- 1MARE - Marine and Environmental Sciences Centre & ARNET - Aquatic Research Network, Faculdade de Ciências, Universidade de Lisboa, Lisboa, Portugal
- 2CIBIO‐InBIO, Research Center in Biodiversity and Genetic Resources, University of Porto, Porto, Portugal
- 3BIOPOLIS Program in Genomics, Biodiversity and Land Planning, CIBIO, Porto, Portugal
- 4IPMA - Instituto Português do Mar e da Atmosfera, Algés, Portugal
- 5Departamento de Biologia Animal, Faculdade de Ciências, Universidade de Lisboa, Lisboa, Portugal
Over one-third of all elasmobranch species are at risk of extinction worldwide. This study aims to contribute to their conservation worldwide through a case study that combines georeferenced data on species presence and abundance with spatial distribution of human activities, through a Spatial Conflict Risk Index (SCRI). The SCRI pinpoints possible risk areas obtained from the spatial overlap of elasmobranch species abundance and distribution with impacting human activities. Data on species presence and abundance around a Marine Protected Area, the Berlengas Natural Reserve (Portugal) were obtained through four non-invasive methods: Baited Remote Underwater Videos (BRUV), Local Ecological Knowledge (LEK), scientific observers onboard longline commercial fishing vessels and citizen science and social media reports. Human activities were mapped based on LEK. Qualitative abundance and distribution data was obtained for 22 species. SCRI highlighted some high-risk areas due to overlap of areas of frequent occurrence of elasmobranchs with potential high impact activities (e.g. longline fishery). This study highlighted the potential of multi-method approaches to estimate the distribution of rare, highly mobile species in data-limited contexts, and assess their exposure to human activities. The SCRI is a useful tool to support the implementation of effective conservation regulations.
1 Introduction
From benthic mesoconsumers to pelagic apex predators, sharks and rays play pivotal roles in the maintenance of ecosystem stability (Rosa et al., 2017). Nevertheless, their low fecundity, delayed maturation, and slow growth rates result in extended recovery periods, rendering these species vulnerable to overexploitation (Dulvy et al., 2008; García et al., 2008; Lucifora et al., 2011; Biery and Pauly, 2012; Worm et al., 2013; Becerril-García et al., 2020; Dulvy et al., 2021).
Despite their key function in marine ecosystem dynamics, elasmobranchs face an array of threats, including fishing-induced mortality (bycatch and targeted capture), habitat degradation, and climate change (Stevens et al., 2000; Campana et al., 2011b; Pennino et al., 2013), particularly the large shallow-water species that are more accessible and exposed to fishing activities (Dulvy and Forrest, 2010; Dulvy et al., 2014). Globally, over 1000 species of sharks and rays have been described, with more than 100 inhabiting the Northeast Atlantic (Dulvy et al., 2014). In mainland Portugal, commercial fisheries commonly capture approximately 44 species of sharks and rays (Correia, 2009). Over half of the species caught in commercial fisheries contribute to a mere 2% of total landings, whether in mainland Portugal or the Azores. In Portugal, rays are predominantly landed in the ports of Peniche and Sesimbra (Figueiredo et al., 2020). While rays and demersal sharks are targeted by several fisheries, pelagic sharks are primarily bycatch in longline fisheries (Batista et al., 2009; Baeta et al., 2010; Coelho et al., 2012a; Figueiredo et al., 2020). In fact, vessels landing pelagic sharks in mainland Portugal predominantly offload their catches in Peniche, with a significant portion of the fleet choosing to land in Vigo, Spain (Coelho et al., 2012b).
The collection of data on these species is therefore imperative for the effective implementation of conservation measures. Georeferenced species occurrence and abundance data are necessary for identifying and safeguarding crucial habitats, such as foraging grounds and nursery areas, a very effective conservation measure for highly mobile species (Maxwell, 2015). In fact, spatial monitoring has proven indispensable in several instances for enforcing stock management and implementing effective conservation strategies such as Marine Protected Areas (MPAs) (Wedding et al., 2011; Pennino et al., 2013; Grorud-Colvert et al., 2021). According to the IUCN criteria, over one-third of elasmobranch species are threatened, yet numerous species lack adequate data or formal risk assessments (IPBES, 2019; Dulvy et al., 2021). A significant obstacle to assessing the population status of these elasmobranchs of high conservation concern arises from the challenge posed by invasive and potentially harmful sampling techniques (e.g. catch and release for tagging, sampling techniques that imply mortality). This complexity compounds the logistical difficulties associated with sampling rare, solitary, and often highly mobile species (Pennino et al., 2013; Gore et al., 2016). In this context, the adoption of non-invasive and non-destructive sampling methods becomes imperative (Dulvy and Forrest, 2010; Pennino et al., 2013).
Onboard scientific observers on fishing vessels with high rates of elasmobranch catch and bycatch are an asset not only to collect georeferenced data on species occurrences, but also to contribute to more accurate assessments of fishing pressure, by ensuring that no information is lost (Baeta et al., 2010; Ewell et al., 2020). While the fishing activity itself is a destructive method, the onboard observer is a passive agent that does not lead to any additional mortality. For this reason, onboard observation can be considered non-destructive sampling.
A commonly used non-invasive, fishery-independent method for shark surveys is the deployment of Baited Remote Underwater Video systems (BRUVs) at different depths, in fixed or drifting setups. This method has consistently proven it can replace destructive alternatives, such as experimental longline fishing (Langlois et al., 2010; Brooks et al., 2011; Santana-Garcon et al., 2014; Harvey et al., 2018; Bruns and Henderson, 2020; Jones et al., 2020).
Another invaluable source of information emanates from Local Ecological Knowledge (LEK), the collective wisdom amassed by residents, visitors, tourism companies and fishers, which can significantly contribute to policymaking and local strategy formulation. Indeed, this principle is a part of the Fisheries and Agriculture Organisation (FAO) Code of Conduct, which stipulates the involvement of all stakeholders in the design and implementation of regulations (Garcia et al., 2008; Pauly et al., 2014; Braga et al., 2018; Silva et al., 2021). Enquiries that gather fishers’ knowledge and observations can yield valuable insights into local extinctions, shifts in distribution patterns, empirical perceptions of migrations, seasonal variations, key habitat locations, and stock statuses (Serra-Pereira et al., 2014). Citizen science projects and platforms are another proven method of leveraging LEK. With the wide availability of smartphones, it is easier than ever for residents and visitors to record images and coordinates of sightings, providing a verifiable and ever-growing database of spatial information (Catlin-Groves, 2012; Fraisl et al., 2022). Finally, even if citizens are not enrolled in any project, they will often post images on social media, and this is particularly true for rare and mediatic species such as pelagic sharks (Giovos et al., 2018, 2019). A thorough compilation of LEK should therefore not disregard this additional source of data, while having to carefully validate each observation (Catlin-Groves, 2012; Giovos et al., 2018; Fraisl et al., 2022).
Besides understanding species distribution patterns, it is indispensable to comprehend the distribution and intensity of impact sources and human activities affecting the area to be able to formulate targeted and effective conservation strategies (Zacharias and Gregr, 2005). Various methodologies have been employed to assess the cumulative impact of overlapping activities on species. In 2008, Halpern and colleagues devised a standardised cumulative impact index by applying a logarithmic function to the multiplication of activity presence or absence by its magnitude and the presence or absence of several habitats within a 1 km2 grid cell (Halpern et al., 2008). Subsequent regional, national and local assessments of human pressure have adopted this approach, including in mainland Portugal (Batista et al., 2014).
This study conducted a pilot assessment in the Berlengas Nature Reserve (Berlengas MPA), a small archipelago off the coast of mainland Portugal, to develop a framework that integrates non-invasive sampling and LEK to generate georeferenced data on the intersection between elasmobranch occurrence and spatial distribution of potentially harmful human activities, providing a quick index to inform management. Such a method can be extremely useful to the conservation of elasmobranchs worldwide that are threatened by the lack of protective measures arising from data scarcity.
2 Methods
2.1 Study area: Berlengas Nature Reserve
The present study was developed in the marine area of the Berlengas MPA and adjacent areas (Figure 1, Supporting Information). First established in 1981, the MPA is located 9.2 km off Peniche in the West coast of Portugal. This protected area includes the Berlenga island, Farilhões islets, small islets of Medas and Estelas and the surrounding marine area (94.56 km2) (Pardal and Azeiteiro, 2001; Mendes et al., 2009). Furthermore, this MPA is located near the Nazaré Canyon which extends for over 170 km with an average depth of 3000 m, and which is responsible for intense upwelling contributing to higher prey densities that attract pelagic predators. This phenomenon is intensified by the strong northwest winds originating from the warm Portugal current that flows from North to South along the Portuguese coast (Wooster et al., 1976; Amado et al., 2007; Mil-Homens et al., 2007). The seabed is composed of granite rock bottom inhabited by algae and sessile invertebrates, flanked by long sandbanks, sheltering, and supporting the life cycles of many species (Amado et al., 2007; Vasco-Rodrigues et al., 2011). Moreover, there are submerged and partially submerged caves, acknowledged by the Habitats Directive of the Natura 2000 Network, contributing to the conservation values of this protected area (Amado et al., 2007; Vasco-Rodrigues et al., 2011).
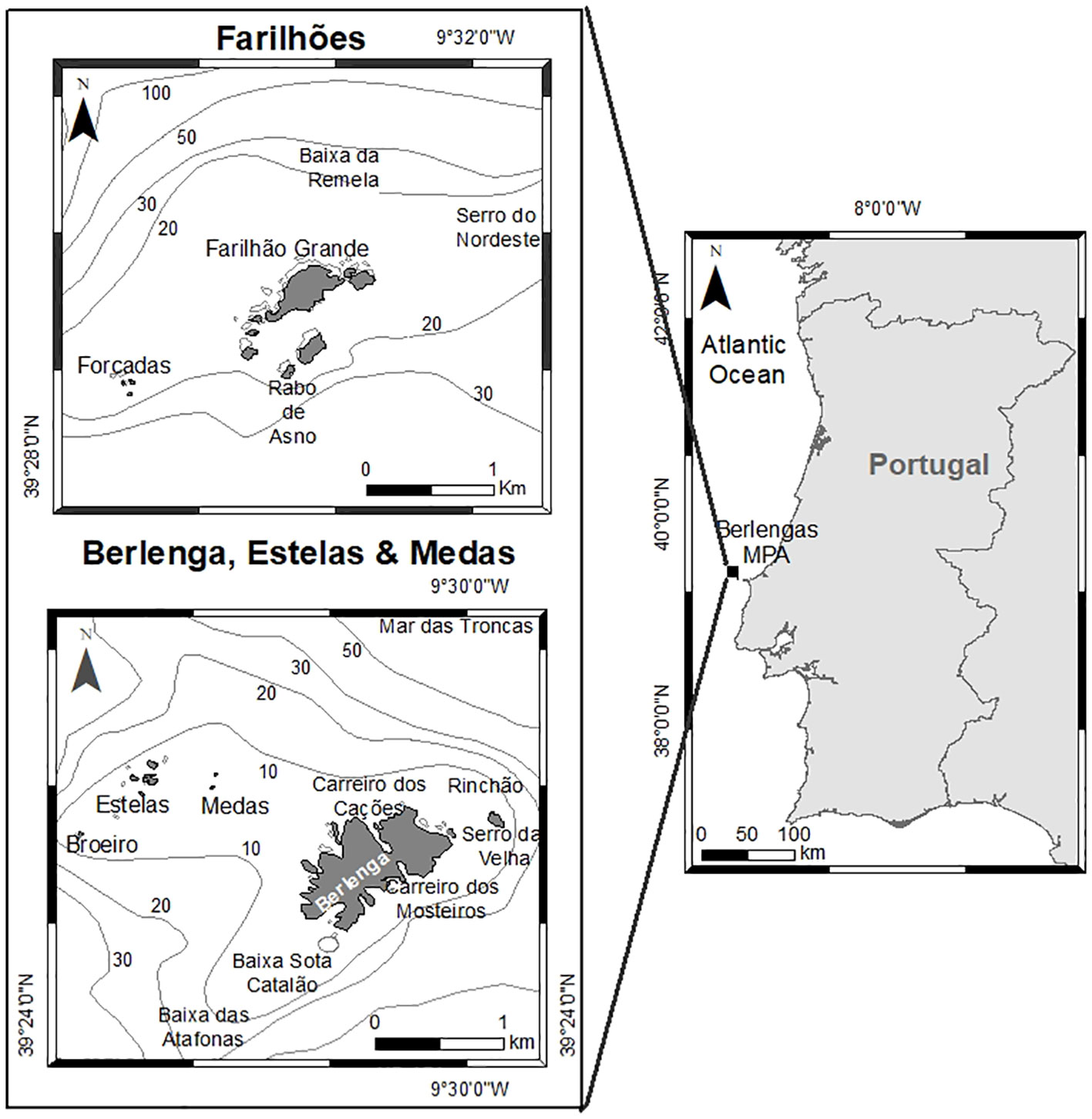
Figure 1 The Berlengas Archipelago (Berlenga island and associated reefs, Farilhões islets, and islets Forcadas, Medas and Estelas), located on the west coast of mainland Portugal.
The Berlengas MPA is divided into different protection zones: two type I partial protection zones, one surrounding Berlenga island, Medas and Estelas islets and the other including the marine area around Farilhões and Forcadas islets; each type I zone is surrounded by a type II partial protection zone; with all type I and type II zones encapsulated within a complementary protection zone (Figure 1, Supplementary Information). Type I partial protection zones encompass valuable biodiversity and landscapes identified by their moderate to high ecological vulnerability. Type II partial protection zones act as buffers or transition zones between Type I areas and the complementary protection zones (the external limit of the MPA).
The management plan of the Berlengas MPA established several conservation measures, such as the prohibition of fishing with trawls, gillnets and refuge traps and spearfishing. Furthermore, only vessels with a special licence to operate in the MPA are allowed. A set of additional restrictions applies to several activities in type I and type II protection zones. In these areas, resource exploitation is allowed with some specific rules. Restrictions to anchoring and navigation are also enforced (in law “Resolução de Conselho de Ministros 180/2008”).
Peniche is the fishing harbour closest to Berlengas MPA and is one of the most important fishing ports in mainland Portugal in terms of landed weight. Peniche’s fishing fleet is composed by purse seiners, trawlers, and multi-gear vessels (Gamito et al., 2016).
2.2 Data collection and processing
The elasmobranch community in Berlengas MPA was assessed using complementary data from different sources and non-invasive sampling methods: stakeholder enquiries, citizen science and social media reporting, scientific observations onboard longline commercial vessels and BRUVs.
2.2.1 Stakeholder enquiries
Primary stakeholder identification was based on previous knowledge of the authors, and consultation of local fishers, dive centres and other research teams working in the study area. Based on this, three groups of stakeholders were identified as operating inside the MPA and in adjacent areas, namely professional fishers, recreational fishers, and nautical activities/tourism operators. Semi-structured map-based enquiries were performed to these three groups to allow for the collection of LEK. The process included a preliminary phase where exploratory interviews were made to key individuals identified by the authors (based in previous studies, contacts with Non-Governmental Organizations and MPA managers) as a reference within each group of identified stakeholders. Based on the results from the preliminary phase, questionnaires and supporting materials (i.e. maps and a catalogue with elasmobranch species known for the area, highlighting key identification characteristics) were applied between January 2019 and September 2020.
The overall structure of the questionnaire was similar for all stakeholder groups, although some questions were adapted according to each group’s particularities. Questionnaires were split into four sections: description of the activity (e.g. vessel characteristics, season, catches - applicable to fishers - and working area), shark and ray observations and ecological patterns, perception of temporal changes of ecological patterns of sharks and rays, and opinion about the Berlengas MPA importance and the impact of sharks and rays on stakeholders´ activities. Both temporal and spatial perceptions on elasmobranchs presence and abundance and working areas were registered with the aid of three maps, containing bathymetry and local reference points: one map of the archipelago (scale 1:50000); one map with the Berlenga island and the Medas and Estelas islets (scale 1:25000); and one map of the Farilhões islets (scale 1:25000). Besides, a catalogue of elasmobranch species was specifically created to accompany all surveys to avoid ambiguity in species identification. The species key identification characteristics and images used were based in “The Field Guide to the Sharks of the World” (Compagno et al., 2005), FishBase database (Froese and Pauly, 2022) and “Rays from Portugal” (IPMA - Instituto Português do Mar e da Atmosfera, 2006).
The snowball sampling strategy (Goodman, 1961) was used, similarly to Ramires et al., 2015. Verbal consent was given by the respondents at the beginning of each questionnaire session, in accordance with the European legislation on data protection and anonymity (Directive 95/46/EC).
In questions related with species observation a scoring system (0-4) was used, as a proxy for presence and abundance, where 0 means “absent species that was once present in the ecosystem”, 1 was assigned to “rare species who appears every other year or less”, 2 for “species whose sightings are occasional (e.g. once a year)”, 3 for “common species, that are observed every month or several times a year”, and 4 for “abundant species that occur on a weekly basis”.
In addition, all respondents were requested to register all observations in the citizen science app (see 2.2.2. for details) or contact the authors if they observed an elasmobranch species (through a phone call or messaging service).
To assess the presence and abundance of each species in the study area, the number of questionnaires that referred a given species and that assigned it any score (not including zero) were quantified, and the median of scores attributed (including zero) was determined.
To probe for factors that might have possibly biased the respondent’s perception, an analysis was made on the influence of age and occupation on score attribution, similarly to Leitão et al. (2020). To test these hypotheses, the normality of the score data was tested through a Shapiro-Wilk test. Afterwards, Kruskal-Wallis tests were made to test for the null hypothesis of no significant differences (p< 0.05) on score attribution between different factors. Due to the wide range of activities and ages of the respondents, the multiple factors tested were age (ranging from 23 to 77), age class [23-43 (n=44); 45-53 (n=40); 55-77 (n=41)], activity (namely set longline for demersal fishes deployed near the bottom to target European conger (Conger conger), and near the surface to target European Seabass (Dicentrarchus labrax), gillnetting, handlining, sightseeing, scuba diving, and dolphin watching) and effect type (split into 2 categories: extractive and non-extractive activities; Table 1). Age class was split into categories that allowed the sample size to be as close as possible to each other (around 40 individuals per group). This evaluation was made for the species most frequently mentioned or with high scores in the enquiries. Questionnaire data was analysed using R software (R Core Team, 2021).
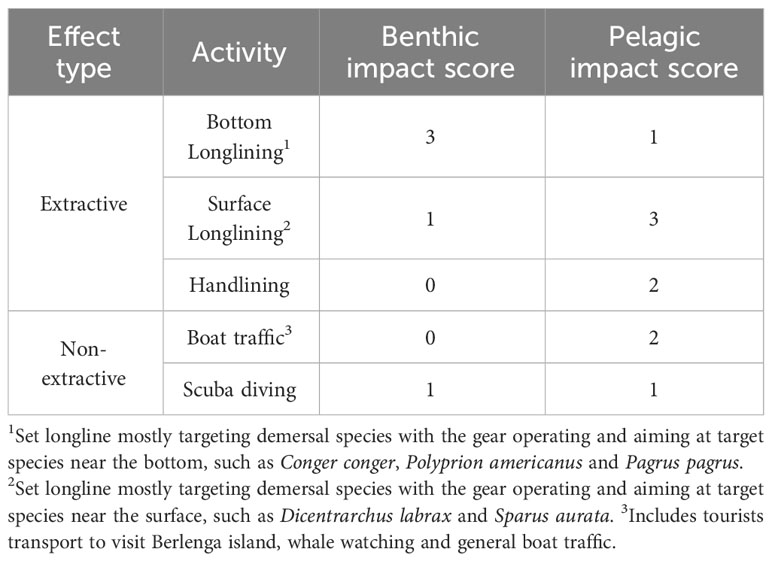
Table 1 Activities occurring in the MPA and adjacent areas by effect type (extractive and non- extractive) and their impact scores on benthic and pelagic habitats (1 – lower impact to 3 - higher impact level); impact scores were used to calculate the Spatial Conflict Risk Index – SCRI - detailed in 2.4).
2.2.2 Citizen science and social media reporting
An umbrella project for elasmobranch sightings in Portugal was created in the iNaturalist citizen science platform (https://www.inaturalist.org/projects/findrayshark), and a campaign was run to teach the community how to report sightings using the iNaturalist mobile phone application. Information about the application and the citizen science project was transmitted with every enquiry with fishers and tourism companies, as well as on social media and in ocean literacy activities in schools. During enquiries, fishers and tourism companies were also encouraged to contact the project team directly to report any sightings, with image support whenever possible.
Additionally, from November 2019 to October 2020, sightings were also compiled from social media, mostly on Facebook, by searching weekly using relevant keywords in Portuguese (shark, ray, sighing, common names of all species of interest in Portuguese, Berlenga). Care was taken to have researchers from this study joining private and public Facebook groups of recreational fishers, spearfishing, SCUBA diving and nautical tourism companies. For each social media post, the date and approximate coordinates were confirmed with the post authors whenever necessary, and any unverifiable or duplicate reports were discarded.
2.2.3 Onboard observations in longline fisheries
Since onboard observations utilise regular fishing operations and do not cause additional mortality, they were considered as a non-invasive method for the purpose of this methodological framework.
Georeferenced data (per haul) of elasmobranch species occurrence in commercial fisheries were obtained by onboard scientific observers working with the “Sociedade Portuguesa para o Estudo das Aves” (SPEA/member of BirdLife International) as part of the LIFE Berlengas project (LIFE13/NAT/PT/000458). These data were made available by request for the purpose of the present study. Surveys occurred from January 2016 to June 2019 on commercial fishing vessels operating set longlines (mostly targeting demersal species at various depths) in the existing Natura 2000 Special Protection Area (SPA) Ilhas Berlengas (PTZPE0009; with near 1027 km2) that includes the Berlengas MPA. The target of SPEA’s study was the characterisation of bycatch of seabirds, and the observers registered all captured species, including elasmobranchs, and the approximate location of the associated fishing gear, which constitutes a rare case of reliable identifications coupled with relatively precise locations.
2.2.4 Baited Remote Underwater Video Systems (BRUVs)
The BRUV rigs used in the present study were composed of two stainless steel bars attached with metal pegs at a 90° angle. The vertical bar held a single action-camera with 170-degree view angle, and the horizontal bar had a 40x10cm PVC perforated tube as a bait box. Care was taken to point the camera so that the bait box was kept at the centre of the field of vision. Videos were recorded at 1080p resolution, 24 frames-per-second to provide the best balance between resolution, battery life and file size.
Three types of BRUV rig setups were used (Figure 2). A benthic setup targeting benthopelagic species, a pelagic fixed setup to detect pelagic species in locations where anchoring the rig was possible (depths< 50 m), and a pelagic drifting setup, used at greater depths without anchoring the rigs.
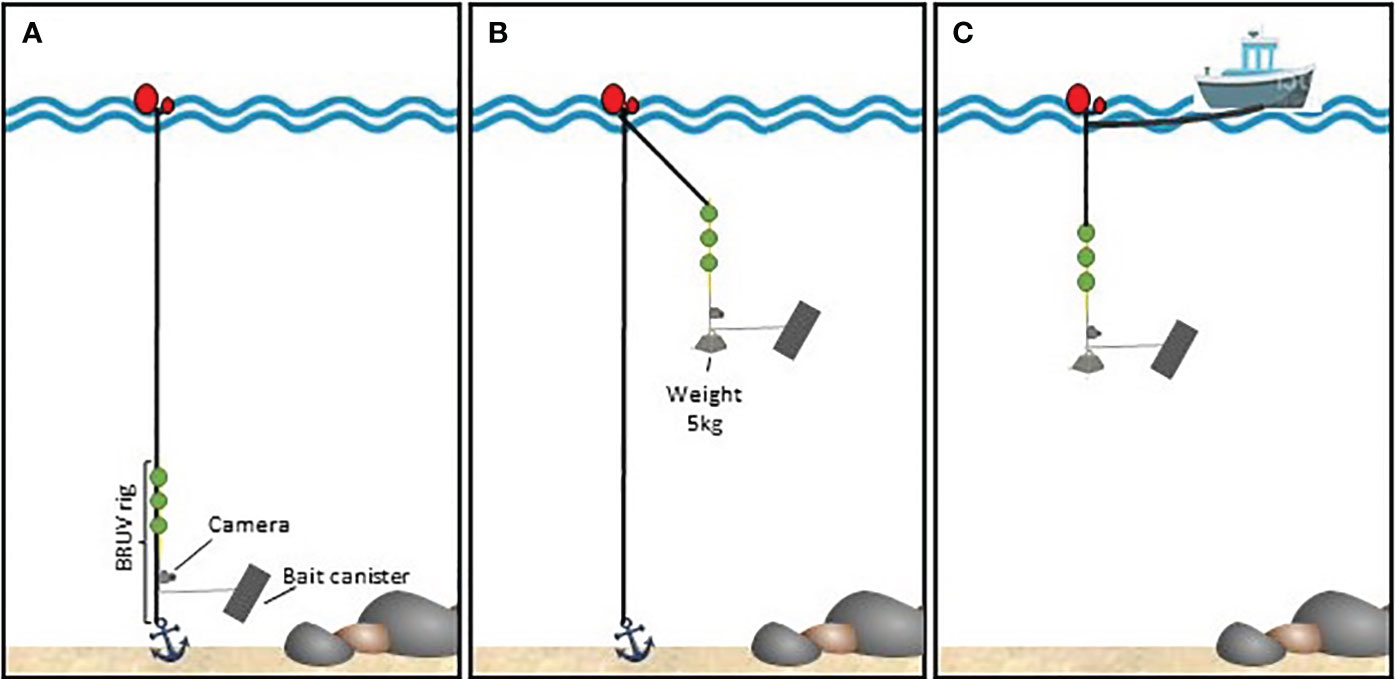
Figure 2 BRUV rig setup schematics. (A) benthic setup; (B) pelagic-fixed setup; (C) pelagic-drift setup.
In benthic BRUVs, only the main rope connected to the anchor was used and the rig was hooked on to this rope to ensure the verticality of the bar carrying the camera. Hollow spheres were also looped into this main rope to keep the rope from dropping in front of the camera. The maximum depth at which benthic BRUV rigs were deployed was limited by sunlight availability and the camera housing rating of 30-40 m.
For pelagic BRUVs, the rig was deployed hanging from a buoy at the surface using a 13-metre rope ending in three rigid, submersible buoys providing 3 kg of lift to straighten the rig and soften oscillation due to waves. To maintain verticality in strong currents, 5 kg of weight were attached to the bottom of the rig. Surface buoys were secured to an anchor at the bottom with a second rope, to keep the rig within a restricted sampling site.
For pelagic drifting deployments, the rig’s surface buoy was tied to the boat using a 70-metre rope, boat engines were cut, and echo sounders turned off to minimise boat interference. This setup also allowed connecting several rigs in sequence, while keeping a minimum of 40 metres between surface buoys, to maximise the probability of detection and the area affected by the bait plume. Depending on sea conditions and marine traffic, up to 4 rigs were attached in a multi-rig drifting setup.
In a first phase, sampling was directed to areas that fishers pointed out as more relevant for elasmobranch captures in the enquiries. In addition, other sites within the study area were sampled to increase area coverage and maximise the probability of finding elasmobranchs, and to include fishery-independent sampling sites.
Each deployment was baited with approximately 1.5 kg of minced Atlantic Chub Mackerel (Scomber colias), a species regarded by local fishers as an effective attractor for elasmobranchs. The bait was stored frozen after being bought fresh from local markets and defrosted 24h before sampling. This procedure permits long-term storage of frozen bait, while providing similar olfactory cues when compared to fresh bait (Becerril-García et al., 2020).
BRUVs were deployed at several times during the day, starting at sunrise to include the period of highest sighting probability (Bouchet et al., 2018). Geographical coordinates, date, time of day, BRUV depth, bottom depth and sea surface temperature were registered for each deployment. A total of 246 valid deployments, 53 benthic and 193 pelagic, were made between May 2019 and June 2021 (Figure 3). In each trip, 8 to 12 deployments were made, with soaking times of 1.5 hours.
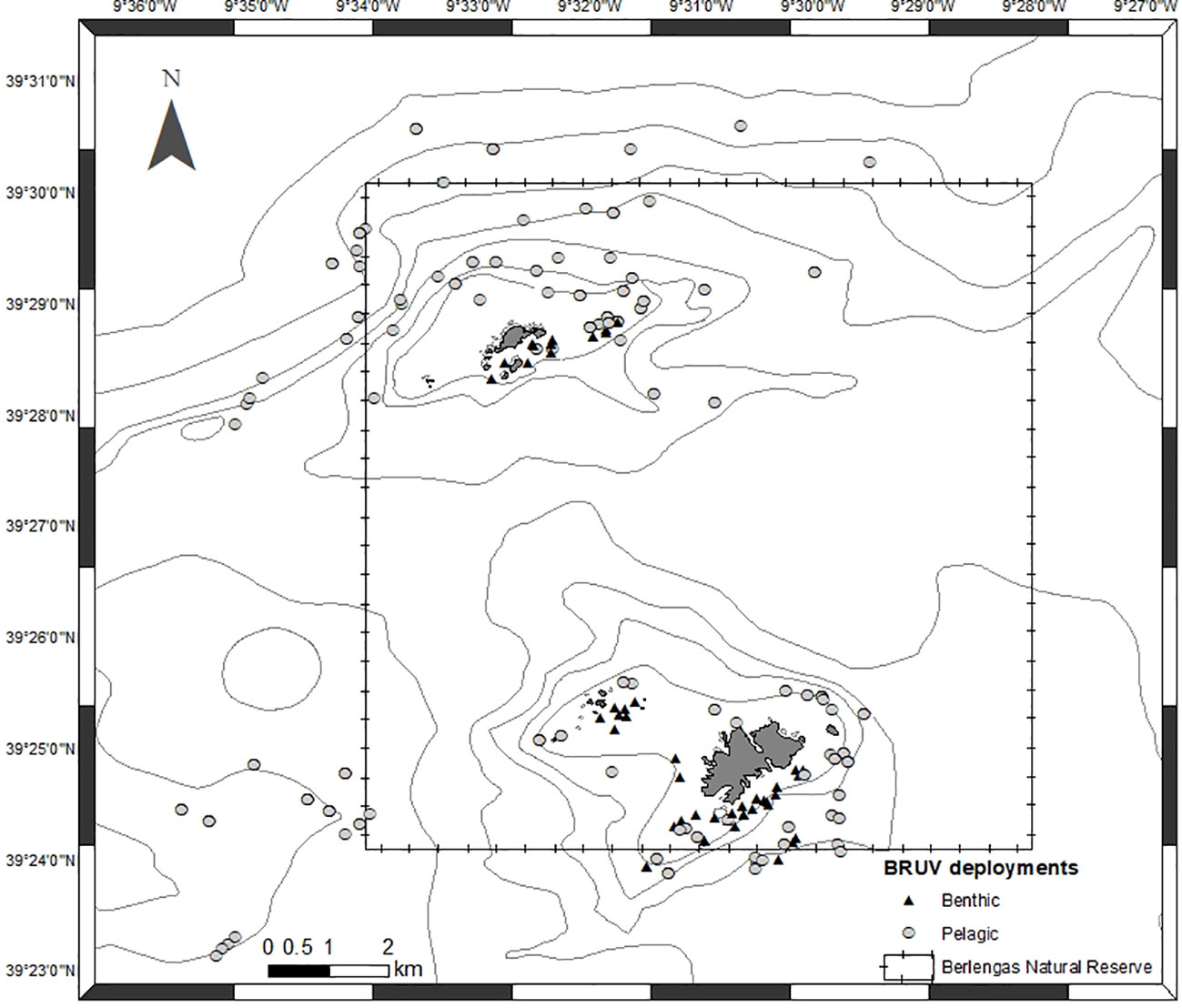
Figure 3 Location of BRUV deployments from May 2019 to June 2021. Triangles represent benthic deployments and circles represent pelagic deployments.
Video analysis was done for the total duration of each video by a single observer using VLC software (VideoLan, 2006) with manual annotation on a spreadsheet. Fifty-five deployments with high turbidity (i.e. the bait canister was not visible) were discarded. For each deployment, elasmobranch species were identified with visual support from the Collins Field Guide (Compagno et al., 2005) and the maximum number of individuals observed of a species in a single frame of video (MaxN) was registered. In multi-rig drifting setups, care was taken to avoid double counting if the same individual appeared on more than one camera. For benthic deployments, the predominant substrate type was also registered (rock, sand, mixed).
2.3 Combining data sources into spatial data: human activities and species distribution
A grid of 1 km2 cells was generated over the map of the study area, providing enough buffer area to compensate for lack of accuracy in some data sources. Two types of data on species presence and abundance were used: 1) observational data with visual confirmation by scientists (BRUVs, onboard fisheries observers, citizen science and social media reports) and 2) non-observational data (enquiries to stakeholders). In each 1 km2 cell on the map, we quantified the number of mentions of a species in that area from enquiries, the number of observations from onboard observers, the number of BRUV sightings, and the number of confirmed sightings from social media reports and citizen science. The number of mentions of human activities per grid cell was also compiled from enquiries.
2.4 Spatial conflict risk assessment
A spatial conflict risk assessment was conducted to map possible risk zones of human activity overlapping with sensitive species. To do so, an index was developed, by attributing relative scores to each 1 km2 grid cell, loosely based on the approaches of Halpern et al. (2008) and Batista et al. (2014), among others.
The perceived abundance (PA) of each species was obtained from the median abundance scores obtained for each species in the stakeholder questionnaires, ranging from 0 to 4 (see 2.2.1 for further details). As some of these species are highly mobile, questionnaires specifically stated that perceived abundance estimates should refer to the whole MPA, and not to a specific cell. For each grid cell, the perceived abundance of all pelagic species (SP) and benthic species (SB) reported for that cell was summed. A species was only counted once per cell, even if mentioned multiple times, to avoid introducing report bias (e.g. if a species was mentioned five times as present in a cell, and the perceived abundance score was 3, the abundance score for that species in that cell is 3, and not 5x3).
As a proxy for the level of anthropogenic impact (I) in each grid cell, a qualitative score was attributed to the impact of each activity in bottom habitats (AB) and in pelagic habitats (AP) in the Berlengas MPA (Table 1), ranging from 0 (negligible impact) to 3 (high impact). The cumulative impact score I for each 1 km2 cell was calculated separately for benthic habitats and pelagic habitats, by summing the corresponding scores of all activities reported for that cell. As with the perceived abundance data, each activity only counts once per cell, even if mentioned multiple times.
Then, the Spatial Conflict Risk Index (SCRI) was calculated for each grid cell as:
Where PA is the perceived abundance score of each benthic (SB) and pelagic (SP) species present in the cell, and I is the impact score of each activity in benthic (AB) and pelagic (AP) habitats. The SCRI is therefore the cumulative impact score multiplied by the total units of perceived abundance per habitat, per cell. All spatial analyses were performed using ArcGIS 10.8.1 (ESRI, 2022).
3 Results
3.1 Species presence and abundance
Twenty-two elasmobranch species were mentioned or observed overall. A total of 128 mentions (in the enquiries) and 52 observations (in citizen science and social media reporting, scientific observations onboard commercial vessels and BRUVs) were obtained. Among the observational data, 69% of the occurrences were from scientific observations onboard commercial vessels, 21% from BRUV footage and 10% from citizen science and social media reporting.
3.1.1 Stakeholder enquiries
A total of 40 questionnaires were applied, of which 16 to professional fishers, 13 to recreational fishers, 10 to marine tourism companies and one to the captain of the National Republican Guard (GNR) “Coastline Control Unit”. All respondents were men between the ages of 20 and 80 with 32.5% being 40 to 50 years old. A total of 43 registered boats were referred during the enquiries, of which 31 were boats operating within and around the Berlengas MPA, 62% of which with five to 10 metres total length, and a maximum of 25 metres. Among the 29 fishing vessels referred (professional and recreational), 24 are fishing in Berlengas MPA area with set longline mostly targeting demersal species (with the gear operating and aiming at target species more towards the surface, such as D. labrax and Gilthead Seabream, Sparus aurata (5) or more towards the bottom such as the Wreckfish (Polyprion americanus) and the Red Porgy, Pagrus pagrus (4), and handlines (13 recreational; 3 professional); whereas outside the MPA, there were four vessels operating with trammel/gill nets, that periodically set traps to catch Octopus (Octopus vulgaris), and one operating with handlines).
Among the marine tourism companies surveyed, nine were ecotourism companies operating within the Berlengas MPA, of which three are recreational diving companies that often operate in the same sites.
During the enquiries, 21 species were mentioned. Blue Shark (Prionace glauca) and Lesser Spotted Dogfish (Scyliorhinus canicula) stand out as the most mentioned (N=23 mentions each), followed by Hammerhead species (Sphyrna spp.; N=11), Shortfin Mako (Isurus oxyrinchus; N=8) and the Thornback Ray (Raja clavata; N=8) (Table 2, Figure 4). In contrast, some species were mentioned only once and thus were not included in the analysis: Sandbar Shark (Carcharhinus plumbeus), Nursehound (Scyliorhinus stellaris), Bull Ray (Aetomylaeus bovinus), Basking Shark (Cetorhinus maximus) and Whale Shark (Rhincodon typus).
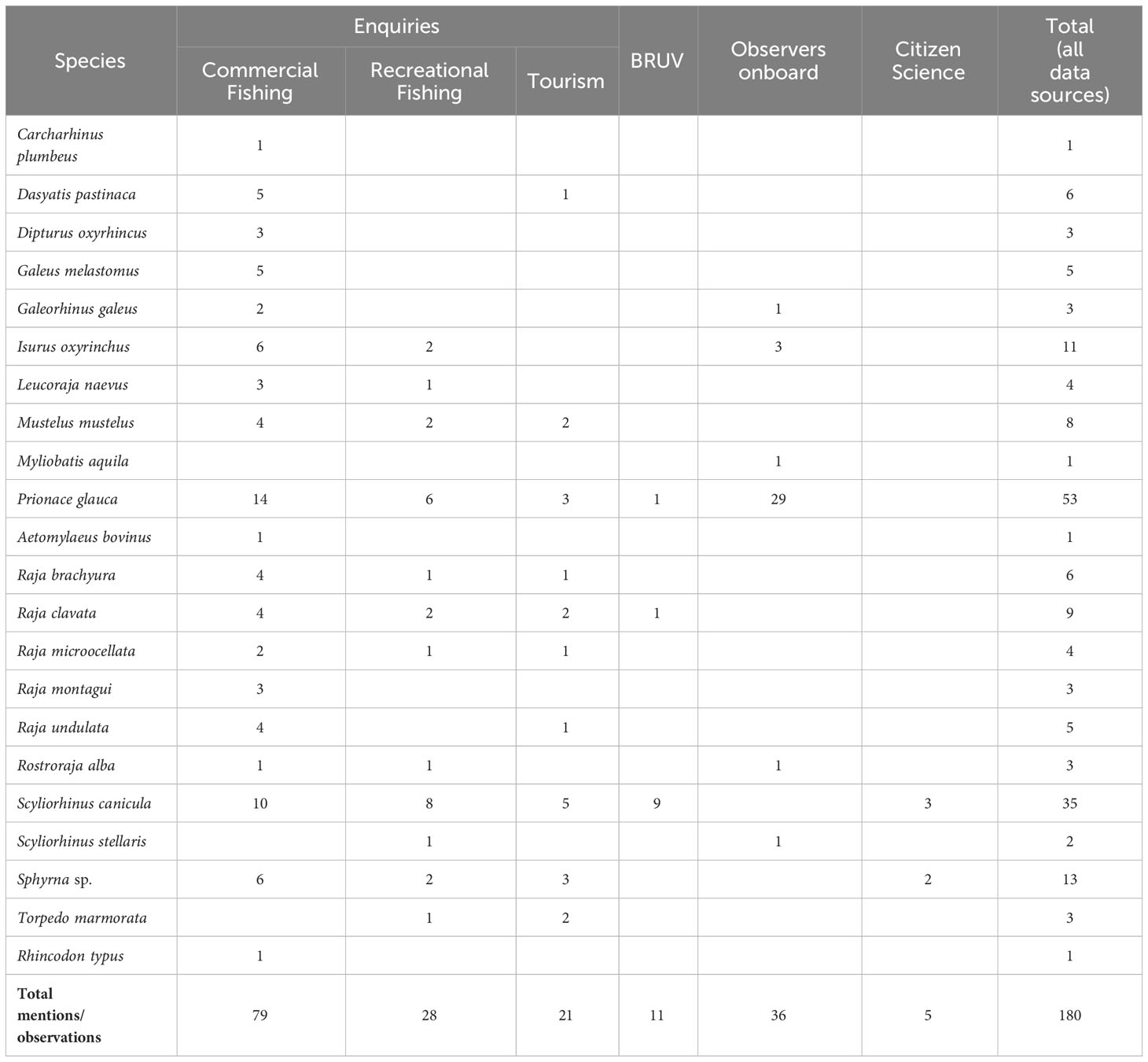
Table 2 Species mentioned in the enquiries (performed to commercial and recreational fishers and to tourism companies) and observed in BRUVs, scientific observations onboard fishing vessels and from citizen science and social media reporting occurrences.
Regarding the species presence and abundance patterns obtained from the enquiries, the Blackmouth Catshark (Galeus melastomus), Long-nosed Skate (Dipturus oxyrinchus), Spotted Ray (Raja montagui) and Undulate Ray (Raja brachyura) had the highest scores (Figure 4). The Smooth-hound (Mustelus mustelus) was one of the most mentioned species, but interviewees differed in their scores: while some considered it as common (score 4) others assumed it to have existed in that ecosystem but is no longer observed (score 0).
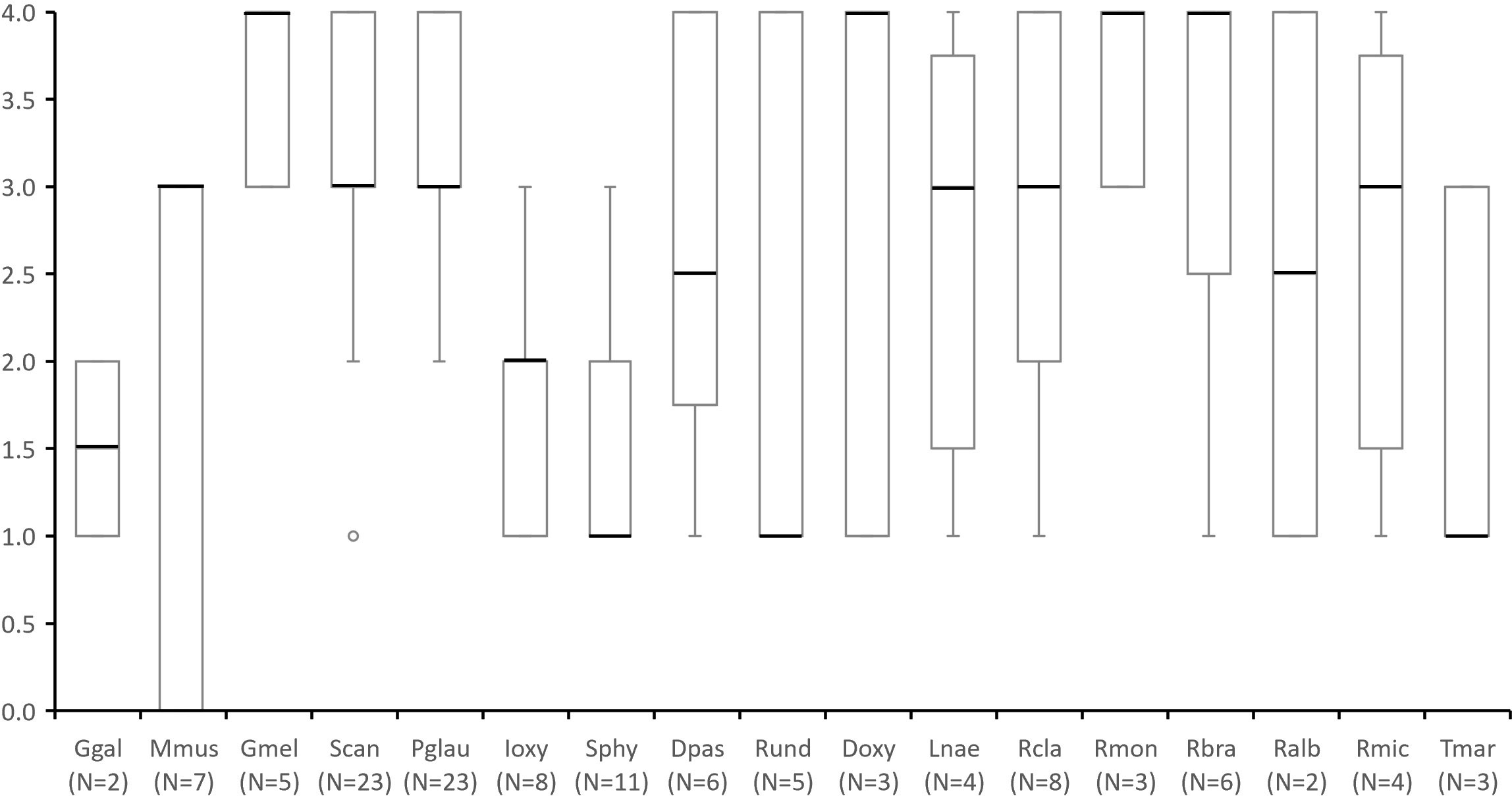
Figure 4 Species presence and abundance through a score from 0 to 4 assigned in questionnaires to stakeholders in which: 0 - “absent species that was once present in the ecosystem”; 1 - “rare species who appears every other year or less”; 2 -”species whose sightings are occasional (e.g. once a year)”; 3 - “common species, that are observed every month or several times a year”, and 4 - “abundant species that occur on a weekly basis”. Number of questionnaires in which each species was mentioned by more than one respondent (including scores of zero) are indicated under species name (N=). Species abbreviations: Ggal (Galeorhinus galeus); Mmus (Mustelus mustelus); Gmel (Galeus melastomus); Scan (Scyliorhinus canicula); Pgla (Prionace glauca); Ioxy (Isurus oxyrinchus); Sphy (Sphyrna spp.); Dpas (Dasyatis pastinaca); Rund (Raja undulata); Doxy (Dipturus oxyrinchus); Lnae (Leucoraja naevus); Rcla (Raja clavata); Rmon (Raja montagui); Rbra (Raja brachyura); Ralb (Rostroraja alba); Rmic (Raja microocelata) and Tmar (Torpedo marmorata). This data considers all mentions, including those whose geographic occurrence occurred outside MPA boundary in surrounding areas.
In general, the respondents’ perception about the presence and abundance of elasmobranchs was not significantly influenced by age or occupation. Exceptions to this are the P. glauca, which had significantly higher abundance scores given by representatives of set longline fishing (Kruskal Wallis: chi-squared = 6.8018: p< 0.05), and by younger respondents (Kruskal Wallis: chi-squared = 8.4543: p< 0.05), and the S. canicula, which had higher scores by respondents from extractive activities (Kruskal Wallis: chi-squared = 6.4196: p< 0.05), namely longline and handline fishing (Kruskal Wallis: chi-squared = 11.131: p< 0.05).
Regarding the stakeholders’ opinion on the MPA implementation, 25% responded positively and 37.5% had a neutral opinion about the impact of the reserve. However, 37.5% of the 40 interviewees had a negative opinion about the MPA efficacy. When probed about their opinion, respondents justified their answers with a need for improvement in monitoring and enforcement strategies.
3.1.2 Citizen science and social media reporting
No new records were registered on the iNaturalist citizen science platform for the study area. Only two records existed at the start of the study, both from 2012, and those were discarded for being outside of the adopted time range. Social media reports, however, recorded the most common species in the area, namely S. canicula and P. glauca, but also provided records of Sphyrna spp. (N=2), whose occurrence was also mentioned in the enquiries (Table 2).
3.1.3 Onboard observations in longline fisheries
A total of 36 individuals of six elasmobranch species were caught (mostly as bycatch) during longline commercial fishing trips with scientific observers onboard in Berlengas MPA or near its limits (Table 2). P. glauca was the most common species with 29 observations and G. melastomus (N=1), I. oxyrinchus (N=3), Common Eagle Ray (Myliobatis aquila; N=1), White skate (Rostroraja alba; N=1) and S. stellaris (N=1) were also observed.
3.1.4 Baited Remote Underwater Video Systems (BRUVs)
In BRUV footage a total of 11 individuals of only three species (S. canicula, P. glauca and R. clavata) were detected (Table 2). The most common observation was S. canicula, in benthic deployments, with a total of nine individuals registered.
3.2 Spatial analysis of human activities and species distribution
Overall, human presence is higher on the eastern side of Berlenga island, on the southern and eastern side of the Farilhões islets and around Estelas and Medas islets (Figure 5). The intensity of human activities was lower in benthic habitats (Figure 5A) than in pelagic habitats (Figure 5B). Bottom-oriented activities occur mainly over southeast and eastern of Berlenga island (on the Rinchão area) (Figure 5A). Most surface-oriented activities occur on the Rinchão area (northeast of Berlenga island), southeastern of Berlengas and around the Estelas islets (Figure 5B), whereas passenger drop-offs on Carreiro do Mosteiro, SCUBA diving in a popular shipwreck, and dolphin-watching tours were more prominent on the south and eastern of Berlenga, strongly contributing to the higher impact scores obtained in this area. In the Farilhões islets, boat tours and SCUBA diving composed all non-extractive pressures.
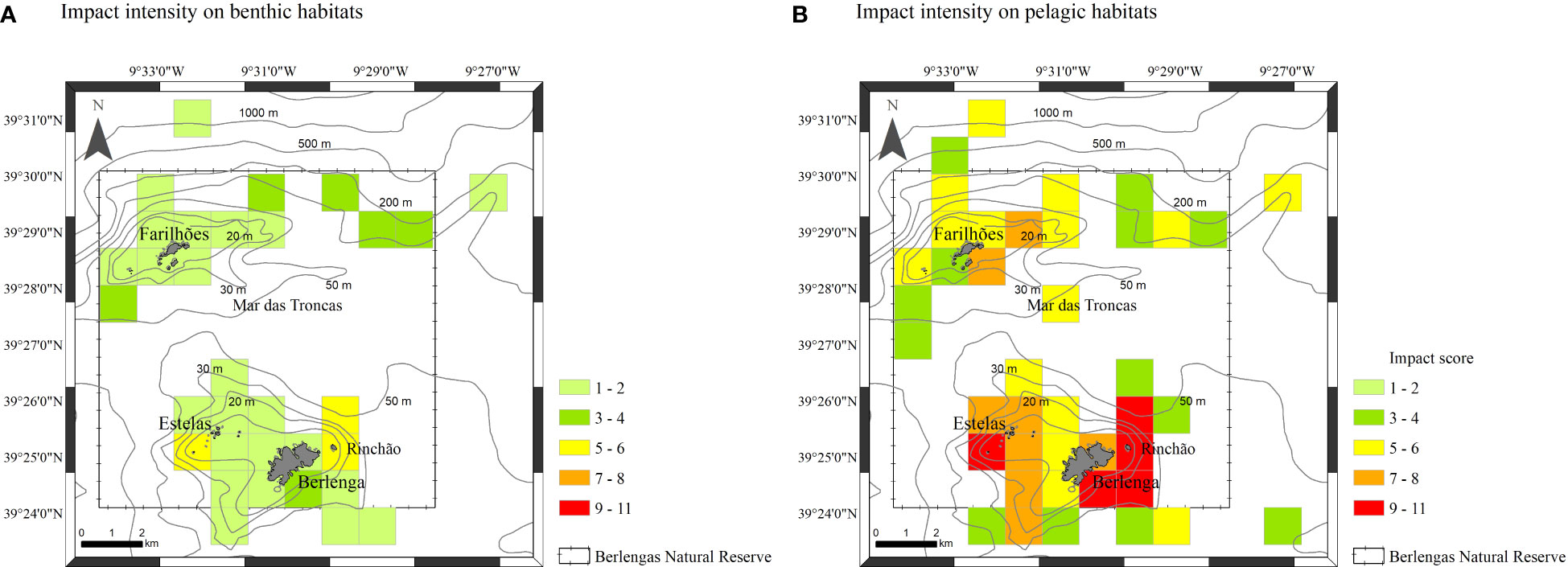
Figure 5 Impact intensity of anthropogenic activities per square km (impact scores from 1 to 11): on benthic habitats (A) and on pelagic habitats (B).
The combination of occurrence and report data from all sources revealed that the perceived abundance of benthic elasmobranch species is high around Berlenga island (south and southeastern sides) but also in Mar das Troncas and at south of Farilhões, namely skates and rays (Figure 6A). Pelagic species, namely P. glauca, showed a marked perceived abundance around Farilhões, but also in some areas around Berlenga (Figure 6B). Pelagic species showed a tendence to occur at higher depths and more distant of the shore than observed for benthic species (Figure 6).
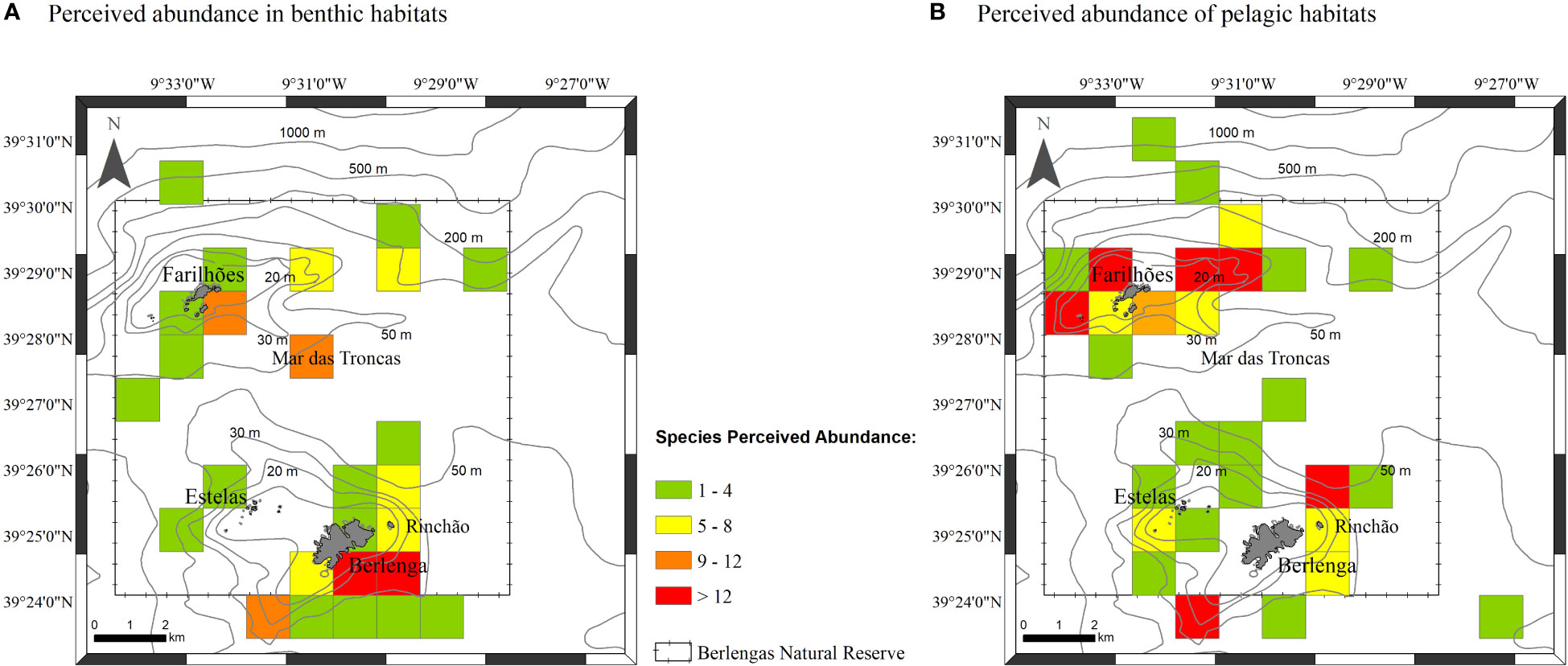
Figure 6 Total perceived abundance of species per square km obtained to: benthic habitats (A) and pelagic habitats (B).
3.3 Spatial conflict risk assessment
After combining species presence and perceived abundance with human activity data into the Spatial Conflict Risk Index (SCRI; see section 2.4), three locations stood out with higher risk of conflict – the eastern-northeastern and side of the Berlenga island, namely on Rinchão, the south of Berlenga and in the areas around Farilhões islets (Figure 7). Rinchão is a sought-after spot for set longline for demersal species as well as for recreational fishing. The surrounding area of Rinchão and south Berlenga encompasses popular diving spots and also important areas for nautical recreation (anchoring area for tourism boats, intense boat traffic). Occurrence of particularly vulnerable species (IUCN, 2024), Sphyrna spp., and the Common Stingray (Dasyatis pastinaca) was recorded for this site. Species such as S. canicula and R. clavata were scored as abundant (score 4) and common (score 3), respectively, by the stakeholders, contributing to higher risk in these areas. The areas around Farilhões islets also scored a high risk, with frequent mentions of P. glauca and extractive activities reported, such as set longlines and handlining.
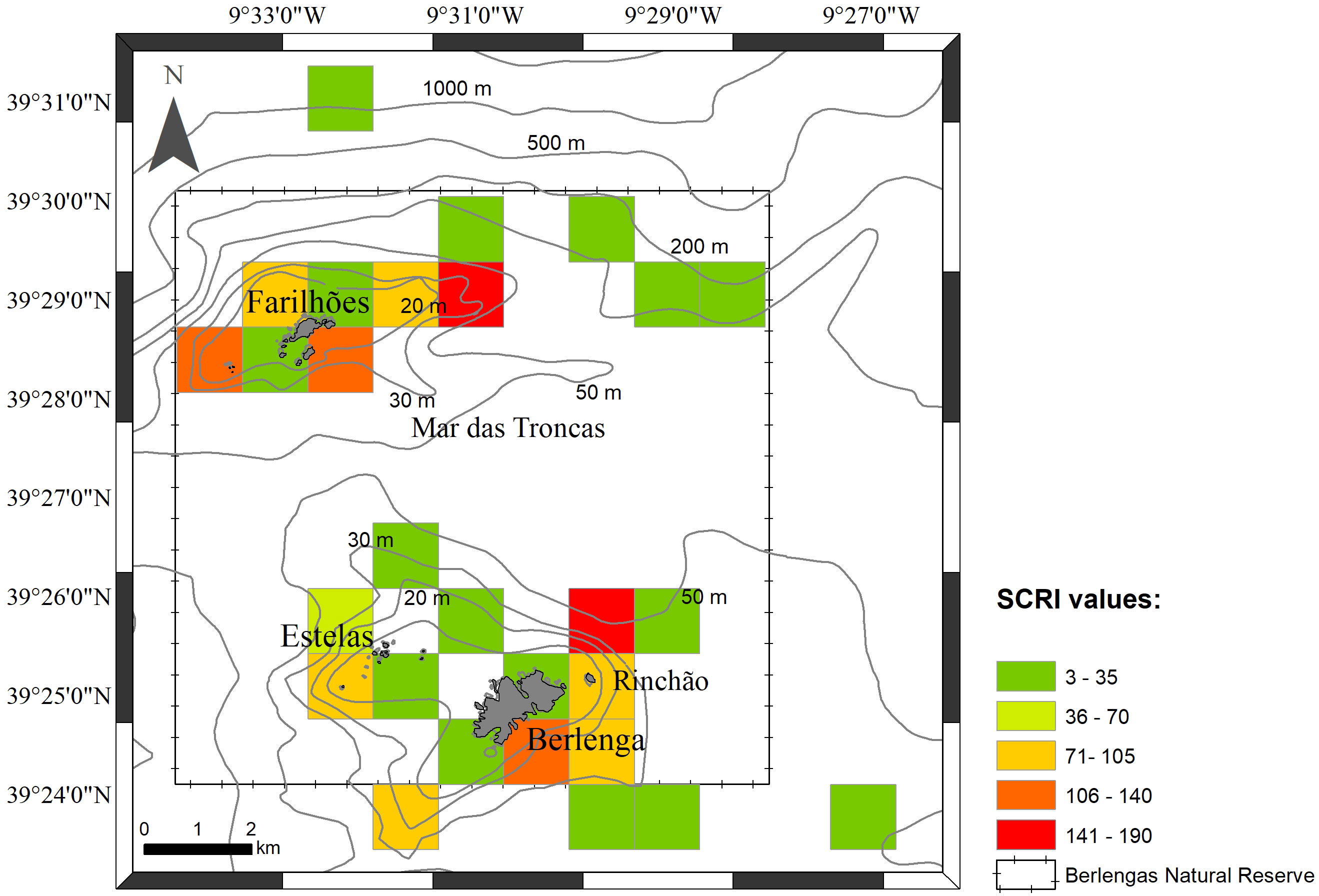
Figure 7 Sensitivity Spatial Conflict Risk Index (SCRI) values obtained per square km in the study area.
4 Discussion
4.1 Advantages and limitations of sampling methods and data sources
In this study, we employed a combination of non-invasive sampling methods to evaluate the presence, abundance, and spatial distribution of elasmobranch species. The establishment of a robust relationship with local stakeholders facilitated the incorporation of Local Ecological Knowledge (LEK), a powerful tool for assessing the human dimension of ecosystems and acquiring valuable ecological data. When combined with complementary data sources, such as questionnaires combined with scientific data from the literature (Bevilacqua et al., 2016), or questionnaires and scientific observations onboard commercial vessels (Lima et al., 2017), LEK becomes a valuable asset in informing policy development, increase data quality and facilitate the support by fishers for future management strategies (Goetz et al., 2014; Dinkel and Sánchez-Lizaso, 2020).
As in the present study, LEK have been providing important knowledge on the characterisation of threats on vulnerable species and habitats worldwide (e.g. cetaceans, elasmobranchs, sea turtles), contributing to the improvement of conservation measures with particular relevance in data-poor regions (Goetz et al., 2014; Serra-Pereira et al., 2014; Sawchuk et al., 2015; Giovos et al., 2018; Cochrane et al., 2019; Dinkel and Sánchez-Lizaso, 2020). This wide use and proven validity of LEK approaches supports the validity of LEK data as applied in the present study. For instance, a study by Dinkel and Sánchez-Lizaso (2020), where semi-structured interviews were used to estimate the conservation status of I. oxyrinchus and P. glauca populations in the Atlantic Ocean, underscores the importance of utilising all available data for decision-support regarding elasmobranch populations; Goetz et al. (2014) quantified cetaceans bycatch levels in multiple sites and fisheries in Galician waters, through face-to-face interviews to local fishers, which allowed the identification of potential conservation measures to minimise bycatch and gears damage (e.g. time and area closures and relocation of some fishing effort) and Giovos et al. (2018) combined unconventional data, including fishers’ LEK to identify critical areas for the endangered guitarfish sympatric species Common Guitarfish (Rhinobatos rhinobatos) and Blackchin Guitarfish (Glaucostegus cemiculus) in Greece.
While questionnaire data may exhibit biases due to factors such as intentional non-disclosure of fishing grounds, reliance on photographic memory, and stakeholder species identification capabilities (Close and Hall, 2006), strategies were employed to enhance data reliability. The use of a species identification catalogue improved respondent accuracy, and visual confirmation by our research team was sought whenever possible, with particular emphasis on ecotourism companies, which often possess and share pertinent information. To address potential trust issues, surveys were conducted anonymously, and an ongoing, proximate relationship was cultivated with the local community, akin to the approach taken by Ramires et al. (2015).
To complement the available information with newly collected field data, non-invasive methods were used to avoid the introduction of unnecessary stressors to the ecosystem when acquiring data. Although the use of BRUV rigs is constrained by factors such as light availability and depth (Bouchet et al., 2018), it provides a cost-effective means of species detection and habitat description. In our study, the BRUV rig reached depths of approximately 30 to 40 metres due to limitations of the waterproof casing. This, regrettably, did not enable a comprehensive study of the sandy bottom of the Mar das Troncas area, with areas up to 70 m deep, and identified as an important skate species habitat by local fishers. In the Nazaré Canyon, we deployed drifting BRUVs to overcome depth limitations, but harsh sea conditions on the northern side of the Farilhões islets limited our deployments in this area, despite it being considered a pelagic shark hotspot by local fishers. In fact, sea conditions limiting the sample size and frequency for pelagic BRUVs may explain the very scarce occurrence of pelagic species in BRUV deployments in this area. Future sampling efforts should focus on the north of the Farilhões area, near the slope of the canyon, and closely guided by input from fishers, in order to better capture the distribution of pelagic sharks. Furthermore, further BRUV studies should experiment with different bait, as different species may exhibit distinct prey preferences (Ghazilou et al., 2016; Jones et al., 2020).
While citizen science can be a very useful method to gather occurrence data on coastal areas (Parretti et al., 2023) it did not produce good results in this area. Despite our efforts to teach all enquiry respondents on how to quickly report a photograph using a mobile phone application, in addition to several activities in schools with students and teachers, new observations reported in the platform were not inside the MPA limits and consisted mostly of egg capsules of skates and S. canicula on beaches, which are much more accessible than islands located 10 km offshore. However, the alternative contact methods that were left with fishers and tourism companies were more effective, as some of them would occasionally contact the authors directly with occurrence reports and photographs. This highlights the critical importance of transparency and communication to establish good relationships with stakeholders based on trust and mutual respect (Batista et al., 2009; Horta e Costa et al., 2013). In addition, social media reports, particularly in local thematic Facebook groups (e.g. fishers, local citizens, nautical tourism companies) provided a few useful observations. This is of course positively biased by the mediatic visibility of sharks, allied to the rarity of sightings in mainland Portugal, so it is expected that other species will rarely be reported on social media, and need to be compensated by other methods, such as local knowledge and scientific observations onboard fishing vessels. Scientific observations onboard fishing vessels is another important source of information that is key for fisheries management purposes, since it provides reliable information on catches composition, including on bycatch, but that can also provide valuable information for conservation, namely elasmobranchs species (Batista et al., 2009; Baeta et al., 2010). In the present study, fisheries observation data was provided by an NGO with a long history of working in seabirds’ conservation (SPEA), which enhanced the results and conclusions obtained and also highlighted the importance of cooperation and data sharing among stakeholders (e.g. NGOs, researchers, managers) to meet conservation objectives.
While all methods have their strengths and limitations, our approach allows for the combination of multiple sources, as long as they report an occurrence with confirmable identification that can be linked to a 1 km2 cell on a grid. This may include acoustic telemetry, satellite tagging, scuba diving visual census, but excludes methods that do not fit the criteria, such as environmental DNA from water samples. While species identification can be very accurate with a good supporting genetic database (Gilbey et al., 2021), strong currents allied to relatively long DNA degradation times in sea water make it very hard to link a species to a cell, or even to the MPA limits at this scale.
4.2 Species distribution in the Berlengas MPA: potential implications for management
The species distribution map evidenced the frequent occurrence of benthic-dependent species such as Raja spp. and S. canicula particularly near Berlenga island and in Mar das Troncas. Although specific measures for elasmobranch conservation are lacking within the Berlengas MPA, some benthic species may benefit from the existing prohibition of bottom-oriented fishing gears. Furthermore, a seasonal fishing closure for skates is in force between May and June (extended to July for R. undulata) on the coast of mainland Portugal. For species such as R. clavata, which can lay eggs from December to August, peaking in June (Holden, 1975), and for R. undulata, which follows a similar reproductive pattern (Serra-Pereira et al., 2015), this seasonal closure may offer protection to gestating females. Despite the insights gained from our study on elasmobranch presence and distribution within the MPA, additional information on species abundance, habitat use (e.g. data collected over subsequent years), and the scale of human activities (e.g. through dedicated studies) is imperative to enhance the efficacy of management strategies, maximizing ecological benefits while minimizing economic impacts, if any.
4.3 Spatial conflict risk assessment
4.3.1 Insights on extractive and non-extractive activities potentially affecting elasmobranchs in the Berlengas MPA
Results from the enquiries and the map of human activities revealed that the primary extractive activities within the MPA involve set longline fishing targeting demersal species, with variations in gear depth and target species. These fishing methods inadvertently result in the incidental capture of species such as P. glauca and I. oxyrinchus (Mandelman et al., 2008; Campana et al., 2009; Campana et al., 2011a), two of the most commonly encountered species in this study. However, higher bycatch rates occur in longlines targeting large pelagic species, as pelagic sharks often trail their prey and get caught when feeding on hooked fish, a phenomenon termed depredation (Mitchell et al., 2018). In the Berlengas MPA, this occurs when pelagic sharks pursue species such as D. labrax and S. aurata, target species of demersal set longlines. Consequently, these two pelagic shark species may experience the greatest impact within the MPA, with the risk index pinpointing the Rinchão area as the most conflicted for these species and extractive activities.
The set longline for demersal species also appears to frequently capture rays and skates, as reported by local fishers during the enquiries. However, the impact of these captures on ray and skate abundance is not expected to be substantial, as the main fishing gears responsible for higher levels of impact are gill and trammel nets, as well as bottom trawls (Baeta et al., 2010; Figueiredo et al., 2020), none of which are permitted within the MPA. It is relevant to note, nonetheless, that species such as R. montagui, R. brachyura, and R. undulata, categorised as “Near Threatened” by the IUCN Red List (Ellis et al., 2007, 2015; McCully et al., 2015; IUCN, 2024), are often landed as “Raja spp.” due to misidentifications, leading to an information gap regarding landing volumes per species (Serra-Pereira et al., 2005, 2015), which poses a significant challenge in species-specific risk assessment.
Besides extractive activities, enquiry data reported the presence of nine ecotourism companies in the area, dedicated to visits to the Berlenga’s caves (by the sea), scuba diving and tourists’ transportation to Berlenga. Among these, three are recreational diving companies frequently operating on the same sites. Even in the absence of more impactful tourism practices like chumming and cage diving in the study area, the presence of non-extractive activities such as marine fauna observation and scuba diving may still influence elasmobranch behaviour (Semeniuk et al., 2009). This was demonstrated in other regions for R. typus and Whitetip Reef Sharks (Triaenodon obesus), where boat presence was shown to induce stress and alter metabolic rates during deep and prolonged dives (Barnett et al., 2016; Montero-Quintana et al., 2020). While there are no reported high magnitude impacts from these non-extractive activities affecting elasmobranch species in the MPA, they should still be monitored and considered in future management decisions.
4.3.2 Implications of the risk index construction and applicability
The Spatial Conflict Risk Index developed in this study is intended to be a simple assessment tool, which was not achievable without assumptions and simplifications. A very complex assessment index would require large amounts of data and information which is usually lacking in data-scarce regions, defeating the purpose of this approach. For this reason, it comes with some implications that branch from its construction. Instead of relying on species-specific impact scores for each activity, which would be difficult to estimate in many cases, it only divides an activity into its pelagic habitat impact and benthic habitat impact (see section 2.4), which assumes that impacting any of those habitats may equally affect all species most associated with it. This means that more vulnerable or more iconic species do not count more than any other species towards risk estimation. In fact, iconic or vulnerable species are often rare, and a single circumstantial sighting would instantly overvalue a cell, yielding misleading results.
What determines the weight of a species in the final calculation of risk is simply its perceived abundance (PA) score (0-4), gathered from enquiries with local stakeholders, as all perceived abundances are summed and then the total abundance score is multiplied by the cumulative impact (I) score. In practice, this means that a cell with two very impactful activities (I = 3 x 2 = 6) and one rare species (PA = 1) will have a risk score of 6 but, if the species is very common (PA = 4), the risk score becomes 24, four times higher. This fact justifies the need for careful assignment of PA scores per species, and that is why the median score of all enquiries was adopted, as the average would be more sensitive to outliers.
Due to the index formulation, an area can be considered of high risk if it has a low but present impact and high species richness and abundance, but it can also reach such high values with few species but many impactful activities. This is particularly evident in the south and eastern side of the Berlenga island. Although there are not many species reported for the area (mostly S. canicula, T. marmorata and R. clavata), the high concentration of human activities in this region is the main contributor towards a high-risk score, highlighting the need for close monitoring. In fact, as a sheltered area and the primary entrance to the island, uncontrolled recreational use may negatively impact habitats crucial to these species (Milazzo et al., 2002; Di Franco et al., 2009).
The SCRI holds promise as a valuable tool for identifying vulnerable areas in a timely manner for data-scarce regions, due to its capability of leveraging local knowledge. Requiring only quantification of impact magnitude for human activities, and a qualitative scale of species abundance, this index can be easily adapted for use in different ecosystems, with different species, and employing different types of data, such as field measurements and analyses for more precise impact and abundance. A study by Queiroz et al. (2016) using satellite tracking has highlighted the importance of mapping fishing effort, and its overlap with shark distribution, to efficiently inform management strategies suggesting implementation of seasonal closures and the establishment of catch quotas. The SCRI can ultimately help identify areas potentially subject to unsustainable impacts or high fishing mortality, providing support for management decisions towards sustainable fisheries practices and conservation measures in MPAs.
4.4 Achievements of the approach and future directions
The methodological approach presented in this study provided a relatively low cost and fast solution to combine several sources of data into a meaningful georeferenced guide on priority areas. Before our study, the Berlengas MPA lacked spatial information on the presence of elasmobranch species, particularly sharks. By combining enquiries and local knowledge with onboard observations, social media reports and remote camera deployments, we were able to generate a map of elasmobranch species in the area with perceived abundances, a map of cumulative pressure from human activities, and a combined map of potentially higher risk areas for elasmobranchs, which constitutes a valuable information package for conservation (Coelho et al., 2015; Queiroz et al., 2019).
Given the well-documented vulnerability of elasmobranch species globally, the limited scientific knowledge regarding their ecology, and the inherent challenges associated with obtaining systematic data on their detailed spatial distribution, our approach assumes critical importance. It serves to inform both scientists and decision-makers, contributing significantly to the support of precautionary conservation measures. It is reasonable to conclude that our approach was able to provide clearer, more organised and more useful data in an area where information was dispersed and long-term data series were not available, particularly for rare and threatened species. While our approach could not yield fine-scale distribution data or evidence of nurseries or aggregation areas, it was able to provide a baseline to direct future monitoring efforts and stakeholder engagement activities to support scientifically informed management decisions. Nonetheless, the underlying data used in this study still has limitations and could be significantly improved with additional, preferably quantitative, and spatially precise information on species abundance, habitat use, and the extent of human activities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MB: Conceptualisation, Data curation, Formal Analysis, Investigation, Methodology, Writing – original draft. CA: Data curation, Formal Analysis, Methodology, Writing – original draft. AV: Writing – review & editing. RV: Writing – review & editing. MP: Conceptualisation, Investigation, Data curation, Formal Analysis, Methodology, Supervision, Writing – original draft. SH: Conceptualisation, Investigation, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Supervision, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was funded by Fundo para a Conservação dos Oceanos, by Oceanário de Lisboa and Oceano Azul Foundation. It also had the support of the Fundação para a Ciência e a Tecnologia (FCT) through MARE (Marine and Environmental Sciences Centre, UIDP/04292/2020, DOI: 10.54499/UIDP/04292/2020 and UIDB/04292/2020, DOI: 10.54499/UIDB/04292/2020) and ARNET (Aquatic Research Infrastructure Network Associated Laboratory, LA/P/0069/2020; DOI: 10.54499/LA/P/0069/2020). AV, MP, and SH were supported by research contracts (DL57/2016/CP1440; DL57/2016/CP1479/CT0020 and DL57/2016/CP1479/CT0021). RV was supported by PNAB – “Programa Nacional de Amostragem Biológica” from IPMA, I.P. -Instituto Português do Mar e da Atmosfera.
Acknowledgments
We are thankful to all members of the Just Dive – Blue Academy team for all the logistical support during BRUV surveys; Sociedade Portuguesa para o Estudo das Aves (SPEA) and Life Berlengas (LIFE13/NAT/PT/000458) for onboard observation data and initial support to collect ecological local knowledge; local fishers for technical advice to set BRUV structures and to all enquiry respondents for their important collaboration; all volunteers that participated in BRUV surveys and enquiries; Ivone Figueiredo and Barbara Serra-Pereira and colleagues (Instituto Português do Mar e da Atmosfera, I.P. - IPMA) for the advice in preparing the ecological knowledge data collection; Docapesca Portos e Lotas S.A. for the support to logistics of bait acquisition; iNaturalist platform, Biodiversity4All and all citizen scientists that uploaded observations; Instituto da Conservação da Natureza e das Florestas (ICNF) and Capitania do Porto de Peniche for their institutional support and licensing of the project field sampling.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1321620/full#supplementary-material
Supplementary Figure 1 | Berlengas MPA limits and zonation.
References
Amado A., Gafeira C., Teixeira A., Preto A. (2007). “Plano de Ordenamento da Reserva Natural Das Berlengas,” in ICNB - Inst. da Conserv. da Nat. e da Biodiversidade, 253. Available at: http://www.cm-peniche.pt/_uploads/PDF_Berlengas_Laboratorio/PO_RNB_Relatorio.pdf.
Baeta F., Batista M., Maia A., Costa M. J., Cabral H. (2010). Elasmobranch bycatch in a trammel net fishery in the Portuguese west coast. Fish. Res. 102, 123–129. doi: 10.1016/j.fishres.2009.10.016
Barnett A., Payne N. L., Semmens J. M., Fitzpatrick R. (2016). Ecotourism increases the field metabolic rate of whitetip reef sharks. Biol. Conserv. 199, 132–136. doi: 10.1016/j.biocon.2016.05.009
Batista M. I., Henriques S., Pais M. P., Cabral H. N. (2014). Assessment of cumulative human pressures on a coastal area: Integrating information for MPA planning and management. Ocean Coast. Manage. 102, 248–257. doi: 10.1016/j.ocecoaman.2014.09.020
Batista M. I., Teixeira C. M., Cabral H. N. (2009). Catches of target species and bycatches of an artisanal fishery: The case study of a trammel net fishery in the Portuguese coast. Fish. Res. 100, 167–177. doi: 10.1016/j.fishres.2009.07.007
Becerril-García E. E., Hoyos-Padilla E. M., Micarelli P., Galván-Magaña F., Sperone E. (2020). Behavioural responses of white sharks to specific baits during cage diving ecotourism. Sci. Rep. 10, 1–12. doi: 10.1038/s41598-020-67947-x
Bevilacqua A. H. V., Carvalho A. R., Angelini R., Christensen V. (2016). More than anecdotes: Fishers’ ecological knowledge can fill gaps for ecosystem modeling. PloS One 11, 1–18. doi: 10.1371/journal.pone.0155655
Biery L., Pauly D. (2012). A global review of species-specific shark-fin-to-body-mass ratios and relevant legislation. J. Fish Biol. 80, 1643–1677. doi: 10.1111/j.1095-8649.2011.03215.x
Bouchet F., Meeuwig J., Huveneers C., Langlois T., Letessier T., Lowry M., et al. (2018). “Marine Sampling Field Manual for Benthic Stereo BRUVS (Baited Remote Underwater Videos),” in Field Manuals for Marine Sampling to Monitor Australian Waters. Eds. Przeslawski R., Foster S. Marine Biodiversity Hub, pp. 105–132. Available at: https://www.researchgate.net/publication/323470393_Marine_sampling_field_manual_for_pelagic_stereo-BRUVS_Baited_Remote_Underwater_Videos.
Braga H., de O., Pardal M.Â., Azeiteiro U. M. (2018). Incorporation of local ecological knowledge (LEK) into biodiversity management and climate change variability scenarios for threatened fish species and fishing communities—Communication patterns among bioResources users as a prerequisite for co-management. Clim. Change Manage. 2, 237–262. doi: 10.1007/978-3-319-70066-3_16
Brooks E. J., Sloman K. A., Sims D. W., Danylchuk A. J. (2011). Validating the use of baited remote underwater video surveys for assessing the diversity, distribution and abundance of sharks in the Bahamas. Endanger. Species Res. 13, 231–243. doi: 10.3354/esr00331
Bruns S., Henderson A. C. (2020). A baited remote underwater video system (BRUVS) assessment of elasmobranch diversity and abundance on the eastern Caicos Bank (Turks and Caicos Islands); an environment in transition. Environ. Biol. Fishes 103, 1001–1012. doi: 10.1007/s10641-020-01004-4
Campana S. E., Brading J., Joyce W. (2011a). Estimation of pelagic shark by catch and associated mortality in Canadian Atlantic fisheries. DFO Can. Sci. Advis. Sec. Res. Doc. vi, 19p.
Campana S. E., Ferretti F., Rosenberg A. (2011b). “Sharks and other elasmobranchs,” in The First Global Integrated Marine Assessment, World Ocean Assessment I. (United Nations). (Cambridge: Cambridge University Press), 781–788. doi: 10.1017/9781108186148.050
Campana S. E., Joyce W., Manning M. J. (2009). Bycatch and discard mortality in commercially caught blue sharks prionace glauca assessed using archival satellite pop-up tags. Mar. Ecol. Prog. Ser. 387, 241–253. doi: 10.3354/meps08109
Catlin-Groves C. L. (2012). The citizen science landscape: From volunteers to citizen sensors and beyond. Int. J. Zool. 2012, 1–14. doi: 10.1155/2012/349630
Close C. H., Hall G. B. (2006). A GIS-based protocol for the collection and use of local knowledge in fisheries management planning. J. Environ. Manage. 78, 341–352. doi: 10.1016/j.jenvman.2005.04.027
Cochrane K. L., Rakotondrazafy H., Aswani S., Chaigneau T., Downey-Breedt N., Lemahieu A., et al. (2019). Tools to enrich vulnerability assessment and adaptation planning for coastal communities in data-poor regions: Application to a case study in Madagascar. Front. Mar. Sci. 5. doi: 10.3389/fmars.2018.00505
Coelho R., Fernandez-Carvalho J., Lino P. G., Santos M. N. (2012a). An overview of the hooking mortality of elasmobranchs caught in a swordfish pelagic longline fishery in the Atlantic Ocean. Aquat. Living Resour. 25, 311–319. doi: 10.1051/alr/2012030
Coelho R., Fernandez-Carvalho J., Santos M. N. (2015). Habitat use and diel vertical migration of bigeye thresher shark: Overlap with pelagic longline fishing gear. Mar. Environ. Res. 112, 91–99. doi: 10.1016/j.marenvres.2015.10.009
Coelho R., Santos M. N., Amorim S. (2012b). Effects of hook and bait in a tropical northeast Atlantic pelagic longline fishery. Bull. Mar. Sci. 88, 449–467. doi: 10.5343/bms.2011.1064
Compagno L. J. V., Dando M., Fowler S. (2005). A Field Guide to the Sharks of the World (London: Collins), 368 pp.
Correia J. P. S. (2009). Pesca comercial de tubarões e raias em Portugal. Tese de doutoramento. Universidade de Aveiro, Portugal. 402pp.
Di Franco A., Milazzo M., Baiata P., Tomasello A., Chemello R. (2009). Scuba diver behaviour and its effects on the biota of a Mediterranean marine protected area. Environ. Conserv. 36, 32–40. doi: 10.1017/S0376892909005426
Dinkel T. M., Sánchez-Lizaso J. L. (2020). Involving stakeholders in the evaluation of management strategies for shortfin mako (Isurus oxyrinchus) and blue shark (Prionace glauca) in the Spanish longline fisheries operating in the Atlantic Ocean. Mar. Policy 120, 104124. doi: 10.1016/j.marpol.2020.104124
Dulvy N. K., Baum J. K., Clarke S., Compagno L. J. V., Cortés E., Domingo A., et al. (2008). You can swim but you can’t hide: The global status and conservation of oceanic pelagic sharks and rays. Aquat. Conserv. Mar. Freshw. Ecosyst. 18, 459–482. doi: 10.1002/aqc.975
Dulvy N. K., Forrest R. E. (2010). Sharks and their relatives II. In Eds. Carrier J. C., Musick J. A., Heithaus M. R. Biodiversity, Adaptative physiology, and conservation (Florida, US: CRC Press). doi: 10.1111/j.1095-8649.2011.03024.x
Dulvy N. K., Fowler S. L., Musick J. A., Cavanagh R. D., Kyne P. M., Harrison L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. Elife 3, 1–34. doi: 10.7554/eLife.00590
Dulvy N. K., Pacoureau N., Rigby C. L., Pollom R. A., Jabado R. W., Ebert D. A., et al. (2021). Overfishing drives over one-third of all sharks and rays toward a global extinction crisis. Curr. Biol. 31, 4773–4787.e8. doi: 10.1016/j.cub.2021.08.062
Ellis J. R., McCully S., Walls R. H. L. (2015). Raja undulata (Europe assessment). The IUCN Red List of Threatened Species 2015. (Gland, Switzerland: IUCN).
Ellis J., Ungaro N., Serena F., Dulvy N., Nertozzi M., Pasolini P., et al. (2007). Raja montagui. The IUCN red list of threatened species 2007: e.T63146A12623141. Gland, Switzerland: IUCN. doi: 10.2305/IUCN.UK.2007.RLTS.T63146A12623141.en
ESRI (2022). ArcGIS 10.8.1. software. https://www.esri.com/
Ewell C., Hocevar J., Mitchell E., Snowden S., Jacquet J. (2020). An evaluation of Regional Fisheries Management Organization at-sea compliance monitoring and observer programs. Mar. Policy 115, 103842. doi: 10.1016/j.marpol.2020.103842
Figueiredo I., Maia C., Lagarto N., Serra-Pereira B. (2020). Bycatch estimation of Rajiformes in multispecies and multigear fisheries. Fish. Res. 232, 105727. doi: 10.1016/j.fishres.2020.105727
Fraisl D., Hager G., Bedessem B., Gold M., Hsing P. Y., Danielsen F., et al. (2022). Citizen science in environmental and ecological sciences. Nat. Rev. Methods Prim. 2, 1–20. doi: 10.1038/s43586-022-00144-4
Froese R., Pauly D. (2022). FishBase. World Wide Web electronic publication. www.fishbase.org. (Version september, 2022).
Gamito R., Pita C., Teixeira C., Costa M. J., Cabral H. N. (2016). Trends in landings and vulnerability to climate change in different fleet components in the Portuguese coast. Fish. Res. 181, 93–101. doi: 10.1016/j.fishres.2016.04.008
Garcia S. M., Allison E. H., Andrew N. J., Béné C., Bianchi G., de Graaf G. J., et al. (2008). Towards integrated assessment and advice in small-scale fisheries: Principles and processes. . FAO Fisheries and Aquaculture Technical Paper No. 515. Rome: FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS.
García V. B., Lucifora L. O., Myers R. A. (2008). The importance of habitat and life history to extinction risk in sharks, skates, rays and chimaeras. Proc. R. Soc B Biol. Sci. 275, 83–89. doi: 10.1098/rspb.2007.1295
Ghazilou A., Shokri M. R., Gladstone W. (2016). Animal v. plant-based bait: Does the bait type affect census of fish assemblages and trophic groups by baited remote underwater video (BRUV) systems? J. Fish Biol. 88, 1731–1745. doi: 10.1111/jfb.12935
Gilbey J., Carvalho G., Castilho R., Coscia I., Coulson M. W., Dahle G., et al. (2021). Life in a drop: Sampling environmental DNA for marine fishery management and ecosystem monitoring. Mar. Policy 124, 1–9. doi: 10.1016/j.marpol.2020.104331
Giovos I., Chatzispyrou A., Doumpas N., Stoilas V.-O., Moutopoulos D. K. (2018). Using unconventional sources of information for identifying critical areas for the endangered guitarfish in Greece. J. Black Sea/Mediterranean Environ. 24, 38–50.
Giovos I., Stoilas V. O., Al-Mabruk S. A. A., Doumpas N., Marakis P., Maximiadi M., et al. (2019). Integrating local ecological knowledge, citizen science and long-term historical data for endangered species conservation: Additional records of angel sharks (Chondrichthyes: Squatinidae) in the Mediterranean Sea. Aquat. Conserv. Mar. Freshw. Ecosyst. 29, 881–890. doi: 10.1002/aqc.3089
Goetz S., Read F. L., Santos M. B., Pita C., Pierce G. J. (2014). Cetacean–fishery interactions in Galicia (NW Spain): results and management implications of a face-to-face interview survey of local fisher. ICES J. Mar. Sci. 71, 604–617. doi: 10.1093/icesjms/fst149
Gore M. A., Frey P. H., Ormond R. F., Allan H., Gilkes G. (2016). Use of photo-identification and mark-recapture methodology to assess basking shark (Cetorhinus maximus) populations. PloS One 11, 1–22. doi: 10.1371/journal.pone.0150160
Grorud-Colvert K., Sullivan-Stack J., Roberts C., Constant V., Horta E Costa B., Pike E. P., et al. (2021). The MPA guide: A framework to achieve global goals for the ocean. Science 373, 1–10. doi: 10.1126/science.abf0861
Halpern B. S., Walbridge S., Selkoe K. A., Kappel C. V., Micheli F., D’Agrosa C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Harvey E. S., Santana-Garcon J. S., Goetze J. S., Saunders B. J., Cappo M. (2018). “The use of stationary underwater video for sampling sharks,” in Shark research: Emerging technologies and applications for the field and laboratory (Boca Raton, Florida: CRC Press), 111–132.
Holden M. J. (1975). The fecundity of raja clavata in british waters. ICES J. Mar. Sci. 36, 110–118. doi: 10.1093/icesjms/36.2.110
Horta e Costa B., Gonçalves L., Gonçalves E. J. (2013). Vessels’ site fidelity and spatio-temporal distribution of artisanal fisheries before the implementation of a temperate multiple-use marine protected area. Fish. Res. 148, 27–37. doi: 10.1016/j.fishres.2013.08.001
IPBES (2019). Global assessment report of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Eds. Brondízio E. S., Settele J., Díaz S. (Bonn, Germany: Ngo IPBES secretariat).
IPMA - Instituto Português do Mar e da Atmosfera (2006) Rays from Portugal. Available online at: https://www.ipma.pt/export/sites/ipma/bin/docs/relatorios/pescas.mar/2016_Guia_de_raias_PT.pdf.
IUCN (2024) The IUCN Red List of Threatened Species. Version 2023-1. Available online at: https://www.iucnredlist.org/ (Accessed Jan 2024).
Jones R. E., Griffin R. A., Januchowski-Hartley S. R., Unsworth R. K. F. (2020). The influence of bait on remote underwater video observations in shallow-water coastal environments associated with the North-Eastern Atlantic. PeerJ 8, 1–21. doi: 10.7717/peerj.9744
Langlois T. J., Harvey E. S., Fitzpatrick B., Meeuwig J. J., Shedrawi G., Watson D. L. (2010). Cost-efficient sampling of fish assemblages: Comparison of baited video stations and diver video transects. Aquat. Biol. 9, 155–168. doi: 10.3354/ab00235
Leitão P., Henriques S., Pérez-Ibarra I., Trujillo M., García-Charton J. A., Vasconcelos R. P. (2020). Shifting baselines in a Mediterranean small-scale fishery. Ocean Coast. Manage. 183, 1–10. doi: 10.1016/j.ocecoaman.2019.104985
Lima M. S. P., Oliveira J. E. L., de Nóbrega M. F., Lopes P. F. M. (2017). The use of Local Ecological Knowledge as a complementary approach to understand the temporal and spatial patterns of fishery resources distribution. J. Ethnobiol. Ethnomed. 13, 1–12. doi: 10.1186/s13002-017-0156-9
Lucifora L., García V. B., Worm B. (2011). Global diversity hotspots and conservation priorities for sharks. PloS One 6, 1–8. doi: 10.1371/journal.pone.0019356
Mandelman J. W., Cooper P. W., Werner T. B., Lagueux K. M. (2008). Shark bycatch and depredation in the U.S. Atlantic pelagic longline fishery. Rev. Fish Biol. Fish. 18, 427–442. doi: 10.1007/s11160-008-9084-z
Maxwell S. M. (2015). The case for mobile marine protected areas the case for mobile marine protected areas. AAAS Annu. Meet., 1–2.
McCully S., Serena F., Walls R. H. L., Morey G., Ellis J. R. (2015). Raja brachyura (Europe assessment). The IUCN Red List of Threatened Species 2015: e.T161691A48907330. 48907330. (IUCN: Gland, Switzerland).
Mendes S., Fernández-Gómez M. J., Galindo-Villardón M. P., Morgado F., Maranhao P., Azeiteiro U., et al. (2009). The Study of bacterioplankton dynamics in the Berlangas Archipelago (West coast of Portugal) by applying the HJ-biplot method. Arquipélago Life Mar. Sci. 1, 25–35.
Milazzo M., Chemello R., Badalamenti F., Riggio R. C., Riggio S. (2002). The impact of human recreational activities in marine protected areas: What lessons should be learnt in the Mediterranean sea? Mar. Ecol. 23, 280–290. doi: 10.1111/j.1439-0485.2002.tb00026.x
Mil-Homens M., Stevens R. L., Cato I., Abrantes F. (2007). Regional geochemical baselines for Portuguese shelf sediments. Environ. pollut. 148, 418–427. doi: 10.1016/j.envpol.2006.12.007
Mitchell J. D., McLean D. L., Collin S. P., Langlois T. J. (2018). Sharkdepredation in commercial and recreational fisheries. Rev. Fish. Biol. Fisheries. 28, 715–748. doi: 10.1007/s11160-018-9528-z
Montero-Quintana A. N., Vázquez-Haikin J. A., Merkling T., Blanchard P., Osorio-Beristain M. (2020). Ecotourism impacts on the behaviour of whale sharks: An experimental approach. Oryx 54, 270–275. doi: 10.1017/S0030605318000017
Pardal M.Â., Azeiteiro U. M. (2001). Zooplankton biomass, abundance and diversity in a shelf area of Portugal (the Berlenga Marine Natural Reserve). Life Mar. Sci. 18-A, 25–33.
Parretti P., Monteiro J. G., Gizzi F., Martínez-Escauriaza R., Alves F., Chebaane S., et al. (2023). Citizen science and expert judgement: A cost-efficient combination to monitor and assess the invasiveness of non-indigenous fish escapees. J. Mar. Sci. Eng. 11, 1–14. doi: 10.3390/jmse11020438
Pauly D., Ulman A., Piroddi C., Bultel E., Coll M. (2014). ‘Reported’ versus ‘likely’ fisheries catches of four Mediterranean countries. Sci. Mar. 78, 11–17. doi: 10.3989/scimar.2014.78s1
Pennino M. G., Muñoz F., Conesa D., López-Qúlez A., Bellido J. M. (2013). Modeling sensitive elasmobranch habitats. J. Sea Res. 83, 209–218. doi: 10.1016/j.seares.2013.03.005
Queiroz N., Humphries N. E., Couto A., Vedor M., da Costa I., Sequeira A. M. M., et al. (2019). Global spatial risk assessment of sharks under the footprint of fisheries. Nature 572, 461–466. doi: 10.1038/s41586-019-1444-4
Queiroz N., Humphries N. E., Mucientes G., Hammerschlag N., Lima F. P., Scales K. L., et al. (2016). Ocean-wide tracking of pelagic sharks reveals extent of overlap with longline fishing hotspots. Proc. Natl. Acad. Sci. U. S. A. 113, 1582–1587. doi: 10.1073/pnas.1510090113
Ramires M., Clauzet M., Barrella W., Rotundo M. M., Silvano R. A. M., Begossi A. (2015). Fishers’ knowledge about fish trophic interactions in the southeastern Brazilian coast. J. Ethnobiol. Ethnomed. 11, 1–11. doi: 10.1186/s13002-015-0012-8
R Core Team (2021). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna. Available at: https://www.R-project.org/.
Rosa R., Rummer J. L., Munday P. L. (2017). Biological responses of sharks to ocean acidification. Biol. Lett. 13, 20160796–20160796. doi: 10.1098/rsbl.2016.0796
Santana-Garcon J., Braccini M., Langlois T. J., Newman S. J., Mcauley R. B., Harvey E. S. (2014). Calibration of pelagic stereo-BRUVs and scientific longline surveys for sampling sharks. Methods Ecol. Evol. 5, 824–833. doi: 10.1111/2041-210X.12216
Sawchuk J. H., Beaudreau A. H., Tonnes D., Fluharty D. (2015). Using stakeholder engagement to inform endangered species management and improve conservation. Mar. Policy 54, 98–107. doi: 10.1016/j.marpol.2014.12.014
Semeniuk C. A. D., Bourgeon S., Smith S. L., Rothley K. D. (2009). Hematological differences between stingrays at tourist and non-visited sites suggest physiological costs of wildlife tourism. Biol. Conserv. 142, 1818–1829. doi: 10.1016/j.biocon.2009.03.022
Serra-Pereira B., Erzini K., Figueiredo I. (2015). Using biological variables and reproductive strategy of the undulate ray Raja undulata to evaluate productivity and susceptibility to exploitation. J. Fish Biol. 86, 1471–1490. doi: 10.1111/jfb.12653
Serra-Pereira B., Erzini K., Maia C., Figueiredo I. (2014). Identification of potential essential fish habitats for skates based on fishers’ knowledge. Environ. Manage. 53, 985–998. doi: 10.1007/s00267-014-0257-3
Serra-Pereira B., Figueiredo I., Farias I., Moura T., Gordo L. S. (2005). Description of Portuguese mixed-fisheries with positive landings of Raja brachyura Lafonr 1910 and Raja montagui Fowle. Ices C. 2005/N18 1–10.
Silva P. M., Teixeira C. M., Pita C., Cabral H. N., França S. (2021). Portuguese artisanal fishers’ Knowledge about elasmobranchs—A case study. Front. Mar. Sci. 8. doi: 10.3389/fmars.2021.684059
Stevens J. D., Bonfil R., Dulvy N. K., Walker P. A. (2000). The effects of fishing on sharks, rays, and chimaeras (chondrichthyans), and the implications for marine ecosystems. ICES J. Mar. Sci. 57, 476–494. doi: 10.1006/jmsc.2000.0724
Vasco-Rodrigues N., Mendes S., Franco J. N., Castanheira M., Castro N., Maranhão P. (2011). Fish diversity in the Berlengas Natural Reserve (Portugal), a marine protected area. Ecol. - SPECO 43, 35–43.
VideoLan (2006) VLC software. Available online at: https://www.videolan.org/.
Wedding L. M., Gibson B. A., Walsh W. J., Battista T. A., Hall S. (2011). Integrating remote sensing products and GIS tools to support marine spatial management in West Hawaiì. J. Conserv. Plan. 7, 60–73.
Wooster W. S., Bakun A., McLain D. R. (1976). The seasonal upwelling cycle along the eastern boundary of the North Atlantic. J. Mar. Res., 131–141.
Worm B., Davis B., Kettemer L., Ward-Paige C. A., Chapman D., Heithaus M. R., et al. (2013). Global catches, exploitation rates, and rebuilding options for sharks. Mar. Policy 40, 194–204. doi: 10.1016/j.marpol.2012.12.034
Keywords: sharks, rays and skates, spatial conflict, non-invasive sampling, local ecological knowledge, Marine Protected Areas
Citation: Batista MI, Abril C, Veríssimo A, Vasconcelos RP, Pais MP and Henriques S (2024) Mapping elasmobranch occurrences and overlap with human activities using local knowledge and non-invasive sampling to identify areas of potential conflict. Front. Mar. Sci. 11:1321620. doi: 10.3389/fmars.2024.1321620
Received: 14 October 2023; Accepted: 25 March 2024;
Published: 17 April 2024.
Edited by:
Antonio Di Franco, Stazione Zoologica Anton Dohrn, ItalyReviewed by:
Natascha Wosnick, Federal University of Paraná, BrazilRachel Ann Hauser-Davis, Oswaldo Cruz Foundation (Fiocruz), Brazil
Copyright © 2024 Batista, Abril, Veríssimo, Vasconcelos, Pais and Henriques. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marisa I. Batista, bWliYXRpc3RhQGZjLnVsLnB0
†These authors share last authorship
 Marisa I. Batista
Marisa I. Batista Catarina Abril
Catarina Abril Ana Veríssimo
Ana Veríssimo Rita P. Vasconcelos
Rita P. Vasconcelos Miguel P. Pais1,5†
Miguel P. Pais1,5†