- 1Department Food Science and Technology, Graphic Era (Deemed to be University), Dehradun, Uttarakhand, India
- 2Department of Pharmacy, Banasthali Vidyapith, Banasthali, Rajasthan, India
- 3Faculty of Agricultural Sciences, GLA University, Mathura, India
- 4Himalayan School of Biosciences, Swami Rama Himalayan University, Dehradun, Uttarakhand, India
- 5Department of Microbiology, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland
- 6Division of Inter-Disciplinary Research Platform (IDRP) – Smart Health Care, Indian Institute of Technology, Jodhpur, Rajasthan, India
- 7Department of Zoology, Akal University, Bhatinda, Punjab, India
- 8Department of Food Technology, School of Applied and Life Sciences, Uttaranchal University, Dehradun, Uttarakhand, India
- 9School of Agriculture, Graphic Hill University, Dehradun, Uttarakhand, India
Marine algae are thought to be a source of various metabolites that have a wide range of positive effects on human health. The pharmacological properties of algal metabolites, including their antioxidant, anti-inflammatory, cholesterol homeostasis, protein clearance, and anti-amyloidergic effects, lend credence to their protective efficacy against oxidative stress, neuroinflammation, mitochondrial dysfunction, and impaired proteostasis, all of which are involved in the pathophysiology of neurodegenerative disorders. There are currently no clinical trials on the effects of marine algae on neuroinflammation; however, considering the significant biological activities that have been established by in vitro and animal research, we expect that there will be clinical trials on this topic in the not-too-distant future. The most recent and important findings on the potentially neuroprotective effects of the anti-inflammatory properties of marine algae were chosen for this study. Next, we conducted a literature review on the neuroprotective potential of algal compounds, along with the underlying pharmacological mechanism, and finally, we evaluated recent advances in therapeutics.
Introduction
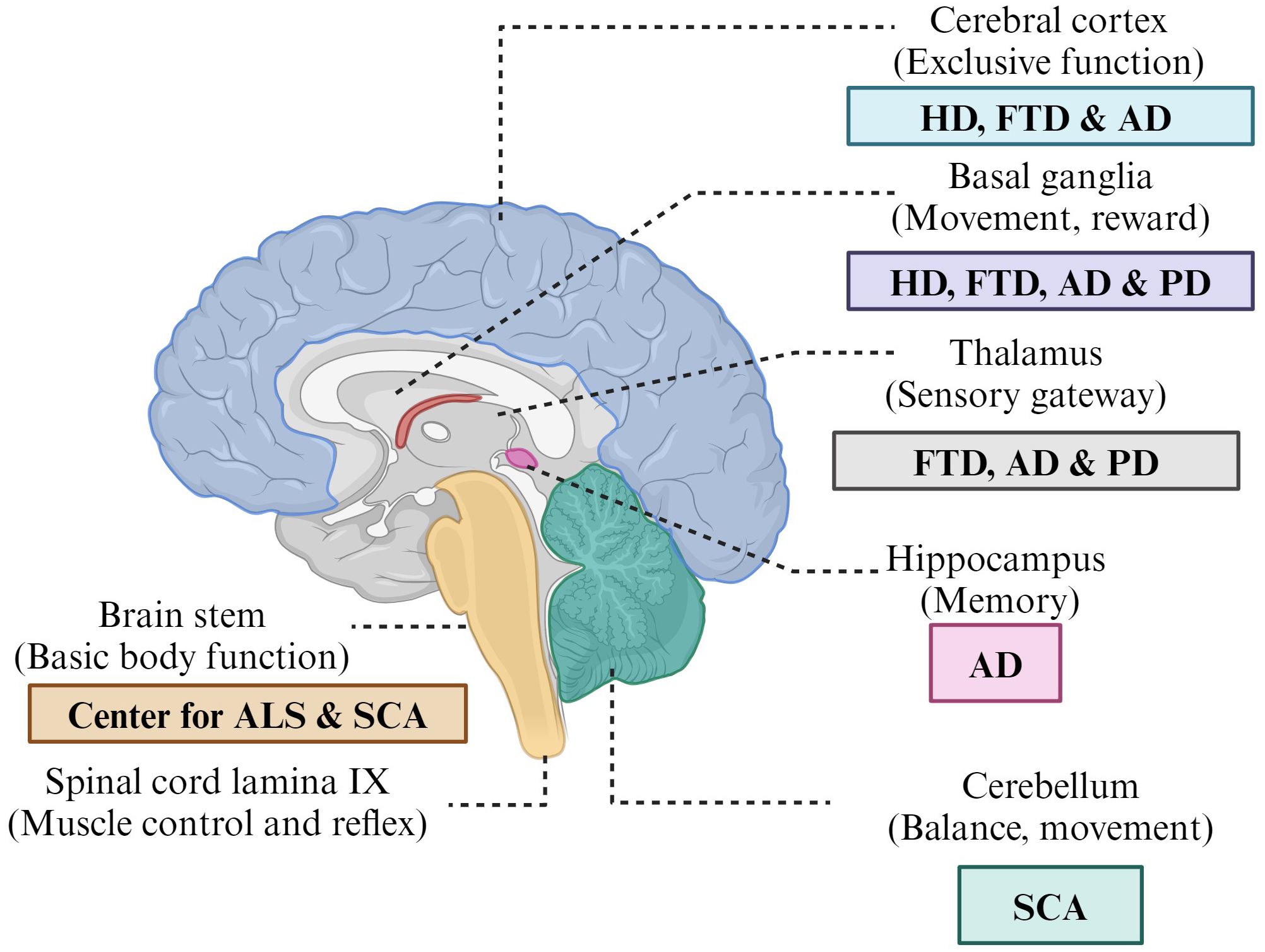
Neurodegenerative diseases (NDDs) are diseases that can progressively arise due to dysfunction of specific neurons. This dysfunction occurs since some neurons undergo structural and functional changes, and thus, their loss leads to neuronal cell death. The word “neurodegeneration” is composed of two different words, i.e., “neuro”, which refers to neurons or nerve cells, and “degeneration”, which refers to “damage”. Examples include Alzheimer’s disease (AD), amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), Friedreich ataxia (FA), Lewy body disease (LBD), Huntington’s disease (HD), spinal muscular atrophy (SMA), prion disease (PrD), progressive supranuclear palsy (PSP), brain trauma (BT), and spinocerebellar ataxia (SCA). Neuronal loss could be due to the deposition or accumulation of extra- and an intracellular misfolded protein that later becomes toxic. The generation of neurotoxic reactive oxygen species (ROS), which exert oxidative stress on mitochondria, is one cause. Another cause could be alterations in mitochondrial function. Nerve cells are highly complicated, specialized, and amitotic in nature, and since neurons are amitotic, they cannot undergo mitotic division; hence, if destroyed, they cannot divide or be replaced. The nervous system acts via neuronal cells by conducting nerve impulses. Disruption of this process causes neurons to stop communicating with one another, resulting in both motor and nonmotor symptoms, such as vision and hearing loss, and symptoms associated with movement, such as speech and language, memory, and others. Affected brain regions and their functions in relation to neurodegenerative disorders are shown in Figure 1.
Alzheimer’s disease
One of the important causes of dementia is AD where dysfunctions of the medial temporal lobe (MTL) and the hippocampus of the brain occur. Patients with AD initially face reduced episodic memory, which leads to gradually affected extensive cognitive dysfunctions like apraxia, amnesia, and visuospatial deficits (Eramudugolla et al., 2017). The pathogenesis of AD is characterized by hyperphosphorylated tau protein (p-tau) in neurofibrillary tangles and the aggregation of the amyloid-β (Aβ) protein in senile plaques in the brain (Moghadas et al., 2020). The abnormal clusters of protein fragments formed between nerve cells are plaques, while the tangles are described as dead nerve cells. The clumping of these small protein fragments is responsible for forming plaques that hamper cell communication by disrupting cell signaling at synapses. However, the tangles are used to destroy a protein from a vital cell transport system. Thus, disease disrupts neuron-related processes and functional networks with their activities, such as communication, metabolism, and repair systems. The sticky beta-amyloid that surrounds nerve cells is present in the lipid bilayer membrane. Another cause of AD progression is an accumulation of Th1 cells and M1 microglial cells in the brain due to the peripheral deposition of phenylalanine and isoleucine by alteration of the gut microbiome (Wang X. et al., 2019). The disease is typically considered a brain disorder that diminishes a large number of neurons in the brain region responsible for learning and memory, i.e., the entorhinal cortex, hippocampus region, and cerebral cortex region involved in speech and social behavior. Therefore, it diminishes memory, thinking skill abilities, and behavioral manifestations, finally leading to dementia with impairment of memory and cognitive judgments.
Amyotrophic lateral sclerosis
ALS is a multisystem neurodegenerative fatal disorder in which loss of muscular control occurs due to progressive degeneration of nerve cells in the brain and in the spinal cord. ALS could be due to the involvement of genetic inheritance (familial: fALS) and environmental factors (sporadic: sALS) as well as aging‐related dysfunctions that affect the motor system due to the loss of both upper and lower motoneurons in the motor cortex of the brainstem and anterior horn of the spinal cord. ALS disease is also termed Lou Gehrig’s disease based on the name of a famous baseball player who was diagnosed initially whereas the term “amyotrophic lateral sclerosis” was given by the French neurologist Jean‐Martin Charcot (Masrori and Van Damme, 2020). The hallmarks of ALS include weakness, stiffness, dysarthria, dysphagia, dysphonia, and hyperreflexia. Muscle weakness first starts in the distal muscles and later in the proximal muscles, and wasting occurs. ALS is mainly spread by Mendelian inheritance-associated autosomal dominant gene mutation of Cu/Zn superoxide dismutase (SOD1) and TAR-DNA binding protein 43 (TDP-43) (Grad et al., 2017).
Friedreich ataxia
FA is an autosomal recessive degenerative disorder caused by damage to the cerebellum, spinal cord, and peripheral or other nerves or defects or mutations in the FXN gene. The FXN gene holds the genetic code for a mitochondrial protein, called frataxin, i.e., a highly conserved protein, and balanced cellular iron homeostasis (Schmucker and Puccio, 2010). This type of gene mutation is responsible for guanine–adenine–adenine (GAA) trinucleotide repeats in both alleles of FXN genes (Campuzano et al., 1996). The repeated expansion of gene expression reduces the transcription of the gene. This could result in diminished energy production within neurons and cardiac cells. Thus, the result causes poor muscle control and clumsy voluntary movements. Two defective copies of the gene obtained from each parent increase the risk of disease to their offspring. FA is thought to be acquired, degenerative, or hereditary. The term “Friedreich ataxia” was first described by Nikolaus Friedreich and is characterized by motor coordination because of dysfunction of the cerebellum and its connections (Campuzano et al., 1996). The symptoms that may arise are fatigue, nystagmus (involuntary eye movements), hearing loss, dysarthria (slurred speech), cardiomyopathy (heart enlargement), heart failure (CHF), diabetes, difficulty in walking, and sometimes deformities in feet like high arches and curvature of the spine (scoliosis).
Lewy body disease
Abnormal protein deposits in brain tissues are called Lewy bodies, which result in the decay of brain tissues due to the excess accumulation of abnormal proteins; this condition is called LBD. The disease arises in particular brain regions involved in thinking, learning, memory, and movement. The disease results in dementia; psychiatric symptoms such as visual hallucinations, changes in attention, and alertness; and motor symptoms such as rigid muscles, slow movement, walking difficulty, and tremors.
Parkinson’s disease
PD is the second most common neurocognitive and chronic neurodegenerative disorder affecting the nervous system and body parts controlled by the nerves of the nigrostriatal pathway; thus, patients with PD develop both motor and nonmotor features and symptoms (Deal et al., 2019). Dr. James Parkinson, an English physician, initially defined paralysis agitans or shaking palsy in 1817, and the pattern is known as PD (Schnabel, 2010). There are various mutations related to PD, i.e., α-synuclein, LRRK2, PINK1, Parkin, DJ-1, VPS35, GBA1, and the loss of the dopaminergic neuron potential (Zeng et al., 2018). The key pathogenic mechanisms of this disease are mainly oxidative stress and the loss of dopaminergic neurons. This dopaminergic degeneration mainly occurs in the substantia nigra part of the brain since nerve cells, mainly in the substantia nigra region, produce dopamine. Dopamine is a chemical that acts as a neurotransmitter that coordinates body movements. This disease results in uncontrollable movements such as shaking, stiffness, and difficulty balancing and coordinating due to the loss of dopamine neurotransmitters, which are responsible for movement (Deal et al., 2019). In PD, symptoms usually begin gradually and worsen over time. It may also cause mental and behavioral changes, disturbances in sleeping patterns, depression, memory difficulties, and fatigue. As the disease progresses, it becomes more serious, and muscle rigidity and tremors grow due to the slowness of voluntary movement and postural instability.
Huntington’s disease
It is a lethal autosomal dominant mutation that causes HD, a neurodegenerative illness of the central nervous system. HD is an inherited disease that progressively breaks down or degenerates nerve cells in the brain due to a mutation in the genetic code of a protein called Huntington. This mutation causes defects in the building blocks of DNA due to the repetition of cytosine, adenine, and guanine (CAG) trinucleotides on the short arm of Huntingtin, i.e., HTT (Parsons and Raymond, 2015). This particular HTT gene mutation reduces the growth of the aberrant polyglutamine protein, which causes neurodegeneration (Andhale and Shrivastava, 2022). The nuclear symptoms of this neuropsychiatric disorder include chorea, i.e., abnormal involuntary movements, behavioral and psychiatric disturbances, dementia, and cognitive decline. Patients also have some other psychiatric symptoms including apathy, suicide tendency, mania, and schizophrenia-like symptoms as well as cognitive defects such as lack of attention, motor skill learning deficits, and organizational deficit.
Spinal muscular atrophy
SMA is an autosomal recessive genetic condition affecting muscles that degenerate anterior horn cells, and alpha motoneurons of the spinal cord present symptoms of weakness, wasting, or thinning paralysis of the proximal muscular mass, and significant disability due to low levels of SMN protein in lower spinal cord motor neurons. Reduced levels of SMN have activated the JNK and ROCK signaling pathways in the in vitro and in vivo SMA models (Ahmad et al., 2016). Anterior horn cells are a type of motoneuron emerging from the anterior portion of the gray matter in the spinal cord toward the skeletal muscle, while alpha motoneurons (α) are the lower motoneurons found in the brainstem and spinal cord and are the largest neurons in the spinal cord with myelinated axons. Both are responsible for motor behavior and thus help to generate movement. The term was given by Werdnig and Hoffmann (D’Amico et al., 2011), and the disease could be recognized by the development of symptoms of weakness in one arm and/or one leg as well as facial weakness, numbness or tingling, difficulty in swallowing or speaking, trouble walking and balancing, and gradual memory loss.
Seaweed bioprospecting has garnered significant attention in recent years due to its potential for discovering novel bioactive compounds with diverse applications (Upadhyay and Singh, 2021). Researchers have focused on identifying seaweed species with promising bioactivities and elucidating the chemical structures and biological properties of their components (Donia and Hamann, 2003). Several studies highlighted the neuroprotective effects of seaweed-derived compounds, particularly in the context of neurodegenerative disorders (Olasehinde et al., 2020; Catanesi et al., 2021; Dhahri et al., 2021; Wang et al., 2022; Nyiew et al., 2023). These investigations have underscored the importance of exploring seaweed resources for the development of therapeutic interventions targeting neurological diseases (Schepers et al., 2020). In India, very few regional research efforts have been intensified in the field of neuroprotection, with a particular emphasis on natural products derived from indigenous seaweeds (Suganthy et al., 2010). However, preclinical and clinical investigations conducted have further validated the neuroprotective properties of seaweed-derived agents, paving the way for their potential therapeutic application in neurological disorders. Overall, the convergence of seaweed bioprospecting and neuroprotection research is a growing interest in harnessing marine resources for addressing neurological health challenges, with promising prospects for the development of novel therapeutic interventions.
Methodology used for data collection
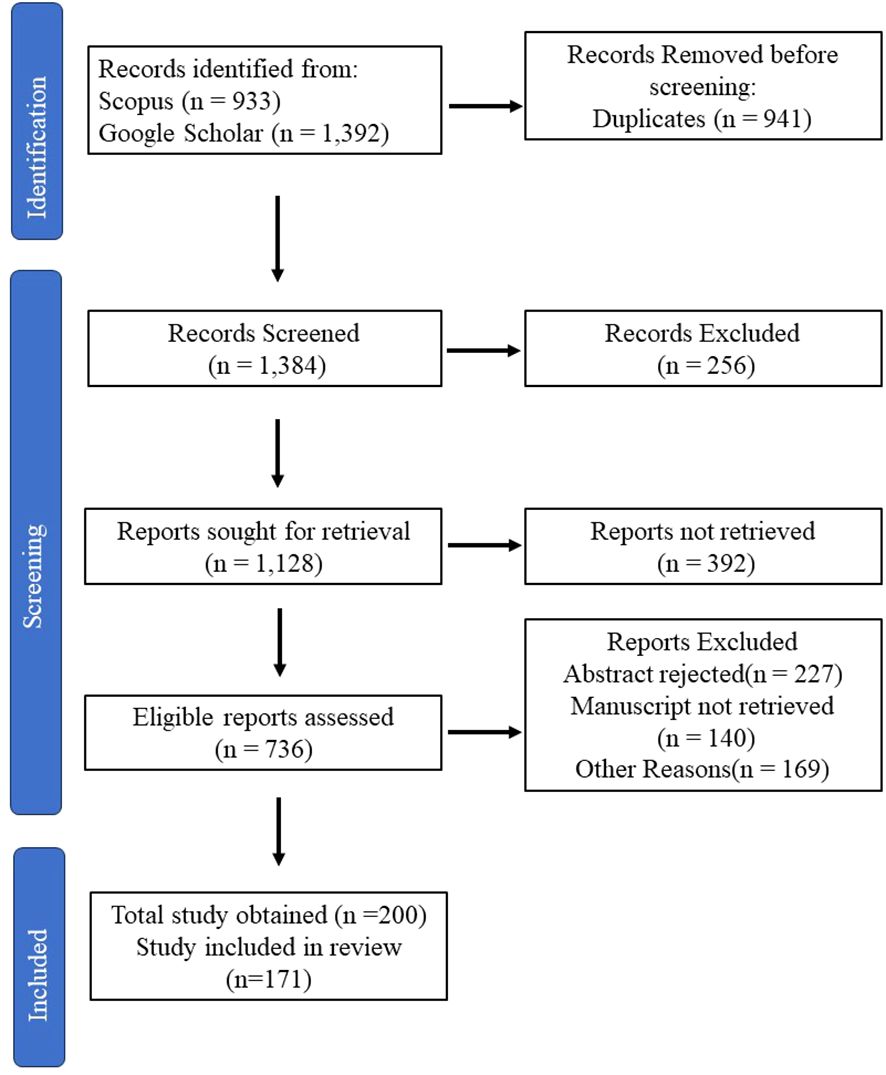
The initial data for this study were collected by keyword search across two databases, Google Scholar and Scopus. Within the Google Scholar database, 7 hits were obtained using (“Marine Algae” AND “Algal Metabolites” AND “Neuroprotection” AND “Neuroinflammation” AND “Therapeutics”), 1,120 hits were obtained using (“Marine Algae” AND “Algal Metabolites), 225 hits were obtained by (“Marine Algae” AND “Algal Metabolites” AND “Therapeutics”), and 40 hits were obtained using (“Marine Algae” AND “Algal Metabolites” AND “Neuroprotection”) keywords. Similarly, within the Scopus database, 818 hits were obtained by (Marine AND Algal AND metabolites), 15 hits were obtained by using (Marine Algae AND Algal Metabolites AND Neuroprotection), 89 hits were obtained by (Marine Algae AND Therapeutics), and 11 hits were obtained using (Marine Algae AND Algal Metabolites AND Therapeutics). The process of further screening is given in Figure 2.
Neurodegenerative algal metabolites
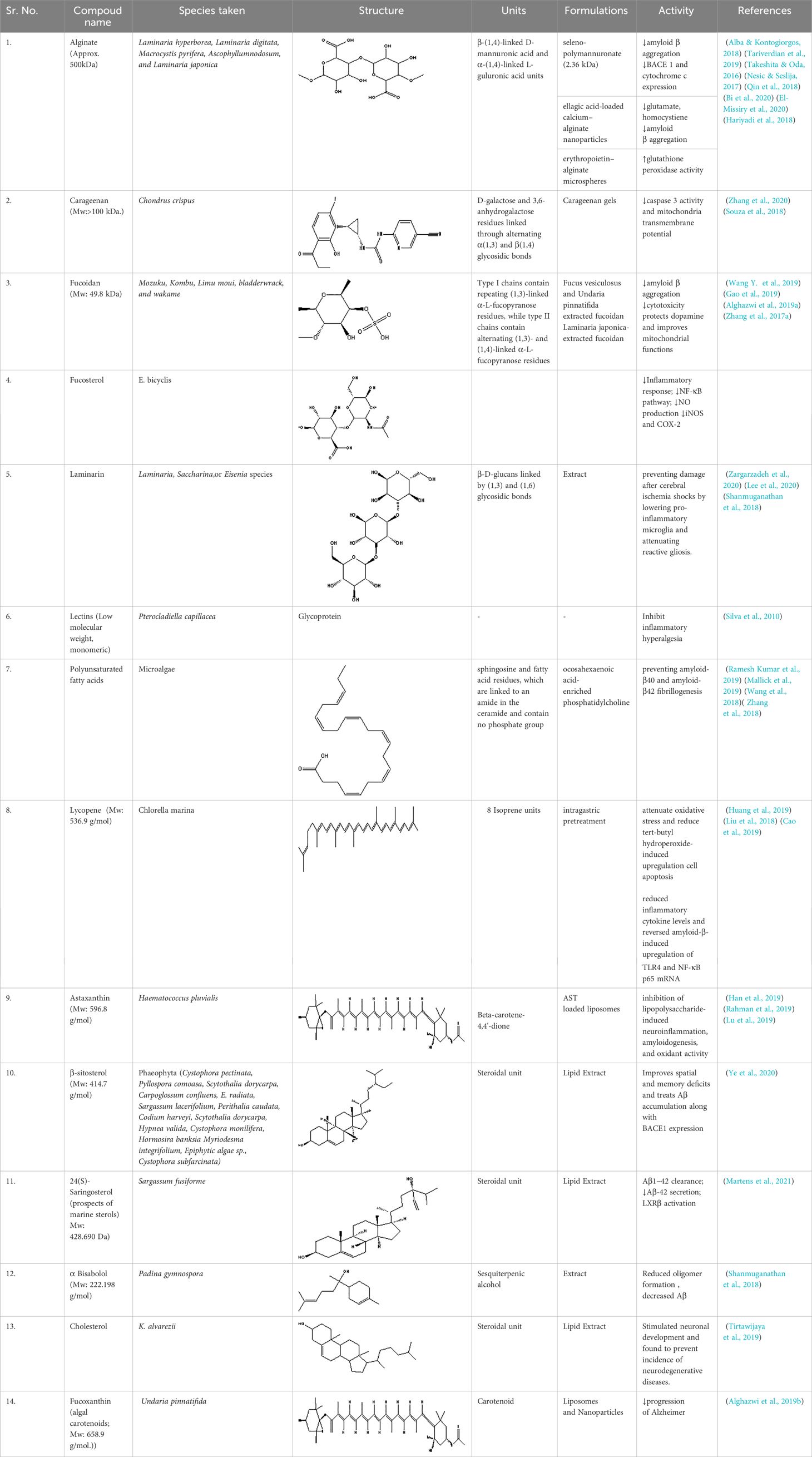
Marine algae have piqued people’s curiosity as possible renewable resources throughout the past few decades. There are more than 8,000 distinct classes of marine algal species that have been discovered worldwide (Probst, 2015; Menaa et al., 2020). The primary and secondary metabolites found in seaweed, such as polysaccharides, proteins, amino acids, dietary fiber, essential fatty acids, pigments, phytosterols, polyphenols, terpenoids, carotenoids, and tocopherols, are known to have cytostatic, antiviral, anthelminthic, antifungal, neuroprotective, and antibacterial effects (Pérez et al., 2016; Cheong et al., 2018; Uzair et al., 2020; Ramos-Romero et al., 2021). Although terrestrial organisms are currently the primary source for discovering natural medicines, there has been an upsurge in interest in focusing on marine organisms (Menaa et al., 2020). There has been an increase in interest in focusing on marine creatures, even though terrestrial organisms are now the main source for producing natural medicines (Gong et al., 2018). The increase in marine variety is due to variations in pressure, temperature, salinity, light, nutrients, oxygen, and ion concentrations, which result in unique adaptations and specializations of the species (Hamed et al., 2015; Carson and Clarke, 2018; Gong et al., 2018). In this review, we discuss several algal metabolites that play crucial roles in neuroprotection. Summarized marine algal metabolites for neuroprotective activity have been given in Table 1.
Lipids
Polyunsaturated fatty acids (PUFAs) make up between 25% and 60% of total lipids. Phytosterols, glycolipids, phospholipids, and fat-soluble vitamins are among the functional lipid fractions present in seaweeds (carotenoids; vitamins A, D, E, and K), which have relatively low concentrations of lipids (between 1% and 5% of dry weight) (Hamed et al., 2015; Menaa et al., 2020). PUFA-like omega-3-rich foods are pharmacologically significant because they can (i) regulate blood pressure, blood clotting, and membrane fluidity; (ii) lower the risk of diabetes, cardiovascular disease (CVD), and osteoporosis; and (iii) correct the growth and function of the brain and the nervous system (Peng et al., 2015). Isochrysis galbana, Ulva fasciata, Laurencia papillosa, Gracilaria salicornia, Dictyota fasciola, Taonia atomaria, Chaetoceros, Tetraselmis, Thalassiosira, and Nannochloropsis are among the marine algae that are known to produce large amounts of PUFA. Undaria pinnatifida has high concentrations of EPA, DHA, and monounsaturated FAs (C12:1 lauroleic acid, C14:1 myristoleic acid, C16:1 palmitoleic acid, C17:1 cis-10-heptadecenoic acid, and C18:1 oleic acid). Algal lipids also benefit from increased bioavailability and a range of health advantages for both people and animals because of these features. Lipid molecules can combine with glycosidic fragments to form glycolipids, which are categorized into three groups: glycosphingolipids, atypical glycolipids, and glycoglycerol (Cheng-Sánchez and Sarabia, 2018).
Lipids play a major role in PD because they have been found to have anti-inflammatory effects. Omega-3 fatty acids are directly correlated with inducible nitric oxide synthase (iNOS) and hence have a neuroprotective effect on rats. Layé et al. (2018) reported the anti-inflammatory effects of omega-3 fatty acids on microglial and neuronal cells and demonstrated their therapeutic efficacy against neurodegenerative diseases.
Proteins
Compared to brown seaweed (5%–24%) (such as Sargassum polycystum), edible green seaweed (such as Caulerpa lentillifera) and red seaweed (such as Eucheuma cottonii) have the highest protein content (10%–47% of dry weight) (Peng et al., 2015; Cherry et al., 2019). Significant amounts of proteins are present in Catenella repens, Polysiphonia mollis, Gelidiella acerosa, Capsosiphon fulvescens, Ulva prolifera, Porphyra sp., Osmundea pinnatifida, Pterocladium capillacea, Sphaerococcus coronopifolius, Gelidium microdon, and Cystoseira abies-marin (Cherry et al., 2019).
The prevention and treatment of neurodegenerative diseases, cancers, and gastric ulcers; DNA replication; the response to stimuli; the transport of molecules; and the catalysis of biochemical reactions are all made possible by proteins having anti-inflammatory, antioxidant, antitumor, antiaging, and protective effects (Menaa et al., 2021). Additionally, amino acids are useful in functional medicines, nutraceuticals, and cosmeceuticals since they are used as natural moisturizing agents for hair and skin (Couteau and Coiffard, 2016). Because of their high nutritional value and high protein content, macroalgal species, including Chlorella sp., Dunaliella salina, Aphanizomenon flos-aquae, Dunaliella tertiolecta, and Spirulina platensis, are frequently employed as human food sources (Menaa et al., 2021).
Histidine and taurine are found in Ulva australis; aspartic and glutamic acid (26%–32% of the total amino acids) are found in Ulva spp.; high levels of serine, alanine, and glutamic acid are found in Palmaria palmata (Dulse) and Himanthalia elongata (sea spaghetti); and methionine is found in Sargassum vulgare. Additionally, MAAs have been found in a wide range of species, particularly in Rhodophyta, including Chondrus crispus, Palmaria palmata, Gelidium spp., Porphyra/Pyropia spp., Curdiea racovitzae, Grateloupia lanceola, Asparagopsis armata, Solieria chordalis, and Gracilaria cornea (Menaa et al., 2021).
Additionally, these algal species have the potential to be employed as UV blockers and cell growth stimulants in cosmetics and toiletries (Couteau and Coiffard, 2016). A protein known as phycobilin (i.e., PC and PE) is covalently joined to chromophores to form phycobiliproteins (Cherry et al., 2019). These water-soluble proteins can be employed as natural food colorants and are effective antioxidants. A549 lung cancer cells are resistant to PC, a blue-colored phycobiliprotein mostly generated by the cyanobacterium Arthrospira spp., and PE, a pink-colored protein pigment produced by the cyanobacterium Lyngbya spp. Various proteins, such as carnosine, taurine, and glutathione, demonstrate antiapoptotic and antioxidant effects in the rat brain (Pérez et al., 2016; Pérez-Andrés et al., 2019). Coelho et al. (2017) reported that lectins are glycoproteins that have antinociceptive, antibiotic, mitogenic, and cytotoxic effects.
Carbohydrates
Carbohydrates or polysaccharides are abundant in several seaweeds and are therapeutically active and have anti-inflammatory and antioxidant effects. Oligosaccharides can be mono- or poly(OH). In brown algae, the monosaccharides present are glucose, galactose, glucuronic acid, mannuronic acid, xylose, and fucose. The polysaccharides used were laminarin, alginate, fucoidan, and mannitol. Red algae include carrageenans, florideans, lignin, and funorans. In addition to these algae, green algae contain ulvan, mannan, xylans, cellulose, and lignin (Barbalace et al., 2019).
Fucoidans, xylans, and ulvans, three minor sulfated polysaccharides, are present in brown, red, and green seaweeds, respectively (Cheong et al., 2018). In Ulva spp., 9% to 36% of the dry mass of algae is made up of sulfated polysaccharides that have been isolated from the intercellular space and the fibrillar wall of green seaweeds (Cherry et al., 2019). Chlorella ellipsoidea has several health advantages, including the ability to improve hemoglobin concentrations, reduce blood sugar levels, and function as a hepatoprotective, neuroprotective, and hypocholesterolemic agent. The most significant component of these goods is β-1,3-glucan, which is a potent immunostimulator, free radical scavenger, and blood lipid reductant (Menaa et al., 2021; Cheong et al., 2018). The ketogenic diet has gained much importance because it contains primarily BHB and ACA, which are used to treat neurological disorders.
The primary polysaccharides of the red algal cell wall, i.e., carrageenans, are categorized into three broad types based on the degree of sulfation: kappa, lambda, and iota (Pérez et al., 2016). Fucoidan polysaccharides are often generated by brown algae such as Sargassum thunbergi, Ascophyllum nodosum, Fucus vesiculosus, Laminaria japonica, Fucus evanescens, and Laminaria cichorioides, which are then exploited to create innovative medications and useful foods (Menaa et al., 2021).
Several studies have investigated the role of saccharides in neurodegeneration. Jin et al. (2017) reported in their study that Gracilaria lemaneiformis extracted from agaro has antioxidant potential when tested on kumming mice. Liu et al. (2019) observed the ability of oligosaccharides from Ulva lactuca and Enteromorpha prolifera to protect brain neurons by reducing oxidative stress and inflammatory factors. Another study by Bi et al. (2020) revealed the neuroprotective effect of seleno-polymannuronate (a selenium derivative of polymannurate) separated from alginate. Selenium is known for guarding neurocytes. He observed the suppression of microglial and astrocytic activation induced by LPS; hence, LPS functions as an anti-neuroinflammatory molecule.
Phenols
A type of chemical compound known as a phenolic compound has hydroxyl groups that are directly joined to aromatic hydrocarbon rings. The simplest compound is phenol, which has just one aromatic ring. Depending on how many phenol units are present in the molecule, phenolic substances can be either single phenols or polyphenols. All members of the plant kingdom contain phenols in varying amounts, but the phenols found in marine algae are distinct from those made by terrestrial plants (Barbalace et al., 2019). Phenolic acids can easily penetrate the brain and can potentially act on PD, HD, and anti-amyotrophic disease.
Phloroglucinol and phlorotannin are two of the most well-known polyphenols found in marine algae. The subclasses of phlorotannins are eckols, fuhalols, fucophlorethols, phlorethols, fucols, and ishofuhalols. Green and red algae have the most phenolic chemicals (bromophenols, phenolic acids, and flavonoids). The only source of phlorotannins is marine brown algae (Menaa et al., 2021).
Nho et al. (2020) studied the role of phlorotannin from the edible seaweed Ecklonia cava and reported that dickol inhibited AChE and BChE and prolonged acetylcholine neurotransmission in rat brain neurons. Paudel et al. (2019) reported the targeting of eckol from Ecklonia stolonifera and revealed that eckol is an agonist of the dopamine receptor and is active against PD. Rengasamy et al. (2015) concluded that a study on phenolic extracts of Amphiroa beauvoisii, G. foliaceum, Codium capitatum, and C. duthieae revealed a decrease in AChE activity, which was better proven in AD.
Isoprenoids and caprotenoids
Major phytosterol derivatives are found in brown algae, including Agarum cribosum, Undaria pinnatifida, and Laminaria japonica (Menaa et al., 2021). Fucosterol makes up 83%–97% of the total phytosterol concentration. Astaxanthin-carotene, lutein, lycopene, and canthaxanthin are among the many common lipophilic-pigmented molecules known as carotenoids (Bajpai et al., 2018). The antioxidant activity, anti-inflammatory effects, anticancer activity, antiobesity activity, antidiabetic activity, hepatoprotective impact, antiangiogenic effect, and cerebrovascular protective effect are some of the possible health-promoting benefits of these carotenoids.
Fucoxanthin in particular has been shown to reduce oxidative damage and inflammation, and astaxanthin has been shown to reduce IL-6 production in activated microglia (Fantonalgo, 2017), all of which have been linked to the etiology of neurodegenerative disorders.
Various pigments, which impart color, are found in marine algae. Algal color was categorized into three categories: brown pigments from Phaeophyta, green pigments from Chlorophyta (lutein, xanthin, and neoxanthin), and red pigments from Rhodophyta (zeaxanthin). These carotenoids have promising antioxidant and neuroprotective effects (Chuyen and Eun, 2017).
In another study performed by Kim et al. (2017), patients with PD had lower serum levels of alpha and beta carotene, lycopene, and tocopherols than did patients in the control group. Browne et al. (2019) also reported decreased levels of alpha tocopherols and vitamin E in patients with AD. Another study focused on the role of carotenoids in neurodegenerative disorders. Lakey-Beitia et al. (2019) conducted a lead-induced PD study and revealed that treating patients with Crocus sativus hydroethanolic extract prevented all damage to the brain. Furthermore, Fernandes et al. (2021) investigated lutein-loaded nanoparticles in a PD model and found that they had a positive impact on restoring dopamine levels. Lycopene was also found to be more active in AD (C.-B. Liu et al., 2018). Lycopene is involved in downregulating the serum levels of TNF-α and IL-β, hence reducing the inflammatory response at the choroid plexus and improving cognitive defects. Sun et al. (2020) investigated the effect of fucoxanthin on MPTP-induced PD and reported that fucoxanthin counteracted neuronal loss by reducing oxidative stress.
Alkaloids
A large number of marine alkaloids can act as neuroprotective agents. Various studies have shown that alkaloids have great potential for treating neurodegenerative disorders. Copmans et al. (2019) reported the specific effects of the isoquinole alkaloids TMC 120 B and TMC 120 B, which were isolated from Aspergillus insuetus, on zebrafish and mice and reported a reduction in seizure duration. Rivanor et al. (2018) investigated the effect of lectin from the green seaweed Caulerpa cupressoides. It reduced the levels of TNF-α and interleukins and hence proved to be a potential anti-inflammatory and antinociceptive agent. Furthermore, Pan et al. (2019) studied the effect of 9-methylfascaplysin from the marine sponge Fascaplysinopsis sp. on a scopolamine-induced AD model, which causes decreased cognitive dysfunction and no Aβ-induced tau hyperphosphorylation.
Phytosterols
These compounds are fatty in nature, are obtained from plants, and contribute to maintaining the integrity of biological membranes. Several phytosterols, such as fucosterol and brassicasterol, have been extracted from brown algae, also known as Phaeophyta. In addition, in red algae, also referred to as Rhodophyta, cholesterol is the main principal component, and it has little phytosteroidal content and is composed of fucosterol, desmosterol, chalinasterol, and sitosterol. Poriferasterol, 28-isofucosterol, beta sitosterol, chondrillasterol, and ergosterol are the major phytosterols found in green algae, i.e., chlorophyta. In addition, phytosterols also have anti-inflammatory, antioxidant, and anti-AD effects. Another study revealed the involvement of phytosterols in AD (Wong et al., 2018). He studied several mechanisms of fucosterol’s antioxidant activity, such as an increase in free radical scavenging enzymes and inhibitory activities against AChE, which contribute to AD. Vanbrabant et al. (2021) stated that 24S saringosterol has greater oxidant properties than fucosterol and hence greater neuroprotective power. Several studies have shown the anti-inflammatory potential of fucosterol, which has synergistic effects on neurodegenerative disorders. Alghazwi et al. (2019) reported that seaweed-derived fucoxanthin and fucoidan improve memory deficits. Wong et al. (2018) reported that fucosterol also reduces the generation of inflammatory mediators in Aβ-induced microglia, protecting against Aβ-associated neuroinflammation. Kheiri et al. (2018) reported that the activation of p38 mitogen-activated protein kinase (MAPK) by the Aβ peptide triggers inflammation.
Terpenes
Brown algae are considered to be a renowned source of terpenoids, primarily diterpenes and meroterpenoids. Sargachromenols are the most well-known terpenoids found in Sargassum species. The genus Caulerpa, which is found in green algae, is a source of monocyclic sesquiterpenes and diterpenes with neuroprotective properties (Yang et al., 2014). Many of these compounds contain chromene groups, which may have cytotoxic and antioxidant properties. Three meroterpenoids from S. macrocarpum, sargaquinoic acid, sargahydroquinoic acid, and tuberatolide B have been the subject of previous research. These compounds demonstrated neuroprotective effects, reduced inflammation, and suppressed cancer growth (Gaysinski et al., 2015). Silva et al. (2019) investigated the antioxidant and neuroprotective potential of diterpenes extracted from Bifurcaria bifurcata (brown seaweed). The use of isolated diterpenes has shown promising results in treating PD. Silva also studied the neuroprotective effect of algal extracts on SH-SY5Y cells and found that neurotoxicity was reduced, hence affecting neurodegeneration. Another study on zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, by Shimizu et al. (2015) revealed that zonarol activates the Nrf2/ARE pathway and hence participates in the protection of neuronal cells from oxidative stress. Furthermore, studies by Wozniak et al. (2015) showed the promising effects of Papenfussiella lutea’s extracted sesquiterpenes involved in the inhibition of AChE, hence decreasing AD symptoms. MaChado et al. (2015) reflected the role of halogenated monoterpenes extracted from Ochtodes secundiramea against AD by inhibiting AChE. Recently, Kwon et al. (2022) reported the neuroprotective effects of two monoterpenoid lactones from Sargassum macrocarpum via the inhibition of the MAO enzyme, which is involved in the physiology of PD.
Mode of action of metabolites in neuroprotection
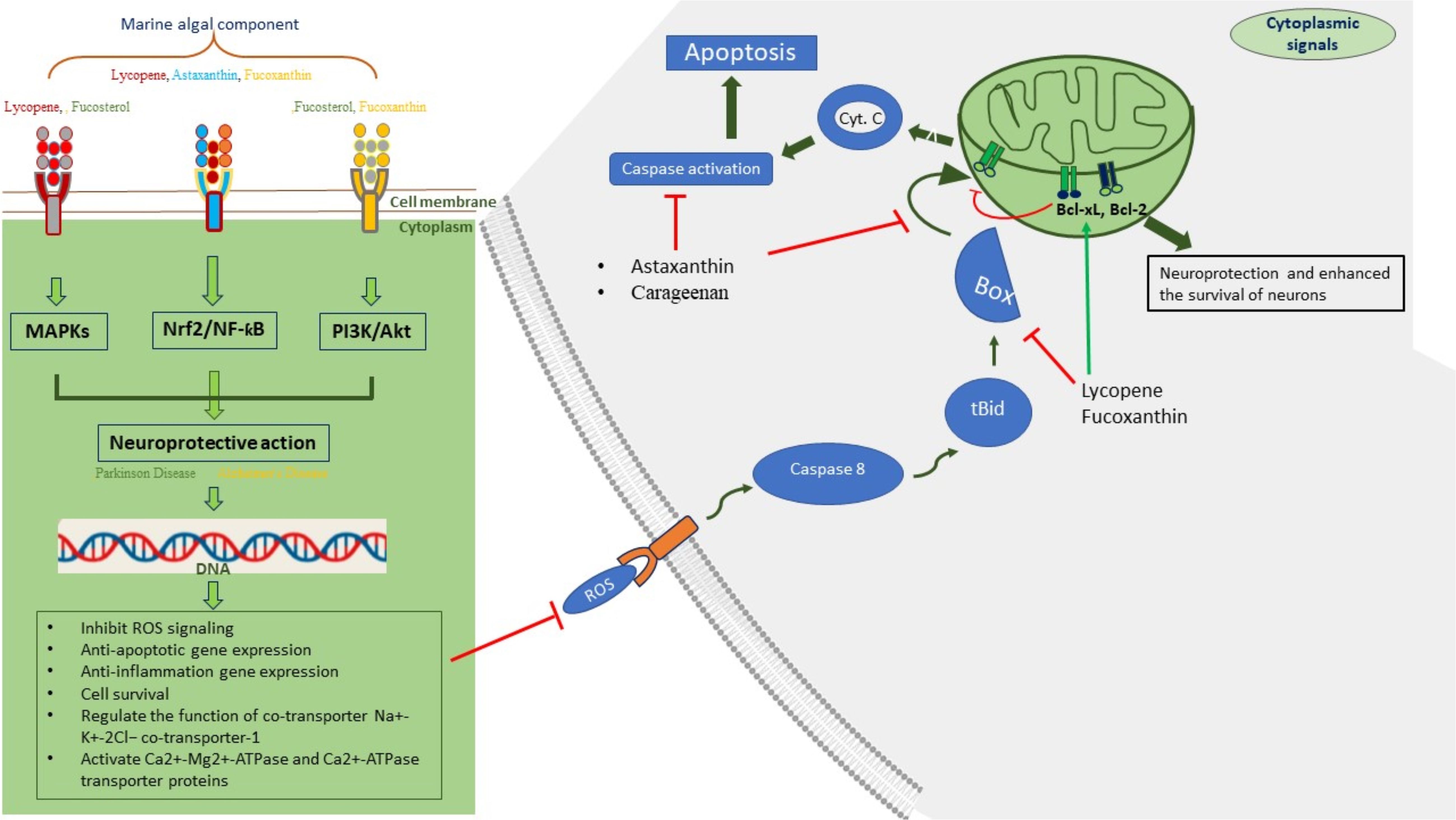
Currently, marine algae play an important role in the field of neuroprotection via various mechanisms, such as anti-neuroinflammatory and antioxidant effects, the inhibition of neuron death, and cholinesterase inhibitors. Therefore, various therapeutic systems, such as nutraceuticals and pharmaceuticals, are believed to have neuroprotective effects on marine algae (Pradhan et al., 2020). Signaling and neuroprotective functions of various components of marine algae are shown in Figure 3. In the biological system, ROS affect human health by damaging macromolecules (Angelova et al., 2018), whereas different lines of experimental evidence have shown that ROS are neutralized by the antioxidant components of various marine algae, such as green, brown, and red algal species, which have lots of beneficial compounds, such as vitamins, carotenoids, astaxanthin, phycocyanin, phycobilin, and polyphenols. These components of marine algae are used to treat different diseases, such as cancer, diabetes, immune responses, and neurological diseases (Pradhan et al., 2020). Different signaling pathways that regulate the survival of brain neurons, like ROS, NF-kB/Akt, and MAPK pathways, balance neurodegeneration, and we will further talk about the regulation of these signaling pathways in terms of metabolites of marine algae. Lycopene is a red–pink carotenoid found in several fruits and vegetables, like guava and watermelon. Lycopene significantly recovers neuronal life by regulating the cyclooxygenase-2 (COX-2) pathway in inflammation, balancing Nrf2 via the NF-κB pathway and stimulating MAPK in the brain (Fradet et al., 2009; Hwang et al., 2017; Zhao et al., 2017). To prevent primary hippocampal neuron apoptosis, lycopene stopped the degradation of the antiapoptotic proteins Bcl-2 and Bcl-xL, enhanced the efflux of calcium from neurons by activating Ca2+-Mg2+-ATPase and Ca2+-ATPase transporter proteins (Zhang F. et al., 2017), and balanced mitochondrial membrane integrity to regulate membrane potential (Qu et al., 2016). During ROS challenge in brain neurons, lycopene also balances the inner membrane potential of mitochondria to increase the life of neurons (Soleymaninejad et al., 2017). Astaxanthin is lipophilic in nature; thus, trans-astaxanthin easily crosses the blood–brain barrier and plays a significant role not only in neuroprotection in the cortex and hippocampus of rats (Manabe et al., 2018) but also in neurological disorders via different pathways: it initially reduces the levels of ROS-generating enzymes, for example, p22phox protein and NADPH oxidase 4; it increases the level of superoxide dismutase, which has antioxidant effects; and it increases Nrf2 protein levels to reduce oxidative stress (Deng et al., 2019; Lin et al., 2020). Astaxanthin prevents the apoptosis of neurons in the brain of rat via reducing the levels of Bax protein and cleaved caspase 3. Unlike initial parameters, it increases the level of Bcl2 by balancing the mitochondrial membrane potential of hippocampal neurons of rat brain (Wang et al., 2018; Deng et al., 2019). On the other hand, astaxanthin also protected the neurons by balancing the function of the Na+-K+-2Cl− cotransporter-1 (NKCC1) of brain cells regulated by the NF-κB pathway to reduce the swelling of brain cells (Zhang J. et al., 2017). Another highly abundant marine carotenoid, fucoxanthin, is found in edible brown algae (Yang et al., 2021) and has antiapoptotic, antioxidant, and anti-inflammatory properties that protect against neuron damage in animals challenged by neurodegenerative diseases and improve motor function. Various studies have indicated that, like astaxanthin, fucoxanthin enhances rodent brain functions by stimulating Nrf2 transcription factors via different pathways including PI3K/Akt pathways that enhance the expression of target molecules, such as superoxide dismutase, heme oxygenase-1, and Bcl-2, to reduce neural damage or apoptosis (Zhang L. et al., 2017). Another significant neuroprotective role of fucoxanthin in Addison’s disease is that it helps reduce neuroinflammation and oxidative stress and regulate amyloid-β to prevent the aggregation of amyloid-β fibrils (Lakey-Beitia et al., 2019). Fecosterol is an important marine algae-derived phytosterol that shows different medicinal properties, such as neuroprotective, antioxidant, and anti-inflammatory properties. Various experimental and in silico studies have indicated that it can be used against neurodegenerative disorders to recover life of the neurons by regulating different pathways, such as the PI3K/Akt, MAPK, and neurotrophin pathways and also via transporter proteins, enzymes, and TLR signaling related to neurons (Hannan et al., 2019). Alginate is a linear marine polysaccharide of brown algae that plays a significant role in the development of dopaminergic (DA) neurons from induced human pluripotent stem cells (hiPSCs) in in vitro culture systems for easier treatment of patients with PD. Alginate plays a neurogenerative function through the mechanical properties responsible for proper culture of human and mouse embryonic stem cells (ESCs, i.e., hESCs and mESCs) into the mature neuron lineage to treat neurodegenerative diseases (Gilmozzi et al., 2021). Various other components of marine algae, such as lectins (Doolen et al., 2020) and PUFAs (Kim et al., 2022b), play significant roles in different neurodegenerative diseases and enhance the survival of neurons by increasing the secretion of neurotransmitters in the human and animal brains.
Another significant marine component of brown algae, i.e., sodium oligomannate, is a linear oligosaccharide of 2–10 carbohydrate moieties that can cross the blood–brain barrier via the GLUT1 transporter to destabilize the Aβ fibril formation in the brain during AD, and because of such significant result, it has reached phase III clinical trial in China (Wang X. et al., 2019). It also suppresses gut dysbiosis with a reduction of phenylalanine/isoleucine accumulation during AD (Seo et al., 2019). Experimental evidence indicates that sodium oligomannate enhances the memory of mouse models of AD (C. Shanghai Green Valley Pharmaceuticals Co, 2019) by not only modifying cellular components of inflammation like proinflammatory Th1 cells and M1 microglial cells via brain cytokine alteration but also preventing Aβ aggregation and phosphorylation of tau protein in the brain (Wang et al., 2019).
The need of the hour
In recent years, a growing number of reports have revealed the serious involvement of the immune system in the beginning and progression of neurodegeneration (Seeley, 2017; Oxtoby et al., 2021) since neurodegeneration is characterized by alterations in cytokine signaling, proliferation of immune cells, movement, reactive gliosis, and altered phagocytosis (Burda and Sofroniew, 2014). Neuroinflammation, or the stimulation of neuroimmune microglia and astrocytes in proinflammatory states, is a powerful endogenous defense that defends the central nervous system from pathogens and injury. It is often a beneficial means aimed at eliminating dangers and restoring balance (Glass et al., 2010). On the other hand, prolonged neuroinflammatory events can cause a chain of events that culminate in the slow neuronal damage that characterizes many neurodegenerative illnesses (Spencer et al., 2012). Astrocytes, glial cells, and microglia play pro- and anti-inflammatory roles and are engaged in phagocytosis, steroid release, free radical reduction, and cell repair under normal and pathological conditions (Dhapola et al., 2021). Glial cells promote inflammation by producing ROS and cytokines, which cause synaptic dysfunction and loss and neural fatality, resulting in damage to the central nervous system. To date, most studies have concentrated on microglia as a major player in neuroinflammation in neurodegeneration, but recent research-based data have demonstrated that astrocytes play a crucial role in the inflammation that characterizes neurodegenerative illnesses (Jäkel and Dimou, 2017; Crespo-Castrillo and Arevalo, 2020; Muzio et al., 2021).
Degenerative brain disorders such as AD and PD are the result of abnormal aging of the brain and are characterized by specific regional cell loss (Cetin et al., 2022). All over the world, these disorders are the leading causes of dementia in elderly people (Azarpazhooh et al., 2018). Although the specific causes of many brain disorders are unknown, they share some pathophysiologies, such as mitochondrial dysfunction, oxidative stress, neuroinflammation, protein misfolding, and defective protein clearance, which complicate these conditions (Picca et al., 2021); however, if not deadly, ischemic, hurtful, and other types of brain damage cause subsequent damage and are significant sources of cognitive problems in patients. Brain injuries, such as neurodegenerative illnesses, have the same pathogenesis (Cruz-Haces et al., 2017). Regardless of the type of dementia one has, the existing treatment options can only relieve symptoms, not stop the disease from progressing.
Unfortunately, no pharmaceutical therapy exists to date that can delay or stop the progression of these deadly illnesses. As a result, research has focused on identifying natural substances that can protect against these ailments. Given the importance of neuroinflammation in the initiation and progression of neurodegenerative diseases, natural substances with anti-inflammatory effects may be promising candidates for the development of successful treatment techniques. Marine organisms provide a significant supply of natural chemicals, some of which have structural properties that differ from those found on land. Natural chemicals derived from the sea may have antidiabetic (Abo-Shady et al., 2023), anti-inflammatory (Barbalace et al., 2019), antioxidant, anticancer (Haq et al., 2019), and antiobesity properties (Wan-Loy and Siew-Moi, 2016; Oh et al., 2022), paving the way for the development of new treatments (Menaa et al., 2021).
Furthermore, current medications are associated with a variety of negative effects. Given the enormous societal and economic cost of these ailments, researchers are focusing their efforts on discovering possible therapeutic molecules that can target disease etiology while generating no adverse effects on patient wellbeing. Although man-made medications have some benefits, such as ease of development, naturally derived chemicals have taken precedence because they are comparatively well tolerated (Atanasov et al., 2021). Natural substances are said to have anti-inflammatory, antioxidant, and immunomodulatory properties (Hahn et al., 2020). Compounds with various pharmacological actions provide better treatment options for neurological illnesses with complicated pathomechanisms.
Macroalgae, often identified as seaweed, are abundant oceanic organisms that may offer renewable sources for nutrition and commercial applications (Leandro et al., 2020; Kumar et al., 2022). In addition, the metabolites of marine algae, such as carotenoids, phytosterols, phenolics, alkaloids, terpenoids, and polysaccharides, have piqued the interest of medicinal chemists owing to their functional and structural diversity (Singh et al., 2017; Bhowmick et al., 2020; Hannan et al., 2020; Catarino et al., 2023). Because of their antioxidant, anti-inflammatory, and immunoregulatory properties, these bio-functional chemicals have been demonstrated to offer neuroprotective effects in preclinical prototypes of neurodegenerative disorders, diabetes, ischemic stroke, BT, and overweightness, among others (Cornish et al., 2017; Sathasivam et al., 2019; Pagarete et al., 2021; Ibrahim et al., 2023). Reports show that metabolites of algal origin, fucosterol, fucoxanthin, and fucoidan, could possibly lead to the development of CNS disease treatments (Koirala et al., 2017; Schepers et al., 2020; Meinita et al., 2022). Sodium oligomannate was discovered and conditionally approved as an anti-AD medicine, despite the slow pace of algal metabolite drug discovery (Syed, 2020), increasing the prospect of future medicines derived from marine algae.
Among the oceanic animals, micro- and macroalgae constitute one of the seas’ most precious sources. Epidemiological research comparing Japanese and Western diets revealed that algal consumption is associated with a lower incidence of chronic degenerative illnesses (Brown et al., 2014). Algae are valuable resources of important biologically active constituents such as minerals, antioxidants, vitamins (Wells et al., 2017), PUFAs (Harwood, 2019), polysaccharides (Xu et al., 2017), proteins, terpenes, sterols, carotenoids, tocopherols (Galasso et al., 2017), phycocyanins, phycobilins, phycocolloids, and soluble dietary fibers (Pradhan et al., 2020). Recently, Elbandy (2023) summarized the most recent information on the possible anti-inflammatory action of oceanic algal metabolites, demonstrating their possible defensive efficacy against neuroinflammation. Marine algae, in particular, have been demonstrated to suppress neuroinflammation at multiple cellular levels, including by blocking proinflammatory enzymes such as iNOS and COX-2 (Jin et al., 2006; Je et al., 2021), regulating MAPK pathways (Jung et al., 2009), and activating NK-kB (Jiang et al., 2016). Currently, there are no medical tests on the effects of marine algal metabolites on neuroinflammation, but considering their essential biological potential, as proven by in vitro studies and research on animals, we anticipate that these studies will be conducted soon. Furthermore, because anti-inflammatory medications can cause problems and serious adverse effects (Scarpignato et al., 2017), finding new anti-inflammatory compounds in marine algal candidates could be a viable answer to these issues. Indeed, because of their extensive use in traditional medicines, natural anti-inflammatory substances have been shown to be harmless (Singh et al., 2017; Hannan et al., 2020).
The biological potential of marine algae, its nutritive value, and the probable advantages for their wellbeing have all been extensively researched and reviewed in recent years. This article, on the other hand, concentrates on the neuroprotective effects of marine algal species and highlights their prospective utility as forthcoming pharmacological agents for preventing neurodegenerative illnesses.
Safety issues of marine algae and its compounds
East Asian countries consume a lot of seaweed, and there have been no health problems associated with its consumption. Its buildup of vital microelements like iron and iodine, as well as heavy metals like cadmium, arsenic, mercury, and lead, is concerning. It is also critical to note that a large number of bioactive substances, particularly terpenoids, have not been subjected to toxicological testing. Consequently, it is essential to carry out in-depth safety assessments for seaweed (Sá Monteiro et al., 2019; Hannan et al., 2020). Furthermore, the toxicity profiles of drugs derived from seaweed need to be carefully examined, even though safety issues come up throughout the therapeutic development process. Numerous investigations have documented the non-toxic characteristics of algal polysaccharides, despite the paucity of information regarding the safety of algal metabolites. Research conducted both in vitro and in vivo has validated the safety of fucoidan, irrespective of the source of the algae (Hwang et al., 2016). Even at high oral dosages, fucoidan derived from Undaria pinnatifida and Laminaria japonica has been shown to be safe in animal models, and human clinical investigations have also shown that it has no harmful effects on health (Chung et al., 2010; Kim et al., 2010). Comparably, studies on the safety of carrageenan have revealed no harmful consequences from sub-chronic or chronic use of this food-grade polysaccharide, nor have they revealed any link to genotoxicity, carcinogenicity, or reproductive abnormalities (Weiner, 2014). Additionally, research on iota-carrageenan taken orally did not find any toxicological reactions (Hebar et al., 2015). The toxicity of another chemical, fucoxanthin, has been studied and shown to be safe in experimental subjects with no discernible negative effects (Beppu et al., 2009). Some of the toxicity profiles of marine metabolites have been evaluated; nevertheless, there are inadequate toxicological data to support the need for thorough investigations using suitable experimental models for additional potentially bioactive metabolites (Rengasamy et al., 2020).
Conclusion and future prospects
In conclusion, an exciting area for further study and practical application is exploring the possibility of algae-derived metabolites as potential therapeutic agents against neurodegenerative diseases. Preclinical research has shown that a wide range of bioactive substances, including fatty acids, polysaccharides, and polyphenols, found in marine algae, possess significant neuroprotective capabilities. These substances have an array of modes of action, such as neurotrophic, anti-inflammatory, and antioxidant properties, all of which are vital in the fight against the intricate pathophysiology of neurodegenerative diseases. Furthermore, using marine algae metabolites has several benefits, such as their sustainability, abundance, and potential for biotechnological optimization. Additionally, the marine environment continues to be a mostly unexplored source for novel bioactive chemicals, offering an enormous reservoir for the discovery and production of novel therapeutic agents.
To fully understand the precise mechanisms of action behind the neuroprotective benefits of marine algae metabolites, more research is required in the future. This entails looking into how they interact with important biological targets connected to neurodegenerative processes and how they might work in concert with current treatments. Furthermore, clinical trials are necessary to assess the efficacy and safety of drugs obtained from marine algae in humans. These studies will yield important information on the drugs’ potential for therapy, the best dosage schedules, and any possible adverse effects. Translating marine algae metabolites from bench to bedside will require close collaboration between researchers, doctors, and industry partners. Additionally, the production and bioavailability of chemicals derived from marine algae that can be used for medicinal purposes can be improved by the use of biotechnological techniques including synthetic biology and bioengineering. This includes developing new delivery methods to improve bioavailability and targeted delivery to the central nervous system, genetically modifying algae to increase metabolite production, and optimizing culture procedures. Overall, the study of marine algae metabolites is a potential area of research in the search for efficient treatments for neurodegenerative illnesses. Harnessing the full potential of these natural chemicals to reduce the increasing burden of neurodegenerative illnesses on global health will require ongoing studies and interdisciplinary collaboration.
Author contributions
BN: Investigation, Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Conceptualization. SRi: Writing – review & editing, Writing – original draft. SB: Writing – review & editing, Writing – original draft. SM: Writing – review & editing, Writing – original draft. VijK: Visualization, Validation, Supervision, Methodology, Formal analysis, Conceptualization, Writing – review & editing, Writing – original draft. VivK: Writing – review & editing, Writing – original draft. PS: Writing – review & editing, Writing – original draft, Validation, Conceptualization. AG: Writing – review & editing, Writing – original draft. RM: Writing – review & editing, Writing – original draft. UG: Writing – review & editing, Writing – original draft, Visualization. SRu: Writing – review & editing, Writing – original draft. MP: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors are thankful to Helsinki University, Helsinki, Finland, for open access support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abo-Shady A. M., Gheda S. F., Ismail G. A., Cotas J., Pereira L., Abdel-Karim O. H. (2023). Antioxidant and antidiabetic activity of algae. Life 13 (2), 460. doi: 10.3390/life13020460
Ahmad S., Bhatia K., Kannan A., Gangwani L. (2016). Molecular mechanisms of neurodegeneration in spinal muscular atrophy. J. Exp. Neurosci. 10, JEN.S33122. doi: 10.4137/JEN.S33122
Alba K., Kontogiorgos V. (2018) Seaweed polysaccharides (agar, alginate carrageenan). Available at: https://espace.library.uq.edu.au/view/UQ:deab024.
Alghazwi M., Smid S., Karpiniec S., Zhang W. (2019a). Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromolecules 122, 255–264. doi: 10.1016/j.ijbiomac.2018.10.168
Alghazwi M., Smid S., Musgrave I., Zhang W. (2019b). In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ1–42) toxicity and aggregation. Neurochemistry Int. 124, 215–224. doi: 10.1016/j.neuint.2019.01.010
Andhale R., Shrivastava D. (2022). Huntington’s disease: A clinical review. Cureus 14 (8), e28484. doi: 10.7759/cureus.28484
Angelova P. R., Abramov A. Y., Angelova P. R., Abramov A. Y. (2018). Role of mitochondrial ROS in the brain: from physiology to neurodegeneration. FEBS Lett. 592, 692–702. doi: 10.1002/1873-3468.12964
Atanasov A. G., Zotchev S. B., Dirsch V. M., Orhan I. E., Banach M., Rollinger J. M., et al. (2021). Natural products in drug discovery: advances and opportunities. Nat. Rev. Drug Discovery 20, 200–216. doi: 10.1038/s41573-020-00114-z
Azarpazhooh M. R., Avan A., Cipriano L. E., Munoz D. G., Sposato L. A., Hachinski V. (2018). Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimer’s Dementia 14, 148–156. doi: 10.1016/j.jalz.2017.07.755
Bajpai V., Shukla S., Kang S. M., Hwang S., Song X., Huh Y., et al. (2018). Developments of cyanobacteria for nano-marine drugs: relevance of nanoformulations in cancer therapies. Mar. Drugs 16, 179. doi: 10.3390/md16060179
Barbalace M. C., Malaguti M., Giusti L., Lucacchini A., Hrelia S., Angeloni C. (2019). Anti-inflammatory activities of marine algae in neurodegenerative diseases. Int. J. Mol. Sci. 20, 3061. doi: 10.3390/ijms20123061
Beppu F., Niwano Y., Tsukui T., Hosokawa M., Miyashita K. (2009). Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicological Sci. 34, 501–510. doi: 10.2131/jts.34.501
Bhowmick S., Mazumdar A., Moulick A., Adam V. (2020). Algal metabolites: An inevitable substitute for antibiotics. Biotechnol. Adv. 43, 107571. doi: 10.1016/j.biotechadv.2020.107571
Bi D., Li X., Li T., Li X., Lin Z., Yao L., et al. (2020). Characterization and neuroprotection potential of seleno-polymannuronate. Front. Pharmacol. 11, 21. doi: 10.3389/fphar.2020.00021
Brown E. M., Allsopp P. J., Magee P. J., Gill C. I., Nitecki S., Strain C. R., et al. (2014). Seaweed and human health. Nutr. Rev. 72, 205–216. doi: 10.1111/nure.2014.72.issue-3
Browne D., McGuinness B., Woodside J. V., McKay G. J. (2019). Vitamin E and Alzheimer’s disease: what do we know thus far? Clin. Interventions Aging 14, 1303–1317. doi: 10.2147/CIA.S186760
Burda J. E., Sofroniew M. V. (2014). Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81, 229–248. doi: 10.1016/j.neuron.2013.12.034
Campuzano V., Montermini L., Moltò M. D., Pianese L., Cossée M., Cavalcanti F., et al. (1996). Friedreich’s ataxia: Autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science 271, 1423–1427. doi: 10.1126/science.271.5254.1423
Cao Z., Wang P., Gao X., Shao B., Zhao S., Li Y. (2019). Lycopene attenuates aluminum-induced hippocampal lesions by inhibiting oxidative stress-mediated inflammation and apoptosis in the rat. J. Inorganic Biochem. 193, 143–151. doi: 10.1016/j.jinorgbio.2019.01.017
Carson M., Clarke S. (2018). Bioactive compounds from marine organisms: potential for bone growth and healing. Mar. Drugs 16, 340. doi: 10.3390/md16090340
Catanesi M., Caioni G., Castelli V., Benedetti E., d’Angelo M., Cimini A. (2021). Benefits under the sea: The role of marine compounds in neurodegenerative disorders. Mar. Drugs 19, 24. doi: 10.3390/md19010024
Catarino M. D., Silva-Reis R., Chouh A., Silva S., Braga S. S., Silva A. M. S., et al. (2023). Applications of antioxidant secondary metabolites of sargassum spp. Mar. Drugs 21 (3), 172. doi: 10.3390/md21030172
Cetin S., Knez D., Gobec S., Kos J., Pišlar A. (2022). Cell models for Alzheimer’s and Parkinson’s disease: At the interface of biology and drug discovery. Biomedicine Pharmacotherapy 149, 112924. doi: 10.1016/j.biopha.2022.112924
Cheng-Sánchez I., Sarabia F. (2018). Chemistry and biology of bioactive glycolipids of marine origin. Mar. Drugs 16, 294. doi: 10.3390/md16090294
Cheong K. L., Qiu H. M., Du H., Liu Y., Khan B. M. (2018). Oligosaccharides derived from red seaweed: production, properties, and potential health and cosmetic applications. Molecules 23, 2451. doi: 10.3390/molecules23102451
Cherry P., O’Hara C., Magee P. J., McSorley E. M., Allsopp P. J. (2019). Risks and benefits of consuming edible seaweeds. Nutr. Rev. 77, 307–329. doi: 10.1093/nutrit/nuy066
Chung H., Jeun J., Houng S., Jun H., Kweon D., Lee S. (2010). Toxicological evaluation of fucoidan from Undaria pinnatifidain vitro and in vivo. Phytotherapy Res. 24, 1078–1083. doi: 10.1002/ptr.3138
Chuyen H., Eun J. B. (2017). Marine carotenoids: Bioactivities and potential benefits to human health. Crit. Rev. Food Sci. Nutr. 57, 2600–2610. doi: 10.1080/10408398.2015.1063477
Coelho L. C. B. B., Silva P. M., dos S., de M. V. L., Pontual E. V., Paiva P. M. G., et al. (2017). Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evidence-Based Complementary Altern. Med. 2017, 1–22. doi: 10.1155/2017/1594074
Copmans D., Kildgaard S., Rasmussen S. A., Ślęzak M., Dirkx N., Partoens M., et al. (2019). Zebrafish-based discovery of antiseizure compounds from the north sea: isoquinoline alkaloids TMC-120A and TMC-120B. Mar. Drugs 17, 607. doi: 10.3390/md17110607
Cornish M. L., Critchley A. T., Mouritsen O. G. (2017). Consumption of seaweeds and the human brain. J. Appl. Phycology 29, 2377–2398. doi: 10.1007/s10811-016-1049-3
Couteau C., Coiffard L. (2016). “Seaweed application in cosmetics,” in Seaweed in Health and Disease Prevention (UK & USA: Elsevier), 423–441. doi: 10.1016/B978–0-12–802772–1.00014–2
Crespo-Castrillo A., Arevalo M. A. (2020). Microglial and astrocytic function in physiological and pathological conditions: estrogenic modulation. Int. J. Mol. Sci. 21 (9), 3219. doi: 10.3390/ijms21093219
Cruz-Haces M., Tang J., Acosta G., Fernandez J., Shi R. (2017). Pathological correlations between traumatic brain injury and chronic neurodegenerative diseases. Trans. Neurodegeneration 6 (1), 20. doi: 10.1186/s40035-017-0088-2
C. Shanghai Green Valley Pharmaceuticals Co., Ltd (2019) Sodium oligomannate capsules: Chinese prescribing information. Available at: https://www.greenvalleypharma.com/En/Index/listView/catid/81.html (Accessed 15 Jan 2020).
D’Amico A., Mercuri E., Tiziano F. D., Bertini E. (2011). Spinal muscular atrophy. Orphanet J. Rare Dis. 6 (1), 71. doi: 10.1186/1750-1172-6-71
Deal L. S., Flood E., Myers D. E., Devine J., Gray D. L. (2019). The Parkinson’s disease activities of daily living, interference, and dependence instrument. Movement Disord. Clin. Pract. 6, 678–686. doi: 10.1002/mdc3.12833
Deng X., Wang M., Hu S., Feng Y., Shao Y., Xie Y., et al. (2019). The neuroprotective effect of astaxanthin on pilocarpine-induced status epilepticus in rats. Front. Cell. Neurosci. 13, 123. doi: 10.3389/fncel.2019.00123
Dhahri M., Alghrably M., Mohammed H. A., Badshah S. L., Noreen N., Mouffouk F., et al. (2021). Natural polysaccharides as preventive and therapeutic horizon for neurodegenerative diseases. Pharmaceutics 14, 1. doi: 10.3390/pharmaceutics14010001
Dhapola R., Hota S. S., Sarma P., Bhattacharyya A., Medhi B., Reddy D. H. K. (2021). Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29, 1669–1681. doi: 10.1007/s10787-021-00889-6
Donia M., Hamann M. T. (2003). Marine natural products and their potential applications as anti-infective agents. Lancet Infect. Dis. 3, 338–348. doi: 10.1016/S1473-3099(03)00655-8
Doolen S., Keyes G. S., Ramsden C. E. (2020). Hydroxy-epoxide and keto-epoxide derivatives of linoleic acid activate trigeminal neurons. Neurobiol. Pain 7, 100046. doi: 10.1016/j.ynpai.2020.100046
Elbandy M. (2023). Anti-inflammatory effects of marine bioactive compounds and their potential as functional food ingredients in the prevention and treatment of neuroinflammatory disorders. Molecules 28 (1), 2. doi: 10.3390/MOLECULES28010002
El-Missiry M. A., Othman A. I., Amer M. A., Sedki M., Ali S. M., El-Sherbiny I. M. (2020). Nanoformulated ellagic acid ameliorates pentylenetetrazol-induced experimental epileptic seizures by modulating oxidative stress, inflammatory cytokines and apoptosis in the brains of male mice. Metab. Brain Dis. 35, 385–399. doi: 10.1007/s11011-019-00502-4
Eramudugolla R., Mortby M. E., Sachdev P., Meslin C., Kumar R., Anstey K. J. (2017). Evaluation of a research diagnostic algorithm for DSM-5 neurocognitive disorders in a population-based cohort of older adults. Alzheimer’s Res. Ther. 9 (1), 15. doi: 10.1186/s13195-017-0246-x
Fantonalgo R. N. (2017). Hypoglycemic and laxative activities of crude ethanolic extracts of brown seaweed sargassum oligocystum. J. Natural Sci. Res. 7, 45–52.
Fernandes E. J., Poetini M. R., Barrientos M. S., Bortolotto V. C., Araujo S. M., Santos Musachio E. A., et al. (2021). Exposure to lutein-loaded nanoparticles attenuates Parkinson’s model-induced damage in Drosophila melanogaster: Restoration of dopaminergic and cholinergic system and oxidative stress indicators. Chemico-Biological Interact. 340, 109431. doi: 10.1016/j.cbi.2021.109431
Fradet V., Cheng L., Casey G., Witte J. S. (2009). Dietary omega-3 fatty acids, Cyclooxygenase-2 genetic variation, and aggressive prostate cancer risk. Clin. Cancer Res. 15, 2559–2566. doi: 10.1158/1078-0432.CCR-08-2503
Galasso C., Corinaldesi C., Sansone C. (2017). Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 6 (4), 96. doi: 10.3390/antiox6040096
Gao Y., Zhang L., Jiao W. (2019). Marine glycan-derived therapeutics in China. Prog. Mol. Biol. Trans. Sci. 163, 113–134. doi: 10.1016/bs.pmbts.2019.02.006
Gaysinski M., Ortalo-Magné A., Thomas O. P., Culioli G. (2015). Extraction, purification, and NMR analysis of terpenes from brown algae. Methods Mol. Biol. (Clifton N.J.) 1308, 207–223. doi: 10.1007/978–1-4939–2684-8_13
Gilmozzi V., Gentile G., Riekschnitz D. A., Von Troyer M., Lavdas A. A., Kerschbamer E., et al. (2021). Generation of hiPSC-derived functional dopaminergic neurons in alginate-based 3D culture. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.708389
Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. doi: 10.1016/j.cell.2010.02.016
Gong H., Luo Z., Chen W., Feng Z. P., Wang G. L., Sun H. S. (2018). Marine compound xyloketal B as a potential drug development target for neuroprotection. Mar. Drugs 16, 516. doi: 10.3390/md16120516
Grad L. I., Guy A. R., John R., Neil R. C. (2017). Clinical spectrum of amyotrophic lateral sclerosis (ALS). Cold Spring Harber Perspective Med. 7, a024117. doi: 10.1101/cshperspect.a024117
Hahn D., Shin S. H., Bae J. S. (2020). Natural antioxidant and anti-inflammatory compounds in foodstuff or medicinal herbs inducing heme oxygenase-1 expression. Antioxidants 9, 1–40. doi: 10.3390/antiox9121191
Hamed I., Özogul F., Özogul Y., Regenstein J. M. (2015). Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 14, 446–465. doi: 10.1111/1541-4337.12136
Han J. H., Lee Y. S., Im J. H., Ham Y. W., Lee H. P., Han S. B., et al. (2019). Astaxanthin ameliorates lipopolysaccharide-induced neuroinflammation, oxidative stress and memory dysfunction through inactivation of the signal transducer and activator of transcription 3 pathway. Mar. Drugs 17 (2), 123. doi: 10.3390/md17020123
Hannan M. A., Dash R., Haque M. N., Al A., Sohag M., Rahman A., et al. (2020). Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs 18, 347. doi: 10.3390/md18070347
Hannan M. A., Dash R., Mamun Sohag A., Moon I. S. (2019). Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Marine 17, 639. doi: 10.3390/md17110639
Haq S. H., Al-Ruwaished G., Al-Mutlaq M. A., Naji S. A., Al-Mogren M., Al-Rashed S., et al. (2019). Antioxidant, anticancer activity and phytochemical analysis of green algae, chaetomorpha collected from the Arabian gulf. Sci. Rep. 9 (1), 18906. doi: 10.1038/S41598–019-55309–1
Hariyadi D. M., Rahmadi M., Rahman Z. (2018). In vivo neuroprotective activity of erythropoietin-alginate microspheres at different polymer concentrations. Asian J. Pharmaceutics (AJP) 12, 255. doi: 10.22377/AJP.V12I04.2833
Harwood J. (2019). Algae: critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 9, 708. doi: 10.3390/biom9110708
Hebar A., Koller C., Seifert J. M., Chabicovsky M., Bodenteich A., Bernkop-Schnürch A., et al. (2015). Non-clinical safety evaluation of intranasal iota-carrageenan. PloS One 10, e0122911. doi: 10.1371/journal.pone.0122911
Huang C., Wen C., Yang M., Gan D., Fan C., Li A., et al. (2019). Lycopene protects against t-BHP-induced neuronal oxidative damage and apoptosis via activation of the PI3K/Akt pathway. Mol. Biol. Rep. 46, 3387–3397. doi: 10.1007/s11033-019-04801-y
Hwang S., Lim J., Kim H. (2017). Inhibitory effect of lycopene on amyloid-β-induced apoptosis in neuronal cells. Nutrients 9, 883. doi: 10.3390/nu9080883
Hwang P. A., Yan M. D., Lin H. T., Li K. L., Lin Y. C. (2016). Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 14, 121. doi: 10.3390/md14070121
Ibrahim T. N. B. T., Feisal N. A. S., Kamaludin N. H., Cheah W. Y., How V., Bhatnagar A., et al. (2023). Biological active metabolites from microalgae for healthcare and pharmaceutical industries: A comprehensive review. Bioresource Technol. 372, 128661. doi: 10.1016/j.biortech.2023.128661
Jäkel S., Dimou L. (2017). Glial cells and their function in the adult brain: A journey through the history of their ablation. Front. Cell. Neurosci. 11. doi: 10.3389/fncel.2017.00024
Je J. G., Lee H. G., Fernando K. H. N., Jeon Y. J., Ryu B. (2021). Purification and structural characterization of sulfated polysaccharides derived from brown algae, sargassum binderi: Inhibitory mechanism of inos and cox-2 pathway interaction. Antioxidants 10 (6), 822. doi: 10.3390/antiox10060822
Jiang X., Chen L., Shen L., Chen Z., Xu L., Zhang J., et al. (2016). Trans-astaxanthin attenuates lipopolysaccharide-induced neuroinflammation and depressive-like behavior in mice. Brain Res. 1649, 30–37. doi: 10.1016/j.brainres.2016.08.029
Jin D., Lim C., Sung J., Choi H., Ha I., letters J. H.-N. (2006). Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglia. Elsevier 402, 154–158. doi: 10.1016/j.neulet.2006.03.068
Jin M., Liu H., Hou Y., Chan Z., Di W., Li L., et al. (2017). Preparation, characterization and alcoholic liver injury protective effects of algal oligosaccharides from Gracilaria lemaneiformis. Food Research International. 100, 186–195. doi: 10.1016/j.foodres.2017.08.032
Jung W., Heo S., Jeon Y., Lee C., Park Y., Byun H., et al. (2009). Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglia. ACS Publications 57, 4439–4446. doi: 10.1021/jf9003913
Kheiri G., Dolatshahi M., Rahmani F., Rezaei N. (2018). Role of p38/MAPKs in Alzheimer’s disease: implications for amyloid beta toxicity targeted therapy. Rev. Neurosci. 30, 9–30. doi: 10.1515/revneuro-2018-0008
Kim J. H., Hwang J., Shim E., Chung E. J., Jang S. H., Koh S. B. (2017). Association of serum carotenoid, retinol, and tocopherol concentrations with the progression of Parkinson’s Disease. Nutrition Research and Practice. 10 (2), 114.
Kim H.-Y., Huang B. X., Spector A. A. (2022). Molecular and signaling mechanisms for docosahexaenoic acid-derived neurodevelopment and neuroprotection. Int. J. Mol. Sci. 23, 4635. doi: 10.3390/ijms23094635
Kim K. J., Lee O. H., Lee B. Y. (2010). Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 48, 1101–1104. doi: 10.1016/j.fct.2010.01.032
Koirala P., Jung H. A., Choi J. S. (2017). Recent advances in pharmacological research on Ecklonia species: a review. Arch. Pharmacal Res. 40, 981–1005. doi: 10.1007/s12272-017-0948-4
Kumar S., Kumar R., Kumari A., Panwar A. (2022). Astaxanthin: A super antioxidant from microalgae and its therapeutic potential. J. Basic Microbiol. 62, 1064–1082. doi: 10.1002/jobm.202100391
Kwon J., Lee K., Hwang H., Kim S. H., Park S. E., Durai P., et al. (2022). New monocyclic terpenoid lactones from a brown algae sargassum macrocarpum as monoamine oxidase inhibitors. Plants 11, 1998. doi: 10.3390/plants11151998
Lakey-Beitia J., Kumar D. J., Hegde M., Rao K. S. (2019). Carotenoids as novel therapeutic molecules against neurodegenerative disorders: chemistry and molecular docking analysis. Int. J. Mol. Sci. 20, 5553. doi: 10.3390/ijms20225553
Layé S., Nadjar A., Joffre C., Bazinet R. P. (2018). Anti-inflammatory effects of omega-3 fatty acids in the brain: physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 70, 12–38. doi: 10.1124/pr.117.014092
Leandro A., Pereira L., Goncalves drugs A. M. M. (2020). Diverse applications of marine macroalgae. Mar. Drugs 18, 17. doi: 10.3390/md18010017
Lee T. K., Ahn J. H., Park C. W., Kim B., Park Y. E., Lee J. C., et al. (2020). Pre-treatment with laminarin protects hippocampal CA1 pyramidal neurons and attenuates reactive gliosis following transient forebrain ischemia in gerbils. Mar. Drugs 18 (1), 52. doi: 10.3390/md18010052
Lin W.-N., Kapupara K., Wen Y.-T., Chen Y.-H., Pan I.-H., Tsai R.-K. (2020). Hematococcus pluvialis-Derived Astaxanthin Is a Potential Neuroprotective Agent against Optic Nerve Ischemia. Mar. Drugs 18, 85. doi: 10.3390/md18020085
Liu X., Liu D., Lin G., Wu Y., Gao L., Ai C., et al. (2019). Anti-aging and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromolecules 139, 342–351. doi: 10.1016/j.ijbiomac.2019.07.195
Liu C.-B., Wang R., Yi Y.-F., Gao Z., Chen Y.-Z. (2018). Lycopene mitigates β-amyloid induced inflammatory response and inhibits NF-κB signaling at the choroid plexus in early stages of Alzheimer’s disease rats. J. Nutr. Biochem. 53, 66–71. doi: 10.1016/j.jnutbio.2017.10.014
Lu Y., Wang X., Feng J., Xie T., Si P., Wang W. (2019). Neuroprotective effect of astaxanthin on newborn rats exposed to prenatal maternal seizures. Brain Res. Bull. 148, 63–69. doi: 10.1016/j.brainresbull.2019.03.009
MaChado L. P., Carvalho L. R., Young M. C. M., Cardoso-Lopes E. M., Centeno D. C., Zambotti-Villela L., et al. (2015). Evaluation of acetylcholinesterase inhibitory activity of Brazilian red macroalgae organic extracts. Rev. Bras. Farmacognosia 25, 657–662. doi: 10.1016/j.bjp.2015.09.003
Mallick R., Basak S., Duttaroy A. K. (2019). Docosahexaenoic acid,22:6n-3: Its roles in the structure and function of the brain. Int. J. Dev. Neuroscience: Off. J. Int. Soc. Dev. Neurosci. 79, 21–31. doi: 10.1016/j.ijdevneu.2019.10.004
Manabe Y., Komatsu T., Seki S., Sugawara T. (2018). Dietary astaxanthin can accumulate in the brain of rats. Bioscience Biotechnol. Biochem. 82, 1433–1436. doi: 10.1080/09168451.2018.1459467
Martens N., Schepers M., Zhan N., Leijten F., Voortman G., Tiane A., et al. (2021). 24(S)-saringosterol prevents cognitive decline in a mouse model for alzheimer’s disease. Mar. Drugs 19 (4), 190. doi: 10.3390/md19040190
Masrori P., Van Damme P. (2020). Amyotrophic lateral sclerosis: a clinical review. Eur. J. Neurol. 27, 1918–1929. doi: 10.1111/ene.14393
Meinita M. D. N., Harwanto D., Choi J. S. (2022). Seaweed exhibits therapeutic properties against chronic diseases: an overview. Appl. Sci. (Switzerland) 12 (5), 2638. doi: 10.3390/app12052638
Menaa F., Wijesinghe U., Thiripuranathar G., Althobaiti N. A., Albalawi A. E., Khan B. A., et al. (2021). Marine algae-derived bioactive compounds: a new wave of nanodrugs?. Marine Drugs 19 (9), 484. doi: 10.3390/md19090484
Menaa F., Wijesinghe P. A. U. I., Thiripuranathar G., Uzair B., Iqbal H., Khan B. A., et al. (2020). Ecological and industrial implications of dynamic seaweed-associated microbiota interactions. Mar. Drugs 18, 641. doi: 10.3390/md18120641
Moghadas S. M., Fitzpatrick M. C., Sah P., Pandey A., Shoukat A., Singer B. H., et al. (2020). The implications of silent transmission for the control of COVID-19 outbreaks. Proc. Natl. Acad. Sci. 117, 17513–17515. doi: 10.1073/pnas.2008373117
Muzio L., Viotti A., Martino G. (2021). Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Front. Neurosci. 15. doi: 10.3389/fnins.2021.742065
Nesic A. R., Seslija S. I. (2017). The influence of nanofillers on physical–chemical properties of polysaccharide-based film intended for food packaging. Food Packaging, 637–697. Academic Press (UK & USA). doi: 10.1016/B978-0-12-804302-8.00019-4
Nho J. A., Shin Y. S., Jeong H. R., Cho S., Heo H. J., Kim G. H., et al. (2020). Neuroprotective effects of phlorotannin-rich extract from brown seaweed ecklonia cava on neuronal PC-12 and SH-SY5Y cells with oxidative stress. J. Microbiol. Biotechnol. 30, 359. doi: 10.4014/jmb.1910.10068
Nyiew K.-Y., Ngu E.-L., Wong K.-H., Goh B.-H., Yow Y.-Y. (2023). “Neuroprotective potential of marine algal antioxidants,” in Marine antioxidants (UK & USA: Academic Press), 341–353. doi: 10.1016/B978-0-323-95086-2.00030-8
Oh S., Kim S., Jung K., Pham T. N. A., Yang S., Ahn B. (2022). Potential prebiotic and anti-obesity effects of codium fragile extract. Appl. Sci. (Switzerland) 12 (3), 959. doi: 10.3390/app12030959
Olasehinde T. A., Olaniran A. O., Okoh A. I. (2020). Sulfated polysaccharides of some seaweeds exhibit neuroprotection via mitigation of oxidative stress, cholinergic dysfunction and inhibition of Zn–induced neuronal damage in HT-22 cells. BMC complementary Med. therapies 20, 1–10. doi: 10.1186/s12906-020-03047-7
Oxtoby N. P., Leyland L. A., Aksman L. M., Thomas G. E. C., Bunting E. L., Wijeratne P. A., et al. (2021). Sequence of clinical and neurodegeneration events in Parkinson’s disease progression. Brain 144, 975–988. doi: 10.1093/brain/awaa461
Pagarete A., Ramos A. S., Puntervoll P., Allen M. J., Verdelho V. (2021). Antiviral potential of algal metabolites—a comprehensive review. Marine Drugs 19 (2), 94. doi: 10.3390/md19020094
Pan H., Qiu H., Zhang K., Zhang P., Liang W., Yang M., et al. (2019). Fascaplysin derivatives are potent multitarget agents against alzheimer’s disease: in vitro and in vivo evidence. ACS Chem. Neurosci. 10, 4741–4756. doi: 10.1021/acschemneuro.9b00503
Parsons M. P., Raymond L. A. (2015). “Huntington disease,” in Neurobiology of Brain Disorders (Elsevier), 303–320. doi: 10.1016/B978–0-12–398270–4.00020–3
Paudel P., Seong S. H., Wu S., Park S., Jung H. A., Choi J. S. (2019). Eckol as a potential therapeutic against neurodegenerative diseases targeting dopamine D3/D4 receptors. Mar. Drugs 17 (2), 108. doi: 10.3390/md17020108
Peng Y., Hu J., Yang B., Lin X.-P., Zhou X.-F., Yang X.-W., et al. (2015). “Chemical composition of seaweeds,” in Seaweed Sustainability (UK & USA: Elsevier), 79–124. doi: 10.1016/B978–0-12–418697–2.00005–2
Pérez M. J., Falqué E., Domínguez H. (2016). Antimicrobial action of compounds from marine seaweed. Mar. Drugs 14, 52. doi: 10.3390/md14030052
Pérez-Andrés J. M., Álvarez C., Cullen P. J., Tiwari B. K. (2019). Effect of cold plasma on the techno-functional properties of animal protein food ingredients. Innovative Food Sci. Emerging Technol. 58, 102205. doi: 10.1016/j.ifset.2019.102205
Picca A., Guerra F., Calvani R., Romano R., Coelho-Júnior H. J., Bucci C., et al. (2021). Mitochondrial dysfunction, protein misfolding and neuroinflammation in Parkinson’s disease: Roads to biomarker discovery. Biomolecules 11 (10), 1508. doi: 10.3390/biom11101508
Pradhan B., Nayak R., Patra S., Jit B., Ragusa A., Molecules M. J. (2020). Bioactive metabolites from marine algae as potent pharmacophores against oxidative stress-associated human diseases: A comprehensive review. Molecules 26 (1), 37. doi: 10.3390/molecules26010037
Probst Y. (2015). A review of the nutrient composition of selected rubus berries. Nutr. Food Sci. 45, 242–254. doi: 10.1108/NFS-07-2014-0063
Qin Y., Jiang J., Zhao L., Zhang J., Wang F. (2018). Applications of alginate as a functional food ingredient. Biopolymers Food Design, 409–429. doi: 10.1016/B978-0-12-811449-0.00013-X
Qu M., Jiang Z., Liao Y., Song Z., Nan X. (2016). Lycopene prevents amyloid [Beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochemical Res. 41, 1354–1364. doi: 10.1007/s11064-016-1837-9
Rahman S. O., Panda B. P., Parvez S., Kaundal M., Hussain S., Akhtar M., et al. (2019). Neuroprotective role of astaxanthin in hippocampal insulin resistance induced by Aβ peptides in animal model of Alzheimer’s disease. Biomedicine Pharmacotherapy = Biomedecine Pharmacotherapie 110, 47–58. doi: 10.1016/j.biopha.2018.11.043
Ramesh Kumar B., Deviram G., Mathimani T., Duc P. A., Pugazhendhi A. (2019). Microalgae as rich source of polyunsaturated fatty acids. Biocatalysis Agric. Biotechnol. 17, 583–588. doi: 10.1016/j.bcab.2019.01.017
Ramos-Romero S., Torrella J. R., Pagès T., Viscor G., Torres J. L. (2021). Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 13, 563. doi: 10.3390/nu13020563
Rengasamy K. R. R., Amoo S. O., Aremu A. O., Stirk W. A., Gruz J., Šubrtová M., et al. (2015). Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycology 27, 1599–1605. doi: 10.1007/s10811-014-0438-8
Rengasamy K. R., Mahomoodally M. F., Aumeeruddy M. Z., Zengin G., Xiao J., Kim D. H. (2020). Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 135, 111013. doi: 10.1016/j.fct.2019.111013
Rivanor R. L., da C., Do Val D. R., Ribeiro N. A., Silveira F. D., de Assis E. L., et al. (2018). A lectin fraction from green seaweed Caulerpa cupressoides inhibits inflammatory nociception in the temporomandibular joint of rats dependent from peripheral mechanisms. Int. J. Biol. Macromolecules, 115, 331–340. doi: 10.1016/j.ijbiomac.2018.04.065
Sá Monteiro M., Sloth J., Holdt S., Hansen M. (2019). Analysis and risk assessment of seaweed. EFSA J. 17 (S2), e170915. doi: 10.2903/j.efsa.2019.e170915
Sathasivam R., Radhakrishnan R., Hashem A., Abd_Allah E. F. (2019). Microalgae metabolites: A rich source for food and medicine. Saudi J. Biol. Sci. 26, 709–722. doi: 10.1016/j.sjbs.2017.11.003
Scarpignato C., Dolak W., Lanas A., Matzneller P., Renzulli C., Grimaldi M., et al. (2017). Rifaximin reduces the number and severity of intestinal lesions associated with use of nonsteroidal anti-inflammatory drugs in humans. Gastroenterology 152, 980–982.e3. doi: 10.1053/j.gastro.2016.12.007
Schepers M., Martens N., Tiane A., Vanbrabant K., Liu H. B., Lütjohann D., et al. (2020). Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regeneration Res. 15, 790–795. doi: 10.4103/1673-5374.268894
Schmucker S., Puccio H. (2010). Understanding the molecular mechanisms of Friedreich’s ataxia to develop therapeutic approaches. Hum. Mol. Genet. 19, R103–R110. doi: 10.1093/hmg/ddq165
Seeley W. W. (2017). Mapping neurodegenerative disease onset and progression. Cold Spring Harbor Perspect. Biol. 9, a023622. doi: 10.1101/cshperspect.a023622
Seo D. O., Boros B. D., Holtzman D. M. (2019). The microbiome: A target for Alzheimer disease? Cell Res. 29, 779–780. doi: 10.1038/s41422–019-0227–7
Shanmuganathan B., Suryanarayanan V., Sathya S., Narenkumar M., Singh S. K., Ruckmani K., et al. (2018). Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Medicinal Chem. 143, 1196–1207. doi: 10.1016/j.ejmech.2017.10.017
Shimizu H., Koyama T., Yamada S., Lipton S. A., Satoh T. (2015). Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 457, 718. doi: 10.1016/j.bbrc.2015.01.059
Silva J., Alves C., Freitas R., Martins A., Pinteus S., Ribeiro J., et al. (2019). Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs, 17 (2), 85. doi: 10.3390/md17020085
Silva L. M. C. M., Lima V., Holanda M. L., Pinheiro P. G., Rodrigues J. A. G., Lima M. E. P., et al. (2010). Antinociceptive and anti-inflammatory activities of lectin from marine red alga Pterocladiella capillacea. Biol. Pharm. Bull. 33, 830–835. doi: 10.1248/bpb.33.830
Singh R., Parihar P., Singh M., Bajguz A., Kumar J., Singh S., et al. (2017). Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: Current status and future prospects. Front. Microbiol. 8. doi: 10.3389/fmicb.2017.00515
Soleymaninejad M., Joursaraei S. G., Feizi F., Jafari Anarkooli I. (2017). The effects of lycopene and insulin on histological changes and the expression level of bcl-2 family genes in the hippocampus of streptozotocin-induced diabetic rats. J. Diabetes Res. 2017, 1–9. doi: 10.1155/2017/4650939
Souza R. B., Frota A. F., Silva J., Alves C., Neugebauer A. Z., Pinteus S., et al. (2018). In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromolecules 112, 1248–1256. doi: 10.1016/j.ijbiomac.2018.02.029
Spencer J. P. E., Vafeiadou K., Williams R. J., Vauzour D. (2012). Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Aspects Med. 33, 83–97. doi: 10.1016/j.mam.2011.10.016
Suganthy N., Pandian S. K., Devi K. P. (2010). Neuroprotective effect of seaweeds inhabiting South Indian coastal area (Hare Island, Gulf of Mannar Marine Biosphere Reserve): Cholinesterase inhibitory effect of Hypnea valentiae and Ulva reticulata. Neurosci. Lett. 468, 216–219. doi: 10.1016/j.neulet.2009.11.001
Sun X., Zhao H., Liu Z., Sun X., Zhang D., Wang S., et al. (2020). Modulation of gut microbiota by fucoxanthin during alleviation of obesity in high-fat diet-fed mice. J. Agric. Food Chem. 68, 5118–5128. doi: 10.1021/acs.jafc.0c01467
Syed Y. Y. (2020). Sodium oligomannate: first approval. Drugs 80, 441–444. doi: 10.1007/s40265-020-01268-1
Takeshita S., Oda T. (2016). Usefulness of alginate lyases derived from marine organisms for the preparation of alginate oligomers with various bioactivities. Adv. Food Nutr. Res. 79, 137–160. doi: 10.1016/bs.afnr.2016.07.003
Tariverdian T., Navaei T., Milan P. B., Samadikuchaksaraei A., Mozafari M. (2019). Functionalized polymers for tissue engineering and regenerative medicines. Advanced Funct. Polymers Biomed. Appl., 323–357. doi: 10.1016/B978-0-12-816349-8.00016-3
Tirtawijaya G., Nazmul Haque M., Choi J. S., Moon I. S., Meinita M. D. N., Choi J. S., et al. (2019). Spinogenesis and synaptogenesis effects of the red seaweed kappaphycus alvarezii and its isolated cholesterol on hippocampal neuron cultures. Prev. Nutr. Food Sci. 24, 418. doi: 10.3746/pnf.2019.24.4.418
Upadhyay S. K., Singh S. P. (2021). Bioprospecting of Plant Biodiversity for Industrial Molecules (USA: John Wiley & Sons). Available at: http://books.google.ie/books?id=Q_w2EAAAQBAJ&printsec=frontcover&dq=Dolui,+A.+K.+(2021).+Marine+Bioprospecting.+Bioprospecting+of+Plant+Biodiversity+for+Industrial+Molecules.&hl=&cd=1&source=gbs_api. doi: 10.1002/9781119718017
Uzair B., Liaqat A., Iqbal H., Menaa B., Razzaq A., Thiripuranathar G., et al. (2020). Green and cost-effective synthesis of metallic nanoparticles by algae: safe methods for translational medicine. Bioengineering 7, 129. doi: 10.3390/bioengineering7040129
Vanbrabant K., Van Meel D., Kerksiek A., Friedrichs S., Dubbeldam M., Schepers M., et al. (2021). 24(R, S)-Saringosterol-From artifact to a biological medical agent. J. Steroid Biochem. Mol. Biol. 212, 105942. doi: 10.1016/j.jsbmb.2021.105942
Wang X., Chen W., Fu X., Ma J., Wang M., Hou Y., et al. (2018). Reversal of homocysteine-induced neurotoxicity in rat hippocampal neurons by astaxanthin: evidence for mitochondrial dysfunction and signaling crosstalk. Cell Death Discovery 4, 50. doi: 10.1038/s41420-018-0114-x
Wang Y., Chen R., Yang Z., Wen Q., Cao X., Zhao N., et al. (2022). Protective effects of polysaccharides in neurodegenerative diseases. Front. Aging Neurosci. 14, 917629. doi: 10.3389/fnagi.2022.917629
Wang X., Sun G., Feng T., Zhang J., Huang X., Wang T., et al. (2019). Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 29, 787–803. doi: 10.1038/s41422-019-0216-x
Wang Y., Xing M., Cao Q., Ji A., Liang H., Song S. (2019). Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 17 (3), 183. doi: 10.3390/md17030183
Wan-Loy C., Siew-Moi P. (2016). Marine algae as a potential source for anti-obesity agents. Mar. Drugs 14, 222. doi: 10.3390/md14120222
Weiner M. L. (2014). Food additive carrageenan: Part II: A critical review of carrageenanin vivosafety studies. Crit. Rev. Toxicol. 44, 244–269. doi: 10.3109/10408444.2013.861798
Wells M. L., Potin P., Craigie J. S., Raven J. A., Merchant S. S., Helliwell K. E., et al. (2017). Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycology 29, 949–982. doi: 10.1007/s10811-016-0974-5
Wong C. H., Gan S. Y., Tan S. C., Gany S. A., Ying T., Gray A. I., et al. (2018). Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglia. J. Appl. Phycology 30, 3261–3270. doi: 10.1007/s10811-018-1495-1
Wozniak M., Bell T., Dénes Á., Falshaw R., Itzhaki R. (2015). Anti-HSV1 activity of brown algal polysaccharides and possible relevance to the treatment of Alzheimer’s disease. Int. J. Biol. Macromolecules 74, 530–540. doi: 10.1016/j.ijbiomac.2015.01.003
Xu S. Y., Huang X., Cheong K. L. (2017). Recent advances in marine algae polysaccharides: Isolation, structure, and activities. Mar. Drugs 15 (12), 388. doi: 10.3390/md15120388
Yang M., Jin L., Wu Z., Xie Y., Zhang P., Wang Q., et al. (2021). PLGA-PEG nanoparticles facilitate in vivo anti-alzheimer’s effects of fucoxanthin, a marine carotenoid derived from edible brown algae. J. Agric. Food Chem. 69, 9764–9777. doi: 10.1021/acs.jafc.1c00569
Yang P., Liu D.-Q., Liang T.-J., Li J., Zhang H.-Y., Liu A.-H., et al. (2014). doi: 10.1016/j.bmc.2014.11.031
Ye J. Y., Li L., Hao Q. M., Qin Y., Ma C. S. (2020). β-Sitosterol treatment attenuates cognitive deficits and prevents amyloid plaque deposition in amyloid protein precursor/presenilin 1 mice. Korean J. Physiol. Pharmacology: Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 24, 39–46. doi: 10.4196/KJPP.2020.24.1.39
Zargarzadeh M., Amaral A. J. R., Custódio C. A., Mano J. F. (2020). Biomedical applications of laminarin. Carbohydr. Polymers 232, 15774. doi: 10.1016/j.carbpol.2019.115774
Zeng X. S., Geng W. S., Jia J. J., Chen L., Zhang P. P. (2018). Cellular and molecular basis of neurodegeneration in parkinson disease. Front. Aging Neurosci. 10. doi: 10.3389/fnagi.2018.00109
Zhang L., Hao J., Zheng Y., Su R., Liao Y., Gong X., et al. (2018). Fucoidan protects dopaminergic neurons by enhancing the mitochondrial function in a rotenone-induced rat model of Parkinson’s disease. Aging Dis. 9, 590. doi: 10.14336/AD.2017.0831
Zhang J., Pu H., Zhang H., Wei Z., Jiang X., Xu M., et al. (2017). Inhibition of Na + -K + -2Cl – cotransporter attenuates blood-brain-barrier disruption in a mouse model of traumatic brain injury. Neurochemistry Int. 111, 23–31. doi: 10.1016/j.neuint.2017.05.020
Zhang L., Wang H., Fan Y., Gao Y., Li X., Hu Z., et al. (2017). Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 7, 46763. doi: 10.1038/srep46763
Zhang F., Xing S., Li Z. (2017). Antagonistic effects of lycopene on cadmium-induced hippocampal dysfunctions in autophagy, calcium homeostatis and redox. Oncotarget 8, 44720–44731. doi: 10.18632/oncotarget.v8i27
Zhang H., Zhang F., Yuan R. (2020). Applications of natural polymer-based hydrogels in the food industry. Hydrogels Based Natural Polymers, 357–410. doi: 10.1016/B978–0-12–816421–1.00015-X
Keywords: marine algae, algal metabolites, neuroprotection, neuroinflammation, therapeutics
Citation: Naik B, Richa S, Bharadwaj S, Mishra S, Kumar V, Kumar V, Saris PEJ, Gupta AK, Mishra R, Gupta U, Rustagi S and Preet MS (2024) Utilizing marine algal metabolites to fight neurodegenerative diseases. Front. Mar. Sci. 11:1370839. doi: 10.3389/fmars.2024.1370839
Received: 15 January 2024; Accepted: 23 April 2024;
Published: 24 May 2024.
Edited by:
Suresh Veeraperumal, Upstate Medical University, United StatesReviewed by:
Perumal Karthick, Sea6 Energy Pvt Ltd., IndiaSachithanandam Veeraragavan, National Centre for Sustainable Coastal Management, India
Arunkumar Venkatesan, Upstate Medical University, United States
Pazhanichamy Kalailingam, Massachusetts General Hospital and Harvard Medical School, United States
Copyright © 2024 Naik, Richa, Bharadwaj, Mishra, Kumar, Kumar, Saris, Gupta, Mishra, Gupta, Rustagi and Preet. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vijay Kumar, dmlqYXlna3BAZ21haWwuY29t; Per Erik Joakim Saris, cGVyLnNhcmlzQGhlbHNpbmtpLmZp
 Bindu Naik
Bindu Naik Shruti Richa2
Shruti Richa2 Shivangi Bharadwaj
Shivangi Bharadwaj Vijay Kumar
Vijay Kumar Vivek Kumar
Vivek Kumar Per Erik Joakim Saris
Per Erik Joakim Saris Arun Kumar Gupta
Arun Kumar Gupta Sarvesh Rustagi
Sarvesh Rustagi Manpreet Singh Preet
Manpreet Singh Preet