- 1Sea Turtle Association of Japan, Hirakata, Osaka, Japan
- 2AQUARIUM x ART átoa, Kobe, Hyogo, Japan
- 3Department of Biosphere-Geosphere Science, Okayama University of Science, Okayama, Japan
- 4Shikoku Aquarium, Utazu-chō, Kagawa, Japan
- 5Graduate School of Information Sciences, Tohoku University, Sendai, Miyagi, Japan
- 6Western Pacific Regional Fishery Management Council, Honolulu, HI, United States
- 7National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Southwest Fisheries Science Center, La Jolla, CA, United States
Genetic characteristics of North Pacific loggerhead turtles captured as bycatch in pound nets operating in Muroto, Kochi, Japan were sampled to identify and estimate stock structure of coastal foraging aggregations. Tissue samples were obtained from juvenile, subadult and adult turtles from 2005–2006 and 2008–2010. For each of the years, 100 samples were processed and approximately 820 bp of mitochondrial DNA control region were sequenced. Straight carapace length of the turtles ranged from 56.3– 99.1 cm and their life stages were identified based on previous estimates of the size at puberty (66.0cm) and maturity (82.1cm). A total of 487 out of the 500 samples yielded sequences of sufficient quality to identify five different haplotypes. We conducted mixed stock analysis (MSA) using Bayesian approaches to estimate the contributions of three potential source nesting Management Units (MU) to the Muroto foraging aggregation. There were no significant differences between haplotype frequencies among the different size classes and life stages, nor among the different years, thus the haplotype frequencies were combined for the MSA. The MSA was run with and without consideration of MU size and distance, which resulted in similar MSA estimates. A >50% contribution was estimated from the Yakushima MU, and 40% from the Mainland MU, with only minor contribution from the Ryukyu MU. The estimated contribution from Mainland MU and Yakushima MU were disproportionately larger than the relative abundance of nesting at these MUs compared with Ryukyu MU, demonstrating that closer MUs had a greater contribution than those from further away. The lack of differences found in haplotype frequency among life stages, suggests that both juvenile and mature loggerhead turtles that remigrate to Japanese waters have the tendency to utilize foraging grounds and migration routes near their natal waters.
1 Introduction
Understanding the life history of marine species is important for conservation, but challenging for migratory vertebrates that have cryptic life histories such as sea turtles. While life history stages related to nesting are well known for sea turtles, much of the biology and ecology of juvenile and subadult stages are still unknown. The loggerhead turtle (Caretta caretta) is one such species of migratory animals living around the waters of Japan. The population found around Japan broadly belongs to the North Pacific Regional Management Unit (RMU) (Wallace et al., 2010), or sub-population (IUCN), or Distinct Population Segment (DPS) (Conant et al., 2009), made up of breeding populations whose nesting sites are restricted primarily to Japan (Kamezaki et al., 2003). As with the South Pacific and Northwest Atlantic populations, North Pacific loggerhead hatchlings are thought to disperse eastward from their natal beaches with the aid of ocean currents. After they grow up in central and eastern oceanic developmental habitats, the juveniles migrate back to foraging areas, where they settle in the regions nearer their natal beaches (Bowen et al., 1995; Bolten et al., 1998; Boyle et al., 2009). It is generally believed that for loggerheads, this shift from distant developmental habitats to residential foraging habitats occurs when turtles reach sizes of approximately 50-70 cm in carapace length (Bjorndal et al., 2000; Limpus and Limpus, 2003; Ishihara et al., 2011).
As for the adult female loggerhead of the North Pacific RMU, previous studies have shown the relationship of foraging grounds, prey items and body size related to their nesting beaches (Hatase et al., 2002a, 2007, 2010). Some adult turtles continue to prey on planktonic organisms in oceanic zones of the Kuroshio extension to the east of mainland Japan, while other adults shift to feeding on benthic prey at neritic foraging areas such as the East China Sea (Hatase et al., 2002a). This foraging dichotomy is thought to affect the body size of individuals as shown in Hatase et al. (2002a) where benthic foraging adults were larger than planktonic adults based on stable isotopes analysis.
Little is known about the life history of subadults and juveniles after they remigrate toward their natal waters, despite the majority (89.4%) of loggerhead turtles found in foraging grounds off Cape Muroto belonging these age groups (Ishihara et al., 2011). Cape Muroto is located adjacent to the Kuroshio Current and thought to be one of the important migration corridors for North Pacific loggerhead turtles. Loggerhead turtles are bycaught mostly alive in large pound nets at Cape Muroto, providing an opportunity to study the biology and life history by addressing knowledge gaps through the collection of samples from foraging animals of various life history stages. Furthermore, adult females are present only during the May-August nesting season, while subadults occur year-round (Sea Turtle Association of Japan, unpublished data). This suggests that adult females use the coastal area around Cape Muroto as a migratory corridor between nesting sites and distant foraging grounds, while subadults appear to use this area primarily to forage. This further suggests that there is a shift in migration routes, migration seasons, foraging ground use, associated with the different life stages.
Given that both neritic and oceanic females nest on same beaches (Hatase et al., 2002a, 2013), foraging preferences of adult females do not appear to be determined by the location of the natal beach. In other words, foraging grounds are likely to be shared by females from multiple natal origins. For the nesting populations however, there is a latitudinal cline in relative composition of nesters based on body size and foraging habitat use, with the southern extent of the nesting sites having more large, neritic foragers and the northern extent having more small, oceanic foragers (Kamezaki et al., 1995; Hatase et al., 2013; Okuyama et al., 2022). Moreover, foraging ground use and migration patterns of subadults may also be affected by their natal beach to some extent. In the Northwest Atlantic, post-pelagic juveniles start to settle into coastal neritic habitat associated with general areas near their natal beaches off the US East coast (Bowen et al., 2005). However, the continental shelf off Japan is narrow and does not have sufficient habitat, so sub-adults in the corridor around Cape Muroto still forage on oceanic planktonic prey.
Fine scale nesting beach population structure has been determined for the North Pacific loggerhead population (Matsuzawa et al., 2016). Management Units (MUs) are defined as rookeries (or groups of rookeries) with significant differences in mitochondrial (mt) DNA haplotype frequencies (Moritz, 1994) and these populations are considered to be demographically isolated with respect to female natal recruitment. Three MUs known as Ryukyu, Yakushima Island, and Mainland MUs were identified in the North Pacific RMU (Matsuzawa et al., 2016).
Genetic studies using mtDNA have been useful for learning about stock structure and aspects of life history, such as reproductive natal homing (e.g. Bowen et al., 1993; Encalada et al., 1998; Hatase et al., 2002b; Bowen and Karl, 2007; Lohmann et al., 2013). Mixed Stock Analysis (MSA) using mtDNA markers has shown that foraging aggregations of loggerheads are often composed of turtles from multiple breeding populations (Engstrom et al., 2002; Witzell et al., 2002; Bowen et al., 2004; Jensen et al., 2013). In this study, MSA was conducted at Cape Muroto to determine the genetic composition of the bycaught turtles to better understand the natal origin of coastal migrating and foraging juvenile and adult loggerheads. This knowledge will lead to improved opportunities for conservation of North Pacific loggerhead turtles from those specific MUs that are most impacted.
2 Methods
2.1 Field sampling and data collection
Loggerhead turtles incidentally caught in pound nets operating in Muroto, Kochi, Japan, were retrieved, measured for Straight Carapace Length (SCL), tagged and released as described by Ishihara et al. (2011). Tissue samples were collected from tag punches obtained while applying flipper tags to live turtles and small piece of muscle collected from dead turtles. Tissue samples were preserved in 99% ethanol at room temperature. A total of 166 samples were collected in 2005, 242 in 2006, 138 in 2008, 178 in 2009 and 127 in 2010. Due to budget constraints, 100 samples were selected randomly for each of the five years for the genetic analysis.
2.2 Laboratory analysis
Samples were processed and sequenced by genetic service laboratories, Leaveanest Co., Ltd., in Tokyo, Japan, Biotechnology Center, Akita Prefectural University, in Akita, Japan and Laboratory of Systematic Zoology, Graduate School of Science, Kyoto University, in Kyoto, Japan. Primers LCM-15382 and H950g (Abreu-Grobois et al., 2006) were used to generate approximately 820bp of mtDNA sequence. Sequences (both forward and reverse fragments) were aligned against a local reference library of ~800 bp haplotype sequences using Geneious Pro 6.0.2 (Drummond et al., 2011). The alignment of each mtDNA segment was checked and edited manually and haplotypes assigned following nomenclature in Matsuzawa et al. (2016).
2.3 Statistical analysis
We tested for differences in haplotype frequencies for various temporal and life history strata as follows: between years, between life history stages and within season combined across years. We used AMOVA as implemented in Arlequin v 3.5.1.2 (Excoffier and Lischer, 2010). We classified turtles into one of the following three life history stages; juvenile, representing the younger juveniles without any visually detectable sexual differentiation; subadults, representing the older juveniles characterized by the presence of secondary sexual characteristics, but prior to maturity (pubescent); and adult, representing fully mature turtles. For the North Pacific loggerhead populations, SCL thresholds for puberty and maturity are shown to be 66.0 cm and 82.1 cm (Ishihara and Kamezaki, 2011), respectively. We therefore regarded turtles with SCL ≤ 66.0 cm as juvenile, 66.1–82.1 cm as subadult, and SCL ≥ 82.2 cm as adult.
We conducted MSA to estimate nesting MU contributions to the Muroto foraging aggregation using Bayesian approaches incorporating Markov Chain Monte Carlo (MCMC) methods implemented in the program BAYES (Pella and Masuda, 2001). Haplotype frequencies for the three MUs (Table 1; Ryukyu: n=147, Yakushima Island: N=108, and Mainland: n=301) identified by Matsuzawa et al. (2016) were used as the baseline for potential source nesting stocks in the MSA. BAYES was run using three models: (1) unweighted priors where the source nesting stocks were treated with equal weightings; and weighted priors, which take into account 2) the relative size and 3) distance of each MU (Figure 1) on the assumption that when genetic information is similar for rookeries, the larger or closer MU is more likely to contribute to the foraging aggregation than the smaller or more distant one. The use of weighted priors can be helpful when genetic diversity is weak, rookeries share common haplotypes that are widely distributed, and the relative size or distance of each rookery differs greatly (Bolker et al., 2007; LaCasella et al., 2013; Dutton et al., 2019). A total of six chains of 30,000 MCMC steps were run each with different starting points. A burn-in of 15,000 runs was used to calculate the posterior distribution. The Gelman and Rubin shrink factor diagnostic was computed to test that all chains had converged (Pella and Masuda, 2001).
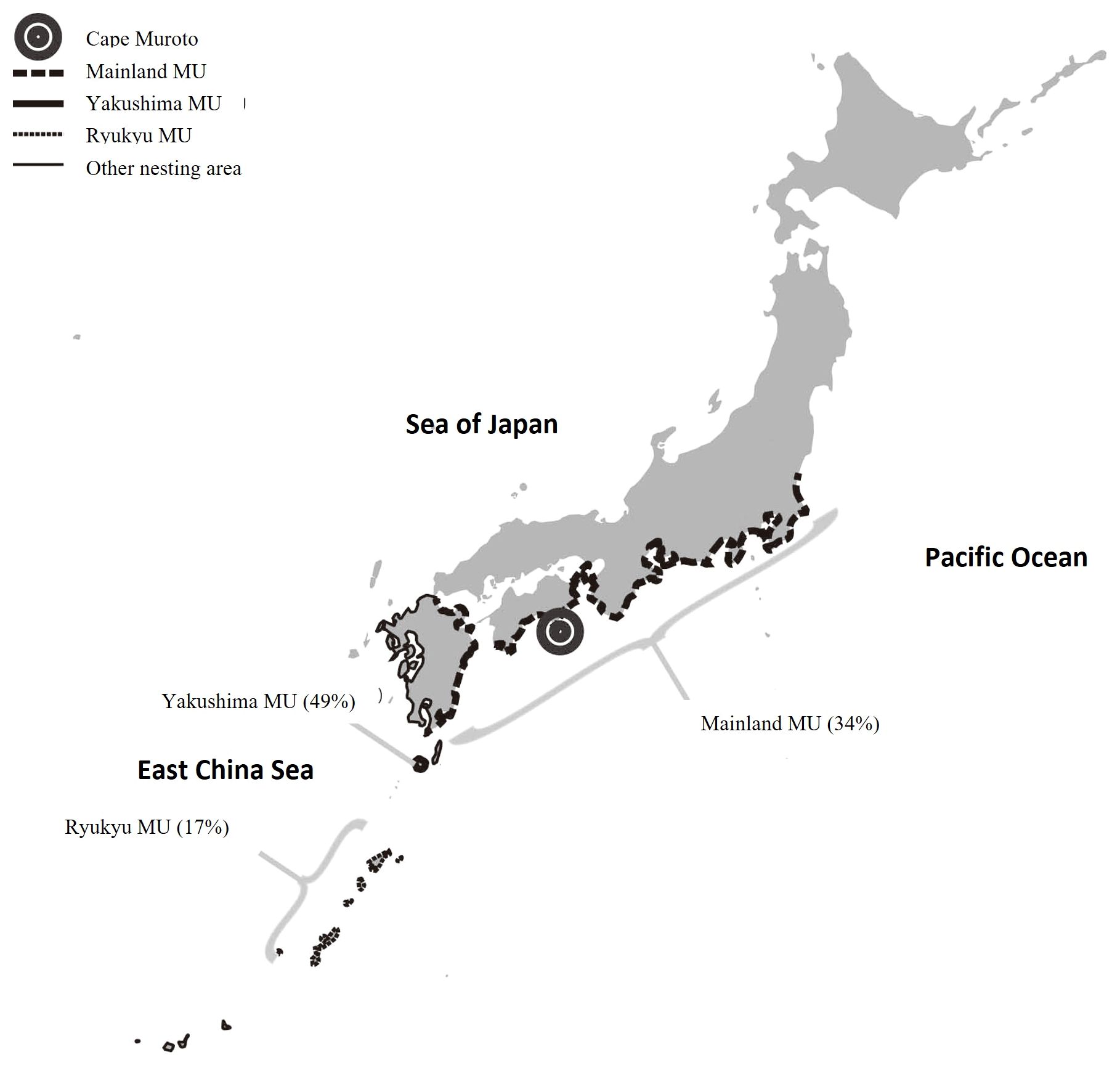
Figure 1 Map showing location of study site (Muroto pound net). Distribution of rookeries belong to 3 MU’s described by Matsuzawa et al. (2016) is shown with relative proportion (in parentheses) of total nesting from 2012 to 2014 (data compiled by Sea Turtle Association of Japan, unpublished).
3 Results
A total of 487 out of the 500 samples analyzed yielded sequences of sufficient quality to identify five different haplotypes, all of which have been previously identified among the Japanese nesting populations. Two haplotypes made up the majority consisting of CcP2.1 (66%), and CcP3.1 (16%) (Table 1). Sizes of the sampled turtles ranged from 56.3–99.1 cm SCL (mean 76.7cm +/- 68.3 SD), with 21 (4.5%) classified as juveniles, 348 as subadults (74.0%), and 101 as adults (21.5%) (Figure 2). The percentages by life stages reflected the tendency of this foraging area where Subadults were the most abundant. There was no significant difference (p>0.05, results not shown) between haplotype frequencies among the different size/age classes, nor among the different years (Appendix 2), therefore the haplotype frequencies were combined for the MSA (Table 1). Results of the MSA estimated that the majority (approx. 97%) of the turtles originated from Yakushima MU and Mainland MU, with minor estimated contribution from the Ryukyu MU (Tables 2, 3). The MSA estimates were similar for models using unweighted (Table 2) and weighted (Table 3) priors.
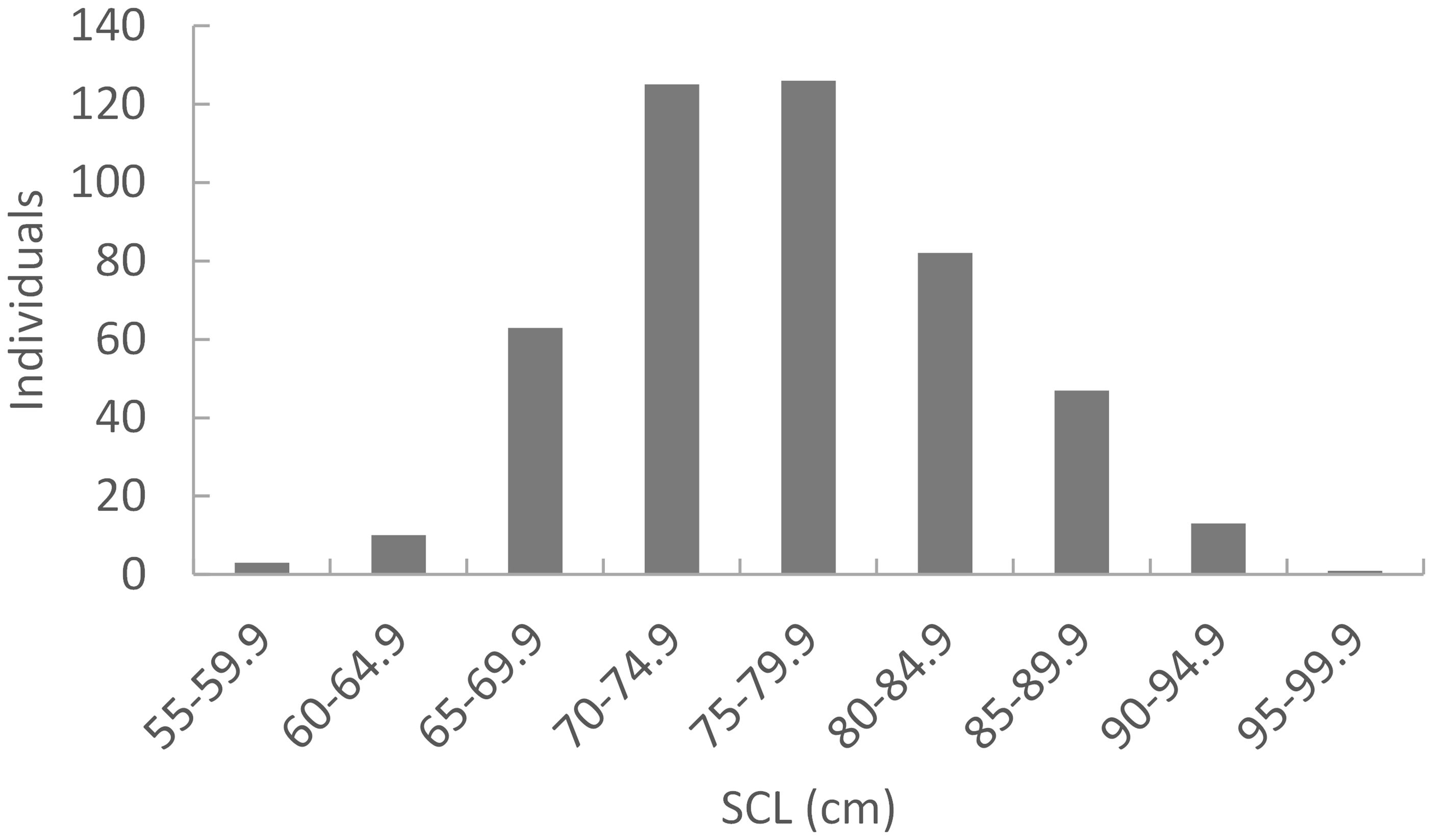
Figure 2 Size distribution of loggerhead turtle bycaught at large pound nets in Muroto, Shikoku, Japan (n=470). These individuals were randomly sampled and analyzed for mtDNA haplotype. Data adapted from Ishihara et al. (2011).
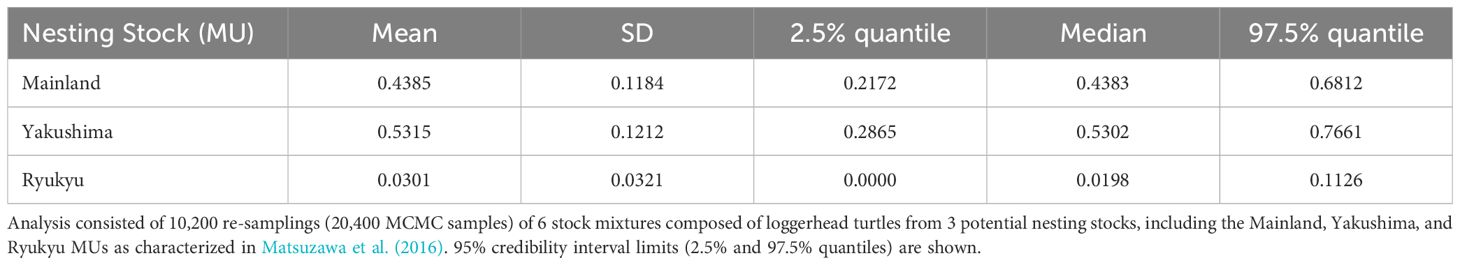
Table 2 Mean estimated stock mixtures of loggerhead turtles captured around Cape Muroto, Japan using BAYES with unweighted priors (Pella and Masuda, 2001).
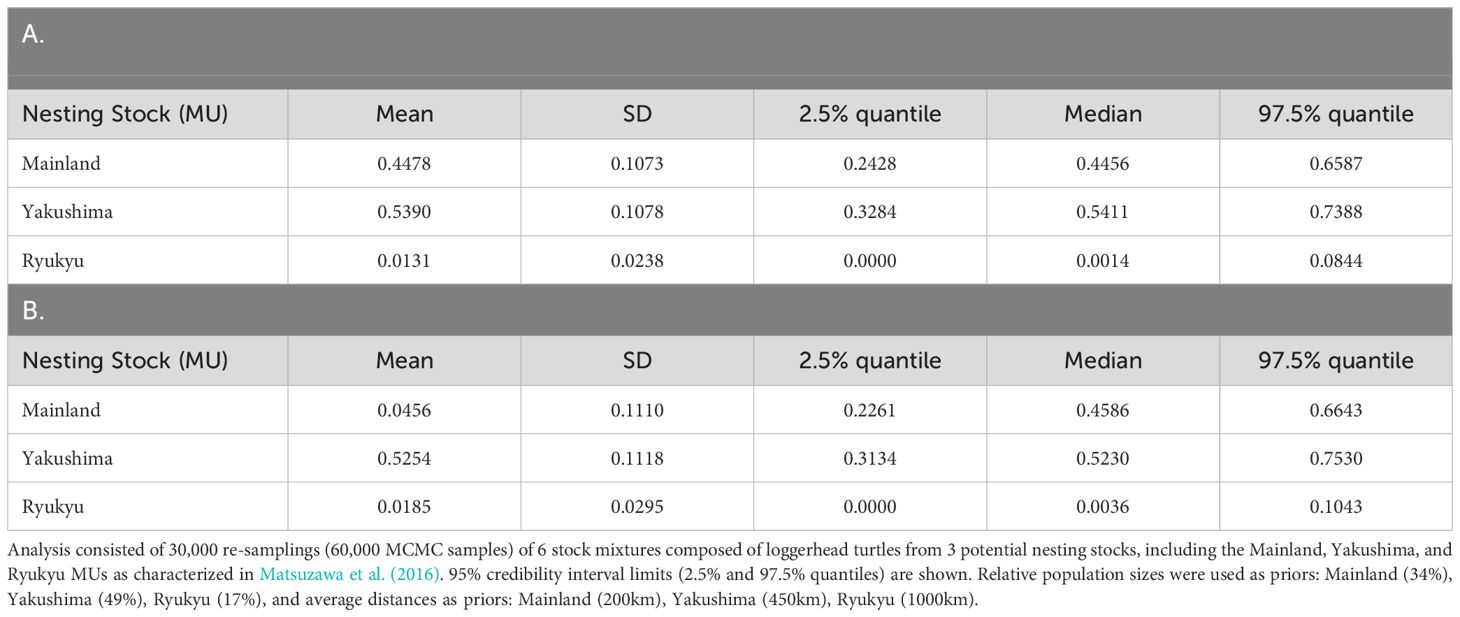
Table 3 Mean estimated stock mixtures of loggerhead turtles captured around Cape Muroto, Japan using BAYES with (A) weighted population size priors, and (B) weighted distance priors (Pella and Masuda, 2001).
4 Discussion
There was no discernable difference between the MSA estimates for the relative MU contributions to the Muroto foraging aggregation produced using unweighted and weighted priors. The point estimates should be interpreted with caution given the large credible interval (CI) around the mean, which is typical for mtDNA data that have several shared common haplotypes (Jensen et al., 2013). Nevertheless, the consistency between the models indicate that the relative patterns are informative.
The Muroto aggregation was found to be almost entirely from the Yakushima and Mainland MUs, with a higher contribution from Yakushima. The Ryukyu MU was under-represented in the Muroto foraging aggregation compared to the relative population size. The nesting population sizes of Ryukyu, Yakushima, and Mainland MUs represents 17, 49, and 34% of the North Pacific loggerhead population, respectively, whereas their contributions to the Muroto foraging aggregation were estimated at 3% (CI 0-11%), 53% (CI 29-77%) and 44% (CI 22-68%), respectively. The difference in the proportion of nesting population size and contribution to the foraging aggregation appears to be related to the distance from the nesting beaches. In contrast, the Sanriku foraging aggregation, a higher latitude feeding area, was found to have a greater proportion of turtles from more distant nesting rookeries than the closer nesting rookeries, whether or not the nesting population size is taken into account (Nishizawa et al., 2014). However, the rookery baseline dataset in Nishizawa et al. (2014) was limited to five potential source populations, which did not include most of the Mainland MU and all of the Ryukyu MU in Matsuzawa et al. (2016), so the studies may not be directly comparable. The three nesting sites that Nishizawa et al. (2014) grouped into distant rookeries are split into two in the Mainland MU and one in the Yakushima MU in this study. The contribution of the Yakushima nesting site alone to the Sanriku aggregation is 72.93% (95% CI = 13.43 to 99.98%) or 51.45% (95% CI = 0.05 to 98.61%) (Nishizawa et al., 2014), which is not inconsistent with the results of this study. This may be due to the fact that most of the Mainland MU and all of the Ryukyu MU were not included in the nesting population baseline used for the MSA by Nishizawa et al. (2014).
The Ryukyu MU is the farthest from Muroto compared to the other two nesting areas, with Cape Muroto located 650–1400 km from the Ryukyu MU rookeries, 400–500 km from the Yakushima MU rookeries, and in the vicinity of the Mainland MU rookeries. The results of this study show that the relative contributions of the Mainland MU rookeries were higher than those from the Ryukyu MU rookeries. This general pattern of foraging preferentially closer to their respective natal rookery locations is consistent with findings for loggerheads in the Northwest Atlantic (Sears et al., 1995; Bowen et al., 2004) and now referred to as natal foraging philopatry also found in hawksbill and green turtles (Gaos et al., 2017).
No significant difference in haplotype frequencies were found among adult males and females. In addition, the months of adult presence at Muroto also shows no significant difference between 38 males and 63 females in this study (p>0.05, Kolmogorov-Smirnov test). The appearance of these adults coincided with the reproductive season, suggesting that they were on their reproductive migration. Our results suggest that these migrating adults for breeding represent a mixed stock of the multiple MUs that might engage in opportunistic mating on their way through the Muroto area. This is consistent with findings by Watanabe et al. (2011), of lack of nDNA (microsatellite) differentiation between Japanese loggerhead nesting sites that show mtDNA structure, and implies connectivity facilitated by male-mediated gene flow. Although mating usually takes place near the nesting beaches with mtDNA indicating natal homing by both males and females, overlap between reproductively active males and receptive females from different MUs at nearby migratory corridors is a likely means for male-mediated gene flow in loggerheads (Bowen et al., 2005; Carreras et al., 2007; Watanabe et al., 2011). Further studies focusing on male reproductive migration and genomic DNA are needed to investigate consequences of opportunistic mating in the Muroto migratory corridor to gene flow among the Japanese MUs.
Similarly, there were no significant differences in haplotype frequency by life stage, sampling year, season, and SCL, although CI range of expected MUs was large. Although it is difficult to draw definitive conclusions given the short timeframe, this suggests that general mixed stock composition of the foraging aggregation in Cape Muroto may remain relatively consistent and turtles in the aggregation maintain their foraging areas as they grow, reach sexual maturity, and as generations turn over. While this result does not reject the hypothesis that there is a shift in migration route and/or foraging ground, a significant difference would be expected in life-stage stock composition if loggerheads shift foraging grounds at sexual maturity. Similarly, significant differences would be observed in sampling year and SCL if migration route and foraging ground are determined by passive factors such as oceanographic conditions, and in season if seasonal change of carapace length is based on shifts of prey resources. Despite lack of long-term data assessing temporal variation and foraging areas, one recent study reported a shift in the composition of the natal rookeries over 20 years for a green turtle foraging aggregation in the Bahamas (Kynoch et al., 2022). Further genetic studies on loggerhead turtles in the North Pacific are warranted, including establishing Muroto as an foraging index site to monitor long-tern temporal and life-stage shifts in stock composition in conjunction with regular monitoring of the demographic trends at the respective natal rookeries to help clarify regional recruitment patterns, as has been done for green turtles in Australia (Jensen et al., 2018).
Based on the results of this study, selection probability of usage and appearance in the migration corridor by each nesting MU is not likely to dramatically change according to growth, sexual maturation, sex, and age. In the Northwest Atlantic, juveniles also showed homing migration to natal waters, however, the homing of juvenile is thought to be less precise than breeding adults (Bowen et al., 2004). There is no significant difference among adults, subadults, and juveniles in this study, thus we cannot conclude that is differences in foraging migration among the life stages of the North Pacific loggerhead turtle population.
Our finding that the loggerheads from the Ryukyu MU are not well represented off Cape Muroto suggests that the foraging grounds and migration routes associated with this MU may extend into the East China Sea and South China Sea. Some nesting females and pound net bycaught adults have been satellite-tracked from the Ryukyu archipelago, and the results indicate a tendency to migrate to the East China Sea (Oki et al., 2019; Okuyama et al., 2022). Although satellite telemetry data for subadult and juvenile loggerheads in the Ryukyu area are limited, there have been telemetry studies tracking movement of subadults or juveniles caught in Taiwanese waters (Kobayashi et al., 2011). Turtles tagged in Taiwanese waters migrate to and are distributed across coastal and pelagic areas in the East China Sea, South China Sea, Sea of Japan and the Pacific Ocean adjacent to Taiwan, China, South Korea and Japan, and their distribution “hot spot” is mainly located in East China Sea (Kobayashi et al., 2011). Haplotypes of these turtles have not been analyzed and the source MU has not been identified. However, if foraging aggregations of loggerheads in waters around Taiwan are primarily linked to the Ryukyu MU, their movements support the hypothesis that the foraging ground and migration route selection relates to natal area even in juvenile and subadult life stages. Natal foraging philopatry (Gaos et al., 2017) of juveniles and subadults were found in the Northwest Atlantic population (Engstrom et al., 2002; Bowen et al., 2004). Based on these studies taken together with the results of our study, we would expect to find a higher proportion of Ryukyu origin turtles foraging around Ryukyu Islands. Further studies, including in other areas of the East China Sea, are warranted to determine whether juveniles and subadults in North Pacific move back from distant high seas areas across the Pacific toward their natal areas.
The lack of differences in haplotype frequency among adults, subadults and juveniles could be explained if the animals of multiple life stages from all three MUs use the Muroto foraging area. Due to the absence of a continental shelf at the Pacific coast, there is no discreet benthic foraging habitat for adults and subadults to settle into or take up residence, as is typical in the Caribbean and Northwestern Atlantic (Engstrom et al., 2002; Bowen et al., 2004). In addition, pelagic juveniles may also occur in the nearshore areas off Japan. Given the regional topography and oceanography around Japan, we hypothesize that the feeding ecology of the foraging loggerhead aggregation represented in our study is more dynamic and adapted to following patches of food depending on local oceanographic conditions. One would expect turtles to move periodically from nearshore to offshore areas, perhaps looping out and back as they follow productive convergence zones around local eddies similar to that described for high seas regions (Polovina et al., 2000, 2004, 2006). This model contrasts that for the Northwestern Atlantic, where loggerhead subadults settle into benthic habitat near their natal beaches (Bowen et al., 2004).
5 Conservation implications
Our results establish the connectivity between the Muroto foraging area and specific MUs, primarily the nearby nesting areas in the Mainland MU, and the Yakushima Island MU. This information enables a more nuanced approach for conducting population and threats assessments that can incorporate nesting population monitoring with at-sea assessments for a more holistic conservation framework (Dutton and Squires, 2011). A comprehensive accurate assessment of at-sea threats to North Pacific loggerhead population will need to account for a broad and complex range of habitat across the entire North Pacific (Bowen et al., 1995; Conant et al., 2009; Bolten et al., 2011). However, our results provide some notable insights of regional relevance to the long term nesting beach conservation efforts. From the perspective of nesting trends in each MU, while the sharp decline in the 1990s was constant in all MUs for the whole of Japan (Kamezaki et al., 2003), the recovery trend through 2013 was mainly in the Yakushima MU and in geographically closer rookeries of the Mainland MU and not in the more distant northern rookeries (Kamezaki and Taniguchi, 2012). This suggests that factors affecting reproductive rates such as mortality, growth and sexual maturity are also different among the MUs. For example, nesting trend data for rookeries in the Yakushima MU, show a surge from 342 nests in 1988 to 2,268 nests in 2008 at Inakahama Beach, and a rapid increase at Maehama Beach from 198 nests in 1999 to 1,609 nests in 2008 (Yakushima Umigame Kan, 2011). These increasing trends continued after 2010 in Yakushima (Yakushima Umigame Kan, 2015), and contributed to a change in the relative sizes of the MUs. The proportion of Yakushima nesting in relation to all MUs increased from 33.9-40.0% in 1998-2001 to 43.0-53.5% in 2006-2009 (Yakushima Umigame Kan, 2011). Mitigating threats from pound nets to the survival of loggerheads, particularly the mature nesters of highest reproductive value (Wallace et al., 2008), will continue to enhance nesting beach conservation actions. Since most loggerheads in Muroto pound nets are released alive, this ensures that new nesters and remigrants are able to continue to lay nests each season. However, most pound nets in Japan and other countries in the region, are submerged and associated with morality from drowning, and the potential cumulative impacts are unknown.
Our results show that the relative nesting population size of the Yakushima MU is generally reflected by the MSA contribution estimates for this MU across all life history stages at Muroto. Our study provides a basis for establishing Muroto waters as an index in-water monitoring site for assessing changes in relative abundance of juvenile and adult turtles from the different Regional MUs over the long-term.
Finally, environmental factors at foraging grounds will also influence nesting population trends and demographics, so understanding the characteristics of the Muroto ecosystem will be relevant to population assessments of the Yakushima and Mainland MUs. For Yakushima, previous studies have suggested that the difference in foraging ground and prey items (benthic prey in neritic foraging grounds or planktonic prey in oceanic foraging grounds) affect carapace length, clutch size, clutch frequency, breeding frequency, and remigration interval for nesting adult females (Hatase et al., 2013).
Therefore, the difference in nesting trend between the MUs may be related to the difference in environmental factors affecting reproduction and mortality. The only loggerhead foraging areas in the North Pacific MSA has been examined are Sanriku (Nishizawa et al., 2014) and Cape Muroto (this study). Further studies are needed to identify and address threats and the extent of impacts specific to each of the different foraging areas used by the North Pacific loggerhead MUs. Additional fine-scale analysis within the currently defined MUs may be necessary using genomic wide analysis with multiple markers (Carreras et al., 2007; Hamabata et al., 2020; Silver-Gorges, 2023), given that nesting trends vary within each MU, especially at Mainland MU nesting beaches. Further studies are necessary to gather information about biological aspects of each foraging ground and migration route, as well as connectivity between multiple foraging grounds.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because specimens were caught as bycatch in the fishery. They were surveyed and sampled before release. They were treated as carefully as possible on board and in port.
Author contributions
TI: Investigation, Project administration, Writing – original draft, Writing – review & editing. NK: Funding acquisition, Project administration, Supervision, Writing – review & editing. SH: Investigation, Writing – review & editing. YM: Funding acquisition, Supervision, Writing – review & editing. TH: Data curation, Methodology, Writing – review & editing. AI: Conceptualization, Funding acquisition, Writing – review & editing. PD: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by the Western Pacific Regional Fishery Management Council under Contract No.: 10-turtle-011, Sea Turtle Association of Japan, and NOAA-Fisheries Southwest Fisheries Science Center.
Acknowledgments
Tissue samples were collected with the great cooperation of Muroto Research Station of Sea Turtle Association of Japan, Takaoka Oshiki Fishery Association, Shiina Oshiki Fishery Association, and Mitsu Oshiki Fishery Association. Especially, Suguru Yamashita, Kunihiko Ebisui, Ken Hashimoto, Yukio Yasuoka, Chiaki Ebisui, Futoshi Iwamoto, Takao Nakamura, Kaname Kusamkabe, Hiroki Tanaka, Koji Saito, Mihoko Hara, Mina Matsumoto, Kizashi Mori, Kei Okamoto, Akane Abe, Hiromasa Mizuno, and Takeshi Nakazatomi, provided great contributions. Michael Jensen and Erin LaCasella provided helpful input to earlier drafts of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2024.1303553/full#supplementary-material
References
Abreu-Grobois A., Horrocks J., Formia A., Dutton P., LeRoux R., Velez-Zuazo X., et al. (2006). “New mtDNA dloop primers which work for a variety of marine turtle species may increase the resolution of mixed stock analyses. Abstract retrieved from the Book of Abstracts,” In: Frick M, Panagopoulou A, Rees AF, Williams K (eds) Proc. 26th Annu. Symp. Sea Turtle Biol. and Conserv., Crete, 3–8 April 2006. International Sea Turtle Society, Athens, p 179.
Bjorndal K. A., Bolten A. B., Martins H. R. (2000). Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of pelagic stage. Mar. Ecol. Prog. Ser. 202, 265–272. doi: 10.3354/meps202265
Bolker B. M., Okayama T., Bjorndal K. A., Bolten A. B. (2007). Incorporating multiple mixed stocks in mixed stock analysis: ‘many-to-many’ analyses. Mol. Ecol. 16, 685–695. doi: 10.1111/j.1365-294X.2006.03161.x
Bolten A. B., Bjorndal K. A., Martins H. R., Dellinger T., Biscoito M. J., Encalada S. E., et al. (1998). Transatlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analysis. Ecol. Appl. 8, 1–7. doi: 10.1890/1051-0761(1998)008[0001:TDMOLS]2.0.CO;2
Bolten A. B., Crowder L. B., Dodd M. G., MacPherson S. L., Musick J. A., Schroeder B. A., et al. (2011). Quantifying multiple threats to endangered species: an example from loggerhead sea turtles. Front. Ecol. Environ. 9, 255–308. doi: 10.1890/090126
Bowen B., Avise J. C., Richardson J. I., Meylan A. B., Margaritoulis D., Hopkins-Murphy S. R. (1993). Population structure of loggerhead turtles (Caretta caretta) in the northwestern Atlantic Ocean and Mediterranean Sea. Conserv. Biol. 7, 834–844. doi: 10.1046/j.1523-1739.1993.740834.x
Bowen B. W., Abreu-Grobois F. A., Balazs G. H., Kamezaki N., Limpus C. J., Ferl R. J. (1995). Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl. Acad. Sci. U.S.A. 92, 3731–3734. doi: 10.1073/pnas.92.9.3731
Bowen B. W., Bass A. L., Chow S. M., Bostrom M., Bjorndal K. A., Bolten A. B., et al. (2004). Natal homing in juvenile loggerhead turtles (Caretta caretta). Mol. Ecol. 13, 3797–3808. doi: 10.1111/j.1365-294X.2004.02356.x
Bowen B. W., Bass A. L., Soares L., Toonen R. J. (2005). Conservation implications of complex population structure: lessons from the loggerhead turtle (Caretta caretta). Mol. Ecol. 14, 2389–2402. doi: 10.1111/j.1365-294X.2005.02598.x
Bowen B. W., Karl S. A. (2007). Population genetics and phylogeography of sea turtles. Mol. Ecol. 16, 4886–4907. doi: 10.1111/j.1365-294X.2007.03542.x
Boyle M. C., FitzSimmons N. N., Limpus C. J., Kelez S., Velez-Zuazo X., Waycott M. (2009). Evidence for transoceanic migrations by loggerhead sea turtles in the southern Pacific Ocean. Proc. R. Soc B 276, 1993–1999. doi: 10.1098/rspb.2008.1931
Carreras C., Pascual M., Cardona L., Aguilar A., Margaritoulis D., Rees A., et al. (2007). The genetic structure of the loggerhead sea turtle (Caretta caretta) in the Mediterranean as revealed by nuclear and mitochondrial DNA and its conservation implications. Conserv. Genet. 8, 761–775. doi: 10.1007/s10592-006-9224-8
Conant T. A., Dutton P. H., Eguchi T., Epperly S. P., Fahy C. C., Godfrey M. H., et al. (2009). Loggerhead sea turtle (Caretta caretta) 2009 status review under the US Endangered Species Act. Rep. Loggerhead Biol. Rev. Team to Natl. Mar. Fish. Serv. Available online at: https://repository.library.noaa.gov/view/noaa/16204.
Drummond A. J., Ashton B., Buxton S., Al. E. (2011). Geneious v.5.4. Available online at: www.geneious.com/.
Dutton P. H., LeRoux R. A., LaCasella E. L., Seminoff J. A., Eguchi T., Dutton D. L. (2019). Genetic analysis and satellite tracking reveal origin of the green turtles in San Diego bay. Mar. Biol. 166, 1–13. doi: 10.1007/s00227-018-3446-4
Dutton P. H., Squires D. (2011). “A holistic strategy for Pacific sea turtle conservation,” in Conservation of Pacific Sea Turtles. Eds. Dutton P. H., Squires D., Ahmed M. (University of Hawaii Press, Honolulu, HI), 37–59. doi: 10.1515/9780824860196-005
Encalada S. E., Bjorndal K. A., Bolten A. B., Zurita J. C., Schroeder B., Possardt E., et al. (1998). Population structure of loggerhead turtle (Caretta caretta) nesting colonies in the Atlantic and Mediterranean as inferred from mitochondrial DNA control region sequences. Mar. Biol. 130, 567–575. doi: 10.1007/s002270050278
Engstrom T. N., Meylan P. A., Meylan A. B. (2002). Origin of juvenile loggerhead turtles (Caretta caretta) in a tropical developmental habitat in Caribbean Panama. Anim. Conserv. 5, 125–133. doi: 10.1017/S1367943002002184
Excoffier L., Lischer H. E. L. (2010). Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 10, 564–567. doi: 10.1111/j.1755-0998.2010.02847.x
Gaos A. R., Lewison R. L., Jensen M. P., Liles M. J., Henriquez A., Chavarria S., et al. (2017). Natal foraging philopatry in eastern Pacific hawksbill turtles. R. Soc Open Sci. 4, 4170153. doi: 10.1098/rsos.170153
Hamabata T., Matsuo A., Sato M. P., Kondo S., Kameda K., Kawazu I., et al. (2020). Natal origin identification of green turtles in the North Pacific by genome-wide population analysis with limited DNA samples. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00658
Hatase H., Kinoshita M., Bando T., Kamezaki N., Sato K., Matsuzawa Y., et al. (2002a). Population structure of loggerhead turtles, Caretta caretta, nesting in Japan: bottlenecks on the Pacific population. Mar. Biol. 141, 299–305. doi: 10.1007/s00227-002-0819-4
Hatase H., Omuta K., Tsukamoto K. (2007). Bottom or midwater: alternative foraging behaviors in adult female loggerhead sea turtles. J. Zool. 273, 46–55. doi: 10.1111/j.1469-7998.2007.00298.x
Hatase H., Omuta K., Tsukamoto K. (2010). Oceanic residents, neritic migrants: a possible mechanism underlying foraging dichotomy in adult female loggerhead turtles (Caretta caretta). Mar. Biol. 157, 1337–1342. doi: 10.1007/s00227-010-1413-9
Hatase H., Omuta K., Tsukamoto K. (2013). A mechanism that maintains alternative life histories in a loggerhead sea turtle population. Ecology 94, 2583–2594. doi: 10.1890/12-1588.1
Hatase H., Takai N., Matsuzawa Y., Sakamoto W., Omuta K., Goto K., et al. (2002b). Size-related differences in feeding habitat use of adult female loggerhead turtles Caretta caretta around Japan determined by stable isotope analyses and satellite telemetry. Mar. Ecol. Prog. Ser. 233, 273–281. doi: 10.3354/meps233273
Ishihara T., Kamezaki N. (2011). Size at maturity and tail elongation of loggerhead turtles (Caretta caretta) in the North Pacific. Chelonian Conserv. Biol. 10, 281–287. doi: 10.2744/CCB-0893.1
Ishihara T., Kamezaki N., Matsuzawa Y., Iwamoto F., Oshika T., Miyagata Y., et al. (2011). Reentery of juvenile and subadult loggerhead turtles into natal waters of Japan. Curr. Herpetol. 30, 63–68. doi: 10.5358/hsj.30.63
Jensen M. P., Allen C. D., Eguchi T., Bell I. P., LaCasella E. L., Hilton W. A., et al. (2018). Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 28, 154–159. doi: 10.1016/j.cub.2017.11.057
Jensen M. P., FitzSimmons N. N., Dutton P. H. (2013). “Molecular genetics of sea turtles,” in Biology of Sea Turtles, vol. Volume III . Eds. Wyneken J., Lohmann K. J., Musick J. A. (CRC Press, Boca Raton, FL), 135–161.
Kamezaki N., Goto K., Matsuzawa Y., Nakashima Y., Omuta K., Sato K. (1995). Carapace length and width of the loggerhead turtle, Caretta caretta, nested in the coast of Japan. Umigame Newsl. 26, 12–13.
Kamezaki N., Matsuzawa Y., Abe O., Asakawa H., Fujii T., Goto K., et al. (2003). “Loggerhead turtles nesting in Japan,” in Loggerhead Sea Turtles. Eds. Bolten A. B., Witherington B. E. (Smithsonian Books, Washington, D.C), 210–217.
Kamezaki N., Taniguchi M. (2012). “Nesting of sea turtles in Japan, (2012),” in Proceedings of 23rd Japanese Sea Turtle Symposium in Shibushi (Osaka: Sea Turtle Association of Japan), 25–26.
Kobayashi D., Cheng I. J., Parker D. M., Polovina J. J., Kamezaki N., Balazs G. H. (2011). Loggerhead turtle (Caretta caretta) movement off the coast of Taiwan: characterization of a hotspot in the East China Sea and investigation of mesoscale eddies. ICES J. Mar. Sci. 68, 707–718. doi: 10.1093/icesjms/fsq185
Kynoch C., Fuentes M. M. P. B., Dutton P. H., LaCasella E. L., Silver-Gorges I. (2022). Origins of juvenile green sea turtles (Chelonia mydas) in the Bahamas: A comparison of recent and historical rookery contributions. Ecol. Evol. 12, 1–12. doi: 10.1002/ece3.9548
LaCasella E., Epperly S., Jensen M., Stokes L., Dutton P. (2013). Genetic stock composition of loggerhead turtles Caretta caretta bycaught in the pelagic waters of the North Atlantic. Endanger. Species Res. 22, 73–84. doi: 10.3354/esr00535
Limpus C. J., Limpus D. J. (2003). “Biology of the loggerhead turtle in Western South Pacific Ocean foraging areas,” in Loggerhead Sea Turtles. Eds. Bolten A. B., Witherington B. E. (Smithsonian Books, Washington, D.C), 93–113.
Lohmann K., Lohmann C. M. F., Brothers J. R., Putman N. F. (2013). “Natal homing and imprinting in sea turtles,” in Biology of Sea Turtles, vol. Volume III . Eds. Wyneken J., Lohmann K. J., Musick J. A. (CRC Press, Boca Raton, Florida), 59–77.
Matsuzawa Y., Kamezaki N., Ishihara T., Omuta K., Takeshita H., Goto K., et al. (2016). Fine scale genetic population structure of loggerhead turtles in the Northwest Pacific. Endanger. Species Res. 30, 83–93. doi: 10.3354/esr00724
Moritz C. (1994). Defining ‘Evolutionarily significant units’ for conservation. Trends Ecol. Evol. 9, 373–375. doi: 10.1016/0169-5347(94)90057-4
Nishizawa H., Narazaki T., Fukuoka T., Sato K., Hamabata T., Kinoshita M., et al. (2014). Genetic composition of loggerhead turtle feeding aggregations: migration patterns in the North Pacific. Endanger. Species Res. 24, 85–93. doi: 10.3354/esr00588
Oki K., Hamabata T., Arata T., Parker D. M., Ng C. K. Y., Balazs G. H. (2019). Inferred adult foraging grounds of two marine turtle species nesting at Amami-oshima, Japan. Chelonian Conserv. Biol. 18, 91–97. doi: 10.2744/CCB-1337.1
Okuyama J., Watabe A., Takuma S., Tanaka K., Shirai K., Murakami-Sugihara N., et al. (2022). Latitudinal cline in the foraging dichotomy of loggerhead sea turtles reveals the importance of East China Sea for priority conservation. Divers. Distrib. 28, 1568–1581. doi: 10.1111/ddi.13531
Pella J., Masuda M. (2001). Bayesian methods for analysis of stock mixtures from genetic characters. Fish. Bull. 99, 151–167.
Polovina J. J., Balazs G. H., Howell E. A., Parker D. M., Seki M. P., Dutton P. H. (2004). Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish. Oceanogr. 13, 36–51. doi: 10.1046/j.1365-2419.2003.00270.x
Polovina J. J., Kobayashi D. R., Parker D. M., Seki M. P., Balazs G. H. (2000). Turtles on the edge: movement of loggerhead turtles (Caretta caretta) along oceanic fronts, spanning longline fishing grounds in the central North Pacific 1997-1998. Fish. Oceanogr. 9, 71–82. doi: 10.1046/j.1365-2419.2000.00123.x
Polovina J., Uchida I., Balazs G., Howell E., Parker D., Dutton P. (2006). The Kuroshio extension bifurcation region: a pelagic hotspot for juvenile loggerhead sea turtles. Deep. Res. 2 53, 326–339. doi: 10.1016/j.dsr2.2006.01.006
Sears C. J., Bowen B. W., Chapman R. W., Galloway S. B., Hopkins-Murphy S. R., Woodley C. M. (1995). Demographic composition of the feeding population of juvenile loggerhead sea turtles (Caretta caretta) off Charleston, South Carolina: evidence from mitochondrial DNA markers. Mar. Biol. 123, 869–874. doi: 10.1007/BF00349132
Silver-Gorges I. (2023). Habitat use and ecological connectivity in imperiled, highly migratory species: case studies with loggerhead sea turtles. Tallahassee, FL, USA: Florida State University, 134pp.
Wallace B. P., DiMatteo A. D., Hurley B. J., Finkbeiner E. M., Bolten A. B., Chaloupka M. Y., et al. (2010). Regional Management Units for marine turtles: a novel framework for conservation and research priorities across multiple scales. PloS One 5, e15465. doi: 10.1371/journal.pone.0015465
Wallace B. P., Heppell S. S., Lewison R. L., Kelez S., Crowder L. B. (2008). Impacts of fisheries bycatch on loggerhead turtles worldwide inferred from reproductive value analyses. J. Appl. Ecol. 45, 1076–1085. doi: 10.1111/j.1365-2664.2008.01507.x
Watanabe K. K., Hatase H., Kinoshita M., Omuta K., Bando T., Kamezaki N., et al. (2011). Population structure of the loggerhead turtle, Caretta caretta, a large marine carnivore that exhibits alternative foraging behaviors. Mar. Ecol. Prog. Ser. 424, 273–283. doi: 10.3354/meps08989
Witzell W. N., Bass A. L., Bresette M. J., Singewald D. A., Gorham J. C. (2002). Origin of immature loggerhead sea turtles (Caretta caretta) at Hutchinson Island, Florida : evidence from mtDNA markers. Fish. Bull. 100, 624–631.
Yakushima Umigame-kan (2011). Report on Sea Turtle Research in Yakushima Island, (1985-2009) (Kagoshima: Yakushima Umigame-kan).
Keywords: mitochondrial DNA, natal rookery, foraging aggregation, mixed stock analysis, Caretta caretta
Citation: Ishihara T, Kamezaki N, Hirai S, Matsuzawa Y, Hamabata T, Ishizaki A and Dutton PH (2024) Genetic characteristics of loggerhead turtles in the coastal corridor of the North West Pacific, around the Cape Muroto, Japan. Front. Mar. Sci. 11:1303553. doi: 10.3389/fmars.2024.1303553
Received: 28 September 2023; Accepted: 20 June 2024;
Published: 05 July 2024.
Edited by:
Mark Meekan, University of Western Australia, AustraliaReviewed by:
Alexander Richard Gaos, Pacific Islands Fisheries Science Center, National Oceanic and Atmospheric Administration, United StatesNicolas James Pilcher, Marine Research Foundation, Malaysia
Copyright © 2024 Ishihara, Kamezaki, Hirai, Matsuzawa, Hamabata, Ishizaki and Dutton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Ishihara, dC1pc2hpaGFyYUBhcXVhbWVudC5jby5qcA==; Peter H. Dutton, cGV0ZXIuZHV0dG9uQG5vYWEuZ292
 Takashi Ishihara
Takashi Ishihara Naoki Kamezaki3
Naoki Kamezaki3 Tomoko Hamabata
Tomoko Hamabata Peter H. Dutton
Peter H. Dutton