- 1Department of Fisheries Biology, College of Fisheries Sciences, Pukyong National University, Busan, Republic of Korea
- 2Marine Science Study Program, Faculty of Science and Agricultural Technology, Universitas Muhammadiyah Semarang, Semarang, Indonesia
- 3Graduate School of Engineering and Science, University of the Ryukyus, Nishihara, Okinawa, Japan
- 4Organization for Research Promotion, University of the Ryukyus, Nishihara, Okinawa, Japan
- 5Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, Sesoko, Motobu, Japan
- 6Department of Marine Life Science, Jeju National University, Jeju, Republic of Korea
- 7Department of Chemistry, Biology and Marine Science, University of the Ryukyus, Nishihara, Okinawa, Japan
Melatonin and dopamine participate in the regulation of reproduction-related hormone/peptide synthesis and secretion at the hypothalamic–pituitary–gonadal axis in fish. Here, we report a unique reproduction-related interaction of melatonin and dopamine in the brain of the sapphire devil Chrysiptera cyanea, a tropical damselfish with a long-day preference. We examined the expression of arylalkylamine N-acetyltransferase 2 (aanat2)—a rate-limiting enzyme of melatonin, dopamine 2b receptor (d2b), gonadotropin-releasing hormone (gnrh1), and β-subunit of follicle-stimulating hormone (fshβ) and luteinizing hormone (lhβ)—in the brain of the sapphire devil. During the reproductive season, mature females were divided into the early vitellogenesis (EV), late vitellogenesis (LV), and post-spawning (PS) stages; a day-low and night-high profile of aanat2 was observed during EV and LV but not during PS. There were nocturnal increases in gnrh1 during EV and LV as well as d2b during LV, suggesting that melatonin has a positive effect on the levels of gnrh1 and d2b transcripts in mature fish. When the brains of females in the non-breeding season were sampled at 4-h intervals, nocturnal increases in the levels of fshβ and lhβ transcripts were observed at 00:00 and 04:00, respectively. The immersion of immature fish in melatonin-containing seawater for 6 h resulted in the upregulation of fshβ and lhβ, as well as downregulation of d2b, in the brain. Additionally, in situ hybridization analysis showed that melatonin treatment lowered the signals of d2b transcripts in the ventral hypothalamus, rostral pars distalis, proximal pars distalis, and pars intermedia, suggesting that melatonin has a negative impact on the levels of d2b transcripts in the hypothalamus and pituitary of immature females; the opposite effect is likely concerning the levels of fshβ and lhβ transcripts in the pituitary. In conclusion, melatonin positively and negatively acted on the hypothalamus and pituitary in the sapphire devil; these opposite effects were related to differences in gonadal development status.
1 Introduction
For seasonal breeders inhabiting aquatic environments, periodic changes in photoperiod and water temperature are proximate cues with important roles in the initiation and termination of reproductive activity (Davies and Bromage, 2002; Mull et al., 2008). Changes in these exogenous factors may be perceived by sensory organs, conveyed as internal stimuli, and then activate the hypothalamic–pituitary–gonadal axis; this process results in the synthesis and release of gonadotropin-releasing hormone (GnRH), gonadotropins (GtHs; follicle-stimulating hormone and luteinizing hormone), and sex steroids (Montero et al., 1994; Levavi-Sivan et al., 2006; Jéhannet et al., 2019).
Putative endogenous transducers include melatonin, an indoleamine synthesized from serotonin through the enzymatic activities of hydroxyindole-O-methyltransferase (HIOMT) and arylalkylamine N-acetyltransferase (aaNAT) in the pineal organ and the retina; aaNAT is the rate-limiting enzyme, which controls the day-low and night-high cycle of melatonin (Coon et al., 1999; Falcón et al., 2007). Because melatonin and its receptor(s) exhibit seasonal variation in accordance with dark-phase duration in certain fish species (Bayarri et al., 2010; Baekelandt et al., 2020; Zhao et al., 2022), melatonin is considered the timekeeper of periodic and seasonal reproduction (Burgerhout et al., 2019). Indeed, seasonal changes in plasma melatonin levels have been associated with reproductive activity in seasonal spawners, including the Senegal sole Solea senegalensis (Vera et al., 2007) and the European sea bass Dicentrarchus labrax (Bayarri et al., 2010). Melatonin is generally regarded as a suppressor of reproductive activity in long-day breeders (Renuka and Joshi, 2010; Imamura et al., 2022). However, debate persists concerning the physiological roles of melatonin in reproduction because stimulatory or inhibitory effects on gonadal development have been reported in fish. For example, melatonin treatment showed a stimulatory effect on gonadal development in female zebrafish Danio rerio (Carnevali et al., 2011), but it failed to prevent secondary sexual characteristic development (kidney hypertrophy) in male three-spined stickleback Gasterosteus aculeatus (Mayer et al., 1997).
Dopamine is a catecholamine synthesized from tyrosine through the activities of tyrosine hydroxylase, the rate-limiting enzyme, and aromatic-l-amino-acid decarboxylase (Ganesh, 2021). The actions of dopamine are mediated by its receptors (the adenylyl cyclase stimulator D1 and the adenylyl cyclase inhibitor D2), which belong to the G protein-coupled seven-transmembrane domain receptor superfamily and are expressed on neurons and cells of target organs (Kebabian and Calne, 1979). In certain fish, dopamine acts as a GtH-release inhibitory factor, with a negative effect on reproductive activity (Dufour et al., 2010; Zohar et al., 2010) . The D2-type receptor, but not D1-type receptor, is likely involved in dopaminergic inhibition of reproduction, considering that treatment of zebrafish with domperidone, a D2 receptor antagonist, induced expression of the β-subunit of luteinizing hormone and vitellogenesis (Fontaine et al., 2013). Oocyte development of the gray mullet Mugil cephalus was reportedly accelerated after treatment with domperidone (Aizen et al., 2005).
There is apparent interplay between melatonin and dopamine in the brains of fish, as demonstrated by several studies where dopaminergic activity decreased in the pituitary of the rainbow trout Oncorhynchus mykiss after melatonin treatment (Hernández-Rauda et al., 2000) and dopamine concentration increased in the hypothalamus of the common carp Cyprinus carpio after melatonin injection (Popek et al., 2006). However, it remains unclear how melatonin is involved in the dopaminergic system in the brains of fish, although neurons synthesizing GnRH in the brain and cells synthesizing GtHs in the pituitary gland are possible targets of melatonin (Amano et al., 2000, 2004; Ghosh and Nath, 2005; Carnevali et al., 2011) , which may mediate the actions of dopamine and its receptors.
The sapphire devil, Chrysiptera cyanea, is a species of tropical damselfish found in the Pomacentridae family and commonly inhabits the West Pacific region (Myers, 1999). Previous research on sapphire devil populations on coral reefs around the Okinawa Islands has shown that ovarian development in females begins in March and peaks in May (Bapary et al., 2009). Similarly, spermatogenesis in males commences in March and intensifies from April to May (Igarashi et al., 2015). Experimental evidence has indicated that vitellogenesis progression can be induced under extended photoperiods with appropriate temperature ranges during the non-spawning season (Bapary et al., 2009; Bapary and Takemura, 2010) . Furthermore, the sapphire devil is known to be a multiple spawner, with individuals releasing their entire egg or sperm load during each spawning event (Bapary and Takemura, 2010).
Previous studies showed that reduced dopaminergic activity in the brain is related to increased reproductive activity in the sapphire devil Chrysiptera cyanea, a tropical damselfish with long-day preference in gonadal development (Badruzzaman et al., 2013, 2015). Moreover, short- and long-term melatonin treatments reduced transcript levels of the β-subunits of GtHs (follicle-stimulating hormone, fshβ; luteinizing hormone, lhβ) and GnRH (gnrh1) in the brain (Imamura et al., 2022). The present study was performed to further explore the interplay between melatonin and dopamine during reproduction in the sapphire devil. Transcription of the dopamine 2b receptor (d2b) in the brains of fish was carefully monitored during the breeding and non-breeding seasons. Day-night differences in transcript levels of reproduction-related genes (gnrh1, fshβ, lhβ, and d2b) and aaNAT (aanat2) were examined in vitellogenic and post-spawning females. The effects of melatonin treatment on the transcript levels of reproduction-related genes were measured in the brains of fish during the non-breeding season.
2 Materials and methods
2.1 Fish and experimental design
Sapphire devils (body mass, 0.77–4.41 g) were collected using hand nets from Odo Beach (26°5′21.81″N, 127°42′26.31″E), Okinawa, Japan. All experiments were conducted in compliance with the guidelines of the Animal Care and Use Committee of the University of the Ryukyus and regulations for the care and use of laboratory animals in Japan.
The first experiment (Experiment I) was designed to determine day-night differences in transcript levels of gnrh1, fshβ, lhβ, d2b, and aanat2 in the brains of mature females during vitellogenesis and after spawning. The fish used in this experiment were collected between March and September; they were housed in aquariums (60-L capacity) with filtered seawater and aeration under ambient water temperature and photoperiod at the Department of Chemistry, Biology and Marine Science, University of the Ryukyus, Okinawa, Japan. After acclimatization and anesthetization with 2-phenoxyethanol (Kanto Chemical, Tokyo, Japan), fish (n = 6–8 per time point) were sampled at 12:00 and 24:00, then euthanized by decapitation. After the body mass and ovarian mass had been recorded, the whole brain with pituitary of each individual was collected, immersed in RNAiso Plus (Takara Bio, Kusatsu Japan), and stored at −80°C. Pieces of the ovaries were preserved in Bouin’s solution. The gonadosomatic index [GSI = (ovarian mass/body mass) × 100] was calculated.
The second experiment (Experiment II) was designed to assess daily changes in transcript levels of fshβ and lhβ in the brains of immature fish. Fish (n = 42) were reared in four plastic tanks (60-L capacity) with running seawater and ambient aeration under natural light (sunrise 06:00, sunset 17:50); they were fed commercial pellets at 5% of body mass, each day at 10:00. After 6 days of acclimatization, fish were sampled at 4-h intervals (6 individuals per time point) beginning at 06:00. At each time point, fish were collected from the tanks and anesthetized. After the body mass had been recorded, the whole brain including the pituitary was removed and preserved in RNAiso Plus. All tissues were stored at −80°C until assessment via molecular analyses.
The third experiment (Experiment III) was designed to examine the effects of melatonin treatment on transcript levels of fshβ, lhβ, and d2b in the brains of fish during the non-breeding season. Fish (n = 20, body mass 0.77–2.83) were acclimatized in aquariums (60-L capacity) with filtered and aerated seawater under conditions of ambient temperature and photoperiod of 12-h light and 12-h dark (LD12:12; lights on at 06:00 and lights off at 18:00). After acclimatization by daily feeding of commercial pellets (Pure Gold EP1; Nisshin-Marubeni, Tokyo, Japan), fish were divided into a control group (n = 10) and a melatonin treatment group (n = 10). Both groups were housed in aquariums (60-L capacity) with filtered and aerated seawater under conditions of ambient temperature and LD12:12 photoperiod. As previously described (Badruzzaman et al., 2013), melatonin (Sigma-Aldrich, St. Louis, MO, USA) dissolved in ethanol (1 mg/mL) was added to the seawater of the melatonin treatment group at 12:00 to a final concentration of 100 μg/L. Vehicle only was added to the seawater of the control group. After 6 h of melatonin treatment before lights out, fish were anesthetized with 2-phenoxyethanol, euthanized by decapitation, and then weighed. The whole brain including the pituitary (n = 6 per group) was collected, preserved in RNAiso Plus, and then stored at −80°C. The heads of the residual individuals (n = 4 per group) were fixed overnight in 4% paraformaldehyde (Nacalai Tesque, Kyoto, Japan) at 4°C.
2.2 Total RNA extraction, cDNA synthesis, and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from tissues using RNAiso Plus, in accordance with the manufacturer’s protocol; quantities of total RNA were assayed using a NanoDrop™ One spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at wavelengths of 260 and 280 nm. Subsequently, cDNA synthesis was performed with a PrimeScript RT reagent Kit and gDNA eraser (Takara Bio), using 1000 ng of total RNA as the template.
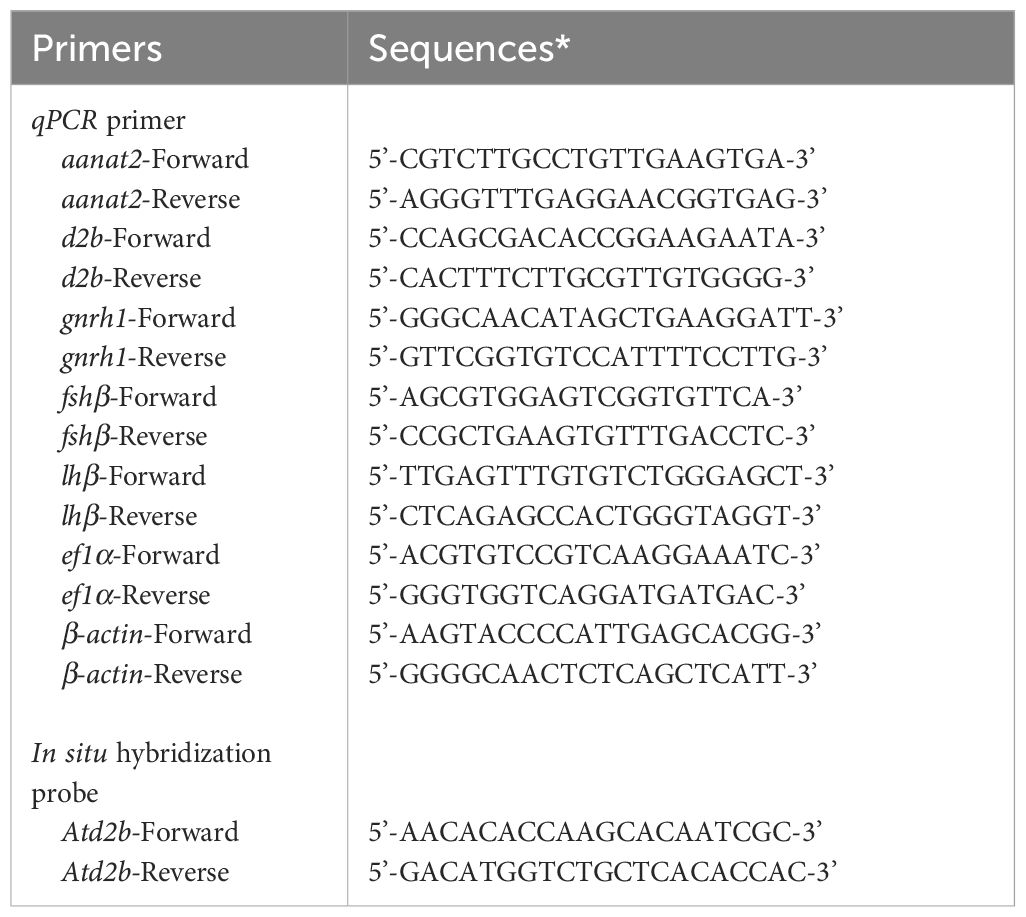
Real-time qPCR was conducted to amplify the mRNA fragments of target genes using a Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) and TB Green Premix Ex Taq II (Tli RNaseH Plus; Takara Bio). Primers (Table 1) were designed using Primer3Plus software. qPCR was performed in a final volume of 10 µL containing 5 µL of TB Green Premix Ex Taq II, 0.3 µL each of forward and reverse primers, 2.4 µL of nuclease-free water, and 2 µL of the cDNA template. The thermocycler protocol comprised initial denaturation for 30 s at 95°C, followed by 40 cycles of denaturation for 5 s at 95°C, annealing and extension for 30 s at 60°C. Melting curve analysis was performed from 65°C to 95°C using increments of 0.5°C for 5 s each. The mRNA abundances of dopamine receptors in each sample were normalized relative to ef1α and β-actin as an internal control.
2.3 Histological procedures
After fixation with Bouin’s solution for 1 day at room temperature, pieces of ovary were subjected to ethanol dehydration. The dehydrated ovaries were embedded in paraffin with a melting point of 56°C (Surgipath Paraplast; Leica Biosystems, Nussloch, Germany) using xylene. Seven-micrometer-thick sections were stained with Mayer’s hematoxylin and eosin for microscopic observations. Based on an existing classification method (Hoque et al., 1998), oocytes in the ovary were divided into 5 stages: perinucleolus stage (PNS), oil droplet stage (ODS), primary yolk stage (PYS), secondary yolk stage (SYS), and tertiary yolk stage (TYS).
Ovarian development was divided into three phases (Imamura et al., 2017; Mahardini et al., 2018): early vitellogenic phase (EV, ovarian condition with vitellogenic oocytes at PYS and SYS in the ovary), late vitellogenic phase (LV, ovarian condition with vitellogenic oocytes at TYS in the ovary), and post-spawning phase (PS, spent ovary occupied by immature oocytes at PNS).
2.4 In situ hybridization
The localization of d2b in the whole brain was confirmed by in situ hybridization. Digoxigenin (DIG)-labeled sense and antisense probes were synthesized by in vitro transcription using partial d2b cDNA fragments (Table 1). Whole brains (in skulls) that had been fixed with 4% paraformaldehyde were decalcified using Morse’s solution (10% sodium citrate and 22.5% formic acid), dehydrated by passage through a graded ethanol series (ethanol diluted with diethyl pyrocarbonate-treated water), embedded in paraffin, and then cut into 5-μm-thick sections.
After deparaffinization and hydration, sections were subjected to digestion with proteinase K (10 μg/mL) at 37°C for 15 min, fixed with 4% paraformaldehyde, and prehybridized with 2 × SSC/50% formamide. Next, sections were hybridized with a hybridization mixture consisting of 500 ng/mL DIG-labeled RNA probes, 10% dextran sulfate, 2 × SSC, 0.02% sodium dodecyl sulfate, and 50% formamide at 60°C for 16 h. Subsequently, sections were washed with 2 × SSC at room temperature for 5 min, washed three times with 1 × SSC/50% formamide at 60°C for 30 min, washed three times with 1 × SSC at 60°C for 30 min, and washed twice with Tris-buffered saline for 5 min. Sections were immersed in blocking solution containing 10% bovine serum albumin and maleic acid for 1 h at room temperature. DIG-labeled RNA probes were detected using anti-DIG-AP antibody (Roche, Basel, Switzerland), diluted 1:1000 with the same blocking solution, for 1 h at room temperature or overnight at 4°C. Signals were visualized using the NBT/BCIP liquid substrate system (Sigma-Aldrich). Sections were observed and photographed under a microscope.
2.5 Statistical analysis
All data are presented as means ± standard errors. GSI was analyzed by one-way analysis of variance, followed by Tukey’s honestly significant difference multiple comparison test. Day-night differences in the expression patterns of aanat2 and reproduction-related genes (gnrh1, fshβ, lhβ, and d2b) and the effects of melatonin treatment on the levels of reproduction-related gene transcripts (fshβ, lhβ, and d2b) were compared using Student’s t-test and the Mann–Whitney U test, respectively. Statistics Resource Pack software (Minipack Copyright Notice, University of Chicago, Chicago, IL, USA) was used for statistical analysis. In all analyses, P < 0.05 was assumed to indicate statistical significance.
3 Results
3.1 Day-night differences in gene expression patterns during the breeding season
Changes in the GSI of females during the breeding season are shown in Figure 1. Fish at EV had low GSI, but their ovaries contained vitellogenic oocytes at PYS and SYS (Figures 1A, D). Fish at LV, in which histological observation of the ovary revealed oocytes at TYS, showed significantly elevated GSI (Figures 1B, D). The GSI of fish at PS returned to the basal levels. Ovaries at this phase were exclusively occupied by immature oocytes at PNS (Figures 1C, D).
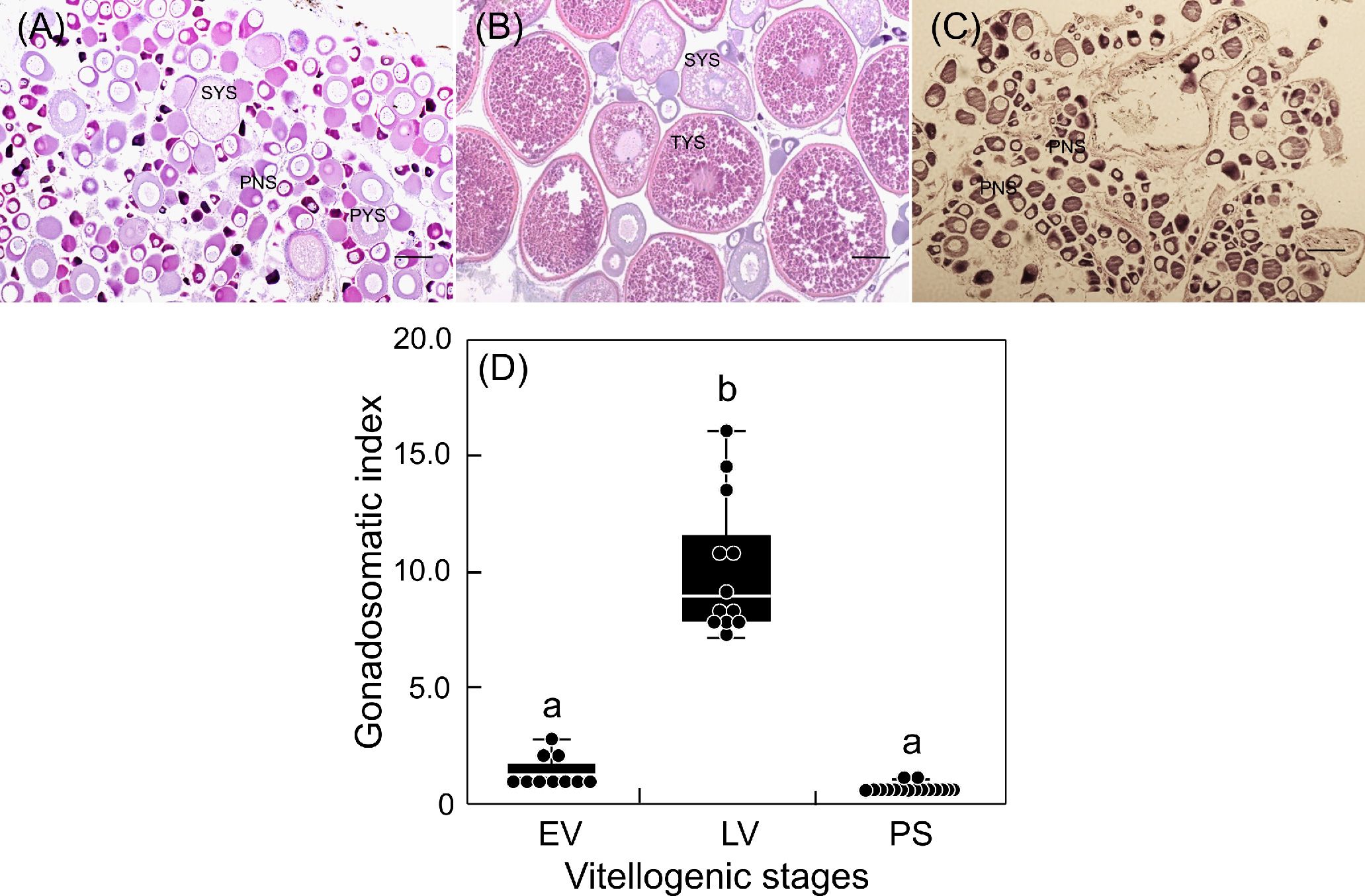
Figure 1 Changes in ovarian development (A-C) and gonadosomatic index (D) of sapphire devil during and after the breeding season. Fish were collected and classified into three phases (EV, early vitellogenic phase (A), LV, late vitellogenic phase (B), PS, post-spawning phase (C)) according to ovarian development. Each point represents mean ± standard error of the mean (SEM). Different letters show significant difference at P < 0.05. Scale bar=50 µm.
The transcript levels of aanat2 in the sapphire devil brain were determined at 12:00 and 24:00 during three phases of ovarian development (Figure 2). There were day-low and night-high differences in the transcript levels of aanat2 at both EV and LV (P < 0.05). However, there were minimal differences in aanat2 transcript levels among the brains of fish at PS.
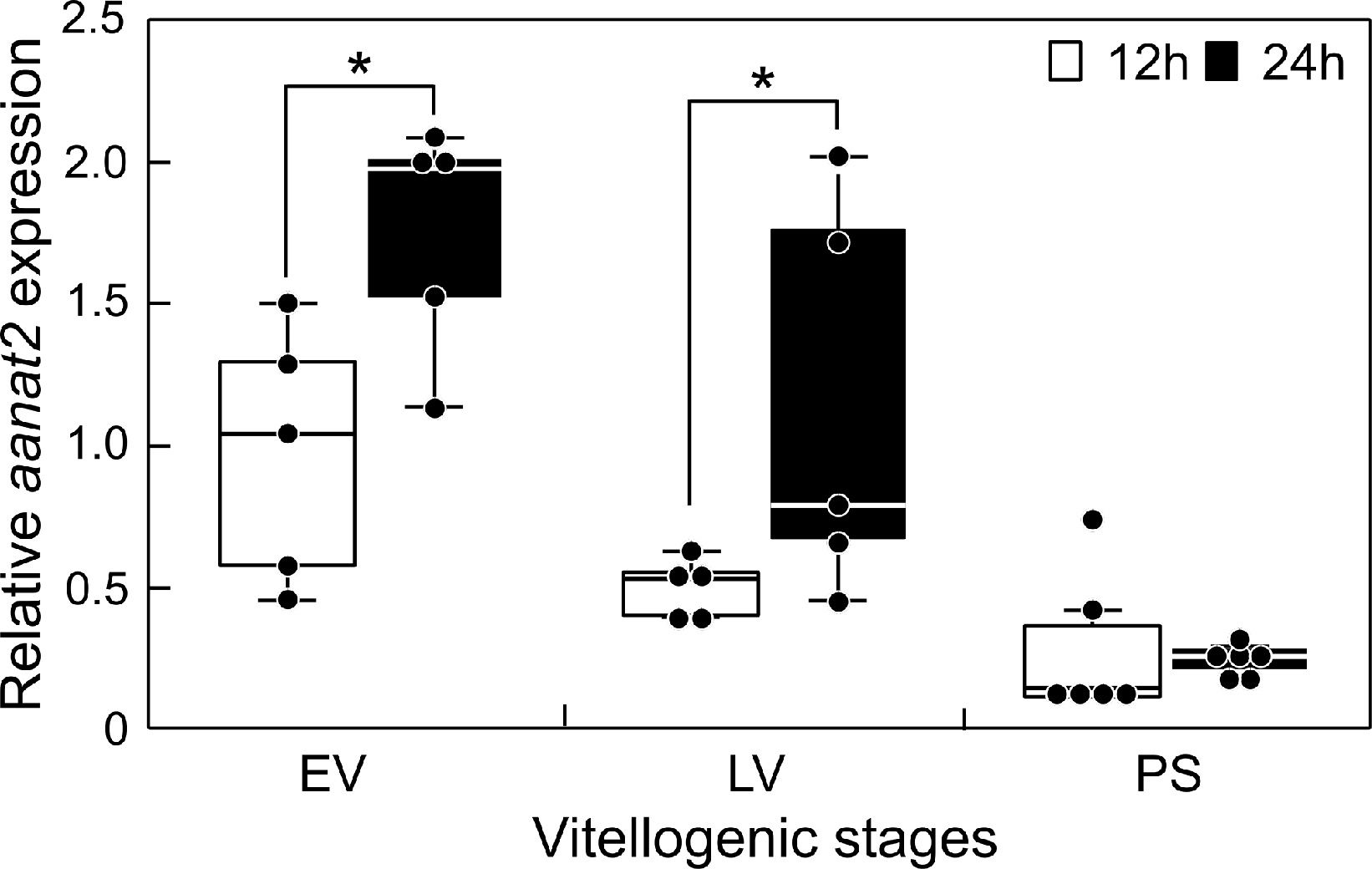
Figure 2 Changes in the mRNA abundance of aanat2 in the brain of sapphire devil during and after the breeding season. Fish were collected at 1200 h and 2400 h during the three phases (EV, early vitellogenic phase; LV, late vitellogenic phase; PS, post-spawning phase) according to ovarian development. The transcript levels of aanat2 in the brain were analyzed using qPCR and normalized against ef1α and β-actin. Open and solid columns represent the light and dark phases, respectively. Each point represents mean ± standard error of the mean (SEM). Asterisks in the figure show significant difference at P < 0.05.
The transcript levels of reproduction-related genes in the sapphire devil brain were measured at 12:00 and 24:00 during three phases of ovarian development (Figure 3). Significantly higher transcript levels (P < 0.05) were observed at 24:00 for gnrh1 at EV and LV (Figure 3A), for fshβ (12:00 = 0.0037 ± 0.00038; 24:00 = 0.0174 ± 0.00342) and lhβ (12:00 = 0.00019 ± 6.66E-05; 24:00 = 0.00091 ± 0.00010) at PS, and for d2b at LV (Figures 3B, C, D).
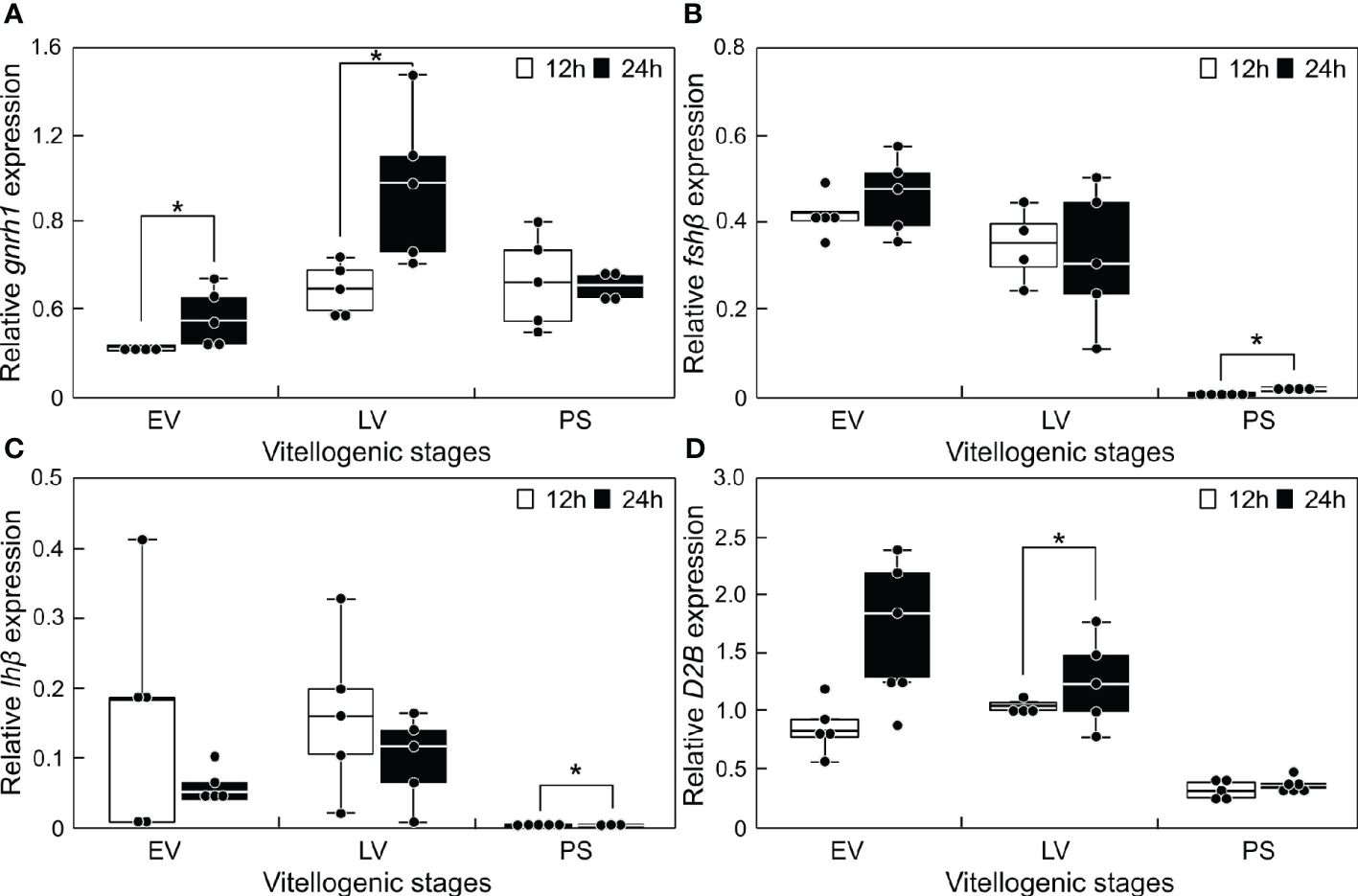
Figure 3 Changes in the mRNA abundance of reproduction-related genes in the brain of sapphire devil during and after the breeding season. Fish were collected at 1200 h and 2400 h during the three phases (EV, early vitellogenic phase; LV, late vitellogenic phase; PS, post-spawning phase) according to ovarian development. (A) gnrh1, (B) fshb, (C) lhb, (D) d2b. The transcript levels of reproduction-related genes in the brain were analyzed using qPCR and normalized against ef1α and β-actin. Open and solid columns represent the light and dark phases, respectively. Each point represents mean ± standard error of the mean (SEM). Asterisks in the figure show significant difference at P < 0.05.
3.2 Daily variation of fshβ and lhβ during the non-breeding season
The transcript levels of fshβ and lhβ in the sapphire devil brain were measured at 4-h intervals (Figure 4). Both fshβ and lhβ expression levels fluctuated daily, however only fshβ showed significant increases at 00:00 (Figure 4A) and 04:00 (Figure 4B), respectively.
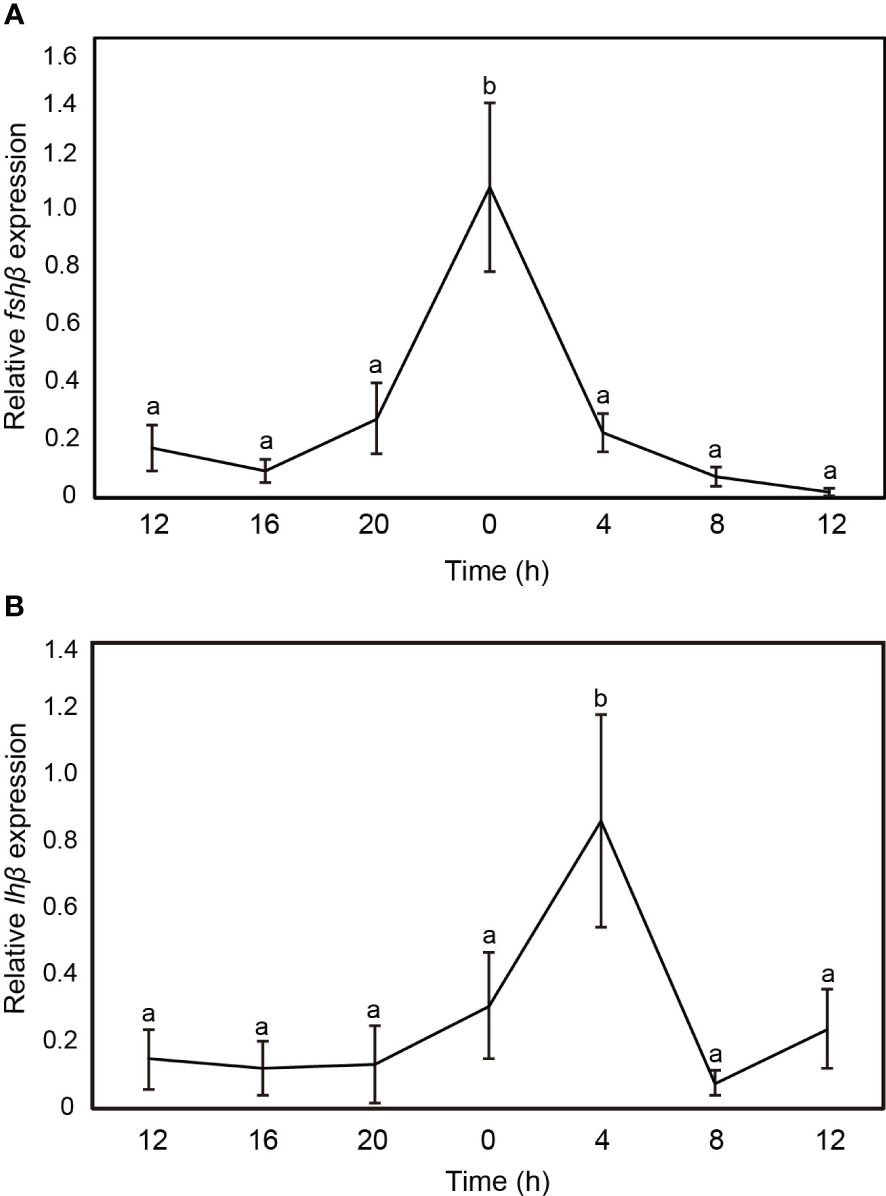
Figure 4 Changes in the mRNA abundance of gonadotropin genes in the brain of sapphire devil during the out-of-breeding season. (A) fshb, (B) lhb. The transcript levels of reproduction-related genes in the brain were analyzed using qPCR and normalized against ef1α and β-actin. Open and solid columns represent the light and dark phases, respectively. Each point represents mean ± standard error of the mean (SEM). Asterisks in the figure show significant difference at P < 0.05.
3.3 Effects of melatonin treatment on reproduction-related gene transcript levels
Fish during the non-breeding season were immersed in melatonin-containing seawater for 6 h. The transcript levels of reproduction-related genes (gnrh1, fshβ, lhβ, and d2b) in the brain were compared between the melatonin treatment and control groups (Figure 5). The results revealed no differences in gnrh1 transcript level (Figure 5A). However, the transcript levels of fshβ (Figure 5B) and lhβ (Figure 5C) were significantly upregulated (P < 0.05), whereas the transcript levels of d2b (Figure 5D) were significantly downregulated, in the melatonin treatment group (P < 0.05).
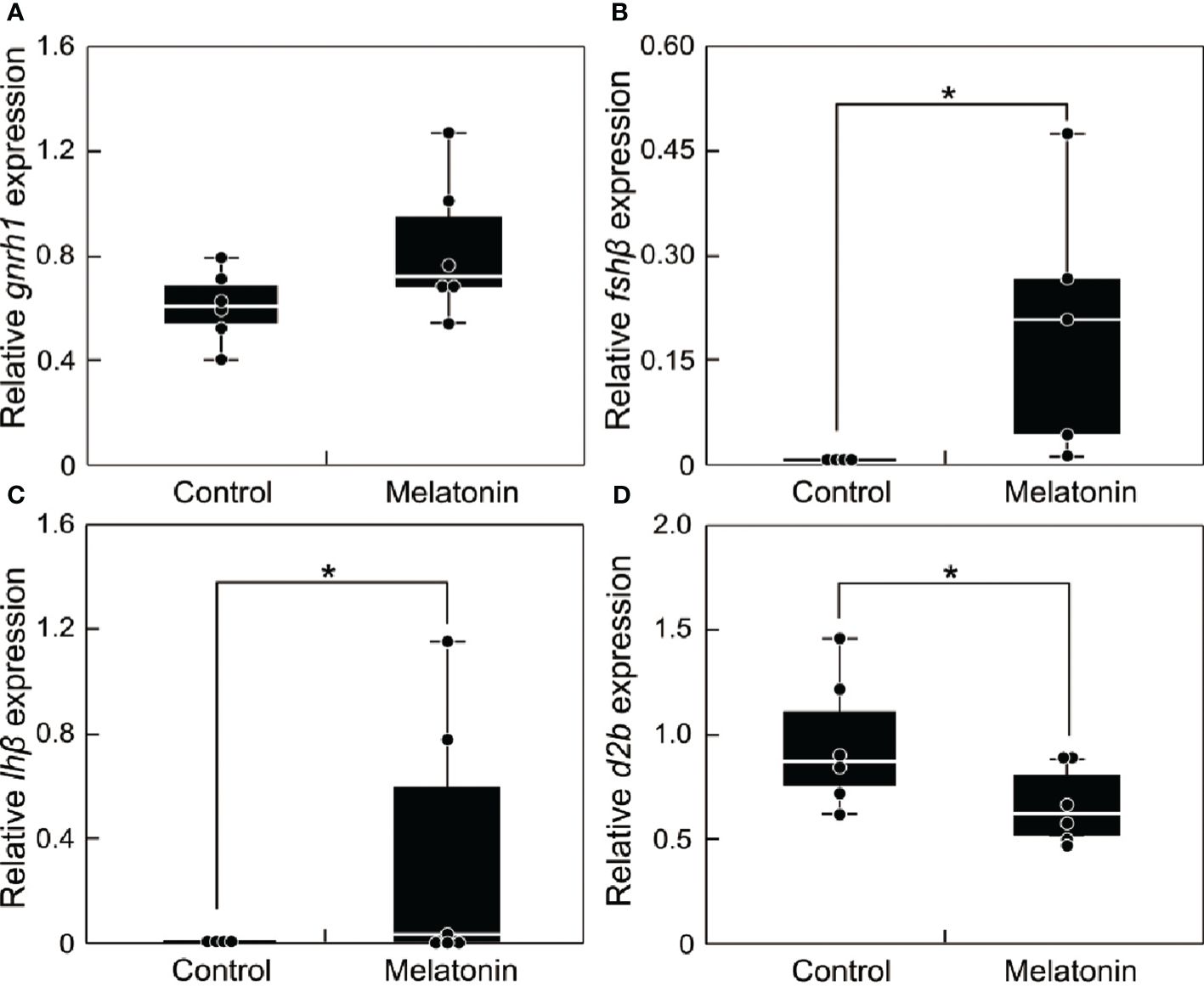
Figure 5 Effect of melatonin treatment on the mRNA abundance of reproduction-related genes in the brain of sapphire devil during the out-of-breeding season. (A) gnrh1, (B) fshb, (C) lhb, (D) d2b. Fish were immersed in seawater containing melatonin or vehicle. The transcript levels of reproduction-related genes in the brain were analyzed using qPCR and normalized against ef1α and β-actin. Each point represents mean ± standard error of the mean (SEM). Asterisks in the figure show significant difference at P < 0.05.
In situ hybridization was performed to examine d2b expression in the brains of fish immersed in seawater with or without melatonin (Figure 6). In the control group, positive signals for d2b expression were detected in the ventral part of the hypothalamus, along with the rostral pars distalis (RPD), proximal pars distalis (PPD), and pars intermedia (PI) of the pituitary gland (Figure 6A). Melatonin treatment reduced the positive signals in these regions (Figure 6B). The sense probe showed no signals in control sections (Figures 6C, D).
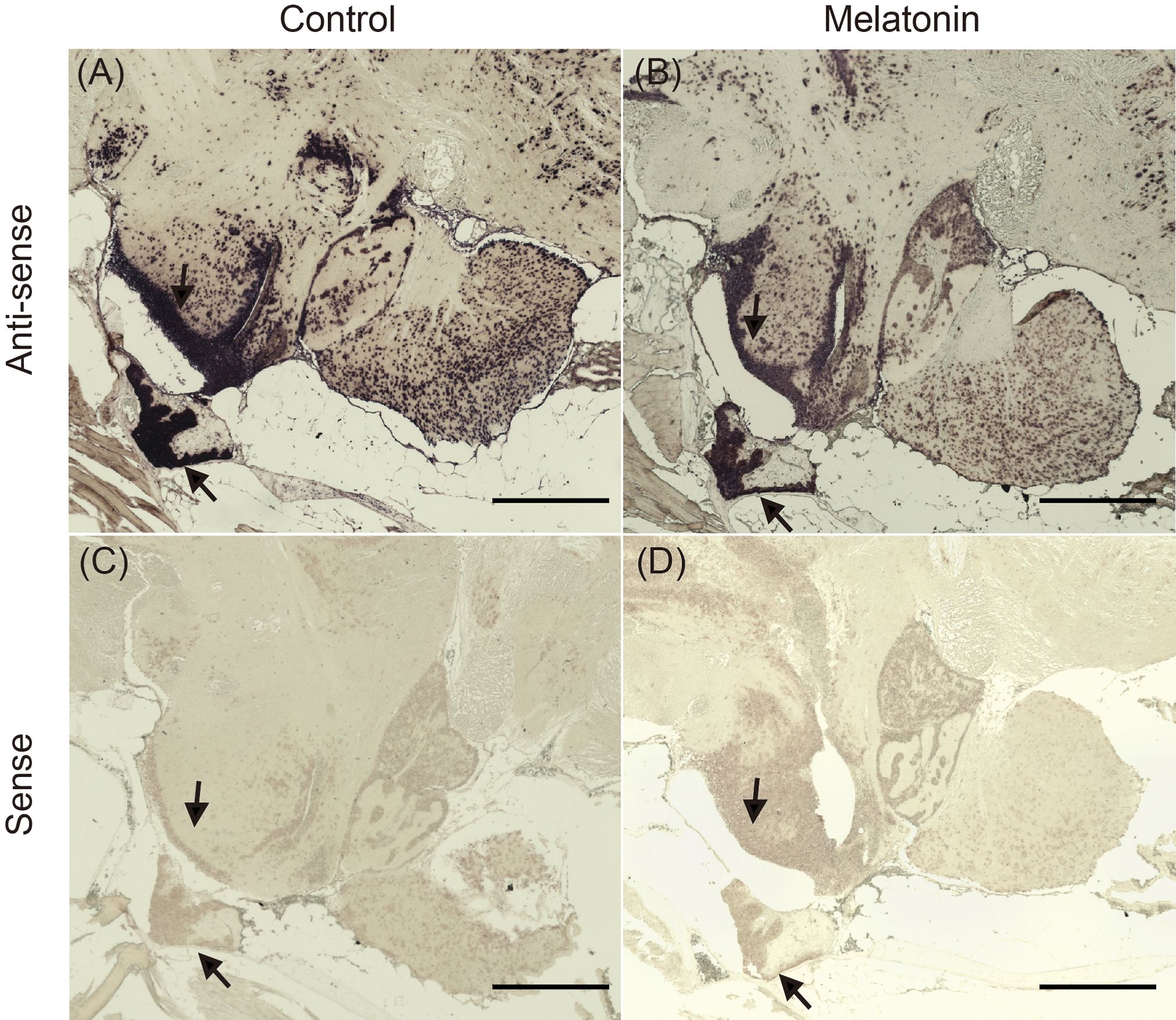
Figure 6 Localization of d2b mRNA in the brain of the sapphire devil. The whole-brain of female during the non-breeding season was fixed in 4% paraformaldehyde and subjected to processes of in-situ hybridization. (A) control (anti-sense probe), (B) melatonin treatment (anti-sense probe), (C) control (sense probe), (D) melatonin treatment (sense probe). Arrows indicate area (positive) with antisense probes. Scale bar=500 µm.
4 Discussion
In the present study, ovarian development was divided into two vitellogenic phases (EV and LV) and a post-spawning phase (PS). Histological observations showed that EV and LV had vitellogenic oocytes at PYS/SYS and SYS/TYS, respectively. These results suggested that fish at EV began vitellogenesis, whereas fish at LV actively incorporated vitellogenin into developing oocytes of their ovaries. In contrast, only immature oocytes at PNS were present in the ovaries of fish at PS, suggesting that fish at this stage have terminated vitellogenesis.
The transcript levels of aanat2 showed day-low and night-high fluctuations in the brains of mature females at EV and LV but not PS. Because aanat2 is specifically expressed in the pineal gland (Coon et al., 1999; Benyassi et al., 2000) and its mRNA abundance parallels melatonin synthesis (Saha et al., 2019), day-night differences in aanat2 transcript level are related to the regulation of vitellogenesis in this species. The direct involvement of melatonin in liver vitellogenin synthesis may be excluded because negligible expression of major melatonin receptor(s) was observed in this tissue in certain fish species (Sauzet et al., 2008; Hong et al., 2014) . Additionally, melatonin treatment failed to upregulate estrogen receptor and vitellogenin gene mRNA abundances in hepatocytes from rainbow trout (Mazurais et al., 2000). However, melatonin likely participates in the synthesis of hormones/peptides at the hypothalamic–pituitary–gonadal axis and regulates vitellogenesis because melatonin receptor gene expression and melatonin binding site localization were observed in the hypothalamus of the turbot Scophthalmus maximus (Zhao et al., 2022) and European sea bass (Bayarri et al., 2004a; Herrera-Pérez et al., 2010) , as well as the pituitary of the pike Esox Lucius (Gaildrat and Falcón, 2000). These findings were confirmed in a previous study, which revealed day-night variation in gnrh1 under the direct action of the melatonin 1 receptor during sexual maturation in the orange-spotted grouper Epinephelus coioides (Chai et al., 2013). A similar situation likely occurs in mature sapphire devil females because the transcript levels of gnrh1 increased in the brains of those sapphire devils at night. Conversely, research on the European sea bass, Dicentrarchus labrax, indicated that melatonin not only suppresses the expression of GnRH-1 but also GnRH-3 and GnRH receptors in the brain (Servili et al., 2013). This suggests that the regulatory impact of melatonin on GnRH expression may differ among species and physiological conditions. Furthermore, no day-night differences in the transcript levels of fshβ and lhβ were observed in the brains of mature females. However, considering that fshβ abundance was significantly higher at EV and LV than at PS, follicle-stimulating hormone may be involved in vitellogenesis in this species during both daytime and nighttime. Intriguingly, a previous study showed that feeding of melatonin-containing pellets (500 μg/g dry pellets) decreased the transcript levels of gnrh1 and lhβ in mature sapphire devils within 3 h (Imamura et al., 2022). This discrepancy may have been partially related to differences in experimental design; positive and negative gonadal activity impacts of melatonin have been reported in certain fish species (Fenwick, 1970; Urasaki, 1972; Sundararaj and Keshavanath, 1976; Chattoraj et al., 2005; Ghosh and Nath, 2005; Sébert et al., 2008; Carnevali et al., 2011) . In the spotted snakehead Channa punctatus, a long-day breeder, gonadal responses to exogenous melatonin varied according to time, mode, and duration of treatment. Specifically, the GSI of fish reared under long-day conditions increased with immersion treatment (100 μg/L) and decreased with injection of melatonin; the GSI of fish reared under long-day conditions increased with treatment for 24 h and decreased with treatment for a restricted period between 17:00 and 08:00 (Renuka and Joshi, 2010). Diurnal changes in melatonin receptor sensitivity may be partially responsible for the differential responses of the gonads to melatonin (Renuka and Joshi, 2010).
Dopamine receptors transmit the effects of dopamine in other physiological pathways, including reproduction (Dufour et al., 2010; Zohar et al., 2010) . In the present study, the transcript levels of d2b in the brains of mature females were significantly higher at EV and LV than at PS. Additionally, upregulation of the d2b transcript level at night was observed in the brain during LV. These results indicated that d2b is involved in vitellogenesis in sapphire devils. The hypothalamus and pituitary are possible targets of this gene product in the brain, considering that in situ hybridization showed strong signals of d2b in the ventral part of the hypothalamus, as well as the RPD, PPD, and PI of the pituitary gland (Figure 6). Dopamine has an inhibitory effect on the synthesis and release of GtHs in certain species (Dufour et al., 2010). Indeed, D2 receptors are reportedly involved in suppressing GtH secretion in female goldfish, zebrafish (Chang and Peter, 1983; Fontaine et al., 2013) . In addition, Byun et al. (2024) reported that melatonin treatment decreased d2b receptor mRNA transcription in the midbrain and increased lhβ mRNA transcription in the pituitary of sexually mature male Japanese eel. Similar inhibitory effects of dopamine have been confirmed in previous studies; pharmacological destruction of dopamine neurons with the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) resulted in decreased levels of dopamine and DOPAC[3,4-Dihydroxyphenylacetic acid], a decreased DOPAC/dopamine ratio, and increased vitellogenic oocytes in the treated fish (Badruzzaman et al., 2015). Therefore, the results of the present study suggest that the relationship between melatonin and dopaminergic signaling may be an important factor in the regulation of fish reproduction. Although our results require careful interpretation regarding the specific role of d2b in sapphire devil reproduction, it is evident that melatonin has complex effects on reproductive processes. These complex effects of melatonin may have influenced our results.
The present study showed that the transcript levels of fshβ and lhβ in the brains of immature female sapphire devils fluctuated daily, with peaks at 00:00 and 04:00, respectively. These results suggested that GtH synthesis and secretion increase at night. This daily pattern also appears to occur in mature females because nocturnal increases in the expression levels of these genes were observed at PS. Therefore, daily expression levels of these genes may vary according to reproductive status. Melatonin is clearly involved in the upregulation of fshβ and lhβ in the brains of immature females, considering that the corresponding transcript levels were significantly increased in immature females that had been immersed in melatonin-containing seawater (Figure 5). Concomitant increases in the plasma levels of melatonin and luteinizing hormone have been observed in European sea bass (Khan and Thomas, 1996; Bayarri et al., 2004b) . Melatonin reportedly affects luteinizing hormone secretion at the levels of the preoptic anterior hypothalamus and the pituitary in the Atlantic croaker Micropogonias undulatus (Khan and Thomas, 1996). Our results emphasized the involvement of dopamine in gth transcription; increases in the transcript levels of fshβ and lhβ and a decrease in the transcript level of d2b concomitantly occurred in the brains of immature females that had been immersed in melatonin-containing seawater. Additionally, in situ hybridization revealed minimal differences in the RPD, PPD, and PI of the pituitary gland between the control and melatonin treatment groups (Figure 6). Therefore, melatonin likely plays a role in GtH synthesis through decreased dopamine activity.
In conclusion, our results showed that melatonin is involved in reproduction in female damselfish C. cyanea via regulation of d2b transcript levels in the brain. Additionally, the interaction between d2b and melatonin differed among sapphire devil sexual stages . Although the sapphire devil is a diurnal species, the increase in melatonin level at night serves as a positive driver of reproduction in the immature stage of this species. Therefore, the preparation of prospective reproduction may begin during the short-day period. Further studies are needed to confirm these observations.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number (s) can be found below: https://www.ncbi.nlm.nih.gov/genbank/, accession numbers: LC011049.1 for aanat2, LC671895.1 for d2b, LC009422.1 for gnrh1, LC011047.1 for fshβ, LC011048.1 for lhβ, LC055098.1 for ef1α, LC493949.1 for β-actin.
Ethics statement
All experiments were conducted in compliance with the guidelines of the Animal Care and Use Committee of the University of the Ryukyus and regulations for the care and use of laboratory animals in Japan. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
JHB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. AM: Data curation, Methodology, Software, Writing – original draft, Writing – review & editing. DR: Data curation, Investigation, Writing – original draft. SU: Data curation, Methodology, Investigation, Writing – original draft. FK: Data curation, Investigation, Methodology, Writing – original draft. ET: Data curation, Investigation, Methodology, Writing – original draft. JK: Formal analysis, Software, Supervision, Writing – review & editing. SPH: Formal analysis, Software, Supervision, Writing – review & editing. AT: Conceptualization, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by the corroborative research (21D7000002 to AT) between University of the Ryukyus and Make-it Co. Ltd., as well as by Japan Science and Technology Agency (JST) Grant Number JPMJPF2012 to AT. Also, this work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2022R1C1C2006913) to JHB. The authors declare that this study received funding from Make-it Co. Ltd. The funder is involved in the maintenance of NAICe, which is the facility to keep our fish. Make-it Co. Ltd. was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Acknowledgments
We gratefully thank the staff of Sesoko Station, Tropical Biosphere Research Center, University of the Ryukyus, and Nakagusuku Aquaculture Innovation Center (NAICe; Contribution No. 2022–004), Okinawa, Japan, for using facilities, respectively. This study was supported in part by COI-NEXT (JPMJPF2012 to AT). The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/EVFqdA.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aizen J., Meiri I., Tzchori I., Levavi-Sivan B., Rosenfeld H. (2005). Enhancing spawning in the grey mullet (Mugil cephalus) by removal of dopaminergic inhibition. Gen. Comp. Endocrinol. 142, 212–221. doi: 10.1016/j.ygcen.2005.01.002
Amano M., Iigo M., Ikuta K., Kitamura S., Okuzawa K. (2004). Disturbance of plasma melatonin profile by high dose melatonin administration inhibits testicular maturation of precocious male masu salmon. Zool. Sci. 21, 79–85. doi: 10.2108/0289-0003(2004)21[79:DOPMPB]2.0.CO;2
Amano M., Iigo M., Ikuta K., Kitamura S., Yamada H., Yamamori K. (2000). Roles of melatonin in gonadal maturation of underyearling precocious male masu salmon. Gen. Comp. Endocrinol. 120, 190–197. doi: 10.1006/gcen.2000.7547
Badruzzaman M., Bapary M. A. J., Takemura A. (2013). Possible roles of photoperiod and melatonin in reproductive activity via changes in dopaminergic activity in the brain of a tropical damselfish, Chrysiptera cyanea. Gen. Comp. Endocrinol. 194, 240–247. doi: 10.1016/j.ygcen.2013.09.012
Badruzzaman M., Imamura S., Takeuchi Y., Ikegami T., Takemura A. (2015). Effects of neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) treatment on ovarian development of the sapphire devil, Chrysiptera cyanea. Fish Physiol. Biochem. 41, 61–71. doi: 10.1007/s10695-014-0006-9
Baekelandt S., Milla S., Cornet V., Flamion E., Ledoré Y., Redivo B., et al. (2020). Seasonal simulated photoperiods influence melatonin release and immune markers of pike perch Sander lucioperca. Sci. Rep. 10, 2650. doi: 10.1038/s41598–020-59568–1
Bapary M. A. J., Fainuulelei P., Takemura A. (2009). Environmental control of gonadal development in the tropical damselfish Chrysiptera cyanea. Mar. Biol. Res. 5, 462–469. doi: 10.1080/17451000802644722
Bapary M. A. J., Takemura A. (2010). Effect of temperature and photoperiod on the reproductive condition and performance of a tropical damselfish Chrysiptera cyanea during different phases of the reproductive season. Fisheries Sci. 76, 769–776. doi: 10.1007/s12562-010-0272-0
Bayarri M. J., Falcón J., Zanuy S., Carrillo M. (2010). Continuous light and melatonin: Daily and seasonal variations of brain binding sites and plasma concentration during the first reproductive cycle of sea bass. Gen. Comp. Endocrinol. 169, 58–64. doi: 10.1016/j.ygcen.2010.07.007
Bayarri M. J., Garcia-Allegue R., Muñoz-Cueto J., Madrid J. A., Tabata M., Sánchez-Vázquez F. J., et al. (2004a). Melatonin binding sites in the brain of European sea bass (Dicentrarchus labrax). Zool. Sci. 21, 427–434. doi: 10.2108/zsj.21.427
Bayarri M. J., Rodríguez L., Zanuy S., Madrid J. A., Kagawa H., Okuzawa K., et al. (2004b). Effect of photoperiod manipulation on the daily rhythms of melatonin and reproductive hormones in caged European sea bass (Dicentrarchus labrax). Gen. Comp. Endocrinol. 136, 72–81. doi: 10.1016/j.ygcen.2003.12.004
Benyassi A., Schwartz C., Coon S. L., Klein D. C., Falcón J. (2000). Melatonin synthesis: arylalkylamine N-acetyltransferases in trout retina and pineal organ are different. Neuroreport 11, 255–258. doi: 10.1097/00001756-200002070-00006
Burgerhout E., Lokman P. M., van den Thillart G. E. E. J. M., Dirks R. P. (2019). The time-keeping hormone melatonin: A possible key cue for puberty in freshwater eels (Anguilla spp.). Rev. Fish Biol. Fish 29, 1–21. doi: 10.1007/s11160-018-9540-3
Byun J. H., Hyeon J. Y., Hettiarachchi S. A., Udagawa S., Mahardini A., Kim J. M., et al. (2024). Effects of dopamine and melatonin treatment on the expression of the genes associated with artificially induced sexual maturation in Japanese eel, Anguilla japonica. J. Exp. Zool. A Ecol. Integr. Physiol. 341, 389–399. doi: 10.1002/jez.2788
Carnevali O., Gioacchini G., Maradonna F., Olivotto I., Migliarini B. (2011). Melatonin induces follicle maturation in Danio rerio. PloS One 6, e19978. doi: 10.1371/journal.pone.0019978
Chai K., Liu X., Zhang Y., Lin H. (2013). Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Mol. Reprod. Dev. 80, 535–548. doi: 10.1002/mrd.22191
Chang J. P., Peter R. E. (1983). Effects of dopamine on gonadotropin Release in female goldfish, Carassius auratus. Neuroendocrinology 36, 351–357. doi: 10.1159/000123480
Chattoraj A., Bhattacharyya S., Basu D., Bhattacharya S., Maitra S. K. (2005). Melatonin accelerates maturation inducing hormone (MIH): Induced oocyte maturation in carps. Gen. Comp. Endocrinol. 140, 145–150. doi: 10.1016/j.ygcen.2004.10.013
Coon S. L., Bégay V., Deurloo D., Falcón J., Klein D. C. (1999). Two arylalkylamine N-acetyltransferase genes mediate melatonin synthesis in fish. J. Biol. Chem. 274, 9076–9082. doi: 10.1074/jbc.274.13.9076
Davies B., Bromage N. (2002). The effects of fluctuating seasonal and constant water temperatures on the photoperiodic advancement of reproduction in female rainbow trout, Oncorhynchus mykiss. Aquaculture 205, 183–200. doi: 10.1016/S0044-8486(01)00665-2
Dufour S., Sebert M. E., Weltzien F. A., Rousseau K., Pasqualini C. (2010). Neuroendocrine control by dopamine of teleost reproduction. J. Fish Biol. 76, 129–160. doi: 10.1111/j.1095-8649.2009.02499.x
Falcón J., Besseau L., Sauzet S., Boeuf G. (2007). Melatonin effects on the hypothalamo–pituitary axis in fish. Trends Endocrinol. Metab. 18, 81–88. doi: 10.1016/j.tem.2007.01.002
Fenwick J. C. (1970). Demonstration and effect of melatonin in fish. Gen. Comp. Endocrinol. 14, 86–97. doi: 10.1016/0016-6480(70)90010-9
Fontaine R., Affaticati P., Yamamoto K., Jolly C. C., Bureau C., Baloche S., et al. (2013). Dopamine inhibits reproduction in female zebrafish (Danio rerio) via three pituitary D2 receptor subtypes. Endocrinology 154, 807–818. doi: 10.1210/en.2012-1759
Gaildrat P., Falcón J. (2000). Melatonin receptors in the pituitary of a teleost fish: mRNA Expression, 2-[125I]iodomelatonin binding and cyclic AMP response. Neuroendocrinology 72, 57–66. doi: 10.1159/000054571
Ganesh C. B. (2021). The stress – Reproductive axis in fish: The involvement of functional neuroanatomical systems in the brain. J. Chem. Neuroanat 112, 101904. doi: 10.1016/j.jchemneu.2020.101904
Ghosh J., Nath P. (2005). Seasonal effects of melatonin on ovary and plasma gonadotropin and vitellogenin levels in intact and pinealectomized catfish, Clarias batrachus (Linn). Indian J. Exp. Biol. 43, 224–232.
Hernández-Rauda R., Miguez J. M., Ruibal C., Aldegunde M. (2000). Effects of melatonin on dopamine metabolism in the hypothalamus and the pituitary of the rainbow trout, Oncorhynchus mykiss. J. Exp. Zool. 287, 440–444. doi: 10.1002/1097–010X(20001101)287:6<440::AID-JEZ5>3.0.CO;2-S
Herrera-Pérez P., Del Carmen Rendón M., Besseau L., Sauzet S., Falcón J., Muñoz-Cueto J. A. (2010). Melatonin receptors in the brain of the European sea bass: An in situ hybridization and autoradiographic study. J. Comp. Neurol. 518, 3495–3511. doi: 10.1002/cne.22408
Hong L. Y., Hong W. S., Zhu W. B., Shi Q., You X. X., Chen S. X. (2014). Cloning and expression of melatonin receptors in the mudskipper Boleophthalmus pectinirostris: Their role in synchronizing its semilunar spawning rhythm. Gen. Comp. Endocrinol. 195, 138–150. doi: 10.1016/j.ygcen.2013.11.004
Hoque M., Takemura A., Hoque M., Takemura A., Takano K. (1998). Annual changes in oocyte development and serum vitellogenin level in the rabbitfish Siganus canaliculatus (Park) in okinawa, southern japan. Fisheries Sci. 64, 44–51. doi: 10.1002/cne.903560105
Igarashi S., Hur S.-P., Takeuchi Y., Takemura A. (2015). Seasonal change in testicular activity of the sapphire devil, Chrysiptera cyanea, inhabiting coral reefs around Okinawa-Jima. The Biological Magazine Okinawa. 53, 1–9.
Imamura S., Hur S.-P., Takeuchi Y., Badruzzaman M., Mahardini A., Rizky D., et al. (2022). Effect of short-and long-term melatonin treatments on the reproductive activity of the tropical damselfish Chrysiptera cyanea. Fish Physiol. Biochem. 48, 253–262. doi: 10.1007/s10695-022-01051-x
Imamura S., Hur S.-P., Takeuchi Y., Bouchekioua S., Takemura A. (2017). Molecular cloning of kisspeptin receptor genes (gpr54-1 and gpr54-2) and their expression profiles in the brain of a tropical damselfish during different gonadal stages. Comp. Biochem. Physiol. -Part A: Mol. Integr. Physiol. 203, 9–16. doi: 10.1016/j.cbpa.2016.07.015
Jéhannet P., Kruijt L., Damsteegt E. L., Swinkels W., Heinsbroek L. T. N., Lokman P. M., et al. (2019). A mechanistic model for studying the initiation of anguillid vitellogenesis by comparing the European eel (Anguilla Anguilla) and the shortfinned eel (A. australis). Gen. Comp. Endocrinol. 279, 129–138. doi: 10.1016/j.ygcen.2019.02.018
Kebabian J. W., Calne D. B. (1979). Multiple receptors for dopamine. Nature 277, 93–96. doi: 10.1038/277093a0
Khan I. A., Thomas P. (1996). Melatonin influences gonadotropin II secretion in the Atlantic croaker (Micropogonias undulatus). Gen. Comp. Endocrinol. 104, 231–242. doi: 10.1006/gcen.1996.0166
Levavi-Sivan B., Biran J., Fireman E. (2006). Sex steroids are involved in the regulation of gonadotropin-releasing hormone and dopamine D2 receptors in female tilapia pituitary. Biol. Reprod. 75, 642–650. doi: 10.1095/biolreprod.106.051540
Mahardini A., Yamauchi C., Takeuchi Y., Rizky D., Takekata H., Takemura A. (2018). Changes in mRNA abundance of insulin-like growth factors in the brain and liver of a tropical damselfish, Chrysiptera cyanea, in relation to seasonal and food-manipulated reproduction. Gen. Comp. Endocrinol. 269, 112–121. doi: 10.1016/j.ygcen.2018.09.001
Mayer I., Bornestaf C., Wetterberg L., Borg B. (1997). Melatonin does not prevent long photoperiod stimulation of secondary sexual characters in the male three-spined stickleback Gasterosteus aculeatus. Gen. Comp. Endocrinol. 108, 386–394. doi: 10.1006/gcen.1997.6985
Myers R. F. (1999). Micronesian reef fishes: A comprehensive guide to the coral reef fishes of micronesia, (Barrigada, Guam: Coral Graphics).
Mazurais D., Porter M., Lethimonier C., Le Dréan G., Le Goff P., Randall C., et al. (2000). Effects of melatonin on liver estrogen receptor and vitellogenin expression in rainbow trout: An in vitro and in vivo study. Gen. Comp. Endocrinol. 118, 344–353. doi: 10.1006/gcen.2000.7472
Montero M., Vidal B., King J. A., Tramu G., Vandesande F., Dufour S., et al. (1994). Immunocytochemical localization of mammalian GnRH (gonadotropin-releasing hormone) and chicken GnRH-II in the brain of the European silver eel (Anguilla Anguilla L.). J. Chem. Neuroanat 7, 227–241. doi: 10.1016/0891-0618(94)90015-9
Mull C. G., Lowe C. G., Young K. A. (2008). Photoperiod and water temperature regulation of seasonal reproduction in male round stingrays (Urobatis halleri). Comp. Biochem. Physiol. A 151, 717–725. doi: 10.1016/j.cbpa.2008.08.029
Popek W., Łuszczek-Trojnar E., Dra̧g-Kozak E., Rza̧sa J., Epler P. (2006). Effect of melatonin on dopamine secretion in the hypothalamus of mature female common carp, Cyprinus carpio L. Acta Ichthyol. Piscat 36, 135–141. doi: 10.3750/AIP2006.36.2.07
Renuka K., Joshi B. N. (2010). Melatonin-induced changes in ovarian function in the freshwater fish Channa punctatus (Bloch) held in long days and continuous light. Gen. Comp. Endocrinol. 165, 42–46. doi: 10.1016/j.ygcen.2009.05.020
Saha S., Singh K. M., Gupta B. B. P. (2019). Melatonin synthesis and clock gene regulation in the pineal organ of teleost fish compared to mammals: Similarities and differences. Gen. Comp. Endocrinol. 279, 27–34. doi: 10.1016/j.ygcen.2018.07.010
Sauzet S., Besseau L., Herrera Perez P., Covès D., Chatain B., Peyric E., et al. (2008). Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol. 157, 186–195. doi: 10.1016/j.ygcen.2008.04.008
Sébert M.-E., Legros C., Weltzien F.-A., Malpaux B., Chemineau P., Dufour S. (2008). Melatonin activates brain dopaminergic systems in the eel with an inhibitory impact on reproductive function. J. Neuroendocrinol. 20, 917–929. doi: 10.1111/j.1365-2826.2008.01744.x
Servili A., Herrera-Pérez P., del Carmen Rendón M., Muñoz-Cueto J. A. (2013). Melatonin inhibits GnRH-1, GnRH-3 and GnRH receptor expression in the brain of the European sea bass, Dicentrarchus labrax. Int. J. Mol. Sci. 14, 7603–7616. doi: 10.3390/ijms14047603
Sundararaj B. I., Keshavanath P. (1976). Effects of melatonin and prolactin treatment on the hypophysial-ovarian system in the catfish, Heteropneustes fossilis (Bloch). Gen. Comp. Endocrinol. 29, 84–96. doi: 10.1016/0016-6480(76)90010-1
Urasaki H. (1972). Effects of restricted photoperiod and melatonin administration on gonadal weight in the Japanese killifish. J. Endocrinol. 55, 619–620. doi: 10.1677/joe.0.0550619
Vera L. M., De Oliveira C., López-Olmeda J. F., Ramos J., Mañanós E., Madrid J. A., et al. (2007). Seasonal and daily plasma melatonin rhythms and reproduction in Senegal sole kept under natural photoperiod and natural or controlled water temperature. J. Pineal. Res. 43, 50–55. doi: 10.1111/j.1600-079X.2007.00442.x
Zhao C., Xu S., Liu Y., Feng C., Xiao Y., Wang Y., et al. (2022). Changes of melatonin and its receptors in synchronizing turbot (Scophthalmus maximus) seasonal reproduction and maturation rhythm. Acta Oceanologica Sin. 41, 84–98. doi: 10.1007/s13131-021-1923-y
Keywords: arylalkylamine N-acetyltransferase, damselfish, gonadotropin, GnRH, hypothalamus, pituitary
Citation: Byun J-H, Mahardini A, Rizky D, Udagawa S, Kodai F, Tan ES, Kim J-M, Hur S-P and Takemura A (2024) Melatonin influences reproduction in the sapphire devil Chrysiptera cyanea via regulation of dopamine 2b receptor transcription. Front. Mar. Sci. 11:1412148. doi: 10.3389/fmars.2024.1412148
Received: 04 April 2024; Accepted: 27 May 2024;
Published: 11 June 2024.
Edited by:
Yung-Che Tseng, Academia Sinica, TaiwanReviewed by:
Joseph Aizen, Ruppin Academic Center, IsraelRania Fahmy Ismail, National Institute of Oceanography and Fisheries (NIOF), Egypt
Guan-Chung Wu, National Taiwan Ocean University, Taiwan
Copyright © 2024 Byun, Mahardini, Rizky, Udagawa, Kodai, Tan, Kim, Hur and Takemura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiro Takemura, dGFrZW11cmFAc2NpLnUtcnl1a3l1LmFjLmpw
†These authors have contributed equally to this work and share first authorship
 Jun-Hwan Byun
Jun-Hwan Byun Angka Mahardini
Angka Mahardini Dinda Rizky3
Dinda Rizky3 Fukunaga Kodai
Fukunaga Kodai Sung-Pyo Hur
Sung-Pyo Hur Akihiro Takemura
Akihiro Takemura