
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Mar. Sci., 19 October 2023
Sec. Marine Megafauna
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.997491
This article is part of the Research TopicWhales and ClimateView all 14 articles
Southern Hemisphere humpback whales (Megaptera novaeangliae) were heavily targeted during modern commercial whaling operations, with some 216,000 individuals killed between 1903 and 1973. That impacted the abundance of all the seven breeding stocks of the species. Most of these stocks have been recovering from whaling pressure although the understanding of the current growth rates of some stocks, and how the rates compare across stocks are lacking. Updated information is fundamental for understanding the species’ current status, and to support the review of management plans promoting its protection and recovery, especially considering current changes in ocean environments due to climate change. This work offers a comprehensive overview of the current knowledge on Southern Hemisphere humpback whales breeding stocks’ status. The aim is to provide information on their post-whaling growth trends and changes in distribution and migration patterns. Within that, records of supplementary feeding records (i.e. feeding beyond their formally described feeding grounds) are described. We have also identified knowledge gaps and note that the establishment of research collaborations, as well as standard methodologies for data collection can be important steps for the acquisition of better comparable data sets for the analysis of the current status of humpback whales and to fill such gaps. The compiled information provided can be used as part of an In-Depth Assessment of the species by the International Whaling Commission.
Humpback whales (Megaptera novaeangliae) are a cosmopolitan cetacean species (Clapham and Mead, 1999) and one of the most studied large baleen whales (IWC, 2006). In the Southern Hemisphere, humpback whales generally migrate seasonally between high-latitude feeding grounds typically used during austral summer and mid to low-latitude breeding grounds for late austral autumn and winter (Clapham and Mead, 1999).
Currently, seven humpback whale breeding stocks (hereafter referred to as stocks) are recognized by the International Whaling Commission (IWC) in the Southern Hemisphere (Figure 1; IWC, 1998). These are referred to stocks ‘A’ to ‘G’ by IWC and each is assigned to a specific breeding area. Based on genetic, mark-recapture or whaling data (Findlay, 2000; Rosenbaum et al., 2009; Fleming and Jackson, 2011), some stocks have been subdivided into sub-stocks. The breeding and feeding grounds used by each stock and sub-stock are indicated in Table 1. Given the connectivity amongst sub-stocks from New Caledonia (E2), Tonga (E3), Cook Islands (F1) and French Polynesia (F2), they have been grouped in the so-called Oceania stock (IWC, 2016a). In this review, as for in many publications in the field, we prioritize referring to the stocks based on their breeding ground location. Throughout the text, we refer to specific areas or locations within the breeding grounds that might not be familiar to the reader, so for a better location of the areas mentioned please see the maps included as supplementary information (Figures S1–S5).
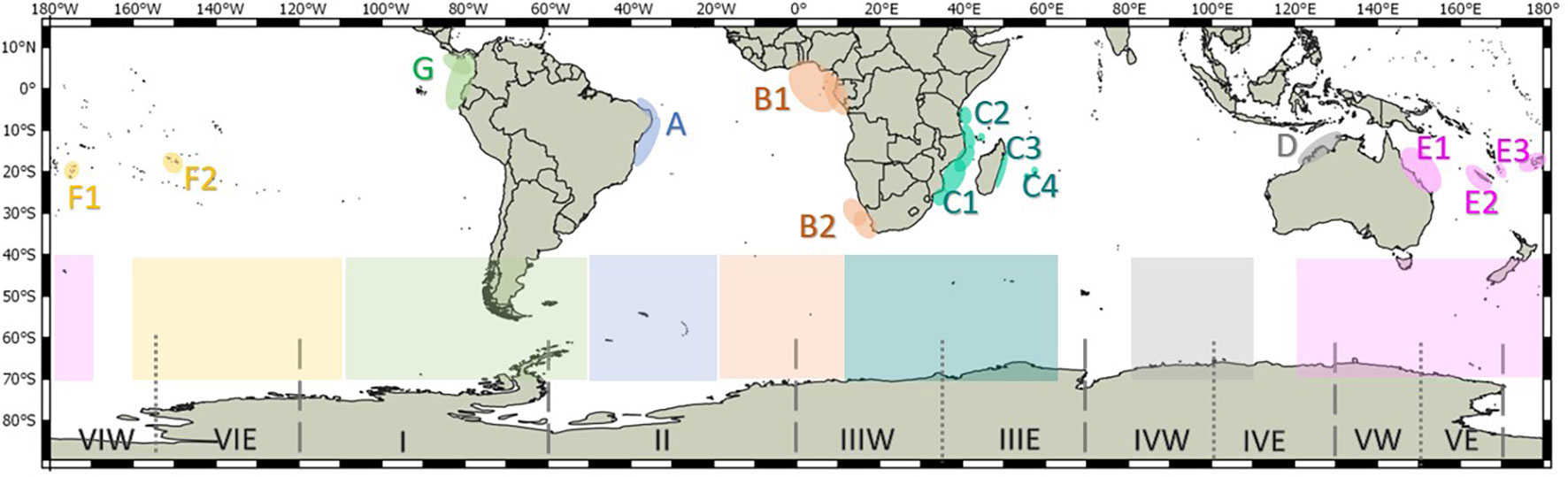
Figure 1 Distribution of the core breeding and primary feeding grounds of the Southern Hemisphere humpback whale Breeding Stocks A – G, and Southern Ocean Management Areas I – VI and sub-areas (Donovan, 1991; IWC, 1998; IWC, 2006). Colors are used to indicate the breeding and feeding grounds used by each breeding stock and sub-stock. Dashed lines indicate the limit of the main Management Areas, whereas dotted lines are marking the limits of sub-areas (W = west and E = east). Areas and sub-areas limits are: I = 120°W–60°W; II = 60°W–0°; III = 0–70°E (IIIW = 0–35°E and IIIE = 35°E–70°E); IV = 70°E–130°E (IVW = 70°E–100°E and IVE = 100°E–130°E); V = 130°E–170°E (VW = 130°E–150°E and VE = 150°E–170°E); and VI = 170°E–120°W (VIW = 170°W–155°W and VIE = 155°W–120°W).
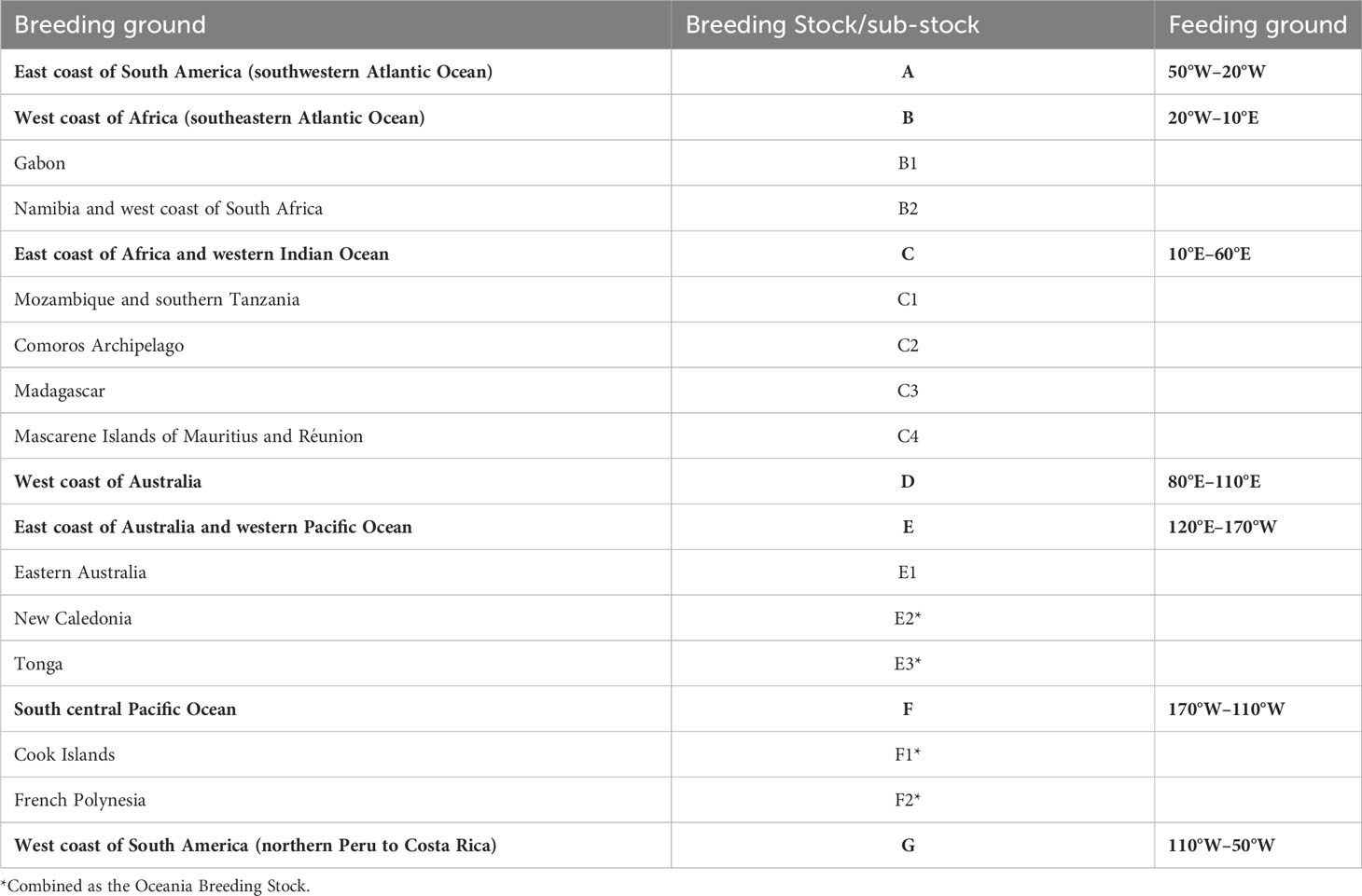
Table 1 Breeding and feeding grounds of the Southern Hemisphere humpback whale Breeding Stocks A – G and sub-stocks based following the definition by the International Whaling Commission and information from IWC (IWC, 2007; IWC, 2016a, 2016b) and Branch (2011).
The species was severely depleted by modern commercial whaling in the Southern Hemisphere. It is estimated that about 216,000 individuals were killed across the region from 1903 to 1973 (Allison, 2020), which reduced its abundance to a very small fraction of their pre-exploitation levels (Findlay, 2000). Although humpback whales have been protected across the Southern Hemisphere since October 1963 (Tønnessen and Johnsen, 1982), illegal Soviet operations continued until 1973 (Clapham et al., 2009). Further protection for the species came into force in 1986 with the Moratorium established by the IWC (Clapham and Baker, 2002).
Based on catch allocations of blue (Balaenoptera musculus) and fin (Balaenoptera physalus) whales, the IWC historically identified six main Management Areas (Areas I–VI) within the Southern Ocean for all baleen whales (Figure 1; Mackintosh, 1942; Donovan, 1991). As new information on the distribution of illegal Soviet catches was gained, some of these areas necessitated further longitudinal division of feeding areas in the Southern Ocean(Figure 1; IWC, 1998). The use of these Management Areas by the different stocks of humpback whale is a vexing question that was historically investigated through the analyses of mark-recapture data and catch histories. For example, high-latitude catches were allocated to particular stocks on the basis of longitudinal dispersal (e.g. Paton and Clapham, 2006). With time, information has been gained on the level of mixing between the stocks in high-latitude areas. Reviews of Discovery Investigation’s whale mark-recapture data (collected using stainless-steel tags deployed during the whaling era – please see Rayner (1940)), movement patterns (Bestley et al., 2019), satellite-tag individuals (e.g. Reisinger et al., 2021) and photo-identification data (e.g. Marcondes et al., 2021) suggest a possible greater degree of mixing among stocks on the feeding grounds than originally agreed by the IWC (IWC, 2016a; Jackson et al., 2015).
A Comprehensive Assessment of Southern Hemisphere humpback whales was developed by the IWC was completed in 2014 and results were synthesised in 2015 (IWC, 2016a, 2016b). It was based on a Bayesian statistical approach including a backward projection (Butterworth and Punt, 1995; Jackson et al., 2015), and estimated the pre-modern whaling abundance of the species in the Southern Hemisphere at 137,972 (95% PI = 111,833-197,781) individuals (IWC, 2016b). The sum of the median abundance projected for each stock for 2015 was of 96,675 (95% PI = 78,041-117,527) individuals. An overall recovery of about 70% for all stocks combined is indicated, although there are marked differences in the rates of increase (ROI) across stocks (IWC, 2016b). As a reflection of the recovery of the stocks, the species is currently listed as “Least Concern” on the IUCN Red List, although low numbers of the Oceania stocks have meant these are still listed as “Endangered” (Childerhouse et al., 2008; Cooke, 2018).
Despite intense research on Southern Hemisphere humpback whales over the last decades, updated information on their population demographic parameters is essential for stock assessments and evaluation of conservation status (e.g. Rodrigues et al., 2006; Punt and Donovan, 2007). Updated estimates of trends in abundance and of absolute stock sizes are essential for the evaluation of the need for management strategies and for effective measures to be developed, if necessary (e.g. Caughley, 1994; Rockwood, 2015). That is particularly important for humpback whales considering the current pressures faced by the species. Such pressures include climate-driven environmental variabilities affecting both their breeding and feeding grounds, as well as migratory corridors (e.g. Derville et al., 2019; Tulloch et al., 2019; Meynecke et al., 2020; Meynecke et al., 2021; Seyboth et al., 2021; van Weelden et al., 2021), and other threats such as ship strikes (Van Waerebeek et al., 2007; Smith et al., 2020), entanglements (Groom and Coughran, 2012; Ott et al., 2016; Félix et al., 2020a; Santora et al., 2020), underwater noise (Rossi-Santos, 2015; Dunlop, 2019; Dunlop et al., 2020), and pollution (Besseling et al., 2015; Das et al., 2017; Casà et al., 2019; Remili et al., 2020). One example of a climate-driven impact is the influence of sea surface temperature (SST) on the abundance and distribution of prey stocks, which has been identified as a threat to different whale populations (e.g. Simmonds and Isaac, 2007). That is mainly related to the decrease of Antarctic krill (Euphausia superba) abundance as a response to warmer ocean conditions (e.g. Loeb and Santora, 2015; Atkinson et al., 2019). This has potential consequences for humpback whale stocks as Antarctic krill is the main prey item in the diet of Southern Hemisphere humpback whales (e.g. Mackintosh, 1965; Santora et al., 2010; Herr et al., 2016) and as food availability may impact their reproductive success (e.g. Seyboth et al., 2021). However, disentangling the effects of these pressures on stock recovery from the effects of exploitation from whaling is complex and requires detailed information on the extent and rates of recovery within and across stocks so their abundance trajectory can be better understood.
Other important aspects to be investigated are potential changes in the distribution of the species including feeding and breeding grounds and migration corridors, and the performance of feeding behaviour in regions beyond the regular feeding grounds. Such changes might be becoming more common as the species increase in abundance, and faces the changes observed in prey populations in the Southern Ocean.
Furthermore, status assessments are important for the understanding of the roles of humpback whales in the trophic ecology of Southern Ocean systems. For example, whales in general may influence both prey populations and community structure through top-down forcing (Croll et al., 2006; Leaper et al., 2008), and the primary production and biogeochemistry of the marine environment through micronutrient fertilisation (Ratnarajah et al., 2014; Ratnarajah et al., 2016). Given the removal of some two million individuals of different whale species from Southern Ocean systems by modern whaling and associated influences on trophic ecology - see for example the krill surplus hypotheses discussion initially introduced by Laws (1977), updated information on population recoveries and status are critical in Southern Ocean management models (e.g. Friedlaender et al., 2006a; Seyboth et al., 2016; Warwick-Evans et al., 2022).
Aiming to contribute to the knowledge of the current status of Southern Hemisphere humpback whale, in this study we review information on their post-whaling abundance and growth rate estimates, and compile information on the stock structure, potential changes in distribution and migration patterns, and records of supplementary feeding (i.e. feeding beyond their regular feeding grounds in high latitudes) for each stock. The information combined in this review may serve as a contribution toward an In-depth Assessment of Southern Hemisphere humpback whale by the IWC.
We collated information relevant to the review from literature made available online until 01 April 2022 in peer-reviewed papers, books, and IWC or project reports. Online searches were conducted using a different combination of the words/terms ‘humpback whale’, ‘population growth’, ‘growth rate’, ‘rate of increase’, ‘abundance’, ‘abundance estimate’, ‘population recovery’, ‘feeding record’, ‘distribution’, and ‘breeding stock’ in Google, Google Scholar, and Web of Science platform, and checked references cited in the publications found relevant during this search.
Information found was grouped by stock whenever possible. The cases for which such classification could not be applied were related to circumpolar data and are presented at the end of the following section as complementary data.
We identified 58 studies in which post-whaling abundance and ROI estimates are presented for Southern Hemisphere humpback whales, published between 1989 and 2022. The number of studies per stock is indicated in Table 2, as well as the number of studies with evidence of feeding beyond the regular feeding grounds of the stocks, which have been reported for the stock related to the east coast of South America, west coast of Africa, east coast of Australia and western Pacific Ocean and west coast of South America. Most studies on abundance were related to stock breeding on the east coast of South America, while most of those investigating ROI were related to eastern Australia.
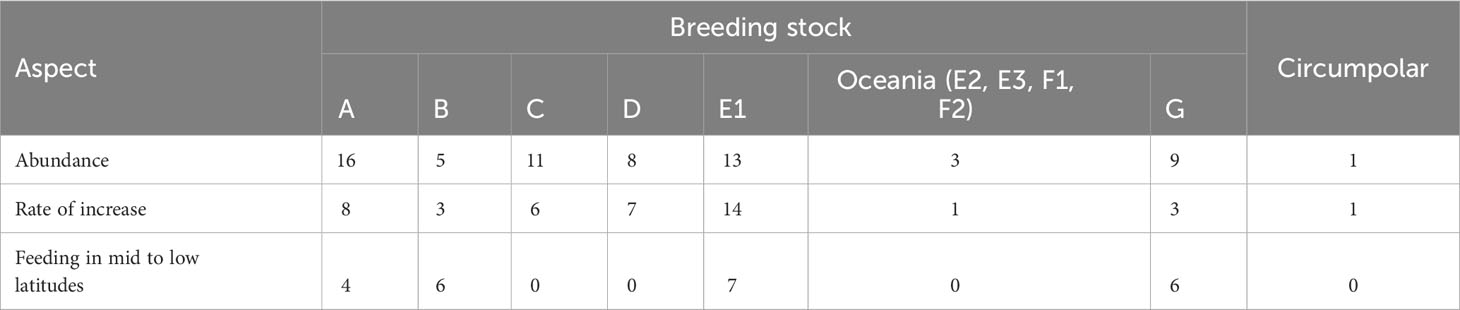
Table 2 Summary of the review survey, indicating the number of studies found for each Southern Hemisphere humpback whale main breeding stock/sub-stock on each aspect aimed to be investigated.
All the estimates found on abundance and ROI for each breeding ground/stock are presented in Tables 3, 4, respectively, and some studies were chosen to be included in the text to describe the recovering trajectory of each stock. Information on changes in distribution and records of supplementary feeding of each stock is described in the text below.
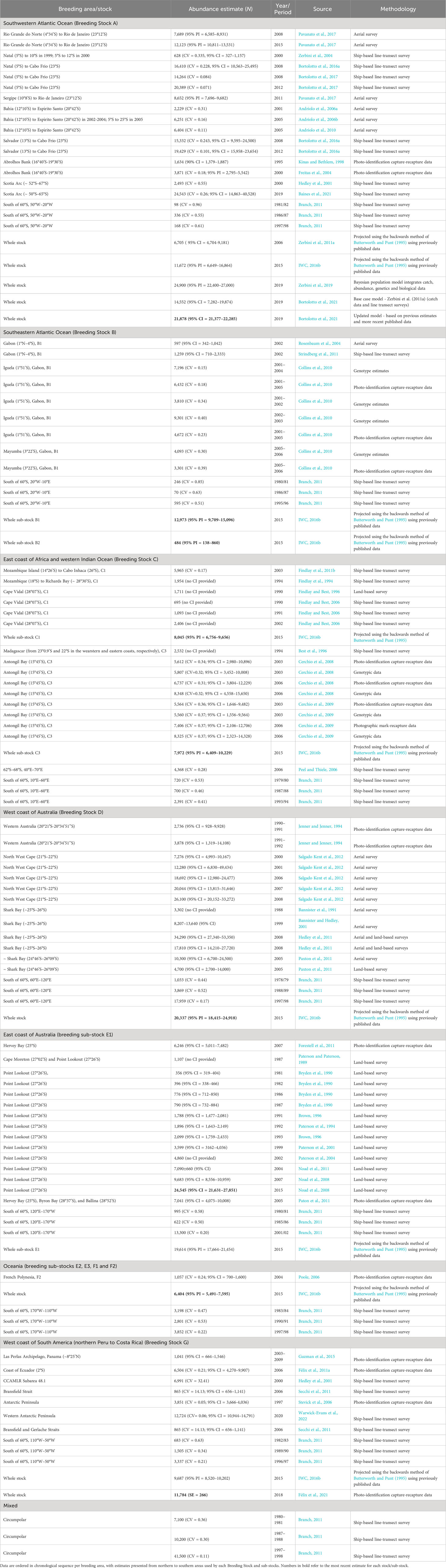
Table 3 A summary of information on abundance (N) for humpback whales published until 01 April 2022, organized by Breeding Stock (A – G).
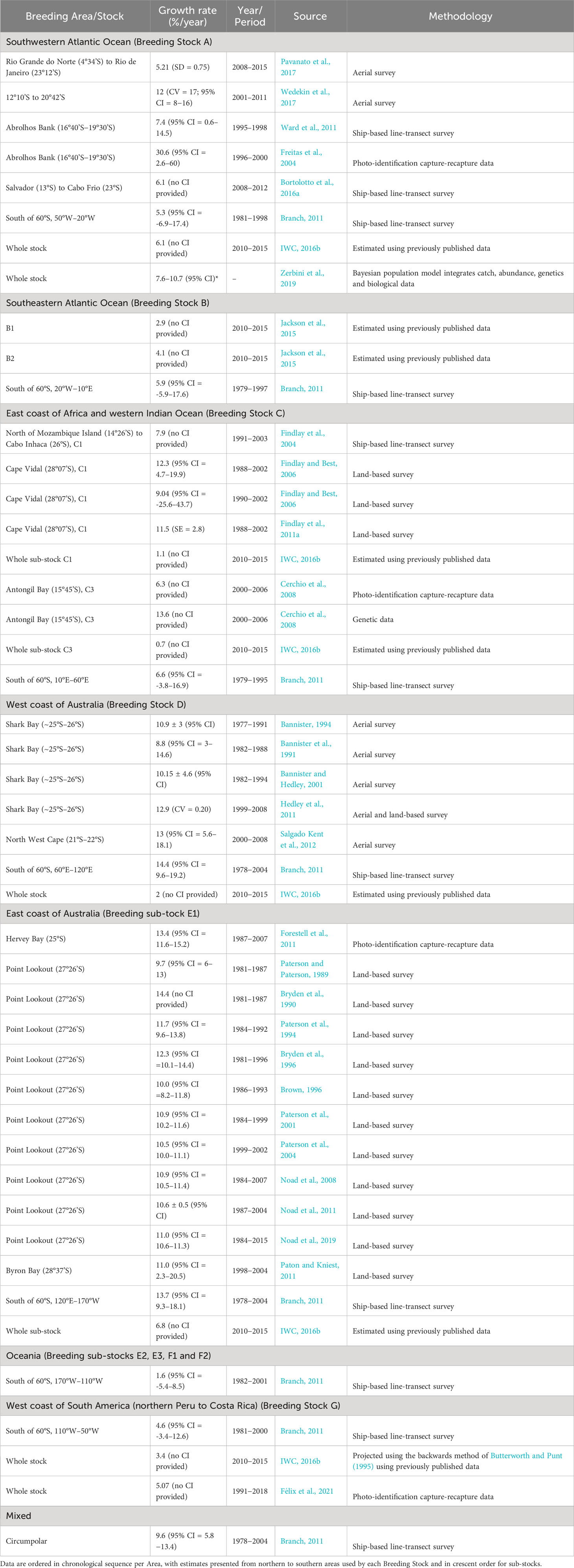
Table 4 A summary of information on stock growth rates (including information found on rate of increase (ROI) and maximum intrinsic rate of increase (rmax)* - both as % per year) estimates for humpback whales published until 01 April 2022, organized by Breeding Stock (A – G).
The breeding area for this stock is located along the coast of Brazil the eastern coast of South America, mainly over the Abrolhos Bank (16°40’S–19°30’S) (Andriolo et al., 2006a; Zerbini et al., 2006; Andriolo et al., 2010). Within this area, higher group densities are found between 140 and 236 km from the coast with relatively shallow waters (< 500 m) (Pavanato et al., 2018), and associated to slower currents, sheltered areas and SSTs of between 24 and 25°C (Bortolotto et al., 2017). Areas surrounding oceanic islands such as the Archipelagos of Fernando de Noronha (3°51’S) and Trindade and Martin Vaz (20°30’S) have been included as part of the breeding ground (Lodi, 1994; Siciliano et al., 1999). The breeding period is from June to November, with a peak in August-September (Martins et al., 2001; Morete et al., 2003).
Off the coast of Brazil, there is evidence that the species have been reoccupying regions of the breeding ground that were known to be used before whaling decimated the stocks and subsequently restricted their range in breeding grounds (Zerbini et al., 2004). For example, the increase of encounter rates on the north coast of Bahia State (around 12°S–13°S) between 2000 and 2006 may represent a post-whaling expansion of the area used by the species around Abrolhos Bank (Rossi-Santos et al., 2008). Observations to the south of Abrolhos Bank are also increasing, as it has been reported for the vicinities of Ilhabela Island (23°55’23.2”S, 45°26 53.7”W), São Paulo State, including records of calves and competitive groups, which can indicate the use of the area as a calving and mating site (Morete et al., 2022). There is also a recent discussion on the expansion of the range of the stock or recolonization of broader areas in northern Brazil (at about 5°04’49’’S, 45°36’03”W), possibly reflecting the post-whaling recovery of the stock (Ristau et al., 2020).
From late austral spring, whales migrate through offshore areas to the Scotia Sea (Zerbini et al., 2006; Zerbini et al., 2011b) and northern Weddell Ridge (Bedriñana-Romano et al., 2022). Individuals tend to concentrate around the South Georgia and the South Sandwich Archipelago (Stevick et al., 2006; Zerbini et al., 2006; Engel et al., 2008; Horton et al., 2020), within IWC Management Area II. However, there are reports of individuals feeding as far west as 42°W (Stevick et al., 2006) and even matches between the coast of Brazil and the vicinity of Bouvet Island (3°E) (Engel and Martin, 2009). There is also an indication of a potential southerly expansion of the area being used during the 2010s, which can be a response to the increase of the SST during this period, with consequences to food availability for the stock (Bedriñana-Romano et al., 2022). New evidence on the permeability of the boundary between Brazilian and western South America stocks on the feeding grounds has been presented based on photo-identification analysis (Marcondes et al., 2021) and satellite tracking data (Reisinger et al., 2021), the latter also indicating an overlap in the feeding area used by the Southwestern and the Southeastern Atlantic Ocean stocks.
The migration paths of the Southwestern Atlantic Ocean stock are believed to be consistent over time, despite changes in environment conditions over the last decades (Zerbini et al., 2011b; Horton et al., 2020). Such conditions include those related to SST, productivity and currents, for instance, known to influence the species distribution and migration (e.g. Derville et al., 2019; Meynecke et al., 2021). The fact that the migration of this stock does not seem to be affected by changes in these conditions might indicate that humpback whale movement decisions include mechanistic responses to stable and predictable exogenous cues, including gravity (Horton et al., 2020).
There is evidence of individuals from this stock feeding in low and mid-latitude waters. For example, the stomach content of a humpback whale stranded on the coast of southern Brazil (29°45’38’’S, 50°00’43’’W) in 2002 contained a large amount of the aviu shrimp Acetes americanus (Decapoda: Sergestidae) and some Brachyura larvae, with preys assumed to have been eaten about 21 nautical miles from the stranding location (Danilewicz et al., 2009). The presence of humpback whales in this coastal area is unusual, as the migratory corridor of the stock that is located offshore in this area (Danilewicz et al., 2009). The distribution of A. americanus ranges from Guayanes Beach, Porto Rico to Rio Grande do Sul State, Brazil, associated with tropical waters (D’Incao and Martins, 2000). A second stranding in southern Brazil (27°26’27.6”S, 48°22’26.4”W) in 2014 reinforced the use of the area for feeding, with the shrimp Peisos petrunkevitchi found in the stomach of the specimen (Bortolotto et al., 2016b). This shrimp species is distributed from Rio de Janeiro, Brazil (22°29’S) to Chubut Province, Argentina (~44°S) (D’Incao and Martins, 2000). Additional feeding evidence for mid-latitude regions come from reports by Siciliano et al. (2019) of plunge-diving feeding behaviour performed by a young individual and other feeding activity by adults of the species in association with gillnet fishery. More evidence has been reported by Pinto de sa Alves et al. (2009), based on observations made in August 2005 from an oil platform located at 19°35’02’’S, 39°14’37’’W of two likely juvenile individuals performing lunge-feeding behaviour to prey on small fishes of an unidentified species.
There is no evidence of stock sub-structure in the Southwestern Atlantic Ocean. Also, individuals from the stock breeding along the coast of Brazil are genetically significantly differentiated from those breeding on western and eastern coasts of Africa, which can be related to maternal site fidelity and ecological and oceanographic features in breeding and feeding grounds (Rosenbaum et al., 2009).
The number of individuals of the stock was estimated to have declined from about 27,000 (95% PI = 22,800–33,000) in 1830 to only 450 (95% PI = 200–1,400) in the mid-1950s (Zerbini et al., 2019). Although this is a substantial decrease in the stock size, studies have indicated no significant decrease in its genetic diversity when analysing samples collected between 1999 and 2007 (Cypriano-Souza et al., 2018).
The first abundance estimate after the end of whaling is for 1995, when 1,634 (90% CI = 1,379–1,887) individuals were estimated to be in the Abrolhos Bank (Kinas and Bethlem, 1998). A continuous recovery of the stock has been reported since then (Table 3), with an absolute estimate of 3,871 (95% PI = 2,795–5,542) individuals for 2000 in the same area (Freitas et al., 2004). For the main area used by the stock, between Natal, Rio Grande do Norte (~ 5°S) and Cabo Frio, Rio de Janeiro (~ 23°S), estimates based on ship-based surveys were of 14,264 (CV = 0.084) individuals for 2008 and 20,389 (CV = 0.071) individuals for 2012 (Bortolotto et al., 2017). Using data from aerial surveys in the same area, abundances of 7,689 (95% PI = 6,585–8,931) individuals for 2008 and 12,123 (95% PI = 10,811–13,531) individuals in 2015 have been estimated (Pavanato et al., 2017). Annual ROIs of 11.08% (2008–2011) and of 1.17% (2011–2015) were estimated for this area, with the difference between periods likely being a result of the stabilization of the population growth towards 2015 (Pavanato et al., 2017). That can also the reason for the decrease in the ROI over a longer period of time, between 1995 and 1998, during which the stock grew at an annual increase of 7.4% (95% CI = 0.5–14.6%) (Ward et al., 2011). A more recent investigation based on combined data from breeding and feeding grounds resulted in the maximum intrinsic rate of increase estimate (rmax) ranging from 7.6 to 10.7% (95% CI) (Zerbini et al., 2019). Currently there is no ROI estimate from feeding ground data due to some limitations on data collection during the IWC IDCR/SOWER cruises as indicate by Leaper et al. (2008) and Branch (2011).
Zerbini et al. (2011a) estimated the size of this stock through modelling parameters using estimates from Andriolo et al. (2010) as an index of relative abundance (as the surveys did not cover the whole range of the breeding stock) and on whaling-derived data. They found it to be 6,705 (95% CI = 4,704–9,181) individuals in 2006, which was about 33% of the pre-exploitation levels. Following up, Zerbini et al. (2019) developed models based on results from Bortolotto et al. (2017) and estimated the stock size as 24,900 individuals in 2019 (95% PI = 22,400–27,000), indicating a recovery of approximately 93% of the pre-exploitation levels. Later on, Bortolotto et al. (2021) run an updated model and provided precise estimates for the stock in 2019, with 21,878 (95% CI = 21,377–22,285) individuals.
The recovery of the stock after whaling is also clear from data from the Scotia Sea, within the feeding ground of its stock (Table 3). For example, 2,493 (CV = 0.55) individuals were estimate in the area in 2000 (Hedley et al., 2001), and 24,543 (CV = 0.26; 95% CI = 14,863–40,528) individuals were estimated in 2019 (Baines et al., 2021), although the confidence interval of this last study is considerably large and consideration should be given to the mixing between stocks in the feeding grounds, which can influence such estimates.
Individuals from this stock concentrate on the western coast of Africa (IWC, 2006). There is limited information on the overall breeding ground used by this stock (Collins et al., 2010). However, it is known that the sub-stock B1 uses the central West African coast and the northern islands of the Gulf of Guinea for breeding purposes, while the genetically distinct sub-stock B2 uses the west coast of South Africa as a feeding site and migratory corridor (IWC, 2006; Barendse et al., 2010; Barendse et al., 2011). The low numbers of calves and competitive groups observed and the lack of singing activity of sub-stock off Namibia suggests that the area is also not used as a breeding ground, but as a migratory route instead (Elwen et al., 2014). The Gulf of Guinea has generally been considered the northern limit of the distribution of the sub-stock B1, but mixing of individuals from Southern and Northern Hemispheres seem to occur in regions up north. That comes from sightings, satellite track data and strandings of individuals on the coast of Northwest Africa (up to 14°20’N) in January-February and August-November periods, most of them during the breeding season of the Southern Hemisphere humpback whales (Acevedo and Smultea, 1995; Van Waerebeek et al., 2001; Bamy et al., 2010; Van Waerebeek et al., 2013; Rosenbaum et al., 2014). The boundary between the two sub-stocks is not clear but proposed to be around 18°S in the region of the Angola-Benguela Front (IWC, 2011). Some individuals observed on the coast of South Africa have been observed in the areas associated with the Gabon sub-stock, but the breeding ground of the sub-stock feeding on the coast of South Africa remains unknown (IWC, 2011).
Areas such as that off São Tomé (0°20′10’’N, 6°43′53’’E), known to be used by the Gabon sub-stock from whaling data (Townsend, 1935), have been repopulated by individuals as shown from data collected in the area since 2002 (Carvalho et al., 2011). Considering the relatively high proportion of mother-calf pairs seen, and the limited number of competitive groups (a behaviour typically associated with mating) registered during fieldwork, the authors believe that the area is used primarily for calving and nursing or for resting.
The detection of non-song calls during austral spring in 2019 in the surroundings of Vema Seamount (31°38’S, 8°20’E), an offshore area off the southwestern coast of South Africa may indicate that the region is part of the migratory route of the stock (Ross-Marsh et al., 2022). However, it is recognized that further research is needed for a validation of these results.
Movements between Western South Africa and Gabon in austral spring and summer have been recorded, but whether individuals stay in the former year-round or use the area intermittently during a year is still not confirmed (e.g. Barendse et al., 2011). Genetic and photographic data indicated that individuals that were sighted in the Western South Africa with their mother have returned there as post-weaned, which seems to be evidence of a maternally derived use of the area for feeding (Barendse et al., 2013).
Although information on the connectivity between west African stock and feeding grounds is scarce (IWC, 2006; Leaper et al., 2008), it has been suggested that individuals feed between the longitudes of 20°W and 10°E, within the IWC Management Areas II and IIIW (IWC, 1998). Data from satellite tagged individuals also support the use of this latitudinal band and indicate the importance of the vicinities of Bouvet Island for individuals from the Gabon sub-stock (Rosenbaum et al., 2014). The low number of sightings in this latitudinal band to the south of 60°S during the IWC IDCR/SOWER cruises (Branch, 2011) can also indicate that the species feeds northerly in Areas II and III than in other Areas. However, the cruises did not cover areas north of 60°S to provide evidence on the presence of individuals in such area.
Seasonal data from whaling stations located in Saldanha Bay, on the west coast of South Africa, show bimodal trends in the presence of humpback whales in the area (Best and Allison, 2010). That reflects the regular migration of the species. However, there are also data indicating the extended presence of the species in the area through the austral summer (Townsend, 1935), with multiple sources reporting on feeding activity as that time of the year, as detailed below. These are all considered evidence that some individuals suppress migration to feeding grounds in high-latitudes, staying in mid to low-latitude regions year-round.
The first evidence of feeding activity on the west coast of South Africa came from whaling records (Matthews, 1938) and records of stranded or entangled individuals with prey items in their stomachs (Findlay and Best, 1995), including mantis shrimp (Pterygosquilla armata capensis). Observation of lunge-feeding behaviour from Cape Columbine (32˚50’S, 17˚51’E) was reported for October–November 1993 at a relative short distance from shore (0.8 to 3.5 km) (Best et al., 1995). During that investigation, the analysis of faecal samples indicated the presence of an unidentified Euphausia species, which was believed to be E. lucens given the sample location. No dominant swimming direction among the observed individuals, and a relatively low mean swim speed (2.8 km h-1), and a residency of up to 20 days in the area were reported (Best et al., 1995). The combination of these factors provided evidence of the use of the west coast of South Africa by non-migratory animals during spring.
Further observations of non-migratory behaviour and feeding activity on the west coast of South Africa were made during land and boat-based monitoring from Saldanha Bay in 2001–2007, with feeding activity on crustacean prey being reported (Barendse et al., 2010). Most groups performing feeding behaviour were composed of two or more individuals, but on some occasions (spring 2001, 2002, and 2007) there were loose aggregations of up to 20 individuals (Barendse et al., 2010). Photo-identification and genetic data collected between 1983 and 2008 have indicated that some 500 individuals might have used the area to feed during spring and summer (September-March) during the period (Barendse et al., 2011).
The occurrence of such loose aggregations seems to have grown over time, and the occurrence of the so called ‘super-groups’ (tightly aggregated groups of 20+ individuals) of humpback whales were recorded during cruises in October and November between Cape Point and St Helena Bay in 2011, 2014, 2015 (Findlay et al., 2017). The origin and destination of the individuals forming these aggregations is unknown. Evidence available so far from satellite tagging data indicate individuals migrating towards the Southern Ocean from early December (IWC, 2016a), but also a considerable spread of the individuals between 15°W and 35°E. It is reported that no calves were encountered within the super-groups, and the relatively small size of the animals encountered suggest a high incidence of non-breeding young animals.
Data on the occurrence of the super-groups have been recorded during cruises, aerial surveys, land and boat-based observations and as citizen-science data every season since 2011, showing consistency on the use of the area as a feeding ground over the last decade (e,g, Findlay et al., 2017; Seafari App, unpublished data). To better understand this behaviour, data from Findlay et al. (2017) was used in association with oceanographic data of the Southern Benguela System. A combination of relatively high chlorophyll-a concentration in the month before the observation of the whales, in association with the decrease in the water export from the area throughout October, seems to support prey availability in the area, composing a scenario favourable to the formation of the super-groups (Dey et al., 2021).
Although no feeding evidence has been reported so far off the coast of Namibia, the occurrence of individuals in the area through austral spring and summer is believed to be a result of the predictable prey availability given the upwelling of the Benguela Current in the region (Papastavrou and Van Waerebeek, 1997). Such evidence comes from catch data (Townsend, 1935) and other observations as described by Best and Shaughnessy (1979). At the same time, an overwinter stay in high latitude areas is also apparent, as the species has been recorded in acoustic monitoring (sounds likely produced by individuals breeding on western Africa given the area of occurrence) for 9 and 11 months over 2008 and 2009, respectively, in the Southern Ocean (70°31’S, 8°13’W) (Van Opzeeland et al., 2013).
Genetic data have indicated substantial (Pomilla et al., 2006; Rosenbaum et al., 2009) to subtle but statistically significant (Carvalho et al., 2014) differentiation between sub-stocks B1 and B2, and there is photographic evidence of interchange between them (Barendse et al., 2011). Potential reasons for the genetic differentiation are maternal site fidelity, the use of two migratory routes (one coastal and other offshore), and spatial or temporal segregation within the Gulf of Guinea breeding ground (Carvalho et al., 2014). More research is needed for the determination of the level of interchange between these stocks and for the identification of the breeding ground used by sub-stock B2. The southeastern Atlantic Ocean stock seems to have an interchange with southwestern Indian Ocean stock (e.g. Best et al., 1998; Rosenbaum et al., 2009; Kershaw et al., 2017), but further investigation is also necessary for the predominant level of connection to be determined.
To date, there is no abundance estimate for the whole range of the sub-stock using Namibian and western South African coasts, although some studies have provided numbers for localised areas within the breeding ground. For example, Collins et al. (2010) used two types of data from different locations used by B1 individuals off coastal Gabon, to provide abundance estimates. Numbers based on photographic data indicated 3,225 (CV = 0.39) individuals for 2001–2002, 3,332 (CV = 0.34) for 2002–2003 and 2,814 (CV = 0.28) for 2003–2004 for Iguela area (1°51’S, 9°20’E), and 3,301 (CV = 0.39) for 2005–2006 for Mayumba region (3°22’S, 10°38’E). The analysis of genotypic data resulted in estimates that ranged from 3,810 (CV = 0.34) for 2001–2002 to 9,301 (CV = 0.40) individuals in 2002–2003 for the Iguela area, and of 4,093 (CV = 0.30) individuals for 2005–2006 at Mayumba. The concurrent use of these two areas by the stock is however not well understood and the estimates presented can be biased (Collins et al., 2010).
A further study for the entire Gabon area - from Equatorial Guinea (1°N) to the Republic of Congo (4°S) - indicated that at least 1,259 individuals (95% PI = 710–2,333) utilised the area during the breeding season in 2002 (Strindberg et al., 2011). A projection of abundance at a rate of 2.9% per year between 2010 and 2015 indicated that about 12,973 (95% PI = 9,709–15,096) individuals constituted the sub-stock B1 in 2015 (IWC, 2016b).
Limited abundance information is also available for the sub-stock B2. Evidence suggests that the population size of this sub-stock is small, as 260 individuals were photo-identified on the west coast of South Africa with a relatively high between-year resighting rate (15.6%) (Barendse et al., 2006). That was further evident from the estimate based on photographic and genetic data, of about 500 individuals on the west coast of South Africa during spring and summer (September to March) each year from 1983 to 2008 (Barendse et al., 2011). A similar number, of 484 (95% PI = 138–860) individuals was projected for 2015 for this sub-stock (IWC, 2016b).
These estimates lead to a projected aggregated sum of 13,457 individuals was projected to constitute the stock in 2015 (IWC, 2016b). However, it is important to highlight that the extend of the breeding ground used by the sub-stock B1 is not fully known, and that there are still uncertainties about the use of such ground by individuals from sub-stock B2 (Collins et al., 2010). Therefore, estimates from this stock should be taken with caution.
Data on abundance and ROI estimates are available from IDCR/SOWER cruises from 1979 to 1997 in the Southern Ocean feeding ground between 20˚W and 10˚E. Data from CPIII (using data from seasons 1992–93 and 1996–97) indicate an estimate of about 595 (CV = 0.51) individuals, and the annual increase rate of 5.9% (-5.9–17.6%) (Branch, 2011).
The overall breeding ground utilized by this stock comprises the eastern coast of Africa and the archipelagos of the Western Indian Ocean (IWC, 1998). The stock has been divided into four sub-stocks, namely C1 (which utilizes the coasts of South Africa, Mozambique and southern Tanzania), C2 (found around Mayotte Island, Comoros Islands and in the Mozambique Channel), C3 (Breeding on the coast of Madagascar, and shown to extend to northern African coastal mainland regions (Cerchio et al., 2016)), and C4 (using the Mascarene islands of Mauritius and Réunion) (IWC, 2006).
Information on the link between breeding and feeding grounds is limited, but the latter is considered to be between 10˚E and 60˚E in the IWC Management Area III (IWC, 2008). One of the few pieces of evidence of this connection come from the animals individually marked in high latitudes at about 54˚S, 10˚E and recaptured south of Madagascar during whaling activities, as part of the Discovery Investigations experiments (Rayner, 1940). Another comes from tagged individuals from the Madagascar sub-stock travelling to Crozet Island (Cerchio et al., 2016).
Based on the review of a combination of data sources, including catch, acoustic, at-sea, land-based and aerial monitoring, three main migratory routes have been proposed for the stock: (i) on the eastern African coast, from South Africa to central Mozambique; (ii) through Madagascar Ridge, with individuals migrating past north Madagascar; and (iii) along the Mozambique Channel (Best et al., 1998).
Although the extents of the feeding ground of western Africa and eastern Africa and western Indian Ocean may suggest that individuals from the west and east coast of Africa might spatially overlap in feeding areas, stable isotope analyses have provided evidence that such mixing does not necessarily happen. Values of carbon (δ13C) and nitrogen (δ15N) stable isotope ratios differed significantly between animals from Gabon and Mayotte, Mozambique Channel and Madagascar (Montanari et al., 2020). This can potentially represent a change in the migration patterns of the stocks, or at least of one of them, but this aspect deserves further investigation. Also, as detailed below, individuals from eastern Africa and western Indian Ocean can be mixing with individuals from Namibia and west coast of South Africa sub-stock to feed on the west coast of South Africa. Studies on photo-identification matches and movement tracking of both studies are highly needed for a better understanding of their movement and potential migration changes.
There is limited information on supplementary feeding events beyond regular feeding grounds for this stock. Individuals may use the productive waters of the west coast of South Africa, joining individuals from western Africa to form the super-groups seen during spring and summer before continuing their migration to high latitude feeding grounds (Findlay et al., 2017). However, this is a topic that deserves further investigation for conclusions to be made.
The level of connectivity amongst these sub-stocks is variable (Leaper et al., 2008), and genetic studies have shown some level of differentiation between Mozambique and southern Tanzania and both Comoros Archipelago and Madagascar (IWC, 2006; Pomilla et al., 2006; Cerchio et al., 2008; Rosenbaum et al., 2009; Ersts et al., 2011; Kershaw et al., 2017). Photographic and satellite tracking data have also been used to investigate this aspect and suggest that although differentiated, there is an interchange between Comoros, Madagascar and Mascarene Islands sub-stocks, and little interchange between Mozambique and southern Tanzania and the other sub-stocks (Cerchio et al., 2008; Dulau-Drouot et al., 2011; Fossette et al., 2014; Dulau et al., 2017).
The recovery of the Mozambique and southern Tanzania sub-stock has been monitored using different methodologies. For example, shore-based surveys of its migration stream were conducted from Cape Vidal, South Africa, from 1988, have indicated a considerable recovery from whaling, as the stock abundance has been increasing over time. For 1990, the estimated abundance of the sub-stock was of 1,711 individuals (Findlay and Best, 1996), while more recent data resulted in estimates of 11,098 (2018) and 13,485 (2019) individuals (Wilkinson et al., 2023). Estimate of ROI are of 12.3% (95% CI = 4.7%–19.9%) (Findlay and Best, 2006), 11.5% (SE = 2.8) (Findlay et al., 2011a) for the period of 1988–2002, and 7.4–8.8% for the period of 1988–2019 (Wilkinson et al., 2023). While the point estimates might suggest a slowdown in the increasing rate of the sub-stock, the range of the estimates overlap and then might not differ significantly from a statistical perspective. In the breeding ground itself, abundance estimate by a line-transect survey along the central-southern coast in August-September 1991 between Quelimane (~18˚S) and Maputo (~26˚S) and from 18.3 to 183 m depths was of 1,954 (CV = 0.38) individuals for that year (Findlay et al., 1994). The authors also found higher densities of humpback whales in the southern region, between 33˚E and 35˚30’E, compared to the northern region. This can be a result of habitat preference considering the orientation of the coast, and wider continental shelf, with the result of the Mozambique Current flowing more offshore in the southern region, meaning individuals can find protection from strong currents closer inshore in this area (Findlay et al., 1994). A second survey in the region was performed during August-September 2003, between Cabo Inhaca (26°00’S) and to the north of Mozambique Island (14°26’S), which allowed the abundance estimation of 5,965 (CV = 0.17) individuals for that year (Findlay et al., 2004; Findlay et al., 2011b), with a ROI of 7.9% per annum for the period of 1991–2003 (Findlay et al., 2004). In both surveys, the high proportion of cow-calf pairs observed confirmed the importance of this region as a breeding area (Findlay et al., 1994; Findlay et al., 2011b). A projection based on a Bayesian multi-stock assessment, resulted in a median abundance estimate of 8,045 (95% PI = 6,756–9,656) individuals across the whole sub-stock C1 in 2015 (IWC, 2016b).
No abundance for the Comoros sub-stock has been estimated to date. For the Madagascar sub-stock, a line-in the southern and eastern portions of the breeding ground resulted in an abundance estimate of 2,532 individuals in 1994 (Best et al., 1996). Later on, Cerchio et al. (2009) estimated the number of individuals using Antongil Bay, northeastern Madagascar, during the 2000–2006 period as 7,406 and 8,325 based on photographic mark-recapture and genotypic surveys, respectively. These numbers were used to project the abundance of the whole sub-stock for 2015, which was 7,972 (95% PI = 6,409–10,228) individuals (IWC, 2016b).
To date, there is no abundance and ROI estimates representative of the stock based on data from the feeding grounds, although numbers are available from IDCR/SOWER cruises as indicated Tables 3, 4.
Individuals of this stock breed on the west coast of Australia, where the Kimberley coastal region is the main concentration between Camden Sound and Broome (15˚S–18˚S) (Jenner et al., 2001). To the south of this region are areas of intense monitoring of this stock, on the Ningaloo coast (~21°5’S), North West Cape (22˚45’S), and Shark Bay (25˚46’S) (400 km distant from each other), used during their migration. Also, there is evidence of North West Cape being used by females with calves over the last decade, which can represent an expansion of the calving area off the western Australian coast, as the species recovers from whaling (Irvine et al., 2018).
During the summer feeding season, individuals utilize the area between 80˚E and 110˚E, aligned with Antarctic Management Area IV (IWC, 2006), with the southern Kerguelen plateau being highlighted as a main area of concentration through satellite tagging data (Bestley et al., 2019). Such information is well aligned with whaling and Discovery mark-recapture data (Bestley et al., 2019). It has been postulated that some individuals probably also use Area IIIE, where they mix with eastern Australia sub-stock (Pastene et al., 2019).
No records of supplementary feeding have been published so far for this stock, but there is evidence that individuals opportunistically do perform such behaviour. When investigating feeding habits of the stock using stable isotope analysis of baleen plates of individuals stranded between 1940 and 2015, Eisenmann et al. (2016) found that the feeding and fasting cycles followed a classical feeding model, with isotope values correlating with those of Antarctic krill. However, one of the individuals sampled showed supplementary discrete feeding in the temperate zone during migration reflected in relatively higher values of δ13C and δ15N in comparison to the other individuals (Eisenmann et al., 2016).
An interchange between individuals from western and eastern Australia sub-stocks has been initially indicated from Discovery Investigation mark-recapture data, based on whales that were marked in the Management Area V and recaptured in Area IV. Two of these ten individuals were also recaptured on the west coast of Australia (Chittleborough, 1965; Dawbin, 1966). Further evidence came from acoustic data suggesting shared songs by whales from western and eastern Australia (Noad et al., 2000). Genetic (Pastene et al., 2019) and photo-identification (Kaufman et al., 2011) evidence reinforce such mixing.
This is recognized to be the largest Southern Hemisphere stock of the species. Estimates have indicated a total of 26,100 (95% CI = 20,152–33,272) individuals in the North West Cape area in 2008 using aerial survey data (Salgado Kent et al., 2012), and some 21,750 individuals (95% CI = 17,550–43,000) migrating northward off Shark Bay in the same year from a combination of aerial survey and land-based data (Hedley et al., 2011). Abundance modelling projection of these numbers resulted in projected estimates of 20,337 (95% PI = 18,415–24,918) whales for 2015 for the whole eastern Australian stock (IWC, 2016b).
Previous estimates for Shark Bay from aerial survey data point to- 8,207–13,640 individuals in 1999 (Bannister and Hedley, 2001) and 10,300 (95% CI = 6,700–24,500) individuals in 2005 (Paxton et al., 2011). It is important to note that the estimate from Paxton et al. (2011) has a broader uncertainty (which can be seen from the confidence interval) and is considered conservative as the duration of the survey did not cover the whole breeding season of the species in the study area.
The trend for the North West Cape area shows a relatively high annual ROI (13%, 95% CI = 5.6–18.1%) between 2000 and 2008 (Salgado Kent et al., 2012). That is similar to the ROI observed for Shark Bay – 12.9% (CV = 0.20) – estimated based on the comparison of data from 2008 and those collected in a 1999 survey in the same area (Hedley et al., 2011). Such a relatively high ROI can indicate that the easter Australian stock is increasing at its maximum capacity over the last decade (Jenner et al., 2019). Alternatively, it can arise from sources of error in the abundance estimates for both Shark Bay and North West Cape, including limited accuracy in g(0) estimates, and in precision on the migration direction of the individuals in immigration models when considering data from aerial surveys (Jenner et al., 2019).
Abundance across the feeding ground was estimated to be of 17,959 (CV = 0.17) individuals from CPIII (1991/92–2003/04), with a ROI of 14.4% (95% CI = 9.6–19.2%) per year for the 1978–2004 period (Branch, 2011).
The sub-stock corresponds to whales breeding in coastal waters off eastern Australia (sub-stock E1) Hervey Bay is considered an important stopover for individuals of the stock coming from the Great Barrier Reef travelling towards the south to Antarctic waters (Forestell et al., 2011), especially for mother-calf pairs (Franklin et al., 2018; McCulloch et al., 2021). The Gold Coast Bay, part of the migratory corridor of eastern Australia sub-stock, has recently been indicated to also potentially be a calving area, as 74 newborns were observed in the area between 2013 and 2016 (Torre-Williams et al., 2019).
The coast of New Zealand is used by individuals of the eastern Australia stock during their migration between breeding and feeding grounds, and there is also evidence of the connectivity with individuals from New Caledonia, Fiji and Tonga has been found from photo-identification, acoustic, satellite tag and genetic data (Chittleborough, 1959; Dawbin, 1964; Franklin et al., 2014; Andrews-Goff et al., 2018; Steel et al., 2018; Warren et al., 2020). Data on the occurrence of the species on the east coast of New Zealand between 1970 and 1999 were compiled (Gibbs and Childerhouse, 2000). Over this 30-year period, only 157 sighting events were made, especially from Kaikoura and Cook Strait with an increase on the numbers over the last four years of the study period (Gibbs and Childerhouse, 2000). Data from annual surveys in the Cook Strait have indicated an increase of 13% (95% CI = 4.9%–21.7%) from 2005 to 2015, which seems to also indicate an influx of individuals from east Australia to this area (Gibbs et al., 2018). Recent acoustic data from 2016 have also supported the use of this migratory corridor between June and August, with song matches with New Caledonia breeding ground (Warren et al., 2020).
Feeding grounds used by the eastern Australian stock have been postulated to be within the longitudes 120˚E and 170˚W, corresponding to the IWC Management Areas IVE, V, and VIW (IWC, 2008). Within this broad area, some specific locations have been identified as important feeding locations for the sub-stock. For example, the Balleny Islands have been indicated to be important for eastern Australia sub-stock through photo-identification data analysis (Franklin et al., 2012). Also, an individual from eastern Australia in Area I during the feeding season, performing one of the longest mammalian migrations ever registered (Acevedo et al., 2022). Another finding relates one individual from the eastern Australia sub-stock to the west of the majority of the stock, widely using Management Area IV during summer (Andrews-Goff et al., 2018). Satellite tracking data of Oceania stock individuals tagged off Raoul Island (29°16′S), Kermadec Islands, New Zealand have shown that females with calves utilized the Ross Sea region during the feeding season, while most adults without calves migrated further east to the Amundsen and Bellingshausen Seas region (Riekkola et al., 2018). Findings indicating a close to year-round presence of the species in the feeding grounds can represent further evidence of possible suppression of migration or adoption of partial migration by some individuals in particular years.
Supplementary feeding for this sub-stock was first raised by Dawbin (1956) on the coast of New Zealand, given the presence of prey items such as the coastal krill Nyctiphanes australis in the stomach of individuals killed during whaling operations. N. australis was also sampled in proximity to two humpback whales feeding on the coast of Tasmania, near Cape Bougainville (~42°30’S) in November 1996 (Gill et al., 1998). That was after another sighting of individuals feeding on the coast of Tasmania off Blackmans Bay (43°S) in October of the same year (Gill et al., 1998).
Additional evidence of low-latitude feeding occurred some decades later, from the observation of an adult humpback whale in apparent feeding behaviour off Cape Moreton, Queensland, during austral summer in 2004 (Stockin and Burgess, 2005). During this event, observed during a whale-watching trip in the region, the whale was seen expanding and contracting the ventral pleats, and having the mouth partially opened close to the surface, with baitfish (likely sardines Sardinops sagax, although not confirmed) seen close to it.
Further south, evidence on feeding in the proximities of Narooma (36°5’S) and Eden (37°16’S), from late September to early November in 2002, 2003, and 2005, also during whale-watching activities, have been described by Stamation et al. (2007). Upwelling does occur in the area (Dawbin, 1956), enhancing marine productivity in the area and possibly contributing to the adoption of feeding activities by the whales of the eastern Australia sub-stock.
The feeding behaviour off Eden has been investigated with the use of digital acoustic recording tagging (DTAGs) of nine individuals between September and October 2011 and 2012 (Owen et al., 2015; Owen et al., 2017). Individuals performed lunge feeding at higher rates when preying upon krill than on fish items. The contribution of energy acquisition from feeding in the area seems significant, especially when individuals prey on N. australis, as they were estimated to then consume 1.2–3.4 times their energy requirements (Owen et al., 2017). A lower contribution comes from feeding on fish items, which likely included the jack mackerel Trachurus declivis, pilchards Sardinops neopilchardus, and redbait Emmelichthy nitidus, as they were observed on the surface during the 2012 surveys. Despite such a significant contribution of this low-latitude feeding to the energetic intake of the individuals, it is still not clear if the behaviour is opportunistic or an important aspect of the migration ecology of the sub-stock (Owen et al., 2017).
More recently, the bubble-netting feeding behaviour by humpback whales has been photographed off the east coast of Australia, adopted by individuals of the species feeding between Narooma and Eden and off the coast off Tasmania in September – October 2020 (Pirotta et al., 2021). It is suggested that this represents the second record of a super-group feeding in the Southern Hemisphere, the first being in South Africa. A total of six super-groups were observed, with sizes ranging from 20 to 90 individuals in each (Pirotta et al., 2021). Environmental conditions such as SST and nutrients availability are suggested to might have created a favourable scenario for the increase of the productivity in the area, then leading to large aggregations of individuals for feeding.
There are levels of interchange amongst individuals from the east coast of Australia and the stock using the west coast of Australia (Kaufman et al., 2011).
Since 1981, several shore-based survey operations have monitored the relative abundance of east Australian sub-stock (e.g. Paterson and Paterson, 1989; Bryden et al., 1990; Paterson et al., 1994; Paterson et al., 2001; Paterson et al., 2004). The sub-stock abundance was estimated to be 1,107 in 1987, having grown 9.7% (95% CI = 6–13%) yearly between 1981 and 1987 (Paterson and Paterson, 1989). By 1992, the abundance estimate of this stock was of 1,900 (± 250) individuals, growing at a rate of about 11.7% (95% CI = 9.6–13.8%) per year during the 1983-1992 period (Paterson et al., 1994). This is similar to the estimate of 2,099 (1,759–2,433) individuals for 1993 and the ROI of 10.0% (95% CI = 8.2–11.8%) per year between 1986 and 1993 (Brown, 1996) and of 12.3% (95% CI = 10.1–14.4%) per year between 1981 and 1996 (Bryden et al., 1996). By 1999, the abundance was estimated at 3,599 (95% CI = 3,162–4,036), with a ROI of 10.9% (95% CI = 10.2–11.6%) per year between 1987 and 1999 (Paterson et al., 2001), and the numbers increased to about 4,860 individuals by 2002 (Paterson et al., 2004). Later on, an absolute abundance of 9,683 (95% CI = 8,556–10,959) individuals was estimated for 2007 from land-based monitoring in Point Lookout, with a ROI of around 10.9% (95% CI = 10.5–11.4%) per year from 1984 to 2007 (Noad et al., 2008). This is similar to the estimate based on photo-identification data from Byron Bay (northern migration) and Hervey Bay and Ballina (southern migration) of 7,041 (95% CI = 4,075–10,008) individuals for 2005 (Paton et al., 2011).
Based on these data, the abundance of the eastern Australia sub-stock was projected to be of 19,614 (95% PI = 17,664–21,454) individuals in 2015 (IWC, 2016b). Another estimate for 2015 indicates 24,545 (95% CI = 21,631–27,851) individuals (Point Lookout), with a ROI of 11.0% (95% CI = 10.6–11.3%) per year across the period of 1984-2015 (Noad et al., 2019). In this study, the authors state that such estimates indicate the sub-stock can be considered closely recovered from the whaling, but still growing at a relatively high rate. Noad et al. (2019) also modelled future abundance for the sub-stock, and indicate two possible scenarios: (i) continuous growth to an abundance of at least 40,000 individuals, which would represent a new carrying capacity and mean that by 2015 it was actually 62% recovered, or (ii) grow to a peak of about 36,000 to 52,000 individuals at some point between 2021 and 2026, and then potentially face variations over the next decade from there.
On the feeding ground, data from the most recent IDCR/SOWER cruises (CPIII, 1998−2004) indicated an abundance estimate of 13,300 (CV = 0.20) individuals for the whole stock, and a ROI of 13.7% (95% CI = 9.3−18.1%) per year for the 1987−2007 period (Branch, 2011). Although the confidence interval indicates some uncertainty in this estimate, it is believed that the surveys covered most of the stock associated feeding ground.
Here we refer to sub-stock breeding in New Caledonia (sub-stock E2), Fiji and Tonga (sub-stock E3), Cook Islands (sub-stock F1) and French Polynesia (sub-stock F2) (IWC, 2007). There is considerably less information on the different aspects of these sub-stocks in comparison to the others (Tables 2–4). For New Caledonia sub-stock, the use of offshore areas is evident from satellite tag data, with seamounts seemingly important in the distribution of the individuals both during breeding and migration (Garrigue et al., 2015; Derville et al., 2020). For Tonga sub-stock, there seems to be a lack in the description of the habitat use of the species, although it is known that nearshore areas are important, attracting the whale-watching and swim-with-whale tourism industries (e.g. Schaffar and Garrigue, 2007; O’Connor et al., 2009). It has been suggested that the distribution of the French Polynesia sub-stock is expanding as a response to the increase in number or changing distribution in response to environmental changes. That comes from sightings of the species off Pitcairn Islands (25°04’S) during land-based surveys in 2007, with individuals apparently using the area for breeding and calving (Horswill and Jackson, 2012). Later on, the use of the area was confirmed, specifically off Henderson Island, from data collected in 2014 (Irving et al., 2018). This area is in the Central South Pacific, and it is indicated that individuals might be to its sub-stock, migrating from the Southern Ocean through Pitcairn to French Polynesia (Horswill and Jackson, 2012). However, further investigations are needed to confirm if there is a connection between the individuals sighted off Pitcairn and any of the currently recognized stocks. Data from dedicated surveys from areas of the Oceania breeding ground between 1996-2017 suggest the distribution of the individuals using it to be influenced by topography, with a preference for shallowest waters areas close to coast or in lagoons or around seamounts in offshore regions (Derville et al., 2019). In addition, a plasticity in its distribution is indicated as well as the potential use of new habitats as the regular ones become unsuitable due to rising sea temperatures (to greater than 28°C) by the end of the 21st century (Derville et al., 2019).
The migration routes are also poorly understood, but the feeding ground of this stock is suggested to be associated with the area between longitudes 170˚W and 110˚W, within Area VI (IWC, 2004; Hauser et al., 2010). For the Cook Islands sub-stock, there is direct evidence of a connection to Area VI E, based on the migration of a satellite-tagged individual (Hauser et al., 2010). In addition, there is also evidence of the connection of an individual from Tonga sub-stock with Area I (Robbins et al., 2011).
It is worth reminding that there is the Oceania stock is comprised by different sub-stocks from western and south central Pacific considering the level of interchange amongst them (e.g. Olavarría et al., 2007; Clapham et al., 2008; Hauser et al., 2010; Garrigue et al., 2011; Jackson et al., 2012; Pastene et al., 2013; Franklin et al., 2014; Derville et al., 2020).
There is limited information on abundance estimates for these sub-stocks, but some studies are available. A preliminary mark-recapture analysis of photo-identification data by the South Pacific Whale Research Consortium provided estimates for the period of 1999−2004 of 472 (CV = 0.18) for New Caledonia sub-stock and 2,311 (CV = 0.22) individuals for Tonga sub-stock (SPWRC et al., 2006). There is additional information on abundance for the Tonga sub-stock based on photo-identification mark-recapture data, indicating that some 1,057 (CV = 0.24) individuals utilized the area of French Polynesia between 1999 and 2004 (Poole, 2006). The Oceania stock as a whole was estimated to be of 4,329 (95% CI = 3,345−5,313) individuals in 2005, based on photo-identification and microsatellite genotype data (Constantine et al., 2012), and predicted to be of about 6,404 (95% PI = 5,491−7,595) individuals in 2015, considering a ROI of 8.2% per year during the 2010-2015 period (IWC, 2016b). These numbers indicate that BSO is the least abundant stock in the Southern Hemisphere.
The only estimated ROI for BSF comes from ICDR/SOWER cruise data and indicates an annual increase of 1.6% (95% CI = -5.4%–8.5%) for the 1982-2001 period (Branch, 2011). Considering the broad confidence interval, there is limited evidence for an actual increase (Leaper et al., 2008). The cruises in the Southern Ocean are also the source of the abundance estimate for BSF feeding grounds of 3,852 (CV = 0.22) individuals (Branch, 2011).
The breeding area for this stock is located on the east coast of Central and South America, including the coasts of Peru, Ecuador, Colombia, Panama, and Costa Rica (Flórez-González, 1991; Clapham and Mead, 1999; Félix and Haase, 2001; Stevick et al., 2004; Pacheco et al., 2009; Guidino et al., 2014; Acevedo et al., 2017; Albertson et al., 2018). Although the breeding ground encompasses a relatively large area, there is not enough current evidence for its sub-division into sub-stocks.
Possible extension of the breeding ground has also been reported, e.g. the presence of the calves in northern Peru potentially indicating the use of new areas with suitable environmental conditions (Guidino et al., 2014), as it increases in number (e.g. Félix et al., 2021). in the use of the Galapagos Archipelago (1°S, 91°W), with evidence of a genetic connection to western South America stock (Félix et al., 2011a), can also be an example of the post-whaling expansion of the area used by the species.
Individuals breeding between the Peruvian and Colombian coasts have been shown to feed in the Southern Ocean at longitudes between 110°W–50°W, mostly in the vicinities of the Western Antarctic Peninsula (e.g. Mackintosh, 1942; Stone et al., 1990; IWC, 2007; Rasmussen et al., 2007; Caballero et al., 2021). A second feeding ground has been identified in the Fuegian Archipelago, in the Magellan Strait (~53°30’S), with connection to individuals breeding on the coast of Panama (Acevedo et al., 2017). Magellan Strait is believed to have been repopulated as the stock recovers from whaling. Although there are historical records of whales using the area for the last six centuries (Gibbons et al., 2003 and references therein), there is evidence of the increase in the use of the area over the last few decades. It is indicated that individuals utilising this area generally do not migrate to the Antarctic Peninsula (Acevedo et al., 2017), although a few photo-identification matches have been found between this area and the Antarctic Peninsula (Acevedo et al., 2021). A third feeding location has been identified in the Gulf of Corcovado, Chilean Patagonia (41–44°S) (Hucke-Gaete et al., 2006), where the number of individuals encountered each year has been increasing since 2000 (Hucke-Gaete et al., 2013). Little is known about the breeding area of whales utilising this region.
There is also evidence that some individuals can suppress migration. In some cases, both evidence of feeding and breeding behaviour was seen in the same area, as in Chile, a feeding area beyond the regular feeding grounds (e.g. Hucke-Gaete et al., 2013), and there are also records of feeding observations were made during austral winter in the Magellan Strait, Chile (Gibbons et al., 2003), or individuals appeared to stay in the breeding ground year-round (e.g. Acevedo et al., 2017).
Although the connection of breeding and feeding grounds of this stock is well known (Stevick et al., 2004), the migratory corridors used by individuals remained unknown until recently. Satellite telemetry has been used for the investigation of this aspect, with the tagging of 16 whales in Antarctic waters between 2012 and 2017 (Modest et al., 2021). Results show two points of convergence of the migratory routes, one from the southern portion of Chile, and another off Peru, towards Colombia and Ecuador and into Panama. The authors indicate that such information provides a baseline for future studies to investigate the potential impacts of climate change and other stressors on the migration of humpback whales breeding on the east coast of South America.
The stock uses two feeding grounds in mid latitudes. Gibbons et al. (2003) provided evidence of the post-whaling use of the Magellan Strait (in the area between 48°50’S and 54°18’S) based on the record of 128 individuals in the area between December and June from 1997-2001, including the performance of lunge and ‘flick’ feeding behaviour. Another investigation on feeding behaviour in the area has demonstrated the adoption of bubble-net feeding, some of which were single straight-line bubble curtains (Acevedo et al., 2011). Gibbons et al. (2003) have indicated the presence of schooling fish as Fuegian sprat Sprattus fueguensis as potential prey, and more recently stable isotope analysis confirmed these to be the main food item of humpback whale individuals in the area (Haro et al., 2016), especially of adults in comparison with juveniles (Haro et al., 2021).
The second additional feeding ground of the stock is recognized to be also in Chilean waters, in the northern Chilean Patagonia (mostly between 41.5°S and 44°S), where aerial, land, and boat-based surveys performed between 2000 and 2010 (Hucke-Gaete et al., 2013) provided data on feeding activities in the area, mainly from 2006 onwards. Observations of group lunge feeding behaviour during boat-based surveys with concurrent prey sampling allowed the identification of krill Euphausia valentinii and lobster krill Munida spp. (M. subrugosa/gregaria).
In addition to these feeding grounds in mid latitude regions along the coast of Chile, feeding behaviour has been observed in low latitudes in the Southeast Pacific Ocean, including lunge and trap feeding behaviour by two humpback whales in Mejillones Bay (~23°S), northern Chile, in March-April 2019, preying upon Peruvian anchovy (Engraulis ringens) (García-Cegarra et al., 2021). One of the two individuals was resighted feeding in the same area in April 2020. In addition, two humpback whales are reported to have performed lunge feeding behaviour in June 2005 off Machalilla National Park (1°18’S), Ecuador, and feacal matter was observed on the surface on different occasions in 2008, 2011, 2016 and 2017. One of the individuals seen in Mejillones Bay in 2019 was also observed feeding on krill Euphausia sp. in the Gulf of Corcovado (42°S), Chile, in 2017, and further resighted at Machalilla National Park in 2020 feeding on E. ringens. García-Cegarra et al. (2021) state that the feeding activities can be a result of a higher competition for food in high latitudes given the increase in the stock’s abundance, changes in prey distribution, or more intense research efforts in regions that were previously poorly investigated.
To date, there is no evidence of stock division. Genetic data from the stock were found to be significantly different from other stocks, which can be related to a high site fidelity to the Antarctic Peninsula feeding ground (Amaral et al., 2016).
Humpback whales have been studied off the coast of Ecuador since 1991 (e.g. Félix et al., 2011a; Félix et al., 2011b). The abundance of the species visiting the area has been estimated to be of 6,504 (CV = 0.21; 95% PI = 4,270–9,907) individuals for 2006, based on photo-identification data collected from 1991 to 2006 (Félix et al., 2011a).
In an investigation around Las Perlas Archipelago, Panama, a stock size of some 1,041 (95% CI = 664–1,546) adults was estimated to have utilized the area across the 2003-2009 period (Guzman et al., 2015). On the coast of Colombia, the Gorgona Natural Park is an important area for breeding and nursing of humpback whales (Ávila et al., 2020). The analysis of a long-term dataset (1988-2018) revealed that the residency of the species in the area during the breeding season has extended over time, with individuals arriving from May and staying until December (Ávila et al., 2020). To date, there is no abundance estimate specific to this area.
An estimated abundance projection of 9,687 (95% PI = 8,520–10,202) individuals was made for 2015 (IWC, 2016b). An updated capture-recapture estimate using data from both feeding and breeding grounds across a 27-years period (1991–2018) indicated an abundance of 11,784 (SE = 266) individuals in 2018 and an estimated ROI of 5.07% (no CI provided) per year (Félix et al., 2021). In comparison to the ROI of other stocks, this increased rate is relatively low, which is attributed to different factors, including potential past overestimates and mixing of different stocks within the feeding grounds (Félix et al., 2021).
Based on the IDCR/SOWER cruises, an abundance of 3,337 (CV = 0.21) individuals was estimated for the Antarctic feeding ground (110°W–50°W) for 1999/2000 season, a number that increased about 4.6% (95% PI = -3.4–12.6%) per year over the period between 1981 and 2000 (Branch, 2011). Also, this feeding ground showed a very high pregnancy rate from sampled females, of 63.5% (95% CI = 57.14–69.57%), of which a considerable number (54.5%) were females with calves, indicating short post-partum periods as part of the reproductive cycles (Pallin et al., 2018). It is stated that such high pregnancy rates are not consistent with the current ROI of this BS, and that environmental factors may be playing a role in its demography. The correlation between the number of calves observed in the breeding area and the density of krill in the feeding area of the stock in the previous summer (Seyboth et al., 2021) can be an indication of this role. This is because food availability for sexually mature individuals is essential for them to be able to breed successfully and sustain a pregnancy (e.g. Reeves et al., 2001).
The IWC developed the International Decade of Cetacean Research (IDCR) and the Southern Ocean Whale Ecosystem Research (SOWER) programmes with the main aim of estimating minke whale abundance in the Southern Ocean (south of 60°S). However, sightings of all baleen whales were recorded during the cruises, between 1978 and 2004, so data have been used for other purposes (e.g. Branch and Butterworth, 2001). The initiative completed three circumpolar surveys (CPs) in 1978/79–1983/84 (CPI), 1985/86–1990/91 (CPII), and 1991/92–2003/04 (CPIII) (Branch, 2011).
Abundance estimates for the approximate mid-point of each CV were 7,100 (CV = 0.36) for 1980/81; 10,200 (CV = 0.30) for 1987/88; and 41,500 (CV = 0.11) for 1997/98, with an average ROI of 9.6% (95% CI = 5.8–13.4%) per year (Branch, 2011). The author states that these abundances are underestimates considering that some surveys did not cover the entire feeding grounds of the stocks. In the Southern Ocean, the area of higher encounter rates of humpback whales is in the Western Antarctic Peninsula (Kasamatsu et al., 1996), which can be related to the considerable amount of prey availability in the area, as indicated by the high density of krill in the area (e.g. Murphy et al., 2017).
This review highlights the successful recovery of humpback whale stocks in the Southern Hemisphere after being subjected to decades of human exploitation. The level of recovery, however, varies amongst different stocks. Most recent data suggest western Australian stock to be the most abundant, followed, in decreasing order, by the stocks and sub-stocks related to the east coast of South America, western Australia, Mozambique and southern Tanzania, west coast of South America, and Madagascar, Gabon, and French Polynesia. The simple sum of latest mean estimated and projected numbers from each sub-stock result in an abundance of around 114,422 individuals (the numbers considered are highlighted in bold in Table 3). Considering the estimate of around 140,000 whales individuals in the early 1900s (IWC, 2016b), current estimates of abundance suggest that Southern Hemisphere humpback whales recovered to nearly 80% of their abundance prior to the onset of modern whaling operations. Based on most recent growth estimates, the stock related to the east coast of South America (rmax = 7.6-10.7%) and Mozambique and southern Tanzania sub-stock (ROI = 7.4-8.8%) seem to be the ones growing at highest rates in comparison to other stocks and sub-stocks. These are however just broad evaluations of the most recent estimates, and we emphasize that in-dep analyses are needed for estimates and comparisons to be conclusive. Therefore, it seems to be important that a possible In-Depth Assessment for humpback whales in the Southern Hemisphere takes into consideration the updated estimates and the influence of modern threats (as climate change, ship-strikes, entanglements, underwater noise, and pollution – please see the Introduction section).
Although information on abundance, ROI, and distribution is available for all Southern Hemisphere humpback whale stocks, it can be very limited and not available at the sub-stock level in some cases. Since the last IWC Comprehensive Assessment (IWC, 2016a), little progress has been made to fill important gaps for most of the sub-stocks. The need for new estimates of abundance and trends for some sub-stocks (Namibia and west coast of South Africa, Comoros Archipelago, Madagascar, New Caledonia, Cook Islands, and French Polynesia) as indicated in such assessment remains relevant to date. Gathering information for the evaluation of both the absolute abundance and the growth rate of each sub-stock will require a collaborative approach and would be ideally based on the use of the same methodology and protocol for data collection so the numbers are highly comparable and the growth estimates more precise. Estimates based on data from feeding grounds are also limited, and as indicated throughout the text, some from IDCR/SOWER cruises should not be considered representative of the whole stock they refer to considering the area covered during the cruises (Miyashita et al., 1995; Ensor et al., 2006).
It is also worth mentioning that some of the growth rate estimates presented in Table 4 for different stocks are not statistically different even though the point estimate is different. It is also important to remind that while most of the values refer to ROIs, one of them, as highlighted on the table, refers to the maximum intrinsic rate of increase (rmax), which needs to be taken in consideration when evaluating the values. Also, the data for their estimation were not necessarily collected using the same protocol and then some comparison should be made with caution. However, they are informative, and we believe they contribute to the knowledge of the stocks trends and therefore are considering such information during the review of the publications.
The methodology adopted for data collection for abundance estimation can impact on the results found (e.g. Cerchio et al., 2008; Paxton et al., 2011). Aerial surveys, with the use of distance sampling methodology (Buckland et al., 2001), have been indicated as one of the most effective methods for the estimation of population abundance and growth, once adequate protocols are established (Andriolo et al., 2006b). In some cases, however, as for the investigation of migration of the western Australia stock using land-based and aerial surveys (Jenner et al., 2019), the combination of methodologies for data collection can increase confidence in the results. Therefore, it seems important that the method applied consider the characteristics of each stock and the resources available for estimating their size and trends. Furthermore, the importance of standardization of data collection in a specific area over time so changes can be properly evaluated has been highlighted by Andriolo et al. (2006b).
Working on the standardization and improving the quality of citizen science data (e.g. Downs et al., 2021) is also important. Although coming with limitations and biases, this type of data has been increasingly used in marine science (e.g. Kelly et al., 2020) and can be highly valuable to provide updated information from different humpback whale stocks and sub-stocks (e.g. Tonachella et al., 2012; Bruce et al., 2014; Lodi and Tardin, 2018; Pirotta et al., 2020). Initiatives to connect the public with research groups, and to increase the use of established platforms such as Happywhale (Cheeseman et al., 2022) are encouraged.
The interchange among breeding stocks and sub-stocks is another relevant factor for the investigation of stock parameters such as abundance and ROI and ignoring this aspect can lead to a misinterpretation of the recovery of stocks (Amaral et al., 2016). There are different levels of interchange between stocks and sub-stocks, and they tend to be higher in the feeding grounds (e.g. Butterworth and Johnston, 2009; Pastene et al., 2013; Stevick et al., 2013; Rosenbaum et al., 2017; Félix et al., 2020b; Marcondes et al., 2021). It is also worth noting that the increase in the number of individuals as the stocks recover from the whaling era is expected to lead to an increase in the mixing between stocks in feeding grounds, suggesting that feeding ground boundaries between stocks might need to be revised (Marcondes et al., 2021), with potential implications to catch allocation. Although in a lesser extent, the connection between breeding sub-stocks in breeding grounds and the different residency patterns of the individuals in such areas should be considered for the development of management and conservation actions in both regional and local scales (Dulau et al., 2017).
A potential factor contributing for relatively high ROI estimates can be an increased reproductive capacity of the individuals (e.g. through a shorter calving interval), as observed for the Oceania stock (Chero et al., 2020). It is important to note that some of the ROI estimates presented are higher than the maximum biologically plausible rate estimated for the species (11.8% per year, Zerbini et al., 2010), which can be a result of measurement errors and/or faulty sampling methods. For the Oceania stock, immigration amongst sub-stocks seems to be an important aspect contributing to relatively high estimates observed and for the relatively low rates estimated for areas that are known to have a substantial abundance before whaling (Clapham and Zerbini, 2015). Also, some abundance and ROI estimates presented have considerable large confidence intervals, and in some cases the ROI range includes the value of 11.8% per annum, so can also be considered with caution as it then falls in values not considered biologically plausible for the species and can indicate limitations in data collection and/or analysis. That also adds uncertainty to some numbers, and the continuity of data collection and investigation of potential causes for the large confidence interval are necessary for a more precise investigation of the status of the species in some areas.
How the recovery of the species might impact prey populations is an additional aspect to be addressed, especially considering the variabilities in biomass that the main prey item of the species is already subjected to under the current climate change scenario (e.g. Atkinson et al., 2019; Veytia et al., 2020; Sylvester et al., 2021). That can influence not just the relationship between humpback whales and krill, but also the interaction of humpback whale and other species that rely on krill as their main prey item. For example, resource partitioning of humpback and minke (Friedlaender et al., 2006b) and fin (Herr et al., 2016) whales has been observed in the Western Antarctic Peninsula, and can potentially be affected as whale species recover from whaling and krill development tend to decrease over time given environmental changes (e.g. Atkinson et al., 2004, Atkinson et al., 2019). Supplementary feeding beyond the Southern Ocean has been reported for baleen whales for a long time (e.g. Kawamura, 1975) but it can potentially increase with the changes being observed in the Southern Ocean and the consequences for krill availability, so the compilation of the records so far for humpback whales might serve as a baseline for comparison in the future. The formation of feeding aggregations (super-groups) in feeding grounds and/or migratory routes in lower latitudes, as on the west coast of South Africa (Findlay et al., 2017) and off Tasmania (Pirotta et al., 2021), might also be a response of some stocks to environmental changes. On this topic, it is worth indicating that information coming from stranded animals should be taken with caution as those animals might not be healthy and not performing their expected behaviour, then providing misleading information.
Another point to be considered in future studies is how the different threats to the species will affect current numbers and possible interaction between stocks, as well as the challenging task of disentangling the effect of each of these multiple stressors. A multidisciplinary approach focused on the understanding of the effects of climate change on humpback whale stocks can be essential for such achievement, as proposed by Meynecke et al. (2020), a process that can also be benefitted from crossdisciplinary analysis (Pirotta et al., 2022). Analysing potential changes in the distribution of the species, considering the multiple environmental drivers of habitat selection (e.g. SST, bathymetry, chlorophyll-a concentration – Meynecke et al., 2021) is also necessary to better evaluate the conservation status of different stocks and the need for their management. That would help in the identification of potential new areas to be used by the species, as well as areas under such a high level of stress that might become unsuitable, which is relevant for the better protection of humpback whales (Meynecke et al., 2021).
The more information gathered on the distribution and behaviour of the species, the more viable it is for researchers to start relating changes to specific causes or threats. Finding the causes of changes in distribution, migration and/or behaviour for the species can be an important aspect for the management of the different stocks. In addition, the adaptability shown by the species, for instance by the return to areas used prior to whaling and the expansion of historical breeding grounds into both breeding and feeding grounds are aspects that can impact the abundance found in these areas, as well as management planning. Such plasticity shown by different stocks makes the investigation of the recovery of the species and interpretation of estimates even more challenging. For example, data collection that has been performed for some time in specific places within the breeding area where individuals tended the concentrate can indicate a decrease in the numbers, but that does not necessarily mean the growth rate decrease, but be a reflect of the stocks expanding their distribution in breeding grounds, spreading out as their return to areas previously used and/or explore new areas. It is also important to note that the inclusion of pre-modern whaling catches (e.g. Zerbini et al., 2019) and the use of correction factors to account for struck-and-lost animals (Baker and Clapham, 2004; Vighi et al., 2020) is critical for the whaling data to be more accurate and the habitat use of the stocks to be better understood. That can influence for instance on the evaluation of catch data when investigating humpback whale plasticity and potential response to climate change in breeding areas.
Despite research efforts on humpback whales in the Southern Hemisphere since the cessation of the commercial whaling activities, there are a number of unknowns. This review may help in updating trends of the different stocks and in giving direction to further efforts to fill current knowledge gaps that affect the conservation of the species.
ES and KF contributed to the conception and design of the study. ES performed the literature review, organized data and information found, and wrote the first draft of the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
This work was supported by a grant to Griffith University from a private charitable trust as part of the Whales & Climate Research Program.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.997491/full#supplementary-material
Supplementary Figure 1 | Map with the indication of specific locations/areas withing the breeding area of the Southwestern Atlantic Ocean stock, along the coast of Brazil.
Supplementary Figure 2 | Map with the indication of specific locations/areas withing the breeding area of the west coast of Africa stock.
Supplementary Figure 3 | Map with the indication of specific locations/areas withing the breeding area of the east coast of Africa and western Indian Ocean stock.
Supplementary Figure 4 | Map with the indication of specific locations/areas withing the breeding area along the west coast of Australia.
Supplementary Figure 5 | Map with the indication of specific locations/areas withing the breeding area of the stock using the west coast of South America.
Acevedo A., Smultea M. A. (1995). First records of humpback whales including calves at Golfo Dulce and Isla del Coco, Costa Rica, suggesting geographical overlap of northern and southern hemisphere populations. Mar. Mammal Sci. 11 (4), 554–560. doi: 10.1111/j.1748-7692.1995.tb00677.x
Acevedo J., Aguayo-Lobo A., Allen J., Botero-Acosta N., Capella J., Castro C., et al. (2017). Migratory preferences of humpback whales between feeding and breeding grounds in the eastern South Pacific. Mar. Mammal Sci. 33, 1035–1052. doi: 10.1111/mms.12423
Acevedo J., Aguayo-Lobo A., Beeman P., Cheeseman T., Olavarría C. (2022). From the Antarctic Peninsula to eastern Australia: the longest migration of a humpback whale through the South Pacific Ocean. Mamm. Biol. 102 (4), 1463–1468. doi: 10.1007/s42991-021-00195-2
Acevedo J., Capella J., Cheeseman T., Monnahan C. C., Southerland K., Acuna P., et al. (2021). First evidence of interchange of humpback whales (Megaptera novaeangliae) between the Magellan Strait and Antarctic Peninsula feeding grounds. Polar Biol. 44, 613–619. doi: 10.1007/s00300-021-02827-2
Acevedo J., Plana J., Aguayo-Lobo A., Pastene L. A. (2011). Surface feeding behavior of humpback whales in the Magellan Strait. Rev. Biol. Mar. Oceanogr. 46, 483–490. doi: 10.4067/S0718-19572011000300018
Albertson G. R., Friedlaender A. S., Steel D. J., Aguayo-Lobo A., Bonatto S. L., Caballero S., et al. (2018). Temporal stability and mixed-stock analyses of humpback whales (Megaptera novaeangliae) in the nearshore waters of the Western Antarctic Peninsula. Polar Biol. 41, 323–340. doi: 10.1007/s00300-017-2193-1
Allison C. (2020). IWC summary large whale catch database Version 7.1. (135 Station Road, Impington, Cambridge: International Whaling Commission). CB24 9NP UK.
Amaral A. R., Loo J., Jaris H., Olavarría C., Thiele D., Ensor P., et al. (2016). Population genetic structure among feeding aggregations of humpback whales in the Southern Ocean. Mar. Biol. 163, 1–13. doi: 10.1007/s00227-016-2904-0
Andrews-Goff V., Bestley S., Gales N. J., Laverick S. M., Paton D., Polanowski A. M., et al. (2018). Humpback whale migrations to Antarctic summer foraging grounds through the southwest Pacific Ocean. Sci. Rep. 8, 12333. doi: 10.1038/s41598-018-30748-4
Andriolo A., Kinas P. G., Engel M. H., Martins C. C. A. (2006b). Monitoring humpback whale abundance (Megaptera novaeangliae) population in the Brazilian breeding ground 2002 to 2005. IWC Paper SC/58/SH15.
Andriolo A., Kinas P. G., Engel M. H., Martins C. C. A., Rufino A. M. (2010). Humpback whales within the Brazilian breeding ground: distribution and population size estimate. Endang. Species Res. 11, 233–243. doi: 10.3354/esr00282
Andriolo A., Martins C. C. A., Engel M. H., Pizzorno J. L., Más-Rosa S., Freitas A. C., et al. (2006a). The first aerial survey of humpback whale (Megaptera novaeangliae) to estimate abundance in the breeding ground, Brazil. J. Cetacean Res. Manage. 8, 307–311. doi: 10.47536/jcrm.v8i3.728
Atkinson A., Hill S. L., Pakhomov E. V., Siegel V., Reiss C. S., Loeb V. J., et al. (2019). Krill (Euphausia superba) distribution contracts southward during rapid regional warming. Nat. Clim. Change. 9, 142–147. doi: 10.1038/s41558-018-0370-z
Atkinson A., Siegel V., Pakhomov E., Rothery P. (2004). Long-term decline in krill stock and increase in salps within the Southern Ocean. Nature. 432, 100–103.
Ávila I. C., Dormann C. F., García C., Payán L. F., Zorrilla M. X. (2020). Humpback whales extend their stay in a breeding ground in the Tropical Eastern Pacific. ICES J. Mar. Sci. 77, 109–118. doi: 10.1093/ICESjms/fsz251
Baines M., Kelly N., Reichelt M., Lacey C., Pinder S., Fielding S., et al. (2021). Population abundance of recovering humpback whales Megaptera novaeangliae and other baleen whales in the Scotia Arc, South Atlantic. Mar. Ecol. Prog. Ser. 676, 77–94. doi: 10.3354/meps13849
Baker C. S., Clapham P. J. (2004). Modelling the past and future of whales and whaling. Trends Ecol. Evol. 19 (7), 365–371. doi: 10.1016/j.tree.2004.05.005
Bamy I. L., Van Waerebeek K., Bah S. S., Dia M., Kaba B., Keita N., et al. (2010). Species occurrence of cetaceans in Guinea, including humpback whales with southern hemisphere seasonality. Mar. Biodivers. Rec. 3, e48. doi: 10.1017/S1755267210000436
Bannister J. L. (1994). Continued increase in humpback whales off Western Australia. Rep. Int. Whal. Commn. 44, 309–310.
Bannister J. L., Hedley S. L. (2001). Southern Hemisphere group IV humpback whales: their status from recent aerial survey. Mem. Queensl. Mus. 47, 587–598.
Bannister J. L., Kirkwood G. P., Wayte S. E. (1991). Increase in humpback whales off Western Australia. Rep. Int. Whal. Commn. 41, 461–465.
Barendse J., Best P. B., Carvalho I., Pomilla C. (2013). Mother knows best: occurrence and associations of resighted humpback whales suggest maternally derived fidelity to a Southern Hemisphere coastal feeding ground. PloS One. 8, e81238. doi: 10.1371/journal.pone.0081238
Barendse J., Best P. B., Thornton M. (2006). Preliminary results of photo-identification of humpback whales on the west coast of South Africa. IWC Paper SC/A06/HW4.
Barendse J., Best P. B., Thornton M., Elwen S. H., Rosenbaum H. C., Carvalho I., et al. (2011). Transit station or destination? Attendance patterns, movements and abundance estimate of humpback whales off west South Africa from photographic and genotypic matching. Afr. J. Mar. Sci. 33, 353–373. doi: 10.2989/1814232X.2011.637343
Barendse J., Best P. B., Thornton M., Pomilla C., Carvalho I., Rosenbaum H. C. (2010). Migration redefined? Seasonality, movements and group composition of humpback whales Megaptera novaeangliae off the west coast of South Africa. Afr. J. Mar. Sci. 32, 1–22. doi: 10.2989/18142321003714203
Bedriñana-Romano L., Zerbini A. N., Andriolo A., Danilewicz D., Sucunza F. (2022). Individual and joint estimation of humpback whale migratory patterns and their environmental drivers in the Southwest Atlantic Ocean. Sci. Rep. 12 (1), 7487. doi: 10.1038/s41598-022-11536-7
Besseling E., Foekema E. M., Van Franeker J. A., Leopold M. F., Kühn S., Rebolledo E. B., et al. (2015). Microplastic in a macro filter feeder: humpback whale Megaptera novaeangliae. Mar. pollut. Bull. 95, 248–252. doi: 10.1016/j.marpolbul.2015.04.007
Best P. B., Allison C. (2010). Catch history, seasonal and temporal trends in the migrations of humpback whales along the west coast of southern Africa. IWC Paper SC/62/SH5.
Best P. B., Findlay K. P., Sekiguchi K., Peddemors V. M., Rakotonirina B., Rossouw A., et al. (1998). Winter distribution and possible migration routes of humpback whales Megaptera novaeangliae in the southwest Indian Ocean. Mar. Ecol. Prog. Ser. 162, 287–299. doi: 10.3354/meps162287
Best P. B., Sekiguchi K., Findlay K. P. (1995). A suspended migration of humpback whales Megaptera novaeangliae on the west coast of South Africa. Mar. Ecol. Prog. Ser. 118, 1–12. doi: 10.3354/meps118001
Best P. B., Sekiguchi K., Rakotonirina B., Rossouw A. (1996). The distribution and abundance of humpback whales off southern Madagascar, August–September 1994. Rep. Int. Whal. Commn. 46, 323–331.
Best P. B., Shaughnessy P. D. (1979). An independent account of Captain Benjamin Morrell’s sealing voyage to the south-west coast of Africa in the Antarctic 1828/29. Fish. Bull. South Afr. 12, 1–19.
Bestley S., Andrews-Goff V., van Wijk E., Rintoul S. R., Double M. C., How J. (2019). New insights into prime Southern Ocean forage grounds for thriving Western Australian humpback whales. Sci. Rep. 9, 1–12. doi: 10.1038/s41598-019-50497-2
Bortolotto G. A., Danilewicz D., Andriolo A., Secchi E. R., Zerbini A. N. (2016a). Whale, whale, everywhere: increasing abundance of western South Atlantic humpback whales (Megaptera novaeangliae) in their wintering grounds. PloS One. 11, e0164596. doi: 10.1371/journal.pone.0164596.t002
Bortolotto G. A., Danilewicz D., Hammond P. S., Thomas L., Zerbini A. N. (2017). Whale distribution in a breeding area: spatial models of habitat use and abundance of western South Atlantic humpback whales. Mar. Ecol. Prog. Ser. 585, 213–227. doi: 10.3354/meps12393
Bortolotto G. A., Kolesnikovas C. K. M., Freire A. S., Simões-Lopes P. C. (2016b). Young humpback whale Megaptera novaeangliae feeding in Santa Catarina coastal waters, Southern Brazil, and a ship strike report. Mar. Biodivers. Rec. 9, 1–6. doi: 10.1186/s41200-016-0043-4
Bortolotto G. A., Thomas L., Hammond P., Zerbini A. N. (2021). Alternative method for assessment of southwestern Atlantic humpback whale population status. PloS One. 16 (11), e0259541. doi: 10.1371/journal.pone.0259541
Branch T. A. (2011). Humpback whale abundance south of 60°S from three complete circumpolar sets of surveys. J. Cetacean Res. Manage. Special Issue. 3, 53–69.
Branch T. A., Butterworth D. S. (2001). Estimates of abundance south of 60°S for cetacean species sighted frequently on the 1978/79 to 1997/98 IWC/IDCR-SOWER sighting surveys. J. Cetacean Res. Manage. 3 (3), 251–270.
Brown M. R. (1996). Population biology and migratory characteristics of east Australian humpback whales Megaptera novaeangliae.. (University of Sydney). 322pp.
Bruce E., Albright L., Sheehan S., Blewitt M. (2014). Distribution patterns of migrating humpback whales (Megaptera novaeangliae) in Jervis Bay, Australia: A spatial analysis using geographical citizen science data. Appl. Geogr. 54, 83–95. doi: 10.1016/j.apgeog.2014.06.014
Bryden M. M., Brown M. R., Field M. S., Clarke E. D., Butterworth D. S. (1996). Survey of humpback whales (Megaptera novaeangliae) off eastern Australia (Canberra: Report to the Australian Nature Conservation Agency). 77pp.
Bryden M. M., Kirkwood G. P., Slade R. W. (1990). “Humpback whales Area V an increase in numbers off Australia’s east coast,” in Antarctic ecosystems, ecological change and conservation. Eds. Kelly K. R., Hempel G. (Berlin, Heidelberg: Springer-Verlag), 271–277.
Buckland S. T., Anderson D. R., Burnham K. P., Laake J. L., Borchers D. L., Thomas L. (2001). Introduction to distance sampling: estimating abundance of biological populations. (London: Oxford University Press).
Butterworth D. S., Johnston S. J. (2009). Report on discussions on modelling studies of possible interchange between the C1 and C3 breeding Substocks of Southern Hemisphere humpback whales. IWC Paper SC/F09/SH1.
Butterworth D. S., Punt A. E. (1995). On the Bayesian approach suggested for the assessment of the Bering-Chukchi-Beaufort Seas stock of bowhead whales. Rep. Int. Whal. Commn. 45, 303–311.
Caballero S., Steel D., Pallin L., Botero-Acosta N., Felix F., Olavarría C., et al. (2021). Migratory connections among breeding grounds off the Eastern Pacific and feeding areas in the Antarctic Peninsula based on genotype matching. Bol. Invest. Mar. Cost. 50, 31–40. doi: 10.25268/bimc.invemar.2021.50.SuplEsp.933
Carvalho I., Brito C., dos Santos M. E., Rosenbaum H. C. (2011). The waters of São Tomé: a calving ground for West African humpback whales? Afr. J. Mar. Sci. 33, 91–97. doi: 10.2989/1814232X.2011.572353
Carvalho I., Loo J., Collins T., Barendse J., Pomilla C., Leslie M. S., et al. (2014). Does temporal and spatial segregation explain the complex population structure of humpback whales on the coast of West Africa? Mar. Biol. 161, 805–819. doi: 10.1007/s00227-013-2379-1
Casà M. V., van Mourik L. M., Weijs L., Mueller J., Nash S. B. (2019). First detection of short-chain chlorinated paraffins (SCCPs) in humpback whales (Megaptera novaeangliae) foraging in Antarctic waters. Environ. Pollut. 250, 953–959. doi: 10.1016/j.envpol.2019.04.103
Caughley G. (1994). Directions in conservation biology. J. Anim. Ecol. 63, 215–244. doi: 10.2307/5542
Cerchio S., Ersts P., Pomilla C., Loo J., Razafindrakoto Y., Leslie M., et al. (2009). Updated estimates of abundance for humpback whale breeding stock C3 off Madagascar 2000–2006. IWC Paper SC/61/SH7.
Cerchio S., Findlay K., Ersts P., Minton G., Bennet D., Meÿer M., et al. (2008). Initial assessment of exchange between breeding stocks C1 and C3 of humpback whales in the western Indian Ocean using photographic mark-recapture data 2000-2006. IWC Paper SC/60/SH33.
Cerchio S., Trudelle L., Zerbini A. N., Charrassin J. B., Geyer Y., Mayer F. X., et al. (2016). Satellite telemetry of humpback whales off Madagascar reveals insights on breeding behavior and long-range movements within the southwest Indian Ocean. Mar. Ecol. Prog. Ser. 562, 193–209. doi: 10.3354/meps11951
Cheeseman T., Southerland K., Park J., Olio M., Flynn K., Calambokidis J., et al. (2022). Advanced image recognition: a fully automated, high-accuracy photo-identification matching system for humpback whales. Mamm. Biol. 102 (3), 915–929. doi: 10.1007/s42991-021-00180-9
Chero G., Pradel R., Derville S., Bonneville C., Gimenez O., Garrigue C. (2020). Reproductive capacity of an endangered and recovering population of humpback whales in the Southern Hemisphere. Mar. Ecol. Prog. Ser. 643, 219–227. doi: 10.3354/meps13329
Childerhouse S., Jackson J., Baker C. S., Gales N., Clapham P. J., Brownell R. L. Jr. (2008) Megaptera novaeangliae (Oceania subpopulation). IUCN Red List Threat Species Version 20132. Available at: http://www.iucnredlist.org/details/132832.
Chittleborough R. G. (1959). Australian marking of humpback whales. Norsk Hvalfangst Tidende (Norwegian Whaling Gazette). 48, 47–55.
Chittleborough R. G. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Mar. Freshw. Res. 16 (1), 33–128. doi: 10.1071/MF9650033
Clapham P. J., Baker C. S. (2002). Modern whaling, in Encyclopedia of Marine Mammals. Eds. Perrin W. F., Würsig B., Thewissen J. G. M. (London: Academic Press), 1328–1332.
Clapham P. J., Garrigue C., Hauser N., Geyer Y., Zerbini A. (2008). Movements of satellite-monitored humpback whales from New Caledonia and the Cook Islands. IWC Paper SC/60/SH34.
Clapham P. J., Mead J. G. (1999). Megaptera novaeangliae. Mamm. Species. 604, 1–9. doi: 10.2307/3504352
Clapham P., Mikhalev Y., Franklin W., Paton D., Baker C. S., Ivashchenko Y. V., et al. (2009). Catches of humpback whales, Megaptera novaeangliae, by the Soviet Union and other nations in the Southern Ocean 1947–1973. Mar. Fish. Rev. 71, 39–43.
Clapham P. J., Zerbini A. N. (2015). Are social aggregation and temporary immigration driving high rates of increase in some Southern Hemisphere humpback whale populations? Mar. Biol. 162, 625–634. doi: 10.1007/s00227-015-2610-3
Collins T., Cerchio S., Pomilla C., Loo J., Carvalho I., Ngouessono S., et al. (2010). Revised estimates of abundance for humpback whale breeding stock B1: Gabon. IWC Paper SC/60/SH28.
Constantine R., Jackson J. A., Steel D., Baker C. S., Brooks L., Burns D., et al. (2012). Abundance of humpback whales in Oceania using photo-identification and microsatellite genotyping. Mar. Ecol. Prog. Ser. 453, 249–261. doi: 10.3354/meps09613
Cooke J. G. (2018) Megaptera novaeangliae. The IUCN Red List of Threatened Species 2018. (Accessed 31 May 2023).
Croll D. A., Kudela R., Tershy B. R. (2006). “Ecosystem impacts of the decline of large whales in the North Pacific,” in Whales, whaling and ocean ecosystems. Eds. Estes J. A., DeMaster D. P., Doak D. F., Williams T. M., Brownell R. L. (Berkeley, CA: University of California Press), 200–212.
Cypriano-Souza A. L., da Silva T. F., Engel M. H., Bonatto S. L. (2018). Effective population size and the genetic consequences of commercial whaling on the humpback whales (Megaptera novaeangliae) from Southwestern Atlantic Ocean. Genet. Mol. Biol. 41, 253–262. doi: 10.1590/1678-4685-gmb-2017-0052
Danilewicz D., Tavares M., Moreno I. B., Ott P. H., Trigo C. C. (2009). Evidence of feeding by the humpback whale (Megaptera novaeangliae) in mid-latitude waters of the western South Atlantic. Mar. Biodivers. Rec. 2, e88. doi: 10.1017/S1755267209000943
Das K., Malarvannan G., Dirtu A., Dulau V., Dumont M., Lepoint G., et al. (2017). Linking pollutant exposure of humpback whales breeding in the Indian Ocean to their feeding habits and feeding areas off Antarctica. Environ. pollut. 220, 1090–1099. doi: 10.1016/j.envpol.2016.11.032
Dawbin W. H. (1956). The migrations of humpback whales which pass the New Zealand Coast. Trans. Proc. R. Soc N. Z. 84, 147–196.
Dawbin W. H. (1964). Movements of humpback whales marked in the southwest Pacific Ocean 1952 to 1962. Norsk Hvalfangst Tidende (Norwegian Whaling Gazette). 53, 68–78.
Dawbin W. H. (1966). “The seasonal migratory cycle of humpback whales,” in Whales, dolphins and porpoises. Ed. Norris K. (Berkeley, CA: University of California Press), 145–170.
Derville S., Torres L. G., Albertson R., Andrews O., Baker C. S., Carzon P., et al. (2019). Whales in warming water: Assessing breeding habitat diversity and adaptability in Oceania’s changing climate. Glob. Change Biol. 25, 1466–1481. doi: 10.1111/gcb.14563
Derville S., Torres L. G., Zerbini A. N., Oremus M., Garrigue C. (2020). Horizontal and vertical movements of humpback whales inform the use of critical pelagic habitats in the western South Pacific. Sci. Rep. 10, 1–13. doi: 10.1038/s41598-020-61771-z
Dey S. P., Vichi M., Fearon G., Seyboth E., Findlay K. P., Meynecke J. O., et al. (2021). Oceanographic anoMalies coinciding with humpback whale super-group occurrences in the Southern Benguela. Sci. Rep. 11, 1–13. doi: 10.1038/s41598-021-00253-2
D’Incao F., Martins S. T. S. (2000). Brazilian species of the genera Acetes H. Milne Edwards 1830 and Peisos Burkenroad 1945 (Decapoda: sergestidae). J. Crust. Biol. 20 (5), 78–86. doi: 10.1163/1937240X-90000010
Donovan G. P. (1991). A review of IWC stock boundaries. Rep. Int. Whal. Commun. Special Issue. 13, 39–68.
Downs R. R., Ramapriyan H. K., Peng G., Wei Y. (2021). Perspectives on citizen science data quality. Front. Clim. 3, 615032. doi: 10.3389/fclim.2021.615032
Dulau V., Pinet P., Geyer Y., Fayan J., Mongin P., Cottarel G., et al. (2017). Continuous movement behavior of humpback whales during the breeding season in the southwest Indian Ocean: on the road again! Mov. Ecol. 5, 1–17. doi: 10.1186/s40462-017-0101-5
Dulau-Drouot V., Cerchio S., Jouannet V., Ersts P., Fayan J., Boucaud V., et al. (2011). Preliminary comparison of humpback whale photographic identifications indicates connectivity between Reunion (BS C4) and Madagascar (BS C3). IWC Paper SC/63/SH28.
Dunlop R. A. (2019). The effects of vessel noise on the communication network of humpback whales. R. Soc Open Sci. 6, 190967. doi: 10.1098/rsos.190967
Dunlop R. A., McCauley R. D., Noad M. J. (2020). Ships and air guns reduce social interactions in humpback whales at greater ranges than other behavioral impacts. Mar. pollut. Bull. 154, 111072. doi: 10.1016/j.marpolbul.2020.111072
Eisenmann P., Fry B., Holyoake C., Coughran D., Nicol S., Bengtson Nash S. (2016). Isotopic evidence of a wide spectrum of feeding strategies in Southern Hemisphere humpback whale baleen records. PloS One. 11, e0156698. doi: 10.1371/journal.pone.0156698
Elwen S. H., Tonachella N., Barendse J., Collins T., Best P. B., Rosenbaum H. C., et al. (2014). Humpback whales off Namibia: occurrence, seasonality, and a regional comparison of photographic catalogs and scarring. J. Mammal. 95, 1064–1076. doi: 10.1644/14-MAMM-A-108
Engel M. H., Fagundes N. J., Rosenbaum H. C., Leslie M. S., Ott P. H., Schmitt R., et al. (2008). Mitochondrial DNA diversity of the Southwestern Atlantic humpback whale (Megaptera novaeangliae) breeding area off Brazil, and the potential connections to Antarctic feeding areas. Conserv. Genet. 9, 1253–1262. doi: 10.1007/s10592-007-9453-5
Engel M. H., Martin A. R. (2009). Feeding grounds of the western South Atlantic humpback whale population. Mar. Mammal Sci. 25, 964–969. doi: 10.1111/j.1748-7692.2009.00301.x
Ensor P., Komiya H., Olson P., Sekiguchi K., Stafford K. (2006). 2005-2006 International Whaling Commission-Southern Ocean Whale and Ecosystem Research (IWC-SOWER) cruise. IWC Paper SC/58/IA1.
Ersts P. J., Pomilla C., Kiszka J., Cerchio S., Rosenbaum H. C., Vély M., et al. (2011). Observations of individual humpback whales utilising multiple migratory destinations in the south-western Indian Ocean. Afr. J. Mar. Sci. 33, 333–338. doi: 10.2989/1814232X.2011.600436
Félix F., Abras D. R., Cheeseman T., Haase B., Santos J. D. A. F., Marcondes M. C. C., et al. (2020a). A new case of interoceanic movement of a humpback whale in the southern hemisphere: the el nino link. Aquat. Mamm. 46, 578–584. doi: 10.1578/AM.46.6.2020.578
Félix F., Acevedo J., Aguayo-Lobo A., Ávila I. C., Botero-Acosta N., Calderón A., et al. (2021). Humpback whale breeding stock g: updated population estimate based on photo-id matches between breeding and feeding areas. IWC Paper SC/68C/ASI/02.
Félix F., Castro C., Laake J. L., Haase B. E. N., Scheidat M. (2011a). Abundance and survival estimates of the southeastern Pacific humpback whale stock from 1991–2006 photo-identification surveys in Ecuador. J. Cetacean Res. Manage. 3, 301–307. doi: 10.47536/jcrm.vi.303
Félix F., Haase B. (2001). The humpback whale off the coast of Ecuador, population parameters and behavior. Rev. Biol. Mar. Oceanogr. 36, 61–74. doi: 10.4067/S0718-19572001000100006
Félix F., Muñoz M., Falconí J., Botero N., Haase B. (2020b). Entanglement of humpback whales in artisanal fishing gear in Ecuador. J. Cetacean Res. Manage. 3, 283–290. doi: 10.47536/jcrm.vi.308
Félix F., Palacios D. M., Salazar S. K., Caballero S., Haase B., Falconí J. (2011b). The 2005 Galápagos humpback whale expedition: a first attempt to assess and characterise the population in the Archipelago. J. Cetacean Res. Manage. 3, 291–299. doi: 10.47536/jcrm.vi.304
Findlay K. P. (2000). A review of humpback whale catches by modern whaling operations in the Southern Hemisphere. Mem. Queensl. Mus. 47, 411–420.
Findlay K. P., Best P. B. (1995). Summer incidence of humpback whales on the west coast of South Africa. S. Afr. J. Mar. Sci. 15, 279–282. doi: 10.2989/02577619509504851
Findlay K. P., Best P. B. (1996). Estimates of the numbers of humpback whales observed migrating past Cape Vidal, South Africa 1988–1991. Mar. Mammal Sci. 12, 354–370. doi: 10.1111/j.1748-7692.1996.tb00589.x
Findlay K., Best P. (2006). The migration of humpback whales past Cape Vidal, South Africa, and a preliminary estimate of the population increase rate. IWC Paper SC/A06/HW16.
Findlay K. P., Best P. B., Meÿer M. A. (2011a). Migrations of humpback whales past Cape Vidal, South Africa, and an estimate of the population increase rate, (1988–2002). Afr. J. Mar. Sci. 33, 375–392. doi: 10.2989/1814232X.2011.637345
Findlay K. P., Best P. B., Peddemors V. M., Gove D. (1994). The distribution and abundance of humpback whales on their southern and central Mozambique winter grounds. Rep. Int. Whal. Commn. 44, 311–320.
Findlay K., Meyer M., Elwen S., Kotze D., Johnson R. M., Truter P., et al. (2004). Distribution and abundance of humpback whales, Megaptera novaeangliae, off the coast of Mozambique. IWC Paper SC/56/SH12.
Findlay K., Meyer M., Elwen S., Kotze D., Johnson R., Truter P., et al. (2011b). Distribution and abundance of humpback whales, Megaptera novaeangliae, off the coast of Mozambique. J. Cetacean Res. Manage. 3, 163–174.
Findlay K. P., Seakamela S. M., Meyer M. A., Kirkman S. P., Barendse J., Cade D. E., et al. (2017). Humpback whale “super-groups”–A novel low-latitude feeding behaviour of Southern Hemisphere humpback whales (Megaptera novaeangliae) in the Benguela Upwelling System. PloS One. 12, e0172002. doi: 10.1371/journal.pone.0172002
Fleming A., Jackson J. (2011). Global review of humpback whales (Megaptera novaeangliae). (La Jolla, California: NOAA Technical Memorandum NMFS-SWFSC-474, Southwest Fisheries Science Center).
Flórez-González L. (1991). Humpback whales, Megaptera novaeangliae, in the Gorgona Island, Colombian Pacific breeding waters: population and pod characteristics. Mem. Queensl. Mus. 30, 291–295.
Forestell P. H., Kaufman G. D., Chaloupka M. (2011). Long term trends in abundance of humpback whales in Hervey Bay, Australia. J. Cetacean Res. Manage. 3, 237–241. doi: 10.47536/jcrm.vi.321
Fossette S., Heide-Jørgensen M. P., Jensen M. V., Kiszka J., Bérubé M., Bertrand N., et al. (2014). Humpback whale (Megaptera novaeangliae) post breeding dispersal and southward migration in the western Indian Ocean. J. Exp. Mar. Biol. Ecol. 450, 6–14. doi: 10.1016/j.jembe.2013.10.014
Franklin W., Franklin T., Brooks L., Gibbs N., Childerhouse S., Smith F., et al. (2012). Antarctic waters (Area V) near the Balleny Islands are a summer feeding area for some eastern Australian breeding stock E(i) humpback whales (Megaptera novaeangliae). J. Cetacean Res. Manage. 12, 321–327. doi: 10.47536/jcrm.v12i3.562
Franklin T., Franklin W., Brooks L., Harrison P. (2018). Site-specific female-biased sex ratio of humpback whales (Megaptera novaeangliae) during a stopover early in the southern migration. Can. J. Zool. 96, 533–544. doi: 10.1139/cjz-2017-0086
Franklin W., Franklin T., Gibbs N., Childerhouse S., Garrigue C., Constantine R., et al. (2014). Photo-identification confirms that humpback whales (Megaptera novaeangliae) from eastern Australia migrate past New Zealand but indicates low levels of interchange with breeding grounds of Oceania. J. Cetacean Res. Manage. 14, 133–140. doi: 10.47536/jcrm.v14i1.530
Freitas A. C., Kinas P. G., Martins C. C. A., Engel M. H. (2004). Abundance of humpback whales on the Abrolhos Bank wintering ground, Brazil. J. Cetacean Res. Manage. 6, 225–230. doi: 10.47536/jcrm.v6i3.764
Friedlaender A. S., Halpin P. N., Qian S. S., Lawson G. L., Wiebe P. H., Thiele D., et al. (2006a). Whale distribution in relation to prey abundance and oceanographic processes in shelf water of the western Antarctic Peninsula. Mar. Ecol. Prog. Ser. 317, 297–310. doi: 10.3354/meps317297
Friedlaender A., Lawson G. L., Halpin P. N. (2006b). Evidence of resource partioning and niche separation between humpbacks and minke whales in Antarctica: implications for interspecific competition. IWC Paper SC/58/E32.
García-Cegarra A. M., Castro C., Van Waerebeek K. (2021). Feeding of humpback whales in low latitudes of the Southeast Pacific Ocean. Neotrop. Biodivers. 7, 421–430. doi: 10.20944/preprints202012.0446.v1
Garrigue C., Clapham P. J., Geyer Y., Kennedy A. S., Zerbini A. N. (2015). Satellite tracking reveals novel migratory patterns and the importance of seamounts for endangered South Pacific humpback whales. R. Soc Open Sci. 2, 150489. doi: 10.1098/rsos.150489
Garrigue C., Franklin T., Constantine R., Russell K., Burns D., Poole M., et al. (2011). First assessment of interchange of humpback whales between Oceania and the east coast of Australia. J. Cetacean Res. Manage. 3, 269–274.
Gibbons J., Capella J. J., Valladares C. (2003). Rediscovery of a humpback whale, Megaptera novaeangliae, feeding ground in the Straits of Magellan, Chile. J. Cetacean Res. Manage. 5, 203–208. doi: 10.47536/jcrm.v5i2.818
Gibbs N., Childerhouse S. (2000). Humpback whales around New Zealand. Vol. 287 (Wellington, New Zealand: Department of Conservation). Conservation Advisory Science Notes32 pp.
Gibbs N. J., Dunlop R. A., Gibbs E. J., Heberley J. A., Olavarría C. (2018). The potential beginning of a postwhaling recovery in New Zealand humpback whales (Megaptera novaeangliae). Mar. Mammal Sci. 34, 499–513. doi: 10.1111/mms.12468
Gill P. C., Evans K. J., Wapstra H. (1998). Feeding by humpback whales in Tasmanian waters. Records Queen Victoria Museum. 107, 1–5.
Groom C. J., Coughran D. K. (2012). Entanglements of baleen whales off the coast of Western Australia between 1982 and 2010: patterns of occurrence, outcomes and management responses. Pac. Conserv. Biol. 18, 203. doi: 10.1071/PC130203
Guidino C., Llapapasca M. A., Silva S., Alcorta B., Pacheco A. S. (2014). Patterns of spatial and temporal distribution of humpback whales at the southern limit of the Southeast Pacific breeding area. PloS One. 9, e112627. doi: 10.1371/journal.pone.0112627
Guzman H. M., Condit R., Pérez-Ortega B., Capella J. J., Stevick P. T. (2015). Population size and migratory connectivity of humpback whales wintering in Las Perlas Archipelago, Panama. Mar. Mammal Sci. 31, 90–105. doi: 10.1111/mms.12136
Haro D., Riccialdelli L., Acevedo J., Aguayo-Lobo A., Montiel A. (2016). Trophic ecology of humpback whales (Megaptera novaeangliae) in the Magellan Strait as indicated by carbon and nitrogen stable isotopes. Aquat. Mamm. 42, 233. doi: 10.1578/AM.42.2.2016.233
Haro D., Sabat P., Acevedo J., Capella J., Cáceres B., Aguayo-Lobo A., et al. (2021). Ontogenetic and seasonal analysis of the diet and isotopic niche of humpback whales in the Magellan Strait, Chile. Mar. Ecol. Prog. Ser. 669, 213–226. doi: 10.3354/meps13733
Hauser N., Zerbini A. N., Geyer Y., Heide-Jørgensen M. P., Clapham P. (2010). Movements of satellite-monitored humpback whales, Megaptera novaeangliae, from the Cook Islands. Mar. Mamm. Sci. 26 (3), 679–685.
Hedley S. L., Bannister J. L., Dunlop R. A. (2011). Abundance estimates of Southern Hemisphere Breeding Stock ‘D’ humpback whales from aerial and land-based surveys off Shark Bay, Western Australia 2008. J. Cetacean Res. Manage. 3, 209–221. doi: 10.47536/jcrm.vi3.326
Hedley S., Reilly S., Borberg J., Holland R., Hewitt R., Watkins J., et al. (2001). Modelling whale distribution: a preliminary analysis of data collected on the CCAMLR-IWC krill synoptic survey 2000. IWC Paper SC/53/E9.
Herr H., Viquerat S., Siegel V., Kock K. H., Dorschel B., Huneke W. G., et al. (2016). Horizontal niche partitioning of humpback and fin whales around the West Antarctic Peninsula: evidence from a concurrent whale and krill survey. Polar Biol. 39, 799–818. doi: 10.1007/s00300-016-1927-9
Horswill C., Jackson J. (2012). Humpback whales wintering at Pitcairn Island, South Pacific. Mar. Biodivers. Rec. 5, e90. doi: 10.1017/S1755267212000693
Horton T. W., Zerbini A. N., Andriolo A., Danilewicz D., Sucunza F. (2020). Multi-decadal humpback whale migratory route fidelity despite oceanographic and geomagnetic change. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00414
Hucke-Gaete R., Haro D., Torres-Florez J. P., Montecinos Y., Viddi F., Bedriñana-Romano L., et al. (2013). A historical feeding ground for humpback whales in the eastern South Pacific revisited: The case of northern Patagonia, Chile. Aquat. Conserv.: Mar. Freshw. Ecosyst. 23, 858–867. doi: 10.1002/aqc.2343
Hucke-Gaete R., Torrez-Florez J. P., Viddi F. A., Cuellar S., Montecinos Y., Ruiz J. A. (2006). A new humpback whale (Megaptera novaeangliae) feeding ground in northern Patagonia, Chile: extending summer foraging ranges. SC/58/SH10.
Irvine L. G., Thums M., Hanson C. E., McMahon C. R., Hindell M. A. (2018). Evidence for a widely expanded humpback whale calving range along the Western Australian coast. Mar. Mammal Sci. 34, 294–310. doi: 10.1111/mms.12456
Irving R. A., O’Keefe S., Warren P., Koldewey H. J., Dawson T. P. (2018). Confirmation of a new breeding ground for humpback whales (Megaptera novaeangliae) in the central South Pacific. J. Cetacean Res. Manage. 18, 119–125. doi: 10.47536/jcrm.v18i1.440
IWC (1998). Annex G. Report of the Sub-Committee on Comprehensive Assessment of Southern Hemisphere humpback whales. Rep. Int. Whal. Comm. 48, 170–182.
IWC (2004). Annex H. Report of the sub-committee on other southern hemisphere whale stocks. J. Cetacean Res. Manage., Supplement 6, 246–271.
IWC (2006). Annual Report of the International Whaling Commission 2005. Covering the 2004-2005 financial year and the 57th Annual Meeting held in Ulsan in 2005. (Cambridge: International Whaling Commission), 182. Available at: https://archive.iwc.int/?r=60
IWC (2007). Annual Report of the International Whaling Commission 2006. Covering the 2005-2006 financial year and the 58th Annual Meeting held in St Kitts and Nevis 2006. (Cambridge: International Whaling Commission), 203. Available at: https://archive.iwc.int/?r=61
IWC (2008). Annual Report of the International Whaling Commission 2007. Covering the 2006-2007 financial year and the 59th Annual Meeting held in Anchorage, Alaska in 2007. (Cambridge: International Whaling Commission), 175. Available at: https://archive.iwc.int/?r=62
IWC (2011). Report of the Scientific Committee. Annex H. Report of the Sub-Committee on Other Southern Hemisphere Whale Stocks. J. Cetacean Res. Manage. Supplement 12, 203–326.
IWC (2016b). Report of the Scientific Committee, Annex H. Report of the Sub-Committee on Other Other Southern Hemisphere Whale Stoks. J. Cetacean Res. Manag. Supplement 17, 250–282.
Jackson J. A., Anderson M., Steel D. S., Brooks L., Braverstock P., Burns D., et al. (2012). Multistate measurements of genotype interchange between East Australia and Oceania (IWC breeding sub-stocks E1, E2, E3 and F2) between 1999 and 2004. IWC Paper SC/64/SH22.
Jackson J. A., Ross-Gillespie A., Butterworth D., Findlay K., Holloway S., Robbins J., et al. (2015). Southern Hemisphere humpback whale Comprehensive Assessment—a synthesis and summary: 2005–2015. IWC Paper SC/66a/SH3.
Jenner K. C. S., Jenner M. N. (1994). A preliminary population estimate of the Group IV breeding stock of humpback whales off Western Australia. Rep. Int. Whal. Commn. 44, 303–307.
Jenner K. C., Jenner M. M., McCabe K. A. (2001). Geographical and temporal movements of humpback whales in Western Australian waters. APPEA J. 41, 749–765. doi: 10.1071/AJ00044
Jenner C., Jenner M., Salgado-Kent C., Bouchet P., Dunlop R., Burton C. (2019). A comparison of southern hemisphere breeding stock ‘D’ humpback whale population estimates from two key locations along the Western Australian coast. (Centre for Whale Research report submitted to the Australian Marine Mammal Centre). 24 pp. doi: 10.31230/osf.io/5skqb
Kasamatsu F., Joyce G., Ensor P., Mermoz J. (1996). Current occurrence of baleen whales in Antarctic waters. Rep. Int. Whal. Commn. 46, 293–304.
Kaufman G., Coughran D., Allen J. M., Burns D., Burton C., Castro C., et al. (2011). Photographic evidence of interchange between East Australia (BS E-1) and West Australia (BS–D) humpback whale breeding populations. IWC Paper SC/63/SH11.
Kawamura A. (1975). A consideration on an available source of energy and its cost for locomotion in fin whales with special reference to the seasonal migrations. Sci. Rep. Whales Res. Instit. 27, 61–79.
Kelly R., Fleming A., Pecl G. T., von Gönner J., Bonn A. (2020). Citizen science and marine conservation: a global review. Philos. Trans. R. Soc Lond. B. 375 (1814), 20190461. doi: 10.1098/rstb.2019.0461
Kershaw F., Carvalho I., Loo J., Pomilla C., Best P. B., Findlay K. P., et al. (2017). Multiple processes drive genetic structure of humpback whale (Megaptera novaeangliae) populations across spatial scales. Mol. Ecol. 26, 977–994. doi: 10.1111/mec.13943
Kinas P. G., Bethlem C. B. P. (1998). Empirical Bayes abundance estimation of a closed population using mark-recapture data, with application to humpback whales, Megaptera novaeangliae, in the Abrolhos Bank. Rep. Int. Whal. Commn. 48, 447–452.
Laws R. M. (1977). Seals and whales of the southern ocean. Philos. Trans. R. Soc Lond. B Biol. Sci. 279, 81–96. doi: 10.1098/rstb.1977.0073
Leaper R. E., Bannister J. L., Branch T. A., Clapham P., Donovan G., Reilly S., et al. (2008). A review of abundance, trends and foraging parameters of baleen whales in the Southern Hemisphere. IWC Paper SC/60/EM3.
Lodi L. (1994). Ocorrências de baleias-jubarte, Megaptera novaeangliae, no Arquipélago de Fernando de Noronha, incluindo um resumo de registros de capturas no Nordeste do Brasil. Biotemas. 7 (1,2), 116–123.
Lodi L., Tardin R. (2018). Citizen science contributes to the understanding of the occurrence and distribution of cetaceans in southeastern Brazil–a case study. Ocean Coast. Manage. 158, 45–55. doi: 10.1016/j.ocecoaman.2018.03.029
Loeb V. J., Santora J. A. (2015). Climate variability and spatiotemporal dynamics of five Southern Ocean krill species. Prog. Oceanogr. 134, 93–122. doi: 10.1016/j.pocean.2015.01.002
Marcondes M. C. C., Cheeseman T., Jackson J. A., Friedlaender A. S., Pallin L., Olio M., et al. (2021). The Southern Ocean Exchange: porous boundaries between humpback whale breeding populations in southern polar waters. Sci. Rep. 11, 1–12. doi: 10.1038/s41598-021-02612-5
Martins C. C. A., Morete M. E., Coitinho M. H. E., Freitas A. C., Secchi E. R., Kinas P. G. (2001). Aspects of habitat use patterns of humpback whales in the Abrolhos Bank, Brazil, breeding ground. Mem. Queensl. Mus. 47.
McCulloch S., Meynecke J. O., Franklin T., Franklin W., Chauvenet A. L. M. (2021). Humpback whale (Megaptera novaeangliae) behaviour determines habitat use in two Australian bays. Mar. Freshw. Res. 72 (9), 1251–1267. doi: 10.1071/MF21065
Meynecke J. O., De Bie J., Barraqueta J. L. M., Seyboth E., Dey S. P., Lee S. B., et al. (2021). The role of environmental drivers in humpback whale distribution, movement and behavior: A review. Front. Mar. Sci. 8, 720774. doi: 10.3389/fmars.2021.720774
Meynecke J. O., Seyboth E., De Bie J., Menzel Barraqueta J. L., Chama A., Prakash Dey S., et al. (2020). Responses of humpback whales to a changing climate in the Southern Hemisphere: Priorities for research efforts. Mar. Ecol. 41, e12616. doi: 10.1111/maec.12616
Miyashita T., Kato H., Kasuya T. (1995). Worldwide map of cetacean distribution based on Japanese sighting data (Volume 1). (Shizuoka, Japan: National Research Institute of Far Seas Fisheries). 140 pp.
Modest M., Irvine L., Andrews-Goff V., Gough W., Johnston D., Nowacek D., et al. (2021). First description of migratory behavior of humpback whales from an Antarctic feeding ground to a tropical calving ground. Anim. Biotelemetry. 9, 1–16. doi: 10.1186/s40317-021-00266-8
Montanari S., Kershaw F., Rosenbaum H. C. (2020). Feeding ecology and population delineation of south-eastern Atlantic and south-western Indian Ocean humpback whales using stable isotope analysis. Aquat. Conserv.: Mar. Freshw. Ecosyst. 30, 486–496. doi: 10.1002/aqc.3266
Morete M. E., Pace R. M., Martins C. C. A., Freitas A. C., Engel M. H. (2003). Indexing seasonal abundance of 590 humpback whales around Abrolhos Archipelago, Bahia, Brazil. Lat. Am. J. Aquat. Mamm. 2, 21–28. doi: 10.5597/lajam00027
Morete M. E., Marques M. L., de Souza R. C., Tristão I. A., Motta M. C., Martins C. C., et al. (2022). Is the reproductive area of the humpback whale (Megaptera novaeangliae) in Brazilian waters increasing? Evidence of breeding and calving activities around Ilhabela, São Paulo, Brazil. Lat. Am. J. Aquat. Mamm. 17 (1), 63–67.
Murphy E. J., Thorpe S. E., Tarling G. A., Watkins J. L., Fielding S., Underwood P. (2017). Restricted regions of enhanced growth of Antarctic krill in the circumpolar Southern Ocean. Sci. Rep. 7 (1), 6963. doi: 10.1038/s41598-017-07205-9
Noad M. J., Cato D. H., Bryden M. M., Jenner M. N., Jenner K. C. S. (2000). Cultural revolution in whale songs. Nature. 408 (6812), 537–537. doi: 10.1038/35046199
Noad M. J., Dunlop R. A., Paton D., Cato D. H. (2008). An update of the east Australian humpback whale population (E1) rate of increase. IWC Paper SC/60/SH31.
Noad M. J., Dunlop R. A., Paton D., Cato D. H. (2011). Absolute and relative abundance estimates of Australian east coast humpback whales (Megaptera novaeangliae). J. Cetacean Res. Manage. 3, 243–252. doi: 10.47536/jcrm.vi.318
Noad M. J., Kniest E., Dunlop R. A. (2019). Boom to bust? Implications for the continued rapid growth of the eastern Australian humpback whale population despite recovery. Popul. Ecol. 61, 198–209. doi: 10.1002/1438-390x.1014
O’Connor S., Campbell R., Cortez H., Knowles T. (2009). Whale Watching Worldwide: tourism numbers, expenditures and expanding economic benefits, a special report from the International Fund for Animal Welfare. (Yarmouth MA, USA: Economists at Large).
Olavarría C., Baker C. S., Garrigue C., Poole M., Hauser N., Caballero S., et al. (2007). Population structure of South Pacific humpback whales and the origin of the eastern Polynesian breeding grounds. Mar. Ecol. Prog. Ser. 330, 257–268. doi: 10.3354/meps330257
Ott P. H., Milmann L., Santos M. D. O., Rogers E. M., Rodrigues D. D. P., Siciliano S. (2016). Humpback whale breeding stock “A”: increasing threats to a recently down-listed species off Brazilian fauna. IWC Paper SC/66b/SH/04.
Owen K., Kavanagh A. S., Warren J. D., Noad M. J., Donnelly D., Goldizen A. W., et al. (2017). Potential energy gain by whales outside of the Antarctic: prey preferences and consumption rates of migrating humpback whales (Megaptera novaeangliae). Polar Biol. 40, 277–289. doi: 10.1007/s00300-016-1951-9
Owen K., Warren J. D., Noad M. J., Donnelly D., Goldizen A. W., Dunlop R. A. (2015). Effect of prey type on the fine-scale feeding behaviour of migrating east Australian humpback whales. Mar. Ecol. Prog. Ser. 541, 231–244. doi: 10.3354/meps11551
Pacheco A. S., Silva S., Alcorta B. (2009). Winter distribution and group composition of humpback whales (Megaptera novaeangliae) off northern Peru. Lat. Am. J. Aquat. Mamm. 7, 33–38. doi: 10.5597/lajam00131
Pallin L. J., Baker C. S., Steel D., Kellar N. M., Robbins J., Johnston D. W., et al. (2018). High pregnancy rates in humpback whales (Megaptera novaeangliae) around the Western Antarctic Peninsula, evidence of a rapidly growing population. R. Soc Open Sci. 5, 180017. doi: 10.1098/rsos.180017
Papastavrou V., Van Waerebeek K. (1997). A note on the occurrence of humpback whales (Megaptera novaeangliae) in tropical and subtropical areas: the upwelling link. Rep. Int. Whal. Commn. 47, 945–947.
Pastene L. A., Goto M., Taguchi M., Matsuoka K. (2019). Distribution and possible areas of spatial mixing of two stocks of humpback whales, a krill predator, in the Indo-Pacific region of the Antarctic revealed by genetic analyses. CCAMLR Paper WG-EMM-2019/67.
Pastene L. A., Kitakado T., Goto M., Kanda N. (2013). Mixing rates of humpback whales of Stocks D, E, and F in the Antarctic feeding grounds based on mitochondrial DNA analysis. IWC Paper SC/65a/SH13.
Paterson R., Paterson P. (1989). The status of the recovering stock of humpback whales Megaptera novaeangliae in East Australian waters. Biol. Conserv. 47, 33–48. doi: 10.1016/0006-3207(89)90018-9
Paterson R., Paterson P., Cato D. H. (1994). The status of humpback whales Megaptera novaeangliae in east Australia thirty years after whaling. Biol. Conserv. 70, 135–142. doi: 10.1016/0006-3207(94)90281-X
Paterson R., Paterson P., Cato D. H. (2001). Status of humpback whales (Megaptera novaeangliae) in east Australia at the end of the 20th Century. Mem. Queensl. Mus. 47, 579–586.
Paterson R., Paterson P., Cato D. H. (2004). Continued increase in east Australian humpback whales in 2001, 2002. Mem. Queensl. Mus. 49, 712.
Paton D. A., Brooks L., Burns D., Franklin T., Franklin W., Harrison P., et al. (2011). Abundance of East coast Australian humpback whales (Megaptera novaeangliae) in 2005 estimated using multi-point sampling and capture-recapture analysis. J. Cetacean Res. Manage. 3, 253–259. doi: 10.47536/jcrm.vi.317
Paton D. A., Clapham P. J. (2006). An assessment of Southern Hemisphere humpback whale population structure and migratory interchange based on Discovery mark data. IWC Paper SC/A06/HW33.
Paton D. A., Kniest R. (2011). Population growth of Australian east coast humpback whales, observed from Cape Byron 1998 to 2004. J. Cetacean Res. Manage. 3, 261–268. doi: 10.47536/jcrm.vi.316
Pavanato H. J., Mayer F. P., Wedekin L. L., Engel M. H., Kinas P. G. (2018). Prediction of humpback whale group densities along the Brazilian coast using spatial autoregressive models. Mar. Mammal Sci. 34, 734–754. doi: 10.1111/mms.12492
Pavanato H. J., Wedekin L. L., Guilherme-Silveira F. R., Engel M. H., Kinas P. G. (2017). Estimating humpback whale abundance using hierarchical distance sampling. Ecol. Modell. 358, 10–18. doi: 10.1016/j.ecolmodel.2017.05.003
Paxton C. G., Hedley S. L., Bannister J. L. (2011). Group IV humpback whales: their status from aerial and land-based surveys off Western Australi. J. Cetacean Res. Manage. 3, 223–234.
Peel D., Thiele D. (2006). An estimate of abundance of humpback whales within Antarctic Area IV from the BROKE survey data. IWC Paper SC/58/SH13.
Pinto de sa Alves L. C., Andriolo A., Zerbini A., Altmayer Pizzorno J. L., Clapham P. (2009). Record of feeding by humpback whales (Megaptera novaeangliae) in tropical waters off Brazil. Mar. Mammal Sci. 25 (2), 416–419. doi: 10.1111/j.1748-7692.2008.00249.x
Pirotta V., Owen K., Donnelly D., Brasier M. J., Harcourt R. (2021). First evidence of bubble-net feeding and the formation of ‘super-groups’ by the east Australian population of humpback whales during their southward migration. Aquat. Conserv.: Mar. Freshw. Ecosyst. 31, 2412–2419. doi: 10.1002/aqc.3621
Pirotta V., Reynolds W., Ross G., Jonsen I., Grech A., Slip D. ,. R., et al. (2020). A citizen science approach to long-term monitoring of humpback whales (Megaptera novaeangliae) off Sydney, Australia. Mar. Mamm. Sci. 36 (2), 472–485. doi: 10.1111/mms.12651
Pirotta E., Thomas L., Costa D. P., Hall A. J., Harris C. M., Harwood J., et al. (2022). Understanding the combined effects of multiple stressors: A new perspective on a longstanding challenge. Sci. Total Environ. 821, 153322. doi: 10.1016/j.scitotenv.2022.153322
Pomilla C., Best P. B., Findlay K. P., Collins T., Engel. M., Minton G., et al. (2006). Population structure and sex-biased gene flow in humpback whales from Wintering Regions A, B, C, and X based on nuclear microsatellite variation. SC/A06/HW38 presented to the IWC Scientific Committee Workshop on the Comprehensive Assessment of Southern Hemisphere humpback whales.
Poole M. M. (2006). An update on the occurrence of humpback whales in French Polynesia. IWC Paper SC/A06/HW60.
Punt A. E., Donovan G. (2007). Developing management procedures that are robust to uncertainty: lessons from the International Whaling Commission. ICES J. Mar. Sci. 64, 603–612. doi: 10.1093/icesjms/fsm035
Rasmussen K., Palacios D. M., Calambokidis J., Saborío M. T., Dalla Rosa L., Secchi E. R., et al. (2007). Southern Hemisphere humpback whales wintering off Central America: insights from water temperature into the longest mamMalian migration. Biol. Lett. 3, 302–305. doi: 10.1098/rsbl.2007.0067
Ratnarajah L., Bowie A. R., Lannuzel D., Meiners K. M., Nicol S. (2014). The biogeochemical role of baleen whales and krill in Southern Ocean nutrient cycling. PloS One. 9, e114067. doi: 10.1371/journal.pone.0114067
Ratnarajah L., Melbourne-Thomas J., Marzloff M. P., Lannuzel D., Meiners K. M., Chever F., et al. (2016). A preliminary model of iron fertilisation by baleen whales and Antarctic krill in the Southern Ocean: sensitivity of primary productivity estimates to parameter uncertainty. Ecol. Modell. 320, 203–212. doi: 10.1016/j.ecolmodel.2015.10.007
Rayner G. W. (1940). Whale marking: progress and results to December 1939. Discovery Rep. 19, 245–284.
Reeves R. R., Rolland R., Clapham P. (2001). Causes of reproductive failure in North Atlantic right whales: new avenues of research. Northeast Fisheries Science Centre Reference Document 01-16. (166 Water St., Woods Role, MA 02543-1026: National Marine Fisheries Service).
Reisinger R. R., Friedlaender A. S., Zerbini A. N., Palacios D. M., Andrews-Goff V., Dalla Rosa L., et al. (2021). Combining regional habitat selection models for large-scale prediction: Circumpolar habitat selection of Southern Ocean humpback whales. Remote Sens. 13, 2074. doi: 10.3390/rs13112074
Remili A., Gallego P., Pinzone M., Castro C., Jauniaux T., Garigliany M. M., et al. (2020). Humpback whales (Megaptera novaeangliae) breeding off Mozambique and Ecuador show geographic variation of persistent organic pollutants and isotopic niches. Environ. pollut. 267, 115575. doi: 10.1016/j.envpol.2020.115575
Riekkola L., Zerbini A. N., Andrews O., Andrews-Goff V., Baker C. S., Chandler D., et al. (2018). Application of a multi-disciplinary approach to reveal population structure and Southern Ocean feeding grounds of humpback whales. Ecol. Indic. 89, 455–465. doi: 10.1016/j.ecolind.2018.02.030
Ristau N. G., Martins C. C., Luvizotto-Santos R., Balensiefer D., Sousa G., Marmontel M., et al. (2020). Sharing the space: Review of humpback whale occurrence in the Amazonian Equatorial Coast. Glob. Ecol. Conserv. 22, e00854. doi: 10.1016/j.gecco.2019.e00854
Robbins J., Dalla Rosa L., Allen J. M., Mattila D. K., Secchi E. R., Friedlaender A. S., et al. (2011). Return movement of a humpback whale between the Antarctic Peninsula and American Samoa: a seasonal migration record. Endang. Species Res. 13, 117–121. doi: 10.3354/esr00328
Rodrigues A. S. L., Pilgrim J. D., Lamoreux J. F., Hoffmann M., Brooks T. M. (2006). The value of the IUCN Red List for conservation. Trends Ecol. Evol. 21, 71–76. doi: 10.1016/j.tree.2005.10.010
Rosenbaum H. C., Kershaw F., Mendez M., Pomilla C., Leslie M. S., Findlay K. P., et al. (2017). First circumglobal assessment of Southern Hemisphere humpback whale mitochondrial genetic variation and implications for management. Endang. Species Res. 32, 551–567. doi: 10.3354/esr00822
Rosenbaum H. C., Maxwell S. M., Kershaw F., Mate B. (2014). Long‐range movement of humpback whales and their overlap with anthropogenic activity in the South Atlantic Ocean. Conserv. Biol. 28 (2), 604–615.
Rosenbaum H. C., Pomilla C., Mendez M., Leslie M. S., Best P. B., Findlay K. P., et al. (2009). Population structure of humpback whales from their breeding grounds in the South Atlantic and Indian Oceans. PloS One. 4, e7318. doi: 10.1371/journal.pone.0007318
Rosenbaum H. C., Strindberg S., Ersts J. (2004). Initial estimates of abundance and distribution of humpback whales on their wintering grounds of Gabon (southeastern Atlantic Ocean, Area B) based on aerial surveys. IWC Paper SC/56/4.
Rossi-Santos M. R. (2015). Oil industry and noise pollution in the humpback whale (Megaptera novaeangliae) soundscape ecology of the southwestern Atlantic breeding ground. J. Coast. Res. 31, 184–195. doi: 10.13140/2.1.2620.2249
Rossi-Santos M. R., Neto E. S., Baracho C. G., Cipolotti S. R., Marcovaldi E., Engel M. H. (2008). Occurrence and distribution of humpback whales (Megaptera novaeangliae) on the north coast of the State of Bahia, Brazil 2000–2006. ICES J. Mar. Sci. 65, 667–673. doi: 10.1093/icesjms/fsn034
Ross-Marsh E. C., Elwen S. H., Fearey J., Thompson K. F., Maack T., Gridley T. (2022). Detection of humpback whale (Megaptera novaeangliae) non-song vocalizations around the Vema Seamount, southeast Atlantic Ocean. JASA Express Lett. 2, 041201. doi: 10.1121/10.0010072
Salgado Kent C., Jenner C., Jenner M., Bouchet P., Rexstad E. (2012). Southern Hemisphere breeding stock ‘D’ humpback whale population estimates from North West Cape, Western Australia. J. Cetacean Res. Manage. 12, 29–38. doi: 10.31230/osf.io/m94xh
Santora J. A., Mantua N. J., Schroeder I. D., Field J. C., Hazen E. L., Bograd S. J., et al. (2020). Habitat compression and ecosystem shifts as potential links between marine heatwave and record whale entanglements. Nat. Commn. 11, 536. doi: 10.1038/s41467-019-14215-w
Santora J. A., Reiss C. S., Loeb V. J., Veit R. R. (2010). Spatial association between hotspots of baleen whales and demographic patterns of Antarctic krill Euphausia superba suggests size-dependent predation. Mar. Ecol. Prog. Ser. 405, 255–269.
Schaffar A., Garrigue C. (2007). Review of commercial humpback whale watching activities in the South Pacific. IWC Paper IWC/59/8.
Secchi E., Dalla-Rosa L., Kinas P. G., Nicolette R. F., Rufino A. M., Azevedo A. F. (2011). Encounter rates and abundance of humpback whales (Megaptera novaeangliae) in Gerlache and Bransfield Straits, Antarctic Peninsula. J. Cetacean Res. Manage. 3, 107–111. doi: 10.47536/jcrm.vi.312
Seyboth E., Félix F., Lea M. A., Dalla Rosa L., Watters G. M., Reid K., et al. (2021). Influence of krill (Euphausia superba) availability on humpback whale (Megaptera novaeangliae) reproductive rate. Mar. Mammal Sci. 37, 1498–1506. doi: 10.1111/mms.12805
Seyboth E., Groch K. R., Dalla Rosa L., Reid K., Flores P. A., Secchi E. R. (2016). Southern right whale (Eubalaena australis) reproductive success is influenced by krill (Euphausia superba) density and climate. Sci. Rep. 6, 28205. doi: 10.1038/srep28205
Siciliano S., Cardoso J., Francisco A., Moreira S. C. (2019). A stop for a snack: apparent humpback whale (Megaptera novaeangliae) feeding behavior and association with gillnets during migration off south-eastern Brazil. Bol. Lab. Hidrobiol. 29, 41–49. doi: 10.18764/1981-6421e2019.6
Siciliano S., Pizzorno J. L. A., Barata P. C. R. (1999). Distribution and possible migratory routes of humpback whales Megaptera novaeangliae in the western South Atlantic. IWC Paper SC/51/CAWS4.
Simmonds M. P., Isaac S. J. (2007). The impacts of climate change on marine mammals: early signs of significant problems. Oryx. 41, 19–26. doi: 10.1017/S0030605307001524
Smith J. N., Kelly N., Childerhouse S., Redfern J. V., Moore T. J., Peel D. (2020). Quantifying ship strike risk to breeding whales in a multiple-use marine park: the great barrier reef. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00067
SPWRC, Baker C. S., Garrigue C., Constantine R., Madon B., Poole M., et al. (2006). Abundance of humpback whales in Oceania (South Pacific), 1999 to 2004. IWC Paper SC/A06/HW51.
Stamation K. A., Croft D. B., Shaughnessy P. D., Waples K. A. (2007). Observations of humpback whales (Megaptera novaeangliae) feeding during their southward migration along the coast of southeastern New South Wales, Australia: identification of a possible supplemental feeding ground. Aquat. Mamm. 33, 165. doi: 10.1578/AM.33.2.2007.165
Steel D., Anderson M., Garrigue C., Olavarría C., Caballero S., Childerhouse S., et al. (2018). Migratory interchange of humpback whales (Megaptera novaeangliae) among breeding grounds of Oceania and connections to Antarctic feeding areas based on genotype matching. Polar Biol. 41, 653–662. doi: 10.1007/s00300-017-2226-9
Stevick P., Aguayo-Lobo A., Allen J., Ávila I. C., Capella J., Castro C., et al. (2004). Migrations of individually identified humpback whales between the Antarctic Peninsula and South America. J. Cetacean Res. Manage. 6, 109–113. doi: 10.47536/jcrm.v6i2.773
Stevick P. T., Allen J. M., Engel M. H., Félix F., Haase B., Neves M. C. (2013). Inter-oceanic movement of an adult female humpback whale between Pacific and Atlantic breeding grounds off South America. J. Cetacean Res. Manage. 13, 159–162. doi: 10.47536/jcrm.v13i2.545
Stevick P. T., Godoy L. P., McOsker M., Engel M. H., Allen J. (2006). Movement of a humpback whale from Abrolhos Bank, Brazil to South Georgia. J. Cetacean Res. Manage. 8, 297–300. doi: 10.47536/jcrm.v8i3.726
Stockin K. A., Burgess E. A. (2005). Opportunistic feeding of an adult humpback whale (Megaptera novaeangliae) migrating along the coast of Southeastern Queensland, Australia. Aquat. Mamm. 31, 120. doi: 10.1578/AM.31.1.2005.120
Stone G. S., Flórez-Gonzalez L., Katona S. (1990). Whale migration record. Nature. 346, 705–706. doi: 10.1038/346705a0
Strindberg S., Ersts P. J., Collins T., Sounguet G. P., Rosenbaum H. C. (2011). Line transect estimates of humpback whale abundance and distribution on their wintering grounds in the coastal waters of Gabon. J. Cetacean Res. Manage. 3, 153–160. doi: 10.47536/jcrm.vi3.324
Sylvester Z. T., Long M. C., Brooks C. M. (2021). Detecting climate signals in Southern Ocean krill growth habitat. Front. Mar. Sci. 8, 669508. doi: 10.3389/fmars.2021.669508
Tonachella N., Nastasi A., Kaufman G., Maldini D., Rankin R. W. (2012). Predicting trends in humpback whale (Megaptera novaeangliae) abundance using citizen science. Pac. Conserv. Biol. 18 (4), 297–309. doi: 10.1071/PC120297
Tønnessen J. N., Johnsen A. O. (1982). The history of modern whaling. (Berkeley: University of California Press).
Torre-Williams L., Martinez E., Meynecke J. O., Reinke J., Stockin K. A. (2019). Presence of newborn humpback whale (Megaptera novaeangliae) calves in Gold Coast Bay, Australia. Mar. Freshw. Behav. Physiol. 52 (5), 199–216. doi: 10.1080/10236244.2019.1671769
Townsend C. H. (1935). The distribution of certain whales as shown by logbook records of American whale ships. Zoologica. 19, 1–50.
Tulloch V. J., Plagányi É.E., Brown C., Richardson A. J., Matear R. (2019). Future recovery of baleen whales is imperiled by climate change. Glob. Change Biol. 25, 1263–1281. doi: 10.1111/gcb.14573
Van Opzeeland I., Van Parijs S., Kindermann L., Burkhardt E., Boebel O. (2013). Calling in the cold: pervasive acoustic presence of humpback whales (Megaptera novaeangliae) in Antarctic coastal waters. PloS One. 8, e73007. doi: 10.1371/journal.pone.0073007
Van Waerebeek K., Baker A. N., Félix F., Gedamke J., Iñiguez M., Sanino G. P., et al. (2007). Vessel collisions with small cetaceans worldwide and with large whales in the Southern Hemisphere, an initial assessment. Lat. Am. J. Aquat. Mamm. 6 (1), 43–69. doi: 10.5597/lajam00109
Van Waerebeek K., Djiba A., Krakstad J. O., Bilal A. S. O., Bamy I. L., Almeida A., et al. (2013). New evidence for a South Atlantic stock of humpback whales wintering on the Northwest African continental shelf. Afr. Zool. 48, 177–186. doi: 10.1080/15627020.2013.11407581
Van Waerebeek K., Tchibozo S., Montcho J., Nobime G., Sohou Z., Sohouhoue P., et al. (2001). The Bight of Benin, a North Atlantic breeding ground of a Southern Hemisphere humpback whale population, likely related to Gabon and Angola substocks. IWC Paper SC/53/IA21.
van Weelden C., Towers J. R., Bosker T. (2021). Impacts of climate change on cetacean distribution, habitat and migration. Clim. Change Ecol. 1, 100009. doi: 10.1016/j.ecochg.2021.100009
Veytia D., Corney S., Meiners K. M., Kawaguchi S., Murphy E. J., Bestley S. (2020). Circumpolar projections of Antarctic krill growth potential. Nat. Clim. Change. 10 (6), 568–575. doi: 10.1038/s41558-020-0758-4
Vighi M., Borrell A., Jackson J. A., Carroll E. L., Pennino M. G., Aguilar A. (2020). The missing whales: relevance of “struck and lost” rates for the impact assessment of historical whaling in the southwestern Atlantic Ocean. ICES J. Mar. Sci. 78 (1), 14–24. doi: 10.1093/icesjms/fsaa205
Ward E., Zerbini A. N., Kinas P. G., Engel M. H., Andriolo A. (2011). Estimates of population growth rates of humpback whales (Megaptera novaeangliae) in the wintering grounds off the coast of Brazil (Breeding Stock A). J. Cetacean Res. Manage. Special Issue. 3, 145–149. doi: 10.47536/jcrm.vi3.323
Warren V. E., Constantine R., Noad M., Garrigue C., Garland E. C. (2020). Migratory insights from singing humpback whales recorded around central New Zealand. R. Soc Open Sci. 7, 201084. doi: 10.1098/rsos.201084
Warwick-Evans V., Kelly N., Dalla Rosa L., Friedlaender A., Hinke J. T., Kim J. H., et al. (2022). Using seabird and whale distribution models to estimate spatial consumption of krill to inform fishery management. Ecosphere. 13, e4083. doi: 10.1002/ecs2.4083
Wedekin L. L., Engel M. H., Andriolo A., Prado P. I., Zerbini A. N., Marcondes M. M. C., et al. (2017). Running fast in the slow lane: rapid population growth of humpback whales after exploitation. Mar. Ecol. Prog. Ser. 575, 195–206. doi: 10.3354/meps12211
Wilkinson C., Seyboth E., Olbers J., Vermeulen E., Kramer R., Findlay K. (2023). Estimating population changes in humpback whales Megaptera novaeangliae migrating past Cape Vidal, South Africa. Afr. J. Mar. Sci. 1-12. doi: 10.2989/1814232X.2023.2193591
Zerbini A. N., Adams G., Best J., Clapham P. J., Jackson J. A., Punt A. E. (2019). Assessing the recovery of an Antarctic predator from historical exploitation. R. Soc Open Sci. 6, 190368. doi: 10.1098/rsos.190368
Zerbini A. N., Andriolo A. R., Da Rocha J. M., Simões-Lopes P. C., Siciliano S., Pizzorno J. L., et al. (2004). Winter distribution and abundance of humpback whales (Megaptera novaeangliae) off Northeastern Brazil. J. Cetacean Res. Manage. 6, 101–107. doi: 10.47536/jcrm.v6i1.796
Zerbini A. N., Andriolo A., Heide-Jorgensen M. P., Moreira S. C., Pizzorno J. L., Maia Y. G., et al. (2011b). Migration and summer destinations of humpback whales (Megaptera novaeangliae) in the Western South Atlantic Ocean. J. Cetacean Res. Manage. 3, 113–118. doi: 10.47536/jcrm.vi.315
Zerbini A. N., Andriolo A., Heide-Jørgensen M. P., Pizzorno J. L., Maia Y. G., VanBlaricom G. R., et al. (2006). Satellite-monitored movements of humpback 661 whales Megaptera novaeangliae in the Southwest Atlantic Ocean. Mar. Ecol. Prog. Ser. 313, 295–304. doi: 10.3354/meps313295
Zerbini A. N., Clapham P. J., Wade P. R. (2010). Assessing plausible rates of population growth in humpback whales from life-history data. Mar. Biol. 157, 1225–1236. doi: 10.1007/s00227-010-1403-y
Keywords: abundance estimate, cetacean, climate change, population trends, Megaptera novaeangliae, population recovery
Citation: Seyboth E, Meynecke J-O, de Bie J, Roychoudhury A and Findlay K (2023) A review of post-whaling abundance, trends, changes in distribution and migration patterns, and supplementary feeding of Southern Hemisphere humpback whales. Front. Mar. Sci. 10:997491. doi: 10.3389/fmars.2023.997491
Received: 19 July 2022; Accepted: 28 August 2023;
Published: 19 October 2023.
Edited by:
Marcos R. Rossi-Santos, Federal University of the Recôncavo of Bahia, BrazilReviewed by:
Anke Kügler, Syracuse University, United StatesCopyright © 2023 Seyboth, Meynecke, de Bie, Roychoudhury and Findlay. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa Seyboth, ZWxpc2Euc2V5Ym90aEB1cC5hYy56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.