- 1Department of Life Sciences, Hemchandracharya North Gujarat University, Patan, Gujarat, India
- 2Department of Zoology, Faculty of Science, The Maharaja Sayajirao University of Baroda, Vadodara, Gujarat, India
- 3Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia
The present study focused on investigating the population structure and breeding biology of a species of Porcellanid crab, Petrolisthes boscii (Audouin, 1826). Evaluating the population ecology of the species is essential considering its crucial role in ecological functions including the nutrition cycle and its potential vulnerability to environmental stressors. The samples were collected over a period of 12 consecutive months (from March 2019 to February 2020) in an area of 500 m2 during low tide, utilizing the catch-per-unit-effort (CPUE) method when the water receded. The collected specimens (859 individuals) were categorized into males (407 individuals), non-ovigerous females (452 individuals), and ovigerous females (303 individuals). The assessment of fecundity was conducted by measuring the egg characteristics, including the total number, size, and weight of the eggs, as well as the carapace width of the ovigerous females. There was an evident difference in size, with males (8.60 ± 2.76) being significantly larger compared to females (8.39 ± 2.46) (p < 0.001). The overall and monthly sex ratios were female-biased (1:1.17). Moreover, the size frequency distribution revealed a bimodal distribution in male as well as female individuals. Ovigerous females occurred in all the months, which shows that the species breeds continuously throughout the year, with peaks in the months of March to May and August to October. There was a significant positive relationship observed among the size of ovigerous females and their total number of eggs (130.39 ± 83.31), egg mass weight (150 ± 110 mg). The study may provide baseline information for future studies on the impacts of a continuously altering environment and the consequences of increasing anthropogenic pressure on coastal areas.
1 Introduction
Petrolisthes boscii inhabits rocky intertidal zones, playing a significant ecological role in influencing the characteristics of the benthic community (Gebauer et al., 2007). The species is essential to the benthic environment, affecting nutrient cycling and acting as a food source for numerous predatory species (Ahmed and Mustaquim, 1974). In its megalopa stage, as a filter feeder, it aids in preserving water quality by ingesting suspended algae, hence enhancing the health of its ecosystem (Gonor and Gonor, 1973). Although P. boscii is necessary for ecological balance, environmental stressors like pollution can impact its populations, potentially affecting their rates of reproduction and survival (Roesijadi et al., 1974).
Research on population structure and breeding biology began in the early 1940s (Flores and Paula, 2002). This research primarily uncovers the patterns of species interactions and their functions within their distinct ecosystems. Such studies try to understand the genetic diversity, spatial distribution of the population, sex ratio, changes occurring in the year-round composition, breeding pattern, recruitment of juveniles, etc. (Litulo, 2005; Saher and Qureshi, 2010; Hu et al., 2015; Manzoor et al., 2016). Knowledge on these aspects of a species or population helps in understanding the competition and interaction among the coexisting species, along with the influence of ecosystem dynamics.
The anomuran crabs of the Porcellanidae family are comprised of more than 280 species (30 genera) (Osawa et al., 2010; Prakash et al., 2013; Baeza, 2016). The majority of the species are found in the tropical and subtropical intertidal region, inhabiting crevices, cobbles, boulders, and rocks, whereas a few species are found in association with some other organisms, including sea urchins, corals, sponges, etc (Baeza and Thiel, 2000; Baeza et al., 2001). The majority of studies on the biology and ecology of porcellanid crab populations have been conducted in the southern hemisphere (Jones, 1977; Baeza and Thiel, 2000; Gebauer et al., 2007; Miranda and Mantelatto, 2009; Baeza et al., 2013; Wehrtmann et al., 2011; Pinheiro et al., 2017), while information on the populations of the northern hemisphere is scanty. Studies focusing on the population structure and breeding biology of a species/population in India are scarce. A total of 32 species (12 genera) belonging to family Porcellanidae are reported from India (Patel et al., 2022a) out of which 16 species (7 genera) are reported from Gujarat. Gujarat state is situated in the northwestern part of India and has the longest coastline (~ 1650 km; 21%) among Indian states (Patel et al., 2022b). The state possesses diverse coastal habitats, therefore supporting a wide variety of marine decapods (Gosavi et al., 2021). Among the different coastal habitats, the Saurashtra coast has a rocky intertidal region where a huge diversity of brachyuran and anomuran crabs has been reported (Trivedi and Vachhrajani, 2013; Trivedi et al., 2018; Patel et al., 2020; Trivedi et al., 2021; Gosavi et al., 2021; Patel et al., 2022a; Padate et al., 2022).
Petrolisthes boscii is a porcellanid crab that is commonly found in the intertidal region of Shivrajpur, on the Saurashtra coast of Gujarat state, India. The species distribution has also been reported from the Red Sea to Mergui Archipelago; Western Pacific from Japan to Malay Archipelago and Australia (Komai, 2000). In India, the species has also been reported from Maharashtra, Goa, Kerala, Tamil Nadu, and Andhra Pradesh (Patel et al., 2022a). Porcellanid crabs, including P. boscii, play a significant ecological role in the benthic community as competitors and/or prey organisms (Hollebone and Hay, 2008). The roles of Petrolisthes boscii render it a crucial species for understanding ecological dynamics in benthic ecosystems, especially in rocky intertidal habitats and coastal biodiversity. Recently, a few studies have been conducted on the population structure and breeding biology of brachyuran crabs (Patel et al., 2024a, b) and hermit crabs (Patel et al., 2023); however, such studies are still not available on the porcellanid crabs, which are the common residents of rocky intertidal regions. The present investigation was conducted with the hypothesis that the population structure of P. boscii has an ideal sex ratio (1:1), with no disparities between the sexes and no temperature influence on the fecundity of the species. The objectives of testing the hypothesis were (1) to investigate the population structure and (2) to elucidate the reproductive biology of P. boscii population that is commonly occurring in the intertidal region of the Saurashtra coast of Gujarat state, India.
2 Materials and methods
2.1 Study site
Samples for the current investigation were collected on the rocky intertidal zone of Shivrajpur (22°12’21.97”N 68°58’31.75”E), Devbhumi Dwarka district located on the Saurashtra coast of Gujarat state, India (Figure 1). Petrolisthes boscii is a porcellanid crab that is commonly found under the cobbles, boulders, and rocks in the study area (Figure 2).
Figure 1. Map of study site: (A) Gujarat state; (B) Shivrajpur (22°12’21.97”N 68°58’31.75”E), Saurashtra coast, Gujarat state, India.

Figure 2. (A) Intertidal region of the study site, (B) Study site Petrolisthes boscii (Audouin, 1826) dorsal view of the specimen in habitat.
2.2 Sampling methods
The samples of P. boscii were collected once every month for a period of 12 consecutive months (March 2019 to February 2020) with each sampling session lasting four hours when the water recedes during low tide. An area of 500 m2 was marked in the intertidal region when the water receded and was thoroughly scanned for the presence of P. boscii. The specimens were collected by a single person by the CPUE method using handpicking collection method for a period of four hours in the marked area. Moreover, the cobbles and boulders in the marked region were also upturned to see whether the crabs were present under them, as the species prefers to hide under them for shelter during low tide. Whenever an individual was encountered, it was collected in a zip-lock bag, kept in ice box and brought to the laboratory for further analysis. Furthermore, ambient temperature was measured using digital thermometer each month during the time of specimen sampling.
2.3 Laboratory analysis
Once the specimens were brought to the laboratory, they were cleaned and identified up to species level with the help of standard identification keys (Garth, 1965; Beleem et al., 2016). The individuals of P. boscii were categorized as male, non-ovigerous female, or ovigerous female. The crabs can be distinguished by the position of their gonopores, which are present on the coxa of the fifth pair of pereiopods for males and on the coxa of the third pair of pereiopods for females. Ovigerous females are the females with egg mass attached to their pleopods. Additionally, a digital vernier caliper with an accuracy of 0.01 mm (Mitutoyo Digimatic Vernier Calliper 500-196-20) was used to measure the carapace width (CW) of the specimens (the broadest part between their lateral margins) as a measure of size during the morphometric analysis. The individuals that were smaller than the smallest ovigerous female among the sample individuals were considered juveniles (<6.47 mm) as established by Baeza et al. (2013).
In order to conduct a fecundity analysis, the eggs were extracted from the pleonal appendages of the ovigerous females (n = 53) using forceps. The eggs were then placed in 20 ml of saline water and stirred gently to ensure even distribution without causing any damage to the eggs. In addition, the measurement of egg size (diameter), egg mass weight, and total egg count was performed using the methodology described by Patel et al. (2023).
2.4 Data analysis
The samples collected were categorized into size classes with intervals of 2 mm, ranging from 2 mm to 16 mm CW to analyze the frequency distribution of the species on a monthly and overall basis. A Shapiro-Wilk test was used to determine if the obtained data was normally distributed. The results showed that the data was not normally distributed (p < 0.001) and hence non-parametric tests were run. A Kruskal-Wallis (KW) test was performed to evaluate the average carapace width among male, non-ovigerous female, and ovigerous female individuals. When significant results (p < 0.05) were obtained, Dunn’s post hoc test was conducted. Month-wise graphs of different sexes of all the size classes were plotted to understand the monthly variation in the percentage occurrence of males, non-ovigerous females, and ovigerous females. A chi-square test (χ2) was performed to find out the difference in the overall male: female (ovigerous and non-ovigerous females) ratio. Monthly data of ambient temperature was plotted against the monthly frequency of occurrence of ovigerous females and juveniles to understand the effect of temperature on the breeding and juvenile settling of P. boscii. Spearman correlation analysis was performed to examine the relationship between monthly ambient temperature and frequency of occurrence of ovigerous females and juveniles.
A regression analysis was employed to ascertain the relationship between the carapace width (CW) of ovigerous females and the morphological characteristics of their eggs. All statistical results were considered significant if the p-value was less than 0.05. Wherever applied, the values were expressed as mean ± standard deviation. The statistical analysis was conducted using MS Excel and Past software (version 4.03).
3 Results
In the present study, a total of 859 individuals were sampled which comprised 407 (47.38%) males and 452 (52.62%) females (303 (35.27%) non-ovigerous females and 149 (17.35%) ovigerous females). The size of individuals ranged from 3.78 mm to 15.12 mm in males, 2.92 mm to 12.64 mm in non-ovigerous females and 6.47 mm to 14.06 mm in ovigerous females. A strong sexual dimorphism was evident, with males exhibiting a substantially greater size than females (Kruskal-Wallis test, H = 112.8; p<0.001). Furthermore, Dun’s post hoc test showed that ovigerous females were considerably larger than non-ovigerous females (Bonferroni corrected, p<0.001) (Table 1).
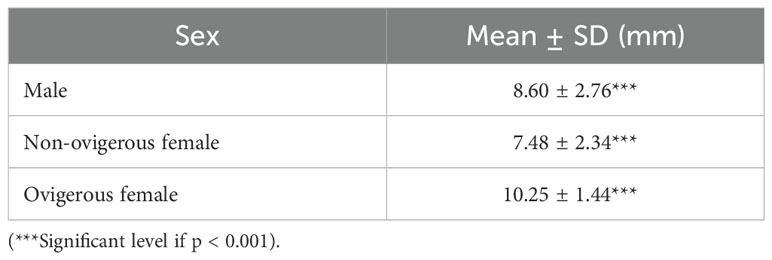
Table 1. Carapace width of samples from different sexes of Petrolisthes boscii from Shivrajpur, Gujarat state, India.
The sex ratio between males and females was somewhat skewed towards females, with a ratio of 1:1.17. However, this deviation from the expected 1:1 ratio was not statistically significant (χ2 = 1.23, p = 0.267) (Table 2). With the exception of January, June, November, and December, the monthly sex ratio was also skewed toward females. The occurrence of ovigerous females in every month suggests that the species has a continuous breeding cycle, with two breeding peaks that take place in the months of August to October and March to May (Table 2).
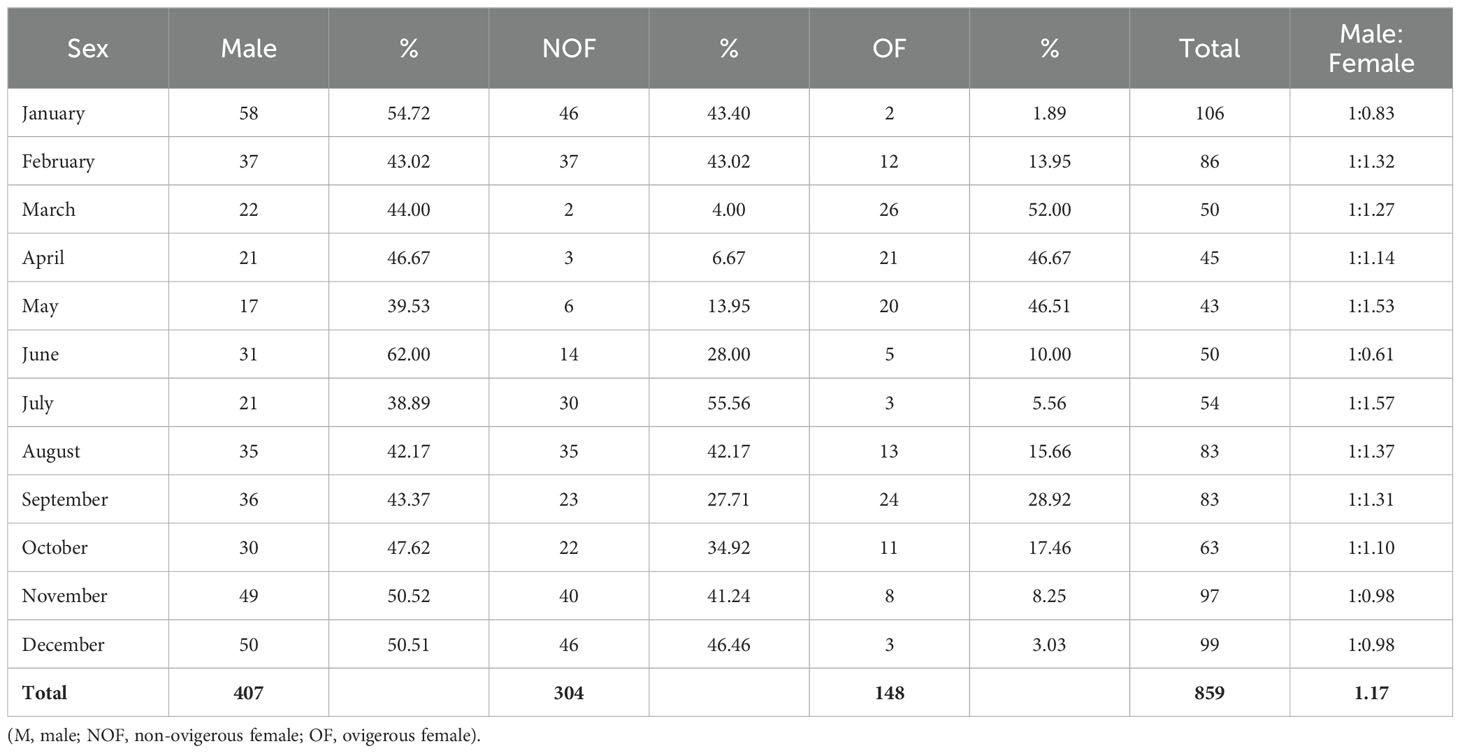
Table 2. Total number of Petrolisthes boscii samples collected from Shivrajpur, Gujarat state, India.
The P. boscii individuals have been recorded in every size class, from 2 mm to 16 mm. The male individuals of P. boscii showed a bimodal pattern in their overall frequency distribution, with the maximal occurrence reported in the 6–8 mm and 14–16 m size classes. In females also, a similar bimodal frequency distribution pattern was found, with peak occurrence reported in the 2–4 mm and 8–10 mm size classes (Figure 3).

Figure 3. Overall size frequency distribution of Petrolisthes boscii samples collected from Shivrajpur, Gujarat state, India.
In case of monthly frequency distribution, a bimodal distribution pattern was observed in male individuals in all the months except in February. Whereas, in case of females, a bimodal frequency distribution was observed in majority of the months except February, March, June, September, and October (Figure 4). The study also revealed occurrence of juvenile individuals in all the months suggesting year-round juvenile settlement (Figure 4). There was a significantly positive relationship observed between the monthly ambient temperature and frequency occurrence of ovigerous females (r = 0.68, p<0.01) suggesting the positive effect of ambient temperature on the breeding efficiency of the species. Moreover, the relationship between the monthly occurrence of ovigerous females and juveniles was significantly negative (r = -0.83, p<0.001) (Figure 5), which suggests that with increasing occurrence of ovigerous females, the frequency of occurrence of juveniles decreases or vice versa.
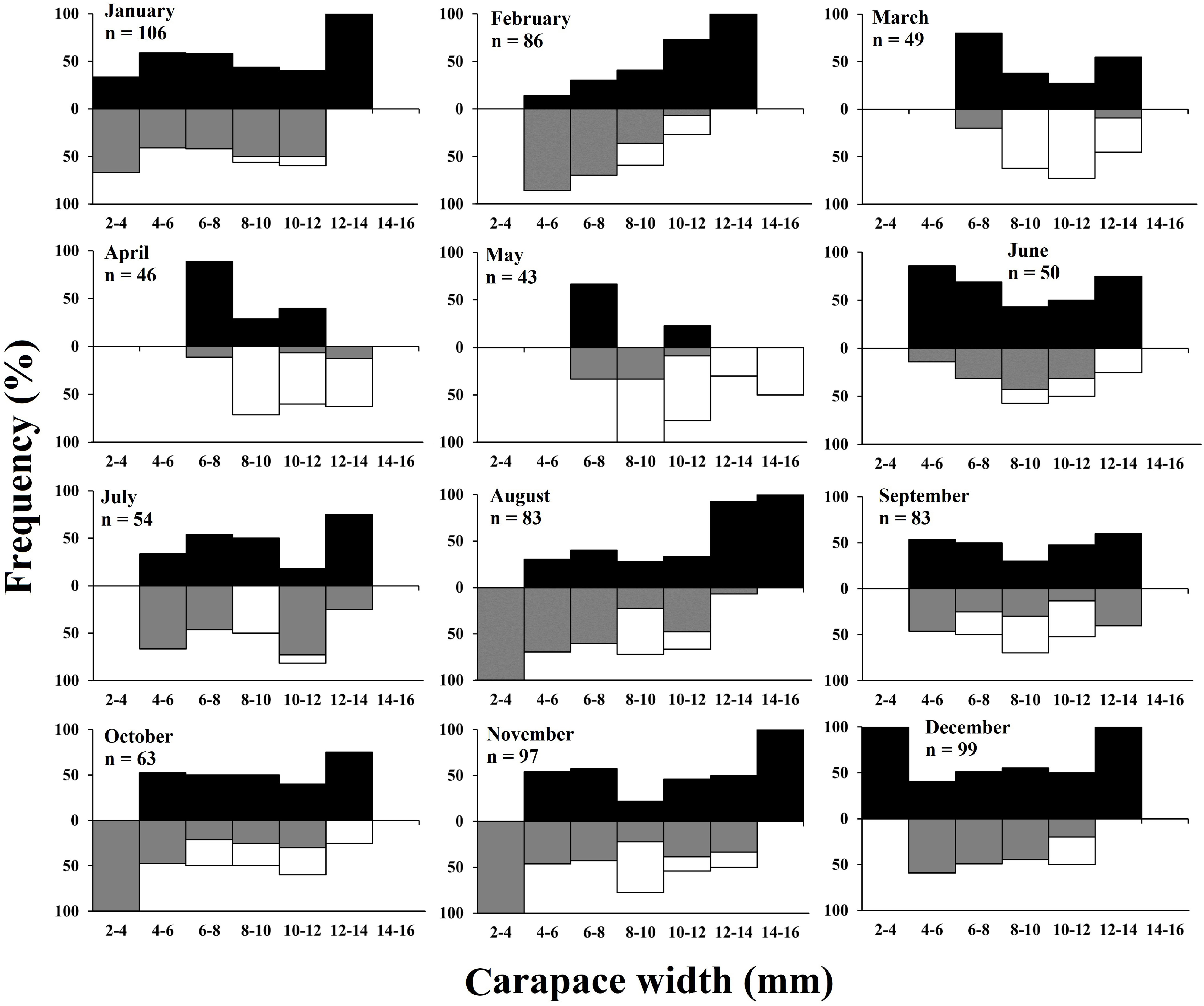
Figure 4. Monthly size–frequency distributions of Petrolisthes boscii samples collected from Shivrajpur, Gujarat state, India.
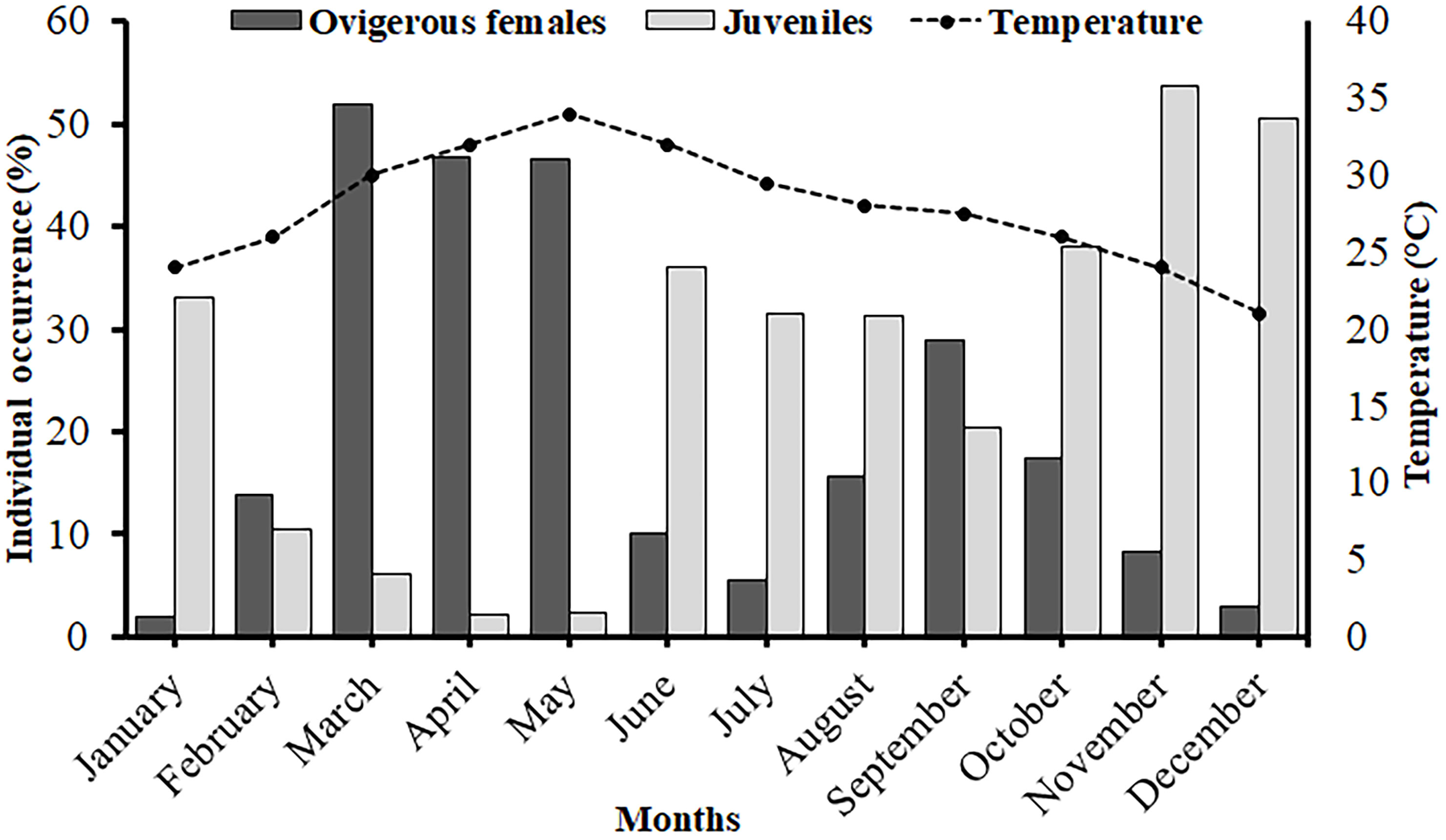
Figure 5. Association between temperature and the occurrence of juveniles (of both the sexes) and ovigerous females of Petrolisthes boscii samples collected from Shivrajpur, Gujarat state, India.
The average number of eggs, weight of egg mass, and size of eggs of the examined ovigerous females were 130.39 ± 83.31, 150 ± 110 mg, and 0.49 ± 0.03 mm, respectively, according to the analysis for fecundity estimation (Table 3). The carapace width of the corresponding ovigerous females was shown to have a significant positive correlation with both the average number of eggs and the weight of egg mass (Figure 6).
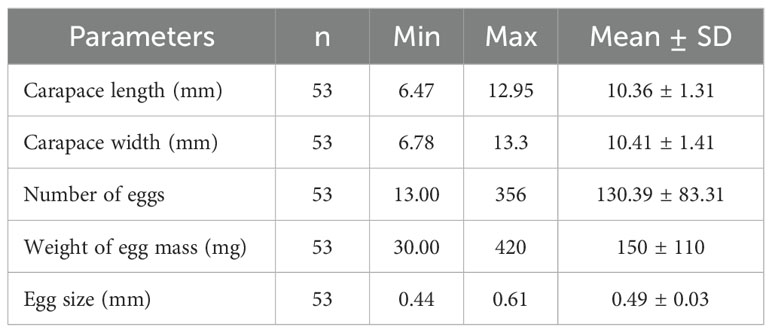
Table 3. Summary of Petrolisthes boscii ovigerous female carapace width, umber of eggs weight of egg mass, egg number and egg size collected from Shivrajpur, Gujarat state, India.
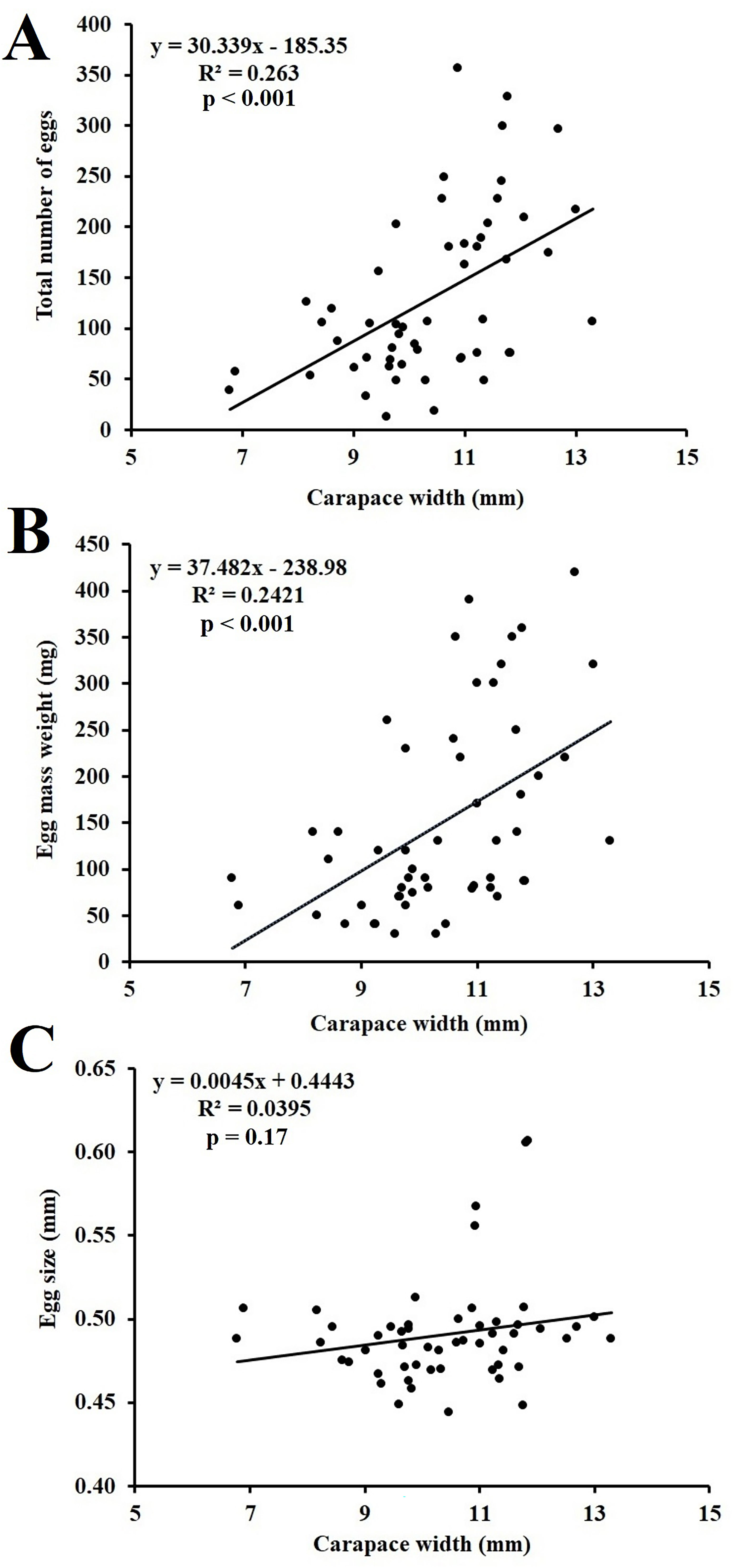
Figure 6. Relationship of ovigerous females of Petrolisthes boscii CW with (A) total number of eggs, (B) weight of egg mass, and (C) size of eggs.
4 Discussion
The present study revealed sexual dimorphism in P. boscii, where males were significantly larger as compared to females. This has been observed in other crab species, including Clibanarius rhabdodactylus (Patel et al., 2023), Carcinoplax vestita (Doi et al., 2007), Hexapanopeus paulensis (Silva et al., 2014), Eriocheir sinensis (Czerniejewski and Wawrzyniak, 2006), Gecarcinus ruricola (Hartnoll et al., 2006), Leptodius exaratus (Patel et al., 2024a), and Dotilla blanfordi (Patel et al., 2024b). Such variance in size is generally linked with the energy investment differences between sexes, where males use the majority of their energy for somatic growth (Silva et al., 2014), while females have to invest most of their energy in gamete production and incubation (Mantelatto and Martinelli, 1999). Several ecological and biological factors like differential mortality between sexes, are affected by various biotic and abiotic factors including predation or competition. Since males are often more conspicuous due to their larger size or behaviors like competition for mates, they experience higher predation risk (Naderi et al., 2018). Moreover, males tend to migrate more compared to females in search of mates or suitable habitats which could also result in reduced occurrence of males (Asakura, 1992).
The overall and monthly sex ratio was deviating from the ideal 1:1 and was biased towards females as previously discovered in other species like Neopanope sayi (Swartz, 1976), Acanthonyx scutiformis (Teixeira et al., 2009), Ocypode rotundata (Naderi et al., 2018), Callinectes sapidus (Lycett et al., 2020), and C. rhabdodactylus (Patel et al., 2023). Female-biased sex ratios can be influenced by various factors, including ecological conditions and limited dispersal abilities, leading to stronger isolation and favoring female dominance (Nijman and Vonk, 2022). The deviation from the ideal 1:1 sex ratio can be caused by a variety of factors, such as competition for local mates (Hamilton, 1967), differences in the amount of time and energy invested in male and female offspring (Kobayashi et al., 2018), differences in the way the sexes use their habitats (Silk, 1984), migration patterns (Allen, 1966), life span, growth rates, and spatiotemporal distribution. A female-biased sex ratio can also be linked to survival and reproductive advantages where female-biased population shows increased egg production and greater survival rate specifically in extreme habitats like intertidal region (Ewers-Saucedo, 2019).
The sex ratio in different life stages was also different, with female biased in smaller to intermediate size classes and male biased in larger individuals. Changes in the proportion of males to females may have a significant effect on the size and structure of a population, making the sex ratio a very significant population feature (Carver et al., 2005; Hjelset et al., 2012). Increased male mortality in intermediate size class may be the cause of female-biased sex ratio (Asakura, 1992). However, in larger size classes, a male-biased sex ratio results from a faster somatic growth rate in males (Wenner, 1972). In larger size classes, a male-biased sex ratio may also result from higher female mortality from higher reproductive costs and lower predation risk in larger males with larger chelas (Johnson, 2003).
In the current study, a bimodal frequency distribution was observed in male and female individuals which has also been found in other species like Pilumnus vespertilio (Litulo, 2005), Aegla franciscana (Gonçalves et al., 2006), Aegla georginae (Copatti et al., 2016), C. rhabdodactylus (Patel et al., 2023), L. exaratus (Patel et al., 2024a), and D. blanfordi (Patel et al., 2024b). This distribution pattern may be explained by a number of variables, including variations in migratory patterns (Flores and Negreiros-Fransozo, 1999), growth rates (Negreiros-Fransozo et al., 2003), and mortality rates (Díaz and Conde, 1989). Unimodality arises in stable populations with equal numbers of immigrants and emigrants, consistent recruitment and mortality throughout the life cycle, and stable demography (Thurman, 1985; Díaz and Conde, 1989). Bimodality, on the other hand, indicates a general tendency toward population expansion. In addition, rapid recruitment of larvae and a high reproduction rate can significantly and gradually change the population size and frequency of dispersion (Thurman, 1985).
The study site exhibited a wide range of ambient temperatures, fluctuating between 21°C and 34°C. These temperatures fall within the tropical-subtropical climatic range, allowing continuous reproduction. This reproductive activity was clearly detected in the current experiment. Moreover, a significant correlation was found between the monthly ambient temperature and the occurrence of ovigerous females (p < 0.05). The temperature is a critical factor in determining the occurrence of ovigerous females in crabs. Studies on various crab species like Scylla tranquebarica (Hidir et al., 2022), Petrolisthes cinctipes (Lam et al., 2022), S. paramamosain and S. serrata (Sanda et al., 2022), Portunus armatus (Nolan et al., 2021), and S. olivacea (Paul et al., 2021) have highlighted the significant impact of temperature on sex determination, gonadal development, reproductive output, and movement patterns showcasing how environmental factors can shape population dynamics. Researchers have found that high sediment temperature correlates with crab density and gonadal ripeness (Saeedi et al., 2018). The intertidal zone of a tropical or subtropical region where temperatures reach extremely high levels, temperature is one of the primary determinants controlling the abundance and distribution of the species (Allen, 1966; Asakura, 1987; Al-Wazzan et al., 2020). Seasonal variation arises from population movement or mortality, leading to an underestimation of the population during the summer (Patel et al., 2024a).
Moreover, there was a significantly negative relation between the frequency occurrence of ovigerous females and juveniles suggesting a decline in juvenile occurrence during increased occurrence of ovigerous females. Followed by the increased frequency occurrence of ovigerous females, there was an increase in the frequency occurrence of juveniles. Similar results have been observed in other crab species including P. armatus (Pinheiro et al., 2017); P. japonicus (Hamasaki et al., 2020), D. japonicus (Oh and Lee, 2020), S. olivacea (Rouf et al., 2021), C. rhabdodactylus (Patel et al., 2023). Certain factors like salinity (Huang et al., 2022), female nutritional quality (Matias et al., 2016), nutrition availability and quality (Viña-Trillos et al., 2023), water temperature (Chou et al., 2019), photoperiod (Zhang et al., 2023), and predation rate (Touchon et al., 2006) could affect the reproductive maxima of the population. Periodicity in reproduction might be caused by a variety of biotic and abiotic variables, including larval ecology (Reese, 1968), food availability (Goodbody, 1965), time to sexual maturity, mating period, gonadal development, incubation duration (Sastry et al., 1983), etc.
There was a significantly positive correlation between the number of eggs and the weight of egg mass with the respective CW of ovigerous females. Such types of outcomes have also been reported in other studies including P. sanguinolentus (Yang et al., 2014), Thalamita crenata (Mustaquim et al., 2022), Uca maracoani (Aviz et al., 2022), C. rhabdodactylus (Patel et al., 2023), L. exaratus (Patel et al., 2024a), and D. blanfordi (Patel et al., 2024b). However, disparities in the total number of eggs and egg mass weight were observed in ovigerous females with the same CW. The possible reasons for such disparity could be variation in the availability of food, differences in egg production or the loss of eggs (Hines, 1982). Intraspecific variation in the fertility of ovigerous females occupying same habitat or different parts of the same habitat can be influenced by several external and internal factors like age of sexual development, availability of nutrition, differences in overall female size, etc. (Zairion et al., 2015). Fecundity may be affected by the energy trade-off between somatic development and egg production (Zairion et al., 2015). Muiño (2002) found that females with greater CW lay more eggs, suggesting that CW plays a significant role in fecundity variability.
5 Conclusion
The population structure and fecundity of a Porcellanid crab P. boscii which is a commonly occurring species on the rocky coast of Shivrajpur, Gujarat State, India. The study revealed a significant sexual dimorphism as a result of differential energy investment among males and females. A female-biased sex ratio of the population was observed that can be due to the combined effect of various factors like ecological conditions, limited dispersal abilities, competition among local mates, investment in male and female offspring, habitat utilization patterns, migration patterns, life span, growth rates, and spatiotemporal distribution. The frequency distribution of males was bimodal whereas unimodal in females that is governed by the ecological factors like differences in their growth and mortality rate affected by several abiotic and biotic factors. Since the study site falls in sub-tropical region, a continuous pattern of reproduction was observed in the population. There was a significant correlation observed between the size of ovigerous females and the parameters of their eggs which is governed by several factors including energy trade-off between somatic development and egg production, age of sexual development, food availability, and differences among overall female size. Such studies unveiling the population dynamics and breeding pattern of a species play important role in understanding the ecology of the species. Moreover, the study can provide a baseline information that can be used in future to study the effects of a continuously changing environment as well as the effects of increasing anthropogenic pressure on coastal regions.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
NP: Investigation, Methodology, Writing – original draft, Writing – review & editing. KP: Investigation, Methodology, Writing – original draft, Writing – review & editing. AP: Conceptualization, Formal analysis, Visualization, Writing – review & editing. DA: Data curation, Funding acquisition, Software, Writing – review & editing. SA: Funding acquisition, Resources, Validation, Writing – review & editing. JT: Formal analysis, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
This research was supported by Researchers Supporting Project number (RSP2024R27), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed M., Mustaquim J. (1974). Population structure of four species of porcellanid crabs (Decapoda: Anomura) occurring on the coast of Karachi. Mar. Biol. 26, 173–182. doi: 10.1007/BF00388887
Allen J. A. (1966). The rhythms and population dynamics of decapod Crustacea. Oceanogr. Mar. Biol. 4, 247–265.
Al-Wazzan Z., Vay L. L., Behbehani M., Giménez L. (2020). Scale-dependent spatial and temporal patterns of abundance and population structure of the Xanthid crab Leptodius exaratus on rocky shores in Kuwait. Regional Stud. Mar. Sci. 37, 101325. doi: 10.1016/j.rsma.2020.101325
Asakura A. (1987). Population ecology of the sand-dwelling hermit crab Diogenes nitidimanus Terao: 3. Mating system. Bull. Mar. Sci. 41, 282–288.
Asakura A. (1992). Population ecology of the sand-dwelling hermit crab Diogenes nitidimanus Terao: 5. Ecological implications in the pattern of molting. J. Crustacean Biol. 12, 537. doi: 10.2307/1548835
Aviz D., Amorim Carmona P., Caroline de Castro Barbosa A., Rannieri Meira dos Santos C. (2022). Fecundity and reproductive patterns of the fiddler crab Uca maracoani Latreille 1802-1803 in an Amazonian estuary in northern Brazil. Invertebrate Reprod. Dev. 66, 197–207. doi: 10.1080/07924259.2022.2125353
Baeza J. A. (2016). Molecular phylogeny of porcelain crabs (Porcellanidae: Petrolisthes and allies) from the southeastern Pacific: the genera Allopetrolisthes and Liopetrolisthes are not natural entities. PeerJ 4, e1805. doi: 10.7717/peerj.1805
Baeza J. A., Furlan M., Almeida A. C., de Barros-Alves S. P., Alves D. F. R., Fransozo V. (2013). Population dynamics and reproductive traits of the ornamental crab Porcellana sayana: implications for fishery management and aquaculture. Sexuality Early Dev. Aquat. Organisms 1, 1–12. doi: 10.3354/sedao00002
Baeza J. A., Stotz W., Thiel M. (2001). Life history of Allopetrolisthes spinifrons, a crab associate of the sea anemone Phymactis clematis. J. Mar. Biol. Assoc. United Kingdom 81, 69–76. doi: 10.1017/S0025315401003411
Baeza J. A., Thiel M. (2000). Host use pattern and life history of Liopetrolisthes mitra, a crab associate of the black sea urchin Tetrapygus Niger. J. Mar. Biol. Assoc. United Kingdom 80, 639–645. doi: 10.1017/s0025315400002460
Beleem I., Poriya P., Gohil B. (2016). Porcelain crabs (Crustacea: Decapoda: Anomura) of western coast of India. Mar. Biodiversity Records 9, 55. doi: 10.1186/s41200-016-0057-y
Carver A. M., Wolcott T. G., Wolcott D. L., Hines A. H. (2005). Unnatural selection: Effects of a male-focused size-selective fishery on reproductive potential of a blue crab population. J. Exp. Mar. Biol. Ecol. 319, 29–41. doi: 10.1016/j.jembe.2004.06.013
Chou C.-C., Head M. L., Backwell P. R. Y. (2019). Effects of temperature on reproductive timing and hatching success in a tropical fiddler crab. Biol. J. Linn. Soc. 128, 225. doi: 10.1093/biolinnean/blz157
Copatti C. E., Legramanti R. P., Trevisan A., Santos S. (2016). Growth, sexual maturity and sexual dimorphism of Aegla georginae (Decapoda: Anomura: Aeglidae) in a tributary of the Ibicuí River in southern Brazil. Zoologia (Curitiba) 33, e20160010. doi: 10.1590/s1984-4689zool-20160010
Czerniejewski P., Wawrzyniak W. (2006). Body weight, condition, and carapace width and length in the Chinese mitten crab (Eriocheir sinensis H. Milne-Edwards 1853) collected from the Szczecin Lagoon (NW Poland) in spring and autumn 2001. Oceanologia 48, 275–285.
Díaz H., Conde J. E. (1989). Population dynamics and life history of the mangrove crab Aratus pisonii (Brachyura, Grapsidae) in a marine environment. Bull. Mar. Sci. 45, 148–163.
Doi W., Lwin T., Yokota M., Strüssmann C. A., Watanabe S. (2007). Maturity and reproduction of goneplacid crab Carcinoplax vestita (Decapoda, Brachyura) in Tokyo Bay. Fisheries Sci. 73, 331–340. doi: 10.1111/j.1444-2906.2007.01339.x
Ewers-Saucedo C. (2019). Evaluating reasons for biased sex ratios in Crustacea. Invertebrate Reprod. Dev. 63, 222–230. doi: 10.1080/07924259.2019.1588792
Flores A., Negreiros-Fransozo M. L. (1999). Allometry of the secondary sexual characters of the shore crab Pachygrapsus transversus (Gibbes 1850) (Brachyura, Grapsidae). Crustaceana 72, 1051–1066. doi: 10.1163/156854099504013
Flores A. A. V., Paula J. (2002). Population dynamics of the shore crab Pachygrapsus marmoratus (Brachyura: Grapsidae) in the central Portuguese coast. J. Mar. Biol. Assoc. United Kingdom 82, 229–241. doi: 10.1017/s0025315402005404
Gebauer P., Paschke K., Moreno C. A. (2007). Reproductive biology and population parameters of Petrolisthes laevigatus (Anomura: Porcellanidae) in southern Chile: consequences on recruitment. J. Mar. Biol. Assoc. United Kingdom 87, 729–734. doi: 10.1017/s0025315407055282
Gonçalves R. S., Castiglioni D. S., Bond-Buckup G. (2006). Ecologia populacional de Aegla franciscana (Crustacea, Decapoda, Anomura) em São Francisco de Paula, RS, Brasil. Iheringia Série Zoologia 96, 109–114.
Gonor S. L., Gonor J. J. (1973). Feeding, cleaning, and swimming behavior in larval stages of porcellanid crabs (Crustacea: Anomura). Mar. Biol. 27, 213–217. doi: 10.1007/BF00391946
Goodbody I. (1965). Continuous Breeding in Populations of Two Tropical Crustaceans, Mysidium columbiae (Zimmer) and Emerita portoricensis Schmidt. Ecology 46, 195–197. doi: 10.2307/1935274
Gosavi S., Purohit B., Mitra S., Patel K., Vachhrajani K., Trivedi J. (2021). “Annotated checklist of marine decapods (Crustacea: Decapoda) of Gujarat state with three new records,” in Proceedings of the “Marine Biology Research Symposium – MBRS 2021. 45–66.
Hamasaki K., Ishii M., Dan S. (2020). Reproductive traits and population structure of the porcellanid crab Petrolisthes japonicus (Decapoda: Anomura: Porcellanidae). Crustacean Res. 49, 121–132. doi: 10.18353/crustacea.49.0_121
Hamilton W. D. (1967). Extraordinary sex ratios. Science 156, 477–488. doi: 10.1126/science.156.3774.477
Hartnoll R. G., Atkin H., James J., Grandas Y., Baine M. S. P. (2006). Population biology of the black land crab, gecarcinus ruricola, in the San Andres archipelago, Western Caribbean. J. Crustacean Biol. 26, 316–325. doi: 10.1651/c-2640.1
Hidir A., Aaqillah-Amr M. A., Mohd-Sabri M., Mohd-Zaidi I., Shahreza M. S., Abualreesh M. H., et al. (2022). Effect of temperature on sex and steroid hormones of purple mud crab, Scylla tranquebarica (Fabricius 1798) during egg incubation, larvae rearing and juvenile production. Aquaculture Res. 53, 4095–4105. doi: 10.1111/are.15911
Hines A. H. (1982). Allometric constraints and variables of reproductive effort in brachyuran crabs. Mar. Biol. 69, 309–320. doi: 10.1007/bf00397496
Hjelset A. M., Nilssen E. M., Sundet J. H. (2012). Reduced size composition and fecundity related to fishery and invasion history in the introduced red king crab (Paralithodes camtschaticus) in Norwegian waters. Fisheries Res. 121-122, 73–80. doi: 10.1016/j.fishres.2012.01.010
Hollebone A. L., Hay M. E. (2008). An invasive crab alters interaction webs in a marine community. Biol. Invasions 10, 347–358. doi: 10.1007/s10530-007-9134-9
Hu M., Kwan B., Wang Y., Cheung S., Shin P. (2015). “Population structure and growth of juvenile horseshoe crabs Tachypleus tridentatus and Carcinoscorpius rotundicauda (Xiphosura) in Southern China,” in Changing Global Perspectives on Horseshoe Crab Biology, Conservation and Management. Eds. Carmichael R., Botton M., Shin P., Cheung S. (Springer Cham, Switzerland), 167–180.
Huang X., He L., Tan R., Feng G., Geng Z., Zhao F., et al. (2022). Effects of salinity on reproductive characteristics and embryo quality of Eriocheir sinensis. Aquaculture Res. 53, 4970–4979. doi: 10.1111/are.15983
Johnson P. (2003). Biased sex ratios in fiddler crabs (Brachyura, Ocypodidae): a review and evaluation of the influence of sampling method, size class, and sex-specific mortality. Crustaceana 76, 559–580. doi: 10.1163/156854003322316209
Jones M. B. (1977). Breeding and seasonal population changes of Petrolisthes elongatus (Crustacea, Decapoda, Anomura) at Kaikoura, New Zealand. J. R. Soc. New Z. 7, 259–272. doi: 10.1080/03036758.1977.10419428
Kobayashi M., Wong Y. H., Oguro-Okano M., Dreyer N., Høeg J. T., Yoshida R., et al. (2018). Identification, characterization, and larval biology of a rhizocephalan barnacle, Sacculina yatsui Boschma 1936, from northwestern Japan (Cirripedia: Sacculinidae). J. Crustacean Biol. 38, 329–340. doi: 10.1093/jcbiol/ruy020
Komai T. (2000). A checklist of thalassinidea and anomura (Crustacea: decapoda) from the South China sea. Raffles Bull. Zool. 8, 343–376.
Lam E. K., Abegaz M., Gunderson A. R., Tsukimura B., Stillman J. H. (2022). Interactions between temperature variability and reproductive physiology across traits in an intertidal crab. Front. Physiol. 13. doi: 10.3389/fphys.2022.796125
Litulo C. (2005). Population structure and breeding biology of the hairy crab Pilumnus vespertilio (Fabricius 1793) (Crustacea: Brachyura: Pilumnidae) in southern Mozambique. J. Natural History 39, 1359–1366. doi: 10.1080/00222930400010070
Lycett K. A., Shields J. D., Chung J. S., Pitula J. S. (2020). Population structure of the blue crab callinectes sapidus in the maryland coastal bays. J. Shellfish Res. 39, 699–713. doi: 10.2983/035.039.0316
Mantelatto F. L. M., Martinelli J. M. (1999). Carapace width-weight relationships of Callinectes ornatus (Brachyura, Portunidae) from Ubatuba Bay, Brazil. Iheringia Série Zoologia 87, 111–116.
Manzoor R., Haider S., Fatima M., Qari R. (2016). Study on abundance, breeding and growth of the ocypodide crab dotilla blanfordi in Karachi Coast, Pakistan. Int. J. Mar. Sci. 6, 1–14. doi: 10.5376/ijms.2016.06.0022
Matias D., Joaquim S., Matias A. M., Leitão A. (2016). Reproductive effort of the European clam Ruditapes decussatus (Linnaeus 1758): influence of different diets and temperatures. Invertebrate Reprod. Dev. 60, 49–58. doi: 10.1080/07924259.2015.1126537
Miranda I., Mantelatto F. L. (2009). Estimating population features of the anomuran crab Petrolisthes armatus (Porcellanidae) in a remaining and impacted mangrove area of the western Atlantic. J. Natural History 43, 2027–2039. doi: 10.1080/00222930903094613
Muiño R. (2002). Fecundity of Liocarcinus depurator (Brachyura: Portunidae) in the Ría de Arousa (Galicia, north-west Spain). J. Mar. Biol. Assoc. United Kingdom 82, 109–116. doi: 10.1017/s0025315402005222
Mustaquim J., Khatoon S., Rashid S. (2022). A note on sex ratio, size at maturity, fecundity and breeding season of the portunid crab, Thranita crenata (Rüppell 1830) from the Pakistani coast. Crustaceana 95, 127–136. doi: 10.1163/15685403-bja10179
Naderi M., Hosseini S. A., Pazooki J., Hedayati A., Zare P., Lastra M. (2018). Reproductive biology of the ghost crab, Ocypode rotundata Miers 1882 (Decapoda, Ocypodidae) at Qeshm Island, Persian Gulf. Crustaceana 91, 1039–1059. doi: 10.1163/15685403-00003804
Negreiros-Fransozo M. L., Costa T. M., Colpo K. D. (2003). Allometric growth in the fiddler crab uca thayeri (Brachyura, ocypodidae) from a subtropical mangrove. J. Crustacean Biol. 23, 273–279. doi: 10.1163/20021975-99990337
Nijman V., Vonk R. (2022). Room for females only? Exploring strongly female-biased sex ratios in ingolfiella (Crustacea: peracarida: ingolfiellida) in relation to ecological condition. J. crustacean Biol. 42, 1–5. doi: 10.1093/jcbiol/ruac049
Nolan S. E. F., Johnson D. D., Hanamseth R., Suthers I. M., Taylor M. D. (2021). Reproductive biology of female blue swimmer crabs in the temperate estuaries of south-eastern Australia. Mar. Freshw. Res. 73, 366–376. doi: 10.1071/mf21191
Oh I.-K., Lee S. (2020). Effects of temperature on the survival and larval development of deiratonotus japonicus (Brachyura, camptandriidae) as a biological indicator. J. Mar. Sci. Eng. 8, 213–213. doi: 10.3390/jmse8030213
Osawa M., McLaughlin P. A., Haworth P., Milne H., Randall P., Henderson P., et al. (2010). Annotated checklist of anomuran decapod crustaceans of the world (exclusive of the Kiwaoidea and families Chirostylidae and Galatheidae of the Galatheoidea) Part II − Porcellanidae. Raffles Bull. Zool. Supplement 23, 109–129.
Padate V. P., Patel K. J., Rivonker C. U., Trivedi J. N. (2022). On Indian species of nanosesarma tweedie 1950 (Decapoda: brachyura: sesarmidae). Nauplius 30, e2022031. doi: 10.1590/2358-2936e2022031
Patel K., Padate V., Osawa M., Tiwari S., Vachhrajani K., Trivedi J. (2022a). An annotated checklist of anomuran species (Crustacea: Decapoda) of India. Zootaxa 5157, 1–100. doi: 10.11646/zootaxa.5157.1.1
Patel K., Patel H., Ali D., Gosavi S., Choudhary N., Yadav V. K., et al. (2024b). On population structure and breeding biology of burrowing crab Dotilla blanfordi Alcock 1900. PeerJ 12, e17065. doi: 10.7717/peerj.17065
Patel K., Patel H., Gosavi S., Vachhrajani K., Trivedi J. (2024a). Population structure and fecundity of the Xanthid crab Leptodius exaratus (H. Milne Edwards 1834) on the rocky shore of Gujarat state, India. PeerJ 12, e16916–e16916. doi: 10.7717/peerj.16916
Patel P., Patel K., Trivedi J. (2020). First record of Hermit crab Clibanarius ransoni Forest 1953 (Crustacea: Anomura: Diogenidae) from India. J. Biol. Stud. 3, 19–23. doi: 10.62400/jbs.v3i1.4601
Patel K. J., Vachhrajani K. D., Trivedi J. N. (2022b). Study on shell utilization pattern of two sympatric hermit crab species on the rocky intertidal region of Veraval, Gujarat, India. Thalassas: Int. J. Mar. Sci. 39, 125–137. doi: 10.1007/s41208-022-00487-5
Patel K. J., Vachhrajani K. D., Trivedi J. (2023). Population structure and reproductive biology of Clibanarius rhabdodactylus Forest 1953 (Crustacea: Anomura: Diogenidae) in Gujarat state, India. Regional Stud. Mar. Sci. 63, 103033. doi: 10.1016/j.rsma.2023.103033
Paul P., Islam M., Khatun S., Bir J., Ghosh A. (2021). Reproductive biology of mud crabs (Scylla olivacea) collected from Paikgachha, Khulna, Bangladesh. J. Advanced Veterinary Anim. Res. 8, 44–50. doi: 10.5455/javar.2021.h483
Pinheiro M. A. A., João M. C. A., Leme M. H. A., Matsunaga A. M. F., Rio J. P. P., Hernáez P. (2017). Insights of the life history in the porcellanid crab Petrolisthes armatus (Gibbes 1850) (Crustacea: Anomura: Porcellanidae) from the Southwestern Atlantic coast. Invertebrate Reprod. Dev. 61, 78–89. doi: 10.1080/07924259.2017.1285818
Prakash S., Kumar T. T. A., Khan S. A. (2013). Checklist of the porcellanidae (Crustacea: decapoda: anomura) of India. Check List 9, 1514. doi: 10.15560/9.6.1514
Reese E. S. (1968). Annual breeding seasons of three sympatric species of tropical intertidal hermit crabs, with a discussion of factors controlling breeding. J. Exp. Mar. Biol. Ecol. 2, 308–318. doi: 10.1016/0022-0981(68)90022-1
Roesijadi G., Petrocelli S. R., Anderson J. W., Presley B. J., Sims R. (1974). Survival and chloride ion regulation of the porcelain crab Petrolisthes armatus exposed to mercury. Mar. Biol. 27, 213–217. doi: 10.1007/BF00391946
Rouf M. A., Shahriar S. I. M., Antu A.-H., Siddiqui M. N. (2021). Population parameters of the orange mud crab Scylla olivacea (Herbst 1796) from the Sundarban mangrove forest in Bangladesh. Heliyon 7, e06223. doi: 10.1016/j.heliyon.2021.e06223
Saeedi H., Kamrani E., Nordhaus I., Diele K. (2018). Sediment temperature impact on population structure and dynamics of the crab austruca Iranica pretzmann 1971 (Crustacea: ocypodidae) in subtropical mangroves of the Persian gulf. Wetlands 38, 539–549. doi: 10.1007/s13157-018-0998-5
Saher N. U., Qureshi N. A. (2010). Zonal distribution and population biology of Ilyoplax frater (Brachyura: Ocypodoidea: Dotillidae) in a coastal mudflat of Pakistan. Curr. Zool. 56, 244–251. doi: 10.1093/czoolo/56.2.244
Sanda T., Shimizu T., Iwasaki T., Dan S., Hamasaki K. (2022). Effect of temperature on survival, intermolt period, and growth of juveniles of two mud crab species, Scylla paramamosain and Scylla serrata (Decapoda: Brachyura: Portunidae), under laboratory conditions. Nauplius 30. doi: 10.1590/2358-2936e2022012
Sastry A., Vernberg F. J., Vernberg W. (1983). “Ecological aspects of reproduction,” in Environmental Adaptations (Academic Press, New York, UK), 1–410.
Silk J. B. (1984). Local resource competition and the evolution of male-biased sex ratios. J. Theor. Biol. 108, 203–213. doi: 10.1016/s0022-5193(84)80066-1
Silva T. E., Fumis P. B., Almeida A. C., Bertini G., Fransozo V. (2014). Morphometric analysis of the mud crab Hexapanopeus paulensis Rathbun 1930(Decapoda, Xanthoidea) from the southeastern coast of Brazil. Latin Am. J. Aquat. Res. 42, 588–597. doi: 10.3856/vol42-issue3-fulltext-16
Swartz R. C. (1976). Sex ratio as a function of size in the Xanthid crab Neopanope sayi. Am. Nat. 110, 898–900.
Teixeira G. M., Fransozo V., Cobo V. J., Hiyodo C. M. (2009). Population features of the spider crab Acanthonyx scutiformis (Dana 1851) (Crustacea, Majoidea, Epialtidae) associated with rocky-shore algae from southeastern Brazil. Pan-American J. Aquat. Sci. 4, 87–95.
Thurman C. L. (1985). Reproductive biology and population structure of the fiddler Crabuca subcylindrical (Stimpson). Biol. Bull. 169, 215–229. doi: 10.2307/1541399
Touchon J. C., Gomez-Mestre I., Warkentin K. M. (2006). Hatching plasticity in two temperate anurans: responses to a pathogen and predation cues. Can. J. Zool. 84, 556–563. doi: 10.1139/z06-058
Trivedi J., Mitra S., Patel P., Maheta N., Patel K., Ng P. K. L. (2021). On the Indian species of eurycarcinus A. Milne-edwards 1867, heteropanope stimpson 1858, and pilumnopeus A. Milne-edwards 1867 (Decapoda: brachyura: pilumnidae). Nauplius 29. doi: 10.1590/2358-2936e2021004
Trivedi J. N., Trivedi D. J., Vachhrajani K. D., Ng P. K. L. (2018). An annotated checklist of the marine brachyuran crabs (Crustacea: Decapoda: Brachyura) of India. Zootaxa 4502, 1–83. doi: 10.11646/zootaxa.4502.1.1
Trivedi J. N., Vachhrajani K. D. (2013). First record of two porcellanid crabs from Gujarat state, India (Crustacea: Decapoda: Porcellanidae). J. Mar. Biol. Assoc. India 55, 55–58. doi: 10.6024/jmbai.2013.55.1.01756-09
Viña-Trillos N., Brante A., Urzúa Ángel (2023). Intraspecific variation in reproductive traits and embryo elemental composition of the crab Hemigrapsus crenulatus (Milne Edwards 1837) across fluctuating coastal environments along Chilean coasts. Mar. Environ. Res. 188, 106023–106023. doi: 10.1016/j.marenvres.2023.106023
Wehrtmann I. S., Miranda I., Lizana-Moreno C. A., Hernáez P., Barrantes-Echandi V., Mantelatto F. L. (2011). Reproductive plasticity in (Anomura, Porcellanidae): a comparison between a Pacific and an Atlantic population. Helgoland Mar. Res. 66, 87–96. doi: 10.1007/s10152-011-0250-7
Wenner A. M. (1972). Sex ratio as a function of size in marine crustacea. Am. Nat. 106, 321–350. doi: 10.1086/282774
Yang C.-P., Xu J., Li L., Li H.-X., Yan Y. (2014). Population structure, morphometric analysis and reproductive biology of Portunus sanguinolentus (Herbst 1783) (Decapoda: Brachyura: Portunidae) in Honghai Bay, South China Sea. J. crustacean Biol. 34, 722–730. doi: 10.1163/1937240x-00002273
Zairion Z., Wardiatno Y., Boer M., Fahrudin A. (2015). Reproductive biology of the blue swimming crab portunus pelagicus (Brachyura: portunidae) in east Lampung waters, Indonesia: fecundity and reproductive potential. Trop. Life Sci. Res. 26, 67.
Keywords: anomuran crab, Porcellanidae, fecundity, rocky coast, Arabian Sea, ecology
Citation: Patel N, Patel KJ, Patel A, Ali D, Alarifi S and Trivedi JN (2024) Unveiling the population structure and breeding patterns of Petrolisthes boscii (Audouin, 1826) a common intertidal inhabitant of Shivrajpur, Saurashtra Coast, Gujarat. Front. Mar. Sci. 11:1438129. doi: 10.3389/fmars.2024.1438129
Received: 31 May 2024; Accepted: 25 October 2024;
Published: 12 November 2024.
Edited by:
Punyasloke Bhadury, Indian Institute of Science Education and Research Kolkata, IndiaReviewed by:
Prakash Sanjeevi, Sathyabama Institute of Science and Technology, IndiaFahmida Tina, Nakhon Si Thammarat Rajabhat University, Thailand
Copyright © 2024 Patel, Patel, Patel, Ali, Alarifi and Trivedi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashish Patel, dW5pLmFzaGlzaEBnbWFpbC5jb20=; Jigneshkumar N. Trivedi, am50cml2ZWRpMjZAeWFob28uY28uaW4=
†These authors share first authorship
 Nayan Patel1†
Nayan Patel1† Krupal J. Patel
Krupal J. Patel Ashish Patel
Ashish Patel Daoud Ali
Daoud Ali Saud Alarifi
Saud Alarifi Jigneshkumar N. Trivedi
Jigneshkumar N. Trivedi