
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 22 July 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1431852
Hot water extract of Chlorella vulgaris (CVE) is a biologically substance that enhances organism’s immune function and antioxidative capacity. This study evaluated the effect of supplementation with various concentrations of CVE on muscle nutritional components, non-specific immunity, antioxidation, and resistance to non-ionic ammonia (NH3-N) stress in Litopenaeus vannamei over 45 days using diets supplemented with CVE at five different concentrations (0%, 0.5%, 1%, 5%, 10%, and 15%). Specifically, fresh and sweet amino acids (Asp, Glu) significantly increased (P < 0.05) in shrimp fed the 1% CVE diet, reaching 18.12 g/kg and 33.08 g/kg, respectively. Bitter amino acids (Leu) and Hypoxanthine (Hx) significantly decreased (P < 0.05) in shrimp fed the 1% CVE diet, at 10.56 g/kg and 10.56 ug/g. CVE supplementary enhanced the activities of acid phosphatase, alkaline phosphatase, and nitric oxide synthase while decreasing malondialdehyde levels. Shrimp fed with a 1% CVE diet exhibited significantly higher enzyme activity than the control group under NH3-N conditions (P < 0.05). Overall, this study demonstrated that 1% CVE as a feed additive significantly improved the muscle mass, boosted immunity and reduced the stress response to NH3-N in L. vannamei. This research provides a valuable reference for the application of CVE as a feed additive in crustacean aquaculture.
The hot water extract of Chlorella vulgaris (CVE) is a nucleotide-peptide complex enriched with amino acids, peptides, vitamins, minerals, nucleic acids, and carbohydrates, which not only strengthens the immune system and improves antioxidant activity but also promotes cell growth and enhances the regenerative capacity of normal cells (Merchant and Andre, 2001; Kang et al., 2013; An et al., 2016; Yamaguchi, 1996; Kumar et al., 2020; Kumar et al., 2020). Chlorella (Chlorella sp.) is a genus of single-cell green algae characterized by relatively easy cultivation, high productivity, and high protein, chlorophyll, lutein, and other essential micronutrients (Buono et al., 2014; Jeon et al., 2012; Choi et al., 2021; Hao et al., 2021). Various Chlorella-based products, including liquid, powdered, and fermented Chlorella and Chlorella extracts, are being introduced as feed additives (An et al., 2016). In recent years, more research has been conducted on powder of Chlorilla as a feed additive (Ma and Hu, 2024) and less on CVE as a feed additive. As a feed additive, CVE has been found to reduce vertebrate plasma peroxidation and increase antioxidant enzyme activity (Hu et al., 2007; Vijayavel et al., 2007), but it has not been extensively studied in aquatic animals. The cell wall of Chlorella vulgaris may limit the entry of digestive enzymes into the cell for appropriate digestion and absorption of components (Nemcova and Kalina, 2000). Therefore, CVE is important as a feed additive in aquatic animals.
Litopenaeus vannamei is one of the most important shrimp species cultured worldwide. Owing to its high nutritional value, fast growth rate, and strong adaptability to the environment, it has high economic value and is widely cultivated worldwide (Aktaş et al., 2014; Eissa et al., 2023). During aquaculture production, with prolonged culture time, the residual feed and waste metabolites of aquaculture animals tend to accumulate, thereby elevating the concentrations of ammonia nitrogen in the water, which, upon reaching or exceeding the tolerance limit of the aquaculture stock, will have the adverse effects of retarding growth and reducing metabolic capacity (Jensen et al., 2013). Shrimp culture is susceptible to various environmental factors, and changes in these factors can cause physiological changes and oxidative stress (Amaya et al., 2007; Wang et al., 2009). Oxidative stress weakens the immune system, making animals susceptible to opportunistic pathogens (Li et al., 2016; Thirugnanasambandam et al., 2019). This leads to a decline in immunity, thereby increasing the likelihood of infection with various pathogens and potentially widespread mortality (Bhoopathy et al., 2021). Therefore, improving the antioxidant and immune systems of shrimp is a practical and urgent problem in the aquaculture industry, especially intensive aquaculture (Huynh et al., 2017). Research has shown that the addition of Caulerpa racemosa (Agardh, 1873) polysaccharides (CRP) to feed can enhance the antioxidant and immune properties of shrimp (Lee et al., 2020). This illustrates that active microalgal substances as feed additives can enhance immunity and stress resistance in aquatic animals (Chen et al., 1988; Pulz and Gross, 2004), and research on active microalgal substances is essential.
In the study of feed additives, amino and fatty acid contents serve as important indicators of the nutritional value and flavor of aquatic products (Peng et al., 2021). The amino acids comprising muscle are not only important nutrients but also make a valuable contribution to determining the color, aroma, and taste of fish meat. During the culture of aquatic organisms, the amino acid content of the muscle can be modified to a certain extent by adding trace elements to the basic feed and optimizing the ratios of proteins, which in turn can enhance the nutritional quality of the muscles of aquatic animals (Long et al., 2020). Inosinic acid is an intermediate metabolite widely distributed in animals, and the freshness of aquatic products is often influenced by the accumulated amount of this acid (Tanimoto et al., 1993). Furthermore, it has been demonstrated that using the alga Chlorella vulgaris instead of fish meal as a protein source can significantly enhance the amino acid and fatty acid content of African catfish (Tikk et al., 2016), and other functional feed additives have been established to enhance the development, immunity, and antioxidant capacity of shrimp.
Currently, relatively little information is available regarding the application of hot water extracts of C. vulgaris (CVE) in crustacean cultures. In this study, we assessed the effects of CVE as functional additives in crustacean aquaculture. We examined the effects of different concentrations of CVE as a feed supplement on the nutritional composition and antioxidant system of muscles in the Litopenaeus vannamei. The optimal CVE concentrations screened were also subjected to ammonia nitrogen stress experiments to verify the immune-protective effects of CVE on shrimp. This study aimed to provide nutritional and immunological support for the application of CVE as a feed additive in aquaculture and to provide a scientific basis for the health of Litopenaeus vannamei.
The method of Hasegawa was used to obtain CVE (Raji et al., 2020). Dried Chlorella vulgaris cells were suspended in distilled water at a concentration of 10%(w/v), boiled at 100°C for 20 min, and centrifuged at 7200 r for 20 min, then the supernatant was lyophilized to obtain CVE. The basic feed (Table 1) formula is shown in the Table 1. CVE was added to basic feed by mass percentage of 0% (control), 0.5%, 1%, 5%, 10% and 15%. Two percent sodium alginate was added to the feed for bonding. After mixing, particles with particle size of 1.2 mm were made, dried in dark, sealed and frozen for standby. The nutritional levels of each treatment group are shown in Table 1.
The aquaculture experiment was carried out in the circulating water aquaculture system of Tianjin Key Laboratory of Aquatic Ecology and Aquaculture, College of Fisheries, Tianjin Agricultural University. The shrimp for the experiment were collected from Tianjin Haile aquaculture Cooperative. The breeding system consists of 60 cm × 30 cm ×50 cm. The test water is sand filtered seawater and aerated tap water. Before the experiment, two weeks of feeding was carried out, during which basic feed was fed. After the end of temporary feeding, the experiment was started after fasting for 24 hours. This study randomly divided 900 juvenile shrimp with an average initial weight of (0.85 ± 0.05) g into 6 groups, each with 3 water tanks (120cm x 120cm x 80cm; 1.0t of water; 50 shrimp). They were fed with CVE feed with the addition ratio of 0% (control), 0.5%, 1%, 5%, 10% and 15% respectively for 45 days. The water temperature is 28–30 °C, the seawater salinity is 23–25, the mass concentration of dissolved oxygen is > 6mg/L, the pH value is 7.6–8.1, the mass concentration of ammonia nitrogen is < 0.1mg/L, and the photo period is 12L: 12D. During the experiment, shrimp were fed four times a day (at 8:00, 12:00, 17:00 and 21:00 respectively), and the number of deaths was recorded. After the experiment, 3 replicates were set up for each group, and 6 shrimps per replicate were measured in their hepatopancreas and muscles to analyse the muscle composition and activity of immune enzyme.
Validation of the appropriate CVE concentration based on the results of previous experiments and using immunological indicators (Hasegawa et al., 1995). According to the previously published results of Litopenaeus vannamei (Supplementary Table 1) (Han et al., 2022), the experimental concentration of NH3-N is 0.5mg/L. 0.5 mg/L was prepared from ammonia chloride ammonia nitrogen stock solution. On pH 8.10, temperature 22°C and salinity level of 25‰, the proportion of NH3-N in ammonia nitrogen was calculated (Whitfield, 1978; Jia et al., 2013). According to this proportion, the stock solution was diluted to the required ammonia-N concentration. In the experiment, only healthy and intact shrimp were used. After fasting for 24 h before the experiment, individuals weighing 0.1–0.15 g were randomly divided into two groups of glass Aquariums, 20 in each group, Five shrimps. After NH3-N stress, four individuals were collected at 1th, 3th, 6th, 12th and 24 h respectively.
At each sampling, shrimps were dried with absorbent paper and then quickly dissected on an ice plate. The hepatopancreas and muscle tissues were collected into a chilled glass homogenizer, diluted with 50 mM phosphate buffer saline solution (PBS, Solar Bio, China) (pH = 6.6, v: w = 9: 1) and centrifuged for 3000r for 15 min. The supernatants were collected and stored at -80°C for measurements of the muscle components and determination of antioxidant and immune parameters.
The samples were dissolved in 0.02 mol/L hydrochloric acid and fixed, and the amino acids in the samples were determined by the external standard method, and finally analysed by a fully automated amino acid analyser.
Muscle quality and other related indexes were detected by high performance liquid chromatography (HPLC), and the content of Hypoxanthine and Adenosine monophosphate were uesd Elisa kits (Jiangsu Kote Biotechnology Co, China).
The Acids phosphatse (ACP), Alkaline phosphatase (AKP), Glutathione peroxidase(GSH-PX), Nitricoxdesynthase (NOS), superoxide dismutase (SOD) Hexokinase (HK), Phosphofructokinase (PK), Lactate dehydrogenase (LDH), Succinate dehydrogenase (SDH) activitiey level and Malondialdehyde (MDA) and contents using reagent kits (Nanjing Jiancheng Bioengineering Institude, China). Samples used for analyses were prepared by grinding 1.0 g of muscle tissue using a high-throughput tissue grinder. To the homogenate thus obtained, we added 10 mL of 0.86% normal saline pre-cooled at 4°C to give a 10% homogenate, which was centrifuged for 15 min at 8000 rpm and 4°C. The resulting supernatants were collected and used for enzyme activity determinations.
The statistics of the experimental data were performed using SPSS26.0 software, analysed by one-way ANOVA, and multiple comparisons were performed using Duncan’s method, with P<0.05 indicating a significant difference (Whitfield, 1978). Independent samples t-test was used to analyse the difference between the overall means of the two groups of 0% and 1% CVE and the differences between the two groups were added. Pictures were made using GraphPad Prism 8 software.
The effects of supplementing feed with different concentrations of CVE on the amino acid contents of L. vannamei muscle are presented in Table 2. The values obtained revealed that the total amino acid content in muscle was significantly influenced by the addition of different concentrations of CVE (P < 0.05). Although we detected differences among the different amino acids, initially increase and subsequently decline with an increase in the amount of supplement of the amino acids showed a tend to CVE, with the values for Asp, Glu. Ser, Gly, Thr, Val, and Lys peaking in shrimps fed a diet containing 1% CVE, which were significantly higher than the value recorded at other concentrations (P < 0.05). Compared with other concentrations, we detected a significantly lower content of the bitter-tasting amino acid Leu in shrimps fed a diet containing 1% CVE (P<0.05).
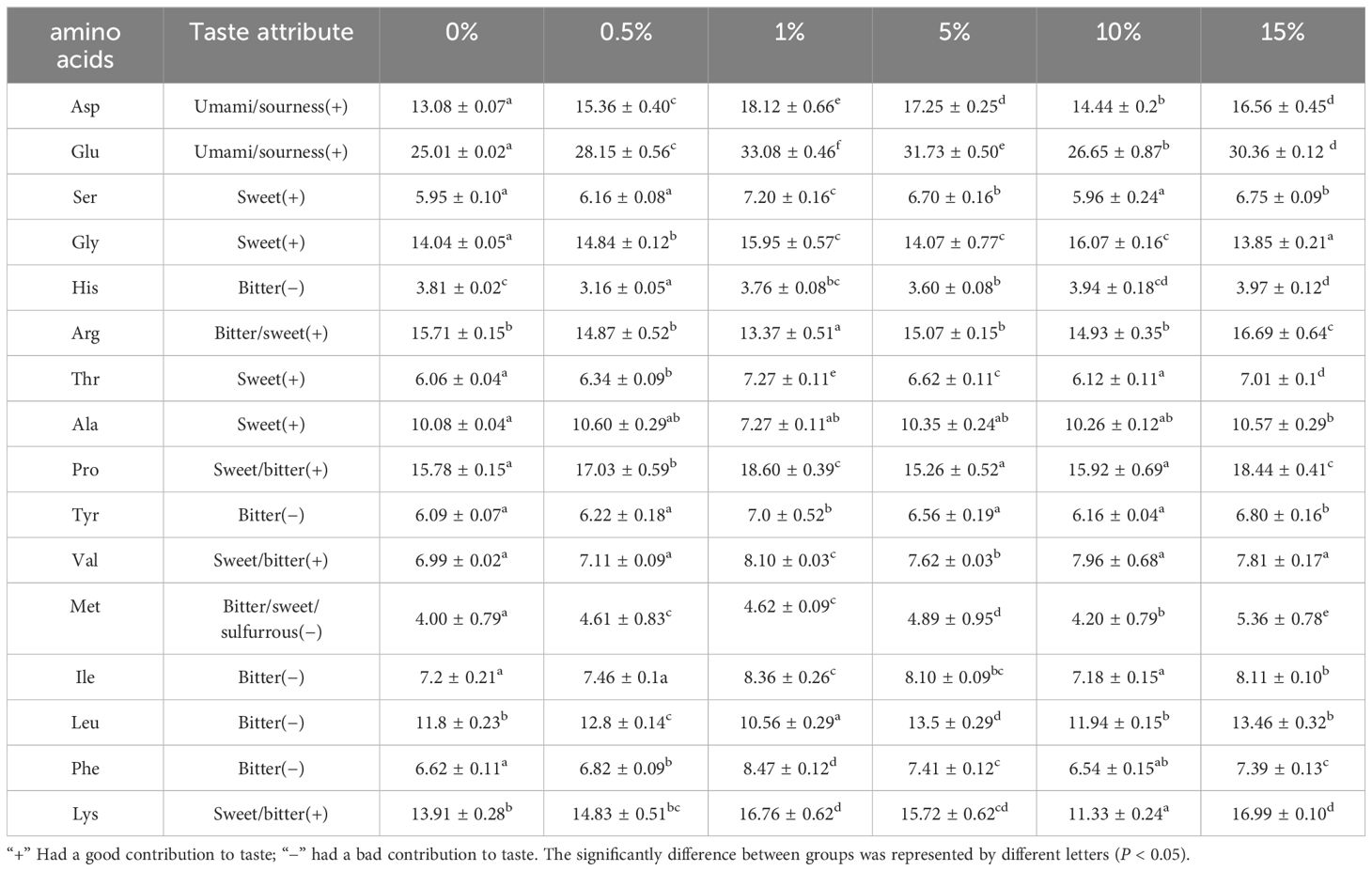
Table 2 Effect of CVE addition to feed on the content of various amino acids in Litopenaeus vannamei (g/kg).
The effects of supplementing feed with different concentrations of CVE on the quality-related indices of L. vannamei muscle are shown in Table 3. Supplementing feed was found to have significant effects on the assessed muscle quality-related indices, with values tending to increase with an increase in the proportion of supplemented CVE. The one exception in this regard was hypoxanthine, the content of which in shrimps fed the 1% CVE-supplemented feed was significantly lower than the in shrimps in the other treatment groups (P <0.05).
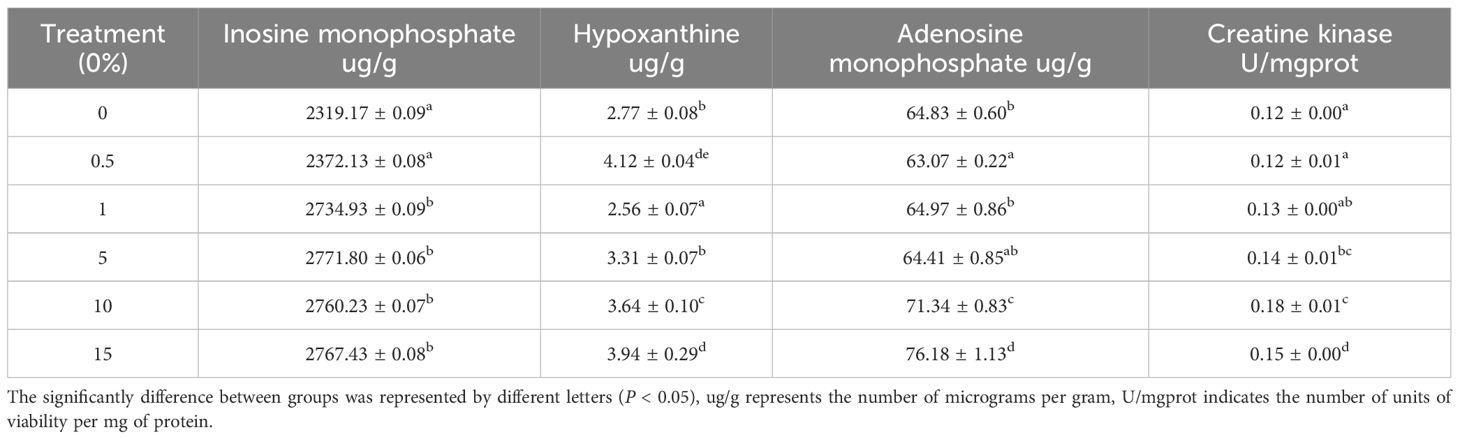
Table 3 Effect of feed supplementation with CVE on muscle quality-related indexes of Litopenaeus vannamei (mg/kg).
As shown in Figures 1A–C, the highest activities of acid phosphatase (ACP), alkaline phosphatase (AKP) and nitric oxide synthase (NOS) were recorded in shrimps fed a diet containing 1% CVE, among which the activities of ACP and AKP were significantly higher than in shrimps fed the control diet (P< 0.05). Consumption of CVE was also found to have a significant influence on the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and the content of malondialdehyde (MDA) (P < 0.05) (Figures 1D–F). With an increase in the dietary content of CVE, there was an initial increase and subsequent decline in the activity of SOD, whereas the contents of MDA were characterized by the opposite trend. The highest activities of SOD and GSH-Px were detected in the 10% and 5% groups, respectively, whereas the lowest contents of MDA were recorded in shrimps fed the 0.5% CVE diet.
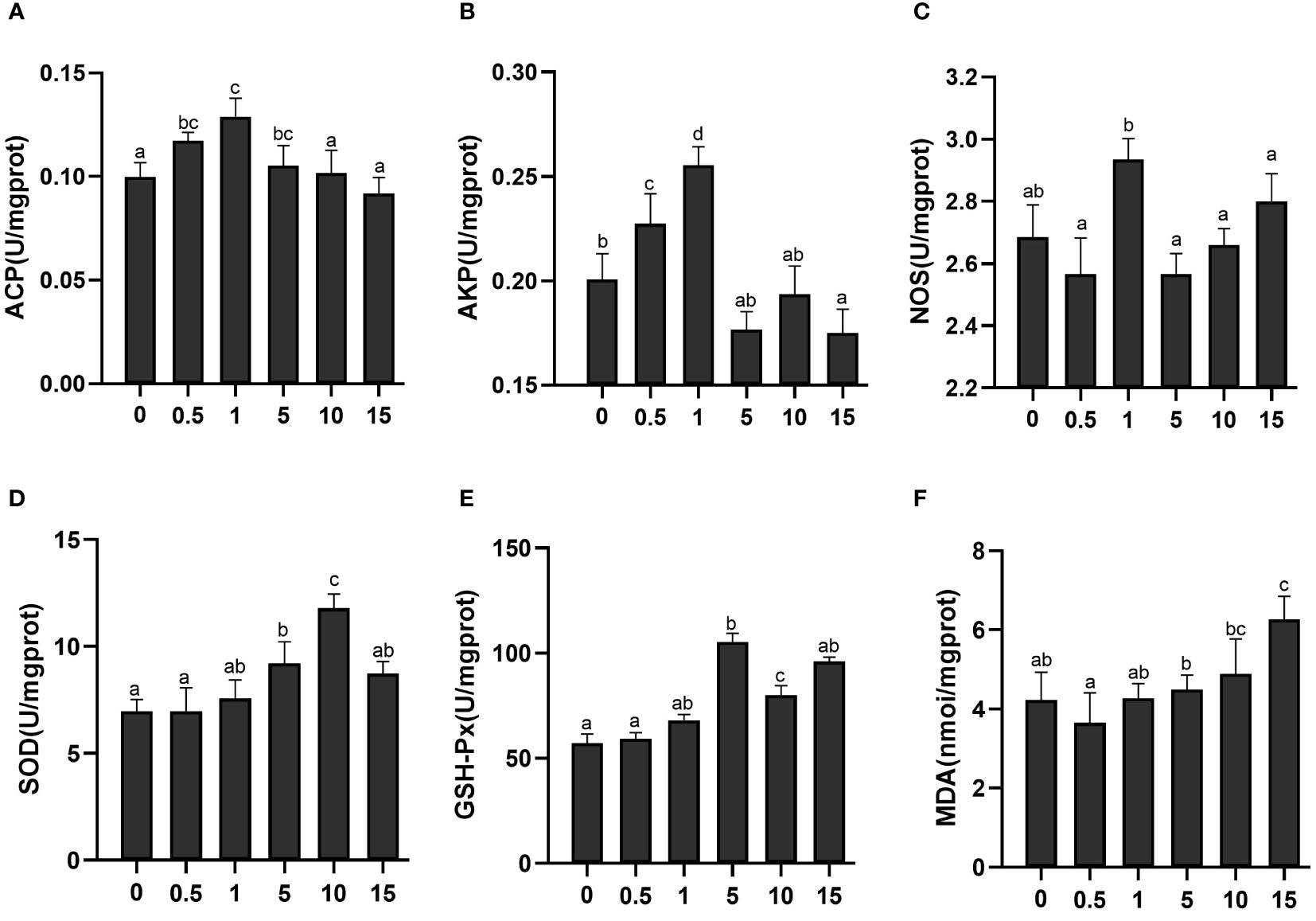
Figure 1 Activitiey level of ACP (A), AKP (B), NOS (C), SOD (D), GSH-Px (E) and the content of MDA (F) in L. vannamei after dietary CVE. Significant difference between the groups was denoted by different letters (P < 0.05).
Figures 2A–C shows the activities of ACP, AKP, and NOS in response to non- ionized ammonia (NH3-N) stress in L. vannamei fed the CVE-supplemented and control diets. In both the CVE and control groups, the activity of ACP initially declined prior to a subsequent increase, whereas the opposite trend was observed regarding the activity of NOS. However, compared with shrimps in the control group, the activity of ACP was significantly higher in those fed diets supplemented with CVE at each of the assessed time point, with the exception of the measurements obtained at 3 h (P > 0.05). On release from the imposed stress after 24 h, the activities ACP, AKP, and NOS in the CVE group shrimps returned to pre-experimental levels, although the activities ACP and AKP in these shrimps remained higher than those in the control group shrimps. As shown in Figures 2D–F), from 0 to 6 h, there were increases in the activity of SOD and contents of MDA in the CVE groups, after which the activities and contents declined. On the relief from stress after 24 h, we detected no significant differences among the CVE groups with respect to the pre-experimental and post-experimental activities of SOD and GSH- Px or MDA contents (P > 0.05). Among shrimps in the CVE groups, we detected elevated MDA contents within 6 h of the onset of NH3-N stress, which had returned to pre-experimental levels at 24 h. In contrast, a marked increase in MDA levels was observed between 6 and 24 h in the control group shrimps (P>0.05). As shown in Figures 3B–D, dietary supplementation with 1% CVE enhanced the overall activities of PK, LDS and SDH in the muscle of L. vannamei exposed to NH3-N stress, the levels of all of which were higher than those in the control group shrimps 12 at 24 h. As shown in Figure 3A, the activity of HK in shrimps fed the supplemented diets was also significantly higher than that during the initial stages of the experiment at 3 h (P > 0.05).

Figure 2 Activity level of ACP (A), AKP (B), NOS (C), SOD (D), GSH-Px (E) and the content of MDA (F) in L. vannamei response to nonionic ammonia stress after dietary 1%CVE. The”*”means the significant differences between control group and the treatment group at the same time. Different lowercase letters mean significant difference(P < 0.05), whereas different capital letters mean significant difference (P < 0.05).
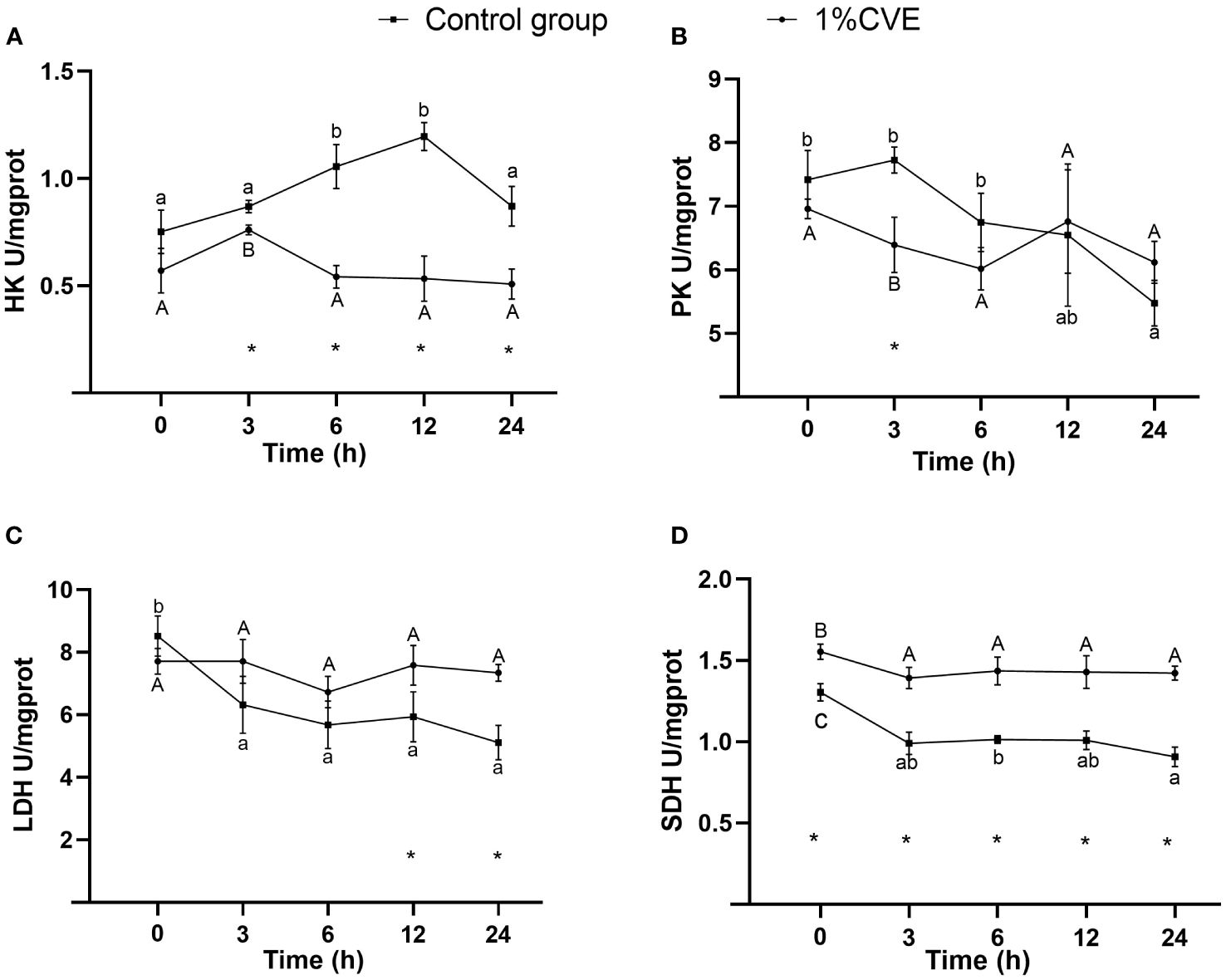
Figure 3 Activity level of HK (A), PK (B), LDH (C), SDH (D) in L. vannamei response to nonionic ammonia stress after dietary 1%CVE. The”*”means the significant differences between control group and the treatment group at the same time. Different lowercase letters mean significant difference(P < 0.05), whereas different capital letters mean significant difference (P < 0.05).
CVE is a compound enrich with amino acids, polysaccharide and other substances, more easily absorbed by the body, so CVE as a feed additive in aquaculture has a positive prospect of application. In previous study, it was found that CVE has significant effects on the growth of Litopenaeus vannamei. Adding 1% CVE to the feed can effectively improve the final weight, gain rate, specific growth rate, and survival rate of Litopenaeus vannamei, and reduce the feed coefficient (Han et al., 2022).
The composition and content of amino acids are important indicators of the nutritional value of proteins, and CVE is rich in amino acids; therefore, its use as a feed additive in the aquatic industry has significant advantages (Chen et al., 2021). In the present study, we found that supplementing feed with different proportions of CVE promoted an increase in amino acid content in the muscle of L. vannamei. Feeding the 1% CVE feed group significantly increased the “fresh sweet” amino acid content and reduced the “bitter” amino acid content of the shrimp. Similarly, replacing fish meal with C. vulgaris in the diet of African catfish has been shown to promote a significant increase the contents of “fresh” amino acids (Arg, Val, Thr, and Lys) in the muscle of these fish (Tikk et al., 2016), and consistently, replacing 20% of fish meal with microalgae in the diet of L. vannamei was found to enhance the content of fresh amino acids (Phe, Arg, Glu) in the muscle of these shrimps (Pês et al., 2016). Collectively, these findings indicate that supplementing the feed of L. vannamei with 1% CVE had the greatest effect on enhancing the flavor and protein quality of shrimp.
Nucleotide degradation-related compounds are important indicators for evaluating the freshness and flavor of aquatic products (Clausen et al., 2018). The degradation of adenosine triphosphate (ATP) in the muscle begins to take place; ATP breaks down into adenosine diphosphate (ADP), ADP breaks down into adenosine monophosphate (AMP) by creatine kinase (CK), AMP generates inosine monophosphate (IMP) by adenosine monophosphate dehydroammonia enzyme, and IMP will be hydrolyzed to produce hypoxanthine (Hx), which has a bitter flavor (Tikk et al., 2016). The content of inosinic acid and its degradation products in aquacultured species is not only determined by breed but is also associated with factors such as feed composition (Bremner et al., 1988; Greene and Bernatt-Byrne, 1990). To date, comparatively few studies have assessed the flavor-enhancing effects of dietary CVE supplementation on aquaculture products. Our findings revealed that supplementing shrimp feed with different concentrations of CVE contributed to increases in the IMP, AMP, and CK contents of shrimp muscle, with levels gradually increasing with an increase in the proportion of supplemented CVE. The hypoxanthine content in shrimp fed the 1% CVE-supplemented diet was significantly lower than that in the other groups (P< 0.05), whereas the levels of IMP, AMP, and CK were significantly higher than those recorded in the control group (P<0.05). Similarly, the addition of glycerol monolaurate (GML) to feed enhances the inosinic acid content of large yellow croaker (Larimichthys crocea) muscle (Zhuang et al., 2022). Glu and Asp could play roles in the nucleotide synthesis and further trigger the generation of inosinic acid (Kurihara, 2015). Therefore, the contents of Glu and Asp were significantly increased after CVE sup plementation which could be the explanation for the increase in the inosinic acid content. Current research on algal additives has revealed that algae contain various active substances, high protein and low fat contents, and multiple vitamins, which, when incorporated into feed, can contribute to enhancing the quality and flavor of animal muscle (Ahmad et al., 2020).
Amino acids and inosine can also be used together as indicators for evaluating the flavor of aquatic animal meat (Yamaguchi, 1998). Research has shown that replacing fishmeal with C. vulgaris in the deit of L. vannamei can effectively improve muscle quality (Li et al., 2022). Application of a dietary lysine to feed can increase the content of amino acids, fatty acids, and inosine in grass carp muscle, thereby improving the muscle quality of grass carp (Ctenopharyngodon Idella) (Tang et al., 2023). CVE as an extract of C. vulgaris enriched with amino acids that has the same positive effect. Subsequent comparative studies will be conducted on aquatic animals by adding C. vulgaris and C. vulgaris extracts.
The activity of immune enzymes in the hepatopancreas influences the metabolism and antioxidant capacity of shrimp and can serve as an important indicator of the immune capacity of aquatic animals. In the present study, ACP, AKP, and NOS enzyme activities were the highest in the CVE 1% group, SOD and GSH-Px increased with increasing CVE addition, and MDA content was the lowest in the 0.5% CVE group. Hot water extracts of Chlorella can stimulate or enhance the immune system of animals (Kotrbacek et al., 2015). Previous studies have shown that the shrimp fed the Panax ginseng polysaccharides (GSP) diet had significantly increased ACP, AKP, T-SOD and GSH-Px activities in L. vannamei (Liu et al., 2011). Increasing of ACP, AKP and NOS by dietary CVE suggested that the CVE might contain some bioactive substances involving in the regulating of fish immune response. Immunostimulants obtained by hot water extraction of Chlorella cells, such as the powerful antioxidants vitamin C, and polysaccharides (Spolaore et al., 2006) have been observed to increase the immune resistance of shrimp and fish (Maliwat et al., 2016). Microalgae in general are considered as potential sources of natural antioxidants (Tsao and Deng, 2004). Chlorella was also reported to have a better activity in inhibiting lipid peroxidation and have antioxidant property (Bengwayan et al., 2010). Research has shown that supplementing Atlantic salmon feed with 10% AquaArom enhances the activities of SOD and other antioxidant enzymes while reducing temperature responsiveness (Kamunde et al., 2019). The activity found that The addition of 10% Chlorella powder to feed promoted a significant increase in SOD content and a reduction in MDA content in rainbow trout (Chen et al., 2021), possibly attributing to the antioxidant activity of polyphenols and flavonoids in the Chlorella (Shibata et al., 2003). Research has shown that adding amino acids to feed can enhance the antioxidant capacity, non-specific immune ability, and ability to resist adverse environments of aquatic animals (Oehme et al., 2010; Cheng et al., 2012). In this study, the addition of CVE significantly increased the amino acid content in L. vannamei. This indicates that CVE as a feed additive can accelerate liver metabolism and reduce lipid peroxidation and enhances antioxidant capacity of L. vannamei.
The most economical and effective CVE additive (1%) was selected for ammonia nitrogen stress experiments on L. vannamei to verify its protective effect of CVE as a feed additive on the organismal immunity of L. vannamei. Among the shrimp exposed to NH3-N stress, we found that the activities of ACP and AKP in those fed the supplemented diet were invariably higher than those in the control group after 6 h. The MDA content in the control group was characterized by an initial decline and subsequent increase, and when measured at 24 h, MDA levels in the 1% CVE group were found to 3 be significantly lower than those in the control group (P < 0.05), with an overall contrasting trend of an initial increase and subsequent decline. Moreover, when measured at 3 h, we detected a significantly higher level of NOS activity in shrimps fed the supplemented diet compared with those in the control shrimps (P<0.05), with the levels of activity reaching a maximum at 12 h. Whereas the research found that NOS activity in L. vannamei initially increased and subsequently declined in response to exposure to heat stress (Jia et al., 2014). These authors similarly observed initial increases and subsequent declines in NOS activity in shrimps exposed to 0.1 mg/L NH3-N stress, whereas in response to treatment with 0.5 mg/L NH3-N, they detected a significant reduction in NOS activity, a reduction in SOD activity, and increases in the content of MDA (Jia et al., 2017). When shrimp are stimulated by environmental factors it leads to disruption of the body’s antioxidant system. Excessive non-ionic ammonia can cause metabolic disorders in aquatic animals, affecting their ionic regulation ability and damaging cellular structure to cause harm to the organism (Chen et al., 1988; Cheng and Chen, 2001). When exposed to oxidative stress, organisms deploy various antioxidative defense mechanisms, including enzymatic (e.g., SOD and glutathione peroxidase) and non-enzymatic (e.g., MDA) (Al-Ghanim et al., 2020). This is similar with previous reports that dietary Chlorella improved survival rate of L. vannamei under hypoxia challenge (Pakravan et al., 2017).The shrimp fed the CVE diet may contribute to these specific enzymes involved in immunological activity to enhance ammonia nitrogen stress resistance. CVE, as a feed additive, enhanced the antioxidant capacity and detoxification activity of L. vannamei in the treated group compared to those in the control group.
Research has shown that the addition of calcium pyruvate to feed impairs glucose utilization in L. crocea, as evidenced by the elevated plasma glucose levels and reduced activities of enzymes associated with glucose metabolism (HK, PFK, PK, and PDH) in the liver and muscles (Zhang et al., 2023). In the present study, we found that the muscle activities of HK, LDH, and SDH showed similar trends in the control and treatment groups, whereas the KH activity increased to different extents during the early stage of treatment. In contrast, the activity of PK in the muscle was characterized by a declining trend in the control group shrimp and an initial decline and subsequent increase in shrimp fed the supplemented diet, thereby indicating the enhanced blood glucose regulatory capacity of these shrimp. In response to the imposition of nonionic ammonia stress, we detected a decline in LDH activity in the muscle of L. vannamei when measured at 6 h, which implies an initial gradual attenuation of anaerobic respiration in these shrimp, although we detected a subsequent recovery of activity at the 12-h time point. The vigor of shrimp fed a supplemented diet was found to be significantly higher than that of the control group shrimp, which is presumed to be associated with the effects of CVE in regulating metabolic levels in L. vannamei, thus contributing to the enhancement of the anaerobic respiratory capacity of these shrimp when exposed to stress conditions. A similar pattern of response was observed for SDH following exposure to non-ionic ammonia stress, with activities in the muscle of the control group shrimp characterized by a declining trend over time, whereas an initial decline in activity followed by a subsequent increase was detected in shrimp fed the supplemented feed. These findings indicate the higher aerobic respiratory capacity of shrimp in the 1% CVE group and an inhibition of aerobic respiration in the control group, which could be ascribed to tissue hypoxia in these control shrimp. Consumption of a diet supplemented with 1% CVE was found to promote the immune capacity of these shrimps, which, compared with those in the control group, incurred less damage when exposed to non-ionic ammonia stress conditions.
The indicator for determining shrimp stress and health status is the immunological enzyme activity. In terms of immune response, we We added different proportions of CVE and selected the most economical and effective 1% CVE as the feed additive and to verify its promoting effect on the immunity of L. vannamei under ammonia nitrogen stress. CVE can enhance the activity of immune enzymes and antioxidant enzymes in L. vannamei.The study has found that adding C. vulgaris to feed can enhance the immunity of L. vannamei and immune biomarker responses, leading to disease resistance in cultured shrimp (Eissa et al., 2024);. CVE as the extract of C. vulgaris has a positive role in enhancing the immunity of L. vannamei.
Our findings indicate that supplementing the feed of the shrimp L. vannamei with CVE may contribute to improving muscle nutrient composition, antioxidant capacity, and non-specific immunity and that under conditions of NH3-Nstress, a diet supplemented with 1% CVE may be beneficial in protecting tissues from the adverse effects of oxidative stress and, to a certain extent, reduce lipid peroxidation. However, supplementation of feed with higher levels of CVE did not appear to be conducive to conferring further beneficial effects. In this study, we obtained evidence indicating that supplementary CVE have a significant influence on the nutrient composition and immune response capacity of L. vannamei. In terms of shrimp health, our findings provide a valuable scientific basis for enhancing the quality of aquaculture environments and important insights into the mechanisms underlying the beneficial effects of CVE on the physiology and ecology of aquatic invertebrates. Moreover, these findings will contribute to the further development of microalgal resources and aquaculture applications of CVE.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The Animal Ethics Committee’s scope of approval is limited to studies involving vertebrates (Laboratory Animal Act, Chapter 5Article 25). Our study is on invertebrate species, and does not need ethical approval in China. The study was conducted in accordance with the local legislation and institutional requirements.
DZ: Formal analysis, Visualization, Writing – review & editing. SS: Investigation, Supervision, Writing – original draft. XJ: Investigation, Supervision, Validation, Writing – review & editing. WZ: Conceptualization, Investigation, Writing – review & editing. XS: Formal analysis, Writing – original draft. CH: Formal analysis, Methodology, Writing – original draft. YL: Investigation, Methodology, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (No. 32303038), Tianjin mariculture industry technology system innovation team construction project (ITTMRS2021000), the Scientific Research Programme of Tianjin Municipal Education Commission (Key Project of Natural Science)(2023ZD001), and the Science and Technology Programme of Tianjin (22ZXBTSN00020).
We are grateful to the Tianjin Agricultural University, Fisheries College, Tianjin Key Laboratory of Aqua-Ecology & Aquaculture for providing technical assistances.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1431852/full#supplementary-material
Supplementary Table 1 | Effects of Dietary CGF Supplementation at Different Concentrations on Growth Performance of Litopenaeus vannamei. The means with different superscripts in the same column are significantly different (P<0.05);the mean with ame superscripts indicate.no significant difference among groups (P>0.05). The same as below.
Ahmad M. T., Shariff M., Yusoff F. M., Goh Y. M., Banerjee S. (2020). Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 12, 328–346. doi: 10.1111/raq.12320
Aktaş M., Ciğer O., Genç E., Genç M. A., Çavdar N. (2014). Effects of mannan oligosaccharide and serotonin on molting, growth, body composition and hepatopancreas histology of white leg shrimp Litopenaeus vannamei (Boone 1931). Turk J. Fish Aquat Sci. 14, 205–211. doi: 10.4194/1303-2712-v14_1_22
Al-Ghanim K. A., Mahboob S., Vijayaraghavan P., Al-Misned F. A., Kim Y. O., Kim H. J. (2020). Sub-lethal effect of synthetic pyrethroid pesticide on metabolic enzymes and protein profile of non-target Zebra fish. Danio rerio. Saudi J. Biol. Sci. 27, 441–447. doi: 10.1016/j.sjbs.2019.11.005
Amaya E. A., Davis D. A., Rouse D. B. (2007). Replacement of fish meal in practical diets for the Pacific white shrimp (Litopenaeus Vannamei) reared under pond conditions. Aquaculture. 262, 393–401. doi: 10.1016/j.aquaculture.2006.11.015
An B. K., Kim K. E., Jeon J. Y., Lee K. W. (2016). Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. Springerplus. 5, 718. doi: 10.1186/s40064-016-2373-4
Bengwayan P. T., Laygo J. C., Pacio A. E., Poyaoan J. L. Z., Rebugio J. F., Yuson A. L. L. (2010). A comparative study on the antioxidant property of Chlorella (Chlorella sp.) tablet and glutathione tablet. Int. Sci. Res. J. 2, 25–35.
Bhoopathy S., Inbakandan D., Rajendran T., Chandrasekaran K., Prabha S B., Reddy B. A., et al. (2021). Dietary supplementation of curcumin-loaded chitosan nanoparticles stimulates immune response in the white leg shrimp Litopenaeus vannamei challenged with Vibrio harveyi. Fish Shellfish Immunol. 117, 188–191. doi: 10.1016/j.fsi.2021.08.002
Bremner H. A., Olley J., Statham J. A., Vail A. M. A. (1988). Nucleotide catabolism: influence on the storage life oftropical species offish from the north west shelf of Australia. J. Food Sci. 53, 6–11. doi: 10.1111/j.1365-2621.1988.tb10165.x
Buono S., Langellotti A. L., Martello A., Rinna F., Fogliano V. (2014). Functional ingredients from microalgae. Food Funct. 5, 1669–1685. doi: 10.1039/C4FO00125G
Chen J. C., Liu P. C., Lin Y. T., Lee C. K. (1988). Super intensive culture of red-tailed shrimp Penaeus penicillatus. J. World Aquac Soc 19, 127–131. doi: 10.1111/j.1749-7345.1988.tb00940.x
Chen W. J., Luo L., Han D. X., Long F. P., Chi Q. L., Hu Q. (2021). Effect of dietary supplementation with Chlorella sorokiniana meal on the growth performance, antioxidant status, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 33, 3113–3122. doi: 10.1007/s10811-021-02541-w
Cheng W., Chen J. C. (2001). Effects of intrinsic and extrinsic factors on the haemoeytes profiles of the prawn, Macrobrachim rosenbergii. Fish Shellfish Immun. 11, 53–63. doi: 10.1006/fsim.2000.0293
Cheng Z., Gatlin D. M. III, Buentello A. (2012). Dietary supplementation of arginine and/or glutamine influences growth performance, immune responses and intestinal morphology of hybrid striped bass (Morone chrysops× Morone saxatilis). Aquaculture. 362, 39–43. doi: 10.1016/j.aquaculture.2012.07.015
Choi J. Y., Shin J. H., An H. J., Oh M. J., Kim S. R. (2021). Analysis of secretome and N-glycosylation of Chlorella species. Algal Res. 59, 102466. doi: 10.1016/j.algal.2021.102466
Clausen M. P., Christensen M., Djurhuus T. H., Duelund L., Mouritsen O. G. (2018). The quest for umami: can sous vide contribute. Int. J. Gastron Food Sci. 13, 129–133. doi: 10.1016/j.ijgfs.2018.03.002
Eissa E.-S. H., Ahmed R. A., Abd Elghany N. A., Elfeky A., Saadony S., Ahmed N. H., et al. (2023). Potential symbiotic effects of -1,3 glucan, and fructooligosaccharides on the growth performance, immune response, redox status, and resistance of Pacific white shrimp, Litopenaeus vannamei to Fusarium solani infection. Fishes 8, 105. doi: 10.3390/fishes8020105
Eissa E. S. H., Aljarari R. M., Elfeky A., Abd El-Aziz Y. M., Munir M. B., Jastaniah S. D., et al. (2024). Protective effects of Chlorella vulgaris as a feed additive on growth performance, immunity, histopathology, and disease resistance against Vibrio parahaemolyticus in the Pacific white shrimp. Aquacult Int. 32, 2821–2840. doi: 10.1007/s10499-023-01298-y
Greene D. H., Bernatt-Byrne E. I. (1990). Adenosine triphosphate catabolites as flavor compounds and freshness indicators in Pacific cod (Gadus macrocephalus) and pollock (Theragra chalcogramma). J. Food Sci. 55, 257–258. doi: 10.1111/j.1365-2621.1990.tb06067.x
Han C. J., Chen Y. Y., He Z. N., Zhang Y. J., Zhou W. L., Deng Y. T. (2022). Effects of Chlorella growth factor (CGF) on growth performance and nutrition composition of pacific white shrimp Litopenaeus vannamei. Fish Sci. 41, 959–966. doi: 10.27717/d.cnki.gtjnx.2021.0000070.16378/j.cnki.1003-1111.21020
Hao B. B., Wu H. P., You J. Q., Xing W., Cai Y. P. (2021). Biomass and physiological responses of green algae and diatoms to nutrient availability differ between the presence and absence of macrophytes. Ecol. Indic. 129, 107987. doi: 10.1016/j.ecolind.2021.107987
Hasegawa T., Okuda M., Makino M., Hiromatsu K., Nomoto K., Yoshikai Y. (1995). Hot water extracts of Chlorella vulgaris reduce opportunistic infection with Listeria monocytogenes in C57BL/6 mice infected with LP-BM5 murine leukemia viruses. Int. J. Immunopharmacol. 17, 505–512. doi: 10.1016/0192-0561(95)00035-Z
Hu Q. H., Pan B. S., Xu J., Sheng J. C., Shi Y. (2007). Effects of supercritical carbon dioxide extraction conditions on yields and antioxidant activity of Chlorella pyrenoidosa extracts. J. Food Eng. 80, 997–1001. doi: 10.1016/j.jfoodeng.2006.06.026
Huynh T. G., Shiu Y. L., Nguyen T. P., Truong Q. P., Chen J. C., Liu C. H. (2017). Current applications, selection, and possible mechanisms of actions of synbiotics in improving the growth and health status in aquaculture: a review. Fish Shellfish Immunol. 64, 367–382. doi: 10.1016/j.fsi.2017.03.035
Jensen M. A., Fitzgibbon Q. P., Carter C. G., Adams L. R. (2013). Recovery periods of cultured spiny lobster, Sagmariasus verreauxi juveniles: effects of handling, force feeding, exercising to exhaustion and anaesthesia on oxygen consumption and ammonia-N excretion rates. Aquaculture. 410–411, 114–121. doi: 10.1016/j.aquaculture.2013.06.020
Jeon J. Y., Kim K. E., Im H. J., Oh S. T., Lim S. U., Kwon H. S., et al. (2012). The production of lutein-enriched eggs with dietary Chlorella. Korean J. Food Sci. Anim. Resour. 32, 13–17. doi: 10.5851/kosfa.2012.32.1.13
Jia X. Y., Wang F., Lu Y. L., Zhang D., Dong S. L. (2014). Immune responses of Litopenaeus vannamei to thermal stress: a comparative study of shimp in freshwater and seawater conditions. Mar. Freshw. Behav. Physiol. 47, 79–92. doi: 10.1080/10236244.2014.894349
Jia X. Y., Wang F., Wang C. S., Dong S. L. (2013). Effects of abrupt change in temperature and non-ion ammonia on survival in Litopenaeus vannamei under seawater and freshwater conditions. Period Ocean Univ China. 43, 33–40. doi: 10.16441/j.cnki.hdxb.2013.10.005
Jia X. Y., Zhang D., Wang F., Dong S. L. (2017). Immune responses of Litopenaeus vannamei to non-ionic ammonia stress: a comparative study on shrimps in freshwater and seawater conditions. Aquacult Res. 48, 177–188. doi: 10.1111/are.2017.48.issue-1
Kamunde C., Sappal R., Melegy T. M. (2019). Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PloS One 14, e0219792. doi: 10.1371/journal.pone.0219792
Kang H. K., Salim H. M., Akter N., Kim D. W., Kim J. H., Bang H. T., et al. (2013). Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult Res. 22, 100–108. doi: 10.3382/japr.2012-00622
Kotrbacek V., Doubek J., Doucha J. (2015). The chlorococcalean alga Chlorella in animal nutrition: a review. J. Appl. Phys. 27, 2173–2180. doi: 10.1007/s10811-014-0516-y
Kumar T. S., Josephine A., Sreelatha T., Azger Dusthackeer V. N., Mahizhaveni B., Dharani G., et al. (2020). Fatty acids-carotenoid complex: An effective anti-TB agent from the chlorella growth factor-extracted spent biomass of Chlorella vulgaris. J. Ethnopharmacol. 249, 112392. doi: 10.1016/j.jep.2019.112392
Kumar T. S., Josephine A., Sreelatha T., Azger Dusthackeer V. N., Mahizhaveni B., Dharani G., et al. (2020). Fatty acids-carotenoid complex: An effective anti-TB agent from the chlorella growth factor-extracted spent biomass of Chlorella vulgaris. J Ethnopharmacol. 249, 112392. doi: 10.1016/j.jep.2019.11239
Kurihara K. (2015). Umami the fifth basic taste: history of studies on receptor mechanisms and role as a food flavor. BioMed. Res. Int. 1, 189402. doi: 10.1155/2015/189402
Lee P. T., Lee M. C., Ho Y. T., Liao Z. H., Huang H. T., Ho Y. S., et al. (2020). Polysaccharides from the green alga Caulerpa racemosa (Agardh 1873) improve the immune response and antioxidant status in the white shrimp Litopenaeus vannamei (Boone 1931)(Dendrobranchiata, penaeidae). Crustaceana 93, 611–632. doi: 10.1163/15685403-bja10045
Li M., Wang J. F., Song S. Q., Li C. W. (2016). Molecular characterization of a novel nitric oxide synthase gene from Portunus trituberculatus and the roles of NO/O2— generating and antioxidant systems in host immune responses to Hematodinium. Fish Shellfish Immunol. 52, 263–277. doi: 10.1016/j.fsi.2016.03.042
Li M. L., Li X. Q., Yao W. X., Wang Y. Y., Zhang X., Leng X. J. (2022). An evaluation of replacing fishmeal with Chlorella Sorokiniana in the diet of pacific white shrimp (Litopenaeus Vannamei): growth, body color, and flesh quality. Aquac Nutr. 2022, 8617265. doi: 10.1155/2022/8617265
Liu X.-L., Xi Q.-Y., Yang L., Li H.-Y., Jiang Q.-Y., Shu G., et al. (2011). The effect of dietary Panax ginseng polysaccharide extract on the immune responses in white shrimp, Litopenaeus vannamei. Fish Shellfish Immun. 30, 495–500. doi: 10.1016/j.fsi.2010.11.018
Long X. W., Guo Q., Wang X. C., Francis D. S., Cheng Y. X., Wu X. G. (2020). Effects of fattening period on ovarian development and nutritional quality of adult female Chinese mitten crab Eriocheir sinensis. Aquaculture. 519, 734748. doi: 10.1016/j.aquaculture.2019.734748
Ma M., Hu Q. (2024). Microalgae as feed sources and feed additives for sustainable aquaculture: prospects and challenges. Rev. Aquacult. 16, 818–835. doi: 10.1111/raq.12869
Maliwat G. C. F., Stephanie F. V., Buluran S. M. D., Tayamen M. M., Ragaza J. A. (2016). Growth and immune response of pond-reared giant freshwater prawn Macrobrachium rosenbergii post larvae fed diets containing Chlorella vulgaris. Aquacult. Fish. 6, 465–470. doi: 10.1016/j.aaf.2020.07.002
Merchant R. E., Andre C. A. (2001). A review of recent clinical trials of the nutritional supplement Chlorella pyrenoidosa in the treatment of fibromyalgia, hypertension, and ulcerative colitis. Altern. Ther. Health Med. 7, 79–91.
Nemcova Y., Kalina T. (2000). Cell wall development, microfibril and pyrenoid structure in type strains of Chlorella vulgaris, C. kessleri, C. sorokiniana compared with C. luteoviridis (trebouxiophyceae, hlorophyta). Algological Stud. 100, 95–105.
Oehme M., Grammes F., Takle H., Zambonino-Infante J. H., Refstie S., Thomassen M. S., et al. (2010). Dietary supplementation of glutamate and arginine to Atlantic salmon (Salmo salar L.) increases growth during the first autumn in sea. Aquaculture. 310, 156–163. doi: 10.1016/j.aquaculture.2010.09.043
Pakravan S., Akbarzadeh A., Sajjadi M. M., Hajimoradloo A., Noori F. (2017). Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile pacific white shrimp Litopenaeus vannamei. Aquacult. Nutr. 24, 1–11. doi: 10.1111/anu.12594
Peng K., Huang W., Zhao H. X., Sun Y. P., Chen B. (2021). Dietary condensed tannins improved growth performance and antioxidant function but impaired intestinal morphology of Litopenaeus vannamei. Aquacult Rep. 21, 100853. doi: 10.1016/j.aqrep.2021.100853
Pês T. S., Saccol E. M., Ourique G. M., Londero É.P., Gressler L. T., Finamor I. A., et al. (2016). Effect of diets enriched with rutin on blood parameters, oxidative biomarkers and pituitary hormone expression in silver catfish (Rhamdia quelen). Fish Physiol. Biochem. 42, 321–333. doi: 10.1007/s10695-015-0140-z
Pulz O., Gross W. (2004). Valuable products from biotechnology of microalgae. Appl. Microbiol. Biot. 65, 635–648. doi: 10.1007/s00253-004-1647-x
Raji A. A., Jimoh W. A., Bakar N. H. A., Taufek N. H. M., Muin H., Alias Z., et al. (2020). Dietary use of Spirulina (Arthrospira) and Chlorella instead of fish meal on growth and digestibility of nutrients, amino acids and fatty acids by African catfish. J. Appl. Phycol. 32, 1763–1770. doi: 10.1007/s10811-020-02070-y
Shibata S., Natori Y., Nishihara T., Tomisaka K., Matsumoto K., Sansawa H., et al. (2003). Antioxidant and anti-cataract effects of Chlorella on rats with streptozotocin-induced diabetes. J. Nutr. Sci. Vitaminol. 49, 334–339. doi: 10.3177/jnsv.49.334
Spolaore P., Cassan J. C., Duran E., Isambert A. (2006). Commercial applications of microalgae. J. Biosci. Bioeng. 101, 87–96. doi: 10.1263/jbb.101.87
Tang Y.-J., Feng L., Jiang W.-D., Wu P., Liu Y., Zhang L., et al. (2023). Enhancements in flavor substances, mouthfeel characteristics and collagen synthesis in the muscle of sub-adult grass carp (Ctenopharyngodon Idella): Application of a dietary lysine nutrition strategy. Aquaculture. 565, 739115. doi: 10.1016/j.aquaculture.2022.739115
Tanimoto S. Y., Koike K., Takahashi S. (1993). Improvement in broiled meat texture of cultured eel by feeding of tochu leaf powder. Biosci. Biotechnol. Biochem. 57, 325–327. doi: 10.1271/bbb.57.325
Thirugnanasambandam R., Inbakandan D., Kumar C., Subashni B., Vasantharaja R., Stanley Abraham L., et al. (2019). Genomic insights of Vibrio harveyi RT-6 strain, from infected “Whiteleg shrimp“ (Litopenaeus vannamei) using Illumina platform. Mol. Phylogenet Evol. 130, 35–44. doi: 10.1016/j.ympev.2018.09.015
Tikk M., Tikk K., Tørngren M. A., Meinert L., Aaslyng M. D., Karlsson A. H., et al. (2016). Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 54, 7769–7777. doi: 10.1021/jf060145a
Tsao R., Deng Z. (2004). Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. 812, 85–99. doi: 10.1016/j.jchromb.2004.09.028
Vijayavel K., Anbuselvam C., Balasubramanian M. P. (2007). Antioxidant effect of the marine algae Chlorella vulgaris against naphthalene-induced oxidative stress in the albino rats. Mol. Cell Biochem. 303, 39–44. doi: 10.1007/s11010-007-9453-2
Wang W. N., Zhou J., Wang P., Tian T. T., Zheng Y., Liu Y., et al. (2009). Oxidative stress, DNA damage and antioxidant enzyme gene expression in the Pacific white shrimp, Litopenaeus vannamei when exposed to acute pH stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 150, 428–435. doi: 10.1016/j.cbpc.2009.06.010
Whitfield M. (1978). The hydrolysis of ammonium ions in sea water-experimental confirmation of predicted constants at one atmosphere pressure. J. Mar. Biol. Assoc. U K. 58, 781–786. doi: 10.1017/S0025315400041436
Yamaguchi K. (1996). Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass metabolites: a review. J. Appl. Phycol. 8, 487–502. doi: 10.1007/BF02186327
Yamaguchi S. (1998). Basic properties of umami and its effects on food flavor. Food Rev. Int. 14, 139–176. doi: 10.1080/87559129809541156
Zhang Y. Q., Lai W. C., Chen Q. C., Wei F., Cui K., He Y. L., et al. (2023). Calcium pyruvate attenuates fat deposition by augmenting fatty acid oxidation and inhibiting glucose oxidation in juvenile large yellow croaker (Larimichthys crocea) consuming a high-fat diet. Aquaculture. 562, 738778. doi: 10.1016/j.aquaculture.2022.738778
Keywords: Litopenaeus vannamei, Chlorella vulgaris, muscle component, nonspecific immunity, antioxidation
Citation: Zhang D, Shi S, Jia X, Zhou W, Sun X, Han C and Lu Y (2024) Effects of dietary hot water extracts of Chlorella vulgaris on muscle component, non-specific immunity, antioxidation, and resistance to non-ionic ammonia stress in Pacific white shrimp Litopenaeus vannamei. Front. Mar. Sci. 11:1431852. doi: 10.3389/fmars.2024.1431852
Received: 13 May 2024; Accepted: 03 July 2024;
Published: 22 July 2024.
Edited by:
Jinghui Fang, Chinese Academy of Fishery Sciences (CAFS), ChinaCopyright © 2024 Zhang, Shi, Jia, Zhou, Sun, Han and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuying Jia, SmlheHV5aW5nQHRqYXUuZWR1LmNu
†These authors contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.