
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 10 July 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1419606
 Yixi Tao1,2
Yixi Tao1,2 Chunying Du1,2
Chunying Du1,2 Shanwen Jiang1,2
Shanwen Jiang1,2 Siling Zhang1,2
Siling Zhang1,2 Jingyun Feng1,2
Jingyun Feng1,2 Xiaomin Miao1,2
Xiaomin Miao1,2 Hao Xu1,2
Hao Xu1,2 Yun Li1,2*
Yun Li1,2*The liver is essential for nutritional balance in fish, and liver damage in farmed fish often arises from factors like overfeeding, causing various health issues. Clinical observations indicate that liver diseases frequently involve spleen dysfunction, and there is evidence to suggest that the spleen has a significant impact on liver function. However, there has been no discussion on the role of the spleen in maintaining liver health in fish. To understand the role of fish spleen in liver metabolism, this study selected Nile tilapia (Oreochromis niloticus) as the experimental material and first established a tilapia splenectomy model. Various biochemical parameters of the liver and serum were measured, and the liver metabolism characteristics of the splenectomy group (SP group) and the sham operation group (SO group) were analyzed using metabolomics. After splenectomy, biochemical parameters of the liver and serum showed abnormalities, including significant increases in total cholesterol (T-CHO) and serum total bile acid (TBA) levels, alongside a significant decrease in liver TBA levels, suggesting impaired metabolic function and cholesterol deposition in the liver. Metabolomics analysis showed that metabolites such as lipids, lipid-like molecules, and organic acids and derivatives were differentially regulated between the SO and SP group. KEGG analysis showed that differential metabolites were enriched in lipid metabolism and amino acid metabolism. The metabolic pathway analysis of differential metabolites showed that after splenectomy, the low-activity urea cycle in the liver may accelerate lipid synthesis, while low concentrations of aromatic amino acids and taurine may inhibit lipid catabolism. These results indicate that after splenectomy, the liver metabolic capacity is impaired, which causes abnormal lipid metabolism by interfering with amino acid metabolism, making splenectomy tilapia liver at risk of liver disease, including cholesterol deposition, hepatic steatosis and nonalcoholic fatty liver disease (NAFLD). Our results show that the spleen is involved in regulating liver lipid and amino acid metabolism, and the spleen may interfere with lipid metabolism by regulating liver amino acid metabolism. Our data can provide support for further research on liver and spleen functions and the immune-nutrient metabolism mechanisms in fish, as well as new ideas for healthy fish farming.
The liver is the metabolic center for nutrients such as proteins, lipids, and carbohydrates, and it plays a decisive role in maintaining nutrient homeostasis (Trefts et al., 2017). To accelerate growth performance, excessive energy-rich diets are often fed to fish, resulting in an excessive burden on the fish liver (Du et al., 2006). Furthermore, improper handling during procurement, transportation, and preservation can lead to lipid peroxidation in the feed. Studies have shown that oxidized oil is one of the important causes of oxidative damage to fish liver cells and abnormal liver function (Chen et al., 2012b). Damage to the health of fish livers can compromise their immunity and stress resistance, ultimately leading to a deterioration in their overall health, and triggering other diseases (Cao et al., 2019). These conditions increase the susceptibility of fish to diseases and mortality, ultimately reducing their growth performance and feed efficiency, thereby driving up the costs of aquaculture. Therefore, protecting the health of fish livers is very important.
The spleen has been reported as an organ closely related to lipid metabolism for decades (He et al., 2022). Clinical evidence suggests that splenectomy is associated with changes in serum lipoproteins (Demuner et al., 2015). In animal models, splenectomy has been shown to exacerbate various diseases caused by high-fat diets, such as obesity, atherosclerosis, and NAFLD (Ai et al., 2018). A study has found that the relative weight of the spleen in broilers experiencing chronic heat stress is positively correlated with the amount of lysine added to their feed (Han et al., 2022). In traditional Chinese medicine, a spleen-deficiency constitution is reported to be associated with disruptions in tryptophan metabolism and the metabolism of cysteine and methionine (Liang et al., 2023). A spleen-deficiency rat model have been found to be highly associated with endogenous metabolic disorders, primarily affecting amino acid and lipid metabolism (Zhang et al., 2021). These findings demonstrate that the spleen is widely involved in lipids and amino acids metabolism.
The spleen is close to the liver anatomically, and bioactive substances secreted by the spleen enter the liver via the portal vein, influencing the progression and prognosis of liver diseases. Cirrhosis is often accompanied by various complications such as splenomegaly and hyperfunction of the spleen in clinical practice (Iwakiri, 2014; Sharma and John, 2024), and splenectomy can improve liver function in cirrhosis patients (Murata et al., 2008). In the process of liver fibrosis, the spleen can promote the secretion of chemotactic factor CCL2 by liver macrophages by upregulating SOCS3 signaling, and ultimately promote fibrosis progression (Li et al., 2018). Oishi et al. (2011) studied rats with nonalcoholic steatohepatitis that had their spleens removed, demonstrating the spleen’s protective role in the occurrence and development of NAFLD. Arakawa et al. (2009) examined the impact of splenectomy on extensive liver resection in rats, showing that splenectomy can mitigate liver injury and promote regeneration. Splenectomy easily inhibits the activity of Kupffer cells in the liver’s reticular-endothelial system, resulting in dyslipidemia (Hoekstra et al., 2005), while the spleen is a core participant in the reticular-endothelial system (Tarantino et al., 2021). These studies indicate that the spleen plays a crucial role in maintaining normal liver function, although the mechanisms are not yet clear.
In fish, there has not yet been a discussion on the role of the spleen in maintaining liver health. Therefore, we hope to explore this by establishing a spleen-deficient model in fish. As an excellent farming fish, tilapia has been studied for many applications, such as tilapia breeding technology optimization (Azim and Little, 2008; Mengistu et al., 2020; Wang et al., 2023), tilapia high-quality feed research (Lin and Luo, 2011), tilapia disease prevention and control (Chen et al., 2012a; Zhu et al., 2015). Some researchers also use tilapia as a drug research model (Valença-Silva et al., 2014; Crivelaro et al., 2019). The development of tilapia genome sequencing and database construction (Katagiri et al., 2005; Joshi et al., 2018) provides sequence resources for the study of tilapia, and also provides a basis for further study of the physiological mechanism of tilapia. These research foundations have made tilapia an excellent model for studying fish physiology (Brown et al., 2014).
Metabolomics is a new “omics” research field, mainly for high-throughput qualitative and quantitative analysis of small and medium-sized molecular metabolites in metabolomics (Nguyen et al., 2018), especially small molecular substances with molecular weight < 1000 (German et al., 2005). Metabolomics focuses on the metabolites of these small molecules. It is easier to detect, does not need a characteristic database, has fewer types than genes and proteins, and the metabolites are universal (Taylor et al., 2002). Through metabolomics, researchers can measure the end products of complex and difficult-to-decipher gene, epigenetic, and environmental interactions, and then obtain a complete description of the body phenotype (Fiehn, 2002; Monteiro et al., 2013). Therefore, metabolomics has been gradually applied in more disciplined research fields, such as biomedical marker screening and disease mechanism analysis, food nutrition analysis, crop characteristic analysis, and environmental monitoring (Moore et al., 2007; Gilany et al., 2014; Mandavi et al., 2015; Kim et al., 2016). At the beginning of metabolomics research, a large number of studies have been carried out in the field of liver disease (Gibelin et al., 2000; Robertson et al., 2000; Nicholson et al., 2002, 2008).
In this study, we established a spleen-deficient model of tilapia by splenectomy, and the serum measured the serum and liver biochemical parameters of tilapia, and hoped to study the effect of splenectomy on tilapia liver metabolism through metabolomics, to understand the role of the spleen in liver metabolism. We found that the levels of lipids, lipid-like molecules, and organic acids and derivatives in the liver of tilapia significantly decreased after splenectomy. Some metabolic pathways changed. These metabolic pathways mainly involved arginine biosynthesis; arginine and proline metabolism; phenylalanine, tyrosine and tryptophan biosynthesis; alanine, aspartate and glutamate metabolism; phenylalanine metabolism; glycine, serine and threonine metabolism; and taurine and hypotaurine metabolism. Our data can provide a reference for the study of fish spleen function and liver-spleen interaction mechanisms.
Healthy Nile tilapia (Oreochromis niloticus) (n = 28, average body length = 14.15 ± 2.03 cm, average weight = 83.21 ± 3.56 cm) were bred in laboratory circulating water culture system. The water temperature was 28.5 ± 1.0°C, pH was 6.8-7.5, fed twice a day (9:00 and 17:00), and water was changed once every two days, and one-half of each time.
Splenectomy (SP group): Feeding was stopped one day before the operation. The scalpel and other instruments were sterilized with high pressure steam before the operation. An absorbable surgical suture packed with vacuum sterilization was used. The fish were anesthetized with MS-222 (100 mg/L) until the fish turned over and stopped completely. After anesthesia, the experimental fish were placed on the sterile gauze pad with the left side upward, and the gills were perfused intermittently with the same concentration of MS-222 solution during the operation. After removing the scales in the posterior part of the pectoral fin, a 1~1.5 cm incision was made 5 mm behind the pectoral fin, and then the incision was opened with a spreader. After the spleen was found, it was carefully removed, and the spreader was removed. The blood stains and other residues were washed away with 0.65% NaCl, and then the incision was sutured with the simple intermittent suture method, each suture was 0.5 cm apart, a total of 3 stitches. The suture line was disinfected with iodophor and then put into the recirculating aquaculture system. The operation time for each fish was not more than 10 minutes.
Sham operation (SO group): For the sham operation, except for no splenectomy, the other steps were the same as those in the SP group.
Among the 28 fish, 16 were randomly selected for the SP group, and 12 for the SO group as the control. No feeding for the first 7 days after the operation to prevent the incision from cracking. Normal feeding was started on the 8th day after the operation.
At 35th day postoperatively, 8 fish were randomly selected from the SP group and SO group, and an appropriate amount of MS-222 was added for anesthesia. Blood samples were collected from the caudal vein of fish (approximately 0.8 mL per fish) and transferred into EP tubes, then kept at room temperature for 30 minutes and stored overnight at 4°C. After centrifugation at 3500 rpm and 4°C for 10 minutes, the isolated serum was obtained and stored at -80°C until analysis. Liver tissues were separated, and each liver tissue was divided into two parts. One for metabolomics analysis, these liver tissues were immediately put into liquid nitrogen for 30 seconds and stored in EP tubes at -80°C until analysis. The other for measuring biochemical parameters, these liver tissues were put in EP tubes, and 0.9% NaCl was added at a ratio of weight (g): volume (mL) = 1: 9, homogenized in an ice bath, centrifuged at 2500 rpm and 4°C for 10 minutes, and the supernatant was extracted and stored at -80°C until analysis.
The cryopreserved serum and liver supernatant were thawed on ice. The contents of total protein (TP), albumin (ALB), total cholesterol (T-CHO), triglyceride (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and total bile acid (TBA) were determined using methods described in the detection kits of Nanjing Jiancheng Bioengineering Institute (Jiangsu, China).
Metabolites extraction, detection, and analysis of the liver samples were performed as described previously (Fang et al., 2022). Briefly, an extract solution was added to the liver sample, and then the supernatant was extracted through homogenization, ultrasound, incubation, and centrifugation. Then the metabolites were detected in positive ion mode (POS) and negative ion mode (NEG) by an UHPLC system of Thermo Fisher Scientific. The raw data were processed by peak detection, extraction, alignment, and integration. Then, the metabolites were annotated using the MS2 database of Shanghai Biotree Biomedical Technology Co., Ltd. (Shanghai, China).
Firstly, the raw data was filtered, input missing values and normalized to a single peak. Then, multivariate analysis was conducted using SIMCA software (version 16.0.2) where the dataset underwent scaling and log transformation to reduce the effects of variable noise and variance. Principal component analysis (PCA) and orthogonal projections to latent structures-discriminant analysis (OPLS-DA) were utilized to assess sample distribution and identify significant metabolic variations. The robustness of the model was evaluated using permutation tests. Additionally, the significance of metabolites was determined by assessing the variable importance in projection (VIP) scores from the OPLS-DA, with metabolites having a VIP score greater than 1 and a p-value less than 0.05 being classified as significantly differential. A Euclidean distance matrix was constructed based on the levels of these metabolites, followed by hierarchical clustering using the complete linkage approach. The Human Metabolome Database (HMDB) was referenced to determine the origins of these differential metabolites. Pathway enrichment analysis was carried out with the aid of KEGG and MetaboAnalyst to elucidate the metabolic pathways involved.
Statistical significance between the two groups was assessed using the t-test. Significance was established at the p-value of less than 0.05. The data are reported as mean values with standard error of mean (SEM). For the data analysis, we employed GraphPad Prism version 9.5.1 (GraphPad Software, San Diego, California, USA).
To understand how the biochemical parameters of tilapia changed after splenectomy, we measured the biochemical indices of serum and liver. In serum (Figure 1A), there was no significant difference in the contents of TP, ALB, HDL, and AST between the SP group and SO group. The contents of T-CHO, TG, LDL, ALT, and TBA in the SP group were significantly higher than those in the SO group. In liver (Figure 1B), there was no significant difference in TP, ALB, TG, HDL, AST, and ALT contents between the SP group and SO group. The contents of T-CHO and LDL in the SP group were significantly higher than those in the SO group, and the content of TBA in the SP group was significantly lower than that in the SO group.
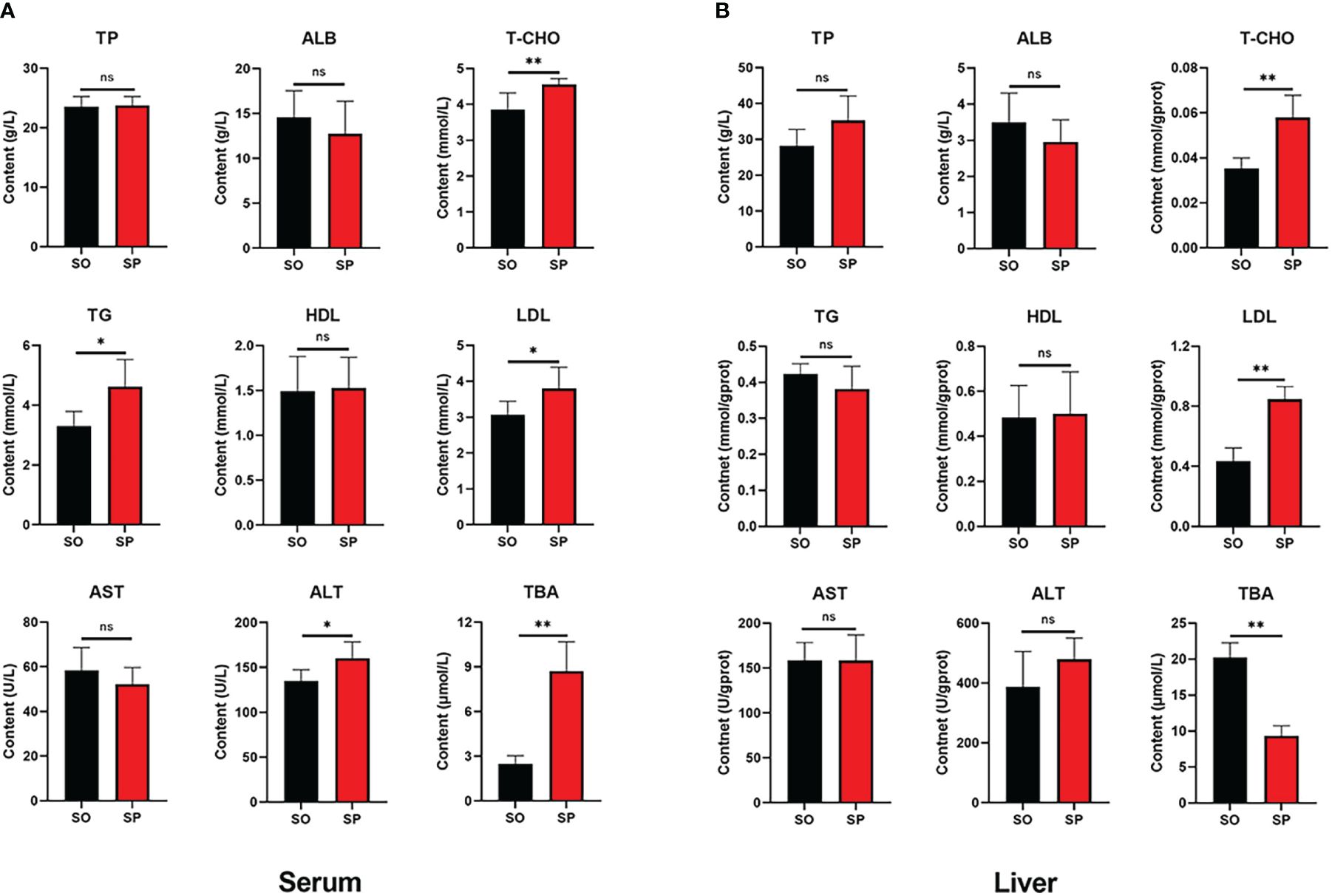
Figure 1 Changes of serum and liver biochemical parameters after splenectomy. (A) Serum; (B) Liver. ns p > 0.05, * p < 0.05, ** p < 0.01.
Following data preprocessing, which included filtering, imputing missing values, and normalizing single peaks, we identified and quantified a total of 5792 valid peaks in POS and 6144 in NEG for liver samples. PCA was employed to uncover the intrinsic data structure and reduce dimensionality, thereby facilitating a more coherent interpretation of the variables. The PCA score scatter plots demonstrated that all samples fell within the 95% Hotelling’s T-squared ellipse for both POS and NEG modes (Figures 2A, B). The PCA model exhibited R2X values of 0.617 for POS and 0.520 for NEG when comparing the SP group and SO group.
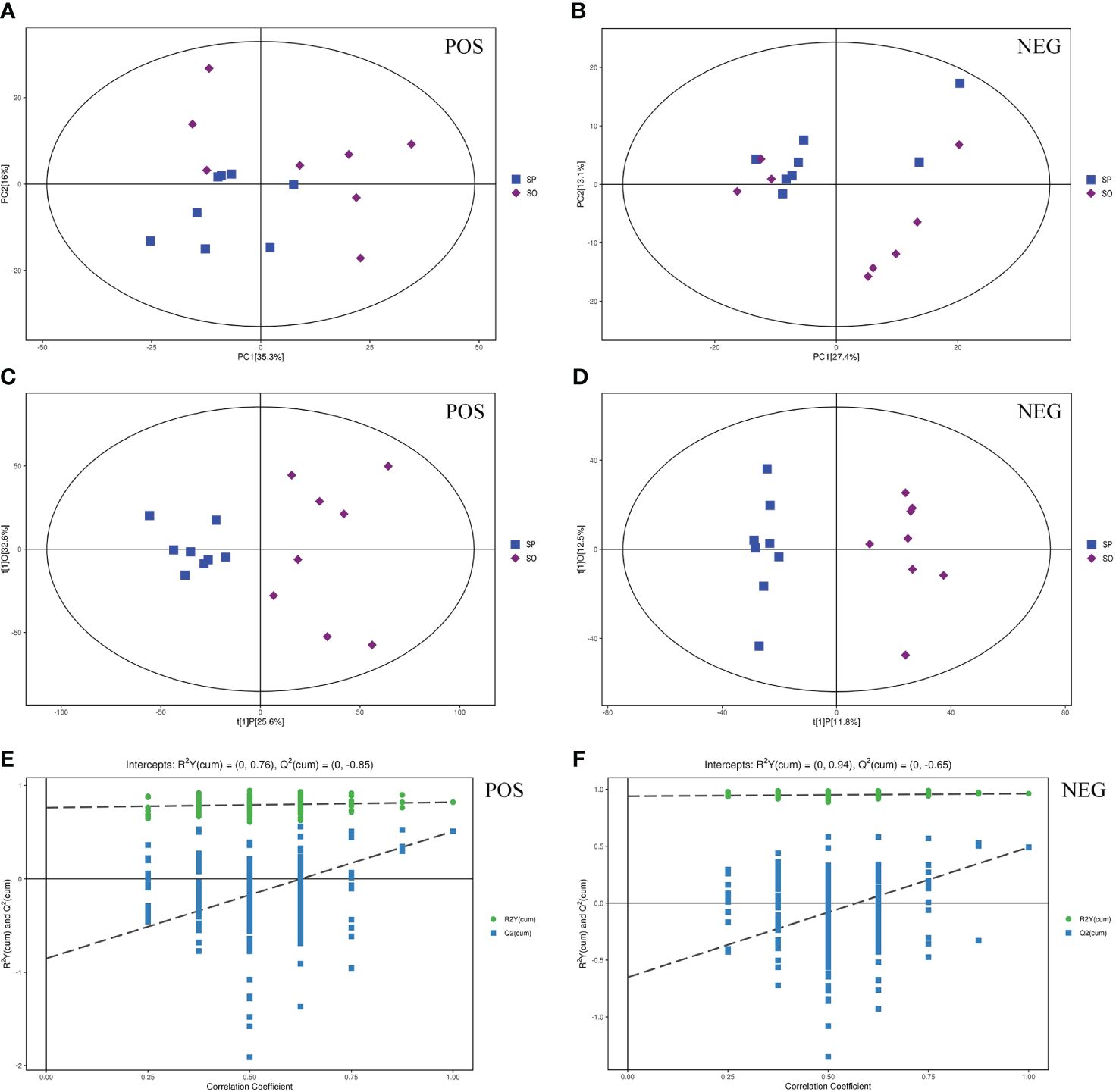
Figure 2 Multivariate analysis of liver metabolites. (A, B) Score scatter plot of PCA model. (C, D) OPLS-DA model for SP vs. SO. (E, F) Permutation test of OPLS-DA model for SP vs. SO. (A, C, E) Positive ion mode. (B, D, F) Negative ion mode.
However, reliance solely on the PCA model for analysis could scatter the differential variables across multiple principal components due to the influence of correlated variables. This dispersion could obscure visualization and hinder further analysis. To address this, OPLS-DA was applied, providing more reliable correlation information regarding differential metabolites between the two groups. All samples in the OPLS-DA score scatter plots were also aligned within the 95% Hotelling’s T-squared ellipse (Figures 2C, D). For the OPLS-DA model in POS, we observed R2X, R2Y, and Q2 values of 0.582, 0.821, and 0.509, respectively. In NEG, the values were 0.243 for R2X, 0.962 for R2Y, and 0.491 for Q2. The model’s validity was further affirmed through permutation testing, yielding R2Y and Q2 values of 0.76 and -0.85 in POS and 0.94 and -0.65 in NEG, respectively (Figures 2E, F). These findings confirm that the OPLS-DA model is robust and free from overfitting, thus qualifying it for more detailed analysis.
In the comparative study of liver metabolites, we detected a total of 1,618 metabolites in POS and 806 in NEG across the two groups. The distribution of these metabolites can be categorized into up-regulated and down-regulated subsets, as depicted in Figures 3A, B. Differential metabolites were identified using the p-value threshold of less than 0.05 and the VIP score greater than 1.
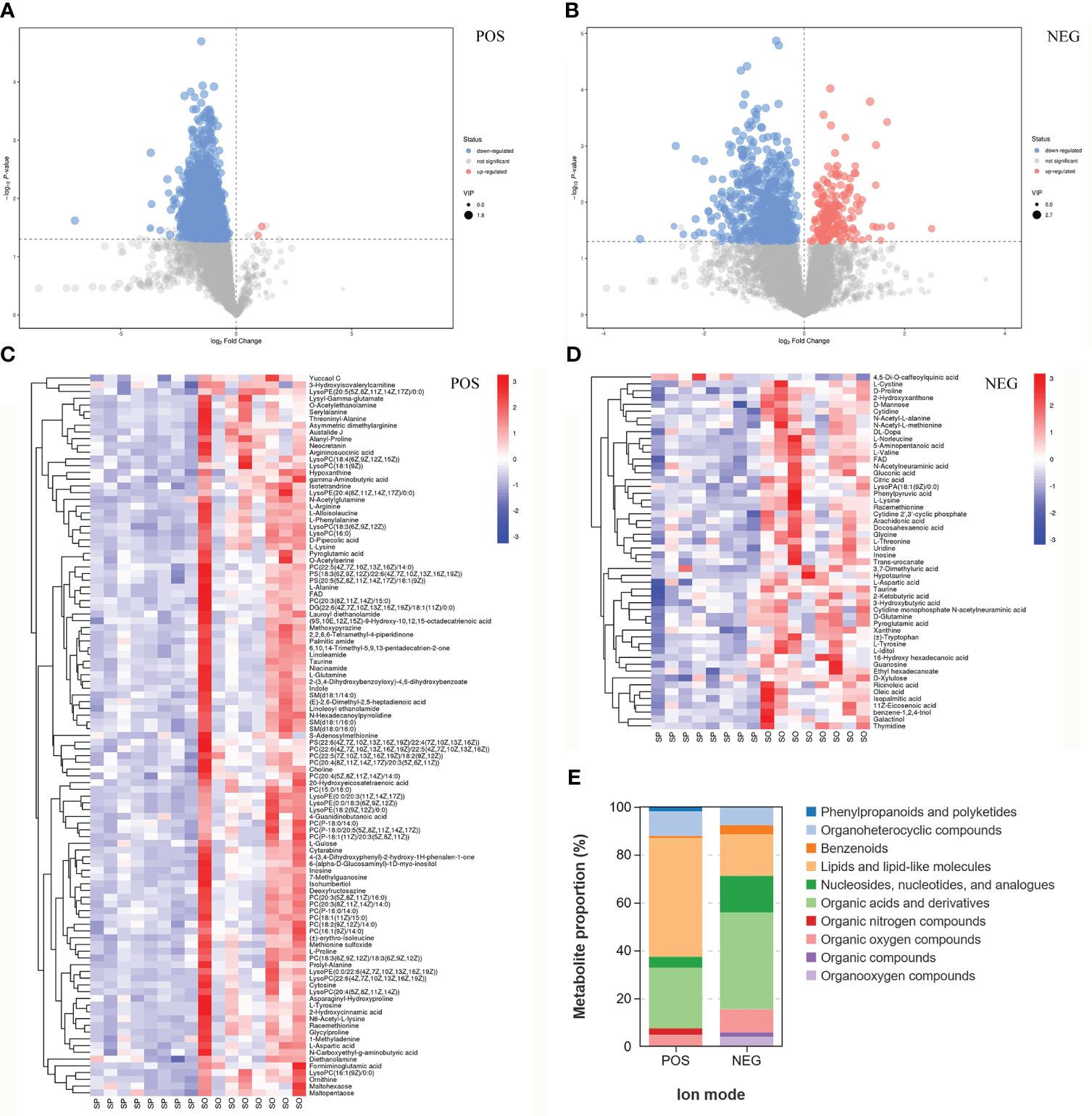
Figure 3 Analysis of differentially expressed metabolites. (A, B) Volcano plot for SP vs. SO. Red and blue indicate significantly up-regulated and down-regulated metabolites respectively, gray indicates non significantly different metabolites. (C, D) Heatmap of hierarchical clustering analysis for SP vs. SO. The color blocks represent the relative expression of metabolites at corresponding positions, while red and blue indicate high and low expression of the metabolites. (E) Classification of differential metabolites in HMDB database. Different colors represent different metabolite classifications. (A, C) Positive ion mode. (B, D) Negative ion mode.
Hierarchical clustering analysis highlighted distinct variations in metabolite profiles between the SP group and the SO group in both ionization modes. Notably, the SP group exhibited significantly lower metabolite levels compared to the SO group, as shown in Figures 3C, D. Specifically, in the POS mode, 107 metabolites were significantly downregulated in the SP group relative to the SO group (Figure 3C and Supplementary Table 1). In the NEG mode, 51 metabolites were significantly downregulated, and one metabolite was significantly upregulated in the SP group compared to the SO group (Figure 3D and Supplementary Table 2).
Further classification of these differential metabolites through the HMDB annotation revealed that they fell into eight distinct categories for both POS and NEG modes, as illustrated in Figure 3E. In POS, the predominant categories were lipids and lipid-like molecules (53 metabolites, accounting for 49.53%), organic acids and derivatives (27 metabolites, accounting for 25.23%), and organoheterocyclic compounds (11 metabolites, accounting for 10.28%), detailed in Supplementary Table 1. In NEG, the predominant categories were organic acids and derivatives (21 metabolites, accounting for 40.38%), lipids and lipid-like molecules (9 metabolites, accounting for 17.31%), and nucleosides, nucleotides, and analogues (8 metabolites, accounting for 15.38%), detailed in Supplementary Table 2.
We carried out KEGG pathway annotation on every differential metabolite to give a more thorough and organized examination of metabolic and regulatory processes. In POS, KEGG annotation pathway of differential metabolites involved 5 pathway types, of which “metabolism” accounts for the largest proportion, including eight metabolic pathways such as amino acid metabolism, lipid metabolism, and metabolism of other amino acids (Figure 4A). In NEG, the KEGG annotation pathway of differential metabolites involved 6 pathway types, of which “metabolism” accounts for the largest proportion, including eight metabolic pathways such as amino acid metabolism, metabolism of other amino acids, and carbohydrate metabolism (Figure 4B). In POS, KEGG enrichment analysis showed that significantly enriched pathways such as biosynthesis of amino acids, choline metabolism in cancer, and glycerophospholipid metabolism (Figure 4C). In NEG, KEGG enrichment analysis showed that significantly enriched pathways such as 2-Oxocarboxylic acid metabolism, biosynthesis of amino acids, and D-Amino acid metabolism (Figure 4D).
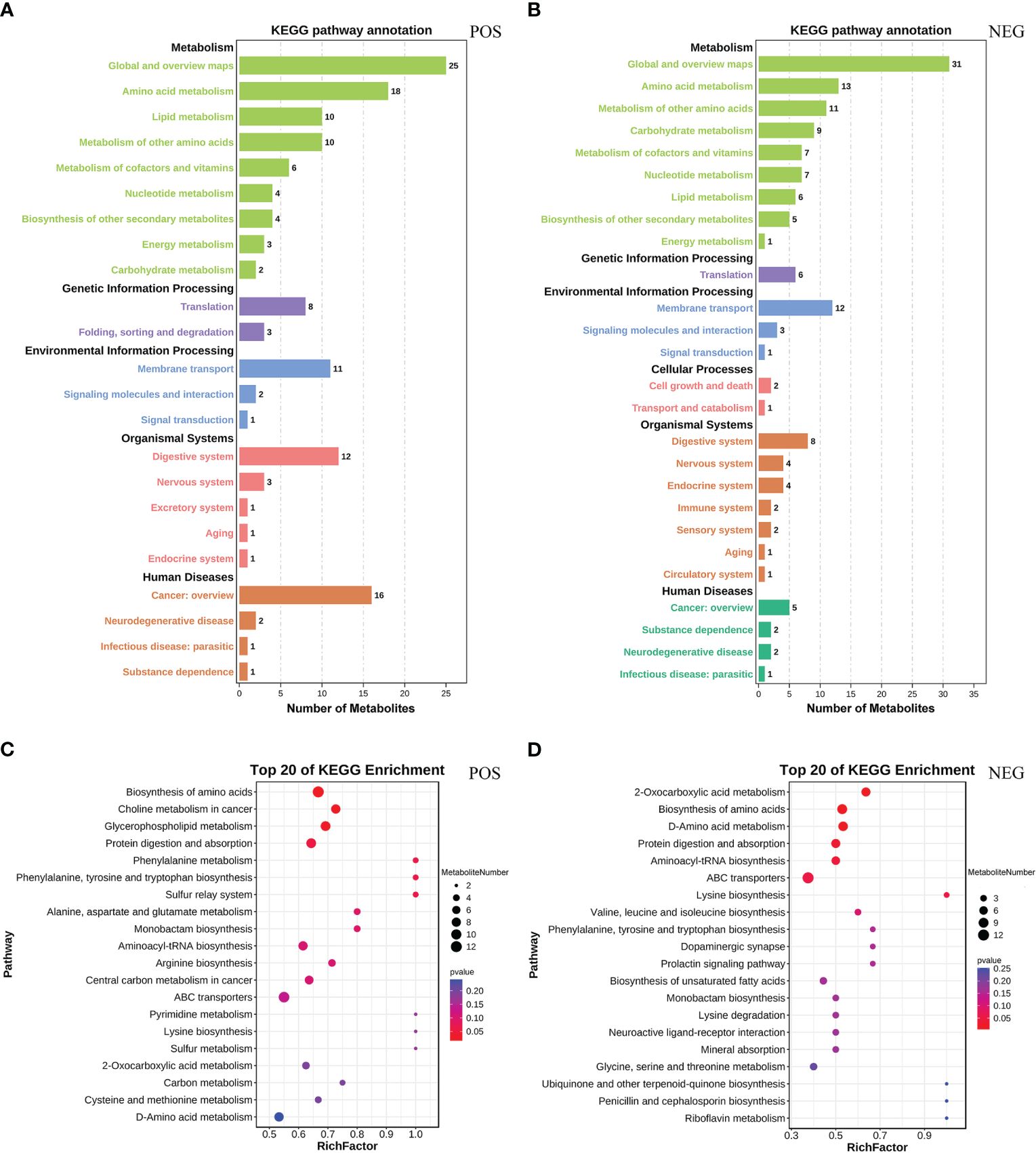
Figure 4 KEGG analysis of differential metabolites. (A, B) KEGG pathway annotation of differential metabolites. The abscissa is the number of differential metabolites. (C, D) KEGG enrichment scatterplot of differential metabolites (top 20). The redder the bubble color, the more significant enrichment. The larger the bubble, the more differential metabolites. (A, C) Positive ion mode. (B, D) Negative ion mode.
Differential metabolites were mapped to the KEGG database, and then the corresponding data was loaded into MetaboAnalyst for search and metabolic pathway analysis to investigate the potential impact of tilapia splenectomy on liver metabolic pathways. There were 6 and 4 main influential metabolic pathways in POS and NEG, respectively (Figures 5A, B). Considering the pathway impact score and p value, the related significant metabolic pathways were determined. In POS, arginine biosynthesis; arginine and proline metabolism; alanine, aspartate and glutamate metabolism; phenylalanine, tyrosine and tryptophan biosynthesis; phenylalanine metabolism; and taurine and hypotaurine metabolism were involved, with further specifics available in Supplementary Table 3. In NEG, phenylalanine, tyrosine and tryptophan biosynthesis; taurine and hypotaurine metabolism; phenylalanine metabolism; and glycine, serine and threonine metabolism were involved, with further specifics available in Supplementary Table 4. To see the changes in metabolic pathways more clearly and intuitively, a summary of the main influential metabolic pathways and mapped differential metabolites was made (Figure 6). The metabolism of aliphatic amino acids (such as arginine, proline and aspartic acid, etc.) and the metabolism of aromatic amino acids (phenylalanine and tyrosine) were significantly disturbed. In addition, the metabolism of sulfur-containing amino acids (taurine and hypotaurine) was also disturbed.
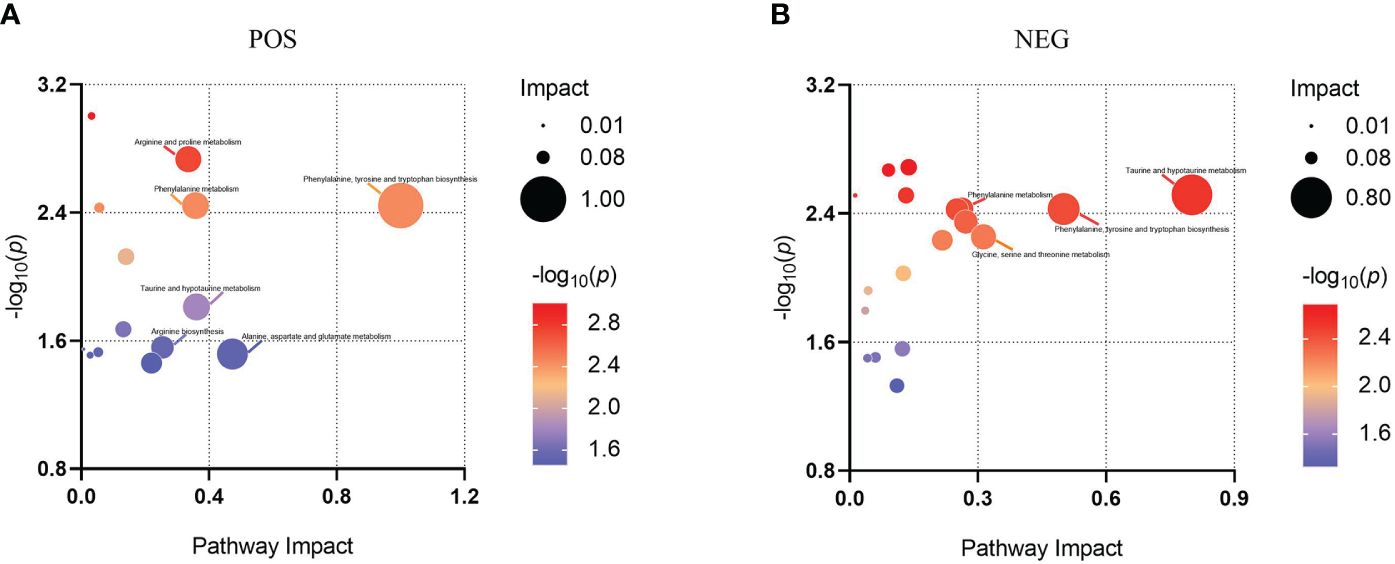
Figure 5 Bubble plot of pathway analysis for SP vs. SO. Each bubble represents a metabolic pathway. The larger the bubble size, the greater the impact score of the pathway in the topological analysis. The redder the bubble color, the more significant the enrichment degree. (A) Positive ion mode. (B) Negative ion mode.
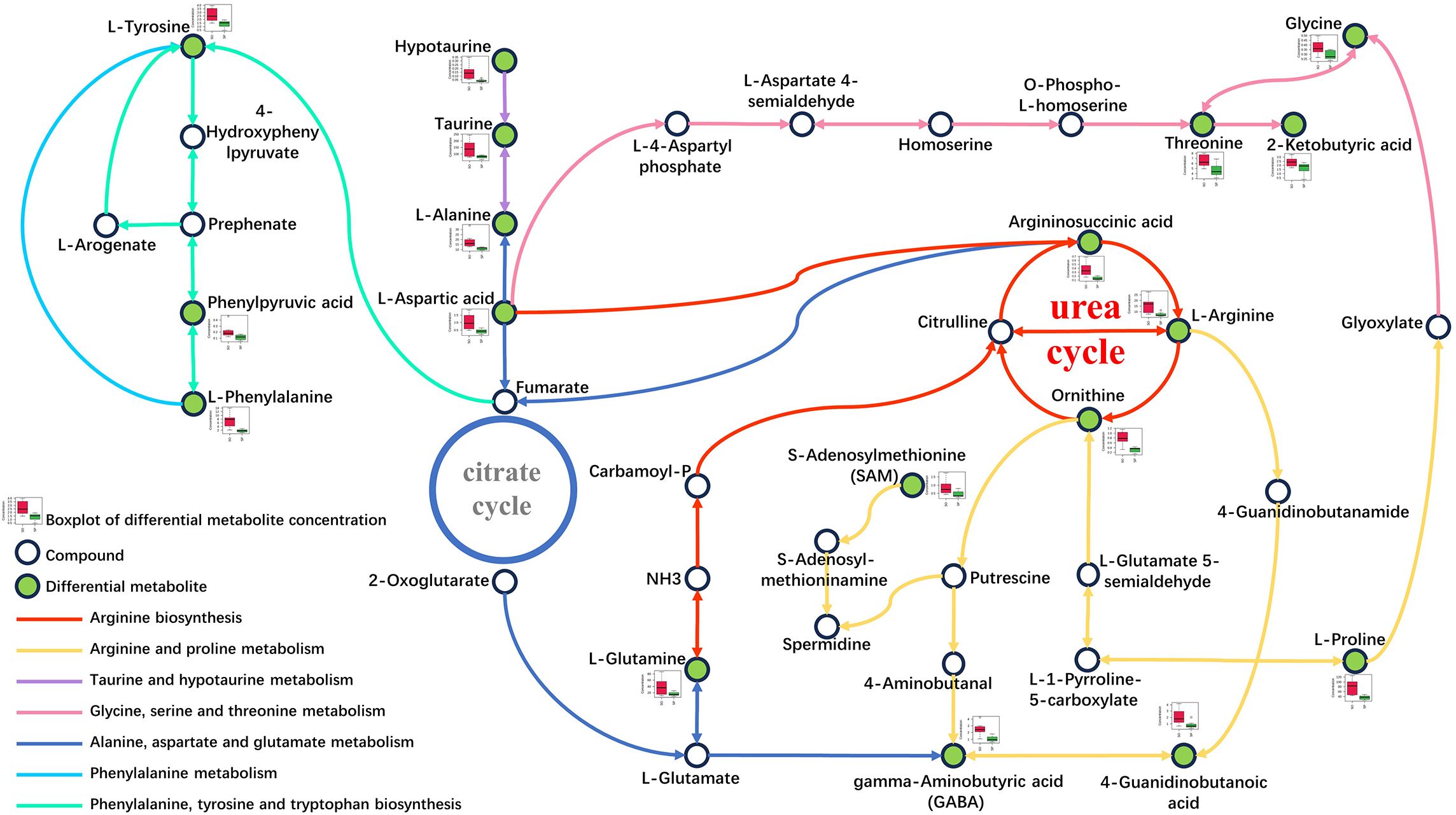
Figure 6 Summary of main influential metabolic pathways and differential metabolites. Different colored lines represent different metabolic pathways. The green dots represent differential metabolites with decreased concentration. The white dots represent metabolites with no change in concentration. In the boxplot, red represents the concentration of this metabolite in the SO group, and green represents the concentration of this metabolite in the SP group.
The changes in serum biochemical parameters can reflect the metabolic status and health status of fish (Xie et al., 2021; Luo et al., 2022). The main components of blood lipids in serum include T-CHO, TG, and lipoproteins, etc. LDL transports T-CHO from the liver to peripheral tissues, while HDL transports T-CHO from peripheral tissues to the liver (Tan et al., 2019). Elevated levels of T-CHO, TG, and LDL, and decreased levels of HDL usually indicate abnormal lipid metabolism (Ma et al., 2009; Wang et al., 2015). AST and ALT are key transaminases in fish, which are usually present in the liver, heart, and muscle (Jin et al., 2013). When liver cells are damaged or necrotic, AST and ALT will enter the blood, so the increase of the two enzymes in the blood is usually regarded as a sign of liver damage (Xu et al., 2010; Yang et al., 2011). The increase of ALT indicates that there is some damage to the liver (Younossi et al., 2018), while a significant increase in AST indicates that there is a risk of substantial damage to the liver (Yu and Keeffe, 2003). Furthermore, because ALT is found in comparatively small amounts in other tissues, it is more specific than AST (Hall and Cash, 2012). Bile acids are biomarkers of liver diseases (Jahnel et al., 2015). Studies have found that elevated serum TBA in patients with liver disease (Jiao et al., 2018; Gottlieb and Canbay, 2019). In this study, serum T-CHO, TG, LDL, ALT, and TBA were significantly increased in the SP group, liver T-CHO and LDL were also significantly increased in the SP group, indicating that after splenectomy, the liver metabolic function of tilapia was affected to some extent, and cholesterol deposition in the liver which may cause cholesterol catabolism to be blocked, and cholesterol could not be converted into bile acids (Chiang and Ferrell, 2018), resulting in a significant decrease in TBA in the liver of the SP group.
In systems biology, metabolomics is a relatively new discipline with high-throughput and high-sensitivity. In this study, to understand the impact of splenectomy on the liver metabolic function of Nile tilapia, we used metabolomics to study the metabolic characteristics of liver metabolites in the SO group and the SP group. The liver is the main place for amino acid metabolism and protein turnover (Trefts et al., 2017), and it is also the central place for lipid metabolism (Nguyen et al., 2008). We found that splenectomy had a great influence on the metabolic characteristics of the tilapia liver. The concentration of most differential metabolites in the SP group was lower than that in the SO group, confirming that the liver metabolic capacity of the SP group tilapia was damaged. Based on the HMDB annotation classification, organic acids and derivatives and lipids and lipid-like molecules were the two most abundant differential metabolite categories. Most of the metabolites in organic acids and derivatives were amino acids (Supplementary Tables 1 and 2). Through KEGG pathway annotation and enrichment analysis, we also found that most differential metabolites were annotated and enriched in amino acid metabolism and lipid metabolism pathway types. These results indicated that the amino acid metabolism and lipid metabolism in the liver of tilapia were greatly affected after splenectomy. The spleen and liver have a close circulatory connection (Barrea et al., 2018). A positive correlation between spleen iron levels and the severity of non-alcoholic steatohepatitis (NASH) expression was reported in mice experimental model study, further confirming the correlation between anatomy and function between the spleen and liver (Murotomi et al., 2016). Another study measuring the splenic metabolic function of NAFLD suggests that the so-called hepato-splenic axis goes beyond simple morphological relationships, where the metabolic activities of two tissues may be interconnected (Keramida et al., 2018). There are also some studies that clearly support the connection between the metabolic activities of the liver and spleen (Tarantino et al., 2021). Our results also confirmed the important role of the spleen in maintaining metabolism function of the tilapia liver.
KEGG analysis only identified the pathways involved in all differential metabolites. To identify pathways closely related to experimental conditions, further MetaboAnalyst analysis was performed on differential metabolites. Through MetaboAnalyst analysis, we found multiple amino acid metabolic pathways significantly affected by splenectomy, and the involved amino acid concentrations were significantly reduced (Figure 6). The spleen is involved in a wide range of metabolic control, including the metabolism of senescent red blood cells (Ai et al., 2018), which clears damaged or senescent red blood cells that are not suitable for continued circulation (Hadidi et al., 2008). Red blood cells are hydrolyzed in the phagosomes of spleen macrophages (Mebius and Kraal, 2005). Hemoglobin is first decomposed into heme and globin (Slusarczyk and Mleczko-Sanecka, 2021). The heme part is further decomposed in macrophages to release iron, which is stored in the spleen or liver, or used to synthesize new hemoglobin (Maines, 1997). The globin is further decomposed into amino acids, which can be reused in the body to synthesize new proteins (Slusarczyk and Mleczko-Sanecka, 2021). In this study, due to the absence of spleen, the damaged or senescent red blood cells in tilapia cannot be fully recovered and reused, which may lead to a decrease in endogenous amino acid concentration. On the other hand, although the spleen undertakes the main task of removing senescent red blood cells, the liver also undertakes this task, and in the case of stress, macrophages will temporarily accumulate in the liver to remove stressed red blood cells (Theurl et al., 2016; Nakayama et al., 2024). The absence of the spleen may promote the compensatory effect of the liver (Nakayama et al., 2024), increase the burden on the liver, and interfere with other metabolic functions of the liver, including amino acids metabolism and lipids metabolism.
Arginine biosynthesis; arginine and proline metabolism; and alanine, aspartate, and glutamate metabolism were closely related to the urea cycle (Figure 6). The urea cycle occurs within liver cells, which can convert toxic ammonia into non-toxic urea and excrete nitrogen-containing waste from the body (Ueno et al., 2022; Patel et al., 2024). The urea cycle is not only the main mechanism for ammonia removal, but also responsible for the production of endogenous amino acids (Lopes et al., 2023). Disruption of the urea cycle often accompanies liver diseases, such as hepatocyte dysfunction, liver failure, and hepatic steatosis (Lichter-Konecki, 2016; Ranucci et al., 2019). There is evidence to suggest that urea cycle activity is reduced in NAFLD and fatty liver during pregnancy (Weber et al., 1979; Rodríguez-Suárez et al., 2010). Aspartic acid, alanine, and arginine related to the urea cycle play a role in regulating lipid metabolism. In a high-fat diet induced obesity model, aspartic acid can inhibit hepatic steatosis in cholesterol fed rabbits (Yanni et al., 2010), alanine intake can reduce the expression of fat synthesis genes (Freudenberg et al., 2013), and arginine can reduce the degree of lipid peroxidation in liver-injured rats (Nanji et al., 2002). The decrease in the concentration of urea cycle-related metabolites in the SP group indicated a decrease in urea cycle activity, which also affected glycine, serine and threonine metabolism, and the concentrations of glycine and threonine decreased significantly. Studies have shown that the level of glycine in the liver was reduced in NAFLD (Mardinoglu et al., 2017), and the reduction of glycine and serine levels can be used for early detection of NAFLD (Tricò et al., 2021). Clinical studies have found a negative correlation between threonine levels and the risk of NAFLD (Li et al., 2024), while a lack of threonine can lead to metabolic diseases such as NAFLD (Viviani et al., 1966). It can be inferred that after splenectomy, the urea cycle activity of tilapia liver decreased, the ability to synthesize endogenous amino acids such as aspartic acid decreased, and low concentrations amino acids could not play their normal physiological functions, which may accelerate liver lipid synthesis and increase the risk of fatty liver in tilapia liver.
Phenylalanine metabolism and taurine and hypotaurine metabolism were also significantly disturbed. Phenylalanine is an essential amino acid mainly metabolized in the liver, and tyrosine is produced via the catabolization of phenylalanine (Zhou et al., 2022; Liu et al., 2023). Tyrosine is a semi-essential amino acid, which is converted into three catecholamines by tyrosine hydroxylase in the organism, including dopamine, epinephrine and norepinephrine (Liu et al., 2023). Catecholamines can mediate the mobilization of free fatty acids from triglycerides stored in adipose tissue (Young and Landsberg, 1977). Epinephrine and norepinephrine have the effect of accelerating lipolysis (Bahnsen et al., 1984). A study in mice has found that lack of tyrosine could induce hepatic steatosis (Sano et al., 2021). Clinical studies have shown that the concentration of tyrosine in patients with NAFLD also decreased (Kawanaka et al., 2015; Masoodi et al., 2021). The decrease of phenylalanine and tyrosine concentrations in the SP group may lead to a decrease in the concentration of its metabolites catecholamines, thereby weakening the catabolism of liver lipids, leading to liver lipid deposition and eventually developing into NAFLD.
In taurine and hypotaurine metabolism, hypotaurine, like taurine, can be obtained through endogenous synthesis and food. In the organism, hypotaurine can be converted into taurine. Taurine is a conditionally essential amino acid that is not involved in protein synthesis. It is crucial for numerous physiological processes within the organism, including osmotic pressure regulation, cell membrane stability, bile acid binding, and improved fat absorption (Belli et al., 1987; Oja and Saransaari, 2007; De Luca et al., 2015; Ping et al., 2019). The most important function of taurine in the liver is to participate in bile metabolism, thereby increasing the ability of organisms to digest and absorb various types of lipids (Petrosian and Haroutounian, 2000). Taurine can increase the degradation of cholesterol to reduce the content of cholesterol in the liver and serum (Mochizuki et al., 1999). In this study, the content of taurine and hypotaurine in the liver of the SP group decreased significantly. Combined with the significant decrease of bile acid content and the significant increase of cholesterol content in the liver, it can be confirmed that the lipid metabolism of tilapia liver was abnormal after splenectomy. Among them, catabolism of cholesterol was hindered by the weakening of taurine and bile binding, resulting in liver cholesterol deposition.
In this study, the spleen was removed by surgical method to establish a spleen-deficient tilapia model. The biochemical parameters of tilapia liver and serum were measured, and the liver metabolic characteristics were studied. We found abnormal liver and serum biochemical parameters, impaired liver metabolic function, and cholesterol deposition in Nile tilapia after splenectomy. Metabolomics analysis has found a significant decrease in the levels of metabolites in the liver of the SP group, which are mainly lipids and lipid-like molecules and organic acids and derivatives. KEGG annotation analysis showed that differential metabolites were significantly enriched in lipid metabolism and amino acid metabolism, suggesting that splenectomy affected lipid and amino acid metabolism in tilapia liver. MetaboAnalyst was used for metabolic pathway analysis, and it was found that in the liver of the SP group, arginine biosynthesis; arginine and proline metabolism; phenylalanine, tyrosine and tryptophan biosynthesis; alanine, aspartate and glutamate metabolism; phenylalanine metabolism; glycine, serine and threonine metabolism; and taurine and hypotaurine metabolism were significantly affected. By analyzing the significantly down-regulated amino acids in the above metabolic pathways and combining the results of previous biochemical parameters and KEGG analysis, it is speculated that after splenectomy, the ability of tilapia to recover and utilize hemoglobin from senescent red blood cells is reduced. On the one hand, it leads to a decrease in the concentration of endogenous amino acids. On the other hand, it increases the burden of the liver to remove senescent red blood cells, resulting in abnormal amino acid metabolism in the liver, which leads to abnormal lipid metabolism. The low-activity urea cycle may accelerate lipid synthesis, and low concentrations of phenylalanine, tyrosine and taurine cannot promote lipid catabolism, which may be the reason for the final deposition of cholesterol in the liver, posing a risk of liver diseases such as hepatic steatosis and NAFLD in tilapia. Our results suggest that tilapia spleen is involved in regulating liver metabolism and may interfere with lipid metabolism by regulating liver amino acid metabolism, which may provide new insights for healthy fish farming. Regular monitoring of liver enzymes, lipids, and other biochemical parameters in fish during daily aquaculture can detect metabolic problems early on, and timely intervention can be achieved by adding amino acids or other diets that protect the liver and spleen. Due to the limitations of this study, the specific molecular mechanisms by which the spleen is involved in regulating liver amino acid metabolism and lipid metabolism require further in-depth investigation. Additionally, the hypothesis that the spleen influences lipid metabolism by regulating amino acid metabolism also needs further experimental validation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author/s.
The animal study was approved by Institutional Animal Care and Use Committee (IACUC) of Southwest University. The study was conducted in accordance with the local legislation and institutional requirements.
YT: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. CD: Writing – review & editing, Data curation, Formal analysis. SJ: Data curation, Formal analysis, Writing – review & editing. SZ: Data curation, Writing – review & editing. JF: Investigation, Writing – review & editing. XM: Investigation, Writing – review & editing. HX: Methodology, Writing – review & editing. YL: Conceptualization, Funding acquisition, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Key Research Project of Chongqing Fishery Technology Innovation Union (CQFTIU2024-08) and Yangtze River Upper Reaches Germplasm Creation and Utilization Engineering Research Center Project by Chongqing Development and Reform Commission (2010423001).
Our acknowledgement goes to all the founders of this study. We would like to express our special thanks to Prof. Deshou Wang and Prof. Minghui Li from the School of Life Sciences, Southwest University, for providing Nile tilapia broodstock for this experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1419606/full#supplementary-material
Ai X.-M., Ho L.-C., Han L.-L., Lu J.-J., Yue X., Yang N.-Y. (2018). The role of splenectomy in lipid metabolism and atherosclerosis (AS). Lipids Health Dis. 17, 186. doi: 10.1186/s12944-018-0841-2
Arakawa Y., Shimada M., Uchiyama H., Ikegami T., Yoshizumi T., Imura S., et al. (2009). Beneficial effects of splenectomy on massive hepatectomy model in rats. Hepatol. Res. 39, 391–397. doi: 10.1111/j.1872-034X.2008.00469.x
Azim M. E., Little D. C. (2008). The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 283, 29–35. doi: 10.1016/j.aquaculture.2008.06.036
Bahnsen M., Burrin J. M., Johnston D. G., Pernet A., Walker M., Alberti K. G. (1984). Mechanisms of catecholamine effects on ketogenesis. Am. J. Physiol. Endocrinol. Metab. 247, E173–E180. doi: 10.1152/ajpendo.1984.247.2.E173
Barrea L., Di Somma C., Muscogiuri G., Tarantino G., Tenore G. C., Orio F., et al. (2018). Nutrition, inflammation and liver-spleen axis. Crit. Rev. Food Sci. Nutr. 58, 3141–3158. doi: 10.1080/10408398.2017.1353479
Belli D. C., Levy E., Darling P., Leroy C., Lepage G., Giguère R., et al. (1987). Taurine improves the absorption of a fat meal in patients with cystic fibrosis. Pediatrics 80, 517–523. doi: 10.1542/peds.80.4.517
Brown C. L., Yang T. B., Fitzsimmons K., Bolivar R. B. (2014). The value of pig manure as a source of nutrients for semi-intensive culture of Nile tilapia in ponds (A Review). Agric. Sci. 5, 1182–1193. doi: 10.4236/as.2014.512128
Cao X.-F., Liu W.-B., Zheng X.-C., Yuan X.-Y., Wang C.-C., Jiang G.-Z. (2019). Effects of high-fat diets on growth performance, endoplasmic reticulum stress and mitochondrial damage in blunt snout bream Megalobrama amblycephala. Aquac. Nutr. 25, 97–109. doi: 10.1111/anu.2019.25.issue-1
Chen M., Li L.-P., Wang R., Liang W.-W., Huang Y., Li J., et al. (2012a). PCR detection and PFGE genotype analyses of streptococcal clinical isolates from tilapia in China. Vet. Microbiol. 159, 526–530. doi: 10.1016/j.vetmic.2012.04.035
Chen Y.-J., Liu Y.-J., Yang H.-J., Yuan Y., Liu F.-J., Tian L.-X., et al. (2012b). Effect of dietary oxidized fish oil on growth performance, body composition, antioxidant defence mechanism and liver histology of juvenile largemouth bass Micropterus salmoides. Aquac. Nutr. 18, 321–331. doi: 10.1111/anu.2012.18.issue-3
Chiang J. Y. L., Ferrell J. M. (2018). Bile acid metabolism in liver pathobiology. Gene Expr. 18, 71–87. doi: 10.3727/105221618X15156018385515
Crivelaro R. M., Thiesen R., Aldrovani M., Silva P. E. S., Sobrinho A. A. F. B., Moraes P. C. (2019). Behavioural and physiological effects of methadone in the perioperative period on the Nile tilapia Oreochromis niloticus. J. Fish Biol. 94, 823–827. doi: 10.1111/jfb.13959
De Luca A., Pierno S., Camerino D. C. (2015). Taurine: the appeal of a safe amino acid for skeletal muscle disorders. J. Transl. Med. 13, 243. doi: 10.1186/s12967-015-0610-1
Demuner B. L., Pinho G. Z., Thomaz J. C., Stegmiller N. P., Mendes R. M., de A., et al. (2015). Effect of total splenectomy in the lipid profile in mice. Acta Cir. Bras. 30, 306–312. doi: 10.1590/S0102-865020150050000001
Du Z.-Y., Liu Y.-J., Tian L.-X., He J.-G., Cao J.-M., Liang G.-Y. (2006). The influence of feeding rate on growth, feed efficiency and body composition of juvenile grass carp (Ctenopharyngodon idella). Aquac. Int. 14, 247–257. doi: 10.1007/s10499-005-9029-7
Fang J., Feng L., Lu H., Zhu J. (2022). Metabolomics reveals spoilage characteristics and interaction of Pseudomonas lundensis and Brochothrix thermosphacta in refrigerated beef. Food Res. Int. 156, 111139. doi: 10.1016/j.foodres.2022.111139
Fiehn O. (2002). Metabolomics – the link between genotypes and phenotypes. Plant Mol. Biol. 48, 155–171. doi: 10.1023/A:1013713905833
Freudenberg A., Petzke K. J., Klaus S. (2013). Dietary l-leucine and l-alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids 44, 519–528. doi: 10.1007/s00726-012-1363-2
German J. B., Hammock B. D., Watkins S. M. (2005). Metabolomics: building on a century of biochemistry to guide human health. Metabolomics 1, 3–9. doi: 10.1007/s11306-005-1102-8
Gibelin H., Eugene M., Hebrard W., Henry C., Carretier M., Hauet T. (2000). A new approach to the evaluation of liver graft function by nuclear magnetic resonance spectroscopy. A comparative study between Euro-Collins and University of Wisconsin solutions. Clin. Chem. Lab. Med. 38, 1133–1136. doi: 10.1515/CCLM.2000.171
Gilany K., Moazeni-Pourasil R. S., Jafarzadeh N., Savadi-Shiraz E. (2014). Metabolomics fingerprinting of the human seminal plasma of asthenozoospermic patients. Mol. Reprod. Dev. 81, 84–86. doi: 10.1002/mrd.22284
Gottlieb A., Canbay A. (2019). Why bile acids are so important in non-alcoholic fatty liver disease (NAFLD) progression. Cells 8, 1358. doi: 10.3390/cells8111358
Hadidi S., Glenney G. W., Welch T. J., Silverstein J. T., Wiens G. D. (2008). Spleen size predicts resistance of rainbow trout to Flavobacterium psychrophilum challenge. J. Immunol. 180, 4156–4165. doi: 10.4049/jimmunol.180.6.4156
Hall P., Cash J. (2012). What is the real function of the liver ‘function’ tests? Ulster Med. J. 81, 30–36. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609680/.
Han G., Cui Y., Shen D., Li M., Ren Y., Bungo T., et al. (2022). In ovo feeding of l-leucine Improves antioxidative capacity and spleen weight and changes amino acid concentrations in broilers after chronic thermal stress. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.862572
He Z., Zhang H., Song Y., Yang Z., Cai Z. (2022). Exposure to ambient fine particulate matter impedes the function of spleen in the mouse metabolism of high-fat diet. J. Hazard. Mater. 423, 127129. doi: 10.1016/j.jhazmat.2021.127129
Hoekstra M., Out R., Kruijt J. K., Van Eck M., Van Berkel T. J. C. (2005). Diet induced regulation of genes involved in cholesterol metabolism in rat liver parenchymal and Kupffer cells. J. Hepatol. 42, 400–407. doi: 10.1016/j.jhep.2004.11.032
Iwakiri Y. (2014). Pathophysiology of portal hypertension. Clin. Liver Dis. 18, 281–291. doi: 10.1016/j.cld.2013.12.001
Jahnel J., Zöhrer E., Alisi A., Ferrari F., Ceccarelli S., De Vito R., et al. (2015). Serum bile acid levels in children with nonalcoholic fatty liver disease. J. Pediatr. Gastroenterol. Nutr. 61, 85–90. doi: 10.1097/MPG.0000000000000774
Jiao N., Baker S. S., Chapa-Rodriguez A., Liu W., Nugent C. A., Tsompana M., et al. (2018). Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 67, 1881–1891. doi: 10.1136/gutjnl-2017-314307
Jin Y., Tian L., Zeng S., Xie S., Yang H., Liang G., et al. (2013). Dietary lipid requirement on non-specific immune responses in juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 34, 1202–1208. doi: 10.1016/j.fsi.2013.01.008
Joshi R., Arnyasi M., Lien S., Gjoen H. M., Alvarez A. T., Kent M. (2018). Development and validation of 58K SNP-array and high-density linkage map in Nile tilapia (O. niloticus). Front. Genet. 9. doi: 10.3389/fgene.2018.00472
Katagiri T., Kidd C., Tomasino E., Davis J. T., Wishon C., Stern J. E., et al. (2005). A BAC-based physical map of the Nile tilapia genome. BMC Genomics 6, 89. doi: 10.1186/1471-2164-6-89
Kawanaka M., Nishino K., Oka T., Urata N., Nakamura J., Suehiro M., et al. (2015). Tyrosine levels are associated with insulin resistance in patients with nonalcoholic fatty liver disease. Hepatic Med. Evid. Res. 7, 29–35. doi: 10.2147/HMER.S79100
Keramida G., Dunford A., Kaya G., Anagnostopoulos C. D., Peters A. M. (2018). Hepato-splenic axis: hepatic and splenic metabolic activities are linked. Am. J. Nucl. Med. Mol. Imaging 8, 228–238. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6056241/.
Kim S., Kim J., Yun E. J., Kim K. H. (2016). Food metabolomics: from farm to human. Curr. Opin. Biotechnol. 37, 16–23. doi: 10.1016/j.copbio.2015.09.004
Li L., Wei W., Li Z., Chen H., Li Y., Jiang W., et al. (2018). The spleen promotes the secretion of CCL2 and supports an M1 dominant phenotype in hepatic macrophages during liver fibrosis. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 51, 557–574. doi: 10.1159/000495276
Li X., Ma W., Yang T., Wang C., Zhang W., Li H., et al. (2024). Higher intakes of lysine, threonine and valine are inversely associated with non-alcoholic fatty liver disease risk: A community-based case-control study in the Chinese elderly. Food Sci. Hum. Wellness 13, 191–197. doi: 10.26599/FSHW.2022.9250016
Liang Z., Fu Q., Li H., Xu X., Ding P., Tang W., et al. (2023). Metabolite comparison between spleen-deficiency and healthy children. Evid. Based Complement. Alternat. Med. 2023, e5937308. doi: 10.1155/2023/5937308
Lichter-Konecki U. (2016). Defects of the urea cycle. Transl. Sci. Rare Dis. 1, 23–43. doi: 10.3233/TRD-160002
Lin S., Luo L. (2011). Effects of different levels of soybean meal inclusion in replacement for fish meal on growth, digestive enzymes and transaminase activities in practical diets for juvenile tilapia, Oreochromis niloticus×O. aureus. Anim. Feed Sci. Technol. 168, 80–87. doi: 10.1016/j.anifeedsci.2011.03.012
Liu P., Wu J., Yu X., Guo L., Zhao L., Ban T., et al. (2023). Metabolomics and network analyses reveal phenylalanine and tyrosine as signatures of anthracycline-induced hepatotoxicity. Pharmaceuticals 16, 797. doi: 10.3390/ph16060797
Lopes F. F., Lamberty Faverzani J., Hammerschmidt T., Aguilar Delgado C., Ferreira de Oliveira J., Wajner M., et al. (2023). Evaluation of oxidative damage to biomolecules and inflammation in patients with urea cycle disorders. Arch. Biochem. Biophys. 736, 109526. doi: 10.1016/j.abb.2023.109526
Luo J., Amenyogbe E., Huang J., Chen G. (2022). Hepatic metabolomics analysis of hybrid grouper (Epinephelus fuscoguttatus♀×Epinephelus polyphekadion♂) fed with quercetin and sodium quercetin-5’-sulfonates. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.891080
Ma M., Liu G., Yu Z., Chen G., Zhang X. (2009). Effect of the Lycium barbarum polysaccharides administration on blood lipid metabolism and oxidative stress of mice fed high-fat diet in vivo. Food Chem. 113, 872–877. doi: 10.1016/j.foodchem.2008.03.064
Maines M. D. (1997). The heme oxygenase system: a regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 37, 517–554. doi: 10.1146/annurev.pharmtox.37.1.517
Mandavi V., Farimani M. M., Fathi F., Ghassempour A. (2015). A targeted metabolomics approach toward understanding metabolic variations in rice under pesticide stress. Anal. Biochem. 478, 65–72. doi: 10.1016/j.ab.2015.02.021
Mardinoglu A., Bjornson E., Zhang C., Klevstig M., Söderlund S., Ståhlman M., et al. (2017). Personal model-assisted identification of NAD+ and glutathione metabolism as intervention target in NAFLD. Mol. Syst. Biol. 13, 916. doi: 10.15252/msb.20167422
Masoodi M., Gastaldelli A., Hyötyläinen T., Arretxe E., Alonso C., Gaggini M., et al. (2021). Metabolomics and lipidomics in NAFLD: biomarkers and non-invasive diagnostic tests. Nat. Rev. Gastroenterol. Hepatol. 18, 835–856. doi: 10.1038/s41575-021-00502-9
Mebius R. E., Kraal G. (2005). Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616. doi: 10.1038/nri1669
Mengistu S. B., Mulder H. A., Benzie J. A. H., Komen H. (2020). A systematic literature review of the major factors causing yield gap by affecting growth, feed conversion ratio and survival in Nile tilapia (Oreochromis niloticus). Rev. Aquac. 12, 524–541. doi: 10.1111/raq.12331
Mochizuki H., Takido J., Yokogoshi H. (1999). Improved suppression by dietary taurine of the fecal excretion of bile acids from hypothyroid rats. Biosci. Biotechnol. Biochem. 63, 753–755. doi: 10.1271/bbb.63.753
Monteiro J. P., Oliveira P. J., Jurado A. S. (2013). Mitochondrial membrane lipid remodeling in pathophysiology: A new target for diet and therapeutic interventions. Prog. Lipid Res. 52, 513–528. doi: 10.1016/j.plipres.2013.06.002
Moore R. E., Kirwan J., Doherty M. K., Whitfield P. D. (2007). Biomarker discovery in animal health and disease: the application of post-genomic technologies. biomark. Insights 2, 185–196. doi: 10.1177/117727190700200040
Murata K., Ito K., Yoneda K., Shiraki K., Sakurai H., Ito M. (2008). Splenectomy improves liver function in patients with liver cirrhosis. Hepatogastroenterology 55, 1407–1411. Available at: https://pubmed.ncbi.nlm.nih.gov/18795700/.
Murotomi K., Arai S., Uchida S., Endo S., Mitsuzumi H., Tabei Y., et al. (2016). Involvement of splenic iron accumulation in the development of nonalcoholic steatohepatitis in Tsumura Suzuki Obese Diabetes mice. Sci. Rep. 6, 22476. doi: 10.1038/srep22476
Nakayama Y., Masuda Y., Mukae T., Mikami T., Shimizu R., Kondo N., et al. (2024). A secretory protein neudesin regulates splenic red pulp macrophages in erythrophagocytosis and iron recycling. Commun. Biol. 7, 1–11. doi: 10.1038/s42003-024-05802-9
Nanji A. A., Jokelainen K., Lau G. K. K., Rahemtulla A., Tipoe G. L., Polavarapu R., et al. (2002). Arginine reverses ethanol-induced inflammatory and fibrotic changes in liver despite continued ethanol administration. J. Pharmacol. Exp. Ther. 299, 832–839. Available at: https://jpet.aspetjournals.org/content/299/3/832.long.
Nguyen P., Leray V., Diez M., Serisier S., Bloc’h J. L., Siliart B., et al. (2008). Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. 92, 272–283. doi: 10.1111/j.1439-0396.2007.00752.x
Nguyen T. V., Alfaro A. C., Young T., Ravi S., Merien F. (2018). Metabolomics study of immune responses of New Zealand greenshell™ mussels (Perna canaliculus) infected with pathogenic Vibrio sp. Mar. Biotechnol. 20, 396–409. doi: 10.1007/s10126-018-9804-x
Nicholson J. K., Connelly J., Lindon J. C., Holmes E. (2002). Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discovery 1, 153–161. doi: 10.1038/nrd728
Nicholson J. K., Lindon J. C., Holmes E. (2008). Metabonomics”: understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 29, 1181–1189. doi: 10.1080/004982599238047
Oishi T., Terai S., Iwamoto T., Takami T., Yamamoto N., Sakaida I. (2011). Splenectomy reduces fibrosis and preneoplastic lesions with increased triglycerides and essential fatty acids in rat liver cirrhosis induced by a choline-deficient L-amino acid-defined diet. Hepatol. Res. 41, 463–474. doi: 10.1111/j.1872-034X.2011.00784.x
Oja S. S., Saransaari P. (2007). “8 taurine,” in Handbook of neurochemistry and molecular neurobiology: amino acids and peptides in the nervous system. Eds. Lajtha A., Oja S. S., Schousboe A., Saransaari P. (Springer US, New York, NY), 155–206. doi: 10.1007/978-0-387-30373-4_8
Patel M., Jaiswal A., Naseer A., Tripathi A., Joshi A., Minocha T., et al. (2024). Amyloidogenic propensity of metabolites in the uric acid pathway and urea cycle critically impacts the etiology of metabolic disorders. ACS Chem. Neurosci. 15, 916–931. doi: 10.1021/acschemneuro.3c00563
Petrosian A. M., Haroutounian J. E. (2000). Taurine as a universal carrier of lipid soluble vitamins: a hypothesis. Amino Acids 19, 409–421. doi: 10.1007/s007260070020
Ping F., Guo Y., Cao Y., Shang J., Yao S., Zhang J., et al. (2019). Metabolomics analysis of the renal cortex in rats with acute kidney injury induced by sepsis. Front. Mol. Biosci. 6. doi: 10.3389/fmolb.2019.00152
Ranucci G., Rigoldi M., Cotugno G., Bernabei S. M., Liguori A., Gasperini S., et al. (2019). Chronic liver involvement in urea cycle disorders. J. Inherit. Metab. Dis. 42, 1118–1127. doi: 10.1002/jimd.12144
Robertson D. G., Reily M. D., Sigler R. E., Wells D. F., Paterson D. A., Braden T. K. (2000). Metabonomics: Evaluation of nuclear magnetic resonance (NMR) and pattern recognition technology for rapid in vivo screening of liver and kidney toxicants. Toxicol. Sci. 57, 326–337. doi: 10.1093/toxsci/57.2.326
Rodríguez-Suárez E., Duce A. M., Caballería J., Arrieta F. M., Fernández E., Gómara C., et al. (2010). Non-alcoholic fatty liver disease proteomics. Proteomics Clin. Appl. 4, 362–371. doi: 10.1002/prca.200900119
Sano A., Kakazu E., Hamada S., Inoue J., Ninomiya M., Iwata T., et al. (2021). Steatotic hepatocytes release mature VLDL through methionine and tyrosine metabolism in a Keap1-Nrf2–dependent manner. Hepatology 74, 1271. doi: 10.1002/hep.31808
Sharma B., John S. (2024).Hepatic cirrhosis. In: StatPearls (Treasure Island (FL: StatPearls Publishing). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK482419/ (Accessed April 12, 2024).
Slusarczyk P., Mleczko-Sanecka K. (2021). The multiple facets of iron recycling. Genes 12, 1364. doi: 10.3390/genes12091364
Tan X., Sun Z., Ye C. (2019). Dietary Lycium barbarum extract administration improved growth, meat quality and lipid metabolism in hybrid grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀) fed high lipid diets. Aquaculture 504, 190–198. doi: 10.1016/j.aquaculture.2019.01.044
Tarantino G., Citro V., Balsano C. (2021). Liver-spleen axis in nonalcoholic fatty liver disease. Expert Rev. Gastroenterol. Hepatol. 15, 759–769. doi: 10.1080/17474124.2021.1914587
Taylor J., King R. D., Altmann T., Fiehn O. (2002). Application of metabolomics to plant genotype discrimination using statistics and machine learning. Bioinformatics 18, S241–S248. doi: 10.1093/bioinformatics/18.suppl_2.S241
Theurl I., Hilgendorf I., Nairz M., Tymoszuk P., Haschka D., Asshoff M., et al. (2016). On-demand erythrocyte disposal and iron recycling requires transient macrophages in the liver. Nat. Med. 22, 945–951. doi: 10.1038/nm.4146
Trefts E., Gannon M., Wasserman D. H. (2017). The liver. Curr. Biol. 27, R1147–R1151. doi: 10.1016/j.cub.2017.09.019
Tricò D., Biancalana E., Solini A. (2021). Protein and amino acids in nonalcoholic fatty liver disease. Curr. Opin. Clin. Nutr. Metab. Care 24, 96. doi: 10.1097/MCO.0000000000000706
Ueno Y., Maeda T., Okamoto S., Taniguchi H. (2022). “Evaluation of urea cycle activity by metabolic flux analysis using mass spectrometry,” in Hepatocytes: Methods and Protocols. Ed. Tanimizu N. (Springer US, New York, NY), 129–144. doi: 10.1007/978-1-0716-2557-6_9
Valença-Silva G., Braz M. G., Barreto R. E., Salvadori D. M. F., Volpato G. L. (2014). Low dose of the anesthetic propofol does not induce genotoxic or mutagenic effects in Nile tilapia. Trans. Am. Fish. Soc 143, 414–419. doi: 10.1080/00028487.2013.856814
Viviani R., Sechi A. M., Lenaz G. (1966). Lipid metabolism in fatty liver of lysine- and threonine-deficient rats. J. Lipid Res. 7, 473–478. doi: 10.1016/S0022-2275(20)39256-7
Wang X., Li Y., Hou C., Gao Y., Wang Y. (2015). Physiological and molecular changes in large yellow croaker (Pseudosciaena crocea R.) with high-fat diet-induced fatty liver disease. Aquac. Res. 46, 272–282. doi: 10.1111/are.2014.46.issue-2
Wang Q., Yan Y., Tao Y., Lu S., Xu P., Qiang J. (2023). Transcriptional knock−down of mstn encoding myostatin improves muscle quality of Nile tilapia (Oreochromis niloticus). Mar. Biotechnol. 25, 951–965. doi: 10.1007/s10126-023-10252-1
Weber F. L., Snodgrass P. J., Powell D. E., Rao P., Huffman S. L., Brady P. G. (1979). Abnormalities of hepatic mitochondrial urea-cycle enzyme activities and hepatic ultrastructure in acute fatty liver of pregnancy. J. Lab. Clin. Med. 94, 27–41. Available at: https://pubmed.ncbi.nlm.nih.gov/469376/.
Xie R., Amenyogbe E., Chen G., Huang J. (2021). Effects of feed fat level on growth performance, body composition and serum biochemical indices of hybrid grouper (Epinephelus fuscoguttatus × Epinephelus polyphekadion). Aquaculture 530, 735813. doi: 10.1016/j.aquaculture.2020.735813
Xu J.-Y., Su Y.-Y., Cheng J.-S., Li S.-X., Liu R., Li W.-X., et al. (2010). Protective effects of fullerenol on carbon tetrachloride-induced acute hepatotoxicity and nephrotoxicity in rats. Carbon 48, 1388–1396. doi: 10.1016/j.carbon.2009.12.029
Yang X., Dong C., Ren G. (2011). Effect of soyasaponins-rich extract from soybean on acute alcohol-induced hepatotoxicity in mice. J. Agric. Food Chem. 59, 1138–1144. doi: 10.1021/jf103749r
Yanni A. E., Agrogiannis G., Nomikos T., Fragopoulou E., Pantopoulou A., Antonopoulou S., et al. (2010). Oral supplementation with l-aspartate and l-glutamate inhibits atherogenesis and fatty liver disease in cholesterol-fed rabbit. Amino Acids 38, 1323–1331. doi: 10.1007/s00726-009-0340-x
Young J. B., Landsberg L. (1977). 4 - Catecholamines and intermediary metabolism. Clin. Endocrinol. Metab. 6, 599–631. doi: 10.1016/S0300-595X(77)80073-X
Younossi Z. M., Henry L., Bush H., Mishra A. (2018). Clinical and economic burden of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Clin. Liver Dis. 22, 1–10. doi: 10.1016/j.cld.2017.08.001
Yu A. S., Keeffe E. B. (2003). Elevated ast or alt to nonalcoholic fatty liver disease: accurate predictor of disease prevalence? Off. J. Am. Coll. Gastroenterol. 98, 955. doi: 10.1111/j.1572-0241.2003.07485.x
Zhang Z., Yang S., Lin X., Huang Y., Wei X., Zhou J., et al. (2021). Metabolomics of Spleen-Yang deficiency syndrome and the therapeutic effect of Fuzi Lizhong pill on regulating endogenous metabolism. J. Ethnopharmacol. 278, 114281. doi: 10.1016/j.jep.2021.114281
Zhou Q., Sun W.-W., Chen J.-C., Zhang H.-L., Liu J., Lin Y., et al. (2022). Phenylalanine impairs insulin signaling and inhibits glucose uptake through modification of IRβ. Nat. Commun. 13, 4291. doi: 10.1038/s41467-022-32000-0
Keywords: metabolomics, liver, spleen, lipids, amino acids
Citation: Tao Y, Du C, Jiang S, Zhang S, Feng J, Miao X, Xu H and Li Y (2024) Metabolomics analysis of splenectomy of Nile tilapia (Oreochromis niloticus) reveals the spleen involved in regulating liver lipids and amino acids metabolism. Front. Mar. Sci. 11:1419606. doi: 10.3389/fmars.2024.1419606
Received: 18 April 2024; Accepted: 27 June 2024;
Published: 10 July 2024.
Edited by:
Xiaodan Wang, East China Normal University, ChinaCopyright © 2024 Tao, Du, Jiang, Zhang, Feng, Miao, Xu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Li, YXF1YXRpY3NAc3d1LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.