- 1Department of Convergence Interdisciplinary Education of Maritime and Ocean Contents, Korea Maritime and Ocean University, Busan, Republic of Korea
- 2Department of Aquaculture, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Gazipur, Bangladesh
- 3Division of Convergence on Marine Science, Korea Maritime and Ocean University, Busan, Republic of Korea
- 4Division of Marine Technology, Chonnam National University, Yeosu, Republic of Korea
The low fish meal (FM) diet commonly causes deteriorated palatability, and eventually leads to lowered feed consumption and growth performance of fish. This study was, therefore, designed to determine inclusion impact of the graded levels of jack mackerel meal (JMM) in olive flounder (P. olivaceus) diets replacing 50% FM with tuna by-product meal (TBM) on growth, feed availability, biochemical composition, blood chemistry, and economic efficiency. Six isonitrogenous (55.5%) and isolipidic (10.0%) diets were formulated. Sixty percent FM was incorporated in the control (Con) diet. In the Con diet, fifty percent of FM was replaced by TBM, and then 10, 20, 30, 40, and 50% JMM were included at the cost of FM, referred as the TJ10, TJ20, TJ30, TJ40, and TJ50 diets, respectively. Three hundred and sixty juvenile fish (18.0 ± 0.01g; mean ± SD) were delivered into 18, 50-L flow-through tanks. All diets were randomly assigned to triplicate groups of fish. Fish were hand-fed to apparent satiation twice daily for 56 days. Weight gain, specific growth rate, and feed consumption of fish fed the TJ30, TJ40, and TJ50 diets were significantly (P < 0.001, P < 0.001, and P < 0.002, respectively) greater than those of fish fed the Con and TJ10 diets, but not significantly (P > 0.05) different from those of fish fed the TJ20 diet. Feed utilization, proximate composition, amino acid profiles, and blood chemistry of fish were not significantly (P > 0.05) altered by dietary treatments. However, the TJ30 diet was the most recommendable to farmers in terms of economic profit index (EPI). Conclusively, 30% inclusion of JMM is the most recommendable in the olive flounder diet substituting 50% FM with TBM based on growth performance, feed availability, and EPI.
1 Introduction
Olive flounder (P. olivaceus) belonging to the order Pleuronectiformes is considered the most economically valuable marine water fish in the Republic of Korea (KOSIS, 2023). Farming olive flounder is becoming popular among fish farmers because of its good flavor and taste, environmental tolerance, disease resistance, and high market demand worldwide (Dar et al., 2021; Stieglitz et al., 2021). The total yield of this fish from aquaculture was shown to be 45,801 metric tons (MT), occupying about 50.6% out of total aquaculture production of 90,545 MT in Korea in 2022 (KOSIS, 2023). Due to the carnivorous feeding nature of olive flounder, it requires a high protein diet (40–65% protein depending on life stage) of which about 50–70% is fish meal (FM) (Tacon and Metian, 2008; Hamidoghli et al., 2020). FM is very expensive in the global market due to its scarcity (Oliva-Teles et al., 2015) and increased processing and transportation cost (Tacon and Metian, 2008), which is ultimately increasing feed cost. FM price will continue to rise because of the high demand for global scale expansion of aquaculture and scarcity of wild sources of fish generally used for producing FM (Kim et al., 2021). Therefore, many efforts are required to look for an appropriate substitute for high-cost FM to achieve sustainable aquaculture production of olive flounder.
Tuna by-product meal (TBM) primarily made of bone, fin, skin, head, blood, viscera, and left over of tuna after the tuna-canning process is a popular replacer for FM in marine fish species in Korea (Kim et al., 2018, 2021, 2022). There is no precise statistical information available on annual production of TBM, however, about 8.2 million metric tons of different tuna species were caught globally in 2019 (FAO, 2022). The amount of solid waste produced by the tuna-canning industry is 70% in relation to the raw material (Guerard et al., 2002). TBM as a replacer for FM not only ensures the use of fish by-product, but also minimizes fishery industry waste (Kim et al., 2022). In olive flounder diet, dietary optimum replacement levels for FM were estimated to be 47.1 and 46.3% for the maximum weight gain and feed consumption, respectively (Kim et al., 2022).
Reduced feed consumption and deteriorated feed palatability are the common concerns caused when FM is substituted by various other protein sources in the fish diet (Cho et al., 2005; Uyan et al., 2006; Oncul et al., 2019; Ha et al., 2021). Therefore, incorporation of feed ingredients having a feed stimulant effect can be an effective approach to resolve the issue. Feed attractants or stimulants are low molecular weight free amino acids (AA), nucleotides, nucleosides, organic acids, and quaternary ammonium (NH4) bases (Morais, 2017; Kim and Cho, 2019), commonly added into fish feed to reduce feeding time and improve acceptability by fish (Peng et al., 2022). Feeding response of fish depends on three chemoreception channel, olfaction and vision, which facilitates smell and location, and gustation, which facilitates taste or consumption (McCrickerd and Forde, 2016; Senzui et al., 2020; Yu et al., 2021). In aquatic surroundings, fish use highly developed chemosensory (olfactory and gustatory) pathways to detect food at distance (Hara, 1994; Morais, 2017). Addition of attractants in feed allows fish to quick access feed, and creates condition for fast ingestion, in other words, minimizes leaching-out ratio of nutrients and deterioration of water quality (Morais, 2017; Jeong et al., 2020). Moreover, attractants also provide supplemental nutrients for protein and energy metabolism (Biswas et al., 2018). Along with the optimum nutrition-balance, palatability is also considered crucial in the development of an artificial diet (Takakuwa et al., 2019). Artificial diets must contain the modest quantity of chemostimulants to be chemically appealing to reveal their location, increase ingestion rates, and elevate feed efficiency (Nunes et al., 2006). Feed stimulants, therefore, have received considerable attention to lower feed waste, enhance feed consumption and growth of fish, and lower pollution source to surrounding water (Li et al., 2019; Yuan et al., 2021; Peng et al., 2022).
A variety of feed ingredients, such as fish products including FM (Singh et al., 2006), fish soluble (Kader et al., 2012), mollusc meals including blue mussel meal (Nagel et al., 2014) and squid meal (Novriadi et al., 2017), crustacean meals including shrimp meal (Kim and Cho, 2019) and krill meal (Smith et al., 2005), animal by-product meals including blood meal (Tusche et al., 2011), plant by-products (Liew et al., 2020), and the synthetic chemicals (Biswas et al., 2018) have been tested to evaluate their attractiveness to different aquatic animals. Especially, jack mackerel (Trachurus japonicus) meal (JMM) has been reported to be the prominent stimulants and attractants in various fish species, such as olive flounder (Jeong et al., 2020, 2022), rockfish (Sebastes schlegeli) (Kim and Cho, 2019; Kim et al., 2019; Baek et al., 2021), and yellowtail (Seirola quinqueradiata) (Hosokawa et al., 2001). AA in the extracts of jack mackerel, especially, histidine has remarkable feeding stimulant activity on olive flounder (Ikeda et al., 2012). Likewise, Takakuwa et al. (2019) proved that muscle extracts of jack mackerel is rich in nucleotide, particularly inosine monophosphate (IMP), which has feed stimulatory effect on greater amberjack (Seriola dumerili). In addition, Kim et al. (2019) unveiled that the most potent feed attractant activity of rockfish was found in JMM among 16 crude feed ingredients. Kim and Cho (2019) reported that the greatest improvement in weight gain and highest feed consumption were attained in rockfish fed a diet supplemented with 5% JMM when rockfish were fed with a 55% anchovy meal-based diet or diets supplemented with 5% JMM, pollock meal, sardine meal, squid meal or shrimp meal at the expense of anchovy meal. Therefore, JMM is not only a rich source of protein for fish, but also exhibits a strong feed attractant and stimulant effect. However, the inclusion effect of a specific ingredient relies profoundly on species of fish, feeding habit of fish, and type and doses of the ingredient (Miyasaki and Harada, 2002; Hancz, 2020; Jeong et al., 2020).
Thus, the primary goal of this study was to elucidate inclusion effect of graded levels of JMM in the olive flounder diets replacing 50% FM by TBM on growth, feed availability, proximate composition, blood chemistry, and economic efficiency.
2 Materials and methods
2.1 Design of the feeding experiment
Six isonitrogenous (55.5%) and isolipidic (10.0%) feeds were formulated (Table 1). In the control (Con) diet, the primary protein sources were 60% FM and 15% fermented soybean meal. In addition, 18.3% wheat flour, and 2.1% each of fish and soybean oils were included as the carbohydrate, and lipid sources, respectively. Fifty percent FM in the Con diet was replaced with TBM, and then graded levels (10, 20, 30, 40, and 50%) of JMM were included at the expense of FM, referred to as the TJ10, TJ20, TJ30, TJ40, and TJ50 diets, respectively. However, a diet replacing 50% FM with TBM without JMM inclusion was not included because dietary optimum FM substitution levels with TBM for the weight gain and specific growth rate (SGR) of olive flounder were reported to be 47.1 and 48.6%, respectively (Kim et al., 2022). All feeds were assigned to triplicate groups of olive flounder.
To prepare a mixture, all feed ingredients were properly blended with water at a 3:1 ratio. The dough was then pelleted by passing through a laboratory type pellet extruder (Dongsung Mechanics, Busan, Korea). Finally, the sinking type pellets were then kept at –20°C until use after being dried at 30°C in an air-forced oven for a 48 h. Each experimental diet was hand-fed to satiation twice (08:30 and 17:30) a day for 56 days. Fish were carefully fed to apparent satiation to reduce feed waste and the pellet size (4–6 mm in diameter) was adjusted with fish growth. The daily feed supply was recorded for each tank and uneaten feeds were not collected. All feed provided to the fish tank was considered consumed.
2.2 Rearing conditions
Healthy juvenile olive flounder were acquired from a commercial fish farm (Taean-gun, Chungcheongnam-do, Korea) and transported to the laboratory. Prior to the feeding trial, fish were acclimated to the rearing conditions for two weeks by feeding a commercial extruded pellet (55% crude protein and 8% crude lipid) twice a day at a ratio of 2–3% biomass. Three hundred and sixty juvenile (initial weight of 18.0 ± 0.01 g; mean ± SE) fish were assigned into 18, 50 L rectangular flow-through tanks (20 fish/tank) in a completely randomized design (CRD). Each tank received sand filtered seawater with a flow rate of 4.2 L/min. A multifunctional water quality meter (AZ-8603, AZ Instrument, Taichung, Tai-wan) was used daily to monitor water quality. Temperature, salinity, dissolved oxygen, and pH ranged from 17.2 to 19.4°C (17.8 ± 0.84°C; mean ± SD), 31.8 to 33.7 g/L (32.6 ± 0.52 g/L), 6.3 to 7.8 mg/L (7.2 ± 0.31 mg/L), and 6.9 to 7.6 (7.3 ± 0.20), respectively. The bottom of fish tanks were cleaned daily by syphoning and the photoperiod (14 L: 10 D, light: dark cycle) followed the natural condition.
2.3 Calculation of growth, feed utilization, and biological indices of fish
After the 56-day rearing experiment, all live fish in each tank were fasted for 24 h, and then anaesthetized by MS-222 (tricaine methanesulfonate) (E10521, Sigma-Aldrich, Gangnam-gu, Seoul, Korea) at the concentration of 100 ppm. All surviving olive flounder from each tank were counted, and weighed collectively. Ten anaesthetized fish chosen from each tank were individually weighed, measured in total length, and then dissected to collect liver and viscera to determine hepatosomatic index (HSI) and viscerosomatic index (VSI). Growth performance, feed utilization, and biological indices of the fish were evaluated by using the following formulas: specific growth rate (SGR, %/day) = (Ln final body weight – Ln initial body weight) × 100/days of feeding trial (56 days), feed efficiency (FE) = weight gain of fish/feed consumption, protein efficiency ratio (PER) = weight gain of fish/protein consumption, protein retention (PR, %) = protein gain × 100/protein consumption, condition factor (K, g/cm3) = body weight of fish (g) × 100/total length of fish (cm)3, viscerosomatic index (VSI, %) = viscera weight of fish × 100/body weight of fish, and hepatosomatic index (HIS, %) = liver weight of fish × 100/body weight of fish.
2.4 Analysis of biochemical composition of experimental feeds and fish
Twenty fish at the beginning and 10 fish after measurement of biological indices of fish were homogenized to analyze the proximate composition by standard AOAC (1990) method. Crude protein content was evaluated by Auto Kjeldahl System (Buchi, Flawil, Switzerland), and crude lipid content was evaluated by ether-extraction method using a Soxhlet Extractor (VELP Scientifica, Milano, Italy). Moisture content was evaluated by oven drying at 105°C for 24 h, and ash content was evaluated by using a muffle furnace at 550°C for 4 h.
Amino acids (apart from methionine and cysteine) in the experimental diets and whole body of fish were analyzed by hydrolyzing the diets with 6 N HCl for 24 h at 110°C, followed by ion exchange chromatography with an amino acid analyzer (L-8800 Auto-analyzer: Hitachi, Tokyo, Japan). For the analysis of methionine and cysteine, the samples were oxidized with performic acid at below 5°C for 24 h to obtain methionine sulphone and cysteic acid, and they were then freeze-dried twice with deionized water. The freeze-dried samples were hydrolyzed and analyzed as per the process used for the other amino acids. Lipid for fatty acid analyses in the experimental diets and whole body of fish were extracted by a mixture of chloroform and methanol (2:1 v/v), and fatty acid methyl esters were prepared by transesterification with14% BF3-MeOH (Sigma, St. Louis, MO, USA). Fatty acid methylesters were analyzed using a gas chromatography (Trace GC, Thermo, Waltham, MA, USA) with flame ionization detector, equipped with SPTM-2560 capillary column (100 m × 0.25 mm I.d. film thickness 0.20 µm; Supelco, Bellefonte, PA, USA).
2.5 Analysis of plasma parameters of the fish
After the 56-day feeding trial, sample of blood were collected by using heparinized syringes from the caudal veins of three anaesthetized fish from each tank. After centrifugation (2,716 × g at 4°C) for 10 min, plasma was collected, and preserved in a freezer at –70°C as separate aliquots for further analysis. Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), total bilirubin (T-BIL), total cholesterol (T-CHO), triglyceride (TG), total protein (TP), and albumin (ALB) were analyzed by using an automatic chemistry system (Fuji Dri-Chem NX500i, Fujifilm, Tokyo, Japan).
2.6 Analysis of serum lysozyme activity and superoxide dismutase of the fish
Blood from three anesthetized fish from each tank were drawn from their caudal veins using syringes. Serum was extracted and kept for subsequent analysis in separate aliquots in a freezer at −70°C after centrifugation (2,716 × g at 4°C) for 10 min. Serum lysozyme activity was determined by the turbidimetric assay according to Lange et al. (2001) and superoxide dismutase (SOD) was determined by using a 19160 SOD Determination Kit (Sigma-Aldrich Inc., St. Louis, MO, USA) following the manufacturer’s direction.
2.7 Economic analysis of the study
Economic analysis of the study was carried out by using the following formula (Martínez-Llorens et al., 2007): Economic conversion ratio (ECR, USD/kg fish) = feed consumption (kg) × feed cost (USD/kg)/weight gain (kg); economic profit index (EPI, USD/fish) = [final weight (kg/fish) × selling price (USD/kg)] – [feed consumption (kg) × diet price (USD/kg)]. The price (USD/kg) of each ingredient was as follows: FM = 2.26, TBM = 1.30, JMM = 2.43, fermented soybean meal = 0.70, wheat flower = 0.55, fish oil = 2.76, soybean oil = 1.79, vitamin mix = 8.28, mineral mix = 6.66, and choline = 1.30. Olive flounder sale price was calculated at 12.44 USD/kg.
2.8 Statistical analysis
SPSS version 24.0 (SPSS Inc., Chicago, IL, USA) was used to conduct one-way ANOVA and Duncan’s multiple range test (Duncan, 1955) to analyze all evaluated variables if there is any significant differences of means in respect to incorporation levels of JMM in diets substituting 50% FM with TBM. The data were tested for normality and homogeneity and percentage data were arcsine-transformed prior to statistical analysis. A follow-up trend analysis using orthogonal polynomial contrasts was carried out to assess if the effect was linear, quadratic or cubic. The difference was considered significant at P < 0.05. Statistical significance was determined (linear, quadratic, and cubic), and then the data were subjected to regression analysis to fit the best model. Additionally, regression analysis was performed between dietary inclusion levels of JMM, and weight gain, SGR, and feed consumption to fit the best model.
3 Results
3.1 AA and FA profiles of the feeds
Leucine and lysine, and aspartic acid and glutamic acid were the abundant essential AA (EAA) and non-essential AA (NEAA), respectively, in all formulated feeds (Table 2). Among EAA, leucine, lysine, threonine, tryptophan, and valine content were found to increase with elevated JMM inclusion levels in TJ diets. All experimental feeds fulfilled dietary arginine, lysine, and threonine requirement for olive flounder, however not for dietary requirement of methionine.
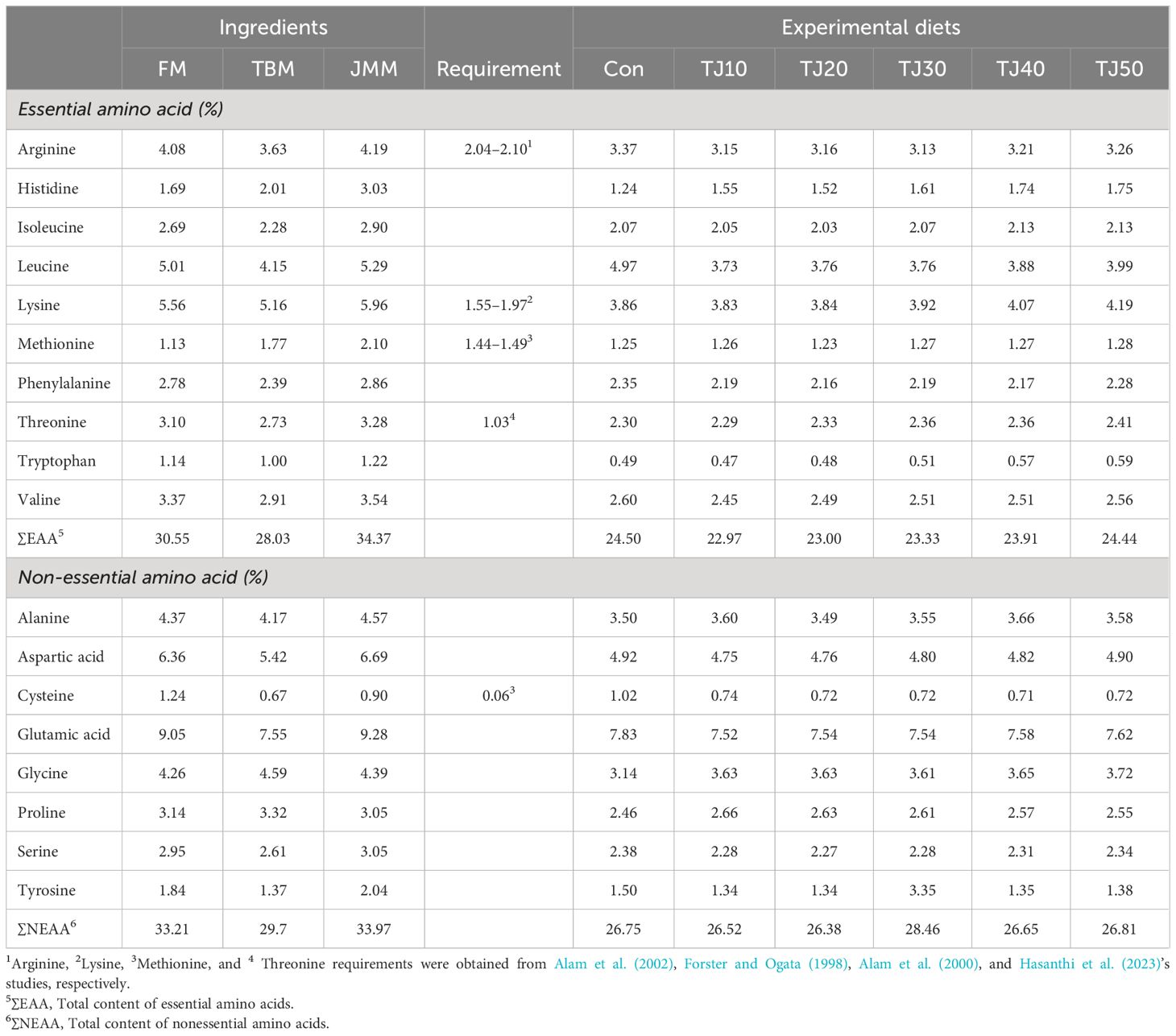
Table 2 Amino acids (AA) profile (% of the diet) of the main feed ingredients and experimental diets.
FM and JMM were rich in eicosapentaenoic acid (EPA) (C20:5n-3) and total content of n-3 highly unsaturated FA (∑n-3 HUFA), while TBM was relatively rich in total content of saturated fatty acids (∑SFA) and monounsaturated fatty acids (∑MUFA) (Table 3). EPA level was found to decrease with increasing JMM inclusion levels in the TJ diets, while ∑MUFA and DHA (C22:6n-3) tended to increase.
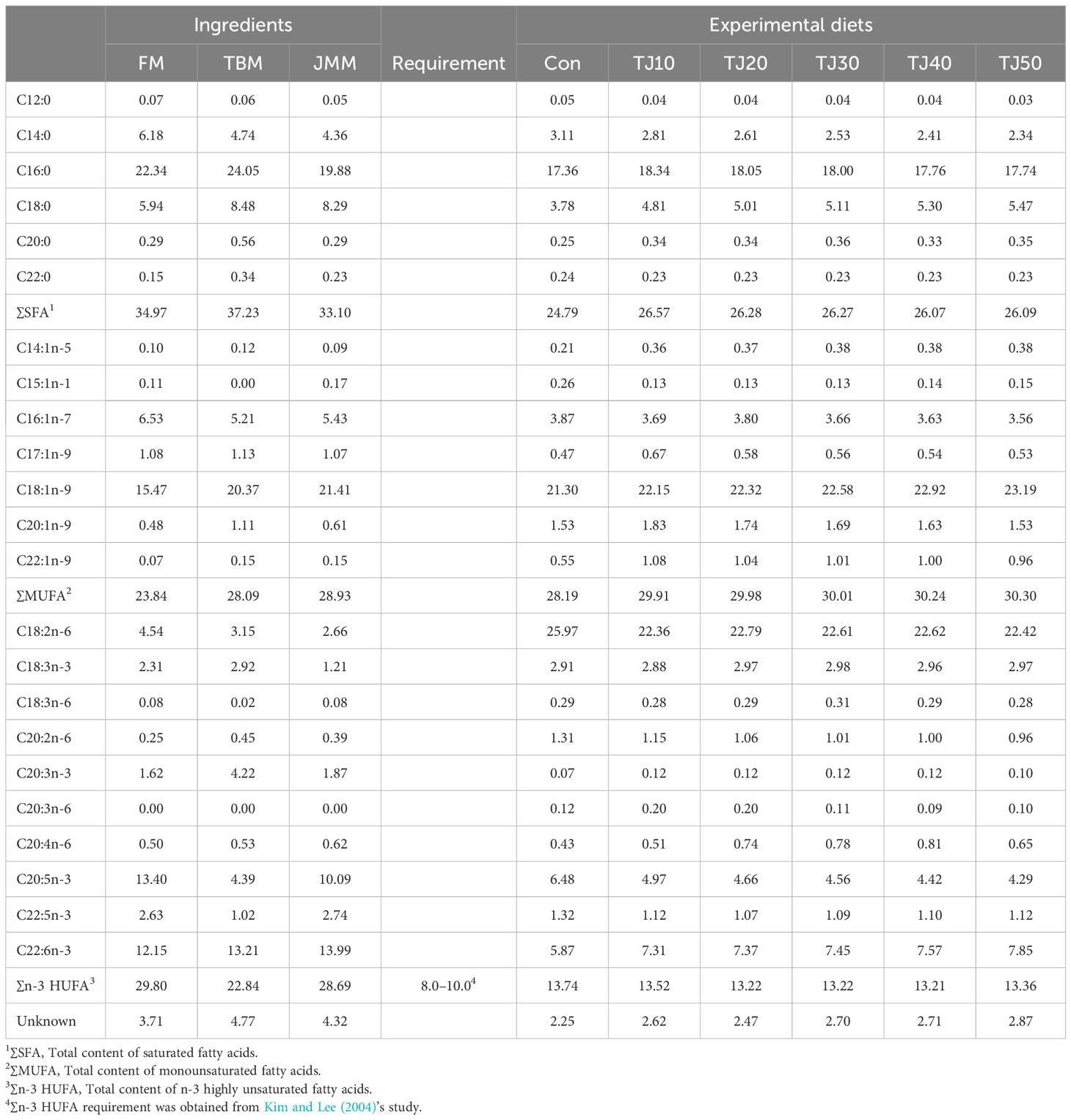
Table 3 Fatty acids (FA) profile (% of total fatty acids) of the feed ingredients and experimental diets.
3.2 Growth, feed availability, and the biological indices of fish
All fish survived at the completion of the 56-day feeding trial (Table 4). Weight gain and SGR of olive flounder fed the TJ30, TJ40, and TJ50 diets were significantly (P < 0.001) greater than those of fish fed the Con and TJ10 diets, but not significantly (P > 0.05) different from those of fish fed the TJ20 diet (Figures 1, 2, respectively). Weight gain and SGR of olive flounder fed the TJ20 diet were also significantly (P < 0.05) greater than those of fish fed the Con diet, but not significantly (P > 0.05) different from those of fish fed the TJ10 diet. In orthogonal polynomial contrast, significant linear (P = 0.002 for both) relationships between weight gain and SGR versus inclusion levels of JMM in diets substituting 50% FM by TBM were observed. In regression analysis, quadratic relationships were found to be the best fit model between inclusion levels of JMM in diets substituting 50% FM with TBM and weight gain (Y = − 0.002095X2 + 0.176381X + 68.06, R2 = 0.6477, P < 0.002, Ymax = X value of 42.1%) and SGR (Y = − 0.00004214X2 + 0.003559X + 2.792733, R2 = 0.6599, P < 0.002, Ymax = X value of 42.2%), respectively (Figures 1, 2, respectively). Feed consumption of olive flounder fed the TJ30, TJ40, and TJ50 diets were significantly (P < 0.002) higher than that of olive flounder fed the Con and TJ10 diets, but not significantly (P > 0.05) different from that of fish fed the TJ20 diet (Figure 3). Feed consumption of olive flounder fed the TJ20 diet was also significantly (P < 0.05) higher than that of olive flounder fed the TJ10 diets, but not significantly (P > 0.05) different from that of olive flounder fed the Con diet. In orthogonal polynomial contrast, a significant linear (P = 0.005) relationship was observed between feed consumption versus dietary inclusion levels of JMM. In regression analysis, quadratic model was found to be the best fit model between dietary JMM inclusion levels and feed consumption (Y = − 0.002162X2 + 0.174448X + 63.600667, R2 = 0.7613, P < 0.001, Ymax = X value of 40.3%).
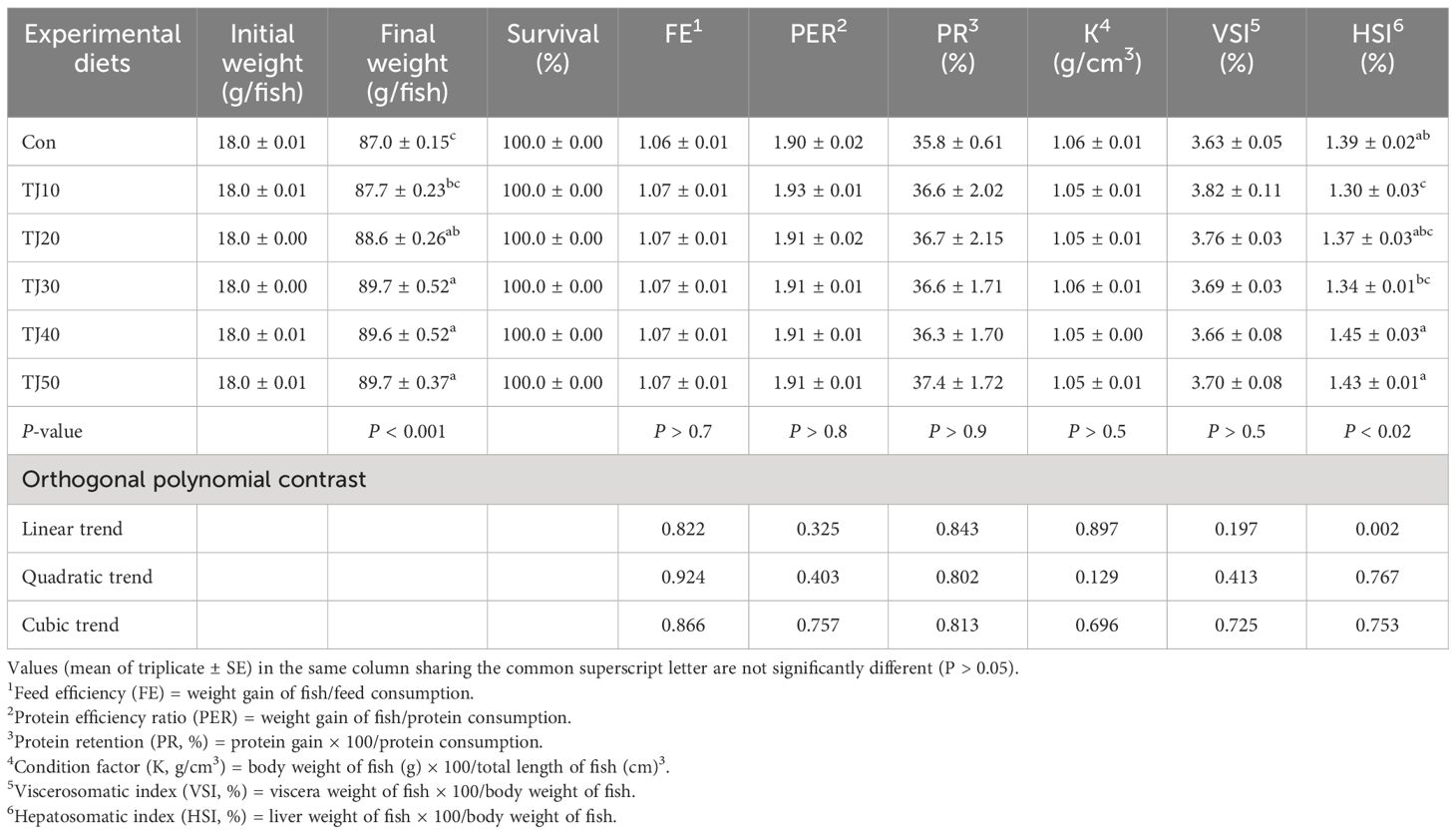
Table 4 Survival (%), feed efficiency (FE), protein efficiency ratio (PER), protein retention (PR), condition factor (K), viscerosomatic index (VSI), and hepatosomatic index (HSI) of olive flounder fed the experimental diets for 56 days.
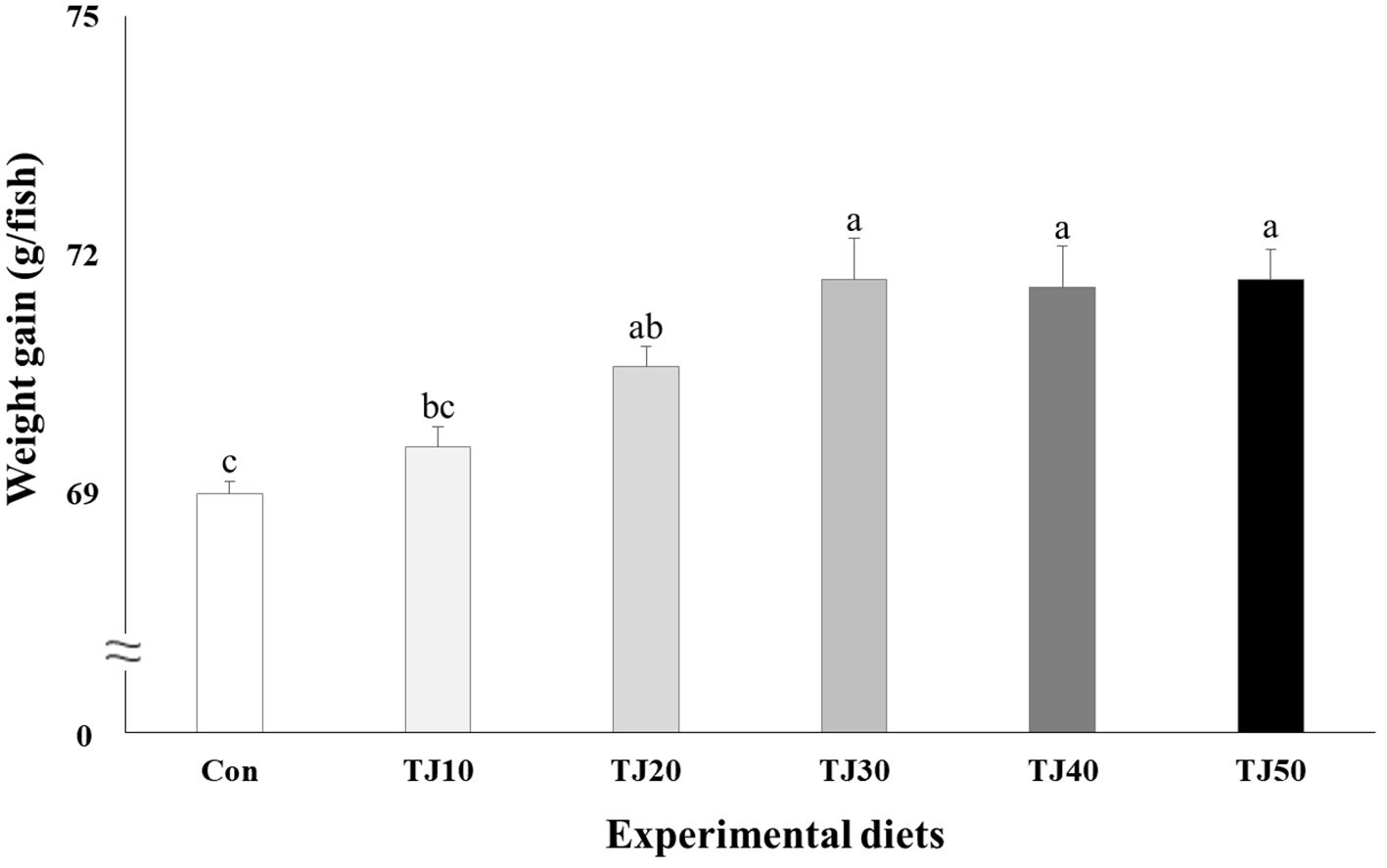
Figure 1 Weight gain (g/fish) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (P < 0.001). Different letters indicate statistically significant differences (P < 0.05).
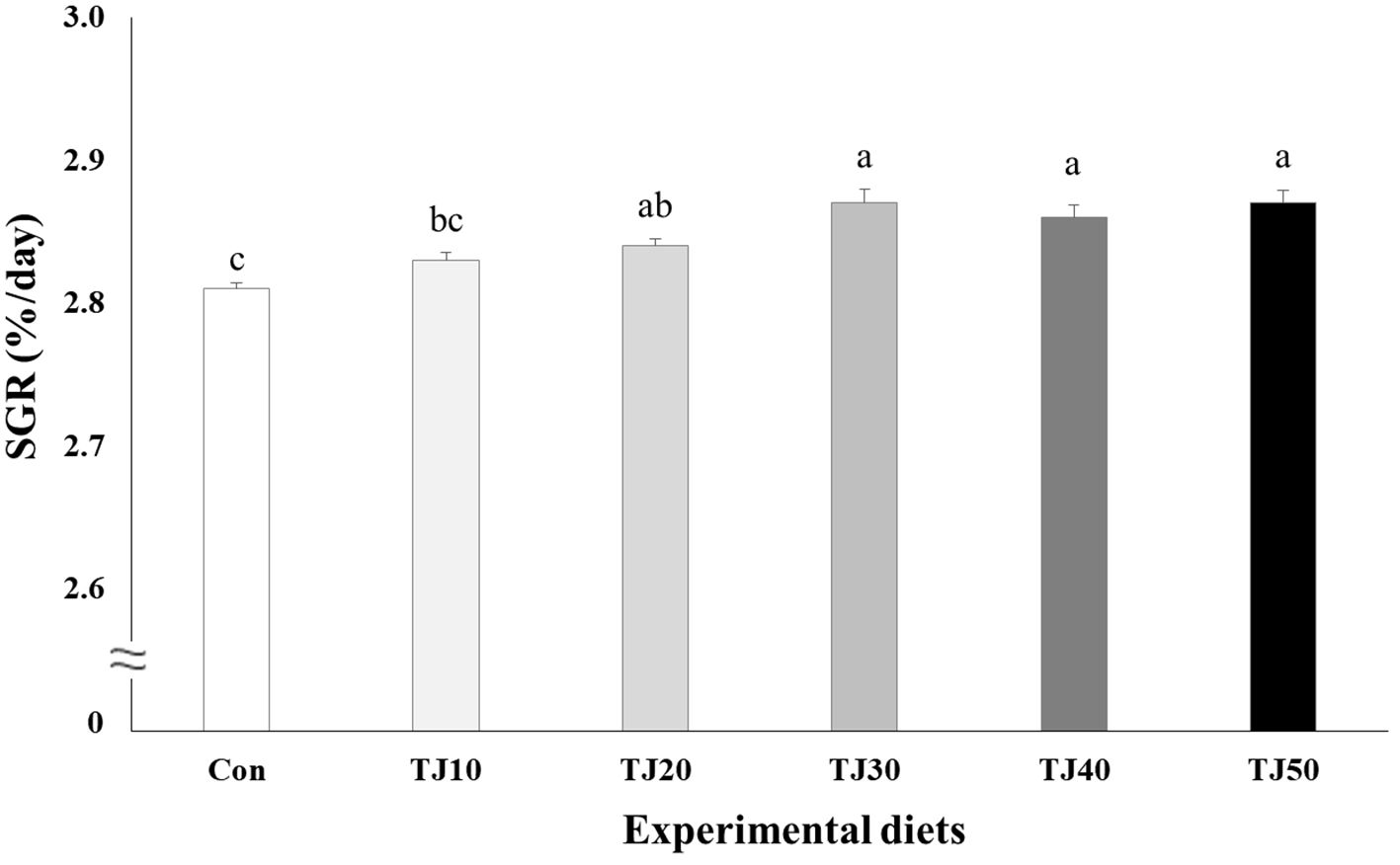
Figure 2 Specific growth rate (SGR, %/day) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (P < 0.001). Different letters indicate statistically significant differences (P < 0.05).
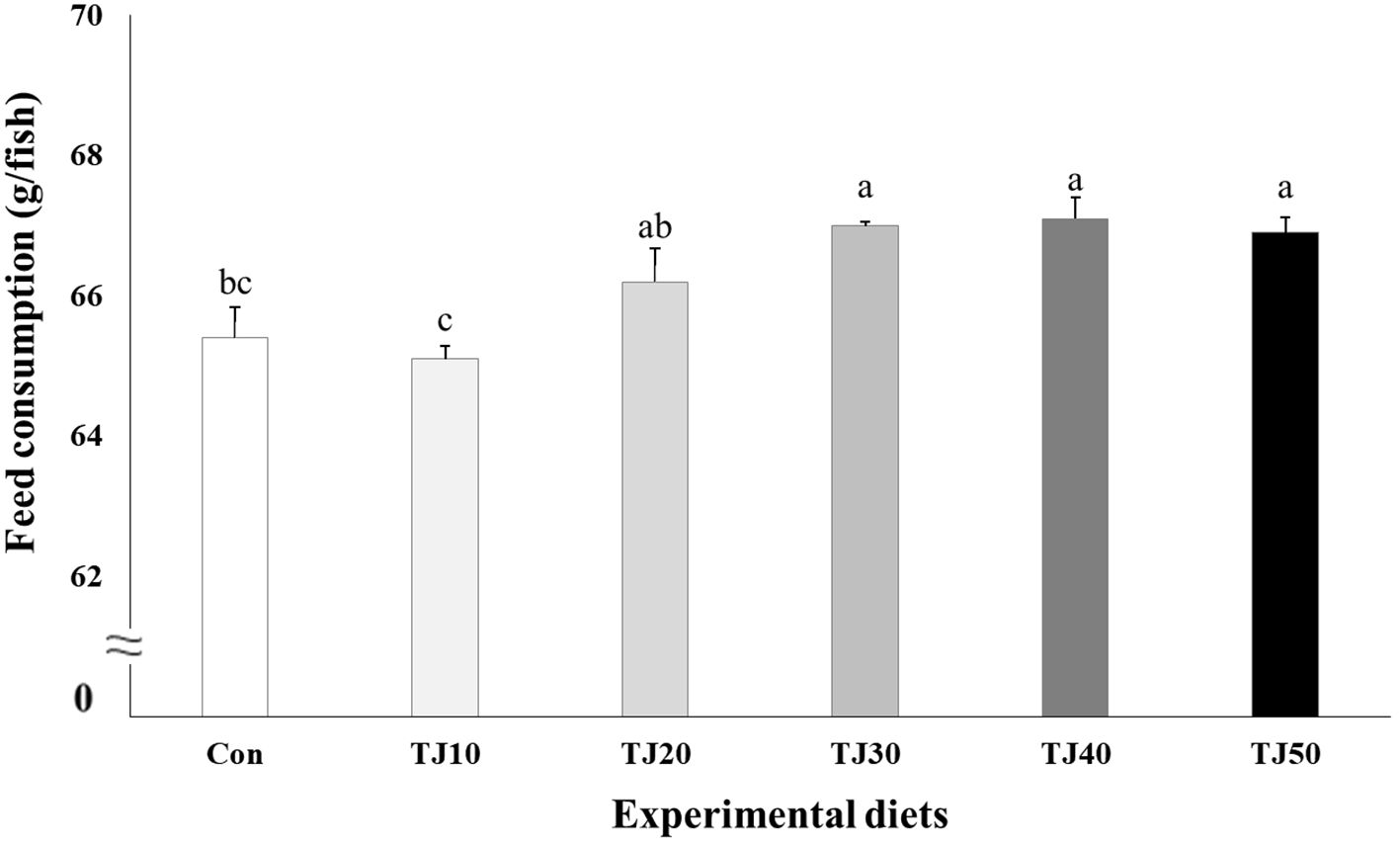
Figure 3 Feed consumption (g/fish) of olive flounder (Paralichthys olivaceus) fed the experimental diets for 56 days (mean of triplicate ± SE) (P < 0.002). Different letters indicate statistically significant differences (P < 0.05).
However, FE of olive flounder varied from 1.06 to 1.07, PER varied from 1.90 to 1.93, and PR varied from 35.8% to 37.4%, respectively, but these parameters were significantly (P > 0.7, P > 0.8, and P > 0.9, respectively) unaffected by dietary treatments.
K and VSI of olive flounder changed from 1.05 to 1.06 g/cm3 and 3.63 to 3.82% respectively, but these indices were not significantly (P > 0.5 for both) altered by dietary treatments. HSI of olive flounder fed the TJ40 and TJ50 diets was significantly (P < 0.02) higher than that of fish fed the TJ10 and TJ30 diets, but not significantly (P > 0.05) different from that of fish fed the Con and TJ20 diets. In orthogonal polynomial contrast, a significant linear (P = 0.002) relationship was observed between HSI versus dietary inclusion levels of JMM.
3.3 Proximate composition of the whole-body olive flounder
Moisture, crude protein, crude lipid, and ash content of the whole-body fish fluctuated from 68.6 to 72.0%, 18.7 to 19.4%, 3.5 to 3.8%, and 3.7 to 4.2%, respectively (Table 5). These parameters were not significantly (P > 0.7, P > 0.9, P > 0.9, and P > 0.5, respectively) changed by dietary treatments
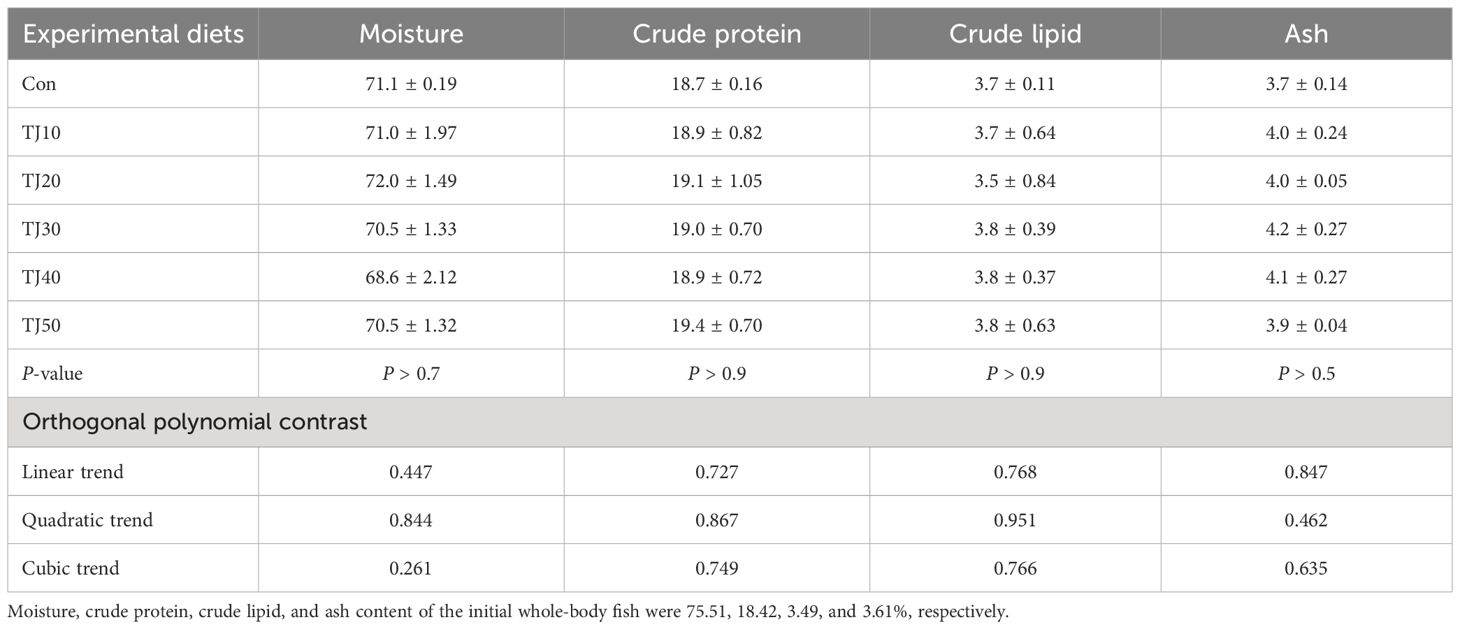
Table 5 Chemical composition (%, wet weight) of whole-body olive flounder fed the experimental diets for 56 days (mean of triplicate ± SE).
3.4 AA and FA profiles of the whole-body fish
The experimental feeds did not show any statistical (P > 0.05) impact on AA profile of the whole-body olive flounder (Table 6).
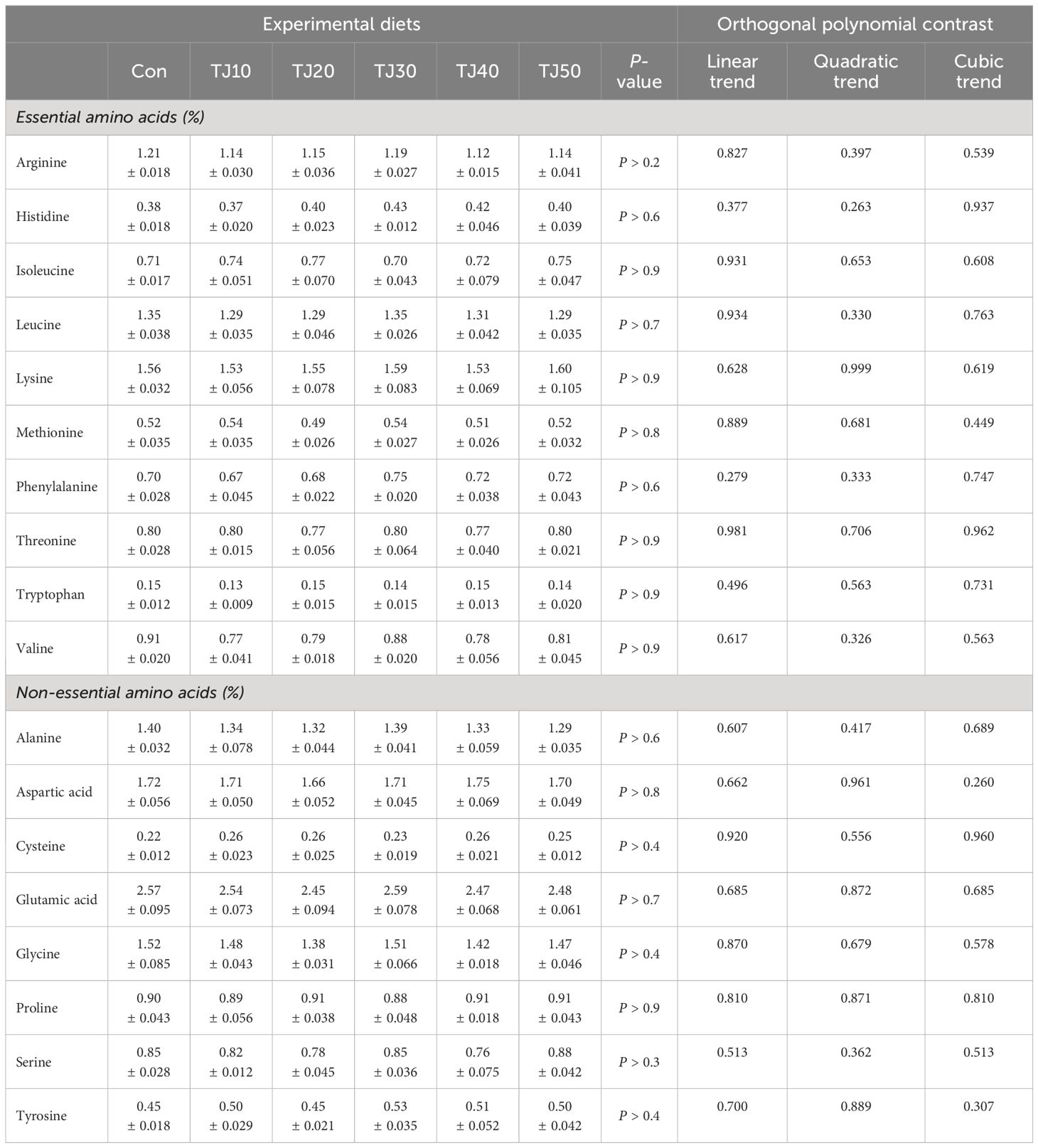
Table 6 Amino acid profiles (% of wet weight) of the whole-body of olive flounder fed the experimental diets for 56 days (mean of triplicate ± SE).
The ∑SFA of olive flounder fed TJ10 and TJ20 diets was significantly (P < 0.001) higher than that of fish fed Con, TJ40, and TJ50 diets, but not significantly (P > 0.05) different from that of fish fed the TJ30 diet (Table 7). In orthogonal polynomial contrast, a significant linear (P = 0.001) relationship was observed between ∑SFA of olive flounder versus dietary inclusion levels of JMM. The ∑MUFA content of olive flounder fed all TJ diets was significantly (P < 0.003) higher than that of fish fed the Con diet. The EPA and ∑n-3 HUFA content of olive flounder fed the Con diet were significantly (P < 0.0001 for both) higher than those of fish fed all other TJ diets. However, EPA content was found to decrease with elevated JMM incorporation levels in all TJ diets. In orthogonal polynomial contrast, a significant linear (P = 0.001) relationship was observed between EPA of olive flounder versus dietary incorporation levels of JMM. The DHA content of olive flounder fed the Con diet was significantly (P < 0.03) lower than that of fish fed the TJ30, TJ40, and TJ50 diets, but not significantly different (P > 0.05) from that of fish fed the TJ10 and TJ20 diets. In orthogonal polynomial contrast, a significant linear (P = 0.023) relationship was observed between DHA of fish versus dietary incorporation levels of JMM.
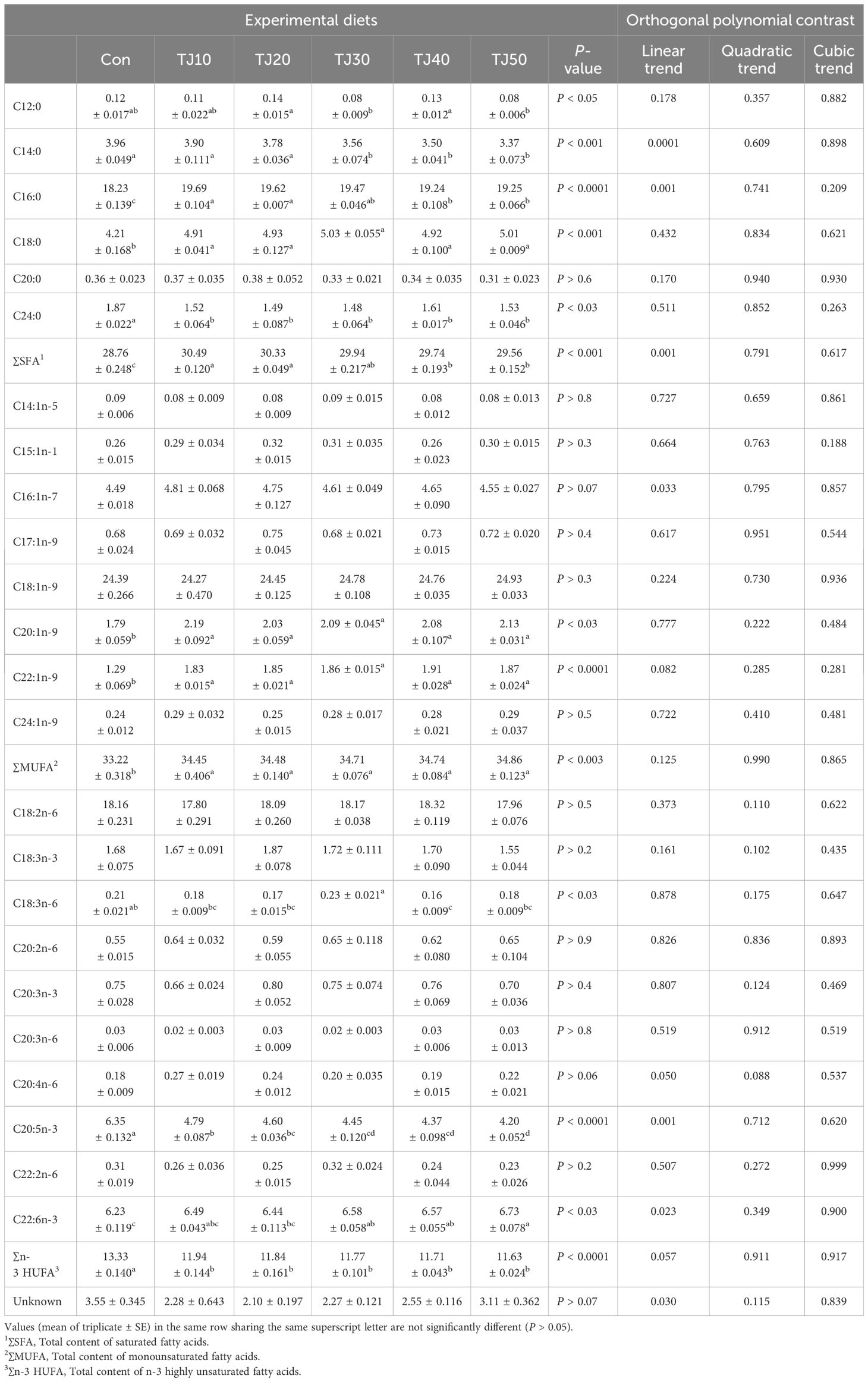
Table 7 Fatty acids profile (% of total fatty acids) of the whole-body of olive flounder the experimental diets for 56 days.
3.5 Plasma parameters of fish
Plasma AST, ALT, ALP, T-BIL, T-CHO, TG, TP, and ALB level fluctuated from 13.1 to 13.2 U/L, 6.2 to 6.4 U/L, 111.1 to 113.6 U/L, 0.8 to 0.9 mg/dL, 347.1 to 368.3 mg/dL, 442.2 to 449.7 mg/dL, 5.5 to 5.6 g/dL, and 1.0 to 1.1 g/dL, respectively (Table 8). The experimental feeds had no significant (P > 0.9, P > 0.9, P > 0.9, P > 0.9, P > 0.7, P > 0.9, P > 0.9 and P > 0.2, respectively) impact on any plasma parameters of fish.
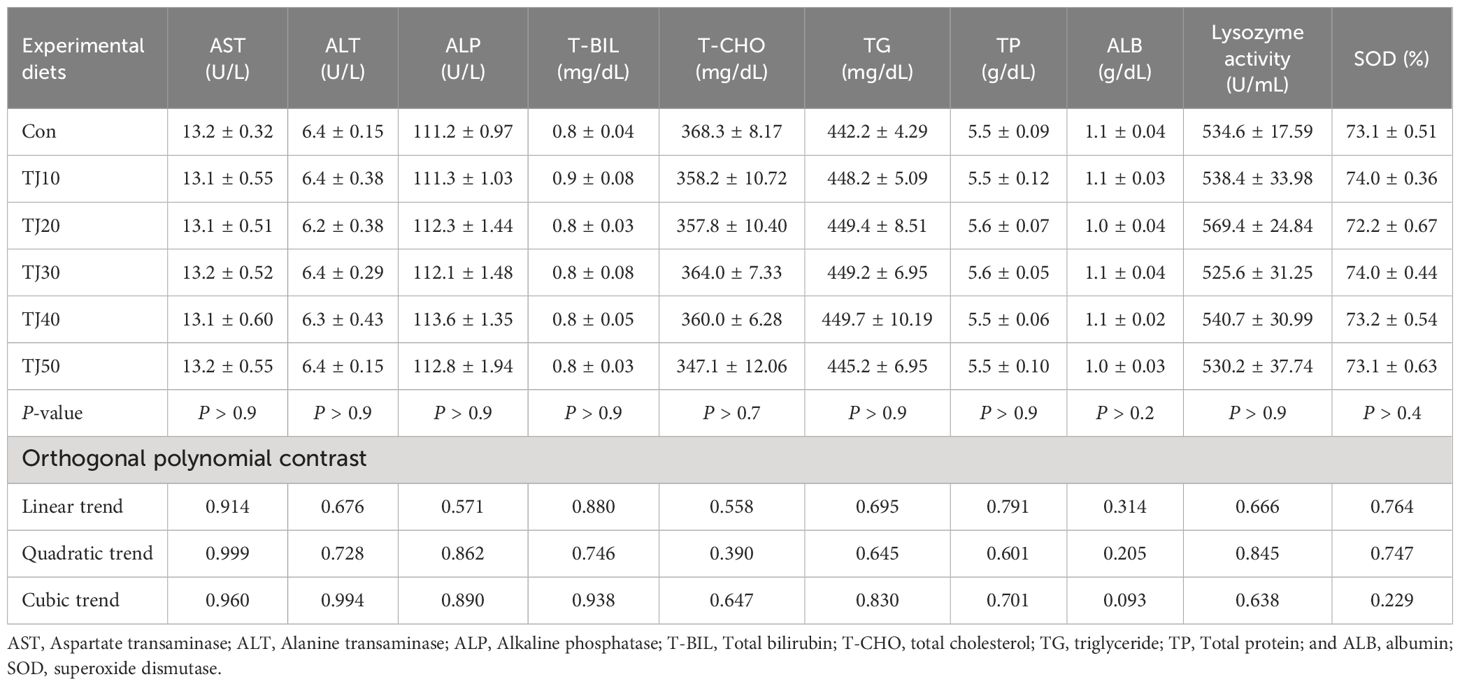
Table 8 Plasma parameters, and serum lysozyme activity and superoxide dismutase (SOD) of olive flounder fed the experimental diets for 56 days (mean of triplicate ± SE).
3.6 Serum lysozyme activity and SOD of fish
The experimental feeds did not show any significant (P > 0.9 and P > 0.4, respectively) difference in either serum lysozyme activity or SOD of fish (Table 8).
3.7 Economic analysis of the study
Replacement of 50% FM with TBM in diets supplemented with graded levels of JMM changed the price and economic parameters (ECR and EPI) (Table 9). The prices of TBM replaced diets with JMM inclusion tended to increase. The ECR of the Con diet was significantly (P < 0.001) higher than that of all TJ diets, but no significantly difference in ECR was found among the all TJ diets. The EPI of the TJ30, TJ40, and TJ50 diets were significantly (P < 0.001) greater than that of the Con and TJ10 diets, but not significantly (P > 0.05) different from that of the TJ20 diet. The significant linear (P = 0.040 and P = 0.003) relationships between ECR and EPI versus dietary incorporated JMM levels in orthogonal polynomial contrast were found.
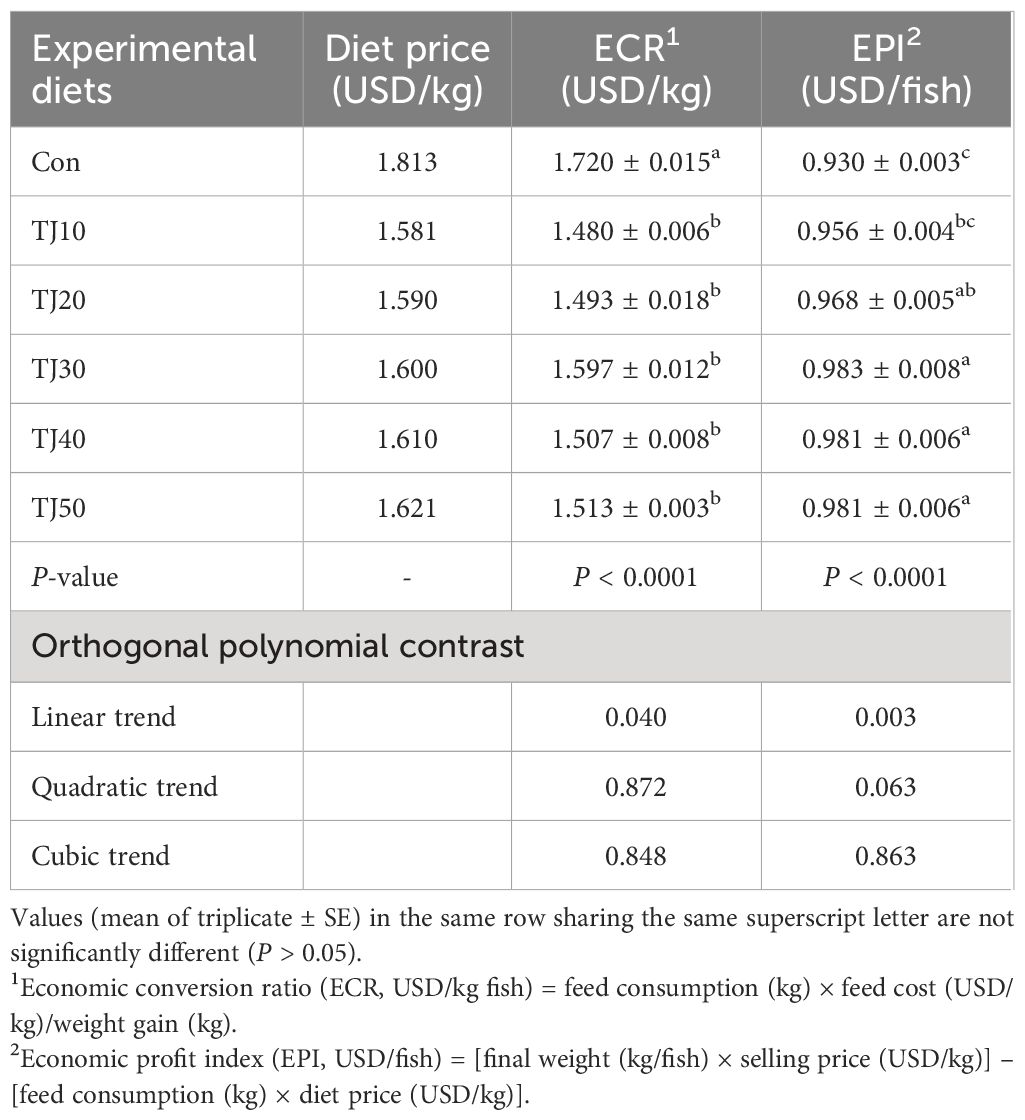
Table 9 Effect of dietary replacement of FM with TBM supplemented with graded level of JMM on economic parameters of the study.
4 Discussion
In aquaculture production, feed is the most expensive component in operating aquaculture systems due to increased price of FM, which is generally utilized as a primary supply of protein in fish feed (Zhao et al., 2021). A variety of studies have been performed to elucidate substitutability of FM in fish diets. Kim et al. (2022) emphasized that the greatest growth performance was achieved in olive flounder fed a diet replacing 40% FM by TBM when olive flounder were fed with a 65% FM-basal diet or diets substituting the graded levels (10–50%) of FM by TBM for 56 days, but growth was slightly lowered in olive flounder fed a diet substituting 50% FM with TBM. Kim et al. (2022) also estimated dietary optimum TBM replacement levels of 47.1, and 46.3% for the maximum weight gain, and feed consumption, respectively. However, in this experiment, all TJ diets substituting 50% FM with TBM supplemented with JMM inclusion, except for the TJ10 diet achieved better weight gain and higher feed consumption by olive flounder compared to fish fed with a 60% FM-based diet, proving that further amount (50%) of FM in the olive flounder diet could be substituted by TBM when JMM was incorporated. Especially, inclusion of 30–50% JMM at the expense of FM in low FM diets substituting 50% FM by TBM brought about superior growth and feed consumption of olive flounder compared to fish fed a 60% FM-basal diet, but not significantly different from those of fish fed the TJ20 diet. Superior growth performance of olive flounder fed the TJ30, TJ40, and TJ50 diets compared to olive flounder fed the Con and TJ10 diets, but comparable to olive flounder fed the TJ20 diet in this experiment were closely associated with higher feed consumption, causing no distinctive variation in feed utilization (FE, PER, and PR). Similarly, elevated growth performance of rockfish, walleye pollock (Gadus chalcogrammus), and olive flounder resulted from elevated feed consumption led to no distinctive difference in feed utilization when 10–60% JMM was included in diets (Choi et al., 2020; Baek et al., 2021; Jeong et al., 2022).
Significantly or slightly, but not significantly, improved feed consumption by olive flounder fed the TJ20, TJ30, TJ40, and TJ50 diets compared to that of fish fed the Con diet in this experiment could be demonstrated by the fact that smell (olfactory cue) and taste (gustatory cue) induced by the incorporation of JMM could have elevated feed consumption and weight gain of rockfish and olive flounder (Kim et al., 2019; Jeong et al., 2020). Similarly, AA, such as histidine, glycine, alanine and proline, and nucleotides, such as inosine, inosine monophosphate, adenosine monophosphate, adenosine diphosphate, and adenosine triphosphate in jack mackerel muscle have shown feeding stimulatory effects on yellowtail, olive flounder, and greater amberjack (Hosokawa et al., 2001; Ikeda et al., 2012; Takakuwa et al., 2019). Abundant EAA and NEAA, except for cysteine and proline in JMM (Table 2) may indicate that JMM has a feed enhancing effect along with being a good quality source of protein in the olive flounder feeds. Likewise, AA and nucleotides having strong chemosensory ability are responsible for flavor and taste in feeds (Nunes et al., 2006; Chotikachinda et al., 2013).
In orthogonal polynomial contrast, linear relationships between weight gain, SGR, and feed intake of fish versus dietary JMM inclusion levels were the most suitable model in this study, however, the quadratic model was the best adjusted model between weight gain, SGR, and feed intake and dietary JMM inclusion levels in regression analysis. The inclusion levels of JMM to induce the maximum weight gain and SGR, and feed consumption were calculated to be 42.1, 42.2, and 40.3% in diets substituting 50% FM by TBM, respectively, according to the regression analysis. As JMM is one of the highest price FM sources in fish feeds, it is recommendable to use JMM inclusion at a minimum level in diets without causing deterioration in feed consumption and growth performance. No distinctive changes in weight gain, SGR, and feed consumption of olive flounder fed the TJ20, TJ30, TJ40, and TJ50 diets in this experiment suggested that 20% of JMM inclusion at the expense of FM was the most recommendable dietary treatment when 50% FM were substituted with TBM in the olive flounder diets. In considering ECR and EPI of the study, however, the TJ30 diet seemed to be the most recommendable to farmer because of higher economic return. Dietary replacement of 50% FM by TBM with all JMM inclusion levels appeared to be economically feasible as prices of all TJ diets were relatively lower than that of the Con diet. Moreover, the lower ECR and higher EPI in all TJ diets, except for EPI of the TJ10 diet led to a greater economic return to the farmers in the grow-out stage (≤ ca. 100 g) of olive flounder (Table 9).
The EAA are important for growth (Elmada et al., 2016), feed efficiency (Tang et al., 2009), and immunity modulation of fish (Pan et al., 2016). Arginine, lysine, and threonine requirements (2.04–2.10%, 1.55–1.97%, and 1.03% of diet, respectively) in all experiment diets were fulfilled for olive flounder (Forster and Ogata, 1998; Alam et al., 2002), but methionine content seemed to be little bit lower than dietary requirement (1.44–1.49%) in the presence of 0.06% cysteine (Alam et al., 2000). However, cysteine could spare 42, 50, and 60% of methionine in the diets of rainbow trout (Oncorhyncus mykiss), yellow perch (Perca flavescens), and channel catfish (Ictalurus punctatus), respectively (Harding et al., 1977; Kim et al., 1992; Twibell et al., 2000). Therefore, EAA requirements known in the olive flounder feeds seemed to be all met in the current study. All TJ diets achieved comparable or superior growth performance compared to olive flounder fed the Con diet in this study because of comparable or superior feed consumption of olive flounder fed the former. Although FA was also very important for many physiological functions of fish, there would be still a big knowledge gap in FA requirement of olive flounder. Dietary ∑n-3 HUFA requirement (8.0–10.0 of total FA) for olive flounder (Kim and Lee, 2004) was also satisfied in all experimental diets in this study. Farmed fish need to supply long chain n-3 HUFA through diets because they have limited capacity to develop them in their body (Lee et al., 2020).
The biological indicators, such as K, VSI, and HSI are widely utilized as they’re representatives of growth, nutritional status, energy reserve, and physiological condition of fish because they are fast and easily ascertainable (Chellappa et al., 1995; Ng et al., 2000). K is an indicator of energy reserve in fish and variation in K signals changes in health and nutritional state, while VSI and HSI are associated with lipid content of viscera and liver (Lee et al., 2000; Wang et al., 2016). In this study, no distinctive variation in K and VSI of olive flounder was observed among dietary treatments, while HSI of olive flounder fed the TJ40 and TJ50 diets was higher than that of fish fed the TJ10 and TJ30 diets. Likewise, dietary inclusion of commercially available stickwater (by-product of fish meal processing), squid paste, and their mixture as feed attractants altered K and HSI in Chinese perch (Siniperca chuatsi), but did not change VSI (Peng et al., 2022). Incorporated feed stimulants in diets may enhance the ingestion, fat metabolism, and nutrient absorption of fish. Unlike this study, however, dietary supplementation of 10% fish soluble, 5% squid meal, 5% krill meal, and their mixture as feed attractants had no distinctive impact on K, VSI, and HSI of red sea bream (Pagrus major) (Kader et al., 2012). This disparity in impact on morphologic indices of fish are possibly due to the type and dose of feed stimulants and attractants (Peng et al., 2022).
No distinctive variation in proximate composition and AA profiles of whole-body olive flounder was found in this experiment, agreeing with other investigations (Jeong et al., 2022; Jeong and Cho, 2023) demonstrating that the proximate composition and AA profile of the whole-body fish were not affected by dietary inclusion of JMM. This could be explained by the fact that body proteins are synthesized based on the genetic information from DNA, and that is why AA profiles of the whole-body protein is the same although there were differences in treatment groups (Yamamoto et al., 2000). Likewise, chemical composition of red sea bream and turbot (Psetta maxima) was not altered by supplementation of fish soluble, squid meal, krill meal, shrimp protein hydrolysate, and mussel meal as feed attractants in low-FM diets (Kader et al., 2012; Nagel et al., 2014; Gunathilaka et al., 2021). Kim et al. (2021) also demonstrated that there was no marked variation in chemical composition and AA profiles of olive flounder when fish were fed with diets substituting 30% FM with different animal protein sources. Higher ∑SFA and ∑MUFA content, but lower ∑n-3 HUFA and EPA content in the whole-body olive flounder fed all TJ diets compared to those of fish fed the Con diet resulted from the FA profiles of the experimental feeds in the current study. This is consistent with other research (Kim and Lee, 2004; Lee et al., 2020; Ha et al., 2021; Kim et al., 2021) that revealed dietary FA profiles had a significant effect on whole-body FA profile of olive flounder.
Plasma parameters of fish are commonly used non-lethal way of monitoring physio-logical condition of farmed fish. In relation to nutritional changes of fish, they can also be used to evaluate the state of fish health (Fazio, 2019). All plasma parameters of olive flounder in this experiment showed no distinctive difference among dietary treatments. This might indicate that the nutritional and physiological condition of olive flounder were not deteriorated when fish were fed with diets substituting 50% FM by TBM supplemented with various levels of JMM. Khosravi et al. (2015) also demonstrated that incorporated krill hydrolysate concentrate or tuna hydrolysate as feed attractants in the 50% and 52% FM-basal diets did not alter blood chemistry of olive flounder and red sea bream, respectively. Supplementation of various (krill, shrimp, and tilapia) protein hydrolysates as feed attractants in low FM diets substituting 50% FM by soy protein concentrate did not alter haematological parameters of olive flounder, except for ALT (Khosravi et al., 2018).
Serum lysozyme activity and SOD are popular biomarkers for innate immune response and oxidative response of fish, respectively (Lange et al., 2001; Ha et al., 2021). No distinctive difference in serum lysozyme activity and SOD in this experiment might indicate the diets substituting 50% FM by TBM supplemented with JMM did not cause any negative impact on serum parameters of olive flounder. Likewise, Jeong et al. (2022) highlighted that lysozyme activity and SOD of olive flounder were not influenced by dietary inclusion of JMM. Unlike this study, however, inclusion of krill and tuna hydrolysate for FM increased nitro blue tetrazolium (NBT) activity and lysozyme activity, and NBT activity, lysozyme activity, and SOD in red sea bream, and olive flounder, respectively (Khosravi et al., 2015). NBT, total immunoglobulin, lysozyme activity, and SOD of olive flounder were increased by low FM diets supplemented with different protein hydrolysates (Khosravi et al., 2018). This contradiction is perhaps because bioactive peptides are produced by enzymatic hydrolysis of marine originated proteins that have immunomodulatory properties (Kang et al., 2019; Muley et al., 2021).
5 Conclusion
Olive flounder fed the TJ30 diet obtained the greatest weight gain, SGR and feed consumption. Nevertheless, feed utilization, biological indices (K and VSI), proximate composition, AA profile, and plasma and serum measurements of olive flounder were unaffected by dietary treatments. Furthermore, the TJ30 diet seemed to be the most recommendable in considering both ECR and EPI. A commercial-scale investigation is required to evaluate the long-term effect and feasibility of using JMM in commercial production system.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Korea Maritime and Ocean University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MI: Data curation, Investigation, Writing – original draft. SC: Conceptualization, Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. TK: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (RS-2018-KS181194).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alam M. S., Teshima S., Ishikawa M., Koshio S. (2000). Methionine requirement of juvenile Japanese flounder Paralichthys olivaceus. J. World Aquaculture Soc. 31, 618–626. doi: 10.1111/j.1749-7345.2000.tb00911.x
Alam M. S., Teshima S., Yaniharto D., Koshio S., Ishikawa M. (2002). Influence of different dietary amino acid patterns on growth and body composition of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 210, 359–369. doi: 10.1016/S0044-8486(01)00892-4
AOAC (1990). “Association of official analytical chemists,” in The official methods of analysis, 15th edition (Association of official analytical chemists, Arlington, VA, USA).
Baek S. I., Cho S. H., Kim H. S. (2021). Dietary inclusion effect of various levels of jack mackerel meal on the growth performance, feed efficiency and whole body composition of rockfish (Sebastes schlegeli). Fisheries Aquat. Sci. 24, 311–317. doi: 10.47853/FAS.2021.e30
Biswas P. R. P., Patel A. B., Saha H. (2018). Effect of dietary incorporation of chemo-attractants on growth and survival during seed rearing of Ompok bimaculatus (Bloch). Turkish J. Fisheries Aquat. Sciences 18, 491–499. doi: 10.4194/1303–2712-v18_3_15
Chellappa S., Huntingford F. A., Strang R. H. C., Thomson R. Y. (1995). Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J. Fish Biol. 47, 775–787. doi: 10.1111/j.1095-8649.1995.tb06002.x
Cho S. H., Lee S. M., Lee S. M., Park B. H., Park I. S., Choi C. Y., et al. (2005). Effect of partial replacement of fish meal with squid liver mealTM in the diet on growth and body composition of Juvenile Olive Flounder (Paralichthys olivaceus) during winter season. Fisheries Aquat. Sci. 8, 65–69. doi: 10.5657/fas.2005.8.2.065
Choi J., Lee K. W., Han G. S., Byun S. G., Lim H. J., Kim H. S. (2020). Dietary inclusion effect of krill meal and various fish meal sources on growth performance, feed utilization, and plasma chemistry of grower walleye pollock (Gadus chalcogrammus, Pallas 1811). Aquaculture Rep. 17, 100331. doi: 10.1016/j.aqrep.2020.100331
Chotikachinda R., Tantikitti C., Benjakul S., Rustad T., Kumarnsit E. (2013). Production of protein hydrolysates from skipjack tuna (Katsuwonus pelamis) viscera as feeding attractants for Asian seabass (Lates calcarifer). Aquaculture Nutr. 19, 773–784. doi: 10.1111/anu.2013.19.issue-5
Dar S. A., Kole S., Shin S. M., Jeong H. J., Jung S. J. (2021). Comparative study on antigen persistence and immunoprotective efficacy of intramuscular and intraperitoneal injections of squalene–aluminium hydroxide (Sq + Al) adjuvanted viral hemorrhagic septicaemia virus vaccine in olive flounder (Paralichthys olivaceus). Vaccine 39, 6866–6875. doi: 10.1016/j.vaccine.2021.10.026
Elmada C. Z., Huang W., Jin M., Liang X., Mai K., Zhou Q. (2016). The effect of dietary methionine on growth, antioxidant capacity, innate immune response and disease resistance of juvenile yellow catfish (Pelteobagrus fulvidraco). Aquaculture Nutr. 22, 1163–1173. doi: 10.1111/anu.2016.22.issue-6
FAO (2022). “The state of world fisheries and aquaculture 2022,” in Towards blue transformation (Rome, Italy: Food and Agricultural Organization). doi: 10.4060/cc0461en
Fazio F. (2019). Fish hematology analysis as an important tool of aquaculture: a review. Aquaculture 500, 237–242. doi: 10.1016/j.aquaculture.2018.10.030
Forster I., Ogata H. Y. (1998). Lysine requirement of juvenile Japanese flounder Paralichthys olivaceus and juvenile red sea bream Pagrus major. Aquaculture 161, 131–142. doi: 10.1016/S0044-8486(97)00263-9
Garling D. L., Wilson R. P. (1976). Optimum dietary protein to energy ratio for channel catfish fingerlings, Ictalurus punctatus. J. Nutr. 106, 1368–1375. doi: 10.1093/jn/106.9.1368
Guerard F., Guimas L., Binet A. (2002). Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catalysis B: Enzymatic 19, 489–498. doi: 10.1016/S1381-1177(02)00203-5
Gunathilaka B. E., Khosravi S., Shin J., Shin J., Herault M., Fournier V., et al. (2021). Evaluation of shrimp protein hydrolysate and krill meal supplementation in low fish meal diet for red seabream (Pagrus major). Fisheries Aquat. Sci. 24, 109–120. doi: 10.47853/FAS.2021.e11
Ha M. S., Cho S. H., Kim T. (2021). Dietary substitution of fish meal by meat meal: Effects on juvenile olive flounder (Paralichthys olivaceus) growth performance, feed utilization, haematology, biochemical profile and disease resistance against Streptococcus iniae. Aquaculture Nutr. 27, 1888–1902. doi: 10.1111/anu.13326
Hamidoghli A., Won S., Lee S., Lee S., Farris N. W., Bai S. C. (2020). Nutrition and feeding of olive flounder Paralichthys olivaceus: A Review. Rev. Fisheries Sci. Aquaculture 28, 340–357. doi: 10.1080/23308249.2020.1740166
Hancz C. (2020). Feed efficiency, nutrient sensing and feeding stimulation in aquaculture: a review. Acta Agraria Kaposváriensis 24, 35–54. doi: 10.31914/aak.2375
Hara T. J. (1994). Olfaction and gustation in fish: an overview. Acta Physiologica Scandinavica 152, 207–217. doi: 10.1111/j.1748-1716.1994.tb09800.x
Harding D. E., Allen O. W., Wilson R. P. (1977). Sulfur amino acid requirement of channel catfish: l-methionine and l-cystine. J. Nutr. 107, 2031–2035. doi: 10.1093/jn/107.11.2031
Hasanthi M., Kim M. G., Lim H., Lim J., Hur S. W., Lee S., et al. (2023). The dietary requirement for threonine in juvenile olive flounder (Paralichthys olivaceus). Fisheries Aquat. Sci. 26, 58–68. doi: 10.47853/FAS.2023.e5
Hosokawa H., Takii K., Shimeno S., Takeda M. (2001). Identification of feeding stimulants for yellowtail in muscle extract of jack mackerel. Aquaculture Sci. 49, 225–229. doi: 10.11233/aquaculturesci1953.49.225
Ikeda I., Okamoto Y., Oda K. (2012). Identification of feeding stimulants for Japanese flounder in muscle extract of jack mackerel. Aquaculture Sci. 60, 195–198. doi: 10.11233/aquaculturesci.60.195
Jeong H. S., Cho S. H. (2023). Inclusion effect of jack mackerel meal as feed stimulants in diets replacing different levels of fish meal with various animal protein sources on growth performance of olive flounder (Paralichthys olivaceus). Aquaculture Rep. 28, 101450. doi: 10.1016/j.aqrep.2022.101450
Jeong H. S., Choi D. G., Lee K. W., Cho S. H., Lim S. G., Lee B. J., et al. (2020). Attractiveness of various crude feed ingredients to juvenile olive flounder (Paralichthys olivaceus, Temminck & Schlegel) and its application to aquaculture. Aquaculture Res. 51, 4517–4532. doi: 10.1016/j.aqrep.2022.101450
Jeong H. S., Kim J., Olowe O. S., Cho S. H. (2022). Dietary optimum inclusion level of jack mackerel meal for olive flounder (Paralichthys olivaceus, Temminck & Schlegel 1846). Aquaculture 559, 738432. doi: 10.1016/j.aquaculture.2022.738432
Kader M. A., Bulbul M., Koshio S., Ishikawa M., Yokoyama S., Nguyen B. T., et al. (2012). Effect of complete replacement of fishmeal by dehulled soybean meal with crude attractants supplementation in diets for red sea bream Pagrus major. Aquaculture 350, 109–116. doi: 10.1016/j.aquaculture.2012.04.009
Kang H. K., Lee H. H., Seo C. H., Park Y. (2019). Antimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides. Mar. Drugs 17, 350. doi: 10.3390/md17060350
Khosravi S., Bui H. T. D., Herault M., Fournier V., Kim K., Lee B., et al. (2018). Supplementation of protein hydrolysates to a low-fishmeal diet improves growth and health status of juvenile olive flounder, Paralichthys olivaceus. J. World Aquaculture Soc. 49, 897–911. doi: 10.1111/jwas.12436
Khosravi S., Bui H. T. D., Rahimnejad S., Herault M., Fournier V., Kim S. S., et al. (2015). Dietary supplementation of marine protein hydrolysates in fish-meal based diets for red sea bream (Pagrus major) and olive flounder (Paralichthys olivaceus). Aquaculture 435, 371–376. doi: 10.1016/j.aquaculture.2014.10.019
Kim J., Baek S. I., Cho S. H., Kim T. (2022). Evaluating the efficacy of partially substituting fish meal with unfermented tuna by-product meal in diets on the growth, feed utilization, chemical composition and non-specific immune responses of olive flounder (Paralichthys olivaceus). Aquaculture Rep. 24, 101150. doi: 10.1016/j.aqrep.2022.101150
Kim H. S., Baek S. I., Lee K. W., Jeong H. S., Cho S. H. (2019). Attractiveness of various protein sources to juvenile rockfish (Sebastes schlegeli, Hilgendorf 1880). J. Appl. Aquaculture 32, 205–220. doi: 10.1080/10454438.2019.1655516
Kim H. S., Cho S. H. (2019). Dietary inclusion effect of feed ingredients showing high feeding attractiveness to rockfish (Sebastes schlegeli Hilgendorf 1880) on the growth performance, feed utilization, condition factor and whole body composition of fish. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 231, 66–73. doi: 10.1016/j.cbpa.2019.01.011
Kim J., Cho S. H., Kim T., Hur S. W. (2021). Substitution effect of fish meal with various sources of animal by-product meals in feed on growth, feed utilization, body composition, haematology and non-specific immune response of olive flounder (Paralichthys olivaceus, Temminck & Schlegel 1846). Aquaculture Res. 52, 2802–2817. doi: 10.1111/are.15132
Kim K. D., Jang J. W., Kim K. W., Lee B. J., Hur S. W., Han H. S. (2018). Tuna by-product meal as a dietary protein source replacing fishmeal in juvenile Korean rockfish Sebastes schlegeli. Fisheries Aquat. Sci. 21, 1–8. doi: 10.1186/s41240-018-0107-y
Kim K. I., Kayes T. B., Amundson C. H. (1992). Requirements for sulphur amino acids and utilization of d-methionine by rainbow trout (Oncorhyncus mykiss). Aquaculture 101, 95–103. doi: 10.1016/0044-8486(92)90235-D
Kim K., Lee S. (2004). Requirement of dietary n-3 highly unsaturated fatty acids for juvenile flounder (Paralichthys olivaceus). Aquaculture 229, 315–323. doi: 10.1016/S0044-8486(03)00356-9
KOSIS (2023) (Korea: Korean Statistical Information Service). Available online at: https://kosis.kr/eng/ (Accessed 25 February 2022).
Lange S., Guđmundsdottir B. K., Magnadottir B. (2001). Humoral immune parameters of cultured Atlantic halibut (Hippoglossus hippoglossus L.). Fish Shellfish Immunol. 11, 523–535. doi: 10.1006/fsim.2000.0333
Lee S., Aya F. A., Won S., Hamidoghli A., Bai S. C. (2020). Effects of replacing dietary fish oil with beef tallow on growth performance, serological parameters, and fatty acid composition in juvenile olive flounder, Paralichthys olivaceus. J. World Aquaculture Soc. 51, 393–406. doi: 10.1111/jwas.12654
Lee S. M., Cho S. H., Kim K. D. (2000). Effects of dietary protein and energy levels on growth and body composition of juvenile flounder (Paralichthys olivaceus). J. World Aquaculture Soc. 31, 306–315. doi: 10.1111/j.1749-7345.2000.tb00882.x
Li L., Fang J., Liang X. F., Alam M. S., Liu L., Yuan X. (2019). Effect of feeding stimulants on growth performance, feed intake and appetite regulation of mandarin fish Siniperca chuatsi. Aquaculture Res. 50, 3684–3691. doi: 10.1111/are.14327
Liew K. S., Yong A. S. K., Lim L. S., Kawamura G. (2020). Dietary sugarcane juice as a feeding stimulant for the purple mud crab Scylla tranquebarica. Aquaculture Res. 51, 2164–2167. doi: 10.1111/are.14545
Martínez-Llorens S., Moñino A. V., Tomás Vidal A., Salvador V. J. M., Pla Torres M., Jover Cerdá M. (2007). Soybean meal as a protein source in gilthead sea bream (Sparus aurata L.) diets: effects on growth and nutrient utilization. Aquaculture Res. 38, 82–90. doi: 10.1111/j.1365-2109.2006.01637.x
McCrickerd K., Forde C. G. (2016). Sensory influences on food intake control: moving beyond palatability. Obes. Rev. 17, 18–29. doi: 10.1111/obr.12340
Miyasaki T., Harada K. (2002). Feeding attractants and stimulants for aquatic animals. Fisheries Sci. 68, 1406–1409. doi: 10.2331/fishsci.68.sup2_1406
Morais S. (2017). The physiology of taste in fish: potential implications for feeding stimulation and gut chemical sensing. Rev. Fisheries Sci. Aquaculture 25, 133–149. doi: 10.1080/23308249.2016.1249279
Muley A. B., Pandit A. B., Singhal R. S., Dalvi S. G. (2021). Production of biologically active peptides by hydrolysis of whey protein isolates using hydrodynamic cavitation. Ultrasonics Sonochemistry 71, 105385. doi: 10.3390/md17060350
Nagel F., Von Danwitz A., Schlachter M., Kroeckel S., Wagner C., Schulz C. (2014). Blue mussel meal as feed attractant in rapeseed protein-based diets for turbot (Psetta maxima L.). Aquaculture Res. 45, 1964–1978. doi: 10.1111/are.2014.45.issue-12
Ng W. K., Lu K. S., Hashim R., Ali A. (2000). Effects of feeding rate on growth, feed utilization and body composition of a tropical bagrid catfish. Aquaculture Int. 8, 19–29. doi: 10.1023/A:1009216831360
Novriadi R., Spangler E., Rhodes M., Hanson T., Davis D. A. (2017). Effects of various levels of squid hydrolysate and squid meal supplementation with enzyme-treated soy on growth performance, body composition, serum biochemistry and histology of Florida pompano Trachinotus carolinus. Aquaculture 481, 85–93. doi: 10.1016/j.aquaculture.2017.08.032
Nunes A. J., Sá M. V., Andriola-Neto F. F., Lemos D. (2006). Behavioral response to selected feed attractants and stimulants in Pacific white shrimp, Litopenaeus vannamei. Aquaculture 260, 244–254. doi: 10.1016/j.aquaculture.2006.06.027
Oliva-Teles A., Enes P., Peres H. (2015). “Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish,” in Feed and feeding practices in aquaculture. Ed. Davis D. A. (Sawston, Cambridge, United Kingdom: Elsevier), 203–233. doi: 10.1016/B978–0-08–100506–4.00008–8
Oncul F. O., Aya F. A., Hamidoghli A., Won S., Lee G., Han K. R., et al. (2019). Effects of the dietary fermented tuna by-product meal on growth, blood parameters, nonspecific immune response, and disease resistance in juvenile olive flounder, Paralichthys olivaceus. J. World Aquaculture Soc. 50, 65–77. doi: 10.1111/jwas.12535
Pan F. Y., Feng L., Jiang W. D., Jiang J., Wu P., Kuang S. Y., et al. (2016). Methionine hydroxy analogue enhanced fish immunity via modulation of NF-κB, TOR, MLCK, MAPKs and Nrf2 signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 56, 208–228. doi: 10.1016/j.fsi.2016.07.020
Peng D., Peng B., Li J., Zhang Y., Luo H., Xiao Q., et al. (2022). Effects of three feed attractants on the growth, biochemical indicators, lipid metabolism and appetite of Chinese perch (Siniperca chuatsi). Aquaculture Rep. 23, 101075. doi: 10.1016/j.aqrep.2022.101075
Senzui A., Masumoto T., Fukada H. (2020). Neuropeptide Y expression in response to sensory organ-detected fish meal soluble components and orally fed fish meal-based diet in yellowtail Seriola quinqueradiata. Aquaculture 514, 734512. doi: 10.1016/j.aquaculture.2019.734512
Singh R. K., Balange A. K., Khandagale P. A., Chavan S. L. (2006). Evaluation of fish meals as natural feed stimulants on the feed behavior of fry and juveniles of Lates calcarifer (Bloch). Asian Fisheries Sci. 19, 97–106. doi: 10.33997/j.afs.2006.19.2.001
Smith D. M., Tabrett S. J., Barclay M. C., Irvin S. J. (2005). The efficacy of ingredients included in shrimp feeds to stimulate intake. Aquaculture Nutr. 11, 263–272. doi: 10.1111/j.1365-2095.2005.00349.x
Stieglitz J. D., Hoenig R. H., Baggett J. K., Tudela C. E., Mathur S. K., Benetti D. D. (2021). Advancing production of marine fish in the United States: Olive flounder, Paralichthys olivaceus, aquaculture. J. World Aquaculture Soc. 52, 566–581. doi: 10.1111/jwas.12804
Tacon A. G. J., Metian M. (2008). Global overview on the use of fish meal and fish oil in industrially com-pounded aquafeeds: Trends and future prospects. Aquaculture 285, 146–158. doi: 10.1016/j.aquaculture.2008.08.015
Takakuwa F., Masumoto T., Fukada H. (2019). Identification of feeding stimulants for greater amberjack Seriola dumerili in muscle tissue of jack mackerel Trachurus japonicus. Fisheries Sci. 85, 387–395. doi: 10.1007/s12562-018-01285-w
Tang L., Wang G. X., Jiang J., Feng L., Yang L., Li S. H., et al. (2009). Effect of methionine on intestinal enzymes activities, microflora and humoral immune of juvenile Jian carp (Cyprinus carpio var. Jian). Aquaculture Nutr. 15, 477–483. doi: 10.1111/anu.2009.15.issue-5
Tusche K., Berends K., Wuertz S., Susenbeth A., Schulz C. (2011). Evaluation of feed attractants in potato protein concentrate based diets for rainbow trout (Oncorhynchus mykiss). Aquaculture 321, 54–60. doi: 10.1016/j.aquaculture.2011.08.020
Twibell R. G., Wilson K. A., Brown P. B. (2000). Dietary sulfur amino acid requirement of juvenile yellow perch fed the maximum cystine replacement value for methionine. J. Nutr. 130, 612–616. doi: 10.1093/jn/130.3.612
Uyan O., Koshio S., Teshima S. I., Ishikawa M., Thu M., Alam M. S., et al. (2006). Growth and phosphorus loading by partially replacing fishmeal with tuna muscle by-product powder in the diet of juvenile Japanese flounder, Paralichthys olivaceus. Aquaculture 257, 437–445. doi: 10.1016/j.aquaculture.2006.02.060
Wang L., Lu Q., Luo S., Zhan W., Chen R., Lou B., et al. (2016). Effect of dietary lipid on growth performance, body composition, plasma biochemical parameters and liver fatty acids content of juvenile yellow drum Nibea albiflora. Aquaculture Rep. 4, 10–16. doi: 10.1016/j.aqrep.2016.05.002
Yamamoto T., Unuma T., Akiyama T. (2000). The influence of dietary protein and fat levels on tissue amino acid levels of fingerling rainbow trout (Oncorhynchus mykiss). Aquaculture 182, 353–372. doi: 10.1016/S0044-8486(99)00277-X
Yu H., Wang X., Kong F., Song X., Tan Q. (2021). The attractive effects of amino acids and some classical substances on grass carp (Ctenopharyngodon idellus). Fish Physiol. Biochem. 47, 1489–1505. doi: 10.1007/s10695-021-00990-1
Yuan Y., Lawrence A. L., Chehade S. B., Jensen K. E., Barry R. J., Fowler L. A., et al. (2021). Feed intake as an estimation of attractability in Pacific white shrimp Litopenaeus vannamei. Aquaculture 532, 736041. doi: 10.1016/j.aquaculture.2020.736041
Zhao W., Liu Z. L., Niu J. (2021). Growth performance, intestinal histomorphology, body composition, hematological and antioxidant parameters of Oncorhynchus mykiss were not detrimentally affected by replacement of fish meal with concentrated dephenolization cottonseed protein. Aquaculture Rep. 19, 100557. doi: 10.1016/j.aqrep.2020.100557
Keywords: tuna by-product meal, jack mackerel meal, Paralichthys olivaceus, alternative protein source, feed enhancing effect
Citation: Islam MR, Cho SH and Kim T (2024) Inclusion effect of jack mackerel meal in olive flounder (Paralichthys olivaceus) diet substituting blended fish meal with tuna by-product meal on growth, feed availability, and economic efficiency. Front. Mar. Sci. 11:1407162. doi: 10.3389/fmars.2024.1407162
Received: 26 March 2024; Accepted: 23 May 2024;
Published: 04 June 2024.
Edited by:
Marty Riche, Florida Atlantic University, United StatesReviewed by:
Marta Monteiro, University of Porto, PortugalMd. Tawheed Hasan, Sylhet Agricultural University, Bangladesh
Copyright © 2024 Islam, Cho and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung Hwoan Cho, Y2hvc3VuaEBrbW91LmFjLmty
 Md. Rabiul Islam
Md. Rabiul Islam Sung Hwoan Cho
Sung Hwoan Cho Taeho Kim4
Taeho Kim4