
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 23 August 2024
Sec. Aquatic Physiology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1452775
Antioxidants play a crucial role in maintaining health by scavenging free radicals that causes cellular damage and accelerates aging and disease. The present study focuses on the antioxidant levels in various organs of Hilsa fish, both male and female, with a weight range of 50 g to 780 g collected from the Hooghly River in the lower Gangetic plain. The study utilized commercially available free radicals such as, viz., 2,2′-azinobis-[3-ethyl-benzothiazoline-6-sulfonic acid] and ferric reducing antioxidant potential to measure the scavenging activity. The results indicated that female Hilsa fish possess higher levels of antioxidants (93.79 ± 0.26) compared to male counterparts (84.50 ± 0.65) in all organs, with levels increasing proportionally with weight. Interestingly, in males, the serum antioxidant profile was higher in the lower weight group (50–100 g), whereas, in females, the highest antioxidant activity was observed in the weight range of 300 g to 400 g. The present study was attempted for the first time to characterize the antioxidant distribution pattern in the different organs of Hilsa. These findings suggest that Hilsa fish organs, including serum, contain high levels of antioxidants that could significantly benefit human nutrition and potentially will contribute to anti-aging effects by incorporating Hilsa to their diet, which needs further clinical study.
Hilsa (Tenualosa ilisha) is not just a delicacy but a vital part of the ecosystem and the livelihoods of millions. Recent studies have indicated a trend in the declining population of the prized Hilsa shad from the waters of India and other Southeast Asian countries.
Antioxidants are compounds capable of disrupting radical chain reactions and preventing the formation of reactive oxidants (Huang et al., 2005), which alleviate oxidative stress, help avoid disease, and improve eye and brain health. They can support healthy aging processes, boost mental wellness, and reduce inflammation. All fish species include a variety of antioxidative chemicals designed to protect their lipids against damage from reactive oxygen species (ROS). ROS are typically reactive molecules containing free radicals generated from molecular oxygen. They are extremely reactive and may damage lipids, proteins, nucleic acids, and other components of cells (Zhang et al., 2016). The complex interaction between ROS is one biomarker for determining the stress in antioxidant enzyme activity (Vranković et al., 2021). These substances come from different chemical categories and employ unique mechanisms to exploit their antioxidative properties. The two most comprehensive methods for assessing the antioxidant activity of fish samples and serum involve reactions with a colored solution of a free radical, namely, 2,2′-azinobis-[3-ethyl-benzothiazoline-6-sulfonic acid] (ABTS) and ferric reducing antioxidant potential (FRAP). FRAP is simple and rapid enough to be performed on freshly collected specimens (Benzie et al., 1999). The objectives of this study were to compare the antioxidant activity of the serum and organs of the Hilsa fish between males and females and weight variation. A crucial component of this study was to survey the consumption pattern of Hilsa, in addition to the numerous health benefits. Further, it also dealt with the role of antioxidants in the anti-aging process. For that, a survey was conducted randomly close to significant landing sites among Hilsa eating fishers including consumption patterns in four districts of West Bengal.
The consumption pattern includes whether they like the whole portion or discard the viscera during dressing. Based on their reponses a hypothesis was formulated to find out the derived antioxidants from Hilsa consumption by the Hilsa fishers of the River Hooghly (4 months available) and the potential health benefits by the fishers. In addition to the consumptive pattern of antioxidants among fishermen, many Hilsa lovers also have their health benefits. Therefore, antioxidant research is crucial as a part of waste to wealth from this anadromous fish as it is a nutri-smart fish containing essential micronutrients along with vitamins, etc.
River Ganga (2,525 km) in India splits into two sections in West Bengal: the Hooghly portion, which flows from Farakka to West Bengal, and the Ganga portion, which flows from Farakka to Bangladesh as river Padma. Hilsa migrates in the Ganga river, from the lower stretch to the upper stretch (up to Farakka, Murshidabad, West Bengal) for breeding purposes. Two types of sampling were done here, like fish sampling, which was done at Farakka, Murshidabad, West Bengal, from River Ganga. Another data collection of Hilsa consumption was surveyed from Hilsa fishers in four primary sampling sites in four districts: North 24 Parganas (22°47′21.85″N; 88°20′8.99″E), South 24 Parganas (22°23′44.39″N; 88°8′14.57″E), Hooghly (22°36’17.97″N; 88°21′53.13″E), and Farakka (24°48′1.34″N; 87°55′21.53″E), respectively (Figure 1).
The fish sample, T. ilisha, the wild stock, was procured from the river Ganga at Farakka, Murshidabad, West Bengal. A total of 72 (n = 6; male and female each group) wild stocks were collected during August to November 2021. The total length and body weight were analyzed using an electric digital balance for each fish. Fish were anesthetized by MS-222 (120 mg/L) following Neiffer and Stamper (2009) and dissected, and different organs were collected for antioxidant analysis. The collected tissues like the serum, brain, kidney, liver, muscle, and gut were stored at 4°C. For sex determination, morphometric characteristics like the size of the fish and abdomen were examined. Matured males were separated on site by the presence of milt by application of pressure on the abdomen and females by oozing out eggs.
Blood was collected by puncturing the caudal vasculature with a 2-mL sterile syringe from a live fish in the field, and collected blood was transferred to a 1.5-mL Eppendorf tube and left for 2–3 h at room temperature for clotting, and the supernatant was collected for analysis. The blood sample, after being brought to the laboratory in an ice bucket for an additional quantity of serum, was centrifuged to 8,000 rpm for 5–8 min, collected from the supernatant, and immediately stored the serum in refrigerator at −40°C. Acetonitrile and sample were taken into a 1:1 ratio for deproteinization before analyzing the antioxidant activities. Therefore, serum deproteinization with acetonitrile during free radicals assay as the protein in the sample made no clash with free radicals (Chrzczanowicz et al., 2008).
The kidney, brain, gut, liver, gill, and muscle samples were homogenized using QIAGEN tissue lyzer II (QIAGEN, Hilden, Germany) and centrifuged at 10,000 rpm for 10 min. Afterward, the supernatant was collected in sterile 2-mL eppendorf tubes and deproteinization with acetonitrile.
Acetonitrile, ascorbic acid, butylated hydroxytoluene (BHT), ferrous sulfate (FeSO4), methanol, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), sodium acetate, and uric acid were purchased from Sigma-Aldrich chemical Pvt Limited (St. Louis, MO, USA). Ethanol was obtained from Merck, Germany.
The antioxidant potential of Hilsa samples (different organs and serum) was evaluated by assessing their ability to scavenge free radicals using the commercial free radicals ABTS and FRAP. The results were compared with commercial antioxidants and presented as mean ± SE. The percentage of free radical scavenging activity was determined using the following method:
The ABTS antioxidant test was conducted following the methodology of Arnao et al. (2001). Briefly, the reaction mixture contained an equal amount of 2.4 mM potassium per sulfate (Sigma-Aldrich) and 7 mM ABTS solution (Sigma-Aldrich). Both the sample and control vials received the diluted solution, i.e., 10 µL of methanol, 280 µL of ABTS solution and 10 µL of each sample. The control was prepared by mixing 280 µL of ABTS solution with 20 µL of methanol. The generation of ABTS•+ from ABTS in the presence of antioxidants was measured at an absorbance of 734 nm. The results were compared to the standards of ascorbic acid used during the assay and expressed as ABTS inhibition (%).
The Fe3+ (ferric) to Fe2+ (ferrous) reduction by antioxidants in each sample is the basis of the FRAP (Sigma-Aldrich) technique. The test was carried out following the steps outlined by Risso et al. (2021). TPTZ (10mM) and the sodium acetate buffer (300 mmol/L) were dissolved in 37% HCl. In 10 mL of millipore water, 20 mM iron(III) chloride hexahydrate (FeCl3·6H2O) was disbanded. The working solution was made both with and without TPTZ solution because the sperm sample had a turbid color. The standard curve produces 2,000 µM of iron(II) sulfate heptahydrate (FeSO4·7H2O), ranging from 0 Parts Per Million (ppm) to 2,000 ppm. The sample blank was made with 10 µL of millipore water and 290 µL of working solution with TPTZ; the sample solution made followed the same method. The absorbance was measured for 15 min at 37°C and again for 5 min at room temperature. The Fe2++ TPTZ complex absorbance was read at 593 nm.
The standard curve, which plots sample absorbance as a function of their final concentration, results in a linear regression equation. By substituting the sample absorbance into this equation (Y-axis), the corresponding X-axis value represents the concentration of Fe2+ in the analyzed sample, measured in µM/L. Finally, the Fe2+/mL of the sample was calculated, and the results are expressed as equivalent µmoles of Fe2+/mL of the sample.
During December 2021 and November 2022, a total of 746 Hilsa fishermen were interviewed (Table 1). The research used stratified random sampling. In accordance with the specified study sites of the National Mission for Clean Ganga Project of Indian Council of Agricultural Research-Central Inland Fisheries Research Institute (ICAR-CIFRI), sampling was conducted in four districts longitudinally from the middle stretch to the lower stretch of the river Hooghly. These areas were include Murshidabad, North 24 Parganas, South 24 Parganas, and Hooghly.
To simplify, the weight ranges were divided into six groups. We conducted experiments using triplicate samples from both males and females. Group A includes weights ranging from 50 g to 100 g, Group B contains weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g.
The data on the antioxidant levels in seven organs of Hilsa fish were subjected to statistical analysis using the R software version 4.0.4. The analysis was carried out through a box plot based on one-way ANOVA (Duncan test) to evaluate the variations and significance in antioxidant levels based on two variables: gender and weight. The analysis graph depicts bars representing the mean values and confidence intervals of ±95% for each provenance, which are identified by different colors. Notably, additional variables were employed in the analysis, and differentiation was made based on the gender and weight of the fish. Overall, the analysis was quite comprehensive, providing valuable insights into the antioxidant levels of Hilsa fish organs based on gender and weight. The detailed analysis reveals exciting insights into the distribution of antioxidants in male and female Hilsa fish and how the distribution varies with size. These findings have important implications for the study of fish nutrition and as well as human health.
Antioxidant activity changes in several organs of Hilsa as serum, muscle, gut, liver, kidney brain, and gill with weight variations using FRAP and ABTS method; details of the result of each organ are described below.
In the analysis of the six weight groups of both the sex experiments, the result showed (Figures 2A, B) an almost similar pattern in both assays. The males in lower (below 100 g) and higher weight groups (500 g and above) carry more antioxidants: 68.18% ± 0.03% in ABTS, 54.16% ± 0.04% in FRAP, and 65.62% ± 0.17% in ABTS, 51.63% ± 0.17% in FRAP. Weight groups B and D were significantly different from each other and with groups A, C, E, and F with p ≤ 0.005 (0), whereas the later groups did not show any significant differences within them. In the females, the antioxidant level was higher in group D: 66.93% ± 0.36% (ABTS) and 64.31% ± 0.07% (FRAP). The results of FRAP radical scavenging activity in various weight groups of male and female Hilsa were displayed in Figure 2A. It was observed that females in weight group B (101 g to 200 g) had fewer antioxidants, with a value of 27.79% ± 0.08%.
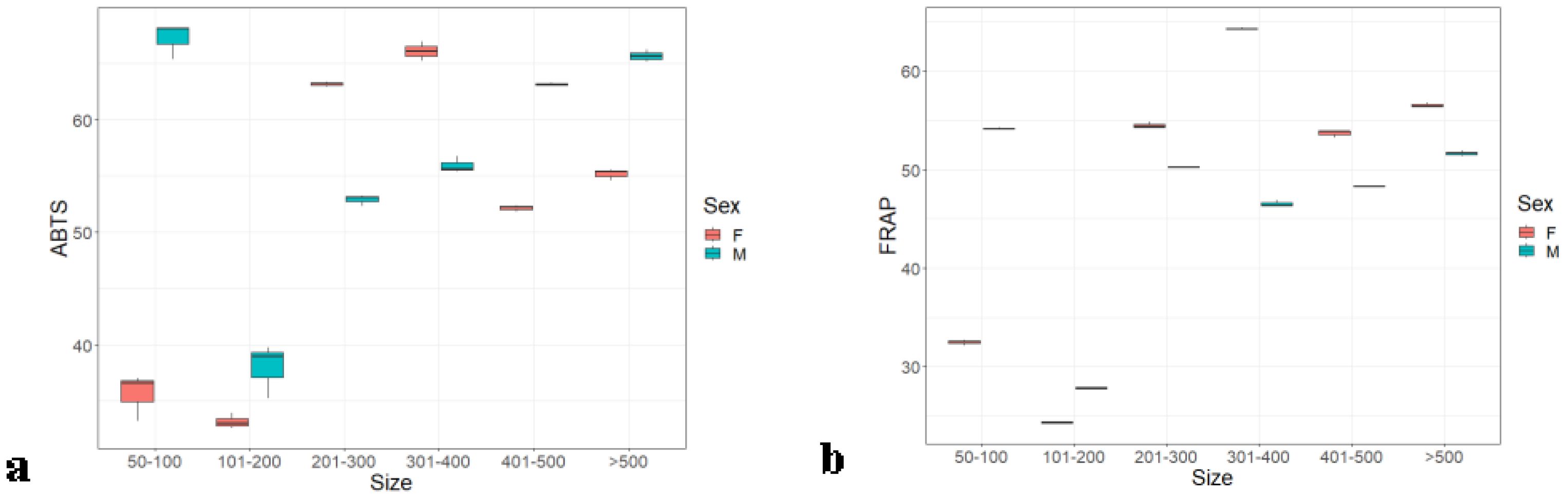
Figure 2. Serum antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
The Hilsa muscle antioxidant activity was higher in the females when done by ABTS free radical assay. However, the results were different from that of the FRAP protocol. The muscle of male fish had a higher value (Figure 3) corresponding to the increasing body weight. Weight groups A, B, and D significantly differed with p ≤ 0.05 (0.028). However, there were no significant differences between groups A, C, E, and F. The antioxidant activity was the highest, corresponding to the highest weight group F (ABTS: 88.14 ± 1.15 and FRAP: 61.65 ± 0.14) in both the assays, as shown in Figures 4A, B.

Figure 3. Gut antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
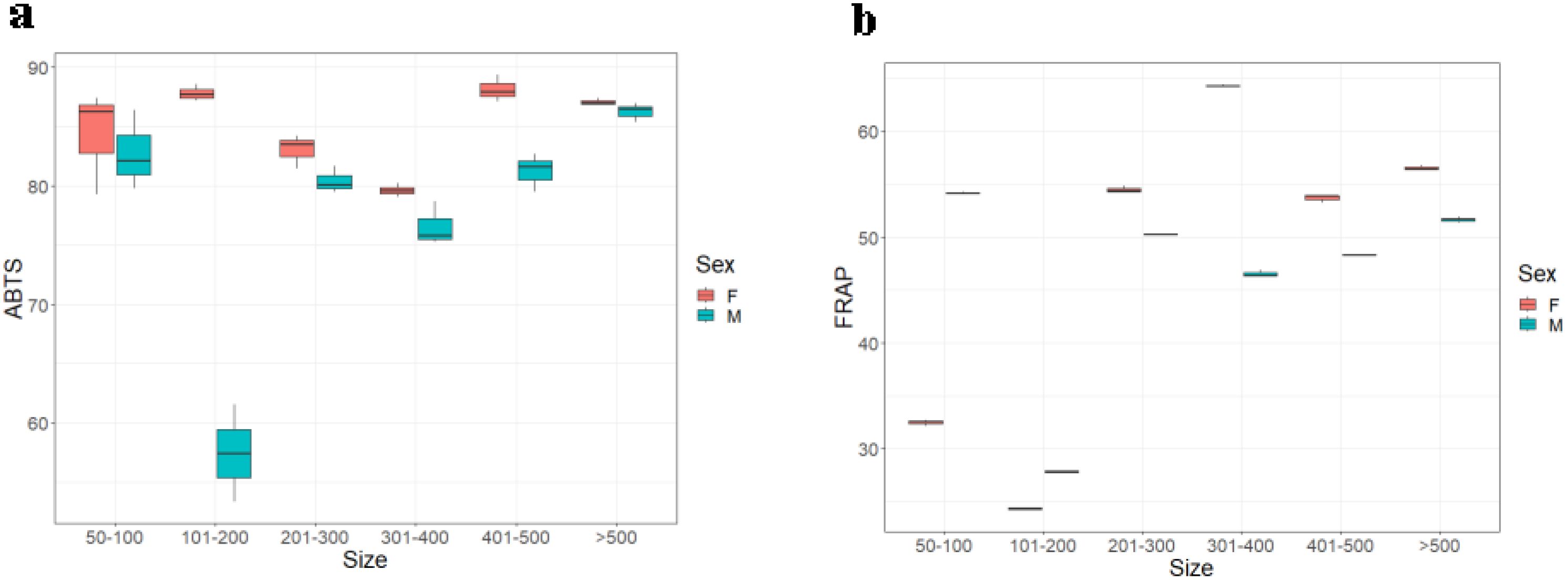
Figure 4. Muscle antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100 g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
The antioxidant assay of the Hilsa gut by ABTS assay followed a dissimilar pattern in Figures 3A, B compared to that of other organs. The gut antioxidant value of the male fishes followed a decreasing trend with an increasing weight group. Weight groups A, B, E, and F were significantly different from each other and with groups C and D with p ≤ 0.5 (0.057), whereas groups C and D did not show any significant differences within them. However, the values corresponding to the female fish were consistent over all six weight groups. The importance of the antioxidant assay by FRAP method is shown in Figure 3 that both males and females followed a synchronized trend, i.e., increasing antioxidant value following a higher body weight gradient.
In the Hilsa liver, the antioxidant follows a similar trend to the other organs, like antioxidant activity that was increasing proportionately to the weight. Females also contain more antioxidants than males in both methods. In the ABTS method (Figure 5A), female and male antioxidants were 96.55% ± 0.57% and 93.70% ± 0.07%, but, in the case of the FRAP method (Figure 5B), it was 64.24% ± 0.1.20% and 62.42% ± 0.1.5%. Weight groups A and E were significantly different from each other and with groups B, C, D, and F with p ≤ 0.5 (0.257), whereas these weight groups did not show any significant differences within them. The ABTS results were closer to the synthetic antioxidant standard BHT.
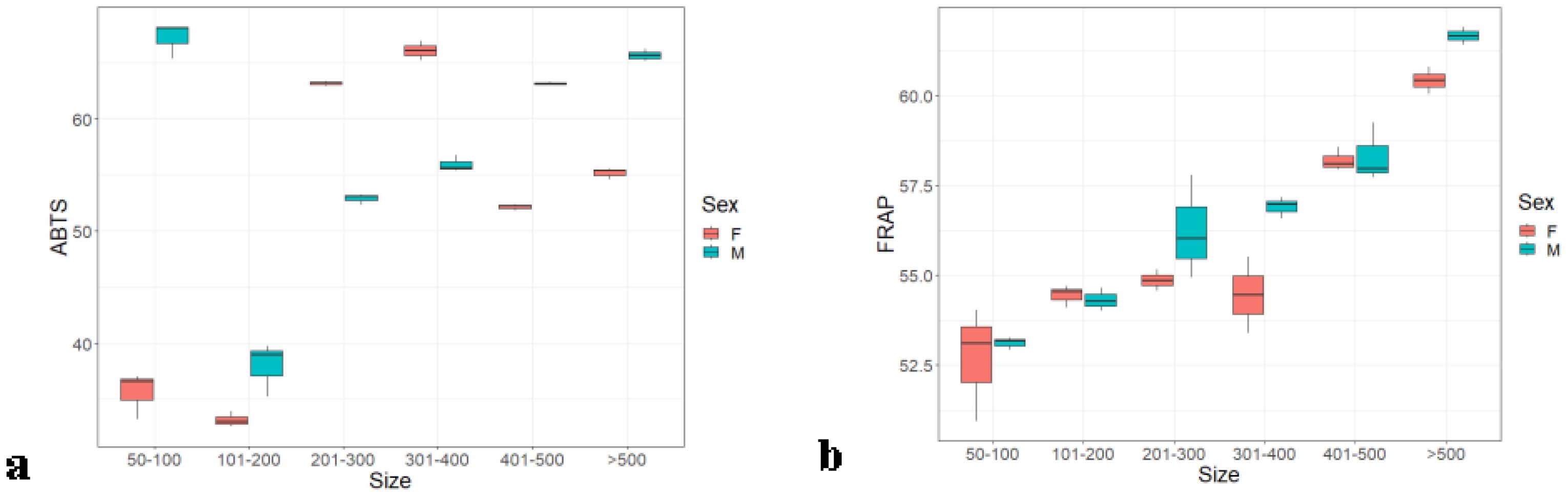
Figure 5. Liver antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 5 g to 100 g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
The kidney of female Hilsa contains more antioxidants than that of males, similar to the other organs. In the ABTS assay, a dissimilar pattern was observed (Figure 6A) between weight group and sex, but, in FRAP, the pattern is uniform Figure 6B. Weight group F was significantly different from all other weight groups with p ≤ 0.05 (0.02), whereas these weight groups did not show any significant differences within them. The highest antioxidant value observed in the kidney was 94.66% ± 0.61% by the ABTS assay and 61.28% ± 0.7% by the FRAP assay.
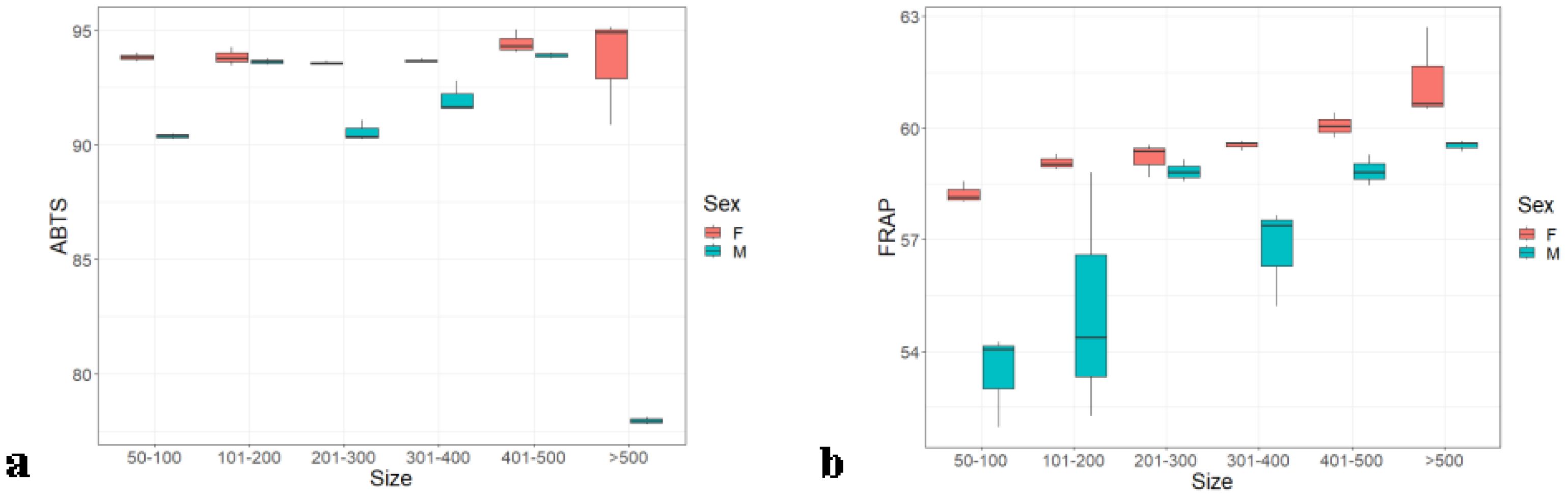
Figure 6. Kidney antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100 g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
In the brain of Hilsa, the antioxidant values exhibited a notable pattern by FRAP assay, corresponding to sex and weight groups. For males, there was a decreasing trend in antioxidant values with increasing weight groups, whereas females showed an increasing trend as weight increased (Figure 7B), and almost remained the same (52%–57%) in all weight groups. Weight groups A, C, and F were significantly different from each other (p ≤ 0.005) (0.005), whereas weight groups B, D, and E did not show any significant differences among them. In the case of ABTS assay, the highest antioxidant value for males (92.15% ± 0.5%) was observed in weight groups D and E; in females, the highest value (92.14% ± 0.19%) was found in weight group C, as shown in Figure 7A.
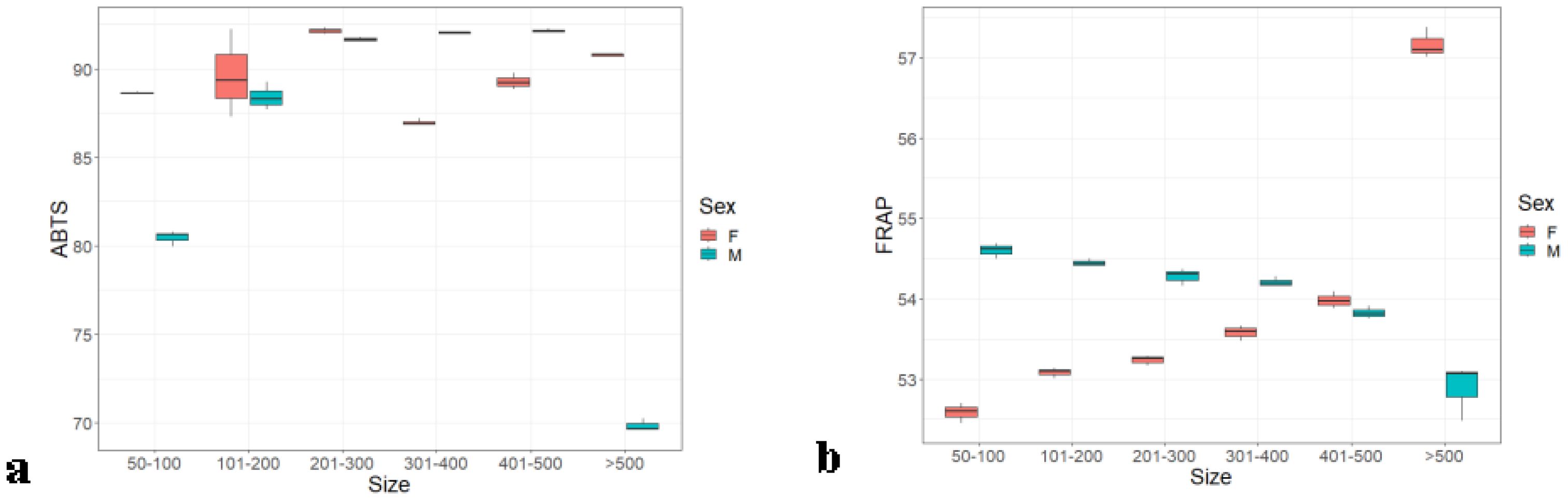
Figure 7. Brain antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100 g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
In the ABTS assay, an upward trend in antioxidant values were observed (Figure 8A) with increasing weight of the Hilsa in both sexes. In FRAP assay, both sexes contained an average of 55%–59% antioxidants, as shown in Figure 8B. All six weight groups, A to F, significantly differed, with p ≤ 0.005 (0). It is noteworthy that the male Hilsa fish exhibits a higher percentage of antioxidants (93.15% ± 0.6%) in its gills compared to the female fish (90.43% ± 0.13%) in the ABTS antioxidant assay, which is similar to the commercial standard BHT.

Figure 8. Gill antioxidants of male and female Hilsa, by the (A) ABTS and (B) FRAP methods (Group A includes weights ranging from 50 g to 100g, Group B includes weights ranging from 101 g to 200 g, Group C includes weights ranging from 201 g to 300 g, Group D includes weights ranging from 301 g to 400 g, Group E includes weights ranging from 401 g to 500 g, and Group F includes weights above 500 g).
Public access to Hilsa fisheries is typically available. Fish holds tremendous value in fishing communities due to its delicious flavor and high nutritious content. Whereas inland waters have produced a steady amount of Hilsa over the past 20 years, the Bay of Bengal regions have shown a rise in marine yields (Hossain et al., 2019). During the previous study on the socio-economic analysis of fishers in the deltaic Ganga region of river Hooghly (Chakraborty et al., 2024). The present study found that, in the season (as Hilsa is available in two seasons—June to September and January to April), Hilsa fishers also consume small-sized Hilsa as a whole and more than 1 kg of Hilsa with dressing and remove the intestine only. As a result, their nutrition requirement must be fulfilled from here, and they are feeding their child during this nutrient-rich fish season. The Hilsa production in India is directly counted as a consumption basis, and, additionally, Hilsa is a nutri-smart fish, one of the reasons behind the Hilsa decline in consumption compared to that in Sahoo et al. (2018); Hilsa production in West Bengal declined from 80,000 t to 20,000 t over 10 years since 2001. Fisheries in Hilsa are usually open to the public for its great taste and high nutritional value (Mukherjee et al., 2019); fish is highly valued in the fishing communities. Whereas inland waters have produced a steady amount of Hilsa over the past 20 years, the Bay of Bengal regions have shown a rise in marine yields (Hossain et al., 2019; Hossain et al., 2020).
However, adult men are predicted to require at least 11,000 The Oxygen Radical Absorbance Capacity (ORAC) units per day, based on their daily caloric intake of roughly 2,500. With an average daily calorie intake of 1,800, women should consume a minimum of 8,000 units. From the Bangladesh Development studies (Toufique, 2015), it was stated that Hilsa was consumed per day at 200 g (2010), so, as per our result, Hilsa contain 2,728 ORAC units, which is highly recommended mainly from fish muscle tissues that must be examined as high in antioxidant, which is mostly consumed by human, where the serum kidney and brain were also consumed in small fish (>500 g). Gill, gut, and liver can be denoted as waste material for big Hilsa (<1,000), but, in the case of smaller ones, the whole fish was consumed mostly in fried conditions, as reported by the survey. The possible benefits of antioxidants from higher weight groups of Hilsa in daily intake are considered as anti-aging capacity.
Antioxidative activity has received more attention nowadays due to the various diseases that a diet high in antioxidants prevents and its anti-aging effects (Janaszewska and Bartosz, 2002). Although antioxidant supplements are frequently regarded as good, too many might cause problems. A balanced diet is a much better way to ensure that your body gets the antioxidants that it needs. The antioxidant systems in living organisms may be divided into two types. One is represented by enzymes, such as superoxide dismutase, catalase, and peroxidases, which remove ROS. The other group of antioxidative compounds scavenges free radicals; they are generally of low molecular weight and may be water or lipid soluble. These antioxidants reduce free radicals and are themselves oxidized. After that, other reducing systems may reduce them to their active forms. In biological systems, the initiation of oxidation is balanced by the presence of natural antioxidants. Lipid-soluble antioxidants donate a hydrogen atom (H+) to a fatty acid–based free radical more readily than an unoxidized fatty acid.
Hilsa is particularly vulnerable to lipid oxidation due to its high amount of unsaturated fatty acids (Ackman and Connell, 1980; 1988; 1989a; 1989b; Morris et al., 1989; Hardy, 1980; Khayat and Schwall, 1983). It is believed to contain more than 9% of the total fatty acids found, such as the physiologically significant polyunsaturated fatty acids eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) (Mahmud et al., 2004). Inside the fish body, pro-oxidative and antioxidative factors are in a balanced condition, which regulates oxidative processes, but, under physiological circumstances, endogenous antioxidant enzymes work in the fish body to balance the pro-oxidant effects of ROS and play a significant role in the redox status of muscle tissue. Understanding these trends can provide insights into how antioxidant defenses are regulated in different tissues and how body weight may impact oxidative stress responses in organisms. This study provides valuable insights into the antioxidant levels in various organs of Hilsa fish, focusing on comparing the antioxidant activity between male and female fish of different weight ranges. In this study, for most tissues (excluding brain and gill), both male and female subjects show a synchronized trend where the antioxidant value increases with higher body weight. This implies that, as the body weight of the subjects increases, their tissues exhibit higher antioxidant capacity. Fish protein peptides are a source of bioactive, having treasures of valuable pharmaceutical potentials (Ren et al., 2008) and health benefits, as well as exhibiting a wide variety of antioxidant activities and increasing immunology and regular blood pressure, calcium binding, anti-diabetic activities, etc (Bougatef et al., 2010; Harnedy and FitzGerald, 2012). The total antioxidant activity (TAC), also known as “total antiradical activity,” of biological material has been measured using a variety of techniques if the test system’s oxidants were free radicals (Janaszewska and Bartosz, 2002). Using commercially available free radicals, ABTS and FRAP, for measuring the scavenging activity provides a standardized and widely used approach to assessing antioxidant capacity. These methods allow for the quantification of antioxidant levels and comparison with known antioxidants, such as ascorbic acid and BHT. Generally, fish protein hydrolysate has been extensively studied for its antioxidant properties. The antioxidants stop the oxidation process by dissipating free radicals. As Hilsa is a proven nutri-smart fish and rich in all essential amino acids, EPA, DHA, and micronutrients (FAO, 1974), the compelling benefits on health have been studied in the present study in organs (liver, kidney, brain, gut, gill, and muscle) and serum to understand its bioavailability and pathway in distribution and the level of intake in human consumption from these organs. Although earlier reports are not available on the role of antioxidant levels in the different organs of this fish and its role in making the availability of a rich source of all micro- and macronutrients as well as its biosynthetic pathway, this study was the first attempt to characterize the antioxidant distribution pattern in different organs of the fish.
In our study, we found that lower body weight Hilsa sometimes exhibited a higher antioxidant capacity using the ABTS method, whereas a different result was noted with the FRAP method, as shown in the figures. A possible explanation could be the ABTS method is sensitive to a broader range of antioxidants (hydrophilic and lipophilic), which may account for the higher antioxidant values in lower–body weight fish with higher metabolic rates. In contrast, the FRAP method primarily measures the reducing power of hydrophilic antioxidants, indicating higher antioxidant values in heavier fish (Thaipong et al., 2006). The unique antioxidant profiles in brain and gill tissues showed a different trend in relation to the methods used, which might be attributed to the types of antioxidants that are bound to the chemicals in our study and need further investigation. The author of the present study did not find any such work, which will substantiate the study. The observed difference in antioxidant levels between male and female fish brain and gill tissues could be attributed to several factors. Hormonal differences in females may enhance their antioxidant defenses. Additionally, variations in metabolic rates and reproductive physiology could influence oxidative stress and antioxidant levels. Environmental factors, genetic differences, and disparities in age and growth rates might also play roles. Collectively, these factors could contribute to the lower antioxidant levels observed in male fish compared to that in females. Additionally, the interaction of brain and gill tissue components with the reagents used in these assays may vary, with FRAP potentially being more reflective of sex-specific oxidative stress markers in humans as well as in animals (Munteanu and Apetrei, 2021). It has been noticed that all the organs of fish exhibited antioxidant levels, the highest being detected in the kidney of males, followed by the gill. The antioxidant capacity of biological fluids has been estimated using a variety of techniques; however, comparing the outcomes of different techniques is not always easy (Rice-Evans et al., 1993; 2000; 2001; Prior and Cao, 1999); the four methods (2,2-diphenyl-1-picrylhydrazyl (DPPH), ABTS, FRAP, and kinetic method) are used to determine and compare the human blood plasma antioxidants, and they showed that the result varied and the main reason was the TAC of blood plasma with respectable signs due to the variations in antioxidant reactivities (Janaszewska and Bartosz, 2002). In this study, the result indicates the variability in the expression pattern from all tissues and serum in both methods using the standards of ascorbic acids and BHT. ABTS assay is an effective method for assessing the antioxidant activity of chain-breaking antioxidants (scavengers of lipid peroxyl radicals) and hydrogen-donating antioxidants (scavengers of aqueous has radicals) (Leong and Shui, 2002). Previous research showed (Das et al., 2023) that the TAC followed the fish weight-dependent gradient. The fish with the greatest TAC, weighing 357.8 g, had a TAC of milt (65%–84%), followed by fish with a TAC of milt (272.61 g). In this study, male Hilsa showed higher trends of antioxidants in the lower-age group, and females showed increasing trends from the lower age group to higher-age group. Except for serum antioxidant profiles, most of the organs showed antioxidant levels of >50% as compared to the standard one. The study showed that migratory nutri-smart fatty Hilsa fish is rich in antioxidants in all the organs and serum, which could be beneficial for human consumption and health.
ABTS free radical scavenging is a standard universal method used commonly to measure antioxidant activities (Binsan et al., 2008) in Litopenaeus vannamei, which is reduced by an antioxidant (Miller et al., 1993); its an antioxidant that can lower the relatively stable radical known as ABTS+ (Miller et al., 1993). In the present study, in the ABTS method, gut activity levels at all the time remained at more than 84% in all male and female age groups. Here, females in the gut showed more than 92% activity. A similar pattern was found in the kidney, liver, muscles, brain, gut, and gills, except for a few samples with more than 500 g of weight. This is consistent with other research papers that have reported higher antioxidant activity in females across various fish species. For instance, a study by Huang et al. (2005) discussed the presence of antioxidants in fish and their role in disrupting radical chain reactions and preventing the formation of reactive oxidants. Another study by Pramanik and Mohanty (2016) on antioxidant enzymes in different tissues of Indian Major Carp reported high in female fish. This might be because very few fish were available to test in this group.
Interestingly, the serum also showed antioxidant ability, and the expression level was above 33% and below 68% in all tested groups in the ascorbic acid standard. Our findings align with previous studies investigating the antioxidant activity of fish species. For instance, a survey by Hossain et al. (2019) and Das et al. (2023) explored the nutritional value of Hilsa fish, and they highlighted its omega-3 fatty acid content, protein content, and presence of vitamins, calcium, and trace minerals (Mukherjee et al., 2019). The reducing power of any tissues or serum is an indicator of antioxidant activities used to evaluate its ability to donate an electron or hydrogen, as reported by Yıldırım et al. (2001). The reduction of ferric 3+/ferric cyanide complex to ferrous ion and its color changes from yellow to green or blue shades depend on the reducing potential, representing the presence of antioxidants. There is a direct relationship between antioxidant activity and the reducing power of the bioactive peptides (Ali et al., 2009).
The level of expression found in the present study measured in the FRAP method was less than that in the ABTS method. However, we noticed an average of 50% and above expression of the antioxidant property in all the organs tested except in a few cases in the serum of female Hilsa up to the weight group of 200 g. The lower expression level of the FRAP method compared to that of the ABTS might be attributed to the low level of antioxidants responsible for the deduction of the ferric ion compared to that of the ferrous ion. In the present study, the nature of antioxidants was not evaluated, so we could not conclude the differential expression between these two methods.
In the case of ABTS compared to FRAP, the reduction was more than 70% higher in most cases. This might be due to the binding capacity of the free radicals available in the tissue samples, including serum. The findings of this study have important implications for human nutrition and potential anti-aging effects. High levels of antioxidants in various organs, including the serum, suggest that consuming Hilsa fish can provide substantial health benefits. It is well recognized that amino acids, peptides, and amines have powerful antioxidant capabilities. In previous studies by Ganguly et al. (2018) and De et al. (2019), the amino acid content of Hilsa from two rivers was compared. The Hooghly stock had essential amino acids in higher amounts, and, in the case of Padma, the total non-essential amino acids were found to have higher cencentration and played a role as principal antioxidants or synergists (Ganguly et al., 2018). This study further showed that the antioxidant represents free radicals capable of donating hydrogen ions that can bind more in ABTS rather than that in FRAP and gives more activity using the standard of BHT and ascorbic acid.
Furthermore, our study revealed a positive correlation between antioxidant levels and weight in both males and females. As weight increased, the antioxidant levels also increased proportionally. This correlation suggests that larger Hilsa fish may have a higher antioxidant capacity. These findings are consistent with studies on other fish species that have observed a positive relationship between body size and antioxidant activity. For example, a study by Chen et al. (2019) on Nile tilapia found that larger fish exhibited higher antioxidant enzyme activities. However, this is an important finding that might be further studied with the differential expression acceptable tuning method and comparing the peptides responsible for bioactive properties and validity in vivo for its health benefits.
The majority of surveyed Hilsa fishers had Hilsa almost alternative days in their diet in Hilsa season. From the Bangladesh Development studies, it was stated that Hilsa was consumed by extremely poor households in Bangladesh from 0.56 kg/person/year in 2000 to 0.2 kg/person/year in 2010 (Toufique, 2015). In another survey of 150 consumers from the World Fish Centre in Dhaka in November 2010, the main and second most significant fish types are medium-sized freshwater capture species and Hilsa (which includes jatka), which are consumed in huge quantities by people across the society. Being the high-value species still, Hilsa is popular in the fish market, and, day by day, its demand is increasing. People are purchasing Hilsa at high prices for its taste and nutritional point of view, and Hilsa indeed contributes to anti-aging factor, as, already stated (DiNicolantonio and O’Keefe, 2019), omega-3 fatty acids are highly available in Hilsa and also Hilsa has more than 60% antioxidants present in all the organs that reflect the anti-aging property by neutralize free radicals and protect the skin from oxidative damages. Like other marine fish and identical fish like salmon, other marine fish trout also contain a high antioxidant named astaxanthin. In order to get 3.6 mg of astaxanthin, one can eat 165 g of salmon per day (Ambati et al., 2014), which is important for skin health. This plays a key role in collagen production, repair of skin, and protection against environmental pollutant damage. On the other hand, the antioxidant properties of Hilsa may have been utilized to prevent oxidative damage in diseased cells. Additional investigation is necessary to fully understand the antioxidants present in Hilsa cells, including which kinds of antioxidants are present and how their levels change throughout migration.
All the organs found rich in antioxidants, which could be beneficial to human health point of view. However, the values of antioxidants corresponding to the female Hilsa were consistent in the whole study in all six weight groups. The antioxidant defenses in various tissues are influenced by body weight, but the influence varies between tissues. Most tissues follow a positive trend where higher body weight is associated with increased antioxidant capacity. However, brain and gill tissues exhibit a unique response, possibly due to differing metabolic demands, exposure to oxidative stress, or other tissue-specific factors. The practice of consuming these organs traditionally by people in eastern India and Bangladesh indicated that, in addition to omega-3 fatty acids, it is contributing antioxidant levels. Further, either whole fish or partial dressed fish contribute to the antioxidants levels, which are to be promoted among the fishers, and organs need to be used for consumptions as study suggested. Recent evidence of the role of antioxidants in maintaining human health has sparked interest in research on antioxidants. In light of this interest, extensive study has been done on defining and identifying the components that have antioxidative action in both biological systems and people. Further research could focus on elucidating the specific antioxidant compounds in Hilsa fish and exploring their potential health benefits in more detail. Additionally, investigating the underlying mechanisms responsible for the observed differences in antioxidant levels between sexes and weight ranges would provide a deeper understanding of the factors influencing antioxidant activity in Hilsa fish. Although Hilsa is an essential high-value, nutritionally dense food fish, its health benefits are from fatty acids and antioxidant properties. More research studies are needed to characterize the nature of the antioxidant and its role in different health benefits, including anti-aging in humans.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The requirement of ethical approval was waived by The Central Inland Fisheries Research Institute (ICAR) ethical committee for the studies involving humans. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
HC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. BD: Conceptualization, Investigation, Supervision, Validation, Visualization, Writing – review & editing. NC: Conceptualization, Formal Analysis, Methodology, Writing – review & editing. AS: Supervision, Writing – review & editing. JM: Conceptualization, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors acknowledged the director, ICAR-CIFRI, for providing the facility to carry out the research work and the supportive members who have helped us during the sampling of Hilsa. The authors would also like to acknowledge Vidyasagar University for providing a library and lab facility for the specific work and give a special thanks to NMCG for providing the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ackman R. G. (1989b). Nutritional composition of fats in seafoods. Prog. Food Nutr. Sci. 13, 161–289.
Ackman R. G., Connell J. J. (1980). Advances in fish science and technology (Surrey: Fishing New Book Farnham), 86–123.
Ambati R. R., Phang S. M., Ravi S., Aswathanarayana R. G. (2014). Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 12, 128–152. doi: 10.3390/md12010128
Arnao M. B., Cano A., Acosta M. (2001). The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 73, 239–244. doi: 10.1016/S0308-8146(00)00324-1
Binsan W., Benjakul S., Visessanguan W., Roytrakul S., Tanaka M., Kishimura H. (2008). Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei). Food Chem. 106, 185–193. doi: 10.1016/j.foodchem.2007.05.065
Bougatef A., Nedjar-Arroume N., Manni L., Ravallec R., Barkia A., Guillochon D., et al. (2010). Purification and identification of novel antioxidant peptides from enzymatic hydrolysates of sardinelle (Sardinella aurita) by-products proteins. Food Chem. 118, 559–565. doi: 10.1016/j.foodchem.2009.05.021
Chakraborty H., DebRoy P., Kunui A., Nandy S. K., Jana C., Sahoo A. K., et al. (2024). Hilsa fisheries in India: a socio-economic analysis of fishers in deltaic Ganga region of river Hooghly. Front. Sustain. Food Syst. 8, 1310077. doi: 10.3389/fsufs.2024.1310077
Chen X. Q., Zhao W., Xie S. W., Xie J. J., Zhang Z. H., Tian L. X., et al. (2019). Effects of dietary hydrolyzed yeast (Rhodotorula mucilaginosa) on growth performance, immune response, antioxidant capacity and histomorphology of juvenile Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 90, 30–39. doi: 10.1016/j.fsi.2019.03.068
Chrzczanowicz J., Gawron A., Zwolinska A., de Graft-Johnson J., Krajewski W., Krol M., et al. (2008). Simple method for determining human serum 2, 2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity–possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 46, 342–349. doi: 10.1515/CCLM.2008.062
Das B. K., Sadhukhan D., Chakraborty N., Ganguly S., Sahoo A. K. (2023). An insight into milt antioxidant, fatty acid, amino acid composition and testis histology of an anadromous euryhaline fish Tenualosa ilisha, Ham. 1822 for its conservation and aquaculture perspectives. Aquaculture 576, 739832. doi: 10.1016/j.aquaculture.2023.739832
De D., Mukherjee S., Anand P. S., Kumar P., Suresh V. R., Vijayan K. K. (2019). Nutritional profiling of hilsa (Tenualosa ilisha) of different size groups and sensory evaluation of their adults from different riverine systems. Scientific Reports. 9 (1), 19306.
DiNicolantonio J. J., O’Keefe J. H. (2019). The benefits of marine omega-3s for the prevention and treatment of cardiovascular disease. Missouri Med. 116, 404.
Ganguly S., Mahanty A., Mitra T., Mohanty S., Das B. K., Mohanty B. P. (2018). Nutrigenomic studies on Hilsa to evaluate flesh quality attributes and genes associated with fatty acid metabolism from the rivers Hooghly and Padma. Food Res. Int. 103, 21–29. doi: 10.1016/j.foodres.2017.10.017
Hardy R. (1980). “Fish lipids. Part 2,” in Advances in Fish Science and Technology. Ed. Connell J. J. (Fishing News Books Ltd, Farnham, Surrey, England103–111).
Harnedy P. A., FitzGerald R. J. (2012). Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. foods 4, 6–24. doi: 10.1016/j.jff.2011.09.001
Hossain M. A., Das I., Genevier L., Hazra S., Rahman M., Barange M., et al. (2019). Biology and fisheries of Hilsa shad in Bay of Bengal. Sci. Total Environ. 651, 1720–1734. doi: 10.1016/j.scitotenv.2018.10.034
Hossain M. S., Sarker S., Sharifuzzaman S. M., Chowdhury S. R. (2020). Primary productivity connects Hilsa fishery in the Bay of Bengal. Sci. Rep. 10, 5659. doi: 10.1038/s41598-020-62616-5
Huang D., Ou B., Prior R. L. (2005). The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53, 1841–1856. doi: 10.1021/jf030723c
Janaszewska A., Bartosz G. (2002). Assay of total antioxidant capacity: comparison of four methods as applied to human blood plasma. Scandinavian J. Clin. Lab. Invest. 62, 231–236. doi: 10.1080/003655102317475498
Leong L. P., Shui G. (2002). An investigation of antioxidant capacity of fruits in Singapore markets. Food Chem. 76, 69–75. doi: 10.1016/S0308-8146(01)00251-5
Mahmud I., Hossain A., Hossain S., Hannan A., Ali L., Hashimoto M. (2004). Effects of Hilsa ilisa fish oil on the atherogenic lipid profile and glycaemic status of streptozotocin-treated type 1 diabetic rats. Clin. Exp. Pharmacol. Physiol. 31, 76–81. doi: 10.1111/j.1440-1681.2004.03953.x
Miller N. J., Rice-Evans C., Davies M. J., Gopinathan V., Milner A. (1993). A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. (London England: 1979) 84, 407–412. doi: 10.1042/cs0840407
Mukherjee S., Anand P. S. S., Kumar P., Suresh V. R., Vijayan K. K. (2019). Nutritional profiling of Hilsa (Tenualosa ilisha) of different size groups and sensory evaluation of their adults from different riverine systems. Sci. Rep. 9, 19306–19306. doi: 10.1038/s41598-019-55845-w
Munteanu I. G., Apetrei C. (2021). Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 22, 3380. doi: 10.3390/ijms22073380
Neiffer D. L., Stamper M. A. (2009). Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J. 50, 343–360. doi: 10.1093/ilar.50.4.343
Pramanik D. S., Mohanty S. S. (2016). Length-weight relationship and biology of some common edible fish species at Chandipur, Bay of Bengal, Odisha. Int. J. Fisheries Aquat. Stud. 4, 335–340.
Prior R. L., Cao G. (1999). In vivo total antioxidant capacity: comparison of different analytical methods1. Free Radical Biol. Med. 27, 1173–1181. doi: 10.1016/S0891-5849(99)00203-8
Ren J., Zhao M., Shi J., Wang J., Jiang Y., Cui C., et al. (2008). Optimization of antioxidant peptide production from grass carp sarcoplasmic protein using response surface methodology. LWT-Food Sci. Technol. 41, 1624–1632. doi: 10.1016/j.lwt.2007.11.005
Rice-Evans C. A. (2000). Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radical Res. 33, S59–S66.
Risso A., Pellegrino F. J., Corrada Y., Schinella G. (2021). Evaluation of total antioxidant activity and oxidative stress in seminal plasma from dogs supplemented with fish oil and vitamin E. Int. J. Fertility Sterility 15, 15.
Sahoo A. K., Wahab M. A., Phillips M., Rahman A., Padiyar A., Puvanendran V., et al. (2018). Breeding and culture status of Hilsa (Tenualosa ilisha, Ham. 1822) in South Asia: A review. Rev. Aquaculture 10, 96–110. doi: 10.1111/raq.12149
Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D. H. (2006). Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Composition Anal. 73, 669–675. doi: 10.1016/j.jfca.2006.01.003
Toufique K. A. (2015). Some thoughts on Hilsa exports and management in Bangladesh. Bangladesh Dev. Stud. 38, 115–127.
Vranković J., Stanković M., Marković Z. (2021). Levels of antioxidant enzyme activities in cultured rainbow trout (Oncorhynchus mykiss) fed with different diet compositions. Bull. Eur. Assoc. Fish Pathologists 41, 135–145.
Yıldırım A., Mavi A., Kara A. A. (2001). Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 49, 4083–4089. doi: 10.1021/jf0103572
Keywords: ABTS, anadromous fish, antioxidant, FRAP, Hilsa
Citation: Chakraborty H, Das BK, Chakraborty N, Sahoo AK and Maity J (2024) Distribution patterns of antioxidants in the organs of anadromous fish Tenualosa ilisha (Hamilton, 1822)—a profiling in different age groups for future application in anti-aging. Front. Mar. Sci. 11:1452775. doi: 10.3389/fmars.2024.1452775
Received: 21 June 2024; Accepted: 29 July 2024;
Published: 23 August 2024.
Edited by:
Monjurul Haq, Jashore University of Science and Technology, BangladeshReviewed by:
Sofia Priyadarsani Das, National Taiwan Ocean University, TaiwanCopyright © 2024 Chakraborty, Das, Chakraborty, Sahoo and Maity. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Basanta Kumar Das, YmFzYW50YWt1bWFyZEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.