
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 10 June 2024
Sec. Deep-Sea Environments and Ecology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1387144
Introduction: The black coral Leiopathes glaberrima is a key component of deep-sea animal forests of the Western Mediterranean and North-Atlantic hard bottoms. Due to its ecological role, biological characteristics and sensitivity to fishing activities, it has been included in the Annex II of the Barcelona Convention, listed as an endangered species in IUCN Red Lists, and recognized as an indicator of Vulnerable Marine Ecosystems by the FAO.
Methods: During a survey conducted in the Strait of Sicily, 140 ROV transects were carried out allowing the characterization of environmental preferences, associated fauna and population structure of L. glaberrima in the study area.
Results and discussion: 1020 colonies were distributed between 165 and 672 meters and arranged in 17 patches and isolated colonies. The average height was determined to be 40.9 ± 1.0 cm, with the range spanning from a minimum of 5 cm up to nearby 200 cm. Pristine colonies accounted for 62.9%, mainly constituted by small-sized individuals densely aggregated (up to 14.5/m2 in a single frame) in the eastern part of the area. 24.5% of the specimens were dead and localized in the western margin where we reported a rare case of mass mortality in the deep-sea environment and the first ever described for black corals, opening new scenarios regarding the possibility that these events can occur in environments previously considered safe from danger. The triggering causes of these events in inaccessible environments are actually only hypothesized, but it is not to be excluded that, sooner or later, a similar effect could potentially result from anthropogenic climate changes.
The black coral Leiopathes glaberrima (Esper, 1788) is widely distributed in the Western Mediterranean Sea and in the Northern Atlantic Ocean at depths ranging from 100 to 2048 meters (Roark et al., 2006; Etnoyer et al., 2018; Massi et al., 2018). Some specimens were also reported from Hawaiian waters (Grigg and Opresko, 1977), but it turned out to be a misidentification of the endemic congeneric Leiopathes annosa (Wagner and Opresko, 2015). L. glaberrima settles on rocky bottoms where it tends to aggregate in patches with three-dimensional colonies which may exceed two-meters height. These aggregations of tree-like organisms represent a key component of deep-sea animal forests (sensu Rossi et al., 2017), communities comparable in complexity, biodiversity, and structuring role to the terrestrial forests, but composed of suspension-feeding organisms, e.g. sponges, anthozoans, bryozoans and bivalves (Bo et al., 2009, 2015; Buhl-Mortensen et al., 2010; Cerrano et al., 2010; Rossi et al., 2017). Despite challenges posed by taxonomic heterogeneity and spatial organization, the term marine animal forest provides an evocative representation of the concept as it was intended to be used. In fact, such a term groups diverse benthic ecosystems including coral gardens, oyster reefs, and sponge grounds, each with characteristic dominant species, yet collectively sharing a common ecological role. Suspension-feeding communities play an important role in benthic-pelagic coupling – thus the transfer of energy, biomass and nutrients between the pelagic and the benthic ecosystems - by recycling particulate organic matter sinking from upper photosynthetic layers (Gili and Coma, 1998; Bo et al., 2014a). The three-dimensionality of marine animal forests provides secondary substrates and niches, forming a spawning, nursery and shelter ground for a rich associated fauna (Cerrano et al., 2010; Bo et al., 2014a; Cau et al., 2017; D’Onghia et al., 2017; Rueda et al., 2019). For these reasons, ecosystems dominated by Cold-Water Corals (CWCs) – a group of habitat-forming azooxanthellate anthozoans (Phylum Cnidaria) with an affinity for cold waters - are considered as biodiversity hotspots (Mastrototaro et al., 2010; Tsounis et al., 2010). Another aspect that these marine ecosystems share with the terrestrial ones is the longevity of the structuring species. The dating of several species of stony corals, black corals and gorgonian octocorals, has shown that those anthozoans can live for hundreds of years (Roark et al., 2006; Prouty et al., 2011; Tracey et al., 2018; Hitt et al., 2020) and the black corals belonging to the Leiopathes genus are among the most longevous marine species known so far. Roark et al. (2009) carried out radiocarbon dating on a specimen of Leiopathes sp. from Hawaiian waters, estimating an age of 4265 ± 44 years old. In the Mediterranean Sea, Bo et al. (2015) carried out the same analysis on a specimen of Leiopathes glaberrima collected off the southwestern Sardinian coasts, estimating an age of 1973 ± 55 years old.
Longevity, as well as other biological characteristics, namely maturation at relatively old ages, slow growth, low natural mortality rates, intermittent recruitment of successful year classes and spawning that may not occur every year, are indicators of highly sensitive species (FAO, 2008).
For these reasons, Leiopathes glaberrima belongs to the “Endangered” category in the IUCN Red List of Mediterranean Anthozoans and is listed in Annex II of the SPA/BD protocol of the Barcelona Convention. In addition, this species is a key component of hard-bottom coral gardens, which are considered VMEs (Vulnerable Marine Ecosystems) in relation to several human activities (FAO, 2008; Aguilar and Marín, 2013; D’Onghia et al., 2017). Its bathymetric distribution greatly enhances the risk of being subjected to locally high fishing pressure (Bo et al., 2009, 2014a, 2014b; Deidun et al., 2010). Among the main threats, bottom trawling can entirely remove colonies while re-suspension of soft sediments increases turbidity, even beyond the trawling footprint, which can lead to smothering of benthic communities (Bo et al., 2014b; D’Onghia et al., 2017; Paradis et al., 2017; Otero and Marin, 2019; Bilan et al., 2023). Numerous other fishing gears such as gillnets, trammel nets, long lines, and traps can cause physical damage and removal of benthic species (Chiappone et al., 2005; Macfadyen et al., 2009; Bo et al., 2014b; Otero and Marin, 2019). Moreover, derelict fishing gears (also referred to as Abandoned, Lost or otherwise Discarded Fishing Gears - ALDFGs) may abrade tissues by either getting entangled or drifting on the seafloor (Laist, 1997; Brown and Macfadyen, 2007; Bo et al., 2014b). In anthozoans, the abrasion of coenenchyme favors the growth of epibionts. which can weaken their skeletons and enhance the detachment of branches (Bavestrello et al., 1997).
Other threats are related to the exploitation of the geological resources of the seabed, as well as the laying of submarine pipelines and cables or the dumping of waste into the sea (Turley et al., 2007; Roberts and Cairns, 2014; Roberts et al., 2016). Moreover, there are the potential effects of climate change on deep-sea communities, including rising temperatures, acidification, deoxygenation, and changes in trophic inputs, which could lead to a reduction in suitable habitats (Thresher et al., 2015; Morato et al., 2020).
Studies on the distribution of antipatharians in the Mediterranean Sea only started in the 2000s, primarily through the work of Bo et al. (2008, 2009, 2011a, 2011b, 2012, 2014a, 2014b, 2015), garnering a growing interest over the years. Published data over black coral distribution in the Mediterranean Sea is reviewed in the work of Chimienti et al. (2019), while a literature review focused on Leiopathes glaberrima populations along Italian waters was undertaken by Ingrassia and Di Bella (2021) and Lauria et al. (2021). The low number of detailed studies describing these populations and the lack of parameters that allow for an unequivocal definition of marine animal forests, make their quantification in L. glaberrima challenging. As a result, the reviews previously mentioned report several findings throughout the Mediterranean Sea but aggregations of L. glaberrima have been described only in few locations.
The conservation status of such forests varies from pristine, as the case of those reported by Bo et al. (2015) in Carloforte Shoal (SW Sardinia) which reached a peak of 8 colonies/m-2, to highly impacted, as the ones described at Marco Bank (NW Sicily) by Bo et al. (2014b). The health status was assessed as a result of the quantification of damaged colonies and ALDFGs, in relation to fishing effort. Fishing activities turned out to be the main contributor to the mortality of this species.
This study is based on a dataset of 1020 colonies of L. glaberrima, used to characterize its environmental preferences, associated fauna and the population structure in terms of distribution, abundance, size and health status. Additionally, a site where almost all the specimens were dead has been observed, reporting a rare case of mass mortality in the deep-sea environment and the first ever described for black corals.
Mass mortality events (MMEs) among benthic species have been documented across coastal and upper coralligenous environments. In the last thirty years the frequency of these events increased exponentially, marking a concerning trend. Garrabou et al. (2019) tracked these events within the Mediterranean, unveiling a predominant impact on Cnidaria (47.4%) and Porifera (37.6%). The causes of MMEs in anthozoans have frequently been linked to strong and recurrent marine heat waves and, only in a few cases, to heightened sedimentation. Temperature anomalies induce physiological stress which can directly result in the death of the organism or in the weakening of its immune response, thereby heightening the susceptibility to pathogenic infections (Garrabou et al., 2001; Vezzulli et al., 2010; Carella et al., 2014). MMEs have been predominantly recorded within the first 50 meters depth, a zone that has historically commanded the focus of scientific exploration conducted by scuba divers. The expansion of research efforts into deeper environments is progressively extending the boundaries of our understanding of mass mortalities. The deepest MMEs have been recorded from 120 to 180 meters depth and confined to the red coral Corallium rubrum off the Provence coastline, the Gulf of Napoli and Salerno and the southeastern shores of Sardinia. In these cases, the possible causes were hypothesized to be current-driven pollutants, unusual drops of summer thermocline and warmwater emissions in areas characterized by volcanic activities (Rivoire, 1991; Bavestrello et al., 2014; Toma et al., 2022).
Despite these recent studies, most of the instances of mass mortalities in the deep Mediterranean Sea are referred to fossil reefs from late Neogene and Quaternary of Madrepora oculata and Desmophyllum pertusum due to changes of the paleo-environmental conditions and tectonic evolutions (Fink et al., 2015; Taviani et al., 2019).
The Strait of Sicily connects the western and eastern Mediterranean basins playing an important role in the physical and dynamical processes evolving in the Mediterranean Sea (Astraldi et al., 2001; García Lafuente et al., 2002). Hydrodynamic circulation and mesoscale structures are characterized by an inflow of relatively fresh surface water of Atlantic origin (Modified Atlantic Water, MAW) and an outflow of salty deeper water formed in the Levantine basin (Levantine Intermediate Water, LIW) towards the western Mediterranean (Napolitano et al., 2003). The complex geomorphology of the area, characterized by several submarine reliefs (including shallows, ridges, knolls, pinnacles and seamounts) composed of sedimentary or volcanic rocks, affect the currents resulting in substantial upwelling, increasing the overall productivity (Civile et al., 2016; Consoli et al., 2016). As a result of the peculiar geomorphologic and oceanographic characteristics, the Strait of Sicily is one of the most important biodiversity hotspots in the Mediterranean Sea (Vega Fernández et al., 2012; Deidun et al., 2015; Di Lorenzo et al., 2018). Furthermore, the rocky bottoms of the Strait represent an important crossroad for the distribution of benthic species between the two Mediterranean basins (Bianchi and Morri, 2000). These features make the area highly sensitive to anthropogenic pressures, including exploitation of the geological resources, laying of submarine pipelines and cables, dumping of waste into the sea, and especially fishing activities.
The Strait of Sicily has historically been an intensively fished area (Garofalo et al., 2003; Vega Fernández et al., 2012; Jarboui et al., 2022). Bottom trawling is the most significant activity, both inshore and offshore, targeting species with high economic demand, such as the crustaceans Aristeomorpha foliacea, Aristeus antennatus, Nephrops norvegicus, Parapenaeus longirostris, the hake Merluccius merluccius and the mullets Mullus barbatus and M. surmuletus (Di Lorenzo et al., 2018; Jarboui et al., 2022). Additionally, the artisanal fishery locally exploits a wide range of species using different fixed gears, e.g., longlines, trammel nets, gillnets (Di Lorenzo et al., 2018).
The study area is located 27 km westwards from Marettimo Island (Aegadian Archipelago, Sicily) and covers a 1651 km² surface. The geomorphological homogeneity of the dominant muddy planes is disrupted in the central zone by a channel descending to a depth of 978 meters and to north by a large bank that rises to 135 meters in the shallower part. The rocky bottoms are spread along the NW-SE-oriented edge of the area, encircling the base of the bank, and in the eastern region (Figure 1).
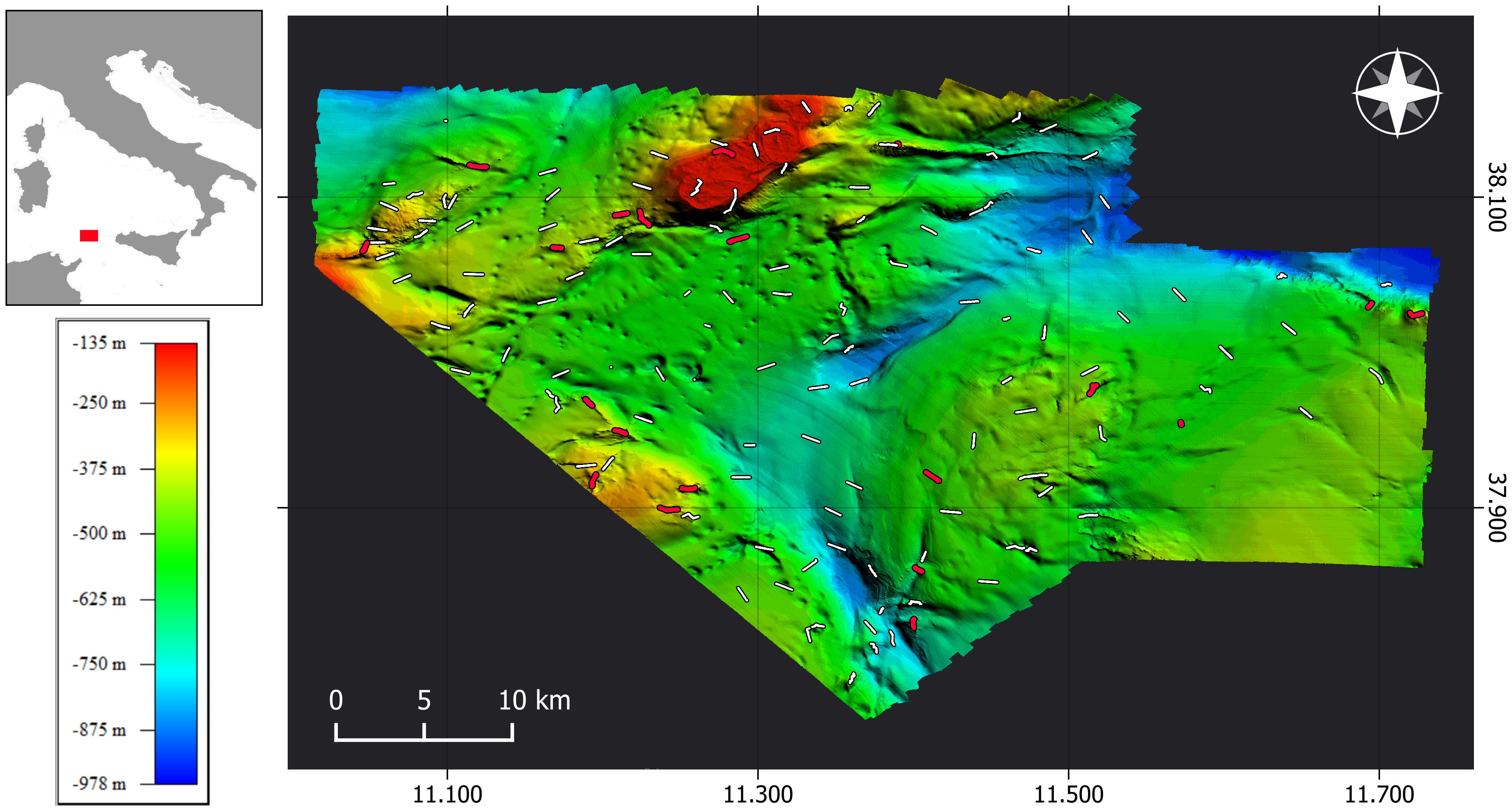
Figure 1 High-resolution morpho-bathymetric map of the study area and locations of ROV transects carried out for image data acquisition. Transects with presence of Leiopathes glaberrima are represented by red lines; otherwise, transects where the species was not observed are represented by white lines.
Data were collected during an oceanographic survey carried out from August to November 2021 aboard the Mainport Geo vessel. The survey aimed at collecting geomorphologic and oceanographic data, assessing the presence of habitats and species of conservation interest and anthropogenic impact within the footprint of the study area.
The seafloor bathy-geomorphology was acquired with a Multibeam echosounder Kongsberg EM2040 for water depths up to 250 m and Kongsberg EM710-MK2 for deeper waters; data were processed and elaborated to produce a DTM of the whole area at a resolution of 5 meters. Oceanographic parameters were sampled in 97 stations through a multiparameter probe Seabird SBE 911 Plus and then processed to identify the vertical distribution of water masses in the area. For this purpose, a co-kriging analysis was performed in R software using salinity and potential temperature. The video survey was carried out using the remotely operated vehicle (ROV) Tomahawk Light Work Class equipped with a Sonardyne Ranger 2 USBL acoustic positioning system, a Rovins iXblue INS, a Teledyne Explorer DVL, two standard definition cameras (for rear and frontal view), a Teledyne Bowtech Explorer high-definition camera, a 6K Cinema (ZCAM E2-F6, China) Full Frame with Canon EF 16–35 mm f/2.8L III USM lens, two lights, two laser beams and two HLK-HD5 hydraulic arms. The laser beams were parallel with 20 cm horizontal spacing providing a reference scale for carrying out the measurements in the video frames.
A total of 140 ROV dives were carried out, amounting to a total of 129.5 km of transects and over 406 hours of video footage. The ROV field of view was estimated using the two laser beams as reference and resulted to be, on average, two meters wide, aligning with estimates from other studies that utilized ROV imaging analysis (Bo et al., 2009; Deidun et al., 2015). The video was analyzed to record the presence of Leiopathes glaberrima and geographic position of every colony, depth, substrate type, tissue loss percentage, entanglement with Abandoned, Lost, or Otherwise Discarded Fishing Gears (ALDFGs), height of the specimens and associated fauna. The substrate type was identified and discriminated between outcropping rock, rock covered by crustose coralline algae, CWC framework and shipwrecks. The tissue loss percentage, considered as partial to complete loss of the coenenchyme, was assessed as partial to complete denudation of the axial skeleton of colonies and then arranged within five classes:
● absence of tissue loss (0%) - indicative of pristine colonies,
● less than 1/3 of exposed skeletal portion,
● between 1/3 and 2/3,
● more than 2/3,
● totally dead (100%).
Since the extreme values conditions (0%, 100%) are clearly distinguishable, they were not included in a class but treated as individual values. The height of each colony was measured through ImageJ software (www.imagej.nih.gov), using the laser beams reference scale, and then organized in classes to delineate the size-frequency distribution of L. glaberrima in the whole area.
The geographic position of each specimen along transects, as recorded by the USBL system and processed with the Kalman filter to reduce positioning errors, was used to calculate the distance among colonies. Then, for every transect the mean colonies distance and the cumulative frequency of distance were calculated. The distance value corresponding to 95% of cumulative frequency was chosen as the threshold for distinguishing between isolated colonies and patch-aggregated colonies. The density of the patches (N°colonies m-2 ± Standard Error) was defined as the number of specimens related to the area calculated multiplying the length of the transect from the first to the last colony by the field of view of the camera. For each patch the Total Tissue Loss (TTL) was calculated adding up the tissue loss values of each colony of a patch and dividing the result by the number of colonies in the patch.
Out of 140 transects, hard bottoms suitable for the settlement of Leiopathes glaberrima were found in 70 transects amounting for approximately 66000 m2 of explored surface.
A total of 1020 specimens were counted during 20 transects. The distribution depth ranged from 165 to 672 m, with the deepest record constituted by an isolated colony in the southern part of the project footprint. A peak of abundance occurred at depths between 350 and 400 m where a substantial 44.4% of the total specimens were found (Figure 2), with most of them observed during TR_002 (Table 1). The species exhibits a diverse settlement pattern on different types of hardgrounds within the study area. 69.3% of the observed colonies were found growing on exposed rock surfaces. Additionally, 27.3% of the population was observed growing directly on bio-constructed cold-water coral frameworks, 2.4% were seen on rocks covered by crustose coralline algae, while a minor proportion of 0.9% was observed on the metal framework of shipwrecks. A single colony (0.1%) was exceptionally found in the middle of a muddy bottom dominated by a typical muddy community and, probably, anchored on a non-visible hard substrate (Figure 3D). In 48.8% of the observations the species was seen on horizontal grounds, while in the 42.6% of the cases it was found on sloping bottoms mainly constituted by coral mounds. Only a few specimens (8.6%) were observed on almost vertical substrates such as rocky cliffs, the edge of the bank and shipwrecks. Based on the analysis of colony height, the average height (± SE) was determined to be 40.9 ± 1.0 cm, with the range spanning from a minimum of 5 cm to a maximum of 200 cm. The height-class frequency distribution is unimodal and asymmetrical, characterized by a peak of individuals ranging from 10 cm up to 30 cm (Figure 4). Concerning the health status in the whole area, 62.9% of the colonies of L. glaberrima were in good health, showing no tissue loss; 24.5% were found to be entirely dead, and the remaining 12.6% fell into intermediate health classes, distributed as follows: 4.7% of the colonies showed one-third of tissue loss, 3.9% showed between one-third and two-thirds, and another 3.9% of the colonies showed more than two-thirds of tissue loss. Only two longlines were counted in proximity of L. glaberrima throughout the area and sixty-five specimens displayed breakage at their trunks.
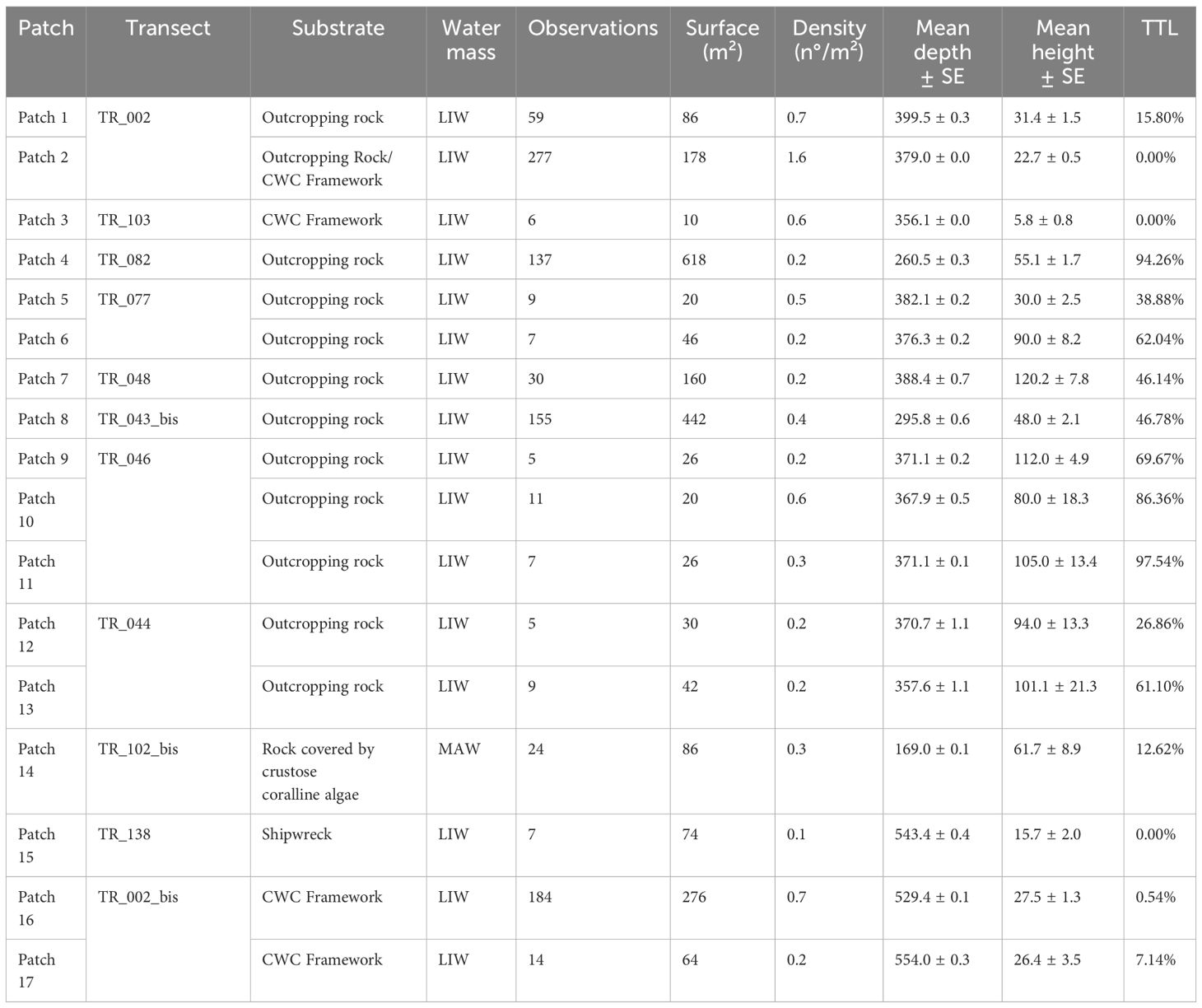
Table 1 Patch details including transect number, substrate type, water mass characterizing the site (LIW, Levantine Intermediate Water; MAW, Modified Atlantic Water), number of observations of Leiopathes glaberrima, extent in square meters, density of colonies, depth range, mean height and percentage of total tissue loss of specimens for each Patch (TTL).
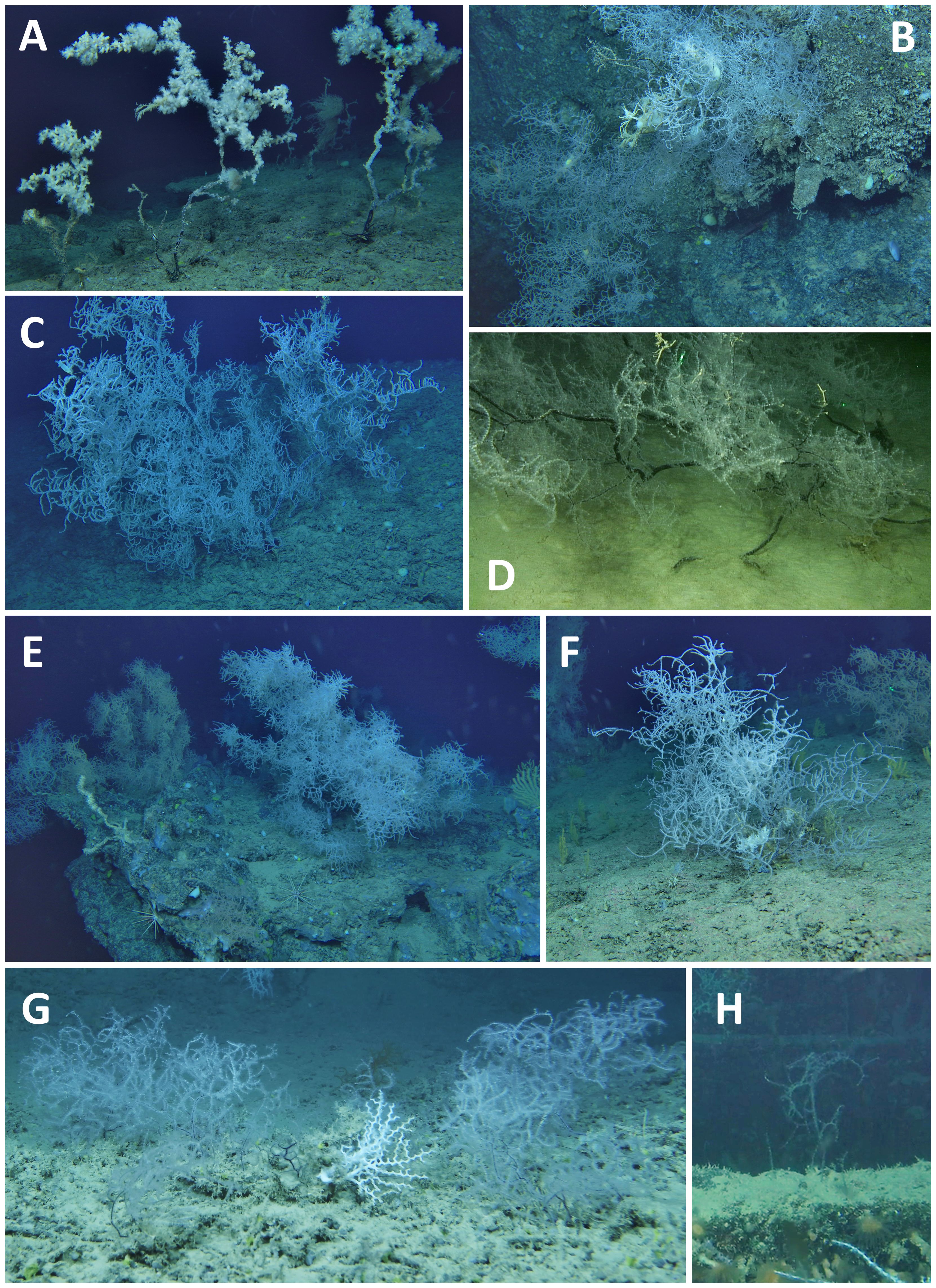
Figure 3 Different types of aggregations of Leiopathes glaberrima in the study area. (A) Dead colonies of L. glaberrima documented in Patch 4; (B) Forest of L. glaberrima thriving on a rocky cliff; (C) A massive, isolated colony on hard ground; (D) A solitary individual developing on a muddy bottom; (E) Colonies of L. glaberrima prospering at the edge of a bench terrace; (F) A forest belonging to Patch 14 discovered on an offshore coralligenous formation; (G) High density of small-sized colonies growing on a CWCs framework in Patch 16; H) Young colony on the hull of a shipwreck in Patch 15.
The average distance between specimens was 6.7 m with a standard error of 0.8, and the cumulative frequency indicated that 95% of colonies were within a 15-meters radius from each other. As a result, colonies located within a 15-meter radius were considered as aggregated and forming patches, while colonies situated at distances greater than 15 m were classified as isolated colonies. 17 patches were counted ranging in surface from 10 m2 in TR_103 to 618 m2 in TR_082 (Table 1, Figure 5). Additionally, in six ROV transects single specimens were found completely isolated (Figure 3C).
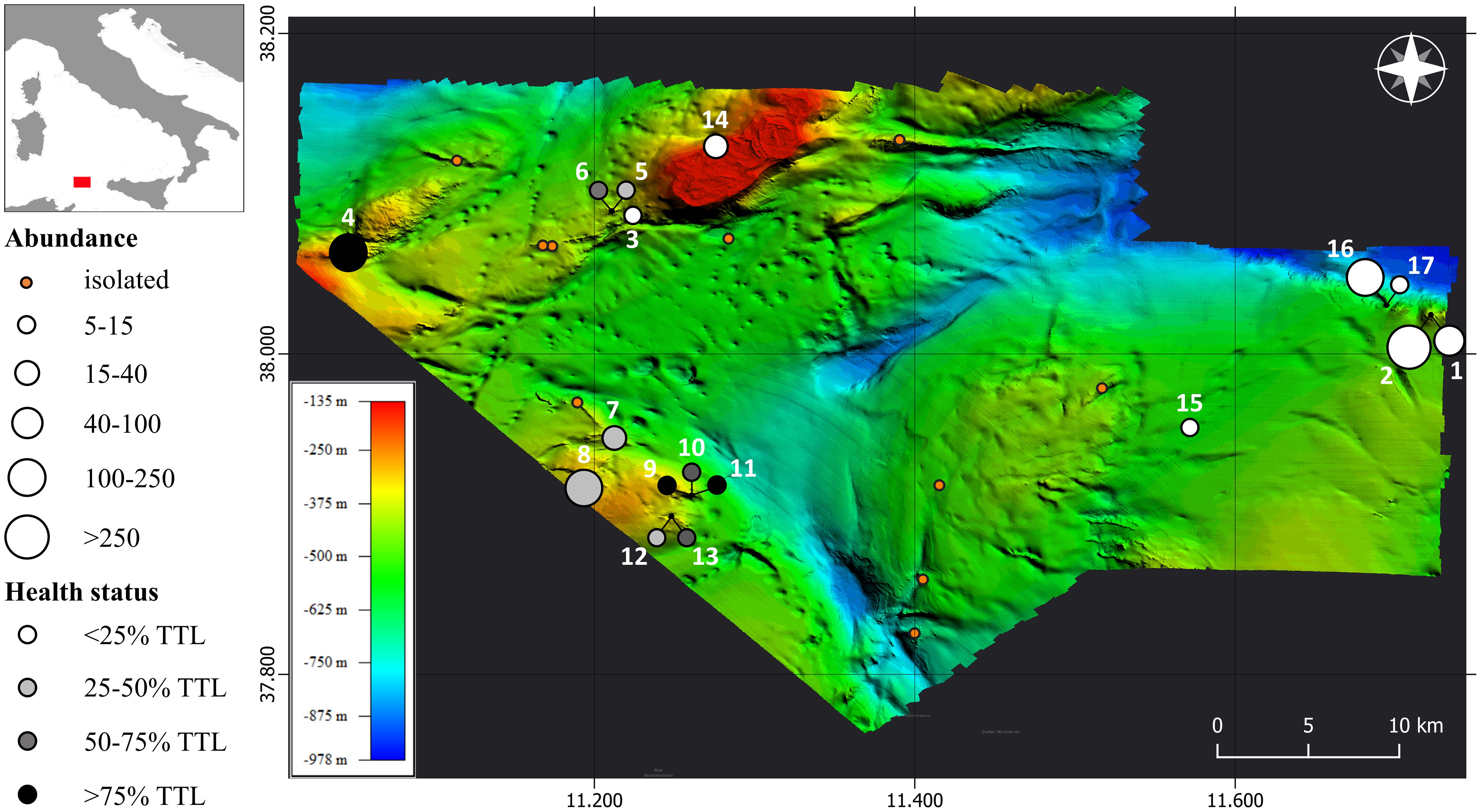
Figure 5 Distribution of isolated colonies and patches in the study area. The isolated colonies are indicated by small orange dots, while patches are represented by dots varying in size, proportionally to the abundance, and in tones of greyscale in function of the Total Tissue Loss (TTL).
Patches 1, 2, 16 and 17 were found on nearby mounds mainly constituted by CWC frameworks. They were characterized by medium/small-sized specimens, healthy condition and high density of colonies (Figure 3G), with the highest density value of 14.5 specimens/m2 in a single frame recorded in Patch 2. The aggregation of the latter patch was completely pristine, whereas Patches 1, 16 and 17 showed 78.0%, 92.9% and 99.5% of pristine colonies, respectively. In Patch 2 a longline was seen laying on the seabed for several meters among thirty-five colonies, entangling three specimens. Nevertheless, none of these colonies had any exposed skeletal portion.
Patches 3, 5 and 6 were located at the base of the western margin of the bank, between 356 and 382 m depth, and all constituted by few specimens. Colonies of Patch 3 were smaller than 10 cm and located on a CWC framework. Patch 5 developed on the edge of a rocky bench terrace with medium-sized individuals showing 38.9% of total tissue loss (Figure 3E). In Patch 6, the average height of colonies measured 90 ± 8 cm with total tissue loss reaching 62%.
Patch 4 was observed on a rocky outcrop that rises from 320 m to 255 m depth on the summit. 86% of the population consisted of completely dead colonies, discovered intact and attached to the substrate, and an additional 6.6% were in very poor status, displaying more than two-third of tissue loss (Figure 3A).
Patches 7, 8, 9, 10, 11, 12, and 13, were all situated within the same geographic region on the western margin of the project footprint. The sub-area was characterized by a rocky bottom with steep locations (Figure 3B). All the above patches, except for Patch 8, fell within the bathymetric range of 350–400 m and showed relatively low values of abundances and medium-sized individuals. Patch 8 was found at shallower depths compared to the other patches, at 289 m depth, and was characterized by a high abundance and medium-sized individuals. Patch 7, Patch 11 and Patch 13 exhibited the highest average heights, measuring 120.2 ± 7.8, 105.0 ± 13.4 and 101.1 ± 21.3 cm, respectively. Particularly, in Patch 7 the smallest of the measured colonies was 70-cm high. In contrast, Patch 3 displayed the lowest average height, with a value of 5.8 ± 0.8 cm. The general health status of the sub-area was poor. In five patches, populations manifest a total tissue loss surpassing 45%, with Patch 11 reaching 97.5%. A total of 48 specimens showed severe breakages at their trunks. A longline was also seen close to seven colonies of Patch 8, entangling three specimens. One entangled colony had less than one-third of tissue loss while another one, although not being directly entangled, was found to be entirely dead.
On the western margin of the bank, Patch 14 flourished on a deep coralligenous substrate at 165 m depth, constituting the shallowest occurrence in the area (Figure 3F). The chemico-physical parameters associated with this site significantly differed in terms of water masses, as it was the only location experiencing the Modified Atlantic Water instead of the Levantine Intermediate Water, ubiquitous in the other sites.
Patch 15 was found on the iron hull of a shipwreck and was constituted by small-sized individuals, ranging from 10 cm to 25 cm and showing no tissue loss (Figure 3H). The shipwreck that hosts Patch 15 was estimated to have sunk around a century before. Assuming that the highest colony settled shortly after the sinking of the ship, the growth rate for the specimen is estimated to be 0.25 cm/year in height. Another case regards a 20-cm-high isolated colony on another shipwreck known to have sunk in 1979 and with a growth rate of 0.48 cm/year in height. In all the specimens inhabiting the wrecks, the diameter of the base was too thin to be measured with video analysis but apparently on the scale of millimeters, in line with the radiant growth rates in literature (Bo et al., 2015).
All the colonies suffering more than one-third of tissue loss or total mortality had epibionts (Figure 6). The most frequent epibionts were non-identified encrusting sponges, small sea barnacles, serpulids, hydrozoans and zoanthids. Other epibiontic associations included the carnivorous sponge Lycopodina hypogea, the sea anemone Amphianthus dohrnii, the stony coral Desmophyllum dianthus and the sea barnacles Lepas sp. and Pachylasma giganteum. The naked stems also hosted some arborescent anthozoans such as the white corals Madrepora oculata and Desmophyllum pertusum (with 29 and 12 observations respectively) the octocorals Acanthogorgia hirsuta, Muriceides lepida and Dendrobrachia bonsai. Furthermore, some vagile fauna were observed in proximity or inside the canopy as in the case of the crabs Anamathia rissoana – with 60 specimens counted – Latreillia elegans and Homola barbata, the sea urchins Cidaris cidaris and Echinidae n.d. and the crinoid Leptometra phalangium. Among fishes, 86 specimens of the robust cask eel Benthocometes robustus were seen swimming vertically within the branches; the boarfish Capros aper, the longspine snipefish Macroramphosus scolopax and the cardinalfish Epigonus sp. were occasionally observed in proximity to the coral canopy. In addition, 86 egg capsules, at different stages of maturation, of the small-spotted catshark Scyliorhinus canicula were found attached to the branches.
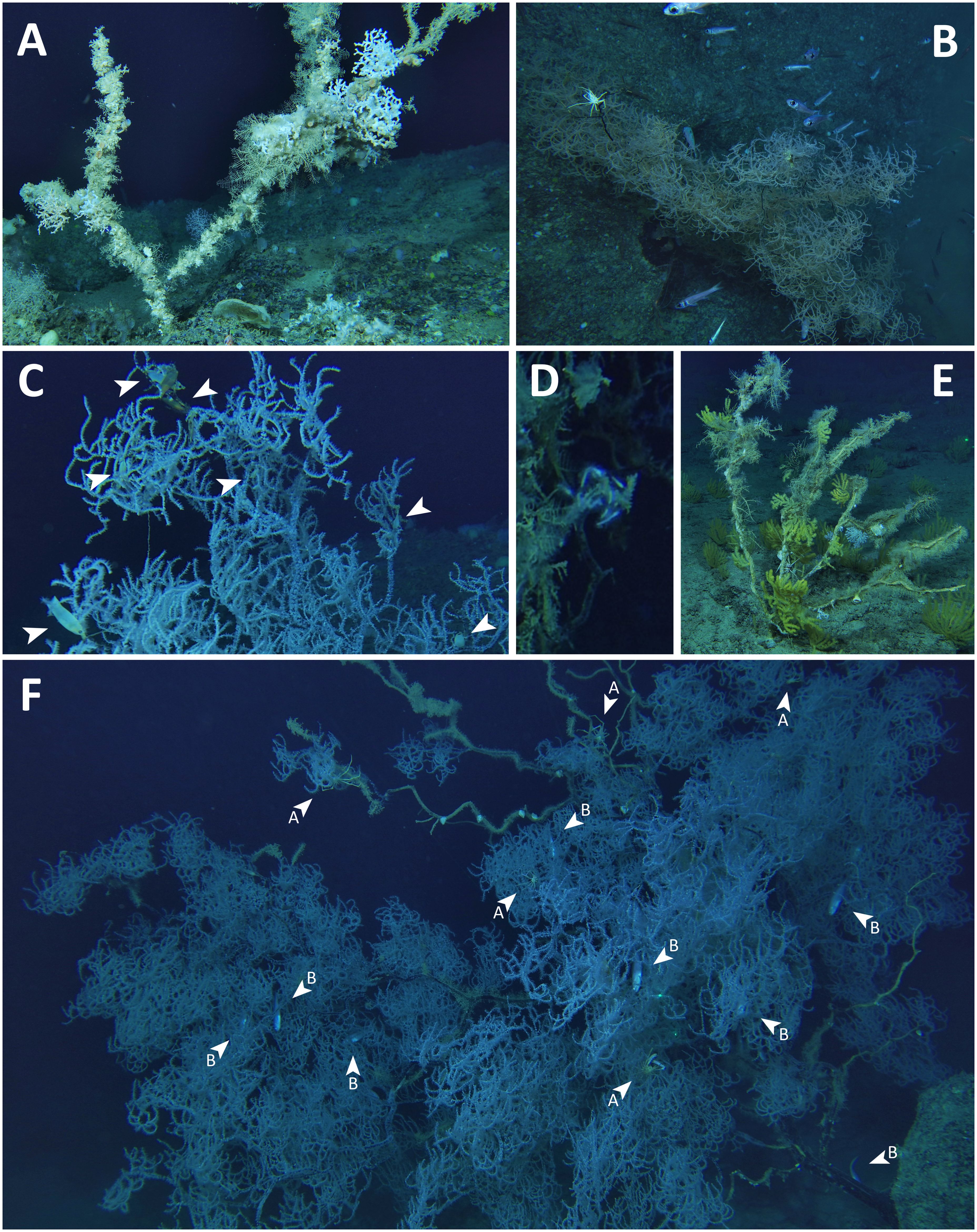
Figure 6 Fauna directly associated with specimens of Leiopathes glaberrima. (A) Skeleton showing later stages of colonization with the sea barnacle Pachylasma giganteum and the anthozoans Dendrobrachia bonsai, Desmophyllum pertusum and Madrepora oculata; (B) A school of Epigonus sp. and a specimen of Anamathia rissoana among the canopy; (C) Close up of Scyliorhinus canicula eggs (arrows) on the branches; (D) A cluster of carnivorous sponges of the species Lycopodina hypogea; (E) A cluster of Acanthogorgia hirsuta, a single colony of Muriceides lepida, the sea-anemone Amphianthus dohrnii and a sea urchin belonging to the family Cidaridae on the dead stems; (F) Several individuals of Anamathia rissoana (arrows with A) and Benthocometes robustus (arrows with B) hiding into a large-sized colony.
The extensive exploration of such a vast area provided us with new information, on a medium to large scale, over the distribution patterns and the types of aggregations of Leiopathes glaberrima (Figure 3). The bathymetric range of this species in this area is comparable with the data reported for the Mediterranean Sea in the review made by Chimienti et al. (2019). The species occurrence on diverse hard bottoms with varying slopes emphasizes its adaptability to such environmental drivers.
The scarcity of Abandoned, Lost, or Otherwise Discarded Fishing Gears (ALDFGs) in the vicinity of the colonies might be interpreted as a low anthropogenic impact. However, the contrasting data revealing breakage at the trunks of specimens, accounting for 6.4% of the total observations, appeared to be an objective indicator of impact. The removal of colonies may be caused by both demersal longlines and bottom trawling, the most common techniques used in many deep-sea fisheries (Clark et al., 2016).
Concerning the spatial distribution, the species can occur as aggregated in dense patches as well as single isolated colonies. While the results of the spatial analysis in the area confirmed the tendency of the species for aggregation, least expected was the presence of several isolated specimens. Despite the individual colonies being fewer, this pattern raises questions about the factors influencing distribution, larval dispersion, and connectivity of this species. The high number of isolated colonies in locations with low or absent fishing impact may be attributed to a challenging accessibility of larvae to such sites or the presence of borderline environmental conditions.
Patches 1, 2, 16 and 17 exhibit similar features except for the substrate composition, indeed the first two predominantly thrive on rocky bottom while the others grow on a CWCs framework. High density of colonies, predominance of medium/small-sized specimens in excellent health condition are representative of relatively young forests. The proximity of such prosperous aggregations implies that the sub-area experiences suitable environmental conditions for the settlement and growth of L. glaberrima, suggesting that additional forests may occur on the surrounding hard bottoms. The mean density values of these aggregations are in line with data available for other pristine populations in literature, while the peak of density of 14.5 colonies/m2 observed in Patch 2 constitutes the highest value recorded so far, compared with the maximum densities ranging from 4 to 11 colonies/m2 registered in several locations of the Central Mediterranean Sea (Bo et al., 2014b, 2015; Deidun et al., 2015; Ingrassia et al., 2016; Angiolillo et al., 2023). It is worth pointing that the patches abundance could have been partially underestimated as the contiguity of the canopies in high density aggregations makes the discrimination between small-sized individuals challenging.
The mass mortality documented in Patch 4 represents a rare case in the deep-sea environment and the first ever described for black corals. The total absence of fishing gears and broken colonies along the transect suggests that the direct impact of fishing, despite being a heavily performed activity in the area and a key factor often associated with mortality in deep-sea environments, can be ruled out as a contributing cause. Other possible causes of mass mortality proposed for the red coral C. rubrum are linked to the transport of urban pollutants by dominant currents and to the unusual dropping of the summer thermocline due to a gravity flow of coastal debris. Both hypotheses are to be excluded given the depth and the offshore location of the site. Putative causes could be related to poisoning from toxic wastes, e.g. barrels of toxic chemicals and batteries, as well as pathogens-related diseases (Garrabou et al., 2001; Vezzulli et al., 2010; Carella et al., 2014; Silva et al., 2016; Merrifield et al., 2023). Another possible explanation could be reconducted to changes in physical and chemical conditions caused by past hydrothermal emissions, as assumed for the red coral bank around Li Galli Island (Gulf of Salerno) by Bavestrello et al. (2014).
Patches from 7 to 13 have been clustered according to geographical proximity and geomorphological features. Moreover, the whole sub-area displays a precarious health state, which is not excluded to be attributable to the events that caused the mass mortality of Patch 4, geographically not far away. The high number of broken specimens and the longline observed throughout the sub-area. let us suppose that fishing activities could potentially act as a cumulative stressor for the populations.
Geographical proximity is also a common factor also for Patches 3, 5 and 6. Nevertheless, they differ in terms of colony height and health status. In Patch 3, the presence of very small individuals suggests that they are new recruits. In contrast, Patch 6 is constituted by large specimens with high percentages of tissue loss, characteristic aspects of a population that has stopped recruiting.
The discovery of L. glaberrima in a different water mass in Patch 14 may hide a potential level of plasticity to environmental parameters, considering that CWCs have usually been reported to settle along the path of the well-ventilated and nutrient-rich LIW (Chimienti et al., 2019; Lauria et al., 2021). However, all the explored sites along the bank share nearly identical environmental conditions, L. glaberrima was observed in a single location situated on the western margin. The absence of this species in other areas of the bank is indicative of how local factors may play a key role for its distribution.
Patch 15 is unique as it was attached to the iron surface of a shipwreck. Those kinds of anthropogenic structures act as artificial reefs and biodiversity hotspot by providing hard substrata, food supply, protection and higher exposure to currents (Frizzel, 2020). Knowing the sinking date of shipwrecks, or at least the approximate period, allows us to estimate the growth rates of sessile fauna without the need for traditional dating techniques like radioisotope analysis.
The framework created by CWCs introduces spatial heterogeneity and potential to locally modify the hydrodynamic conditions. This phenomenon is particularly pronounced in Leiopathes glaberrima due to its high three-dimensionality and its tendency to form aggregations. Both living colonies and dead skeletons host a diverse fauna spanning multiple trophic levels, creating a small-scale ecosystem that significantly enhances the overall diversity of the surrounding environment. This chitinous skeleton constitutes a suitable secondary substrate for sessile species, while the intricate network of branches is exploited by vagile fauna as a shelter, feeding, spawning and nursery area (Gori et al., 2017; D’Onghia, 2019; Rueda et al., 2019). Suspension feeding species can use the opportunity to settle on black coral branches to rise from the seabed and ensure themselves a greater exposure to current flow and trophic resources (Bo et al., 2015). The presence of arborescent anthozoans on the stems - especially the slow-growing white corals M. oculata and D. pertusum - is indicative of later stages of colonization, suggesting that the colonies have been dead for a relatively long period of time. Interestingly, in certain instances, white corals were observed as epibionts, whereas they were absent on the adjacent rocky bottoms.
The presence of several fishes and small-spotted catshark egg capsules in close association with L. glaberrima underscores the significance of cold-water corals forests as an Essential Fish Habitat (EFH) (D’Onghia, 2019). The deposition of eggs on black corals offers environmental benefits in terms of ventilation and protection, as well as for the capability of the flexible organic skeleton to mitigate the potential negative effects of turbulent hydrodynamic conditions (Cau et al., 2017).
B. robustus and A. rissoana exhibited a strong association with the black coral canopy, being commonly hosted by this coral species. Robust cusk-eels were often seen in aggregations – with up to ten individuals on a single colony – while the crab A. rissoana usually occurs in pairs, suggesting a key role of L. glaberrima in the reproductive ecology of these species. Despite being commonly hosted by this coral species, they are not exclusive, as both were also observed in association with the bamboo coral Isidella elongata and the white coral Madrepora oculata but with less frequency (D’Onghia, 2019; Rueda et al., 2019).
This work describes the distribution pattern, health status, associated fauna and types of aggregations of Leiopathes glaberrima populations in the northern part of the Strait of Sicily, laying out fresh insights about the ecology of the species and its conservation.
Extensive and pristine forests have been documented, achieving density values never reported before for the species. Such pristine populations are rare, representing a true biodiversity gem of the Mediterranean Sea.
In contrast, the study also provides the description of a rare case of mass mortality in a deep-sea environment, which leaves room for another consideration: large-scale mortality events due to anthropogenic climate change are now common in shallower environments, as sadly observed for tropical corals and temperate mesophotic ecosystems (Cerrano et al., 2019), while the stability of deep-sea environments coupled with the long water sinking times have led to the idea that such phenomena were virtually non-existent at great depths and their impact was negligible. In such a context, this study opens new scenarios regarding the possibility that mass mortality events can occur in deep environments too, even if they were considered safe from danger. The triggering causes of these events in inaccessible environments are actually only hypothesized, but it is not to be excluded that, sooner or later, a similar effect could potentially result from anthropogenic climate changes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
AG: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. SC: Conceptualization, Investigation, Supervision, Writing – original draft, Writing – review & editing, Project administration. BD: Data curation, Investigation, Writing – review & editing. TR: Project administration, Resources, Supervision, Writing – review & editing. SG: Funding acquisition, Project administration, Resources, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The data were collected during an oceanographic campaign funded by the MedWind company aimed at an environmental impact study for the construction of an offshore wind farm. MedWind company was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
The authors are glad to the reviewers for sharing our enthusiasm for the topic and for the precious comments, which have significantly improved the quality of the manuscript. The authors would like to thank all the people involved in the oceanographic survey, including the crew members, the technicians of the survey and ROV pilots. A special regard for our colleagues Pietro Battaglia, Frine Cardone, Valentina Costa, Bruna Giordano, Giacomo Milisenda, Valeria Palummo, Augusto Passarelli, Daniela Pica, Eva Salvati, Francesco Stenico, Margherita Toma.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aguilar R., Marín P. (2013). Mediterranean deep-sea corals, reasons for protection under Barcelona Convention (Spain: Oceana). Available at: https://europe.oceana.org/wp-content/uploads/sites/26/d_files/oceana_brief_deep-sea_corals.pdf.
Angiolillo M., Bo M., Toma M., Giusti M., Salvati E., Giova A., et al. (2023). A baseline for the monitoring of Mediterranean upper bathyal biogenic reefs within the marine strategy framework directive objectives. Deep-Sea Res. I: Oceanogr. Res. Pap. 194, 103963. doi: 10.1016/j.dsr.2023.103963
Astraldi M., Gasparini G. P., Gervasio L., Salusti E. (2001). Dense water dynamics along the strait of sicily (Mediterranean sea). J. Phys. Oceanogr. 31, 3457–3475. doi: 10.1175/1520–0485(2001)031<3457:DWDATS>2.0.CO;2
Bavestrello G., Bo M., Canese S., Sandulli R., Cattaneo-Vietti R. (2014). The red coral populations of the gulfs of Naples and Salerno: human impact and deep mass mortalities. Ital. J. Zool. 81, 552–563. doi: 10.1080/11250003.2014.950349
Bavestrello G., Cerrano C., Zanzi D., Cattaneo-Vietti R. (1997). Damage by fishing activities to the Gorgonian coral Paramuricea clavata in the Ligurian Sea. Aquat. Conserv.: Mar. Freshw. Ecosyst. 7, 253–262. doi: 10.1002/(SICI)1099–0755(199709)7:3<253::AID-AQC243>3.0.CO;2–1
Bianchi C. N., Morri C. (2000). Marine biodiversity of the mediterranean sea: situation, problems and prospects for future research. Mar. pollut. Bull. 40, 367–376. doi: 10.1016/S0025-326X(00)00027-8
Bilan M., Gori A., Grinyó J., Biel-Cabanelas M., Puigcerver-Segarra X., Santín A., et al. (2023). Vulnerability of six cold-water corals to sediment resuspension from bottom trawling fishing. Mar. pollut. Bull. 196, 115423. doi: 10.1016/j.marpolbul.2023.115423
Bo M., Bava S., Canese S., Angiolillo M., Cattaneo-Vietti R., Bavestrello G. (2014a). Fishing impact on deep Mediterranean rocky habitats as revealed by ROV investigation. Biol. Conserv. 171, 167–176. doi: 10.1016/j.biocon.2014.01.011
Bo M., Bavestrello G., Angiolillo M., Calcagnile L., Canese S., Cannas R., et al. (2015). Persistence of pristine deep-sea coral gardens in the mediterranean sea (SW sardinia). PloS One 10, e0119393. doi: 10.1371/journal.pone.0119393
Bo M., Bavestrello G., Canese S., Giusti M., Cerrano C., Salvati E., et al. (2011a). Coral assemblages off the Calabrian Coast (South Italy) with new observations on living colonies of Antipathes dichotoma. Ital. J. Zool. 78, 231–242. doi: 10.1080/11250001003652619
Bo M., Bavestrello G., Canese S., Giusti M., Salvati E., Angiolillo M., et al. (2009). Characteristics of a black coral meadow in the twilight zone of the central Mediterranean Sea. Mar. Ecol. Prog. Ser. 397, 53–61. doi: 10.3354/meps08185
Bo M., Bavestrello G., Kurek D., Paasch S., Brunner E., Born R., et al. (2012). Isolation and identification of chitin in the black coral Parantipathes larix (Anthozoa: Cnidaria). Int. J. Biol. Macromol. 51, 129–137. doi: 10.1016/j.ijbiomac.2012.04.016
Bo M., Bertolino M., Borghini M., Castellano M., Covazzi Harriague A., Di Camillo C. G., et al. (2011b). Characteristics of the mesophotic megabenthic assemblage of the Vercelli Seamount (North Tyrrhenian Sea). PloS One 6, e16357. doi: 10.1371/journal.pone.0016357
Bo M., Cerrano C., Canese S., Salvati E., Angiolillo M., Santangelo G., et al. (2014b). The coral assemblages of an off-shore deep Mediterranean rocky bank (NW Sicily, Italy). Mar. Ecol. 35, 332–342. doi: 10.1111/maec.12089
Bo M., Tazioli S., Spanò N., Bavestrello G. (2008). Antipathella subpinnata (Antipatharia, Myriopathidae) in Italian seas. Ital. J. Zool. 75, 185–195. doi: 10.1080/11250000701882908
Brown J., Macfadyen G. (2007). Ghost fishing in European waters: Impacts and management responses. Mar. Policy 31, 488–504. doi: 10.1016/j.marpol.2006.10.007
Buhl-Mortensen L., Vanreusel A., Gooday A. J., Levin L. A., Priede I. G., Buhl-Mortensen P., et al. (2010). Biological structures as a source of habitat heterogeneity and biodiversity on the deep ocean margins: Biological structures and biodiversity. Mar. Ecol. 31, 21–50. doi: 10.1111/j.1439-0485.2010.00359.x
Carella F., Aceto S., Saggiomo M., Mangoni O., De Vico G. (2014). Gorgonian disease outbreak in the Gulf of Naples: pathology reveals cyanobacterial infection linked to elevated sea temperatures. Dis. Aquat. Org. 111, 69–80. doi: 10.3354/dao02767
Cau A., Follesa M. C., Moccia D., Bellodi A., Mulas A., Bo M., et al. (2017). Leiopathes glaberrima millennial forest from SW Sardinia as nursery ground for the small spotted catshark Scyliorhinus canicula: catshark nursery ground in a millennial black coral forest. Aquat. Conserv.: Mar. Freshw. Ecosyst. 27, 731–735. doi: 10.1002/aqc.2717
Cerrano C., Bastari A., Calcinai B., Di Camillo C., Pica D., Puce S., et al. (2019). Temperate mesophotic ecosystems: gaps and perspectives of an emerging conservation challenge for the Mediterranean Sea. Eur. Zool. J. 86, 370–388. doi: 10.1080/24750263.2019.1677790
Cerrano C., Danovaro R., Gambi C., Pusceddu A., Riva A., Schiaparelli S. (2010). Gold coral (Savalia savaglia) and gorgonian forests enhance benthic biodiversity and ecosystem functioning in the mesophotic zone. Biodivers Conserv. 19, 153–167. doi: 10.1007/s10531-009-9712-5
Chiappone M., Dienes H., Swanson D. W., Miller S. L. (2005). Impacts of lost fishing gear on coral reef sessile invertebrates in the Florida Keys National Marine Sanctuary. Biol. Conserv. 121, 221–230. doi: 10.1016/j.biocon.2004.04.023
Chimienti G., Bo M., Taviani M., Mastrototaro F. (2019). “19 occurrence and biogeography of mediterranean cold-water corals”, in Mediterranean Cold-Water Corals: Past, Present and Future Coral Reefs of the World. Eds. Orejas C., Jiménez C. (Springer International Publishing, Cham), 213–243. doi: 10.1007/978–3-319–91608-8_19
Civile D., Lodolo E., Caffau M., Baradello L., Ben-Avraham Z. (2016). Anatomy of a submerged archipelago in the Sicilian Channel (central Mediterranean Sea). Geol. Mag. 153, 160–178. doi: 10.1017/S0016756815000485
Clark M. R., Althaus F., Schlacher T. A., Williams A., Bowden D. A., Rowden A. A. (2016). The impacts of deep-sea fisheries on benthic communities: a review. ICES J. Mar. Sci. 73, i51–i69. doi: 10.1093/icesjms/fsv123
Consoli P., Esposito V., Battaglia P., Altobelli C., Perzia P., Romeo T., et al. (2016). Fish distribution and habitat complexity on banks of the strait of sicily (Central mediterranean sea) from remotely-operated vehicle (ROV) explorations. PloS One 11, e0167809. doi: 10.1371/journal.pone.0167809
Deidun A., Andaloro F., Bavestrello G., Canese S., Consoli P., Micallef A., et al. (2015). First characterisation of a Leiopathes glaberrima (Cnidaria: Anthozoa: Antipatharia) forest in Maltese exploited fishing grounds. Ital. J. Zool. 82, 1–10. doi: 10.1080/11250003.2014.986544
Deidun A., Tsounis G., Balzan F., Micallef A. (2010). Records of black coral (Antipatharia) and red coral (Corallium rubrum) fishing activities in the Maltese Islands. Mar. Biodivers. Rec. 3, e90. doi: 10.1017/S1755267210000709
Di Lorenzo M., Sinerchia M., Colloca F. (2018). The North sector of the Strait of Sicily: a priority area for conservation in the Mediterranean Sea. Hydrobiologia 821, 235–253. doi: 10.1007/s10750-017-3389-7
D’Onghia G. (2019). “30 Cold-Water corals as shelter, feeding and life-history critical habitats for fish species: ecological interactions and fishing impact”, in Mediterranean Cold-Water Corals: Past, Present and Future Coral Reefs of the World. Eds. Orejas C., Jiménez C. (Springer International Publishing, Cham), 335–356. doi: 10.1007/978–3-319–91608-8_30
D’Onghia G., Calculli C., Capezzuto F., Carlucci R., Carluccio A., Grehan A., et al. (2017). Anthropogenic impact in the Santa Maria di Leuca cold-water coral province (Mediterranean Sea): Observations and conservation straits. Deep-Sea Res. II: Top. Stud. Oceanogr. 145, 87–101. doi: 10.1016/j.dsr2.2016.02.012
Etnoyer P. J., Wagner D., Fowle H. A., Poti M., Kinlan B., Georgian S. E., et al. (2018). Models of habitat suitability, size, and age-class structure for the deep-sea black coral Leiopathes glaberrima in the Gulf of Mexico. Deep-Sea Res. II: Top. Stud. Oceanogr. 150, 218–228. doi: 10.1016/j.dsr2.2017.10.008
FAO. (2008). Annex F of the Report of the Technical Consultation on International Guidelines for the Management of Deepsea Fisheries in the High Seas (Rome: FAO), 17.
Fink H. G., Wienberg C., De Pol-Holz R., Hebbeln D. (2015). Spatio-temporal distribution patterns of Mediterranean cold-water corals (Lophelia pertusa and Madrepora oculata) during the past 14,000 years. Deep-Sea Res. I: Oceanogr. Res. Pap. 103, 37–48. doi: 10.1016/j.dsr.2015.05.006
Frizzel T. J. (2020). In-Situ Preservation of Deep-Sea Shipwrecks: Understanding Biological Interactions and Environmental Impacts. Texas A&M University, College Station (TX.
García Lafuente J., García A., Mazzola S., Quintanilla L., Delgado J., Cuttita A., et al. (2002). Hydrographic phenomena influencing early life stages of the Sicilian Channel anchovy: Hydrographic relations with the Sicilian Channel anchovy. Fish. Oceanogr. 11, 31–44. doi: 10.1046/j.1365-2419.2002.00186.x
Garofalo G., Gristina M., Fiorentino F., Fulgosi F. C., Norrito G., Sinacori G. (2003). Distributional pattern of rays (Pisces, Rajidae) in the Strait of Sicily in relation to fishing pressure. Hydrobiology 503, 245–250. doi: 10.1023/B:HYDR.0000008487.25578.d4
Garrabou J., Gómez-Gras D., Ledoux J.-B., Linares C., Bensoussan N., López-Sendino P., et al. (2019). Collaborative database to track mass mortality events in the Mediterranean Sea. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00707
Garrabou J., Perez T., Sartoretto S., Harmelin J. (2001). Mass mortality event in red coral Corallium rubrum populations in the Provence region (France, NW Mediterranean). Mar. Ecol. Prog. Ser. 217, 263–272. doi: 10.3354/meps217263
Gili J. M., Coma R. (1998). Benthic suspension feeders: their paramount role in littoral marine food webs. Trends Ecol. Evol. 13, 316–321. doi: 10.1016/S0169-5347(98)01365-2
Gori A., Bavestrello G., Grinyó J., Dominguez-Carrió C., Ambroso S., Bo M. (2017). “Animal Forests in Deep Coastal Bottoms and Continental Shelf of the Mediterranean Sea,”, in Marine Animal Forests. Eds. Rossi S., Bramanti L., Gori A., Orejas C. (Springer International Publishing, Cham), 1–27. doi: 10.1007/978–3-319–17001-5_5–1
Grigg R. W., Opresko D. M. (1977). Order Antipatharia: black corals. Reef and shore fauna of Hawaii. Section 1: Protozoa through Ctenophora. Eds. Devaney D. M., Eldredge L. G. (Honolulu, HI: Bishop Museum Press), 242–261.
Hitt N. T., Sinclair D. J., Fallon S. J., Neil H. L., Tracey D. M., Komugabe-Dixson A., et al. (2020). Growth and longevity of New Zealand black corals. Deep-Sea Res. I: Oceanogr. Res. Pap. 162, 103298. doi: 10.1016/j.dsr.2020.103298
Ingrassia M., Di Bella L. (2021). Black coral distribution in the Italian seas: A review. Diversity 13, 334. doi: 10.3390/d13070334
Ingrassia M., Macelloni L., Bosman A., Chiocci F. L., Cerrano C., Martorelli E. (2016). Black coral (Anthozoa, Antipatharia) forest near the western Pontine Islands (Tyrrhenian Sea). Mar. Biodivers. 46, 285–290. doi: 10.1007/s12526-015-0315-y
Jarboui O., Ceriola L., Fiorentino F. (2022). “Current fisheries management in the strait of Sicily and progress towords an ecosystem approach,” in Transition towards an ecosystem approach to fisheries in the Mediterranean Sea - lessons learned through selected case studies. Eds. Vasconcellos M., Ünal V. (FAO, Rome), 147–162. Available at: http://www.fao.org/3/cb8268en/cb8268en.pdf.
Laist D. W. (1997). “Impacts of Marine Debris: Entanglement of Marine Life in Marine Debris Including a Comprehensive List of Species with Entanglement and Ingestion Records,” in Marine Debris Springer Series on Environmental Management. Eds. Coe J. M., Rogers D. B. (Springer New York, New York, NY), 99–139. doi: 10.1007/978–1-4613–8486-1_10
Lauria V., Massi D., Fiorentino F., Milisenda G., Cillari T. (2021). Habitat suitability mapping of the black coral Leiopathes glaberrima to support conservation of vulnerable marine ecosystems. Sci. Rep. 11, 15661. doi: 10.1038/s41598–021-95256–4
Macfadyen G., Huntington T., Cappell R. (2009). Abandoned, lost or otherwise discarded fishing gear (Rome: United Nations Environment Programme: Food and Agriculture Organization of the United Nations).
Massi D., Vitale S., Titone A., Milisenda G., Gristina M., Fiorentino F. (2018). Spatial distribution of the black coral Leiopathes glaberrima (Esper 1788) (Antipatharia: Leiopathidae) in the Mediterranean: a prerequisite for protection of Vulnerable Marine Ecosystems (VMEs). Eur. Zool. J. 85, 169–178. doi: 10.1080/24750263.2018.1452990
Mastrototaro F., D’Onghia G., Corriero G., Matarrese A., Maiorano P., Panetta P., et al. (2010). Biodiversity of the white coral bank off Cape Santa Maria di Leuca (Mediterranean Sea): An update. Deep-Sea Res. II: Top. Stud. Oceanogr. 57, 412–430. doi: 10.1016/j.dsr2.2009.08.021
Merrifield S. T., Celona S., McCarthy R. A., Pietruszka A., Batchelor H., Hess R., et al. (2023). Wide-area debris field and seabed characterization of a deep ocean dump site surveyed by Autonomous Underwater Vehicles. Environ. Sci. Technol. 57, 18162–18171. doi: 10.1021/acs.est.3c01256
Morato T., González-Irusta J., Dominguez-Carrió C., Wei C., Davies A., Sweetman A. K., et al. (2020). Climate-induced changes in the suitable habitat of cold-water corals and commercially important deep-sea fishes in the North Atlantic. Glob. Change Biol. 26, 2181–2202. doi: 10.1111/gcb.14996
Napolitano E., Sannino G., Artale V., Marullo S. (2003). Modeling the baroclinic circulation in the area of the Sicily channel: The role of stratification and energy diagnostics. J. Geophys. Res. 108, 3230. doi: 10.1029/2002JC001502
Otero M. D. M., Marin P. (2019). “46 Conservation of Cold-Water Corals in the Mediterranean: Current Status and Future Prospects for Improvement,”, in Mediterranean Cold-Water Corals: Past, Present and Future Coral Reefs of the World. Eds. Orejas C., Jiménez C. (Springer International Publishing, Cham), 535–545. doi: 10.1007/978–3-319–91608-8_46
Paradis S., Puig P., Masqué P., Juan-Díaz X., Martín J., Palanques A. (2017). Bottom-trawling along submarine canyons impacts deep sedimentary regimes. Sci. Rep. 7, 43332. doi: 10.1038/srep43332
Prouty N., Roark E., Buster N., Ross S. (2011). Growth rate and age distribution of deep-sea black corals in the Gulf of Mexico. Mar. Ecol. Prog. Ser. 423, 101–115. doi: 10.3354/meps08953
Rivoire G. (1991). “Mortalité de corail et de gorgones en profondeur au large des côtes provençales,” in Les espèces marines à protéger en Méditerranée. Eds. Boudouresque C. F., Avon M., Gravez V. (France: GIS Posidonies), 53–59.
Roark E. B., Guilderson T. P., Dunbar R. B., Ingram B. L. (2006). Radiocarbon-based ages and growth rates of Hawaiian deep-sea corals. Mar. Ecol. Prog. Ser. 327, 1–14. doi: 10.3354/meps327001
Roark E. B., Guilderson T. P., Dunbar R. B., Fallon S. J., Mucciarone D. A. (2009). Extreme longevity in proteinaceous deep-sea corals. Proc. Natl. Acad. Sci. U.S.A. 106, 5204–5208. doi: 10.1073/pnas.0810875106
Roberts J. M., Cairns S. D. (2014). Cold-water corals in a changing ocean. Curr. Opin. Environ. Sustain. 7, 118–126. doi: 10.1016/j.cosust.2014.01.004
Roberts J. M., Murray F., Anagnostou E., Hennige S., Gori A., Henry L.-A., et al. (2016). “Cold-Water Corals in an Era of Rapid Global Change: Are These the Deep Ocean’s Most Vulnerable Ecosystems?,”, in The Cnidaria, Past, Present and Future. Eds. Goffredo S., Dubinsky Z. (Springer International Publishing, Cham), 593–606. doi: 10.1007/978–3-319–31305-4_36
Rossi S., Bramanti L., Gori A., Orejas C. (2017). “An Overview of the Animal Forests of the World,”, in Marine Animal Forests. Eds. Rossi S., Bramanti L., Gori A., Orejas C. (Springer International Publishing, Cham), 1–26. doi: 10.1007/978–3-319–17001-5_1–1
Rueda J. L., Urra J., Aguilar R., Angeletti L., Bo M., García-Ruiz C., et al. (2019). “29 Cold-Water Coral Associated Fauna in the Mediterranean Sea and Adjacent Areas”, in Mediterranean Cold-Water Corals: Past, Present and Future Coral Reefs of the World. Eds. Orejas C., Jiménez C. (Springer International Publishing, Cham), 295–333. doi: 10.1007/978–3-319–91608-8_29
Silva M., Etnoyer P. J., MacDonald I. R. (2016). Coral injuries observed at mesophotic reefs after the Deepwater Horizon oil discharge. Deep-Sea Res. II: Top. Stud. Oceanogr. 129, 96–107. doi: 10.1016/j.dsr2.2015.05.013
Taviani M., Vertino A., Angeletti L., Montagna P., Remia A. (2019). “2 Paleoecology of Mediterranean Cold-Water Corals,”, in Mediterranean Cold-Water Corals: Past, Present and Future Coral Reefs of the World. Eds. Orejas C., Jiménez C. (Springer International Publishing, Cham), 15–30. doi: 10.1007/978–3-319–91608-8_2
Thresher R. E., Guinotte J. M., Matear R. J., Hobday A. J. (2015). Options for managing impacts of climate change on a deep-sea community. Nat. Clim. Change 5, 635–639. doi: 10.1038/nclimate2611
Toma M., Bo M., Cattaneo-Vietti R., Canese S., Canessa M., Cannas R., et al. (2022). Basin-scale occurrence and distribution of mesophotic and upper bathyal red coral forests along the Italian coasts. Medit. Mar. Sci. 23, 484–498. doi: 10.12681/mms.28052
Tracey D., Bostock H., Shaffer M. (2018). Ageing methods for protected deep-sea corals: A review and recommendation for an ageing study. DOC Contract 4527 GMC - Age & Growth of coral (POP2017–07). NIWA Client Report No. 2018035WN 40 p. Available at: https://www.doc.govt.nz/globalassets/documents/conservation/marine-and-coastal/marine-conservation-services/reports/pre-2019-annual-plans/methodology-report-age-and-growth-of-coral.pdf.
Tsounis G., Rossi S., Grigg R., Santangelo G., Bramanti L., Gili J.-M. (2010). The exploitation and conservation of precious corals. Oceanogr. Mar. Biol. 48, 161–212. doi: 10.1201/EBK1439821169-c3
Turley C. M., Roberts J. M., Guinotte J. M. (2007). Corals in deep-water: will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 26, 445–448. doi: 10.1007/s00338-007-0247-5
Vega Fernández T., Pace M. L., Badalamenti F., D’Anna G., Fiorentino F., Garofalo G., et al. (2012). Application of the MESMA framework. Case study: Strait of Sicily. MESMA report. Available at: http://eprints.bice.rm.cnr.it/15735/1/D3.1-D3.2%20MESMA%20-%20Annex%20Strait%20of%20Sicily.pdf.
Vezzulli L., Previati M., Pruzzo C., Marchese A., Bourne D. G., Cerrano C., et al. (2010). Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environ. Microbiol. 12, 2007–2019. doi: 10.1111/j.1462-2920.2010.02209.x
Keywords: black corals, mass mortality, deep sea, Mediterranean Sea, ROV-imaging
Citation: Giova A, Canese S, Donelli BZ, Romeo T and Greco S (2024) Structural diversity of Leiopathes glaberrima populations in the strait of Sicily: from pristine to declining forests. Front. Mar. Sci. 11:1387144. doi: 10.3389/fmars.2024.1387144
Received: 16 February 2024; Accepted: 27 May 2024;
Published: 10 June 2024.
Edited by:
Lorenzo Angeletti, IRBIM-CNR, ItalyReviewed by:
Giorgio Bavestrello, University of Genoa, ItalyCopyright © 2024 Giova, Canese, Donelli, Romeo and Greco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Giova, YW50b25pby5naW92YUBzem4uaXQ=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.