
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 18 September 2024
Sec. Coral Reef Research
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1454887
 Emily Walton1,2
Emily Walton1,2 Lindsey Badder1
Lindsey Badder1 Claudia Tatiana Galindo-Martínez1,2
Claudia Tatiana Galindo-Martínez1,2 David B. Berry3
David B. Berry3 Martin Tresguerres2
Martin Tresguerres2 Daniel Wangpraseurt1,2*
Daniel Wangpraseurt1,2*In the face of escalating threats posed by human-induced climate change, urgent attention to coral reef restoration is imperative due to ongoing reef degradation. Here, we explored the potential of generating coral micropropagates as a tool to rapidly generate coral tissue for reef restoration and reef engineering. We developed a hypersalinity-induced polyp bailout protocol and a simple attachment device to support the growth of micropropagates on commonly used restoration substrates. We found that hypersalinity induction, at a rate of < 1 ppt hr-1, produced healthy micropropagates of the coral Stylophora pistillata. The highest attachment success (~74%) was achieved in CaCO3 substrate devices, which outperformed PVC (~48%) and Portland cement (~5%). Settled micropropagates displayed rapid growth rates on both CaCO3 (0.037 mm²/day ± 0.002 SE) and PVC (0.057 mm²/day ± 0.008 SE) substrates, while Portland cement induced tissue degradation. Our study provides a detailed methodology for reliably generating, attaching, and growing coral micropropagates and underscores the potential of polyp bailout as a viable technique supporting coral propagation efforts.
Reef-building corals are the primary architects of tropical reef ecosystems that harbor a diversity of life forms spanning from microorganisms to large marine vertebrates (Hughes et al., 2017). Coral reefs are a hotspot for ocean biodiversity and provide crucial goods and services via fisheries, shoreline protection, nutrient cycling, and tourism activities, as well as societal and cultural importance for indigenous communities (Eddy et al., 2021; Eliff and Iracema., 2017; Rädecker et al., 2015). However, coral reefs are degrading at an unprecedented rate due to anthropogenic stressors such as climate change, overfishing, and pollution (Pandolfi et al., 2003; van Hooidonk et al., 2016; Heron et al., 2016; Hoegh-Guldberg et al., 2017).
The rapid decline of coral reefs highlights the urgency of comprehensive conservation and restoration efforts (Suggett et al., 2023; Roger et al., 2023a) including coral gardening approaches and the development of artificial or hybrid reef structures (Burt et al., 2009; Lima et al., 2019; Rinkevich, 2021; Roger et al., 2023b). Coral gardening (Rinkevich, 2021) involves the cultivation of coral fragments under protected conditions in nurseries until they reach a certain size and maturation, and their subsequent outplanting onto existing degraded reefs (Forrester et al., 2012). Artificial and hybrid reefs (A&HR) involve the deployment of human-made structures that mimic the structure of natural reefs (Burt et al., 2009; Lima et al., 2019). There has been a growing interest in the deployment of A&HRs since the 1980s, as these structures reduce coastal erosion and enhance eco-tourism activities (Rossi and Rizzo, 2020; Higgins et al., 2022). A key challenge for creating A&HRs is to rapidly cover the man-made reef structures with beneficial benthic coral reef communities, including stony corals. Space competition for bare substrate is high on coral reefs and it has thus been of interest to rapidly cover or ‘re-skin’ bare substrates with live corals. By creating small fragments, usually between 1-4 cm2 in size, one can generate rapid two-dimensional spreading of coral tissue at rates several orders of magnitude higher than under standard field conditions (Forsman et al., 2015, 2017). Such microfragments are now widely used in restoration activities (Tortolero-Langarica et al., 2020; Knapp et al., 2022; Mostrales et al., 2022). However, as corals are modular animals that are composed of repeated ‘building blocks’ called polyps that can bud off asexually, it is theoretically possible to seed and propagate individual polyps.
A potential option for propagating individual polyps is via polyp bailout, a natural response to stress that results in the separation of individual polyps from the coral colony (Sammarco, 1982). This process serves as an escape response to extreme stressors such as hypersalinity, extreme high and low temperatures, and acidification (Kvitt et al., 2015; Shapiro et al., 2016). Importantly, bailed out polyps retain their symbionts and can re-attach themselves to a substrate and grow into a new colony (Shapiro et al., 2016; Chuang and Mitarai, 2020). Polyp bail out has been used to create the coral-on-a-chip system, a promising microscale platform to study coral biology in the laboratory (Shapiro et al., 2016; Pang et al., 2020). While this system has been used to characterize the reattachment of bailed out polyps on a glass surface and to monitor subsequent individual polyp growth (Shapiro et al., 2016), the potential of using polyp bailout as a method for reef restoration remains largely unexplored.
Polyp bailout typically produces dozens of micropropagates from a singular coral fragment (Shapiro et al., 2016; Schweinsberg et al., 2021), an output that is much larger than traditional fragmentation methods. Thus, coral bailout holds great potential as a method to rapidly re-skin A&HR substrates or aid in the production of coral fragments for gardening applications. The aims of this study are to develop a reliable technique for rapidly generating coral micropropagates and inducing successful settlement/attachment and growth of micropropagates on commonly used restoration substrates. Specifically, we focused on the model species Stylophora pistillata (Shefy and Rinkevich, 2021) and evaluate attachment success and tissue growth rates in response to CaCO3, polyvinyl chloride (PVC), and Portland cement.
Colonies of S. pistillata from Birch aquarium (San Diego, USA) were cultivated in experimental aquaria at Scripps Institution of Oceanography (UC San Diego, USA), which provides flow-through seawater at constant temperature (25 °C); an aquarium heater (EnjoyRoyal, USA) was used to further maintain temperature in each tank. Downwelling irradiance was provided at 100 μmol photons m-2 s-1 at a 10hr/14hr light-dark cycle that included moonlight simulation (4h during dark cycle) (Orbit Marine LED Current Loop). For polyp bailout experiments, we used fragments of S. pistillata that were about 2 cm in length.
Polyp bailout was evaluated throughout a range of hypersalinity regimes and experimental protocols. To create different salinity gradients over time, we evaluated the use of a peristaltic pump, manual adjustments in water salinity, as well as natural evaporation of water for different water volumes. Preliminary experiments with manual increases in salinity with the addition of high salinity water over a set time and the use of a peristaltic pump resulted in inconsistent salinity gradients and proved difficult to replicate. Thus, we focused on creating salinity gradients induced by natural evaporation of water. Six different volumes (25 mL, 50 mL, 75 mL, 100 mL, 150 mL, 200 mL) of filtered natural seawater (0.35 µm) (FSW) at 35 PPT were evaporated in a small glass container (8 cm diameter) to create different rates of salinity increase over time. Evaporation experiments were performed in a 25 °C room with the container placed on a magnetic stirrer at 40 rpm to create gentle water movement.
Incident irradiance was provided by a light emitting diode (LED) panel that delivered a downwelling irradiance of 100 μmol photons m-2 s-1 (400-700 nm, PAR). An air pump was connected to a small pipette to ensure that the O2 content of the ambient water was air saturated. Salinity was measured hourly using a refractometer (Agriculture Solutions, USA). For each salinity gradient, we exposed 3 fragments to hypersalinity stress. Each fragment was visually monitored for polyp detachment. S. pistillata required gentle agitation for the polyps to be released from the skeleton. This was done by utilizing a plastic pipette to gently agitate the fragment with water. The generated coral micropropagates were then transferred to a petri dish filled with 35 ppt seawater to re-acclimate to normal seawater conditions.
The health and viability of micropropagates was assessed by examining polyp morphology using a stereoscope (Olympus SZ61, Japan) and PSII photochemistry via pulse amplitude modulated (PAM) chlorophyll (chl) a fluorescence imaging (Imaging-PAM). We categorized a healthy polyp morphology based on the presence of intact tentacles, a well-defined tissue structure surrounding the mouth opening, and the presence of the stomach (Schweinsberg et al., 2021). Polyps that were characterized as degraded were lacking one or more of these characteristics. Viable and healthy polyps also often demonstrated a spinning behavior, as noticed in other studies (Supplementary Material S1, Shapiro et al., 2016).
For chl a fluorimetry, we used an imaging pulse amplitude-modulated chl a fluorometer (Imaging PAM, mini version; WALZ GmbH, Effeltrich, Germany) that employs a blue measuring light (460 nm). Following a dark acclimation period of 20 min, we measured the maximum quantum yield (FV/Fm) of photosystem II (PSII) following a saturation pulse (Wangpraseurt et al., 2019a). We also measured relative electron transport rates (rETR) of coral micropropagates during the attachment period to artificial substrates, to evaluate the light-use efficiency (ɑ) and maximum relative electron transport rate (rETRmax) over time (Ralph and Gademann, 2005). For this, rETR curves were performed spanning an irradiance regime from 0 to 783 μmol photons m-2 s-1 (0, 1, 23, 43, 81, 145, 222, 269, 321, 403, 492, 783 μmol photons m-2 s-1) with an incubation period of 30 s at each light step (Ralph et al., 2008). Five micropropagates from each substrate were measured at days 7, 14, and 21 following bail out. Data was fit to the Platt equation (Platt et al., 1980).
For our attachment tests, we used tiles made from CaCO3 (Ocean Wonders, USA), PVC and Portland cement (HardieBacker, USA). CaCO3 tiles were left at manufactured dimensions (3.2 cm x 3.2 cm) while PVC and Portland cement tiles were cut to 10 cm x 5 cm in size. Preliminary attachment experiments suggested that the creation of small crevices improved attachment success, and we therefore fabricated crevices that were 2 mm deep and 1 mm wide with 2.5 cm spacings and 2 crevices per substrate using a handsaw.
To ensure sufficient gas and nutrient exchange between the micropropagates and the ambient water and to provide replication, we developed 18 laminar flow chambers (LFCs) (12 cm x 6 cm x 3 cm). These LFCs were designed using CAD software (OnShape) and 3-D printed (PRUSA MK4 3D Printer) using black PLA filament. LFCs were fitted with laminar flow dividers (6 cm x 3 cm) that were laser cut from acrylic paneling. The LFCs were then placed on top of holding tanks filled with 10 liters of FSW (35 ppt) that provided flowthrough aerated seawater at a laminar flow velocity of 1 cm s-1. Flow velocity was measured via tracking the movement of small particles in the water. The water temperature was set to 25°C via a submersible aquarium heater (EnjoyRoyal, USA). Tiles were deployed in the LFCs 3 days prior to micropropagate attachment.
To facilitate attachment to the restoration substrates, 7 micropropagates were carefully dispersed within the crevice of each tile. Attachment experiments were performed with a total of 6 tiles distributed over 3 tanks per treatment (PVC, CaCO3, Portland cement), resulting in a total of 126 tested micropropagates in 18 laminar flow chambers. Experiments were performed with FSW at 25°C, 35 ppt salinity, and an incident irradiance of 100 μmol photons m-2 s-1 (10hr/14hr light-dark cycle). To maintain salinity levels, FSW was manually added to compensate for any evaporative losses. Attachment experiments were performed only with healthy micropropagates, as defined based on morphological characteristics and Fv/Fm values > 0.3 (see above). Attachment was observed for 7 days and categorized as attached, detached or degraded (polyp lysis).
To determine the growth rate of attached micropropagates, close-up images were taken over 14 days using a stereoscope (Olympus SZ61, Japan). To minimize physical disturbance, images were taken directly in the LFCs. Lateral tissue growth was approximated using ImageJ (version 1.53, USA) by manually segmenting the planar surface area (SA, mm2) covered by coral tissue. The growth rate of micropropagates was analyzed as the % change in the SA of the micropropagates over a 14-day growth period.
Optical Coherence Tomography (OCT) imaging was used to non-invasively characterize the surface structure of the artificial substrates and the attachment of coral micropropagates. OCT imaging was performed as described previously (Wangpraseurt et al., 2017, 2019b) using a Ganymede spectral domain OCT system (GAN311C1, 930 nm) with an axial resolution of 5.5 µm, an imaging depth of 2.9 mm and a lateral resolution of 8 µm (in air). Briefly, bare substrates were imaged in a black acrylic chamber filled with seawater. To calculate surface roughness, 3D OCT images were binarized in ImageJ (NIH, Bethesda, MD) and imported into Matlab (Mathworks, Natick, MA) as a tiff stack. The voxels on the surface of the substrate were identified as the binary voxel closest to the probe for each stack of voxels. Then the surface voxels were fit to a plane by simple linear regression. The difference between the surface and the best-fit plane was calculated for all points. Surface roughness is reported as the mean difference between the surface and best-fit plane for each sample (Equation 2).
Wherein Ra is surface roughness, S(x,y,z) is the surface, and t(x,yz) is the best-fit plane.
All statistical analyses were performed using Origin Pro (2023) and Microsoft Excel (2022). We used an alpha level of 0.05 to determine statistical significance.
We successfully generated viable S. pistillata bailed polyps using a simple evaporation-based hypersalinity gradient (Figure 1A). These bailed polyps looked intact at salinity rates of 0.9 ppt/hr or less, while tissue disintegration occurred at faster rates (Figure 1A). At a salinity increase rate of 0.9 ppt/hr, > 82% of micropropagates appeared healthy as indicated by intact tentacles, stomach and mouth opening (Figure 1A). Variable chlorophyll a fluorescence imaging revealed no significant difference in the maximum quantum yield (Fv/Fm) of photosystem II (PSII) of micropropagates compared to intact coral fragments (0.45 ± 0.04 vs. 0.47 ± 0.08; ANOVA, p = 0.208; Figures 1C–E). The sequence of morphological changes over the salinity gradient was reproducible and we typically observed polyp retraction when salinities of 44-46 ppt were reached, which was followed by the separation and thinning of the coenosarc at about 48-51 ppt, and total separation of polyps from the connecting tissue and skeleton at about 52-56 ppt. This process resulted in the total bailout of polyps after a period of 24-26 hours.
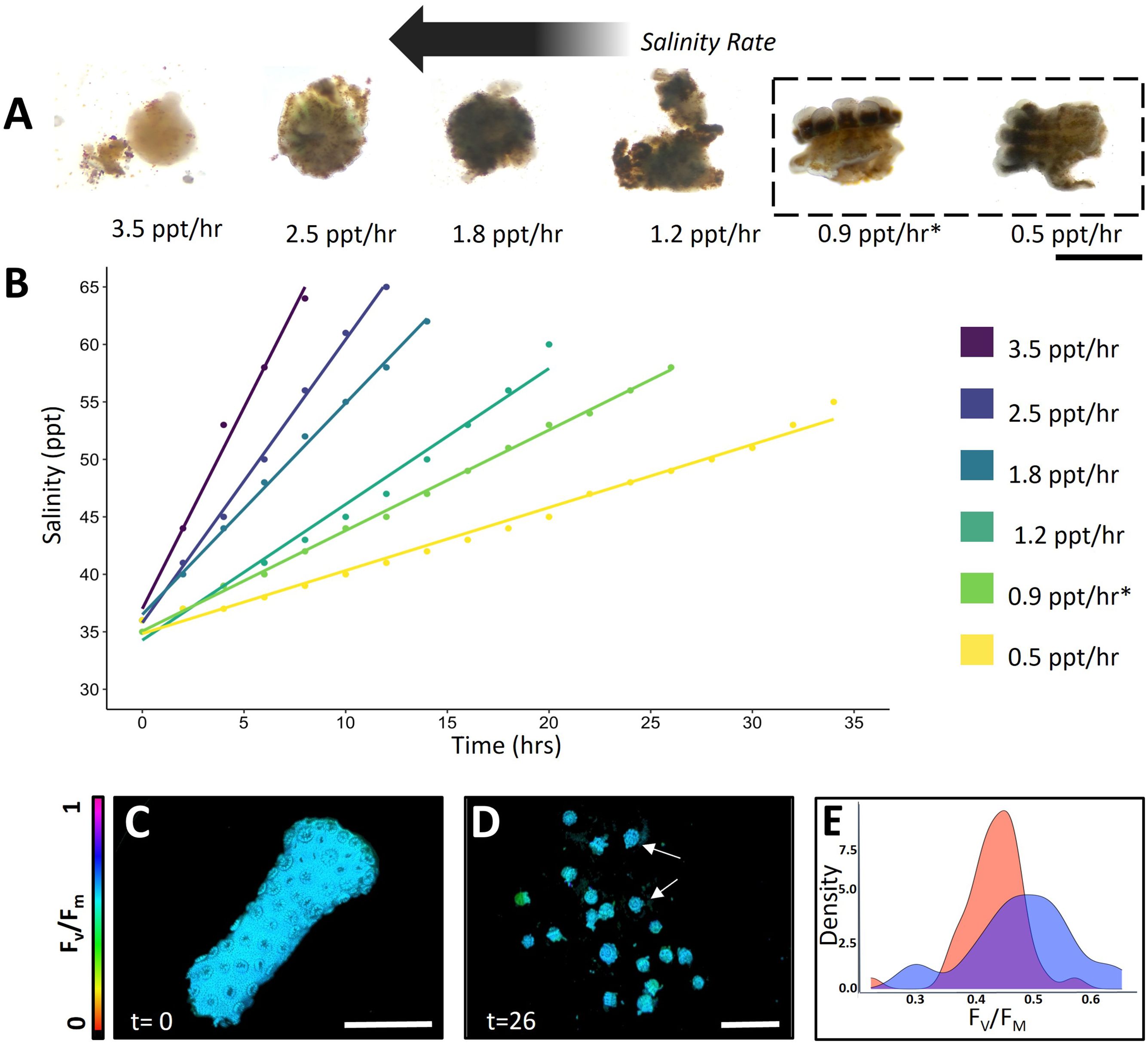
Figure 1. Stylophora pistillata micropropagate viability (A, B) Generated micropropagates in response to evaporation-based hypersalinity gradients (scale bar = 1 mm). (C, D) Representative images of the maximum quantum yield (Fv/Fm) of photosystem II (PSII) of healthy coral fragments and generated coral micropropagates following 26 h of hypersalinity treatment (scale bar = 0.5 cm). (E) Histogram of Fv/Fm of healthy S. pistillata polyps from intact fragments (dark blue) and generated micropropagates (red) induced via a salinity gradient of 0.9 ppt/hr (n= 20 polyps).
Our study builds upon the work of Shapiro et al. (2016), which demonstrated that two other Pocilloporidae coral species (Pocillopora damicornis and Seriatopora hystrix) responded to hypersalinity stress with viable polyp bailout. However, our study developed a simple and replicable method protocol for inducing polyp bailout in S. pistillata. Consistent with Shapiro et al. (2016), we observed polyp bailout occurring at salinity levels 17-21 ppt above that of ambient seawater. It is noteworthy that, in contrast to previous observations in P. damicornis, the induction of viable micropropagates in S. pistillata required agitation and water circulation, presumably to alleviate hypoxic stress during darkness (Wangpraseurt et al., 2012). The similarities in polyp bailout responses across P. damicornis, S. hystrix, and S. pistillata suggest that this may be a common response to hypersalinity stress in corals, or at least within the Pocilloporidae family.
The use of coral micropropagates for restoration has been hampered by the inability of micropropagates to successfully attach and grow over extended periods of time (Schweinsberg et al., 2021). Here, we developed a simple attachment device that uses an aerated, laminar flow-through system equipped with micropropagate chips (Figures 2A–D, see methods) to successfully induce attachment of S. pistillata bailed out polyps.
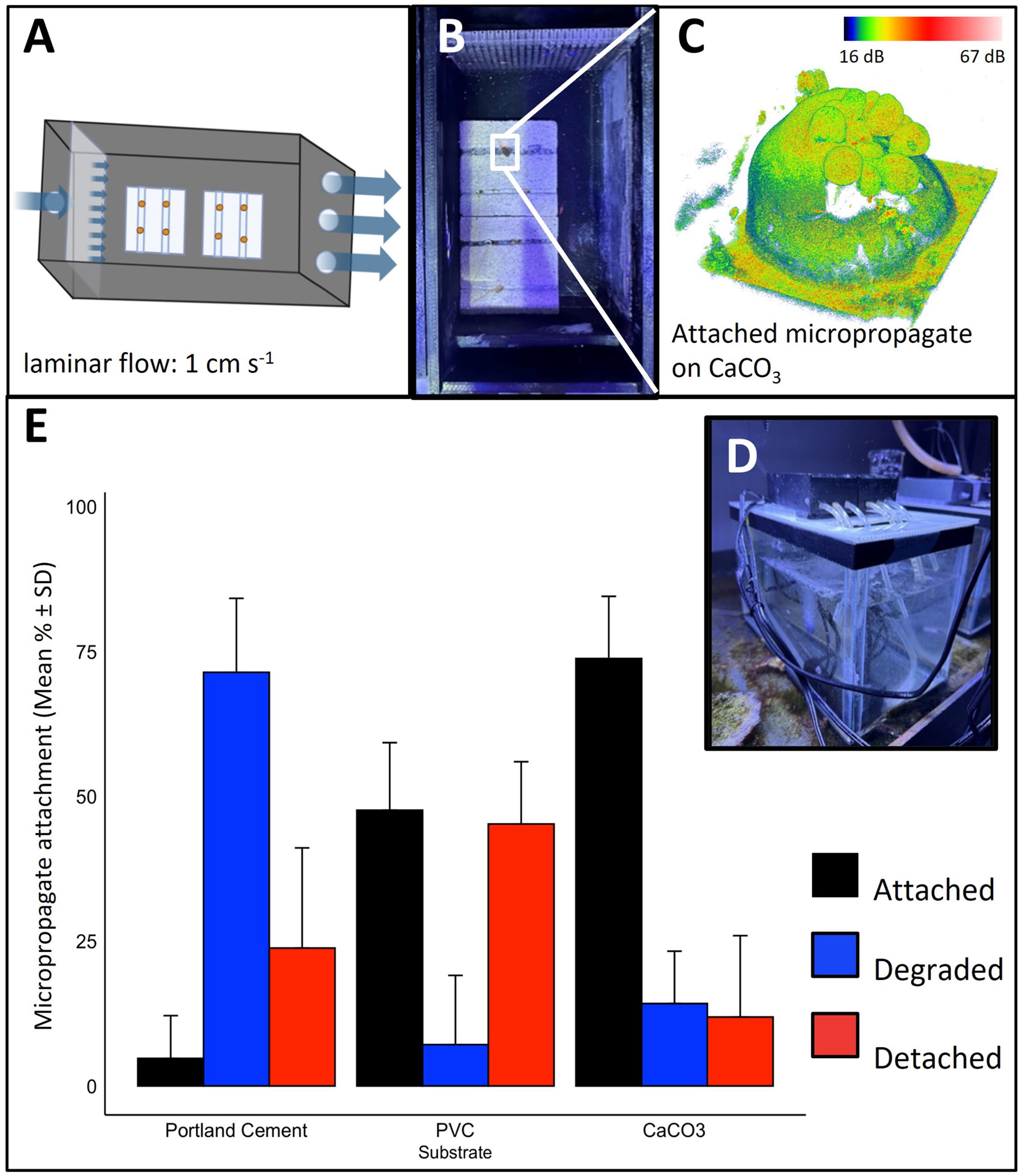
Figure 2. Attachment of micropropagates to restoration substrates. (A–D) Micropropagate chip device. (A, B) Schematic and image of the 3D printed acrylic chambers. Slow, laminar flow of aerated seawater is delivered to the micropropagates. Crevices (1 mm in width and height) facilitate tissue attachment. (C) Example 3D optical coherence tomography image of attached micropropagate and (D) image of entire recirculating system. (E) Micropropagate attachment success for chips made from Portland cement, PVC and CaCO3.
We evaluated the suitability of commonly used restoration materials, including CaCO3, PVC, and Portland cement as micropropagate chip substrates (Adey and Michael Vassar, 1975; Boström-Einarsson et al., 2020; Levenstein et al., 2022). CaCO3 is often used as a substrate in coral gardening efforts and is a preferred substrate as it is also the building material of coral skeletons (Levenstein et al., 2022). PVC-based substrates have been extensively used in coral nurseries and the formation of AR frameworks, due to their low cost and ease of installation (Mallela et al., 2017). Likewise, cement serves as an inexpensive and scalable substrate that has become increasingly popular in the development of ARs (Burt et al., 2009; Boström-Einarsson et al., 2020). We found significant differences in settlement and attachment rates between CaCO3, PVC and Portland cement using our custom-made micropropagate attachment device (Two-way ANOVA, p = 2.39E-10 (Figures 2A–D). The highest attachment rate was observed for CaCO3 (73.81% ± 10.7%) which was > 15-fold higher compared to Portland cement (4.76% ± 7.38%) and about 1.4-fold higher than for PVC (47.62% ± 11.66%, Figure 2E). For the first seven days, micropropagate degradation was highest for Portland cement (71.4% ± 12.8%), which was nearly 5-fold greater than for CaCO3 (14.2% ± 9.1%) and 10-fold greater compared to PVC (7.1% ± 12.0%, Figure 2E). Together, these results underscore the importance of material choice for optimizing attachment success of micropropagates.
We hypothesized that the observed differences in attachment success are related to surface roughness and substrate microarchitecture (Fujiwara et al., 2022). We therefore used OCT, an optical analog to ultrasound (Jaffe et al., 2022), to characterize the 3D surface structure of restoration substrates with micrometer resolution. We found no clear relationship between microscale surface roughness (Ra, see methods) p = 0.287 and attachment success, (Figure 3D, Supplementary Figure S1). Ra was highest for CaCO3 (18.5 μm ± 1.3 SE) which was 1.5-fold higher compared to Portland cement (12.2 μm ± 1.5 SE) and about 6.5-fold greater than for PVC (2.8 μm ± 0.3 SE) (Figures 3A–D). Although it is known that larger roughness elements (e.g. mm scale crevices, Randall et al., 2021), provide protection and can enhance attachment success in a flow-driven environment, smaller roughness elements analyzed in this study (Ra < 20 μm) did not affect micropropagate performance. These results suggest that other factors have a more prominent role in modulating attachment success and micropropagate degradation within the scope of our experiment. For instance, Portland cement is known to be highly alkaline (pH ~13, e.g. Guilbeau et al., 2003) and such enhancements in surface pH could have negative effects on coral growth.
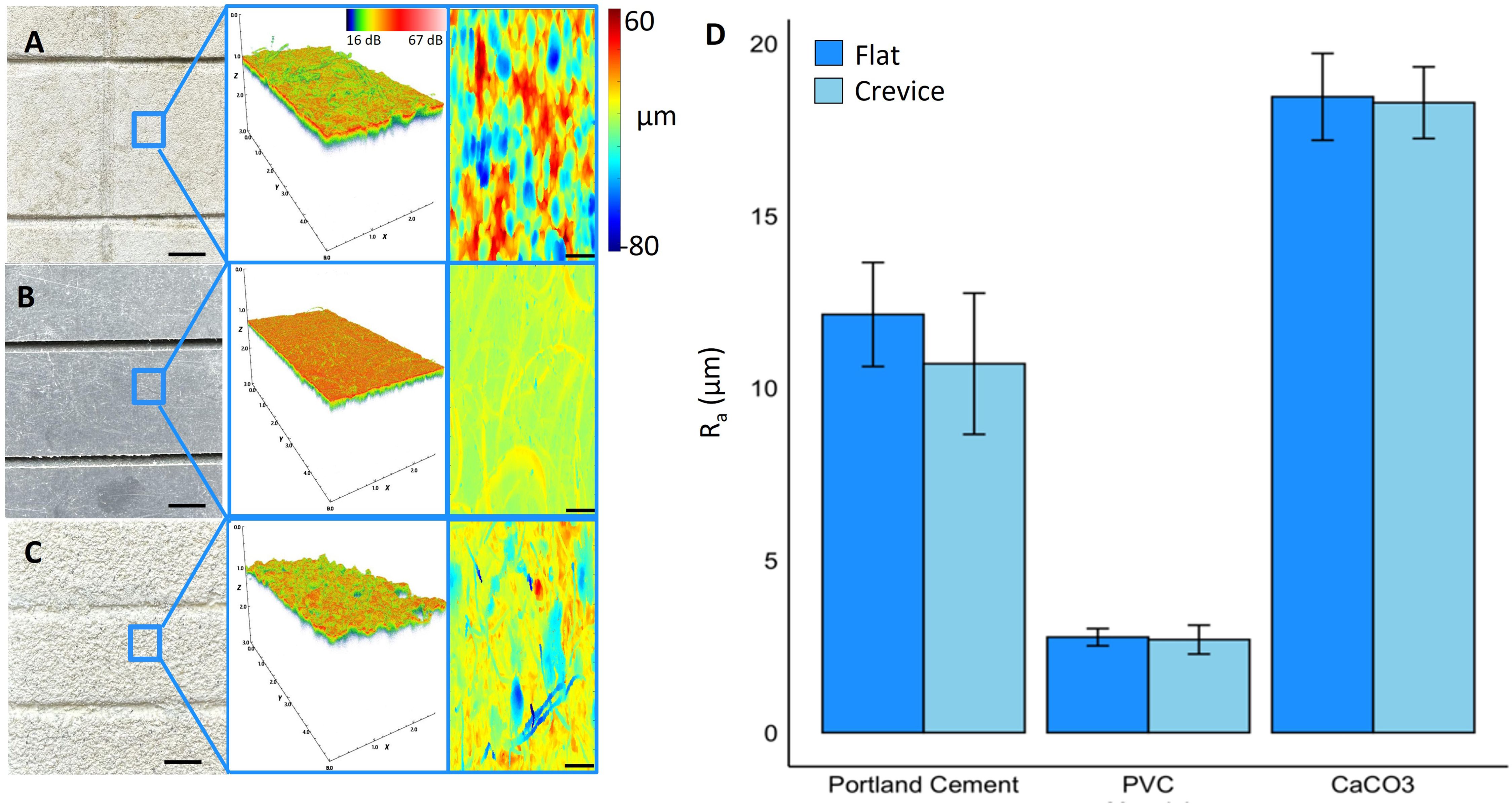
Figure 3. Optical coherence tomography imaging and microstructural characterization of restoration substrates. (A) Portland cement (B) PVC and (C) calcium carbonate substrates used in attachment experiments. Top view image (left, scale bar = 5 mm) and associated 3D OCT scan of area highlighted in blue (middle). Analyzed 2D projections of the difference in distance between the tissue height and a plane fit to the tissue surface (right, scale bar = 100 μm). (D) Surface roughness (Ra) for the tested restoration substrates.
Using our micropropagate chip device, we successfully created growing micropropagates on PVC and CaCO3 chips (Figures 4A–I). We observed the highest growth rate of micropropagates on PVC (0.057 mm2 ± 0.008 SE) which was 1.5-fold higher than for CaCO3 (0.037 mm2 ± 0.002 SE, Figures 4E, H). In contrast, micropropagates growing on Portland cement experienced significant tissue degradation and did not grow laterally (Figures 4A, B). Variable chl a fluorescence imaging revealed highest Fv/Fm values for day 7, day 14, and day 21 for micropropagates growing on CaCO3 (Day 7 = 0.49 ± 0.06, Day 14 = 0.46 ± 0.09, Day 21 = 0.48 ± 0.08) which was nearly 1.5-fold higher than for PVC (Day 7 = 0.36± 0.04, Day 14 = 0.38 ± 0.03, Day 21 = 0.35 ± 0.05) and almost 2-fold greater compared to Portland cement (Day 7 = 0.28 ± 0.04, Day 14 = 0.22 ± 0.06, Day 21 = 0.20 ± 0.3) (Supplementary Figure S2). Analysis of rETR showed an increase in both the initial slope of the curve (α- light use efficiency) and the rETRmax over time for CaCO3 and PVC, eventually yielding rates similar to healthy control fragments (Figures 4C, F, I). In contrast, micropropagates on Portland cement did not recover and showed continuous loss of photosynthetic potential over time (Figures 4C, Supplementary Figure S2).
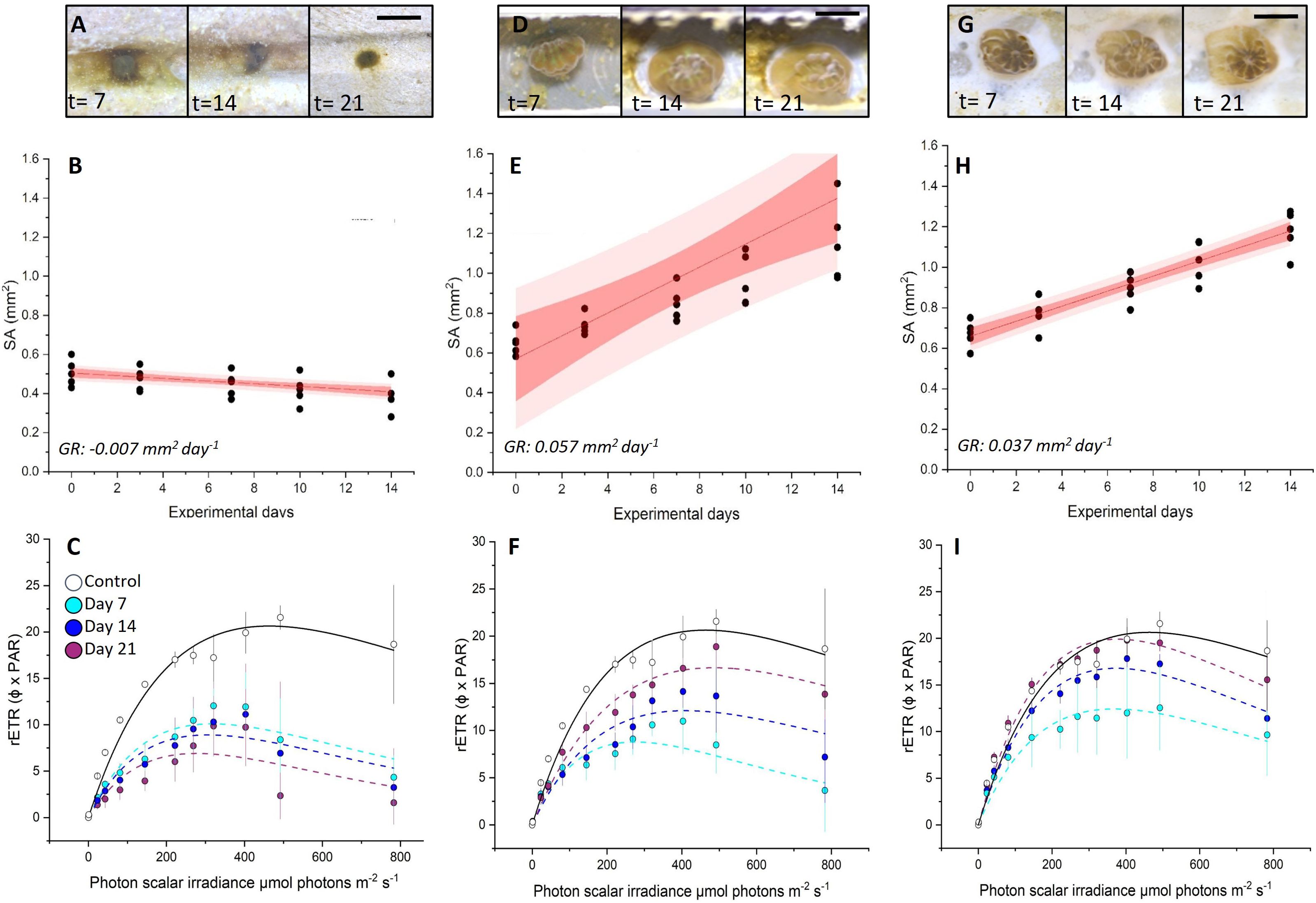
Figure 4. Growth rates and viability of settled coral micropropagates for (A–C) Portland cement, (D–F) PVC and (G–I) CaCO3 substrates. Microscope images of morphological changes at day 7, 14 and 21 (A, D, G). Planar tissue surface area changes (mm-2) per micropropagate over 14 days (B, E, H). Best fits (solid red line), 95% confidence intervals (red area), 95% prediction intervals (light red area) and average growth rates (GR) are shown (R2= 0.93, 0.93 and 0.99 for B, E and H, respectively). (C, F, I) rETR of micropropagates after 7, 14 and 21 days of cultivation in micropropagate chip set-up. Control measurements (healthy fragments) were performed before bailout. On Day 21 at PAR of 492, CaCO3 = 19.5 ± 0.9, PVC = 18.9 ± 2.2, Portland cement = 2.3 ± 2.5. Data are means ± SE (n= 45). Best fits (dotted and solid lines) are based on the Platt equation (see Equation 1).
The results of this study highlight the potential of the polyp bailout approach to enhance the production of individual polyps, underscored by the ability to generate viable and growing micropropagates from a single small coral fragment (Figure 1). We developed a simple, transferable method to generate and grow micropropagtaes from S. pistillata in laboratory set-ups. Using a custom-made micropropagate chip device, our results suggest that attachment and growth are substantially affected by substrate material properties, whereby CaCO3- or PVC-based substrates are more suitable than cement. Our study focused on the first month after attachment, and material behavior as well as coral physiology might change over time and affect these results within the first year (e.g. Leonard et al., 2022; Yus et al., 2024). Additionally, the success of this technique for coral outplanting and gardening applications will strongly depend on competitive pressure from adjacent benthos. Indeed, and similar to settled coral larvae, micropropagates may be very susceptible to overgrowth by competitive macroalgae (e.g. turf algae) (e.g. Lirman, 2000). Therefore, the choice of substrate should also consider settlement and growth of competitive organisms (Leonard et al., 2022). Another concern is the lack of genetic variability of micropropagates, as it is an asexual mode of propagation (Shearer et al., 2009). A potential approach is to use micropropagates obtained by coral bail out in ex situ coral nurseries (e.g. Vizel and Kramarsky-Winter, 2015) and leverage their rapid initial tissue growth rate, similar to current microfragment cultivation approaches (Mostrales et al., 2022). Outplanting of tissue-covered chips, if economically viable, could then supplement the re-skinning of artificial or hybrid reefs together with assisted sexual reproduction (Koch et al., 2022; Roger et al., 2023a) and larval seeding approaches (Dela Cruz and Harrison, 2020).
In conclusion, polyp bailout offers the potential for large-scale production of viable micropropagates, representing a valuable addition to coral propagation efforts. Future research should aim to assess the performance of micropropagates produced through this technique under different environmental conditions and how micropropagates compare to other fragmentation techniques under in situ conditions. This will contribute to a more comprehensive understanding of the advantages and limitations of polyp bailout in the broader landscape of coral restoration strategies. Additionally, due to their similar properties to settled coral larvae, micropropagates can also be used as a suitable model system for studying mechanisms that control post-settlement mortality (e.g. competition, predation, detachment).
The original contributions in the study are included in the article/Supplementary Material. Additional Raw data is deposited on Figshare https://doi.org/10.6084/m9.figshare.26543872.v1.
The manuscript presents research on animals that do not require ethical approval for their study.
EW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LB: Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CG-M: Data curation, Investigation, Methodology, Writing – review & editing. DB: Formal analysis, Investigation, Methodology, Writing – review & editing. MT: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. DW: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by grants from the US National Science Foundation (NSF-BSF IOS award # 2149925 to DW and 2149926 to MT, award NSF DBI award # 2316391 to DW) as well as the Defense Advanced Research Projects Agency under the Reefense Program, BAA HR001121S0012 to DW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The views, opinions, and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the US government.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1454887/full#supplementary-material
Adey W. H., Michael Vassar J. (1975). Colonization, succession and growth rates of tropical crustose coralline algae (Rhodophyta, cryptonemiales). Phycologia 14, 55–69. doi: 10.2216/i0031-8884-14-2-55.1
Boström-Einarsson L., Babcock R. C., Bayraktarov E., Ceccarelli D., Cook N., Ferse S. C., et al. (2020). Coral restoration – a systematic review of current methods, successes, failures and future directions. PloS One 15. doi: 10.1371/journal.pone.0226631
Burt J., Bartholomew A., Bauman A., Saif A., Sale P. F. (2009). Coral recruitment and early benthic community development on several materials used in the construction of artificial reefs and breakwaters. J. Exp. Mar. Biol. Ecol. 373, 72–78. doi: 10.1016/j.jembe.2009.03.009
Chuang P.-S., Mitarai S. (2020). Signaling pathways in the coral polyp bail-out response. Coral Reefs 39, 1535–1548. doi: 10.1007/s00338-020-01983-x
Dela Cruz D. W., Harrison P. L. (2020). Enhancing coral recruitment through assisted mass settlement of cultured coral larvae. PloS One 15, e0242847. doi: 10.1371/journal.pone.0242847
Eddy T. D., Lam V. W. Y., Reygondeau G., Cisneros-Montemayor A. M., Greer K., Palomares M. L. D. (2021). Global decline in capacity of coral reefs to provide ecosystem services. One Earth 4, 1278–1285. doi: 10.1016/j.oneear.2021.08.016
Elliff C. I., Iracema RS. (2017). Coral reefs as the first line of Defense: Shoreline Protection in face of climate change. Mar. Environ. Res. 127, 148–154. doi: 10.1016/j.marenvres.2017.03.007
Forrester G. E., Maynard A., Schofield S., Taylor K. (2012). Evaluating causes of transplant stress in fragments of acropora palmata used for coral reef restoration. Bull. Mar. Sci. 88, 1099–1113. doi: 10.5343/bms.2012.1016
Forsman Z. H., Christopher A., Toonen R. J., David VaughanForsman Z. H., Page C. A., Vaughan D. (2017). “Coral fusion: harnessing coral clonality for reef restoration,” in Active Coral Restoration: Techniques for a Changing Planet. Ed. Vaughan D. (J. Ross Publishing, Plantation, Florida), 172–201.
Forsman Z. H., Page C. A., Toonen R. J., Vaughan D. (2015). Growing coral larger and faster: micro-colony-fusion as a strategy for accelerating coral cover. PeerJ 3. doi: 10.7717/peerj.1313
Fujiwara S., Kezuka D., Hagiwara K., Ishimori H., Tabata H. (2022). Effect of substratum structural complexity of coral seedlings on the settlement and post-settlement survivorship of coral settlers. Oceans 4, 1–12. doi: 10.3390/oceans4010001
Guilbeau B. P., Harry F. P., Gambrell R. P., Knopf F. C., Dooley K. M. (2003). Algae attachment on carbonated cements in fresh and brackish waters—preliminary results. Ecol. Eng. 20, 309–319. doi: 10.1016/S0925-8574(03)00026-0
Heron S. F., Maynard J. A., van Hooidonk R., Eakin C.M. (2016). Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 6. doi: 10.1038/srep38402
Higgins E., Metaxas A., Scheibling R. E. (2022). A systematic review of artificial reefs as platforms for coral reef research and conservation. PloS One 17. doi: 10.1371/journal.pone.0261964
Hoegh-Guldberg O., Poloczanska E. S., Skirving W., Dove S. (2017). Coral reef ecosystems under climate change and ocean acidification. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00158
Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., Jackson J. B., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1038/nature22901
Jaffe J. S., Schull S., Kühl M., Wangpraseurt D. (2022). Non-invasive estimation of coral polyp volume and surface area using optical coherence tomography. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.1049440
Knapp I. S. S., Forsman Z. H., Greene A., Johnston E. C., Bardin C. E., Chan N., et al. (2022). Coral micro-fragmentation assays for optimizing active reef restoration efforts. doi: 10.7717/peerj.13653
Koch H. R., Matthews B., Leto C., Engelsma C., Bartels E. (2022). Assisted sexual reproduction of Acropora cervicornis for active restoration on Florida’s Coral Reef. Front. Mar. Sci. 9, 959520. doi: 10.3389/fmars.2022.959520
Kvitt H., Kramarsky-Winter E., Maor-Landaw K., Zandbank K., Kushmaro A., Rosenfeld H., et al. (2015). Breakdown of Coral Colonial Form under Reduced Ph Conditions Is Initiated in Polyps and Mediated through Apoptosis. Proc. Natl. Acad. Sci. 112, 2082–2086. doi: 10.1073/pnas.1419621112
Leonard C., Hédouin L., Lacorne M. C., Dalle J., Lapinski M., Blanc P., et al. (2022). Performance of innovative materials as recruitment substrates for coral restoration. Restor. Ecology. 30, e13625. doi: 10.1111/rec.13625
Levenstein M. A., Gysbers D. J., Marhaver K. L., Kattom S., Tichy L., Quinlan Z., et al. (2022). Millimeter-scale topography facilitates coral larval settlement in wave-driven oscillatory flow. PloS One 17. doi: 10.1371/journal.pone.0274088
Lima J. S., Zalmon I. R., Love M. (2019). Overview and trends of ecological and socioeconomic research on artificial reefs. Mar. Environ. Res. 145, 81–96. doi: 10.1016/j.marenvres.2019.01.010
Lirman D. (2000). Fragmentation in the branching coral acropora palmata (Lamarck): growth, survivorship, and reproduction of colonies and fragments. J. Exp. Mar. Biol. Ecol. 251, 41–57. doi: 10.1016/s00220981(00)00205-7
Mallela J., Milne B. C., Martinez-Escobar D. (2017). A comparison of epibenthic reef communities settling on commonly used experimental substrates: PVC versus ceramic tiles. J. Exp. Mar. Biol. Ecol. 486, 290–955. doi: 10.1016/j.jembe.2016.10.028
Mostrales T. P., Rollon R. N., Licuanan W. Y. (2022). Evaluation of the performance and cost-effectiveness of coral microfragments in covering artificial habitats. Ecol. Eng. 184, 106770. doi: 10.1016/j.ecoleng.2022.106770
Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., et al. (2003). Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958. doi: 10.1126/science.1085706
Pang A.-P., Luo Y., He C., Lu Z., Lu X. (2020). A polyp-on-chip for coral long-term culture. Sci. Rep. 10. doi: 10.1038/s41598-020-63829-4
Platt T., Gallegos C. L., Harrison W. G. (1980). Photoinhibition of photosynthesis in natural assemblages of marine phytoplankton. J. Mar. Res. 38, (4).
Rädecker N., Pogoreutz C., Voolstra C. R., Wiedenmann J., Wild. C. (2015). Nitrogen cycling in corals: the key to understanding holobiont functioning? Trends Microbiol. 23, 490–497. doi: 10.1016/j.tim.2015.03.008
Ralph P. J., Gademann R. (2005). Rapid light curves: A powerful tool to assess photosynthetic activity. Aquat. Bot. 82, 222–237. doi: 10.1016/j.aquabot.2005.02.006
Ralph P. J., Bhagooli R., Baird A. H. (2008). “Does the coral host protect its algal symbionts from heat and light stresses?” 11th International Coral Reef Symposium. Ft. Lauderdale 7–11.
Randall C. J., Giuliano C., Heyward A. J., Negri A. P. (2021). Enhancing coral survival on deployment devices with microrefugia. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.662263
Rinkevich B. (2021). Augmenting coral adaptation to climate change via coral gardening (the nursery phase). J. Environ. Manage. 291, 112727. doi: 10.1016/j.jenvman.2021.112727
Roger L., Lewinski N., Putnam H., Chen S., Roxbury D., Tresguerres M., et al. (2023b). Nanotechnology for coral reef conservation, restoration and rehabilitation. Nat. Nanotechnology 18, 831–833. doi: 10.1038/s41565-023-01402-6
Roger L. M., Lewinski N. A., Putnam H. M., Roxbury D., Tresguerres M., Wangpraseurt D. (2023a). Nanobiotech engineering for future coral reefs. One Earth 6, 778–789. doi: 10.1016/j.oneear.2023.05.008
Rossi S., Rizzo L. (2020). Marine animal forests as carbon immobilizers or why we should preserve these three-dimensional alive structures. Perspect. Mar. Anim. Forests World, 333–400. doi: 10.1007/978-3-030-57054-5_11
Sammarco P. W. (1982). Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar. Ecol. Prog. Ser. 10, 57–655. doi: 10.3354/meps010057
Schweinsberg M., Gösser F., Tollrian R. (2021). The history, biological relevance, and potential applications for polyp bailout in corals. Ecol. Evol. 11, 8424–8440. doi: 10.1002/ece3.7740
Shapiro O. H., Kramarsky-Winter E., Gavish A. R., Stocker R., Vardi. A. (2016). A coral-on-a-chip microfluidic platform enabling live-imaging microscopy of reef-building corals. Nat. Commun. 7. doi: 10.1038/ncomms10860
Shearer T. L., Porto Hannes I., Zubillaga A. L. (2009). Restoration of coral populations in light of genetic diversity estimates. Coral Reefs 28, 727–733. doi: 10.1007/s00338-009-0520-x
Shefy D., Rinkevich B. (2021). “Stylophora pistillata—a model colonial species in basic and Applied Studies,” in Handbook of Marine Model Organisms in Experimental Biology, Boca Raton, FL: CRC Press 195–216. doi: 10.1201/9781003217503-11
Suggett D. J., Edwards M., Cotton D., Hein M., Camp E. F. (2023). An integrative framework for sustainable coral reef restoration. One Earth 6, 666–681. doi: 10.1016/j.oneear.2023.05.007
Tortolero-Langarica J.J.A., Rodríguez-Troncoso A. P., Cupul-Magaña A. L., Rinkevich B. (2020). Micro-fragmentation as an effective and applied tool to restore remote reefs in the eastern tropical pacific. Int. J. Environ. Res. Public Health 17. doi: 10.3390/ijerph17186574
van Hooidonk R., Maynard J., Tamelander J., Gove J., Ahmadia G., Raymundo L., et al. (2016). Local-scale projections of coral reef futures and implications of the paris agreement. Sci. Rep. 6. doi: 10.1038/srep39666
Vizel M., Kramarsky-Winter E. (2015). “Coral tissue culture and micropropagation,” in Diseases of Coral, Hoboken, NJ: John Wiley & Sons, 482–488.
Wangpraseurt D., Jacques S., Lyndby N., Holm J. B., Pages C. F., Kühl M. (2019b). Microscale light management and inherent optical properties of intact corals studied with optical coherence tomography. J. R. Soc. Interface 16, 20180567. doi: 10.1098/rsif.2018.0567
Wangpraseurt D., Lichtenberg M., Jacques S. L., Larkum A. W., Kühl M. (2019a). Optical properties of corals distort variable chlorophyll fluorescence measurements. Plant Physiol. 179, 1608–1619. doi: 10.1104/pp.18.01275
Wangpraseurt D., Weber M., Røy H., Polerecky L., de Beer D., Suharsono, et al. (2012). In situ oxygen dynamics in coral-algal interactions. PloS One 7. doi: 10.1371/journal.pone.0031192
Wangpraseurt D., Wentzel C., Jacques S. L., Wagner M., Kühl M. (2017). In vivo imaging of coral tissue and skeleton with optical coherence tomography. J. R. Soc. Interface 14, 20161003. doi: 10.1098/rsif.2016.1003
Keywords: coral propagation, bail out, micropropagates, asexual reproduction, coral restoration
Citation: Walton E, Badder L, Galindo-Martínez CT, Berry DB, Tresguerres M and Wangpraseurt D (2024) Advancing the coral propagation toolkit via hypersalinity induced coral micropropagates. Front. Mar. Sci. 11:1454887. doi: 10.3389/fmars.2024.1454887
Received: 25 June 2024; Accepted: 26 July 2024;
Published: 18 September 2024.
Edited by:
Baruch Rinkevich, Israel Oceanographic and Limnological Research, IsraelReviewed by:
Robert H. Richmond, University of Hawaii at Manoa, United StatesCopyright © 2024 Walton, Badder, Galindo-Martínez, Berry, Tresguerres and Wangpraseurt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daniel Wangpraseurt, ZHdhbmdwcmFzZXVydEB1Y3NkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.