- 1Department of Marine Ecology and Environment, Gangneung-Wonju National University, Gangneung, Republic of Korea
- 2Marine Environment Research Division, National Institute of Fisheries Science, Busan, Republic of Korea
- 3Department of Oceanography, Chonnam National University, Gwangju, Republic of Korea
- 4Dokdo Research Center, Korea Institute of Ocean Science and Technology, Uljin, Republic of Korea
The objective of this study was to characterize the trophic structure of fish assemblages on the coasts of offshore islands and the eastern mainland of the Korean Peninsula. We compared the seasonal variability in the trophic structure of fish assemblages between the coasts of two island sites (Ulleungdo and Dokdo) and one mainland site (Hupo), which are on a similar latitude. We analyzed the stable carbon and nitrogen isotope ratios (δ13C and δ15N) of fish assemblages during spring (April) and summer (August) 2021. No temporal differences in the isotope values of fish and basal resources (i.e., suspended particulate organic matter (SPOM)) were found over the sampling period at the Hupo site. In contrast, at the Ulleungdo and Dokdo sites, the fishes and SPOM showed seasonal differences in the δ13C and δ15N values between the two seasons. In particular, the fish δ15N values at the island sites were relatively higher in summer compared to those in spring, suggesting the seasonal variation in the food chains and/or trophic status between consumers and their dietary sources. These regional isotopic variations also result in differences in the seasonal tendencies of the isotopic niche parameters of fish assemblages between the mainland and island coasts. Such differences in the seasonal isotopic patterns of fish assemblages suggest a relatively substantial shift in the dietary resources available to fish consumers on island coasts compared to those on the mainland coast. Overall, our results suggest that fish assemblages in offshore island coasts have distinct seasonal variability in trophic characteristics in response to changing environmental conditions, including basal resources, compared with fish food webs on the mainland coast at similar latitudes.
1 Introduction
Ulleungdo and Dokdo are volcanic islands in the East Sea, located at the eastern extremity of the Korean peninsula. Because these islands are far from the mainland, they have contributed to preserving relatively pristine ecosystems with lower human impacts than more populated coastal areas. The coastal areas of Ulleungdo and Dokdo exhibit unique geological and geomorphological features, including rocky shores, kelp forests, and offshore reefs that provide diverse habitats and support a wide range of marine organisms (Ryu et al., 2012; Choi and Seong, 2021). In addition, these islands are known to have unique environmental and biological characteristics that are considerably different from those of mainland coastal regions at the same latitude (Kang et al., 2013; Kang et al., 2019; Chung et al., 2020; Kim et al., 2020). In particular, the coastal ecosystems of Ulleungdo and Dokdo are characterized by high biodiversity and are home to several endangered and endemic species (usually, marine invertebrates and plants) (Song et al., 2017; Kim and Yu, 2021; Kim et al., 2023). Thus, to understand the ecological features of these islands as biological hotspots, it is necessary to characterize the structure and function of marine ecosystems.
In general, island and reef topography is of great importance within marine ecosystems because it provides essential habitats and spawning grounds for marine organisms. The coastal zones adjacent to the islands are known to induce flow disturbances, such as upwelling and turbulence, which positively impact biological productivity owing to the influence of the island mass effect (Doty and Oguri, 1956; Gove et al., 2016). Changes in ocean currents around the island may alter the dynamics of organic matter and/or nutrients, which have significant implications for the quality of coastal waters and fluctuations in oceanic productivity (De Carlo et al., 2007). Furthermore, spatiotemporal variations in both biotic and abiotic factors in near-island ecosystems can propagate changes in the entire community structure, spanning from primary producers (e.g., micro- and macroalgae) to higher trophic levels (e.g., fish and marine mammals) through trophic cascades (Frank et al., 2005; Kortsch et al., 2015). Understanding the pathways and rates of organic matter transfer among different trophic levels can help researchers unravel the complex interactions and dependencies within the marine ecosystems around island waters (Polis and Hurd, 1996; Stanek et al., 2022). Accordingly, this knowledge is vital for managing and conserving marine resources, including fisheries and protected species, as well as for addressing broader environmental challenges such as climate change and biodiversity loss.
Stable isotope analysis is a valuable method for elucidating organic matter transfer through food webs in diverse aquatic ecosystems, including freshwater, estuaries, and oceans (Fry and Sherr, 1984; Peterson and Fry, 1987; Michener and Schell, 1994). This method offers several advantages for assessing consumer organisms by providing integrated information on the long-term assimilation of diets (Hobson and Sealy, 1991). In addition, it allows the elucidation of energy flow pathways with greater efficiency and less time investment compared to traditional techniques (i.e., stomach content analysis) for quantifying the dietary composition of consumer species (Boecklen et al., 2011). Specifically, stable carbon isotopes (δ13C) are utilized for tracing the origin of dietary sources for consumers, as they typically exhibit an enrichment of approximately 1‰ between prey and predator (Fry and Sherr, 1984; Layman et al., 2012). In contrast, stable nitrogen isotopes (δ15N) in consumer tissues tend to increase by 2−4‰ through isotopic fractionation from prey to predator, enabling assessment of the trophic position of predators (Post, 2002; McCutchan et al., 2003). Overall, these stable isotope ratios offer useful insights into trophic dynamics and the ecological roles of organisms within food webs, thereby providing a foundation for comprehensive ecological studies of marine ecosystems.
In this study, we assessed the spatial and temporal variability in the trophic structure of coastal fish assemblages in the East Sea of Korea by comparing the coastal regions of two islands, Ulleungdo and Dokdo, and the mainland, at similar latitudes. In general, spatial differences in a variety of oceanic factors, including currents, sea level, temperature, salinity, wind conditions, upwelling intensity, mixing layer thickness, and predator behavior, have the potential to significantly alter breeding habitats and food availability for fish species, consequently affecting the abundance of fish populations and their trophic interactions (Galarza et al., 2009; Riccialdelli et al., 2020). Accordingly, we hypothesized that divergent marine environmental conditions resulting from geographical dissimilarity between the mainland and island coasts due to the island mass effect would affect the trophic structure of fish assemblages. Our analysis involved examining the carbon and nitrogen isotope ratios of coastal fish assemblages encompassing three study sites in the East Sea during two distinct seasons. By evaluating these parameters, we attempted to elucidate the potential effects of temporal and spatial factors on the trophic dynamics of coastal fish assemblages in unique island regions.
2 Materials and methods
2.1 Study sites
The study was conducted at the Hupo site (St. H), located on the southwestern margin of the Ulleung Basin in the East Sea (EJS), and at the Ulleungdo (St. U) and Dokdo (St. D) islets situated in the central Ulleung Basin (Figure 1). The water depths at the sampling sites ranged from 60 to 120 m at Hupo and 20 to 30 m at Ulleungdo and Dokdo. The sampling site at Hupo is a continental margin that is 7–11 km wide and is known to be an upwelling area (Yoo and Park, 2009). Ulleungdo and Dokdo are islets 130 km and 80 km from the Korean Peninsula, respectively. Environmental conditions are generally affected by two currents, the East Korea Warm Current (EKWC) and the North Korea Cold Current (NKCC), which form subpolar fronts by seasonal expansion and contraction. The tidal amplitude in the Ulleung Basin is low (less than 40 cm) (Teague et al., 2005).
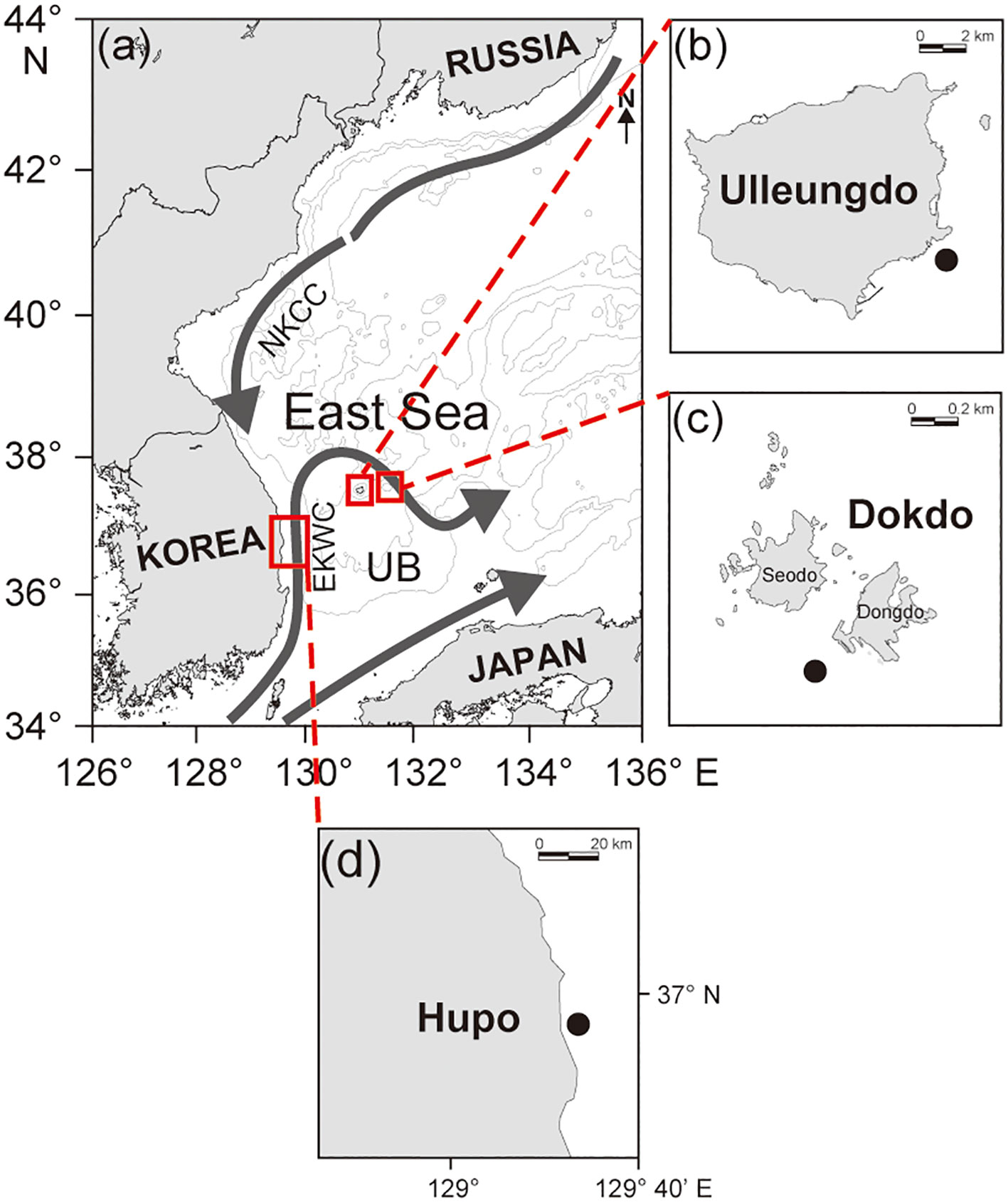
Figure 1 Map of the three sampling areas in the eastern part (A) of Korean peninsula. The sampling sites were located at the Ulleungdo (B) and Dokdo (C) sites in the central Ulleung Basin and the Hupo (D) site in the southwestern margin of the Ulleung Basin in the East Sea.
2.2 Sample collection and processing
Seawater, suspended particulate organic matter, and fish samples were collected in April (spring) and August (summer) 2021 using a commercial fishing boat. For the collection of suspended particulate organic matter (SPOM) samples, Aliquots of 20−40 L of surface water were collected at each sampling site using a van Dorn water sampler and then filtered using a 200-μm mesh net to avoid the possible remains of zooplankton and large particles. The pre-filtered water samples were filtered through a pre-combusted (4 h, 450°C) Whatman GF/F glass fiber filter (pore size: 0.7 μm) in the laboratory. The collected SPOM was acidified with 1 N HCl to eliminate inorganic carbonate content and oven-dried at 60°C for 24 h. The filter samples were preserved at −80°C until isotope analyses. Water samples for chlorophyll a (chl-a) concentration were filtered through Whatman GF/F glass fiber filters after removing large particles and zooplankton using a 200-μm mesh net and kept at −80°C. The sample collection of zooplankton was performed by oblique towing with a Bongo net (2.0 m2 mouth opening, 350 μm mesh). The zooplankton samples of calanoid copepods for the isotope analysis were identified and collected using a stereomicroscope.
All animal samples from the coastal sites of the mainland (Hupo) and islets (Ulleungdo and Dokdo) were collected using a coastal gillnet at a depth of 80 m. The fish specimens were immediately stored in an icebox and transferred to the laboratory. The specimens were identified to the lowest possible taxonomic level using an illustrated book on Korean fish (Kim et al., 2005). The size of individual specimens was measured as total length to the nearest 0.1 cm and weighed to the nearest 0.1 g. White muscle tissue was dissected from the anterior dorsal regions of fish specimens for stable isotope analysis. All fish samples were lyophilized for 72 h and ground into a homogeneous powder using a ball mill (MM200; Retsch GmbH, Haan, Germany).
2.3 Stable isotope analyses
Powdered samples (0.5–1 mg) were encapsulated into tin cups. The prepared filter samples were enclosed in tin disks for the stable isotope analysis. Subsequently, all samples were introduced into an automated CHN element analyzer (vario MICRO cube, Hanau, Germany) to combust at 1030°C. The carbon and nitrogen stable isotope ratios of the CO2 and N2 gases produced during combustion were examined using a continuous flow-through mass spectrometer (Isoprime CF-IRMS; Micromass, UK) connected to an elemental analyzer. The isotope abundances were expressed in delta (δ) notation and deviation in parts per 1000 (‰) relative to the differences between isotopic ratios of the sample and international standard material for carbon and nitrogen as follows: δX (‰) = [(Rsample/Rstandard) − 1] × 1000, where X is 13C or 15N and R is the corresponding proportions, 13C/12C or 15N/14N. Pee Dee Belemnite (PDB) and atmospheric N2 are the international standards for carbon and nitrogen, respectively. To calibrate the analysis results, International Atomic Energy Agency (IAEA) CH-6 (sucrose) and IAEA-N1 (ammonium sulfate) were used as international standard reference materials. The analytical precision of the analysis was approximately 0.2‰ for δ13C and 0.3‰ for δ15N, obtained by repeated measurements (> 20) of urea. Fish species generally contain high levels of lipids, which can lead to intraspecific differences in the concentration of 13C-depleted lipids, thereby causing a bias in their 13C values (Sweeting et al., 2006). Therefore, δ13C values of specimens with C/N ratios higher than 3.5 were lipid-normalized using the following equation of Post et al. (2007): δ13Cnormalized = δ13Cuntreated − 3.32 + 0.99 × C:N (ratios), where δ13C untreated is δ13C values of the not defatted sample. The TP for fish was calculated according to the equation: TPi = (δ15Ni − δ15Nbaseline)/Δ15N + 2, where δ15Ni represents the mean δ15N of the target species, δ15Nbaseline is the mean δ15N of trophic baseline consumers (i.e., calanoid copepods), Δ15N is the enrichment factor (3.4‰) in δ15N per TP, and 2 represents the baseline TP (Post, 2002).
2.4 Data analyses
Prior to statistical analyses, all data were tested for normality and homogeneity of variance using Shapiro–Wilk and Levene’s tests, respectively, using IBM SPSS software (ver. 21.0, IBM Corp., Armonk, NY, USA). Significant differences in the isotopic values of SPOM, zooplankton, and fish among the sampling sites and seasons were tested using a permutational multivariate analysis of variance (PERMANOVA). Two-way analysis of variance (ANOVA), followed by Tukey’s honest significant difference (HSD) multiple comparison post hoc test, were used to identify significant differences in the TP values of fish among sampling sites and seasons. The PERMANOVA test was conducted using PRIMER version 6 (PRIMER-e, Auckland, New Zealand) with the PERMANOVA + PRIMER add-on (PRIMER-e, Auckland, New Zealand).
Isotopic niche areas (‰2) of fish were compared between sites, seasons, and years using the package Stable Isotope Bayesian Ellipses in R (SIBER; Jackson et al., 2011) to assess the difference in their trophic pathways. Isotopic niche areas were compared using total area (TA) and small sample size-corrected standard ellipse area (SEAc), a quantitative proxy for the trophic diversity of consumer species, based on the spread and extent of isotopic data points (Layman et al., 2007; Newsome et al., 2012). The TA and SEAc were estimated to quantify the maximum potential overlap in the isotopic δ-space, considering uncertainty and biases by the occurrence of smaller sample sizes and errors due to the sampling processes (Jackson et al., 2011).
3 Results
3.1 Environmental conditions
The surface water temperatures were similar between the sampling areas (14.3°C–26.8°C in the Ulleungdo/Dokdo areas and 14.1°C–29.6°C in the Hupo area) (Table 1). The water temperatures at the bottom layer in the two island areas were relatively high (12.8°C in April and 16.5°C in August) compared to those in the Hupo area (7.1°C in April and 12.0°C in August). The salinities of the bottom water were very similar between the sampling areas and seasons, ranging from 34.2 (Hupo in April) to 34.5 (Ulleungdo/Dokdo in August). In contrast, the salinities of the surface water were relatively high in the Ulleungdo/Dokdo areas (32.5–34.7) compared to those in the Hupo area (31.7–34.3). The chlorophyll a concentrations of the surface water varied between 0.89 μg/L (Hupo) and 1.90 μg/L (Ulleungdo/Dokdo) in April and between 0.7 μg/L (Hupo) and 0.28 μg/L (Ulleungdo/Dokdo) in August.
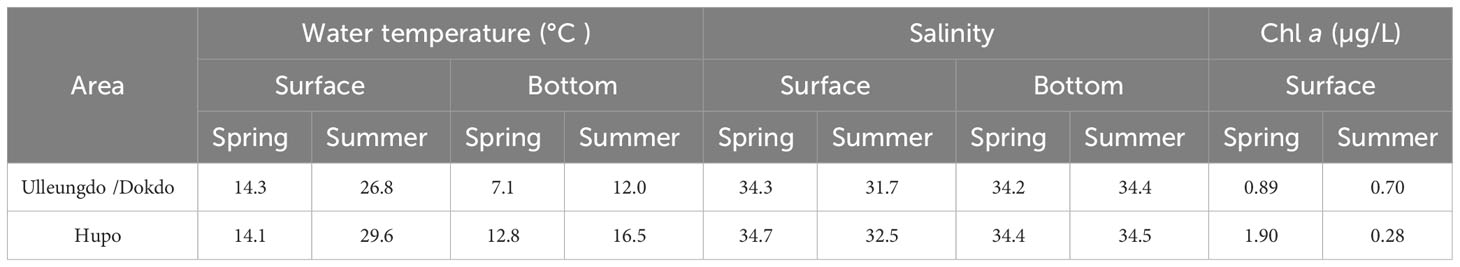
Table 1 The surface and bottom environmental conditions (water temperature, salinity, and chlorophyll a) in the Ulleungdo/Dokdo and Hupo areas located in the eastern part of the Korean peninsula during April (spring) and August (summer) 2021.
3.2 Stable isotope values of organic matter sources and zooplankton
The δ13C and δ15N values of the SPOM differed significantly among the three sampling sites (PERMANOVA, pseudo-F 2, 25 = 16.69, p = 0.001) and between the two seasons (pseudo-F 1, 25 = 39.77, p = 0.001), and a significant effect of the interaction term (site × season, pseudo-F 2, 25 = 5.12, p = 0.007) was observed (Table 2). The mean δ13C and δ15N values of SPOM at the three sites ranged from –22.1 ± 0.4‰ (Site D in April) to –21.1 ± 0.3‰ (Site H in August) and from 4.9 ± 0.4‰ (Site D in April) to 7.2 ± 0.4‰ (Site H in August), respectively. There were no significant differences in the δ13C and δ15N values for SPOM between the Ulleungdo and Dokdo sites (pseudo-F 1, 15 = 0.64, p = 0.546), whereas the isotope values differed significantly between the seasons (pseudo-F 1, 15 = 40.64, p = 0.001). In contrast, no significant difference in the isotopic values of SPOM at the Hupo site was found between seasons (pseudo-F 1, 9 = 0.83, p = 0.437).
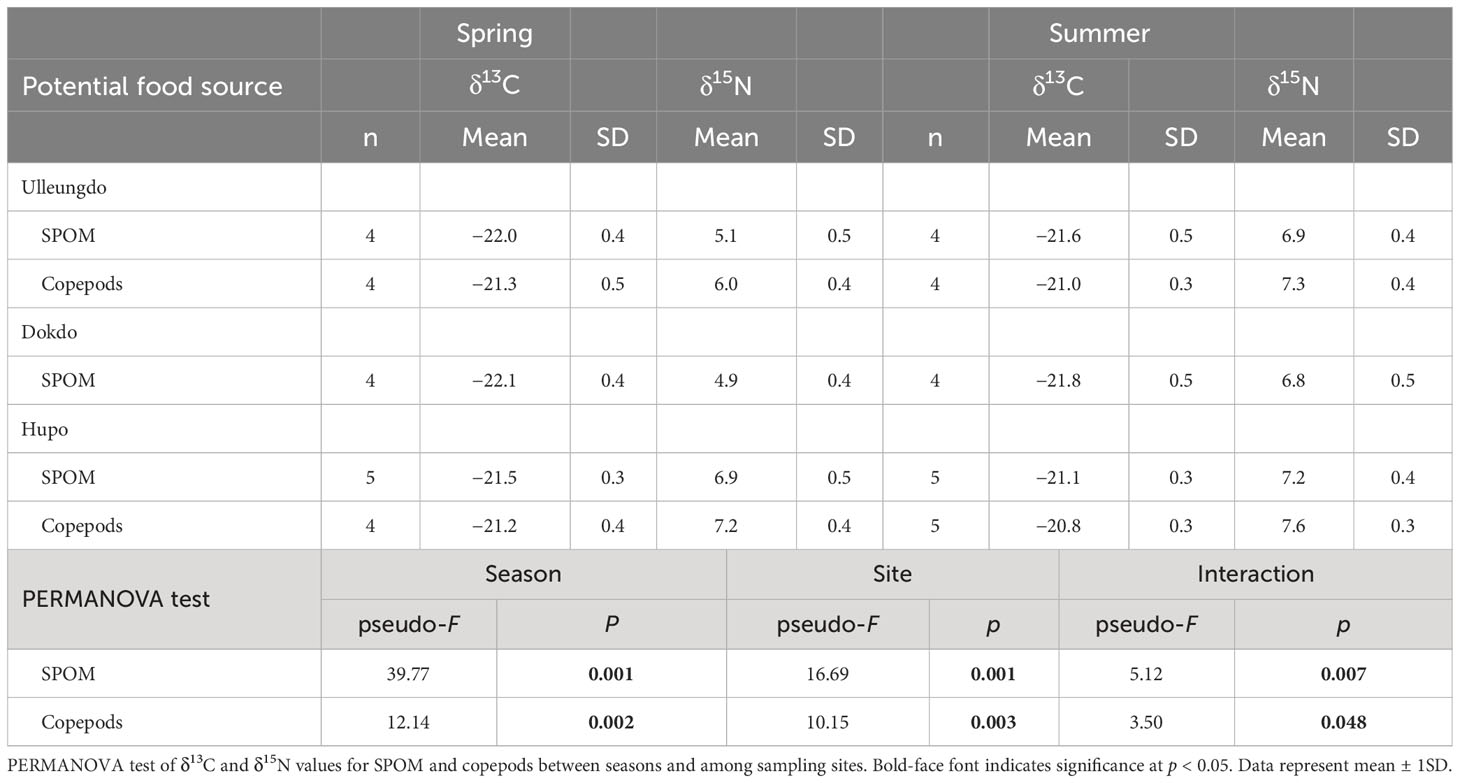
Table 2 δ13C and δ15N values of organic matter (SPOM, suspended particulate organic matter) and zooplankton (calanoid copepods) collected at the Ulleungdo, Dokdo, and Hupo sites located in the eastern part of the Korean peninsula during April (spring) and August (summer) 2021.
Significant differences in the δ13C and δ15N values of the copepods were found among the sampling sites (pseudo-F 1, 16 = 10.15, p = 0.003) and between seasons (pseudo-F 1, 16 = 12.14, p = 0.002), with a significant effect of the interaction term (site × season, pseudo-F 1, 16 = 3.50, p = 0.048). The overall mean δ13C and δ15N values for copepods ranged from –21.3 ± 0.5‰ (Site U in April) to –20.8 ± 0.3‰ (Site H in August) and 6.0 ± 0.4‰ (Site U in April) to 7.6 ± 0.3‰ (Site H in August).
3.3 Stable isotope values of fish
A total of 9 and 17, 13 and 11, and 13 and 8 fish species were collected at three sampling sites (Ulleungdo, Dokdo, and Hupo) in April and August, respectively. The rock fish (family Scorpaenidae) dominated at the Ulleungdo and Dokdo sites (five species), whereas flat fish (family Pleuronectidae) was the dominant group at the Hupo site (six species).
The δ13C and δ15N values of fish were significantly different among the three sampling sites (pseudo-F 2, 177 = 44.92, p = 0.001) and between the two seasons (pseudo-F 1, 177 = 188.92, p = 0.001), and significant effect of the interaction term (site × season, pseudo-F 2, 177 = 43.65, p = 0.001) was also found (Figure 2 and Tables 3 and 4). In contrast, no significant differences in δ13C and δ15N values were observed between the island areas (pseudo-F 1, 114 = 3.38, p = 0.072), whereas their isotope values varied significantly between seasons (pseudo-F 1, 114 = 247.25, p = 0.001). At the Ulleungdo and Dokdo sites, the mean δ13C values of fishes showed similar ranges from –19.4‰ to –16.9‰ and –20.0‰ to –18.2‰ in April and –19.8‰ to –16.7‰ and –19.9‰ to –16.3‰ in August, respectively. For the mean δ15N values, there was a similar seasonal pattern at both the Ulleungdo and Dokdo sites, which were relatively high in August (11.4‰ to 13.9‰ and 12.5‰ to 15.9‰, respectively) compared to those in April (7.6‰ to 10.9‰ and 7.4‰ to 10.8‰, respectively). In contrast, very similar ranges of the mean δ13C and δ15N values for fishes at the Hupo site were observed between the two seasons, from –20.3‰ to –16.5‰ and 11.3‰ to 14.7‰ in April and –19.6‰ to –17.1‰ and 11.5‰ to 15.8‰ in August, respectively.
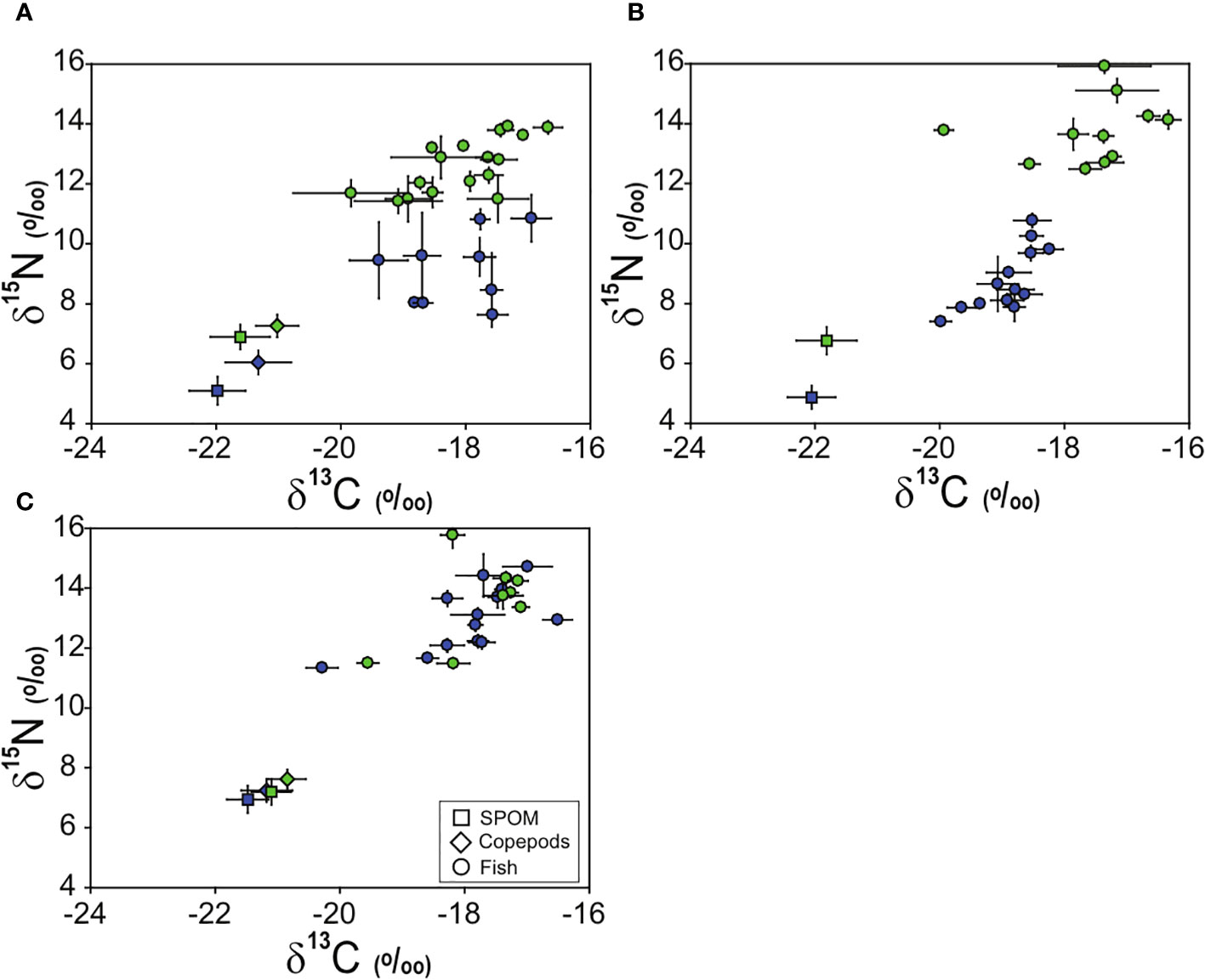
Figure 2 Dual isotope plots of δ13C and δ15N values of zooplankton and fish and their basal resource (suspended particulate organic matter, SPOM) at the Ulleungdo (A), Dokdo (B), and Hupo (C) sites in April (blue colors) and August (green colors) 2021. Values are presented as mean δ13C and δ15N (‰ ± 1 SD). Species codes indicate the fish consumers listed in Tables 3, 4.
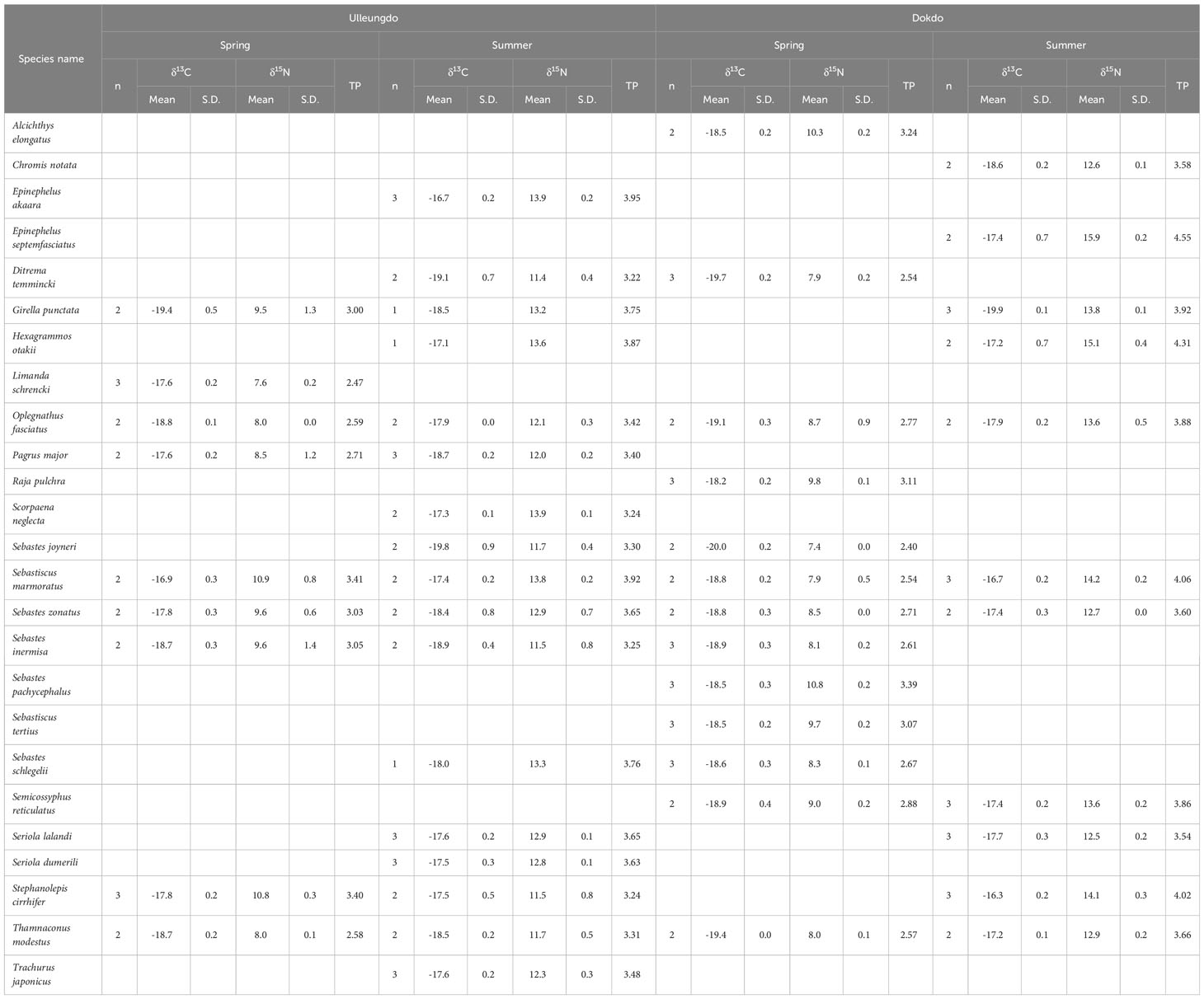
Table 3 δ13C and δ15N values and trophic position (TP) of fish assemblages collected at the Ulleungdo and Dokdo sites located in the eastern part of the Korean peninsula during April (spring) and August (summer) 2021. Data represent mean ± 1SD.
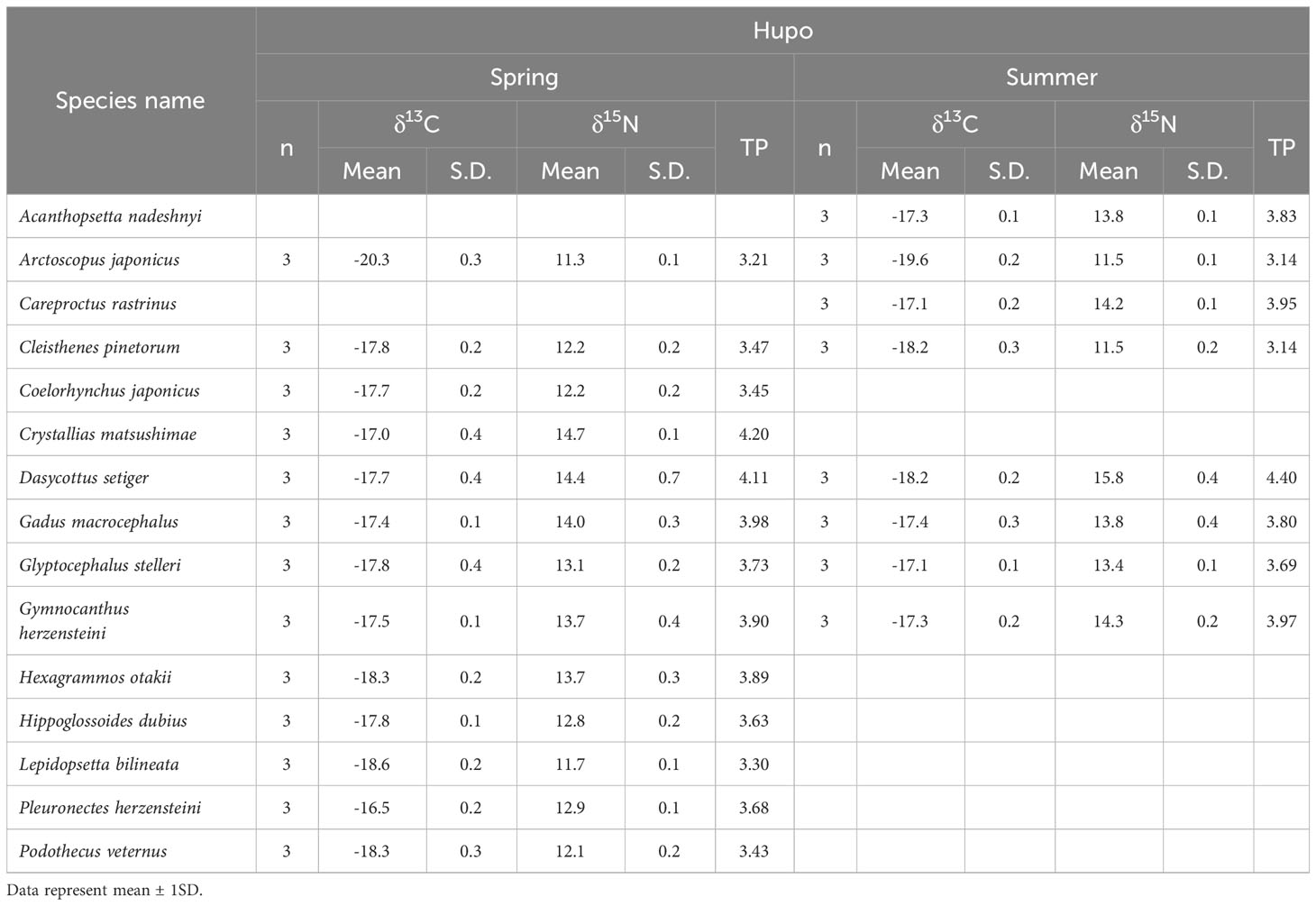
Table 4 δ13C and δ15N values and trophic position (TP) of fish assemblages collected at the Hupo site located in the eastern part of the Korean peninsula during April (spring) and August (summer) 2021.
3.4 Isotopic niche areas and trophic positions of fish
The isotopic niche areas of fish at the three sites during April and August were assessed using TA and SEAc values (Figure 3). The TA and SEAc values showed different seasonal patterns at Ulleungdo (8.78 and 3.63 in April and 6.75 and 1.97 in August, respectively) and Dokdo (3.67 and 1.23 in April and 11.02 and 3.96 in August, respectively) sites. In contrast, the TA and SEAc values of fish at the Hupo site were relatively similar between the two seasons (6.93 and 2.39 in April and 7.62 and 3.27 in August, respectively).
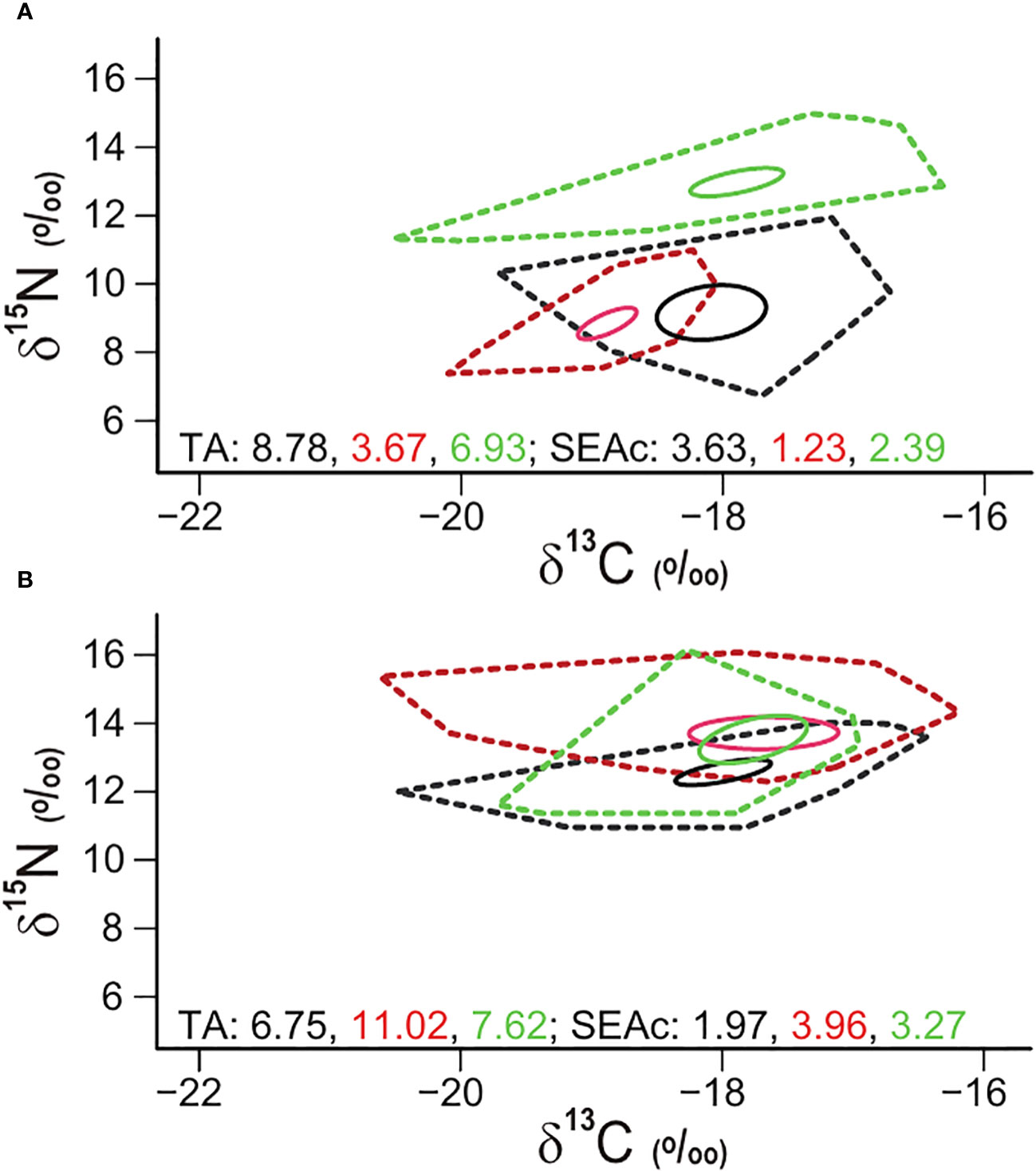
Figure 3 The isotopic niche areas (‰2) of fish assemblages collected at the Ulleungdo (black colors) and Dokdo (red colors) sites and the Hupo (green colors) site in April (A) and August (B) 2021 estimated as total area (TA, dotted line) and standard ellipse area (SEAc, solid line).
The calculated TP values of fish differed significantly among sites (Tukey’s HSD test, F2, 71 = 12.27, p = 0.01) and seasons (Tukey’s HSD test, F1, 71 = 58.78, p = 0.01) (Tables 3, 4). The fish TP values at the Ulleungdo and Dokdo sites were relatively high in August (3.22–3.96 and 3.54–4.55, respectively) compared to those in April (2.47–3.41 and 2.40–3.39, respectively). At the Hupo site, there were similar ranges in TP values between the two seasons (3.21–4.20 in April and 3.14–4.40 in August).
4 Discussion
Here, we describe the characteristics of fish trophic structure on the coasts of Ulleungdo and Dokdo by comparing the carbon and nitrogen stable isotope ratios of fish assemblages between the mainland coast and the island coast of the East Sea during two seasons. The results showed that the isotopic ratios and niches of fish assemblages on the island coasts showed more distinct seasonality than those on the land coast, with clear spatial differences in species composition. These results may be related to the specific oceanographic features of the regional island effects, leading to seasonal variations in prey availability and diet for fish consumers and their trophic interactions. Our study highlights the importance of stable isotope signatures in identifying temporal resource-use patterns of fish assemblages on offshore island coasts, which provide information on the fish trophic structure and functioning of marine ecosystems under unique environmental conditions.
4.1 Effect of environmental factors on fish community in the offshore islands
Ulleungdo and Dokdo are well known for their distinctive marine ecosystems, which are geographically far from mainland and have relatively little influence from human activities. Geographical effects may be reflected by a noticeable difference in fish composition between the mainland and island coasts. The composition of the fish species collected at the Hupo site was mainly resident and benthic temperate fish, such as Glyptocephalus stelleri and Gymnocanthus herzensteini, which is consistent with a previous report on the eastern coast of the Korean peninsula (Choi et al., 2012; Park et al., 2020a). The fish communities on the east coast of the Korean peninsula are characterized by a high proportion of the endemic and resident species (Sohn et al., 2015). In contrast, relatively few species of tropical and subtropical fish taxa, such as Thamnaconus modestus and Sebastes zonatus were found in assemblages collected from the Ulleungdo and Dokdo sites. The reef-associated fish species on these island coasts are a major group resulting from the geological traits of the rocky terrain (Chung et al., 2015). The geological type of bottom habitats can affect the species composition and diversity of fish assemblages (Gaertner et al., 1999; Farré et al., 2015). Considering that the bottom type of mainland coastal zones, including the sampling area, consists mainly of muddy sand and sand (Kim and Kim, 2001), such regional differences in species composition and taxonomy may be substantially influenced by differences in the bottom habitat environments. In contrast, physical factors such as water temperature and currents may also lead to contrasting species compositions of fish assemblages between areas. In particular, migratory fishes such as the genera Trachurus and Seriola on the coasts of Ulleungdo and Dokdo were likely influenced by the warm Tsushima Current, which is supported by the similarity of fish assemblages between the coastal areas of Dokdo and Jeju (Lee et al., 2010). Therefore, regional environmental variations in geological and physical factors may contribute to spatial differences in species composition of fish assemblages between the mainland and island coasts.
4.2 Food web characteristics in the offshore islands
In the present study, the δ13C and δ15N values for SPOM at all the sampling sites were certainly within previously reported isotopic ranges (–24 to –18‰ for δ13C and 2–10‰ for δ15N) of general marine phytoplankton and SPOM in coastal waters of the eastern Korean peninsula and other temperate regions (Fry and Sherr, 1984; Park et al., 2020a; Shin et al., 2022). These results indicate that organic matter derived from phytoplankton may be the greatest source of the SPOM pool on both the mainland and island coasts. However, our study showed the spatial differences in the δ13C and δ15N values for SPOM between the mainland and island coasts and also the dissimilar seasonal pattern. Although no temporal variation in the isotopic values of SPOM was observed at the Hupo site, the two island sites displayed significant seasonal differences. Isotopic ranges are generally influenced by physical/chemical (e.g., water temperature and availability of dissolved inorganic carbon and nitrogen) and biological (e.g., phytoplankton taxonomy and physiology) factors (Cifuentes et al., 1988; Goering et al., 1990; Kurle and McWhorter, 2017). Thus, such dissimilar patterns in spatial and seasonal variability in the isotopic values of SPOM suggest different environmental conditions between sampling areas. Furthermore, our results suggest that the spatial isotopic differences of SPOM as a trophic base can result in the difference from lower to higher trophic levels along the food chain between the mainland and island coasts.
Similarly, the stable isotope values of fish in our study showed different seasonal patterns between the mainland and island sites. No temporal differences in the isotope values of the fish were found during the sampling period at the Hupo site. These results are consistent with those of a previous study on the limited seasonal isotopic variations in resident fish in the Hupo coastal area during spring and summer (Park et al., 2020a). In the Hupo coastal area, the presence of migrating pelagic fishes, despite their relatively low abundance and species numbers, during specific seasons (i.e., fall and winter) has been reported to result in seasonal variation in the trophic structure of fish with changing environmental conditions (Park et al., 2020a). However, because our study was conducted during spring and summer and only benthic fishes were collected, we did not determine seasonal variations in the fish trophic structure within the community structure. It is worth noting that the δ15N values of fish at the Hupo site were relatively higher than those at the two island sites, suggesting the differences in the environmental conditions and, thus, trophic baselines (Goering et al., 1990; Sato et al., 2006). Moreover, anthropogenic effects may influence mainland coasts more than the offshore island coasts (Kim and Park, 2014). The δ15N of consumers generally increases with the human population density due to the high δ15N of sewage from anthropogenic activities (Cabana and Rasmussen, 1996; McClelland et al., 1997).
In contrast, at the Ulleungdo and Dokdo sites, the fish with a basal resource (i.e., SPOM) showed seasonal differences in the δ13C and δ15N values between spring and summer. In particular, their δ15N values were relatively higher in summer compared to those in spring, suggesting the seasonal variation in the food chains and/or trophic status between consumers and their dietary sources. In general, seasonal variability in the trophic structure of marine ecosystems is likely to be closely related to various environmental factors, resulting in the utilization of available resources and diversity of dietary items (Cresson et al., 2020; Park et al., 2020a). Complex trophic pathways may support food web structures involving diverse biotic and abiotic components within marine ecosystems. The interactions between environmental factors (especially water temperature and food availability) and organisms can have important effects on seasonal variability in the species composition and trophic structure of fish assemblages (Wilson and Sheaves, 2001). Moreover, the dynamics of the phytoplankton concentration and community as a basal resource can lead to changes in the trophic interactions between organisms and their trophic levels in marine ecosystems through bottom-up processes (Ullah et al., 2018; Park et al., 2020b). In this respect, the phytoplankton community around Dokdo Island has been reported to respond sensitively to ambient environmental conditions and thus shift seasonally from a high biomass of micro-sized phytoplankton in spring to a small biomass of nano-sized phytoplankton (more than 70% proportion) in summer (Lee et al., 2022). Because of the trophic importance of phytoplankton as a fundamental component of marine food webs, seasonal changes in the dominance of micro-phytoplankton over nano-phytoplankton can affect the dynamics of trophic structures on island coasts (Rolff, 2000; Park et al., 2020b). Therefore, the seasonal shift in the isotopic values of fish assemblages at the Ulleungdo and Dokdo sites may have been influenced by the alteration in the basal resources supporting the food web, which is related to the different oceanographic conditions between spring and summer.
4.3 Trophic niche characteristics in the offshore islands
The isotopic niche parameters of TA and SEAc have been used to elucidate the spatial and seasonal variability in the food web structure by altering prey-predator relationships due to ambient environmental changes (Layman et al., 2007; Abrantes et al., 2014; Park et al., 2020a). In our study, the differences in the seasonal tendencies of TA and SEAc of fish assemblages between the mainland and island coasts likely reflect the regional isotopic variation in the different prey-consumer relationships due to differing environmental conditions and food availability, as mentioned above (Cherel et al., 2007). Some studies have reported that spatial differences in isotopic niche variability occur due to the alteration of basal resources for fish consumers and their trophic relationships within the community under different environmental conditions (Kingsbury et al., 2020; Shin et al., 2022; Park et al., 2023). In addition, spatial environmental differences related to various physicochemical and biological factors can change fish components within a community, which may lead to regional distinctions in TA and SEAc (Krumsick and Fisher, 2019; Wang et al., 2021). Our results also suggested that clear spatial differences in fish composition between the mainland and island coasts may influence the different temporal patterns in isotopic niche areas. Accordingly, seasonal patterns in the isotopic niche indices likely result from regional differences in environmental conditions and nutritional resources supporting food webs, with a distinction in the species composition of fish assemblages.
In conclusion, our isotopic data on fish assemblages demonstrated differences in the seasonal pattern of the fish food web structure between the mainland and the offshore island coasts of the Korean Peninsula. Given that basal resources supporting fish food webs and environmental conditions showed seasonally different patterns between the sampling areas, the seasonal differences in the isotopic signatures and isotopic niche indices for fish assemblages suggest a relatively substantial shift in dietary resources available to fish on the island coasts compared to those on the mainland coast. Overall, our results suggest that fish assemblages in offshore island coasts have distinct seasonal variability in trophic characteristics in response to changing environmental conditions, including basal resources, compared with fish food webs on the mainland coast at similar latitudes. Further studies on annual and long-term variations in the trophic structure of fish assemblages based on stable isotope techniques, coupled with regular monitoring of environmental factors and fish communities, are needed to better understand the ecological functioning of unique island marine ecosystems.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by Ministry of Oceans and Fisheries. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
THP: Data curation, Methodology, Writing – original draft, Writing – review & editing. D-YL: Formal analysis, Investigation, Writing – review & editing. HYK: Data curation, Validation, Writing – review & editing. JMP: Investigation, Validation, Writing – review & editing. DK: Visualization, Writing – review & editing. HJP: Conceptualization, Project administration, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was part of the project titled “A sustainable research and development of Dokdo (PG52911),” funded by the Ministry of Oceans and Fisheries, Korea. This research was also supported by the National Research Foundation of Korea (NRF-2021R1A2C1012537) funded by Ministry of Science and ICT and the Korea Institute of Marine Science and Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries (20220214), Korea.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrantes K. G., Barnett A., Bouillon S. (2014). Stable isotope-based community metrics as a tool to identify patterns in food web structure in east African estuaries. Funct. Ecol. 28, 270–282. doi: 10.1111/1365-2435.12155
Boecklen W. J., Yarnes C. T., Cook B. A., James A. C. (2011). On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 42, 411–440. doi: 10.1146/annurev-ecolsys-102209-144726
Cabana G., Rasmussen J. B. (1996). Comparison of aquatic food chains using nitrogen isotopes. Proc. Nati. Acad. Sci. 93, 10844–10847. doi: 10.1073/pnas.93.20.10844
Cherel Y., Hobson K. A., Guinet C., Vanpe C. (2007). Stable isotopes document seasonal changes in trophic niches and winter foraging individual specialization in diving predators from the Southern Ocean. J. Anim. Ecol. 76, 826–836. doi: 10.1111/j.1365-2656.2007.01238.x
Choi K. H., Han M. H., Kang C. K., Park J. M., Choi J. H., Park J. H., et al. (2012). Seasonal variations in species composition of fish assemblage collected by trammel net in coastal waters of the East Sea. J. Korean Soc Fish. Res. 48, 415–427. doi: 10.3796/KSFT.2012.48.4.415
Choi K. H., Seong Y. B. (2021). Constraining the long-term lowering rates of shore platforms on volcanic islands in the East Sea of the Korean Peninsula, using cosmogenic 36Cl. Geosci. J. 25, 267–281. doi: 10.1007/s12303-020-0030-y
Chung S., Cha H. K., Lee J. B., Lee H. W., Yang J. H. (2015). Species composition of the catches collected by trammel net in the coastal waters off Ulleungdo of Korea. J. Korean Soc Fish. Res. 51, 567–575. doi: 10.3796/KSFT.2015.51.4.567
Chung J. M., Shin J. K., Kim H. M. (2020). Diversity of vascular plants native to the Ulleungdo and Dokdo Islands in Korea. J. Asia-Pac. Biodivers. 13, 701–708. doi: 10.1016/j.japb.2020.08.001
Cifuentes L. A., Sharp J. H., Fogel M. L. (1988). Stable carbon and nitrogen isotope biogeochemistry in the Delaware estuary. Limnol. Oceanogr. 33, 1102–1115. doi: 10.4319/lo.1988.33.5.1102
Cresson P., Chouvelon T., Bustamante P., Bănaru D., Baudrier J., Le Loc'h F., et al. (2020). Primary production and depth drive different trophic structure and functioning of fish assemblages in French marine ecosystems. Prog. Oceanogr. 186, 102343. doi: 10.1016/j.pocean.2020.102343
De Carlo E. H., Hoover D. J., Young C. W., Hoover R. S., Mackenzie F. T. (2007). Impact of storm runoff from tropical watersheds on coastal water quality and productivity. Appl. Geochem. 22, 1777–1797. doi: 10.1016/j.apgeochem.2007.03.034
Doty M. S., Oguri M. (1956). The island mass effect. ICES J. Mar. Sci. 22, 33–37. doi: 10.1093/icesjms/22.1.33
Farré M., Lombarte A., Recasens L., Maynou F., Tuset V. M. (2015). Habitat influence in the morphological diversity of coastal fish assemblages. J. Sea Res. 99, 107–117. doi: 10.1016/j.seares.2015.03.002
Frank K. T., Petrie B., Choi J. S., Leggett W. C. (2005). Trophic cascades in a formerly cod-dominated ecosystem. Science 308, 1621–1623. doi: 10.1126/science.1113075
Fry B., Sherr E. B. (1984). δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib. Mar. Sci. 27, 13–47. doi: 10.1007/978-1-4612-3498-2_12
Gaertner J. C., Mazouni N., Sabatier R., Millet B. (1999). Spatial structure and habitat associations of demersal assemblages in the Gulf of Lions: a multicompartmental approach. Mar. Biol. 135, 199–208. doi: 10.1007/s002270050617
Galarza J. A., Carreras-Carbonell J., Macpherson E., Pascual M., Roques S., Turner G. F., et al. (2009). The influence of oceanographic fronts and early-life-history traits on connectivity among littoral fish species. Proc. Nati. Acad. Sci. 106, 1473–1478. doi: 10.1073/pnas.0806804106
Goering J., Alexander V., Haubenstock N. (1990). Seasonal variability of stable carbon and nitrogen isotope ratios of organisms in a North Pacific Bay. Estuar. Coast. Shelf. Sci. 30, 239–260. doi: 10.1016/0272-7714(90)90050-2
Gove J. M., McManus M. A., Neuheimer A. B., Polovina J. J., Drazen J. C., Smith C. R., et al. (2016). Near-island biological hotspots in barren ocean basins. Nat. Commun. 7, 10581. doi: 10.1038/ncomms10581
Hobson K. A., Sealy S. G. (1991). Marine protein contributions to the diet of northern saw-whet owls on the Queen Charlotte Islands: a stable-isotope approach. Auk 108, 437–440.
Jackson A. L., Inger R., Parnell A. C., Bearhop S. (2011). Comparing isotopic niche widths among and within communities: SIBER–Stable Isotope Bayesian Ellipses in R. J. Anim. Ecol. 80, 595–602. doi: 10.1111/j.1365-2656.2011.01806.x
Kang S. M., Lee H. G., Kim S. L., Choi J. W., Park C. H., Yu O. H. (2019). Species composition and community structure of macrobenthos during fall on the Dokdo coast, Korea. Ocean Polar Res. 41, 47–61. doi: 10.4217/OPR.2019.41.2.047
Kang D. W., Seo S. Y., Kang J. S., Paek W. K. (2013). Diversity of intertidal benthic invertebrate of Dokdo and Ulleung-do Island from Korea. J. Asia-Pac. Biodivers. 6, 157–164. doi: 10.7229/jkn.2013.6.1.157
Kim I. S., Choi Y., Lee C. L., Lee Y. J., Kim B. J., Kim J. H. (2005). Illustrated book of Korean fishes Vol. 615 (Seoul: Kyo-Hak Publishing Co.).
Kim G. Y., Kim D. C. (2001). Comparison and correlation of physical properties from the plain and slope sediments in the Ulleung Basin, East Sea (Sea of Japan). J. Asian Earth Sci. 19, 669–681. doi: 10.1016/S1367-9120(00)00062-6
Kim B., Lee Y., Koh B., Jhang S. Y., Lee C. H., Kim S., et al. (2023). Distinctive origin and evolution of endemic thistle of Korean volcanic island: Structural organization and phylogenetic relationships with complete chloroplast genome. PloS One 18, e0277471. doi: 10.1371/journal.pone.0277471
Kim C. H., Park C. H. (2014). Detailed bathymetry and seabed characteristics of wangdol-cho, hupo bank in the east sea. Econ. Environ. Geol. 47, 533–540. doi: 10.9719/EEG.2014.47.5.533
Kim H. G., Song S. J., Lee H., Park C. H., Hawkins S. J., Khim J. S., et al. (2020). A long-term ecological monitoring of subtidal macrozoobenthos around Dokdo waters, East Sea, Korea. Mar. pollut. Bull. 156, 111226. doi: 10.1016/j.marpolbul.2020.111226
Kim S. L., Yu O. H. (2021). Understanding the spatial and temporal distribution and environmental characteristics of polychaete assemblages in the coastal waters of Ulleungdo, East Sea of Korea. J. Mar. Sci. Eng. 9 (11), 1310. doi: 10.3390/jmse9111310
Kingsbury K. M., Gillanders B. M., Booth D. J., Nagelkerken I. (2020). Trophic niche segregation allows range-extending coral reef fishes to co-exist with temperate species under climate change. Glob. Change Biol. 26, 721–733. doi: 10.1111/gcb.14898
Kortsch S., Primicerio R., Fossheim M., Dolgov A. V., Aschan M. (2015). Climate change alters the structure of arctic marine food webs due to poleward shifts of boreal generalists. Proc. R. Soc B. 282, 20151546. doi: 10.1098/rspb.2015.1546
Krumsick K. J., Fisher J. A. (2019). Spatial and ontogenetic variation in isotopic niche among recovering fish communities revealed by Bayesian modeling. PloS One 14, e0215747. doi: 10.1371/journal.pone.0215747
Kurle C. M., McWhorter J. K. (2017). Spatial and temporal variability within marine isoscapes: implications for interpreting stable isotope data from marine systems. Mar. Ecol. Prog. Ser. 568, 31–45. doi: 10.3354/meps12045
Layman C. A., Araujo M. S., Boucek R., Hammerschlag-Peyer C. M., Harrison E., Jud Z. R., et al. (2012). Applying stable isotopes to examine food-web structure: an overview of analytical tools. Biol. Rev. 87, 545–562. doi: 10.1111/j.1469-185X.2011.00208.x
Layman C. A., Arrington D. A., Montaña C. G., Post D. M. (2007). Can stable isotope ratios provide for community-wide measures of trophic structure? Ecology 88, 42–48. doi: 10.1890/0012-9658(2007)88[42:CSIRPF]2.0.CO;2
Lee H. W., Hong B. K., Sohn M. H., Chun Y. Y., Lee D. W., Choi Y. M., et al. (2010). Seasonal variation in species composition of fish collected by trammel net around Dokdo, East Sea of Korea. Korean J. Fish. Aquat. Sci. 43, 693–704. doi: 10.5657/kfas.2010.43.6.693
Lee M., Kim Y. B., Park C. H., Baek S. H. (2022). Characterization of seasonal phytoplankton pigments and functional types around offshore island in the East/Japan Sea, based on HPLC pigment analysis. Sustainability 14, 5306. doi: 10.3390/su14095306
McClelland J. W., Valiela I., Michener R. H. (1997). Nitrogen-stable isotope signatures in estuarine food webs: A record of increasing urbanization in coastal watersheds. Limnol. Oceanogr. 42, 930–937. doi: 10.4319/lo.1997.42.5.0930
McCutchan J. H. Jr., Lewis W. M. Jr., Kendall C., McGrath C. C. (2003). Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102, 378–390. doi: 10.1034/j.1600-0706.2003.12098.x
Michener R. H., Schell D. M. (1994). “Stable isotope ratios as tracers in marine aquatic food webs,” in Stable isotopes in ecology and environmental science. Eds. Lajtha K., Michener R. H. (Oxford: Blackwell Scientific), 138–157.
Newsome S. D., Yeakel J. D., Wheatley P. V., Tinker M. T. (2012). Tools for quantifying isotopic niche space and dietary variation at the individual and population level. J. Mammal. 93, 329341. doi: 10.1644/11-MAMM-S-187.1
Park H. J., Kwak J. H., Kang H. Y., Kwon K. Y., Lim W., Kang C. K. (2020b). Incorporation of Cochlodinium bloom-derived organic matter into a temperate subtidal macrobenthic food web as traced by stable isotopes. Mar. pollut. Bull. 154, 111053. doi: 10.1016/j.marpolbul.2020.111053
Park T. H., Lee C. I., Kang C. K., Kwak J. H., Lee S. H., Park H. J. (2020a). Seasonal variation in food web structure and fish community composition in the East/Japan sea. Estuar. Coast. 43, 615629. doi: 10.1007/s12237-019-00530-4
Park T. H., Lee C. I., Kim T. H., Kim D., Park H. J. (2023). Trophic response of fishes to rainfall variability in a temperate estuarine system of Korea: A stable isotope approach. Mar. pollut. Bull. 193, 115183. doi: 10.1016/j.marpolbul.2023.115183
Peterson B. J., Fry B. (1987). Stable isotopes in ecosystem studies. Annu. Rev. Ecol. Syst. 18, 293–320. doi: 10.1146/annurev.es.18.110187.001453
Polis G. A., Hurd S. D. (1996). Linking marine and terrestrial food webs: allochthonous input from the ocean supports high secondary productivity on small islands and coastal land communities. Am. Nat. 147, 396–423. doi: 10.1086/285858
Post D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703718. doi: 10.1890/0012-9658(2002)083[0703:USITET]2.0.CO;2
Post D. M., Layman C. A., Arrington D. A., Takimoto G., Quattrochi J., Montana C. G. (2007). Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152, 179–189. doi: 10.1007/s00442-006-0630-x
Riccialdelli L., Becker Y. A., Fioramonti N. E., Torres M., Bruno D. O., Rey A. R., et al. (2020). Trophic structure of southern marine ecosystems: a comparative isotopic analysis from the Beagle Channel to the oceanic Burdwood Bank area under a wasp-waist assumption. Mar. Ecol. Prog. Ser. 655, 127. doi: 10.3354/meps13524
Rolff C. (2000). Seasonal variation in δ13C and δ15N of size-fractionated plankton at a coastal station in the northern Baltic proper. Mar. Ecol. Prog. Ser. 203, 47–65. doi: 10.3354/meps203047
Ryu S. H., Jang K. H., Choi E. H., Kim S. K., Song S. J., Cho H. J., et al. (2012). Biodiversity of marine invertebrates on rocky shores of Dokdo, Korea. Zool. Stud. 51, 710–726.
Sato T., Miyajima T., Ogawa H., Umezawa Y., Koike I. (2006). Temporal variability of stable carbon and nitrogen isotopic composition of size-fractionated particulate organic matter in the hypertrophic Sumida River Estuary of Tokyo Bay, Japan. Estuar. Coast. Shelf Sci. 68, 245–258. doi: 10.1016/j.ecss.2006.02.007
Shin D., Park T. H., Lee C. I., Jeong J. M., Lee S. J., Kang S., et al. (2022). Trophic ecology of largehead hairtail Trichiurus japonicus in the South Sea of Korea revealed by stable isotope and stomach content analyses. Front. Mar. Sci. 9, 910436. doi: 10.3389/fmars.2022.910436
Sohn M. H., Park J. H., Yoon B. S., Choi Y. M., Kim J. K. (2015). Species composition and community structure of demersal fish caught by a danish seine fishery in the coastal waters of the middle and southern East Sea, Korea. Korean J. Fish. Aquat. Sci. 48, 529–541. doi: 10.5657/KFAS.2015.0529
Song S. J., Park J., Ryu J., Rho H. S., Kim W., Khim J. S. (2017). Biodiversity hotspot for marine invertebrates around the Dokdo, East Sea, Korea: Ecological checklist revisited. Mar. pollut. Bull. 119, 162–170. doi: 10.1016/j.marpolbul.2017.03.068
Stanek A. E., von Biela V. R., Laske S. M., Taylor R. L., Dunton K. H. (2022). Barrier islands influence the assimilation of terrestrial energy in nearshore fishes. Estuar. Coast. Shelf Sci. 278, 108094. doi: 10.1016/j.ecss.2022.108094
Sweeting C. J., Polunin N. V. C., Jennings S. (2006). Effects of chemical lipid extraction and arithmetic lipid correction on stable isotope ratios of fish tissues. Rapid Commun. Mass Spectrom. 20, 595–601. doi: 10.1002/rcm.2347
Teague W. J., Tracey K. L., Watts D. R., Book J. W., Chang K. I., Hogan P. J., et al (2005). Observed deep circulation in the Ulleung Basin. Deep Sea Res. Part II: Topical Studies in Oceanography 52 (11-13), 1802–1826. doi: 10.1016/j.dsr2.2003.10.014
Ullah H., Nagelkerken I., Goldenberg S. U., Fordham D. A. (2018). Climate change could drive marine food web collapse through altered trophic flows and cyanobacterial proliferation. PloS Biol. 16, e2003446. doi: 10.1371/journal.pbio.2003446
Wang S., Luo B. K., Qin Y. J., Zhao J. G., Wang T. T., Stewart S. D., et al. (2021). Fish isotopic niches associated with environmental indicators and human disturbance along a disturbed large subtropical river in China. Sci. Total Environ. 750, 141667. doi: 10.1016/j.scitotenv.2020.141667
Wilson J., Sheaves M. (2001). Short-term temporal variations in taxonomic composition and trophic structure of a tropical estuarine fish assemblage. Mar. Biol. 139, 787–796. doi: 10.1007/s002270100624
Keywords: Ulleungdo, Dokdo, fish assemblages, trophic structure, stable isotopes, isotopic niches, environmental change
Citation: Park TH, Lee D-Y, Kang HY, Park JM, Kim D and Park HJ (2024) Trophic structure of fish assemblages in two offshore islands (Ulleungdo and Dokdo) of Korea revealed using stable isotope analysis. Front. Mar. Sci. 11:1293542. doi: 10.3389/fmars.2024.1293542
Received: 13 September 2023; Accepted: 08 January 2024;
Published: 25 January 2024.
Edited by:
Susana Carvalho, King Abdullah University of Science and Technology, Saudi ArabiaReviewed by:
Pei Qu, Ministry of Natural Resources, ChinaGoutam Kumar Kundu, University of Dhaka, Bangladesh
Rodrigo Silvestre Martins, Federal University of São Paulo, Brazil
Copyright © 2024 Park, Lee, Kang, Park, Kim and Park. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hyun Je Park, cGhqMTM1NzlAZ3dudS5hYy5rcg==
 Tae Hee Park
Tae Hee Park Dong-Young Lee
Dong-Young Lee Hee Yoon Kang
Hee Yoon Kang Joo Myun Park
Joo Myun Park Dongyoung Kim
Dongyoung Kim Hyun Je Park
Hyun Je Park