- 1Ministry of Education Key Laboratory for Ecology of Tropical Islands, Key Laboratory of Tropical Animal and Plant Ecology of Hainan Province, College of Life Sciences, Hainan Normal University, Haikou, China
- 2Hainan Sansha Provincial Observation and Research Station of Sea Turtle Ecology, Sansha, China
- 3Marine Protected Area Administration of Sansha City, Sansha, China
Introduction: Sea turtles are ideal flagship and umbrella species for marine biodiversity conservation. The quality of nesting grounds is crucial for the successful reproduction of sea turtles, as it determines whether they can successfully nest and hatch. The Xisha Islands represent the largest remaining nesting grounds for green turtles in China. However, they face numerous threats and lack management guidelines for nesting ground restoration.
Methods: In this study, we continuously monitored the beach changes and coastal erosion on North Island, which is located in the northern region of the Xisha Islands, from 2019 to 2022.
Results: From 2020 to 2022, the beach area on North Island decreased annually by 11,800 m2, accounting for 24.39% of the beach area in 2020. The proportion of suitable nesting sand types (including coarse and medium sands) also decreased annually, with a reduction of 40–70% on the southern beach of North Island. The peak nesting period of green turtles on North Island coincides with the frequent occurrence of typhoons in the South China Sea, causing tidal surges that inundate green turtle nests, resulting in an average nest loss rate of 35.25%.
Discussion: Based on the above threats, it is recommended to promptly initiate habitat restoration in severely eroded areas of green turtle nesting grounds to prevent further declines in nesting area and quality. Additionally, measures such as nest relocation should be implemented to enhance green turtle reproductive success.
1 Introduction
Sea turtles rely on suitable beaches for reproduction (Miller, 1989), and they exhibit high fidelity to their birth beaches (Ackerman, 1997). Adult female sea turtles return to their birth beaches to nest, yet they are highly sensitive to environmental changes in their nesting grounds (Rumaida et al., 2021). Nesting beaches for sea turtles face severe erosion worldwide (Maneja et al., 2021), with diminished historical nesting grounds and the remaining beach areas at risk of further loss due to human activity (McClenachan et al., 2006; Liles et al., 2015). The reduction in suitable nesting beaches could negatively affect the stability of sea turtle populations (Maneja et al., 2021).
The loss or degradation of nesting grounds is currently the greatest threat to sea turtles (Jensen et al., 2013; Venter et al., 2016). Environmental changes, including short- and long-term sea level rise, increased intensity and frequency of typhoons, and water flow induced by tides and waves, lead to beach morphological changes and erosion (Maneja et al., 2021), thereby adversely affecting the critical nesting efforts of sea turtles (Veelenturf et al., 2020). The availability of suitable nesting beaches is predicted to decrease, particularly on small islands, because of rising sea levels (Fish et al., 2005). The enhanced frequency and intensity of typhoons have also been reported to increase the loss of nesting beaches and decrease the success of hatching and emergence (Fuentes et al., 2011; Poloczanska et al., 2009; Fujisaki et al., 2018).
Protecting nesting grounds is an important and effective conservation strategy for wild green turtles (Serafini et al., 2009; Rumaida et al., 2021). Concurrent conservation and restoration studies support the development of management strategies to maximize local green turtle reproductive potential. They also aid in determining the availability of suitable nesting areas for green turtles under potential spatiotemporal changes caused by global climate change and island development (Rumaida et al., 2021). For instance, owing to effective conservation efforts over the past 20–30 years, breeding populations and nest numbers of green turtles in Japan, Australia, Hawaii, Florida, Costa Rica, and Ascension Island (an affiliated island of Saint Helena in the South Pacific) have steadily increased (Chaloupka et al., 2008; Weber et al., 2014).
The Xisha Islands in the South China Sea are currently China’s largest nesting grounds for green sea turtles, having ecological characteristics that provide natural, functional, and suitable areas for green turtle nesting activities (Zhang et al., 2024). Previous ecological and molecular biology studies showed that green sea turtles breeding on the Xisha Islands have formed a new geographic population with unique conservation importance (Gaillard et al., 2021; Li et al., 2023). However, basic information, such as the threatening factors and habitat conditions of green turtle nesting grounds on the Xisha Islands, remains scarce. In this study, we aimed to determine the spatiotemporal distribution of green turtle nests on the Xisha Islands. To this end, we conducted field surveys and used satellite image mapping to assess changes in the nesting ground areas of green turtles on North Island of the Xisha Islands from 2019 to 2022. We also assessed the effects of coastal erosion and typhoons on green turtle reproduction. Our findings elucidate the variation trends of and threats faced by the green turtle nesting grounds on the Xisha Islands. Based on our results, we provided specific recommendations for sea turtle nesting ground management and conservation.
2 Materials and methods
2.1 Study area
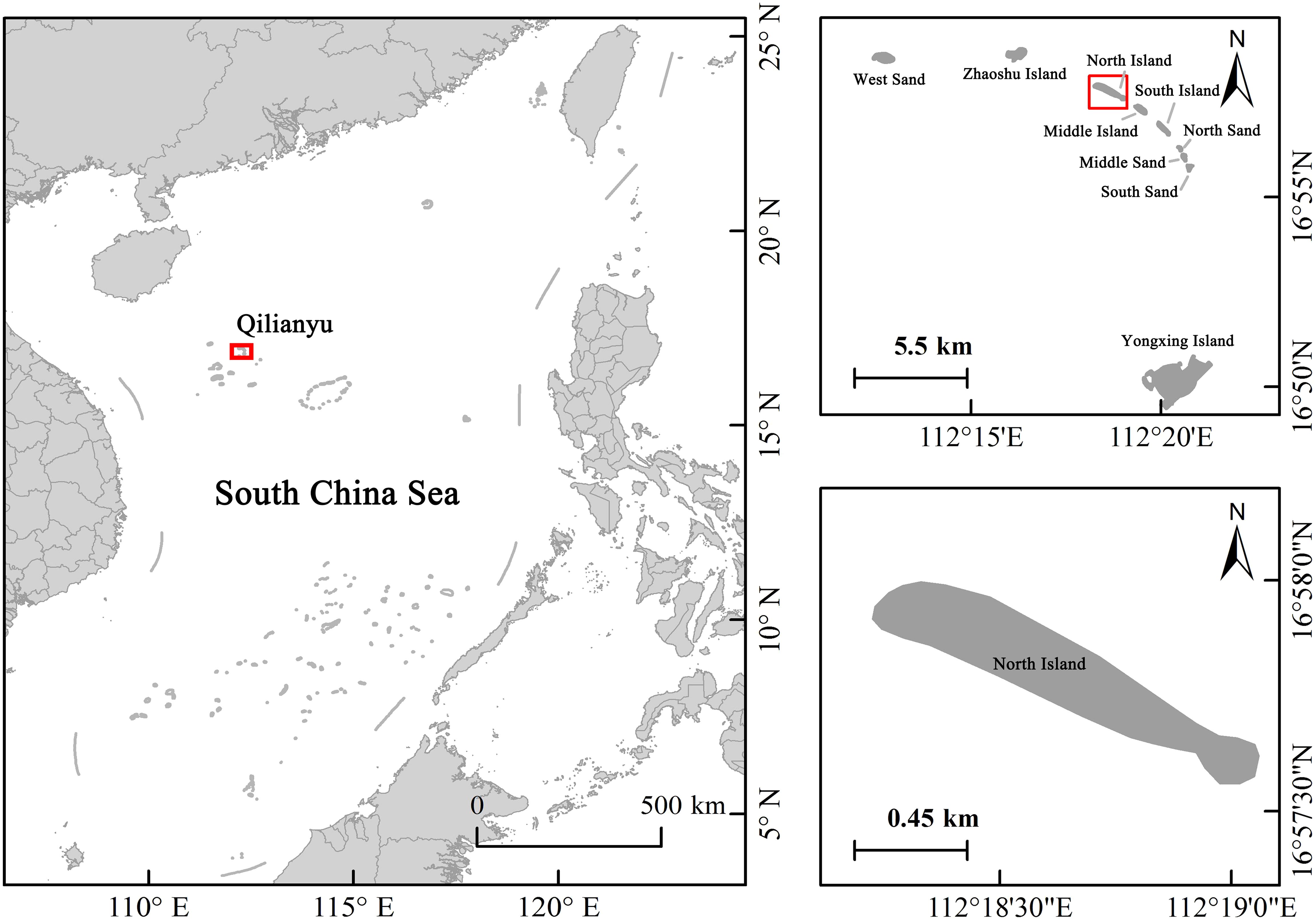
North Island is located in the northern region of the Xisha Islands. It is long and narrow, running northwest to southeast, with an area of approximately 0.4 km2 (Figure 1). The Xisha Sea has a high temperature year-round, with an annual average air temperature of 26–27°C and an annual average relative humidity of approximately 81%. In summer, the sea current flows northeast, while in winter, it flows southwest. The sea water temperature is generally high and relatively uniform, with an annual average surface sea water temperature of 27.5°C and annual surface sea salinity of 33.6 ‰ (Ye, 1996; Zhao et al., 2017; Zhang et al., 2024). North Island is also known as “Sea Turtle Island,” as sea turtles frequently come ashore to lay eggs, with the number of sea turtle nests accounting for 37% of the total on the Xisha Islands in recent years (Zhang et al., 2024). However, green turtle nesting beaches on North Island are exposed to extreme climatic conditions, such as high summer temperatures (Luo et al., 2018) and intense seasonal winds (Wang et al., 2012). These conditions can affect the characteristics of nesting beaches, leading to low sand moisture content, high temperatures, increased salinity, inundation by seawater, and beach erosion. During the southwest monsoon season, which is the peak season for sea turtle nesting, the prevailing wind is southwest, with an average wind force of 4–6 m/s. During the northeast monsoon season, the prevailing wind is northeast, with an average wind force of 6–9 m/s. During tropical cyclones, the prevailing wind direction is southwest, with an average wind force of 6–7 m/s (Ye, 1996; Zhang et al., 2024).
2.2 Temporal and spatial distribution of green turtle nesting grounds
We conducted nighttime (19:00–2:00) and subsequent daytime (7:00–8:00) patrols and marked all confirmed green turtle nesting grounds with tags (including laying dates and nest numbers). The coordinates of the nests were recorded using a handheld GPS device (ZL-188; ZL Electronic Technology, Beijing, China), and a nest distribution map was created. Kernel density estimation was used for recording the nests. Using ArcGIS 10.4.1 software and its Spatial Analyst® extension (Silva et al., 2020), nesting grounds and the distances between them were calculated based on the total number per km² and previous counts.
2.3 Island morphological and area changes
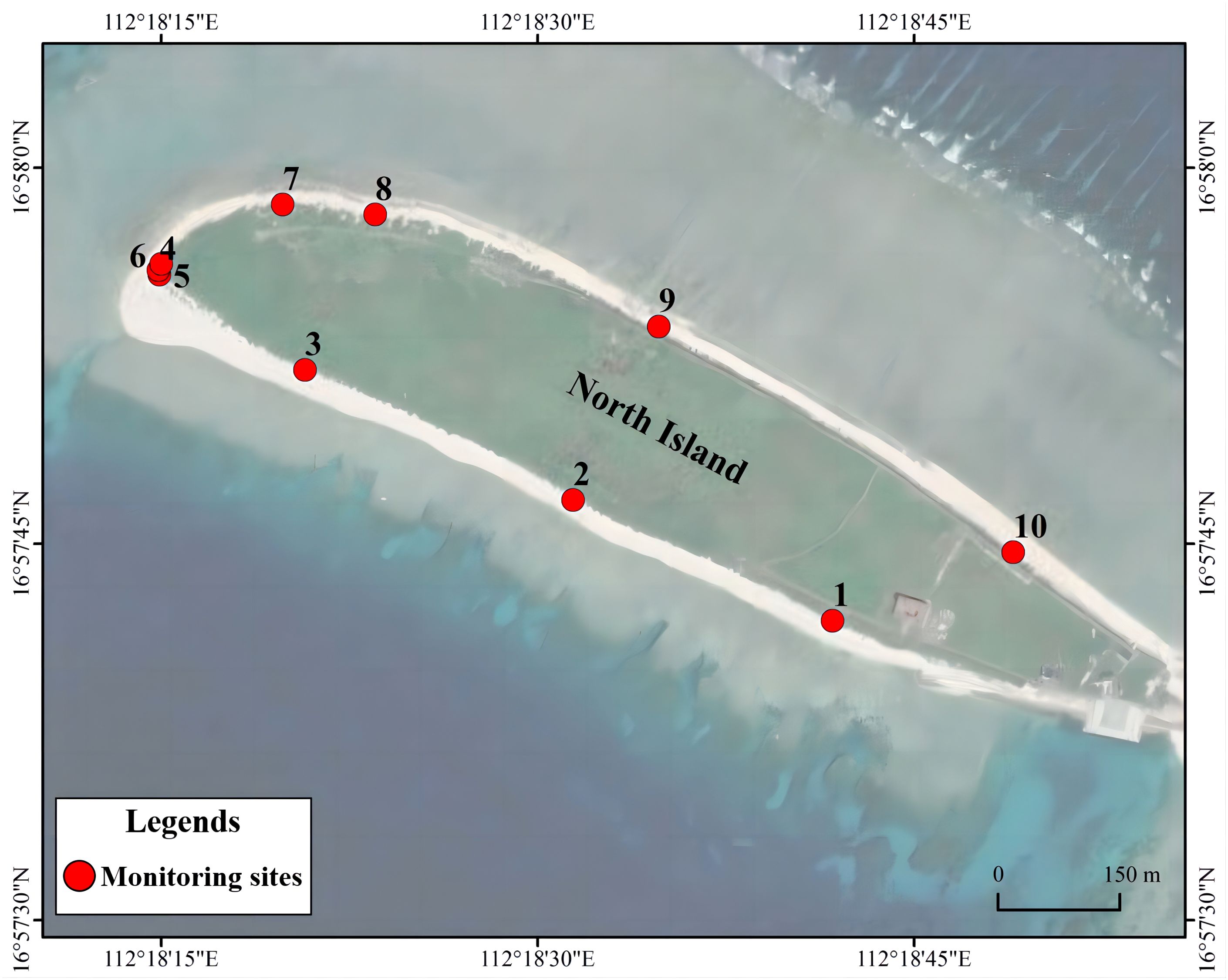
Combining satellite imagery with traditional ground surveys allows for a more comprehensive and accurate observation of island morphological changes (Casale and Ceriani, 2019). Ten fixed benchmarks were selected as the monitoring points on North Island of Qilianyu (Figure 2). Measurements of beach width, slope, grain size, the presence of scarps, and reef flats were conducted every three months from 2018 to 2022. Remote sensing satellite images and ArgGIS analysis were used to statistically analyze the dynamic changes in nesting beach width, slope, area, and morphology over different years during the same period and in different quarters within the same year. Continuous monitoring of beach morphology was conducted on the southwest- and northwest-facing beaches of North Island in May, July, September, and November 2021 and March 2022 to observe monthly trends in nesting site morphology in these areas.
Surveys were conducted before and after Typhoon Sinlaku in September 2020, Typhoon Cempaka in July 2021, Typhoon Chaba in 2022, and Typhoon Noru in 2022 to assess the impact of storm surges on green turtle nests.
2.3.1 Beach slope
Emery’s survey method (Emery, 1961) was employed to measure the slope of the beach from the nesting point to the lowest tide point, using a leveling device.
Using field-recorded beach profile data, the cumulative vertical elevation (y-axis) was plotted as a function of the cumulative horizontal position (x-axis) to reveal the actual beach profile. The slope (%) was calculated by dividing the difference in elevation between two adjacent points by the difference in horizontal distance between these points.
2.3.2 Beach sand characteristics
Samples of beach sands were collected from various monitoring points. The dried samples were sieved using a standard series of seven mesh sizes (4.0, 2.0, 1.0, 0.425, 0.250, 0.125, and 0.063 mm) (OLOEY; Hai Chuang Instruments Co., Ltd., China) using a vibrating sieve (CHENGJIA Hai Chuang Instruments Co., Ltd., China). Materials coarser than 4 Φ (phi) (> 63 μm, sand fraction) were analyzed at Φ intervals (− 2 to 4 Φ), while samples smaller than 63 μm were analyzed using liquid displacement. The sand retained on each sieve and the silt and clay analyzed using a pipette were dried again and weighed (± 0.01 g) to determine their mass fractions relative to the total mass. Data were described using the Wentworth Scale to classify sand composition on the nesting beaches (Wentworth, 1922), dividing sand grains by size into: pebbles (> 4 mm); granules (2–4 mm); very coarse sand (1–2 mm); coarse sand (0.425–1 mm); medium sand (0.250–0.425 mm); fine sand (0.125–0.250 mm); very fine sand (0.063–−0.125 mm); and clay (< 0.063 mm).
Zhang et al. (2024) indicated that green turtles tend to prefer coarse (0.425–1 mm) and medium (0.250–0.425 mm) sand for nesting. Therefore, the proportions of coarse (0.425–1 mm) and medium (0.250–0.425 mm) sand at different monitoring points were used to assess variations in nesting beach sand grain sizes.
2.4 Data analysis
All data were tested for normal distribution and homogeneity of variance prior to statistical analysis. Descriptive statistics, including the mean, maximum, minimum, and standard deviation, were used to report the slope of the monitoring points, and the coefficient of variation was used to report the change in the beach slope. Pearson’s correlation test was used to analyze the changing trend of beach area from 2019 to 2022 and to determine the relationship between the beach area of the west end of North Island and the percentage of green turtle nests. The relevant data are shown as mean ± standard deviation. A significance level of P < 0.05 was considered statistically significant according to the two-tailed test. All statistical analyses were performed using SPSS 19.0 (IBM Corp., USA).
3 Results
3.1 Distribution of green turtle nests on North Island
The nest kernel density distribution of green turtles on North Island from 2016 to 2022 (Figure 3) showed an extremely high use ratio of nesting grounds by green turtles, with nesting grounds distributed extensively along the island’s circumferential beaches. Nesting grounds were concentrated mainly on the southern, southwestern, and northwestern sides of North Island, with the highest nesting kernel density observed on the southwestern side.
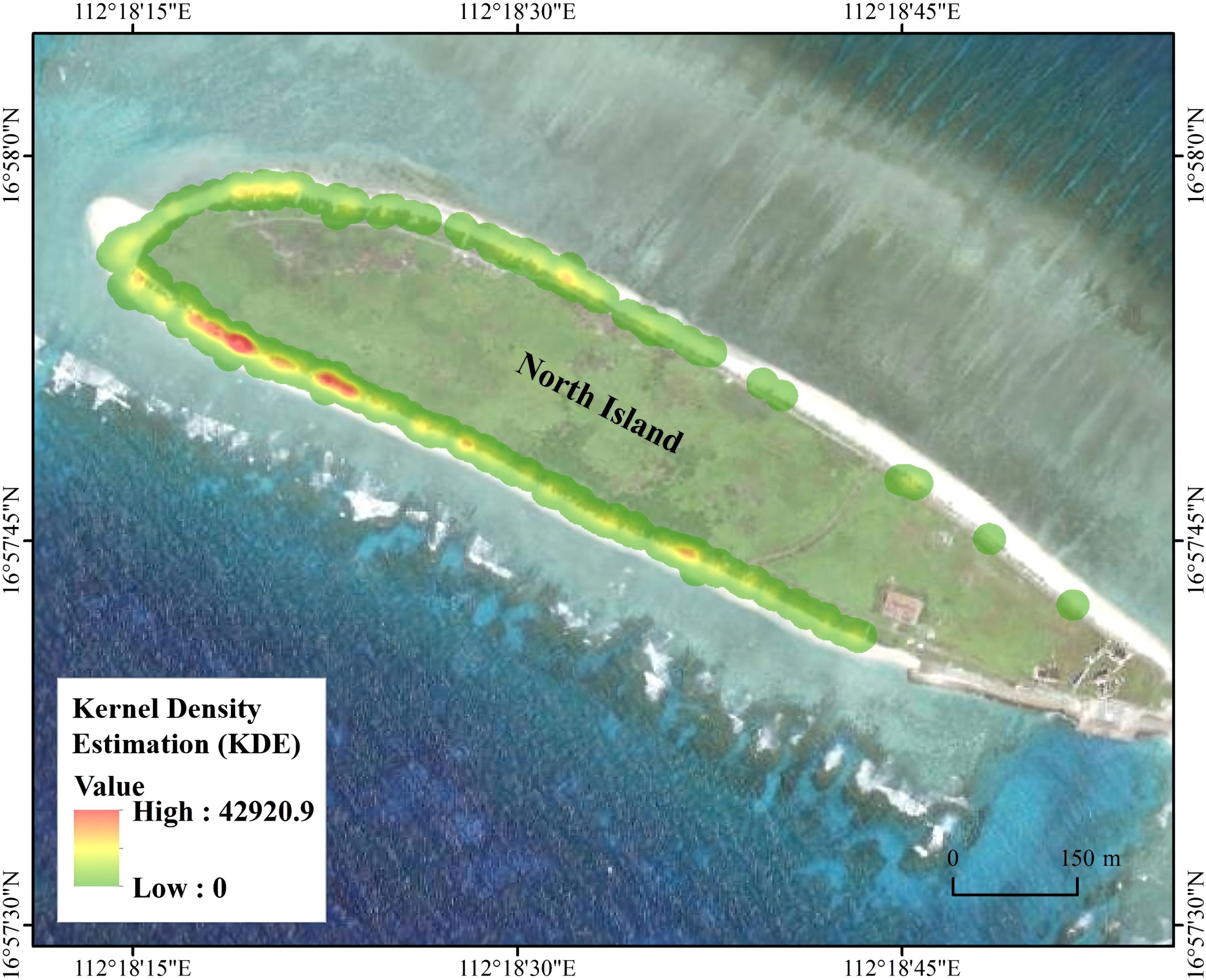
Figure 3. Estimated distribution of nest kernel density of green turtles on North Island from 2016 to 2022.
From 2021 to 2022, 165 green turtle nests were located on North Island (Figures 4A, B). The analysis revealed that in 2021, nests were relatively scattered, with fewer nests compared to 2022 in the southwestern region, where the core density of green turtle nests was highest. In 2022, there was an increase in the nest number on the southern and northern sides of North Island, while the southwestern region (where the highest core density of green turtle nest) had few nests.
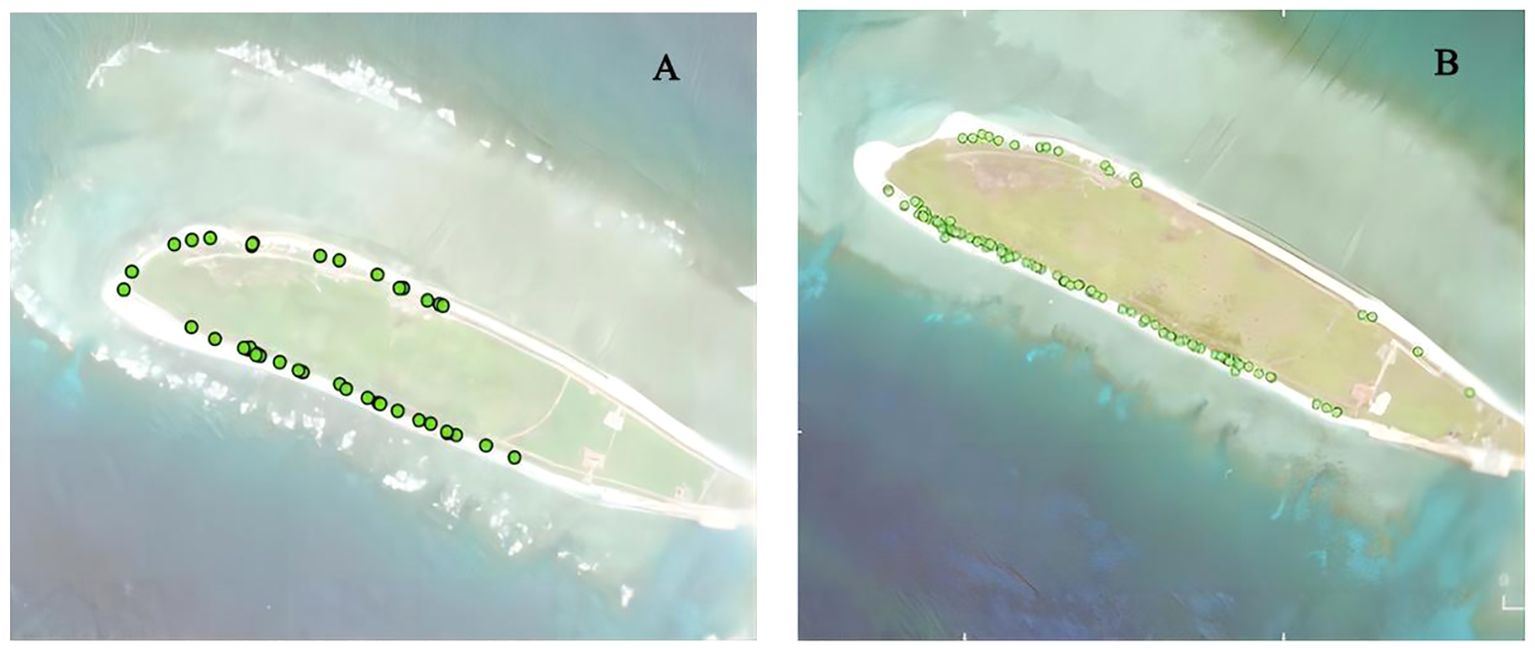
Figure 4. Nest distribution of green turtles on North Island, Qilianyu from 2021 to 2022. (A) January to September 2021 (n = 40); (B) January to September 2022 (n = 125).
3.2 Island morphological and area changes
3.2.1 Annual dynamic changes in nesting ground morphology
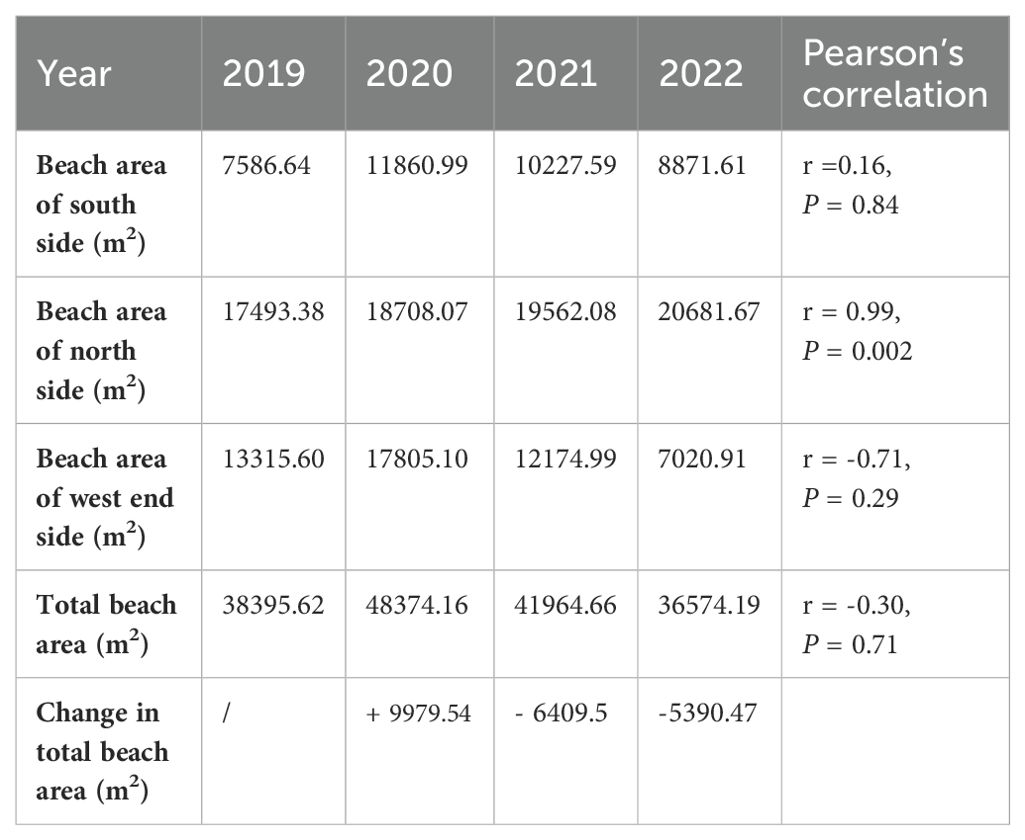
From 2020 to 2022, the beach area of North Island decreased, but not significantly (r = -0.30, P = 0.71). In 2022, it decreased by 11,800 m2 compared to the area in 2020, accounting for 24.39% of the area in 2020 (Table 1). However, the reduced area was mainly located on the western side of North Island (r = -0.71, P = 0.29), and the beach area on the northern side increased significantly (r = 0.99, P = 0.002) (Table 1). Furthermore, the proportion of nests on the western side out of the total number on the island also declined from 38.89% to 12.00%. This changing trend was consistent with that of the beach area on the western side of North Island (r = 0.94, P = 0.058) (Table 2, Figure 5).
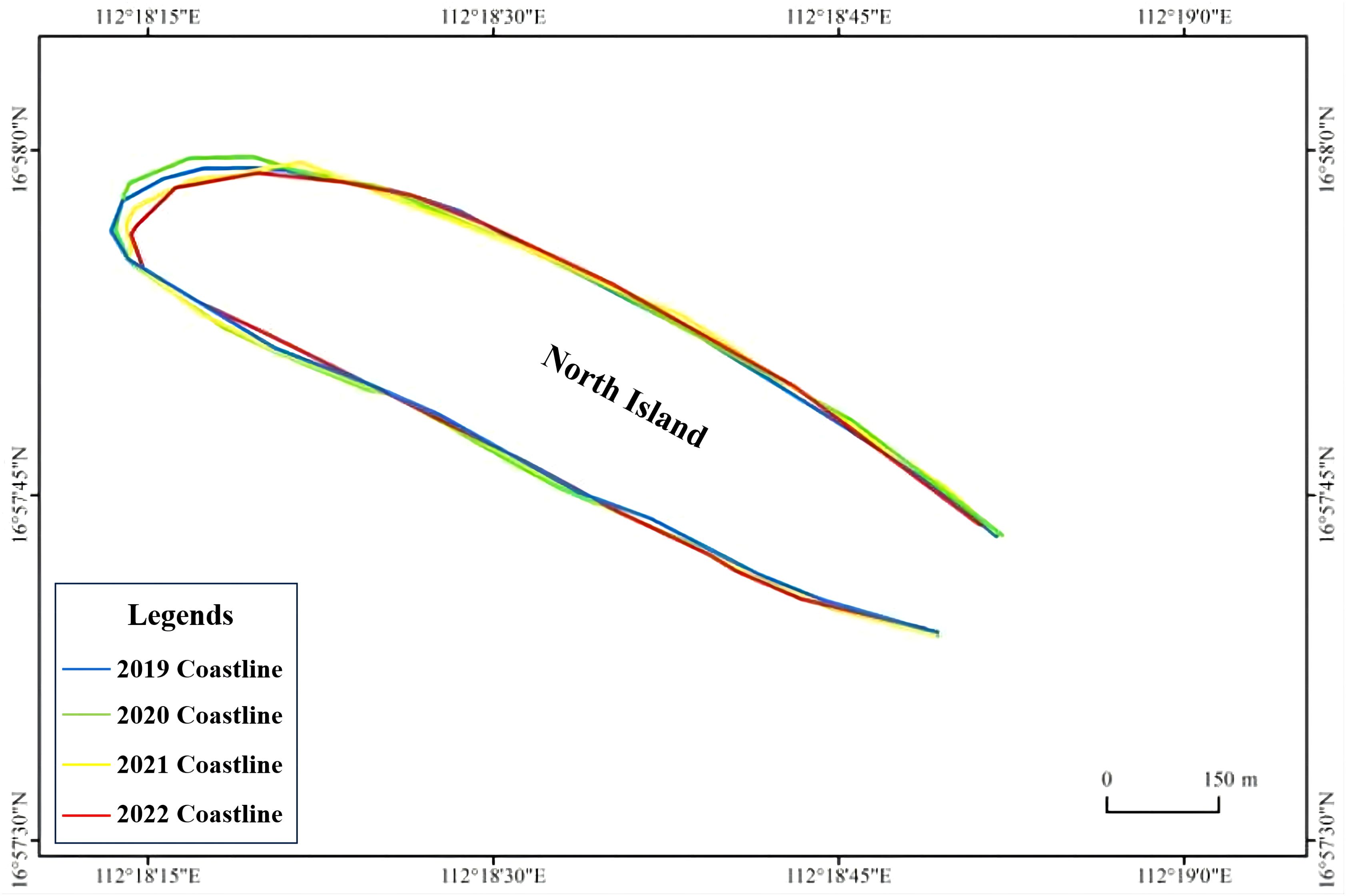
Figure 5. Distribution of and changes in the coastline of North Island, Qilianyu in July from 2019 to 2022.
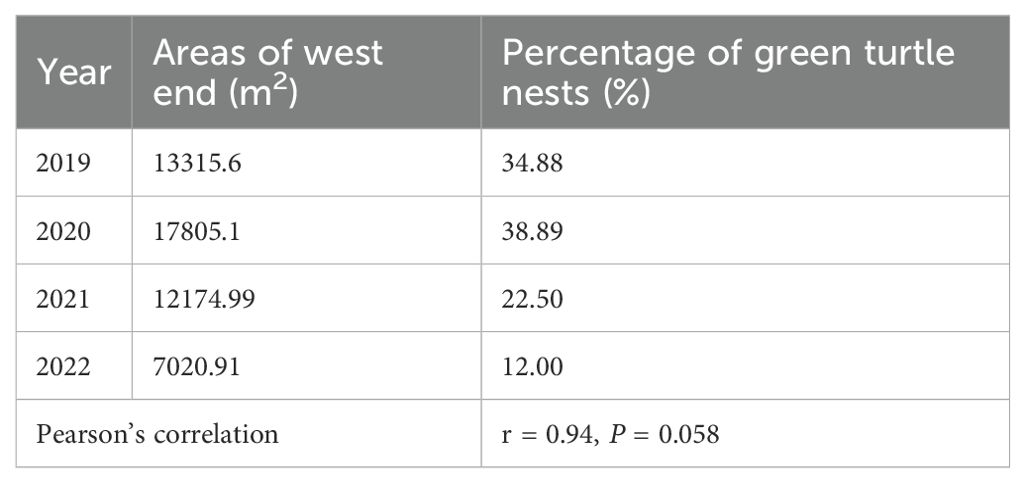
Table 2. Correlation between area and the percentage of nests at west end (southwest and northwest).
3.2.2 Monthly dynamics of nesting ground morphology
The morphology of the beaches on the western side of North Island varied widely across different months (Figure 6). From July to September, sand gradually accumulated on the northwestern beaches of North Island, covering reef rocks and providing nesting areas for green turtles. However, from September to March of the following year, reef rocks were extensively exposed, with heights ranging from 30 cm to 50 cm, preventing green turtles from crossing these rocks to nest on the beaches.
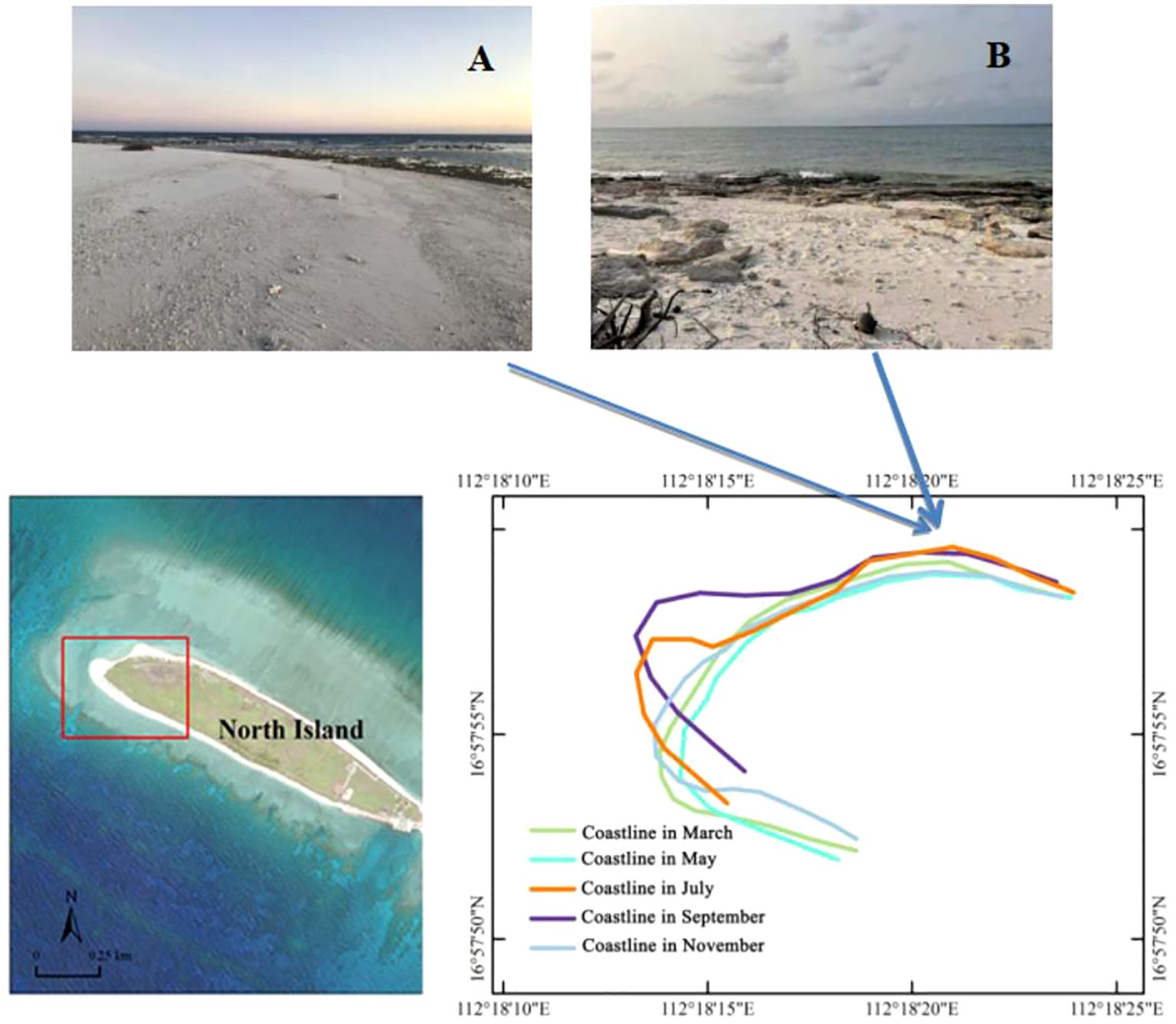
Figure 6. Dynamic changes in beach morphology at the southwestern and northwestern corners of North Island, Qilianyu in different months of 2020. (A) Northwestern North Island in July 2021; (B) Northwestern North Island in November 2021.
3.2.3 Slope of nesting grounds
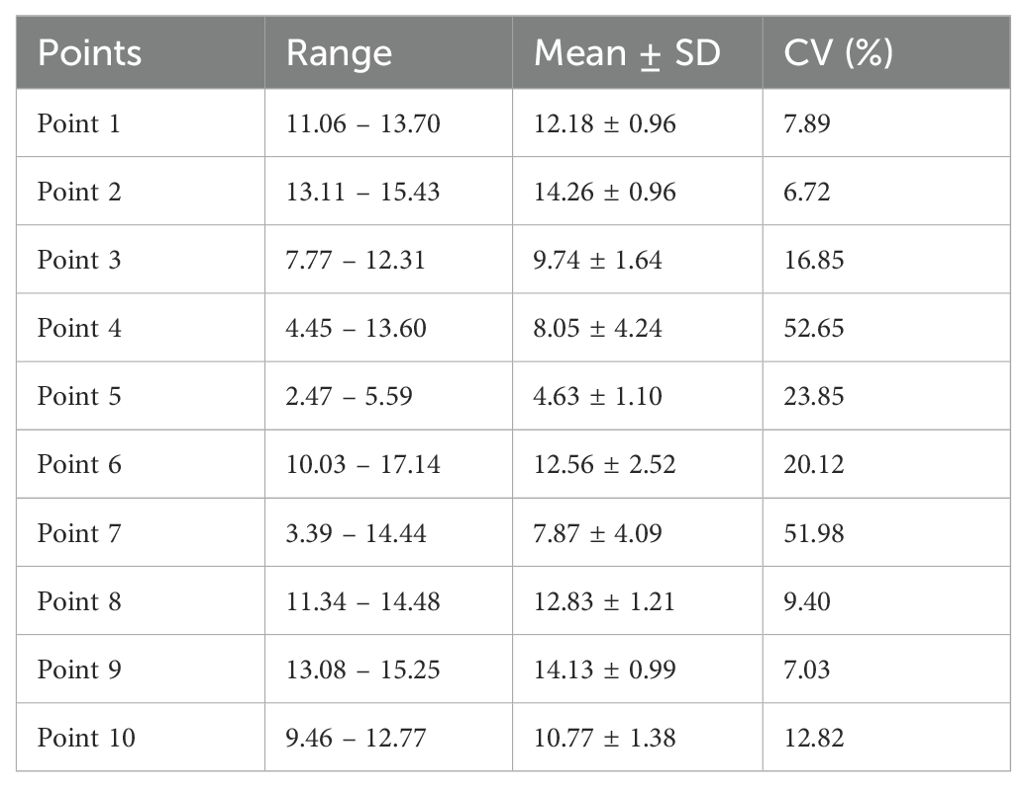
From 2019 to 2022, the overall slope of the beaches on North Island was relatively uniform, ranging of average slope from 8% to 14%, except at Point 5 (Table 3, Figure 7). The coefficient of variation of the slope at Points 1, 2, 8, and 9 was < 10%, indicating that the slope changes at these monitoring points were very small. However, Points 4 and 7 on the southwestern and northwestern sides of North Island exhibited pronounced slope variations (coefficient of variation > 50%) (Table 3). In some cases, these variations formed very steep scarps, preventing green turtles from climbing up to the nesting site. For instance, the beach scarp at Point 7 in 2022 (Figure 8) reduced the available nesting areas for green turtles.
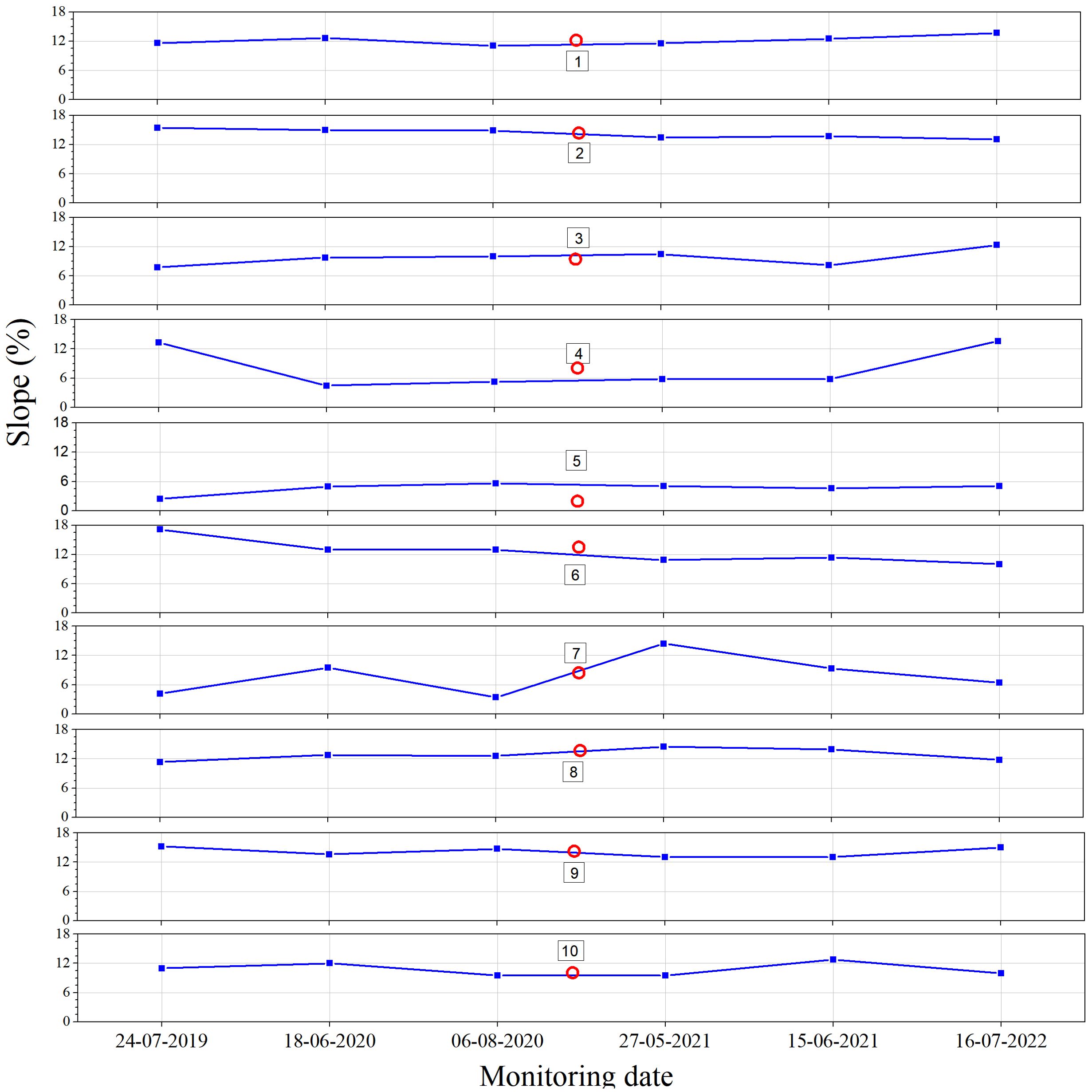
Figure 7. Slope variation map of the monitoring sites on North Island, Qilianyu from 2019 to 2022. Empty red circles indicate average values.

Figure 8. Sandy beach vertical scarp in the southwestern corner (between monitoring Point 6 and 7) of North Island in 2022; the green turtle was forced to return to the sea.
3.2.4 Sand grain size of nesting beaches
The proportions of coarse and medium sands at most monitoring points remained relatively stable, each above 70%, from 2020 to 2022. However, Points 1, 2, and 3 exhibited a decreasing trend in the annual proportions of coarse and medium sands. Point 10 (located in northern North Island near residential areas) had approximately 60% pebbles (> 4 mm), with proportions of coarse (0.425–1 mm) and medium (0.250–0.425 mm) sand below 20%. This type of sand is not suitable for green turtle nesting, and no green turtle nest sites were observed at this location from 2020 to 2022 (Figure 9).
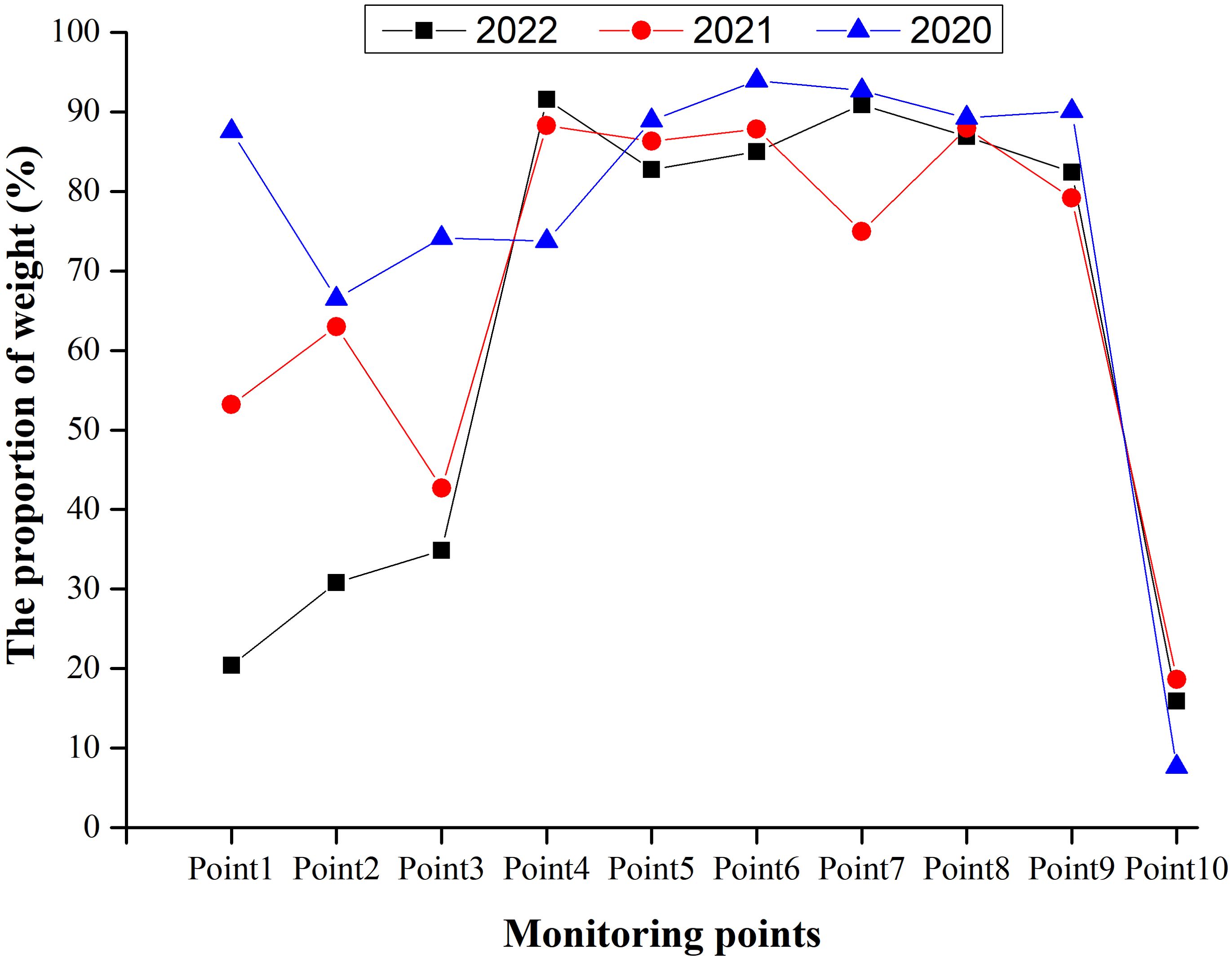
Figure 9. Variation trends of coarse and medium sand (particle size range: 0.250–1 mm) at different monitoring sites on North Island, Qilianyu.
3.2.5 Effects of typhoons on green turtle nests
The peak period for green turtle nesting on North Island coincides with the typhoon season in the South China Sea. During Typhoon “Sinlaku” on September 2020, nests within 20.7 m from the coastline were submerged by seawater, with Nest No. 91 buried under sand up to 78 cm deep (Figures 10, 11). Investigations during Typhoon “Cempaka” in July 2021 revealed that nests within 20.1 m from the coastline were submerged, and 6 of the 14 hatching nests on North Island were submerged, resulting in a high loss rate of 42.9%.

Figure 10. Nest No. 91 was deeply buried due to Typhoon “Sinlaku” in 2020. (A) Pre-typhoon; (B) Post-typhoon.

Figure 11. Impact of typhoon on the southwestern corner beach of North Island. (A) Normal weather; (B) Typhoon “Sinlaku” in 2020.
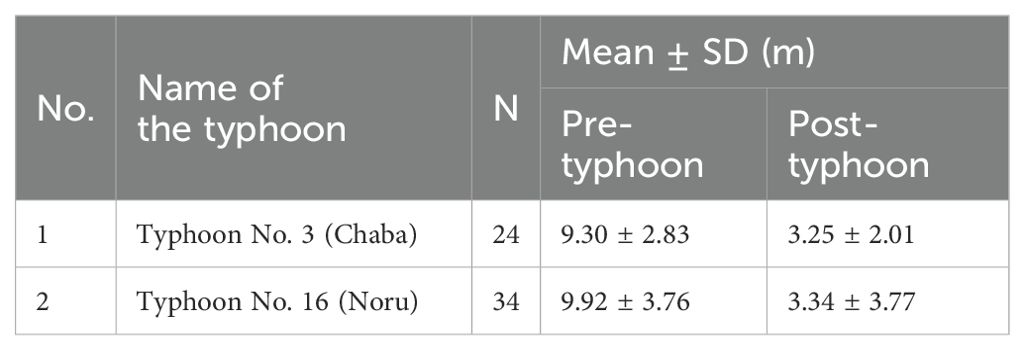
In 2022, during Typhoon No. 3 “Chaba” and Typhoon No. 16 “Noru,” the high tide level rose by more than 6 m. Nests with a distance from the high tide line of less than 6 m were submerged by seawater. Sixteen green turtle nests were soaked during these two typhoons (loss rate of 27.59%), resulting in the failed development and reproduction of green turtles (Table 4).
In summary, the average loss rate of green turtle nests monitored from 2021 to 2022 owing to typhoon impacts was 35.25%.
4 Discussion
Nest site selection and distribution have substantial biological implications for sea turtle reproductive success (Resetarits, 1996). North Island has the largest number of green turtle nests in the Xisha Islands each year (Zhang et al., 2024). Turtle nests were mainly concentrated on the southern, southwestern, and northwestern sides of North Island. Although a beach slope is necessary for protecting nests from tidal impacts (Fish et al., 2005; Fuentes et al., 2010), Maurer and Johnson (2017) found that steep slopes can adversely affect sea turtle nesting. Green turtles encountering steep, erosion-induced beach slopes tend to return to the sea or nest in the tidal zone (Silva et al., 2020). During 2021 and 2022, the nest core density of green turtles tended to shift southward on North Island as sea turtles selected less favorable nesting areas, such as Points 1, 2, and 3. In contrast, the southwestern area of North Island, where green turtles have historically nested most frequently, had fewer nests over time. However, the sand types on the southwestern side of North Island consisted predominantly of medium and coarse grains, meeting the green turtle nesting requirement of good sand quality. The reason that fewer nests were distributed on the southwestern side of North Island may be the steeper slopes (scarps) that formed due to erosion and typhoons in 2021, hindering nesting green turtles from accessing nesting sites (Wood and Bjorndal, 2000; Maison et al., 2010).
Natural cycles of erosion and accretion in nesting grounds are common phenomena, in which sand erodes from one area and is deposited in another. However, persistent sand loss can lead to net sediment loss on coastlines and islands, critically affecting the nesting efforts of sea turtles (Veelenturf et al., 2020). Coastal erosion can lead to the loss of suitable nesting areas for green turtles (Schlacher et al., 2008; Reece et al., 2013; Fujisaki et al., 2018). Our monitoring data indicated changes in the morphology, area, and slope of the sandbars on the southwestern and northwestern sides of North Island from November to May of the following year, resulting in reduced nesting areas and exposed reefs and causing shrinkage of turtle nesting grounds. Loss rates of nesting grounds for various sea turtle species worldwide range from 24% to 89% under different sea level rise scenarios (ranging from 0.18 m to 1.3 m) (Fuentes et al., 2010; Katselidis et al., 2014; Hiebert et al., 2017). If the sea level rises by >1 m by 2100, some nesting beaches may be completely submerged (Turner and Batianoff, 2007), whereas others may be partially submerged. However, this reduction in available nesting areas may lead to density-related issues, such as nest infections due to increased bacterial activity between adjacent nests (Fish et al., 2008) and nest destruction by conspecifics (Limpus et al., 2003), which could reduce hatching success and affect population dynamics (Fuentes et al., 2010). Exposed reefs may also obstruct nesting turtles from accessing the beach or returning to the sea (Basyoni, 1999; McClenachan et al., 2006). Additionally, hatchling turtles may become trapped in crevices between beach rocks during low tides. Therefore, in the routine management of green turtle nesting beaches, patrol personnel should assist distressed or threatened nesting female green turtles in turtle conservation areas.
With beach erosion, changes in beach morphology pose specific threats to sea turtles as they select suitable locations for nesting (Veelenturf et al., 2020). From 2020 to 2022, green turtle nests shifted annually toward vegetated areas on North Island, possibly due to beach erosion, necessitating longer crawls to prevent nest inundation by seawater (Stokes et al., 2024). Sea water rise during typhoons put nests near the tidal line at risk of flooding and erosion (Kasparek et al., 2001). In this study, the coastal erosion caused by typhoon events resulted in changes in beach morphology and sand composition. Additionally, the rising tides submerged the green turtle nests. Previous studies have suggested that the relocation of nests at high risk of flooding is a worthwhile conservation activity (Yalçın Ozdilek, 2007; Sönmez and Yalçın-Ozdilek, 2013). Therefore, conservation efforts during the typhoon season are crucial for the successful reproduction of sea turtles. It is recommended to conduct pre-typhoon assessments to relocate green turtle eggs in the early incubation stages from lower to higher beach areas to continue incubation. Greenhouses can be used to protect the nests in different areas of the island in the future, thereby enhancing overall hatch rates during the typhoon season and promoting sustained recovery of green turtle populations.
Habitat conditions play a crucial role in the nesting activities of sea turtles (Rumaida et al., 2021). Changes in beach morphology at nesting grounds affect the selection process for sea turtle nesting. To the best of our knowledge, this is the first study to monitor the morphology and area of green turtle nesting beaches on the Xisha Islands, China. Our findings highlight the impact of coastal erosion and typhoons on green turtle nesting and hatching. This study raises concerns about the vulnerability of sea turtle nesting habitats and provides guidance for the conservation and restoration of nesting grounds on the Xisha Islands. Future recommendations include continuous monitoring and expansion of the study area to other islands to comprehensively analyze the resilience of island beaches and the impact of climate change on sea turtle nesting site morphology.
North Island of the Xisha Islands is one of the most important nesting beaches for green turtles in China, with over 500 nests over the past five years. Therefore, protective measures are required to safeguard beaches and ensure green turtle survival. The most urgent actions are to prevent further habitat loss, especially on the southern and southwestern sides of North Island, and to restore the beach habitat. This can be addressed by protecting and restoring the dune ecosystems behind the beaches. Scientific efforts should focus on addressing the current issues facing the nesting grounds of green turtles on North Island, such as clearing large rocks and accumulated dead organic matter, providing more suitable gravel types for nesting, and conducting artificial repair of beach fractures. The relocation of green turtle nests located at low tide or the use of greenhouses during typhoons should also be implemented as part of management and rescue measures (Mazaris et al., 2009).
5 Conclusions
In conclusion, owing to the influence of climatic factors (e.g., cycles of erosion), the shape and area of North Island have changed. The monitoring results of green turtle nesting grounds from 2021 to 2022 indicate that the beach area of North Island had a downward trend, with noticeable changes in slope. Scarps were formed in some cases, preventing turtles from reaching suitable areas for nesting. The peak nesting period of green turtles coincides with the frequent typhoon season in the South China Sea. Typhoon-induced surges can lead to the inundation of turtle eggs, thereby halting their development. Therefore, protective measures are required to safeguard beaches and ensure the survival of green sea turtles.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author contributions
TZ: Writing – original draft, Methodology, Investigation, Formal Analysis, Conceptualization. CZ: Writing – review & editing, Formal Analysis. YL: Writing – review & editing. YY: Writing – review & editing, Methodology, Investigation. XA: Writing – review & editing, Investigation. YJ: Writing – review & editing, Investigation. JW: Writing – review & editing, Formal Analysis, Conceptualization. LL: Writing – review & editing, Conceptualization. H-TS: Writing – review & editing, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (Grant Numbers 32471736, 32400408, 32170532, 32160135), the China Postdoctoral Science Foundation (Grant Number 2024M750703), the Hainan Science Popularisation Project (Project No. KPHD202406), and the Research Project on Education and Teaching Reform in Higher Education Institutions in Hainan Province (Project No. Hnjg-2024-47).
Acknowledgments
We are grateful to Meimei Li, Deqin Li, and Rui Huang for their assistance with sample collection, and Huaiqing Chen for his assistance with providing advice on statistical analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ackerman R. A. (1997). “The nest environment and the embryonic development of sea turtles,” in The Biology of Sea Turtles. Eds. Lutz P. L., Musick J. A. (CRC Press, Boca Raton, FL), 83–106.
Basyoni M. H. (1999). Sedimentology and geochemistry of the Karan Island sediments, Arabian gulf, Saudi Arabia. J. King Abdulaziz Univ. 10, 89–106. doi: 10.4197/mar.10-1.6
Casale P., Ceriani S. A. (2019). Satellite surveys: a novel approach for assessing sea turtle nesting activity and distribution. Mar. Biol. 166, 47. doi: 10.1007/s00227-019-3494-4
Chaloupka M., Bjorndal K. A., Balazs G. H., Bolten A. B., Ehrhart L. M., Limpus C. J., et al. (2008). Encouraging outlook for recovery of a once severely exploited marine mega herbivore. Global Ecol. Biogeogr. 17, 297–304. doi: 10.1111/j.1466-8238.2007.00367.x
Emery K. O. (1961). A simple method of measuring beach profiles. Limnol. Oceanogr 6, 90–93. doi: 10.2307/2832794
Fish M. R., Cote I. M., Gill J. A., Jones A. P., Renshoff S., Watkinson A. R. (2005). Predicting the impact of sea-level rise on Caribbean sea turtle nesting habitat. Conserv. Biol. 19, 482–491. doi: 10.1111/j.1523-1739.2005.00146.x
Fish M. R., Cote I. M., Horrocks J. A., Mulligan B., Watkinson A. R., Jones A. P. (2008). Construction setback regulations and sea level rise: Mitigating Sea turtle nesting beach loss. Ocean & Coastal Manage. 51, 330–341. doi: 10.1016/j.ocecoaman.2007.09.002
Fuentes M. M. P. B., Limpus C. J., Hamann M. (2011). Vulnerability of sea turtle nesting grounds to climate change. Global Change Biol. 17, 140–153. doi: 10.1111/j.1365-2486.2010.02192.x
Fuentes M. M. P. B., Limpus C. J., Hamann M., Dawson J. (2010). Potential impacts of projected sea-level rise on sea turtle rookeries. Aquat. Conserv. 20, 132–139. doi: 10.1002/aqc.v20:2
Fujisaki I., Lamont M., Carthy R. (2018). Temporal shift of sea turtle nest sites in an eroding barrier island beach. Ocean Coast. Manage. 155, 24–29. doi: 10.1016/j.ocecoaman.2017.12.032
Gaillard D., Yeh F. C., Lin L., Chen H., Zhang T., Luo S., et al. (2021). Lost at sea: determining geographic origins of illegally traded green sea turtles (Chelonia mydas) rescued on Hainan Island, China. Wildlife Res. 48, 55–63. doi: 10.1071/WR19127
Hiebert K., Shaker J., Llamas I., Fish M., Santos K. (2017). A methodological framework for assessing sea level rise effects on a marine turtle nesting beach: a case study at Mayto, Jalisco, Mexico. Global Ecol. Conserv. 6, 93–100.
Jensen M. P., FitzSimmons N. N., Dutton P. H. (2013). “Molecular genetics of sea turtles,” in The Biology of Sea Turtles, vol. III . Eds. Wyneken J., Lohmann K. J., Musick J. A. (CRC Press, Boca Raton, FL), 135–161.
Kasparek M., Godley B. J., Broderick A. C. (2001). Nesting of green turtle, Chelonia mydas, in the Mediterranean: a review of status and conservation needs. Zool. Middle East 24, 45–74. doi: 10.1080/09397140.2001.10637885
Katselidis K. A., Schofield G., Stamou G., Dimopoulos P., Pantis J. D. (2014). Employing Sea level rise scenarios to strategically select sea turtle nesting habitat important for long term management at a temperate breeding area. J. Exp. Mar. Biol. Ecol. 450, 47–54. doi: 10.1016/j.jembe.2013.10.017
Li M. M., Zhang T., Liu Y., Li Y., Fong J. J., Yu Y., et al. (2023). Revisiting the genetic diversity and population structure of the endangered Green Sea Turtle (Chelonia mydas) breeding populations in the Xisha (Paracel) Islands, South China Sea. PeerJ 11, e15115. doi: 10.7717/peerj.15115
Liles M. J., Peterson M. J., Seminoff J. A., Altamirano E., Henríquez A. V., Gaos A. R., et al. (2015). One size does not fit all: Importance of adjusting conservation practices for endangered hawksbill turtles to address local nesting habitat needs in the eastern Pacific Ocean. Biol. Conserv. 184, 405–413. doi: 10.1016/j.biocon.2015.02.017
Limpus C. J., Miller J. D., Parmenter C. J., Limpus D. J. (2003). The green turtle, Chelonia mydas, population of Raine Island and the northern great barrier reef: 1843–2001. Memoirs Queensland Museum 49, 349–440.
Luo H., Fu C. B., Ouyang H. X. (2018). The change of precipitation and rainy days in Xisha Waters of South China. Torrential Rain Disasters 37, 90–96.
Maison K. A., King R., Lloyd C., Echert S. (2010). Leatherback nest distribution and beach erosion pattern at Levera Beach, Grenada, West Indies. Mar. Turtle Newslett. 127, 9–12.
Maneja R. H., Miller J. D., Li W., Thomas R., El-Askary H., Perera S., et al. (2021). Multidecadal analysis of beach loss at the major offshore sea turtle nesting islands in the Northern Arabian gulf. Ecol. Indic. 121, 107146. doi: 10.1016/j.ecolind.2020.107146
Maurer A. S. E., Johnson M. W. (2017). Loggerhead nesting in the northern Gulf of Mexico: importance of beach slope to nest site selection in the Mississippi Barrier Islands. Chelonian Conserv. Biol. 16, 250–254. doi: 10.2744/CCB-1256.1
Mazaris A. D., Matsinos Y. G., Pantis J. D. (2009). Evaluating the impacts of coastal squeeze on sea turtle nesting. Ocean Coast. Manage. 52, 139–145. doi: 10.1016/j.ocecoaman.2008.10.005
McClenachan L., Jackson J. B., Newman M. J. (2006). Conservation implications of historic sea turtle nesting beach. Front. Ecol. and the Environ. 4, 290–296. doi: 10.1890/1540-9295(2006)4[290:CIOHST]2.0.CO;2
Miller J. D. (1989). Marine Turtles Volume 1: An assessment of the conservation status of marine turtles in the Kingdom of Saudi Arabia. Technical Report, MEPA Coastal and Marine Management Series, Report No.9. 209.
Poloczanska E. S., Limpus C. J., Hays G. C. (2009). Vulnerability of marine turtles to climate change. Adv. Mar. Biol. 56, 151–211. doi: 10.1016/S0065-2881(09)56002-6
Reece J. S., Passeri D., Ehrhart L., Hagen S. C., Hays A., Long C., et al. (2013). Sea level rise, land use, and climate change influence the distribution of loggerhead turtle nests at the largest USA rookery (Melbourne Beach, Florida). Mar. Ecol. Prog. Ser. 493, 259–274. doi: 10.3354/meps10531
Resetarits W. J. (1996). Oviposition site choice and life history evolution. Am. Zoologist 36, 205–215. doi: 10.1093/icb/36.2.205
Rumaida M. Y., Putra S. A., Mulyadi A., Nasution S. (2021). Nesting habitat characteristics of green sea turtle (Chelonia mydas) in the Tambelan archipelago, Indonesia. J. Coast. Conserv. 25, 6. doi: 10.1007/s11852-021-00798-4
Sönmez B., Yalçın-Ozdilek S. (2013). Conservation technique of the green turtle (Chelonia mydas L. 1758) nests under the risk of tidal inundation with hatcheries, on Samanda˘ g beach. Turkey. Russian J. Herpetol. 20, 19–26.
Schlacher T. A., Schoeman D. S., Dugan J., Lastra M., Jones A., Scapini F., et al. (2008). Sandy beach ecosystems: key features, sampling issues, management challenges and climate change impacts. Mar. Ecol. 29, 70–90. doi: 10.1111/j.1439-0485.2007.00204.x
Serafini T. Z., Lopez G. G., Bernardo da Rocha P. L. (2009). Nest site selection and hatching success of hawksbill and loggerhead sea turtles (Testudines, Cheloniidae) at Arembepe Beach, northeastern Brazil. Phyllomedusa J. Herpetol. 8, 3–17. doi: 10.11606/issn.2316-9079.v8i1p03-17
Silva I. S. S., Arantes M. O., Hackradt C. W., Schiavetti A. (2020). Environmental and anthropogenic factors affecting nesting site selection by sea turtles. Mar. Environ. Res. 162, 105090. doi: 10.1016/j.marenvres.2020.105090
Stokes H. J., Esteban N., Graeme C., Hays G. C. (2024). Nest site selection in sea turtles shows consistencies across the globe in the face of climate change. Anim. Behav. 208, 59–68. doi: 10.1016/j.anbehav.2023.12.001
Turner M., Batianoff G. (2007). “Vulnerability of island flora and fauna in the Great Barrier Reef to climate change,” in Climate Change and the Great Barrier Reef: A Vulnerability Assessment. Eds. Johnson J., Marshall P. (Great Barrier Reef Marine Park Authority and Green house Office, Townsville), 621–666.
Veelenturf C. A., Sinclair E. M., Paladino F. V., Honarvar S. (2020). Predicting the impacts of sea level rise in sea turtle nesting habitat on Bioko Island, Equatorial Guinea. PloS One 15, e0222251. doi: 10.1371/journal.pone.0222251
Venter O., Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., et al. (2016). Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558. doi: 10.1038/ncomms12558
Wang D., Shi M., Nan F. (2012). Study on the characteristics of tides and residual currents in Xisha Islands. J. Ocean Univ. China 42, 1–9.
Weber S. B., Weber N., Ellick J., Avery A., Frauenstein R., Godley B. J., et al. (2014). Recovery of the South Atlantic’s largest green turtle nesting population. Biodiversity Conserv. 23, 3005–3018. doi: 10.1007/s10531-014-0759-6
Wentworth C. K. (1922). A scale of grade and class terms for clastic sediments. J. Geol. 30, 377–392. doi: 10.1086/622910
Wood D. W., Bjorndal K. A. (2000). Relation of temperature, moisture, salinity, and slope to nest site selection in Loggerhead Sea Turtles. Copeia 2000, 119–128. doi: 10.1643/0045-8511(2000)2000[0119:ROTMSA]2.0.CO;2
Yalçın Ozdilek S. (2007). Status of sea turtles (Chelonia mydas and Caretta caretta) on Samanda˘ g Beach, Turkey: evaluation of five-year monitoring study. Annales Zoologici Fennici 44, 333–347.
Ye J. Z. (1996). Environmental hydrological characteristics of Xisha Islands. Acta Scientiarum Naturalium Universitatis Sunyatseni 35, 19–25.
Zhang T., Zhang C. L., Liu Y., Li Y., Yu Y., Wang J., et al. (2024). Selection characteristics and utilization of nesting grounds by green sea turtles on Xisha Islands, South China Sea. Global Ecol. Conserv. 54, e03091. doi: 10.1016/j.gecco.2024.e03091
Keywords: beach erosion, green turtle, hatching success, island morphological changes, nesting ground
Citation: Zhang T, Zhang C, Li Y, Yu Y, An X, Jiang Y, Wang J, Lin L and Shi H-T (2024) Beach erosion and typhoons reduce green turtle nesting grounds on the Xisha Islands, South China Sea. Front. Mar. Sci. 11:1470777. doi: 10.3389/fmars.2024.1470777
Received: 26 July 2024; Accepted: 13 November 2024;
Published: 29 November 2024.
Edited by:
Eduardo Cuevas, Autonomous University of Baja California, MexicoReviewed by:
Nancy Pérez Morga, Universidad Autónoma del Carmen, MexicoMarisol Escorza-Reyes, Universidad Autónoma del Carmen, Mexico
Maria Mónica Lara Uc, Universidad Autónoma de Baja California Sur, Mexico
Copyright © 2024 Zhang, Zhang, Li, Yu, An, Jiang, Wang, Lin and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liu Lin, a3lsZWxpbmxpdUAxNjMuY29t; Hai-Tao Shi, aGFpdGFvLXNoaUAyNjMubmV0
 Ting Zhang
Ting Zhang Chenglong Zhang2,3
Chenglong Zhang2,3 Jichao Wang
Jichao Wang Liu Lin
Liu Lin