
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci., 31 July 2024
Sec. Discoveries
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1451808
This article is part of the Research TopicNew Observations on the Behavior, Ecology, and Biology of Sharks and RaysView all 18 articles
The white shark, Carcharodon carcharias, is an iconic apex predator, playing an important ecological role across its range. Persistent bycatch and overfishing led to white shark declines, but recent studies in the North Western Atlantic (NWA) revealed evidence for regional recovery, and highlighted the importance of Southeastern Florida and the Gulf of Mexico as overwintering grounds for maturing white sharks. However, despite its proximity to Florida and comparably productive habitats, records of white sharks in The Bahamas are extremely rare, with a comprehensive survey of sightings and captures describing only one white shark between 1800 - 2010. Here, we reveal acoustic tracking detections of ten white sharks from 2020 - 2024 along the western edge of the Tongue of the Ocean off Central Andros Island, The Bahamas. White sharks were originally tagged off the coast of the United States and Canada, and detected off Andros Island, The Bahamas from November-May. White sharks were detected along the drop-off zone of the reef at ca. 25 m, exclusively between dusk and dawn, with the number of detections suggesting transient behavior. These findings expand our knowledge of white shark distribution in the NWA, highlighting data gaps in The Bahamas and underlining the importance of collaborative protective measures for species recovery.
The white shark Carcharodon carcharias is a large-bodied, wide-ranging species with broadly distributed populations in temperate and subtropical waters worldwide (Compagno, 2001). As upper-trophic-level predators, they play an important ecological role (Carrier et al., 2010), primarily feeding on small sharks and rays, squid, and benthic fishes as small juveniles (< 2.5 m, Estrada et al., 2006; Clark et al., 2023) and incorporating marine mammals into their diet as they grow (Tricas and McCosker, 1984; Hussey et al., 2012). Large juvenile, subadult, and adult white sharks seasonally aggregate near pinniped colonies often when water temperatures correspond to their preferred range (Klimley et al., 2001; Bruce and Bradford, 2015; Hewitt et al., 2018; Kock et al., 2022; Winton et al., 2023). Much of our understanding of white shark movement ecology comes from studies where animals are tracked with acoustic or satellite tags at these sites (e.g., Neptune Islands, South Australia; Guadalupe Island, Mexico; California and Cape Cod, U.S.A), which have documented philopatric behaviors, such as high site fidelity, seasonal residency as well as long-distance return migrations (Bonfil et al., 2005; Jorgensen et al., 2010; Bruce and Bradford, 2015; Skomal et al., 2017; Huveneers et al., 2018; Bastien et al., 2020).
White sharks also occur in the tropics (Tirard et al., 2010), but information on their distribution and occurrence in these regions are limited, particularly for populations that experienced dramatic declines, including the population in the North Western Atlantic (NWA; Curtis et al., 2014). White sharks were never targeted commercially in the region, but bycatch and capture in commercial and recreational fisheries were sufficient to reduce abundance by as much as 73% in the 1970s and 1980s (Curtis et al., 2014). Consequently, harvest was prohibited in the US Atlantic in 1997 (NMFS 1997). As a result of this protective measure, alongside other international measures (i.e., in 2002 they were listed on Appendix I and II of the Convention on the Conservation of Migratory Species of Wild Animals (CMS), in 2004 they were added to the Convention on International Trade in Endangered Species (CITES) Appendix II), the NWA population appears to be recovering (Curtis et al., 2014; Rigby et al., 2019).
Despite these measures and evidence of recovery, only recently have we begun to learn about their horizontal and vertical space use in the NWA, through various biotelemetry studies (Skomal et al., 2017; Bastien et al., 2020; Lowerre-Barbieri et al., 2021; Franks et al., 2021; Winton et al., 2021; Bowlby et al., 2022). Adult white sharks have been shown to exhibit an ontogenetic shift in their space use from near-coastal, shelf-oriented waters to pelagic habitat, with frequent excursions to mesopelagic depths (Skomal et al., 2017). White sharks migrate to the southeast shelf waters of North Carolina to Florida during the late fall when water temperatures generally drop below 12°C in the NWA (Casey and Pratt, 1985; Curtis et al., 2014; Skomal et al., 2017; Bowlby et al., 2022), with some individuals traveling as far as the Gulf of Mexico in winter and early spring (Skomal et al., 2017; Franks et al., 2021). They show strong site fidelity to the NWA, returning seasonally to specific regions (e.g., Massachusetts and Atlantic Canada), with most white sharks exhibiting an annual migratory cycle, spending the majority of their time over the continental shelf (Franks et al., 2021; Bowlby et al., 2022). Movements between regions are rapid and directed, with white sharks exhibiting no stop-over behavior, which is common for other large coastal-pelagic sharks and fishes (Skomal et al., 2017; Lowerre-Barbieri et al., 2021; Franks et al., 2021). More recently, there has been an increase in white shark sightings and detections via acoustic tracking data in the Canadian Atlantic (Bastien et al., 2020; Bowlby et al., 2022). This trend matches reports from Massachusetts and the U.S. north Atlantic (Curtis et al., 2014) and is probably a result of regional trends in recovery of the white shark population, indicative of a return to their former range where they have long been rare or absent (Winton et al., 2023). However, it is likely that other factors have played a role, including climate change, increasing prey abundance (e.g., due to pinniped recovery), and/or insufficient sampling and monitoring preventing their documentation in certain parts of their range (Bowlby et al., 2022).
Despite annual overwintering in Florida’s Gulf and Atlantic shelf waters, records of white sharks in nearby Bahamian waters are very rare. In their catalog of elasmobranch fishes in the region, Bigelow and Schroeder (1948) included one record of a “positively identified capture of a great white shark in the vicinity of the Bahamas near Nassau”. More recently, Curtis et al. (2014) compiled comprehensive capture and sightings records from the NWA from the years 1800–2010; of those 649 verified records, only one white shark was documented in Bahamian waters. This shark, estimated at ~ 4 m total length, washed ashore on the west coast of Grand Bahama, 6th June 2008. Here we report the first records of white sharks in the central Bahamas using shelf edge habitat along the reef wall of the Tongue of the Ocean (TOTO), in an area adjacent to the northern bight of eastern Andros Island. We discuss the implications of these new records within the context of the species recent recovery in the NWA, and consider whether these are a result of species’ recovery, inability to observe them due to inadequate sampling and monitoring, or a combination of the above.
The Bahamas are a group of about 700 islands and cays in the NWA. The Atlantic basin extends within the archipelago, forming a semi-enclosed deep-sea trench (40 km wide, 200 km long) known as the Tongue of the Ocean (TOTO), which is bordered by Andros Island to the west and Exuma cays to the east. An extensive fringing reef runs along the western edge of the TOTO, quickly dropping to a deep pelagic zone (~1500 – 3000 m depth) with gullied slopes beyond 400 m of the shelf (Buchan, 2000). The three main islands of Andros (North, Mangrove Cay and South) are separated by bights, large shallow channels 1 - 5 m deep and 3 - 6 km wide that trifurcate the island from east to west.
Omnidirectional acoustic receivers (VR2W; Innovasea systems®) were initially deployed in 2019 (n = 9) to monitor the space use of sharks and rays on the flats (depth: 3 m) and backreef (depth: 12 m) of the north bight of Andros Island (Figure 1). Coverage expanded annually to include the reef wall (depth: 25 m) from High Cay (24.6482°, -77.6894°) to Driggs Hill (24.2317°, -77.59986°) as well as the deep pelagic zone (depth: 300 to 500 m) adjacent the AUTEC U.S. Navy base outside of Cargill Creek (24.4862°, -77.7125°) (Table 1). The receivers were retrieved for data download and returned to the recording location in the same 24 h period every 6 - 12 months for 5 years. All receivers were either moored to augers, screwed into soft sediment, or secured to a line and float weighted by an 18 kg concrete-filled cone. Data were filtered to remove spurious detections, which were defined as any single transmitter detection occurring alone within a 24 h period. All other detections were treated as genuine and were assigned a tidal phase (low, high or mid; semi-diurnal) and a lunar illumination percentage (0 = new; 100% = full). High and low tide times were obtained from NOAA (National Oceanic and Atmospheric Administration; Fresh Creek TEC4613) and detections were assigned phases defined by: high and low tide = 2 h either side of high and low tide times, respectively, and mid-tide = the 2 h in the middle. Lunar illumination percentage was obtained from the U.S. Astronomical Applications Department (https://aa.usno.navy.mil). Participation in a data-sharing network (Ocean Tracking Network, OTN) allowed us to detect tagged animals. We calculated minimum straight-line distance between tagging location and detecting receivers to determine individual white shark movements in the region. Furthermore, we included additional information on individuals that were recorded in other arrays pre-and-post their detections in Andros.
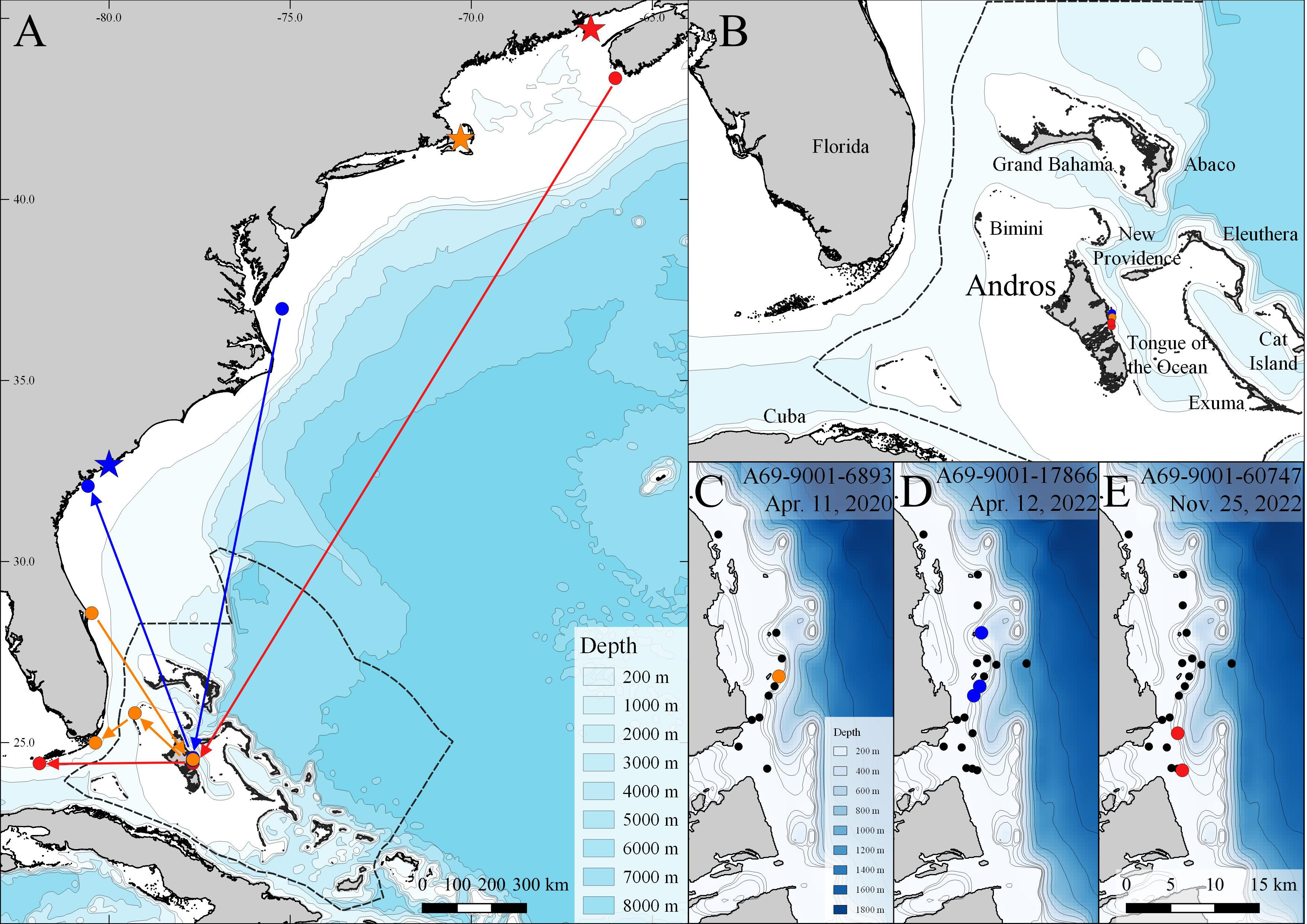
Figure 1 Acoustic detections highlighting regional movements of three (n = 3) white sharks, Carcharodon carcharias pre-and-post movement to Andros Island, The Bahamas. Colored stars, circles, and arrows represent different individuals (orange, blue, red). Stars indicate tagging locations (see Table 1) and colored circles in region-wide map (A) indicate detection locations in Andros Island, and the last detection of each individual prior to and after detection off Andros Island. Arrows show least cost paths based on detection sequences but do not represent travel routes based on variation in the timing of detections prior and subsequent to detections off the Andros Island array (B). Colored circles in Andros-specific maps (C–E) indicate the detection locations for each individual; black dots indicate locations of acoustic receivers where white sharks were not detected.
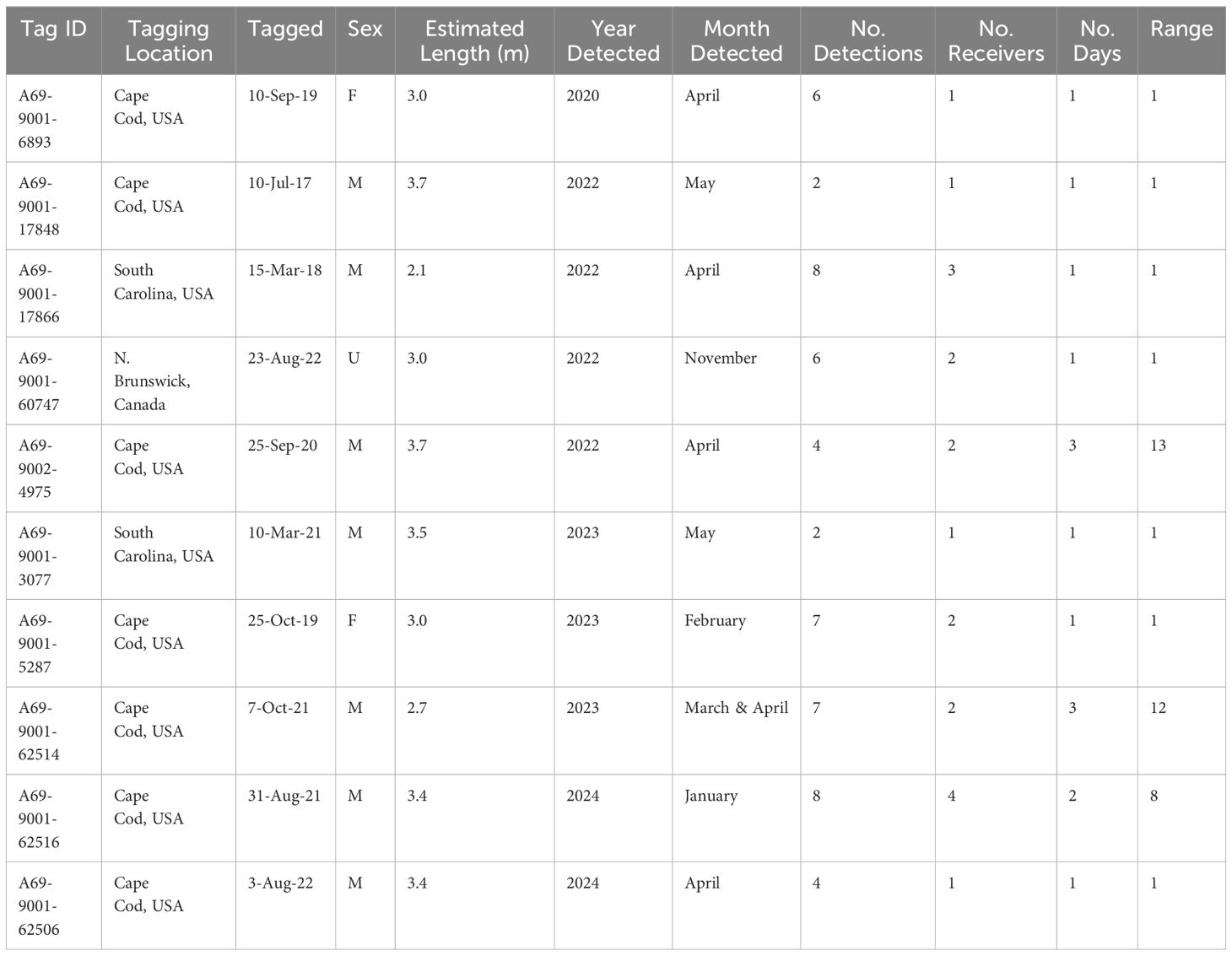
Table 1 Acoustic detection summary for ten white sharks, Carcharodon carcharias, recorded in Andros Island, The Bahamas, from 2020 - 2024.
White sharks (n = 331) were equipped with acoustic transmitters (V16-4x, V16-6x, or V16TP-6x; power output 158 dB; Innovasea Systems®) during dedicated research trips from 2010 to 2023 (for details see Skomal et al., 2017; Winton et al., 2023). These were conducted throughout the year off the east coast of North America from Jacksonville, U.S., to New Brunswick, Canada. Transmitters deployed on the white sharks included in this study all had random transmission intervals of 60 - 120s (estimated battery life 3321 days) and were tagged externally using a tagging pole (Table 1). At the time of tagging, the total length (TL) of each shark was visually estimated to the nearest 0.3 m via expert consensus using the vessel pulpit length (3.2 m) for scale (Skomal et al., 2017). Underwater video of each shark was also captured allowing for sex determination (Winton et al., 2023). All tagging was conducted under Exempted Fishing Permits (SHK-EFP-11-04, SHK-EFP-12-08, SHK-EFP-13-01, SHK-EFP-14-03) issued by the NMFS Highly Migratory Species Management Division and permits issued by the Massachusetts Division of Marine Fisheries.
Ten white sharks (size range at tagging = 2.1 - 3.7 m [estimate total length]; 2 females, 7 males, 1 unknown sex) were detected from 2020 to 2024 on acoustic receivers deployed along a 27 km stretch of fringing reef wall (Green Cay to Gibson Cay; depth 25 m) off the east coast of Andros Island (Figures 2, 3; Table 1). Detected white sharks were tagged at two sites in the U.S., Cape Cod, Massachusetts (n = 7), and Hilton Head, South Carolina (n = 2), with one individual tagged off the coast of New Brunswick, Canada. Their time at liberty since tagging ranged from 5 months to 6 years at the time of detection off Andros Island (Table 1).
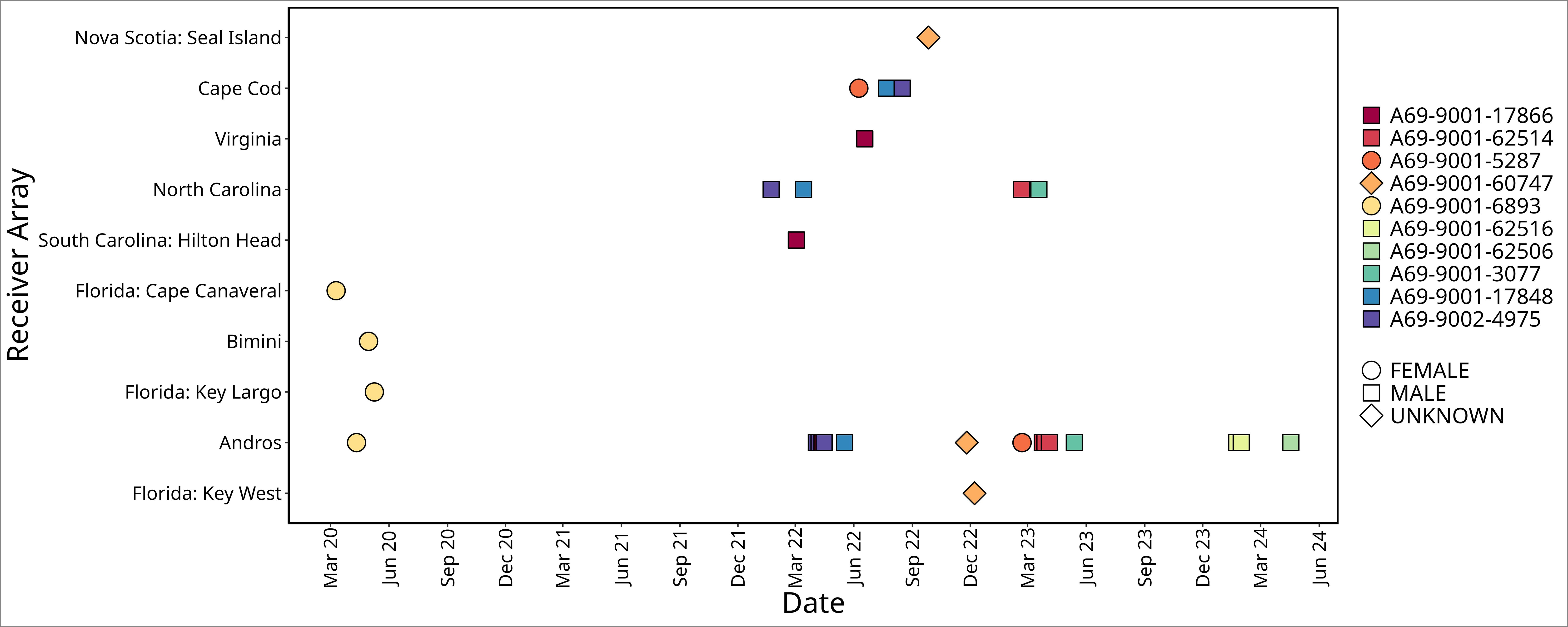
Figure 2 Time series of acoustic detections for all ten white sharks, Carcharodon carcharias, prior to, during, and after detections on Andros Island receivers. Detection data from other receiver arrays, ordered by latitude, were provided via the Ocean Tracking Network. Point shape denotes sex; point fill color denotes animal ID.
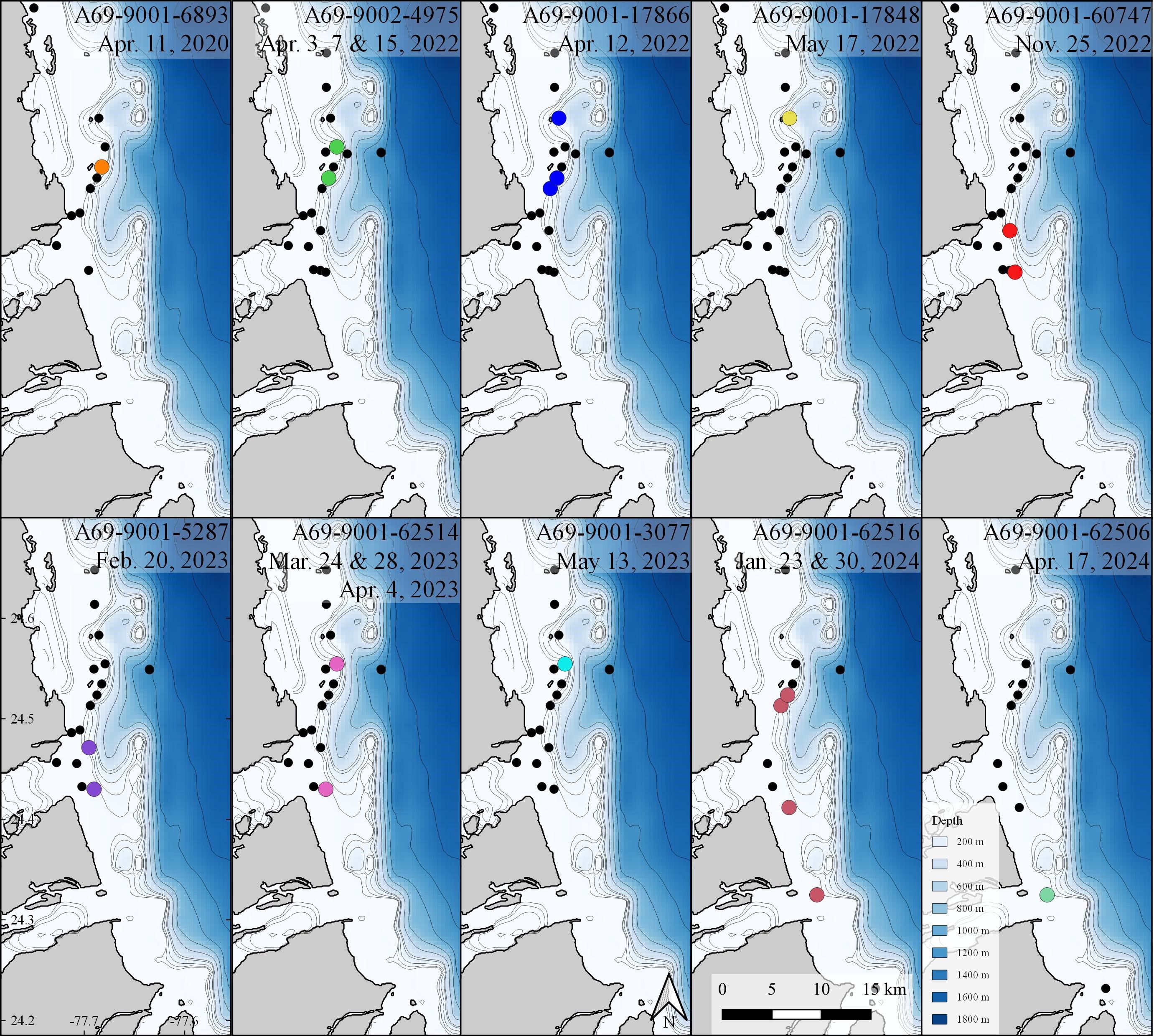
Figure 3 Acoustic detections of all white sharks (n=10), Carcharodon carcharias off Andros Island, The Bahamas. Black dots indicate locations of acoustic receivers where white sharks were not detected.
No sharks were detected in Andros on receivers < 25 m deep, nor at deepwater receivers (300 - 500 m offshore of fringing reef wall; Figures 2, 3). Detections were recorded between November 25 and May 17 during all sampling years, with five white sharks detected in April and two in May. Seven of the ten sharks were detected on a single day on 1 - 4 of the receivers (Table 1). Three sharks were detected on receivers multiple days: A69-9002-4975 was detected across 13 days (3 to 15 April 2022), A69-9001-62506 was detected across 8 days (23 to 30 January 2024), and A69-9001-62514 was detected across 12 days (28 March to 4 April 2023) (Figures 2, 3). All white shark detections were between 20:00 and 07:00, across different lunar phases (range: 0 – 98% lunar illumination; median 54.5%) and throughout the tidal cycle (high 13%, low 41%, and mid-tide 46%).
White shark A69-9001-60747 was tagged on 26 September 2022 off New Brunswick, Canada, and was detected 60 days later off Andros Island on 25 November 2022. This represents a minimum southern movement of ~ 2300 km (38 km per day). This shark was subsequently detected 12 days later off the Florida Keys on 7 December 2022, after another ~ 490 km movement (45 km per day) (Figure 1). Female white shark A69-9001-6893 was tagged on 10 September 2019 off Cape Cod, MA, U.S., and was detected on 9 March 2020 off Cape Canaveral, FL, U.S., a movement of ~ 1700 km. Next, she was detected off Andros on 11 April 2020, a southern movement of ~ 520 km, and then off North Bimini, Bahamas, 18 days later on 29 April 2020, a further ~ 200 km. Finally, she was detected on 8 May 2020 off Key Largo, FL, U.S. after crossing the Gulf Stream, totaling a minimum travel distance of 900 km in 60 days (Figure 1). Male white shark A69-9001-17866 was detected on 2 March 2022 off Savannah, GA, U.S. and detected off Andros 40 days later on 12 April 2022. He was then detected on 18 June 2022 off Virginia Beach, VA, U.S. ~ 65 days later, after a northern movement of ~ 1400 km (Figure 1).
This study provides the first evidence of white sharks using waters in the Tongue of the Ocean (TOTO), Andros Island, The Bahamas. Across the last 70 years, only two records exist of the species in this region of the NWA, with none in the central Bahamas. Our acoustic detections of ten white sharks of varying size and sex along the TOTO near-shore shelf across four years (2020-2024) represent an important finding that expands the current known distribution of white sharks in this part of the world. This also broadens our understanding of elasmobranch diversity in The Bahamas, which is a shark sanctuary where all sharks are protected through national legislation, and supports previously published and anecdotal evidence of white sharks in Bahamian waters.
White shark detections in our array were across short-time periods (1 to 13 days) during the late fall, winter, and early spring. They were recorded in other arrays (e.g., A69-9001-60747 in FL Keys and A69-9001-6893 in Bimini) soon after being detected in Andros (12 and 18 days), suggesting transitory-type behavior to/through the TOTO. Other tracking studies also found that white sharks exhibited rapid migrations in winter and spring along near-shore shelf waters of the southeastern U.S. Atlantic and Gulf of Mexico (GOM) (Skomal et al., 2017; Lowerre-Barbieri et al., 2021). Franks et al. (2021) found that nearly 40% (14 of 36) of the sharks tracked between 2013 and 2019 spent time in waters around the Florida Keys or in the GOM during the overwintering period, with nine returning to the GOM in subsequent periods. Philopatric behavior for white sharks is common across their range (Jorgensen et al., 2010; Huveneers et al., 2018), however despite four years of coverage in the TOTO, we did not detect individuals across multiple years. This, coupled with most white sharks detected in our array being larger juveniles or sub-adults (2.5 – 3.5 m), might indicate that our detections are a result of exploratory behavior exhibited by this life stage. Tagged juvenile white sharks are nearly 5 times more likely to be observed in Canadian waters than a tagged adult (Bowlby et al., 2022), which is hypothesized to be a result of younger animals dispersing more widely across suitable coastal habitats than older age classes. Consequently, immature white sharks are more likely to be detected on acoustic monitoring arrays outside of tagging areas (Bowlby et al., 2022). Given recent evidence for recovery in the NWA, our detections are also likely a result of white sharks returning to historically important parts of their range and the increased numbers of younger life stages being tagged off Cape Cod. However, it’s important to consider the limited fishery monitoring capacity in The Bahamas compared to the east coast of the US and that detections were exclusively along the drop off, which could mean white sharks have always used the habitat, but due to deepwater use and no commercial longlining, have gone undetected. It will be important to expand acoustic tracking coverage within the TOTO and broader Bahamas archipelago to determine the scope and drivers of their space and habitat use in the region.
Despite most detections in our array spanning only one or two days, three individuals were recorded across almost two weeks. Given the limited receiver coverage in the TOTO (< 47 km linear range), perhaps some white sharks partially overwinter in the area, like those in the southeastern U.S. Atlantic and GOM. White shark detections were exclusively overnight on the fore-reef edge (25 m) along the interface between the reef and deepwater habitats, where the depth rapidly increases to 40 m then to 400 m. This steep slope is ideal habitat for ambush predators like white sharks (Klimley et al., 2001; Martin et al., 2005; Hammerschlag et al., 2006). One study in Guadalupe Island, Mexico, a location notorious for steep drop-offs close to shore, observed approaching, bumping, and biting of an autonomous underwater vehicle at depths of 53 to 90 m, providing direct evidence of predatory behavior at depth (Skomal et al., 2015). Although pinnipeds, which are a key prey species across its range, do not exist in the Bahamas (but see McClenachan and Cooper, 2008 for extinction of the Caribbean monk seal Monachus tropicalis), there is an abundance of coastal sharks and rays (e.g., Caribbean reef, Carcharhinus perezi, and silky sharks Carcharhinus falciformis; Talwar et al., 2022; Shipley et al., 2023), as well as reef (e.g., Nassau grouper Epinephelus striatus; Stump et al., 2017) and pelagic fishes (e.g., dolphinfish Coryphaena hippurus; Merten et al., 2015) that are known to use this transition zone in the TOTO, and could provide an important food source for white sharks. Caribbean reef sharks making night time excursions to > 50 m during the winter months (Shipley et al., 2017; Guttridge pers. obs.) would conceivably be targets for white sharks cruising the shelf.
The TOTO is also recognized as an important habitat for a variety of cetaceans (e.g., bottlenose dolphins, Tursiops truncatus, and short-finned pilot whales, Globicephala macrorhynchus; Claridge et al., 2015), particularly Blainsville’s beaked whales, Mesoplodon densirostris, that are resident to the area (Hin et al., 2023). The presence of a U.S. Navy base, Atlantic Undersea Test and Evaluation Center (AUTEC) that regularly conducts sonar testing, has resulted in two decades of research on the distribution, abundance, and behavioral responses of cetaceans (Hazen et al., 2011; Claridge et al., 2015; Joyce et al., 2020; Benoit-Bird et al., 2020). Skomal et al. (2017) hypothesized that during their overwintering residency period, white sharks may feed on whales off the southeastern U.S. coast and in the GOM. Beaked whales and bottlenose dolphins have been identified as part of white sharks’ diet in other regions (Long and Jones, 1996; Heithaus, 2001; Celona et al., 2006), and the population of Blainville’s beaked whales in the TOTO is one of the highest densities to have been estimated (Hin et al., 2023). When examining the deep scattering layer, the western TOTO was characterized by intense, relatively thin layers compared to other parts of the region, offering high quality foraging opportunities for these whales (Hazen et al., 2011). In turn, the foraging effort of Blainville’s beaked whales matched this with 2.5x more clicks in the Western TOTO compared to the Eastern TOTO (Benoit-Bird et al., 2020), with surveys revealing a higher density of squid with higher mean mantle lengths (i.e. larger size) in this zone, leading to much more profitable foraging habitat. Thus, there is horizontal overlap between where white sharks were detected and beaked whale foraging grounds. Further, after a deep dive the whales spend an extensive period (66 – 155 min) in the upper 50 m of the water column (Baird et al., 2006; Joyce et al., 2020), which is the vertical zone white sharks spent 95% of their time when overwintering in the SE U.S. and GOM (Skomal et al., 2017). Thus, these local cetaceans, or perhaps the squid beak whales are hunting, could provide an important prey source and motivate white sharks to use the TOTO.
Despite a growing body of knowledge about the movement ecology of white sharks in the NWA, we have provided the first evidence of their presence in the western TOTO in central Bahamas. The Bahamas declared its waters a Shark Sanctuary in 2011 and has banned commercial longlining and gillnetting since 1993, thus The Bahamas is an important refuge for this iconic and vulnerable species. However, despite these new records, the spatial and temporal extent to which this species utilizes these waters remains unclear. While our findings are most likely a result of regional recovery, it is possible that white sharks exploit the productive mesopelagic zone whilst in the TOTO but have remained undetected when using this deepwater habitat because of insufficient monitoring. Clearly further research is required to learn more about white shark presence in The Bahamas including continued long-term collaborative monitoring and tagging programs which allowed our team to reveal these novel records.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because tagging was conducted under Special Permits issued to GS.
GT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. MP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. GA: Funding acquisition, Project administration, Resources, Writing – original draft, Writing – review & editing. WM: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. DS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. SG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding for this work was provided by Saving the Blue, Ocean Tracking Network, Discovery Communications, Fahlo, Lackland Foundation, Endangered Species Chocolate, ProShot, Earth Breeze, Bonnie and David Epstein, the Atlantic White Shark Conservancy, National Geographic, and the Massachusetts Division of Marine Fisheries. All funders supported either acoustic receiver acquisition, deployment and maintenance or acoustic tag acquisition or deployment.
We thank Saving the Blue board of Directors, Advisors, Science Committee and team for continued support, guidance, and expertise. Special mention goes to Sarontaa Bain, Gabby Lozada and Captain Cole McVay, and the Andros Island Bonefish Club in Cargill Creek. We thank Saving the Blue volunteers for joining us on expeditions to Andros and for assisting with acoustic receiver maintenance. We thank spotter pilot Wayne Davis and Captain John King, Brian Hanson, Ken Johnson, Cynthia Wigren, and other crew members of the F/V Aleutian Dream for their help tagging white sharks off Cape Cod, MA; H. Bowlby and W. Joyce of the Canadian Atlantic Shark Research Laboratory of Fisheries and Oceans Canada and Captain Dale Mitchell and the crew of the F/V Groundskeeper for tagging work conducted off New Brunswick, Canada; and Captain Chip Michalove of Outcast Sport Fishing for his tagging efforts off South Carolina. We also thank Ashleigh Novak and Victoria Migneco for identifying and cataloging the individual white sharks tracked. We would like to thank the Ocean Tracking Network and iTAG Network for supporting receivers, and receiver array owners for white shark post-tagging detection data including Mike Arendt and Bryan Frazier of the South Carolina Department of Natural Resources. We are grateful to the Department of Environmental Planning and Protection (DEPP) particularly Dr. Rhianna Neely and Dr. Lester Gittens of the Department of Marine Resources (DMR) in The Bahamas for continued support and for issuing research permits.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1451808/full#supplementary-material
Baird R. W., Webster D. L., McSweeney D. J., Ligon A. D., Schorr G. S., Barlow J. (2006). Diving behaviour of Cuvier’s (Ziphius cavirostris) and Blainville’s (Mesoplodon densirostris) beaked whales in Hawai ‘i. Can. J. Zool. 84, 1120–1128. doi: 10.1139/z06-09
Bastien G., Barkley A., Chappus J., Heath V., Popov S., Smith R., et al. (2020). Inconspicuous, recovering, or northward shift: status and management of the white shark (Carcharodon carcharias) in Atlantic Canada. Can. J. Fish. Aquat. Sci. 77, 1666–1677. doi: 10.1139/cjfas-2020-0055
Benoit-Bird K., Southall B., Moline M., Claridge D., Dunn C., Dolan K., et al. (2020). Critical threshold identified in the functional relationship between beaked whales and their prey. Mar. Ecol. Prog. Ser. 654, 1–16. doi: 10.3354/meps13521
Bonfil R., Meÿer M., Scholl M. C., Johnson R., O’Brien S., Oosthuizen H., et al. (2005). Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science 310, 100–103. doi: 10.1126/science.1114898
Bowlby H. D., Joyce W. N., Winton M. V., Coates P. J., Skomal G. B. (2022). Conservation implications of white shark (Carcharodon carcharias) behaviour at the northern extent of their range in the Northwest Atlantic. Can. J. Fish. Aquat. Sci. 79, 1843–1859. doi: 10.1139/cjfas-2021-0313
Bruce B., Bradford R. (2015). Segregation or aggregation? Sex-specific patterns in the seasonal occurrence of white sharks Carcharodon carcharias at the Neptune Islands, South Australia. J.Fish. Biol. 87, 1355–1370. doi: 10.1111/jfb.12827
Carrier J. C., Musick J. A., Heithaus M. R. (2010). Sharks and their relatives II: biodiversity, adaptive physiology, and conservation (Boca Raton, FL: CRC Press/Taylor & Francis).
Casey J. G., Pratt H. L. (1985). Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. Mem South Calif Acad Sci 9, 2–14.
Celona A., De Maddalena A., Comparetto G. (2006). Evidence of predatory attack on a bottlenose dolphin Tursiops truncatus by a great white shark Carcharodon carcharias in the Mediterranean. Ann. Ser. Hist. Nat. 16, 164.
Claridge D., Dunn C., Ylitalo G., Herman D., Durban J., Parsons K. (2015). Behavioral Ecology of Deep- diving Odontocetes in The Bahamas. SERDP Project Number: RC-2114 Bahamas Marine Mammal Research Organisation, NOAA Alaska Fisheries Science Center – Final Technical Report Vers. 2 (Washington, DC: NOAA).
Clark Z. S. R., Fish J. J., Butcher P. A., Holland O. J., Sherman C. D. H., Rizzari J., et al. (2023). Insights into the diet and trophic ecology of white sharks (Carcharodon carcharias) gained through DNA metabarcoding analyses of cloacal swabs. Environ.DNA 5, 1362–1377. doi: 10.1002/edn3.454
Compagno L. J. V. (2001). “Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Volume 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes),” in FAO Species Catalogue for Fishery Purposes (FAO, Rome).
Curtis T. H., McCandless C. T., Carlson J. K., Skomal G. B., Kohler N. E., Natanson L. J., et al. (2014). Seasonal distribution and historic trends in abundance of white sharks, Carcharodon carcharias, in the western North Atlantic Ocean. PloS One 9, e99240. doi: 10.1371/journal.pone.0099240
Estrada J. A., Rice A. N., Natanson L. J., Skomal G. B. (2006). Use of Isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecol. 87, 829–834. doi: 10.1890/0012-9658(2006)87[829:UOIAOV]2.0.CO;2
Franks B. R., Tyminski J. P., Hussey N. E., Braun C. D., Newton A. L., Thorrold S. R., et al. (2021). Spatio-temporal variability in white shark (Carcharodon carcharias) movement ecology during residency and migration phases in the Western North Atlantic. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.744202
Hammerschlag N., Martin R. A., Fallows C. (2006). Effects of environmental conditions on predator–prey interactions between white sharks (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus) at Seal Island, South Africa. Environ. Biol. Fish. 76, 341–350. doi: 10.1007/s10641-006-9038-z
Hazen E. L., Nowacek D. P., St. Laurent L., Halpin P. N., Moretti D. J. (2011). The relationship among oceanography, prey fields, and beaked whale foraging habitat in the Tongue of the Ocean. PloS One 6, p.e19269. doi: 10.1371/journal.pone.0019269
Heithaus M. R. (2001). Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies, and attack seasonality. Mar. Mam. Sci. 17, 526–539. doi: 10.1111/j.1748-7692.2001.tb01002.x
Hewitt A. M., Kock A. A., Booth A. J., Griffiths C. L. (2018). Trends in sightings and population structure of white sharks, Carcharodon carcharias, at Seal Island, False Bay, South Africa, and the emigration of subadult female sharks approaching maturity. Environ. Biol. Fish. 101, 39–54. doi: 10.1007/s10641-017-0679-x
Hin V., De Roos A. M., Benoit-Bird K. J., Claridge D. E., DiMarzio N., Durban J. W., et al. (2023). Using individual-based bioenergetic models to predict the aggregate effects of disturbance on populations: A case study with beaked whales and Navy sonar. PloS One 18, p.e0290819. doi: 10.1371/journal.pone.0290819
Hussey N., McCann H., Cliff G., Dudley S., Wintner S., Fisk A. (2012). Size-Based Analysis of Diet and Trophic Position of the White Shark, Carcharodon carcharias, in South African Waters, in: Global Perspectives on the Biology and Life History of the White Shark (Boca Raton: CRC Press), 27–50. doi: 10.1201/b11532-5
Huveneers C., Apps K., Becerril-García E. E., Bruce B., Butcher P. A., Carlisle A. B., et al. (2018). Future research directions on the “Elusive” White Shark. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00455
Jorgensen S. J., Reeb C. A., Chapple T. K., Anderson S., Perle C., Van Sommeran S. R., et al. (2010). Philopatry and migration of Pacific white sharks. Proc. R. Soc B. 277, 679–688. doi: 10.1098/rspb.2009.1155
Joyce T. W., Durban J. W., Claridge D. E., Dunn C. A., Hickmott L. S., Fearnbach H., et al. (2020). Behavioral responses of satellite tracked Blainville’s beaked whales (Mesoplodon densirostris) to mid-frequency active sonar. Mar. Mam. Sci. 36, 29–46. doi: 10.1111/mms.12624
Klimley A. P., Le Boeuf B. J., Cantara K. M., Richert J. E., Davis S. F., Van Sommeran S. (2001). Radio-acoustic positioning as a tool for studying site-specific behavior of the white shark and other large marine species. Mar. Biol. 138, 429–446. doi: 10.1007/s002270000394
Kock A. A., Lombard A. T., Daly R., Goodall V., Meÿer M., Johnson R., et al. (2022). Sex and size influence the spatiotemporal distribution of white sharks, with implications for interactions with fisheries and spatial management in the southwest Indian ocean. Front. Marine Sci. 9, 811985.
Long D. J., Jones R. E. (1996). “White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean,” in Great white sharks: the biology of Carcharodon carcharias (San Diego: Academic Press), 293–307.
Lowerre-Barbieri S. K., Friess C., Griffin L. P., Morley D., Skomal G. B., Bickford J. W., et al. (2021). Movescapes and eco-evolutionary movement strategies in marine fish: Assessing a connectivity hotspot. Fish.Fish 22, 1321–1344. doi: 10.1111/faf.12589
Martin R. A., Hammerschlag N., Collier R. S., Fallows C. (2005). predatory behaviour of white sharks (Carcharodon carcharias) at seal island, South Africa. J. Mar. Biol. Ass. 85, 1121–1135. doi: 10.1017/S002531540501218X
McClenachan L., Cooper A. B. (2008). Extinction rate, historical population structure and ecological role of the Caribbean monk seal. P Roy Soc B-Biol Sci 275 (1641), 1351–1358.
Merten W. B., Schizas N. V., Craig M. T., Appeldoorn R. S., Hammond D. L. (2015). Genetic structure and dispersal capabilities of dolphinfish (Coryphaena hippurus) in the western central Atlantic. J. Fish. Biol. 113, 419–429. doi: 10.7755/FB.113.4.5
Rigby C., Barreto R., Carlson J., Fernando D., Fordham S., Francis M., et al. (2019). “White Shark (Carcharodon carcharias),” in IUCN Red List of Threatened Species E.T3855A2878674. doi: 10.2305/IUCN.UK.2019-3.RLTS.T3855A2878674.en
Shipley O. N., Howey L. A., Tolentino E. R., Jordan L. K. B., Ruppert J. L. W., Brooks E. J. (2017). Horizontal and vertical movements of Caribbean reef sharks (Carcharhinus perezi): conservation implications of limited migration in a marine sanctuary. R. Soc Open Sci. 4, 160611. doi: 10.1098/rsos.160611
Shipley O. N., Matich P., Hussey N. E., Brooks A. M. L., Chapman D., Frisk M. G., et al. (2023). Energetic connectivity of diverse elasmobranch populations – implications for ecological resilience. Proc. R. Soc B. 290, 20230262. doi: 10.1098/rspb.2023.0262
Skomal G., Braun C., Chisholm J., Thorrold S. (2017). Movements of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar. Ecol. Prog. Ser. 580, 1–16. doi: 10.3354/meps12306
Skomal G. B., Hoyos-Padilla E. M., Kukulya A., Stokey R. (2015). Subsurface observations of white shark Carcharodon carcharias predatory behaviour using an autonomous underwater vehicle. J. Fish. Biol. 87, 1293–1312. doi: 10.1111/jfb.12828
Stump K., Dahlgren C., Sherman K., Knapp C. (2017). Nassau grouper migration patterns during full moon suggest collapsed historic fish spawning aggregation and evidence of an undocumented aggregation. bms 93, 375–389. doi: 10.5343/bms.2016.1042
Talwar B. S., Bradley D., Berry C., Bond M. E., Bouyoucos I. A., Brooks A. M. L., et al. (2022). Estimated life-history traits and movements of the Caribbean reef shark (Carcharhinus perezi) in The Bahamas based on tag-recapture data. Mar. Biol. 169, 55. doi: 10.1007/s00227-022-04044-9
Bigelow H. B., Schroeder W. C. (1948). Fishes of the western North Atlantic. Part 1 (lancelets, cyclostomes, sharks). Yale UP, New Haven, CT.
Tirard P., Manning M. J., Jollit I., Duffy C., Borsa P. (2010). Records of great white sharks (Carcharodon carcharias) in New Caledonian waters. Pac. Sci. 64, 567–576. doi: 10.2984/64.4.567
Tricas T. C., McCosker J. E. (1984). Predatory behavior of the white shark (Carcharodon carcharias), with notes on its biology. Proc. California Acad. Sci. 43, 221–238.
Winton M., Fay G., Skomal G. (2023). An open spatial capture-recapture framework for estimating the abundance and seasonal dynamics of white sharks at aggregation sites. Mar. Ecol. Prog. Ser. 715, 1–25. doi: 10.3354/meps14371
Keywords: white shark, acoustic tracking, Andros, range expansion, Carcharodon carcharias, Bahamas
Citation: Guttridge TL, Matich P, Guttridge AE, Winton M, Dedman S and Skomal G (2024) First evidence of white sharks, Carcharodon carcharias, in the tongue of the ocean, central Bahamas. Front. Mar. Sci. 11:1451808. doi: 10.3389/fmars.2024.1451808
Received: 19 June 2024; Accepted: 09 July 2024;
Published: 31 July 2024.
Edited by:
Charlie Huveneers, Flinders University, AustraliaReviewed by:
Alison Towner, Rhodes University, South AfricaCopyright © 2024 Guttridge, Matich, Guttridge, Winton, Dedman and Skomal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: T. L. Guttridge, dHJpc3Rhbmd1dHRyaWRnZUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.