- 1Coastal Oregon Marine Experiment Station, Oregon State University, Newport, OR, United States
- 2Fisheries Wildlife and Conservation Sciences, Oregon State University, Corvallis, OR, United States
- 3Washington Department of Fish and Wildlife, Olympia, WA, United States
- 4National Marine Fisheries Service, National Oceanic and Atmospheric Association, Department of Commerce, Lacey, WA, United States
1 Introduction
High trophic-order predators play a critical role in ecosystems, exerting top-down control on prey species populations through direct predation (Terborgh et al., 1999; Pinnegar et al., 2000; Feit et al., 2019) and modify the behavior and habitat utilization of lower trophic level species through their presence in these systems (Ripple and Beschta, 2004; Heithaus et al., 2007). Therefore, shifts in the abundance and distribution of top predator species within an ecosystem can have cascading effects on lower trophic levels, changing trophic dynamics and ecosystem function (Heithaus et al., 2008; Baum and Worm, 2009; Newsome and Ripple, 2015). Thus, identifying shifts in the spatiotemporal habitat utilization of predators can be critical to the management of ecologically and economically valuable systems.
As a highly migratory marine predator, the soupfin shark (Galeorhinus galeus; aka school shark, tope) occupies a high trophic position (Bizzarro et al., 2017) and can exert top-down effects through direct predation upon forage fish, commercially valuable fish species, and smaller sharks (Nakatsu, 1957), significantly influencing trophic dynamics and biodiversity of their system. This coastal-pelagic species possesses an amphitropical distribution, with several geographically and genetically distinct populations throughout temperate waters (Chabot and Allen, 2009; Chabot, 2015) and seasonally inhabits a diverse array of habitats, spanning from shallow estuarine systems to the mid-continental slope (Thorburn et al., 2019; COSEWIC, 2021).
The northeast Pacific (from British Columbia, Canada, to Baja California, Mexico) once supported an extensive but brief fishery for soupfin sharks in the 1930s-40s (COSEWIC, 2021), and this species is still regularly encountered in fisheries and fishery surveys throughout these coastal waters. However, while common in estuarine systems in other regions (Walker et al., 2020; Nosal et al., 2021), soupfin sharks were not thought to inhabit the Salish Sea (Pietsch and Orr, 2015; Blaine et al., 2020; Lowry et al., 2022). The Salish Sea is an expansive inland waterway covering 16,925 km² across Northwestern Washington State and Southern British Columbia (Figure 1) that serves as a nexus of economic, environmental, and cultural interests (Gaydos et al., 2008; Jones et al., 2021). Despite consistent and extensive research and fishing efforts, apart from two recent strandings and a reported commercial catch in its northernmost extent in 2016 (Figure 1), soupfin sharks have not been previously described in the Salish Sea. However, in the last few years, there have been a growing number of anecdotal reports, by recreational fishers, of soupfin sharks and the largely sympatric broadnose sevengill shark (Notorynchus cepedianus) in the southernmost extent of the Salish Sea (aka South Puget Sound ~about 300km from previous strandings; Schulte et al., 2024).
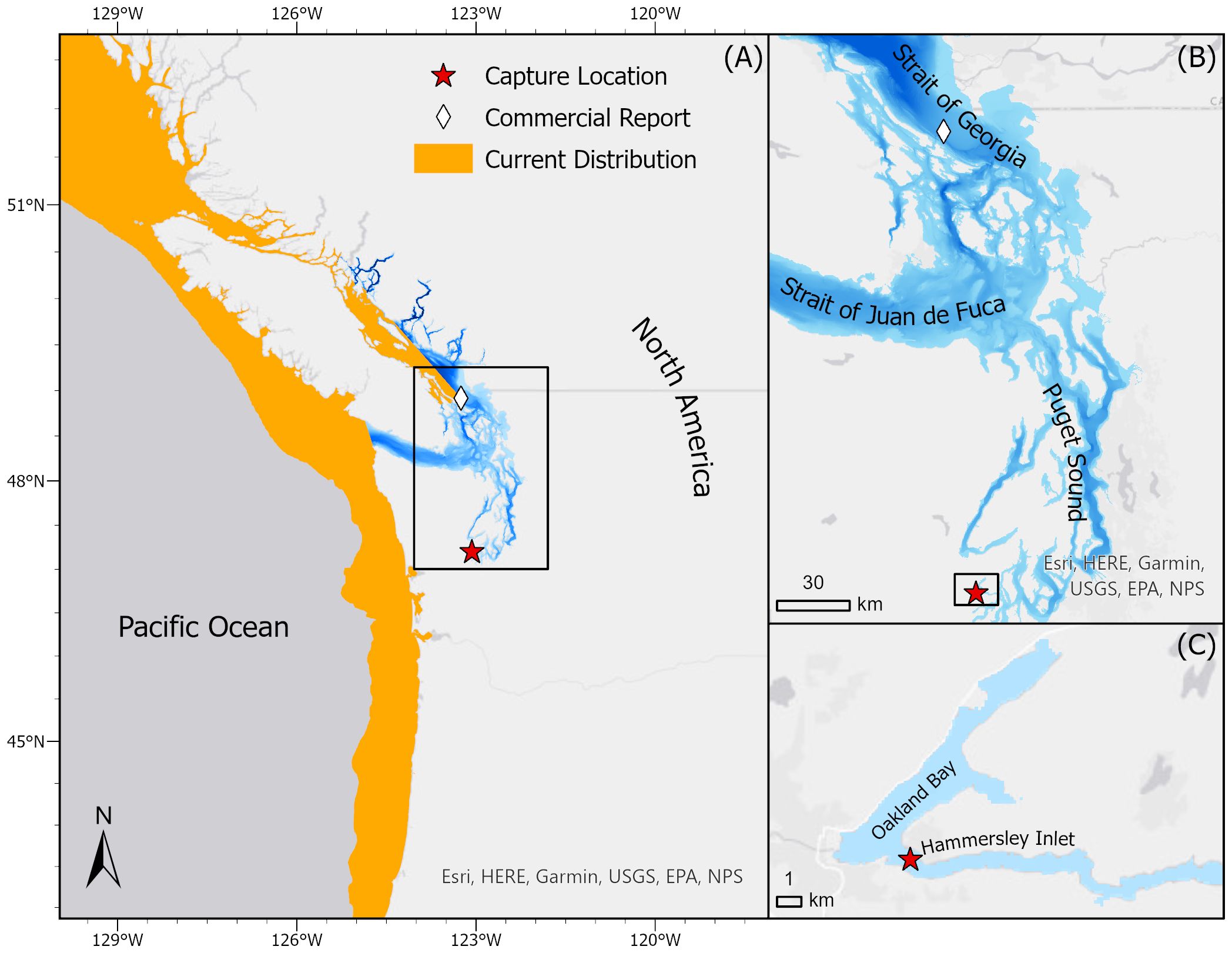
Figure 1 (A) Map showing the previously reported distribution of the soupfin shark (Galeorhinus galeus) in orange, the location of the reported commercial capture (diamond) (COSEWIC, 2021), and the capture location of the specimen reported here (star). The extent of the Salish Sea, encompassing the Strait of Juan de Fuca, Strait of Georgia, and Puget Sound, is represented in blue (https://wp.wwu.edu/salishseaatlas/). (B) Map showing the 3 major sub-basins of the Salish Sea; Strait of Juan de Fuca, Strait of Georgia, and Puget Sound. (C) Map showing the sampling site; Hammersley Inlet, Oakland Bay, and the capture location of the specimen described. Each black square represents the extent of the subsequent map.
At sufficient population levels, the presence of a novel marine predator species in the Salish Sea may influence system trophic dynamics and could necessitate consideration in ecosystem-based fisheries models currently utilized in the region (Morzaria-Luna et al., 2022). In order to address the anecdotal reports of soupfin sharks in South Puget Sound, we launched a multi-agency collaborative project aimed to: 1) confirm the presence of this species at a single site; and 2) evaluate whether reported encounters were merely stray occurrences (akin to the sporadic observations of juvenile gray whales in the Mediterranean Sea; Manfrini et al., 2023) or likely to be the result of a range extension driven by favorable climatic conditions and prey species abundance.
2 Methods
2.1 Site description — Hammersley Inlet
Hammersley Inlet is a narrow channel at the western edge of the South Salish Sea, near Shelton, WA, spanning approximately 10 kilometers in length with an average depth of 7 meters. Hammersley Inlet serves as the only connection between Oakland Bay — a shallow, highly productive, fjord-like estuary — and the greater Salish Sea (Figure 1C). Hammersley Inlet is characterized by significant current exchange, with peak flow velocities reaching 2.2 m/s, resulting in river-like hydrology throughout much of the inlet (Albertson et al., 2012). By water course distance, the eastern mouth of Hammersley Inlet is > 300 km from the mouth of the Strait of Juan de Fuca, providing extremely limited connection to open ocean waters, and the inlet is characterized by prolonged water residency times due to a combination of complex topography and intrabasin sills at several locations along this pathway (Khangaonkar et al., 2011). The west end of Hammersley Inlet is occupied by an industrial lumber mill, a marina, and other urban infrastructure.
2.2 Sampling
In response to anecdotal reports from recreational fishers, we employed a systematic approach to confirm the presence of soupfin sharks in Hammersley Inlet. We integrated fishing effort with opportunistic sampling of salinity and temperature within the inlet to identify environmental covariates associated with their occurrence outside of their previously known distribution. We used two primary fishing methods; multi-hook droplines deployed via research vessel and shore-based fishing using heavy tackle fishing rods. Hooks were baited with farmed Atlantic salmon (Salmo salar) and striped bonito (Sarda orientalis). These species were chosen because they are assumed prey in other systems but do not occur naturally within the study region (Klemetsen et al., 2003; Viñas et al., 2010); they can be excluded from any dietary analyses if observed in recovered stomach contents.
Each dropline consisted of a 8mm 3-strand nylon line, and from 1-3 PVC-coated wire leaders with baited circle hooks. This configuration was anchored with a 5kg weight then suspended from an inflatable PVC buoy and is designed to oscillate vertically signaling the presence of an animal on the line when deployed in calm inland waters. The baited hook was positioned between one and three meters above the anchor weight. This placement allowed water currents to impart a more lifelike movement to the bait, enhancing its attractiveness, and serving to minimize interactions with the benthic scavengers that also inhabit the inlet. For each sampling trip, the dropline sets were initially limited to a 30-minute duration and then were adjusted based on daily shark activity. This minimized the risk of predation on captured animals.
From shore, we utilized 3m casting rods rigged with 45kg breaking-strength monofilament fishing line, a 100g sinker, and leaders specially designed to target medium shark species. These leaders consisted of a 1m strand of braided steel wire covered in a translucent green nylon coating and a single barbless circle hook. The nylon coating’s semi-transparent nature helps reduce the visibility of the leader underwater and protect the sharks from wire abrasions.
2.3 Data collection
Captured sharks were brought to the surface, restrained alongside the research vessel, and positioned into the direction of the tidal current flow to better irrigate seawater over the gills. Once properly positioned, we collected morphometric data (length, sex, clasper state, and biological tissue samples) from each individual. Sharks were measured (precaudal length, fork length, and total length [TL]). For male sharks, claspers were measured from their insertion to tip, and sexual maturity was determined based on the degree of ossification in the inner structure and clasper length in relation to the pelvic fins (ICES, 2018). A small sample of tissue from the second dorsal fin was collected for genetic cataloging. Directly prior to release sharks were then tagged with uniquely numbered ID tags to aid in the identification of recaptured individuals.
Environmental covariates measured during sampling consisted of salinity (ppt) and temperature (°C), which were recorded using a YSI Pro30 (Yellow Spring Instruments Inc., USA). These measurements were taken at both the sea surface and sea floor to address potential vertical stratification and the effect of solar surface warming. Additionally, these data were collected opportunistically during periods within the inlet without soupfin shark catches. Tidal stage data was also recorded for each captured shark.
3 Results
During this exploratory study, we conducted 146 hook hours of effort over 8 field excursions to Hammersley Inlet, resulting in the capture of one soupfin shark, confirming the presence of this species outside of their known distribution. Despite the effectiveness of shore-based fishing efforts in capturing other elasmobranch species within the inlet, this method did not result in the capture of any soupfin sharks. Research vessel-based techniques were solely responsible for the capture of this species during our sampling efforts. The captured individual was a mature male measuring 153 cm in TL and was identified as G. galeus based on species-specific distinct morphological features. These include the specimen’s large body size, small second dorsal fin which closely resembled the size and shape of the anal fin, and long terminal caudal lobe which markedly distinguishes the species from other members of Triakidae (Figure 2). Capture occurred at a depth of 8 m, ~2 m above the seafloor, with a sea surface temperature and sea surface salinity of 17.6°C and 22.5 ppt, respectively, and a seafloor temperature and seafloor salinity of 16.8°C and 22.8 ppt, respectively. The individual was encountered during a rising tide, 2.88 hours before high water. Genetic material, photos, capture location coordinates, and other encounter metadata were submitted to the University of Washington’s Burke Museum fish collection (accession number 2023-003, catalog number UW202516).
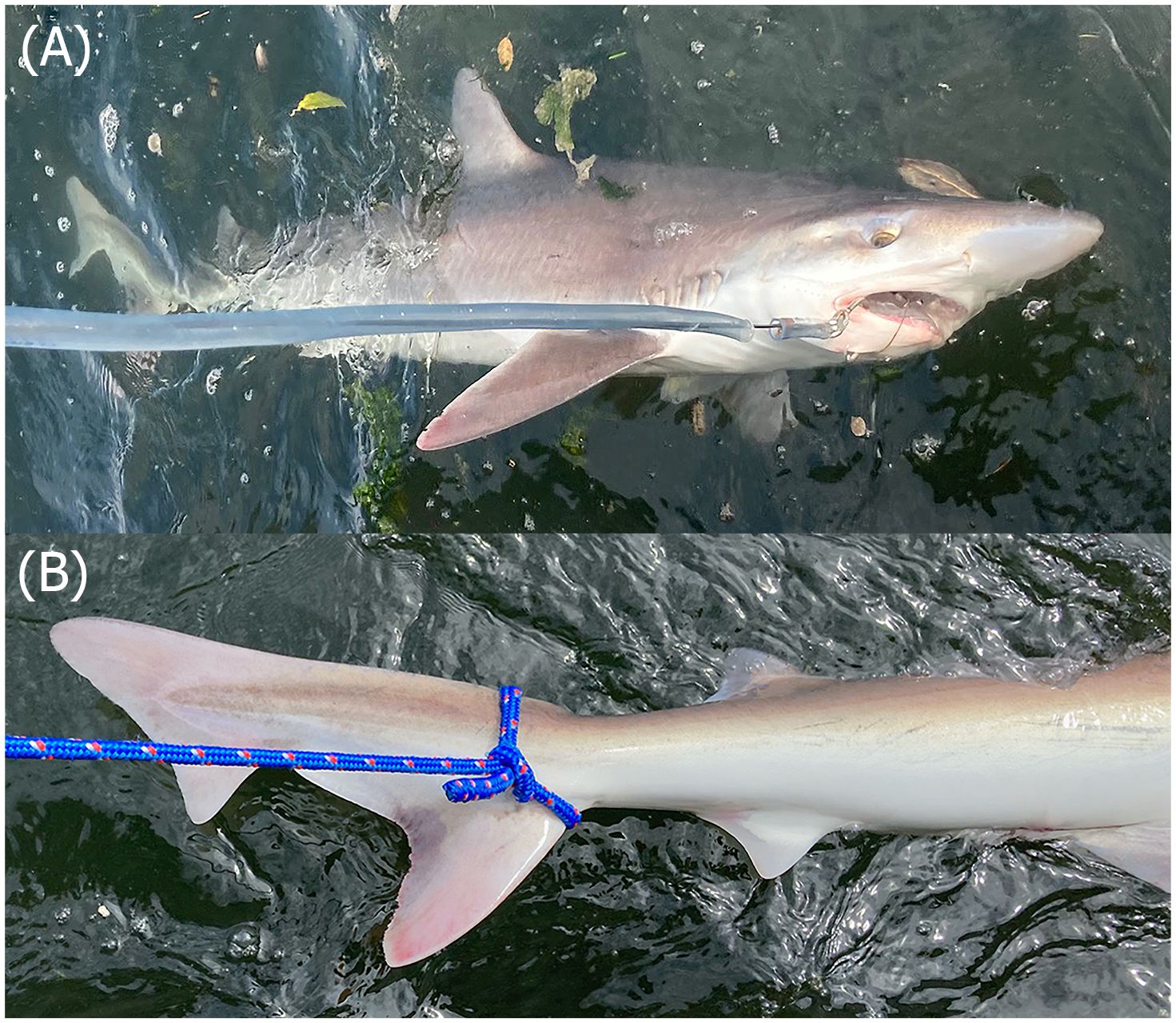
Figure 2 Photos of the captured 153 cm TL male soupfin shark (Galeorhinus galeus) in Hammersley Inlet, highlighting distinctive morphological characteristics of the (A) head, (B) tail, and second dorsal fin. Photos: (A) D. Lowry & (B) M. English.
4 Discussion
We present the first scientifically confirmed observation and capture of a soupfin shark in South Puget Sound, the southernmost extent of the Salish Sea, well outside of their previously described distribution (~300 km). These findings suggest that soupfin sharks are likely more pervasive in the Salish Sea than previously considered. There are two likely scenarios why the soupfin shark was not previously described in the Salish Sea: 1) this observation represents the first documentation of a species that has thus far evaded scientific surveys and significant fishing effort in a highly urbanized waterway, or, 2) this observation represents a potential emergence of the soupfin shark as a novel marine predator species in the Salish Sea.
It is conceivable that our observation of the soupfin shark in South Puget Sound represents the initial detection of an established but cryptic population of this highly mobile species within the Salish Sea (Scenario 1). However, we find this scenario unlikely. In this case, soupfin sharks would have to utilize habitats or exhibit behaviors that make them inaccessible to fishing gear (e.g., recreational, trawl, gillnet, longline gear), though these methods have been employed in the Salish Sea for over a century and are effective at catching soupfin in other regions. Further, soupfin sharks have not been observed on underwater video surveys within the Salish Sea, despite the efficacy of these methods in recording other shark species inhabiting the system (Lowry et al., 2022).
Instead, we suggest the presence of soupfin sharks likely represents an expansion of the seasonally occupied foraging habitat of this species (Scenario 2), potentially mediated by ongoing shifts in thermal conditions and prey species community composition within the Salish Sea. As a result of significant local anthropogenic pressure and influenced by broader climatological regimes and human-caused climate change, the Salish Sea has experienced pervasive shifts in trophic structure and species composition (Harvey et al., 2010; Greene et al., 2015; Ruggerone et al., 2019). For example, over the past century, the Salish sea forage fish community has been dominated by Pacific herring (Clupea pallasii), Pacific sand lance (Ammodytes personatus), surf smelt (Hypomesus pretiosus) and other smelt species (Osmeridae) (Kincaid, 1919; Therriault et al., 2009; Greene et al., 2015; Frick et al., 2022), while earlier accounts indicate periodic spikes in the abundance of Northern anchovy (Engraulis mordax) that were closely correlated with increased sea surface temperature and likely primary productivity throughout the Salish Sea (Morin et al., 2023). Forage fish play a critical role in marine systems, facilitating the flow of energy from basal trophic levels to higher-order predators, while simultaneously exerting top-down effects through predation upon planktonic species and the early life stages of their predators (Cury et al., 2000; Hallfredsson and Pedersen, 2009; Minto and Worm, 2012; Pikitch et al., 2014). The most recent significant spike in anchovy abundance in the Salish Sea began around 2014, aligning with a period of elevated sea surface temperatures in the region (Amos et al., 2014; Duguid et al., 2019), simultaneous with the increase in anecdotal reports of soupfin sharks by recreational fishers, which prompted this investigation.
Though we only report one capture, it is important to note that our catch per unit effort (CPUE) may be understated; we balanced our sampling between soupfin sharks and broadnose sevengill sharks, which were also recently discovered to inhabit the inlet (Schulte et al., 2024). For example, the 14/0 and 16/0 hooks, which are sufficiently large to minimize bycatch, likely discriminated against smaller soupfin sharks which are more selective feeders. Bait quality and fishing gear conspicuity may have been limiting factors as well. Expanding upon this initial exploration, future work should use smaller size 5/0 and 10/0 hooks which have proven effective in capturing an increased number of soupfin sharks across numerous size and age classes in other regions (Elías et al., 2005). Additionally, long-term acoustic tagging and monitoring has been utilized to identify highly migratory behavior and the periodicity of interannual site fidelity in soupfin sharks in other regions (Nosal et al., 2021). Therefore, we recommend additional sampling efforts for this species, incorporating both satellite and acoustic tagging, to understand the residency and habitat utilization of soupfin sharks within the Salish Sea and connectivity to the broader Northeast Pacific.
Our work contributes to a preliminary understanding of soupfin shark distribution and habitat preferences within the Salish Sea ecosystem that can both inform our understanding of changing regional ecosystem dynamics and the management of this critically endangered shark species.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Oregon State University IACUC-2023-0373. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
EP: Conceptualization, Investigation, Visualization, Writing – original draft, Writing – review & editing, Data curation, Project administration. JS: Conceptualization, Investigation, Writing – review & editing, Data curation, Project administration, Writing – original draft. DL: Conceptualization, Investigation, Writing – review & editing, Data curation, Funding acquisition, Project administration, Resources, Writing – original draft. LH: Investigation, Writing – review & editing, Funding acquisition, Project administration, Resources, Writing – original draft. ME: Investigation, Writing – original draft, Writing – review & editing, Data curation, Visualization. TC: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing, Data curation, Funding acquisition, Project administration, Resources, Supervision.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding for this research was provided by the Washington Department of Fish and Wildlife and Oregon State University.
Acknowledgments
The authors would like to acknowledge C. Seifert, whose reports spurred the survey efforts that documented the occurrence of this species in South Puget Sound. We are also thankful for the diligent fieldwork performed by K. Inch, E. Farias, and F. Chamberlain. We would like to thank JCH Cornapple and A. Nosal for supporting ongoing research on soupfin sharks in the Northeast Pacific.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Albertson S., Fox B., Meriwether F., Newton J. (2012). A dye, current meter, and modeling study in south puget sound — a highly refluxed semi-enclosed estuary. Estuar. Coast. Modeling, 1035–1052. doi: 10.1061/40990(324)56
Amos C. L., Martino S., Sutherland T. F., Al Rashidi T. (2014). Sea surface temperature trends in the coastal zone of british columbia, Canada. J. Coast. Res. 31, 434–446. doi: 10.2112/JCOASTRES-D-14-00114.1
Baum J. K., Worm B. (2009). Cascading top-down effects of changing oceanic predator abundances. J. Anim. Ecol. 78, 699–714. doi: 10.1111/j.1365-2656.2009.01531.x
Bizzarro J. J., Carlisle A. B., Smith W. D., Cortés E. (2017). Diet composition and trophic ecology of northeast pacific ocean sharks. Adv. Mar. Biol. 77, 111–148. doi: 10.1016/bs.amb.2017.06.001
Blaine J., Lowry D., Pacunski R. (2020). 2002-2007 WDFW Scientific Bottom Trawl Surveys in the Southern Salish Sea: Species Distributions, Abundance, and Population Trends (No. FPT 20-01) (Olympia, WA: Washington Department of Fish and Wildlife), i–237.
Chabot C. L. (2015). Microsatellite loci confirm a lack of population connectivity among globally distributed populations of the tope shark galeorhinus galeus (Triakidae): population connectivity of galeorhinus galeus. J. Fish Biol. 87, 371–385. doi: 10.1111/jfb.12727
Chabot C. L., Allen L. G. (2009). Global population structure of the tope (Galeorhinus galeus) inferred by mitochondrial control region sequence data. Mol. Ecol. 18, 545–552. doi: 10.1111/j.1365-294X.2008.04047.x
COSEWIC (2021). COSEWIC assessment and status report on the Tope Galeorhinus galeus in Canada. Committee on the Status of Endangered Wildlife in Canada. Available online at: https://www.Canada.ca/en/environment-climate-change/services/species-risk-public-registry.html.
Cury P., Bakun A., Crawford R. J. M., Jarre A., Quiñones R. A., Shannon L. J., et al. (2000). Small pelagics in upwelling systems: patterns of interaction and structural changes in “wasp-waist” ecosystems. ICES J. Mar. Sci. 57, 603–618. doi: 10.1006/jmsc.2000.0712
Duguid W. D. P., Boldt J. L., Chalifour L., Greene C. M., Galbraith M., Hay D., et al. (2019). Historical fluctuations and recent observations of Northern Anchovy Engraulis mordax in the Salish Sea. Deep Sea Res. Part II: Topical Stud. Oceanography Drivers dynamics small pelagic fish resources: Environ. control long-term changes 159, 22–41. doi: 10.1016/j.dsr2.2018.05.018
Elías I., Rodriguez A., Hasan E., Reyna M. V., Amoroso R. (2005). Biological observations of the tope shark, galeorhinus galeus, in the northern patagonian gulfs of Argentina. J. Northw. Atl. Fish. Sci. 35, 261–265. doi: 10.2960/J.v35.m487
Feit B., Feit A., Letnic M. (2019). Apex predators decouple population dynamics between mesopredators and their prey. Ecosystems 22, 1606–1617. doi: 10.1007/s10021-019-00360-2
Frick K. E., Kagley A. N., Fresh K. L., Samhouri J. F., Ward L. S., Stapleton J. T., et al. (2022). Spatiotemporal variation in distribution, size, and relative abundance within a salish sea nearshore forage fish community. Mar. Coast. Fisheries 14, e10202. doi: 10.1002/mcf2.10202
Gaydos J. K., Dierauf L., Kirby G., Brosnan D., Gilardi K., Davis G. E. (2008). Top 10 principles for designing healthy coastal ecosystems like the Salish Sea. EcoHealth 5, 460–471. doi: 10.1007/s10393-009-0209-1
Greene C., Kuehne L., Rice C., Fresh K., Penttila D. (2015). Forty years of change in forage fish and jellyfish abundance across greater Puget Sound, Washington (USA): anthropogenic and climate associations. Mar. Ecol. Prog. Ser. 525, 153–170. doi: 10.3354/meps11251
Hallfredsson E. H., Pedersen T. (2009). Effects of predation from juvenile herring (Clupea harengus) on mortality rates of capelin (Mallotus villosus) larvae. Can. J. Fish. Aquat. Sci. 66, 1693–1706. doi: 10.1139/F09-105
Harvey C. J., Bartz K., Davies J., Francis T., Good T., Guerry A., et al. (2010). A Mass-balance Model for Evaluating Food Web Structure and Community-scale Indicators in the Central Basin of Puget Sound (NOAA Technical Memorandum No. NMFS-NWFSC-106) (Seattle, WA: U.S. Department of Commerce), ii–180.
Heithaus M. R., Frid A., Wirsing A. J., Dill L. M., Fourqurean J. W., Burkholder D., et al. (2007). State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 76, 837–844. doi: 10.1111/j.1365-2656.2007.01260.x
Heithaus M. R., Frid A., Wirsing A. J., Worm B. (2008). Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. doi: 10.1016/j.tree.2008.01.003
ICES (2018). Report of the Workshop for Advancing Sexual Maturity Staging in Fish (WKASMSF) Vol. 38 (Copenhagen, Denmark: ICES CM/EOSG), 1–75.
Jones J., Keller P., van der Flier Keller E. (2021). Review of official responsibility for the Salish Sea marine environment. Ocean Coast. Manage. 211, 105748. doi: 10.1016/j.ocecoaman.2021.105748
Khangaonkar T., Yang Z., Kim T., Roberts M. (2011). Tidally averaged circulation in Puget Sound sub-basins: Comparison of historical data, analytical model, and numerical model. Estuarine Coast. Shelf Sci. 93, 305–319. doi: 10.1016/j.ecss.2011.04.016
Kincaid T. (1919). An Annotated List of Puget Sound Fishes (Olympia, Wash. USA: State printer), 1–52.
Klemetsen A., Amundsen P.-A., Dempson J. B., Jonsson B., Jonsson N., O’Connell M. F., et al. (2003). Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshw. Fish 12, 1–59. doi: 10.1034/j.1600-0633.2003.00010.x
Lowry D., Pacunski R., Hennings A., Blaine J., Tsou T., Hillier L., et al. (2022). Assessing bottomfish and select invertebrate occurrence, abundance, and habitat associations in the U.S. Salish Sea with a small, remotely operated vehicle: results of the 2012-13 systematic survey (Fish Program Technical Report No. 22– 03) (Seattle, WA: Washington Department of Fish and Wildlife), i–67.
Manfrini V., Fioravanti T., Madonna A., Maio N. (2023). First sighting of Gray Whale Eschrichtius robustus (Lilljeborg 1861) (Cetartiodactyla: Eschrichtiidae) in Italian waters and review of Mediterranean Sea records. Hystrix It. J. Mamm. 34, 147–150. doi: 10.4404/hystrix-00666-2023
Minto C., Worm B. (2012). Interactions between small pelagic fish and young cod across the North Atlantic. Ecology 93, 2139–2154. doi: 10.1890/10-2036.1
Morin J., Evans A. B., Efford M. (2023). The rise of vancouver and the collapse of forage fish: A story of urbanization and the destruction of an aquatic ecosystem on the salish sea, (1885–1920 CE). Hum. Ecol. 51, 303–322. doi: 10.1007/s10745-023-00398-w
Morzaria-Luna H. N., Kaplan I. C., Harvey C. J., Girardin R., Fulton E. A., MacCready P., et al. (2022). Design and Parameterization of a Spatially Explicit Atlantis Ecosystem Model for Puget Sound. doi: 10.25923/TNP6-MF67
Nakatsu L. M. (1957). A Review of the Soupfin Shark Fishery of the Pacific Coast. Pacific Coast Comm. Fish. Rev, Vol. 19. 5–8.
Newsome T., Ripple W. (2015). A continental scale trophic cascade from wolves through coyotes to foxes. J. Anim. Ecol. 84, 49–59. doi: 10.1111/1365-2656.12258
Nosal A. P., Cartamil D. P., Ammann A. J., Bellquist L. F., Ben-Aderet N. J., Blincow K. M., et al. (2021). Triennial migration and philopatry in the critically endangered soupfin shark Galeorhinus galeus. J. Appl. Ecol. 58, 1570–1582. doi: 10.1111/1365-2664.13848
Pietsch T., Orr J. (2015). Fishes of the Salish Sea: a compilation and distributional analysis (Seattle, WA: The scientific publications office, National Marine Fisheries Service, NOAA), iii–106.
Pikitch E. K., Rountos K. J., Essington T. E., Santora C., Pauly D., Watson R., et al. (2014). The global contribution of forage fish to marine fisheries and ecosystems. Fish Fisheries 15, 43–64. doi: 10.1111/faf.12004
Pinnegar J. K., Polunin N. V. C., Francour P., Badalamenti F., Chemello R., Harmelin-Vivien M.-L., et al. (2000). Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ. Conserv. 27, 179–200. doi: 10.1017/S0376892900000205
Ripple W. J., Beschta R. L. (2004). Wolves and the ecology of fear: can predation risk structure ecosystems? BioScience 54, 755–766. doi: 10.1641/0006-3568(2004)054[0755:WATEOF]2.0.CO;2
Ruggerone G. T., Springer A. M., Shaul L. D., van Vliet G. B. (2019). Unprecedented biennial pattern of birth and mortality in an endangered apex predator, the southern resident killer whale, in the eastern North Pacific Ocean. Mar. Ecol. Prog. Ser. 608, 291–296. doi: 10.3354/meps12835
Schulte J., Personius E. M., Lowry D., Hillier L., McInturf A. G., Chapple T. K. (2024). Advancing the ecological narrative: Documentation of broadnose sevengill sharks (Notorynchus Cepedianus) in South Puget Sound, Washington, USA. Front. Mar. Sci. 11, 1–6. doi: 10.3389/fmars.2024.1430962
Terborgh J., Estes J. A., Paquet P., Ralls K., Boyd-Herger D., Miller B. J., et al. (1999). “The Role of Top Carnivores in Regulating Terrestrial Ecosystems,” in Continental conservation: Scientific foundations of regional reserve networks. Eds. Soule M. E., Terborgh J. (Island Press, Washington, DC), 39–64.
Therriault T. W., Hay D. E., Schweigert J. F. (2009). Biological overview and trends in pelagic forage fish abundance in the salish sea (Strait of Georgia, british columbia). Mar. Ornithology 37, 3–8.
Thorburn J., Neat F., Burrett I., Henry L.-A., Bailey D. M., Jones C. S., et al. (2019). Ontogenetic variation in movements and depth use, and evidence of partial migration in a benthopelagic elasmobranch. Front. Ecol. Evol. 7. doi: 10.3389/fevo.2019.00353
Viñas J., Alvarado Bremer J. R., Pla C. (2010). Phylogeography and phylogeny of the epineritic cosmopolitan bonitos of the genus Sarda (Cuvier): inferred patterns of intra- and inter-oceanic connectivity derived from nuclear and mitochondrial DNA data. J. Biogeography 37, 557–570. doi: 10.1111/j.1365-2699.2009.02225.x
Keywords: marine ecology, Salish Sea, elasmobranch, forage fish, species distribution, marine conservation
Citation: Personius EM, Schulte JM, Hillier L, Lowry D, English M and Chapple TK (2024) Observation of the critically endangered soupfin shark (Galeorhinus galeus) in the Changing Salish Sea. Front. Mar. Sci. 11:1420721. doi: 10.3389/fmars.2024.1420721
Received: 03 May 2024; Accepted: 01 July 2024;
Published: 18 July 2024.
Edited by:
Austin Gallagher, Beneath the Waves, Inc., United StatesReviewed by:
Pablo Del Monte-Luna, Centro Interdisciplinario de Ciencias Marinas (IPN), MexicoOwen Nichols, Center for Coastal Studies, United States
Copyright © 2024 Personius, Schulte, Hillier, Lowry, English and Chapple. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ethan M. Personius, ZXRoYW4ucGVyc29uaXVzQG9yZWdvbnN0YXRlLmVkdQ==
 Ethan M. Personius
Ethan M. Personius Jessica M. Schulte
Jessica M. Schulte Lisa Hillier
Lisa Hillier Dayv Lowry
Dayv Lowry Maddie English
Maddie English Taylor K. Chapple
Taylor K. Chapple