
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci., 22 July 2024
Sec. Marine Fisheries, Aquaculture and Living Resources
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1325120
Determination of bar spacing of bycatch reduction devices (BRDs) should consider species composition and morphometric characteristics (particularly width) of target species krill and bycatch. This study conducted a scientific investigation of the finfish bycatch in the Antarctic krill (Euphausia superba) trawl fishery by the fishing vessel SHEN LAN in the waters surrounding the South Orkney Islands from December 24, 2022, to February 20, 2023. The results show that scientific observers sampled 676 individuals of finfish bycatch. Of these, 665 were identified to species (17 species from 8 families), while the remaining 11 specimen were juveniles of the Nototheniidae family that could not be identified to the species level. IRI (index of relative importance) calculations showed three dominant (IRI value greater than 1,000) finfish bycatch species (Champsocephalus gunnari, Pseudochaenichthys georgianus, and C. aceratus from the Channichthyidae family) and four important (IRI value between 1,000 and 100) finfish bycatch species (Electrona carlsbergi and Gymnoscopelus nicholsi from the Myctophidae family, Gobionotothen gibberifrons from the Nototheniidae family, and Notolepis coatsi from the Paralepididae family). Our study provides morphometric data (particularly body width) that is crucial to model the potential for bycatch reduction by use of bycatch reduction devices (BRDs) and to determine the appropriate candidate bar spacings for BRD sea trials. Predictions suggest that a 10 mm (the maximum body width of krill) bar spacing releases a significant amount of dominant and important bycatch species (93.94% of C. gunnari, 53.99% of P. georgianus, 76.25% of C. aceratus, and 100% of G. gibberifrons). Reduced fishing pressure would reduce the risks to dominant and important bycatch species to make the krill fishery sustainable. We recommend that future BRD sea trials should initially test a 10 mm bar spacing. If marked loss of krill is observed, wider spacings (e.g. 15 mm) must be tested.
Antarctic krill (Euphausia superba, hereafter “krill”) is abundant and widely distributed in the Southern Ocean (Atkinson et al., 2008). It also has the largest catchable biomass in the world. As a source of protein for human and animal consumption, commercial fishing for krill is expected to increase in the future with new and efficient fishing technologies (Gigliotti et al., 2008; Nicol et al., 2012). The annual krill catch has increased from around 100,000 tons at the beginning of the 21st century to 400,000 tons in recent years (CCAMLR, 2022a). The total allowable catch for the southwest Atlantic is currently about 5.61 million tons annually, according to CCAMLR.
Krill swarms are typically found at depths of 0-200 m. The midwater trawls are the most effective fishing gear for krill (Everson et al., 1992; Nicol and Endo, 1999; Siegel and Watkins, 2016). Commercial krill trawls typically use double netting because of the small size (<60 mm length) of the krill. Small-mesh liners are fitted in a trawl body and codend to reduce krill catch loss through meshes (Everson et al., 1992; Wang et al., 2021; CCAMLR, 2023a). This design makes it challenging for non-target organisms to escape once they enter the trawl. Therefore, the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) mandated that all krill trawls must have an “exclusion device” to prevent the accidental catch of whales, seals, and other large marine organisms (CCAMLR 2023b).
An “exclusion device” usually involves one of two main types of separator netting panels. The first type covers the mouth of the trawl to prevent large marine organisms from entering the trawl. The other type is a slanting net panel placed in the forward part of the belly that guides large marine organisms towards a release hole in the top or side panels. The mesh size of the separator netting is usually 200-300 mm, which balances the towing resistance and the performance to prevent large marine organisms from entering the trawl (CCAMLR 2023c). However, smaller organisms, such as finfish can pass through the separator netting and enter the codend. Large quantities of finfish bycatch may obstruct the transportation pipeline and cause stoppage during the fishing process. Additionally, it is challenging to separate finfish bycatch from the krill in both the capture and onboard processing phases.
We have developed an anti-blocking exclusion device to reduce finfish bycatch in krill trawls (Figure 1). This device is placed in the extension section of the trawl and permits the entry of krill into the codend through the bar spacings. In contrast, larger-sized bycatch cannot pass through bar spacing and will escape by activating a pressure spring to open a releasing vent. Determination of bar spacing should consider morphometric characteristics (particularly width) of target species krill and bycatch, particularly the vulnerable species (Hornborg et al., 2013; Gamaza et al., 2015; Tokac et al., 2016; De Juan et al., 2020).
Therefore, we collected finfish bycatch in krill trawls by a commercial F/V SHEN LAN in the waters of the South Orkney Islands during the 2022/23 fishing season. We analyzed the morphometric characteristics of the bycatch species to determine the appropriate bar spacing of rigid bycatch exclusion devices. The implementation of such devices is crucial for reducing the fishing impact on vulnerable finfish species and thereby ensuring a responsible krill fishery.
Scientific observers collected finfish bycatch specimens from the conveyor belt outlet in the processing room onboard F/V SHEN LAN in the waters of South Orkney Islands. The vessel used conventional and continuous fishing methods, with a 400 mm mesh-sized netting panel covering the trawl mouth opening to prevent the accidental catch of large marine organisms. The entire trawl aft of the marine mammal exclusion device (Figure 2) were equipped with small-mesh (16 mm) liners.
Continuous fishing operations were carried out in 404 hauls between December 22, 2022, and January 15, 2023. Hauls are defined as the catch taken during a 2-hour period (i.e., 00.00-02.00 hours, 02.00-04.00 hours, etc.), except for the first haul and last haul based on the procedure of CCAMLR (CCAMLR, 2019). The daily krill catch was estimated using a flow scale and allocated to each haul based on its holding tank volume. Conventional trawling operations were carried out in 249 hauls from January 20, 2023, to February 25, 2023. Finfish bycatch specimens were collected from 56 hauls; 15 hauls of continuous fishing and 41 hauls of conventional trawling. Sampling positions (Figure 3) were plotted using the package “ggOceanMaps” (Vihtakari, 2024) in R version 4.1.0 (R Core Team, 2024). Detailed information on each haul is provided in Appendix 1.
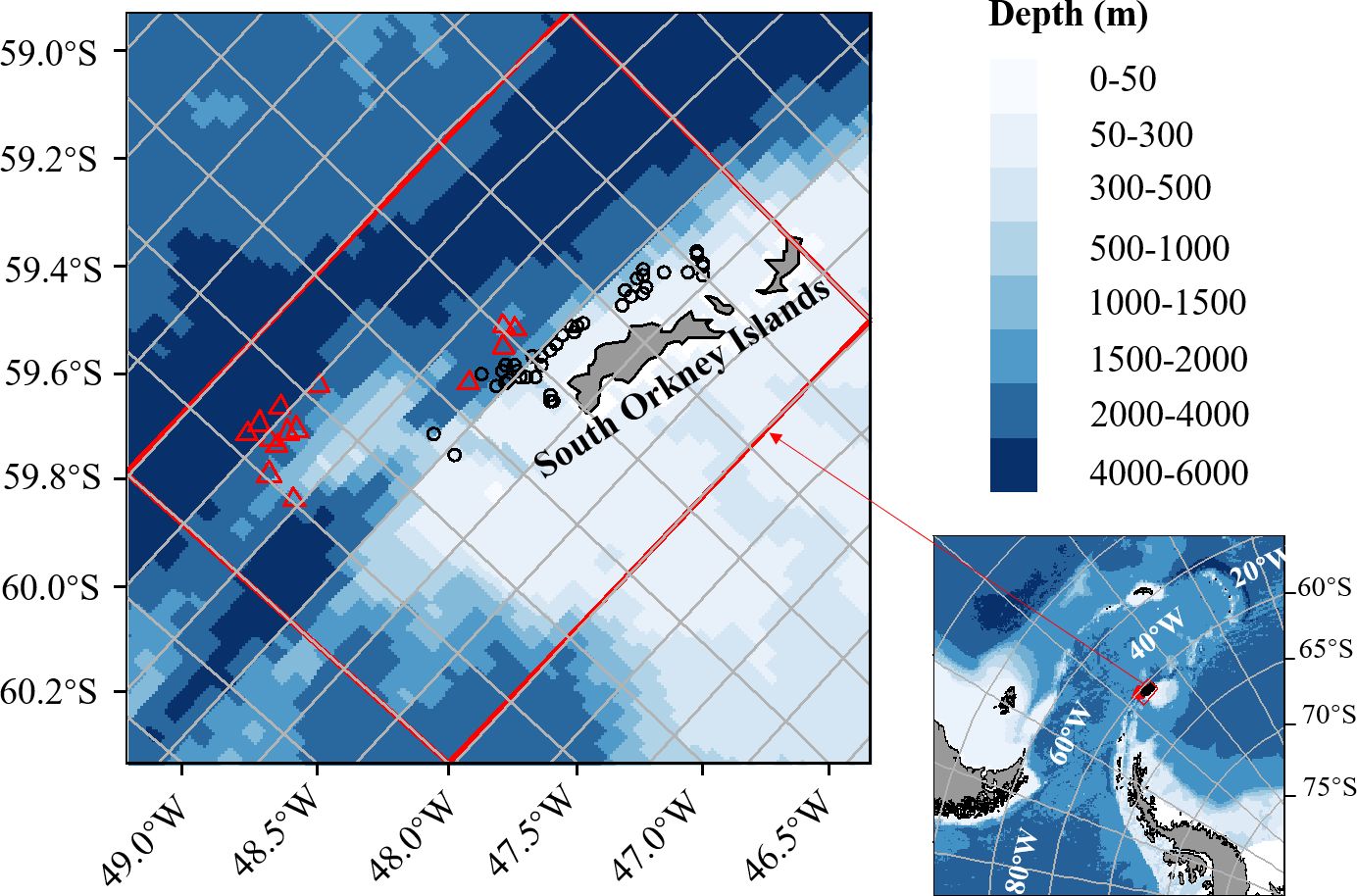
Figure 3 Sampling positions for continuous fishing (red triangles) and conventional trawling (black dots) carried out by F/V SHEN LAN in the waters of the South Orkney Islands. The position on the map is the median of the starting and ending longitudes and latitudes of each haul.
The identification of species followed the manual “By-catch identification and educational material for use by observers on vessels” (https://www.ccamlr.org/en/science/information-technical-coordinators-and-scientific-observers) and other related observer training materials. Fishing vulnerability is the intrinsic extinction vulnerability of marine fishes to fishing that is calculated based on life history and ecological characteristics (e.g., maximum length, age at first maturity, longevity, fecundity, etc.) available through FishBase (Froese and Pauly, 2024. www.fishbase.org) by retrieval using scientific names. Two scientific observers onboard F/V SHEN LAN measured morphometric characteristics-body length, height, width, and weight -of fresh krill and finfish bycatch specimens. Krill body lengths were measured from the front of the eye to the tip of the telson based on CCAMLR standard protocols (CCAMLR, 2022b). Finfish bycatch fork lengths were measured from the snout tip to the base of the caudal fin’s median rays (Mazhirina, 1990). Body widths and heights correspond to the maximum sections (Herrmann et al., 2009; Tokac et al., 2016) by moving and adjusting the vernier caliper while barely touching the body. Body lengths less than 150 mm, widths, and heights were measured using a digital vernier caliper with a range of 0-150 mm and a resolution of 0.01 mm (Ningbo Deli Tools Co., ltd). Body lengths ranging from 150 mm to 350 mm were measured using a grid ruler with a range of 0-350 mm and a resolution of 1 mm. Body lengths greater than 350 mm were measured using a tape with a range of 0-100 cm and a resolution of 1 mm. Body weights of less than 1,000 g were measured using a traditional balancing hanging scale (scale 1 with a range of 0-10 g and a resolution of 0.1 g; scale 2 with a range of 0-1,000 g and a resolution of 5 g). Body weights over 1,000 g were measured using a Marel marine scale with a range of 0-30 kg and a resolution of 10 g.
The IRI (index of relative importance) was calculated to determine the importance of each bycatch species using the following Equation (1):
where N is the numerical percentage, W is the weight percentage, and F is the frequency of occurrence percentage (Hart et al., 2002). Dominant bycatch species have an IRI value greater than 1,000, important bycatch species have an IRI value between 1,000 and 100, common bycatch species have an IRI value between 100 and 10, and rare bycatch species have an IRI value less than 10 (Chen et al., 2022; Luo et al., 2023).
Morphometric characteristics of krill were analyzed along with dominant and important finfish bycatch. These analyses were conducted using Microsoft Office Excel (2016) software, which provided minimum, maximum, mean value, and standard deviation statistics. The length distributions and the relationships of body height and width at length were plotted using the “GGally” package (Schloerke et al., 2021) in R version 4.1.0 (R Core Team, 2024).
The release ratio is the bycatch species body width ratio wider than the bar spacings. We modelled the release ratio for different bar spacings solely based on individual fish width measurements of dominant and important bycatch species by using the following Equations (2) and (3):
where Rnis is the release ratio in terms of number for bycatch species s and bar spacing i; Nis is the number of bycatch species s and bar spacing i; Ns is the total number of bycatch species s; Rwis is the release ratio in terms of weight for bycatch species s and bar spacing i; Wis is the weight of bycatch species s and bar spacing i; Ws is the total weight of bycatch species s.
The observers recorded 676 specimens of finfish bycatch in the waters around South Orkney Islands during the study period. Of these, 665 individuals were identified as belonging to 17 species from 8 families, while 11 juveniles of the Nototheniidae family could not be classified to a lower taxonomic level. There were six species of the Channichthyidae family, four or five species of the Nototheniidae family, two species of the Myctophidae family, and only one species each of Paralepididae, Artedidraconidae, Macrouridae, Gempylidae, and Centrolophidae families. The IRI calculations showed three dominant finfish bycatch species (Champsocephalus gunnari, Pseudochaenichthys georgianus, Chaenocephalus aceratus) and four important bycatch species (Electrona carlsberg, Gymnoscopelus nicholsi, Gobionotothen gibberifrons, Notolepis coatsi) (Table 1; Figure 4).
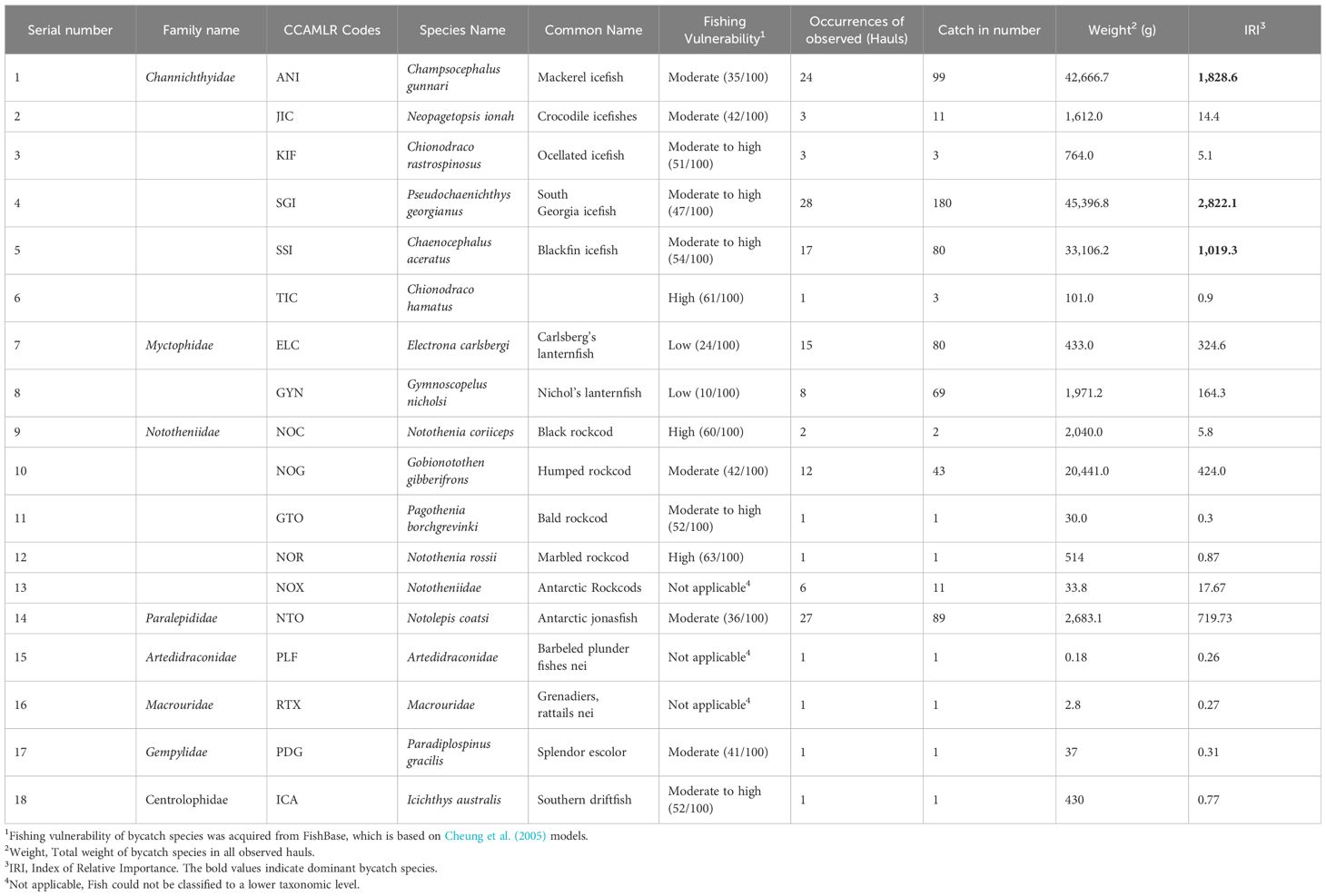
Table 1 Species composition, fishing vulnerability, and index of relative importance of finfish bycatch in the krill trawl fishery in the waters of South Orkney Islands from December 24, 2022, to February 20, 2023.
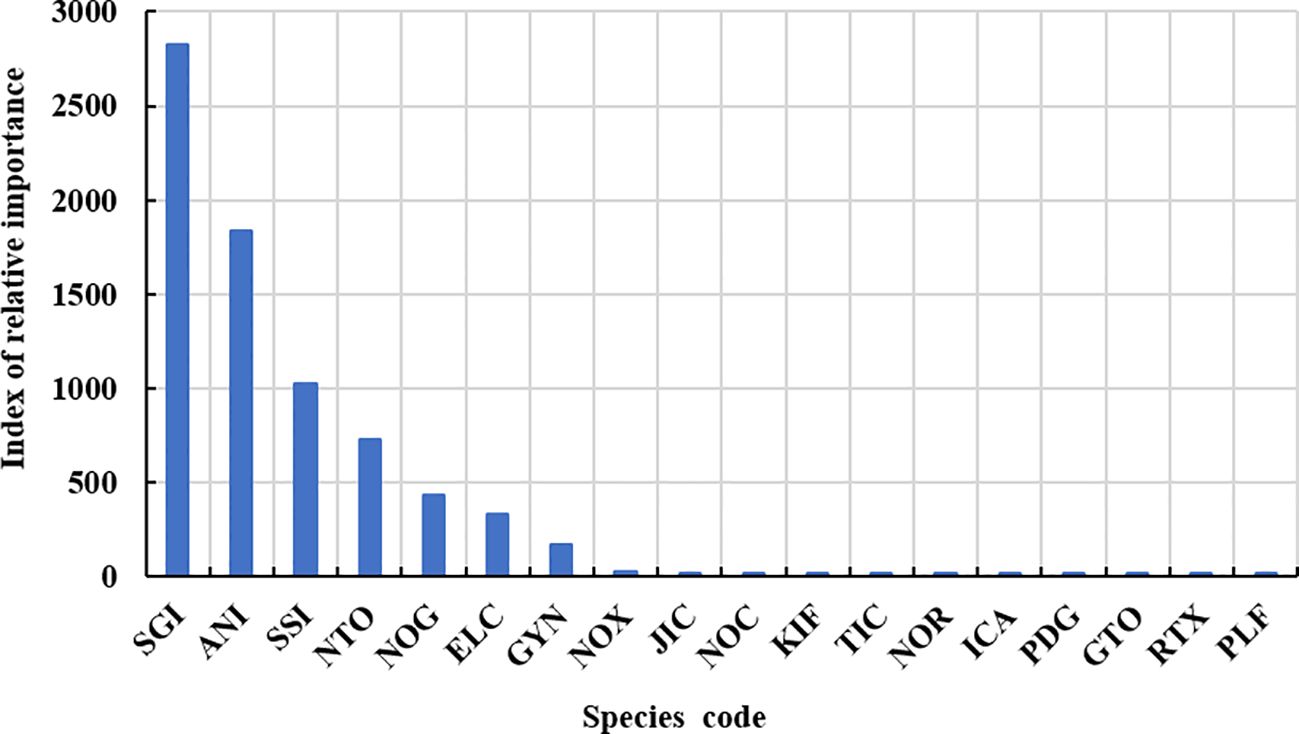
Figure 4 Index of relative importance for each finfish bycatch species caught in the krill trawl fishery by F/V SHEN LAN in the waters of South Orkney Islands from December 24, 2022, to February 20, 2023.
According to the index of relative importance analysis, dominant and important species of finfish bycatch differed between conventional trawling and continuous fishing (Table 2). Fishing depth of conventional trawling operations ranged from the surface to nearly 300 meters in shallow areas. Dominant bycatch were three species (C. gunnari, P. georgianus, and C. aceratus) of the Channichthyidae family. These species were mainly caught in hauls at fishing depths deeper than 50 meters. Important bycatch species, G. gibberifrons was caught primarily in the hauls taken at fishing depths deeper than 120 meters, G. nicholsi was caught in the hauls taken at fishing depths shallower than 120 meters, while N. coatsi was caught in the hauls taken at all fishing depths. In comparison, continuous trawling operations ranged from the surface to nearly 120 meters due to the depth limit set by the conveyor hose. Dominant and important bycatch species caught in continuous fishing were mostly mesopelagic E. carlsbergi, N. coatsi, and P. georgianus (Figure 5).
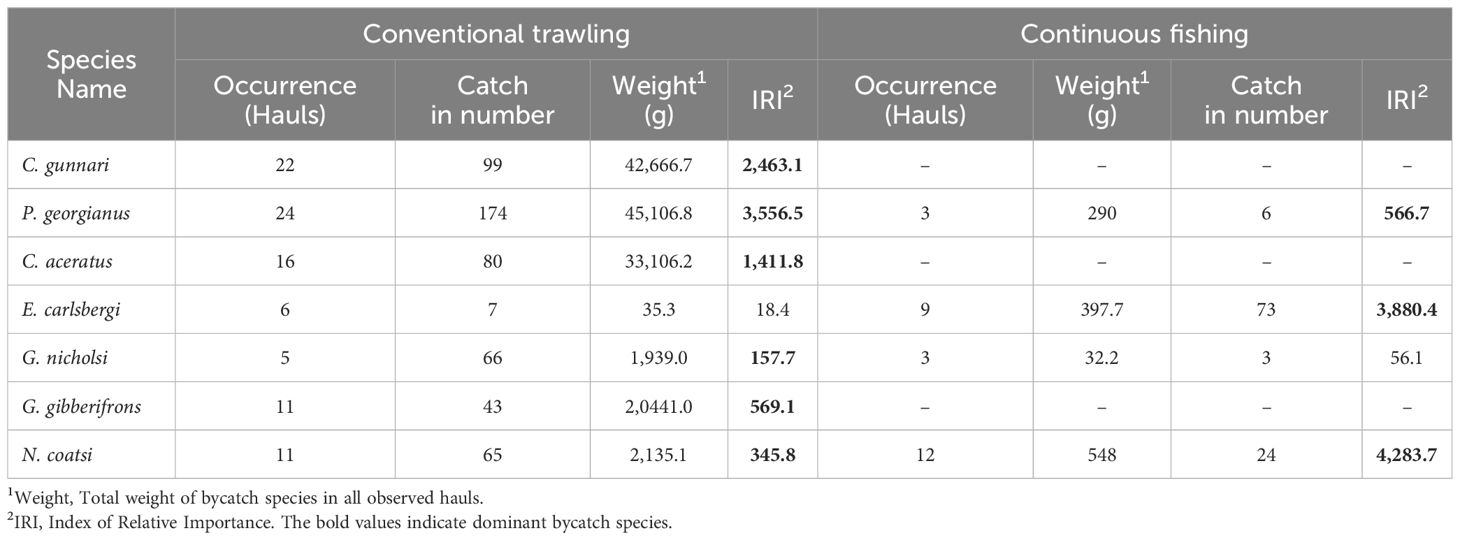
Table 2 Dominant and important bycatch species caught in conventional and continuous fishing by F/V SHEN LAN in the waters of South Orkney Islands from December 24, 2022, to February 20, 2023.
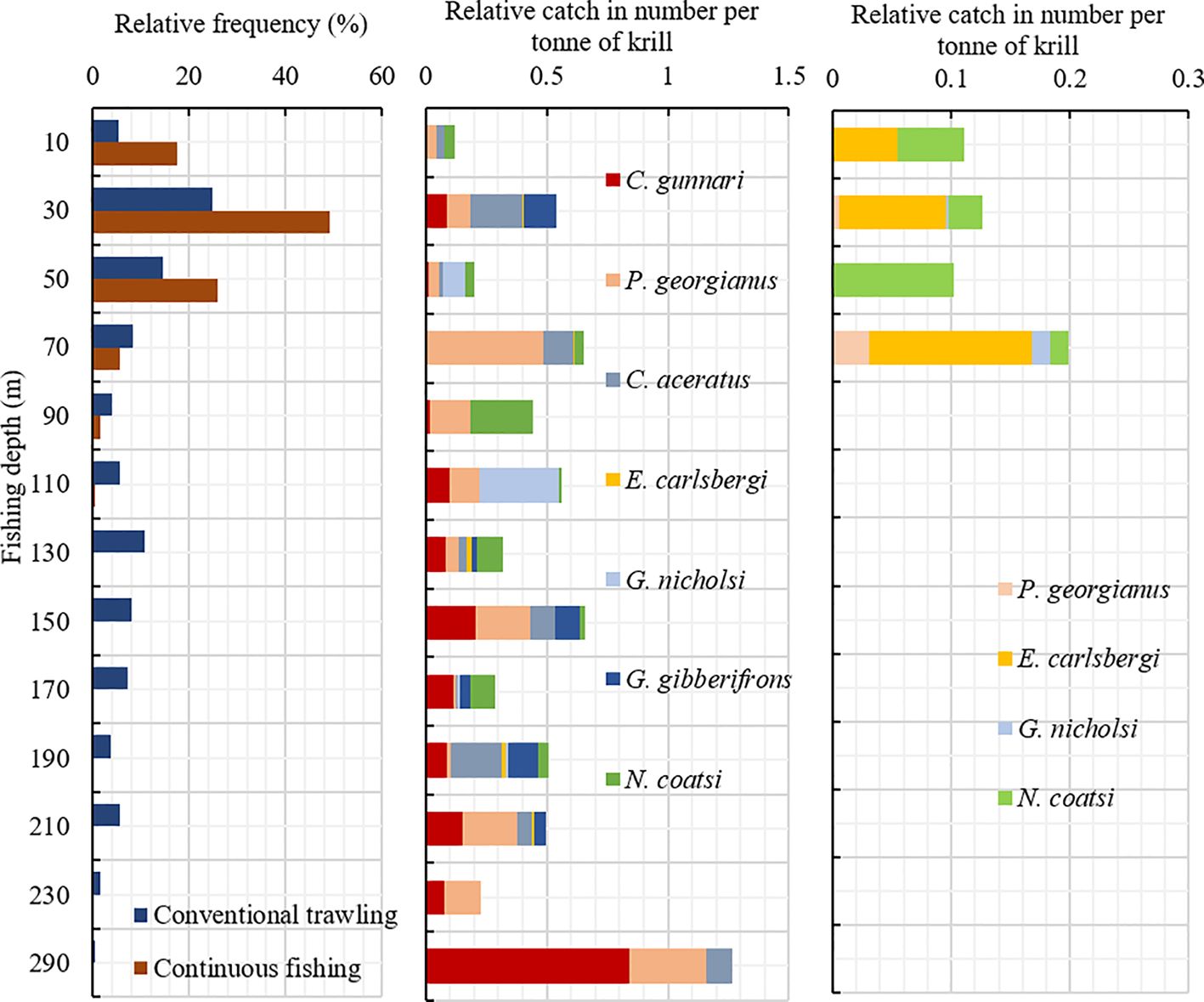
Figure 5 Left: Relative distribution of fishing depth for hauls taken by conventional trawling and continuous fishing. Relative catch in number per ton of krill of dominant and important finfish bycatch species caught in conventional (Middle) and continuous trawling (Right) at different fishing depths in waters of South Orkney Islands from December 24, 2022, to February 20, 2023.
Krill had a unimodal size distribution. Body lengths ranged from 30.64 mm to 59.19 mm, with a mean value of 48.31 mm (± 5.64 mm, Standard deviation). Body heights ranged from 3.53 mm to 12.10 mm, with a mean body height of 6.72 mm (± 1.44 mm). Body widths ranged from 2.81 mm to 9.92 mm, with a mean body width of 5.39 mm (± 0.36 mm). Contrary, dominant finfish bycatch species (C. gunnari, P. georgianus, and C. aceratus) had wide and multimodal length distributions, from the smallest fish (in their first year) to largest, reaching approximately 50 cm in body length. For C. gunnari, body lengths ranged from 29.4 mm to 452.0 mm, heights ranged from 4.7 mm to 78.6 mm, and widths ranged from 3.1 mm to 60.0 mm. For P. georgianus, body lengths ranged from 25.2 mm to 471.0 mm, heights ranged from 4.3 mm to 107.0 mm, and widths ranged from 3.3 mm to 76.8 mm. For C. aceratus, body length ranged from 21.0 mm to 495.0 mm, heights ranged from 2.6 mm to 82.9 mm, and widths ranged from 1.7 mm to 69.3 mm. Of the important bycatch species, E. carlsbergi, G. nicholsi and G. gibberifrons had unimodal length distributions with body lengths of 74.3 mm (± 9.8 mm), 138.5 mm (± 12.4 mm) and 321.9 mm (± 20.6 mm), respectively, while N. coatsi had a bimodal length distribution with bimodal length distributions. Body lengths ranged from 86.4 mm to 356.0 mm, heights ranged from 6.1 mm to 19.3 mm, and widths ranged from 3.1 mm to 13.1 mm (Table 3; Figure 6).
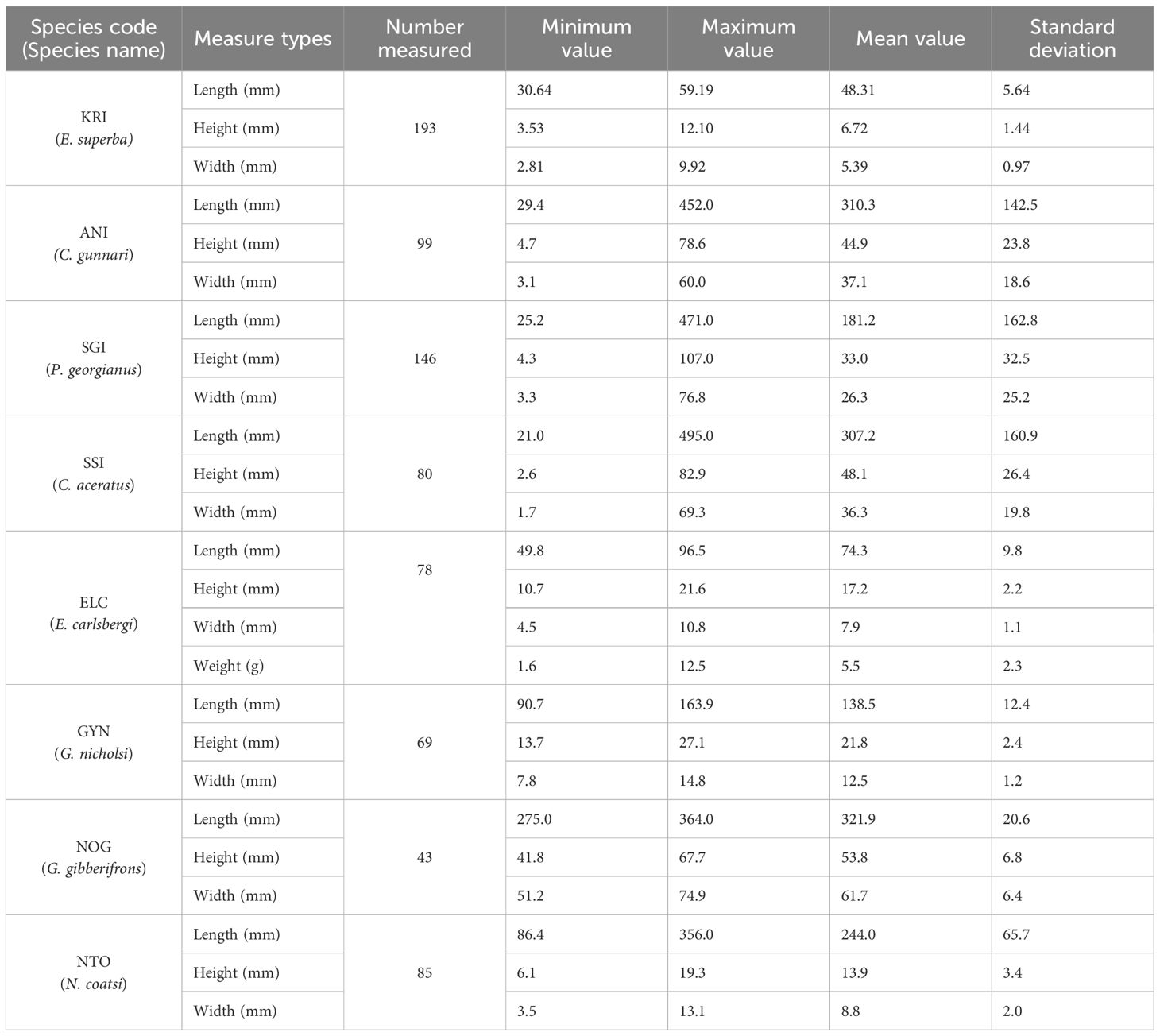
Table 3 Body lengths, heights, and widths of dominant and important bycatch species caught in the krill trawls taken by F/V SHEN LAN in the waters of the South Orkney Islands from December 24, 2022, to February 20, 2023.
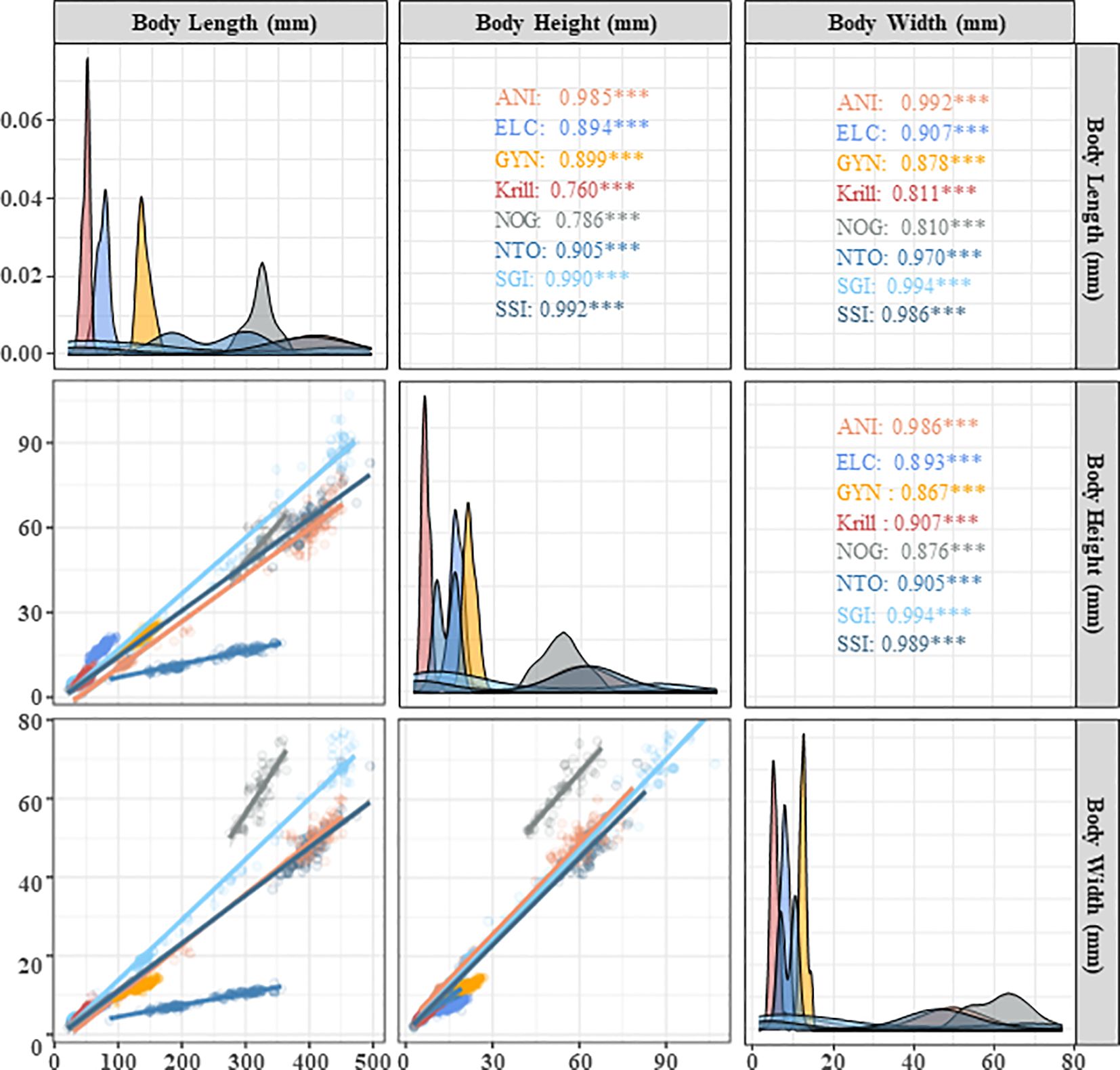
Figure 6 Length distributions and relationships of body height and width at length for krill and dominant and important bycatch species caught during krill trawls taken by F/V SHEN LAN in the waters of the South Orkney Islands from December 24, 2022, to February 20, 2023. Notes: The species names corresponding to the codes in the legend are C. gunnari (ANI), P. georgianus (SGI), C. aceratus (SSI), E. carlsbergi (ELC), G. nicholsi (GYN), G. gibberifrons (NOG), N. coatsi (NTO). The horizontal axis legend is located at the top. The vertical axis legend is located on the right side. Diagonal figures represent body length distribution. Scatter plot and linear fitting of the relationship between body length, height, and width at the bottom left corner of the diagonal figures. Pearson correlation coefficient and its significance between body length, height, and width are located at the upper right corner of the diagonal figures (*** - mean significance level<0.001).
Modelled release ratios for different species under various bar spacings are as follows: C. gunnari was 93.94%, 71.72%, 69.70%, 66.67%, and 66.67% in number, while 99.97%, 99.54%, 99.39%, 99.02%, and 99.02% in weight for 10 mm, 15 mm, 20 mm, 25 mm, and 30 mm bar spacings, respectively. P. georgianus was 53.99%, 52.15%, 34.36%, 30.06%, and 28.22% in number, while 99.95%, 99.85%, 98.18%, 97.68%, and 97.16% in weight for 10 mm, 15 mm, 20 mm, 25 mm, and 30 mm bar spacings, respectively. C. aceratus was 76.25% in number and 99.99 in weight for 10 mm bar spacing, while 73.55% in number and 99.1% in weight for bar spacings over 15 mm to 30 mm. G. gibberifrons was all 100% for bar spacings below 30 mm. E. carlsbergi, G. nicholsi, and N. coatsi were 1.25%, 97.10%, and 43.02% in number, while 2.89%, 99.38%, and 75.21% in weight for 10 mm bar spacing, and 0% for bar spacing larger than 15 mm for these three species (Table 4; Figure 7).
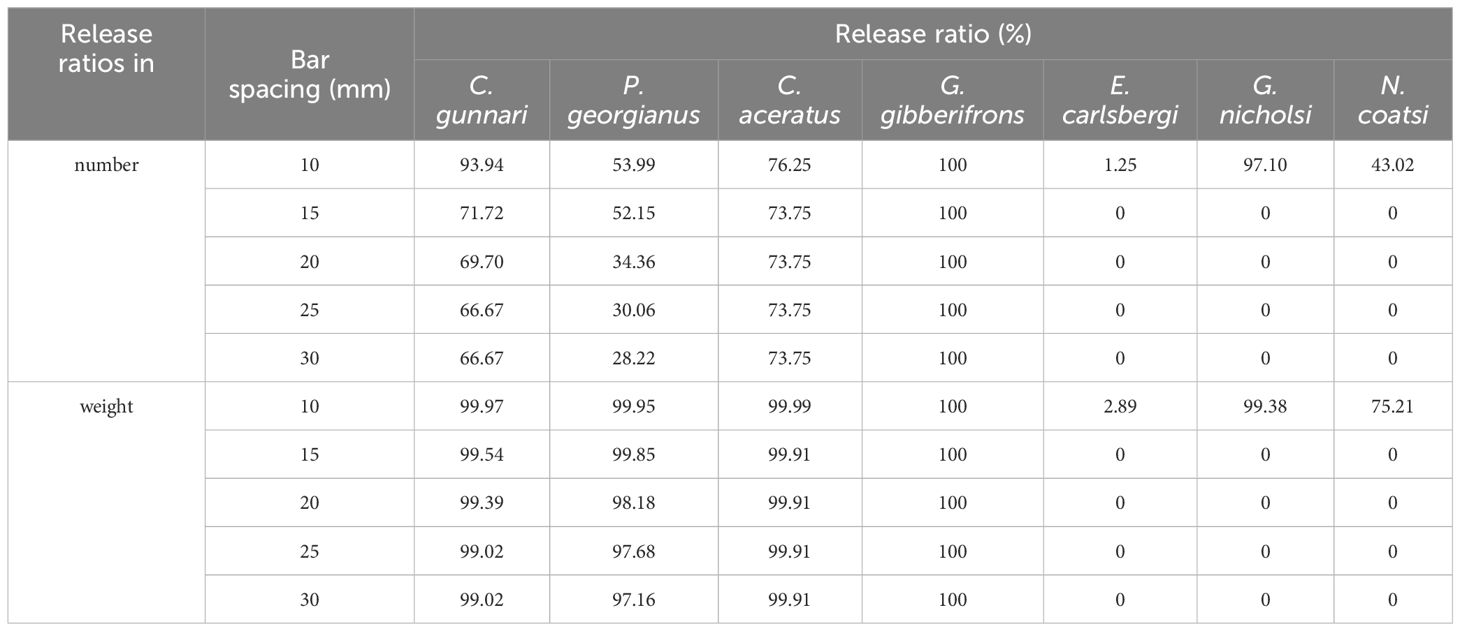
Table 4 Release ratios for different bar spacing options of exclusion devices for dominant and important bycatch species during the study period carried out by F/V SHEN LAN in the waters of the South Orkney Islands.
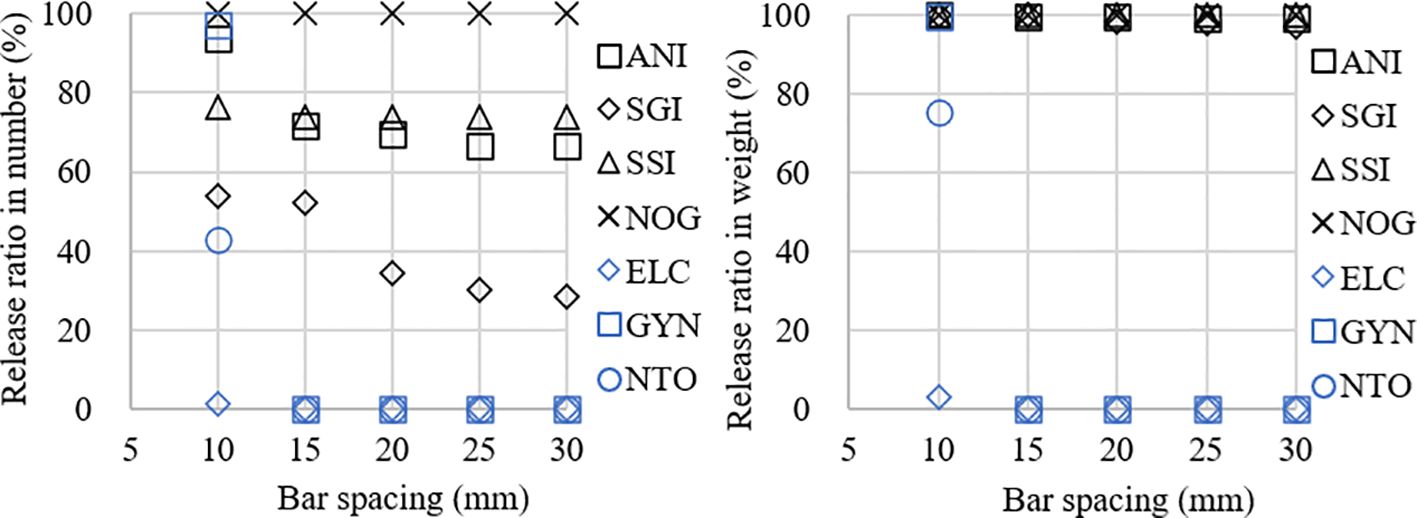
Figure 7 Modelled release ratios in number (left) and weight (right) of the bycatch exclusion device for bar spacings of 10, 15, 20, 25, and 30 mm. Calculations are made for dominant and important bycatch species C. gunnari (ANI), P. georgianus (SGI), C. aceratus (SSI), E. carlsbergi (ELC), G. nicholsi (GYN), G. gibberifrons (NOG), and N. coatsi (NTO) during the study period carried out by F/V SHEN LAN in the waters of the South Orkney Islands.
The waters around the South Orkney Islands are a primary location for krill fishing in Subarea 48.2 of CCAMLR. Commercial krill trawling is concentrated in this area from December to March or April of the following year (Atkinson et al., 2008; Krafft et al., 2023). Bycatch levels have been observed to increase with the increase in krill catch (Kock et al., 2012; CCAMLR, 2022a). Krafft et al. (2023) reported that the bycatch ratio ranged from 0.1-0.3% and was dominated by finfish during the fishing season in Subareas 48.1, 48.2, and 48.3. The bycatch ratio differences in krill fisheries are possibly caused by seasonal and spatial variation (Kozlov et al., 1990; Krafft et al., 2023). The highest bycatch ratios were observed in February-March in Subarea 48.2 (Krafft et al., 2023). This subarea hosts a considerable proportion of high-Antarctic finfish species due to the influence of Weddell Sea water (Kock et al., 2000). Our study found that the finfish bycatch ratio ranged from 0-0.1% in this subarea during a two-month period.
Information on bycatch composition and mitigation by species is crucial for the responsible management of the krill fishery in an EBFM framework, including the impact of krill fishery on bycatch species. This study identified three dominant bycatch species (C. gunnari, P. georgianus, and C. aceratus) from the Channichthyidae family. Additionally, four important bycatch species were identified: G. gibberifrons from the Nototheniidae family, E. carlsbergi and G. nicholsi from the Myctophidae family, and N. coatsi from the Paralepididae family. Our results are consistent with previous studies that found the bycatch to be dominated by demersal species from the families Nototheniidae and Channichthyidae, pelagic species from the family Myctophidae, and bathypelagic species from the family Paralepididae in this area (Williams, 1985; Kock et al., 2000, 2012; Hill et al., 2007).
This study revealed that the finfish bycatch differed between conventional trawling and continuous fishing is most likely a result of gear differences, fished in different areas, and different depths (Jones et al., 2000; Collins et al., 2012). Conventional trawling takes place in the layer from the surface to depths of nearly 300 meters, predominantly in shelf areas. In comparison, continuous fishing was done in the layer shallower than 120 meters in a relatively deepwater area.
Our study found that except for P. georgianus, the demersal species (C. gunnari, P. georgianus, C. aceratus, and G. gibberzfrons) were dominant and important bycatch in the hauls of conventional trawling rather than continuous fishing (Kock et al., 2000, 2012; Jones et al., 2000). The specimens of the pelagic species E. carlsbergi were caught in the continuous fishery from the surface to depths of 100 meters in deepwater areas. In contrast, G. nicholsi were caught in conventional trawling at fishing depths around 100 meters in the shelf area. Those results are consistent with previous studies. E. carlsbergi tends to live in deepwater and move up to the epipelagic zone (50 to 200 m) during the spring and summer seasons (Filin et al., 1990; Kozlov et al., 1990; Everson et al., 1992; Kozlov, 1995). In comparison, the pelagic stock of G. nicholsi comprises juveniles and sub-adults living in offshore waters (Linkowski, 1985; Saunders and Tarling, 2018).
Additionally, our study found that N. coatsi were caught at almost all fishing depths. This result is consistent with previous studies that N. coatsi has a predominantly oceanic distribution where it is found from the surface to depths of more than 2000 meters (Williams, 1985; Torres and Somero, 1988; Gon and Heemstra, 1990; Hoddell et al., 2000), although specimen larger than 55 mm are mainly caught in continental slope areas (Hoddell et al., 2000).
Understanding the species composition and the physical characteristics of the finfish bycatch is essential to design effective exclusion devices for the krill trawl fishery. These devices are meant to release the finfish bycatch while retaining the targeted krill. Broadhurst et al. (2018) suggested that the bar spacing for reducing larger bycatch species can be fixed at a size approaching the maximum body width of the targeted species. Araya-Schmidt et al. (2023) found that using a Nordmøre grid with a 17 mm or 15 mm bar spacing effectively reduced redfish bycatch without significant loss of specimens of the target species Northern shrimp with carapace lengths up to 30 mm and carapace widths up to 15 mm. This study investigation found that the target species, krill, had a unimodal size distribution, and the maximum body width was 9.92 mm in the collected specimens. Therefore, the bar spacing should be no less than 10 mm.
We modelled release ratios solely based on morphometric measurements of dominant bycatch species (C. gunnari, P. georgianus, and C. aceratus) and important bycatch species (E. carlsbergi, G. nicholsi, and G. gibberifrons) for different bar spacing. A 10 mm bar spacing can release a significant amount of dominant bycatch species (93.94% of C. gunnari, 53.99% of P. georgianus, and 76.25% of C. aceratus) and important bycatch species (100% of G. gibberifrons) according to the body size composition in our investigation. In comparison, a 15 mm bar spacing will retain more juveniles of the dominant bycatch species (28.28% of C. gunnari, 47.85% of P. georgianus, and 26.25% of C. aceratus) and almost all important bycatch species E. carlsbergi, G. nicholsi, and N. coatsi. In future sea trials of a sorting grid, we recommend to test a bar spacing of 10 mm. If the trials demonstrate loss of krill, particularly at high catch rates of krill, a wider bar spacing of 15 mm should be tested. This will result in higher bycatch of less vulnerable species (i.e., E. carlsbergi, G. nicholsi, and N. coatsi).
This study reveals generally low bycatch rates in the krill fishery, but three dominant and vulnerable species (C. gunnari, P. georgianus, and C. aceratus) may suffer from overexploitation of krill fishery. We measured morphometric data (particularly body width) of krill and finfish bycatch, which is crucial to model release ratios of bycatch reduction devices for these species. Calculations suggest that a large fraction of dominant and vulnerable species can be excluded using a grid type BRD within a 15 mm bar spacing.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The manuscript presents research on animals that do not require ethical approval for their study.
ZW: Data curation, Investigation, Visualization, Writing – original draft, Writing – review & editing, Formal analysis, Funding acquisition. SM: Writing – review & editing, Conceptualization, Formal analysis, Methodology. YW: Writing – review & editing, Investigation. LW: Writing – review & editing, Funding acquisition, Project administration, Supervision.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the Marine S&T Fund of Shandong Province for Qingdao Marine Science and Technology Center (No. 2022QNLM030002-2) and the Shanghai Sailing Program (23YF1459700).
The authors thank the scientific observers who collected the finfish bycatch from the outlet of catches in the processing room. The authors would also like to thank Jiangsu Sunline Deep Sea Fishery Co., Ltd and the captain for allowing access to data collected on the F/V SHEN LAN with personnel on the vessel that assisted the scientific observers.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1325120/full#supplementary-material
Araya-Schmidt T., Bayse S. M., Winger P. D., Frank C. H. (2023). Smaller bar spacings in a Nordmøre grid reduces the bycatch of redfish (Sebastes spp.) in the offshore Northern shrimp (Pandalus borealis) fishery of eastern Canada. Aquac. Fish. 8, 661–671. doi: 10.1016/j.aaf.2023.01.009
Atkinson A., Siegel V., Pakhomov E. A., Rothery P., Loeb V., Ross R. M., et al. (2008). Oceanic circumpolar habitats of Antarctic krill. Mar. Ecol. Prog. Ser. 362, 1–23. doi: 10.3354/meps07498
Broadhurst M. K., Millar R. B., Dingle A. (2018). Minimizing Nordmøre grid bar spaces in an Australian penaeid fishery. Mar. Fish. Rev. 80, 29–33. doi: 10.7755/MFR.80.1.2
CCAMLR (2019). Procedure for estimating two-hour catches during continuous trawl fishing for krill using daily flow-scale records split according to the distribution of two-hour catches derived from holding tank volume monitoring. Report of the thirty-eighth meeting of the scientific committee (Hobart, Australia: SC-CAMLR-38, Annex 9). Available online at: https://meetings.ccamlr.org/system/files/e-sc-38-rep_2.pdf (Accessed April 10, 2024).
CCAMLR (2022a). CCAMLR Statistical Bulletin. Available online at: https://www.ccamlr.org/en/publications/statistical-bulletin (Accessed October 7, 2023).
CCAMLR (2022b). 2022 Observer Krill Trawl Logbook - Instructions Version: OKv2022a. Available online at: https://www.ccamlr.org/en/science/information-technical-coordinators-and-scientific-observers (Accessed April 10, 2024). Covered by Scientific Observer's Manual – Krill Fisheries - Version 2023.
CCAMLR (2023a). Fishery Report 2022: Euphausia superba in Area 48. Available online at: https://fishdocs.ccamlr.org/FishRep_48_KRI_2022.pdf (Accessed October 7, 2023).
CCAMLR (2023b). Krill - biology, ecology and fishing. Available online at: https://www.ccamlr.org/en/fisheries/krill (Accessed October 7, 2023).
CCAMLR (2023c). Fishing Gear information of List of authorised vessels. Available online at: https://www.ccamlr.org/en/compliance/licensed-vessels (Accessed October 7, 2023).
Chen B. J., Sun X., Wang H. J., Zhang Z., Gu Y. X., Luo J., et al. (2022). Heavy metal content and health risk assessment of fish in 4 reservoirs of Dalian. Appl. Ecol. Environ. Res. 20 (4), 2963–2983. doi: 10.15666/aeer/2004_29632983
Cheung W. W., Pitcher T. J., Pauly D. (2005). A fuzzy logic expert system to estimate intrinsic extinction vulnerabilities of marine fishes to fishing. Biol. Conserve. 124, 97–111. doi: 10.1016/j.biocon.2005.01.017
Collins M. A., Stowasser G., Fielding S., Shreeve R., Xavier J. C., Venables H. J., et al. (2012). Latitudinal and bathymetric patterns in the distribution and abundance of mesopelagic fish in the Scotia Sea. Deep-Sea Res. Pt II. 59, 189–198. doi: 10.1016/j.dsr2.2011.07.003
De Juan S., Hinz H., Sartor P., Vitale S., Bentes L., Bellido J. M., et al. (2020). Vulnerability of demersal fish assemblages to trawling activities: A traits-based index. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00044
Everson I., Neyelov A., Permitin Y. E. (1992). Bycatch of fish in the South Atlantic krill fishery. Antarct. Sci. 4, 389–392. doi: 10.1017/S0954102092000579
Filin A. A., Gorchinsky K. V., Kiseleva V. M. (1990). Biomass of myctophids in the Atlantic sector of the Southern Ocean as estimated by acoustic surveys (CCAMLR Sel. Sci. Pap. WG-FSA-90/19). Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/25-Filin-et-al.pdf (Accessed October 7, 2023).
Froese R., Pauly D. (Eds.) (2024). FishBase (World Wide Web electronic publication). Available at: www.fishbase.org. 02/2024.
Gamaza M., Sobrino I., Erzini K. (2015). Testing Nordmøre grids on the target and by-catch species of the commercial bottom trawl fishery in the Gulf of Cadiz. Sci. Mar. 79, 465–477. doi: 10.3989/scimar.2015.79n4
Gigliotti J. C., Jaczynski J., Tou J. C. (2008). Determination of the nutritional value, protein quality and safety of krill protein concentrate isolated using an isoelectric solubilization/precipitation technique. Food Chem. 111, 209–214. doi: 10.1016/j.foodchem.2008.03.03
Gon O., Heemstra P. C. (1990). Fishes of the Southern Ocean (Grahamstown: J.L.B. Smith Institute of Ichthyology), 462. pp. 141 pls.
Hart R. K., Calver M. C., Dickman C. R. (2002). The index of relative importance: an alternative approach to reducing bias in descriptive studies of animal diets. Wildlife Res. 29, 415–421. doi: 10.1080/15627020.2011.11407511
Herrmann B., Krag L. A., Frandsen R. P., Madsen N., Lundgren B., Stæhr K. J. (2009). Prediction of selectivity from morphological conditions: methodology and a case study on cod (Gadus morhua). Fish. Res. 97, 59–71. doi: 10.1016/j.fishres.2009.01.002
Hill S. L., Reid K., Thorpe S. E., Hinke J., Watters G. M. (2007). A compilation of parameters for ecosystem dynamics models of the Scotia Sea-Antarctic Peninsula region. CCAMLR Sci. 14, 1–25. Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/01hill-etal.p (Accessed July 13, 2023).
Hoddell R. J., Crossley A. C., Williams R., Hosie G. W. (2000). The distribution of Antarctic pelagic fish and larvae (CCAMLR division 58.4. 1). Deep-Sea Res. Pt II. 47, 2519–2541. doi: 10.1016/S0967-0645(00)00034-5
Hornborg S., Svensson M., Nilsson P., Ziegler F. (2013). By-catch impacts in fisheries: utilizing the IUCN red list categories for enhanced product level assessment in seafood LCAS. Environ. Manage. 52, 1239–1248. doi: 10.1007/s00267-013-0096-7
Jones C. D., Kock K.-H., Balguerias E. (2000). Changes in biomass of eight species of finfish around the South Orkney Islands (Subarea 48.2) from three bottom trawl surveys. CCAMLR Sci. 7, 53–74. Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/01hill-etal.pdf (Accessed July 13, 2023).
Kock K. H., Barrera Oro E., Belchier M., Collins M. A., Duhamel G., Hanchet S., et al. (2012). The role of fish as predators of krill (Euphausia superba) and other pelagic resources in the Southern Ocean (CCAMLR Sci). Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/Kock-et-al.pdf (Accessed October 7, 2023).
Kock K. H., Jones C. D., Wilhelms S. (2000). Biological characteristics of Antarctic fish stocks in the southern Scotia Arc region. CCAMLR Sci. 7, 1–41.
Kozlov A. (1995).A review of the trophic role of mesopelagic fish of the family Myctophidae in the Southern Ocean ecosystem (CCAMLR Sci). Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/05kozlov.pdf (Accessed October 7, 2023).
Kozlov A. N., Shust K. V., Zemsky A. V. (1990). Seasonal and inter-annual variability in the distribution of Electrona carlsbergi in the Southern Polar Front area (the area to the north of South Georgia is used as an example) (CCAMLR Sel. Sci. Pap. WG-FSA-90/35). Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/21-Kozlov-et-al.pdf (Accessed October 7, 2023).
Krafft B. A., Lowther A., Krag L. A. (2023). Bycatch in the Antarctic krill (Euphausia superba) trawl fishery. Fisheries Manage. Ecol. 30, 154–160. doi: 10.1111/fme.12607
Linkowski T. B. (1985). Population biology of the myctophid fish Gymnoscopelus nicholsi (Gillbert 1911) from the western South Atlantic. J. fish Biol. 27, 683–698. doi: 10.1111/j.1095-8649.1985.tb03213.x
Luo Z., Yang C., Wang L., Liu Y., Shan B., Liu M., et al. (2023). Relationships between fish community structure and environmental factors in the nearshore waters of Hainan Island, south China. Diversity 15, 901. doi: 10.3390/d15080901
Mazhirina G. P. (1990). On reproduction of Electrona carlsbergi tanning (CCAMLR Sel. Sci. Pap. WG-FSA-90/20). Available online at: https://www.ccamlr.org/en/system/files/science_journal_papers/23-Mazhirina.pdf (Accessed October 7, 2023).
Nicol S., Endo Y. (1999). Krill fisheries: development, management and ecosystem implications. Aquat. Living Resour. 12, 105–120. doi: 10.1016/S0990-7440(99)80020-5
Nicol S., Foster J., Kawaguchi S. (2012). The fishery for Antarctic krill-recent developments. Fish Fish. 13, 30–40. doi: 10.1111/j.1467-2979.2011.00406.x
R Core Team (2024). R: A Language and Environment for Statistical Computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed May 8, 2024).
Saunders R. A., Tarling G. A. (2018). Southern Ocean mesopelagic fish comply with Bergmann’s rule. Am. Nat. 191, 343–351. doi: 10.1086/695767
Schloerke B., Cook D., Larmarange J., Briatte F., Marbach M., Thoen E., et al. (2021). GGally: Extension to 'ggplot2'. R package version 2.1.2. Available online at: https://CRAN.R-project.org/package=GGally (Accessed May 8, 2024).
Siegel V., Watkins J. L. (2016). Distribution, biomass and demography of antarctic krill, Euphausia superba. Adv. Polar Ecol. 21–100. doi: 10.1007/978-3-319-29279-3_2
Tokac A., Herrmann B., Gökçe G., Krag L. A., Nezhad D. S., Lök A., et al. (2016). Understanding the size selectivity of red mullet (Mullus barbatus) in Mediterranean trawl codends: A study based on fish morphology. Fish. Res. 174, 81–93. doi: 10.1016/j.fishres.2015.09.002
Torres J. J., Somero G. N. (1988). Vertical distribution and metabolism in Antarctic mesopelagic fishes. Comp. Biochem. Phys. B. 90, 521–528. doi: 10.1016/0305-0491(88)90291-X
Vihtakari M. (2024). ggOceanMaps: Plot Data on Oceanographic. Maps using 'ggplot2'. R package version 2.2.0. Available online at: https://mikkovihtakari.github.io/ggOceanMaps/ (Accessed January 27, 2024).
Wang Z., Tang H., Herrmann B., Xu L. (2021). Catch Pattern for Antarctic krill (Euphausia superba) of Different Commercial Trawls in Similar Times and Overlapping Fishing Grounds. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.670663
Williams R. (1985). “Trophic relationships between pelagic fish and euphausiids in Antarctic waters,” in Antarctic Nutrient Cycles and Food Webs. Eds. Siegfried W. R., Condy P. R., Laws R. M. (Springer-Verlag, Berlin), 452–459. doi: 10.1007/978-3-642-82275-9_63
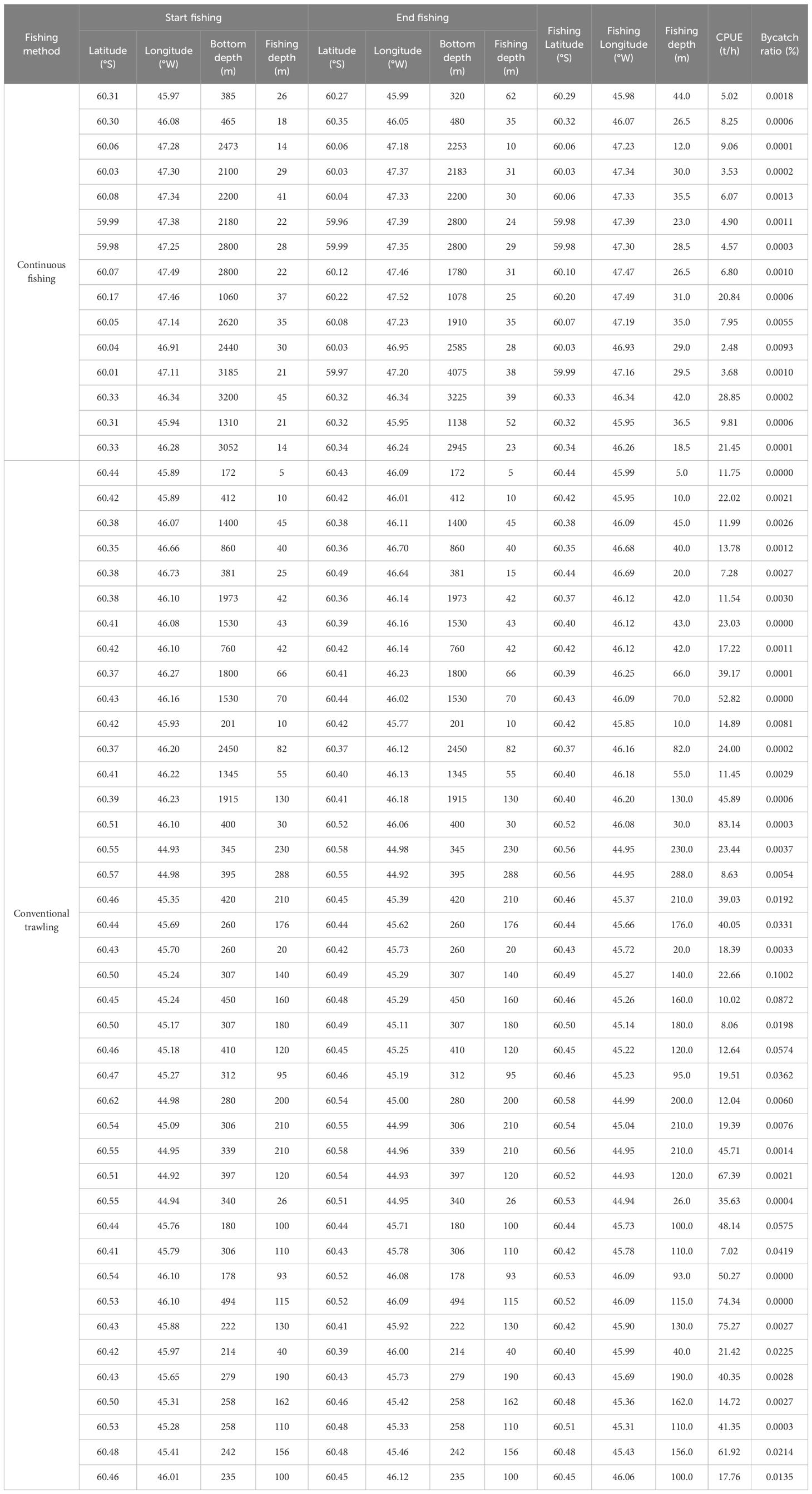
Fishing information on each haul of finfish bycatch was observed in the krill trawl onboard F/V SHEN LAN in the waters of South Orkney Islands (Notes: The fishing latitude, longitude, and depth are the median of the starting and ending fishing latitudes, longitudes, and depths; The bycatch ratio is by weight).
Keywords: Southern Ocean, trawling, icefish, lanternfish, bycatch exclusion device
Citation: Wang Z, Ma S, Wang Y and Wang L (2024) Morphometric characteristics of Antarctic krill (Euphausia superba) and finfish bycatch in the krill fishery in the waters of South Orkney Islands during the 2022/23 fishing season. Front. Mar. Sci. 11:1325120. doi: 10.3389/fmars.2024.1325120
Received: 20 October 2023; Accepted: 08 July 2024;
Published: 22 July 2024.
Edited by:
Bin Wang, Zhejiang Ocean University, ChinaReviewed by:
Marcelo Ândrade, Federal University of Maranhão, BrazilCopyright © 2024 Wang, Ma, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuo Ma, bWFzaHVvX2Vjc2ZAMTI2LmNvbQ==; Yongjin Wang, d2FuZ3lqQGVjc2YuYWMuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.