- 1Doctoral Program of Environmental Science, Postgraduate School, Padjadjaran University, Bandung, Indonesia
- 2Department of Aquatic Resources, Faculty of Agriculture, Lampung University, Bandar Lampung, Indonesia
- 3Marine Research Laboratory (MEAL), Department of Marine Sciences, Faculty of Fisheries and Marine Sciences, Universitas Padjadjaran, Jatinangor, Sumedang, Indonesia
- 4Department of Chemistry, Faculty of Mathematics and Natural Science, Padjadjaran University, Jatinangor, Sumedang, Indonesia
- 5Department of Agronomy, Faculty of Agriculture, Universitas Gadjah Mada, Yogyakarta, Indonesia
- 6Departement of Applied Geology, Faculty of Geological Engineering, Padjadjaran University, Jatinangor, Sumedang, Indonesia
In response to the escalating demand for blue swimming crabs (BSC) and the consequential impact on their habitat and population, this study advocates for sustainable management through a holistic approach. Our comprehensive framework integrates ecological conservation, socioeconomic well-being, and governance principles, aiming to establish a policy framework for the sustainable management of BSC in the Eastern Coast of Lampung. The research emphasizes the urgent issue of overexploitation, with an exploitation rate of 0.71, requiring immediate attention to prevent stock depletion and maintain ecosystem health. Findings reveal that male BSC, measured by carapace width, typically reaches the length at first catch (Lc50) at approximately 118.69 mm, while for females, it is about 122.70 mm—indicating that BSC is often caught larger than the 10 cm legal requirement set by the Ministry of Marine and Fisheries of Indonesia. Economic analysis demonstrates the profitability of the blue swimming crab business on Lampung's east coast, with a favorable crab capture per trip (4.63) and total costs ratio (1.18). The study area, featuring an extensive mangrove forest, hosts a crucial crustacean population, contributing significantly to BSC's diet. Beyond ecological significance, mangrove conservation enhances our understanding of environmental sustainability, particularly in carbon stock contributions. Addressing sustainability challenges requires a multi-faceted approach, including precise fishing technologies, effective regulation enforcement, and improved monitoring. Collaborative efforts among government authorities, fishing communities, and conservation groups are essential for balancing economic interests with the long-term ecological health of BSC populations in the study area.
1 Introduction
Indonesia, as a maritime country with a coastline spanning over 270 kilometers, has enormous potential in marine diversity, abundance, and natural products, which play a pivotal role in coastal communities' economic growth and prosperity, particularly those engaged in maritime activities (Farhan and Lim, 2010). However, increasing fishing and aquaculture activities to meet marine resources demand has also resulted in unintended consequences, mainly marine ecosystem damage due to overexploitation and destructive fishing methods (Sloan and Ugandhy, 1994; Farhan and Lim, 2010; Siry, 2011). For example, using illegal fishing gear and excessive fishing efforts can result in bycatch and non-target species, depleting diversity and abundance in natural habitats, which may lead to long-term ecological consequences. Moreover, converting coastal areas into fish farms can also lead to ecosystem degradation, especially by destroying mangroves and other critical habitats (Sukardjo, 2002). Mangroves serve as essential functions and services in coastal areas, providing nursery areas for various marine species, coastal protection, carbon sequestration, and support for local livelihoods (Alongi, 2012; Soegianto et al., 2022). Water quality parameters such as salinity, dissolved oxygen (DO), nutrient level, turbidity, fluorescence and temperature are critical factors for the survival and growth of mangrove vegetation (Field, 1999; Kristensen et al., 2008; Pawar, 2013; Soegianto et al., 2022). For example, excessive nutrient inputs from anthropogenic activities, such as agriculture or urban development in coastal areas, can lead to eutrophication, which may favor the growth of certain plant species over others (Trott and Alongi, 2000; Pawar, 2013). Therefore, protecting and preserving critical habitats, such as mangrove ecosystems, is essential for maintaining ecological integrity and restoration to ensure coastal ecosystems' long-term health and sustainability.
Indonesia contributed 108,583.90 tons of global BSC production (FAO, 2022), and make it as the third-largest seafood export (FAO, 2020; Soegianto et al., 2022) which mostly BSC fishery take place in Jawa (Java), Sulawesi, and Sumatera (Sumatra) (Monterey Bay Aquarium Seafood Watch, 2023). Lampung Province, as one of the provinces in Sumatera Island with an extensive coastline area and favorable conditions for the growth and abundance of marine resources, is renowned for its diversity of marine products, including blue swimming crab (BSC) (Portunus pelagicus), a highly sought-after seafood delicacy both locally and internationally (Zairion et al., 2015; Kembaren et al., 2018). The BSC, (Portunus pelagicus Linnaeus 1758), also known as sand crab, is widely distributed in Asian countries and the regions of Australia and Oceania (Carpenter and Niem, 2001). It could be easily encountered in marine ecosystems, usually at depths of up to 40 meters, residing on sandy to sandy-muddy substrates near diverse habitats such as mangrove ecosystems (Soegianto et al., 2022). Due to the high demand for the BSC as a result of its reputation as a healthy and delicious seafood option, there has been a notable surge in annual catching activities (Wu et al., 2010; Rabaoui et al., 2021). Consequently, fishing and aquaculture activities targeting BSC have intensified to meet the rising market requirements (Yanti et al., 2022; Ervinia et al., 2023). According to Fahmi et al. (2015), the BSC ranked as the most significant fish export from Indonesia, following tuna and shrimp, with a substantial total value of US$153 million (Fahmi et al., 2015). This substantial contribution to Indonesia's fishery exports highlights the economic importance and demand for BSC in local and international markets (Yanti et al., 2022). However, with such high export value and increasing demand, ensuring the sustainable management of BSC becomes even more critical to maintain its long-term viability and preserve the health of its habitat and population.
As one of the central hubs for the BSC fishery in Indonesia, Lampung Province plays an important role in meeting the growing export demand for this prized seafood. This condition was supported by the ecological supported in Lampung province in terms of the development of BSC. As highlighted by Kembaren et al. (2018), water quality parameters in this area were suitable for the development of BSC larvae, but some oceanographic profiles might have essential roles for the larvae distribution, with the highest abundance of BSC larvae found in mid-shore (Kembaren et al., 2018). In 2019, Lampung Province demonstrated its significance in the BSC industry, with the total export volume of BSC reaching 12,749 tones and an export value surpassing 259 million, solidifying its position as the third-highest fishery product export value of BSC (Monterey Bay Aquarium Seafood Watch, 2018). Lampung province contributed 10-15 % of national blue swimming crab production, concentrated in three regencies: Tulang Bawang Regency, Central Lampung Regency, and East Lampung Regency, employing over 4000 fishermen in these areas (APRI, 2022). While capitalizing on economic opportunities, it is essential to prioritize sustainable management practices by involving stakeholders, government, and local communities.
Nevertheless, several problems have been reported in BSC production in these areas, such as overfishing, mangrove degradation, water pollution, and use of fishing gear, depleting the BSC stocks and disrupting the balance of the ecosystem (Zairion et al., 2015; Kembaren et al., 2018; Madduppa et al., 2021). These issues can have adverse economic and ecological consequences, especially on the growth and survival of BSC (Johnston et al., 2011; Soegianto et al., 2022). In addition, increasing temperature and changing rainfall patterns due to climate change effects will affect the favorable conditions of BSC, since the temperature and rainfall have been reported to have a positive correlation with catch per unit of effort (CPUE), with the highest CPUE at the temperature of 22 – 25 °C, and slightly higher CPUE at wet condition (Kunsook et al., 2014; Ansari et al., 2021; Johnston et al., 2021; Lin et al., 2022). Apart from those problems, social-economic conflicts related to BSC production, such as the competition for accessing to BSC among different groups, the establishment of aquaculture farms or the expansion of fishing operations and, lack of clear and inclusive governance mechanisms can exacerbate the sustainable management of BSC resources (Zairion et al., 2015; Kembaren et al., 2018; Monterey Bay Aquarium Seafood Watch, 2018). Understanding and addressing these challenges are essential for the sustainable management of BSC resources in Lampung Province for fostering a more inclusive and equitable approach to resource management as well as to enhance social well-being, economic prosperity, and the long-term sustainability of BSC production in the region. Therefore, the objective of this study is to formulate a policy framework for sustainable BSC management in the coastline area of East Lampung through a holistic approach by assessing the stock status of BSC, water quality, the condition of the mangrove ecosystem, socioeconomic conditions, and analyzing the sustainability status of BSC from a multidimensional perspective. This research will contribute to the development of a comprehensive policy framework that promotes the sustainability of crab fisheries in the coastal areas of East Lampung by integrating ecological, socioeconomic, and governance considerations.
2 Materials and methods
2.1 Study area
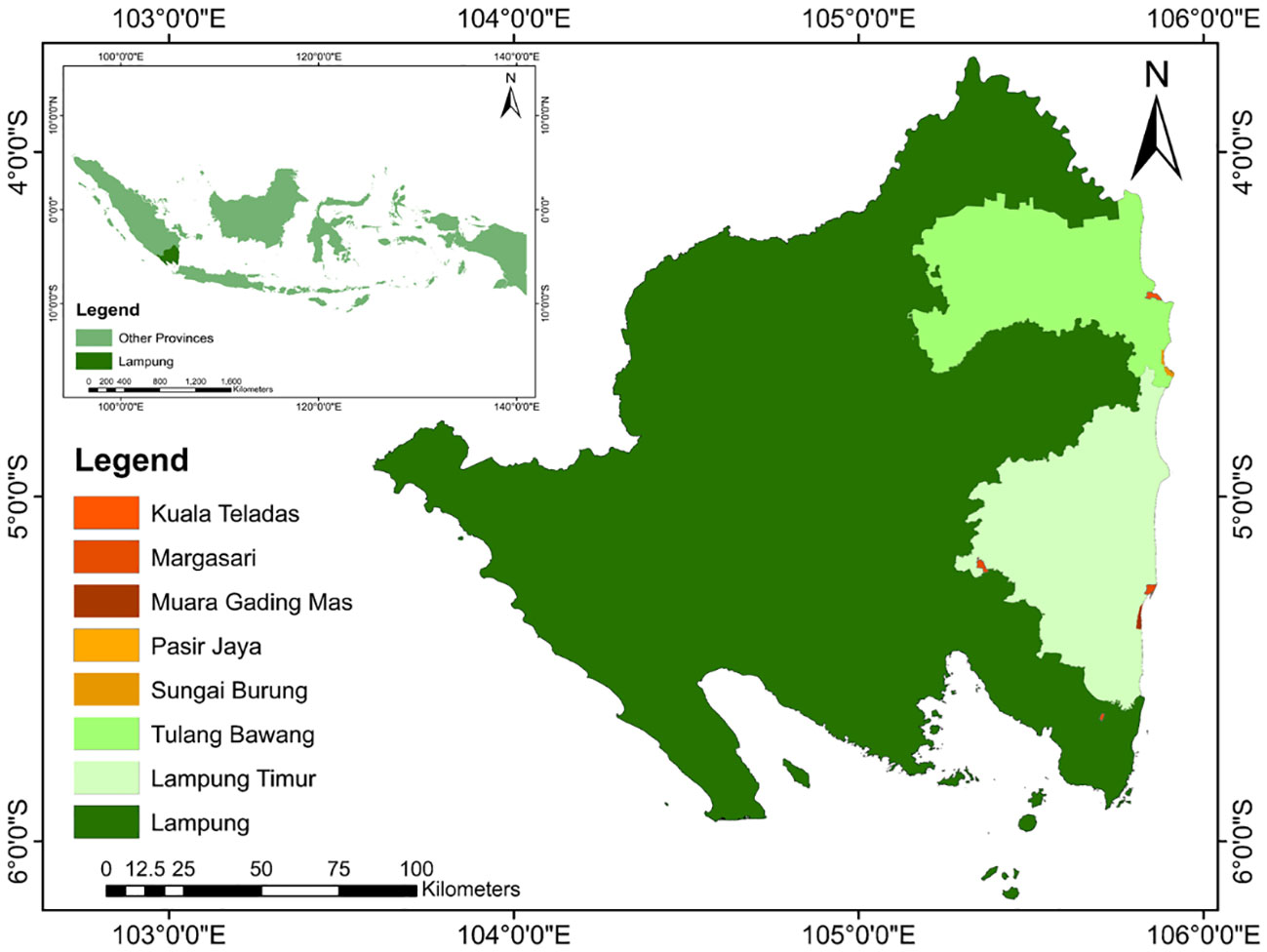
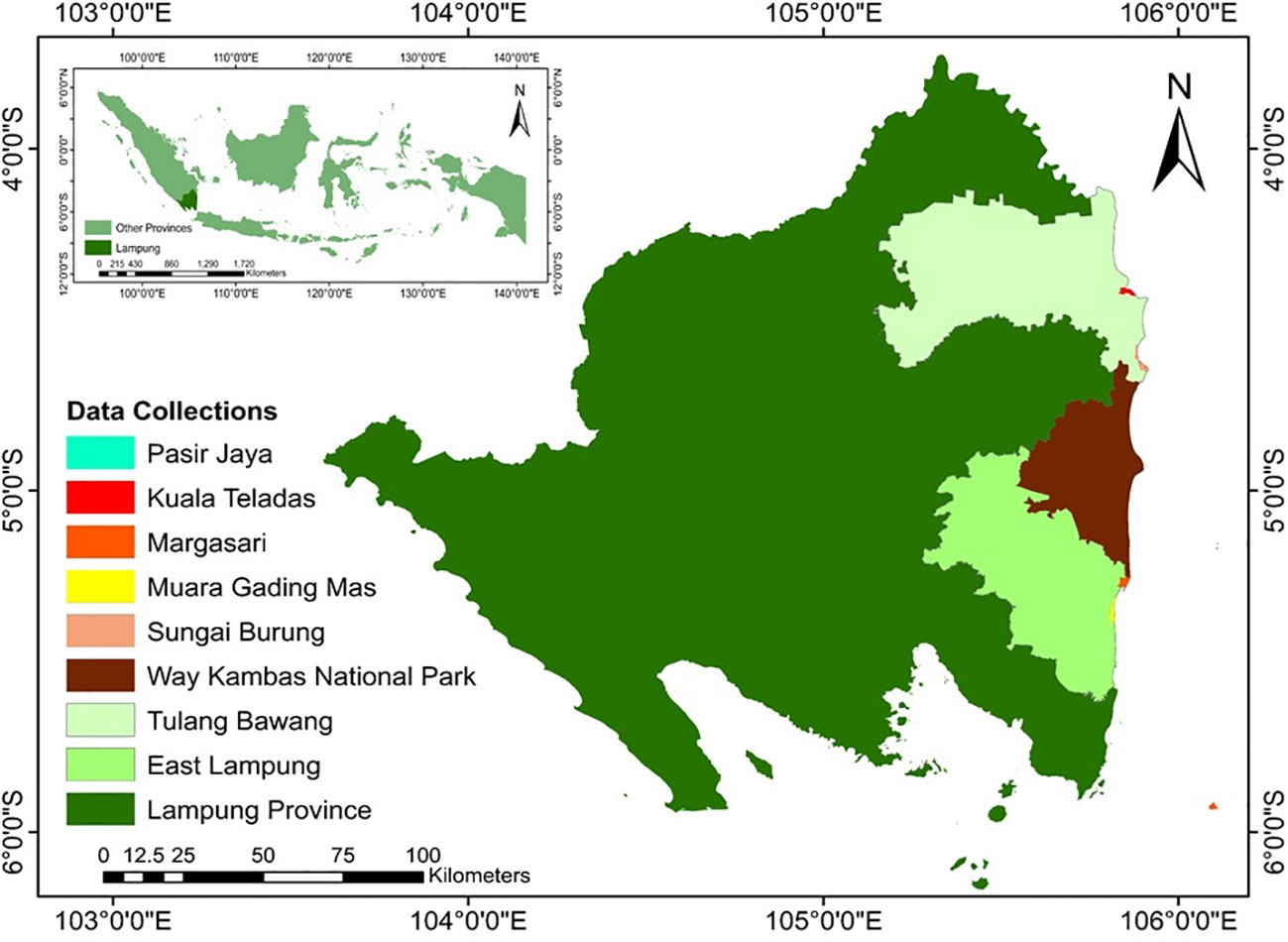
This study was conducted in the East Lampung and Tulang Bawang Regency of Lampung Province, Indonesia, specifically focused on five key landing areas, namely, Pasir Jaya, Kuala Teladas, Margasari, Muara Gading Mas, and Sungai Burung (Figure 1). These areas are known as centers of BSC production in Lampung Province. They are located in close proximity to each other and share the common characteristics of being situated within the mangrove ecosystem. Moreover, these study sites are adjacent to the Way Kambas National Park (4°37' –5°16' S and 105°33' – 105°54' E). The selection of these specific areas is due to the consideration of valuable insights into ecological dynamics, habitat quality, and interactions between BSC and the surrounding mangrove ecosystem. Additionally, the proximity to the Way Kambas National Park offers an opportunity to explore potential ecological connections and spillover effects between the protected area and the surrounding mangrove ecosystem. According to Köppen-Geiger Climate Classification (Kottek et al., 2006), the study area is characterized as a tropical region with a climate classified as Af (tropical rainforest climate). It represents high humidity and relatively consistent temperatures with significant yearly rainfall, even during the driest months (July-August). The average temperature ranges from 24 to 34 °C, while the average annual rainfall ranges from 2000 to 2500 mm with six wet months and two to three dry moths based on Oldeman climate classification.
2.2 Study framework
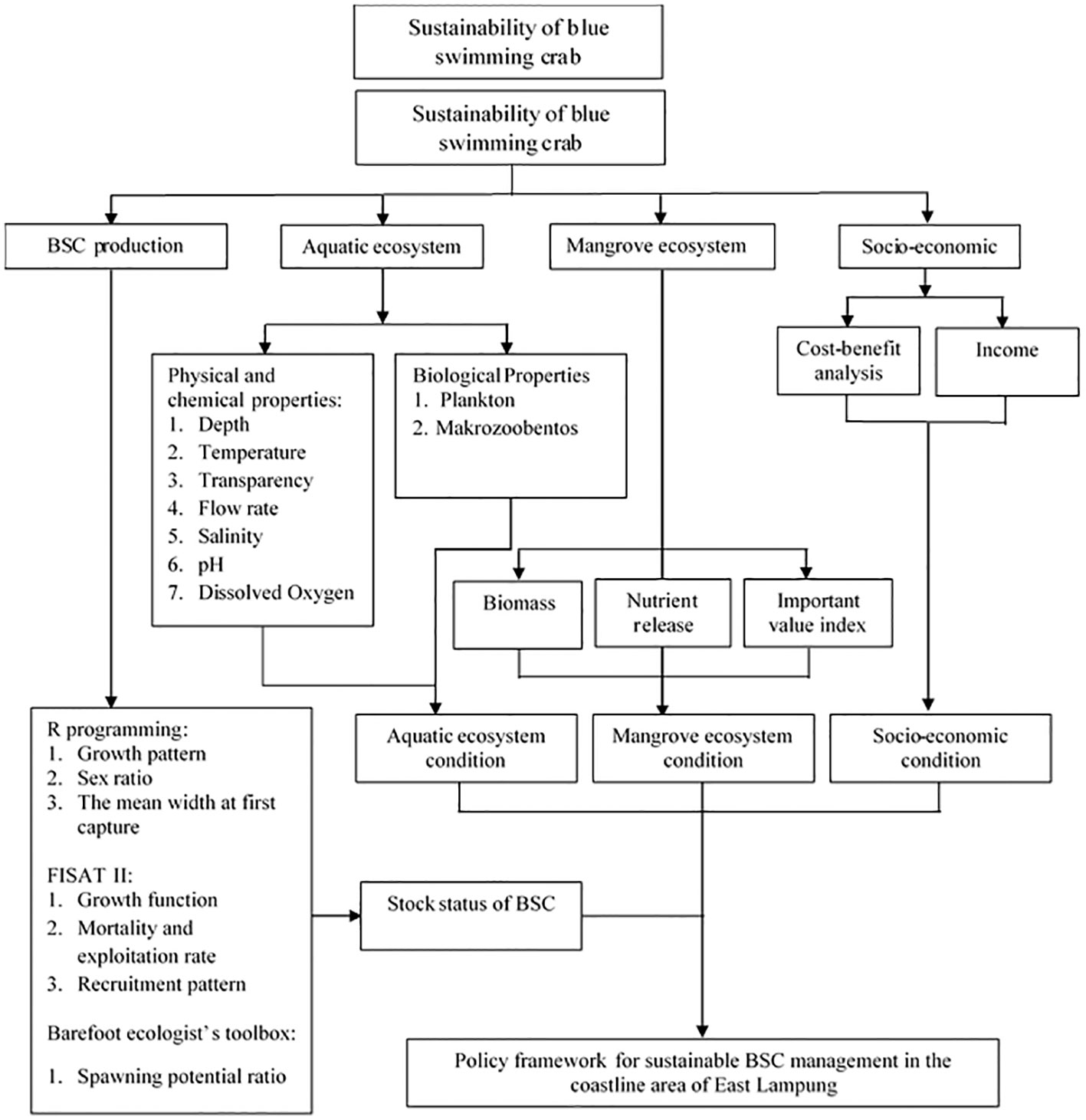
This study encompassed a holistic approach to analyze the sustainability of BSC in the eastern Lampung, consisting of BSC fishery production, aquatic ecosystem assessment, mangrove ecosystem assessment, and socioeconomic assessment, as illustrated in Figure 2. The BSC production assessment examined various aspects related to BSC fisheries, such as catch volume, fishing practices, and stock status. This assessment aimed to provide insight into the current state of BSC production and its sustainability. The aquatic assessment focused on evaluating the key ecological factors influencing the BSC population, including physical, chemical, and biological parameters. This assessment aimed to understand the ecological dynamics and potential impact on BSC populations within the aquatic environment. The mangrove assessment focused on the health and condition of mangrove habitats, which serve as critical breeding grounds and habitats of BSC. This assessment aimed to understand the state of the current mangrove ecosystem and its importance for the sustainability of BSC populations. The socioeconomic assessment included analyzing the livelihoods and socioeconomic contributions of local communities engaged in BSC fishing and assessing the social and cultural values associated with BSC in the study area. This holistic approach ensured that ecological, socioeconomic, and governance aspects were considered in formulating effective management strategies and policy recommendations to enhance BSC populations' long-term viability and sustainability in the Eastern Coastline of Lampung.
2.3 Data collection
We collected the data through a field survey, which involved direct observations and measurements in the study areas as well as interviews. A detailed description of data collection is presented in the following section:
2.3.1 BSC sampling
Daily data of BSC were collected, including information on the vessel, fishing effort, duration of fishing trips, day per trip, fishing location, fishing gear employed, catch weight, species identification, vessel type, soak time, fishing license number, start and end times of fishing, number of pots lifted, and number of crew members present (Xiao and Kumar, 2004). These data were collected daily from June 2019 to July 2020 at each designated site. The BSC samples obtained during this period underwent various measurements and assessments. Each crab was identified by its gender, the widths of its carapace, and the condition of the crabs upon capture (whether they were live, dead, or in a soft-shelled state). To understand the population dynamics of BSC, males, and females from each landing site were separated and measured to determine population size structure, growth parameters, recruitment rates, and exploitation levels.
2.3.2 Measurement of mangrove ecosystem
The mangrove ecosystem assessment was conducted in Way Kambas National Park, involving various measurements to evaluate its health and condition, including vegetation assessment, biomass measurement, soil characteristics, fauna surveys, and carbon stocks. In each study area, observational stations were conceptually designated based on the representation of study locations in the intertidal area. Within each observation station, line transects were established from the sea towards the land (perpendicular to the coastline along the entire zoning of the mangrove forest). Sampling in the research locations was conducted using the strip plot method. The initial lines and plots were randomly determined, and subsequent lines and plots were systematically selected. Observation plots were created with dimensions of 20 m x 20 m, with a 20 m distance between plots. A comprehensive assessment of the total biomass and organic matter content was involved through measurements of the tree height, diameter at breast height (DBH), and calculation of vegetation density and diversity indices, considering above-ground biomass, below-ground biomass, litter, and soil organic matter. Mangrove biomass was estimated by sampling and weighing different components such as leaves, branches, and roots to assess carbon storage capacity and overall productivity. Stem diameter measurements were taken at breast height (1.3 m) for stems with a diameter ≥ 5 cm, in accordance with the (Penman et al., 2003) guidelines, considering various conditions such as branched trees, aerial roots, and abnormal trunks. Leaf litter biomass data were collected from 0.5 m x 0.5 m subplots within each main plot. These samples were preserved through proper labeling and documentation, ensuring their integrity for further analysis. The type of plot used in this research is the strip plot, as follows (Figure 3).
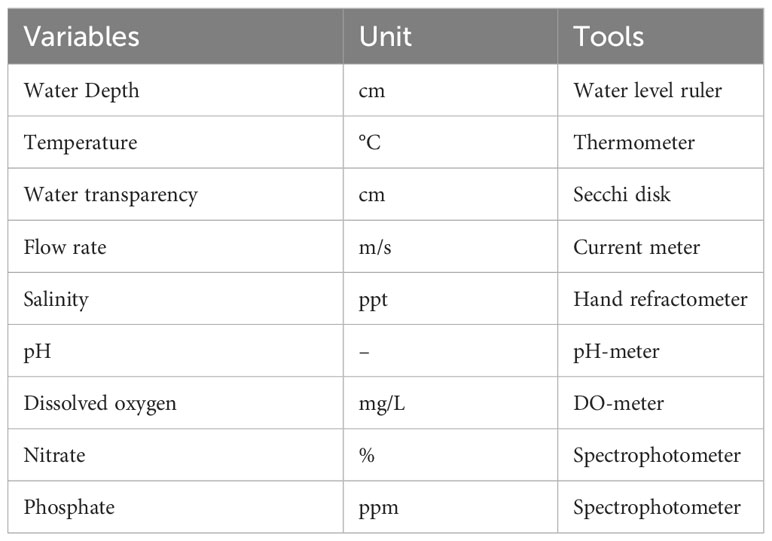
Soil samples were collected from various depths to analyze their properties, such as texture, organic matter, pH, salinity, and nutrient levels, using soil core samples. Water quality sampling processes were conducted at three distinct locations: river, swamp, and sea, which they selected as predetermined observation points to indicate water suitability for mangrove growth and identify stressors. The measurements were conducted three times in the west and east monsoon seasons at each mangrove observation location. The water quality variables were measured using various tools, as outlined in Table 1. The fauna measurements within the mangrove ecosystem, inclusive of plankton and benthic organisms, were conducted using a comprehensive methodology. In our comprehensive study of aquatic ecosystems, meticulous sampling procedures were implemented at predetermined observation points to ensure a representative assessment of the diverse environments under investigation. For the analysis of plankton samples, a sophisticated approach was employed. The contents of each sample bottle were stirred to achieve a homogeneous distribution of plankton. Subsequently, 1 ml of the sample was meticulously extracted using a dropper and carefully deposited into the Sedgewick Rafter Counting Cell (SRCC). Microscopic observations ensued, conducted at a magnification of 10 x 10, allowing for a detailed examination of planktonic organisms. Species identification was facilitated by referencing a comprehensive plankton identification book. Concurrently, macrozoobenthos sampling was carried out utilizing the Line Transect and Sample Plot method. This involved strategically placing quadratic transects, constructed from iron plates measuring 25 x 25 x 30 cm, at each station. The sampling process incorporated the deployment of a modified paralon pipe with a diameter of 5 inches (0.127 m) and a length of 25 cm, immersed in the water substrate at the predetermined sampling points. The macrozoobenthos samples obtained underwent a meticulous filtration process. Two sequential filtration stages were implemented, initially using a filter with a mesh size of 5 x 5 mm for stage I, followed by a filter with a mesh size of 1 x 1 mm for stage II. The collected samples were then preserved in 10% formalin to facilitate subsequent identification and analysis in the laboratory. Numerous parameters are under scrutiny in our investigation, including species composition, individual abundance, diversity index, evenness, and dominance index.
2.3.3 Interview processes
We used the purposive sampling method, which involved selecting participants directly and indirectly involved in BSC along the study areas based on specific criteria aligning with the research objectives to ensure unbiased research results (Tongco, 2007). A total of 60 respondents were interviewed from August to September 2020, encompassing individuals from Pasir Jaya, Kuala Teladas, Margasari, Muara Gading Mas, and Sungai Burung. The selection of these locations aimed to capture a diverse range of perspectives and experiences within the communities involved in BSC activities. To enhance participant convenience and foster open communication, the interviews were conducted in local languages. This approach aimed to create a comfortable environment for respondents to express their insights and experiences related to BSC. Prior to the initiation of data collection, rigorous measures were undertaken to ensure the reliability and validity of the interview process. The questionnaire used in the interviews underwent thorough validation and reliability tests. Subject matter experts in aquaculture and fisheries reviewed the questionnaire to confirm the accuracy of the indicators and their relevance to the research objectives. The internal consistency of the questionnaire was assessed through Cronbach's alpha, with a value exceeding 0.6 considered acceptable for research purposes. This rigorous validation process aimed to guarantee the precision of the measurements and the consistency of results (Tongco, 2007).
2.4 Data analysis
2.4.1 BSC analysis
Once all of the BSC data were collected, it would be analyzed based on the relationship between carapace width and weight, sex ratio, growth parameter, size at first captured, mortality rate, and spawning potential ratio (SPR). The relationship between carapace width and weight was determined using the Hartnoll equation, followed by a t-test (Equations 1, 2) (Hartnoll et al., 1982). Variables a and b were obtained through linear regression analysis which further a hypothesis test was performed where the t-count will be compared with the t-table with a 95% confidence interval. The condition factor (Equation 3 = isometric or Equation 4 = allometric) was calculated using a metric system based on the relationship between carapace width and weight. The sex ratio was determined by comparing a total of males and females, followed by the Khi (χ2) test (Equations 5, 6) (Xiao and Kumar, 2004; Zairion et al., 2015; Madduppa et al., 2021). Growth parameter, coefficient of growth and asymptotic (Equations 7–9) were determined based on Von Bertalanffy growth function, incorporated into FISAT II, while the theoretical age of the crab when carapace width = 0 (Equation 10) was determined using the empirical equation from previous study (Pauly et al., 1984). Size at first captured was determined based on Boutson et al. (2009) (Equation 11) The mortality rate was determined based on Pauly et al. (1984). SPR was determined through analysis of the Barefoot Ecologist Toolbox based on Prince et al. (2020) (Equation 12), then the exploitation rate was analyzed by using Sparre and Venema equation (Equation 13) (Sparre and Venema, 1995; Prince et al., 2015a). Proportion of reproductive potential at age t and Reproduction of output at age t were estimated based on Prince et al. (2015a) (Equations 14, 15).
Where W represents BSC’s weight, L represents BSC’s width, a is the initial growth coefficient, b is the growth coefficient, Lt represents carapaces width, represents asymptotic of carapaces width, K represents coefficient of growth rate, t represents the age of carapace width at the time, t0 represents the age of BSC at 0, SLc represents the size of BSC at first captures, exp (a + bL) represents BSC with carapaces retained in gear, T represents mean sea temperature, F represent mortality due to catching, Z represents total mortality, M represents natural mortality, E represents exploitation rate, SPRt represents potential proportion production at age t, EPt represents reproduction output at age t, Nt represents total BSC at age t with N0 equals to 1000, ft represents mean fecundity
2.4.2 Mangrove ecosystem analysis
Once the required data were obtained, a mathematical analysis was conducted, following the calculation stages by Bengen (2002), specifically designed for calculating important value index (IVI) (Wardiatno et al., 2021). The IVI is a quantitative measure that assesses species’ relative importance or dominance within an ecosystem, ensuring a systematic and rigorous approach. The stage of calculating IVI involved evaluating various parameters such as relative density, relative frequency, and relative coverage (Equation 16).
Where; Rdi represents relative density, Rfi represents relative frequency, and Rci represents relative coverage.
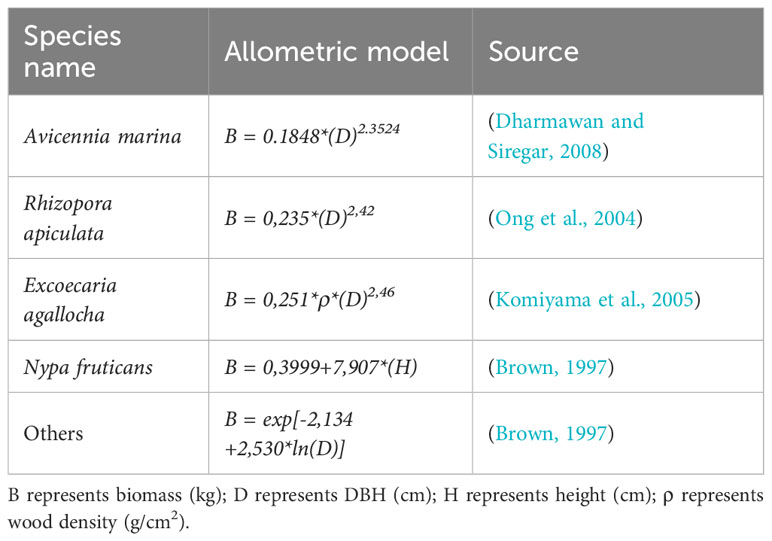
Measuring and analyzing above-ground biomass in the mangrove ecosystem involves systematically sampling mangrove trees within representative plots. The quantification of carbon stored above ground in mangrove ecosystems involves a precise process of allometric calculations, as outlined in Table 2. The determination of plant biomass is derived from the specific gravity of the plant, multiplied by the diameter of the tree trunk. This involves taking into account the plant biomass and then applying a conversion factor of 0.47, as stipulated by the SNI 7724:2011 standard.
Measuring and analyzing below-ground biomass in a mangrove ecosystem involved soil sampling and root collection. The roots are cleaned, dried, and weighed to calculate biomass using conversion factors of (SNI 7724:2011). The total below-ground biomass was based on Caims et al. 1997 in Equation 17 (Cairns et al., 1997). The Litter biomass was calculated using the formula provided by (Hairiah et al., 2011) which takes into consideration both the dry weight of the sub-sample (BK) and the wet weight of the sub-sample (BB).
Where; RBD represents root biomass, and AGB represents above-ground biomass.
Carbon stocks were analyzed using the loss on ignition method (LOI). This method involved heating soil samples to high temperatures (60 °C ± 48 hours) to remove organic matter, thereby determining the amount of carbon present. The carbon content can be quantified by measuring the weight loss after ignition (Equations 18–20). Moreover, we also measured nutrient release (Nitrogen and Phosphate) to identify the relationship between the degradation rate of organic matter, nitrogen, and phosphate as an incubation function based on Nga et al. (2006) (Equation 21) (Nga et al., 2016).
The calculation of plankton abundance and diversity index followed the formula specified by (Eaton et al., 2005) and (Shannon and Weaver, 1949), as follow in (Equations 22, 23):
N = Plankton abundance (cell/m3 or ind/M3); Vd = volume of filtered water sample (m3); Vt = volume of filtered sample (ml); Vcg = concentrate volume of Sedgwick Rafter Counting Cell (ml); n = number of observed plankton.; ASRC: area of SRC (1000 mm2); Aobserved :observed area; H’ = Shannon-Wiener diversity index; Pi: ni/N; ni : number of individual species-ith; N: total number of individuals.
2.4.3 Social-economic analysis
The result from the interview processes is subjected to descriptive analysis to assess the level of community participation in the research using the Likert Scale Method. The Likert Scale allows for quantifying community engagement and attitudes toward the research regarding the socioeconomic conditions of traditional fishing communities. The analysis involves comparing the actual and ideal scores using a specific formula. This comparison provides insights into the alignment between the current conditions and the desired or optimal conditions for the fishing communities. Moreover, a modified RAPFISH software with a Multidimensional Scaling (MDS) approach was utilized for analyzing the sustainability of BSC in the study area. The modified version of RAPFISH adapted for the blue swimming crab fishery in Lampung includes specific parameters and indicators relevant to this particular fishery. The MDS approach is a statistical method used to visualize and analyze similarities or dissimilarities between multiple variables in a multidimensional space. In the context of the blue swimming crab fishery assessment, MDS would aid in identifying patterns and relationships between different aspects of sustainability, such as ecological health, community engagement, and economic viability. The ratio of male and female sex varies every month, with more proportions of male crabs compared to females. The theory states that if the number of male blue swimming crabs is more than the female, there can be competition between males to mate with females. Males can mate more than once per mating season. Finally, the feasibility of BSC was conducted by comparing the income generated by the fisherman with the costs incurred during their fishing activities (Equation 24) (Chávez, 2007).
3 Results
3.1 BSC production in East Coast Lampung
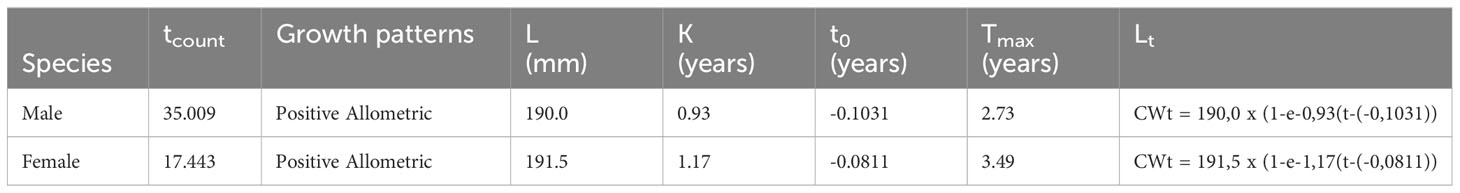
The distribution of BSC was meticulously categorized into ten distinct classes to gain valuable insight into species size distribution. The most frequent carapace width was observed in the 110-119 mm class for male BSC, while the lowest distribution was found in female BSC, specifically in the 180-189 mm class, indicating a concentration of individuals within the size range (Figure 4A). The sex ratio of BSC exhibits monthly variations, with a higher proportion of males compared to females. Interestingly, only November and December showed equality between males and females, as indicated by the value of 2 (Figure 4B). The growth pattern of both male and female BSC exhibited positive allometry (Table 3), indicating that their weight increased faster than their carapace width. On the other hand, the growth pattern of male and female BSC showed a positive correlation, (male = (R2=0.902); female = (R2= 0.875), between their carapace width and weight (Figure 4C). Utilizing the Von Bertalanffy growth model, the analysis revealed that the maximum carapace width (CW∞) for males reached 190 mm, while for females, it was 191.5 mm (Figure 4D). The growth analysis further indicated that male BSC took approximately 12 months to reach their maximum carapace width, while female BSC achieved the same milestone in around nine months. However, if the blue swimming crab reaches its maximum carapace width faster, then it will indicate that its lifespan is also getting shorter. Indeed, the growth rate (K) analysis reveals exciting differences between males and females which females showed a relatively faster growth rate (K=1.17) compared to their male counterparts (K= 0.93). This disparity in growth rates between the sexes is a crucial aspect of their population dynamics and reproductive strategies, which can influence the overall stability and sustainability of this area’s BSC population. The comparison between berried female (BEF) and non-berried female (NBF) (Figure 4E) showed NBF has higher percentage rather than BEF, especially in August and September which showed 100 % of NBF. The relationship between the size and SLc (Figure 4F) showed that male and female have similar trends using logistic function method.
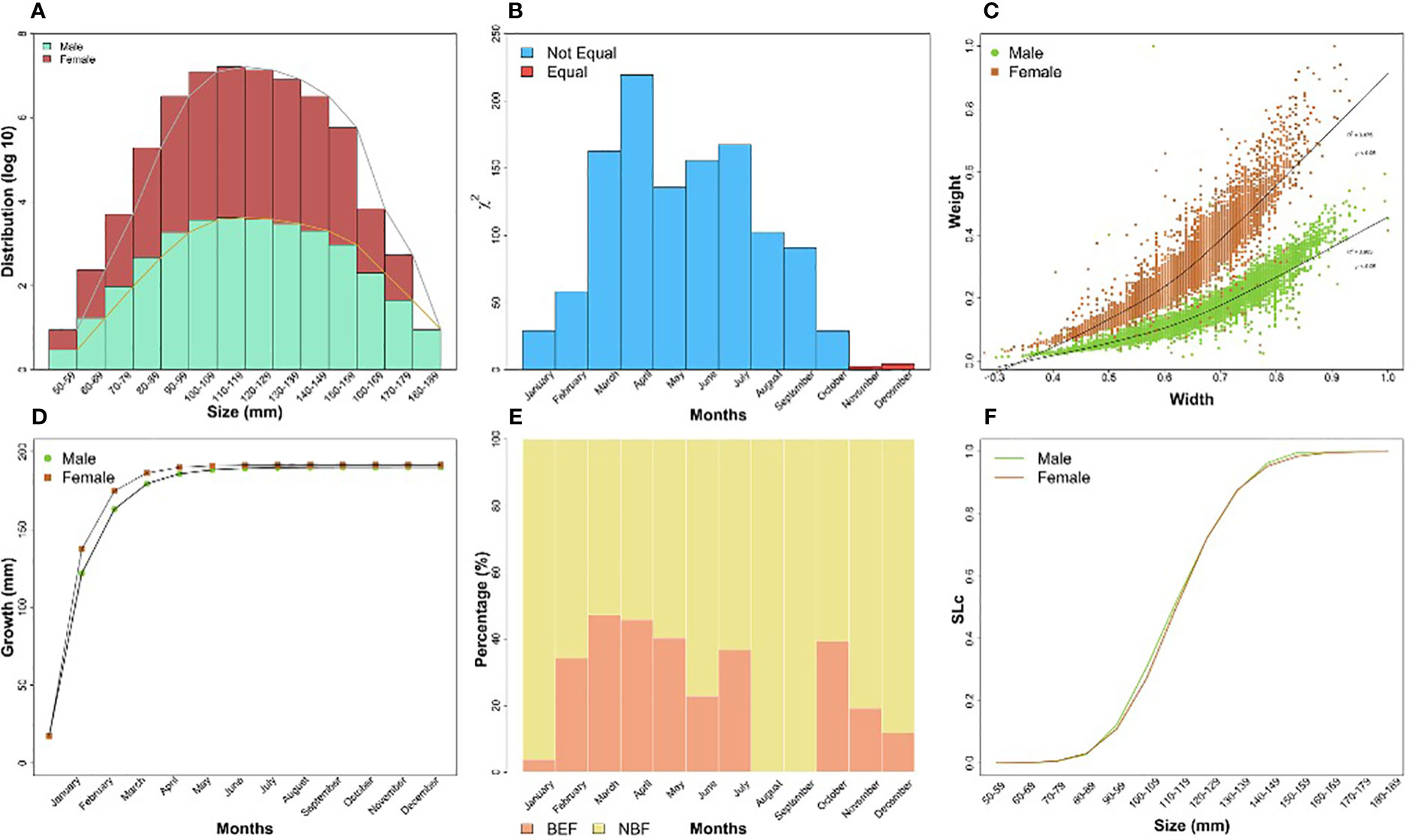
Figure 4 BSC fishery production Production. (A) BSC fishery production based on their sizes; (B) comparison between male and female populations throughout the year; (C) the relationship between width and weight of BSC; (D) Comparison of growth rate between male and female BSC; (E) Comparison between berried female and non-berried female; (F) the relationship between the size and SLc.
The study found that the length at first caught (Lc50) of male BSC, measured using carapace width, was approximately 118.69 mm, while for females, it was approximately 122.70 mm. These values indicated that the BSC was typically caught at a size larger than the minimum legal-size requirement by the Ministry of Marine and Fisheries of Indonesia, which is 10 cm or equivalent to 100 mm. This finding suggests that the BSC population in the coastal waters of East Lampung tends to reach maturity and become eligible for capture before reaching the minimum legal size, potentially enhancing the sustainability of the fishery by allowing individuals to reproduce and contribute to the population before being caught. The peak recruitment season of BSC occurred throughout the year, with the highest take placed in July (male) and May 2020 (female), respectively, indicating only one age group in the BSC stock (one cohort).
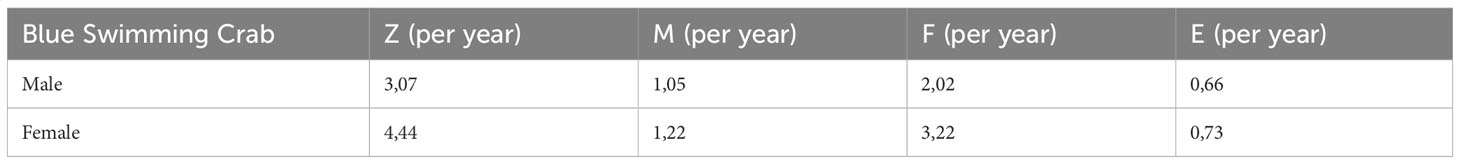
The results from the mortality estimation indicate that fishing is the primary reason for BSC mortality, as evidenced by the F value (fishing mortality) value being higher than the M (natural mortality) value (Table 4). This suggests that fishing activities were exerting significant pressure on the BSC population. The estimated relative fishing pressure (F/M) value was relatively high (3.75), which was a considerably more significant level of SPR for being conserved. Moreover, the exploitation rate of 0.71 indicated that the level of fishing is in a state of overexploitation. As per the classification by Sparre (1998), an exploitation rate (E) of less than 0.5 would be considered under-exploited, E equals 0.5 as moderate, and E is greater than 0.5 as over-exploitation. With an E value of 0.71, it is evident that the BSC fishery was being heavily exploited, posing a risk to the sustainability of the population. Moreover, the length-based Spawning Potential Ratio (SPR) analysis revealed that the BSC’s SPR in the study area was 19 %, indicating that the fishing activities in the study area have led to overfishing. Given that the calculated SPR value for the BSC in this study area is 19 %, it is evident that the current fishing practices have resulted in an overfishing situation. In addition, the SPR value is below the biological reference limit of 20 % and the biological reference target of 50 %. Based on the results of the SPR analysis, the average caught size of blue swimming crab (Lc50) in the coastal waters of East Lampung is 109.54 m. This situation suggests that the current stock was below the critical level required for sustainable reproduction, potentially affecting recruitment. Given that the SPR is below the biological reference limit, there is a risk of compromising the reproductive potential of the BSC production, which can have adverse consequences for the overall stock abundance.
3.2 Mangrove ecosystem
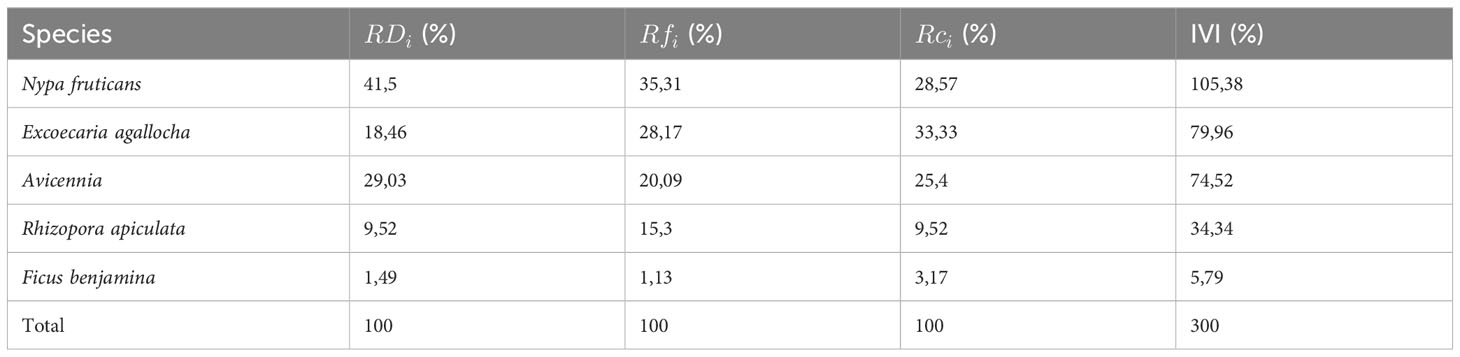
The mangrove ecosystem in the study area consists of five true mangroves (Avicennia marina, Nypa fruticans, Rhizophora apiculata, Exoecaria agallocha, Sonneratia alba) and one mangrove associate (Ficus benjamina). Table 5 shows that Nypa fruticans exhibit the highest dominance in the coastal area, indicating the most significant influence on the stability of the mangrove ecosystem. However, the dominance is not absolute, as Exoesceria agallocha and Avicennia marina also show relatively high dominance in the study area. This suggests that the dominant territorial control in this area is fairly evenly distributed among these three species. This distribution is considered favorable since no single species shows extreme dominance (with an IVI value > 150). Although Rhizophora apiculata has a lower IVI index (34.34 %) than the highest three species, it can also be considered to have an essential role in terms of the stability of the mangrove ecosystem, compared to Ficus benjamina, which has the lowest IVI index (5.79 %). According to the Shannon Diversity Index, the mangrove ecosystem in the study area is classified as moderate, with a value of 2.72. A moderate diversity in the study area indicated that the community in this area possesses a moderate complexity level. It suggests that interactions between species occur with moderate intensity, and in other words, energy transfer (food webs), predation, competition, and niche partitioning theoretically occur within a moderate level of complexity.
The aquatic organisms living in the mangrove ecosystem consist of plankton and benthic. Plankton consists of Bacillariophyceae class (Trybilionella, Thalassiothrix, Bicapitata, Guinardia, Proboscia, and Toaxarium), Chlorophyceae class (Imbricata), and Dinophyceaea class (Carriense), which the highest found in the Bacillariophyceae class, accounting for 81.90 %, followed by Chlorophyceae, and Dinophyceae. The diversity and evenness indexes of plankton are 1.352 and 0.317, respectively, indicating a low diversity of phytoplankton and relatively uniform abundance among different plankton species. The analysis of diversity indices did not identify any plankton species with blooming potential, indicating that this area was still in good condition and had the potential for increased productivity. The benthic organisms in this area include Bivalva class (Nuculana taphira), Malacostrata class (Acetes indicus, llyoplax sp.), Gastropoda class (Asiminera sp., Batillaria zonalis, Clithon oualeniaense, Ophicardelus omatus, Odostomia laevigata, Pomacea canalicuta, Telescopium Telescopium, Voluthapa amulacacea), Crustacea class (Episersarma sp.), and Rhabditophora class (Planaria torva), with the highest and the lowest percentage was observed in Gastropoda and Rhabditophora classes, respectively, accounting for 68.4 %, and 5.3 %, for both classes. The benthic diversity index ranges from 1.046 to 1.03, indicating low benthic diversity and suggesting relatively low community stability. The evenness and dominance index ranged from 0.358 to 0.297 and 0.372 to 0.471, respectively, indicating uneven distribution and a lack of dominance within the benthic community in the study area. Moreover, this area experienced a wide range of salinity levels, resulting in diverse plankton species that can adapt to varying salinity conditions. Additionally, the average value of water depth, temperature, water transparency, flow rate, salinity, pH, DO, nitrate, phosphate, and soil texture were 130 cm; 27.75 °C; 17.3 cm; 1.16 km/hour;15.12 ppt; 7.3;3.81 mg/L, 0.114 %; 8.05 ppm, sandy clay respectively.
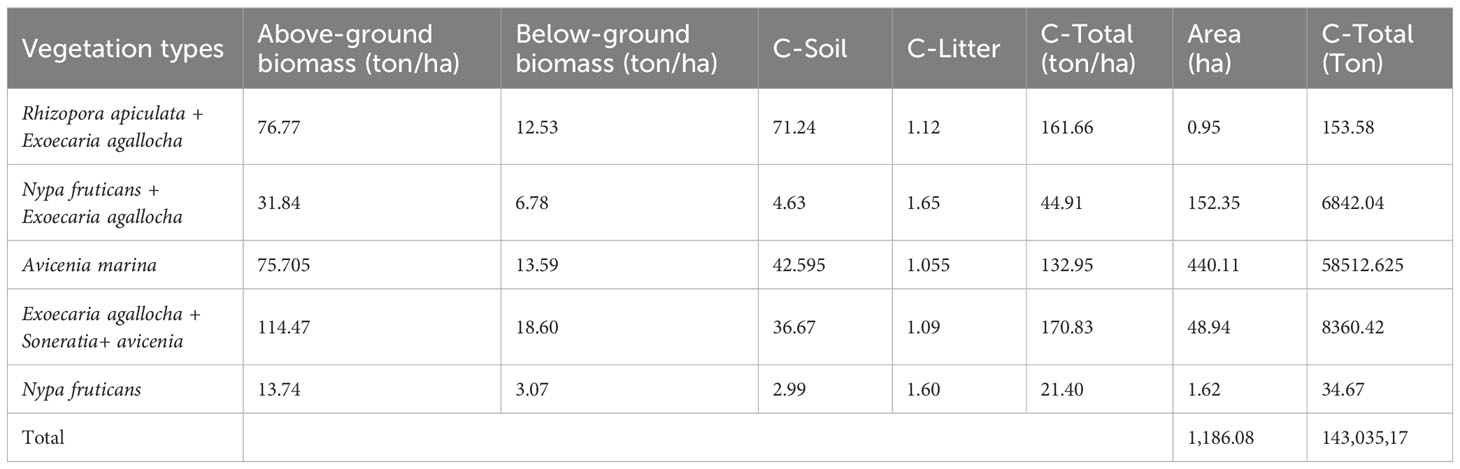
Table 6 presents the carbon stocks in various vegetation types in the coastal mangrove forest area in East Lampung. The vegetation type of Exoecaria agallocha + Soneratia+ Avicenia marina showed the highest above-ground and below-ground biomass, totaling 114.47 and 18.60 tons/ha, respectively. Surprisingly, the vegetation type comprising Rhizopora apiculata + Exoecaria agallocha, had the highest C-soil content, despite covering the smallest area compared to other vegetation types. Moreover, the results revealed that the carbon stocks in this area range from 21.40 to 170.83 tons per hectare. These carbon stocks were relatively low and can be attributed to the young age of the trees in the area. The plants’ relatively small diameter, with most being less than 20 cm, and the average height of 14-14 meters, indicate that they are still in the early stages of growth and far from reaching their adult size. Despite having the highest vegetation density among other vegetation (1,775 individuals/ha), the relatively lower carbon stock in areas with Nypa fruticans−can reaches 30 meters− can be attributed to its limited carbon storage potential. The trees’ young age and limited growth contribute to the lower carbon stocks in the coastal area. Exoecaria + Soneratia+ Avicenia exhibited the highest carbon stocks because these vegetation type thrives in favorable biophysical conditions, with Exoecaria plants requiring an ample supply of fresh water. These species are commonly found at the back of the mangrove forest, where the salinity level is lower or above the highest tide limit. The presence of these vegetation types contributes to higher carbon sequestration due to their ability to efficiently capture and store carbon dioxide from the atmosphere.
3.3 Socioeconomic assessment
The respondents in this comprised both male and female participants, however, it is essential to note that the sample size did not deliberately differentiate based on gender. Despite this, the study’s findings revealed that male respondents constituted a significant majority, accounting for 95 % of the participants, while female respondents represented only 5 percent. This imbalance can be attributed to BSC fisherman being traditionally male-dominated, as fishing is commonly perceived as a physically demanding occupation less suited for women. The age distribution of the respondents is as follows: 15 %are aged 20-30 years old, 34 % fall within the 31-40 years old range, approximately 43 % are between 41-60 years old, and only 8 % are older than 61 years old. The majority of respondents are in the 41-60 age group, indicating that they are still in the productive age range. Regarding education levels, the respondent’s achievements varied. It was found that 2 % of respondents did not attend formal schooling, approximately 34 % completed elementary school, 48 % graduated from middle school, and 33 % completed high school.
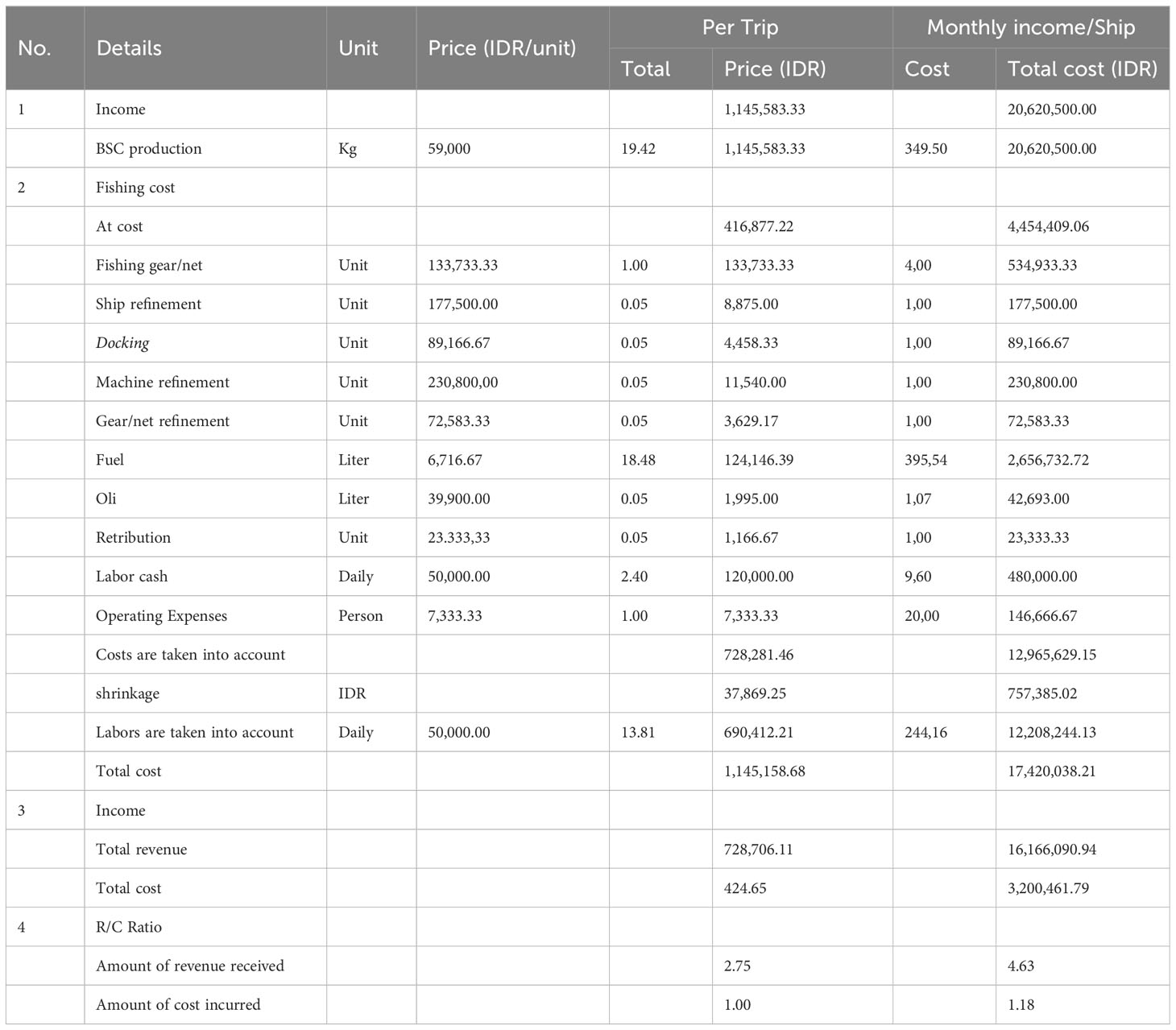
The production (catch) obtained and the average price of BSC generate an income of IDR 1,1,45,583.33 per trip (Table 7). It is assumed that the production yield (caught BSC) per trip and the price per kilo was the same as the number of trips per week on average five times with a total of 4 weeks per month will be IDR 20,620,500.00. The cast cost incurred in one trip amounts to IDR 416,877.22, with the most significant portion allocated to fishing gear/nets at IDR 133,733.33. The total costs for the month amount to IDR 4,454,409.06, with the highest cost being diesel fuel at IDR 2,656,732.72. The calculated costs are IDR 728,281.46 per trip, with labor costs being the highest at IDR 690,412.21 per trip, resulting in a total monthly cost per ship of IDR 12,208,244.13 and a total of IDR 17,420,038.21 per month for one ship. The R/C ratio for BSC fishing per trip and total costs were 4.63 and 1.19, respectively, where R/C > 1, indicating that the BSC business yields positive benefits and was economically viable, as the revenue generated was significantly higher than the cost incurred.
4 Discussion
BSC landed in our study areas exhibit a year-round presence, with the abundance peaking in various months or seasons, suggesting a pattern of seasonal-continuous-spawning. The spawning season reaches its peak in September-October 2019 (late east season and early west season) and June-July 2020 (beginning of the east season). These findings align with Zairion’s research (2015) in the coastal waters of East Lampung, where blue swimming crabs are found throughout the year, indicating a continuous-seasonal (seasonal-continuous) reproductive pattern with peak spawning seasons in May and September (beginning and end of the east season) and October (beginning of the west season). The observations indicate that the fishing season’s peak, as evidenced by the catch, spans from December 2019 to July 2020, resulting in an overlapping peak between the fishing and spawning seasons, particularly in June-July 2020. Ekawati (2019) notes that the peak season occurs in November/December to early March, the middle season spans March-June/July, and the lean season occurs from late July to November. In this scenario, the likelihood of catching blue swimming crabs in the BEF (Berried Egg Female) stage is high due to the presumed high fishing intensity. It is estimated that BEF blue swimming crabs with a chance to escape capture are those located outside the fishing catchment area. The size of the blue swimming crabs caught can be influenced by the fishing ground. Panggabean and Pane (2018) note that adult blue swimming crabs tend to inhabit deeper waters, while smaller crabs (<10 cm) are commonly found in coastal areas. In our study areas, the average carapace width aligns with the Minister of Maritime Affairs and Fisheries’ Decree No. 12 of 2020, Article 8, stipulating that the minimum catchable size for blue swimming crabs is more than 10 cm or equal to 100 mm, with a body weight exceeding 60 grams per head. Additionally, the mesh size used in crab fishing plays a role in determining the caught carapace width. In June and July, there is a suspicion that a significant portion of female blue swimming crabs are undergoing gonad maturity, with an anticipation that the subsequent month will witness the initiation of the spawning process. As (Damora and Nurdin, 2016) asserts, July records the highest peak in the condition factor, indicating a substantial number of female blue swimming crabs undergoing gonad maturity, and the expectation is that they will spawn in the following month. This spawning process may lead to a reduction in the body weight of the blue swimming crabs, resulting in a lower condition factor (Tanod et al. (2000) supports this by stating that during the spawning season, there is an increase in the population of mature blue swimming crabs with gonads, leading to a rise in body weight compared to the increase in carapace width. The blue swimming crab falls into the category of short-lived species, with an estimated age to reach the maximum carapace width (Tmax or longevity). The Tmax values for male and female blue swimming crabs are reported as 2.73 years (equivalent to 32.75 months) and 3.49 years (equivalent to 41.88 months), respectively. These Tmax values are crucial in understanding the life history and growth patterns of blue swimming crabs. The differences between male and female Tmax values may reflect biological variations in growth rates and maturation processes between the sexes.
Our analysis showed that the business feasibility indicates that the blue swimming crab business on the east coast of Lampung is economically profitable and viable, with a favorable R/C ratio of crab capture per trip (4.63) and the R/C of total costs (1.18). The cash income received by crab fishermen amounts to IDR 728,706.11 per trip and IDR 16,166,090.94 per ship per month. The blue swimming crab fishery business in this region has witnessed substantial expansion, as blue swimming crabs are highly valued in the market and can be easily caught in the nearby waters (Bahtiar et al., 2016; (Monterey Bay Aquarium Seafood Watch, 2018; Monterey Bay Aquarium Seafood Watch, 2023). Despite the significant economic impact on BSC fishery, however, it is worth noting that there were concerning signs of habitat degradation and declining BSC stocks, potentially vulnerable to BSC extinction (Bahtiar et al., 2016; (Warren and Steenbergen, 2021; Monterey Bay Aquarium Seafood Watch, 2023). The findings from our study revealed that the most frequently caught BSC fell within the size range of 120-129 mm. However, we also observed a concerning trend of capturing smaller BSC sizes ranging from 50-59 mm. Moreover, a high exploitation rate in this area (0.71) due to high demand for this would potentially reduce the stock of BSC due to declining population size, reduced reproductive success, and ecosystem disruption (Kunsook et al., 2014; Zairion et al., 2015; Prince et al., 2020; Rabaoui et al., 2021). We can see from the SPR value, which shows 19 %, indicating significant overfishing in East Lampung (Prince et al., 2015b). A recent report by Indonesian Blue Swimming Crab Associations have indicated a gradual decline in the production of blue swimming crabs in the last several years (APRI, 2022) due to illegal fishing, which attributed to significant threat to the sustainability of blue swimming crab populations, contributing to overexploitation and habitat degradation (Chapsos and Hamilton, 2019; Awaliyah et al., 2020; Mackay et al., 2020).Despite the regulations set by the Marine and Fisheries Ministry of Indonesia regarding the Placement of Fishing Equipment and Fishing Aid in the State Fisheries Management Areas of the Republic of Indonesia and the High Sea, as well as the Arrangement of Fishing Andon (Ministry of Marine Affairs and Fisheries Indonesia, 2020a), there have been several cases from illegal fishing where inadequate monitoring and low enforcement capabilities have led to non-compliance (Chapsos and Hamilton, 2019; Mackay et al., 2020; Dirhamsyah et al., 2022). Moreover, the “Minister of Maritime Affairs and Fisheries Regulation Number 12/PERMEN-KP/2020 of 2020 concerning Management of Lobsters (Panulirus Spp.), Crabs (Scylla Spp.), and Crab (Portunus Spp.) in the Territory of the Republic of Indonesia” outlines specific conditions for the catching and removal of crabs (Portunus spp.) from the territory (Ministry of Marine Affairs and Fisheries Indonesia, 2020b). These conditions encompass criteria related to spawning conditions, carapace width, weight, quotas, fishing gear, and supervision by fisheries authorities (Ministry of Marine Affairs and Fisheries Indonesia, 2020b). However, there have been widespread instances of abuse and violations related to this statute, as documented in reports by Chapsos and Hamilton (2019) and Dirhamsyah et al. (2022). Moreover, the effect of climate change might affect to the BSC availability in the ocean due to increasing surface sea temperature (Champion et al., 2020; Ansari et al., 2023a).
Therefore, to address this critical issue and safeguard the future of BSC fisheries in this area, implementing sustainable fishing practices, such as minimum size limits and seasonal closures, can help maintain the reproductive potential of BSC populations (Johnston et al., 2011; Prince et al., 2020; Madduppa et al., 2021). The study emphasizes a concerning trend in capturing smaller BSC sizes (50-59 mm), highlighting the need for innovative technologies or strategic approaches to prevent the unintended capture of undersized crabs and ensure the conservation of juvenile populations. The region contends with a soaring exploitation rate (0.71) driven by fervent demand, amplifying the risk of depleting BSC stocks. This heightened exploitation poses a direct threat to reproductive success, potentially leading to an ecosystem imbalance and hampering the long-term sustainability of BSC populations (Gilman et al., 2016; Do and Armstrong, 2023). A critical revelation from the study is the Spawning Potential Ratio (SPR) value of 19%, indicating significant overfishing in East Lampung. This poses an immediate threat to the sustainable future of BSC populations, warranting urgent intervention to safeguard reproductive capacities. The unregulated and continuous use of traps or nets in BSC fishing practices poses the risk of ghost fishing (Matsuoka et al., 2005; Beneli et al., 2020). This unintended consequence results in the unnecessary mortality of BSC and other marine species, inflicting damage on vital habitats like mangroves and coral reefs, exacerbating ecological challenges. An additional layer of complexity emerges from unclear law enforcement, indicating a gap in effectively enforcing regulations. This leaves room for uncontrolled fishing practices, contributing to sustainability challenges faced by BSC populations (Awaliyah et al., 2020; Dirhamsyah et al., 2022). Clarifying and reinforcing legal frameworks is imperative for effective conservation measures and sustainable resource management (Chapsos and Hamilton, 2019; Dirhamsyah et al., 2022). Educating fisherman and raising awareness about the consequences of abandoned gear can play a crucial role in preventing ghost fishing (Susilo et al., 2021).
Beyond regulatory measures, the conservation of mangroves emerges as a pivotal strategy to enhance the availability of the blue swimming crab (BSC). Mangrove, as a unique coastal ecosystem through their close interaction with various abiotic and biotic factors, significantly plays a crucial role in coastal areas, supporting fishing activities, including aquaculture and capture fisheries (Sukardjo, 2002; Alongi, 2012; Pawar, 2013). The importance of mangroves in these activities stems from their roles as crucial spawning grounds, nursery areas, and feeding habitats for diverse aquatic species and other land animals. One of the most significant contributions of mangrove forests to coastal waters is the shedding of their fallen leaves into the water, influencing water fertility and becoming food for various aquatic biota (Ye et al., 2013; Liu et al., 2017). Mangrove ecosystems play a vital role in influencing the biological conditions of the blue swimming crab fishery. For instance, the condition of swimming crabs can indicate excellent or poor crab growth. In a study conducted by Nurdin and Haser (2018) on Salemo Island, South Sulawesi, the condition factors of blue swimming crabs (Portunus pelagicus) caught in different ecosystems showed mangrove ecosystems had higher condition factors compared to those caught in seagrass and coral reef ecosystems (Nurdin and Haser, 2018). Our study area, which covers an extensive mangrove forest alongside east Lampung coastal, contains a lot of crustaceans, which are the dominant components of the diet of BSC (Zainal, 2013). The study area provided a favorable environment for the thriving crustacean population, which in turn plays a significant role in sustaining the BSC population. In addition to its ecological significance, mangrove conservation holds implications for carbon stocks, contributing to the broader understanding of environmental sustainability. Mangrove ecosystems are renowned for their capacity to sequester and store substantial amounts of carbon, playing a vital role in mitigating climate change (Kristensen et al., 2008; Alongi, 2012). The dense vegetation, particularly the intricate root systems, traps organic matter and facilitates the accumulation of carbon in both above-ground and below-ground biomass, as well as in the sediment. The mangrove’s blue carbon reservoir, encompassing carbon stored in living biomass, detritus, and soil, holds immense value in climate change mitigation strategies. Studies have demonstrated that mangrove forests have higher carbon sequestration potential compared to other terrestrial ecosystems (Alongi, 2012; Hilmi et al., 2017; Hamilton and Friess, 2018).Therefore, the conservation of mangroves not only benefits the blue swimming crab fishery but also contributes to global efforts to combat climate change by preserving crucial carbon sinks. The interconnectedness of mangrove conservation, aquatic biodiversity, and the well-being of the blue swimming crab highlights the need for integrated coastal management practices to ensure the resilience and sustainability of these ecosystems (Johnston et al., 2021; Marine and Fisheries Ministry of Indonesia, 2022; Ansari et al., 2023b).
To promote the sustainability of blue swimming crab fisheries in the eastern coastal area of Lampung, a comprehensive set of recommendations is proposed. First and foremost, there is a critical need to strengthen conservation efforts and implement sustainable management practices for mangrove ecosystems. Recognizing their pivotal role in supporting fisheries, especially for species like blue swimming crabs, these ecosystems should be safeguarded as vital spawning grounds, nursery areas, and feeding habitats. Monitoring and regulation of fishing practices are essential components of sustainable management, emphasizing the enforcement of regulations on minimum catchable sizes, mesh sizes, and seasonal closures to protect the reproductive potential of blue swimming crab populations and prevent the capture of undersized individuals. Furthermore, economic diversification strategies should be explored to reduce dependence solely on blue swimming crab fisheries, mitigating potential ecological and economic risks associated with overreliance on a single resource. Encouraging the adoption of new technologies and sustainable fishing practices is crucial to minimize unintentional captures and reduce bycatch. Combatting ghost fishing is paramount, necessitating measures to retrieve and properly dispose of lost fishing gear, along with raising awareness among fishermen about the consequences of abandoned gear. A robust framework for ongoing research and data collection is recommended to monitor blue swimming crab populations, habitat health, and ecosystem dynamics. The collected data should inform adaptive management strategies to ensure the sustainability of blue swimming crab fisheries. Community engagement and education play pivotal roles in fostering a sense of responsibility among local fishermen, encouraging them to embrace sustainable fishing practices. Collaborative management involving local communities, government agencies, and non-governmental organizations is essential for holistic and inclusive approaches. Regular stock assessments are proposed to determine the health and abundance of blue swimming crab populations, enabling the adjustment of management measures based on scientific findings. Advocacy for policies prioritizing sustainable fishing practices, habitat conservation, and the protection of blue swimming crab populations is crucial. Working closely with policymakers to integrate scientific recommendations into fisheries management regulations will contribute to the long-term conservation and sustainable development of blue swimming crab fisheries in the region.
5 Conclusions
In conclusion, the comprehensive assessment of the Blue Swimming Crab (BSC) fishery in the study area reveals a complex interplay of economic gains, environmental challenges, and regulatory issues. The habitat degradation and diminishing BSC stocks, despite their significant economic impact, present a precarious situation that could lead to the extinction of BSC populations. The capture of undersized BSC, coupled with a high exploitation rate and overfishing, further intensifies the risks to the sustainability of this crucial marine resource. The study emphasizes the need for technological interventions to precisely manage the capture of undersized crabs and highlights the importance of balancing demand with ecological impact. The Spawning Potential Ratio (SPR) value indicates a state of significant overfishing, demanding urgent interventions to safeguard the reproductive capacities of BSC populations. The hazards of ghost fishing, compounded by unclear law enforcement, pose additional challenges that contribute to the sustainability concerns faced by BSC populations. Despite regulations set by the Marine and Fisheries Ministry of Indonesia, inadequate monitoring and low enforcement capabilities have led to non-compliance, as evidenced by reported cases of illegal fishing. The regulatory framework, including specific regulations on the management of crabs, faces challenges related to abuse and violations, emphasizing the need for strengthened enforcement mechanisms. Addressing the critical sustainability challenges affecting BSC resources requires a multi-faceted approach. This involves implementing precise fishing technologies, enforcing existing regulations more effectively, and enhancing monitoring capabilities. Collaboration between stakeholders, including government authorities, fishing communities, and conservation groups, is crucial to strike a balance between economic interests and the long-term ecological health of BSC populations. In doing so, the study underlines the importance of sustainable fishing practices, community involvement in mangrove protection, and government support to ensure the preservation of this vital marine resource for future generations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
HY: Conceptualization, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. YI: Visualization, Writing – review & editing, Supervision, Validation. DS: Supervision, Validation, Writing – review & editing. AA: Formal analysis, Visualization, Writing – review & editing. H: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1304838/full#supplementary-material
References
Alongi D. M. (2012). Carbon sequestration in mangrove forests. Carbon Manag 3, 313–322. doi: 10.4155/cmt.12.20
Ansari A., Lin Y.-P., Lur H.-S. (2021). Evaluating and adapting climate change impacts on rice production in Indonesia: A case study of the Keduang Subwatershed, Central Java. Environments 8, 117. doi: 10.3390/environments8110117
Ansari A., Pranesti A., Telaumbanua M., Alam T., Taryono, Wulandari R. A., et al. (2023a). Evaluating the effect of climate change on rice production in Indonesia using multimodelling approach. Heliyon 9, e19639. doi: 10.1016/j.heliyon.2023.e19639
Ansari A., Pranesti A., Telaumbanua M., Ngadisih, Hardiansyah M. Y., Alam T., et al. (2023b). Optimizing water-energy-food nexus: achieving economic prosperity and environmental sustainability in agriculture. Front. Sustain Food Syst. 7. doi: 10.3389/fsufs.2023.1207197
APRI (2022). Annual report 2022 (Surabaya, Indonesia: The Indonesian Blue Swimming Crab Management Association). Available at: https://www.apri.or.id/full-width-page-layout/downloads/download-info/apri-annual-report-2022/.
Awaliyah S., Mangku D. G. S., Yuliartini N. P. R., Suastika I. N. (2020). Enforcement of illegal fishing laws that was done by foreign ships in the Indonesian sea region, viewed from international sea law. Int. J. 9, 1165. doi: 10.6000/1929-4409.2020.09.137
Bahtiar R., Nuva, Anggraeni D., Hidayat N. K. (2016). Economic evaluation of implementing minimum legal size on blue swimming crab fishery in Indonesia. In: Olewiler N., Francisco H., Ferrer A. Eds. Marine and Coastal Ecosystem Valuation, Institutions, and Policy in Southeast Asia (Singapore: Springer), 341–363. doi: 10.1007/978-981-10-0141-3_17
Beneli T. M., Pereira P. H. C., Nunes J., Barros F. (2020). Ghost fishing impacts on hydrocorals and associated reef fish assemblages. Mar. Environ. Res. 161, 105129. doi: 10.1016/j.marenvres.2020.105129
Bengen D. G. (2002). Introduction and management of mangrove ecosystems. center for coastal and marine resource study (Bogor: Bogor Institute of Agriculture).
Boutson A., Mahasawasde C., Mahasawasde S., Tunkijjanukij S., Arimoto T. (2009). Use of escape vents to improve size and species selectivity of collapsible pot for blue swimming crab Portunus pelagicus in Thailand. Fisheries Sci. 75, 25–33. doi: 10.1007/s12562-008-0010-z
Brown S. (1997). Estimating biomass and biomass change of tropical forests: A primer forest forestry papers (Rome Italy: FAO) 134, 55. Retrieved from http://www.fao.org/docrep/w4095e/w4095e00.htm.
Cairns M. A., Brown S., Helmer E. H., Baumgardner G. A. (1997). Root biomass allocation in the world’s upland forests. Oecologia 111, 1–11. doi: 10.1007/s004420050201
Carpenter K. E., Niem V. H. (1998). FAO species identification guide for fishery purposes. The living marine resources of the Western Central Pacific. v. 1. Seaweeds, corals, bivalves and gastropods. v. 2. Cephalopods, crustaceans, holothurians and sharks. v. 3. Batoid fishes, chimaeras and bony fishes part 1 (Elopidae to Linophrynidae). v. 4. Bony fishes part 2 (Mugilidae to Carangidae) (No. FAO 589.4 F686). FAO, Rome (Italia).
Champion C., Broadhurst M. K., Ewere E. E., Benkendorff K., Butcherine P., Wolfe K., et al. (2020). Resilience to the interactive effects of climate change and discard stress in the commercially important blue swimmer crab (Portunus armatus). Mar. Environ. Res. 159, 105009. doi: 10.1016/j.marenvres.2020.105009
Chapsos I., Hamilton S. (2019). Illegal fishing and fisheries crime as a transnational organized crime in Indonesia. Trends Organ Crime 22, 255–273. doi: 10.1007/s12117-018-9329-8
Chávez E. A. (2007). “Socio-economic assessment for the management of the Caribbean spiny lobster,” in Proceedings of the 60th Gulf and Caribbean Fisheries Institute. 60, 193–196.
Damora A., Nurdin E. (2016). Some biological aspects of blue swimming crab (Portunus pelagicus) in Labuhan Maringgai waters, East Lampung. Bawal J. 8, 13–20. Retrieved from http://ejournal-balitbang.kkp.go.id/index.php/bawal/article/view/2013/1632.
Dharmawan I. W. S., Siregar C. A. (2008). Soil carbon and carbon estimation of Avicennia marina (Forsk.) Vierh. stand at Ciasem, Purwakarta. J. Penelit. Hutan dan Konservasi Alam 5, 317–328. doi: 10.20886/jphka.2008.5.4.317-328
Dirhamsyah D., Umam S., Arifin Z. (2022). Maritime law enforcement: Indonesia’s experience against illegal fishing. Ocean Coast. Manag 229, 106304. doi: 10.1016/j.ocecoaman.2022.106304
Do H.-L., Armstrong C. W. (2023). Ghost fishing gear and their effect on ecosystem services–Identification and knowledge gaps. Mar. Policy 150, 105528. doi: 10.1016/j.marpol.2023.105528
Eaton A. D., Clesceri L. S., Rice E. W., Greenberg A. E., Franson M. (2005). APHA: standard methods for the examination of water and wastewater (Washington, DC: APHA, AWWA, WEF). Centennial Edition.
Ekawati A. K. (2019). Spatial and temporal bioeconomic analysis of crab (Portunus pelagicus) fisheries in the east coastal waters of lampung (Doctoral dissertation, IPB University).
Ervinia A., Nugroho K. C., Setioko W. (2023). “Life history and spawning potential of blue swimming crab Portunus pelagicus (Linnaeus 1758) in Pamekasan, Madura Island, Indonesia,” in IOP Conference Series: Earth and Environmental Science. 1251 (1), 012042. (IOP Publishing). doi: 10.1088/1755-1315/1251/1/012042
Fahmi A. S., Maksum M., Suwondo E. (2015). USFDA import refusal and export competitiveness of Indonesian crab in US market. Agric. Agric. Sci. Proc. 3, 226–230. doi: 10.1016/j.aaspro.2015.01.044
FAO (2020). FishStatJ-Software for fishery and aquaculture statistical time series. FAO Fisheries Division [online]. Rome. Updated, 22.
Farhan A. R., Lim S. (2010). Integrated coastal zone management towards Indonesia global ocean observing system (INA-GOOS): Review and recommendation. Ocean Coast. Manag 53, 421–427. doi: 10.1016/j.ocecoaman.2010.06.015
Field C. D. (1999). Rehabilitation of mangrove ecosystems: an overview. Mar. pollut. Bull. 37, 383–392. doi: 10.1016/S0025-326X(99)00106-X
Gilman E., Chopin F., Suuronen P., Kuemlangan B. (2016). Abandoned, lost and discarded gillnets and trammel nets: methods to estimate ghost fishing mortality, and the status of regional monitoring and management. FAO Fisheries and Aquaculture Technical Paper, (600), I. Rome, Italy: FAO.
Hairiah K., Dewi S., Agus F., Velarde S., Andree E., Rahayu S., et al (2011). Measuring carbon stocks. Bogor, Indonesia: World Agroforestry Centre. p. 95.
Hamilton S. E., Friess D. A. (2018). Global carbon stocks and potential emissions due to mangrove deforestation from 2000 to 2012. Nat. Clim Chang 8, 240–244. doi: 10.1038/s41558-018-0090-4
Hartnoll R. G., Bliss D. E., Abele L. G. (1982). The Biology of Crustacea: Volume 2: Embryology, morphology and genetics (New York: Academic Press).
Hilmi E., Vikaliana R., Kusmana C., Sari L. K. (2017). The carbon conservation of mangrove ecosystem applied REDD program. Reg. Stud. Mar. Sci. 16, 152–161. doi: 10.1016/j.rsma.2017.08.005
Johnston D., Harris D., Caputi N., Thomson A. (2011). Decline of a blue swimmer crab (Portunus pelagicus) fishery in Western Australia-History, contributing factors and future management strategy. Fish Res. 109, 119–130. doi: 10.1016/j.fishres.2011.01.027
Johnston D. J., Yeoh D. E., Harris D. C. (2021). Environmental drivers of commercial blue swimmer crab (Portunus armatus) catch rates in Western Australian fisheries. Fish Res. 235, 105827. doi: 10.1016/j.fishres.2020.105827
Kembaren D. D., Zairion Z., Kamal M. M., Wardianto Y. (2018). Abundance and spatial distribution of blue swimming crab (Portunus pelagicus) larvae during east monsoon in the East Lampung waters, Indonesia. Biodiversitas 19, 1326–1333. doi: 10.13057/biodiv/d190420
Komiyama A., Poungparn S., Kato S. (2005). Common allometric equations for estimating the tree weight of mangroves. J. Trop. Ecol. 21, 471–477. doi: 10.1017/S0266467405002476
Kottek M., Grieser J., Beck C., Rudolf B., Rubel F. (2006). World map of the Köppen-Geiger climate classification updated. Meteorologische Zeitschrift. Gebrüder Borntraeger Science Publishers, Stuttgart. doi: 10.1127/0941-2948/2006/0130
Kristensen E., Bouillon S., Dittmar T., Marchand C. (2008). Organic carbon dynamics in mangrove ecosystems: a review. Aquat Bot. 89, 201–219. doi: 10.1016/j.aquabot.2007.12.005
Kunsook C., Gajaseni N., Paphavasit N. (2014). A stock assessment of the blue swimming crab portunus pelagicus (Linnaeus 1758) for sustainable management in Kung Krabaen Bay, gulf of Thailand. Trop. Life Sci. Res. 25, 41–59.
Lin Y.-P., Ansari A., Ngoc-Dan Cao T., Shiau Y.-J., Lur H.-S., Muzaffar A., et al. (2022). Using inhibitors to trade greenhouse gas emission for ammonia losses in paddy soil: A zero-sum game. Environ. Technol. Innov. 28, 102547. doi: 10.1016/j.eti.2022.102547
Liu X., Xiong Y., Liao B. (2017). Relative contributions of leaf litter and fine roots to soil organic matter accumulation in mangrove forests. Plant Soil 421, 493–503. doi: 10.1007/s11104-017-3477-5
Mackay M., Hardesty B. D., Wilcox C. (2020). The intersection between illegal fishing, crimes at sea, and social well-being. Front. Mar. Sci. 7, 589000. doi: 10.3389/fmars.2020.589000
Madduppa H., Martaulina R., Zairion Z., Renjani R. M., Kawaroe M., Anggraini N. P., et al. (2021). Genetic population subdivision of the blue swimming crab (Portunus pelagicus) across Indonesia inferred from mitochondrial DNA Implication to sustainable fishery. PloS One 16, 1–14. doi: 10.1371/journal.pone.0240951
Matsuoka T., Nakashima T., Nagasawa N. (2005). A review of ghost fishing: scientific approaches to evaluation and solutions. Fisheries Sci. 71, 691–702. doi: 10.1111/j.1444-2906.2005.01019.x
Ministry of Marine Affairs and Fisheries Indonesia (2020a). Minister of maritime affairs and fisheries regulation number 12/PERMEN-KP/2020 of 2020 concerning management of lobsters (Panulirus spp.), crabs (Scylla spp.), and crab (Portunus spp.) in the territory of the republic of Indonesia (Jakarta). Accessed on 13 December 2023. Available at: www.peraturan.or.id.
Ministry of Marine Affairs and Fisheries Indonesia (2020b). Management of lobsters (Panulirus spp.), crabs (Scylla spp.), and crab (Portunus spp.) in the territory of the republic of Indonesia. BN. 2020 no. 454, jdih.kkp.go.id, Indonesia.
Monterey Bay Aquarium Seafood Watch (2018). Blue swimming crab (Portunus pelagicus)-Indonesia Bottom gillnet (Accessed 15 December 2023), 1–65. Available at: www.seafoodwatch.org.
Monterey Bay Aquarium Seafood Watch (2023) Blue swimming crab (Portunus pelagicus)-indonesia bottom gillnet (Accessed 15 December 2023).
Nga B. T., Roijackers R., Scheffer M. (2016). “Effects of decomposition and Nutrient release of Rhizophora apiculata Leaves on the MangroveShrimp System in the Camau Province Vietnam,” in Intenational Symposium on Southeast Asian Water Environment. 67–72. doi: 10.1007/s10499-006-9049-y
Nga B. T., Roijackers R., Nghia T. T., Ut V. N., Scheffer M. (2006). Effects of decomposing rhizophora apiculata leaves on larvae of the shrimp penaeus monodon. Aquaculture Int. 14, 467–477. doi: 10.1007/s10499-006-9049-y
Nurdin M. S., Haser T. F. (2018). Faktor kondisi rajungan (Portunus pelagicus) yang tertangkap pada ekosistem mangrove, lamun, dan terumbu karang di pulau salemo sulawesi selatan. Jurnal Ilmiah Samudra Akuatika 2, 9–13.
Ong J. E., Gong W. K., Wong C. H. (2004). Allometry and partitioning of the mangrove, Rhizophora apiculata. For Ecol. Manage 188, 395–408. doi: 10.1016/j.foreco.2003.08.002
Panggabean A. S., Pane A. R. P. (2018). Population dynamics and level of utilization of blue crab (Portunus pelagicus linnaeu) in jakarta bay waters. Jurnal Penelitian Perikanan Indonesia 24, 73–85. Available at: http://ejournal-balitbang.kkp.go.id/.
Pauly D., Ingles J., Neal R. (1984). Application to shrimp stocks of objective meathods for the estimation of growth, mortality and recruitment-related parameters from length-frequency data (ELEFAN I and II). Fishing News Books Ltd: London, United Kingdom.
Pawar P. R. (2013). Monitoring of impact of anthropogenic inputs on water quality of mangrove ecosystem of Uran, Navi Mumbai, west coast of India. Mar. pollut. Bull. 75, 291–300. doi: 10.1016/j.marpolbul.2013.06.045
Penman J., Gytarsky M., Hiraishi T., Krug T., Kruger D., Pipatti R., et al (2003). Good practice guidance for land use, land-use change and forestry. Kanagawa, Japan. p.593.
Prince J., Creech S., Madduppa H., Hordyk A. (2020). Length based assessment of spawning potential ratio in data-poor fisheries for blue swimming crab (Portunus spp.) in Sri Lanka and Indonesia: Implications for sustainable management. Reg. Stud. Mar. Sci. 36, 101309. doi: 10.1016/j.rsma.2020.101309
Prince J., Hordyk A., Valencia S. R., Loneragan N., Sainsbury K. (2015a). Revisiting the concept of Beverton–Holt life-history invariants with the aim of informing data-poor fisheries assessment. ICES J. Mar. Sci. 72, 194–203. doi: 10.1093/icesjms/fsu011
Prince J., Victor S., KloulChad V., Hordyk A. (2015b). Length based SPR assessment of eleven Indo-Pacific coral reef fish populations in Palau. Fish Res. 171, 42–58. doi: 10.1016/j.fishres.2015.06.008
Rabaoui L., Yacoubi L., Lin Y. J., Joydas T. V., Maneja R. H., Dagoy J., et al. (2021). Distribution, abundance, and life history traits of the blue swimming crab Portunus segnis (Forskå 1775) in the Saudi waters of the Arabian gulf. Reg. Stud. Mar. Sci. 46, 101895. doi: 10.1016/j.rsma.2021.101895
Shannon C. E., Weaver W. (1949). The mathematical theory of communication, by CE Shannon (and recent contributions to the mathematical theory of communication) W. Weaver (Illinois, United States: University of illinois Press).
Siry H. Y. (2011). In search of appropriate approaches to coastal zone management in Indonesia. Ocean Coast. Manag 54, 469–477. doi: 10.1016/j.ocecoaman.2011.03.009
Sloan N. A., Ugandhy A. S. (1994). An overview of Indonesian coastal environmental management. Coast. Manage. 22, 215–233. doi: 10.1080/08920759409362233
Soegianto A., Nurfiyanti P. E., Saputri R. N. R., Affandi M., Payus C. M. (2022). Assessment of the health risks related with metal accumulation in blue swimming crab (Portunus pelagicus) caught in East Java coastal waters, Indonesia. Mar. pollut. Bull. 177, 113573. doi: 10.1016/j.marpolbul.2022.113573
Sparre P., Venema S. C. (1995). Introducción a la evaluación de recursos pesqueros tropicales Parte I øManual (Valparaíso (Chile: FAO).
Sparre P. (1998). Introduction to tropical fish stock assessment. part 1. Manual. FAO Fish. Tech. Paper. 306, 1–407.
Sukardjo S. (2002). Integrated coastal zone management (ICZM) in Indonesia: a view from a mangrove ecologist. Japanese J. Southeast Asian Stud. 40, 200–218.
Susilo E., Purwanti P., Fattah M., Qurrata V. A., Narmaditya B. S. (2021). Adaptive coping strategies towards seasonal change impacts: Indonesian small-scale fisherman household. Heliyon 7, e06919. doi: 10.1016/j.heliyon.2021.e06919
Tanod A., Sulistiono, Watanabe S. (2000). “Reproduction and growth of three species mudcrab (Scylla serrata, S. tranquebarica, S. oceanica) in Segara Anakan Lagoon, Indonesia,” in JSPS-DGHE International Symposium. 10 (4), 347–351.
Tongco M. D. C. (2007). Purposive sampling as a tool for informant selection. Ethnobotany Research and Applications 5, 147–158. Retrieved from https://ethnobotanyjournal.org/index.php/era/article/view/126.
Trott L. A., Alongi D. M. (2000). The impact of shrimp pond effluent on water quality and phytoplankton biomass in a tropical mangrove estuary. Mar. pollut. Bull. 40, 947–951. doi: 10.1016/S0025-326X(00)00035-7
Wardiatno Y., Yulianda F., Rusmana I., Bengen D. G. (2021). Methods and Analysis of Mangrove Ecosystem Studies. (Bogor, Indonesia: PT Penerbit IPB Press), 10.
Warren C., Steenbergen D. J. (2021). Fisheries decline, local livelihoods and conflicted governance: An Indonesian case. Ocean Coast. Manag 202, 105498. doi: 10.1016/j.ocecoaman.2020.105498
Wu X., Zhou B., Cheng Y., Zeng C., Wang C., Feng L. (2010). Comparison of gender differences in biochemical composition and nutritional value of various edible parts of the blue swimmer crab. J. Food Composition Anal. 23, 154–159. doi: 10.1016/j.jfca.2009.08.007
Xiao Y., Kumar M. (2004). Sex ratio, and probability of sexual maturity of females at size, of the blue swimmer crab, Portunus pelagicus Linneaus, off southern Australia. Fish Res. 68, 271–282. doi: 10.1016/j.fishres.2003.11.012
Yanti N. D., Kurnia R., Mashar A., Mardyani Y., Lindawati L. (2022). “Management status of blue swimming crab (Portunus pelagicus Linnaeus 1758) based on EAFM in the coastal of Pangkep Regency, South Sulawesi,” in IOP Conference Series: Earth and Environmental Science. 967 (1), 012022. (IOP Publishing). doi: 10.1088/1755-1315/967/1/012022
Ye Y., Chen Y. P., Chen G. C. (2013). Litter production and litter elemental composition in two rehabilitated Kandelia obovata mangrove forests in Jiulongjiang Estuary, China. Mar. Environ. Res. 83, 63–72. doi: 10.1016/j.marenvres.2012.10.011
Zainal K. A. Y. (2013). Natural food and feeding of the commercial blue swimmer crab, Portunus pelagicus (Linnaeus 1758) along the coastal waters of the Kingdom of Bahrain. J. Assoc. Arab Universities Basic Appl. Sci. 13, 1–7. doi: 10.1016/j.jaubas.2012.09.002
Keywords: blue swimming crab, marine, conservation, mangrove, socio-economic
Citation: Yulianto H, Ihsan YN, Sumiarsa D, Ansari A and Hendarmawan (2024) Assessing the sustainability of the blue swimming crab (Portunus pelagicus) on the Eastern Coast of Lampung: a holistic approach to conservation and resource stewardship. Front. Mar. Sci. 11:1304838. doi: 10.3389/fmars.2024.1304838
Received: 30 September 2023; Accepted: 05 January 2024;
Published: 24 January 2024.
Edited by:
Øivind Bergh, Norwegian Institute of Marine Research (IMR), NorwayReviewed by:
Periyadan K. Krishnakumar, King Fahd University of Petroleum and Minerals, Saudi ArabiaEduardo Grimaldo, UiT The Arctic University of Norway, Norway
Copyright © 2024 Yulianto, Ihsan, Sumiarsa, Ansari and Hendarmawan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Herman Yulianto, aGVybWFuLnl1bGlhbnRvQGZwLnVuaWxhLmFjLmlk; Hendarmawan, aGVuZGFybWF3YW5AdW5wYWQuYWMuaWQ=
 Herman Yulianto
Herman Yulianto Yudi Nurul Ihsan
Yudi Nurul Ihsan Dadan Sumiarsa1,4
Dadan Sumiarsa1,4 Andrianto Ansari
Andrianto Ansari