- 1Research Center for Conservation of Marine and Inland Water Resources, National Research and Innovation Agency, Bogor, West Java, Indonesia
- 2Research Center for Fishery, National Research and Innovation Agency, Bogor, Indonesia
- 3Research Center for Oceanography, National Research and Innovation Agency, Jakarta Utara, DKI Jakarta, Indonesia
- 4Department of Marine Science and Technology, Faculty of Fisheries and Marine Science, Institute Pertanian Bogor (IPB) University, Bogor, Indonesia
- 5Aquatic Research Facility, Environmental Sustainability Research Centre, University of Derby, Derby, United Kingdom
- 6Leibniz Centre for Tropical Marine Research (ZMT), Bremen, Germany
Black band disease (BBD) is one of the oldest recognized diseases of scleractinian corals. This disease is little known on the variation of progression rates across relatively small spatial scales and how local variations in the environment can impact prevalence and spread. The purpose of this study was to explore the progression of BBD on genus Montipora in relation to spatio-temporal environmental parameters in two islands of the Seribu Islands, North Jakarta, Indonesia during dry season and rainy season. Monthly underwater pictures were taken for determining the progress of disease level. Interestingly, the progression rate of the disease recorded at Pramuka Island was higher (8.10 cm2 day-1) than the one at Pari Island (3.79 cm2 day-1). In Pramuka Island, the infected corals had almost 89% of the dead surface, compared to only 68% at Pari Island. Similar to other studies in the region, we confirmed that the disease progressed faster during the dry season, where the environmental parameters, such as temperature, light intensity, and phosphate, were starting to increase, while total organic matter, current flow rates, and turbidity were lower. Progression of the disease was significantly different between seasons (p<0.001), but not between sites (p=0.118). Therefore, the progress of BBD has a higher impact at the more populated Pramuka island than at the less populated Pari Island, in addition to the influence of environmental parameters on coral vulnerability to diseases.
1 Introduction
Indonesia houses approximately 51% of all coral reefs in Southeast Asia and 18% of the coral reefs in the world (Burke et al., 2002). Furthermore, Indonesian reefs are the most diverse with over 569 species of scleractinian corals (described to date), from 83 genera (Burke et al., 2002; Hadi et al., 2020). However, in a similar trend to that seen across the globe, Indonesian reefs are in decline and suffer from bleaching and disease outbreaks (Carpenter et al., 2008; Johan et al., 2014; Plass-Johnson et al., 2015). Indeed, approximately 33.82% of Indonesia’s reefs have been classified as “in a poor state” in a recent nationwide survey (Hadi et al., 2020). In addition to the global trends of increasing sea surface temperatures and ocean acidification, brought about by climate change (Smith and Smith, 2009), Indonesian reefs suffer greatly from more regional and local stressors such as overexploitation, destructive fishing practices, and pollution (Fadila and Idris, 2009).
Combined these factors are known to cause a decline in reef health (Carpenter et al., 2008), yet the scale of the impacts is largely unknown, as baseline surveys and longer-term monitoring of tagged colonies are not widely undertaken within the country. Only relatively recently have disease surveys been conducted (Haapkylä et al., 2007; Yusri and Estradivari, 2007; Sabdono and Radjasa, 2008; Haapkylä et al., 2009; Delpopi et al., 2015; Johan et al., 2016; Johan and Idris, 2022; Ulfah et al., 2022 ; Haya et al., 2023). These earlier surveys indicated that nine diseases are prevalent in Indonesian waters infecting eight coral species. The particular note is the apparent prevalence of black band disease (BBD), one of the earliest bacterial coral diseases recorded (first in 1972) and arguably one of the most well studied (Sato et al., 2011; Sato et al., 2016).
Coral disease surveys are important and need to be included in the monitoring of coral health conditions throughout Indonesia, such as those being carried out by the Coral Reefs Rehabilitation and Management Project by RCO-LIPI (Research Center for Oceanography, Indonesian Institute of Sciences). Up to now, there are almost no studies that have explored spatio-temporal variations in the progression of diseases such as BBD throughout Indonesia that could act as a baseline study to monitor reef health in the future. In general, data and reports about BBD are some reports from around Indonesia, but very rare data related with the BBD progress in Indonesia, including this site research. Mostly research related with disease abundance and prevalence (Delpopi et al., 2015; Johan et al., 2016; Johan and Idris, 2022; Ulfah et al., 2022; Haya et al., 2023).
Therefore, this study aimed to observe and describe the progress of BBD and to link it to environmental factors, which may explain any observed variations in the progression rates. Moreover, the data were collected from two islands and two seasons to figure out the spatio-temporal variation relation to BBD. In addition, this is the first time that time series with underwater (UW) photos are used in BBD study.
2 Materials and methods
2.1 Research site
Observation of the progression rates of black band disease (BBD) was conducted at two islands of the Seribu Islands, Indonesia: Pari Island and Pramuka Island. These islands were chosen based on the presence of BBD on Montipora corals and the distance to Java mainland, so they have different levels of impacts from anthropogenic activities such as the capital city if Indonesia knows with high pollution into Jakarta Bay (Purbonegoro et al., 2018). Pramuka island is further away ( ± 29 km) and within the national park on two sites, south and north of Pramuka Island as site 1 (05°45’01.9” S, 106°36’41.5”E) and site 2 (05°44’24.9” S, 106°37’14.5”E). while Pari Island is closer to the coast of Java ( ± 16 km) and more exposed to land-based pollution (Baum et al., 2015), on two sites, south and north of Pari Island as site 3 (05°52’14.0” S, 106°36’38.58 E) and site 4 (05°51’61.1” S, 106°37’25.0”E), respectively (Figure 1).
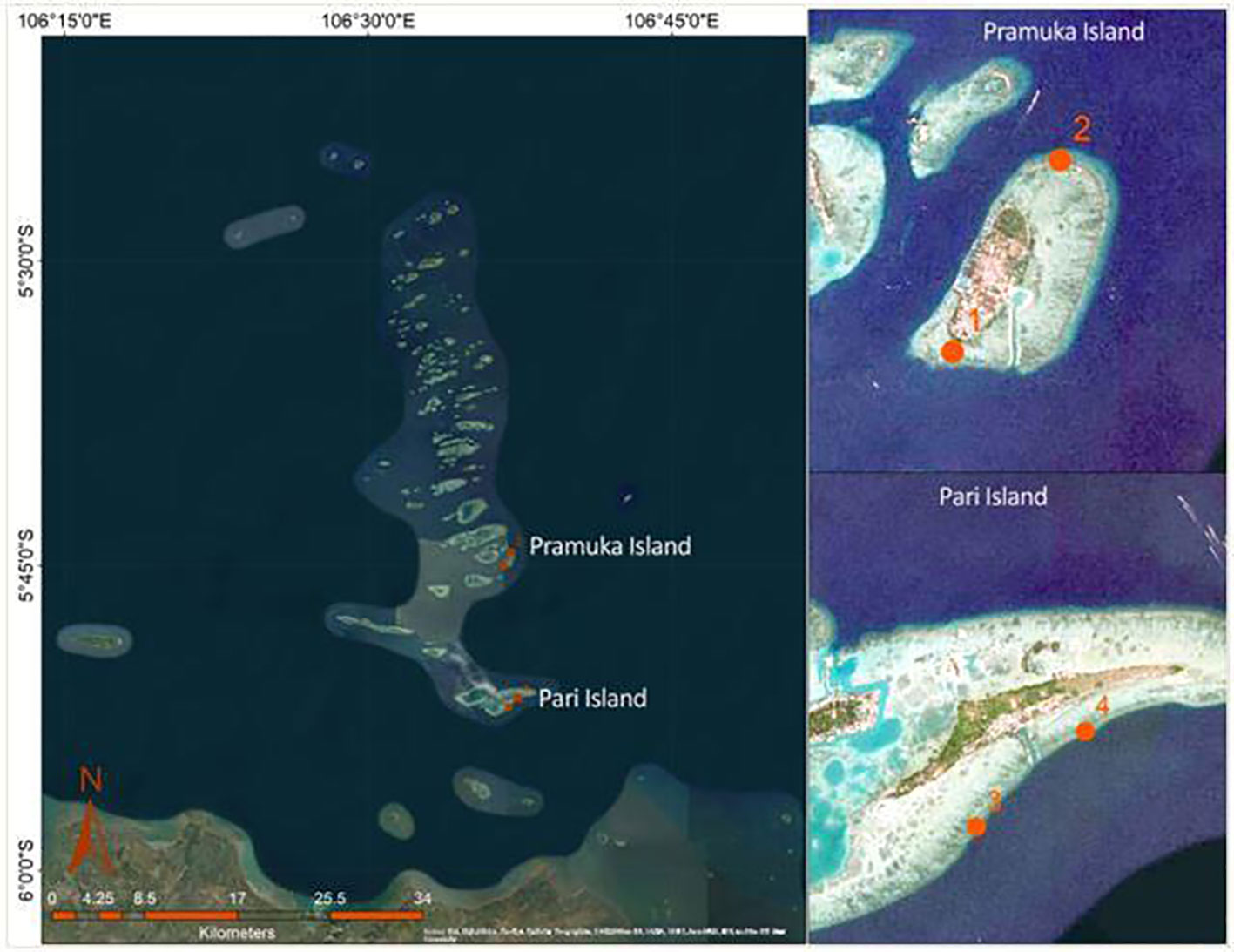
Figure 1 Research location and position of permanent transect placement at two islands (Pramuka and Pari), with different distances from Java mainland. Each island has two replication sites (1-4).
However, both islands are inhabited, so local stress with regard to the effects of a small population on the reefs should be comparable. This study was carried out over one year to include a continuous data set (time series) encompassing the dry (June-September 2011) and wet seasons (December 2011 - March 2012), and the ‘transitional’ part in between (October-November 2011). All surveys were undertaken by snorkeling and using a Canon IXUS 120 underwater camera to take photos of the colonies with an attached ruler for the scale.
2.2 BBD progression rates
A total of 28 colonies showing signs of BBD were tagged at the two islands, comprising two sites in the south of Pari Island, and two sites-one each in north and south of Pramuka Island. The progress data at Pari island were collected from different seasons, comprising dry (3 samples), transition (7 samples) and rainy (18 samples) seasons. Whereas at Pramuka island, the data were collected in dry (6 samples), transition (15 samples) and rainy (7 samples) seasons.
Selected colonies for time series monitoring were marked with serial numbers. Photos were taken in a vertical position with a static scale (cm) to avoid data bias. The same procedure was carried out throughout the data collection. Every two weeks these colonies were visited and photographed for the one-year duration of the study.
Analysis of the images was undertaken using the software ImageJ (Kurniawan et al., 2011; Schneider et al., 2012), giving a progression rate in cm2 day-1 following the edge of dead colonies area by digitation to get the width of disease progress (Delpopi et al. (2015; Wada et al., 2017). Colonies with initial signs of BBD were classified into three categories of vitality level at the end of observation: dead (after contraction of the disease), still infected (an active, progressive lesion), and survived (no evidence of BBD lesions after initial contraction). The criteria of infection progress were indicated by percentage of colony-die-off, which was classified into four categories, as follows: 0-25%, 26-50%, 51-75%, and 76-100%.
2.3 Environmental parameters
Some environmental parameters were measured at the study sites. They are water depth (m), water current (m s-1), water temperature (°C), light intensity (Lux), Total Dissolved Solids (TDS, mg L-1), salinity (‰), dissolved oxygen (DO, mg L-1), pH, phosphate (mg L-1), nitrate (mg L-1), TOM (mg L-1), and turbidity (NTU). Water depth data were taken using a dive computer. Current data were taken using a manual method by floating an object using an extension rope and recording the duration needed to reach a certain distance to calculate the current speeds (Soeboer et al., 2018; Rambe et al., 2022). Water temperature and light intensity were recorded every two hours for one year using a datalogger from Hobo (Onset, USA). In situ water quality measurements were taken every month with a YSI 556 MPS-Multiprobe System (USA), including TDS, salinity, DO, pH, TOM, turbidity, and water current. Phosphate and Nitrate concentrations were analyzed in the laboratory of the Research Institute of Ornamental Fish Culture in Depok, West Java.
2.4 Statistical analyses
Data of disease progression (between islands, between seasons, among the health state of the colony and among the surrounding water quality parameters) were analyzed using IBM SPSS statistics version 20 software. For identifying differences between seasons, a pairwise analysis (Tukey TSD test) was used, with the statistically significant p<0.05.
3 Results
3.1 Progression rates of black band disease
The diseased colonies at Pari Island showed an average progression rate of 3.79 cm2 day-1, ranging from 0.041 to 20.80 cm2 day-1 with standard deviation of 4.90 cm2 day-1. The diseased colonies at Pramuka island had been progressing at a much faster rate with an average of 8.10 cm2 day-1, ranging from 0.05 to 51.53 cm2 day-1 with standard deviation of 10.95 cm2 day-1 (Figure 2). Based on temporal observations among seasons, the highest progression rates were shown to occur in the dry season with an average of 15.98 cm2 day-1, followed by the transition period with a progression rate of 6.52 cm2 day-1, and the rainy season had the lowest rate of 1.84 cm2 day-1. The progression data of BBD on Montipora spp. are shown in Table 1.
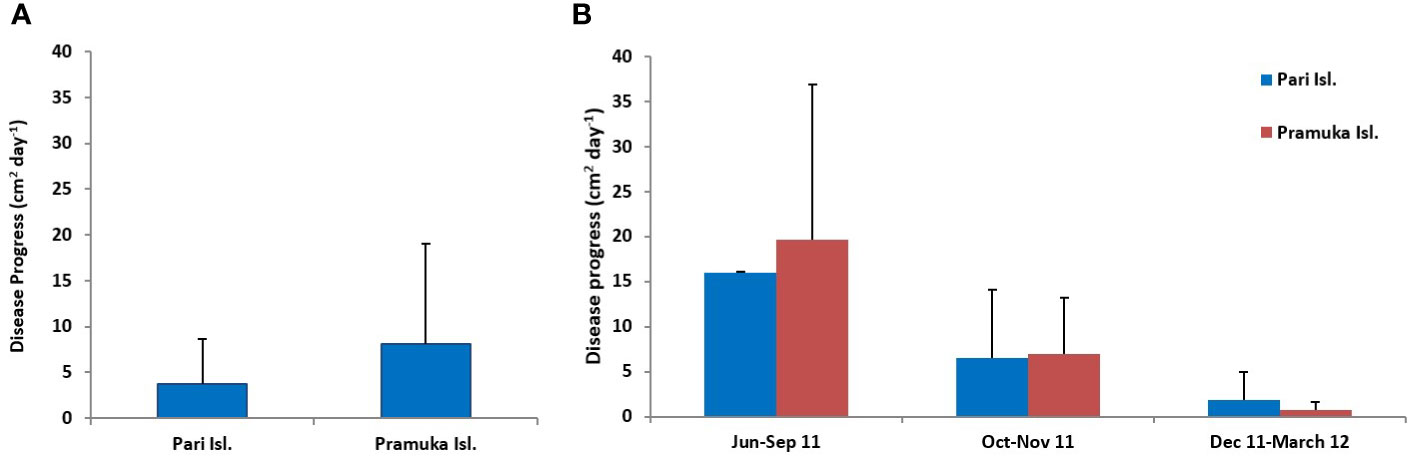
Figure 2 Disease progression at two sites (average for one year) (A) and for three seasons (B) dry (Jun-Sep 2011), transition (Oct-Nov 2011), and rainy (Dec 2011-Mar 2012). Values are given as mean ± SD, n= 28.
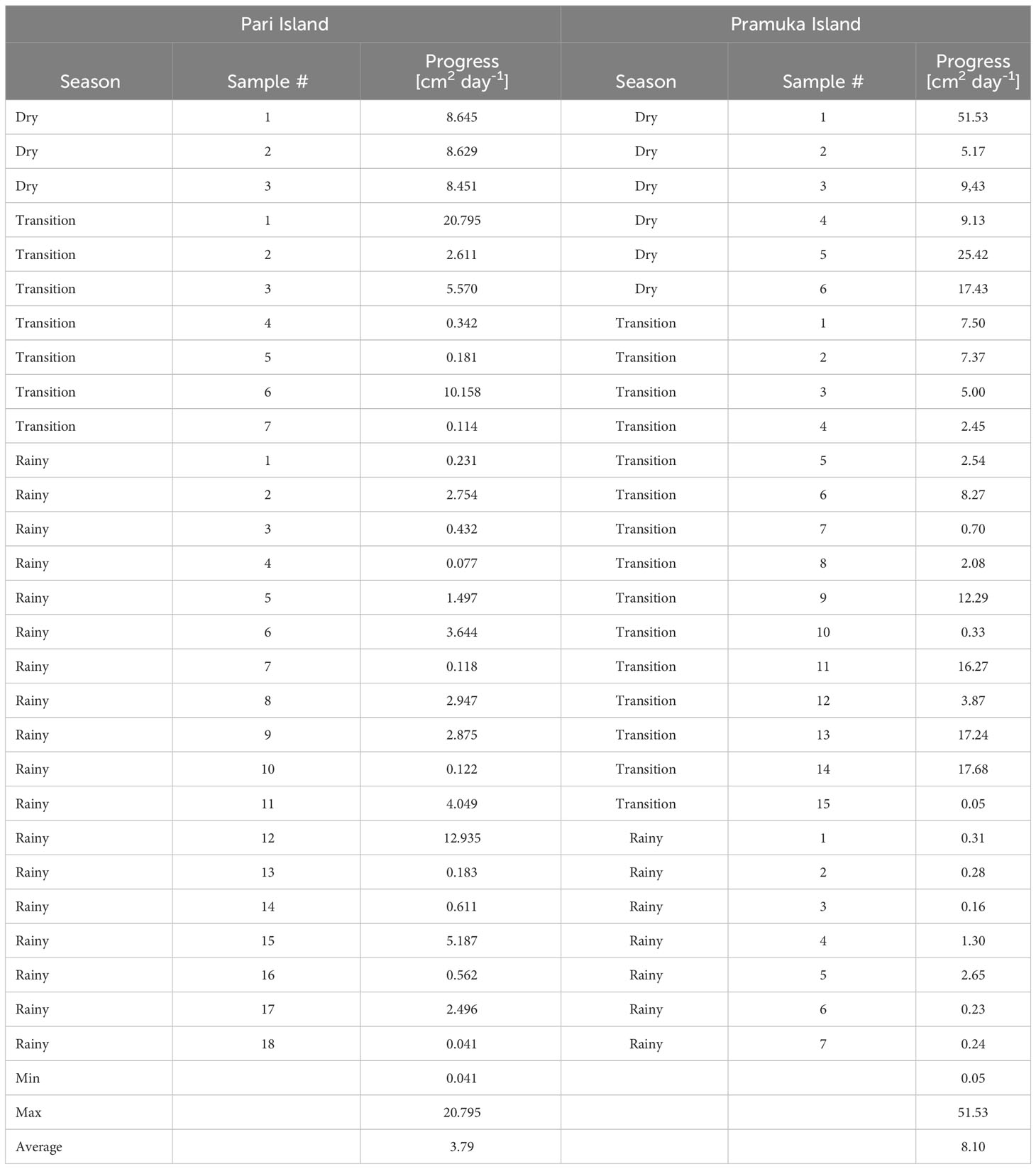
Table 1 The progression rates of Black Band Disease, which impacted Montipora spp. at Pari Island and Pramuka Island throughout the duration of this study.
The progression of BBD was significantly different between seasons (F = 9.140, p <0.001), but not between sites (F = 2.531, p = 0.118). The disease progression based on sites and seasons are shown in Tables 1, 2, and Figure 2.
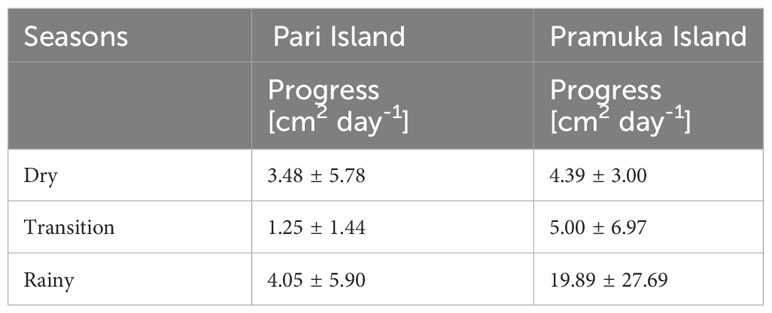
Table 2 The average values of the progression rate of Black Band Disease, which impacted Montipora spp. during three seasons of the study. Data represent mean ± SD of 28 samples (n).
The disease progress is presented as a percentage of the dead area (as a result of the disease) in comparison with the colony’s width, by comparing 28 colonies.
During our study, we observed that BBD first appears on colonies in small multi-focal spots (usually between 1 and 3), which appear simultaneously, then radiate from any parts of the colony (see Figure 3). Often, these small spots progress and join into one larger lesion. These larger lesions then progress faster and finally appear to spread to other coral colonies. The level of progression of the disease is characterized by the development of the characteristics of black band (or cyanobacterial mat), which leaves behind an exposed coral skeleton devoid of tissue.
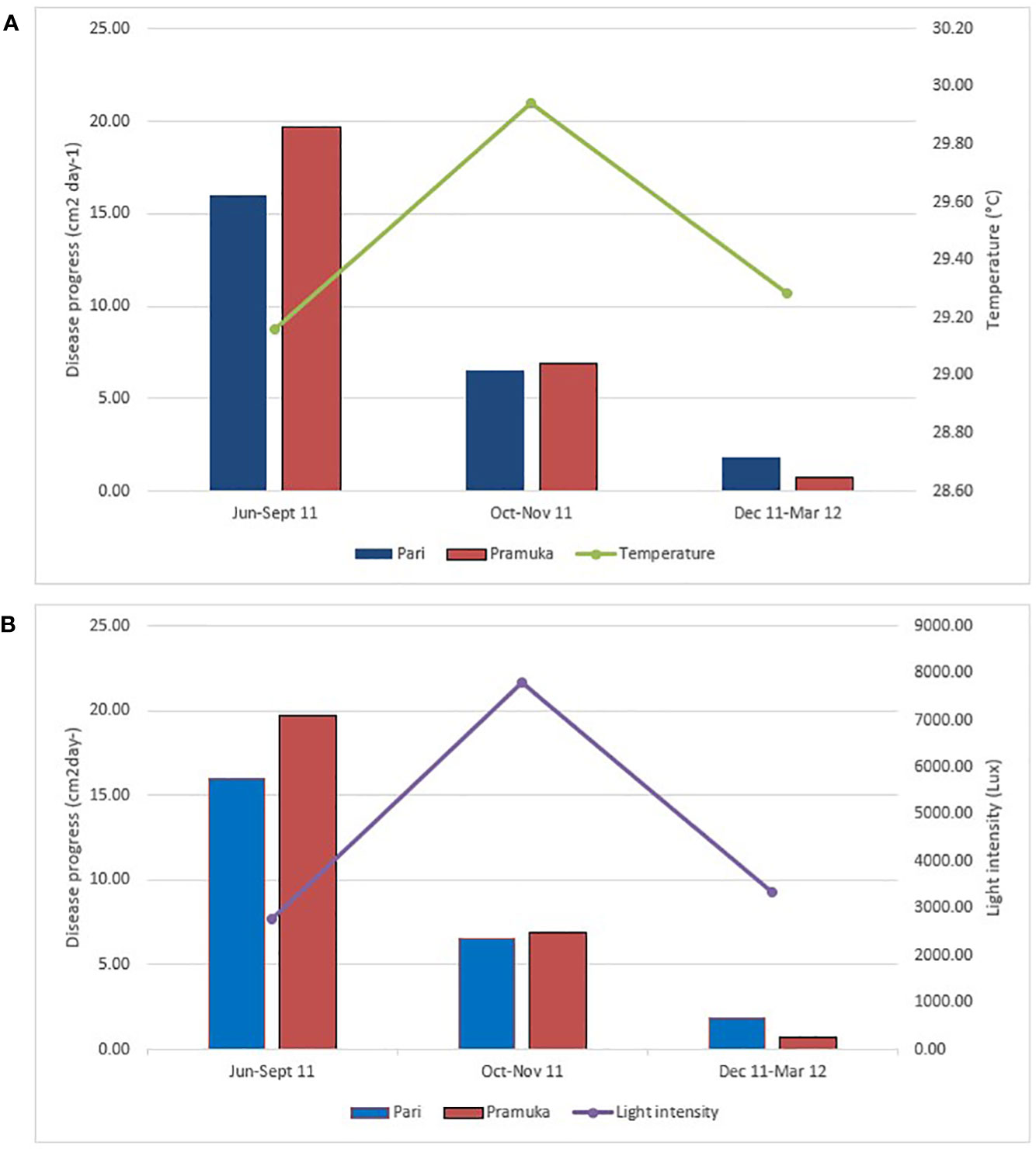
Figure 3 The disease progression in relation to the average temperature (A) and light intensity (B) during the study period from June 2011 to March 2012. The light and temperature data were recorded every 2 hours during this dry season (Jun-Sep 2011), transition season (Oct-Nov 2011), and rainy season (Dec 2011-Mar 2012). Sample number (n) was 6 for each season and site.
Dead areas of coral colonies were identified by visual estimation underwater and via photographs (Figure 4). For this estimation, we only used colonies that at least partly survived. From a total of 28 impacted colonies (i.e. the number of colonies that suffered from the disease), most colonies displayed up to 89% of the dead coral area at Pramuka Island, as compared to 68% at Pari Island. There were significant differences between the various percent categories (0-25%, 26-50%, 51-75% and 76-100% of dead colony area) (F = 23.79, p < 0.0001).
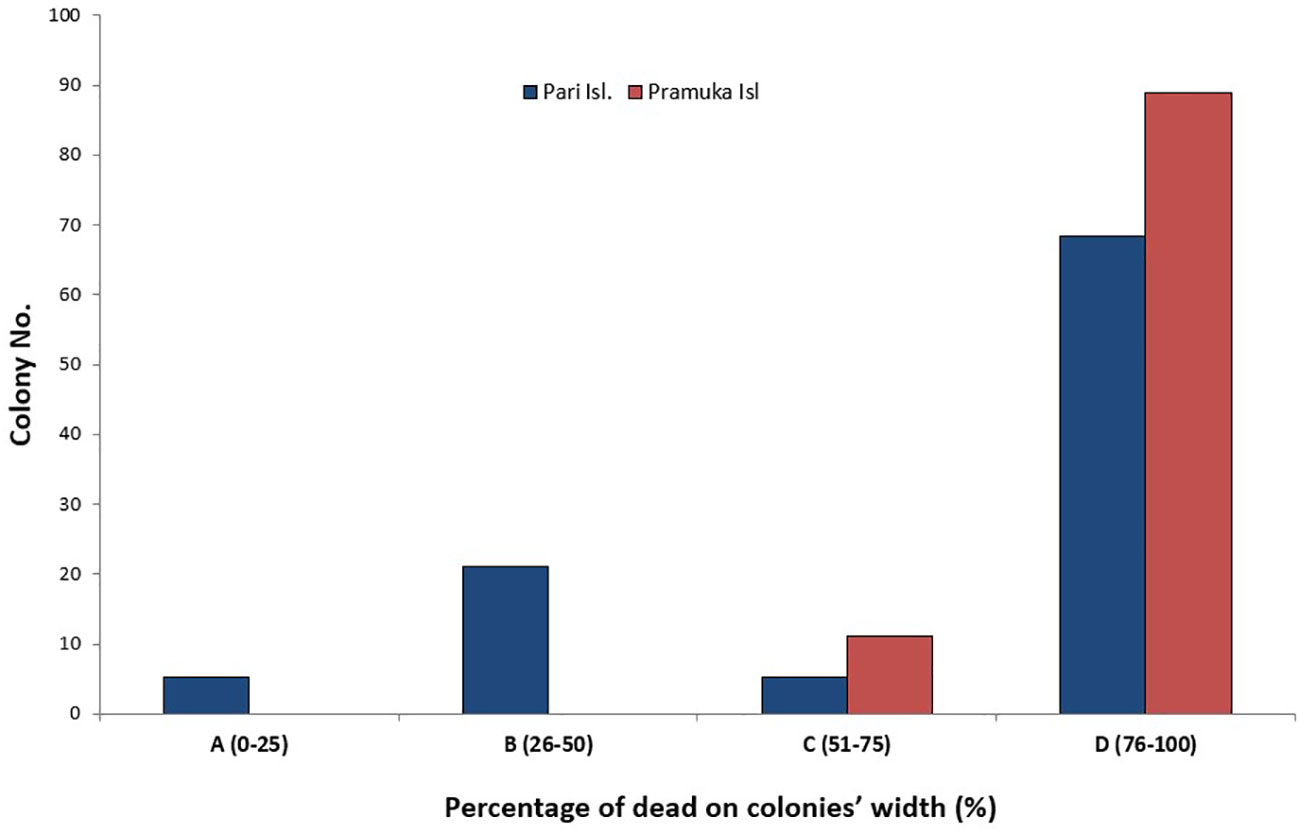
Figure 4 The percentage of dead areas that had contracted BBD progress. The percentage area of the infected colony was split into percentiles (0-25, 26-50, 51-75 and 76-100% tissue loss or dead part of colony) at Pari Island and Pramuka Island.
We observed instances where certain corals, like Pocillopora verrucae, were apparently resistant to contraction of the disease, despite the lesion progressing directly adjacent to it (Figure 5).
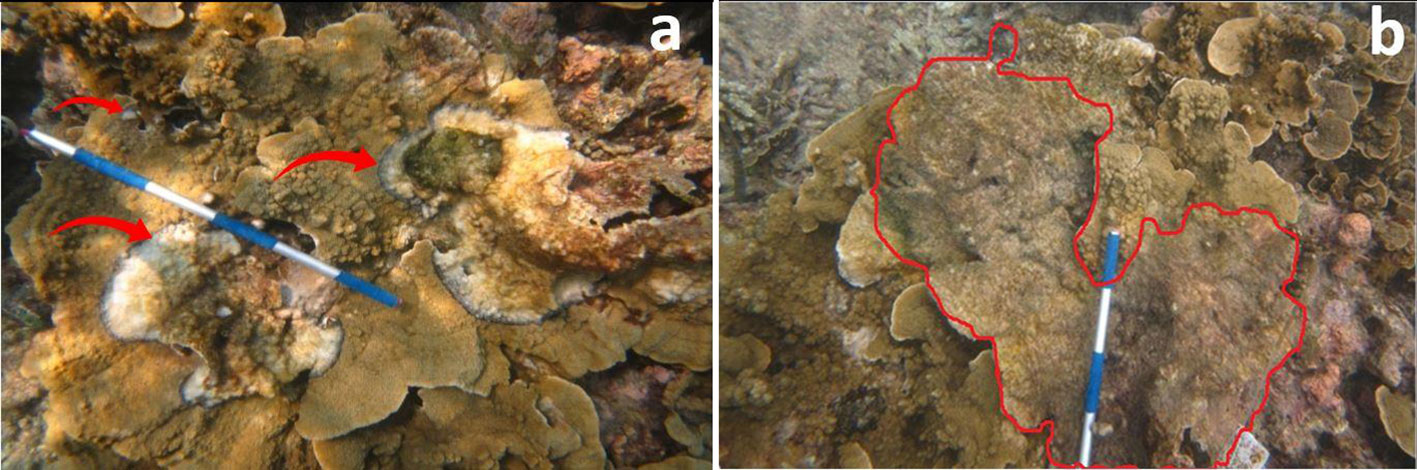
Figure 5 BBD starts from three multi focal spots, apparently simultaneously, radiating to all parts of the coral colony. (A) indicates three site origins of the disease and (B) highlights the fate of the colony after 20 days showing consolidation of the original three isolated starting points.
3.2 Environmental parameters around the black band disease colonies
Temperatures varied between 28 and 31.7°C at both locations with a clear peak in the transition period between October and November (Figure 3A). The highest average temperature recorded during our observation period was 30.18˚C at Pari Island and 29.71°C at Pramuka Island. Likewise, the light intensity increased to almost 8000 Lux during the transition period in October-November (Figure 3B) and then decreased again during the rainy season to a low in April-May.
There was a significant difference in the majority of the water parameters between either site and/or season except for turbidity, current, and DO. The phosphate was different between dry versus rainy season, and between transition-rainy season as shown in Table 3.
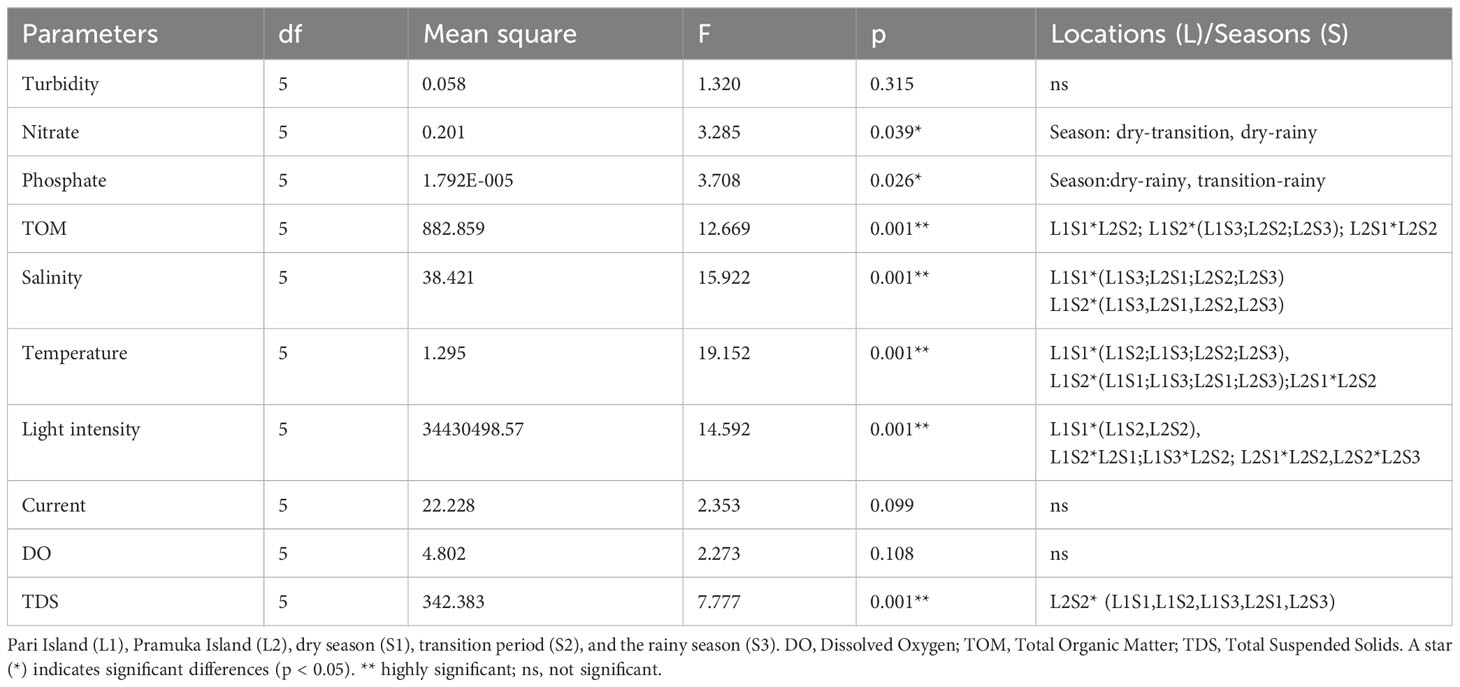
Table 3 Analysis of variance test (Tukey HSD) of the water quality parameters at two sites and across the seasons.
There were several factors which influenced the increasing progression of Black Band Disease (BBD). These correlates to an increase in temperature, light intensity, and phosphate concentration in the dry season until the transition season, then a decrease in the rainy season.
Other environmental parameters such as water current and TOM decreased from a maximum in the dry season to a lower value in the rainy season. The water current peaked during the dry season, where also the disease progress was the highest, and then it was continuously decreasing to a low current during the second transition time until the rainy season, when also the coral disease progress decreased to a low level in the rainy season (compare Figures 2, 3, and 6).
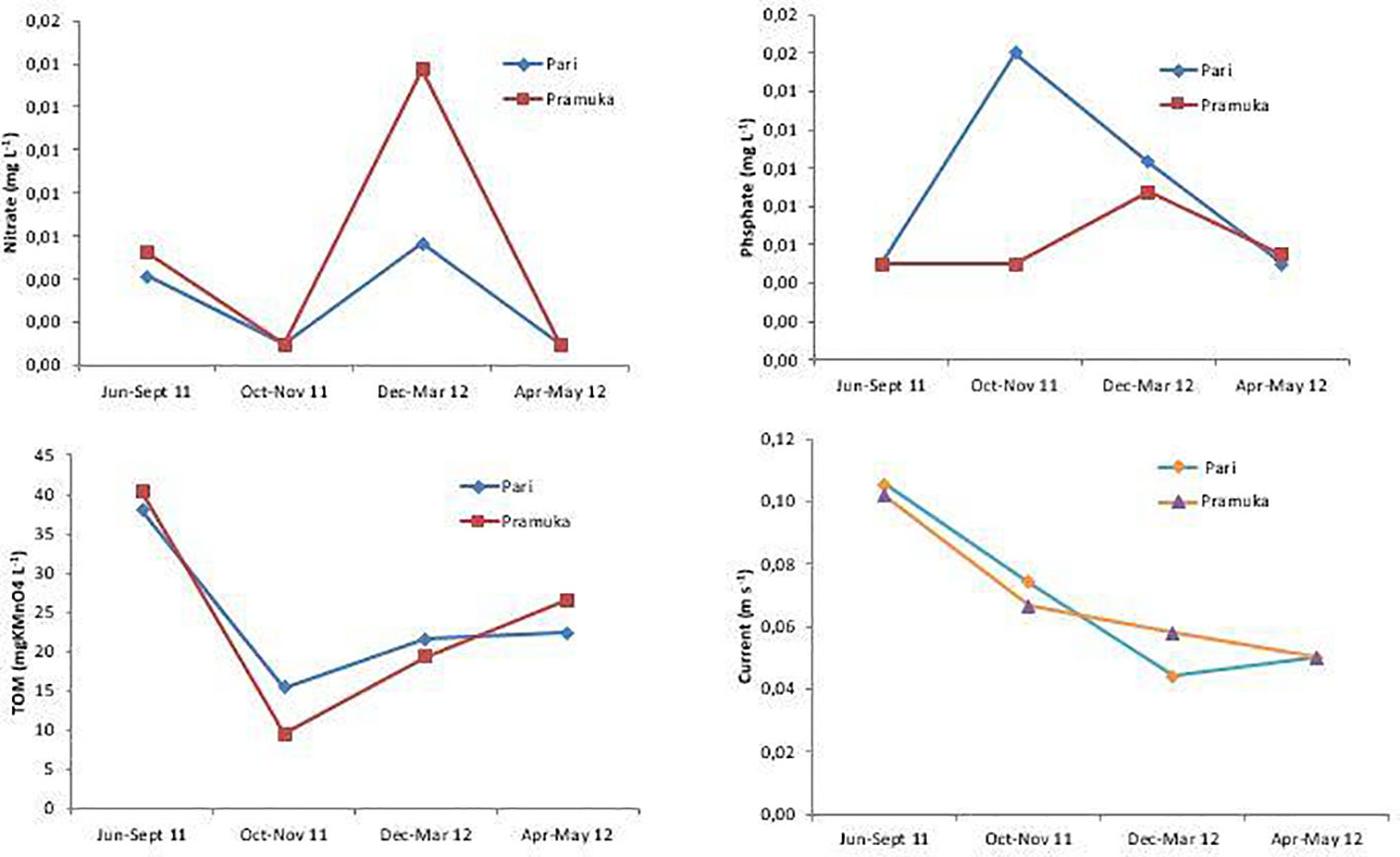
Figure 6 Water quality (nitrate, phosphate, TOM) and water velocity at Pari and Pramuka Island from June 2011 to May 2012. All measurements are means with three replications. Data were collected during four seasons: dry (June-Sept 2011), transition 1 (Oct-Nov 2011), rainy (Dec 2011-Mar 2012), and transition 2 (Apr-May 2012).
4 Discussion
In this paper, the progression rate of the black band disease (BBD) in hard corals was studied at four sites of both Pari Island and Pramuka Island with increasing distance to the mainland coast of Java and in relation to environmental data. The highest progression rate of BBD on the genus Montipora spp. was found with 8.10 cm2 day-1 on Pramuka Island, a site which is more far compared with with Pari Island from Jakarta Bay or the central city of Jakarta. Disease progress was faster during the dry season due to temperature, light intensity, and phosphate were increased.
4.1 Progression rate of BBD
At the end of observations, most of the coral colonies infected with BBD died at Pramuka Island. The progress rate of BBD (3.79 cm2 day-1 in Pari Island – 8.10 cm2 day-1 in Pramuka Island) on Montipora spp is lower than the progress of White Band Dry Rocks (13 cm2 day-1) in Florida on Acropora cervicornis (Williams and Miller, 2005). In comparison, the progress rate of BBD in Pari Island was 0.46 cm2 day-1, where the data were collected in 5 months from March to July in 2014 (Delpopi et al., 2015). Other research on the same genus of Montipora in around Sesoko island, Okinawa, found a progress rate of 2 – 2.5 mm day-1 (Das et al., 2022), it is interesting that the highest progress rate was found in this study in Seribu islands.
Some coral colonies can survive disease attacks if the corals such as Montipora spp have a bulge (branch) upwards. The part of the branch is not degraded by bacteria, because bacteria seem to only spread out on parts of the colony in a horizontal manner. Similar genus on Montipora in Seseko Island, Okinawa, the progress rate was influenced by environmental conditions by increasing the temperature and light intensity (Das et al., 2022). The impact of increasing the temperature and light intensity, BBD outbreak in the transition season after the high progress of the BBD (Johan et al., 2016). Infected colonies of corals in Montipora spp apparently do not transmit the disease to other nearby coral colonies, even from the same coral species, as supported by Edmunds (1991) who found that infected colonies are not infectious between nearby colonies. The disease progress was fast when water parameters such as temperature, light or nutrients were elevated. When environmental parameters recover to normal values, the progress of the disease can stop, and many coral colonies can survive the disease. In some cases, BBD outbreaks in the Seribu Islands are only visible for two weeks, and most of the corals survived. High abundance and outbreaks of BBD also happened on encrusting Montipora sp in south of Sesoko Island (Das et al., 2022); also in Taiwan 23 genera of corals including Montiopora presented the disease (Huang et al., 2021). BBD also infected other corals on foliose and branching Montipora, Acropora hyacinthus sp. complex, Acropora cytherea, and Gardineroseris planulata (Das et al., 2022); from 16 genera which were susceptible to several kinds of diseases in the Persion Gulf, only the genus Acropora was impacted by BBD (Hazraty-Kari et al., 2021).
The colonies that had contracted the disease were able to recover (5.26-21.05% of those monitored). This occurred more commonly in the transition period. Most corals succumbed to the disease during the dry season when environmental parameters such as temperature increased as a kind of trigger until reaching a peak in the transition season, where the outbreak of BBD happened at both sites. BBD is thought to be contracted via direct coral to coral contact (Zvuloni et al., 2009; Randall et al., 2016).
It is interesting that although several specimens of Montipora sp. were obviously infected with BBD, the disease did not move to other colonies, even though the same species. In one case we even found that the coral Pocillopora verrucae was not infected by BBD, even though it was completely surrounded by diseased coral area from Montipora spp. This also happened in Sesoko island and in Taiwan where BBD only impacted specific genera (Huang et al., 2021; Das et al., 2022). The coral Montipora spp. was more susceptible due to the foliose growth form of the species, where a larger area of the colony is exposed to sunlight, so it is easier stressed than other species. The impact of high temperature and light intensity was more impactful in the transition season (dry - wet season) with low current that made sunlight optimal reach the bottom of the sea in shallow waters (Johan et al., 2016).
Black band disease was mostly found in Montipora spp, but we also found other infected species such as Pachyseris sp., Pavona sp, and Astreopora sp. outside of the permanent observation transect. Montipora corals seem to be more sensitive than other species due to a large horizontal colony area that is exposed to the light. High light intensities can be a trigger of stress for Montipora. From regional studies it is known that different geographical areas can host species with completely different sensitivity levels toward a BBD infection, such as in the Caribbean with Montastrea annularis (Goreau et al., 1998), or Diploria clivosa, D. strigosa, Montastrea annularis, M. cavernosa and Siderastrea siderea in Jamaica (Bruckner et al., 1997).
The disease spread usually begins with 1-3 small spots, which are separated from each other, then spots are developing throughout the colony. If environmental conditions as potential triggers were stressful, the small spots have developed and joined into one large spot. Progress rate was much faster then and the disease established on the coral.
Among potential environmental triggers we identified e.g. nutrient enrichment, which could be caused by nutrient pollution from fish culture close to our observation site at Pari Island. Another potential source is the higher population on Pramuka Island. This is also reported by Voss and Richardson (2006) from the Bahamas, where fertilizer and increased terrestrial runoff due to anthropogenic activities seemed to enhance black band disease progress.
In this study, the highest progress level at the two islands was found in a colony in the dry season reaching 51.53 cm2 day-1, while the average range was between 1.84-15.98 cm2 day-1 during the observation period. A comparison with the results of other studies shows progression rates of 1.3-100 cm2 day-1 in M. annularis in Puerto Rico (Bruckner, 2002) and 2 cm day-1 in other Atlantic coral reefs and the Caribbean (Rutzler et al., 1983). In contrast, Sutherland et al. (2004) described the average progress with 3 mm day-1.
An interesting observation was, that although BBD is thought to be contracted via direct coral to coral contact (Zvuloni et al., 2009; Randall et al., 2016), we observed instances where in certain corals, like Pocillopora verrucae for example, which were apparently resistant to contraction of the disease, the lesion was progressing directly adjacent to it (Figure 6).
4.2 Potential impact of environmental parameters on the progression of black band disease
An increase in four environmental factors (temperature, light intensity, current, phosphate concentration) in the dry season (Jun-Sep) seem to co-occur with an increased disease progress. An outbreak of BBD seems to be triggered by the peak values of above-mentioned parameters, particularly sharp increases of temperature and light (Johan et al., 2014). Solar radiation data from the entire area of the Seribu Islands show that in October 2011 high intensities were reached during almost 7 hours per day.
An additional factor promoting the disease progress is proximity to human settlements, as can be seen at the site on Pramuka Island, where higher population and aquaculture facilities are correlated with an increased disease progress. Sato et al. (2011) reported from the Great Barrier Reef, Australia that light intensity seems to have a greater effect than the temperature in triggering BBD in the summer. A similar condition was also reported by Bruckner et al. (1997), when the coral species Siderastrea siderea was impacted by BBD, additionally increased due to terrestrial run-off associated with abnormally high rainfall in Jamaica. Research conducted by Boyett et al. (2007) in aquaria and field experiments also concluded that high temperatures and light intensities play an important role in increasing the progress of BBD, where the summer progress rate is much faster (1.7-2.4 times) compared to winter. Furthermore, it was explained that the increase in temperature and light intensity simultaneously (i.e. the combination of both) had a greater impact on the level of progress of black band disease than the effect of temperature alone.
One explanation could be that under these peak conditions, upper temperatures close to the thermal limit of corals paired with high intensity of light (i.e. short but very intensive stress) can easily cause the fast progress of the coral disease in the dry season, which then could explain that in the following transition season we record the highest prevalence of BBD.
5 Conclusions
The progression of the BBD disease at Pramuka Island, far from the mainland, with an average of 8.10 cm2 day-1 was faster than 3.79 cm2 day-1 at Pari Island, which is closer to the mainland. The highest progression rates were shown to occur in the dry season with an average of 15.98 cm2 day-1, then followed by a transition and rainy season. In Pramuka Island infected corals had almost 89% of dead surface, as compared to only 68% on Pari Island. Progression of the disease was significantly different between seasons, but not between sites.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
OO designed and ran the coral disease progress research. OO, RS, MA, and G collected the photos of progress status and identified the species of corals. OO, RG, IA, LS, RR, NS, NZ, MS, and AK analyzed the data and wrote-revised the manuscript. MS and AK advised the data acquisition, analyses, and interpretation, and revised the manuscript. G collected photos in the field and analyzed the data and wrote-revised the manuscript. AS assisted in collecting data of water quality in the field and revised the manuscript. NU and PR helped in analyzing the water quality and revised manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are grateful for the close collaboration with the Marine National Park of Seribu Islands, the local government for Marine and Fisheries of Seribu Island, and Bogor Agriculture University in completing this study. The personal support of Abdul Rasyid, the ship’s captain (Andi), and the local dive center ELANG in Seribu Islands is highly appreciated.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Baum G., Januar H. I., Ferse S. C. A., Kunzmann A. (2015). Local and regional impacts of pollution on coral reefs along the thousand islands north of the megacity Jakarta, Indonesia. PloS One 10 (9), 1–26. doi: 10.1371/journal.pone.0138271
Boyett H. V., Bourne D. G., Willis B. L. (2007). Elevated temperature and light enhance progression and spread of black band disease on staghorn corals of the Great Barrier Reef. Mar. Biol. 151 (5), 1711–1720. doi: 10.1007/s00227-006-0603-y
Bruckner A. W. (2002). Priorities for effective management of coral diseases, (Issue August) NOAA Technical Memorandum NMFS-OPR-22. U.S. Department of Commerce. National Oceanic and Atmospheric Administration National Marine Fisheries Service. 41 p.
Bruckner A. W., Bruckner R. J., Williams E. H. (1997). Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bull. Mar. Sci. 61 (3), 919–928.
Burke L., Selig E., Spalding M. (2002) Reefs at Risk in Southeast Asia. Available at: http://www.citeulike.org/group/342/article/484868.
Carpenter K. E., Abrar M., Aeby G., Aronson R. B., Banks S., Bruckner A., et al. (2008). One-Third of reef-Building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Das R. R., Wada H., Masucci G. D., Singh T., Tavakoli-Kolour P., Wada N., et al. (2022). Four-Year Field Survey of Black Band Disease and Skeletal Growth Anomalies in Encrusting Montipora spp. Corals around Sesoko Island, Okinawa. Diversity 14, 32. doi: 10.3390/d14010032
Delpopi M., Zamani N. P., Soedarma D., Johan O. (2015). Prevalence, incidence, and progression of black-band disease on scleractinian coral (Montipora spp) in shallow water of pari islands. ILMU KELAUTAN: Indonesian J. Mar. Sci. 20 (1), 52. doi: 10.14710/ik.ijms.20.1.52-60
Edmunds P. J. (1991). Extent and effect of Black Band Disease on a Caribbean reef. Coral Reefs 10 (3), 161–165. doi: 10.1007/BF00572175
Fadila, Idris (2009). Perbandingan dua tahun persentase penutupan karang di Kepulauan Seribu, (2003, 2005, dan 2007). Terumbu karang Jakarta: Pengamatan jangka panjang terumbu karang Kepulauan Seribu, (2003-2007). Eds. Estradivari, Setyawan E., Yusri S. (Yayasan Terumbu Karang Indonesia).
Goreau T. J., Cervino J., Goreau M., Hayes R., Hayes M., Richardson L., et al. (1998). Rapid spread of diseases in caribbean coral reefs.pdf. Rev. Biol. Trop. 46 (5), 157–171.
Haapkylä J., Seymour A. S., Trebilco J., Smith D. (2007). Coral disease prevalence and coral health in the Wakatobi Marine Park, south-east Sulawesi, Indonesia. J. Marine Biol. Assoc. United Kingdom. 87 (2), 403–414. doi: 10.1017/S0025315407055828
Haapkylä J., Unsworth R. K. F., Seymour A. S., Melbourne-thomas J., Flavell M., Willis B. L., et al. (2009). Spatio-temporal coral disease dynamics in the Wakatobi Marine National Park, South-East Sulawesi, Indonesia. Dis. Aquat. Organ. 87(1-2):105–15. doi: 10.3354/dao02160
Hadi T. A., Abrar M., Giyanto B. P., Johan O., Budiyanto A., Dzumalek A. R., et al. (2020). Research Center for Oceanography-Indonesian Institute of Sciences, Jakarta. Vi+88 p. Available at: http://indocoasting.id.
Haya L. O. M. Y., Palupi R. D., Subhan S., Rahmadani R. (2023). Patterns of coral diseases linked to the impact of climate change: a case study of scleractinia corals inSoutheast Sulawesi, Indonesia’s coral triangle. Model. Earth Syst. Environ. 9:4265–4277. Springer Science and Business Media LLC. doi: 10.1007/s40808-023-01745-y
Hazraty-Kari S., Tavakoli-Kolour P., Das R. R., Farhadi M., Barkhordari-Ahmadi A., Yahyavi M., et al. (2021). Baseline assessment of coral diseases in an environmentally extreme environment of the northern Persian Gulf. Marine Pollut. Bullet. 171, 112707. doi: 10.1016/j.marpolbul.2021.112707
Huang C. Y., Hwang J. S., Yamashiro H., Tang S. L. (2021). Spatial and cross-seasonal patterns of coral diseases in reefs of Taiwan: high prevalence and regional variation. Dis. Aquat. Organisms 146, 145–156. doi: 10.3354/dao03624
Johan O., Idris (2022). “The abundance of coral disease and compromisedhealth in Sabu Raijua Waters, East Nusa Tenggara,” IOP Conf. Series: Earth and Environmental Science, Vol. 967. 012020. IOP Publishing Ltd. doi: 10.1088/1755-1315/967/1/012020
Johan O., Kristanao A. H., Haryadi J., Radiarta I. N. (2014). Puncak prevalensi penyakit karang jenis sabuk hitam (Black Band Disease) di Kepulauan Seribu, Jakarta. Jurnal Riset Akuakultur 9 (2), 307–317.
Johan O., Zamany N. P., Smith D., Sweet M. J. (2016). Prevalence and incidence of black band disease of scleractinian corals in the Kepulauan Seribu Region of Indonesia. Diversity 8 (11), 1–9. doi: 10.3390/d8020011
Kurniawan C., Waluyo T. B., Sabayang P. (2011). “Particle size analysis usingfree-software imageJ,” in Conference: Seminar Nasional Fisika 2011. Pusat Penelitian Fisika-LIPI, Serpong. 8p.
Plass-Johnson J. G., Cardini U., Van Hoytema N., Bayraktarov E., Burghardt I., Naumann M. S., et al. (2015). “Coral bleaching,” in Environmental indicators (Netherlands: Springer), 117–146. doi: 10.1007/978-94-017-9499-2_9
Purbonegoro T., Cordova M. R., Puspitasari R., Hindarti D. (2018). Inhibition Effects of Jakarta Bay Sediments to the Growth of Marine Diatom (Chaetoceros Gracilis). Bulletin of the Marine Geology 33(2):77–78. 276951-inhibition-effects-of-jakarta-bay-sedime-7080801e.
Rambe A. R., Suhendra T., Kusuma H. A. (2022). Drifter buoy design for measuring ocean currents using the lagrangin method. Student Online J. Universitas Maritim Raja Ali Haji. 3 (1), 1–3.
Randall C. J., Jordan-Garza A. G., Muller E. M., Van Woesik R. (2016). Does dark-spot syndrome experimentally transmit among caribbean corals’. PloS One 11 (1), e0147493. doi: 10.1371/journal.pone.0147493
Rutzler K., Santavy D. L., Antonius A. (1983). The black band disease of atlantic reef corals. Mar. Ecol. 4 (4), 329–358. doi: 10.1111/j.1439-0485.1983.tb00118.x
Sabdono A., Radjasa O. (2008) The black band disease of Indonesian coral reefs. Available at: https://nsuworks.nova.edu/occ_icrs/2.
Sato Y., Bourne D. G., Willis B. L. (2011). Effects of temperature and light on the progression of black band disease on the reef coral, Montipora hispida. Coral Reefs 30 (3), 753–761. doi: 10.1007/s00338-011-0751-5
Sato Y., Civiello M., Bell S. C., Willis B. L., Bourne D. G. (2016). Integrated approach to understanding the onset and pathogenesis of black band disease in corals. Environ. Microbiol. 18 (3), 752–765. doi: 10.1111/1462-2920.13122
Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). “NIH Image to ImageJ: 25 years of image analysis”. Nat. Methods 9, 671–675.
Smith G. W., Smith M.-A. (2009). “Coral disease and global climate change,” in Proceedings of the 2007 National Conference on Environmental Science and Technology. National Conference on Environmental Science and Technology. 233–237. doi: 10.1007/978-0-387-88483-7
Soeboer D. A., Iskandar B. H., Jaya I., Imron M. (2018). Design and construction of the bouy for detecting surface current movement using GPS instrument. Albacore 2 (3), 263–277.
Sutherland K. P., Porter J. W., Torres C. (2004). Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Marine Ecology Progress Series 266, 273–302. http://www.jstor.org/stable/2486757
Ulfah M., Turnip I. N., Octavina C., Dewiyanti I., Purnawan S. (2022). Prevalence and abundance of coral disease in Aceh Besar, Aceh regency, Indonesia. Depik Jurnal Ilmu-Ilmu Perairan Pesisir dan Perikanan. 11 (3), 490–496. doi: 10.13170/depik.11.3.26593
Voss J. D., Richardson L. L. (2006). Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 25, 569–576. doi: 10.1007/s00338-006-0131-8
Wada N., Mano N., Yanagisawa Y., Mori T. (2017). Occurrence of coral diseases at Akajima, Okinawa, Japan in 2010 and 2011. Galaxea, Journal of Coral Reef Studies 19, 35–44.
Williams D. E., Miller M. W. (2005). Coral disease outbreak: Pattern, prevalence and transmission in Acropora cervicornis. Mar. Ecol. Prog. Ser. 301, 119–128. doi: 10.3354/meps301119
Yusri S., Estradivari (2007). Distribusi Infeksi Penyakit White Syndromes dan Karang Memutih (Coral Bleaching) pada Komunitas Karang Keras di Pulau Petondan Timur, Kepulauan Seribu. Berita Biologi 8 (4), 223–229. doi: 10.14203/beritabiologi.v8i4.2112
Keywords: coral reef, death, disease trigger, season, survival
Citation: Ofri OJ, Ginanjar R, Rustam A, Ardi I, Sholichah L, Rachmawati R, Siringoringo RM, Abrar M, Sari NP, Giyanto, Samusamu AS, Undap NI, Rachmawati PF, Zamani NP, Sweet M and Kunzmann A (2024) Spatio-temporal variation in progression rates of black band disease between Pramuka Island and Pari Island of the Seribu Islands, Indonesia. Front. Mar. Sci. 11:1239956. doi: 10.3389/fmars.2024.1239956
Received: 14 June 2023; Accepted: 12 January 2024;
Published: 12 April 2024.
Edited by:
Thamasak Yeemin, Ramkhamhaeng University, ThailandReviewed by:
Renato Crespo Pereira, Fluminense Federal University, BrazilParviz Tavakoli-Kolour, University of the Ryukyus, Japan
Copyright © 2024 Ofri, Ginanjar, Rustam, Ardi, Sholichah, Rachmawati, Siringoringo, Abrar, Sari, Giyanto, Samusamu, Undap, Rachmawati, Zamani, Sweet and Kunzmann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ofri Johan, b2ZyaS5qb2hhbkBicmluLmdvLmlk
 Ofri Johan Ofri
Ofri Johan Ofri Rendy Ginanjar1
Rendy Ginanjar1 Lili Sholichah
Lili Sholichah Rita Rachmawati
Rita Rachmawati Rikoh Manogar Siringoringo
Rikoh Manogar Siringoringo Muhammad Abrar
Muhammad Abrar Neviaty Putri Zamani
Neviaty Putri Zamani Michael Sweet
Michael Sweet Andreas Kunzmann
Andreas Kunzmann