
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 31 July 2024
Sec. Coastal Ocean Processes
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1432106
This article is part of the Research Topic Processes, Mechanisms and Solutions in Coastal Wetland to Adapt to Changing Environment View all 10 articles
Zostera japonica (Z. japonica), the most widely distributed seagrass species in temperate estuaries, has experienced a dramatic decline of nearly 75% over the past decade. While previous research has investigated the adaptation of seagrass individuals and populations to single stress factors, the molecular mechanisms underlying the interaction of multiple stressors remain poorly understood. This study conducted laboratory experiments to examine the response of Z. japonica at different life stages to environmental pressures, specifically salinity and turbidity, as indicated by changes in free amino acids (FAAs). The results demonstrate that Z. japonica exhibits stronger adaptability to high salinity environments but displays weaker adaptability to freshwater conditions. Through single stress experiments, the salinity and turbidity thresholds for FAA homeostatic disturbance in Z. japonica were determined at seedling, juvenile, and mature stages. As Z. japonica matures, its metabolic pathways expand and diversify, allowing the regulation of key FAAs to enhance stress resistance. Turbidity stress exerts a more pronounced negative impact on the cellular homeostasis of Z. japonica compared to salinity stress, and when turbidity levels exceed 150 NTU, they significantly intensify the negative effects of salinity stress on the seagrass. Furthermore, under strong salinity-turbidity interactions, the concentration of key FAAs generally decreases by 20-30%, indicating inhibition of growth and development in Z. japonica. These findings have important implications for the conservation of intertidal seagrass beds and estuarine ecosystems in the face of multiple human activities and environmental stressors. The study provides valuable insights into the molecular mechanisms underlying Z. japonica’s adaptations to salinity and turbidity stress, contributing to the development of targeted strategies to mitigate the impacts of environmental pressures on seagrass populations and promote the resilience of these critical marine ecosystems.
Seagrass, a marine angiosperm that thrives in the intertidal and subtidal zones of temperate and tropical coasts, plays a pivotal role as one of the most important primary producers in coastal ecosystems. Seagrass beds are among the most productive and ecologically valuable coastal ecosystems, providing a wide range of essential services (Orth et al., 2006; Short et al., 2007). These underwater meadows contribute significantly to water quality improvement by reducing suspended particles and removing excess nutrients from the water column and sediments. Moreover, seagrass beds act as natural barriers that attenuate wave energy and mitigate coastal erosion (Cornelisen and Thomas, 2006). In addition to their physical benefits, seagrass beds serve as critical habitats for a diverse array of marine species and provide food sources in the form of leaves, detritus, and epiphytes (Barbier et al., 2011).
Zostera japonica, a eurythermal seagrass species, exhibits a wide distribution along the northwestern Pacific Coast, spanning from the temperate regions of Russia to the tropical waters of southern Vietnam. The Yellow River Delta in China boasts the largest intertidal Z. japonica meadow in the country, covering an impressive area of over 1000 hectares (Zhang et al., 2020). This species is characterized by its well-developed and creeping rhizome, which enables both asexual reproduction through underground stems and sexual reproduction via seed germination. As a perennial angiosperm, Z. japonica predominantly inhabits the upper reaches of the intertidal zone. The ecological significance of Z. japonica lies in its ability to sequester carbon dioxide, store nutrients in sediments, and enhance dissolved oxygen levels in the water column (Park et al., 2011). Furthermore, this species serves as a valuable bioindicator for assessing water eutrophication levels (Han et al., 2017).
Recent studies have highlighted the importance of understanding the factors that influence the growth and distribution of Z. japonica in the Yellow River Delta. Environmental variables such as temperature, salinity, light availability, and sediment characteristics have been identified as key drivers of seagrass productivity and spatial patterns (Han et al., 2017; Zhang et al., 2020). Additionally, anthropogenic pressures, including coastal development, pollution, and climate change, pose significant threats to the long-term stability and resilience of these valuable ecosystems (Orth et al., 2006; Short et al., 2007).
Despite their ecological importance, seagrass beds have experienced a significant decline at an alarming annual rate of 7% worldwide since the 1990s, primarily due to the impacts of global warming and human activities (Waycott et al., 2009). Z. japonica, in particular, has been widely threatened and has undergone a dramatic decrease in its native range, especially in China (Zhang et al., 2020). In the last decade, the Z. japonica seagrass bed area in the Yellow River Delta has degraded by nearly 75% due to increasing human activities, the invasion of Spartina alterniflora, and extreme climate events (Zhou et al., 2022; Gao et al., 2023). Consequently, the protection and restoration of seagrass beds have become extremely pressing and challenging.
Previous studies have demonstrated that Z. japonica possesses strong self-defense mechanisms to mitigate the damage caused by salinity stress, with its salt tolerance mainly dependent on various physiological and biochemical processes (Canalejo et al., 2014). High salinity poses a significant obstacle to the survival and growth of seagrass by reducing the water potential of leaves and increasing the difficulty of maintaining growth (Cambridge et al., 2017; Blanco-Murillo et al., 2023). Prolonged exposure to saline stress can alter the photosynthetic and respiratory metabolism in seagrass, impairing the carbon balance and leading to complex photosynthetic adjustments (Sandoval-Gil et al., 2014).
In addition to salinity, light is another critical eco-factor influencing the growth of Z. japonica. Sediments from land can cause changes in light transmittance (Cussioli et al., 2020), and Hou et al. (2020) found a clear linear relationship between the height and biomass of Z. japonica and water turbidity. However, compared to subtidal species, Z. japonica exhibits a stronger adaptability to turbidity stress (Shafer and Kaldy, 2014). Under low light conditions, Z. japonica increases shoot height and chlorophyll concentration to enhance photosynthetic performance (Kim et al., 2020).
Free amino acids (FAAs) are sensitive to environmental stresses such as drought and cold and can serve as biological indicators to measure the effect of environmental disturbances. Song et al. (2012) discovered that Z. japonica typically increases the content of relevant amino acids to stimulate the accumulation of stress-responsive proteins, thereby enhancing its tolerance to stress. Chou et al. (2019) also used FAAs to monitor the stoichiometric characteristics of aquatic Z. japonica, finding that the free amino acid content in Z. japonica is related to the availability of carbon dioxide and light in the water. These 17 FAAs likely contribute to different aspects of plant defense mechanisms. For instance, proline (Pro) generally increases in response to osmotic and salt stress, while leucine, a branched-chain amino acid, plays a crucial role as an osmotic regulator in plant stress resistance. Methionine (Met) from the aspartate pathway can stimulate metabolic and transcriptomic responses associated with drought stress and mitochondrial energy metabolism, enhancing tolerance to abiotic stressors. Furthermore, the metabolism of cysteine (Cys) is associated with biological pathways such as protein synthesis, DNA repair, and antioxidant responses. Therefore, exploring the response mechanisms of seagrasses to environmental stress by analyzing the metabolism of amino acid components is essential for understanding their adaptations and resilience.
The Yellow River Estuary (YRE) is characterized by complex flow, sediment, and salinity conditions, and is home to an extensive seagrass bed. The annual distribution of water and sediment fluxes from the Yellow River (YR) is extremely uneven. During the flood season, surged runoff with high sediment is injected into the estuary, dramatically reducing salinity and increasing turbidity in the YRE. This unique environment makes the YRE an ideal experimental field for exploring the adaptive mechanisms of Z. japonica to salinity and turbidity stress. Through laboratory control experiments, this study aims to answer the following questions: 1) What is the effect mechanism of individual eco-factors on amino acid homeostasis in Z. japonica? 2) What is the synergistic influence mechanism of multiple eco-factors on Z. japonica at the molecular level? and 3) What are the responses of Z. japonica at different growth stages? To address these questions, Z. japonica plants were collected at seedling, juvenile, and mature stages. Stress experiments were conducted under six salinity gradients (0 ppt [fresh water], 5 ppt, 10 ppt, 20 ppt [control group], 25 ppt, and 35 ppt [seawater]) and six turbidity gradients (0 NTU [control group], 50 NTU, 100 NTU, 150 NTU, 200 NTU, and 250 NTU). The concentration of each FAA was determined, and the response mechanisms of Z. japonica at different growth stages to external stresses (salinity and turbidity) were further analyzed. This comprehensive approach will provide valuable insights into the adaptations and resilience of Z. japonica to the dynamic environmental conditions in the Yellow River Estuary (YRE), contributing to the development of effective conservation and management strategies for this important seagrass species.
YRE is located in Dongying, Shandong Province, where the Yellow River discharges into the Bohai Sea (Figure 1). In 2015, the largest seagrass bed of Z. japonica in China, covering an area of 1031.8 hectares, was discovered in the intertidal zone of the YRE (Zhou et al., 2015, Zhou et al., 2016). This seagrass bed is interspersed with the invasive species Spartina alterniflora and stretches 25-30 km along the coast on both the north and south sides of the estuary, with a width of 200-500 m from the shoreline to the sea. The Z. japonica bed provides essential food sources (Li et al., 2019) and habitats for various wetland birds, such as Cygnus, Grus, and Ciconia, as well as fishes and invertebrates.
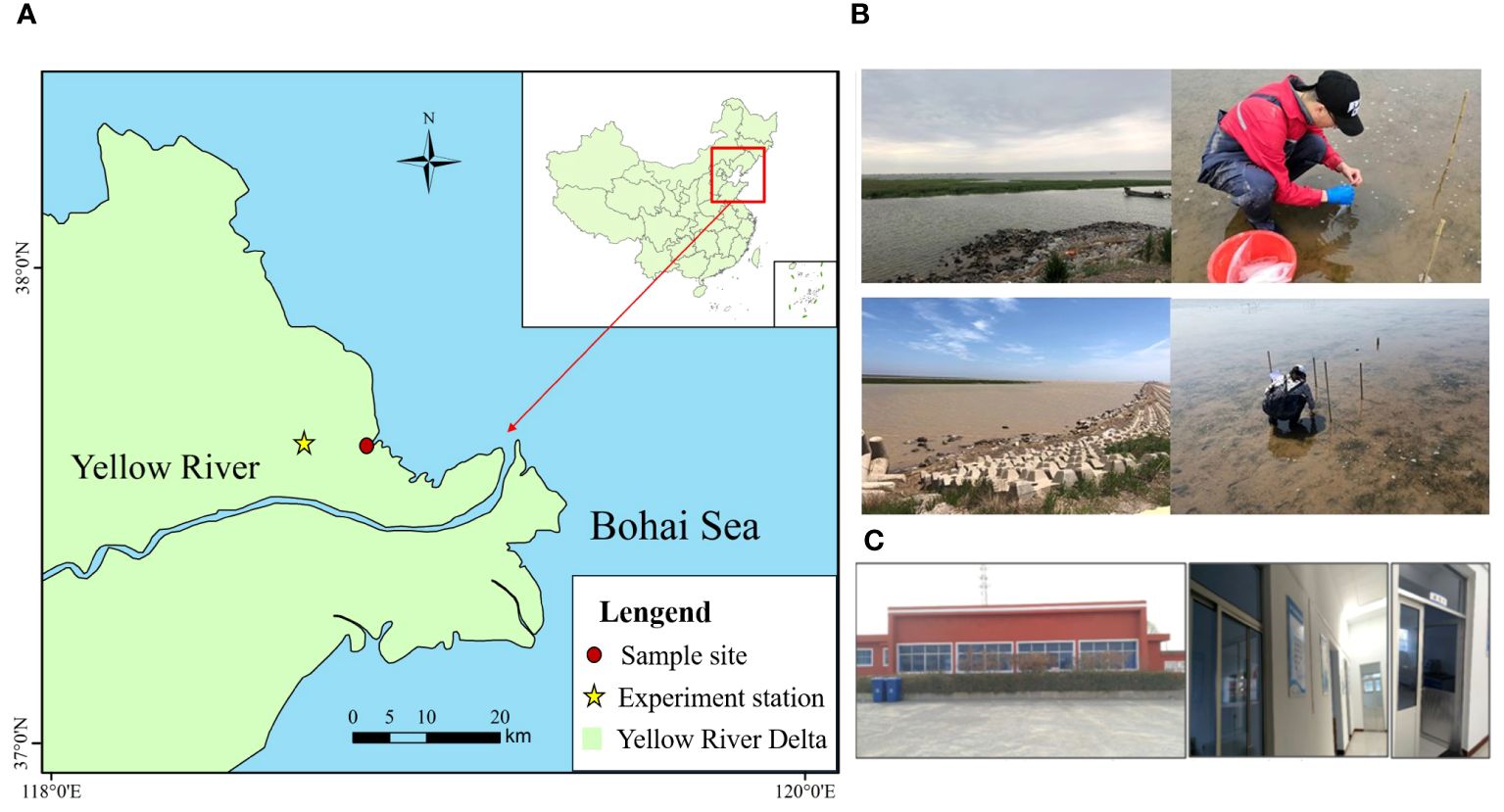
Figure 1 Location of the Zostera japonica sampling site and experiment station in the Yellow River Delta. (A) Study area; (B) The sampling setting of Zostera japonica; (C) Experiment station.
Seagrasses with similar growth (approximately 1000 Z. japonica/m2) were collected from less disturbed tidal flats on the northern shore of the YRE (119°15′10.25′′E, 37°44′17.04′′N), in an area with minimal influence from inflow, as shown in Figure 1. Monitoring of water salinity and turbidity was conducted on-site using the YSI (EXO multiparameter water quality monitor) (Supplementary Table S1). The sampling zone consisted of three belts, each with three sampling points evenly set 50 m apart. At each sampling point, a 1m×1m quadrat was arranged (Figures 1B, 2). Samples were collected during three growth stages: seedling stage (March to April), juvenile stage (May to June), and mature stage (July to August). To minimize ecological damage in the sample area and ensure practicality, the sampling number was kept small. Reusable PP planting basins with 0.8 mm thickness, 6.0 cm bottom diameter, and 8.0 cm height were selected based on the size of the sample area. During sampling, whole Z. japonica plants were collected to maintain the integrity of the root system, with approximately 10 plants in each pot covered with topsoil. The pots were placed indoors for a week of preculture, and Z. japonica plants with better adaptation were selected and cultivated under improved ventilation and light conditions for a ten-day control experiment.
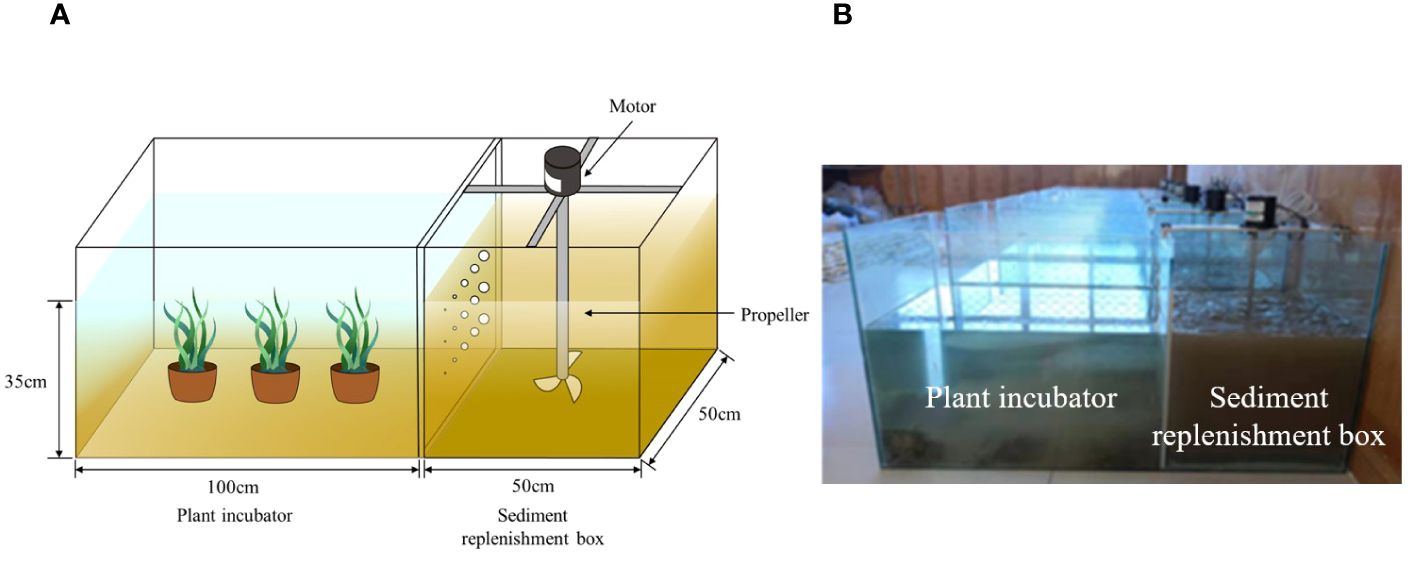
Figure 2 Experimental installation (A) Schematic diagram of the equipment and experiment. (B) Photo of the equipment and experiment.
In this experiment, locally produced marine crystals (origin: Jinan Huili Chemical Technology Co., Ltd., Gudao Town, Hekou District, Dongying City, Shandong Province, China) were used to prepare experimental water with different salinity levels. The marine crystals did not contain nutrients such as nitrogen and phosphorus. Surface sediments were collected from the growing area of Z. japonica on the northern shore of the YRE. The sediments were dried, crushed, and sieved through a 100-mesh sieve (< 150 μm) to set different turbidity gradients. This approach ensured that the experimental conditions closely mimicked the natural environment of Z. japonica, allowing for a more accurate assessment of the species’ adaptations to salinity and turbidity stress.
The laboratory control experiment was conducted at the Yellow River Estuary Wetland Ecosystem Field Scientific Observation and Research Station in Dongying City, Shandong Province (Figure 1). The experimental setup consisted of a water-sand mixing box and an experimental reaction box, both made of 5-mm-thick glass with good transparency. A propeller at the bottom of the glass box, driven by an engine, rotated to ensure thorough mixing of water and sediment. The suspended sediment from the disturbed water flow entered the reaction box through pores, forming different turbidity gradients. During the experiment, the water depth was maintained at 35 cm, and the turbidity in the reaction chamber was adjusted every 4 h. The motor speed was set at 110 r/min, and 15 holes were evenly distributed in a 5/row × 3 rows pattern in the glass between the water-sand mixing box and the experimental reaction box. The hole diameters were 0.2 cm, 0.5 cm, 1.0 cm, 1.2 cm, and 1.5 cm. Figures 2A and 2B illustrate the experimental apparatus.
Based on the actual possible stress range, the experiment included six salinity gradients and six turbidity gradients. The control group was grown in water with a salinity of 20 ppt and a turbidity of 0 NTU, which is considered the most suitable salinity for Z. japonica growth (Shafer et al., 2011; Hou et al., 2020). The salinity gradients were divided into two groups: low salinity (0 ppt, 5 ppt, and 10 ppt) and high salinity (25 ppt and 35 ppt). The turbidity gradients were set at 50 NTU, 100 NTU, 150 NTU, 200 NTU, and 250 NTU. Throughout the experiment, the water temperature was maintained at approximately 25°C, consistent with the local temperature, and no nutrients were added. After treating the experimental plants, three planting pots were collected from each experimental device, and the number of surviving Z. japonica in each pot was counted. The surviving Z. japonica were isolated and washed with distilled water to determine the composition and content of FAAs. The use of multiple salinity and turbidity gradients, along with the inclusion of a control group, allows for a comprehensive assessment of Z. japonica’s adaptations to these stressors.
The collected Z. japonica samples were dried in an oven at 70°C until they reached a constant weight. The dried samples were then crushed and filtered through a 250 μm (60 mesh) sieve. A 50 mg portion of each sample was weighed and placed into a 3 ml centrifuge tube, followed by the addition of 1.5 ml of 5% sulfosalicylic acid solution. The samples were centrifuged at 10,000 r/min for 20 min at 20°C. The supernatant was collected and diluted twice with 0.02 mol·L-1 hydrochloric acid solution. The content of FAAs was determined using a Biochrom 30+ automatic amino acid analyzer (BIOCHROM, UK). The experiment utilized the 17 kinds of amino acid mixture standard material GBW(E)100062 (China, China Institute of Metrology) as the standard solution. The 17 kinds of FAAs in Z. japonica tissue were determined, as shown in Supplementary Table S2.
One-way ANOVA with the least significant difference (LSD) test was employed to assess the significant differences in FAAs of Z. japonica under different turbidity and salinity gradients at 95% (P < 0.05) and 99% confidence levels (P < 0.01). Additionally, two-way analysis of variance was used to analyze the differences in the effects of turbidity-salinity interactions on FAAs in Z. japonica at 95% (P < 0.05) and 99% confidence levels (P < 0.01). These statistical analyses were performed using SPSS 24.0 software (SPSS Inc., Chicago, IL, USA).
Changes in the 17 FAAs of Z. japonica at different growth stages under critical salinity/turbidity conditions were analyzed. First, the top 6 FAAs with the highest concentrations in the control group were identified. Then, the key FAAs that played major coordinating roles were determined based on their fold change (FC) values under critical stress conditions. Linear and non-linear trend tests for the key FAAs were conducted to reveal the regulatory mechanisms of Z. japonica under single and interactive stresses using the R package “ggtrendline” (Version 4.3.1) (Greenwell and Kabban, 2014).
Based on their biosynthetic pathways, the 17 FAAs measured in the experiment were divided into five groups: (1) glutamic-group amino acids (Glu group), containing Glutamic (Glu), Proline (Pro), and Arginine (Arg); (2) aspartate-group amino acids (Asp group), containing Aspartate (Asp), Lysine (Lys), Methionine (Met), and Threonine (Thr); (3) branched-chain amino acids (BCAAs), containing Valine (Val), Leucine (Leu), and Isoleucine (Ile); (4) aromatic-group amino acids (AAAs), containing L-Phenylalanine (Phe), Tyrosine (Tyr), and Histidine (His); and (5) serine-group amino acids (Ser group), containing Glycine (Gly), Serine (Ser), Alanine (Ala), and L-Cysteine (Cys). The composition and content of amino acids in Z. japonica tissues under different salinity and turbidity treatments were further analyzed to gain insights into the species’ adaptive responses.
The content and composition of FAAs in Z. japonica varied significantly across the three growth stages. As a starting point for investigating the regulatory mechanisms during salinity/turbidity stress, the FAA content and composition of the control group, grown under optimal conditions (salinity at 20 ppt, turbidity at 0 NTU), were analyzed to establish a baseline understanding of their status in the absence of stress (Figure 3). Under optimal conditions, the total amount of FAAs in Z. japonica was lowest (12.16 mg/g) at the seedling stage and highest (31.30 mg/g) at the juvenile stage. The composition of FAAs in Z. japonica was mainly composed of the Glu group, Asp group, and Ser group. However, the proportion of each amino acid group varied greatly at different growth stages. During the transition from the seedling stage to the mature stage, there was a gradual decrease in the proportion of the Glu group, while that of the Ser group increased.
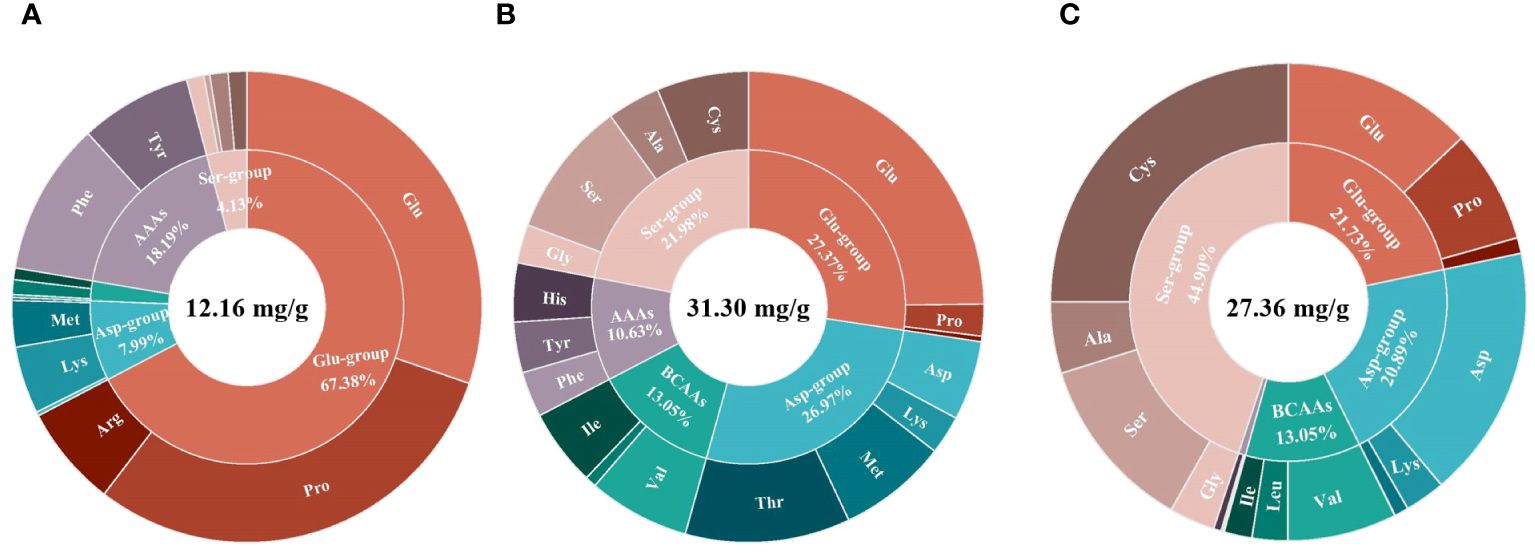
Figure 3 The proportion of FAAs in Z. japonica under different salinity and turbidity treatments. (A) Seedling stage. (B) Juvenile stage. (C) Mature stage.
Compared to the control group, the stress threshold of Z. japonica at different growth stages was determined. When the salinity was 10 ppt, the content of FAAs in the seedling stage significantly decreased (p < 0.01), indicating the occurrence of salinity stress (Figure 4A). However, Z. japonica at the mature stages only showed salinity stress when the salinity decreased to 0 ppt (Figures 4B, C). Under high salt treatment, FAAs of seedling Z. japonica significantly increased at a salinity of 25 ppt (p < 0.01) (Figure 4A), indicating hypersaline stress. Interestingly, there were no significant changes in FAAs in juvenile and mature Z. japonica under high-salinity treatment (Figures 4B, C). Under the optimal salinity condition (20 ppt), the differences in the total content of FAAs in Z. japonica were analyzed between turbidity-free and different turbidity gradients. When the turbidity reached 100 NTU, FAAs in Z. japonica significantly increased, indicating an apparent turbidity stress (Figure 4). It indicated that a turbidity level of 100 NTU represents a critical threshold for Z. japonica, irrespective of growth stage.
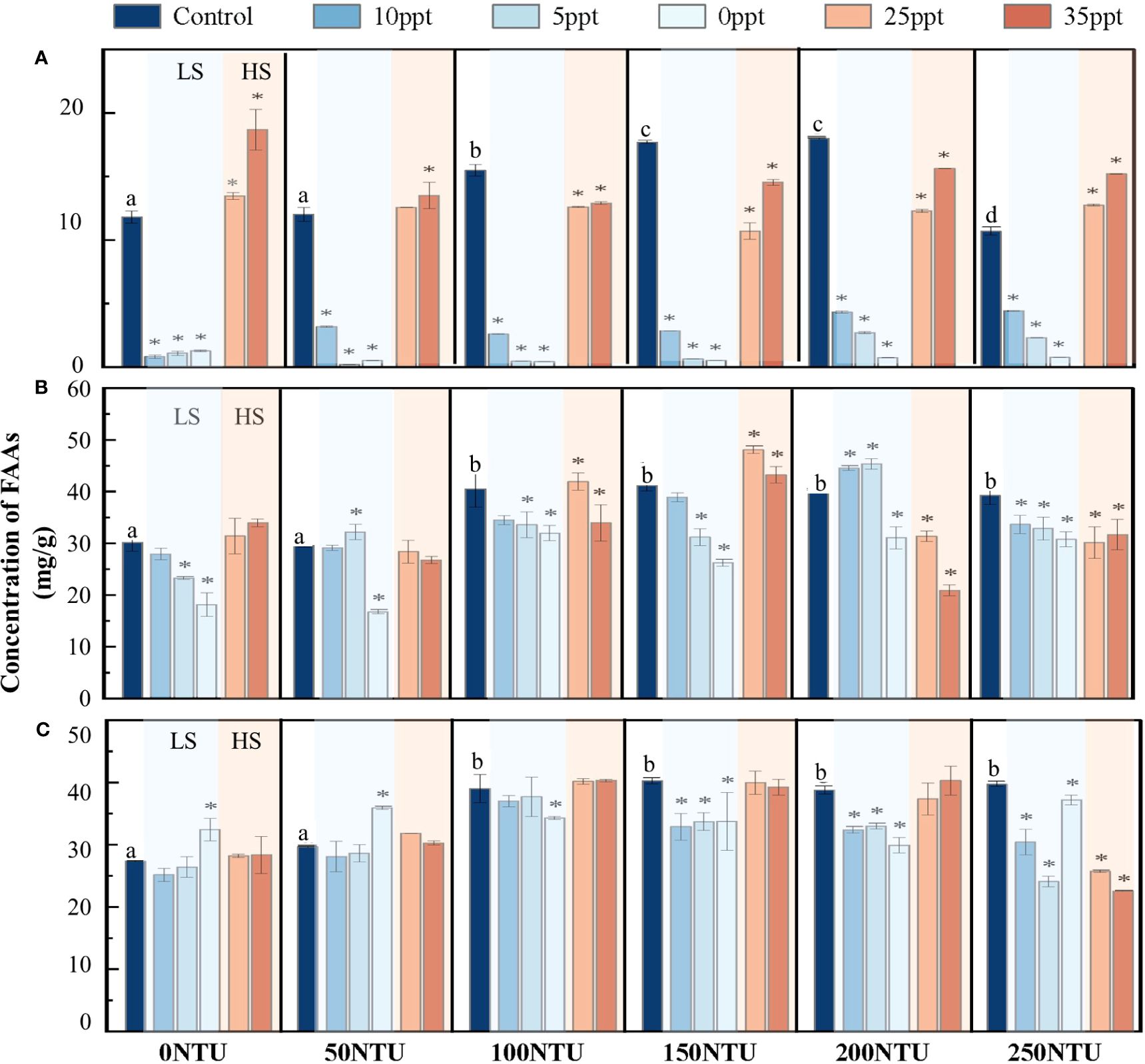
Figure 4 Changes of total concentration of FAAs in Z. Japonica at different growth stages under different salinity gradients. (A) Seedling stage. (B) Juvenile stage. (C) Mature stage. One-way ANOVA was constructed between control group and each experiment group: “⁎” represents FAAs concentration in this salinity condition significantly different from that in control group [95% confidence level]; Alphabet (e.g. a, b, c, …) represents FAAs concentration in this turbidity condition significantly different from that in control group [95% confidence level].
The interaction between salinity and turbidity had a cumulative effect on Z. japonica. Two-way ANOVA (Table 1) was used to determine the effect size of salinity, turbidity, and salinity-turbidity interaction, represented by partial eta squared (η2). Compared to the single-stress treatments, salinity-turbidity interaction had a stronger explanatory power for the variations in FAAs of Z. japonica at the seedling and juvenile stages. At the seedling stage, the content of FAAs in Z. japonica was the lowest and fluctuated sharply with changes in salinity and turbidity (Figure 4A). At the juvenile stage, increased water turbidity exacerbated the stress of salinity on Z. japonica. With a 100 NTU turbidity treatment, FAAs of Z. japonica in the juvenile stage showed a significant decrease under 25 ppt salinity (p < 0.05), which was not observed under turbidity-free conditions. For mature Z. japonica, turbidity stress contributed more to the changes in FAAs than salinity stress and salinity-turbidity interaction (Table 1). FAAs were relatively higher under the treatment of high salinity-medium turbidity (Figure 4C). Furthermore, at a turbidity of 150 NTU, the impact of salt stress on mature Z. japonica was intensified.
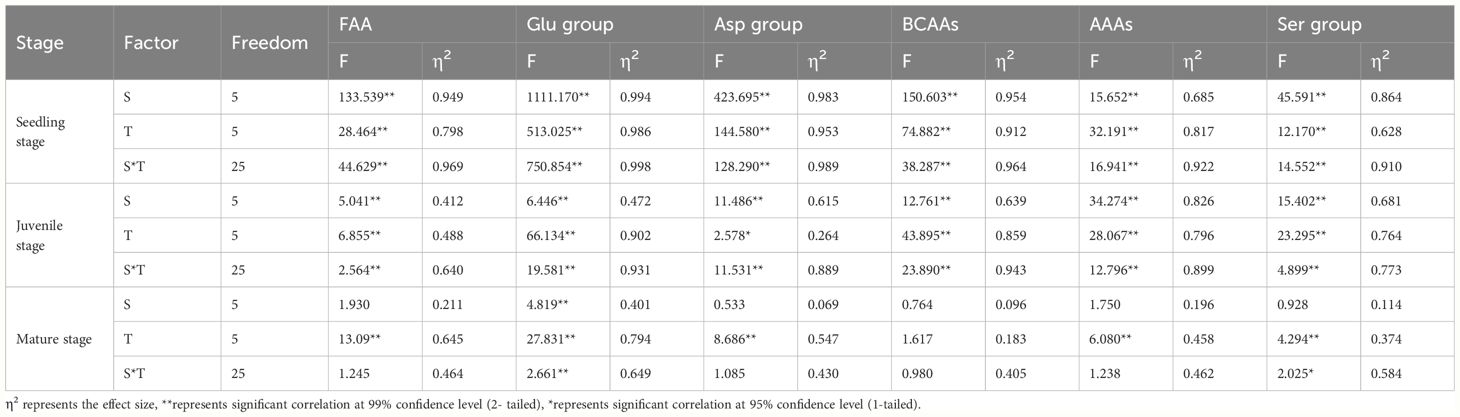
Table 1 Statistical results of the two-way ANOVA analyses examining the effects of Salinity (S), Turbidity (T) and Salinity-turbidity interaction (S*T) treatment on the content of FAAs in Z. Japonica.
These findings highlight the complex interplay between salinity and turbidity in shaping the stress responses of Z. japonica at different growth stages. The stronger explanatory power of salinity-turbidity interaction for FAA variations in seedling and juvenile plants suggests that these earlier life stages are more sensitive to the cumulative effects of multiple stressors. The sharp fluctuations in FAA content under varying salinity and turbidity levels at the seedling stage further support this notion of heightened sensitivity during early development. The observation that increased turbidity exacerbates the impact of salinity stress on juvenile Z. japonica underscores the importance of considering the synergistic effects of environmental factors when assessing the adaptive capacity of this species. The significant decrease in FAAs under high salinity (25 ppt) and moderate turbidity (100 NTU) conditions, which was not detected under turbidity-free conditions, indicates that the combined stress exceeds the tolerance threshold of juvenile plants, leading to a more pronounced metabolic response.
In contrast, the greater contribution of turbidity stress to FAA changes in mature Z. japonica suggests a shift in the relative importance of individual stressors as the plants age. The higher FAA levels observed under high salinity-medium turbidity and medium salinity-high turbidity treatments in mature plants may reflect an adaptive response to maintain osmotic balance and protect cellular functions under these challenging conditions.
Considering that high turbidity treatment exacerbated the effect of salinity stress on Z. japonica, the differences in the change trends of regulatory FAAs under single salinity stress and interactive stress were compared to reveal the regulatory mechanisms of Z. japonica under interactive stress. For Z. japonica at the seedling stage, Pro, Met, and Val served as the key regulatory FAAs (Figure 5). Under turbidity stress, the concentrations of Met (FC=2.63) and Val (FC=3.15) initially increased and then decreased with increasing turbidity, exceeding twice that of the control group at the critical point (100 NTU) (Figures 5, 6A). Under low salinity conditions, all FAAs decreased to nearly 0. Under high salinity stress, the concentrations of 14 FAAs, primarily Val (FC=1.54), Pro (FC=1.58), and Met (FC=1.79), increased (Figure 6B). Under salinity-turbidity interaction, the contents of the three key FAAs decreased overall and exhibited a decreasing trend with increasing intensity of salinity stress (Figure 6C).
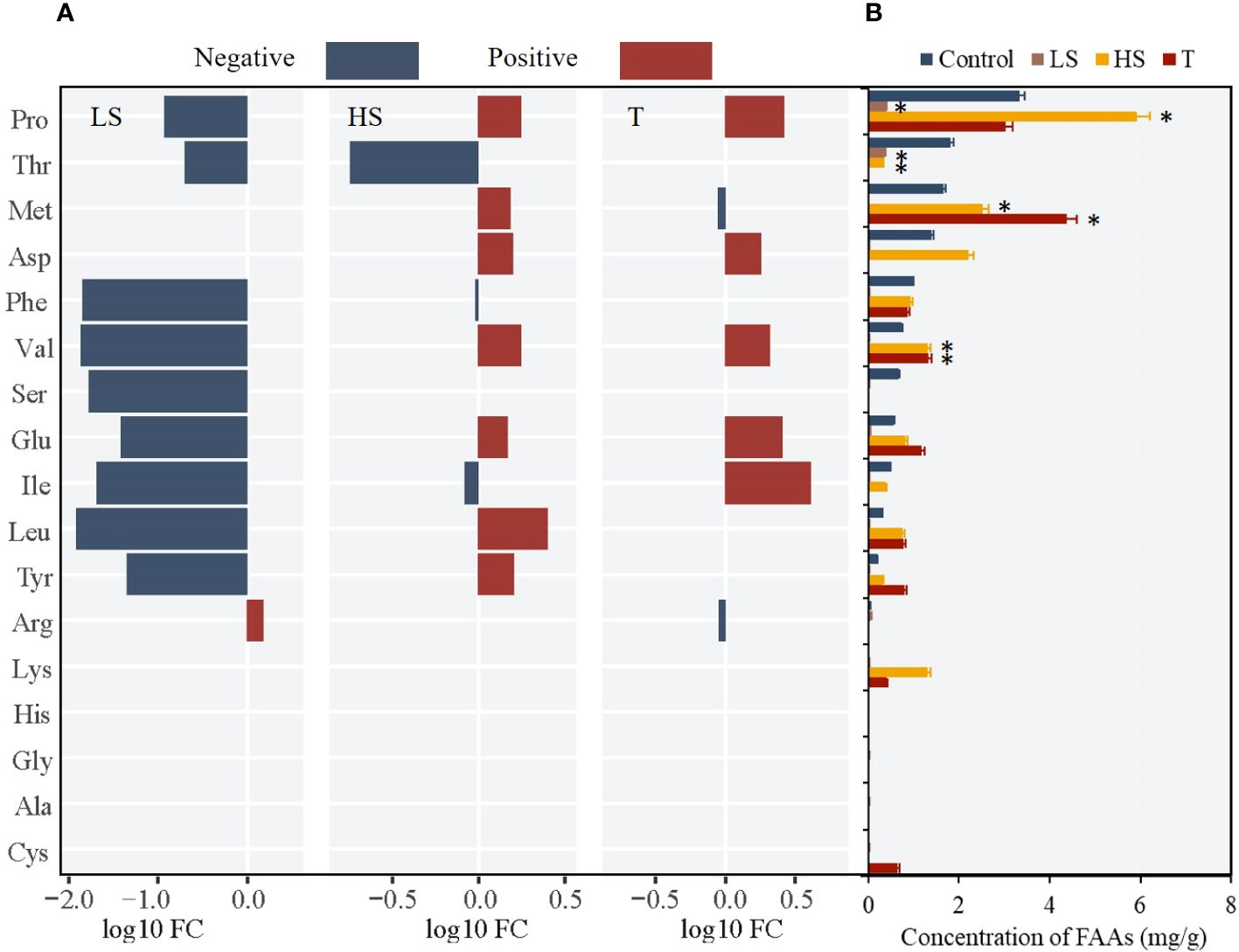
Figure 5 Changes of 17 FAAs in seedling Z. Japonica with low salinity (LS), high salinity (HS) and turbidity(T) treatments comparing with control group (* represents significant differences of major FAAs contents between experimental group and control group).
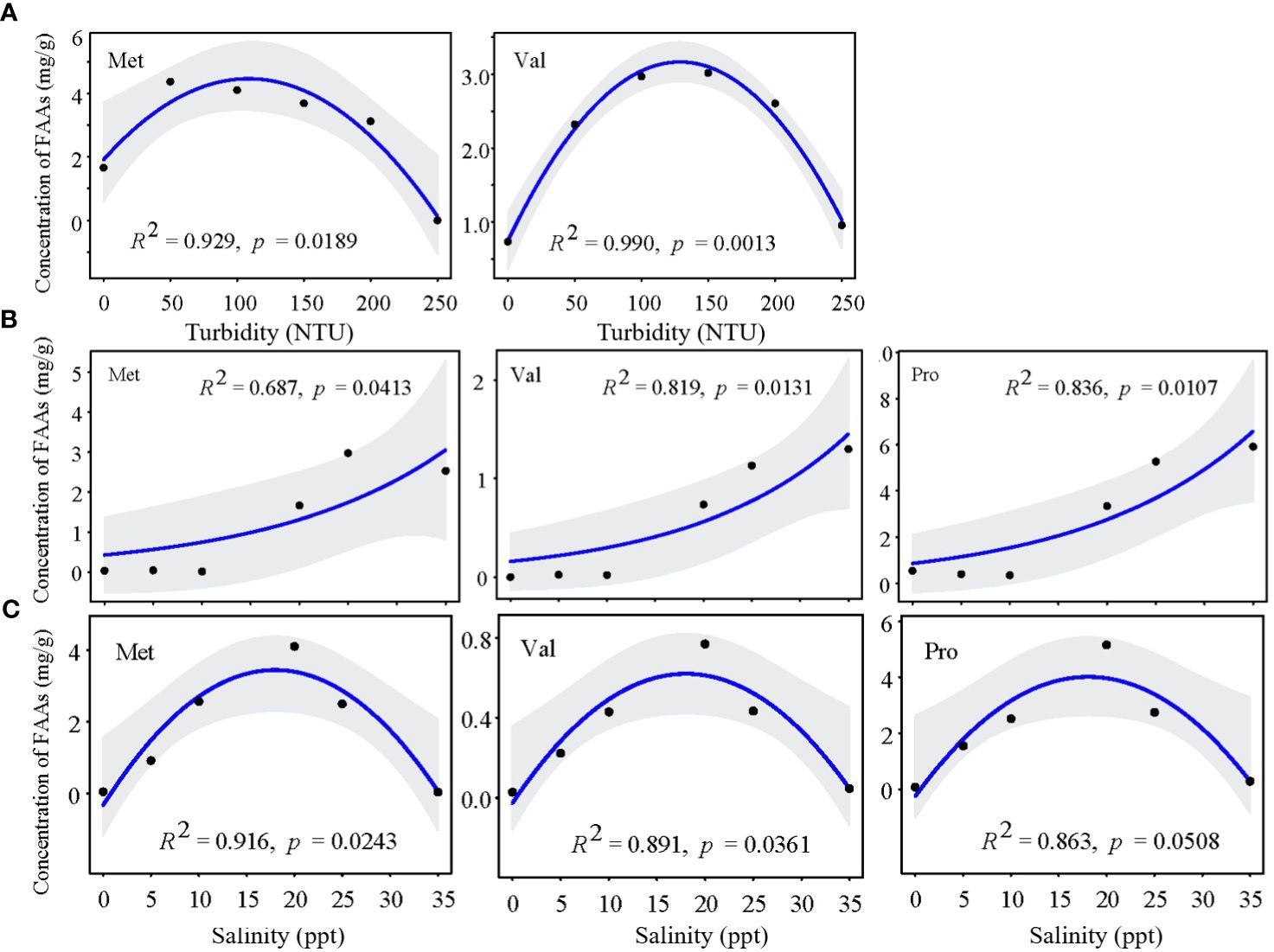
Figure 6 Change trend in key FAAs of seedling Z. Japonica with (A) turbidity, (B) salinity and (C) salinity-turbidity interaction treatments(Turbidity = 100NTU).
For Z. japonica at the juvenile stage, the key regulatory FAAs were Cys, Thr, Val and Glu. Under the critical turbidity condition (100 NTU), Cys (FC=7.20) and Met (FC=2.13) concentrations were up-regulated, while Glu concentration was down-regulated by 2/3 (Figure 7). Under single turbidity stress, Cys concentration first increased and then decreased with increasing turbidity, while Glu concentration showed an opposite trend, with a turning point at 150 NTU (Figure 8A). Under single salinity stress, FAA concentrations generally decreased (Figure 8B). Under freshwater conditions, only a few FAAs showed an increase, with Cys (FC=2.43) concentration reaching more than double that of the control group (Figure 7). Under high salinity stress, concentrations of Val (FC=1.89) and Thr (FC=1.82) increased, while Cys showed an opposite trend (Figure 8B). With the salinity-turbidity interaction treatment (turbidity at 150 NTU), the variations in Cys and Val concentrations were significantly influenced by turbidity stress, exhibiting altered patterns. Cys concentration showed a significant decreasing trend in response to hypersaline stress, while Val concentration showed an opposite trend (Figure 8C).
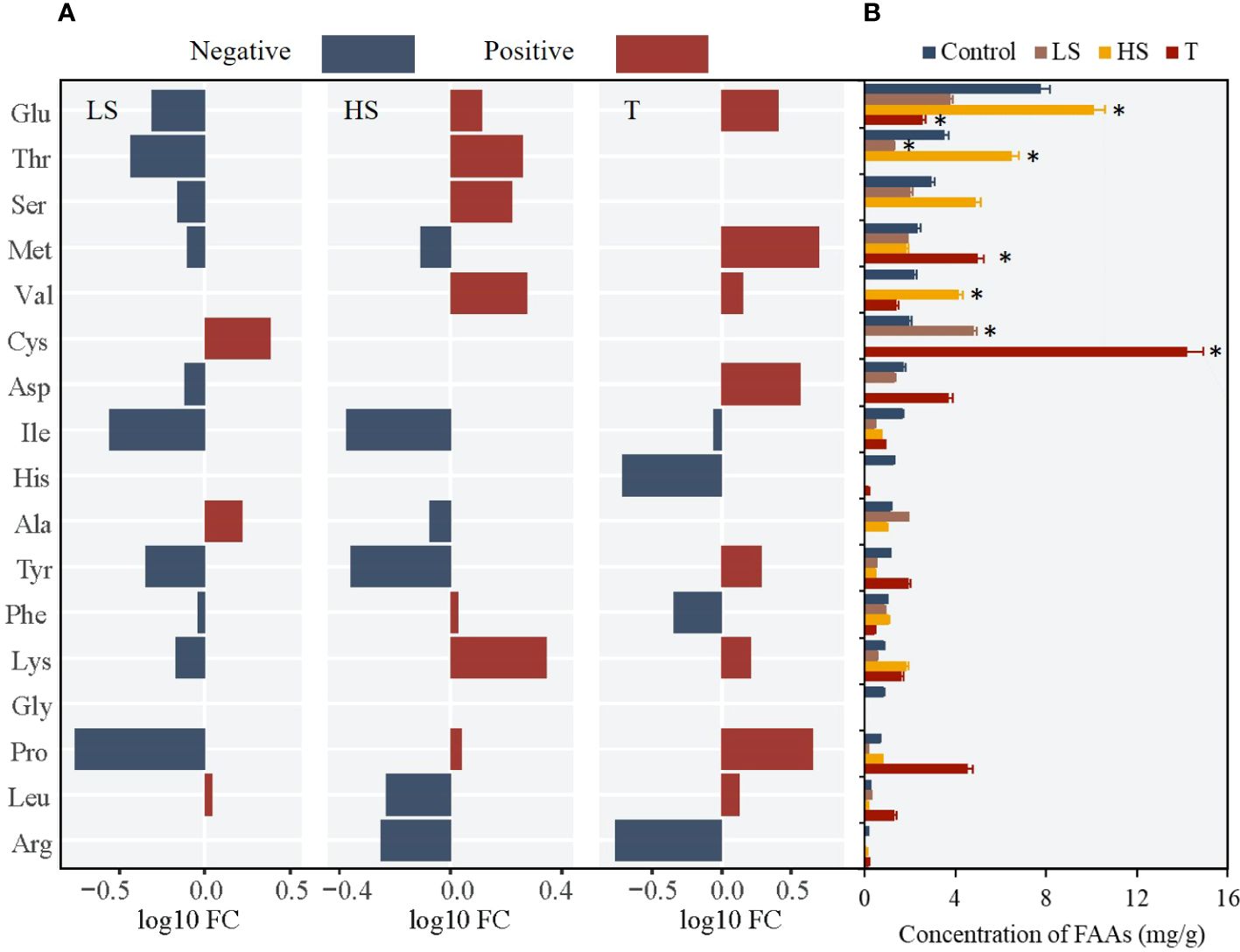
Figure 7 Changes of 17 FAAs in juvenile Z. Japonica with low salinity (LS), high salinity (HS) and turbidity(T) treatments comparing with control group (* represents significant differences of major FAAs contents between experimental group and control group).
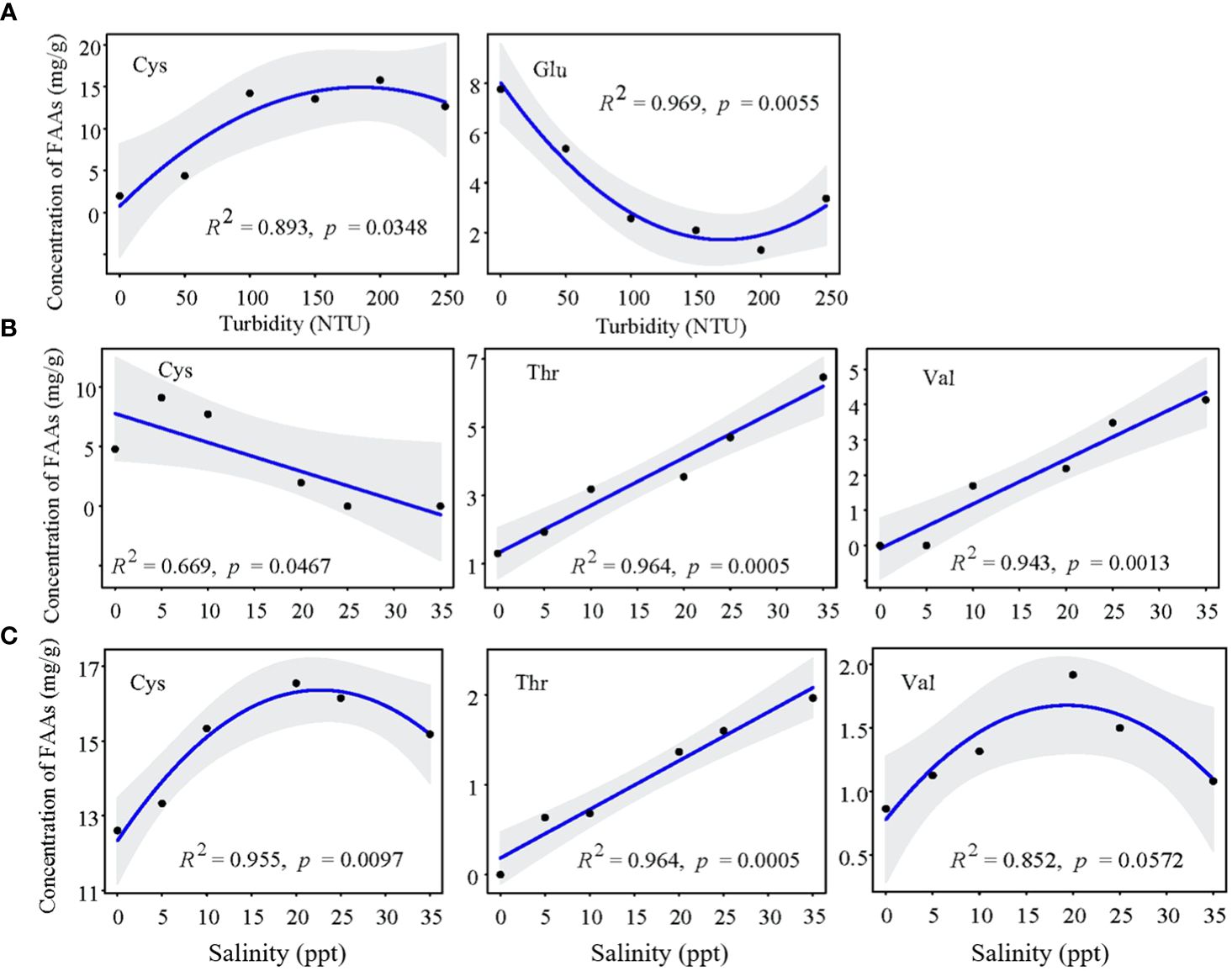
Figure 8 Change tendency of key FAAs concentration in juvenile Z. Japonica with (A) turbidity, (B) salinity and (C) salinity-turbidity interaction treatments (Turbidity = 150NTU).
For mature Z. japonica, the key regulatory FAAs were Ser, Glu and Pro. When turbidity stress (150 NTU) occurred, Pro (FC=2.84) played a major regulating role and was positively correlated with water turbidity (Figures 9, 10A). The concentrations of other main FAAs fluctuated less. When salinity stress occurred at 0 ppt, most FAAs exhibited an upward trend. Notably, Ser (FC=2.44) and Glu (FC=1.91) played crucial roles in the resistance to salinity stress, as they were up-regulated with decreasing salinity levels (Figure 10B). However, the interaction exacerbated the impact of salinity stress, as evidenced by the contrasting trends in Ser and Glu concentrations and an overall decrease (Figure 10C). Interestingly, high turbidity-high salinity conditions increased the performance levels of key FAAs.
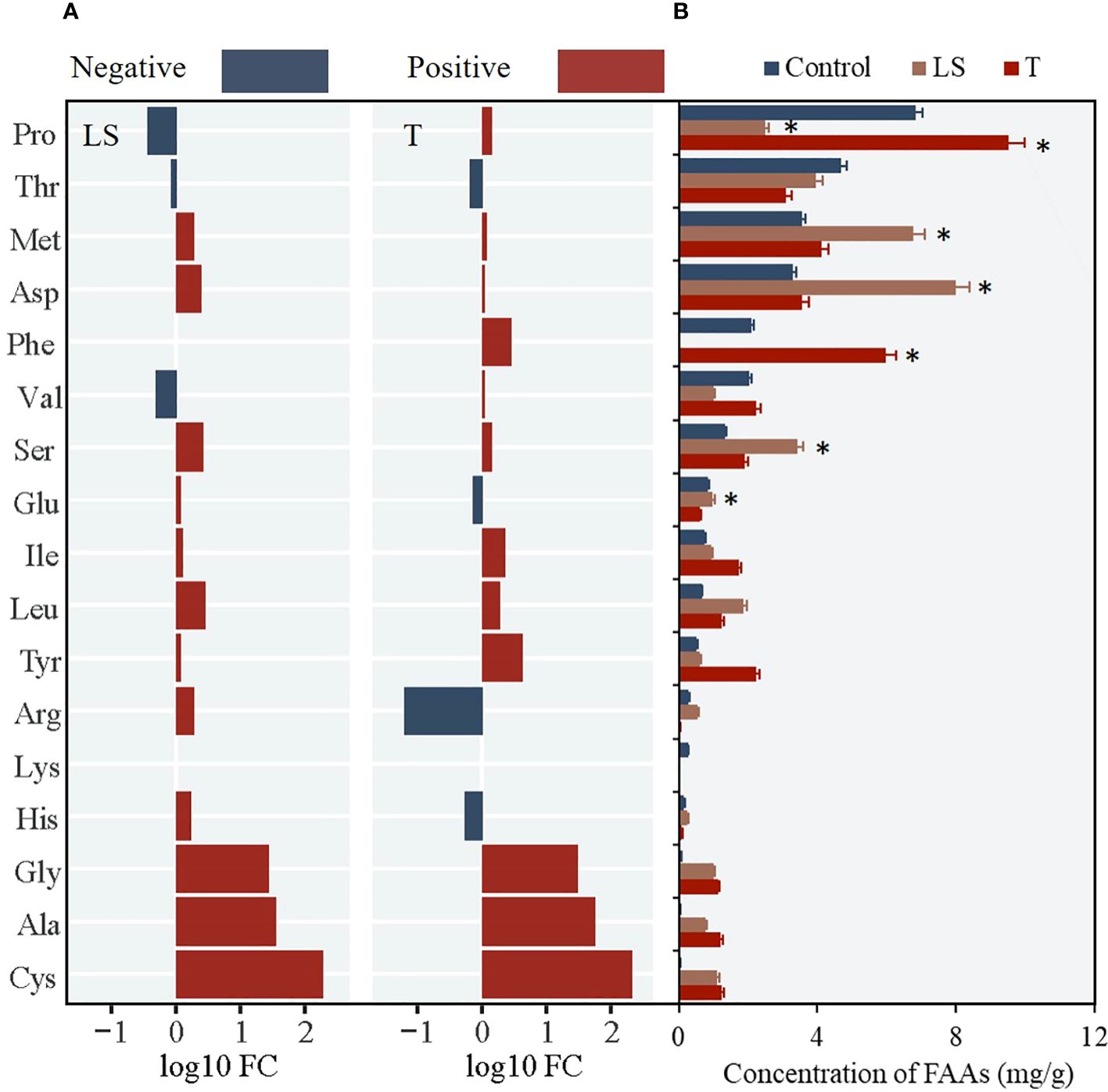
Figure 9 Changes of 17 FAAs in juvenile Z. Japonica with low salinity (LS), high salinity (HS) and turbidity(T) treatments comparing with control group (* represents significant differences of major FAAs contents between experimental group and control group).
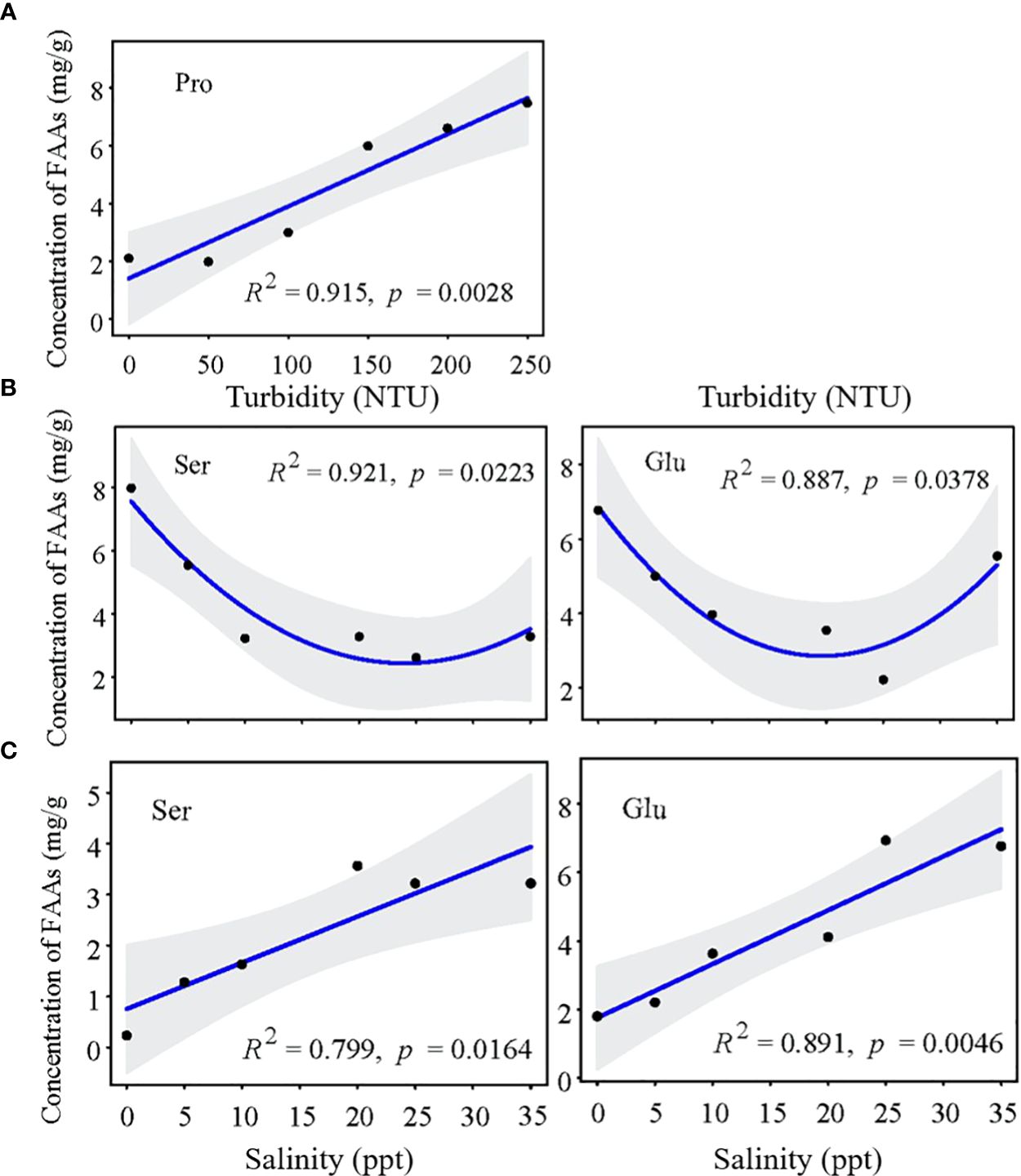
Figure 10 Change tendency of key FAAs concentration in mature Z. Japonica with (A) turbidity, (B) salinity and (C) salinity-turbidity interaction treatments (Turbidity = 150NTU).
Z. japonica, an intertidal seagrass species, has evolved remarkable adaptations to the highly variable environmental conditions in its habitat, exhibiting high desiccation tolerance, salinity adaptation, and light adaptation (Shafer and Kaldy, 2014; Kim et al., 2020). As crucial organic solutes, amino acids play a pivotal role in Z. japonica’s ability to cope with stress conditions by participating in osmotic pressure adjustments, photogrammetry responses, and nitrogen metabolism maintenance (Sandoval-Gil et al., 2014; Cambridge et al., 2017). The composition of amino acid groups in Z. japonica varies significantly across its life cycle, reflecting the changing metabolic demands and physiological processes associated with each growth stage. In the seedling stage, glutamic acid group (Glu group) constitutes more than 30% of the total amino acid content (Figure 3), which can be attributed to the high organic nitrogen requirements for the rapid development of leaves and stems. Glutamic acid serves as a primary amino donor for the synthesis of other amino acids and nitrogen-containing compounds, facilitating the seedlings’ growth and development (Griffiths et al., 2020). As Z. japonica matures, the proportion of serine group amino acids (Ser group) becomes the highest (Figure 3), supporting chlorophyll synthesis and maintaining homeostasis in the fully developed plants. The varying amino acid composition across growth stages highlights the seagrass’s ability to fine-tune its metabolic processes in response to changing developmental needs and environmental conditions. Z. japonica exhibits distinct adjustment mechanisms to external stress at different life stages, which can be attributed to the morphological and physiological variations associated with its development. When exposed to salinity and/or turbidity stress, mature Z. japonica maintains metabolic balance by altering the composition of FAAs within a narrow range (Figure 3), demonstrating an enhanced tolerance to these environmental challenges compared to earlier growth stages. This adaptive capacity allows mature plants to better cope with the dynamic conditions in their intertidal habitat, ensuring their survival and persistence.
As intermediates in specific metabolic pathways, FAAs serve a crucial regulatory function in plants by influencing multiple metabolic, physiological, and biochemical processes. Consequently, changes in FAA content and composition can act as reliable indicators of cellular homeostasis and abiotic stress in plants. In this study, molecular analysis was employed to determine the environmental threshold at which cellular homeostasis is disturbed in Z. japonica (Figure 4). Z. japonica exhibits varying stress thresholds for salinity and turbidity at different growth stages. The seedling stage appears to be the most sensitive to salinity stress, with significant changes in FAA content observed at both low (10 ppt) and high (25 ppt) salinity levels. In contrast, juvenile and mature plants only showed significant responses to extremely low salinity (0 ppt), suggesting a higher tolerance to salinity fluctuations at these later growth stages. Remarkably, this threshold was found to be narrower than the range of conditions that elicit significant morphological changes at each growth stage (Hou et al., 2020). This finding underscores the potential of FAAs as highly sensitive indicators of abiotic stress in seagrasses inhabiting the intertidal zones of high-turbidity estuaries. By detecting stress responses at the molecular level before visible morphological changes occur, FAA analysis can provide early warning signals of environmental perturbations and facilitate timely management interventions to protect these valuable coastal ecosystems.
Z. japonica exhibits a remarkable capacity to adapt to high salinity, which strengthens as the plant matures. The absence of significant changes in FAA content under high-salinity treatment in juvenile and mature plants further supports this notion of increased salinity tolerance with age. Salinity affects seagrass metabolism by altering osmotic pressure and ion concentrations in Z. japonica (Galvan-Ampudia and Testerink, 2011; Canalejo et al., 2014; Julkowska and Testerink, 2015). FAAs play a crucial role in maintaining the metabolic balance of Z. japonica under different salinity conditions through continuous synthesis, accumulation, and transformation. Under freshwater conditions, a notable decrease in FAA concentration was observed in both seedling and juvenile Z. japonica. However, under hypersaline stress, seedling-stage Z. japonica adapted by upregulating FAAs related to osmotic regulation (e.g., Pro) and cellular energy metabolism (e.g., Val, Met) (Cohen et al., 2014). The increased expression of ornithine ω-transaminase under osmotic stress leads to Pro synthesis (Delauney et al., 1993), which prevents ion outflow. Additionally, due to the increased demand for proteins and energy under adverse conditions, BCAAs like Val, Leu, and Ile participate in the tricarboxylic acid cycle (TAC) for mitochondrial respiration. Val degradation, in particular, produces energy comparable to carbohydrate metabolism (Heinemann et al., 2021). As Z. japonica matures, its metabolic pathways expand and diversify, enhancing stress resistance by upregulating certain FAAs involved in chlorophyll synthesis (e.g., Glu, Ser, Thr), thereby improving photosynthetic rates (Figure 6B, Figure 8B, Figure 10B) (Xu et al., 2020). Simultaneously, the conversion from Gly to Ser can provide ATP for plants, enhancing photorespiration and protecting the seagrass against external stress (Ehlting et al., 2007).
Compared to saline stress, light suppression might trigger more severe inhibition of Z. japonica’s metabolism. Underwater light conditions affect the photosynthetic rate of Z. japonica, and increased water turbidity can lead to water hypoxia, allowing gaseous H2S to invade the seagrass (Borum et al., 2005), inhibiting seaweed metabolism and increasing mortality (Pérez-Pérez et al., 2012; Lamers et al., 2013). However, Z. japonica, known as a highly light-adapted species with significant morphological and physiological plasticity, can thrive under highly variable environmental conditions. Under moderate turbidity (100~150 NTU), key FAAs in Z. japonica accumulate to maintain homeostasis by participating in various metabolic pathways. During energy deficiency, Z. japonica relies on BCAA catabolism pathways (e.g., Val, Leu, and Ile) to supply energy and maintain basic cellular metabolism. Juvenile and mature Z. japonica accumulate specific FAAs (e.g., Pro and Cys) related to protein synthesis and detoxification of organic peroxides to sustain cellular function and enhance resistance to turbidity stress (Figure 6A, Figure 8A, Figure 10A) (Yang et al., 2020). This indicates that Z. japonica demonstrates increased physiological plasticity in response to external stress as it matures. The long-term survival of Z. japonica in highly turbid water may lead to genetic improvements that enhance resistance to turbidity stress. However, when water turbidity exceeds 150 NTU, it becomes unsuitable for Z. japonica growth. The concentration of FAAs in Z. japonica generally declines, indicating a disruption in the resistance response and potential impacts on cellular homeostasis (Figure 4) (Heinemann et al., 2021).
Turbidity exacerbates the negative effects of salinity stress on Z. japonica. In highly turbid water, the limited photosynthesis of Z. japonica results in a restricted energy supply, rendering it more susceptible to salinity changes. The significant increase in FAA content at this turbidity level suggests that the plants are experiencing stress and adjusting their metabolic processes in response to the reduced light availability and altered sediment conditions. The identification of key regulatory FAAs at each growth stage provides valuable insights into the specific metabolic pathways and adaptive strategies employed by the plant to cope with these environmental challenges. The up-regulation of Pro in mature Z. japonica under turbidity stress and the increased concentrations of Ser and Glu under low salinity conditions underscore the importance of these FAAs in maintaining metabolic homeostasis and coping with environmental challenges in later life stages. Through FAA synthesis and transformation, Z. japonica continuously supplies energy to activate resistance responses to salinity stress, maintaining homeostasis, growth, and development. This includes the catabolism of BCAAs and the Asp group, as well as the conversion between the Ser group and Asp group (Song et al., 2013). However, increased turbidity imposes more severe light restrictions on Z. japonica, intensifying the inhibition of the photosynthesis pathway and blocking the organism’s energy generation pathway, leading to an insufficient energy supply. Moreover, Z. japonica requires more energy for resistance responses to maintain homeostasis, depleting organic matter within the cell and impeding normal physiological functions. These combined pressures degrade the adaptability of Z. japonica to salt stress, and even slight changes in salinity can cause drastic fluctuations in FAA composition and content within the seagrass, altering the environmental threshold (Figure 4). The exacerbated impact of salinity stress under salinity-turbidity interaction, as evidenced by the contrasting trends in key FAAs concentrations, suggests that the combined stress may strain the mature plants’ adaptive capacity. It is worth noting that juvenile and mature Z. japonica mitigate the negative effects of other side effects by elevating the content of FAAs associated with antioxidant responses and activating protective mechanisms, including Cys and Glu (Figure 8C, Figure 10C). This suggests that salinity-turbidity interaction may induce a progressive increase in glutathione synthesis (γ-glutathione + glycine, GSH), which can remove reactive oxygen species (ROS) to maintain redox status and increase chlorophyll content to restore photosynthesis (Gill and Tuteja, 2010; Son et al., 2014; Capó et al., 2020). However, the increased performance levels of key FAAs under high turbidity-high salinity conditions indicate a potential synergistic effect that enhances the plant’s stress tolerance.
To better understand the mechanisms underlying these stage-specific responses to salinity and turbidity stress, future studies should focus on integrating metabolomic, transcriptomic, and proteomic data to develop a more comprehensive understanding of the complex interplay between amino acid metabolism and stress responses in seagrasses. Comparative analyses of the metabolomic and transcriptomic profiles of Z. japonica at different growth stages and under various stress conditions could provide valuable insights into the adaptive strategies employed by this species throughout its life cycle. Furthermore, YRE has complex hydrodynamic and water environmental conditions, with many factors affecting the growth and development of Z. japonica. Assessing the impact of other environmental factors, such as total nitrogen, total phosphorus and dissolved oxygen, could help further elucidate the resistance mechanism of external stress on Z. japonica.
This study utilized laboratory control experiments to investigate the physiological mechanisms of Z. japonica under varying salinity and turbidity conditions. The results provide valuable insights into the adaptive capacity and vulnerability of Z. japonica to environmental stressors, highlighting the potential use of FAAs as sensitive biomarkers for monitoring seagrass health in high-turbidity estuaries. These findings contribute to effective conservation and management strategies for protecting these important marine ecosystems. The three main findings of this study are as below:
(1) FAAs can serve as highly sensitive indicators of abiotic stress in seagrasses within the intertidal zone of high-turbidity estuaries. Through single stress experiments, the salinity and turbidity thresholds for FAA homeostatic disturbance in Z. japonica were determined at seedling, juvenile, and mature stages.
(2) Z. japonica exhibits strong adaptability to high salinity environments but displays weaker adaptability to freshwater conditions. Interaction experiments further revealed that turbidity has a more pronounced negative impact on the cellular homeostasis of Z. japonica compared to salinity, and the two stressors have a cumulative effect. The negative effects of salinity stress on Z. japonica were significantly intensified when turbidity levels exceeded 150 NTU.
(3) Under the interaction of high turbidity and high salinity, the concentration of key FAAs in Z. japonica generally showed a downward trend. This decline in FAA concentration could contribute to the degradation of cellular energy metabolism, protein synthesis, stress resistance, and other physiological processes, ultimately inhibiting the growth and development of Z. japonica.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
YY: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. FZ: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CH: Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. CZ: Writing – original draft, Writing – review & editing. CT: Formal analysis, Validation, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (U2243236, 52025092), and the National Key Research and Development Program of China (2022YFC3202002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1432106/full#supplementary-material
Barbier E. B., Hacker S. D., Kennedy C., Koch E. W., Stier A. C., Silliman B. R. (2011). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Blanco-Murillo F., Díaz M. J., Rodríguez-Rojas F., Navarrete C., Celis-Plá P. S. M., Sánchez-Lizaso J. L., et al. (2023). A risk assessment on Zostera chilensis the last relict of marine angiosperms in the South-East Pacific Ocean, due to the development of the desalination industry in Chile. Science of the Total Environment 883, 163538. doi: 10.1016/j.scitotenv.2023.163538
Borum J., Pedersen O., Greve T. M., Frankovich T. A., Zieman J. C., Fourqurean J. W., et al. (2005). The potential role of plant oxygen and sulphide dynamics in die-off events of the tropical seagrass, Thalassia testudinum. J. Ecol. 93, 148–158. doi: 10.1111/j.1365-2745.2004.00943.x
Cambridge M. L., Zavala-Perez A., Cawthray G. R., Mondon J., Kendrick G. A. (2017). Effects of high salinity from desalination brine on growth, photosynthesis, water relations and osmolyte concentrations of seagrass Posidonia australis. Mar. pollut. Bull. 115, 252–260. doi: 10.1016/j.marpolbul.2016.11.066
Canalejo A., Martínez-Domínguez D., Córdoba F., Torronteras R. (2014). Salt tolerance is related to a specific antioxidant response in the halophyte cordgrass. Estuar. Coast. Shelf S 146, 68–75. doi: 10.1016/j.ecss.2014.05.017
Capó X., Tejada S., Ferriol P., Pinya S., Mateu-Vicens G., Montero-González I., et al. (2020). Hypersaline water from desalinization plants causes oxidative damage in Posidonia oceanica meadows. Sci. Of Total Environ. 736, 139601. doi: 10.1016/j.scitotenv.2020.139601
Chou Q. C., Cao T., Ni L. Y., Xie P., Jeppesen E. (2019). Leaf soluble carbohydrates, free amino acids, starch, total phenolics, carbon and nitrogen stoichiometry of 24 aquatic macrophyte species along climate gradients in China. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00442
Cohen H., Israeli H., Matityahu I., Amir R. (2014). Seed-specific expression of a feedback-insensitive form of CYSTATHIONINE-γ-SYNTHASE in arabidopsis stimulates metabolic and transcriptomic responses associated with desiccation stress. Plant Physiol. 166, 1575–1592. doi: 10.1104/pp.114.246058
Cornelisen C. D., Thomas F. I. M. (2006). Water flow enhances ammonium and nitrate uptake in a seagrass community. Mar. Ecol. Prog. Ser. 312, 1–13. doi: 10.3354/meps312001
Cussioli M. C., Seeger D., Pratt D. R., Bryan K. R., Bischof K., de Lange W. P., et al. (2020). Spectral differences in the underwater light regime caused by sediment types in New Zealand estuaries: implications for seagrass photosynthesis. Geo-Mar Lett. 40, 217–225. doi: 10.1007/s00367-020-00640-0
Delauney A. J., Hu C. A., Kishor P. B., Verma D. P. (1993). Cloning of ornithine delta-aminotransferase cDNA from Vigna aconitifolia by trans-complementation in Escherichia coli and regulation of proline biosynthesis. J. Biol. Chem. 268 (25), 18673–18678. doi: 10.1016/S0021-9258(17)46682-8
Ehlting B., Dluzniewska P., Dietrich H., Selle A., Teuber M., Hansch R., et al. (2007). Interaction of nitrogen nutrition and salinity in Grey poplar (Populus tremula x alba). Plant Cell Environ. 30, 796–811. doi: 10.1111/j.1365-3040.2007.01668.x
Galvan-Ampudia C. S., Testerink C. (2011). Salt stress signals shape the plant root. Curr. Opin. In Plant Biol. 14, 296–302. doi: 10.1016/j.pbi.2011.03.019
Gao Y., Yi Y., Chen K., Xie H. (2023). Simulation of suitable habitats for typical vegetation in the Yellow River Estuary based on complex hydrodynamic processes. Ecol. Indic. 154, 110623. doi: 10.1016/j.ecolind.2023.110623
Gill S. S., Tuteja N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch 48, 909–930. doi: 10.1016/j.plaphy.2010.08.016
Greenwell B. M., Kabban C. M. S. (2014). investr: an R package for inverse estimation. R J. 6, 90–100. doi: 10.32614/RJ-2014-009
Griffiths L. L., Melvin S. D., Connolly R. M., Pearson R. M., Brown C. J. (2020). Metabolomic indicators for low-light stress in seagrass. Ecol. Indic. 114, 106316. doi: 10.1016/j.ecolind.2020.106316
Han Q. Y., Soissons L. M., Liu D. Y., van Katwijk M. M., Bouma T. J. (2017). Individual and population indicators of Zostera japonica respond quickly to experimental addition of sediment-nutrient and organic matter. Mar. pollut. Bull. 114, 201–209. doi: 10.1016/j.marpolbul.2016.08.084
Heinemann B., Künzler P., Eubel H., Braun H. P., Hildebrandt T. M. (2021). Estimating the number of protein molecules in a plant cell: protein and amino acid homeostasis during drought. Plant Physiol. 185, 385–404. doi: 10.1093/plphys/kiaa050
Hou C. Y., Song J., Yan J. G., Wang K., Li C. H., Yi Y. J. (2020). Growth indicator response of Zostera japonica under different salinity and turbidity stresses in the Yellow River Estuary, China. Mar. Geology 424, 106169. doi: 10.1016/j.margeo.2020.106169
Julkowska M. M., Testerink C. (2015). Tuning plant signaling and growth to survive salt. Trends In Plant Sci. 20, 586–594. doi: 10.1016/j.tplants.2015.06.008
Kim S. H., Kim J. W., Kim Y. K., Park S. R., Lee K. S. (2020). Factors controlling the vertical zonation of the intertidal seagrass, Zostera japonica in its native range in the northwestern Pacific. Mar. Environ. Res. 157, 104959. doi: 10.1016/j.marenvres.2020.104959
Lamers L. P. M., Govers L. L., Janssen I., Geurts J. J. M., van der Welle M. E. W., Van Katwijk M. M., et al. (2013). Sulfide as a soil phytotoxin-a review. Front. Plant Sci. 4. doi: 10.3389/fpls.2013.00268
Li X. W., Hou X. Y., Song Y., Shan K., Zhu S. Y., Yu X. B., et al. (2019). Assessing changes of habitat quality for shorebirds in stopover sites: a case study in yellow river delta, China. Wetlands 39, 67–77. doi: 10.1007/s13157-018-1075-9
Orth R. J., Carruthers T. J. B., Dennison W. C., Duarte C. M., Fourqurean J. W., Heck K. L., et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Park S. R., Kim Y. K., Kim J. H., Kang C. K., Lee K. S. (2011). Rapid recovery of the intertidal seagrass Zostera japonica following intense Manila clam (Ruditapes philippinarum) harvesting activity in Korea. J. Of Exp. Mar. Biol. And Ecol. 407, 275–283. doi: 10.1016/j.jembe.2011.06.023
Pérez-Pérez M. E., Lemaire S. D., Crespo J. L. (2012). Reactive oxygen species and autophagy in plants and algae. Plant Physiol. 160, 156–164. doi: 10.1104/pp.112.199992
Sandoval-Gil J. M., Ruiz J. M., Marin-Guirao L., Bernardeau-Esteller J., Sanchez-Lizaso J. L. (2014). Ecophysiological plasticity of shallow and deep populations of the Mediterranean seagrasses Posidonia oceanica and Cymodocea nodosa in response to hypersaline stress. Mar. Environ. Res. 95, 39–61. doi: 10.1016/j.marenvres.2013.12.011
Shafer D. J., Kaldy J. E. (2014). Comparison of photosynthetic characteristics of the seagrass congeners Zostera marina L. and Zostera japonica Ascher. & Graeb. Aquat. Bot. 112, 91–97. doi: 10.1016/j.aquabot.2013.09.002
Shafer D. J., Kaldy J. E., Sherman T. D., Marko K. M. (2011). Effects of salinity on photosynthesis and respiration of the seagrass Zostera japonica: A comparison of two established populations in North America. Aquat. Bot. 95, 214–220. doi: 10.1016/j.aquabot.2011.06.003
Short F., Carruthers T., Dennison W., Waycott M. (2007). Global seagrass distribution and diversity: A bioregional model. J. Of Exp. Mar. Biol. And Ecol. 350, 3–20. doi: 10.1016/j.jembe.2007.06.012
Son J. A., Narayanankutty D. P., Roh K. S. (2014). Influence of exogenous application of glutathione on rubisco and rubisco activase in heavy metal-stressed tobacco plant grown in vitro. Saudi J. Biol. Sci. 21, 89–97. doi: 10.1016/j.sjbs.2013.06.002
Song Q., Cao F., Gong Y., Cheng X., Bi X., Liu L. (2012). Current research progresses of amino acids uptake,transport and their biological roles in higher plants. Plant Nutr. Fertitizer Sci. 18, 1507–1517.
Song S. K., Hou W. S., Godo I., Wu C. X., Yu Y., Matityahu I., et al. (2013). Soybean seeds expressing feedback-insensitive cystathionine -synthase exhibit a higher content of methionine. J. Exp. Bot. 64 (7), 1917–1926. doi: 10.1093/jxb/ert053
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. P Natl. Acad. Sci. U.S.A. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Xu S. C., Wang P. M., Wang F., Liu P., Liu B. J., Zhang X. M., et al. (2020). In situ Responses of the Eelgrass Zostera marina L. @ to Water Depth and Light Availability in the Context of Increasing Coastal Water Turbidity: Implications for Conservation and Restoration. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.582557
Yang Q. Q., Zhao D. S., Liu Q. Q. (2020). Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00928
Zhang X. M., Zhou Y., Adams M. P., Wang F., Xu S. C., Wang P. M., et al. (2020). Plant morphology and seed germination responses of seagrass (Zostera japonica) to water depth and light availability in Ailian Bay, northern China. Mar. Environ. Res. 162, 105082. doi: 10.1016/j.marenvres.2020.105082
Zhou Q., Ke Y., Wang X., Bai J., Zhou D., Li X. (2022). Developing seagrass index for long term monitoring of Zostera japonica seagrass bed: A case study in Yellow River Delta, China. Isprs J. Photogramm 194, 286–301. doi: 10.1016/j.isprsjprs.2022.10.011
Zhou Y., Xu J., Wang D., Zhao X., Yang C. (2015). Rechecking the characteristic flood levels of a built reservoir after extending the hydrologic time series. River Basin Manage. Viii 197, 81–90. doi: 10.2495/RM150081
Keywords: Zostera japonica, free amino acids, salinity, turbidity, cumulative effects, seagrass conservation
Citation: Yi Y, Zhao F, Hou C, Zhang C and Tang C (2024) Mechanism and threshold of environmental stressors on seagrass in high-turbidity estuary: case of Zostera japonica in Yellow River Estuary, China. Front. Mar. Sci. 11:1432106. doi: 10.3389/fmars.2024.1432106
Received: 13 May 2024; Accepted: 24 June 2024;
Published: 31 July 2024.
Edited by:
Qin Zhu, Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou), ChinaCopyright © 2024 Yi, Zhao, Hou, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yujun Yi, eWl5dWp1bkBibnUuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.