- 1Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
- 2Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou, China
- 3School of Life Sciences, Guangzhou University, Guangzhou, China
- 4Dalian Ocean Development Affairs Service Center, Dalian, China
- 5College of Energy Environment and Safety Engineering, China Jiliang University, Hangzhou, China
Introduction: Invasive species can cause ecological and economic damage in various areas, including nature reserves. The invasion risks of aquatic invasive vertebrates in nature reserves, however, remain unclear since this group often hides under the water and is frequently neglected in ecological surveys based on traditional methods.
Methods: Environmental DNA (eDNA) provides a promising alternative way to conduct biodiversity surveys in aquatic ecosystems. Here, we collected aquatic eDNA samples from eight nature reserves in Guangdong Province, China to mainly investigate the diversity of aquatic invasive vertebrates and inform their invasion risks in these nature reserves.
Results and discussion: We detected a total of 104 aquatic vertebrate species belonging to three classes (Actinopteri, Amphibia and Reptilia), 12 orders, 32 families, and 71 genera, among which nine were invasive species (8.65% of all aquatic vertebrates detected), i.e., Coptodon zillii, Sarotherodon galilaeus, Oreochromis niloticus, Oreochromis tanganicae, Gambusia affinis, Clarias gariepinus, Chelydra serpentina, Trachemys scripta elegans, and Rana catesbeiana. Surprisingly, 55.56% of these aquatic invasive vertebrates (i.e., five species) were found in at least 75.00% samples, and both C. zillii and S. galilaeus were detected in all samples (100%), suggesting that most invasive species were widely distributed in these nature reserves. In addition, all aquatic invasive vertebrate species ranked very high (top 66 of aquatic vertebrates detected) regarding their relative abundance of sequences, and three of the top 10 species with the highest number of sequences were invasive species (i.e., C. zillii, S. galilaeus, and O. niloticus), suggesting high population size of these invasive vertebrates. Moreover, we also detected 16 endangered/threatened species (15.38% of all vertebrates detected), which demonstrated notable overlaps of geographic distribution with invasive species. The reality of high abundance, wide geographical distribution and overlaps with the endangered/threatened species indicated considerable risks of aquatic invasive vertebrates in nature reserves in Guangdong Province, which calls for urgent needs for effective management. Our study would provide fundamental insights for the formulation of effective management measures to reduce losses caused by invasive species and promote the protection of endangered/threatened species in nature reserves.
1 Introduction
Biological invasion is one of the biggest concerns worldwide, as invasive species can cause huge ecological and economic damage to local ecosystems and economic industries by competing for space and food, preying on native species and transmitting disease (Simberloff et al., 2013; Bellard et al., 2016). Currently, various areas are threatened by invasive species, of which invasive plants and invertebrates can be easily found in farmlands, parks and even protected areas. Whereas invasive vertebrates are hardly found in these areas, since most of them are aquatic species with high mobility and often hide under the water (global invasive species database, GISD) (http://www.iucngisd.org/gisd/). Aquatic invasive vertebrates can invade streams, pools, reservoirs and wetlands, threatening rare or endangered species, because the majority of the most endangered/threatened amphibians and reptiles also live in these habitats (Ficetola et al., 2015; Xin et al., 2024) (http://www.amphibiachina.org/). In addition, many aquatic invasive vertebrates are often carnivorous or omnivorous, even a few individuals can prey on a mass of native and even endangered species, leading to high invasion risks, such as the notorious Atractosteus spatula (Li and Zhang, 2023). Hence, to formulate effective management measures, it is critical to understand the composition and distribution of aquatic invasive vertebrates in a given area, especially for areas with endangered/threatened species.
Many endangered/threatened species are protected in nature reserves, which can provide relatively undisturbed environments (Watson et al., 2014; Mi et al., 2023). Whereas many nature reserves have been disturbed by invasive species and an increasing numbers of invasive species have been reported in nature reserves, especially in countries that are highly populated, such as China (Guo et al., 2017). For example, previous investigations using traditional transects and plot surveys revealed a total of 14 and 23 invasive plant species in the Dinghushan National Nature Reserve in Guangdong Province and Shiwandashan National Natural Reserve in Guangxi Zhuang Autonomous Region, respectively (Wei et al., 2006; Song et al., 2009). Similar findings were also reported in many other nature reserves, such as the Bawangling Nature Reserves in Hainan Province, Jinfoshan Nature Reserve in Chongqing City and Lishan Nature Reserve in Shanxi Province (Guo et al., 2017). In addition, a few aquatic invasive vertebrate species (e.g. Neovison vison, Oncorhynchus mykiss and Rana catesbeiana) were also reported in nature reserves in northern China, following traditional observation-based surveys (Gong et al., 2017). However, the traditional observation-based survey methods may underestimate the diversity of aquatic invasive species due to their dwelling under the water (Zhan et al., 2017; Bailey et al., 2020). In addition, only very few studies have focused on aquatic invasive vertebrate species in nature reserves in southern China, which may suffer from severe biological invasions due to intensive anthropogenic disturbances and favorable climates (Yan et al., 2017). The above limitations or facts suggest that the composition of this group and their invasion risks in nature reserves in southern China remain poorly understood.
Environmental DNA (eDNA) technique can provide a cost-efficient way to investigate species’ richness and abundance based on high-throughput sequencing. This technique has proven effective in detecting species diversity of various aquatic groups using group-universal primers (Thomsen and Willerslev, 2015; Valentini et al., 2016; Wang et al., 2021). For example, based on 109 aquatic sampling sites from river systems in Beijing, a total of 75 fish taxa including 23 alien taxa were detected using the fish-universal primer set Ac12S, and the relative sequence abundance of these alien taxa was high, especially in areas that were highly populated (Zhang et al., 2022). Similar studies based on eDNA technique were also performed in plankton (Xiong et al., 2017; Wang et al., 2024) and other groups (Li et al., 2022). Recently, multiple vertebrate-universal primer sets have been developed, such as V12S-U (Wang et al., 2023b), MarVer1 (Valsecchi et al., 2020) and Vert-16S (Vences et al., 2016). These universal primers showed high PCR amplification success and taxonomic discrimination ability, among which V12S-U seems more suitable than other primers and has been used in diversity investigations of aquatic vertebrate species (Wang et al., 2023a, b). In addition, there are abundant 12S sequences of vertebrate species in public database, such as NCBI (National Center for Biotechnology Information) and BOLD (Barcode of Life Data System). Thus, available vertebrate-universal primers combined with sufficient reference sequences of 12S in public databases provide foundations for the reliable investigation of aquatic vertebrate species using eDNA technique.
In the present study, we collected aquatic eDNA samples from eight nature reserves in Guangdong Province with high-density populations to mainly investigate the diversity of aquatic invasive vertebrates using vertebrate-universal primers and inform their invasion risks in these nature reserves. Our study is of critical importance in making effective management measures targeting invasive species and protecting species diversity in nature reserves.
2 Material and methods
2.1 Sample collection and filtration
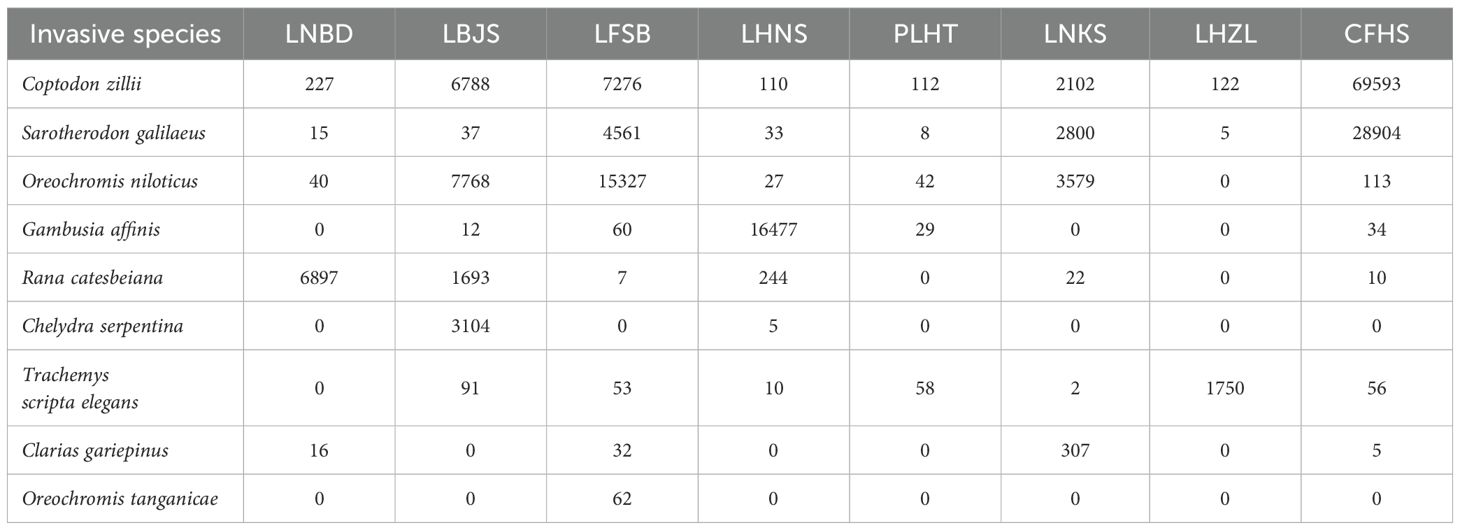
We collected aquatic eDNA samples from streams, pools or reservoirs of eight typical provincial nature reserves (Figure 1A) distributed in Guangdong Province in April 2023, i.e., Lianshan Bijiashan Nature Reserve (LBJS), Liannan Bandong Nature Reserve (LNBD), Lianping Huangniushi Nature Reserve (LHNS), Longchuan Fengshuba Nature Reserve (LFSB), Pingyuan Longwen-Huangtian Nature Reserve (PLHT), Chaoan Fenghuangshan Nature Reserve (CFHS), Longmen Nankunshan Nature Reserve (LNKS) and Luhe Nanwan Hongzhuilin Nature Reserve (LHZL). A total of 1 L mixed water was collected at three representative locations of water body from each nature reserve using brand new plastic bottles, and all water samples were placed on ice in coolers and transported to laboratory. We preserved samples at 4°C until filtration, which was completed within 24h of collection.
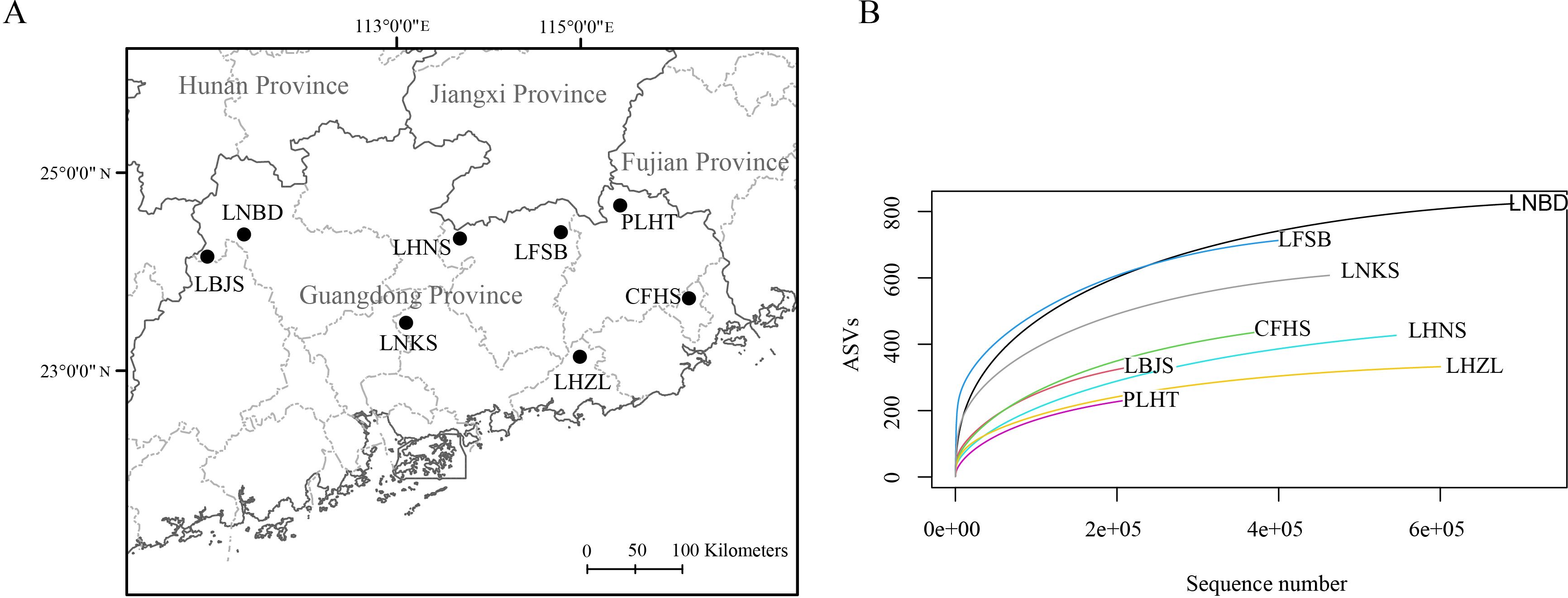
Figure 1. Sampling sites from eight nature reserves (A) and their rarefaction curves based on eDNA analysis (B).
Each water sample was filtered through 0.45 μm microporous filter membranes (Millipore, USA) with a circulating water vacuum pump in a clean laboratory in Institute of Zoology, Guangdong Academy of Sciences. In addition, 1 L double-distilled H2O (ddH2O) was also filtered and used as filtration blank. Filtration units were rinsed with 10% commercial bleach before ddH2O to remove potential cross-contamination between samples. The obtained filters were placed in 1.5 mL centrifugal tubes and preserved at -20°C until DNA extraction.
2.2 DNA extraction and high throughput sequencing
The filter-based eDNA was extracted using DNeasy Blood & Tissue Kits (QIAGEN Ltd., West Sussex, United Kingdom) according to the manufacturer’s protocol with slight modifications. Briefly, the filters were cut into small pieces using clean surgical scissors in a 2 ml centrifuge tube with 360 μm ATL buffer and 40 μm proteinase K. The obtained mixtures were incubated at 56°C for 2 h, and then 400 μm AL buffer and 400 μm ethanol were added. The new mixture was centrifuged at 6000 rpm for 1 min, the obtained supernate was transferred into a DNeasy Mini spin column and centrifuged at 8000 rpm for 1 min. The eDNA reserved in the DNeasy Mini spin column was successively washed using 500 μm AW1 buffer and 500 μm AW2 buffer by centrifuging at 8000 rpm for 1 min and 13000 rpm for 1 min, respectively. The eDNA was eluted using 80 μm AE buffer by centrifuging at 13000 rpm for 3 min, and the concentration and quality were determined using a Nanodrop One spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA).
Metabarcoding of aquatic invasive vertebrate species in nature reserves was performed using the vertebrate-universal primer pair V12S-U (Forward primer: GTGCCAGCNRCCGCGGTYANAC, Reverse primer: ATAGTRGGGTATCTAATCCYAGT), which targets an ~208 bp 12S ribosomal RNA gene fragment and has shown efficient detection of vertebrate diversity in aquatic realms (Wang et al., 2023a, b). We performed three PCR replicates for each extract of eDNA samples and filtration blanks to avoid biased amplification. Each PCR in a 25 μl total volume included 12.5 μl of 2 × Taq PCR Master Mix (Cat: B639295, Sangon Biotech, Shanghai, China), 10 pmol each of forward and reverse primers, 1 μl DNA that was diluted 5-fold to reduce the PCR inhibition. The PCR program consisted of an initial denaturation step at 95°C for 5 min; then 35 cycles of 95°C for 30 sec, 53°C for 30 sec, and 72°C for 30 sec; and a final elongation step at 72°C for 5 min. PCR products were checked using 2.0% agarose gel and no bands were detected for the filtration blanks. PCR products of the three replicates for each sample were pooled and purified using the SanPrep Column PCR Product purification kit (Cat: B518141, Sangon Biotech, Shanghai, China). The purified PCR products-based sequencing libraries were sequenced using 2 × 150 bp paired end sequencing on an Illumina NovaSeq 6000 Sequencing System (Illumina, San Diego, CA, USA).
2.3 Bioinformatics processing
Raw sequence reads were filtered to remove adapters and low-quality reads (average Phred quality score per read < 20) using Trimmomatic (Bolger et al., 2014). Reads shorter than 100 bp were also discarded. The obtained clean pair-end reads were then analyzed using the program VSEARCH v 2.27.1 (Rognes et al., 2016). The pair-end reads were merged into sequences using “–fastq_mergepairs” command with default settings. The primers and sequences with possible sequencing errors were removed from merged sequences using “–fastx_filter” command with the expected error threshold of 0.5. The “–derep_fulllength” command was used to remove redundant sequences. Unique amplicon sequence variants (ASVs) were generated using the “–cluster-unoise” command with minimum abundance of 2 (–minsize = 2). Chimeras were removed using the “–uchime_ref” command and ASV table was generated by running the “–usearch_global” command. To determine whether the sequencing data for each sample was sufficient to cover the biodiversity, the ASVs-based rarefaction curve was plotted using R package Vegan (Dixon, 2003) and ggplot2 (Ginestet, 2011).
The relative proportion of each ASV was calculated in each sample and used as the proxy of relative ASV abundance for subsequent analyses (Hirai et al., 2015). The ASVs were annotated according to the best-hit by searching against the NR database from NCBI using SEED v1.46 (Větrovský and Baldrian, 2013) with the parameters of e value <10 −80, minimum query coverage >99% and similarity >98% (Cilleros et al., 2019). ASVs assigned to non-freshwater vertebrate species were discarded. Here, aquatic invasive vertebrate species and endangered/threatened species were determined according to “List of Invasive Alien Species in China” and “China’s Red List of Biodiversity: Vertebrates (2020)”, respectively, published by the Chinese government.
3 Results
3.1 Composition of freshwater vertebrates
High throughput sequencing produced a total of 4,258,886 raw paired end reads (NCBI SRA sample accession: SAMN42018080-SAMN42018087). After quality filtering, de-replication, and ASV detection, a total of 2130 ASVs were retained, and rarefaction curves were saturated for all samples (Figure 1B). Among the 2130 ASVs, 1216 ASVs were successfully annotated as vertebrates. After removing ASVs assigned to non-freshwater species and ASVs with similarity < 98%, a total of 211 ASVs were kept for downstream analyses (Supplementary Table S1).
Based on the 211 ASVs, we identified a total of 104 freshwater vertebrate species (Supplementary Table S2), belonging to three classes: Actinopteri, Amphibia and Reptilia, of which Actinopteri was the most abundant class (87 species), followed by Amphibia (11 species) and Reptilia (6 species). From the three classes, we identified 12 orders, of which Cypriniformes (56 species), Siluriformes (11 species), Anura (10 species), Gobiiformes (7 species) and Testudines (6 species) were identified as the first five dominant orders. A total of 32 families were retrieved, and the top six were Xenocyprididae (13 species), Cyprinidae (9 species), Gobionidae (8 species), Acheilognathidae (7 species), Gobiidae (6 species) and Bagridae (6 species). A total of 71 genera were detected, and the top six were Rhinogobius (6 species), Acheilognathus (5 species), Tachysurus (5 species), Channa (4 species), Mauremys (3 species) and Hemiculter (3 species).
Among the 104 freshwater vertebrate species, we detected a total of nine (8.65%) invasive species: Coptodon zillii, Sarotherodon galilaeus, Oreochromis niloticus, Oreochromis tanganicae, Gambusia affinis, Clarias gariepinus, Chelydra serpentina, Trachemys scripta elegans, and Rana catesbeiana (Table 1). In addition, we also detected 16 (15.38%) endangered/threatened species, including four critically endangered species (Acipenser schrenckii, Mauremys mutica, Mauremys nigricans, and Mauremys sinensis), two endangered species (Pelodiscus sinensis, Hoplobatrachus chinensis), five vulnerable species (Xenocypris fangi, Silurus soldatovi, Odontobutis haifengensis, Onychostoma macrolepis, and Sinocrossocheilus labiatus) and five near threatened species (Channa striata, Pterocryptis cochinchinensis, Sylvirana guentheri, Sylvirana maosonensis, and Paramesotriton hongkongensis) (Table 2).
3.2 Distribution and abundance of invasive species
These invasive species distributed widely with five (55.56%) (C. zillii, S. galilaeus, O. niloticus, T. s. elegans, and R. catesbeiana) detected in at least six (75.00%) nature reserves (Figure 2A). Both C. zillii and S. galilaeus were detected in all eight nature reserves (LBJS, LNBD, LHNS, LFSB, PLHT, CFHS, LNKS, and LHZL). O. niloticus and T. s. elegans were detected in all nature reserves except for LHZL and LNBD, respectively. R. catesbeiana, G. affinis, and C. gariepinus were detected in six (LBJS, LNBD, LHNS, LFSB, CFHS, and LNKS), five (LBJS, LHNS, LFSB, PLHT, and CFHS) and four (LNBD, LFSB, CFHS, and LNKS), respectively. C. serpentine and O. tanganicae were only detected in two (LBJS and LHNS) and one (LFSB), respectively (Table 1). The highest invasive species richness was found in LFSB (eight species), followed by LBJS (seven), LHNS (seven), CFHS (seven), LNKS (six), PLHT (five) and LNBD (five), while LHZL (three) had the lowest number of aquatic invasive vertebrate species (Figure 2B; Table 1).
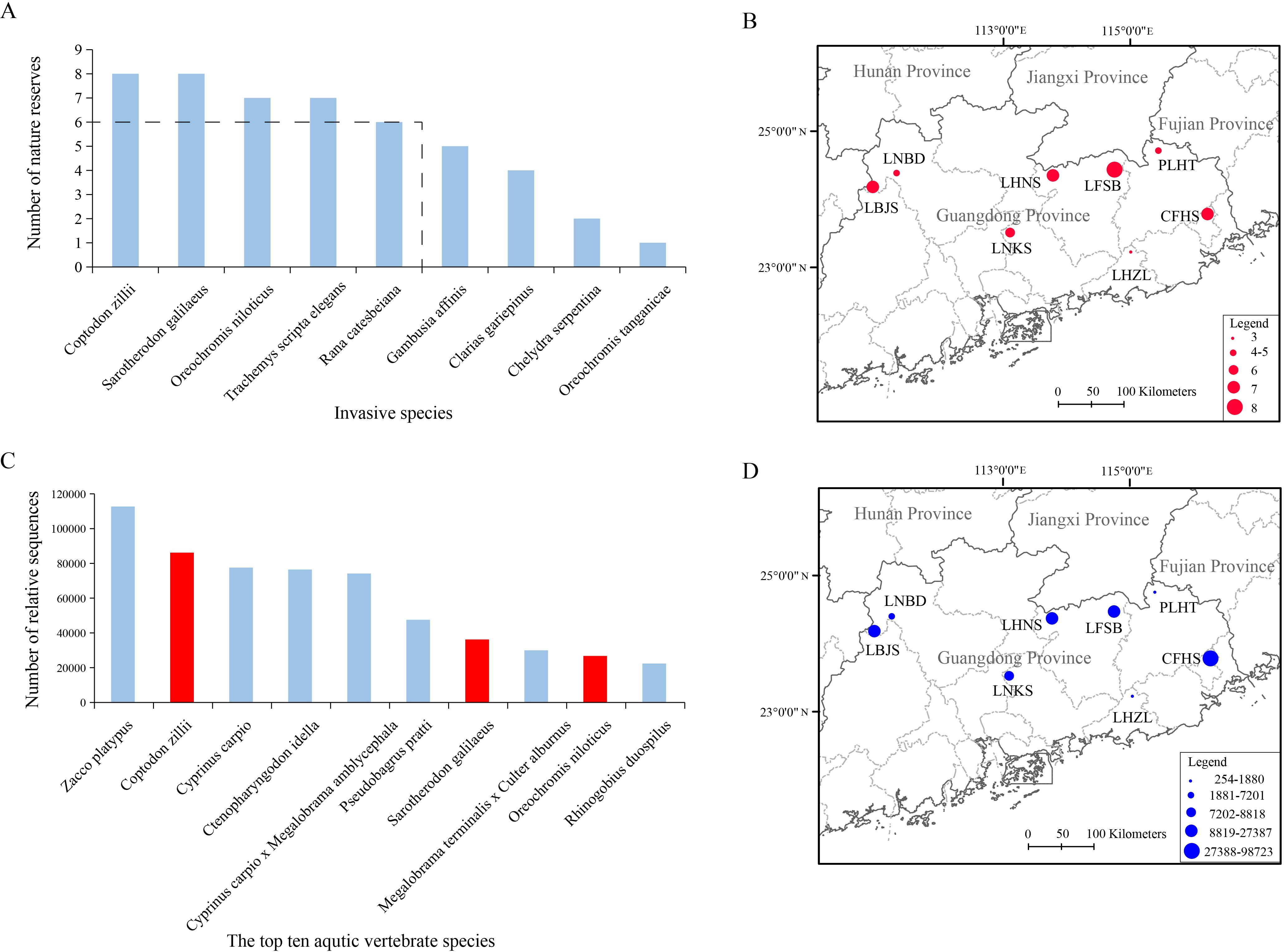
Figure 2. The number of nature reserves for each aquatic invasive vertebrate species (A); The whole invasive species richness in each nature reserve (B); The relative sequence number of the top ten aquatic vertebrate species with three invasive species (red box) (C); The whole invasive species abundance in each nature reserve (D).
These invasive species ranked top 66 (63.46%) of aquatic vertebrate species regarding their relative sequences, and three invasive species (C. zillii, S. galilaeus and O. niloticus) were found among the top 10 aquatic vertebrate species, suggesting high abundances of these invasive species (Figure 2C; Table 1). The average of relative sequence reads per invasive species was 20,070, ranging from 62 relative sequence reads for O. tanganicae to 86,331 relative sequence reads for C. zillii. The top four invasive species were C. zillii (86,331), S. galilaeus (36,363), O. niloticus (26,896) and G. affinis (16,613), followed by R. catesbeiana (8,873), C. serpentine (3,109) and T. s. elegans (2,021), and the relative sequence reads of both C. gariepinus (361) and O. tanganicae (62) were relatively less. Both C. zillii (69,593) and S. galilaeus (28,904) were detected with the most sequence reads in CFHS, followed by LFSB, where both O. niloticus and O. tanganicae were detected with the most sequence reads. Other five invasive species were detected with the highest abundance in different nature reserves, G. affinis, R. catesbeiana, C. serpentine, T. s. elegans, and C. gariepinus were detected with the highest abundance in LHNS (16,477), LNBD (6,897), LBJS (3,104), LHZL (1,750) and LNKS (307), respectively (Table 1). The highest abundance of all invasive species was found in CFHS (98,723), followed by LFSB (27,387), LBJS (19,501), LHNS (16,913), LNKS (8,818), LNBD (7,201) and LHZL (1,880), and the lowest abundance were found in LHNS with only 254 relative sequence reads of all invasive species (Figure 2D).
3.3 Distribution and abundance of endangered/threatened species
Unlike invasive species, only two (12.50%) endangered species (M. mutica and M. nigricans) were detected in at least six (75.00%) nature reserves (Table 2). Most (11, 68.75%) endangered/threatened species (P. cochinchinensis, X. fangi, O. macrolepis, S. labiatus, P. sinensis, C. striata, P. hongkongensis, S. soldatovi, A. schrenckii, M. sinensis, and O. haifengensis) were distributed in less than five nature reserves, such as P. cochinchinensis detected in LBJS, LHNS, PLHT, and CFHS, O. haifengensis detected in LBJS, LFSB and CFHS. Both H. chinensis and S. maosonensis were detected in LNBD and LNKS, while S. guentheri was only found in LNKS. For the richness of endangered/threatened species, the top two highest nature reserves were LFSB and CFHS, both had 12 endangered/threatened species, followed by LHNS (11), PLHT (9), LBJS (8), LNKS (6) and LNBD (4), the lowest richness of endangered/threatened species was detected in LHZL, which had only one endangered species (M. mutica) (Figure 3A).
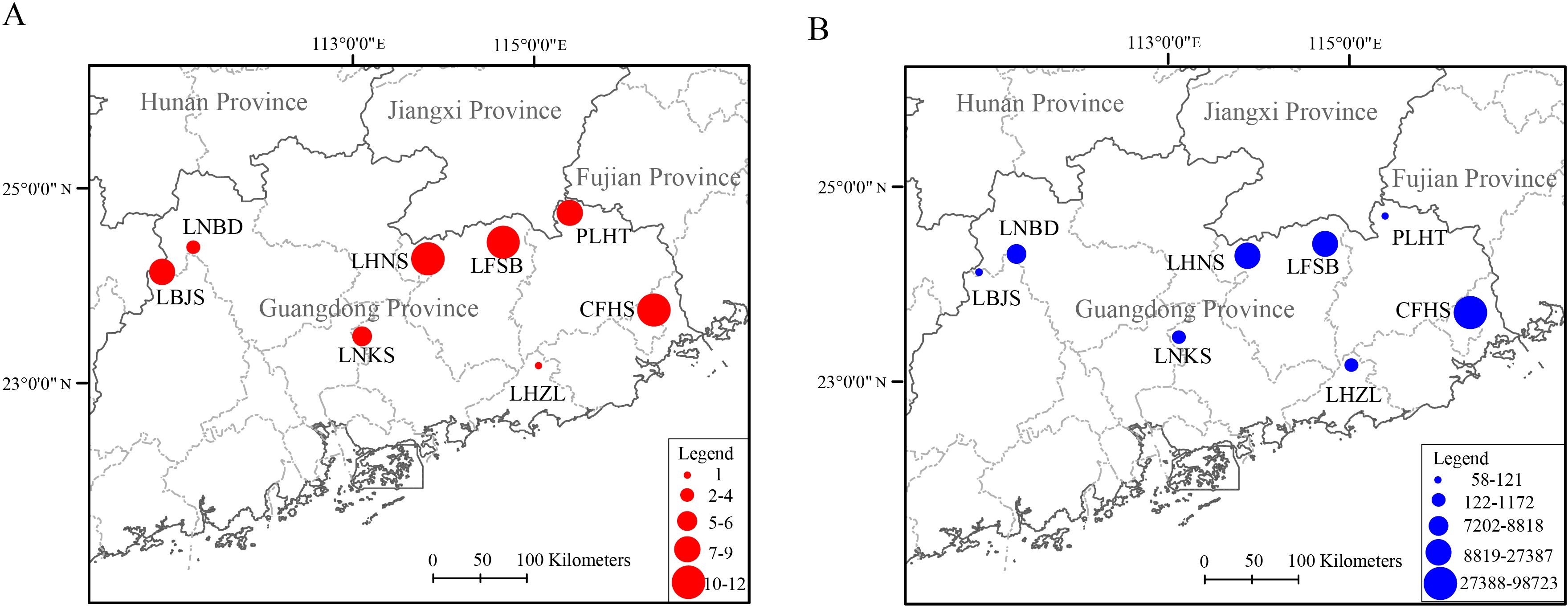
Figure 3. The whole endangered/threatened species richness (A) and abundance (B) in each nature reserve.
O. macrolepis and S. labiatuswas were identified as the top two highest abundant endangered/threatened species with 14,589 and 10,209 sequence reads in total, respectively, followed by M. mutica (2,870), X. fangi (2,846), C. striata (2,379), H. chinensis (1,959), A. schrenckii (1,511) and S. maosonensis (746). The rest endangered/threatened species had extremely low abundance with less than 100 sequence reads in total for each species, the top two lowest abundant species were P. sinensis (15) and S. guentheri (10). The most endangered/threatened species with highest abundance were distributed in different nature reserves, while the rest endangered/threatened species with highest abundance were found in the same nature reserves. For example, CFHS was found with the highest abundance of O. macrolepis with 14,470 sequence reads, LHNS was detected with the highest abundance of S. labiatuswas with 10,106 sequence reads, and LNBD was found with the highest abundance of H. chinensis with 1,941 sequence reads. While, six species (M. mutica, X. fangi, C. striata, A. schrenckii, M. sinensis, and P. sinensis) were detected with the highest abundances in LFSB (Figure 3B).
3.4 The comparison between invasive species and endangered/threatened species
To investigate the potential threats of invasive species to local endangered/threatened species, we compared the richness and abundances of both groups in the same nature reserves. We found high overlaps of geographic distribution between endangered/threatened species and invasive species (Figures 2, 3). Nature reserves with both high richness and abundances of invasive species were also detected with both high richness and abundances of endangered/threatened species, and vice versa. For example, the top three highest nature reserves in endangered/threatened species diversities [LFSB (12), CFHS (12) and LHNS (11)] were also detected with the top three largest invasive species richness [LFSB (8), CFHS (7) and LHNS (7)]. While, the top two lowest nature reserves in endangered/threatened species diversities [LNBD (4) and LHZL (1)] were also detected with the top two lowest invasive species richness [LNBD (5) and LHZL (3)].
4 Discussion
Aquatic invasive vertebrates often hide under the water and are frequently neglected by traditional ecological surveys in nature reserves, leading to witting invasion risks exerted on nature reserves. Here, based on eDNA technique, we detected nine invasive species and 16 endangered/threatened species in eight nature reserves in Guangdong Province. Surprisingly, these invasive species had a wide distribution and high abundance in nature reserves. In addition, we found high overlaps of geographic distribution between invasive species and endangered/threatened species. These observations indicated high invasion risk in nature reserves in Guangdong Province, highlighting urgent needs to manage these invasive species.
Invasive species can cause huge damages to local biodiversity and ecosystems, and are often characterized by wide distributions and high abundances (Fristoe et al., 2021). Here, eDNA analysis detected a total of nine aquatic invasive vertebrate species, among which tilapia species (C. zillii, S. galilaeus, and O. niloticus) showed both the widest distribution and highest abundance. Tilapias are notorious invaders and their invasions can alternative aquatic communities and ecosystem functions by preying on native aquatic species’ eggs, larvae, and even adults, competing for food resources and habitat, and deteriorating water quality, potentially causing population decline and even extinction of native species (Canonico et al., 2005; Martin et al., 2010; Sanches et al., 2012; Yongo et al., 2023). For example, previous field investigations showed that the invasion of O. niloticus could reduce the food resources available for local species and can significantly reduce the abundance and body size of native fishes, such as Cirrhinus molitorella, Megalobrama terminalis and Hemiculter leucisculus (Gu et al., 2015; Shuai et al., 2019; Shuai and Li, 2022). Further manipulative experiments revealed the inhibited growth of native C. molitorella in the presence of O. niloticus (Gu et al., 2015), thus providing experimental evidence of serious damages caused by tilapia species to native species. Hence, the wide distribution and high abundance of these tilapia species in the present study indicated that their invasions may have caused population decline and even extinction of native species in nature reserves in southern China.
Although both T. s. elegans and R. catesbeiana had lower abundance (less than 10,000 relative sequence numbers) compared to the above three tilapia species (more than 25,000 relative sequence numbers), they had wide distributions and were detected in at least 75.00% nature reserves. They are also notorious invaders and have been listed as the world’s 100 worst invasive species compiled by the International Union for the Conservation of Nature (IUCN) Invasive Species Specialist Group (ISSG) of the Species Survival Commission. Mounting evidence showed that both invasive species can cause huge damages to native species by preying on them (Kraus, 2015), competing for food and basking sites with native species (Cadi and Joly, 2003, 2004), compromising integrity of native species through hybridizing with native genetically closed species (Kraus, 2015), and spreading pathogens (Daszak et al., 2004; Gong et al., 2014; Martínez-Ríos et al., 2022; Hossack et al., 2023). For example, T. s. elegans has invaded into most wetlands in Europe where the native specie European pond turtle (Emys orbicularis) also lives, when E. orbicularis were cultured together with T. s. elegans for three years, the weight of E. orbicularis lost significantly (p < 0.05) and its mortality was significantly higher (p < 0.001) than control groups (Cadi and Joly, 2004). Hence, its reasonable to speculate that the introduction of T. s. elegans into these nature reserves may have caused decreased weight and increased mortality of native endangered turtles, such as M. mutica and M. nigricans in the present study. In addition, one investigation of frog community in 65 permanent lentic waters on islands showed that sites with R. catesbeiana invasion had lower native frog abundance and species richness than sites without R. catesbeiana, and these indexes of native frogs were negatively related to post-metamorphosis R. catesbeiana abundance, suggesting that post-metamorphosis R. catesbeiana can negatively influence native frog communities, and that the influence extents are proportional to post-metamorphosis R. catesbeiana abundance (Li et al., 2011). Hence, the low abundances of both T. s. elegans and R. catesbeiana in the present study suggested that their damages to native common species may be not severe in nature reserves, but potential huge damages to endangered amphibians, reptiles and other rare species, given their wide distributions in nature reserves, high overlaps of geographic distribution with endangered/threatened species, and carnivorous diets with the big appetites (Wang et al., 2008; Nishizawa et al., 2014). There were similar concerns for other aquatic invasive vertebrate species at least in a few nature reserves, such as G. affinis in LHNS, since G. affinis was abundant in LHNS.
In conclusion, based on eDNA technique, we detected a total of nine aquatic invasive vertebrate species, most of which showed high abundances and were widely distributed in nature reserves in southern China. In addition, we also detected 16 endangered/threatened species, which showed high overlaps of geographic distribution with invasive species. These findings indicated high invasion risks of these invasive species in nature reserves in Guangdong Province, highlighting urgent measures to manage these invasive species and prevent their further spread. Our study would provide fundamental insights for the formulation of effective management measures to curb potential damage and losses caused by invasive species and protect endangered/threatened species in nature reserves. Moreover, our study detected a total of four endangered turtle species in eight nature reserves based on eDNA technique, which was also successfully used for the detection of endangered turtle Platysternon megacephalum in nature reserves in Hong Kong (Lam et al., 2022), thus providing evidence for that eDNA technique is a promising tool for the conservation of endangered turtle species. However, although some species such as O. macrolepis (VU species) were detected in some nature reserves based on eDNA technique in the present study, their presence based on morphological characteristics was absent in these nature reserves (Zhao et al., 2011). Hence, further investigations based on both eDNA technique and morphological methods can provide more comprehensive insights into invasion risks of alien species and protection of endangered/threatened species in nature reserves.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because DNA sequences were collected from water samples.
Author contributions
YG: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. YW: Data curation, Investigation, Writing – original draft, Writing – review & editing. CW: Data curation, Writing – review & editing. SZ: Formal analysis, Software, Writing – review & editing. ZX: Methodology, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the National Natural Science Foundation of China (32371753), the Guangdong Basic and Applied Basic Research Foundation (2024A1515010914), GDAS Special Project of Science and Technology Development (2022GDASZH-2022010106) and the Scientific and Technological Program of Guangzhou (2023A04J0844).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1462123/full#supplementary-material
References
Bailey S. A., Brown L., Campbell M. L., Canning-Clode J., Carlton J. T., Castro N., et al. (2020). Trends in the detection of aquatic non-indigenous species across global marine, estuarine and freshwater ecosystems: A 50-year perspective. Diversity Distributions. 26, 1780–1797. doi: 10.1111/ddi.13167
Bellard C., Genovesi P., Jeschke J. M. (2016). Global patterns in threats to vertebrates by biological invasions. Proc. Biol. Sci. 283, 20152454. doi: 10.1098/rspb.2015.2454
Bolger A. M., Lohse M., Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120. doi: 10.1093/bioinformatics/btu170
Cadi A., Joly P. (2003). Competition for basking places between the endangered European pond turtle (Emys orbicularis galloitalica) and the introduced red-eared slider (Trachemys scripta elegans). Can. J. Zool. 81, 1392–1398. doi: 10.1139/z03–108
Cadi A., Joly P. (2004). Impact of the introduction of the red-eared slider (Trachemys scripta elegans) on survival rates of the European pond turtle (Emys orbicularis). Biodivers. Conserv. 13, 2511–2518. doi: 10.1023/B:BIOC.0000048451.07820.9c
Canonico G. C., Arthington A., McCrary J. K., Thieme M. L. (2005). The effects of introduced tilapias on native biodiversity. Aquat. Conserv.: Mar. Freshw. Ecosyst. 15, 463–483. doi: 10.1002/aqc.699
Cilleros K., Valentini A., Allard L., Dejean T., Etienne R., Grenouillet G., et al. (2019). Unlocking biodiversity and conservation studies in high-diversity environments using environmental DNA (eDNA): A test with Guianese freshwater fishes. Mol. Ecol. Resour. 19, 27–46. doi: 10.1111/1755–0998.12900
Daszak P., Strieby A., Cunningham A. A., Longcore J. E., Brown C. C., Porter D. (2004). Experimental evidence that the bullfrog (Rana catesbeiana) is a potential carrier of chytridiomycosis, an emerging fungal disease of amphibians. Herpetol. J. 14, 201–207.
Dixon P. (2003). VEGAN, a package of R functions for community ecology. J. Vegetation. Sci. 14, 927–930. doi: 10.1658/1100–9233(2003)014[0927:vaporf]2.0.co;2
Ficetola G. F., Rondinini C., Bonardi A., Baisero D., Padoa-Schioppa E. (2015). Habitat availability for amphibians and extinction threat: a global analysis. Diversity Distributions. 21, 302–311. doi: 10.1111/ddi.12296
Fristoe T. S., Chytrý M., Dawson W., Essl F., Heleno R., Kreft H., et al. (2021). Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. U.S.A. 118, e2021173118. doi: 10.1073/pnas.2021173118
Ginestet C. (2011). ggplot2: Elegant graphics for data analysis. J. R. Stat. Soc. Ser. a-Statistics. Soc. 174, 245–245. doi: 10.1111/j.1467–985X.2010.00676_9.x
Gong L., Li J., Liu X., Zhao X., Zhao C. (2017). Analysis of invasive alien species in Chinese national nature reserves. Ecol. Sci. 36, 210–216.
Gong S., Wang F., Shi H., Zhou P., Ge Y., Hua L., et al. (2014). Highly pathogenic Salmonella Pomona was first isolated from the exotic red-eared slider (Trachemys scripta elegans) in the wild in China: Implications for public health. Sci. Total. Environ. 468–469, 28–30. doi: 10.1016/j.scitotenv.2013.08.025
Gu D. E., Ma G. M., Zhu Y. J., Xu M., Luo D., Li Y. Y., et al. (2015). The impacts of invasive Nile tilapia (Oreochromis niloticus) on the fisheries in the main rivers of Guangdong Province, China. Biochem. Syst. Ecol. 59, 1–7. doi: 10.1016/j.bse.2015.01.004
Guo H., Mazer S. J., Xu X., Luo X., Huang K., Xu X. (2017). “Biological invasions in nature reserves in China,” in Biological Invasions and Its Management in China: Volume 1. Eds. Wan F., Jiang M., Zhan A. (Springer Netherlands, Dordrecht), 125–147.
Hirai J., Yasuike M., Fujiwara A., Nakamura Y., Hamaoka S., Katakura S., et al. (2015). Effects of plankton net characteristics on metagenetic community analysis of metazoan zooplankton in a coastal marine ecosystem. J. Exp. Mar. Biol. Ecol. 469, 36–43. doi: 10.1016/j.jembe.2015.04.011
Hossack B. R., Hall D., Crawford C. L., Goldberg C. S., Muths E., Sigafus B. H., et al. (2023). Successful eradication of invasive American bullfrogs leads to coextirpation of emerging pathogens. Conserv. Lett. 16, 2151–2162. doi: 10.1111/conl.12970
Kraus F. (2015). Impacts from invasive reptiles and amphibians. Annu. Rev. Ecol. Evol. Syst. 46, 75–97. doi: 10.1146/annurev-ecolsys-112414–054450
Lam I. P. Y., Sung Y. H., Fong J. J. (2022). Using eDNA techniques to find the endangered big-headed turtle (Platysternon megacephalum). PloS One 17, e0262015. doi: 10.1371/journal.pone.0262015
Li F., Wang S., Zhang Y., Zhang N., Cai Y., Yang Z. (2022). DNA metabarcoding reveals human impacts on macroinvertebrate communities in polluted headwater streams: Evidence from the Liao River in northeast China. Environ. pollut. 300, 118929. doi: 10.1016/j.envpol.2022.118929
Li M., Zhang H. (2023). Predicting the distribution of the invasive species Atractosteus spatula, the Alligator gar, in China. Water 15, 4291. doi: 10.3390/w15244291
Li Y., Ke Z., Wang Y., Blackburn T. M. (2011). Frog community responses to recent American bullfrog invasions. Curr. Zool. 57, 83–92. doi: 10.1093/czoolo/57.1.83
Martin C. W., Valentine M. M., Valentine J. F. (2010). Competitive interactions between invasive nile tilapia and native fish: the potential for altered trophic exchange and modification of food webs. PloS One 5, e14395. doi: 10.1371/journal.pone.0014395
Martínez-Ríos M., Martín-Torrijos L., Diéguez-Uribeondo J. (2022). The invasive alien red-eared slider turtle, Trachemys scripta, as a carrier of STEF-disease pathogens. Fungal Biol. 126, 113–121. doi: 10.1016/j.funbio.2021.11.003
Mi C., Ma L., Yang M., Li X., Meiri S., Roll U., et al. (2023). Global Protected Areas as refuges for amphibians and reptiles under climate change. Nat. Commun. 14, 1389. doi: 10.1038/s41467–023-36987-y
Nishizawa H., Tabata R., Hori T., Mitamura H., Arai N. (2014). Feeding kinematics of freshwater turtles: what advantage do invasive species possess? Zoology 117, 315–318. doi: 10.1016/j.zool.2014.04.005
Rognes T., Flouri T., Nichols B., Quince C., Mahé F. (2016). VSEARCH: a versatile open source tool for metagenomics. PeerJ 4, e2584. doi: 10.7717/peerj.2584
Sanches F. H. C., Miyai C. A., Costa T. M., Christofoletti R. A., Volpato G. L., Barreto R. E. (2012). Aggressiveness overcomes body-size effects in fights staged between invasive and native fish species with overlapping niches. PloS One 7, e29746. doi: 10.1371/journal.pone.0029746
Shuai F., Li J. (2022). Nile tilapia (Oreochromis niloticus Linnaeus 1758) invasion caused trophic structure disruptions of fish communities in the South China river-Pearl River. Biology 11, 1665. doi: 10.3390/biology11111665
Shuai F., Li X., Liu Q., Zhu S., Wu Z., Zhang Y. (2019). Nile tilapia (Oreochromis niloticus) invasions disrupt the functional patterns of fish community in a large subtropical river in China. Fisheries. Manage. Ecol. 26, 578–589. doi: 10.1111/fme.12368
Simberloff D., Martin J.-L., Genovesi P., Maris V., Wardle D. A., Aronson J., et al. (2013). Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28, 58–66. doi: 10.1016/j.tree.2012.07.013
Song L., Cao F., He Y., Qiang S., Qin W., Jiang M. (2009). A survey of invasive alien plant species in Dinghushan National Nature Reserve. J. Zhejiang. For. Coll. 26, 538–543.
Thomsen P. F., Willerslev E. (2015). Environmental DNA - An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 183, 4–18. doi: 10.1016/j.biocon.2014.11.019
Valentini A., Taberlet P., Miaud C., Civade R., Herder J., Thomsen P. F., et al. (2016). Next-generation monitoring of aquatic biodiversity using environmental DNA metabarcoding. Mol. Ecol. 25, 929–942. doi: 10.1111/mec.13428
Valsecchi E., Bylemans J., Goodman S. J., Lombardi R., Carr I., Castellano L., et al. (2020). Novel universal primers for metabarcoding environmental DNA surveys of marine mammals and other marine vertebrates. Environ. DNA 2, e72. doi: 10.1002/edn3.72
Vences M., Lyra M. L., Perl R. G. B., Bletz M. C., Stanković D., Lopes C. M., et al. (2016). Freshwater vertebrate metabarcoding on Illumina platforms using double-indexed primers of the mitochondrial 16S rRNA gene. Conserv. Genet. Resour. 8, 323–327. doi: 10.1007/s12686–016-0550-y
Větrovský T., Baldrian P. (2013). Analysis of soil fungal communities by amplicon pyrosequencing: current approaches to data analysis and the introduction of the pipeline SEED. Biol. Fertil. Soils. 49, 1027–1037. doi: 10.1007/s00374–013-0801-y
Wang S., Gu S., Zhang Y., Deng Y., Qiu W., Sun Q., et al. (2024). Microeukaryotic plankton community dynamics under ecological water replenishment: Insights from eDNA metabarcoding. Environ. Sci. Ecotechnol. 20, 100409. doi: 10.1016/j.ese.2024.100409
Wang Q., Han X., Wang Z., Zheng K., Dong Z., Zhang P., et al. (2023a). Eurasian otters prefer to prey on religious released non-native fish on the Qinghai-Tibetan Plateau. Curr. Zool. zoad025. doi: 10.1093/cz/zoad025
Wang Z., Liu X., Liang D., Wang Q., Zhang L., Zhang P. (2023b). VertU: universal multilocus primer sets for eDNA metabarcoding of vertebrate diversity, evaluated by both artificial and natural cases. Front. Ecol. Evol. 11. doi: 10.3389/fevo.2023.1164206
Wang Y., Wang Y., Lu P., Zhang F., Li Y. (2008). Diet composition of post-metamorphic bullfrogs (Rana catesbeiana) in the Zhoushan archipelago, Zhejiang Province, China. Front. Biol. China 3, 219–226. doi: 10.1007/s11515–008-0036–8
Wang S., Yan Z., Hänfling B., Zheng X., Wang P., Fan J., et al. (2021). Methodology of fish eDNA and its applications in ecology and environment. Sci. Total. Environ. 755, 142622. doi: 10.1016/j.scitotenv.2020.142622
Watson J. E. M., Dudley N., Segan D. B., Hockings M. (2014). The performance and potential of protected areas. Nature 515, 67–73. doi: 10.1038/nature13947
Wei Y., Ye D., Wen Y., Men Y. (2006). Invasive plant species in Shiwandashan Mountain National Natural Reserve, Guangxi, China. China For. Sci. Technol. 20, 23–26.
Xin Y., Yang Z., Du Y., Cui R., Xi Y., Liu X. (2024). Vulnerability of protected areas to future climate change, land use modification, and biological invasions in China. Ecol. Applications.: Publ. Ecol. Soc. America 34, e2831. doi: 10.1002/eap.2831
Xiong W., Ni P., Chen Y., Gao Y., Shan B., Zhan A. (2017). Zooplankton community structure along a pollution gradient at fine geographical scales in river ecosystems: The importance of species sorting over dispersal. Mol. Ecol. 26, 4351–4360. doi: 10.1111/mec.14199
Yan Y., Xian X., Jiang M., Wan F. (2017). “Biological invasion and its research in China: an overview,” in Biological Invasions and Its Management in China: Volume 1. Eds. Wan F., Jiang M., Zhan A. (Springer Netherlands, Dordrecht), 3–19.
Yongo E., Zhang P., Mutethya E., Zhao T., Guo Z. (2023). The invasion of tilapia in South China freshwater systems: A review. Lakes. Reservoirs.: Sci. Policy Manage. Sustain. Use 28, e12429. doi: 10.1111/lre.12429
Zhan A., Ni P., Xiong W., Chen Y., Lin Y., Huang X., et al. (2017). “Biological invasions in aquatic ecosystems in China,” in Biological Invasions and Its Management in China: Volume 1. Eds. Wan F., Jiang M., Zhan A. (Springer Netherlands, Dordrecht), 67–96.
Zhang S., Zheng Y., Zhan A., Dong C., Zhao J., Yao M. (2022). Environmental DNA captures native and non-native fish community variations across the lentic and lotic systems of a megacity. Sci. Adv. 8, eabk0097. doi: 10.1126/sciadv.abk0097
Keywords: nature reserves, aquatic vertebrate species, invasive species, invasion risk, eDNA
Citation: Wei Y, Wu C, Zhang S, Xia Z and Gao Y (2024) eDNA analysis reveals high invasion risks in nature reserves in Guangdong Province, China. Front. Mar. Sci. 11:1462123. doi: 10.3389/fmars.2024.1462123
Received: 09 July 2024; Accepted: 31 July 2024;
Published: 16 August 2024.
Edited by:
Shuping Wang, Chinese Research Academy of Environmental Sciences, ChinaReviewed by:
Yiyong Chen, Chinese Academy of Sciences (CAS), ChinaFanrong Xiao, Hainan Normal University, China
Copyright © 2024 Wei, Wu, Zhang, Xia and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yangchun Gao, Z2FveWMwNDEyQDE2My5jb20=
 Yufeng Wei1,2
Yufeng Wei1,2 Yangchun Gao
Yangchun Gao