). RIACON: Risk analysis of coastal nourishment techniques in the Netherlands. (The Hague, Netherlands: RIKZ (Rijksinstituut voor Kust en Zee)).
van Denderen P. D., Hintzen N. T., Rijnsdorp A. D., Ruardij P., van Kooten T. (2014). Habitat-specific effects of fishing disturbance on benthic species richness in marine soft sediments. Ecosystems 17, 1216–1226. doi: 10.1007/s10021-014-9789-x
van der Heide T., Tielens E., van der Zee E. M., Weerman E. J., Holthuijsen S., Eriksson B. K., et al. (2014). Predation and habitat modification synergistically interact to control bivalve recruitment on intertidal mudflats. Biol. Conserv. 172, 163–169. doi: 10.1016/j.biocon.2014.02.036
van der Meer J., Craeymeersch J., Glorius S., Tulp I. (2024). No evidence that the Atlantic jackknife clam Ensis leei profits from shrimp fisheries. J. Sea Res. in press.
van der Molen J., Ruardij P., Greenwood N. (2016). Potential environmental impact of tidal energy extraction in the Pentland Firth at large spatial scales: results of a biogeochemical model. Biogeosciences 13, 2593–2609. doi: 10.5194/bg-13-2593-2016
van der Reijden K. J., Hintzen N. T., Govers L. L., Rijnsdorp A. D., Olff H. (2018). North Sea demersal fisheries prefer specific benthic habitats. PloS One 13, e0208338. doi: 10.1371/journal.pone.0208338
van der Veer H. W., Dapper R., Henderson P. A., Jung A. S., Philippart C. J. M., Witte J. I. J., et al. (2015). Changes over 50 years in fish fauna of a temperate coastal sea: Degradation of trophic structure and nursery function. Estuarine Coast. Shelf Sci. 155, 156–166. doi: 10.1016/j.ecss.2014.12.041
van der Veer H. W., Feller R. J., Weber A., Witte J. I. (1998). Importance of predation by crustaceans upon bivalve spat in the intertidal zone of the Dutch Wadden Sea as revealed by immunological assays of gut contents. J. Exp. Mar. Biol. Ecol. 231, 139–157. doi: 10.1016/S0022-0981(98)00090-2
van der Veer H. W., Tulp I., Witte J. I. J., Poiesz S. S. H., Bolle L. J. (2022). Changes in functioning of the largest coastal North Sea flatfish nursery, the Wadden Sea, over the past half century. Mar. Ecol. Prog. Ser. 693, 183–201. doi: 10.3354/meps14082
van de Wolfshaar K. E., Brinkman A. G., Benden D. L. P., Craeymeersch J. A., Glorius S., Leopold M. F. (2023). Impact of disturbance on common scoter carrying capacity based on an energetic model. J. Environ. Manage. 342, 118255. doi: 10.1016/j.jenvman.2023.118255
van Leeuwen S., Tett P., Mills D., van der Molen J. (2015). Stratified and nonstratified areas in the North Sea: Long-term variability and biological and policy implications. J. Geophys. Res.: Oceans 120, 4670–4686. doi: 10.1002/2014JC010485
Verween A., Vincx M., Degraer S. (2007). The effect of temperature and salinity on the survival of Mytilopsis leucophaeata larvae (Mollusca, Bivalvia): The search for environmental limits. J. Exp. Mar. Biol. Ecol. 348, 111–120. doi: 10.1016/j.jembe.2007.04.011
Wakelin S. L., Artioli Y., Butenschön M., Allen J. I., Holt J. T. (2015). Modelling the combined impacts of climate change and direct anthropogenic drivers on the ecosystem of the northwest European continental shelf. J. Mar. Syst. 152, 51–63. doi: 10.1016/j.jmarsys.2015.07.006
Wang X., Yue Y., Faraway J. J. (2018). Bayesian regression modeling with INLA (Boca Raton FL. USA: CRC press).
Watanabe S. (2010). Asymptotic equivalence of bayes cross validation and widely applicable information criterion in singular learning theory. J. Mach. Learn. Res. 11, 3571–3594. doi: 10.5555/1756006.1953045
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Weerman E. J., Eriksson B. K., Olff H., van der Heide T. (2014). Predation by native brown shrimp on invasive Pacific oyster spat. J. Sea Res. 85, 126–130. doi: 10.1016/j.seares.2013.04.010
Weijerman M., Lindeboom H., Zuur A. F. (2005). Regime shifts in marine ecosystems of the North Sea and Wadden Sea. Mar. Ecol. Prog. Ser. 298, 21–39. doi: 10.3354/meps298021
Weinberg J. R. (2005). Bathymetric shift in the distribution of Atlantic surfclams: response to warmer ocean temperature. ICES J. Mar. Sci. 62, 1444–1453. doi: 10.1016/j.icesjms.2005.04.020
Weissberger E. J., Grassle J. P. (2003). Settlement, first-year growth, and mortality of surfclams, Spisula solidissima. Estuarine Coast. Shelf Sci. 56, 669–684. doi: 10.1016/S0272-7714(02)00218-4
Wijsman J. W. M., Prins T. C., Moons J. J. S., Herman P. M. J. (2023). Changed sediment composition prevents recovery of macrobenthic community four years after a shoreface nourishment at the Holland coast. Estuarine Coast. Shelf Sci. 293, 108521. doi: 10.1016/j.ecss.2023.108521
Witbaard R., Duineveld G. ,. C. A., Amaro T., Bergman M. ,. J. N. (2005). Growth trends in three bivalve species indicate climate forcing on the benthic ecosystem in the southeastern North Sea. Climate Res. 30, 29–38. doi: 10.3354/cr030029
Ysebaert T., Walles B., Haner J., Hancock B. (2019). Habitat modification and coastal protection by ecosystem-engineering reef-building bivalves. Goods Serv. Mar. bivalves 253-273.
Zhang Q., Warwick R. M., McNeill C. L., Widdicombe C. E., Sheehan A., Widdicombe S. (2015). An unusually large phytoplankton spring bloom drives rapid changes in benthic diversity and ecosystem function. Prog. Oceanogr. 137, 533–545. doi: 10.1016/j.pocean.2015.04.029
Keywords: anthropogenic impacts, bayesian statistics, climate change, Dutch North Sea, population dynamics, Spisula subtruncata
Citation: de Fouw J, van Horssen PW, Craeymeersch J, Leopold MF, Perdon J, Troost K, Tulp I, van Zwol J and Philippart CJM (2024) Spatio-temporal analysis of potential factors explaining fluctuations in population size of Spisula subtruncata in the Dutch North Sea. Front. Mar. Sci. 11:1476223. doi: 10.3389/fmars.2024.1476223
Received: 05 August 2024; Accepted: 28 October 2024;
Published: 20 November 2024.
Edited by:
Stelios Katsanevakis, University of the Aegean, GreeceReviewed by:
Steven Degraer, Royal Belgian Institute of Natural Sciences, BelgiumCopyright © 2024 de Fouw, van Horssen, Craeymeersch, Leopold, Perdon, Troost, Tulp, van Zwol and Philippart. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jimmy de Fouw, amltLmRlZm91d0BydS5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.