- 1Freshwater Institute, Fisheries and Oceans Canada, Winnipeg, MB, Canada
- 2Department of Biological Sciences, University of Manitoba, Winnipeg, MB, Canada
- 3Conservation and Research Department, Assiniboine Park Zoo, Winnipeg, MB, Canada
- 4Animal Health and Nutrition Department, Assiniboine Park Zoo, Winnipeg, MB, Canada
Biologging tools can provide invaluable information on the movement and behaviour of animals, facilitating the elucidation of ecological dynamics, especially for wide-ranging species, and supporting conservation and management efforts. Harbour seals (Phoca vitulina) exhibit extensive habitat plasticity in their vast range across the northern hemisphere, with likely recent increases in abundance at northern latitudes, yet details of their movement behaviour in subarctic areas remain largely unknown. We used satellite-telemetry data, including nearly 5,000 locations and over 12,000 dives, obtained from six harbour seals tagged in western Hudson Bay from 2021 to 2023, to address the knowledge gap on their movement behaviour between marine and freshwater habitats in subarctic regions. We document the behavioural patterns, transit speeds, and diverse aquatic system usage, including detailed records of a harbour seal track traversing over 170 km upriver on three separate trips along the Seal River, Canada. Notably, we observed a rapid downstream transit from the Seal River to Hudson Bay, covering 214 km within a single day. Additionally, we highlight the prevalence of short dive durations in the Seal and Churchill Rivers, in contrast to longer dive durations in Hudson Bay. These insights complement existing evidence of harbour seal occurrences and river use at northern latitudes, as well as enhance our understanding of harbour seal movement ecology within Hudson Bay which can be used to better inform conservation and management strategies between connected freshwater and marine environments in the Arctic.
1 Introduction
Animals travel extensively for various reasons, including thermoregulatory benefits, foraging opportunities, and predator avoidance (e.g., Nathan et al., 2008; Hebblewhite and Merrill, 2009; Avgar et al., 2013). Additionally, many species exhibit versatility in habitat use, transitioning between disparate environments that can vary in resource productivity (e.g., Steller sea lions Eumetopias jubatus move between marine and freshwater habitats for seasonal prey resources, Wright et al., 2010). Biologging tools can provide extensive insights into animal movement and behaviour patterns, particularly for wide-ranging species, providing a better understanding of ecological mechanisms that govern animal spatial ecology (Nathan et al., 2008; Hussey et al., 2015). These data are also critical for planning protected areas and mitigating the impacts of environmental changes (Block et al., 2011).
Harbour seals (Phoca vitulina) demonstrate extensive habitat flexibility in aquatic ecosystems across the northern hemisphere (e.g., Mansfield, 1967; Merkel et al., 2013; Lubinsky-Jinich et al., 2017). Over their range, they occupy diverse habitats that can include marine coastal waters as well as rivers and lakes (e.g., Beck et al., 1970; Lesage et al., 2004; van Neer et al., 2023). Harbour seal freshwater-habitat use in Hudson Bay may be influenced by foraging opportunities, similar to harbour seals in the Pacific Northwest (Wright et al., 2007) and bearded seals (Erignathus barbatus) and spotted seals (Phoca largha Pallas) in Alaska (Gryba et al., 2021).
The historical and current presence of harbour seals in freshwater habitats in Hudson Bay underscores the need for detailed research on their movement patterns, behaviour, and habitat use to inform effective management strategies. In Hudson Bay, European place names like Seal River (Manitoba) and Ranger Seal Bay (Nunavut), and Inuit place names like Qasigiaqsit (Nunavut) and Qasigialik (Nunavut) indicate that harbour seals have a longstanding presence in freshwater habitats in the region (see Inuit Heritage Trust Place Names Program; Mansfield, 1967). Limited tagging efforts in western Hudson Bay have provided insights into harbour seal use of Hudson Bay and nearby watersheds, including observations 30 km upriver in the Seal River (Bajzak et al., 2013). The likely increase in harbour seal abundance in the Churchill River in relation to diminishing sea ice (Florko et al., 2018) underscores the importance of quantifying their movement patterns, behaviour, and habitat use between inter-connected marine and freshwater environments to inform management efforts. To address the knowledge gap regarding harbour seal movements, behaviours, and habitat usage in nearby rivers, we used biologging data from six harbour seals to describe and compare their move-persistence and dive duration between freshwater and marine habitats. We also discuss in detail one harbour seal’s relatively high-speed movements between disparate environments and highlight the important connections between marine and freshwater habitats.
2 Methods
2.1 Transmitter deployment
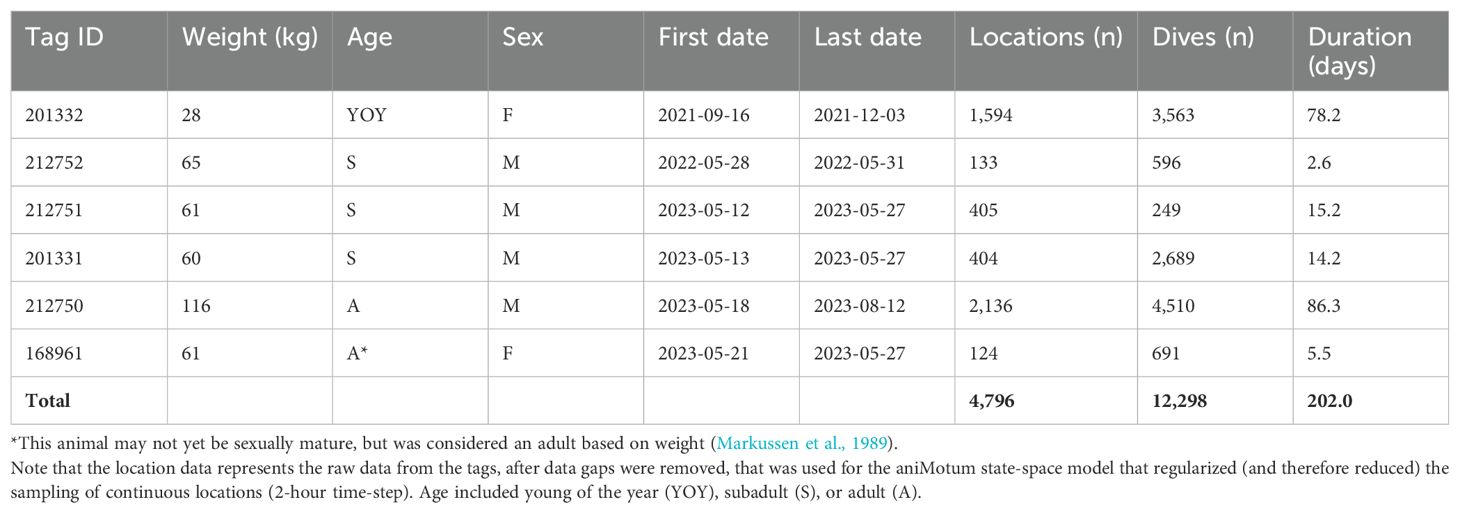
We captured and tagged six harbour seals in August 2021 and May of 2022 and 2023 near Churchill, Manitoba, Canada (Figure 1A; Table 1). In August 2021, we captured one harbour seal using a hoop net near the Churchill River weir, similar to Bajzak et al. (2013). In May of 2022 and 2023, we captured five harbour seals using monofilament mesh nets that were set perpendicular to the floe edge near the mouth of the Churchill River estuary. All seals were weighed and equipped with an Argos satellite telemetry SPLASH10-297 tag (MK10, tagwear version 1.26w, Wildlife Computers Ltd, Redmond, Washington, USA) attached to the fur mid-dorsally with quick-setting epoxy glue. These tags were programmed to transmit data on the seal’s movement and dive patterns at a 10 second interval in both marine and freshwater until the moult in June-July (subadults) or August-September (adults; Daniel et al., 2003).
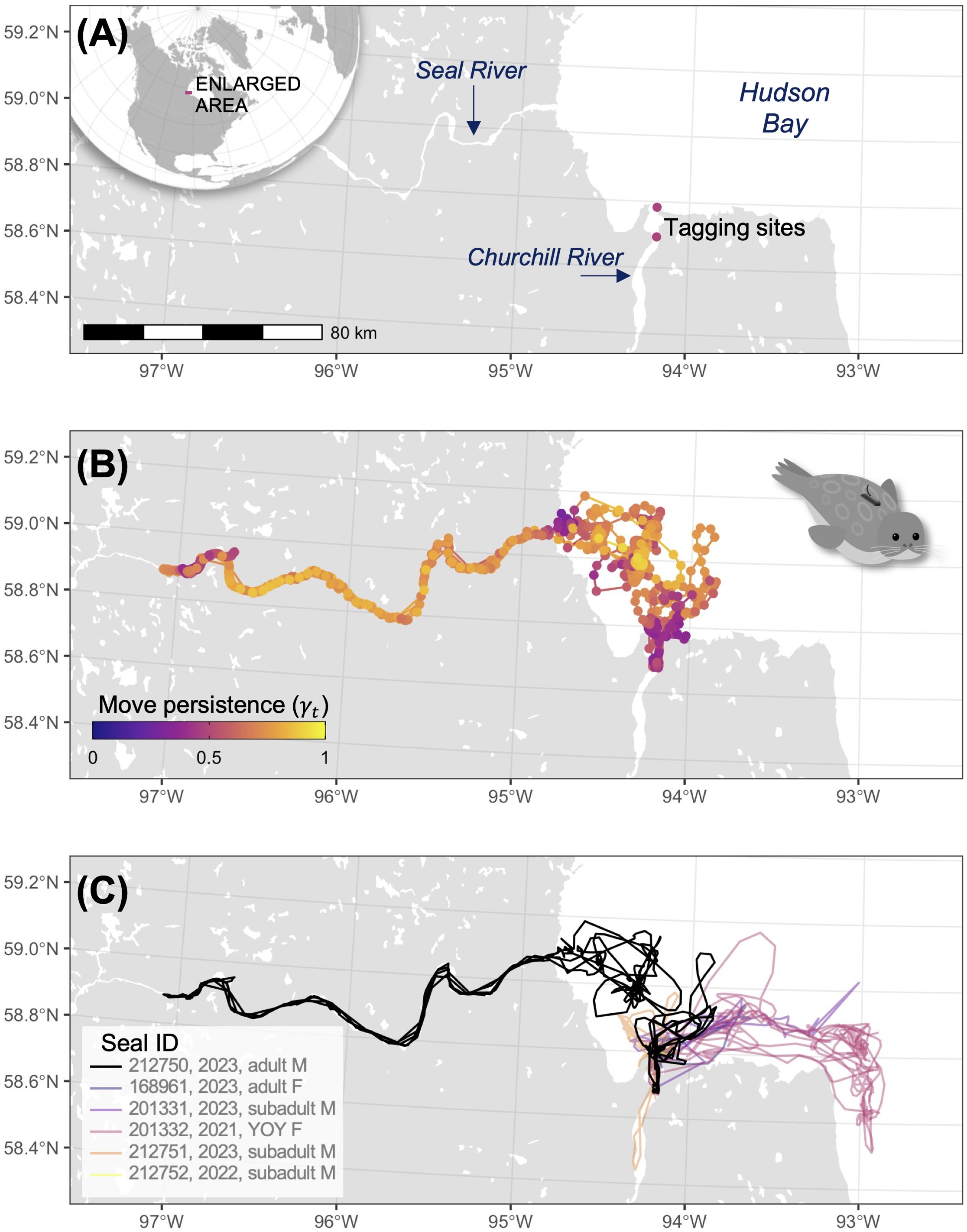
Figure 1. (A) Study area, (B) movement track of one male harbour seal, coloured by move persistence, (C) movement track of one male harbour seal (black) who traveled up the Seal River three times, and five additional harbour seals who stayed in Hudson Bay and the Churchill River.
2.2 Data preparation
To mitigate the inherent error and irregular sampling associated with Argos locations (Costa et al., 2010), we fit a state-space model to predict more accurate locations (Auger-Méthé et al., 2021). We fit a continuous-time correlated random walk state-space model to the Argos tag data using the fit_ssm() function in the aniMotum R package (Jonsen et al., 2023). This model generated predicted location data at a 2-hour interval. Prior to model fitting, we partitioned tracks into smaller segments when transmission ceased for over 12 hours and removed segments with <100 locations to overcome convergence issues. The state-space model applied to this dataset (4,796 raw locations) yielded 2,123 predicted locations over a total of 202 seal days from 16 Aug 2021 to 12 Aug 2023 (range = 2-86 days per seal), where the adult male seal traveled up the Seal River three times contributed to 2,136 of the raw locations and 1,027 of the predicted locations over 18 May 2023 to 12 Aug 2023 (86 days, Table 1).
Creating a path between seal locations to measure trip distance led to land crossings due to the two-hour interval between predicted locations. To ensure the seal’s trajectory remained exclusively within the water, we used the route_path() function in the pathroutr R package (London, 2020).
Our tags also recorded time-depth dive data (n = 12,298 dives). For the dives to have an associated location, for each seal, we matched their dive data to the nearest (in time) predicted movement location.
2.3 Statistical analyses
We fitted a move-persistence model on predicted harbour seal locations using the fit_mpm() function in the aniMotum R package. The model provides an estimate of directional persistence along the path of movement and is presented as a continuous value between zero and one. Lower values indicated low directional persistence, typically associated with behaviours like area-restricted search (e.g., residency behaviours such as foraging and resting), while higher values indicated high directional persistence, typically associated with traveling. We were limited to fitting a move-persistence model solely to the seal that traveled upriver (tag ID: 212750, see Figure 1), as attempts to fit models to other seal tracks failed to converge.
To assess differences in dive duration and move-persistence between the different regions (Seal River, Churchill River, and Hudson Bay), we used linear mixed-effects models (LMM) using the glmmTMB() function in the glmmTMB R package. The dive duration models included data from all six seals, whereas the move-persistence models included data from just one adult male seal (see above). Each model included seal identification number as a random effect, and a first order (AR1) autocorrelation term to account for the time-series nature of biologging data. We also included bathymetry as a model term to account for regional differences in water depth. We extracted the bathymetry (m, 0.01 ° resolution) associated with each predicted seal location from the National Oceanic and Atmospheric Administration (NOAA) Environmental Research Division Data Access Program (ERDDAP) data servers from the etopo180 dataset (Amante and Eakins, 2009) using the rerddapXtracto R package (Mendelssohn, 2019).
3 Results
Harbour seals primarily used Hudson Bay near the tagging site, with some individuals using the Churchill River and Seal River (Figure 1). Our tags revealed one of the six tagged seals, an adult male harbour seal, demonstrated substantial use of the Seal River, embarking on three trips upriver (Movie S1). The first trip commenced on 11 June 2023. By 14 June 2023, the seal reached a location approximately 173 km upriver (Figure 1). The seal departed 15 June 2023, and returned to Hudson Bay on 18 June 2023, resulting in a total trip duration of 7 days (Figure 2). The second trip commenced on 23 June 2023. By 28 June 2023, the seal reached a location approximately 182 km upriver, approximately 9 km farther upriver than the previous trip. The seal departed 24 July 2023, and returned to Hudson Bay on 25 July 2023, resulting in a total trip duration of 32 days. Notably, the seal traveled approximately 214 kms on 25 July 2023 (average speed = 8.91 km/hr) including the outbound trip from the Seal River (Figure 2). The third trip commenced on 8 August 2023. By 12 August 2023, the seal reached a location approximately 173 km upriver, near the distal location of the first trip. The tag stopped transmitting new data on 12 August 2023, suggesting that it had detached from the seal.
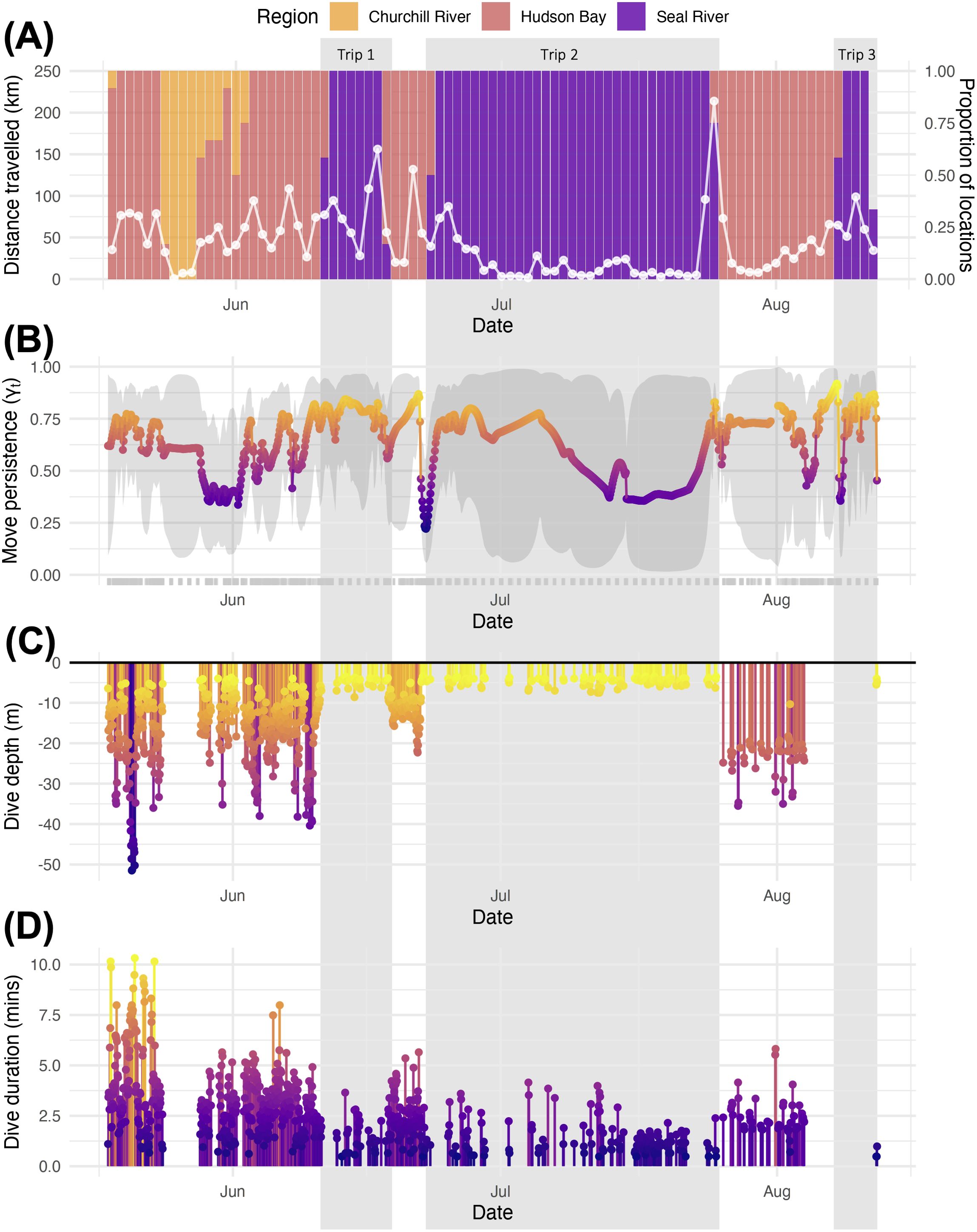
Figure 2. Movement and diving data through time from the one adult male harbour seal who traveled up the Seal River three times during one summer: (A) proportion of locations in the Churchill River, Hudson Bay, and the Seal River, with the daily distance traveled (white circle) overlaid; (B) move-persistence estimates; (C) hourly mean dive depth; (D) hourly mean dive duration. The three trips up the Seal River are highlighted in gray, and circles in B-D are coloured by the y-axis variable.
We found patterns in harbour seal dive and move-persistence behaviour between the three regions in our study area, Seal River, Churchill River, and Hudson Bay (Figure 3). Dives were shorter in Seal River (mean = 1.4 min, range = 0.5-5.5 min) than in Churchill River (mean = 2.1 min, range = 0.5-8.9 min; est. = 0.496, SE = 0.164, p = 0.003) and Hudson Bay (mean = 2.5 min, range = 0.5-11.0 min; est = 0.812, SE = 0.149, p < 0.001). Dive duration was also affected by bathymetry, where after accounting for area, dives were longer in shallower waters (est = 0.006, SE = 0.002, p = 0.007).
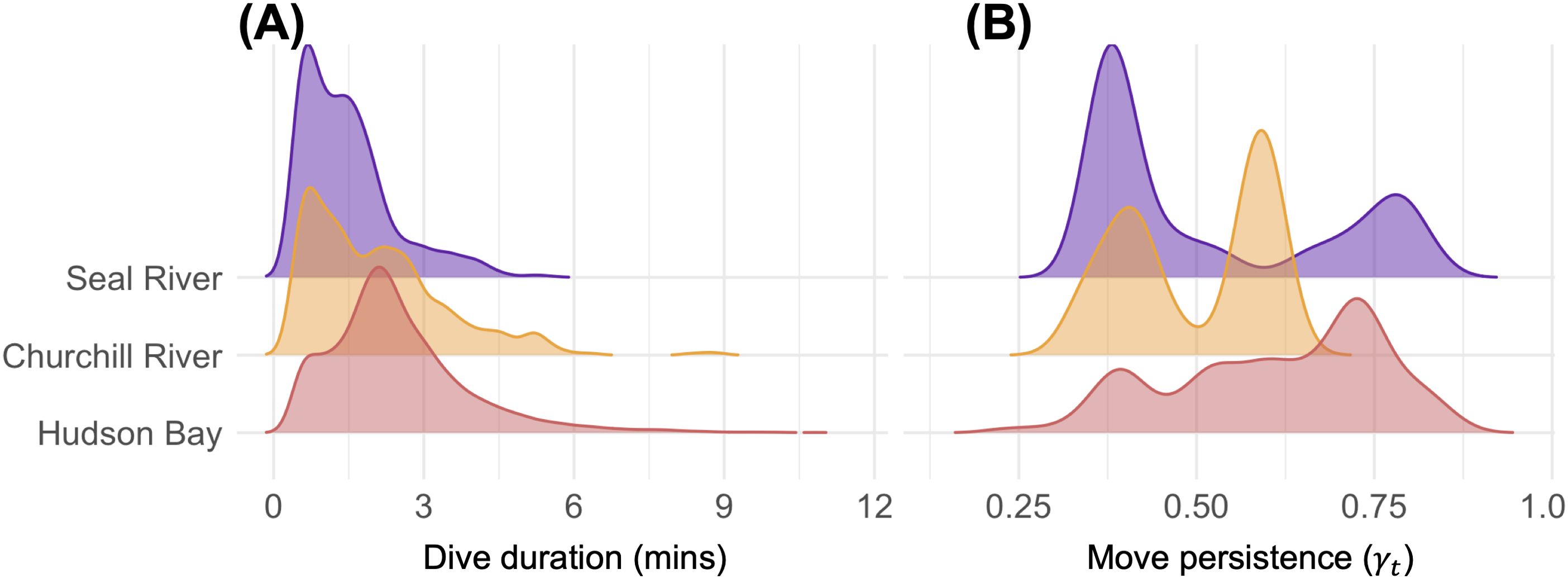
Figure 3. Distribution of (A) dive duration data from all six harbour seals and (B) move persistence data from the one male harbour seal that embarked on three trips up the Seal River. Note that we were limited to fitting a move-persistence model solely to the seal that traveled upriver (tag ID: 212750, see Figure 1), as attempts to fit models to other seal tracks failed to converge.
The move-persistence of the adult male was not statistically different between Seal River and Churchill River (est. = -0.075, SE = 0.052, p = 0.148) or Hudson Bay (est = -0.089, SE = 0.052, p = 0.089). Variation in move-persistence was best explained by bathymetry, where move-persistence was lower in shallower waters (est. = -0.002, SE = 0.001, p < 0.001). However, move-persistence in Seal River appeared to consist of mostly low and high move-persistence, whereas in Churchill River move-persistence consisted of mostly low and moderate move-persistence, and in Hudson Bay was well distributed with relatively more values of moderate-high move-persistence (Figure 3B).
4 Discussion
The use of rivers by harbour seals in Hudson Bay has long been noted. Our tracking study provides novel details of the behavioural patterns, transit speed, and the multifaceted use of interconnected marine and freshwater systems. We observed harbour seals’ use of the Churchill River Estuary and Hudson Bay near the tagging site, as well as the movement and dive behaviour exhibited by an adult male harbour seal undertaking three excursions up the Seal River (Figure 1). While previous studies have recorded harbour seals typically making short trips (average 5 ± 4 days; Bajzak et al., 2013) from haul-out locations to forage in Hudson Bay, we recorded a month-long stay far inland suggesting potential foraging in freshwater habitats. This was followed by a particularly rapid and sustained outbound movement of this seal covering approximately 214 km in one day, which to our knowledge is both the longest and fastest daily movement distance and rate of a harbour seal to date (equating to 8.9 km/hr). In other geographic areas, harbour seals have been observed making many repeated trips from marine waters to upriver waters, albeit to shorter distances overall (maximum movement was approximately 72 km over the course of one day, equating to 3 km/hr; van Neer et al., 2023).
We speculate that favourable outflow conditions from the river, combined with moderate wind and relatively high water discharge, may have facilitated this rapid movement despite the absence of extraordinary weather events. Specifically, the weather on this day was characterized by average conditions, with a mean temperature of 11.4°C, no precipitation, maximum gust speeds of 42 km/hr, and a wind direction of 350° (i.e., North wind; Churchill Weather Station, www.climate.weather.gc.ca). However, the water discharge during this outbound trip was higher (673 m2/s) than during the initial outbound trip (634 m2/s), which took three days to complete (Supplementary Figure S1, Seal River Below Great Island Station 06GD001, https://wateroffice.ec.gc.ca/report/historical_e.html?stn=06GD001). Additionally, the long duration of this seal’s tag transmissions (86 days) facilitated our documentation of these trips upriver (see Table 1).
Our results reveal regional patterns in harbour seal movement and diving duration related to water depth. We observed shorter dives observed in Seal River, in contrast with longer dives in Churchill River and Hudson Bay (Figure 3). The moderate dive durations recorded in the Churchill River likely occur within the deeper estuary (included in our “Churchill River” region). However, after accounting for region, our findings suggest that harbour seals dove longer and had lower move-persistence in shallow areas. While low move-persistence likely reflects a composite of residency-related behaviours (e.g., hauling out, resting, foraging; Breed et al., 2012), the additional evidence of longer dives may suggest indications of resting dives or area-restricted search in shallow waters (although see Florko et al., 2023).
Harbour seals use freshwater environments for a variety of reasons including prey availability, predator avoidance, thermoregulation, and reproduction (Smith et al., 1996). While harbour seal diet information and relative prey abundance in our study area is unknown, harbour seals likely forage on freshwater species, as local river estuaries also attract dense aggregations of seabirds and beluga whales (Delphinapterus leucas) due to their productivity (Stewart and Lockhart, 2005; Stewart and Barber, 2010). Within these freshwater systems, harbour seals may forage particularly in shallow waters as suggested by our observations of longer dives and lower move-persistence in these waters. Additional benefits of using freshwater habitats in western Hudson Bay could be to avoid marine predators such as killer whales (Orcinus orca) or polar bears (Ursus maritiumus, Ferguson et al., 2012; Westdal et al., 2016; Florko et al., 2018). Further, harbour seals could benefit from warmer water and rocks in river systems to facilitate molting (Paterson et al., 2012) and avoid predators while pupping, which likely occurs in June as observed in the Churchill River (Florko et al., 2018).
While our findings of river use supplement our understanding of the speed, frequency of visits, and diving behaviour in rivers, our documentation of harbour seal occurrence in the Seal River aligns with previous research by Bajzak et al. (2013), who reported similar riverine explorations (albeit 30 km upriver) by seals tagged in the Churchill River estuary in 2001 and 2002. Additionally, Beck et al. (1970), and Bajzak et al. (2013) document the occurrence of harbour seals in Thlewiaza River, Nunavut (approx. 875 km north of Seal River), which includes Sealhole Lake and Ranger Seal Lake.
Our detailed movement data of harbour seals in this area aligns with local knowledge of harbour seal occurrence in these systems and adds to the growing knowledge of how marine and terrestrial ecosystems are connected. The repeated movements over 170 km upriver show that these links can occur at various trophic levels (St. George et al., 2023) and must be factored into conservation planning and wildlife management. There are current proposals to create an Indigenous Protected and Conserved Area encompassing the Seal River Watershed (see feasibility assessment, https://parks.canada.ca/pn-np/cnpn-cnnp/riviere-seal-river) and additional discussions to evaluate a western Hudson Bay marine protected area. The movement of this harbour seal emphasizes the importance of protected areas planning that combines interconnected ecosystems.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: Movebank and Github (https://github.com/kflorko/riverseals).
Ethics statement
The animal study was approved by Freshwater Institute Animal Care Committee (OPA-ACC-2021-15; OPA-ACC-2022-17; OPA-ACC-2023-16) and Fisheries and Oceans Canada’s License to Fish for Scientific Purposes (S-21/22-1025-NU; S-22/23-1015-NU; S-23/24-1010-NU). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
KF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing – original draft, Writing – review & editing. DY: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing. CB: Investigation, Project administration, Writing – review & editing, Methodology. SF: Conceptualization, Investigation, Writing – review & editing. HG: Investigation, Writing – review & editing. AG: Investigation, Project administration, Writing – review & editing. AN: Investigation, Project administration, Writing – review & editing. CS: Writing – review & editing. SP: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. We gratefully acknowledge financial support from Assiniboine Park Conservancy, Churchill Northern Studies Centre, Fisheries and Oceans Canada, and Manitoba Hydro.
Acknowledgments
It is essential to acknowledge and honor the invaluable local knowledge contributed by community members in Churchill and surrounding areas. Local insights have enriched our research, enhancing our understanding of harbour seal ecology. We thank Jack Batstone for his guidance and sharing his local knowledge on the sea ice. Additionally, we thank Brendan McEwan, Mike Stover, and Sheldon Oliver for safe trips on the sea ice, and Deborah Sharpe and Jillian St. George for their assistance in the field. Animal handling was approved by the Freshwater Institute Animal Care Committee (OPA-ACC-2021-15; OPA-ACC-2022-17; OPA-ACC-2023-16) and Fisheries and Oceans Canada’s License to Fish for Scientific Purposes (S-21/22-1025-NU; S-22/23-1015-NU; S-23/24-1010-NU).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1435206/full#supplementary-material
References
Amante C., Eakins B. W. (2009). ETOPO1 arc-minute global relief model: procedures, data sources and analysis. NOAA Technical Memorandum NESDIS NGDC-24.
Auger-Méthé M., Newman K., Cole D., Empacher F., Gryba R., King A. A., et al. (2021). A guide to state–space modeling of ecological time series. Ecol. Monogr. 91, e01470. doi: 10.1002/ecm.1470
Avgar T., Street G., Fryxell J. M. (2013). On the adaptive benefits of mammal migration. Can. J. Zool. 92, 481–490. doi: 10.1139/cjz-2013-0076
Bajzak C. E., Bernhardt W., Mosnier A., Hammill M. O., Stirling I. (2013). Habitat use by harbour seals (Phoca vitulina) in a seasonally ice-covered region, the western Hudson Bay. Polar Biol. 36, 477–491. doi: 10.1007/s00300-012-1274-4
Beck B., Smith T. G., Mansfield A. W. (1970). Occurrence of the harbour seal, Phoca vitulina, Linnaeus in the Thlewiaza River, NWT. Can. Field-Naturalist 84, 297–300. doi: 10.5962/p.342974
Block B. A., Jonsen I. D., Jorgensen S. J., Winship A. J., Shaffer S. A., Bograd S. J., et al. (2011). Tracking apex marine predator movements in a dynamic ocean. Nature 475, 86–90. doi: 10.1038/nature10082
Breed G. A., Costa D. P., Jonsen I. D., Robinson P. W., Mills-Flemming J. (2012). State-space methods for more completely capturing behavioral dynamics from animal tracks. Ecol. Model. 235, 49–58. doi: 10.1016/j.ecolmodel.2012.03.021
Costa D. P., Robinson P. W., Arnould J. P., Harrison A. L., Simmons S. E., Hassrick J. L., et al. (2010). Accuracy of ARGOS locations of pinnipeds at-sea estimated using Fastloc GPS. PloS One 5, e8677. doi: 10.1371/journal.pone.0008677
Daniel R. G., Jemison L. A., Pendleton G. W., Crowley S. M. (2003). Molting phenology of harbor seals on Tugidak Island, Alaska. Mar. Mammal Sci. 19, 128–140. doi: 10.1111/j.1748-7692.2003.tb01097.x
Ferguson S. H., Higdon J. W., Westdal K. H. (2012). Prey items and predation behavior of killer whales (Orcinus orca) in Nunavut, Canada based on Inuit hunter interviews. Aquat. Biosyst. 8, 1–16. doi: 10.1186/2046-9063-8-3
Florko K. R. N., Bernhardt W., Breiter C. J. C., Ferguson S. H., Hainstock M., Young B. G., et al. (2018). Decreasing sea ice conditions in western Hudson Bay and an increase in abundance of harbour seals (Phoca vitulina) in the Churchill River. Polar Biol. 41, 1187–1195. doi: 10.1007/s00300-018-2277-6
Florko K. R. N., Shuert C. R., Cheung W. W., Ferguson S. H., Jonsen I. D., Rosen D. A., et al. (2023). Linking movement and dive data to prey distribution models: new insights in foraging behaviour and potential pitfalls of movement analyses. Movement Ecol. 11, 17. doi: 10.1186/s40462-023-00377-2
Gryba R., Huntington H. P., Von Duyke A. L., Adams B., Frantz B., Gatten J., et al. (2021). Indigenous Knowledge of bearded seal (Erignathus barbatus), ringed seal (Pusa hispida), and spotted seal (Phoca largha) behaviour and habitat use near Utqiaġvik, Alaska, USA. Arctic Sci. 7, 832–858. doi: 10.1139/as-2020-0052
Hebblewhite M., Merrill E. H. (2009). Trade-offs between predation risk and forage differ between migrant strategies in a migratory ungulate. Ecology 90, 3445–3454. doi: 10.1890/08-2090.1
Hussey N. E., Kessel S. T., Aarestrup K., Cooke S. J., Cowley P. D., Fisk A. T., et al. (2015). Aquatic animal telemetry: a panoramic window into the underwater world. Science 348, 1255642. doi: 10.1126/science.1255642
Jonsen I. D., Grecian W. J., Phillips L., Carroll G., McMahon C., Harcourt R. G., et al. (2023). aniMotum, an R package for animal movement data: Rapid quality control, behavioural estimation and simulation. Methods Ecol. Evol. 14, 806–816. doi: 10.1111/2041-210X.14060
Lesage V., Hammill M. O., Kovacs K. M. (2004). Long-distance movements of harbour seals (Phoca vitulina) from a seasonally ice-covered area, the St. Lawrence River estuary, Canada. Can. J. Zool. 82, 1070–1081. doi: 10.1139/z04-084
London J. M. (2020). pathroutr: an R Package for (Re-)Routing Paths Around Barriers (Version v0.1.1-beta) (Zenodo). doi: 10.5281/zenodo.4321827
Lubinsky-Jinich D., Schramm Y., Heckel G. (2017). The Pacific harbor seal’s (Phoca vitulina richardii) breeding colonies in Mexico: abundance and distribution. Aquat. Mammals 43, 73–81. doi: 10.1578/AM.43.1.2017.73
Mansfield A. W. (1967). Distribution of the harbour seal, Phoca vitulina Linnaeus, in Canadian Arctic waters. J. Mammal. 48, 249–257. doi: 10.2307/1378028
Markussen N. H., Bjørge A., Øritsland N. A. (1989). Growth in harbour seals (Phoca vitulina) on the Norwegian coast. J. Zool. 219, 433–440. doi: 10.1111/j.1469-7998.1989.tb02591.x
Mendelssohn R. (2019). rerddapXtracto: extracts environmental data from ‘ERDDAP’web services (R package).
Merkel B., Lydersen C., Yoccoz N. G., Kovacs K. M. (2013). The world’s northernmost harbour seal population–how many are there? PloS One 8, e67576. doi: 10.1371/journal.pone.0067576
Nathan R., Getz W. M., Revilla E., Holyoak M., Kadmon R., Saltz D., et al. (2008). A movement ecology paradigm for unifying organismal movement research. Proc. Natl. Acad. Sci. 105, 19052–19059. doi: 10.1073/pnas.0800375105
Paterson W., Sparling C. E., Thompson D., Pomeroy P. P., Currie J. I., McCafferty D. J. (2012). Seals like it hot: Changes in surface temperature of harbour seals (Phoca vitulina) from late pregnancy to moult. J. Thermal Biol. 37, 454–461. doi: 10.1016/j.jtherbio.2012.03.004
Smith R. J., Hobson K. A., Koopman H. N., Lavigne D. M. (1996). Distinguishing between populations of fresh-and salt-water harbour seals (Phoca vitulina) using stable-isotope ratios and fatty acid profiles. Can. J. Fisheries Aquat. Sci. 53, 272–279. doi: 10.1139/f95-192
Stewart D. B., Barber D. G. (2010). “The ocean-sea ice-atmosphere system of the Hudson Bay complex,” in A little less Arctic. Eds. Ferguson S. H., Loseto L. L., Mallory M. L. (Dordrecht: Springer). doi: 10.1007/978-90-481-9121-5_1
Stewart D. B., Lockhart W. L. (2005). An overview of the Hudson Bay marine ecosystem. Can. Tech. Rep. Fish. Aquat. Sci. 2586, vi + 487 p.
St. George J. R., Petersen S. D., Roth J. D., Ferguson S. H., Yurkowski D. J. (2023). Habitat coupling dynamics of mobile consumers along a freshwater and marine resource gradient in a sub-Arctic estuarine system. Estuarine Coast. Shelf Sci. 292, 108449. doi: 10.1016/j.ecss.2023.108449
van Neer A., Nachtsheim D., Siebert U., Taupp T. (2023). Movements and spatial usage of harbour seals in the Elbe estuary in Germany. Sci. Rep. 13, 6630. doi: 10.1038/s41598-023-33594-1
Westdal K. H., Davies J., McPherson A., Orr J., Ferguson S. H. (2016). Behavioural changes in belugas (Delphinapterus leucas) during a killer whale (Orcinus orca) attack in southwest Hudson Bay. Can. Field-Naturalist 130, 315–319. doi: 10.22621/cfn.v130i4.1925
Wright B. E., Riemer S. D., Brown R. F., Ougzin A. M., Bucklin K. A. (2007). Assessment of harbor seal predation on adult salmonids in a Pacific Northwest estuary. Ecol. Appl. 17, 338–351. doi: 10.1890/05-1941
Keywords: animal tracking, Arctic, habitat use, harbour seal (Phoca vitulina), long-distance movement, Hudson bay, movement ecology, subarctic
Citation: Florko KRN, Yurkowski DJ, Breiter C-JC, Ferguson SH, Gamblin HEL, Grottoli A, Nace A, Shuert CR and Petersen SD (2024) Biologging reveals rapid movements of harbour seals between freshwater and marine habitats in the subarctic. Front. Mar. Sci. 11:1435206. doi: 10.3389/fmars.2024.1435206
Received: 23 May 2024; Accepted: 09 September 2024;
Published: 30 September 2024.
Edited by:
Vitor H. Paiva, University of Coimbra, PortugalReviewed by:
Won Young Lee, Korea Polar Research Institute, Republic of KoreaCecile Vincent, Université de la Rochelle, France
Copyright © 2024 Florko, Yurkowski, Breiter, Ferguson, Gamblin, Grottoli, Nace, Shuert and Petersen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katie R.N. Florko, a2F0aWVmbG9ya29AZ21haWwuY29t
 Katie R.N. Florko
Katie R.N. Florko David J. Yurkowski
David J. Yurkowski C-Jae C. Breiter
C-Jae C. Breiter Steven H. Ferguson
Steven H. Ferguson Holly E.L. Gamblin2
Holly E.L. Gamblin2 Adam Grottoli
Adam Grottoli Andrea Nace
Andrea Nace Courtney R. Shuert
Courtney R. Shuert