
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Mar. Sci. , 01 February 2023
Sec. Marine Pollution
Volume 10 - 2023 | https://doi.org/10.3389/fmars.2023.965059
This article is part of the Research Topic Advances in Quantification, Degradation and Ecotoxicology of Microplastics in Marine Resources View all 10 articles
Microplastics (MPs) are emerging contaminants that pose a global threat to the environment. Mangrove ecosystems, which contribute to biogeochemical cycles, are vulnerable to various anthropogenic disturbances and chemical pollutants. In this study, the abundance and the characteristics of MPs were investigated in 10 species of benthic organisms, including crabs, bivalves, and snails, from seven typical mangrove distribution areas, with a total of 15 sampling sites in Hainan, South China. The abundance of MPs in each sampling site ranged between 0.83 ± 1.32 and 12.00 ± 0.00 items/individual, with an average of 3.90 ± 3.31 items/individual, while the abundance of MPs varied between 0.17 and 2.00 items/individual for the different species. Fibers (80.13%) were the most abundant MPs, most of the MPs were brown (37.18%) or blue (26.64%), and more than 80% were small-sized plastic (<2 mm). Raman analysis showed that polypropylene (94.44%) was the most dominant type of polymer. In addition, crabs (with an average abundance of 1.10 ± 0.59 items/individual) showed a higher potential for accumulating MPs than the other species investigated in the present study. This study shows that MPs are widely distributed throughout benthic species in the mangrove wetlands of Hainan.
Microplastic (MP; particles <5 mm) pollution is one of the emerging threats to both aquatic and terrestrial ecosystems, even in polar regions, due to their long-distance migration in the environment (Wright et al., 2013; de Souza Machado et al., 2018). Their widespread presence in different environmental media, including atmospheric (Cai et al., 2017; Liu et al., 2019), terrestrial (He et al., 2018; Zhou et al., 2018; Corradini et al., 2019; Chia et al., 2021), freshwater (Wang et al., 2017; Fu and Wang, 2019; Mintenig et al., 2019; Meng et al., 2020; Yang et al., 2021), and marine (Cincinelli et al., 2017; Wang et al., 2019; Zhang et al., 2020; Gao et al., 2022; Jiang et al., 2022) ecosystems, has been increasingly reported. MPs are ingested directly and indirectly by fish, bivalves, crustaceans, and other animals (Chan et al., 2019; Teng et al., 2019; Carlin et al., 2020; Savoca et al., 2020; Sequeira et al., 2020; Pequeno et al., 2021; Yin et al., 2022). MPs may transfer from lower to higher trophic levels along the food chain and may cause potential threats to human health (Santillo et al., 2017).
Mangrove forests are saline and tidal habitats and are considered one of the most carbon-dense ecosystems on Earth (Bai et al., 2021). Mangrove wetlands provide numerous ecological services and functions, including water purification, coastal protection, and marine animal habitats (Lovelock and Duarte, 2019). Several studies have reported the distribution of MPs in global mangrove forests. Li et al. (2018) found that the abundance of MPs ranged from 15 to 12,852 items/kg in sandy beaches and mangrove wetlands. The average abundance of MPs in mangrove sediments in Singapore was 9.2 ± 5.9 particles/250 g (Mohamed Nor and Obbard, 2014) and varied from 0.6 to 8.0 items/individual in fish species collected from the mangrove wetland of Zhanjiang (Huang et al., 2020). In addition, Zhou et al. (2020) investigated the distribution of MPs along the coast of China and found that the abundance of MPs in mangrove sediments was 8.5 times higher than that in mangrove-free sediments. Nevertheless, in the Muara Angke Wildlife Reserve of Indonesia, the concentrations of MPs in sediments were higher outside than inside mangrove areas (Cordova et al., 2021). However, few studies have focused on MP pollution in benthic species, especially invertebrates, in mangrove wetlands. Hainan Island represents nearly 20% of the mangrove forest areas in China, and these areas are distributed in Dongfang, Danzhou, Lingao, Chengmai, Dongzhaigang, Wenchang, and Sanya. Among them, the Dongzhaigang National Mangrove Reserve, which was established in 1986, was designated as one of the most important wetlands in the world in 1992 (Qiu et al., 2011; Wu et al., 2013).
In this study, wild benthic species were collected from 15 sites along seven typical mangrove wetlands in Hainan Island, and their abundance, morphotype, size, color, and polymer composition were investigated. The main objectives were to quantify and characterize the MPs in benthic organisms from mangrove areas. This study provides basic data on the contamination level of MPs in benthic organisms in the mangrove wetlands of Hainan.
Seven typical mangrove distribution areas around Hainan Island, including Danzhou, Lingao, Chengmai, Dongzhaigang, Wenchang, Sanya, and Dongfang, were chosen as the sampling sites for mangrove benthos. A total of 15 sites were included: Huachong, Caiqiao, Fuli mangrove bay, Beijie, Kunshan, Wuli, He Harbor, Changwan, Nanjie, Dongye, Pai Harbor, Xiachang, Sanya River, Qingmei Harbor, and Dongfang. The layout of the stations in each area is shown in Figure 1.
A total of 10 species of mangrove benthos were collected from the Hainan mangrove area in April 2019. All the benthos were sieved from sediments collected with a Van Veen grab at 30 cm depth from a ~10 m × 10 m area in each sampling site, using a five-point sampling method according to previous studies, with slight modifications (Ryan, 2004). A total of 135 individuals were identified down to the species level (Table 1) by referring to the Atlas of molluscs in Dongzhai bay, Hainan, and the Atlas of marine animals in mangrove wetland in Beibu Gulf, Guang Xi. There were six species of crabs (n = 80), three species of bivalves (n = 49), and one species of snails (n = 6). To avoid contamination of MPs during transportation, the collected samples were packed in aluminum foil, transported to the laboratory under −4°CC, and stored at −20°CC.
The MPs were extracted as described by Munno et al. (2018), with some modifications. Briefly, the soft tissues of animals (crabs, bivalves, and snails) were collected and weighed and then placed in 500-ml glass beakers individually. Subsequently, 180 ml of 10% (m/v) KOH and 20 ml of 30% H2O2 were added for the digestion process. Each beaker was covered with aluminum foil and placed in an oven at 60°CC for at least 48 h. To ensure complete digestion, the beaker was shaken every 6 h. The digestate was then cooled and vacuum filtered through a GF/F glass microfiber filter (0.7 mm pore size, 47 mm diameter; Whatman plc, Maidstone, UK). Afterward, the filters were placed in clean Petri dishes and dried at room temperature (25°CC) until analysis.
Suspected plastic particles were observed using a stereoscopic microscope (GL6545T; Guilin, China) equipped with a high-resolution digital camera. The MPs were classified and counted according to shape (classified into fibers, granules, fragments, pellets, and films), size (classified into <1, 1–2, 2–3, 3–4, and 4–5 mm) (Cui et al., 2022), and color (Nie et al., 2019). In addition, a laser confocal microscope and a Raman spectrometer (DXR2; Thermo Fisher Scientific, Waltham, MA, USA) were used to analyze the suspected plastic particles according to the methods of Di and Wang (2018). To identify the chemical composition, a spectral database based on OMNIC software (Thermo Fisher Scientific) was used to compare the spectra of the samples, and the level of certainty was set to 60% (Woodall et al., 2014; European Commission, 2013).
All of the experimental equipment used in this study were made of non-plastic materials and were rinsed carefully with filtered distilled water several times to avoid potential contamination from other sources. All solutions, including the distilled water, KOH, and H2O2, were filtered through a 0.45 μm filter paper under vacuum before use. During all experimental processes, all containers were covered with aluminum foil, and polymer-free gloves and cotton lab coats were worn. In addition, the blank samples were corrected for potential procedural contamination.
A location map of the sampling areas was drawn using ArcGIS 10.2. All data were analyzed using Microsoft Excel and are shown as the mean ± standard deviation (SD). The abundance of MPs in benthic species at each site was expressed as items per individual (items/individual). In addition, the characteristics of the MPs were plotted using GraphPad Prism software, and SPSS 16.0 was used for statistical analysis. Differences in the abundance of MPs were determined using one-way ANOVA with Dunnett’s test, and significance was set at p < 0.05.
MP pollution in the benthos of Hainan’s mangrove wetlands was studied for the first time. A total of 135 benthic organisms, including six species of crab, three species of bivalves, and one species of snail from 15 sampling sites in seven typical mangrove wetlands of Hainan, were analyzed to determine the abundance and characteristics of MP contamination. The different sizes, shapes, colors, and chemical compositions of the MPs were examined in the benthos samples from different mangrove wetland areas.
The abundance of MPs in the benthos from different mangrove areas is shown in Table 2. The abundance of MPs ranged between 0.83 ± 1.32 and 12 ± 0.00 items/individual (average, 3.90 ± 3.31 items/individual), with the highest abundance in Changwan, Dongzhaigang (12.00 ± 0.00 items/individual), while the lowest abundance (0.83 ± 1.32 items/individual) was found in Fuli mangrove bay, Chengmai. This result was similar to the number of MPs observed in organisms collected from the mangrove region of Zhanjiang (0.6–8.0 items/individual) (Huang et al., 2021). Our results also demonstrated that there were differences in the MP abundance between each sampling site (Figure 2A). The abundance of MPs in the benthos collected from Changwan was significantly higher than that from other sites in the Hainan wetlands (p < 0.05), which was largely due to the input of plastic debris from the tourism industry and the semi-closed bay with weak hydrodynamic conditions. It has been reported that Dongzhaigang is a mangrove wetland nature reserve in China and is the biggest bay in Hainan Island (Li et al., 2020). Furthermore, the abundance of MPs in Pai Harbor, He Harbor, and Sanya River was relatively high, with values of 9.00 ± 8.08, 7.50 ± 0.58, and 6.33 ± 6.66, respectively, which may due to port transportation and the urban communities around these areas.
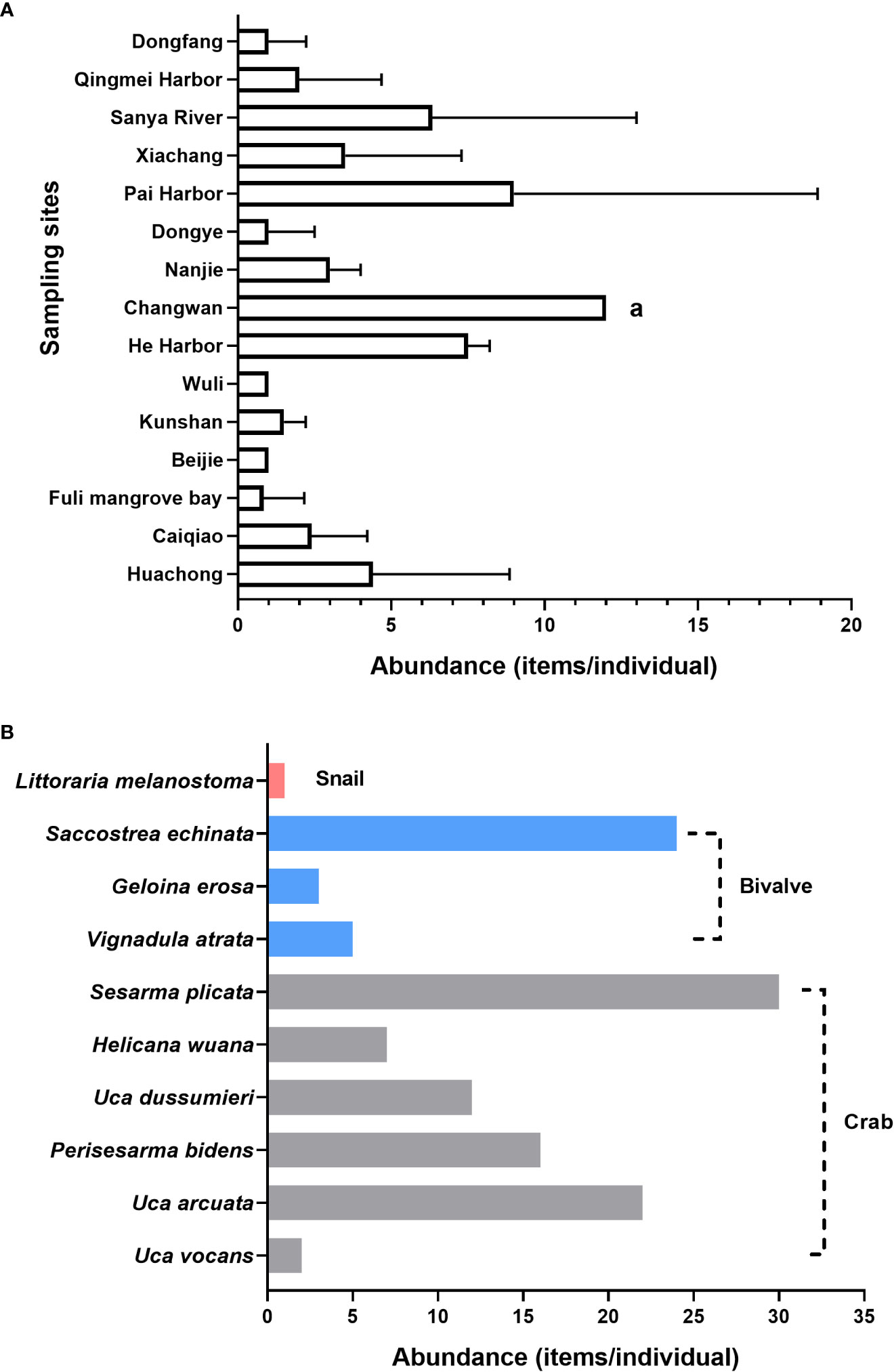
Figure 2 Microplastic abundance in the different sample sites (A) and in each benthic species (B) from the mangrove areas of Hainan. Lowercase letter "a" indicate significant differences between sampling sites.
In addition, we calculated the abundance of MPs in the different benthic species (Table 1 and Figure 2B) and found that it varied from 0.17 to 2.00 items/individual in each species, with the highest MP abundance found in crabs (including Uca vocans, Uca arcuata, Perisesarma bidens, Uca dussumieri, Helicana wuana, and Sesarma plicata), which showed an average abundance of 1.10 ± 0.59 items/individual; among them, S. plicata had the highest abundance (1.36 items/individual), followed by U. arcuata and P. bidens (1.16 items/individual for both). The average abundance of MPs in bivalves (including Vignadula atrata, Geloina erosa, and Saccostrea echinata) was 0.77 ± 0.94 items/individual, with the highest found in S. echinata (1.85 items/individual), followed by G. erosa (0.23 items/individual) and V. atrata (0.22 items/individual); the snails Littoraria melanostoma had the lowest MP abundance (average, 0.17 items/individual). We compared our results with those of previous studies that focused on the abundance of MPs in benthos from different areas (see Table 3). The MP abundance values found in our study were consistent with those detected in crabs from the English Channel and the Atlantic Ocean (Welden et al., 2018), but the concentrations of MPs in crabs and bivalves from the Arctic and sub-Arctic regions (Fang et al., 2018) were slightly lower than those in the present study. Moreover, the bivalve species from Qingdao (Ding et al., 2021), Shanghai’s biggest fishery market (Li et al., 2015), and the coastal areas of China (Teng et al., 2019) have been reported to have considerably higher MP abundance compared to this study. The average abundance of MPs in snails from the Hainan mangrove areas was lower than that in predatory snails from the Persian Gulf (Naji et al., 2018). Our results suggest that the levels of MPs in biota species from Hainan’s mangrove wetlands were low to moderate compared to those reported in previous studies.
Five different morphotypes of MPs—fibers, granules, fragments, pellets, and films—were observed in the sampled benthic organisms from these mangrove areas. The most common type of MP in all collected benthic species was fiber (80.13%) (Figure 3A), which was consistent with those detected in benthic organisms from other areas (Table 3) and in fish species from the mangrove wetland of Zhanjiang (70%) (Huang et al., 2020), as well as in mussels from 25 sites along the coastal waters of China (Qu et al., 2018). In addition, the site proportion of fibrous MPs in Sanya River, Dongye, Wuli, Beijie, Fuli mangrove bay, and Caiqiao accounted for 100% (Figure 3D), all of which are close to urban communities and fishing areas. It was speculated that the high levels of fibrous MPs may be associated with human activities such as the disposal of municipal wastewater and the fishery.
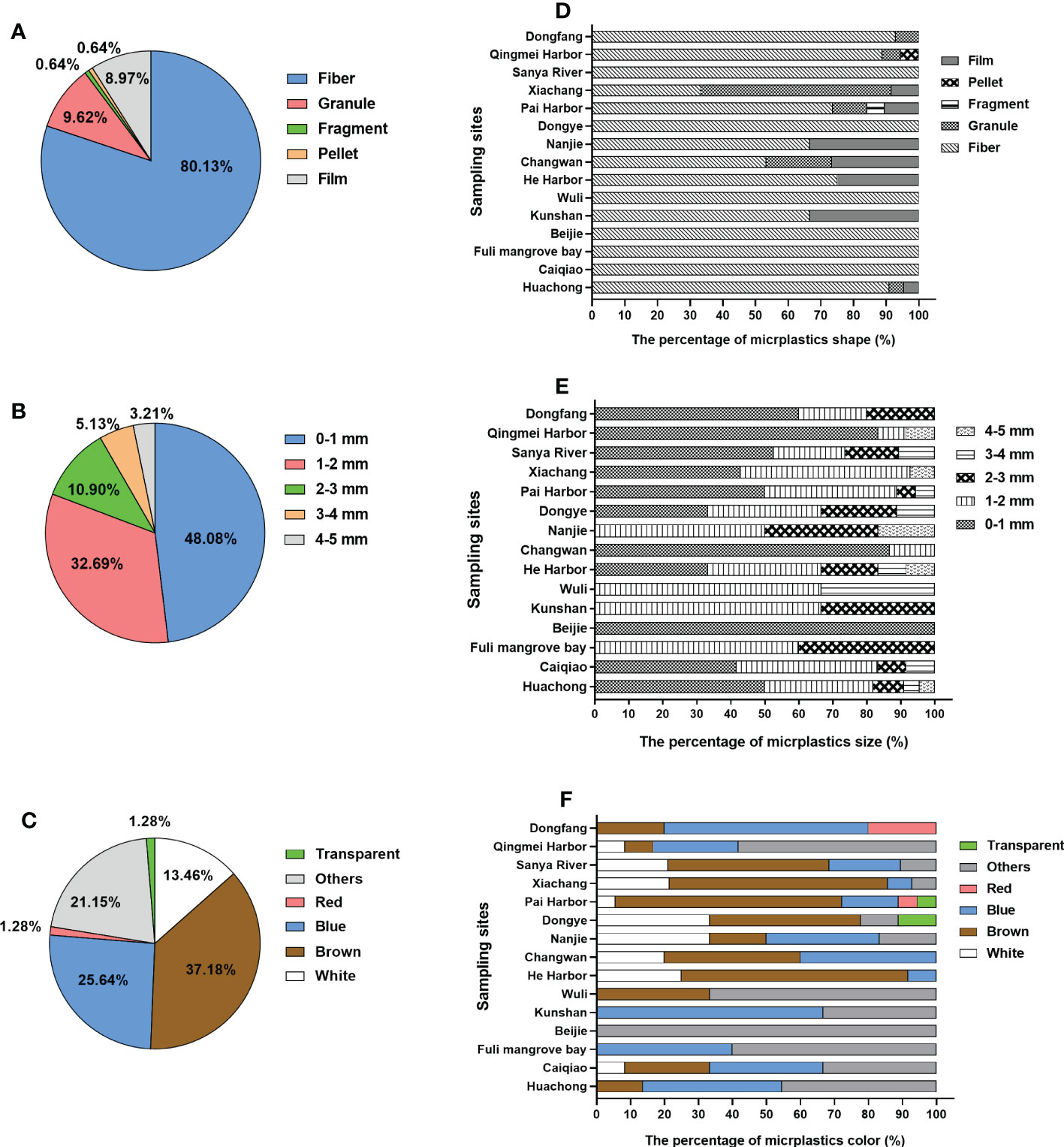
Figure 3 (A–C) Shape (A), size (B), and color (C) of the microplastics (MPs) in the benthos. (D–F) Distributions of the shape (D), size (E), and color (F) of the MPs in benthic species from the different sample sites in Hainan’s mangrove areas.
The sizes of the MPs in the present study were classified into five ranges, i.e., <1, 1–2, 2–3, 3–4, and 4–5 mm, which accounted for 48.08%, 32.69%, 10.90%, 5.13%, and 3.21% of the MPs, respectively (Figure 3B). The proportion of MP size in benthos from the Beijie site accounted for 100%, while Changwan contained more smaller-sized MPs (<1 mm, 86.61%), which also had the highest abundance of MP in benthic species (Figure 3E). The main size ranges were <1 mm (48.08%) and <2 mm (>80%), which were similar to the proportions of small MPs found in benthic species from other mangrove and coastal areas of China (Courtene-Jones et al., 2017; Wang et al., 2019; Filgueiras et al., 2020). The size range of the MPs can be explained by the feeding habits of benthic species (Bour et al., 2018). It has been reported that mussels are more likely to ingest smaller MPs (Qu et al., 2018).
In addition, the color can affect the ingestion of MPs by aquatic species (Filgueiras et al., 2020). MPs of five different colors—brown (37.18%), blue (25.64%), white (13.46%), red (1.28%), transparent (1.28%), and other artificial colors (21.15%)—were observed (Figure 3C). Among the examined species, brown and blue MPs were the predominant ingested items, similar to other studies on mussels (Digka et al., 2018) and sea snails (Courtene-Jones et al., 2017). The differences in the colors of the MPs in each site are shown in Figure 3F, with multicolor or brown and blue MPs being the most prevalent. Moreover, in the present study, crabs were the dominant species with the highest proportion of MPs (72.95%), followed by bivalves (22.95%) and snails (4.10%) (Figure 4A). The higher intake of small and colorful particles may be explained by their feeding habits, as crabs are visual predators and may confound plastic particles with their natural food (Nanninga et al., 2020).
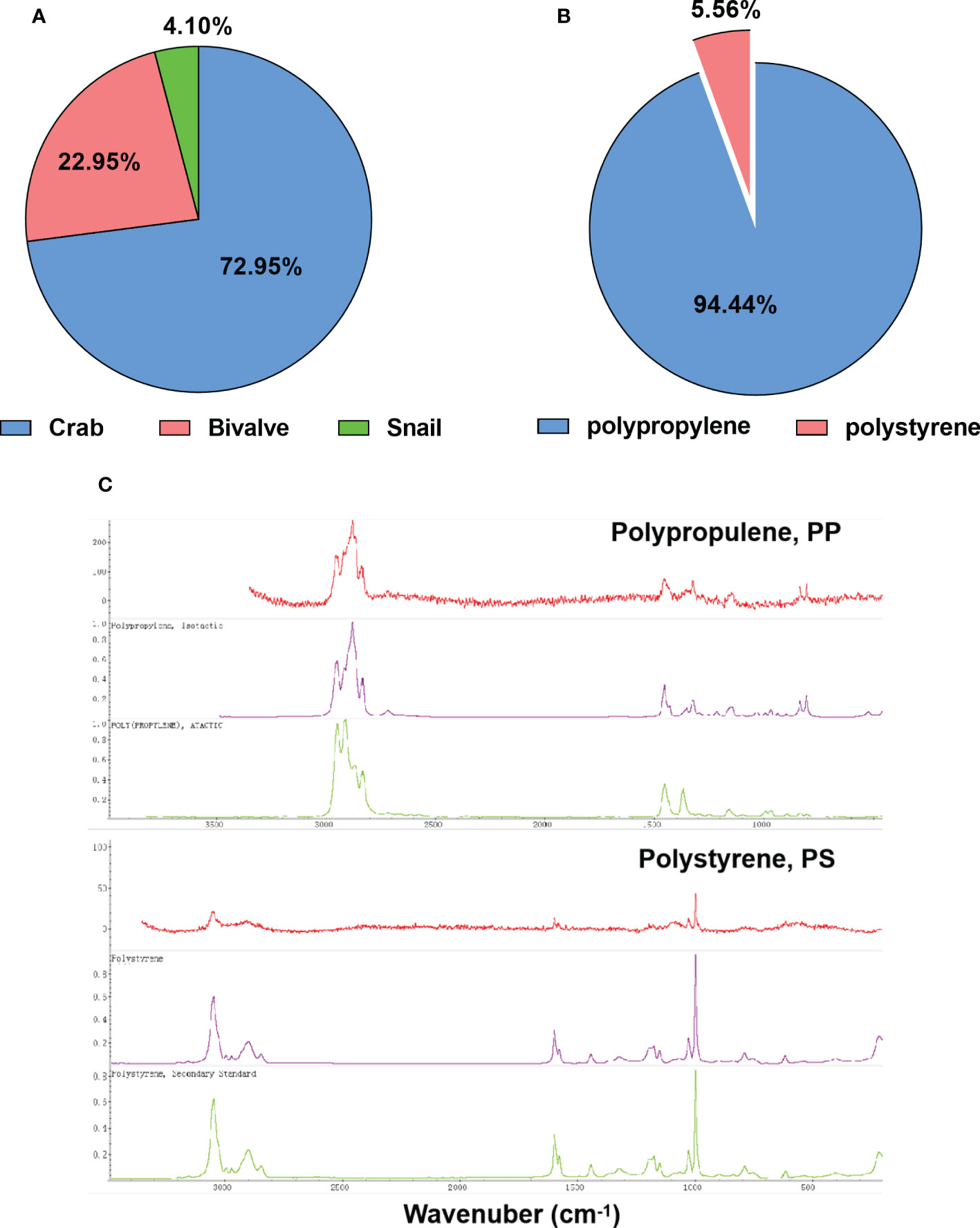
Figure 4 (A, B) Number and percentage of abundance of microplastics (MPs) (A) and polymer types (B) in the different benthic species. (C) Raman spectra of the selected MPs in benthic species.
A laser confocal microscope and a Raman spectrometer were used to identify the polymer types of the MPs ingested by the benthic species in Hainan’s mangrove wetland areas. As shown in Figures 4B, C, two types of MPs, polypropylene (PP) and polystyrene (PS), were identified. PP accounted for 94.44% of the MPs in benthic species, which was inconsistent with previous studies reporting that polyethylene (PE), polyamide (PA), and/or polyethylene terephthalate (PET) were the major polymer types of MPs in benthic organisms collected from other areas (Fang et al., 2018; Naji et al., 2018). On the contrary, other studies reported that PP fibers were the most abundant in sediments from the Beibu Gulf Sea (Xue et al., 2020). PP is commonly used in packaging, containers, pipes, textiles, and fishing equipment (Park et al., 2004; Cai et al., 2018). Around the mangrove region, there are a number of fish ports and mariculture sites in the harbor; concurrently, the mangrove wetlands are tourist areas. The present study suggests that artificial disturbance, including urban wastewater treatment, mariculture, and port transportation, might be the sources of MP contamination in the Hainan mangrove wetlands.
Mangrove ecosystems are important coastal resources that create unique ecological environments hosting various species. This study is the first to quantify MP pollution in the benthic species from Hainan’s mangrove wetlands. In this study, the MPs were extensively characterized in 10 benthic species collected from 15 sampling sites within seven typical mangrove wetlands in Hainan Island. The average abundance of MPs ranged between 0.83 ± 1.32 and 12.00 ± 0.00 items/individual in each sampling site. According to Raman analysis, most detected MPs were PP, with an abundance rate of 94.44%, mainly in the form of fibers (80.13%). Ingesting MPs and associated contaminants in other organisms through the food chain is a great risk to human health. Our results are indicative of the bioavailability of MPs to benthic marine organisms. Future studies on the abundance and distribution of MPs in various organisms from different geographical locations are needed to assess the risk of MPs to public health and ecosystems.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
QZ: Conceptualization, data curation, formal analysis, and writing—original draft. JX: Conceptualization, data curation, formal analysis, and writing—reviewing and editing. SM: Investigation, methodology, and data curation. YC and FL: Investigation. XD: Conceptualization, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
This project was funded by the Ministry of Science and Technology of the People’s Republic of China (no. 2017FY100703); the Key Research and Development Project of Hainan Province (nos. ZDYF2018122 and ZDYF2020178); the Key Science and Technology Program of Haikou City (no. 2017052); and the Initial Fund from Hainan University for R&D [KYQD(ZR)1870].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
de Souza Machado A. A., Kloas W., Zarfl C., Hempel S., Rillig M. C. (2018). Microplastics as an emerging threat to terrestrial ecosystems. Glob Chang Biol. 24 (4), 1405–1416. doi: 10.1111/gcb.14020
Bai J., Meng Y., Gou R., Lyu J., Dai Z., Diao X., et al. (2021). Mangrove diversity enhances plant biomass production and carbon storage in hainan island, China. Functional Ecology 35 (3), 774–786. doi: 10.1111/1365-2435.13753
Bour A., Avio C. G., Gorbi S., Regoli F., Hylland K. (2018). Presence of microplastics in benthic and epibenthic organisms: Influence of habitat, feeding mode and trophic level. Environ. pollut. 243, 1217–1225. doi: 10.1016/j.envpol.2018.09.115
Cai M., He H., Liu M., Li S., Tang G., Wang W., et al. (2018). Lost but can’t be neglected: Huge quantities of small microplastics hide in the south China Sea. Sci. Total Environ. 633, 1206–1216. doi: 10.1016/J.SCITOTENV.2018.03.197
Cai L., Wang J., Peng J., Tan Z., Zhan Z., Tan X., et al. (2017). Characteristic of microplastics in the atmospheric fallout from dongguan city, China: preliminary research and first evidence. Environ Sci Pollut Res. 24 (32), 24928–24935. doi: 10.1007/s11356-017-0116-x
Carlin J., Craig C., Little S., Donnelly M., Fox D., Zhai L., et al. (2020). Microplastic accumulation in the gastrointestinal tracts in birds of prey in central Florida, USA. Environ. pollut. 264, 114633. doi: 10.1016/J.ENVPOL.2020.114633
Chan H. S. H., Dingle C., Not C. (2019). Evidence for non-selective ingestion of microplastic in demersal fish. Mar. pollut. Bull. 149, 110523. doi: 10.1016/j.marpolbul.2019.110523
Chia R. W., Lee J.-Y., Kim H., Jang J. (2021). Microplastic pollution in soil and groundwater: a review. Environ. Chem. Lett. 19, 4211–4224. doi: 10.1007/s10311-021-01297-6
Cincinelli A., Scopetani C., Chelazzi D., Lombardini E., Martellini T., Katsoyiannis A., et al. (2017). Microplastic in the surface waters of the Ross Sea (Antarctica): Occurrence, distribution and characterization by FTIR. Chemosphere 175, 391–400. doi: 10.1016/J.CHEMOSPHERE.2017.02.024
Cordova M. R., Ulumuddin Y. I., Purbonegoro T., Shiomoto A. (2021). Characterization of microplastics in mangrove sediment of muara angke wildlife reserve, Indonesia. Mar. pollut. Bull. 163, 112012. doi: 10.1016/J.MARPOLBUL.2021.112012
Corradini F., Meza P., Eguiluz R., Casado F., Huerta-Lwanga E., Geissen V. (2019). Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 671, 411–420. doi: 10.1016/J.SCITOTENV.2019.03.368
Courtene-Jones W., Quinn B., Gary S. F., Mogg A. O. M., Narayanaswamy B. E. (2017). Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the rockall trough, north Atlantic ocean. Environ. pollut. 231, 271–280. doi: 10.1016/J.ENVPOL.2017.08.026
Cui Y., Liu M., Selvam S., Ding Y., Wu Q., Pitchaimani V. S., et al. (2022). Microplastics in the surface waters of the south China sea and the western pacific ocean: Different size classes reflecting various sources and transport. Chemosphere 299, 134456. doi: 10.1016/J.CHEMOSPHERE.2022.134456
Digka N., Tsangaris C., Torre M., Anastasopoulou A., Zeri C. (2018). Microplastics in mussels and fish from the northern Ionian Sea. Mar. pollut. Bull. 135, 30–40. doi: 10.1016/J.MARPOLBUL.2018.06.063
Ding J., Sun C., He C., Li J., Ju P., Li F. (2021). Microplastics in four bivalve species and basis for using bivalves as bioindicators of microplastic pollution. Sci. Total Environ. 782, 146830. doi: 10.1016/J.SCITOTENV.2021.146830
Di M., Wang J. (2018). PP microplastics in surface waters and sediments of the three gorges reservoir, China. Sci. Total Environ. 616–617, 1620–1627. doi: 10.1016/J.SCITOTENV.2017.10.150
European Commission. (2013). Green Paper on a European Strategy on Plastic Waste in the Environment. (European Commission: Brussels) 123, European Commission 7.3.2013 COM (2013) (2013), 1–20.
Fang C., Zheng R., Zhang Y., Hong F., Mu J., Chen M., et al. (2018). Microplastic contamination in benthic organisms from the Arctic and sub-Arctic regions. Chemosphere 209, 298–306. doi: 10.1016/J.CHEMOSPHERE.2018.06.101
Filgueiras A. V., Preciado I., Cartón A., Gago J. (2020). Microplastic ingestion by pelagic and benthic fish and diet composition: A case study in the NW Iberian shelf. Mar. pollut. Bull. 160, 111623. doi: 10.1016/J.MARPOLBUL.2020.111623
Fu Z., Wang J. (2019). Current practices and future perspectives of microplastic pollution in freshwater ecosystems in China. Sci. Total Environ. 691, 697–712. doi: 10.1016/J.SCITOTENV.2019.07.167
Gao L., Wang Z., Peng X., Su Y., Fu P., Ge C., et al. (2022). Occurrence and spatial distribution of microplastics, and their correlation with petroleum in coastal waters of hainan island, China. Environ. pollut. 294, 118636. doi: 10.1016/j.envpol.2021.118636
He D., Luo Y., Lu S., Liu M., Song Y., Lei L. (2018). Microplastics in soils: Analytical methods, pollution characteristics and ecological risks. TrAC Trends Anal. Chem. 109, 163–172. doi: 10.1016/J.TRAC.2018.10.006
Huang J. S., Koongolla J. B., Li H. X., Lin L., Pan Y. F., Liu S., et al. (2020). Microplastic accumulation in fish from zhanjiang mangrove wetland, south China. Sci. Total Environ. 708, 134839. doi: 10.1016/j.scitotenv.2019.134839
Huang D., Tao J., Cheng M., Deng R., Chen S., Yin L., et al. (2021). Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 407, 124399. doi: 10.1016/J.JHAZMAT.2020.124399
Jiang Y., Yang F., Hassan Kazmi S. S. U., Zhao Y., Chen M., Wang J. (2022). A review of microplastic pollution in seawater, sediments and organisms of the Chinese coastal and marginal seas. Chemosphere 286, 131677. doi: 10.1016/J.CHEMOSPHERE.2021.131677
Liu K., Wang X., Fang T., Xu P., Zhu L., Li D. (2019). Source and potential risk assessment of suspended atmospheric microplastics in shanghai. Sci. Total Environ. 675, 462–471. doi: 10.1016/J.SCITOTENV.2019.04.110
Li J., Yang D., Li L., Jabeen K., Shi H. (2015). Microplastics in commercial bivalves from China. Environ. pollut. 207, 190–195. doi: 10.1016/J.ENVPOL.2015.09.018
Li J., Zhang H., Zhang K., Yang R., Li R., Li Y. (2018). Characterization, source, and retention of microplastic in sandy beaches and mangrove wetlands of the qinzhou bay, China. Mar. pollut. Bull. 136, 401–406. doi: 10.1016/J.MARPOLBUL.2018.09.025
Li R., Zhang S., Zhang L., Yu K., Wang S., Wang Y. (2020). Field study of the microplastic pollution in sea snails (Ellobium chinense) from mangrove forest and their relationships with microplastics in water/sediment located on the north of beibu gulf. Environ. pollut. 263, 114368. doi: 10.1016/J.ENVPOL.2020.114368
Lovelock C. E., Duarte C. M. (2019). Dimensions of blue carbon and emerging perspectives. Biol. Lett. 15, 23955–26900. doi: 10.1098/rsbl.2018.0781
Meng Y., Kelly F. J., Wright S. L. (2020). Advances and challenges of microplastic pollution in freshwater ecosystems: A UK perspective. Environ. pollut. 256, 113445. doi: 10.1016/J.ENVPOL.2019.113445
Mintenig S. M., Löder M. G. J., Primpke S., Gerdts G. (2019). Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 648, 631–635. doi: 10.1016/J.SCITOTENV.2018.08.178
Mohamed Nor N. H., Obbard J. P. (2014). Microplastics in singapore’s coastal mangrove ecosystems. Mar. pollut. Bull. 79, 278–283. doi: 10.1016/J.MARPOLBUL.2013.11.025
Munno K., Helm P. A., Jackson D. A., Rochman C., Sims A. (2018). Impacts of temperature and selected chemical digestion methods on microplastic particles. Environ. Toxicol. Chem 37 (1), 91–98. doi: 10.1002/etc.3935
Naji A., Nuri M., Vethaak A. D. (2018). 18 microplastics contamination in molluscs from the northern part of the Persian gulf. Environ. pollut. 235, 113–120. doi: 10.1016/J.ENVPOL.2017.12.046
Nanninga G. B., Horswill C., Lane S. M., Manica A., Briffa M. (2020). Microplastic exposure increases predictability of predator avoidance strategies in hermit crabs. J. Hazard. Mater. Lett. 1, 100005. doi: 10.1016/J.HAZL.2020.100005
Nie H., Wang J., Xu K., Huang Y., Yan M. (2019). Microplastic pollution in water and fish samples around nanxun reef in nansha islands, south China Sea. Sci. Total Environ. 696, 134022. doi: 10.1016/J.SCITOTENV.2019.134022
Park C. H., Kang Y. K., Im S. S. (2004). Biodegradability of cellulose fabrics. J Appl Polym Sci. 94 (1), 248–253. doi: 10.1002/app.20879
Pequeno J., Antunes J., Dhimmer V., Bessa F., Sobral P., Panti C., et al. (2021). Microplastics in marine and estuarine species from the coast of Portugal. Front. Environ. Sci. | www.frontiersin.org 9. doi: 10.3389/fenvs.2021.579127
Qiu Y. W., Yu K. F., Zhang G., Wang W. X. (2011). Accumulation and partitioning of seven trace metals in mangroves and sediment cores from three estuarine wetlands of hainan island, China. J. Hazard. Mater. 190, 631–638. doi: 10.1016/J.JHAZMAT.2011.03.091
Qu X., Su L., Li H., Liang M., Shi H. (2018). Assessing the relationship between the abundance and properties of microplastics in water and in mussels. Sci. Total Environ. 621, 679–686. doi: 10.1016/J.SCITOTENV.2017.11.284
Ryan D. A. J. (2004). Point sampling strategies for estimating coverage from benthic video transects. Environmetrics 15, 193–207. doi: 10.1002/env.634
Santillo D., Miller K., Johnstony P. (2017). Microplastics as contaminants in commercially important seafood species. Integr. Env. Assess. Manag 13, 516–521. doi: 10.1002/ieam.1909
Savoca S., Bottari T., Fazio E., Bonsignore M., Mancuso M., Luna G. M., et al. (2020). Plastics occurrence in juveniles of engraulis encrasicolus and sardina pilchardus in the southern tyrrhenian Sea. Sci. Total Environ. 718, 137457. doi: 10.1016/J.SCITOTENV.2020.137457
Sequeira I. F., Prata J. C., da Costa J. P., Duarte A. C., Rocha-Santos T. (2020). Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 160, 111681. doi: 10.1016/j.marpolbul.2020.111681
Teng J., Wang Q., Ran W., Wu D., Liu Y., Sun S., et al. (2019). Microplastic in cultured oysters from different coastal areas of China. Sci. Total Environ. 653, 1282–1292. doi: 10.1016/J.SCITOTENV.2018.11.057
Wang W., Ndungu A. W., Li Z., Wang J. (2017). Microplastics pollution in inland freshwaters of China: A case study in urban surface waters of wuhan, China. Sci. Total Environ. 575, 1369–1374. doi: 10.1016/J.SCITOTENV.2016.09.213
Wang J., Wang M., Ru S., Liu X. (2019). High levels of microplastic pollution in the sediments and benthic organisms of the south yellow Sea, China. Sci. Total Environ. 651, 1661–1669. doi: 10.1016/J.SCITOTENV.2018.10.007
Welden N. A., Abylkhani B., Howarth L. M. (2018). The effects of trophic transfer and environmental factors on microplastic uptake by plaice, pleuronectes plastessa, and spider crab, maja squinado. Environ. pollut. 239, 351–358. doi: 10.1016/J.ENVPOL.2018.03.110
Woodall L. C., Sanchez-Vidal A., Canals M., Paterson G. L. J., Coppock R., Sleight V., et al. (2014). The deep sea is a major sink for microplastic debris. R. Soc Open Sci. 1 (4), 140317. doi: 10.1098/rsos.140317
Wright S. L., Rowe D., Thompson R. C., Galloway T. S. (2013). Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 23, R1031–R1033. doi: 10.1016/J.CUB.2013.10.068
Wu P., Zhang J., Ma Y., Li X. (2013). Remote sensing monitoring and analysis of the changes of mangrove resource in China in the past 20 years. Adv. Mar. Sci, 31, 406–414.
Xue B., Zhang L., Li R., Wang Y., Guo J., Yu K., et al. (2020). Underestimated microplastic pollution derived from fishery activities and “Hidden” in deep sediment. Environ. Sci. Technol. 54, 2210–2217. doi: 10.1021/acs.est.9b04850
Yang L., Zhang Y., Kang S., Wang Z., Wu C. (2021). Microplastics in freshwater sediment: A review on methods, occurrence, and sources. Sci. Total Environ. 754, 141948. doi: 10.1016/J.SCITOTENV.2020.141948
Yin J., Li J. Y., Craig N. J., Su L. (2022). Microplastic pollution in wild populations of decapod crustaceans: A review. Chemosphere 291, 132985. doi: 10.1016/J.CHEMOSPHERE.2021.132985
Zhang D., Liu X., Huang W., Li J., Wang C., Zhang D., et al. (2020). Microplastic pollution in deep-sea sediments and organisms of the Western pacific ocean. Environ. pollut. 259, 113948. doi: 10.1016/J.ENVPOL.2020.113948
Zhou Q., Tu C., Fu C., Li Y., Zhang H., Xiong K., et al. (2020). Characteristics and distribution of microplastics in the coastal mangrove sediments of China. Sci. Total Environ. 703, 134807. doi: 10.1016/J.SCITOTENV.2019.134807
Keywords: microplastics, mangrove wetland, invertebrates, contaminates, Hainan Island
Citation: Zhang Q, Xie J, Ma S, Chen Y, Lin F and Diao X (2023) Occurrenceand characteristics of microplastics in benthic species from mangrove wetlands of Hainan, South China. Front. Mar. Sci. 10:965059. doi: 10.3389/fmars.2023.965059
Received: 09 June 2022; Accepted: 13 January 2023;
Published: 01 February 2023.
Edited by:
Guangxu Liu, Zhejiang University, ChinaReviewed by:
Jinfeng Ding, Ocean University of China, ChinaCopyright © 2023 Zhang, Xie, Ma, Chen, Lin and Diao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia Xie, anhpZUBoYWluYW51LmVkdS5jbg==; Xiaoping Diao, ZGlhb3hpcEBoYWluYW51LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.