- 1Key Laboratory of Applied Biology and Aquaculture of Northern Fishes in Liaoning Province, Dalian Ocean University, Dalian, China
- 2College of Fisheries and Life Sciences, Dalian Ocean University, Dalian, China
During the suspension cage culture procedure, a substantial quantity of attachments from the suspension cage will amass in the tidal flats, resulting in environmental pollution and resource wastage. The aim of the present study was to explore the feasibility of using attachments of suspension cage (ASC) as a raw feed material for sea cucumbers. Different ASC concentrations (0, 25, 50, 75, and 100%) were used in sea cucumber (Apostichopus japonicus) fed for 60 d. Replacing sea mud with 50% ASC significantly improved sea cucumber growth, reduced the feed coefficient, significantly increased the activity of intestinal digestive enzymes, improved the activities of acid phosphatase and alkaline phosphatase, improved the intestinal structure, and enriched intestinal microbiome diversity (P<0.05). Replacing sea mud with 75% and 100% ASC significantly improved sea cucumber total antioxidant capacity and total superoxide dismutase (P<0.05).Furthermore, expressions of c-myc gene, mapk-7 gene and fgfr-1 gene, which all three growth genes, significantly increased in the 50% ASC treatment compared to that in the control; whereas, that of gdf-8 gene, a negative growth regulator, was inhibited (P<0.05). Based on the quadratic regression analysis for the weight gain rate, the appropriate levels of ASC substitution were estimated as to be 44.28%. The results suggested the potential of ASC as a new raw feed material for sea cucumbers.
1 Introduction
The sea cucumber, Apostichopus japonicus, is renowned for its medicinal properties and use as a traditional tonic. It is highly popular in China and other Asian nations influenced by Chinese cultural traditions, including Japan, South Korea, Vietnam, and Singapore (Han et al, 2016). In 2022, A. japonicus production in China reached 248,508 tons (Ru et al., 2022). However, the demand for sea cucumbers is increasing owing to China’s growing population. A critical factor influencing aquaculture success is the quality of the artificial feed (Han et al., 2016). Nonetheless, the high cost of raw feed materials poses a significant challenge for sustainable aquaculture. Addressing this issue is crucial for the ongoing success and growth of the sea cucumber industry (Wu et al., 2015).
Sea mud is a crucial component of sea cucumber feed. However, commercial sea mud’s nutritional components vary, resulting in suboptimal feed effects (Gong et al., 2012). Consequently, identifying viable alternatives to reduce reliance on marine sediments has become an urgent challenge in sustainable sea cucumber aquaculture development.
During the suspension cage culture procedure, regular cleaning to remove epiphytic organisms from the cage is requisite to avert economic losses caused by the cage's sinking. However, subsequent to cleaning, a substantial quantity of attachments from the suspension cage will amass in the tidal flats, resulting in environmental pollution and resource wastage. Attachments of suspension cage (ASC) is a complex mixture consisting of approximately 30 different types of attachments, including algae, mosses, barnacles, small protozoa, silt, and shell fragments. However, ASC is currently discarded after oysters are harvested, representing a significant waste of resources. Some studies have reported that farming wastes from shellfish aquaculture industry could be reused by sea cucumber (Yuan et al., 2006; Lu et al., 2018). Chen et al. (2015) observed that wet shrimp waste can serve as a nutritious food source for sea cucumber. However, there is a significant absence of prior research exploring ASC as a potential feed ingredient.
In the present study, ASC was identified as a viable substitute for sea mud in the formulation of sea cucumber feed. The results provide valuable reference data for the advancement of sustainable and eco-friendly approaches to sea cucumber farming. These findings are also anticipated to furnish a scientific basis for the development of novel raw feed materials and processing methods.
2 Materials and methods
2.1 Ethical statement
The study protocol and procedures were approved by the Ethics Committee of Dalian Ocean University.
2.2 Experimental diets
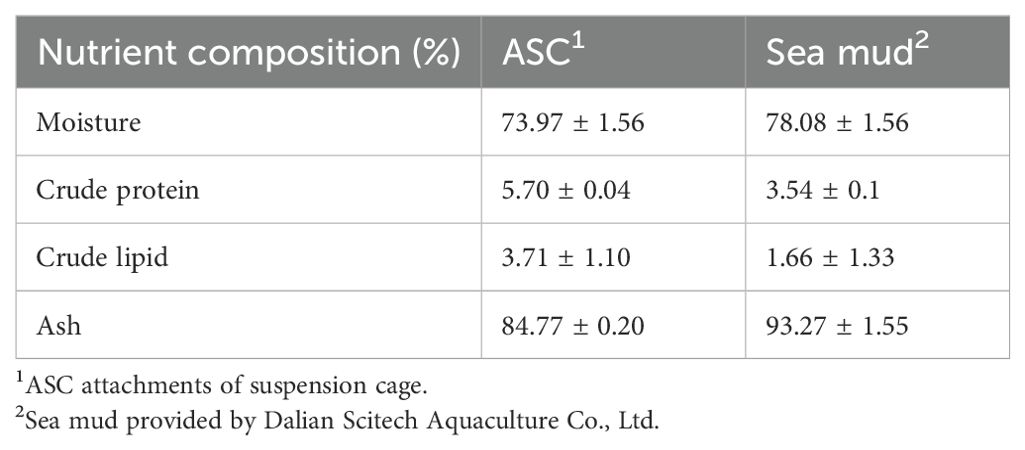
The ASC used in this study was sourced from Dalian Shang Pin Tang Marine Biological Co., Ltd. and collected in April 2023 from Shicheng Island, Dalian (39°54’N, 112°95’E). The general nutritional components of sea mud and ASC are shown in Table 1.
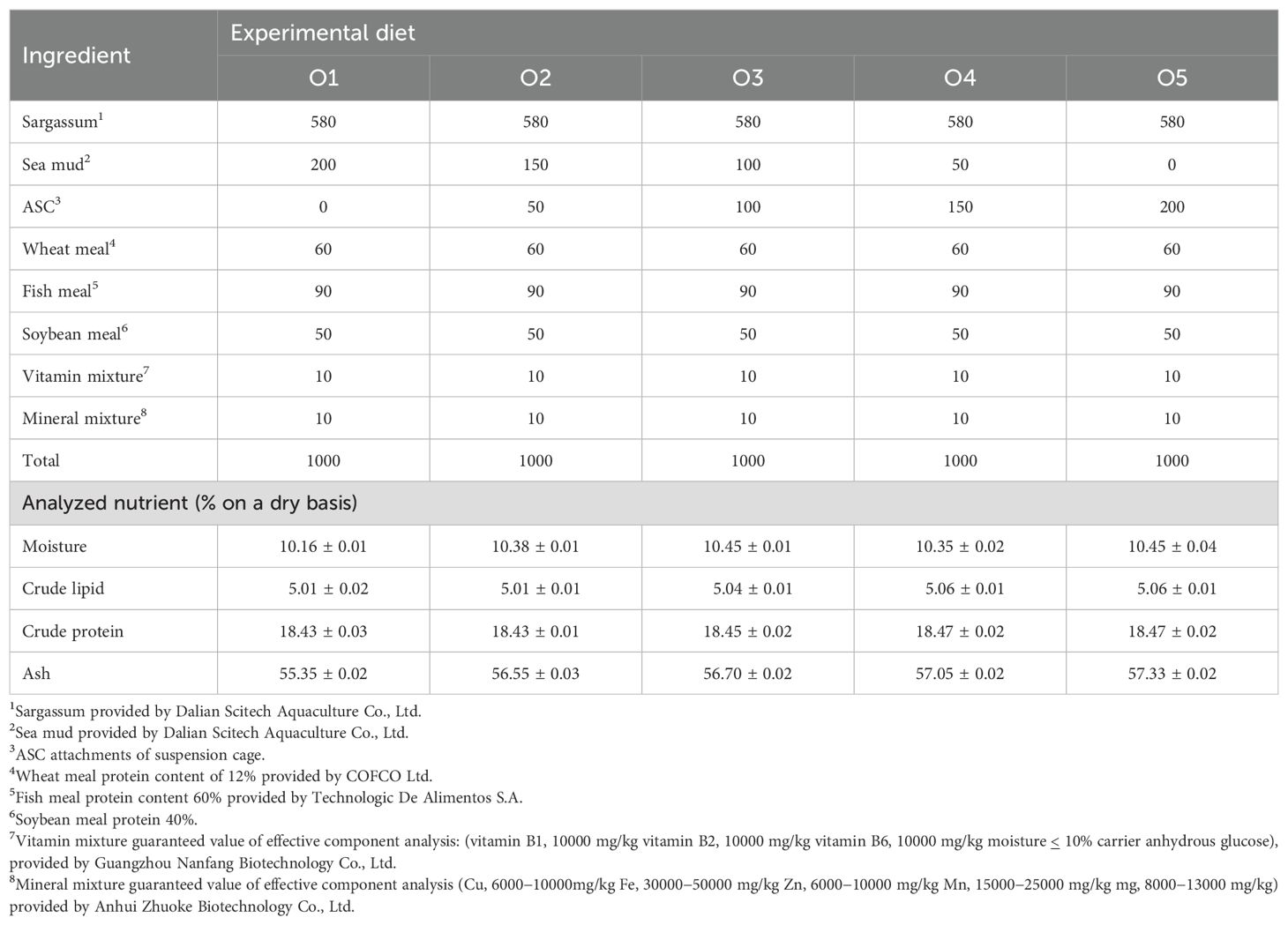
Five distinct diets were prepared, each incorporating varying concentrations of ASC as a substitute for sea mud. The diets comprised 0% (O1, control group containing sea mud without ASC), 25% (O2), 50% (O3), 75% (O4), and 100% (O5) ASC replacement for sea mud. The feed formula and general nutrient compositions are detailed in Table 2. All raw materials were ground and sieved through a 60-mesh sieve. A meticulous mixing process was performed, and the blended materials were introduced into a granulator (JLA-125; Pinzheng Equipment Co., Ltd., Shenzhen, China) where they were shaped into pellet feed with a standardized particle size of 1 mm, air-dried, and stored at -20°C.
2.3 System for rearing sea cucumbers and feeding experiment
Sea cucumbers were obtained from Dalian Feikun Aquaculture Co. Ltd. To facilitate acclimation to warm and saline conditions of the seawater laboratory, all sea cucumbers were domesticated in a pool for 2 weeks. Subsequently, 300 sea cucumbers weighing 10 ± 0.06 g were divided into three replicate groups and randomly assigned to 15 100-L tanks and a recirculating aquaculture system capable of producing 10 L of water per minute. A 60-d feeding study was conducted. At 17:00, they were fed 3% of their total body weight; the amount of feed was modified based on the gradual increase in their body weight. All tanks were aerated constantly throughout the experiment. The average water temperature was maintained at 17 ± 2°C, salinity levels ranged from 28−30‰, and dissolved oxygen concentration was > 5 mg/L.
2.4 Sample collection
Sea cucumber samples were collected as described by Li et al. (2020). After the feeding experiment, all sea cucumbers were starved for 24 h. All animals were then removed from their tanks, body surface moisture was immediately wiped with medical gauze, and individual weights were determined. A total of 10 sea cucumbers were randomly selected from each tank for sampling. Each sea cucumber had all of its tissues collected, including coelomic fluid, body wall, and intestines. Coelomic fluid was obtained from the selected sea cucumbers by puncturing the body wall with a 1mL disposable syringe. The coelomic fluid was centrifuged at 3000 × g for 15 min at 4°C (Shanghai Medical Analytical Instrument Factory, China). After centrifugation, the supernatant was stored in sterile microcentrifuge tubes at -80°C. The body wall and intestines of sea cucumbers were dissected on ice and stored in centrifuge tubes at -80°C for preservation. No mixed samples were utilized in subsequent studies.
2.5 Growth performance
The growth performance of each group was calculated based on the average body weight of 10 sea cucumbers in each tank. Equations 1–4 (Li et al., 2020) were used to obtain the following growth performance parameters:
where Wm is the mean final weight, Ws is the mean initial weight, t is the feeding trial duration (days), F is the mean feed intake, Wi is the mean intestinal quality, Fs is the mean final number of sea cucumber and Is is the mean initial number of sea cucumber.
2.6 Biochemical analysis of sea cucumbers
The AOAC standard method (AOAC, 1990) was employed for the proximate analysis of the sea cucumber body wall. The crude protein content was measured using the Kjeldahl technique (Kjeltec KDN-1000 Auto Analyzer, Shanghai, China). Crude lipids were quantified using the Soxhlet method after ether extraction (QW-SZF-06A; Hangzhou Qiwei Instrument Co., Ltd., Hangzhou, China). The ash content was determined using a muffle furnace (Shanghai Yuejin Medical Instrument Co., Ltd., Shanghai, China) at 550°C for 6 h. Moisture content was measured by drying samples in an oven at 105°C until a consistent weight was achieved.
The levels of lipase (LPS), amylase (AMS), and pepsin (PEP) activity in the intestines of sea cucumbers were measured using corresponding enzyme assay kits (LOT NO. A054-1-1, C016-1-1, and A080-1-1, respectively; Nanjing Jiancheng Bioengineering Institute, China), in compliance with the manufacturer's instructions. In addition, using corresponding enzyme assay kits (LOT NO. A060-1-1, A059-1-1, A015-1-2, A001-1-2, A007-1-1, and A003-1-2, respectively; Nanjing Jiancheng Bioengineering Institute), immune and antioxidant enzymes in sea cucumber coelomic fluid, including acid phosphatase (ACP), alkaline phosphatase (AKP), total antioxidant capacity (T-AOC), total superoxide dismutase (T-SOD), catalase (CAT), and lysozyme (LZM), were determined according to the manufacturer's instructions. To perform gut histology, the procedures outlined by Zeng et al. (2021) were followed. Briefly, the intestinal sample was fixed using 4% paraformaldehyde solution at 4°C for 24 h and dehydrated using a series of graded ethanol concentrations. To prepare slices for hematoxylin-eosin (HE) staining, standard paraffin embedding methods were used. Measurements were performed using the S-viewer software, and observations were made using a light microscope equipped with an image analyzer. For every sample, 10 or more intact villi were selected, and their average values were used to calculate the muscle layer thickness (MT), villus height (VH), and villus width (VW).
2.7 Growth gene expression analysis
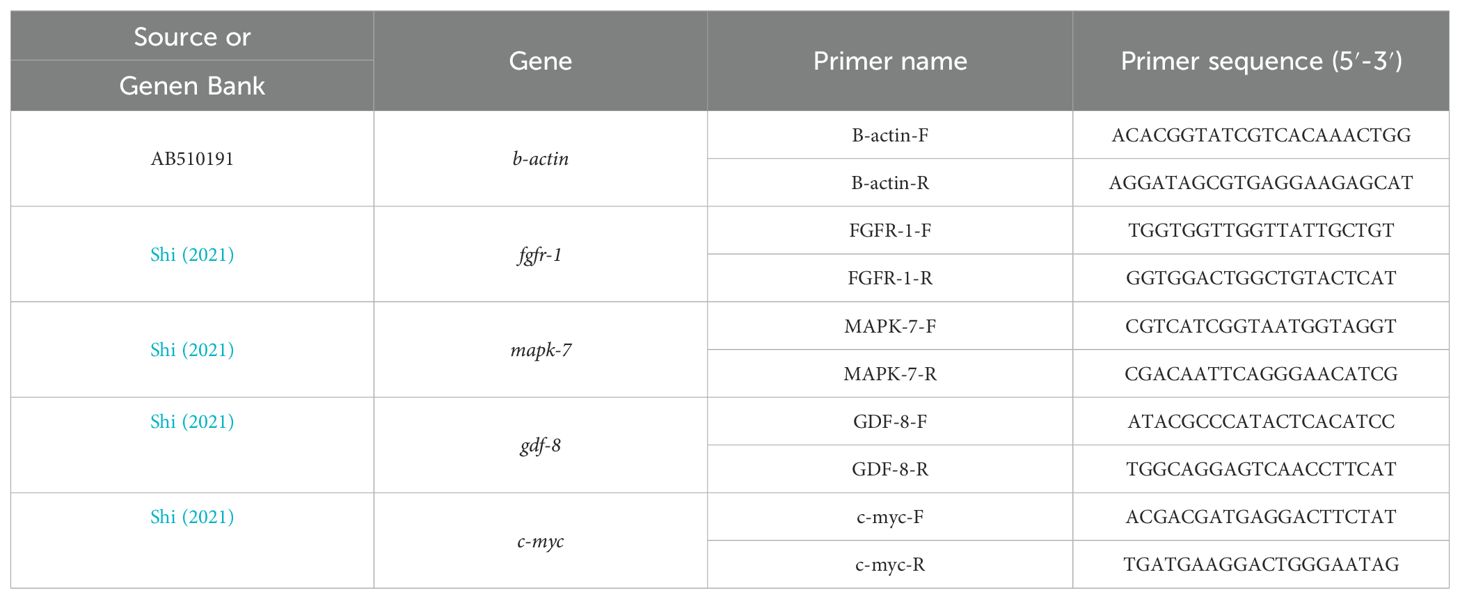
Total RNA was extracted from sea cucumber body walls using the method described by Shi et al. (2021). Table 3 lists the quantitative primers for growth genes. Total RNA from the sea cucumber body walls was extracted according to the method described by Shi et al., using the RNA Prep Pure Tissue Kit (LOT NO. DP451; Tiangen Biotech, Co., Ltd, Beijing, China). RNA concentration and purity were determined using a protein-nucleic acid spectrophotometer (P330-31; Implen Nano Photometer).
2.8 Sea cucumber intestinal microflora sequencing
After fasting for 24-h, three healthy sea cucumbers were randomly selected from each tank and disinfected with 70% ethanol, and their intestines were removed using aseptic surgical scissors. Samples were then transferred to Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (China) for gut microbial sequencing. An E.Z.N.A. soil DNA Kit (Omega Bio-teak, Norcross, GA, USA) was used to extract DNA, which was then identified on a 1% agarose gel, as directed by the manufacturer. A NanoDrop 2000 UV-vis spectrophotometer was used to detect DNA integrity. The V3–V4 hyper-variable region of the 16S rRNA gene was amplified using polymerase chain reaction with barcode-specific primers (341F:5′CCTACGGGNGGCWGCAG-3′; 806R:5′-GGACTACHVGGGTATCTAAT-3′). The purified amplicons were pooled in equimolar proportions, and paired-end sequencing was carried out on the NovaSeq PE250 platform (Illumina, San Diego, CA, USA) according to standard protocols of Shanghai Majorbio Bio-pharm Technology Co., Ltd., Shanghai, China. The original 16SRNA gene sequencing findings were examined, and Fastp v0.19.6 was used to ensure quality. Flash v1.2.11 was used for stitching, and the similarity criteria exceeded 97%. Uparse v11 and RDP v11.5 were used to conduct operational taxonomic unit (OTU) clustering analysis. Mothur v1.0.2 was used to conduct an alpha diversity study.
2.9 Statistical analysis
The experimental data were analyzed using one-way analysis of variance in IBM SPSS Statistics 29 (IBM Corp., Armonk, NY, USA). Data are expressed as mean values ± standard error of the mean. Data from each group were compared using Duncan’s test, and a significant difference was set at P < 0.05. Orthogonal polynomial contrasts were used to test linear and quadratic response of dietary ASC levels.
Spearman's correlation coefficient was used to determine the relationships between growth performance and digestive enzymes, intestinal morphology, and proximate makeup. The analysis was carried out in R using the “psych” and “reshape2” packages. Unless otherwise noted, all analyses were carried out using R v3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
3 Results
3.1 Growth performance
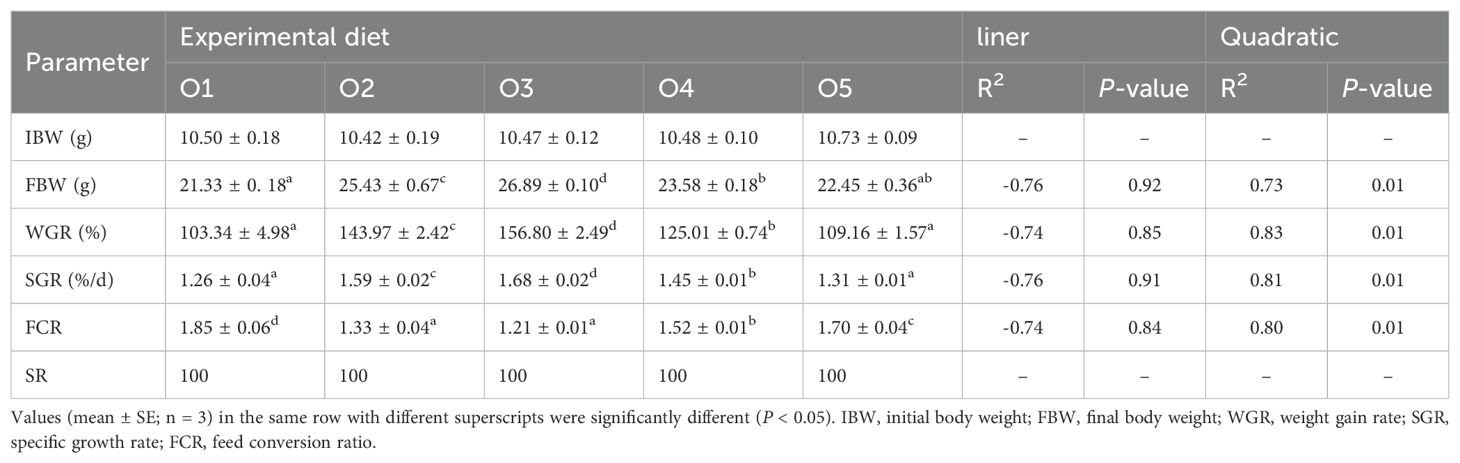
The effects of the different diets used during the 60-d feeding trial on the growth performance of A. japonicus are shown in Table 4. The final body weight (FBW), WGR, and SGR of A. japonicus in the O2, O3 and O4 groups were significantly higher than those in the control group (P < 0.05), exhibiting a significant quadratic association (P < 0.05). The FCR was significantly decreased in the O2, O3, O4 and O5 groups than in the control group (P < 0.05). In Figure 1, the association between dietary ASC level and WGR was: (R2 = 0.83). Based on this equation, the maximum WGR was achieved with a dietary ASC substitution of 44.28%.
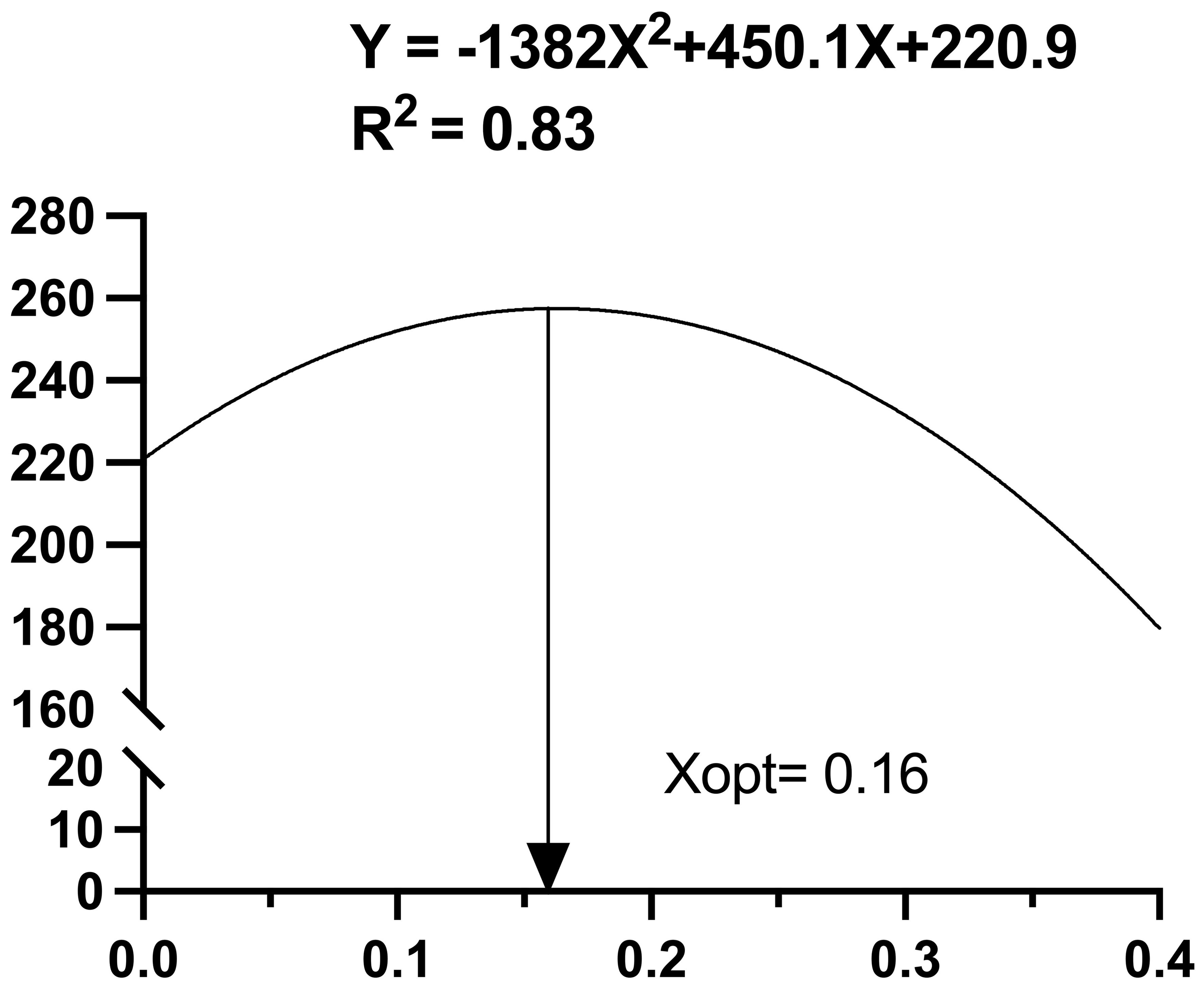
Figure 1. Regression analysis illustrating the relationship between attachments of suspension cage (ASC) content and Weight gain rate of Apostichopus japonicus.
3.2 Approximate component analysis of sea cucumber body wall
The effects of ASC-based diets on the approximate composition of the A. japonicus body wall are presented in Table 5. The inclusion of ASC into the feed did not notable alter the moisture and ash contents of the A. japonicus body wall (P > 0.05), with no significant linear and quadratic differences observed among all dietary groups (P > 0.05). However, as the ASC replacement ratio increased, the levels of crude fat and protein in the body wall also increased. Notably, the O5 group exhibited significantly higher crude fat and protein contents compared to the O1 group (P < 0.05). The crude lipid and protein contents of A. japonicus increased linearly with increasing ASC concentrations (replacement ratio) (P < 0.05).
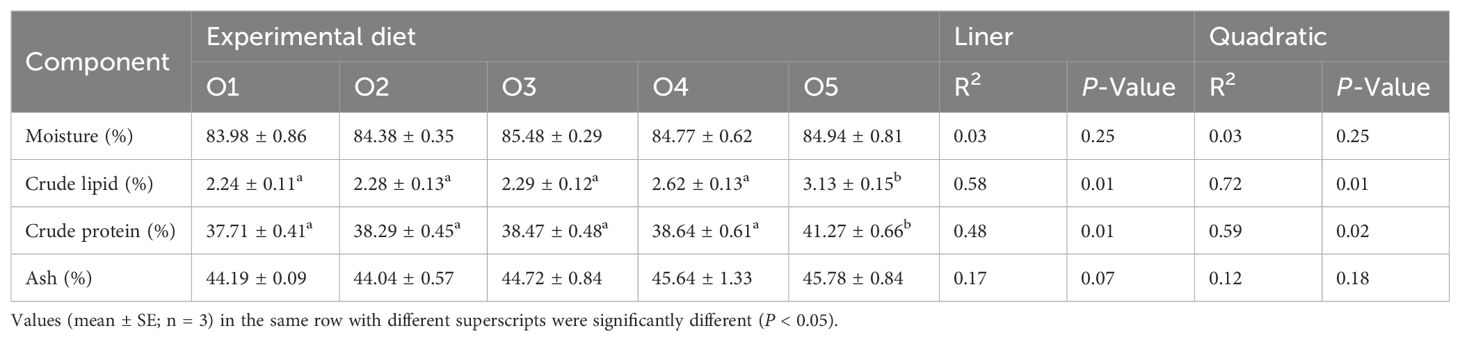
Table 5. Effects of different ASC diets on approximate component (dry matter) analysis of the Apostichopus japonicus body wall.
3.3 Growth gene expression in body wall
Replacing sea mud with ASC significantly upregulated the expression of mapk-7, c-myc and fgfr-1 (P < 0.05; Figure 2), while significantly downregulated that of gdf-8. The expression levels of mapk-7, c-myc and fgfr-1 in the sea cucumber body wall increased with ASC addition, followed by a decline. The O3 group exhibited significantly increased expression compared to the O1 group (P < 0.05). In contrast, the expression of gdf-8 decreased initially and then increased.
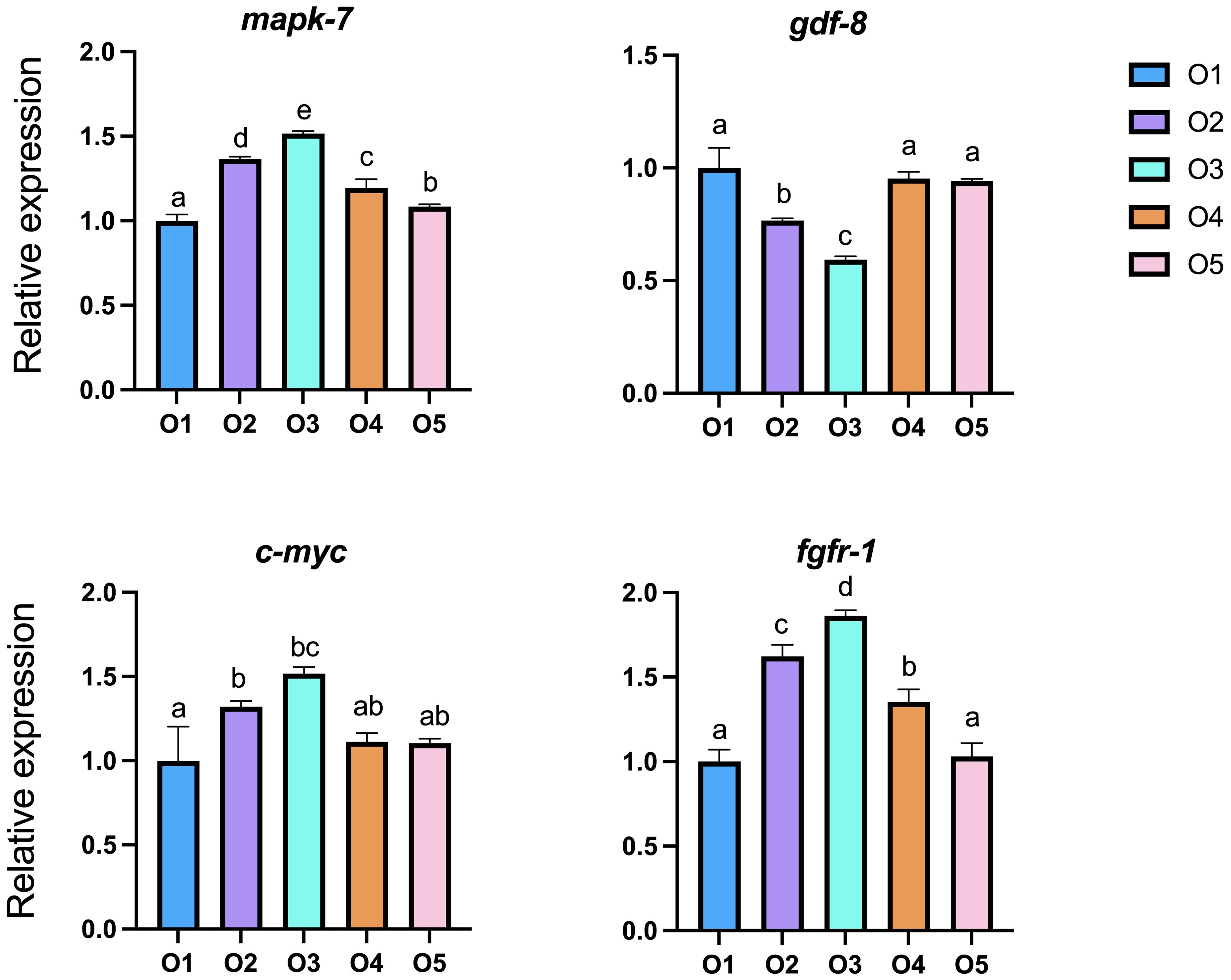
Figure 2. Effects of dietary attachments of suspension cage (ASC) on the expression of growth-related genes in Apostichopus japonicus. O1: 0% ASC, O2: 25% ASC, O3: 50% ASC instead, O4: 75% ASC, AND O5: 100% ASC instead of sea mud. A significant difference (P < 0.05) is indicated by values (mean ± SE) with different superscripts.
3.4 Intestinal digestive enzyme activities
ASC significantly enhanced the activity of intestinal digestive enzymes compared to sea mud (O1 group) (P < 0.05), and the intestinal digestive activity increased initially before decreasing with the addition of ASC. The intestinal AMS levels were significantly higher in the O3 and O4 groups compared to the O1 control group (P < 0.05; Figure 3A). Intestinal PEP levels increased with the proportion of ASC, with significantly higher levels observed in the O2 and O3 group compared to the O1 control group (P < 0.05; Figure 3B). Intestinal LPS levels were significantly higher in the O2, O3 and O4 group than in the O1 control group (P < 0.05; Figure 3C).
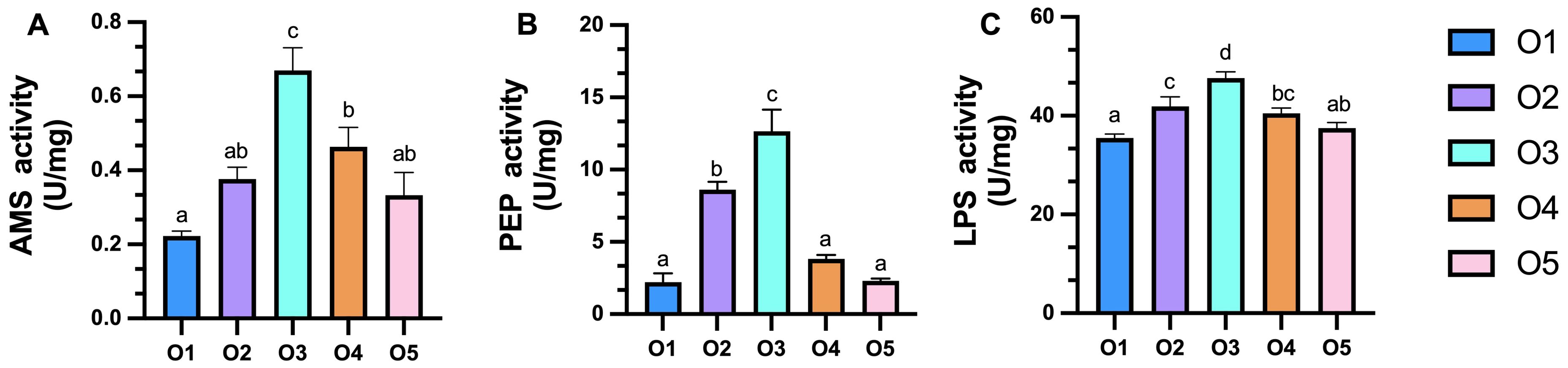
Figure 3. (A–C) Effects of dietary attachments of suspension cage (ASC) intestinal digestive enzyme activities in Apostichopus japonicus. O1: 0% ASC, O2: 25% ASC, O3: 50% ASC instead, O4: 75% ASC, AND O5:100% ASC instead of sea mud. A significant difference (P < 0.05) is indicated by values (mean ± SE) with different superscripts.
3.5 Coelomic fluid enzyme activities
The ACP and AKP enzyme activity in the coelomic fluid in the O3 group was significantly higher than that in the O1 group, and the ACP and AKP enzyme activity increased first and then decreased with the proportion ASC (P < 0.05 Figures 4A, B). Replacing sea mud with ASC significantly increased the activity of antioxidant enzymes (P < 0.05). T-AOC enzyme activity in the coelomic fluid of the O4 and O5 groups was significantly higher than that in the O1 group (P < 0.05; Figure 4C). Similarly, the T-SOD activity in the coelomic fluid of the O4 and O5 groups was significantly higher than that in the O1 group (P < 0.05; Figure 4D). There were no significant differences in the LZM enzyme activity among the groups; however, the activity increased with ASC addition (Figure 4E). The CAT activity in the coelomic fluid of the O3, O4, and O5 groups was significantly higher than that in the O1 group (P < 0.05; Figure 4F).
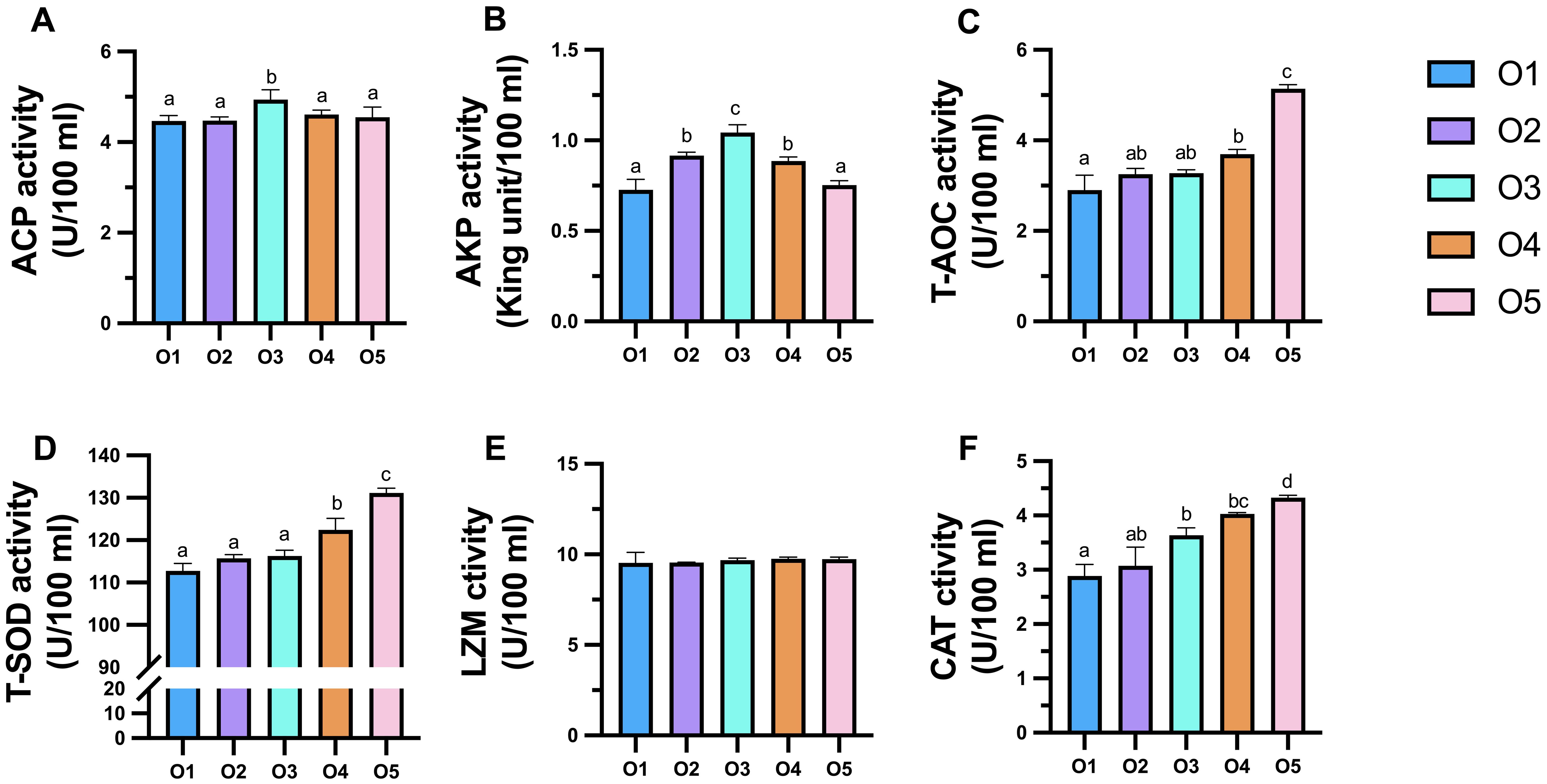
Figure 4. (A–F) Effects of dietary attachments of suspension cage (ASC) on coelomic fluid enzyme activities in Apostichopus japonicus. A significant difference (P < 0.05) is indicated by values (mean ± SE) with different superscripts.
3.6 Intestinal histomorphometrically
The effect of ASC on the intestinal (midgut) morphology of sea cucumbers is shown in Figure 5. Notably, the VH and MT of the A. japonicus intestine exhibited an upward and then downward trend corresponding to increasing ASC levels. Specifically, compared to the control group, the VH of the sea cucumber intestines in the O3 group demonstrated significant increases; the MT of sea cucumber intestines in the O4 group demonstrated significant increases (P < 0.05). In contrast, as the ASC level increased, the VW of the A. japonicus intestine exhibited a downward trend before ascending. Furthermore, the VW of sea cucumber intestines in the O3 and O4 groups was significantly reduced compared to that in the O1 group (P < 0.05).
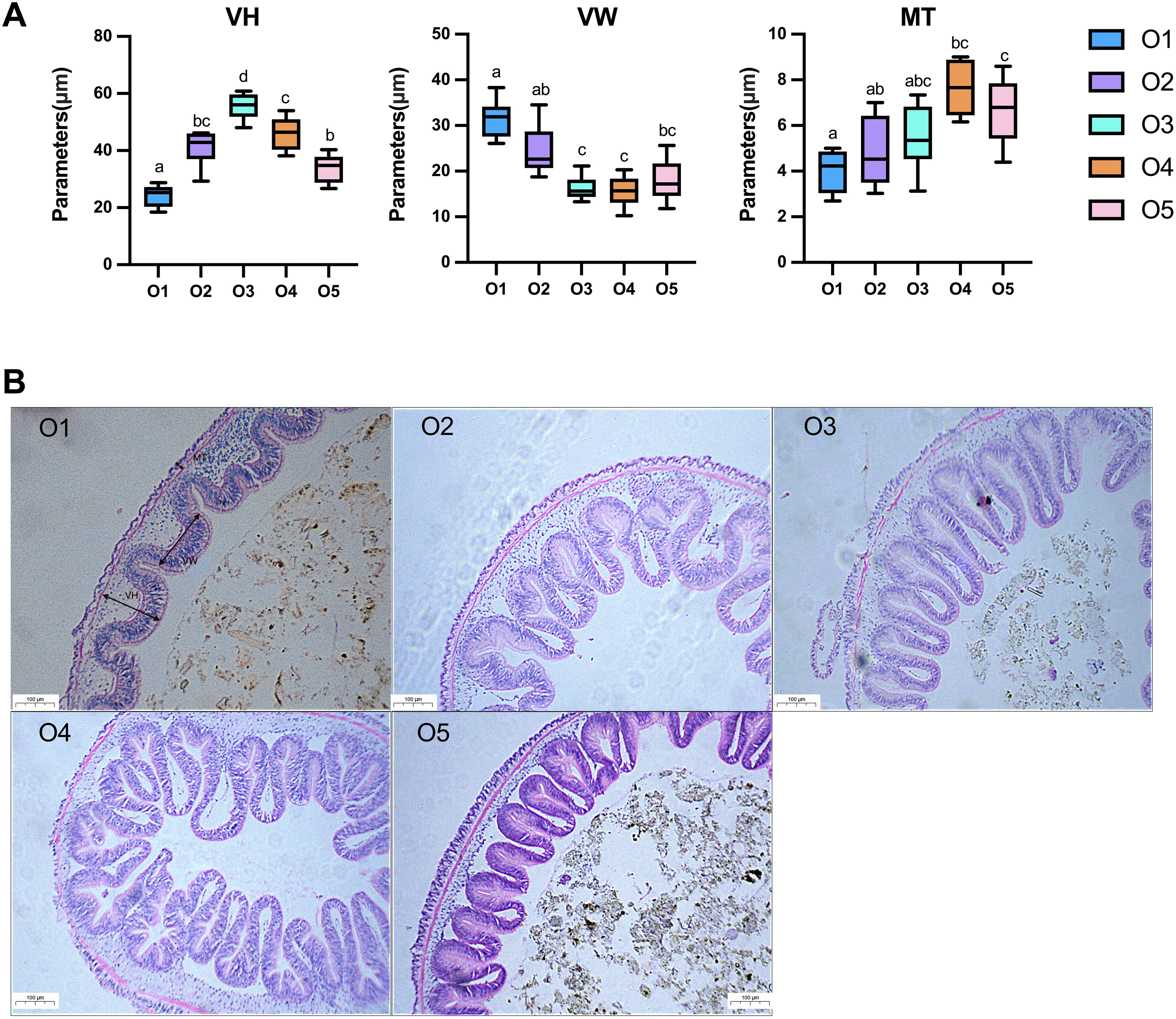
Figure 5. Effect of replacement of sea mud with dietary attachments of suspension cage (ASC) on intestinal morphology of Apostichopus japonicus. (A) Midgut morphological characteristics of A. japonicus after receiving an experimental diet for 8 weeks. MT, muscle layer thickness; VH, villus height; VW, villus width. The error bar is min to max. Mean values with different superscripts are significantly different (P < 0.05). n = 10. (B) Effects of dietary ASC on the intestinal (midgut) morphology of A. japonicus. Magnification = 40×.
3.7 Correlation analysis
A comprehensive correlation analysis was conducted based on the strong interactions observed among growth performance, digestive enzyme activity, intestinal morphology, and similar components of A. japonicus. The key factors influencing the growth performance of A. japonicus after the addition of ASC compared to sea mud (control group, O1) were identified (Figure 6). The results revealed significant positive correlations (P < 0.05) between FBW, WGR, and SGR with crucial parameters such as VW and intestinal digestive enzymes (AMS, PEP, and LMS). Furthermore, FCR exhibited a significant negative correlation with VW and intestinal digestive enzymes (AMS, PEP, and LMS, P < 0.05).
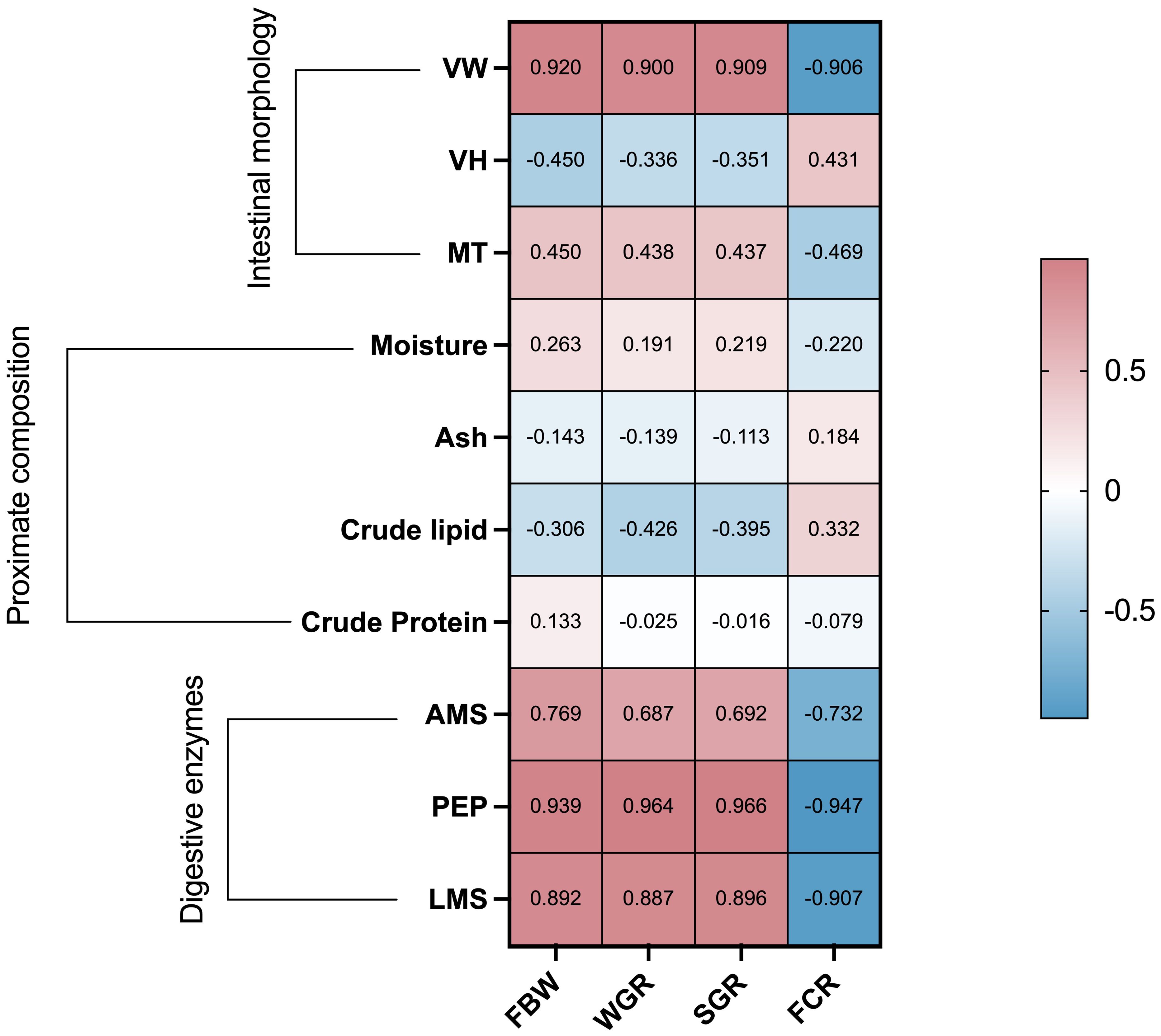
Figure 6. Correlation analysis of growth performance with digestive enzymes, intestinal shape, and proximate composition utilizing Spearman’s correlation coefficient. Colors and numbers indicate Spearman’s correlations. VW, villus width; VH, villus height; MT, muscle layer thickness; AMS, amylase; PEP, pepsin; FBW, final body weight; WGR, weight gain rate; SGR, specific growth rate; FCR, feed conversion ratio.
3.8 Gut microflora
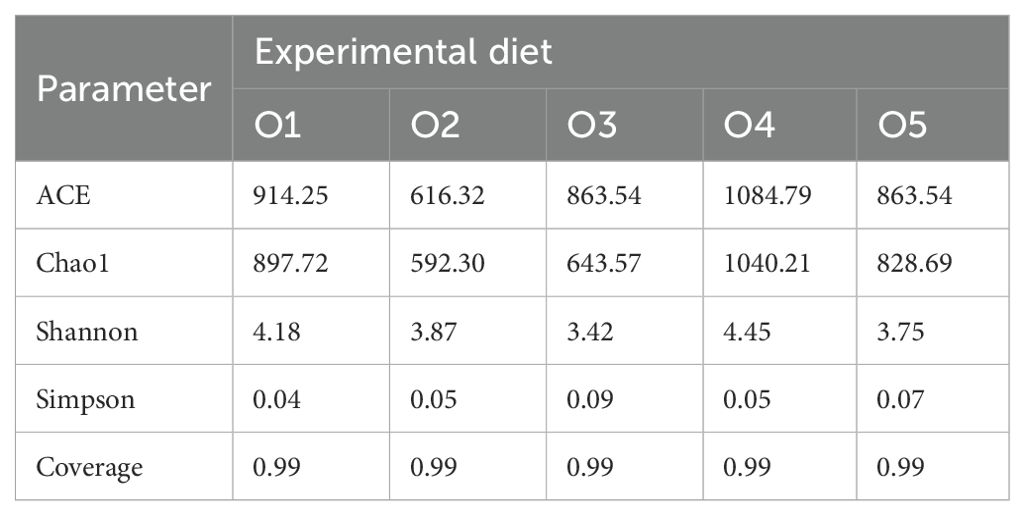
A total of 306,930 high-quality sequences (59,3002 reads per sample) were analyzed, and 1,541 OTUs were identified across 15 A. japonicus gut microbiota samples. OTUs were further categorized into 27 phyla, 57 classes, 167 orders, 276 families, 547 genera, and 800 species to provide a comprehensive taxonomic overview of the gut microbiota. Venn diagram analysis revealed a shared pool of 190 OTUs among the five experimental groups. Notably, the control group (O1) and the treatment groups O2, O3, O4, and O5 groups harbored 136, 81, 67, 230, and 85 unique OTUs, respectively (Figure 7A). The ACE, Chao1, and Shannon indices of the O4 group surpassed those of the control group, indicating increased microbial diversity in the O4 group (Table 6). Additionally, the Simpson index of the O2, O3, O4 and O5 groups were higher than that of the control group, indicating variation in community evenness between the groups.
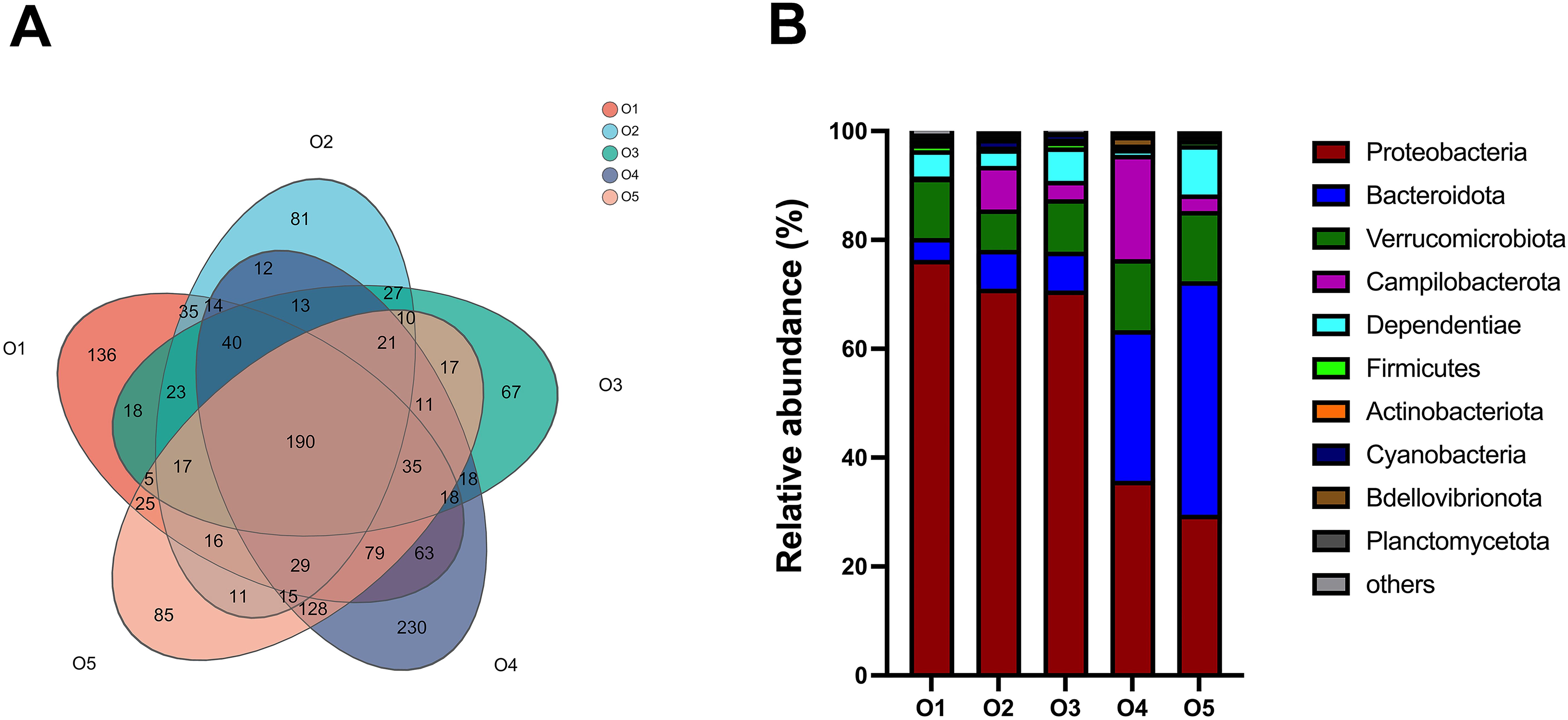
Figure 7. Effect of replacement of sea mud with dietary attachments of suspension cage (ASC) on gut microflora of Apostichopus japonicus. (A) Venn diagram of bacterial community operational taxonomic unit (OTU) distribution in A. japonicus intestines. (B) Composition and relative phylum abundance levels for the dominant microbes in Apostichopus japonicus intestine. Data represent the mean values of nine A. japonicus samples.
The intestinal flora of sea cucumbers was primarily composed of Proteobacteria, Bacteroidetes, Verrucomicrobia, Campylobacter, and other taxa. Notably, the relative abundance of Proteobacteria in the O2 (71.04%), O3 (70.71%), O4 (35.77%), and O5 (29.51%) groups exhibited a discernible decrease compared to that in the O1 group (76.34%). Conversely, the relative abundance of Bacteroidetes in the O2 (7.14%), O3 (7.14%), O4 (27.66%), and O5 (42.90%) groups was increased compared to that in the O1 group (4.00%). The proportion of Verrucomicrobia was lower in the O2 (7.43%) and O3 (9.64%) groups than in the O1 group (11.09%). Furthermore, the relative abundance of Verrucomicrobia in the O4 (13.06%) and O5 (12.91%) groups surpassed that in the O1 group (11.09%). The emergence of Campylobacter was evident in the treatment groups O2 (7.98%), O3 (3.35%), O4 (19.12%), and O5 (3.03%, Figure 7B).
4 Discussion
In China, sea mud is typically added to sea cucumber feed to accommodate their dietary requirements (Qiu et al., 2014). Previous researches showed that the primary functions of sea mud have been conclusively established, including providing microorganisms, mineral components, and specific nutrients essential for optimal sea cucumber growth (Gong et al., 2012; Qiu et al., 2014; Yuan et al., 2006). Furthermore, sea mud serves as a digestive regulator, diluting feed nutrients to facilitate their assimilation by sea cucumbers (Shi et al., 2015). In this study indicated that replacing sea mud with ASC not only stimulated sea cucumber growth but also exerted a positive influence on their intestinal flora structure. This result indicates that ASC has the same function as sea mud. However, a more in-depth investigation is still required to explore the underlying mechanisms.
The optimal proportion of sea mud in sea cucumber feed is suggested to be 20% (Rahman and Verdegem, 2007; Yuan et al., 2006), with the highest SGR observed in sea cucumbers fed with 20% sea mud or yellow mud (Liu et al., 2009). In the present study, the substitution of sea mud with ASC exhibited a significant positive impact and promoted A. japonicus growth. Notably, the most pronounced growth performance was observed with the 50% ASC replacement diet. These findings highlight the potential of using ASC exclusively as a substitute for sea mud. This finding is consistent with a previous study reporting that the use of 50% wet waste from shrimp with 50% sea mud for enhances the growth performance of sea cucumbers (Chen et al., 2015).
The genes gdf-8, c-myc, mapk-7, and fgfr-1 play crucial roles in regulating A. japonicus growth (Gao et al., 2019). Among these, gdf-8 exerts a negative regulatory effect on animal skeletal muscle growth, with higher gdf-8 expression levels observed in the body walls of A. japonicus (Aiello et al., 2018); whereas, c-myc regulates cell proliferation, differentiation, and apoptosis and promotes uncontrolled cell proliferation (Pelengaris and Khan, 2003). Moreover, ERK5, a component of the mitogen-activated protein kinase pathway which is pivotal for regulating cell function and gene expression, is closely associated with cell proliferation and differentiation. Notably, mapk-7 can directly activate the ERK5 pathway, thereby influencing sea cucumber growth (Cuenda et al., 2017). Additionally, fgfr-1 plays a role in tissue regeneration, metabolic function, and repair (Orntiz and Itoh, 2015). In the present study, expression of gdf-8 was significantly lower in the O3 group than in the O1 group; whereas, the expression levels of mapk-7, c-myc and fgfr-1 were significantly higher in the O3 group than in the O1 group. These findings suggested that ASC addition to feed inhibited the negative regulation of gdf-8 and promoted expression of mapk-7, c-myc and fgfr-1. Collectively, these results suggested that substituting sea mud in feed with ASC may enhance the sea cucumber growth performance by stimulating the expression of growth-related genes.
The body walls of sea cucumbers serve as the primary edible component. A previous study highlighted the correlation between the nutrients present in the sea cucumber body wall, the type of feed, and the nutrient content (Bao et al., 2018). In the present study, gradient ASC substitutions for sea mud resulted in no significant differences in the water and ash content among the groups, maintaining a consistently stable range of 83.98–85.48%. Similarly, the ash content was stable within the range of 44.19–45.78%. Notably, the O5 group exhibited a significant increase in crude fat and crude protein contents. Lu et al. (2018) observed comparable results when using sea mud from scallop cultures and loess as replacements for sea mud, thereby affecting the general nutrient composition of sea cucumbers. Thus, our analysis of the general nutritional components of the sea cucumber body wall substantiated the notion that replacing sea mud with ASC is a viable and feasible approach.
Sea cucumbers primarily rely on nonspecific immunity to resist pathogenic microorganisms, encompassing both cells and coelomic fluid (Wang et al., 2013). The coelomic fluid of sea cucumbers can phagocytize foreign biomass and secrete nonspecific immune factors with bactericidal activity (Yang et al., 2015). Various nonspecific immune enzymes, such as ACP, AKP, SOD, T-AOC, LZM, and CAT, play pivotal roles in maintaining antioxidant functions and eliminating harmful pathogens (Zhang et al., 2021). In this study, the O5 group exhibited a significant increase in the activities of antioxidant enzymes. Notably, the results of our study are consistent with the reported antioxidative and anti-aging effects of oyster shell extract on the skin (Liu et al., 2006; Ma, 2008). Therefore, substituting sea mud with ASC exhibited a positive effect on the nonspecific immunity and antioxidant capacity of sea cucumbers.
The activity of intestinal digestive enzymes largely reflects the digestion and absorption efficiency of animals and affects their growth and development (Wu et al., 2015). Intestinal digestive enzyme activity is a significant indicator of animal digestion and absorption efficiency, exerting a profound influence on overall growth and development. The digestive enzyme activity of sea cucumbers is affected by many factors such as food composition, culture temperature, water quality, and intestinal flora (Gao et al., 2011; Wang et al., 2017). In the present study, a noteworthy observation was the significant increase in the activities of proteases, lipases, and amylases in the sea cucumber intestine corresponding to an increase in the substitution ratio of ASC. We speculate that the direct stimulation of the intestine by ASC fragments may contribute to increase the area of microbial attachment in the intestine. This finding is consistent with a previous study reporting that the use of sea mud from scallop cultures and loess as replacements for sea mud (Lu et al., 2018). Moreover, the shift in sea cucumber intestinal flora is likely a potential mechanism driving increased digestive enzyme activity in the intestine (Yuan et al., 2006).
Sea cucumbers predominantly rely on rich bacterial populations in the esophagus and intestines, along with bacteria ingested through organic debris, to effectively disrupt their diet (Fenchel and Blackburn, 1979). These microorganisms play a pivotal role in maintaining host health (Hou et al., 2018; Xie et al., 2016). Certain probiotics can adhere to and colonize the intestine, subsequently enhancing intestinal health and contributing to growth, immunity, and disease resistance (Gatesoupe, 1999; Li et al., 2019; Liu et al., 2024; Nayak, 2010; Yan et al., 2014; Zhao et al., 2012). The findings revealed that the substitution of sea mud with ASC led to increased diversity in A. japonicus intestinal flora. This substitution reduced the relative abundance of Proteobacteria and increased that of Firmicutes, with Firmicutes dominating the O5 group. As demonstrated previously (Foysal et al., 2020; Li et al., 2020), an increase in certain Firmicutes can enhance host immunity. Consequently, the incorporation of ASC into feed not only enhanced the diversity of intestinal flora but also optimized the overall structure of the intestinal microbiota. This alteration in the intestinal microbiota exerted a subsequent influence on the intestinal morphology of sea cucumbers.
The intestine is the primary site food digestion and nutrient absorption in sea cucumbers, and their growth status is closely linked to intestinal health (Yang et al., 2015). The most objective manifestation of intestinal health is intestinal morphology. Changes in the intestinal VH and VW are directly correlated with the size of intestinal absorption area, making these parameters valuable indicators of the digestive ability of an animal (Safari et al., 2014). Furthermore, an increase in intestinal muscle wall thickness can strengthen intestinal peristalsis and bolster the overall digestive capacity. Consequently, the assessment of intestinal VH, VW, and MW is a reliable method for gauging the digestion and absorption abilities of animals. In this study, the substitution of sea mud with ASC in feed resulted in a significant increase in the intestinal VH and MT of sea cucumbers, reaching peak values in the O3 and O4 group. These findings effectively demonstrated that replacing sea mud in the feed with ASC led to an enhanced intestinal absorption area for sea cucumbers, thus improving intestinal morphology. Intestinal flora can enhance the intestinal mucosa by regulating gene expression, and many beneficial bacteria in the intestine can improve intestinal morphology and promote overall health (Hess and Greenberg, 2012; Cheng et al., 2011). Consistent with these insights, the results of the present study indicated that ASC increased the prevalence of beneficial bacteria, which, in turn, maintained intestinal health by optimizing the intestinal flora structure, thereby fostering continued sea cucumber growth.
In the present study, a correlation analysis was conducted to identify the key factors influencing the growth performance of sea cucumbers fed with ASC, as compared to those fed sea mud. A significant positive correlation was observed between growth performance indicators (FBW, WGR, and SGR) and various factors, including intestinal digestive enzymes (AMS, LPS, and PEP). This finding aligns with the results of previous studies, indicating that improvements in the digestive process and intestinal morphology can lead to changes in host growth (Cahu et al., 1998; Yu et al., 2016). However, intestinal morphology (VW) exhibited a significant negative correlated with growth indicators. These outcomes emphasize the complex and variable nature of factors influencing host growth performance and suggest that replacing sea mud in feed with ASC can improve the digestion process and intestinal morphology, thereby enhancing sea cucumber growth. However, it should be noted that the ASC used in this study was collected from the northern part of the Yellow Sea during spring, and its approximate composition may have been slightly different due to factors such as seasonal sea areas. Consequently, further comparative experiments should be conducted based on the findings of this study to assess the feasibility of ASC as a novel raw feed material for sea cucumbers.
5 Conclusion
Overall, this study has demonstrated that ASC represents a viable raw feed material for sea cucumbers. Specifically, the use of ASC promoted sea cucumber growth, reduced the feed coefficient, and positively influenced intestinal flora. According to the quadratic regression analysis for WGR, the appropriate levels of ASC substitution are estimated as to be 44.28%. Thus, the incorporation of ASC as a substitute for sea mud will significantly contribute to the development of new raw feed materials for sea cucumbers.
Data availability statement
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1153790.
Ethics statement
The animal study was approved by Animal Ethics Committee of Dalian Ocean University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
ZG: Writing – original draft, Conceptualization, Data curation, Formal analysis, Investigation. XM: Formal analysis, Investigation, Writing – original draft. YZ: Formal analysis, Investigation, Writing – original draft. DW: Investigation, Writing – original draft. RZ: Formal analysis, Writing – original draft. XZ: Funding acquisition, Project administration, Writing – review & editing. TR: Funding acquisition, Project administration, Writing – review & editing. YH: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Key Research and Development Program of China (grant number 2022YFE0117900).
Acknowledgments
We thank Dalian Shang Pin Tang Marine Biological Co., Ltd. for the experimental materials provided for this study. We also thank Wanqi Li, Zexin Liu, and Lin Wu for their assistance with the sea cucumber sampling process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ACP, acid phosphatase; AKP, alkaline phosphatase; AMS, amylase; ASC, attachments of suspension cage; CAT, catalase; FBW, final body weight; O1, control group comprising sea mud with no ASC; O2, 25% ASC; O3, 50% ASC; O4, 75% ASC; O5, 100% ASC; FCR, feed conversion ratio; IBW, initial body weight; LPS, lipase; LZM, lysozyme; MT, muscle layer thickness; PEP, pepsin; SGR, specific growth rate; T-AOC, total antioxidant capacity; T-SOD, total superoxide dismutase; VH, villus height; VW, villus width; WGR, weight gain rate.
References
Aiello D., Patel K., Lasagna E. (2018). The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim. Genet. 49, 505–519. doi: 10.1111/age.12696
AOAC. (1990). Association of Official Analytical Chemists: Official Methods of Analysis. (Arlington, VA, USA: Association of Official Analytical Chemists International).
Bao P., Li. X., Xu Y. (2018). An evaluation on the ratio of plant to animal protein in the diet of juvenile sea cucumber (Apostichopus japonicus): Growth, nutrient digestibility and nonspecific immunity. J. Ocean Univ. China 17, 479–1486. doi: 10.1007/s11802-018-3725-1
Cahu C. L., Zambonino Infante J. L. Z., Péres A., Quazuguel P., Le Gall M. M. (1998). Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: Effect on digestive enzymes. Aquaculture 161, 479–489. doi: 10.1016/S0044-8486(97)00295-0
Chen Y., Hu C., Ren C. (2015). Application of wet waste from shrimp (Litopenaeus vannamei) with or without sea mud to feeding sea cucumber (Stichpous monotuberculatus). J. Ocean Univ. China 14, 114–120. doi: 10.1007/s11802-015-2348-z
Cheng Z., Buentello A., Gatlin D. M. 3rd (2011). Dietary nucleotides influence immune responses and intestinal morphology of red drum Sciaenops ocellatus. Fish& Shellfish Immunol. 30, 143–147. doi: 10.1016/j.fsi.2010.09.019
Cuenda A., Lizcano J. M., Lozano J. (2017). Editorial: mitogen activated protein kinases. Front. Cell Dev. Biol. 5. doi: 10.3389/fcell.2017.00080
Foysal J., Alam M., Kawser R., Hasan H., Rahman M., Tay C. Y., et al. (2020). Meta-omits technologies reveals beneficiary effects of Lactobacillus plantarum as dietary supplements on gut microbiota, immune response and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 520, 734974. doi: 10.1016/j.aquaculture.2020.734974
Gao Q.-F., Wang Y., Dong S., Sun Z., Wang F. (2011). Absorption of different food sources by sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea): Evidence from carbon stable isotope. Aquaculture 319, 272–276. doi: 10.1016/j.aquaculture.2011.06.051
Gao K., Wang Z., Qiu X., Song J., Wang H., Zhao C., et al. (2019). Transcriptome analysis of body wall reveals growth difference between the largest and smallest individuals in the pure and hybrid populations of Apostichopus japonicus. Comp. Biochem. Physiol. Part D: Genomics Proteomics 31, 100591. doi: 10.1016/j.cbd.2019.05.001
Gatesoupe F. J. (1999). The use of probiotics in aquaculture. Aquaculture 180, 147–165. doi: 10.1016/S0044-8486(99)00187-8
Gong K., Wang B. J., Liu M., Jiang K. Y., Sun S. J., Qiu C. W., et al. (2012). A preliminary study on the estimation of dietary sea mud in sea cucumber (Apostichopus japonicus). Feed Industry 33, 38–41.
Han Q., Keesing J. K., Liu D. (2016). A review of sea cucumber aquaculture, ranching, and stock enhancement in China. Rev. Fisheries Science& Aquaculture 24, 326–341. doi: 10.1080/23308249.2016.1193472
Hess J. R., Greenberg N. A. (2012). The role of nucleotides in the immune and gastrointestinal systems: Potential clinical applications. Nutr. Clin. Pract. 27, 281–294. doi: 10.1177/0884533611434933
Hou D., Huang Z., Zeng S., Liu J., Wei D., Deng X., et al. (2018). Intestinal bacterial signatures of white feces syndrome in shrimp. Appl. Microbiol. Biotechnol. 102, 3701–3709. doi: 10.1007/s00253-018-8855-2
Lu J-j., Chunlong Z., Ran G., Zhaojin C., Hui X. (2018). Effects of dietary sea mud from coastal mudflat and scallop culture and loess on growth, digestion and immune indicators of sea cucumber Apostichopus japonicus. J. Dalian Ocean Univ. 33, 14–18. doi: 10.16535/j.cnki.dlhyxb.2018.01.003
Li X., Ringø E., Hoseinifar S. H., Lauzon H. L., Birkbeck H., Yang D. (2019). The adherence and colonization of microorganisms in fish gastrointestinal tract. Rev. Aquaculture 11, 603–618. doi: 10.1111/raq.12248
Li Z., Xue X., Yang H., Liao M., Han Y., Jiang Z., et al. (2020). Effect of dietary carbohydrate levels on growth performance, non-specific immune enzymes and acute response to low salinity and high temperature of juvenile sea cucumber Apostichopus japonicus. Aquaculture Nutr. 26, 683–692. doi: 10.1111/anu.13027
Liu L., Liu Y., Qin G., Wei C., Li Y., Cui L., et al. (2024). Evaluation of regulatory capacity of three lactic acid bacteria on the growth performance, non-specific immunity, and intestinal microbiota of the sea cucumber Apostichopus japonicus. Aquaculture 740156. doi: 10.1016/j.aquaculture.2023.740156
Liu Y., Dong S., Tian X., Wang F., Gao Q. (2009). Effects of dietary sea mud and yellow soil on growth and energy budget of the sea cucumber Apostichopus japonicus (Selenka). Aquaculture 286, 266–270. doi: 10.1016/j.aquaculture.2008.09.029
Liu Y. C., Natsui N., Hasegawa Y. (2006). Promotion of lypolysis activity in mouse C3H10T1/2 adipocyte cells by components from scallop shells. Fisheries Sci. 72, 702–704. doi: 10.1111/j.1444-2906.2006.01204.x
Ma J. H. (2008). New active organic substance in oyster shell capable of scavening oxygen free radicals with high efficiency. Chem. Res. Chin. Universities 24, 171–174. doi: 10.1016/S1005-9040(08)60035-5
Nayak S. K. (2010). Probiotics and immunity: A fish perspective. Fish& Shellfish Immunol. 29, 2–14. doi: 10.1016/j.fsi.2010.02.017
Orntiz D. M., Itoh N. (2015). The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip. Reviews-Developmental Biol. 4, 215–266. doi: 10.1002/wdev.176
Pelengaris S., Khan M. (2003). The many faces of c-MYC. Arch. Biochem. Biophysics 416, 129–136. doi: 10.1016/s0003-9861(03)00294-7
Qiu T., Zhang L., Zhang T., Yang H. (2014). Effects of mud substrate and water current on the behavioral characteristics and growth of the sea cucumber Apostichopus japonicus in the Yuehu lagoon of northern China. Aquaculture Int. 22, 423–433. doi: 10.1007/s10499-013-9650-9
Rahman M. M., Verdegem M. C. J. (2007). “Multi-species fishpond and nutrients balance,” in Fishponds in Farming Systems. Eds. van der Zijpp A. J., Verreth J. A. J., van Mensfoort M. E. F., Bosma R. H., Beveridge M. C. M. (Wageningen Academic Publisher, Place of Publication, Wageningen), 79–88.
Ru X., Feng Q., Zhang S., Liu S., Zhang L., Yang H. (2022). Eco-friendly method forrearing sea cucumber (Apostichopus japonicus) larvae. Aquaculture Res. 53, 3759–3766. doi: 10.1111/are.15880
Safari O., Shahsavani D., Paolucci M., Atash M. M. S. (2014). Single or combined effects of fructo- and mannan oligosaccharide supplements on the growth performance, nutrient digestibility, immune responses and stress resistance of juvenile narrow clawed crayfish, Astacus leptodactylus leptodactylus Eschscholtz 1823. Aquaculture 432, 192–203. doi: 10.1016/j.aquaculture.2014.05.012
Shi L. (2021). Effects of two nutritional additives on the growth, immunity and antioxidant capacity of Apostichopus japonicus. Master (dissertation Dalian Ocean University). doi: 10.27821/d.cnki.gdlhy.2021.000039
Shi C., Dong S., Wang F., Gao Q., Tian X. (2015). Effects of the sizes of mud or sand particles in feed on growth and energy budgets of young sea cucumber (Apostichopus japonicus). Aquaculture 440, 6–11. doi: 10.1016/j.aquaculture.2015.01.028
Shi L., Zhao Y., Wei L., Zhai H., Ren T., Han Y. (2021). An evaluation on taurine addition in the diet of juvenile sea cucumber (Apostichopus japonicus): Growth, biochemical profiles and immunity genes expression. Aquaculture Nutr. 27, 1315–1323. doi: 10.1111/anu.13270
Wang J. H., Guo H., Zhang T.-r., Wang H., Liu B.-n., Xiao S. (2017). Growth performance and digestion improvement of juvenile sea cucumber Apostichopus japonicus fed by solid-state fermentation diet. Aquaculture Nutrition 23, 1312–1318. doi: 10.1111/anu.12506
Wang T., Sun Y., Jin L., Thacker P., Li S., Xu Y. (2013). Aj-rel and Aj-p105, two evolutionary conserved NF-κB homologues in sea cucumber (Apostichopus japonicus) and their involvement in LPS induced immunity. Fish& Shellfish Immunol. 34, 71–22. doi: 10.1016/j.fsi.2012.09.006
Wu B., Xia S., Rahman M. M., Rajkumar M., Fu Z., Tan J., et al. (2015). Substituting seaweed with corn leaf in diet of sea cucumber (Apostichopus japonicus): Effects on growth, feed conversion ratio and feed digestibility. Aquaculture 444, 88–92. doi: 10.1016/j.aquaculture.2015.03.026
Xie X., Zhang L., Liu S., Zhang T., Yang H. (2016). Aerated sea mud is beneficial for post-nursery culture of early juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture Int. 24, 211–224. doi: 10.1007/s10499-015-9920-9
Yan F. J., Tian X., Dong S., Fang Z., Yang G. (2014). Growth performance, immune response, and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus fed a supplementary diet of the potential probiotic Paracoccus marcusii DB11. Aquaculture. 420–421, 105–111. doi: 10.1016/j.aquaculture.2013.10.045
Yang G., Tian X., Dong S., Peng M., Wang D. (2015). Effects of dietary Bacillus cereus G19, B. cereus BC-01, and Paracoccus marcusii DB11 supplementation on the growth, immune response, and expression of immune-related genes in coelomocytes and intestine of the sea cucumber (Apostichopus japonicus Selenka). Fish& Shellfish Immunol. 45, 800–807. doi: 10.1016/j.fsi.2015.05.032
Yu H., Gao Q., Dong S., Lan Y., Ye Z., Wen B. (2016). Regulation of dietary glutamine on the growth, intestinal function, immunity and antioxidant capacity of sea cucumber Apostichopus japonicus (Selenka). Fish& Shellfish Immunol. 50, 56–65. doi: 10.1016/j.fsi.2016.01.024
Yuan X., Yang H., Zhou Y., Mao Y., Zhang T., Liu Y. (2006). The influence of diets containing dried bivalve feces and/or powdered algae on growth and energy distribution in sea cucumber Apostichopus japonicus (Selenka) (Echinodermata: Holothuroidea). Aquaculture 256, 457–467. doi: 10.1016/j.aquaculture.2006.01.029
Zeng F., Wu L., Ren X., Xu B., Cui S., Li M., et al. (2021). ). Effects of chronic prometryn exposure on antioxidative status, intestinal morphology, and microbiota in sea cucumber (Apostichopus japonicus). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 250, 109187. doi: 10.1016/j.cbpc.2021.109187
Zhang W., Li C., Guo M. (2021). Use of ecofriendly alternatives for the control of bacterial infection in aquaculture of sea cucumber Apostichopus japonicus. Aquaculture 545, 737185. doi: 10.1016/j.aquaculture.2021.737185
Zhao Y., Zhang W., Xu W., Mai K., Zhang Y., Liufu Z. (2012). Effects of potential probiotic Bacillus subtilis T13 on growth, immunity and disease resistance against Vibrio splendidus infection in juvenile sea cucumber Apostichopus japonicus. Fish& Shellfish Immunol. 32, 750–755. doi: 10.1016/j.fsi.2012.01.027
Keywords: Apostichopus japonicus, digestive enzyme, attachments suspension cage, intestinal flora, intestinal structure
Citation: Guo Z, Meng X, Zhang Y, Wu D, Zhang R, Zhao X, Ren T and Han Y (2024) Replacing sea mud with attachment of suspension cage can improve growth and gut health for sea cucumber Apostichopus japonicus. Front. Mar. Sci. 11:1452166. doi: 10.3389/fmars.2024.1452166
Received: 20 June 2024; Accepted: 19 August 2024;
Published: 02 October 2024.
Edited by:
Kwaku Amoah, Guangdong Ocean University, ChinaReviewed by:
Bin Yin, Zhuhai Modern Agriculture Development Center, ChinaSahya Maulu, Centre for Innovative Approach Zambia (CIAZ), Zambia
Copyright © 2024 Guo, Meng, Zhang, Wu, Zhang, Zhao, Ren and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuzhe Han, aHl6QGRsb3UuZWR1LmNu
 Zhixu Guo1,2
Zhixu Guo1,2 Yuzhe Han
Yuzhe Han