
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 24 July 2024
Sec. Marine Evolutionary Biology, Biogeography and Species Diversity
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1411111
This article is part of the Research TopicMediterranean Coastal Fish Biology and EcologyView all 6 articles
The Mediterranean Sea is a biodiversity hotspot, being home to a vast array of marine species. Furthermore, seawater warming is facilitating the arrival and spread of new thermophilic species, posing a severe threat to biodiversity. Among the species currently extending their range and increasing in abundance in Mediterranean waters, sea chubs (genus Kyphosus) are one of the most enigmatic. One challenge arises from the high phenotypic similarity between the two congeneric species documented in the basin: Kyphosus vaigiensis and Kyphosus sectatrix. Their resemblance has often led to identification challenges, resulting in incorrect or omitted species-level classifications. Therefore, despite the growing presence of these fish in the Mediterranean, it remains unclear whether only one or both species are experiencing a demographic increase and range extension. To date, there have been 26 reports of Kyphosus individuals in the Mediterranean Sea, documented in 24 separate papers. Here, we reviewed the history of the genus in the basin and provided 13 new records of these fish from multiple localities along Mediterranean coasts. In addition, we sequenced the entire mitogenomes of two specimens, assessed their phylogenetic relationships with published Kyphosus mitochondrial DNAs from around the world, and conducted detailed morphological and meristic analyses on one of them, allowing us to provide accurate species-level identifications. Our results indicate that K. vaigiensis is the species currently expanding its range in the Mediterranean Sea, while K. sectatrix is still very rare and only sporadically reported. Notably, our mitogenome data indicate that Mediterranean K. vaigiensis individuals most likely came from Atlantic waters, while there is no evidence to support an entrance through the Red Sea or any other anthropogenic vector. Finally, the potential ecological and fishing impacts associated with the proliferation of these fish in the region are discussed.
The distribution and population dynamics of many marine species are constantly varying due to climate change and anthropogenic activities. This shifting process is particularly evident in the Mediterranean Sea, where the emerging scenario is of critical concern (Chatzimentor et al., 2023). In fact, albeit the basin hosts a rich and unique biodiversity, its communities are undergoing rapid changes due to many factors, with the increase and spread of thermophilic and non-indigenous species being among the major ones (Bianchi et al., 2019; Tiralongo et al., 2020).
Among the species currently expanding in Mediterranean waters, sea chubs (Kyphosidae) are one of the most intriguing taxonomic groups. In fact, due to the high phenotypic similarities between some Kyphosus species, their identification is often problematic, and involves the assessment of several characters, including counting of soft rays in the anal and dorsal fins, scale rows along the body, gill rakers, as well as the evaluation of colour patterns, relative body proportions, and relative size and placement of fins (Knudsen and Clements, 2013). Despite numerous taxonomic studies and reviews attempting to establish a clear and unique classification for the genus (Sakai and Nakabo, 1995, 2004, 2006; Knudsen and Clements, 2013, 2016), conflicting results persist, leaving the taxonomy of the genus very elusive.
From an ecological perspective, sea chubs are strictly herbivorous fishes, feeding primarily on macroalgae, on both temperate and tropical reef areas. In some areas such as Western Australia, they represent a large proportion of coastal fishes, considerably impacting algal biomass (Knudsen and Clements, 2016). Sea chubs are also considered highly valued as food, being generally caught with handline, gillnet and spear (Sakai, 2003). However, the fishing statistics for species of the Kyphosidae family are generally included in broader fisheries reports rather than being reported separately.
In the Mediterranean Sea, two species belonging to the genus Kyphosus have been reported so far: Kyphosus sectatrix (Linnaeus, 1758) and Kyphosus vaigiensis (Quoy & Gaimard, 1825). In European Atlantic waters both species are present, but K. vaigiensis seems to be the most common, probably due to its greater mobility (Bañón and de Carlos, 2022). These two taxa have often been reported under different names, later recognised as junior synonyms or misspelled names (Knudsen and Clements, 2013, 2016); for example, K. incisor for K. vaigiensis (Orsi Relini et al., 2010; Azzurro et al., 2013; Bilecenoglu et al., 2013), or K. sectator for K. sectatrix (Šoljan, 1963; Merella et al., 1998; Hemida et al., 2004; Francour and Mouine, 2008). Besides, specimens have often been misidentified; for example, original observations of K. sectatrix have later been attributed to K. vaigiensis (Ligas et al., 2011 and Elbaraasi et al., 2013; Knudsen and Clements, 2013 and Goren et al., 2016).
Table 1 lists the 26 Kyphosus spp. records reported in Mediterranean waters so far, with details on the date of observation, locality, number of specimens, size, depth, and fishing gear. Despite the wealth of literature, most articles report sightings of a single individual, and all are referred to a single locality. Overall, the large majority of sea chub observations are attributed to K. vaigiensis. One study also proposed the presence of a third species (Knudsen and Clements, 2013): the authors indicated that the (alleged) K. sectatrix specimen from Palermo (Italy) reported by Doderlein (1883) could be Kyphosus bigibbus Lacepède, 1801. The number of scales on the lateral line of this specimen (80) slightly falls outside the variability of both K. bigibbus and K. sectatrix, while the other morphometric and morphological characteristics are coherent with both (Doderlein, 1883; Knudsen and Clements, 2013). However, the same study refers to K. bigibbus as “not known from the Mediterranean”, leaving doubts about the status of this specimen. Nevertheless, a later study on the same specimen, preserved at the Museum of Zoology, University of Palermo, refers to it as K. sectatrix (Lo Brutto, 2017). To date, no concrete evidence has been provided about the presence of a third Kyphosus species in the Mediterranean Sea.
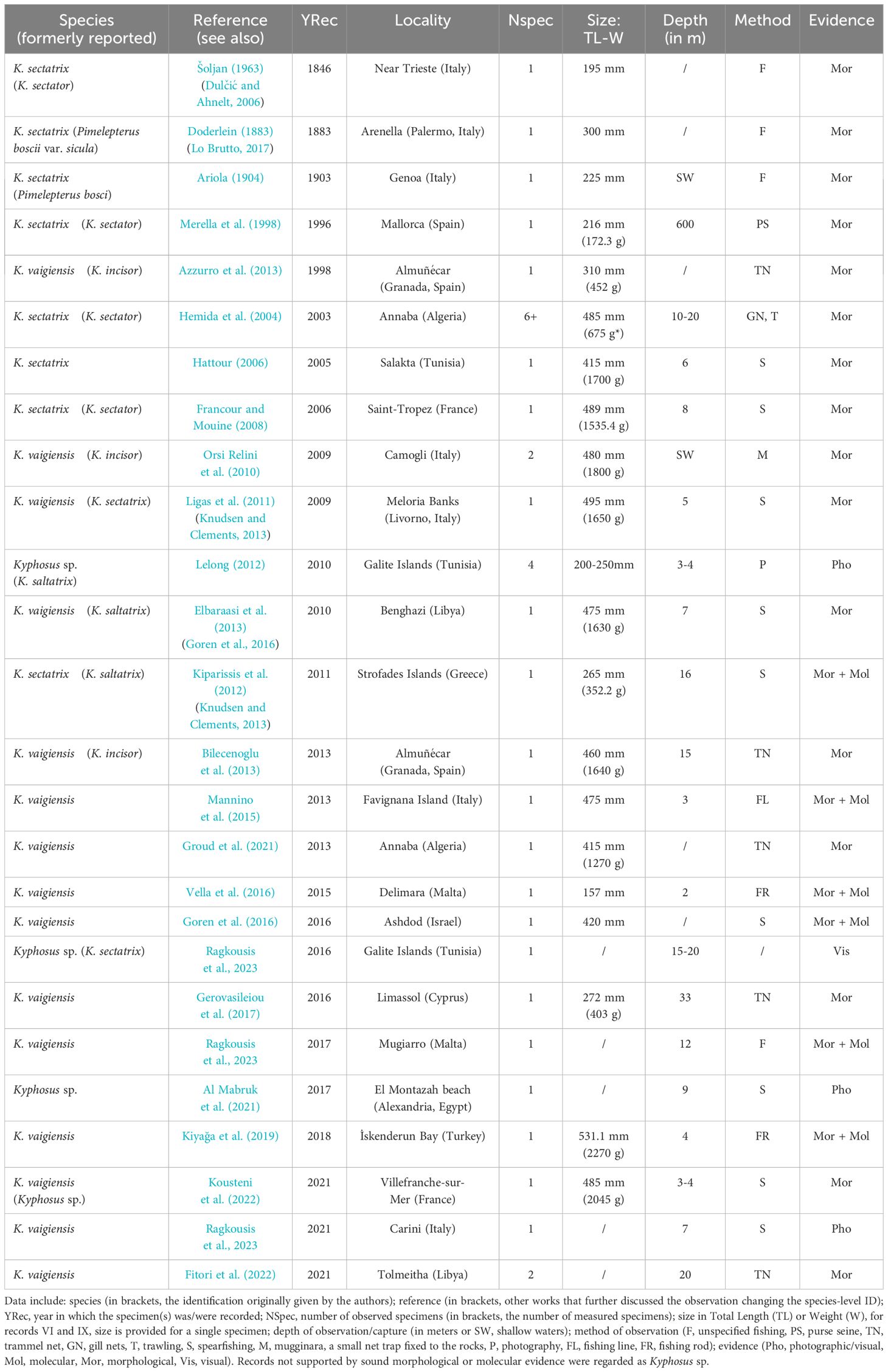
Table 1 Previous records of Kyphosus species in the Mediterranean Sea, and associated relevant data (updated up to February 2024).
In addition to morphometric and morphological studies, molecular analyses of mitochondrial DNA (mtDNA) have been also employed to study Kyphosus species. All of these studies have assessed mtDNA sequence variation at the level of a single gene or a few genes: cytochrome oxidase subunit 1 (COI), cytochrome b, and ND2 genes, as well as 12S and 16S RNA genes (Yagishita et al., 2002; Kiparissis et al., 2012; Mannino et al., 2015; Vella et al., 2016). These genes have often been used as useful barcoding regions to identify fish species, particularly the COI gene (Ardura et al., 2013; Mohanty et al., 2015; Bingpeng et al., 2018). However, while this multi-gene approach allows comparisons across multiple mtDNA regions, it also makes it difficult to compare published data from specimens of diverse global populations.
In this work, we present additional records of Kyphosus specimens from numerous localities along the Mediterranean coasts, with related relevant data on the species, date, locality, size, depth, and technique of observation. Moreover, to facilitate comprehensive mtDNA comparisons with previously published data, we completely sequenced the mitogenome of two of the Kyphosus specimens. This multidisciplinary approach allowed us to perform a species-level identification of reported individuals, to document a large-scale increase of the species Kyphosus vaigiensis in Mediterranean Sea, to define that Mediterranean K. vaigiensis specimens are of Atlantic origin and to compare the Mediterranean emerging scenario with the one earlier proposed for Galician (Atlantic) waters (Bañón and de Carlos, 2022).
Our new records were collected during the AlienFish campaign (specimens I-XIII, Table 2). The AlienFish project was launched in 2012, aiming to monitor the distribution of rare, thermophilic, and non-indigenous fish species along the Mediterranean coasts. The initiative relies on contributions from citizens, who often use social networks to provide interesting and otherwise unavailable information (Tiralongo et al., 2020). As the project is based in Italy, most of the new records here reported were collected through Italian Facebook groups, including both marine biology and fishing groups. In particular, we used these groups to search for photos and videos of Kyphosus species, and hence contacted the authors to ask information about the observation, and the permission to use the published photographic material. With this approach data about the date, locality, number of specimens, size, depth and fishing technique were gathered (Table 2).
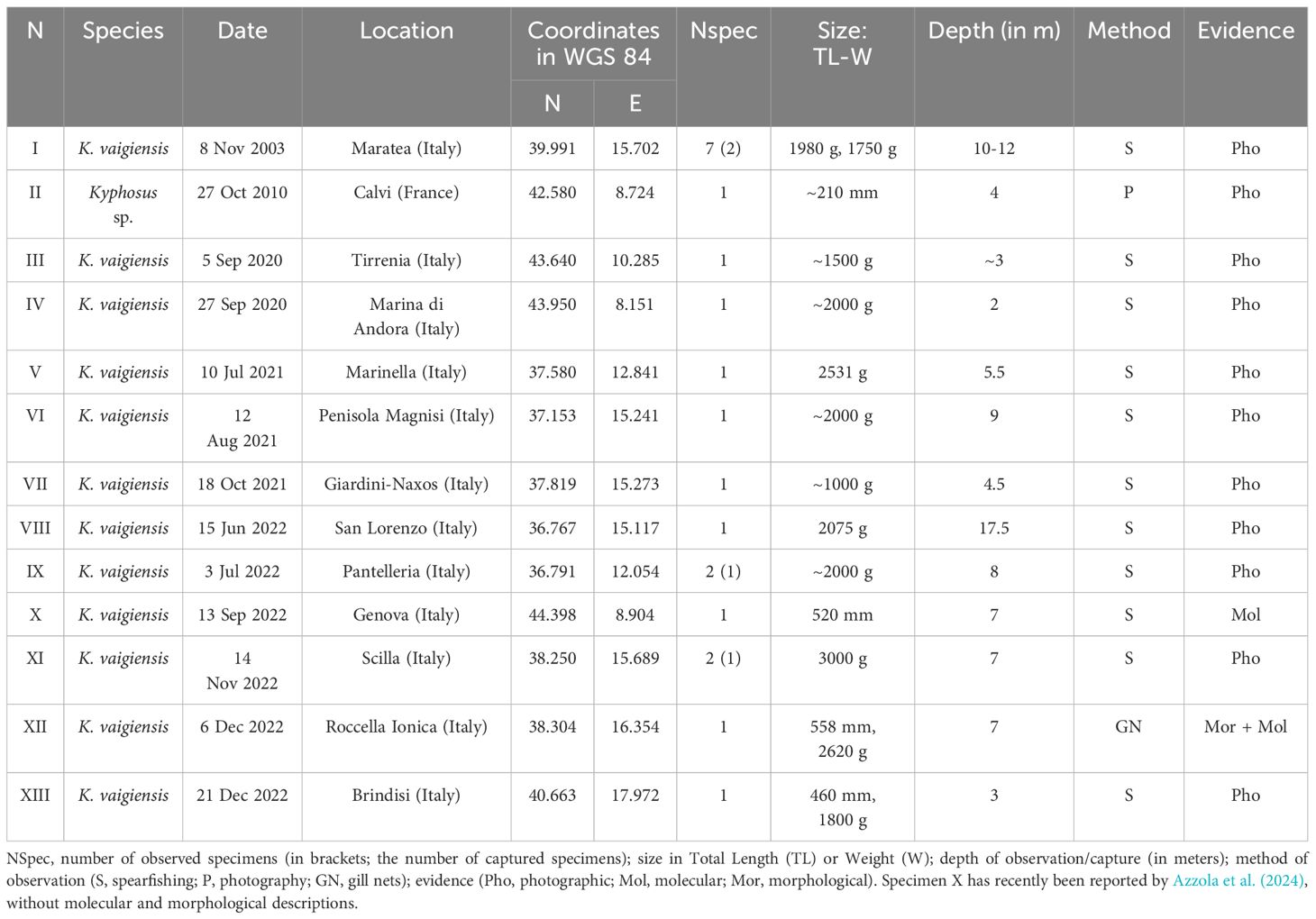
Table 2 New records of Kyphosus specimens in the Mediterranean Sea reported in this study, with associated relevant data.
One specimen (specimen XII) was physically collected and examined by the authors. It was weighed to the nearest 1 g and subjected to an accurate morphological and meristic analyses. Sex determination was performed through macroscopic analysis of the mature gonads present in specimen XII. Stomach content was also inspected. Part of another fish (specimen X) was kept frozen by the fisherman and later collected and analysed by the authors; morphological measurements were collected as well for this fish. Molecular analyses, including DNA extraction and whole mitogenome sequencing, were conducted for both specimens X and XII.
Small portions of the fish were stored in absolute alcohol. Using clean tweezers, the tissue was picked, washed with Milli-Q® water, and transferred to a 1.5 mL tube. Samples were then pestled and DNA was extracted using phenol-chloroform as in Lombardo et al. (2022).
We designed two primer pairs on the basis of Kyphosus mtDNA sequences available in GenBank to amplify two partially overlapping amplicons covering the entire mitogenome. Primer: (i) forward: 5’-TTGTTCACTGATTCCCGCTGT-3’, reverse: 5’-ATTGTGTGAGGGGTCGGAATG-3’; (ii) forward: 5’-CACCTGCCAACCCACTAGTAAC-3’, reverse: 5’-TGTAGGCGTCTGGGTAGTCTG-3’. PCR reactions were performed in a volume of 25 μL, containing 12.5 µL of GoTaq® Long PCR Master Mix (Promega, USA), 0.75 µL of each primer (final concentration of 0.3 µM), 1 µL of template DNA (107 ng/µL for specimen X, 64 ng/µL for specimen XII) and 10 µL of nuclease-free water. DNA amplification started with 2 min of initial denaturation (94°C) followed by 30 cycles of: 30 sec at 94°C, 30 sec at 57°C, 9 min at 65°C; at the end, a final extension cycle of 10 min at 72°C was performed. 3 uL of the amplification products were checked by 1% agarose gel electrophoresis and ethidium bromide staining.
Amplified mtDNA amplicons were purified using QIAquick spin columns (Qiagen, Redwood City, CA, USA) and then quantified by the Quantus™ fluorometer (Promega) using the QuantiFluor® ONE dsDNA system. About 50 nanograms for each of the two amplicons were combined for library preparation.
The two complete mitogenome sequences were obtained by Next Generation Sequencing on a MiSeq Illumina platform. Nextera™ DNA Flex Library Prep was used to prepare the sequencing libraries, following the steps described in the manufacturer’s protocol: tagmentation of input DNA, amplification of tagmented DNA with the addition of premixed dual-indexed adapters (Nextera™ DNA CD Indexes), and PCR clean-up. Libraries were then quantified by the Qubit 4 Fluorometer (Thermo Fisher). We then run the pooled normalized library on the 4150 TapeStation System (Agilent) and diluted to 4nM with RSB resuspension buffer. Five microliters of pooled libraries were denatured with 5 μl of NaOH (0.2N), diluted to the loading concentration of 6 pM (600 μl final volume) using HT1 hybridization buffer and sequenced on a MiSeq system (Illumina) using paired-end sequencing with a MiSeq Reagent Kit v. 2 (2 × 250 cycles).
The raw MiSeq sequencer data were output in BCL format, demultiplexed, and converted to FASTQ format with the Illumina® bash package, bcl2fastq2 Conversion Software v2.20. FASTQ files were analysed with Geneious v. 8.1.9 (https://www.geneious.com) and initially aligned to a published mitogenome of K. vaigiensis (GenBank accession number OP035071). This alignment allowed us to export the consensus of sample number X (Table 2), which was then employed as the reference sequence in this study. Variant calling was performed by setting the threshold for heteroplasmies at 30% of reads and considering only mutations in the range 30%–70% as heteroplasmic in phylogenetic analyses. The two mitogenomes were completely sequenced with an average depth of 9481X and 11166X for samples X and XII, respectively. Finally, mitogenome sequences were exported in the standard FASTA format and deposited in GenBank (accession numbers: PP178641 for specimen X, PP178642 for specimen XII).
In order to assess mtDNA affinities and ancestral sources of the two sequenced fish, previously published COI sequences (614 bp from nucleotide position (np) 5564 to np 6177) belonging to the species K. vaigiensis were downloaded from GenBank and added to our database to build a phylogenetic tree including worldwide specimens. Sequences labeled as K. incisor were included as well due to their synonymy with K. vaigiensis (Knudsen and Clements, 2013), also verified in this article. Besides, we also included, when available, one COI sequence for other Kyphosus species to assess the relationships of K. vaigiensis with its congeneric counterparts. The choice of the COI gene reflects the wealth of available sequences in GenBank.
Sequences were aligned with Sequencher v. 4.9 (Sequencher® version 4.9 DNA sequence analysis software, Gene Codes Corporation, Ann Arbor, MI USA), and a maximum parsimony tree was built manually, indicating nucleotide positions that differed among mtDNAs, with respect to the newly established K. vaigiensis reference sequence (specimen X). The same dataset of COI sequences used to build the MP tree was employed for the construction of a Median-Joining network with the software Network 10.2.0.0 (www.fluxusengineering.com).
We also constructed a maximum parsimony (MP) tree with the whole coding-region of five K. vaigiensis mitogenomes - the two that we sequenced and three previously published (GenBank accession numbers OP057015, OP035071 and OP035143). Their control-region sequences (876 base pairs from np 15668 to np 16543) were excluded from this tree, similarly to previous mitogenome studies of other animal species (Battaglia et al., 2022; Lombardo et al., 2022) due to the particularly high mutation rate of mtDNA control regions, which often limits their phylogenetic informativeness. The Kyphosus cinerascens (Forsskål, 1775) complete mitogenome (GenBank accession number NC_013138) was employed as outgroup.
Thirteen new records were collected (I-XIII, Table 2), involving a total of 21 specimens, captured or spotted from 2003 to 2022. Interestingly, despite the AlienFish campaign is still ongoing, no Kyphosus record was collected during 2023. As most of the exploited Facebook groups were Italian, the majority of records came from Italy (12 records). At the same time, we also report one record from a French locality (specimen II), dating back to 2010. Figure 1 shows the geographical source of each new and previous record on a Mediterranean scale, while specific information about each new observation is provided in Table 2.
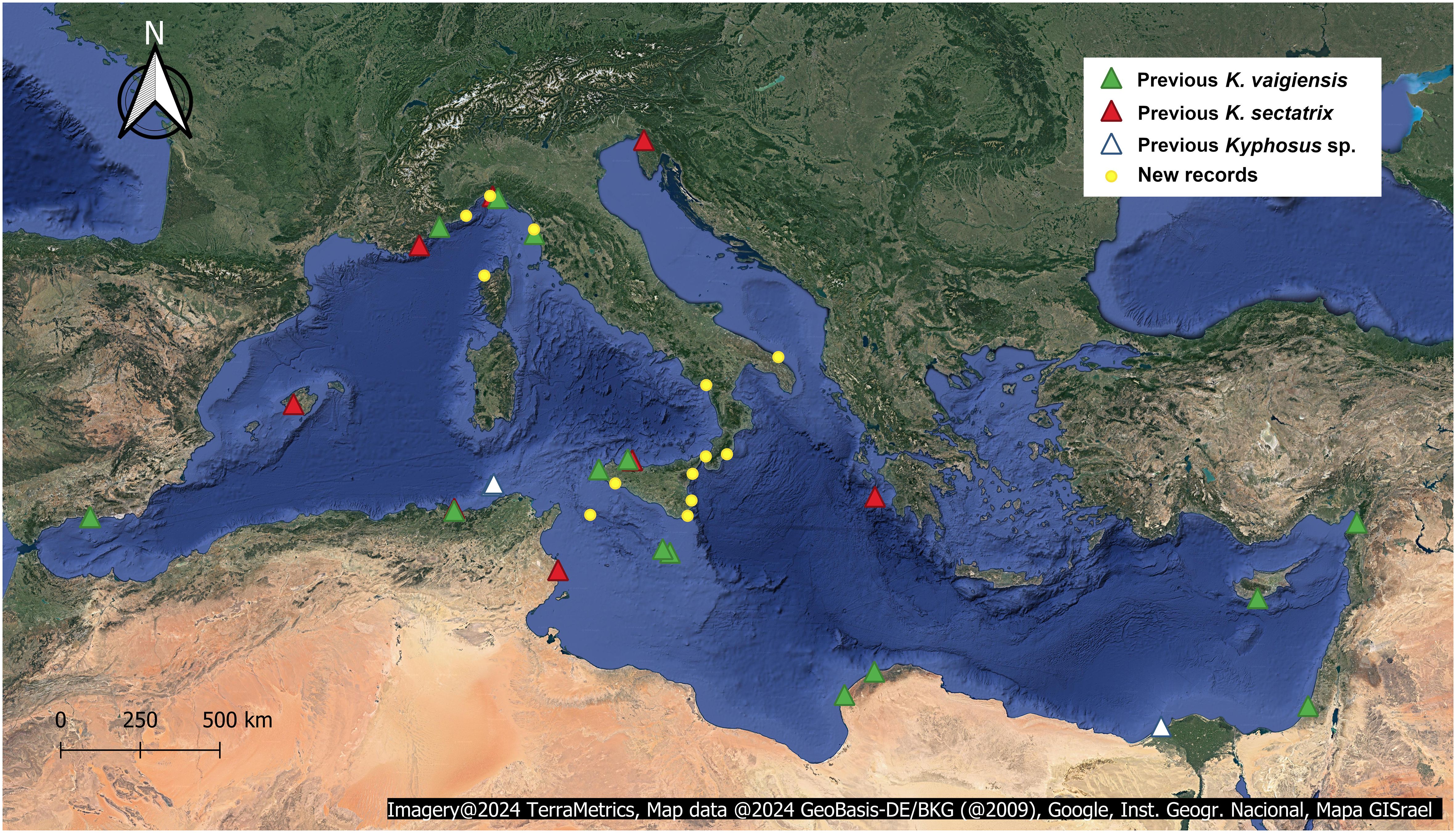
Figure 1 Previous records of K. vaigiensis (green triangle) and K. sectatrix (red triangle), and new Kyphosus records hereby reported (yellow dot). We considered as “Kyphosus sp.” the records reported by Lelong (2012) and Al Mabruk et al. (2021) (white triangle), since the provided photographic material is not adequate to allow species-level identifications. Map in Spherical Mercator (EPSG:3857).
The clear horizontal golden rows observable in almost all our specimens (examples in Figure 2) indicate their belonging to the species K. vaigiensis (Knudsen and Clements, 2013).

Figure 2 Seven of our reported Kyphosus specimens: (A) record I; (B) record VII; (C) record VIII; (D) record IX; (E) record X; (F) record XIII. The golden horizontal rows that are typical of K. vaigiensis are evident in the photos.
Morphological and meristic traits measured for specimens X and XII (Table 3) also corroborate the classification as K. vaigiensis (Figure 3). The fish showed a head profile that was gently convex in front of the eye, 14 dorsal rays, 12 anal rays, 9 and 23 gill rakers on the upper and lower limbs of the first gill arch, respectively; it had an eviscerated weight of 2400 g (vs. 2620 g of full total weight). Furthermore, the analysis of the stomach content revealed the presence of 80 g of algal material. Macroscopic analysis of gonads indicated that it was a mature male.
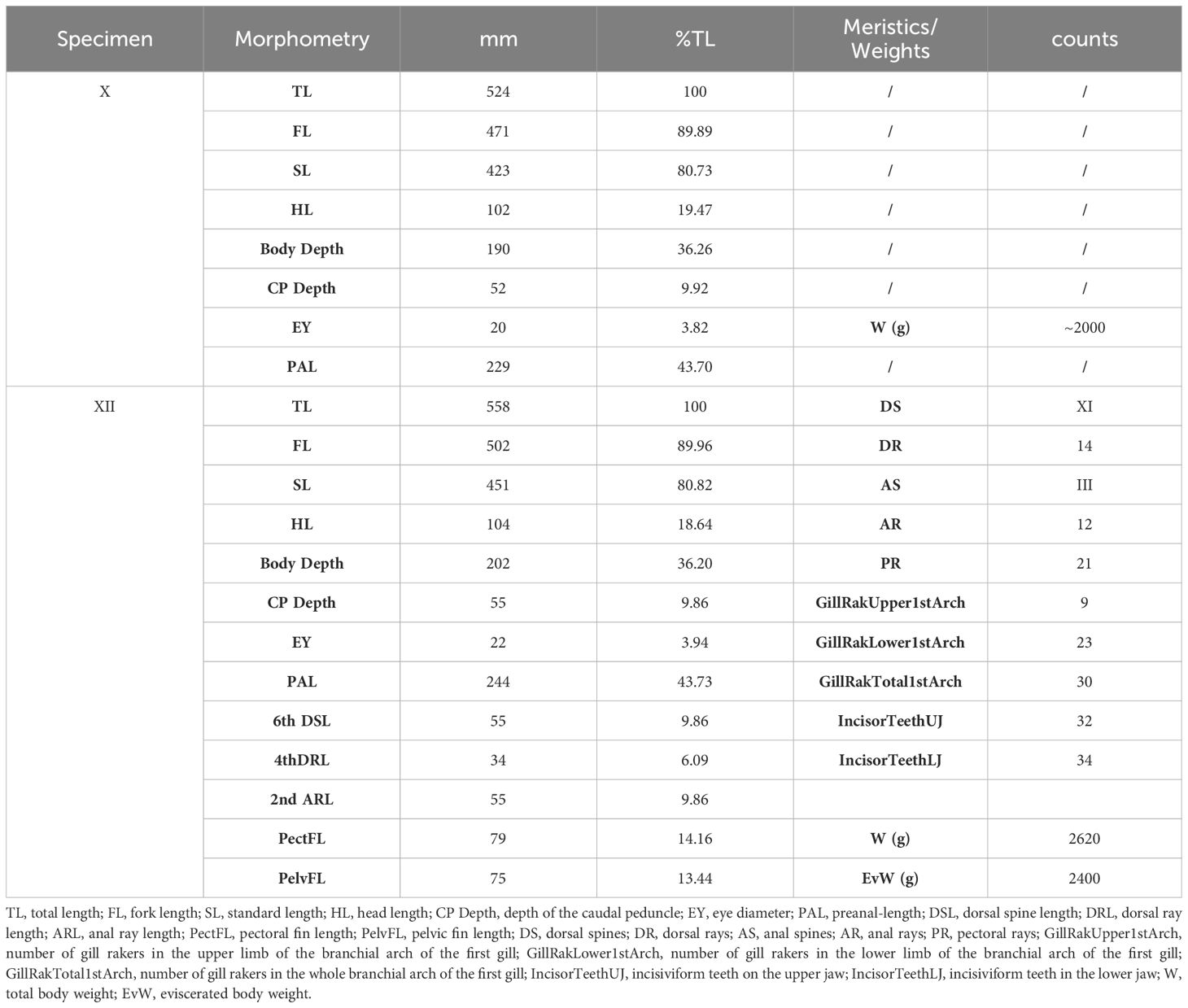
Table 3 Morphometric and meristic data collected on specimen X and XII caught at Genoa (Italy, Ligurian Sea) in September 2022 and at Roccella Ionica (Italy, Ionian Sea) in December 2022.
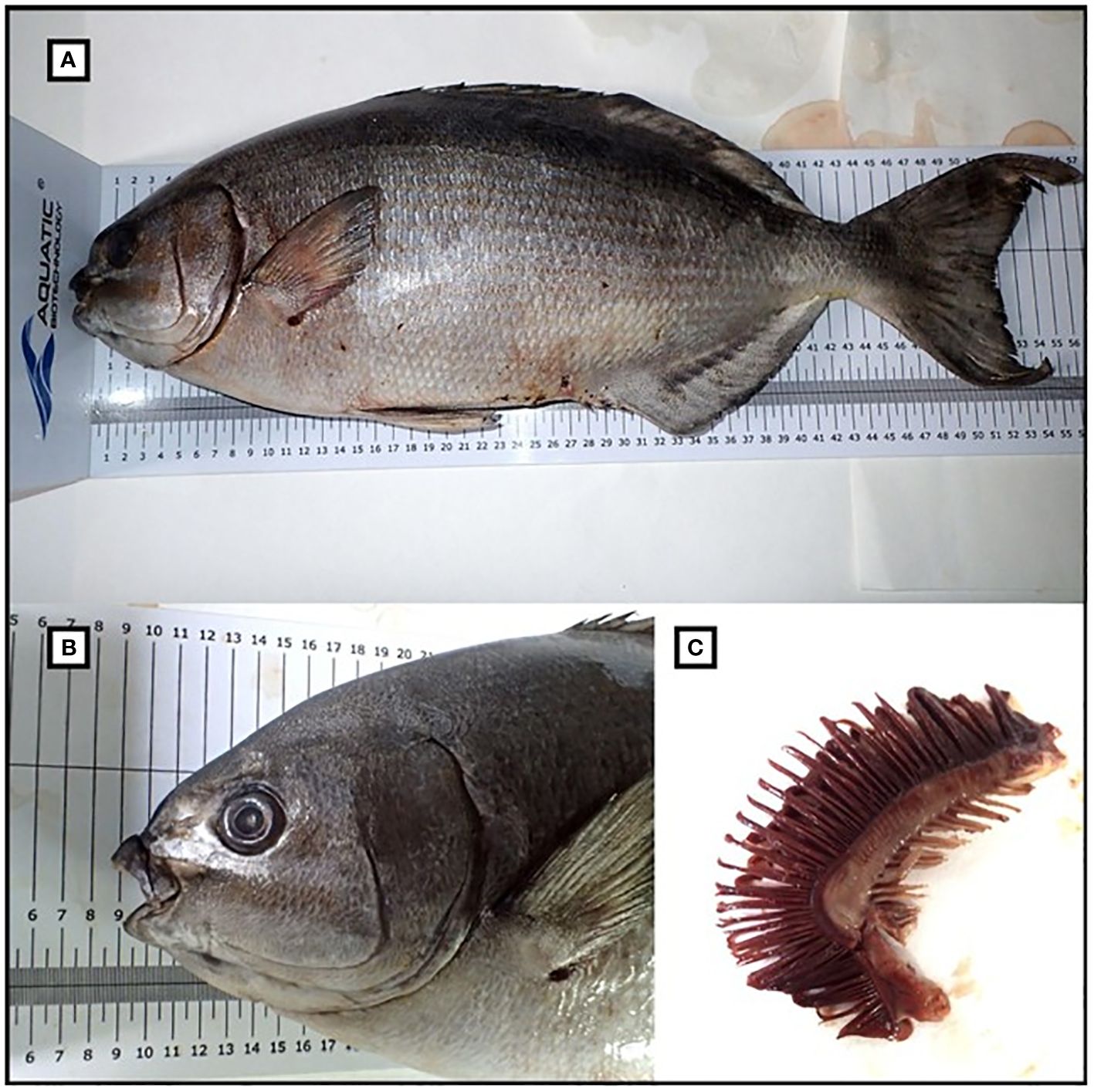
Figure 3 (A) Kyphosus vaigiensis specimen XII caught at Roccella Ionica (Italy, Ionian Sea) on December 2022; (B) particular of the head; (C) first gill arch with gill filaments (left side on the photo) and gill rakers (right side).
The MP tree of COI sequences reported in Figure 4 and the network in Supplementary Figure 1 confirm that K. vaigiensis mtDNAs share nine distinctive COI transitions and form a well defined species-specific branch. However, it should be also noted that two mtDNAs from Japan (n. 44, JF952771) and Myanmar (n. 45, MH235651) cluster in the K. cinerascens branch despite their postulated classification as K. vaigiensis. As shown in the tree, K. cinerascens is the closest congeneric species to K. vaigiensis, with sequence divergence values for different mtDNA regions in the range 2.5% - 3.0% (Table 4), which are close to the postulated interspecies COI thresholds for fish species (Ward et al., 2009). In contrast, K. sectatrix, the other Mediterranean species, is most closely related to other species (K. hawaiiensis and K. bigibbus).

Figure 4 Unrooted MP phylogenetic tree based on a segment of the COI gene sequence (614 bp from np 5564 to np 6177). The tree encompasses 51 specimens and was built using sample 1 (specimen X, GenBank accession number PP178641) as reference sequence. Mutations are shown on the branches; they are transitions unless a base is explicitly indicated. Recurrent mutations within the phylogeny are underlined, while retromutations are indicated with @. Samples present also in Figure 5 are thickly circled. GenBank accession numbers, localities and authors are provided for each sample in Supplementary Materials (Supplementary Table S1). Two sequences (KJ632827 and KJ632831) were excluded from the tree due to the presence of numerous transversions and uncertain geographic origin.
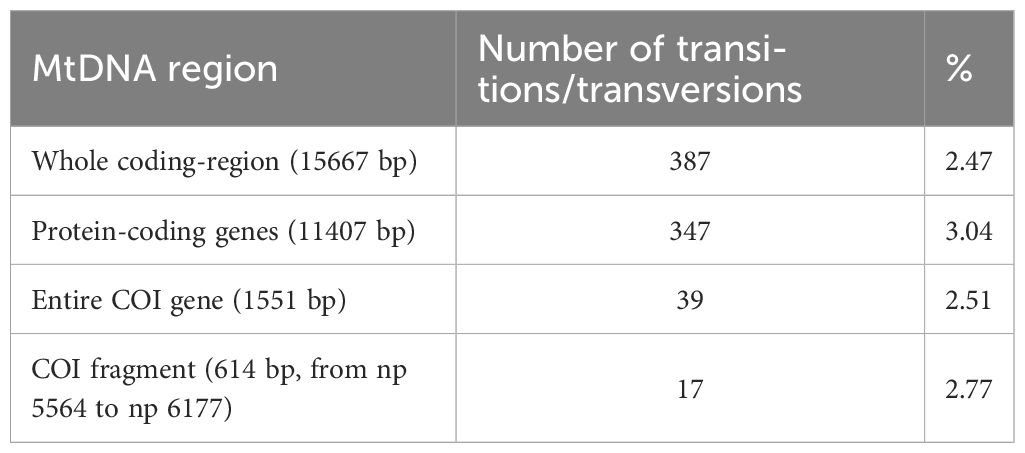
Table 4 Sequence divergence between K. vaigiensis (specimen X) and K. cinerascens (GenBank accession number NC_013138).
The COI sequences of our specimens X and XII are members of the K. vaigiensis branch, thus fully supporting morphological and meristic evidence. The COI sequence from specimen XII (reported as n. 33 in Figure 4) is identical to two K. vaigiensis sequences (n. 31-32) from Galician waters (Bañón and de Carlos, 2022) and belongs to a branch, defined by the transition at np 5919, which also includes mtDNAs (n. 29-30) from Brazil, thus indicating a likely Atlantic ancestral source for specimen XII (Figure 4). In contrast, the sequence of the specimen X (n. 1 in Figure 4) showed 100% identity with 25 previously published sequences, including not only the Mediterranean specimen (n. 22) reported by Mannino et al. (2015) in Sicily and specimens from European (n. 15-20) and American Atlantic waters (n. 8-9, 23-26), but also specimens from the Red Sea (n. 4) and many other extremely distant locations (South Africa, South East Asia, Polynesia, Hawaii) (Supplementary Table S1).
The phylogenetic relationships of Kyphosus mtDNAs were also assessed at a much higher level of molecular and phylogenetic resolution by comparing whole mtDNA coding regions (15667 bp) (Figure 5). The MP tree reported in Figure 5 is much more informative than the one in Figure 4, albeit including only five mtDNAs (n. 1-2, 24, 27 and 33). Each of the five mitogenomes bears a distinct haplotype, clustering into two well-defined haplogroups that we termed Kv1 and Kv2. The latter includes two Hawaiian mitogenomes (n. 2 and 27), while haplogroup Kv1 encompasses our two rather divergent (22 transitions/transversions) Mediterranean mitogenomes (n. 1 and 33) together with mitogenome n. 24, which is from the Atlantic (Belize) (Figure 5), thus further supporting the Atlantic Ocean as ancestral source for the Mediterranean K. vaigiensis population.
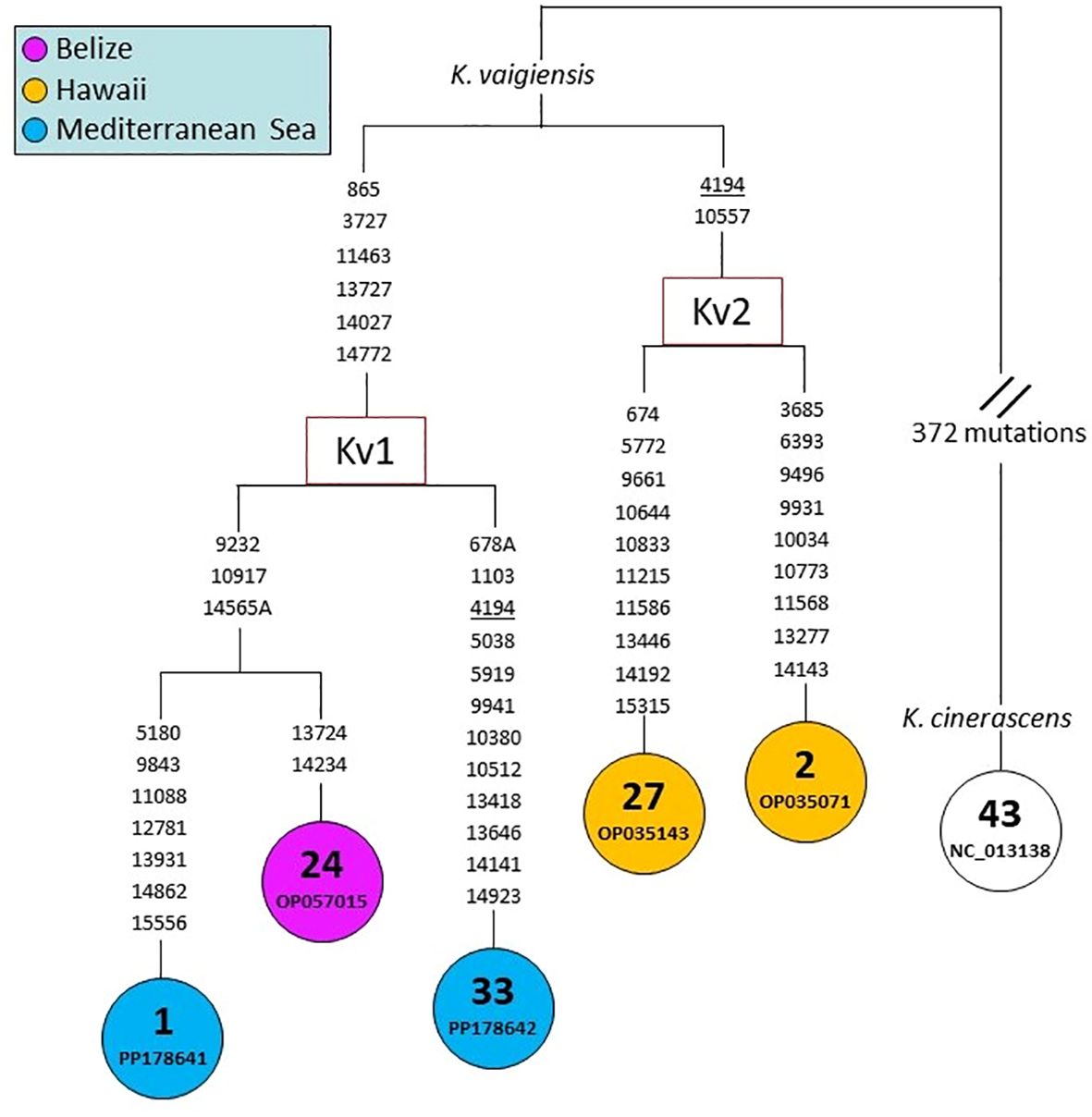
Figure 5 MP tree based on the whole mtDNA coding-region (15667 bp). This tree was built using the mitogenome sequence of specimen X (n. 1 in this figure) as reference sequence (GenBank accession number PP178641). MtDNA numbers correspond to those in Figure 4. The mtDNA of K. cinerascens (n. 43) was employed as outgroup. Mutations are shown on the branches; they are transitions unless a base is explicitly indicated. Recurrent mutations within the phylogeny are underlined. The GeneBank accession number is reported for each sample. Additional information for the samples is provided in Supplementary Materials (Supplementary Table S1).
The recent expansion of sea chubs in Mediterranean waters has posed several challenges in accurately identifyingspecies due to the high morphological similarity between K. vaigiensis and K. sectatrix (Knudsen and Clements, 2013), and has prevented authors from giving species-specific identifications (Kousteni et al., 2022). At the same time, several specimens have been misidentified or reported under a synonymous name (see Table 1). Our data, based on a combined approach encompassing citizen science, morphological and molecular methods, appear to fully address this issue and might represent a valuable approach for other studies concerning similar and difficult-to-identify species.
As for our morphometric and morphological data, an important character, very useful for visual identification from photos, is the presence of yellow/golden horizontal rows along the sides of K. vaigiensis (Knudsen and Clements, 2013), well visible in fresh and sometimes in preserved specimens and not present in adults of the congeneric K. sectatrix. Specimen II (Table 2) has been photographed underwater and the apparent absence of the horizontal golden rows casts doubt on its species-level identification: were these rows missing or simply not visible in that photo due to the particular exposure, angle, and brightness? Similarly, for specimen VI, we only have a frame of a spearfishing video, where the golden rows are not appreciable. In our opinion, this is likely due to the lack of definition of the image, and to a general difficulty in observing the rows in underwater photos. Indeed, this specimen was spotted in Sicily in 2021, and all the other specimens spotted in the same region and period correspond to K. vaigiensis.
Therefore, there is no concrete morphometric/morphological evidence to support the presence of K. sectatrix among our records, and it is highly likely that all specimens here reported correspond to K. vaigiensis.
The recent record in the bay of Villefranche-sur-Mer, reported as “Kyphosus sp.” (Kousteni et al., 2022), is probably referred to K. vaigiensis as well. In fact, morphometric and meristic counts match the variability of the species observed by other authors (Knudsen and Clements, 2013; Mannino et al., 2015; present study). Besides, the clear yellow/golden rows present in the original figure (Kousteni et al., 2022) further support this hypothesis (Knudsen and Clements, 2013).
As for our mtDNA data, the two sequences from Japan (n. 44, JF952771) and Myanmar (n. 45, MH235651) labeled as K. vaigiensis, but clustering within the K. cinerascens clade in the COI-based phylogeny (Figure 4), could result from an event of hybridization between the two species, as suggested by other authors (Bañón et al., 2017); K. vaigiensis and K. cinerascens are indeed genetically very close to each other (Table 4). However, to the best of our knowledge, no study has ever evaluated the presence of hybridization between the two species. The COI-based tree shows a general lack of geographic clustering for K. vaigiensis populations. Indeed 26 out of the 42 mtDNA sequences (~62%) in the K. vaigiensis branch, including the Mediterranean specimen X, are represented by the same COI haplotype, despite the geographical sources of the specimens encompass all oceans (Atlantic, Pacific and Indian Ocean). One exception is represented by a branch (defined by the transition at np 5919) that includes five mtDNAs, four from Atlantic sources, plus one from the Mediterranean (our specimen XII), thus suggesting a Gibraltar entry route for the latter. A general lack of genetic structure of K. vaigiensis mtDNA sequences from all over the world has been also reported in previous studies (Vella et al., 2016). This might be interpreted as supportive of the notion that K. vaigiensis covers very long distance migrations (Sakihara et al., 2015). However, it most likely indicates that, as previously shown for many other animal species, the assessment of only short segments of mtDNA does not permit the identification of structure at the population level and the understanding of a species’ origin and spread (Lombardo et al., 2022).
The latter scenario is supported by our entire coding-region tree, despite the fact that it encompasses only five mitogenome sequences. MtDNAs n. 1 and 33 differ for only one mutation in the COI phylogeny (Figure 4), but are 22 mutations apart in the coding-region phylogeny (Figure 5). Moreover, the two Mediterranean mitogenomes cluster together in the same haplogroup (Kv1) with a Belize specimen, while the two from Hawaii form a distinct sister haplogroup (Kv2), further suggesting an Atlantic origin of Mediterranean sea chubs. An Atlantic source fits also with the timing of the recent expansion of K. vaigiensis in Galician waters (Bañón and de Carlos, 2022), which appears to be simultaneous to the expansion of the species in the Mediterranean Sea.
Overall, our combined morphological and molecular results indicate that K. vaigiensis is the sea chub species currently expanding in Mediterranean waters, while K. sectatrix, albeit present, is to date very rare. This is also partially explained by the different mobility of the two species. Indeed, while K. vaigiensis is capable of undertaking extensive migrations along coastal waters, K. sectatrix exhibits a markedly more limited range of movements (Eristhee and Oxenford, 2001; Sakihara et al., 2015; Bañón and de Carlos, 2022). Furthermore, in agreement with other authors that evidenced the difficulty to evaluate whether old records of K. sectatrix in the basin are valid or not (Knudsen and Clements, 2013), we hypothesise that other records of K. vaigiensis might be present among them.
According to available literature (Knudsen and Clements, 2016; Knudsen et al., 2019), the closest species to K. vaigiensis are in the order Kyphosus ocyurus (Jordan and Gilbert, 1882), Kyphosus elegans (Peters, 1869), and K. cinerascens. The limited divergence between K. vaigiensis and K. cinerascens (Figure 4; Table 4) shows that further mitochondrial analyses on K. ocyurus and K. elegans are also needed. Their complete mitogenome sequences would help to confirm or dismiss the distinction as separated species.
Unfortunately, it was not possible to physically obtain all the specimens here reported and thus provide their molecular and meristic data. This limitation is intrinsic to all citizen science-based projects. However, citizen science is probably the only available approach for documenting a large-scale expansion of similar fish species, and combined with meristic and molecular data of the obtained specimens, allows to reach sound results (Castejón-Silvo et al., 2023).
Interestingly, the great majority of our observations (11 out of 13 in Table 2) are from spearfishermen. As previously reported in other studies (Tiralongo et al., 2020 and references therein), this underlines the importance of recreational fishing, and in particular spearfishing, in monitoring the expansion of certain species, which could travel unnoticed by professional fishermen, divers and other sea-user categories. Furthermore, being these species mainly herbivorous, sport techniques that use hooks and baits or artificial baits are probably not very efficient in catching these fish, which are instead much more easily captured with spearguns, due to their rather considerable size.
Despite the several K. vaigiensis records here presented, which have never similarly been reported in Mediterranean waters, in our opinion, even this Kyphosus species still remains quite rare in the basin. In fact, the 13 collected records are the result of a collection campaign of the AlienFish project that involves hundreds of professional and recreational fishermen. Nevertheless, it is likely that the ongoing seawater warming will favour the expansion of K. vaigiensis in the Mediterranean Sea in the next future, similarly to what is already happening for other fish species of Atlantic origin, namely Parapristipoma octolineatum (Valenciennes, 1833) (Tiralongo et al., 2023).
The native status of K. vaigiensis in the Mediterranean requires special attention. In fact, the species has often incorrectly been considered “alien” (Groud et al., 2021; Azzola et al., 2024, and references therein), probably based on the assumption that the first records in the basin are referred to the last three decades (Table 1). However, following widely accepted rules regarding terminology in the science of biological invasions (Pyšek et al., 2004; Crees and Turvey, 2015; Essl et al., 2018), naturally expanding species must be considered part of the native biota, and not alien taxa. Some authors proposed that K. vaigiensis could be able to follow vessels and that this could facilitate its introduction in the basin (Azzurro et al., 2013; Lo Brutto, 2017), and mentioned Nelson (1994) as support for such a possibility. However, neither this version, nor the latest version of Nelson’s book “Fishes of the World” report this particular behaviour for the species (Nelsonet al., 2016). In all cases, the spread and increasing abundance of K. vaigiensis in the Mediterranean has been substantially witnessed only in the last decade (Table 1, Table 2), and thus vessel-following cannot explain alone the expansion of this fish in the basin. Other authors even suggested that the species might have been introduced by ballast waters (Groud et al., 2021). In our opinion, this is rather unlikely, and unnecessary to explain the current expansion of the fish. Indeed, previous research suggested a thermophilic nature of K. vaigiensis: for example, in Maltese waters, the species was caught in an area with increased sea temperature due to thermal effluent discharges from a power station (Vella et al., 2016). The high number of individuals captured in southern Italian regions between 2021 and 2022 now suggests the presence of a stable population of the species in the southern (warmer) Mediterranean sectors, indicating a natural expanding phenomenon and subsequent establishment of this species in the area. This is perfectly in agreement with the ability of a species able to migrate 251 km in 33 days (Sakihara et al., 2015). Hence, current data suggest the natural range expansion from the western Atlantic to Mediterranean waters of this thermophilic species.
Since all the available data support the notion that K. vaigiensis populations represent a natural expansion from the Atlantic Ocean through the Strait of Gibraltar, the species must not be considered “alien” nor “exotic” for the Mediterranean, but just part of the native fauna of the basin (Essl et al., 2018). Alternatively, following Essl et al. (2019), the species can be called “neonative” (i.e. a taxon that expanded its range due to biophysical changes in the environment, but not as a result of human transport or dispersal corridors creation).
The possible ecological impacts of a substantial expansion of K. vaigiensis are difficult to predict. Kyphosus species are typically herbivorous (Clements and Choat, 1997), and their diet significantly contributes to the structure and dynamics of marine benthic communities, as their grazing can influence the composition and abundance of algae and associated species. Additionally, the feeding behaviour of Kyphosus species can affect the marine ecosystems indirectly, influencing habitat availability and resources for other species. However, given the currently limited abundance of the fish in the Mediterranean, its impacts are far from being comparable to the ones caused by other expanding species such as the herbivorous rabbitfish species (Siganus spp.) (Escalas et al., 2022; Vella et al., 2023).
In a broader context, the increasing proliferation of thermophilic fish species in the Mediterranean Sea raises concerns about potential ecological and economical effects on ecosystem and fishing activities (Coco et al., 2022; Nota et al., 2023). As sea temperatures continue to rise, the Mediterranean ecosystem faces unpredicted shifts in species distribution and biodiversity. As these ecological dynamics evolve, it is imperative to prioritize initiatives aimed at understanding the drivers and consequences of species expansion in the basin.
To conclude, the recent proliferation of sea chubs in the Mediterranean Sea presents a challenge in species recognition, primarily due to the morphological similarity between K. vaigiensis and K. sectatrix. This has led to misidentification and the reporting of specimens under synonymous names. Here we proposed a multidisciplinary approach, incorporating both citizen science, morphological and molecular methods, which can be applied also to the study of other expanding species (Pires et al., 2023; Michail et al., 2024). This approach provided valuable insights into the ongoing expansion of K. vaigiensis, indicating this species as the sea chub currently expanding its range in the Mediterranean Sea. The results obtained by other authors in Galician waters, together with the golden rows clearly visible on the majority of our reported specimens and our morphological and molecular evidence, altogether support the scenario that the species currently expanding in the Mediterranean is K. vaigiensis, while to date, there is no concrete reason to hypothesize a similar expansion pattern for K. sectatrix. Genetic analyses suggest that the origin of these K. vaigiensis specimens has to be considered Atlantic, and albeit a human introduction of the species or the entry/exit through the Suez Canal cannot be excluded, there is no evidence in this regard for the moment. In the current scenario of global change and water warming of the Mediterranean Sea, the increase of thermophilic species puts the attention on ecological and economic risks associated with their presence.
Moreover, several biological and ecological aspects of K. vaigiensis remain still largely unknown, hence the urgent need to orient further research in aspects such as the life span and larval planktonic phases of the species, especially in response to changing ocean temperatures. The adaptation of invading species to the new habitats usually involves many species-specific ecological and genetic processes (Nota et al., 2024). Therefore, understanding these and others life history traits of this thermophilic species is crucial to know and predict its distribution and possible ecological impacts.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the two sequenced fish were collected already dead from the fishermen.
AN: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. FT: Writing – review & editing, Writing – original draft, Resources, Project administration, Formal analysis, Data curation, Conceptualization. AS: Writing – review & editing, Validation, Methodology, Investigation. AT: Writing – review & editing, Writing – original draft, Validation, Resources, Funding acquisition. AO: Writing – review & editing, Validation, Supervision, Resources, Funding acquisition, Formal analysis.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Italian Ministry of University and Research (MUR) project PRIN2017 2017CWHLHY and the European Union - Next Generation EU under the programs of the National Biodiversity Future Center, PNRR Project CN00000033 (to AO), PRIN2022 PNRR P2022JZKPY (to AT), and PRIN2022 2022SNEBJY (to AT).
We would like to thank the fishermen who took part in this study, and in particular, Giacomo Bernardi, Gennaro Bisceglie, Matteo Bottero, Stefano Castronovo, Luca Colaciello, Ciro Esposito, Daniele Farinello, Francesco Franzò, Fabio Pasquale Lia, Piermario Mangili, Antonino Martino, and Salvatore Tagliaferri. We are also grateful to Daniele Frigerio and Emanuele Deagostino for their help with the lab activities.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1411111/full#supplementary-material
Al Mabruk S. A. A., Abdulghani A., Nour O. M., Adel M., Crocetta F., Doumpas N., et al. (2021). The role of social media in compensating for the lack of field studies: five new fish species for Mediterranean Egypt. J. Fish. Biol. 99, 673–678. doi: 10.1111/jfb.14721
Ardura A., Planes S., Garcia-Vazquez E. (2013). Applications of DNA barcoding to fish landings: authentication and diversity assessment. ZooKeys 365, 49–65. doi: 10.3897/zookeys.365.6409
Ariola V. (1904). Pesci nuovi o rari per il Golfo di Genova. Annali del Museo Civico di Storia Naturale di Genova 3, 153–168.
Azzola A., Bianchi C. N., Merotto L., Nota A., Tiralongo F., Morri C., et al. (2024). The changing biogeography of the Ligurian Sea: seawater warming and further records of southern species. Diversity 16(3), 159. doi: 10.3390/d16030159
Azzurro E., Peña-Rivas L., Lloris D., Bariche M. (2013). First documented occurrence of Kyphosus incisor in the Mediterranean Sea. Mar. Biodivers. Rec. 6, e98. doi: 10.1017/S1755267213000717
Bañón R., Barros-García D., De Carlos A. (2017). Integrative taxonomy supports the presence of two species of Kyphosus (Perciformes: Kyphosidae) in Atlantic European waters. Scientia Marina 81 (4), 467–475. doi: 10.3989/scimar.04601.08A
Bañón R., de Carlos A. (2022). Preliminary evidence about the colonisation process of Kyphosus species (Perciformes: Kyphosidae) in the subtropical–temperate Northeast Atlantic Ocean and Mediterranean Sea. J. Mar. Sci. Eng. 10, 9. doi: 10.3390/jmse10091237
Battaglia V., Agostini V., Moroni E., Colombo G., Lombardo G., Rambaldi Migliore N., et al. (2022). The worldwide spread of Aedes albopictus: new insights from mitogenomes. Front. Genet. 13. doi: 10.3389/fgene.2022.931163
Bianchi C. N., Azzola A., Bertolino M., Betti F., Bo M., Cattaneo-Vietti R., et al. (2019). Consequences of the marine climate and ecosystem shift of the 1980-90s on the Ligurian Sea biodiversity (NW Mediterranean). Eur. Zool. J. 86, 458–487. doi: 10.1080/24750263.2019.1687765
Bilecenoglu M., Alfaya J. E. F., Azzurro E., Baldacconi R., Boyaci Y. Ö., Circosta V., et al. (2013). New Mediterranean marine biodiversity records (December 2013). Mediterr. Mar. Sci. 14, 463–480. doi: 10.12681/mms.676
Bingpeng X., Heshan L., Zhilan Z., Chunguang W., Yanguo W., Jianjun W. (2018). DNA barcoding for identification of fish species in the Taiwan Strait. PloS One 13, e0198109. doi: 10.1371/journal.pone.0198109
Castejón-Silvo I., Terrados J., Morales-Nin B. (2023). Citizen science in the study of marine biodiversity: the case of iconic and cryptic syngnathids. Thalassas 39, 679–686. doi: 10.1007/s41208-023-00590-1
Chatzimentor A., Doxa A., Katsanevakis S., Mazaris A. D. (2023). Are Mediterranean marine threatened species at high risk by climate change? Glob. Change Biol. 29, 1809–1821. doi: 10.1111/gcb.16577
Clements K. D., Choat J. H. (1997). Comparison of herbivory in the closely-related marine fish genera Girella and Kyphosus. Mar. Biol. 127, 579–586. doi: 10.1007/s002270050048
Coco S., Roncarati A., Tiralongo F., Felici A. (2022). Meridionalization as a possible resource for fisheries: the case study of Caranx rhonchus Geoffroy Saint-Hilaire 1817, in southern Italian waters. J. Mar. Sci. Eng. 10, 2. doi: 10.3390/jmse10020274
Crees J. J., Turvey S. T. (2015). What constitutes a ‘native’ species? Insights from the Quaternary faunal record. Biol. Conserv. 186, 143–148. doi: 10.1016/j.biocon.2015.03.007
Doderlein P. (1883). Rinvenimento di una specie di pesce dell’esotico genere Pimelepterus, Lac. nelle acque del Golfo di Palermo. Naturalista Sicil. 3, 81–86.
Dulčić J., Ahnelt H. (2006). On validity of the record of the Bermuda sea chub Kyphosus sectator (Kyphosidae) from the Adriatic Sea. Period. Biol. 108, 231–233.
Elbaraasi H., Bograra O., Elsilini O., Bojwari J. (2013). First record of the Bermuda sea chub, Kyphosus saltatrix (Actinopterygii: Perciformes: Kyphosidae), in the coastal waters of Libya. Acta Ichthyol. Piscat. 43, 3. doi: 10.3750/AIP2013.43.3.09
Eristhee N., Oxenford H. A. (2001). Home range size and use of space by Bermuda chub Kyphosus sectatrix (L.) in two marine reserves in the Soufrière Marine Management Area, St Lucia, West Indies. J. @ Fish. Biol. 59, 129–151. doi: 10.1111/j.1095-8649.2001.tb01383.x
Escalas A., Avouac A., Belmaker J., Bouvier T., Clédassou V., Ferraton F., et al. (2022). An invasive herbivorous fish (Siganus rivulatus) influences both benthic and planktonic microbes through defecation and nutrient excretion. Sci. Total. Environ. 838, 156207. doi: 10.1016/j.scitotenv.2022.156207
Essl F., Bacher S., Genovesi P., Hulme P. E., Jeschke J. M., Katsanevakis S., et al. (2018). Which taxa are alien? Criteria, applications, and uncertainties. BioScience 68, 496–509. doi: 10.1093/biosci/biy057
Essl F., Dullinger S., Genovesi P., Hulme P. E., Jeschke J. M., Katsanevakis S., et al. (2019). A conceptual framework for range-expanding species that track human-induced environmental change. BioScience 69, 908–919. doi: 10.1093/biosci/biz101
Fitori A., El-Fituri A., Golani D. (2022). First record of the Brassy Chub Kyphosus vaigiensis (Pisces: Kyphosidae) from the Mediterranean coast of Libya. Acta Adriat. 63, 123–126. doi: 10.32582/aa.63.1.12
Francour P., Mouine N. (2008). First record of Kyphosus sectator (Linnaeus 1758) (Kyphosidae) along the French Mediterranean coast. Cybium 32, 275–276.
Gerovasileiou V., Akel E. H. K., Akyol O., Alongi G., Azevedo F., Babali N., et al. (2017). New Mediterranean biodiversity records (July 2017). Mediterr. Mar. Sci. 18, 2. doi: 10.12681/mms.13771
Goren M., Galil B. S., Gevili R., Stern N. (2016). First record of the Brassy Chub Kyphosus vaigiensis (Quoy and Gaimard 1825) in the eastern Mediterranean (Osteichthyes: perciformes: kyphosidae). Zool. Middle East. 62, 319–322. doi: 10.1080/09397140.2016.1250710
Groud L., Chaoui L., Kara H. (2021). A new record of the brassy chub, Kyphosus vaigiensis (Actinopterygii: Perciformes: Kyphosidae), from the Mediterranean Sea. Acta Ichthyol. Piscat. 51, 219–223. doi: 10.3897/aiep.51.64069
Hattour A. (2006). Premiere observation de la calicagere blanche Kyphosus sectatrix (Linnaeus 1758) sur les cotes tunisiennes. INSTM. Bull.: Mar. Freshw. Sci. 33, 123–125.
Hemida F., Kanoun N., Golani D., Souissi J. B., Guélorget O., Capapé C. (2004). Records of the Bermuda sea chub, Kyphosus sectator (Linnaeus 1758) (Osteichthyes: Kyphosidae) from the coastal waters of Algeria (southern Mediterranean). Ann. Ser. Hist. Nat. 14, 49–52.
Kiparissis S., Loukovitis D., Batargias C. (2012). First record of the Bermuda sea chub Kyphosus saltatrix (Pisces: Kyphosidae) in Greek waters. Mar. Biodivers. Rec. 5, 1. doi: 10.1017/S1755267211001199
Kiyağa V., Mavruk S., Özyurt C., Akamca E., Coşkun Ç. (2019). Range extension of Kyphosus vaigiensis (Quoy and Gaimard 1825) in the northeastern Mediterranean, Iskenderun Bay, Turkey. Turk. J. Zool. 43, 644–649. doi: 10.3906/zoo-1901-1
Knudsen S. W., Choat J. H., Clements K. D. (2019). The herbivorous fish family Kyphosidae (Teleostei: Perciformes) represents a recent radiation from higher latitudes. J. Biogeogr. 46, 2067–2080. doi: 10.1111/jbi.13634
Knudsen S., Clements K. (2013). Revision of the fish family Kyphosidae (Teleostei: Perciformes). Zootaxa 3751, 1–101. doi: 10.11646/zootaxa.3751.1.1
Knudsen S. W., Clements K. D. (2016). World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes). Mol. Phylogenet. Evol. 101, 252–266. doi: 10.1016/j.ympev.2016.04.037
Kousteni V., Anastasiadis A., Bariche M., Battaglia P., Bonifazi A., Ćetković I., et al. (2022). New records of rare species in the Mediterranean Sea (May 2022). Mediterr. Mar. Sci. 23, 417–446. doi: 10.12681/mms.25295
Lelong P. (2012). A new record of Bermuda sea chub, Kyphosus saltatrix (Linnaeus 1758) (Osteichthyes, kyphosidae) from Galite Islands (Tunisia, Southern Mediterranean). Mar. Life 18, 3–7.
Ligas A., Sartor P., Sbrana M., Ranieri S. (2011). A new record of Kyphosus saltatrix (Pisces: Kyphosidae) along the Italian coasts (north-western Mediterranean). Mar. Biodivers. Rec. 4, e6. doi: 10.1017/S1755267210001211
Lo Brutto S. (2017). The case of a rudderfish highlights the role of natural history museums as sentinels of bio-invasions. Zootaxa 4254, 3. doi: 10.11646/zootaxa.4254.3.8
Lombardo G., Rambaldi Migliore N., Colombo G., Capodiferro M. R., Formenti G., Caprioli M., et al. (2022). The mitogenome relationships and phylogeography of barn swallows (Hirundo rustica). Mol. Biol. Evol. 39, msac113. doi: 10.1093/molbev/msac113
Mannino A., Balistreri P., Iaciofano D., Galil B., Lo Brutto S. (2015). An additional record of Kyphosus vaigiensis (Quoy and Gaimard 1825) (Osteichthyes, Kyphosidae) from Sicily clarifies the confused situation of the Mediterranean kyphosids. Zootaxa 3963, 45–54. doi: 10.11646/zootaxa.3963.1.3
Merella P., Massutí E., Deudero S. (1998). On the occurrence of Kyphosus sectator (Osteichthyes: Kyphosidae) in the western Mediterranean. J. Mar. Biol. Assoc. U. K. 78, 687–690. doi: 10.1017/S0025315400041771
Michail C., Tanduo V., Crocetta F., Giovos I., Litsiou S., Kleitou P. (2024). Engagement of fishers in citizen science enhances the knowledge on alien decapods in Cyprus (eastern Mediterranean Sea). Aquat. Ecol. 58, 107–116. doi: 10.1007/s10452-023-10046-6
Mohanty M., Jayasankar P., Sahoo L., Das P. (2015). A comparative study of COI and 16S rRNA genes for DNA barcoding of cultivable carps in India. Mitochondrial DNA 26, 79–87. doi: 10.3109/19401736.2013.823172
Nelson J. S., Grande T. C., Wilson M. V. H. (2016). Fishes of the World (Hoboken, New Jersey: Wiley), 752 pp. doi: 10.1002/9781119174844
Nota A., Bertolino S., Tiralongo F., Santovito A. (2024). Adaptation to bioinvasions: when does it occur? Glob. Change Biol. 30, e17362. doi: 10.1111/gcb.17362
Nota A., Ignoto S., Bertolino S., Tiralongo F. (2023). First record of Caranx crysos (Mitchill 1815) in the Ligurian Sea (northwestern Mediterranean Sea) suggests northward expansion of the species. Ann. Ser. Hist. Nat. 33, 55–60. doi: 10.19233/ASHN.2023.09
Orsi Relini L., Costa M., Relini M. (2010). First record of the yellow sea chub Kyphosus incisor in the Mediterranean. Mar. Biodivers. Rec. 3, e4. doi: 10.1017/S1755267209991096
Pires R. F., Froufe E., Secci-Petretto G., dos Santos A. (2023). Report on the occurrence of the hydromedusa Odessia maeotica (Ostroumoff 1896) in the north-eastern Atlantic revealed by citizen science and integrative taxonomy. Aquat. Ecol. 58, 323–334. doi: 10.1007/s10452-023-10071-5
Pyšek P., Richardson D. M., Rejmánek M., Webster G. L., Williamson M., Kirschner J. (2004). Alien plants in checklists and floras: towards better communication between taxonomists and ecologists. Taxon 53, 131–143. doi: 10.2307/4135498
Ragkousis M., Zenetos A., Souissi J. B., Hoffman R., Ghanem R., Taşkın E., et al. (2023). Unpublished Mediterranean and Black Sea records of marine alien, cryptogenic, and neonative species. Bioinvasions Rec. 12, 339–369. doi: 10.3391/bir.2023.12.2.01
Sakai K. (2003). “Kyphosidae,” in FAO Species Identification Guide for Fishery Purpose. The Living Marine Resources of the Western Central Pacific. Volume 5. Bony Fishes Part 3 (Menidae to Pomacentridae) (FAO, Rome, Italy), 3290–3205.
Sakai K., Nakabo T. (1995). Taxonomic review of the Indo-Pacific Kyphosid fish, Kyphosus vaigiensis (Quoy and Gaimard). Jpn. J. Ichthyol. 42, 61–70. doi: 10.11369/jji1950.42.61
Sakai K., Nakabo T. (2004). Two new species of Kyphosus (Kyphosidae) and a taxonomic review of Kyphosus bigibbus Lacepède from the Indo-Pacific. Ichthyol. Res. 51, 20–32. doi: 10.1007/s10228-003-0186-2
Sakai K., Nakabo T. (2006). Taxonomic reviews of two Indo-Pacific sea chubs, Kyphosus cinerascens (Forsskål 1775) and Kyphosus sydneyanus (Günther 1886). Ichthyol. Res. 53, 337–356. doi: 10.1007/s10228-006-0355-1
Sakihara T., Nishiura L., Shimoda T., Shindo T., Nishimoto R. (2015). Brassy chubs Kyphosus vaigiensis display unexpected trans-island movement along inshore habitats. Environ. Biol. Fishes. 98, 155–163. doi: 10.1007/s10641-014-0245-8
Šoljan T. (1963). Fishes of the Adriatic (Ribe Jadrana). Revised and enlarged for the English edition. Belgrade: NOLIT Pub. House (Washington: Office of Technical Services, U.S. Dept. of Commerce). Available at: https://search.library.wisc.edu/catalog/999637442602121.
Tiralongo F., Crocetta F., Riginella E., Lillo A. O., Tondo E., Macali A., et al. (2020). Snapshot of rare, exotic and overlooked fish species in the Italian seas: a citizen science survey. J. Sea. Res. 164, 101930. doi: 10.1016/j.seares.2020.101930
Tiralongo F., Pappalardo A. M., Ignoto S., Lombardo B. M., Ferrito V., Sosa A. C., et al. (2023). The african striped grunt, Parapristipoma octolineatum (Valenciennes 1833), in the Mediterranean Sea: The third record with biological and ecological notes, and identification key for Haemulidae recorded in the Mediterranean. J. Mar. Sci. Eng. 11, 1688. doi: 10.3390/jmse11091688
Vella A., Scicluna Y., Mifsud C. M., Monaco C., Peri I., Tibullo D., et al. (2023). The first record of the marbled spinefoot, Siganus rivulatus Forsskål & Niebuhr 1775 and further records of the dusky spinefoot, Siganus luridus (Rüppell 1829) from Malta. BioInvasions Rec. 12, 417–426. doi: 10.3391/bir.2023.12.2.06
Vella N., Vella A., Darmanin S. A. (2016). The first record of the lowfin chub Kyphosus vaigiensis (Quoy and Gaimard 1825) from Malta. J. Black. Sea/Medit. Environ. 22, 175–181.
Ward R. D., Hanner R., Hebert P. D. N. (2009). The campaign to DNA barcode all fishes, FISH-BOL. J. Fish. Biol. 74, 329–356. doi: 10.1111/j.1095-8649.2008.02080.x
Keywords: Kyphosus vaigiensis, Mediterranean Sea, mitochondrial genome, morphological analyses, taxonomy, thermophilic species
Citation: Nota A, Tiralongo F, Santovito A, Torroni A and Olivieri A (2024) Chronicles of Kyphosus in the Mediterranean Sea: new records and complete mitogenomes support the scenario of one expanding fish species. Front. Mar. Sci. 11:1411111. doi: 10.3389/fmars.2024.1411111
Received: 03 April 2024; Accepted: 02 July 2024;
Published: 24 July 2024.
Edited by:
Anna Rita Rossi, Sapienza University of Rome, ItalyReviewed by:
Adriana Vella, University of Malta, MaltaCopyright © 2024 Nota, Tiralongo, Santovito, Torroni and Olivieri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessandro Nota, YWxlc3NhbmRyby5ub3RhQHVuaXB2Lml0
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.