
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 30 May 2024
Sec. Marine Ecosystem Ecology
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1334235
This article is part of the Research Topic Understanding the Response of Ecosystems to Increasing Human Pressures and Climate Change – Management Options View all 24 articles
 Ali M. Ussi1*
Ali M. Ussi1* Mohammed S. Mohammed1
Mohammed S. Mohammed1 Rashid J. Rashid1
Rashid J. Rashid1 Mohammed A. Sheikh1
Mohammed A. Sheikh1 Peter A. Staehr2
Peter A. Staehr2 Christopher A. Muhando3
Christopher A. Muhando3 Saleh Yahya3
Saleh Yahya3 Karsten Dahl2
Karsten Dahl2Introduction: Coral reefs as key ecosystems in Zanzibar are exposed to several anthropogenic and natural stressors.
Methods: The benthic composition and coverage of coral reefs were investigated on three data sets involving ten coral reefs monitored from 1992 to 2016. Firstly, we investigated differences in the reef composition using data from seven reefs in 2015. Secondly, we analyzed communities on three distinctive reefs (2010 to 2012) to understand the importance of seasons and reef zones (slope, crest and flat) on species abundance. Finally, we investigated long-term changes (1992 to 2016) of five reefs.
Results: Branching Porites and Acropora, and soft coral order Corallimorpharia, characterized sheltered reef communities. Soft corals and algal species characterized the reef communities exposed to strong hydrodynamic conditions, which also displayed greater cover of rocks and rubbles. The average dissimilarity between reefs ranged between 60% and 75%. The seasonal changes in community structure for reefs near Stone Town were mostly associated with soft coral Corallimorpharia. Indeed, the bare rock and algae distinguished the northern exposed reef from more sheltered reefs. Acropora was a key genus for the sheltered Chumbe reef, which explained between 14% and 18% of the dissimilarities among the three reefs. Hard corals covered between 40% and 70% in most years, with severe declines following El Niño events in 1998 and 2016. The dominating genus Acropora showed a strong decline from the late 1990s’ with signs of recovery at remote reefs compared to reefs closer to human residence.
Discussion: Our results highlight the importance of seasonality and spatial differences, reflecting differences in human impact and physical exposure and significant long-term changes in coral communities. Continued monitoring of reef health is essential to evaluate the success of ongoing management to sustain the reef services.
Coral reefs are among the most valuable coastal ecosystems in Zanzibar (Johnstone et al., 1998). The reefs host high biodiversity, are productive (Jiddawi and Öhman, 2002; Lange and Jiddawi, 2009), providing significant support to human livelihoods in the coastal zones, and are a key selling point for the prosperous tourist industry (Muhando and Jiddawi, 1998; Wolanski et al., 2003). The reef ecosystems in the recent decade, however, have been exhibiting signs of increasing degradation (Muhando, 2008; Staehr et al., 2018; Ussi et al., 2019). Very strong El-Niño events occurred in 1998, 2007 and 2016, resulting in massive coral bleaching due to global warming in this region (Wilkinson et al., 1999; McClanahan et al., 2007; Gudka et al., 2018; Ussi et al., 2019). Furthermore, severe outbreaks of the invasive crown-of-thorns starfish destroying corals through predation were observed from 2002 to 2008 (Muhando and Lanshammar, 2008; Ussi, 2009). The ongoing destructive fishing practices such as the use of blast fishing (Chevallier, 2017; Gray et al., 2017; Raycraft, 2018), and drag-net fishing (Wallner-Hahn et al., 2016) continue to affect the reef communities negatively (Wells, 2009; Raycraft, 2018). In addition, declining water quality in the near shore areas due to pollution and poorly managed tourism have been reported to further exacerbate the negative impact on coral reefs off Stone Town (McClanahan et al., 1999; Muhando et al., 2002; Obura, 2002; Wells, 2009; Muzuka et al., 2010). The consistent increase in coral-reef ecosystem degradation, attributed to these threats has resulted in a substantial change in coral reef community structure at different magnitudes among reefs. Coral reef degradation is projected to continue in response to the intensity of existing impacts (Porter et al., 2017). It is therefore important to periodically assess the health status of existing reef communities, and evaluate the impacts of long-term threats at the community level for proper management.
Located in the Inter-Tropical Convergence Zone, Zanzibar experiences seasonality driven mostly by the northeast monsoons (NEM) from October-March and the southeast monsoons (SEM) from April-September. Four seasons can be identified; 1) From December to February, conditions are calm and hot (related to summer); followed by 2) a heavy rain season from March to May (related to spring); 3) a cold windy season from June-August (related to winter) and finally 4) another wet period referred to as the short rainy season from September-November (related to autumn). Given these clear seasonal changes in weather conditions, we expect some seasonal variability in the cover of reef benthic communities as indicated by previous studies in the region (Nzali et al., 1998). The knowledge of this seasonality will be important for planning future monitoring and for setting proper management targets for the coral reefs around Zanzibar.
Coral reef monitoring around Zanzibar started in 1992. However, the regular monitoring was interrupted from 2009 to 2015 due to a lack of financial resources. Fortunately, a series of research studies carried out by postgraduate students ensured data on coral health status during these periods. Together with these resources, this study provides three supplementing analyses of the coral reef health around Zanzibar. Firstly, we gave a systematic description of the reef structure and differences between seven important coral reef sites around Zanzibar islands based on data collected in 2015. Secondly, we explored the extent to which community structure is sensitive to seasonality and or to the reef habitat orientation of the monitored zone using data from three distinct reefs monitored from 2010 to 2012. This information was important for interpreting the existing data collected during different seasons and for future planning of monitoring activities. Finally, we updated previous reports on long-term changes in key biological and physical structures using a unique dataset from 1992 to 2016 on five reefs. This also made it possible to evaluate the importance of the different environmental conditions explaining these changes to guide management efforts.
Ten reef sites with varying amounts of data (year and season) and sampling frequency were studied (Figure 1). To improve the quality of data and meet the statistical validity, data were standardized using the method described by Holmes and Johnstone (2010) (Holmes and Johnstone, 2010). Variations in data quantity among reef sites were mostly an outcome of differences in the monitoring starting at the different periods. For example, four reef sites (Changuu, Chapwani, Bawe and Misali) were monitored from 1992, two reefs (Chumbe and Mnemba) were monitored from 1994, and monitoring of Kwale reef began in 1996. All these seven reefs were monitored at different intervals until 2016. Kizimkazi, Murogo and Nungwi reefs were the newest sites with the most recent monitoring data available for 2015-2016. These reef sites were selected based on expected differences related to the human pressure and management regimes. Chapwani, Bawe and Murogo reefs are located at increasing distances (3.8, 6.2 and 7.1 km respectively) from Stone Town, the most populated area of Unguja Island. The reef sites off Stone Town are relatively sheltered from the effects of monsoons (Figure 1). The northern reef sites off Nungwi and Mnemba are particularly exposed to strong wind conditions during the North-East Monsoon (NEM), whereas the reefs off Kizimkazi at the southern tip and Kwale on the southwest of Unguja Island are exposed to the South-East monsoon (SEM). Misali site is located off the west coast of the less populated Pemba Island. Mnemba reef is located on the North-East coast of Unguja (Figure 1, Table 1), and is a national protected area, where fishing restrictions are applied in the core zone of the reef area with permission for selective fishing gear in the periphery of the reef. Chumbe reef is a private marine protected area with restricted access. Kizimkazi and Kwale reefs are located within marine conservation areas with fishing gear restrictions, whereas reefs off Stone Town (Changuu, Chapwani, Murogo and Bawe reefs) are freely accessible.
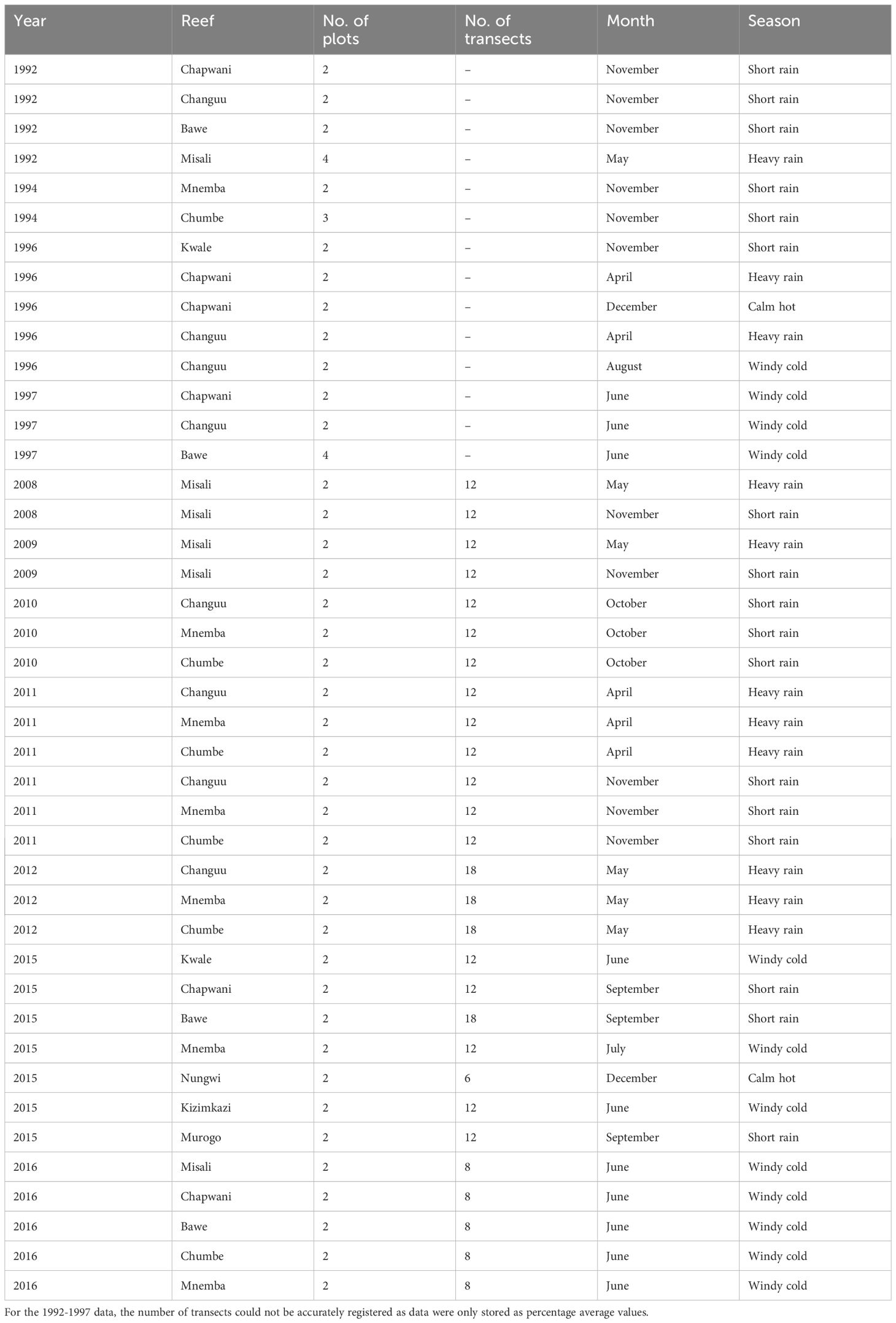
Table 1 Overview of the investigated reefs around Unguja and Pemba Island, with information on the number of transects, sampling month and season.
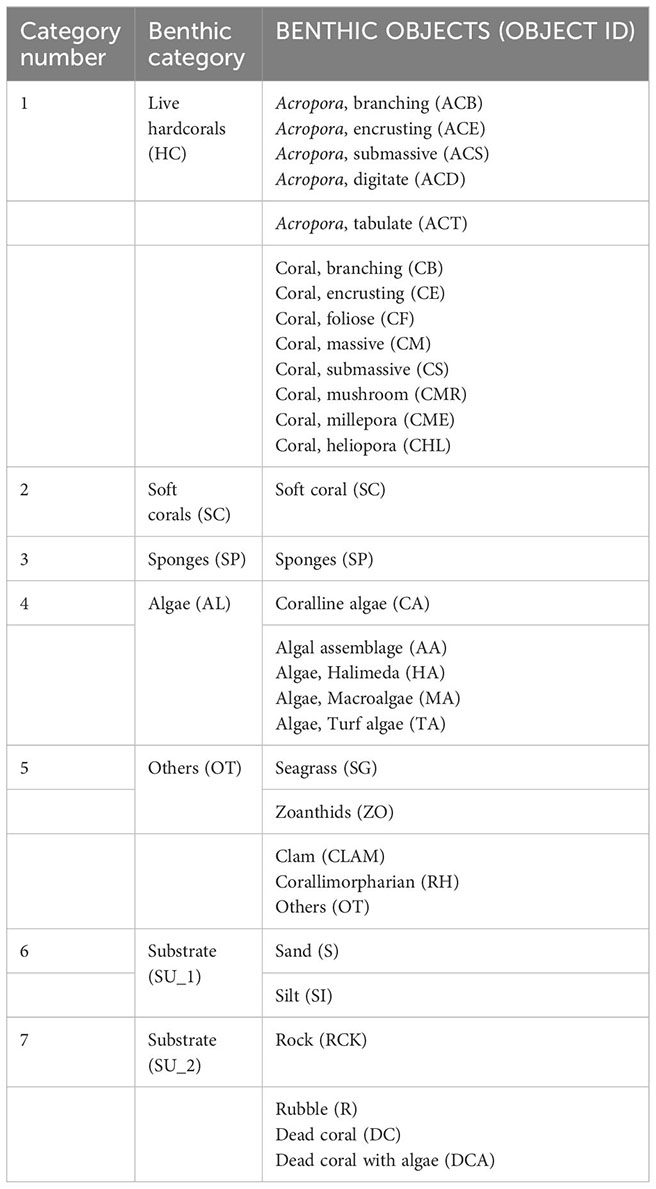
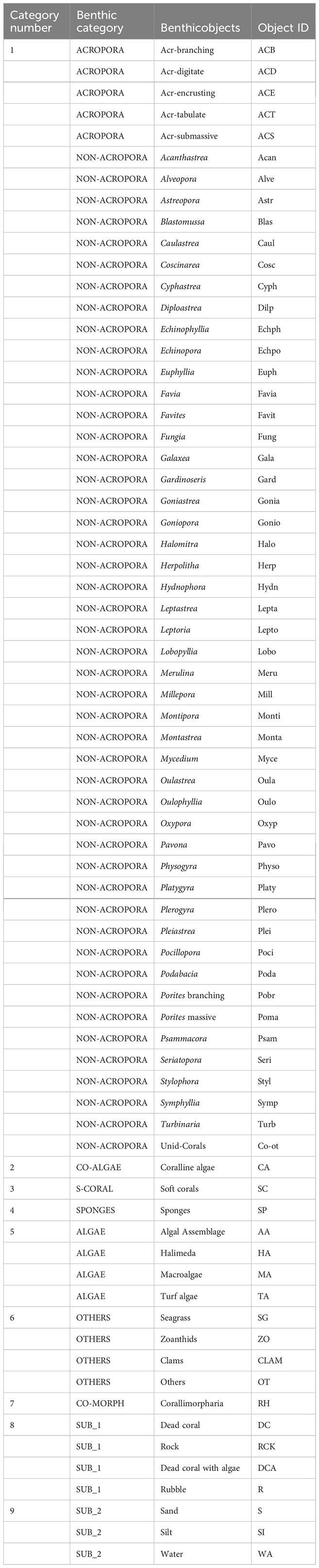
At each reef site, two to four permanent monitoring plots were established, each of approximately 75m x 50m. The plots were used for the ongoing monitoring and were separated by approximately 300 m. In each plot, 6 to 12 twenty-meter line-intercept transects (Table 1) were set on the reef flat (1 - 5m deep), the reef crest (3-4m deep) and the reef slope (5 - 15m deep), all parallel to the reef front. The transects within plots were not fixed but established haphazardly during each survey. The benthic assessment was done by taxonomically skilled divers, who measured all benthic categories underlying the 20-m long line-intercept transect (LIT) (English et al., 1997) with modifications. The modifications involved dentification of hard corals at the genus level instead of growth forms only and upgrading the coralline algae and Corallimorpharia from sub-categories into full categories because of their substantially increased dominance in the community. This modification resulted in benthic cover descriptions of nine categories instead of the previous seven Appendixes A, B).
Until 1998, coral reef benthic cover was described using seven categories (Appendix A) (Muhando, 2010); (1) Live hard corals (Acropora and non-Acropora), (2) Corallimorpharia, (3) Sponges, (4) Algae, (5) Others, (6) Soft non-biotic substrate (SU_1) and (7) Hard non-biotic substrate (SU_2). From 1999, the number of reef benthic cover categories was expanded by including specific species or species groups. In addition, coralline algae and hexacorallian soft corals (the order Corallimorpharia) were changed from sub-categories into full respective categories. This change allowed harmonization with previous categories (Appendix B) (Muhando, 2010). The number of sites, plots and transects investigated over the years and across seasons have changed over time (Table 1). In addition, the original transect data collected from 1992 to 1997 could not be obtainable and only aggregated data representing average values of cover data were available, and hence standardized.
Three data sets were used in our analysis. One dataset collected in 2015 included a more detailed description (Appendix B) of the biological diversity as well as non-biotic benthic elements of seven reefs (Table 1). This dataset was used to provide a detailed and the most recent characterization of the coral reef’s health. The second dataset was collected in 2010, 2011 and 2012 and was based on the detailed description (Appendix B), and was used to evaluate the effects of year, season, sampling zone and site on community structure. The third dataset consisted of a long-term time series (1992-2016) used to explore long-term changes in overall reef structure and health. Here we categorized data into nine groups consisting of (1) Live hard corals (Acropora species and other non-Acropora hard corals), (2) Coralline algae, (3) Soft corals, (4) Sponges, (5) Algae, (6) Others, (7) Corallimorpharia, (8) Hard non-biotic substrate (SUB_1) and (9) Soft non-biotic substrate (SUB_2). The constituent sub-categories for each group are shown in Appendix B.
A One-way ANOSIM test based on Bray-Curtis similarities (Bray and Curtis, 1957) from non-parametric multivariate statistical software PRIMER (Clarke and Gorley, 2015) was used to detect overall spatial differences in benthic community structure between reef sites in the 2015 data subset. Two replicates of each zone per location were considered. Possible seasonal effects were neglected as data from the different reef sites were collected over three seasons.
A Permutational Analysis of Variance (PERMANOVA from the PRIMER add-on package (Anderson, 2008) based on the Bray-Curtis similarity matrix was performed for the matrix of benthic cover studying the effect of location, year, season and zone in the subset of data covering three years (2010, 2011 and 2012), and two seasons. Data were unbalanced as both seasons were only present in 2011 (Table 1). The benthic covers were used without transformation of the data. Location, year, season and reef zone (slope, crest and flat) were included as fixed effects with interactive terms. We ran PERMANOVA using sums of squares (SS) Type III to account for the unbalanced design (Anderson, 2008). Highly insignificant terms (p>50%) were removed from the final model and those terms were pooled with residuals, as they were considered sources of error.
Similarities among sample groups (reefs, seasons, years, zones) are visualized in Multi-Dimensional Scaling plots (MDS-plots) from the PRIMER software package (Clarke et al., 2014; Clarke, 2015). A Beta Flexible Cluster analysis was performed on the nine key biological and structural categories and the results were used as an overlay on one MDS plot to visualize clusters of observation from the different reefs (Clarke et al., 2014). The SIMPER analysis in PRIMER was used to calculate the average similarity within groups of samples and the dissimilarity between groups. This procedure also provided the percentage of each category that contributed to the similarity and dissimilarity, and they were ranked according to their importance. No transformation on data was used as there was no need to down weight any category given the percentage cover scale used.
The long-term development of coral health was investigated for five reef sites; Chapwani, Changuu, Bawe, Chumbe and Misali, with data from the early 1990s. We used the non-parametric Spearman rank correlation analysis to describe changes over the years of five selected key elements; total hard coral cover, dead coral cover, algal cover and the cover of the more sensitive Acropora genus of corals compared to the less sensitive “non-Acropora” species group.
Investigation of differences in the coral community structure on reefs around Zanzibar in 2015 revealed an overall significant distinction between reef sites (One-way ANOSIM test, global R = 0.711, p = 0.001). Only two reef sites Nungwi and Mnemba at the northern tip of Unguja could not be distinguished from each other in the pairwise test (R statistics 0.124 and p = 0.0014). Although all other reef sites differed significantly, the sites near Stone Town (Chapwani, Bawe and Murogo) formed a cluster with pairwise R statistics between 0.232 and 0.409, and this group of reefs differed from the reefs at the northern and southern part of the island (Mnemba, Nungwi and Kizimkazi). Kwale was the only reef found in both of the two major clusters (Figure 2).
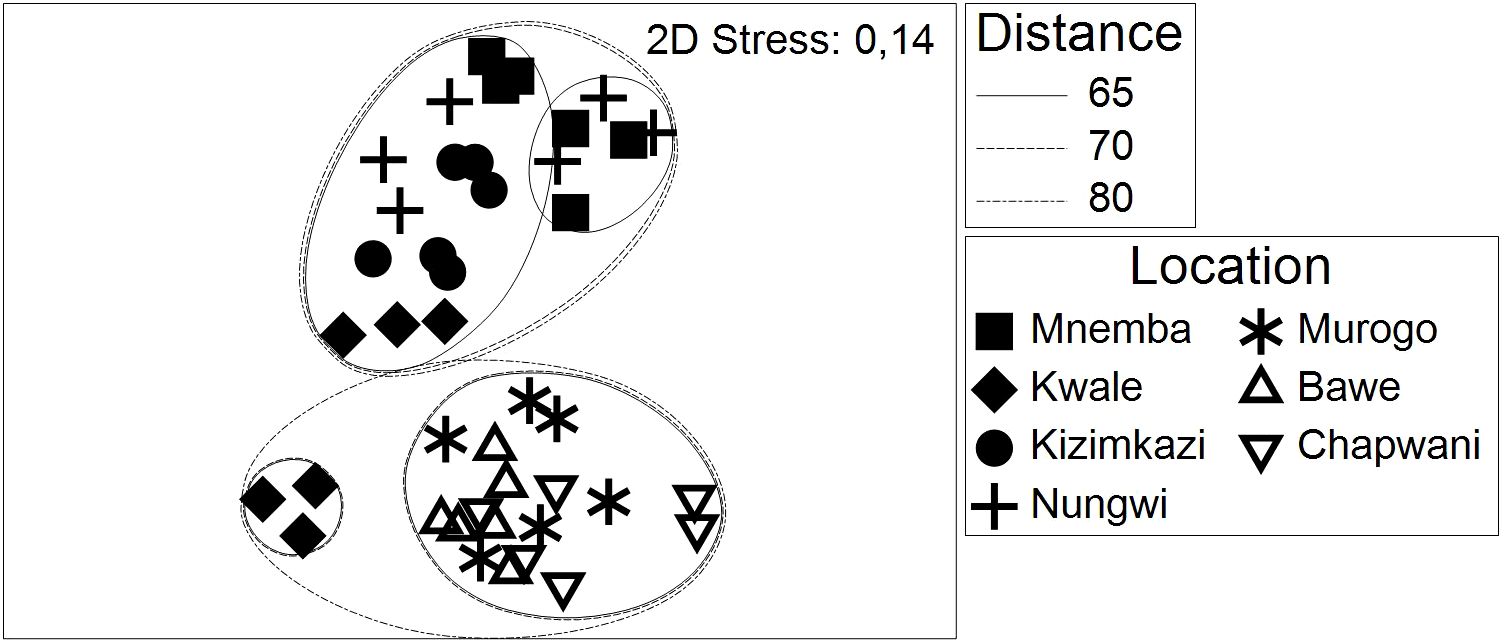
Figure 2 MDS plot reflecting key elements on coral reefs collected at seven different sites in 2015 around the Island Unguja. Six transects were monitored on each reef site. Clusters of observations generated by a beta flexible Cluster analysis are shown for three different distances and then overlaid the MDS plot.
To visualize the importance of the key components describing the different reef sites, we provided multidimensional scaling MDS plots where the sizes of the circles represent the cover of the eight most important biological and structural elements (Figure 3). The branching hard coral genus Porites and the soft coral Corallimorpharia were present in all transects on three reef sites near Stone Town (Chapwani, Murogo and Bawe). At other reef sites; Mnemba, Kwale, Kizimkazi and Nungwi, the Corallimorpharia cover was very minimal on most transects investigated The hard corals belonging to the genus Acropora had considerably higher cover at the offshore Kwale reef on the eastern side compared to all other reef sites. Soft corals, except Corallimorpharians, and algal species were present at all transects investigated at Mnemba, Nungwi and off Kizimkazi and reached a cover of up to 55% and 22% respectively, whereas soft corals other than Corallimorpharia and algae had relatively low coverage at the reef sites off Stone Town. The percentage of “rock” and “rubble” representing solid seabed from old dead coral reefs were considerably higher at the two northern reef sites Nungwi and Mnemba as well as the southern reef off Kizimkazi.
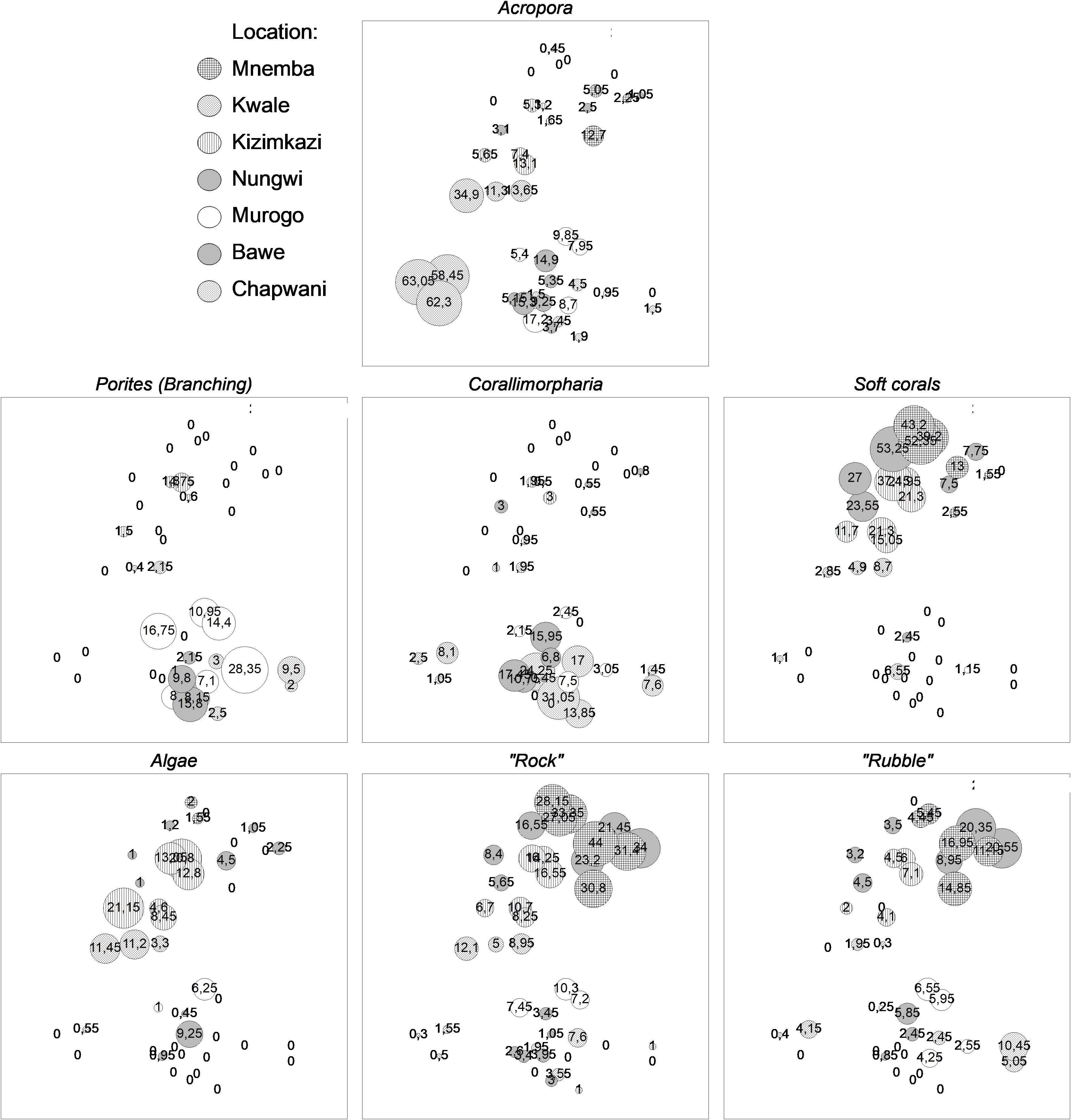
Figure 3 MDS plots reflecting similarities in cover of eight key biological and structural elements representing the seven different coral reefs in 2015 around Zanzibar. The size of the bubbles represents the percent cover with the actual value given within bubbles or as zero.
The datasets available for investigating differences among years, seasons zones on the reef, and reef sites were collected at Chumbe, Changuu and Mnemba reefs in 2010, 2011 and 2012. All four factors site, year, zone and season differed significantly among themselves and site, zone and year in combination with one another (Table 2). In total 51.2% of the total variation were explained with location being the far most important predictor of benthic habitat structure. Figure 4 illustrates the importance of location and sampling zones for data collected in each of the two seasons.
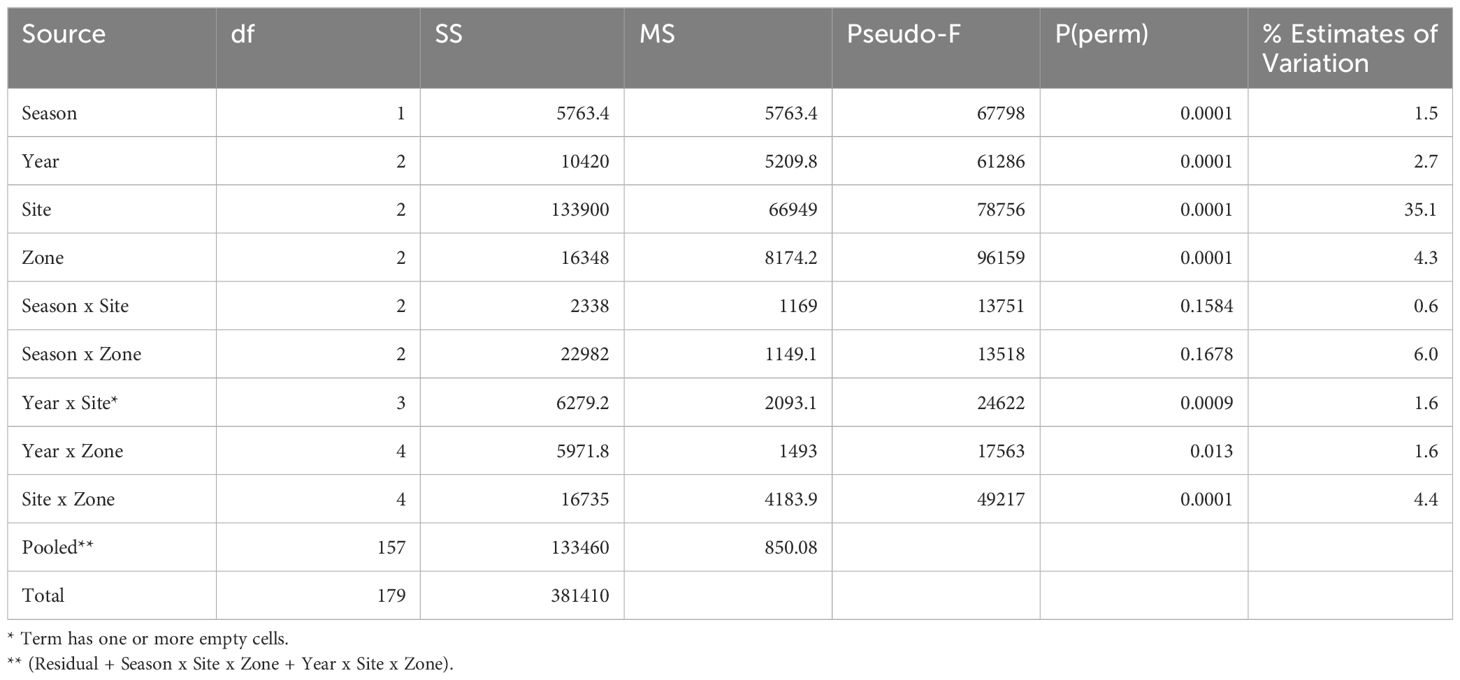
Table 2 PERMANOVA results on benthic community structure on three reefs (Chumbe, Changuu and Mnemba) in 2010, 2011 and 2012.
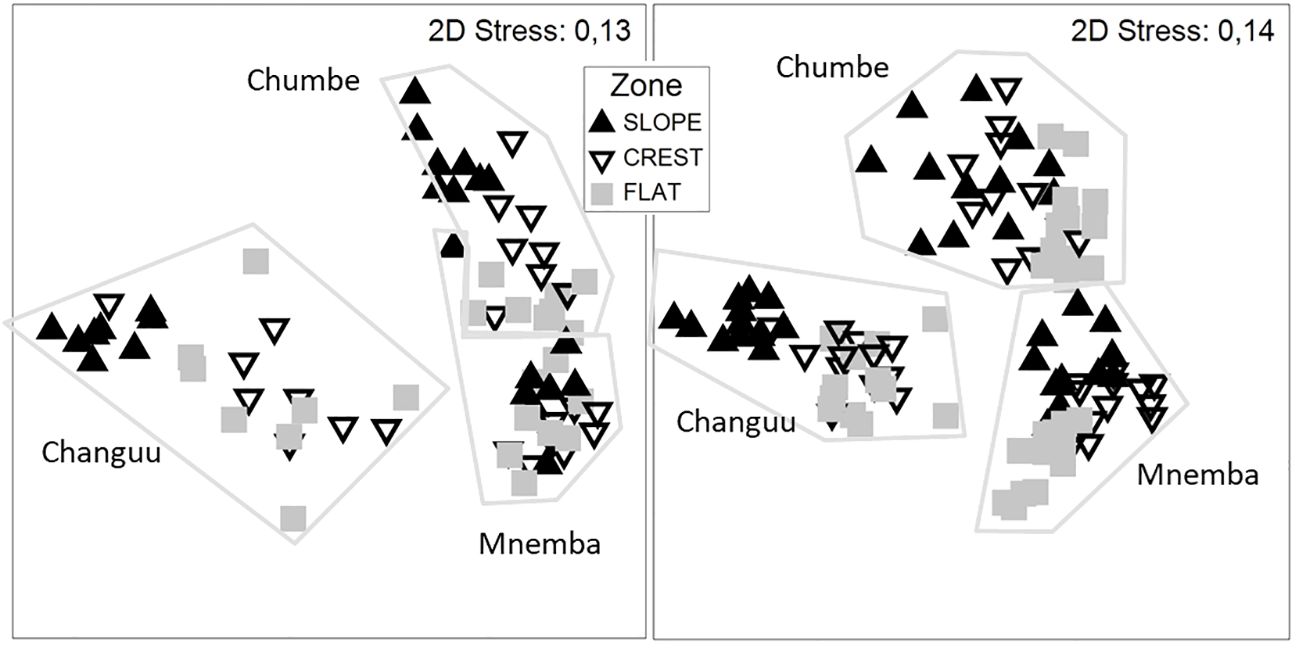
Figure 4 MDS plots showing the coral reef community structure of the different locations and sampling zones. This illustrates the importance of location and sampling zones (Flat, Crest and Slope) when considering reef characteristics. he left figure presents data collected in 2010 and 2011 during the short rain autumn season and the right figure data collected in 2011 and 2012 during the heavy rain spring season. Symbols indicate whether observations were made on the slope, crest or top flat part of the reef sites.
The SIMPER analysis showed that the most important benthic categories across years, zones and seasons that distinguished the Changuu reef from the other reefs was a substantially higher cover of the hard coral Porites and the soft coral Corallimorpharia (Table 3). The presence of a high amount of bare rock together with high algal coverage and almost no hard coral Porites, distinguished Mnemba from the other two reefs. There was a negative association between the algal overgrowths and the hard coral coverage at Chumbe. The hard coral Acropora was found to be a key genus for Chumbe compared with the other two sites. Altogether, the five key benthic categories (Porites, Acropora, Corallimorpharia, Algae and Rock) explained 50. 5 to 71.9% of the dissimilarity between the reefs for both seasons.
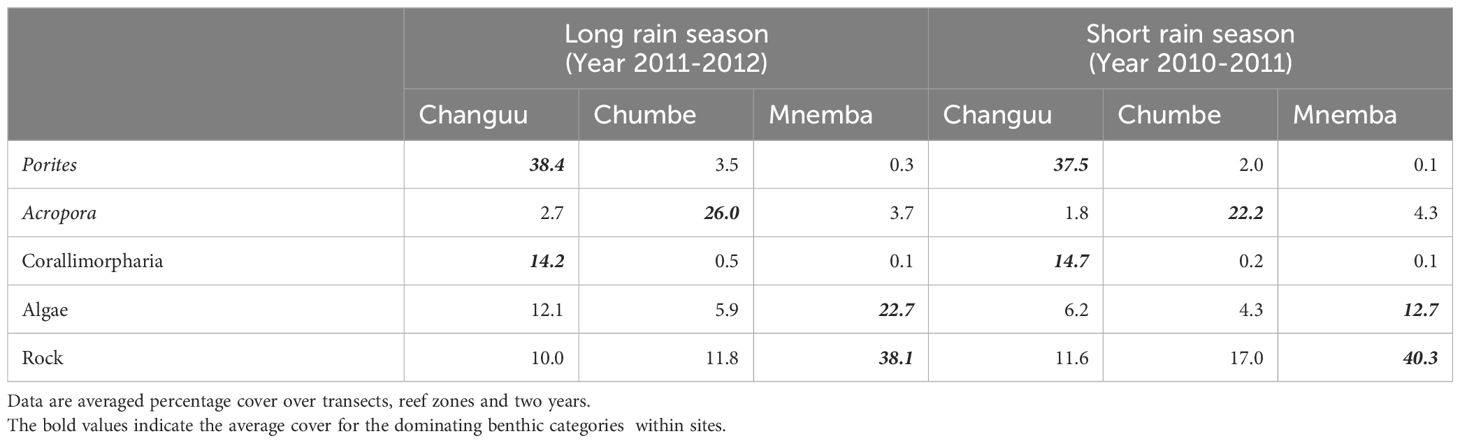
Table 3 Mean percentage cover of the most important key elements separating the community on three different reefs at two different seasons.
Data collected in 2011 at Chumbe, Changuu and Mnemba were most suitable to investigate differences between seasons. Algal cover and bare rock were the two most important benthic categories distinguished the two seasons. The average algal cover on the three reef sites was 17.2% in the heavy rainy spring season and only 4.9% in the short rainy autumn season in 2011. The average cover of bare rock shows the opposite pattern with 17.6% in the heavy rain season and 26.4% in the short rain season.
In early 1990’s, the observed total cover of hard coral species has ranged between 30% and 70% in most years at the four reef sites off Stone Town (Changuu, Chapwani, Bawe and Chumbe), with no significant trend (Figure 5, Table 4). At Misali reef west of Pemba Island, the total cover of hard coral used to be above 70% in the 1990s’ but four observations since 2003 were considerably lower at 20 to 30% cover (Figure 5), suggesting a significant decline in hard coral cover (Table 4). Common for all above mentioned five reef sites, except Chapwani, is a significant increase in dead coral over the 25 years of investigation from 1992-2016. The most dramatic increase in dead coral from almost zero to approximately 50% was observed at Misali corresponding to the decrease in total hard coral cover. Changes in algal cover were not significant for any of the reefs, but showed a tendency towards a decrease at the reef site off Chumbe but increased at all other sites (Figure 5, Table 4).
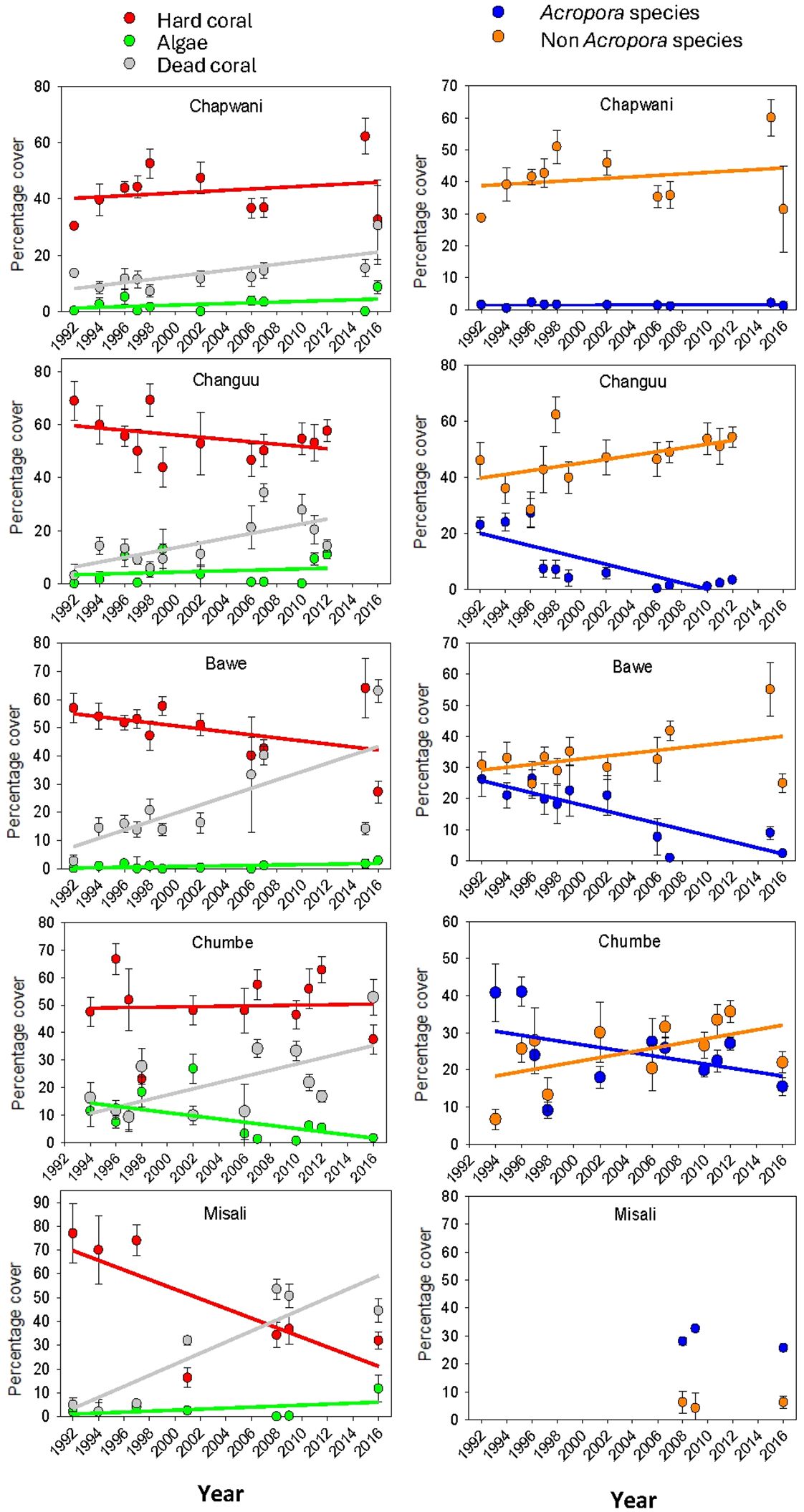
Figure 5 Cover of selected key elements ( ± Standard deviation) from 1992 until 2016 at four reef sites on the sheltered west coast of Unguja Island (Chumbe, Chapwani, Chunguu and Bawe) and one sheltered site (Misali) located off the coast of the northern Island Pemba. Three sites near Stone Town, (Chapwani, Chunguu and Bawe), represent reefs with increasing westward distance from Zanzibar City, with the small island Chumbe south of Stone Town even further away (See Figure 1). The left panel shows the cover of hard corals, dead corals and algae and the right panel shows the overall cover of Acropora species and the remaining cover of other hard corals. A full dataset for Acropora and non-Acropora does not exist for Misali. While the significance of trends is analyzed with Spearman rank correlation analysis (Table 4), linear regression lines are inserted to indicate trends.
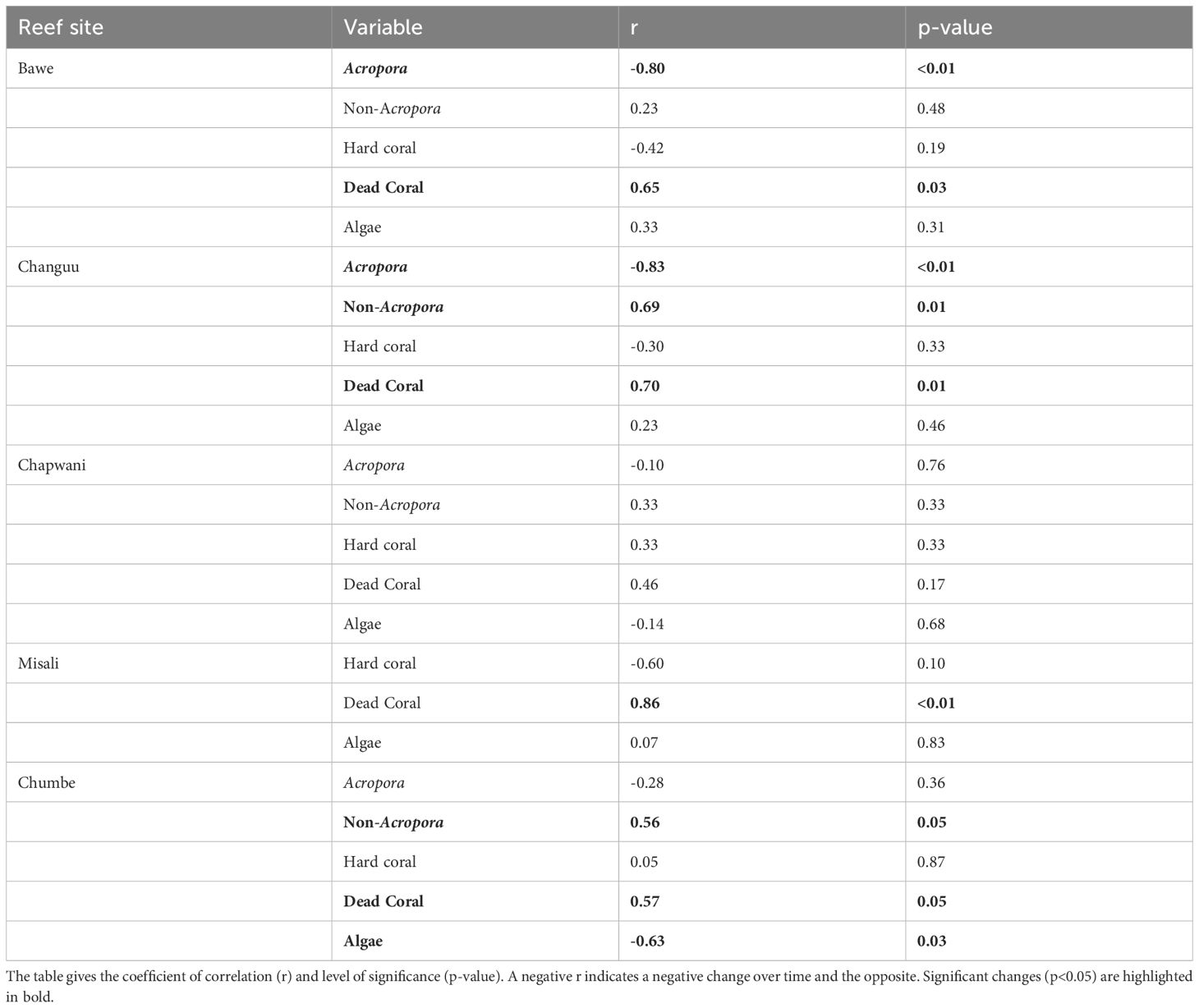
Table 4 Spearman rank correlation analysis on changes in five selected key elements (Acropora, non-Acropora, hard coral, dead coral and algae) on five reef sites off the coast of Zanzibar.
The more sensitive species belonging to the genus Acropora have become rare at the three reef sites closest to the Stone Town; Changuu, Chapwani and Bawe (Figure 5). There was a significant decline observed at Changuu and Bawe reefs (Table 4). The coverage of the non-Acropora hard coral species have increased significantly at Changuu and Chumbe, and is today dominating the coral community on most reefs around Unguja Island. However, this development is still not observed at Misali reef off Pemba Island.
Distinct differences in coral community structures were found among the seven monitored coral reefs in 2015 (Figure 2) and the three studied in 2010-2012. Results from Chumbe, Changuu and Mnemba indicated that some of the deviations identified in the 2015 investigation could be related to comparing different seasons. However, our result supports previous findings (Connell et al., 1997) that composition and coverage of corals and associated flora and fauna could be strongly influenced by physical disturbance and prevailing hydrodynamic regimes. The clear clustering of sites near the main population center, Stone Town, also supports the earlier studies on the importance of human pressure on reef community structure (Johnstone et al., 1998; Staehr et al., 2018). Reef clusters from the northern side of Unguja Island (Nungwi and Mnemba) were very different from the western side (Murogo, Bawe and Chapwani). Even though there were no physical variables measured in this study, which makes it very difficult to draw any conclusions other than based on literature, this finding suggests that protection from strong waves and strong currents (McClanahan et al., 2000) and proximity to human influence such as pollution, fishing pressure and large number of tourists visiting the sites over long time largely defined coral communities near Stone Town. Correspondingly, the similarity of Kwale and Kizimkazi reefs with the reefs from the northern side suggests the resemblances in exposure to physical disturbance and hydrodynamic regimes driven by monsoonal wind patterns, though in an opposite direction. Kizimkazi Reefs were highly exposed to the SE monsoon and were directly affected by strong waves from April to September, and Kwale reef to a lesser degree. Similarly, northern cluster reefs were highly exposed to the NE monsoon and were disturbed mostly from October to March. The partial overlap of the Kwale reef with the western cluster suggested that the southwest orientation provided some protection from the NE monsoon. Similar reef compositions amongst NE and SE monsoon exposed reefs accordingly, indicated the importance of differences in exposure level more than the location of the reef around the Island in defining the reef communities.
As reef communities develop in response to a combination of environmental, biological, chemical, physical and anthropogenic conditions (Veron and Stafford-Smith, 2000; Bell et al., 2015), it was difficult to relate and isolate the observed differences in 2015 to physical conditions alone. Nevertheless, the observed pattern of key biological and structural elements gave some important insights into the more specific differences in community structure amongst coral reefs (Figure 3). For instance, hard coral species of branching Porites, which were predominant in a relatively sheltered cluster of reefs off the Stone Town area, are recognized to thrive best in shallow leeward-sided reefs, as they are sensitive to strong waves and currents (Porter et al., 2017). Likewise, branching hard corals, such as Acropora species, are common on sheltered reefs with less wave exposure (Veron and Stafford-Smith, 2000), and indeed Kwale Reefs specifically on the leeward side supported higher cover of this genus. Higher cover of branching Acropora was also reported to dominate the sheltered Changuu and Bawe reefs, in particular off Stone Town, until the late 1990s (Obura, 2002; Muhando, 2003) when they were strongly reduced by the El Niño event in 1998 that caused massive coral bleaching. Soft corals and algae are known to thrive best in areas with strong water currents and wave exposure (Fabricius, 2005; Bronstein and Loya, 2014). High contributions from soft corals and algae at exposed reefs of Mnemba, Nungwi and Kizimkazi, along with exposed solid aragonite seabed (rock) and rubbles (Figure 3), were therefore expected. Growth of algae and soft corals such as Corallimorpharia were also expected to flourish in areas with high nutrient levels (McCook, 1999; Miller et al., 1999; Szmant, 2002; Islam and Tanaka, 2004; Fabricius, 2005). While algae did not contribute significantly, the cover of Corallimorpharia was higher on reef clusters near Stone Town (Chapwani, Bawe and Murogo). The findings support the recent observations of elevated levels of nutrients and organic pollution near Stone Town on the west coast of Unguja (Staehr et al., 2018), suggesting that these reefs are more affected by human induced changes compared to more remote reefs.
Differences in the distribution of corals have previously been documented in this region (Mbije et al., 2002). Our detailed sampling from 2010 to 2012 (Changuu, Mnemba and Chumbe reefs) similarly revealed significant differences in coral cover between individual reefs and between reef zones, but also between seasons and years (Figure 4, Table 3). Similar to (Zvuloni et al., 2010), our sampling supports expectations that reefs in well managed marine protected areas such as Chumbe, were in a better state with a higher cover of the sensitive Acropora corals, compared to non-protected reefs, such as Changuu or less managed reefs such as Mnemba, that were characterized by a low coral cover (Table 3). Regardless, all three reefs had almost twice as much cover of algae during the heavy rain season compared to other seasons (Table 3). This is because algae proliferate during periods of high sea-surface temperatures and elevated nutrient run-off thus supporting algal growth during the heavy rain season, before being abraded during the cold and windy SE monsoon period in June to August (Shunula, 1988; Ussi, 2014). There was a slight negative association between the algal overgrowths and the hard coral cover at Chumbe, which suggests that seasonal differences in algal overgrowth could have affected estimates of hard coral cover. Although there were significant differences in the cover of algae and hard coral cover between the two main seasons at Chumbe (Table 4), the overall projection revealed a positive trend in hard coral cover over the years and a negative trend in macroalgal cover (Figure 5). The results imply that with our current monitoring approach and the available dataset, we fail to conclude with confidence if this observed algal growth cover seasonality at Chumbe could affect coral cover. This possible bias therefore highlights the necessity of optimizing the coral reef monitoring methodology, which should account more accurately for hard corals and algae, and the relationship/association between these two. Furthermore, a consistent sampling period is highly recommended.
Similar to the 2015 data set, we observed a higher cover of soft coral Corallimorpharia at the Changuu Reef from 2010 to 2012. This reef was very close to the untreated sewage water effluents from Stone Town, indicating that the site was affected by elevated nutrient levels (Muhando et al., 2002; Staehr et al., 2018). In comparison, reefs near Mnemba on the north coast had a higher cover of bare rock and algae but lower cover of soft corals and Porites. It seemed likely that this difference resulted from a higher exposure to strong waves and currents (Richmond and Francis, 2001; Mbije et al., 2002; Bronstein and Loya, 2014), and that only the substrate became occupied seasonally by resistant sessile benthic species, like certain macroalgal species.
Historically, reef communities close to Stone Town, specifically Changuu and Bawe, were more similar to the remote reefs further away from Stone Town such as Chumbe and Kwale. Being geographically located on the sheltered western side of the major Unguja Island, these reefs were initially dominated by dense populations of branching Acropora coral, which develop well in sheltered areas (Muhando, 2003). Our results showed that from 1992 to 2016, the cover of hard corals ranged between 40% and 80% in the five monitored reef sites. There was, however, an overall decline in Acropora at four of the reef sites with an annual average loss of 0.5-2.3% since 1997. The loss of Acropora was mostly profound at Bawe and Changuu (Figure 5). Devastation by the invasive crown-of-thorns starfish that started in 1997 (Obura, 2002; Ussi, 2009), followed by a massive coral bleaching event in 1998, resulted in a widespread mortality of Acropora (Wilkinson, 1998; Obura, 2002). The crown-of-thorns starfish problem reappeared from 2004 to 2009, and resulted in even higher mortality of recovering Acropora. A crown-of-thorns starfish removal program was introduced at Chumbe from 2004 (Muhando and Lanshammar, 2008). Interestingly, the cover of Acropora, with a persistent crown-of-thorns starfish removal program, increased considerably compared to other sites like Bawe and Changuu (Muhando and Lanshammar, 2008) until the 2016 El Niño bleaching event (Gudka et al., 2018) (Figure 5). The findings suggest that the establishment of marine protected conservation areas with active management including consistent removal of the reef predator like the invasive crown-of-thorns starfish, together with control of fishing activities (Staehr et al., 2018) could have enhanced the recovery of Acropora corals. In a similar way, the coral reef at Misali, Pemba was severely damaged by the El Niño event in 1998, diminishing the hard coral cover by a factor of 4 (Figure 5). Establishment of a non-extraction zone (~protected area) within a Marine Conservation Area Reef helped hard corals recovery (Poonian, 2008). Our data furthermore documented recovery of Acropora during the last three investigations in 2008, 2009 and 2016 at Misali reefs. However, prior to 2007, there was no record of Acropora in Misali because the benthic cover categories during data collection did not consider specific species or species groups. In comparison, non Acropora species had increased on all our reef sites around Unguja Island, although not significantly. The other more remote reef among surveyed sites of Unguja, Kwale, showed some stability in hard coral cover. The observed stability could explain the impact of less exposure to human pressure due to a greater distance from human residence and better exchange of clean waters. In summary, these results highlighted the recovery potential of Zanzibar reefs, given proper management of human related pressures, allowing reefs to remain at proper thriving conditions.
According to (Vytopil and Willis, 2001) the loss in Acropora cover could have negative impact on reef communities due to loss of important microhabitats. Branching, bushy and tabulate Acropora corals are well known to significantly contribute to reef complexity, which importantly provides microhabitats for juveniles of many fish species and other invertebrates (Stella et al., 2010; Graham and Nash, 2013). Likewise, branching Acropora is known to contribute to increased modifications to the local hydrodynamic environment (Holmes and Johnstone, 2010), and increased potential for niche separation (Ricklefs and Schluter, 1993). Hence, the loss of Acropora on reefs off Zanzibar has likely caused important negative changes in reef ecosystem functions.
The highest cover of algae was observed on Chumbe reef in the 1990s and early 2000s, with a gradual decrease in recent years where reef management intervention has been in place (Muhando and Lanshammar, 2008). In comparison, algal cover seemed to be gradually increasing at Changuu lately and at Chapwani and Misali in 2016. This change in algal cover could have been influenced by changes in local conditions in terms of water quality (D’Angelo and Wiedenmann, 2014; Risk, 2014; Silbiger et al., 2018). Changuu and Chapwani were prone to nutrient pollution from Stone Town (Staehr et al., 2018). However, the varying sampling season likely played an important role as well, as shown in Table 3. Regardless, the results highlight the necessity of detailed monitoring of water quality along the coast of Zanzibar to strengthen coastal ecosystem management through pollution control.
The global coral reef ecosystems have experienced substantial fluctuations over the past times due to numerous environmental pressures, including climate change, overfishing, and pollution (Hughes et al., 2017). Recent assessments reveal that about half of the world’s coral reefs are considered degraded due to these environmental stressors. Live hard coral cover was comparatively stable until the first massive coral bleaching event in 1998. The event led to a substantial 8% loss in global coral cover. Between 2009 and 2018, there was an additional decline of approximately 14% in coral cover, mostly due to recurrent large-scale coral bleaching events exacerbated by deficient recovery periods between events (Souter et al., 2021). The observed trend in coral cover in this study contributes to the reported global decline. However, efforts to improve reef management, increase resilience through reef restoration techniques, and global initiatives to decrease greenhouse gas emissions are critical to reversing some of these trends and safeguarding future coral health. Overall, the health of the world’s coral reefs is a combination of challenging declines and hopeful signs of resilience, underscoring the need for continued conservation and intervention efforts to preserve these vital ecosystems.
In conclusion, we provide the first documentation of long-term degradation of coral reefs in Zanzibarian waters with significant increase of areas with dead corals at the investigated sites. For most reefs, particularly sites off Stone Town, we report a significant decline of Acropora species being replaced by non-Acropora species and the soft coral Corallimorpharia, which were favored by elevated nutrient concentrations (Muhando et al., 2002). El Nino events and invasion by crown-of-thorn starfish likely also contributed to this decline, with physical disturbance from fisheries and tourism further exacerbated the degradation (Staehr et al., 2018). These findings call for concern and the need for better management of human influence. In particular, attention should be given to control of coastal pollution with nutrients and toxic substances. Attention should also be given to destructive fishing efforts and other physical damage on reef structures, such as underwater tourism. Such management is vital for development of a long-term sustainable local food supply and tourist industry. Promising results were provided from the protected reef site at Chumbe, where enforced restriction of tourists and fishing as well as active efforts to reduce the numbers of invasive crown of thorn starfish have been undertaken. These initiatives showed a strong recovery potential of Zanzibar coral reefs even after severe El Nino events, with regrowth of sensitive Acropora species and a flourishing fish community. To better understand the causes and effects of these human mediated pressures, and possible mitigation success, a long-term monitoring program needs to be implemented. To evaluate the success of urgently needed management to ensure continued health function of these highly valuable coastal habitats, it is essential to strengthen the currently fragmented monitoring on reef health around Zanzibar. The monitoring of coral reef communities should apply a methodology that accounts for effects of seasonality by describing algal cover and fauna as an add-on cover on top of the hard substrate of living and dead coral structures and other sessile faunal organisms.
Consistent reef monitoring is important to document the changes of corals and associated communities to climate change, and other anthropogenic and/or natural disturbances. Such knowledge is essential for identifying areas of concern, initiate conservation and restoration actions to protect and restore endangered reef sites.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The manuscript presents research on animals that do not require ethical approval for their study.
AU: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. MM: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. RR: Writing – review & editing. MS: Writing – review & editing. PS: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. CM: Investigation, Writing – review & editing. SY: Investigation, Writing – review & editing. KD: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Danish Ministry of Foreign Affairs supported this work through the Building Stronger Universities Project—Phases two and three (BSU-II & III) under DANIDA.
We greatly appreciate constructive comments from two reviewers and help from Sanjina Upadhyay to improve language and structure of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Anderson M. (2008). PERMANOVA+ for PRIMER: guide to software and statistical methods (Plymouth Marine Laboratory: Primer-E Limited).
Bell J. J., McGrath E., Biggerstaff A., Bates T., Bennett H., Marlow J., et al. (2015). Sediment impacts on marine sponges. Mar. Pollut. Bull. 94, 5–13. doi: 10.1016/j.marpolbul.2015.03.030
Bray J. R., Curtis J. T. (1957). An ordination of the upland forest communities of southern Wisconsin. Ecol. Monographs. 27, 326–349. doi: 10.2307/1942268
Bronstein O., Loya Y. (2014). Echinoid community structure and rates of herbivory and bioerosion on exposed and sheltered reefs. J. Exp. Mar. Biol. Ecology. 456, 8–17. doi: 10.1016/j.jembe.2014.03.003
Chevallier R. (2017). Safeguarding Tanzania's coral reefs: the case of illegal blast fishing. South African Institute of International Affairs, 31.
Clarke K. (2015). PRIMER v7: user manual/tutorial (Plymouth, plymouth marine laboratory: PRIMER-E) 20(1).
Clarke K. R., Gorley R. N., Somerfield P. J., Warwick R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation. 2, 1–168.
Connell J. H., Hughes T. P., Wallace C. C. (1997). A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol. Monographs. 67, 461–488. doi: 10.1890/0012-9615(1997)067[0461:AYSOCA]2.0.CO;2
D’Angelo C., Wiedenmann J. (2014). Impacts of nutrient enrichment on coral reefs: new perspectives and implications for coastal management and reef survival. Curr. Opin. Environ. Sustainability. 7, 82–93. doi: 10.1016/j.cosust.2013.11.029
English S., Wilkinson C., Baker V. (1997). Survey manual for tropical marine resources. Australian Institute of Marine Science, Townville, 34–49.
Fabricius K. E. (2005). Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bulletin. 50, 125–146. doi: 10.1016/j.marpolbul.2004.11.028
Graham N. A., Nash K. L. (2013). The importance of structural complexity in coral reef ecosystems. Coral Reefs. 32, 315–326. doi: 10.1007/s00338-012-0984-y
Gray N. J., Bennett N. J., Day J. C., Gruby R. L., Wilhelm T. A., Christie P. (2017). Human dimensions of large-scale marine protected areas: advancing research and practice. Coast. Management. 45, 407–415. doi: 10.1080/08920753.2017.1373448
Gudka M., Obura D., Mwaura J., Porter S., Yahya S., Mabwa R. (2018). Impact of the 3rd global coral bleaching event on the Western Indian Ocean in 2016 (Global Coral Reef Monitoring Network (GCRMN)/Indian Ocean Commission), 1–67.
Holmes G., Johnstone R. (2010). Modelling coral reef ecosystems with limited observational data. Ecol. Modelling. 221, 1173–1183. doi: 10.1016/j.ecolmodel.2010.01.010
Hughes T. P., Barnes M. L., Bellwood D. R., Cinner J. E., Cumming G. S., Jackson J. B. C., et al. (2017). Coral reefs in the anthropocene. Nature 546, 82–90. doi: 10.1038/nature22901
Islam M. S., Tanaka M. (2004). Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar. Pollut. Bull. 48, 624–649. doi: 10.1016/j.marpolbul.2003.12.004
Jiddawi N. S., Öhman M. C. (2002). Marine fisheries in Tanzania. Ambio: J. Hum. Environment. 31, 518–527. doi: 10.1579/0044-7447-31.7.518
Johnstone R. W., Muhando C. A., Francis J. (1998). The status of the coral reefs of Zanzibar: one example of a regional predicament. Ambio 27 (8), 700–707. doi: I10.2307/4314818
Lange G.-M., Jiddawi N. (2009). Economic value of marine ecosystem services in Zanzibar: Implications for marine conservation and sustainable development. Ocean Coast. Management. 52, 521–532. doi: 10.1016/j.ocecoaman.2009.08.005
Mbije N. E., Wagner G. M., Francis J., Öhman M. C., Garpe K. (2002). Patterns in the distribution and abundance of hard corals around Zanzibar Island. AMBIO: A J. Hum. Environment. 31, 609–611. doi: 10.1579/0044-7447-31.7.609
McClanahan T. R., Ateweberhan M., Muhando C. A., Maina J., Mohammed M. S. (2007). Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecol. Monographs. 77, 503–525. doi: 10.1890/06-1182.1
McClanahan T., Muthiga N., Kamukuru A., Machano H., Kiambo R. (1999). The effects of marine parks and fishing on coral reefs of northern Tanzania. Biol. Conserv. 89, 161–182. doi: 10.1016/S0006-3207(98)00123-2
McClanahan T. R., Sheppard C. R., Obura D. O. (2000). Coral reefs of the Indian Ocean: their ecology and conservation (New York, NY: Oxford University Press). doi: 10.1093/oso/9780195125962.001.0001
McCook L. J. (1999). Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs. 18, 357–367. doi: 10.1007/s003380050213
Miller M., Hay M., Miller S., Malone D., Sotka E., Szmant A. (1999). Effects of nutrients versus herbivores on reef algae: a new method for manipulating nutrients on coral reefs. Limnology Oceanography. 44, 1847–1861. doi: 10.4319/lo.1999.44.8.1847
Muhando C. (2003). Enhanced coral larval settlement and coral transplantation as means of promoting coral replenishment in Tanzania. PhD Theses, Dar es Salaam, Tanzania: University of Dar es Salaam.
Muhando C. A. (2008). Approaches to coral reef monitoring in Tanzania. In: Obura D. O., Tamelander J., Linden O. (Eds) Ten years after bleaching facing the consequences of climate change in the Indian Ocean. CORDIO Status Report 2008. CORDIO (Mombasa: Coastal Oceans Research and Development in the Indian Ocean)/Sida-SAREC) 489 pp. Available at: http//:www.cordioea.org.
Muhando C. A. (2010). Calibration of community-based coral reef monitoring protocols: Tanzanian case study. Western Indian Ocean J. Mar. Science. 9, 103–114.
Muhando C. A., Jiddawi N. (1998). “Fisheries resources of Zanzibar: Problems and recommendations,” in Large marine ecosystems of the Indian Ocean: assessment, sustainability and management, 247–254.
Muhando C. A., Kuguru B. L., Wagner G. M., Mbije N. E., Öhman M. C. (2002). Environmental effects on the distribution of corallimorpharians in Tanzania. AMBIO: A J. Hum. Environment. 31, 558–561. doi: 10.1579/0044-7447-31.7.558
Muhando C. A., Lanshammar F. (2008). Ecological effects of the crown-of-thorns starfish removal programme on Chumbe Island Coral Park, Zanzibar, Tanzania. Proceedings of the 11th International Coral Reef Symposium, Ft. Lauderdale, Florida.
Muzuka A. N., Dubi A. M., Muhando C. A., Shaghude Y. W. (2010). Impact of hydrographic parameters and seasonal variation in sediment fluxes on coral status at Chumbe and Bawe reefs, Zanzibar, Tanzania. Estuarine Coast. Shelf Science. 89, 137–144. doi: 10.1016/j.ecss.2010.05.007
Nzali L. M., Johnstone R. W., Mgaya Y. (1998). Factors affecting scleractinian coral recruitment on a nearshore reef in Tanzania. Ambio 27 (8), 717–722. doi: 10.2307/4314820
Obura D. (2002). Status of coral reefs in Eastern Africa: Kenya, Tanzania, Mozambique and South Africa. In: Clive Willinson (ed.) Status of Coral Reefs of the World: 2002 Australian Institute of Marine Science. 63–78pp.
Poonian C. (2008). The influence of protected area management on the status of coral reefs at Misali Island, Tanzania following the 1998 bleaching event in the western Indian Ocean. Afr. J. Ecology. 46, 471–478. doi: 10.1111/j.1365-2028.2007.00873.x
Porter S. N., Branch G. M., Sink K. J. (2017). Changes in shallow-reef community composition along environmental gradients on the East African coast. Mar. Biol. 164, 101. doi: 10.1007/s00227-017-3130-0
Raycraft J. (2018). Marine protected areas and spatial fetishism: A viewpoint on destructive fishing in coastal Tanzania. Mar. Pollut. Bulletin. 133, 478–480. doi: 10.1016/j.marpolbul.2018.06.008
Richmond M., Francis J. (Eds.). (2001). Marine science development in Tanzania and eastern Africa. 20th Anniversary Conference on Advances in Marine Science in Tanzania (1999: Zanzibar, Tanzania) (Zanzibar, Tanzania: Institute of Marine Sciences, University of Dar es Salaam and Western Indian).
Ricklefs R. E., Schluter D. (1993). Species diversity: regional and historical influences. Species Diversity Ecol. communities. 1, 350–363.
Risk M. J. (2014). Assessing the effects of sediments and nutrients on coral reefs. Curr. Opin. Environ. Sustainability. 7, 108–117. doi: 10.1016/j.cosust.2014.01.003
Shunula J. (1988). Seasonal growth and reproduction of two species of Sargassum at Pange Island, Zanzibar, Tanzania. J. Mar. Biol. Ass India. 30, 160–163.
Silbiger N. J., Nelson C. E., Remple K., Sevilla J. K., Quinlan Z. A., Putnam H. M., et al. (2018). Nutrient pollution disrupts key ecosystem functions on coral reefs. Proc. R. Soc. B 285, 20172718. doi: 10.1098/rspb.2017.2718
Souter D., Planes S., Wicquart J., Logan M., Obura D., Staub F. (eds). (2021). Status of coral reefs of the world: 2020 report. Global Coral Reef Monitoring Network (GCRMN) and International Coral Reef Initiative (ICRI). doi: 10.59387/WOTJ9184
Staehr P., Sheikh M., Rashid R., Ussi A., Suleiman M., Kloiber U., et al. (2018). Managing human pressures to restore ecosystem health of zanzibar coastal waters. J. Aquac Mar. Biol. 7, 59–70. doi: 10.15406/jamb
Stella J. S., Jones G. P., Pratchett M. S. (2010). Variation in the structure of epifaunal invertebrate assemblages among coral hosts. Coral reefs. 29, 957–973. doi: 10.1007/s00338-010-0648-8
Szmant A. M. (2002). Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuaries 25, 743–766. doi: 10.1007/BF02804903
Ussi A. M. (2009). Population distribution and impact of Crown-of thorns starfish, Acanthaster planci (L), on some coral reefs of Zanzibar. Dar es Salaam, Tanzania: University of Dar es Salaam.
Ussi A. (2014). Recruitment and growth of scleractinian corals in relation to bio-physical processes in reefs of Unguja island, Zanzibar, Tanzania. Dar es Salaam, Tanzania: University of Dar es Salaam.
Ussi A., Mohammed M., Muhando C., Yahya S. (2019). “Ecological impact of thermal stress in reefs of Zanzibar following the 2016 elevated higher sea surface temperatures,” in Climate change and coastal resources in Tanzania: studies on socio-ecological systems’ Vulnerability, resilience and governance, 93–115.
Veron J., Stafford-Smith M. (2000). Corals of the world Australian institute of marine science (Townsville: CCR Old Ptv Ltd).
Vytopil E., Willis B. (2001). Epifaunal community structure in Acropora spp.(Scleractinia) on the Great Barrier Reef: implications of coral morphology and habitat complexity. Coral Reefs 20, 281–288. doi: 10.1007/s003380100172
Wallner-Hahn S., Molander F., Gallardo G., Villasante S., Eklöf J. S., Jiddawi N. S., et al. (2016). Destructive gear use in a tropical fishery: Institutional factors influencing the willingness-and capacity to change. Mar. Policy. 72, 199–210. doi: 10.1016/j.marpol.2016.07.001
Wells S. (2009). Dynamite fishing in northern Tanzania–pervasive, problematic and yet preventable. Mar. pollut. Bulletin. 58, 20–23. doi: 10.1016/j.marpolbul.2008.09.019
Wilkinson C. (1998). Status of coral reefs of the world (Townsnville: Australian Institute of Marine Science). Internal Report.
Wilkinson C., Lindén O., Cesar H., Hodgson G., Rubens J., Strong A. E. (1999). Ecological and socioeconomic impacts of 1998 coral mortality in the Indian Ocean: An ENSO impact and a warning of future change? Ambio 28, 188–196.
Wolanski E., Richmond R., McCook L., Sweatman H. (2003). Mud, marine snow and coral reefs: the survival of coral reefs requires integrated watershed-based management activities and marine conservation. Am. Scientist. 91, 44–51. doi: 10.1511/2003.11.44
Zvuloni A., van Woesik R., Loya Y. (2010). Diversity partitioning of stony corals across multiple spatial scales around Zanzibar Island, Tanzania. PloS One 5, e9941. doi: 10.1371/journal.pone.0009941
Keywords: coral reef, community structure, zonation, seasonality, long-term changes, Zanzibar
Citation: Ussi AM, Mohammed MS, Rashid RJ, Sheikh MA, Staehr PA, Muhando CA, Yahya S and Dahl K (2024) Status and long-term changes of coral reefs around Zanzibar. Front. Mar. Sci. 11:1334235. doi: 10.3389/fmars.2024.1334235
Received: 06 November 2023; Accepted: 15 May 2024;
Published: 30 May 2024.
Edited by:
Heliana Teixeira, University of Aveiro, PortugalReviewed by:
Maria Eggertsen, Swedish University of Agricultural Sciences, SwedenCopyright © 2024 Ussi, Mohammed, Rashid, Sheikh, Staehr, Muhando, Yahya and Dahl. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali M. Ussi, YW1hdTA0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.