
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Mar. Sci. , 05 July 2024
Sec. Discoveries
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1401258
This article is part of the Research Topic New Observations on the Behavior, Ecology, and Biology of Sharks and Rays View all 18 articles
Data on the reproductive biology of elasmobranchs are essential for understanding their life history. Published studies on batoid ray reproductive biology are comparatively scarce, leading to limited understanding and data gaps. The Oceania fantail ray, Taeniura lessoni, is a good example. This Data Deficient nearshore stingray is restricted to Melanesia, with lacking biological and ecological data, including reproduction. To expand upon the limited life-history data for this species, this short paper provides observational data on the reproductive condition in female T. lessoni, at Drawaqa Island, Fiji. Field work involved direct observations and ocean temperature measurements. Over 40 days spanning three months, 105 surveys were conducted across five sites, resulting in 71 sightings of the species. Based on spot patterns and body markings, four female individuals were identified. Between January and March 2024, these females exhibited convex dorsa indicating advanced gestation, transitioning to concave dorsa suggesting parturition. The presence of neonates from early March onwards coincided with the estimated parturition period inferred from the rays' condition. Furthermore, a female previously pregnant was photographed with a dermal abrasion around her pectoral fin, possibly indicating pre-copulatory biting, suggesting a continuous reproductive cycle. The average monthly water temperature at the surveyed sites remained relatively stable throughout the study. Collectively, our findings suggest that Drawaqa Island provides suitable habitat niches for reproductive activities in female T. lessoni. Repeated and long-term data is certainly needed to confirm either a continuous reproductive cycle or seasonal peaks. While preliminary, our observational data represents the first documentation on female reproductive condition in a stingray in Fiji.
Elasmobranchs (sharks, skates, and rays) are an evolutionarily conserved, diverse, and threatened vertebrate group (Dulvy et al., 2021; Sherman et al., 2023). Threats encompass direct and indirect fisheries activities (Worm et al., 2024), ocean warming (Rosa et al., 2017; Osgood et al., 2021), and habitat degradation, including disturbances to coastal breeding and pupping grounds (Sherman et al., 2020; Simpfendorfer et al., 2023).
Elasmobranchs have developed nine distinct reproductive strategies (Awruch, 2015) over their 400-million-year evolutionary history (Kriwet et al., 2008). Batoids, the most diverse group of cartilaginous fishes (Aschliman et al., 2012), comprise four orders, 23 families and 663 species, with many more yet to be described (Last et al., 2016a). While the largest order, Rajiformes (skates), is strictly oviparous, the remaining orders (Myliobatiformes, Rhinopristiformes, and Torpediniformes) are viviparous, with lecithotrophic and matrotrophic modes of reproduction (Conrath and Musick, 2012; Awruch, 2015). Myliobatiformes, including the family Dasyatidae (stingrays), rely on lipid histotrophy via trophonemata. Stingrays contain 19 genera, 86 extant species (Last et al., 2016a), and represent the most abundant group of rays occurring in tropical and subtropical coastal waters (White and Dharmadi, 2007). Stingrays produce broods of one to 10 with gestation periods up to 11 months, while smaller tropical species have shorter gestation periods of three to six months (Fahy et al., 2007; Pierce et al., 2009; Mull et al., 2010; Furumitsu et al., 2019). The duration of reproductive cycles varies among stingrays (Walker, 2020), and annual and biannual cycles have been confirmed in wild populations (Ramírez-Mosqueda et al., 2012; Schieber et al., 2023). However, batoid ecology and life history are comparably less understood than in sharks (Bräutigam et al., 2016; Martins et al., 2018; Jorgensen et al., 2022), with more than 250 Data Deficient species in the International Union for Conservation of Nature (IUCN) Red List (IUCN, 2023).
The Oceania fantail ray, Taeniura lessoni, (Last, White & Naylor, 2016) was described in 2016 (Last et al., 2016b), marking the second species within the genus alongside the widely-distributed bluespotted lagoon ray, T. lymma, (Forsskål, 1775). The disc width (DW), defined as the maximum distance between the wingtips (Serra-Pereira et al., 2010), ranges between 18 cm to 22 cm in female T. lessoni paratypes (Last et al., 2016b). The mature male holotype has a 20.9 cm DW, with large claspers measuring 21.2% of this width, while the immature paratype male measured 18.5 cm (Last et al., 2016b). T. lessoni is smaller than T. lymma and lacks the pair of vivid blue longitudinal stripes found along the tail. Also, the species appears to be restricted to Melanesia, including Papua New Guinea, Solomon Islands, Vanuatu, and Fiji (Last et al., 2016a; Hylton et al., 2017). T. lessoni inhabits shallow-water coral reefs, usually at depths of 20 m and less, typically shelters in caves during the day, and forages at night (Last et al., 2016a). In T. lymma, the embryos are initially nourished by the yolk sac and subsequently feed on uterine secretions (Hamlett et al., 2005; Musick et al., 2005; Abel and Grubbs, 2020). Fecundity has been reported with brood sizes of one to seven in wild individuals (Ferreira, 2013; Pereira et al., 2017). However, reproductive biology data for T. lessoni is lacking, and this species is classified as Data Deficient by the IUCN (Kyne and Finucci, 2018). In Fiji, T. lessoni is regularly observed during snorkeling trips and dives. Sightings are reported from across the archipelago (Glaus et al., 2024a), and the species is caught in small-scale fishing activities (Glaus et al., 2024b). To expand upon the limited life-history data for this range-restricted species, this study represents preliminary observational data focusing on the distribution and reproductive condition of female T. lessoni.
The study area was located approximately between 17° South latitude and 177° East longitude (Figure 1) and focused on the coastal waters off Drawaqa Island within the Yasawa Island Group in western Fiji. Besides a single tourism operator (Barefoot Manta Island Resort), Drawaqa Island is uninhabited and belongs to the traditional landowners of Mua-ira on the nearby island of Naviti (Murphy et al., 2018). The northern point of Drawaqa Island includes five beaches, which were surveyed: Goat, Lagoon, Manta, Sunrise, and Sunset Beach (Figure 1).
Since January 2024, research on Drawaqa Island has been exploring environmental factors influencing spatiotemporal variation in ray species abundance and distribution, with the data presented here being part of a larger dataset. Fieldwork involved direct observations and ocean temperature measurements using three HOBO water temperature Pro V2 (U22) data loggers deployed on-site. Over 40 days across January to March, 105 surveys were conducted to observe rays. Each survey lasted 45 min for standardization, and were conducted as roving explorations, including 53 snorkel surveys, 32 beach walks, and 20 SCUBA dives: Sunrise Beach (68 surveys: 39 snorkels, 20 dives, 9 walks), Lagoon Beach (11 surveys: 10 walks, 1 snorkel), Goat Beach (10 snorkels), Manta Beach (9 surveys: 2 snorkels, 7 walks), and Sunset Beach (7 surveys: 6 walks, 1 snorkel). Snorkel surveys were conducted at all sites along 100 m line transects parallel to the shoreline. Maximum depth was 12 m, with visibility extending to the seafloor. SCUBA dives, performed only at Sunrise Beach due to sufficient depth, followed predetermined 100 m line transects perpendicular to the shore, from the shallows to a maximum depth of 21 m, with visibility ranging from 8 to 20m. For each line transect, the surrounding visibility was scanned for the presence of T. lessoni within the maximum visible range on either side of the line. The line transects followed the reef structure along the same route for each snorkel and dive survey. Beach walks were carried out at four of the five sites.
Whenever possible, sighted T. lessoni were photographed using either a GoPro12 or Olympus TG7. Images of T. lessoni were recorded to identify distinct spotting patterns and body markings (McIvor et al., 2023). Photographs were also inspected for signs of advanced gestation (i.e., convex dorsum), recent parturition (i.e., concave dorsum) (Henningsen, 2000; Spieler et al., 2013), and evidence of mating behaviour (i.e., fresh bite wounds) (Kajiura et al., 2000). The sex of rays was determined by the presence or absence of claspers (Awruch et al., 2008), and the DW was estimated in situ and from images (Supplementary Figure 1). T. lessoni with a DW greater than 20 cm were classified as mature, and rays less than 20 cm DW were considered immature (Last et al., 2016b). Size at birth for T. lymma is 13 cm to 14 cm DW (Last et al., 2016b). As T. lessoni is smaller and due to the uncertainty associated with in situ estimates, only the smallest individuals, with an estimated DW of 10 cm (roughly palm-sized), were considered as neonates.
The data were recorded, with individuals whose sex could not be determined denoted as “nA”. Plots were generated using the ggplot2 package (Wickham, 2011; Wickham and Bryan, 2023) in R (R Development Core Team, 2005). Catch per unit effort (CPUE) was calculated by dividing total T. lessoni sightings at a site by the number of surveys conducted there.
T. lessoni were observed in 71 surveys (67.6% of the total number surveys). Number of sightings by location varied: Sunrise Beach (n=47), Goat Beach (n=9), Lagoon Beach (n=6), Manta Beach (n=6), and Sunset Beach (n=3). At Sunrise Beach, mature females were sighted 38 times, six times at Goat Beach, and once each at Manta Beach and Sunset Beach. Mature males were observed three times each at Sunrise Beach and Goat Beach, and once at Sunset Beach. Neonates were sighted from early March onwards: six times at Lagoon Beach, two times at Manta Beach, and one time each at Sunrise and Sunset Beach (Supplementary Figure 2). CPUE was highest at Goat Beach (0.9 T. lessoni observed per survey), followed by Sunrise Beach (0.7 T. lessoni observed per survey), Manta Beach (0.7 T. lessoni observed per survey), Lagoon Beach (0.6 T. lessoni observed per survey), and Sunset Beach (0.4 T. lessoni observed per survey).
Snorkelling recorded the highest number of sightings (n=48), followed by beach walks (n=15), and dives (n=8) (Table 1). Mature females and males were mostly observed during snorkelling surveys, while immature specimens were predominantly sighted during beach walks (Table 1).
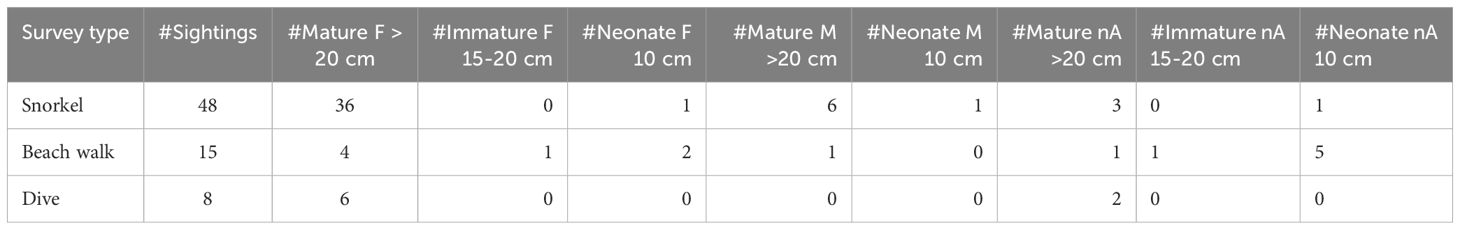
Table 1 Sightings of T. lessoni across different survey methods, including the number of sightings per sex, and likely maturity level based on in situ DW estimates.
At Sunrise Beach, four females in advanced gestation with conspicuously convex dorsa (Figure 2) were recorded, occurring in shallow waters down to eight m depth. These four females were collectively recorded 24 times at the same site from the beginning of January until the end of March 2024. Individual 1 was recorded 13 times, Individual 2 six times, Individual 3 four times, and Individual 4 once, accounting for 63.2% of all female T. lessoni sightings at Sunrise Beach (n=38, Supplementary Figure 2). The DW of Individual 1 was estimated to be 24 cm to 25 cm, DW of the remaining three individuals in advance gestation was estimated between 21 to 25 cm.
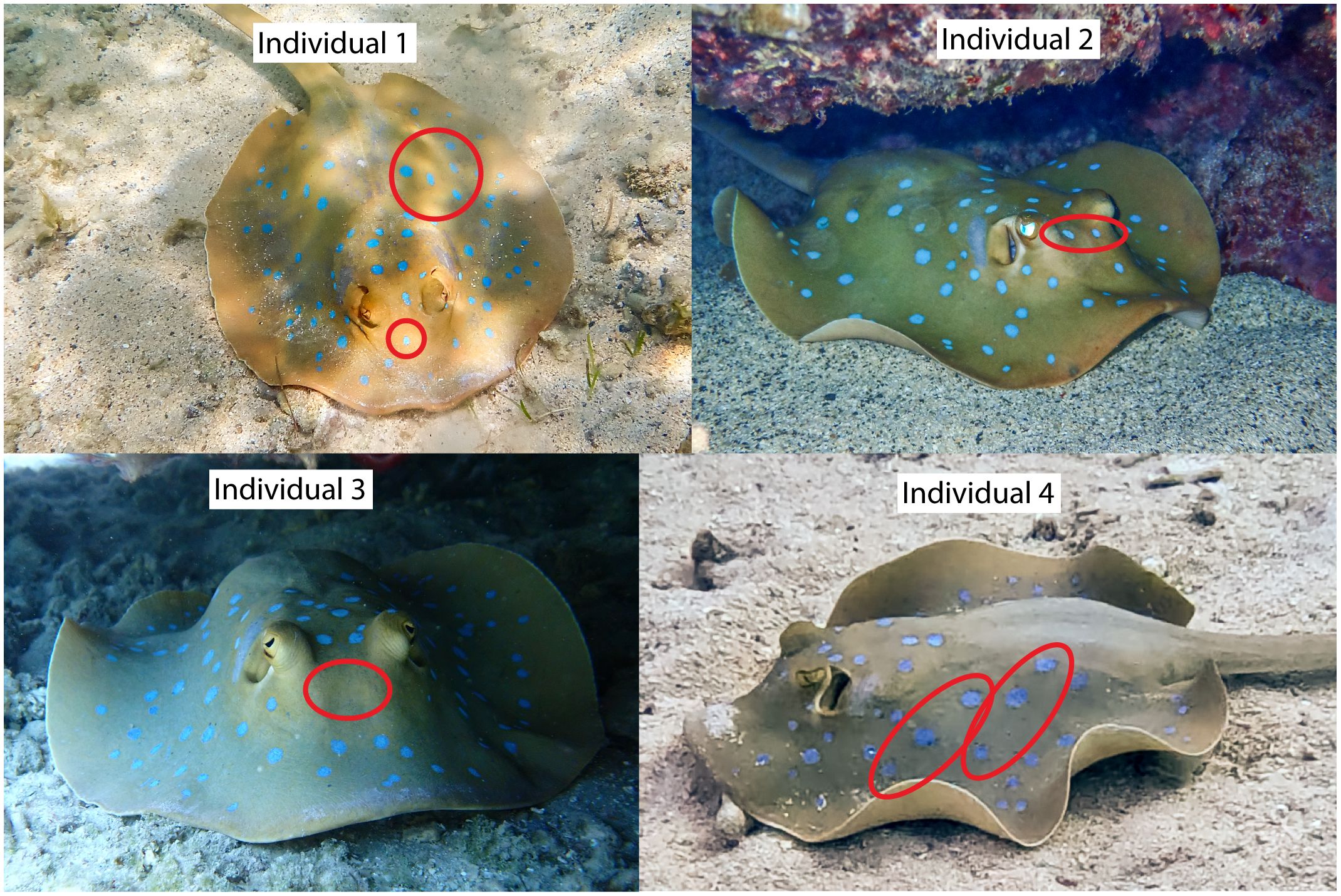
Figure 2 Photographs of individual T. lessoni in advanced gestation observed at Sunrise Beach, Drawaqa Island, with convex dorsa, indicative for advanced gestation. Encircled in red are selected differentiating features: Individual 1 displays single antorbital blue spots, and a row of three prominent vertical blue spots followed by a row of horizontal blue spots posterolateral; Individual 2 has two antorbital blue spots; Individual 3 features a plain antorbital area, including a tail partly cut (shown in Figure 3F), and Individual 4 has scapular and posterolateral rows of three blue spots.
The first indication of parturition was noted on February 29, 2024, when Individual 1 was photographed with a concave dorsum (Figures 3A, B). Over the following two weeks, Individual 3 (March 4) and Individual 2 (March 12) followed suit (Figures 3C-F). Additionally, Individual 1 exhibited a visible dermal abrasion on the left pectoral fin (Figure 3B; Supplementary Figure 3).
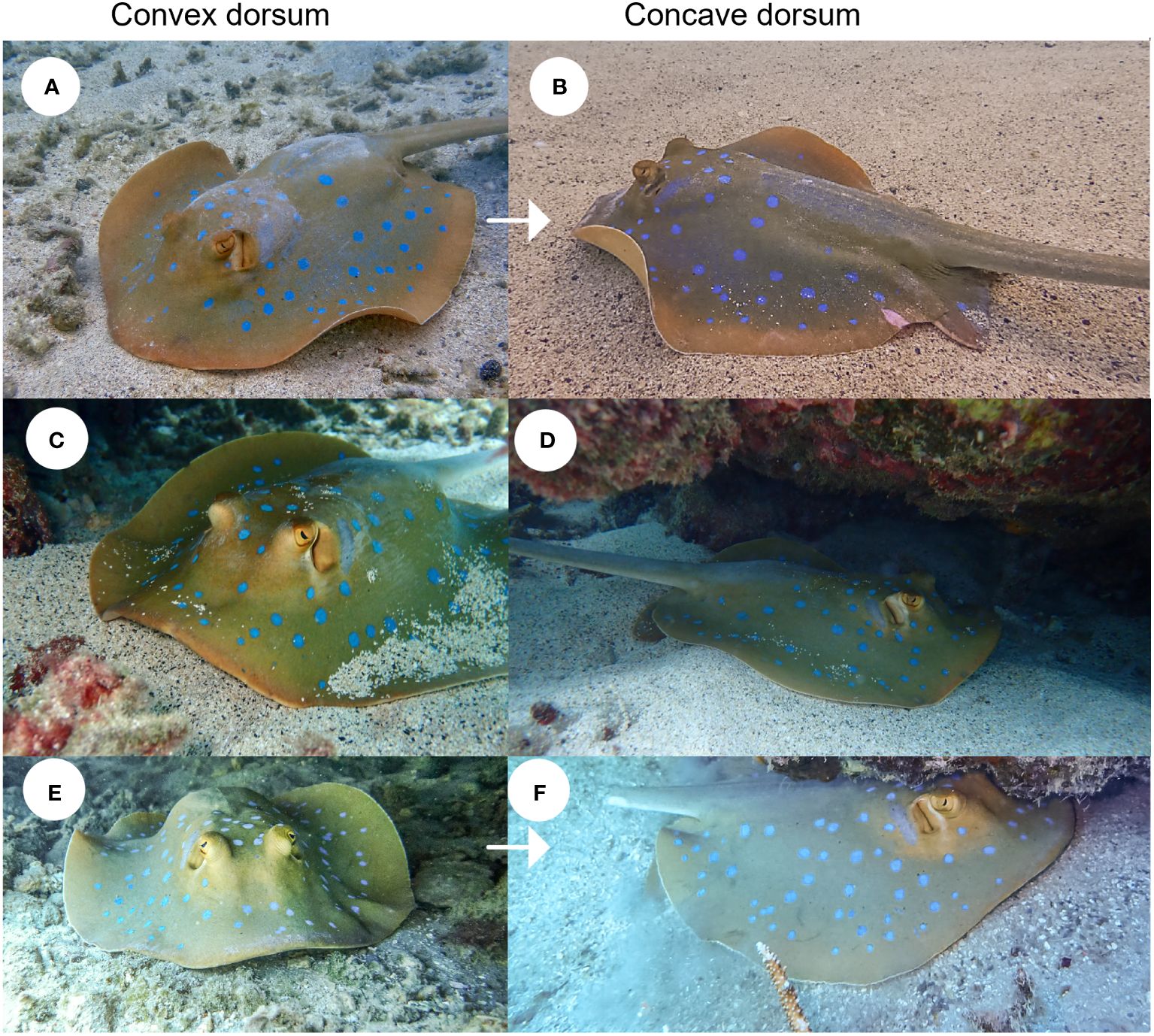
Figure 3 Visual comparison of Individuals 1, 2, and 3 during advanced gestation (A, C, E) and post-partum (B, D, F) stages. Image (A) of individual 1 was taken on February 3, 2024, during advanced gestation, while (B) was captured on February 29, 2024, post-partum. Image (C) shows the individual 2 on January 13, 2024, during advanced gestation, and (D) on March 12, 2024, post-partum. Image (E) shows the individual 3 on February 11, 2024, during pregnancy, and (F) on March 4, 2024, post-partum.
During the study period, the water temperature ranged from 28.22°C to 33.08°C. The lowest temperature was recorded at Sunrise Beach in January with an average of 29.94°C, while the highest temperature was measured at Sunset Beach with an average of 30.52°C in March.
This study represents the first documentation of advanced gestation in a stingray species in Fiji. We recorded four pregnant T. lessoni (Figure 2), of which three gave birth between the end of February and mid-March, based upon photographic evidence (Figure 3). The presence of neonates from early March onwards coincided with the estimated parturition period inferred from the rays’ condition. We were unable to determine the periodicity of the reproductive cycle and the gestation period, but T. lessoni may reproduce asynchronously and aseasonally. In a captive stingray species (formerly known as Dasyatis kuhlii and Neotrygon kuhlii) mating was observed immediately after parturition, suggesting the absence of a specific breeding season (Janse and Schrama, 2010). Based on bycatch data from eastern Indonesia, neither distinct seasonal reproductive cycles nor synchronicity in three stingray species could be determined, indicating a continuous reproductive cycle (White and Dharmadi, 2007). The dermal wound around the pectoral fin in Individual 1 (Figure 3B; Supplementary Figure 3) suggests a bite or abrasion from a pectoral grip or pre-copulatory biting, indicating that copulation may occur soon or immediately after parturition, as observed in captive T. lymma (Smith et al., 2017). Therefore, a continuous cycle with the potential for multiple pregnancies annually seems likely. However, long-term data, ideally combined with ultrasound diagnostics (Murakumo et al., 2020), are required to document gestation and to determine whether T. lessoni follows a continuous reproductive cycle or exhibits seasonal peaks around Drawaqa Island. To ensure practicality and minimal invasiveness, we determined maturity levels based solely on in situ size estimates. Assaying sex steroid hormones instead, enables accurate determination of maturity levels and depiction of cumulative proportions across different developmental stages (Mull et al., 2010). The surveyed sites on Drawaqa Island exhibit similar average temperatures ranging from 29.94°C to 30.52°C. Neonates and presumably immature T. lessoni were mostly observed during beach walks (Table 1), and at Manta Beach and Lagoon Beach (Supplementary Figure 2). Manta Beach and Lagoon Beach have a similar habitat composition with areas of sandy patches, rubbles, and seagrass assemblages, which could provide shelter for neonate T. lessoni (Dabruzzi et al., 2013). Sunrise Beach, predominantly frequented by mature females, has narrower expanses of sandy patches. Except for Individual 4, which was sighted only once, the other three females remained at Sunrise Beach, indicating repeated use of the area. Interestingly, mature males were only encountered seven times (Table 1). One possibility to explain the skewed sex ratio towards females could be attributed to the increased energetic requirements of females during gestation (Jirik and Lowe, 2012), and thus the selection of areas that offer favourable conditions, including prey availability (Delpiani et al., 2013) and protection from predators (Martins et al., 2018).
Moving forward, a small-scale passive acoustic telemetry study, combined with ongoing environmental data collection, could help better understand presence, activity patterns, sexual segregation (Simpson et al., 2021), and seasonal or long-term site fidelity (Schlaff et al., 2014; Elston et al., 2021; Kraft et al., 2023). The waters surrounding Drawaqa Island are home to at least nine batoid species: T. lessoni (Data Deficient), Mobula birostris (Endangered), Aetobatus ocellatus, M. alfredi, Pateobatis fai, Taeniurops meyeni and Urogymnus asperrimus (all Vulnerable), Rhynchobatus australiae (Critically Endangered), and Neotrygon sp (Gordon and Vierus, 2022; Glaus et al., 2024a). Our preliminary data indicate that Drawaqa’s nearshore waters likely serve as pupping grounds for T. lessoni. Considering this, along with the diversity of species, the easy accessibility, and the continued monitoring setup, this location is ideal for further studies to deepen our understanding of ray ecology and biology.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation upon reasonable request.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study was non-invasive and did not include any handling of living animals. The findings solely derive from visual observations.
KG: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. TV: Conceptualization, Data curation, Validation, Visualization, Writing – original draft, Writing – review & editing. RM: Data curation, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding was provided by the DrawaqaMarine Conservation Trust and by the Deutsche Stiftung Meeresschutz. The Funders are Drawaqa Marine Conservation Trust and Deutsche Stiftung Meeresschutz.
Special thanks go to Victor Bonito of Reef Explorers Fiji for sharing the water temperature data, to James Szymankiewicz for the picture used in Figure 3B, and to Peter Last for his expertise on the photographs of the individual female rays. We acknowledge the Directors of Barefoot Manta Island Resort, and Mirko Rossi, Rafal Jaklik, and Martin Eberle for their continuous support during the surveys. We thank the anonymous reviewers for their constructive comments and valuable suggestions, which greatly improved the quality of this paper.
RM is a board member of the Drawaqa Marine Conservation Trust.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1401258/full#supplementary-material
Supplementary Figure 1 | In situ DW estimate of Individual1.
Supplementary Figure 2 | Number of T. lessoni sightings and estimated maturity levels across the surveyed sites.
Supplementary Figure 3 | Individual1 feeding, showing visible dermal abrasion on the left side.
Abel D. C., Grubbs R. D. (2020). Shark Biology and Conservation: essentials for educators, students, and enthusiasts (Johns Hopkins University Press).
Aschliman N. C., Claeson K. M., Mceachran J. D. (2012). Phylogeny of batoidea. Biol. sharks their relatives 2, 57–96.
Awruch C. A. (2015). Reproduction strategies. Fish Physiol Academic Press 34, 225–310. doi: 10.1016/B978-0-12-801289-5.00007-9
Awruch C. A., Frusher S. D., Pankhurst N. W., Stevens J. D. (2008). Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar. Ecol. Prog. Ser. 355, 277–285. doi: 10.3354/meps07227
Bräutigam A., Callow M., Campbell I. (2016). Global priorities for conserving sharks and rays: a 2015-2025 strategy. IUCN: International Union for Conservation of Nature. Global Sharks and Rays Initiative, IUCN Species Survival Commission (SSC), Shark Specialist Group, The Shark Trust, UK, TRAFFIC International, Wildlife Conservation Society (WCS), US, WWF. Retrieved from https://policycommons.net/artifacts/1375898/global-priorities-for-conserving-sharks-and-rays/1990160/.
Conrath C. L., Musick J. A. (2012). Reproductive biology of elasmobranchs. Biol. sharks their relatives 2, 291–311.
Dabruzzi T. F., Bennett W. A., Rummer J. L., Fangue N. A. (2013). Juvenile ribbontail stingray, Taeniura lymma (Forsskål 1775)(Chondrichthyes, Dasyatidae), demonstrate a unique suite of physiological adaptations to survive hyperthermic nursery conditions. Hydrobiologia 701, 37–49. doi: 10.1007/s10750-012-1249-z
Delpiani G. E., Spath M. C., Figueroa D. E. (2013). Feeding ecology of the southern thorny skate, Amblyraja doellojuradoi on the Argentine Continental Shelf. J. Mar. Biol. Assoc. United Kingdom 93, 2207–2216. doi: 10.1017/S0025315413000787
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31, 4773–4787.e8. doi: 10.1016/j.cub.2021.08.062
Elston C., Cowley P. D., von Brandis R. G., Lea J. (2021). Residency and habitat use patterns by sympatric stingrays at a remote atoll in the Western Indian Ocean. Mar. Ecol. Prog. Ser. 662, 97–114. doi: 10.3354/meps13632
Fahy D. P., Spieler R. E., Hamlett W. C. (2007). Preliminary observations on the reproductive cycle and uterine fecundity of the yellow stingray, Urobatis jamaicensis (Elasmobranchii: Mylioba tiformes: Urolophidae) in Southeast Florida, USA. Raffles Bull. Zoology 131, 131–139.
Ferreira A. S. (2013). Breeding and juvenile growth of the ribbontail stingray Taeniura lymma at Oceanário de Lisboa Doctoral dissertation. (Repositório da Universidade de Lisboa).
Furumitsu K., Wyffels J. T., Yamaguchi A. (2019). Reproduction and embryonic development of the red stingray Hemitrygon akajei from Ariake Bay, Japan. Ichthyological Res. 66, 419–436. doi: 10.1007/s10228-019-00687-9
Glaus K., Gordon L., Vierus T., Marosi N. D., Sykes H. (2024a). Rays in the shadows: batoid diversity, occurrence, and conservation status in Fiji. Biology 13, 73. doi: 10.3390/biology13020073
Glaus K., Savou R., Brunnschweiler J. M. (2024b). Characteristics of Fiji’s small-scale ray fishery and its relevance to food security. Mar. Policy 163, 106082. doi: 10.1016/j.marpol.2024.106082
Gordon L., Vierus T. (2022). First photographic evidence of oceanic manta rays (Mobula birostris) at two locations in the Fiji islands. PeerJ 10, e13883. doi: 10.7717/peerj.13883
Hamlett W. C., Jones C. J., Paulesu L. (2005). “Placentatrophy in sharks,” in Reproductive biology and phylogeny of Chondrichthys, Sharks, Batoids, and Chimaeras, (Routledge, Taylor & Francis Group) 3, 463–502.
Henningsen A. D. (2000). Notes on reproduction in the southern stingray, Dasyatis americana (Chondrichthyes: Dasyatidae), in a captive environment. Copeia 2000, 826–828. doi: 10.1643/0045-8511(2000)000[0826:NORITS]2.0.CO;2
Hylton S., White W., Chin A. (2017). The sharks and rays of the Solomon Islands: a synthesis of their biological diversity, values and conservation status. Pacific Conserv. Biol. 23, 324–334. doi: 10.1071/PC17012
IUCN (2023). The IUCN Red List of Threatened Species (IUCN). Available online at: www.iucnredlist.org (Accessed 03.03 2024).
Janse M., Schrama J. W. (2010). Reproductive cycle, nutrition and growth of captive blue spotted stingray, Dasyatis kuhlii (Dasyatidae). J. Mar. Biol. Assoc. United Kingdom 90, 353–360. doi: 10.1017/S002531540999035X
Jirik K., Lowe C. (2012). An elasmobranch maternity ward: female round stingrays Urobatis halleri use warm, restored estuarine habitat during gestation. J. Fish Biol. 80, 1227–1245. doi: 10.1111/j.1095-8649.2011.03208.x
Jorgensen S. J., Micheli F., White T. D., van Houtan K. S., Alfaro-Shigueto J., Andrzejaczek S., et al. (2022). Emergent research and priorities for shark and ray conservation. Endangered species Res. 47, 171–203. doi: 10.3354/esr01169
Kajiura S. M., Sebastian A. P., Tricas T. C. (2000). Dermal bite wounds as indicators of reproductive seasonality and behaviour in the Atlantic stingray, Dasyatis sabina. Environ. Biol. Fishes 58, 23–31. doi: 10.1023/A:1007667108362
Kraft S., Winkler A., Abecasis D. (2023). Small coastal marine protected areas offer recurring, seasonal protection to the common stingray (Dasyatis pastinaca). Ocean Coast. Manage. 246, 106891. doi: 10.1016/j.ocecoaman.2023.106891
Kriwet J., Witzmann F., Klug S., Heidtke U. H. (2008). First direct evidence of a vertebrate three-level trophic chain in the fossil record. Proc. R. Soc. B: Biol. Sci. 275, 181–186. doi: 10.1098/rspb.2007.1170
Kyne P. M., Finucci B. (2018). Taeniura lessoni (International Union for Conservation of Nature). Available online at: https://www.iucnredlist.org/species/116855477/116855482 (Accessed 6 March 2024).
Last P., Naylor G., Séret B., White W., de Carvalho M., Stehmann M. (2016a). Rays of the World (CSIRO publishing). doi: 10.1071/9780643109148
Last P. R., White W. T., Naylor G. (2016b). Three new stingrays (Myliobatiformes: Dasyatidae) from the Indo-West Pacific. Zootaxa 4147, 377–402. doi: 10.11646/zootaxa.4147.4
Martins A., Heupel M., Chin A., Simpfendorfer C. (2018). Batoid nurseries: definition, use and importance. Mar. Ecol. Prog. Ser. 595, 253–267. doi: 10.3354/meps12545
McIvor A. J., Williams C. T., Rich W. A., Knochel A. M., Burns N. M., Berumen M. L. (2023). Mark-recapture validates the use of photo-identification for the widely distributed blue-spotted ribbontail ray, Taeniura lymma. doi: 10.21203/rs.3.rs-2952109/v1
Mull C. G., Lowe C. G., Young K. A. (2010). Seasonal reproduction of female round stingrays (Urobatis halleri): steroid hormone profiles and assessing reproductive state. Gen. Comp. Endocrinol. 166, 379–387. doi: 10.1016/j.ygcen.2009.12.009
Murakumo K., Matsumoto R., Tomita T., Matsumoto Y., Ueda K. (2020). The power of ultrasound: observation of nearly the entire gestation and embryonic developmental process of captive reef manta rays (Mobula alfredi). Fishery Bull. 118 (1), p1-7. 19p. doi: 10.7755/FB
Murphy S. E., Campbell I., Drew J. A. (2018). Examination of tourists’ willingness to pay under different conservation scenarios; Evidence from reef manta ray snorkeling in Fiji. PloS One 13, e0198279. doi: 10.1371/journal.pone.0198279
Musick J. A., Ellis J. K., Hamlett W. (2005). “Reproductive evolution of chondrichthyans,” in Reproductive biology and phylogeny of chondrichthyes: sharks, batoids and chimaeras (Routledge: Taylor & Francis Group), vol. 3, 45–80.
Osgood G. J., White E. R., Baum J. K. (2021). Effects of climate-change-driven gradual and acute temperature changes on shark and ray species. J. Anim. Ecol. 90, 2547–2559. doi: 10.1111/1365-2656.13560
Pereira N., Batista H., Baylina N. (2017). “Ultrasound assessment of pregnant ribbontail stingrays, Taeniura lymma (Forsskål 1775),” in The Elasmobranch Husbandry Manual II, vol. 325.
Pierce S., Pardo S., Bennett M. (2009). Reproduction of the blue-spotted maskray Neotrygon kuhlii (Myliobatoidei: Dasyatidae) in south-east Queensland, Australia. J. Fish Biol. 74, 1291–1308. doi: 10.1111/j.1095-8649.2009.02202.x
Ramírez-Mosqueda E., Pérez-Jiménez J. C., Mendoza-carranza M. (2012). Reproductive parameters of the southern stingray Dasyatis americana in southern gulf of Mexico. Latin Am. J. Aquat. Res. 40, 335–344. doi: 10.3856/vol40-issue2-fulltext-8
R Development Core Team (2005). R: A language and environment for statistical computing. R Foundation for Statistical Computing (Vienna, Austria). Available at: www.R-project.org.
Rosa R., Rummer J. L., Munday P. L. (2017). Biological responses of sharks to ocean acidification. Biol. Lett. 13R, 20160796. doi: 10.1098/rsbl.2016.0796
Schieber J. J., Fahy D. P., Carlson J. K., Kerstetter D. W. (2023). Age, growth and maturity of the yellow stingray (Urobatis jamaicensis), a biannually reproductive tropical batoid. J. Fish Biol. 102, 1281–1295. doi: 10.1111/jfb.15374
Schlaff A. M., Heupel M. R., Simpfendorfer C. A. (2014). Influence of environmental factors on shark and ray movement, behaviour and habitat use: a review. Rev. Fish Biol. Fisheries 24, 1089–1103. doi: 10.1007/s11160-014-9364-8
Serra-Pereira B., Farias I., Moura T., Gordo L. S., Santos M., Figueiredo I. (2010). Morphometric ratios of six commercially landed species of skate from the Portuguese continental shelf, and their utility for identification. ICES J. Mar. Sci. 67, 1596–1603. doi: 10.1093/icesjms/fsq056
Sherman C. S., Heupel M. R., Moore S. K., Chin A., Simpfendorfer C. A. (2020). When sharks are away, rays will play: effects of top predator removal in coral reef ecosystems. Mar. Ecol. Prog. Ser. 641, 145–157. doi: 10.3354/meps13307
Sherman C. S., Simpfendorfer C. A., Pacoureau N., Matsushiba J. H., Yan H. F., Walls R. H., et al. (2023). Half a century of rising extinction risk of coral reef sharks and rays. Nat. Commun. 14, 15. doi: 10.1038/s41467-022-35091-x
Simpfendorfer C. A., Heithaus M. R., Heupel M. R., Macneil M. A., Meekan M., Harvey E., et al. (2023). Widespread diversity deficits of coral reef sharks and rays. Science 380, 1155–1160. doi: 10.1126/science.ade4884
Simpson S. J., Humphries N. E., Sims D. W. (2021). Habitat selection, fine-scale spatial partitioning and sexual segregation in Rajidae, determined using passive acoustic telemetry. Mar. Ecol. Prog. Ser. 666, 115–134. doi: 10.3354/meps13701
Smith M., Warmolts D., Thoney D., Hueter R., Murray M., Ezcurra J. (2017). The Elasmobranch Husbandry Manual II: Recent Advances in the Care of Sharks, Rays and their Relatives. Special Publication of the Ohio Biological Survey. viii + 504 p.
Spieler R. E., Fahy D. P., Sherman R. L., Sulikowski J. A., Quinn T. P. (2013). The yellow stingray, Urobatis jamaicensis (Chondrichthyes: Urotrygonidae): a synoptic review. Caribbean J. Sci. 47, 67–97. doi: 10.18475/cjos.v47i1.a8
Walker T. I. (2020). “Chapter 10 Reproduction of Chondrichthyans,” In Yoshida M., Asturiano J. (eds) Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology (Singapore: Springer), 193–223. doi: 10.1007/978-981-15-2290-1_11
White W., Dharmadi (2007). Species and size compositions and reproductive biology of rays (Chondrichthyes, Batoidea) caught in target and non-target fisheries in eastern Indonesia. J. Fish Biol. 70, 1809–1837. doi: 10.1111/j.1095-8649.2007.01458.x
Wickham H. (2011). ggplot2. Wiley Interdiscip. reviews: Comput. Stat 3, 180–185. doi: 10.1002/wics.147
Keywords: batoids, reproductive biology, Melanesia, data deficient species, range restricted, Dasyatidae
Citation: Glaus K, Vierus T and Macfarlane R (2024) Observational data on the reproductive condition of female Oceania fantail rays, Taeniura lessoni, from Drawaqa Island, Fiji. Front. Mar. Sci. 11:1401258. doi: 10.3389/fmars.2024.1401258
Received: 15 March 2024; Accepted: 14 June 2024;
Published: 05 July 2024.
Edited by:
Brendan Shea, Beneath the Waves, Inc., United StatesReviewed by:
Alfonsina E. Romo-Curiel, The University of Texas at Austin, United StatesCopyright © 2024 Glaus, Vierus and Macfarlane. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerstin Glaus, a2Vyc3Rpbi5nbGF1c0B1c3AuYWMuZmo=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.