- 1School of Marine Sciences, Ningbo University, Ningbo, China
- 2Mangrove Conservation Research Center, Southern Marine Science and Engineering Guangdong Laboratory (Zhanjiang), Zhanjiang, China
- 3Yueqing Branch of Wenzhou Ecological Environment Bureau, Wenzhou, China
- 4Yuzhi Environmental Technology Co., Ltd, Ningbo, China
Stock enhancement activities have many benefits but also negative impacts. The macrobenthic diversity index and the M-AMBI index were adopted to assess the effects of stock enhancement of Phascolosoma esculenta on macrobenthic communities and the local ecosystem in Yueqing Bay. The results revealed that the Shannon–Wiener diversity index (H’) and Margalef richness index (d) increased in October 2022 after stock enhancement, indicating a positive ecological restoration effect. The average M-AMBI in the intertidal zone of Yueqing Bay was 0.59, indicating good benthic ecological quality. Excluding seasonal factors, the M-AMBI in the intertidal zone increased after stock enhancement, indicating that stock enhancement by Phascolosoma esculenta had a certain positive effect on the intertidal ecological environment of Yueqing Bay. The increase in the M-AMBI occurred mainly in the low-tide zone, increased slightly in the mid-tide zone, and remained basically unchanged in the high-tide zone. On the one hand, Phascolosoma esculenta can accumulate heavy metals from the environment and release a large amount of organic matter throughout its lifecycle, promoting nutrient cycling, which plays a positive role in the environment. On the other hand, Phascolosoma esculenta is an economic species; however, the planting of mangroves in the low tide zone causes fishers to fish in the mid- and high-tide zones, so the changes in the M-AMBI values in each tidal zone are related mainly to Phascolosoma esculenta and anthropogenic disturbances.
1 Introduction
Yueqing Bay is located on the southern coast of Zhejiang Province, north of the Oujiang River Estuary. It is an important marine aquaculture base in Zhejiang Province and its largest semi enclosed bay (Committee, C.G.C.C, 1993; Wang et al., 2018). In recent years, although the development of the Yueqing Bay Economic Circle has led to the rapid development of the local economy, it has also brought great potential threats to the marine ecological environment, especially in the intertidal zone at the land−sea boundary, which is the area most severely affected by human activities and disturbances (Zhuang et al., 2003). Factors such as industrial and agricultural wastewater discharge, pond aquaculture, land reclamation, and the invasion of alien species such as Spartina alterniflora (Chen, 2016) have led to a fragmented landscape in many intertidal areas, resulting in the decline and succession of macrobenthic communities (Peng et al., 2011).
Stock enhancement is the most common method for marine biological resource restoration in land reclamation and ecological restoration projects (Liu et al., 2022), while the key to ecological restoration is maintaining species diversity. To protect the mangrove ecosystem, relevant departments in Yueqing Bay have implemented a stock enhancement project in the mangrove area, releasing 6,000 kg of Phascolosoma esculenta to promote the recovery of biodiversity in marine wetlands. Countries worldwide have conducted numerous stock enhancement practices (Molony et al., 2005), but only a few of them have been able to directly demonstrate an increase in resources or other positive impacts. Conversely, there are numerous negative impacts associated with these activities, as they can pose various ecological risks to wild resource species and water released into the ecosystems (Araki and Schmid, 2010; Jang et al., 2014; Qi et al., 2016).
Macrobenthos constitute an essential part of marine ecosystems. The marine ecological environment determines the abundance, dominant species, species composition, and community structure of macrobenthos. Moreover, macrobenthos directly or indirectly affect marine ecosystems through activities such as feeding, being preyed upon, tube-building, and burrowing (Sun et al., 2011; Zhong et al., 2018). Owing to their long lifespan, rich diversity, ease of collection, and sensitivity to environmental disturbances, macrobenthos are usually used as indicator species to reflect the health status of marine ecosystems and the recovery status of degraded habitats (Borja et al., 2010; Zhang et al., 2022). When evaluating the effectiveness of mangrove restoration, the diversity indices of macrobenthic communities are considered essential (Wu et al., 2023). Furthermore, both domestic and international scientists generally believe that macrobenthic and biotic indices can be used for assessing ecological environmental quality (Tang et al., 2019). By analyzing the changes in the diversity indices of macrobenthic communities and biotic indices, the impact of restocking programs on macrobenthic communities and the effectiveness of mangrove restoration programs can be evaluated.
On the basis of the response of biotic indices to multiple stresses, the M-AMBI is at the forefront (Borja et al., 2015). The M-AMBI combines the AMBI, species richness, and Shannon−Wiener diversity index (H’); furthermore, compared with a single index, the use of the M-AMBI can more accurately reflect the actual environmental conditions (Borja and Tunberg, 2011). This index has been widely used in studies of the ecological quality of China’s estuaries, bays, and coastal waters (Cai et al., 2013a; Ding et al., 2021; Han et al., 2013; Li et al., 2017; Ni et al., 2019; Wu et al., 2013; Yan et al., 2020). Additionally, it is applicable to the assessment of intertidal benthic ecological quality in China (Liu et al., 2018; Liu et al., 2019; Song et al., 2017; Yan et al., 2019; Zhang et al., 2022).
Currently, there is a lack of in-depth research on the impact of stock enhancement on macrobenthic communities. According to existing reports, after stock enhancement of Thais luteostoma and Mytilus coruscus, the diversity index (H’) and richness index (d) of macrobenthic communities clearly increased (Peng et al., 2012). The stock of Crassostrea sp. greatly increased the species number, abundance, and biomass of the macrobenthic species in the release waters (Chen et al., 2007). To our knowledge, research on the impact of stock enhancement on benthic ecological quality is extremely scarce, and research on the benthic ecological quality of the intertidal zone in Yueqing Bay is insufficient. Existing studies have used the MPI (Long et al., 2008) and ABC curve (Peng et al., 2011) for evaluations, but these studies are relatively old and have relatively low reference values. This study aims to investigate the ecological impacts of stock enhancement on macrobenthic communities within the intertidal zone of Yueqing. The specific objectives are as follows: (1) to validate whether macrobenthic assemblages undergo alterations in response to stock enhancement practices of Phascolosoma esculenta; (2) to evaluate the potential impact of stock enhancement of Phascolosoma esculenta on the ecological status by utilizing macrobenthic biodiversity indices alongside the biological index M-AMBI as assessment tools.
2 Materials and methods
2.1 Study area and sampling design
In November 2021, seedlings of Phascolosoma esculenta were released in Yueqing Bay in six mangrove ecological restoration areas, with 1000 kg of Phascolosoma esculenta seedlings released at each location. In accordance with the living habits of Phascolosoma esculenta, the seeds were released mainly in the high- and mid-tide zones. Six monitoring sections were set up at the same locations in the release area, with each section consisting of clayey silt. Each section was divided into high-tide, mid-tide, and low-tide zones, totaling 18 stations (Figure 1).
In accordance with the schedule of the stock enhancement process, combined with the season of the mangrove restoration project and the environmental characteristics of the intertidal zone, water quality and macrobenthos surveys were conducted in the intertidal zone before stock enhancement in October 2021 (autumn) and after stock enhancement in January 2022 (winter), April 2022 (spring), July 2022 (summer), and October 2022 (autumn). Sediment samples were additionally collected at the same locations in October 2021 and October 2022.
2.2 Sampling procedure
At each sampling time and area, sampling plots were chosen at a similar elevation, and quartic sediment samples of 25 cm × 25 cm × 30 cm (length × width × depth) were randomly selected from the middle zones of the 18 study stations. The sediment samples were washed and sieved through a 0.5 mm mesh, and the retained fauna were preserved in 75% alcohol solution before being identified and counted.
The taxonomic classification of species referred to the latest classification system of WoRMS (www.marinespecies.org). In this study, Phascolosoma esculenta is classified under the phylum Annelida. All macrobenthic organisms were sorted into five AMBI ecological groups (E1: sensitive species; E2: indifferent species; E3: tolerant species; E4: second-order opportunistic species; and E5: first-order opportunistic species) on the basis of the species list provided in the latest AMBI v6.0, and macrobenthos not included in the species list were substituted with other species of the same genus or family in conjunction with expert advice (Borja et al., 2008).
Water temperature and salinity were measured via a YSI water quality meter (YSI, Ohio, USA); pH was measured via a pH meter; dissolved oxygen (DO) was measured via the iodometric method; organic carbon (TOC) was measured via the potassium dichromate oxidation−reduction capacity method; sulfide (S) was measured via the methylene blue spectrophotometric method; oil was measured via ultraviolet spectrophotometry; copper (Cu), lead (Pb), zinc (Zn), cadmium (Cd), and chromium (Cr) were measured via flameless atomic absorption spectrophotometry; and total mercury (Hg) and arsenic (As) were measured via atomic fluorescence spectrometry.
2.3 Community diversity indices
The diversity characteristics of the macrobenthic communities were analyzed via the Shannon–Wiener diversity index (H’) (Shannon, 1948), Pielou’s evenness index (J’) (Pielou, 1969), and Margalef richness index (d) (Margalef, 1951).
where H’ represents the value of the species diversity index, S represents the total number of species in the sample, and Pi represents the ratio of the individual abundance (ni) of the i-th species to the total abundance (N) (ni/N). J’ represents the evenness index value; d represents the richness index value. d represents Margalef’s richness index value.
2.4 M-AMBI
Before the M-AMBI was calculated, vertebrates such as fish needed to be removed. Macrobenthic invertebrates not listed in the species list were assigned to the same group as other species (Borja et al., 2008). The calculation of the M-AMBI was based on the AMBI, species richness, and Shannon−Wiener diversity index (H’). According to historical studies in the intertidal zones of China, we set the maximum AMBI to 6 (if no species were present, AMBI = 7). The reference conditions were 115% of the maximum H’ and species richness (S) values (Liu et al., 2018; Liu et al., 2019; Song et al., 2017). The threshold standards for M-AMBI values are “High” > 0.77, “Good” = 0.53–0.77, “Moderate” = 0.38–0.53, “Poor” = 0.20–0.38, and “Bad”< 0.20.
2.5 Data analysis
The sampling station map was drawn via ArcGIS Desktop 10.8. The coordinate system used was GCS_WGS_1984, and the base image was downloaded from the National Geomatic Center of China (https://www.ngcc.cn/ngcc/html/1/index.html). The Shannon−Wiener diversity index (H’), Pielou’s evenness index (J’), and Margalef richness index (d) were calculated via PRIMER 6.0 (Plymouth Routines in Multivariate Ecological Research). The multivariate AMBI (M-AMBI) for each station was calculated via the AMBI 6.0 software package (www.azti.es). One-way ANOVA and Tukey’s HSD post hoc tests were conducted via SPSS 22.0 software. The relationships between the M-AMBI and environmental factors were analyzed via Spearman correlation analysis with the “psych” package in R.
3 Results
3.1 Environmental factors of the intertidal zone in Yueqing Bay
The environmental factors of the intertidal zone in Yueqing Bay from October 2021 to October 2022 are shown in Table 1. In general, in terms of seawater, the change in pH was not significant (P>0.05), whereas temperature, salinity, and DO showed greater variations at different times. In terms of sediments, compared with those in October 2021, the contents of Cu, Zn, and Cr significantly decreased in October 2022 (P<0.05); the contents of Pb, TOC, and S slightly decreased; the contents of Cd, Hg, and oil significantly increased (P<0.05); and the content of As slightly increased. The sediment content in the intertidal zone of Yueqing Bay reached China’s first-class marine sediment quality standards in October 2021 and October 2022.

Table 1. Environmental factors in the intertidal zone of Yueqing Bay from October 2021 to October 2022.
3.2 Number and abundance of macrobenthic species
From October 2021 to October 2022, a total of 121 macrobenthic species belonging to 8 phyla and 76 families were collected from the intertidal zone of Yueqing Bay. Mollusks were the most abundant, with 48 species (39.67%), followed by arthropods with 30 species (24.79%), annelids with 29 species (23.97%), and 9 other species (7.44%).
In terms of species number, the results were as follows (Figure 2A): April 2022 (59 species) > October 2022 (55 species) > July 2022 (42 species) > October 2021 (40 species) > January 2022 (38 species). In terms of abundance, the results were as follows (Figure 2B): April 2022 (1924.00 ind./m²) > July 2022 (614.00 ind./m²) > October 2022 (606.67 ind./m²) > January 2022 (598.00 ind./m²) > October 2021 (248.00 ind./m²).
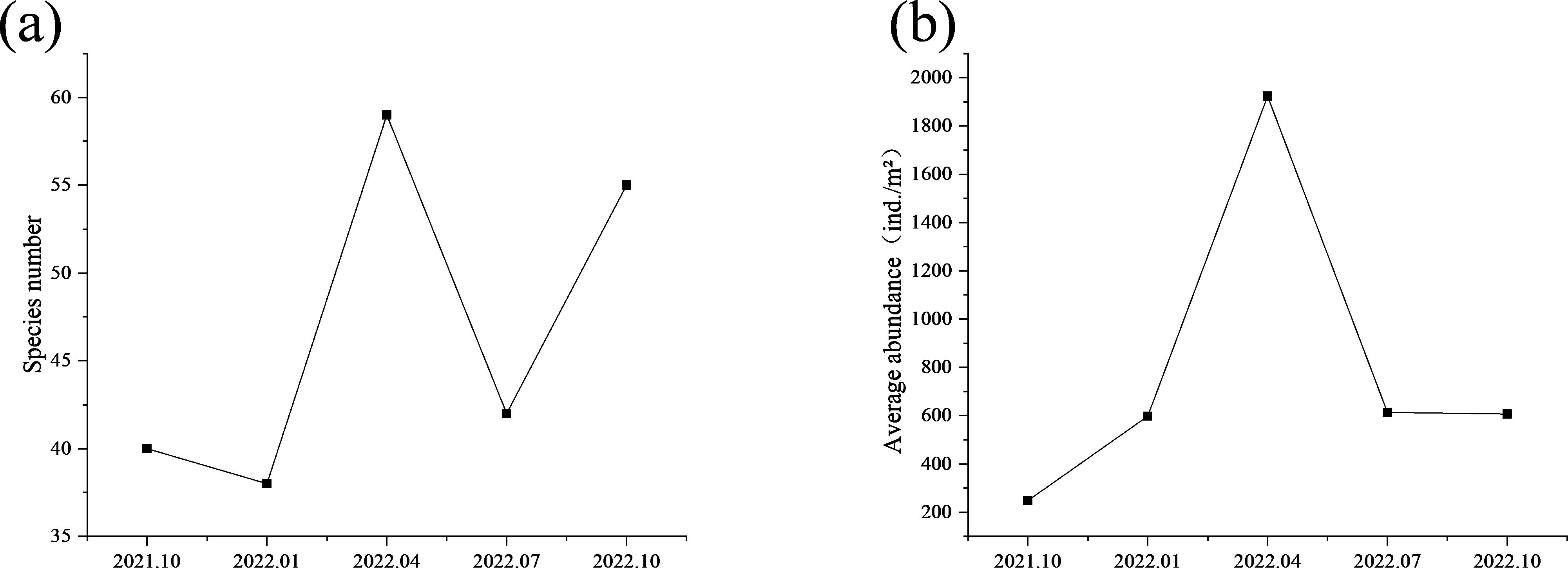
Figure 2. Species number (A) and average abundance (B) of macrobenthos from October 2021 to October 2022.
3.3 Community diversity index
One-way ANOVA was conducted with time and section as factors, and the results revealed that there were no significant differences in d, J’, or H’ among the different time points or among the different sections (P>0.05). The spatiotemporal distributions of the diversity indices of macrobenthos in the intertidal zone of Yueqing Bay are shown in Figure 3.
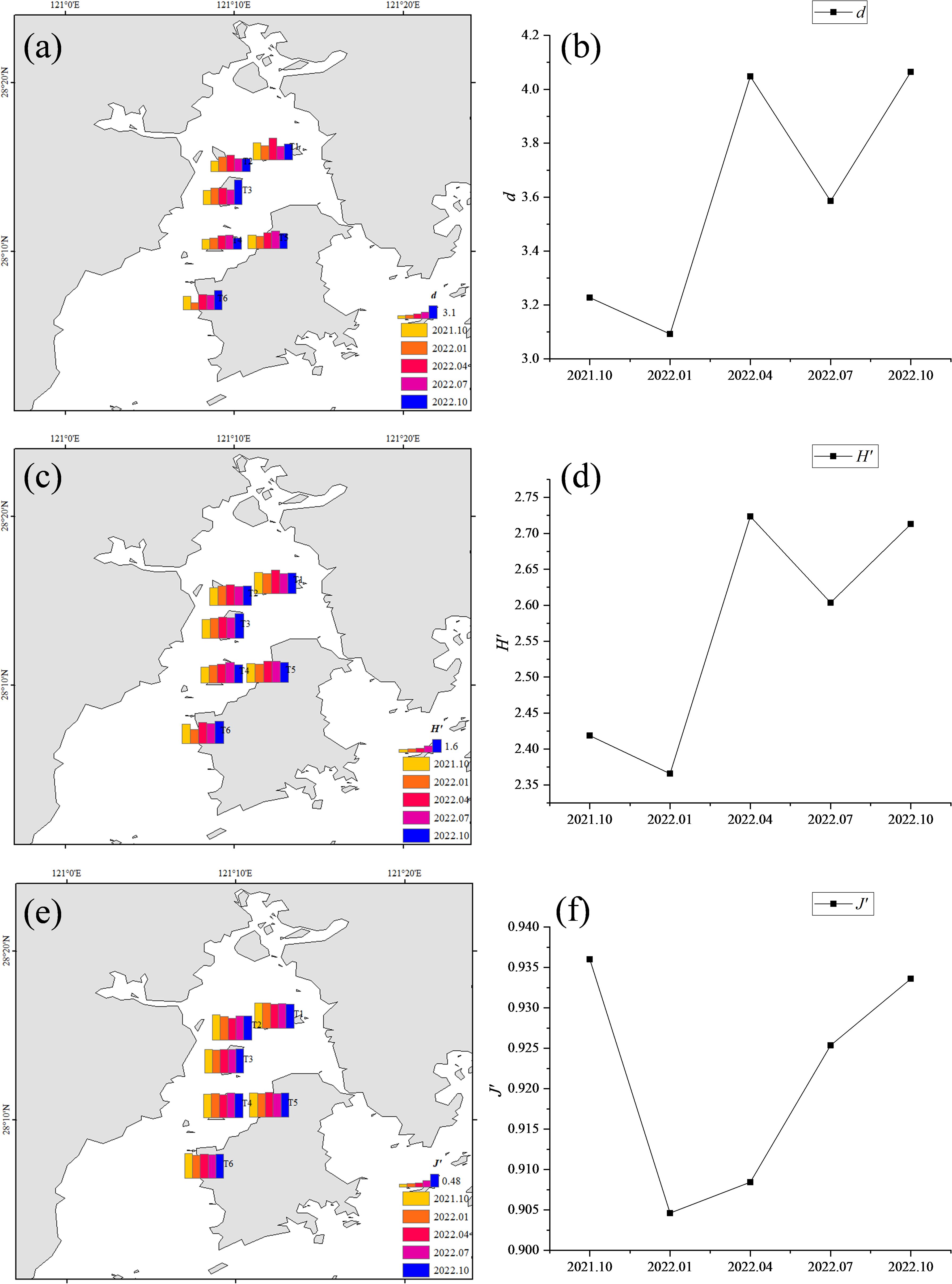
Figure 3. Distribution and average variation in the diversity indices of macrobenthos in the intertidal zone of Yueqing Bay from October 2021 to October 2022; (A, B): d; (C, D): H’; (E, F): J’.
The Margalef richness index (d) was calculated according to the mean value from the time scale as follows: 2022.10 (4.065) > 2022.04 (4.048) > 2022.07 (3.586) > 2021.10 (3.227) > 2022.01 (3.092) (Figure 3B). The Shannon−Wiener index (H’), which is based on the mean values from the time scale, was as follows: month 2022.04 (2.724) > month 2022.10 (2.713) > month 2022.07 (2.604) > month 2021.10 (2.419) > month 2022.01 (2.366) (Figure 3D). d and H’ showed similar spatial patterns (Figures 3A, C). In the T1 section, the highest values of both d and H’ appeared in October 2021, with values of d (4.144) and H’ (2.757), respectively. In the T2 section, the lowest value of d (2.555) was noted. In the T4 section, the lowest value of H’ (2.058) was noted. In the T2 section, the highest value of d (4.134) and the highest value of H’ (2.587) in the 2022.01 section were noted. In the T3 section, the lowest value of d (1.555) was noted. In the T4 section, the lowest value of d (1.587) was noted. In the T6 section, the d minimum (1.694) and H’ minimum (1.839) were noted. In the T1 section, d maximum (5.353) and H’ (3.036) maximum values in 2022 04 were noted. In the T4 section, d minimum (3.294) and H’ minimum (2.424) were noted. In the T1 section, the d maximum (4.321) and H’ maximum (2.773) in 2022 07 were noted. In the T5 section, the H’ maximum (2.773) were noted. In the T2 section, the H’ maximum (3.114) and H’ minimum (2.403) were noted. In the T3 section, the d maximum (6.177) and H’ maximum (3.270) in October 2022 were noted. In the T4 section, the d minimum (2.676) and H’ minimum (2.321) were noted.
The average values of J’ (Figures 3E, F) decreased in the following order: October 2021 (0.936) > October 2022 (0.934) > July 2022 (0.925) > April 2022 (0.908) > January 2022 (0.905). Across spatial scales, the highest J’ value in October 2021 was in the T1 section (0.973), and the lowest was in the T2 section (0.894). In January 2022, the highest J’ value was in the T1 section (0.955), and the lowest was in the T3 section (0.879). In April 2022, the highest J’ value was in the T6 section (0.944), and the lowest was in the T2 section (0.839). In July 2022, the highest J’ value was in the T1 section (0.952), and the lowest was in the T2 section (0.910). In October 2022, the highest J’ value was in the T3 section (0.944), and the lowest was in the T6 section (0.928).
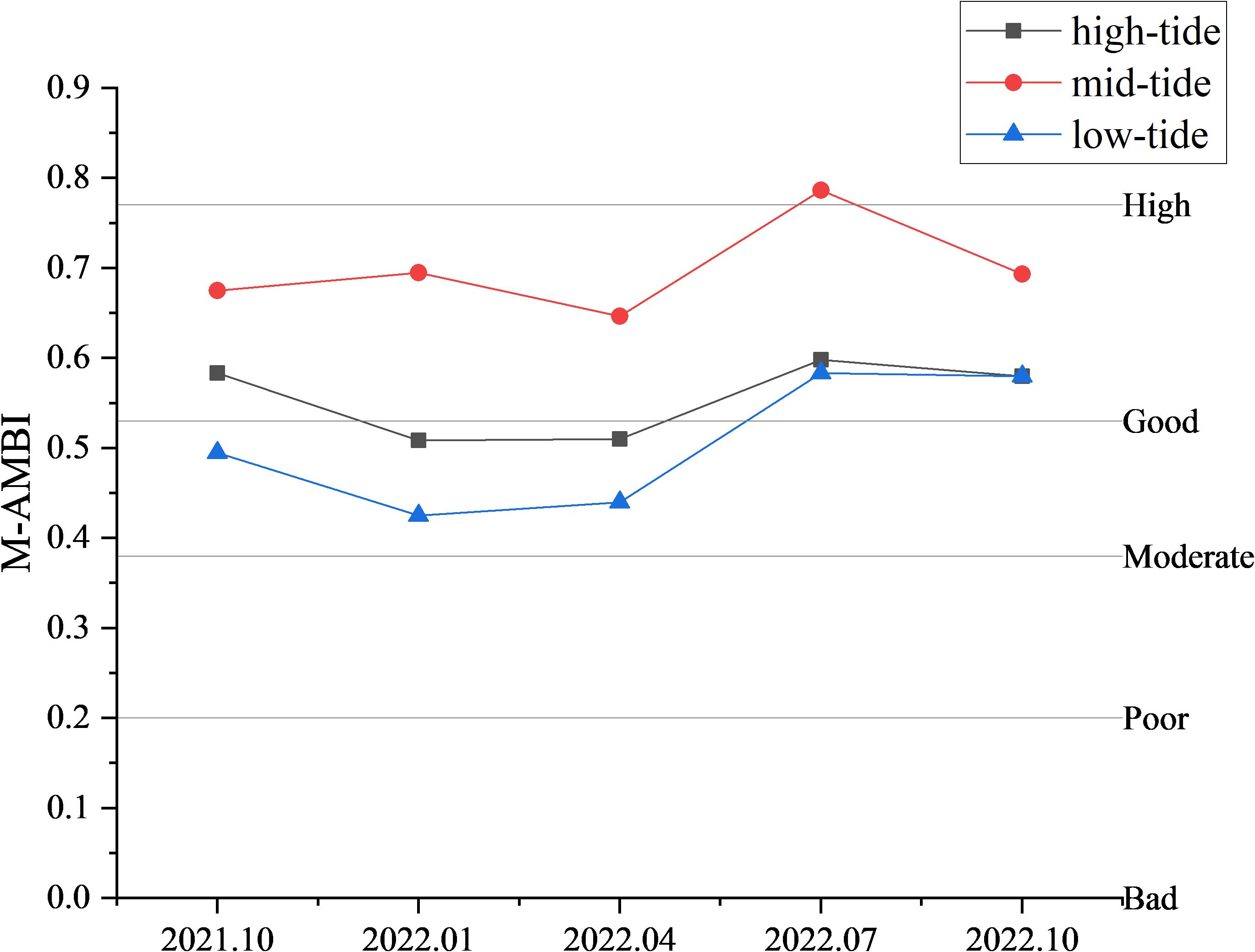
3.4 M-AMBI
In this study, the proportions of EG I-EG V were 31.97%, 32.30%, 29.77%, 0.71%, and 5.26%, respectively. Overall, the macrobenthos in the intertidal zone of Yueqing Bay were represented mainly by nonsensitive species (EG II), followed by sensitive species (EG I) and tolerant species (EG III).
The spatial and temporal distributions of the macrobenthic M-AMBI index in the intertidal zone of Yueqing Bay are shown in Figure 4. In terms of the mean value, the M-AMBI index of macrobenthos before and after stock enhancement fluctuated in a wave-like manner, but the overall level was still above the “good” level. From the temporal scale, the highest value of the M-AMBI index (0.66) occurred in July 2022, and the lowest value (0.53) occurred in April 2022. From the spatial scale, in October 2021, the lowest value of the M-AMBI (0.19) was found in the low-tide area of the T4 section, and the highest value of the M-AMBI (0.80) was found in the mid-tide area of the T6 section. In January 2022, the lowest value of the M-AMBI (0.00) was found in the low-tide area of the T5 section and the high-tide area of the T6 section, and the highest value of the M-AMBI (0.79) was found in the mid-tide area of the T5 section. In April 2022, the lowest M-AMBI value (0.27) was found in the high-tide area of the T6 section, and the largest M-AMBI value (0.83) was found in the mid-tide area of the T1 section. In July 2022, the lowest M-AMBI value (0.42) was found in the high-tide area of the T6 section, and the largest M-AMBI value (0.83) was found in the mid-tide area of the T5 section. In October 2022, the lowest M-AMBI value (0.42) was found in the high-tide area of the T6 section, and the largest M-AMBI value (0.42) was found in the mid-tide area of the T3 section. Overall, the lowest M-AMBI value (0.42) and the largest M-AMBI value (0.84) in the mid-tide zone were found in the T3 section. One-way ANOVA was conducted using time, section, and tidal area as factors, and the results showed that there were no significant differences in the M-AMBI values among the different times and sections. The M-AMBI values in the mid-tidal areas were significantly greater than those in the high tidal areas and low tidal areas (P<0.05). After stock enhancement, the average M-AMBI values for the remaining years, except for January 2022 and April 2022, were greater than those in October 2021. Apart from seasonal factors, there was no obvious pattern in the variation in the M-AMBI values in each section. The increase in M-AMBI values occurred mainly in the low-tide zone, with a slight increase in the mid-tide zone and no significant change in the high-tide zone (Figure 5).
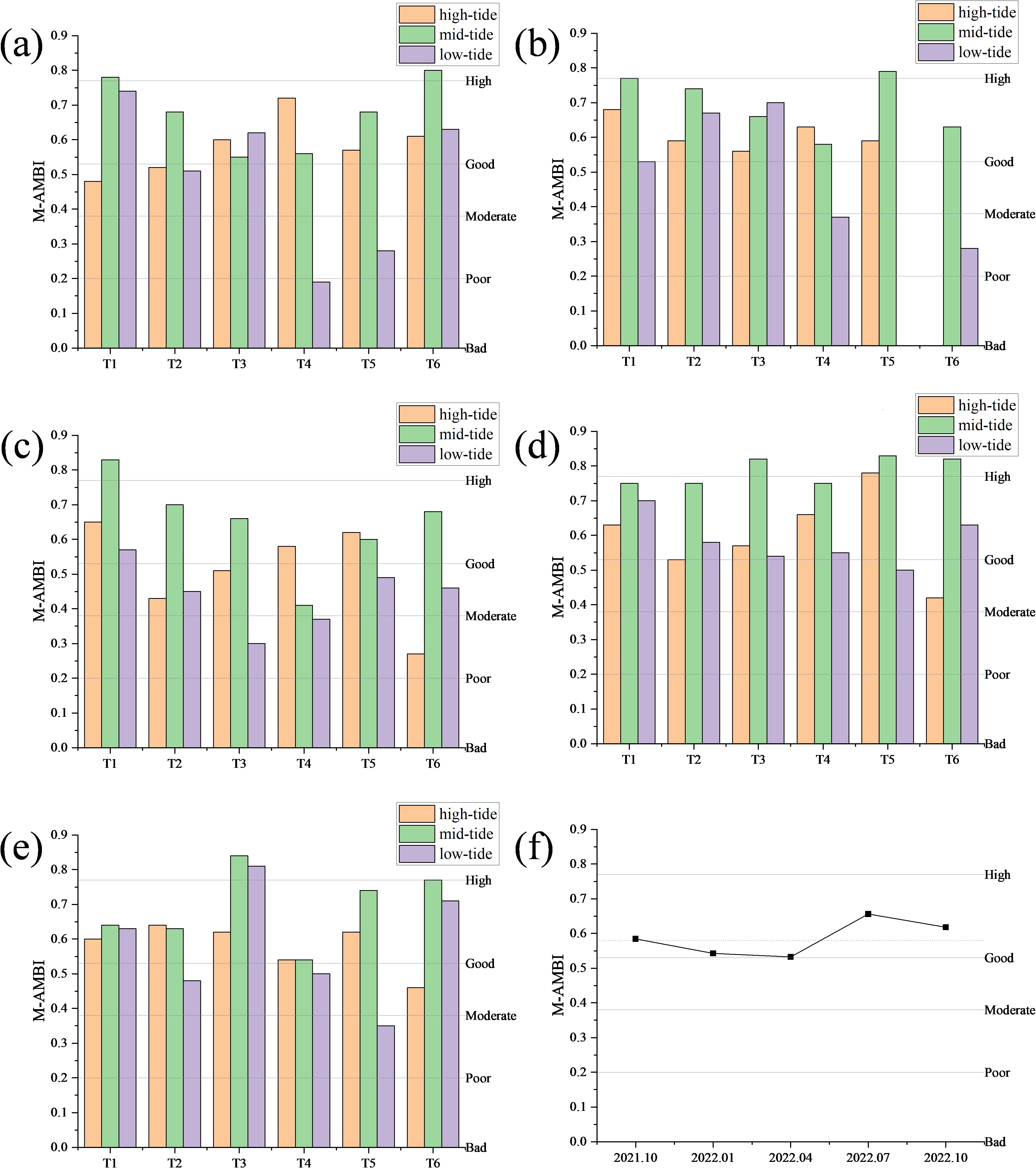
Figure 4. Distribution of the M-AMBI in the intertidal zone of Yueqing Bay: (A–E) October 2021 to October 2022; (F) average M-AMBI.
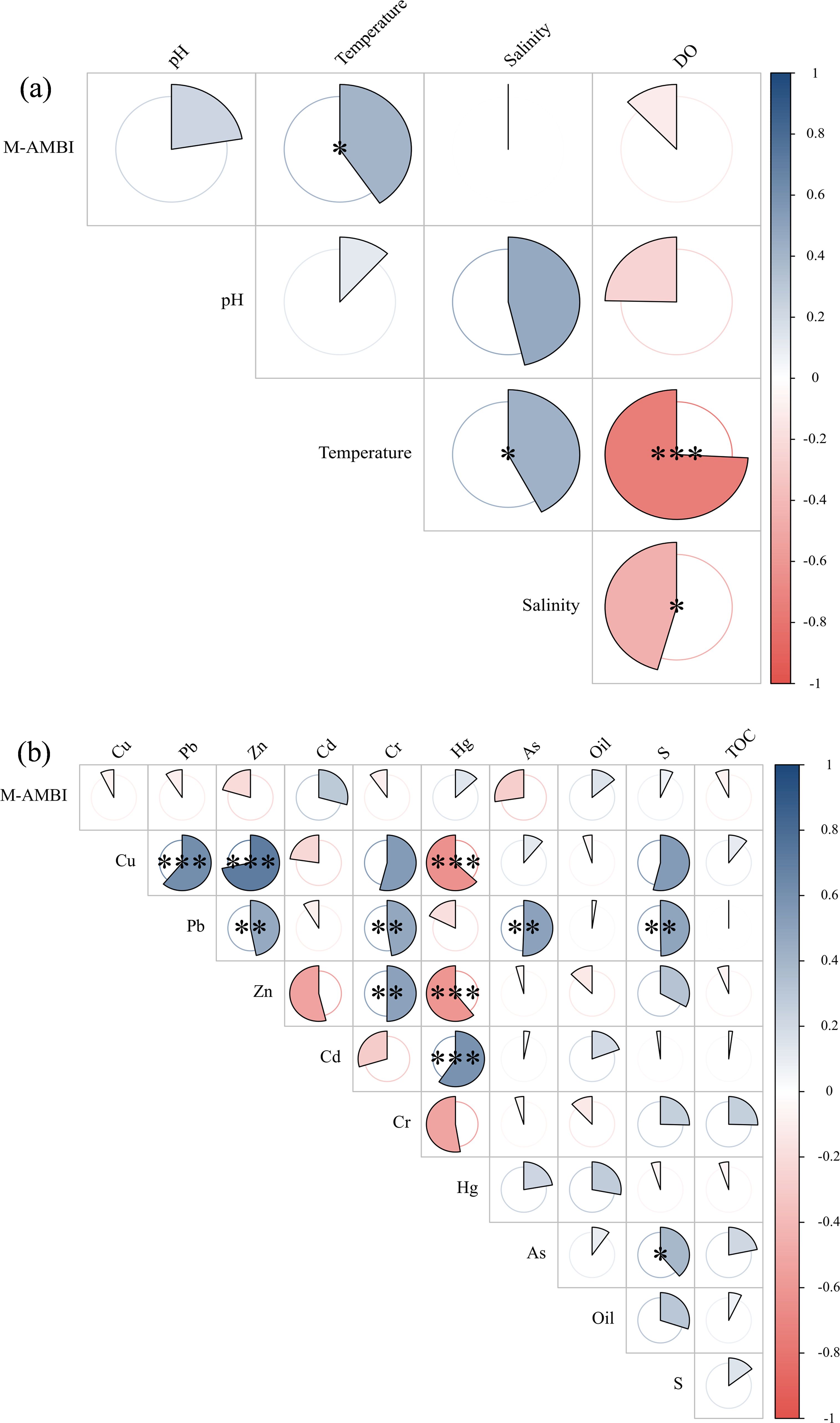
Figure 5. Spearman correlation analysis between the M-AMBI and the environmental factors of water quality (A) and sediment (B) in Yueqing Bay.
3.5 Correlation between the M-AMBI and environmental factors in the intertidal zone of Yueqing Bay
Spearman correlation analysis (Figure 5) revealed the following relationships between the M-AMBI and environmental factors in the intertidal zone of Yueqing Bay: the M-AMBI was significantly positively correlated with water temperature (P<0.05), whereas there was no significant correlation between the M-AMBI and sediment factors.
4 Discussion
4.1 Applicability of the M-AMBI
This study evaluated the benthic ecological quality of the intertidal zone in Yueqing Bay before and after stock enhancement via the M-AMBI. The M-AMBI, developed on the basis of the AMBI, combines the diversity index and species richness as two indicators and introduces a reference state to evaluate results in a more comprehensive and objective manner, thereby accurately reflecting the actual environmental conditions (Borja and Tunberg, 2011), with a stronger coupling with other biotic indices (Muxika et al., 2007). The software for calculating this index can be freely downloaded from the internet, making calculations convenient and simple and making the evaluation results easy to understand. Its greatest advantage lies in fully considering the ecological adaptation strategies and environmental sensitivity of macrobenthos, but this is also the main limiting factor for the application of this index, as it requires a rich foundation in macrobenthic taxonomy and biotic indices, which can be challenging when used in countries or regions with relatively scarce benthic ecological research. Since the M-AMBI has been used to assess the ecological quality of the intertidal zone in Shenzhen Bay (Cai et al., 2011), the M-AMBI has been widely applied in China’s harbors, coastal waters, intertidal zones, and estuarine areas for benthic ecological quality assessment (Ding et al., 2020; Jia et al., 2022; Li et al., 2017; Liu et al., 2014; Ni et al., 2019; Qiu et al., 2018; Song et al., 2017; Tang et al., 2019; Yan et al., 2020; Zhang et al., 2022), while reference conditions have also been used for long-term development. Many studies have indicated that setting the reference conditions to 115% of the maximum values of H′ and S can enable the M-AMBI to be accurately applied in intertidal sea areas (Liu et al., 2014; Liu et al., 2018; Liu et al., 2019; Ni et al., 2019); thus, this study also used these reference conditions. Among the macrobenthic samples investigated in this study, 26 were not included in the species list provided by the latest AMBI v6.0 and were replaced by other species of the same genus or family. The results revealed that six species were categorized as EG I, 11 as EG II, six as EG III, and one as EGIV, and the results of the evaluation were reliable (Cai et al., 2013b). When there are multiple types of habitats, the application of this index may be limited (De Paz et al., 2008; Teixeira et al., 2008), while the sediment types in the sampling areas of this study were all clayey silt. In conclusion, the M-AMBI is considered suitable for the evaluation of benthic ecological quality in the intertidal zone of Yueqing Bay.
4.2 Diversity indices and M-AMBI analysis of macrobenthos in the intertidal zone of Yueqing Bay
Community diversity indices integrate information on the relative abundance of species within a community and are commonly used to describe the ecological characteristics of biological communities and monitor changes in the structure of macrobenthic communities (Huang et al., 2019; Zhu et al., 2018). From October 2021 to October 2020, the mean values of d, J’, and H’ for macrobenthos in the intertidal zone of Yueqing Bay were 3.60, 0.92, and 2.57, respectively. In a study conducted from 2006-2007, these values were 4.29, 0.62, and 2.85, respectively (Peng et al., 2011). Compared with those in the latter study, the values of d and H’ in this study were lower, whereas the value of J’ was greater. This situation occurred because the survey locations in the latter study were all set in representative areas with relatively uniform beach substrate types, intact tidal areas, and relatively stable conditions, including the Ximen Island Marine Special Reserve (Liao et al., 2013), which elevated the average values of d and H’. The lower value of J’ may be attributed to the rapid economic development phase of Yueqing Bay during the 2006-2007 study period, when the intertidal zone was still frequently affected by human activities, resulting in a fragmented landscape in the intertidal zone (Zhao et al., 2009). This encroachment reduced the living space for intertidal biota and increased the degree of homogeneity of macrobenthic species, leading to a decrease in uniformity and exacerbation of the imbalance (Wang et al., 2021).
Historical research on the benthic ecological quality of the intertidal zone in Yueqing Bay has been relatively scarce. Previous reports have used MPI and ABC curves to evaluate the benthic ecological quality of the intertidal zone in Yueqing Bay. The results revealed that the benthic ecological quality of the intertidal zone in Yueqing Bay was basically stable from 2004 to 2006, ranging from light to moderate pollution (Long et al., 2008). The community structure of macrobenthos was moderately disturbed from 2006 to 2007, with poor stability (Peng et al., 2011). The ABC curve, as a single indicator, cannot be used to distinguish between the environmental effects of natural changes and human disturbances. The MPI was built on the ABC curve (Cai, 2003). It has the advantages of easy calculation and easy mastery, but its evaluation results are lagging and cannot accurately reflect key ecological information within the community (Long et al., 2008), such as the pollution tolerance and sensitivity characteristics of species within the community. This study used the M-AMBI method and reported that the overall average M-AMBI of the intertidal zone in Yueqing Bay was 0.59. The overall benthic ecological quality was good, which was obviously better than that reported in historical research. In addition, the bioindicator species of marine pollution, Capitella capitata, appeared in the macrobenthos collected in the 2004-2006 study (Wang et al., 2021) and became the dominant species. However, this species was not included in this study, This situation also verified that the benthic ecological quality of the intertidal zone in Yueqing Bay had improved. This may be attributed to recent coastal environmental management policies in Yueqing Bay and the planting of mangroves. On the one hand, in recent years, in Yueqing Bay, special rectification of the mudflat aquaculture environment has been carried out, which has provided strong support for continuous improvements in marine ecological environmental protection and the safety and quality of aquatic products. In 2021, the Yueqing Municipal Government issued the “Yueqing Bay (Yueqing Section) ‘One Bay One Policy’ Rectification Implementation Plan” to address the prominent ecological and environmental problems in Yueqing Bay. These coastal environmental management policies are important for improving benthic ecological quality (Wang et al., 2021). On the other hand, mangrove vegetation affects the macrobenthic community by changing soil types, providing habitats, improving adverse intertidal environments, etc., and promoting the biodiversity and stability of intertidal communities (Chen et al., 2013; Huang et al., 2017), thereby improving the benthic ecological quality of the intertidal zone in Yueqing Bay. A previous study in Yueqing Bay revealed that the mid-tide zone and the low-tide zone are submerged in seawater for a longer time than is the high-tide zone, which is more conducive to the growth and habitation of marine organisms, so the benthic ecological environment is better (Long et al., 2008). However, this study revealed that the M-AMBI values of the high-tide zone and the low-tide zone in Yueqing Bay were significantly lower than those of the mid-tide zone. The M-AMBI was highly correlated with H’. A previous study showed that the planting of mangroves increased the biodiversity of the high- and mid-tide zones and reduced the biodiversity of the low-tide zone (Tian et al., 2018); thus, this phenomenon occurred.
4.3 Effects of stock enhancement on the community diversity indices and M-AMBI in the intertidal zone of Yueqing Bay
According to the results of the community diversity indices (Figure 3), the H’ and d values of the macrobenthos fluctuated between October 2021 and October 2022, with consistent trends, reaching their maximum values in October 2022 and April 2022, respectively, whereas J’ first tended to decrease but then increased. After stock enhancement, except for January 2022, the average d and average H’ were greater than those in October 2021, whereas the average J’ was slightly lower than that in October 2021. The month of January had relatively low temperatures in Yueqing Bay, and the temperature constraint resulted in the lowest average values of all diversity indices occurring in that month. Generally, for a community with a certain number of species, the greater the evenness (J’) is, the greater the community diversity index (H’) is (Li et al., 2014), indicating a positive correlation between the two indices. This study did not reveal this phenomenon, which could be because the release of many Phascolosoma esculenta into the macrobenthic community reduced J’; however, J’ remained relatively high (average values all greater than 0.90). Subsequently, owing to the self-regulation of the macrobenthic community, evenness gradually recovered, leading to a trend of first decreasing and then increasing in the average J’. Apart from seasonal factors, d and H’ in October 2022 were greater than those in October 2021, whereas J’ was slightly lower than that in October 2021. There were no significant changes in any of the diversity indices before and after stocking, indicating that stock enhancement has not yet affected the complexity and stability of the intertidal macrobenthic community in Yueqing Bay (Wang, 2016). After stock enhancement, the diversity and richness of macrobenthos in the intertidal zone of Yueqing Bay improved, resulting in good ecological restoration (Peng et al., 2012).
According to the M-AMBI results (Figure 4), the average M-AMBI values of macrobenthos fluctuated between October 2021 and October 2022, reaching their maximum in July 2022. Except for January 2022 and April 2022, the average M-AMBI values after stock enhancement were greater than those in October 2021. Apart from seasonal factors, the average M-AMBI value in October 2022 was greater than that in October 2021, indicating that the increase in the stock of Phascolosoma esculenta has played a role in improving the intertidal ecological environment of Yueqing Bay. Diversity indices are commonly used as benthic indices to assess ecological status and the extent of pollution (Cai et al., 2002). However, some scholars have argued that diversity indices are meaningful only for comparative purposes and should not be used to assess benthic ecological status (Borja et al., 2019; Shade, 2017). Therefore, in this study, H’ was used to compare the differences in benthic ecological quality before and after stock enhancement. According to the H’ criteria (Cai et al., 2002), the benthic ecological quality of the intertidal zone of Yueqing Bay is good, with the benthic ecological quality in October 2022 being greater than that in October 2021. Water temperature is a significant environmental factor influencing the M-AMBI values in the intertidal zone of Yueqing Bay (Figure 3A), and the trend of the M-AMBI changes is closely related to the trend of the water temperature changes, indicating a strong positive correlation. Different seasons present significant variations in water temperature, which affects the number, biomass, and distribution range of species by influencing the growth, development, and reproduction of organisms (Liu et al., 2022), thereby impacting the ecological grouping of macrobenthos. Despite lower water temperatures in October 2022 than in October 2021, the M-AMBI value was greater, indicating better benthic ecological quality in the intertidal zone of Yueqing Bay in October 2022 and further highlighting the role of stocking Phascolosoma esculenta in improving the ecological environment of the intertidal zone of Yueqing Bay.
In October 2021, the benthic ecological quality of the intertidal zone in Yueqing Bay exhibited the following pattern: mid-tide zone > high-tide zone > low-tide zone. In October 2022, there was a pattern of a mid-tide zone > high-tide zone = low-tide zone. The increase in M-AMBI values occurred mainly in the low-tide zone, with a slight increase in the mid-tide zone and no significant change in the high-tide zone (Figure 6). An analysis of the reasons is as follows: in this study, the M-AMBI values showed no significant relationships with the various sediment factors. This may be because the stocking areas are close to areas where humans live, and the levels of different metals in the sediments may fluctuate as a result of disturbances caused by human activities. The sediment contents in the monitoring area in both October 2021 and October 2022 met China’s first-class marine sediment quality standards, indicating that the pollutant content may not be sufficient to determine its relationship with benthic ecological quality (Pitacco et al., 2021). Therefore, the sediment content is only a small part of the reason for the variation in M-AMBI values (Umehara et al., 2022). The main reasons are related to the increase in the stock of Phascolosoma esculenta and human disturbances. On the one hand, Phascolosoma esculenta can enrich heavy metals in sediment, which has a positive impact on the environment (Gao et al., 2012; Wu and Xu, 2018), and ecological protection and restoration projects can alter biological connectivity and hydrological connectivity (Jiang et al., 2020). An increase in the stock of Phascolosoma esculenta increases the biomass in high- and mid-tide zones, releasing a large amount of organic matter during their life and death processes. The decomposition of organic matter is a core process in the energy and material cycles of all ecosystems (Barreiro et al., 2011; Gomez et al., 2018), and increasing organic matter in the high-tide zone can increase the nutrient cycle in the entire intertidal ecosystem and thus promote improvements in ecological and environmental quality. On the other hand, currently, fisher’s harvesting activities have far exceeded traditional self-sufficiency, and the harvesting of economic species is relatively intense (Liu et al., 2018). Phascolosoma esculenta is an economic species, and the increase in the stock of Phascolosoma esculenta and the planting of mangroves to some extent hinder the ability of fishers to harvest low-tide zones (Zhang, 2019); instead, the harvesting range is concentrated in high- and mid-tide zones. The combination of all of these reasons resulted in a slow or even no increase in benthic ecological quality in the high- and mid-tide zones after stock enhancement, whereas the disturbance level decreased in the low-tide zone, leading to an increase in benthic ecological quality.
5 Conclusion
(1) The Shannon–Wiener diversity index (H’) and Margalef richness index (d) increased in October 2022 after stock enhancement, indicating a positive ecological restoration effect. The average M-AMBI of the intertidal zone in Yueqing Bay from October 2021 to October 2022 was 0.59, indicating good benthic ecological quality.
(2) Because Phascolosoma esculenta can accumulate heavy metals from the environment and because increasing the stock of these organisms increases their biomass, Phascolosoma esculenta releases a large amount of organic matter throughout its lifecycle, which to increases the nutrient cycle of the ecosystem and promotes improvements in environmental ecological quality. However, owing to the planting of mangroves in the low-tide zone, people tend to fish for Phascolosoma esculenta in the mid- and high-tide zones, so the M-AMBI values in the intertidal zone of Yueqing Bay increased in the low-tide zone, slightly increased in the mid-tide zone, and basically did not change in the high-tide zone.
(3) Water temperature varies greatly in different seasons, and temperature affects the number, biomass and distribution range of species by influencing the growth, development and reproduction of organisms, as well as the ecological grouping of macrobenthos. Therefore, the trend of the M-AMBI changes is basically consistent with the trend of the water temperature changes, indicating a strong positive correlation.
(4) The macrobenthic diversity index in the intertidal zone of Yueqing Bay was high, and the M-AMBI was able to assess the ecological status of the intertidal zone of Yueqing Bay because of its easy usage and distinct threshold.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
YY: Conceptualization, Methodology, Software, Validation, Visualization, Writing – original draft. CS: Writing – original draft, Conceptualization, Data curation, Formal analysis, Software. PC: Data curation, Investigation, Writing – review & editing. WL: Writing – review & editing, Data curation, Investigation, Resources. QH: Writing – review & editing, Conceptualization, Funding acquisition, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the National Natural Science Foundation of China (42076156) and was also partly sponsored by K.C. Wong Magna Fund in Ningbo University.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (42076156) and was also partly sponsored by K.C. Wong Magna Fund in Ningbo University.
Conflict of interest
Author WL was employed by the company Yuzhi Environmental Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Araki H., Schmid C. (2010). Is hatchery stocking a help or harm? Aquaculture 308, S2–S11. doi: 10.1016/j.aquaculture.2010.05.036
Barreiro F., Gomez M., Lastra M., Lopez J., Huz R. D. L. (2011). Annual cycle of wrack supply to sandy beaches: Effect of the physical environment. Mar. Ecol. Prog. Ser. 433, 65–74. doi: 10.3354/meps09130
Borja A., Chust G., Muxika I. (2019). Forever young: the successful story of a marine biotic index. Adv. Mar. Biol. 82, 93–127. doi: 10.1016/bs.amb.2019.05.001
Borja A., Dauer D. M., Diaz R., Llansó R. J., Muxika I., Rodríguez J. G., et al. (2008). Assessing estuarine benthic quality conditions in Chesapeake Bay: a comparison of three indices. Ecol. Indic. 8, 395–403. doi: 10.1016/j.ecolind.2007.05.003
Borja Á., Elliott M., Carstensen J., Heiskanen A., van de Bund W. (2010). Marine management–towards an integrated implementation of the European Marine Strategy Framework and the Water Framework Directives. Mar. pollut. Bull. 60, 2175–2186. doi: 10.1016/j.marpolbul.2010.09.026
Borja Á., Marín S. L., Muxika I., Pino L., Rodríguez J. G. (2015). Is there a possibility of ranking benthic quality assessment indices to select the most responsive to different human pressures? Mar. pollut. Bull. 97, 85–94. doi: 10.1016/j.marpolbul.2015.06.030
Borja A., Tunberg B. G. (2011). Assessing benthic health in stressed subtropical estuaries, eastern Florida, USA using AMBI and M-AMBI. Ecol. Indic. 11, 295–303. doi: 10.1016/j.ecolind.2010.05.007
Cai L. (2003). Macrozoobenthos pollution index (MPI). Acta Scientiae Circumstantiae. 23, 625–629. doi: 10.1007/s11769-003-0089-1
Cai L., Chen X., Wu C., Peng X., Cao J., Fu S. (2011). Temporal and spatial variation of macrofaunal communities in Shenzhen Bay intertidal zone between 1995 and 2010. Biodiv Sci. 19, 702–709. doi: 10.3724/SP.J.1003.2011.08124
Cai L., Ma L., Gao Y., Zheng T., Lin P. (2002). Analysis on assessing criterion for polluted situation using species diversity index of marine macrofauna. J. Xiamen Univ. (Natural Science). 41, 641–646.
Cai W., Meng W., Liu L., Zhu Y., Zhou J. (2013a). Assessing the benthic ecological status in Yangtze river estuary using AMBI and M-AMBI. Environ. Science. 34, 1725–1734. http://ir.yic.ac.cn/handle/133337/6754.
Cai W., Meng W., Zhu Y., Zhou J., Liu L. (2013b). Assessing benthic ecological status in stressed Liaodong Bay (China) with AMBI and M-AMBI. Chin. J. Oceanol Limn. 31, 482–492. doi: 10.1007/s00343-013-2177-0
Chen Z. (2016). On mangrove plantation and tidal flats ecosystem restoration of maoyandao in southern Zhejiang. Ocean Dev. Management. 33, 40–44. doi: 10.20016/j.cnki.hykfygl.2016.04.008
Chen Y., Shi L., Quan W. (2007). The proliferation and release of benthic animal communities in the ecological restoration project of the Yangtze River Estuary and its effectiveness evaluation. Fishery Modernization. 34, 35–39. doi: 10.3969/j.issn.1007-9580.2007.02.013
Chen G., Yu D., Ye Y., Chen B. (2013). Impacts of mangrove vegetation on macro-benthic faunal communities. Acta Ecologica Sinica. 33, 327–336. doi: 10.5846/stxb201111091699
Committee, C.G.C.C (1993). China Gulf Chronicle Vol. 6 (Southern Bay of Zhejiang Province: China Ocean Press).
De Paz L., Patrício J., Marques J. C., Borja A., Laborda A. J. (2008). Ecological status assessment in the lower Eo estuary (Spain). The challenge of habitat heterogeneity integration: a benthic perspective. Mar. pollut. Bull. 56, 1275–1283. doi: 10.1016/j.marpolbul.2008.04.027
Ding J., Li J., Xue S., Zhang W., Huo E., Ma Z., et al. (2021). Health assessment for b benthos in the sea area adjacent to the Xiaoqing River estuary, Laizhou Bay. Acta Ecologica Sinica. 41, 4806–4817. doi: 10.5846/stxb202002290369
Ding J., Zhang W., Li Y., Xue S., Li J., Jiang Z., et al. (2020). Health assessment of the benthic ecosystem in Jiaozhou Bay: Ecological characteristics of the macrobenthos. Prog. Fishery Sci. 41, 20–26. doi: 10.19663/j.issn2095-9869.20181104001
Gao Y., Pan L., Wu H., Wang Z. (2012). Contents and correlationship of heavy metals in Phascolosoma esculentas and their habitat sediments. Mar. Sci. 36, 54–60.
Gomez M., Barreiro F., Lopez J., Lastra M. (2018). Effect of upper beach macrofauna on nutrient cycling of sandy beaches: metabolic rates during wrack decay. Mar. Biol. 165, 131–133. doi: 10.1007/s00227-018-3392-1
Han Q., Wang Y., Zhang Y., Keesing J., Liu D. (2013). Effects of intensive scallop mariculture on macrobenthic assemblages in Sishili Bay, the northern Yellow Sea of China. Hydrobiologia. 718, 1–15. doi: 10.1007/s10750-013-1590-x
Huang K., Chen L., Fu T., Huang Z., Chen K. (2019). Spatial distribution of macrobenthic diversity factors in Xiamen Bay and its relationship with environmental factors. J. Fisheries Res. 41, 293–301. doi: 10.14012/j.cnki.fjsc.2019.04.004
Huang X., Ren P., Li H., Bao Y., Afli A. (2017). Niche analysis of dominant species of macro benthos in different mangrove habitats on Maoyan Island. J. Zhejiang Normal Univ. (Natural Sciences). 40, 446–452. doi: 10.16218/j.issn.1001-5051.2017.04.014
Jang Y., Lin N., Yang L., Cheng J. (2014). The ecological risk of stock enhancement and the measures for prevention and control. J. Fishery Sci. China. 21, 413–422.
Jia H., Cao L., Chai X. (2022). The changes of macrobenthic community structure and cause analysis in the Yangtze Estuary during summer from 2016 to 2019. Mar. Environ. Science. 41, 180–186. doi: 10.13634/j.cnki.mes.2022.02.015
Jiang Y., Wang Y., Zhou D., Ke Y., Yan J. (2020). The impact assessment of hydro-biological connectivity changes on the estuary wetland through the ecological restoration project in the Yellow River Delta, China. Sci. Total Environ. 758, 143706. doi: 10.1016/j.scitotenv.2020.143706
Li B., Li X., Bouma T. J., Soissons L. M., Cozzoli F., Wang Q., et al. (2017). Analysis of macrobenthic assemblages and ecological health of Yellow River Delta, China, using AMBI & M-AMBI assessment method. Mar. pollut. Bull. 119, 23–32. doi: 10.1016/j.marpolbul.2017.03.044
Li Z., Qiu S., Zhang J., Geng B. (2014). Preliminary analysis of fishery community structure in southern Shandong Peninsula before and after portunus trituberctdatus releasing. J. Yantai Univ. (Natural Sci. Eng. Edition). 27, 184–190. doi: 10.13951/j.cnki.37-1213/n.2014.03.013
Liao Y., Shou L., Ceng J., Gao A., Tang Y., Yan X., et al. (2013). Functional groups of marine macrobenthos in relation to environmental factors around the Ximen Island National Marine Special Reserve, Zhejiang. Biodiv Sci. 21, 3–10. doi: 10.3724/SP.J.1003.2013.08151
Liu Z., Fan B., Huang Y., Yu P., Li Y., Chen M., et al. (2019). Assessing the ecological health of the Chongming Dongtan Nature Reserve, China, using different benthic biotic indices. Mar. pollut. Bull. 146, 76–84. doi: 10.1016/j.marpolbul.2019.06.006
Liu L., Li B., Lin K., Cai W., Wang Q. (2014). Assessing benthic ecological status in coastal area near Changjiang River estuary using AMBI and M-AMBI. Chin. J. Oceanol Limn. 32, 290–305. doi: 10.1007/s00343-014-3125-3
Liu X., Qi Y., Sha J., Xu Z., Jian X., Du X., et al. (2022). Community characteristics and ecological health assessment of macrobenthos in nügukou of Jiaozhou bay. Guangxi Sci. 29, 1197–1205. doi: 10.13656/j.cnki.gxkx.20230110.020
Liu Y., Wang H., Wu S., Yue Q. (2022). A study of ecological restoration techniques in the reclamation area. J. Ocean Technology. 41, 112–120. doi: 10.3969/j.issn.1003-2029.2022.04.012
Liu Z., Yu P., Chen M., Cai M., Fan B., Lv W., et al. (2018). Macrobenthic community characteristics and ecological health of a constructed intertidal oyster reef in the Yangtze Estuary, China. Mar. pollut. Bull. 135, 95–104. doi: 10.1016/j.marpolbul.2018.07.019
Liu G., Yuan X., Guan C., Luo H., Gu Y. (2018). Influence of reclamation on off-shore macrobenthic community in the Qingduizi bay. Mar. Environ. Science. 37, 663–669. doi: 10.1016/j.marpolbul.2018.07.019
Long H., Yu J., Zhou Y. (2008). Using macrozoobenthos pollution index to assess Yueqingwan Bay tideland environmental quality. J. Mar. Sci. 26, 97–104. doi: 10.3969/j.issn.1001-909X.2008.04.015
Margalef R. (1951). Diversidad de especies en las comunidades naturales. Publicaciones del Instituto Biol. Aplicada. 6, 59–72.
Molony B. W., Lenanton R., Jackson G., Norriss J. (2005). Stock enhancement as a fisheries management tool. Rev. Fish Biol. Fisheries 13, 409–432. doi: 10.1007/s11160-005-1886-7
Muxika I., Borja A., Bald J. (2007). Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Mar. pollut. Bull. 55, 16–29. doi: 10.1016/j.marpolbul.2006.05.025
Ni D., Zhang Z., Liu X. (2019). Benthic ecological quality assessment of the Bohai Sea, China using marine biotic indices. Mar. pollut. Bull. 142, 457–464. doi: 10.1016/j.marpolbul.2019.03.055
Peng X., Wu H., Gao P., Chen Q., Chou J., Cai J., et al. (2012). Ecological response of intertidal macrobenthos to the construction of stock enhancement and protection area: A case of Dachen Island. J. Mar. Sci. 30, 19–26. doi: 10.3969/j.issn.1001-909X.2012.01.003
Peng X., Xie Q., Chen S., Huang X., Chou J., Zhong W., et al. (2011). The community distribution pattern of intertidal macrozoobenthos and the responses to human activities in Yueqing Bay. Acta Ecologica Sinica. 31, 954–963. doi: CNKI:SUN:STXB.0.2011-04-007
Pitacco V., Mistri M., Granata T., Moruzzi L., Meloni M. L., Massara F., et al. (2021). Habitat heterogeneity: A confounding factor for the effect of pollutants on macrobenthic community in coastal waters. Mar. Environ. Res. 172, 105499. doi: 10.1016/j.marenvres.2021.105499
Qi J., Zeng Z., Ning Y., Wu Q. (2016). Construction of ecological risk assessment system for stock enhancement in zoobenthos. J. Fisheries China. 40, 1099–1105. doi: 10.11964/jfc.20150509899
Qiu B., Zhong X., Liu X. (2018). Assessment of the benthic ecological status in the adjacent waters of Yangtze River Estuary using marine biotic indices. Mar. pollut. Bull. 137, 104–112. doi: 10.1016/j.marpolbul.2018.10.006
Shade A. (2017). Diversity is the question, not the answer. ISME J. 11, 1–6. doi: 10.1038/ismej.2016.118
Shannon C. E. (1948). A mathematical theory of communication. Bell system Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Song Q., Zou X., Zhang H., Yu W., Zang Z., Wang C. (2017). An approach based on M-AMBI for assessing benthic ecological status of a broad intertidal zone: A case study in the Jiangsu intertidal zone, China. Mar. pollut. Bull. 116, 87–94. doi: 10.1016/j.marpolbul.2016.12.066
Sun D., Li C., Wu Q., Zhang H. (2011). Characteristics of macrobenthos community in Nanpeng island water. Hubei Agric. Sci. 50, 2084–2087. doi: 10.14088/j.cnki.issn0439-8114.2011.10.062
Tang Y., Wang J., Cheng H., Zheng B., Ma Z. (2019). Eco-environment quality assessment of macrobenthic community in the East Ningde sea waters. Mar. Environ. Science. 38, 278–285. doi: 10.13634/j.cnki.mes.2019.02.017
Teixeira H., Salas F., Neto J. M., Patrício J., Pinto R., Veríssimo H., et al. (2008). Ecological indices tracking distinct impacts along disturbance-recovery gradients in a temperate NE Atlantic Estuary–Guidance on reference values. Estuar. Coast. Shelf S. 80, 130–140. doi: 10.1016/j.ecss.2008.07.017
Tian J., Wang Y., Tian K., Zhang M., Qiu J., Shui B., et al. (2018). Study on the Characteristics of the Macrobenthic Community in the Intertidal Zone along the Yanpu Bay before and after Kandelia candel Planting. J. Zhejiang Ocean Univ. (Natural Science). 37, 114–122. doi: 10.3969/j.issn.1008-830X.2018.02.004
Umehara A., Borja Á., Ishida A., Nakai S., Nishijima W. (2022). Responses of the benthic environment to reduction in anthropogenic nutrient loading in the Seto Inland Sea (Japan), based on M-AMBI assessment. Mar. Environ. Res. 173, 105509. doi: 10.1016/j.marenvres.2021.105509
Wang L. (2016). Impacts on natural stock genetic diversity and fish composition by Sebastes schlegelii enhancement in Lidao bay. Institute Oceanology Academia Sin.
Wang G., Liu X., Feng C., Dan C., Ma C. (2021). Research progress on evaluation of ecosystem health based on benthic index. Anhui Agric. Sci. Bulletin. 27, 169–172. doi: 10.16377/j.cnki.issn1007-7731.2021.13.05
Wang X., Xiao X., Xu X., Zou Z., Chen B., Qin Y., et al. (2021). Rebound in China’s coastal wetlands following conservation and restoration. Nat. Sustainability. 4, 1076–1083. doi: 10.1038/s41893-021-00793-5
Wang H., Zou Q., Liu Y., Lin Y., Lv B., Yao W. (2018). The characteristics and changes of the species and quantity of macrobenthos in Yueqing Bay. Mar. Sci. 42, 78–87. doi: CNKI:SUN:HYKX.0.2018-06-011
Wu Y., Ai Y., Zheng Z., Zhuang M., Ding Z. (2023). Evaluation for the effectiveness of four mangrove wetland restoration projects in Xiamen. Chin. J. Ecology. 42, 2419–2424. doi: 10.13292/j.1000-4890.202310.020
Wu H., Chen K., Zhang P., Fu S., Hou J., Chen Q. (2013). Eco-environmental quality assessment of Luoyuan Bay, Fujian Province of East China based on biotic indices. Chin. J. Appl. Ecology. 24, 825–831. doi: 10.13287/j.1001-9332.2013.0228
Wu Y., Xu R. (2018). Sipuncula phascolosoma esculenta: research progress. Fisheries Sci. 37, 855–861. doi: 10.16378/j.cnki.1003-1111.2018.06.022
Yan J., Sui J., Xu Y., Li X., Wang H., Zhang B. (2020). Assessment of the benthic ecological status in adjacent areas of the Yangtze River Estuary, China, using AMBI, M-AMBI and BOPA biotic indices. Mar. pollut. Bull. 153, 111020. doi: 10.1016/j.marpolbul.2020.111020
Yan R., Zhu F., Han Q., Huang C., Chen Q., Han Q. (2019). Research on intertidal macrobenthic community in the yellow river estuary. Chin. J. Zoology. 54, 835–844. doi: 10.13859/j.cjz.201906010
Zhang M. (2019). Study on the changes of community structure and secondary productivity of macrobenthos before/after planting of Kandelia candel in Yanpu Bay, Cangnan (Zhoushan, China: Zhejiang Ocean University).
Zhang A., Gu Y., Yuan X., Brustolin M. C., Yang X., Zhang R., et al. (2022). Benthic habitat quality assessment in estuarine intertidal flats based on long-term data with focus on responses to eco-restoration activity. Water-Sui. 14, 3846. doi: 10.3390/w14233846
Zhao Y. Q., Zeng J. N., Chen Q. Z., Gao A. G., Liao Y. B., Shou L. (2009). Macrozoobenthos community pattern in the intertidal zone alongside Daxie development region in spring. Chin. J. Zoology. 44, 78–83. doi: 10.13859/j.cjz.2009.02.010
Zhong H., Qu F., Sui J., Zhang M., Tu L., Zhao F., et al. (2018). Abundance, biomass and community structure of macrobenthos in southern coast of Shandong Province in spring. Mar. pollut. Bull. 37, 88–98. doi: 10.11840/j.issn.1001-6392.2018.01.011
Zhu X., Chen B., Yu W., Lin J., Huang Y., Liao J. (2018). Discussion on taxonomic diversity indices and taxonomic sufficiency for macrobenthos in Xiamen Bay. Acta Ecologica Sinica. 38, 5554–5565. doi: 10.5846/stxb201708121452
Keywords: macrobenthos, diversity indices, M-AMBI, stock enhancement, Yueqing Bay
Citation: Yang Y, Song C, Chen P, Lu W and Han Q (2024) Evaluation of the stock enhancement effect of Phascolosoma esculenta on macrobenthic communities using diversity and biotic indices in Yueqing Bay, East China Sea. Front. Mar. Sci. 11:1457599. doi: 10.3389/fmars.2024.1457599
Received: 01 July 2024; Accepted: 13 September 2024;
Published: 02 October 2024.
Edited by:
Xiaoshou Liu, Ocean University of China, ChinaReviewed by:
Anguo Zhang, National Marine Environmental Monitoring Center, ChinaXu Yong, Chinese Academy of Sciences (CAS), China
Wenzhe Xu, Tianjin University of Science and Technology, China
Copyright © 2024 Yang, Song, Chen, Lu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingxi Han, aGFucWluZ3hpQG5idS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Yushu Yang1†
Yushu Yang1† Chen Song
Chen Song Qingxi Han
Qingxi Han