- 1Department of Environmental Protection and Regeneration, Red Sea Global (RSG-Red Sea Zone), Umluj, Saudi Arabia
- 2Island Biodiversity and Conservation Centre, University of Seychelles, Victoria, Seychelles
- 3Island Avian Surveys Ltd., Orkney, United Kingdom
- 4Reef Ecology Lab, Biological and Environmental Science and Engineering Division, Red Sea Research Center, King Abdullah University of Science and Technology, Thuwal, Saudi Arabia
Introduction: Seabirds and other insular birds are an important part of marine ecosystems and are increasingly threatened worldwide. Phenology, abundance, distribution, and breeding success are important baseline parameters needed to evaluate population trends and dynamics, identify biodiversity hotspots and potential breeding sites, and assess habitat selection. In the Red Sea basin, reliable and complete data on birds are lacking for islands in most of the Important Bird Areas (IBA). Such data are now especially important since development projects have started at several of these locations. Here, we assess the distribution, abundance, phenology, and population trends of 13 bird species that breed in the Duba Islands, Al Wajh Bank, and Al Lith Islands. Breeding success and habitat selection for six species were also assessed.
Methods: Between December 2020 and August 2023, more than 90 islands were visited multiple times with different frequencies. All nests were counted, and the area occupied by the different colonies was georeferenced. Breeding success was assessed by visiting selected colonies multiple times until the chicks were ready to fledge. Habitat selection was determined by analyzing the habitat found in the georeferenced colonies.
Results: More than 25,000 nests were counted each year, mainly in the summer, on more than 75 islands. Of the 13 species studied, the most abundant and widespread species was the White-cheeked tern, and the second most widespread was the Osprey.
Discussion: We estimate that the Al Wajh Bank hosts the following percentages of global breeding populations: Crab plover: 5%, Sooty gull: 17%–35%, White-eyed gull: 15%, Bridled tern: 1%, White-cheeked tern: 4%, and Lesser crested tern: 2%, making the area a regional and global hotspot for these species. Some of the islands occupied by breeding birds are slated for development for tourism activities as part of the Kingdom’s tourism expansion plans. At the same time, there are several ambitious conservation programs underway in these areas. To be effective, such programs require reliable and comprehensive data of the kind presented here.
1 Introduction
Seabirds and other insular avifauna are an important component of the marine ecosystems (Schreiber and Burger, 2001; Gaston, 2004) and, being top predators, indicators of its health. They have been well studied in relation to marine ecosystem functions (Frederiksen et al., 2006; Zador et al., 2013), used to evaluate the impact of climate change (Barbraud et al., 2008), fisheries (Einoder, 2009; Le Corre et al., 2012), and as indicators of prey stock (Piatt et al., 2007; Lyday et al., 2015). Knowledge about seabirds and other insular avifauna is therefore essential for the conservation of marine ecosystems (Bibby et al., 2012). In particular, knowledge of population sizes allows for determining the status of species and trends. The changes in numbers and range can be analyzed in relation to environmental features, direct threats (e.g., poaching of adults, young, and eggs), and the success or failure of conservation management policies in protected areas (Sutherland et al., 2004; Bibby et al., 2012).
Seabirds are now more threatened than any other group of birds. Of the 346 seabird species, 97 (28%) are globally threatened, and a further 10% are listed as near-threatened. Almost half of all seabird species are known or suspected to be experiencing population declines (Dias et al., 2019).
Other insular avifauna, such as waders and piscivorous raptors, are also strictly dependent on marine insular ecosystems and at risk if the delicate balance of islands changes (Bond et al., 2019).
Phenology, abundance, and distribution of breeding birds, as well as breeding success, are important baseline parameters needed to assess population trends and dynamics, identify biodiversity hotspots and potential breeding sites, and are useful for understanding habitat selection (Koleček et al., 2020; Kamp et al., 2021; Vitasse et al., 2021; Lees et al., 2022).
In the Red Sea, seabirds have generally been understudied, and data are lacking (http://datazone.birdlife.org/site). Such data are now especially important since tourism development projects have started at several of these locations (Chalastani et al., 2020).
In Saudi Arabia, an objective of Vision 2030 (a series of goals to be achieved by Saudi Arabia by 2030, https://www.vision2030.gov.sa/v2030/overview/) is to create a more diverse and sustainable economy, which includes many tourism projects aiming to host international sustainable and luxury tourism.
Concurrently, the Saudi Green Initiative aims to increase the environmental preservation efforts in the country and improve the conservation status of many species of animals and plants. This will primarily be achieved with initiatives focusing on (i) energy transition, including emissions reduction across sectors and boosting renewable energy capacity; (ii) habitat rehabilitation; and (iii) commitments to increase protected areas to increase biodiversity and safeguard Saudi Arabia’s diverse natural environments (https://www.greeninitiatives.gov.sa/about-sgi/).
To achieve these ambitious objectives, baseline knowledge on biodiversity, abundance, and distribution of species is fundamental to allowing for informed spatial planning, especially in areas that will be the object of development and enhancement/protection.
Among the areas being developed are several islands that are potentially important for seabird biodiversity. These include one out of three islands in the Duba region (hereafter called Duba islands), notably An Numan island, several islands in the Important Bird Area (IBA) Al Wajh Bank, established in 1994 by BirdLife International (Evans, 1994), and one out of four islands located north of the Farasan Bank IBA (offshore the town of Al Lith, hereafter called Al Lith islands). For the Duba and Al Lith islands, prior to this study, there was little information available on breeding species and population abundance (Newton and Al Suhaibany, 1996). Within the Al Wajh Bank, 16 out of the 92 islands were visited in 2011 (Shobrak and Aloufi, 2014), but there is no previous comprehensive and comparable data available for the whole lagoon. The largest-scale surveys conducted previously, limited to summer, took place in the Al Wajh Bank and at An Numan Island between 2018 and 2021 as part of an environment impact assessment (Red Sea Global unpublished reports). The methods used for that work were mainly flush counts, and the survey data are not published. Therefore, for the four species triggering the Al Wajh IBA (Crab plover [Dromas ardeola], Sooty gull [Ichthyaetus hemprichii], White-eyed gull [Ichthyaetus leucophthalmus], and Sooty falcon [Falco concolor], not included here) and for other species, the most recent published estimates still date back to the 1990s and did not include comprehensive numbers for all islands (Newton and Al Suhaibany, 1996). In addition, the breeding season for some of these species had not previously been systematically assessed anywhere in the Red Sea.
The aim of this study was to fill several information gaps by assessing phenology, distribution, and abundance and setting the baseline numbers for future population trends of 13 priority seabird species that breed in the Duba islands, Al Wajh Bank, and Al Lith islands. These species are Osprey (Pandion haliaetus haliaetus), Caspian tern (Hydroprogne caspia), Brown booby (Sula leucogaster plotus), Saunders’s tern (Sternula saundersi), Great crested tern (Thalasseus bergii velox), Eurasian spoonbill (Platalea leucorodia archeri), Crab plover, Sooty gull, White-eyed gull, Brown noddy (Anous stolidus plumbeigularis), Bridled tern (Onychoprion anaethetus fuligula), White-cheeked tern (Sterna repressa), and Lesser crested tern (Thalasseus bengalensis bengalensis). These species were selected based on their global and local conservation importance, their level of endemism, and other special local considerations (Table 1). Most of them are subspecies locally distributed in the Arabian region. Breeding success and habitat selection for some of these species were also assessed for the first time in the Al Wajh Bank. To facilitate narration and ease of reading, the order in which the species are listed in the text is based on their time of breeding (from early breeders to late breeders across the year), unless logic requires otherwise.
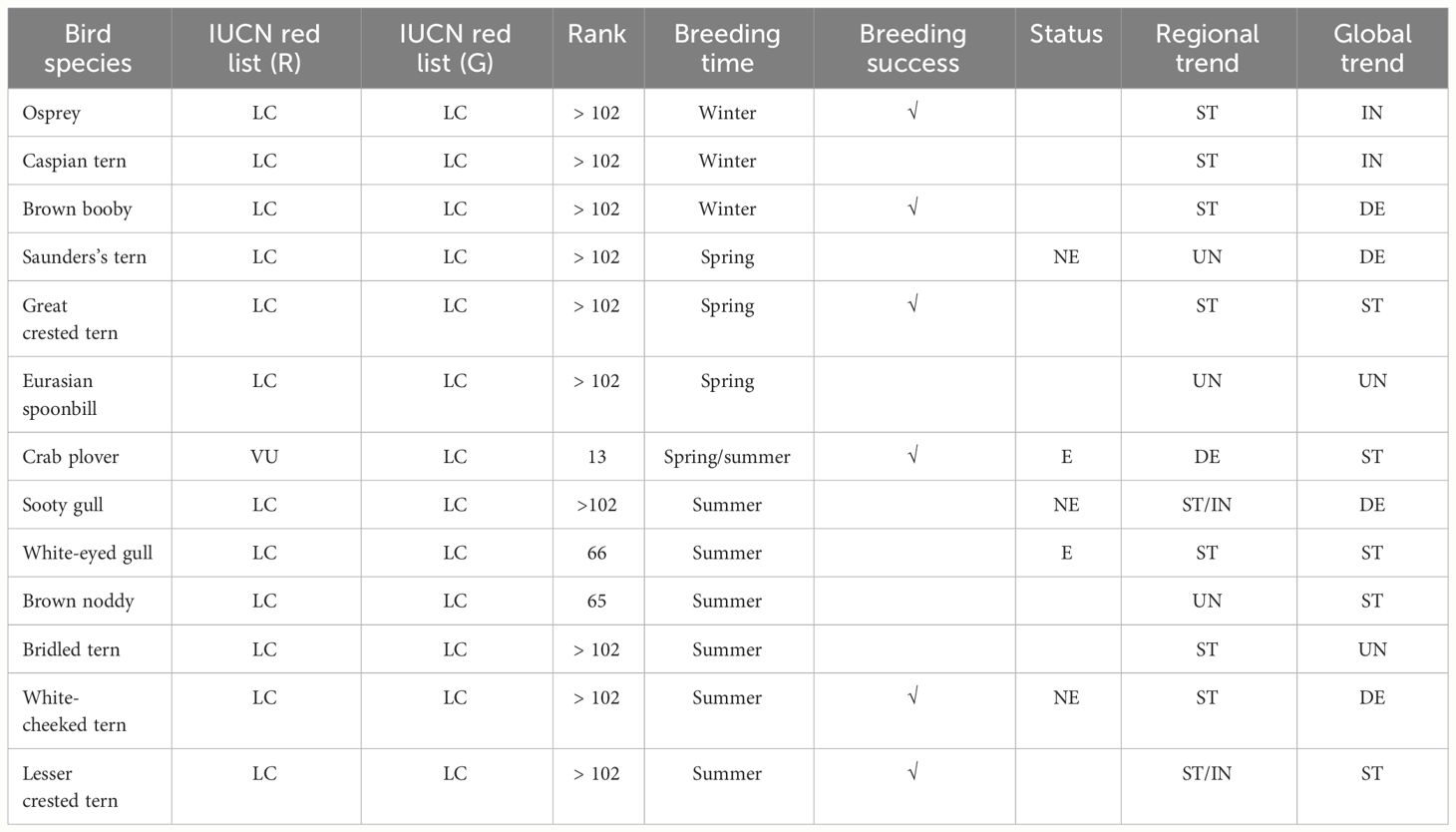
Table 1 Study species with status according to the IUCN Regional (R) and Global (G) lists, rank, which is the position within the high conservation priority bird species list in Saudi Arabia (Boland and Burwell, 2020), breeding time (beginning of the breeding season), breeding success (ticked if assessed in the current study), breeding status in the Arabian peninsula according to Jennings, 2010 [near-endemic (NE), endemic (E)], and regional and global trend [increasing (IN), stable (ST), decreasing (DE), unknown (UN)] (Boland and Burwell, 2020 and Symes et al., 2015).
2 Materials and methods
2.1 Study sites
The three surveyed sites are shown in Figure 1. From the north, these are three Duba islands, which include: An Numan, a large rocky outcrop surrounded by lowland with salt-tolerant shrubs, and Nabqiyah and Awandia, which are low sandy islands with salt-tolerant shrubs. They are respectively, about 558, 2.3, and 1.5 hectares (ha). The Al Wajh Bank includes 92 islands; the smallest ones have an area of less than 1 ha, while the biggest is 1,658 ha. Most of the islands are low-lying, with sandy substrate and salt-tolerant shrubs. Of these islands, 52 have different levels of mangrove coverage, and only 12 present rocky outcrops and areas with sabkha, which is a dry, salt-encrusted, former wet zone (Vincent, 2008). Among the Al Lith islands, Jabal Al Lith is the biggest, with 393 ha of rocky outcrops, sabkha, and low-lying sandy beaches with salt-tolerant vegetation. The size of the other three islands varies between 5 ha and 15 ha. Mar Mar Island is the only island almost completely covered with relatively tall vegetation (1.5 m to 1.8 m, halophyte Suaeda sp.), while the other two islands are low-lying sandy islands with salt-tolerant shrubs.

Figure 1 Distribution of the study sites across the Saudi Arabian Red Sea. Abundance and distribution of seabirds in the Duba (A), Al Wajh (B), and Al Lith (C) islands. The abundance is shown as the average over 2, 3, and 1 year, respectively.
2.2 Breeding season, peak, and species counts
The number of breeding pairs, identified by counting nests, was selected as the best parameter to assess the bird abundance since it gives a good indication of the capacity of the population to sustain itself. It is also the easiest and least biased way to count, since counting nests is easier and more reliable than counting birds (Bibby et al., 2012).
Each of the three island groups was visited several times across 3 years of the study (2021−2023) and across all seasons (see Supplementary Material 1 for detailed periods). During 2021, the islands in the Al Wajh Bank were visited and surveyed on a regular basis to provide an accurate phenology of the species, which was then used to identify key breeding periods and inform future fieldwork (see below). The Duba islands were visited less often, but surveys were carried out in February, May, July, and September in 2022 and 2023 to cover all seasons. The Al Lith islands were visited only twice: in June and July 2021 to count summer breeders and in October 2022 to assess any other breeding species. The islands were visited early in the morning, and in the summer, visits were limited from sunrise to late morning/midday to avoid disturbing the nesting birds during the warmest part of the day. The colony counts were conducted as fast as possible, and birds moved rapidly back to the nest after the count was done, normally within a few minutes. Occasional sightings of other breeding species were also recorded, but population abundance for these was not assessed.
For species breeding in winter (Osprey and Caspian tern), the best time to survey the islands was from December to March since the first eggs were found in December. In 2021, nests were found at all stages; therefore, breeding attempts that failed at the early stage may have been missed (especially for the Caspian tern, which does not build a prominent nest like the Osprey). In 2023, a specific survey for the Osprey was carried out aimed at counting all breeding pairs shortly after the peak of the egg-laying period, in February.
Counts of species nesting in the spring were conducted from March to May. A good population estimate was made for the Brown booby and the Eurasian spoonbill, while for the Saunders’s tern, since breeding pairs are more difficult to locate, only a minimum number of breeding pairs could be estimated.
Summer-breeding species were counted shortly after the peak of the egg-laying period, when late breeders had also laid their eggs. To identify this window of time, from April 2021 to the end of June 2021, some sampling islands where colonies were historically present (Red Sea Global unpublished reports) were surveyed regularly and the behavior of the birds recorded. The beginning of the summer census was scheduled for 2 to 3 weeks after the first egg of gulls or terns was observed. All islands were visited, giving priority to gull colonies (which generally breed earlier than most terns). After the gulls were counted, the census continued from south to north to keep the field methods standardized across years. The islands with gull colonies were revisited a second time for the White-cheeked tern and a third time for the Lesser crested tern based on their egg-laying period. The Bridled tern breeding pairs could not be counted as they were nested in thick vegetation, and the egg and/or chick was not visible; as a proxy, the number of mobbing adults flushed from the eggs was counted while crossing the colony. Since at least one adult was expected to always be present with the egg, but both could also be present, the minimum number of pairs was calculated by dividing the number of counted adults by two, and the maximum number of pairs was the number of counted adults.
Once the appropriate period was determined, the islands were extensively surveyed on foot by two to four observers for each survey.
To assess the number of breeding pairs, two different counting methods were used, depending on the species. For strictly colonial species, such as the Crab plover and terns (apart from Caspian and Saunders’s terns since they tended to breed as individuals or in loose colonies), the perimeter of the colony was marked with GPS by one observer while the other was counting the number of eggs, chicks, and scrapes (Apparently Occupied Nests [AON]) found in the colony (for Crab plovers, only the number of burrows was counted from the perimeter of the colony as to enter the colony area would destroy the nests). For loosely colonial and single nest species (Osprey, Caspian, and Saunders’s Tern, and Sooty and White-eyed gull), every nest was georeferenced and the content recorded (scrape or active nest, egg, chick). For the White-eyed gull, from single nest positions, a colony area was also extracted with GIS software (QGIS Association, 2023). The total number of breeding pairs was obtained by summing scrapes/active nests can also be called AON with eggs and chicks.
Observations at the islands and nest contents were used to refine the phenology of each species based on what is known of their breeding cycle (i.e., average number of days for incubation, chick rearing). Each fortnight was identified as (i) the prebreeding stage: the courtship phase, before the first eggs are laid; (ii) the egg stage: the period when eggs can be found (in colonies, found together with chicks); and (iii) chick stage: the period when chicks can be found before fledging.
2.3 Breeding success
Breeding success was assessed for six species only at the Al Wajh Bank (Table 1). For all species, except the Crab plover, several colonies and/or nests were visited at least twice to assess the number of chicks that reached an age close to fledge. Since once fledged, the birds may leave the colony or nest, a nest or egg was considered successful when the chick reached approximately: 40 days for Ospreys (Eriksson and Wallin, 1994); 42 days for Brown boobies; and 17 to 27 days for Great crested, White-cheeked, and Lesser crested terns (following methods used by Nisbet and Drury, 1972 and Nisbet et al., 2020 for other tern species, but with different numbers of days according to different species). At this stage, the chicks were deemed able to fledge. Breeding success was therefore a measure of the number of large nestlings for each colony or nest seen on the last visit. In addition, where possible, additional breeding information was also reported (e.g., the number of adults).
The breeding success of Crab plovers is difficult to assess due to their burrow-nesting behavior. We deployed camera traps in 2023 to monitor the fledging success of a subset of burrows. A total of 27 burrows were monitored from two colonies on separate islands (N = 15 Um Rumah 1 and N = 12 Al Numaniat 3). Initially, burrows were checked using a burrowscope camera to assess occupancy. The camera’s settings were set to record 2 h in the morning, 2 h in the afternoon, and 2 h in the evening at the beginning of the breeding season. At approximately 20 days from the expected fledging events, they were put on motion sensors to capture all birds approaching the monitored nests and identify fledglings. Chicks regularly emerge from the burrows when they are close to fledging, as observed in 2022 from camera traps (Calabrese unpublished data); therefore, this method can be considered a valid tool to assess fledging success. The camera footage was reviewed at the end of the breeding season, and all chicks seen at approximately 50 days of age were considered to have fledged (Tayefeh et al., 2013).
2.4 Habitat use
Habitat use was described by overlapping georeferenced colonies with the island satellite imagery (ArcGis online maps from World Imagery, 2022) showing the habitat. The habitat categories identified were the following: sandy substrate with no vegetation, sandy substrate with scattered shrubs (less than 50% coverage), sandy substrate with dense shrubs (more than 50% coverage), sabkha, limestone rock, and Avicennia marina mangrove cover (less than 50%). Observations on the habitat were also made on the field. For the White-cheeked tern, the different types of materials used to make the nests were also noted, and pictures of different typologies of nests were taken.
3 Results
3.1 Phenology, abundance, and distribution
The phenology of each species is described in Figure 2, and the number of breeding pairs of each species and in each area is summarized in Table 2. The earliest breeding species was the Osprey, for which the first eggs were found in December, but the peak of egg-laying was late January or early February. The Caspian tern also bred in winter, and the egg-laying peak was found in January. The Brown booby and the Saunders’s tern started breeding in spring, although eggs of the first were found also in summer. The Great crested tern was the first summer breeding seabird, with egg-laying starting in May, together with the Eurasian spoonbill and the Crab plover. The Sooty and White-eyed gull started laying eggs at the end of May and the beginning of June, with the peak of the egg-laying being at the end of June. White-cheeked and Bridled terns’ peak of egg-laying is during the second week of July, while for Lesser crested terns, it occurred in the third week of July.
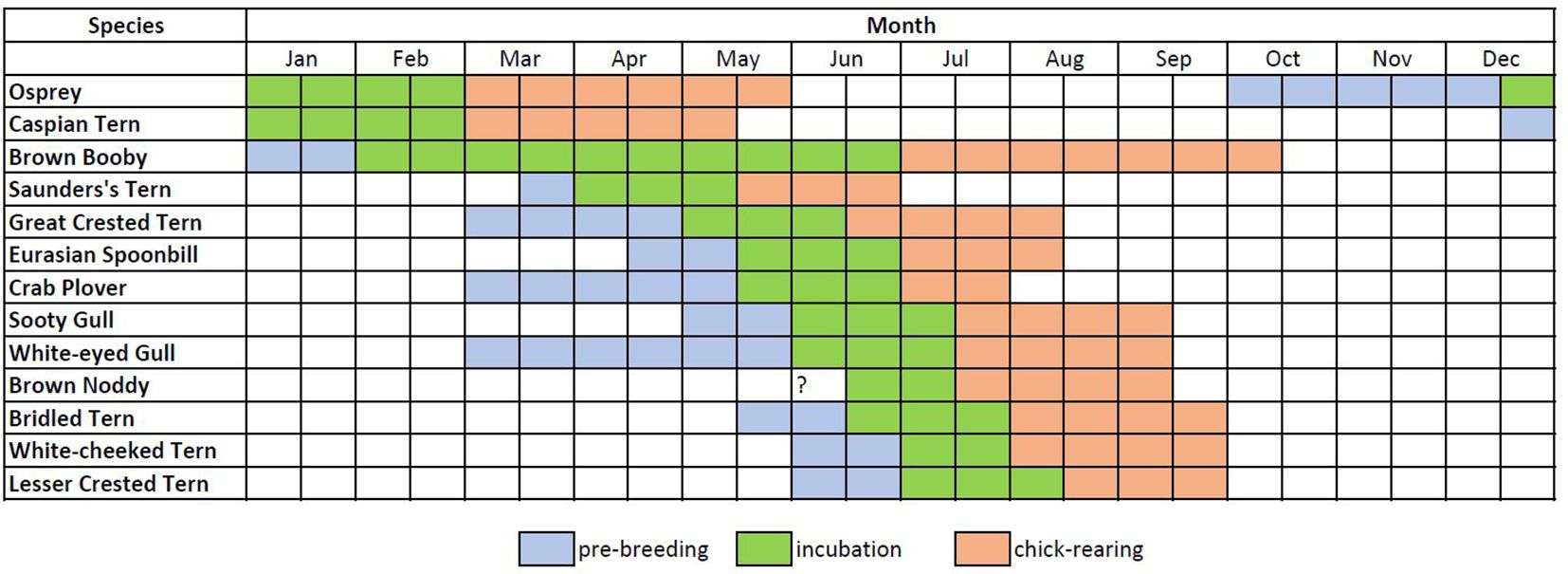
Figure 2 Phenology of target species. Blue indicates the prebreeding phase when breeding pairs inspect the area, select a suitable area, and nest-building begins; green is the incubation phase when adults are incubating the eggs; pink indicates the chick-rearing phase that lasts from the hatching to the fledging of the chicks.
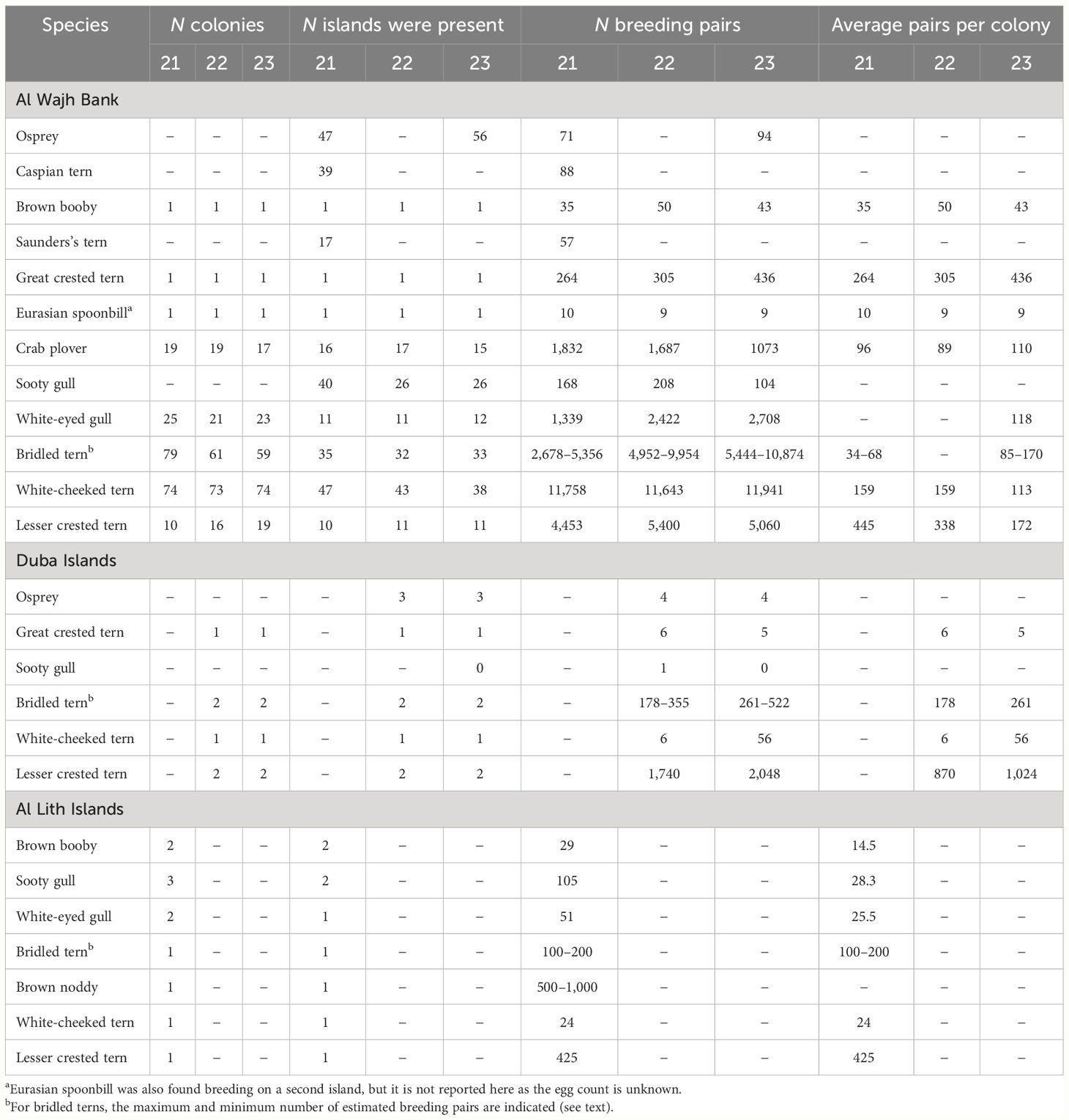
Table 2 Summary of the number of breeding pairs counted for the 3 years (2021–2023, in the table indicated as 21, 22, and 23) of monitoring at the three different island groups of the Saudi Arabian Red Sea.
A total of five, 12, and seven priority species of birds were found breeding on the Duba, Al Wajh, and Al Lith islands, respectively. The Red-billed tropicbird (Phaethon aethereus indicus) was also found to be breeding, but it is the object of another manuscript (Calabrese in prep.) since it was the first breeding record in the north of the Saudi Arabian Red Sea.
The highest abundance of birds was recorded in the summer when the Crab plover and all the colonial seabirds (Eurasian spoonbill, Sooty and White-eyed gull, Great crested, White-cheek, Bridled, and Lesser crested tern) except the Brown booby breed. The number of colonies for each species for the different survey areas is indicated in Table 2 and the number of breeding pairs on each island and in each year is reported in Supplementary Material 2, together with the total of breeding attempts excluding the number of AON (i.e., eggs plus chicks). The latest parameter is indicated to provide additional baseline information for further surveys. In summer, in the Duba islands, a total of six colonies were found in both 2022 and 2023, for a total of 1,917 breeding pairs of six species counted in 2022 and 2,442 in 2023, while in the Al Wajh Bank, a total of 210, 199, and 207 seabird colonies were found and counted in 2021, 2022, and 2023, respectively, for a total of more than 25,000, 30,000, and 30,000 breeding pairs belonging to 12 species. In Al Lith, 11 colonies were counted on four islands in 2021, for a total of about 1,500 breeding pairs and seven species.
In winter, in the Duba islands, the Osprey was found breeding on three islands, for a total of four breeding pairs counted both in 2022 and 2023. In the Al Wajh Bank, it bred on 47 and 56 islands in 2021 and 2023, respectively, for a total of 71 and 94 pairs. In the Al Lith islands, Ospreys were not surveyed.
Lesser crested tern, White-cheeked tern, and Brown noddy were the most abundant species found in the Duba, Al Wajh, and Al Lith islands, respectively (Table 2). The Brown noddy is only a rare visitor to the Al Wajh Bank (Jennings pers. comm.).
In the Al Wajh Bank, in 2023, the Lesser crested tern had the highest colony density with 5.78 nests/m2 (Table 3) and formed the biggest colonies with an average of 514 breeding pairs for each colony (Table 2). This was closely followed by Great crested terns with a density of 5.34 nests/m2 (Table 3). The White-cheeked tern and Crab plover were the most colonial after the crested terns with 0.25 nests/m2 and 0.15 nests/m2, respectively. The White-eye gull and the Brown booby were found to be loosely colonial, and the breeding pairs were scattered across the colony area with low density (Table 3). White-eyed gull colonies had between one and 689 pairs, while Sooty gulls, Caspian, and Saunders’s terns were found to be mainly noncolonial, with breeding pairs typically scattered across the islands, with often only one pair on an island (Tables 2, 3). Two small colonies of Saunders’s terns were also found and consisted of up to 10 pairs.

Table 3 Average (± standard deviation), minimum, and maximum colony densities for the Crab plover, Great crested tern, White-eyed gull, White-cheeked tern, and Lesser crested tern counted in the 2023 season in the Al Wajh Bank.
In the Al Wajh Bank, the number of islands where at least one of the 13 target species was breeding was 75 in 2021, 63 in 2022, and 68 in 2023 (however, Caspian and Saunders’s terns were not censused in 2022 and 2023). In general, 87% of the islands had at least one species breeding over the 3 years of surveys. All of the Duba islands hosted at least one of the target species in both 2022 and 2023, while four of the five Al Lith islands (80%) had at least one species breeding in 2021. Accounts for each species are reported in Table 2. The distribution of breeding birds across the three areas and the number of species and breeding pairs for each island are indicated in Figure 1. For the Al Wajh Bank and the Duba islands, an average of the counting years is provided. Other bird species were confirmed to be breeding on the islands (excluding passerines), such as Western reef-heron (Egretta gularis schistacea), Kentish plover (Anarhynchus alexandrinus alexandrinus), and Common kestrel (Falco tinnunculus, only found on An Numan island). Purple heron (Ardea purpurea purpurea) and Goliath heron (Ardea goliath) were recorded all year round, but evidence of breeding was not found, even if they are likely to breed in the Al Wajh Bank (Jennings, 2010).
3.2 Breeding success
The breeding success of six species is summarized in Table 4. Overall, the Great and Lesser crested tern had the highest breeding success in 2022, with 74% and 82%, respectively. The other two species monitored in 2022 were the Brown booby and the White-cheeked tern, both with breeding success of 20% and 33%, respectively. Some species showed a drop in productivity in 2023 compared with 2022, with a reduction from 82% to 47% for Great crested tern, 33% to 18% for White-cheeked tern, and 74% to 71% for Lesser crested tern (Table 4).
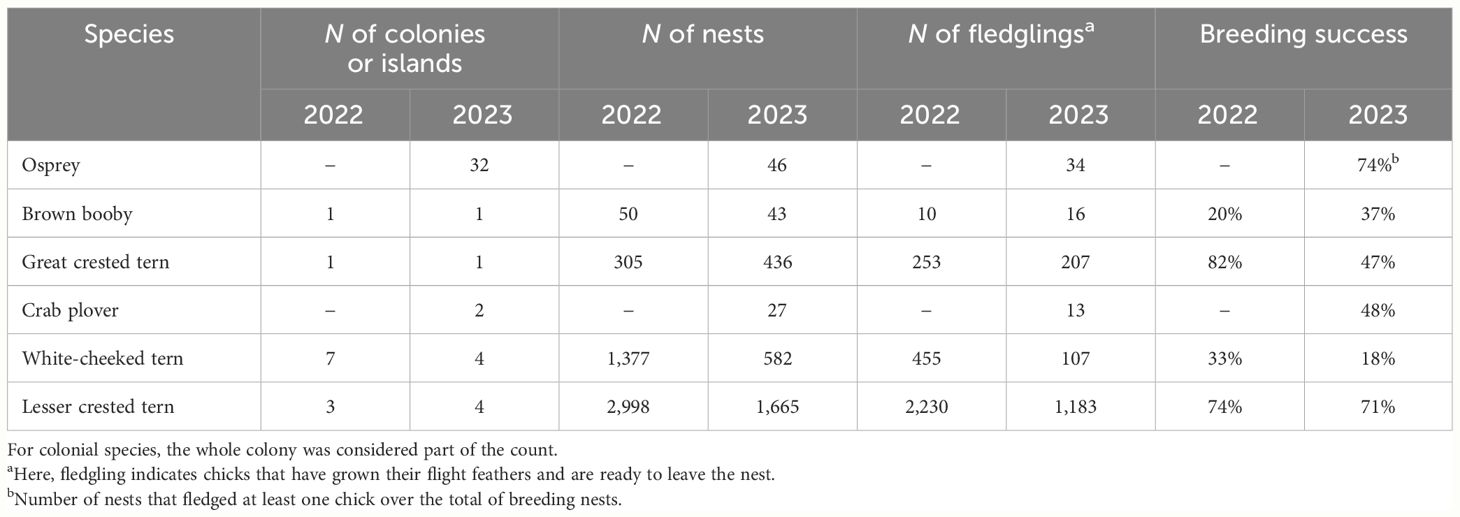
Table 4 Summary of the number of nests and fledglings and the breeding success for Osprey, Brown booby, Great crested tern, Crab plover, White-cheeked tern, and Lesser crested tern surveyed in the 2022 and 2023 seasons.
The Crab plover nests monitored in two colonies in 2023 had very different outcomes: the ones in the Um Rumah 1 colony had 87% breeding success (N = 15), while the ones monitored on Al Numaniat 3 fledged zero chicks (N = 12) (Table 4).
In 2023, 46 Osprey breeding pairs were monitored and assessed for productivity. Clutch size ranged from one to three eggs, with a mean (± SD) of 2.58 (± 0.67). Brood size varied from one to three chicks, with a mean (± SD) of 2.03 (± 0.76). An observation of a nest on An Numan (Duba island) reported a brood of four chicks, which successfully fledged in 2022. Egg failures (18 eggs) were more evident than chick mortality (four chicks), and an average (± SD) of 2.59 (± 0.62) eggs hatched and 1.48 (± 0.89) of chicks fledged per nest. Of the monitored nests, 37 (80%) hatched at least one chick, and, out of these, 34 (92%) nests contained at least one large chick that could fledge. Breeding success (the number of nests that fledged at least one chick over the total of nests where breeding was recorded) was therefore 0.74 (Table 4).
3.3 Habitat use
Sandy substrate with scarce vegetation was the most selected habitat type by all assessed species. The White-cheeked tern was the most flexible species in terms of habitat use, utilizing all the habitat categories for nesting (Table 5). It also used a variety of materials to build the nest, depending on item availability. In sandy substrates (beaches), it mainly used shells and pebbles and also dry seaweed washed ashore; in sandy substrates with scattered or dense shrubs, leaves and sticks were mainly used, while on sabkha and limestones, the pairs used small pieces of rock but also seaweed in the limestone that was in proximity to the beach. If nests were in mangroves, the materials used were mainly mangrove leaves and sticks. The Lesser crested tern and the Crab plover both used three habitat types, with most of their colonies being found on sandy substrates with scattered shrubs. The former, however, also largely selected sandy substrate with no vegetation, while the latter’s second choice was sandy substrate with dense shrubs. Bridled terns were selecting almost equally dense and scattered shrubs, the same as the two colonies of Great crested terns. The one Brown booby colony was found in an area with scattered shrubs.
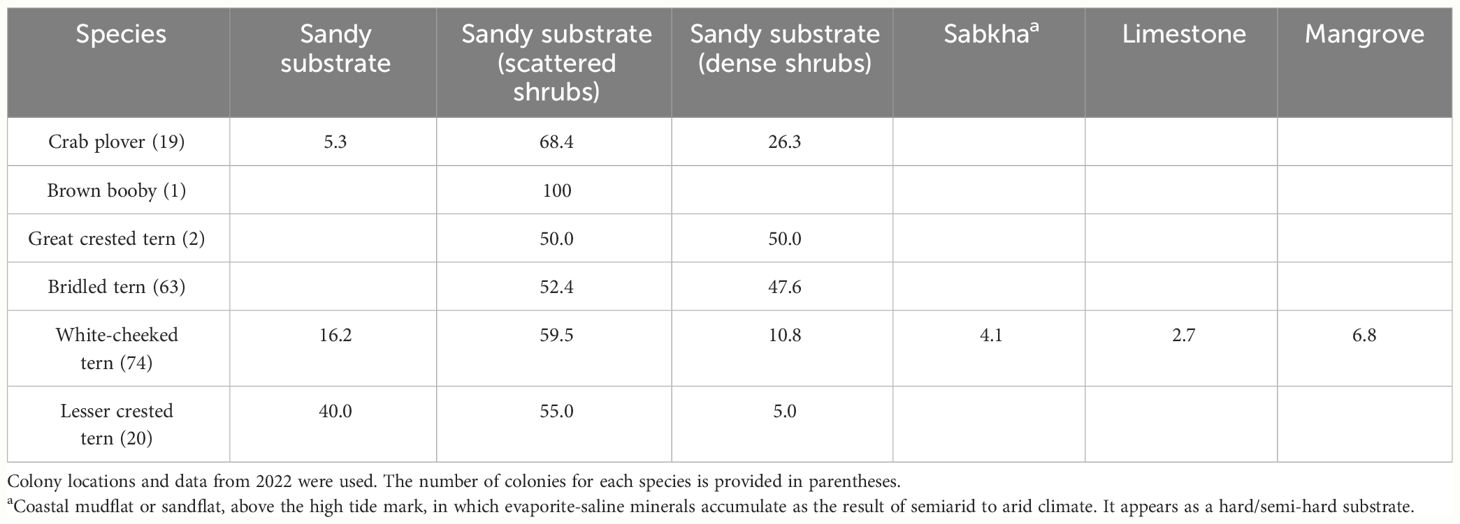
Table 5 Percentage of colonies found in different habitat types for each of the surveyed colonial species.
4 Discussion
Here, for the first time, we describe the phenology, abundance, distribution, breeding success, and habitat selection of seabirds and other insular avifauna breeding at several locations across the Saudi Arabian Red Sea, between 19.75°N and 27.14°N. The phenology observed on the islands in the north (Duba and Al Wajh Bank islands) is slightly delayed compared with the one on the south islands, by at least 1 to 2 weeks. Little is known about the phenology of these species in other areas of the Red Sea; however, earlier studies (Jennings, 2010; Shobrak and Aloufi, 2014) report generally similar breeding times for most species with a few differences. Caspian terns were reported to lay eggs from February to May in the Red Sea and from April to July and September to January in the Persian Gulf (Jennings, 2010) and in spring in the Al Wajh Bank (Shobrak and Aloufi, 2014), while in this study, egg-laying started in December until the end of February (with two nests with eggs also found in May, Jennings pers. comm.). This, however, aligns with the Caspian tern phenology observed on the other side of the Red Sea, in North Egypt (Habib, 2022). Brown booby egg laying started earlier (February) than described by Jennings (2010, May and October, with a likelihood of eggs in February in the south) and Shobrak and Aloufi (2014, March), but at similar times in the North Egyptian islands (Habib, 2022). The Great crested tern in the North Red Sea study areas bred 1 month earlier (May) than described in Arabia (Jennings, 2010), while in the Egyptian North Red Sea islands, the species was observed with eggs 2 months later (Habib, 2022). The Eurasian spoonbill and the Lesser crested tern egg laying happens a month later than described in the northern Red Sea (Jennings, 2010). In addition, the Eurasian spoonbill has a significant resident population along the Arabian Red Sea (Jennings, 2010), but it was never recorded breeding in the Al Wajh Bank; this study is the first published record. To summarize, we observed different breeding times among different populations of the same species in our study areas compared with other sites across the Arabian Peninsula. Further work may clarify these differences. This parameter is important as accurate phenology allows for surveying the islands at appropriate times and collecting meaningful information to inform construction planning in relevant areas. Having a baseline phenology survey will also allow us to detect future changes.
In total, between 25,000 and 30,000 breeding pairs were estimated each year across the island groups, mainly in the summer, when most of the eggs or small chicks were present. In the Al Wajh Bank, the most abundant and widespread species was the White-cheeked tern, and the second most widely widespread species, although in much smaller numbers, was the Osprey. In the Duba and Al Lith islands, the most abundant species was the Lesser crested tern, which also had the highest density of breeding pairs across the three breeding sites.
Global population estimates provided by the International Union for Conservation of Nature and Natural Resources Red List (IUCN, 2024) are not fully reliable and probably incomplete for most of these species, but, based on these figures, we estimated that the Al Wajh Bank alone hosts the following percentages of global populations: Crab plover: 5%, Sooty gull: 17%–35%, White-eyed gull: 15%, Bridled tern: 1%, White-cheeked tern: 4%, and Lesser crested tern: 2%, making the area a regional and global hotspot for these species. The populations of the Duba and Al Lith islands are less numerous as they have fewer islands than the Al Wajh Bank; however, the first was found to be an important breeding ground for the Lesser crested tern and the latter for the Brown noddy. The coastal area in front of the Al Wajh Bank and of Al Lith was also identified as having high species richness (Almalki et al., 2015), probably due to their vicinity to seabird colonies.
Our numbers could not be compared with the population assessment made by Shobrak and Aloufi (2014) in Al Wajh Bank because different methods were used and only a subset of the islands were surveyed in the earlier study. The choice of using the walk-over survey with the direct count of all the nests was preferred to methods previously used as it gives better estimates of the number of breeding pairs. Replicating the methods used by Shobrak and Aloufi in addition to our methods was not feasible, as it would have greatly increased the time spent and consequent disturbance to the colonies. Regardless of the methods used, colonies can move between islands from 1 year to the next; thus, meaningful comparisons with previous estimates would only be possible if surveys were carried out on all or nearly all the islands. The data we present for three consecutive years are not sufficient to meaningfully assess species trends, for which longer-term monitoring is required. However, they can give an indication of population variation during the period we surveyed and will be used to establish trends from ongoing and future monitoring. From our data, all censused species except the Crab plover seem to be stable over the monitored 2 and 3 years in the Duba and Al Wajh Bank islands, respectively. The Crab plover population indicated an aberration in the Al Wajh Bank between the counts of the first and the third years, from 1,832 pairs in 2021 to 1,073 pairs in 2023. This species is important as it is one of the species that triggered the identification of the IBA by BirdLife International in 1994 (BirdLife International, 2023), it is classified as vulnerable in the Regional IUCN Red List (Symes et al., 2015), and it is ranked 13 among the bird priority species for conservation in Saudi Arabia (Boland and Burwell, 2020). Therefore, this apparent reduction needs to be further investigated at this site and elsewhere using standardized methods and a long-term trend assessed through more years of counts to improve the estimation of the conservation status of the species.
A recent survey on seabirds on the Egyptian side of the Red Sea, at the same latitude as the Duba islands and Al Wajh Bank (Habib, 2022), found generally similar species breeding as on the Saudi Arabian Red Sea side. Ashrafi archipelago, Zabargad island, and Hurghada and Sayal archipelagoes were surveyed for a total of 16 islands. The densities of colonies calculated in our study for most species are comparable with counts reported by Habib (2022) in Egypt and are consistent with the breeding biology of the species in Arabia (Jennings, 2010), and in the rest of their range. Only the Caspian and the Saunders’s tern appeared to be more solitary nesters in the Al Wajh Bank than in Egypt and the rest of Arabia (Jennings, 2010; Habib, 2022).
The breeding success of six species was assessed in the Al Wajh Bank across 2022 and 2023 (four species in 2022 and six species in 2023). Obtaining data on breeding success for tern species is challenging because chicks become very mobile after a few days of hatching and tend to leave the nest (Nisbet and Drury, 1972). Conversely, Osprey nests can be followed quite easily until the chicks are ready to fledge (Forys et al., 2021). Here, we gave an indication of how many chicks reached the age before the first flight took place. This was the first time breeding success was described for the study species in the Al Wajh Bank, and our results can be used as a baseline to compare future surveys.
The breeding success of some species was assessed elsewhere and can be compared with our results. Osprey nesting success assessed by Clancy (2006) in Australia was 0.6, slightly lower than assessed in this study (0.74). Our fledging success (number of fledglings out of hatched eggs: 92%) is also higher than reported in previous studies throughout the Red Sea (82%, Jennings, 2010). Brown boobies nesting in the eastern tropical Pacific had lower breeding success (17.3%, Ospina-Alvarez, 2008) than the average reported in this study (28.5%). Great crested terns monitored in Australia and Iran (Langham and Hulsman, 1986; Ghasemi et al., 2011) showed a breeding success of 63.8% and 66.6%, respectively, very similar to the 64.5% average over 2 years in our study. The average breeding success of Lesser crested tern in this study (72.5%) was very similar to the previously assessed value in Iran of 74.43% (Ghasemi et al., 2011). This parameter is very important to assess the productivity of the bird colonies and to identify threats or stress factors that may lead to lower breeding success. Tracking this parameter will help identify key areas for intervention and provide early indications of future population trends.
Low-density vegetated habitats were found to be very important for breeding birds, probably because low shrubs provide important shelter from the sun and predators for the chicks, as found in other tern species (Arnold et al., 2020). The distribution of the breeding birds across the Al Wajh Bank could be driven by different factors. The presence of suitable breeding habitat could play a major role, especially for the most selective species. For more opportunistic species, like the White-cheeked tern, other factors such as food availability, philopatry, and the absence of specific threats could be more important in determining the colonies’ location. Distribution drivers should be better described and identified for all the priority species nesting in the study areas and elsewhere in Saudi Arabia to better understand and protect the seabird species nesting in the Red Sea.
Other insular avian species were found breeding on some of the investigated islands in the Al Wajh Bank, notably herons and plovers. These species should also be further investigated in terms of population size, trends, and threats identified across the Red Sea.
4.1 Conservation implications
With this study, we found that the Al Wajh Bank hosts important portions of the world population for regionally endemic and regionally threatened species, notably the White-eyed gull (Jennings, 2010) and the Crab plover (Symes et al., 2015). Given that both large-scale development projects, which could have significant impacts on bird populations, and large-scale conservation planning are ongoing in the region, our data are particularly important to provide a baseline and a foundation on which future studies, as well as spatial planning and mitigation measures, can build upon. Developing and focusing on future wildlife surveys is indeed particularly important to have more reliable trends and to fill the knowledge gaps in seabird research. At the same time, well-established conservation techniques can and should be applied to the study sites to support and enhance the existing populations and minimize development impacts. Such techniques are notable: (i) removal of invasive species, which can favor the settlement of new seabird populations and avoid the extirpation of existing ones by egg and chick predation; (ii) access restriction of sensitive areas during critical stages of seabird reproduction to avoid disturbance that can result in a detrimental interruption of the breeding attempts; (iii) hunting and fishing regulations and enforcement to reduce the mortality by direct killing and bycatch but also to restore fish prey stock; (iv) continuous monitoring and assessment of new threats, such as climate change; (v) initiation of habitat protection strategies and enhancement plans to assure the availability of suitable nesting habitat; (vi) implementation of biosecurity plans on development islands; and (vii) spatial conservation planning. Such measures are in line with what is recommended at the regional (PERSGA, 2004) and international levels (Dias et al., 2019; Hays et al., 2020) to protect and facilitate the recovery and enhancement of the seabird population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because the study involved observational measures only.
Author contributions
LC: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. JR: Data curation, Investigation, Methodology, Writing – original draft, Writing – review & editing. IL: Data curation, Investigation, Methodology, Writing – original draft. AF: Investigation, Methodology, Writing – review & editing. TC: Data curation, Investigation, Writing – review & editing. JC: Data curation, Investigation, Writing – review & editing. YA: Data curation, Investigation, Writing – review & editing. EA: Data curation, Investigation, Writing – review & editing. PB: Data curation, Investigation, Writing – review & editing. IW: Conceptualization, Project administration, Supervision, Writing – review & editing. OA: Conceptualization, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Department of Environmental Protection and Regeneration (Red Sea Zone - Red Sea Global). AF is funded by the KAUST PhD program.
Acknowledgments
The authors would like to acknowledge the Department of Environmental Protection and Regeneration operation team that facilitated the numerous boat rides to the islands and the other colleagues of the monitoring team who took part in or contributed to the surveys. We would also like to acknowledge the Saudi Green Initiative for their support with the Protected Species and Biodiversity Assessments and Monitoring Initiative. This bird assessment plays a crucial part in this initiative.
Conflict of interest
Authors JR, IL, TC, JC, and PB were employed by the company Island Avian Surveys Ltd. Authors LC, YA, EA, IW, and OA were employed by the company Red Sea Global (Red Sea Zone).
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1379601/full#supplementary-material
References
Almalki M., Alrashidi M., O’Connell M. J., Shobrak M., Székely T. (2015). Modelling the distribution of wetland birds on the Red Sea coast in the Kingdom of Saudi Arabia. Appl. Ecol. Environ. Res. 13, 67–84. doi: 10.15666/aeer/1301_067084
Arnold J. M., Oswald S. A., Nisbet I. C. T., Pyle P., Patten M. A. (2020). “Common Tern (Sterna hirundo), version 1.0,” in Birds of the world. Ed. Billerman S. M. (Cornell Lab of Ornithology, Ithaca, NY, USA). doi: 10.2173/bow.comter.01
Barbraud C., Marteau C., Ridoux V., Delord K., Weimerskirch H. (2008). Demographic response of a population of white-chinned petrels Procellaria aequinoctialis to climate and longline fishery bycatch. J. Appl. Ecol. 45, 1460–1467. doi: 10.1111/j.1365-2664.2008.01537.x
Bibby C. J., Burgess N. D., Hill D. A. (2012). Bird census techniques (Elsevier 84 Theobald's road, London, UK: Academic press).
BirdLife International (2023) Important Bird Area factsheet (Al-Wajh Bank). Available online at: http://datazone.birdlife.org/site/factsheet/al-wajh-bank-iba-saudi-arabia (Accessed August 6, 2023).
Boland C. R. J., Burwell B. O. (2020). Ranking and mapping the high conservation priority bird species of Saudi Arabia. Avian Conserv. Ecol. 15, 1–13. doi: 10.5751/ACE-01705-150218
Bond A. L., Carlson C. J., Burgio K. R. (2019). Local extinctions of insular avifauna on the most remote inhabited island in the world. J. Ornithology 160, 49–60. doi: 10.1007/s10336-018-1590-8
Chalastani V. I., Manetos P., Al-Suwailem A. M., Hale J. A., Vijayan A. P., Pagano J., et al. (2020). Reconciling tourism development and conservation outcomes through marine spatial planning for a Saudi giga-project in the red sea (The red sea project, vision 2030). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00168
Clancy G. P. (2006). The breeding biology of the Osparey Pandion haliaetus on the North coast of New South Wales. J. Aust. Bird Stucty Assoc. 30, 1–8. Available at: https://hdl.handle.net/1959.11/3822.
Dias M. P., Martin R., Pearmain E. J., Burfield I. J., Small C., Phillips R. A., et al. (2019). Threats to seabirds: A global assessment. Biol. Conserv. 237, 525–537. doi: 10.1016/j.biocon.2019.06.033
Einoder L. D. (2009). A review of the use of seabirds as indicators in fisheries and ecosystem management. Fish Res. 95, 6–13. doi: 10.1016/j.fishres.2008.09.024
Eriksson M. O. G., Wallin K. (1994). Survival and breeding success of the Osprey Pandion haliaetus in Sweden. Bird Conserv. Int. 4, 263–277. doi: 10.1017/S0959270900002835
Forys E. A., Hindsley P. R., Bryan S. (2021). Predictors of osprey nest success in a highly urbanized environment. J. Raptor Res. 55, 485–495. doi: 10.3356/JRR-20-97
Frederiksen M., Edwards M., Richardson A. J., Halliday N. C., Wanless S. (2006). From plankton to top predators: Bottom-up control of a marine food web across four trophic levels. J. Anim. Ecol. 75, 1259–1268. doi: 10.1111/j.1365-2656.2006.01148.x
Ghasemi S., Tayefeh F. H., Hoveizeh N. M. (2011). Breeding success of lesser Crested Tern and swift tern at Shidvar Island, Iran. J. Am. Sci. 7, 633–638. doi: 10.7537/marsjas070111.69
Habib M. (2022). Status of breeding birds on the Red Sea Island of Egypt 2021 to 2021. Ornithologischer Beobachter 119, 308–317.
Hays G. C., Koldewey H. J., Andrzejaczek S., Attrill M. J., Barley S., Bayley D. T. I., et al. (2020). A review of a decade of lessons from one of the world’s largest MPAs: conservation gains and key challenges. Mar. Biol. 167, 1–22. doi: 10.1007/s00227-020-03776-w
IUCN (2024) The IUCN red list of threatened species. Version 2023–1. Available online at: https://www.iucnredlist.org.
Kamp J., Frank C., Trautmann S., Busch M., Dröschmeister R., Flade M., et al. (2021). Population trends of common breeding birds in Germany 1990–2018. J. Ornithol 162, 1–15. doi: 10.1007/s10336-020-01830-4
Koleček J., Adamík P., Reif J. (2020). Shifts in migration phenology under climate change: temperature vs. abundance effects in birds. Clim Change 159, 177–194. doi: 10.1007/s10584-020-02668-8
Langham N. P., Hulsman K. (1986). The breeding biology of the crested tern sterna bergii. Emu 86, 23–32. doi: 10.1071/MU9860023
Le Corre M., Jaeger A., Pinet P., Kappes M., Weimerskirch H., Catry T., et al. (2012). Tracking seabirds to identify potential Marine Protected Areas in the tropical western Indian Ocean. Biol. Conserv. 156, 83–93. doi: 10.1016/j.biocon.2011.11.015
Lees A. C., Haskell L., Allinson T., Bezeng S. B., Burfield I. J., Renjifo L. M., et al. (2022). Annual review of environment and resources state of the world’s birds. Annual review of Environment and Resources 47, 231–260. doi: 10.1146/annurev-environ-112420
Lyday S. E., Ballance L. T., Field D. B., Hyrenbach K. D. (2015). Shearwaters as ecosystem indicators: Towards fishery-independent metrics of fish abundance in the California Current. J. Mar. Syst. 146, 109–120. doi: 10.1016/j.jmarsys.2014.08.010
Newton S. F., Al Suhaibany A. H. (1996). Distribution and abundance of summer breeding seabirds in the Saudi Arabian Red Sea (Riyadh: NCWCD).
Nisbet I. C. T., Drury W. H. (1972). Measuring breeding success in common and roseate terns. Bird-banding 43, 97–106. doi: 10.2307/4511853
Nisbet I. C. T., Iles D., Kaneb A., Mostello C. S., Jenouvrier S. (2020). Breeding performance of Common Terns (Sterna hirundo) does not decline among older age classes. Auk 137, 1–17. doi: 10.1093/auk/ukaa022
Ospina-Alvarez A. (2008). Coloniality of Brown Booby (Sula leucogaster) in Gorgona national natural park, eastern tropical pacific. Ornitol Neotrop 19, 517–529.
PERSGA (2004) The Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden (PERSGA) Regional Action Plan for the Conservation of Breeding Seabirds and their Habitats in the Red Sea and Gulf of Aden. Available online at: http://www.persga.org.
Piatt J. F., Sydeman W. J., Wiese F. (2007). Introduction: A modern role for seabirds as indicators. Mar. Ecol. Prog. Ser. 352, 199–204. doi: 10.3354/meps07070
Schreiber E. A., Burger J. (2001). Biology of marine birds (Boca Raton: CRC Press). doi: 10.1201/9781420036305
Shobrak M. Y., Aloufi A. A. (2014). Status of breeding seabirds on the Northern Islands of the Red Sea, Saudi Arabia. Saudi J. Biol. Sci. 21, 238–249. doi: 10.1016/j.sjbs.2013.11.002
Sutherland W. J., Pullin A. S., Dolman P. M., Knight T. M. (2004). The need for evidence-based conservation. Trends Ecol. Evol. 19, 305–308. doi: 10.1016/j.tree.2004.03.018
Symes A., Taylor J., Mallon D., Porter R., Simms C., Budd K. (2015). The conservation status and distribution of the breeding birds of the Arabian Peninsula (UAE: The IUCN Red List of Threatened Species - Regional Assessment). Available at: www.iucn.org.
Tayefeh F. H., Zakaria M., Amini H., Ghasemi M., Amini A., Jafari H., et al. (2013) Monitoring of populations of breeding terns and crab plovers on the Iranian islands of the Persian Gulf. Available online at: www.wesca.net.
Vitasse Y., Ursenbacher S., Klein G., Chittaro Y., Delestrade A., Monnerat C., et al. (2021). Phenological and elevational shifts of plants, animals and fungi under climate change in the European Alps. Biol. Rev. 96, 1816–1835. doi: 10.1111/brv.12727
World Imagery (2022). Sources: Esri, DigitalGlobe, GeoEye, i-cubed, USDA FSA, USGS, AEX, Getmapping, Aerogrid, IGN, IGP, swisstopo, and the GIS User Community.
Keywords: phenology, population estimate, breeding success, habitat selection, tern, gull, crab plover, conservation
Citation: Calabrese L, Riordan JA, Lloyd IA, Foster AD, Collier TE, Chambon JA, Aljohani YW, Alhamdi EA, Beaumont PR, Williams ID and Al-Attas O (2024) A sea of birds: first bird population assessments in the Saudi Arabian Red Sea. Front. Mar. Sci. 11:1379601. doi: 10.3389/fmars.2024.1379601
Received: 31 January 2024; Accepted: 06 May 2024;
Published: 28 May 2024.
Edited by:
Nicolas James Pilcher, Marine Research Foundation, MalaysiaReviewed by:
Abdullah Alsuhaibany, Bird Protection Society, Saudi ArabiaGeorgios Karris, Ionian University, Greece
Copyright © 2024 Calabrese, Riordan, Lloyd, Foster, Collier, Chambon, Aljohani, Alhamdi, Beaumont, Williams and Al-Attas. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Licia Calabrese, Y2FsYWJyZXNlLmxpY2lhQGdtYWlsLmNvbQ==
 Licia Calabrese
Licia Calabrese Julie Ann Riordan3
Julie Ann Riordan3 Imogen Anne Lloyd
Imogen Anne Lloyd Alexa Darby Foster
Alexa Darby Foster Ivor Douglas Williams
Ivor Douglas Williams