- 1College of Marine Living Resource Sciences and Management, Shanghai Ocean University, Shanghai, China
- 2Jiangsu Marine Fisheries Research Institute, Nantong, China
- 3State Key Laboratory of Estuarine and Coastal Research, East China Normal University, Shanghai, China
Introduction: The implementation of the 10-year fishing ban in the Yangtze River has provided a crucial opportunity for the recovery of rare and endangered diadromous species, such as Coilia nasus.
Methods: In this study, we utilized electronic length–frequency analysis (ELEFAN) and length-based Bayesian biomass estimation (LBB) method to fit the body length data of C. nasus from the Yangtze River Estuary and its adjacent sea areas before and after the fishing ban (2019-2023), and the resource changes of C. nasus population were evaluated. Additionally, combined the catch production monitoring data from 2020 to 2022, we comprehensively analyzed the impact of the Yangtze River fishing ban on the recovery of C. nasus resources.
Results: The results showed that: (1) The proportion of quantity, weight and occurrence frequency of C. nasus in catches showed a significant increasing trend year by year. (2) 4,994 C. nasus were caught from 2021–2023, with body lengths ranging from 13–410 mm. In 2023, the average body length and weight of C. nasus had increased by 39.93% and 133.89%, respectively, from those in 2021. (3) ELEFAN estimated that the growth parameters after fishing ban, including asymptotic length, growth coefficient, and the theoretical age at length zero, were determined to be 42.92 cm, 0.43 year-1, and -0.31 year, respectively. The total mortality rate, fishing mortality rate, and exploitation rate were determined to be 1.47 year-1, 0.79 year-1, and 0.54, respectively. (4) LBB estimated that the relative fishing mortality of C. nasus before the fishing ban increased from 1.22 in 2019 to 2.65 in 2020, while the relative biomass decreased from 0.34 to 0.22. After the fishing ban, the relative fishing mortality decreased from 0.85 in 2021 to 0.06 in 2023, and the relative biomass increased from 0.26 in 2021 to 0.90 in 2023, with a significant increase in 2022, indicating a clear recovery trend in C. nasus resources.
Discussion: By quantifying the resource characteristics of C. nasus before and after the 10-year fishing ban on the Yangtze River, this research revealed the impact of the ban and provided a reference for future systematic evaluations of the C. nasus population.
1 Introduction
The Yangtze River, the longest river in Asia with a basin rich in fishery resources, is not only a representative of biodiversity but also a cradle of freshwater fisheries in China. In recent decades, aquatic biological resources have sharply declined owing to long-term overfishing, construction of water conservancy projects, and water pollution (Chen et al., 2009; Liu et al., 2019). Consequently, the biological integrity index once plummeted to the lowest level, indicating a state of “no fish” (Zhang et al., 2020; Chen T. et al., 2020; Dong et al., 2023). The fish community within the river basin has undergone significant changes, and many endemic and rare aquatic species such as Lipotes vexillifer, Psephurus gladius, Acipenser sinensis, and Acipenser dabryanus have become functionally extinct. Since the late 1990s, the Yangtze River is in a vicious cycle of ‘the fewer resources are caught, the worse the ecology is caught, and the poorer the fishermen are caught’ (Chen et al., 2009; He et al., 2019; Chen et al., 2021). Thus, as part of China’s intensive efforts to protect the Yangtze River and prevent large-scale development, the 10-year fishing ban was officially launched on January 1, 2020 and fully implemented on January 1, 2021 (Mei et al., 2020; Ma et al., 2022). This ban primarily targeting the Yangtze River Basin, including the main stream, the Yangtze Estuary, Poyang Lake, Dongting Lake, and the seven tributaries that connect to it: the Dadu, Min, Tuo, Chishui, Jialing, Wu, and Han River. The ban represents the most extensive and strict management measures ever implemented in the basin. Among them, the Yangtze River Estuary plays a crucial role in the resource recovery of diadromous species. The ban is a critical initiative for China to restore aquatic biological resources and protect the ecosystem, and it can also serve as a reference for global fisheries management and ecological conservation efforts. Therefore, long-term monitoring and objective evaluation of aquatic biological resources in the Yangtze River basin have become important needs to assess the effectiveness of the fishing ban. Currently, the effect of the 10-year ban on fishing in the Yangtze River has been widely publicized in the world.
Coilia nasus, an anadromous migratory fish species, has historically been a significant target in the Yangtze River Basin, known as one of the ‘Three Delicacies of the Yangtze River’ (Jiang et al., 2023). In China, the Yangtze River Estuary and its adjacent sea areas serve as the main habitats for C. nasus (Yuan, 1988; Wang and Cui, 2019). According to ecotypes, the population of C. nasus in the Yangtze River can be divided into two phenotypes: freshwater residents (Coilia brachygnathus) and anadromous migrants (C. nasus). Anadromous C. nasus has developed a unique flavor and taste because of its distinct lifestyle habits, which also makes it highly esteemed in the aquatic product market and very popular among consumers. Therefore, this type has the highest economic value and is the primary fishing target (Jiang et al., 2020). Anadromous C. nasus grows and fattens in coastal areas and undergoes spawning migration after reaching sexual maturity. Mature adults initiate this journey in February each year, moving from the near-sea waters toward the Yangtze River Estuary, then ascending through the estuary to the spawning grounds where they reproduce and lay eggs (Yuan et al., 1980). The spawning period of C. nasus extends from late May to early October each year. After completing the spawning process, C. nasus adults move downstream with the current, and return to the ocean. After hatching, juveniles live for a time at the spawning ground, before eventually entering the ocean in batches, carried by water currents (Yuan et al., 1980; Zhang et al., 2005; Ge and Zhong, 2010; Guan et al., 2010). Historically, the Yangtze River was abundant in C. nasus, with the catch reaching 3,750 t in the 1970s (Zhang et al., 2005). However, multiple factors such as long-term high-intensity fishing, blocked migratory channels, and intensified water pollution have resulted in a sharp decline in C. nasus resources in the Yangtze River (Zhang et al., 2005; Jiang et al., 2022). The migratory range has also greatly shortened, the distribution area has progressively shrunk, and the catch in various river sections has continued to significantly decline (Shi et al., 2009; Guan et al., 2010; Ma et al., 2022). By 2016, the catch of C. nasus had decreased to 3.7 × 103kg, a decrease of 99.06% from the historical peak catch (Zhang et al., 2005; Xuan et al., 2021). Accordingly, the management department implemented a series of measures to alleviate fishing pressure, including reducing fishing time, issuing fewer C. nasus fishing licenses since 2004, and establishing the C. nasus National Aquatic Germplasm Resources Protection Area on December 7, 2012 (Zhang et al., 2005; Dai et al., 2020). However, stimulated by high profits, these measures have not effectively reduced the fishing intensity. To protect C. nasus, the Ministry of Agriculture and Rural Affairs stopped issuing special fishing licenses for C. nasus, Coilia mystus, and Eriocheir sinensis on February 1, 2019, and banned productive fishing of these species. Subsequently, on Jan. 1, 2021, the comprehensive implementation of the 10-year fishing ban and the extension of the Yangtze River Estuary Fishing Ban Zone provided greater protection space for the population restoration of endangered species such as C. nasus. An objective evaluation of the impact of the 10-year fishing ban in the Yangtze River on the recovery of the C. nasus resources is crucial for further optimizing management measures. However, due to the high similarity in phenotypes and mixed habitat of Coilia brachygnathus and C. nasus, accurately assessing the resource status of C. nasus presents a significant challenge. Therefore, it is very important to select a reasonable research area and apply appropriate assessment methods.
The scientific and accurate assessment of fishery resources is crucial for formulating fishery management policies. Traditional fishery assessment methods, such as the Beverton–Holt method, often require multiple life-history parameters and age data (Ralston et al., 2011; Hordyk et al., 2015). However, since more than 90% of global fishery populations lack sufficient data, making scientific assessments with traditional methods challenging (Kindong et al., 2020). Over the past decade, several fishery resource assessment methods based on limited data have been developed and implemented. To some extent, these methods have alleviated the pressure on fishery resource assessment due to the lack of data to some extent (Goodwin et al., 2006; Cope and Punt, 2009; Punt et al., 2011; Phillips et al., 2015; Armelloni et al., 2021). Among these, the electronic length–frequency analysis (ELEFAN) and length-based Bayesian biomass estimation method (LBB) are the simplest in terms of data requirements, requiring only representative length-frequency data (LFD) to estimate biological parameters and population resource status (Froese et al., 2018; Barman et al., 2021; Zhang et al., 2021a; Al-Mamun et al., 2022; Kumar et al., 2022). These methods offer a rational basis for the sustainable development of commercial fisheries and effective fishery management (Cui et al., 2020; Ju et al., 2020). In China, the ELEFAN method and LBB method have been widely applied to assess the resources of various fish populations in different maritime areas (Cui et al., 2020; Wang L. et al., 2020; Wang Y. et al., 2020; Zhai et al., 2020). For an endangered species such as C. nasus, traditional methods requiring extensive catch data are not suitable, whereas the ELEFAN method and LBB method can assess the resource status with limited data. At the same time, by leveraging the life history characteristics of C. nasus, the resource assessment in the Yangtze River Estuary and its adjacent sea areas can fundamentally exclude the influence of Coilia brachygnathus, thereby enabling a scientifically rigorous and effective reflection of the resource status of C. nasus, while also evaluating the impact of the 10-year fishing ban in the Yangtze River. In addition, following implementation of the 10-year fishing ban in the Yangtze River, no reference catch production monitoring data are available for the basin. Therefore, the supplement of marine fishing data is very important for a complete understanding of the impact of the fishing ban.
The purpose of this study is to objectively evaluate the effect of the policy by analyzing the recovery of anadromous C. nasus population from the perspective of the ocean outside the Yangtze River estuary after the implementation of the 10-year fishing ban policy in the Yangtze River, and to provide basic data for future research on C. nasus population. The technical route is shown in Figure 1.
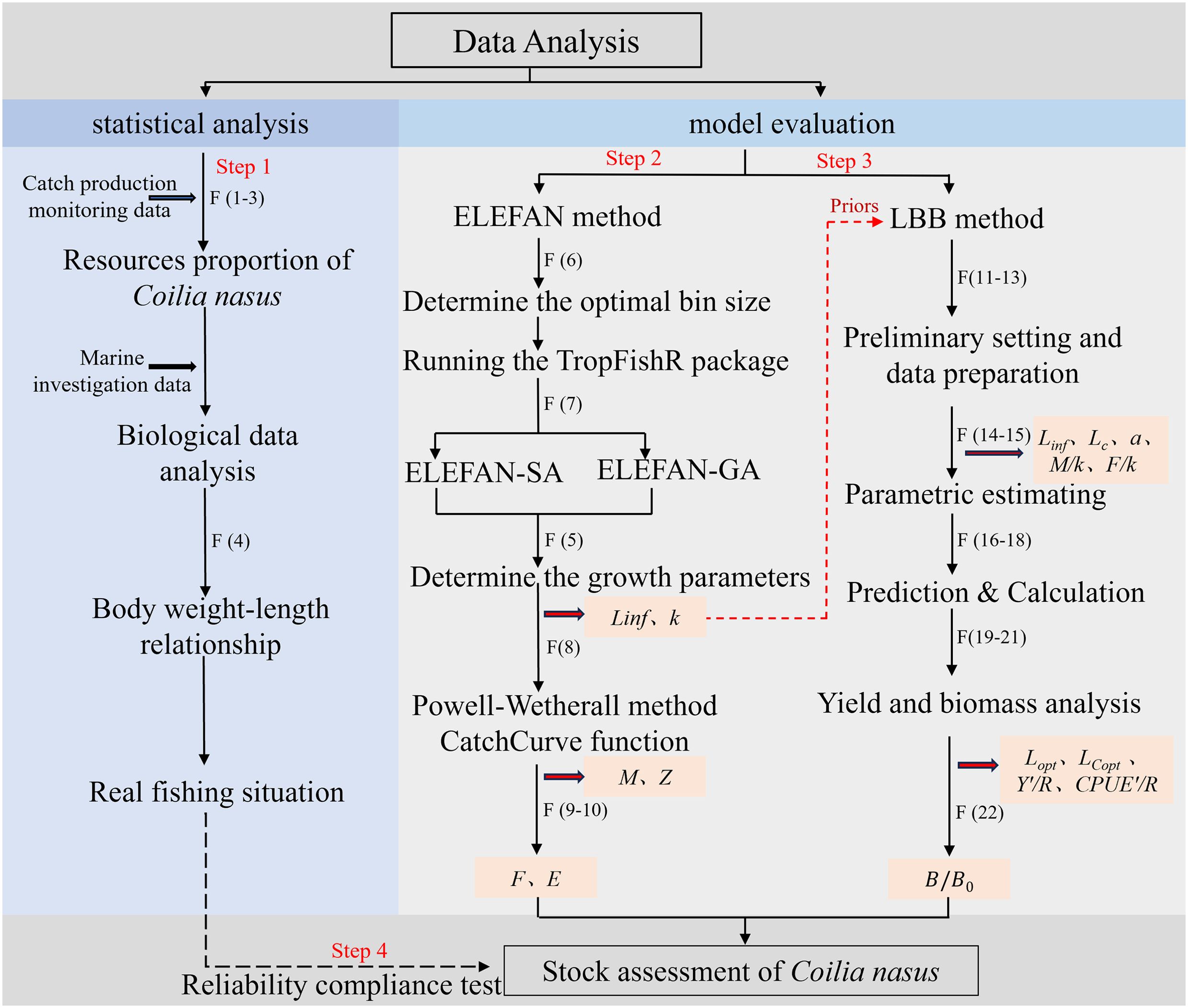
Figure 1. The technical route of this study. The black bold arrows indicate the introduction of data sources or the introduction of formulas here. The red dotted arrow indicates that the output result is fed into the new model as initial data. The red bold arrows indicate the output result. F represents the formula used in the fitting. Such as, F (6) denotes the calculation according to Formula 6.
2 Materials and methods
2.1 Survey area
The Yangtze River Estuary (Figure 2), extending inward, is connected to the Yangtze River and its tributaries, as well as the lakes and rivers that flow into it. Outward, it communicates with the waters of the East China and Yellow Seas to form an area where land runoff and seawater converge. Moreover, the estuary represents a junction of various marine current systems, including the Subei coastal current, the Taiwan warm current, and the Yellow Sea cold water masses. Sea areas are rich in nutrients and bait organisms that integrate the functions of spawning grounds, feeding grounds, and nurseries (Li et al., 2015). The unique natural environment of the Yangtze River Estuary fosters rich diversity of fishery biological resources, including estuarine, marine, freshwater, and migratory fishes (Chen Y. et al., 2020; Chen et al., 2021). As a vital migration corridor for diadromous species in the Yangtze River basin, the estuary is bifurcated by islands like Chongming Island into distinct northern and southern branches. These branches show notable variations in topography, hydrological features, and environmental factors, which substantially affect the distribution and abundance of fishery resources (Zhai et al., 2023).
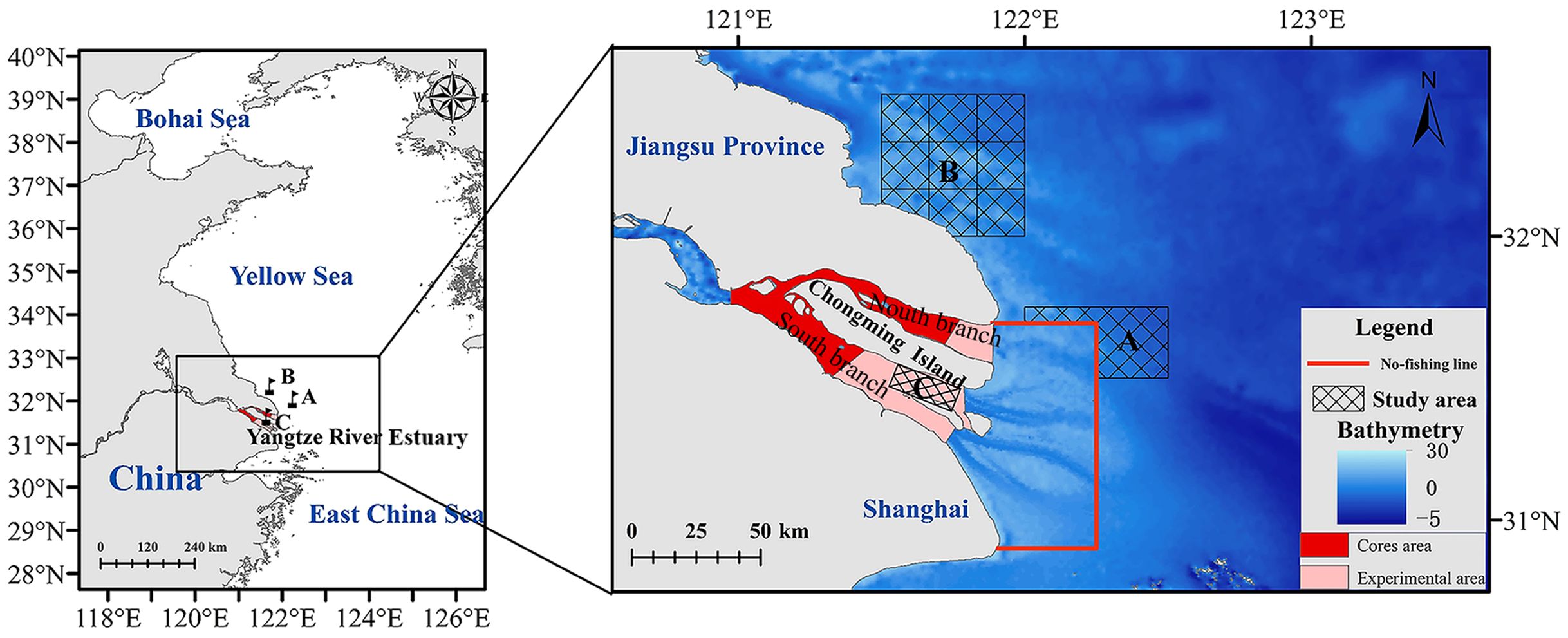
Figure 2. Map of the survey area. Grid delineates the survey area. The red area designates the core zone of the C. nasus reserve; the pink area signifies the experimental zone of the same reserve. The area bounded by the red line and to the left of the red line shows the Yangtze River Estuary Fishing Ban Zone; the area to the right of the red line shows the allowable fishing area.
2.2 Data sources
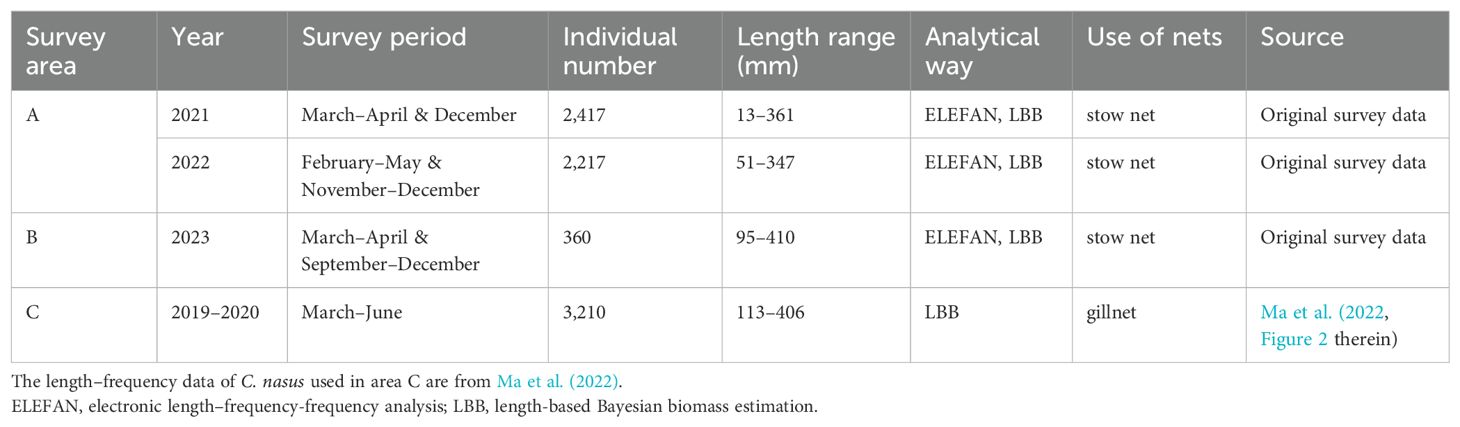
The body length data for this study were collected from sample collections and scientific papers. Sample collection areas A and B utilized stow nets for fishing. The length of the opening rope was 26 m and 22 m, the length of the side rope was 24 m and 5.5 m, the total stretched length of the net was 60 m and 32 m, and the mesh size was 20 mm and 25 mm for areas A and B, respectively. The nets were secured by a stake that was firmly wedged into the seabed, and they were equipped with two bamboo beams to regulate the horizontal expansion of the net mouth. The vertical expansion of the net mouth was sustained by the buoyancy of the top beam and the adhesive force of the bottom beam (Xiong et al., 2017a; Qin et al., 2024). The data for area C were read off from scientific papers (Ma et al., 2022). The sampling gear used were gillnets, consisting of multiple rectangular nets linked together and typically set up in fish migratory channels, which catch fish by winding around or piercing (Li et al., 2009; Xiong et al., 2017b). The gillnet used in this study had a mesh size of 40 mm, a length of 150 m, and a height of 12 m (Ma et al., 2022).
C. nasus samples were collected in accordance with the Technical Specifications for Marine Fishery Resources Survey. The collected samples were cryogenically preserved and transported to the laboratory for biological analyses, which included measuring body length and body weight, determining sex, and assessing gonadal development stages (I-VI). Body length and weight measurements accurate to 1 mm and 0.1 g, respectively. A total of 4,994 samples of C. nasus were collected. And the specific data sources and body length data were recorded in detail in Table 1 and Supplementary Material S1. In addition, we also collected catch production monitoring records from the same commercial fishing vessel in area B in April each year from 2020 to 2022, and extracted the relevant data of C. nasus. Details of the catch production monitoring data can be found in Supplementary Material S2.
2.3 Data analysis
2.3.1 Catch production monitoring data analysis
The number percentage, weight percentage, and occurrence frequency of C. nasus captured from 2020 to 2022 were calculated as follows:
where N% is the percentage of the total number of C. nasus in relation to the total number of fish species caught, W% is the percentage of the total weight of C. nasus in relation to the total weight of all fish species caught, and F% is the percentage of occurrences of C. nasus in relation to the total number of fishing trips.
2.3.2 Marine investigation data analysis
The body length–weight relationship of C. nasus was calculated using the following formula:
where W is the body weight of C. nasus, L is the body length of C. nasus, a is the growth condition factor, and b is the power exponent.
2.3.3 Electronic length–frequency analysis
ELEFAN is a method for deriving von Bertalanffy growth function parameters from the LFD (Mildenberger et al., 2017). ELEFAN has been integrated with R statistical computing software to create the TropFishR package, which offers both traditional and updated versions of ELEFAN, as well as new optimization techniques. In this study, two optimized ELEFAN algorithms were used to fit the seasonally oscillating von Bertalanffy growth function: “Simulated Annealing” (ELEFAN-SA), and “Genetic Algorithm” (ELEFAN-GA). The best fit method (Rn_max) was used for further analyses:
where ESP is the sum of the explainable peaks, ASP is the sum of the available peaks, and is the best fit (Pauly, 1985).
The optimal bin size (OBS), which can effectively reduce the estimation bias, was determined using the following formula (Wang K. et al., 2020):
where OBS is the optimal bin size, and is the maximum body length of the fish.
The ELEFAN-SA and ELEFAN-GA methods use restructured data in combination with the seasonally oscillating von Bertalanffy growth function for analysis (Pauly and Morgan, 1987):
where and . C is the constant for the amplitude of the oscillation (with a value range of 0–1), and TS is the phase that regulates the seasonal oscillation (with a value range of 0–1).
The total mortality rate (Z) was estimated based on the length-converted catch curve, and the natural mortality coefficient (M) was determined using Pauly’s empirical formula (Pauly, 1980):
where Linf is the asymptotic body length, K is the growth parameter, and T is the annual average temperature of the habitat for C. nasus. The average habitat temperature for C. nasus in 2021 and 2022 was 14.2°C. The average habitat temperature was obtained from National Oceanic and Atmospheric Administration (2022) Ocean Watch, with a spatial resolution of 1° and a temporal resolution of months (https://oceanwatch.pifsc.noaa.gov).
The fishing mortality coefficient (F) was calculated as follows (Wang et al., 2021):
The fishery development rate (E)was calculated as follows (Wang et al., 2021):
2.3.4 Length-based Bayesian biomass estimation
Compared to ELEFAN, the LBB method is more accurate, using the ratios of total mortality and fishing mortality to growth rate in the estimation process instead of fixed values for K, M, and Z. Here, we only list the main reference formulas; the R code was downloaded from http://oceanrep.gemar.de/44832/. Similar to ELEFAN, we assumed that fish growth followed the von Bertalanffy growth equation (von Bertalanffy, 1938; Beverton and Holt, 1957), as follows:
where is the asymptotic length at which the fish reaches maturity, K is the growth coefficient, t is the age of the fish, and t0 is the theoretical age at which the fish would be at zero length.
When the commercial catch is fully selected by a specific fishing gear, the length–frequency curve of the catch can be described as a function of Z in relation to the length growth rate:
where is the number of fish surviving at length L, is the number of fish fully selected at length , with all individuals entering the net being retained, and Z/K is the ratio of Z to the growth parameter.
Here, assuming that fish were caught in a stow net, the selectivity can be expressed by the following functions:
where SL is the proportion of individuals of length L captured by the fishing gear, Lc is the length at first capture, and a indicates the steepness of the net’s selectivity curve.
The parameters , , a, M/K, and F/K were estimated using the following equations (Froese et al., 2018):
where is the number of individuals in the length group , is the number of individuals in the previous length group, and is the number of individuals caught in the length group .
The length distribution predicted by the LBB model was expressed by the following equation:
where is a function of the estimable population dynamic determents .
By substituting , M/k, and M/F into Equations 17, 18, we obtained the optimal length for unexploited generations with maximum biomass, as well as the optimal catch length :
The relative unit supplement yield Y’/R was given by the following equation:
Assuming that the catch per unit of fishing effort was proportional to the population biomass, and that fishing mortality was directly proportional to fishing effort, dividing both sides of Equation 19 by F/M resulted in the following equation:
When F=0, the relative biomass expression is:
where B0 is the initial biomass. The biological reference point B/B0 of the developed stock can be expressed as follows:
where B is the current biomass. When F, M, Lc, and are equal, the ratio of the biomass corresponding to the maximum sustainable yield to the initial biomass can be obtained by recalculating Equations 19–22.
3 Results
3.1 Statistical results
3.1.1 Resource proportion of C. nasus
According to the survey data in April from the Yangtze River Estuary and adjacent sea areas, the number percentage, weight percentage, and occurrence frequency of C. nasus showed a continuous upward trend from 2020 to 2022, indicating recovery of C. nasus resources (Table 2).
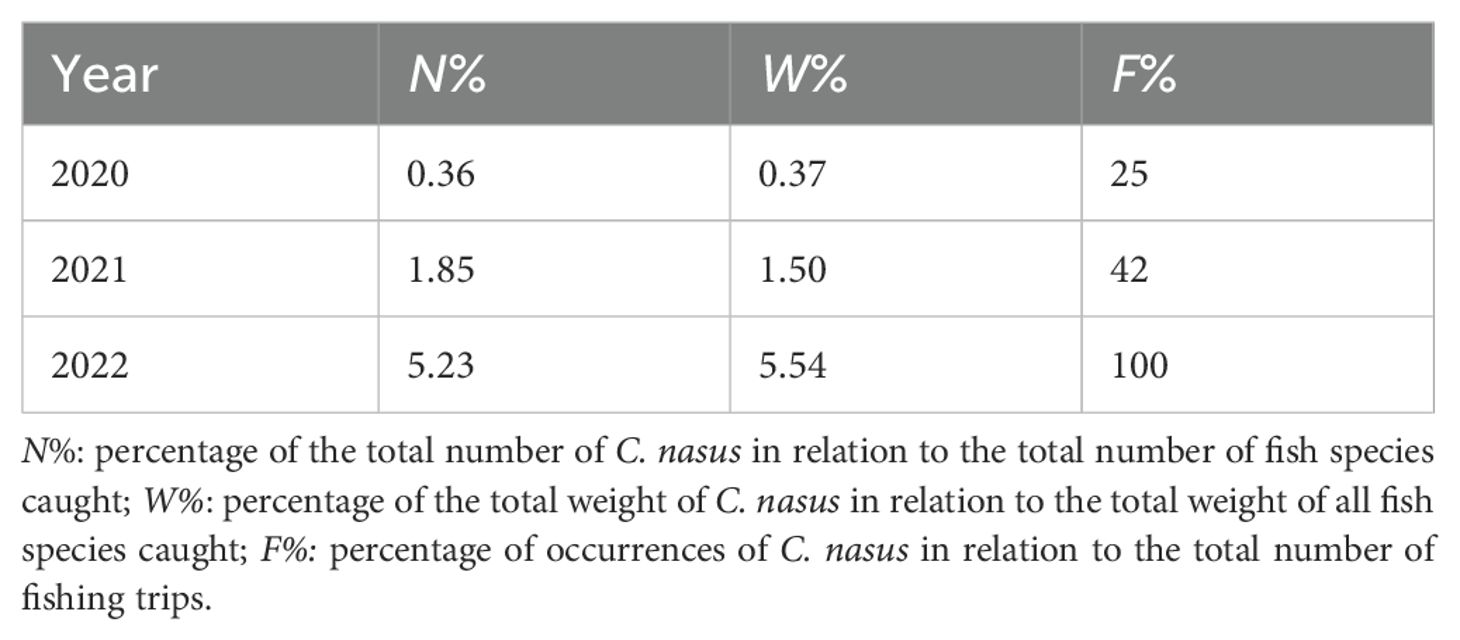
Table 2. The proportion of C. nasus caught during the fishing season (April) outside the Yangtze River Estuary Fishing Ban Zone from 2020–2022.
3.1.2 Body length and weight characteristics of C. nasus
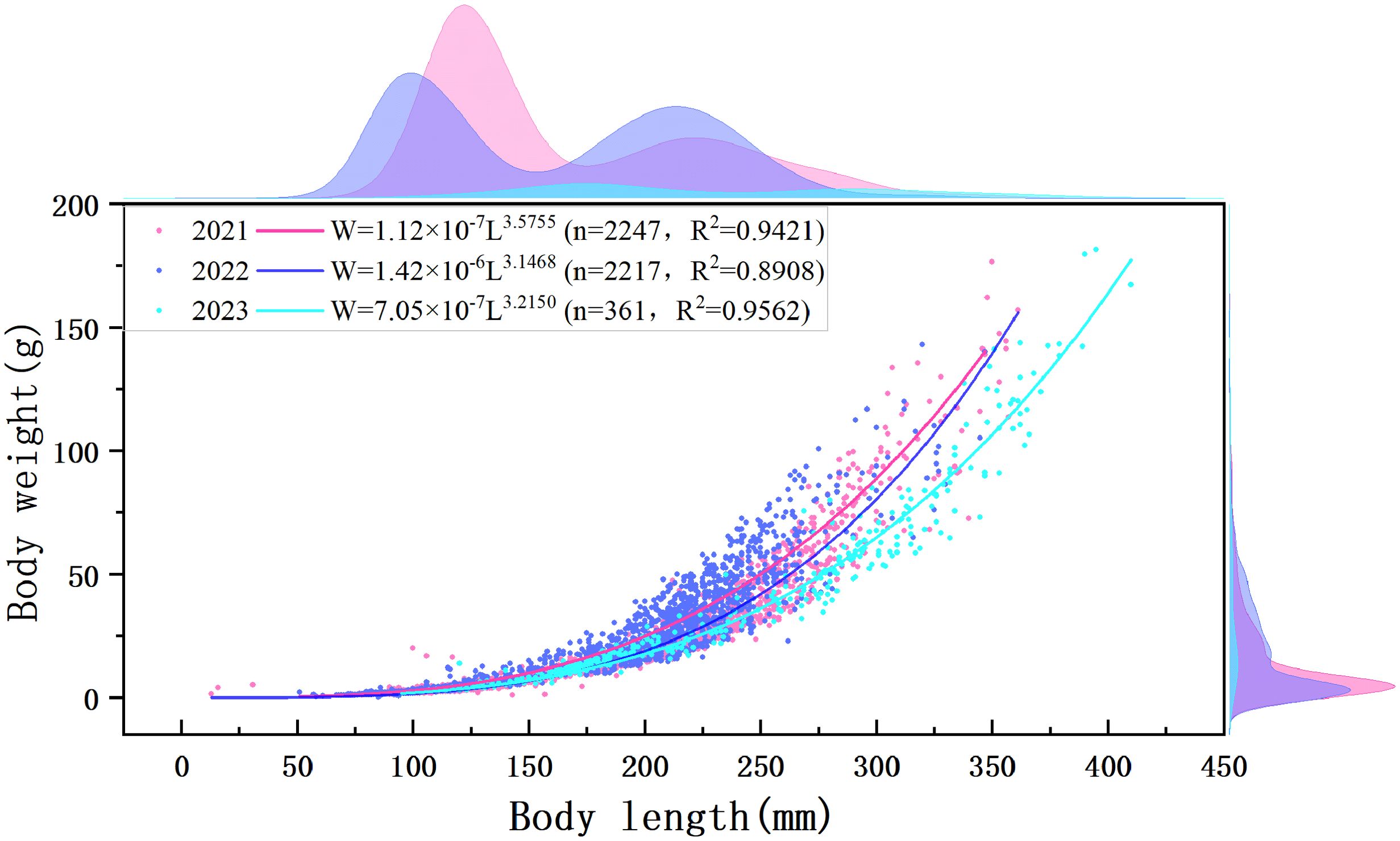
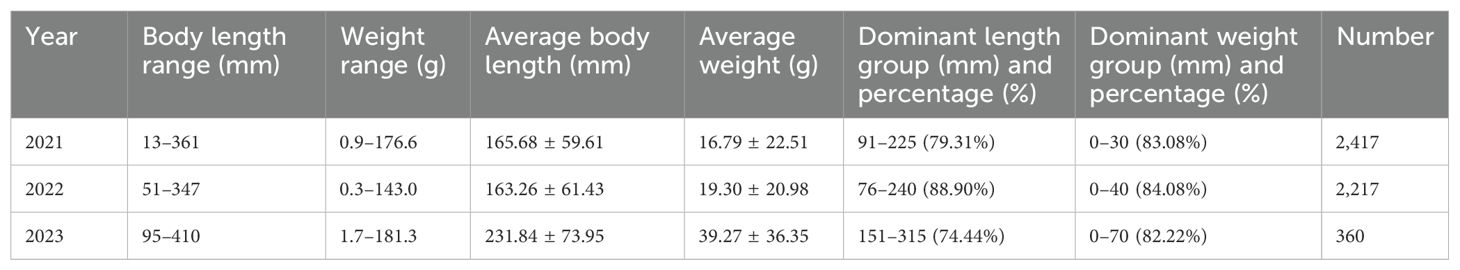
Based on measure body length and weight data, fitting was performed to derive the length–weight relationship curves of C. nasus for 2021–2023. The regression parameter b for the length–weight relationship was consistently greater than 3, indicating a positive allometric growth pattern. All fitting degrees of determination were greater than 0.89, suggesting a strong correlation and a good fit (Figure 3). During the three-year period, the average body length and weight of C. nasus increased annually (Table 3). The gonadal development stage was mainly in stage II, and the proportion of stage II individuals in 2021, 2022, and 2023 was 83.7%, 86.1%, and 79.5%, respectively. The ratio of female to male was 1:1.28, 1:1.14, and 1.87:1, respectively.
3.2 Model Evaluation
3.2.1 Population parameters estimated based on ELEFAN
According to the calculation results of Equation 6, the OBS for the LFD of C. nasus was determined to be 1 cm. After setting the moving average to 11 (Figure 4), and applying both methods, the ELEFAN-GA ( =0.32) fitting outcome was superior (Figure 5). The ELEFAN-GA results from 2021–2023 indicated that was 42.92 cm, K was 0.43 year-1, and the theoretical age at length zero (t0) for 2021–2022 was -0.31 year. Additionally, Z from 2021–2022 was 1.47 year-1, F was 0.79 year-1, E was 0.54.
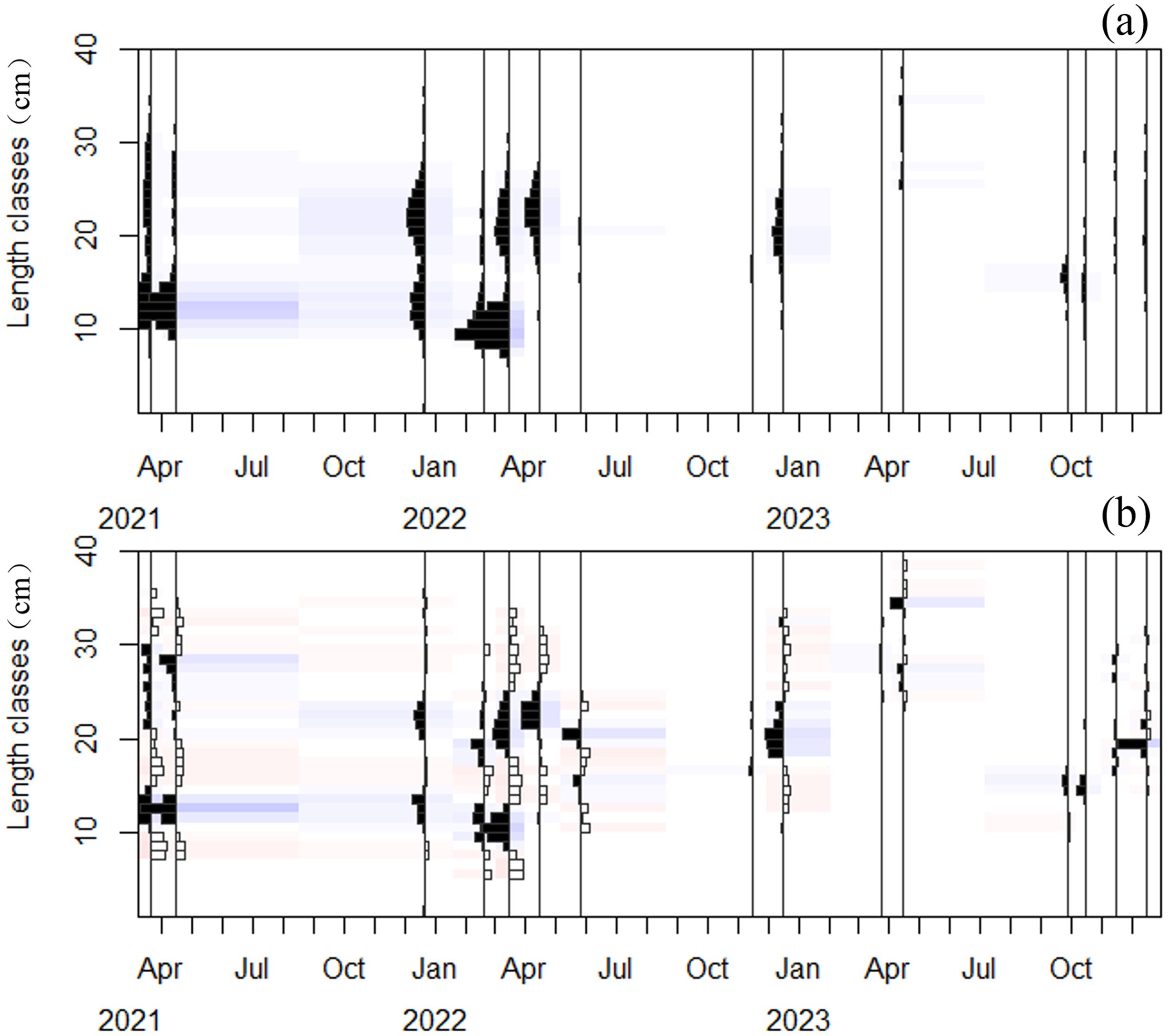
Figure 4. Visualization of C. nasus length–frequency data. (A) Originally measured body length frequency data (B) Recombined body length frequency data for MA = 11.
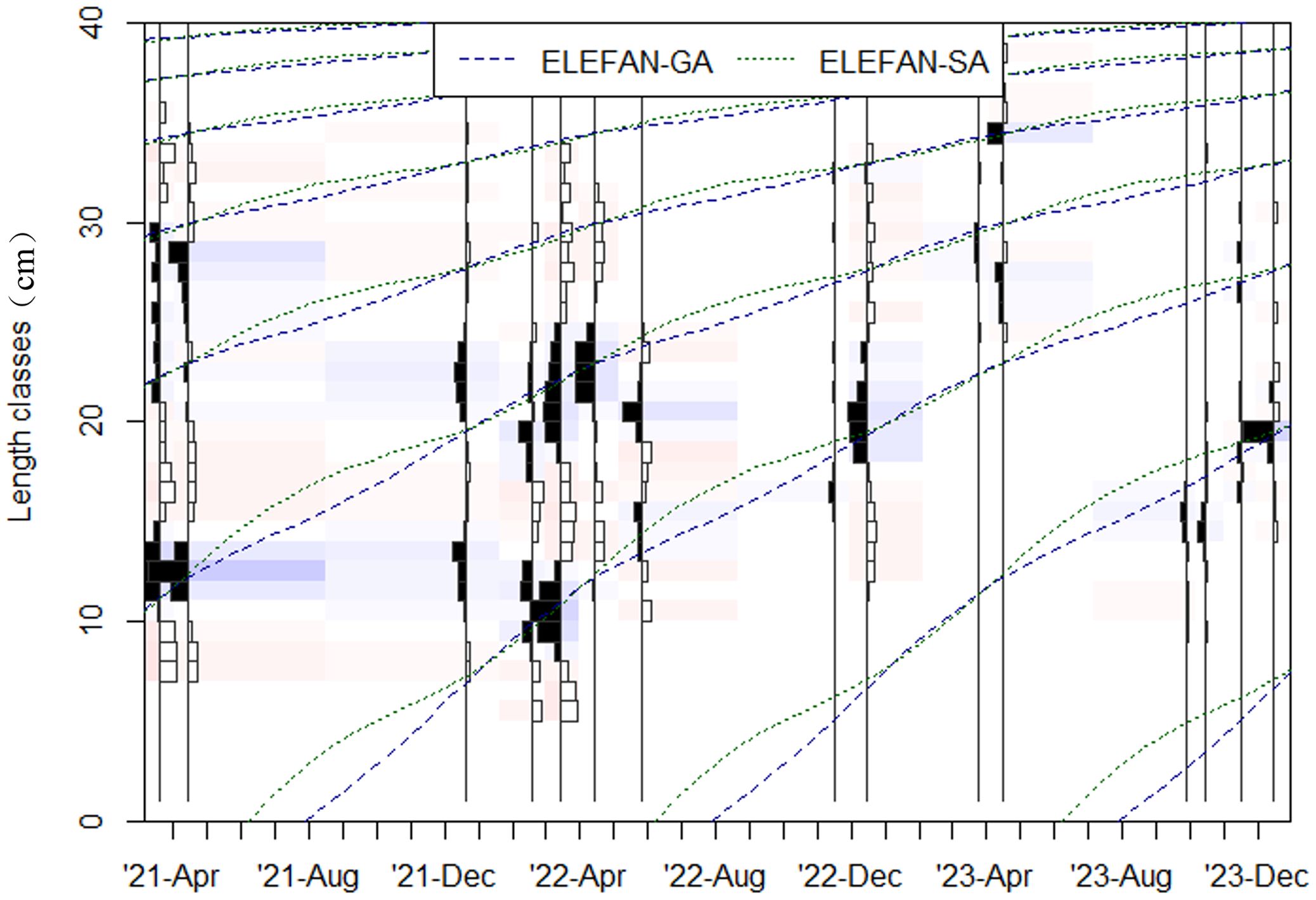
Figure 5. The seasonal VBGF fitted to length frequency distribution of C. nasus using ELEFAN-SA and ELEFAN-GA algorithms.
3.2.2 Population parameters estimated based on LBB
We then combined the = 42.92 cm and M/K = 1.57 values obtained by the ELEFAN method, as prior information, with the LBB method, and estimated that the asymptotic length () of C. nasus captured from 2019–2023 was 38.7–43.3 cm, and were 19–26 cm (Table 4; Figure 6). Z/K, F/K and M/K generally declined, indicating a decreasing impact of fishing pressures. The relative biomass (B/B0) of the C. nasus population gradually increased, indicating a recovery trend. The ratio of biomass at maximum sustainable yield to initial biomass (B/BMSY) increased from 0.5 in 2019 to 2.4 in 2023, indicating that the C. nasus population gradually recovered from an overfished state to healthy (Table 4; Figure 7).
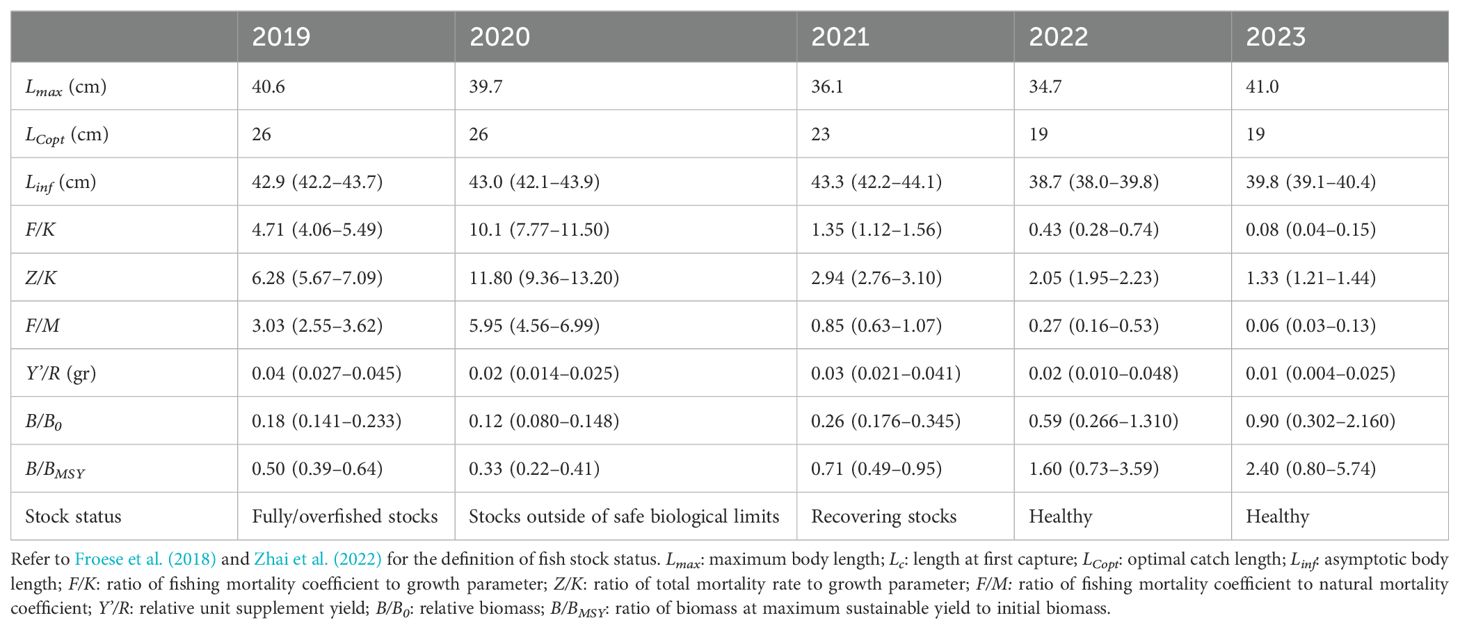
Table 4. Parameter estimates and 95% confidence intervals for each year were obtained using the LBB method in this study.
Figure 6. Estimation results of the length-based Bayesian biomass estimation (LBB) method for C. nasus from 2019–2023 (A–E). Left figure shows the LFD, a priori asymptotic body length (Linf), and ratio of total mortality to growth rate (Z/K) estimated from LFD. Right figure shows the results of fitting the LBB master equation to LFD.
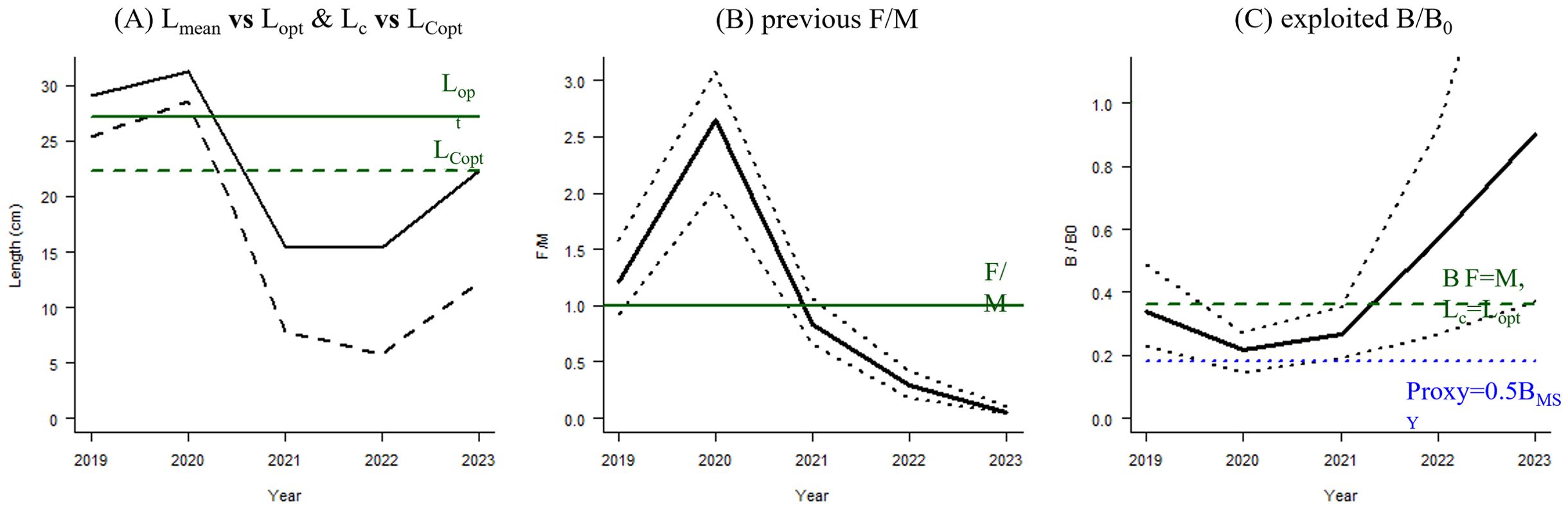
Figure 7. Continuous estimation results of C. nasus from 2019–2023 using the LBB method. (A) Average capture length/optimal length for unexploited generations with maximum biomass (Lmean/Lopt; solid black line) and length at first capture/optimal catch length (Lc/LCopt; dashed black line). (B) Fishing mortality coefficient/natural mortality coefficient (F/M; solid black line) and its 95% confidence interval (dashed black line), with a reference line (solid green line) indicating the point where F = M. (C) Relative biomass of the C. nasus population (B/B0; solid black line) and its 95% confidence interval (dashed black line). Biomass (B) value for F = M and optimal Lc is shown by the dashed green line, whereas that for an alternative ratio of biomass at maximum sustainable yield to initial biomass of 0.5 is shown by the dashed blue line.
4 Discussion
Both the ELEFAN and LBB methods are advantageous in that they minimize data requirements (Shi et al., 2022), enabling a reasonable assessment of the population status of C. nasus. The ELEFAN method is a technique for inferring the stock status of a population by analyzing the length–frequency distribution of captured fish. Since its development, FiSAT II software has become the preferred tool for conducting ELEFAN analyses and has been widely applied across various fisheries worldwide (Ye et al., 2014; Osei et al., 2021). After the development of the TropFishR package, fixed applications within the FiSAT II software were optimized, leading to the formation of a comprehensive suite of methods for fishery analysis using LFD, such as: ELEFAN-SA and ELEFAN-GA (Korkmaz et al., 2023). Similar to the ELEFAN method, the LBB method use LFD as the initial input for population assessment (Zhang et al., 2021b). Moreover, the ELEFAN method can offer effective prior information for the LBB method, thereby improving the precision of its estimation outcomes (Ziegler et al., 2011). In the LBB method, the default M/K values can be replaced by alternative values according to different life histories among populations. Choosing M/K values within the range of 0.3–3.0 has a minimal impact on relative biomass estimates (Froese et al., 2018). Therefore, we applied the management reference points (42.92 cm), M (0.68 year-1), and K (0.432 year-1) estimated using the ELEFAN method as prior information for LBB estimation, thereby correcting the fixed default value of M/K and enhancing the accuracy and credibility of the LBB estimation results. In this study, we measured the whole length data of 4,494 individuals of C. nasus over a period of 3 years, representing the recent population composition of this species. Consequently, the collected data meet the requirements of both methods. In addition, the estimation results are suitable for fishery management and can be used directly as prior information for other assessment methods.
Fishery production data reflect the growth conditions of organisms to a certain extent (Geng et al., 2019). The data collected during April over three consecutive years (2020–2022) indicate that, after implementation of the 10-year fishing ban on the Yangtze River, the relative proportion of C. nasus in the catch increased. Both its weight proportion and number of individuals increased considerably, from less than 1% to over 5%. The average body length and weight also showed a consistent annual increase, indicating a clear resource recovery trend (Table 2). Fish growth parameters indicate not only their growth and survival status but also habitat suitability to a certain degree (Han et al., 2017). The biological data of C. nasus from 2021–2023 also revealed consistent outcomes: the distribution of body length and weight became more uniform. An increase in the number of larger-sized individuals was also observed, alongside an increase in both the average body length and average weight of the population. C. nasus has a relatively short life cycle and is highly sensitive to fishing pressure, necessitating a lengthy recovery period following resource depletion (Zhang et al., 2005). However, following implementation of the 10-year fishing ban and the expansion of the Yangtze River Estuary Fishing Ban Zone, the high-pressure fishing activities that previously threatened the resources of C. nasus in the Yangtze River Basin have completely ceased. This has greatly reduced fishing pressure on C. nasus, allowing breeding adults to smoothly enter the spawning grounds, thereby increasing the recruitment of the population. Concurrently, the mortality rate of young fish attributed to bycatch in eel fry nets has decreased considerably. Furthermore, we observed the fish season of C. nasus, which was not observed during overfishing. In the study, the C. nasus had an obvious fishing season, which began at the end of February and extended through May. This finding aligns with historical data on the timing of the fishing season (Yuan et al., 1980; Liu et al., 2012). The reappearance of migratory C. nasus in the Xiangjiang River of Hunan Province in 2023 substantiates the profound impact of the decade-long fishing moratorium on the conservation of this species.
In this study, sample data of C. nasus, collected following implementation of the fishing ban, were used to estimate the population status via the ELEFAN method. With the fishery development rate of 0.54, indicating that the effects of historical overfishing are still present. Although the early phase of the Yangtze River’s 10-year fishing moratorium demonstrated a nascent recovery in the number and resource status of C. nasus, a prolonged period of recovery is still essential (Jiang et al., 2022; Ma et al., 2022). Moreover, the estimation results of the LBB method provided further details. Between 2019 and 2023, C. nasus resources exhibited a fluctuating upward trend. Except for a minor decline in 2020, the relative biomass of C. nasus has increased annually, demonstrating a positive recovery trend. Although the issuance of special fishing licenses for C. nasus ended in the Yangtze River Basin in 2019, the resource volume did not significantly increase in the subsequent period, which may be attributed to the high-intensity fishing activities that still persist in the Yangtze River Basin. Fishing activities, such as eel fry fishing, may impede the recovery of C. nasus populations (Zhang et al., 2005). In China, the period in which eel fry are usually caught, between January and April, significantly overlaps with the migration of young C. nasus for feeding (Ge et al., 2013). The Yangtze River Estuary is the main migration channel and primary area for eel fry fishing (Wang S. et al., 2023). During the eel fry fishing season, numerous eel fry nets are deployed in the Yangtze River Estuary area, where young C. nasus enter the nets with the tide, resulting in a large number of deaths (Zhang et al., 2005). Multiple studies have indicated that young C. nasus often become a dominant bycatch species during eel fry fishing (Ge et al., 2013; Deng et al., 2023; Yan et al., 2024). Consequently, despite the cessation of specialized fishing for C. nasus, eel fry fishing activities may still affect the recovery of the C. nasus population to some extent. After the full implementation of the 10-year fishing ban in the Yangtze River in 2021, eel fry fishing was prohibited within the Yangtze River Estuary Fishing Ban Zone, allowing the early recruits of C. nasus to pass through the area smoothly improving its survival rate and increasing the amount of C. nasus resources.
Fish resources are affected by many factors, such as fish adaptability, germplasm characteristics and habitat environment (Meffe, 1986; Yang et al., 2014; Duarte et al., 2020). The population of C. nasus is primarily affected by its own reproductive capacity and environmental factors (Zhang et al., 2005). C. nasus individuals can reach sexual maturity at two years of age and have a strong reproductive capacity, allowing rapid population increases and demonstrating significant potential for resource recovery (Song et al., 2022). Since the Yangtze River Protection Strategy was proposed in 2016, there has been a strengthening of river management along the Yangtze River and water source management, which collectively contributed to a significant and comprehensive improvement in the ecological environment of the Yangtze River Basin (Dong et al., 2024). The spawning and hatching habitats of C. nasus are located within the Poyang Lake and Dongting Lake (Xuan et al., 2020; Jiang et al., 2022). The improved habitat not only boosts the survival rate of C. nasus eggs but also provides an excellent feeding environment for larvae and juveniles, thereby increasing the resources of the early supplementary population of C. nasus. Moreover, as a high trophic level consumer in the Yangtze River, the trophic level of C. nasus increases with body length (Deng, 2023; Liu et al., 2023). There are fewer fish that prey on C. nasus in the river, and the resources for adult C. nasus are well protected. These factors contribute to the possibility of maintaining a stable population structure and a continuous increase in resources as showed in this study. The relative biomass of C. nasus in 2022 doubled from that in 2021, likely because of the accumulation of population resources prior to this period. Additionally, the expansion policy implemented in the Yangtze River Estuary Fishing Ban Zone on November 20, 2021, provided a wider range of protection for young C. nasus that year. In 2022 and 2023, the relative biomass increased by 3.3 times and 4 times than in 2021, respectively, and the population assessment results of C. nasus were in a healthy state. However, the proportion of young C. nasus below age 1 caught during 2022 and 2023 was low, so the lack of assessment of young C. nasus possibly cause an overestimation of the population state in our study. In the future, it is necessary to increase the survey frequency of young C. nasus as they return from the spawning grounds to the sea, in order to better assess the population dynamics.
Table 5 illustrates that Zhai et al. (2022) used the LBB method to evaluate the historical resource status of C. nasus. In contrast, the results of this study on the population status of C. nasus in 2021 are similar to those of 2011 (Tables 4, 5), which reflects that the utilization intensity of C. nasus resources has decreased and its resource sustainability has increased. However, the F/M in 2011 was significantly higher than that in 2021, suggesting that the excessive fishing pressure during 2011 was the primary cause for the population decline, a factor closely tied to the geographical locations of the two study areas. Numerous studies have shown that the distribution of C. nasus is significantly influenced by environmental factors such as temperature, water depth, and salinity. Specifically, in the Yangtze River Estuary, C. nasus is predominantly found in areas with water depths exceeding 10 meters, where the temperature is relatively high and salinity fluctuations are minimal (Guo et al., 2016; Tong et al., 2018). The northern branch of the Yangtze River Estuary is characterized by shallow water depths and significant salinity changes. Therefore, the resources of C. nasus in the southern branch of the Yangtze River Estuary are usually significantly higher than those in the northern branch, and fishing vessels are more inclined to catch C. nasus in the southern branch of the Yangtze River Estuary. The survey area in 2011 of Zhai et al. (2022) for C. nasus was situated in the southern branch of the Yangtze River Estuary, where fishing pressure is higher. This aligns with our research findings. Moreover, the study also indicates that the implementation of the 10-year fishing ban along the Yangtze River and the expansion of the Fishing Ban Zone in the Yangtze Estuary have had a positive impact on the recovery of diadromous fish species, including C. nasus. Currently, in the early stages of the fishing ban, aquatic biological resources have been effectively protected and have shown notable recovery. The index of aquatic biological integrity has improved markedly from pre-ban levels (Meng et al., 2023; Wang Y. et al., 2023; Luo, 2024; Yang et al., 2024). Therefore, the study advocates for the implementation of long-term monitoring and research on diadromous fish populations with a particular emphasis on important migration channels as the adjacent marine areas of Yangtze River Estuary Fishing Ban Zone. It will enable a scientific assessment of resource changes and facilitate the timely adjustment of management measures in ocean, especially for C. nasus, and help to accelerate the recovery of C. nasus from its endangered status.
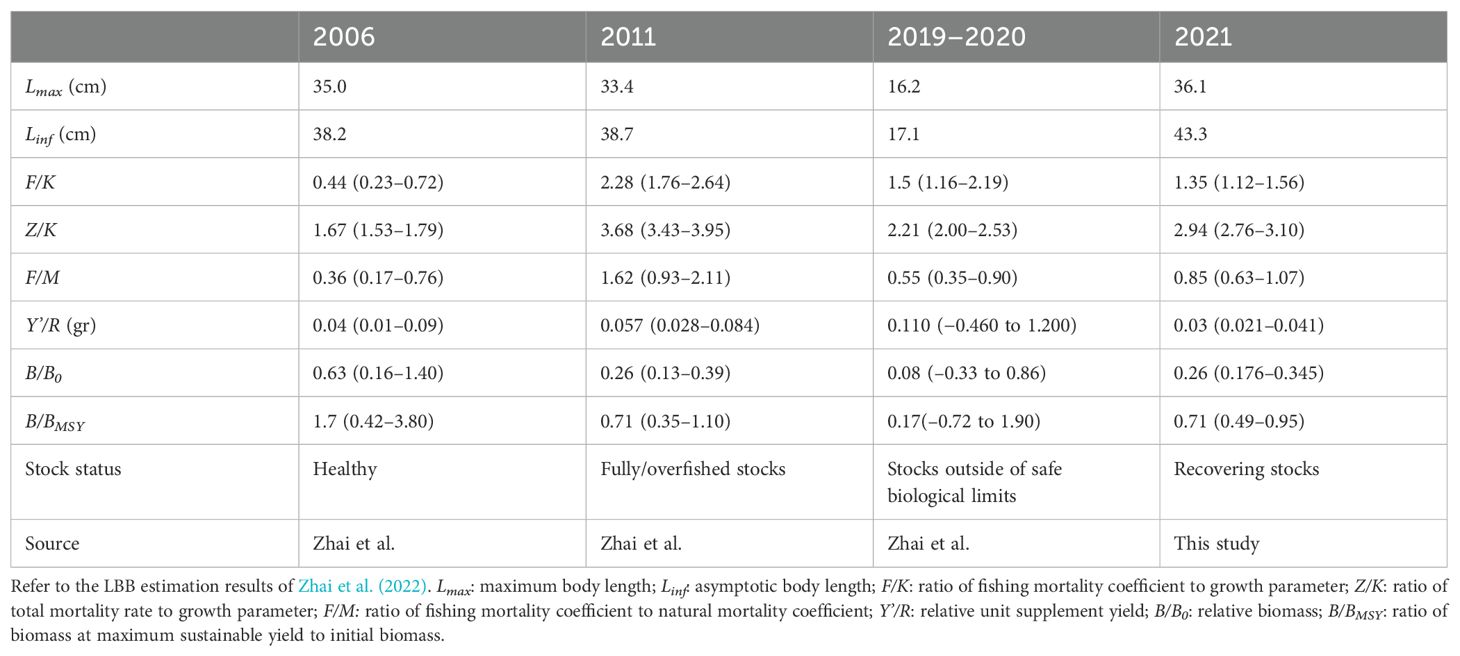
Table 5. Parameter estimates and 95% confidence intervals obtained using the length-based Bayesian biomass estimation (LBB) method in previous studies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was reviewed and approved by Marine Fisheries Research Institute of Jiangsu Province.
Author contributions
SW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – original draft, Writing – review & editing. YX: Conceptualization, Funding acquisition, Resources, Writing – review & editing. HZ: Formal analysis, Investigation, Visualization, Writing – review & editing. DS: Formal analysis, Visualization, Writing – review & editing. YW: Data curation, Investigation, Resources, Writing – review & editing. HG: Data curation, Investigation, Resources, Writing – review & editing. CZ: Formal analysis, Visualization, Writing – review & editing. LL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing. XZ: Conceptualization, Funding acquisition, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Nature Science Foundation of China (Grant No. 32473169) and the Fisheries Ecology and Resources Monitoring Projects of Agricultural Ecological Protection and Resource Utilization in Jiangsu Province (2021–SJ–110–02 and 2022–SJ–061–01).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1474996/full#supplementary-material
References
Al-Mamun M. A., Shamsuzzaman M. M., Schneider P., Mozumder M. M. H., Liu Q. (2022). Estimation of stock status using the LBB and CMSY methods for the Indian Salmon Leptomelanosoma indicum (Shaw 1804) in the Bay of Bengal, Bangladesh. J. Mar. Sci. Eng. 10, 336. doi: 10.3390/jmse10030366
Armelloni E. N., Scanu M., Masnadi F., Coro G., Angelini S., Scarcella G. (2021). Data poor approach for the assessment of the main target species of Rapido Trawl fishery in Adriatic Sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.552076
Barman P. P., Liu Q., Al-Mamun M. A., Schneider P., Mozumder M. M. H. (2021). Stock assessment of exploited sardine populations from northeastern Bay of Bengal Water, Bangladesh using the Length-Based Bayesian Biomass (LBB) Method. J. Mar. Sci. Eng. 9, 1137. doi: 10.3390/jmse9101137
Beverton R. J. H., Holt S. J. (1957). On the Dynamics of Exploited Fish Populations (London: Ministry of Agriculture, Fisheries and Food).
Chen D., Xiong F., Wang K., Chang Y. (2009). Status of research on Yangtze fish biology and fisheries. Environ. Biol. Fishes. 85, 337–357. doi: 10.1007/s10641-009-9517-0
Chen J., Wang X., Tian S., Wu J., Dai L., Gao C., et al. (2021). A review of the development of fishery resources monitoring in the Yangtze River Estuary and its adjacent waters. Resour. Environ. Yangtze Val. 30, 122–136. doi: 10.11870/cjlyzyyhj202101012
Chen T., Wang Y., Gardner C., Wu F. (2020). Threats and protection policies of the aquatic biodiversity in the Yangtze River. J. Nat. Conserv. 58, 125931. doi: 10.1016/j.jnc.2020.125931
Chen Y., Liu S., He Y., Qin Y., Ji X., Zhang H., et al. (2020). Study on ecosystem health and variation trend at the Changjiang River Estuary in the past 30 years. Acta Oceanol. Sin. 42, 55–65. doi: 10.3969/j.issn.0253-4193
Cope J. M., Punt A. E. (2009). Length-based reference points for data-limited situations: Applications and restrictions. Mar. Coast. Fish. 1, 169–186. doi: 10.1577/C08-025.1
Cui L., Xian W., Liu S., Pauly D. (2020). Assessments of 14 exploited fish and invertebrate stocks in chinese waters using the LBB Method. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00314
Dai P., Yan Y., Zhu X., Tian J., Ma F., Liu K. (2020). Status of Coilia nasus resources in the National Aquatic Germplasm Resources Conservation Area in the Anqing Section of the Yangtze River. J. Fish. Sci. China 27, 1267–1276. doi: 10.3724/SP.J.1118.2020.20130
Deng Y. (2023). Study on feeding ecology of migratory Coilia nasus in the lower reaches of the Yangtze River. (master’s thesis). Shanghai Ocean University, Shanghai.
Deng Y., Tang W., Zhang Y., Zhao Z. (2023). Species diversity and assemblage structure of fishes bycatch in elver nets in Yangtze River Estuary. Acta Hydrobiol. Sin. 47, 168–176. doi: 10.7541/2023.2022.0016
Dong F., Fang D., Zhang H., Wei Q. (2023). Protection and development after the ten-year fishing ban in the Yangtze River. J. Fish. China 47, 245–259. doi: 10.11964/jfc.20221013724
Dong H., Hu Y., Qian L., Yan J., Gao L., Mei W., et al. (2024). Preliminary manifestation of the Yangtze River Protection Strategy in improving the carbon sink function of estuary wetlands. iScience 27, 108974. doi: 10.1016/j.isci.2024.108974
Duarte C. M., Agustí S., Barbier E. B., Britten G. L., Castilla J. C., Gattuso J., et al. (2020). Rebuilding marine life. Nature 580, 39–51. doi: 10.1038/s41586-020-2146-7
Froese R., Winker H., Coro G., Demirel N., Tsikliras A. C., Dimarchopoulou D., et al. (2018). A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 75, 2004–2015. doi: 10.1093/icesjms/fsy078
Ge C., Zhong J., Ge K., Li A., Liu B., Wang M., et al. (2013). Analysis on the composition of by-catch in elver nets and the suggestions on the management of elver nets in Yangtze River Estuary. J. Shanghai Ocean Univ. 22, 391–397.
Ge K., Zhong J. (2010). Daily-age structure and growth characteristics of coilia nasus larvae and juveniles in the surf zone of Yangtze River Estuary. Acta Hydrobiol. Sin. 36, 716–721. doi: 10.3724/SP.J.1035.2010.00716
Geng Y., Zhang C., Han D., Ren Y. (2019). Estimation of growth parameters of Oratosquilla oratoria based on fishery-independent and -dependent data collected from the coast of Shandong Province. J. Fish. Sci. China 26, 756–764. doi: 10.3724/SP.J.1118.2019.19028
Goodwin N. B., Grant A., Perry A. L., Dulvy N. K., Reynolds J. D. (2006). Life history correlates of density-dependent recruitment in marine fishes. Can. J. Fish. Aquat. Sci. 63, 494–509. doi: 10.1139/f05-234
Guan W., Chen H., Ding H., Xuan F., Dai X. (2010). Reproductive characteristics and conditions of anadromous Coilia ectenes (Engraulidae) in Yangtze Estuary. Mar. Fish. 32, 73–81. doi: 10.3969/j.issn.1004-2490.2010.01.011
Guo H., Zhang X., Tang W., Li F., Shen L., Zhou T., et al. (2016). Temporal variations of coilia nasus catches at jingjiang section of the Yangtze river in fishing season in relation to environmental factors. Resour. Environ. Yangtze Val. 25, 1850–1859. doi: 10.11870/cjlyzyyhj201612008
Han Y., Shi J., Wu W., Lei J., Kong B. (2017). Characteristics and changes in the growth and biology of major fish species in Guangxi inland waters. Jiangsu Agric. Sci. 45, 155–160. doi: 10.15889/j.issn.1002-1302.2017.16.040
He Y., Yuan Y., Wang T., Zhang H., Chen Y. (2019). Integrated assessment of marine ecological vulnerability in the Yangtze River Estuary using GIS. Acta Ecol. Sin. 39, 3918–3925. doi: 10.5846/stxb201807091491
Hordyk A. R., Loneragan N. R., Prince J. D. (2015). An evaluation of an iterative harvest strategy for data-poor fisheries using the length-based spawning potential ratio assessment methodology. Fish. Res. 171, 20–32. doi: 10.1016/j.fishres.2014.12.018
Jiang T., Li H., Yang J., Chen X., Xue J., Liu H. (2023). Reappearance of anadromous Coilia nasus in the Xiangjiang River of Hunan Province. J. Fish. Sci. China 30, 1409–1416. doi: 10.12264/JFSC2023-0307
Jiang T., Liu H., Xuan Z., Chen X., Yang J. (2020). Classification of ecomorphotypes of from the middle and lower reaches of the Yangtze River Basin. J. Lake Sci. 32, 518–527. doi: 10.18307/2020.0220
Jiang T., Yang J., Xuan Z., Chen X., Liu H. (2022). Preliminary report on the effects of resource recovery on anadromous Coilia nasus in Poyang Lake under the national 10-year fishing ban. Prog. Fish. Sci. 43, 24–30. doi: 10.19663/j.issn2095-9869.20210119001
Ju P., Chen M., Tian Y., Zhao Y., Yang S., Xiao J. (2020). Stock status estimating of 5 shark species in the waters around Taiwan using a Length-Based Bayesian Biomass Estimation (LBB) Method. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00632
Kindong R., Gao C., Pandong N. A., Ma Q., Tian S., Wu F., et al. (2020). Stock status assessments of five small pelagic species in the Atlantic and Pacific oceans using the Length-Based Bayesian Estimation (LBB) Method. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.592082
Korkmaz B., Bolat Y., Cilbiz M. (2023). Length-based stock assessment for the Data-poor Crayfish fishery from the Eğirdir Lake, Turkiye. Turk. J. Fish. Aquat. Sci. 23, TrjfaS22354. doi: 10.4194/TRJFAS22354
Kumar R., Dineshbabu A. P., Rahangdale S., Vase V. K., Gohel J., Solanki V. (2022). Assessing low value crustacean bycatch species using Length Based Bayesian Biomass (LBB) Method, a Tool for Data Poor Fish Stock Assessment. Turk. J. Fish Aquat. Sci. 23, TrjfaS22189. doi: 10.4194/TRJFAS22189
Li J., Hu F., Lin N. (2015). Ecological distribution of fish larvae and juveniles in the Yangtze River Estuary and its adjacent waters in spring. South China Fish. Sci. 11, 1–8. doi: 10.3969/j.issn.2095-0780.2015.01.001
Li L., Wang L., Huang H., Zhang X., Feng C., Xu Y., et al. (2009). Study on the component of gill-net catches around the south region of the Yellow Sea in summer. Mar. Sci. 33, 36–40.
Liu F., Lin P., Li M., Gao X., Wang C., Liu H. (2019). Situations and conservation strategies of fish resources in the Yangtze River Basin. Acta Hydrobiol. Sin. 43, 144–156. doi: 10.7541/2019.177
Liu K., Duan J., Xu D., Zhang M., Fang D., Shi W. (2012). Present situation of Coilia nasus population features and yield in Yangtze River Estuary waters in fishing season. Chin. J. Ecol. 31, 3138–3143. doi: 10.13292/j.1000-4890.2012.0407
Liu S., Shen M., Liu X. (2023). Trophic niche analysis of fish in Taihu Lake using stable isotopes of carbon and nitrogen. J. Hydroecol. 44, 76–83. doi: 10.15928/j.1674-3075.202201240027
Luo B. (2024). Studies on fish resources of Chaohu Lake in the early period of Ten-year fishing ban in the Yangtze River. (master’s thesis). Guizhou University, Guizhou.
Ma F., Yang Y., Fang D., Ying C., Xu P., Liu K., et al. (2022). Characteristics of Coilia nasus resources after fishing ban in the Yangtze River. Acta Hydrobiol. Sin. 46, 1580–1590. doi: 10.7541/2023.2022.0070
Meffe G. K. (1986). Conservation genetics and the management of endangered fishes. Fisheries 11, 14–23. doi: 10.1577/1548-8446(1986)011<0014:CGATMO>2.0.CO;2
Mei Z., Cheng P., Wang K., Wei Q., Barlow J., Wang D. (2020). A first step for the Yangtze. Science 367, 1314. doi: 10.1126/science.abb5537
Meng Z., Yang D., Hu F., Chen K., Liu L., Xiang M., et al. (2023). Fish resources spatio–temporal distribution patterns and controlling factors in Zhelin reservoir, Lake Poyang Basin during early fishing ban period. J. Lake Sci. 35, 2071–2083. doi: 10.18307/2023.0635
Mildenberger T. K., Taylo M. H., Wolff M. (2017). TropFishR: an R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 8, 1520–1527. doi: 10.1111/2041-210X.12791
National Oceanic and Atmospheric Administration (2022).E. coli. Available online at: https://oceanwatch.pifsc.noaa.gov (Accessed December 25, 2022).
Osei I. K., Yankson K., Obodai E. A., Okyere I. (2021). Implications of overlooked seasonal growth dynamics in tropical fisheries assessment: A test case of an oyster (Crassostrea tulipa) fishery in the Densu Delta, Ghana. Fish. Res. 244, 106118. doi: 10.1016/j.fishres.2021.106118
Pauly D. (1980). On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 39, 175–192. doi: 10.1093/icesjms/39.2.175
Pauly D. (1985). On improving operation and use of the elefan programs. part 1, avoiding” drift” of K towards low values. Fishbyte 3, 13–14.
Phillips S. R. M., Scott F., Ellis J. R. (2015). Having confidence in productivity susceptibility analyses: A method for underpinning scientific advice on skate stocks? Fish. Res. 171, 87–100. doi: 10.1016/j.fishres.2015.01.005
Punt A. E., Smith D. C., Smith A. D. M. (2011). Among-stock comparisons for improving stock assessments of data-poor stocks: the “Robin Hood” approach. ICES J. Mar. Sci. 68, 972–981. doi: 10.1093/icesjms/fsr039
Qin X., Zhang C., Cui X., Song M., Gong D., Wu w., et al. (2024). The selectivity about separating mesh for fishes and shrimps of multi-anchored monolithic trap in Liaodong Bay. Modern Fish. 51, 106–112. doi: 10.3969/j.issn.1007-9580.2024.01.013
Ralston S., Punt A. E., Hamel O. S., DeVore J. D., Conser R. J. (2011). A meta-analytic approach to quantifying scientifc uncertainty in stock assessments. Fish. Bull. 109, 217–231.
Shi D., Zhang K., Cai Y., Xu Y., Sun M., Nan S., et al. (2022). Stock assessment of Collichthys lucidus in the Pearl River Estuary in data-limited conditions. Mar. Fish. 44, 435–445. doi: 10.13233/j.cnki.mar.fish.2022.04.005
Shi W., Zhang M., Liu k., Xu D., Duan J. (2009). Stress of hydraulic engineering on fisheries in the lower reaches of the Yangtze River and compensation. J. Lake Sci. 21, 10–20. doi: 10.3321/j.issn:1003-5427.20
Song C., Li Y., Zhao F., Liu R., Feng G., Huang X., et al. (2022). Reproductive population composition and reproductive performance of Coilia nasus from the Yangzte Estuary. J. Fish. Sci. China 29, 951–959. doi: 10.12264/JFSC2021-0412
Tong J., Chen J., Gao C., Dai L., Wang X. (2018). Temporal—spatial distribution of Coilia nasus in the Yangtze River Estuary based on habitat suitability index. J. Shanghai Ocean Univ. 27, 584–593. doi: 10.12024/jsou.20180202224
von Bertalanffy L. (1938). A quantitative theory of organic growth (inquiries on growth laws. II.). Hum. Biol. 10, 181–213. doi: 10.2307/41447359
Wang K., Zhang C., Sun M., Xu B., Ji Y., Xue Y., et al. (2021). Fishing pressure and lifespan affect the estimation of growth parameters using ELEFAN. Fish. Res. 238, 105903. doi: 10.1016/j.fishres.2021.105903
Wang K., Zhang C., Xu B., Xue Y., Ren Y. (2020). Selecting optimal bin size to account for growth variability in Electronic LEngth Frequency ANalysis (ELEFAN). Fish. Res. 225, 105474. doi: 10.1016/j.fishres.2019.105474
Wang L., Lin L., Li Y., Xing Y., Kang B. (2020). Sustainable exploitation of dominant fishes in the largest estuary in southeastern China. Water 12, 3390. doi: 10.3390/w12123390
Wang M., Cui M. (2019). Research progress on Coilia nasus in the Yangtze River of China. J. Anhui Agric. Sci. 47, 10–12. doi: 10.3969/j.issn.0517-6611.2019.24.004
Wang S., Song C., Zhang T., Gao Y., Zhuang P., Zhao F. (2023). Spatiotemporal distribution and catching status of elver (Anguilla japonica) in the Yangtze River Estuary. J. Fish. China 47, 171–182. doi: 10.11964/jfc.20220313355
Wang Y., Deng Y., Liu S., Li P., Liu K. (2023). Status analysis of fish community in the lower reaches of the Yangtze River at the beginning of 10-year fishing ban and assessment of fishing ban effect. J. Fish. China 47, 206–218. doi: 10.11964/jfc.20220913698
Wang Y., Wang Y., Liu S., Liang C., Zhang H., Xian W. (2020). Stock assessment using LBB method for eight fish species from the Bohai and Yellow seas. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00164
Xiong Y., Yang J., Jiang T., Liu H., Zhong X., Tang J. (2017a). Early life history of the small yellow croaker (Larimichthys polyactis) in sandy ridges of the South Yellow Sea. Mar. Biol. Res. 13, 993–1002. doi: 10.1080/17451000.2017.1319067
Xiong Y., Zhong X., Tang J., Jian Y., Li L. (2017b). Gillnet selectivity on the small yellow croaker Larimichthys polyactis in the Southern Yellow Sea. Turk. J. Fish. Aquat. Sci. 17, 1287–1296. doi: 10.4194/1303-2712-v17_6_22
Xuan Z., Jiang T., Liu H., Chen X., Yang J. (2021). Mitochondrial DNA and microsatellite analyses reveal strong genetic differentiation between two types of estuarine tapertail anchovies (Coilia) in Yangtze River Basin, China. Hydrobiologia 848, 1409–1431. doi: 10.1007/s10750-021-04541-w
Xuan Z., Jiang T., Liu H., Qiu C., Chen X., Yang J. (2020). Are there still anadromous the estuarine tapertail anchovies coilia nasus in Dongting Lake? Acta Hydrobiol. Sin. 44, 838–843. doi: 10.7541/2020.100
Yan X., Wang C., Shi J., Ge H., Wang Y., Zhu H. (2024). Tidal distribution characteristics of main economic fish species collected by elver net. J. Aquacult. 45, 22–27. doi: 10.3969/j.issn.1004-2091.2024.01.004
Yang S., Ye S., Xu J., Li M., Liu H. (2024). Evaluation of the effects on “The YEAR fishing ban” in Poyang Lake ecosystem based on Ecopath model. Acta Hydrobiol. Sin. 48, 1402–1420. doi: 10.7541/2024.2023.0429
Yang Y., Wang L., Li L., He S., Li X., Chen Y., et al. (2014). The influence factors of the resource of an endangered fish-silurus lanzhouensis and the technique and measures of natural population recovery. China Fish. Econ. 31, 144–150. doi: 10.3969/j.issn.1009-590X.2013.05.022
Ye S., Moreau J., Zeng W., Zhang T., Liu J., Li Z. (2014). Growth and mortality of two small fishes, Toxabramis swinhonis Günther 1873 and Hyporhamphus intermedius (Cantor 1842), in a Yangtze shallow lake (China) assessed by length frequency analysis. J. Appl. Ichthyol. 30, 479–484. doi: 10.1111/jai.12405
Yuan C. (1988). The changes in the resources and population composition of the knife fish in the middle and lower reaches of the Yangtze River and the reasons behind them. Chin. J. Zool., 12–15. doi: 10.13859/j.cjz.1988.03.005
Yuan C., Qin A., Liu R., Lin J. (1980). On the classification of the anchovies, Coilia, from the lower Yangtze River and the southeast coast of China. Acta Univ. Nankin. Sci. Nat., 67–82.
Zhai L., Li Z., Hu Y., Huang C., Tian S., Wan R., et al. (2022). Assessment of Coilia mystus and C. nasus in the Yangtze River Estuary, China, using a length-based approach. Fishes 7, 95. doi: 10.3390/fishes7030095
Zhai L., Li Z., Wan R., Tian S., Song P., Lin J. (2023). Effects of estuarine environmental heterogeneity on the habitat of Gobiidea species larvae. Mar. Coast. Fish. 15, e10241. doi: 10.1002/mcf2.10241
Zhai L., Liang C., Pauly D. (2020). Assessments of 16 exploited fish stocks in chinese waters using the CMSY and BSM Methods. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.483993
Zhang H., Kang M., Shen L., Wu J., Li J., Du H., et al. (2020). Rapid change in Yangtze fisheries and its implications for global freshwater ecosystem management. Fish Fish. 21, 601–620. doi: 10.1111/faf.12449
Zhang K., Li J., Hou G., Huang Z., Shi D., Chen Z., et al. (2021a). Length-based assessment of fish stocks in a Data-poor, jointly exploited (China and Vietnam) fishing ground, northern south China sea. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.718052
Zhang K., Zhang J., Shi D., Chen Z. (2021b). Assessment of Coral Reef fish stocks from the Nansha islands, south China sea, using length-based bayesian biomass estimation. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.610707
Zhang M., Xu D., Liu K., Shi W. (2005). Studies on biological characteristics and change of resource of coilia nasus schlegel in the lower reaches of the Yangtze River. Environ. Yangtze Val. 14, 694–698. doi: 10.3969/j.issn.1004-8227.2005.06.005
Keywords: 10-year fishing ban, Yangtze River, Coilia nasus, ELEFAN, LBB, resource recovery
Citation: Wang S, Xiong Y, Zhang H, Song D, Wang Y, Ge H, Zhang C, Liang L and Zhong X (2024) Recovery of Coilia nasus resources after implementation of the 10-year fishing ban in the Yangtze River: implied from the Yangtze River Estuary and its adjacent sea areas. Front. Mar. Sci. 11:1474996. doi: 10.3389/fmars.2024.1474996
Received: 02 August 2024; Accepted: 11 November 2024;
Published: 05 December 2024.
Edited by:
Hui Zhang, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Oumar Sadio, Institut de recherche pour le développement, SenegalChao Song, Chinese Academy of Fishery Sciences (CAFS), China
Yuan Li, Ministry of Natural Resources, China
Copyright © 2024 Wang, Xiong, Zhang, Song, Wang, Ge, Zhang, Liang and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Xiong, eXhpb25nc2hmdUAxMjYuY29t; Xiaming Zhong, b2NlYW54bXpoQDE2My5jb20=
 Shuyan Wang1,2
Shuyan Wang1,2 Ying Xiong
Ying Xiong Hushun Zhang
Hushun Zhang