- 1Department of Natural Resources Science, University of Rhode Island, Kingston, RI, United States
- 2Independent Researcher, Sea Cow’s Bay, Tortola, British Virgin Islands
- 3Ministry of Environment, Natural Resources, Climate Change, Road Town, Tortola, British Virgin Islands
- 4Independent Researcher, Kaneohe, HI, United States
Stony coral tissue loss disease (SCTLD) causes severe mortality in many hard corals and is now present in most of the Caribbean. The application of amoxicillin paste is currently the most successful local intervention to treat SCTLD lesions in nature, but the potential development of antibiotic resistance makes alternatives valuable. In a preliminary field trial (n = 84 corals), we compared two treatments against SCTLD, (1) amoxicillin paste and (2) chlorine mixed with cocoa butter paste and covered with a clay band. We found that amoxicillin and chlorine treatments both significantly reduced the rate of tissue loss in SCLTD-affected corals as compared to controls. Amoxicillin treatment was the most effective and effectively halted tissue loss in 78% of colonies. Even so, chlorine treated colonies lost tissue at approximately half the rate of untreated controls. The non-specific antiseptic nature of chlorine treatments may also be useful for other tissue loss diseases of unknown etiologies. Although, not perfect, the chlorinated cocoa butter treatment can be added to the growing list of methods to reduce mortality from disease in the field.
1 Introduction
Coral diseases contribute substantially to ongoing coral community declines (Rogers and Miller, 2013), and their future impacts are likely to be exacerbated by increasing ocean temperatures and eutrophication (Harvell et al., 2007; Maynard et al., 2015). In the tropical Atlantic, the impacts of stony coral tissue loss disease (SCTLD) are particularly severe (Papke et al., 2024) because of its broad host range (at least 22 species infected, Roth et al., 2024), efficient transmission (Aeby et al., 2019; Muller et al., 2020) and high rate of mortality (e.g. Precht et al., 2016). SCTLD was first reported in 2014 in Florida, USA (Precht et al., 2016) and has subsequently spread to most other parts of the region, reaching the southernmost parts of the Caribbean in 2023 (Roth et al., 2024).
In general, efforts to develop and refine management actions to limit the spread of coral diseases aim to reduce pathogen loads in infected colonies, control local stressors that might intensify impacts, and promote coral population recovery after an outbreak (Beeden et al., 2011). Several methods have been used to treat coral diseases in-situ (summarized by Neely et al., 2021). Approaches include shading to slow disease growth, aspiration to remove diseased tissue, creating a barrier to disease spread by excavating a trench around the infection or applying materials like modelling clay and epoxy. A complementary approach, often used in combination with barriers, uses antibiotics, phages, or disinfectants like chlorine to treat infections. There is still uncertainty surrounding the etiology of SCTLD, and causal agents may include viruses or bacteria (reviewed by Papke et al., 2024). Nonetheless, the contagious transmission of SCTLD and the consistent changes in microbial community associated with disease progression (Rosales et al., 2023; Papke et al., 2024) support the use of disinfectants and/or antibiotics to reduce pathogen loads.
The application of antibiotics by divers is the most widely used method to treat corals infected with SCTLD (Papke et al., 2024). Following successful laboratory tests showing that antibiotics halted SCTLD lesions (Aeby et al., 2019; Muller et al., 2020), topical amoxicillin pastes were developed that could be applied around the perimeter of SCTLD lesions on colonies (Neely et al., 2020). Field trials in Florida showed that this approach healed or halted the spread of most active lesions, though new lesions sometimes appeared on treated colonies (Neely et al., 2020; Shilling et al., 2021; Walker et al., 2021). With periodic retreatment, the probability of reinfection was predicted to decrease through time (Neely et al., 2021). As a result, in-water treatment programs are active in at least 12 Caribbean locations (Roth et al., 2024) and treating even a fraction of corals at a site benefits the overall coral community (Forrester et al., 2022).
Despite its effectiveness, the potential for reduced effectiveness with long-term use underscores the importance of developing alternative, non-antibiotic treatments. First, the efficacy of amoxicillin treatment may vary. As example, Walker and colleagues (2021) report a 58.8% success rate of antibiotic treatment on Montastraea cavernosa whereas Neely and colleagues (2021) report effectiveness exceeding 95% on multiple other coral species, and so alternatives may be valuable for less-responsive species. Second, antibiotic pollution in the environment is a major global problem affecting human health as it can speed up the development of antibiotic resistant pathogens (CDC, 2024). Hence, using antibiotics on corals is of concern, and chlorine is a potential alternative based on its successful use to control aquatic microorganisms (Tebbutt, 1997) and wildlife diseases (Langwig et al., 2015). Chlorinated epoxy barriers were successful at treating corals with black band disease (Aeby et al., 2015), but were less effective at treating SCTLD in Florida (Neely et al., 2021; Shilling et al., 2021; Walker et al., 2021). Our objective was thus to test an alternative method of chlorine treatment - application in cocoa butter paste with a clay barrier. We hypothesized this approach might allow for a concentrated exposure of lesions to the chlorine, with the clay band also preventing treatment beyond the covered area. We compared the effectiveness of chlorine treatment in reducing the progression of SCTLD to that of amoxicillin-treated colonies and untreated controls. This study was part of a broader effort to manage impacts of SCTLD across the British Overseas Territories (Dosell et al., 2021; Meakins, 2022).
2 Materials and methods
2.1 Study sites
The study was performed at the Eastern end of Horseshoe Reef, near Anegada in the British Virgin Islands (BVI) (18°44' L, 64°20' W). Anegada and its surrounds formed as an extensive reef platform during the last interglacial highstand (roughly 130,000 years ago), distinguishing it from the other islands in the Puerto Rico/Virgin Islands platform, all of which are volcanic (Gore, 2013). Horseshoe Reef is the third largest contiguous reef in the Eastern Caribbean (133 km2). It comprises a high energy windward barrier reef plus an extensive network of shallow leeward patch reefs, both of which supported coral cover (often exceeding 50%) and diversity in the 1960-1970s (Ogden, 1977; Dunne and Brown, 1979; Brown and Dunne, 1980). Because of its biological richness and valuable fisheries, a large part of Horseshoe Reef was declared a Fisheries Protected Area in 1990 under the Virgin Islands Fisheries Ordinance. It thus exemplifies a site of high conservation values and for this reason was an area of high priority for the BVI SCTLD treatment program.
SCTLD was first discovered in the BVI in 2020 and had transitioned to endemic status (as defined in Neely, 2018a) by the start of the study. Perhaps because of its separation from other islands and local efforts to mitigate the spread of SCTLD (Forrester et al., 2022), the disease was not observed around Anegada until January 2022 and our study sites were still in epidemic phase (as defined in Neely, 2018a) at the start of the study. Corals were treated at six sites, each approximately 40 x 40 m (Supplementary Table 1).
2.2 Study design and treatments
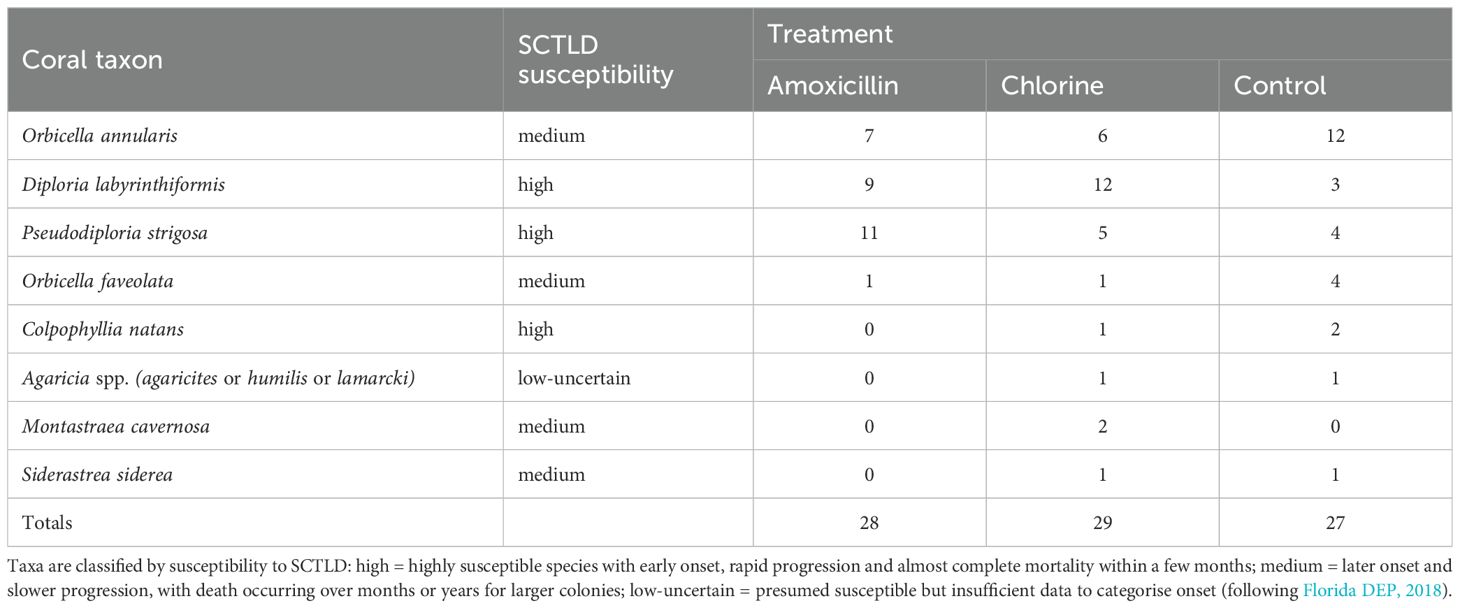
We used a simple experimental design, in which corals with signs of SCTLD lesions were haphazardly assigned to one of three treatments: (i) chlorine, (ii) amoxicillin, or (iii) control - no treatment. We treated 84 corals of several species, and sample sizes were unequal across species (Table 1) because field time was limited and the abundance and diversity of corals that could be treated varied at the sites.
Most experimental corals (77 of 84) were initially treated between 09-23 January, and the 5 remaining corals were treated between 22 February and 02 March 2023. All corals were tagged, and their location mapped relative to a permanently marked 30 m linear transect. Control colonies were left untreated. For the antibiotic treatment, amoxicillin paste (Coral Cure Base2b, Ocean Alchemists LLC) was applied to SCTLD lesions using a syringe (see Neely, 2018b). For the chlorine treatment, a cocoa butter/mineral oil paste (7:1 by volume) was used as base. Chlorine granules (68% calcium hypochlorite) were ground into a finer powder using a mortar and pestle and mixed into the cocoa butter base (ratio 1:1 by mass). The paste was placed into 60 ml syringes and applied to SCTLD lesions. A roughly 5 mm thick sheet of non-hardening modelling clay (Sargent Art plasticina) was applied over the paste, overlapping 3-5 cm into the adjacent live tissue, to contain the chlorine (Supplementary Figure 1).
Any new lesions that appeared during the study were treated as encountered, and existing lesions were retreated if the clay barrier was dislodged, or the disease progressed past the barrier. We compared the retreatment rate for amoxicillin- and chlorine-treated colonies by recording the percentage of visits during which re-application was needed.
Coral disease prevalence, spread and virulence can be influenced by biotic and abiotic factors (Harvell et al., 2007) and SCTLD is no exception. Studies have reported differences in virulence among species (Sharp et al., 2020; Alvarez-Filip et al., 2022), regions (Aeby et al., 2019; Sharp et al., 2020) and with heat stress (Meiling et al., 2020). As such, sites were surveyed on SCUBA from December 2022 - February 2023 (pre-treatment) to provide background on the coral species composition and general state of the coral reef where treatments would be conducted. A permanent 30 m transect centered at each site was used for the surveys and as a reference to help relocate treated corals. Coral community composition was described by recording the species of all colonies counted within a 30 x 2 m belt (60 m2) centered on the tape. Percent cover of benthic substrata was estimated by recording the substrate underlying the tape every 0.5 m. Coral diseases (SCTLD & other endemic diseases) were documented by recording and photographing all colonies with visible disease lesions along the same 30 m transect but the width was extended out to 6 meters (180 m2).
2.3 Monitoring corals
We measured, photographed, and described SCTLD lesions for all tagged corals approximately every 4 weeks (4 or 5 occasions per colony) until the end of the study (03 May 2023). Each colony was measured in length (L) width (W) and height (H) in cm, and colony surface area (CSA) was estimated as
assuming colonies were hemispherical in shape (Fisher et al., 2008). Treated corals were variable in size, but sizes were generally similar across treatments so this should not have biased the outcome (Supplementary Figure 2).
During each visit, a single observer (AH) visually estimated the percent of tissue that was live (PL) for each colony. The surface area of live tissue (CSAL) was calculated as
and the daily rate of tissue loss (TL) was calculated from change in CSAL as
where time is the number of days between the initial and final measurement.
We calculated the difference between the initial and final percentage of live tissue on each colony as an approximate index of whether the treatments halted, or substantially slowed, the overall progression of the disease. Visual estimates of PL are typically accurate to within 10% (Neely, 2024), so the progression of disease was classified as halted for surviving colonies with <10% change in PL.
On each visit, we recorded the number of lesions on each living colony and compared the rate at which colonies developed lesions (new lesions per colony per day) among the three treatments.
2.4 Data analysis
Statistical modelling was done in the R programming environment, using the packages survival (Therneau, 2024) and stats (R Core Team, 2024). Differences among treatments in all coral responses except survival were tested using Welch’s one-way ANOVA followed by pairwise comparisons using the Games-Howell method (when data were normally distributed but heteroscedastic), or Kruskal-Wallis one-way ANOVA followed by Dunn’s test for pairwise comparisons (when data were not normally distributed nor homoscedastic). We compared survival probabilities among treatments using the Kaplan-Meier method for interval- and right-censored data, with differences indicated by lack of overlap in 95% confidence intervals. All p-values were adjusted for multiple comparisons using Holm’s method.
3 Results
3.1 Condition of coral reefs at study areas
Pre-treatment surveys found up to 12 species of hard coral within transects with the numerically dominant coral genera being Orbicella spp. and Porites spp. (Supplementary Table 3). Mean coral cover was 7.2% (SD ± 4.2%) and mean macroalgae cover was 35.3% (SD ± 17.9%) (Supplementary Table 2). SCTLD was found at all six sites, and SCTLD lesions were observed on nine species (Supplementary Table 4). The mean prevalence of SCTLD was 2.8% (SD ± 2.0%) (Supplementary Table 2), and it was the most frequently observed disease (71% of all disease lesions). Seven other, presumably endemic, diseases were observed and included Porites focal bleaching and chronic tissue loss disease, Siderastraea siderea dark spot and chronic tissue loss disease, Orbicella focal bleaching and growth anomalies, and Diploria labyrinthiformis growth anomalies. Mean endemic disease prevalence (excluding SCTLD) was 0.65% (SD ± 0.46%) (Supplementary Table 5).
3.2 Treatment effects on experimental corals
Reapplication of amoxicillin paste was required on 44% of visits, whereas the chlorine-treated corals required reapplication of the paste and/or clay band on 79% of visits (tWelch = 5.91, p <0.0001).
Most treated corals initially had a single SCTLD lesion, with a maximum of six lesions on a single colony (Supplementary Figure 4). Neither of the treatment methods prevented the development of new lesions and the rate at which they appeared on chlorine-treated corals (mean ± SD = 0.005 ± 0.004 lesions per coral per day) was indistinguishable from that on corals treated with amoxicillin (mean ± SD = 0.002 ± 0.004 lesions per coral per day) (tWelch = 1.54, p = 0.13).
At the start of the experiment, the amount of live tissue (%) was variable among treated colonies but similar overall across the three treatments (Supplementary Figure 3). Once treatment commenced, rates of tissue loss differed significantly among the treatments (χ2Kruskall-Wallis = 32.2, p < 0.0001). With data from all coral species pooled, compared to untreated controls, tissue loss was significantly slower in chlorine-treated colonies (p = 0.02) and amoxicillin-treated colonies (p < 0.0001). amoxicillin-treated colonies lost tissue at a significantly slower rate than chlorine-treated colonies (p = 0.016) (Figure 1). The absolute amount of tissue lost is time-dependent but, for descriptive purposes, we note that roughly two thirds of the way through the experiment (after 80 days) the median percent of tissue lost was 74.4% for controls, 17.6% for chlorine-treated colonies and 1.7% for amoxicillin-treated colonies.
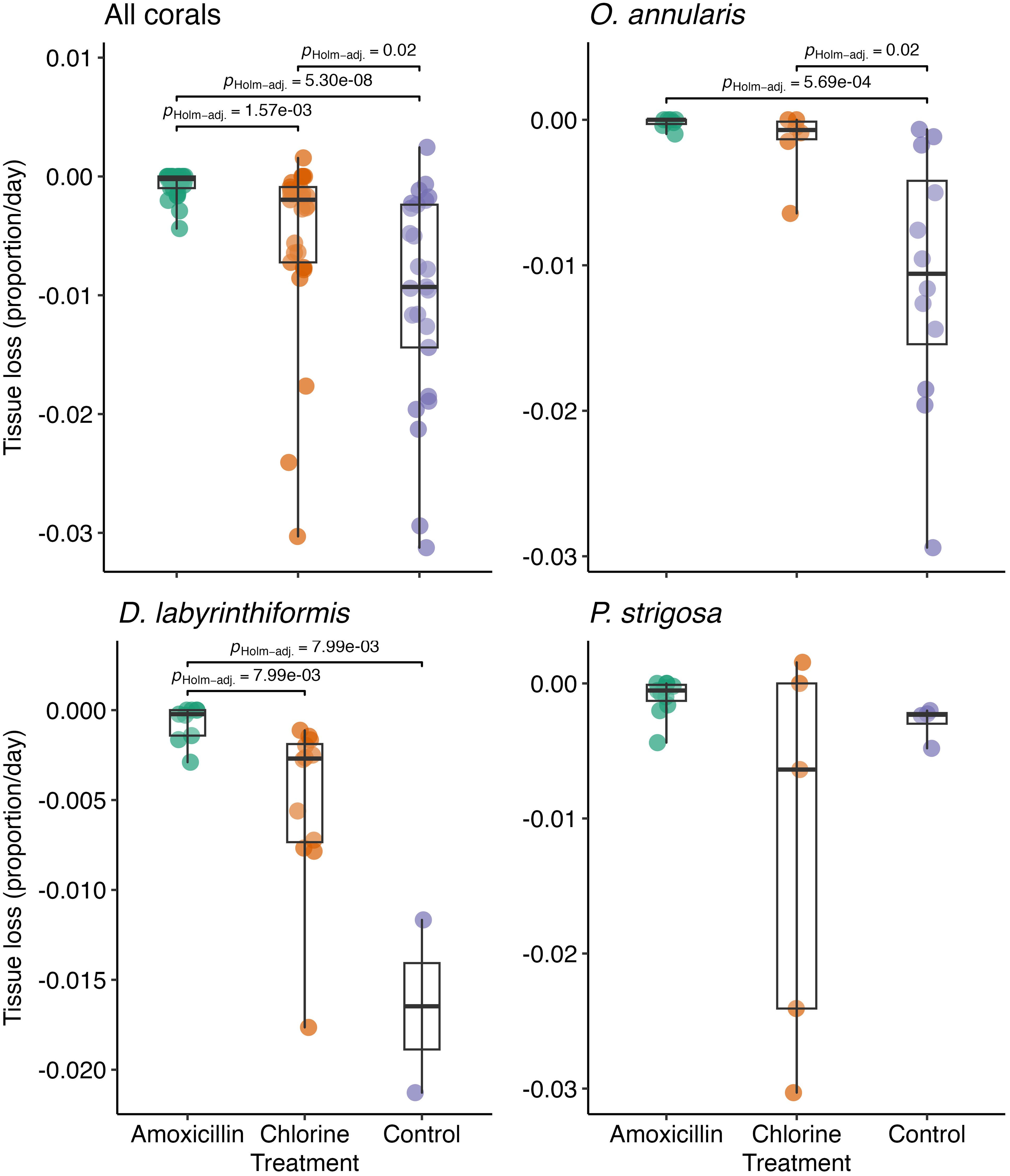
Figure 1. Boxplots displaying the rate of tissue loss for corals in each treatment with p-values for significant pairwise differences between treatments. Separate plots show data for all corals, plus each of the three most common coral species.
Qualitatively similar patterns of median tissue loss were observed when data for the three most common coral species were plotted separately, although variability among species was evident (Figure 1). Relative to control colonies, amoxicillin significantly reduced tissue loss in O. annularis (p = 0.0005) and D. labyrinthiformis (p = 0.008), chlorine treatment significantly reduced tissue loss in O. annularis (p = 0.02) and no significant differences were found with either treatment in P. strigosa (χ2Kruskall-Wallis = 3.94, p = 0.14).
A lesion was considered successfully halted over time if a colony lost <10% tissue from the beginning to the end of the study. For amoxicillin treatment, 22 of 28 colonies lost <10% of initial live tissue (78%), for chlorine treatment 6 of 29 (21%) of colonies lost <10% and in control colonies 4 of 25 colonies (16%) lost <10% tissue.
We detected no significant differences in predicted colony survival probability among treatments (p always > 0.05). Nonetheless, the data were suggestive of higher survival of the amoxicillin-treated colonies because all colonies survived the study and, consistent with the data on tissue loss, the survival rate of chlorine-treated corals appeared to be intermediate between that of the controls and amoxicillin-treated colonies (Figure 2).

Figure 2. Survival curves for corals in each treatment. Separate plots show data for all corals, plus each of the three most common coral species.
4 Discussion
We found that amoxicillin and chlorine treatments were both effective at reducing tissue loss in SCLTD-affected corals relative to controls, but not surprisingly, amoxicillin treatment was the most effective. Amoxicillin has been shown to be effective at treating SCTLD in previous studies in Florida (Neely et al., 2020, Neely et al., 2021; Shilling et al., 2021; Walker et al., 2021) and Belize (Lee Hing et al., 2022). The chlorine treatment we tested (chlorine mixed with cocoa butter and covered with a clay band) was less successful than amoxicillin, but can slow down SCTLD lesions. Chlorine mixed with marine epoxy was tested on SCTLD-affected colonies in prior studies, but comparisons with our results are difficult because they focused on whether lesions were stopped rather than emphasizing rates of tissue loss (Neely et al., 2021; Walker et al., 2021). However, a consistent finding is that amoxicillin is a more effective treatment for SCTLD than chlorine, whether delivered in epoxy or cocoa butter.
Although our sample sizes were small, our results suggest possible differences among species in response to treatments may allow future efforts for SCTLD to be refined. Orbicella annularis had a significant reduction in tissue loss when treated with antibiotics and the chlorine mixture, Diploria labyrinthiformis only responded to antibiotics and there was no effect of either treatment on Pseudodiploria strigosa. Neely and colleagues (2021) also noted some species-specific differences in antibiotic treatment for SCTLD, but these were not statistically significant. Similarly, Shilling and coworkers (2021) tested different treatment methods (amoxicillin and chlorine mixed with marine epoxy) for SCTLD in Montastrea cavernosa and found that lesions on approximately 40% of their control colonies naturally quiesced after 46 weeks. This highlights the potentially species-specific nature of SCTLD in corals which can be integrated into treatment strategies.
Continually improving our understanding on how mortality from disease can be managed in the field may also be useful for other tissue loss diseases of unknown etiology or if long-term use of antibiotics diminishes their effectiveness. Repetitive use of antibiotics may have unintended side effects, such as the risk of the development of antibiotic-resistant bacteria (Bengtsson-Palme et al., 2018; Griffin et al., 2020; Liu et al., 2020). Antibiotic resistance a global problem that is impacting human health (CDC, 2024) and so application of antibiotics in the environment should always be done with caution to limit ecological side-effects (Hatosy and Martiny, 2015; Gomez-Olivan et al., 2016). Highlighting these challenges, a recent study in Florida found tissue loss lesions, grossly consistent with SCTLD, that were not responsive to amoxicillin treatment (Neely, 2023). Coral disease cannot be diagnosed in the field from gross lesions (Work and Aeby, 2006; Raymundo et al., 2008) and so this lack of response to amoxicillin could be due to development of antibiotic-resistant SCTLD pathogens or the emergence of a new coral pathogen.
From our pre-treatment surveys, we found up to 12 coral species at our study sites, from 5 to13% hard coral cover and prevalence of non SCTLD coral disease lesions to be < 1%. This suggests our Horseshoe Reef study area is in better condition than heavily impacted reefs in Florida where most SCTLD research has been done (Souter et al., 2022) and, as SCTLD spreads, further studies should consider whether corals in varying condition upon disease onset respond differently to treatment. We also note that our study was partly motivated by a practical constraint of the amoxicillin method - the need for regular reapplication (Neely et al., 2021). We hypothesized that a potential advantage of the chlorine treatment might be a reduced need for re-treatment, but strong currents and tidal surge at the study sites created practical difficulties applying both the chlorine and antibiotic treatments, and the clay barriers were particularly vulnerable to dislodgement in between visits. Future trials could thus also test if applying chlorine in paste with a clay barrier is more durable, and so more effective, at low energy sites.
The treatment of individual coral colonies by divers, whether with antibiotics, chlorine or other agents, is not practical as a long-term region-wide solution to controlling the impact of SCTLD or other future coral diseases. Nonetheless, it may represent a valuable component of management plans, particularly at sites of high conservation value like our study site at Horseshoe Reef. Although amoxicillin treatment is currently the most effective local intervention method to mitigate SCTLD outbreaks, it may have unintentional side effects via influences on the microbiomes of healthy coral (Connelly et al., 2022) and/or, the development of antibiotic resistance bacteria (Griffin et al., 2020; Liu et al., 2020). As SCTLD outbreaks occur and the disease becomes endemic in more Caribbean locations, alternatives to amoxicillin treatment will become increasingly useful.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
GF: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. LA: Writing – review & editing, Resources, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. AH: Writing – review & editing, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. GA: Writing – review & editing, Validation, Supervision, Resources, Methodology, Investigation, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for the study was provided by a UK Government Darwin Plus Grant Scheme Award to the Joint Nature Conservation Committee (Project DPLUS147) and the Falconwood Foundation.
Acknowledgments
We thank the Government of the Virgin Islands for permission to conduct the treatment study within the Fisheries Protected Area, Beyond the Reef for the boat and equipment use, Margy Church, Katie Nickles, and Rebecca O’keefe-Davis for help with fieldwork and the peer reviewers for thoughtful feedback.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1465173/full#supplementary-material
References
Aeby G. S., Ushijima B., Campbell J. E., Jones S., Williams G. J., Meyer J. L., et al. (2019). Pathogenesis of a tissue loss disease affecting multiple species of corals along the florida reef tract. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00678
Aeby G. S., Work T. M., Runyon C. M., Shore-Maggio A., Ushijima B., Videau P., et al. (2015). First record of black band disease in the hawaiian archipelago: response, outbreak status, virulence, and a method of treatment. PloS One 10, e0120853. doi: 10.1371/journal.pone.0120853
Alvarez-Filip L., González-Barrios F. J., Pérez-Cervantes E., Molina-Hernández A., Estrada-Saldívar N. (2022). Stony coral tissue loss disease decimated Caribbean coral populations and reshaped reef functionality. Commun. Biol. 5, 440. doi: 10.1038/s42003-022-03398-6
Beeden R., Maynard J. A., Marshall P. A., Heron S. F., Willis B. L. (2011). A framework for responding to coral disease outbreaks that facilitates adaptive management. Environ. Manage. 49, 1–13. doi: 10.1007/s00267-011-9770-9
Bengtsson-Palme J., Kristiansson E., Larsson D. G. J. (2018). Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 42, fux053. doi: 10.1093/femsre/fux053
Brown B. E., Dunne R. P. (1980). Environmental controls of patch-reef growth and development. Mar. Biol. 56, 85–96. doi: 10.1007/BF00390598
CDC (2024).Antimicrobial resistance. In: Antimicrobial resistance. Available online at: https://www.cdc.gov/antimicrobial-resistance/index.html (Accessed August 24, 2024).
Connelly M. T., McRae C. J., Liu P.-J., Martin C. E., Traylor-Knowles N. (2022). Antibiotics alter pocillopora coral-symbiodiniaceae-bacteria interactions and cause microbial dysbiosis during heat stress. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.814124
Dosell A., Aeby G., Britton A., Meakins B. (2021). Stony coral tissue loss disease: practical handbook (Peterborough, United Kingdom: Joint Nature Conservation Committee).
Dunne R. P., Brown B. E. (1979). Some aspects of the ecology of reefs surrounding Anegada, British Virgin Islands. Atoll Res. Bull. 236, 1–80. doi: 10.5479/si.00775630.236.1
Fisher W. S., Fore L. S., Hutchins A., Quarles R. L., Campbell J. G., LoBue C., et al. (2008). Evaluation of stony coral indicators for coral reef management. Mar. pollut. Bull. 56, 1737–1745. doi: 10.1016/j.marpolbul.2008.07.002
Florida DEP (2018). Stony coral tissue loss disease case definition. Available online at: https://floridadep.gov/sites/default/files/Copy%20of%20StonyCoralTissueLossDisease_CaseDefinition%20final%2010022018.pdf (Accessed September 15, 2020).
Forrester G. E., Arton L., Horton A., Nickles K., Forrester L. M. (2022). Antibiotic treatment ameliorates the impact of stony coral tissue loss disease (SCTLD) on coral communities. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.859740
Gomez-Olivan L. M., Elizalde-Velazquez A., Galar-Martinez M., Islas-Flores H., Garcia O. D., SanJuan-Reyes N. (2016).Amoxicillin in the aquatic environment, its fate and environmental risk. In: Environmental health risk - hazardous factors to living species (IntechOpen) (Accessed August 24, 2024).
Gore S. (2013). “Anegada: an emergent pleistocene reef island,” in Coral reefs of the United Kingdom overseas territories. Ed. Sheppard C. R. (Springer, Dordrecht), 47–60.
Griffin D. W., Banks K., Gregg K., Shedler S., Walker B. K. (2020). Antibiotic resistance in marine microbial communities proximal to a florida sewage outfall system. Antibiotics 9, 118. doi: 10.3390/antibiotics9030118
Harvell D., Jordán-Dahlgren E., Merkel S., Rosenberg E., Raymundo L., Smith G., et al. (2007). Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanog. 20, 172–195. doi: 10.5670/oceanog.2007.91
Hatosy S. M., Martiny A. C. (2015). The ocean as a global reservoir of antibiotic resistance genes. Appl. Environ. Microbiol. 81, 7593–7599. doi: 10.1128/AEM.00736-15
Langwig K. E., Voyles J., Wilber M. Q., Frick W. F., Murray K. A., Bolker B. M., et al. (2015). Context-dependent conservation responses to emerging wildlife diseases. Front. Ecol. Environ. 13, 195–202. doi: 10.1890/140241
Lee Hing C., Guifarro Z., Dueñas D., Ochoa G., Nunez A., Forman K., et al. (2022). Management responses in Belize and Honduras, as stony coral tissue loss disease expands its prevalence in the Mesoamerican reef. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.883062
Liu S., Su H., Pan Y.-F., Xu X.-R. (2020). Spatial and seasonal variations of antibiotics and antibiotic resistance genes and ecological risks in the coral reef regions adjacent to two typical islands in South China Sea. Mar. pollut. Bull. 158, 111424. doi: 10.1016/j.marpolbul.2020.111424
Maynard J., van Hooidonk R., Eakin C. M., Puotinen M., Garren M., Williams G., et al. (2015). Projections of climate conditions that increase coral disease susceptibility and pathogen abundance and virulence. Nat. Clim Change 5, 688–694. doi: 10.1038/nclimate2625
Meakins B. (2022). Collaborative approach to managing impacts of coral disease/Coral Conservation in the Overseas Territories Working Group (C-COT) (Peterborough, United Knngdom: Joint Nature Conservation Committee). Available online at: https://hub.jncc.gov.uk/assets/fcc00fd2-5ce9-4fce-a782-63d2ae17562b (Accessed July 10, 2024).
Meiling S., Muller E. M., Smith T. B., Brandt M. E. (2020). 3D photogrammetry reveals dynamics of stony coral tissue loss disease (SCTLD) lesion progression across a thermal stress event. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.597643
Muller E. M., Sartor C., Alcaraz N. I., van Woesik R. (2020). Spatial epidemiology of the stony-coral-tissue-loss disease in florida. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00163
Neely K. (2018a). Coral disease intervention plan (Miami, Florida, USA: Florida Department of Environmental Protection).
Neely K. (2018b). Ex-Situ disease treatment trials (Miami, Florida, USA: Florida Department of Environmental Protection).
Neely K. (2023). Observations of rapidly progressing lesions on corals within the Florida Keys National Marine Sanctuary: a Quicklook report (Florida, USA: Nova Southesatern University). Available at: https://www.agrra.org/coral-disease-resources/.
Neely K. L. (2024). Measuring coral disease lesions: a comparison of methodologies. Front. Mar. Sci. 11. doi: 10.3389/fmars.2024.1348929
Neely K. L., Macaulay K. A., Hower E. K., Dobler M. A. (2020). Effectiveness of topical antibiotics in treating corals affected by Stony Coral Tissue Loss Disease. PeerJ 8, e9289. doi: 10.7717/peerj.9289
Neely K. L., Shea C. P., Macaulay K. A., Hower E. K., Dobler M. A. (2021). Short- and long-term effectiveness of coral disease treatments. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.675349
Ogden J. (1977).Binder 10: anegada patch reefs. In: University of south florida research | Digital commons. Available online at: https://digitalcommons.usf.edu/ogden10/ (Accessed August 11, 2024).
Papke E., Carreiro A., Dennison C., Deutsch J. M., Isma L. M., Meiling S. S., et al. (2024). Stony coral tissue loss disease: a review of emergence, impacts, etiology, diagnostics, and intervention. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1321271
Precht W. F., Gintert B. E., Robbart M. L., Fura R., van Woesik R. (2016). Unprecedented disease-related coral mortality in southeastern florida. Sci. Rep. 6, 31374. doi: 10.1038/srep31374
Raymundo L., Work T. M., Bruckner A. W., Willis B. (2008). A coral disease handbook: Guidelines for assessment, monitoring, and management Coral reef targeted research and capacity building for management program (Melbourne, Australia). Available online at: https://pubs.usgs.gov/publication/70197913 (Accessed September 8, 2024).
R Core Team. (2024). “R: A language and environment for statistical computing,” in R foundation for statistical computing. (Vienna, Austria: R Foundation for Statistical Computing). Available at: https://www.R-project.org/.
Rogers C. S., Miller J. (2013). Coral diseases cause reef decline. Science 340, 1522. doi: 10.1126/science.340.6140.1522-a
Rosales S. M., Huebner L. K., Evans J. S., Apprill A., Baker A. C., Becker C. C., et al. (2023). A meta-analysis of the stony coral tissue loss disease microbiome finds key bacteria in unaffected and lesion tissue in diseased colonies. Isme Commun. 3, 1–14. doi: 10.1038/s43705-023-00220-0
Roth L., Kramer P. R., Doyle E., O’Sullivan C. (2024). Caribbean SCTLD dashboard (ArcGIS online). Available online at: https://www.agrra.org (Accessed June 19, 2024).
Sharp W. C., Shea C. P., Maxwell K. E., Muller E. M., Hunt J. H. (2020). Evaluating the small-scale epidemiology of the stony-coral-tissue-loss-disease in the middle Florida Keys. PloS One 15, e0241871. doi: 10.1371/journal.pone.0241871
Shilling E. N., Combs I. R., Voss J. D. (2021). Assessing the effectiveness of two intervention methods for stony coral tissue loss disease on Montastraea cavernosa. Sci. Rep. 11, 8566. doi: 10.1038/s41598-021-86926-4
Souter D., Planes S., Wicquart J., Logan M., Obura D., Staub F., et al. (2022). Status and trends of coral reefs of the Caribbean region. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI). doi: 10.59387/WOTJ9184
Tebbutt T. H. Y. (1997). Principles of water quality control. (Oxford, United Kingdom: Elsevier Science & Technology). Available online at: http://ebookcentral.proquest.com/lib/uri/detail.action?docID=318390 (Accessed June 26, 2024).
Therneau T. (2024). A package for survival analysis in R. Available online at: https://CRAN.R-project.org/package=survival (Accessed June 28, 2024).
Walker B. K., Turner N. R., Noren H. K. G., Buckley S. F., Pitts K. A. (2021). Optimizing stony coral tissue loss disease (SCTLD) intervention treatments on montastraea cavernosa in an endemic zone. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.666224
Keywords: amoxicillin, clay barrier, cocoa butter, epidemic, mortality
Citation: Forrester GE, Arton L, Horton A and Aeby G (2024) The relative effectiveness of chlorine and antibiotic treatments for stony coral tissue loss disease. Front. Mar. Sci. 11:1465173. doi: 10.3389/fmars.2024.1465173
Received: 15 July 2024; Accepted: 16 September 2024;
Published: 14 November 2024.
Edited by:
Christina A. Kellogg, United States Department of the Interior, United StatesReviewed by:
Abigail S. Clark, Boy Scouts of America, United StatesKaren Lynn Neely, Nova Southeastern University, United States
Copyright © 2024 Forrester, Arton, Horton and Aeby. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Graham E. Forrester, Z2ZvcnJlc3RlckB1cmkuZWR1
†Present address: Laura Arton, Centre for Applied Marine Studies, H. Lavity Stoutt Community College, Paraquita Bay, British Virgin Islands
‡These authors have contributed equally to this work
 Graham E. Forrester
Graham E. Forrester Laura Arton
Laura Arton Argel Horton
Argel Horton Greta Aeby
Greta Aeby