- 1Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes of Ministry of Education, Hohai University, Nanjing, China
- 2College of Environment, Hohai University, Nanjing, China
- 3The National Key Laboratory of Water Disaster Prevention, Hohai University, Nanjing, China
Silver nanoparticles (AgNPs) are ubiquitous in the aquatic environment and have attracted extensive attention to their toxic effects on aquatic species. However, responses of the nervous system to AgNPs are little known, especially co-existing with the ubiquitous natural organic matter (NOM), which is critical for the ability to act in aquatic species. Here, this study investigated the neurotoxicology of environmentally relevant AgNPs with or without bovine serum albumin (BSA; a classical NOM) to zebrafish (Danio rerio) using visualized transgenic zebrafish. Exposure to AgNPs reduced the locomotor behavior of zebrafish by 28%–45%, including swimming distance and velocity, exhibiting obvious behavioral inhibition. The visualized transgenic zebrafish treated with AgNPs showed developmental retardation in the early development of the heart, central nervous, and motor nerve, as well as the related neurodevelopment genes, which may be responsible for the lowered locomotor behavior. In addition, AgNPs can specifically interfere with the cholinergic system and affect neuronal signaling, ultimately leading to behavioral abnormalities. However, the co-existing BSA alleviated the neurotoxicity of AgNPs in zebrafish, which may partially be attributed to the increased size and electronegativity of AgNPs caused by BSA, thus reducing the direct interaction of AgNPs with cells. The interaction between BSA and the released Ag+ from AgNPs may also be responsible for the alleviation of the neurodevelopment dysfunction in zebrafish. These findings provide valuable insights into the toxicity and risks of AgNPs in natural aquatic environments.
1 Introduction
Due to the unique physicochemical properties and excellent antimicrobial performance, silver nanoparticles (AgNPs) are widely used in daily life, including textiles, personal care products, medical devices, and building materials (Liu et al., 2023). It reports that more than 1,800 consumer products claim to contain AgNPs, with an annual production of over 800 tons (Khoshnamvand et al., 2020; Yan et al., 2023b). The widespread utilization of AgNPs has led to their abundant presence in different environments (Liu et al., 2023). The aquatic environment is an essential destination for AgNPs, which could inevitably interact with various aquatic biotas and thus cause varying degrees of damage to microbial systems, algae, invertebrates, and fish, including lipid peroxidation, inflammation, dysfunction, and even death (Gao et al., 2021; Shen et al., 2023). Increasing the environmental concentration of AgNPs in aquatic environments, concerns about their toxic effects on aquatic species are sharply enhanced.
Among the toxic effects, the neurotoxicity of different NPs has only recently come to light, because NPs can cross the blood–brain barrier and enter different nervous systems (Zhao et al., 2022). In aquatic organisms, the nervous system is critical for their ability to act, including locomotor behavior, social behavior, and anxiety and aggression; thus, the neurotoxicity of NPs may result in poor survival of aquatic organisms. Previous studies have shown the neurotoxicity of different NPs in aquatic organisms, including silica NPs, carbon nanodots, graphene oxide, and several metal-based NPs (Zhao et al., 2022). The neurotoxicity of AgNPs is only beginning to be revealed. Following exposure to AgNPs at environmentally relevant concentrations, the developing zebrafish (Danio rerio) exhibited transient hyperexcitability (González et al., 2018). In addition, a series of neurodevelopmental anomalies, including a small-eye phenotype, neuronal morphology defects, and inhibition of athletic abilities, were observed in zebrafish following exposure to AgNPs (Lu et al., 2023). Because AgNPs have size-dependent toxic effects on aquatic organisms (Xin et al., 2015), smaller ones could accumulate in the head and heart of Arctic char (Salvelinus alpinus), thus disrupting the cardiac pacemaker function and inhibiting the Na+/K+-ATPase activity, as well as the neuronal development in aquatic organisms (Parker et al., 2024). Zhao et al. (2019) suggested that the damage to the fish touch responses and the formation of neural circuits caused by AgNPs may be attributed to their release of Ag+. Janzadeh et al. (2022) proposed that Ag+ could act through increasing oxidative stress that produces apoptosis in the brain, leading to neurotoxicity in different animals. All of these studies suggested that AgNPs have the potential to induce neurotoxicity, which may further delay the social behavior of individuals and hinder the development of the population. Hence, understanding the potential neurotoxicity of AgNPs to aquatic organisms from different pathways is an important part of assessing their ecological risks prior to their safety applications.
Once entering into organisms, NPs have to encounter immediate and directly interact with many proteins (e.g., enzyme and transporter), forming a protein–NP complex and deciding the final biological effects of NPs on organisms (Tomak et al., 2021). Hence, exploring the influences of proteins on the effects caused by NPs on organisms and their interactions are topics of high relevance for their future applications in biomedicine. In addition, proteins also ubiquitously exist in natural water (Docter et al., 2015) as an important composition of natural organic matter (NOM). It has been reported that the stability, transformation, and toxicity of NPs could be altered by different NOM due to their interaction, forming “eco-coronas” on the surface of NPs (Ren et al., 2023). For instance, the presence of humic acid at low concentrations could improve the stability of AgNP suspension in waters by increasing the negative surface charge of AgNPs but leading to the aggregation and settling of AgNPs via the bridging effect at high concentrations (Dong et al., 2020). In the related biological effects, the forming eco-coronas could prevent NPs from directly interacting with biological cells, thus decreasing the toxicity of different NPs (e.g., graphene oxide NPs, polystyrene NPs, and copper NPs) toward Daphnia magna (Liang et al., 2020). In particular, the presence of NOM could combine with the Ag+ cation released from AgNPs and even transform it into homologous NPs, thereby attenuating the toxicity of AgNPs (Wirth et al., 2012; Liu et al., 2018). On the contrary, the presence of NOM may enhance the biocompatibility of NPs, leading to more NPs entering into organisms. Wang et al. (2015a) also reported that the occurrence of NOM caused more copper ions released from copper NPs and posed a higher toxicity to aquatic organisms. These contrasting effects highlight the importance of NOM in regulating the toxicity of NPs in aquatic organisms. However, research on the toxicity and mechanism of NPs in aquatic organisms in the presence of NOM is still limited, especially for the neurotoxicity of AgNPs.
Recently, studies on the neurotoxicity induced by different pollutants have sharply risen, and the zebrafish embryo has become a popular model organism for investigating neurodevelopmental and neurobehavioral toxicology (Zhao et al., 2022; Lin et al., 2023). In addition, zebrafish transgenic lines are also an extremely useful tool for determining the neurotoxicity of different NPs, which allows the visualization of neuronal development (Zhou et al., 2023). Therefore, in this study, zebrafish embryo and their different transgenic lines were selected as model organisms to study the neurotoxicity of AgNPs. Given the high proportion of proteins in aquatic NOM and their wide applications in stabilizing different engineered NPs, the interaction between proteins and NPs in aquatic environments is inevitable. Among these proteins, bovine serum albumin (BSA) is one of the most abundant proteins found in different waters and has been commonly used as a model protein (Yan et al., 2019; Ma et al., 2022). Hence, BSA was chosen as a representative NOM in this study. The main objective of the present study is to investigate the neurotoxicology of AgNPs at environmentally relevant concentrations to non-transgenic and transgenic zebrafish embryos in the presence of BSA and the underlying mechanism, focusing on the alterations in the neural and behavioral development using transgenic zebrafish lines. The responses of neurotransmitter and neurodevelopment-related genes in zebrafish at the early life stage were determined to reflect the underlying mechanism. The findings will provide valuable information for understanding the AgNPs neurotoxicity, especially in natural aquatic environments containing NOM.
2 Materials and methods
2.1 Chemicals
AgNPs solutions were purchased from XFNANO Materials Tech (Nanjing, China) with a concentration of 100 mg/L and a size of 15 ± 5 nm. Ag+ solutions (1 g/L) were obtained from Central Iron & Steel Research Institute (Beijing, China). BSA was purchased from Solarbio Science & Technology (Beijing, China). All exposure solutions were freshly prepared in dilution with sonication according to Organization for Economic Co-operation and Development (OECD) 203 before each test to obtain well-dispersed suspensions. Among them, the exposure solutions of BSA-coated AgNPs and Ag+ were prepared following the methods of Yi et al. (2016) with some modifications. Briefly, the stock solutions of AgNPs and Ag+ were diluted with ultrapure water and then mixed with BSA immediately. After mixing thoroughly, the combined solutions were shaken in an oscillation chamber for 1 h to obtain the solutions of BSA-coated AgNPs and BSA-coated Ag+, respectively. The morphology of AgNPs alone or in combined with BSA was characterized by transmission electron microscopy (TEM) (JEM-2100F, JEOL, Japan). Their zeta potentials and hydrodynamic diameters were examined using a Zetasizer NanoZS90 (Malvern, UK) during the water exchange periods.
Based on the probabilistic material flow analysis from a life-cycle perspective, the predicted environmental concentrations of AgNPs in surface water may be up to 10 μg/L (Maurer-Jones et al., 2013), with level in wastewater potentially as high as 65 μg/L (Tiede et al., 2012). Meanwhile, these predicted values were obtained two decades ago, and it is quite possible that they will have increased if evaluated again (Shevlin et al., 2018). Hence, AgNPs with a concentration of 50 μg/L were selected for this study, reflecting an environmentally relevant concentration as reported in previous studies (Huang et al., 2020; Tiede et al., 2012; Maurer-Jones et al., 2013), and have no acute toxicity in zebrafish larvae in our previous study (Yan et al., 2023b). Based on the detected concentrations of NOM in freshwater (1–10 mg/L) (Solomon et al., 2015) and the high concentration of related proteins (8.6 mg/L) (Zhu et al., 2018), the used concentration of BSA in this study was set at 5 mg/L, which is also environmentally relevant. In addition, because the Ag+ would be inevitably released from AgNPs and pose risks to different organisms, a comparative study with Ag+ was carried out to understand the underlying mechanisms governing the neurotoxicity of AgNPs to zebrafish. Our previous study showed that not more than 2.5 μg/L of Ag+ were released from AgNPs over 48 h (Yan et al., 2023b), a concentration of 2.5 μg/L Ag+ was used in this study to represent the highest release ratio of Ag+ from AgNPs.
2.2 Test organisms
The non-transgenic parent zebrafish (AB strain) were obtained from EzeRinka Biotechnology (Nanjing, China) and maintained in our laboratory for at least 6 months in a recirculation water system under controlled conditions, including temperature (28 ± 0.5°C), pH (7.0 ± 0.1), dissolved oxygen (DO; more than 6.5 mg/L), and a photoperiod of 14/10 h (light/dark). After spawning under light stimulation, embryos were collected and checked using a stereomicroscope (Changfang, China). The healthy embryos at 4 h post-fertilization (hpf) were used for further exposure. Meanwhile, three kinds of transgenic zebrafish embryos labeled with green fluorescence—Tg (cmlc2: EGFP), Tg (HuC: EGFP), and Tg (hb9: EGFP)—were selected to visualize the development of zebrafish heart, central nervous, and motor nerve. All these transgenic zebrafish embryos at 4 hpf were purchased from EzeRinka Biotechnology (Nanjing, China) and used for subsequent exposure. All exposure procedures involving fish were approved by the Animal Ethics Committee of Hohai University (Approval No. HH20230015).
2.3 Exposure experiment
In this study, four treatments were carried out, including control treatment (control), AgNPs treatment (AgNPs; spiked with AgNPs of 50 μg/L), BSA and AgNPs co-treatment (BSA-coated AgNPs; spiked with AgNPs of 50 μg/L and BSA of 5 mg/L), and BSA and Ag+ co-treatment (BSA-coated Ag+; spiked with Ag+ of 2.5 μg/L and BSA of 5 mg/L). For each treatment, 360 non-transgenic embryos were divided into three replicates (120 embryos per replicate) and exposed in glass petri dishes. The exposure experiments were conducted in an illumination incubator with a temperature of 28 ± 0.5°C, a photoperiod of 14/10-h light/dark, pH 7.0 ± 0.1, and DO of more than 6.5 mg/L. During exposure periods, all exposure solutions were replaced daily with a ratio of 90% v/v to maintain a constant exposure condition. The heart rate (48 hpf), hatching rate (72 hpf), and abnormalities (120 hpf) of embryos were recorded regularly using a stereomicroscope. In addition, the locomotor behavior of zebrafish larvae at 120 hpf was also analyzed. Subsequently, the remaining zebrafish larvae (120 hpf) from each replicate were taken and quickly frozen in liquid nitrogen for further determination of the neurodevelopmental and neurobehavioral gene expression, as well as the neurotransmitter contents.
For each kind of transgenic zebrafish, 120 embryos at 4 hpf were randomly assigned to the abovementioned four solutions containing 1-phenyl 2-thiourea (200 μM) to inhibit the production of melanin. Each treatment was carried out with three replicates simultaneously (30 embryos per replicate, 90 embryos in total per treatment). The rest of the exposure conditions were identical to the non-transgenic embryo treatment. At 96 hpf, transgenic zebrafish larvae were photographed by a fluorescence microscope (Nikon, DS-Qi2, Japan) to obtain the development images of zebrafish heart, central nervous, motor nerve, skeletal muscle, and fast muscle. The green fluorescence intensity of different transgenic larvae was measured at an excitation wavelength of 450 nm and an emission wavelength of 490 nm and calculated through the ImageJ software (National Institutes of Health, Maryland, USA).
2.4 Locomotor behavior test
The locomotor behavior of zebrafish larvae from each treatment was recorded in accordance with Yan et al. (2023a) using a ViewPoint ZebraLab behavior monitoring station (Life Sciences, France). Briefly, 20 non-transgenic zebrafish larvae from each replicate were selected. The fish were transferred into a 24-well plate with one larva per well. After acclimation for 10 min in the dark, the locomotor behavior of each larva was recorded under light-to-dark transitions (5 min illumination followed by 5 min dark) with three cycles without any shock or noise interference. The swimming speed (mm/s) and distance (mm) for each larva were analyzed using a video tracking system.
2.5 Neurotransmitter analyses
The contents of the neurotransmitter acetylcholine (ACh) and dopamine (DA) in non-transgenic zebrafish were determined using a diagnostic enzyme-linked immunosorbent assay kit obtained from Jiangsu Meimian Industrial Co., Ltd. (Nanjing, China). Briefly, 50 non-transgenic larvae from each replicate were homogenized in phosphate buffered saline solution with a ratio of 1: 9 (w: v). After centrifugation at 9,000 g for 10 min at 4°C, the supernatant was used for neurotransmitter determination. All measurement processes were strictly performed according to the manufacturer’s instructions.
2.6 Expression of nerve function genes
Another 50 non-transgenic zebrafish larvae from each replicate were collected and used for determination of the gene expression related to neurodevelopment in zebrafish, including neuronal differentiation (elav13 and ngn1), neuron axon and myelin sheath development (gap43, syn2a, and mbp), center nerve development (gfap and nestin), cholinergic system (chat, vacht, and ache), and dopaminergic system (th, dat, and mao). The expression of each target gene was quantified by the quantitative real-time polymerase chain reaction (qRT-PCR).
Total RNA extraction and cDNA synthesis were consistent with our previous studies (Yan et al., 2020, 2023a). The primer sequences of each target gene for qRT-PCR analysis are listed in Supplementary Table S1. The primer pairs were designed by Shanghai Biozeron (Shanghai, China). The qRT-PCR reactions were performed using the SsoFast EvaGreen Supermixes kit (Bio-Rad, USA) through a CFX96 Touch qRT-PCR determination system (Bio-Rad, USA). A total of 20-μL final volume was used for the amplification of each target gene, including 10 μL of SYBR Green Mix, 2 μL of Primer Mix, 3 μL of Nuclease-free water, and 5 μL of cDNA. The detailed reaction parameters and standard curve for each target gene are shown in the Supplementary Materials (Supplementary Table S2, Supplementary Figure S1). The relative expression level of all target genes was calculated by the method of 2−ΔΔCt, and the gene expression was presented as the normalized value against the reference gene (β-actin) and the control treatment.
2.7 Statistical analysis
Statistical analysis was carried out using SPSS 22.0 (Chicago, USA). All data were presented as mean ± standard deviation, and the Shapiro–Wilk test and Levene’s test were used to verify the normality and variance homogeneity. One-way analysis of variance was used to assess the statistical difference among all treatments, followed by Tukey’s post-hoc test (α = 0.05). A p < 0.05 was considered statistically significant.
3 Results and discussion
3.1 Properties of AgNPs
The TEM images revealed the morphology and size of the AgNPs alone or combined with BSA. As shown in Figure 1A, the AgNPs alone exhibited a spherical shape and dispersed well without significant agglomeration, with a diameter of 15 nm. In the presence of BSA, visible shadows were observed around AgNPs (Figure 1), indicating the existence of protein corona on the surface of AgNPs. In addition, the hydrodynamic sizes and zeta potentials of AgNPs alone or in combination with BSA were also determined (Figure 1). The hydrodynamic sizes of naked AgNPs were mostly distributed around 13 nm. In the presence of BSA, the hydrodynamic size of AgNPs sharply increased to 31 nm, suggesting that the surface of AgNPs was coated well by BSA. Meanwhile, the zeta potential of BSA-coated AgNPs was about −43 mV, whereas that of the naked AgNPs was about −29 mV. It suggests a significant decrease in the surface charge of BSA-coated AgNPs, which may lead to an increase in the electrostatic steric resistance between AgNPs and organism cells. During the water exchange periods (24 h), their zeta potentials and hydrodynamic diameters showed a stable behavior (Supplementary Figure S2). This finding demonstrates that the coated BSA could enhance the dispersity and stability of AgNPs in waters.
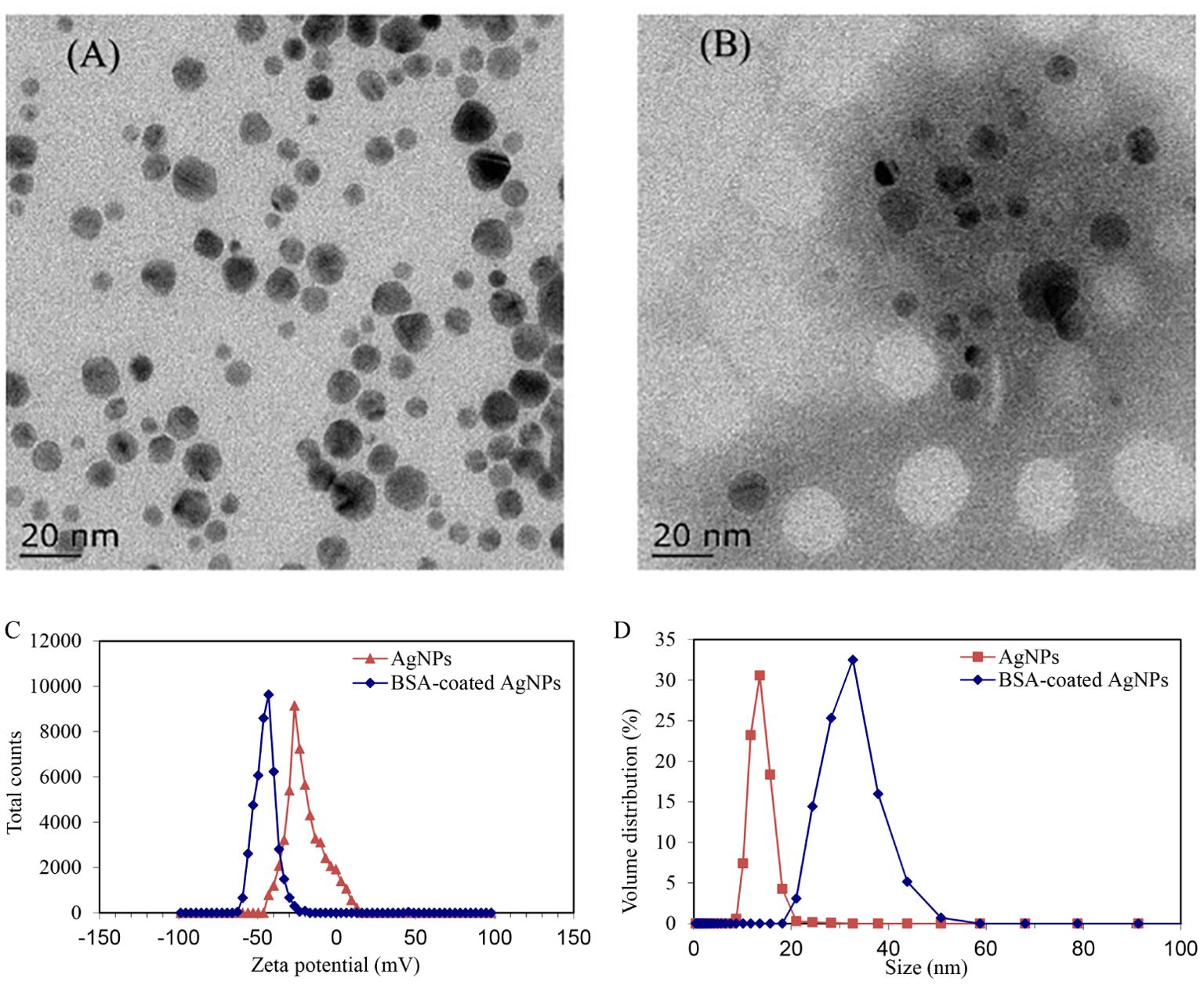
Figure 1. TEM characterization images of AgNPs (A) and the BSA-coated AgNPs (B), and the zeta potential (C) and size (D) of AgNPs before and after coating with BSA.
3.2 Development of zebrafish larvae
The development of zebrafish larvae in different treatments is shown in Supplementary Figure S3, including the 72 hpf hatching rate, 72 hpf heart rate, 120 hpf malformation rate, and 120 hpf body length. Compared to the control, exposure to AgNPs or Ag+ did not significantly alter the hatching rate, heart rate, abnormalities, and body length of zebrafish embryos. Although many studies have reported the negative effects of AgNPs on the development of zebrafish larvae, exposure to AgNPs at the environmentally relevant concentration may have no developmental toxicity in zebrafish larvae, which has been also observed in our previous study (Yan et al., 2023b).
3.3 Locomotor behavior of zebrafish larvae
Locomotor behavior is an essential tool to study the developmental neurotoxicity of zebrafish larvae, including the distance of movement and the mean velocity (Figure 2). Compared with the control, the total swimming distance of zebrafish was significantly reduced by AgNPs, with a decrease of 28%–45% in light and 21%–35% in dark. Similar inhibition was also observed in the swimming velocity. Because the locomotor behavior of zebrafish larvae was regulated by the central nervous system (CNS) (Zhao et al., 2022), this changed locomotor behavior suggests that AgNPs at the environmentally relevant concentration can cause impaired neurological function at the early stage of zebrafish. Chen et al. (2017) suggested that the suppressed behavior of zebrafish larvae may be attributed to reduced acetylcholinesterase activity or enhanced oxidative stress. Zhou et al. (2023) also found a correlation between abnormal neuronal development and the inhibition of swimming behavior. Therefore, the abnormal behavioral activity of zebrafish larvae caused by AgNPs may be regulated through a series of pathways, including neuron development, neurodevelopmental gene expression, and neurotransmitters. However, in the presence of BSA, the lowered swimming distance and velocity of zebrafish larvae caused by AgNPs were enhanced to control levels, suggesting a protective role of BSA. The increased particle size of AgNPs caused by BSA may further inhibit their uptake and accumulation in zebrafish, thus leading to an alleviative effect. In addition, co-exposure to BSA and Ag+ also resulted in a significant inhibition in the locomotor behavior of zebrafish, suggesting that the abnormal behavior of zebrafish caused by AgNPs may be not mainly attributed to their releasing Ag+.

Figure 2. Locomotor activity of zebrafish larvae in different treatments (control, AgNPs, BSA-coated AgNPs, and BSA-coated Ag+). Different lowercase letters (a, b) indicate significant differences (p < 0.05) between different treatments in the same time period.
3.4 Neural and behavioral development of zebrafish larvae
The transgenic zebrafish larvae are established tools to provide a visualization of neural development during their early development (Wu et al., 2019). To examine the structural and functional changes of neural and behavioral development of zebrafish larvae, several transgenic zebrafish lines involving heart development, central nervous, and motor nerves were used to probe the effects of AgNPs and the related influences of BSA.
3.4.1 Development of heart
The heart development is an essential endpoint for toxicity evaluation in zebrafish larvae, which is the first organ with fully developed function in the early stage of zebrafish and dominates the subsequent tissue development, including the nervous system (Peguera et al., 2021; Huang et al., 2022). Hence, the aim of this experiment was to investigate whether exposure to AgNPs could disturb the neural and behavioral development in zebrafish larvae by impacting heart development. As shown in Figure 3, exposure to AgNPs induced an increase of 7.3% in the values of fluorescence intensity in zebrafish heart when compared to the control, showing cardiac dysplasia caused by AgNPs. Similarly, Bowman et al. (2012) found that exposure to 100 μg/L AgNPs resulted in a buildup of fluid around the heart, cardiac hypertrophy, and heart rate reduction in zebrafish. In the presence of BSA, the enhanced fluorescence intensity in the heart by AgNPs was reduced to the control level, indicating that the formation of BSA corona on AgNPs could alleviate their toxic effect on zebrafish heart development. Such alleviation may be also attributed to the increased size of AgNPs caused by BSA coating, which can hinder the transport of AgNPs in zebrafish larvae across the blood–brain barrier. As a comparison, co-exposure to BSA and Ag+ resulted in an increase of 10.8% in the values of fluorescence intensity in the zebrafish heart, which was significantly higher than that co-exposure to BSA and AgNPs, suggesting that AgNPs may not mainly interfere with the zebrafish cardiac development through releasing Ag+.
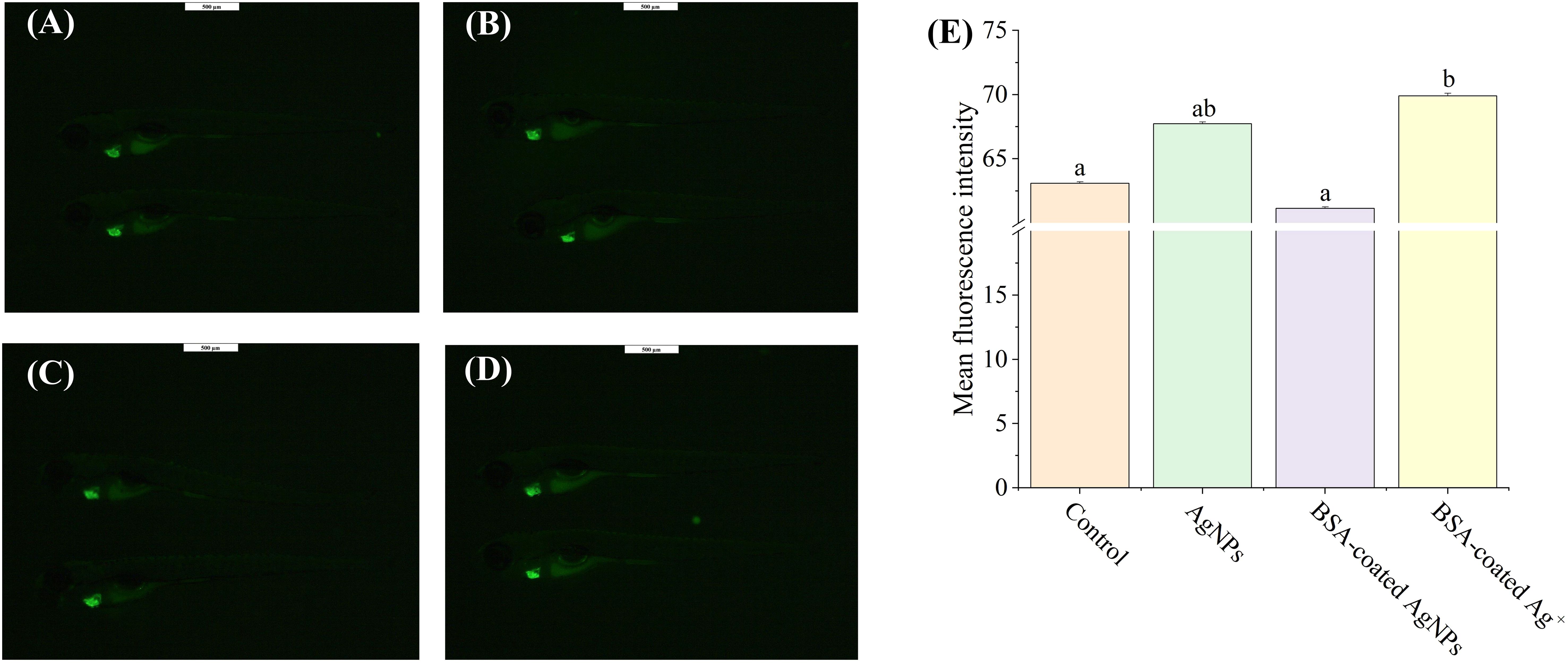
Figure 3. The images of heart development in Tg (cmlc2: EGFP) zebrafish larvae in the treatment of control (A), AgNPs (B), BSA-coated AgNPs (C), and BSA-coated Ag+ (D), and the fluorescence intensity of heart in zebrafish larvae in four treatments (E). Different letters indicate significant differences between different treatments (p < 0.05).
3.4.2 Development of central nervous
In zebrafish, neurogenesis is the process by which undifferentiated neural progenitor cells generate mature and functional neurons, which will eventually populate the mature CNS. In previous studies, the transgenic zebrafish Tg (HuC: EGFP) has been widely used to study the effects of pollutants on the development of CNS (Gu et al., 2022; Zhou et al., 2023). In this zebrafish, the EGFP expressed in the CNS is integrated into the promoter sequence of the elavl3 gene, which encodes the neuron-specific RNA-binding protein HuC, one of the first neuronal markers in zebrafish. Thus, the aim of this experiment was to investigate the influences of AgNPs on the neurogenesis in zebrafish larvae by examining their impact on the development of central nervous. In this study, the fluorescence intensity values of neurons in zebrafish larvae was significantly decreased by AgNPs (22.6%) compared to the control, indicative of an obvious inhibition for neuron growth and a delay in the neurogenesis (Figure 4). Similar results have been resulted from the small-sized NPs of titanium dioxide and plastics, which could also cross the blood–brain barrier and accumulate in the zebrafish brain, thus reducing the fluorescence in HuC-EGFP transgenic zebrafish and the number of neurons (Gu et al., 2022; Zhou et al., 2023). The reduced neurons caused by AgNPs in zebrafish may be attributed to the formation of hypoxic microenvironment in the embryo (Sun et al., 2021), which could further alter the development of the cardiovascular system and lead to brain hypoxia and death. The oxidative damage caused by AgNPs in zebrafish may also damage neuronal cells, resulting in a decrease in neuronal numbers (Liu et al., 2022). In addition, the apoptosis and autophagy pathways triggered by AgNPs may also be responsible for the neuronal death in zebrafish (Gupta et al., 2021; Rohde et al., 2021). After co-exposure to BSA, the fluorescence intensity of neurons was significantly enhanced by 12.0% compared to AgNPs alone, implying a retarding effect of BSA on the delaying development of central nerve in zebrafish caused by AgNPs. Given that the fluorescence intensity of zebrafish exposure to BSA-coated AgNPs was markedly higher than that treated with BSA-coated Ag+, the Ag+ released from AgNPs may be not the dominant factor for the reduction of neurons in zebrafish larvae.
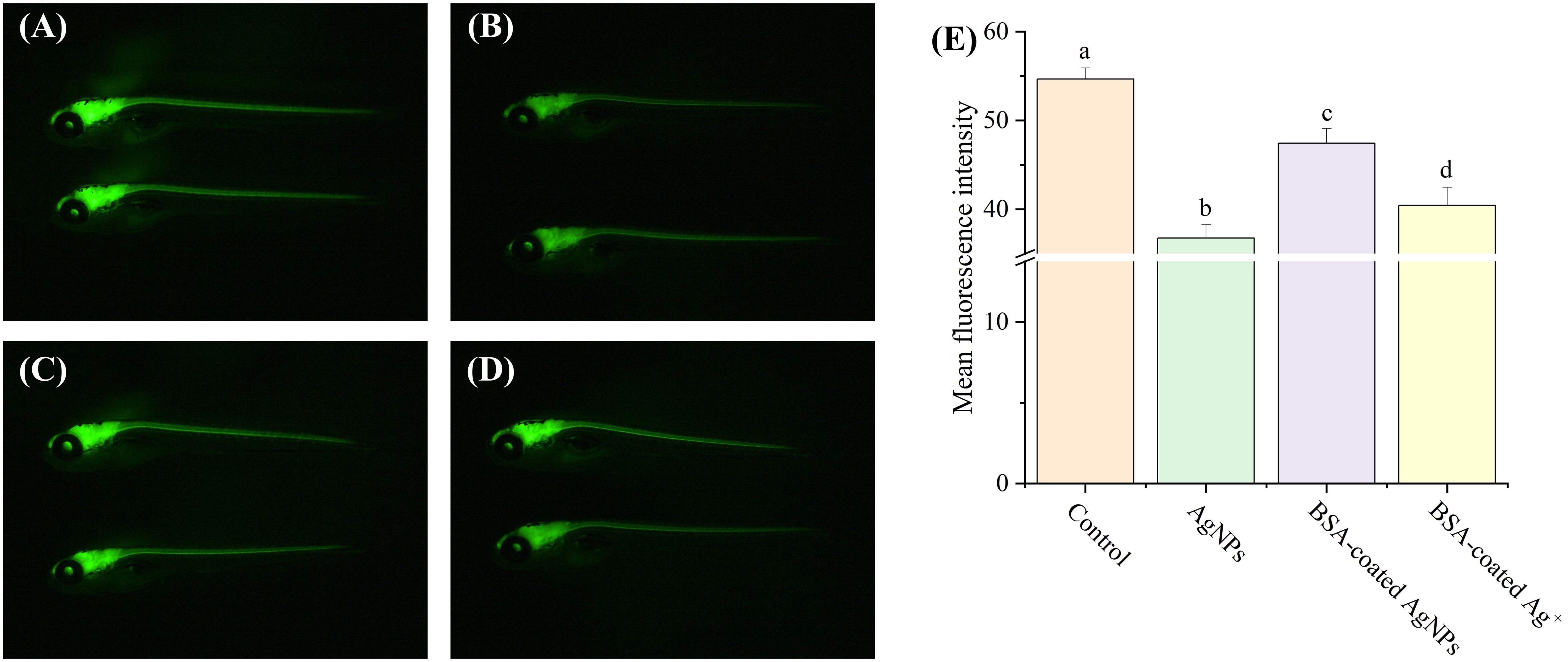
Figure 4. Development of neurons in transgenic Tg (HuC: eGFP) zebrafish larvae in the treatment of control (A), AgNPs (B), BSA-coated AgNPs (C), and BSA-coated Ag+ (D), and the fluorescence intensity of neuron in zebrafish larvae in four treatments (E). Different letters indicate significant differences between different treatments (p < 0.05).
3.4.3 Development of motor neurons
As one of the most important neurons in creatures, motor neurons directly participate in behavioral control (Fan et al., 2022). However, it remains unclear whether exposure to AgNPs could disrupt the behavioral control of zebrafish larvae by impairing motor neuron function. In this study, the Tg (hb9: EGFP) transgenic zebrafish embryos were selected to investigate motor nerve development. Compared to the control, the fluorescence intensity of the motor nerve was significantly lowered by AgNPs (Figure 5), which was further elevated by BSA-coated AgNPs. It indicates that AgNPs have a direct effect on axonal developmental damage in motor neurons, which could be alleviated by the co-existence of BSA. In zebrafish, the lowered locomotor behavior may be attributed to various factors, including impaired motor neuron development, changes in muscles or synapses (Berg et al., 2018). The inhibition of motor neuron development caused by AgNPs may partially explain the reduced locomotor behavior of zebrafish larvae. In addition to the increased size of AgNPs, the coated BSA could also enhance the electrostatic steric resistance between AgNPs and organisms, thus reducing AgNPs uptake and the related motor neurological dysfunction, as reported by Ding et al (Ding et al., 2019). Similar to BSA-coated AgNPs, co-exposure to BSA and Ag+ also enhanced the fluorescence intensity of motor nerve in zebrafish to the control levels, implying that the released Ag+ from AgNPs may be partially responsible for the reduction of motor neuron development in zebrafish larvae. A similar result has been reported by Taylor et al (Taylor et al., 2020), who found that the movement behavior of fish could be altered by metal ions through neurological dysfunction.
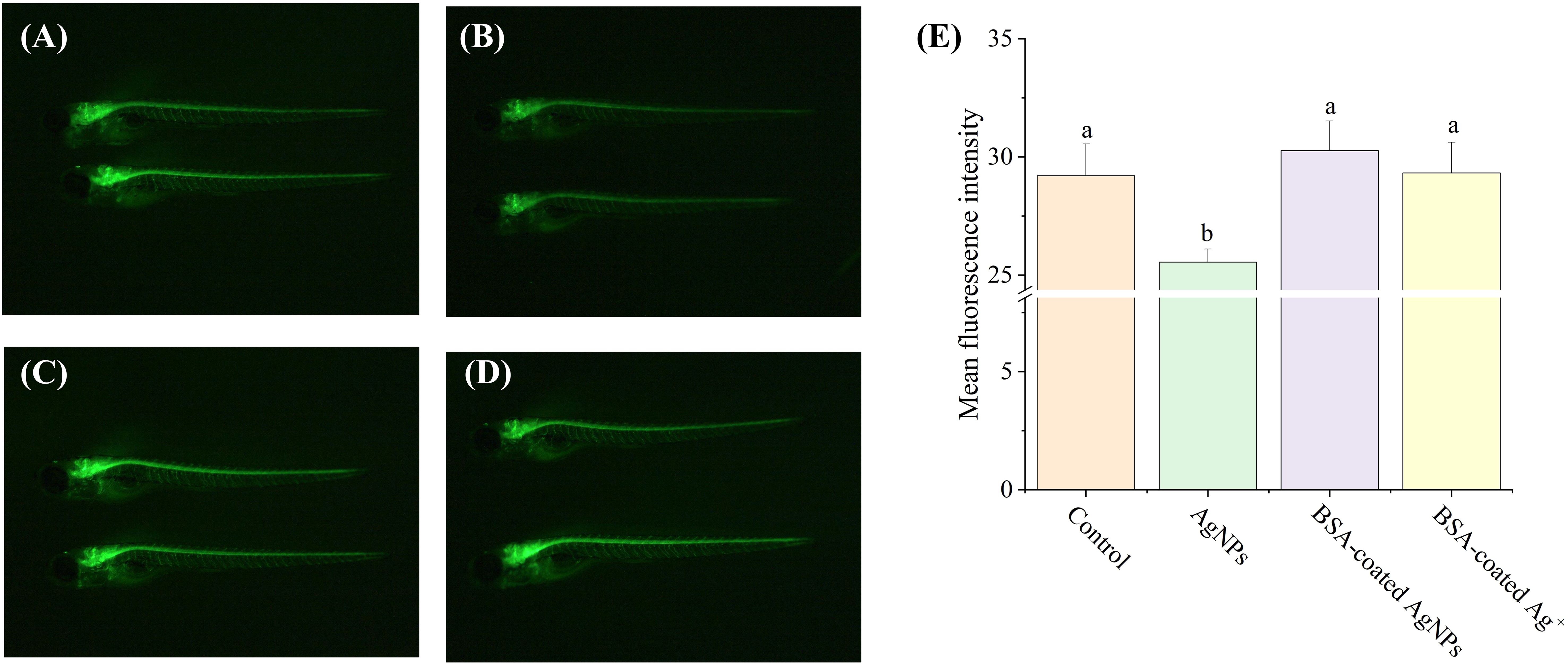
Figure 5. The fluorescence images of motor neurons in Tg (hb9: EGFP) transgenic zebrafish larvae in the treatment of control (A), AgNPs (B), BSA-coated AgNPs (C), and BSA-coated Ag+ (D), and the fluorescence intensity of motor nerve in zebrafish larvae in four treatments (E). Different letters indicate significant differences between different treatments (P < 0.05).
3.5 Expression of neurodevelopment genes
The expression of several functional genes related to neuronal differentiation, neuron axon and myelin sheath development, and nerve center development in zebrafish larvae are shown in Figure 6. Following exposure to AgNPs, the expression of gene elav like neuron-specific RNA binding protein 3 (elav13), neurogenin 1 (ngn1), and neuroepithelial stem cell protein (nestin) in zebrafish larvae were significantly decreased compared with the control, whereas the expression of glial fibrillary acidic protein (gfap) and growth associated protein 43 (gap43) gene was much higher than that in the control. The gene expressions of synapsin IIa (syn2a) and myelin basic protein (mbp) were not significantly different from that of the control. After co-exposure to BSA, these altered gene expressions were recovered gradually, showing an alleviative effect. In the combined treatment with BSA and Ag+, the expression of these target genes showed similar variation as that in the AgNPs treatment in most cases. These results indicate that the AgNPs may not only interfere with the expression of neurodevelopment genes in zebrafish larvae through the release of Ag+.
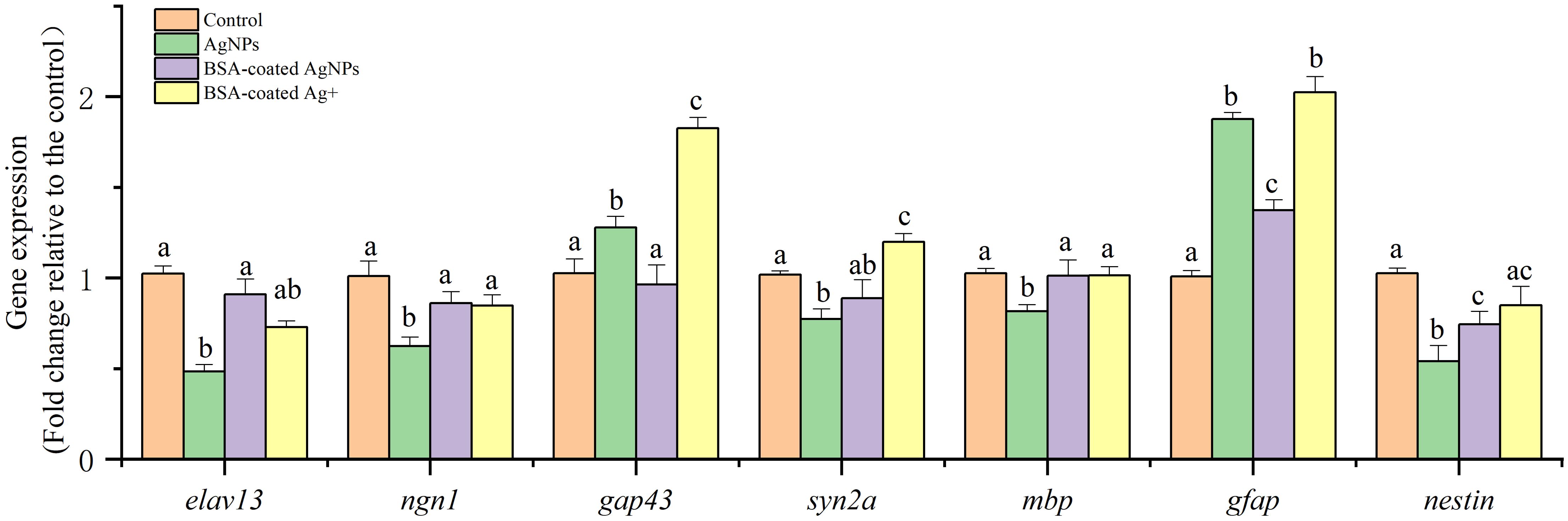
Figure 6. The expression of different functional genes related to nervous system development in zebrafish larvae. Different letters indicate statistically significant differences between different treatment groups in the same gene (p < 0.05).
During the early stage of zebrafish, the gene elav13 encodes the neural-specific RNA binding protein HuC, and ngn1 is a critical regulator of helix-loop transcription in neuronal differentiation (Kim et al., 2023). Both of two genes are considered as the biomarkers of early neurons in zebrafish (Wang et al., 2022; Kim et al., 2023). Hence, the lowered expression of gene elav13 and ngn1 caused by AgNPs suggests that exposure to AgNPs could disrupt the differentiation of neuronal cells from neurons, which is consistent with the neuroimaging data in the transgenic zebrafish Tg (HuC: EGFP) larval. Subsequently, the presence of BSA alleviated the inhibition of AgNPs on the gene expression elav13 and ngn1, showing protection in neuronal differentiation.
After neuronal differentiation, the genes of gap43, syn2a, and mbp were transcribed to regulate the synaptic/axonogenesis in motor neurons and have been used as markers to reflect the growth and development of motor neurons (Zhou et al., 2023). Among these genes, gap43 gene regulates neuronal regeneration, helping to reconnect the damaged neurons. Hence, the enhanced expression of gene gap43 implies damage of motor neurons in zebrafish after exposure to AgNPs. This result is consistent with the decreased fluorescence intensity in the Tg (hb9: EGFP) transgenic zebrafish. In addition, the gene of syn2a is a marker of synaptogenesis (Wang et al., 2015b), and the mbp gene is a crucial marker to show the myelination of axons in the developing CNS (Song et al., 2022). Because the gene expressions of syn2a and mbp in larvae were not significantly changed, exposure to AgNPs may have no effect on the synaptogenesis and myelination of axons. Hence, exposure to AgNPs may eventually lead to damage in motor neurons and abnormal motor behavior in zebrafish larvae through regulating the gene expression of gap43, but not by the syn2a and mbp. Consistent with neuronal differentiation, the presence of BSA showed an alleviated effect on the motor neuron development suppressed by AgNPs.
In addition, the gene expression of gfap and nestin was tested to investigate the center nerve development. In which, the gfap gene is crucial a biomarker of central nervous astrocytes, which could provide mechanical support in the CNS (Hu et al., 2022). In the CNS, the protein encoded by gfap gene could inhibit the proliferation of brain neurons and axon extension, thus isolating the damaged cells by forming a physical barrier (Brenner, 2014). Hence, the activated gene expression of gfap in zebrafish caused by AgNPs also suggests damage in the center nerve cells. Given the gene nestin is highly expressed in neural precursor cells, its expression has been used as one of the fast and sensitive indicators for nervous system lesions and injuries (Zheng et al., 2023). In this study, the significantly downregulated nestin gene expression in zebrafish caused by AgNPs implies a developmentally deficient of the center nerve.
Therefore, exposure to AgNPs could change the neurodevelopment of zebrafish larvae by inhibiting neuronal differentiation, regulating the synaptic/axonogenesis in motor neurons, and disturbing the nerve center development, thereby leading to motor neuron damage and abnormal motor behavior. However, these abovementioned gene changes were significantly alleviated by the presence of BSA, which could enhance the size of AgNPs and the electrostatic steric resistance with organism cells, thus lowering their uptake in cells and related neurodevelopment dysfunction. The similar gene changes in zebrafish treated with AgNPs and Ag+ in the presence of BSA in most cases also indicate that the released Ag+ may not be the only way to interfere with the neurodevelopment genes by AgNPs.
3.6 Development of neurotransmitter systems
In vertebrates, neurotransmitters play a crucial role in regulating electrical signals by controlling the activity of ion channels in neurons and mediate intercellular communication through surface receptors, making them ideal molecules to regulate behaviors (Li et al., 2020). To explore the regulation of neurotransmitter systems in the neural and behavioral abnormalities in zebrafish, the responses of cholinergic and dopaminergic systems were analyzed, including the contents of acetylcholine (ACh) and dopamine (DA) and the related regulatory genes in different treatments. As shown in Figure 7, the ACh content of zebrafish larvae treated with AgNPs was significantly decreased by 35% when compared to the control. In the presence of BSA, the decreased ACh content caused by AgNPs in zebrafish returned to the baseline, as well as that in the Ag+ treatment. For the DA content in zebrafish, there are no significant changes in any treatments.
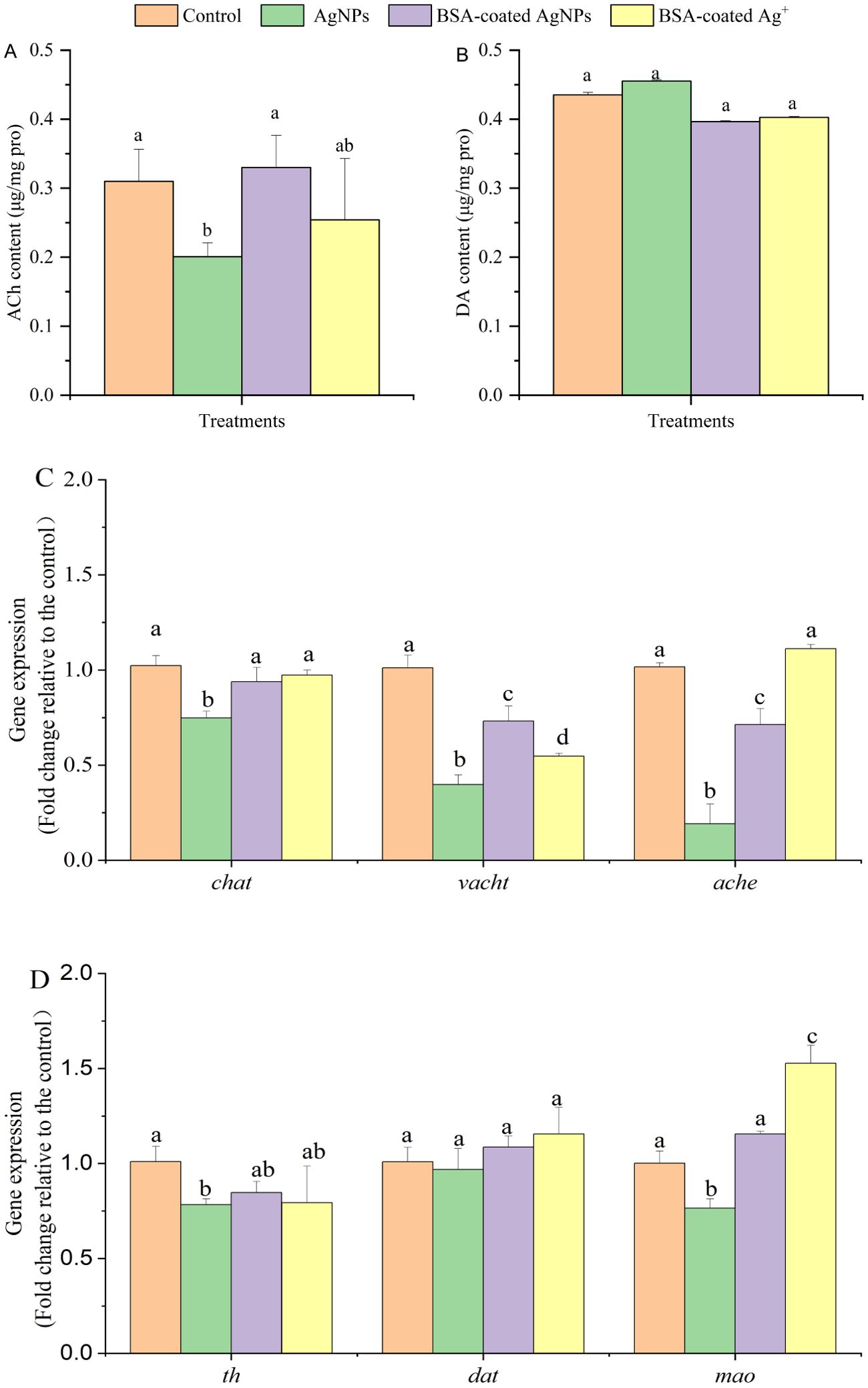
Figure 7. The content of Ach (A) and DA (B) and the related gene expression (C, D) in zebrafish larvae in different treatments. Different letters indicate significant differences between different treatments in the same gene (p < 0.05).
It has been reported that the alterations in the contents of ACh and DA are directly connected to the neurobehavioral changes in aquatic organisms, including learning, memory, and motor behavior (Wang et al., 2023). The decreased ACh content in organisms has been connected with the deterioration of cognitive, autonomic, and neuromuscular function, which may lead to Alzheimer’s disease (Jiang et al., 2023). Hence, the decreased ACh content caused by AgNPs may be partly responsible for the lowered locomotor behavior of zebrafish. In addition to regulate the neuronal pathways, the ACh may also work as a signaling molecule in non-neuronal cells, including O2 chemosensing and immunomodulation (Capillo et al., 2021). The lowered ACh content also suggests negative effects of AgNPs on the non-neuronal pathways in zebrafish. In aquatic organisms, the content of ACh is synthesized by choline acetyltransferase (chat), transported by vesicular Ach transporter (vacht), and degraded by acetylcholinesterase (ache), thus regulating the neurobehavioral through the cholinergic system (Ding et al., 2023). In this study, the gene expression of chat, vacht, and ache was downregulated by 26%, 60%, and 81% by AgNPs when compared with the control (Figure 7). The results suggest that AgNPs may decrease the ACh content in zebrafish larvae through inhibiting the synthesis by chat, thereby leading to a lowered transport of ACh by vacht and degradation by ache in nerve cells. When the BSA was coated, the lowered gene expressions were enhanced, although they were still lower than those in the control. The returned ACh content caused by BSA suggested that not all of these changed genes could be transcribed in the cholinergic system of zebrafish. The presence of BSA may alleviate the declined locomotor behavior of zebrafish resulting from AgNPs by stimulating the synthesis and transport of ACh. Considering that the recovery of cholinergic genes in the Ag+ treatment was similar to that in the AgNPs treatment with BSA, the released Ag+ may be partly responsible for the inhibition in the cholinergic system by AgNPs.
Similar to ACh, DA is the primary neurotransmitter of the dopaminergic system in the central nervous and directly regulates the learning, cognition, and motor functions in organisms (Tao et al., 2022). Barsagade (2020) also suggested that the presence of DA in the fish brain could alter the reproductive activity of fish by regulating estrogen synthesis through its receptors. Previous studies have reported that the reduced DA contents could decrease the swimming speed of zebrafish and even lead to the motor symptoms of classic Parkinson’s disease (Stednitz et al., 2015; Rademacher and Nakamura, 2024). In the present study, the DA content of zebrafish larvae was not changed by the exposed AgNPs alone or in combination with BSA, indicating that AgNPs at this environmentally relevant concentration may not induce neurotoxicity and behavioral abnormalities through regulating the dopaminergic system. However, the related gene expression of DA in zebrafish was markedly changed by AgNPs. Similar to the cholinergic system, the synthesis, transport, and degradation of DA in organisms were regulated by the genes of tyrosine hydroxylase (th), dopamine transporter (dat), and encoding monoamine oxidase (mao), respectively (Zhou et al., 2023). Compared with the control, the expression of th and mao was downregulated by AgNPs, with a decrease of 22% and 24%, respectively, whereas the dat gene expression was not changed. It suggests that there may be a balance between the simultaneous decrease of DA synthesis and degradation in zebrafish, thus maintaining the stability of DA content and, therefore, not altering the related transport process. In addition, these decreased gene expression was also enhanced by the presence of BSA, further indicating an alleviative effect on the dopaminergic system in zebrafish larvae. Because similar enhancement in the dopaminergic genes was also observed in the treatment with BSA and Ag+, the presence of BSA may alleviate the inhibition in the dopaminergic system through interacting with the released Ag+ from AgNPs. Considering that toxicity of AgNPs in natural aquatic environments could be also altered by various environmental factors, such as pH and illumination, research should prioritize investigating the influences of BSA on the neurotoxicity of AgNPs under different environmental factors and reveal the underlying mechanisms. Additionally, more attention should be paid to the key neural pathways in future studies, given the neurodevelopment is extremely complex in aquatic organisms.
4 Conclusions
This study investigated the influences of BSA on the neurotoxicity of AgNPs in zebrafish larvae. The results suggest that exposure to AgNPs at environmentally relevant concentration significantly lowered the locomotor behavior of zebrafish larvae. The development of the heart, central nervous, and motor nerve of zebrafish larvae were also inhibited significantly by AgNPs through inhibiting the neuronal differentiation, regulating the synaptic/axonogenesis in motor neurons, and disturbing the nerve center development, which may partially be responsible for the lowered locomotor behavior. In addition, AgNPs may also result in neural and behavioral abnormalities in zebrafish by regulating the neurotransmitter cholinergic systems. However, the neurotoxicity of AgNPs could be markedly alleviated by the ubiquitous BSA in waters. The increased size and electronegativity of AgNPs caused by BSA may reduce the uptake and residual of AgNPs in cells, thereby alleviating the neurodevelopment dysfunction. The similar variations in neurotoxicity of zebrafish treated with AgNPs and Ag+ in the presence of BSA suggest that BSA could also improve the neurodevelopment and behavioral development of zebrafish larvae through interacting with the released Ag+. These findings imply that future risk assessments and safety evaluations of AgNPs should incorporate the influences of BSA or other NOMs in natural aquatic environments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the Animal Ethics Committee of Hohai University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZY: Writing – review & editing, Writing – original draft. YC: Writing – original draft. YY: Writing – review & editing, Methodology, Investigation. YZ: Writing – review & editing. PS: Writing – review & editing. SY: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the National Key Research and Development Program of China (Grant 2022YFC3202602), the National Natural Science Foundation of China (42377389, U2340221), the Fundamental Research Funds for the Central Universities (B230201057), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX24_0899).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1473054/full#supplementary-material
References
Barsagade V. G. (2020). Dopamine system in the fish brain: A review on current knowledge. J. Entomol. Zool. Stud. 8, 2549–2555. doi: 10.22271/j.ento.2020.v8.i5ag.9098
Berg E. M., Björnfors E. R., Pallucchi I., Picton L. D., El Manira A. (2018). Principles governing locomotion in vertebrates: Lessons from zebrafish. Front. Neural Circuits 12. doi: 10.3389/fncir.2018.00073
Bowman C. R., Bailey F. C., Elrod-Erickson M., Neigh A. M., Otter R. R. (2012). Effects of silver nanoparticles on zebrafish (Danio rerio) and Escherichia coli (ATCC 25922): A comparison of toxicity based on total surface area versus mass concentration of particles in a model eukaryotic and prokaryotic system. Environ. Toxicol. Chem. 31, 1793–1800. doi: 10.1002/etc.1881
Brenner M. (2014). Role of GFAP in CNS injuries. Neurosci. Lett. 565, 7–13. doi: 10.1016/j.neulet.2014.01.055
Capillo G., Zaccone G., Cupello C., Fernandes J. M. O., Viswanath K., Kuciel M., et al. (2021). Expression of acetylcholine, its contribution to regulation of immune function and O2 sensing and phylogenetic interpretations of the African butterfly fish Pantodon buchholzi (Osteoglossiformes, Pantodontidae). Fish Shellfish Immun. 111, 189–200. doi: 10.1016/j.fsi.2021.02.006
Chen Q. Q., Gundlach M., Yang S. Y., Jiang J., Velki M., Yin D. Q., et al. (2017). Quantitative investigation of the mechanisms of microplastics and nanoplastics toward zebrafish larvae locomotor activity. Sci. Total Environ. 584, 1022–1031. doi: 10.1016/j.scitotenv.2017.01.156
Ding P., Xiang C. D., Li X. T., Chen H. B., Shi X. X., Li X., et al. (2023). Photoaged microplastics induce neurotoxicity via oxidative stress and abnormal neurotransmission in zebrafish larvae. Sci. Total Environ. 881, 163480. doi: 10.1016/j.scitotenv.2023.163480
Ding Y. Y., Bai X., Ye Z. F., Gong D. Q., Cao J. J., Hua Z. L. (2019). Humic acid regulation of the environmental behavior and phytotoxicity of silver nanoparticles to Lemna minor. Environ. Sci. Nano 6, 3712–3722. doi: 10.1039/c9en00980a
Docter D., Strieth S., Westmeier D., Hayden O., Gao M. Y., Knauer S. K., et al. (2015). No king without a crown - impact of the nanomaterial-protein corona on nanobiomedicine. Nanomedicine 10, 503–519. doi: 10.2217/nnm.14.184
Dong B., Liu G. F., Zhou J. T., Wang J., Jin R. F. (2020). Transformation of silver ions to silver nanoparticles mediated by humic acid under dark conditions at ambient temperature. J. Hazard. Mater. 383, 121190. doi: 10.1016/j.jhazmat.2019.121190
Fan B. Y., Dai L. L., Liu C. S., Sun Q., Yu L. Q. (2022). Nano-TiO2 aggravates bioaccumulation and developmental neurotoxicity of triphenyl phosphate in zebrafish larvae. Chemosphere 287, 132161. doi: 10.1016/j.chemosphere.2021.132161
Gao Y. F., Wu W. R., Qiao K. X., Feng J. F., Zhu L., Zhu X. S. (2021). Bioavailability and toxicity of silver nanoparticles: Determination based on toxicokinetic-toxicodynamic processes. Water Res. 204, 117603. doi: 10.1016/j.watres.2021.117603
González E. A., Carty D. R., Tran F. D., Cole A. M., Lein P. J. (2018). Developmental exposure to silver nanoparticles at environmentally relevant concentrations alters swimming behavior in zebrafish (Danio rerio). Environ. Toxicol. Chem. 37, 3018–3024. doi: 10.1002/etc.4275
Gu J., Guo M., Yin X. G., Huang C. X., Qian L. L., Zhou L. J., et al. (2022). A systematic comparison of neurotoxicity of bisphenol A and its derivatives in zebrafish. Sci. Total Environ. 805, 150210. doi: 10.1016/j.scitotenv.2021.150210
Gupta R., Ambasta R. K., Pravir K. (2021). Autophagy and apoptosis cascade: Which is more prominent in neuronal death? Cell Mol. Life Sci. 78, 8001–8047. doi: 10.1007/s00018-021-04004-4
Hu B., Lelek S., Spanjaard B., El-Sammak H., Simoes M. G., Mintcheva J., et al. (2022). Origin and function of activated fibroblast states during zebrafish heart regeneration. Nat. Genet. 54, 1227-1237. doi: 10.1038/s41588-022-01129-5
Huang Y., Wang Z. Q., Peng Y. Y., Xu R., Yan J. J., Xiong C., et al. (2022). Carboxin can induce cardiotoxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 233, 113318. doi: 10.1016/j.ecoenv.2022.113318
Huang Z. Z., Zeng Z. T., Song Z. X., Chen A. W., Zeng G. M., Xiao R., et al. (2020). Antimicrobial efficacy and mechanisms of silver nanoparticles against Phanerochaete chrysosporium in the presence of common electrolytes and humic acid. J. Hazard. Mater. 383, 121153. doi: 10.1016/j.jhazmat.2019.121153
Janzadeh A., Behroozi Z., Saliminia F., Janzadeh N., Arzani H., Tanha K., et al. (2022). Neurotoxicity of silver nanoparticles in the animal brain: a systematic review and meta-analysis. Forensic Toxicol. 40, 49–63. doi: 10.1007/s11419-021-00589-4
Jiang N., Li X. X., Wang Q., Baihetiyaer B., Fan X. T., Li M. S., et al. (2023). Ecological risk assessment of environmentally relevant concentrations of propofol on zebrafish at early life stage: Insight into physiological, biochemical, and molecular aspects. Chemosphere 316, 137846. doi: 10.1016/j.chemosphere.2023.137846
Khoshnamvand M., Hao Z. N., Fadare O. O., Hanachi P., Chen Y. S., Liu J. F. (2020). Toxicity of biosynthesized silver nanoparticles to aquatic organisms of different trophic levels. Chemosphere 258, 127346. doi: 10.1016/j.chemosphere.2020.127346
Kim J., Bang J., Ryu B., Kim C. Y., Park J. H. (2023). Flubendazole exposure disrupts neural development and function of zebrafish embryos. Sci. Total Environ. 898, 165376. doi: 10.1016/j.scitotenv.2023.165376
Li F., Eriksen J., Finer-Moore J., Chang R., Nguyen P., Bowen A. (2020). Ion transport and regulation in a synaptic vesicle glutamate transporter. Science 368, 893–897. doi: 10.1126/science.aba9202
Liang D. Y., Wang X. R., Liu S., Zhu Y., Wang Y., Fan W. H., et al. (2020). Factors determining the toxicity of engineered nanomaterials to Tetrahymena thermophila in freshwater: the critical role of organic matter. Environ. Sci. Nano 7, 304–316. doi: 10.1039/c9en01017c
Lin W. T., Huang Z. S., Zhang W. Q., Ren Y. (2023). Investigating the neurotoxicity of environmental pollutants using zebrafish as a model organism: A review and recommendations for future work. Neurotoxicology 94, 235–244. doi: 10.1016/j.neuro.2022.12.009
Liu S. Q., Miao L. Z., Li B. L., Shan S. J., Li D. P., Hou J. (2023). Long-term effects of AgNPs on denitrification in sediment: Importance of AgNPs exposure ways in aquatic ecosystems. Water Res. 242, 120283. doi: 10.1016/j.watres.2023.120283
Liu Y. X., Wang Y. H., Li N., Jiang S. N. (2022). Avobenzone and nanoplastics affect the development of zebrafish nervous system and retinal system and inhibit their locomotor behavior. Sci. Total Environ. 806, 150681. doi: 10.1016/j.scitotenv.2021.150681
Liu Y. L., Yang T., Wang L., Huang Z. S., Li J., Cheng H. J., et al. (2018). Interpreting the effects of natural organic matter on antimicrobial activity of Ag2S nanoparticles with soft particle theory. Water Res. 145, 12–20. doi: 10.1016/j.watres.2018.07.063
Lu C. J., Liu Y., Liu Y., Kou G. H., Chen Y., Wu X. W., et al. (2023). Silver nanoparticles cause neural and vascular disruption by affecting key neuroactive ligand-receptor interaction and VEGF signaling pathways. Int. J. Nanomed. 18, 2693–2706. doi: 10.2147/Ijn.S406184
Ma L. F., Peng F. Y., Dong Q. Q., Li H. P., Yang Z. G. (2022). Identification of the key biochemical component contributing to disinfection byproducts in chlorinating algogenic organic matter. Chemosphere 296, 133998. doi: 10.1016/j.chemosphere.2022.133998
Maurer-Jones M. A., Gunsolus I. L., Murphy C. J., Haynes C. L. (2013). Toxicity of engineered nanoparticles in the environment. Anal. Chem. 85, 3036–3049. doi: 10.1021/ac303636s
Parker K. S., El N., Buldo E. C., Maccormack T. J. (2024). Mechanisms of PVP-functionalized silver nanoparticle toxicity in fish: Intravascular exposure disrupts cardiac pacemaker function and inhibits Na/K-ATPase activity in heart, but not gill. Comp. Biochem. Physiol. 277, 109837. doi: 10.1016/j.cbpc.2024.109837
Peguera B., Segarra M., Acker-Palmer A. (2021). Neurovascular crosstalk coordinates the central nervous system development. Curr. Opin. Neurobiol. 69, 202–213. doi: 10.1016/j.conb.2021.04.005
Rademacher K., Nakamura K. (2024). Role of dopamine neuron activity in Parkinson’s disease pathophysiology. Exp. Neurol. 373, 114645. doi: 10.1016/j.expneurol.2023.114645
Ren J. Y., Bao Q. D., Yang Y., Tang Y. Q., Zhang N., Liu G. L., et al. (2023). Nano-eco interactions: a crucial principle for nanotoxicity evaluation. Environ. Sci. Nano 10, 3253–3270. doi: 10.1039/d3en00617d
Rohde M. M., Snyder C. M., Sloop J., Solst S. R., Donati G. L., Spitz D. R., et al. (2021). The mechanism of cell death induced by silver nanoparticles is distinct from silver cations. Part. Fibre. Toxicol. 18, 37. doi: 10.1186/s12989-021-00430-1
Shen L., Li Q. Q., Kang Y. H., Xiang Q. Q., Luo X., Chen L. Q. (2023). Metabolomics reveals size-dependent persistence and reversibility of silver nanoparticles toxicity in freshwater algae. Aquat. Toxicol. 258, 106471. doi: 10.1016/j.aquatox.2023.106471
Shevlin D., O’Brien N., Cummins E. (2018). Silver engineered nanoparticles in freshwater systems-Likely fate and behaviour through natural attenuation processes. Sci. Total Environ. 621, 1033–1046. doi: 10.1016/j.scitotenv.2017.10.123
Solomon C. T., Jones S. E., Weidel B. C., Buffam I., Fork M. L., Karlsson J., et al. (2015). Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: Current knowledge and future challenges. Ecosystems 18, 376–389. doi: 10.1007/s10021-015-9848-y
Song Y., Liu S. Y., Jiang X., Ren Q. Y., Deng H. Y., Paudel Y. N., et al. (2022). Benzoresorcinol induces developmental neurotoxicity and injures exploratory, learning and memorizing abilities in zebrafish. Sci. Total Environ. 834, 155268. doi: 10.1016/j.scitotenv.2022.155268
Stednitz S. J., Freshner B., Shelton S., Shen T., Black D., Gahtan E. (2015). Selective toxicity of L-DOPA to dopamine transporter-expressing neurons and locomotor behavior in zebrafish larvae. Neurotoxicol. Teratol. 52, 51–56. doi: 10.1016/j.ntt.2015.11.001
Sun M. Q., Ding R. Y., Ma Y. M., Sun Q. L., Ren X. K., Sun Z. W., et al. (2021). Cardiovascular toxicity assessment of polyethylene nanoplastics on developing zebrafish embryos. Chemosphere 282, 131124. doi: 10.1016/j.chemosphere.2021.131124
Tao M. Z., Dou K. X., Xie Y. J., Hou B. H., Xie A. M., In P. S. P. M. (2022). The associations of cerebrospinal fluid biomarkers with cognition, and rapid eye movement sleep behavior disorder in early Parkinson’s disease. Front. Neurosci. 16. doi: 10.3389/fnins.2022.1049118
Taylor C. A., Tuschl K., Nicolai M. M., Bornhorst J., Gubert P., Varao A. M., et al. (2020). Maintaining translational relevance in animal models of manganese neurotoxicity. J. Nutr. 150, 1360–1369. doi: 10.1093/jn/nxaa066
Tiede K., Westerhoff P., Hansen S. F., Fern G. J., Hankin S. M., Aitken R. J., et al. (2012). Review of the risks posed to drinking water by man-made nanoparticels. Available online at: https://orbit.dtu.dk/en/publications/review-of-the-risks-posed-to-drinking-water-by-man-made-nanoparti. (Accessed January 1, 2012).
Tomak A., Cesmeli S., Hanoglu B. D., Winkler D., Karakus C. O. (2021). Nanoparticle-protein corona complex: understanding multiple interactions between environmental factors, corona formation, and biological activity. Nanotoxicology 15, 1331–1357. doi: 10.1080/17435390.2022.2025467
Wang W. J., Chen F., Zhang L., Wen F. L., Yu Q., Li P., et al. (2023). Neurotransmitter disturbances caused by methylmercury exposure: Microbiota-gut-brain interaction. Sci. Total Environ. 873, 162358. doi: 10.1016/j.scitotenv.2023.162358
Wang L. F., Habibul N., He D. Q., Li W. W., Zhang X., Jiang H., et al. (2015a). Copper release from copper nanoparticles in the presence of natural organic matter. Water Res. 68, 12–23. doi: 10.1016/j.watres.2014.09.031
Wang S. H., Han X., Yu T. T., Liu Y. L., Zhang H. Y., Mao H. L., et al. (2022). Isoprocarb causes neurotoxicity of zebrafish embryos through oxidative stress-induced apoptosis. Ecotoxicol. Environ. Saf. 242, 113870. doi: 10.1016/j.ecoenv.2022.113870
Wang Q. W., Lai N. L. S., Wang X. F., Guo Y. Y., Lam P. K. S., Lam J. C. W., et al. (2015b). Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 49, 5123–5132. doi: 10.1021/acs.est.5b00558
Wirth S. M., Lowry G. V., Tilton R. D. (2012). Natural organic matter alters biofilm tolerance to silver nanoparticles and dissolved silver. Environ. Sci. Technol. 46, 12687–12696. doi: 10.1021/es301521p
Wu T. S., Cheng Y. C., Chen P. J., Huang Y. T., Yu F. Y., Liu B. H. (2019). Exposure to aflatoxin B interferes with locomotion and neural development in zebrafish embryos and larvae. Chemosphere 217, 905–913. doi: 10.1016/j.chemosphere.2018.11.058
Xin Q., Rotchell J. M., Cheng J. P., Yi J., Zhang Q. (2015). Silver nanoparticles affect the neural development of zebrafish embryos. J. Appl. Toxicol. 35, 1481–1492. doi: 10.1002/jat.3164
Yan Z. H., Chen Y. F., Zhang X. D., Lu G. H. (2023a). The metabolites could not be ignored: A comparative study of the metabolite norfluoxetine with its parent fluoxetine on zebrafish (Danio rerio). Aquat. Toxicol. 257, 106467. doi: 10.1016/j.aquatox.2023.106467
Yan C., Cheng T., Shang J. (2019). Effect of bovine serum albumin on stability and transport of kaolinite colloid. Water Res. 155, 204–213. doi: 10.1016/j.watres.2019.02.022
Yan Z. H., Zhang X. D., Bao X. H., Ling X., Yang H. H., Liu J. C., et al. (2020). Influence of dissolved organic matter on the accumulation, metabolite production and multi-biological effects of environmentally relevant fluoxetine in crucian carp (Carassius auratus). Aquat. Toxicol. 226, 105581. doi: 10.1016/j.aquatox.2020.105581
Yan Z. H., Zhou Y. X., Zhu P. Y., Bao X. H., Su P. P. (2023b). Polystyrene nanoplastics mediated the toxicity of silver nanoparticles in zebrafish embryos. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1195125
Yi F., Chen G. Q., Zeng G. M., Guo Z., Liu W. W., Huang Z. Z., et al. (2016). Influence of cysteine and bovine serum albumin on silver nanoparticle stability, dissolution, and toxicity to Phanerochaete chrysosporium. Rsc Adv. 6, 106177–106185. doi: 10.1039/c6ra23675h
Zhao G., Wang Z. Y., Xu L., Xia C. X., Liu J. X. (2019). Silver nanoparticles induce abnormal touch responses by damaging neural circuits in zebrafish embryos. Chemosphere 229, 169–180. doi: 10.1016/j.chemosphere.2019.04.223
Zhao Y., Yang Q. X., Liu D., Liu T. Q., Xing L. Y. (2022). Neurotoxicity of nanoparticles: Insight from studies in zebrafish. Ecotoxicol. Environ. Saf. 242, 113896. doi: 10.1016/j.ecoenv.2022.113896
Zheng C. Q., Gao Y., Zhu J. L., Gan L., Wang M. M., Zhang W., et al. (2023). Prolonged electrolysis injures the neural development of zebrafish (Danio rerio). Environ. Sci. pollut. Res. 30, 25863–25872. doi: 10.1007/s11356-022-23864-2
Zhou R. R., Zhou D., Yang S. X., Shi Z. Q., Pan H., Jin Q. J., et al. (2023). Neurotoxicity of polystyrene nanoplastics with different particle sizes at environment-related concentrations on early zebrafish embryos. Sci. Total Environ. 872, 162096. doi: 10.1016/j.scitotenv.2023.162096
Keywords: AgNPs, neurotoxicity, transgenic zebrafish, neurodevelopment, neurotransmitter
Citation: Yan Z, Chen Y, Yang Y, Zhou Y, Su P and Yuan S (2024) Alleviation of bovine serum albumin on the neurotoxicity of silver nanoparticles in zebrafish (Danio rerio) larvae. Front. Mar. Sci. 11:1473054. doi: 10.3389/fmars.2024.1473054
Received: 30 July 2024; Accepted: 27 August 2024;
Published: 19 September 2024.
Edited by:
Gisele Cristina Favero, Universidade Federal de Minas Gerais, BrazilReviewed by:
D. K. Meena, Central Inland Fisheries Research Institute (ICAR), IndiaSofia Priyadarsani Das, National Taiwan Ocean University, Taiwan
Copyright © 2024 Yan, Chen, Yang, Zhou, Su and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenhua Yan, aHdhaHVlckBoaHUuZWR1LmNu
 Zhenhua Yan
Zhenhua Yan Yufang Chen1,2
Yufang Chen1,2