
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 27 February 2024
Sec. Marine Conservation and Sustainability
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1337392
 Donghyun Hong1
Donghyun Hong1 Jeong-Soo Gim1
Jeong-Soo Gim1 Gea-Jae Joo2
Gea-Jae Joo2 Dong-Kyun Kim3
Dong-Kyun Kim3 Daehyun Choi4
Daehyun Choi4 Hak-Young Lee5
Hak-Young Lee5 Kwang-Seuk Jeong6
Kwang-Seuk Jeong6 Hyunbin Jo1,7*
Hyunbin Jo1,7*Estuary reopening is a means of restoring estuarine habitats, which has recently been implemented in a few developed countries. The regeneration of a brackish zone in the Nakdong River Estuary (NRE), South Korea, were tested through a series of barrage reopening. During the same period, we conducted extensive fish surveys in the upper part of the NRE barrage on a monthly basis from 2017 to 2021, and subsequently determined whether fish populations and communities were affected by the reopening. The results showed that the reopening of the NRE hardly affected the fish community structure, as non-native species such as Erythroculter erythropterus and Lepomis macrochirus maintained their dominance. Still, we discovered that certain euryhaline species are positively affected by estuary reopening, as total 46 Japanese eels (Anguilla japonica) were captured after the reopening, which had not been detected before the reopening. By comparing the size structure of various fish species, we discovered that size distribution of native and migratory species presented more positively skewed pattern after the reopening, while size structure in non-native species remained relatively unchanged normally distributed pattern. Piecewise structural equation modelling revealed that the NRE had become more complex ecosystem, as migratory fish species abundance and biomass started to show a positive correlation with hydraulic factors such as discharge and negative correlation with seasonality after the reopening. We concluded that estuary reopening created some changes in migratory and native freshwater species but such changes were not notably detected in non-native species. Therefore, appropriate sluice operation methodologies, such as considering the migration seasons of migratory species, should be developed. Additionally, human-involved management policies are required to regulate non-native species populations.
Estuaries are the only ecosystems in which saltwater and freshwater exchange biological, physical, and chemical substances (Pinckney et al., 2001). Therefore, estuaries form diverse habitat types, such as brackish zones, tidal flats, and salt marshes. These multiple associated habitat types can play important roles in the diverse biota. They provide protection from predators (Whitfield, 1999; McLusky and Elliott, 2004), nursery grounds (Yagi et al., 2011), reproductive grounds, refugia, and feeding areas where survival, development, and growth are potentially optimised (Elliott and Dewailly, 1995; Able, 2005; Martinho et al., 2007). Regardless of their ecological value, estuaries are vulnerable to various disturbances. The primary factor in many of these disturbances is anthropogenic activities due to economic activities, land reclamation, and the construction of estuarine barrages (Connell et al., 1981; Yoon et al., 2016).
The construction of artificial transverse structures has significantly contributed to human development (World Commission on Dams, 2000) but has had significant impacts on hydrological processes, such as increasing the residence time of contaminants (Dynesius and Nilsson, 1994; Wright and Worrall, 2001), and biological responses, such as species composition shifts (Gao et al., 2019). The properties of closed estuaries created by the construction of vertical structures have created disconnections between rivers and seas, converting estuaries into lake-like systems (Almodóvar and Nicola, 1999; Agostinho et al., 2004), with implications for their physicochemical properties and species assemblages, often leading to biodiversity degradation (Roshni et al., 2021).
One solution to restore biodiversity in closed estuaries is to remove the estuarine barrage and reopen the estuary to satisfy sea–freshwater circulation (van Puijenbroek et al., 2019). Such attempts are gaining global attention as they improve biodiversity (Barnes et al., 2008) and remove physical obstacles, thus enhancing the mobility of migratory species (van Wichelen et al., 2021). Indeed, the permanent opening of the Haringvliet sluices in the Netherlands led to the successful regain of historical biota (Baas et al., 2020; Beekelaar et al., 2020).
In South Korea, estuarine barrages have been established for water management (i.e., droughts and floods) and the prevention of salt intrusion to facilitate agricultural and industrial water supplies. In the Nakdong River Estuary (NRE), the longest river in South Korea, a barrage was built in 1987, along with eight additional large-scale weirs in the upper region. This led to a decrease in the downstream brackish water zone and habitat alterations owing to a shift from lotic to lentic ecosystems (Jo et al., 2019; Ko et al., 2022).
Moreover, freshwater fish species in the upper part of estuarine barrages are often threatened by poor connectivity between habitats, the emergence of non-native species and the disappearance of native and migratory species (Yoon et al., 2016). Non-native species has begun to dominate the freshwater-estuary ecosystem through trophic and spatial alterations (Jang et al., 2002) as they are typically better at adapting to disconnected habitats which are characterised as lotic and eutrophic environments (Jo et al., 2011). Together with the destruction of lotic and brackish zones, biodiversity degradation induced by non-native species has eventually led to a decrease in fisheries in NRE. The total fish catch has been steadily decreasing since the construction of the NRE barrage (Busan Metropolitan City, 2023).
To address habitat fragmentation, the Ministry of Environment and the Ministry of Oceans and Fishes in South Korea adopted new legislation, including minimal development around the estuary, and designated it as a protected area (Choi et al., 2021). However, the introduced efforts have not been effective because of the excessive demand for development and the lack of specific laws to protect the estuary as a singular ecosystem (Nam et al., 2010). Consequently, the Korea Water Resource Corporation (K-water) and Busan Metropolitan City (located around the Nakdong River and particularly close to the NRE) took measures to restore the estuarine habitat by promoting sea-freshwater circulation in the NRE and began to transiently open the sluice of the barrage (total of seven opening tests until 2021; minimum of 38 min and maximum of ~1 month).
Previous studies have shown that population shifts such as the emergence or disappearance of certain species may require a dense survey span (monthly or seasonally, Magilligan et al., 2016; Vasconcelos et al., 2021). Therefore, we set a monthly survey span with a relatively longer study period (five years) to obtain population shifts and resultant community changes. This study aimed to (1) identify changes at the population level (i.e. size structure changes, emergence of new species) and community level after the reopening of the NRE, (2) determine which physiochemical factors (e.g. temperature and aquatic conductivity) contributed to the fish community changes, and (3) offer appropriate suggestions for better estuarine barrage opening management to promote more natural migrations for fishes.
The Nakdong River is the longest river in South Korea, with a drainage area of approximately 23,384 km2 and length of 510 km. The NRE barrage, located at the lowest reach of the Nakdong River system (35°08’20.3”, 128°57’26.2”, in the south-eastern part of the Korean Peninsula) and 2-3km from the river mouth, was constructed from 1983 to 1987 to provide a sustainable water supply and control salt intrusion. Additionally, the NRE barrage functions as a bridge connecting the western and eastern regions, reducing traffic congestion. It is approximately 2.4 km long and 18.7 m high and can retain five million tons of water. The NRE barrage includes four regulating sluices (length: 47.5 m, height: 8.3 m) and six main sluices (length: 47.5 m, height: 9.2 m). The purpose of regulating the sluices is to release freshwater downstream during the rainy season (in most cases, July–August) and to maintain the water level at low tide (Figure 1). A boat pass and two fishways were constructed along the edge of the NRE barrage (Supplementary Figure S1A).
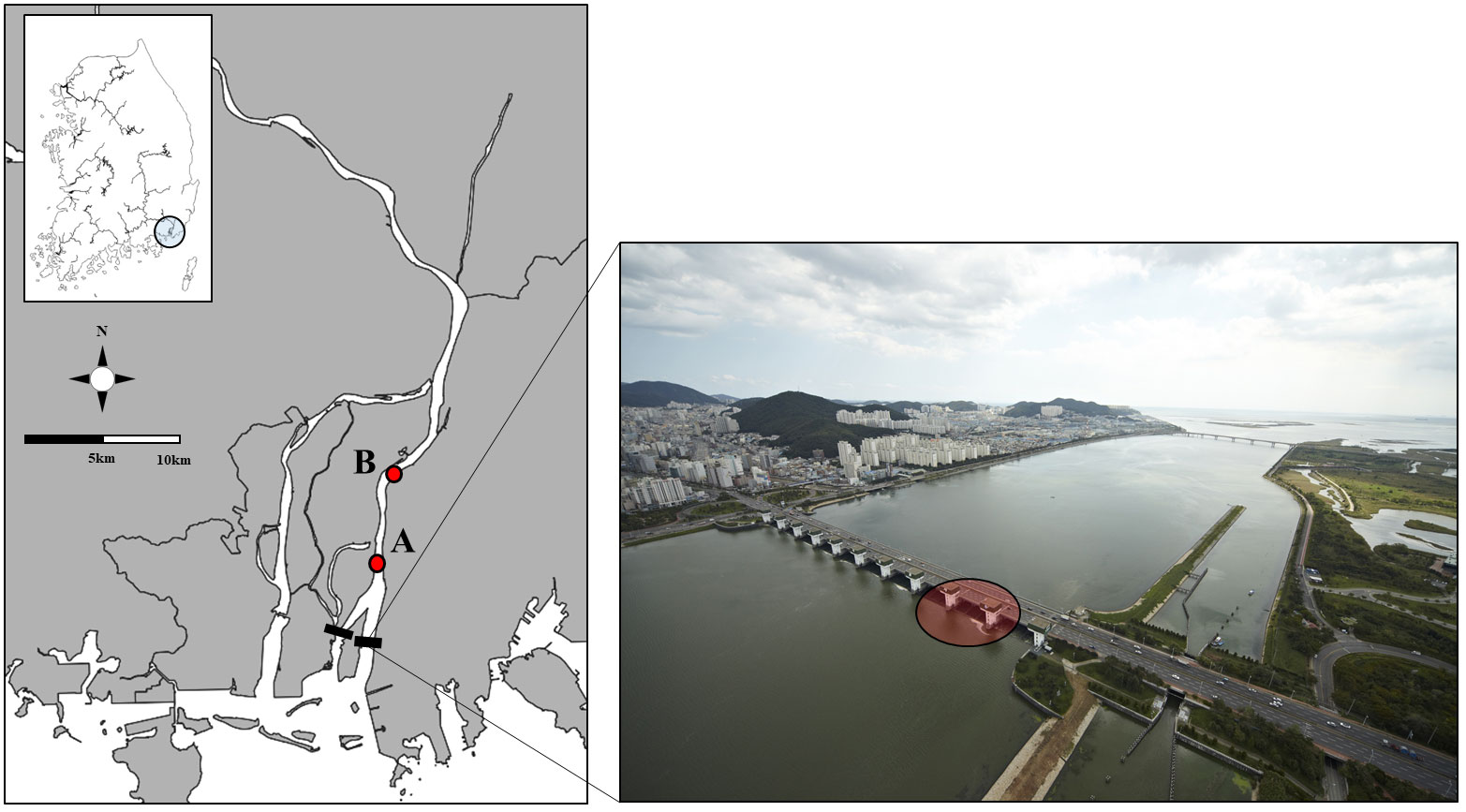
Figure 1 Illustrations of the Nakdong River Estuarine barrage and fish sampling sites (A, B). Left: Study sites (A) and (B) are situated 3.9 and 7.8 km away from the Nakdong River Estuarine barrage. Right: Regulating sluice (red circle) is utilised to regulate seawater intrusion.
To analyse the spatiotemporal changes in fish communities resulting from the reopening of the NRE, we conducted fish sampling at two study sites located upstream of the barrage. As described in Figure 1, Sites A and B were 3.9 and 7.8 km away from the barrage, respectively. Fish sampling was conducted monthly from 2017 to 2021. A considerable number of winter (December–February) samples were excluded because the study area is protected as a cultural heritage site for overwintering birds (total 8-9 samplings were conducted annually). Sampling was conducted at site A starting in 2017 and 41 samplings were conducted. In contrast, at site B, sampling began in 2020 and 11 samplings were conducted. This was because site B was set as a reference area for site A and surveys were only conducted after the NRE had been reopened. At each sampling site, fish were collected by local fishermen using a commercial fyke net (mesh:10 × 10 mm; three sides; leader net:10m) that was deployed in the water for 48 h (Supplementary Figure S2A). A fyke net was deployed in the riparian zone and fish were collected from different preferred environments (bushes, rocks, etc.). To further detect the impact of the estuary reopening, we conducted longline fishing before and during the reopening period, with a line of approximately 200 m in length and fishing hooks at 3-m intervals deployed at the same location as the fyke nets and left in the water for 24 h (Supplementary Figure S2B). Starting in 2020, one longline fishing gear was deployed at sites A and B and was deployed 10 times during this period (Figure 1).
The morphological parameters (length ± 0.1 cm, weight ± 0.1 g) of the collected fish were measured after capture. If a particular species was captured too many, only about 30 individuals were randomly measured for that species per each survey. All species were released afterwards except for non-native species, such as largemouth bass (Micropterus salmoides) and blue gill (Lepomis macrochirus), as their release is prohibited by the act on protecting native and endemic species (Act on the Conservation and Use of Biological Diversity, Act No. 14513, December 27, 2016, by the Ministry of the Environment).
The opening procedure of the NRE barrage comprises several phases and methods. Only one regulatory sluice (sluice 9) was used to open the NRE (Figure 1 and Supplementary Figure S1B), operated by opening the lower (underflow method, Supplementary Figure S1C) or upper (overflow method, Figures A1-d) chamber of the sluice. Seven openings were conducted from 2019 to 2021 (Table 1), with 19,460,000 tons of intruded seawater. In 2019, the first sluice opening began on 6th June and lasted for 38 min using the underflow method (640,000 tons, 3.3%). The second opening lasted for 1 h, using a combination of overflow and underflow (1,010,000 tons, 5.2%).
Starting in 2020, the sluice openings proceeded with a significantly greater magnitude and duration. For the reopening in 2020, a total of 9,300,000 tons of seawater intruded into the inner part of the NEB, which accounted for approximately 47.8% of the total amount of seawater intruded during the study period. The NRE reopening in 2020 lasted for approximately one month, and different methodologies were applied (Supplementary Figure S3). During the first spring tide, the regulatory sluice stopped when the seawater level surpassed the freshwater level, intermittently introducing 2,580,000 tons of seawater using the overflow method. After the first spring tide, the overflow method ceased and the underflow method was used for the remaining tides (two neaps and another spring tide in between). In this case, although the regulatory sluice was continuously open, seawater did not flow into the freshwater part of the estuarine barrage because the level of seawater was lower than that of the river water. Seawater can only flow into the river during the second spring tide (1–3 h/day), totalling 6,720,000 tons. During both open periods, we anticipated that fish migration would ease through seawater inflow. In 2021, the sluice was opened four times. The methodology for opening the regulatory sluice was similar to that applied in 2020. Therefore, we assumed that the magnitude of seawater intrusion significantly increased from the 3rd sluice opening (June 2020) and compared the changes in the fish community caused by seawater intrusion based on the 3rd sluice opening event.
The fish species collected were classified into two lifestyle types: freshwater and migratory. Freshwater species were further categorised into two subcategories (native and non-native) for more detailed analysis. Lifestyle classification was performed using FishBase (Froese and Pauly, 2022; http://www.Fishbase.Org/). All water quality variables were obtained from the Water Environment Information System (https://water.nier.go.kr/web). Specifically, we utilised eight water quality parameters; water temperature (°C), dissolved oxygen (DO, mg/L), pH, total nitrogen (TN, mg/L), total phosphorus (TP, mg/L), biological oxygen demand (BOD; mg/L), chemical oxygen demand (COD, mg/L) and suspended solid (SS, mg/L). For hydraulic data conductivity (μS cm/L) and discharge (m3/s), we referred to K-water (K-water, 2022).
To detect size structural changes in fish species before and after the reopening of the NRE, we drew size-frequency distribution histograms on the most abundant fish species from all subcategories using Excel 2013. Chi-square distance (x2d) analysis was used to verify the significance of the distance. The x2d equation is as follows:
Where n is the number of bins, is the value in the first bin, and is the value in the second. This is a simple and fast approach to rank our histogram bins and was chosen because it is a suitable model for examining distributional changes when comparing two or more clusters and for measuring the similarity between two groups (Chung et al., 1989).
To elucidate the relationships between abiotic environmental, hydraulic, and fish community before and after the reopening of the NRE, we conducted piecewise structural equation modelling (piecewise SEM; Lefcheck, 2016) using R software version 4.21 using the lavaan tool (Gana and Broc, 2019). For the environmental factors, we applied principal component analysis (PCA) to the eight water quality parameters mentioned and investigated the coefficient loading of the eight water quality parameters using Paleontological Statistics software (PAST, version 4.05, Hammer et al., 2001). We selected principal component axes with an eigenvalue of 1 and above, and therefore chose three axes (PC1, PC2, and PC3). We found that seasonality (summer season feature) best described PC1, whereas productivity and nutrients best described PC2 and PC3, respectively (Supplementary Figure S4). Therefore, to simplify the model, we used three variables including eight water quality parameters. For the hydraulic factors, we used two variables: conductivity and discharge. Furthermore, fish community parameters such as freshwater and migratory species abundance/biomass were utilised, and the Shannon diversity index (Shannon, 1948) was utilised simultaneously. All variables used in the piecewise SEM analyses were standardised.
The direction of the model pathways was constructed considering aquatic ecosystem factors known to influence fish population structure (Wetzel, 1995). Insignificant pathways were sequentially deleted or altered until the final model with the lowest AIC was obtained. We classified all variables into three levels: ecosystem functioning groups (conductivity, discharge, seasonality, productivity, and nutrients), abundance and biomass (freshwater species abundance, migratory species abundance, freshwater species biomass, and migratory species biomass), and species diversity. We checked for multicollinearity based on each predictor’s variance inflation factor (VIF) for each predictor. No predictor showed a VIF > 3.0. The fit of paths in the models was obtained by maximum likelihood and the model fit was evaluated using Shipley’s test of d-separation and Fisher’s C-statistic, with P > 0.05, indicating an adequate model. Bivariate scatter plots were examined to determine the linear relationships between fish sampling data, ecosystem functioning components, and species diversity. Pearson correlations derived from the preliminary analyses were also utilised in the model development process. Prior to building the SEM models, identified outliers were removed (n ≤ 4).
Figure 2 shows the seawater intrusion effect owing to the opening of the regulatory sluice. Before seawater intrusion occurred, the salinity inside the NER barrage (freshwater part) was approximately 0.1 psu, whereas at the 0–1 km upstream barrage, it was approximately 0.5 psu. In the first seawater intrusion event, where only 640,000 tons of seawater were intruded, a 1–2-m-thick saline (>2 psu) layer formed at the riverbed up to 6–8 km upstream of the barrage. However, during the third seawater intrusion, 9,300,000 tons of seawater intruded as far as 12.4 km upstream from the barrage. In both seawater intrusion cases, although the magnitudes of the vertical salinity distributions were different, they showed relatively similar patterns; thus, high salinity was recorded in the bottom layer, creating a 1–4 m thick salinity layer. After every seawater intrusion, the intruded seawater was gradually diluted or washed away because of heavy rain. Additionally, Water quality parameters were compared before and after the reopening. All water quality parameters except for TN were not significantly changed (Table A1).
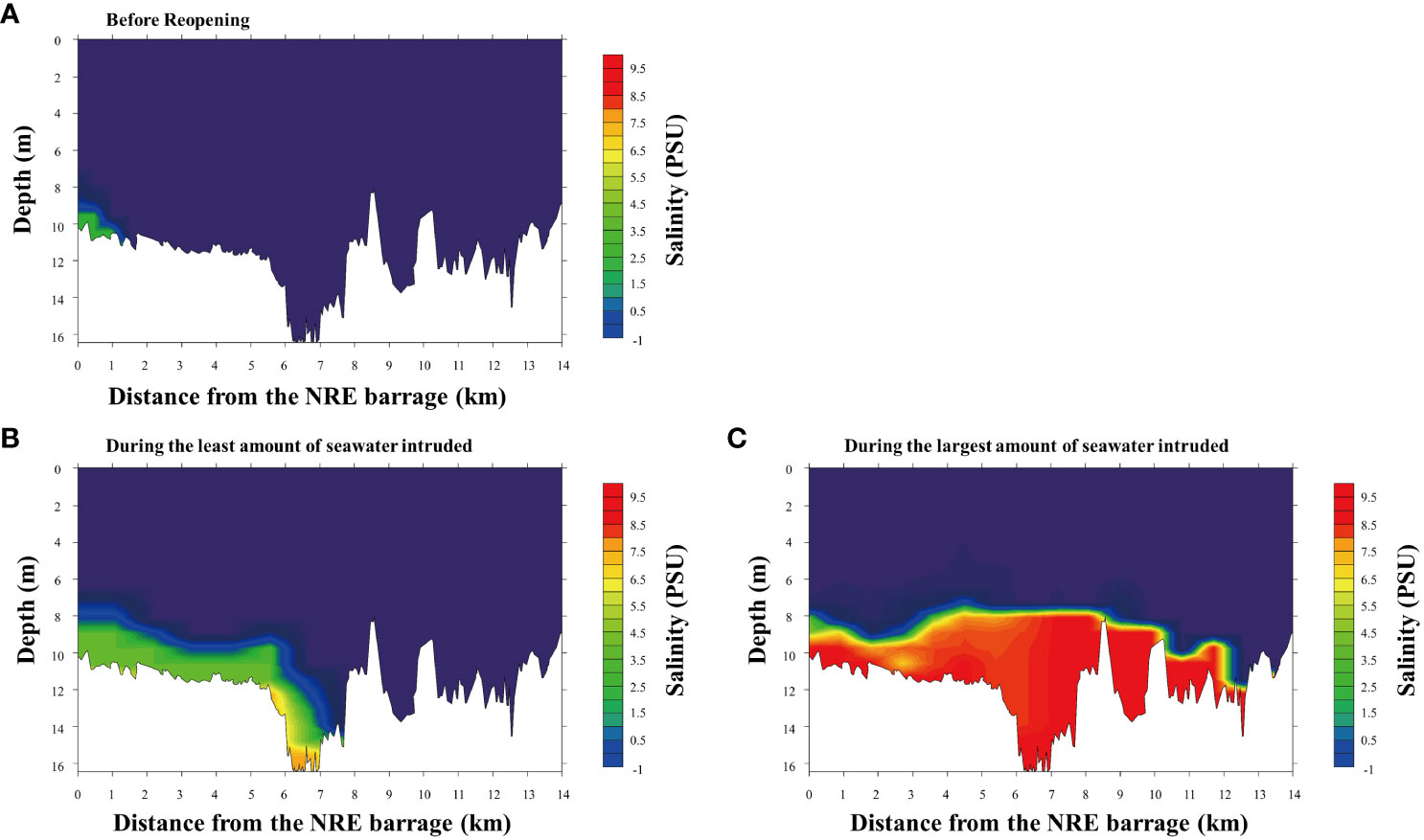
Figure 2 Salinity upstream of the Nakdong River Estuary barrage after the intrusion of different amounts of seawater. (A) Before the seawater intrusion (low salinity near the barrage and salinity below 0.1 psu in other areas). (B) After the intrusion of 640,000 tons of seawater. (C) After the intrusion of 9,300,000 tons of seawater.
Using fyke nets, total 11,119 fish belonging to 12 families and 25 species were collected and identified from 2017 to 2021 (Table A2). Before reopening, 7,143 fish belonging to 10 families and 21 species were collected. Among them, Erythroculter erythropterus, a non-native species, was dominant in the lower NRE (Figure 3 and Supplementary Figure S2C), showing the relative abundance (RA) of 90.3%. The RA was followed by another non-native species, Lepomis macrochirus, with a RA of 4.4%. Among the native species, Hemibarbus labeo had the highest RA (1.6%) followed by Hemiculter eigenmanni (0.36%). Additionally, the migratory species with the highest RA was Lateolabrax maculatus (0.95%), followed by Mugil cephalus (0.17%; Table A3). After reopening, 3,976 fish belonging to 10 families and 23 species were collected. The RA of E. erythropterus slightly decreased to 80.8%, but RA of L. macrochirus increased to 7.2%. For native species, RA of both H. labeo and H. eigenmanni marked 3.4%. The RA of both migratory species L. maculatus and M. celphalus slightly increased to 1.2% and 0.88% respectively (Table A3). The average Shannon diversity index slightly increased after the reopening but not significant (Table A1).
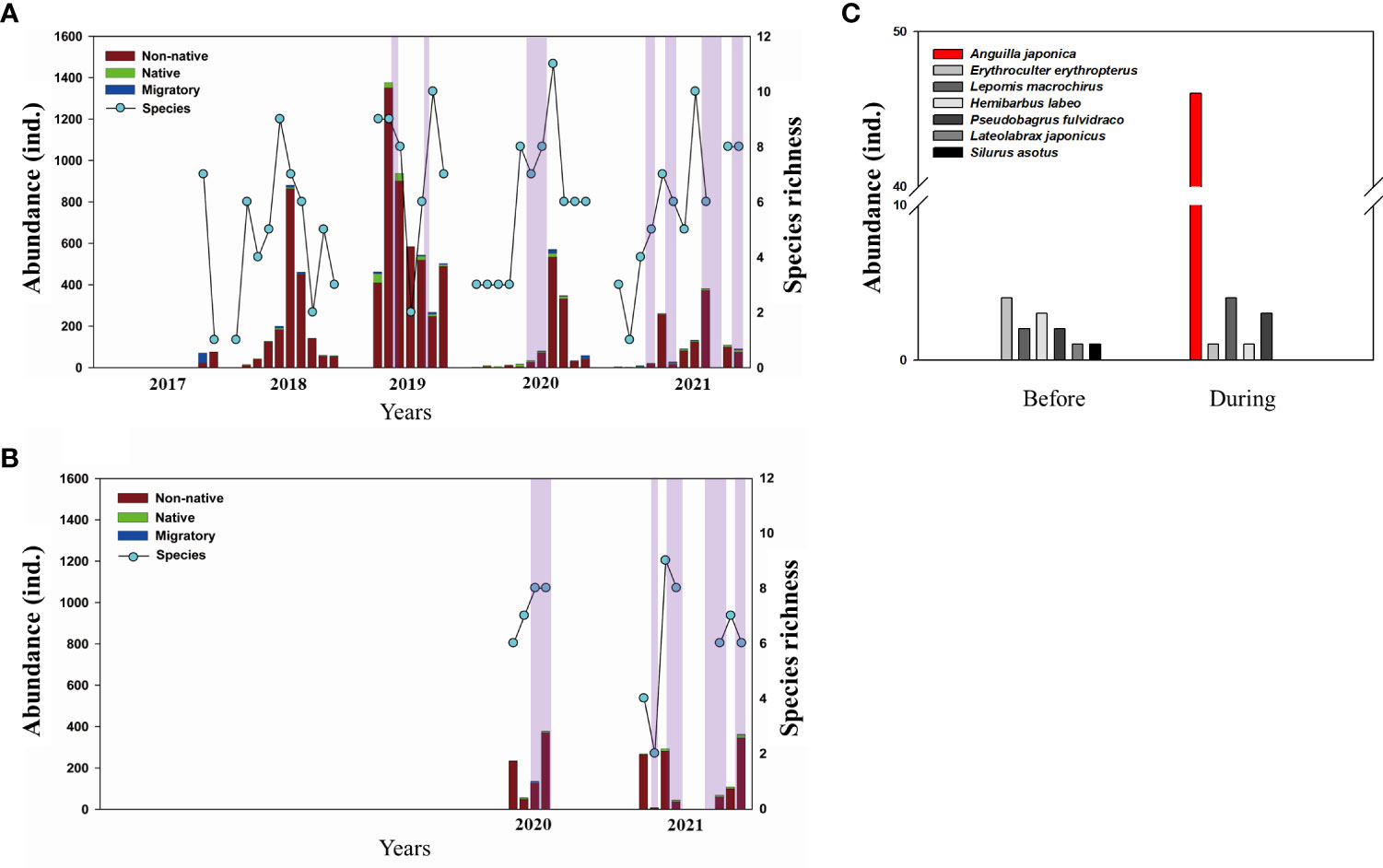
Figure 3 Fish community trends during the survey. The purple shaded areas indicate the period of the reopening Nakdong River Estuary: (A, B) show fish abundance collected from the fyke net samplings at study sites A and B, respectively; (C) results of longline fishing conducted before and during the opening at study sites A and B (before n = 10, during n = 10). A total of 46 Japanese eels (Anguilla japonica) in the yellow eel phase were captured when the Nakdong River Estuary is reopen.
In general, the abundance of fish at site A displayed a slightly decreasing pattern after the reopening and more individuals tended to be collected during the summer (Figure 3A). Meanwhile, the abundance of fish at site B fluctuated and did not display decreasing patterns during the sampling period of site B (Figure 3B). Non-native species accounted for more than 85%, whereas relatively small proportions of native and migratory species were collected during all field surveys at both sites.
Field surveys of longline fishing exhibited a different pattern from that of fyke nets (Figure 3C). A total of 46 Japanese eels (Anguilla japonica) were captured during the opening, whereas no Japanese eels were detected before. All captured Japanese eels were in the yellow eel phase, which is an intermediate phase between the juvenile (glass eel) and adult (silver eel) phases (Supplementary Figure S2D).
We analysed the size structures of the six most common species collected from fyke net sampling. The patterns differed among species. For the native species, the frequency of the smaller H. labeo and Hemiculter eigenmann increased notably after the opening began (Figures 4A, B). The size distribution trend of both H. labeo and H. eigenmanni displayed more truncated normal distribution, as the trend of size is positively (right) skewed after the reopening. On the other hand, the size distribution of the non-native species E. erythropterus and L. macrochirus displayed more distinct normal distribution patterns after the reopening, that is, middle-sized individuals were captured more often than before the reopening (Figures 4C, D). For migratory species, M. cephalus and L. maculatus, exhibited a higher frequency of smaller specimens, which is relatively similar to the size distribution pattern of native species (Figures 4E, F). The x2d value among the six species varied from 6.9 to 80.5, being highest for H. Labeo and lowest for L. macrulatus.
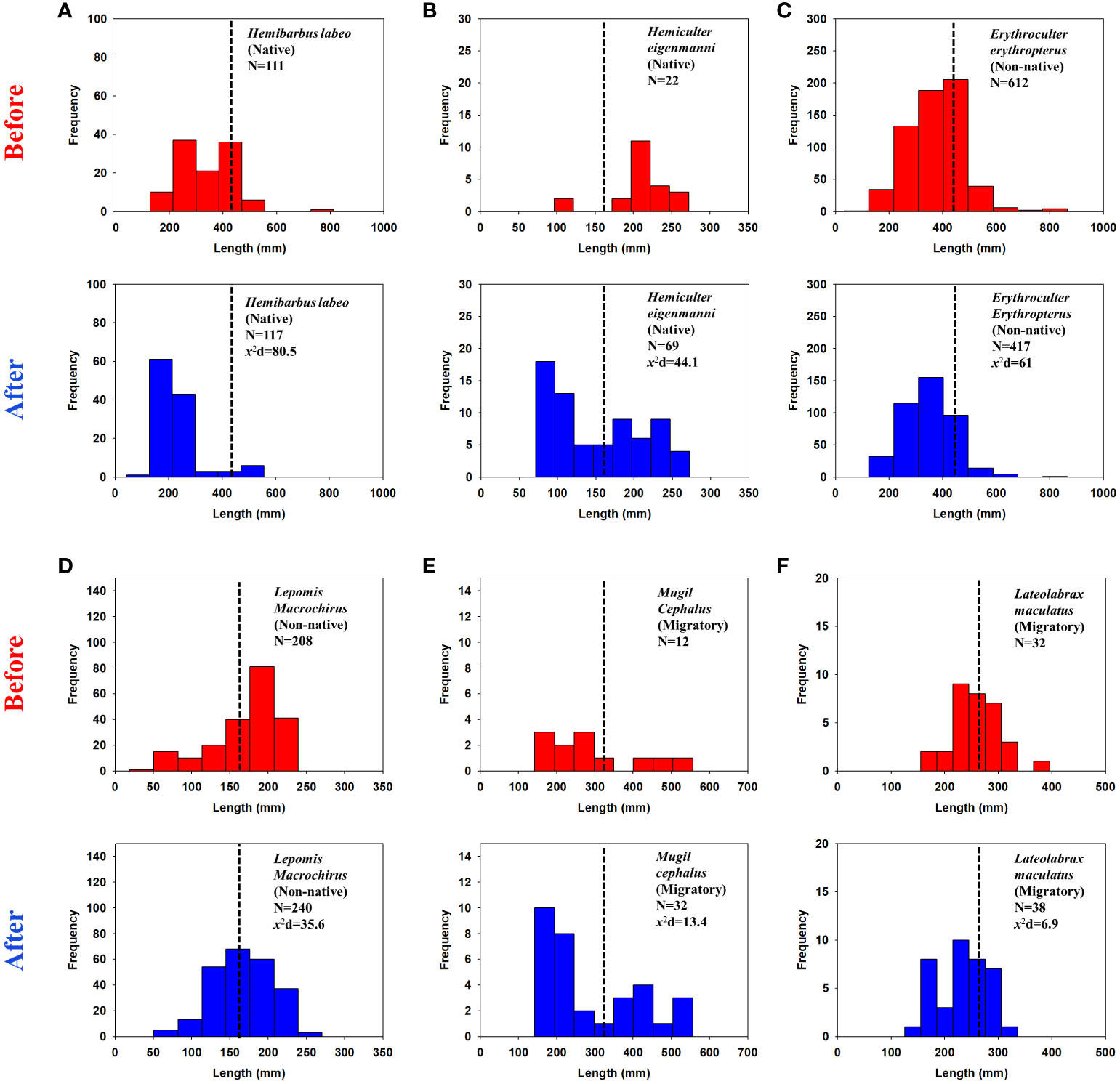
Figure 4 Total length frequency distributions of the six most common fish species in accordance with life-history classifications. Histogram bins in red show the size structure before the reopening of Nakdong River Estuary (2017-June 2020) and the blue bins after the opening (June 2020-2021). Black dotted lines are the median of the number of bins that representing the size distribution. x2d is the Chi-Square distance of the bin distribution from before to after the opening. (A, B) is total length frequency of native species Hemibarbus labeo and Hemiculter eigenmanni; (C, D) is non-native species Erythroculter erythropterus and Lepomis macrochirus, respectively; (E, F) is migratory species Mugil cephalus and Lateolabrax maculatus, respectively.
We further analysed the annual differences in total length for the six species (Supplementary Figure S5). The annual mean total length of the six species displayed fluctuating patterns; however, H. labeo, H. eigenmann, and L. macrochirus displayed more distinctive decreasing trends after reopening.
Using a bivariate correlation matrix, the fish community parameters showed strong relationships with ecosystem functioning components (Supplementary Figure S6). In addition, the relationship differed depending on whether the NRE was reopened (Tables 2 and A4).
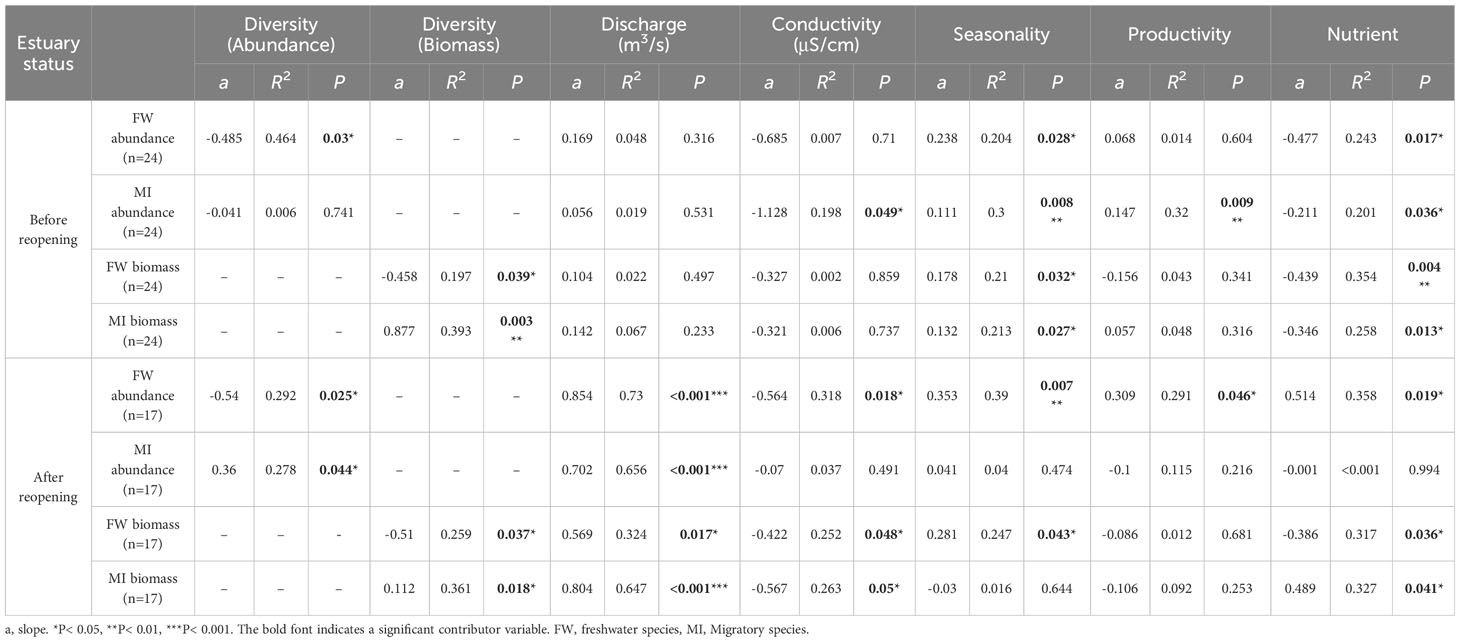
Table 2 Pearson correlation coefficients between fish community parameters and ecosystem functioning groups before and after the reopening of the Nakdong River Estuary.
To illustrate whether the relationship between abiotic environmental and hydraulic factors changed after reopening, we fitted a piecewise SEM composed of hydraulic factors, environmental factors, and fish community parameters. The model revealed that seasonality acts as a factor with a positive correlation to both freshwater species abundance and biomass, which is also negatively correlated with diversity (Figures 5A, B). After the reopening of the NRE, the positive correlation between seasonality and freshwater species abundance disappeared but discharge (hydraulic factor) started showing a positive correlation with both freshwater and migratory species abundance/biomass, whereas conductivity showed a negative correlation with freshwater species biomass (Figures 5C, D). Productivity and nutrients were negatively correlated with the abundance of migratory species and freshwater species biomass respectively. Furthermore, unlike before the reopening, migratory species properties showed correlation with diversity; both migratory species abundance and biomass showed a positive correlation with diversity, while freshwater species abundance and biomass after the reopening began to show a negative correlation or were stronger than before.
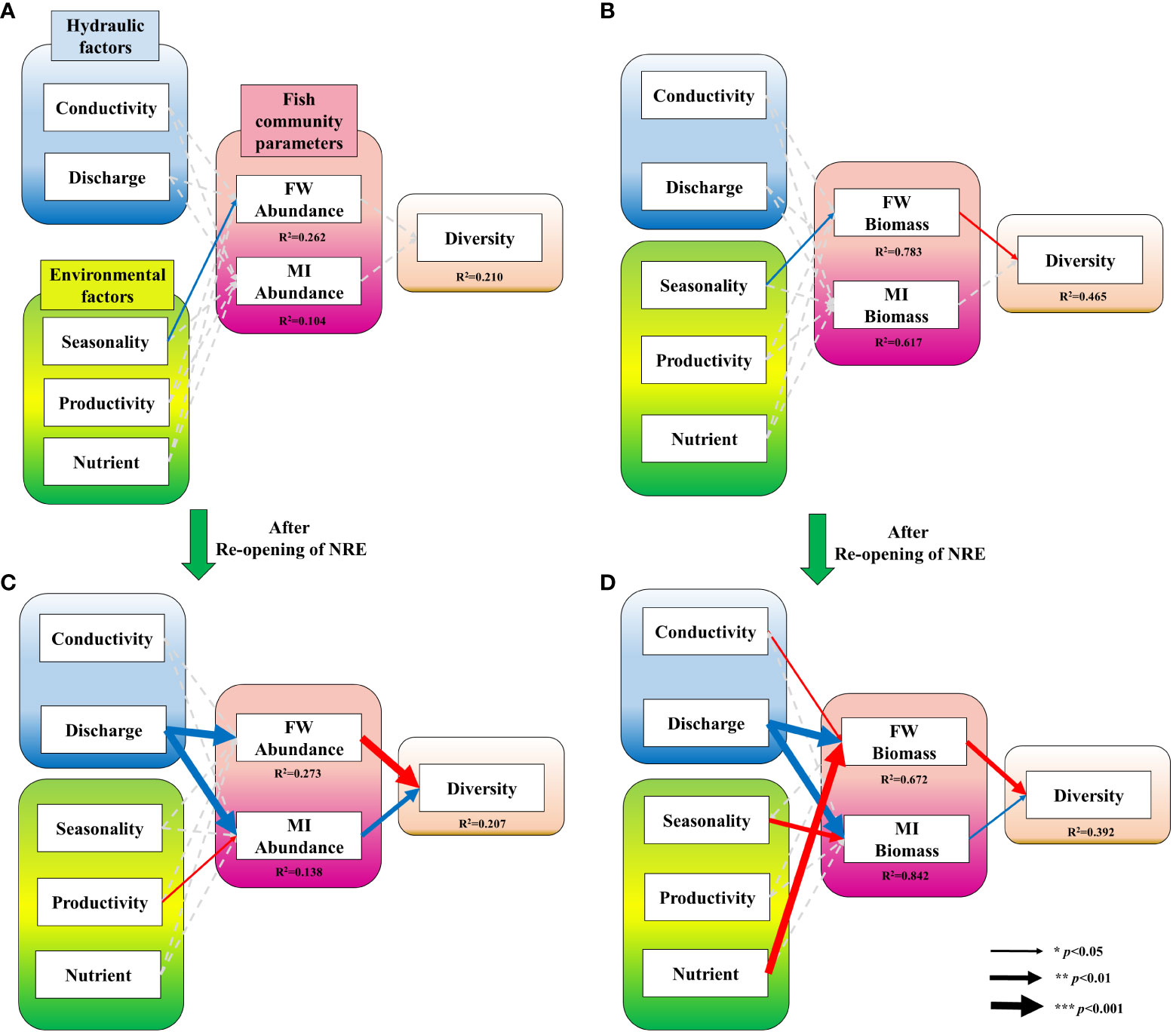
Figure 5 Piecewise structural equation models (piecewise SEM) exploring the effects of environmental factors (seasonality, productivity, and nutrients) and hydraulic factors (conductivity and discharge) on fish community parameters (freshwater species (FW) abundance/biomass and migratory species (MI) abundance/biomass) and diversity. Blue arrows represent positive correlations, red arrows negative correlations, and grey broken arrows no significant correlation. (A, C) depict the effect on FW and MI abundance, while (B, D) represent the effect on biomass.
Our results show the monthly fish community structure for five consecutive years before and during the reopening of the NRE. The overall size structure of the abundant species showed an increasing trend towards smaller sizes. However, the community structure in the NRE did not show notable responses to the reopening, with the relative abundance of the assorted fish species remaining generally stable. In other words, the reopening circumstance led to changes in fish size distribution, which can be interpreted as population changes, while community structure displayed little change. Only one correlation was identified between environmental factors and fish community parameters before the reopening: a positive correlation between seasonality and freshwater species abundance/biomass. However, the estuarine ecosystem after reopening showed more complex relationships, with other environmental and hydraulic factors, such as discharge and conductivity, showing a correlation with fish community parameters. The magnitude of seawater intrusion and recovered habitat connectivity could be relatively small compared to other studies (Bennett et al., 1985; Raat, 2001) as only a single sluice had been utilised to reopen the estuary. Nevertheless, some of findings in fish species make it worthy as merely partial opening of the estuary was able to create fish fauna changes.
Seawater intrusion plays an important role in the inducing of fish communities and spatiotemporal distribution changes (Love et al., 2008; Kantoussan et al., 2012; Mohamed and Hameed, 2019; Alam et al., 2020; Kim et al., 2020). However, we observed that the fish community structure in the NRE did not change remarkably despite a series of reopening circumstances. This unchanged community structure can be attributed to the dominance of non-native species that are less vulnerable to environmental changes. Furthermore, previous studies have shown that non-native species are better adaptors in fragmented habitats (Han et al., 2008; Jo et al., 2019), which suggests that temporarily opening the regulatory sluice seems unable to fully resolve the pre-occurring habitat fragmentation created by the construction of the estuarine barrage. However, although there seems to be little prominent change in the community structure, notable changes have been discovered, as we revealed the emergence of Japanese eels in the yellow eel phase when the NRE was reopened (Figure 4). The Japanese eels are listed as endangered (EN) on the IUCN Red List and show a declining pattern in Korea (Hong et al., 2017). They have principal life stages: the leptocephalus, glass, elver, yellow, and silver eel phases (Arai, 2014). Japanese eels in the yellow phase are immature and known to inhabit a relatively broad range of habitats, from freshwater tributaries to seawater, which is known as facultative catadromy (Tsukamoto and Arai, 2001; Kutzer et al., 2020). The captured individuals might have originated from the upper river and tributaries, as several studies have reported on the salinity preferences of Japanese eels (Fukuda et al., 2019; Shuai et al., 2023), while some of them still have possibilities of emigrating from the sea when the sluice remained open. It is thus reasonable to assume that the eels sensed the seawater intrusion event in 2019-2021, and the series of reopening issues successfully created a ‘temporary-brackish zone’ enough to attract euryhaline and brackish-preferring species.
Data on fish length can provide important clues regarding habitat alteration (Sarkar et al., 2013). We found that the size distribution of certain species changed starting from the reopening of the NRE (Figure 4). Though the x2d showed relatively various values from 6.9 to 80.5, it is not recommended to directly compare these values to others since the x2d values are sensitive to sample sizes (Bergh, 2015). In this case, although x2d values might be able to utilised as fundamental information in future study, interpreting and discussing the shape and trend of the histogram seems to be more desirable. The histogram illustrated that native and migratory species displayed an increasing trend in smaller sized individuals, presenting positive skewed pattern to some extent, while size structure for non-native species E. erythropterus and L. macrochirus remained relatively unchanged. As Griffiths (2010); Griffiths (2012) have reported, factors that can explain such changes in fish size structure is biology and habitat suitability. For positively skewed pattern, we found that H. labeo had a significantly higher frequency of smaller individuals (Figure 4A) after the NRE reopening. H. labeo is a benthic fish species that feeds on benthic invertebrates (http://www.fishbase.org/). Because seawater intrudes into freshwater along the riverbed (Figure 2), H. labeo may have experienced more severe hyperosmotic stress (Martin and Leberg, 2011) as well as loss/changes in food diversity (Herbert et al., 2015). Migratory species mostly migrate to brackish areas to utilise as nursery grounds during the stage of larvae and juveniles (Fujita, 2005; Whitfield et al., 2012). Juvenile migratory species using such strategies can migrate more easily in physically open estuaries than disconnected estuaries, which indicates that the migratory species locomotion would gain more ease when NRE remain reopen. On the other hand, non-native species maintained their normal distribution patterns. As mentioned at the outset, the most likely reason for the maintenance of the size structure of non-native species is that they are able to endure relatively well in such reopened estuary. This also advocate that subordinate native species are less compatible with non-native species in reopened estuary. However, since there is no control groups or study sites that share distinctive characteristics with the NRE (fish species composition, water quality, presence of large estuarine barrage, etc.) in common, it would be more reasonable to conduct additional monitoring to see the substantial cause of the decreasing pattern of size structures.
Yoon et al. (2016) reported that fish assemblages in freshwater areas gradually changed after the NRE barrage construction. Similarly, we found that hydraulic and environmental factors in freshwater areas displayed different relationships with freshwater and migratory species abundance/biomass (Figure 5). Before reopening, the only significant influencing factor on the fish community was seasonality, indicating that the summer-like environment had an indirect positive impact on the abundance and biomass of freshwater species. The following negative correlations between freshwater species biomass and diversity may be accounted for by Shannon’s diversity index, as greater dominance decreases diversity (Nagendra, 2002).
After the reopening, hydraulic factors were correlated with fish community parameters. Increased conductivity has created an increase in diversity by reducing the biomass of freshwater species. Conductivity has been reported as one of the most controlling factors in freshwater fish species (Copp, 2003) and we observed that a few freshwater species increased in frequency on smaller size after the reopening. Discharge itself can have positive effects on fish communities, as greater discharge of organic matter provides a source of potential prey upstream and downstream as tidal movements distribute the material (Hall et al., 1997). The effect of this mechanism would be maximised if the NRE reopened, as the barrage sluice remained open and induced the natural mixing of seawater consistently. Therefore, it is conceivable that sea-freshwater circulation under reopening circumstances is one of the most powerful explanatory factors that influence fish community parameters and diversity.
The correlation between environmental factors and fish communities changed over time. Under reopening conditions, decreased seasonality, which can be interpreted as more winter characteristics, can promote an increase in diversity by increasing migratory species biomass. Migratory species, such as M. cephalus and L. maculatus have seasonal migration patterns; they spawn in the sea during winter (Watanabe, 1965) and would inhabit in estuaries until they achieve recruitment. Therefore, such winter migratory behaviours might be more clearly identified under reopening circumstances. Additionally, productivity and nutrients affect fish community parameters; however, each environmental parameter is influenced by the different lifestyles of the fish species. The ecological niche of a species is a determining factor in how nutrients affect the species (Lenat and Crawford, 1994). The nutritional impact can differ based on properties such as the prey-predator relationship (Sánchez‐Hernández et al., 2021) and size distribution (Wilson, 1975).
The estuary reopening successfully resulted in attracting euryhaline species, as well as changed effects of hydraulic and environmental factors on migratory species. Indeed, physical disturbances in estuarine ecosystems are the main cause of migratory species population declines and estuary reopenings are a key environment management strategy used to mitigate such physical disturbances, particularly as related to habitat disconnection. The effect of habitat connection recovery and seawater intrusion on fish community may be intensified as it gets closer to the NRE barrage, but such research related to the NRE is limited and have been conducted by different approaches (different sampling methodology and magnitude, Busan Metropolitan City, 2021; Jeong et al., 2022). Understanding how the degree of influence varies depending on the distance should also be identified as well. One of the potential study area is the fishways, as more than 30 species are known to utilise these fishways (Yoon et al., 2016; Park et al., 2020). Furthermore, an additional study site at the lower part of the NRE barrage should be established to identify the downward movement of migratory fishes.
As of 2021, there were several reopenings with further reopening plans being planned. Therefore, additional monitoring of reopening is expected in the future as fish community shifts require a long period (>10 years) to show and be identified (Kiernan et al., 2012; Gao et al., 2019). If increased amount of seawater would intrude in the future reopening, it may intensify not only the currently revealed results, but also more significant changes on community structure such as relative abundances. According to past research, large brackish area from the river mouth to 40km upstream was developed before the NRE barrage had been constructed (Jang and Kim, 2006). This record indicates that deliberate reopening strategies are necessary, as indiscreet reopening may contaminate freshwater and groundwater that should be used as agriculture and industrial water, as well as at the water intake station which is 25km away from the NRE barrage; increasing reopening (seawater intrusion) magnitude practically subject to many restrictions. Therefore, in order to increase the diversity while maintaining the magnitude of seawater intrusion, sluice management considering species biology should be carried on. We look forward to creating a more developed brackish zone after a series of future reopenings, which should be accompanied with adequate conservation management plans.
Since seasonality is the most explicit explanatory variable for migratory species, it requires additional effort that maximise its impact on the migratory fish community (Figure 5). We desire a wise solution for sluice management be developed by considering diverse species-specific migrating seasons of resident migratory species, as well as globally important species, to maximise the effect of seasonality and ecological connectivity. For instance, glass eels (juvenile phase of the Japanese eel) born in the Philippine Sea (Tsukamoto, 2006) can utilize tidal currents on the surface layer for transport during the night (Tesch and Bartsch, 2008) from February to April (Cheng and Tzeng, 1996) when entering the mouths of rivers in East Asian countries. Therefore, a species-specific strategy for glass eels should utilize the overflow method to promote sea-freshwater exchange at the surface layer (Supplementary Figure S1D) during the night from February to April. This effort would eventually increase the habitat connectivity and seasonality for important migratory species simultaneously. Other strategies should be applied for chum salmon (Oncorhynchus keta) as they prefer bottom layer movement (Ueno, 1992). In this case, the underflow method (Supplementary Figure S1C) is required to promote natural migration. Such flexible management of estuarine barrages can increase fish diversity in estuarine ecosystems.
As a secondary effect, we have originally anticipated that estuary reopening would reduce the dominance of freshwater species, especially non-native species in the NRE. Though Shannon diversity value did not significantly change after reopening until now, the results of the piecewise SEM and the size distribution pattern indicates that the native and migratory species have been partly affected by reopening. Such changes could be able to potentially alter diversity in the long run. On the other hand, relative abundance and size structure of non-native species remained relatively unchanged. To promote increased diversity, reducing the number of non-native species could be an important issue, as they are the top predators in the NRE ecosystem (Yoon et al., 2016). If more seawater intruded, significant changes in the community structure in NRE may be induced, but as mentioned earlier, there are practical limitations. This suggests that there should be anthropogenic efforts to decrease the dominance and density of non-native species, if it is difficult to help naturally. One solution is to adopt a purchase loan program for diverse non-native species to control their populations. Policies for non-native species management in South Korea have been implemented to encourage fishermen to catch those (Park et al., 2021). By introducing such human-involving effort, the diversity in NRE can be effectively increased.
Based on the reopening of the NRE, we found that seawater intrusion led to a vertically separated layer with high salinity near the riverbed. In the field survey, the community structure remained relatively unchanged, with an extreme dominance of the non-native species Erythroculter erythropterus regardless of the series of estuary reopenings. However, total 46 Japanese eels (Anguilla japonica) were collected during the opening period, whereas no eels were collected before that. The size structures of native and migratory species illustrated relatively different pattern after reopening, by exhibiting more frequent observations of smaller individuals, whereas size structure of non-native species remained relatively unchanged. These results may suggest that non-native FW species colonising in the NRE are relatively less negatively affected than native species by reopening of the estuarine barrage. Piecewise SEM revealed that the correlation between hydraulic and environmental factors and fish community parameters changed significantly, as most driving factors changed from seasonal factors to hydraulic factors, such as discharge. Therefore, species-specific protocols for sluice management to aid migratory species and human-involved population management of non-native species are required.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
DH: Data curation, Investigation, Methodology, Writing – original draft. J-SG: Data curation, Investigation, Writing – review & editing. G-JJ: Conceptualization, Funding acquisition, Writing – review & editing. D-KK: Data curation, Formal analysis, Writing – review & editing. DC: Investigation, Writing – review & editing. H-YL: Conceptualization, Writing – review & editing. K-SJ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. HJ: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by 1) a Basic Research (NRF- 2016R1D1A1B01009492) grant from the National Research Foundation (NRF) of Korea and 2) a Project Open Innovation R&D grant (20-D-W-003) from K-water.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1337392/full#supplementary-material
Able K. W. (2005). A re-examination of fish estuarine dependence: evidence for connectivity between estuarine and ocean habitats. Estuar. Coast. Shelf Sci. 64, 5–17. doi: 10.1016/j.ecss.2005.02.002
Agostinho A. A., Luiz C. G., Samuel V., Edson K. O. (2004). Flood regime, dam regulation and fish in the Upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Re. Fish Biol. Fish. 14, 11–19. doi: 10.1007/s11160-004-3551-y
Alam M. R., Sharmin S., Islam S. M. M., Alam M. A., Ehiguese F. O., Pattadar S. N., et al. (2020). Salinity intrusion affects early development of freshwater aquaculture species pabda, Ompok pabda. Aquac. Rep. 18, 100476. doi: 10.1016/j.aqrep.2020.100476
Almodóvar A., Nicola G. G. (1999). Effects of a small hydropower station upon brown trout Salmo trutta L. in the River Hoz Seca (Tagus basin, Spain) one year after regulation. Regul. Rivers Res. Manage. 15, 477–484. doi: 10.1002/(SICI)1099-1646(199909/10)15:5<477::AID-RRR560>3.0.CO;2-B
Arai T. (2014). How have spawning ground investigations of the Japanese eel Anguilla japonica contributed to the stock enhancement? Rev. Fish Biol. Fish. 24, 75–88. doi: 10.1007/s11160-013-9318-6
Baas V., Clabbers N., Moens J., Schuurke E., Spierings J., Termaat E., et al. (2020). Opening of the Haringvliet, a stream of possibilities. Available online at: https://www.delta21.nl/wp-content/uploads/2020/12/GWF-Final-Consultancy-Report.pdf.
Barnes N., Bamber R. N., Moncrieff C. B., Sheader M. (2008). Meiofauna in closed coastal saline lagoons in the United Kingdom: Structure and biodiversity of the nematode assemblage. Estuar. Coast. Shelf Sci. 79, 328–340. doi: 10.1016/j.ecss.2008.03.017
Beekelaar H., Vernoolj W., van Lier W., de Wolf A. (2020). The potential influence of the Delta21 plan on the recovery of a tidal ecosystem and fish migration in the Haringvliet. Delta21, 1–36.
Bennett B. A., Hamman K. C. D., Branch G. M., Thorne S. C. (1985). Changes in the fish fauna of the Bot River Estuary in relation to opening and closure of the estuary mouth. Trans. R. Soc S. Afr. 45, 459–464. doi: 10.1080/00359198509519503
Bergh D. (2015). Chi-squared test of fit and sample size-a comparison between a random sample approach and a chi-square value adjustment method. J. Appl. Meas. 16, 204–217.
Busan Metropolitan City. (2021). Monitoring the Ecosystem of Nakdong River Estuary. Available online at: https://www.busan.go.kr/depart/ahnaturalsurvey02 (Accessed January 2, 2024).
Busan Metropolitan City. (2023). Monitoring the Ecosystem of Nakdong River Estuary. Available online at: https://www.busan.go.kr/depart/ahnaturalsurvey02 (Accessed January 23, 2024).
Cheng P. W., Tzeng W. N. (1996). Timing of metamorphosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 131, 87–96. doi: 10.3354/meps131087
Choi Y. E., Oh C. O., Chon J. H. (2021). Applying the resilience principles for sustainable ecotourism development: A case study of the Nakdong Estuary, South Korea. Tour. Manage. 83, 104237. doi: 10.1016/j.tourman.2020.104237
Chung J. K., Kannappan P. L., Ng C. T., Sahoo P. K. (1989). Measures of distance between probability distributions. J. Math. Anal. 138, 280–292. doi: 10.1016/0022-247X(89)90335-1
Connell D. W., Bycroft B. M., Miller G. J., Lather P. (1981). Effects of a barrage on flushing and water quality in the Fitzroy River estuary, Queensland. Mar. Freshw. Res. 32, 57–63. doi: 10.1071/MF9810057
Copp G. H. (2003). Is fish condition correlated with water conductivity? J. Fish Biol. 63, 263–266. doi: 10.1046/j.1095-8649.2003.00145.x
Dynesius M., Nilsson C. (1994). Fragmentation and flow regulation of river systems in the northern third of the world. Science. 266, 753–762. doi: 10.1126/science.266.5186.753
Elliott M., Dewailly F. (1995). The structure and components of European estuarine fish assemblages. Neth. J. Aquat. Ecol. 29, 397–417. doi: 10.1007/BF02084239
Froese R., Pauly D. (2022) FishBase (World Wide Web Electronic Publication). Available online at: http://www.fishbase.org (Accessed 20 July 2022).
Fujita S. (2005). Ecological study on larvae and juveniles of the two sea basses and the three sparines occuurring in the Shimanto estuary, Japan. Japan Bull. Mar. Sci. Fish Kochi Univ. 23, 1–57.
Fukuda N., Yokouchi K., Yamamoto T., Kurogi H., Yada T. (2019). “Insight into Japanese eel divergence into sea-estuaries and rivers,” in Eels: biology, monitoring, management, culture and exploitation. Eds. Don A., Coulson P. (Sheffield, UK: 5M Publishing).
Gana K., Broc G. (2019). Structural equation modeling with lavaan (Hoboken, New Jersy: John Wiley & Sons).
Gao X., Fujiwara M., Winemiller K. O., Lin P., Li M., Liu H. (2019). Regime shift in fish assemblage structure in the Yangtze River following construction of the Three Gorges Dam. Sci. Rep. 9, 1–11. doi: 10.1038/s41598-019-38993-x
Griffiths D. (2010). Pattern and process in the distribution of North American freshwater fish. Biol. J. Linn. Soc 100, 46–61. doi: 10.1111/j.1095-8312.2010.01404.x
Griffiths D. (2012). Body size distributions in North American freshwater fish: large-scale factors. Glob. Ecol. Biogeogr. 21, 383–392. doi: 10.1111/j.1466-8238.2011.00680.x
Hall J. A., Frid C. L. J., Gill M. E. (1997). The response of estuarine fish and benthos to an increasing discharge of sewage effluent. Mar. pollut. Bull. 34, 527–535. doi: 10.1016/S0025-326X(97)00156-2
Hammer Ø., Harper D. A., Ryan P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 4, 9.
Han M. D., Fukushima M., Kameyama S., Fukishima T., Matsushita B. (2008). How do dams affect freshwater fish distributions in Japan? Statistical analysis of native and nonnative species with various life histories. Ecol. Res. 23, 735–743. doi: 10.1007/s11284-007-0432-6
Herbert E. R., Boon P., Burgin A. J., Neubauer S. C., Franklin R. M., Ardón M., et al. (2015). A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere. 6, 1–43. doi: 10.1890/ES14-00534.1
Hong Y. K., Kim J. E., Lee J. H., Song M. Y., Park H. W., Lee W. O. (2017). Occurrence of exotic eels in natural waters of South Korea. Anim. Cells Syst. (Seoul). 21, 341–348. doi: 10.1080/19768354.2017.1377108
Jang S. T., Kim K. C. (2006). Change of oceanographic environment in the nakdong estuary. Sea: J. Korean Soc. Oceanography. 11, 11–20.
Jang M. H., Kim J. G., Park S. B., Jeong K. S., Cho G. I., Joo G. J. (2002). The current status of the distribution of introduced fish in large river systems of South Korea. Int. Rev. Hydrobiol. 87, 319–328. doi: 10.1002/1522-2632(200205)87:2/3<319::AID-IROH319>3.0.CO;2-N
Jeong S. I., Han J. H., Lee J. Y., Kim H. Y. (2022). The gate operation for bolstering up fish migration in the nakdong river estuary. Korean J. Environ. Ecol. 36, 468–480. doi: 10.13047/KJEE.2022.36.5.468
Jo H. B., Jang M. H., Jeong K. S., Do Y. N., Joo G. J., Yoon J. D. (2011). Long-term changes in fish community and the impact of exotic fish, between the Nakdong River and Upo Wetlands. J. Ecol. Environ. 31, 59–68. doi: 10.5141/JEFB.2011.008
Jo H. B., Jeppesen E., Ventura M., Buchaca T., Gim J. S., Yoon J. D., et al. (2019). Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total. Environ. 657, 1334–1342. doi: 10.1016/j.scitotenv.2018.11.446
Kantoussan J., Ecoutin J. M., Simier M., de Morais L. T., Laë R. (2012). Effects of salinity on fish assemblage structure: An evaluation based on taxonomic and functional approaches in the Casamance estuary (Senegal, West Africa). Estuar. Coast. Shelf Sci. 113, 152–162. doi: 10.1016/j.ecss.2012.07.018
Kiernan J. D., Moyle P. B., Crain P. K. (2012). Restoring native fish assemblages to a regulated California stream using the natural flow regime concept. Ecol. Appl. 22, 1472–1482. doi: 10.1890/11-0480.1
Kim J. H., Park S. H., Baek S. H., Jang M. H., Yoon J. D. (2020). Changes in fish assemblages after dike construction in the Saemangeum area. Ocean. Sci. J. 55, 129–142. doi: 10.1007/s12601-020-0008-8
Ko E. J., Jung. E. S., Do Y. N., Joo G. J., Kim H. W., Jo H. B. (2022). Impact of river-reservoir hybrid system on zooplankton community and river connectivity. Sustainability. 14, 5184. doi: 10.3390/su14095184
Kutzer A., Lavergne E., Kume M., Wada T., Terashima Y., Yamashita Y. (2020). Foraging behavior of yellow-phase Japanese eels between connected fresh-and brackish water habitats. Environ. Biol. Fishes. 103, 1061–1077. doi: 10.1007/s10641-020-01002-6
K-water (2022). Nakdong estuary water quality and aquatic ecosystem monitoring (in korean). Busan, Korea.
Lefcheck J. S. (2016). piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 7, 573–579. doi: 10.1111/2041-210X.12512
Lenat D. R., Crawford J. K. (1994). Effects of land use on water quality and aquatic biota of three North Carolina Piedmont streams. Hydrobiologia. 294, 185–199. doi: 10.1007/BF00021291
Love J. W., Gill J., Newhard J. J. (2008). Saltwater intrusion impacts fish diversity and distribution in the Blackwater River drainage (Chesapeake Bay watershed). Wetlands. 28, 967–974. doi: 10.1672/07-238.1
Magilligan F. J., Nislow K. H., Kynard B. E., Hackman A. M. (2016). Immediate changes in stream channel geomorphology, aquatic habitat, and fish assemblages following dam removal in a small upland catchment. Geomorphology (Amst). 252, 158–170. doi: 10.1016/j.geomorph.2015.07.027
Martin S. B., Leberg P. L. (2011). Influence of environmental stress on age-and size-at-maturity: genetic and plastic responses of coastal marsh fishes to changing salinities. Can. J. Fish. Aquat. Sci. 68, 2121–2131. doi: 10.1139/f2011-11
Martinho F., Leitão R., Viegas I., Dolbeth M., Neto J. M., Cabral H. N., et al. (2007). The influence of an extreme drought event in the fish community of a southern Europe temperate estuary. Estuar. Coast. Shelf Sci. 75, 537–546. doi: 10.1016/j.ecss.2007.05.040
McLusky D. S., Elliott M. (2004). The estuarine ecosystem: ecology, threats and management, third ed (Oxford: Oxford university press).
Mohamed A. R. M., Hameed E. K. (2019). Impacts of saltwater intrusion on the fish assemblage in the middle part of Shatt Al-Arab River, Iraq. Asian J. Appl. Sci. 7, 577–586. doi: 10.24203/ajas.v7i5.5917
Nagendra H. (2002). Opposite trends in response for the Shannon and Simpson indices of landscape diversity. Appl. Geogr. 22, 175–186. doi: 10.1016/S0143-6228(02)00002-4
Nam J. H., Ryu J. S., Fluharty D., Koh C. H., Dyson K., Chang W. K., et al. (2010). Designation processes for marine protected areas in the coastal wetlands of South Korea. Ocean Coast. Manage. 53, 703–710. doi: 10.1016/j.ocecoaman.2010.10.002
Park S. C., Lee K. Y., Choi K. S., Han M. S., Ko M. H. (2021). Inhabitat status and gastric contents of invasive fish species and the effect on fish fauna at three reservoirs in national parks of Korea. Korean. J. Ichthyol. 33, 84–94. doi: 10.35399/ISK.33.2.5
Park J. M., Riedel R., Ju H. H., Choi H. C. (2020). Fish assemblage structure comparison between freshwater and estuarine habitats in the lower Nakdong River, South Korea. J. Mar. Sci. Eng. 8, 496. doi: 10.3390/jmse8070496
Pinckney J. L., Paerl H. W., Tester P., Richardson T. L. (2001). The role of nutrient loading and eutrophication in estuarine ecology. Envrion. Health Perspect. 109, 699–706. doi: 10.1289/ehp.01109s5699
Raat A. J. P. (2001). Ecological rehabilitation of the Dutch part of the River Rhine with special attention to the fish. Regul. Rivers. 17, 131–144. doi: 10.1002/rrr.608
Roshni K., Renjithkumar C. R., Raghavan R., Ranjeet K. (2021). Fish distribution and assemblage structure in a hydrologically fragmented tropical estuary on the south-west coast of India. Reg. Stud. Mar. Sci. 43, 101693. doi: 10.1016/j.rsma.2021.101693
Sánchez-Hernández J., Finstad A. G., Arnekleiv J. V., Kjærstad G., Amundsen P. A. (2021). Beyond ecological opportunity: Prey diversity rather than abundance shapes predator niche variation. Freshw. Biol. 66, 44–61. doi: 10.1111/fwb.13606
Sarkar U. K., Khan G. E., Dabas A., Pathak A. K., Mir J. I., Rebello S. C., et al. (2013). Length weight relationship and condition factor of selected freshwater fish species found in River Ganga, Gomti and Rapti, India. J. Environ. Biol. 34, 951–956.
Shannon C. E. (1948). A mathematical theory of communication. Bell. Syst. Tech. J. 27, 379–423. doi: 10.1002/j.1538-7305.1948.tb01338.x
Shuai F., Li H., Li J., Jiang T., Yang J., Yang W. (2023). Unravelling the life history patterns and habitat preferences of Japanese eel (Anguilla japonica) in the Pearl River China. J. Fish Biol. early view. doi: 10.1111/jfb.15303
Tsukamoto K., Arai T. (2001). Facultative catadromy of the eel Anguilla japonica between freshwater and seawater habitats. Mar. Ecol. Prog. 220, 265–276. doi: 10.3354/meps220265
Ueno Y. (1992). Deepwater migrations of chum salmon (Oncorhynchus keta) along the Pacific coast of northern Japan. Can. J. Fish. Aquat. Sci. 49, 2307–2312. doi: 10.1139/f92-25
van Puijenbroek P. J. T. M., Buijse A. D., Kraak M. H. S., Verdonschot P. F. M. (2019). Species and river specific effects of river fragmentation on European anadromous fish species. River Res. Appl. 35, 68–77. doi: 10.1002/rra.3386
van Wichelen J., Verheist P., Buysse D., Belpaire C., Vlietinck K., Coeck J. (2021). Glass eel (Anguilla Anguilla L.) behaviour after artificial intake by adjusted tidal barrage management. Estuar. Coast. Shelf Sci. 249, 107127. doi: 10.1016/j.ecss.2020.107127
Vasconcelos L. P., Alves D. C., Câmara L. F., Hahn L. (2021). Dams in the Amazon: The importance of maintaining free-flowing tributaries for fish reproduction. Aquat. Conserv.: Mar. Freshw. 31, 1106–1116. doi: 10.1002/aqc.3465
Watanabe T. (1965). Ecological distribution of eggs of common sea bass, Lateolabrax japonicas (Cuvier) in Tokyo Bay. Bull. Jpn. Soc Sci. Fish. 31, 385–590.
Wetzel R. G. (1995). Death, detritus, and energy flow in aquatic ecosystems. Freshw. Biol. 33, 83–89. doi: 10.1111/j.1365-2427.1995.tb00388.x
Whitfield A. K. (1999). Ichthyofaunal assemblages in estuaries: a South African case study. Rev. Fish Biol. Fish. 9, 151–186. doi: 10.1023/A:1008994405375
Whitfield A. K., Panfili J., Durand J.-D. (2012). A global review of the cosmopolitan flathead mullet Mugil cephalus Linnaeus 1758 (Teleostei: Mugilidae), with emphasis on the biology, genetics, ecology and fisheries aspects of this apparent species complex. Rev. Fish Biol. Fish. 22, 641–681. 10.1007/s11160-012-9263-9
Wilson D. S. (1975). The adequacy of body size as a niche difference. Am. Nat. 109, 769–784. doi: 10.1086/283042
World Commission on Dams (2000). Dams and development: A new framework for decision-making: The report of the world commission on dams. Earthscan London.
Wright J., Worrall F. (2001). The effects of river flow on water quality in estuarine impoundments. Phys. Chem. Earth Part B: Hydrology Oceans Atmosphere. 26, 741–746. doi: 10.1016/S1464-1909(01)00079-X
Yagi Y., Kinoshita I., Fujita S., Aoyama D., Kawamura Y. (2011). Importance of the upper estuary as a nursery ground for fishes in Ariake Bay, Japan. Environ. Biol. Fishes. 91, 337–352. doi: 10.1007/s10641-011-9790-6
Keywords: estuary reopening, seawater intrusion, migratory species, non-native species, piecewise structural equation modelling, size structure
Citation: Hong D, Gim J-S, Joo G-J, Kim D-K, Choi D, Lee H-Y, Jeong K-S and Jo H (2024) Effects of estuary reopening management on the fish community in the Nakdong River Estuary. Front. Mar. Sci. 11:1337392. doi: 10.3389/fmars.2024.1337392
Received: 13 November 2023; Accepted: 01 February 2024;
Published: 27 February 2024.
Edited by:
Lorenzo Mari, Polytechnic University of Milan, ItalyReviewed by:
Emily Chen, University of California, Berkeley, United StatesCopyright © 2024 Hong, Gim, Joo, Kim, Choi, Lee, Jeong and Jo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyunbin Jo, cHJvemV2YUBwdXNhbi5hYy5rcg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.