
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 25 January 2024
Sec. Marine Megafauna
Volume 11 - 2024 | https://doi.org/10.3389/fmars.2024.1164026
This article is part of the Research Topic The ACCOBAMS Survey Initiative (ASI): Implementing Large Scale Surveys for Marine Megafauna in the Mediterranean and Black Seas View all 13 articles
Acoustic surveys for sperm whales (Physeter macrocephalus) were conducted in the Mediterranean Sea in summer 2018 as part of the vessel-based component of the ACCOBAMS Survey Initiative (ASI). Equal-spaced zigzag transects provided uniform coverage of key sperm whale habitats and were surveyed using a towed hydrophone array deployed from a research vessel at speeds of 5-8 knots. A total of 14,039 km of tracklines were surveyed in the western basin, Hellenic Trench and Libyan waters, with an acoustic coverage of 10% realised for sperm whales. During these surveys, 254 individual sperm whales were detected on the trackline, with an additional 66 individuals off-track. Sperm whales were only seen ten times on-track, with an additional 16 off-track sightings. Estimates of slant range to echolocating whales were used to derive density estimates through both design- and model-based distance sampling methodologies. An acoustic availability of 0.912 (sd = 0.036) was derived from via published models. When correcting for availability bias, a design-based abundance estimates of 2,673 individuals (95% CI 1,739-4,105; CV = 0.21) was derived for the surveyed blocks, which incorporated most known sperm whale habitat in the Mediterranean Sea. The equivalent model-based estimate was 2,825 whales (2,053-3,888; CV = 0.16). Over 97% of detected whales were in the western basin, with highest densities in the Algerian and Liguro-Provencal Basins between Algeria and Spain/France. In the eastern basin, detections were sparse and concentrated along the Hellenic Trench. A density surface modelling (DSM) exercise identified location and benthic aspect as being the most instructive covariates for predicting whale abundance. Distance sampling results were used in a power analysis to quantify the survey effort required to identify population trends. In the most extreme scenario modelled (10% per annum decline with decennial surveys), the population could have dropped by 90% before the decline was identified with high statistical power. Increasing the regularity of surveys would allow population trends to be detected more expediently. Mediterranean sperm whales are listed as Endangered on the IUCN’s Red List and the need for urgent conservation measures to reduce injury and mortality remains paramount for this unique sub-population.
Sperm whales (Physeter macrocephalus, Linnaeus, 1758) are the largest of the toothed whales (Whitehead, 2017), and are found in all deep waters of the Mediterranean Sea, from the Strait of Gibraltar to the Levantine Basin (Ryan et al., 2014; Rendell and Frantzis, 2016; Lewis et al., 2018). Although it is unclear how a viable breeding population of sperm whales first established itself in the region, sperm whale vertebrae have been discovered in excavations of a Phoenician colony in western Sicily dating from the sixth to fifth centuries BCE (Reese, 2005). The first written account of sperm whales appears to come from Aristotle’s description in the fourth century BCE of a whale with the “air passage in its forehead” (Balme, 2011). Although the Mediterranean Sea is connected to the neighbouring North Atlantic Ocean, the shallow Camarinal Sill to the west of the Strait of Gibraltar may act as a significant barrier to the passage of sperm whales, essentially containing the Mediterranean individuals as a discrete sub-population. This is supported by genetic studies (Drouot et al., 2004a; Engelhaupt et al., 2009) that indicate little genetic flow between the Mediterranean Sea and Atlantic Ocean. Additional evidence of population segregation comes from acoustic studies investigating the variation of ‘codas’, stereotyped patterns of broadband clicks used in communicative contexts. Mediterranean sperm whale codas are distinctive compared with those in other regions: they are broadly dominated by the 3 + 1 type (67-98% of all codas recorded; Pavan et al., 2000; Drouot et al., 2004b; Teloni, 2005). As codas appear to be acquired via cultural transmission (Rendell et al., 2012), the relatively homogeneous repertoire in the Mediterranean provides further evidence of an isolated population.
Robust baseline information on the abundance and density of sperm whales in the Mediterranean is required to ensure they are protected appropriately. Although the presence of sperm whales in the Mediterranean has been established for several centuries, estimating the size of the population has proved challenging. This is partly due to their routine deep-diving behaviour (to 800 m, Zimmer et al., 2005), prolonged submergence time (97% of the time, Watwood et al., 2006), and widespread distribution (across at least 21 separate national jurisdictions, Notarbartolo di Sciara and Tonay, 2021). Where they do exist, density estimates are typically confined to sovereign waters (e.g. Frantzis et al., 2014). There have been few large scale, multi-jurisdiction surveys. Gannier et al. (2002) conducted surveys from 5°W to 30°E over four years and derived acoustic and visual encounter rates for sperm whales. Rendell et al. (2014) used photo-identification to estimate the abundance of individuals (approximately 400) in Balearic, French, and Italian waters in the northwest Mediterranean. Laran et al. (2017) derived abundance estimates (95% CI 80–2,600) using aerial surveys for the waters of France, Monaco and Italy in the northwest Mediterranean. Lewis et al. (2007) estimated acoustic abundances (95% CI 24–165) from line-transect surveys in the northern Ionian Sea, Sicilian and Malta Channels. The only estimate of total population size in the Mediterranean comes from a series of acoustic line-transect surveys within the eastern and western basins that were extrapolated to unsurveyed areas to derive an estimate of 1,842 individuals (Lewis et al., 2018). These studies, in conjunction with inferred population declines due in part to bycatch and ship strike, have contributed to the Mediterranean sub-population of sperm whales being assessed as Endangered C2a(ii) on the IUCN Red List (Pirotta et al., 2021).
Deep-diving cetaceans may be under-recorded by traditional visual surveys as they have proportionally low surface availability to observers (Barlow and Taylor, 2005). Passive acoustic techniques can offer several advantages over visual methods for detecting submerged individuals, including extended strip widths, and detection at night or during periods of bad weather (Leaper et al., 1992; Barlow and Taylor, 2005). Sperm whales are particularly well suited for acoustic surveying as they generate regular loud clicks that can be detected up to 20 km away (apparent source levels up to 236 dB re: 1µPa rms; Møhl et al., 2003; Zimmer et al., 2005). Furthermore, they are vocal throughout 60-80% of their dive cycles (Douglas et al., 2005; Watwood et al., 2006; Teloni et al., 2008; Fais et al., 2016). As sperm whale clicks have rapid onsets, the time-of-arrival differences between two or more hydrophone elements can be used to derive bearing information; the triangulation of bearing lines for successive clicks in a click train can allow robust distance estimates to be derived (Leaper et al., 1992; Matthews, 2014). Thus, acoustic detections of sperm whales lend themselves well to distance sampling techniques for estimating density. Such estimates have been derived for the central islands of the Azores (Leaper et al., 1992), waters of South Georgia (Leaper et al., 2000), the Faroes Shetland Trough (Hastie et al., 2003), a section of the Eastern North Pacific (Barlow and Taylor, 2005), a naval range in the Bahamas (Ward et al., 2012), the Canary Islands (Fais et al., 2016), the Mediterranean Sea (Lewis et al., 2007; Lewis et al., 2018), offshore Irish waters (Gordon et al., 2020) and the northern Gulf of Mexico (Li et al., 2021).
As several cetacean populations in the Mediterranean and Black Seas are threatened by human activities (Reeves and Notarbartolo di Sciara, 2006), robust information on population trends is necessary to evaluate the effectiveness of conservation measures. For sperm whales, entanglement in nets (Notarbartolo di Sciara and Tonay, 2021) and collisions with ships (Frantzis et al., 2019) continue to be significant causes of mortality. In addition, pollution (including chemical and noise), ingestion of plastic debris and disturbance from vessels all contribute to the species’ assumed decline in the region (Rendell and Frantzis, 2016; Pirotta et al., 2021). Responding to the urgent need for improved knowledge of cetacean populations in the region, the ACCOBAMS (Agreement on the Conservation of Cetaceans of the Black Sea, Mediterranean Sea and continuous Atlantic area) Secretariat coordinated the first ever large-scale survey of marine megafauna in the Mediterranean Sea during the summer of 2018. In light of increasing human activities at sea, the ACCOBAMS Survey Initiative (ASI) was organised with the participation of range states to generate robust assessments of the status of cetacean populations. Approximately 75% of the Mediterranean basin was surveyed by aerial teams (Cañadas et al., 2023; Panigada et al., 2023), while simultaneous vessel-based surveys prioritised areas not surveyed by plane and known to be important for deep-diving cetaceans that may be under-represented by aerial surveys. The majority of the vessel-based component of the ASI was conducted from the research vessel Song of the Whale and the results of these combined visual and passive acoustic surveys are presented here. The primary aim of this work was to enable improved detection of sperm whales during the ASI and generate both design- and model-based estimates of local density and basin-wide abundance.
Distance sampling methodologies can provide robust estimates of the density and abundance of a species in a defined space and time (Buckland et al., 2004) and can also detect potential trends (Taylor et al., 2007). Standard line transects methods assume the density of animals on the surveyed tracks is representative of the density in the entire study area; this will typically be true if the transects are designed systematically with a random component (such as a random start) and each part of the study area has an equal probability of being surveyed. Therefore, transects for the ASI vessel surveys were designed as equal-spaced zigzags using Distance 7.3 software (Thomas et al., 2010) to provide almost uniform coverage probability. Transects were designed within the same survey blocks used for the ASI aerial surveys, with minor modifications made due to logistical constraints (such as security considerations and permit restrictions). Transects were designed to provide acoustic coverage of at least 6% based on an estimated strip half-width (ESHW) of 10 km for sperm whales (based on similar research conducted from the same research vessel; Lewis et al., 2018). A total of 17,272 km of transects were designed for the vessel-based surveys conducted by the Song of the Whale team (Figure 1).
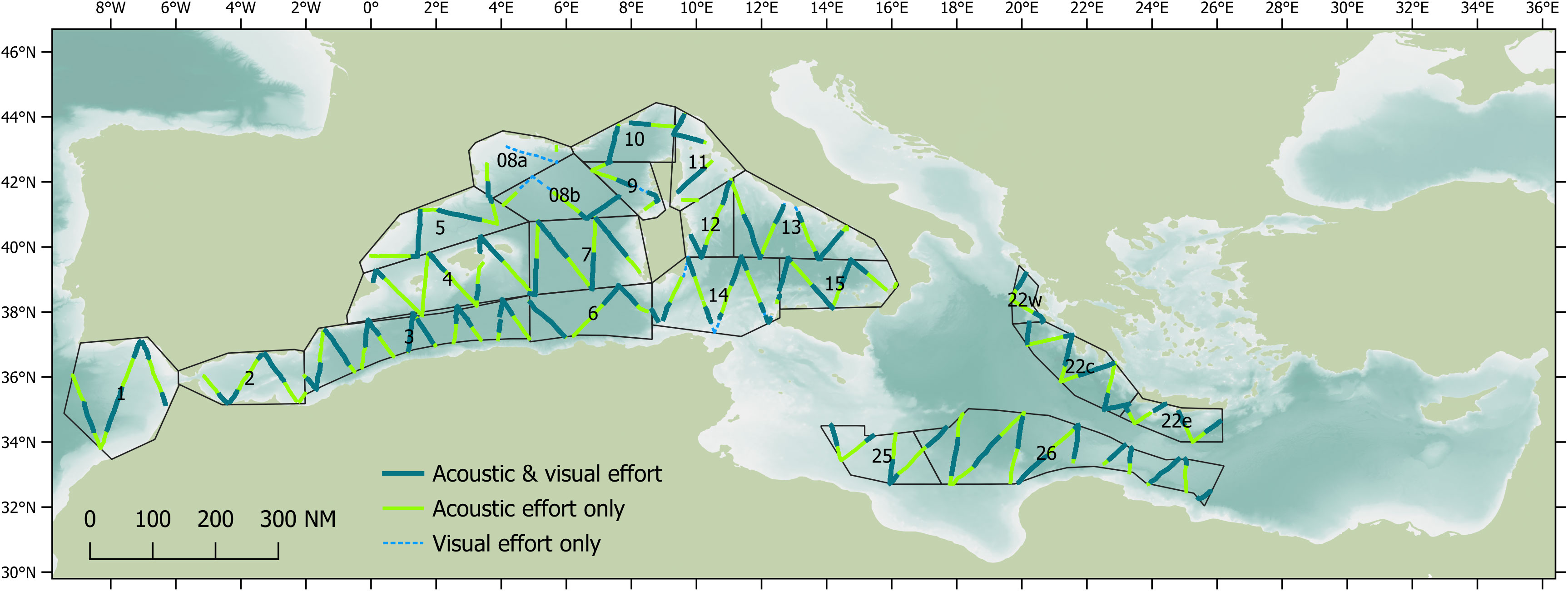
Figure 1 Acoustic and visual survey effort realised by the Song of the Whale team in 2018. Survey blocks are based on those also surveyed by ASI aerial teams.
Surveys were conducted from R/V Song of the Whale, a 21 m auxiliary-powered cutter-rigged sailing research vessel. Acoustic effort was conducted 24 hours a day when the depth was sufficient to tow a hydrophone array (> 50 m). Survey speeds of 5 to 8 knots were optimal for both minimising cable strum and biases related to animal movement, being 2-3 times faster than the speed of the target animals (Buckland et al., 2015); the mean speed of sperm whales is typically 2.1 knots (Whitehead, 2017). The towed array consisted of a 400 m tow cable attached to multiple hydrophone elements in an oil-filled tube. The array incorporated a pair of AQ-4 elements (Teledyne Benthos) with a flat frequency response ( ± 1.5 dB) from 1 Hz to 30 kHz and receiving sensitivity of -201 dB re 1V/µPa. Pre-amplifiers with 29 dB gain were used to prevent voltage drop between the array and the research vessel. Each hydrophone element was separated by 3 m. The array outputs were digitised at 500 kHz by a SAIL DAQ cards (SA Instrumentation) after a 10 Hz high pass filter and 12 dB gain had been added to the signal; the high sample rate was chosen to allow for the detection of the ultrasonic clicks of beaked whales (see ACCOBAMS, 2021). Signals from the two elements were decimated to 48 kHz using a low pass 4th order Chebyshev filter with a cut off frequency of 20 kHz (i.e. approximately 0.8 times the Nyquist frequency to avoid aliasing) and monitored in real-time using a click detector module in PAMGuard (Gillespie et al., 2008) configured to detect candidate sperm whale clicks. Recordings were written to disk as 16-bit wav files. The 400 m tow cable provided typical array depths of 29-33 m (see Figure 7 in Boisseau et al., 2023 for the towing profile). As the mixed layer depth is typically shallower than 20 m in summer (Houpert et al., 2015), the array was assumed to tow below any thermocline which could refract upwelling clicks from sperm whales.
Visual effort was conducted by two dedicated observers from an elevated observation platform (mean eye height of 5.4 m). Effort was separated in two quadrants, with observers primarily scanning the trackline ahead of the vessel with the naked eye; a starboard observer scanned the sector from 340-90° and a port observer from 270-20° degrees. Observers used 7x50 binoculars to confirm details of sightings. Observers reported species identity, range (estimated by eye), bearing (from angle boards) and group size to another team member acting as a dedicated data recorder. The data recorder saved the information to a survey database using Logger software (www.marineconservationresearch.org). Logger also logged the vessel’s GPS stream with the heading from a GPS gyro sensor to the database; various other parameters, including wind speed and direction measured by masthead instruments, were also logged automatically every 10 seconds. Environmental information (including sea state, wave and swell height, cloud cover and glare) were logged manually every hour, or when there was a significant change in conditions (Lewis et al., 2018).
Recordings made in the field were independently re-examined in PAMGuard by two experienced analysts (OB and JR) to identify candidate sperm whale click trains. Sperm whale clicks have stereotypical spectral properties (with most energy at or below 12 kHz), waveforms (with rapid onset and offset and evidence of multiple pulses within each click) and inter-click intervals (a regular click being produced every 1-2 seconds (Leaper et al., 1992; Møhl et al., 2003). Candidate clicks were identified as forming part of a click train, i.e. with similar bearings and regular inter-click intervals. Differences in bearing information were used to identify individual click trains (Lewis et al., 2018); the standard deviation of consecutive clicks from a focal animal is typically less than one degree (when bearings are greater than 15°; Rankin et al., 2008). Thus, acoustic detections were made at the individual level rather than the group level. Estimates of slant range to individual whales were made in PAMGuard using the target motion analysis (TMA) module. A towed array will detect multiple sequential clicks from a focal animal; if the source is assumed stationary, then each click will be detected with a time differential on the two hydrophone elements. Successive sets of time delays can be visualised as 2D bearings converging on the likely sperm whale location. To overcome the left/right ambiguity inherent in a linear array, PAMGuard calculates a chi-squared goodness-of-fit between the expected and observed bearings, and the side with the smaller value is considered the best convergence point.
Slant ranges to sperm whale clicks detected when the survey vessel was ‘on-track’ and following the survey protocol (i.e. traveling at 5-8 knots) were used to generate acoustic detection functions and density estimates, using multiple covariates distance sampling (MCDS) in Distance 7.3. Without information available to determine the depth of vocalising animals (for example through the use of time-depth recorder tags), slant ranges could not be accurately converted to perpendicular distances. Thus, as most sperm whales are detected at depth, using uncorrected slant ranges does not incorporate the vertical component of their location (Westell et al., 2022). To increase the robustness of the analysis, the distance data were right-truncated where the probability of detection was estimated to be approximately 0.15 (Buckland et al., 2001). Detection functions were modelled using a key function (either half-normal or hazard rate) with an adjustment term (null, cosine, simple polynomial or Hermite polynomial). C ovariates that could modify the noise field around the hydrophone array, and thus affect the likelihood of detecting clicks, were included in the analysis to modify the scale of the detection function without affecting its shape. These covariates were logged at least every hour in the field and included sea state (Beaufort scale), wave height (m), swell height (m) and rain condition (heavy, light or none); in addition, instruments on the research vessel logged wind speed (knots), sea surface temperature (SST; °C), engine speed (rpm), vessel heading (° true) and vessel speed (knots) every 10 seconds. These covariates were investigated for collinearity using Pearson’s correlation coefficient to remove any redundancy; all remaining covariates were subsequently incorporated into model generation. Models were initially generated with single covariates; the best-fitting detection function was selected using Akaike’s Information Criterion (AIC). Forward stepwise selection was then conducted by adding one additional covariate at a time to a model containing the one(s) already selected until there was no decrease in AIC. Densities could then be estimated using traditional design-based approaches (Lewis et al., 2018), both with and without a correction for availability. Availability for detection is influenced by both whale behaviour (specifically the proportion of time sperm whales spend clicking) and by survey protocol (as survey speed affects the length of the time window during which whales can be detected). In the absence of detailed information of the vocal behaviour of individual sperm whales during the ASI surveys, for example via the application of suction-cup tags, the acoustic availability of sperm whales was taken from a Monte Carlo simulation performed for tagged sperm whales in the Azores (Fais et al., 2016). ESHW and mean vessel speed were used to determine availability bias.
In addition to design-based estimates, the survey transects were sub-divided into short segments of homogeneous effort type and detection probabilities for individual sperm whales were used in subsequent density surface modelling (DSM). To derive model-based estimates of density, the encounter rate data were fitted to density covariates using a generalised additive model (GAM; Wood, 2006), assuming local density varied in space and in response to specific environmental covariates. In addition to latitude and longitude, several bathymetric and oceanographic parameters were used to generate the DSM (Table 1) and were selected on the basis of their potential to influence sperm whale distribution and their availability for the whole survey area. These parameters have been linked to sperm whale distribution in other studies, and included depth (Cañadas et al., 2005; Pirotta et al., 2011; Mannocci et al., 2017b; Pace et al., 2018; Pirotta et al., 2020), slope (Cañadas et al., 2005; Praca and Gannier, 2008; Pirotta et al., 2011; Pirotta et al., 2020), aspect (Pirotta et al., 2011; Pirotta et al., 2020), SST (Cañadas et al., 2005; Praca and Gannier, 2008; Pirotta et al., 2011; Pirotta et al., 2020), chlorophyll (Jaquet et al., 1996; Praca and Gannier, 2008; Mannocci et al., 2017b), distance to isobath (including 0, 200 and 1,000 m; Praca and Gannier, 2008; Pace et al., 2018; Sahri et al., 2020; Avila et al., 2022), distance to bathymetric features (such as canyons, escarpments, ridges, seamounts, shelves, slopes, terraces and troughs; Mannocci et al., 2017b; Sahri et al., 2020; Vachon et al., 2022), mixed layer thickness (Avila et al., 2022) and local currents (Vachon et al., 2022). Dynamic oceanographic parameters, such as SST, chlorophyll, depth of mixed layer and water speed/direction, can vary at time-scales from seconds to decades. As animal associations with large scale and persistent oceanographic features are best modelled with climatological covariates (Mannocci et al., 2017a), composites of dynamic covariates were used that approximately overlapped with the duration of the survey. ‘Instantaneous’ covariates were not used, as these are typically more useful for modelling associations with ephemeral and/or fine-scale features.
In addition to generating density estimates for the surveyed blocks, DSM allowed extrapolation to the entire Mediterranean Sea. To achieve this, a prediction grid was generated for the Mediterranean Sea by dividing the region into 3,634 grid cells with a resolution of 8 km latitude by 8 km longitude (Lambert azimuthal equal-area projection); grid resolution was selected to correspond with the approximate lowest resolution of the available covariates. Segments should be small enough such that neither whale density nor covariate values vary markedly within a segment (Miller et al., 2013); making segments approximately square is usually sufficient to achieve this. As each segment is no wider than twice the truncation distance (4 km in this study), using a segment length of 8 km ensured segments were approximately square. Increasing the size of segments can reduce the number of ‘empty’ segments (i.e. those without detections); however, if segment size becomes too large, the ability of the model to identify associations with persistent oceanographic features can become compromised. The segment length of 8 km in this study was larger than that used in similar modelling exercises in nearby regions (e.g. means of 6.96 and 5.84 km respectively for the North-East Atlantic; Cañadas et al., 2009; Rogan et al., 2017), but was deemed unlikely to reduce model performance as it approximately matched the resolution of the covariates. Only regions with waters deeper than 200 m were considered for the prediction grid to exclude those regions not likely to provide suitable sperm whale habitat (Pirotta et al., 2011; Lewis et al., 2018). The covariates described in Table 1 were averaged over each grid cell. Survey effort was sub-divided into segments approximately twice the truncation distance of the dataset (i.e. 8 km), making the two-dimensional outline of a segment approximately square. The centroid of each segment was assigned to a cell in the prediction grid, and the average values of all covariate in that cell were assigned to that segment. The response variable used to model sperm whale distribution was the count of individuals in each segment, once corrected using the detection function generated during MCDS; the effective area of each segment (defined as the actual area multiplied by the estimated probability of detection using the selected detection function) served as an offset in the model. When taking availability bias into account, this offset was divided by the correction factor for availability. Spatial location was included in the model as a bivariate smooth of x and y (metres east and north respectively). As smoothing over areas with complicated boundaries, such as islands and peninsulas, can lead to the inappropriate linking of different regions (Wood et al., 2008; Miller et al., 2013), a realistic spatial model should be fitted to the data to provide valid inference. A soap film smoother was used to allow boundary conditions to be estimated for the complex study area and to be incorporated in to a bivariate smooth function of location (Wood et al., 2008); the complexity of the soap film was set to 10 knots. Smooth functions of the environmental covariates were constructed using thin plate regression splines with shrinkage, except for the circular variables aspect and water direction which used cyclic cubic regression splines. The Tweedie distribution with logarithmic link function was assumed for the response variable, an approach that adequately handles zero-inflated spatial models (Miller et al., 2013). GAMs were fitted using the “dsm” R package (R Core Team, 2021). Model selection was conducted by adding one candidate explanatory variable at a time in a forward approach. The model selected at each step was chosen by looking for an improvement in the Restricted Maximum Likelihood (REML) score and percentage of variation explained; randomised quantile residuals and quantile-quantile plots were also examined for normality, auto-correlation and homoscedasticity. REML was used for model selection as it derives less variable estimates of the smoothing parameter than other criteria (Wood, 2011); comparing REML scores is appropriate for models that use shrinkage in the smoothing penalty. Maps showing extrapolated densities for the whole Mediterranean were created in QGIS using the outputs from the DSM procedure for the surveyed study area. Variance estimates of abundance were derived by combining the variances of the GAM and detection function using the delta method.
Repeated survey effort allows population trends to be identified; the greater the survey effort, the more rapidly any changes can be identified. To investigate the power of repeated surveys to detect significant changes, the general inequality model of Gerrodette (1987) was used, whereby:
where r is the annual rate of population change, n is the number of surveys, CV is the coefficient of variation for the population estimate, zα/2 is the one-tailed probability of a Type 1 error (false positive) and zβ is the probability of making a Type II error (false negative). For subsequent calculations, the corrected population size estimated by model-based distance sampling was used, along with the attendant CV. The influence of different levels of survey effort was investigated by varying the inter-survey interval from one year (i.e. annual surveys) to 10 years (i.e. decennial surveys). Following Taylor et al. (2007), statistical power was assessed at both a high level (i.e. 0.95) and an acceptable level (i.e. 0.80).
Between 28th May and 29th September 2018, the Song of the Whale team completed almost 22,000 km of survey effort in both the eastern and western basins as part of the ACCOBAMS Survey Initiative (Figure 1). Approximately 14,039 km (66%) was “on-track”, following pre-determined survey transects at 5-8 knots with acoustic effort; visual effort was conducted during daylight hours when weather conditions were appropriate. A total acoustic coverage of 8.3% was realised, based on an ESHW of 3.5 km. Sperm whales were detected acoustically throughout the western basin of the Mediterranean Sea, with additional detections in the Hellenic Trench in the eastern basin, and in the Atlantic approaches to the Strait of Gibraltar region (Figure 2). A total of 254 individual sperm whales were detected on- track, with an additional 66 individuals detected off- track (i.e. when off a transect or faster/slower than 5-8 knots). Sperm whales were seen only ten times on the trackline, with 16 sightings made off-track; observed group sizes ranged from one to seven individuals.
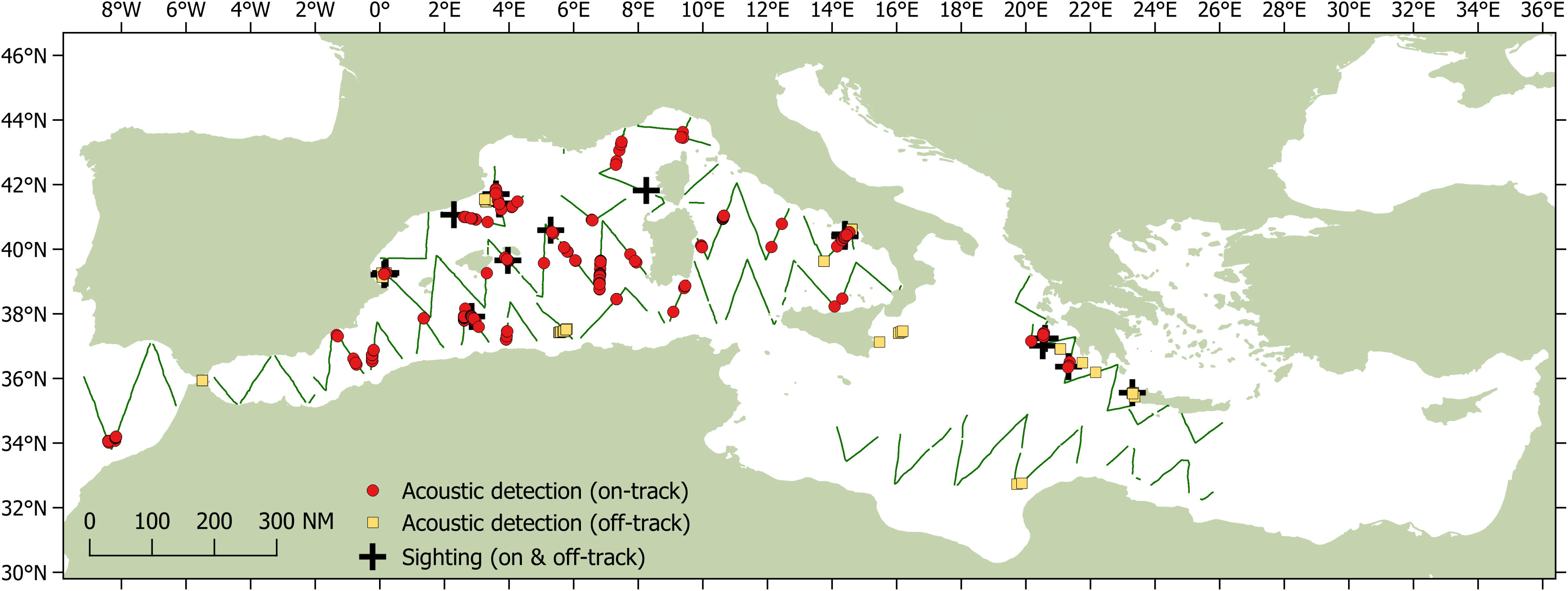
Figure 2 Sperm whale acoustic detections from Song of the Whale during the ASI survey. Individual whales detected on the track line are shown as red circles (n = 254); whales off-track are shown as orange squares (n = 66). Both on- and off-track sightings are shown as black crosses. Those sections of track survey using acoustic effort are shown as green lines.
Slant ranges estimated via TMA in PAMGuard were imported into the Distance software to generate acoustic detection functions and density estimates using MCDS. Only the 254 on-track detections were used. Distance data were right-truncated where the probability of detection was approximately 0.15 (Buckland et al., 2001); this excluded detections beyond 5000 m prior to analysis, representing 19% of the largest distance estimates. Prior to including covariates in subsequent analysis, they were first investigated for correlation using Pearson’s correlation. Wind speed and wave height were found to be strongly correlated (r 2 = 0.566, p<0.001); as wind speed was logged by a sensor on board R/V Song of the Whale, it was used in MCDS in lieu of the subjective estimates of wave height. The remaining covariates (vessel heading, vessel speed, engine revs, wind speed, wind direction, sea surface temperature, sea state, swell height and rain condition) were used to modify the detection function. A hazard rate key function without an adjustment term generated a detection function with the closest fit to the slant range estimates based on AIC scores. Inclusion of wind speed had the most pronounced effect on the detection function, deriving the lowest AIC score. Inclusion of additional covariates did not improve the fit of the model and thus only this covariate was included in the final model. A goodness-of-fit test suggested the detection function incorporating wind speed adequately represented the slant ranges (χ2(3,205) = 1.158, p = 0.763). The ESHW was 3,442 m (Figure 3). A quantile-quantile plot suggested model fit was adequate and randomised quantile residuals did not exhibit heteroscedasticity.
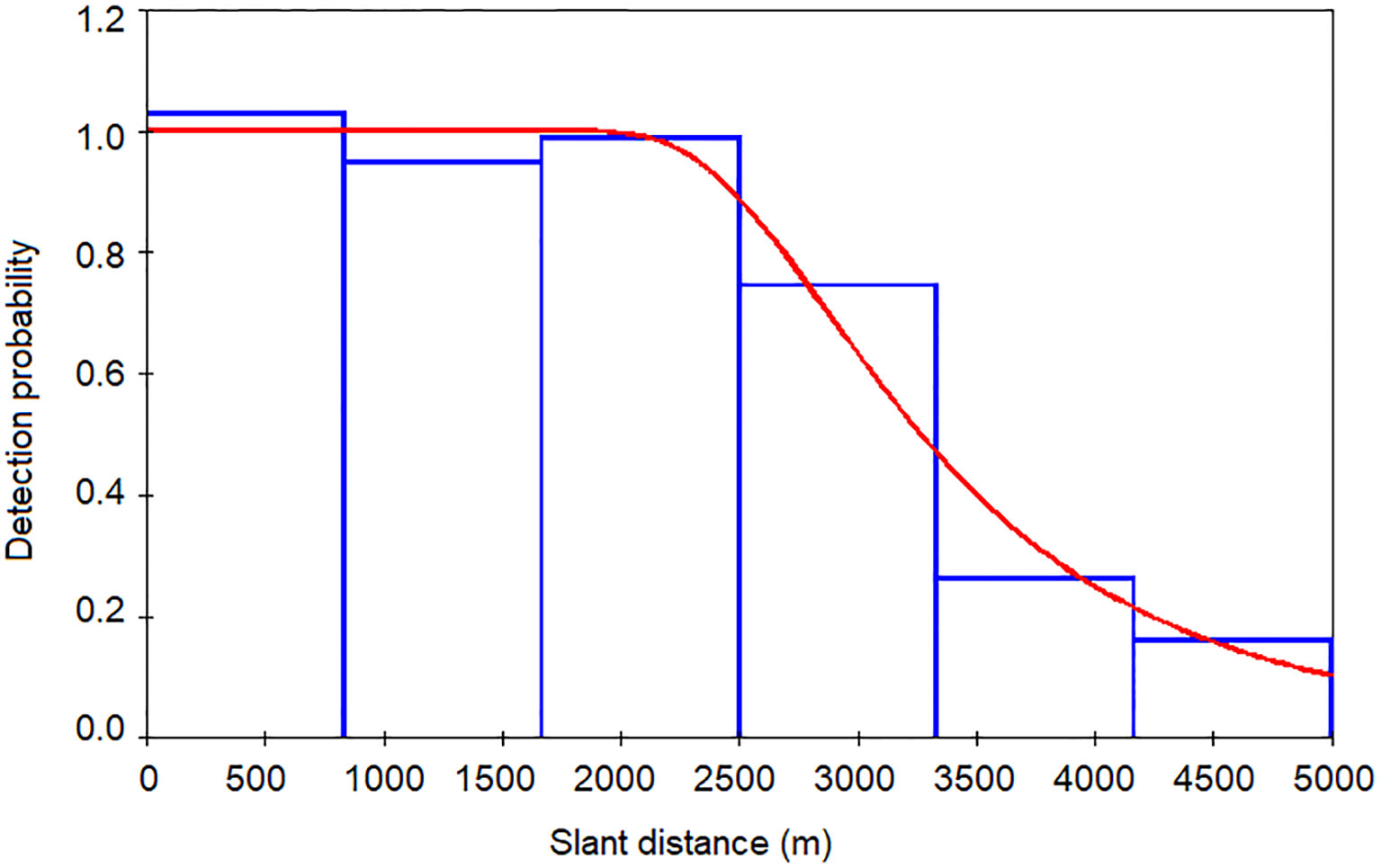
Figure 3 The detection function generated using MCDS (hazard rate key without adjustment). The covariate wind speed was used in the final model. Effective strip half-width was estimated as 3,442 m.
MCDS was used to generate density estimates for those blocks with a sufficient number of on-track detections (Table 2). Without an adjustment for g(0), the uncorrected total estimate was 2,439 whales (95% CI 1,598-3,717) which included most of the known habitat for sperm whales in the Mediterranean Sea. The acoustic availability of sperm whales was taken from a Monte Carlo simulation performed by Fais et al., 2016 for tagged sperm whales recorded in the Azores. An estimate for g(0) of 0.912 (sd = 0.036) was derived using an ESHW of 3.5 km and average survey speed of 6 knots. By incorporating this estimate of availability bias, a corrected abundance estimate of approximately 2,673 individual sperm whales was derived for the blocks surveyed (95% CI 1,739-4,105; CV = 0.212) (Table 3).
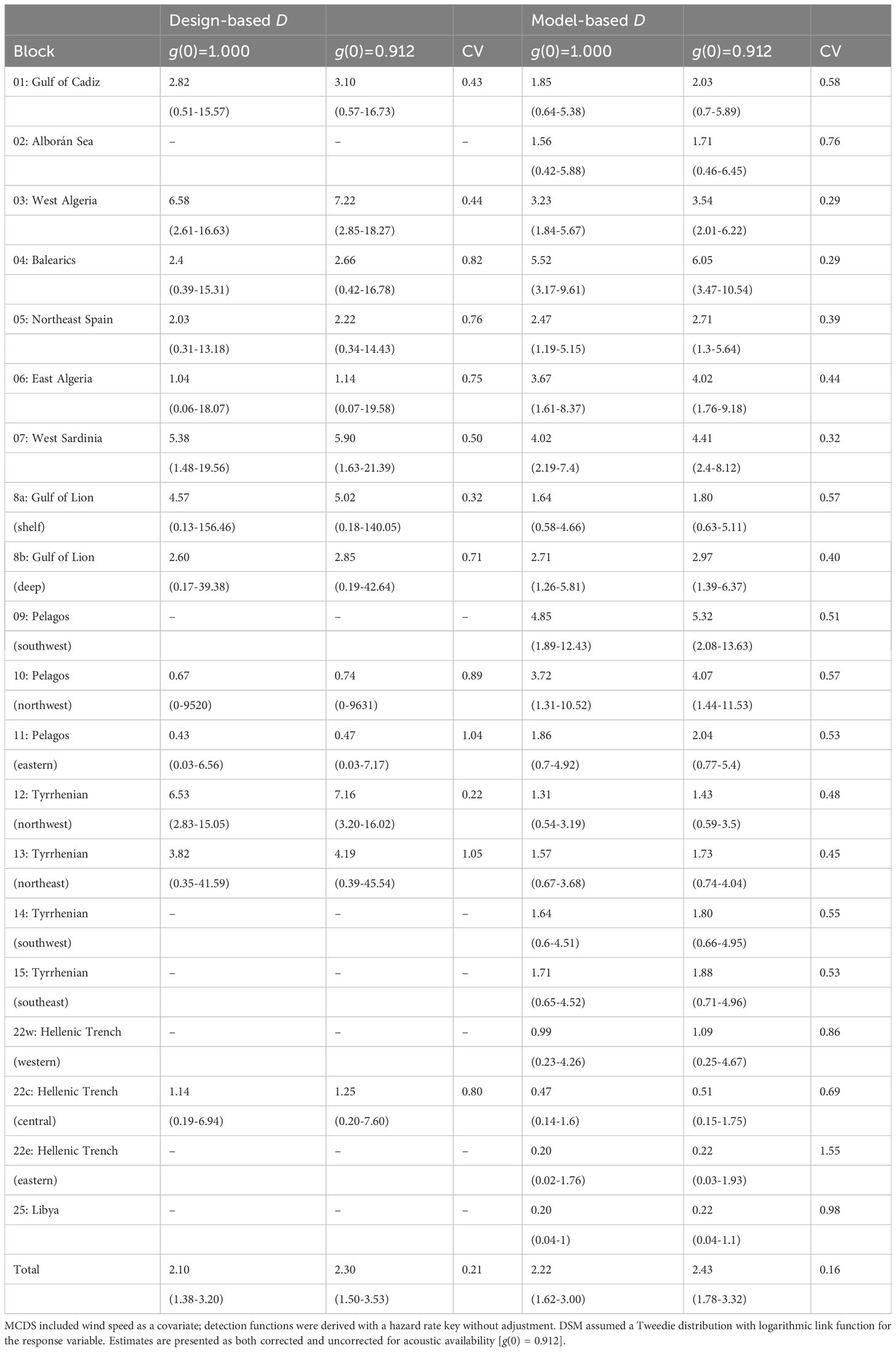
Table 2 Density (D) derived from design- and model-based approaches for each survey block expressed as the number of individuals per 1000 km2.
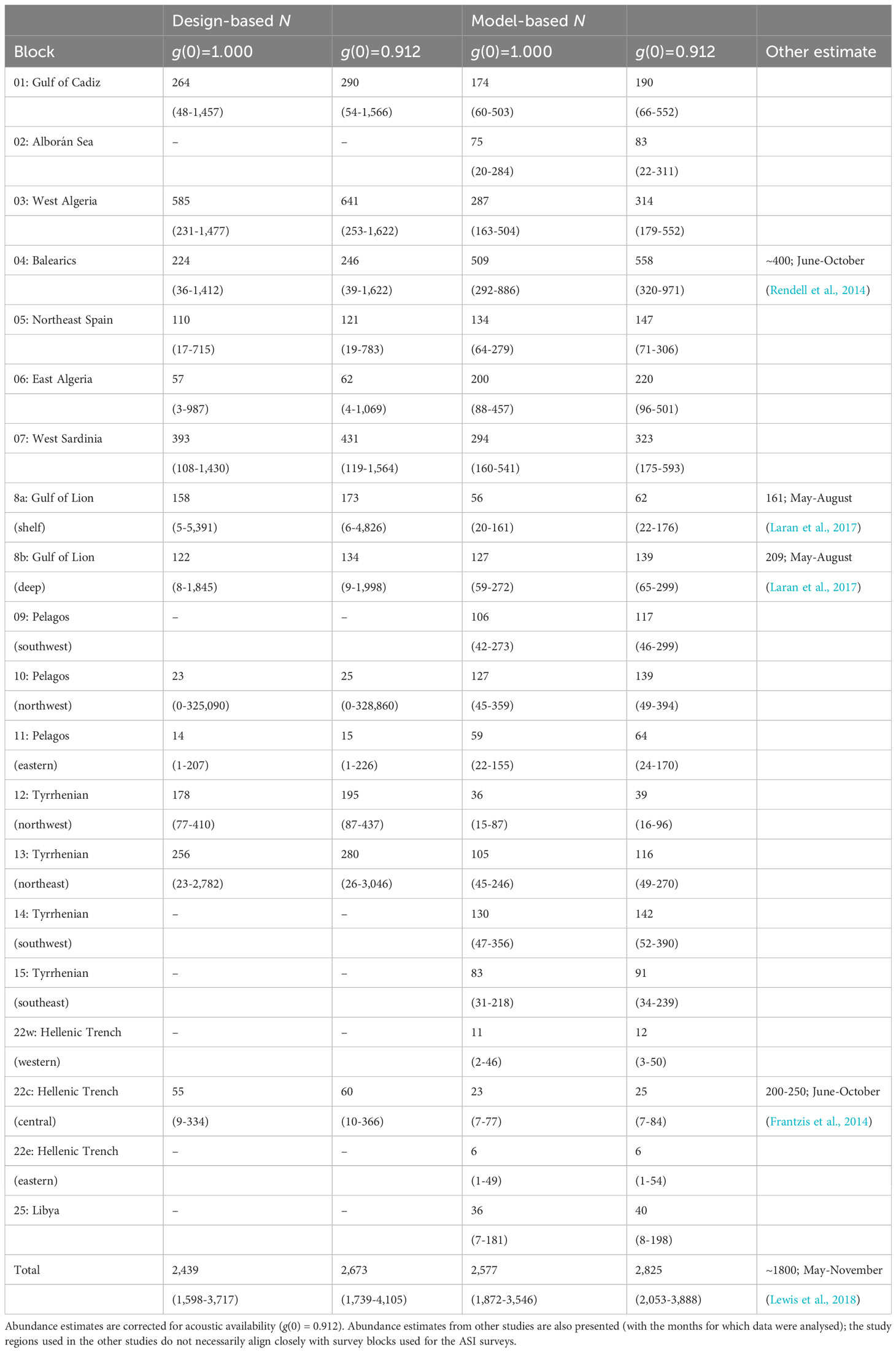
Table 3 Outputs from design- and model-based approaches to abundance (N) estimation for each survey block using wind speed as a covariate; detection functions were derived with a hazard rate key without adjustment.
The DSM procedure applied the detection function generated during MCDS (i.e. a hazard rate key function without an adjustment term incorporating wind speed as a covariate) to 720 segments of homogeneous effort type. As sperm whales tend to aggregate in clusters, and the study area was orders of magnitude larger the average segment size, many segments were ‘empty’ (n = 678). However, if larger segments were used, many may have had very similar covariate values, which could have reduced the utility of the model. The Tweedie distribution used in the model can be useful when modelling count data with a high proportion of zeros in the dataset (Miller et al., 2013). Using a simple bivariate smooth of location showed signs of ‘leakage’, particularly between the Tyrrhenian, Adriatic, and Aegean. To help address this leakage, the final DSM model selected included a soap film bivariate smooth of location (xy) along with a cyclic cubic regression of mean aspect, the former having the most pronounced effect on the model. Both covariates were considered significant (p< 0.01) and explained 32.2% of the deviance in the model. Densities were highest in the Algerian and Liguro-Provencal Basins (Table 2) and in regions of west-facing aspect (>180°; Figure 4). The DSM derived an uncorrected abundance estimate of 3,268 (95% CI 2,499-7,540; CV = 0.287) sperm whales for Mediterranean waters deeper than 200 m; the estimated abundance was 3,583 (95% CI 1,881-5,677) if corrected with a g(0) of 0.912 (Figure 5). If considering only the blocks surveyed by the Song of the Whale team, the uncorrected abundance estimate was 2,577 (95% CI 1,872-3,546; CV = 0.164); the estimated abundance was 2,825 (95% CI 2,053-3,888) if corrected with a g(0) of 0.912 (Table 3). The coefficients of variation associated with the DSM predictions are shown in Figure 6.
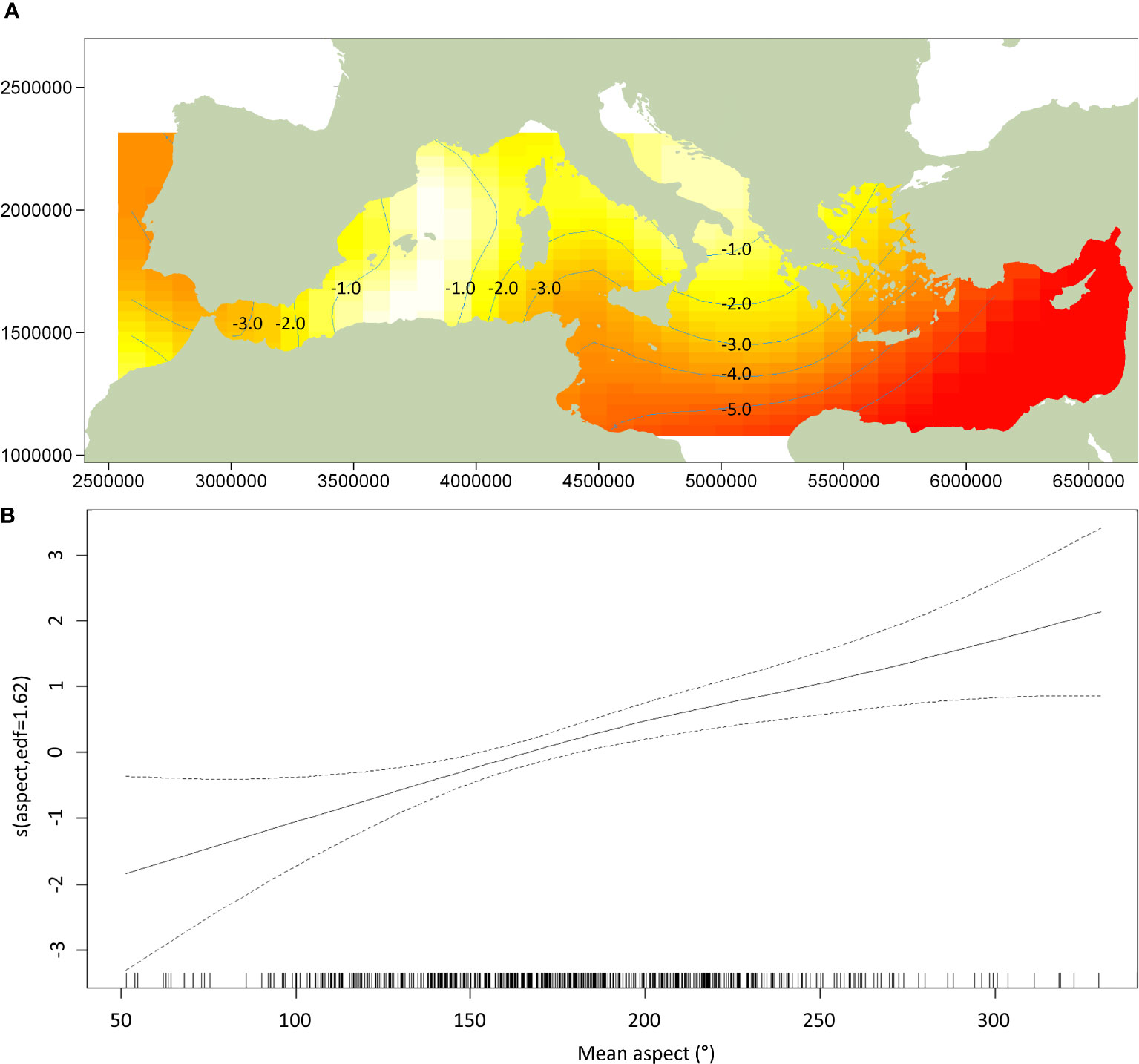
Figure 4 Plot of the GAM smooth fit of density across (A) location and (B) mean aspect. Values in plot (A) of the soap film spatial smooth are relative abundances; yellow indicates high values, red low indicates low values (Lambert azimuthal equal-area projection). In plot (B) of mean aspect, the solid line represents the best fit with dashed lines representing 95% confidence intervals; vertical lines on the x-axis are observed data values.
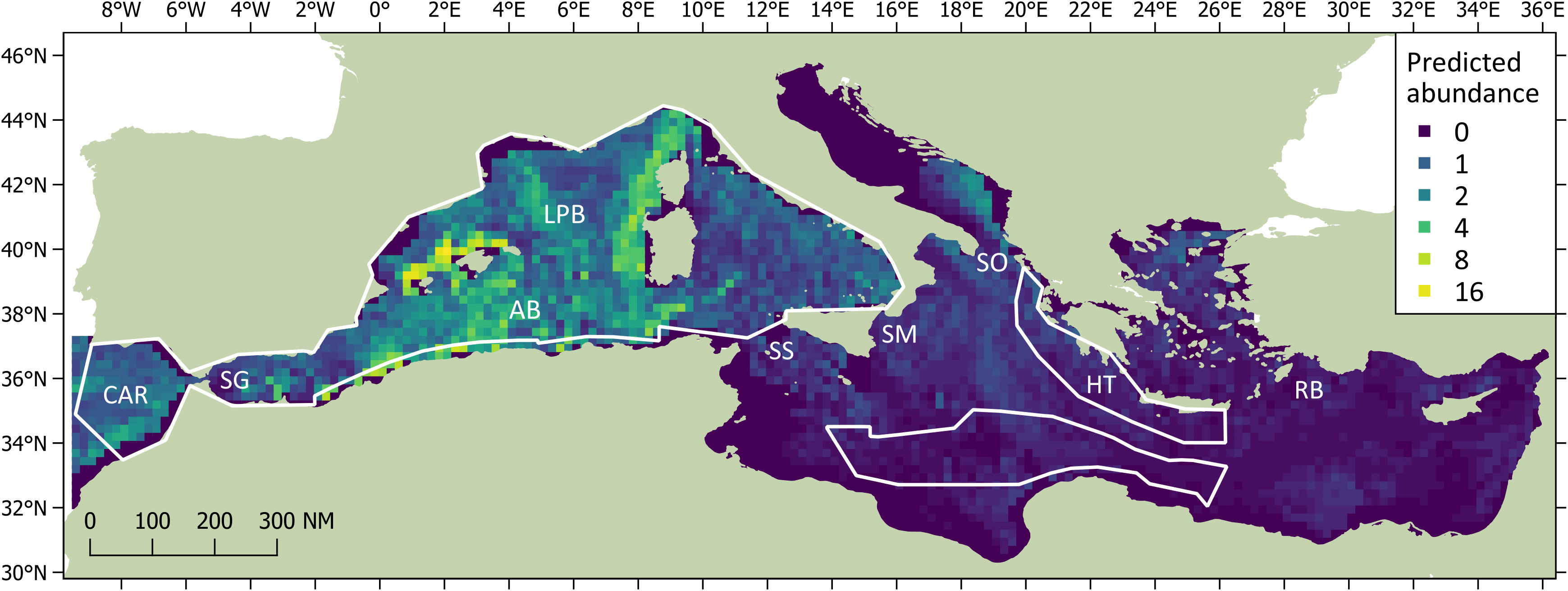
Figure 5 Predicted abundances of sperm whales (expressed as number of animals per grid cell) derived from Density Surface Modelling. The surveyed blocks are shown as white outlines. Significant regions discussed in the text are labelled as CAR (contiguous Atlantic region), SG (Strait of Gibraltar), AB (Algerian Basin), LPB (Liguro-Provencal Basin), SC (Strait of Sicily), SM (Strait of Messina), SO (Strait of Otranto), HT (Hellenic Trench) and RB (Rhodes Basin).
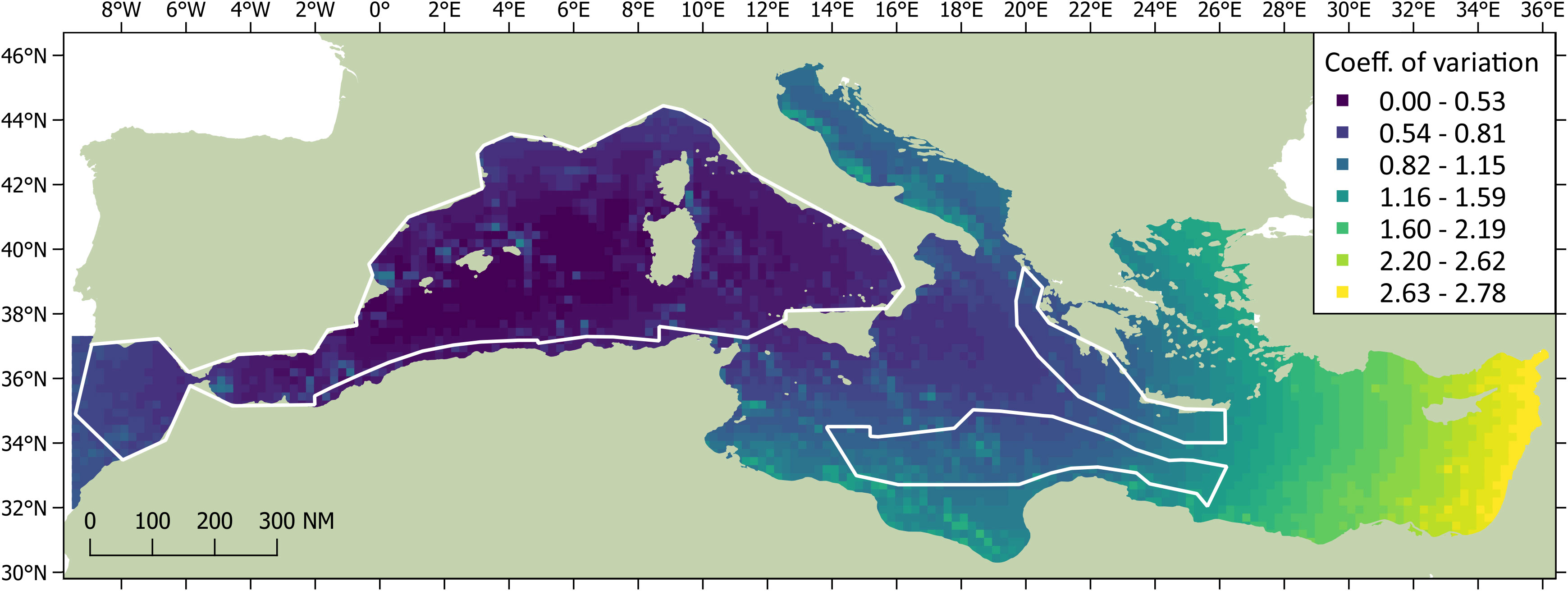
Figure 6 The coefficients of variation associated with the DSM predictions of sperm whale abundance. CVs for the surveyed blocks are shown in colour for waters deeper than 200 m; CVs for the unsurveyed regions are shown in grayscale. The darkest areas show highest precision.
Repeated surveys are required to detect statistically robust population trends. The power analyses suggested that the shorter the interval between surveys, the sooner that significant declines can be detected (Table 4). Although larger population declines (such as 10% per annum) can be detected more quickly than smaller population declines (such as 1% per annum), by the time they are detected with sufficient statistical power, the population could have dropped by up to 90% (for example, decennial surveys identifying a 10% per annum decline with high power). Although annual surveys would be considered extremely effective, they are financially and logistically unfeasible. Other large-scale survey efforts for cetaceans have been conducted decennially (e.g. SCANS; Hammond et al., 2013), and the modelled outputs for decennial surveys for sperm whales in the Mediterranean are shown in Figure 7. For comparative purposes, outputs for sexennial surveys are also shown, as suggested by the Long Term Monitoring Programme adopted at the ACCOBAMS Meeting Of Parties (Malta, November 2022).

Table 4 The ability to detect population decline with two separate levels of statistical power; high (0.95) and acceptable (0.80).
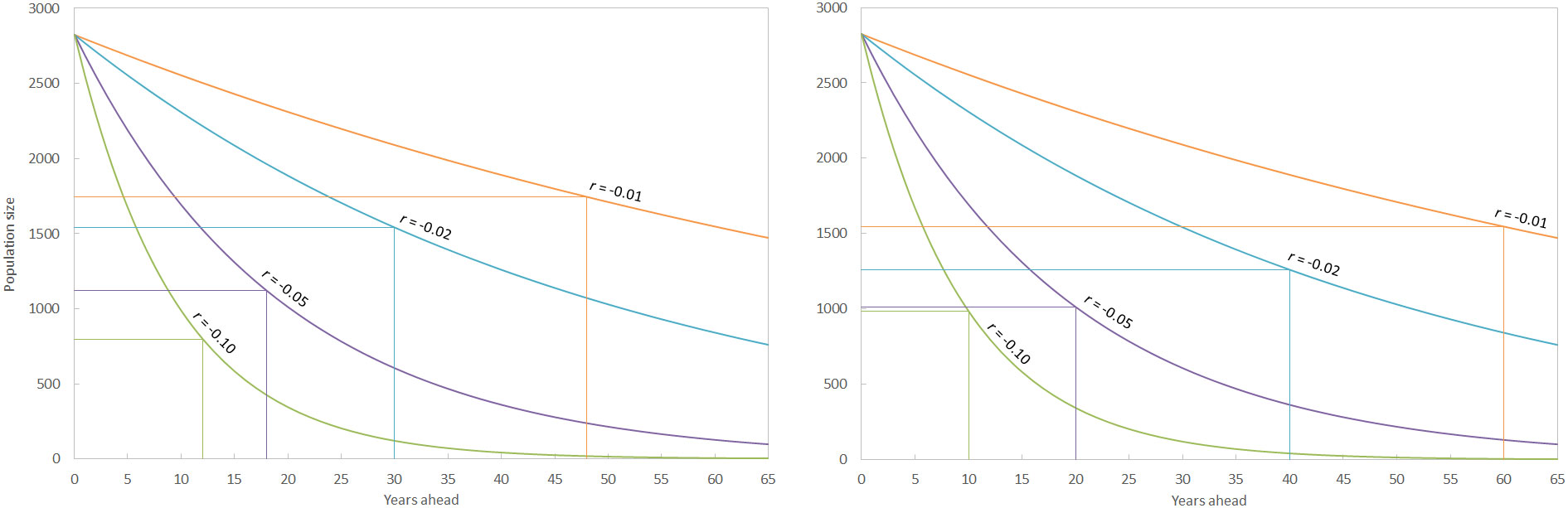
Figure 7 The time taken to detect different rates of decline of the sperm whale population in the Mediterranean Sea with an acceptable level of power (0.80) if estimates of population size are made sexennially (left) and decennially (right). The vertical lines represent the time taken for a significant decline to be detected, with the corresponding population size marked by the horizontal lines, for each hypothetical rate of decline (r). The corrected model-based abundance of 2,825 whales (CV = 0.16) is used for calculations. As an example, running surveys every six years might detected a 10% per annum decline in the population after two surveys with acceptable statistical power (by which point the total number of animals would have reduced by 72%).
This study presents acoustic density estimates for sperm whales in the Mediterranean Sea and contiguous Atlantic region derived using both design-based and model-based methods. Although the precise estimates varied between the two approaches, they generally agreed on approximately 2,500 whales in the surveyed blocks (2,439 and 2,577 whales using design- and model-based approaches respectively), rising to 2,800 individuals if a correction for acoustic availability is applied (2,673 and 2,825 whales respectively). Although the distribution of sperm whales in the Mediterranean may vary by sex and age (Caruso et al., 2015; Reid, 2019), these abundance estimates include all individuals, regardless of sex, age or size.
In keeping with other studies (Rendell et al., 2014; Laran et al., 2017; Lewis et al., 2018), the western basin was found to provide habitat for the majority of sperm whales in the Mediterranean, with blocks 1-15 accounting for over 97% of total abundance. Both design- and model-based approaches found highest densities in the Algerian and Liguro-Provencal Basins between Algeria and Spain/France. Densities were generally 2-6 whales per 1,000 km2 in the western basin, although they declined close to the Straits of Gibraltar, Sicily and Messina. These straits are characterised by relatively shallow water sills that may restrict the movements of deeper water cetaceans. The Strait of Gibraltar provides the only natural connection between the Mediterranean Sea and the North Atlantic Ocean; however, the low densities encountered near the 290 m deep Camarinal Sill supports the theory that it acts as a migratory barrier to sperm whales (Drouot et al., 2004a; de Stephanis et al., 2008; Engelhaupt et al., 2009). The Strait of Sicily provides a 160 km wide interface between the western and eastern basins, and the maximum depth of the area is only 316 m; the nearby Strait of Messina is only 3 km wide with a maximum depth of 80 m. Despite the noted year-round presence of sperm whales in the Ionian Sea (Pavan et al., 2008; Caruso et al., 2015), it is likely that both straits may also act as a significant deterrent to the movement of sperm whale groups between east and west (Lewis et al., 2007; Boisseau et al., 2010), with inter-basin movements possibly restricted to adult males (Frantzis et al., 2011).
Most detections, and the highest modelled densities, were in an approximately rectangular region bounded by Algeria, Spain, France and Sardinia. All sightings except one made by the aerial component of the ASI in the western basin were also made in this region (Cañadas et al., 2023; Panigada et al., 2023). The region has long been noted for its importance to Mediterranean sperm whales (Pavan et al., 2000; Gannier et al., 2002; Praca and Gannier, 2008; Rendell et al., 2014; Lewis et al., 2018), although there had previously been little systematic survey effort off the Algerian coast. Numerous detections were made in regions of steep slope, such as off the Spanish mainland, the Balearic Islands and Sardinia. However, sperm whale encounter rates were just as high in expanses of open water with relatively uniform bathymetry, such as in the Algerian and Liguro-Provencal Basins. Outside of the central region of highest density, predicted abundances were also high in the eastern Tyrrhenian and off the Moroccan Atlantic coast. Although regional patches of high sperm whale density have been noted off Italy’s west coast (e.g. Mussi et al., 2014; Pace et al., 2018), only two vessel surveys covering the whole Tyrrhenian Sea had been conducted prior to the ASI (Gannier et al., 2002), both being conducted over 20 years ago. In keeping with these previous studies, sperm whales were clustered near the Italian coast to the south of Ischia. Likewise, surveys for sperm whales in the contiguous Atlantic region have been rarely undertaken, in part because of challenging swell and weather conditions. As in a similar previous acoustic/visual study (Boisseau et al., 2010), sperm whales were encountered off Morocco’s Atlantic coast but not towards the Iberian peninsula. These waters once supported seemingly high densities of sperm whales (Sanpera and Aguilar, 1992; Aguilar and Borrell, 2007), but 19th and 20th century whaling removed significant numbers of animals over several decades and it is not clear to what extent this has affected the local distribution patterns seen today.
The surveys conducted in the eastern basin found very low densities of sperm whales except for the Hellenic Trench; both design- and model-based approaches estimated only 40-60 animals present in block 22 during the ASI survey. This finding is supported by other studies that have found moderately high year-round densities of sperm whales in the Hellenic Trench, Rhodes Basin and south Aegean Sea (Öztürk et al., 2013; Frantzis et al., 2014; Lewis et al., 2018; Akkaya et al., 2020), but in few other places in the eastern basin. Lower densities have been reported around the Republic of Cyprus and reported group sizes are typically small (Boisseau et al., 2010; Ryan et al., 2014; Boisseau et al., 2017; Lewis et al., 2018; Snape et al., 2020); however, a larger social unit has been encountered in Cypriot waters at least once (Kerem et al., 2012). Although there was one off-track detection of a single sperm whale in Libyan waters during the ASI, it seems this area may only be used occasionally by sperm whales, with only a single individual encountered in a similar acoustic/visual survey conducted in 2007 (approximately 500 km east of the 2018 detection; Boisseau et al., 2010). Likewise, visual surveys conducted from other vessels during ASI rarely documented sperm whale encounters, with only two groups seen off Egypt (a group of two plus a group of three to five) and a solitary whale seen off Syria (ACCOBAMS, 2021). It should be noted, however, that the species identification for all three of these encounters was not considered ‘definite’. The aerial surveys conducted during ASI documented sperm whale sightings in the Hellenic Trench and southern Aegean Sea, but also a group of four in the Ionian Sea and a group of three in Turkey’s Gulf of Antalya (Cañadas et al., 2023; Panigada et al., 2023). Although strandings have been documented for most eastern basin states, including Italy (Bearzi et al., 2011; Pace et al., 2019), Greece (Frantzis et al., 2019), Turkey (Tonay et al., 2021), Syria (Gonzalvo and Bearzi, 2008), Israel (Kerem et al., 2012), Egypt and Libya (Farrag et al., 2019), and Tunisia (Karaa et al., 2016), aggregations of live sperm whales are rarely encountered outside of Greek, Turkish and Cypriot waters. In Israeli waters before 2012, for example, there had only been a single acoustic detection documented plus seven sightings by non-experts of unsexed animals of which most were solitary (Kerem et al., 2012). Since 2017, there have been at least 28 sightings of unsexed sperm whales in the waters of Israel, Lebanon and Cyprus, most of which were of solitary animals with occasional pairs being reported (D Kerem & O Galili, pers. comm. August 2022)1. These patchy encounters and the results from DSM modelling reinforce the theory that the core sperm whale habitats in the eastern basin are concentrated near the Hellenic Trench (Frantzis et al., 2014). Although during the ASI some sperm whale sightings were made in deep waters, a large dataset of sightings collected over the last two decades indicates that sperm whales and particularly social units have a strong preference for waters close to the 1000 m contour of the Hellenic Trench where the density of marine traffic is often highest (Frantzis et al., 2014; Frantzis et al., 2019).
The selected model used for the DSM analysis suggested location and aspect were the most instructive covariates for predicting sperm whale abundance. The GAM soap film smooth fit of density using latitude and longitude highlighted the central region in the western basin as having notably high sperm whale densities. In the eastern basin, the highest- density region identified by the soap film smooth of location was the southern Adriatic Sea. Although this region was not surveyed by vessel during the ASI, the aerial surveys did not encounter any sperm whales in the Adriatic (Cañadas et al., 2023; Panigada et al., 2023). Previous surveys have similarly not found evidence that the Adriatic Sea provides suitable habitat for sperm whales (Bearzi et al., 2009; Lewis et al., 2018), and it is therefore incongruous that the model suggested high densities in this region. DSM outputs that make predictions outside the range of the input data should be treated with caution (Miller et al., 2013). The Adriatic Sea connects to the Ionian Sea via the relatively narrow (72 km) yet deep (780 m) Strait of Otranto (Širović and Holcer, 2020). Although deeper than the other notable straits in the Mediterranean, and therefore unlikely to present a barrier to the free movement of sperm whales, the deepest point of the southern Adriatic Sea is 1,233 m (Širović and Holcer, 2020). As the northern Adriatic is essentially a shallow continental shelf, it is unlikely that sperm whales can find enough suitable habitat in the broader Adriatic Sea, particularly when considering those in the nearby Hellenic Trench may be found in waters 2,500 m deep (Frantzis et al., 2014). In addition to providing sub-optimal foraging conditions, anthropogenic pressures in the Adriatic Sea may also prevent the region from supporting significant numbers of sperm whales. Illegal, unreported and unregulated fisheries (IUU), including driftnets, have been reported for Italian waters (Piroddi et al., 2015), and it is not clear to what extent these may have affected Adriatic sperm whales. As the Adriatic Sea is essentially a shallow, enclosed basin, it is susceptible to noise; as an important shipping route with high densities of recreational boating (Širović and Holcer, 2020), this region may present considerable risks to sperm whales in terms of both noise and ship-strike risk (Bearzi et al., 2011) and may account for their local absence.
The other covariate retained in the final DSM model was mean aspect. The smooth fit of aspect suggested areas with west- to south-facing slopes were of particular importance to the sperm whales encountered during the ASI. This was particularly evident off the Atlantic coast of Morocco, Sardinia, the Ligurian Sea, the Tyrrhenian Sea and the Hellenic Trench. However, in other regions, such as the Alborán Sea, Algerian Basin and Liguro-Provencal Basin, this did not appear to be the case. It should be noted that although considered a significant smooth term, including mean aspect with location in the final model only explained an additional 0.4% of deviance. The importance of mean aspect in the DSM output should therefore not be over-interpreted. Other studies have found aspect to be an important covariate; for example, analysis of a long-term dataset from the Balearic Islands suggested sperm whales were encountered less often when the seafloor was oriented west to northwest (Pirotta et al., 2011; Pirotta et al., 2020). It is likely that the orientation of slope aspect preferred by Mediterranean sperm whales varies by region in response to local bathymetry, currents and prey density, and a snapshot DSM analysis is not granular enough to capture this heterogeneity. Where slope aspect does play a role in sperm whale distribution, it is likely to interact with the local circulation of water to drive downwelling/upwelling events that influence the availability and density of bathypelagic cephalopods that predominate the diet of Mediterranean individuals (Foskolos et al., 2019).
The analysis process involved generating detection functions using slant ranges to vocalising individuals as a proxy for perpendicular distances. Although this approach can lead to overestimation of perpendicular distances which may in turn lead to underestimation of abundance, a previous modelling exercise for Mediterranean sperm whales found that for hazard rate detection functions with high values (i.e. > 1,000) of the scale parameter, σ, and values of β between 1 and 5, this bias is negligible (Lewis et al., 2018). As the hazard rate detection function used in the final ASI model had parameter estimates of σ = 3,000 and β = 4.3, it is likely that any errors introduced in to the estimate of detection probability were minimal. The addition of wind speed as a covariate improved the fit of the detection function. Higher wind speeds tended to be associated with more distant detections of sperm whales (i.e. a broadening of the detection function). Although high winds at the sea surface increase ambient noise levels, and thus may make it harder to detect sperm whale clicks, they may also promote mixing of the water column. This mixing action may remove any thermoclines that could have the potential to reflect or refract clicks produced at depth, thus modifying estimated slant ranges.
The uncorrected abundance estimate derived from a design-based approach was 2,439 whales for the surveyed regions; the model-based approach derived a slightly higher number of 2,577 whales. When correcting for availability bias, these estimates rose to 2,673 and 2,825 respectively. A noticeable difference in the two approaches was that the design-based approach could not be used in those survey blocks without detections, whereas the model-based approach derived estimates for these regions. An uncorrected model-based estimate that excludes the ‘blank’ blocks of the design-based approach (namely blocks 2, 9, 14, 22w, 22e and 25) is 2,466, a number much closer to the design-based estimate of 2,439. Although these ‘blank’ blocks all had detections of sperm whales, these were typically removed from the design-based analysis as the right-truncation distance was set as 5,000 m (i.e. probability of detection > 0.15). This truncation distance was used to avoid a resulting long tail of low detectability in the detection function, as detections a long way from the line contribute little to abundance estimates (Buckland et al., 2001).
Abundance estimates increase with the inclusion of corrections for availability. The estimate of 0.912 for acoustic g(0) was based on the diving behaviour of seven tagged whales off the Azores in the mid-Atlantic (Fais et al., 2016). Although dive data exists for five sperm whales tagged in the Ligurian Sea (Miller et al., 2004), that dataset only generated 21 complete dive cycles compared with the 80 in the Azorean dataset. For that reason, the Azorean dataset was used; however, considering the published summaries (Zimmer et al., 2005; Fais et al., 2016), it does not appear that Mediterranean whales perform radically different foraging dives to those in the Azores. If the results from DSM were extended to include those regions not surveyed by the Song of the Whale team in 2018, the uncorrected abundance estimate rose by almost 40% to 3,268 whales. Although this estimate excluded shallow water habitats (only 2% of all encounters were in waters shallower than 200 m), it included 56 individuals in the seemingly unsuitable habitat in the Adriatic Sea. As discussed above, this figure does not seem realistic. The total estimate also included 197 whales in the contiguous Atlantic region outside block 1. This block was surveyed during ASI in part due to its importance to smaller odontocete species (such as killer whales Orcinus orca and long-finned pilot whales Globicephala melas; Cañadas et al., 2023; Panigada et al., 2023); as sperm whales along Morocco’s Atlantic seaboard are likely to belong to a separate sub-population, it is not appropriate to consider these animals as part of the core Mediterranean assemblage as there is little evidence of movement between the two populations (Drouot et al., 2004a; Engelhaupt et al., 2009). Therefore, the estimate for the surveyed regions alone (i.e. a corrected value of 2,825) is likely to provide the most accurate approximation of the total sperm whale population size for the Mediterranean Sea.
The ASI results showed some agreement with the few regional abundance estimates that exist from previous survey effort. Aerial surveys in 2011/12 derived corrected summer estimates of 161 (95% CI 44-583; CV = 0.74) and 209 (95% CI 39-1,108; CV = 1.03) sperm whales for regions approximately equivalent to ASI blocks 8a and 8b (Laran et al., 2017). Despite the high CVs for these estimates, the figures are little higher than the ASI model-based figures for blocks 8a (62 whales; 95% CI 22-176; CV = 0.57) and 8b (139 whales; 95% CI 65-299; CV = 0.42). A photo-identification study conducted in the Balearic Islands and Ligurian Sea from 1990 to 2008 estimated no more than 400 sperm whales in the western Mediterranean basin (Rendell et al., 2014). This figure is radically different from the corrected ASI estimates of 1,833 (design-based) and 2,102 (model-based) for blocks 2 to 10. However, if considering the Balearic and Ligurian individuals as separate populations, the respective estimates of 320 (95% CI 241-541) and 112 (95% CI 76-180) were closer to the model-based ASI estimates for the analogous blocks 4 (558; 95% CI 320-971) and 10 (139; 95% CI 46-299). A comparison of this nature is perhaps more appropriate as distance sampling approaches assume whales are stationary and do not move between survey blocks. In addition, the discovery curve for the photo-ID study did not show signs of becoming asymptotic, suggesting the population had not been fully characterised. In the Hellenic Trench, a photo-ID study from 1998 to 2009 derived “an advisable working” estimate of 200-250 sperm whales (Frantzis et al., 2014). However, more recent estimates by the same research team suggest numbers in the Hellenic Trench have decreased to below 200 (A Frantzis & P. Alexiadou, pers. comm. December 2022). The corrected ASI estimates of 60 whales (design-based) in block 22c and/or 43 whales (model-based) in blocks 22e, 22c and 22w are less than the more recent photo-ID estimates for the region; however, as the Hellenic Trench may provide at least temporary habitat for all sperm whales in the eastern basin, a snapshot survey in this region may be expected to estimate fewer individuals than a multi-year photo-ID study.
Other survey effort in the Mediterranean has derived density estimates for sperm whales. The northwest Pelagos Sanctuary has received a great degree of research effort over the last few decades; visual density estimates have ranged from 0.39 (CV = 0.39; Laran et al., 2010) to 1.0 sperm whales per 1000 km2 (Gannier, 1995) for summer months, while an acoustic density estimate of 1.69 whales per 1000 km2 (Poupard et al., 2022) has been derived from a static recorder deployed from 2015-18. The ASI results for block 10 showed some variability, with a corrected design-based estimate of 0.74 whales per 1000 km2 (CV = 0.89) contrasting a model-based figure of 4.07 (CV = 0.57). The high CVs for these ASI results suggests caution should be taken when interpreting these densities, but when considering the neighbouring Pelagos Sanctuary blocks 9 and 11, densities were similarly high (5.32 and 2.04 respectively for model-based estimates). It is possible sperm whale densities have been increasing in the Pelagos Sanctuary over recent years, i.e. from 0.39 in 2001-04 (Laran et al., 2010) to 1.69 in 2015-18 (Poupard et al., 2022) to 2.04 in 2018 (this study). However, as the estimates from the other studies were not corrected for availability, it is likely they represent underestimates, and as such direct comparisons are challenging. Juxtapositions such as these are useful for detecting any potential trends, but is should be borne in mind that the survey areas under discussion often do not closely align, either spatially or temporally.
Prior to the ASI project, the most comprehensive effort to characterise the population size of Mediterranean sperm whales was conducted using the same field and analysis protocols from the same research vessel (Lewis et al., 2018). This multi-year survey used design-based methods to derive a corrected total estimate of 1,842 whales (95% CI = 1,173-2,892 if using CV = 0.23 reported for vessel surveys) when extrapolating density estimates to unsurveyed regions. Although the equivalent design-based ASI estimate (2,673 whales) fell within the confidence interval of the composite study by Lewis et al., the ASI estimates of density and abundance for all blocks tended to be higher than the composite study. One interpretation of this difference could be that the number of sperm whales in the Mediterranean is increasing. However, it is unlikely that the results from the two studies actually provide evidence of this. The study by Lewis et al. used the best available data at the time that had been collected over several years (2003-2013), and used extrapolation and/or aerial survey data to characterise densities in unsurveyed regions. The long dive time of sperm whales led to high uncertainty in the aerial survey estimates for the Ligurian Sea, for example, with CVs of 0.76-1.05 reported (Laran et al., 2017). Pooling together surveys conducted over a decade may mask any shifts in distribution or introduce biases in to models exploring habitat preferences. Photo-identification studies have suggested the area between the Strait of Gibraltar and the Liguro-Provencal Basin is characterised by the fluid movements of individuals (Carpinelli et al., 2014; Rendell et al., 2014). Thus the ASI snapshot survey, incorporating the entire western basin over the course of several weeks, may be more likely to faithfully characterise the population of the western basin than episodic surveys conducted over non-contiguous periods and locations. Any perceived population increase since the Lewis et al. (2018) estimate may rather be the result of the above confounding factors.
When investigating the amount of survey effort required to identify population trends, it is assumed not only that the population is closed, but also that surveys are taken at regular intervals, the field protocols are the same, and the abundance estimates are independent. Some of these assumptions may be logistically difficult (e.g. ensuring repeat surveys take place at the same interval) but the analysis is robust to mild violations of these assumptions (Gerrodette, 1987). The power analysis suggested that conducting regular surveys every six years would detect significant population declines much sooner than surveys every 10 years, with the exception of the most precipitous decline modelled (10% per annum detected after 12 years vs. 10 years under sexennial and decennial surveys respectively; Figure 7). These anomalies are rare however, and more intense effort will normally detect significant trends sooner than less intense survey regimes. The Mediterranean sub-population of sperm whales is currently listed as endangered C2a(ii) (Pirotta et al., 2021). The related listing of Endangered C1 pertains to small populations declining by 20% in five years or two generations. Using the former parameter (i.e. an annual decrease of 4.4%), sexennial surveys would only detect this decline with acceptable power (0.80 after Taylor et al., 2007) after three surveys (i.e. after 18 years), at which point the population would have more than halved to 1,265 whales. Decennial surveys would take 20 years to detect this decline, at which point the population would have declined by approximately 60% to 1,157 whales. Currently, any trajectory in the population size of Mediterranean sperm whales is unknown. A regular censusing regime is essential to characterise any trends, with more frequent surveys (e.g. every six years) more powerful than less frequent surveys (every ten years plus). Additional survey effort, such as by vessel or via static acoustic recorders, can also be important for indicating changes in distribution at a finer temporal and regional resolution.
Sperm whales in the Mediterranean are exposed to direct human-induced mortality risks, such as bycatch in illegal driftnets and ship strike, as well as cumulative stressors, including underwater noise, chemical and plastic pollution, prey depletion and the effects of climate change (Rendell and Frantzis, 2016; Notarbartolo di Sciara and Hoyt, 2020). In part due to these threats, sperm whales are protected by their listing on the Bonn Convention, (CMS Appendices I and II), the Bern Convention (Appendix II), CITES (Appendix I), ACCOBAMS (a priority species for conservation action) and the Protocol on Specially Protected Areas and the Biological Diversity in the Mediterranean of the Barcelona Convention (Annex II) (Pirotta et al., 2021). In addition, the EU’s Marine Strategy Framework Directive requires Member States to achieve or maintain ‘Good Environmental Status’ (GES) of their waters. In 2017, six areas within the Mediterranean region were designated as Important Marine Mammal Areas (IMMAs) as they provide discrete portions of habitat of particular importance to sperm whales; the Alborán Corridor and Alborán Deep, the Balearic Islands Shelf and Slope, the North West Mediterranean Sea, Slope and Canyon System, the Campanian and Pontino Archipelago, and the Hellenic Trench (IUCN Marine Mammal Protected Areas Task Force, 2017). An additional candidate IMMA has been proposed for East Sicily and the Strait of Messina due to evidence of the routine presence of sperm whales (Pavan et al., 2008; Caruso et al., 2015). Although IMMAs do not confer any legal protections, they provide impetus for marine mammal and wider ocean conservation measures (Tetley et al., 2022). Despite these various designations, there is an inferred continuing decline in the Mediterranean sub-population of sperm whales (Pirotta et al., 2021), and the threats listed above may therefore threaten their continued survival in the region. As an example of how these mechanisms may fail, the Hellenic Trench IMMA has recently been impinged upon by a large area granted by the Greek government as a concession to the oil and gas industry for hydrocarbon exploitation (Notarbartolo di Sciara and Hoyt, 2020). In the west, a security area limiting maximum vessel speeds to 13 knots was established in the Strait of Gibraltar in 2007 (Notarbartolo Di Sciara, 2014), with the aim of reducing collisions with sperm whales. However, the measure is only implemented from April to August, despite evidence that sperm whales use the area year-round (Gauffier et al., 2012), and there is little evidence of compliance by mariners (Silber et al., 2012). Oversights such as these highlight the inconsistency with which protective measures are implemented and enforced. The ASI results suggest many regions of core sperm whale habitat, such as the waters off Algeria and the Atlantic seaboard of Morocco, may remain excluded from any targeted protection such as those provided through the European Union. A wider network of effectively managed and monitored protected areas is required to improve conservation outcomes for sperm whales in the Mediterranean Sea, in tandem with further population censusing to determine trends in abundance.
The ASI vessel surveys in 2018 allowed a snapshot survey to determine the density of sperm whales using acoustic techniques, as deep-diving cetaceans may be under-represented by aerial surveys. Both design- and model -based approaches broadly agreed on a total estimate of approximately 2,800 individuals using a correction for acoustic availability. As for previous research effort, density was not homogenous, with model results suggesting most sperm whales detected were in the western basin. Densities were highest in the Algerian and Liguro-Provencal Basins between Algeria and Spain/France. Although few whales were detected in the eastern basin, the Hellenic Trench, Rhodes Basin and south Aegean Sea appeared to provide the core habitat, as noted in previous studies. Although comparisons with previous surveys are challenging, the ASI results were broadly in keeping with other density estimates. Importantly, the ASI project allowed a synoptic survey to be conducted of all key sperm whale habitats within the same year and same season, thus overcoming any biases introduced by the long-range movement of individuals. Repeat survey effort is required to determine any population trends, and using the parameters estimated in this study, undertaking systematic surveys every six years allows a much faster identification of any significant population decline than other regimes (e.g. decennially). As Mediterranean sperm whales are currently listed as Endangered on the IUCN’s Red List, and they are known to suffer significant mortality risk from anthropogenic stressors including fisheries interactions and ship strike, there is an urgent need to reduce anthropogenic mortalities to improve the conservation status of this vulnerable population.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://accobams.org/asi-data-access-request/.
Ethical approval was not required for the study involving animals in accordance with the local legislation and institutional requirements because this research was not conducted through a University or governmental organization; as such, no relevant animal ethics committee was available. However, all research was conducted under permits issued by the relevant countries involved, and every measure was taken to minimize disturbance to the animals studied (for example, via the use of passive acoustic detection techniques deployed from a research vessel specifically designed to be quiet).
OB, AM, RM and SP contributed to the conception and design of the study. OB, AM and RM prepared field protocols and secured relevant permissions. OB, JR, CR and RM conducted fieldwork. OB, JR and CR performed acoustic analysis; subsequent analyses were conducted by OB. Manuscript preparation was overseen by OB. All authors contributed to the article and approved the submitted version.
The authors thank the funding bodies that facilitated ASI: Mava Foundation, Prince Albert II Foundation, the Spanish Ministry of Agriculture, Fisheries, Food and Environmental Affairs, the French Agency for Biodiversity, the Italian Ministry for Environment and Protection of Land and Sea, the Principality of Monaco and the International Fund for Animal Welfare. ASI was implemented with contributions from all ACCOBAMS parties.
The authors would like to thank the ACCOBAMS Permanent Secretariat, the ASI Steering Committee, National Focal Points and the ASI Contact Group. Many thanks also to the relevant government bodies for providing permissions and/or logistical support in the field; the UK Foreign & Commonwealth Office also lent invaluable assistance in this regard, with particular thanks to Colin Glen, Ravinder Lota and Clive Hughes of the Maritime Policy Unit. The vessel survey of the Hellenic Trench blocks was facilitated as a collaboration between MCR, IFAW and Pelagos Cetacean Research Institute. The surveys described in this report would not have been possible without the hard work of the following team members and participants: Brian Morrison, Niall MacAllister, Mat Jerram, Jack Fabricious, Nick Carter, Hannah Stowe, Judith Matz, Nienke van Geel, Denise Risch, Enrico Pirotta, Simon Ingram, Aixa Morata, Alexandros Frantzis, Paraskevi Alexiadou, Niki Koutouzi, Amalia Alberini, Souad Lamouti, Mohammed Bouaicha, Abdelmadjid Gherdis, Mohamed Laid, Rabah Selmani, Abdelkader Mehraz, El-Houari Erroukrma, Youcef Bouzid, Laura Mannocci, Mathieu Cellard, Yotam Zuriel, Yaly Mevorach, Almokhtar Saied, Mustafa Almuntasri, Salih Diryaq, Ali Berbash, Alhassn Mansor, Mihailo Jovicevic, Jelena Popovic, Badreddine Mekyassi, Abdelkrim Kelmouni, Ali Rahmani, Said Ait Taleb, Sidahmed Baibat, Rimel Benmessaoud, Mourad Cherif, Sami Karaa and İlayda Destan Öztürk.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACCOBAMS (2021). Estimates of abundance and distribution of cetaceans, marine mega-fauna and marine litter in the Mediterranean Sea from 2018-2019 surveys. Eds. Panigada S., Boisseau O., Canadas A., Lambert C., Laran S., McLanaghan R., Moscrop A. (Monaco: ACCOBAMS).
Aguilar A., Borrell A. (2007). Open-boat whaling on the Straits of Gibraltar ground and adjacent waters. Mar. Mammal Sci. 23, 322–342. doi: 10.1111/j.1748-7692.2007.00111.x
Akkaya A., Lyne P., Schulz X., Awbery T., Capitain S., Rosell B. F., et al. (2020). Preliminary results of cetacean sightings in the eastern Mediterranean Sea of Turkey. J. Black Sea/Mediterranean Environ. 26, 26–47.
Avila I. C., Farías-Curtidor N., Castellanos-Mora L., Do Amaral K. B., Barragán-Barrera D. C., Orozco C. A., et al. (2022). The Colombian Caribbean Sea: a tropical habitat for the Vulnerable sperm whale Physeter macrocephalus? Oryx 56, 814–824. doi: 10.1017/S0030605321001113
Balme D. M. (2011). Aristotle: Historia Animalium (Volume I Books I-X) (Cambridge, UK: Cambridge University Press).
Barlow J., Taylor B. L. (2005). Estimates of sperm whale abundance in the northeastern temperate Pacific from a combined acoustic and visual survey. Mar. Mammal Sci. 21, 429–445. doi: 10.1111/j.1748-7692.2005.tb01242.x
Bearzi G., Costa M., Politi E., Agazzi S., Pierantonio N., Tonini D., et al. (2009). Cetacean records and encounter rates in the northern Adriatic Sea during the years 1988-2007. Annales Ser. Hist. Nat. 19, 145–150.
Bearzi G., Pierantonio N., Affronte M., Holcer D., Maio N., Notarbartolo Di Sciara G. (2011). Overview of sperm whale Physeter macrocephalus mortality events in the Adriatic Sea, 1555-2009. Mammal Review 41, 276–293.
Boisseau O., Frantzis A., Petrou A., Van Geel N., McLanaghan R., Alexiadou P., et al. (2017). Visual and passive acoustic survey report. Report submitted to Cyprus’ Department of Fisheries and Marine Research by the AP Marine Environmental Consultancy Consortium. 49 pages.
Boisseau O., Lacey C., Lewis T., Moscrop A., Danbolt M., McLanaghan R. (2010). Encounter rates of cetaceans in the Mediterranean Sea and contiguous Atlantic area. J. Mar. Biol. Assoc. 90, 1589–1599. doi: 10.1017/S0025315410000342
Boisseau O., Nowacek D., Roberts J., Pabst D. A., Clabaugh A., Moscrop A., et al. (2023). Acoustic density estimates of beaked whales off the mid-Atlantic coast of the USA in winter and spring. Deep Sea Res. Part I: Oceanogr. Res. Papers 199, 104108. doi: 10.1016/j.dsr.2023.104108
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., Thomas L. (2001). Introduction to distance sampling: estimating abundance of biological populations (Oxford, UK: Oxford University Press).
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., Thomas L. (2004). Advanced distance sampling (Oxford, UK: Oxford University Press).
Buckland S. T., Rexstad E. A., Marques T. A., Oedekoven C. S. (2015). Distance sampling: methods and applications (Cham, Switzerland: Springer).
Cañadas A., Burt M. L., Macleod K., Rogan E., Santos B., Vázquez J. A., et al. (2009). “CODA Appendix II: Model-based estimates of cetacean abundance in offshore European Atlantic waters,” in Cetacean Offshore Distribution and Abundance (CODA). Eds. Hammond P. S., Macleod K., Gillespie D., et al (St. Andrews: SMRU), 19 pages.
Cañadas A., Pierantonio N., Araújo H., David L., Di-Méglio N., Ghislain D., et al. (2023). Distribution patterns of marine megafauna density in the Mediterranean Sea assessed through the ACCOBAMS Survey Initiative. Front. Mar. Sci. Manuscript ID: 1270917. doi: 10.3389/fmars.2023.1270917
Cañadas A., Sagarminaga R., De Stephanis R., Urquiola E., Hammond P. S. (2005). Habitat preference modelling as a conservation tool: proposals for marine protected areas for cetaceans in southern Spanish waters. Aquat. Conserv.: Mar. Freshw. Ecosyst. 15, 495–521. doi: 10.1002/aqc.689
Carpinelli E., Gauffier P., Verborgh P., Airoldi S., David L., Di-Méglio N., et al. (2014). Assessing sperm whale (Physeter macrocephalus) movements within the western Mediterranean Sea through photo-identification. Aquat. Conserv.: Mar. Freshw. Ecosyst. 24, 23–30. doi: 10.1002/aqc.2446
Caruso F., Sciacca V., Bellia G., De Domenico E., Larosa G., Papale E., et al. (2015). Size distribution of sperm whales acoustically identified during long term deep-sea monitoring in the Ionian Sea. PloS One 10, e0144503. doi: 10.1371/journal.pone.0144503
de Stephanis R., Cornulier T., Verborgh P., Salazar Sierra J., Gimeno N. P., Guinet C. (2008). Summer spatial distribution of cetaceans in the Strait of Gibraltar in relation to the oceanographic context. Mar. Ecol. Prog. Ser. 353, 275–288. doi: 10.3354/meps07164
Douglas L. A., Dawson S. M., Jaquet N. (2005). Click rates and silences of sperm whales at Kaikoura, New Zealand. J. Acoustical Soc. America 118, 523–529. doi: 10.1121/1.1937283
Drouot V., Bérubé M., Gannier A., Goold J. C., Reid R. J., Palsbøll P. J. (2004a). A note on genetic isolation of Mediterranean sperm whales (Physeter macrocephalus) suggested by mitochondrial DNA. J. Cetacean Res. Manage. 6, 29–32. doi: 10.47536/jcrm.v6i1.787
Drouot V., Goold J. C., Gannier A. (2004b). Regional diversity in the social vocalizations of sperm whale in the Mediterranean Sea. Rev. D Ecologie-La Terre Et La Vie 59, 545–558. doi: 10.3406/revec.2004.1226
Engelhaupt D., Hoelzel A. R., Nicholson C., Frantzis A., Mesnick S., Gero S., et al. (2009). Female philopatry in coastal basins and male dispersion across the North Atlantic in a highly mobile marine species, the sperm whale (Physeter macrocephalus). Mol. Ecol. 18, 4193–4205. doi: 10.1111/j.1365-294X.2009.04355.x
Fais A., Lewis T. P., Zitterbart D. P., Álvarez O., Tejedor A., Soto N. A. (2016). Abundance and distribution of sperm whales in the Canary Islands: Can sperm whales in the archipelago sustain the current level of ship-strike mortalities? PloS One 11, e0150660. doi: 10.1371/journal.pone.0150660
Farrag M. M. S., Ahmed H. O., Toutou M. M. M., Eissawi M. M. (2019). Marine mammals on the Egyptian Mediterranean Coast “records and vulnerability”. Int. J. Ecotoxicol. Ecobiol. 4, 8–16. doi: 10.11648/j.ijee.20190401.12
Foskolos I., Koutouzi N., Polychronidis L., Alexiadou P., Frantzis A. (2019). A taste for squid: the diet of sperm whales stranded in Greece, Eastern Mediterranean. Deep Sea Res. Part I: Oceanogr. Res. Papers 155, 103164. doi: 10.1016/j.dsr.2019.103164
Frantzis A., Airoldi S., Notarbartolo-Di-Sciara G., Johnson C., Mazzariol S. (2011). Inter-basin movements of Mediterranean sperm whales provide insight into their population structure and conservation. Deep-Sea Res. I 58, 454–459. doi: 10.1016/j.dsr.2011.02.005
Frantzis A., Alexiadou P., Gkikopoulou K. C. (2014). Sperm whale occurrence, site fidelity and population structure along the Hellenic Trench (Greece, Mediterranean Sea). Aquat. Conserv.: Mar. Freshw. Ecosyst. 24, 83–102. doi: 10.1002/aqc.2435
Frantzis A., Leaper R., Alexiadou P., Prospathopoulos A., Lekkas D. (2019). Shipping routes through core habitat of endangered sperm whales along the Hellenic Trench, Greece: Can we reduce collision risks? PloS One 14, e0212016. doi: 10.1371/journal.pone.0212016
Gannier A. (1995). Les cétacés de Méditerranée nord-occidentale: estimation de leur abondance et mise en relation de la variation saisonnière de leur distribution avec l’écologie du milieu. PhD thesis submitted to Ecole Preactique des Hautes-Etudes, Montpellier. 437 p.
Gannier A., Drouot V., Goold J. C. (2002). Distribution and relative abundance of sperm whales in the Mediterranean Sea. Mar. Ecol. Prog. 243, 281–293. doi: 10.3354/meps243281
Gauffier P., Moral Cendón M., Blasi A., Carpinelli E., Jiménez Torres C., Giménez Verdugo J., et al. (2012). “Winter presence of large cetaceans in the Strait of Gibraltar,” in 26th Annual Meeting European Cetacean Society (26th – 28th March 2012 in Galway, Ireland). Available at: www.europeancetaceansociety.eu/sites/default/files/ecs_2012_galway_abstract_book.pdf.
Gerrodette T. (1987). A power analysis for detecting trends. Ecology 68, 1364–1372. doi: 10.2307/1939220
Gillespie D., Gordon J., McHugh R., Mclaren D., Mellinger D., Redmond P., et al. (2008). PAMGUARD: Semiautomated, open source software for real-time acoustic detection and localisation of cetaceans. Proc. Institute Acoust. 30, 54–62.
Gonzalvo J., Bearzi G. (2008). “Action Plan for the conservation of cetaceans in Syria,” in Regional Activity Centre for Specially Protected Areas, contract 39/2007_RAC/SPA, 45 pp.
Gordon J., Gillespie D., Leaper R., Lee A., Porter L., O’Brien J., et al. (2020). A first acoustic density estimate for sperm whales in Irish offshore waters. J. Cetacean Res. Manage. 21, 123–133. doi: 10.47536/jcrm.v21i1.187
Hammond P. S., Macleod K., Berggren P., Borchers C. L., Burt L., Cañadas A., et al. (2013). Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biological Conservation. 164, 107–122. doi: 10.1016/j.biocon.2013.04.010
Hastie G. D., Swift R. J., Gordon J. C. D., Slesser G., Turrell W. R. (2003). Sperm whale distribution and seasonal density in the Faroe Shetland Channel. J. Cetacean Res. Manage. 5, 247–252. doi: 10.47536/jcrm.v5i3.804
Houpert L., Testor P., De Madron X. D., Somot S., D’ortenzio F., Estournel C., et al. (2015). Seasonal cycle of the mixed layer, the seasonal thermocline and the upper-ocean heat storage rate in the Mediterranean Sea derived from observations. Prog. Oceanogr. 132, 333–352. doi: 10.1016/j.pocean.2014.11.004
Hu C., Feng L., Lee Z., Franz B. A., Bailey S. W., Werdell P. J., et al. (2019). Improving satellite global chlorophyll a data products through algorithm refinement and data recovery. J. Geophysi. Res.: Oceans 124, 1524–1543. doi: 10.1029/2019JC014941
IUCN Marine Mammal Protected Areas Task Force (2017). Final report of the workshop: First IMMA regional workshop for the Mediterranean (Greece: Chania). 24-28 October 2016.
Jaquet N., Whitehead H., Lewis M. (1996). Coherence between 19th century sperm whale distributions and satellite derived pigments in the tropical Pacific. Mar. Ecol. Progress Ser. 145, 1–10. doi: 10.3354/meps145001
Karaa S., Saadaoui A., Bradai M. N. (2016). First record of live stranded sperm whales Physeter macrocephalus in the Gulf of Gabes, Tunisia. Cahiers Biol. Mar. 57, 329–333.
Kerem D., Hadar N., Goffman O., Scheinin A., Kent R., Boisseau O., et al. (2012). Update on the Cetacean fauna of the mediterranean Levantine basin. Open Mar. Biol. J. 6, 6–27. doi: 10.2174/1874450801206010006
Laran S., Joiris C., Gannier A., Kenney R. D. (2010). Seasonal estimates of densities and predation rates of cetaceans in the Ligurian Sea, northwestern Mediterranean Sea: an initial examination. J. Cetacean Res. Manage 11, 31–40. doi: 10.47536/jcrm.v11i1.628
Laran S., Pettex E., Authier M., Blanck A., David L., Dorémus G., et al. (2017). Seasonal distribution and abundance of cetaceans within French waters-Part I: The North-Western Mediterranean, including the Pelagos sanctuary. Deep Sea Res. Part II: Topical Stud. Oceanogr. 141, 20–30. doi: 10.1016/j.dsr2.2016.12.011
Leaper R., Chappell O. P., Gordon J. (1992). The development of practical techniques for surveying sperm whale populations acoustically. Rep. Int. Whal. Commn. 42, 549–559.
Leaper R., Gillespie D., Papastavrou V. (2000). Results of passive acoustic surveys for odontocetes in the Southern Ocean. J. Cetacean Res. Manage. 2, 187–196. doi: 10.47536/jcrm.v2i3.505
Lewis T., Boisseau O., Danbolt M., Gillespie D., Lacey C., Leaper L., et al. (2018). Abundance estimates for sperm whales in the Mediterranean Sea from acoustic line-transect surveys. J. Cetacean Res. Manage. 18, 103–117. doi: 10.47536/jcrm.v18i1.437
Lewis T., Gillespie D., Lacey C., Matthews J., Danbolt M., Leaper R., et al. (2007). Sperm whale abundance estimates from acoustic surveys of the Ionian Sea and Straits of Sicily in 2003. J. Mar. Biol. Assoc. UK 87, 353–357. doi: 10.1017/s0025315407054896
Li K., Sidorovskaia N. A., Guilment T., Tang T., Tiemann C. O. (2021). Decadal assessment of sperm whale site-specific abundance trends in the Northern Gulf of Mexico using passive acoustic data. J. Mar. Sci. Eng. 9, 454. doi: 10.3390/jmse9050454
Mannocci L., Boustany A. M., Roberts J. J., Palacios D. M., Dunn D. C., Halpin P. N., et al. (2017a). Temporal resolutions in species distribution models of highly mobile marine animals: Recommendations for ecologists and managers. Diversity Distrib. 23, 1098–1109. doi: 10.1111/ddi.12609
Mannocci L., Roberts J. J., Miller D. L., Halpin P. N. (2017b). Extrapolating cetacean densities to quantitatively assess human impacts on populations in the high seas. Conserv. Biol. 31, 601–614. doi: 10.1111/cobi.12856
Matthews J. (2014). Method for estimating sperm whale abundance from an acoustic strip-transect survey with a two-element towed hydrophone. J. Cetacean Res. Manage. 14, 159–170. doi: 10.47536/jcrm.v14i1.532
Miller D. L., Burt M. L., Rexstad E. A., Thomas L., Gimenez O. (2013). Spatial models for distance sampling data: recent developments and future directions. Methods Ecol. Evol. 4, 1001–1010. doi: 10.1111/2041-210x.12105
Miller P. J., Johnson M. P., Tyack P. L. (2004). Sperm whale behaviour indicates the use of echolocation click buzzes “creaks” in prey capture. Proc. Biol. Sci. 271, 2239–2247. doi: 10.1098/rspb.2004.2863
Møhl B., Wahlberg M., Madsen P. T., Heerfordt A., Lund A. (2003). The monopulsed nature of sperm whale clicks. J. Acoust. Soc Am. 114, 1143–1154. doi: 10.1121/1.1586258
Mussi B., Miragliuolo A., Zucchini A., Pace D. S. (2014). Occurrence and spatio-temporal distribution of sperm whale (Physeter macrocephalus) in the submarine canyon of Cuma (Tyrrhenian Sea, Italy). Aquat. Conserv.: Mar. Freshw. Ecosyst. 24, 59–70. doi: 10.1002/aqc.2460
Notarbartolo Di Sciara G. (2014). Sperm whales, Physeter macrocephalus, in the Mediterranean Sea: a summary of status, threats, and conservation recommendations. Aquat. Conserv.: Mar. Freshw. Ecosyst. 24, 4–10. doi: 10.1002/aqc.2409
Notarbartolo di Sciara G., Hoyt E. (2020). Healing the wounds of marine mammals by protecting their habitat. Ethics Sci. Environ. Politics 20, 15–23. doi: 10.3354/esep00190
Notarbartolo di Sciara G., Tonay A. (2021). Conserving whales, dolphins and porpoises in the Mediterranean Sea, Black Sea and adjacent areas: an ACCOBAMS status report (Monaco: ACCOBAMS).
Öztürk A. A., Tonay A. M., Dede A. (2013). Sperm whale (Physeter macrocephalus) sightings in the Aegean and Mediterranean part of Turkish waters. J. Black Sea/Mediterr. Environ. 19, 168–176.
Pace D. S., Arcangeli A., Mussi B., Vivaldi C., Ledon C., Lagorio S., et al. (2018). Habitat suitability modeling in different sperm whale social groups. J. Wildlife Manage. 82, 1062–1073. doi: 10.1002/jwmg.21453
Pace D. S., Giacomini G., Campana I., Paraboschi M., Pellegrino G., Silvestri M., et al. (2019). An integrated approach for cetacean knowledge and conservation in the central Mediterranean Sea using research and social media data sources. Aquatic Conservation: Marine and Freshwater Ecosystems. 29, 1302–1323. doi: 10.1002/aqc.3117
Panigada S., Pierantonio N., Araújo H., David L., Di Meglio N., Dorémus G., et al. (2023). The ACCOBAMS Survey Initiative: the first synoptic assessment of cetacean abundance in the Mediterranean Sea through aerial surveys. Front. Mar. Sci. Manuscript ID: 1270513. doi: 10.3389/fmars.2023.1270513
Pavan G., Hayward T. J., Borsani J. F., Priano M., Manghi M., Fossati C., et al. (2000). Time patterns of sperm whale codas recorded in the Mediterranean Sea 1985-1996. J. Acoust. Soc Am. 107, 3487–3495. doi: 10.1121/1.429419
Pavan G., La Manna G., Zardin F., Internullo E., Kloeti S., Cosentino G., et al. (2008). “Short term and long term bioacoustic monitoring of the marine environment. Results from NEMO ONDE experiment and way ahead,” in Computational bioacoustics for assessing biodiversity. Proceedings of the International Expert meeting on IT-based detection of bioacoustical patterns (Bonn, Germany: Federal Agency for Nature Conservation), 7–14.
Piroddi C., Gristina M., Zylich K., Greer K., Ulman A., Zeller D., et al. (2015). Reconstruction of Italy’s marine fisheries removals and fishing capacity 1950–2010. Fisheries Res. 172, 137–147. doi: 10.1016/j.fishres.2015.06.02
Pirotta E., Brotons J. M., Cerdà M., Bakkers S., Rendell L. E. (2020). Multi-scale analysis reveals changing distribution patterns and the influence of social structure on the habitat use of an endangered marine predator, the sperm whale Physeter macrocephalus in the Western Mediterranean Sea. Deep Sea Res. Part I 155, 103169. doi: 10.1016/j.dsr.2019.103169
Pirotta E., Carpinelli E., Frantzis A., Gauffier P., Lanfredi C., Pace D. S., et al. (2021). Physeter macrocephalus (Mediterranean subpopulation). The IUCN Red List of Threatened Species: e.T16370739A50285671. doi: 10.2305/IUCN.UK.2021-3.RLTS.T16370739A50285671.en
Pirotta E., Matthiopoulos J., Mackenzie M., Scott-Hayward L., Rendell L. (2011). Modelling sperm whale habitat preference: a novel approach combining transect and follow data. Mar. Ecol. Prog. Ser. 436, 257–272. doi: 10.3354/meps09236
Poupard M., Ferrari M., Best P., Glotin H. (2022). Passive acoustic monitoring of sperm whales and anthropogenic noise using stereophonic recordings in the Mediterranean Sea, North West Pelagos Sanctuary. Sci. Rep. 12, 1–13. doi: 10.1038/s41598-022-05917-1
Praca E., Gannier A. (2008). Ecological niche of three teuthophageous odontocetes in the northwestern Mediterranean Sea. Ocean Sci. 4, 49–59. doi: 10.5194/os-4-49-2008
Rankin S., Barlow J., Oswald J. N. (2008). An assessment of the accuracy and precision of localization of a stationary sound source using a two-element towed hydrophone array. NOAA Technical Memorandum NMFS NOAA-TM-NMFS-SWFSC-416. (National Marine Fisheries Service, NOAA). 29 pages.
R Core Team (2021). R: A language and environment for statistical computing (Vienna: R Foundation for Statistical Computing).
Reese D. S. (2005). Whale bones and shell purple-dye at Motya (western Sicily, Italy). Oxford J. Archaeol. 24, 107–114. doi: 10.1111/j.1468-0092.2005.00227.x
Reeves R., Notarbartolo di Sciara G. (2006). The status and distribution of cetaceans in the Black Sea and Mediterranean Sea (Malaga, Spain: IUCN Centre for Mediterranean Cooperation).
Reid J. (2019). Measuring the length of sperm whales (Physeter macrocephalus) in the Mediterranean using acoustics (MSc thesis, University of St. Andrews: 47 pages).
Rendell L., Frantzis A. (2016). “Mediterranean sperm whales, Physeter macrocephalus: the precarious state of a lost tribe,” in Advances in marine biology. Eds. Notarbartolo Di Sciara G., Podestà M., Curry B. E. (London: Elsevier), 37–74. doi: 10.1016/bs.amb.2016.08.001
Rendell L., Mesnick S. L., Dalebout M. L., Burtenshaw J., Whitehead H. (2012). Can genetic differences explain vocal dialect variation in sperm whales, Physeter macrocephalus? Behav. Genet. 42, 332–343. doi: 10.1007/s10519-011-9513-y
Rendell L., Simião S., Brotons J. M., Airoldi S., Fasano D., Gannier A. (2014). Abundance and movements of sperm whales in the western Mediterranean basin. Aquat. Conserv.: Mar. Freshw. Ecosyst. 24, 31–40. doi: 10.1002/aqc.2426
Rogan E., Cañadas A., Macleod K., Santos M. B., Mikkelsen B., Uriarte A., et al. (2017). Distribution, abundance and habitat use of deep diving cetaceans in the North-East Atlantic. Deep Sea Res. Part II: Topical Stud. Oceanogr. 141, 8–19. doi: 10.1016/j.dsr2.2017.03.015
Ryan C., Cucknell A.-C., Romagosa M., Boisseau O., Moscrop A., Frantzis A., et al. (2014). A visual and acoustic survey for marine mammals in the eastern Mediterranean Sea during summer 2013 (report to the International Fund for Animal Welfare) (Kelvedon, UK: Marine Conservation Research International).
Sahri A., Putra M. I., Mustika P. L., Murk A. J. (2020). A treasure from the past: Former sperm whale distribution in Indonesian waters unveiled using distribution models and historical whaling data. J. Biogeogr. 47, 2102–2116. doi: 10.1111/jbi.13931
Sanpera C., Aguilar A. (1992). Modern whaling off the Iberian Peninsula during the 20th century. Rep. Int. Whaling Commission 42, 723–730.
Silber G. K., Vanderlaan A. S., Arceredillo A. T., Johnson L., Taggart C. T., Brown M. W., et al. (2012). The role of the International Maritime Organization in reducing vessel threat to whales: process, options, action and effectiveness. Mar. Policy 36, 1221–1233. doi: 10.1016/j.marpol.2012.03.008
Širović A., Holcer D. (2020). ”Ambient noise from seismic surveys in the Southern Adriatic Sea,” in The Montenegrin Adriatic Coast: Marine Biology, eds. A. Joksimović, M. Đurović, I. S. Zonn, A.G. Kostianoy, A.V. Semenov. (Switzerland: Springer Nature). 109, 497–514. doi: 10.1007/698_2020_710
Snape R. T. E., Çiçek B. A., Hadjioannou L., Öztürk A. A., Beton D. (2020). Two sperm whale (Physeter macrocephalus) sightings in Cyprus from social media. J. Black Sea/Medit. Environ. 26, 238–248.
Taylor B. L., Martinez M., Gerrodette T., Barlow J., Hrovat Y. N. (2007). Lessons from monitoring trends in abundance of marine mammals. Mar. Mammal Sci. 23, 157–175. doi: 10.1111/j.1748-7692.2006.00092.x
Teloni V. (2005). Patterns of sound production in diving sperm whales in the northwestern Mediterranean. Mar. Mammal Sci. 21, 446–457. doi: 10.1111/j.1748-7692.2005.tb01243.x
Teloni V., Mark J. P., Patrick M. J. O., Peter M. T. (2008). Shallow food for deep divers: Dynamic foraging behavior of male sperm whales in a high latitude habitat. J. Exp. Mar. Biol. Ecol. 354, 119–131. doi: 10.1016/j.jembe.2007.10.010
Tetley M. J., Braulik G. T., Lanfredi C., Minton G., Panigada S., Politi E., et al. (2022). The important marine mammal area network: a tool for systematic spatial planning in response to the marine mammal habitat conservation crisis. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.841789
Thomas L., Buckland S. T., Rexstad E. A., Laake J. L., Strindberg S., Hedley S. L., et al. (2010). Distance software: design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 47, 5–14. doi: 10.1111/j.1365-2664.2009.01737.x
Tonay A. M., Öztürk A. A., Salman A., Dede A., Danyer I. A., Danyer E., et al. (2021). Stranding records of sperm whale (Physeter macrocephalus) on the Turkish coast in 2019-2020 with a note on the opportunistic sampling of stomach content. J. Black Sea/Mediterranean Environ. 27, 281–293.
Vachon F., Eguiguren A., Rendell L., Gero S., Whitehead H. (2022). Distinctive, fine-scale distribution of Eastern Caribbean sperm whale vocal clans reflects island fidelity rather than environmental variables. Ecol. Evol. 12, e9449. doi: 10.1002/ece3.9449
Ward J. A., Thomas L., Jarvis S., Dimarzio N., Moretti D., Marques T. A., et al. (2012). Passive acoustic density estimation of sperm whales in the Tongue of the Ocean, Bahamas. Mar. Mammal Sci. 28, E444–E455. doi: 10.1111/j.1748-7692.2011.00560.x
Watwood S. L., Miller P. J., Johnson M., Madsen P. T., Tyack P. L. (2006). Deep-diving foraging behaviour of sperm whales (Physeter macrocephalus). J. Anim. Ecol. 75, 814–825. doi: 10.1111/j.1365-2656.2006.01101.x
Westell A., Sakai T., Valtierra R., Van Parijs S. M., Cholewiak D., Deangelis A. (2022). Sperm whale acoustic abundance and dive behaviour in the western North Atlantic. Sci. Rep. 12, 1–13. doi: 10.1038/s41598-022-20868-3
Whitehead H. (2017). “Sperm whale,” in The Encyclopedia of Marine Mammals. Eds. Würsig B., Thewissen J., Kovacs K. M. (Cambridge, MA: Academic Press), 919–925. doi: 10.1016/B978-0-12-804327-1.00242-9
Wood S. N. (2006). Generalized additive models: an introduction with R. Chapman and Hall/CRC. Texts Stat. Sci. 67, 391. doi: 10.1201/9781315370279
Wood S. N. (2011). Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Society: Ser. B (Statistical Methodology), 73, 3–39.
Wood S. N., Bravington M. V., Hedley S. L. (2008). Soap film smoothing. J. R. Stat. Soc. Ser. B: Stat. Method. 70, 931–955. doi: 10.1111/j.1467-9868.2008.00665.x
Keywords: passive acoustic monitoring, density estimation, sperm whale, Mediterranean Sea, density surface modelling, Physeter macrocephalus
Citation: Boisseau O, Reid J, Ryan C, Moscrop A, McLanaghan R and Panigada S (2024) Acoustic estimates of sperm whale abundance in the Mediterranean Sea as part of the ACCOBAMS Survey Initiative. Front. Mar. Sci. 11:1164026. doi: 10.3389/fmars.2024.1164026
Received: 11 February 2023; Accepted: 09 January 2024;
Published: 25 January 2024.
Edited by:
Leslie New, Ursinus College, United StatesReviewed by:
Gianni Pavan, University of Pavia, ItalyCopyright © 2024 Boisseau, Reid, Ryan, Moscrop, McLanaghan and Panigada. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oliver Boisseau, b2JvaXNzZWF1QG1jci10ZWFtLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.