- 1South Sea Fisheries Research Institute, National Institute of Fisheries Science, Yeosu, Republic of Korea
- 2Department of Marine Biology and Aquaculture, College of Marine Science, Gyeongsang National University, Tongyoung, Republic of Korea
- 3Ocean Climate and Ecology Research Division, National Institute of Fisheries Science, Busan, Republic of Korea
- 4Fisheries Engineering Research Division, National Institute of Fisheries Science, Busan, Republic of Korea
- 5Distant Water Fisheries Resources Division, National Institute of Fisheries Science, Busan, Republic of Korea
Background: The brown croaker, Miichthys miiuy, is an economically important fish species in Korean waters. Research on the spawning ecology of M. miiuy in Korea has primarily focused on specimens from the southwestern waters, assessing parameters, such as spawning season and mature body length through histological examination. However, a comprehensive histological analysis across various body sizes during the spawning season is imperative to accurately ascertain these factors, as well as for efficient resource management amidst potential environmental variations. Therefore, this study was undertaken to investigate the spatiotemporal distribution and reproductive biology of M. miiuy in the southwestern waters of Korea.
Methods: Monthly changes in gonadal maturity stages, gonadosomatic index (GSI), egg diameter, and fecundity were analyzed. Additionally, total length at sexual maturity at 50%, 75%, and 97.5%, as well as the sex ratio, were determined.
Results: M. miiuy, captured using offshore gillnets and stow nets, exhibited a seasonal migration pattern: a winter presence near Jeju Island, followed by a northward movement in spring, a summer shift to the shallow waters of Shinan, and an autumnal migration to the waters around Jeju Island for overwintering. The overall female-to-male sex ratio was 1:0.8. GSI values peaked in September and August for both sexes; however, microscopic analysis of gonadal tissue revealed a spawning period from July to September 2023, with the primary spawning season for the entire population occurring in August and September. The fork length at 50% maturity was 57.4 cm for females and 54.6 cm for males.
Conclusion: Overall, these results suggest an earlier shift in the spawning period of brown croakers in the southwestern waters of Korea compared to past observations. This shift is potentially attributable to higher water temperatures, increased resource levels, and alterations in the species composition of major prey items in the spawning grounds. Further research is imperative for a comprehensive understanding of the factors influencing the reproductive ecology of this important fish species.
1 Introduction
The brown croaker, Miichthys miiuy, is a semi–benthic fish belonging to the Perciformes and Sciaenidae families. Typically inhabiting muddy or sandy bottoms, it thrives at depths of 15–100 m within the temperate waters of the northwestern Pacific Ocean (Zhu et al., 1963; Kim et al., 2005; Zhong et al., 2005; Zhang et al., 2008). In Korea, the brown croaker represents a single population inhabiting the East China Sea (Xu et al., 2014), with its primary catchment area encompassing the western expanse of the South Sea, including the West Sea and Jeju Island. The migration pattern of M. miiuy unfolds as follows: commencing in May, the species migrates northward from the southwestern waters of Jindo to the vicinity of Deokjeokdo, Incheon, situated in the northern part of the West Sea, where spawning occurs from August to October (Kim et al., 1994). Subsequent to spawning, adult and juvenile brown croakers, having grown post-hatching, winter in the deep-sea regions of the southwestern sector of Jeju Island and the northern domain of the East China Sea. This period, spanning from December to February, is characterized by water temperatures above 12 °C. In the spring, these croakers migrate northward along the coast of the Yellow Sea (Kim et al., 1994; Seo, 2004).
Miichthys miiuy, an economically significant fish species highly valued, particularly in Korea and China, has been extensively studied (Chikuni, 1985; Kim et al., 2004; Peng et al., 2010, 2020). In China, the rapid depletion of multiple fishing grounds harboring brown croaker populations has led to a significant decline in catches, attributed to overfishing and habitat destruction (Lian et al., 2007; Peng et al., 2020). Conversely, in Korea, the average annual catch of M. miiuy during the 1990s was less than 2,000 tonnes, which surged to 2,327 tonnes in the 2000s. In recent years, fluctuation in catches at approximately 4,000 tonnes has been observed, reaching a peak of 6,324 tonnes in 2022 (KOSIS, 2024, Figure 1A). Annual variations in catches were analyzed by fishing method in the southwestern fishing grounds, where M. miiuy specimens were collected from 2006 to 2022. Until 2014, predominant catches were associated with Southwest Coast Danish Seine fishing gear. However, a notable shift occurred thereafter, with escalating catches observed with coastal gillnets and large trawls (KOSIS, 2024, Figure 1B). Despite these changes, the only existing management measure for M. miiuy resource conservation remains a minimum length limit of 33 cm, which falls short of the length of a mature body size. Consequently, more robust resource management strategies, such as the implementation of a closed season, have yet to be established.
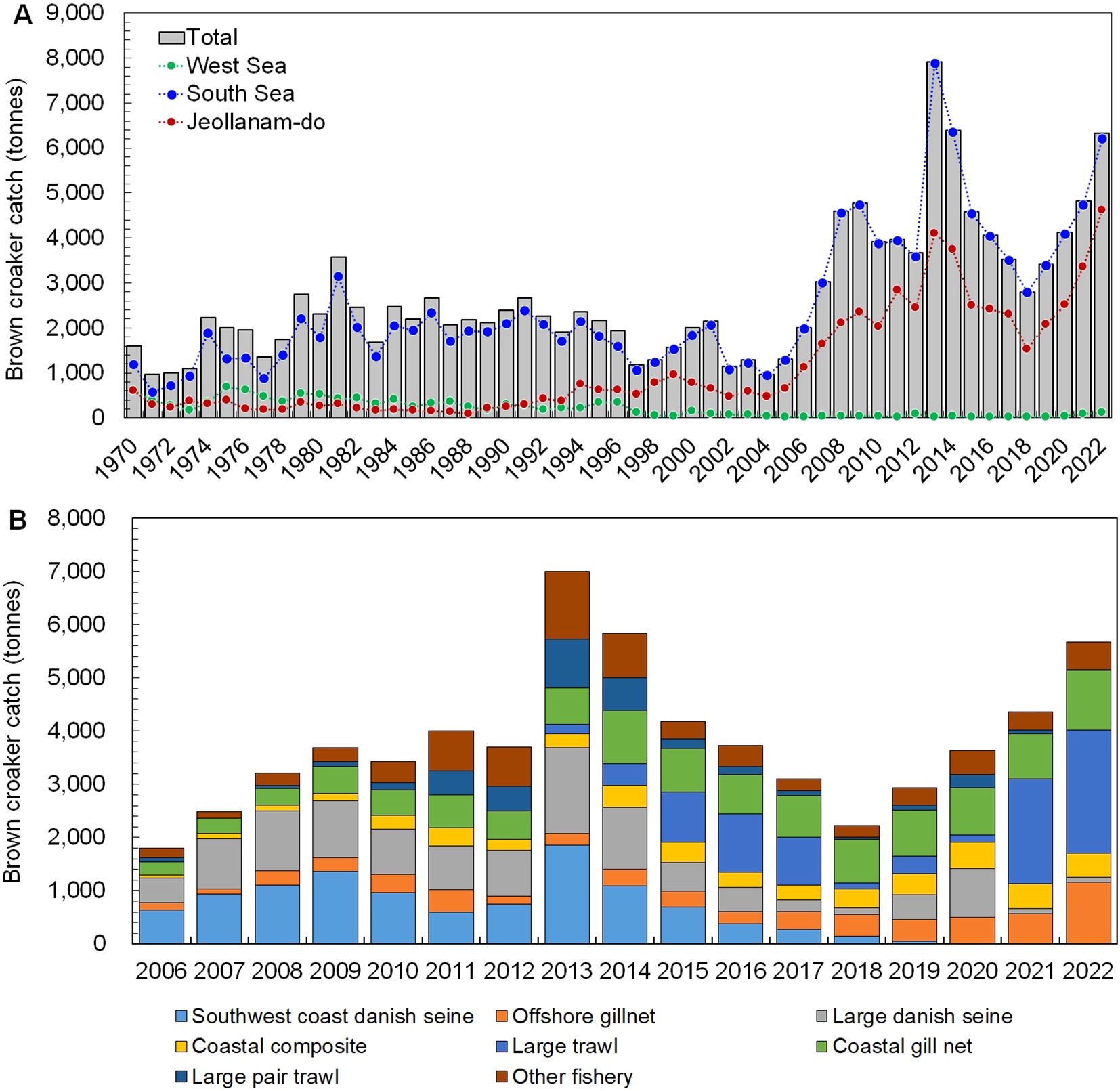
Figure 1. Annual changes on catch of the brown croaker Miichthys miiuy in the southwestern waters of Korea. (A), catch by region; (B), catch by fishing type.
Recognized as an important fishery resource in the northwestern Pacific region, including Korea, the brown croaker has been the subject of various biological studies. These studies have explored aspects such as maturation and spawning (Zhao et al., 1990; Yamada and Yamada, 1999; Lee et al., 2017a; Peng et al., 2020), reproductive ecology and early growth (Seo, 2004; Zhen et al., 2007; Zheng et al., 2008), age and growth (Lee et al., 2017b; Peng et al., 2020), feeding habits (Zhang et al., 2005; Jeong et al., 2019; Peng et al., 2020), and genetic population structure (Cheng et al., 2011; Xu et al., 2014; Gwak and Roy, 2023). The spawning season of M. miiuy varies depending on geographical location. In the coastal waters of Zhejiang and Jiangsu provinces in China, spawning occurs from August to September (Peng et al., 2020), whereas in the Yangtze River Estuary, it occurs from August to October (Zhao et al., 1990; Yamada and Yamada, 1999). In Korea, research on the spawning ecology of M. miiuy has been focused on specimens collected from the southwestern waters of Korea. The spawning season and mature body length have been analyzed through visual and histological examination of reproductive organs (Lee et al., 2017a). However, to accurately determine these parameters, it is imperative to histologically analyze the reproductive organs of brown croakers of various body sizes during the spawning season. Furthermore, for efficient resource management, re-examining the spawning season and mature body length of brown croaker is deemed necessary. This is particularly important as these factors may have undergone changes owing to variations in water temperature and resource levels within brown croaker habitats.
In this study, we aimed to understand the spawning characteristics of M. miiuy by analyzing monthly length distributions, gonadosomatic index (GSI), developmental stage, and maturation rates of individuals caught monthly in the southwestern waters of Korea. Additionally, we sought to ascertain the spatiotemporal distribution characteristics of M. miiuy based on surface water temperature (SST). Furthermore, we aimed to secure biological baseline information for the rational management of M. miiuy resources through comparison with past results on maturation and spawning characteristics.
2 Materials and methods
2.1 Sea surface temperature and spatiotemporal distribution of M. miiuy
Monthly global Operational SST and Sea Ice Analysis (OSTIA) data from January to December 2023 were obtained from the European Copernicus program (http://marine.copernius.eu, accessed on 4 March 2024). The spatial resolution of the OSTIA global SST data is 5 km. To understand the relationship between SST and spatiotemporal distribution characteristics of M. miiuy in the southwestern waters of Korea, monthly catch data from 2023, organized into 0.5°×0.5° grid cells, were analyzed alongside SST data. These data were derived from coastal gillnet and coastal purse-seine fisheries, obtained from a fishery sampling survey conducted by the National Institute of Fisheries Science in Korea. To understand the relation between the SST and spawning season of brown croakers throughout the study period, we used daily water temperature data from the Chuja Island Tide Observation Station, the primary collection site of the samples used in this study, sourced from the National Marine Research Institute’s Badanuri Marine Information Service (Ocean Data in Grid Framework) (KHOA, 2024).
2.2 Fish measurement
The spawning ecology of M. miiuy in the southwestern waters of Korea from January to December 2023 was analyzed. The M. miiuy specimens used were obtained from those caught in the southwestern waters (Jindo Island, Geomundo Island, and the shallow waters of Shinan) using shrimp beam trawls, offshore and coastal gillnets, and offshore stow nets (Figure 2). In total, 671 specimens were collected during the survey period, comprising 377 females and 294 males. Upon transportation to the laboratory, their total length (TL) was measured to 0.1 cm to determine monthly length frequency. Additionally, their body weight (BW) was measured to 0.1 g. Gonads were separated by individuals and weighed to 0.01 g before fixation in 10% neutral formalin. The sex ratio was expressed monthly as the ratio of females to males for specimens collected during the survey period. Sex ratio analysis was performed using the chi-squared test.
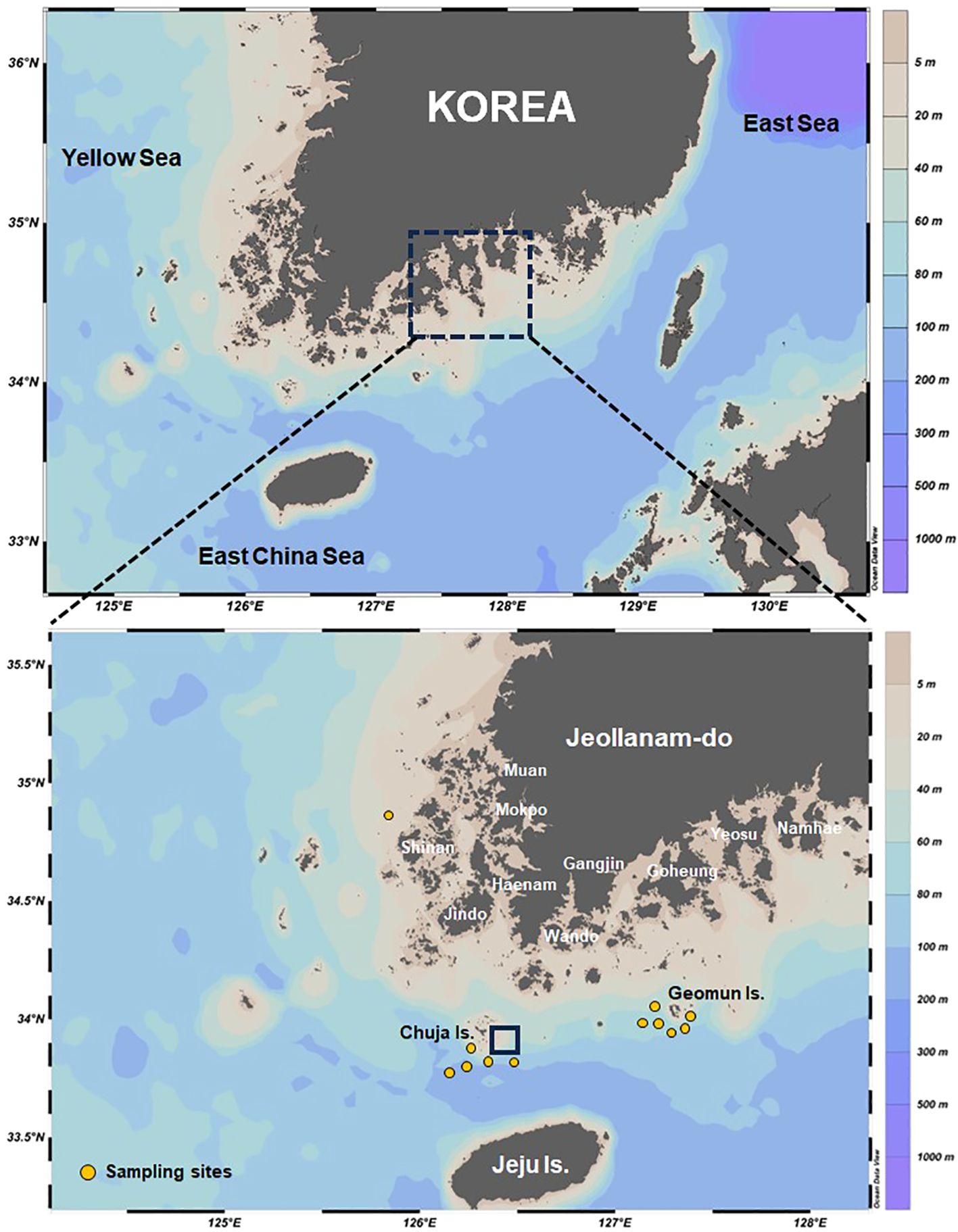
Figure 2. Map of the sampling locations of the brown croaker Miichthys miiuy in the southwestern waters of Korea. Real-time coastal information system for sea surface temperature by the National Marine Research Institute’s Badanuri Marine Information Service (black square) at the Chuja Island, Korea.
Monthly variations of the gonadosomatic index (GSI) were calculated using the following formula:
where, GW represents the weight of the gonads (g), and BW denotes the weight of the body (g).
2.3 Gonad histology and sexual maturity stages
To confirm the maturity and spawning of brown croakers, a visual examination of the gonads was conducted, assessing external characteristics such as shape and color to observe gonad development phase. Subsequently, monthly changes in maturity were analyzed for a total of 575 individuals (310 females and 265 males) from January to October, excluding November and December, when mature females and males were absent. To distinguish the internal structure of the gonads and histological stages of gonad development, extracted gonads were fixed in Bouin’s solution for 24 h, followed by washing, dehydration, and embedding in paraplast. Serial sections of 4–6 μm thickness were prepared, followed by Mayer’s hematoxylin and eosin staining and embedding in Marinol. Finally, the prepared gonad tissue specimens were examined under a microscope (LEICA DMIL LED; Leica Microsystems, Watzlar, Germany).
2.4 Sexual maturation stages
Gonad maturity stages were determined through histological analysis, with detailed microscopic descriptions provided (Grier, 1981; Selman and Wallace, 1989; Brown-Peterson et al., 2011). In the case ovaries, the stages were categorized into primary-growth (O1), cortical alveolus (O2), vitellogenic (O3), ovarian lumen (OL), oil globule (OG), and post-ovulatory follicles (POF) (Grier, 1981; Selman and Wallace, 1989). Conversely, for tests, spermatogenic cysts were classified into five developmental stages: spermatogonia (SG), spermatocytes (SC), spermatids (ST), and sperm (SP) (Wallace and Selman, 1981). Sexual maturity stages were delineated based on the presence of the most advanced oocyte stage and the occurrence of O1, O2, O3, and POF in ovaries, along with the relative proportions of SC and ST and the appearance of SP in the sperm duct (SD) in tests. Consequently, each ovary was assigned to one of the five maturity stages in females: immature, maturing, mature, ripe, and spawning and spent stages; each testis in males: immature, maturing, mature, ripe, and spawning and spent stages (Brown-Peterson et al., 2011).
2.5 Size at sexual maturity
To determine the maturation length of female brown croakers that participated in reproduction, we conducted a survey on 59 females and 91 males from July to September, the estimated spawning season. Maturity length was estimated using the following logistic equation (King, 2007) after calculating the ratio of mature or more individuals to the total population during the spawning season:
where Pi represents the maturity ratio in the length class, FLi denotes the length of class i, and b is stands as a constant. Maturity length serves as a vital biological factor used to assess the resource status of target fish species. Although 50% maturity length is typically used, this study encompassed not only the 50% maturity length but also the 75% and 97.5% maturity lengths to provide more detailed criteria for the efficient resource management of M. miiuy.
2.6 Data analysis
The chi-square (χ2) test was used to determine whether the overall and monthly sex ratio differed from the expected 1:1 ratio. All statistical analyses were conducted at a significance level of p ≤ 0.05. Analyses were conducted using Microsoft Excel and IBM SPSS Statistics version 18.0.
3 Results
3.1 Spatiotemporal distribution of M. miiuy in relation to SST
Monthly catch values for offshore gillnets and stow nets fluctuated between 0.1 and 170.9 tonnes, with the lowest recorded in September and the highest in March (Figure 3). However, upon meticulous examination of the spatial and temporal distribution characteristics of seasonal M. miiuy catches using offshore gillnets and stow nets, it became apparent that, during winter, brown croakers predominantly inhabited the Chuja Island area off the western coast of Jeju Island. Progressing into spring, a distinct northward movement towards the central part of the western coast of Korea was observed. In the summer, their distribution expanded to the shallow waters of Shinan. Conversely, during autumn, M. miiuy exhibited a migratory pattern from the southwestern coast to Jeju waters, indicating a preference for overwintering in this region.
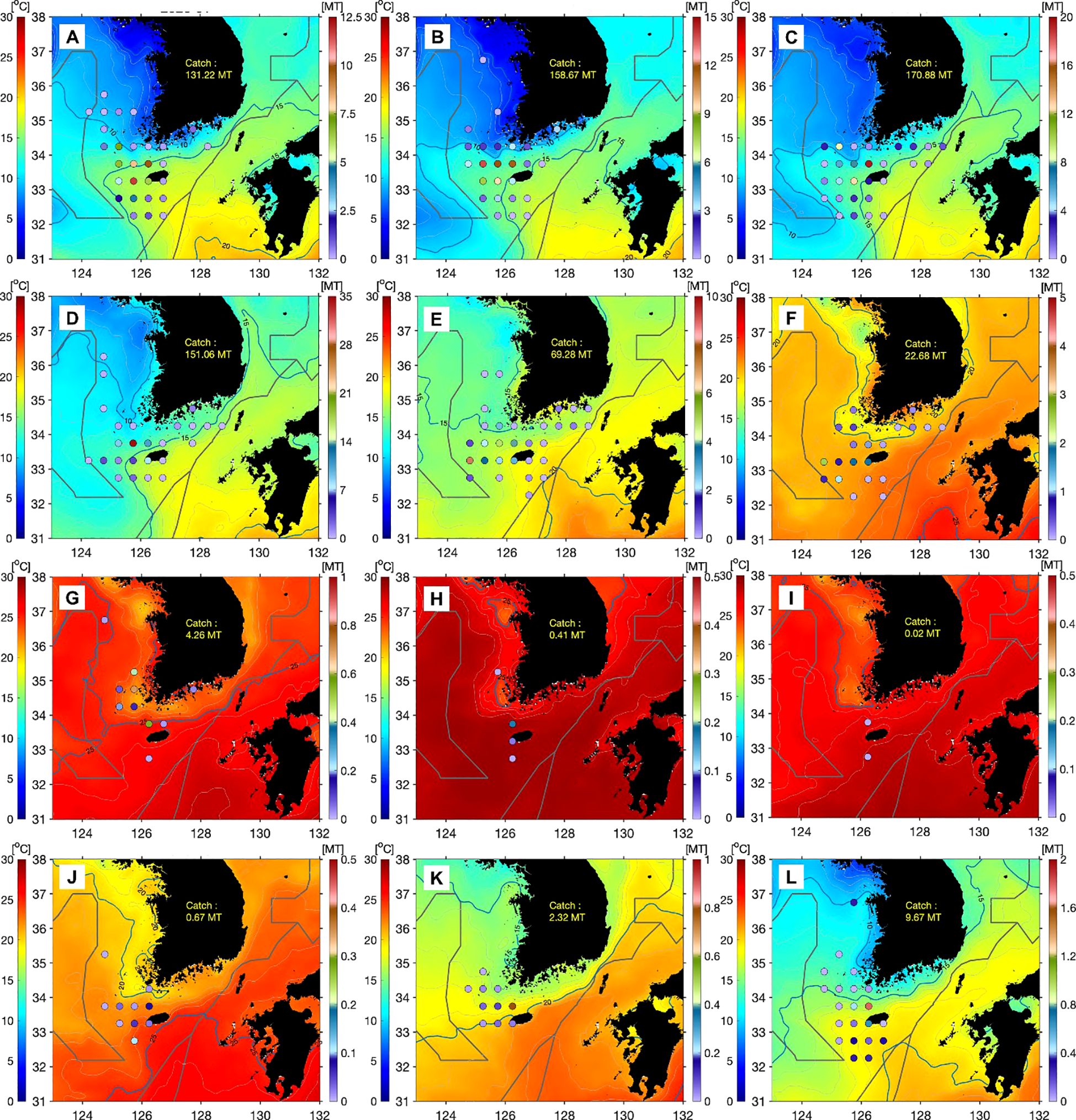
Figure 3. Spatial and temporal distribution and sea surface temperature of the brown croaker Miichthys miiuy captured by offshore gill and stow nets from January to December 2023 in the southwestern waters of Korea. (A), January; (B), February; (C), March; (D), April; (E), May; (F), June; (G), July; (H), August; (I), September; (J), October; (K), November; (L), December 2023. The circle symbol indicated of M. miiuy caught (tonnes).
An analysis of the spatial and temporal distribution characteristics of brown croaker based on monthly SST and catch data from offshore gillnets and stow nets spanning January to December 2023, which represent the primary methods of harvesting M. miiuy, revealed variability in both spatial and temporal catch patterns (Figure 3). An examination of the monthly distribution patterns in relation to SST revealed that during winter (January, February, and December), brown croaker was predominantly distributed in the Chuja Island waters, within an SST range of 8–18°C. In spring (March–May), their distribution expanded from the south of Jeju Island to the central Yellow Sea, encompassing an SST range of 10–23°C. Conversely, during summer (June–August), their distribution range began to shrink from June onwards. By August, with the formation of high SST exceeding 27°C, their distribution was limited to Jeju Island and shallow waters of Shinan. In September, the distribution range was limited owing to the persistence of high SST. However, from October onwards, their distribution gradually expanded, encompassing the southern Yellow Sea to the western waters of Jeju Island by November, within an SST range of 10–24°C.
3.2 Body length and sex ratio
In total, 671 brown croaker individuals inhabiting the coastal waters of Korea were subjected to FL measurements, which yielded values of 33.8–105.2 cm for females and 33.0–88.0 cm for males (Table 1). The average length (± SD) across the population was 58.6 cm (± 13.7), at 57.4 cm (± 13.4) for females and 61.2 cm (± 13.9) for males. Analysis of monthly body length composition during the survey period revealed dynamic patterns. In January, the 60–70 cm length group constituted the highest proportion, accounting for 61% of the population. In February, the 55–60 cm length group accounted for 50%, surpassing that of the other size groups. In March, the 70–80 cm length group accounted for 40%. However, from April to June, over 95% of individuals were below 65 cm. After July, the proportion of size groups with lengths exceeding 65 cm exhibited a continuously increasing trend till September. However, from October, the proportion of size groups with lengths below 75 cm was over 100%, which decreased again in December (Figure 4). The female-to-male sex ratio was 1:0.8, indicating a higher proportion of females (Table 1). Chi-square test analysis indicated a significant difference in the sex ratio of brown croaker each month (p< 0.05).

Table 1. Size distribution and sex ratio of the Brown croaker Miichthys miiuy collected from January to December 2023 in the southwestern waters of Korea.
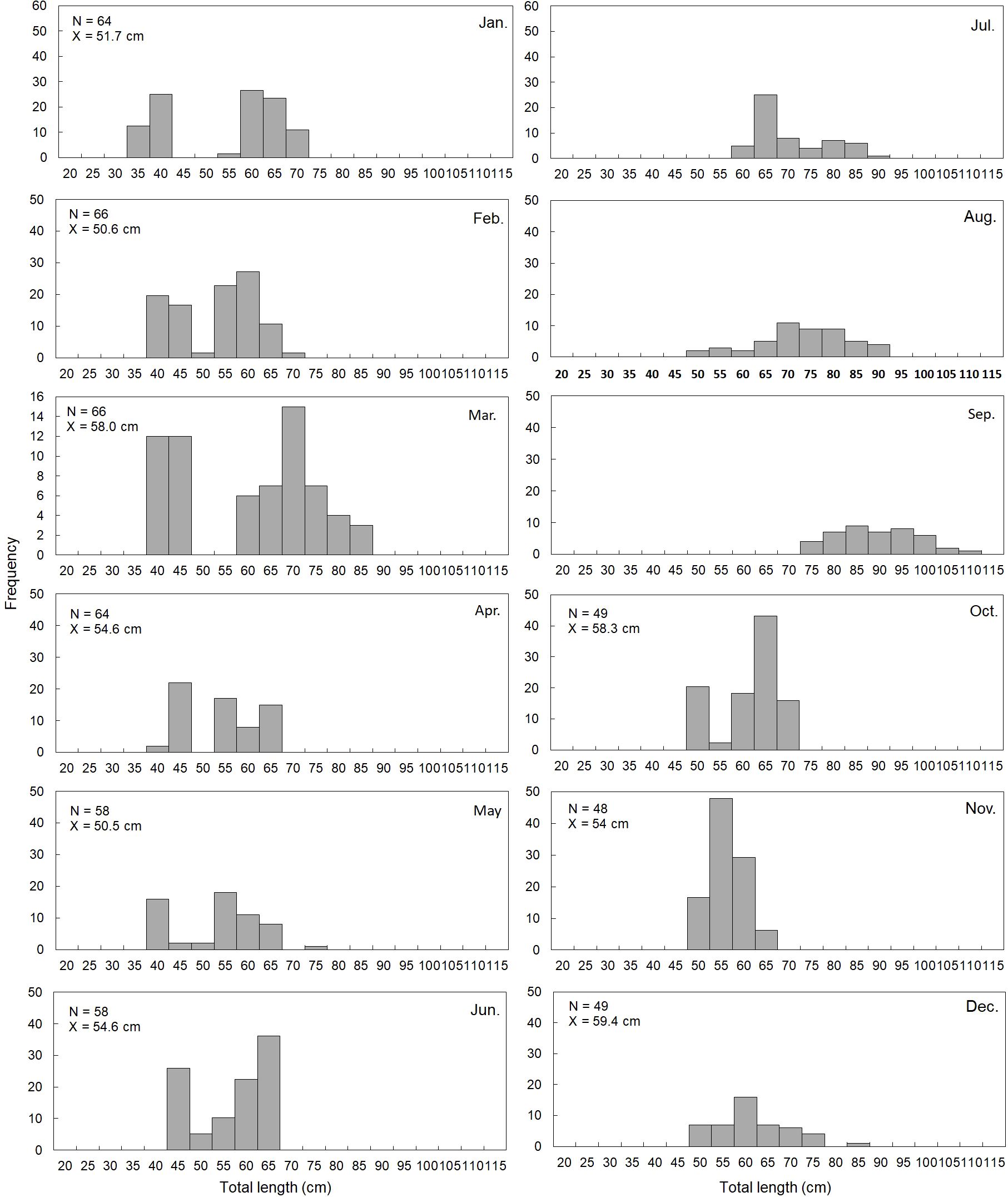
Figure 4. Monthly changes in total length frequency of the brown croaker Miichthys miiuy collected from January to December 2023 in the southwestern waters of Korea. N, number of individuals; X, average of total length.
3.3 Gonadosomatic index
Analysis of monthly changes in the GSI of female and male brown croakers revealed an average GSI range of 0.30–9.55 for females and 0.04–1.01 for males (Figure 5). The highest GSI value was observed in August, with slightly higher values observed for females. The GSI of both females and males exhibited a similar pattern; however, the GSI of females remained low until December, after a sharp increase from July to September.
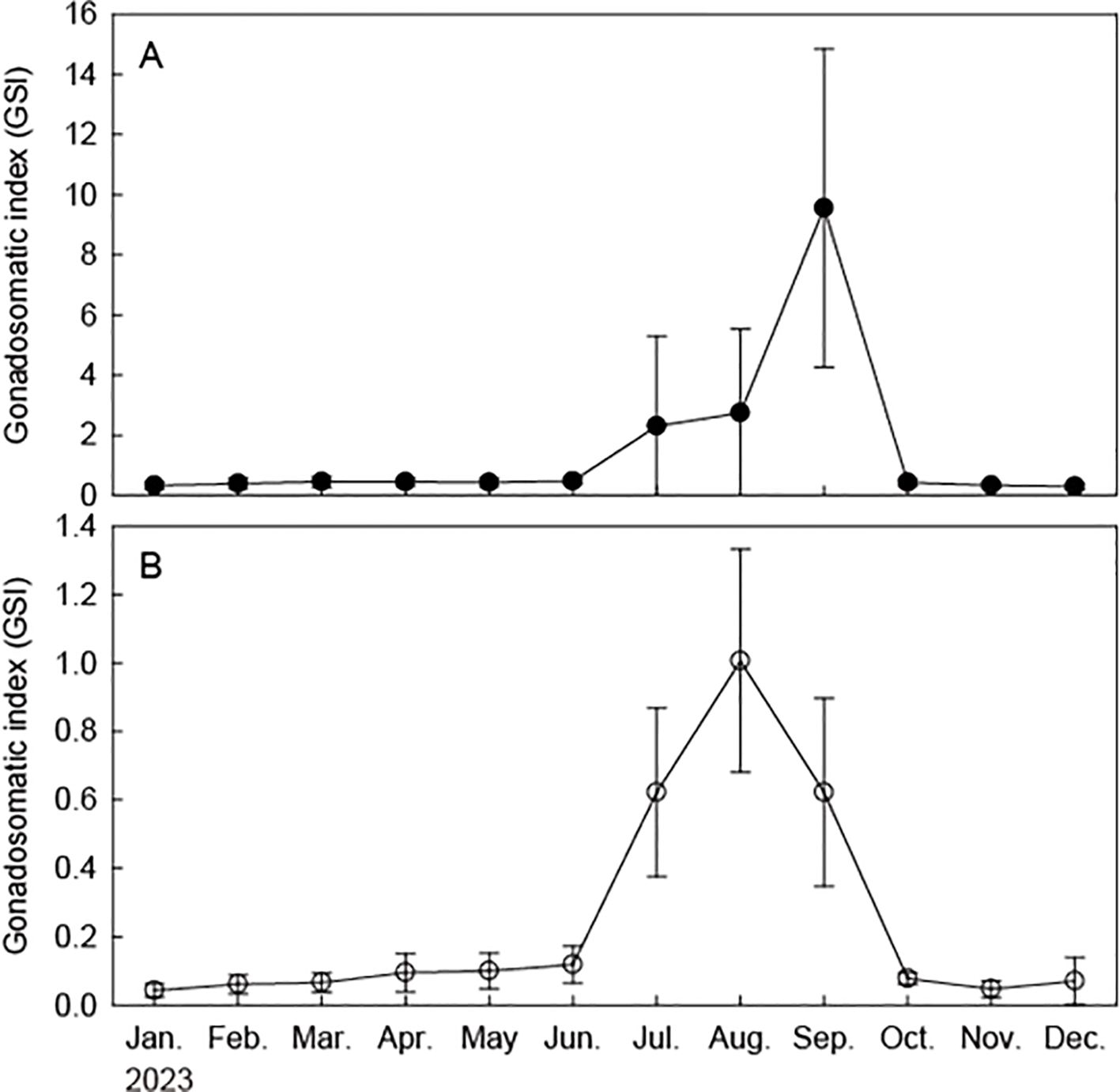
Figure 5. Monthly changes in gonadosomatic index of the brown croaker Miichthys miiuy collected from January to December 2023 in the southwestern waters of Korea. (A), female; (B), male; Bar: Standard deviation.
3.4 Sexual maturity stages
All five sexual maturity stages for females and males of M. miiuy were observed (Figures 6, 7). Regarding female ovaries (Figure 6), immature stage individuals possessed extremely poor ovaries and O1 with nuclei occupying most of the cells were observed in the ovarian lobules (Figure 6A). In the maturing stage individuals, most development oocytes at the O2 are found alongside O1, exhibiting yolk vesicles and granules within the ovarian tissue (Figure 6B). In the mature stage individuals, the most developed oocytes, at O3 show thicker zona radiata and yolk globule fusion, indicating ongoing ovary development with irregular nuclei and notable yolk accumulation prior to nucleus migration (Figure 6C). In ripe stage individuals, the O3 oocytes exhibit nucleus migration or a single yolk mass, with mature eggs containing yolk granules and vacuoles in the cytoplasm. (Figures 6D, E). In spawning and spent stages individuals have undischarged oocyte (UDC) and some POF (Figure 6F).
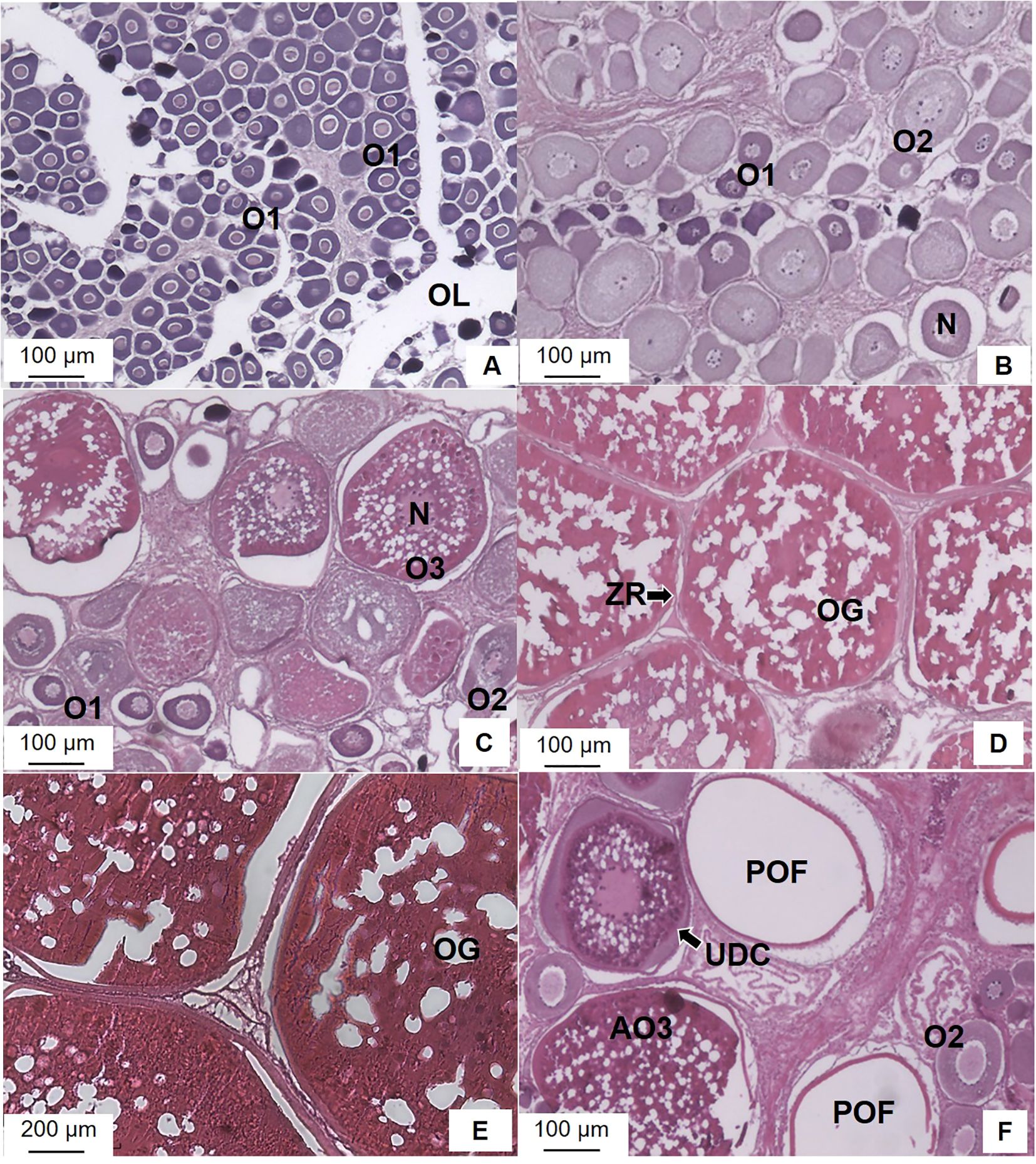
Figure 6. Photomicrographs of ovarian development phases of the brown croaker Miichthys miiuy female collected from January to December 2023 in the southwestern waters of Korea. (A), Immature stage; (B), Maturing stage; (C), Mature stage; (D, E), Ripe stage; (F), Spawning and spent stages. N, nucleus; O1, primary growth stage oocyte; O2, cortical-alveolar stage oocyte; O3, vitellogenic stage oocyte; OL, ovarian lumen; OG, oil globule; POF, post-ovulatory follicles; UDC, undischarged oocyte; ZR, zona radiate.

Figure 7. Photomicrographs of the testis development phases of the brown croaker Miichthys miiuy male collected from January to December 2023 in the southwestern waters of Korea. (A), Immature stage; (B), Maturing stage; (C), Mature stage; (D, E), Ripe stage; (F), Spawning and spent stage. SC, spermatocyte; SD, sperm duct; SG, spermatogonia; SP, sperm; ST, spermatids; UDS, undischarged sperm.
Regarding male testes (Figure 7), immature stage individuals possessed only SG, with no sperm found in the sperm duct (Figure 7A). In the maturing stage, a large amount of SC is observed, but no sperm is found in the sperm duct (Figure 7B). In the mature stage, large numbers of ST and SP are present in both the peripheral and central tubules (Figure 7C). In ripe stage, a large amount of SP is found in the central tubules. The sperm duct is filled with sperm, accompanied by a large amount of ST. (Figures 7D, E). In the spent stage, the lumina of the tubules and SD are either empty or contain residual sperm (Figure 7F).
In females (Figure 8A), the immature-stage was present in all months except July and September, with peak occurrence observed from January to May and October at 73.5–100.0%. The maturing- stage occurred from April to July, peaking in June, with occurrences ranging from 7.9% to 63.6% between April and July. The mature-stage occurred in July and August, peaking in July. The ripe-stage occurred from July to September, peaking in August and September. The spawning- and spent- stages occurred in August and September, peaking in September.
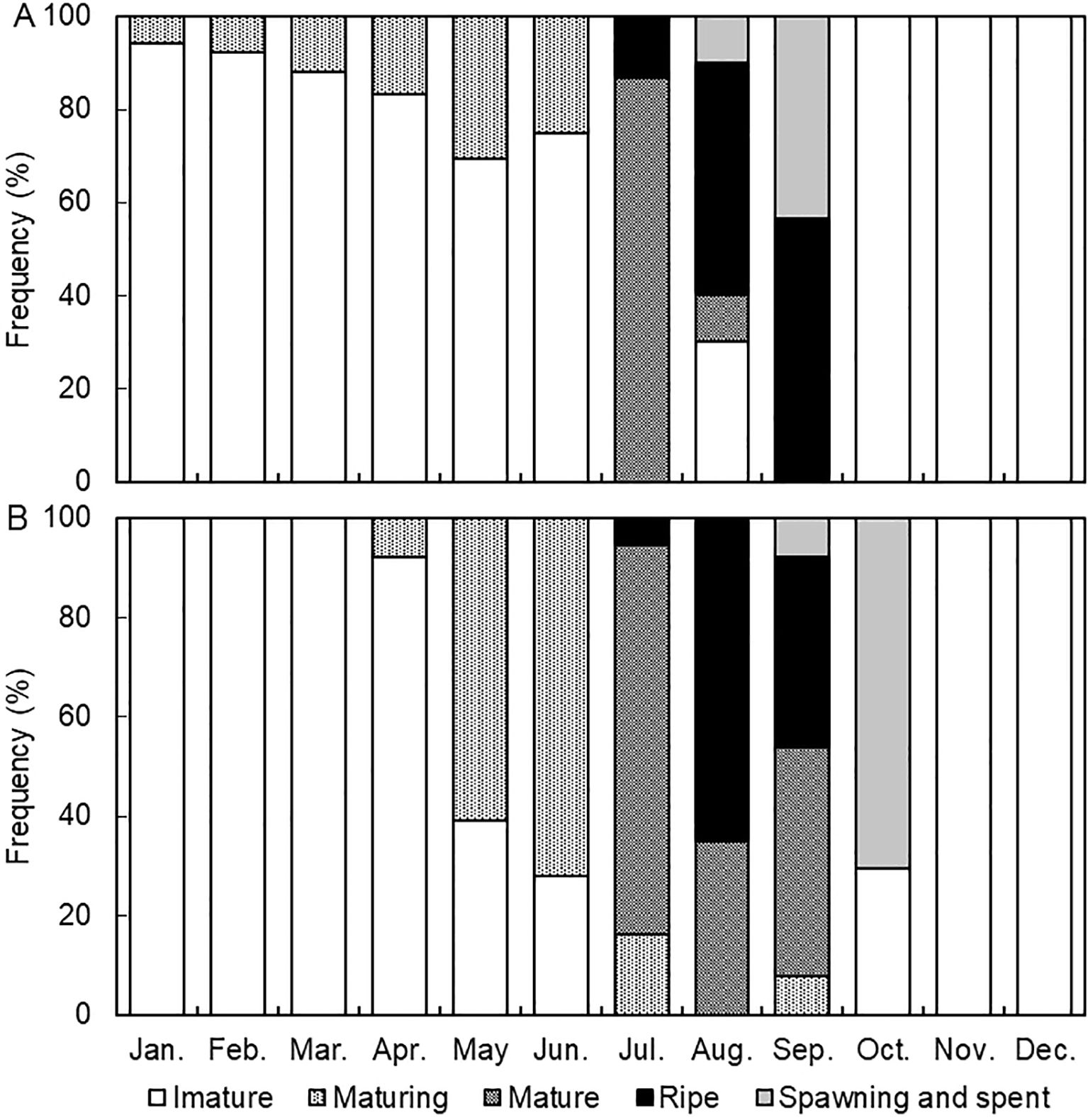
Figure 8. Monthly changes of maturity stages of the brown croaker Miichthys miiuy collected from January to December 2023 in the southwestern waters of Korea. (A), female; (B), male.
In males (Figure 8B), the immature-stage was present in all months except July and September, peaking from January to April at 92.3% to 100.0%. The maturing-stage was evident from April to July and September, with values ranging from 7.7% to 72.0% and peaking notably in May and June. The mature stage occurred from July to September, with values ranging from 35.0% to 78.4%, peaking in July. The ripe-stage occurred from July to September, with values ranging from 5.4% to 65.0%, peaking in August. The spawning- and spent-stages occurred in September and October, with values of 7.7% and 70.6%, respectively. The highest values were recorded in October. The results of the analysis of monthly changes in the maturation stages observed through histological examination of the gonads suggest that the spawning season of brown croakers occurred from July to September, with the main spawning season being between August and September.
3.5 Spawning temperature
Results regarding SST during the spawning season, revealed from 11.8 °C to 27.4 °C (Figure 9). The main spawning season, spanning August to September, exhibited SST values of 20.9–27.2 °C. Analysis of daily SST data collected from the M. miiuy collection zone indicated notable temperature fluctuations towards the end of July and throughout August. This observed variability was substantiated by SST data acquired from satellite imagery, confirming a consistent pattern of fluctuation.
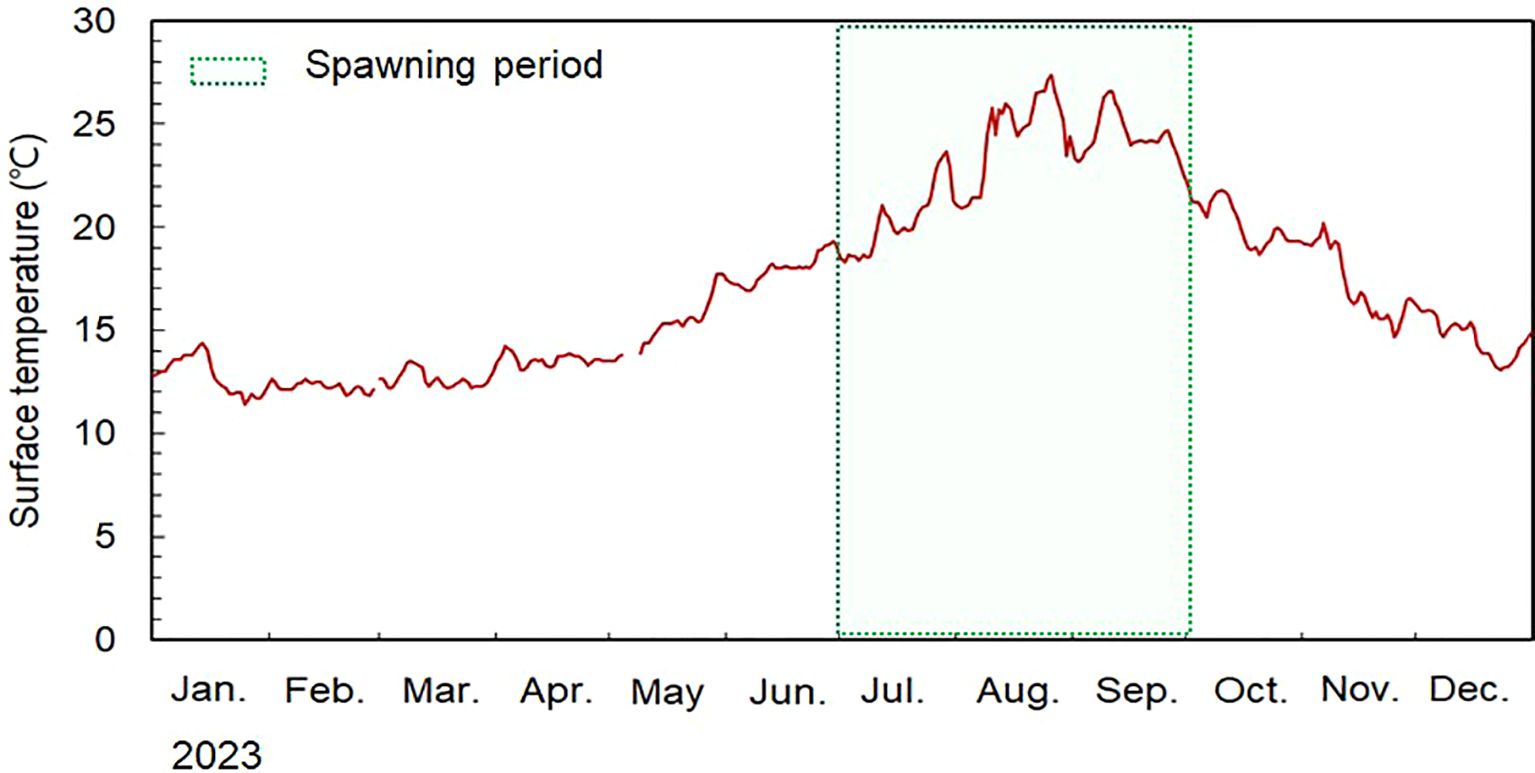
Figure 9. Variation in daily of sea surface temperature from June to September 2023 off Chuja Island coast of Korea.
3.6 Ovary size and egg number
Mature individuals were subjected to ovary size measurement from July to September, which coincided with the estimated spawning season (Figure 10). The average ovary size ranged from 0.56 mm to 0.92 mm. Analysis of the ovary size distribution mode suggested that brown croakers exhibited characteristics of being multiple spawning species. The egg number was measured from July to September, corresponding with the estimated spawning season (Table 2). The average absolute egg numbers were 143,400 for the 50.0–59.9 cm length group. As length increased, egg number also increased, reaching 7,920,660 for the 100.0–109.9 cm length group. The average relative egg numbers were 2,812 for the 50.0–59.9 cm length group. As length increased, relative egg number also increased, reaching 76,676 for the 100.0–109.9 cm length group. The relationship between length and egg number was F=2E–05TL5.6251 (Figure 11).
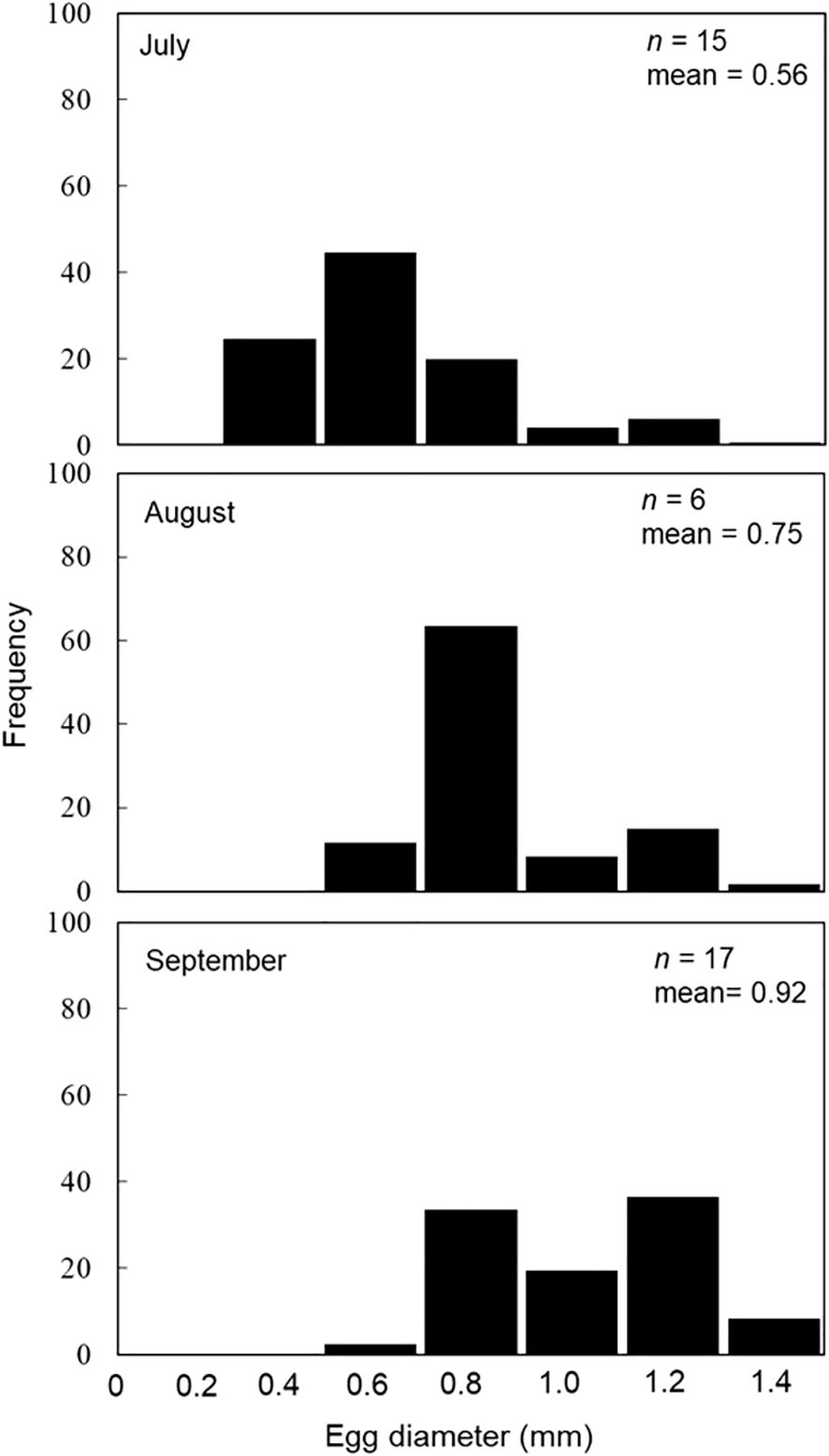
Figure 10. Monthly changes of egg diameter (long view) of the brown croaker Miichthys miiuy collected from July to September 2023 in the southwestern waters of Korea. N, number of individuals; X, average of egg diameter.
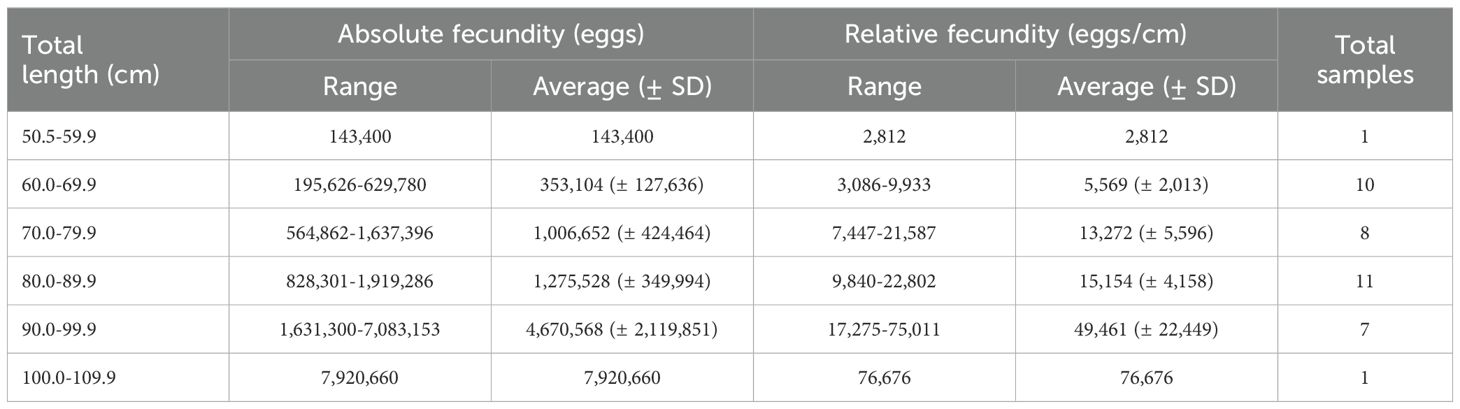
Table 2. Absolute and relative fecundity of the Brown croaker Miichthys miiuy collected July to September 2023 in the southwestern waters of Korea.
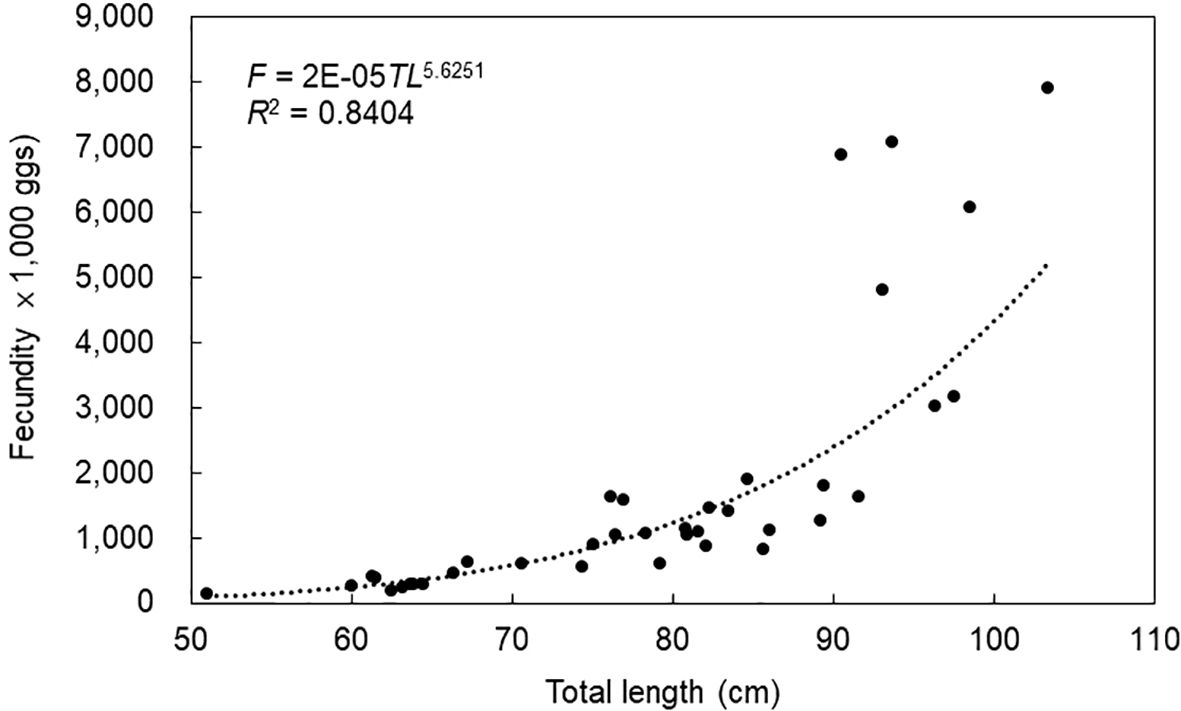
Figure 11. Relationship between total length and fecundity of the brown croaker Miichthys miiuy collected from July to September 2023 in the southwestern waters of Korea.
3.7 Maturation length
The occurrence rate of brown croakers that were at least mature was determined (Figure 12). According to the logistic equation, the 50%, 75%, and 97.5% maturation lengths of female brown croakers were estimated to be 57.4 cm, 62.9 cm, and 75.6 cm, respectively (Figure 12A). The maturation length of males was estimated to be 54.6 cm, 63.7 cm, and 85.0 cm for 50%, 75%, and 97.5% maturation, respectively (Figure 12B).

Figure 12. Relationship between fork length and group maturity of the brown croaker Miichthys miiuy collected from July to September 2023 in the southwestern waters of Korea. (A), female; (B), male; L50 = 50% group maturation; L75 = 75% group maturation; L97.5 = 97.5% group maturation.
4 Discussion
4.1 Spatiotemporal distribution and spawning ecology of M. miiuy
This study represents the first investigation into the spatial distribution and spawning ecology of M. miiuyi in the southwestern waters of Korea, emphasizing seasonal variations influenced by SST. During winter, M. miiuy primarily occupied cooler waters near Chuja Island, where the SST ranged from 8–18°C. As SST rose to between 10 and 23°C in spring, the brown croaker expanded its range northward toward the central Yellow Sea, likely to exploit optimal conditions for reproduction. In summer, when SST exceeded 27°C, M. miiuy retreated to smaller areas around Jeju Island and shallow waters of Shinan, a behavior likely driven by thermal stress, which has been well-documented in marine fishes (Currie and Schulte, 2014; Zhang et al., 2024). By autumn, as SST decreased to 10–24°C, the species expanded its distribution again, moving southward, consistent with research into the small yellow croaker’s migrations behaviors and environmental adaptations (Baik et al., 2004; Xiong et al., 2016). These findings highlight the crucial role of SST in driving the seasonal distribution and migration of M. miiuy in Korean waters. In addition, the results of this study highlight the importance of SST as a key environmental factor influencing not only the spatial distribution but also the reproductive timing of M. miiuy. As global temperatures continue to rise owing to climate change, it is expected that the migratory and reproductive patterns of M. miiuy are expected to shift. This necessitates adaptive management strategies that account for potential changes in habitat availability and spawning timing. Understanding these patterns is essential for sustainable fisheries management, as changes in SST could significantly impact the timing of migration and availability of key spawning habitats for this species.
The spawning season for both female and male M. miiuy, as determined by the GSI and histological analysis of reproductive organs, was identified to be from July to September, with the main spawning season occurring from August to September. Mature ovaries included late stages of oocyte development, occasionally with the occurrence of hydrated oocytes or post-ovulatory follicles. Various stages of hydrated oocytes were observed in the ovaries for several months before active spawning began (Sadovy et al., 1994; Brown-Peterson et al., 2011). The presence of hydrated oocytes and post-ovulatory follicles until September, the late period was estimated to be the spawning season, which is consistent with the GSI data. The GSI in both male and female fish serves as a crucial indicator for identifying the spawning season (Yamaguchi et al., 2006; Tuuli et al., 2011). The findings of this study suggested that GSI is a reliable and cost-effective method for monitoring reproductive activity in M. miiuy, serving as a viable alternative to more labor-intensive gonad histology methods.
Daily SST data collected from the Chuja Island tide observation station, the primary specimen collection site, revealed a wide range of SST conducive to spawning, spanning from 11.8–27.4°C, with the confirmed range during the main spawning season (August to September) being between 20.9–27.2°C. Environmental factors, such as photoperiod and water temperature, play a significant role in fish maturation and spawning (De Vlaming, 1972; Tobin and Wright, 2011). Aida (1991) divided the fish reproductive cycle into six spawning types based on water temperature and photoperiod: spring, spring–summer, summer, spring–autumn, autumn, and winter spawners. The primary spawning season of M. miiuy was estimated to be July to September based on the analysis of monthly changes in GSI and gonadal development stages. In key habits where M. miiuy predominantly captured, the water temperature ranged from 20.9 °C to 27.2 °C, correlating consistently with monthly fluctuations in GSI. Specifically, the reproductive organs of M. miiuy developed rapidly during the period of increasing temperature, and spawning was concluded upon reaching the optimal temperature for the primary spawning season. Consequently, brown croakers were inferred to be summer-to-early autumn spawning species.
The spatial and temporal distribution of M. miiuy catches through offshore gillnets in Korean coastal waters revealed a notable migratory pattern from January to December 2023. Catches were predominantly concentrated in the southwestern waters, particularly in the northwestern region of Jeju Island, with a decline in catches from June onwards, accompanied by a northward shift in distribution from January to May. These observations collectively suggest a seasonal migration of brown croakers from the West Sea coastline to the shallow waters of Shinan, likely motivated by spawning purpose. However, disparities were observed when comparing the results of this study with those of previous research on the spawning ecology of M. miiuy conducted both domestically and internationally. Spawning seasons have been reported to occur in August and September in the coastal areas of Shandong and Guangxi in China (Peng et al., 2020) and from August to October in the Yangtze River Estuary (Zhao et al., 1990; Yamada and Yamada, 1999). Conversely, an analysis of M. miiuy in the southwestern waters of Korea from 2015 to 2016 indicated a spawning season from August to October, with a peak occurring in September (Lee et al., 2017a). The one-month difference in spawning seasons observed in this study versus previous finding can likely be attributed to the difference in sampling timeframes and locations. Spawning temperatures observed in this study fell within the range of 21.4–24.0°C, a range suitable for spawning and hatching of M. miiuy (Shan et al., 2010). The spawning temperature range determined in this study is consistent with the generally accepted temperature range for successful spawning of M. miiuy inhabiting the adjacent seas of Korea. The observed discrepancies in spawning seasons across different regions around Korea may be influenced by rising SST caused by climate change (Han and Lee, 2020; Han et al., 2023). Ultimately, increasing SST associated with climate change is presumed to affect the reproductive dynamics, including M. miiuy maturation and spawning (Pankhurst and Munday, 2011; Rogers and Dougherty, 2019; Slesinger et al., 2021).
4.2 Conservation strategy for Korean brown croaker
The 50%, 75%, and 97.5% maturity sizes of female M. miiuy in the southwestern waters of Korea were identified as 57.4 cm, 62.9 cm, and 75.6 cm, respectively. The length at initial participation in spawning was 47.1 cm, with all individuals engaging in spawning at lengths exceeding 85 cm. Based on age and growth data from a previous study on M. miiuy (Lee et al., 2017b), the estimated age for spawning onset was 3 years, with all individuals aged 8 and older confirmed to participate in spawning. Conversely, the 50% maturity size for M. miiuy in the southwestern waters of Korea was determined to be 54.8 cm in another study (Lee et al., 2017a), indicating a 3 cm difference from the value obtained in the current study. These discrepancies can be attributed to various environmental factors, such as resource conditions, prey availability (Shoji and Tanaka, 2006; Zhang et al., 2022), and variations in spawning temperatures (Pankhurst and Munday, 2011; Fincham et al., 2013).
The brown croaker holds significant commercial importance in Korea. In this study, we investigated its spawning ecology in Korean coastal waters, revealing alterations in the reproductive cycle likely attributed to rising SST. Since the 2000s, the escalating demand for M. miiuy has prompted the implementation of the fry releasing program in regions such as Jeollanam-do (FIRA, 2024). However, inadequate research on M. miiuy ecology hinders effective resource management, potentially leading to population decline. This study highlights the crucial role of determining spawning and maturation size in ensuring the sustainable management and conservation of Korean M. miiuy resources. The catch rates of individuals below 50% maturity size highlight the need to better understand the reproduction biology of this species. Effective management strategies should include the protection of juveniles, adjustments to minimum catch sizes, and the establishment of closed seasons during the spawning period. The data on spawning and maturation sizes provided by this study offer valuable biological insights that can inform resource management policies, such as fishing bans or increases in minimum size limits, which are critical for ensuring the sustainable conservation and management of M. miiuy resources in Korea.
In addition, the broader ecological implications of rising SST due to climate change must be considered, not only in relation to the spawning period of M. miiuy, but also in terms of its ecological interactions and trophic dynamics. As SST continues to rise, shifts in the distribution and availability of prey species are likely, which could have cascading effects on the reproductive success of M. miiuy. Additionally, potential changes in interspecific competition and the introduction of novel predators into the ecosystem must be considered. Future research should prioritize investigations into how climate change influences species interactions, food web structure, and the reproductive success of M. miiuy. Such studies are essential for developing adaptive management strategies aimed at ensuring the sustainable conservation of M. miiuy populations and their ecosystems.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical approval was not required for this study involving animals, as it complied with local legislation and institutional guidelines. The study utilized the gonads of deceased brown croaker specimens collected from commercial fishing vessels to analyze reproductive biology, ensuring adherence to animal ethics standards. Exact sampling locations were recorded based on detailed fishing operation information provided by the vessels.
Author contributions
SM: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. GB: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing – review & editing. JJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. HC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing. CK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Writing – review & editing. MK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. JP: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a grant from the National Institute of Fisheries Science (NIFS) of Korea (R2024010).
Acknowledgments
We express our gratitude to the researchers at the Fisheries Resources Laboratory in the South Sea Fisheries Research Institute for collecting gonad samples from brown croaker for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FL, total length; BW, body weight; GSI, gonadosomatic index.
References
Aida K. (1991). Environmental regulation of reproductive rhythms in teleosts. Boll. Inst. Zool. Acad. Sin. Monogra. 16, 173–187.
Baik C. I., Cho K. D., Lee C. I., Choi K. H. (2004). Oceanographic conditions of fishing ground of yellow croaker (Pseudosciaena polyactis) in Korean waters. J. Kor. Fish. Soc 37, 232–248. doi: 10.5657/kfas.2004.37.3.232
Brown-Peterson N. J., Wyanski D. M., Saborido-Rey F., Macewicz B. J., Lowerre-Barbieri S. K. (2011). A standardized terminology for describing reproductive development in fishes. Mar. Coast. Fish.: Dyn Manage. Ecosyst. Sci. 3, 52–70. doi: 10.1080/19425120.2011.555724
Cheng Y., Jin X., Shi G., Wang R., Xu T. (2011). Genetic diversity and population structure of miiuy croaker populations in East China Sea revealed by the mitochondrial DNA control region sequence. Biochem. Syst. Ecol. 39, 718–724. doi: 10.1016/j.bse.2011.06.009
Chikuni S. (1985). The Fish Resources of the Northwest Pacific Vol. 266 (Rome, Italy: Food and Agriculture Organization).
Currie S., Schulte P. M. (2014). “Thermal stress,” in The physiology of fishes, 4th edn. Eds. Evans D. H., Claiborne J., Currie S. (CRC Press, Boca Raton, FL), 257–279.
De Vlaming V. L. (1972). Environmental controlo f teleost reproductive cycles: A brief review. J. Fish Biol. 4, 131–140. doi: 10.1111/j.1095-8649.1972.tb05661.x
Fincham J. I., Rijnsdorp A. D., Engelhard G. H. (2013). Shifts in the timing of spawning in sole liked to warming sea temperatures. J. Sea Res. 75, 69–76. doi: 10.1016/j.seares.2012.07.004
FIRA (Korea Fisheries Resources Agency) (2024). Korea fisheries resources information system. Available online at: http://seed.fira.or.kr/index.jsp (Accessed 20 January 2024).
Grier H. J. (1981). Cellular organization of the testis and spermatogenesis in fishes. Am. Zool. 21, 345–357. doi: 10.1093/icb/21.2.345
Gwak W. S., Roy A. (2023). Genetic diversity and population structure of Brown croaker (Miichthys miiuy) in Korea and China inferred from mtDNA control region. Genes 14, 1692. doi: 10.3390/genes14091692
Han I. S., Lee J. S. (2020). Change the annual amplitude of sea surface temperature due to climate change in a recent decade around the Korean peninsula. J. Korean Soc Mar. Environ. Saf. 26, 233–241. doi: 10.7837/kosomes.2020.26.3.233
Han I. S., Lee J. S., Jung H. K. (2023). Long-term pattern change of sea surface temperature during summer and winter due to climate change in the Korean waters. Fish. Aquat. Sci. 26, 639–648. doi: 10.47853/FAS.2023.e56
Jeong J. M., Kim Y., Song S. H., Park J. M. (2019). Feeding patterns of Brown croaker, Miichthys miiuy (Basilewsky 1855) from the south–western waters off Korea: Size–related and seasonal trends. Int. J. Mar. Sci. 3, 413–420. doi: 10.1007/s41208–019–00156–0
KHOA (Korea Hydrographic and Oceanographic Agency) (2024).Ocean data in grid framework. Available online at: http://www.khoa.go.kr/oceangrid/khoa/intro.do (Accessed 20 January, 2023).
Kim I. S., Choi Y., Lee C. H., Lee Y. J., Kim B. J., Kim J. H. (2005). Illustrated Book of Korean Fishes (Seoul, Republic of Korea: Kyohak Publishing Co).
Kim Y. S., Han K. H., Kang C. B., Kim J. B. (2004). Commercial Fishes of the Coastal and Offshore Waters in Korea (Busan, Republic of Korea: Hanguel Publishing Co).
Kim Y. U., Kim Y. M., Kim Y. S. (1994). Commercial Fish of the Coastal and Offshore water in Korea (Korea: Nat. Fish. Res. Dev. Agency).
King M. G. (2007). Fisheries Biology Assessment and Management (Oxford: UK: Blackwell Publication Co).
KOSIS (Korean Statistical Information Service) (2024).Statistic databased for fishery production survey. Available online at: http://kosis.kr/ (Accessed 20 January 2024).
Lee S. H., Chung S. D., Kim Y. H., Yoo J. T. (2017a). Maturity and spawning of Brown croaker Miichthys miiuy in the south–western water of Korea. Korean J. Ichthyol. 29, 109–116.
Lee S. H., Chung S. D., Yoo J. T., Kim Y. H. (2017b). Age and growth of Brown croaker Miichthys miiuy in the south–western water of Korea. Korean J. Ichthyol. 29, 69–74.
Lian Q. P., Zhong J. H., Lou B. (2007). Histological studies on the development of digestive system in larval and juvenile Miichthys miiuy. J. Shanghai Ocean Univ. 16, 212–218.
Pankhurst N. W., Munday P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026. doi: 10.1071/MF10269
Peng M., Chen F., Fang Z. (2020). Review on fishery biology of Miichthys miiuy. Fish. Inf. Strateg. 34, 273–278. doi: 10.13233/j.cnki.fishis.2020.04.005
Peng Z. L., Liu M. H., Fu R. B., Luo H. Z., Xia F. F., Xu Z. J. (2010). Comparative studies on the molecular genetic diversities between the Zhoushan wild Miichthys miiuy and their early filial generation by AFLP markers. J. Shanghai Ocean Univ. 19, 172–177.
Rogers L. A., Dougherty A. B. (2019). Effects of climate and demography on reproductive phenology of a harvested marine fish population. Glob. Change Biol. 25, 708–720. doi: 10.1111/gcb.14483
Sadovy Y., Rosario A., Román A. (1994). Reproduction in an aggregating grouper, the red hind, Epinephelus guttatus. Environ. Biol. Fishes. 41, 269–286. doi: 10.1007/BF02197849
Selman K., Wallace R. A. (1989). Cellular aspects of oocytes growth in teleosts. Zool. Sci. 6, 211–231.
Seo D. C. (2004). Developmental ecology and early life growth of Brown croaker Miichthys miiuy [Ph.D. thesis] (Yosu Nat’l (Yeosu, Korea: Univ).
Shan L., Xie Q., Shao X., Yan M. (2010). Study on embryonic development and morphological characteristic habitual behavior for larvae, juvenile and young of Miichthys miiuy. China Mar. Sci. 34, 75–80.
Shoji J., Tanaka M. (2006). Growth–selective survival in piscivorous larvae of Japanese Spanish mackerel Scomberomorus niphonius: Early selection and significance of ichthyoplankton prey supply. Mar. Ecol. Prog. Ser. 321, 245–254. doi: 10.3354/meps321245
Slesinger E., Jensen O. P., Saba G. (2021). Spawning phenology of a rapidly shifting marine fish species throughout its range. ICES J. Mar. Sci. 78, 1010–1022. doi: 10.1093/icesjms/fsaa252
Tobin D., Wright P. J. (2011). Temperature effects on female maturation in a temperate marine fish. J. Exp. Mar. Biol. Ecol. 403, 9–13. doi: 10.1016/j.jembe.2011.03.018
Tuuli C. D., Mitcheson Y. S., Liu M. (2011). Reproductive biology of the greyfin croaker Pennahia anea in the northern South China Sea. Ichthyol. Res. 58, 302–309. doi: 10.1007/s10228–011–0228–8
Wallace R. A., Selman K. (1981). Cellular and dynamic aspects of oocyte growth in teleosts. Am. Zool. 21, 325–343. doi: 10.1093/icb/21.2.325
Xiong Y., Zhong X., Tang J., Yang J. (2016). Migration and population structure characteristics of the small yellow croaker Larimichthys polyactis in the southern Yellow Sea. Acta Oceanol. Sin. 35, 34–41. doi: 10.1007/s13131-016-0844-7
Xu H., Zhang Y., Xu D., Lou B., Guo Y., Sun X., et al. (2014). Genetic population structure of miiuy croaker (Miichthys miiuy) in the Yellow and East China Seas base on mitochondrial COI sequences. Biochem. Syst. Ecol. 54, 240–246. doi: 10.1016/j.bse.2014.01.013
Yamada H., Yamada U. (1999). Descriptive morphology of juvenile stages of two sciaenids, Miichthys miiuy and Pennahia macrocephalus, from the East China Sea. Ichthyol. Res. 46, 93–99. doi: 10.1007/BF02674952
Yamaguchi A., Todoroki T., Kume G. (2006). Reproductive cycle, sexual maturity and diel–reproductive periodicity of white croaker, Pennahia argentata (Sciaenidae), in Ariake Sound, Japan. Fish. Res. 82, 95–100. doi: 10.1016/j.fishres.2006.08.012
Zhang B., Tang Q. S., Jin X. S., Xue Y. (2005). Feeding competition of the major fish in the East China Sea and the Yellow Sea. Acta Zool. Sin. 51, 616–623.
Zhang X. J., Tang J., Xiong Y., Wu L., Zhong X., Liu P., et al. (2008). The biological characteristics and spatial distribution Chinese durm Miichthys miiuy along Jiangsu coastal areas in summer. J. Dalian Ocean Univ. 23, 376–381.
Zhang Z., Wang Y., Liang C., Zheng L., Xian W. (2024). Seasonal resilience of temperate estuarine fish in response to climate change. Ecol. Ind. 158, 111518. doi: 10.1016/j.ecolind.2023.111518
Zhang W., Ye Z., Tian Y., Yu H., Ma S., Ju P., et al. (2022). Spawning overlap of Japanese anchovy Engraulis japonicus and Japanese Spanish mackerel Scomberomorus niphonius in the coastal Yellow Sea: A prey–predator interaction. Fish. Oceanogr. 31, 456–469. doi: 10.1111/fog.12595
Zhao C. Y., Liu X. S., Zeng B. G. (1990). Marine Fishery Resources of China (Hangzhou, China: Zhejiang Scientific and Technical Publishers).
Zhen X. U., Li M., Chen H. (2007). Study on the embryonic development of Miichthys miiuy. Mar. Sci. 31, 93–97.
Zheng Z., Jin C., Li M., Bai P., Dong S. (2008). Effects of temperature and salinity on oxygen consumption and ammonia excretion of juvenile miiuy croaker, Miichthys miiuy (Basilewsky). Aquacult. Int. 16, 581–589. doi: 10.1007/s10499-008-9169-7
Zhong J., Lou B., Yuan J. (2005). Study on the early development in larvae and juveniles of Miichthys miiuy. J. Shanghai Fish. Univ. 14, 231–237.
Keywords: brown croaker, spatiotemporal distribution, sexual maturity, gonadosomatic index, spawning period
Citation: Moon SY, Baeck GW, Jung JH, Choi H, Kim C, Koo MS and Park J-H (2024) Spatiotemporal distribution and reproductive biology of the brown croaker (Miichthys miiuy) in the southwestern waters of Korea. Front. Mar. Sci. 11:1416771. doi: 10.3389/fmars.2024.1416771
Received: 13 April 2024; Accepted: 18 November 2024;
Published: 12 December 2024.
Edited by:
Renato Crespo Pereira, Fluminense Federal University, BrazilReviewed by:
Morufu Olalekan Raimi MNES, REHO, LEHO, FAIWMES, Federal University, NigeriaJoana Cruz, University of Algarve, Portugal
Copyright © 2024 Moon, Baeck, Jung, Choi, Kim, Koo and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seong Yong Moon, bW9vbnN5Nzc0NEBnbWFpbC5jb20=; Myung Sung Koo, a21zMjczNkBrb3JlYS5rcg==
 Seong Yong Moon
Seong Yong Moon Gun Wook Baeck
Gun Wook Baeck Jin Ho Jung
Jin Ho Jung Haeyoung Choi
Haeyoung Choi Changsin Kim
Changsin Kim Myung Sung Koo
Myung Sung Koo Jeong-Ho Park
Jeong-Ho Park