
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 20 March 2024
Sec. Marine Pollution
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1379150
This article is part of the Research Topic EVOLMAR 2023: r-Evolutionary routes in the Sea View all articles
 Davide Asnicar1,2*
Davide Asnicar1,2* Lisa Locatello1,3
Lisa Locatello1,3 Laura Zanovello1
Laura Zanovello1 Riccardo Minichino4,5
Riccardo Minichino4,5 Luciano Masiero1
Luciano Masiero1 Marco Munari6,7
Marco Munari6,7 Maria Gabriella Marin1
Maria Gabriella Marin1Introduction: Due to their relevant ecological position and well-studied biology, sea urchins are reference organisms for ocean acidification studies, at both within- and trans-generational levels. In this study, we examined gamete quality in specimens exposed to future predicted (-0.4 units) pH conditions during gametogenesis.
Methods: Egg physical characteristics, biochemical composition, and fatty acid profiles were assessed after two and six months of exposure, while sperm viability and velocity were analyzed after six months of exposure. Considering the documented intraspecific variability in response to ocean acidification, this study involved two populations of Paracentrotus lividus. One population was sampled from the highly variable lagoon of Venice (Site 1), while the other was obtained from a coastal area (Site 2) characterized by more stable environmental conditions and facing minimal anthropogenic stress.
Results: A different response was highlighted in the two sites. Noteworthy trends emerged, especially in the fatty acid profile and sperm traits. Although adults were fed the same diet, Site 1 eggs contained more high-energetic fatty acids than Site 2, potentially boosting the survival odds for the next generation. Moreover, Site 1 sperms displayed higher viability but slower motility compared to those from Site 2. Within sites, a significant difference between time points and a change in the fitness strategy of sea urchin females emerged when comparing eggs spawned after two and six months of exposure to reduced pH. The effects of time and exposure pH are more pronounced in animals from Site 1, suggesting a higher adaptability of this population rather than negative effects of ocean acidification.
Discussion: Overall, our findings suggest that sea urchins have the potential to acclimate to reduced pH and to produce gametes of the same quality as controls held at the currently natural pH. Our findings emphasize the relevance of combining investigations of gamete quality characteristics, particularly egg biochemistry and fatty acid composition, and considering site variability to fully understand the transgenerational response potential of sea urchins to ocean acidification.
External fertilization is a common mode of reproduction in marine organisms. However, spawning in the water column poses a risk to eggs and sperm, which are directly exposed to environmental variability and anthropogenic stressors. The increase of atmospheric carbon dioxide (CO2) and other greenhouse gases is influencing the climate of the planet (IPCC, 2019), as well as the marine environment (Sabine et al., 2004; Doney et al., 2009). Ocean acidification (OA) linked to a climate change scenario is changing the seawater chemistry (Caldeira and Wickett, 2003), increasing the concentration of hydrogen ions in the seawater, disrupting the metabolism and intra-/extra-cellular pH compensation of organisms, in particular at early life stages, which are the most vulnerable (Foo and Byrne, 2017).
Among marine organisms, calcifying invertebrates, such as sea urchins, are the most sensitive to OA scenario and a shift in their presence can alter profoundly the community (Hall-Spencer et al., 2008; Fabricius et al., 2011; Kroeker et al., 2011, 2013).
Gametes have limited ability to regulate intracellular pH when facing changes in pH of seawater (Ciapa and Philippe, 2013; Bögner et al., 2014; Nishigaki et al., 2014). Indeed, after spawning, eggs of external fertilizers absorb the water they are in contact with to increase jelly coat size (Podolsky, 2001), thus being directly influenced by changes in seawater physico-chemical features. The egg size and energy content (lipids and fatty acids in particular) are two important factors related to fitness, offspring development and survival (Tocher, 2003; Byrne et al., 2008; Foo et al., 2018), with the quality of eggs probably being a better predictor of offspring quality rather than their size (Byrne et al., 2008). Egg size and composition are the most studied parameters when assessing the effects of environmental changes (Moran and McAlister, 2009; Foo and Byrne, 2017). Parental environmental history and diet are also fundamental factors to determine survival chances for the following generation as they influence egg nutrient content, in particular energetic lipids (López et al., 1998; Lamare and Barker, 1999; Liu et al., 2007; Clark et al., 2009; Braun et al., 2013; Feiner et al., 2016; Gago et al., 2016; Gosselin et al., 2019; Hoshijima and Hofmann, 2019). OA effects on egg quality are still unclear, with neutral (Uthicke et al., 2013; Parker et al., 2017; Karelitz et al., 2020) or negative (Bögner et al., 2014; Suckling et al., 2015) responses. It seems, however, that with prolonged exposure, animals have the potential to acclimate to the experimental condition (Suckling et al., 2015; Asnicar and Marin, 2022). As egg quality defines the success of the following generation, it is a crucial endpoint deserving investigation, especially in studies on the impact of OA where inter- and intra-specific variations in physiological parameters have been previously documented.
Sperm quality (e.g. morphology, velocity and viability) is a crucial determinant of fertilization success (Benzie and Dixon, 1994; Levitan, 2000; Au et al., 2002), and many studies underline the usefulness of these endpoints to assess effects of potential stressors on external fertilizers (Moschino and Marin, 2002; Fitzpatrick et al., 2008; Fabbrocini et al., 2010; Fitzpatrick et al., 2012; Suquet et al., 2012). A large majority of studies reports detrimental effects of OA on sperm quality, including impairment of the polyspermy block (Reuter et al., 2011; Bögner et al., 2014), as well as a reduction of sperm velocity, motility and viability (Havenhand et al., 2008; Schlegel et al., 2012; Campbell et al., 2016). Contrasting species-specific differences in OA impact have also shown both positive and absence of effect on sperm quality (Havenhand and Schlegel, 2009; Caldwell et al., 2011; Marčeta et al., 2022). Under stressful conditions sperm quality can be also highly variable among individuals, with both improving and impairing intra-specific effects of OA (Gallo et al., 2020b).
Most of the studies aimed at predicting the impact of OA on gametes did not consider the exposure of parental generations to OA. However, when adults were exposed to OA during gametogenesis, a generally positive effect on gamete quality and on offspring emerged (Thor and Dupont, 2015; Uthicke et al., 2020). Considering these experimental limitations and mixed results due to intra-specific variability, more in-depth investigation is certainly needed (Gallo et al., 2020a; Parker et al., 2017).
This study aims to experimentally investigate the impact of OA (pH reduction of 0.4 units) on gamete quality in sea urchins, Paracentrotus lividus. To test for intra-specific variability in relation to different ecological histories, adult specimens from two populations (lagoon vs. coastal environment) experienced both mid-term (two months) and a long-term (six months) exposure. Specifically, the effect of reduced pH on the size and biochemical composition of the eggs (carbohydrates, proteins, lipids, and fatty acids), and on the viability and velocity of sperm are evaluated.
Detailed information on sampling sites’ characteristics and animal collection are given in Asnicar et al. (2021). Briefly, approximately 200 sea urchins (4.5 cm average test diameter) were collected by scuba divers (two-meter depth) in two sites in the Northern Adriatic Sea selected on the base of their different environmental features:
“Site 1”, located in the southern basin of the Lagoon of Venice (45°13’41” N 12°16’13” E), is characterized by high levels of anthropogenic pressure and environmental variability. “Site 2”, located in a more stable environment along the coast of the Gulf of Trieste (45°43’30” N 13°41’31” E), is far from remarkable freshwater inputs and shows little direct human impact.
Using cooling boxes, sea urchins were transported as quickly as possible to the Hydrobiological Station “U. D’Ancona” in Chioggia for experimental exposure.
Two experiments were carried out, one for each of the two collection sites, using the same experimental setup.
A 5000-litre reservoir, two 120-litre mixing tanks, and a custom-designed mesocosm consisting of six independent flow-through 60-litre aquaria were established at the Hydrobiological Station facility. Seawater from the lagoon was pumped into the reservoir to facilitate sediment deposition. Subsequently, the water was filtered (5 μm polypropylene filters) and transferred to the two mixing tanks for temperature acclimation, using two aquarium chiller/heaters (TK 500, TECO, Italy). The exposure tanks were then gravity fed from the mixing tanks at a flux of ~12 L/h.
In each experiment three replicate tanks were set up for the control (natural condition, no pH manipulation) and three for the reduced pH treatment (achieved through CO2 insufflation). The pH was adjusted using a pH-controlling setup (ACQ110 Aquarium Controller Evolution, Aquatronica, Italy). Each tank was provided with an aerator to keep proper water oxygenation and mixing. Temperature, salinity, dissolved oxygen and oxygen saturation were checked daily with a multiparametric probe (Hi 9829, Hanna, Italy). pH was double-checked daily using a Basic 20 benchtop pH meter (Crison, Spain) calibrated daily with Crison buffer solutions. The pH values throughout the experiments are reported on the total scale (pHT).
For each experiment (Site 1 and Site 2, respectively), upon arrival at the Hydrobiological Station, sea urchins were distributed randomly and equally into the six experimental tanks and were allowed to acclimate to the laboratory conditions for seven days (Site 1: temperature 26.10 ± 0.27°C, salinity 34.07 ± 0.52; Site 2: temperature 26.41 ± 0.90, salinity 34.20 ± 0.32) fed ad libitum with Ulva sp. Subsequently, sea urchins were fasted to stimulate energy consumption and, thus, gonads’ reabsorption (Grosjean et al., 1998; Shpigel et al., 2004). This allowed to level the gametogenesis stage for all specimens before treatment. Four animals per tank were sacrificed weekly to check the gonadal status. Starvation lasted four weeks for the Site 1 specimens and three weeks for the Site 2 specimens.
A week prior to the beginning of the exposure, temperature and pH were gradually regulated. To promote gonadal growth and gametogenesis, photoperiod and temperature were set at 10:14 L:D and 18-19°C (Spirlet et al., 2000; Shpigel et al., 2004). To simulate the RCP 8.5 scenario (IPCC, 2019), pH was lowered of 0.4 units compared to the control condition (Supplementary Table 1).
During exposure sea urchins were fed ad libitum with defrost spinach and canned maize, which enhance gonadal maturation (Sartori et al., 2016). Food was renewed and tanks were cleaned thoroughly by syphoning every two days. Mortality was checked daily.
After two and six months of exposure, gametes were collected for the assessment of endpoints of interest. Before spawning induction for gamete collection, animals were gently rinsed under tap water and dried with paper to prevent sperm activation. Twenty individuals per experimental condition were randomly selected and spawning was induced by injecting 1.5 mL of KCl 0.5 M solution (in distilled water) with a sterile syringe through the peristomal membrane. After injection, animals were gently shaken by hand until spawning started.
For egg collection sea urchin females were placed mouth-up on a beaker filled with 0.45 µm filtered artificial seawater (ASW, salinity 34; Ocean Fish, Prodac International S.r.l., Padova, Italy) to collect eggs. ASW was aerated and brought to temperature overnight, and pH was adjusted according to the female exposure condition. Physico-chemical characteristics of ASW are reported in Supplementary Table 2.
Each female was let spawn for 30 minutes to estimate fertility. Eggs were subsequently filtered on a 200 µm nylon mesh to remove impurities (spines and pedicles) and resuspended in a known volume of ASW. The total number of eggs spawned by each female was estimated by counting subsamples of eggs in triplicate under a stereo-microscope (Leica DM 750). Subsamples of eggs were collected in 2 mL microcentrifuge tubes and centrifuged (1 min at 17000 g at 4°C) to concentrate them and remove supernatant ASW. Samples were stored at -80°C until biochemical analyses.
Eggs were collected after 2 months of exposure (Site 1: 5 control females; 6 -0.4 pH females; Site 2: 6 control females; 5 -0.4 pH females) and after 6 months of exposure (Site 1: 9 control females; 7 -0.4 pH females; Site 2: 9 control females; 9 -0.4 pH females).
Sperm were dry collected after 6 months of exposure from nine males per pH condition in both Sites and preserved in a microcentrifuge tube on ice until viability and velocity measures.
Egg diameter was measured on 150 eggs per female on digital images obtained with a stereomicroscope (Leica DM 750) equipped with a camera (Leica DFC 295). Images were analyzed with the software ImageJ 1.51k (Wayne Rasband. National Institutes of Health, USA).
Total content of egg proteins, carbohydrates and lipids was evaluated spectrophotometrically in each female. Samples preserved at -80°C were freeze-dried, grounded with mortar and pestle, and stored again at -80°C until use. The day of use, eggs were dried in an oven at 40-50°C for 60 minutes to remove potential moisture and kept in a desiccator. Five mg of sample were weighed (Mettler Toledo, XS105 Dual Range analytical balance, 0.01 mg readability) and used for each analysis in duplicate. Total proteins were quantified using the Lowry assay (Lowry et al., 1951). Total carbohydrates content was measured following the phenol-sulfuric acid method described in DuBois et al. (1956). Total lipid content was determined through a chloroform-methanol lipid extraction (Mann and Gallager, 1985) followed by the colorimetric method of Pande et al. (1963).
Egg energy content (µJ egg−1) was calculated by multiplying each constituent by its oxyenthalpic energy equivalent (39.5 kJ g−1 for lipid, 24 kJ g−1 for protein, and 17.5 kJ g−1 for carbohydrate; energy combustion values from Bayne et al., 1976) and summing the resultant values.
Eggs were analyzed for fatty acid (FA) composition. The lipid component of the eggs was extracted following the methyl-tert-butyl ether (MTBE) method of Matyash et al. (2008) and following Locatello et al. (2018). Fatty acids were identified by comparing the retention time of the sample (Cromatograph Agilent Technologies 7890 A, Agilent Technologies, Santa Clara, CA, USA; First capillary column Supelco SP 2560, Sigma-Aldrich, St. Louis, MO, USA; Secondary capillary column J&W HP 5 ms, Agilent Technologies), with that of a mixture of 52 standard Fatty Acid Methyl Esters [FAMEs; GLC reference standard: 674 (Nu-Chek Prep, Inc., MN, USA), and Menhaden Fish Oil (Supelco, Bellefonte, PA, USA)]. Individual FAME presence was reported as a percentage of the total area of eluted FAMEs.
Sperm viability and velocity were tested right after collection (see Section 2.4) in sperm from males from the two sites (Site 1 or Site 2) reared for six months at the two conditions (natural pH or -0.4 pH) (“pH of adult exposure”). For each male, sperm were analyzed after activation with ASW at both natural and acidified pH (“pH of sperm activation”). Each sample was analyzed at two time-points after sperm activation: immediately after activation (T0) and 30 minutes later (T30) (“time post-activation”). Measures were repeated twice at each time point to estimate repeatability within samples.
Sperm viability was measured as the proportion of alive sperm using a Live/Dead Sperm Viability Kit (Molecular Probes). The kit is based on a membrane-permeable nucleic acid stain (SYBR14) which labels alive sperm in green, and a membrane impermeable stain (propidium iodide, PI) which labels dead sperm in red. In a dark room, 5 μL of ejaculate were activated in 300 μL of 0.45 μm filtered ASW. 10 μL of the activated sperm solution were stained with 3 μL of SYBR Green, incubated for five minutes at 36°C, then stained with 1 μL of PI (Propidium Iodide) followed by other five minutes of incubation. Five mL of stained sample were deposited on a slide, gently covered with a coverslip, and examined under a fluorescence microscope (Leica DMLB) at 630X magnification. The proportion of alive sperm was calculated for at least 100 spermatozoa. Cell counts were always performed by the same operator blind to the identity of the subject.
1 µL of preserved ejaculate was activated by adding 900 µL of filtered ASW at room temperature and gently mixing. 2.5 µL of activated sperm were then placed in separate wells on a 12-well multitest slide (MP Biomedicals, Aurora, OH) previously coated with 1% of polyvinyl alcohol (Sigma-Aldrich) in distilled water, to prevent sperm from sticking to the glass slide. Each slide was gently covered with a coverslip and sperm velocity was measured using a CEROS Sperm Tracker (CASA, Hamilton Thorne Research, Beverly, MA). For each video, mean speed was measured on at least 100 sperm individual tracks. We focused on curvilinear velocity (VCL, μm s−1), as this measure is a reliable predictor of the fertilization success in many external fertilizing organisms, including the sea urchin (Au et al., 2002; Locatello et al., 2013; Gallego et al., 2017).
All data were analyzed using R and RStudio software (version 4.2.1, R Core Team, 2023). The significance threshold was fixed at p < 0.05.
The combined effect of treatment (natural vs. acidified treatment) and time of exposure (2 vs. 6 months) on egg quality variables (number, size, weight), biochemical components (lipids, proteins, and carbohydrates), energetic content, and sum of saturated (SAFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids were analyzed with linear models (LM) (“Anova” function in the “car” package Fox and Weisberg, 2019) followed by Tukey post-hoc tests for pairwise comparisons.
A multivariate analyses of variance through a permutation based strategy PERMANOVA (“adonis” function in the “vegan” package; Oksanen et al., 2019) and a Principal Component Analysis (PCA; “prcomp” function in the “stats” package) was also applied to summarize and visualize the overall information.
The effect of “pH of adult exposure”, “pH of sperm activation” and “time post-activation” on sperm velocity were analyzed with a linear mixed model (LMM) (“glmer” function in the “lmerTest” package) followed by a Tukey post-hoc for pairwise comparisons (“glht” function in the “multcomp” package; Hothorn et al., 2008). Sperm viability was analyzed with a generalized linear mixed effect model (GLMM, “glmer” function of the “lme4” package; Bates et al., 2015) assuming a binomial error distribution and logit link function followed by Tukey post-hoc tests. In both models “male identity” was included as a random factor to account for repeated measures within subject at different times post-activation (T0 and T30).
In LMs and LMMs, assumptions of residuals’ normal distribution and variance homoscedasticity were checked with Shapiro-Wilk’s and Bartlett’s test, respectively, and by inspection of diagnostic plots. If assumptions were not matched, a log-transformation was applied to the raw data. In GLMM an observation level random effect was included to account for overdispersion.
Within sample repeatability of sperm traits was assessed using the function “rpt” of the package “rptR” (Stoffel et al., 2017).
The number of eggs released in 30 minutes differed between two and six months of exposure for both sites of origin (Site 1: F1,23 = 12.656, p = 0.002; Site 2: F1,25 = 30.190, p < 0.001) but there was neither an effect of the pH of exposure (Site 1: F1,23 = 0.170, p = 0.684; Site 2: F1,25 = 0.157, p = 0.696) nor of the interaction between the two factors (Site 1: F1,23 = 0.126, p = 0.726; Site 2: F1,25 = 0.161, p = 0.691). Both sea urchin groups produced more eggs after longer exposure (Table 1). In Site 1, eggs spawned by animals at low pH were more abundant after six months than two months (p < 0.05). In Site 2, eggs spawned after two months from females at both exposure pH were significantly fewer than the eggs released after six months (p < 0.05).
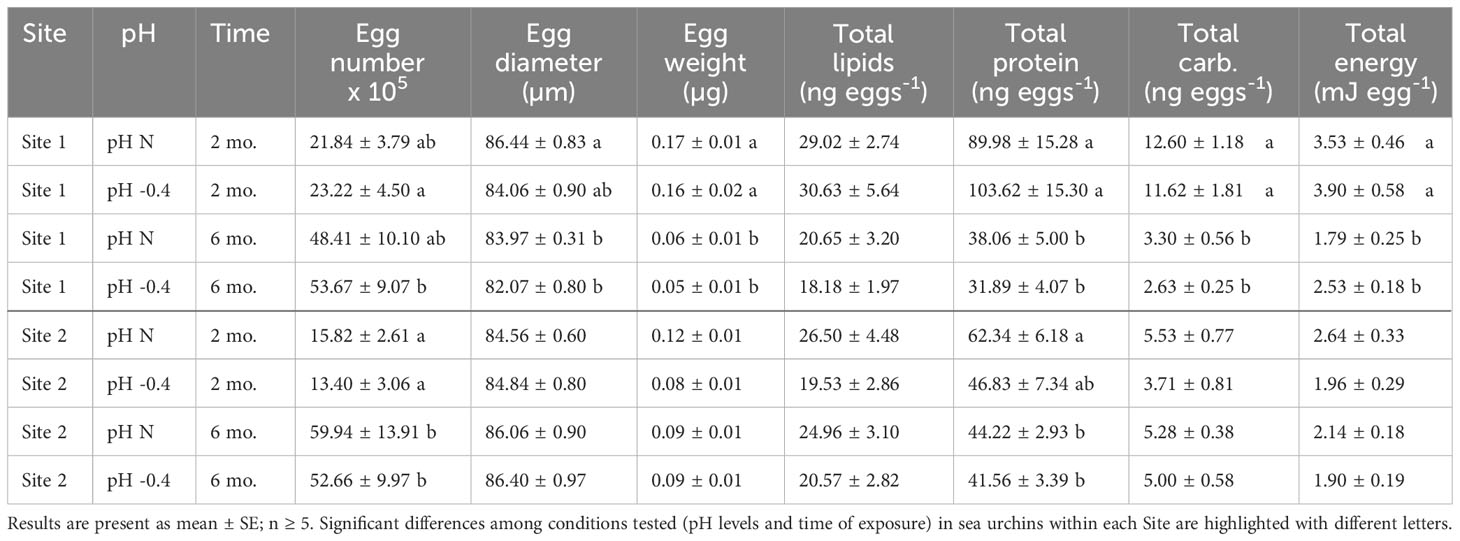
Table 1 Summary of the number of eggs and physical, biochemical and energetical characteristics of eggs released by P. lividus from either lagoon (Site 1) or sea coastal (Site 2) areas after two or six months (mo.) of exposure to either natural or 0.4 units reduced pH.
Both the pH of exposure and the exposure time influenced egg size of Site 1 females (pH: F1,23 = 7.307, p = 0.013; exposure time: F1,23 = 9.008, p = 0.006), but there was no significant interaction between the two factors. The post-hoc analysis highlighted that eggs spawned after two months of exposure by females at natural pH were significantly larger than those spawned after six months by females at both pH (p = 0.019). In females from Site 2, instead, only a marginal effect of the time of maintenance was observed (F1,25 = 4.035, p = 0.056). Following an increase of the exposure time, the diameter of eggs from Site 1 showed an average reduction of 2.23 µm, whereas animals from Site 2 showed an average increase in the egg diameter of about 1.53 µm (Table 1).
The egg weight decreased significantly with the time of exposure for both Sites. The reduction was of approximately 0.11 µg for Site 1 (factor ‘time of exposure’, F1,23 = 62.670, p < 0.001). The post-hoc analysis revealed that eggs spawned after two months by Site 1 females, exposed to both pH conditions, were significantly heavier than the eggs released after six months (p < 0.001). The reduction was less sizable for site 2 (factor ‘time of exposure’, F1,25 = 4.298, p = 0.049), with a reduction of only 0.1 µg. Indeed, no differences were highlighted by the post-hoc tests. The weight of eggs from sea urchins from both sites was not influenced by the pH of exposure nor by the interaction between pH and time.
Lipid content of eggs produced by females from Site 1 was affected by the time of exposure (F1,23 = 8.891, p = 0.007), with eggs produced after two months having more lipids than those produced after six months. No differences between the pH levels nor the interaction between pH and time of exposure were highlighted. Post-hoc analysis did not reveal significant differences between pH or time of exposure. Lipid content of eggs produced by sea urchins from Site 2 was not affected neither by pH nor by the time of exposure or by the interaction between the two factors (Table 1).
Egg protein content differed significantly between the eggs collected after two or six months of exposure for both Site 1 (F1,23 = 46.472, p < 0.001) and Site 2 (F1,25 = 6.754, p = 0.016), but did not differ between pH of exposure nor was it influenced by the interaction between factors. Animals from both sites showed a decrease in egg total protein content with the prolonged exposure (Table 1).
The carbohydrate content of eggs produced by females from Site 1 decreased significantly with the prolongation of the exposure (F1,23 = 94.960, p < 0.001). No differences between pH nor interaction between pH and time of exposure were highlighted. Post-hoc pairwise comparisons showed a significant difference between eggs produced at different pH levels after both two months and six months (p < 0.001). The carbohydrate content of eggs from Site 2 females, instead, was similar between time-points and pH of exposure or rather increased, although not significantly, in the case of females exposed to reduced pH (Table 1).
The total energetic content of the eggs released by Site 1 females was significantly influenced by the time of maintenance (F1,23 = 33.693, p < 0.001), but similar significant effect was not found for Site 2. In both Site 1 and Site 2 females, the energy content per egg decreased with the time of exposure, at both pH tested. However, as highlighted by the post-hoc analysis, only for Site 1 females at both pH levels, the egg energy content was significantly (p < 0.05) higher at two months compared to six months of exposure (Table 1).
For both Site 1 and Site2, the results of the Principal Component analysis performed on the multiple egg quality variables are shown in Figure 1. Loadings values are reported in Supplementary Table 3 in Supplementary Material. The first (PC1) and second (PC2) principal components explained over 85% and 82% of the variance for Site 1 and Site 2, respectively. A clear separation between times of exposure is revealed in both biplots. The first principal component (PC1; 70.69% of the overall variance explained for Site 1 and 65.15% for Site 2) is associated with the egg weight, the biochemical composition and the total energy content, while the second (PC2; 14.72% of the overall variance explained for Site 1 and 17.09% for Site 2) is mainly associated with the number of spawned eggs and their diameter. The time-dependent separation regards PC1 for the Site 1 animals and PC2 for the Site 2 ones. A separation between pH conditions is not evident.
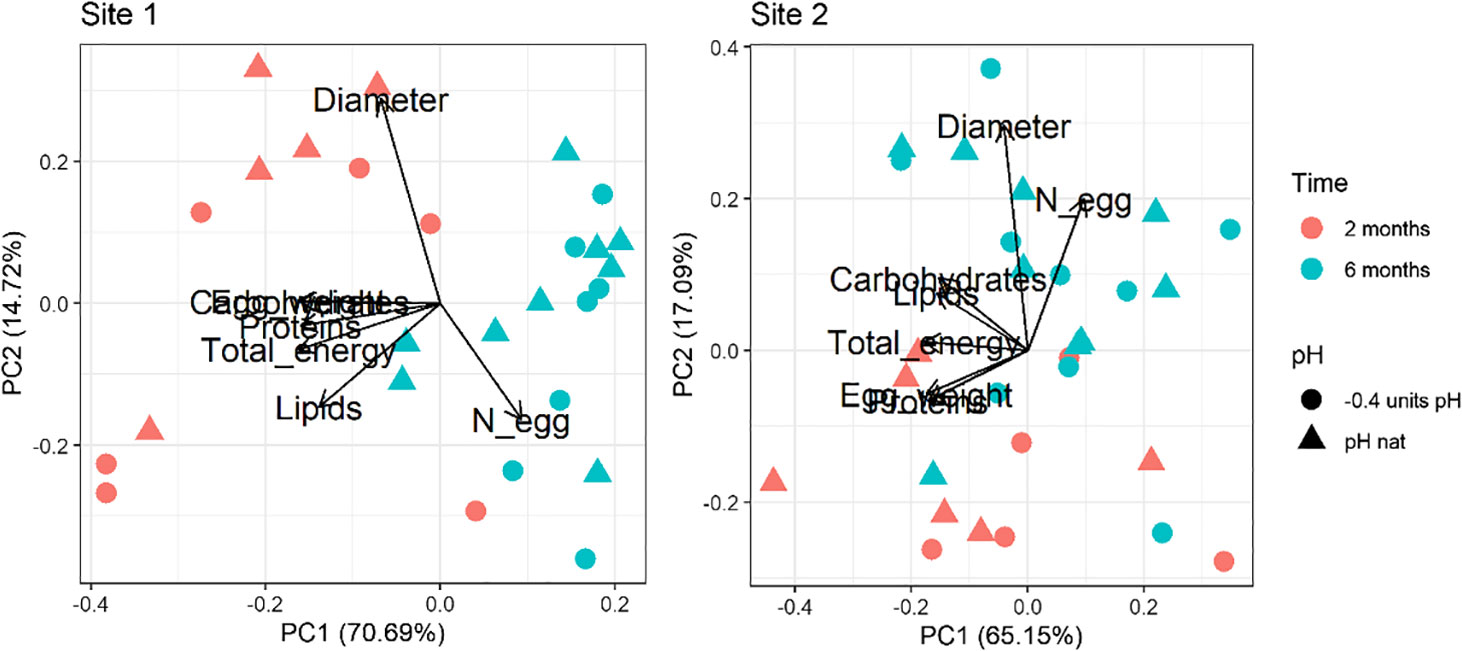
Figure 1 Principal component analysis of the egg dataset (number, diameter, weight, proteins, carbohydrates, lipids and total energy) in sea urchins from lagoon (Site 1) and coastal (Site 2) areas, reared for two and six months at both natural and 0.4 units reduced pH.
The PERMANOVA analysis highlighted the same pattern with significant differences between time points considered (two and six months), for both Site 1 and Site 2 sea urchins (Table 2). Egg quality was not significantly affected by pH of exposure or the interaction between pH and time of exposure.

Table 2 PERMANOVA results on the egg dataset (number, diameter, weight protein, carbohydrates, lipids and total energy) in sea urchins from lagoon (Site 1) and coastal area (Site 2), reared for two and six months at both natural and 0.4 units reduced pH.
Significant changes in total saturated (SAFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acid (FA) profiles due to time, pH, and their interaction were detected in both sites (Table 3). Eggs of females maintained at low pH had different fatty acid composition compared to those of females maintained at natural pH, in particular for animals from Site 1. Site 2 eggs showed significant alteration due to pH only for PUFAs (Table 3).
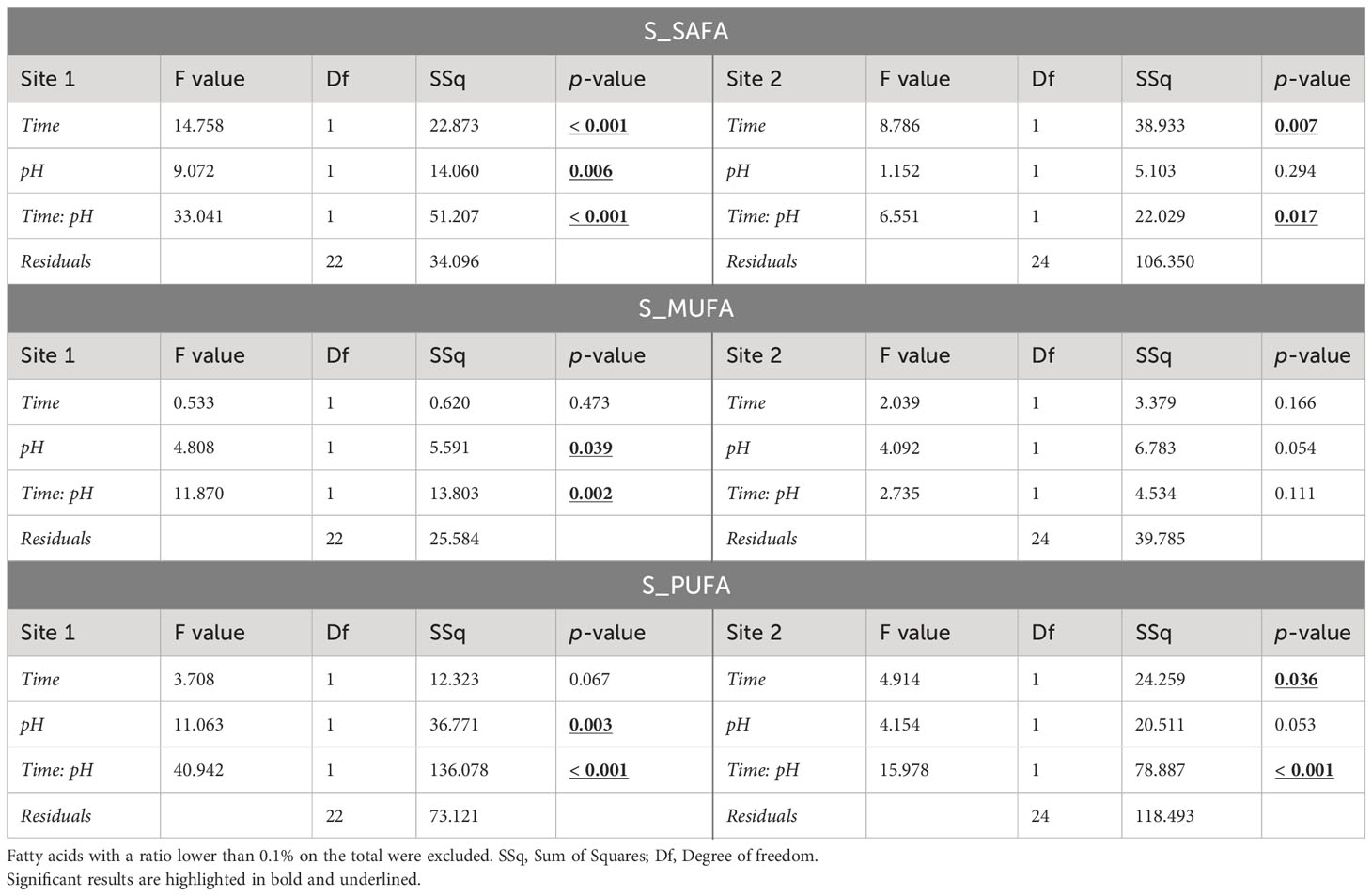
Table 3 ANOVA results on the sum of saturated fatty acids (SAFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs) of eggs in sea urchins from lagoon (Site 1) and coastal area (Site 2), reared for two and six months at both natural and 0.4 units reduced pH.
In Site 1, significant differences were found between the two pH levels after 2 months of exposure, while no differences were detected after 6 months. In particular, SAFA proportion of the total FA profile was ~4% higher (p<0.05) in eggs of sea urchins at low pH after 2 months respect to the other conditions (i.e. 2-months eggs at natural pH and 6-months eggs at both pH values). MUFAs showed the same pattern of variation, with an approximately 2-2.5% higher level in 2-months eggs at reduced pH. PUFAs instead were lower of 5-8% in eggs of sea urchins at low pH after 2 months compared to the other conditions. On the other hand, fatty acid values found after 6 months of exposure at both pH were similar to those of the control after 2 months (Table 3; Supplementary Table 4). However, the control pH at 6 months had a significantly lower level of PUFAs compared to the control at 2 months (p<0.05).
Regarding Site 2, significant differences were observed in SAFAs and PUFAs content. A significant effect of pH was revealed for PUFAs after two months of sea urchin exposure, whereas time of exposure significantly affected both SAFAs and PUFAs at natural pH (Table 3; Supplementary Table 4).
An opposite trend between the two sites was found as FA profiles of eggs of Site 2 sea urchins at 6 months were interestingly more similar to those obtained after 2 months at reduced pH than those at natural pH (Supplementary Table 4).
The first two PCs of the PCA performed on the whole fatty acid dataset explained over 83% and 82% of the variance for Site 1 and Site 2, respectively. In the PCA plot a clear separation can be seen between the two times of exposure (along the PC1; 62.80% and 70.28% of the overall variance explained for Site1 and Site2, respectively). The separation between 2-months and 6-months Site 2 samples was less evident than that observed for Site 1 (Figure 2). A separation is also present between the two pH levels (along the PC2: 21.15% and 12.44% explained variance for Site1 and Site 2, respectively). For both sites, the distinction between pH levels is sensibly clearer in the 2-months compared to the 6-months samples (Figure 2). Multivariate analysis highlighted that the pH caused a significant variation in the fatty acid composition of eggs from Site 1 sea urchins, but not in those from Site 2, as the results of the two pH levels and the two sampling times are more overlapping compared to Site 1. Notably, in Site 1, the distinction between pH levels along the PC2 is opposite if animals were kept for 2 or 6 months. Indeed, the eggs’ fatty acid composition of urchins kept at low pH for 6 months was similar to the control at 2 months. On the other hand, the position of the urchins at low pH for two months is far from the control at 6 months. PCA loadings values are reported in Supplementary Table 5 in Supplementary Material.
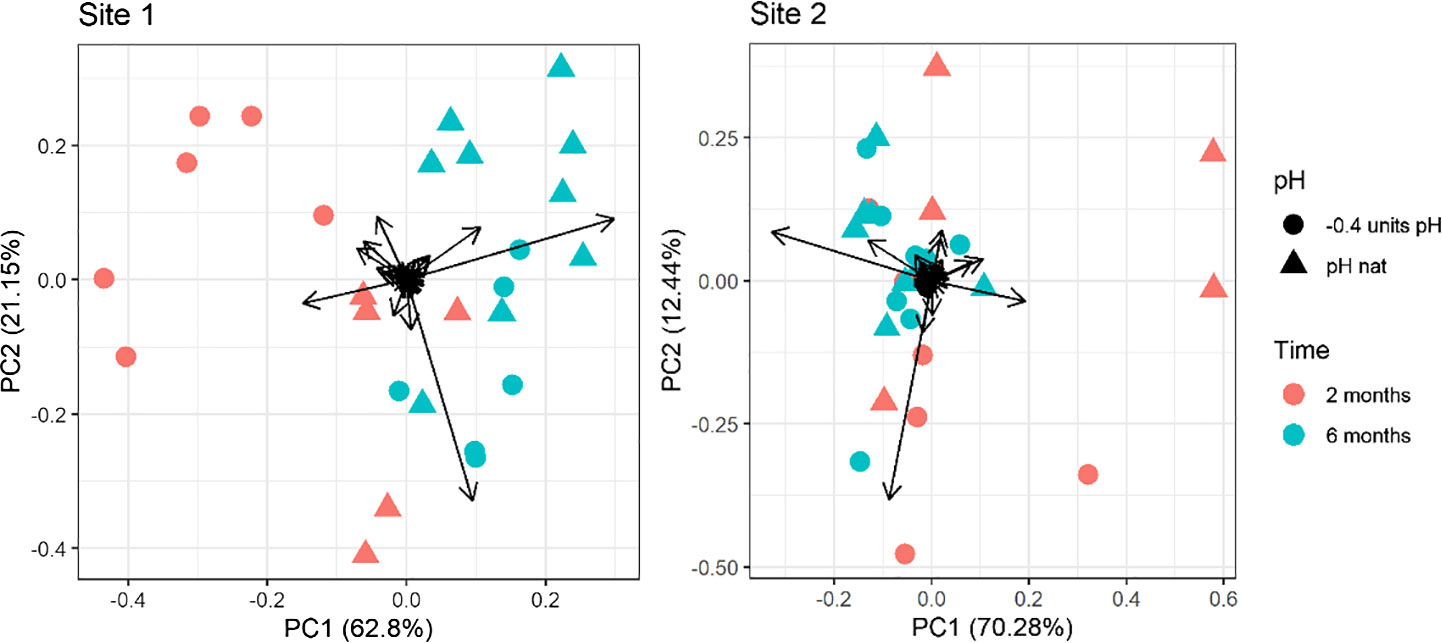
Figure 2 Principal component analysis of the fatty acid profile in eggs of sea urchins from lagoon (Site 1) and coastal area (Site 2) reared for two and six months at both natural and 0.4 units reduced pH. Fatty acids with a ratio lower than 0.1% on the total were excluded.
The PERMANOVA results confirmed a significant effect of exposure time, pH and time/pH interaction (Table 4) for the urchins from Site1. Conversely, in Site 2 sea urchins, a significant difference in egg fatty acid profiles was detected only between the two sampling times (Table 4).
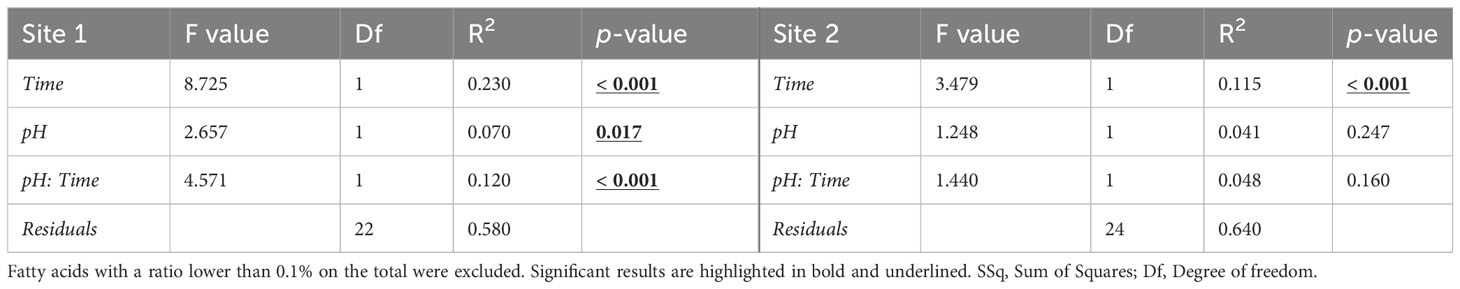
Table 4 PERMANOVA results on the egg fatty acid profile (% of total FAME) in sea urchins from lagoon (Site 1) and coastal area (Site 2), reared for two and six months at both natural and 0.4 units reduced pH.
Repeatability of sperm results are reported in Supplementary Material (Supplementary Table 6). Sperm viability of males from Site 1 significantly differed between T0 and T30 (χ2 = 7.242, p = 0.007) and the significant interaction between activation pH and time of analysis (χ2 = 5.155, p = 0.023) was also found. In Figure 3, the significant pairwise comparisons are highlighted. Interestingly, at T0 the sperm viability of males maintained at natural pH was significantly higher if activation occurred at pH -0.4 compared to pH N (p = 0.035). However, in the latter case, sperm viability remained constant from T0 to T30, whereas in the former a significant reduction from T0 and T30 (p = 0.001) was observed, with values dropping from 87.38% to 77.16% on average (Figure 3). The viability of sperm from adults maintained at pH -0.4 was not influenced by the activation pH at T0, but the viability of sperm activated at pH -0.4 showed a reduction over time, although not significant. No significant effect of the three factors was found for the viability of sperm from Site 2 males.
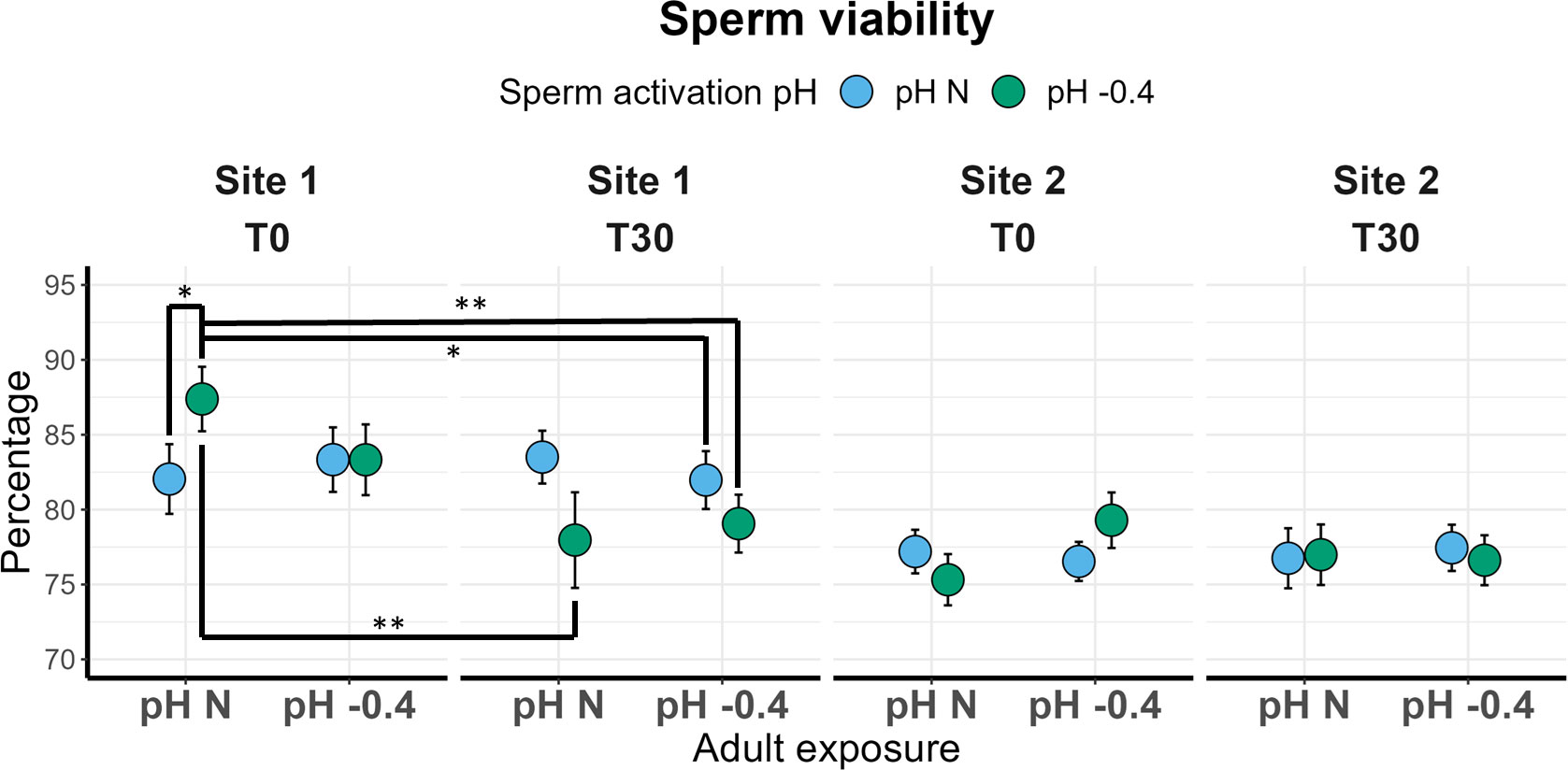
Figure 3 Sperm viability in sea urchins from lagoon (Site 1) and coastal (Site 2) areas, reared for six months at both natural and 0.4 units reduced pH. Sperm was activated at both natural and 0.4 units reduced pH and viability was checked immediately after activation (T0) and after 30 minutes (T30). Data are shown as mean ± SE; n = 9. Significant pair-wise comparisons are represented with asterisks (*: p<0.05; **: p<0.01)
In both sites, the sperm VCL was not significantly influenced by any of the factors investigated. Sperm was slightly faster in the Site 2 sea urchins; however, values were quite similar among all the conditions tested, ranging from 202 to 216 µm s-1 (Figure 4).
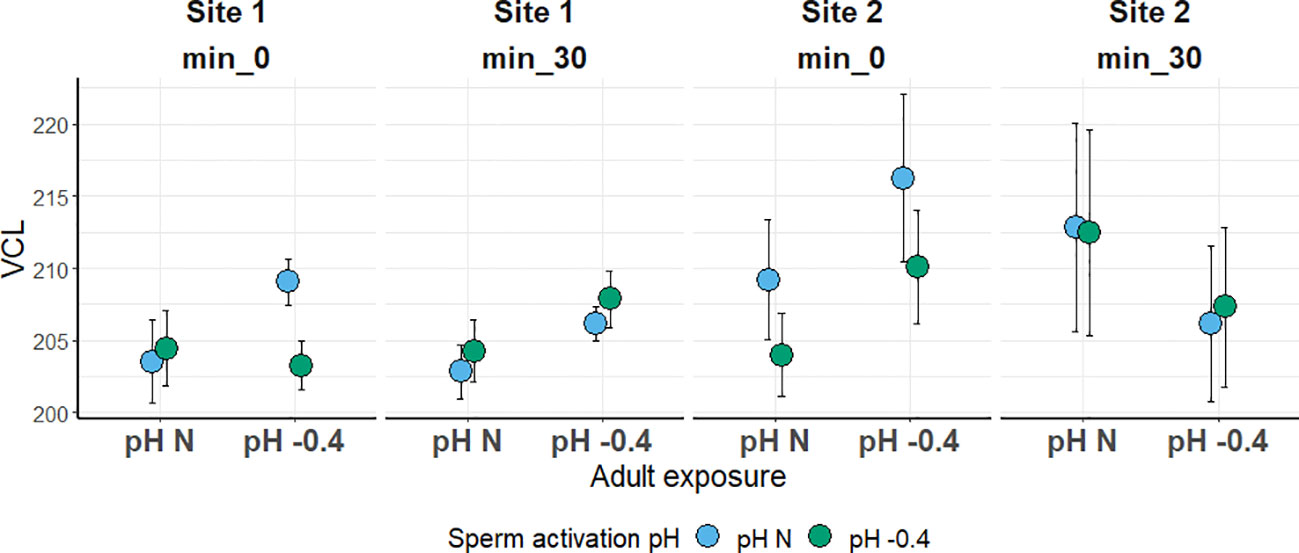
Figure 4 Sperm curvilinear velocity (VCL; in µm s-1) in sea urchins from lagoon (Site 1) and coastal (Site 2) areas, reared for six months at both natural and 0.4 units reduced pH. Sperm was activated at both natural and 0.4 units reduced pH and velocity was checked after 0 and 30 minutes. Data are shown as mean ± SE; n = 9.
In this study, we investigated potential variations in gamete quality of sea urchins P. lividus with different ecological history after long-term exposure to future OA scenario. Parental exposure pH showed little influence on gamete quality, whereas a greater difference emerged in egg quality at different time-points (i.e. eggs spawned after two and six months of parents’ exposure) and in the specific responses of each site of origin (i.e. lagoon and coastal area).
Except for the fatty acid profile (discussed below), the only significant difference due to pH of exposure was found in the diameter of eggs from Site 1 females. Eggs of specimens maintained at lower pH, indeed, were smaller than those from females at natural pH. These findings are different from those obtained in other sea urchin species where egg size was not affected by exposure to lower pH conditions: Echinometra mathaei maintained for seven weeks at pH 7.5 (Uthicke et al., 2013); Strongylocentrotus purpuratus kept at upwelling conditions (-0.3 pH units and -4°C compared to the control) for four months (Hoshijima and Hofmann, 2019); Sterechinus neumayeri specimens, kept for six and seventeen months at pH 7.7 (Suckling et al., 2015). However, in this latter study an effect emerged after seventeen months at pH 7.5, with larger eggs compared to the control (Suckling et al., 2015). In addition to the variability in response to lower pH across sea urchin species, an intraspecific variability in egg size is also observed in natural populations (George et al., 1990; Moran and McAlister, 2009).
In the present study, the number of eggs spawned in 30 minutes was very different between the time-points considered, with the eggs collected after six months being up to four times more abundant than those collected after two months. This is reasonably due to the accumulation of eggs during the 6 months of rearing in captivity during which females continued to produce eggs but did not have the chance to spawn. The pH of exposure, however, did not influence egg production. This is in line with a previous study on Hemicentrotus pulcherrimus exposed for nine months to pH 7.8 (Kurihara et al., 2013).
The eggs from Site 1 females kept at low pH were slightly lighter and with slightly lower carbohydrate content, although this difference was not statistically significant.
The total energetic content did not show a clear influence of the exposure pH, suggesting that embryo development started at the same energetic conditions for all eggs. Also eggs from Site 2 at reduced pH were slightly less abundant, lighter, with lower content of all three biochemical components and with a lower total energy content (although differences between treatments were not statistically significant). However, it is noteworthy that differences in the egg biochemical composition, based on different pH of maternal exposure, decreased with the prolongation of the exposure, suggesting acclimation and better starting condition for the offspring growth.
It has been hypothesized that producing lighter eggs might represent an advantage since lighter eggs can remain longer in the water column, reaching greater distances, and colonizing new areas (Arai et al., 1993; Harii et al., 2007). However, the lower content of energetic components associated to lighter eggs might force the embryo to enter the exotrophic stage faster, possibly resulting in smaller larvae. As a consequence, embryos could be exposed to a higher risk of predation, a reduced possibility to find food and to reach the right substrate for the settlement on time, since all these aspects are related to larval size (Marshall and Keough, 2003; Allen, 2008). Eggs and sperm analyzed in the present study were used for in vitro fertilization trials in a parallel study regarding OA effects on larvae (Asnicar et al., 2022). Larvae obtained from eggs with a lower energetic content were significantly smaller and reached the exotrophic stage (four-armed echinopluteus) faster. Moreover, differences in the larval size also decreased from two to six months of parental exposure (Asnicar et al., 2022). In Heliocidaris erythrogramma, smaller eggs with lower lipid content produce larvae that need more time to develop and metamorphose, have less chance to reach the juvenile stage and result smaller in size (Emlet and Hoegh-Guldberg, 1997). These and our findings emphasize the key role of gamete quality as a good predictor of offspring performance. To the best of our knowledge, the present study investigates for the first time the effect of ocean acidification on Paracentrotus lividus eggs’ biochemical composition and energetic content while standardizing the exposure conditions (seawater parameters, gonadal development).
Although we did not find a significant effect of the pH of exposure on the egg quality, a higher influence of pH in Site 2 specimens than in specimens from Site 1 was highlighted. This can be possibly related to the different ecological features of the two Sites. Site 1, in the lagoon of Venice, shows a highly variable and inhabited environment subject to relevant levels of anthropogenic stress compared to Site 2. Our results suggest that specimens from the lagoon have higher tolerance to OA, possibly reflecting better adaptation to changing environmental conditions.
In both sites, regardless of the pH, females produced fewer but more energy-rich eggs, after two months of exposure and, at the opposite, more eggs but with a lower energy content after six months of exposure. Therefore, maternal plasticity in reproductive strategy can be hypothesized as a tool to optimize fitness gain over time. Indeed, egg energy content is favored over number after two months (when the first eggs are spawned), whereas significantly more eggs are produced after six months, increasing the likelihood that a good number of offspring would survive on the long term. Further investigations are necessary to support this hypothesis.
Our results also confirm that, as suggested by previous studies (Grosjean et al., 1998; Spirlet et al., 2000; Shpigel et al., 2004; Sartori et al., 2016), the maintenance at 18-19°C, 14:10 L:D hours, with a diet based on spinach and corn is optimal to promote sea urchin gametogenesis and produce mature gametes, even after two months of exposure. In fact, in vitro fertilization trials that were carried out with eggs and sperm of the present work (Asnicar et al., 2022) led to a fertilization success always exceeding 90%.
Of course the diet can also influence the lipid metabolism and storage (e.g. Schiopu et al., 2006; Trocino et al., 2012) and in our study the diet was different from a natural one. P. lividus lives in a natural environment, where several food sources, including brown, red, green algae, and seagrass, are available (Boudouresque and Verlaque, 2020). Our unvariegated spinach-corn diet, necessary for the standardization of the procedure, could have influenced the eggs’ lipid profile and could contribute to explain the difference observed in the FA profile between 2 and 6 months of exposure.
However, despite this difference in eggs’ FA composition across the time of exposure, we interestingly observed an effect of the pH of exposure only in animals from Site 1, whereas the effect is very limited in animals from Site 2. The fatty acid profile importance in diet is very well studied, with much evidence that higher MUFA and especially PUFA levels, compared to SAFA, in food intake are beneficial for the health of animals and humans (Dewhurst et al., 2003; Ramsden et al., 2015). It is known that environmental changes and pollution may alter the FA composition of organisms and gametes (Filimonova et al., 2016). Changes in the fatty acid composition of the eggs may be linked to adjustments that the adults make to favor the appropriate growth of embryos or give them extra protection. For example, non-methylene-interrupted (NMI) fatty acids seem to have a protective function from lipid peroxidation processes, although the mechanism of action is not fully understood (Garrido and Medina, 2002). The lack of long chain unsaturated fatty acids, such as linolenic [C18:3(n-3)] and docosahexanoic acid [DHA C22:6(n-3)], may have deleterious effects, as reported for larvae of the sand dollar Dendraster excentricus (Schiopu et al., 2006). Compared to Site 2, in Site 1 eggs we detected a higher content of PUFAs (heneicosapentaenoic acid (HPA), docosahexaenoic acid (DHA), linolenic acid) that play a key role in the health and functioning of animals. After two months of exposure, DHA made up 0.380% of the total FA in the eggs of control animals, while it was three times higher in those from animals kept at reduced pH (1.117%). This difference disappeared after 6 months. Linolein (C18:2) and linolenic (C18:3) acids are precursors of the eicosapentaenoic acid (EPA; C20:5_n3) which also has an important role in animal health (Cohen et al., 1993). In the present study, at two months, linolein acid % was reduced at low pH, while the linolenic acid % was increased. Overall, the EPA % did not change demonstrating compensatory mechanism in the animals.
The larvae from Site 1 urchins, obtained in a further study with these gametes, performed better than those from Site 2 (Asnicar et al., 2022). This suggest that the changes detected at reduced pH may be compensatory changes, with females from Site 1, already accustomed to a more variable environment, providing more nutritious content in the eggs for the future generation. The egg fatty acid profile represents, therefore, a powerful tool in predicting the performance of the following generations in response to environmental stressors.
In the present study, seawater pH affected only slightly the P. lividus sperm endpoints considered. The only significant differences in the sperm viability and velocity were driven by the activation pH, rather than the exposure pH, and they were found in sperm from males maintained at natural pH. For both Site 1 and Site 2, sperm from sea urchins maintained at low pH and activated at the same low pH, showed a similar or even enhanced response if compared to the corresponding one activated at natural pH and also to sperm from adults maintained at natural pH and activated at natural pH. Our results suggest that exposure to reduced pH for six months does not affect the ability of P. lividus to produce gametes of good quality, potentially guaranteeing a successful fertilization irrespective of the pH of parental maintenance.
Previous studies on the effects of OA exposure on sea urchin sperm viability and velocity gave mixed results, possibly due to differences in ecological history of analyzed population or to different methodologies applied. A pH reduction of 0.2-0.3 units enhanced velocity and motility in Psammechinus miliaris sperm (Caldwell et al., 2011); exposure to pH 7.7 significantly reduced velocity and motility in sperm of H. erythrogramma (Havenhand et al., 2008), but in another study on the same species only sperm motility, and not velocity, was affected by reduced seawater pH values (7.8 and 7.6) (Schlegel et al., 2012). Previous results in P. lividus showed that the exposure of sperm to pH reduced of 0.4 units negatively affected the velocity in the first five minutes post-activation (Munari et al., 2022). Although after 15 and 30 minutes from activation treated sperm reached the same performance of controls, the first 5 minutes gap was enough to reduce the fertilization success of treated individuals (Munari et al., 2022). These studies however, did not include exposure of the parents to OA conditions before their gamete analyses. In the only previous study in which P. lividus adults were maintained at pH conditions of 8.0, 7.7, and 7.4 for two months, sperm produced by treated animals did not differ in motility and oxygen consumption from the control (Marčeta et al., 2022). Sperm velocity, instead, was affected by parental pH: sperm from males reared at pH 7.7 show a lower average initial velocity with no changes over time (0-60 minutes). Whereas sperm ATP content was significantly higher in males reared at low pH (both 7.7 and 7.4), ATP consumption was affected by both parental pH and activation pH (Marčeta et al., 2022).
It has been suggested that sperm speed and longevity are negatively correlated in broadcast spawners (Levitan, 2000; Burness et al., 2004) and that sperm longevity, more than velocity, influences the ejaculate fertilization ability (Fitzpatrick et al., 2012). Our data suggests higher sperm viability in Site 1 males, that might be related to a higher reproductive fitness for sea urchins from the site with higher environmental variability and anthropogenic stress, although this deserves further specific analysis to be confirmed.
Overall, our results suggest a negligible effect of seawater acidification on the quality of P. lividus gametes spawned after long-term parental exposure. In particular, OA levels expected for the next century did not cause severe alterations in the quality of eggs and sperm, especially with the prolongation of the adult exposure (six months vs. two months). This limited effect could be related to a general great tolerance to environmental variations in P. lividus sea urchins. In fact, this species has a very wide distribution range, even latitudinally, suggesting a very high adaptation potential. However, our results suggest a different response toward OA in the two sites of origin, in particular in relation to the eggs’ FA composition. An intraspecific variability related to the ecological history of the animals is suggested, with specimens from the coastal area more strongly suffering the effect of OA compared to animals from the lagoon area, and, then, possibly requiring longer time to acclimate to stressful conditions. Our observations concur with recent evidence of robustness of P. lividus to end-of-century acidification conditions. However, here we provide further evidence that local populations subjected to higher levels of environmental stress may play an important role in promoting adaptation and resilience to future reduction in seawater pH. Indeed, considering that the effects of time and exposure pH are more pronounced in animals from Site 1 (lagoon), coupled with the fact that their offspring were of higher quality compared to Site 2 (coastal area), the detected variations might be linked to higher adaptability of the lagoon population rather than to negative effects of ocean acidification. Moreover, our results highlight the importance of integrating the analyses of parameters of gamete quality, in particular eggs’ biochemical and fatty acid composition, to fully understand the transgenerational adaptation ability of sea urchins to ocean acidification.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
DA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. LL: Conceptualization, Formal analysis, Methodology, Supervision, Validation, Writing – review & editing. LZ: Formal analysis, Investigation, Writing – original draft. RM: Formal analysis, Investigation, Writing – original draft. LM: Formal analysis, Investigation, Writing – original draft. MM: Conceptualization, Formal analysis, Investigation, Supervision, Writing – review & editing. MGM: Conceptualization, Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1379150/full#supplementary-material
Allen J. D. (2008). Size-specific predation on marine invertebrate larvae. Biol. Bull. 214, 42–49. doi: 10.2307/25066658
Arai I., Kato M., Heyward A., Ikeda Y., Iizuka T., Maruyama T. (1993). Lipid composition of positively buoyant eggs of reef building corals. Coral Reefs 12, 71–75. doi: 10.1007/BF00302104
Asnicar D., Marin M. G. (2022). Effects of seawater acidification on echinoid adult stage: A review. J. Mar. Sci. Eng. 10, 477. doi: 10.3390/jmse10040477
Asnicar D., Novoa-Abelleira A., Minichino R., Badocco D., Pastore P., Finos L., et al. (2021). When site matters: Metabolic and behavioural responses of adult sea urchins from different environments during long-term exposure to seawater acidification. Mar. Environ. Res. 169, 105372. doi: 10.1016/j.marenvres.2021.105372
Asnicar D., Zanovello L., Badocco D., Munari M., Marin M. G. (2022). Different ecological histories of sea urchins acclimated to reduced pH influence offspring response to multiple stressors. Environ. Res. 212, 113131. doi: 10.1016/j.envres.2022.113131
Au D. W. T., Chiang M. W. L., Tang J. Y. M., Yuen B. B. H., Wang Y. L., Wu R. S. S. (2002). Impairment of sea urchin sperm quality by UV-B radiation: Predicting fertilization success from sperm motility. Mar. Pollut. Bull. 44, 583–589. doi: 10.1016/S0025-326X(01)00288-0
Bates D., Maechler M., Bolker B., Walker S. (2015). Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 67 (1), 1–48. doi: 10.18637/jss.v067.i01
Bayne B. L., Widdows J., Thompson T. J. (1976). “Physiological integrations,” in Marine Mussels: Their Ecology and Physiology (New York, NY, USA: Cambridgre University Press), 121–206).
Benzie J. A. H., Dixon P. (1994). The effects of sperm concentration, sperm:egg ratio, and gamete age on fertilization success in crown-of-thorns starfish (Acanthaster planci) in the laboratory. Biol. Bull. 186, 139–152. doi: 10.2307/1542048
Bögner D., Bickmeyer U., Köhler A. (2014). CO2-induced fertilization impairment in Strongylocentrotus droebachiensis collected in the Arctic. Helgoland Mar. Res. 68, 341–356. doi: 10.1007/s10152-014-0394-3
Boudouresque C. F., Verlaque M. (2020). “Paracentrotus lividus,” in Sea Urchins: Biology and Ecology, 4th ed. Ed. Lawrence J. M. (Elsevier B.V), 447–485). doi: 10.1016/B978-0-12-819570-3.00026-3
Braun D. C., Patterson D. A., Reynolds J. D. (2013). Maternal and environmental influences on egg size and juvenile life-history traits in Pacific salmon. Ecol. Evol. 3, 1727–1740. doi: 10.1002/ece3.555
Burness G., Casselman S. J., Schulte-Hostedde A. I., Moyes C. D., Montgomerie R. (2004). Sperm swimming speed and energetics vary with sperm competition risk in bluegill (Lepomis macrochirus). Behav. Ecol. Sociobiol. 56, 65–70. doi: 10.1007/s00265-003-0752-7
Byrne M., Sewell M. A., Prowse T. A. A. (2008). Nutritional ecology of sea urchin larvae: Influence of endogenous and exogenous nutrition on echinopluteal growth and phenotypic plasticity in Tripneustes gratilla. Funct. Ecol. 22, 643–648. doi: 10.1111/j.1365-2435.2008.01427.x
Caldeira K., Wickett M. E. (2003). Anthropogenic carbon and ocean pH. Nature 425, 365. doi: 10.1038/425365a
Caldwell G. S., Fitzer S., Gillespie C. S., Pickavance G., Turnbull E., Bentley M. G. (2011). Ocean acidification takes sperm back in time. Invertebr. Reprod. Dev. 55, 217–221. doi: 10.1080/07924259.2011.574842
Campbell A. L., Levitan D. R., Hosken D. J., Lewis C. (2016). Ocean acidification changes the male fitness landscape. Sci. Rep. 6, 31250. doi: 10.1038/srep31250
Ciapa B., Philippe L. (2013). Intracellular and extracellular pH and Ca are bound to control mitosis in the early sea urchin embryo via ERK and MPF activities. PloS One 8, e66113. doi: 10.1371/journal.pone.0066113
Clark D., Lamare M., Barker M. (2009). Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: A comparison among a tropical, temperate, and a polar species. Mar. Biol. 156, 1125–1137. doi: 10.1007/s00227-009-1155-8
Cohen Z., Norman H. A., Heimer Y. M. (1993). Potential use of substituted pyridazinones for selecting polyunsaturated fatty acid overproducing cell lines of algae. Phytochemistry 32, 259–264. doi: 10.1016/S0031-9422(00)94978-1
Dewhurst R., Scollan N., Lee M., Ougham H., Humphreys M. (2003). Forage breeding and management to increase the beneficial fatty acid content of ruminant products. Proc. Nutr. Soc. 62, 329–336. doi: 10.1079/PNS2003241
Doney S. C., Fabry V. J., Feely R. A., Kleypas J. A. (2009). Ocean acidification: The other CO2 problem. Annu. Rev. Mar. Sci. 1, 169–192. doi: 10.1146/annurev.marine.010908.163834
DuBois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. (1956). Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356. doi: 10.1021/ac60111a017
Emlet R. B., Hoegh-Guldberg O. (1997). Effects of egg size on postlarval performance: experimental evidence from a sea urchin. Evolution 51, 141–152. doi: 10.1111/j.1558-5646.1997.tb02395.x
Fabbrocini A., Di Stasio M., D’Adamo R. (2010). Computerized sperm motility analysis in toxicity bioassays: A new approach to pore water quality assessment. Ecotoxicol. Environ. Saf. 73, 1588–1595. doi: 10.1016/j.ecoenv.2010.05.003
Fabricius K. E., Langdon C., Uthicke S., Humphrey C., Noonan S., De’ath G., et al. (2011). Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Climate Change 1, 29. doi: 10.1038/NCLIMATE1122
Feiner Z. S., Wang H., Einhouse D. W., Jackson J. R., Rutherford E. S., Schelb C., et al. (2016). Thermal environment and maternal effects shape egg size in a freshwater fish. Ecosphere 7 (5), e01304. doi: 10.1002/ecs2.1304
Filimonova V., Gonçalves F., Marques J. C., De Troch M., Gonçalves A. M. M. (2016). Fatty acid profiling as bioindicator of chemical stress in marine organisms: A review. Ecol. Indic. 67, 657–672. doi: 10.1016/j.ecolind.2016.03.044
Fitzpatrick J. L., Nadella S., Bucking C., Balshine S., Wood C. M. (2008). The relative sensitivity of sperm, eggs and embryos to copper in the blue mussel (Mytilus trossulus). Comp. Biochem. Physiol. - C Toxicol. Pharmacol. 147, 441–449. doi: 10.1016/j.cbpc.2008.01.012
Fitzpatrick J. L., Simmons L. W., Evans J. P. (2012). Complex patterns of multivariate selection on the ejaculate of a broadcast spawning marine invertebrate. Evolution. 66 (8), 2451–2460. doi: 10.1111/j.1558-5646.2012.01627.x
Foo S. A., Byrne M. (2017). Marine gametes in a changing ocean: Impacts of climate change stressors on fecundity and the egg. Mar. Environ. Res. 128, 12–24. doi: 10.1016/j.marenvres.2017.02.004
Foo S. A., Byrne M., Gambi M. C. (2018). Residing at low pH matters, resilience of the egg jelly coat of sea urchins living at a CO2 vent site. Mar. Biol. 165, 97. doi: 10.1007/s00227-018-3359-2
Fox J., Weisberg S. (2019). An R Companion to Applied Regression, Third edition. Sage, Thousand Oaks CA. Available at: https://socialsciences.mcmaster.ca/jfox/Books/Companion/.
Gago J., Martins T., Luís O. J. (2016). Protein content and amino acid composition of eggs and endotrophic larvae from wild and captive fed sea urchin Paracentrotus lividus (Echinodermata: Echinoidea). Aquacult. Res. 47, 114–127. doi: 10.1111/are.12475
Gallego V., Cavalcante S. S., Fujimoto R. Y., Carneiro P. C. F., Azevedo H. C., Maria A. N. (2017). Fish sperm subpopulations: changes after cryopreservation process and relationship with fertilization success in tambaqui (Colossoma macropomum). Theriogenology 87, 16–24. doi: 10.1016/j.theriogenology.2016.08.001
Gallo A., Boni R., Tosti E. (2020a). Gamete quality in a multistressor environment. Environ. Int. 138, 105627. doi: 10.1016/j.envint.2020.105627
Gallo A., Esposito M. C., Cuccaro A., Buia M. C., Tarallo A., Monfrecola V., et al. (2020b). Adult exposure to acidified seawater influences sperm physiology in Mytilus galloprovincialis: Laboratory and in situ transplant experiments. Environ. pollut. 265, 115063. doi: 10.1016/j.envpol.2020.115063
Garrido J. L., Medina I. (2002). Identification of minor fatty acids in mussels (Mytilus galloprovincialis) by GC–MS of their 2-alkenyl-4,4-dimethyloxazoline derivatives. Anal. Chimica Acta 465, 409–416. doi: 10.1016/S0003-2670(02)00207-6
George S. B., Cellario C., Fenaux L. (1990). Population differences in egg quality of Arbacia lixula (Echinodermata: Echinoidea): Proximate composition of eggs and larval development. J. Exp. Mar. Biol. Ecol. 141, 107–118. doi: 10.1016/0022-0981(90)90217-Z
Gosselin L. A., Gallego R., Peters-Didier J., Sewell M. A. (2019). Field evidence of interpopulation variation in oocyte size of a marine invertebrate under contrasting temperature and food availability. Mar. Ecol. Prog. Ser. 619, 69–84. doi: 10.3354/meps12959
Grosjean P., Spirlet C., Gosselin P., Vaïtilingon D., Jangoux M. (1998). Land-based, closed-cycle echiniculture of Paracentrotus lividus (Lamarck) (Echinoidea: Echinodermata): A long-term experiment at a pilot scale. J. Shell. Res. 17, 1523–1531.
Hall-Spencer J. M., Rodolfo-Metalpa R., Martin S., Ransome E., Fine M., Turner S. M., et al. (2008). Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454, 96–99. doi: 10.1038/nature07051
Harii S., Nadaoka K., Yamamoto M., Iwao K. (2007). Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar. Ecol. Prog. Ser. 346, 89–96. doi: 10.3354/meps07114
Havenhand J. N., Buttler F.-R., Thorndyke M. C., Williamson J. E. (2008). Near-future levels of ocean acidification reduce fertilization success in a sea urchin. Curr. Biol. 18, R651–R652. doi: 10.1016/j.cub.2008.06.015
Havenhand J. N., Schlegel P. (2009). Near-future levels of ocean acidification do not affect sperm motility and fertilization kinetics in the oyster Crassostrea gigas. Biogeosciences 6, 3009–3015. doi: 10.5194/bg-6-3009-2009
Hoshijima U., Hofmann G. E. (2019). Variability of seawater chemistry in a kelp forest environment is linked to in situ transgenerational effects in the purple sea urchin, Strongylocentrotus purpuratus. Front. Mar. Sci. 6. doi: 10.3389/fmars.2019.00062
Hothorn T., Bretz F., Westfall P. (2008). Simultaneous inference in general parametric models. Biometric. J. 50, 346–363. doi: 10.1002/bimj.200810425
IPCC (2019). IPCC Special Report on the Ocean and Cryosphere in a Changing Climate. Eds. Pörtner H.-O., Roberts D. C., Masson-Delmotte V., Zhai P., Tignor M., Poloczanska E., et al. (Cambridge, UK and New York, NY, USA: Cambridge University Press), 755 pp. doi: 10.1017/9781009157964
Karelitz S., Lamare M., Patel F., Gemmell N., Uthicke S. (2020). Parental acclimation to future ocean conditions increases development rates but decreases survival in sea urchin larvae. Mar. Biol. 167, 2. doi: 10.1007/s00227-019-3610-5
Kroeker K. J., Micheli F., Gambi M. C. (2013). Ocean acidification causes ecosystem shifts via altered competitive interactions. Nat. Climate Change 3, 156–159. doi: 10.1038/nclimate1680
Kroeker K. J., Micheli F., Gambi M. C., Martz T. R. (2011). Divergent ecosystem responses within a benthic marine community to ocean acidification. Proc. Natl. Acad. Sci. 108, 14515–14520. doi: 10.1073/pnas.1107789108
Kurihara H., Yin R., Nishihara G., Soyano K., Ishimatsu A. (2013). Effect of ocean acidification on growth, gonad development and physiology of the sea urchin Hemicentrotus pulcherrimus. Aquat. Biol. 18, 281–292. doi: 10.3354/ab00510
Lamare M., Barker M. (1999). In situ estimates of larval development and mortality in the New Zealand sea urchin Evechinus chloroticus (Echinodermata:Echinoidea). Mar. Ecol. Prog. Ser. 180, 197–211. doi: 10.3354/meps180197
Levitan D. R. (2000). Sperm velocity and longevity trade off each other and influence fertilization in the sea urchin Lytechinus variegatus. Proceedings of the Royal Society of London. Ser. B: Biol. Sci. 267, 531–534. doi: 10.1098/rspb.2000.1032
Liu H., Kelly M. S., Cook E. J., Black K., Orr H., Zhu J. X., et al. (2007). The effect of diet type on growth and fatty-acid composition of sea urchin larvae, I. Paracentrotus lividus (Lamarck 1816) (Echinodermata). Aquaculture 264, 247–262. doi: 10.1016/j.aquaculture.2006.12.021
Locatello L., Bertotto D., Cerri R., Parmeggiani A., Govoni N., Trocino A., et al. (2018). Sperm quality in wild-caught and farmed males of the European eel (Anguilla Anguilla). Anim. Reprod. Sci. 198, 167–176. doi: 10.1016/j.anireprosci.2018.09.016
Locatello L., Poli F., Rasotto M. B. (2013). Tactic-specific differences in seminal fluid influence sperm performance. Proc. R Soc Lond. B Biol. Sci. 280, 20122891. doi: 10.1098/rspb.2012.2891
López S., Turon X., Montero E., Palacín C., Duarte C., Tarjuelo I. (1998). Larval abundance, recruitment and early mortality in Paracentrotus lividus (Echinoidea). Interannual variability and plankton-benthos coupling. Mar. Ecol. Prog. Ser. 172, 239–251. doi: 10.3354/meps172239
Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275.
Mann R., Gallager S. M. (1985). Physiological and biochemical energetics of larvae of Teredo navalis L. and Bankia gouldi (Bartsch) (Bivalvia: Teredinidae). J. Exp. Mar. Biol. Ecol. 85, 211–228. doi: 10.1016/0022-0981(85)90159-5
Marčeta T., Locatello L., Alban S., Hassan M. S. A., Azmi N.-N. N. M., Finos L., et al. (2022). Transgenerational effects and phenotypic plasticity in sperm and larvae of the sea urchin Paracentrotus lividus under ocean acidification. Aquat. Toxicol. 248, 106208. doi: 10.1016/j.aquatox.2022.106208
Marshall D., Keough M. (2003). Variation in the dispersal potential of non-feeding invertebrate larvae: The desperate larva hypothesis and larval size. Mar. Ecol. Prog. Ser. 255, 145–153. doi: 10.3354/meps255145
Matyash V., Liebisch G., Kurzchalia T. V., Shevchenko A., Schwudke D. (2008). Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146. doi: 10.1194/jlr.D700041-JLR200
Moran A. L., McAlister J. S. (2009). Egg size as a life history character of marine invertebrates: Is it all it’s cracked up to be? Biol. Bull. 216, 226–242. doi: 10.1086/BBLv216n3p226
Moschino V., Marin M. G. (2002). Spermiotoxicity and embryotoxicity of triphenyltin in the sea urchin Paracentrotus lividus Lmk. Appl. Organometal. Chem. 16, 175–181. doi: 10.1002/aoc.285
Munari M., Devigili A., Dalle Palle G., Asnicar D., Pastore P., Badocco D., et al. (2022). Ocean acidification, but not environmental contaminants, affects fertilization success and sperm motility in the sea urchin Paracentrotus lividus. J. Mar. Sci. Eng. 10, 247. doi: 10.3390/jmse10020247
Nishigaki T., José O., González-Cota A. L., Romero F., Treviño C. L., Darszon A. (2014). Intracellular pH in sperm physiology. Biochem. Biophys. Res. Commun. 450, 1149–1158. doi: 10.1016/j.bbrc.2014.05.100
Oksanen J., Blanchet F. G., Friendly M., Kindt R., Legendre P., McGlinn D., et al. (2019). Vegan: Community Ecology Package. R package version 2.5-2.
Pande S. V., Khan R. P., Venkitasubramanian T. A. (1963). Microdetermination of lipids and serum total fatty acids. Anal. Biochem. 6, 415–423. doi: 10.1016/0003-2697(63)90094-0
Parker L. M., O’Connor W. A., Byrne M., Coleman R. A., Virtue P., Dove M., et al. (2017). Adult exposure to ocean acidification is maladaptive for larvae of the Sydney rock oyster Saccostrea glomerata in the presence of multiple stressors. Biol. Lett. 13, 20160798. doi: 10.1098/rsbl.2016.0798
Podolsky R. D. (2001). Evolution of egg target size: An analysis of selection on correlated characters. Evolution 55, 2470–2478. doi: 10.1111/j.0014-3820.2001.tb00761.x
Ramsden C. E., Zamora D., Makriyannis A., Wood J. T., Mann J. D., Faurot K. R., et al. (2015). Diet-induced changes in n-3 and n-6 derived endocannabinoids and reductions in headache pain and psychological distress. J. Pain 16, 707–716. doi: 10.1016/j.jpain.2015.04.007
R Core Team (2023). R: A language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing).
Reuter K. E., Lotterhos K. E., Crim R. N., Thompson C. A., Harley C. D. G. (2011). Elevated pCO2 increases sperm limitation and risk of polyspermy in the red sea urchin Strongylocentrotus franciscanus. Global Change Biol. 17, 163–171. doi: 10.1111/j.1365-2486.2010.02216.x
Sabine C. L., Feely R. A., Gruber N., Key R. M., Lee K., Bullister J. L., et al. (2004). The oceanic sink for anthropogenic CO2. Science 305, 367–371. doi: 10.1126/science.1097403
Sartori D., Pellegrin D., Macchia S., Gaion A. (2016). Can echinoculture be a feasible and effective activity? Analysis of fast reliable breeding conditions to promote gonadal growth and sexual maturation in Paracentrotus lividus. Aquaculture 451, 39–46. doi: 10.1016/j.aquaculture.2015.08.037
Schiopu D., George S. B., Castell J. (2006). Ingestion rates and dietary lipids affect growth and fatty acid composition of Dendraster excentricus larvae. J. Exp. Mar. Biol. Ecol. 328, 47–75. doi: 10.1016/j.jembe.2005.06.019
Schlegel P., Havenhand J. N., Gillings M. R., Williamson J. E. (2012). Individual variability in reproductive success determines winners and losers under ocean acidification: A case study with sea urchins. PloS One 7, 1–8. doi: 10.1371/journal.pone.0053118
Shpigel M., McBride S. C., Marciano S., Lupatsch I. (2004). The effect of photoperiod and temperature on the reproduction of European sea urchin Paracentrotus lividus. Aquaculture 232, 343–355. doi: 10.1016/S0044-8486(03)00539-8
Spirlet C., Grosjean P., Jangoux M. (2000). Optimization of gonad growth by manipulation of temperature and photoperiod in cultivated sea urchins, Paracentrotus lividus (Lamarck) (Echinodermata). Aquaculture 185, 85–99. doi: 10.1016/S0044-8486(99)00340-3
Stoffel M. A., Nakagawa S., Schielzeth H. (2017). rptR: repeatability estimation and variance decomposition by generalized linear mixed-effects models. Methods Ecol. Evol. 8, 1639–1644. doi: 10.1111/2041-210X.12797
Suckling C. C., Clark M. S., Richard J., Si. A., Thorne M. A. S., Harper E. M., et al. (2015). Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short-term exposures. J. Anim. Ecol. 84, 773–784. doi: 10.1111/1365-2656.12316
Suquet M., Le Mercier A., Rimond F., Mingant C., Haffray P., Labbe C. (2012). Setting tools for the early assessment of the quality of thawed Pacific oyster (Crassostrea gigas) D-larvae. Theriogenology 78, 462–467. doi: 10.1016/j.theriogenology.2012.02.014
Thor P., Dupont S. (2015). Transgenerational effects alleviate severe fecundity loss during ocean acidification in a ubiquitous planktonic copepod. Global Change Biol. 21, 2261–2271. doi: 10.1111/gcb.12815
Tocher D. R. (2003). Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107–184. doi: 10.1080/713610925
Trocino A., Xiccato G., Majolini D., Tazzoli M., Bertotto D., Pascoli F., et al. (2012). Assessing the quality of organic and conventionally-farmed European sea bass (Dicentrarchus labrax). Food Chem. 131, 427–433. doi: 10.1016/j.foodchem.2011.08.082
Uthicke S., Patel F., Karelitz S., Luter H., Webster N., Lamare M. (2020). Key biological responses over two generations of the sea urchin Echinometra sp. A under future ocean conditions. Mar. Ecol. Prog. Ser. 637, 87–101. doi: 10.3354/meps13236
Keywords: ocean acidification, sea urchins, reproduction, gamete quality, sperm viability, sperm velocity, egg biochemical composition, fatty acid profile
Citation: Asnicar D, Locatello L, Zanovello L, Minichino R, Masiero L, Munari M and Marin MG (2024) How do sea urchins prepare offspring to face ocean acidification? Gamete intraspecific differences and adaptability. Front. Mar. Sci. 11:1379150. doi: 10.3389/fmars.2024.1379150
Received: 31 January 2024; Accepted: 06 March 2024;
Published: 20 March 2024.
Edited by:
Matteo Oliva, Centro Interuniversitario di Biologia Marina ed Ecologia Applicata “G. Bacci” di Livorno (CIBM), ItalyReviewed by:
Matilde Vieira Sanches, University of Aveiro, PortugalCopyright © 2024 Asnicar, Locatello, Zanovello, Minichino, Masiero, Munari and Marin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Davide Asnicar, ZGF2aWRlLmFzbmljYXJAaHVudHNtYW5tYXJpbmUuY2E=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.